Abstract
Background:
Follow-up at a regional adult congenital heart disease (ACHD) center is recommended for all ACHD patients at least once per the 2018 ACC/AHA guidelines. Other specialties have demonstrated poorer follow-up and outcomes correlating with increased distance from health care providers, but driving time to regional ACHD centers has not been examined in the U.S. population.
Objective:
To identify and characterize potential disparities in access to ACHD care in the U.S. based on drive time to ACHD centers and compounding sociodemographic factors.
Methods:
Mid- to high-volume ACHD centers with ≥500 outpatient ACHD visits and ≥20 ACHD surgeries annually were included based on self-reported, public data. Geographic Information System mapping was used to delineate drive times to ACHD centers. Sociodemographic data from the 2011–2015 American Community Survey (U.S. Census) and the Environmental Systems Research Institute was analyzed based on drive time to nearest ACHD center. Previously established CHD prevalence estimates were used to estimate the similarly located U.S. ACHD population.
Results:
Nearly half of the continental U.S. population (45.1%) lives >1 hour drive to an ACHD center. Overall, 39.7% live 1–4 hours away, 3.4% live 4–6 hours away, and 2.0% live >6 hours away. Hispanics were disproportionately likely to live >6 hour drive to a center (p<0.001). Compared to people with <1 hour drive, those living >6 hours away have higher proportions of uninsured adults (29% vs. 18%; p<0.001), households below the federal poverty level (19% vs. 13%; p<0.001), and adults with less than college education (18% vs. 12%; p<0.001).
Conclusions:
We estimate that ~45% of the continental U.S. population lives >1 hour to an ACHD center, with 5.4% living >4 hours away. Compounding barriers exist for Hispanic, uninsured, lower socioeconomic status, and less educated patients. These results may help drive future policy changes to improve access to ACHD care.
Keywords: Adult congenital heart disease, Access to care, Disparities
Introduction:
Due to improving medical and surgical care, more than 90% of children born with congenital heart disease (CHD) survive to adulthood.1 This change in survivorship has led to a rapidly growing adult congenital heart disease (ACHD) population, which increased by more than 50% from 2000 to 2010.2 Using Canadian population-based data sources, Marelli et al reported that, by 2010, 66% of all CHD patients in Quebec were adults. Using these Canadian prevalence estimates and adjusting for U.S. racial/ethnic differences, Gilboa et al estimated the 2010 U.S. adult population of CHD patients in the United States to be approximately 1.4 million.2
The large and continually-growing ACHD population has a unique set of medical needs due to the intersection of CHD, a traditionally pediatric group of diseases, and internal medicine, with acquired adult disease playing an increasingly significant role as patients age. Care for this population is additionally challenging given the dearth of formally-trained ACHD physicians. The American Board of Internal Medicine jointly with the American Board of Pediatrics recognized ACHD as a formal, board-recognized subspecialty in 2015. Since that time, however, only 308 U.S. physicians have been certified.3 Based on the 2010 estimated ACHD population of 1.4 million, the expected ratio of board-certified ACHD physicians to ACHD patients in the U.S. is ~1:4,500. For comparison, according to 2018 board certification data, the ratio of pediatric cardiologists to pediatric CHD patients was ~1:300, and that of adult cardiologists to adults with cardiovascular disease (including hypertension, obesity, etc. which are often cared for solely by primary care physicians) was ~1:950. 1,3–5
To help address the problems of ACHD-trained physician scarcity and the simultaneous increase in the volume of ACHD patients, the American College of Cardiology (ACC) and the American Heart Association (AHA), in their 2008 and 2018 ACHD management guidelines, recommended two tiers of cardiac care follow-up at an ACHD regional center. First, ACHD patients with simple disease should follow up at least once to establish future need for follow-up; and second, those with moderate and complex CHD should follow up at least every 12 to 24 months.6,7 The ACC and AHA list extensive criteria defining the resources which should be available at an ACHD regional center including 24/7 access to ACHD specialists, congenital heart surgeons, and cardiac anesthesiologists. Additionally, other CHD subspecialties (interventional cardiology, electrophysiology, advanced heart failure, etc.), all imaging and diagnostic modalities, and support staff including social work should be readily accessible.6,7 While no comprehensive list of centers fulfilling these criteria exists, the Adult Congenital Heart Association (ACHA) – an organization originally founded as a patient advocacy group – began offering comprehensive ACHD center accreditation in 2017 using a similar list of criteria. As of March 2019, 26 centers have been ACHD accredited.8
Despite the recommendations for specialized follow-up, nearly half of all ACHD patients experience a gap in care of at least 3 years.9 Lapses in CHD care are associated with poorer symptom control and needing urgent cardiac surgical and catheter-based interventions.10 Other chronic diseases such as cystic fibrosis and spina bifida have also shown increased morbidity in those with less frequent routine follow up.11–14
According to the HEART-ACHD (The Health, Education, and Access Research Trial) national multicenter patient study, issues related to access to care, such as “insurance problems” or “relocating”, were the most commonly reported reasons for gaps in care in patients with moderate and complex CHD.9 This study showed that specific areas in the U.S. were more likely to have patients who had experienced a lapse in care (Mountain West and Pacific Northwest), suggestive of geographic disparities in access to lifesaving care.9 Increased distance to a cardiac care center has been shown to be associated with poorer survival in pediatric CHD.15 Similarly, longer travel time to care correlates with poorer disease control in cystic fibrosis as well as increased morbidity and long-term mortality in adult surgery patients.14,16,17 However, improvement in timely follow up has been shown after enrollment in programs that help eliminate transportation and cost barriers to care for socioeconomically disadvantaged adolescents with diabetes.18
This study was designed to contribute to the body of work on specific challenges in access to ACHD care. Our primary goals were to estimate an individual American’s driving time to the nearest ACHD center and examine the relationship between key sociodemographic characteristics and proximity to ACHD centers. To achieve these goals, we identified mid- to high-volume ACHD centers and delineated a series of ACHD care catchment areas based on driving times to the centers. Subsequently, we compared racial/ethnic and socioeconomic characteristics across the catchment areas. Ultimately, we hope that our findings may be used as a surrogate for an individual ACHD patient’s proximity to appropriate specialty care.
Methods:
Defining ACHD Centers
Given that no list of ACHD regional centers exists in the U.S., and that there is evidence of superior CHD outcomes at higher volume centers, we used the publicly available ACHA directory to identify the top 50% of registered centers based on high clinical and ACHD surgical volumes.8,19–22 We identified the top 50% highest volume centers as those with both ≥500 clinic visits and ≥20 surgeries annually, which we refer to as ACHD centers.
Geographic information system mapping
Geographic information system (GIS)28 was used to compare the sociodemographic characteristics of U.S. residents based on their drive times to an ACHD center. GIS mapping has been used in the healthcare sector for different purposes, including efforts to describe and understand the changing landscape of health care and access.11,23 For this study, we used GIS to (1) geocode ACHD centers, (2) delineate drive time catchment areas, (3) summarize essential sociodemographic data, and (4) tabulate relevant data for further analysis. All GIS analyses were limited to the continental U.S. because no ACHD centers were identified in either Alaska or Hawaii. The selected mid- to high-volume ACHD centers were successfully geocoded in ArcMap 10.5 (Environmental Service Research Institute (Esri®) Corporation, Redlands, CA). Using the Network Analyst Extension in ArcMap 10.5 and Esri’s street network dataset 2007, we delineated the boundaries of the areas (catchment areas) within which we could reasonably expect people to drive to any ACHD center based on typical weather and road conditions. Drive time breakdowns reflected overall time commitment needed to attend a clinic visit: <1 hour suggests completing a visit in a half-day or less, 1–4 hours suggests a relatively facile ability to drive to and from a visit the same day, 4–6 hours suggests a trip likely necessitating an overnight stay vs. one long day, and >6 hours requires at least one overnight stay.
Notably, the delineation of catchment areas produced four mutually exclusive collections of geographic zones that span the continental U.S., one for each specified drive time. These catchment areas were “user-created areas of interest,” therefore, their boundaries do not align neatly with those of standard U.S. Census geographies (e.g., block groups). Therefore, it was necessary to make an estimation of the population that fall within each catchment area, and also produce summary estimates on other relevant sociodemographic data. We used the default Data Allocation method in Esri’s Community Analyst Software as a Service (SaaS) mapping solution to address this challenge – the method allocates data to user-created areas by automatically applying population weights to the computation of the necessary estimates in real time. Once the Data Allocation method was executed, we could extract accurate estimates of the relevant data for each catchment area separately. We tabulated data on the following: total population, race/ethnicity, insurance status, household income, and educational attainment. All estimates were based on the U.S. Census 2012–2016 American Community Survey (ACS) 5-year estimates.24
Statistical Analysis
Descriptive statistics were calculated to determine the proportion of individuals by driving time. Specifically, cross-tabulations were performed to observe differences between categorical drive time variables and selected sociodemographic variables. Differences in proportions were evaluated using chi-square tests. Statistical significance was defined as p<0.05. Analyses were performed in Stata version 14.2.
Results:
ACHD center location
We identified 56 mid- to high-volume ACHD centers which provide well over 90% of clinical care (see Figure 1). These 56 centers and the U.S. population density by county are shown in Figure 2. In general, the identified ACHD centers are located in higher population density areas such as large cities and the coastal regions.
Figure 1:
ACHA-registered ACHD center clinical volumes, 2016–2017
Figure 2:
ACHD center location and continental U.S. population density by county, 2012–2016
Drive time-based catchment areas
Drive times to ACHD centers from any location in the continental U.S. are shown in Figure 3. Overall, 54.9% of the US population lived within the <1 hour drive-time catchment area (half-day visit) while 39.7% lived within the 1–4 hour drive-time catchment area (full-day visit); this left 3.4% and 2.0% of the US population within the 4–6 hour drive-time catchment area (likely overnight visit) and >6 hour drive-time catchment area (definitely overnight visit), respectively (Table 1).
Figure 3:
Mid- to high-volume ACHD centers and driving time to nearest center
Table 1.
US sociodemographic characteristics based on drive time to nearest ACHD center. Breakdown of the US adult 1 population by race/ethnicity, insurance status, poverty level, and highest attained educational level into four geographic catchment areas based on driving time to the nearest ACHD center
| Sociodemographic Characteristics | US Population ≥18 years | Drive-time Catchment Areas (hours) |
P-valuea | |||
|---|---|---|---|---|---|---|
| <1 | 1–4 | 4–6 | >6 | |||
| Total | 241 027 591 | 132 224 744 | 95 801 393 | 8 162 701 | 4 838 753 | <.001 |
| 54.9% | 39.7% | 3.4% | 2.0% | |||
| Race/ethnicity | <.001 | |||||
| White (Non-Hispanic) | 60.8% | 54.9% | 69.2% | 67.5% | 43.9% | |
| Black (Non-Hispanic) | 12.4% | 14.0% | 11.1% | 8.3% | 1.8% | |
| Hispanic | 18.2% | 20.3% | 13.8% | 18.8% | 47.1% | |
| Other | 9.0% | 10.8% | 5.9% | 5.4% | 7.2% | |
| Insuranceb | <.001 | |||||
| Insured | 70.7% | 72.2% | 70.4% | 63.0% | 48.1% | |
| Uninsured | 29.3% | 27.8% | 29.6% | 37.0% | 51.9% | |
| Povertyc | <.001 | |||||
| Above FPL | 85.6% | 86.8% | 84.5% | 82.8% | 81.1% | |
| Below/ FPL | 14.4% | 13.2% | 15.5% | 17.2% | 18.9% | |
| Educationd | <.001 | |||||
| College degree/higher | 31.1% | 35.7% | 25.5% | J 22.9% | 24.3% | |
| Less than college | 08.9% | 64.3% | 74.5% | 77.1% | 75.7% | |
Testing whether populations in the four catchment areas differ significantly in terms of selected sociodemographic characteristics.
Insurance status was defined as any type of health insurance including both private and public (Medicare or Medicaid) coverage.
The 2015 Federal Poverty Line (FPL) Guidelines were used. The federal poverty level is determined based on number of persons in a household; for example, in 2015 the federal poverty level was $27 890 for a family of four.
College degree or greater includes 4-year undergraduate degree or equivalent and any further graduate education. Less than college degree includes less than high school, high school degree or GED. trade school, and some college without degree completion.
Selected sociodemographic characteristics
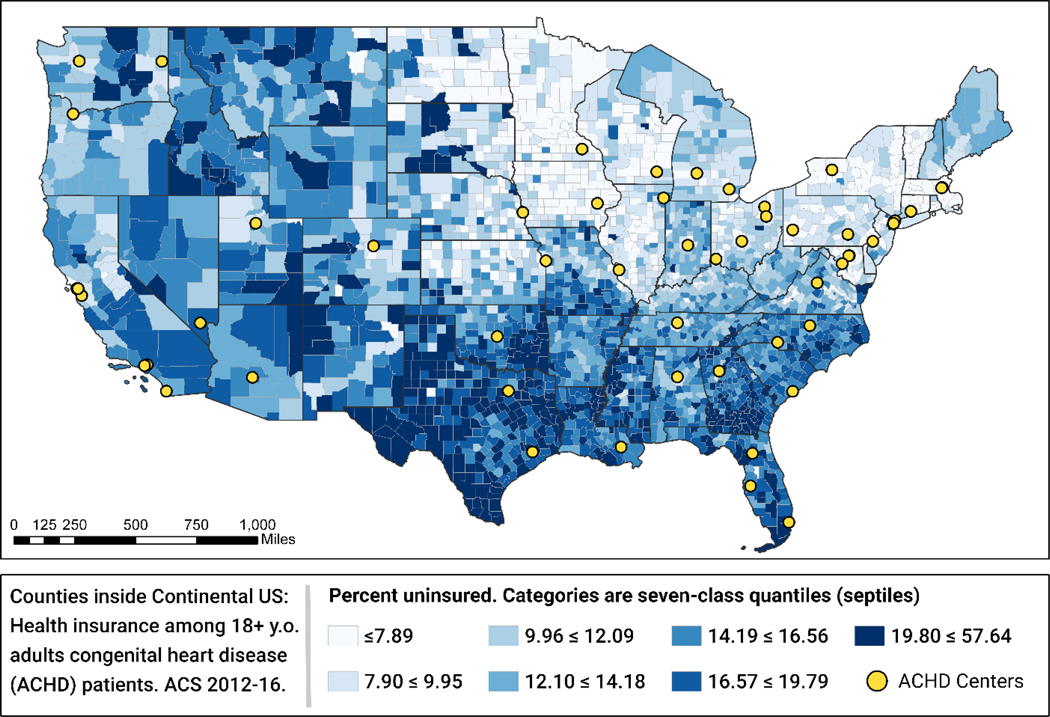
The comparisons of people living in the four drive-time catchment areas are detailed in Table 1. Overall, the distributions of the selected sociodemographic features across the four catchment areas are significantly different (p<0.001) for all features. In terms of race/ethnicity, people who lived in the >6 hours catchment area were more likely to be Hispanic (47.1%) and less likely to be Black, Non-Hispanic (1.8%) compared to their overall population representation of 18.2% and 11.4% respectively. People living farther from an ACHD center were significantly more likely to be uninsured: 27.8% of those living within a one hour drive of an ACHD center were uninsured compared with 51.9% of those living >6 hours away (see Figure 4). Those living farther from an ACHD center were also more likely to live below the federal poverty level: 13.2% of those living <1 hour drive of an ACHD center had household incomes below the federal poverty level compared with 18.9% of those living >6 hours away. Finally, people living farther from an ACHD center were less likely to have graduated from college: 24.3% of those living >6 hour drive from an ACHD center were college graduates compared with 35.7% of those living < 1 hour drive.
Figure 4:
Percent of U.S. population without health insurance by county, 2012–2016
Discussion:
While we estimate that more than half of the continental U.S. lives <1 hour drive to a mid- to high-volume ACHD center, millions live in locations where drive times are a potential barrier to accessing care. Those facing the longest drives are more likely to be Hispanic and of lower socioeconomic status, and their ability to access appropriate care may be compounded with language barriers and the inability to take time off of work if they need >1 day for an ACHD appointment.
Currently, there is no national ACHD registry in the U.S., therefore, there are no authoritative data on ACHD prevalence and the geographic distribution of ACHD patients. The most recent U.S. ACHD prevalence estimate from 2010 of 6.16 patients per 1,000 adults can be applied to the census data that we used in our study, but it is likely an underestimate given the known ongoing increase in this patient population.1,2 It may be reasonable to assume the ACHD population distribution is similar to that of the general population based on birth defect registries showing similar urban vs rural incidence of specific CHD lesions.25,26 It may also be reasonable to assume similar socioeconomic characteristics for ACHD patients compared to the U.S. adult population; a single-center study showed similar insured rates between ACHD and general adult cardiology patients after institution of the Affordable Care Act, although this accounted only for patients seen in clinic.27 If these population distribution assumptions are combined with the 2010 U.S. ACHD prevalence estimate, nearly 700,000 ACHD patients live >1 hour drive from an ACHD center, more than 60,000 of whom have complex disease. This corresponds to 80,000 patients requiring more than a day’s commitment to attend a clinic visit, more than 8,000 of whom have complex disease – we suspect that many of those facing the longest drives to access care suffer compounding sociodemographic disparities. Regardless of the exact patient numbers and distribution, however, it is without question that this large group of patients will continue to face challenges in access to care due to the overwhelming shortage of ACHD physicians and centers.
Progress has finally been made in decreasing disparities in postoperative outcomes for children with CHD, but the scarcity of ACHD physicians will likely shift these disparities to the ACHD population.28 When resources are limited – in this case, access to comprehensive ACHD care centers – disparities, often compounding each other, are bound to exist. We know that chronic disease patients and postoperative patients have better outcomes when they live closer to care, just as we know that receiving care at an ACHD center decreases mortality.14–17,29 In Europe, it is estimated that only 7.1% of ACHD patients receive care at ACHD centers; it is not known if the US does any better.30
ACHD patients historically remained with their pediatric cardiologists who understand congenital cardiac physiology but they lack the experience with general internal medicine practices and age-related cardiovascular disease. Unfortunately, however, simply transferring care to an adult cardiologist is not the answer; this is particularly true given the increased lapses in care during the transition/transfer process.9,31 Current adult cardiology fellowship training guidelines state that while a general adult cardiologist will ideally have at least a month of exposure to ACHD, to graduate they must only have 6 hours of didactic ACHD education; they should be able to review ACHD guidelines to determine which patients require ACHD follow up, but they are not expected to care for this population independently.32 Additionally, these guidelines did not exist for the thousands of adult cardiologists who trained before the need for ACHD care was recognized and incorporated in any manner into training. It is critical that we provide not just care, but high quality care to this burgeoning, high-risk population. Right now, there is significant room for improvement – from 1998 to 2011, ACHD admissions for heart failure increased by 91% compared to a 21% increase for non-ACHD heart failure admissions.33 Further work is needed to determine how to optimize care of this challenging population.
Limitations:
Given the lack of nationally identified regional centers or other centers of excellence, the definition of ACHD centers and the data used to define them is based on unverified, self-reported data. While it has been shown that higher CHD surgical volume centers have superior outcomes, it is unclear if this extends to clinical practice as well. 19–21 Other factors in defining a regional center such as access to subspecialists, diagnostic and therapeutic modalities other than surgery, and social work and support staff were not considered as there is no source for reviewing this data. ACHA accreditation is the closest surrogate to defining a regional center, but as previously mentioned, only 26 centers have been accredited as of March 2019.
We described distribution, driving time to ACHD centers, and sociodemographic characteristics of the U.S. population but acknowledge that this is unlikely to be fully accurate of the characteristics of the ACHD population. Regardless of the precise numbers and distribution, this study highlights large regions of the country in which access to ACHD care is challenging – areas in which the population is more likely to face compounding sociodemographic hardships.
Future directions:
The ultimate goal of this field of research is to identify barriers to care so they can be preemptively addressed before deleteriously affecting health outcomes.34 Given the significant disparities identified in this study, more detailed investigation is needed into access-to-care barriers in the ACHD population.
Ideally, this study’s methods will be repeated in the future using a known population and distribution of ACHD patients via a registry or other definitive data source. Sommerhalter et al were able to do describe true CHD population distribution in an 11-county area in New York using known addresses of adolescent CHD patients, but expanding their methods to a national scale will be challenging; they were able to link adolescent patients to a statewide birth defects registry, which not all states have, and expanding track-and-trace methods to adults adds another layer of complexity.35
In addition to more accurately describing the true ACHD population, our methodology could be repeated using a different definition of ACHD centers or using board-certified ACHD physicians. As ACHA accreditation continues, using accredited centers in place of mid- to high-volume centers will be a more accurate assessment of access to the specific follow up recommended by the ACC/AHA. Examining access to board-certified ACHD physicians and understanding the proportion of ACHD patients under no care, general adult cardiology care, and/or pediatric cardiology care are other important considerations. The ultimate goal of these types of investigations is to improve access to care for the ACHD population by making it easier for them to identify and access high-quality care, especially as they transition, transfer care, or relocate.
Finally, a reevaluation of the recommendation of the ACC/AHA follow-up guidelines may be needed if the number of ACHD-boarded physicians and accredited centers remains low. If studies repeatedly show that appropriate care is not being provided, other methods of care delivery such as satellite clinics or telehealth must be considered. Additionally, specific CHD-lesion specific guidelines can be clarified and refined to guide adult and pediatric cardiologists in managing simple CHD in order to help channel complex patients most in need of ACHD-specific care to ACHD centers.
Acknowledgments:
This project was supported by grant number K23 HL127164 (principal investigator: KNL) from the National Institutes of Health National Heart Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additionally, the ACHA’s database and assistance were instrumental in collecting data about ACHD care in the U.S.
Footnotes
The authors have no conflicts of interest
References:
- 1.Gilboa SM, Devine OJ, Kucik JE, et al. Congenital Heart Defects in the United States: Estimating the Magnitude of the Affected Population in 2010. Circulation. 2016;134(2):101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130(9):749–756. [DOI] [PubMed] [Google Scholar]
- 3.“Number of Certificates Issued - All Candidates”. Published 2018. <https://www.abim.org/about/statistics-data/candidates-certified.aspx>. Accessed 17 June 2018.
- 4.“Interactive ABP Workforce Data”. Published 2018. <https://www.abp.org/content/workforce>. Accessed 17 June 2018.
- 5.“National Center for Health Statistics: FastStats - Heart Disease”. Published 2018. <https://www.cdc.gov/nchs/fastats/heart-disease.htm>. Accessed 17 June 2018.
- 6.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52(23):e143–263. [DOI] [PubMed] [Google Scholar]
- 7.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018. [Google Scholar]
- 8.“ACHA ACHA Accreditation Program”. Published 2017. https://www.achaheart.org/provider-support/accreditation-program/. Accessed 24 August 2017.
- 9.Gurvitz M, Valente AM, Broberg C, et al. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART-ACHD (The Health, Education, and Access Research Trial). J Am Coll Cardiol. 2013;61(21):2180–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeung E, Kay J, Roosevelt GE, Brandon M, Yetman AT. Lapse of care as a predictor for morbidity in adults with congenital heart disease. Int J Cardiol. 2008;125(1):62–65. [DOI] [PubMed] [Google Scholar]
- 11.Delmelle EM, Cassell CH, Dony C, et al. Modeling travel impedance to medical care for children with birth defects using Geographic Information Systems. Birth Defects Res A Clin Mol Teratol. 2013;97(10):673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann JR, Royer JA, Turk MA, et al. Inpatient and emergency room visits for adolescents and young adults with spina bifida living in South Carolina. PM R. 2015;7(5):499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radcliff E, Delmelle E, Kirby RS, Laditka SB, Correia J, Cassell CH. Factors Associated with Travel Time and Distance to Access Hospital Care Among Infants with Spina Bifida. Matern Child Health J. 2015. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JM, Wilcox PG, Quon BS. Evaluating Adult Cystic Fibrosis Care in BC: Disparities in Access to a Multidisciplinary Treatment Centre. Can Respir J 2016;2016:8901756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fixler DE, Nembhard WN, Xu P, Ethen MK, Canfield MA. Effect of acculturation and distance from cardiac center on congenital heart disease mortality. Pediatrics. 2012;129(6):1118–1124. [DOI] [PubMed] [Google Scholar]
- 16.Mehaffey JH, Hawkins RB, Mullen MG, et al. Access to Quaternary Care Surgery: Implications for Accountable Care Organizations. J Am Coll Surg. 2017;224(4):525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehaffey JH, Michaels AD, Mullen MG, Meneveau MO, Pender JR, Hallowell PT. Patient travel for bariatric surgery: does distance matter? Surg Obes Relat Dis. 2017;13(12):2027–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walders-Abramson N, Anderson B, Larkin ME, et al. Benefits and barriers to participating in longitudinal research of youth-onset type 2 diabetes: Results from the TODAY retention survey. Clin Trials. 2016;13(2):240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karamlou T, Diggs BS, Person T, Ungerleider RM, Welke KF. National practice patterns for management of adult congenital heart disease: operation by pediatric heart surgeons decreases in-hospital death. Circulation. 2008;118(23):2345–2352. [DOI] [PubMed] [Google Scholar]
- 20.Kalfa D, Chai P, Bacha E. Surgical volume-to-outcome relationship and monitoring of technical performance in pediatric cardiac surgery. Pediatr Cardiol. 2014;35(6):899–905. [DOI] [PubMed] [Google Scholar]
- 21.Davies RR, Russo MJ, Hong KN, et al. Increased short- and long-term mortality at low-volume pediatric heart transplant centers: should minimum standards be set? Retrospective data analysis. Ann Surg. 2011;253(2):393–401. [DOI] [PubMed] [Google Scholar]
- 22.“ACHD Clinic Directory”. Published 2017. <https://www.achaheart.org/your-heart/clinic-directory/clinic-listings/>. Accessed 17 April 2017.
- 23.McLafferty SL. GIS and health care. Annu Rev Public Health. 2003;24:25–42. [DOI] [PubMed] [Google Scholar]
- 24.“American Community Survey (ACS): Data”. Published 2017. <https://www.census.gov/programs-surveys/acs/data.html>. Accessed 8 June 2017.
- 25.Langlois PH, Scheuerle A, Horel SA, Carozza SE. Urban versus rural residence and occurrence of septal heart defects in Texas. Birth Defects Res A Clin Mol Teratol. 2009;85(9):764–772. [DOI] [PubMed] [Google Scholar]
- 26.Langlois PH, Jandle L, Scheuerle A, Horel SA, Carozza SE. Occurrence of conotruncal heart birth defects in Texas: a comparison of urban/rural classifications. J Rural Health. 2010;26(2):164–174. [DOI] [PubMed] [Google Scholar]
- 27.Lin CJ, Novak E, Rich MW, Billadello JJ. Insurance access in adults with congenital heart disease in the Affordable Care Act era. Congenit Heart Dis. 2018;13(3):384–391. [DOI] [PubMed] [Google Scholar]
- 28.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103(19):2376–2381. [DOI] [PubMed] [Google Scholar]
- 29.Mylotte D, Pilote L, Ionescu-Ittu R, et al. Specialized adult congenital heart disease care: the impact of policy on mortality. Circulation. 2014;129(18):1804–1812. [DOI] [PubMed] [Google Scholar]
- 30.Moons P, Meijboom FJ, Baumgartner H, et al. Structure and activities of adult congenital heart disease programmes in Europe. Eur Heart J. 2010;31(11):1305–1310. [DOI] [PubMed] [Google Scholar]
- 31.Hays L. Transition to Adult Congenital Heart Disease Care: A Review. J Pediatr Nurs. 2015;30(5):e63–69. [DOI] [PubMed] [Google Scholar]
- 32.Warnes CA, Bhatt AB, Daniels CJ, Gillam LD, Stout KK. COCATS 4 Task Force 14: Training in the Care of Adult Patients With Congenital Heart Disease. J Am Coll Cardiol. 2015;65(17):1887–1898. [DOI] [PubMed] [Google Scholar]
- 33.Burchill LJ, Gao L, Kovacs AH, et al. Hospitalization Trends and Health Resource Use for Adult Congenital Heart Disease-Related Heart Failure. J Am Heart Assoc. 2018;7(15):e008775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurvitz M, Burns KM, Brindis R, et al. Emerging Research Directions in Adult Congenital Heart Disease: A Report From an NHLBI/ACHA Working Group. J Am Coll Cardiol. 2016;67(16):1956–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sommerhalter KM, Insaf TZ, Akkaya-Hocagil T, et al. Proximity to Pediatric Cardiac Surgical Care among Adolescents with Congenital Heart Defects in 11 New York Counties. Birth Defects Res. 2017;109(18):1494–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]