Abstract
Endotoxemia-induced acute kidney injury (AKI) is a common clinical condition that lacks effective treatments. Elabela (ELA) is a recently discovered kidney peptide hormone, encoded by the gene apela, and has been reported to improve cardio-renal outcomes in sepsis. However, ELA is a small peptide and is largely unsuitable for clinical use because of its short in vivo half-life. In the present study, we evaluated the potential renoprotective effects of a long-acting constant fragment (Fc)-ELA fusion protein in liposaccharide (LPS)-induced AKI in mice. LPS administration in mice for 5 days greatly lowered the gene expression of apela and impaired kidney function, as evidenced by elevated serum creatinine and the ratio of urine protein to creatinine. In addition, renal inflammation and macrophage infiltration were apparent in LPS-challenged mice. Treatment with the Fc-ELA fusion protein partially restored apela expression and attenuated the kidney inflammation. Moreover, LPS treatment induced reactive oxygen species (ROS) production and apoptosis in kidney HK-2 cells as well as in the mouse kidney, which were mitigated by ELA or Fc-ELA treatment. Finally, we found that ELA promoted the survival of HK-2 cells treated with LPS, and this action was abolished by LY204002, a PI3K/Akt inhibitor. Collectively, we have demonstrated that the Fc-ELA fusion protein has significant renoprotective activities against LPS-induced AKI in mice.
Keywords: acute kidney injury (AKI), Elabela (ELA), fusion protein, liposaccharide (LPS)
Introduction
Sepsis is a common severe clinical condition, often resulting in multiple organ injury and leading to significant mortality [1,2]. Sepsis-induced acute kidney injury (AKI) is a leading cause of deaths in hospitalized patients with mortality rate of up to 48% [3,4]. Currently, no effective pharmacological interventions for septic AKI are available [5] and thus, there is an unmet urgent need for development of novel therapeutics to treat this severe medical complication.
Liposaccharide (LPS)-induced AKI is a commonly used animal model for the study of sepsis-associated kidney injury. It is well-established that LPS activates the innate immune system through pattern recognition receptors such as toll-like receptors (TLRs), leading to a cascade of inflammatory responses, physiological perturbations and tissue damage [6]. For example, the interaction of LPS with TLRs activate tubular cells, as well as interstitial macrophages or dendritic cells, in turn stimulating local production of cytokines and chemokines in the kidney [7]. Inflammatory responses will also lead to increased production of nitric oxide (NO) and reactive oxygen species (ROS) and manifest as inflammatory cell infiltration, tissue destruction and cell death via necrosis and apoptosis [8,9]. Renal dysfunction [1,10,11] ensues as a result.
The apelinergic system is composed of the apelin receptor (APJ) with two endogenous peptide ligands/hormones: apelin and the newly discovered Elabela (ELA, Apela or Toddler) [12,13] and is emerging as a protective system against tissue damage in ischemia and inflammation [14]. Many studies have shown that apelin can attenuate multiple organ injuries following hemorrhagic shock [15] and in sepsis [16–18]. We have previously reported that constant fragment (Fc)-apelin, a long-acting form of apelin, can ameliorate LPS-induced liver damage in mice [19]. Likewise, ELA has recently been reported to be protective against sepsis-induced organ damage [18]. However, whether ELA is renoprotective against sepsis-induced AKI is yet to be evaluated.
ELA is a peptide of 32 amino acids and is presumed to be cleaved to peptide fragments of ELA-21, ELA-14 and ELA-11 [12–14]. Therapeutic peptides of less than 50 amino acids are usually unsuitable for clinical use because of short in vivo half-life [20]. To extend ELA’s half-life, we fused the Fc domain of the human immunoglobulin IgG to ELA-21, resulting in Fc-ELA-21 (referred to hereafter as Fc-ELA unless otherwise specified) which has an extended plasma half-life of ∼44 h (vs.∼13 min of the native peptide [21]) and is biologically active. Administration of Fc-ELA successfully mitigated cardiac dysfunction in a model of myocardial infarction [21]. We hypothesized that the long-acting Fc-ELA, which is clinically applicable, might also mitigate sepsis-induced kidney inflammation and injury. We thus tested this hypothesis by investigating the effects of Fc-ELA on LPS-induced AKI in mice.
Materials and methods
Reagents
LPS from Salmonella enterica was purchased from Sigma–Aldrich (St. Louis, MO, U.S.A.), LY294002 from Cell Signaling Technology (Danvers, MA) and dihydroethidium (DHE) from Invitrogen (Eugene, OR). ELA-21 peptide was made by GenScript (Piscataway, NJ) and Fc-ELA was made by conjugating the human IgG Fc fragment with ELA-21 at the N-terminus as previously described [21].
Animal studies
The animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine and the animal studies were performed at the University of Maryland School of Medicine. C57/BL6 female mice, at 6 weeks old, were obtained from Charles River (Wilmington, MA) and used after 1 week of quarantine and acclimatization. Experimental animals were randomly divided into four groups: (1) The control group (CON) received PBS (vehicle of LPS) intraperitoneally (i.p.) and subcutaneously (s.c.); (2) The Fc-ELA group was treated with Fc-ELA (1 mg/kg/day), s.c. and PBS, i.p.; (3) The LPS group was challenged with LPS (1 mg/kg) i.p. and PBS, s.c.; and (4); The LPS/Fc-ELA group received LPS (1 mg/kg), i.p. and Fc-ELA (1 mg/kg), s.c. Mice were administered with vehicle, LPS and/or Fc-ELA daily for five consecutive days. Six hours after final injection, the mice were anesthetized by CO2 gas and killed by cervical dislocation. Blood samples were collected and serum samples were separated and stored at −20°C until analysis. Kidney specimens were embedded in the optimal cutting temperature compound Tissue-Tek (Sakura, CA), fixed in 10% neutral buffered formalin for histological analysis or snap-frozen in liquid nitrogen.
Quantitative real-time PCR
Total RNAs were prepared from the snap-frozen tissue specimens using TRIzol (Invitrogen), and reverse transcription was carried out in a reaction containing 1 μg of total RNA, poly(dT) primer, and Moloney murine leukemia virus reverse transcriptase using the Transcriptor First Strand cDNA Synthesis kit (Promega). Quantitative real-time PCR (qRT-PCR) was conducted on a LightCycler480 using the LightCycler 480 SYBR Green I Master Mix (Roche Diagnostics). The amplification protocol was as follows: 95ºC/5 min, then (95ºC/10 s, 60ºC/20 s, and 72ºC/30 s) × 45 cycles. Following amplification, a dissociation curve analysis was performed to ensure purity of the PCR product. qPCR primers were 5′-tgcattccacttcattctcg-3′ (forward) and 5′-gttgccatcctgaggttgtt-3′ (reverse) for apela, 5′-actatggggctgacaaccag-3′ (forward) and 5′-ggcaaagtcaccacaaaggt-3′ (reverse) for APJ, 5′-gaggaaatttcgcagacagc-3′ (forward) and 5′-gaggaacttggtgggtgaga for apelin (reverse), and 5′-tggaccttccaggatgaggaca-3′ (forward) and 5′-gttcatctcggagcctgtagtg-3′ (reverse) for IL-1β. Primers for TNFα and IL-6 were described previously [14]. β-actin mRNA was used for normalization of cDNA loading as an internal control. The relative expression of the target genes was determined by the 2−ΔΔCT method [22].
Protein and creatinine assays
At the end of the animal experiment, serum and urine were collected for total protein and creatinine measurements. Total protein concentrations were determined using the Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL, U.S.A.) according to manufacturer’s protocol.
Urine was collected by micturition on a Petri dish and subsequent aspiration with a 200-μl pipette [23]. In brief, pressure was applied in a massaging fashion at both sides of the lower back near the tail with the thumb on one side and the fore and middle fingers on the other side of a mouse, rubbing up and down of the caudal area of the back of the mouse to facilitate urination. Once the mouse started urinating, the experimenter gently pulled the skin at the lower back upward with the massaging fingers to immobilize the mouse and to prevent premature halt of urination. The voided urine was aspirated using a 200-μl pipette. Creatinine was measured by using a creatinine assay kit (Crystal Chem Inc., IL, U.S.A.).
Cell studies
The immortalized human proximal tubular epithelial cell lines HK-2 (ATCC CRL-2190, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (complete medium) at 37°C. For apoptosis studies, cells were plated and grown on cover slips in a well of a six-well plate in complete medium to approximately 80% confluence and then changed to serum-free medium with ELA-21 (2 μM) and/or LPS (2 μg/ml) for 12 h before fixation with 4% paraformaldehyde for 30 min.
Immunostaining and histology studies
Fixed kidney tissues were processed routinely, embedded in paraffin, cut into 5-μm thickness slices and stained with Hematoxylin and Eosin (H/E). For immunohistochemistry (IHC), the paraffin-embedded sections were subjected to deparaffination, 3% H2O2 treatment and antigen retrieval, and then sequential incubation with the primary antibody F4/80 (BM8, eBioscience) and HRP-conjugated goat anti-rabbit IgG (KPL, Gaithersburg, MD), as described previously [19]. Kidney tissue apoptosis was assessed by terminal deoxynucleotidyl transferase-mediated deoxyuridintriphosphate nick-end labeling (TUNEL) on tissue sections or HK2 cells grown on cover slides. The TUNEL staining and Methyl Green counterstaining were performed using DeadEnd™ Fluorometric TUNEL System (Promega, Madison, WI, U.S.A.), according to the manufacturer’s protocol. Fluorescein-labeled TUNEL positive cells were counted under ×400 magnification with a microscope (Olympus IX-51). The apoptotic index was calculated as the percentage of TUNEL-positive cells/total number of cells.
DHE staining
The production of superoxide in oxidative stress were measured in the frozen kidney tissue sections and HK-2 cell cultures using the oxidative fluorescent dye DHE. Kidney cryosections at 5-μm thickness were stained with the superoxide-sensitive dye DHE (1 μmol/l) in a light-protected and humidified chamber for 30 min at 37°C. Three nonoverlapping images per sample were averaged with a fluorescence microscope (Olympus IX-51) and the signal was quantified by Image-Pro Plus 6.0 (Bethesda, MD, U.S.A.).
Western blotting
Kidney tissues were homogenized and HK-2 cells were sonicated in RIPA buffer (TekNova, CA, U.S.A.). After centrifugation, supernatants were dissolved in Laemmli Buffer. The dissolved proteins were separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) and transferred to polyvinylidene difluoride membranes (Millipore, MA, U.S.A.). The membranes were blocked with 5% nonfat milk for 1 h and then were incubated overnight at 4°C with the following primary antibodies: Bcl-2, Bax (Cell Signaling, Danvers, MA, U.S.A.), ELA (AbboMax, San Jose, CA), or GAPDH (Proteintech, Chicago, IL, U.S.A.). After washing, membranes were incubated with the appropriate secondary antibodies and developed by enhanced chemiluminescence (Luminata, Millipore, Billerica, MA, U.S.A.). Protein bands were imaged and quantified by using Alpha View-Fluor ChemQ software (Alpha Innotech Gel Imaging System, San Jose, CA).
MTT assay
Cell viability and proliferation were measured by using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide (MTT) (Acros Organic, Geel, Belgium) according to the manufacturer’s protocol. In brief, cells were seeded at 8000 cells per well into 96-well plates. At 80% confluence, cells were pretreated with Fc-ELA (2 μM) and/or LY294002, then incubated with LPS (2 μg/ml) for 12 h, followed by incubation with MTT (5 mg/ml) for an additional 4 h. The absorbance at the 490-nm wavelength was measured using a microplate reader after medium removal and the addition of 100 μl DMSO. The control group consisting of untreated cells was considered to be 100% viable. Results are expressed as the percentage of viable cells when compared with the control groups.
Data analysis
All data are presented as mean ± SEM. Statistical analyses were performed with one-way ANOVA followed by post-hoc Bonferroni’s test. Differences were considered statistically significant at the level of P<0.05.
Results
Expression of apela is decreased in LPS-treated mice
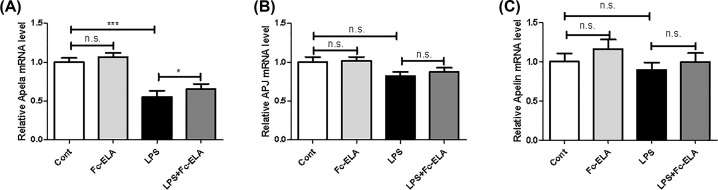
The apela gene, encoding ELA, is known to be selectively expressed in the kidney. To assess whether the expression of apela was affected by LPS and/or exogenous ELA administration, we measured apela mRNA expression in the kidney of the mice receiving LPS and/or Fc-ELA treatment. As shown in Figure 1A, Fc-ELA treatment increased apela expression slightly, but not significantly in the control group. However, Apela expression was significantly decreased by 44.8% (P<0.001) after LPS challenge and co-treatment with Fc-ELA restored its expression level by 18.1% (P<0.05). On the other hand, no significant changes in APJ or apelin expression was noted in any of the treatment groups (Figure 1B,C).
Figure 1. Effect of Fc-ELA on kidney expression of Apela, APJ, and apelin in LPS-treated mice.
qPCR was conducted for apela (A), APJ (B), and apelin (C) in the kidney of mice receiving PBS (Cont) or Fc-ELA (1 mg/kg) with or without LPS (1 mg/kg) for 5 days. Data are expressed as mean + SE (n=8). *P<0.05, ***P<0.001 and n.s., no statistical significance.
Fc-ELA alleviates kidney injury in LPS-treated mice
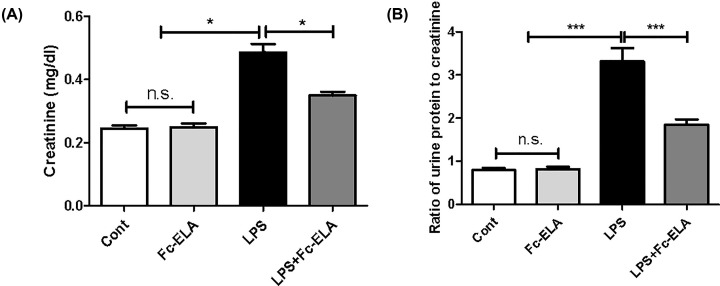
We then assessed kidney function by measuring serum creatinine and the urine protein to creatinine ratio, two widely used indicators of renal function [24]. As shown in Figure 2A, compared with the control group, LPS administration significantly elevated serum creatinine levels from 0.24 to 0.48 mg/dl (P<0.001). Co-treatment with Fc-ELA significantly attenuated the LPS-induced increase to only 0.35 mg/dl (P<0.05), though the creatinine levels remained higher than the control group. Fc-ELA alone had no effect on serum creatinine in mice. Furthermore, LPS administration significantly increased the ratio of urine protein to creatinine (P<0.001), while co-treatment with Fc-ELA significantly attenuated the ratio of protein to creatinine in LPS-challenged mice (P<0.05; Figure 2B). The present study demonstrated that LPS-induced impairment of kidney function was partially reversed by Fc-ELA co-administration.
Figure 2. Effect of Fc-ELA on serum creatinine and the ratio of urine protein to creatinine in different experimental groups.
(A) Effect on serum creatinine. (B) Effect on the ratio of urine protein to creatinine. Mice were administered PBS (Cont) or Fc-ELA (1 mg/kg) with or without LPS (1 mg/kg) daily for 5 days. Serum and urine sample were collected in different experimental groups. Data are expressed as mean + SE (n=9). *P<0.05, ***P<0.001 and n.s., no statistical significance.
Fc-ELA attenuates kidney injury and inflammatory response in LPS-treated mice
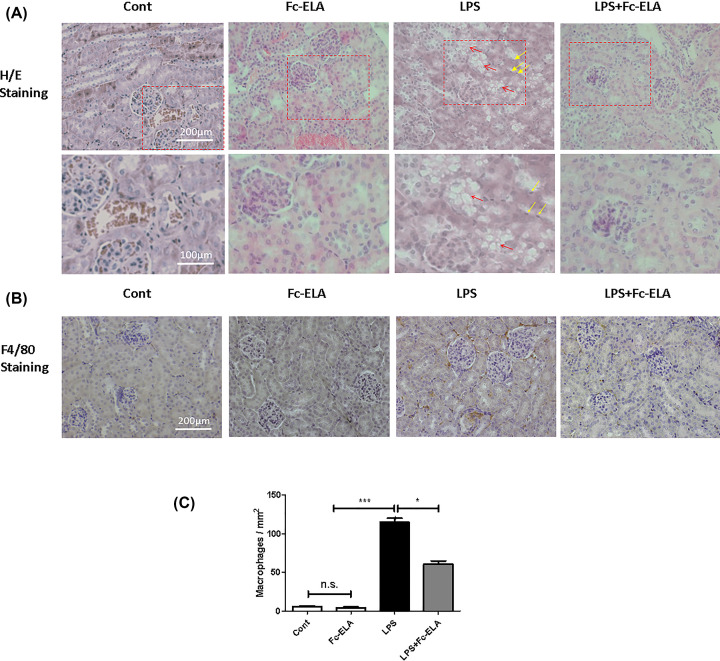
We next conducted histological studies to examine the effect of Fc-ELA on the murine kidney. Renal morphology appeared normal in H/E staining in the mice receiving PBS and Fc-ELA. However, in the LPS-treated mice, renal tubular structure appeared vacuolar with some tubular epithelial cells appearing to show nuclear pyknosis (Figure 3A), a finding significantly attenuated by co-treatment of Fc-ELA. The degree of macrophage infiltration, an indicator of the inflammatory response, was quantified by the staining of F4/80, a macrophage-specific marker [25,26]. As shown in Figure 3B,C, LPS remarkably increased the F4/80 labeling, but this effect was substantially blunted by Fc-ELA treatment. Nevertheless, the extent of macrophage staining in the LPS + Fc-Ela group remained higher than the PBS or Fc-ELA-treated group. These data indicated that the Fc-ELA fusion protein had a renoprotective and anti-inflammatory effect.
Figure 3. Fc-ELA treatment ameliorates LPS-induced kidney damage and macrophage infiltration.
(A) Representative H/E staining images of kidney tissue sections. Red arrows indicate vacuolation and yellow arrows indicate nuclear pyknosis in the LPS group. (B) Representative tissue section images of macrophage marker F4/80 staining and quantification (C). Data are expressed as mean + SE (n=5). *P<0.05, ***P<0.001 and n.s., no statistical significance.
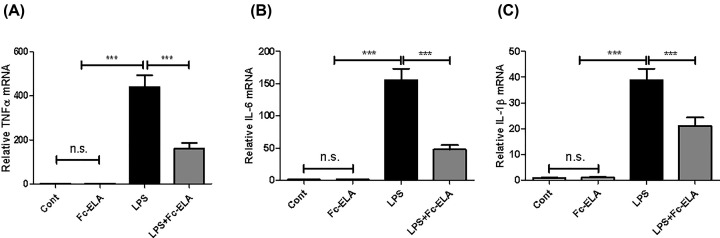
Given the pronounced increase in kidney macrophages of mice receiving LPS and Fc-ELA treatment, we measured mRNA expression of the inflammatory cytokines TNFα, IL-6, and IL-1β. As shown in Figure 4, compared with the PBS or Fc-ELA controls, the TNFα, IL-6, and IL-1β. Transcript levels were significantly elevated after the administration of LPS, and the degree of elevation was attenuated by approximately 63.27, 69.31, and 45.5%, respectively, with Fc-ELA co-administration.
Figure 4. Effect of Fc-ELA on renal expression of TNFα and IL-6 genes.
Quantitative PCR (qPCR) of proinflammatory cytokines TNFα (A), IL-6 (B), and IL-1β (C). Gene expression is normalized with β-actin. Data are expressed as mean + SE (n=5). ***P<0.001 and n.s., no statistical significance.
ELA reduces LPS-induced ROS production in kidneys and HK-2 cells
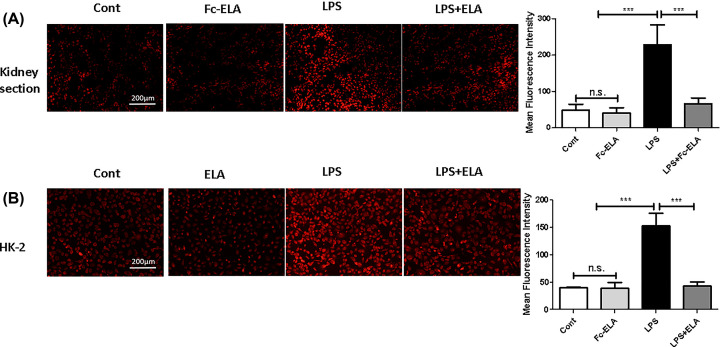
ROS overproduction is a hallmark of inflammation. To determine whether ROS were implicated in ELA’s anti-inflammatory activity [27], we measured ROS levels in kidney tissue sections and in HK-2 cells by DHE fluorescence (Figure 5). Compared with the control group, mice injected with LPS showed a significant increase (P<0.01) in kidney DHE staining. Co-treatment with Fc-ELA markedly lowered the DHE intensity (Figure 5A). In contrast, PBS or Fc-ELA alone had no effect on ROS production. In the human kidney cell line HK-2, ROS production was also induced by LPS and ameliorated by ELA-21 co-treatment (Figure 5B). Thus, ELA suppressed LPS-induced ROS production both in vivo and in vitro.
Figure 5. Detection of ROS in kidneys and HK-2 cells by DHE staining.
(A) Kidney tissue sections of mice receiving PBS, Fc-ELA (1 mg/kg) and/or LPS were stained with DHE and the fluorescence intensity was quantified by fluorescence microscopy. Quantification of ROS production was done by measuring fluorescence intensity. (B) HK-2 cells were pre-treated with PBS/albumin (Cont) or Fc-ELA (0, 5 μM) for 30 min and then with or without LPS (2 μg/ml) for 1 h. The cells were incubated with DHE for 20 min and quantified by microscopy. Quantification of ROS production was done by measuring fluorescence intensity. Data are expressed as mean + SE (n=9). ***P<0.001 and n.s., no statistical significance.
ELA protects against LPS-induced apoptosis in the murine kidney and in HK-2 cells
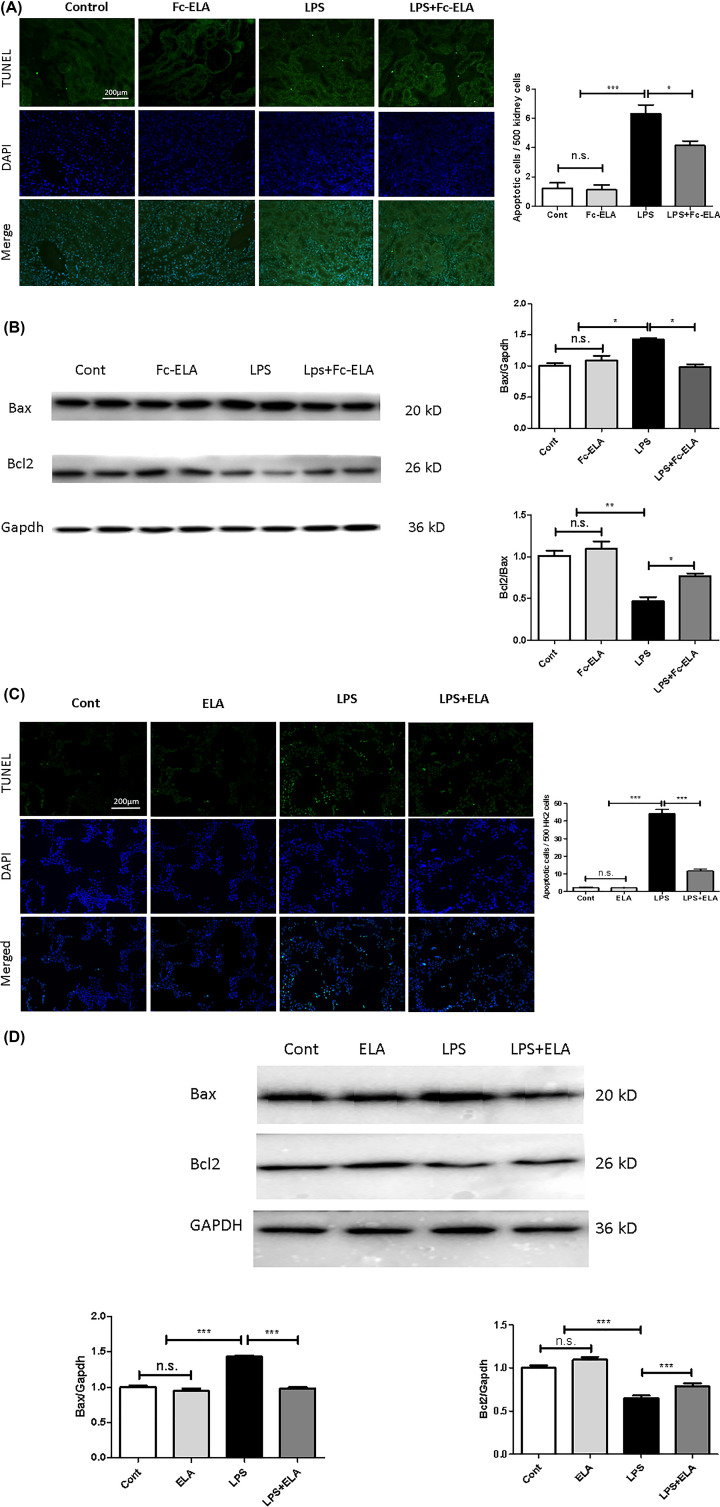
As nuclear pyknosis (Figure 3A) is a sign of cell death, which could be due to apoptosis, we next quantified apoptotic cells by performing a TUNEL assay in kidney tissue slides. As depicted in Figure 6A, very few apoptotic cells were seen in the control or Fc-ELA-treated animals. Remarkably, LPS administration increased the number of apoptotic cells by five-fold, most of which appeared to be in tubular cells. Co-treatment with Fc-ELA significantly reduced the number of apoptotic cells. We next measured the apoptotic proteins Bcl-2 and Bax by immunoblotting. Figure 6B showed that Bax expression was increased significantly, whereas Bcl2 expression was decreased in LPS-challenged kidneys compared with the expression levels in untreated mice. Notably, Fc-ELA co-treatment antagonized the LPS effect by decreasing Bax expression and increasing Bcl2 expression (Figure 6B). We further showed whether or not ELA’s anti-apoptotic action was direct in HK-2 cells by TUNEL assay (Figure 6C). Bax expression was significantly elevated in LPS-treated HK-2 cells, whereas ELA-21 peptide pretreatment decreased Bax expression. Bcl-2 expression was significantly decrease in LPS treated HK-2 cells, whereas ELA-21 peptide pretreatment increased the Bcl2 expression (Figure 6D). Thus, ELA was protective against LPS-induced renal apoptosis both in vitro and in vivo.
Figure 6. ELA suppresses LPS-induced apoptosis in kidneys and HK-2 cells.
Apoptosis analyses of kidney tissues. (A) Upper panels, representative TUNEL staining images (green color); lower panels, DAPI staining (blue color). (B) Representative Western blots of the apoptosis related proteins Bax and Bcl2, in HK-2 cells. (C) Upper panels, representative TUNEL staining images (green color); lower panels, DAPI staining (blue color). (D) Western blot analyses. Data are expressed as mean + SE (n=5 for A,C,D; n=6 for B). *P<0.05, **P<0.01, ***P<0.001, and n.s., no statistical significance.
Implication of the PI3K/Akt signaling pathway in ELA-mediated cell survival
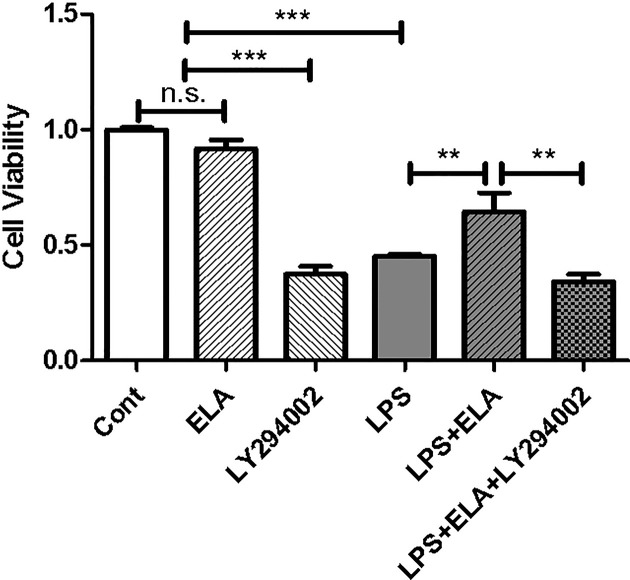
Activation of the PI3K/Akt signaling pathway is known to be critical for cell survival [28] and ELA is a potent activator of the pathway [29]. Thus, we assessed cell viability in response to LPS and/or ELA in the presence or absence of the PI3K inhibitor LY294002. As shown in Figure 7, LPS treatment reduced cell viability by 54.7% (P<0.001), and co-treatment with ELA partially restored the viability to 64.4% of the control (P<0.01), an effect blocked by LY294002 treatment, indicating that the PI3K/Akt signaling is involved in ELA-mediated cytoprotection.
Figure 7. Protection of LPS-induced cell death by ELA.
A cell viability assay was conducted in HK-2 cells treated with LPS, Fc-ELA and/or the PI3K/Akt inhibitor LY294002 and measured by MTT. Data are expressed as mean + SE (n=5). **P<0.01, ***P<0.001 and n.s., no statistical significance.
Discussion
ELA is a newly discovered endogenous ligand of APJ and is selectively expressed in the kidney, though its local and systemic functions remain to be fully understood. Recently, Conquerl et al. [18] have reported that, in a rat model of CLP (cecal ligation puncture)-induced sepsis, continuous infusion of ELA alleviates cardio-renal dysfunction by improving cardiac hemodynamics and urinary output. Notably, ELA appears to be more effective than apelin-13 in its protective effects [18]. However, the ELA peptide has a very short in vivo half-life and is not applicable clinically without modification. Our group has produced Fc-ELA-32 and Fc-ELA-21 fusion proteins and found that Fc-ELA-32 is cleaved whereas Fc-ELA-21 remains intact during in vitro production. Further studies show that Fc-ELA-21 (Fc-ELA) has an in vivo half-life of ∼44 h and can significantly improve heart function in a model of myocardial infarction [21] with daily administration. Since ELA appears to exert an organ protective effect in sepsis [18], we investigated the renoprotective effects of Fc-ELA in LPS-induced endotoxemia.
LPS-induced kidney injury is known to cause renal dysfunction [30] associated with pathological changes through a complex mechanism involving overproduction of ROS [31,32], macrophage infiltration and inflammation, and cell death by apoptosis [1,33]. In this study, mice receiving LPS administration for 5 days developed renal dysfunction as demonstrated by increased levels of serum creatinine and the urine albumin/creatinine ratio, which is associated with inflammation and structural damage to the kidney. Concomitant treatment of Fc-ELA significantly improved kidney function and reduced tissue damage. At the histological level, LPS-treated kidneys showed increased macrophage infiltration, ROS overproduction and apoptosis, all of which were reduced by Fc-ELA co-treatment in the mice. Our in vitro experiments revealed that ELA was capable of suppressing LPS-induced ROS production [27] and apoptosis, and promoting cell survival in human kidney HK-2 cells. These observations are consistent with publications wherein exogenous administration of apelin or ELA can decrease inflammation in vitro and in vivo [14,34] and promote cell survival [19,35,36].
It is interesting to note that apela (the elabela gene) expression was reduced by LPS, but APJ was not in LPS-treated mice. Although both apela and APJ are highly expressed in the kidney, their expression patterns are different; apela is mostly localized to tubular structures whereas APJ and apelin are more widely distributed [37–39]. LPS appeared to selectively cause apoptosis in the tubular cells (Figure 6A), which may explain the significantly decrease in whole tissue apela, but not APJ and apelin. Our finding that apela expression is decreased by LPS and partially restored by Fc-ELA suggests that ELA levels may be a marker of kidney function, which is in line with recent reports that circulatory ELA levels correlate with the ratio of albumin to creatinine in patients with diabetic nephropathy [40]. In light of the high tissue expression of apela in the kidney, ELA may play a protective role locally against injuries by, e.g. inflammation and ischemia [14], which warrants further investigation.
Overall, our work has shown Fc-ELA’s comprehensive renoprotective effects against LPS-induced kidney injury. Given Fc-ELA’s prolonged in vivo half-life, it may be a therapeutic candidate for septic kidney injuries.
Abbreviations
- AKI
acute kidney injury
- APJ
apelin receptor
- Bax
Bcl2 associated X
- Bcl2
B-cell lymphoma 2
- DHE
dihydroethidium
- ELA
Elabela
- Fc
constant fragment
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- H/E
Hematoxylin and Eosin
- HRP
horseradish peroxidase
- IL
interleukin
- i.p.
intraperitoneally
- LPS
liposaccharide
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide
- ROS
reactive oxygen species
- s.c.
subcutaneously
- TLR
toll-like receptor
- TUNEL
terminal deoxynucleotidyl transferase-mediated deoxyuridintriphosphate nick-end labeling
Contributor Information
Da-Wei Gong, Email: dgong@som.umaryland.edu.
Lining Miao, Email: miaolining55@163.com.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was partly supported by the Jilin Province Science and Technology Development Program [grant number 20160414020GH (to L.M.)]; the National Natural Science Foundation of China [grant number #81700723 (to H.Z.)]; and a Maryland Stem Cell Research Fund to D.-W.G.
Author Contribution
F.X., H.Z., H.Z., Q.Z. and R.B. conducted the experiments, analyzed the data and prepared the figures. F.X., M.W., Y.Z., R.B., D.G., and L.M. designed the study and wrote the manuscript. All authors have read and approved the manuscript.
References
- 1.Schrier R.W. and Wang W. (2004) Acute renal failure and sepsis. N. Engl. J. Med. 351, 159–169 10.1056/NEJMra032401 [DOI] [PubMed] [Google Scholar]
- 2.Ng S.W., Zhang H., Hegde A. and Bhatia M. (2008) Role of preprotachykinin-A gene products on multiple organ injury in LPS-induced endotoxemia. J. Leukoc. Biol. 83, 288–295 10.1189/jlb.0807575 [DOI] [PubMed] [Google Scholar]
- 3.Shum H.P., Kong H.H., Chan K.C., Yan W.W. and Chan T.M. (2016) Septic acute kidney injury in critically ill patients - a single-center study on its incidence, clinical characteristics, and outcome predictors. Renal Fail. 38, 706–716 10.3109/0886022X.2016.1157749 [DOI] [PubMed] [Google Scholar]
- 4.Zarbock A., Gomez H. and Kellum J.A. (2014) Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr. Opin. Crit. Care 20, 588–595 10.1097/MCC.0000000000000153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuk A. and Bonventre J.V. (2016) Acute kidney injury. Annu. Rev. Med. 67, 293–307 10.1146/annurev-med-050214-013407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hultstrom M., Becirovic-Agic M. and Jonsson S. (2018) Comparison of acute kidney injury of different etiology reveals in-common mechanisms of tissue damage. Physiol. Genomics 50, 127–141 10.1152/physiolgenomics.00037.2017 [DOI] [PubMed] [Google Scholar]
- 7.Shahin R.D., Engberg I., Hagberg L. and Svanborg Eden C. (1987) Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J. Immunol. 138, 3475–3480 [PubMed] [Google Scholar]
- 8.Mittal M., Siddiqui M.R., Tran K., Reddy S.P. and Malik A.B. (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20, 1126–1167 10.1089/ars.2012.5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aliprantis A.O., Yang R.B., Mark M.R., Suggett S., Devaux B., Radolf J.D. et al. (1999) Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285, 736–739 10.1126/science.285.5428.736 [DOI] [PubMed] [Google Scholar]
- 10.Jaeschke H. (2011) Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J. Gastroenterol. Hepatol. 26, 173–179 10.1111/j.1440-1746.2010.06592.x [DOI] [PubMed] [Google Scholar]
- 11.Wu L. and Mayeux P.R. (2007) Effects of the inducible nitric-oxide synthase inhibitor L-N(6)-(1-iminoethyl)-lysine on microcirculation and reactive nitrogen species generation in the kidney following lipopolysaccharide administration in mice. J. Pharmacol. Exp. Ther. 320, 1061–1067 10.1124/jpet.106.117184 [DOI] [PubMed] [Google Scholar]
- 12.Chng S.C., Ho L., Tian J. and Reversade B. (2013) ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev. Cell 27, 672–680 10.1016/j.devcel.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 13.Pauli A., Norris M.L., Valen E., Chew G.L., Gagnon J.A., Zimmerman S. et al. (2014) Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science 343, 1248636 10.1126/science.1248636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H., Wang L., Wang W., Cheng C., Zhang Y., Zhou Y. et al. (2017) ELABELA and an ELABELA Fragment Protect against AKI. J. Am. Soc. Nephrol. 28, 2694–2707 10.1681/ASN.2016111210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soliman M. and Arafah M. (2015) Apelin protect against multiple organ injury following hemorrhagic shock and decrease the inflammatory response. Int. J. Appl. Basic Med. Res. 5, 195–199 10.4103/2229-516X.165377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo K., Long H., Xu B. and Luo Y. (2015) Apelin attenuates postburn sepsis via a phosphatidylinositol 3-kinase/protein kinase B dependent mechanism: a randomized animal study. Int. J. Surg. 21, 22–27 10.1016/j.ijsu.2015.06.072 [DOI] [PubMed] [Google Scholar]
- 17.Pan C.S., Teng X., Zhang J., Cai Y., Zhao J., Wu W. et al. (2010) Apelin antagonizes myocardial impairment in sepsis. J. Card. Fail. 16, 609–617 10.1016/j.cardfail.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 18.Coquerel D., Chagnon F., Sainsily X., Dumont L., Murza A., Cote J. et al. (2017) ELABELA improves cardio-renal outcome in fatal experimental septic shock. Crit. Care Med. 45, e1139–e1148 10.1097/CCM.0000000000002639 [DOI] [PubMed] [Google Scholar]
- 19.Zhou H., Yang R., Wang W., Xu F., Xi Y., Brown R.A. et al. (2018) Fc-apelin fusion protein attenuates lipopolysaccharide-induced liver injury in mice. Sci. Rep. 8, 11428 10.1038/s41598-018-29491-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strohl W.R. (2015) Fusion proteins for half-life extension of biologics as a strategy to make biobetters. BioDrugs 29, 215–239 10.1007/s40259-015-0133-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xi Y., Yu D., Yang R., Zhao Q., Wang J., Zhang H. et al. (2019) Recombinant Elabela-Fc fusion protein has extended plasma half-life and mitigates post-infarct heart dysfunction in rats. Int. J. Cardiol. 292, 180–187 10.1016/j.ijcard.2019.04.089 [DOI] [PubMed] [Google Scholar]
- 22.Sun X., Yuan X., Chen L., Wang T., Wang Z., Sun G. et al. (2017) Histamine induces bovine rumen epithelial cell inflammatory response via NF-kappaB pathway. Cell. Physiol. Biochem. 42, 1109–1119 10.1159/000478765 [DOI] [PubMed] [Google Scholar]
- 23.Chew J.L. and Chua K.Y. (2003) Collection of mouse urine for bioassays. Lab. Anim. (N.Y.) 32, 48–50 10.1038/laban0803-48 [DOI] [PubMed] [Google Scholar]
- 24.Beynon R.J. and Hurst J.L. (2004) Urinary proteins and the modulation of chemical scents in mice and rats. Peptides 25, 1553–1563 10.1016/j.peptides.2003.12.025 [DOI] [PubMed] [Google Scholar]
- 25.Dos Anjos Cassado A. (2017) F4/80 as a major macrophage marker: the case of the peritoneum and spleen. Results Probl. Cell Differ. 62, 161–179 10.1007/978-3-319-54090-0_7 [DOI] [PubMed] [Google Scholar]
- 26.Leenen P.J., de Bruijn M.F., Voerman J.S., Campbell P.A. and van Ewijk W. (1994) Markers of mouse macrophage development detected by monoclonal antibodies. J. Immunol. Methods 174, 5–19 10.1016/0022-1759(94)90005-1 [DOI] [PubMed] [Google Scholar]
- 27.Kamran Rakhshan Y.A., Naderi N., Afousi A.G. and Aboutaleb N. (2019) ELABELA (ELA) peptide exerts cardioprotection against myocardial infarction by targeting oxidative stress and the improvement of heart function. Int. J. Pept. Res. Ther. 25, 613–621 [Google Scholar]
- 28.Manning B.D. and Cantley L.C. (2007) AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 10.1016/j.cell.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho L., Tan S.Y., Wee S., Wu Y., Tan S.J., Ramakrishna N.B. et al. (2015) ELABELA is an endogenous growth factor that sustains hESC self-renewal via the PI3K/AKT pathway. Cell Stem Cell 17, 435–447 10.1016/j.stem.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 30.Gomez H., Ince C., De Backer D., Pickkers P., Payen D., Hotchkiss J. et al. (2014) A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 41, 3–11 10.1097/SHK.0000000000000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vacas E., Bajo A.M., Schally A.V., Sanchez-Chapado M., Prieto J.C. and Carmena M.J. (2012) Antioxidant activity of vasoactive intestinal peptide in HK2 human renal cells. Peptides 38, 275–281 10.1016/j.peptides.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 32.Xiao L., Ge Y., Sun L., Xu X., Xie P., Zhan M. et al. (2012) Cordycepin inhibits albumin-induced epithelial-mesenchymal transition of renal tubular epithelial cells by reducing reactive oxygen species production. Free Radic. Res. 46, 174–183 10.3109/10715762.2011.647688 [DOI] [PubMed] [Google Scholar]
- 33.Doi K., Leelahavanichkul A., Yuen P.S. and Star R.A. (2009) Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Invest. 119, 2868–2878 10.1172/JCI39421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day R.T., Cavaglieri R.C. and Feliers D. (2013) Apelin retards the progression of diabetic nephropathy. Am. J. Physiol. Renal Physiol. 304, F788–F800 10.1152/ajprenal.00306.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou Y., Wang B., Fu W., Zhou S., Nie Y. and Tian S. (2016) Apelin-13 protects PC12 cells from corticosterone-induced apoptosis through PI3K and ERKs activation. Neurochem. Res. 41, 1635–1644 10.1007/s11064-016-1878-0 [DOI] [PubMed] [Google Scholar]
- 36.Zeng X., Yu S.P., Taylor T., Ogle M. and Wei L. (2012) Protective effect of apelin on cultured rat bone marrow mesenchymal stem cells against apoptosis. Stem Cell Res. 8, 357–367 10.1016/j.scr.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Carroll A.M., Salih S., Griffiths P.R., Bijabhai A., Knepper M.A. and Lolait S.J. (2017) Expression and functional implications of the renal apelinergic system in rodents. PLoS ONE 12, e0183094 10.1371/journal.pone.0183094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pope G.R., Roberts E.M., Lolait S.J. and O’Carroll A.M. (2012) Central and peripheral apelin receptor distribution in the mouse: species differences with rat. Peptides 33, 139–148 10.1016/j.peptides.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Wang Y., Lou Y., Luo M., Lu Y., Li Z. et al. (2018) Elabela, a newly discovered APJ ligand: similarities and differences with Apelin. Peptides 109, 23–32 10.1016/j.peptides.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 40.Zhang H., Gong D., Ni L., Shi L., Xu W., Shi M. et al. (2018) Serum Elabela/Toddler levels are associated with albuminuria in patients with type 2 diabetes. Cell. Physiol. Biochem. 48, 1347–1354 10.1159/000492093 [DOI] [PubMed] [Google Scholar]