Abstract
Promoter proximal pausing (PPP) of RNA polymerase II has emerged as a crucial rate-limiting step in the regulation of gene expression. Regulation of PPP is brought about by complexes 7SK snRNP, P-TEFb (Cdk9/cycT), and the negative elongation factor (NELF), which are highly conserved from Drosophila to humans. Here, we show that RNAi-mediated depletion of bin3 or Hexim of the 7SK snRNP complex or depletion of individual components of the NELF complex enhances Yki-driven growth, leading to neoplastic transformation of Drosophila wing imaginal discs. We also show that increased CDK9 expression cooperates with Yki in driving neoplastic growth. Interestingly, overexpression of CDK9 on its own or in the background of depletion of one of the components of 7SK snRNP or the NELF complex necessarily, and specifically, needed Yki overexpression to cause tumorous growth. Genome-wide gene expression analyses suggested that deregulation of protein homeostasis is associated with tumorous growth of wing imaginal discs. As both Fat/Hippo/Yki pathway and PPP are highly conserved, our observations may provide insights into mechanisms of oncogenic function of YAP—the ortholog of Yki in humans.
Keywords: tumorigenesis, Drosophila, Hippo pathway, promoter proximal pausing, transcription regulation in growth and cancer
REGULATION of growth is arguably the most critical phenomenon that establishes size and shape of all tissues, organs, and overall body size in metazoan animals . It is also an important homeostatic process, failure of which is linked to diseases and disorders, particularly cancer in humans. Regulated growth is achieved by an intricate interplay between factors promoting growth (oncogenes) and those suppressing it (tumor suppressors).
Yorkie (Yki), the Drosophila ortholog of the Yes-Associated Protein 1 (YAP1), acts as a transcriptional cofactor that mediates the effects of the Hippo tumor suppressor pathway. The Hippo pathway is highly conserved from Drosophila to humans (Pan 2010). The Hippo (Hpo)/MST kinases and the Warts (Wts)/LATS kinases and their cofactors form kinase cassettes that directly phosphorylate Yki (YAP/TAZ) to regulate protein stability and activity (Zhao et al. 2011). Members of this pathway were initially found to limit tissue growth in Drosophila by limiting Yki activity (Huang et al. 2005; Dong et al. 2007). Consistent with this, YAP overexpression has been reported as a driver of tissue growth and cancer in a mouse model (Dong et al. 2007; Zanconato et al. 2015). In humans, the YAP1 locus was found to be amplified in different types of cancer (Overholtzer et al. 2006; Zender et al. 2006). These findings have sparked a great deal of interest in understanding of regulation of Yki/YAP function.
In Drosophila, Yki regulates expression of regulators of cell growth and survival such as Diap1, dMyc, bantam, etc. Targets of YAP in humans include the EGFR-ligand AREG as well as CTGF, Cyr61 (Johnson and Halder 2014). While these target genes are necessary for growth induced by Yki/YAP activity, they are not sufficient to phenocopy effects of Yki/YAP. This indicates possibility of more regulators that are involved in Yki/YAP induced growth.
We have reported an in vivo screen in Drosophila (Groth et al. 2020), wherein we have identified a large number of genes, which, when depleted, enhanced growth induced by Yki and EGFR. More importantly, these genes function like classical tumor suppressors as, when downregulated in the background of overexpressed Yki or EGFR, we observed neoplastic growth. Among these, we identified a number of genes involved in the control of promoter proximal transcriptional pausing.
Promoter proximal pausing (PPP) of RNA polymerase (Pol) II has been identified as a key step in transcriptional regulation for many genes, during development and in stem cells (Guenther et al. 2007; Muse et al. 2007; Zeitlinger et al. 2007). At paused loci, after initiation, RNA Pol II first passes through the promoter but then stops at ∼30–60 bp from the transcription start site (Kwak and Lis 2013). Productive transcription then requires release from the paused state. PPP is brought about by the negative transcription elongation factor (NELF) and 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB)-sensitivity inducing factor (DSIF) protein complexes, which were identified as factors responsible for DRB sensitivity of transcription elongation (Wada et al. 1998; Yamaguchi et al. 1999). These complexes bind RNA Pol II and halt its progress downstream of the promoter. This pause is alleviated by a positive transcription elongation factor complex (P-TEFb) (Figure 1A), which consists of cyclin T and cyclin dependent kinase-CDK9 (Marshall and Price 1995). Once recruited to the paused complex, CDK9 phosphorylates NELF and DSIF leading to ejection of NELF from the paused complex while DSIF assists Ser-5 phosphorylated RNA Pol II in productive elongation (Jonkers and Lis 2015). The PTEFb complex is, in turn, regulated through sequestration by 7SK snRNP complex. P-TEFb is required for release paused RNA polymerase II into productive elongation (Kwak and Lis 2013). Sequestration of P-TEFb by 7SK snRNP leads to its unavailability for mediating pause release, which, in turn, regulates transcription elongation via sustained pause of RNA Pol II. Mammalian 7sk-snRNP complex consists of 7sk RNA, Hexim1/2, Larp7, and MePCE. Drosophila homologs of components of mammalian 7sk-snRNP complex were identified and characterized recently (Nguyen et al. 2012). These include Bin3 (MePCE ortholog), Larp (Larp7 ortholog), Hexim (HEXIM1/2 ortholog), and d7SK RNA. All are highly conserved at functional levels with their mammalian counterparts. Signaling events of pathways such as ERK, TCR, etc., trigger liberation of P-TEFb. Thus, making sequestration and liberation of P-TEFb a context dependent process that is critical for regulating expression of gene regulation depending on the context.
Figure 1.
Identification of complexes involved in promoter proximal pausing as tumor suppressors. (A) A schematic representing known function of two complexes we identified as candidate tumor suppressors. The 7SK snRNP complex regulates promoter proximal pausing by sequestring the P-TEFb complex, while the NELF complex is involved in the formation of a stall of RNA Pol II at the promoter proximal region. As dictated by surrounding cues, P-TEFb is released by the 7SK snRNP complex. Thus, freed P-TEFb is recruited to stalled RNA Pol II, where it brings about release of RNA Pol II from the paused state. (B) Larval images showing wing imaginal discs expressing GFP at low magnification. Dimensions of GFP-expressing tissue is indication of growth in imaginal discs. Top row: Larvae overexpressing only Yki (crossed to UAS-GFP as control) and those in combination with RNAi-mediated knockdown of 7sksnRNP components: Hexim or bin3 using GAL80TS; ap-GAL4; UAS-GFP. Bottom row: wing discs overexpressing Yki in combination with RNAi-mediated knockdown of NELF components (from left to right) NelfA, NelfB, and NelfD (also known as TH1) using GAL80TS; ap-GAL4; UAS-GFP. Note significantly larger GFP expressing-wing discs (green) in larvae that are overexpressing Yki and also depleted for a component of PPP.
Interestingly, CDK9 has been shown to be important for transcription of target genes of oncogenes such as Myc (Kanazawa et al. 2003) and YAP (Galli et al. 2015). Here, we present evidence of tumor suppressor function of various components involved in PPP, specifically in the context of elevated Yki activity. Our findings show that factors involved in PPP and its regulation are important to restrict Yki driven growth and to prevent neoplastic transformation in vivo.
Material and Methods
Drosophila strains
The following Drosophila strains are used in this study: ap-Gal4 (Cohen et al. 1992) and UAS-Yki (Huang et al. 2005). The following RNAi stocks were obtained from the Vienna Drosophila RNAi Center and Bloomington Drosophila stock Center: UAS-NelfARNAi (KK106245, TRiP #32897), UAS-NelfBRNAi (KK108441, TRiP #42547), UAS-NelfERNAi (TRiP # 32835), UAS-NelfDRNAi (KK100009, TRiP # 38934, #42931), UAS-bin3RNAi (KK101090, TRiP #41527), UAS-HeximRNAi (KK100500). UAS-CDK9 was obtained from FlyORF (#F001571).
Spatio-temporal regulation of transgene expression in wing imaginal disc
The apterous enhancer was used to drive expression of Gal4 conditionally in dorsal compartment of wing imaginal discs. Gal4 activity was regulated using Gal80TS, which allows expression of transgenes at permissive temperature of 29° as against restrictive 18° temperature. In all experiments, tubulin-Gal80TS was used. Drosophila crosses were allowed to lay eggs for 3 days at 18°, and were then flipped or discarded. Larvae were then allowed to grow for additional 5 days before switching to temperature of 29°. At 29° they were maintained for 4–14 days. All crosses were using tubulin-GAL80TS; ap-GAL4; UAS-GFP. Thus, all experimental crosses had one copy of GFP, while control crosses had two copies of GFP. Detailed methodology is provided in Groth et al. (2020). Larval images were taken in bright field and in GFP channel with a Leica stereomicroscope. Image processing was done using Adobe Photoshop 6 and ImageJ.
Immunohistochemistry
The following primary antibodies were used: rat anti-Ecadherin, mouse anti-MMP1 (Developmental Studies Hybridoma Bank). Rhodamine-phalloidin (ThermoFisher Scientific, Cat no R415) was used to stain actin in tissue.
Third instar larvae were dissected in PBS. Samples were fixed in 4% PFA for 20 min, followed by three 10-min washes in PBT (PBS-Tween20) at room temperature. Then, 5% BSA in PBS was used for blocking followed by overnight incubation in primary antibody at 4°. Next day, the samples were washed with PBT, three times for 10 min each followed by incubation with secondary antibody for 2 hr at room temperature. Samples were then washed with PBT and stained for DNA using 4′,6-diamidino-2-phenylindole (DAPI; Sigma Aldrich) for 5 min. Wing disc tissue was then mounted on slides in Antifade Gold mountant (ThermoFisher Scientific). Imaging was done on a Leica SP8 confocal laser-scanning microscope. Image processing was done using ImageJ and Adobe Photoshop 6. Measurement MMP1 intensities and comparison between different genotypes was carried out using ImageJ, statistical analysis (one-way ANOVA) was done using Prism-Graphpad 5.
RNA-seq
Induction procedure for transgenes was followed as mentioned earlier. Wing imaginal disc tissue was dissected on 4th–5th day after induction for ap > GFP, ap > UAS-Yki, ap > Nelf-A RNAi (KK106245), ap > UAS-Yki, UAS Nelf-A RNAi. Larvae were washed in RNase-, DNase-free ultrapure water (GiBCO), and then dissections were done in RNase-, DNase-free PBS (GiBCo). Number of wing imaginal discs collected was 150, 70, 150, 25, respectively for ap > GFP, ap > UAS-Yki, ap > Nelf-A RNAi (KK106245), ap > UAS-Yki, and UAS Nelf-A RNAi. Collection was done in TRiZOL reagent (ThermoFisher Scientific). Each genotype was collected in three biological replicates. RNA sequencing was done on an Illumina platform.
RNA-seq data analysis
RNA-seq analysis was performed using the HISAT 2.0 package protocol as explained in Pertea et al. (2016). To identify significantly differentially expressing genes in different combinations of comparisons, DEseq package and EdgeR were used (Anders and Huber 2010). The entire RNA-seq data set is available on GEO database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE151935).
The list of genes obtained was then used as input for the web-based tool venny (http://bioinfogp.cnb.csic.es/tools/venny/index.html) to obtain a list of genes that are unique to each genotype, overlapping between all three or combination of any two genotypes.
Gene ontology analysis
For gene ontology (GO) and pathway enrichment analysis, we utilized STRING10 (Szklarczyk et al. 2017). We used gene lists that are significantly differentially expressed in single genotype or a combination of genotypes as mentioned in the results section, as input to the STRING. The output files were downloaded as interaction network and list of genes from input that are enriched in different GO categories or as KEGG pathways.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. All Drosophila stocks are available upon request. RNA-seq data are available at GEO with the accession number: GSE151935. Supplemental material available at figshare: https://doi.org/10.25386/genetics.12689318.
Results
Depletion 7SK snRNP complex components cooperates with Yki in causing tumorous growth
Studies using Drosophila tumor models have found that larvae containing proliferating tumors are unable to enter pupariation and continue to grow (Gateff et al. 1993). The resulting giant larva phenotype can be used in genetic screens to identify tumor-causing genotypes. We made use of this property to identify candidate genes in a genetic screen for tumor suppressors cooperating with Yki [the entire screen is published elsewhere (Groth et al. 2019)]. We found that RNAi-mediated depletion of bin3 or Hexim, components of the 7SK snRNA complex in combination with Yki overexpression led to massive overgrowth in wing disc tissue and giant larval phenotype (Figure 1B). Wing discs expressing Yki alone show only moderate overgrowth phenotype, and larvae eventually pupate (Figure 1B). Depletion of 7SK snRNP components did not produce overgrowth on their own (Supplemental Material, Figure S1), but only did so when coupled with Yki overexpression. We also did not observe wing disc overgrowth when depletion of 7SK snRNP components in combination with overexpression of other well-known oncoproteins such as epidermal growth factor receptor (EGFR) or notch intracellular domain (NICD) (Figure S2). Thus, our observations suggest that, Drosophila 7SK snRNP complex may function, specifically, to repress tumorigenic potential of Yki in vivo in an epithelial tumor model.
Components of the NELF complex may function as tumor suppressors
The NELF complex is composed of four subunits: NELF-A, -B, -C/-D and -E. Depletion of each of the NELF components using RNAi in combination with Yki also produced a giant larval phenotype (Figure 1B) and massively overgrown wing disc tissue compared to the larvae overexpressing only Yki (Figure 1B). Depletion of the NELF components on their own did not cause such giant larval phenotype or overgrowth of the wing disc tissue (Figure S1). These components too did not show any tumor phenotype in the context of overexpressed EGFR or NICD (Figure S2).
It was intriguing to find multiple components of the two spatio-temporally separated protein complexes, involved in the regulation of transcription elongation, among the tumor suppressors identified in a genome-wide screen for factors cooperating with Yki in growth regulation (Groth et al. 2019).
Neoplastic transformation induced by Yki combined with depletion of 7SK snRNP or NELF complexes
Yki is known to promote cell proliferation and cell survival. Thus, it is possible that larger size of the wing disc tissue observed upon loss of either 7SK snRNP or NELF complex is a result of enhancement of growth and survival effect of Yki, and not a neoplastic transformation. To distinguish between the two possibilities, we analyzed the tumor tissue using markers that indicate neoplastic transformation.
First, we examined epithelial cell polarity. Neoplastic transformation of an epithelial tissue is accompanied by the loss of their characteristic apico-basal cell polarity. E-cadherin (E-Cad) is a subapically localized protein that provides a convenient marker for epithelial polarization (Tanos and Rodriguez-Boulan 2008). Wing discs overexpressing Yki alone showed localization of E-Cad, in a pattern similar to the wild-type wing discs, although the former discs are much larger (Figure 2A). This indicated that Yki overexpression caused overgrowth of the epithelium without perturbation of epithelial cell polarity. In contrast, when Yki overexpression was combined with depletion of a component of the 7SK snRNP complex or the NELF complex, subapical localization of E-cad was lost or perturbed (Figure 2A). Additionally, we analyzed F-Actin, which localizes near the apical junctions of the wing disc epithelial cells, using rhodamine-labeled phalloidin. As with E-Cad, we observed loss of apical localization of F-actin in the Yki expressing tissue depleted of a component of the 7SK snRNP or the NELF complex, but not in wing disc tissue expressing Yki alone (Figure S3). We did not observe any change in cell polarity, as indicated by E-Cad or F-Actin localization in wing discs with depletion of components of 7SK snRNP and NELF complexes alone (Figure S4A; data not shown for F-Actin).
Figure 2.
Characterization of tumors induced in the wing disc. (A) Disruption of characteristic epithelial apico-basal polarity in tumor discs. Images of wing discs overexpressing Yki alone (crossed to UAS-GFP as control) or in combination with RNAi-mediated knockdown of Hexim, bin3, NelfA or NelfB using GAL80TS; ap-GAL4; UAS-GFP (Bar, 10 µm). Discs are stained for E-Cadherin (white) expression and localization. Bottom panel of each image shows orthogonal optical section of respective genotype. Note delocalization of E-Cad in tumorous tissues caused by the depletion of a component of PPP and Yki overexpression (higher magnification images are shown for few genotypes). All discs are also stained with DAPI (blue) to visualize nuclei. (B) Increased expression of MMP1 is observed in tumor discs. Images of wing discs overexpressing Yki alone (crossed to UAS-GFP as control) or in combination with RNAi-mediated knockdown of Hexim, bin3, NelfA, or NelfB using GAL80TS; ap-GAL4; UAS-GFP (Bar, 100 µm). Wing discs are stained for MMP1 (white). Note increased MMP1 staining in tumorous tissues caused by the depletion of a component of PPP and Yki overexpression. All discs are also stained with DAPI (blue) to visualize nuclei.
The matrix metallo-protease MMP1 has been used as a marker of epithelial to mesenchymal transition (EMT) and neoplastic transformation in Drosophila tumor models. MMP1expression is elevated in tumor models and its depletion by RNAi has been reported to block metastasis (Uhlirova and Bohmann 2006; Beaucher et al. 2007; Miles et al. 2011). We examined the effects of depleting components of 7SK snRNP and NELF complexes in Yki-expressing tissue on the levels of MMP1 by immunohistochemistry. We observed significantly elevated levels of MMP1 in wing discs overexpressing Yki and depleted for Bin3, Hexim, or the NELF complex (Figure 2B, Figure S5A). We observed only marginal increase (statistically insignificant) in MMP1 levels in wing discs expressing Yki alone (Figure 2B, Figure S5A). We did not observe any detectable change in the intensity of MMP1 levels in the wing discs depleted for the components of 7SK snRNP and NELF complexes alone (Figure S4B and Figure S5B).
Taken together, tumors formed upon depletion of 7SK snRNP or NELF complex components in combination with Yki exhibit neoplastic characters. As neither genetic change alone produced these results, it appears that they act in combination to promote neoplasia, a classical mechanism of cooperative tumorigenesis as known in mammals. These observations provide evidence that the activity of 7SK snRNP and NELF complexes may have a tumor-suppressing function, but only in the context of elevated Yki activity.
CDK9 is required for Yki-mediated tumorigenesis
The 7SK snRNP and NELF complexes help in maintaining the paused state of RNA Pol II. Our findings raised the question of whether pausing of RNA Pol II per se served to limit the tumor promoting potential of Yki activity. If this is the case, we reasoned that using an alternative means to release RNA Pol II should also lead to tumorigenesis in the context of Yki overexpression. The P-TEFb complex, comprising cycT/CDK9, is required for release of paused RNA Pol II and effective elongation of mRNA. CDK9 phosphorylates the NELF complex, leading to eviction of NELF from the pause site. This in turn facilitates release of paused RNA Pol II, aiding in productive elongation. CDK9 also acts directly on RNA Pol II, phosphorylating it on S5 in the C-terminal domain, a known mark of elongating RNA Pol II (Jennings 2013). As the P-TEFb complex is normally rendered inactive through sequestration by 7SK snRNP complex, we hypothesized that overexpressing CDK9 might bypass normal regulation of pausing, leading to inactivation of NELF complex and RNA Pol II release. Consistent with this hypothesis, we indeed observed massive tissue overgrowth when Yki was co-expressed with CDK9, while overexpression of CDK9 alone did not cause any such phenotype (Figure 3A). Such overgrowth phenotype was not observed when CDK9 was overexpressed in the background of elevated activities of EGFR or Notch (Figure S2). This suggests that PPP-mediated regulation of growth is Yki-specific.
Figure 3.
CDK9 cooperates with Yki in tumorigenesis. (A) Larval images showing growth observed in combination of UAS-CDK9 and UAS-Yki as compared to UAS-CDK9 alone (crossed to UAS-GFP as control) using GAL80TS; ap-GAL4; UAS-GFP. The combined overexpression phenocopies the phenotypes observed in Figure 2B. (B) Characterization of tumor tissue caused by combined overexpression of CDK9 and Yki using GAL80TS; ap-GAL4. Top row of images shows wing disc tissue overexpressing CDK9 alone, while the bottom row shows combined overexpression of CDK9 and Yki. Discs in the left column are stained for E-Cadherin (white) (Bar, 10 µm) and those in the right column are stained for MMP1 (white) expression (Bar, 100 µm). Please note deregulated E-cad localization (optical z-sections and two different magnification levels are shown below the discs) and increased MMP1 expression in tissues that overexpress both CDK9 and Yki, suggesting their neoplastic tumor state. All discs are also stained with DAPI (blue) to visualize nuclei. Both the discs stained for MMP1 are imaged at lower magnification (10X) for better comparison, as tumorous disc is too large to show at higher magnification.
Wing discs expressing UAS-CDK9 together with UAS-Yki also showed loss of apically localized E-Cad as well as elevated MMP1 expression (Figure 3B), compared to tissue expressing UAS-CDK9 alone or UAS-Yki alone. This indicates neoplastic transformation in wing discs co-expressing Yki and CDK9, similar to the transformation caused by depletion of 7SK snRNP and NELF complex components in combination with overexpressed Yki.
As further test of this model, we asked whether CDK9 is essential for tumorigenic cooperation between depletion of 7SK snRNP complex components and Yki. Depletion of cdk9 effectively suppressed the tissue overgrowth caused by depleting bin3 or Hexim in Yki expressing tissue (Figure 4A). Those wingdiscs also showed normal apical localization of E-Cad and wildtype levels of MMP1 expression, suggesting complete suppression of tumorous growth (Figure 4, B and C).
Figure 4.
CDK9 is necessary for Yki-mediated tumorigenesis. (A) Loss of CDK9 rescues tumor phenotype. The images show GFP-expressing wing discs of various genotypes as indicated. Size of the wing discs may be discerned by the amount of larval space occupied by GFP-expressing tissue. RNAi-mediated depletion of cdk9 inhibited tumor formation caused by a combination of overexpression of Yki and depletion of a component of the PPP. The GFP-marked wing tissue is of the same size as in controls. All crosses were using GAL80TS; ap-GAL4; UAS-GFP. (B) Tumorous wing disc phenotypes caused by the overexpression of Yki in the background of depletion of bin3 or NelfA shown here again as a control to (A). (C) Restoration of apico-basal polarity in wing disc tissue. The images show wing discs of various genotypes as indicated stained for E-Cad (red). RNAi-mediated depletion of cdk9 restored normal apical localization of E-Cad (optical z-sections are shown below the discs) in wing discs that overexpress Yki and are also depleted for a component of the PPP. All discs are also stained with DAPI (blue) to visualize nuclei (Bar, 10 µm). (D) Tumorous wing discs (stained for E-Cad) of larvae overexpressing Yki in the background of depletion of bin3 or NelfA shown here again as a control to (C). (E) Restoration of MMP1 levels. The images show wing discs of various genotypes as indicated stained MMP1 (white). RNAi-mediated depletion of cdk9 restored normal levels of MMP1 in wing discs that overexpress Yki and also depleted for a component of the PPP. All discs are also stained with DAPI (blue) to visualize nuclei (Bar, 100 µm). (F) Tumorous wing discs (stained for MMP1) of larvae overexpressing Yki in the background of depletion of bin3 or NelfA shown here again as a control to (E).
Given that CDK9 is known to act directly on both NELF proteins and RNA Pol II, we wondered whether CDK9 activity would be required in the absence of the NELF complex. As shown above in the case of removing the 7SK snRNP complex, depletion of cdk9 suppressed overgrowth caused by RNAi-mediated depletion of NelfA and overexpression of Yki (Figure 4A). This was accompanied by restoration of apico-basal polarity and MMP1 expression to wild-type levels (Figure 4, B and C). This finding provides evidence that alleviation of pausing by removal of NELF complex is not sufficient without CDK9 activity. This presumably reflects an importance of activation of RNA Pol II by CDK9-mediated phosphorylation.
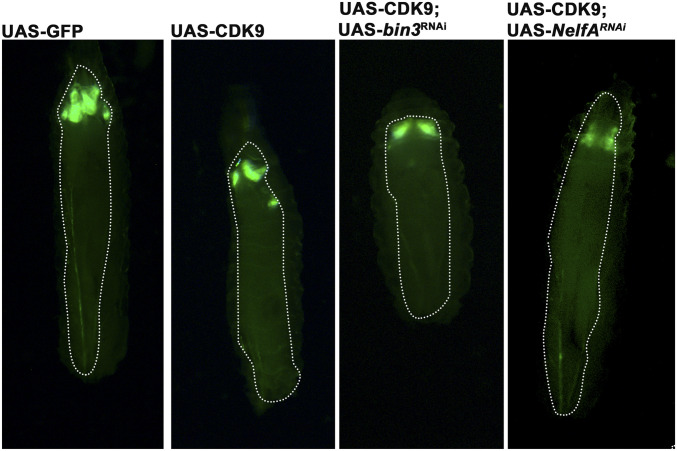
We then examined if depletion of the complexes associated with PPP and increased CDK9 levels are sufficient to cause overgrowth phenotype, or whether the growth is tightly coupled to the presence of a growth driver such as Yki. Depletion of components of 7SK snRNP or NELF complexes in the background of overexpressed CDK9 did not cause any growth phenotype or morphological alteration in wing disc epithelium (Figure 5). This suggests that deregulation of RNA Pol II pausing is not sufficient on its own to produce an overgrowth or neoplastic phenotype; yet it does so in the context of Yki overexpression. In the context of elevated Yki activity, there appear to be two brakes, each of which must be removed by CDK9 activity to allow excess Yki to produce tumors in Drosophila wing disc tissue.
Figure 5.
Yki is the driver of tumorigenesis. Larval images showing phenotype of UAS GFP in combination with (left to right) UAS-GFP, UAS-CDK9 followed by UAS-CDK9 and UAS-bin3RNAi, UAS-CDK9, and UAS-NelfARNAi. None of them show overgrowth phenotype as observed when Yki is overexpressed, suggesting CDK9 may induce tumorous growth only in the context of overexpressed Yki. All crosses were using GAL80TS; ap-GAL4; UAS-GFP.
Tumorigenesis induced by alleviation of pausing is associated with deregulated proteostasis
As overexpression of Yki was essential, although not sufficient, to cause neoplastic tumors, genetic experiments above provided an opportunity to distinguish between Yki-activated genes that cause simple hyperplastic growth of the discs (when Yki is overexpressed in a wild-type background) vs. causing neoplastic growth (when Yki is overexpressed along with depletion of bin3, Hexim, or NELFs).
We carried out RNA-seq to identify differentially expressed genes in discs depleted for NelfA and overexpressing Yki as well as both individual treatments. We also carried out RNA-seq for GFP expressing wild-type wing discs as a control. We find that transcripts corresponding to 776 genes were uniquely upregulated (Figure 6A) and 1009 genes were uniquely downregulated (Figure 6B) in the tumorous wing discs (ap > UAS-Yki; UAS-NelfARNAi), compared to all other genotypes including wild-type discs (noncoding transcripts are not included in this estimation). When compared to the list of direct targets of Yki (reported by based on ChIP-seq data), we find 38 (4.9%) of the upregulated genes and 84 (8.3%) of the downregulated genes are presumptive direct targets of Yki (Table S1).
Figure 6.
Identification of genes potentially involved in Yki-mediated tumorigenesis. (A) Venn diagram showing number of common and unique genes, who expression is upregulated in comparison with ap > GFP from different genotypes as indicated in the figure. (B) Venn diagram depicting number of common and unique genes downregulated in comparison with ap > GFP from different genotypes.
We also observed an enhancement of effect of Yki (compared to wildtype discs) in a subset of transcripts that were common to nontumorous tissue overexpressing Yki alone (ap > UAS-Yki) and tumorous ap > UAS-Yki; UAS-NelfARNAi tissue. We reasoned that since PPP functions to attenuate expression of genes, identifying transcripts whose expression is further up- or downregulated in ap > UAS-Yki; UAS-NelfARNAi tissue (compared to ap > UAS-Yki) may give a better indication of the role of PPP in Yki-mediated growth. We find that transcripts corresponding to 155 genes that are upregulated in both nontumorous ap > UAS-Yki discs and tumorous ap > UAS-Yki; UAS-NelfARNAi discs, but degree of enhancement was higher in tumorous tissue. Likewise, these transcripts corresponding to 160 genes, whose expression was downregulated compared to wildtype discs, were common to both nontumorous and tumorous tissue, but degree of downregulation was higher in tumorous tissue. Interestingly, 31 (20%) of these upregulated genes (n = 155) and 35 (21.9%) of downregulated genes were presumptive direct targets of Yki, suggesting that we indeed have captured many targets of Yki that are regulated by PPP and misregulated due to RNAi medicated knockdown of many components of the pausing machinery.
We used genes corresponding to these transcripts to perform GO analysis in order to explore gene sets that show enrichment and might indicate pathways or processes that are involved in tumorigenesis. For this purpose, the STRING tool was utilized (Szklarczyk et al. 2017). STRING output is based on statistical enrichment score of interactions obtained from the input compared to a random set of genes from the genome of the organism, in this case D. melanogaster. STRING also collates data from manually curated databases of interactions such as Kyoto Encyclopedia of Genes and Genomes (KEGG) and GO terms.
We observed enrichment for pathways involved in ribosome and its biogenesis in the upregulated set (Table 1 and Figure 6). Interestingly, protein processing in endoplasmic reticulum, regulators of proteasome function, and different components of proteasome were enriched among genes downregulated in tumorous tissues (Table 2). These observations indicate overall deregulation of protein homeostasis (proteostasis) in tumors caused by depletion of NelfA in combination with Yki overexpression, consistent with recent data on human cancers (Ruggero 2013; Pelletier et al. 2017).
Table 1. List of genes whose expression is upregulated in the wing discs of ap-GAL4/UAS-NelfARNAi; UAS-Yki.
| Aminoacyl-tRNA biosynthesis | Ribosome | Ribosome biogenesis in eukaryotes | |||
|---|---|---|---|---|---|
| Gene name | logFC | Gene name | logFC | Gene name | logFC |
| Slimp | 2.124626 | RpL24-like | 1.256082 | Non1 | 2.292457 |
| Aats-leu | 1.413973 | RpL5 | 1.202779 | Ns2 | 0.900983 |
| Aats-thr | 0.912237 | RpL15 | 1.130382 | RIOK1 | 1.354352 |
| Aats-cys | 0.813931 | mRpL28 | 0.969423 | CG12301 | 0.997310 |
| Aats-tyr-m | 1.047768 | mRpL9 | 0.799329 | Bka | 0.876333 |
| Aats-pro | 1.07342 | RpS17 | 0.82042 | eIF6 | 0.745744 |
| Aats-ile | 0.73741 | mRpL35 | 0.997681 | l(3)72Dn | 0.800876 |
| CG4573 | 1.138148 | RpS23 | 0.782542 | CG8064 | 0.778604 |
| CG1750 | 1.487797 | RpS4 | 0.775449 | Nmd3 | 0.716537 |
| CG6796 | 0.925494 | RpL27A | 0.716573 | Mat89Ba | 0.713426 |
| CG7441 | 0.884721 | RpL32 | 0.681588 | CG11920 | 0.750235 |
| CG17259 | 0.726080 | RpL40 | 0.674508 | CG3071 | 0.713535 |
| Aats-trp | 0.732224 | RpS29 | 0.743389 | CG33158 | 0.595732 |
| Aats-asp | 0.747097 | RpL26 | 0.620538 | CG13185 | 0.823244 |
| Aats-gly | 0.613889 | mRpL10 | 0.692489 | CG7246 | 0.798345 |
| CG5463 | 1.037030 | mRpL3 | 0.671734 | CG8549 | 0.593618 |
| Aats-ala-m | 0.602770 | RpL35 | 0.631348 | ||
| CG5660 | 0.663614 | RpL27 | 0.594275 | ||
| RpL28 | 0.629209 | ||||
| RpL21 | 0.600412 | ||||
| RpL22-like | 1.081389 | ||||
| RpS3A | 0.587288 | ||||
| RpL37A | 0.3662 | ||||
| mRpL11 | 0.624297 | ||||
Table 2. List of genes whose expression is downregulated in the wing discs of ap-GAL4/UAS-NelfARNAI; UAS-Yki.
| Proteasome | Protein processing in endoplasmic reticulum | ||
|---|---|---|---|
| Gene name | logFC | Gene name | logFC |
| Rpn7 | −1.084698 | prtp | −1.46289 |
| Rpn13 | −0.949757 | Sec61gamma | −1.65593 |
| Rpn2 | −0.900781 | Sec61beta | −1.31964 |
| Prosalpha3 | −0.910536 | CG5885 | −1.28449 |
| Rpn3 | −0.850895 | Sec61alpha | −1.25184 |
| Rpn1 | −0.879125 | TRAM | −1.3381 |
| Pomp | −0.795409 | Pdi | −1.145 |
| Prosalpha5 | −0.834712 | SsRbeta | −1.23571 |
| Prosbeta4 | −0.76685 | Sec13 | −1.01315 |
| Prosbeta7 | −0.717883 | Sec63 | −0.97489 |
| Prosbeta2 | −0.706638 | CG14476 | −1.03296 |
| Prosbeta5 | −0.687177 | Sec24CD | −0.87522 |
| Prosalpha4 | −0.694159 | Ostgamma | −0.97374 |
| Prosbeta6 | −0.631819 | Ost48 | −0.88256 |
| Rpn10 | −0.592037 | CG4164 | −1.21065 |
| Rpn12 | −0.597364 | ergic53 | −0.86625 |
| Plap | −0.86843 | ||
| OstStt3 | −0.90274 | ||
| Gp93 | −0.89197 | ||
| l(1)G0320 | −0.88951 | ||
| CG33303 | −0.8065 | ||
| Hsc70-3 | −0.9493 | ||
| CG5510 | −0.81474 | ||
| p47 | −0.78553 | ||
| Crc | −0.86323 | ||
| CG6453 | −0.81869 | ||
| Sec23 | −0.73903 | ||
| ERp60 | −0.76882 | ||
| Der-1 | −0.80252 | ||
| Csp | −0.64369 | ||
| CaBP1 | −0.61193 | ||
| CG1597 | −0.67306 | ||
Discussion
PPP has emerged as a critical regulatory step in gene expression (Core and Adelman 2019). It involves stalling of RNA Pol II 20–60 nucleotides downstream of the transcription start site, and controlled release of RNA Pol II when triggered by signals from the surroundings. Many studies in recent years have elucidated mechanisms by which RNA Pol II is stalled and the factors that bring about pausing as well as release of the paused RNA Pol II. Our in vivo model for tumorigenesis has allowed us to elucidate the functions of the NELF, 7SKsnRNP, and P-TEFb complexes in the context of growth control in vivo. Previous studies have implicated NELF in regulating the response of embryonic stem cells to signaling cues such as fibroblast growth factor (FGF; Williams et al. 2015). Furthermore, PPP has been shown to be important for coordination of expression genes involved in morphogenesis of Drosophila embryo (Lagha et al. 2013). Our findings provide direct evidence that PPP can limit tumor formation in the context of the Hippo tumor suppressor pathway. Depletion of these factors alone, or even in combination with overexpression of CDK9, was not sufficient to induce tumorous growth but did so when combined with overexpression of Yki. This cooperation appears to be specific to Yki-induced tumors as there was no cooperation with other oncogenic drivers such as EGFR or activated Notch in wing disc tumor models. This suggests that pausing plays a previously unappreciated role regulating the output of Hippo pathway in growth control, thereby limiting its tumorigenic potential.
We were intrigued by the finding that CDK9 activity is required for Yki-driven tumor formation, even when the upstream and downstream pausing complex factors have been removed. These observations suggest that CDK9 activity is required not only to remove the “brake” exerted by NELF pausing complex, but also required to increase RNA Pol II activity through direct phosphorylation. Neither alone is sufficient. This suggests an overlapping “belt and suspenders” regulation to ensure that expression of Yki targets is maintained at appropriate levels for normal growth control, while preventing overexpression, which may lead to tumorigenesis. A mechanism of this sort allows for the possibility that other growth regulatory or metabolic homeostasis pathways might impact on the outcome of Yki activity via regulation of the CDK9. Indeed, evidence of a role for CDK9 in YAP/TAZ-mediated cell growth via regulation of a subset of YAP/TAZ target genes in mammalian liver cells has been demonstrated (Galli et al. 2015). Inhibition of CDK9 activity using flavopiridol nullified the effect of YAP S127A mutant form (the constitutively active form of YAP) on the expression of YAP target genes studied (Galli et al. 2015). Although this observation is not validated in fly tissues, perhaps PPP (including 7skRNP-, CDK9-, and NELFs)-dependent regulation of Yki is independent of the phosphorylation status of Yki, which implies a parallel function for PPP rather than it being upstream of Yki.
Our genetic model is also useful to study the importance of PPP in attenuating transcriptional output at genome wide scale. Preliminary observations of data generated by RNA-seq suggest that most genes that are differentially expressed when Yki is overexpressed show further changes in the same direction (up or down regulation) in combination of Yki overexpression with depletion of Nelf-A. Furthermore, we also report deregulation of proteostasis uniquely in tumor tissue. This is consistent with recent reports that deregulation of translation and deregulation of protein processing are important factors in progression of cancers and might be target for therapy (Ruggero 2013; Pelletier et al. 2017).
To conclude, our study has highlighted additional regulatory module on Yki driven tumorigenic activity, which impinges directly on transcription. It will be interesting to see the role of the PPP machinery, which has been reported to be highly conserved from Drosophila to humans (Peterlin and Price 2006), in the context of highly conserved Hippo pathway effectors YAP/TAZ. Considering the reported function of CDK9 in YAP-driven transcription, and the therapeutic accessibility of CDK9 activity (Galli et al. 2015; Blake et al. 2019), it is critical to understand the function of 7SK snRNP and NELF complexes in this context.
Acknowledgments
We thank G. Deshpande and members of the LSS and SMC laboratories for critical input. This work was supported primarily by an Indo-Danish research grant from the Department of Biotechnology, Government of India to L.S.S. and from the Innovation fund Denmark, Novo Nordisk Foundation NNF12OC0000552 and Neye Foundation to SMC; a JC Bose Fellowship and grant from the Department of Science and Technology, Government of India to LSS; and a University Grants Commission (UGC) Research Fellowship to SN.
Author contributions: S.N., P.G. and R.W. carried out all fly experiments. S.N. did RNA-seq and its analysis, all image analyses, and wrote the MS. L.S.S. and SM conceived the project and wrote the MS. We declare “no-conflict-of-interest”.
Note added in proof: See Groth et al. 2020 (pp. 2999–3008) in G3 10:9 for a related work.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.12689318.
Communicating editor: P. Geyer
Literature Cited
- Anders S., and Huber W., 2010. Differential expression analysis for sequence count data. Genome Biol. 11: R106 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaucher M., Hersperger E., Page-McCaw A., and Shearn A., 2007. Metastatic ability of Drosophila tumors depends on MMP activity. Dev. Biol. 303: 625–634. 10.1016/j.ydbio.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Blake D. R., Vaseva A. V., Hodge R. G., Kline M. P., Gilbert T. S. K. et al. , 2019. Application of a MYC degradation screen identifies sensitivity to CDK9 inhibitors in KRAS-mutant pancreatic cancer. Sci. Signal. 12: eaav7259 10.1126/scisignal.aav7259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., McGuffin M. E., Pfeifle C., Segal D., and Cohen S. M., 1992. apterous, a gene required for imaginal disc development in Drosophila encodes a member of the LIM family of developmental regulatory proteins. Genes Dev. 6: 715–729. 10.1101/gad.6.5.715 [DOI] [PubMed] [Google Scholar]
- Core L., and Adelman K., 2019. Promoter-proximal pausing of RNA polymerase II : a nexus of gene regulation. Genes Dev. 33: 960–982. 10.1101/gad.325142.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Feldmann G., Huang J., Wu S., Zhang N. et al. , 2007. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130: 1120–1133. 10.1016/j.cell.2007.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G. G., Carrara M., Yuan W. C., Valdes-Quezada C., Gurung B. et al. , 2015. YAP drives growth by controlling transcriptional pause release from dynamic enhancers. Mol. Cell 60: 328–337. 10.1016/j.molcel.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateff E., Löffler T., and Wismar J., 1993. A temperature-sensitive brain tumor suppressor mutation of Drosophila melanogaster: developmental studies and molecular localization of the gene. Mech. Dev. 41: 15–31. 10.1016/0925-4773(93)90052-Y [DOI] [PubMed] [Google Scholar]
- Groth C., Vaid P., Khatpe A., Prashali N., Ahiya A. et al. , 2020. Genome-wide screen for context-dependent tumor suppressors identified using in vivo models for neoplasia in Drosophila. G3 (Bethesda) DOI: 10.1534/g3.120.401545. [DOI] [PMC free article] [PubMed]
- Guenther M. G., Levine S. S., Boyer L. A., Jaenisch R., and Young R. A., 2007. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130: 77–88. 10.1016/j.cell.2007.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Wu S., Barrera J., Matthews K., and Pan D., 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122: 421–434. 10.1016/j.cell.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Jennings B. H., 2013. Pausing for thought: disrupting the early transcription elongation checkpoint leads to developmental defects and tumourigenesis. BioEssays 35: 553–560. 10.1002/bies.201200179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., and Halder G., 2014. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 13: 63–79. 10.1038/nrd4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers I., and Lis J. T., 2015. Getting up to speed with transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 16: 167–177. 10.1038/nrm3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa S., Soucek L., Evan G., Okamoto T., and Peterlin B. M., 2003. c-Myc recruits P-TEFb for transcription, cellular proliferation and apoptosis. Oncogene 22: 5707–5711. 10.1038/sj.onc.1206800 [DOI] [PubMed] [Google Scholar]
- Kwak H., and Lis J. T., 2013. Control of transcriptional elongation. Annu. Rev. Genet. 47: 483–508. 10.1146/annurev-genet-110711-155440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagha M., Bothma J. P., Esposito E., Ng S., Stefanik L. et al. , 2013. Paused Pol II coordinates tissue morphogenesis in the drosophila embryo. Cell 153: 976–987. 10.1016/j.cell.2013.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N. F., and Price D. H., 1995. Purification of P-TEFb, a transcription factor required for the transition into productive elongation. J. Biol. Chem. 270: 12335–12338. 10.1074/jbc.270.21.12335 [DOI] [PubMed] [Google Scholar]
- Miles W. O., Dyson N. J., and Walker J., 2011. Modeling tumor invasion and metastasis in Drosophila. Dis. Model. Mech. 4: 753–761. 10.1242/dmm.006908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muse G. W., Gilchrist D. A., Nechaev S., Shah R., Parker J. S. et al. , 2007. RNA polymerase is poised for activation across the genome. Nat. Genet. 39: 1507–1511. 10.1038/ng.2007.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D., Krueger B. J., Sedore S. C., Brogie J. E., Rogers J. T. et al. , 2012. The Drosophila 7SK snRNP and the essential role of dHEXIM in development. Nucleic Acids Res. 40: 5283–5297. 10.1093/nar/gks191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholtzer M., Zhang J., Smolen G. A., Muir B., Li W. et al. , 2006. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. USA 103: 12405–12410. 10.1073/pnas.0605579103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., 2010. The hippo signaling pathway in development and cancer. Dev. Cell 19: 491–505. 10.1016/j.devcel.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin B. M., and Price D. H., 2006. Controlling the elongation phase of transcription with P-TEFb. Mol. Cell 23: 297–305. 10.1016/j.molcel.2006.06.014 [DOI] [PubMed] [Google Scholar]
- Pelletier J., Thomas G., Volarevic S., and Volarević S., 2017. Nrc.2017.104. Nature 18: 51. [Google Scholar]
- Ruggero D., 2013. Translational control in cancer etiology. Cold Spring Harb. Perspect. Biol. 5: a012336 [Corrigenda: Cold Spring Harb. Perspect. Biol. 4 (2012)]. 10.1101/cshperspect.a012336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Morris J. H., Cook H., Kuhn M., Wyder S. et al. , 2017. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 45: D362–D368. 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanos B., and Rodriguez-Boulan E., 2008. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene 27: 6939–6957. 10.1038/onc.2008.345 [DOI] [PubMed] [Google Scholar]
- Uhlirova M., and Bohmann D., 2006. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 25: 5294–5304. 10.1038/sj.emboj.7601401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T., Takagi T., Yamaguchi Y., Ferdous A., Imai T. et al. , 1998. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 12: 343–356. 10.1101/gad.12.3.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. H., Fromm G., Gokey N. G., Henriques T., Muse G. W. et al. , 2015. Pausing of RNA polymerase II regulates mammalian developmental potential through control of signaling networks. Mol. Cell 58: 311–322. 10.1016/j.molcel.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y. Y., Takagi T. T., Wada T. T., Yano K. K., Furuya A. A. et al. , 1999. NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell 97: 41–51. 10.1016/S0092-8674(00)80713-8 [DOI] [PubMed] [Google Scholar]
- Zanconato F., Forcato M., Battilana G., Azzolin L., Quaranta E. et al. , 2015. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 17: 1218–1227. 10.1038/ncb3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlinger J., Stark A., Kellis M., Hong J.-W. W., Nechaev S. et al. , 2007. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat. Genet. 39: 1512–1516. 10.1038/ng.2007.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L., Spector M. S., Xue W., Flemming P., Cordon-Cardo C. et al. , 2006. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125: 1253–1267. 10.1016/j.cell.2006.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Tumaneng K., and Guan K. L., 2011. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 13: 877–883. 10.1038/ncb2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. All Drosophila stocks are available upon request. RNA-seq data are available at GEO with the accession number: GSE151935. Supplemental material available at figshare: https://doi.org/10.25386/genetics.12689318.