Abstract
Information on Lyme borreliosis (LB) during pregnancy is limited. In the present study, the course and outcome of erythema migrans (EM) in 304 pregnant women, diagnosed in the period 1990–2015, was assessed and compared with that in age-matched non-pregnant women. The frequency of unfavorable outcome of pregnancies was also evaluated. The pregnant women reported constitutional symptoms less frequently than the non-pregnant women (22.4% vs. 37.2%, p < 0.001). Pregnant women diagnosed with EM later during pregnancy had a lower probability of reporting constitutional symptoms (odds ratio = 0.97 for 1-week difference in gestation week at diagnosis of EM, 95% CI: 0.94–0.99, p = 0.02). The outcome of pregnancy was unfavorable in 42/304 (13.8%) patients: preterm birth in 22/42 (52.4%), fetal/perinatal death in 10/42 (23.8%), and/or anomalies in 15/42 (35.7%). Several patients had potential explanation(s) for the unfavorable outcome. In conclusion, the course of early LB during pregnancy is milder than in age-matched non-pregnant women. The outcome of pregnancy with the treatment approach used in the present study (i.v. ceftriaxone 2 g once daily for 14 days) is favorable.
Keywords: erythema migrans, Lyme borreliosis, gestation, pregnancy outcome, Borrelia burgdorferi sensu lato
1. Introduction
Lyme borreliosis (LB) usually presents as the skin lesion erythema migrans (EM). The lesion, which is the result of tick bite inoculation of Borrelia burgdorferi sensu lato (s.l.) into the skin, develops early in the course of the disease. The causative agent can disseminate in some patients, resulting in secondary skin lesions and involvement of the nervous system, joints, heart, and/or eye [1].
Information on LB during pregnancy is limited. According to general belief, there are no differences in the course of the disease in pregnant and non-pregnant women. However, a PubMed literature search has found no straightforward data on the course of the infection in pregnant women, and information on the outcome of their pregnancies is limited [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23].
The aim of our study was to evaluate and compare the course and outcome of early LB in non-pregnant and pregnant women and to assess the outcomes of the pregnancies.
2. Patients and Methods
2.1. Selection of Patients
Prospectively acquired data on women with EM who were examined at the LB Outpatient Clinic of the Department of Infectious Diseases, University Medical Center Ljubljana, Slovenia, in the period 1990–2015 were analyzed. All pregnant women diagnosed with EM according to the European criteria [24] were enrolled in the study.
2.2. Clinical Evaluation
Patients were evaluated before commencing treatment with antibiotics, again two weeks later, and then at two, six, 12, and 18 months. Pre-treatment characteristics were assessed using a standardized questionnaire that included demographic, epidemiologic, and clinical data as follows: the number, location, appearance, and diameter of EM; the presence of symptoms at the site of EM (itching, burning, pain); constitutional symptoms (fatigue, headache, myalgia, arthralgia, dizziness, nausea, fever) defined as complaints that newly developed or intensified since the beginning of EM, and the presence of extracutaneous manifestations of LB.
2.3. Laboratory and Microbiological Evaluation
Basic laboratory tests were performed at the first visit and two weeks later.
Serum antibodies to B. burgdorferi s.l. were determined at baseline and at follow-up examinations using either an indirect immunofluorescent assay with a local isolate of B. afzelii as antigen [25] or an indirect chemiluminescence immunoassay (LIAISON®) with antigens OspC and VlsE for the detection of IgM antibodies and VlsE for IgG antibodies.
As of 1994, a sample of citrated blood (5 mL until 2000, 9 mL subsequently) was taken at presentation for cultivation of borreliae in modified Kelly Pettenkofer medium [26]. Isolates were identified to species level using pulsed-field gel electrophoresis after MluI restriction of genomic DNA or by PCR-based restriction fragment length polymorphism of the intergenic region [27,28].
2.4. Treatment
At the first visit, patients received either i.v. ceftriaxone 2 g once daily, i.v. penicillin G 10,000,000 units twice daily, or oral phenoxymethylpenicillin 1 g three times per day. The duration of antibiotic treatment was 14 days.
2.5. Assessment of Outcome
The course and outcome of early LB in the pregnant women and the frequency of unfavorable outcome of their pregnancies (appraised by fetal death, pre-term birth, offspring malformations) were evaluated. The medical history was taken at each visit, including information on the presence of subjective symptoms, and patients were examined clinically. A gynecologist regularly monitored the course of gestation. At the first visit after delivery, detailed information about the birth and the infant was collected. A pediatrician monitored the babies at birth and after 6 months; however, several babies had more frequent and/or longer follow-ups.
2.6. Control Group
The course and outcome of EM in pregnant women was compared with that in age-matched non-pregnant woman diagnosed with EM at our institution in the same year.
2.7. Statistical Analysis
The characteristics of the pregnant women and the control group were compared using the Mann–Whitney test for numeric covariates and Fisher’s exact test for categorical covariates. Categorical variables were summarized with frequencies and percentages and 95% confidence intervals (CI), numeric variables with medians and interquartile ranges. To control for false positives, the p values shown in Table 1 were adjusted using a multivariate permutation procedure [29].
Table 1.
Basic demographic, clinical, and laboratory characteristics of 304 pregnant women before antibiotic treatment of erythema migrans in comparison with 304 sex- and age-matched patients diagnosed with erythema migrans in the same year.
| Pregnant Women | Control Group | p | Adjusted p | |
|---|---|---|---|---|
| No. of patients | 304 | 304 | ||
| Age (years) | 29.5 (27–33) | 29 (26–33) | ||
| Tick bite a | 191 (62.8; 57.1–68.3) | 190 (62.5; 56.8–68.0) | >0.99 | >0.99 |
| Interval from bite to EM onset (days) b | 14 (7–24) | 13 (8–21) | 0.92 | >0.99 |
| Interval from EM onset to diagnosis and treatment (days) | 7 (4–18) | 8 (4–22) | 0.10 | 0.55 |
| Location of EM c: | 0.009 | 0.062 | ||
| Extremities | 256 (84.2; 79.6–88.1) | 227 (74.7; 69.4–79.5) | ||
| Trunk | 43 (14.1; 10.4–18.6) | 73 (24.0; 19.3–29.2) | ||
| Head, neck | 5 (1.6; 0.5–3.8) | 4 (1.3; 0.4–3.3) | ||
| Size of EM c (cm) | 10 (7–16) | 10 (7–17) | 0.54 | >0.99 |
| Ring-like appearance of EM c: | 129 (42.4; 36.8–48.2) | 168 (55.3; 49.5–60.9) | 0.002 | 0.016 |
| Local symptoms | 170 (55.9; 50.1–61.6) | 182 (59.9; 54.1–65.4) | 0.37 | 0.97 |
| Itching d | 154 (50.7; 44.9–56.4) | 163 (53.5; 47.8–59.3) | ||
| Burning d | 26 (8.6; 5.7–12.3) | 33 (10.9; 5.6–14.9) | ||
| Pain d | 26 (8.6; 5.7–12.3) | 39 (12.8; 9.3–17.1) | ||
| Constitutional symptoms | 68 (22.1; 17.8–27.5) | 113 (37.2; 31.7–42.9) | <0.001 | 0.002 |
| Fatigue d | 29 (9.5; 6.5–13.4) | 62 (20.4; 16.0–25.4) | ||
| Headache d | 33 (10.9; 7.6–14.9) | 62 (20.4; 16.0–25.4) | ||
| Myalgias d | 12 (4.0; 2.1–6.8) | 24 (7.9; 5.1–11.5) | ||
| Arthralgias d | 18 (5.9; 3.5–9.2) | 32 (10.5; 7.3–14.5) | ||
| Fever d | 5 (1.6; 0.5–3.8) | 8 (2.6; 1.1–5.1) | ||
| Dizziness d | 7 (2.3; 0.9–4.7) | 11 (3.6; 1.8–6.4) | ||
| Multiple EM | 14 (4.6; 2.5–7.6) * | 16 (5.3; 3.0-8.4) ** | 0.71 | >0.99 |
| Other manifestations of LB e | 2 | 0 |
Data are medians (interquartile range) or frequencies (percentage; 95% confidence interval). p values were obtained with Mann–Whitney tests for numeric variables and chi-squared Fisher’s exact tests for categorical variables. Abbreviations: EM, erythema migrans; LB, Lyme borreliosis. a At site of later EM skin lesion; b data for 189/191 pregnant and 185/190 non-pregnant women who recalled a tick bite at the site of later EM; c findings for the primary lesion in patients with multiple EM; d number (%) of patients reporting an individual symptom; e one patient with associated borrelial lymphocytoma, one with transitory cardiac conduction disorder; * number of skin lesions: 2–13 (IQR 2–6); ** number of skin lesions: 2–12 (IQR 2–3).
The association between gestation week at diagnosis of EM and the presence of constitutional symptoms was investigated using a logistic regression model. A possible departure from the assumption of a linear association between gestation week at which EM was diagnosed and the logit of probability of reporting the symptoms was assessed by fitting an additional model that included a restricted cubic spline transformation (with five knots) of the covariate.
The risk of an adverse outcome of pregnancy varies with week of gestation, and this was used as the timescale. We compared the risk for women in the same gestation week with respect to gestation week at diagnosis of EM. Four additional preselected possible confounders (age, duration of EM at diagnosis in weeks, multiple EM or borreliae isolated from blood, presence of constitutional symptoms) were included in univariable and multivariable Cox regression models.
To account for a possible selection bias of the pregnant patients, 39 patients enrolled in the first eight weeks of pregnancy were included in the risk set only after week 9 of their pregnancy.
R statistical language was used for all the analyses [30].
2.8. Ethical Considerations
The study was performed according to the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Ministry of Health of the Republic of Slovenia (35/04/09). The Ethics Committee waived the need for written informed consent.
3. Results
Among 14,010 patients diagnosed with EM at our institution during a 26-year period, 307 were pregnant, and, for 304 (99%), the natural outcome of the pregnancy was available; 105 of these 304 patients have been reported previously [18,20,23].
3.1. Pre-Treatment Characteristics
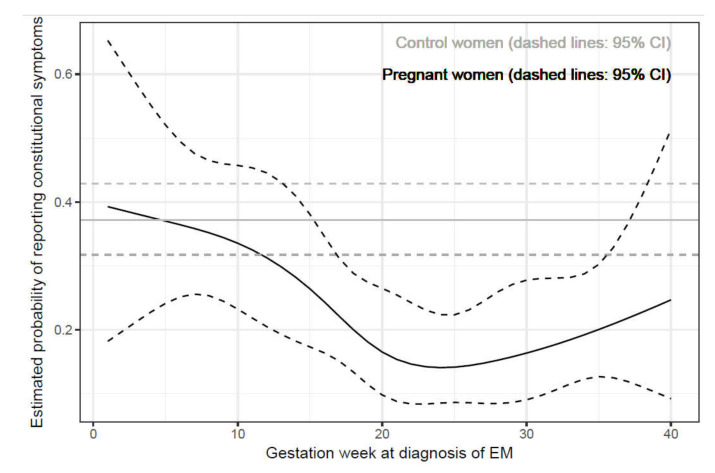
In the comparison of basic clinical characteristics of pregnant and non-pregnant women with EM, findings were analogous for the majority of parameters, with the exceptions that the pregnant women less often had a ring-like EM (42.4% vs. 55.3%, p = 0.002), less often had EM located on the trunk (14.1% vs. 24.0%, p = 0.009), and less often reported constitutional symptoms (22.4% vs. 37.2%, p < 0.001). When the analyses were adjusted for multiple comparisons, the differences remained significant for ring-like EM and the presence of constitutional symptoms (Table 1). Patients who were diagnosed in the later stages of pregnancy had a lower probability of reporting constitutional symptoms (OR = 0.97 for 1-week difference in gestation week at diagnosis of EM, 95% CI: 0.94–0.99, p = 0.02). Our data do not prove a non-linear association between gestation week and the logit of probability (p = 0.20). When including the non-linear term, the estimated prevalence of constitutional symptoms decreased very markedly only in the first 20 weeks of pregnancy but afterwards remained low (Figure 1).
Figure 1.
Estimated probability of reporting constitutional symptoms in pregnant and non-pregnant women with erythema migrans. EM, erythema migrans.
In both groups of women, the proportion of patients with borrelial IgM and/or IgG serum antibodies at presentation (63/295, 21.4% vs. 72/289, 24.9%), the isolation rate of borreliae from blood (8/216, 3.7% vs. 4/187, 2.1%), and the ratio between the isolated Borrelia species (six B. afzelii and two B. garinii vs. three B. afzelii and one B. garinii) were similar.
At the initial visit, dissemination of borreliae was identified in 22 pregnant women: 14/304 (4.6%) had multiple EM; B. burgdorferi s.l. was isolated from the blood in 8/216 (3.7%) patients (all had solitary EM).
3.2. Treatment
Of 304 patients, 299 (98.4%) were treated with i.v. ceftriaxone 2 g once daily, three (1.0%) were treated with i.v. penicillin G 10,000,000 units twice daily, and two (0.7%) with oral phenoxymethylpenicillin 1 g three times per day.
3.3. Post-Treatment Course and Outcome
3.3.1. Comparison of Pregnant and Non-Pregnant Women
After antibiotic treatment, the course and outcome of borrelial infection was uneventful in both groups of women: the EM disappeared in 14 (5–18) days in pregnant women and in 11 (3.5–15) days in non-pregnant women (p = 0.25). None of the 304 patients in either group developed any new objective manifestation of LB following treatment. Persistence of EM, defined as visible EM at follow-up 2–3 months after the beginning of antibiotic treatment, was found in 3/304 (1%) patients in each group. At the 2-month visit, only 5/304 (1.6%) pregnant women reported symptoms that newly developed or intensified after the beginning of EM; at 6 months, 1/300 (0.3%) reported such symptoms. The corresponding findings for the control group were 2/293 (0.7%) and 0/287, respectively.
3.3.2. Outcome of Pregnancies
The outcome of pregnancy was unfavorable in 42/304 (13.8%) patients: preterm birth in 22/42 (52.4%), fetal/perinatal death in 10/42 (23.8%), and/or anomalies detected at birth or within the first year of life in 15/42 (35.7%). Several patients had a potential explanation for the unfavorable outcome (Table 2). At a significance level of alpha = 0.01, none of the chosen factors were associated with a higher risk of unfavorable outcome (Table 3).
Table 2.
Unfavorable outcome of pregnancy in 42 patients after antibiotic treatment of erythema migrans.
| Pregnancy Trimester | Case No. (Year of Diagnosis) | Tick Bite | Onset of EM | Diagnosis of EM | Preterm Birth | Fetal a/Perinatal b Death | Anomalies (Evident at Birth or Later c), Other Abnormalities | Potential Explanations for Unfavorable Outcome |
|---|---|---|---|---|---|---|---|---|
| Week of Gestation | ||||||||
| First trimester | 1 (1998) | no | 2 | 3 | 11 a | |||
| 2 (1996) | 2 | 3 | 4 | syndactyly | anomaly present in family members | |||
| 3 (2002) | 3 | 4 | 4 | 16 a | uterus septus | |||
| 4 (2008) | no | 1 | 4 | 10 a | * | |||
| 5 (1997) | 3 | 5 | 5 | 33 d | pre-eclampsia at week 31 | |||
| 6 (1997) | no | 2 | 5 | 10 a | ||||
| 7 (1999) | −2 e | 4 | 5 | lacrymal canal stenosisc | ||||
| 8 (2008) | 2 | 5 | 6 | 10 a | multipara (6 children, 2 spontaneous abortions previously) * | |||
| 9 (1996) | 6 e | −2 e | 6 | 25 | 25 b | chorioamnionitis, vasculitis of umbilical vessels | ||
| 10 (2010) | 4 | 6 | 7 | 10 a | ||||
| 11 (1993) | no | 6 | 7 | 9 a | uterus bicornus | |||
| 12 (2013) | no | 4 | 8 | 32 d | ||||
| 13 (1997) | 9 | 9 | 10 | 25 | 25 b | |||
| 14 (1996) | 9 | 9 | 10 | 36 | thyroiditis during pregnancy | |||
| 15 (2010) | 8 | 9 | 10 | 35 d | partus imminens (hospitalization from gestational week 24) | |||
| 16 (2006) | 8 | 10 | 12 | 30 d | preterm premature rupture of membranes | |||
| 17 (2008) | 8 | 10 | 12 | 34 d | ||||
| 18 (2009) | no | 10 | 12 | 12 a | ||||
| 19 (1997) | no | 11 | 16 | ureteral stenosis, hydronephrosis, hydroureter dex c (apparent 10 months after birth) | ||||
| 20 (2010) | no | 11 | 16 | fetal growth retardation, CVI in perinatal period with consequent spasticity, left ovarian cyst | ||||
| 21 (2001) | 11 | 12 | 13 | 36 | ||||
| Summary | 13/21 (62%) | 6 (−2–12) | 7 (3–16) | 9/69 (13%) | 10/69 (14%) | 4/69 (6%) | 9/69 (13%) | |
| Second trimester | 22 (2005) | no | 13 | 14 | 36 | |||
| 23 (2000) | 12 | 15 | 16 | stenosis of pulmonary arteria, open foramen ovale | ||||
| 24 (2004) | 15 | 15 | 16 | 36 | hypospadia | |||
| 25 (1994) | 12 | 15 | 16 | 36 d | ASD, VSD | cervical insufficiency | ||
| 26 (1993) | no | 15 | 16 | 36 | ||||
| 27 (2001) | no | 18 | 19 | - | extrasystoles (shortly after birth, duration 3 weeks) | |||
| 28 (2006) | 16 | 20 | 20 | ASD, open foramen ovale, stenosis of pulmonary artery c (apparent 3 months after birth) | ||||
| 29 (2000) | 17 | 20 | 20 | VUR | ||||
| 30 (2001) | 18 | 19 | 20 | hearing deficit | ||||
| 31 (1993) | 19 | 20 | 21 | 26 d | uterus septus; cervical insufficiency | |||
| 32 (1994) | 18 | 19 | 22 | 36 | ||||
| 33 (2010) | 21 | 23 | 23 | 36 | month: ASD | |||
| 34 (2010) | no | 23 | 24 | 36 | ||||
| 35 (1992) | 22 | 23 | 24 | 36 | ||||
| 36 (2001) | no | 24 | 30 | 36 | * | |||
| 37 (2003) | 21 | 25 | 25 | 36 | ||||
| 38 (2004) | 15 | 26 | 28 | 35 f | ||||
| 39 (2004) | 26 | 28 | 28 | 36 | ** | |||
| Summary | 13/18 (72%) | 19 (13–28) | 20 (14–28) | 13/150 (9%) | − | 8/150 (5%) | 2/150 (1%) | |
| Third trimester | 40 (1995) | 31 | 32 | 32 | 5th month: VUR c | |||
| 41 (2007) | 33 | 34 | 34 | ASD, VSD, open ductus arteriosus Botalli | ||||
| 42 (1992) | no | 32 | 35 | 7th month: bilateral ureteral stenosis, hydronephrosis c | ||||
| Summary | 2/3 (67%) | 32 (32–34) | 34 (32–35) | 0/85 | − | 3/85 (4%) | 0/85 | |
Abbreviations: EM, erythema migrans; CVI, cerebrovascular insult; ASD, atrial septum defect; VSD, ventricular septum defect; VUR, vesicoureteral reflux. a Fetal death due to missed abortion (six patients) or spontaneous abortion (two patients); b live at birth, death occurred within few minutes; c later diagnosis of anomalies (within the first year after birth); d respiratory difficulties at birth; e weeks prior to conception; f respiratory distress syndrome and severe icterus at birth; * patient had multiple EM; ** isolation of Borrelia afzelii from blood.
Table 3.
Factors associated with unfavorable outcome of pregnancy.
| Covariate | Univariable Analysis HR (95% CI); p |
Multivariable Analysis HR (95% CI); p |
|---|---|---|
| Gestation week at diagnosis (1-week difference) | 0.97 (0.93–1.00); 0.067 | 0.95 (0.92–0.99); 0.013 |
| Age (10-year difference) | 1.35 (0.76–2.40); 0.31 | 1.43 (0.78–2.62); 0.25 |
| Duration of EM before diagnosis (10 days difference) | 0.82 (0.65–1.04); 0.11 | 0.79 (0.61–1.02); 0.07 |
| Multiple EM or borreliae isolated from blood | 1.52 (0.54–4.28); 0.43 | 1.88 (0.65–5.41); 0.24 |
| Presence of systemic symptoms | 0.53 (0.22–1.26); 0.15 | 0.46 (0.19–1.11); 0.08 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
4. Discussion
Several mechanical and pathophysiologic changes occur during pregnancy, and immune adaptations develop to accommodate the fetus [31]. As pregnancy progresses, hormone levels (estradiol, progesterone) increase markedly in association with several immunologic changes, including a shift from Th1 to Th2 immunity, which results in decreasing robustness of cell mediated immunity [32]. Consequently, the acquisition, clinical presentation, and course of infectious diseases in pregnant women may be altered [33]. Although clinical findings indicate that the course of most infections in pregnant and non-pregnant women are similar, some diseases (for example, listeriosis and malaria) are more frequent during gestation, and several (such as influenza, hepatitis E, herpes simplex virus infections, malaria) are more severe in pregnant women [31]. While it is well known that some non-infectious diseases, such as multiple sclerosis, have a milder course during pregnancy [34], no infectious disease has been reported to have a less severe course during gestation. There is a concept that pregnancy does not affect the course and outcome of early LB; however, studies that directly compare the course of LB in pregnant and in non-pregnant women are lacking.
Our study has shown that the majority of basic clinical and epidemiologic characteristics of EM before treatment with antibiotics were analogous in the two groups of women and consonant with previous findings in Slovenian patients with EM [35,36,37,38,39,40,41,42] and that the outcome after antibiotic treatment was excellent regardless of pregnancy. No subsequent objective manifestations of LB were established in either of the two groups, and the proportion of patients with symptoms at follow-up visits was even lower than found in other recent studies from Slovenia [37,38,39,40,41,42], possibly because only young, previously healthy patients were included in the present study.
Nevertheless, there were also several differences. We do not have a reliable explanation for the observation that the pregnant women less often had ring-like EM despite similar duration of the skin lesion before treatment, but we stress that the findings in our control group are in agreement with previous reports in Slovenian patients with EM [35,36,37,38,39,40,41,42]. Furthermore, the proportion of reported constitutional symptoms accompanying EM was lower in the pregnant women, indicating that the course of EM during pregnancy was milder than in the age-matched non-pregnant women (Table 1, Figure 1), as also shown in previous reports on EM from the same region [35,36,37,38,39,40,41,42]. Our results indicate that the probability of reporting constitutional symptoms systematically decreases with gestation week at diagnosis of EM (EM was diagnosed a median 7 days after the appearance of the skin lesion) and that women infected during the later stages of pregnancy report fewer constitutional symptoms compared with those infected during the early phases of pregnancy, who are more similar to non-pregnant women.
Since B. burgdorferi s.l. does not produce its own toxins or extracellular matrix-degrading proteases, most manifestations of LB result from inflammation generated by the host immune response to the spirochete [43]. Thus, fewer symptoms, as found in the present study of pregnant women with EM, may be associated with lower levels of inflammation. This assumption parallels findings in animal models where, for example, Lyme arthritis in pregnant mice was less severe. The amelioration of arthritis was associated with a shift in inflammatory responses—that is, the down-regulation of Th1 responses, most likely via the progesterone-mediated up-regulation of Th2 cytokine production, resulting in the reduction in pathogenic inflammatory responses during gestation in the mice [44]. In addition, our previous work has shown that higher numbers of constitutional symptoms in EM patients are associated with greater Th1 or Th17-associated cytokine responses, which is consistent with findings that immune responses shift towards a Th2 response in the course of pregnancy [45].
Information on the outcomes of pregnancy in women who develop LB during gestation is limited [46]. Findings of a PubMed literature search for the period of 1985 to January 2020 are shown in Table 4. The search found several individual case reports and a few series on unfavorable outcome of pregnancy in patients who were treated or not treated with antibiotics, the large majority of studies being from the two decades after recognition of LB [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. The described cases show no uniform pattern of abnormalities. In the majority of cases, only a temporal association with maternal LB was described but no causal relationship was confirmed or searched for, and, in some articles, the proof of borrelial infection was imprecise by present standards. In addition, in patients in whom spirochetes were identified microscopically or by culture of placenta or autopsied infants, no signs of inflammation, granuloma formation, or necrosis in the affected tissues were detected. Moreover, Mather et al. reported the absence of the transplacental transmission of Lyme disease spirochetes from reservoir mice to their offspring [47], though the human placenta could be different to that of the mouse. Thus, the association between maternal borrelial infection and unfavorable outcome of pregnancy remains unclear, and the risk of adverse outcomes from maternal Lyme borreliosis has been interpreted to be negligible.
Table 4.
Reports on unfavorable outcomes of pregnancy in patients with Lyme borreliosis during gestation.
| Author(s) and Year Type of Study | Pregnant Women | Outcome of Pregnancy | |||||
|---|---|---|---|---|---|---|---|
| Tick Bite Gestational Week of LB Onset/Diagnosis | LB Signs/LB Symptoms/Antibiotic Treatment Other Clinical Data | LB Serology | Preterm Birth: Week (Weight) | Fetal or Perinatal Death: Week (Weight) | Anomalies, Other Abnormalities | Evidence of Borrelia burgdorferi Sensu Lato Infection of Fetus or Child | |
| Schlesinger et al. 1985 Case report |
No 6–7/8 |
MEM, stiff neck/headache, malaise, arthralgias/no No |
Positive | 35 (ND) | 35 (ND) Respiratory distress, death 39 h after delivery |
Aortic valvular stenosis, patent ductus arteriosus, coarctation of aorta, tubular hypoplasia of aorta and aortic arch, endocardial fibroelastosis No |
A few spirochetes in spleen, renal tubule, bone marrow seen in paraffin block sections stained using modified Dieterle method. No evidence of inflammation, necrosis, or granuloma in any organ. Placenta not available. Later examination: immunohistochemical detection of spirochetes in cardiac tissue |
| MacDonald 1986, 1989 MacDonald et al. 1987 Retrospective analysis of perinatal autopsies 1978–1985; prospective study on perinatal deaths 1985–1988 |
No 1–2/LB not diagnosed |
EM, arthritis/no/no No |
Positive | No | At term (2500 g) Stillbirth | VSD Retardation of intrauterine growth |
Culture of spirochetes from fetal liver tissue; IFA detection of spirochetes in fetal liver, heart, adrenal glands, subarachnoid space; silver stains: spirochetes in myocardium, brain, liver, placenta. No inflammation in fetal tissues, rare plasma cells in isolated placental villi. |
| No ND/LB not diagnosed |
No/no/no Toxemia in w 17 |
Negative | 19 (514 g) | 19 (514 g) Stillbirth | ASD No |
Culture of spirochetes from fetal liver tissue; IFA detection of spirochetes in fetal liver and placenta. | |
| ND ND/LB not diagnosed |
No/arthralgias/no Toxaemia in w 22 |
Negative | 23 (490 g) | 23 (490 g) Stillbirth | Coarctation of aorta No |
Culture of spirochetes from fetal liver tissue; IFA detection of spirochetes in fetal liver and placenta. No tissue inflammation. |
|
| ND ND/LB not diagnosed |
No/no/no No |
Negative | 15 (85 g) | 15 (85 g) Stillbirth | No No |
Culture of spirochetes from fetal liver tissue; IFA detection of spirochetes in fetal liver, placentaNo tissue inflammation | |
| ND ND/LB not diagnosed |
No/no/no Vaginal bleeding in 1st trimester |
ND | No | 39 (2250 g) Respiratory distress, death in 4 h | VSD, hydrocephalus, meningomyelocele, omphalocele, spina bifida, club foot No |
Immunohistochemical detection of spirochetes in fetal tissue | |
| ND ND/LB not diagnosed |
No/no/no No |
ND | No | 40 (1950 g) Respiratory distress, death in 30 min | VSD, absent left hemidiaphragm Retardation of intrauterine growth, cardiac dysfunction |
Indirect IFA detection of spirochetal fragments in fetal tissue | |
| ND ND/LB not diagnosed |
No/no/no Vaginal bleeding in 2nd trimester |
Negative | 17 (30 g) | 17 (30 g) | Hydrocephalus No |
Indirect IFA detection of spirochetes in fetal brain | |
| ND ND/LB not diagnosed |
No/no/no Vaginal bleeding in 2nd trimester |
Negative | 16 (150 g) | 16 (150 g) | No No |
Spirochetes identified in fetal brain using immunohistochemical technique with monoclonal antibodies. No inflammation found in fetal viscera | |
| ND ND/LB not diagnosed |
No/no/no No |
ND | 12 (294 g) | 12 (294 g) | No No |
Culture of fetal viscera in BSK medium yielded B. burgdorferi and other bacteria from fetal kidney; immunohistochemistry negative for spirochetes in fetal viscera | |
| ND ND/LB not diagnosed |
No/arthralgias, myalgias, headache/no No |
Negative | 25 (ND) | 25 (ND) Intrauterine fetal death |
VSD No |
Indirect IFA detection of spirochetes in fetal tissue | |
| ND ND/LB not diagnosed |
No/no/no No |
ND | No | 40 (3746 g) | No Neonatal sepsis, respiratory distress in 1st hour of life |
Rare spirochetes found in “normal” placental villi | |
| ND ND/LB not diagnosed |
No/no/no Toxemia in w 37 |
ND | 37 (2157 g) | No | No Neonatal sepsis, respiratory distress in 1st day of life |
Many spirochetes identified in placenta using Warthin–Starry silver impregnation technique | |
| Markowitz et al. 1986 Retrospective study on 19 pregnant women with LB |
ND 6/8 |
EM, stiff neck, arthritis/headache/penicillin V 10 days No |
Positive | 20 (ND) | 20 (ND) Intrauterine fetal death |
No No |
Culture and indirect IFA negative. No inflammation found in fetal tissues. |
| ND 10/LB not diagnosed |
Facial palsy, arthritis/headache/no No |
ND | 36 (2100 g) | No | No Hyperbilirubinemia |
ND | |
| ND 20/21 |
EM, stiff neck/headache, arthralgia/ erythromycin 10 days (in w 21), penicillin V 10 days (in w 27) No |
ND | No | No | Syndactyly No |
ND | |
| ND 27/27 |
EM/no/penicillin V 10 days No |
ND | No | No | Cortical blindness Developmental delay |
ND | |
| ND 39/39 |
EM, meningitis/no/no No |
ND | No | No | No Generalized rash, hyperbilirubinemia |
ND | |
| Ciesielski et al.1987 Prospective study on 17 pregnant patients with LB |
ND ND/4 |
Not specified/not specified/data on prescribed antibiotic not available ND |
Positive | 13 (ND) | 13 (ND) Spontaneous abortion |
No No |
No evidence of borrelial infection on stains or cultures of fetal tissues. |
| ND ND/7 |
Not specified/not specified/data on prescribed antibiotic not available ND |
Positive | No | No | Syndactyly No |
ND | |
| Weber et al. 1988 Case report |
Yes 10/12 |
EM/no/penicillin 1g tid 7 days No |
Seroconversion | No | At term (3400 g) Respiratory distress, death 23 h after delivery |
No No |
Silver stain and monoclonal antibody identification of spirochetes in brain and liver. No significant inflammation found in any organ. |
| Andrásová et al. 1988 Case report |
ND ND/23 |
EM, facial palsy/arthralgias, low fever/yes (antibiotic not specified) Vaginal bleeding in 1st trimester |
Positive | 32 (1450 g) | No | No Respiratory distress, anemia |
Placenta: no spirochetes and no inflammation. |
| Nadal et al. 1989 Serologic study on 1416 pregnant women and their offspring |
Yes 1st trimester/LB not diagnosed |
EM, arthritis/no/no No |
Positive | No | No | VSD No |
ND |
| Lavoie et al. 1990 Case report |
No ND/LB not diagnosed |
No/arthralgia, malaise/no No |
Negative | No | At term (ND)Respiratory distress, myocardial dysfunction, death 8 days after delivery | No No |
Isolation of B. burgdorferi from frontal cerebral cortex; silver staining: spirochetes in brain and heart. |
| Hercogová et al. 1993, 1994 Prospective studies on 15 and 19 pregnant patients with EM |
No 6/10 |
EM/arthralgia, paresthesias/penicillin V 5 days (in w 10), penicillin V retreatment (in w 14) No |
Positive | 15 (ND) | 15 (ND) Intrauterine fetal death | No No |
Borrelia-like organism in ultrathin sections of the decidua detected using monoclonal antibody H9724 against flagellin. |
| Yes 16/18 |
EM/fatigue/ampicillin 21 days No |
Positive | 18 (ND) | 18 (ND) | Spina bifida, hydrocephalus No |
ND | |
| Yes 4/8 |
EM/no/penicillin V 24 days No |
Negative | No | No | Unclosed ductus arteriosus Botalli No |
ND | |
| Yes 25/30 |
EM/no/penicillin V 14 days No |
Negative | No | No | Cryptorchidism (established at 2 years) No |
ND | |
| ND ND/ND |
EM/ND/yes (antibiotic not specified) No |
ND | No | No | No Hypotrophia |
ND | |
| ND ND/ND |
EM/ND/yes (antibiotic not specified) No |
ND | No | No | No Hyperbilirubinemia |
ND | |
| No 18/24 |
EM/no/penicillin V 14 days, benzanthine penicillin No |
Negative | No | No | Enamel defect No |
ND | |
| No 31/33 |
EM/low fever/penicillin V 14 days No |
Positive | No | No | Enamel defect No |
ND | |
| Yes 20/23 |
EM/no/penicillin V14 days No |
Positive | No | No | No Developmental delay |
ND | |
| Williams et al. 1995 Serologic study on 5011 newborns and their mothers |
ND | ND/ND/yes (antibiotic not specified) ND |
Positive | No | No | Hypospadia No |
ND |
| Maraspin et al. 1996, 1999 Prospective cohort studies on 58 and 105 pregnant patients with EM |
Yes Before conception/6 |
EM/no/ceftriaxone 2 g i.v. 14 days No |
Negative | 25 (610 g) | 25 (610 g)Death in few minutes | No No |
Warthin–Starry silver impregnation of fetal tissues: no spirochetes. No inflammation. Normal placenta. Chorioamnionitis and vasculitis of umbilical vessels. |
| Yes 9/10 |
EM/no/ceftriaxone 2 g i.v. 14 days No |
Negative | 25 (450 g) | 25 (450g)Death in few minutes | No No |
Warthin–Starry silver impregnation of fetal tissues: no spirochetes. No inflammation. Normal placenta. | |
| Yes 5/5 |
EM/no/ceftriaxone 2 g i.v. 14 days Preeclampsia in w 31 |
Negative | 33 (1720 g) | No | No Respiratory distress, hyperbilirubinemia |
Not tested | |
| Yes 20/21 |
EM/no/ceftriaxone 2 g i.v. 14 days No |
Negative | 26 (840 g) | No | No Respiratory distress, bilateral ventricular and periventricular bleeding |
Not tested | |
| Yes 23/24 |
EM/no/ceftriaxone 2 g i.v. 14 days No |
Negative | 36 | No | No No |
Not tested | |
| Yes 15/16 |
EM/no/ceftriaxone 2 g i.v. 14 days No |
Negative | 36 (2940 g) | No | ASD, VSD Respiratory distress, pneumothorax |
Not tested | |
| No 32/35 |
EM/no/ceftriaxone 2 g i.v. 14 days No |
Negative | No | No | Bilateral ureteral stenosis No |
Not tested | |
| Yes 32/32 |
EM/no/ceftriaxone 2 g i.v. 14 days No |
Negative | No | No | Vesicoureteral reflux No |
Not tested | |
| No 11/16 |
EM/no/ceftriaxone 2 g i.v. 14 days No |
Negative | No | No | Ureteral stenosis, hydronephrosis, hydroureter dex No |
Not tested | |
| Yes 3/4 |
EM/no/ceftriaxone 2 g i.v. 14 days No |
Negative | No | No | Syndactyly No |
Not tested | |
| No 6/7 |
EM/no/ceftriaxone 2 g i.v. 14 days No |
Negative | 9 (ND) | 9 (ND) Missed abortion |
No No |
Not tested | |
| No 2/5 |
EM/no/ceftriaxone 2 g i.v. 14 days No |
Negative | 10 (ND) | 10 (ND) Spontaneous abortion |
No No |
Not tested | |
| Lakos et al. 2010 Clinical experiences obtained using miscellaneous approaches |
a ND 1–5/ND |
EM/ND/ND/ 4/6 pts oral antibiotic (ND on antibiotic type); 2/6 untreated |
ND | 8 (ND)—5 pts, 13 (ND)—1 pt |
8–13 (ND) Spontaneous abortion | No No |
ND |
| ND 5/ND |
EM/ND/No ND |
ND | 22 (ND) | 22 (ND) Stillbirth | No No |
ND | |
| ND 17/ND |
EM/ND/cefuroxime axetil ND |
ND | 35 (ND) | No | No No |
ND | |
| ND 13/ND |
EM/ND/ceftriaxone 2 g i.v. 15 days ND |
ND | No | No | No Small for date (2200 g at 39 w) |
ND | |
|
b ND 13–27/ND |
EM/ND/penicillin G i.v. or ceftriaxone iv ND |
ND | No | No | Cavernous hemangioma No |
ND | |
| ND/13/ND | EM/ND/ceftriaxone 2 g i.v. 15 days ND |
ND | No | No | NoHyperbilirubinemia * | ND | |
| ND/38/ND | EM/ND/erythromycin 150 mg qid 30 days ** ND |
ND | No | No | Dysplasia coxae Hyperbilirubinemia * |
ND | |
| ND 18/ND |
EM/ND/ceftriaxone 2 g i.v. 15 days ND |
ND | No | No | Dysplasia coxae No |
ND | |
| ND Before conception/ND |
EM/ND/ceftriaxone 2 g i.v. 15 days ND |
ND | No | No | Pyloric stenosis, dysplasia coxae No |
ND | |
| ND 38/ND |
EM/ND/No ND |
ND | No | No | No Papulovesicular eruption at birth |
ND | |
| ND 4/ND |
EM/ND/No ND |
ND | No | No | No Cerebral bleeding, developmental delay |
ND | |
| ND 27/ND |
EM/ND/No ND |
ND | No | No | Hypospadia, cavernous hemangioma No |
ND | |
| ND 13/ND |
EM/ND/ceftriaxone 2 g i.v. 15 days ND |
ND | No | No | Skeletal anomaly No |
ND | |
| Maraspin et al. 2011 Study on 7 pregnant patients with EM and borreliae isolated from blood |
Yes 28/28 |
EM/No/ceftriaxone 2 g i.v., 14 days No |
Negative | 36 (2500 g) | No | No No |
Not tested |
Abbreviations: LB, Lyme borreliosis; MEM, multiple erythema migrans; EM, erythema migrans; ND, no data; pt, patient; pts, patients; VSD, ventricular septum defect; w, week; ASD, atrial septum defect; IFA, immunofluorescence assay; tid, three times daily; quid, four times daily. a Summarized data for six patients with spontaneous abortion; b summarized data for three patients with cavernous hemangioma; * exchange transfusion required; ** persistent EM, therefore patient treated after delivery with ceftriaxone 2 g i.v. for 15 days.
In the present study, 42/304 (13.8%) patients had an unfavorable outcome of pregnancy. However, several of these patients had a potential explanation for the unfavorable outcome (Table 2), and none of the tested parameters were associated with unfavorable pregnancy outcome (Table 3). Although our multivariable analyses showed an association between the week of pregnancy in which EM was diagnosed and unfavorable outcome, suggesting that patients infected earlier during pregnancy might have a higher risk of such an outcome, the diminishment of the odds of unfavorable outcome with the duration of pregnancy is an expected finding that is valid for the overall population of pregnant women. Thus, unfavorable outcome of pregnancy in women who had LB during pregnancy does not in any way signify that borrelia infection was the cause of the adverse result. Furthermore, the frequency of unfavorable outcome of pregnancy as found in the present study is comparable to the findings described in the literature for the general population, with the exception that the miscarriage rate in our study (11.6%) is somewhat lower than in the majority of previous reports (15–20%) [48,49,50,51] yet still in agreement with a Swedish study [51]. The findings of the present study are also in accord with the pregnancy outcomes reported for the general Slovenian population in the corresponding time frame [52], including miscarriage rate (11.6% in the present study versus 12% in the general population), preterm birth (7.2% versus 6.1%), and anomalies detected at birth (2.6% versus 1.7%). However, we would like to stress that we did not perform a cohort study, where all women enter the study at the beginning of their pregnancy, and therefore we did not attempt to analyze the approximate prevalence of unfavorable outcomes by trimester or to compare them with values observed in the overall population of pregnant women. In our study, pregnant women were enrolled at the gestation week when they were diagnosed with EM and left the study at delivery or at the gestation week of an unfavorable event. Consequently, women experiencing abortion or early delivery were less likely to be included in the sample of pregnant women diagnosed with EM compared with those who did not experience such unfavorable events.
All but five pregnant women were treated for their EM with i.v. ceftriaxone for 14 days. According to current knowledge, this is clearly overtreatment of EM in non-pregnant and possibly also in pregnant women. However, 30 years ago, the understanding of LB was somewhat rudimentary, and information on the course and outcome of pregnancy in patients with early LB was limited to individual case reports, several of them indicating unfavorable outcomes after treatment of EM with oral antibiotics [3,10]. At that time, we decided on a treatment protocol that would achieve high enough levels of antibiotics not only in skin but also in the placenta and fetus; the decision being based on the premise that damage to the fetus probably results from the direct dissemination of borreliae or indirectly through damage to the placenta. In the years since, a concept has been developed in which the outcome of pregnancy with this treatment approach is similar to that in the general population [20]. However, because cases of LB during gestation are not numerous, we did not alter our approach and have been waiting to see whether studies from other research groups would confirm that the same outcome would follow oral antibiotic treatment, as recommended for EM in the non-pregnant population. A report published in 2010 (the most recent available information) states that the proportion of unfavorable pregnancy outcomes in patients with LB was the highest in patients who received no antibiotic treatment for their LB, followed by those who received oral antibiotics, and it was the lowest in patients treated with parenteral antibiotics [22]. The report, although it has several methodologic drawbacks, has influenced us to not change our treatment approach. The consequence of this “wait and watch” tactic is that we know that the outcome of pregnancies is relatively favorable using our treatment protocol, but we do not know whether the same results could be obtained with oral treatment, as is usually recommended for EM. Furthermore, since we did not demonstrate the direct detection of borreliae in fetal tissue or umbilical blood, etc., which is a substantial limitation of the present study, we do not know whether a relatively favorable outcome of pregnancy is the result of our efficacious antibiotic treatment or a consequence of very rare or perhaps even non-existent borrelial involvement in the offspring.
5. Conclusions
The course of early LB during pregnancy is milder than in age-matched non-pregnant women. The smaller proportion of pregnant patients reporting constitutional symptoms at the time of EM diagnosis might be the result of immunologic changes during gestation.
The outcome of pregnancy with the treatment approach used in the present study (i.v. ceftriaxone 2 g once daily for 14 days) is favorable. Multivariable analyses showed that patients who develop EM in the early stages of pregnancy might have a higher risk of unfavorable outcome.
Author Contributions
Conceptualization, V.M. and F.S.; methodology, V.M. and F.S.; validation, V.M. and F.S.; formal analysis, V.M., T.B., E.R.-S., and F.S.; data curation, V.M., T.B., L.L., M.P.P., E.R.-S., and F.S.; writing—original draft preparation, V.M. and F.S.; writing—review and editing, V.M., T.B., L.L., M.P.P., E.R.-S., and F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Slovenian Research Agency, grant number P3-0296. The funding source had no role in the study design, data collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflicts of Interest
Franc Strle served on the scientific advisory board for Roche on Lyme disease serological diagnostics, received research support from the Slovenian Research Agency (grant numbers P3-0296, J3-1744 and J3-8195), and is an unpaid member of the steering committee of the ESCMID Study Group on Lyme Borreliosis/ESGBOR. All other authors (Vera Maraspin, Lara Lusa, Tanja Blejec, Eva Ružić-Sabljić, Maja Pohar Perme) report no potential conflicts.
References
- 1.Stanek G., Wormser G.P., Gray J., Strle F. Lyme borreliosis. Lancet. 2012;379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 2.Schlesinger P.A., Duray P.H., Burke S.A., Steere A.C., Stillman M.T. Maternal-Fetal transmission of the Lyme disease spirochete, Borrelia burgdorferi. Ann. Intern. Med. 1985;103:67–68. doi: 10.7326/0003-4819-103-1-67. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz L.E., Steere A.C., Benach J.L., Slade J.D., Broome C.V. Lyme disease during pregnancy. JAMA. 1986;255:3394–3396. doi: 10.1001/jama.1986.03370240064038. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald A.B. Human fetal borreliosis, toxemia of pregnancy and fetal death. Zbl. Bakt. Hyg. 1986;263:189–200. doi: 10.1016/S0176-6724(86)80122-5. [DOI] [PubMed] [Google Scholar]
- 5.Ciesielski C.A., Russel H., Johnson S. Program and Abstracts of the 27th Interscience Conference on Antimicrobial Agents and Chemotherapy (New York) Volume 39. American Society for Microbiology; Washington, DC, USA: 1987. Prospective study of pregnancy outcome in women with Lyme disease; p. 103. [Google Scholar]
- 6.MacDonald A.B., Benach J.L., Burgdorfer W. Stillbirth following maternal Lyme disease. N. Y. State J. Med. 1987;87:615–616. [PubMed] [Google Scholar]
- 7.Andrásová V., Svárovský J., Matousek B. Lyme disease in pregnancy. Ceska Gynecol. 1988;53:39–41. [PubMed] [Google Scholar]
- 8.MacDonald A.B. Gestational Lyme borreliosis: Implications for the fetus. Rheum. Dis. Clin. N. Am. 1989;15:657–677. [PubMed] [Google Scholar]
- 9.Nadal D., Hunziker U.A., Bucher H.U., Hitzig W.H., Duc G. Infants born to mothers with antibodies against Borrelia burgdorferi at delivery. Eur. J. Pediatr. 1989;148:426–427. doi: 10.1007/BF00595903. [DOI] [PubMed] [Google Scholar]
- 10.Weber K., Bratzke H.J., Neubert U., Wilske B., Duray P.H. Borrelia burgdorferi in a newborn despite oral penicillin for Lyme borreliosis during pregnancy. Pediatr. Infect. Dis. J. 1988;7:286–289. doi: 10.1097/00006454-198804000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Lavoie P.E., Lattner B.P., Duray P.H., Malawista S.E., Barbour A.G., Johnson R.C. Culture positive, seronegative, transplacental Lyme borreliosis infant mortality; Proceedings of the Abstracts (Book B) of the 4th International Conference on Lyme Borreliosis; Stockholm, Sweden. 18–21 June 1990; p. 128. [Google Scholar]
- 12.Bracero L.A., Wormser G.P., Leikin E., Tejani N. Prevalence of seropositivity to the Lyme disease spirochete during pregnancy in an epidemic area. A preliminary report. J. Matern. Fetal. Investig. 1992;2:265–268. [Google Scholar]
- 13.Hercogová J., Tománková M., Frösslová D., Janovská D. Early-Stage Lyme borreliosis during pregnancy: Treatment in 15 women with erythema migrans. Ceska Gynekol. 1993;58:229–232. [PubMed] [Google Scholar]
- 14.Strobino B.A., Williams C.L., Abid S., Chalson R., Spierling P. Lyme disease and pregnancy outcome: A prospective study of two thousand prenatal patients. Am. J. Obstet. Gynecol. 1993;169:367–374. doi: 10.1016/0002-9378(93)90088-Z. [DOI] [PubMed] [Google Scholar]
- 15.Hercogová J., Moidlová M., Živný J., Hulínská D., Rychterova V., Janovska D. Could borreliae found in the placenta influence the fetus? Study of 19 women with erythema migrans during pregnancy; Proceedings of the Program and Abstracts of the 6th International Conference on Lyme Borreliosis; Bologna, Italy. 19–22 June 1994; Bologna, Italy: Societa Editrice Esculapio; p. 76. POO6T. [Google Scholar]
- 16.Lakos A. Lyme borreliosis and pregnancy; Proceedings of the Symposium on the Therapy and Prophylaxis for Lyme Borreliosis; Portorož, Slovenia. 13–16 May 1995; Portorož, Slovenia: Austrian Society for Hygiene and Slovenian Society for Infectious Diseases; p. 43. [Google Scholar]
- 17.Williams C.L., Strobino B., Weinstein A., Spierling P., Medici F. Maternal Lyme disease and congenital malformations: A cord blood serosurvey in endemic and control areas. Paediatr. Perinat. Epidemiol. 1995;9:320–330. doi: 10.1111/j.1365-3016.1995.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 18.Maraspin V., Cimperman J., Lotric-Furlan S., Pleterski-Rigler D., Strle F. Treatment of erythema migrans in pregnancy. Clin. Infect. Dis. 1996;22:788–793. doi: 10.1093/clinids/22.5.788. [DOI] [PubMed] [Google Scholar]
- 19.Strobino B., Abid S., Gewitz M. Maternal Lyme disease and congenital heart disease: A case-control study in an endemic area. Am. J. Obstet. Gynecol. 1999;180:711–716. doi: 10.1016/S0002-9378(99)70277-2. [DOI] [PubMed] [Google Scholar]
- 20.Maraspin V., Cimperman J., Lotric-Furlan S., Pleterski-Rigler D., Strle F. Erythema migrans in pregnancy. Wien Klin. Wochenschr. 1999;111:933–940. [PubMed] [Google Scholar]
- 21.Maraspin V., Strle F. How do I manage tick bites and Lyme borreliosis in pregnant women? Curr. Probl. Dermatol. 2009;37:183–190. doi: 10.1159/000213076. [DOI] [PubMed] [Google Scholar]
- 22.Lakos A., Solymosi N. Lyme borreliosis and pregnancy outcome. Intern. J. Infect. Dis. 2010;14:e494–e498. doi: 10.1016/j.ijid.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Maraspin V., Ružić-Sabljić E., Pleterski-Rigler D., Strle F. Pregnant women with erythema migrans and isolation of borreliae from blood: Course and outcome after treatment with ceftriaxone. Diagn. Microbiol. Infect. Dis. 2011;71:446–448. doi: 10.1016/j.diagmicrobio.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Stanek G., Fingerle V., Hunfeld K.P., Jaulhac B., Kaiser R., Krause A., Kristoferitsch W., O’Connell S., Ornstein K., Strle F., et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 2011;17:69–79. doi: 10.1111/j.1469-0691.2010.03175.x. [DOI] [PubMed] [Google Scholar]
- 25.Cerar T., Ruzic-Sabljic E., Cimperman J., Strle F. Comparison of immunofluorescence assay (IFA) and LIAISON® in patients with different clinical manifestations of Lyme borreliosis. Wien Klin. Wochenschr. 2006;118:686–690. doi: 10.1007/s00508-006-0696-9. [DOI] [PubMed] [Google Scholar]
- 26.Ružić-Sabljić E., Arnež M., Lotrič-Furlan S., Maraspin V., Cimperman J., Strle F. Genotypic and phenotypic characterisation of Borrelia burgdorferi sensu lato strains isolated from human blood. J. Med. Microbiol. 2001;50:896–901. doi: 10.1099/0022-1317-50-10-896. [DOI] [PubMed] [Google Scholar]
- 27.Postic D., Assous M.V., Grimont P.A.V., Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)–rrl (23S) intergenic spacer amplicons. Int. J. Syst. Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 28.Ruzić-Sabljić E., Maraspin V., Lotric-Furlan S., Jurca T., Logar M., Pikelj-Pecnik A., Strle F. Characterization of Borrelia burgdorferi sensu lato strains isolated from human material in Slovenia. Wien Klin. Wochenschr. 2002;114:544–550. [PubMed] [Google Scholar]
- 29.Westfall P.H., Young S.S. Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment. 1st ed. Wiley-Interscience; NewYork, NY, USA: 1993. [Google Scholar]
- 30.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation; Vienna, Austria: 2016. [(accessed on 10 February 2017)]. Available online: http://www.R-project.org. [Google Scholar]
- 31.Kourtis A.P., Read J.S., Jamieson D.J. Pregnancy and infection. N. Engl. J. Med. 2014;370:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pazos M., Sperling R.S., Moran T.M., Kraus T.A. The influence of pregnancy on systemic immunity. Immunol. Res. 2012;54:254–261. doi: 10.1007/s12026-012-8303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müllegger R.R., Häring N.S., Glatz M. Skin infections in pregnancy. Clin. Dermatol. 2016;34:368–377. doi: 10.1016/j.clindermatol.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 34.McKay K.A., Jahanfar S., Duggan T., Tkachuk S., Tremlett H. Factors associated with onset, relapses or progression in multiple sclerosis: A systematic review. Neurotoxicology. 2017;61:189–212. doi: 10.1016/j.neuro.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Strle F., Nelson J.A., Ruzic-Sabljic E., Cimperman J., Maraspin V., Lotric-Furlan S., Cheng Y., Picken M.M., Trenholme G.M., Picken R.N. European Lyme borreliosis: 231 culture-confirmed cases involving patients with erythema migrans. Clin. Infect. Dis. 1996;23:61–65. doi: 10.1093/clinids/23.1.61. [DOI] [PubMed] [Google Scholar]
- 36.Strle F., Nadelman R.B., Cimperman J., Nowakowski J., Picken R.N., Schwartz I., Maraspin V., Aguero-Rosenfeld M.E., Varde S., Lotric-Furlan S., et al. Comparison of culture-confirmed erythema migrans caused by Borrelia burgdorferi sensu stricto in New York State and by Borrelia afzelii in Slovenia. Ann. Intern. Med. 1999;130:32–36. doi: 10.7326/0003-4819-130-1-199901050-00006. [DOI] [PubMed] [Google Scholar]
- 37.Cerar D., Cerar T., Ruzić-Sabljić E., Wormser G.P., Strle F. Subjective symptoms after treatment of early Lyme disease. Am. J. Med. 2010;123:79–86. doi: 10.1016/j.amjmed.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Strle F., Ružić-Sabljić E., Logar M., Maraspin V., Lotrič-Furlan S., Cimperman J., Ogrinc K., Stupica D., Nadelman R.B., Nowakowski J., et al. Comparison of erythema migrans caused by Borrelia burgdorferi and Borrelia garinii. Vector Borne Zoonotic Dis. 2011;11:1253–1258. doi: 10.1089/vbz.2010.0230. [DOI] [PubMed] [Google Scholar]
- 39.Stupica D., Lusa L., Ruzić-Sabljić E., Cerar T., Strle F. Treatment of erythema migrans with doxycycline for 10 days versus 15 days. Clin. Infect. Dis. 2012;55:343–350. doi: 10.1093/cid/cis402. [DOI] [PubMed] [Google Scholar]
- 40.Strle F., Lusa L., Ružić-Sabljić E., Maraspin V., Lotrič Furlan S., Cimperman J., Ogrinc K., Rojko T., Videčnik Zorman J., Stupica D. Clinical characteristics associated with Borrelia burgdorferi sensu lato skin culture results in patients with erythema migrans. PLoS ONE. 2013;8:e82132. doi: 10.1371/journal.pone.0082132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stupica D., Maraspin V., Bogovic P., Ogrinc K., Blagus R., Cerar T., Strle F. Comparison of Clinical Course and Treatment Outcome for Patients with Early Disseminated or Early Localized Lyme Borreliosis. JAMA Dermatol. 2018;154:1050–1056. doi: 10.1001/jamadermatol.2018.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boršič K., Blagus R., Cerar T., Strle F., Stupica D. Clinical Course, Serologic Response, and Long-Term Outcome in Elderly Patients with Early Lyme Borreliosis. J. Clin. Med. 2018;7:e506. doi: 10.3390/jcm7120506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steere A.C., Strle F., Wormser G.P., Hu L.T., Branda J.A., Hovius J.W., Li X., Mead P.S. Lyme borreliosis. Nat. Rev. Dis. Primers. 2016;2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moro M.H., Bjornsson J., Marietta E.V., Hofmeister E.K., Germer J.J., Bruinsma E., David C.S., Persing D.H. Gestational attenuation of Lyme arthritis is mediated by progesterone and IL-4. J. Immunol. 2001;166:7404–7409. doi: 10.4049/jimmunol.166.12.7404. [DOI] [PubMed] [Google Scholar]
- 45.Strle K., Stupica D., Drouin E.E., Steere A.C., Strle F. Elevated levels of IL-23 in a subset of patients with post-lyme disease symptoms following erythema migrans. Clin. Infect. Dis. 2014;58:372–380. doi: 10.1093/cid/cit735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waddell L.A., Greig J., Lindsay L.R., Hinckley A.F., Ogden N.H. A systematic review on the impact of gestational Lyme disease in humans on the fetus and newborn. PLoS ONE. 2018;13:e0207067. doi: 10.1371/journal.pone.0207067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mather T.N., Telford S.R., III, Adler G.H. Absence of transplacental transmission of Lyme disease spirochetes from reservoir mice (Peromyscus leucopus) to their offspring. J. Infect. Dis. 1991;164:564–567. doi: 10.1093/infdis/164.3.564. [DOI] [PubMed] [Google Scholar]
- 48.Wilcox A.J., Weinberg C.R., O’Connor J.F., Baird D.D., Schlatterer J.P., Canfield R.E., Armstrong E.G., Nisula B.C. Incidence of early loss of pregnancy. N. Engl. J. Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 49.Ellish N.J., Saboda K., O’Connor J., Nasca P.C., Stane E.J., Boyle C. A prospective study of early. pregnancy loss. Hum. Reprod. 1996;11:406–412. doi: 10.1093/HUMREP/11.2.406. [DOI] [PubMed] [Google Scholar]
- 50.Cohain J.S., Buxbaum R.E., Mankuta D. Spontaneous first trimester miscarriage rates per woman among. parous women with 1 or more pregnancies of 24 weeks or more. BMC Pregnancy Childbirth. 2017;17:437. doi: 10.1186/s12884-017-1620-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blohm F., Friden B., Milsom I. A prospective longitudinal population-based study of clinical miscarriage. in an urban Swedish population. BJOG. 2008;115:176–183. doi: 10.1111/j.1471-0528.2007.01426.x. [DOI] [PubMed] [Google Scholar]
- 52.Nacionalni Inštitut za Javno Zdravje, Perinatalni Informacijski System. [(accessed on 30 June 2020)]; Available online: https://www.nijz.si/sites/www.nijz.si/files/uploaded/podatki/podatkovne_zbirke_raziskave/pis/peris-metodoloska-navodila-2017_v1-9.pdf.