Abstract
An efficient construction of imidazole ring by a Cs2CO3-promoted annulation of amidoximes with terminal alkynes in DMSO has been developed. This protocol provides a simple synthetic route with high atom-utilization for the synthesis of 2,4-disubstituted imidazoles in good yields under transition-metal-free and ligand-free conditions. Internal alkynes can also undergo the annulation to give 2,4,5-trisubstituted imidazoles.
Keywords: base-promoted; annulation; amidoximes; alkynes; 2,4-disubstituted imidazoles
1. Introduction
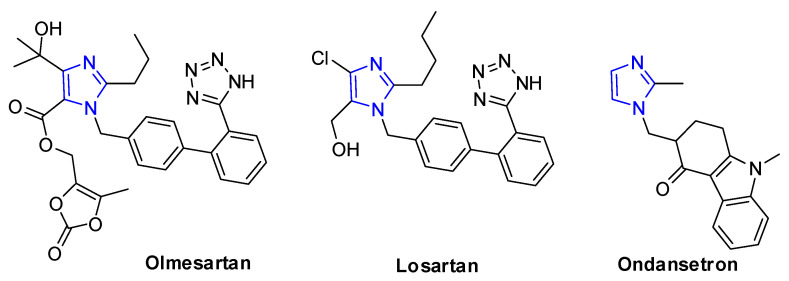
Imidazole ring is the important class of nitrogen-heterocyclic structural motif that has been found in several commercial drugs, such as olmesartan [1,2,3,4] and losartan [5,6,7], for the treatment of hypertension, and ondansetron [8,9,10,11] for reducing nausea and emesis, which have become the best-selling five-membered ring heterocyclic pharmaceuticals [12] (Figure 1). Molecules having this nitrogen-heterocyclic structure often exhibit important and interesting physiological and biological activities [13,14,15]. In addition, imidazoles have been also used as ligands in organometallic complexes [16,17]. Therefore, there has been increasing interest in the developments of efficient methodologies for the construction of imidazole ring [18,19,20].
Figure 1.
Examples of commercial drugs with imidazole skeleton.
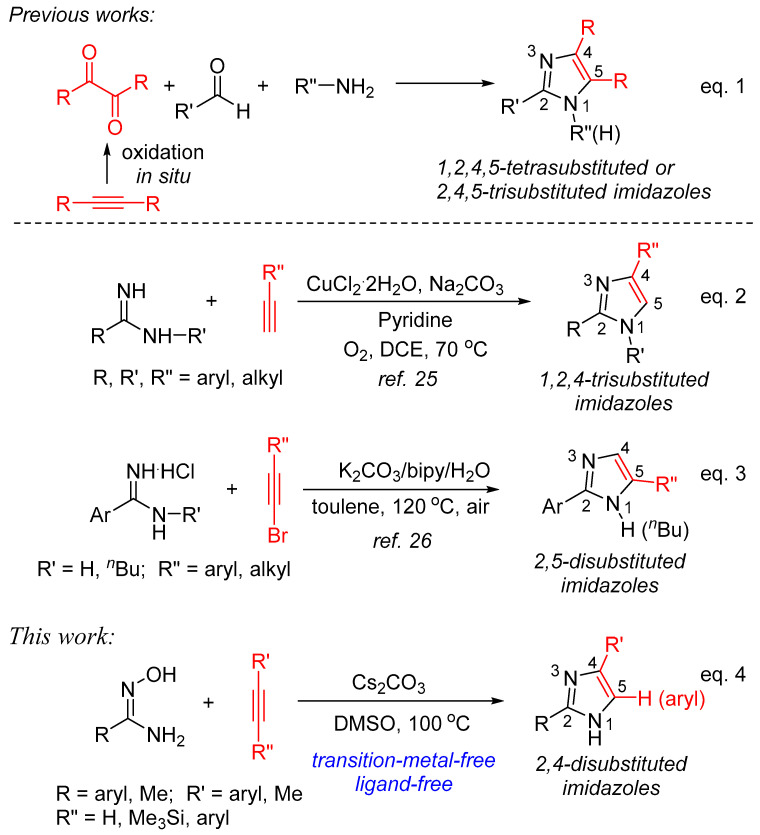
Among them, the construction of imidazole starting from alkyne as one of the reactants has been well developed. As depicted in Scheme 1, one of the classic approaches to synthesize 1,2,4,5-tetrasubstituted or 2,4,5-trisubstituted imidazoles is the three-component cyclization of α-diketone, aldehyde, and amine or ammonia sources catalyzed by transition metal complexes or under acidic conditions (Equation (1)). Recently, this protocol has been evolved using internal alkynes as starting materials via generating 1,2-diketones in situ by oxidation reaction [21,22,23,24]. On the other hand, the formal [3+2] annulation of substituted amidines with alkyne forming imidazole ring usually shows high atom-utilization, and two representative procedures are concluded in Scheme 1. One of the known procedures is the annulation of amidines with terminal alkynes catalyzed by CuCl2·2H2O in pyridine in the presence of Na2CO3 under atmospheric oxygen to afford 1,2,4-trisubstituted imidazoles in modest to good yields (Equation (2)) [25]. The other involves the cyclocondensation of amidine hydrochlorides with bromoacetylenes promoted by K2CO3 under air in the presence of 2,2′-bipyridine and water affording various 2,5-disubstituted imidazoles in good yields (Equation (3)) [26]. It is readily apparent to find that, in addition to the requirement of CuCl2·2H2O and/or ligand (pyridine and 2,2′-bipyridine), in both cases, inorganic bases were used as the additives, indicating that base is the key promoter to realize the formation of imidazoles. Therefore, in continuation of our interest in developing alkyne annulation in the synthesis of nitrogen-heterocyclic compounds [27,28,29,30] and base/DMSO-promoted C-N bond formation [31,32,33,34], we decided to explore the possibility of constructing an imidazole ring with the use of inorganic bases as the promoters, without the use of ligands under transition-metal-free conditions. In this paper, we would like to report a new, simple and efficient procedure to afford a variety of 2,4-disubstituted imidazoles starting with amidoximes and terminal alkynes in the presence of Cs2CO3 in DMSO (Equation (4)) [35].
Scheme 1.
Synthesis of diverse position-substituted imidazoles from alkynes.
2. Results and Discussion
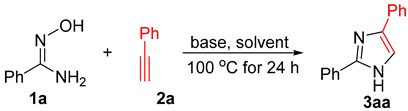
We firstly examined the reaction of benzamidoxime (N′-hydroxybenzimidamide) (1a) with phenyl acetylene (2a, 2.0 equivalent) in the presence of Na2CO3 (4.0 equivalent) in DMSO at 100 °C for 24 h; fortunately, 2,4-diphenylimidazole (3aa) could be isolated from the reaction mixture in a 10% yield (Table 1, entry 1). The structure of 3aa was characterized by its 1H- and 13C-NMR spectral data, which are the same as the reported ones. In addition, 3aa was recrystallized in a mixed solvents of petroleum/EtOAc/EtOH as white crystals, its X-ray diffraction studies confirm the structure unambiguously [36].
Table 1.
Optimizing reaction conditions for imidazole synthesis a.
| Entry | 2a (equiv) | Base(equiv) | Solvent | Yield(%)b |
|---|---|---|---|---|
| 1 | 2.0 | Na2CO3(4) | DMSO | 10 |
| 2 | 2.0 | K2CO3(4) | DMSO | 34 |
| 3 | 2.0 | KOH(4) | DMSO | 53 |
| 4 | 2.0 | KOtBu(4) | DMSO | 41 |
| 5 | 2.0 | Cs2CO3(4) | DMSO | 75 |
| 6 | 2.0 | Cs2CO3(4) | THF | 10 |
| 7 | 2.0 | Cs2CO3(4) | Dioxane | 14 |
| 8 | 2.0 | Cs2CO3(4) | DMF | 21 |
| 9 | 1.0 | Cs2CO3(4) | DMSO | 59 |
| 10 | 1.5 | Cs2CO3(4) | DMSO | 68 |
|
11 12 13 14 |
2.0 2.0 2.0 2.0 |
Cs2CO3(2.5) Cs2CO3(1.0) Cs2CO3(0.5) -- |
DMSO DMSO DMSO DMSO |
73 33 19 0 |
a The reactions were carried out using 1a (1.0 mmol), 2a (1.0~2.0 equiv), and base in 4.0 mL of solvent in a sealed tube at 100 oC for 24 h. b yields of 3aa are isolated yields.
Several other inorganic bases, such as K2CO3, KOH, KOtBu and Cs2CO3, were then examined, and 3aa could be obtained in a 34~75% yield (Table 1, entries 2–5). These results indicate that, in DMSO, Cs2CO3 is the best base for the present transformation, thus, the influence of other solvents and the amounts of 1a and Cs2CO3 were investigated. As shown in entries 6–8, when THF, 1,4-dioxane and DMF were used as solvents to replace DMSO, the yields of 3aa were decreased significantly. In addition, decreasing amounts of 2a (from 2.0 equivalent to 1.5 equivalent or 1.0 equivalent) resulted in the considerable decrease of yields (Table 1, entries 9–10). Although the use of 2.5. of Cs2CO3 also gave results similar to those in entry 5 (Table 1, entry 11 vs. entry 5), the yields of 3aa were reduced when 1.0 equivalent or 0.5. of Cs2CO3 were used (Table 1, entries 12–13). In addition, as discussed above, the base is the key promoter to promote the formation of imidazoles, the absence of Cs2CO3 led to no 3aa formation at all (Table 1, entry 14).
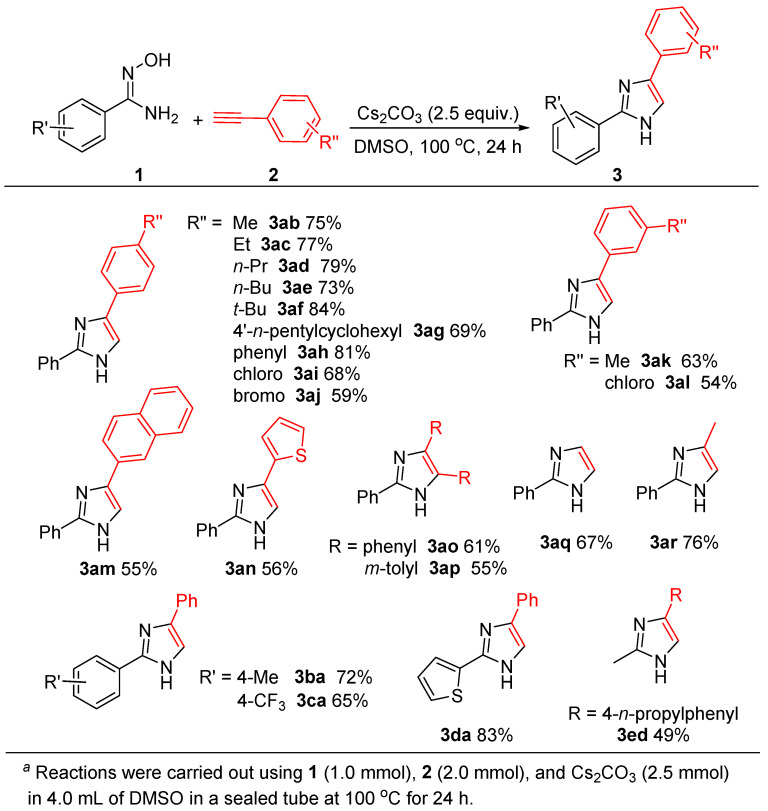
With the optimized conditions established (Table 1, entry 11), we then investigated the scope and generality of the imidazole formation with the use of various alkynes bearing electron-donating and electron-withdrawing groups, as well as several amidoximes, and the obtained results are concluded in Scheme 2. The reactions of 1a with various aromatic terminal alkynes bearing electron-donating groups and electron-withdrawing groups could occur smoothly, to give the corresponding imidazoles in moderate to good yields. It was noted that para-alkyl-substituted aromatic alkynes (R″ = Me, 2b; Et, 2c; n-Pr, 2d; n-Bu, 2e; t-Bu, 2f; 4′-n-pentylcyclohexyl, 2g) showed high reactivity to produce the desired products (3ab ~ 3ag) in 69–84% yields. para-Phenyl phenyl acetylene (2h) reacted with 1a afforded 3ah in a high yield (81%). The reaction of para-chlorophenyl acetylene (2i), an electron-poor alkyne, with 1a gave the product 3ai in a moderate yield (68%). In addition, the reaction of 1a with para-bromophenyl acetylene (2j) afford 3aj in a 59% yield. These results were apparent that the electron-donating group on aromatic terminal alkynes would benefit the formation of imidazoles.
Scheme 2.
Substrate scope of imidazole synthesis a.
When meta-substituted aromatic terminal alkynes were used, the reactions gave slight decrease in yields as compared to para-substituted ones. For example, the reactions of 1a with (3-methylphenyl)ethyne (2k), or with (3-chlorophenyl)ethyne (2l) gave 3ak in a 63% yield (vs. 3ab 75%) and 3al in a 54% yield (vs. 3ag 68%), respectively. It was noted that, in these cases, alkyne bearing electron-donating group also show relatively high reactivity (3ak vs. 3al), similar to the results as R″ at para-position.
In addition, the present reaction conditions are also suitable for the annulation of 1a with 2-naphthylacetylene (2m), and with 2-thienylacetylene (2n), a heteroaromatic terminal alkyne, afforded 3am and 3an in 55% and 56% yields, respectively.
Moreover, under the similar conditions, internal alkynes can also undergo the cyclocondensation with 1a to produce the corresponding 2,4,5-trisubstituted imidazoles. For instance, the reactions of 1a with 1,2-diphenyacetylene (2o), or with 1,2-bis(m-tolyl)acetylene (2p) gave 3ao and 3ap in 61% and 55% yields.
Interestingly, when 1-(trimethylsilyl)acetylene (2q) and 1-methyl-2-trimethylsilylacetylene (2r) were employed, the reactions with 1a produced 2-phenylimidazole (3aq, 67%) and 2-phenyl-4- methylimidazole (3ar, 76%), indicating that desilylation took place smoothly under the used basic conditions.
On the other hand, the substituent (R′) effect on the aryl group of 1 was also investigated, and the results from two representative examples with the use of either electron-donating group (para-Me, 1b) or electron-withdrawing group (para-CF3, 1c) were reported. As shown in Scheme 2, the corresponding products of 3ba and 3ca could be obtained in 72% and 65% yields, respectively. Again, the electron-donating group is favorable for the formation of imidazole ring.
Additionally, the present reaction conditions could be applied to heteroarylamidoximes and alkylamidoximes. For example, the reaction between 2-thienylamidoxime (1d) and 2a afford 3da in a 83% yield, and the reaction of acetamidoxime (1e) with 2d produced the expected product of 3ed in a 49% yield.
It is worth noting that the present reaction conditions are tolerant to C(sp2)-Cl and C(sp2)-Br bonds, the obtained products bearing C(sp2)-X bonds have a highly potential application in organic synthesis via their cross-coupling reactions.
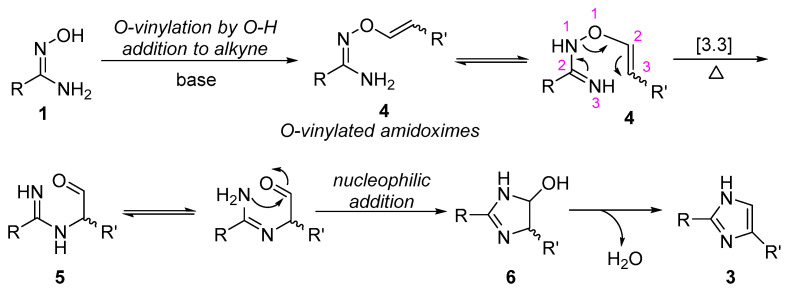
Amidoximes have been well known to be the useful building blocks for the construction of nitrogen-heterocyclic compounds, and the five-membered nitrogen-heterocyclic compounds formation is usually proposed to involve the step of N-O bond cleavage via 3,3-sigmatropic rearrangement [37,38,39]. Therefore, on the basis of our results and the known chemistry of amidoximes, a possible reaction mechanism for the formation of imidazole ring is shown in Scheme 3. It involves the regioselective nucleophilic addition of O-H bond to alkyne under basic conditions, giving an o-vinylated amidoxime (4), which undergoes sequential 3,3-sigmatropic rearrangement forming intermediate 5, and intramolecular nucleophilic addition of nitrogen atom to aldehyde affording five-membered heterocyclic intermediate 6, followed by dehydration to form imidazole ring.
Scheme 3.
Proposed mechanism for the formation of imidazoles.
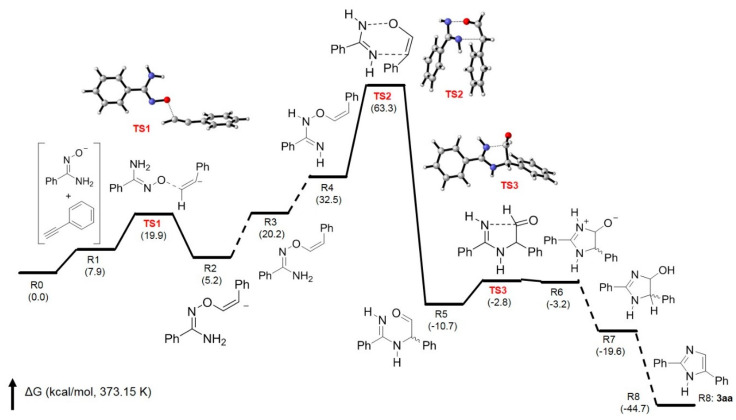
In order to support the proposed mechanism, a theoretical calculation was conducted by using the quantum chemistry program Gaussian 16 [40], and all structures were optimized by using M06-2X Minnesota functional with the 6-31G(d,p) basis set [41]. Figure 2 shows the free energy changes in the base-promoted annulation of 1a with 2a forming 3aa with the transition states for the formation of key intermediates 4–6. It clearly indicates that the transition state for the formation of intermediates 4 (from TS1) and 6 (from TS3) can be found with low activation energies. The key step for the formation of imidazole ring is the 3,3-sigmatropic rearrangement to give intermediate 5. The transition state (TS2) between R4 (intermediate 4) and R5 (intermediate 5) is not very high in activation energy, with 30.8 kcal/mol (TS2-R4), and the Gibbs free energy change in this step is −43.2 kcal/mol. These results have confirmed that at 100 °C (373.15 K, reaction temperature), 3,3-sigmatropic rearrangement can occur smoothly to construct imidazole ring as the proposed routes shown in Scheme 3.
Figure 2.
Free energy profile for the base-promoted annulation of 1a with 2a affording 3aa.
3. Materials and Methods
3.1. General Methods
All commercial reagents are analytically pure and used directly without further purification. Nuclear magnetic resonance (NMR) spectra were recorded on an ECA-400 spectrometer (JEOL, Tokyo, Japan) using DMSO-d6 as solvent at 298 K. 1H-NMR (400 MHz) chemical shifts (δ) were referenced to internal standard TMS (for 1H, δ = 0.00 ppm). 13C-NMR (100 MHz) chemical shifts were referenced to internal solvent DMSO-d6 (for 13C, δ = 39.52 ppm). Mass spectra (MS) were obtained on a GC-MS-QP2010S (Shimadzu, Tokyo, Japan), and the high-resolution mass spectra (HRMS) with electron spray ionization (ESI) were obtained with a micrOTOF-Q spectrometer (Agilent, California, CA, USA). Single crystals of 3aa were obtained by slow evaporation of their solution in a mixture solvents of petroleum, ethyl acetate and EtOH.
3.2. Typical Experimental Procedure for the Synthesis of 2,4-Diphenyl-1H-imidazole (3aa)
A mixture of benzamidoxime (1a, 136.1 mg, 1.0 mmol), phenylacetylene (2a, 204.1 mg, 2.0 mmol) and Cs2CO3 (815.1 mg, 2.5 mmol) in DMSO (4.0 mL), in a 25 mL screw-capped thick-walled Pyrex tube was stirred at 100 °C for 24 h in an oil bath. After the reaction mixture was cooled to room temperature, it was poured into a solvent mixture of water (50.0 mL) and ethyl acetate (20.0 mL), and the two phases were then separated. The aqueous layer was extracted with ethyl acetate (3 × 20.0 mL). The combined organic extracts were dried over anhydrous Na2SO4. After removal of the solvent under reduced pressure, the residue was purified by column chromatography on silica gel with petroleum ether/ethyl acetate (gradient mixture ratio from 100:0 to 70:20) as eluent, to afford 3aa as a white solid (160.4 mg, 73%).
The characterization data for known products of 3aa, 3ab, 3af, 3ah-3al, 3an, 3ao, 3aq, 3ar, 3ba, 3ca and 3da reported in the Supplementary Materials. Each of 3ac, 3ad, 3ae, 3ag, 3am, 3ap and 3ed are new compounds, and their spectroscopic data are reported below.
3.3. Characterization Data of Products
4-(4-Ethylphenyl)-2-phenyl-1H-imidazole (3ac):
White solid (190.4 mg, 77%). 1H-NMR (400 MHz, DMSO-d6) δ 12.71 (s, 1H), 8.17 (d, J = 7.6 Hz, 2H), 7.87 (d, J = 7.6 Hz, 2H), 7.70 (s, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.37 (t, J = 7.3 Hz, 1H), 7.25 (d, J = 8.0 Hz, 2H), 2.61 (q, J = 7.5 Hz, 2H), 1.20 (t, J = 7.5 Hz, 3H); 13C-NMR (100 MHz, DMSO-d6) δ 146.1, 141.9, 130.8, 128.7, 128.0, 127.9, 125.0, 124.5, 28.0, 15.6 HRMS (ESI) m/z: [M + H]+ calcd for C17H16N2, 249.1386; found 249.1388.
2-Phenyl-4-(4-n-propylphenyl)-1H-imidazole (3ad):
White solid (206.7 mg, 79%). 1H-NMR (400 MHz, DMSO-d6) δ 12.64 (sbr, 1H), 8.15 (d, J = 7.7 Hz, 2H), 7.85 (d, J = 7.7 Hz, 2H), 7.66 (s, 1H), 7.49 (t, J = 7.5 Hz, 2H), 7.38 (t, J = 7.3 Hz, 1H), 7.22 (d, J = 7.8 Hz, 2H), 2.56 (t, J = 7.4 Hz, 2H), 1.69–1.53 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H); 13C-NMR (100 MHz, DMSO-d6) δ 146.0, 140.2, 130.8, 129.3, 128.5, 128.4, 127.9, 125.0, 124.4, 118.7, 115.3, 37.0, 24.0, 13.5; HRMS (ESI) m/z: [M + H]+ calcd for C18H18N2, 263.1543; found 263.1540.
4-(4-Butylphenyl)-2-phenyl-1H-imidazole (3ae):
White solid (201.3 mg, 73%). 1H NMR (400 MHz, DMSO-d6) δ 12.64 (sbr, 1H), 8.14 (d, J = 7.4 Hz, 2H), 7.83 (d, J = 7.3 Hz, 2H), 7.65 (s, 1H), 7.48 (t, J = 7.2 Hz, 2H), 7.36 (t, J = 7.0 Hz, 1H), 7.21 (d, J = 7.5 Hz, 2H), 2.58 (t, J = 7.3 Hz, 2H), 1.63–1.50 (m, 2H), 1.40–1.22 (m, 2H), 0.90 (t, J = 7.1 Hz, 3H); 13C-NMR (100 MHz, DMSO-d6) δ 146.0, 140.3, 130.8, 129.3, 128.5, 128.3, 127.8, 124.9, 124.4, 34.6, 33.1, 21.7, 13.7; HRMS (ESI) m/z: [M + H]+ calcd for C19H20N2, 277.1699; found 277.1698.
4-(4-(4-n-Pentylcyclohexyl)phenyl)-2-phenyl-1H-imidazole (3ag):
White Solid (256.7 mg, 69%). 1H-NMR (400 MHz, DMSO-d6) δ 12.58 (s, 1H), 8.00 (d, J = 7.6 Hz, 2H), 7.74 (d, J = 7.8 Hz, 2H), 7.63 (s, 1H), 7.46 (t, J = 7.6 Hz, 2H), 7.35 (t, J = 7.3 Hz, 1H), 7.22 (d, J = 8.0 Hz, 2H), 2.50–2.38 (m, 1H), 1.83–1.80 (m, 4H), 1.52–1.37 (m, 2H), 1.35–1.13 (m, 10H), 1.10–0.95 (m, 2H), 0.88 (t, J = 6.6 Hz, 3H); 13C-NMR (100 MHz, DMSO-d6) δ 145.8, 145.4, 130.6, 128.6, 128.0, 126.7, 124.8, 124.3, 43.9, 36.8, 36.6, 33.8, 33.1, 31.6, 26.0, 22.1, 13.9; HRMS (ESI) m/z: [M + H]+ calcd for C26H32N2, 373.2638; found 373.2635.
4-(Naphthalen-2-yl)-2-phenyl-1H-imidazole (3am):
White solid (148.3 mg, 55%). 1H-NMR (400 MHz, DMSO-d6) δ 12.72 (s, 1H), 8.39 (s, 1H), 8.09–7.85 (m, 7H), 7.55–7.34 (m, 5H); 13C-NMR (100 MHz, DMSO-d6) δ 146.0, 140.9, 133.3, 132.1, 131.8, 130.5, 128.6, 128.1, 127.7, 127.6, 127.4, 126.1, 125.1, 125.0, 124.9, 123.7, 121.7, 114.9; HRMS (ESI) m/z: [M + H]+ calcd for C19H14N2, 271.1230; found 271.1235.
2-Phenyl-4,5-di-m-tolyl-1H-imidazole (3ap):
White solid (178.1 mg, 55%). 1H-NMR (400 MHz, DMSO-d6) δ 12.59 (s, 1H), 8.08 (d, J = 8.3 Hz, 3H), 7.50–7.43 (m, 3H), 7.40–7.24 (m, 5H), 7.22–7.12 (m, 2H), 7.04 (d, J = 6.9 Hz, 1H), 2.34 (s, 3H), 2.27 (s, 3H); 13C-NMR (100 MHz, DMSO-d6) δ 145.2, 137.6, 137.0, 135.0, 130.9, 130.3, 129.5, 128.8, 128.5, 128.3, 128.2, 128.1, 128.0, 127.8, 127.6, 127.0, 125.5, 125.1, 124.1, 21.0, 20.9; HRMS (ESI) m/z: [M + H]+ calcd for C23H20N2, 325.1699; found 325.1697.
2-Methyl-4-(4-n-propylphenyl)-1H-imidazole (3ed):
Waxy oil (98.1 mg, 49%). 1H-NMR (400 MHz, DMSO-d6) δ 7.55 (d, J = 8.1 Hz, 2H), 7.28 (s, 1H), 7.09 (d, J = 8.2 Hz, 2H), 2.50–2.41 (m, 2H), 2.26 (s, 3H), 1.60–1.46 (m, 2H), 0.85 (t, J = 7.3 Hz, 3H); 13C-NMR (100 MHz, DMSO-d6) δ 144.1, 1, 139.5, 131.5, 128.4, 123.9, 36.9, 24.0, 13.9, 13.6; HRMS (ESI) m/z: [M + H]+ calcd for C13H16N2, 201.0681; found 201.0687.
4. Conclusions
In summary, we developed a simple and efficient method to prepare 2,4-disubstituted imidazoles in moderate to good yields from easily available starting materials of amidoximes and terminal alkynes promoted by Cs2CO3 in DMSO. The significant advantages of the present procedure include the formation of imidazoles under transition-metal-free and ligand-free conditions, high atom-utilization, and a broad substrate scope. 2-substituted and 2,4,5-trisubstituted imidazoles could be also prepared under the similar conditions by using 1-(trimethylsilyl)acetylene and internal alkynes.
Acknowledgments
We thank Tsinghua Xuetang Talents Program for computational hardware and software support.
Supplementary Materials
The following are available online. The characterization data of the known products, copies of 1H- and 13C-NMR charts of all products, X-ray structural details (including CIF files) of 3aa, and computational predicted energies and cartesian coordinates.
Author Contributions
Investigation, writing—original draft preparation, H.M.; investigation, M.A.I. & L.L.; conceptualization, supervision, writing—review and editing, R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (21673124). Hina Mehmood and Muhammad Asif Iqbal thank the China Scholarship Council (CSC) for generous support for their study in Tsinghua University as PhD candidates.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the products are not available from the authors.
References and Notes
- 1.Ward K.E., Hume A.L. Olmesartan (Benicar) for hypertension. Am. Fam. Physician. 2005;72:673–674. [Google Scholar]
- 2.Ianiro G., Bibbo S., Montalto M., Ricci R., Gasbarrini A., Cammarota G. Systematic review: Sprue-like enteropathy associated with olmesartan. Aliment. Pharmacol. Ther. 2014;40:16–23. doi: 10.1111/apt.12780. [DOI] [PubMed] [Google Scholar]
- 3.Choi E.Y.K., McKenna B.J. Olmesartan-associated enteropathy: A review of clinical and histologic findings. Arch. Pathol. Lab. Med. 2015;139:1242–1247. doi: 10.5858/arpa.2015-0204-RA. [DOI] [PubMed] [Google Scholar]
- 4.Redon J., Weber M.A., Reimitz P.-E., Wang J.-G. Comparative effectiveness of an angiotensin receptor blocker, olmesartan medoxomil, in older hypertensive patients. J. Clin. Hypertens. 2018;20:356–365. doi: 10.1111/jch.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntyre M., Caffe S.E., Michalak R.A., Reid J.L. Losartan, an orally active angiotensin (AT1) receptor antagonist: A review of its efficacy and safety in essential hypertension. Pharmacol. Ther. 1997;74:181–194. doi: 10.1016/S0163-7258(97)82002-5. [DOI] [PubMed] [Google Scholar]
- 6.Conlin P.R. Efficacy and safety of angiotensin receptor blockers: A review of Losartan in essential hypertension. Curr. Ther. Res. 2001;62:79–91. doi: 10.1016/S0011-393X(01)80019-9. [DOI] [Google Scholar]
- 7.Rubio-Guerra A.F., Garro-Almendaro A.K., Elizalde-Barrera C.I., Suarez-Cuenca J.A., Duran-Salgado M.B. Effect of losartan combined with amlodipine or with a thiazide on uric acid levels in hypertensive patients. Ther. Adv. Cardiovasc. Dis. 2017;11:57–62. doi: 10.1177/1753944716678538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oxford A.W., Bell J.A., Kilpatrick G.J., Ireland S.J., Tyers M.B. Ondansetron and related 5-HT, antagonists: Recent advances. Prog. Med. Chem. 1992;29:239–270. doi: 10.1016/s0079-6468(08)70010-9. [DOI] [PubMed] [Google Scholar]
- 9.Del Favero A., Roila F., Tonato M. Reducing chemotherapy-induced nausea and vomiting. Current perspectives and future possibilities. Drug Saf. 1993;9:410–428. doi: 10.2165/00002018-199309060-00004. [DOI] [PubMed] [Google Scholar]
- 10.Christofaki M., Papaioannou A. Ondansetron: A review of pharmacokinetics and clinical experience in postoperative nausea and vomiting. Expert. Opin. Drug Metab. Toxicol. 2014;10:437–444. doi: 10.1517/17425255.2014.882317. [DOI] [PubMed] [Google Scholar]
- 11.Dewinter G., Staelens W., Veef E., Teunkens A., Van de Velde M., Rex S. Simplified algorithm for the prevention of postoperative nausea and vomiting: A before-and-after study. Br. J. Anaesth. 2018;120:156–163. doi: 10.1016/j.bja.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Baumann M., Baxendale I.R., Ley S.V., Nikbin N. An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J. Org. Chem. 2011;7:442–495. doi: 10.3762/bjoc.7.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rani N., Sharma A., Singh R. Imidazoles as promising scaffolds for antibacterial activity: A review. Mini Rev. Med. Chem. 2013;13:1812–1835. doi: 10.2174/13895575113136660091. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L., Peng X.-M., Damu G.L.V., Geng R.-X., Cheng-He Zhou C.-H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014;34:340–437. doi: 10.1002/med.21290. [DOI] [PubMed] [Google Scholar]
- 15.Ali I., Lonea M.N., Aboul-Enein H.Y. Imidazoles as potential anticancer agents. Med. Chem. Commun. 2017;8:1742–1773. doi: 10.1039/C7MD00067G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauman J.E., Jr., Wang J.C. Imidazole complexes of Nickel (II), Copper (II), Zinc (II) and Silver (I) Inorg. Chem. 1963;3:368–373. doi: 10.1021/ic50013a014. [DOI] [Google Scholar]
- 17.Gómez E., Huertos W.A., Pérez J., Riera L., Menéndez-Velázquez A. Organometallic complexes with terminal imidazolato ligands and their use as metalloligands. Inorg. Chem. 2010;49:9527–9534. doi: 10.1021/ic1012395. [DOI] [PubMed] [Google Scholar]
- 18.Kamijo S., Yamamoto Y. Recent progress in the catalytic synthesis of imidazoles. Chem. Asian J. 2007;2:568–578. doi: 10.1002/asia.200600418. [DOI] [PubMed] [Google Scholar]
- 19.Bellina F., Cauteruccio S., Rossi R. Synthesis and biological activity of vicinal diaryl-substituted 1H-imidazoles. Tetrahedron. 2007;63:4571–4624. doi: 10.1016/j.tet.2007.02.075. [DOI] [Google Scholar]
- 20.Daraji D.G., Prajapati N.P., Patel H.D. Synthesis and applications of 2-substituted imidazole and its derivatives: A review. J. Heterocycl. Chem. 2019;56:2299–2317. doi: 10.1002/jhet.3641. [DOI] [Google Scholar]
- 21.Chen C.-Y., Hu W.-P., Yan P.-C., Senadi G.C., Wang J.-J. Metal-free, acid-promoted synthesis of imidazole derivatives via a multicomponent reaction. Org. Lett. 2013;15:6116–6119. doi: 10.1021/ol402892z. [DOI] [PubMed] [Google Scholar]
- 22.Lee C.F., Holownia A., Bennett J.M., Elkins J.M., St. Denis J.D., Adachi S., Yudin A.K. Oxalyl boronates enable modular synthesis of bioactive imidazoles. Angew. Chem. Int. Ed. 2017;56:6264–6267. doi: 10.1002/anie.201611006. [DOI] [PubMed] [Google Scholar]
- 23.Dubovtsev A.Y., Dar’in D.V., Krasavin M., Kukushkin V.Y. Gold-catalyzed oxidation of internal alkynes into benzils and its application for one-pot synthesis of five-, six- and seven-membered azaheterocycles. Eur. J. Org. Chem. 2019:1856–1864. doi: 10.1002/ejoc.201900108. [DOI] [Google Scholar]
- 24.Naidoo S., Jeena V. One-pot, two-step metal and acid-free synthesis of trisubstituted imidazole derivatives via oxidation of internal alkynes using an iodine/DMSO system. Eur. J. Org. Chem. 2019:1107–1113. doi: 10.1002/ejoc.201801584. [DOI] [Google Scholar]
- 25.Li J., Neuville L. Copper-catalyzed oxidative deamination of terminal alkynes by amidines: Synthesis of 1,2,4-trisubstituted imidazoles. Org. Lett. 2013;15:1752–1755. doi: 10.1021/ol400560m. [DOI] [PubMed] [Google Scholar]
- 26.Chen X.Y., Englert U., Bolm C. Base-mediated syntheses of di- and trisubstituted imidazoles from amidine hydrochlorides and bromoacetylenes. Chem. Eur. J. 2015;21:13221–13224. doi: 10.1002/chem.201502707. [DOI] [PubMed] [Google Scholar]
- 27.Su J., Chen Q., Lu L., Ma Y., Auyoung G.H.L., Hua R. Base-promoted nucleophilic fluoroarenes substitution of C-F bonds. Tetrahedron. 2018;74:303–307. doi: 10.1016/j.tet.2017.11.067. [DOI] [Google Scholar]
- 28.Iqbal M.A., Lu L., Mehmood H., Khan D.M., Hua R. Quinazolinone synthesis through base-promoted SNAr reaction of ortho-fluorobenzamides with amides followed by cyclization. ACS Omega. 2019;4:8207–8213. doi: 10.1021/acsomega.9b00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iqbal M.A., Mehmood H., Lv J., Hua R. Base-promoted SNAr reactions of fluoro- and chloroarenes as a route to N-aryl indoles and carbazoles. Molecules. 2019;24:1145. doi: 10.3390/molecules24061145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q., Wang Y., Hua R. Base-promoted chemodivergent formation of 1,4-benzoxazepin-5(4H)-ones and 1,3-benzoxazin-4(4H)-ones switched by solvents. Molecules. 2019;24:3773. doi: 10.3390/molecules24203773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nizami T.A., Hua R. Cycloaddition of 1,3-butadiynes: Efficient synthesis of carbo- and heterocycles. Molecules. 2014;19:13788–13802. doi: 10.3390/molecules190913788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hua R., Nizami T.A. Synthesis of heterocycles by using propargyl compounds as versatile synthons. Mini-Rev. Org. Chem. 2018;15:198–207. doi: 10.2174/1570193X14666171114122235. [DOI] [Google Scholar]
- 33.Zheng L., Hua R. C–H activation and alkyne annulation via automatic or intrinsic directing groups: Towards high step economy. Chem. Rec. 2018;18:556–569. doi: 10.1002/tcr.201700024. [DOI] [PubMed] [Google Scholar]
- 34.Khan D.M., Hua R. Isoquinolone synthesis via Zn (OTf)2-catalyzed aerobic cyclocondensation of 2-(1-alkynyl) benzaldehydes with arylamines. Catalysts. 2020;10:683. doi: 10.3390/catal10060683. [DOI] [Google Scholar]
- 35.One similar structural substrate reaction between ethyl amino-oximinoacetate (in 1, R = COOEt) and methyl propiolate (an active alkyne) in the presence of Et3N under reflux xylene giving 2-carbethoxy-4-carbomethoxyimidazole in 70% yield was reported, see: Branco, P.S.; Prabhakar, S.; Lobo, A.M.; Wiliams, D.J. Reactions of hydroxylamines with ethyl cyanoformate. Preparation of aminonitrones and their synthetic applications. Tetrahedron 1992, 48, 6335–6360. Very recently, a microwave-assisted synthesis of imidazoles via nucleophilic catalysis has been reported by the addition of amidoximes to activated alkynes followed by a thermally induced rearrangement of the in situ generated O-vinylamidoxime intermediates, see: Shabalin, D.A.; Dunsford, J.J.; Ngwerume, S.; Saunders, A.R.; Gill, D.M.; Camp, J.E. Synthesis of 2,4-disubstituted imidazoles via nucleophilic catalysis. Synlett 2020, 31, 797–800.
- 36.The structure of 3aa was further confirmed by its x-ray diffraction studies (CCDC2012403), and the x-ray structural details are reported in Supplementary Materials.
- 37.Tabolin A.A., Ioffe S.L. Rearrangement of N-oxyenamines and related reactions. Chem. Rev. 2014;114:5426–5476. doi: 10.1021/cr400196x. [DOI] [PubMed] [Google Scholar]
- 38.Bolotin D.S., Bokach N.A., Demakova M.Y., Kukushkin V.Y. Metal-involving synthesis and reactions of oximes. Chem. Rev. 2017;117:13039–13122. doi: 10.1021/acs.chemrev.7b00264. [DOI] [PubMed] [Google Scholar]
- 39.Sridhar Madabhushi S., Vangipuram V.S., Mallu K.K.R., Chinthala N., Beeram C.R. Europium (III) triflate-catalyzed trofimov synthesis of polyfunctionalized pyrroles. Adv. Synth. Catal. 2012;354:1413–1416. doi: 10.1002/adsc.201200036. [DOI] [Google Scholar]
- 40.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., et al. Gaussian 16, Revision B.01. Gaussian, Inc.; Wallingford, CT, USA: 2016. [Google Scholar]
- 41.Zhao Y., Truhlar D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008;120:215–241. doi: 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.