Abstract
Endothelial dysfunction is an early abnormality in the process of atherosclerosis and cardiovascular disease and has been associated with worse clinical outcome. Cardiac rehabilitation (CR) has been reported to be helpful to reduce cardiovascular events in various types of cardiac disease, but the mechanisms of its beneficial effects remain only partially known. In this article, we review the studies that assessed the effect of CR on endothelial function in patients with various cardiac conditions. Available data show that CR significantly improves impaired endothelial function in these patients, which may contribute to the beneficial effects of CR on clinical outcome.
Keywords: cardiac rehabilitation, endothelial function, clinical outcome
1. Introduction
Cardiac rehabilitation (CR) is a program of physical and respiratory exercises, handled by an interdisciplinary team, aimed to improve cardiovascular function and physical capacity mainly after an acute cardiac event or intervention. CR programs have been reported to decrease morbidity and improve quality of life in these patients; furthermore, they have also been suggested to improve survival. These beneficial effects are likely mainly achieved through a tighter control of cardiovascular risk factors (CVRFs) as well as adherence to optimal medical therapy, which prevent the progression of atherosclerosis and reduce complications of atherosclerotic lesions [1,2,3,4,5]. CR programs, indeed, mainly focus on exercise training, but appropriate treatment of CVRFs, optimization of medical therapy, psychosocial interventions and patients’ education also constitute crucial integrative measures [5,6,7,8,9].
Based on the evidence of their beneficial cardiovascular effects, CR has a Class I indication in several American and European guidelines for patients with various heart conditions, including heart surgery, acute myocardial infarction, stable angina, heart failure (HF) with reduced left ventricular function, as well as peripheral artery disease [10,11,12,13,14]. Yet, despite the beneficial properties of CR have widely been documented and are widely accepted, the mechanisms through which it achieves its clinical benefits are still incompletely known.
This article is mainly focused on briefly reviewing the evidence of a favorable effect of CR programs on endothelial function in cardiac patients. An impairment of endothelial function is indeed considered a major cause of susceptibility to vascular complications and an improvement in endothelial function may therefore constitute a major mechanism through which CR may improve vascular function and, consequently, clinical outcome. We will precede the review of the available data on the effects of CR on endothelial function by a comment on the function of the normal endothelium and the implications of its dysfunction and by a short review of the main methods applied to assess endothelial function in clinical studies.
2. Endothelial Function and Dysfunction
The vascular endothelium has an outstanding role in maintaining vascular homeostasis and regulating blood vessel function. Thus, in normal people, the endothelium is a major regulator of blood flow through its vasodilator properties. These are mainly exerted by the release of nitric oxide (NO), but also of prostacyclin (PGI2) and endothelium-derived hyperpolarizing factor (EDHF), in response to various chemical substances (e.g., bradykinin, acetylcholine, serotonin), but also physical stimuli (e.g., shear stress). Furthermore, the normal endothelium also exerts anti-platelet, anti-coagulant, fibrinolytic, anti-inflammatory and antiproliferative actions [15]. Again, endothelial NO production and release play a major role in these beneficial effects, in particular in preserving the anti-thrombogenic property of the endothelial lining [15].
Accordingly, a dysfunctional endothelium is associated with a reduction of these beneficial effects. An impairment of NO production is an early consequence of endothelial cell dysfunction. The reduced NO release by endothelial cells results in an increase of basal vascular tone and in a reduced dilator response to stimuli that exert their vascular dilator effect by inducing NO release from endothelial cells. Of note, a dysfunctional endothelium may also display prevalent vasoconstrictor effects, due to the increased release of vasoconstrictor agents, such as endothelin-1. Furthermore, the impaired NO release also results in a reduction of the protective antithrombotic and anti-inflammatory effects of the endothelium. Moreover, the dysfunctional endothelium may also assume pro-aggregate and proinflammatory properties [16].
These changes in endothelial cell function are now considered the first step through which CVRFs lead to the formation of atherosclerotic plaques [17,18,19,20]. All CVRFs, indeed, have been shown to cause endothelial cell dysfunction, mainly through noxious chemical stimuli, including modified lipoproteins, constituents of cigarette smoke, high glucose levels and inflammatory cytokines. These factors induce the synthesis and expression on endothelial cell surface of adhesion molecules (e.g., E-selectin, vascular cell adhesion molecule [VCAM-1]), procoagulant substances (e.g., tissue factor) and proinflammatory chemokines (e.g., interleukin-8), and eventually cause endothelial cell injury that results in focal endothelial cell desquamation. This triggers platelet adhesion and aggregation with local release of growth factors, which induce migration, proliferation and changes of smooth muscle cells that eventually result in the formation of fibromuscular atherosclerotic plaques [21]. Importantly, both functional and structural changes similar to those described for the abnormal chemical stimuli can be also caused by exposure to disturbed blood flow, as it may occur in hypertension. Turbulent flow, indeed, seems the be able to activate important pathophysiological endothelial genes (such as those for platelet derived growth factor and VCAM-1).
In agreement with its deleterious effects, endothelial dysfunction has been shown to predict cardiovascular events in various clinical settings, including patients with CVRFs, those with acute or chronic coronary artery disease (CAD) and HF. Of note, the prognostic implications of endothelial dysfunction have been reported for both coronary and peripheral, as well as macrovascular and microvascular, circulation [22,23,24,25,26,27,28,29].
Importantly, the control of cardiovascular risk factors with some drugs, such as statins and antihypertensive drugs, improves endothelial function [30,31], which likely contributes to their beneficial clinical effects. Among non-pharmacological therapy, aerobic exercise training has been shown to improve endothelial function in patients with CVRFs, including hypertension and diabetes, both in the large arteries and microcirculation [32,33]. Aerobic exercise may exert its beneficial effects by modifying the pattern of flow at the level of arterial branch points, leading to less turbulent flow, which would favor the expression of atheroprotective genes, such as NO synthase (eNOS), with the restoration of a vasodilator and vasoprotective phenotype of the endothelium [33]. Of note, also an increase of other protective factors, such as PGI2 and EDHF may contribute to the improved physiologic effects of the endothelium by exercise [34,35]. The improvement of autonomic tone, a reduction of inflammation and oxidative stress all may contribute to the positive effects of exercise on endothelial cell function [33,34,35].
3. Assessment of Endothelial Function
Several methods have been described and are used to assess the functional state of the endothelium. Most of these methods are devoted at exploring systemic endothelial function through the assessment of endothelium-mediated dilator function both in large peripheral arteries and microcirculation and include flow-mediated dilatation (FMD), venous occlusion plethysmography, peripheral arterial tonometry (PAT) and laser Doppler flowmetry. Furthermore, indications on the functional state of the vascular endothelium can be obtained from in vivo or in vitro measurements of markers of endothelial activity.
Importantly, methods have also been developed to specifically assess endothelial function in the coronary circulation, although their application in clinical practice is challenged by the need of invasive investigation. Yet, in cardiac diseases it would be important to specifically assess coronary, rather peripheral, endothelial function, as the relation between the presence and severity of endothelial dysfunction in peripheral and coronary circulation has been shown to be less than optimal [36,37].
A detailed review of the methods applied to assess endothelial function can be found elsewhere [38,39,40]. In the next paragraphs, we will only briefly review the methods that have most often been used in clinical studies on the effects of CR programs on endothelial function.
3.1. Peripheral Circulation
3.1.1. Flow-Mediated Dilatation
FMD has become one of the most used techniques to assess systemic endothelial function non-invasively. It consists in measuring by ultrasound the diameter change of a peripheral artery in response to a maximal increase of blood flow consequent to reactive hyperemia. The increase in blood flow velocity, indeed, leads to stimulation of endothelial cells to release NO by the increased shear stress, which results in the dilatation of the vessel.
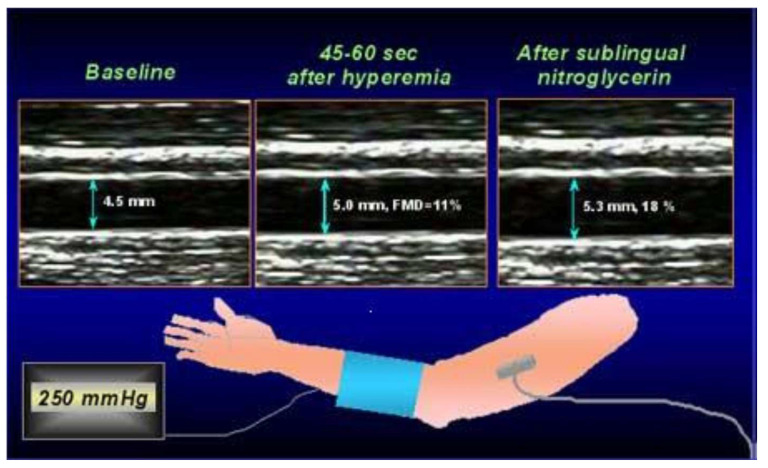
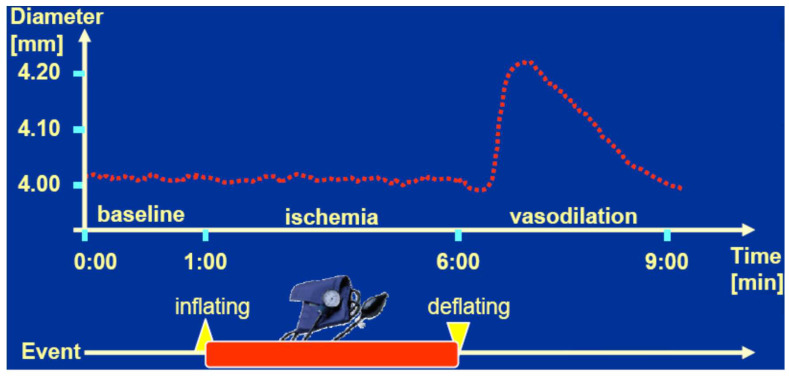
In clinical practice, FMD is usually assessed in a brachial artery (Figure 1). To this scope, images of the artery are first obtained at rest by a high frequency (10 MHz) probe attached to a high-resolution ultrasound system machine and basal diameter of the vessel is measured. Then, a forearm cuff, positioned one centimeter under the antecubital fossa, is inflated at 50 mmHg above the systolic blood pressure, thus inducing forearm ischemia. The cuff is quickly released after five minutes of ischemia to elicit forearm reactive hyperemia. FMD is then calculated as the maximum percent change of the brachial artery diameter during hyperemia compared with the basal diameter.
Figure 1.
Illustration of the method to assess flow-mediated dilation (FMD). Brachial artery diameter is measured at baseline and during hyperemia consequent to 5-min forearm ischemia; FMD is calculated as the percent increase of the artery diameter during hyperemia compared to baseline.
FMD values obtained manually by operators are subject to some variability of the measurements, which affects the comparability of the data and has until now limited a wide applicability in the clinical setting and precluded a recommendation of its use in clinical guidelines [41]. The use of a mechanical support to maintain the probe in a fixed position and the use of software that automatically detect the vessel edges and allow continuous measurements of the vessel diameter throughout the entire examination improve the reproducibility of the data and may allow appropriate standardization of the method and wider applicability both in the research and clinical field [42,43].
FMD has been reported to be impaired in several clinical conditions, including subjects with various CVRFs, patients with stable CAD or HF, as well as in acute settings of CAD [38,44]. Furthermore, FMD has been shown to predict cardiovascular events, particularly in patients with established cardiovascular diseases or at high cardiovascular risk [45,46,47].
Measurement of forearm blood flow velocity at baseline and during reactive hyperemia may also give some insight in the status of peripheral microcirculation. A limited decrease of peripheral resistances during hyperemia, indeed, may indicate an impairment of the microvascular dilatation function, which may also involve the endothelium. Accordingly, an impairment of FMD may, in fact, depend on a reduced microvascular dilatation, which limits forearm blood flow increase, rather than an impaired NO-mediated dilatation of the brachial artery.
3.1.2. Peripheral Arterial Tonometry
This technique investigates peripheral microcirculatory dilatation by measuring pulsatile arterial volume changes by finger plethysmography (EndoPAT) (Figure 2). Practically, a pressure cuff is placed on a forearm and inflated to suprasystolic pressure levels to produce five minutes of ischemia, followed by reactive hyperemia after cuff deflation. A pneumatic probe applied to the fingertip of the same arm, by recording the digital pulse wave amplitude (PWA), allows measuring the changes in arterial blood volume at baseline and during hyperemia. The increase in blood flow during hyperemia is expressed by the reactive hyperemia index (RHI), i.e., the ratio between the post- and preocclusion PWA values. The RHI is normalized by the measurement of the opposite arm, which serves as control of the effect of hyperemia.
Figure 2.
Illustration of the method of peripheral arterial tonometry. Modified from [40].
RHI is considered to reflect microvascular endothelial function [48] However, digital microvessel dilatation during hyperemia is only in part dependent on NO availability [49], whereas it is rather a marker of comprehensive peripheral microvascular capacity. RHI has been found decreased in patients with CVRFs [50,51] and may also predict cardiovascular events, similarly to FMD [52]. However, a poor correlation between RHI and FMD has been found in various settings, suggesting that the two methods explore different, although complementary, aspects of the vascular function [53,54]. The reproducibility of PAT has been reported to be rather low, which makes difficult to standardize its application in clinical practice [55,56], and has until now also precluded its inclusion in clinical guidelines [41].
3.1.3. Laboratory Markers
The functional state of the endothelium can also be derived by measuring blood concentrations of substances that are typically or mainly, released by endothelial cells following endothelial activation and dysfunction, as previously described. An increase in the blood of a large number of biomarkers reflecting endothelial activation, in particular in CAD populations, has been described [39], but those most frequently used in clinical studies include some soluble cell adhesion molecules, as intercellular adhesion molecule-1, VCAM-1, E-selectin and the von Willebrand factor. Serum biomarkers are rather easy to be obtained, but a large variability and the multiple factors that influence their concentrations may limit their clinical applicability.
More sophisticated laboratory methods to assess functional endothelial state include the measurement of endothelial progenitor cells (EPCs) concentrations, which are crucial for repairing endothelial lesions [57], and the development in vitro of cultures of endothelial progenitor cells [58]. These methods, however, are poorly available and may present various technical issues.
3.2. Coronary Circulation
In coronary circulation, endothelial function can adequately be assessed only by invasive methods. Although other stimuli may be used (e.g., atrial pacing, serotonin, substance P), at present it is usually assessed by intracoronary administration of low to medium doses of acetylcholine, which in normal subjects dilate coronary vessels by inducing NO release from endothelial cells [59]. The endothelium-mediated dilator effect of Ach infusion on epicardial vessels is obtained by measuring the changes in vessel diameter by quantitative coronary angiography or intravascular ultrasound. The effect on coronary microcirculation is obtained by measuring coronary blood flow (CBF) by either intracoronary Doppler recording of blood flow velocity or the thermodilution method [60,61]. An increase of 50% or more of CBF is considered as normal.
An alternative stimulus to assess endothelial function in coronary circulation is cold pressor test (CPT), which is performed by putting a hand in ice for 90–120 s. In normal subjects, CPT induces a mild adrenergic activation which results in NO release through alpha–receptor stimulation on endothelial cells [62]. In dysfunctional vessels, however, CPT may trigger sympathetic-mediated vascular constriction [63,64]. Several studies have assessed coronary microvascular endothelial function by assessing the CBF increase in response to CPT, also using non-invasive methods (e.g., positron emission tomography, cardiac magnetic resonance and transthoracic Doppler recording) [55]. The reliability of this method, however, is limited by the impossibility to exclude some direct coronary constriction in the limitation of CBF increase.
4. Cardiac Rehabilitation and Endothelial Function
CR programs mainly focus on exercise training, but they involve a careful implementation of integrative measures, including appropriate treatment of CVRFs, optimization of medical therapy, psychosocial interventions and patients’ education [1,2,3,4,5,6,7,8,9]. CR programs, indeed, involve three phases: (1) an in-hospital phase, in which patient’s health situation and goals, education and cardiovascular risk factors are discussed by a multidisciplinary medical team; (2) a second phase, which can be performed in a residential (inpatient) setting or an ambulatory (outpatient) setting, in which the patient undergo a program of exercises, aggressive risk-factor modifications and education classes; (3) a long-term maintenance phase, in which the patient is encouraged to continue to maintain optimal control of cardiovascular risk factors and to practice an individualized, but regular physical activity [65]. CR rehabilitation programs are therefore expected to improve endothelial function through multiple mechanisms. The emphasis and surveillance on the tight control of CVRFs, diet and adherence to medical therapy certainly play a relevant role to this scope and in the beneficial effects on clinical outcome of CR programs, in particular in CAD patients, even when treated with contemporary evidence-based forms of therapy [66,67]. However, as discussed in the paragraph on endothelial function, aerobic exercise programs may add further favorable effects in cardiac patients [1,2,3].
Several studies have investigated the effects of CR programs on endothelial function, mainly in those recovering from an acute myocardial infarction (AMI), but also in those with stable CAD or HF. A summary of the main characteristics and results of these studies is shown in Table 1.
Table 1.
Summary of the main studies that assessed the effects of cardiac rehabilitation (CR) on endothelial function.
| Study | Population | No. Patients | Study Design | Assessment of ED | Exercise Program | Results |
|---|---|---|---|---|---|---|
| Vona [68] | Recent AMI | 52 | RCT | FMD, CPT | 3 months of moderate aerobic ET | CR significantly improved ED vasodilation (p < 0.01) |
| Lee [69] | Previous AMI or coronary revascularization | 81 | RCT | FMD, vWF | 3 months of home/hospital-based CR program | CR improved FMD and reduced vWF (all p ≤ 0.001) |
| Peller [70] | Recent AMI | 25 | Prospective uncontrolled | RHI-PAT | 4 weeks (12–24 ET session) | CR did not significantly improve endothelial function (p = 0.14) |
| Oliveira [71] | Recent AMI | 96 | RCT | Endothelial biomarkers | 8 weeks of aerobic ET at 70–85% of maximal HR during 3 weekly sessions | CR did not reduce the markers of ED |
| Cornelissen [72] | Stable CAD | 146 | Prospective uncontrolled | FMD, RHI-PAT | 12 weeks (3 sessions per week at an intensity of 80% of HR reserve | CR improved FMD (p < 0.0001), but not RHI (p = 0.47) |
| Edwards [73] | Stable CAD | 18 | Prospective controlled | FMD, nitrites, nitrates | 12 weeks (3 times per week of treadmill walking and stationary cycling at an intensity of 40–50%, to a maximum 70–85% of HR reserve. | CR improved FMD and increase nitrate and nitrite levels |
| Gocke [74] | Stable CAD | 58 | Prospective controlled | FMD | 10 weeks of leg exercise of moderate intensity (30 min 3 times per week) | CR improved FMD (p = 0.02), in particular in the exercised limbs |
| Gagliardi [75] | Stable CAD | 21 | RCT | VEGF, EPCs | 12 weeks (three weekly exercise bout) | CR determined a reduction of EPC and an increase in VEGF |
| SAINTEX-CAD study [76,77] | Stable CAD | 200 | RCT | FMD, EPCs | 12 weeks of aerobic interval vs. continuous ET on a bicycle | Both ET programs improved FMD, but had no effect on EPCs |
| Hambrect [78] | Stable CAD | 19 | RCT | Coronary epicardial and MV response to Ach | 4 weeks (10 min 6 time a day on a bicycle ergometer to the 80% of HR) | CR improved epicardial and MV endothelial response to Ach |
| Tanaka [79] | HFrEF/HFpEF | 78 | Retrospective study | FMD | 5 months (2–3 times per week of training on a cycle ergometer for 20 min at anaerobic threshold level) | CR did not improve FMD, but improved exercise capacity in patients with ED at baseline |
| Legallois [80] | HF in DCM | 29 | Prospective study | MBF response to CPT | 12 weeks (36 aerobic ET sessions on the basis of ventilatory threshold) | CR improved MBF response to CPT (p < 0.001) |
Ach—acetylcholine; AMI—acute myocardial infarction; CAD—coronary artery disease; CPT—cold pressor test; CR—cardiac rehabilitation; DCM—dilated cardiomyopathy; ED—endothelial dysfunction; EPCs—endothelial progenitors cells; ET—exercise training; FMD—flow mediated dilation; HF—heart failure; HFpEF—HF with preserved ejection fraction; HFrEF—HF with reduced ejection fraction; HR—heart rate; MBF—myocardial blood flow; MV—microvascular; RCT—randomized controlled trial; RHI-PAT-reactive hyperemia index on peripheral arterial tonometry; VEGF—vascular endothelial growth factor; vWF—von Willebrand factor.
5. Acute Myocardial Infarction
Several studies investigated the effects of CR programs on endothelial function in patients recovering from AMI. Vona et al. [68], randomized 52 patients with a recent uncomplicated AMI to CR, consisting of moderate aerobic training on a cycle ergometer three times per week or usual care. FMD, CPT and nitrate-mediated dilation (NMD) were assessed at baseline and after 3 months of treatment. While no changes in NMD were observed in both groups, a significant greater improvement of FMD was found in the CR group (from 1.66% ± 4.11% to 9.39% + 4.87%) compared to controls (from 2.04% + 3.4% to 4.4% + 3.9%; p < 0.01). Of note, CPT caused a constriction of the brachial artery at baseline in both groups; at follow-up, however, no changes in brachial artery diameter was observed in the CR group, whereas vasoconstriction still occurred in controls. The improvement of endothelial function was associated with a significant increase in exercise tolerance. Importantly, these benefits of CR disappeared in the treated group after detraining, suggesting that continuous exercise training may be necessary to maintain the improvement of endothelial function.
Lee et al. [69] randomized 81 patients with AMI or coronary revascularization to comprehensive hospital-based or home-based cardiac rehabilitation. FMD was assessed at baseline and after three months of a CR program, together with 24-h ambulatory blood pressure monitoring and measurement of plasma levels of von Willebrand factor, D-dimer, fibrinogen, soluble P-selectin and plasma viscosity. A significant increase in FMD was observed in the whole group (from 4.16% [1.46–7.42%] to 6.9% [2.34–11.5%]; p < 0.001), together with a significant reduction of plasma levels of vWF. No changes in FMD and plasma markers of endothelial function were observed in 20 matched control patients who did not undergo CR. No significant differences in endothelial function improvement were found between home-based and hospital-based cardiac rehabilitation programs.
Peller et al. [70] assessed reactive hyperemia by PAT in 29 patients with AMI before and after CR, started at least four weeks after AMI and consisting of 12 or 24 rehabilitation sessions (3–4 per week) according to patient’s preference. At the end of CR, the lnRHI increased from 0.49 (0.36–0.67) to 0.58 (0.43–0.74), but the difference did not achieve statistical significance (p = 0.14). However, in the subgroup of patients with endothelial dysfunction at baseline (n = 16 or 55.2%), as diagnosed by an lnRHI lower than 0.51, lnRHI improved significantly at follow-up from 0.37 (0.27–0.42) to 0.53 (0.43–0.66) (p = 0.002), thus suggesting that CR may have beneficial effects on endothelial function only when it is significantly depressed at baseline. However, patients training for 24 sessions (n = 16) had similar lnRHI changes to those of patients training for 12 sessions (n = 9; p = 0.44).
Finally, Oliveira et al. [71] randomized 96 AMI patients to an 8-week exercise-based CR program, consisting of aerobic exercise at 70–85% of maximal heart rate during three weekly sessions or controls. Markers of endothelial dysfunction, arterial stiffness, investigated through carotid-femoral pulse wave velocity (cf-PWV), and markers of inflammation were assessed at baseline and at the end of treatment. No significant changes were found between groups in endothelial dysfunction biomarkers, as well as cf-PWV and inflammatory biomarkers, although a decrease of cf-PWV was noticed in patients who attended at least 80% of exercise sessions. In this study, however, the exercise program was relatively short and only adhesion molecules VCAM-1 and ICAM-1 were measured as markers of endothelial function.
6. Stable Coronary Artery Disease
In patients with clinically stable CAD, the effects of CR programs on endothelial function were investigated by a few studies. Cornelissen et al. [72] assessed FMD and RHI by PAT at baseline and after a 12-week cardiac rehabilitation program in 146 patients with stable CAD. FMD showed a significant improvement (average, from 10.0% to 13.0%; p < 0.001), whereas no significant change was observed in RHI (average, from 1.92 to 1.96; p = 0.47).
Edwards et al. [73] assigned 18 stable CAD patients to a 12-week standard CR program or control. Endothelial function was assessed by FMD at baseline and at the end of CR. FMD increased from 7.9% ± 2.2% to 11.1% ± 3.1% (p < 0.05) in the CR group but showed no change in the control group (8.5% ± 2.3% at baseline and 8.2% + 2.3% at follow-up). In addition, an increase in plasma nitrite and nitrate levels was observed in the CR group, confirming the improvement of endothelial function. Moreover, an increase of superoxide dismutase levels, an antioxidant enzyme, was found in the CR group, but not in controls, suggesting that the favorable effects of endurance exercise training as part of a CR program on endothelial function may be related to decreased oxidative stress.
Gokce et al. [74] assessed the effects of a 10-week supervised CR exercise program with moderate leg intensity exercise of FMD on both brachial artery and posterior tibial artery of 40 stable CAD patients. Exercise training was conducted at an intensity of 45% to 85% of heart rate reserve, derived from an initial exercise test and performed 30–40 min three times/week. Data were compared with those of a control group of 18 matched CAD patients with a sedentary lifestyle. Exercise was associated with an increase in functional capacity and significant improvement in FMD in the posterior tibial artery (from 9.7% ± 2.1% to 11.7% ± 2.1%) compared to controls (from 9.9% ± 2.1% to 9.1% ± 2.1%; p < 0.05). No significant difference in the changes of FMD was observed between the two groups in the brachial artery (p = 0.23), thus suggesting that exercise may improve endothelial function only in peripheral arteries of the exercised limbs. However, FMD in the brachial artery improved by 1.9 points in CR patients (6.4% ± 3.4% vs. 8.3% ± 3.4%), but only by 0.3 points in controls (7% ± 3.5% vs. 7.3% ± 3.5%), suggesting that the lack of statistical difference in brachial FMD between the two groups could be related to a limited statistical power of the study.
Gagliardi et al. [75] randomized 21 patients with chronic stable CAD to a rehabilitation program with three exercise bouts weekly over 12 weeks (11 patients) or no rehabilitation program (10 patients). They measured EPCs and vascular endothelial growth factor (VEGF) at baseline. A significant reduction of EPCs and an increase in VEGF at one and three months of follow-up was found, suggesting improvement of endothelial function, but no differences were observed between the two groups.
The multicenter SAINTEX study compared the effects of aerobic interval training (AIT) and aerobic continuous training (ACT) on peak VO2, FMD, CVRFs and quality of life in patients with stable CAD [76]. In this trial, 200 patients were randomized to a supervised 12-week CR program of three weekly sessions of either AIT (90–95% of peak HR) or ACT (70–75% of peak HR) on a bicycle. A similar improvement of FMD was observed in the two groups, together with a similar improvement of peak VO2, CVRFs and quality of life. The improvement of FMD showed a significant correlation with the peak VO2 (r = 0.17; p = 0.035). No effects of both types of ET programs were observed on the blood levels of EPCs, angiogenic T cells and endothelial microparticles [77].
While the previous studies investigated peripheral endothelial function, in a study Hambrecht et al. assessed coronary endothelial function [78]. They randomized 19 patients with documented obstructive CAD and coronary endothelial dysfunction, defined as either epicardial vasoconstriction or diameter dilation < 5% in response to Ach, to an exercise-training group (10 patients) or a control group (9 patients). The coronary response to increasing doses of intracoronary acetylcholine (0.072, 0.72 and 7.2 μg per minute) was assessed at baseline and after four weeks of treatment. The diameter of epicardial coronary vessels and mean peak flow velocity were measured by quantitative coronary angiography and Doppler velocimetry, respectively. At baseline, the two groups showed similar responses to acetylcholine. At the follow-up study the responses to Ach remained unchanged in the control group. In the exercise training group, however, a significant reduction of the vasoconstrictor effect of Ach on epicardial vessels was observed (diameter reduction of 15.2% ± 2.2% vs. 6.8% ± 2.5% at the maximal Ach dose; p for change vs controls < 0.05). Mean peak flow velocity in response to Ach in the exercise group showed a significant improvement (from 78.1 ± 15.5 vs. 141.6 ± 27.7 cm/s at the highest dose; p for change < 0.05). Thus, this study showed that exercise training improves endothelial function both in epicardial and resistance coronary vessels in patients with coronary artery disease.
7. Heart Failure
Several studies have shown that an abnormal endothelial function is present in patients with HF [81,82], likely as a result of the activation of the adreno–medullary neuro–humoral axis. Endothelial dysfunction in HF patients may cause increased vascular resistances and decreased organ perfusion, leading to exercise intolerance and contributing to HF progression. Some studies, indeed, have reported that endothelial dysfunction in HF patients is associated with a worse clinical outcome [83,84]. Accordingly, an improvement of endothelial function may favor the regulation of organ blood flow, improve physical performance and delay the progression of the disease. Exercise-based CR programs have been shown to improve exercise capacity and quality of life in HF patients [85] and an improvement of endothelial function might contribute to these favorable effects.
Two studies investigated the effects of CR on endothelial function of patients with HF. Tanaka et al. [79] retrospectively reviewed the effect of 5-month CR on endothelial function, as assessed by brachial FMD, in 30 patients with HF with reduced left ventricle ejection fraction and 30 patients with HF with preserved ejection fraction. FMD did not improve significantly at follow-up in both groups. CR improved exercise capacity, however, only in patients who, at baseline, showed endothelial dysfunction (defined as FMD ≤ 5%), but this seemed independent of significant changes in FMD values.
Legallois et al. [80] assessed the effect of CR on coronary endothelial function in 29 clinically stable patients with HF related to non-ischemic dilated cardiomyopathy. They measured myocardial blood flow (MBF) by positron emission tomography at rest and during CPT at baseline and after 12 weeks of a CR program, including 36 supervised exercise sessions, diet therapy, medical advice, patient education and medical therapy optimization. At baseline, MBF showed no changes during CPT (1.16 ± 0.41 vs. 1.14 ± 0.31 mL/min/g, respectively). After the completion of the CR program, MBF increased significantly at rest (1.31 ± 0.38 mL/min/g; p = 0.04) and after CPT (1.51 ± 0.38 mL/min/g; p < 0.001) compared to basal values. Furthermore, after CR, MBF increased significantly in response to CPT, whereas no significant change had been observed at the baseline test (0.19 ± 0.22 vs. −0.03 ± 0.22 mL/min/g, respectively; p < 0.001).
8. Conclusions
In conclusion, although with some variable results, the complex of available data supports the notion that, in cardiac patients, CR programs improve endothelial function, particularly when it is significantly impaired. This beneficial effect is likely mediated by a combination of factors, including exercise training programs, lifestyle changes and a tighter control of CVRFs through a better adherence to medical therapy.
The improvement of endothelial function found in the published studies seems largely independent of the type of aerobic exercise training programs, including walking on treadmill vs. bicycle, as well as continuous vs. interval training. Thus, rather than recommending for specific exercise programs, what seems important to achieve beneficial effects on the cardiovascular system and endothelial function, is that the supervised exercise training allows to reach an adequate exercise intensity (e.g., 70–80% of maximal HR for 30 min) and that sessions are performed on a regular basis (e.g., three times per week) and for an adequate period (e.g., 12 weeks). The beneficial effects of CR in cardiovascular patients, indeed, strongly depend on the volume and intensity of exercise program delivery [86]. Importantly, the maintenance of an adequate physical activity after the end of the supervised ET in CR programs is crucial to also maintain the improvement of endothelial function, although the optimal frequency, type and intensity of long-term physical activity remains to be established.
According to available data, the assessment of endothelial function could, therefore, be useful to assess whether CR programs result in benefits on vascular function in individual patients. To this scope, FMD (with forearm blood flow assessment) seems the most simple and comprehensive method, but it still suffers from center and operator variability; efforts should be done, therefore, to achieve a standardization of this (as well as other) method for endothelial function assessment that may allow a large application in clinical practice.
Importantly, while an improvement of endothelial function by CR programs in cardiac patients has been suggested by several studies and is expected to portend beneficial effects on cardiovascular complications, whether it actually translates into improved clinical outcome remains to be demonstrated. To this scope, adequately designed and sized randomized multicenter clinical trials should be planned, using both standardized CR exercise training programs and methods for the assessment of endothelial function.
Author Contributions
Conceptualization, G.A.L., M.G., A.V.; methodology, G.A.L., A.F., M.L.; data curation, G.A.L., P.L., O.L.; writing—original draft preparation, G.A.L., M.G.; writing—review and editing, G.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Anderson L., Thompson D.R., Oldridge N., Zwisler A.D., Rees K. Exercise-based rehabilitation for coronary heart disease (Review) J. Am. Coll. Cardiol. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 2.McMahon S.R., Ades P.A., Thompson P.D. The role of cardiac rehabilitation in patients with heart disease. Trends Cardiovasc. Med. 2017;27:420–425. doi: 10.1016/j.tcm.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosetti M., Abreu A., Corrà U., Davos C.H., Hansen D., Frederix I., Iliou M.C., Pedretti R.F., Schmid J.P., Vigorito C., et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2020 doi: 10.1177/2047487320913379. (in press) [DOI] [PubMed] [Google Scholar]
- 4.Prescott E., Mikkelsen N., Holdgaard A., Eser P., Marcin T., Wilhelm M., Gil C.P., González-Juanatey J.R., Moatemri F., Iliou M.C., et al. Cardiac rehabilitation in the elderly patient in eight rehabilitation units in Western Europe: Baseline data from the EU-CaRE multicentre observational study. Eur. J. Prev. Cardiol. 2019;26:1052–1063. doi: 10.1177/2047487319839819. [DOI] [PubMed] [Google Scholar]
- 5.van Halewijn G., Deckers J., Tay H.Y., van Domburg R., Kotseva K., Wood D. Lessons from contemporary trials of cardiovascular prevention and rehabilitation: A systematic review and meta-analysis. Int. J. Cardiol. 2017;232:294–303. doi: 10.1016/j.ijcard.2016.12.125. [DOI] [PubMed] [Google Scholar]
- 6.Lawler P.R., Filion K.B., Eisenberg M.J. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Am. Heart J. 2011;162:571–584. doi: 10.1016/j.ahj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Heinl R.E., Dhindsa D.S., Mahlof E.N., Schult W.M., Ricketts J.C. Comprehensive cardiovascular risk reduction and cardiac rehabilitation in diabetes and the metabolic syndrome. Can. J. Cardiol. 2016;32(Suppl. 2):S349–S357. doi: 10.1016/j.cjca.2016.07.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotseva K., Wood D., de Bacquer D. Determinants of participation and risk factor control according to attendance in cardiac rehabilitation programmes in coronary patients in Europe: EUROSPIRE IV survey. Eur. J. Prev. Cardiol. 2018;25:1242–1251. doi: 10.1177/2047487318781359. [DOI] [PubMed] [Google Scholar]
- 9.Lavie C.J., Menezes A.R., de Schutter A., Milani R.V., Blumenthal J.A. Impact of cardiac rehabilitation and exercise training on psychological risk factors and subsequent prognosis in patients with cardiovascular disease. Can. J. Cardiol. 2016;31(Suppl. 2):S365–S373. doi: 10.1016/j.cjca.2016.07.508. [DOI] [PubMed] [Google Scholar]
- 10.Leon A.S., Franklin B.A., Costa F., Balady G.J., Berra K.A., Stewart K.J., Thompson P.D., Williams M.A., Lauer M.S. Cardiac rehabilitation and secondary prevention of coronary heart disease: An American Heart Association Scientific Statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in Collaboration With the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2005;111:369–376. doi: 10.1161/01.CIR.0000151788.08740.5C. [DOI] [PubMed] [Google Scholar]
- 11.Balady G.J., Ades P.A., Bittner V.A., Franklin B.A., Gordon N.F., Thomas R.J., Tomaselli G.F., Yancy C.W. Referral, enrollment and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: A presidential advisory from the American Heart Association. Circulation. 2011;124:2951–2960. doi: 10.1161/CIR.0b013e31823b21e2. [DOI] [PubMed] [Google Scholar]
- 12.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST segment elevation. Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 13.Ponikowski P., Voors A.A., Anker S.D., Bueno H., John G.F.C., Coats A.J.S., Falk V., Gonzalez-Juanatey J.R., Harjola V.P., Jankowska E.A., et al. ESC Guidelines for diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 14.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., Cooney M.T., Corrà U., Cosyns B., Deaton C., et al. European Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godo S., Shimokawa H. Endothelial functions. Arter. Thromb. Vasc. Biol. 2017;37:e108–e114. doi: 10.1161/ATVBAHA.117.309813. [DOI] [PubMed] [Google Scholar]
- 16.Deanfield J.E., Halcox J.P., Rabelink T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 17.Konukoglu D., Uzun H. Endothelial dysfunction and hypertension. Adv. Exp. Med. Biol. 2017;956:511–540. doi: 10.1007/5584_2016_90. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y., Vanhoutte P.M. Macro and microvascular endothelial dysfunction in diabetes. J. Diabetes. 2017;9:434–449. doi: 10.1111/1753-0407.12521. [DOI] [PubMed] [Google Scholar]
- 19.Messner B., Bernhard D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arter. Thromb. Vasc. Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 20.Landmesser U., Hornig B., Drexler H. Endothelial dysfunction in hypercholesterolemia: Mechanisms, pathophysiological importance, and therapeutic interventions. Semin. Thromb. Hemost. 2000;26:529–537. doi: 10.1055/s-2000-13209. [DOI] [PubMed] [Google Scholar]
- 21.Gimbrone M.A., García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shechter M., Issachar A., Marai I., Koren-Morag N., Freinark D., Shahar Y., Shechter A., Feinberg M.S. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int. J. Cardiol. 2009;134:52–58. doi: 10.1016/j.ijcard.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Ras R.T., Streppel M.T., Draijer R., Zock P.L. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. Int. J. Cardiol. 2013;168:344–351. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 24.Matsuzawa Y., Kwon T.G., Lennon R.J., Lerman L.O., Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: A systematic review and metaanalysis. J. Am. Heart Assoc. 2015;4:e002270. doi: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gokce N., Keaney J.F.J., Hunter L.M., Watkins M.T., Menzoian J.O., Vita J.A. Risk stratification for postoperative cardiovascular events via non invasive assessment of endothelial function: A prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.CIR.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 26.Schächinger V., Britten M.B., Zeiher A.M. Prognostic impact of coronary vasodilator dysfunction and adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.CIR.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 27.Halcox J.P.J., Schenk W.H., Zalos G., Zalos G., Mincemoyer R., Prasad A., Waclawiw M.A., Nour K.R.A., Quyyumi A.A. Prognostic value of coronary vascular endothelial function. Circulation. 2002;106:653–658. doi: 10.1161/01.CIR.0000025404.78001.D8. [DOI] [PubMed] [Google Scholar]
- 28.Heitzer T., Schlinzig T., Krohn K., Meinertz T., Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 29.Neunteufl T., Heher S., Katzenschlager R., Wölfl G., Kostner K., Maurer G., Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am. J. Cardiol. 2000;86:207–210. doi: 10.1016/S0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 30.Silva I.V.G., de Figueiredo R.C., Rios D.R.A. Effect of Different Classes of Antihypertensive Drugs on Endothelial Function and Inflammation. Int. J. Mol. Sci. 2019;20:3458. doi: 10.3390/ijms20143458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson T.J., Meredith I.T., Yeung A.C., Frei B., Selwyn A.P., Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium dependent coronary vasomotion. N. Engl. J. Med. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 32.Green D.J., Maiorana A., O’Driscoll G., Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. Pt 1J. Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips S.A., Mahmoud A.M., Brown M.D., Haus J.M. Exercise interventions and peripheral arterial function: Implications for cardiac metabolic disease. Progr. Cardiovasc. Dis. 2015;57:521–534. doi: 10.1016/j.pcad.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Zoladz J.A., Majerczak J., Duda K., Chłopicki S. Endurance training increases exercise-induced prostacyclin release in young, healthy men-relationship with VO2max. Pharmacol. Rep. 2010;62:494–502. doi: 10.1016/S1734-1140(10)70305-4. [DOI] [PubMed] [Google Scholar]
- 35.Green D.J., Hopman M.T.E., Padilla J., Laughlin M.H., Thijssen D.H.J. Vascular adaptation to exercise in humans: Role of hemodynamic stimuli. Physiol. Rev. 2017;97:495–528. doi: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonetti P.O., Pumper G.M., Higano S.T., Holmes D.R.J., Kuvin J.T., Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 37.Takase B., Uehata A., Akima T., Nagai T., Nishioka T., Hamabe A., Satomura K., Ohsuzu F., Kurita A. Endothelium-dependent flow mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am. J. Cardiol. 1998;82:1535–1539. doi: 10.1016/S0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 38.Flammer A.J., Anderson T., Celermajer D.S., Creager M.A., Deanfield J., Ganz P., Hamburg N.M., Luscher T.F., Shechter M., Taddei S., et al. The Assessment of Endothelial Function. From Research Into Clinical Practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storch A.S., de Mattos J.D., Alves R., Dos Santos Galdino I., Rocha H.N.M. Methods of endothelial function assessment: Description and applicaions. Int. J. Card. Sci. 2017;30:262–273. doi: 10.5935/2359-4802.20170034. [DOI] [Google Scholar]
- 40.Widmer R.J., Lerman A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014;3:291–308. doi: 10.5339/gcsp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlachopoulos C., Xaplanteris P., Aboyans P., Brodmann M., Cífková R., Cosentino F., de Carlo M., Gallino A., Landmesser U., Laurent S., et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241:507–532. doi: 10.1016/j.atherosclerosis.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Ghiadoni L., Faita F., Salvetti M., Cordiano C., Biggi A., Puato M., di Monaco A., de Siati L., Volpe M., Ambrosio G., et al. Assessment of flow-mediated dilation reproducibility: A nationwide multicenter study. J. Hyperten. 2012;30:1399–1405. doi: 10.1097/HJH.0b013e328353f222. [DOI] [PubMed] [Google Scholar]
- 43.Nerla R., Tarzia P., Sestito A., di Monaco A., Infusino F., Matera D., Greco F., Tacchino R.M., Lanza G.A., Crea F. Effect of bariatric surgery on peripheral flow-mediated dilation and coronary microvascular function. Nutr. Metab. Cardiovasc. Dis. 2012;22:626–634. doi: 10.1016/j.numecd.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Gutiérrez E., Flammer A.J., Lerman L.O., Elízaga J., Lerman A., Fernández-Avilés F. Endothelial dysfunction over the course of coronary artery disease. Eur. Heart J. 2013;34:3175–3181. doi: 10.1093/eurheartj/eht351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson T.J., Phillips S.A. Assessment and prognosis of peripheral artery measures of vascular function. Prog. Cardiovasc. Dis. 2015;57:497–509. doi: 10.1016/j.pcad.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Yeboah J., Folsom A.R., Burke G.L., Johnson C., Polak J.F., Post W., Lima J.A., Crouse J.R., Herrinton D.M. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villano A., Mencarelli E., Melita V., Rizzi A., Lamendola P., de Vita A., Manfredonia L., Ravenna S.E., Pitocco D., Lanza G.A., et al. Endothelial dysfunction and cardiovascular outcome in asymptomatic patients with type 2 diabetes: A pilot study. Diabetes Metab. Res. Rev. 2020;36:e3215. doi: 10.1002/dmrr.3215. [DOI] [PubMed] [Google Scholar]
- 48.Moerland M., Kales A.J., Schrier L., van Dongen M.G.J., Brandock D., Burggraaf J. Evaluation of the EndoPAT as a tool to assess endothelial function. Int. J. Vasc. Med. 2012;2012:904141. doi: 10.1155/2012/904141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nohria A., Gerhard-Herman M., Creager M.A., Hurley S., Mitra D., Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J. Appl. Physiol. 2006;101:545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 50.Hamburg N.M., Keyes M.J., Larson M.G., Vasan R.S., Schnabel R., Pryde M.M., Mitchell G.F., Sheffy J., Vita J.A., Benjamin E.J. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuvin J.T., Mammen A., Mooney P., Alsheikh-Ali A.A., Karas R.H. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc. Med. 2007;12:13–16. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- 52.Rubinshtein R., Kuvin J.T., Soffler M., Lennon R.J., Lavi S., Nelson R.E., Pumper G.M., Lerman L.O., Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart. J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 53.Allan R.B., Vun S.V., Spark J.I. A comparison of measures of endothelial function in patients with peripheral arterial disease and age and gender matched controls. Int. J. Cardiovasc. Med. 2016;2016:2969740. doi: 10.1155/2016/2969740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woo J.S., Jang W.S., Kim H.S., Lee J.H., Choi E.Y. Comparison of peripheral arterial tonometry and flow-mediated vasodilation for assessment of the severity and complexity of coronary artery disease. Coron. Artery Dis. 2014;25:421–426. doi: 10.1097/MCA.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 55.Weisrock F., Fritschka M., Beckmann S., Litmeier S., Wagner J., Tahirovic E., Radenovic S., Zelenak C., Hashemi D., Busjahn A., et al. Reliability of peripheral arterial tonometry in patients with heart failure, diabetic nephropathy and arterial hypertension. Vasc. Med. 2017;22:292–300. doi: 10.1177/1358863X17706752. [DOI] [PubMed] [Google Scholar]
- 56.Nil M., Schafer D., Radtke T., Saner H., Wilhelm M., Eser P. Reproducibility of peripheral arterial tonometry measurements in male cardiovascular patients. Eur. J. Clin. Investig. 2014;44:1065–1071. doi: 10.1111/eci.12341. [DOI] [PubMed] [Google Scholar]
- 57.Fujita Y., Asahara T. Evaluation of circulating endothelial progenitor cells in cardiovascular risk. Circ. J. 2011;75:2541–2542. doi: 10.1253/circj.CJ-11-1050. [DOI] [PubMed] [Google Scholar]
- 58.Marin V., Kaplanski G., Gres S., Farnarier C., Bongrand P. Endothelial cell culture: Protocol to obtain and cultivate human umbilical endothelial cells. J. Immunol. Methods. 2001;254:183–190. doi: 10.1016/S0022-1759(01)00408-2. [DOI] [PubMed] [Google Scholar]
- 59.Quyyumi A.A., Dakak N., Mulcahy D., Andrews N.P., Husain S., Panza J.A., Cannon R.O. Nitric oxide activity in the atherosclerotic human coronary circulation. J. Am. Coll. Cardiol. 1997;29:308–317. doi: 10.1016/S0735-1097(96)00472-X. [DOI] [PubMed] [Google Scholar]
- 60.Joye J.D., Schulman D.S. Clinical application of coronary flow reserve using an intracoronary Doppler guide wire. Cardiol. Clin. 1997;15:101–129. doi: 10.1016/S0733-8651(05)70321-0. [DOI] [PubMed] [Google Scholar]
- 61.Fearon W.M., Kobayashi Y. Invasive assessment of the coronary microvasculature. The index of microcirculatory resistance. Circ. Cardiovasc. Interv. 2017;10:e005361. doi: 10.1161/CIRCINTERVENTIONS.117.005361. [DOI] [PubMed] [Google Scholar]
- 62.Dubois-Randè J.L., Dupouy P., Aptecar E., Bhati A., Teiger E., Hittinger L., Berdeaux A., Castaigne A., Geschwind H. Comparison of the effects of exercise and cold pressure test on the vasomotor response of normal and atherosclerotic coronary arteries and their relation to the flow-mediated mechanism. Am. J. Cardiol. 1995;76:467–473. doi: 10.1016/S0002-9149(99)80132-5. [DOI] [PubMed] [Google Scholar]
- 63.Raizner A.E., Chahine R.A., Ishimori T., Verani M.S., Zacca N., Jamal N., Miller R.R., Luchi R.J. Provocation of coronary artery spasm by the cold pressor test. Hemodynamic, arteriographic and quantitative angiographic observations. Circulation. 1980;62:925–932. doi: 10.1161/01.CIR.62.5.925. [DOI] [PubMed] [Google Scholar]
- 64.Pham I., Nguyen M.T., Valensi P., Rousseau H., Nitenberg A., Vicaut E., Cosson E. Noninvasive study of coronary microcirculation response to a cold pressure test. Eur. J. Clin. Investig. 2015;45:135–143. doi: 10.1111/eci.12389. [DOI] [PubMed] [Google Scholar]
- 65.Dibben G.O., Dalal H.M., Taylor R.S., Doherty P., Tang L.H., Hillsdon M. Cardiac rehabilitation and physical activity: Systematic review and meta-analysis. Heart. 2018;104:1394–1402. doi: 10.1136/heartjnl-2017-312832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rauch B., Davos C.H., Doherty P., Saure D., Metzendorf M.I., Salzwedel A., Völler H., Jensen K., Schmid J.P. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: A systematic review and meta-analysis of randomized and non-randomized studies—The Cardiac Rehabilitation Outcome Study (CROS) Eur. J. Prev. Cardiol. 2016;23:1914–1939. doi: 10.1177/2047487316671181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salzwedel A., Jensen K., Rauch B., Doherty P., Metzendorf M.I., Hackbusch M., Völler H., Schmid J.P., Davos C.H. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: Update of the Cardiac Rehabilitation Outcome Study (CROS-II) Eur. J. Prev. Cardiol. 2020 doi: 10.1177/2047487320905719. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vona M., Rossi A., Capodaglio P., Rizzo S., Servi P., de Marchi M., Cobelli F. Impact of physical training and detraining on endothelium-dependent vasodilation in patients with recent acute myocardial infarction. Am. Heart J. 2004;147:1039–1046. doi: 10.1016/j.ahj.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 69.Lee K.W., Blann A.D., Jolly K., Lip G.Y. BRUM Investigators. Plasma haemostatic markers, endothelial function and ambulatory blood pressure changes with home versus hospital cardiac rehabilitation: The Birmingham Rehabilitation Uptake Maximisation Study. Heart. 2006;92:1732–1738. doi: 10.1136/hrt.2006.092163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peller M., Balsam P., Główczyńska R., Ossoliński K., Gilarowska A., Kołtowski Ł., Grabowski M., Filipiak K.J., Opolski G. The impact of physical training on endothelial function in myocardial infarction survivors: Pilot study. Kardiol. Pol. 2016;74:439–446. doi: 10.5603/KP.a2015.0177. [DOI] [PubMed] [Google Scholar]
- 71.Oliveira N.L., Ribeiro F., Silva G., Alves A.J., Silva N., Guimarães J.T., Teixeira M., Oliveira J. Effect of exercise-based cardiac rehabilitation on arterial stiffness and inflammatory and endothelial dysfunction biomarkers: A randomized controlled trial of myocardial infarction patients. Atherosclerosis. 2015;239:150–157. doi: 10.1016/j.atherosclerosis.2014.12.057. [DOI] [PubMed] [Google Scholar]
- 72.Cornelissen V.A., Onkelinx S., Goetschalckx K., Thomaes T., Janssens S., Fagard R., Verhamme P., Vanhees L. Exercise-based cardiac rehabilitation improves endothelial function assessed by flow-mediated dilation but not by pulse amplitude tonometry. Eur. J. Prev. Cardiol. 2014;21:39–48. doi: 10.1177/2047487312460516. [DOI] [PubMed] [Google Scholar]
- 73.Edwards D.G., Schofield R.S., Lennon S.L., Pierce G.L., Nichols W.W., Braith R.W. Effect of Exercise Training on Endothelial Function in Men with Coronary Artery Disease. Am. J. Cardiol. 2004;93:617–620. doi: 10.1016/j.amjcard.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 74.Gokce N., Vita J.A., Bader D.S., Sherman D.L., Hunter L.M., Holbrook M., O’Malley C., Keaney J.F.J., Balady G.J. Effect of Exercise on Upper and Lower Extremity Endothelial Function in Patients with Coronary Artery Disease. Am. J. Cardiol. 2002;90:124–127. doi: 10.1016/S0002-9149(02)02433-5. [DOI] [PubMed] [Google Scholar]
- 75.Gagliardi J.A., Maciel N., Castellano J.L., Masoli O., Miksztowicz V., Berg G., Bermejo E., Lazzari M., Gelpi R.J. Relationship between endothelial progenitor cells and vascular endothelial growth factor and its variation with exercise. Thromb. Res. 2016;137:92–96. doi: 10.1016/j.thromres.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 76.Conraads V.M., Pattyn N., de Maeyer C., Beckers P.J., Coeckelberghs E., Cornelissen V.A., Denollet J., Frederix G., Goetschalckx K., Hoymans V.Y., et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: The SAINTEX-CAD study. Int. J. Cardiol. 2015;179:203–210. doi: 10.1016/j.ijcard.2014.10.155. [DOI] [PubMed] [Google Scholar]
- 77.Van Craenenbroeck E.M., Frederix G., Pattyn N., Beckers P., van Craenenbroeck A.H., Gavaert A., Possemiers N., Cornelissen V., Goetschalckx K., Vrints C.J., et al. Effects of aerobic interval training and continuous training on cellular markers of endothelial integrity in coronary artery disease: A SAINTEX-CAD substudy. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H1876–H1882. doi: 10.1152/ajpheart.00341.2015. [DOI] [PubMed] [Google Scholar]
- 78.Hambrecht R., Wolf A., Gielen S., Linke A., Hofer J., Erbs S., Schoene N., Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N. Engl. J. Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka S., Sanuki Y., Ozumi K., Harada T., Tasaki H. Heart failure with preserved vs reduced ejection fraction following cardiac rehabilitation: Impact of endothelial function. Heart Vessel. 2018;33:886–892. doi: 10.1007/s00380-018-1128-2. [DOI] [PubMed] [Google Scholar]
- 80.Legallois D., Belin A., Nesterov S.V., Milliez P., Parienti J.J., Knuuti J., Abbas A., Tirel O., Agostini D., Manrique A. Cardiac rehabilitation improves coronary endothelial function in patients with heart failure due to dilated cardiomyopathy: A positron emission tomography study. Eur. J. Prev. Cardiol. 2016;23:129–136. doi: 10.1177/2047487314565739. [DOI] [PubMed] [Google Scholar]
- 81.Katz S.D., Schwarz M., Yuen J., LeJemtel T.H. Impaired acetylcholine mediated vasodilation in patients with congestive heart failure. Role of endothelium-derived vasodilating and vasoconstricting factors. Circulation. 1993;88:55–61. doi: 10.1161/01.CIR.88.1.55. [DOI] [PubMed] [Google Scholar]
- 82.Kubo S.H., Rector T.S., Bank A.J., Williams R.E., Heifetz S.M. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. doi: 10.1161/01.CIR.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 83.Katz S.D., Hryniewicz K., Hriljac I., Balidemaj K., Dimayuga C., Hudaihed A., Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310–314. doi: 10.1161/01.CIR.0000153349.77489.CF. [DOI] [PubMed] [Google Scholar]
- 84.Meyer B., Mörtl D., Strecker K., Hülsmann M., Kulemann V., Neunteufl T., Pacher R., Berger R. Flow-mediated vasodilation predicts outcome in patients with chronic heart failure: Comparison with B-type natriuretic peptide. J. Am. Coll. Cardiol. 2005;46:1011–1018. doi: 10.1016/j.jacc.2005.04.060. [DOI] [PubMed] [Google Scholar]
- 85.Bjarnason-Wehrens B., Nebel R., Jensen K., Hackbusch M., Grilli M., Gielen S., Schwaab B., Rauch B. German Society of Cardiovascular Prevention and Rehabilitation (DGPR). Exercise-based cardiac rehabilitation in patients with reduced left ventricular ejection fraction: The Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF): A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2020;27:929–952. doi: 10.1177/2047487319854140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Arauro Pio C.S., Marzolini S., Pakosh M., Grace S.L. Effect of cardiac rehabilitation dose on mortality and morbidity: A Systematic Review and Meta-regression Analysis. Mayo Clin. Proc. 2017;92:1644–1659. doi: 10.1016/j.mayocp.2017.07.019. [DOI] [PubMed] [Google Scholar]