Abstract
Background:
Recent studies have suggested that vaccine-induced protection against influenza may decline within one season. We reanalyzed data from a study of influenza vaccine effectiveness to determine if time since vaccination was an independent predictor of influenza A (H3N2).
Methods:
Patients with acute respiratory illness were actively recruited during the 2007–2008 season. Respiratory swabs were tested for influenza, and vaccination dates were determined by a validated immunization registry. The association between influenza RT-PCR result and vaccination interval (days) was examined using multivariable logistic regression, adjusting for calendar time, age and other confounders.
Results:
There were 629 vaccinated participants, including 177 influenza A (H3N2) cases and 452 test negative controls. The mean (SD) interval from vaccination to illness onset was 101.7 (25.9) days for influenza cases and 93.0 (29.9) days for controls. There was a significant association between vaccination interval and influenza result in the main effects model. The adjusted odds ratio (aOR) for influenza was 1.12 (CI 1.01, 1.26) for every 14 day increase in the vaccination interval. Age modified the association between vaccination interval and influenza (p = 0.005 for interaction). Influenza was associated with increasing vaccination interval in young children and older adults, but not in adolescents or non-elderly adults. Similar results were found when calendar week of vaccine receipt was assessed as the primary exposure variable.
Conclusions:
Identification of influenza A (H3N2) was associated with increasing time since vaccination among young children and older adults during a single influenza season.
Keywords: Influenza, Influenza vaccine, Immunity, Vaccine effectiveness
1. Introduction
Annual influenza vaccination is a key component of influenza prevention and control efforts in the United States. In most seasons, trivalent inactivated influenza vaccine (TIV) provides moderate protection against influenza illness in healthy adults [1], and a hemagglutination inhibition (HI) titer of 1:40 or greater has been associated with clinical protection [2-4]. A systematic review of published studies found that seroprotective titers against influenza A were maintained for >4 months after immunization in almost all studies [5], but recent reports have raised concerns that vaccine induced protection against influenza illness may decline over the course of a single season [6-8].
The goal of this study was to assess evidence for waning protection against influenza A (H3N2) in a community cohort. To do this, we reanalyzed data from an observational study of influenza vaccine effectiveness that was performed during the 2007–2008 influenza season. Vaccine effectiveness against influenza A (H3N2) was 41% in the study population during that season [9]. The Marshfield Clinic Research Foundation has conducted annual assessments of influenza vaccine effectiveness in Wisconsin since 2005, but this analysis focused on the 2007–2008 season because the number of influenza A (H3N2) cases was substantially higher compared to other seasons.
2. Methods
The source population included community-dwelling residents ≥6 months old living in or near Marshfield, Wisconsin [10]. Patients in this population were screened and enrolled by trained research coordinators during or after an encounter for acute respiratory illness with symptoms of feverishness, chills, or cough. Potential participants with illness duration >7 days were excluded to minimize false negative influenza test results. Enrollment occurred in primary care departments at the Marshfield Clinic main campus, a nearby satellite clinic, and an acute care hospital.
Each participant or parent was interviewed to determine illness onset date. Nasopharyngeal (adults and adolescents) or nasal swabs (children< 12 years) were obtained and tested for influenza by reverse transcription polymerase chain reaction (RT-PCR). Participants were classified as having a high-risk health condition if they had ≤2 medical visits during 2007 with a relevant ICD-9 diagnosis code (list of codes available on request).
Enrollment in the study began on January 21, 2008 based on laboratory identification of influenza at the local clinical laboratory and the Wisconsin State Laboratory of Hygiene. Enrollment continued for 10 weeks, ending on March 28, 2008.
2.1. Laboratory methods
Swabs were placed in M4-RT viral transport media and delivered to the Marshfield Clinic Research Foundation laboratory on the same day. Samples were routinely processed within one day, and weekend samples were tested on Monday. Nucleic acid was extracted using the Roche MagNA Pure Total Nucleic Acid Kit (Roche Diagnostics, Indianapolis, Indiana), and RT-PCR was performed using the LightCycler® Real-Time PCR System (Roche Diagnostics, Basel, Switzerland). The U.S. Centers for Disease Control and Prevention provided sequence information for RT-PCR primers and probes. The TaqMan®-based RT-PCR assay detects two highly-conserved influenza genes: the matrix gene of influenza A and the non-structural gene of influenza B. A human RNase P gene served as a positive control for human nucleic acid. Virus subtyping by RT-PCR was performed on all samples with a positive influenza A result.
2.2. Influenza vaccination status and dates
Vaccination status and dates were determined by a real-time, internet-based registry used by all immunization providers serving the local population (www.recin.org). The capture of the registry was validated during the 2006–2007 and 2007–2008 influenza seasons, and after adjudication it was found that the registry captured 95% of all influenza vaccinations received by study participants [11]. For this analysis, participants were considered immunized if a dose of vaccine was received ≥14 days before illness onset. Children under the age of nine were recommended to receive two doses of influenza vaccine. Partially vaccinated children who received only one of two recommended doses were excluded from the analysis. For fully vaccinated children, the most recent dose received prior to illness onset was used to determine the interval from vaccination to illness. Only trivalent inactivated vaccine was evaluated, and the Marshfield Clinic did not administer live attenuated influenza vaccine during the 2007–2008 season.
The study was approved by the Marshfield Clinic Institutional Review Board and all participants provided written informed consent.
2.3. Analytic approach
We tested the hypothesis that RT-PCR confirmed influenza A was independently associated with a longer interval from influenza vaccination to illness onset after adjustment for calendar time, age and other potential confounders. The analyses were restricted to vaccinated adults and children because a vaccination interval cannot be calculated for unvaccinated individuals. We did not attempt to directly calculate vaccine effectiveness for different vaccination intervals because it would require inclusion of the unvaccinated group, and the precision of vaccine effectiveness estimates was expected to be low for time windows before and after the epidemic peak. In contrast, the analysis of vaccination intervals allowed for detection of small differences in time from vaccination to illness onset for cases and controls after adjusting for the effect of calendar time and age.
The outcome variable was a positive RT-PCR test result for influenza A (H3N2) (cases) vs. a negative result (test negative controls). We excluded individuals with influenza B infection because there were relatively few cases of influenza B, and differences in the temporal occurrence of influenza A and B could be a source of confounding. Although the study design allowed multiple enrollments per person for distinct illness episodes, we included only the first enrollment for each person unless influenza was detected on a subsequent enrollment. In that case, we included the influenza positive enrollment and excluded other enrollments for the same person. We also repeated the primary analysis after exclusion of all individuals with multiple enrollments to ensure that results were not biased by including influenza infections that occurred during a second illness episode.
The multivariable logistic regression model assessed the association between vaccination interval (days from vaccination to illness onset) and probability of influenza. The main predictor was the interval (days) from vaccination to illness onset. The relationship between age and influenza result was nonlinear (Supplemental Figure S1), and we included covariates for age and age squared in the model. The timing of illness onset was analyzed as a series of indicator variables representing 2 week time periods in the model. Each period was compared to the referent period of weeks 7–8 (representing the peak of influenza occurrence in the community).
We examined the association between vaccination interval and influenza using two different models. A main effects model included the primary exposure (days from vaccination to illness onset) and potential confounders. These included sex, interval between illness onset and enrollment (days), and presence or absence of a high-risk health condition. Effect modification was examined with the addition of interaction terms for vaccination interval (days) and each covariate. Each interaction term was evaluated separately, and covariate and interaction terms were included in the final reduced model if they were significantly associated with influenza (p< 0.05) or changed the point estimate for the primary exposure by more than 10%. Since age was modeled as a continuous variable in this analysis, we used the model results to illustrate the relationship between vaccination interval and log-odds of influenza at six arbitrarily selected ages.
We performed a secondary analysis using calendar week of influenza vaccine receipt rather than vaccination interval as the exposure of interest. The model covariates were the same as in the primary analysis. All analytical procedures were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
3. Results
There were 1972 enrollments representing 1955 unique patients during the 2007–2008 influenza season. For the analysis of waning protection against influenza A (H3N2), we excluded study participants with influenza B (n = 233), unvaccinated individuals (n = 1088), and five participants who received influenza vaccine within 14 days before illness onset. During this season there were an additional 17 individuals (<1%) who were enrolled twice for independent illness episodes, including 4 who were positive for influenza during the second enrollment only. The second enrollment was included in the analysis for those four individuals and the first enrollment was used for the other 13. The final analysis included 629 vaccinated study participants; 599 (95%) were enrolled in the outpatient setting and 30 (5%) were enrolled as inpatients.
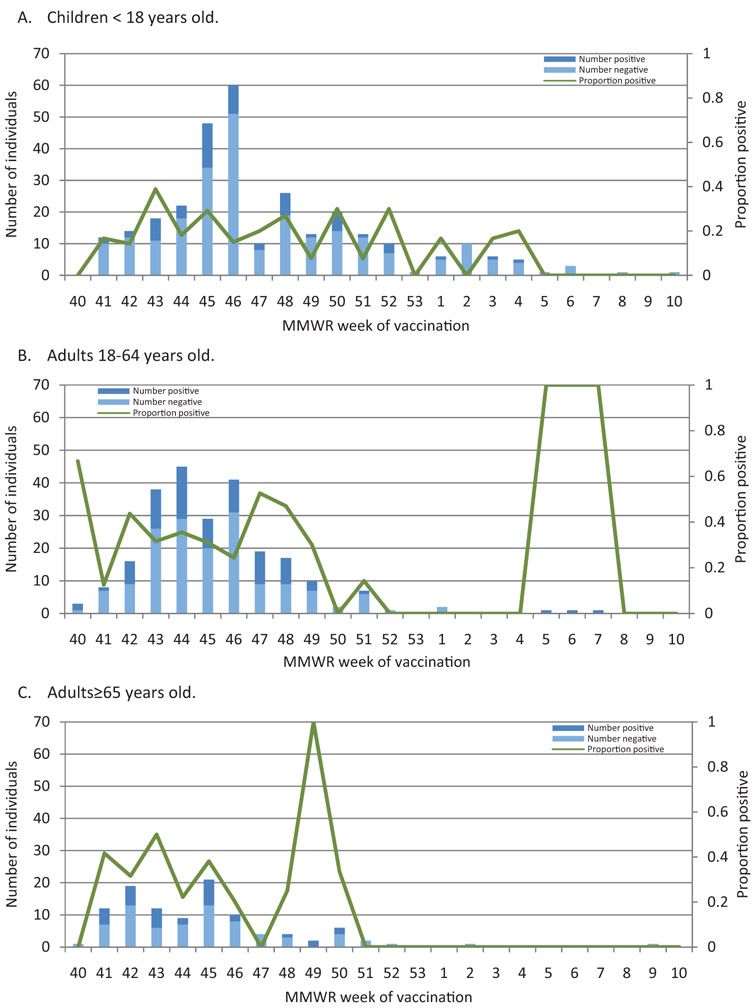
Vaccination dates ranged from 10/1/2007 (week 40) to 3/4/2008 (week 10); vaccine administration peaked in mid-November 2007. The mean age (±SD) was 30.6 (±27.9) years, and the mean interval from illness onset to enrollment was 3.3 (±1.9) days. There were 177 (28%) influenza A cases confirmed by RT-PCR. Study enrollment was initiated in January before influenza activity increased in the community, and the epidemic peak occurred in February (Supplemental Figure S2). Fig. 1 shows the number of influenza positives and negatives by week of vaccination in different age groups.
Fig. 1.
Number of influenza positives and negatives by week of vaccination, according to age.
(A) Children < 18 years old. (B) Adults 18–64 years old. (C) Adults ≥65 years old.
Influenza cases and test negative controls differed significantly by age, interval from vaccination to illness onset, interval from study initiation (January 21) to illness onset, and timing of illness onset relative to the epidemic peak (Table 1). The median date of vaccination was November 6, 2007 for those with RT-PCR confirmed influenza and November 12, 2007 for test negative controls.
Table 1.
Demographic and clinical features of study participants with RT-PCR confirmed influenza A (H3N2) (cases) and non-influenza respiratory illness (controls) who received influenza vaccine at least 14 days before illness onset.
| Characteristic | Vaccinated cases (N = 177) | Vaccinated test negative controls (N = 452) | P-valuea |
|---|---|---|---|
| Age, years, N (%) | |||
| 0–2 | 18 (10.2) | 113 (25.0) | 0.0004 |
| 3–5 | 23 (13.0) | 59 (13.1) | |
| 6–20 | 21 (11.9) | 56 (12.4) | |
| 21–50 | 57 (32.2) | 94 (20.8) | |
| 51–65 | 26 (14.7) | 68 (15.0) | |
| 66–75 | 19 (10.7) | 26 (5.8) | |
| >75 | 13 (7.3) | 36 (8.0) | |
| Male, N (%) | 77 (43.5) | 197 (43.6) | 0.99 |
| High risk condition, N (%) | 53 (29.9) | 134 (29.7) | 0.94 |
| Illness onset to swab interval, days (mean ± SD) | 3.0 ±1.8 | 3.4 ±2.0 | 0.005 |
| Vaccination to illness onset interval, days (mean ± SD) | 101.7 ±25.9 | 93.0 ± 29.9 | 0.0008 |
| Study onset to illness onset interval, days (mean ± SD) | 30.5 ±15.7 | 26.7 ±18.6 | 0.02 |
| MMWR week of vaccine receipt (mean ± SD) | 45.6 ± 3.3 | 46.3 ±3.7 | 0.03 |
| Illness onset period: N (%) | |||
| Influenza peak period (Feb 3-Mar 1) | 114 (64.4) | 207 (45.8) | <0.0001 |
| Pre-peak period (Jan 14-Feb 2) | 22 (12.4) | 125 (27.7) | |
| Post-peak period (Mar 2-Mar 27) | 41 (23.2) | 120 (26.6) | |
P-values shown are chi-square or t-test values for categorical or continuous variables, respectively.
In the univariate analysis, influenza A (H3N2) was significantly associated with the interval (days) from vaccination to illness onset. The unadjusted odds ratio for influenza was 1.16 (CI 1.06, 1.27; p = 0.001) for every 14 day increase in the vaccination interval. There was also a significant association between influenza and interval from vaccination to illness onset in the main effects model without interaction terms. The adjusted odds ratio (aOR) for influenza was 1.12 (CI 1.01, 1.26) for every 14 day increase in the vaccination interval. In the primary multivariable model that included significant interactions, influenza was independently associated with illness onset week, interval from illness onset to enrollment, age (quadratic function represented by age and age squared), and interval from vaccination to illness onset (Table 2). Effect modification by age was observed with significant interaction terms for age-by-vaccination interval (p = 0.005) and age-squared-by-vaccination interval (p = 0.005). There were no other significant interactions. The association between vaccination interval and influenza was nearly identical in the main effects model after excluding the 17 individuals who were each enrolled twice, and the same effect modification by age was observed (data not shown).
Table 2.
Multivariable logistic regression model for confirmed influenza A (H3N2) and days from vaccination to illness onset (primary exposure). The final reduced model is shown with inclusion of all variables significantly associated with influenza (p < 0.05).
| Model parameters | Rl-PCRconfirmed influenza A (H3N2) |
|||
|---|---|---|---|---|
| Ba | OR | CI | p | |
| Intercept | −2.022 | - | - | 0.002 |
| Illness onset week | ||||
| 1–2 | −14.355 | 0.000 | 0.000, >999.0 | 0.975 |
| 3–4 | −0.773 | 0.461 | 0.254, 0.837 | 0.011 |
| 5–6 | 0.021 | 1.021 | 0.634, 1.644 | 0.933 |
| 7–8 | Ref | Ref | Ref | Ref |
| 9–10 | −0.820 | 0.441 | 0.246, 0.789 | 0.006 |
| 11–12 | −0.356 | 0.701 | 0.351, 1.401 | 0.314 |
| Days from illness onset to enrollment | −0.130 | 0.878 | 0.796, 0.970 | 0.010 |
| Age (years) | 0.166 | 1.181 | 1.068, 1.305 | 0.001 |
| Age-squared | −0.002 | 0.998 | 0.996, 0.999 | 0.002 |
| Vaccination interval (days)b | 0.015 | 1.016 | 1.003, 1.028 | 0.016 |
| Interaction: Vaccination interval × Age | −0.001 | 0.999 | 0.998, 1.000 | 0.005 |
| Interaction: Vaccination interval × Age-squared | <0.001 | 1.000 | 1.000, 1.000 | 0.005 |
B values are equal to (natural) logarithmic odds ratio of RT-PCR confirmed influenza A infection relative to the reference category. Positive values indicate that as the predictor variable changes (relative to the reference category for categorical predictors or a 1-unit increase for continuous predictors), the odds of RT-PCR confirmed influenza A increases. Negative values indicate that as the predictor variable changes (relative to the reference category for categorical predictors or a 1-unit increase for continuous predictors), the odds of RT-PCR confirmed influenza A decreases.
Vaccination interval = number of days from vaccination to illness onset.
Since age was included in the model as a continuous variable, we arbitrarily selected six different ages to illustrate the observed association between increasing vaccination interval and odds of influenza (Fig. 2). This demonstrated that individuals at the extremes of age had greater odds of influenza with increasing vaccination interval, but this effect was not observed for older children or non-elderly adults.
Fig. 2.
Adjusted odds ratios and 95% confidence intervals for influenza A positive result per 14 day increase in vaccination interval at selected ages. Models were adjusted for calendar time (illness onset week) and interval from illness onset to swab collection.
To minimize the potential for disease misclassification (false negative RT-PCR), we performed a sensitivity analysis that excluded 161 patients who were enrolled ≥5 days after illness onset. Results were similar to the primary analysis, and significant effect modification between age and vaccination interval was observed. We also performed a sensitivity analysis that excluded 30 individuals who were enrolled as hospital inpatients, since the relationship between vaccination interval and influenza may not be the same for outpatients and inpatients. When the analysis was restricted to outpatient enrollments, the results were nearly identical to the primary analysis that included all participants, and significant effect modification between age and vaccination interval was still present.
We repeated the primary analysis with calendar week of vaccination as the exposure of interest rather than vaccination interval. In this analysis, the exposure was based entirely on calendar time and differences in the date of illness onset did not affect the exposure variable (although the model adjusted for timing of illness onset). Results from this multivariable model were similar to those from the primary analysis. Influenza vaccinations received during earlier weeks were associated with a higher odds of influenza compared to vaccinations received during later weeks after adjustment for potential confounders. A significant interaction was observed between vaccination week and age.
4. Discussion
In this study we identified a significant association between influenza A (H3N2) positive medically attended ARI visits and increasing time since vaccination among young children and elderly adults. The magnitude of the effect was modest, and it was observed only at the extremes of age. For example, the adjusted odds of influenza increased by 1.2 for each two week increase in the vaccination interval in two year old children. Similarly, for a 75 year old adult, the odds of influenza increased by 1.3 for each two week increase in the vaccination interval. We did not see any significant association between influenza and vaccination interval in older children, adolescents, and non-elderly adults. We observed a similar association between calendar week of vaccination and risk of influenza, with the highest risk in people who were vaccinated earlier in the season. These results are consistent with a linear relationship between vaccination interval and risk of influenza, at least at the extremes of age, although we cannot rule out a nonlinear relationship with a threshold effect.
The increasing risk of influenza over time may be related to changes in host immune response or a combination of host and virus-related characteristics. The H3N2 component of the 2007–2008 northern hemisphere influenza vaccine was A/Wisconsin/67/2005-like, and a minor antigenic variant (distinct from the vaccine strain) was identified in the United States and our study population. In the United States, this minor variant (A/Brisbane/10/2007-like) comprised 71% of A (H3N2) viruses characterized in 2007–2008, (http://www.cdc.gov/flu/weekly/weeklyarchives2007-2008/weekly14.htm). This variant was present in 14 of 24 viruses that were evaluated from study participants. All but one of the characterized viruses were obtained before February 10, suggesting that the A/Brisbane/10/2007-like virus was circulating in our study population during the early season, rather than emerging during or after the epidemic peak. However, the number of characterized viruses in our study population was small, and we could not assess changes in the prevalence of antigenic variant viruses during the season. We cannot rule out the possibility that antigenic drift contributed to the increased risk of influenza with increasing time since vaccination. However, we would not expect to see differential effects by age group if virus evolution was the only factor contributing to the changing risk of influenza over time.
Evidence from randomized clinical trials suggests that vaccine induced protection from TIV can diminish within one season, at least in young children. A re-analysis of three pediatric clinical trials comparing TIV and LAIV has shown that the relative efficacy of TIV against antigenically similar strains declined with increasing time since vaccination [12]. The relative efficacy of LAIV vs. TIV for preventing illness due to matched strains increased from 0 to 4 months post-vaccination (range, 25–60%) to >4–8 months post-vaccination (range, 49–89%). However, similar efficacy was seen in each time interval for mismatched strains.
During the 2011–2012 season, observational studies of vaccine effectiveness against A (H3N2) infections in Europe have also provided some evidence consistent with waning protection. In the United Kingdom, adjusted vaccine effectiveness was 53% for individuals vaccinated less than three months before illness onset, and 12% for those vaccinated three months or more before illness onset (p = 0.02, test for trend) [7]. In the Navarre region of Spain, adjusted vaccine effectiveness against A (H3N2) was 61% in the first 100 days after vaccination, 39% between 100 and 119 days, and zero after 120 days [8]. Among patients ≥65 years old, vaccine effectiveness against all influenza strains was 85% and 24%, respectively, for those who became ill <100 days after vaccination and 100–119 days after vaccination. However, the sample size was sparse and the confidence interval included zero for both estimates. The I-MOVE project assessed waning immunity during the 2011–2012 season based on enrollments in eight European Union member states [6]. In a pooled analysis, the adjusted vaccine effectiveness against A (H3N2) was 46.8% and 10.5%, respectively, among patients with illness onset less than 93 days after vaccination and those with illness beginning 93 days or more after vaccination. The interpretation of waning immunity all three studies was complicated by the emergence of antigenic variant A (H3N2) viruses in Europe as the season progressed.
The biologic mechanisms that may contribute to waning protection are uncertain, but they likely differ for children and older adults. Most young children have not been primed by natural infection and the immune system is immature. In older adults, immunosenescence occurs with reduced cytotoxicity of natural killer (NK) cells, reductions in macrophages and dendritic cells, reduced pathogen sensor expression and function, decreased pools of naive T and B cells, and greater numbers of memory and effector T and B cells [13,14]. It is uncertain how these age-related changes in immune response might contribute to waning protection over a period of weeks or months. Although HI antibody titers are maintained for several months in older adults, changes in T cell function may also influence the duration of vaccine mediated protection. Influenza vaccination stimulates a cellular immune response in the elderly, and there is some evidence that measures of cellular immunity may be better correlates of vaccine protection in the elderly compared to HI antibody titers [15,16]. However, it is not known if those responses vary over the course of an influenza season.
The CDC Advisory Committee on Immunization Practices (ACIP) currently recommends that vaccine providers in the United States should begin offering vaccination soon after vaccines becomes available and, if possible, by October [17]. There has been a trend toward earlier vaccination since the 2009 pandemic, and influenza vaccines may be available for administration as early as August [18]. As a result, the interval from vaccination to the peak of the influenza season (i.e., time of highest risk of influenza exposure) could be as long as 7 or 8 months for individuals who are vaccinated very early. Results from this study, which was restricted to a single community and season, do not justify changes to the current recommendations. However, the findings from this study and others indicate the need for further research on waning protection in different seasons and populations.
Strengths of this study include the systematic recruitment from a population cohort using well-defined screening criteria in the out-patient and hospital setting, ascertainment of illness onset dates, use of a highly sensitive and specific molecular test for influenza detection, restriction to a specific influenza A subtype, and access to detailed information on vaccine administration. The major limitations include a relatively small number of cases for age stratified analyses, and the co-circulation of both vaccine matched and antigenic variant A (H3N2) viruses in the study population. We cannot rule out the possibility of residual confounding due to unmeasured characteristics associated both with early vaccination receipt and risk of influenza. We were also unable to assess heterogeneity in the study population as a potential source of bias. It is uncertain if the findings can be generalized to other H3N2 seasons or infections due to other types or subtypes. Analysis of waning protection across multiple seasons was not feasible due to fewer cases in other seasons, changing vaccine formulations, and virus antigenic drift.
In conclusion, we observed a significant association with influenza A (H3N2) positive medically attended ARI visits with increasing time since vaccination over the course of a single season in children and older adults. Antigenic drift and host factors may have contributed to this phenomenon, and further research is needed to confirm these findings.
Supplementary Material
Acknowledgements
We thank the following individuals for their contributions to this work: Megan Elderbrook, Stephanie Irving, David Parfitt, Pam Squires, Sarah Kopitzke, Mary Vandermause, Donna David, Tamara Koepel, and Lynn Ivacic. We also thank the research coordinators who enrolled patients in the study, and the clinical staff at Marshfield Clinic for their support of this study.
Funding
This research was funded by a cooperative agreement from the U.S. Centers for Disease Control and Prevention (5U01IP000471). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Abbreviations:
- HI
hemagglutinin antibody inhibition
- TIV
trivalent inactivated influenza vaccine
- RT-PCR
reverse transcription polymerase chain reaction
- NK
natural killer
- ACIP
Advisory Committee on Immunization Practices
Footnotes
Conflict of interest
Dr. Belongia, Dr. Meece and Ms. Sundaram receive research support from MedImmune LLC. Other authors have no financial disclosures.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2014.06.052.
References
- [1].Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:36–44. [DOI] [PubMed] [Google Scholar]
- [2].Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis 2011;204(December (12)):1879–85. [DOI] [PubMed] [Google Scholar]
- [3].Petrie JG, Ohmit SE, Johnson E, Cross RT, Monto AS. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis 2011;203(May(9)):1309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011;30(December((12)):1081–5. [DOI] [PubMed] [Google Scholar]
- [5].Skowronski DM, Tweed SA, Serres GD. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant. J Infect Dis 2008;197:490–502. [DOI] [PubMed] [Google Scholar]
- [6].Kissling E, Valenciano M, Larrauri A, Oroszi B, Cohen JM, Nunes B, et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case-control study. Eurosurveillance 2013;18(5):pii = 20390. [DOI] [PubMed] [Google Scholar]
- [7].Pebody RG, Andrews N, McMenamin J, Durnall H, Ellis J, Thompson CI, et al. Vaccine effectiveness of 2011/12 trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: evidence of waning intra-seasonal protection. Eurosurveillance 2013;18(5):pii = 20389. [DOI] [PubMed] [Google Scholar]
- [8].Castilla J, Martínez-Baz I, Martínez-Artola V, Reina G, Pozo F, García Cenoz M, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre,Spain, season 2011/12. Eurosurveillance 2013;18(5):pii = 20388. [DOI] [PubMed] [Google Scholar]
- [9].Belongia EA, Kieke BA, Donahue JG, Coleman LA, Irving SA, Meece JK, et al. Influenza vaccine effectiveness in Wisconsin during the 2007-08 season: comparison of interim and final results. Vaccine 2011;29(September (38)):6558–63. [DOI] [PubMed] [Google Scholar]
- [10].Greenlee RT. Measuring disease frequency in the Marshfield Epidemiologic Study Area (MESA). Clin Med Res 2003;1(4):273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Irving SA, Donahue JG, Shay DK, Ellis-Coyle TL, Belongia EA. Evaluation of self-reported and registry-based influenza vaccination status in a Wisconsin cohort. Vaccine 2009;27(47):6546–9. [DOI] [PubMed] [Google Scholar]
- [12].Ambrose CS, Wu X, Belshe RB. The efficacy of live attenuated and inactivated influenza vaccines in children as a function of time postvaccination. Pediatr Infect Dis J 2010;29(September (9)):806–11. [DOI] [PubMed] [Google Scholar]
- [13].Cao W, Kim JH, Chirkova T, Reber AJ, Biber R, Shay DK, et al. Improving immuno-genicity and effectiveness of influenza vaccine in older adults. Expert Rev Vaccines 2011;10(11):1529–37. [DOI] [PubMed] [Google Scholar]
- [14].McElhaney JE. Influenza vaccination in the elderly: seeking new correlates of protection and improved vaccines. Aging Health 2008;4(6):603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol 2002;37(2–3):427–39. [DOI] [PubMed] [Google Scholar]
- [16].McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol 2006;176(10):6333–9. [DOI] [PubMed] [Google Scholar]
- [17].Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)-United States, 2012-13 influenza season. Morbid MortalWeekly Rep 2012;61(32):613–8. [PubMed] [Google Scholar]
- [18].Toback SL, Herley J, Edelman L, Ambrose CS. Trends in US pediatric influenza vaccination from 2006 to 2010 among children with private insurance. Vaccine 2011;29(25):2445–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.