Abstract
Mitochondrial DNA (mtDNA) molecules are packaged into compact nucleo-protein structures called mitochondrial nucleoids (mt-nucleoids). Their compaction is mediated in part by high-mobility group (HMG)-box containing proteins (mtHMG proteins), whose additional roles include the protection of mtDNA against damage, the regulation of gene expression and the segregation of mtDNA into daughter organelles. The molecular mechanisms underlying these functions have been identified through extensive biochemical, genetic, and structural studies, particularly on yeast (Abf2) and mammalian mitochondrial transcription factor A (TFAM) mtHMG proteins. The aim of this paper is to provide a comprehensive overview of the biochemical properties of mtHMG proteins, the structural basis of their interaction with DNA, their roles in various mtDNA transactions, and the evolutionary trajectories leading to their rapid diversification. We also describe how defects in the maintenance of mtDNA in cells with dysfunctional mtHMG proteins lead to different pathologies at the cellular and organismal level.
Keywords: mitochondria, mitochondrial nucleoid, HMG-box protein, Abf2, TFAM, DNA-binding, mitochondrial disease
1. Introduction
According to the widely accepted endosymbiotic theory [1,2], mitochondria are descendants of a singular merger between an archaeal and an α-proteobacterial cell [3,4,5]. Although the nature of the selection pressure favoring this symbiosis is still being discussed [6,7], it is clear that during the more than 1.0–1.9 billion years of the relationship [8], most of the genes of the original endosymbiont were either lost or transferred into the nucleus. Yet, with the exception of highly specialized mitochondria such as the mitosomes and most hydrogenosomes, a handful of protein-coding genes as well as part of the genes for the RNAs involved in their translation, have been retained in the organelle. The reasons why mitochondria keep their own genome are the subject of ongoing debate [9,10]. Although this question is still unanswered, there is a vast amount of literature on how the mitochondrial genome is maintained, expressed, and segregated during organellar and cellular division (for review see [11,12,13]). This review focuses on one particular issue related to both mtDNA maintenance and inheritance, how the DNA is compacted into higher-order structures.
2. Mitochondrial DNA Forms Higher-Order Structures Called Mitochondrial Nucleoids
One characteristic feature of mitochondrial genetics is the polyploid nature of the mitochondrial genomes. Although the individual molecules are several orders of magnitude shorter than the nuclear chromosomes, the total amount of mtDNA per cell can reach substantial lengths. For example, standard haploid yeast Saccharomyces cerevisiae cells contain on average about 50 copies of a 75–86 kbp long mtDNA molecule [14,15,16,17]. A mature human oocyte contains more than 150,000 copies of a 16.6 kbp mtDNA [18,19]; if the mtDNA circles were stacked one atop the other, they would form a column 300 μm high and almost 3 m of mtDNA would need to be packed into a cell only 100–200 μm across [20]. Even human cells with relatively lower mtDNA copy numbers, such as fibroblasts (2000 mtDNA copies per cell [21]) and leucocytes (ca. 360 mtDNA molecules per cell [22]), face a challenge in fitting their mitochondrial genomes into a limited organellar volume.
To circumvent this problem, the mtDNA is compacted into condensed nucleo-protein structures called mitochondrial nucleoids (mt-nucleoids) [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Considering the diameter of the mt-nucleoids in S. cerevisiae (0.3–0.6 μm) [24], yeast mtDNA must undergo a compaction of roughly three orders of magnitude. The size, shape, number of mt-nucleoids per cell (50–100) as well as the number of mtDNA copies per nucleoid (3.9–20) in S. cerevisiae depend on its physiological conditions [23]. Mammalian mt-nucleoids contain a relatively small number of mtDNA molecules (e.g., mouse fibroblasts contain about 3 mtDNA molecules per nucleoid) and are more diffuse compared to their yeast counterparts [25,39,40].
The mt-nucleoid protein composition has been examined in detail especially for yeast and mammalian cells [25,27,34,41,42,43,44,45,46,47,48,49,50]. It has been shown that mitochondria harbor morphologically distinct subpopulations of nucleoids associated with the inner membrane [40,51], which may differ in their involvement in various types of mtDNA transactions [52]. Somewhat unexpectedly, in addition to the proteins involved in mtDNA replication, transcription, recombination (e.g., DNA and RNA polymerases, topoisomerases, DNA helicases), and translation (e.g., ribosomal protein Mnp1, RNA helicases), mt-nucleoids were also shown to contain a substantial number of proteins involved in metabolism, membrane transport, cytoskeletal dynamics, and protein quality control [25,48,53]. Although the roles of individual components in the maintenance of mt-nucleoids are unknown in most cases, the great diversity of proteins associated with the nucleoid shows that there is an intimate connection between DNA transactions and the other biochemical activities taking place in mitochondria. On the other hand, the major mt-nucleoid components are DNA-binding proteins dedicated to mtDNA compaction. Indeed, all mt-nucleoids examined have one major group of DNA-packaging proteins which contain 1–2 so-called high-mobility group (HMG)-box domains. These proteins, termed mitochondrial HMG-box containing proteins (mtHMG proteins for short) are the primary subject of this review.
3. Setting the Stage: The Identification of HMG-Box Proteins in Yeast and Human Mitochondria
The first representative of this large and heterogeneous family of proteins was identified by Caron et al. in yeast by biochemical means [54]. Using the DNA-cellulose chromatography setup employed for the purification of the Escherichia coli protein called HU (known to be the principal E. coli chromosome packaging protein [55]), the authors identified a lysine-rich, thermostable ~20 kDa polypeptide which they named HM (for histone-like in mitochondria). Using petite mutants lacking mtDNA, they inferred that the protein is encoded by a nuclear gene. The abundance of HM was great enough to enable it to organize the structure of the mtDNA. By measuring the introduction of negative superhelical turns in relaxed DNA in the presence of topoisomerase they showed that HM, similar to E. coli HU, forms compact structures with DNA. Comparison of the molar amino-acid composition of HM with the HU, HMG2, and histone proteins yielded ambiguous results, so it was impossible to infer a homology to any of these protein classes. Yet, in hindsight it is astounding how this pioneering study correctly described most of the principal biochemical properties of the protein.
Naturally, there was one essential missing piece, the amino-acid sequence of HM. In the 1980s, the reports on HM were limited to resolving whether or not it represented the yeast H1 histone (it does not) [56,57] and to hints that HM belongs to a group of HMG proteins [56]. This was unequivocally demonstrated by Diffley and Stillman [58] by cloning the gene. Originally aiming for proteins recognizing replication origins (autonomously replicating sequences, ARS) in S. cerevisiae, they purified two ARS-binding factors, Abf1 and Abf2 [59]. Whereas Abf1 was demonstrated to be a bona fide ARS-binding factor [59,60], Abf2 was shown to be primarily localized to the mitochondria [58]. Later it was found that the genome of S. cerevisiae encodes a paralogue of Abf2, Ixr1, that serves as a transcriptional repressor regulating hypoxic genes during normoxia [61]; it also participates in recognizing DNA cross-links [62]. The ABF2 and IXR1 genes are products of whole-genome duplication [63], yielding HMG-box proteins localized to the mitochondria and nucleus, respectively. Analogously in mammals, a testis-specific HMG-box protein was shown to be a nuclear isoform of mitochondrial transcription factor A (TFAM; see below) [64], yet it plays a role in the spermatid nucleus during the replacement of histones with nucleoprotamines [65].
Analysis of its amino-acid sequence revealed that, in addition to a 26-residue N-terminal mitochondrial targeting signal [58], Abf2 contains two tandem HMG-boxes, presumably mediating its DNA binding, either of which is sufficient to stabilize mtDNA in vivo [66]. Based on the sequence of these HMG-boxes, Abf2 was classified as an HMG-1 box/HMGB protein [58,67,68] (there are six additional HMG-box containing proteins in S. cerevisiae: Hmo1, Hmo2 (Nhp10), Nhp6A, Nhp6B, Rox1, and Ixr1). The amino-acid composition of Abf2 and its ability to supercoil DNA in vitro both resembled the findings of Caron et al. [54]; subsequently, Abf2 and HM were shown to be encoded by the same gene [58,69]. Their differences in sequence indicate that although both yeast Abf2 and E. coli HU (and other bacterial nucleoid-associated proteins [70]) participate in the packaging of DNA into nucleoids, they do not share a common ancestor. It also implies that the protein involved in DNA packaging in the original α-proteobacterial endosymbiont was replaced by an HMG protein from the host. This is in contrast to chloroplasts where the packaging of the organellar genome is mediated by an HU-like protein [71]. The involvement of Abf2 in mtDNA maintenance was demonstrated by the analysis of a mutant lacking a functional copy of the ABF2 gene. When grown on media containing a fermentable carbon source, abf2− cells rapidly lost mtDNA and became respiratory deficient. The abf2− strains also exhibit other mutant phenotypes (https://www.yeastgenome.org/locus/S000004676#phenotype), such as sensitivity to osmotic stress [72], but these are mostly related to secondary defects in mtDNA maintenance rather than to the direct participation of Abf2 in the corresponding cellular processes. When spores formed by ABF2/abf2− diploids were transferred directly onto plates containing only a nonfermentable carbon source (glycerol), the abf2− haploid cells were able to grow and maintain their mtDNA, indicating that S. cerevisiae possesses an Abf2-independent mechanism of mtDNA packaging (see below) [58]. A follow-up study provided insights into the biochemical characteristics of Abf2 [73]. It (i) confirmed the ability of Abf2 to introduce negative supercoils into relaxed double-stranded (ds) DNA, (ii) showed that Abf2 prefers supercoiled rather than relaxed or linear DNA substrates, and (iii) demonstrated that Abf2 binding to DNA is nonrandom and possibly mediated by the phased distribution of short stretches of poly(dA), which prevent Abf2 binding. In total, this suggested that Abf2 might bind preferentially to mtDNA regions that are important for replication, transcription, or recombination. The involvement of Abf2 in mtDNA packaging was also demonstrated by complementation studies where the instability of mtDNA in abf2− cells was partially suppressed by the ectopic expression of E. coli HU [69]. Furthermore, the ability of the yeast nuclear HMG protein Nhp6A (when fused to the mitochondrial targeting sequence of Abf2) to functionally replace Abf2 indicated that the function(s) of Abf2 in mitochondria may be similar to the function(s) of nuclear HMG proteins [66].
At the same time as Diffley and Stillman were identifying Abf2 as the yeast mtHMG protein, David Clayton’s laboratory was studying a mammalian protein that promoted mtDNA transcription in vitro, originally called mammalian mitochondrial transcription factor 1 (mtTF1, mtTFA) and later renamed mitochondrial transcription factor A (TFAM) [74,75]. The cloning and sequence analysis of the TFAM-encoding gene revealed that, similar to Abf2 in yeast, it is an HMG-box containing protein [76,77]. Its role in transcription initiation has been extensively studied and was found to be quite complex; it is dependent on the corresponding promoter as well as on additional proteins [78,79].
Comparing the DNA-binding properties of human TFAM and S. cerevisiae Abf2 revealed several similarities [80]. Biochemical experiments indicated that both proteins bind and bend DNA molecules and actively participate in mtDNA compaction into mt-nucleoid-like structures [29,81,82,83]. Although TFAM and Abf2 share some common characteristics, Abf2 does not seem to act as a transcription factor in S. cerevisiae mitochondria [84,85]. It was shown that the addition of the 29-residue C-terminal tail of human TFAM to Abf2 was sufficient to convert the latter into a transcription activator in vitro [86] indicating that it is the absence of the tail that makes Abf2 incompetent to regulate mtDNA transcription; the mitochondrial transcription factor role in yeast mitochondria is performed by the Mtf1 protein [84]. (Of note, the Drosophila homologue of mammalian TFAM is also dispensable for transcription [87]). Despite this difference, both human and mouse TFAM were able to complement abf2−- associated phenotypes in S. cerevisiae, indicating that the functions of these proteins overlap [88,89]. Interestingly, it was recently reported that S. cerevisiae mitochondria are able to stably maintain mouse mtDNA when they also contain TFAM [90]. The discovery of the functional conservation of mtHMG proteins was very important because it implied that studies on the HMG-box proteins of various eukaryotes can be instrumental not only for understanding their evolution, but also for providing important insights into the general principles of mtDNA maintenance.
4. Genetic Studies on mtHMG Proteins Reveal Their Role in Regulating mtDNA Copy Number and Mt-Nucleoid Morphology and Dynamics
The fact that cells lacking Abf2 rapidly lose their mtDNA when grown on glucose [58] was not surprising considering that Abf2 was proposed to be the principal mtDNA-packaging protein. However, there were two issues that were the subject of intensive experimental effort: (i) The relative stability of mutant derivatives of mtDNA in abf2− mutants and (ii) the retention of the wild-type mitochondrial genomes in cells lacking the ABF2 gene when grown on nonfermentable carbon sources.
First, in S. cerevisiae, mitochondria can harbor wild-type (rho+) mtDNA, carrying a full complement of the genes required for cellular respiratory competence, or they can possess its deletion variants (rho−), containing amplified fragments of mtDNA that are stably propagated. Initial studies clearly showed that Abf2 was essential for maintaining rho+ mtDNA; however, Zelenaya-Troitskaya et al. [91] found that the rho− genomes remained relatively stable in abf2− cells. Moreover, they showed that a moderate (2-3-fold) increase in ABF2 expression yielded a 50–150% increase in mtDNA copy number in both rho+ and rho− cells and that the increase in mtDNA copy number in these cells accelerated nuclear DNA replication, cell proliferation, and mitigated the Sir2-dependent repression of genes, suggesting that mitochondrial processes have an active role in the control of cell division [92]. This crosstalk between the nuclear and mitochondrial genomes is reciprocal. Increasing ribonucleotide reductase activity by overexpressing RNR1 not only results in increased mtDNA copy number [93], but also partially rescues the mtDNA depletion in the abf2− strain [94]. Interestingly, the Abf2 paralogue Ixr1 is involved in regulating RNR1 expression [95].
While modest ABF2 overexpression leads to an increase in the amount of mtDNA, when its transcription is driven by a strong GAL1 promoter, the result is rapid loss (in 2–4 generations) of rho+ mtDNA but only slow mtDNA loss in rho− strains [91]. The different effect on maintenance of rho+ vs. rho− mtDNA in abf2− cells could be explained by the participation of Abf2 in the recombination-mediated replication of S. cerevisiae mtDNA. Whereas Abf2 would facilitate replication by binding to recombination intermediates in wild-type mitochondria, the rho− mtDNA genomes often contain tandem repeats that have an enhanced propensity to recombine independently of Abf2 and thus could stably propagate in its absence. This model was supported by studies demonstrating (i) the preferential binding of Abf2 to various types of recombination intermediates in vitro [96]; (ii) the suggested interaction of Abf2 with the Holliday junction resolvase Cce1 [97] and genetic interaction with the recombinase Mhr1 [98]; (iii) the recombination-dependent mechanism of replication and segregation of yeast mtDNA [99,100,101,102,103]; (iv) the negative effect of an absence of Abf2 on the level of mtDNA recombination in vivo [91,104]; and (v) an increase in recombination intermediates in cells overexpressing ABF2 [104]. The engagement of Abf2 in recombination may explain in part the defects of mtDNA partitioning within zygotes lacking a functional ABF2 gene [105], although this phenotype can also be due to the involvement of Abf2 in the segregation of mtDNA, which is independent of recombination.
The second problem requiring an explanation was the ability of abf2− cells to retain their genome when grown on a nonfermentable carbon source. A series of studies from Ron Butow’s laboratory provided some clues to this enigma. An analysis of mt-nucleoids from abf2− cells revealed that the pattern of mtDNA staining differs from that of wild-type cells [106]. In permeabilized mitochondria, some regions of mtDNA became hypersensitive to DNase I digestion, while other loci remained unaffected [106]. This indicated that in addition to Abf2, there are other mt-nucleoid components that play a role in compacting mtDNA and that these proteins can participate in maintaining the mt-nucleoids in cells lacking Abf2.
The search for proteins capable of replacing Abf2 in mtDNA maintenance was initiated by screening a yeast episomal library for genomic DNA fragments able to prevent the loss of mtDNA from abf2− cells grown on glucose. The screen resulted in the identification of ILV5 as a multicopy suppressor of the abf2− phenotype. ILV5 encodes an acetohydroxy acid reductoisomerase, which catalyzes a step in branched-chain amino-acid biosynthesis. For efficient suppression, a 2–3-fold increase in the ILV5 gene copy number was required. Moreover, the stability of mtDNA in abf2− cells was increased on media lacking amino-acids, accompanied by an increased Gcn4-dependent expression of ILV5 [107]. In addition, it was shown that the parsing of mtDNA into nucleoids is regulated by the general amino-acid control pathway and could be separated into one or more activities affecting the recombination of mtDNA and an activity of ILV5 that controls the organization of mtDNA molecules in nucleoids [108]. It was also observed that the increased number of nucleoids resulting from the activation of the general amino-acid control pathway dramatically increases the transmission of mtDNA, suggesting that this pathway operates on mtDNA organization to increase mtDNA transmission under starvation conditions. On the other hand, the involvement of Ilv5 protein in mtDNA stabilization did not depend on the functioning of the branched-chain amino-acid biosynthesis pathway, indicating bifunctionality of the Ilv5 protein. The amino-acid residues responsible for the catalytic activity and mtDNA maintenance reside in distinct regions of the protein [109]. Although it was shown that Ilv5 binds DNA in vitro [110], how exactly Ilv5 promotes mtDNA compaction remains a mystery.
Mitochondrial nucleoids undergo rapid changes not only when Abf2 is absent, but also under various physiological conditions. Therefore, it was of interest to assess the possible involvement of Abf2 in nucleoid dynamics in vivo. To this end, Kucej et al. [43] performed an in organello ChIP-on-chip assay and demonstrated that Abf2 binds to most of the mitochondrial genome with a preference for GC-rich sequences. Under respiring conditions, mt-nucleoids formed open nucleo-protein structures with a lower ratio of Abf2 to mtDNA. The bifunctional nucleoid proteins Hsp60 (a chaperone) and Ilv5 were recruited to mt-nucleoids during glucose repression and amino-acid starvation, respectively. Using co-immunoprecipitation experiments, the authors showed that Aco1 (aconitase), Ald4 (aldehyde dehydrogenase), Idh1, Idh2 (subunits of isocitrate dehydrogenase), and Kgd1 (a subunit of α-ketoglutarate dehydrogenase) are the major protein partners of Abf2 in vivo [43], further supporting the role of bifunctional metabolic enzymes in the maintenance of mt-nucleoids in yeasts [34] and highlighting the general phenomenon of the multitasking or “moonlighting” of proteins originally thought to have dedicated functions in cellular metabolism [111,112].
Another screen identifying multicopy suppressor was based on the selection of abf2− strains containing an episomal plasmid carrying a fragment of genomic DNA able to support growth on glycerol media at 37 °C [69,113]. Subsequently, three genes were identified. The first, SHM1 [114], was later shown to encode a mitochondrial GDT/GTP transporter and renamed GGC1 [115]. The deletion of GGC1 in an abf2− background resulted in synthetic lethality on glycerol, probably due to the combined negative effect of the absence of Abf2 on mtDNA stability and the deficient nucleic acid and protein synthesis caused by an ineffective mitochondrial supply of guanine nucleotides [114]. The second multicopy suppressor, YHM2, also encodes an inner mitochondrial membrane carrier protein (for citrate and oxoglutarate [116]) which co-purifies with mt-nucleoids and exhibits DNA-binding properties in vitro [117]. Finally, the overexpression of TIM17, which encodes a component of the inner mitochondrial membrane import channel, prevents the complete loss of mtDNA in abf2− cells [118]. Conversely, the overexpression of ABF2 was able to rescue the petite-negative phenotype of some TIM17 mutants [119]. The fact that increased levels of inner membrane proteins such Ggc1, Yhm2, or Tim17 can rescue cells from defects caused by the absence of Abf2 is intriguing. It is known that Abf2 is part of a large (~900 kDa) complex containing the DNA helicase Pif1 and DNA polymerase γ (Mip1) [120] and that there are two distinct subpopulations of mt-nucleoids associated with different regions of the mitochondrial membrane [52] (this is similar to some mammalian cell types, which exhibit distinct subpopulations of mt-nucleoids differing in the amount of TFAM [45,46,121]). Nevertheless, a direct link between Ggc1, Yhm2 and Tim17, and the membrane attachment of the mt-nucleoid is still lacking.
A breakthrough in understanding how the mt-nucleoid is preserved in the absence of Abf2 came from a study demonstrating that aconitase (Aco1) is required for stabilizing mtDNA in abf2− cells [47]. When grown on glycerol, abf2− cells may retain their mtDNA concomitantly with elevated HAP complex-dependent ACO1 expression under respiratory conditions [122]. The mt-nucleoid functions of Aco1 are independent of aconitase catalytic activity: The introduction of point mutations in the Fe-S cluster required for the aconitase enzymatic activity in the Krebs cycle did not result in the impairment of its ability to stabilize mtDNA in abf2− cells [47]. The DNA-binding activity of Aco1 also resides in a distinct region [123]. Such bifunctionality has also been described for the cytosolic form of mammalian aconitase, which oscillates between enzymatic and RNA-binding forms based on the assembly and disassembly of the [4Fe-4S] cluster [124,125]. The involvement of Aco1 in mtDNA packaging in S. cerevisiae also indicates that mt-nucleoids exist in different states depending on the metabolic condition of the cells. Whereas Abf2 is essential for mtDNA maintenance under glucose repression, in de-repressed cells with robust oxidative metabolism or in response to retrograde signals, mtDNA packaging may also involve Aco1 [126,127]. It was shown that Aco1 binds to mtDNA (with a preference for GC-rich sequences, similar to Abf2), protects it from an excessive accumulation of point mutations and ssDNA breaks and suppresses the reductive recombination of mtDNA [123]. The participation of aconitase in the formation of the mt-nucleoid not only explains the ability of abf2− cells to retain their mtDNA when grown on glycerol [58], but also provides clues about the mechanisms involved in mt-nucleoid remodeling as part of a strategy for adjusting mtDNA maintenance to changes in cellular metabolism [48,128].
Another interesting observation on the Abf2-independent mechanism of mtDNA maintenance resulted from an investigation into the cellular responses to the inhibition of the processing peptidase cleaving the mitochondrial N-terminal targeting sequence. It was shown that retaining the N-terminal sequences on the imported proteins makes them insoluble and triggers a specific type of unfolded protein response (mtUPR). Depletion of Abf2 under such conditions was compensated for by the relocalization of the nuclear HMG-box transcription factor Rox1 to the mitochondria, where it binds mtDNA and ensures its maintenance and gene expression [129].
Genetic studies were also instrumental for determining the role of mammalian TFAM in mtDNA maintenance in vivo. Whereas heterozygous TFAM+/− mice have a 34 ± 7% [130] or ca. 50% [131] reduction in mtDNA copy number, homozygous knock-outs (TFAM−/−) die in mid-gestation [130]. Manipulation of the levels of TFAM causes changes in mtDNA copy number and defects in mtDNA segregation [132,133,134]; the removal of TFAM using a conditional knock-out system (cre-loxP) resulted in the depletion of mtDNA and mitochondrial transcripts as well as in severe respiratory chain deficiency [130,135,136,137,138,139]. Based on these studies, it was clear that TFAM performs several important functions related to mtDNA packaging, replication, transcription, and segregation and thus is an essential player in the regulation of mtDNA maintenance in mammalian cells [83].
5. mtHMG Proteins Are Rapidly Evolving
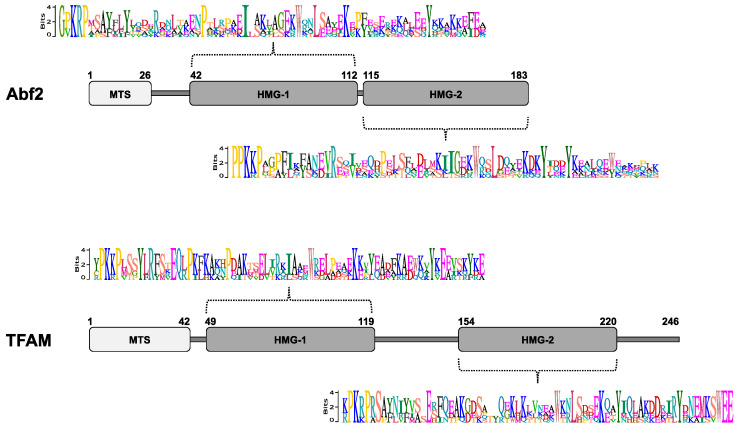
Comparing the biochemical properties and the in vivo roles of Abf2 and mammalian TFAM reveals that some mtHMG protein functions are shared, but many others seem to be species-specific. In contrast to the extremely conserved canonical core of the histones involved in nuclear DNA packaging [140], the amino-acid sequences of the mtHMG proteins are highly divergent even between species within the same taxonomic group, such as ascomycetous yeasts [49,50,58,141,142,143,144,145], making it difficult to identify them from a simple genomic search. The only common features seem to be the presence of at least one HMG-box for mediating mtDNA binding and a cleavable N-terminal signal peptide for mitochondrial import (Figure 1 and Figure S1).
Figure 1.
The domain organization of yeast (Abf2) and human (mitochondrial transcription factor A (TFAM)) HMG-box containing proteins (mtHMG) proteins. The amino-acid sequences of the two HMG-box domains of Abf2 (from S. cerevisiae, S. uvarum, Candida glabrata, Naumovozyma castellii, N. dairenensis, and Vanderwaltozyma polyspora) and TFAM (from human, mouse, rat, Xenopus laevis, and Drosophila melanogaster) were predicted using Simple Modular Architecture Research Tool (SMART) [148] and aligned using multiple sequence alignment software MAFFT (multiple alignment using fast Fourier transform) v7.450 [149]. The sequence logos were created using Geneious v11.1.5 (Biomatters). The mitochondrial targeting sequences (MTS) are based on experimental evidence [58,76].
For example, the Kluyveromyces lactis Abf2 homologue was first identified as a component of purified mt-nucleoids [142] and was later found to exhibit only ~30% sequence identity to S. cerevisiae Abf2 [50]. Investigating the mt-nucleoids from several species of Saccharomyces, Pichia, and Williopsis (the latter two now classified as Cyberlindnera) did reveal the presence of Abf2-like DNA-binding proteins, but these did not cross-react with antibodies raised against S. cerevisiae Abf2, which again highlight the fast evolution of the corresponding ancestral genes [143,146,147].
Considering the affinity of Abf2 for recombination intermediates, it was of interest to search for mtHMG proteins in yeasts with linear mitochondrial genomes, such as Candida parapsilosis, whose mitochondrial telomeres are maintained by a recombination-dependent mechanism involving the generation and rolling-circle replication of telomeric repeats [150,151,152]. Purified mt-nucleoids from C. parapsilosis contained Gcf1, a protein exhibiting some biochemical similarities to S. cerevisiae Abf2, and the corresponding gene complemented the mtDNA stability defect of the S. cerevisiae abf2− mutant. In contrast to Abf2, an in silico analysis of Gcf1 predicted the presence of a coiled-coil domain and two HMG-boxes, one of which is weakly conserved (Figure 1 and Figure S1), suggesting that it represents a novel type of mtHMG protein [49]. Gcf1 from C. parapsilosis has orthologues in Candida albicans and a closely related species from the genus Debaryomyces [144]. Depletion of C. albicans Gcf1 results in a 3000-fold decrease in GCF1 mRNA levels, which is correlated with a substantial decrease in the number of both mtDNA copies and recombination intermediates. The absence of Gcf1 did not result in the loss of cell viability even though C. albicans and C. parapsilosis are petite-negative species unable to grow without a functional mitochondrial genome. This suggests that, similar to S. cerevisiae Abf2, there is a back-up mechanism for mtDNA packaging and maintenance [144].
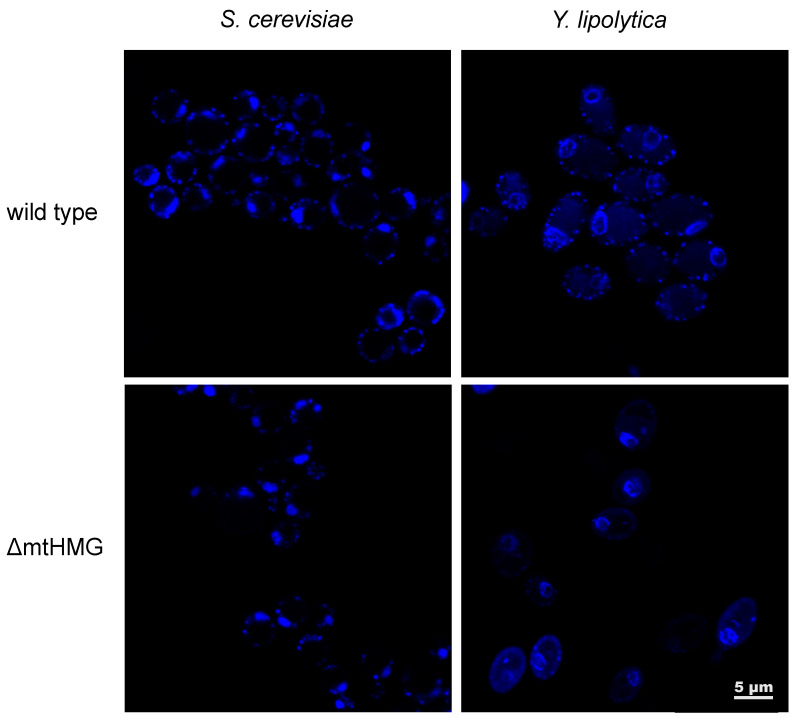
Another type of mtHMG protein, Mhb1, was found in the yeast Yarrowia lipolytica. The protein was identified as the major component of purified mt-nucleoids and was found to compact DNA in vitro [145]. Although Y. lipolytica is a strictly aerobic yeast [153], deletion of the Mhb1-encoding gene did not result in loss of viability. Additionally, the mutant exhibits clear differences in mt-nucleoids (Figure 2) accompanied by an increased sensitivity to ethidium bromide, a DNA intercalating agent known to inhibit the synthesis of mtDNA and to make it prone to degradation [154,155]. The mutant had fewer copies of mtDNA and mtDNA-derived transcripts. Interestingly, its respiratory characteristics and growth under most of the conditions tested were indistinguishable from those of the wild-type strain. On the other hand, the level of mtDNA-encoded proteins in the mutant was similar to the wild-type cells indicating that the cells are able to circumvent the potential imbalance between the subunits of the respiratory chain encoded by the nuclear and mitochondrial genomes [145].
Figure 2.
Visualization of DNA in wild-type cells vs. mutants lacking mtHMG protein (ΔmtHMG). Nuclear DNA (large blue dots) and mt-nucleoids (smaller blue spots) were visualized in yeast cells using confocal microscopy (Olympus IX81) after DAPI staining. As in the S. cerevisiae abf2− mutant, the deletion of mtHMG genes in Y. lipolytica, a strictly aerobic yeast, resulted in a decreased number of mt-nucleoids.
The existence of compensatory mechanism(s) for dealing with decreased levels of mtDNA or mtDNA-derived transcripts was also indicated by studies of S. cerevisiae abf2− mutants and Gcf1-deficient C. albicans cells [91,144]. Various explanations of its still enigmatic molecular nature have been suggested [145]. The translational plasticity of human mitochondrial ribosomes has been shown to contribute to preserving a balance between the nuclear and mitochondrial-encoded respiratory complex subunits. Specifically, defects in the assembly of respiratory chain complexes caused by an insufficient supply of subunits translated by the cytosolic ribosomes result in the arrest of mitochondrial translation [156]. Although mechanistically different, such regulatory crosstalk systems have also been described in yeast, showing that the regulation of mitochondrial and cytosolic translation is coordinated [157]. In addition, the accuracy of yeast mitochondrial translation is monitored by the cytosolic proteostasis system in a manner that shares many characteristics with the metazoan mitochondrial unfolded protein response [158].
So far, no mtHMG protein has been identified in the fission yeast Schizosaccharomyces pombe. Its genome encodes a S. cerevisiae Ixr1 homologue, Cmb1, which has been shown to participate in mismatch repair [159] and to colocalize with mitochondria [160]; bioinformatics tools indicate that it should localize to the mitochondria [161] and that it possesses a cleavable targeting sequence (as predicted by mitochondrial protein targeting software MitoProt II; [162]). However, so far no differences between nucleoids in a cmb1− mutant compared to the wild-type S. pombe cells have been reported. There are other fission yeast HMG proteins with putative mitochondrial localization (such as an Nhp6 homologue), but their involvement in maintaining mt-nucleoids has not been investigated yet. Perhaps the proteomic analysis of purified mt-nucleoids that is underway in the Miyakawa laboratory [23] will pinpoint the protein responsible for mtDNA packaging in fission yeasts.
In the filamentous fungus Podospora anserina, a mtHMG protein was identified as the product of a gene that suppressed premature cell death caused by the accumulation of deletions in mtDNA [141]. Although the protein was not characterized biochemically, it was shown to reside in the mitochondria. In addition, it was the first example of a mtHMG protein combining the AT-hook (a DNA-binding motif consisting of a conserved proline-arginine-glycine-arginine-proline core sequence) [163] and HMG-box DNA-binding domains. In Aspergillus nidulans, the protein HmgB is located primarily in mitochondria, but also exhibits nuclear localization. In addition to a canonical HMG-box at the C-terminus, it also contains two structurally related domains called Shadow-HMG-boxes. An HmgB deletion strain exhibited a decrease in conidial and ascospore viability, probably due to the importance of HmgB in maintaining mtDNA in spores [164]. Furthermore, it was also shown that HmgB plays a role in cellular protection against oxidative stress agents, although it is possible that this role is associated with its nuclear functions [165].
A divergent mtHMG protein termed Glom was identified in the condensed mt-nucleoids of the true slime mold, Physarum polycephalum [166]. In addition to two HMG-boxes at its C-terminus, Glom also has a lysine-rich region with a proline-rich domain in its N-terminal half, and all three domains seem to participate in DNA-binding. Interestingly, the lysine-rich region alone was sufficient for its intense mtDNA condensation in vitro as well as for condensing the E. coli chromosome into nucleoid structures in vivo. On the other hand, the proline-rich region was necessary for keeping the nucleoid accessible to the transcription machinery and the HMG-boxes were required for complementing the defects associated with the absence of the HU protein. Thus, Glom is a distinct type of mtHMG protein which employs three DNA-binding domains and, in coordination with the S. cerevisiae Mgm101 homologue Glom2, plays a complex role in the organization and dynamics of the P. polycephalum mt-nucleoid [166,167].
The kinetoplast DNA (kDNA) of unicellular flagellates from the order Kinetoplastida is composed of a complex, mixed population of maxi- and minicircles [168], and presents a particularly challenging mitochondrial genome for packaging. In Crithidia fasciculata, several basic polypeptides, called kinetoplastid-associated proteins (KAPs), were identified by biochemical means [169,170], and some of them were shown to play a role in kDNA maintenance [171]. In Trypanosoma brucei, the KAP6 protein, containing two HMG-boxes, was shown to be involved in kDNA replication [172] and to possess DNA-binding properties similar to other mtHMG proteins [173].
Although mt-nucleoids have been studied in higher plants [174,175], their principal mtDNA packaging protein(s) remain elusive. The fact that no plant mtHMG protein has been identified to date may be either due to its distant relationship to known mtHMG proteins or because mtDNA packaging in plants does not require a mtHMG protein [176,177]. The resolution of this issue will require the detailed proteomic analysis of purified plant mt-nucleoids.
The rapid evolutionary sequence diversification of mtHMG proteins may be due to the fact that amino-acid substitutions outside the HMG-boxes are generally neutral. A pairwise comparison of the amino-acid sequences of the other protein components of the mitochondrial nucleoids of C. albicans and C. parapsilosis showed that they are more similar than those of their corresponding HMG proteins [49], yet mtHMG proteins retain their ability to form functional complexes with the latter, indicating that their faster diversification do not affect functional protein–protein interactions [178]. Alternatively (or concomitantly) changes in mtHMG proteins may reflect their species-specific biochemical roles in mtDNA maintenance and segregation, or possibly differences in mtDNA base composition and topology. To explore this possibility we compared the biochemical properties of the mtHMG proteins from three distantly related yeast species: S. cerevisiae (Abf2), Y. lipolytica (Mhb1), and C. parapsilosis (Gcf1) and observed several differences [96], but two major areas of commonality. We found that (i) all three proteins exhibit relatively weak binding to intact dsDNA, as demonstrated by the fact that Abf2 and Mhb1 bound to this type of substrate only at very high protein:DNA ratios while Gcf1 showed only negligible binding; and that (ii) all three proteins exhibited a high preference for recombination/replication intermediates such as Holliday junctions and replication forks. These observations highlight the involvement of yeast mtHMG proteins in the maintenance and compaction of mtDNA in vivo. The relatively high affinity of Gcf1 for these types of DNA structures suggests that the protein may be actively involved in the maintenance of mitochondrial telomeres that rely on the recombination-dependent formation of extragenomic circular DNA (telomeric circles or t-circles) [151,152,179]. Mammalian TFAM was also shown to have a high affinity for recombination intermediates [180], thereby contributing to the peculiar organization of the mtDNA in complex junctional networks in human heart muscle as well as in mouse and human brain tissue [181,182].
6. mtHMG Proteins Protect mtDNA against Damage and the Accumulation of Mutations
mtDNA molecules are primary targets of reactive oxygen species (ROS) and therefore must be protected against oxidative damage. mtHMG proteins appear to represent one of the first lines of defense against ROS.
Spontaneous petite colony formation in the abf2− strain was partially suppressed by malonic acid treatment, which decreases ROS formation by the electron transport chain (ETC). ROS generation by the ETC therefore likely contributes to mitochondrial disfunction in abf2− cells. The abf2−, ntg1− double null mutant (which also lacks the DNA N-glycosylase and apurinic/apyrimidinic lyase Ntg1), also exhibits a synthetic petite phenotype if untreated. Importantly, the negative effect of ABF2 deletion is not due to compromised recombination, which indicates that S. cerevisiae possesses recombination-independent mechanisms to cope with oxidative mtDNA damage [183]. Deletion of either ABF2 or NTG1 results in the activation of Rad53 checkpoint signaling and the reduction of the mitochondrial ROS-mediated adaptive extension of chronological lifespan [184].
Another study assessed the types of mutations accumulating in abf2− strains and found an increase in the frequency of frameshifts and direct-repeat mediated deletions with no change in the rate of mtDNA point mutations. Treatment of abf2− cells with UV light resulted in a relative increase in respiratory-deficient mutants when compared to the wild-type [185]. These results highlight the different architecture of the mt-nucleoids in abf2− cells, which renders their mtDNA more vulnerable to genomic instability. A propensity to higher rates of mtDNA mutations was also observed for the Y. lipolytica mhb1− strain [145]. This indicates that, although the absence of mtHMG proteins is well tolerated in the short term even in strictly aerobic species, the increased frequency of mutagenic events accumulating within the mitochondrial genome results in lower fitness overall, and such strains are thus subject to negative selection. TFAM was also involved in DNA repair where it was shown to preferentially bind to mtDNA damage hot spots in rat cells, thus making them less accessible to DNA repair factors. The import of p53 under oxidative stress conditions was suggested to facilitate the weakening of the affinity of TFAM for the damaged sites thereby enabling their repair [186,187,188]. Yoshida et al. [189] observed a significant increase in cisplatin-damaged DNA-binding by TFAM upon interaction with p53 in vitro, however.
7. On the Mechanism of mtDNA Compaction by mtHMG Proteins
Abf2 binds DNA with modest cooperativity and affinity. The reported KD varies between 0.04–1.5 μM [190,191,192], although it was suggested that the affinity of Abf2 for DNA changes by an order of magnitude during its compaction (from 0.28 to 2 μM), indicating that DNA accessibility to Abf2 decreases significantly with increasing compaction [193]. Several studies have determined the number of Abf2 molecules per cell with a median abundance of 15,000 ± 7000 [194] and a range from 3307 [195] to 51,132 [196]. These large differences are partly due to different growth conditions, to the use of different strains, and to different means of quantification. This needs to be considered when extrapolating the results of in vitro studies to the situation in vivo. With the total mitochondrial volume in a haploid cell being about 0.2–1.5 μm3 [15,197], the concentration of Abf2 would range from 27.5–425 μM (for 0.2 μm3) to 4–55 μM (for 1.5 μm3), making the lowest estimated concentration higher than the greatest apparent KD estimate.
Using optical and atomic force microscopy (AFM) it was shown that Abf2 is able to compact dsDNA linear molecules. Based on a fast Abf2 off-rate (koff = 0.014 ± 0.001 s−1) the packaging of DNA was relatively weak and the forces stabilizing the condensed DNA–protein complex were small (<0.6 pN). The visualization of individual complexes by AFM revealed 190-nm structures that were loosely packaged compared to nuclear chromatin, indicating that they are accessible for transcription and replication and, at the same time, more vulnerable to damage [191]. However, such an arrangement does not pose a threat to yeast mitochondrial genome integrity, as other mitochondrial proteins participate with Abf2 in mtDNA stabilization in vivo [34]. A follow-up study showed that Abf2 binding induces sharp bends (of about 78°) in the DNA backbone for both linear and circular DNA and that at a high Abf2 concentration, the DNA suddenly collapses into a tight nucleo-protein complex. Based on these results, a “bent-worm-like chain model” was created in which the introduction of bends into the DNA is sufficient to promote compaction without the need for super-twisting [192]. It may be noted here that structural studies on bacterial HU, which is not an HMG-box protein, demonstrated that it also bends the DNA by 106–124° [198,199]. This suggests that bending is a fairly common means for compacting DNA. However, both studies used a distorted DNA template containing three T–T mismatches and four unpaired Ts. A more recent structure using native DNA shows that HU may actually straighten rather than bend its DNA substrates [200].
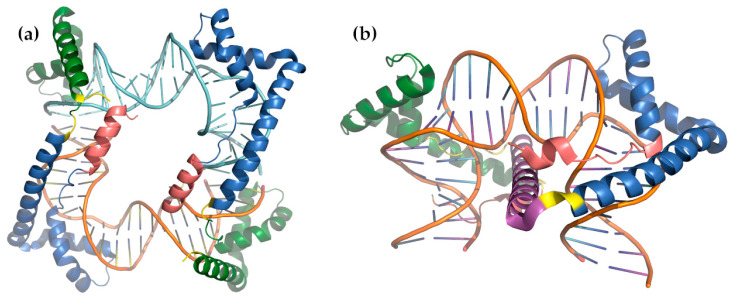
The essential information for a detailed understanding of the mechanism of mtDNA compaction was provided by the determination of the 3D structures of mtHMG proteins. HMG proteins normally contain one or two HMG-box domains. Structurally, an HMG-box domain typically contains three α-helices arranged in an L-shape. The short leg of the L is comprised of two short, anti-parallel α-helices (α1 and α2) while the longer leg consists of α3 together with an elongated N-terminal segment of around 6–7 residues. Structures of HMG-box domains in complex with DNA show that the protein binds to the DNA minor groove through the concave surface of the L with widening and flattening of the minor groove and pronounced bending of the DNA substrate. This bending is stabilized by several polar and non-polar interactions together with the intercalation of non-polar residues from either or both α1 and α2, which disrupt the DNA base-pair stacking. In the Abf2–DNA complex [201] residues Phe-51 (from α1 of HMG-box 1) and Ile-124 (α1 of HMG-box 2) intercalate into the DNA. Important polar contacts were identified for residues Lys-44 and Arg-45 (box 1) and Lys-117 and Lys-118 (box 2). DNA backbone-interacting residues included Tyr-50, Tyr-53, Arg-77, Trp-81, and Lys-89 from box 1 and Phe-123, Tyr-126, Ile-150, Trp-154, and Lys-162 from box 2. Overall, each Abf2 molecule bound to two different DNA substrates using its two HMG-box domains, thereby “stapling” them together (Figure 3). This study also confirmed that the protein displays a distinct “phased-binding” at DNA sequences containing poly-adenine tracts (A-tracts) [58,73]. The two crystal structures of Abf2 in complex with mtDNA-derived fragments bearing A-tracts showed that each Abf2 HMG-box induces a 90° bend in the contacted DNA, causing an overall U-turn. Furthermore, it was demonstrated that an N-terminal flag and α-helix are crucial for mtDNA maintenance. They promote the initial binding of the protein to DNA and facilitate the subsequent interactions with the HMG-boxes which are accompanied by DNA bending. The structure also indicated that Abf2 might be excluded from the A-tracts because these tracts have a narrow minor groove which might make them inaccessible for Abf2-binding [201]. Since the mtDNA of S. cerevisiae is rich in A-tracts, this property could be important for setting the overall nucleoid architecture. On the other hand, there are presently no structures of either Abf2 bound to GC-rich sequences or to recombination intermediates, both of which are its preferred substrates [43,96]. Such studies could shed more light on one additional paradox: It has been shown that for efficient compaction in vitro, the ratio of Abf2 to mtDNA should be one protein molecule per ~10–20 bp [58,201]. In addition, 50–100 molecules of ~80 kbp mtDNA per yeast cell [23] yields 2–8 × 105 binding sites, yet even the largest estimate is about 5 × 104 molecules of Abf2 per cell [196], i.e., almost one order of magnitude lower than that needed to reach the optimal ratio. It is possible that the compaction of mtDNA is achieved by the combined effect of Abf2 and other mt-nucleoid proteins, but it is also likely that GC-rich regions or recombination intermediates play a more important role in the compaction [96].
Figure 3.
Structures of Abf2 and TFAM in complex with DNA. (a) Abf2 in complex with DNA (PDB ID 5JH0; [201]); HMG-box 1 is colored blue, HMG-box 2 is colored green. The two separate DNA molecules are colored differently to differentiate them. The pink α-helices are the additional N-terminal α-helices known so far only from Abf2. (b) TFAM in complex with DNA (PDB ID 3TMM; [207]); HMG-box 1 is colored blue, HMG-box 2 is colored green, the 30-residue linking helix is colored magenta. The N-terminal extension is pink.
TFAM is present in mammalian mitochondria in a ratio of about 1000 molecules per mtDNA (or 1 TFAM per 15–18 bp of mtDNA) making it abundant enough to coat the entire mitochondrial genome [39]. The affinity of TFAM for nonspecific DNA substrates, as determined by surface plasmon resonance (4 nM) [29] and other methods [83], seems to be higher than that of Abf2 and it is even higher for promoter sequences (0.16–1.6 nM), indicating a distinct difference in their function and possibly reflecting the role of TFAM as a transcription factor. TFAM could also contribute to the regulation of mtDNA transactions through G-quadruplex binding. The in vitro affinity of TFAM for certain G-quadruplex structures, which might form in the GC-rich regions of human mitochondrial genomes, was higher than for the corresponding B-DNA [202].
Similar to Abf2, TFAM contains two HMG-boxes, but now they are separated by a longer, 30-residue linker, followed by a C-terminal extension involved in regulating transcription. Moreover, similar to Abf2, TFAM binds to the DNA minor groove using its HMG-box domains, but in this case the two domains bind to the same DNA substrate. Both domains bind to the convex surface of the resulting bent DNA by passing the 30-residue α-helical linker through the bend (Figure 3). This results in a conformation in which the N-terminal, short legs of the HMG-box domains are oriented towards one another across the complex. The linker helix is positively charged, which helps to offset the electrostatic strain introduced by bringing the negatively charged phosphate backbone atoms of the DNA closer to one another. TFAM HMG-box 1 intercalates Leu-58 and partially Tyr-57 (both from α1) while Thr-77, Thr-78, and Ile-81 from α2 make important contacts. HMG-box 2 intercalates Leu-182 (which corresponds to Ile-81 from box 1) while Asn-163 (topologically equivalent to Leu-58) hydrogen-bonds to two T nucleotides, imparting a shear rather than intercalating and Tyr-162 partially intercalates. Trp-88 from box 1 and Trp-189 from box 2, together with a number of other residues from the α3 helices, stabilize the phosphate backbone through polar contacts and positive charges [203,204]. More detailed experiments showed a stepwise binding mechanism where DNA-binding to HMG-box 1 initially bends the DNA into a V-shape reducing the distance between HMG-boxes and eventually facilitating the binding of HMG-box 2. Each HMG-box induces a 90° bending of the minor groove and inserts two leucines (Leu-58 from HMG-box 1 and Leu-182 from HMG-box 2) into two sites separated by 10 bp, thereby completing the typical U-shaped conformation [204]. TFAM functions as a homodimer and generally binds DNA in a sequence-non-specific manner with the exception of sequence-specificity to sites upstream (−15 to −35) of the heavy strand (HSP1) and light strand (LSP) promoters [205]. When bound to the LSP, the U-turn bending of TFAM allows the C-terminal tail, which recruits the transcription machinery, to approach the initiation site [206,207]. The crystal structures of TFAM bound to HSP1 showed that it binds in the opposite direction compared to LSP thereby explaining the different modes of transcription activation at these two sites. While it is unnecessary for DNA bending and transcriptional activation, TFAM dimerization was suggested to be important for mtDNA compaction, possibly by promoting DNA looping [208]. In particular, all TFAM–DNA complexes solved to date feature an interface between the α3 helices of HMG-box 1 from two different complexes. The convex outside surfaces come into contact, with each α3 paired in an anti-parallel orientation. The interface buries a surface area of approximately 1180 Å2 and is characterized by a series of polar and electrostatic interactions involving especially residues Lys-95, Tyr-99, Glu-106, Glu-112, and Arg-116. Mutants which disrupt this interface are still able to bind DNA and initiate transcription, but cannot compact DNA as efficiently as the wild-type protein [208]. A more recent study using a combination of biochemical methods and super-resolution and electron microscopies showed that a mutant TFAM unable to dimerize still compacted DNA. It also revealed that TFAM-mediated nucleoid formation in vitro is a multistep process. It is initiated by TFAM binding single DNA duplexes as beads on a string followed by the TFAM molecules bridging two DNA duplexes to form loops [209]. A cooperative manner of DNA binding was proposed, meaning that the first TFAM binding event influences the second—a characteristic that increases the affinity of TFAM for DNA and drives DNA compaction to completion [29]. Removal of the C-terminal tail only slightly decreased non-sequence-specific DNA binding, and the X-ray crystal structure of HMG-box 2 revealed unusual features for an HMG-box, including interactions of the HMG-box with other regions of TFAM [210].
8. Regulation of mtHMG Proteins
Despite sharing no sequence homology with eukaryotic nuclear histones or HMG-box proteins, bacterial nucleoid-associated proteins (NAPs) are primarily responsible for the dynamic spatial organization of the bacterial nucleoid, the homeostasis of DNA supercoiling, and global gene regulation. In general, NAPs are highly conserved within specific bacterial families, some even highly conserved among all prokaryotes, and all bacterial species possess at least one NAP [211,212]. Bacterial NAPs provide higher-order superstructures that link gene expression and the architecture of the bacterial genome. Their DNA-binding properties allow distant domains to be brought into proximity or two distinct parts of the DNA to be bridged, which significantly influences life-dependent DNA transactions [212]. It is also known that the structure of the nucleoid and global transcription patterns are both influenced by environmental conditions [213]. A more recent E. coli study confirmed that nucleoids undergo visible changes during cell growth with the DNA more tightly compacted during the stationary phase than exponential growth; this has been partly attributed to changes in the expression levels of genes encoding the NAPs that were more abundant during the growth phase [214].
Similar to their bacterial counterparts, mt-nucleoids are sensitive to changes in the levels of active mtHMG proteins. As discussed above, a decreased amount of mtDNA-bound Abf2 was found to increase the transcriptional activity of mt-nucleoids in S. cerevisiae [43] whereas the strong overexpression of Abf2 leads to a rapid loss of mtDNA [91]. These results underline the importance of regulating the levels of mtHMG proteins for maintaining cellular functions.
High-throughput transcriptomic analyses showed several conditions that change in response to changes in ABF2 expression (https://www.yeastgenome.org/). For example, it was shown that ABF2 transcription is under the control of Hcm1, whose overexpression or deletion leads to altered Abf2 levels accompanied by changes in mitochondrial biogenesis [215]. Similarly, expression of the ABF2 gene is decreased in cells lacking its paralogue Ixr1 when they are exposed to hypoxia [61]. A whole chromatin immunoprecipitation analysis revealed that the ABF2 promoter interacts with the transcription factors Fhl1 and Rsf1 when cells are exposed to heat [216]. Despite this, the relevance of the regulation of ABF2 expression on a transcriptional level has not been investigated in sufficient detail. The ratio of Abf2 to mtDNA changes more as a result of fluctuations in mtDNA copy number rather than changes in ABF2 expression [43]. The possibility that Abf2 might be regulated post-transcriptionally emerged from a study demonstrating that about half the ABF2 mRNA transcripts are associated with mitochondria, and thus could potentially provide a direct source of Abf2 to the organelle [217]. Furthermore, Abf2 levels increased in cells treated with rapamycin [218], a selective inhibitor of mTOR (the mechanistic target of rapamycin), a protein kinase that acts as a central integrator of the nutrient signaling pathways [218]. In contrast, deletion of the TOR1 gene resulted in increased mitochondrial respiration through the stimulation of mtDNA translation without affecting the mtDNA copy number [219].
The intracellular levels of TFAM must be finely tuned to prevent the dysregulation of mtDNA transactions that can result in various pathologies (see Section 9; Table 1 and Table S1). Expression of the gene encoding TFAM is transcriptionally controlled by peroxisome proliferator-activated receptor-γ coactivator-1α/nuclear respiratory factor-1 [220,221]. In patients with glioma, KLF16 serves as a key regulator of glioma cell proliferation by binding to the TFAM promoter, leading to the reduction of TFAM expression [222]. TFAM can also be downregulated as a result of hypermethylation of the promoter, which can be induced by cigarette smoke, as in the case of chronic obstructive pulmonary disease [223].
In mouse spermatocytes and elongating spermatids, specific transcripts encode a protein isoform that is imported into the nucleus [224]. Testis-specific isoforms were also found in human germ cells, but they were shown to be transported into the mitochondria [225]. The authors showed that TFAM and mtDNA are present in high levels in undifferentiated male germ cells but in low levels or are absent in differentiated spermatocytes. Testis-specific transcripts exhibited the opposite pattern, suggesting that they may play a role in downregulating mitochondrial biogenesis during spermatogenesis.
In addition, the TFAM mRNA is subject to complex post-transcriptional regulation (Figure 4), including stabilization by RNA-binding proteins [226] and various microRNAs [227,228,229,230]. For example, microRNA-494 (mir494) was shown to downregulate TFAM expression in proliferating myoblasts by binding to 3′-UTR within the mRNA sequence. On the other hand, exercise in mice resulted in decreased expression of mir494 and elevated expression of TFAM accompanied by the stimulation of mitochondrial biogenesis [231], although mir494 was also shown to act upstream of TFAM via a transcriptional coactivator in some cases [232,233]. The TFAM pre-mRNA also undergoes alternative splicing resulting in the production of two major TFAM isoforms: The full-length polypeptide and a version lacking the last 32 amino-acids, which corresponds to most of the second HMG-box [234,235,236]. Both isoforms seem to form the active mitochondrial transcriptional complex [237]. However, the interplay between these two (and other minor putative, tissue-specific) isoforms in vivo has not been studied in detail.
Figure 4.
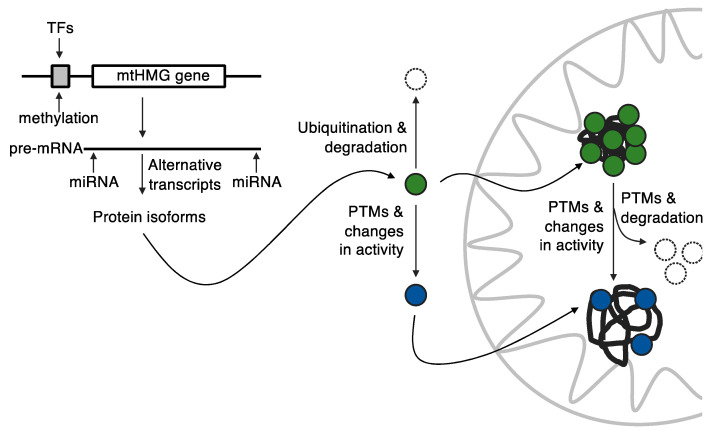
Possible means of regulating mtHMG proteins. The levels of mtHMG proteins can be controlled at the level of transcription by employing specific transcription factors (TFs) as well as by epigenetic markers such as DNA methylation. In the case of TFAM, alternative splicing generates isoforms differing in the number of functional HMG-boxes. Prior to or following their import into mitochondria, mtHMG proteins can undergo various post-translational modifications (PTMs) that can affect their susceptibility to proteolytic degradation, propensity to interact with protein partners (not shown), and DNA-binding activity. As a result, mitochondrial DNA (mtDNA) can be either tightly compacted or more relaxed and thus more accessible to the components of the replication, transcription, recombination, and translation machineries. See text for more details.
In addition to regulation at the levels of gene expression, translation and functional substitution, post-translational modification (PTM) also plays an important role by providing an extra layer of flexibility for protein regulation according to actual need (Figure 4). In eukaryotes, the most studied and the most influential of these proteins are histones, which can be acetylated, methylated or ubiquitinylated and thus influence nuclear DNA replication, chromatin stability, the degree of DNA compaction and the level of DNA transcription [238,239]. Not so widely studied but still fairly common are PTMs in prokaryotes [240,241,242,243]. Mass spectrometry data from 11 independent proteomic studies revealed over 100 unique PTMs in the four most abundant E. coli nucleoid-associated proteins (HU, H-NS, IHF, and FIS) emphasizing the potential impact of these modifications on their in vivo functions [244]. It is worth noting, however, that a large proportion of the PTMs identified in this study were acetylated lysines. Only a few protein-modifying enzymes have been discovered in bacteria, therefore it seems likely that most of this acetylation occurs non-enzymatically [244].
To date, several PTMs have been described for mitochondrial HMG-box proteins. For S. cerevisiae Abf2, it was found that the N-terminal extended segment of the first HMG-box is phosphorylated by a cAMP-dependent protein kinase (PKA) in vitro resulting in a decrease in its DNA-binding activity [190]. An Abf2 mutant lacking the corresponding phosphorylation sites exhibited a severe defect in the regulation of mtDNA content during glucose repression in vivo. It was shown that the first HMG-box of TFAM is also a substrate for PKA in human mitochondria and that the modified protein has a decreased binding to DNA as well as reduced transcriptional activity [245]. Another TFAM phosphorylation site, found in the second HMG-box, is modified by the extracellular signal-regulated protein kinase ERK1/2. A phosphorylation-mimicking mutant showed a reduced affinity for the LSP, although binding to non-specific mtDNA sequences was not affected [246].
PTMs could regulate mtHMG proteins at the levels of protein–protein interactions, substrate binding, and stability. Moreover, it has been shown that phosphorylated TFAM is a substrate for the ATP-dependent protease Lon [245]. S. cerevisiae Abf2 was also demonstrated to be subject to Lon-mediated degradation. When bound to DNA, TFAM and Abf2 are protected from Lon-mediated proteolytic degradation thus providing a possible mechanism for adjusting the stoichiometry of mtHMG proteins to the available mtDNA substrate [247]. TFAM was shown to be phosphorylated in cells treated with rapamycin, although its effect on TFAM DNA-binding activity was not tested [248]. Therefore, the potential involvement of Abf2 and TFAM in TOR signaling is currently unclear.
In addition to phosphorylation, other post-translational modifications can alter the DNA-binding properties of mtHMG-box proteins. For example, mammalian TFAM was shown to be acetylated in vivo [249] and later in vitro studies demonstrated that acetylated TFAM has a significantly lower dsDNA-binding affinity [250]. In contrast, a more recent study showed that acetylation significantly decreased the DNA-unwinding ability of TFAM, while its DNA-binding ability was largely unaffected [251]. It is possible that different combinations of acetylated lysines have different effects on the biochemical properties of TFAM. In clear cell renal cell carcinoma, the ability of TFAM to interact with mtDNA is impaired when it is acetylated at Lys-154 and therefore the protein deacetylase SIRT3 regulates the TFAM function and mitochondrial biogenesis [252]. Additionally, acetylated Abf2 [253] and succinylated forms of both Abf2 and TFAM have been detected in vivo (in HeLa cells for TFAM) [254], although the biological significance of these modifications has not been assessed.
Recently, it was demonstrated that phosphorylation and acetylation have different effects on the kinetics of TFAM binding to DNA [250]. In general, phosphorylation introduces a negative charge and adds steric bulk, which likely causes an electrostatic repulsion with the phosphate backbone of DNA. On the other hand, acetylation neutralizes the positive charges of lysine side-chains and adds a similar steric bulk. The authors proposed that lysine acetylation reduces DNA-binding due to a lower on-rate, whereas the serine phosphorylation results in a decreased on-rate and an increased off-rate [250]. Another interesting finding is that the PTMs of eukaryotic mitochondrial proteins may also occur outside of the mitochondria prior to their import into the organelle. For example, TFAM may undergo ubiquitination [255,256,257], which, in the retina cells of diabetic patients, prevents its entry into the mitochondria and causes retinal cell dysfunction and subsequent sight loss [258]. Furthermore, TFAM can be modulated by O-linked β-N-acetylglucosamine glycosylation [259], although its effect on the activities of TFAM remains unclear. It is likely that TFAM and other mtHMG proteins undergo additional modifications to provide means for the epigenetic regulation of mtDNA maintenance [38] (Figure 4).
9. Involvement of TFAM in Cellular Pathologies
Considering the roles of TFAM, it is not surprising that defects in its functions result in pathologies collectively named mitochondrial DNA maintenance defects (MDMDs). These represent a group of diseases caused by pathogenic variants in several nuclear genes involved in mtDNA maintenance [260]. These pathologies can be caused by impaired mtDNA synthesis leading to qualitative (mtDNA mutations) and quantitative (mtDNA depletion [261]) defects in mtDNA (Table 1 and Table S1).
Table 1.
Examples of human diseases whose pathologies involve TFAM.
| Disease | Model | Variant | mRNA | Protein | mtDNA | Ref. |
|---|---|---|---|---|---|---|
| Mitochondrial DNA depletion syndrome 15 | patient | +/+ Pro178Leu | ↑ in primary fibroblasts |
↓ in primary fibroblasts |
↓ copies in liver and skeletal muscle |
[266] |
| Extrahepatic cholestasis | cell line rat, patient |
gene disruption | ↓ in liver |
(↑)↓ in liver |
↓ copies mtDNA deletions in liver |
[267,268,269] |
| Cardiomyopathy | mouse | KO in germline | ↓ | ↓ | ↓copy in +/−depletion in +/+ |
[130] |
| Cardiomyopathy | mouse | KO in heart |
– | ↓ in heart |
↓ transcripts in heart |
[135,138,264] |
| Myopathy | mouse | KO in skeletal muscle |
– | ↓ in muscle |
↓ copies ↓ transcripts depletion in muscle |
[138,139] |
| Infantile mitochondrial myopathy | cell line patient |
– | ↑ 1 ↓ 3 |
↓ |
depletion 1,3 skeletal muscle |
[270] |
| Carotid artery injury | rat | KD | ↑after injury ↓ KD |
↑ after injury ↓ KD |
↑ mass after injury depletion KD |
[271] |
| Chronic obstructive pulmonary disease (with squamous cell lung cancer) |
cell line patient |
hyper-methylation of promoter | ↓ in lungs 3 |
↓ in lungs, skeletal muscle 3 |
– | [223,272,273] |
| Gastric cancer | cell line | KD | ↓ | ↓ | ↓ copies | [274] |
| Colorectal cancer with microsatellite instability | cell line patient |
Leu149Stop frameshift | – | ↓ | ↓ copies | [275] |
| Colon cancer | mouse | KO +/− |
– | ↓ in intestinal tissue 2 |
depletion ↓ copy ↓mass |
[262] |
| Colon cancer | cell line patient |
↑ miRNA-590-3p |
↑ | ↑ | – | [229] |
| Colon cancer | cell line patient |
↓ miRNA-214 |
↑ | ↑ | – | [276] |
| Epidermoid cancer/ Colon adenocarcinoma |
cell line patient |
chemo-therapeutics treatment | ↓↑ | ↓↑ | – | [189,277] |
| Bladder cancer | cell line | ↓ miRNA-590-3p |
– | ↑ | – | [230] |
| Clear cell renal cell carcinoma | cell line patient |
KD 1 | ↓ 1 | ↓ 1 | ↓ copies | [278] |
| Clear cell renal cell carcinoma | cell line | OE SIRT3 | ↑ | ↑ ↓K154 acetylation |
↑biogenesis | [252] |
| Non-small cell lung cancer | cell line patient |
KD 1 | ↓ 1 ↑ 3 |
↓ 1 ↑ 3 |
↓ copies 1 ↑ copies 3 |
[279] |
| Lung adenocarcinoma/ Breast cancer |
cell line | – | ↑in lactic acidosis in long term estradiol treatment |
– | ↑ copies ↑ mass in lactic acidosis in long term estradiol treatment |
[280,281] |
| Lung adenocarcinoma | cell line | KD, α-irradiation (α-IR) |
– | ↑ in α-IR |
↑ copies in α-IR ≈ copies KD in α-IR |
[282] |
| Lung adenocarcinoma | cell line patient |
– | – | ↓ 1 |
↓ biogenesis ↓ volume |
[283] |
| Glioma | cell line patient |
↓ miRNA-23b |
↑ correlation with malignancy |
↑ correlation with malignancy |
– | [227,284] |
| Glioma | cell line patient |
interaction with KLF16 | ↓ in KLF16 OE |
↓ in KLF16 OE |
– | [222] |
| Glioma | cell line | melatonin treatment | ↓ | ↓ | ↓ transcripts ≈ copies |
[285] |
| Diffusely infiltrating astrocytoma (a type of glioma) | patient | – | ↑ correlation with malignancy |
– | ↓ copies correlation with malignancy |
[286] |
| Arsenic-induced Bowen’s disease | cell line patient |
arsenic exposure 1,3 RNA interference 1 |
↑ | ↑ | ↑ copies | [287] |
| Melanoma | cell line patient |
– | ↓↑ | – | mutations | [288] |
| Prostate cancer | cell line | – | ↑ in arsenic exposure |
↑ in arsenic exposure |
mtDNA point mutation in arsenic exposure |
[289] |
| Cervical cancer | cell line | miRNA-214 OE |
↓ | ↓ | – | [228] |
| Ovarian cancer | patient | – | – | ↑ in nuclei orrelation with grade |
– | [290] |
| Endometrial cancer (the estrogen-related type I) |
patient | – | – | ↑ correlation with stage |
↑ copies | [291,292] |
| Breast cancer (estrogen-positive type) | cell line patient |
KD 1 | ↑ 3 | ↑ 3 | – | [293,294] |
| Hepatocellular carcinoma | patient | – | – | ↑ | ↓ copies mtDNA deletions |
[295] |
| Esophageal squamous cell carcinoma | cell line patient |
KD 1 | – | ↓ 1 | ↑ copies 3 ↓ copies 1 |
[296,297] |
| Esophageal squamous cell carcinoma | cell line patient |
KD 1 | ↓ 1 | ↓ 1 | ↓ copy correlation with malignancy ↓ copies 1 |
[298] |
↑: Increase; ↓: Decrease; +/+: Homozygous; +/−: Heterozygous; 1: Cell line; 2: Mouse; 3: Patient; KO: Knockout; KD: Knockdown; OE: Overexpression; see Table S1 for more details.
The MDMDs are often accompanied by changes in the cellular levels of TFAM. Pioneering studies in mice demonstrated the importance of TFAM in mtDNA homeostasis (see Section 4) [130]. Furthermore, heterozygous mice lacking one copy of the TFAM gene were more prone to metastasis in an intestinal cancer model [262], and heterozygous cells produced more inflammatory cytokines, most likely due to higher levels of mitochondrial stress signaling [131]. Increased levels of cytokines were shown to participate in chronic inflammation accompanying accelerated aging in T cells with dysfunctional mitochondria caused by TFAM deficiency [263]. Tissue-specific knockout in the skeletal muscle and heart produced some fraction of viable offspring, however, these individuals developed a dilated cardiomyopathy similar to the human Kearns-Sayre syndrome during their life [135,138,264]. Mouse muscles depleted of TFAM exhibited decreased levels of Ca2+ in the sarcoplasmic reticulum and increased levels of Ca2+ in the mitochondria. The latter was explained by an acute stimulation of mitochondrial metabolism, which results in the long-term cell damage observed in these mice [265].
Overexpression of TFAM increases the mtDNA copy number [132] and also causes significant modifications in mtDNA transcription and replication in mt-nucleoids reconstituted in vitro [299] and in cultured human cells [133]. Neurodegenerative model studies showed that the overall mtDNA levels were reduced by ~30% with an even more significant reduction (~50%) of TFAM protein levels. However, in both cell and animal neurodegenerative models (Alzheimer’s, Parkinson’s, and Huntington’s diseases), increasing TFAM levels by mild overexpression or enzyme replacement considerably improved neural function and content [300]. In addition to neuroprotection, a TFAM overexpression-induced increase in mtDNA levels also facilitates a cardioprotection associated with limited mitochondrial oxidative stress or decreased cardiac aging and cardiac remodeling [301,302,303].
The variability in mtDNA copy number among different cancers was reviewed recently by Yuan et al. [304] and is summarized in Table 1. Microsatellite (in)stability was linked to differential mtDNA copy numbers in colorectal cancer [305]. Another example of high variability is in esophageal squamous cell carcinoma. Lin et al. [297] showed an increased copy number in patient samples, while Masuike et al. [298] showed a decreased mtDNA copy number in correlation with malignancy. In some cases (e.g., liver in extrahepatic cholestasis and hepatocellular carcinoma, melanoma cell lines, or prostatic cancer cell lines treated with arsenic), compromised levels of TFAM may result in mtDNA mutations or deletions [267,288,289,295].
TFAM expression does not have the same effect on the prognosis of all types of TFAM-associated cancers. Expression of TFAM at higher levels was associated with longer overall survival time in cases of diffusely infiltrating astrocytomas [286]. Conversely, in cases of epidermoid cancer, normal-small cell lung carcinoma, ovarian cancer, endometrial cancer, and breast cancer patients with TFAM-positive tumors had poorer overall survival [277,279,290,291,293]. Prognosis of disease development was also negatively affected with lower mtDNA copy number in esophageal squamous cell carcinoma and with low expression of KLF16 in glioma cells [222,298].
In addition to pathological states caused by altered levels of wild-type TFAM, there are several examples of dysfunctional mitochondria associated with single-nucleotide substitutions within both the coding and noncoding regions of the TFAM-encoding gene (Table 1 and Table S1). For example, rare variants −91 C→A in 5′-UTR exon 1 and the Ala105Thr missense mutation of TFAM were shown to be involved in the pathogenesis of cardiac hypertrophy [306] and variants rs10826175 (A→G) SNP upstream and rs1937 (G→C) SNP in exon 1 of TFAM were associated with diffuse-type gastric cancer [274]. Genetic variants of TFAM (SNPs rs1049432; rs1937) can have an active role in host response to Mycobacterium leprae, causing leprosy, and affect the risk of infection [135,307]. Pro-178 within HMG-box 2 is an important residue for intercalating into the DNA minor groove and creating contacts between TFAM and mtDNA [208]. Manifestations of mtDNA depletion syndrome 15 are caused by a Pro178Leu substitution. The phenotypic manifestations identified include decreased mtDNA copy number and basal respiration, decreased number of nucleoids, and the presence of abnormal nucleoid aggregates. Patients exhibited the neonatal onset of rapidly progressive liver failure, resulting in death in infancy. The primary patient fibroblasts showed increased TFAM mRNA but decreased protein levels, indicating a compensatory mechanism [266]. Possibly, mutant TFAM accumulates inside mitochondria and is recognized and degraded by the Lon protease [245].
In a human prostate cancer cell line and in tissue samples of ovarian cancer, TFAM was detected not only in the mitochondria, but also in nuclear chromatin, where it could regulate the expression of nuclear genes in addition to those in the mitochondria [290,308]. This example underlines the pleiotropic effect of TFAM on cellular functions making interpretations of the molecular mechanisms of TFAM-dependent pathologies quite difficult. On the other hand, it makes this small mitochondrial protein and its counterparts in other eukaryotes even more fascinating subjects for future investigation.
In addition to serving as a prognostic marker, TFAM might also be employed as a candidate therapeutic agent. Mitochondrial dysfunction has been reported in various forms of heart failure and TFAM overexpression showed an increased mtDNA copy number and cardioprotective effects against ROS-mediated mitochondrial oxidative stress in transgenic mice [301,309]. A positive effect on survival was observed after myocardial infarction leading to pathological hypertrophy in the case of transgenic mice with TFAM overexpression and even in the cardiac myocytes of a transgenic mouse model with overexpressed recombinant human TFAM [310,311]. TFAM also exhibited therapeutic potential in the reduction of protease expression involved in heart failure [302].
The overexpression of TFAM also has a potentially synergistic effect with exercise training in enhancing mitochondrial function in order to prevent skeletal muscle atrophy [312]. TFAM overexpression also has a positive impact on maintaining the mtDNA copy number before decline in the dorsal root ganglia of experimental rat diabetic neuropathy [313]. This is probably due to its role in the SIRT1-PGC-1α-TFAM signaling pathway, which is impaired in diabetic peripheral neuropathy [314].
A TFAM-mediated “ProtoFection” was employed to deliver mtDNA into the mitochondria of cells representing a model of Parkinson’s disease and resulted in the restoration of mtDNA levels accompanied by the activation of mitochondrial biogenesis [315]. Similarly, recombinant TFAM reduced the level of reactive oxygen species and amyloid β accumulation in a cell model of Alzheimer’s disease [316].
Since some cellular pathologies are caused by an increased level of mitochondrial biogenesis, some interventions are aimed at decreasing the level of TFAM. For example, antisense oligonucleotides were used to decrease the expression of TFAM in injured rat carotid arteries [271] and knockdown of TFAM in gastric cancer cells resulted in decreased cell proliferation [274].
10. Conclusions
The symbiosis that gave rise to modern eukaryotic organisms required fine-tuning the coordination between the genomes of the original host and the endosymbiont. This led to an apparatus for maintaining the mtDNA able to protect, replicate, and express its genetic information in response to intra- and extracellular stimuli. The principal players in the mitochondrial genome maintenance are mtHMG proteins that mediate the compaction of mtDNA molecules into mt-nucleoids. The properties and regulators of mtHMG proteins have been adapted for the unique characteristics of the mitochondrial genomes and are pivotal in these transactions. This review provides a comprehensive analysis of mtHMG proteins highlighting the following key points: (1) The mechanism of DNA compaction by mtHMG proteins is relatively conserved and is primarily mediated by the bending of DNA by tandemly arranged HMG-boxes; (2) with the exception of the HMG-boxes, the amino-acid sequences of the mtHMG proteins exhibit faster evolutionary diversification than the other mt-nucleoid protein components; (3) the activity of the mtHMG proteins (and thus indirectly the accessibility of mtDNA to biochemical transactions) is regulated at all levels of gene expression, including transcription activation/repression, alternative splicing, regulation by miRNAs, posttranslational modifications, and proteolytic cleavage; (4) in many (even strictly aerobic) microorganisms, the deletion of a gene for a mtHMG protein is in part compensated for by employing other molecular means of mtDNA compaction; and (5) compromising the functions of the mammalian mtHMG protein TFAM results in a variety of pathological states whose nature depends on the particular mutation and the affected tissue.
Although research on mtHMG proteins during the last four decades has provided substantial insight into their functions and evolution, there are still important questions that need to be addressed. For example, how does the metabolic state of a cell or modifications to the mtDNA affect the DNA-mtHMG protein interactions? Which additional posttranslational modifications affect mtHMG proteins? What are the effects of these modifications on the structure and stability of mtHMG proteins? Can combining different posttranslational modifications provide the means for producing a heterogeneous intramitochondrial population of mtHMG proteins with distinct DNA- or protein-binding properties? What are the mechanisms of co-evolution of the conserved protein components of the mt-nucleoid and the rapidly evolving mtHMG proteins? How is mtDNA compacted in cells lacking mtHMG proteins? Are there other possibilities for using TFAM as a prognostic tool or a therapeutic target for pharmacological interventions in human diseases with aberrant mtDNA maintenance? Answering these and other related questions will be instrumental for understanding the molecular bases of pathologies linked to the dysfunction of mtHMG proteins and to mitochondria in general.
Acknowledgments
We thank the members of our laboratories for helpful discussions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-273X/10/8/1193/s1. Table S1: Examples of diseases correlating with changes in TFAM levels and/or functions; Figure S1: Amino-acid sequence alignment of HMG-box domains 1 and 2 from yeast (Abf2, Gcf1, Mhb1) and human (TFAM) mtHMG proteins.
Author Contributions
Conceptualization, Ľ.T. and J.N.; investigation, V.V., N.K., J.A.B., J.F., V.K., K.P., V.D., E.K., V.P., J.N. and Ľ.T.; writing—original draft preparation, Ľ.T., N.K., J.A.B., V.K., V.V. and J.N.; writing review and editing, V.V., N.K., J.A.B., J.F., V.K., K.P., V.D., E.K., V.P., J.N. and Ľ.T.; preparation of figures, J.N., K.P., J.A.B. and Ľ.T.; preparation of tables, V.V.; comprehensive checking of the literature, J.F.; English editing, J.A.B; project administration, Ľ.T., J.N., E.K. and V.P.; funding acquisition, Ľ.T., J.N., E.K., V.P. and V.V. All authors have read and agreed to the published version of the manuscript.
Funding
Research in our laboratories is funded by the Slovak Research and Development Agency (APVV) [APVV-15-0022 and APVV-19-0068 (to L.T.), APVV-18-0239 (to J.N.), APVV-15-0375 and APVV-19-0298 (to E.K.)], Scientific Grant Agency of the Ministry of Education, Science, Research, and Sport of the Slovak republic (VEGA) [1/0061/20 (to L.T.), 1/0027/19 (to J.N.), 2/0075/18 (to E.K.)] and the Comenius University in Bratislava [UK/206/2020 (to V.V.)].
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sagan L. On the origin of mitosing cells. J. Theor. Biol. 1967;14:255–274. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 2.Margulis L. Origin of Eukaryotic Cells; Evidence and Research Implications for a Theory of the Origin and Evolution of Microbial, Plant, and Animal Cells on the Precambrian Earth. 1st ed. Yale University Press; New Haven, NH, USA: 1970. [Google Scholar]
- 3.Archibald J.M. One Plus one Equals One: Symbiosis and the Evolution of Complex Life. 1st ed. Oxford University Press; New York, NY, USA: 2016. [Google Scholar]
- 4.Archibald J.M. Endosymbiosis and eukaryotic cell evolution. Curr. Biol. 2015;25:R911–R921. doi: 10.1016/j.cub.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 5.Martijn J., Vosseberg J., Guy L., Offre P., Ettema T.J.G. Deep mitochondrial origin outside the sampled alphaproteobacteria. Nature. 2018;557:101–105. doi: 10.1038/s41586-018-0059-5. [DOI] [PubMed] [Google Scholar]
- 6.Martin W.F., Garg S., Zimorski V. Endosymbiotic theories for eukaryote origin. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140330. doi: 10.1098/rstb.2014.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaremba-Niedzwiedzka K., Caceres E.F., Saw J.H., Bäckström D., Juzokaite L., Vancaester E., Seitz K.W., Anantharaman K., Starnawski P., Kjeldsen K.U., et al. Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature. 2017;541:353–358. doi: 10.1038/nature21031. [DOI] [PubMed] [Google Scholar]
- 8.Eme L., Spang A., Lombard J., Stairs C.W., Ettema T.J.G. Archaea and the origin of eukaryotes. Nat. Rev. Microbiol. 2017;15:711–723. doi: 10.1038/nrmicro.2017.133. [DOI] [PubMed] [Google Scholar]
- 9.Johnston I.G., Williams B.P. Evolutionary inference across eukaryotes identifies specific pressures favoring mitochondrial gene retention. Cell Syst. 2016;2:101–111. doi: 10.1016/j.cels.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Allen J.F. Why chloroplasts and mitochondria retain their own genomes and genetic systems: Colocation for redox regulation of gene expression. Proc. Natl. Acad. Sci. USA. 2015;112:10231–10238. doi: 10.1073/pnas.1500012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labbé K., Murley A., Nunnari J. Determinants and functions of mitochondrial behavior. Annu. Rev. Cell Dev. Biol. 2014;30:357–391. doi: 10.1146/annurev-cellbio-101011-155756. [DOI] [PubMed] [Google Scholar]
- 12.Klecker T., Westermann B. Asymmetric inheritance of mitochondria in yeast. Biol. Chem. 2020;401:779–791. doi: 10.1515/hsz-2019-0439. [DOI] [PubMed] [Google Scholar]
- 13.Friedman J.R., Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendich A.J. Structural analysis of mitochondrial DNA molecules from fungi and plants using moving pictures and pulsed-field gel electrophoresis. J. Mol. Biol. 1996;255:564–588. doi: 10.1006/jmbi.1996.0048. [DOI] [PubMed] [Google Scholar]
- 15.Grimes G.W., Mahler H.R., Perlman P.S. Nuclear gene dosage effects on mitochondrial mass and DNA. J. Cell Biol. 1974;61:565–574. doi: 10.1083/jcb.61.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foury F., Roganti T., Lecrenier N., Purnelle B. The complete sequence of the mitochondrial genome of Saccharomyces cerevisiae. FEBS Lett. 1998;440:325–331. doi: 10.1016/S0014-5793(98)01467-7. [DOI] [PubMed] [Google Scholar]
- 17.Williamson D. The curious history of yeast mitochondrial DNA. Nat. Rev. Genet. 2002;3:475–481. doi: 10.1038/nrg814. [DOI] [PubMed] [Google Scholar]
- 18.Wai T., Ao A., Zhang X., Cyr D., Dufort D., Shoubridge E.A. The role of mitochondrial DNA copy number in mammalian fertility. Biol. Reprod. 2010;83:52–62. doi: 10.1095/biolreprod.109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson S., Bankier A.T., Barrell B.G., De Bruijn M.H.L., Coulson A.R., Drouin J., Eperon I.C., Nierlich D.P., Roe B.A., Sanger F., et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 20.Marquard K.L., Stephens S.M., Jungheim E.S., Ratts V.S., Odem R.R., Lanzendorf S., Moley K.H. Polycystic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil. Steril. 2011;95:2146–2149. doi: 10.1016/j.fertnstert.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Hara R., Tedone E., Ludlow A., Huang E., Arosio B., Mari D., Shay J.W. Quantitative mitochondrial DNA copy number determination using droplet digital PCR with single-cell resolution. Genome Res. 2019;29:1878–1888. doi: 10.1101/gr.250480.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liou C.-W., Lin T.-K., Chen J.-B., Tiao M.-M., Weng S.-W., Chen S.-D., Chuang Y.-C., Chuang J.-H., Wang P.-W. Association between a common mitochondrial DNA D-loop polycytosine variant and alteration of mitochondrial copy number in human peripheral blood cells. J. Med. Genet. 2010;47:723–728. doi: 10.1136/jmg.2010.077552. [DOI] [PubMed] [Google Scholar]
- 23.Miyakawa I. Organization and dynamics of yeast mitochondrial nucleoids. Proc. Japan Acad. Ser. B. 2017;93:339–359. doi: 10.2183/pjab.93.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyakawa I., Sando N., Kawano S., Nakamura S., Kuroiwa T. Isolation of morphologically intact mitochondrial nucleoids from the yeast, Saccharomyces cerevisiae. J. Cell Sci. 1987;88:431–439. doi: 10.1242/jcs.88.4.431. [DOI] [PubMed] [Google Scholar]
- 25.Miyakawa I., Kanayama M., Fujita Y., Sato H. Morphology and protein composition of the mitochondrial nucleoids in yeast cells lacking Abf2p, a high mobility group protein. J. Gen. Appl. Microbiol. 2010;56:455–464. doi: 10.2323/jgam.56.455. [DOI] [PubMed] [Google Scholar]
- 26.Williamson D.H., Fennell D.J. Visualization of yeast mitochondrial DNA with the fluorescent stain “DAPI”. Methods Enzymol. 1979;56:728–733. doi: 10.1016/0076-6879(79)56065-0. [DOI] [PubMed] [Google Scholar]
- 27.Williamson D.H., Fennell D.J. The use of fluorescent DNA-binding agent for detecting and separating yeast mitochondrial DNA. Methods Cell Biol. 1975;12:335–351. doi: 10.1016/S0091-679X(08)60963-2. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman B.A., Newman S.M., Hallberg R.L., Slaughter C.A., Perlman P.S., Butow R.A. In organello formaldehyde crosslinking of proteins to mtDNA: Identification of bifunctional proteins. Proc. Natl. Acad. Sci. USA. 2000;97:7772–7777. doi: 10.1073/pnas.140063197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman B.A., Kolesar J.E., Perlman P.S., Butow R.A. A function for the mitochondrial chaperonin Hsp60 in the structure and transmission of mitochondrial DNA nucleoids in Saccharomyces cerevisiae. J. Cell Biol. 2003;163:457–461. doi: 10.1083/jcb.200306132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto S., Inai T., Miyakawa I. Morphology of mitochondrial nucleoids in respiratory-deficient yeast cells varies depending on the unit length of the mitochondrial DNA sequence. FEMS Yeast Res. 2016;16:fow055. doi: 10.1093/femsyr/fow055. [DOI] [PubMed] [Google Scholar]
- 31.Miyakawa I., Aoi H., Sando N., Kuroiwa T. Fluorescence microscopic studies of mitochondrial nucleoids during meiosis and sporulation in the yeast, Saccharomyces cerevisiae. J. Cell Sci. 1984;66:21–38. doi: 10.1242/jcs.66.1.21. [DOI] [PubMed] [Google Scholar]
- 32.Farge G.É.R., Falkenberg M. Organization of DNA in mammalian mitochondria. Int. J. Mol. Sci. 2019;20:2770. doi: 10.3390/ijms20112770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bogenhagen D.F. Mitochondrial DNA nucleoid structure. Biochim. Biophys. Acta—Gene Regul. Mech. 2012;1819:914–920. doi: 10.1016/j.bbagrm.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Bonekamp N.A., Larsson N.G. SnapShot: Mitochondrial nucleoid. Cell. 2018;172:388. doi: 10.1016/j.cell.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 35.Garrido N., Griparic L., Jokitalo E., Wartiovaara J., Van der Bliek A.M., Spelbrink J.N. Composition and dynamics of human mitochondrial nucleoids. Mol. Biol. Cell. 2003;14:1583–1596. doi: 10.1091/mbc.e02-07-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilkerson R., Bravo L., Garcia I., Gaytan N., Herrera A., Maldonado A., Quintanilla B. The mitochondrial nucleoid: Integrating mitochondrial DNA into cellular homeostasis. Cold Spring Harb. Perspect. Biol. 2013;5:a011080. doi: 10.1101/cshperspect.a011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufman B.A., Durisic N., Mativetsky J.M., Costantino S., Hancock M.A., Grutter P., Shoubridge E.A. The mitochondrial transcription factor TFAM coordinates the assembly of multiple DNA molecules into nucleoid-like structures. Mol. Biol. Cell. 2007;18:3225–3236. doi: 10.1091/mbc.e07-05-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prachař J. Ultrastructure of mitochondrial nucleoid and its surroundings. Gen. Physiol. Biophys. 2016;35:273–286. doi: 10.4149/gpb_2016008. [DOI] [PubMed] [Google Scholar]
- 39.Kukat C., Wurm C.A., Spåhr H., Falkenberg M., Larsson N.G., Jakobs S. Super-resolution microscopy reveals that mammalian mitochondrial nucleoids have a uniform size and frequently contain a single copy of mtDNA. Proc. Natl. Acad. Sci. USA. 2011;108:13534–13539. doi: 10.1073/pnas.1109263108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown T.A., Tkachuk A.N., Shtengel G., Kopek B.G., Bogenhagen D.F., Hess H.F., Clayton D.A. Superresolution fluorescence imaging of mitochondrial nucleoids reveals their spatial range, limits, and membrane interaction. Mol. Cell. Biol. 2011;31:4994–5010. doi: 10.1128/MCB.05694-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bogenhagen D.F., Wang Y., Shen E.L., Kobayashi R. Protein components of mitochondrial DNA nucleoids in higher eukaryotes. Mol. Cell. Proteomics. 2003;2:1205–1216. doi: 10.1074/mcp.M300035-MCP200. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y., Bogenhagen D.F. Human mitochondrial DNA nucleoids are linked to protein folding machinery and metabolic enzymes at the mitochondrial inner membrane. J. Biol. Chem. 2006;281:25791–25802. doi: 10.1074/jbc.M604501200. [DOI] [PubMed] [Google Scholar]
- 43.Bogenhagen D.F., Rousseau D., Burke S. The layered structure of human mitochondrial DNA nucleoids. J. Biol. Chem. 2008;283:3665–3675. doi: 10.1074/jbc.M708444200. [DOI] [PubMed] [Google Scholar]
- 44.Chen X.J., Wang X., Kaufman B.A., Butow R.A. Aconitase couples metabolic regulation to mitochondrial DNA maintenance. Science. 2005;307:714–717. doi: 10.1126/science.1106391. [DOI] [PubMed] [Google Scholar]
- 45.Chen X.J., Butow R.A. The organization and inheritance of the mitochondrial genome. Nat. Rev. Genet. 2005;6:815–825. doi: 10.1038/nrg1708. [DOI] [PubMed] [Google Scholar]
- 46.Miyakawa I., Okamuro A., Kinsky S., Visacka K., Tomaska L., Nosek J. Mitochondrial nucleoids from the yeast Candida parapsilosis: Expansion of the repertoire of proteins associated with mitochondrial DNA. Microbiology. 2009;155:1558–1568. doi: 10.1099/mic.0.027474-0. [DOI] [PubMed] [Google Scholar]
- 47.Nosek J., Tomaska L., Bolotin-Fukuhara M., Miyakawa I. Mitochondrial chromosome structure: An insight from analysis of complete yeast genomes. FEMS Yeast Res. 2006;6:356–370. doi: 10.1111/j.1567-1364.2005.00016.x. [DOI] [PubMed] [Google Scholar]
- 48.Contamine V., Picard M. Maintenance and integrity of the mitochondrial genome: A plethora of nuclear genes in the budding yeast. Microbiol. Mol. Biol. Rev. 2000;64:281–315. doi: 10.1128/MMBR.64.2.281-315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajala N., Hensen F., Wessels H.J.C.T., Ives D., Gloerich J., Spelbrink J.N. Whole cell formaldehyde cross-linking simplifies purification of mitochondrial nucleoids and associated proteins involved in mitochondrial gene expression. PLoS ONE. 2015;10:e0116726. doi: 10.1371/journal.pone.0116726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kucej M., Kucejova B., Subramanian R., Chen X.J., Butow R.A. Mitochondrial nucleoids undergo remodeling in response to metabolic cues. J. Cell Sci. 2008;121:1861–1868. doi: 10.1242/jcs.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S.R., Han J. Mitochondrial nucleoid: Shield and switch of the mitochondrial genome. Oxid. Med. Cell. Longev. 2017;2017:8060949. doi: 10.1155/2017/8060949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meeusen S., Nunnari J. Evidence for a two membrane-spanning autonomous mitochondrial DNA replisome. J. Cell Biol. 2003;163:503–510. doi: 10.1083/jcb.200304040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gustafsson C.M., Falkenberg M., Larsson N.-G. Maintenance and expression of mammalian mitochondrial DNA. Annu. Rev. Biochem. 2016;85:133–160. doi: 10.1146/annurev-biochem-060815-014402. [DOI] [PubMed] [Google Scholar]
- 54.Caron F., Jacq C., Rouviere Yaniv J. Characterization of a histone-like protein extracted from yeast mitochondria. Proc. Natl. Acad. Sci. USA. 1979;76:4265–4269. doi: 10.1073/pnas.76.9.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rouvière-Yaniv J., Gros F. Characterization of a novel, low molecular weight DNA binding protein from Escherichia coli. Proc. Natl. Acad. Sci. USA. 1975;72:3428–3432. doi: 10.1073/pnas.72.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Certa U., Colavito-Shepanski M., Grunstein M. Yeast may not contain histone H1: The only known “histone H1-like” protein in Saccharomyces cerevisiae is a mitochondrial protein. Nucleic Acids Res. 1984;12:7975–7985. doi: 10.1093/nar/12.21.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sommer A. Yeast chromatin: Search for histone H1. Mol. Gen. Genet. 1978;161:323–331. doi: 10.1007/BF00331008. [DOI] [PubMed] [Google Scholar]
- 58.Diffley J.F.X., Stillman B. A close relative of the nuclear, chromosomal high-mobility group protein HMG1 in yeast mitochondria. Proc. Natl. Acad. Sci. USA. 1991;88:7864–7868. doi: 10.1073/pnas.88.17.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diffley J.F.X., Stillman B. Purification of a yeast protein that binds to origins of DNA replication and a transcriptional silencer. Proc. Natl. Acad. Sci. USA. 1988;85:2120–2124. doi: 10.1073/pnas.85.7.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diffley J.F.X., Stillman B. Similarity between the transcriptional silencer binding proteins Abf1 and Rap1. Science. 1989;246:1034–1038. doi: 10.1126/science.2511628. [DOI] [PubMed] [Google Scholar]
- 61.Vizoso-Vázquez Á., Lamas-Maceiras M., Becerra M., González-Siso M.I., Rodríguez-Belmonte E., Cerdán M.E. Ixr1p and the control of the Saccharomyces cerevisiae hypoxic response. Appl. Microbiol. Biotechnol. 2012;94:173–184. doi: 10.1007/s00253-011-3785-2. [DOI] [PubMed] [Google Scholar]
- 62.Brown S.J., Kellett P.J., Lippard S.J. Ixr1, a yeast protein that binds to platinated DNA and confers sensitivity to cisplatin. Science. 1993;261:603–605. doi: 10.1126/science.8342024. [DOI] [PubMed] [Google Scholar]
- 63.Byrne K.P., Wolfe K.H. The Yeast Gene Order Browser: Combining curated homology and syntenic context reveals gene fate in polyploid species. Genome Res. 2005;15:1456–1461. doi: 10.1101/gr.3672305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pierro P., Capaccio L., Gadaleta G. The 25 kDa protein recognizing the rat curved region upstream of the origin of the L-strand replication is the rat homologue of the human mitochondrial transcription factor A. FEBS Lett. 1999;457:307–310. doi: 10.1016/S0014-5793(99)01055-8. [DOI] [PubMed] [Google Scholar]
- 65.Alami-Ouahabi N., Veilleux S., Meistrich M.L., Boissonneault G. The testis-specific high-mobility-group protein, a phosphorylation-dependent DNA-packaging factor of elongating and condensing spermatids. Mol. Cell. Biol. 1996;16:3720–3729. doi: 10.1128/MCB.16.7.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kao L.R., Megraw T.L., Chae C.B. Essential role of the HMG domain in the function of yeast mitochondrial histone HM: Functional complementation of HM by the nuclear nonhistone protein NHP6A. Proc. Natl. Acad. Sci. USA. 1993;90:5598–5602. doi: 10.1073/pnas.90.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baxevanis A.D., Landsman D. The HMG-1 box protein family: Classification and functional relationships. Nucleic Acids Res. 1995;23:1604–1613. doi: 10.1093/nar/23.9.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bustin M. Revised nomenclature for high mobility group (HMG) chromosomal proteins. Trends Biochem. Sci. 2001;26:152–153. doi: 10.1016/S0968-0004(00)01777-1. [DOI] [PubMed] [Google Scholar]
- 69.Megraw T.L., Chae C.-B. Functional complementarity between the HMG1-like yeast mitochondrial histone HM and the bacterial histone-like protein HU. J. Biol. Chem. 1993;268:12758–12763. [PubMed] [Google Scholar]
- 70.Dillon S.C., Dorman C.J. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol. 2010;8:185–195. doi: 10.1038/nrmicro2261. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi T., Takahara M., Miyagishima S.Y., Kuroiwa H., Sasaki N., Ohta N., Matsuzaki M., Kuroiwa T. Detection and localization of a chloroplast-encoded HU-like protein that organizes chloroplast nucleoids. Plant. Cell. 2002;14:1579–1589. doi: 10.1105/tpc.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pastor M.M., Proft M., Pascual-Ahuir A. Mitochondrial function is an inducible determinant of osmotic stress adaptation in yeast. J. Biol. Chem. 2009;284:30307–30317. doi: 10.1074/jbc.M109.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diffley J.F., Stillman B. DNA binding properties of an HMG1-related protein from yeast mitochondria. J. Biol. Chem. 1992;267:3368–3374. [PubMed] [Google Scholar]
- 74.Fisher R.P., Clayton D.A. Purification and characterization of human mitochondrial transcription factor 1. Mol. Cell. Biol. 1988;8:3496–3509. doi: 10.1128/MCB.8.8.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fisher R.P., Parisi M.A., Clayton D.A. Flexible recognition of rapidly evolving promoter sequences by mitochondrial transcription factor 1. Genes Dev. 1989;3:2202–2217. doi: 10.1101/gad.3.12b.2202. [DOI] [PubMed] [Google Scholar]
- 76.Parisi M.A., Clayton D.A. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science. 1991;252:965–969. doi: 10.1126/science.2035027. [DOI] [PubMed] [Google Scholar]
- 77.Fisher R.P., Lisowsky T., Breen G.A.M., Clayton D.A. A rapid, efficient method for purifying DNA-binding proteins. J. Biol. Chem. 1991;266:9153–9160. [PubMed] [Google Scholar]
- 78.Barshad G., Marom S., Cohen T., Mishmar D. Mitochondrial DNA transcription and its regulation: An evolutionary perspective. Trends Genet. 2018;34:682–692. doi: 10.1016/j.tig.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 79.Bouda E., Stapon A., Garcia-Diaz M. Mechanisms of mammalian mitochondrial transcription. Protein Sci. 2019;28:1594–1605. doi: 10.1002/pro.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fisher R.P., Lisowsky T., Parisi M.A., Clayton D.A. DNA wrapping and bending by a mitochondrial high mobility group-like transcriptional activator protein. J. Biol. Chem. 1992;267:3358–3367. [PubMed] [Google Scholar]
- 81.Takamatsu C., Umeda S., Ohsato T., Ohno T., Abe Y., Fukuoh A., Shinagawa H., Hamasaki N., Kang D. Regulation of mitochondrial D-loops by transcription factor A and single-stranded DNA-binding protein. EMBO Rep. 2002;3:451–456. doi: 10.1093/embo-reports/kvf099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kanki T., Ohgaki K., Gaspari M., Gustafsson C.M., Fukuoh A., Sasaki N., Hamasaki N., Kang D. Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol. Cell. Biol. 2004;24:9823–9834. doi: 10.1128/MCB.24.22.9823-9834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell C.T., Kolesar J.E., Kaufman B.A. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta—Gene Regul. Mech. 2012;1819:921–929. doi: 10.1016/j.bbagrm.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 84.Xu B., Clayton D.A. Assignment of a yeast protein necessary for mitochondrial transcription initiation. Nucleic Acids Res. 1992;20:1053–1059. doi: 10.1093/nar/20.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Dyck E., Clayton D.A. Transcription-dependent DNA transactions in the mitochondrial genome of a yeast hypersuppressive petite mutant. Mol. Cell. Biol. 1998;18:2976–2985. doi: 10.1128/MCB.18.5.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dairaghi D.J., Shadel G.S., Clayton D.A. Addition of a 29 residue carboxyl-terminal tail converts a simple HMG box-containing protein into a transcriptional activator. J. Mol. Biol. 1995;249:11–28. doi: 10.1006/jmbi.1995.9889. [DOI] [PubMed] [Google Scholar]
- 87.Goto A., Matsushima Y., Kadowaki T., Kitagawa Y. Drosophila mitochondrial transcription factor A (d-TFAM) is dispensable for the transcription of mitochondrial DNA in Kc167 cells. Biochem. J. 2001;354:243–248. doi: 10.1042/bj3540243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parisi M.A., Xu B., Clayton D.A. A human mitochondrial transcriptional activator can functionally replace a yeast mitochondrial HMG-box protein both in vivo and in vitro. Mol. Cell. Biol. 1993;13:1951–1961. doi: 10.1128/MCB.13.3.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoon Y.G., Koob M.D., Yoo Y.H. Mitochondrial genome-maintaining activity of mouse mitochondrial transcription factor A and its transcript isoform in Saccharomyces cerevisiae. Gene. 2011;484:52–60. doi: 10.1016/j.gene.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoon Y.G. Transfer of xenomitochondria containing the entire mouse mitochondrial genome into a genetically modified yeast expressing mitochondrial transcription factor A. J. Microbiol. Biotechnol. 2020 doi: 10.4014/jmb.2004.04033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zelenaya-Troitskaya O., Newman S.M., Okamoto K., Perlman P.S., Butow R.A. Functions of the high mobility group protein, Abf2p, in mitochondrial DNA segregation, recombination and copy number in Saccharomyces cerevisiae. Genetics. 1998;148:1763–1776. doi: 10.1093/genetics/148.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blank H.M., Li C., Mueller J.E., Bogomolnaya L.M., Bryk M., Polymenis M. An increase in mitochondrial DNA promotes nuclear DNA replication in yeast. PLoS Genet. 2008;4:e1000047. doi: 10.1371/annotation/89355dbd-390e-463f-b804-c6cf1296402e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taylor S.D., Zhang H., Eaton J.S., Rodeheffer M.S., Lebedeva M.A., O’Rourke T.W., Siede W., Shadel G.S. The conserved Mec1/Rad53 nuclear checkpoint pathway regulates mitochondrial DNA copy number in Saccharomyces cerevisiae. Mol. Biol. Cell. 2005;16:3010–3018. doi: 10.1091/mbc.e05-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lebedeva M.A., Shadel G.S. Cell cycle- and ribonucleotide reductase-driven changes in mtDNA copy number influence mtDNA inheritance without compromising mitochondrial gene expression. Cell Cycle. 2007;6:2048–2057. doi: 10.4161/cc.6.16.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsaponina O., Barsoum E., Åström S.U., Chabes A. Ixr1 is required for the expression of the ribonucleotide reductase Rnr1 and maintenance of dNTP pools. PLoS Genet. 2011;7:e1002061. doi: 10.1371/journal.pgen.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bakkaiová J., Marini V., Willcox S., Nosek J., Griffith J.D., Krejci L., Tomáška Ľ. Yeast mitochondrial HMG proteins: DNA-binding properties of the most evolutionarily divergent component of mitochondrial nucleoids. Biosci. Rep. 2016;36:e00288. doi: 10.1042/BSR20150275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Samoilova E.O., Krasheninnikov I.A., Levitskii S.A. Interaction between Saccharomyces cerevisiae mitochondrial DNA-binding protein Abf2p and Cce1p resolvase. Biochem. 2016;81:1111–1117. doi: 10.1134/S0006297916100096. [DOI] [PubMed] [Google Scholar]
- 98.Ling F., Bradshaw E., Yoshida M. Prevention of mitochondrial genomic instability in yeast by the mitochondrial recombinase Mhr1. Sci. Rep. 2019;9:1–2. doi: 10.1038/s41598-019-41699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lockshon D., Zweifel S.G., Freeman-Cook L.L., Lorimer H.E., Brewer B.J., Fangman W.L. A role for recombination junctions in the segregation of mitochondrial DNA in yeast. Cell. 1995;81:947–955. doi: 10.1016/0092-8674(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 100.Gerhold J.M., Aun A., Sedman T., Jõers P., Sedman J. Strand invasion structures in the inverted repeat of Candida albicans mitochondrial DNA reveal a role for homologous recombination in replication. Mol. Cell. 2010;39:851–861. doi: 10.1016/j.molcel.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Gerhold J.M., Sedman T., Visacka K., Slezakova J., Tomaska L., Nosek J., Sedman J. Replication intermediates of the linear mitochondrial DNA of Candida parapsilosis suggest a common recombination based mechanism for yeast mitochondria. J. Biol. Chem. 2014;289:22659–22670. doi: 10.1074/jbc.M114.552828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bendich A.J. The end of the circle for yeast mitochondrial DNA. Mol. Cell. 2010;39:831–832. doi: 10.1016/j.molcel.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 103.Bendich A.J. Reaching for the ring: The study of mitochondrial genome structure. Curr. Genet. 1993;24:279–290. doi: 10.1007/BF00336777. [DOI] [PubMed] [Google Scholar]
- 104.MacAlpine D.M., Perlman P.S., Butow R.A. The high mobility group protein Abf2p influences the level of yeast mitochondrial DNA recombination intermediates in vivo. Proc. Natl. Acad. Sci. USA. 1998;95:6739–6743. doi: 10.1073/pnas.95.12.6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Okamoto K., Perlman P.S., Butow R.A. The sorting of mitochondrial DNA and mitochondrial proteins in zygotes: Preferential transmission of mitochondrial DNA to the medial bud. J. Cell Biol. 1998;142:613–623. doi: 10.1083/jcb.142.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Newman S.M., Zelenaya-Troitskaya O., Perlman P.S., Butow R.A. Analysis of mitochondrial DNA nucleoids in wild-type and a mutant strain of Saccharomyces cerevisiae that lacks the mitochondrial HMG box protein Abf2p. Nucleic Acids Res. 1996;24:386–393. doi: 10.1093/nar/24.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zelenaya-Troitskaya O., Perlman P.S., Butow R.A. An enzyme in yeast mitochondria that catalyzes a step in branched-chain amino acid biosynthesis also functions in mitochondrial DNA stability. EMBO J. 1995;14:3268–3276. doi: 10.1002/j.1460-2075.1995.tb07330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.MacAlpine D.M., Perlman P.S., Butow R.A. The numbers of individual mitochondrial DNA molecules and mitochondrial DNA nucleoids in yeast are co-regulated by the general amino acid control pathway. EMBO J. 2000;19:767–775. doi: 10.1093/emboj/19.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bateman J.M., Perlman P.S., Butow R.A. Mutational bisection of the mitochondrial DNA stability and amino acid biosynthetic functions of Ilv5p of budding yeast. Genetics. 2002;161:1043–1052. doi: 10.1093/genetics/161.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Macierzanka M., Plotka M., Pryputniewicz-Drobinska D., Lewandowska A., Lightowlers R., Marszalek J. Maintenance and stabilization of mtDNA can be facilitated by the DNA-binding activity of Ilv5p. Biochim. Biophys. Acta—Mol. Cell Res. 2008;1783:107–117. doi: 10.1016/j.bbamcr.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 111.Huangyang P., Simon M.C. Hidden features: Exploring the non-canonical functions of metabolic enzymes. DMM Dis. Model. Mech. 2018;11:dmm033365. doi: 10.1242/dmm.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gancedo C., Flores C.-L. Moonlighting proteins in yeasts. Microbiol. Mol. Biol. Rev. 2008;72:197–210. doi: 10.1128/MMBR.00036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Megraw T.L., Kao L.R., Chae C.B. The mitochondrial histone HM: An evolutionary link between bacterial HU and nuclear HMG1 proteins. Biochimie. 1994;76:909–916. doi: 10.1016/0300-9084(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 114.Kao L.R., Megraw T.L., Chae C.B. SHM1: A multicopy suppressor of a temperature-sensitive null mutation in the HMG1-like abf2 gene. Yeast. 1996;12:1239–1250. doi: 10.1002/(SICI)1097-0061(19960930)12:12<1239::AID-YEA17>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 115.Vozza A., Blanco E., Palmieri L., Palmieri F. Identification of the mitochondrial GTP/GDP transporter in Saccharomyces cerevisiae. J. Biol. Chem. 2004;279:20850–20857. doi: 10.1074/jbc.M313610200. [DOI] [PubMed] [Google Scholar]
- 116.Castegna A., Scarcia P., Agrimi G., Palmieri L., Rottensteiner H., Spera I., Germinario L., Palmieri F. Identification and functional characterization of a novel mitochondrial carrier for citrate and oxoglutarate in Saccharomyces cerevisiae. J. Biol. Chem. 2010;285:17359–17370. doi: 10.1074/jbc.M109.097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cho J.H., Ha S.J., Kao L.R., Megraw T.L., Chae C.-B. A novel DNA-binding protein bound to the mitochondrial inner membrane restores the null mutation of mitochondrial histone Abf2p in Saccharomyces cerevisiae. Mol. Cell. Biol. 1998;18:5712–5723. doi: 10.1128/MCB.18.10.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Iacovino M., Granycome C., Sembongi H., Bokori-Brown M., Butow R.A., Holt I.J., Bateman J.M. The conserved translocase Tim17 prevents mitochondrial DNA loss. Hum. Mol. Genet. 2009;18:65–74. doi: 10.1093/hmg/ddn313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matta S.K., Pareek G., Bankapalli K., Oblesha A., D’Silva P. Role of Tim17 transmembrane regions in regulating the architecture of presequence translocase and mitochondrial DNA stability. Mol. Cell. Biol. 2017;37:e00491-16. doi: 10.1128/MCB.00491-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheng X., Ivessa A.S. Association of the yeast DNA helicase Pif1p with mitochondrial membranes and mitochondrial DNA. Eur. J. Cell Biol. 2010;89:742–747. doi: 10.1016/j.ejcb.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 121.Wai T., Teoli D., Shoubridge E.A. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat. Genet. 2008;40:1484–1488. doi: 10.1038/ng.258. [DOI] [PubMed] [Google Scholar]
- 122.DeRisi J.L., Iyer V.R., Brown P.O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 123.Chen X.J., Wang X., Butow R.A. Yeast aconitase binds and provides metabolically coupled protection to mitochondrial DNA. Proc. Natl. Acad. Sci. USA. 2007;104:13738–13743. doi: 10.1073/pnas.0703078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klausner R.D., Rouault T.A. A double life: Cytosolic aconitase as a regulatory RNA binding protein. Mol. Biol. Cell. 1993;4:1–5. doi: 10.1091/mbc.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kennedy M.C., Mende-Mueller L., Blondin G.A., Beinert H. Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc. Natl. Acad. Sci. USA. 1992;89:11730–11734. doi: 10.1073/pnas.89.24.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gangloff S.P., Marguet D., Lauquin G.J. Molecular cloning of the yeast mitochondrial aconitase gene (ACO1) and evidence of a synergistic regulation of expression by glucose plus glutamate. Mol. Cell. Biol. 1990;10:3551–3561. doi: 10.1128/MCB.10.7.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Z., Butow R.A. A transcriptional switch in the expression of yeast tricarboxylic acid cycle genes in response to a reduction or loss of respiratory function. Mol. Cell. Biol. 1999;19:6720–6728. doi: 10.1128/MCB.19.10.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shadel G.S. Mitochondrial DNA, aconitase “wraps” it up. Trends Biochem. Sci. 2005;30:294–296. doi: 10.1016/j.tibs.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 129.Poveda-Huertes D., Matic S., Marada A., Habernig L., Licheva M., Myketin L., Gilsbach R., Tosal-Castano S., Papinski D., Mulica P., et al. An early mtUPR: Redistribution of the nuclear transcription factor Rox1 to mitochondria protects against intramitochondrial proteotoxic aggregates. Mol. Cell. 2020;77:180–188. doi: 10.1016/j.molcel.2019.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Larsson N.G., Wang J., Wilhelmsson H., Oldfors A., Rustin P., Lewandoski M., Barsh G.S., Clayton D.A. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 131.West A.P., Khoury-Hanold W., Staron M., Tal M.C., Pineda C.M., Lang S.M., Bestwick M., Duguay B.A., Raimundo N., MacDuff D.A., et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ekstrand M.I., Falkenberg M., Rantanen A., Park C.B., Gaspari M., Hultenby K., Rustin P., Gustafsson C.M., Ran Larsson N.-G. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- 133.Pohjoismäki J.L.O., Wanrooij S., Hyvärinen A.K., Goffart S., Holt I.J., Spelbrink J.N., Jacobs H.T. Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. Nucleic Acids Res. 2006;34:5815–5828. doi: 10.1093/nar/gkl703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kasashima K., Sumitani M., Endo H. Human mitochondrial transcription factor A is required for the segregation of mitochondrial DNA in cultured cells. Exp. Cell Res. 2011;317:210–220. doi: 10.1016/j.yexcr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 135.Li H., Wang J., Wilhelmsson H., Hansson A., Thorén P., Duffy J., Rustin P., Larsson N.G. Genetic modification of survival in tissue-specific knockout mice with mitochondrial cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2000;97:3467–3472. doi: 10.1073/pnas.97.7.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Silva J.P., Köhler M., Graff C., Oldfors A., Magnuson M.A., Berggren P.O., Larsson N.G. Impaired insulin secretion and β-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat. Genet. 2000;26:336–340. doi: 10.1038/81649. [DOI] [PubMed] [Google Scholar]
- 137.Sörensen L., Ekstrand M., Silva J.P., Lindqvist E., Xu B., Rustin P., Olson L., Larsson N.G. Late-onset corticohippocampal neurodepletion attributable to catastrophic failure of oxidative phosphorylation in MILON mice. J. Neurosci. 2001;21:8082–8090. doi: 10.1523/JNEUROSCI.21-20-08082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang J., Wilhelmsson H., Graff C., Li H., Oldfors A., Rustin P., Brüning J.C., Kahn C.R., Clayton D.A., Barsh G.S., et al. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat. Genet. 1999;21:133–137. doi: 10.1038/5089. [DOI] [PubMed] [Google Scholar]
- 139.Wredenberg A., Wibom R., Wilhelmsson H., Graff C., Wiener H.H., Burden S.J., Oldfors A., Westerblad H., Larsson N.G. Increased mitochondrial mass in mitochondrial myopathy mice. Proc. Natl. Acad. Sci. USA. 2002;99:15066–15071. doi: 10.1073/pnas.232591499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Buschbeck M., Hake S.B. Variants of core histones and their roles in cell fate decisions, development and cancer. Nat. Rev. Mol. Cell Biol. 2017;18:299–314. doi: 10.1038/nrm.2016.166. [DOI] [PubMed] [Google Scholar]
- 141.Dequard-Chablat M., Alland C. Two copies of mtHMG1, encoding a novel mitochondrial HMG-like protein, delay accumulation of mitochondrial DNA deletions in Podospora anserina. Eukaryot. Cell. 2002;1:503–513. doi: 10.1128/EC.1.4.503-513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miyakawa I., Sato H., Maruyama Y., Nakaoka T. Isolation of the mitochondrial nucleoids from yeast Kluyveromyces lactis and analyses of the nucleoid proteins. J. Gen. Appl. Microbiol. 2003;49:85–93. doi: 10.2323/jgam.49.85. [DOI] [PubMed] [Google Scholar]
- 143.Miyakawa I., Yawata K. Purification of an Abf2p-like protein from mitochondrial nucleoids of yeast Pichia jadinii and its role in the packaging of mitochondrial DNA. Antonie van Leeuwenhoek, Int. J. Gen. Mol. Microbiol. 2007;91:197–207. doi: 10.1007/s10482-006-9105-7. [DOI] [PubMed] [Google Scholar]
- 144.Višacká K., Gerhold J.M., Petrovičová J., Kinský S., Jõers P., Nosek J., Sedman J., Tomáška Ľ. Novel subfamily of mitochondrial HMG box-containing proteins: Functional analysis of Gcf1p from Candida albicans. Microbiology. 2009;155:1226–1240. doi: 10.1099/mic.0.025759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bakkaiova J., Arata K., Matsunobu M., Ono B., Aoki T., Lajdova D., Nebohacova M., Nosek J., Miyakawa I., Tomaska L. The strictly aerobic yeast Yarrowia lipolytica tolerates loss of a mitochondrial DNA-packaging protein. Eukaryot. Cell. 2014;13:1143–1157. doi: 10.1128/EC.00092-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miyakawa I., Kitamura Y., Jyozaki T., Sato H., Umezaki T. Simple detection of a yeast mitochondrial DNA-binding protein, Abf2p, on SDS-DNA gels. J. Gen. Appl. Microbiol. 2000;46:311–316. doi: 10.2323/jgam.46.311. [DOI] [PubMed] [Google Scholar]
- 147.Umezaki T., Miyakawa I. Use of SDS-DNA PAGE for detection of mitochondrial Abf2p-like proteins and mitochondrial nuclease in Saccharomyces yeasts and Arxiozyma telluris. Cytologia (Tokyo) 2002;67:423–428. doi: 10.1508/cytologia.67.423. [DOI] [Google Scholar]
- 148.Letunic I., Bork P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2017;46:493–496. doi: 10.1093/nar/gkx922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nosek J., Dinouël N., Kovac L., Fukuhara H. Linear mitochondrial DNAs from yeasts: Telomeres with large tandem repetitions. Mol. Gen. Genet. 1995;247:61–72. doi: 10.1007/BF00425822. [DOI] [PubMed] [Google Scholar]
- 151.Tomáška Ľ., Nosek J., Makhov A.M., Pastoráková A., Griffith J.D. Extragenomic double-stranded DNA circles in yeast with linear mitochondrial genomes: Potential involvement in telomere maintenance. Nucleic Acids Res. 2000;28:4479–4487. doi: 10.1093/nar/28.22.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nosek J., Ryčovská A., Makhov A.M., Griffith J.D., Tomáška Ľ. Amplification of telomeric arrays via rolling-circle mechanism. J. Biol. Chem. 2005;280:10840–10845. doi: 10.1074/jbc.M409295200. [DOI] [PubMed] [Google Scholar]
- 153.Christen S., Sauer U. Intracellular characterization of aerobic glucose metabolism in seven yeast species by 13C flux analysis and metabolomics. FEMS Yeast Res. 2011;11:263–272. doi: 10.1111/j.1567-1364.2010.00713.x. [DOI] [PubMed] [Google Scholar]
- 154.Goldring E.S., Grossman L.I., Krupnick D., Cryer D.R., Marmur J. The petite mutation in yeast. Loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J. Mol. Biol. 1970;52:323–335. doi: 10.1016/0022-2836(70)90033-1. [DOI] [PubMed] [Google Scholar]
- 155.Perlman P.S., Mahler H.R. Molecular consequences of ethidium bromide mutagenesis. Nat. New Biol. 1971;231:12–16. doi: 10.1016/0006-291X(71)90152-5. [DOI] [PubMed] [Google Scholar]
- 156.Richter-Dennerlein R., Oeljeklaus S., Lorenzi I., Ronsör C., Bareth B., Schendzielorz A.B., Wang C., Warscheid B., Rehling P., Dennerlein S. Mitochondrial protein synthesis adapts to influx of nuclear-encoded protein. Cell. 2016;167:471–483. doi: 10.1016/j.cell.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Couvillion M.T., Soto I.C., Shipkovenska G., Churchman L.S. Synchronized mitochondrial and cytosolic translation programs. Nature. 2016;533:499–503. doi: 10.1038/nature18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Suhm T., Kaimal J.M., Dawitz H., Peselj C., Masser A.E., Hanzén S., Ambrožič M., Smialowska A., Björck M.L., Brzezinski P., et al. Mitochondrial translation efficiency controls cytoplasmic protein homeostasis. Cell Metab. 2018;27:1309–1322. doi: 10.1016/j.cmet.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 159.Fleck O., Kunz C., Rudolph C., Kohli J. The high mobility group domain protein Cmb1 of Schizosaccharomyces pombe binds to cytosines in base mismatches and opposite chemically altered guanines. J. Biol. Chem. 1998;273:30398–30405. doi: 10.1074/jbc.273.46.30398. [DOI] [PubMed] [Google Scholar]
- 160.Matsuyama A., Arai R., Yashiroda Y., Shirai A., Kamata A., Sekido S., Kobayashi Y., Hashimoto A., Hamamoto M., Hiraoka Y., et al. ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 2006;24:841–847. doi: 10.1038/nbt1222. [DOI] [PubMed] [Google Scholar]
- 161.Horton P., Park K.-J., Obayashi T., Fujita N., Harada H., Adams-Collier C.J., Nakai K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007;35:585–587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Claros M.G., Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 1996;241:779–786. doi: 10.1111/j.1432-1033.1996.00779.x. [DOI] [PubMed] [Google Scholar]
- 163.Reeves R., Nissen M.S. The AT-DNA-binding domain of mammalian high mobility group I chromosomal proteins. J. Biol. Chem. 1990;265:8573–8658. [PubMed] [Google Scholar]
- 164.Karácsony Z., Gácser A., Vágvölgyi C., Scazzocchio C., Hamari Z. A dually located multi-HMG-box protein of Aspergillus nidulans has a crucial role in conidial and ascospore germination. Mol. Microbiol. 2014;94:383–402. doi: 10.1111/mmi.12772. [DOI] [PubMed] [Google Scholar]
- 165.Karácsony Z., Gácser A., Vágvölgyi C., Hamari Z. Further characterization of the role of the mitochondrial high-mobility group box protein in the intracellular redox environment of Aspergillus nidulans. Microbiology (United Kingdom) 2015;161:1897–1908. doi: 10.1099/mic.0.000139. [DOI] [PubMed] [Google Scholar]
- 166.Sasaki N., Kuroiwa H., Nishitani C., Takano H., Higashiyama T., Kobayashi T., Shirai Y., Sakai A., Kawano S., Murakami-Murofushi K., et al. Glom is a novel mitochondrial DNA packaging protein in Physarum polycephalum and causes intense chromatin condensation without suppressing DNA functions. Mol. Biol. Cell. 2003;14:4758–4769. doi: 10.1091/mbc.e03-02-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Itoh K., Izumi A., Mori T., Dohmae N., Yui R., Maeda-Sano K., Shirai Y., Kanaoka M.M., Kuroiwa T., Higashiyama T., et al. DNA packaging proteins Glom and Glom2 coordinately organize the mitochondrial nucleoid of Physarum polycephalum. Mitochondrion. 2011;11:575–586. doi: 10.1016/j.mito.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 168.Jensen R.E., Englund P.T. Network news: The replication of kinetoplast DNA. Annu. Rev. Microbiol. 2012;66:473–491. doi: 10.1146/annurev-micro-092611-150057. [DOI] [PubMed] [Google Scholar]
- 169.Xu C., Ray D.S. Isolation of proteins associated with kinetoplast DNA networks in vivo. Proc. Natl. Acad. Sci. USA. 1993;90:1786–1789. doi: 10.1073/pnas.90.5.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu C.W., Hines J.C., Engel M.L., Russell D.G., Ray D.S. Nucleus-encoded histone H1-like proteins are associated with kinetoplast DNA in the trypanosomatid Crithidia fasciculata. Mol. Cell. Biol. 1996;16:564–576. doi: 10.1128/MCB.16.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Avliyakulov N.K., Lukeš J., Ray D.S. Mitochondrial histone-like DNA-binding proteins are essential for normal cell growth and mitochondrial function in Crithidia fasciculata. Eukaryot. Cell. 2004;3:518–526. doi: 10.1128/EC.3.2.518-526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang J., Pappas-Brown V., Englund P.T., Jensen R.E. TbKAP6, a mitochondrial HMG box-containing protein in Trypanosoma brucei, Is the first trypanosomatid kinetoplast-associated protein essential for kinetoplast DNA replication and maintenance. Eukaryot. Cell. 2014;13:919–932. doi: 10.1128/EC.00260-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rajakumara E., Satish M., Abhishek S. In vitro studies on non-canonical DNA binding specificities of KAP6 and HMO1 and mechanistic insights into DNA bound and unbinding dynamics of KAP6. Int. J. Biol. Macromol. 2020 doi: 10.1016/j.ijbiomac.2020.05.228. [DOI] [PubMed] [Google Scholar]
- 174.Fey J., Vermel M., Grienenberger J.M., Maréchal-Drouard L., Gualberto J.M. Characterization of a plant mitochondrial active chromosome. FEBS Lett. 1999;458:124–128. doi: 10.1016/S0014-5793(99)01140-0. [DOI] [PubMed] [Google Scholar]
- 175.Dai H., Lo Y.-S., Litvinchuk A., Wang Y.-T., Jane W.-N., Hsiao L.-J., Chiang K.-S. Structural and functional characterizations of mung bean mitochondrial nucleoids. Nucleic Acids Res. 2005;33:4725–4739. doi: 10.1093/nar/gki783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sakai A., Takano H., Kuroiwa T. Organelle nuclei in higher plants: Structure, composition, function, and evolution. Int. Rev. Cytol. 2004;238:59–118. doi: 10.1016/S0074-7696(04)38002-2. [DOI] [PubMed] [Google Scholar]
- 177.Gualberto J.M., Kühn K. DNA-binding proteins in plant mitochondria: Implications for transcription. Mitochondrion. 2014;19:323–328. doi: 10.1016/j.mito.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 178.Tomáška Ľ., Nosek J. Co-evolution in the jungle: From leafcutter ant colonies to chromosomal ends. J. Mol. Evol. 2020;88:293–318. doi: 10.1007/s00239-020-09935-3. [DOI] [PubMed] [Google Scholar]
- 179.Tomáška Ľ., Nosek J., Kramara J., Griffith J.D. Telomeric circles: Universal players in telomere maintenance? Nat. Struct. Mol. Biol. 2009;16:1010–1015. doi: 10.1038/nsmb.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ohno T., Umeda S., Hamasaki N., Kang D. Binding of human mitochondrial transcription factor A, an HMG box protein, to a four-way DNA junction. Biochem. Biophys. Res. Commun. 2000;271:492–498. doi: 10.1006/bbrc.2000.2656. [DOI] [PubMed] [Google Scholar]
- 181.Chen X.J. Mechanism of homologous recombination and implications for aging-related deletions in mitochondrial DNA. Microbiol. Mol. Biol. Rev. 2013;77:476–496. doi: 10.1128/MMBR.00007-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pohjoismäki J.L.O., Goffart S., Tyynismaa H., Willcox S., Ide T., Kang D., Suomalainen A., Karhunen P.J., Griffith J.D., Holt I.J., et al. Human heart mitochondrial DNA is organized in complex catenated networks containing abundant four-way junctions and replication forks. J. Biol. Chem. 2009;284:21446–21457. doi: 10.1074/jbc.M109.016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.O’Rourke T.W., Doudican N.A., Mackereth M.D., Doetsch P.W., Shadel G.S. Mitochondrial dysfunction due to oxidative mitochondrial DNA damage is reduced through cooperative actions of diverse proteins. Mol. Cell. Biol. 2002;22:4086–4093. doi: 10.1128/MCB.22.12.4086-4093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schroeder E.A., Shadel G.S. Crosstalk between mitochondrial stress signals regulates yeast chronological lifespan. Mech. Ageing Dev. 2014;135:41–49. doi: 10.1016/j.mad.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sia R.A., Carrol S., Kalifa L., Hochmuth C., Sia E.A. Loss of the mitochondrial nucleoid protein, Abf2p, destabilizes repetitive DNA in the yeast mitochondrial genome. Genetics. 2009;181:331–334. doi: 10.1534/genetics.108.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chimienti G., Picca A., Sirago G., Fracasso F., Calvani R., Bernabei R., Russo F., Carter C.S., Leeuwenburgh C., Pesce V., et al. Increased TFAM binding to mtDNA damage hot spots is associated with mtDNA loss in aged rat heart. Free Radic. Biol. Med. 2018;124:447–453. doi: 10.1016/j.freeradbiomed.2018.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huang J.C., Zamble D.B., Reardon J.T., Lippard S.J., Sancar A. HMG-domain proteins specifically inhibit the repair of the major DNA adduct of the anticancer drug cisplatin by human excision nuclease. Proc. Natl. Acad. Sci. USA. 1994;91:10394–10398. doi: 10.1073/pnas.91.22.10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kazak L., Reyes A., Holt I.J. Minimizing the damage: Repair pathways keep mitochondrial DNA intact. Nat. Rev. Mol. Cell Biol. 2012;13:659–671. doi: 10.1038/nrm3439. [DOI] [PubMed] [Google Scholar]
- 189.Yoshida Y., Izumi H., Torigoe T., Ishiguchi H., Itoh H., Kang D., Kohno K. p53 physically interacts with mitochondrial transcription factor A and differentially regulates binding to damaged DNA. Cancer Res. 2003;63:3729–3734. [PubMed] [Google Scholar]
- 190.Cho J.H., Lee Y.K., Chae C.B. The modulation of the biological activities of mitochondrial histone Abf2p by yeast PKA and its possible role in the regulation of mitochondrial DNA content during glucose repression. Biochim. Biophys. Acta—Gene Struct. Expr. 2001;1522:175–186. doi: 10.1016/S0167-4781(01)00333-5. [DOI] [PubMed] [Google Scholar]
- 191.Brewer L.R., Friddle R., Noy A., Baldwin E., Martin S.S., Corzett M., Balhorn R., Baskin R.J. Packaging of single DNA molecules by the yeast mitochondrial protein Abf2p. Biophys. J. 2003;85:2519–2524. doi: 10.1016/S0006-3495(03)74674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Friddle R.W., Klare J.E., Martin S.S., Corzett M., Balhorn R., Baldwin E.P., Baskin R.J., Noy A. Mechanism of DNA compaction by yeast mitochondrial protein Abf2p. Biophys. J. 2004;86:1632–1639. doi: 10.1016/S0006-3495(04)74231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Stigter D. Packaging of single DNA molecules by the yeast mitochondrial protein Abf2p: Reinterpretation of recent single molecule experiments. Biophys. Chem. 2004;110:171–178. doi: 10.1016/j.bpc.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 194.Ho B., Baryshnikova A., Brown G.W. Unification of protein abundance datasets yields a quantitative Saccharomyces cerevisiae proteome. Cell Syst. 2018;6:192–205. doi: 10.1016/j.cels.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 195.Davidson G.S., Joe R.M., Roy S., Meirelles O., Allen C.P., Wilson M.R., Tapia P.H., Manzanilla E.E., Dodson A.E., Chakraborty S., et al. The proteomics of quiescent and nonquiescent cell differentiation in yeast stationary-phase cultures. Mol. Biol. Cell. 2011;22:988–998. doi: 10.1091/mbc.e10-06-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lahtvee P.J., Sánchez B.J., Smialowska A., Kasvandik S., Elsemman I.E., Gatto F., Nielsen J. Absolute quantification of protein and mRNA abundances demonstrate variability in gene-specific translation efficiency in yeast. Cell Syst. 2017;4:495–504. doi: 10.1016/j.cels.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 197.Rafelski S.M., Viana M.P., Zhang Y., Chan Y.H.M., Thorn K.S., Yam P., Fung J.C., Li H., Costa L.D.F., Marshall W.F. Mitochondrial network size scaling in budding yeast. Science. 2012;338:822–824. doi: 10.1126/science.1225720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Swinger K.K., Lemberg K.M., Zhang Y., Rice P.A. Flexible DNA bending in HU–DNA cocrystal structures. EMBO J. 2003;22:3749–3760. doi: 10.1093/emboj/cdg351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim D.H., Im H., Jee J.G., Jang S.B., Yoon H.J., Kwon A.R., Kang S.M., Lee B.J. β-Arm flexibility of HU from Staphylococcus aureus dictates the DNA-binding and recognition mechanism. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014;70:3273–3289. doi: 10.1107/S1399004714023931. [DOI] [PubMed] [Google Scholar]
- 200.Hammel M., Amlanjyoti D., Reyes F.E., Chen J.H., Parpana R., Tang H.Y.H., Larabell C.A., Tainer J.A., Adhya S. HU multimerization shift controls nucleoid compaction. Sci. Adv. 2016;2:e1600650. doi: 10.1126/sciadv.1600650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chakraborty A., Lyonnais S., Battistini F., Hospital A., Medici G., Prohens R., Orozco M., Vilardell J., Solà M. DNA structure directs positioning of the mitochondrial genome packaging protein Abf2p. Nucleic Acids Res. 2017;45:951–967. doi: 10.1093/nar/gkw1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lyonnais S., Tarrés-Soler A., Rubio-Cosials A., Cuppari A., Brito R., Jaumot J., Gargallo R., Vilaseca M., Silva C., Granzhan A., et al. The human mitochondrial transcription factor A is a versatile G-quadruplex binding protein. Sci. Rep. 2017;7 doi: 10.1038/srep43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Reeves R. High mobility group (HMG) proteins: Modulators of chromatin structure and DNA repair in mammalian cells. DNA Repair (Amst) 2015;36:122–136. doi: 10.1016/j.dnarep.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 204.Rubio-Cosials A., Battistini F., Gansen A., Cuppari A., Bernadó P., Orozco M., Langowski J., Tóth K., Solà M. Protein flexibility and synergy of HMG domains underlie U-turn bending of DNA by TFAM in solution. Biophys. J. 2018;114:2386–2396. doi: 10.1016/j.bpj.2017.11.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gaspari M., Falkenberg M., Larsson N.-G., Gustafsson C.M. The mitochondrial RNA polymerase contributes critically to promoter specificity in mammalian cells. EMBO J. 2004;23:4606–4614. doi: 10.1038/sj.emboj.7600465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rubio-Cosials A., Sidow J.F., Jiménez-Menéndez N., Fernández-Millán P., Montoya J., Jacobs H.T., Coll M., Bernadó P., Solà M. Human mitochondrial transcription factor A induces a U-turn structure in the light strand promoter. Nat. Struct. Mol. Biol. 2011;18:1281–1289. doi: 10.1038/nsmb.2160. [DOI] [PubMed] [Google Scholar]
- 207.Ngo H.B., Kaiser J.T., Chan D.C. The mitochondrial transcription and packaging factor Tfam imposes a U-turn on mitochondrial DNA. Nat. Struct. Mol. Biol. 2011;18:1290–1296. doi: 10.1038/nsmb.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ngo H.B., Lovely G.A., Phillips R., Chan D.C. Distinct structural features of TFAM drive mitochondrial DNA packaging versus transcriptional activation. Nat. Commun. 2014;5:1–12. doi: 10.1038/ncomms4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kukat C., Davies K.M., Wurm C.A., Spåhr H., Bonekamp N.A., Kühl I., Joos F., Polosa P.L., Park C.B., Posse V., et al. Cross-strand binding of TFAM to a single mtDNA molecule forms the mitochondrial nucleoid. Proc. Natl. Acad. Sci. USA. 2015;112:11288–11293. doi: 10.1073/pnas.1512131112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gangelhoff T.A., Mungalachetty P.S., Nix J.C., Churchill M.E.A. Structural analysis and DNA binding of the HMG domains of the human mitochondrial transcription factor A. Nucleic Acids Res. 2009;37:3153–3164. doi: 10.1093/nar/gkp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dorman C.J. Function of nucleoid-associated proteins in chromosome structuring and transcriptional regulation. J. Mol. Microbiol. Biotechnol. 2014;24:316–331. doi: 10.1159/000368850. [DOI] [PubMed] [Google Scholar]
- 212.Krogh T.J., Møller-Jensen J., Kaleta C. Impact of chromosomal architecture on the function and evolution of bacterial genomes. Front. Microbiol. 2018;9:2019. doi: 10.3389/fmicb.2018.02019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Atlung T., Ingmer H. H-NS: A modulator of environmentally regulated gene expression. Mol. Microbiol. 1997;24:7–17. doi: 10.1046/j.1365-2958.1997.3151679.x. [DOI] [PubMed] [Google Scholar]
- 214.Talukder A.A., Ishihama A. Growth phase dependent changes in the structure and protein composition of nucleoid in Escherichia coli. Sci. China Life Sci. 2015;58:902–911. doi: 10.1007/s11427-015-4898-0. [DOI] [PubMed] [Google Scholar]
- 215.Rodriguez-Colman M.J., Reverter-Branchat G., Sorolla M.A., Tamarit J., Ros J., Cabiscol E. The forkhead transcription factor Hcm1 promotes mitochondrial biogenesis and stress resistance in yeast. J. Biol. Chem. 2010;285:37092–37101. doi: 10.1074/jbc.M110.174763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Venters B.J., Wachi S., Mavrich T.N., Andersen B.E., Jena P., Sinnamon A.J., Jain P., Rolleri N.S., Jiang C., Hemeryck-Walsh C., et al. A comprehensive genomic binding map of gene and chromatin regulatory proteins in Saccharomyces. Mol. Cell. 2011;41:480–492. doi: 10.1016/j.molcel.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Corral-Debrinski M., Blugeon C., Jacq C. In yeast, the 3′ untranslated region or the presequence of ATM1 is required for the exclusive localization of its mRNA to the vicinity of mitochondria. Mol. Cell. Biol. 2000;20:7881–7892. doi: 10.1128/MCB.20.21.7881-7892.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bandhakavi S., Xie H., O’Callaghan B., Sakurai H., Kim D.H., Griffin T.J. Hsf1 activation inhibits rapamycin resistance and TOR signaling in yeast revealed by combined proteomic and genetic analysis. PLoS ONE. 2008;3:e1598. doi: 10.1371/journal.pone.0001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bonawitz N.D., Chatenay-Lapointe M., Pan Y., Shadel G.S. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yoshino M., Naka A., Sakamoto Y., Shibasaki A., Toh M., Tsukamoto S., Kondo K., Iida K. Dietary isoflavone daidzein promotes Tfam expression that increases mitochondrial biogenesis in C2C12 muscle cells. J. Nutr. Biochem. 2015;26:1193–1199. doi: 10.1016/j.jnutbio.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 221.Virbasius J.V., Scarpulla R.C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: A potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl. Acad. Sci. USA. 1994;91:1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chen X., Li S., Ke Y., Wu S., Huang T., Hu W., Fu H., Guo X. KLF16 suppresses human glioma cell proliferation and tumourigenicity by targeting TFAM. Artif. Cells Nanomedicine Biotechnol. 2018;46:608–615. doi: 10.1080/21691401.2018.1431654. [DOI] [PubMed] [Google Scholar]
- 223.Peng H., Guo T., Chen Z., Zhang H., Cai S., Yang M., Chen P., Guan C., Fang X. Hypermethylation of mitochondrial transcription factor A induced by cigarette smoke is associated with chronic obstructive pulmonary disease. Exp. Lung Res. 2019;45:101–111. doi: 10.1080/01902148.2018.1556748. [DOI] [PubMed] [Google Scholar]
- 224.Larsson N.G., Garman J.D., Oldfors A., Barsh G.S., Clayton D.A. A single mouse gene encodes the mitochondrial transcription factor A and a testis-specific nuclear HMG-box protein. Nat. Genet. 1996;13:296–302. doi: 10.1038/ng0796-296. [DOI] [PubMed] [Google Scholar]
- 225.Larsson N.-G., Oldfors A., David Garman J., Barsh G.S., Clayton D.A. Down-regulation of mitochondrial transcription factor A during spermatogenesis in humans. Hum. Mol. Genet. 1997;6:186–191. doi: 10.1093/hmg/6.2.185. [DOI] [PubMed] [Google Scholar]
- 226.Zhang R., Wang J. HuR stabilizes TFAM mRNA in an ATM/p38-dependent manner in ionizing irradiated cancer cells. Cancer Sci. 2018;109:2446–2457. doi: 10.1111/cas.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jiang J., Yang J., Wang Z., Wu G., Liu F. TFAM is directly regulated by miR-23b in glioma. Oncol. Rep. 2013;30:2105–2110. doi: 10.3892/or.2013.2712. [DOI] [PubMed] [Google Scholar]
- 228.Wen Z., Lei Z., Jin-An M., Xue-Zhen M., Xing-Nan Z., Xiu-Wen D. The inhibitory role of miR-214 in cervical cancer cells through directly targeting mitochondrial transcription factor A (TFAM) Eur. J. Gynaecol Oncol. 2014;35:676–682. doi: 10.3892/or.2013.2712. [DOI] [PubMed] [Google Scholar]
- 229.Wu K., Zhao Z., Xiao Y., Peng J., Chen J., He Y. Roles of mitochondrial transcription factor A and microRNA-590-3p in the development of colon cancer. Mol. Med. Rep. 2016;14:5475–5480. doi: 10.3892/mmr.2016.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mo M., Peng F., Wang L., Peng L., Lan G., Yu S. Roles of mitochondrial transcription factor A and microRNA-590-3p in the development of bladder cancer. Oncol. Lett. 2013;6:617–623. doi: 10.3892/ol.2013.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yamamoto H., Morino K., Nishio Y., Ugi S., Yoshizaki T., Kashiwagi A., Maegawa H. MicroRNA-494 regulates mitochondrial biogenesis in skeletal muscle through mitochondrial transcription factor A and Forkhead box j3. Am. J. Physiol—Endocrinol. Metab. 2012;303 doi: 10.1152/ajpendo.00097.2012. [DOI] [PubMed] [Google Scholar]
- 232.Lemecha M., Morino K., Imamura T., Iwasaki H., Ohashi N., Ida S., Sato D., Sekine O., Ugi S., Maegawa H. MiR-494-3p regulates mitochondrial biogenesis and thermogenesis through PGC1-α signalling in beige adipocytes. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-33438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sun Y., Cui D., Zhang Z., Zhang Q., Ji L., Ding S. Voluntary wheel exercise alters the levels of miR-494 and miR-696 in the skeletal muscle of C57BL/6 mice. Comp. Biochem. Physiol. Part—B Biochem. Mol. Biol. 2016;202:16–22. doi: 10.1016/j.cbpb.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 234.Bruno S., De Virgilio C., Gadaleta G. The smaller isoform of the mitochondrial transcription factor A has a role in the mitochondrial transcription. Ital. J. Biochem. 2007;56:315–318. [PubMed] [Google Scholar]
- 235.Reyes A., Mezzina M., Gadaleta G. Human mitochondrial transcription factor A (mtTFA): Gene structure and characterization of related pseudogenes. Gene. 2002;291:223–232. doi: 10.1016/S0378-1119(02)00600-5. [DOI] [PubMed] [Google Scholar]
- 236.Tominaga K., Hayashi J.I., Kagawa Y., Ohta S. Smaller isoform of human mitochondrial transcription factor 1: Its wide distribution and production by alternative splicing. Biochem. Biophys. Res. Commun. 1993;194:544–551. doi: 10.1006/bbrc.1993.1854. [DOI] [PubMed] [Google Scholar]
- 237.De Virgilio C., Pousis C., Bruno S., Gadaleta G. New isoforms of human mitochondrial transcription factor A detected in normal and tumoral cells. Mitochondrion. 2011;11:287–295. doi: 10.1016/j.mito.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 238.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 239.Tessarz P., Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 2014;15:703–708. doi: 10.1038/nrm3890. [DOI] [PubMed] [Google Scholar]
- 240.Macek B., Mijakovic I., Olsen J.V., Gnad F., Kumar C., Jensen P.R., Mann M. The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol. Cell. Proteomics. 2007;6:697–707. doi: 10.1074/mcp.M600464-MCP200. [DOI] [PubMed] [Google Scholar]
- 241.Soufi B., Jers C., Hansen M.E., Petranovic D., Mijakovic I. Insights from site-specific phosphoproteomics in bacteria. Biochim. Biophys. Acta—Proteins Proteomics. 2008;1784:186–192. doi: 10.1016/j.bbapap.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 242.Yu J.B., Kim J.A., Moon J.H., Pan S.E.R.J. The diversity of lysine-acetylated proteins in Escherichia coli. J. Microbiol. Biotechnol. 2008;18:1529–1536. [PubMed] [Google Scholar]
- 243.Cao X.J., Dai J., Xu H., Nie S., Chang X., Hu B.Y., Sheng Q.H., Wang L.S., Ning Z.B., Li Y.X., et al. High-coverage proteome analysis reveals the first insight of protein modification systems in the pathogenic spirochete Leptospira interrogans. Cell Res. 2010;20:197–210. doi: 10.1038/cr.2009.127. [DOI] [PubMed] [Google Scholar]
- 244.Dilweg I.W., Dame R.T. Post-translational modification of nucleoid-associated proteins: An extra layer of functional modulation in bacteria? Biochem. Soc. Trans. 2018;46:1381–1392. doi: 10.1042/BST20180488. [DOI] [PubMed] [Google Scholar]
- 245.Lu B., Lee J., Nie X., Li M., Morozov Y.I., Venkatesh S., Bogenhagen D.F., Temiakov D., Suzuki C.K. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease. Mol. Cell. 2013;49:121–132. doi: 10.1016/j.molcel.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wang K.Z.Q., Zhu J., Dagda R.K., Uechi G., Cherra S.J., Gusdon A.M., Balasubramani M., Chu C.T. ERK-mediated phosphorylation of TFAM downregulates mitochondrial transcription: Implications for Parkinson’s disease. Mitochondrion. 2014;17:132–140. doi: 10.1016/j.mito.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kunová N., Ondrovičová G., Bauer J.A., Bellová J., Ambro L., Martináková L., Kotrasová V., Kutejová E., Pevala V. The role of Lon-mediated proteolysis in the dynamics of mitochondrial nucleic acid-protein complexes. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-00632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chen R.Q., Yang Q.K., Lu B.W., Yi W., Cantin G., Chen Y.L., Fearns C., Yates J.R., Lee J.D. CDC25B mediates rapamycin-induced oncogenic responses in cancer cells. Cancer Res. 2009;69:2663–2668. doi: 10.1158/0008-5472.CAN-08-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Dinardo M.M., Musicco C., Fracasso F., Milella F., Gadaleta M.N., Gadaleta G., Cantatore P. Acetylation and level of mitochondrial transcription factor A in several organs of young and old rats. Biochem. Biophys. Res. Commun. 2003;301:187–191. doi: 10.1016/S0006-291X(02)03008-5. [DOI] [PubMed] [Google Scholar]
- 250.King G.A., Hashemi Shabestari M., Taris K.K.H., Pandey A.K., Venkatesh S., Thilagavathi J., Singh K., Krishna Koppisetti R., Temiakov D., Roos W.H., et al. Acetylation and phosphorylation of human TFAM regulate TFAM-DNA interactions via contrasting mechanisms. Nucleic Acids Res. 2018;46:3633–3642. doi: 10.1093/nar/gky204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fang Y., Akimoto M., Mayanagi K., Hatano A., Matsumoto M., Matsuda S., Yasukawa T., Kang D. Chemical acetylation of mitochondrial transcription factor A occurs on specific lysine residues and affects its ability to change global DNA topology. Mitochondrion. 2020;53:99–108. doi: 10.1016/j.mito.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 252.Liu H., Li S., Liu X., Chen Y., Deng H. SIRT3 overexpression inhibits growth of kidney tumor cells and enhances mitochondrial biogenesis. J. Proteome Res. 2018;17:3143–3152. doi: 10.1021/acs.jproteome.8b00260. [DOI] [PubMed] [Google Scholar]
- 253.Henriksen P., Wagner S.A., Weinert B.T., Sharma S., Bačinskaja G., Rehman M., Juffer A.H., Walther T.C., Lisby M., Choudhary C. Proteome-wide analysis of lysine acetylation suggests its broad regulatory scope in Saccharomyces cerevisiae. Mol. Cell. Proteomics. 2012;11:1510–1522. doi: 10.1074/mcp.M112.017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Weinert B.T., Schölz C., Wagner S.A., Iesmantavicius V., Su D., Daniel J.A., Choudhary C. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013;4:842–851. doi: 10.1016/j.celrep.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 255.Santos J.M., Mishra M., Kowluru R.A. Posttranslational modification of mitochondrial transcription factor A in impaired mitochondria biogenesis: Implications in diabetic retinopathy and metabolic memory phenomenon. Exp. Eye Res. 2014;121:168–177. doi: 10.1016/j.exer.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Shi Y., Chan D.W., Jung S.Y., Malovannaya A., Wang Y., Qin J. A data set of human endogenous protein ubiquitination sites. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wagner S.A., Beli P., Weinert B.T., Nielsen M.L., Cox J., Mann M., Choudhary C. A Proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Mol. Cell. Proteomics. 2011;10:M111.013284. doi: 10.1074/mcp.M111.013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Santos A.R.C., Dvoriantchikova G., Li Y., Mohammad G., Abu El-Asrar A.M., Wen R., Ivanov D. Cellular mechanisms of High Mobility Group 1 (HMGB-1) protein action in the diabetic retinopathy. PLoS ONE. 2014;9:e87574. doi: 10.1371/journal.pone.0087574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Suarez J., Hu Y., Makino A., Fricovsky E., Wang H., Dillmann W.H. Alterations in mitochondrial function and cytosolic calcium induced by hyperglycemia are restored by mitochondrial transcription factor A in cardiomyocytes. Am. J. Physiol—Cell Physiol. 2008;295 doi: 10.1152/ajpcell.00076.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.El-Hattab A.W., Craigen W.J., Scaglia F. Mitochondrial DNA maintenance defects. Biochim. Biophys. Acta—Mol. Basis Dis. 2017;1863:1539–1555. doi: 10.1016/j.bbadis.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 261.Suomalainen A., Isohanni P. Mitochondrial DNA depletion syndromes: Many genes, common mechanisms. Neuromuscul. Disord. 2010;20:429–437. doi: 10.1016/j.nmd.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 262.Woo D.K., Green P.D., Santos J.H., D’Souza A.D., Walther Z., Martin W.D., Christian B.E., Chandel N.S., Shadel G.S. Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APCMin/+ mice. Am. J. Pathol. 2012;180:24–31. doi: 10.1016/j.ajpath.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Desdín-Micó G., Soto-Heredero G., Aranda J.F., Oller J., Carrasco E., Gabandé-Rodríguez E., Blanco E.M., Alfranca A., Cussó L., Desco M., et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. 2020;368:1371–1376. doi: 10.1126/science.aax0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang J., Silva J.P., Gustafsson C.M., Rustin P., Larsson N.G. Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc. Natl. Acad. Sci. USA. 2001;98:4038–4043. doi: 10.1073/pnas.061038798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Aydin J., Andersson D.C., Hä Nninen S.L., Wredenberg A., Tavi P., Park C.B., Ran Larsson N.-G., Bruton J.D., Kan Westerblad H. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Hum. Mol. Genet. 2009;18:278–288. doi: 10.1093/hmg/ddn355. [DOI] [PubMed] [Google Scholar]
- 266.Farge G., Mehmedovic M., Baclayon M., VandenWildenberg S.M.J.L., Roos W.H., Gustafsson C.M., Wuite G.J.L., Falkenberg M. In vitro-reconstituted nucleoids can block mitochondrial DNA replication and transcription. Cell Rep. 2014;8:66–74. doi: 10.1016/j.celrep.2014.05.046. [DOI] [PubMed] [Google Scholar]
- 267.Kang I., Chu C.T., Kaufman B.A. The mitochondrial transcription factor TFAM in neurodegeneration: Emerging evidence and mechanisms. FEBS Lett. 2018;592:793–811. doi: 10.1002/1873-3468.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ikeda M., Ide T., Fujino T., Arai S., Saku K., Kakino T., Tyynismaa H., Yamasaki T., Yamada K., Kang D., et al. Overexpression of TFAM or Twinkle increases mtDNA copy number and facilitates cardioprotection associated with limited mitochondrial oxidative stress. PLoS ONE. 2015;10:e0119687. doi: 10.1371/journal.pone.0119687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kunkel G.H., Kunkel C.J., Ozuna H., Miralda I., Tyagi S.C. TFAM overexpression reduces pathological cardiac remodeling. Mol. Cell. Biochem. 2019;454:139–152. doi: 10.1007/s11010-018-3459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Picca A., Sirago G., Pesce V., Lezza A.M.S., Calvani R., Bossola M., Villani E.R., Landi F., Leeuwenburgh C., Bernabei R., et al. Administration of enalapril started late in life attenuates hypertrophy and oxidative stress burden, increases mitochondrial mass, and modulates mitochondrial quality control signaling in the rat heart. Biomolecules. 2018;8:177. doi: 10.3390/biom8040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Stiles A.R., Simon M.T., Stover A., Eftekharian S., Khanlou N., Wang H.L., Magaki S., Lee H., Partynski K., Dorrani N., et al. Mutations in TFAM, encoding mitochondrial transcription factor A, cause neonatal liver failure associated with mtDNA depletion. Mol. Genet. Metab. 2016;119:91–99. doi: 10.1016/j.ymgme.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 272.Arduini A., Serviddio G., Escobar J., Tormos A.M., Bellanti F., Viña J., Monsalve M., Sastre J. Mitochondrial biogenesis fails in secondary biliary cirrhosis in rats leadingto mitochondrial DNA depletion and deletions. Am. J. Physiol—Gastrointest. Liver Physiol. 2011;301 doi: 10.1152/ajpgi.00253.2010. [DOI] [PubMed] [Google Scholar]
- 273.Xu S.C., Chen Y.B., Lin H., Pi H.F., Zhang N.X., Zhao C.C., Shuai L., Zhong M., Yu Z.P., Zhou Z., et al. Damage to mtDNA in liver injury of patients with extrahepatic cholestasis: The protective effects of mitochondrial transcription factor A. Free Radic. Biol. Med. 2012;52:1543–1551. doi: 10.1016/j.freeradbiomed.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 274.Tiao M.M., Lin T.K., Liou C.W., Wang P.W., Chen J.B., Kuo F.Y., Huang C.C., Chou Y.M., Chuang J.H. Early transcriptional deregulation of hepatic mitochondrial biogenesis and its consequent effects on murine cholestatic liver injury. Apoptosis. 2009;14:890–899. doi: 10.1007/s10495-009-0357-3. [DOI] [PubMed] [Google Scholar]
- 275.Poulton J., Morten K., Freman-Emmerson C., Potter C., Sewry C., Dubowitz V., Kidd H., Stephenson J., Whitehouse W., Hansen F.J., et al. Deficiency of the human mitochondrial transcription factor h-mtTFA in infantile mitochondrial myopathy is associated with mtDNA depletion. Hum. Mol. Genet. 1994;3:1763–1769. doi: 10.1093/hmg/3.10.1763. [DOI] [PubMed] [Google Scholar]
- 276.Yoshida T., Azuma H., Aihara K.I., Fujimura M., Akaike M., Mitsui T., Matsumoto T. Vascular smooth muscle cell proliferation is dependent upon upregulation of mitochondrial transcription factor A (mtTFA) expression in injured rat carotid artery. Atherosclerosis. 2005;178:39–47. doi: 10.1016/j.atherosclerosis.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 277.Remels A.H., Schrauwen P., Broekhuizen R., Willems J., Kersten S., Gosker H.R., Schols A.M. Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur. Respir. J. 2007;30:245–252. doi: 10.1183/09031936.00144106. [DOI] [PubMed] [Google Scholar]
- 278.Peng H., Yang M., Chen Z.Y., Chen P., Guan C.X., Xiang X.D., Cai S., Chen Y., Fang X. Expression and methylation of mitochondrial transcription factor a in chronic obstructive pulmonary disease patients with lung cancer. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0082739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Lee W.R., Na H., Lee S.W., Lim W.J., Kim N., Lee J.E., Kang C. Transcriptomic analysis of mitochondrial TFAM depletion changing cell morphology and proliferation. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-18064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Guo J., Zheng L., Liu W., Wang X., Wang Z., Wang Z., French A.J., Kang D., Chen L., Thibodeau S.N., et al. Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res. 2011;71:2978–2987. doi: 10.1158/0008-5472.CAN-10-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wu K., Ma J., Zhan Y., Liu K., Ye Z., Chen J., Xu K., Huang H., He Y. Down-regulation of microRNA-214 contributed to the enhanced mitochondrial transcription factor A and inhibited proliferation of colorectal cancer cells. Cell. Physiol. Biochem. 2018;49:545–554. doi: 10.1159/000492992. [DOI] [PubMed] [Google Scholar]
- 282.Yoshida Y., Hasegawa J., Nezu R., Kim Y.K., Hirota M., Kawano K., Izumi H., Kohno K. Clinical usefulness of mitochondrial transcription factor A expression as a predictive marker in colorectal cancer patients treated with FOLFOX. Cancer Sci. 2011;102:578–582. doi: 10.1111/j.1349-7006.2010.01835.x. [DOI] [PubMed] [Google Scholar]
- 283.Lin C.S., Lee H.T., Lee M.H., Pan S.C., Ke C.Y., Chiu A.W.H., Wei Y.H. Role of mitochondrial DNA copy number alteration in human renal cell carcinoma. Int. J. Mol. Sci. 2016;17:814. doi: 10.3390/ijms17060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Xie D., Wu X., Lan L., Shangguan F., Lin X., Chen F., Xu S., Zhang Y., Chen Z., Huang K., et al. Downregulation of TFAM inhibits the tumorigenesis of non-small cell lung cancer by activating ROS-mediated JNK/p38MAPK signaling and reducing cellular bioenergetics. Oncotarget. 2016;7:11609–11624. doi: 10.18632/oncotarget.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Mattingly K.A., Ivanova M.M., Riggs K.A., Wickramasinghe N.S., Barch M.J., Klinge C.M. Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol. Endocrinol. 2008;22:609–622. doi: 10.1210/me.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Romero-Garcia S., Prado-Garcia H., Valencia-Camargo A.D., Alvarez-Pulido A. Lactic acidosis promotes mitochondrial biogenesis in lung adenocarcinoma cells, supporting proliferation under normoxia or survival under hypoxia. Front. Oncol. 2019;9 doi: 10.3389/fonc.2019.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yu J., Wang Q., Chen N., Sun Y., Wang X., Wu L., Chen S., Yuan H., Xu A., Wang J. Mitochondrial transcription factor A regulated ionizing radiation-induced mitochondrial biogenesis in human lung adenocarcinoma A549 cells. J. Radiat. Res. 2013;54:998–1004. doi: 10.1093/jrr/rrt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bellance N., Benard G., Furt F., Begueret H., Smolková K., Passerieux E., Delage J.P., Baste J.M., Moreau P., Rossignol R. Bioenergetics of lung tumors: Alteration of mitochondrial biogenesis and respiratory capacity. Int. J. Biochem. Cell Biol. 2009;41:2566–2577. doi: 10.1016/j.biocel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 289.Lee H., Park J., Tran Q., Kim D., Hong Y., Cho H., Kwon S.H., Brazil D., Kim S.H. Mitochondrial transcription factor a (TFAM) is upregulated in glioma. Mol. Med. Rep. 2017;15:3781–3786. doi: 10.3892/mmr.2017.6467. [DOI] [PubMed] [Google Scholar]
- 290.Franco D.G., Moretti I.F., Marie S.K.N. Mitochondria transcription factor A: A putative target for the effect of melatonin on U87MG malignant glioma cell line. Molecules. 2018;23:1129. doi: 10.3390/molecules23051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Correia R.L., Oba-Shinjo S.M., Uno M., Huang N., Marie S.K.N. Mitochondrial DNA depletion and its correlation with TFAM, TFB1M, TFB2M and POLG in human diffusely infiltrating astrocytomas. Mitochondrion. 2011;11:48–53. doi: 10.1016/j.mito.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 292.Lee C.H., Wu S.B., Hong C.H., Liao W.T., Wu C.Y., Chen G.S., Wei Y.H., Yu H.S. Aberrant cell proliferation by enhanced mitochondrial biogenesis via mtTFA in arsenical skin cancers. Am. J. Pathol. 2011;178:2066–2076. doi: 10.1016/j.ajpath.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Araujo L.F., Siena A.D.D., Plaça J.R., Brotto D.B., Barros I.I., Muys B.R., Biagi C.A.O., Peronni K.C., Sousa J.F., Molfetta G.A., et al. Mitochondrial transcription factor A (TFAM) shapes metabolic and invasion gene signatures in melanoma. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-31170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Singh K.P., Kumari R., Treas J., Dumond J.W. Chronic exposure to arsenic causes increased cell survival, DNA damage, and increased expression of mitochondrial transcription factor A (mtTFA) in human prostate epithelial cells. Chem. Res. Toxicol. 2011;24:340–349. doi: 10.1021/tx1003112. [DOI] [PubMed] [Google Scholar]
- 295.Kurita T., Izumi H., Kagami S., Kawagoe T., Toki N., Matsuura Y., Hachisuga T., Kohno K. Mitochondrial transcription factor A regulates BCL2L1 gene expression and is a prognostic factor in serous ovarian cancer. Cancer Sci. 2012;103:239–444. doi: 10.1111/j.1349-7006.2011.02156.x. [DOI] [PubMed] [Google Scholar]
- 296.Toki N., Kagami S., Kurita T., Kawagoe T., Matsuura Y., Hachisuga T., Matsuyama A., Hashimoto H., Izumi H., Kohno K. Expression of mitochondrial transcription factor A in endometrial carcinomas: Clinicopathologic correlations and prognostic significance. Virchows Arch. 2010;456:387–393. doi: 10.1007/s00428-010-0895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Cormio A., Guerra F., Cormio G., Pesce V., Fracasso F., Loizzi V., Cantatore P., Selvaggi L., Gadaleta M.N. The PGC-1α-dependent pathway of mitochondrial biogenesis is upregulated in type I endometrial cancer. Biochem. Biophys. Res. Commun. 2009;390:1182–1185. doi: 10.1016/j.bbrc.2009.10.114. [DOI] [PubMed] [Google Scholar]
- 298.Gao W., Wu M., Wang N., Zhang Y., Hua J., Tang G., Wang Y. Increased expression of mitochondrial transcription factor a and nuclear respiratory factor-1 predicts a poor clinical outcome of breast cancer. Oncol. Lett. 2018;15:1449–1458. doi: 10.3892/ol.2017.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Gao W., Wu M.H., Wang N., Ying M.Z., Zhang Y.Y., Hua J., Chuan L., Wang Y.J. Mitochondrial transcription factor A contributes to cisplatin resistance in patients with estrogen receptor-positive breast cancer. Mol. Med. Rep. 2016;14:5304–5310. doi: 10.3892/mmr.2016.5881. [DOI] [PubMed] [Google Scholar]
- 300.Qiao L., Ru G., Mao Z., Wang C., Nie Z., Li Q., Huang-yang Y., Zhu L., Liang X., Yu J., et al. Mitochondrial DNA depletion, mitochondrial mutations and high TFAM expression in hepatocellular carcinoma. Oncotarget. 2017;8:84373–84383. doi: 10.18632/oncotarget.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lin C.S., Lee H.T., Lee S.Y., Shen Y.A., Wang L.S., Chen Y.J., Wei Y.H. High mitochondrial DNA copy number and bioenergetic function are associated with tumor invasion of esophageal squamous cell carcinoma cell lines. Int. J. Mol. Sci. 2012;13:11228–11246. doi: 10.3390/ijms130911228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lin C.S., Chang S.C., Wang L.S., Chou T.Y., Hsu W.H., Wu Y.C., Wei Y.H. The role of mitochondrial DNA alterations in esophageal squamous cell carcinomas. J. Thorac. Cardiovasc. Surg. 2010;139 doi: 10.1016/j.jtcvs.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 303.Masuike Y., Tanaka K., Makino T., Yamasaki M., Miyazaki Y., Takahashi T., Kurokawa Y., Nakajima K., Mori M., Doki Y. Esophageal squamous cell carcinoma with low mitochondrial copy number has mesenchymal and stem-like characteristics, and contributes to poor prognosis. PLoS ONE. 2018;13:e0193159. doi: 10.1371/journal.pone.0193159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yuan Y., Ju Y.S., Kim Y., Li J., Wang Y., Yoon C.J., Yang Y., Martincorena I., Creighton C.J., Weinstein J.N., et al. Comprehensive molecular characterization of mitochondrial genomes in human cancers. Nat. Genet. 2020;52:342–352. doi: 10.1038/s41588-019-0557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sun X., Zhan L., Chen Y., Wang G., He L., Wang Q., Zhou F., Yang F., Wu J., Wu Y., et al. Increased mtDNA copy number promotes cancer progression by enhancing mitochondrial oxidative phosphorylation in microsatellite-stable colorectal cancer. Signal. Transduct. Target. Ther. 2018;3:1–9. doi: 10.1038/s41392-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Alonso-Montes C., Castro M.G., Reguero J.R., Perrot A., Ozcelik C., Geier C., Posch M.G., Morís C., Alvarez V., Ruiz-Ortega M., et al. Mitochondrial transcription factors TFA, TFB1 and TFB2: A search for DNA variants/haplotypes and the risk of cardiac hypertrophy. Dis. Markers. 2008;25:131–139. doi: 10.1155/2008/575323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wang D., Li G.D., Fan Y., Zhang D.F., Bi R., Yu X.F., Long H., Li Y.Y., Yao Y.G. The mtDNA replication-related genes TFAM and POLG are associated with leprosy in Han Chinese from Southwest China. J. Dermatol. Sci. 2017;88:349–356. doi: 10.1016/j.jdermsci.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 308.Han B., Izumi H., Yasuniwa Y., Akiyama M., Yamaguchi T., Fujimoto N., Matsumoto T., Wu B., Tanimoto A., Sasaguri Y., et al. Human mitochondrial transcription factor A functions in both nuclei and mitochondria and regulates cancer cell growth. Biochem. Biophys. Res. Commun. 2011;408:45–51. doi: 10.1016/j.bbrc.2011.03.114. [DOI] [PubMed] [Google Scholar]
- 309.Kunkel G.H., Chaturvedi P., Thelian N., Nair R., Tyagi S.C. Mechanisms of TFAM-mediated cardiomyocyte protection. Can. J. Physiol. Pharmacol. 2018;96:173–181. doi: 10.1139/cjpp-2016-0718. [DOI] [PubMed] [Google Scholar]
- 310.Ikeuchi M., Matsusaka H., Kang D., Matsushima S., Ide T., Kubota T., Fujiwara T., Hamasaki N., Takeshita A., Sunagawa K., et al. Overexpression of mitochondrial transcription factor A ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation. 2005;112:683–690. doi: 10.1161/CIRCULATIONAHA.104.524835. [DOI] [PubMed] [Google Scholar]
- 311.Fujino T., Ide T., Yoshida M., Onitsuka K., Tanaka A., Hata Y., Nishida M., Takehara T., Kanemaru T., Kitajima N., et al. Recombinant mitochondrial transcription factor A protein inhibits nuclear factor of activated T cells signaling and attenuates pathological hypertrophy of cardiac myocytes. Mitochondrion. 2012;12:449–458. doi: 10.1016/j.mito.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 312.Theilen N.T., Kunkel G.H., Tyagi S.C. The role of exercise and TFAM in preventing skeletal muscle atrophy. J. Cell. Physiol. 2017;232:2348–2358. doi: 10.1002/jcp.25737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Chandrasekaran K., Muragundla A., Demarest T.G., Choi J., Sagi A.R., Najimi N., Kumar P., Singh A., Ho C.Y., Fiskum G., et al. mGluR2/3 activation of the SIRT1 axis preserves mitochondrial function in diabetic neuropathy. Ann. Clin. Transl. Neurol. 2017;4:844–858. doi: 10.1002/acn3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Chandrasekaran K., Anjaneyulu M., Choi J., Kumar P., Salimian M., Ho C.Y., Russell J.W. International Review of Neurobiology. Volume 145. Academic Press Inc.; Cambridge, MA, USA: 2019. Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD + -dependent SIRT1–PGC-1α–TFAM pathway; pp. 177–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Keeney P.M., Quigley C.K., Dunham L.D., Papageorge C.M., Iyer S., Thomas R.R., Schwarz K.M., Trimmer P.A., Khan S.M., Portell F.R., et al. Mitochondrial gene therapy augments mitochondrial physiology in a Parkinson’s disease cell model. Hum. Gene Ther. 2009;20:897–907. doi: 10.1089/hum.2009.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Oka S., Leon J., Sakumi K., Ide T., Kang D., LaFerla F.M., Nakabeppu Y. Human mitochondrial transcriptional factor A breaks the mitochondria-mediated vicious cycle in Alzheimer’s disease. Sci. Rep. 2016;6 doi: 10.1038/srep37889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.