Abstract
Background: Three previous clinical trials have found that thermometry use reduced diabetic foot ulcers (DFUs) incidence four- to ten-fold among individuals with diabetes at high-risk of developing a DFU. However, these benefits depend on patient adherence to self-assessment. Therefore, novel approaches to improve self-management thermometry adherence are needed. Our objective was to compare incidence of DFUs in the thermometry plus mobile health (mHealth) reminders intervention arm vs. thermometry-only control arm.
Methods: We conducted a randomized trial, enrolling adults with type 2 diabetes mellitus at risk of foot ulcers (risk groups 2 or 3) but without foot ulcers at the time of recruitment and allocating them to control (instruction to use a liquid crystal-based foot thermometer daily) or intervention (same instruction supplemented with text and voice messages with reminders to use the device and messages to promote foot care) groups and followed for 18 months. The primary outcome was time to occurrence of DFU. A process evaluation was also conducted.
Results: A total of 172 patients (63% women, mean age 61 years) were enrolled; 86 to each study group. More patients enrolled in the intervention arm had a history of DFU (66% vs. 48%). Follow-up for the primary endpoint was complete for 158 of 172 participants (92%). DFU cumulative incidence was 24% (19 of 79) in the intervention arm and 11% (9 of 79) in the control arm. After adjusting for history of foot ulceration and study site, the Hazard Ratio (HR) for DFU was 1.44 (95% CI 0.65, 3.22). Adherence to ≥80% of daily temperature measurements was 87% (103 of 118) among the study participants who returned the logbook, with no difference between the intervention and control arms.
Conclusions: This trial contributes to the evidence about the value of mHealth in preventing diabetes foot ulcers.
Trial registration: ClinicalTrials.gov NCT02373592 (27/02/2015)
Keywords: type 2 diabetes mellitus, diabetic foot ulcer, prevention, implementation, mHealth
Background
The prevalence of type 2 diabetes mellitus in the adult population worldwide has doubled from 4.7% in 1980 to 8.5% in 2014 1. Low- and middle-income countries (LMICs) are disproportionally affected by diabetes, since diabetes-related complications, such as diabetic foot ulcer (DFU), are more frequent in these contexts 1, 2. In the US, 60%–70% of people with diabetes will develop peripheral neuropathy 3. This is important since one in four patients with peripheral neuropathy will develop a DFU, which will increase the risk of foot amputation significantly 4.
Thermometry is a tool that can identify early signs of foot inflammation, thus providing early signals to enact management and reduce the incidence of DFU and amputation 5. Three previous clinical trials have found that thermometry use reduced DFU incidence four- to ten-fold among individuals with diabetes at high-risk of developing a DFU 6– 8. However, these benefits depend on patient adherence to self-assessment, and foot temperature should be evaluated on at least half of the days to effectively reduce the risk of foot ulceration 7. Yet, adherence could be challenging, especially in LMIC settings. Therefore, novel approaches to improve self-management thermometry adherence are needed. In this context, interventions using short message service (SMS) for diabetes management have been found to be useful to improve self-efficacy, social support 9, and clinical diabetes-related outcomes 10.
We propose to evaluate the efficacy of a combination of foot thermometry plus mobile health (mHealth)-delivered reminders, using SMS and voice messaging, in reducing DFU in Peru. Our objective was to compare incidence of DFU in the thermometry plus mHealth reminders intervention arm vs. thermometry-only control arm.
Methods
Trial design
This was a physician- and evaluator-blinded, 18-month, randomized clinical trial with two parallel arms and a 1:1 allocation. Details of the intervention and the study protocol have been published elsewhere 11. We followed the extension of the CONSORT 2010 statement for reporting pragmatic trials 12.
Although initially planned to follow participants for 12 months, we decided to extent the follow-up period to 18 months to accrue enough DFU events, as we noticed that the frequency of DFU at six months was lower than we expected. Thus, only the extension of the trial follow-up was changed without affecting randomization or assessment rates. There were no other deviations from the original trial protocol.
Participants
Participants were recruited at the outpatient clinics of two third-level public hospitals in Lima, Peru; Hospital Nacional Cayetano Heredia and Hospital Nacional Arzobispo Loayza. In some cases, physicians referred the patient to the study fieldworkers to perform a foot evaluation and in other cases fieldworkers conducted an active search for potential participants in the waiting room of the Endocrinology clinic.
Patients were eligible if they: had a diagnosis of type 2 diabetes mellitus; were between 18 and 80 years of age; were in risk group 2 or 3 using the diabetic foot risk classification system as specified by the International Working Group on the Diabetic Foot (neuropathy and deformity = category 2, history of ulcer and/or amputation = category 3) 13– 15; had a palpable dorsalis pedis pulse in both feet; had an operating cell phone or a caregiver with an operating cell phone; and had the ability to provide informed consent. Patients were considered not eligible if they had current foot ulcers, active Charcot osteoarthropathy, severe peripheral arterial disease, or foot infection.
Our eligibility criteria differ from previous studies that used thermometry devices, which only included diabetic foot risk group 3 (previous ulceration), because we wanted to pursue a pragmatic approach for the prevention of DFU among people with diabetes in risk group 2 or higher.
Interventions
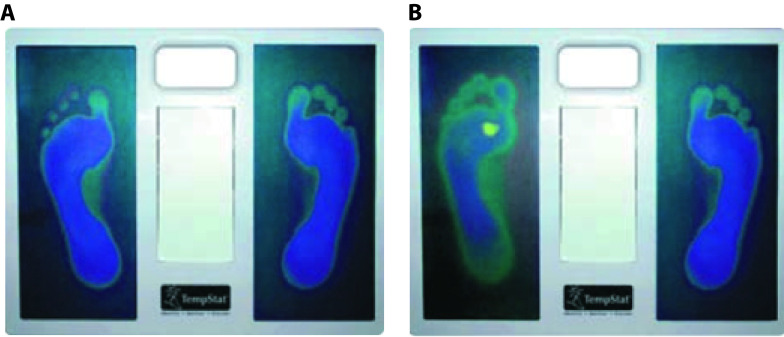
At the initiation visit, all participants received education about foot care, i.e. etiology and risk factors for the development of neuropathy and ulcers, as well as recommendations for foot care practices and early signs of ulceration; and instructions on the use of the TempStat™ device (see Extended data). This was done through two videos that were validated by physicians and patients with type 2 diabetes mellitus, as detailed elsewhere 11. The device uses liquid crystal technology to provide a visual image of the temperatures (e.g. yellow image represents a higher temperature than blue image) ( Figure 1).
Figure 1. TempStat.
A) Normal appearance. B) Alarm sign (yellow spot)
One week after enrollment, the TempStat™ was provided to each participant. Fieldworkers instructed the participants to use the device daily and to contact them by phone or SMS if one of the alarm signs appeared in the pads of the TempStat™: two different colors in the contralateral areas of the feet or a yellow spot in any area for two consecutive days. In these cases, the nurse asked about any lesions in the feet as well as the participant’s activity in the last two weeks and provided recommendations on how to decrease activity until foot temperature normalized. Also, in cases where the alarm sign persisted more than one week, an in-person evaluation was performed to assess the patient for infection and/or a masked injury. Additionally, participants were trained to contact the study nurse in cases of dermal lesion of the foot and they were asked to be evaluated promptly by a nurse who was blind to the intervention. When a DFU was confirmed, the study nurse referred the patients to follow the standard protocol.
In the intervention arm, additional to the TempStat™ participants received the mHealth component (two reminder messages and six foot-care promotion messages) during the 18-month study period via both SMS and voice messaging. The content of these eight messages was developed and validated with 19 people with type 2 diabetes mellitus. Messages were tested using short open surveys to evaluate the clarity and appropriateness of the messages. These messages were constructed based on a literature review about the characteristics of health education messages and advice from a specialist in health communication who suggested taking into account the reading level of our population and use short messages with one single idea. Before testing them with the patients we asked colleagues with previous experience on the use of SMS for health issues to review them. Changes were introduced after their revision.
We printed each of the nine messages in one whole page, which we gave it to the participant to read it by himself/herself. Afterwards, we evaluated each message using the following six questions: (1) Is the message clear? (2) Could you tell me how would you explain the content of the message to another person? (3) Is there any word that is difficult to understand? (4) Is there something that you do not like about the message? (5) Is there any suggestion to improve the message? (6) Would you prefer to be addressed in a formal way “usted” or an informal way “tu”? (see Extended data 16).
The final version of the developed and validated messages 17 were sent at 8am approximately and daily reminders to use the TempStat™ from Monday to Friday were sent during the first two weeks of the intervention. Thereafter, for the remaining 76 weeks, patients received only two messages per week at the same time: the content alternating between reminders to use the TempStat™ and promotion of foot care (one SMS and one voice message). Messages were delivered to the participant or caregiver’s cell phones through an automated software system developed by the study team (see Software availability 18). Every week the system was evaluated by the study coordinator to verify its functionality.
Study procedures
At baseline, enrolled participants provided information to the fieldworker through questionnaires on lifestyle, history of cardiovascular disease and diabetes, current diabetes treatment, use of insoles, use of orthopedic shoes and mobile phone literacy and underwent a demographic evaluation (age, gender, educational level), socioeconomic evaluation (working status), depression assessment (Patient Health Questionnaire-9), anthropometric evaluation (weight, height and body mass index) and blood pressure measurements (see Extended data 19).
Periodic assessments of the participants involving a general checkup and lower extremity evaluation was conducted every two months by the nurse evaluator. Additionally, the nurse collected data about diabetes treatment, caregiver presence, use of insoles and/or orthopedic shoes, and had their weight and blood pressure measured ( Extended data 19). In some cases, participants could not attend to the hospital for the checkup; in those cases, we completed the visit by phone or by domiciliary visits. In the last visit at 18 months, participants were asked to return their logbook of temperature measurements. In general, participants were encouraged to maintain regular visits with their treating physician in the outpatient clinic.
Glycated hemoglobin (HbA1c) was measured at baseline, six, 12 and 18 months. Measurements at baseline and 18 months were used for the study and measurements at six and 12 months were for standard of care. HbA1c was measured using high-performance liquid chromatography (D10, BioRad, Munich, Germany). The blood sample was collected in the endocrinology clinic by the nurse evaluator during the periodic assessment at the time periods specify above. All samples were transported to be analyzed in a single facility and were checked with regular external standards and internal duplicate assays and monitored by BioRad for quality control.
Outcomes
The primary outcome was DFU. The definition was based on the American Diabetes Association criteria 20, 21 and for this study it was considered as the presence of DFU occurring at any point during the 18-month study period after randomization. The evaluator was a trained nurse blind to the intervention allocation. The identification of a DFU was through three ways: during the bimonthly clinical nurse evaluations; if an alarm sign had been noted and prompted the participant to seek clinical evaluation; or if the participant identifies a dermal lesion and seeks clinical evaluation.
The following were pre-defined as secondary outcomes: adherence to daily temperature measurement, defined as the participants having recorded their temperature measurements in the logbook on ≥80% of days, and ≥1% reduction in HbA1c when comparing the 18-month with baseline values.
Our protocol 11 considered two additional pre-defined secondary outcomes: frequency of alarm signs reported to the study nurse and alarm signs registered in the logbook. These were not analyzed because of their low frequency. The dose-response analysis of SMS and voice messaging, pre-specified as a secondary outcome in the protocol, was included as part of the process evaluation.
Sub-group analyses
Our a priori sub-group analyses were i) previous foot ulceration and ii) caregiving status, considering assistance provided to the patient with basic activities of daily living, or in the identification, prevention, or treatment of diabetes or any disability. Also, within the intervention-arm only, the type of recipient of the messaging (patient vs. caregivers) was considered for sub-group analyses. In our protocol 11, we also considered sub-group analyses of participants that use insoles and/or orthopedic shoes, but these were not analyzed due to low frequency.
Sample size
The sample size was estimated using data from previous randomized trials in study populations similar to our study population 7, 8. We expected an absolute change of 21% between the intervention arm and the control arm (9% vs 30%) and with a power of 0.9 and an alpha of 0.05, we required a sample size of 78 participants. We planned to enroll 86 participants in each study arm, anticipating a 10% dropout rate.
Randomization
We conducted stratification using hospital as a single stratum and blocks of six to generate a random allocation sequence. Sealed envelopes with codes were used to randomize participants. An independent researcher prepared the envelopes and the study nurses assigned the codes to each of the enrolled participants. The study coordinator was responsible for opening the envelopes and informing participants about their assigned study arm as per the random list.
Blinding
The participants were instructed not to discuss their treatment assignment with the blinded evaluator. Physicians providing care to study participants, nurses and the field coordinators were blind to treatment allocation.
Process evaluation
Additionally, we performed a process evaluation during the 18-month follow-up visit to a random group of participants of the two study sites. We obtained information through a set of questions and direct observation of the use of the TempStat™ with 102 participants. In addition, with 39 participants, we asked close and open questions about the messages received in the week prior to the 18-month follow-up visit. As part of this process evaluation, we aimed to know: i) if participants knew how to use the TempStat™; ii) how many SMS and voice messages were delivered by the automated system to study participants according to the automated system; iii) how many SMS and voice messages were received by study participants according to the automated system; iv) if participants understood the messages (only if participants reported that they had received a message in the previous two weeks); and v) opinions from the participants about their preferences in SMS vs. voice messages.
The process evaluation was performed by two fieldworkers different to those who delivered the intervention and data collection was conducted through observation (participants were asked to show how they used the TempStat™), questionnaire (about nursing consultation, report of communication with study nurses, reasons for communication, alarm sign detection) and open questions (related to SMS or voice messaging preferences, use of TempStat™, suggestions about how to improve the intervention) 19.
Statistical methods
To compare the rates of DFU between study arms we performed a time-to-event approximation using Cox’s regression, having time to DFU at 18 months as an outcome. Hazard ratios (HR) and their respective 95% confidence intervals (95% CI) were estimated for the primary outcome of DFU and for the a priori defined sub-group analyses. These analyses included all retained participants, regardless of the number of visits attended, following the intention-to-treat principle. The model was adjusted by site and history of previous ulcer. Evaluation of secondary outcomes of interest was performed using logistic regression analysis to calculate odds ratios (OR) and 95% CI. Data analysis was conducted in STATA V.14.0 (StataCorp, College Station, TX, USA).
For the process evaluation, frequencies and percentages are presented. Also, open-ended questions were transcribed, and then a codebook was created, themes were derived from the data. Coding was performed manually and patterns of answers are described.
Ethics
The study protocol, informed consent templates, and questionnaires were reviewed and approved by the Institutional Review Board (IRB) at Universidad Peruana Cayetano Heredia (UPCH) in Lima, Peru (SIDISI 61482). In addition, participating hospitals (Hospital Cayetano Heredia and Hospital Nacional Arzobispo Loayza) in the study received the protocol and consent form for approval 12. The extension in the follow-up period was also approved by the IRB at UPCH and the participants re-consented. The fieldworker explained the study procedures, then the potential participant read the informed consent form and asked questions. After that, if they accepted, they signed the informed consent form. The trial was registered at ClinicalTrials.gov with the identifier NCT02373592 (27/02/2015).
Results
The recruitment was conducted between October 2015 and March 2016 and the follow-up period lasted until October 2017.
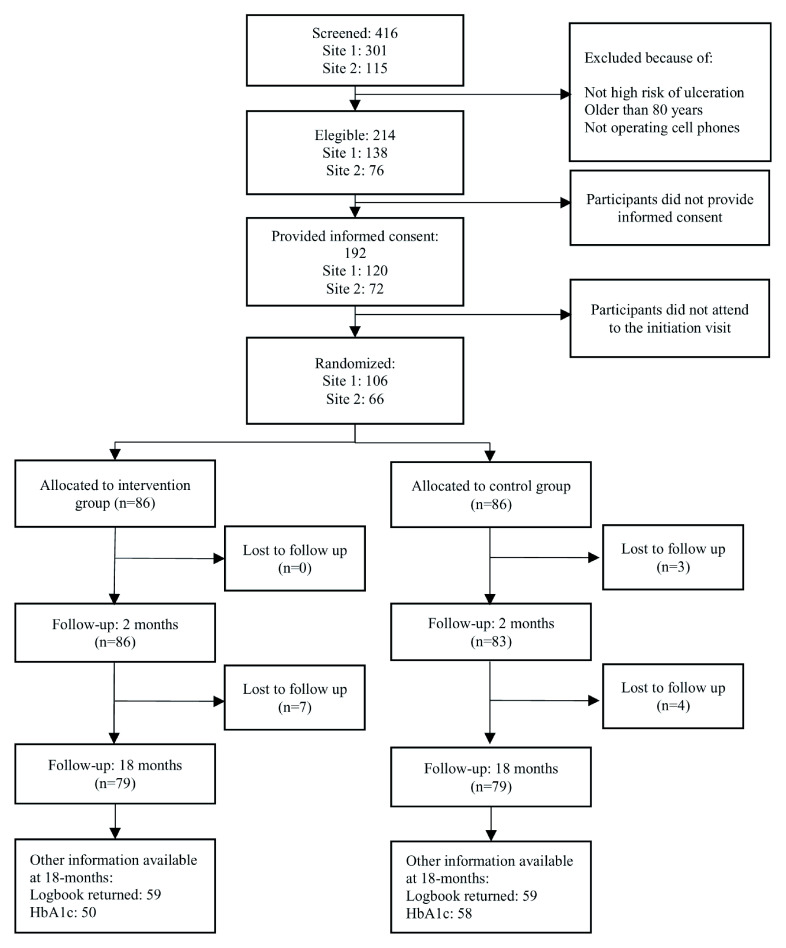
In total, 416 participants were screened and 214 were eligible for the study. Of these, 192 gave informed consent and 172 attended the initiation visit and were allocated to the control (n=86) or intervention (n=86) arms ( Figure 2). Only 79/86 (91.9%) participants in each arm completed the 18-month follow-up.
Figure 2. Flowchart.
Baseline characteristics
The baseline characteristics were similar between the intervention and control arms, with few exceptions ( Table 1). History of previous foot ulcers was reported with more frequency in the intervention arm; 65.9% vs. 48.2% in the control arm (p-value 0.02). Mean HbA1c was 8.9% in the intervention arm and 8.2% among the controls (p-value 0.03). In terms of mHealth literacy, there were no major differences between study arms, with the exception that participants in the intervention arm reported more frequently never having problems with cellphone coverage (89.5% vs. 74.4% in the control arm, p-value 0.01).
Table 1. Baseline characteristics.
| Control arm | Intervention arm | |
|---|---|---|
| (N=86)
n (%) |
(N=86)
n (%) |
|
| Site | ||
| Site 1 | 53 (61.6) | 53 (61.6) |
| Site 2 | 33 (38.4) | 33 (38.4) |
| Sociodemographic variables | ||
| Age, mean (SD) * | 62.1 (9.8) | 60.3 (9.2) |
| Sex (female) | 56 (65.1) | 52 (60.5) |
| Level of education | ||
| <7 years | 30 (34.9) | 30 (34.9) |
| 7 to 11 years | 40 (46.5) | 42 (48.8) |
| 12 or more years | 16 (18.6) | 14 (16.3) |
| Marital status: married or
cohabitant |
63 (73.3) | 59 (68.6) |
| Currently working | 34 (39.5) | 33 (38.4) |
| Had a caregiver | 35 (40.7) | 36 (41.9) |
| Clinical variables | ||
| Body mass index, mean
(SD) |
27.9 (4.8) | 28.0 (4.4) |
| Depression (>9 points in
PHQ-9) |
23 (27.1) | 22 (25.6) |
| Co-morbidities | ||
| Hypertension
diagnosis |
37 (43.0) | 41 (47.7) |
| Previous myocardial
infarction |
3 (3.5) | 4 (4.7) |
| Other cardiac
problems |
3 (3.5) | 3 (3.5) |
| Previous stroke | 3 (3.5) | 5 (5.8) |
| High cholesterol | 48 (55.8) | 41 (47.7) |
| Behavioral variables | ||
| Current smoker (self-
reported) |
4 (4.7) | 11 (12.9) |
| Binge drinking at least
once during the last year |
28 (32.6) | 20 (23.3) |
| Physical activity
(moderate/vigorous, three or more days a week) |
7 (8.2) | 13 (15.3) |
|
Variables related to
diabetes |
||
| Years since diabetes
diagnosis, mean (SD) * |
12.7 (7.9) | 13.3 (8.5) |
| HbA1c at baseline %,
mean (SD) |
8.2 (1.9) | 8.9 (2.3) |
| Current pharmacological
treatment for diabetes |
||
| Metformin | 67 (77.9) | 72 (83.7) |
| Insulin | 35 (40.7) | 47 (54.7) |
| Consultations in the last
12 months |
||
| Ophthalmology | 48 (56.5) | 45 (52.3) |
| Nephrology | 16 (18.6) | 21 (24.4) |
| Cardiology | 30 (35.7) | 35 (41.2) |
| Complications | ||
| Diabetic retinopathy | 13 (15.3) | 21 (24.4) |
| Diabetic
nephropathy |
5 (6.0) | 9 (10.6) |
| Hospitalization in the last
year due to diabetes |
10 (11.6) | 9 (10.5) |
| Current use of orthopedic
shoes ** |
0 (0) | 4 (4.7) |
| Current use of insoles ** | 2 (2.3) | 8 (9.3) |
| mHealth literacy | ||
| The patient receives
messages (instead than the caregiver) |
45 (52.3) | 45 (52.3) |
| The patient knows how to
make calls ** |
81 (96.4) | 85 (98.8) |
| The patient knows how to
answer to calls ** |
82 (97.6) | 85 (100.0) |
| The patient knows how to
send SMS |
77 (91.7) | 75 (89.3) |
| The patient knows how to
read SMS ** |
82 (97.6) | 78 (91.8) |
| Never have problems
with cellphone coverage |
61 (74.4) | 77 (89.5) |
| Foot examination | ||
| Previous foot ulcers | 40 (48.2) | 56 (65.9) |
| Previous foot amputation | 10 (12.1) | 14 (16.5) |
| Any deformity in foot | 53 (63.9) | 54 (63.5) |
| Any alteration in
monophilament test |
71 (85.5) | 70 (82.4) |
| Any alteration in
biotensiometer (≥25) |
65 (78.3) | 75 (88.2) |
* T-test; **Fisher’s exact text.
SD, standard deviation; PHQ, patient health questionnaire; mHealth, mobile health; SMS, short message service.
Primary outcome
The cumulative incidence of DFU in the entire sample was 17.7% (28/158), and it was higher among participants with a history of previous ulceration (27.8%, 25/90) 22.
The incidence of DFU was 11.4% (95% CI 5.2% – 21.6%) in the control arm and 24.1% (95% CI 14.5% – 37.6%) in the intervention arm. Compared to the thermometry-only control arm, the adjusted hazard ratio (aHR) of DFU in the thermometry + mHealth intervention arm adjusted by site was 2.12 (95% CI 0.96 – 4.68), and 1.44 (95% CI 0.65 – 3.22) adjusted by site and previous foot ulceration ( Table 2). The incidence of DFU in participants with previous foot ulceration was 23.7% (9/38) in the control arm and 30.8% (16/52) in the intervention arm, whereas in the participants without previous foot ulceration, incidence was 0% (0/38) in the control arm and 7.7% (2/26) in the intervention arm. Four participants did not have information related to their previous foot ulceration status (three from the control arm and one from the intervention arm).
Table 2. DFU incidence and effect of the intervention on primary and secondary outcomes.
| Incidence | Effect estimates * | |||
|---|---|---|---|---|
| Control arm | Intervention arm | |||
| n/N (%) | n/N (%) | HR (95% CI) | OR (95% CI) | |
| Primary outcome: DFU | ||||
| Overall population | 9/79 (11.4) | 19/79 (24.1) | ||
| Adjusted by site | 2.12 (0.96 – 4.68) | -- | ||
| Adjusted by previous foot ulceration | 1.47 (0.66 – 3.30) | -- | ||
| Adjusted by site and previous foot ulceration | 1.44 (0.65 – 3.22) | -- | ||
| Secondary outcomes | ||||
| ≥80% daily temperature measurements | ||||
| Crude | 54/59 (91.5%) | 49/59 (83.1%) | 0.45 (0.15 – 1.42) | |
| Adjusted by site | 0.46 (0.15 – 1.43) | |||
| Adjusted by site and previous foot ulceration | 0.43 (0.13 – 1.40) | |||
| Reduction of ≥1% of glycosylated hemoglobin | ||||
| Crude | 20/58 (34.5%) | 14/50 (28.0%) | 0.74 (0.33 – 1.68) | |
| Adjusted by site | 0.73 (0.32 – 1.67) | |||
| Adjusted by site and previous foot ulceration | 0.64 (0.28 – 1.51) | |||
* HRs were calculated among the 169 participants that had at least one follow-up evaluation during the 18-month study period. ORs were calculated among the 158 participants that finished the 18-months follow-up and had complete data to analysis. All effect estimates were calculated using the thermometry-only arm as the reference group.
DFU, diabetic foot ulcer; HR, hazard ratio; CI, confidence interval, OR, odds ratio.
Secondary outcomes
The frequency of ≥80% of adherence to daily temperature measurement was 87.2% (103/118) among the study participants that returned the logbook. There was no evidence of a difference between study arms in the secondary outcomes of adherence to daily temperature measurements or reduction of HbA1c ( Table 2).
Sub-group analyses in intervention vs. control arms
No effects of the intervention were found according to a priori pre-defined sub-groups. Among participants that did not have a caregiver (n=96), the aHR of developing a DFU was 3.34 (95% CI 0.94 – 11.92), adjusted by site and previous ulcer. Other results for sub-group analyses are shown in Table 3.
Table 3. DFU incidence and effect of the intervention by a priori defined sub-groups.
| DFU incidence | Effect estimate * | ||
|---|---|---|---|
| Control arm | Intervention arm | ||
| n/N (%) | n/N (%) | HR (95% CI) | |
| Among those who had a caregiver | |||
| Crude | 6/31 (19.4) | 7/31 (22.6) | 1.11 (0.37 – 3.32) |
| Adjusted by site and previous foot ulceration | 0.43 (0.13 – 1.48) | ||
| Among those who did not had a caregiver | |||
| Crude | 3/48 (6.3) | 12/48 (25.0) | 4.13 (1.16 – 14.63) |
| Adjusted by site and previous foot ulceration | 3.34 (0.94 – 11.92) | ||
* HRs were calculated among the 169 participants that had at least one follow-up evaluation during the 18-month study period. All effect estimates were calculated using the thermometry-only arm as the reference group
DFU, diabetic foot ulcer; CI, confidence interval; HR, hazard ratio.
Table 4. Process evaluation of the use of thermometer at the 18-month follow-up.
| Characteristics | N=102
n (%) |
|---|---|
| TempStat™ Use: step by step | |
| Use of TempStat™ in an illuminated area | 8 (7.8%) |
| Use of TempStat™ immediately after wake up | 100 (97.1%) |
| Use of the TemStat™ without socks and with warm feet | 34 (33.0%) |
| Use of the TempStat™ seated in a chair, with the
feet on the device and with the hands in the knees applying a little pressure |
46 (44.7%) |
| Stay with the feet on the TempStat™ during
60 seconds |
68 (66.0%) |
| Correct alarm sign identification | 84 (81.6%) |
| Daily registration in the logbook | 54 (52.4%) |
| Logbook use | |
| Participants using their logbooks | 93 (90.3%) |
| Nursing consultation | |
| Report of communication with the study nurse | 69 (66.9%) |
| Reason of the communication | |
| Consultation about TempStat™ use | 1 (1.5%) |
| Alarm sign detection | 5 (7.3%) |
| Schedule consultation | 63 (91.2) |
| You consider that the nurse solved effectively
your doubts or problems |
66 (97.9%) |
Table 5. Process evaluation of the mHealth strategy at the 18-month follow-up.
| Characteristics | N=39
n (%) |
|---|---|
| Messages | |
| Always read the messages | 27 (69.2%) |
| The messages help you a lot to improve your
foot care |
29 (74.4%) |
| The messages help you a lot to remember to
use the device |
27 (69.2%) |
| Understanding of the messages | |
| Daily thermometer usage | 37 (94.9%) |
| Use of the TempStat™ during the morning | 39 (100.0%) |
| Correct identification of alarm sign | 30 (76.9%) |
| Correct actions if an alarm sign was detected | 30 (76.9%) |
| Use of warm water to wash your feet | 32 (82.1) |
| Avoid utilization of tight shoes | 39 (100%) |
Sub-group analysis within the intervention arm
Participants were arranged according to the recipient of the mHealth reminders; the participants themselves (45/86) or the caregiver (41/86). We found no evidence of a difference in DFU incidence between these two groups in crude (HR 1.09, 95% CI 0.44 - 2.70), and adjusted analyses (aHR 1.72, 95% CI 0.65 – 4.54, adjusted by site and previous ulcer).
Process evaluation indicators
Some process evaluation indicators for TempStat™ use and understanding of the messages are shown in Table 4 and Table 5. This data was obtained at the 18-month follow-up visit 23, 24.
Dose of the mHealth component. The total number of messages to be sent to the patients in the intervention group during the study period was intended to be 86 text messages and 76 voice messages. The automated software system sent <50% of the intended SMS and voice messages to 1/86 (1.2%) of the participants, between 50–75% of the messages to 18/86 (20.9%) of the participants, and ≥75% of the messages to 67/86 (77.9%) of the participants. In contrast, text and voice messages received by the participants was <50% for 42/86 (48.8%) of the participants, and between 50% and 75% for 44/86 (51.2%) of the participants.
Preferences of SMS or voice messages. Among 101 interviewees (one participant did not answer), 42.6% preferred text messaging, whereas 57.4% preferred voice messages. Those who preferred voice messaging over SMS generally had that preference because they had difficulty reading text messages on the cell phone screen. Other participants with this preference mentioned that they have quicker access to the information with a voice message. Those who preferred SMS for reminders cited the fact that SMS can be read at their convenience. Some mentioned that they prefer SMS because they don’t want to have to listen for phone calls and/or pay attention to their phone at certain times.
Some participants commented that regardless of the reminder system (SMS or voice messaging), it was necessary to receive help from other people to read or listen to the messages. Their children were most commonly cited as the people to whom the participants would turn for help.
Use of TempStat™. Some participants mentioned that they had some periods during which they did not use the device. Among the reasons provided were that the device had technical problems or because they did not have the logbook to record their measurements.
Suggestions. Among the suggestions to improve the device and its use, technical comments were the most common. Participants mentioned that they preferred a smaller size and lighter weight device. Furthermore, of the 8% of participants that had to replace the TempStat™ because of technical problems, some mentioned that an improved design could increase the lifetime of the device. Additionally, participants found the reinforcement of the logbook and device utilization by the nurses to be very important, and some commented that more frequent communication with the nurse could improve compliance with device use.
Discussion
Main findings
This study was designed to compare the 18-month incidence of DFU between those receiving thermometry + mHealth reminders versus thermometry-only. The uptake of the thermometry was high in this study, nearly 90% of the participants who returned the logbook had achieved ≥80% of the daily feet temperature measurements. At baseline, we unexpectedly found a higher prevalence of previous foot ulceration in the intervention arm, and the incidence of DFU was higher in this arm. The additional value of SMS and phone-based reminders and communications did not appear to provide further benefit.
Comparison to previous studies
In our cohort, according to the process evaluation results, adherence to temperature measurement was good, procedures about how to use the TempStat™ were regular (some steps have less than 50% of correct answers) and correct alarm sign detection was good (81%). One previous study using thermometry found that 80% of participants who developed an ulcer did not comply with 50% of the temperature assessments, in contrast with the group that did not develop an ulcer, where 92% of participants recorded their foot temperatures at least half the time 7.
Health interventions using SMS for diabetes management have been found to be useful for improving self-efficacy and social support 9, as well as clinical diabetes-related outcomes 10. However, most of the mHealth studies were conducted in high-income countries, with a young population and with outcomes related to HbA1c measurements or questionnaires, without evaluating patient important outcomes like mortality, complications or quality of life. Despite the perceived benefit of mHealth in the elderly population 25, very few studies with this population have been conducted in LMICs. Our automatic system delivered >75% of the messages to two-thirds of the participants only and it did not have a human support component, factors that may have affected the effective engagement with the mHealth intervention 26, 27. For example, a previous study using tailored motivational phone calls followed by SMS in people with pre-hypertension found a larger effect on bodyweight and waist circumference reduction in participants that received ≥75% of the calls 28. Additionally, our system was automatic and did not allow direct bilateral communication. In a previous qualitative study from Canada, conducted to explore the views of patients in using mHealth to monitor and prevent DFU 29, patients expressed interest in a two-way communication system to facilitate sharing of medical data, scheduling appointments and using of alerts to get access to medical attention. Finally, compared to previous mHealth studies where the focus has been on laboratory parameters or questionnaires 30, 31, we measured the impact of mHealth on DFU, an outcome of patient importance.
Limitations and strengths
Our study has some limitations. At baseline, the intervention arm was at higher risk of DFU, and the ulceration rate in the participants was lower than expected. Together these reduced the precision of our estimates despite extending the study from 12 to 18 months. Another limitation was that we did not collect information about duration since the most recent wound healed. Recent research suggests ~10% of wounds recur within a month and 40% within a year of entering diabetic foot remission. Additionally, adherence to measurements was self-reported, which potentially introduced bias. Finally, it is possible that those who did not return the logbook (~30%) may be less conscientious and thus have lower adherence.
The study also has some strengths; namely, it is a practical and pragmatic trial, well protected from bias, measuring an outcome of importance to patients and inclusive of low-income patients over 60 years-old attending public hospitals in a middle-income country.
Relevance to public health
The experience of introducing a device to engage with self-care behaviors for the prevention of DFU in a LMIC setting showed good adherence rates in both study arms, nearly reaching 90%, signaling that mHealth had little room to further exert an impact. Future studies could pre-select participants with low adherence and explore if mHealth appears as a good supplement to prevent DFU.
Maintaining such DFU prevention efforts in routine clinical settings may be difficult to sustain, yet this study demonstrates that adequate promotion of foot care can be achieved.
Conclusions
This randomized trial, conducted in a LMIC setting, did not produce evidence in favor of the use of mHealth to improve adherence to thermometry or reduce DFU incidence in patients with type 2 diabetes at high risk of ulceration. This trial contributes to the body of evidence about the value of mHealth to prevent DFU.
Data availability
Underlying data
Figshare: Database main analysis. https://doi.org/10.6084/m9.figshare.11310827.v2 22
Figshare: Dictionary main database. https://doi.org/10.6084/m9.figshare.11478003.v1 32
Figshare: Database process evaluation. https://doi.org/10.6084/m9.figshare.11317601.v2 23
Figshare: Dictionary process evaluation. https://doi.org/10.6084/m9.figshare.11477985.v1 33
Figshare: Transcripts. https://doi.org/10.6084/m9.figshare.11310740.v1 24
Extended data
Figshare: Validation of mHealth messages. https://doi.org/10.6084/m9.figshare.11310620.v1 16
Figshare: Messages used in the mHealth component. https://doi.org/10.6084/m9.figshare.11310665.v1 17
Figshare: Questionnaires. https://doi.org/10.6084/m9.figshare.11310548.v2 19
Reporting guidelines
Figshare: CONSORT checklist. https://doi.org/10.6084/m9.figshare.11310512.v1 34
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Software availability
Source code available from: https://github.com/dgnest/foottrial
Archived source code at time of publication: https://doi.org/10.5281/zenodo.3628824 18
License: Apache 2.0
Acknowledgments
Our acknowledgments go to the fieldworkers Carmen Cisneros, Yvonne Huaylinos, Edith Rojas and Angela Roncal for their work and support in the implementation of the intervention. Also, we would like to thanks to the health professionals including physicians, nurses and technicians from the Endocrinology Services of the Hospital Cayetano Heredia and Hospital Nacional Arzobispo Loayza. This study would not have been possible without the involvement of the participants, and we appreciate their time and commitment to the study.
We are also grateful to Sol Abarca, Jorge Chachaima Mar, Gianpier Rojas and Bridgette Zarzosa Mezzich for their collaboration in data quality control and the revision of the participant’s logbook. Finally, we would like to thank to Jill Portocarrero for her support in the process evaluation of the study, Miguel Moscoso Porras for his initial support in the design of the messaging system and to the engineers Jorge Estrada and Oscar Giraldo for the development and monitoring of the messaging system.
Funding Statement
AB-O was funded by the Wellcome Trust as a Research Training Fellow in Public Health and Tropical Medicine [103994]. This project is funded by the Fogarty International Center, National Institutes of Health [R21TW009982], under the Global Alliance for Chronic Diseases (GACD) Diabetes Program. JJM currently receives or has received during the planning and conduction of this study further support from the Alliance for Health Policy and Systems Research [HQHSR1206660], Consejo Nacional de Ciencia y Tecnología (CONCYTEC), DFID/MRC/Wellcome Global Health Trials [MR/M007405/1], Grand Challenges Canada [0335-04], the International Development Research Centre Canada [106887-001], the Inter-American Institute for Global Change Research [IAI CRN3036], the Medical Research Council [MR/P008984/1, MR/P024408/1, MR/P02386X/1], the National Cancer Institute [1P20CA217231], the National Heart, Lung and Blood Institute [5U01HL114180, HHSN268200900028C-3-0-1], the National Institute of Mental Health [1U19MH098780], the Swiss National Science Foundation [40P740-160366], Universidad Peruana Cayetano Heredia, and the Wellcome Trust [GR074833MA, WT093541AIA, 074833, 205177].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved with reservations]
References
- 1. American Diabetes Association: Statistics about Diabetes.2016. Reference Source [Google Scholar]
- 2. World Health Organization: Diabetes.2016. Reference Source [Google Scholar]
- 3. Dyck PJ, Davies JL, Wilson DM, et al. : Risk factors for severity of diabetic polyneuropathy: intensive longitudinal assessment of the Rochester Diabetic Neuropathy Study cohort. Diabetes Care. 1999;22(9):1479–86. 10.2337/diacare.22.9.1479 [DOI] [PubMed] [Google Scholar]
- 4. Singh N, Armstrong DG, Lipsky BA: Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–28. 10.1001/JAMA.293.2.217 [DOI] [PubMed] [Google Scholar]
- 5. Bharara M, Schoess J, Armstrong DG: Coming events cast their shadows before: detecting inflammation in the acute diabetic foot and the foot in remission. Diabetes Metab Res Rev. 2012;28 Suppl 1:15–20. 10.1002/dmrr.2231 [DOI] [PubMed] [Google Scholar]
- 6. Armstrong DG, Holtz-Neiderer K, Wendel C, et al. : Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007;120(12):1042–6. 10.1016/j.amjmed.2007.06.028 [DOI] [PubMed] [Google Scholar]
- 7. Lavery LA, Higgins KR, Lanctot DR, et al. : Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007;30(1):14–20. 10.2337/dc06-1600 [DOI] [PubMed] [Google Scholar]
- 8. Lavery LA, Higgins KR, Lanctot DR, et al. : Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004;27(11):2642–7. 10.2337/diacare.27.11.2642 [DOI] [PubMed] [Google Scholar]
- 9. De Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, et al. : Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev. 2012;12:CD007459. 10.1002/14651858.CD007459.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krishna S, Boren SA: Diabetes self-management care via cell phone: a systematic review. J Diabetes Sci Technol. 2008;2(3):509–17. 10.1177/193229680800200324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lazo-Porras M, Bernabe-Ortiz A, Sacksteder KA, et al. : Implementation of foot thermometry plus mHealth to prevent diabetic foot ulcers: study protocol for a randomized controlled trial. Trials. 2016;17(1):206. 10.1186/s13063-016-1333-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zwarenstein M, Treweek S, Gagnier JJ, et al. : Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337:a2390. 10.1136/bmj.a2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shahbazian H, Yazdanpanah L, Latifi SM: Risk assessment of patients with diabetes for foot ulcers according to risk classification consensus of International Working Group on Diabetic Foot (IWGDF). Pak J Med Sci. 2013;29(3):730. 10.12669/pjms.293.3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Apelqvist J, Bakker K, Van Houtum W, et al. : Practical guidelines on the management and prevention of the diabetic foot: based upon the International Consensus on the Diabetic Foot (2007) Prepared by the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2008;24 Suppl 1:S181–S7. 10.1002/dmrr.848 [DOI] [PubMed] [Google Scholar]
- 15. Peters EJ, Lavery LA, International Working Group on the Diabetic FOot.: Effectiveness of the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care. 2001;24(8):1442–7. 10.2337/diacare.24.8.1442 [DOI] [PubMed] [Google Scholar]
- 16. Lazo Porras M: Validation of mHealth messages. figshare.Poster.2019. 10.6084/m9.figshare.11310620.v1 [DOI] [Google Scholar]
- 17. Lazo Porras M, Miranda JJ, Bernabe-Ortiz A: Messages used in the mHealth component. figshare. 2019. 10.6084/m9.figshare.11310665.v1 [DOI] [Google Scholar]
- 18. Estrada J: dgnest/foottrial: v1.0.0 (Version v1.0.0). Zenodo. 2020. 10.5281/zenodo.3628824 [DOI] [Google Scholar]
- 19. Lazo Porras M, Miranda JJ, Bernabe-Ortiz A: Questionnaires. figshare. 2019. 10.6084/m9.figshare.11310548.v2 [DOI] [Google Scholar]
- 20. American Diabetes Association: Consensus Development Conference on Diabetic Foot Wound Care: 7-8 April 1999, Boston, Massachusetts. American Diabetes Association. Diabetes Care. 1999;22(8):1354–60. 10.2337/diacare.22.8.1354 [DOI] [PubMed] [Google Scholar]
- 21. Armstrong DG, Lavery LA, Harkless LB: Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855–9. 10.2337/diacare.21.5.855 [DOI] [PubMed] [Google Scholar]
- 22. Lazo Porras M: Database main analysis. figshare. 2019. 10.6084/m9.figshare.11310827.v2 [DOI] [Google Scholar]
- 23. Lazo Porras M, Miranda JJ, Bernabe-Ortiz A: Database process evaluation. figshare. 2019. 10.6084/m9.figshare.11317601.v2 [DOI] [Google Scholar]
- 24. Lazo Porras M, Miranda JJ, Bernabe-Ortize A: Transcripts. figshare. 2019. 10.6084/m9.figshare.11310740.v1 [DOI] [Google Scholar]
- 25. Changizi M, Kaveh MH: Effectiveness of the mHealth technology in improvement of healthy behaviors in an elderly population-a systematic review. mHealth. 2017;3:51. 10.21037/mhealth.2017.08.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baumeister H, Reichler L, Munzinger M, et al. : The impact of guidance on Internet-based mental health interventions—A systematic review. Internet Interv. 2014;1(4):205–15. 10.1016/j.invent.2014.08.003 [DOI] [Google Scholar]
- 27. Michie S, Yardley L, West R, et al. : Developing and evaluating digital interventions to promote behavior change in health and health care: recommendations resulting from an international workshop. J Med Internet Res. 2017;19(6):e232. 10.2196/jmir.7126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rubinstein A, Miranda JJ, Beratarrechea A, et al. : Effectiveness of an mHealth intervention to improve the cardiometabolic profile of people with prehypertension in low-resource urban settings in Latin America: a randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(1):52–63. 10.1016/S2213-8587(15)00381-2 [DOI] [PubMed] [Google Scholar]
- 29. Boodoo C, Perry JA, Hunter PJ, et al. : Views of patients on using mHealth to monitor and prevent diabetic foot ulcers: qualitative study. JMIR Diabetes. 2017;2(2):e22. 10.2196/diabetes.8505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lipska KJ, Krumholz HM: Is hemoglobin A1c the right outcome for studies of diabetes? JAMA. 2017;317(10):1017–8. 10.1001/JAMA.2017.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gandhi GY, Murad MH, Fujiyoshi A, et al. : Patient-important outcomes in registered diabetes trials. JAMA. 2008;299(21):2543–9. 10.1001/JAMA.299.21.2543 [DOI] [PubMed] [Google Scholar]
- 32. Lazo Porras M: Dictionary main database. figshare. 2019. 10.6084/m9.figshare.11478003.v1 [DOI] [Google Scholar]
- 33. Lazo Porras M, Miranda JJ, Bernabe-Ortiz A: Dictionary process evaluation.pdf. figshare. 2019. 10.6084/m9.figshare.11477985.v1 [DOI] [Google Scholar]
- 34. Lazo Porras M: CONSORT checklist. figshare. 2019. 10.6084/m9.figshare.11310512.v1 [DOI] [Google Scholar]