Heasley, Watson, and Argueso present findings demonstrating that transient and punctuated destabilizing events can promote the rapid formation of extraordinarily reconfigured karyotypes....
Keywords: aneuploidy, genomic instability, punctuated evolution, genome evolution, copy number alteration variation
Abstract
Remarkably complex patterns of aneuploidy have been observed in the genomes of many eukaryotic cell types, ranging from brewing yeasts to tumor cells. Such aberrant karyotypes are generally thought to take shape progressively over many generations, but evidence also suggests that genomes may undergo faster modes of evolution. Here, we used diploid Saccharomyces cerevisiae cells to investigate the dynamics with which aneuploidies arise. We found that cells selected for the loss of a single chromosome often acquired additional unselected aneuploidies concomitantly. The degrees to which these genomes were altered fell along a spectrum, ranging from simple events affecting just a single chromosome, to systemic events involving many. The striking complexity of karyotypes arising from systemic events, combined with the high frequency at which we detected them, demonstrates that cells can rapidly achieve highly altered genomic configurations during temporally restricted episodes of genomic instability.
WHOLE chromosome copy number alterations (CCNAs) (e.g., aneuploidies) are an important source of phenotypic variation and adaptive potential (Hickman et al. 2015; Selmecki et al. 2015; Sansregret and Swanton 2017; Forche et al. 2018; Gilchrist and Stelkens 2019). CCNAs usually arise from defects in chromosome segregation (Musacchio 2015), but, because such errors occur rarely (∼10−6/cell/division) (Klein 2001; Kumaran et al. 2013), the patterns by which cells accumulate extensive collections of CCNAs remain poorly understood (Sansregret and Swanton 2017). Conventional paradigms of genome evolution posit that mutations (e.g., CCNAs) are acquired gradually and independently over many successive generations (Nowell 1976; Podlaha et al. 2012). Cancer-centric models have proposed that tumor cells can gain numerous mutations during punctuated and transient bursts of genomic instability (Eldredge and Gould 1972; Gao et al. 2016; Sampaio et al. 2017; Field et al. 2018), or that they become chronically destabilized and acquire mutations at elevated rates (i.e., mutator phenotype) (Loeb 2016; Coelho et al. 2019). Yet, because cancer genome evolution is retrospectively inferred many generations after neoplastic initiation, our understanding of how these mutagenic patterns contribute to the acquisition of CCNAs remains incomplete.
Results and Discussion
We used the tractable budding yeast model system to determine the patterns by which CCNAs arise. To recover aneuploid clones arising spontaneously from populations of diploid cells grown under normal culture conditions (i.e., rich medium, 30C, <35 cell divisions), we introduced the counter-selectable marker CAN1 onto the right arm of chromosome V (Chr5R) in the haploid strain JAY291 (Argueso et al. 2009). Because the endogenous copy resides on Chr5L, the resulting strain had two copies of CAN1 on Chr5, one on each arm. We crossed this haploid to the S288c reference strain to form a heterozygous diploid. To select for cells that had lost the JAY291 homolog of Chr5 (jChr5), we grew independent cultures for ≤35 generations in rich medium and plated each onto selective medium containing canavanine (CAN) (Larimer et al. 1978). When we visually inspected CAN-resistant (CANR) colonies, we noted that while the majority had a normal smooth appearance; 1 in ∼450 colonies displayed a distinctive rough morphology (Figure 1A). Previously, we reported that this morphological switch is precipitated by interhomolog mitotic recombination (MR), resulting in loss of the wild-type allele of the ACE2 gene encoded on sChr12R and homozygosis of the mutant ace2-A7 allele on jChr12R (Rodrigues Prause et al. 2018). ace2-A7 cells fail to separate after cytokinesis and consequently form rough colonies (Nelson et al. 2003; Rodrigues Prause et al. 2018). In this previous study, rough colonies appeared on nonselective media at a frequency of 1 in ∼10,000 colonies, and were always caused by MR events spanning ACE2 on Chr12R (Sampaio et al. 2017; Rodrigues Prause et al. 2018). Rough colonies resulting from complete loss of Chr12 were never observed (0/67 genotyped clones).
Figure 1.
Clones selected for a single CCNA are enriched for additional CCNAs. (A) Images of smooth and rough colonies. (B) A schematic illustrating the genotypic and phenotypic outcomes of selection for loss of jChr5 and homozygosis of ace2-A7 on jChr12. jChr5-encoded CAN1 markers, yellow boxes; jChr12-encoded ace2-A7 mutation, light blue box; sChr12-encoded ACE2 allele, dark blue box; sChr12-encoded URA3 marker, red box. (i) the parental diploid, (ii) 21% of rough CANR colonies were Ura+ and homozygous for ace2-A7 due to MR, (iii) 79% of rough CANR were Ura− and hemizygous for ace2-A7 due to loss of sChr12. (C) Percentage of rough CANR isolates with 0 (white), 1 (black), and ≥2 (yellow) unselected CCNAs. (D) Karyotypes of the parent strain and five rough Ura- CANR isolates. For each chromosome, yellow bars denote the S288c homolog and black bars denote the JAY291 homolog. Thin bars with a depth equal to those depicted in the parent represent single copies of a given homolog. Thick bars represent two copies of a given homolog. Colored boxes denote the indicated karyotypic events.
Our finding that rough colonies appeared >22-fold more frequently on CAN selection plates than in nonselective conditions led us to hypothesize that a shared mutational process could have caused the concomitant loss of jChr5 and loss-of-heterozygosity on Chr12R. To investigate this, we introduced a URA3 marker onto sChr12L [Figure 1B, (1)]. Rough CANR clones resulting from MR spanning ACE2 would likely retain this URA3 marker and grow on medium lacking uracil (Ura+) [Figure 1B, (2)], while rough clones caused by loss of the sChr12 homolog would be Ura− [Figure 1B, (3)]. We plated cultures to CAN media, screened CANR colonies to identify rough clones, and determined the Ura+/− phenotype of each. In contrast to the rough colonies recovered from nonselective conditions (Sampaio et al. 2017; Rodrigues Prause et al. 2018), 79% (41/52) of rough CANR colonies had lost sChr12 in addition to jChr5 (Figure 1B). Our finding that the selected loss of jChr5 markedly shifted the mutational spectrum of LOH on Chr12R to CCNA was consistent with our above prediction, and indicated that clones harboring one aneuploidy were enriched for the presence of additional unselected aneuploidies.
We performed whole genome sequence (WGS) analysis to comprehensively define the genomic structure of 20 rough CANR Ura− clones (Table S3). The even distribution of heterozygous sites across the genome of the S288c/JAY291 hybrid enabled us to detect CCNAs of each homolog and changes in overall ploidy based on sequencing read depth. Remarkably, the majority (65%) of the sequenced clones harbored unselected CCNAs of chromosomes other than jChr5 and sChr12 (Figure 1C and Table S3). Some clones had lost numerous chromosomes (LRH279), while others displayed systemic gains (LRH266 and LRH280) (Figure 1D). Intriguingly, one clone (LRH271) had acquired CCNAs of nearly every chromosome, such that both copies of one homolog had been retained while both copies of the other homolog had been lost—a state known as uniparental disomy (UPD) (Andersen and Petes 2012). As a result of this UPD-type CCNA, this clone had cumulatively gained and lost 32 homologs, and was fully homozygous for either parental haplotype of all chromosomes except Chr1, Chr3, and Chr9, which were tetrasomies (Figure 1D). The acquisition of such numerous genomic alterations over the limited growth period of ≤35 generations suggested that these clones likely acquired all CCNAs during a temporally restricted episode of chromosomal instability. The homogeneity of WGS read coverage depths observed in the copy number analyses of these clones supported this conclusion. All CCNAs identified within each clonal population were detected at discrete copy numbers; intermediate levels were not observed (data not shown). This demonstrated that CCNAs did not arise continuously during the expansion of the colony, and instead indicated that the instability underlying the formation of these complex genomic alterations was short-lived.
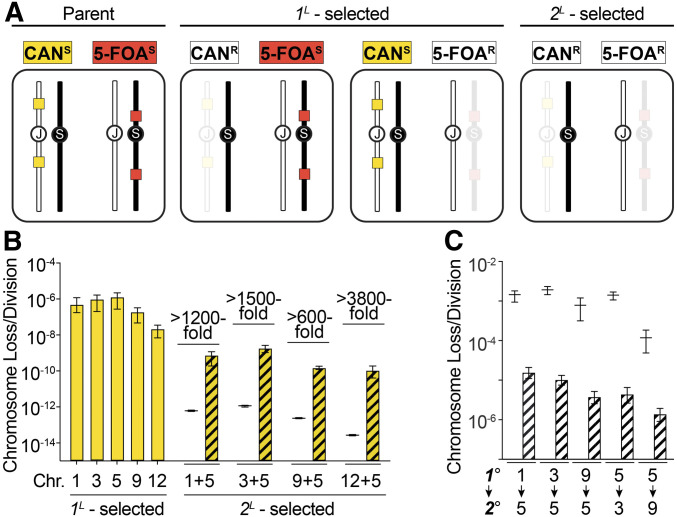
Models of gradual mutation accumulation predict that the rate at which cells independently lose two chromosomes (2L) should be the multiplicative product of the rates at which each individual chromosome is lost (1L), referred to here as the theoretical 2L rate. Our initial results challenged this premise of gradual acquisition and instead suggested that multiple CCNAs could be acquired nonindependently. To quantitatively test this gradual model, we constructed a suite of strains in which jChr5 was marked with two copies of CAN1 and each of several S288c homologs (sChr1, sChr3, sChr9, sChr12) was marked on both arms with copies of URA3 (Figure 2A). Plating cultures of these strains to media containing CAN selected for 1L cells that had lost jChr5, and plating to media containing 5-FOA selected for 1L cells that had lost the URA3-marked homolog (Boeke et al. 1984). 2L cells that had lost both marked homologs were recovered by plating on media containing both CAN and 5-FOA. Notably, neither CAN nor 5-FOA have been found to promote aneuploidization in yeast (Forche et al. 2011; Shor et al. 2013), and aneuploidies in these clones would necessarily have occurred during the initial culture, prior to exposure to these drugs.
Figure 2.
Clones with multiple CCNAs arise more often than predicted by gradual models. (A) Schematic illustrating our quantitative CCNA selection approach. jChr5-encoded CAN1 markers, yellow boxes, S288c homolog-encoded URA3 markers, red boxes. (B) Empirically derived rates of each 1L-selection (yellow) and 2L-selection (yellow striped). Black lines denote theoretical 2L rates. Fold change between each theoretical 2L rate and empirically derived 2L rate is noted. (C) Empirically derived rates at which cells with a primary existing CCNA (1°) lose a second chromosome (2°) (striped). Black lines denote the theoretical rates at which each 2°C CNA should occur if 2L clones arise by sequential acquisition.
We used fluctuation analysis to determine the rates at which 1L and 2L clones arose in ≤35 generation-cultures (Table S8). Consistent with previous reports (Klein 2001; Kumaran et al. 2013), 1L clones arose at rates of 10−7–10−6/division (Figure 2B, yellow bars). Consequently, the theoretical 2L rates for each pair of aneuploidies were exceedingly low (10−15–10−13/division; Figure 2B, black lines). We found that the empirically derived 2L rates were 600- to 3800-fold higher than these theoretical 2L rates (Figure 2B, striped bars), demonstrating that 2L clones arise far more frequently than predicted by a gradual model of CCNA acquisition. These results were corroborated by similar experiments in two additional strains (another heterozygous strain S288c/YJM789, and an isogenic strain S288c/S288c; Figure S1 and Table S8), indicating that the higher-than-expected incidence of 2L clones was a feature common to strains from diverse genetic backgrounds.
In haploids, single aneuploidies (i.e., disomies) can impair chromosomal stability and cause elevated rates of subsequent CCNA acquisition (Sheltzer et al. 2011). We considered the possibility that the 2L clones recovered in our experiments could have resulted from a similar sequential process, and tested whether cells aneuploid for a single chromosome exhibited substantially elevated rates of ensuing chromosome loss. If the empirically derived 2L rates calculated above reflected such a process, then the expected rates at which secondary CCNAs should be acquired would be 1100-fold greater on average (1.2 × 10−4–1.9 × 10−3/division, Figure 2C, black lines) than the empirically derived rates of a primary CCNA (Figure 2B, yellow bars). However, we found that 1L clones (monosomic for sChr1, sChr3, jChr5, or sChr9) lost a second chromosome (jChr5, sChr3, or sChr9) at rates only 2- to 12-fold greater than the euploid parent, and far lower than would be expected if 2L clones arose through a process of accelerated sequential accumulation (Table S8). Thus, this effect alone cannot explain the high rates at which 2L clones were recovered in our fluctuation analysis.
We performed WGS analysis of 146 1L and 2L isolates, as well as 15 control clones that had been isolated from nonselective conditions (e.g., YPD). We detected no structural abnormalities in the genomes the control clones. By contrast, and in agreement with our earlier results (Figure 1), we again observed a remarkable number of 1L and 2L clones harboring additional unselected CCNAs (1L: 39.0%; 2L: 47.9%) (Figure 3A). Of these unselected CCNAs, each of the 16 S. cerevisiae chromosomes was affected at similar frequencies, and we found no evidence that specific CCNAs co-occurred with any particular selected aneuploidy (Figure 3B). This indicates that unselected CCNAs did not arise subsequently as compensatory suppressors. Additionally, while CCNAs were by far the most prevalent unselected structural genomic alteration, several clones (13/146) had also acquired tracts of LOH resulting from mitotic recombination (Tables S3–S5).
Figure 3.
1L and 2L clones display a spectrum of CCNA levels. (A) Percentage of 1L and 2L isolates with 0 (white), 1 (black), and ≥2 (yellow) unselected CCNAs. (B) Graph depicting the proportion of unselected CCNAs that affected each chromosome. (C) Karyotypes of the parent strain and nine clones representing CCNA classes 1–5. Details as in Figure 1D. (D) Plot depicting a model of gradual CCNA accumulation (black dashed line) and the projected number of generations required to generate class 1–5 clones described in (C) (yellow circles). (E) A model illustrating how mitosis with impaired checkpoint activity could generate cells with varying numbers of CCNAs. Gray line, division plane. Red circles, mis-segregated chromosomes.
We classified all 146 sequenced clones by the degree to which their genomes had been altered by CCNAs (Figure 3C). Class 1 clones lost only the selected chromosome(s) and represented 58.2% of the dataset (LRH180, 85/146). The remaining 41.8% of clones contained at least one unselected CCNA (61/146) and were classified as follows: Class 2 clones had additionally gained a second copy of the matched homolog resulting in a UPD-type CCNA (LRH183, 21/61, 34.4%); Class 3 clones harbored one additional CCNA (LRH209, 19/61, 31.1%); Class 4 clones harbored multiple additional CCNAs (LRH225, LRH140, LRH187, LRH85, 19/61, 31.1%); and Class 5 clones harbored UPD-type CCNAs of every homolog (LRH11 and LRH159, 2/61, 3.9%).
We also sequenced the genomes of 86 1L and 2L isolates derived from the S288c/YJM789 hybrid. Surprisingly, WGS analysis revealed that the parent strain was already trisomic for Chr12 (Figure S2 and Table S5). Despite this pre-existing CCNA, empirically derived 1L rates for sChr1, sChr3, and yChr5 in this background were comparable to the euploid S288c/JAY291 and S288c/S288c strains (Figure S1 and Table S8). Similar to the clones derived from the S288c/JAY291 hybrid, numerous S288c/YJM789-derived clones contained unselected CCNAs (1L, 27%; 2L, 40%) (Table S5). Together, CCNA analysis in this background corroborated our above finding that a single pre-existing CCNA, even of a chromosome as large as Chr12, did not substantially perturb genomic stability, nor did it alter the patterns by which derivative clones acquired unselected CCNAs.
We modeled the number of generations required to produce class 1–5 karyotypes shown in Figure 3C if each CCNA was acquired independently at the average 1L rate of 1.5 × 10−6/division (Figure 3D, black dashed line). Contrary to our experimental results, this model projected that class 2–5 karyotypes would have required >35 generations to develop sequentially (41–656 generations) (Figure 3D, yellow circles). Collectively, the conventional gradual model does not effectively explain the remarkable genomic complexity detected in clones from the datasets above, nor does it account for the frequency at which we recovered such clones. Instead, our results are best explained by a model in which multiple CCNAs are acquired during a punctuated and transient burst of genomic instability.
Taken together, our results demonstrate the remarkable swiftness with which CCNAs can accumulate to profoundly alter the structure and heterozygosity of a diploid genome. Indeed, cells can and do acquire individual CCNAs independently, indicating that gradual accumulation of CCNAs occurs. But, nearly as often, cells acquire numerous CCNAs coincidentally. This indicates that a broad spectrum of complex karyotypes can arise during stochastic and short-lived episodes, not as the result of gradualism or chronic genomic instability. Our results in S. cerevisiae are directly analogous to recent studies that suggest that it is through this punctuated mode of mutagenesis that cancer cells acquire numerous copy number alterations early in tumorigenesis (Gao et al. 2016; Casasent et al. 2018; Field et al. 2018).
For many yeasts, including S. cerevisiae and Candida albicans, chronic exposure to environmental stresses such as heat- or drug-treatment can rapidly induce de novo structural genomic alterations and aneuploidies, which often results in the generation of stress-adapted derivative clones (Hickman et al. 2015; Forche et al. 2018). For example, exposure of C. albicans to the antifungal drug fluconazole is sufficient to increase the rate of whole chromosome aneuploidy by fivefold (Forche et al. 2018). Because all aneuploid clones recovered and characterized in this study were isolated from normal growth conditions in rich media, it is unlikely that the punctuated acquisition of CCNAs that we observed can be attributed to stress-induced mutagenesis. Instead, any “stress” that might have promoted aneuploidization of multiple chromosomes simultaneously must have occurred at the single-cell level.
What cellular events might contribute to this process of punctuated copy number evolution (PCNE) (Casasent et al. 2018)? Perturbation of many integral cellular processes including DNA damage repair (Craven et al. 2002), replication (Wilhelm et al. 2019), sister chromatid cohesion (Daum et al. 2011; Covo et al. 2014), spindle assembly (Mattiuzzo et al. 2011; Maiato and Logarinho 2014), and mitotic checkpoint activity (Musacchio 2015) are known to affect the maintenance and inheritance of chromosomes, and failure of any of these pathways has the potential to affect all chromosomes equally and simultaneously (Weaver and Cleveland 2006; Nicholson and Cimini 2011; Musacchio 2015). For instance, even a transient failure to activate the mitotic checkpoint enables a cell to enter anaphase with incorrect chromosome-spindle attachments. Such an erroneous mitosis could produce daughter cells harboring any of the aberrant karyotypic classes described in this study (Figure 3E) (Musacchio 2015). Our experimental approach provides a promising model system with which to meticulously define the causal mechanisms of PCNE as well as to assess the phenotypic consequences and adaptive potential of the remarkable karyotypes that can arise from this process.
Materials and Methods
Strain construction and culture media
All Saccharomyces cerevisiae strains used in this study are listed in Supplemental Material, Table S1 and were derived from the S288c, JAY291 (Argueso et al. 2009), or YJM789 (McCusker et al. 1994) backgrounds. Plasmids used for PCR-based amplification of selectable markers (Wach et al. 1994; Goldstein and McCusker 1999; Zhang et al. 2013) are listed in Table S2. Strain construction was performed using standard transformation, crossing, and sporulation procedures. Specific descriptions of the construction of experimental strains are outlined below. To ensure that each strain used in these studies was unable to initiate meiosis and undergo a return-to-growth (RTG) process, we replaced the IME1 locus on each homolog of Chr10 with HPHMX selectable markers. RTG is a process in which diploid yeast cells initiate meiotic programs, introduce Spo11-mediated double-strand breaks throughout the genome and then return to vegetative growth (Laureau et al. 2016). This process can lead to extensive mitotic recombination-derived loss-of-heterozygosity.
Construction of CAN1-marked chromosomes (jChr5, yChr5, sChr5)
A PCR product consisting of CAN1-KANMX amplified from genomic DNA was integrated into the HOM3 locus on Chr5R. Resulting strains had the endogenous CAN1 gene on Chr5L (31694–33466) and the newly introduced CAN1-KANMX cassette on Chr5R (256375–257958). Coordinates are derived from the S. cerevisiae reference genome build R64-1-1 (yeastgenome.org).
Construction of URA3-marked chromosomes (sChr1, sChr3, sChr9, sChr12)
The CORE3 cassette (pJA95) encodes tandem URA3 genes from S. cerevisiae (ScURA3), Kluyveromyces lactis (KlURA3) and a KANMX cassette. With the exception of Chr1 (see below), the full CORE3 marker was introduced on the left arm of each S288c chromosome at the coordinate listed in Table S1. Into an isogenic strain of the opposite mating type, a single KlURA3 marker was inserted into the right arm of the same chromosome at the coordinate listed in Table S1. The two resulting strains were crossed, sporulated, and spores were dissected to recover a haploid derivative with both the left-arm CORE3 and right-arm KlURA3 markers. For construction of URA3-encoding sChr1, a KlURA3 marker was inserted into both the left and right arms.
Construction of the TRP1-marked chromosome (sChr3)
To select for loss of sChr3 in the S288c/YJM789 hybrid, the TRP1 gene was amplified from genomic DNA and integrated into Chr3L and Chr3R at the coordinates listed in Table S1 in the intermediate strains that were used to make sChr1 (above). These strains were then crossed, sporulated, and spores were dissected to recover a haploid derivative encoding both TRP1 markers and both KlURA3 markers. This strain was crossed to JAY2593 to form a heterozygous diploid in which chromosomes sChr1, sChr3, and yChr5 were each marked with counter-selectable markers. Although efficacy of TRP1 counterselection was strong in the S288c/YJM789 genetic background, we found it to be variable in other genetic backgrounds. For example, we discovered that this selection regime was not effective in an SK1-derived background. Due to the variability of counter-selection efficiency, we used only the URA3 and CAN1 counterselection regimes for all experiments in the S288c/JAY291 background.
Media used to select CCNA clones
Counterselection of URA3 was performed by plating cells on synthetic complete media (20 g/liter glucose, 5 g/liter ammonium sulfate, 1.7 g/liter yeast nitrogen base without amino acids, 1.4 g/liter complete drop-out mix, 20 g/liter bacteriological agar) supplemented with 1 g/liter 5-fluoroorotic Acid (5-FOA). Counterselection of TRP1 was performed by plating cells on synthetic complete media supplemented with 0.75 g/liter 5-fluoroanthranilic Acid (5-FAA). 5-FAA counterselection was only used in plating assays and experiments in the S288c/YJM789 background. Counterselection against CAN1 was performed by plating cells on synthetic media lacking arginine (20 g/liter glucose, 5 g/liter ammonium sulfate, 1.7 g/liter yeast nitrogen base without amino acids, 1.4 g/liter arginine dropout mix, 20 g/liter bacteriological agar) supplemented with 0.06 g/liter canavanine sulfate (CAN). Selection of 2L clones was performed by plating cells to appropriate media supplemented with 1 g/liter 5-FOA and 0.06 g/liter CAN, 1 g/liter 5-FOA, and 0.75 g/liter 5-FAA (S288c/YJM789 only), or 0.75 g/liter 5-FAA and 0.06 g/liter CAN (S288c/YJM789 only). Because most S288c chromosomes in the isogenic experiments were marked with URA3 cassettes, selection of the 2L combinations sChr1/sChr3, sChr1/sChr9, and sChr1/sChr12 was conducted by plating cells to medium supplemented with 1 g/liter 5-FOA.
Rough colony screening and analysis
Diploid yeast cells of the strain JAY2775 were streaked on solid YPD media and incubated at 30° for 32 hr to allow single colonies to grow. Single colonies were each inoculated into 5 or 7 ml liquid YPD cultures and incubated at 30° for another 24 hr on a rotating drum. Each culture was then diluted appropriately, plated onto CAN-supplemented media, and incubated at 30° for 4 days. Plates were then visually screened for the presence of rough colonies. Rough colonies were isolated with a sterile toothpick and streaked onto both CAN-supplemented media (to preserve a stock) and uracil-dropout media (20 g/liter glucose, 5 g/liter ammonium sulfate, 1.7 g/liter yeast nitrogen base without amino acids, 1.4 g/liter uracil drop-out mix, 20 g/liter bacteriological agar). Plates were incubated at 30° for 24 hr. After 24 hr, each clone was assessed for its ability to grow on uracil-dropout medium.
Genome sequencing and analysis
The genomes of 276 unselected, 1L, and 2L clones from either the S288c/JAY291 or S288c/YJM789 hybrid backgrounds were sequenced using Illumina short-read whole-genome sequencing (WGS). Genomic DNA from each clone was isolated using the Yeastar Genomic DNA kit from Zymo Research. Pooled, barcoded libraries of 96 individual genomes were generated using Seqwell plexWell-96 kits. Each 96-sample library was sequenced on a single Illumina HiSeq lane. Using CLC Genomics Workbench software (Qiagen), the following processing pipeline was utilized to analyze each sequenced genome: Illumina reads for each genome were imported into CLC and mapped to the most recent release of the yeast reference genome (R64-2-1, yeastgenome.org). Each resulting read mapping file was then imported into the Nexus Copy Number software (Biodiscovery). Each file was subjected to copy number and single nucleotide polymorphism (SNP) variant analysis to identify the copy number of each chromosome (relative to the diploid parent) and heterozygosity at >20,000 individual sites distributed across the genome. From this, we identified the following structural variations: whole chromosome gains/losses, segmental duplications/deletions, and tracts of (LOH). LOH breakpoints identified in Nexus were confirmed manually in CLC (Tables S3–S5).
Two different approaches were used to define CCNAs, and the analysis of each sequenced dataset are presented in Table S6: (1) the 16-chromosome pairs method; aneuploidy was defined as the deviation of overall ploidy away from 2n. Using this method, uniparental disomy was not scored as an aneuploidy, despite loss of one homolog and gain of the other homolog, (2) the 32-homologs method; aneuploidy was defined as the deviation in copy number of each individual homolog away from 1n. Using this method, UPDs were scored as two CCNAs. Graphs in Figures 1C and 3A, and Figure S2A depict the results from the 32-homologs method of analysis. Results from both the 16-chromosome pairs and 32-homologs analyses for each sequenced dataset are presented in Table S6.
Graphs in Figure 3B and Figure S2B depict the proportion of total unselected aneuploidies that affected each yeast chromosome. To determine if there was a bias toward any chromosome in terms of gains/losses, we used the chi-square goodness-of-fit test to compare the distribution of observed frequencies of CCNA for each chromosome to the test distribution of expected frequencies of 6.25% per chromosome (100% divided by 16 chromosomes). From this test, we calculated a P-value of 0.109, which indicated that there was no significant difference between each chromosome. Because we found no evidence of biases favoring specific chromosomes, we pooled the total number of unselected aneuploidies in the complete S288c/JAY291 or S288c/YJM789 dataset regardless of primary selection (e.g., selection for loss of sChr1). These data are presented in Table S7.
Quantitative chromosome loss assays
Cultures of S288c/JAY291 diploid strains were prepared from single colonies in a manner identical to that used to select for rough CANR clones (see above). Each culture was serially diluted and plated onto YPD (nonselective), 5-FOA- and CAN-supplemented medias (1L selection), and 5-FOA+CAN-supplemented medium (2L selection). For the experiments using the S288c/YJM789 diploid strains, cultures were also plated onto 5-FAA-supplemented medium (sChr3 1L selection), and onto 5-FOA+5-FAA- and CAN+5-FAA-supplemented media (2L selection). Colonies on nonselective and 1L-selected plates were counted after 4 days of growth. Colonies on 2L-selected plates were counted after 6 days of growth. Colony count data were used to calculate rates and 95% confidence intervals of chromosome loss using Flucalc, a MSS-MLE (Ma-Sandri-Sarkar Maximum Likelihood Estimator) calculator for Luria-Delbrück fluctuation analysis (flucalc.ase.tufts.edu) (Radchenko et al. 2018). To determine the theoretical rates at which 2L clones should arise if each chromosome was lost independently, the multiplicative product of both observed 1L rates (and corresponding 95% confidence intervals) was calculated as follows: theoretical rate 2L(ChrA+ChrB) = empirically-derived rate 1L(ChrA) x empirically-derived rate 1L(ChrB). The following rationale was used to calculate the theoretical rates of sequential secondary CCNA acquisition depicted in Figure 2C (black lines). Using empirically-derived 1L and 2L rates (Figure 2B and Table S8), we calculated the rate at which a secondary chromosome (ChrB) would be expected to be lost following loss of a primary chromosome (ChrA) if due to sequential process: theoretical sequential rate 1L(ChrB) = empirically derived rate 2L(ChrA+ChrB)/empirically derived rate 1L(ChrA). All empirically derived and theoretical rates, 95% confidence intervals, and number of cultures used to calculate each rate are listed in Table S8.
Modeling gradual acquisition of CCNAs
We modeled the generations associated with the gradual acquisition of CCNAs using the equation #gen = Log2((1.5 × 106)#A) in which #gen equals the number of generations, #A equals number of CCNAs, and 1.5 × 106 defines a representative and constant rate of chromosome loss.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Sequence files for each clone in this study as well as all strains and other data will be shared upon request. Sequence files are also available through NCBI under the BioProject accession number PRJNA657826. Supplemental material available at figshare: https://doi.org/10.25386/genetics.12749273.
Acknowledgments
We are grateful to Eric Alani, Michael McMurray, Dmitry Gordenin, and Tom Petes for valuable comments on the manuscript. This study was supported by National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) awards 1K99GM13419301 to L.R.H. and R35GM11978801 to J.L.A.
Author contributions: Conceptualization: L.R.H. and J.L.A.; Methodology: L.R.H., R.A.W., and J.L.A.; Investigation: L.R.H. and R.A.W.; Resources: J.L.A.; Writing: L.R.H. and J.L.A.; Funding acquisition: J.L.A. and L.R.H. The authors declare no competing interests.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.12749273.
Communicating editor: F. Cole
Literature Cited
- Andersen S. L., and Petes T. D., 2012. Reciprocal uniparental disomy in yeast. Proc. Natl. Acad. Sci. USA 109: 9947–9952. 10.1073/pnas.1207736109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso J. L., Carazzolle M. F., Mieczkowski P. A., Duarte F. M., Netto O. V. et al. , 2009. Genome structure of a Saccharomyces cerevisiae strain widely used in bioethanol production. Genome Res. 19: 2258–2270. 10.1101/gr.091777.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., and Fink G. R., 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol gen genet. 197: 345–346. 10.1007/BF00330984 [DOI] [PubMed] [Google Scholar]
- Casasent A. K., Schalck A., Gao R., Sei E., Long A. et al. , 2018. Multiclonal invasion in breast tumors identified by topographic single cell sequencing. Cell 172: 205–217.e12. 10.1016/j.cell.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho M. C., Pinto R. M., and Murray A. W., 2019. Heterozygous mutations cause genetic instability in a yeast model of cancer evolution. Nature 566: 275–278. 10.1038/s41586-019-0887-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covo S., Puccia C. M., Argueso J. L., Gordenin D. A., and Resnick M. A., 2014. The sister chromatid cohesion pathway suppresses multiple chromosome gain and chromosome amplification. Genetics 196: 373–384. 10.1534/genetics.113.159202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven R. J., Greenwell P. W., Dominska M., and Petes T. D., 2002. Regulation of genome stability by TEL1 and MEC1, yeast homologs of the mammalian ATM and ATR genes. Genetics 161: 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum J. R., Potapova T. A., Sivakumar S., Daniel J. J., Flynn J. N. et al. , 2011. Cohesion fatigue induces chromatid separation in cells delayed at metaphase. Curr. Biol. 21: 1018–1024. 10.1016/j.cub.2011.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldredge, N., and S. J. Gould, 1972 Punctuated equilibria: an alternative to phyletic gradualism, pp. 82–115 in Models of Paleobiology, edited by T. J. M. Schopf. Freeman, Cooper and Co., San Francisco. [Google Scholar]
- Field M. G., Durante M. A., Anbunathan H., Cai L. Z., Decatur C. L. et al. , 2018. Punctuated evolution of canonical genomic aberrations in uveal melanoma. Nat. Commun. 9: 116 10.1038/s41467-017-02428-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forche A., Abbey D., Pisithkul T., Weinzierl M. A., Ringstrom T. et al. , 2011. Stress alters rates and types of loss of heterozygosity in Candida albicans. MBio 2: e00129-11 10.1128/mBio.00129-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forche A., Cromie G., Gerstein A. C., Solis N. V., Pisithkul T. et al. , 2018. Rapid phenotypic and genotypic diversification after exposure to the oral host niche in. Genetics 209: 725–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Davis A., McDonald T. O., Sei E., Shi X. et al. , 2016. Punctuated copy number evolution and clonal stasis in triple-negative breast cancer. Nat. Genet. 48: 1119–1130. 10.1038/ng.3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist C., and Stelkens R., 2019. Aneuploidy in yeast: segregation error or adaptation mechanism? Yeast 36: 525–539. 10.1002/yea.3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., and McCusker J. H., 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15: 1541–1553. [DOI] [PubMed] [Google Scholar]
- Hickman M. A., Paulson C., Dudley A., and Berman J., 2015. Parasexual ploidy reduction drives population heterogeneity through random and transient aneuploidy in Candida albicans. Genetics 200: 781–794. 10.1534/genetics.115.178020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H. L., 2001. Spontaneous chromosome loss in Saccharomyces cerevisiae is suppressed by DNA damage checkpoint functions. Genetics 159: 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran R., Yang S. Y., and Leu J. Y., 2013. Characterization of chromosome stability in diploid, polyploid and hybrid yeast cells. PLoS One 8: e68094 10.1371/journal.pone.0068094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer F. W., Ramey D. W., Lijinsky W., and Epler J. L., 1978. Mutagenicity of methylated N-nitrosopiperidines in Saccharomyces cerevisiae. Mutat. Res. 57: 155–161. 10.1016/0027-5107(78)90262-2 [DOI] [PubMed] [Google Scholar]
- Laureau R., Loeillet S., Salinas F., Bergström A., Legoix-Né P. et al. , 2016. Extensive recombination of a yeast diploid hybrid through meiotic reversion. PLoS Genet. 12: e1005781 (erratum: PLoS Genet. 12: e1005953). 10.1371/journal.pgen.1005781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., 2016. Human cancers express a mutator phenotype: hypothesis, origin, and consequences. Cancer Res. 76: 2057–2059. 10.1158/0008-5472.CAN-16-0794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H., and Logarinho E., 2014. Mitotic spindle multipolarity without centrosome amplification. Nat. Cell Biol. 16: 386–394. 10.1038/ncb2958 [DOI] [PubMed] [Google Scholar]
- Mattiuzzo M., Vargiu G., Totta P., Fiore M., Ciferri C. et al. , 2011. Abnormal kinetochore-generated pulling forces from expressing a N-terminally modified Hec1. PLoS One 6: e16307 10.1371/journal.pone.0016307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker J. H., Clemons K. V., Stevens D. A., and Davis R. W., 1994. Genetic characterization of pathogenic Saccharomyces cerevisiae isolates. Genetics 136: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., 2015. The molecular biology of spindle assembly checkpoint signaling dynamics. Curr. Biol. 25: R1002–R1018 (erratum: Curr. Biol. 25: 3017). 10.1016/j.cub.2015.08.051 [DOI] [PubMed] [Google Scholar]
- Nelson B., Kurischko C., Horecka J., Mody M., Nair P. et al. , 2003. RAM: a conserved signaling network that regulates Ace2p transcriptional activity and polarized morphogenesis. Mol. Biol. Cell 14: 3782–3803. 10.1091/mbc.e03-01-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. M., and Cimini D., 2011. How mitotic errors contribute to karyotypic diversity in cancer. Adv. Cancer Res. 112: 43–75. 10.1016/B978-0-12-387688-1.00003-X [DOI] [PubMed] [Google Scholar]
- Nowell P. C., 1976. The clonal evolution of tumor cell populations. Science 194: 23–28. [DOI] [PubMed] [Google Scholar]
- Podlaha O., Riester M., De S., and Michor F., 2012. Evolution of the cancer genome. Trends Genet. 28: 155–163. 10.1016/j.tig.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radchenko E. A., McGinty R. J., Aksenova A. Y., Neil A. J., and Mirkin S. M., 2018. Quantitative analysis of the rates for repeat-mediated genome instability in a yeast experimental system. Methods Mol. Biol. 1672: 421–438. 10.1007/978-1-4939-7306-4_29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues Prause A., Sampaio N. M. V., Gurol T. M., Aguirre G. M., Sedam H. N. C. et al. , 2018. A case study of genomic instability in an industrial strain of Saccharomyces cerevisiae. G3 (Bethesda) 8: 3703–3713. 10.1534/g3.118.200446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio N. M. V., Rodrigues Prause A., Ajith V. P., Gurol T. M., Chapman M. J. et al. , 2017. Mitotic systemic genomic instability in yeast. bioRxiv (Preprint posted September 17, 2017) 10.1101/161869 [DOI] [Google Scholar]
- Sansregret L., and Swanton C., 2017. The role of aneuploidy in cancer evolution. Cold Spring Harb. Perspect. Med. 7: a028373 10.1101/cshperspect.a028373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki A. M., Maruvka Y. E., Richmond P. A., Guillet M., Shoresh N. et al. , 2015. Polyploidy can drive rapid adaptation in yeast. Nature 519: 349–352. 10.1038/nature14187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer J. M., Blank H. M., Pfau S. J., Tange Y., George B. M. et al. , 2011. Aneuploidy drives genomic instability in yeast. Science 333: 1026–1030. 10.1126/science.1206412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor E., Fox C. A., and Broach J. R., 2013. The yeast environmental stress response regulates mutagenesis induced by proteotoxic stress. PLoS Genet. 9: e1003680 10.1371/journal.pgen.1003680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network , 2008. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455: 1061–1068 [Corrigenda: Nature 494: 506 (2013)]. 10.1038/nature07385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A., Brachat A., Pöhlmann R., and Philippsen P., 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10: 1793–1808. 10.1002/yea.320101310 [DOI] [PubMed] [Google Scholar]
- Weaver B. A., and Cleveland D. W., 2006. Does aneuploidy cause cancer? Curr. Opin. Cell Biol. 18: 658–667. 10.1016/j.ceb.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Wilhelm T., Olziersky A. M., Harry D., De Sousa F., Vassal H. et al. , 2019. Mild replication stress causes chromosome mis-segregation via premature centriole disengagement. Nat. Commun. 10: 3585 10.1038/s41467-019-11584-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zeidler A. F. B., Song W., Puccia C. M., Malc E. et al. , 2013. Gene copy-number variation in haploid and diploid strains of the yeast Saccharomyces cerevisiae. Genetics 193: 785–801. 10.1534/genetics.112.146522 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Sequence files for each clone in this study as well as all strains and other data will be shared upon request. Sequence files are also available through NCBI under the BioProject accession number PRJNA657826. Supplemental material available at figshare: https://doi.org/10.25386/genetics.12749273.