Abstract
This study evaluated the effect of adding a new step, termed conditioning, to the traditional processing of leaves from Morus alba var. zolwinska wielkolistna grown in Poland (WML-P). This step, modeled on tea leaves processing, was conducted in a controlled environment on a semi-technical scale. The primary goal was to evaluate the effect of the WML-P conditioning for 1–4 h at 32–35 °C on the content of bioactive compounds (total phenolics, phenolic acids, flavonols, 1-deoxynojirimycin) and antioxidant activity (radical scavenging against DPPH, antioxidant capacity, chelating activity and ferric reducing antioxidant potential) of the lyophilized extracts. For the first time WML-P extracts content was comprehensively characterized by assessing dietary fiber fractions, fatty acids, amino acids, macro- and microelements and chlorophyll content. Compared to the traditional process, adding the conditioning step to WML-P processing resulted in an increased total phenolics content, radical scavenging capacity, ability to quench 2,2-diphenyl-1-picrylhydrazyl (DPPH•) and iron-chelating ability in the lyophilized extracts. The beneficial effect depended on conditioning time. The highest flavonols and phenolic acids content were found after 2-h conditioning. We concluded that adding a 2-h conditioning step to traditional WML-P processing results in getting WML-P lyophilized extract with increased bioactive compounds content and high antioxidant activity.
Keywords: Morus alba, antioxidant activity, conditioning, phenolic acids, flavonols, DNJ, polyphenols, semi-technical processing, ABTS test, DPPH test
1. Introduction
There are about 30 species of mulberry trees in the world, half of which are native to China. White mulberry (Morus alba L., Folium Mori) (WM) belongs to the Moraceae family [1]. In central Poland, a Polish variety of WM, var. zolwinska wielkolistna (WML-P) is cultivated. Typically, only fruits and leaves from the mulberry tree are utilized due to the ease of harvesting and low acquisition costs.
Traditionally, Morus alba, as other Morus species, has been utilized in Chinese, Japanese, Indian and Korean traditional medicine. Mulberry products have been used to nourish blood and benefit kidneys and as a treatment for weakness and fatigue, urinary incontinence, dizziness, tinnitus, constipation and in more recent years, are considered helpful in the treatment of some cancers [2]. Over time, mulberry products have also been used as a food component. Compared with other morphological parts, fruits are the most popular [3] and white mulberry leaves (WML) show the highest antioxidant activity.
It has been reported that antioxidants present in the WML products could potentially balance free radicals and protect against their destructive effects on genetic material and physiological processes.
Numerous reports from animal studies have documented strong antidiabetic properties of the WML [4]. In human studies, consumption of food products enriched with the WML extract added into diet resulted in lower glycemic increase compared to non-enriched products in both, patients with diabetes type 2 and healthy controls and reduced excessive body weight [5]. The compound responsible for the lowering of blood glucose concentration is 1-deoxynojirimycin (DNJ) and its derivatives, which are inhibitors of intestinal α-glucosidase.
WML products consumed with a diet are thought to protect against liver cancer, to prevent obesity-related non-alcoholic fatty liver, hypolipidemic or lipid-regulating properties in blood protect against atherosclerosis, to show anti-aggregative properties in platelet and anti-inflammatory effects or to reduce anxiety [6,7].
It has been reported that some medicinal plants show a neuroprotective effect [8]. For example, extracts from young leaves and mulberry shoots rich in oxyresveratrol are utilized in the treatment of neurodegenerative diseases. Most studies, did not find the toxic effects of WML preparations. Reported beneficial health and therapeutic properties of WML products are most likely associated with the presence of bioactive compounds, including flavonoids, phenolic acids, iminosugars, tannins, polysaccharides and alkaloids [9]
In many countries, WML products are commonly used as drought or tea infusion with a specific tonic, low toxicity and excellent therapeutic properties. Currently, many new technologies use WML products as a component of functional foods, often based on recipes derived from folk cuisine. Mulberry products are also used as supplements of traditional foods, such as dairy, bakery, bread and snacks [10,11].
In recent years, the mulberry tree is experiencing an increase in reproduction and cultivation, progress in pest management and finding or confirming the therapeutic properties of WML components [1]. The WML products on the market include a variety of teas, similar to ‘regular’ yellow, red or black tea made from Camellia sinensis, which very often use conditioning as part of processing [12]. To best of our knowledge, there are no studies, reporting if conditioning of WML would affects characteristics, properties and antioxidant capacity of WML products.
The WML processing in practice usually involves minimal processing but differences and especially drying and shredded temperatures affect the chemical composition, antioxidative properties and potential health benefits of the product [13].
Most of the currently available data relate to Asian raw material and do not include the description of mulberry leaves preparation. In one study, Chon et al. [14] used the fermentation step in the processing of Korean WML leaves form Korean trees. In their study, a methanol extract from fermented leaves showed higher than unfermented leaves antiproliferative activity in the human stomach and colon cancer cells. Although the study may indicate that increased antioxidant properties of fermented extract. The study did not report the effect of fermentation on changes in the antioxidant activity if the extracts.
In previous work, we showed that differences in WML-P antioxidant activity depend on drying temperatures [13] and the WML-P extracts included in diet reduce hyperglycemia in diabetic rats [15]. However, we did not use leaves conditioning process or assess the content of antioxidants or certain compounds with certain potential health benefits in the dried extracts.
Therefore, this study aimed to investigate if adding of processing step termed conditioning to traditional WML-P processing would affect chemical composition and characteristics of the lyophilized extracts and improve its nutritional quality and functional properties, including antioxidant capacity.
Our primary hypothesis was that WML-P conditioning will cause an increase in the antioxidant capacity of the lyophilized extract. Our secondary hypothesis was that changes in the functional properties of WML-P will depend on conditioning time.
2. Material and Methods
2.1. Plant Material
2.1.1. Conditioning
Fresh white mulberry (Morus alba L.) leaves form Polish var. zolwinska wielkolistna (WML-P) were picked manually at the mulberry tree farm in Pętkowo (Institute of Natural Fibres & Medicinal Plants), near Poznan, central Poland, in June and July 2011.
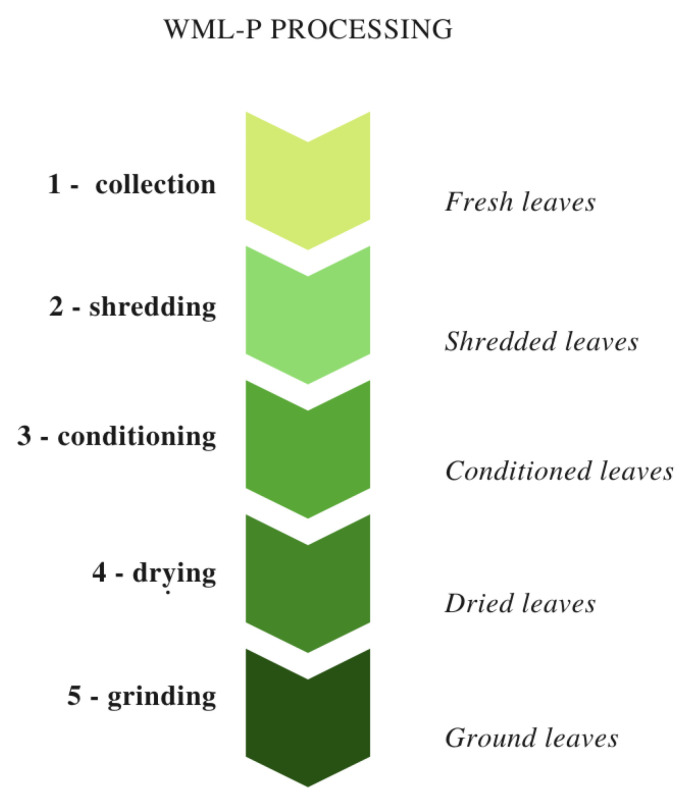
Technological processing of WML-P was conducted at the Institute of Agricultural and Food Biotechnology in Poznan, Poland (IAFB) (Figure 1). The raw leaves were shredded using a chopper/crusher (Stihl, Viking GE 103, Tengen, Germany) into particles measuring approximately 50–70 mm in length and 10–20 mm in width, divided into 5 batches and placed on wooden sieve trays in prisms (100 cm long, 40 cm wide, 10–15 cm high). Leaves from on one tray were not conditioned and used as a control sample. The remaining 4 trays were conditioned in the industrial dryer (SSO, Izoterma, Łany, Poland) for 1 h, 2 h, 3 h or 4h at 32.0 °C ± 3.0. Each of WML-P samples was air-dried in the tunnel dryer (IAFB, Poznań, Poland) at inlet temperature 90 °C and outlet temperature 60 °C. Dried WML-P from each batch were grounded (400 rpm for 15 s) in the laboratory mill (Retsch, GM200, Haan, Germany) to a powder (0.8–0.08 mm) and stored in polyethylene bags at room temperature for processing and analyzes.
Figure 1.
Technological processing of var. zolwinska wielkolistna (WML-P), including controlled conditioning, a novel step in traditional Morus alba leaf processing.
2.1.2. Extraction Process
Aqueous extracts were prepared from 10.0 g ± 0.2 of shredded WML-P. Each WML-P sample was extracted two times by mixing with boiling distilled water (100 mL and 40 mL) and heat for 15 min in 100 °C (EM, Thermo Scientific, Waltham, MA, USA). The extract was separated from the sediment and filtered using the Büchner funnel and Whatman filter paper (No.1, 1–11 µm). Filtered samples were lyophilized (Christ Alpha 1–4 LSC, p < 1 mBa, capacitor temperature −50 °C, shelf temperature 20 °C) and the dried extracts stored in plastic bags (in refrigerator at 4.0 °C) for further analyzes.
2.2. Chemical Composition
2.2.1. Basic Chemical Composition
Total protein, fat, ash and dry matter contents were determined the AOAC methods [16]. The total carbohydrates content (g/100 g) was calculated using the equation:
| (1) |
2.2.2. Dietary Fiber Fractions
The contents of total dietary fiber (TDF), soluble dietary fiber (SDF) and insoluble dietary fiber (IDF) were measured using Fibertec System E 1023 (Foss Tecator, Hilleroed, Sweden). The content of neutral dietary fiber (NDF), acid detergent fiber (ADF), acid detergent lignin (ADL) and cellulose (C) was determined using the detergent [17]. The content of hemicellulose (H) was calculated as follows:
| (2) |
Analyses were conducted using the Fibertec System M 1020 (Foss Tecator, Hilleroed, Sweden).
2.2.3. Fatty Acids (FA) Composition
The fatty acids composition was determined according to the standard methodology [18] using Agilent 7820A GC (Agilent Technologies, Santa Clara, CA, USA) equipped with a flame ionization detector (FID) and SP-2560 (100 m, 0.25 mm, 0.20 µm) column (Supelco, Bellefonte, PA, USA). The FA methyl esters (FAME) were separated under the following conditions—the initial oven temperature 150 °C and increased to 200 °C at 1.5 °C/min; the injector and detector temperatures were 250 °C; split 1:10; injection volume 0.5 µL and the helium at flow rate 1 mL/min was used as carrier gas. The FAME were identified by comparing with commercially available standards (Grain Fatty Acid Methyl Ester Mix, Supelco, Bellefonte, PA, USA). The results were expressed as % of total FA [19].
2.2.4. Amino Acids (AA)
The AA content was determined with HPLC (Nexera X2 Shimadzu LC-30AD, Kyoto, Japan) equipped with a DAD detector (SPD-M30A, Shimadzu, Kyoto, Japan), using Waters column (ACCQ-TAG Ultra C18, 100 mm, 2.1 mm, 1.7 µm) at λ = 260 nm). First, samples were hydrolyzed according to AOAC methods [20,21]. The AA were separated under the following conditions—the injection volume was 1 µL; flow rate 0.6 mL/min; separation temperature 55 °C; total method time 22 min; separation time 18 min; the mobile phases were based on C2H3N (ready-to-use Waters eluents) as 100% solution of AssQ-Taq Ultra (eluent A) and 5% solution of AssQ-Taq Ultra (eluent B); elution was initiated at 100% of eluent A and 0% eluent B. Non-linear gradient separation was a gradual increase in the ratio of phase B to phase A. The level of eluent B increased to a maximum of 59.6% and then decreased to 0%. Qualitative and quantitative analyses were conducted by comparison with commercially available standards (Waters, Elstree, UK) dissolved in water. The results were expressed as g/100 g.
2.2.5. Macro- and Microelements
The atomic absorption spectrometer equipped with a flame atomizer (AAS-3, Carl-Zeiss Jena, Jena, Germany) was used to determine contents of magnesium (Mg), calcium (Ca), manganese (Mn), iron (Fe), copper (Cu), zinc (Zn), sodium (Na) and potassium (K). The WML-P samples were mineralized in a microwave oven (Mars-5, CEM Co., Charlotte, NC, USA) with nitric acid (65%) [22]. The results were expressed in µg/g or mg/g of dried leaves. Certificated materials Soy Bean Flour INCT-SBF-4 and Bovine Liver-Trace Elements NIST-1577 c (Institute of Nuclear Chemistry and Technology in Warsaw, Poland) were used as reference standards.
2.2.6. Chlorophyll a and b
The contents of chlorophyll a and b were determined using the Eder method [23]. Absorbance in the methanol fraction was measured using a Metertech SP-830 PLUS (Nangang, Taipei, Taiwan) spectrophotometer at a wavelength of 645 nm (chlorophyll b) and 663 nm (chlorophyll a).
2.2.7. Phenolic Acids and Flavonols
Phenolic acids: gallic acid (GAL), protocatechuic acid (PRO), 4-hydroxybenzoic acid (HYD), vanillic acid (VAN), caffeic acid (CAF), chlorogenic acid (CHL), p-coumaric acid (CUM), ferullic acid (FER), sinapic acid (SIN) and flavonols: rutin (RUT), 3-β-glucoside (isoquercitrin) (ISQ), quercetin 3-O-(6”-O-malonyl)-β-d-glucoside (MAL), kaempferol 3-glucoside (astragalin) (AST), myricetin (MYR), quercetin (QUE), kaempferol (KEM), isorhamnetin (ISR) contents were determined using HPLC/DAD (Agilent Infinity 1290, CA, USA). A filtered (0.45 µm PTFE filters, Millipore) sample (20 µL) was injected into the thermostated C18 Zorbax SB column (15 mm × 3.9 mm I.D., 5 µm; Agilent Technology, Santa Clara, CA, USA) according to a method in References [24,25] with our own modifications. The mobile phases were H3PO4 (pH = 2.7) (solvent A) and C2H3N (50%) (solvent B) at a flow rate of 1.0 mL/min (average flow rate 1.99 min, ranging from 6.10 to 76.00 min) and 0.6 mL/min (from 2.00 to 6.00). Elution was initiated at 90% of solvent A, maintained for 27 min and then decreased to 60% in 29 min and maintained for 5 min and then decreased to 56% in 2 min and maintained for 8 min. Finally, starting at 71 min, the concentration of solvent A was increased to 90% in 1 min and maintained until 76 min. Chromatograms were recorded at a wavelength of 260 nm, 310 nm, 370 nm. The identification of the compounds was performed by comparing the retention times and photodiode array spectra with those obtained for reference standards. Quantification of identified phenolic acids and flavonols was made by comparing the area under the peaks with those of the standard (Sigma-Aldrich, St. Louis, MO, USA). Accuracy of the method in our laboratory was 96.99–101.95%, limit of detection (LOD) was 3.3 ng/mL and the limit of quantification (LOQ) was 10.0 ng/mL.
2.2.8. Total Phenolics
Total phenolics content in the dried WML-P was determined using the Folin-Ciocalteu colorimetric method [26]. Results were expressed as gallic acid equivalent (mg GAE/g of dried leaves).
2.2.9. 1-Deoxynojirimycin (DNJ)
High-performance liquid chromatography with fluorescence detector (Ex: 254 nm, Em: 322 nm) (Agilent Infinity 1260, Santa Clara, CA, USA) was used to determine the content of DNJ. The separation was done with TSKgel Tosoh Amide-80 (250 mm × 4.6 mm ID × 5 µm) column (Tosoh Bioscience, Tokyo, Japan), according to Kim et al. [27] with slight modifications. The sample (200 mg) was mixed with HCl (1 mL, 0.05 M), vortexed and centrifuged (15 min, Eppendorf Centrifuge 5702R, 4400 rpm). The supernatant was separated and 10 µL sample was mixed with potassium borate buffer (0.4 M, pH 8.5, 10 µL), mixed with 2 µL of FMOC-Cl (5 mM) in 1.5 mL microtube and allowed to react at 20 °C for 20 min in a water circulator. The reaction was terminated by adding 10 µL of glycine (0.1 M) and the mixture was diluted with 968 µL of CH3COOH (0.1%) and filtered through 0.45 µm syringe PTFE filter (Millipore). The sample (5 µL) was injected into the thermostated (30 °C ± 0.8) column by the thermostated autosampler (30 °C ± 1.0). The mobile phases were methanol (eluent A) and C2H3N with CH3COOH (0.1%) (v/v, 1:1) (eluent B) at flow rate of 1.0 mL/min from 0.00 min to 2.00 min, 0.5 mL/min from 2.00 min to 4.50 min and 1.2 mL/min from 7.00 min to 10.00 min. The elution was initiated with 55% of eluent A, increased to 75% for 2 min, maintained for 2.50 min, decreased to 55% eluent A in 2.10 min and maintained for the next 3 min. DNJ was identified by comparing the retention times and spectra with methanol-diluted reference standard and quantified by comparing the area under the peak to the standard. The accuracy of the method was 97.95% and the limit of detection (LOD) and quantification (LOQ) were 6.7 ng/mL and 20.0 ng/mL, respectively
2.3. Antioxidant Activity
2.3.1. Radical Scavenging Capacity Against DPPH
The free-radical scavenging potential of WML-P extracts was measured in the methanol solution of DPPH• (2,2-diphenyl-1-picrylhydrazyl) according to a method described by Amarowicz et al. [28]. Results were expressed as Trolox (TE) equivalent (µM TE/g of dried leaves).
2.3.2. Total Antioxidant Capacity with ABTS•+
The method was based on the SET (single electron transfer) mechanism, according to Re et al. [29] method and expressed as µM TE/g of dried leaves.
2.3.3. Chelating Activity
The chelating activity of WML-P extracts was determined using a method described by Tang et al. [30]. Deionized water was used as a control and ferrozine as a reference standard. The chelating activity was calculated as follows:
| (3) |
and expressed as a percentage (%) of reference standard activity.
2.3.4. Ferric Reducing Antioxidant Potential (FRAP) Assay
FRAP assay was conducted using Benzie and Strain [31] method. The results were expressed as FeCl3 equivalent (µM Fe2+) per mg of dried leaves.
2.4. Reagents
All the chemicals were of analytical grade or chromatographic grade, purchased from POCH, Gliwice, Poland or Merck, Darmstadt, Germany.
2.5. Statistical Analysis
All experiments were performed in triplicate and the results are mean ± SD. The amino acid content of WML-P analysis was performed in duplicate. Data were analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test. Statistical differences were calculated at the significance level p < 0.05 and represented by superscript letters. The statistical analysis was performed using Statistica software, version 13 (StatSoft, Kraków, Poland).
3. Results and Discussion
Similarly to WML cultivated in China, Korea, India, Japan and Thailand, WML-P extracts have excellent nutritional quality [32,33] and have been used in the prevention and/or treatment of chronic diseases, in particular diabetes [15]. This study reports the effects of a new step termed conditioning added to traditional WML-P processing on chemical composition, the content of bioactive components and antioxidative activity in the lyophilized WML-P extracts for the first time. This process simulated withering, fermentation and fixation processes used in tea productions. The conditioning parameters were previously tested in our laboratory and adapted to a semi-technical scale [13].
3.1. Chemical Composition
Dried WML-P contained approximately 99% of dry matter, 11–13% of protein, 1–2% of fat and 2–16% of ash (Table 1A). Noteworthy is protein content in the WML-P was 2–6% lower than the content found in Pakistan WML [34]. Conditioning for up to 3 h did not reduce the protein content of extract. This finding is of practical importance because in other studies [35], the WML were used as protein supplements replacing oilseed meal in the diet of mono-gastric animals. This might suggest that mulberry foliage could be also used as a diet component for farm animals.
Table 1.
The content of basic compounds, amino acids, minerals, dietary fiber fractions, fatty acids profile and chlorophyll in dried Polish var. zolwinska wielkolistna leaves (WML-P) before (control) and after conditioning.
| Component | Derived Products of WML-P | ||||
|---|---|---|---|---|---|
| 0 h (Control) | 1 h | 2 h | 3 h | 4 h | |
| A. Basic Components [g/100 g] | |||||
| Dry matter | 99.01 a ± 0.09 | 98.95 a ± 0.08 | 98.73 a ± 0.36 | 98.88 a ± 0.11 | 99.09 a ± 0.02 |
| Ash | 15.77 e ± 0.12 | 13.29 b ± 0.05 | 12.37 a ± 0.11 | 13.57 c ± 0.06 | 13.83 d ± 0.03 |
| Lipids | 1.79 b ± 0.16 | 1.24 a,b ± 0.47 | 1.14 a ± 0.19 | 1.12 a ± 0.16 | 1.02 a ± 0.03 |
| Proteins | 12.78 b ± 0.33 | 13.35 b ± 0.16 | 12.50 a,b ± 0.49 | 13.42 b ± 0.29 | 11.50 a ± 0.73 |
| Carbohydrates | 69.67 | 72.12 | 73.99 | 71.88 | 73.64 |
| B. Amino Acids (AA) [g/100 g] | |||||
| Phenylalanine (Phe) | 1.333 ± 0.044 | 0.944 ± 0.023 | 0.957 ± 0.031 | 1.022 ± 0.053 | 0.849 ± 0.161 |
| Isoleucine (Ile) | 1.126 ± 0.132 | 0.867 ± 0.038 | 0.795 ± 0.008 | 0.895 ± 0.086 | 0.697 ± 0.098 |
| Leucine (Leu) | 2.291 ± 0.177 | 1.714 ± 0.089 | 1.588 ± 0.017 | 1.714 ± 0.040 | 1.425 ± 0.221 |
| Lysine (Lys) | 1.271 ± 0.111 | 0.920 ± 0.008 | 0.812 ± 0.022 | 0.854 ± 0.025 | 0.686 ± 0.125 |
| Threonine (Thr) | 1.353 ± 0.194 | 1.085 ± 0.051 | 0.959 ± 0.080 | 0.945 ± 0.009 | 0.905 ± 0.127 |
| Valine (Val) | 1.431 ± 0.098 | 1.052 ± 0.062 | 1.013 ± 0.005 | 1.132 ± 0.084 | 0.837 ± 0.124 |
| Arginine (Arg) | 2.099 ± 0.162 | 1.726 ± 0.087 | 1.521 ± 0.039 | 1.554 ± 0.033 | 1.371 ± 0.100 |
| Histidine (His) | 0.730 ± 0.021 | 0.510 ± 0.033 | 0.477 ± 0.047 | 0.534 ± 0.026 | 0.416 ± 0.030 |
| Total EAA | 8.805 | 6.582 | 6.124 | 6.562 | 5.399 |
| Total EAA + semi-EAA | 11.634 | 8.818 | 8.122 | 8.650 | 7.186 |
| Alanine (Ala) | 1.883 ± 0.242 | 1.295 ± 0.093 | 1.148 ± 0.038 | 1.213 ± 0.048 | 1.031 ± 0.115 |
| Glycine (Gly) | 1.659 ± 0.073 | 1.259 ± 0.049 | 1.298 ± 0.171 | 1.249 ± 0.006 | 1.104 ± 0.069 |
| Aspartic acid (Asp) | 3.179 ± 0.493 | 2.584 ± 0.219 | 2.281 ± 0.093 | 2.472 ± 0.061 | 2.100 ± 0.124 |
| Glutamic acid (Glu) | 3.605 ± 0.483 | 2.959 ± 0.428 | 2.537 ± 0.043 | 2.733 ± 0.023 | 2.301 ± 0.303 |
| Proline (Pro) | 1.298 ± 0.078 | 0.947 ± 0.033 | 0.887 ± 0.032 | 0.935 ± 0.013 | 0.766 ± 0.120 |
| Serine (Ser) | 1.418 ± 0.116 | 1.116 ± 0.043 | 1.038 ± 0.077 | 1.082 ± 0.006 | 0.920 ± 0.102 |
| Total NEAA | 13.042 | 10.160 | 9.189 | 9.684 | 8.222 |
| EAA/NEAA | 67.5% | 64.8% | 66.6% | 67.8% | 65.7% |
| Total amino acids | 24.676 | 18.978 | 17.311 | 18.334 | 15.408 |
| C. Fatty Acids (FA) Profile [% of total] | |||||
| Palmitic acid C16:0 | 27.01 a,b,c ± 0.65 | 27.65 c ± 0.49 | 27.24 b,c ± 0.26 | 25.78 a ± 0.90 | 26.23 a,b ± 0.68 |
| Palmitoleic acid C16:1 n-9 c | 2.51 a ± 0.13 | 2.39 a ± 0.46 | 2.53 a ± 0.21 | 2.66 a ± 0.20 | 2.61 a ± 0.21 |
| Margaric acid C17:0 | 0.66 c ± 0.01 | 0.59 b,c ± 0.10 | 0.49 a,b ± 0.06 | 0,45 a ± 0.03 | 0.43 a ± 0.01 |
| Stearic acid C18:0 | 6.05 a ± 0.21 | 5.16 a ± 0.30 | 5.57 a ± 0.89 | 4.84 a ± 0.56 | 5.55 a ± 0.63 |
| Elaidic acid C18:1 n-9t | n.d. | n.d. | n.d. | n.d. | n.d. |
| Oleic acid C18:1 n-9 c | 4.46 a ± 0.38 | 4.33 a ± 0.62 | 4.58 a ± 0.29 | 5.41 b ± 0.24 | 4.49 a ± 0.22 |
| Linoleic acid C18:2 n-6 c | 10.46 a ± 0.40 | 10.94 a,b ± 0.27 | 11.68 c ± 0.26 | 11.49 b,c± 0.43 | 11.12 a,b,c ± 0.14 |
| α-linolenic acid C18:3 n-3 | 45.60 b ± 0.57 | 45.81 b ± 0.64 | 44.15 a ± 0.95 | 45.66 b ± 0.31 | 45.73 b ± 0.10 |
| Arachidic acid C20:0 | 0.57 a,b ± 0.10 | 0.47 a ± 0.08 | 0.66 b,c ± 0.05 | 0.73 c ± 0.02 | 0.74 c ± 0.02 |
| Cis-1-eicosenoic acid C20:1 | 0.55 b ± 0.13 | 0.31 a ± 0.02 | 0.35 a ± 0.04 | 0.30 a ± 0.03 | 0.41 a ± 0.02 |
| Behenic acid C22:0 | 0.48 a ± 0.11 | 0.59 a ± 0.08 | 0.59 a ± 0.06 | 0.57 a ± 0.02 | 0.58 a ± 0.02 |
| Erucic acid C22:1 n-9 | 0.72 a ± 0.11 | 0.69 a ± 0.13 | 1.15 b ± 0.12 | 1.08 b ± 0.11 | 1.16 b ± 0.15 |
| Nervonic acid C24:1 | 0.93 a ± 0.17 | 1.07 a ± 0.03 | 1.01 a ± 0.03 | 1.03 a ± 0.09 | 0.95 a ± 0.03 |
| UFA/SFA | 1.87 | 1.90 | 1.89 | 2.09 | 1.98 |
| D. Minerals | |||||
| Macroelements [mg/g] | |||||
| Mg | 3.49 a ± 0.17 | 3.44 a ± 0.18 | 3.37 a ±0.14 | 3.54 a,b ± 0.21 | 3.51 a,b ± 0.09 |
| Ca | 27.92 b ± 0.44 | 25.04 a ± 0.68 | 24.72 a ± 1.63 | 24.15 a ± 1.31 | 25.74 a,b ± 0.54 |
| K | 67.79 a ± 3.25 | 64.52 a ± 2.93 | 70.96 a ± 3.17 | 69.67 a ± 2.34 | 64.63 a ± 2.98 |
| [µg/g] | |||||
| Na | 88.73 a ± 0.34 | 95.54 b ± 0.62 | 92.99 a ± 2.25 | 100.78 b ± 4.40 | 94.96 a ± 6.11 |
| Microelements [µg/g] | |||||
| Fe | 79.87 a ± 1.11 | 79.73 a ± 8.69 | 78.74 a ± 1.15 | 78.30 a ± 5.33 | 80.28 a ± 7.75 |
| Mn | 27.01 a ± 1.33 | 27.11 a ± 1.77 | 26.81 a ± 0.35 | 27.54 a ± 1.09 | 25.03 a ± 2.18 |
| Zn | 24.09 a ± 0.74 | 23.57 a ± 0.69 | 24.20 a ± 0.79 | 25.54 a ± 1.38 | 24.14 a ± 1.04 |
| Cu | 4.98 a ± 0.89 | 5.22 a ± 0.35 | 5.02 a ± 0.26 | 4.95 a ± 0.32 | 5.09 a ± 0.69 |
| E. Dietary Fiber and Fractions [g/100 g] | |||||
| NDF | 26.42 a ± 1.16 | 27.91 a,b ± 0.98 | 27.83 a,b ± 0.56 | 30.01 b ± 0.36 | 29.73 b ± 1.07 |
| ADF | 19.34 a ± 0.40 | 20.06 a ± 0.45 | 20.30 a ± 1.01 | 20.67 a ± 0.98 | 19.92 a ± 0.78 |
| ADL | 0.10 a ± 0.01 | 0.09 a ± 0.01 | 0.13 b ± 0.01 | 0.18 c ± 0.01 | 0.16 c ± 0.00 |
| Hemicelluloses | 7.09 a ± 0.98 | 7.84 a,b,c ± 0.91 | 7.53 a,b ± 0.73 | 9.33 b,c ± 0.69 | 9.81 c ± 0.48 |
| Celluloses | 19.23 a ± 0.40 | 19.97 a ± 0.45 | 20.17 a ± 1.00 | 20.49 a ± 0.98 | 19.76 a ± 0.78 |
| IDF | 54.57 a ± 0.91 | 58.57 b ± 0.47 | 58.61 b ± 1.29 | 59.75 b ± 1.24 | 58.74 b ± 0.47 |
| SDF | 4.44 a ± 0.13 | 4.49 a ± 0.24 | 4.91 a ± 0.17 | 4.47 a ± 0.42 | 4.44 a ± 0.23 |
| TDF | 59.01 a ± 0.84 | 63.06 b ± 0.70 | 63.52 b ± 1.12 | 64.23 b ± 1.43 | 63.18 b ± 0.46 |
| F. Chlorophyll [mg/g] | |||||
| Chlorophyll a | 1.834 b ± 0.023 | 1.216 a ± 0.010 | 0.945 a ± 0.064 | 1.121 a ± 0.297 | 1.217 a ± 0.135 |
| 66% | 59% | 61% | 60% | 55% | |
| Chlorophyll b | 0.919 a ± 0.082 | 0.834 a ± 0.172 | 0.602 a ± 0.140 | 0.747 a ± 0.312 | 1.015 a ± 0.405 |
| 34% | 41% | 39% | 40% | 45% | |
| Total | 2.753 | 2.050 | 1.546 | 1.868 | 2.232 |
0 h—control leaves; 1 h—leaves conditioned for 1 h; 2 h—leaves conditioned for 2 h; 3 h—leaves conditioned for 3 h; 4 h—leaves conditioned for 4 h; Total EAA—total essential amino acids; Total NEAA—total non-essential amino acids; semi–EAA—semi–essential amino acids; UFA—unsaturated fatty acids; SFA—saturated fatty acids; NDF—neutral dietary fiber; ADF—acid detergent fiber; ADL—detergent lignin; IDF—insoluble dietary fiber; SDF—soluble dietary fiber; TDF—total dietary fiber; chlorophyll %—percentage fraction in total chlorophyll; a, b, c, d, e—different letters show statistically significant differences in Tukey’s test (α < 0.05).
The highest amount of AA (24.676 g/100 g), including EAA (8.805 g/100 g) were found in the control sample. During conditioning, AA content decreased by 23.1% (1 h), 29.9% (2 h), 25.7% (3 h), 37.6% (4 h) (Table 1B). The ratio of EAA to NEAA was ranging from 65% to 67% and was similar to the ratio in Chinese WML reported by Yao et al. [35]. Compared to Chinese WML, the WML-P contained more Gly, Lys, Val, Phe, Ile, Arg and Thr [35].
The total fat content was 1.02–1.79% in WML-P. Among FA, combined MUFA and PUFA contributed to almost ⅔ of all FA (Table 1C). Among PUFAs, α-linolenic acid accounted for almost half and linoleic acid for ~10% of total FA, indicating that the FA profile in WML-P is nutritionally beneficial. The UFA/SFA ratio regardless of the conditioning time, was ~1.9–2.0. This ratio was higher than reported by Radojkovic et al. in Serbian WML [36]. FA are generally well tested in seeds and oils [37]. Sánchez-Salcedo et al. [38] analyzed the FA profile in Morus alba fruits and found the higher content of linoleic acid than α-linolenic acid.
Of note, both the AA composition and FA profile of WML-P are reported for the first time.
The minerals content of WML-P is in Table 1D. Among minerals, Ca and Mg were present in the highest amounts and were similar to those found in Thai and Serbian WML [39,40]. Content of Fe and Mn, cofactors of many enzymes, including catalase in photosynthetic cells, did not change after conditioning.
The dietary fiber content in conditioned WML-P samples was 4–5% higher than in control (Table 1E). The dominant fraction was IDF (92%). Compared to our study, Srivastava et al. [41] found in Indian WML about 1–5% more total fiber but a similar amount of NDF. The NDF is important in maintaining a balanced composition of human intestinal microflora. The amount of fiber in WML-P was comparable to those in other leafy vegetables such as dill, parsley and cabbage. The lignin content in WML-P increased during conditioning from 0.10 to 0.18 g/100 g). It is possible is the increase was caused by lignin’s ability to bind polyphenols to indeterminable complexes with lower antioxidant activity but it requires future studies.
The ratio of chlorophyll a to b was 3:2 and did not change after conditioning. Crushing tissue during the initial steps of WML-P processing most likely caused tissue maceration of both control and conditioned samples. Although conditioning increased both, pH (from 7.2 to 7.9–8.0) and temperature inside the prism (from ~26 °C to ~34 °C), it did not cause any further changes to chlorophyll content.
3.2. Antioxidant Activity
The efficiency of the aqueous WML-P extraction process was 8.2% and was twice lower than that reported by Kim et al. [42]. The discrepancy between the studies was most likely caused by the extraction method (hot plate versus autoclave) and the source of extract (i.e., leaves Polish versus Korean Morus alba trees). In previous studies, various extraction methods or extractants and conditions were used [1,34,36,43]. We have previously shown that the best extractant for WML-P is 65% acetone or 65% ethanol [4]. However, in the present study we used only the aqueous extraction process that could be used on semi-technical scale production of dietary supplements and functional foods.
Total phenolics content, measured using Folin-Ciocalteu test, in control WML-P was 2.468 mg GAE/g of dried leaves (Table 2). Conditioning increased phenolic content by 37% (to 3.376 mg GAE/g) and 46% (to 3.591 mg GAE/g) after 2 and 3 h, respectively. Arabshahi-Delouee and Urooj [44] found twice as much total phenolics in aqueous extract of Indian WML. The difference between the studies might be related to differences in extraction temperature (40 °C versus 100 °C).
Table 2.
Antioxidant activity of dried Polish var. zolwinska wielkolistna leaves (WML-P) before (control) and after conditioning.
| Test | Derived Products of WML-P | ||||
|---|---|---|---|---|---|
| 0 h (Control) | 1 h | 2 h | 3 h | 4 h | |
| Total Phenolics [mg GAE/g] | 2.468 a ± 0.500 | 2.352 a ± 0.074 | 3.376 b ± 0.034 | 3.591 b ± 0.028 | 2.175 a ± 0.067 |
| ABTS•+ Test [µM TE/g] | 23.630 d ± 0.019 | 23.892 d ± 0.004 | 17.235 b ± 0.011 | 16.033 a ± 0.004 | 20.110 c ± 0.003 |
| DPPH• Test [µM TE/g] | 49.420 a ± 0.005 | 53.241 c ± 0.014 | 62.517 d ± 0.004 | 51.323 b ± 0.009 | 51.546 b ± 0.052 |
| Chelating Activity [%] | 40.383 a ± 0.164 | 53.174 d ± 0.388 | 54.129 d ± 0.842 | 50.471 c ± 0.827 | 43.085 b ± 0.623 |
| FRAP Test [µM Fe2+/mg] | 0.221 a ± 0.042 | 0.248 a ± 0.034 | 0.250 a ± 0.013 | 0.187 a ± 0.023 | 0.190 a ± 0.042 |
0 h—control leaves; 1 h—leaves conditioned for 1 h; 2 h—leaves conditioned for 2 h; 3 h—leaves conditioned for 3 h; 4 h—leaves conditioned for 4 h; a, b, c, d—different letters show statistically significant differences in Tukey’s test (α < 0.05).
Compared to results reported by Sánchez-Salcedo et al. [43], WML-P conditioned for 3 or 4 h contained three to four, and control extract five to six times, less polyphenols. However, they [43] used methanol, as an extraction solvent. In this study, we used aqueous extraction in a semi-technical scale production that could contribute to lowering the content of polyphenols.
The antioxidant activity of WML-P was determined using four tests, based on the single electron transfer (SET) mechanism. In the DPPH• test, conditioning for 1 or 2 h increased antiradical activity by 4% to 26%. The ability to quench DPPH• in WML-P was lower than that measured by Iqbal et al. [34] in Spanish WML and more than twice higher than measured in another variety of Polish WML by Tajner-Czopek et al. [45].
In the ABTS•+ test we found that conditioning decreased antioxidant activity by up to 32% (after 3 h to 16.033 µM TE/g). Iqbal et al. [34] reported higher ABTS•+ values than we found for WML. In the Spanish WML [43] the ABTS•+ quenching ability was 10.82–12.00 mg TE/g. In the mentioned earlier study, Tajner-Czopek et al. [45]. Reported three times higher ABTS•+ values for aqueous extracts and eight times higher for aqueous-ethanol extracts from leaves of unknown variety and origin. The method of determining antiradical activity using ABTS cation is similar to the DPPH radical method but the test is considered more inclusive. The reason is that ABTS compound is soluble in both aqueous and organic solvents, which ensures a quick reaction with lipophilic and hydrophilic compounds.
The FRAP method allows us to determine the ability to reduce Fe3+ from the iron-2,4,6-tripyridyl-S-thiazine (TPTZ) complex by antioxidants present in the sample. Conditioning did not significantly affect the Fe3+ reducing ability in WML-P. In the iron chelation test, the conditioning resulted in an increase in chelating ability by 34% (2 h) and 32% (3 h) after conditioning. These results were similar to those reported by Wanyoet et al. in WML from Thai trees [46].
In summary, WML-P conditioning resulted in increased DPPH• quenching capacity, polyphenol content and iron-chelating ability and the highest values were found after 2 and 3 h of conditioning.
3.3. Bioactive Compounds
The content of bioactive compounds is presented in Table 3.
Table 3.
The content of bioactive compounds in dried Polish var. zolwinska wielkolistna leaves (WML-P) before (control) and after conditioning.
| Bioactive Compounds | Derived Products of WML-P | ||||
|---|---|---|---|---|---|
| 0 h (Control) | 1 h | 2 h | 3 h | 4 h | |
| A. Phenolic Acids [mg/g] | |||||
| Gallic Acid (GAL) | 0.190 a ± 0.005 | 0.268 c ± 0.001 | 0.261 c ± 0.007 | 0.231 b ± 0.006 | 0.197 a ± 0.001 |
| Protocatechuic Acid (PRO) | 0.137 a ± 0.009 | 0.256 d ± 0.002 | 0.259 d ± 0.004 | 0.181 b ± 0.012 | 0.215 c ± 0.003 |
| 4-hydroxybenzoic Acid (HYD) | 0.100 a ± 0.001 | 0.111 a,b ± 0.001 | 0.119 b ± 0.003 | 0.112 a,b ± 0.010 | 0.125 b ± 0.005 |
| Vanillic Acid (VAN) | 0.160 a ± 0.003 | 0.219 b ± 0.002 | 0.234 b,c ± 0.002 | 0.245 c ± 0.015 | 0.311 d ± 0.004 |
| Chlorogenic Acid (CAF) | 0.375 b ± 0.003 | 0.528 c ± 0.012 | 0.727 d ± 0.015 | 0.358 b ± 0.017 | 0.162 a ± 0.011 |
| Caffeic Acid (CHL) | 0.378 b ± 0.007 | 0.527 c ± 0.012 | 0.726 d ± 0.015 | 0.347 b ± 0.018 | 0.164 a ± 0.012 |
| p-coumaric Acid (CUM) | 0.047 a ± 0.007 | 0.065 a ± 0.003 | 0.061 a ± 0.012 | 0.159 b ± 0.068 | 0.098 a,b ± 0.003 |
| Ferullic Acid (FER) | 0.139 a ± 0.006 | 0.231 c ± 0.005 | 0.265 d ± 0.009 | 0.200 b ± 0.007 | 0.160 a ± 0.013 |
| Sinapic Acid (SIN) | 0.204 b ± 0.012 | 0.179 a ± 0.008 | 0.206 b ± 0.004 | 0.277 c ± 0.007 | 0.309 d ± 0.004 |
| Total | 1.730 | 2.384 | 2.858 | 2.110 | 1.741 |
| B. Flavonols [mg/g] | |||||
| Rutin (RUT) | 0.283 a ± 0.014 | 0.550 c ± 0.049 | 0.580 c ± 0.001 | 0.429 b ± 0.021 | 0.421 b ± 0.075 |
| Isoquercetin (ISQ) | 0.209 a ± 0.009 | 0.283 b ± 0.011 | 0.320 c ± 0.001 | 0.283 b ± 0.006 | 0.287 b ± 0.018 |
| Quercetin 3-O-(6′′-O-malonyl)-β-d-glucoside (MAL) | 0.207 b ± 0.025 | 0.258 c ± 0.033 | 0.274 c ± 0.009 | 0.206 b ± 0.010 | 0.157 a ± 0.004 |
| Astragalin (AST) | 0.203 a ± 0.012 | 0.342 b ± 0.011 | 0.435 c ± 0.004 | 0.321 b ± 0.044 | 0.313 b ± 0.078 |
| Myricetin (MYR) | 0.062 a ± 0.001 | 0.145 d ± 0.011 | 0.156 e ± 0.001 | 0.119 c ± 0.002 | 0.089 b ± 0.002 |
| Quercetin (QUE) | 0.032 b ± 0.005 | 0.022 a ± 0.004 | 0.025 a,b ± 0.000 | 0.049 c ± 0.009 | 0.064 d ± 0.000 |
| Kaempferol (KEM) | 0.008 c ± 0.001 | 0.004 a,b ± 0.001 | 0.003 a ± 0.000 | 0.004 b ± 0.000 | 0.008 c ± 0.000 |
| Isorhamnetin (ISR) | 0.001 b ± 0.000 | 0.001 b ± 0.000 | 0.000 a ± 0.000 | 0.002 c ± 0.000 | 0.000 a ± 0.000 |
| Total | 1.005 | 1.605 | 1.793 | 1.413 | 1.339 |
| C. Iminosugar [mg/g] | |||||
| 1-deoxynojirimycin (DNJ) | 0.617 b ± 0.038 | 0.472 a ± 0.017 | 0.461 a ± 0.030 | 0.596 b ± 0.017 | 0.394 a ± 0.058 |
0 h—control leaves; 1 h—leaves conditioned for 1 h; 2 h—leaves conditioned for 2 h; 3 h—leaves conditioned for 3 h; 4 h—leaves conditioned for 4 h; a, b, c, d, e—different letters show statistically significant differences in Tukey’s test (α < 0.05).
The phenolic acids and flavonols belong to the polyphenols and their presence in the raw material largely determines its antioxidant activity. The content of flavonols increased in WML-P extracts after 2 h of conditioning by 70% and then decreased after 3 h and 4 h, although not to the control (0 h) level. Compared to control, phenolic acids content increased after 1 h by 65% from 1.730 to 2.384 mg/g) and 2 h by 38% (to 2.858 mg/g) of conditioning (Table 3A). The predominant phenolic acids were CHL and CAF, which content almost doubled after 2 h of conditioning (from 0.375 to 0.727 mg CHL/g and from 0.378 to 0.726 mg CAF/g). Similar trends were observed for the other phenolic acids. The increase probably was caused by the release of phenolic acids from more complex compounds during conditioning. Among other phenolic acids (GAL, PRO, VAN, FER, SIN) conditioning lowered the CHL and CAF extract concentration. It has been shown that CHL was also dominant in aqueous extracts from other varieties of WML [1,14] and acetone extract from WML-P [6].
Sánchez-Salcedo et al. [43] found CHL content in Spanish WML ranging from 5.29 to7.18 mg/g of dry matter in one study and 12.98 mg/g in another study [46]. In another study, Zhang et al. [47] found CHL in WML from Chinese trees ranging from 1.768 mg/g (Zhejiang, Hangzhou) to 9.617 mg/g (Anhui, Huangshan) in var. Qiangsang1. In this study, GAL and PRO contents in all preparations were similar to those reported in WML from Serbian trees [36]. The increasing amount of CAF and CHL in WML-P indicates that biochemical changes occur during conditioning for 1 and 2 h. Further conditioning causes CAF to break down into caffeic acid and quinic acid. As a result of processing, CHL may be hydrolyzed into two acids, which were quantified in the test sample. The quantitative advantage of caffeic acid over chlorogenic acid in mulberry leaves maybe a marker of the intensity of changes in leaves during processing. It is plausible that the ratio of caffeic acid to chlorogenic acid can be used as a qualitative marker for mulberry leaves.
In the WML-P samples, we identified eight flavonols (Table 3B). The largest amount of flavonols (1.793 mg/g) was found in WML-P conditioned for 2 h. After conditioning for 3 or 4 h, total flavonols content decreased to 1.413 and 1.339 mg/g, respectively) in the total flavonols content. The dominant flavonols were RUT (from 0.283 to 0.580 mg/g), ISQ (from 0.209 to 0.320 mg/g), AST (from 0.203 to 0.435 mg/g), MAL (from 0.157 to 0.274 mg/g). The results were similar to those found in WML from Korean trees [48]. After conditioning for 2 h the RUT content was approximately 100% higher and for 3 or 4 h, approximately 50% higher than in control. This trend may indicate the gradual release of flavonols from bounded forms.
The flavonol quercetin 3-O-(6”-O-malonyl)-β-d-glucoside (MAL), present in WML, is a bioactive ingredient with antiatherosclerotic and antihyperglycemic capacity. The content of MAL increased by 31% after 2 h of conditioning (to 0.274 mg/g). The further conditioning resulted in a significant reduction in the MAL content (to 0.157 mg/g), while the QUE content increased (to 0.064 mg/g). A plausible explanation is that endogenous esterases released during leaf shredding caused MAL hydrolysis, which resulted in the release of the QUE aglycone. Lee et al. [48] reported that quantitative changes in phenolics content of WML depend on variety, harvest period and heat processing conditions. Similarly, conditioning WML-P for 2 h caused an increase in ISQ (to 0.320 mg/g) and AST (to 0.435 mg/g) content by 53% and 114%, respectively. ISQ was also one of the major flavonols in other studies of WML-P [4,15,49] and in WML from Korean trees [42]. Lee and Choi [48] measured similar amounts of AST.
QUE content in WML-P doubled after conditioning for 4 h, which can be explained by the breakdown of other flavonols containing QUE in their structure, that is, RUT, MAL, ISQ, ISR and was similar to those reported by Kim and Jang [42]. It is speculated that up to 50% of the antidiabetic effect of WML extracts depends of the presence of chlorogenic acid and rutin. Chlorogenic acid can weaken glycogenolysis and reduce glucose uptake and has strong antioxidant properties [1,42]. Rutin protects against cancer and inhibits the peroxidation of LDL cholesterol. The content of rutin in conditioned WML-P was close to those found in WML var. Yun711, Qiangsang1, 7946, Fengtian5 from eastern China [47]. We found that CHL and RUT are present in WML-P in significant quantities. However, CHL can easily disintegrate during processing so that every technological process can change the ratio of caffeic acid to chlorogenic acid.
Another important bioactive component of mulberry leaves is the alkaloid DNJ—1-deoxynojirimycin (Table 3C)—a glucose analogue with a substituted NH group in the pyranose ring with α-glucosidase. This compound has known antioxidant, anti-bacterial and anti-inflammatory inhibitory properties and might also participate in regulating/modulating gut microbiota and having a neuroprotective effect in Alzheimer’s patients [27]. Conditioning did not significantly affect the amounts of DNJ, except for the sample 3 h (to 0.596 mg/g). A similar content of DNJ was found in a few Chinese varieties of WML [47].
In summary, grinding, conditioning and drying caused qualitative and quantitative changes of bioactive components and caused the increased of antioxidant activity of WML samples. These changes were most likely facilitated by enzymes present in the WML such as polyphenol oxidases, hydrolases, synthetases. In addition, the impact of the matrix changes, such as changes in the structural connections within the raw material, including the presence of fiber and its structure, could be also significant. In further studies, the time of conditioning (e.g., 0.5, 1.5, 2.5 h) should be tested and parameters refined. The quantitative relation found between chlorogenic and caffeic acids in products of WML-P may serve as a marker of changes occurring during conditioning of WML-P.
4. Conclusions
The conditioning process was successfully utilized in the processing of WML-P. The conditioning process, at ~32–35 °C, caused changes in functional properties and antioxidant activity of WML-P that were time-dependent. Among tested times, conditioning for 1 or 2 h caused the highest increase of the bioactive compounds in the WML-P products. Conditioning of WML-P for 3 and 4 h caused the decrease of the activity of the biologically active WML-P compounds.
Acknowledgments
We thank the Institute of Agricultural and Food Biotechnology in Poznan, Poland for infrastructure support in semi-technical scale processing.
Author Contributions
Conceptualization, M.P. and E.F.; methodology, M.P., J.K.-C.; validation, M.P., D.K., A.T.-G., A.G.-M., H.S.; J.F.-F.; investigation, M.P., A.T.-G., H.S., D.K.; resources, M.P., E.F.; writing—original draft preparation, M.P. and E.F.; writing—review and editing, M.P. and M.S.B.; visualization, M.P.; supervision, E.F.; funding acquisition, M.P., A.G.-M., J.K.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a European Union project POIG 01.01.02-00-061/09 “New Bioactive Food with Designed Functional Properties” and Department of Gastronomy Science and Functional Foods, Poznan University of Life Sciences, Poland. The APC was co-financed within Department of Gastronomy Science and Functional Foods, Poznan University of Life Sciences, Poland (statutory fundings).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Thabti I., Elfalleh W., Hannachi H., Ferchichi A., Campos M.D.G. Identification and quantification of phenolic acids and flavonol glycosides in Tunisian Morus species by HPLC-DAD and HPLC-MS. J. Funct. Foods. 2012;4:367–374. doi: 10.1016/j.jff.2012.01.006. [DOI] [Google Scholar]
- 2.Yang M.-Y., Wu C.-H., Hung T.-W., Wang C.-J. Endoplasmic reticulum stress-induced resistance to doxorubicin is reversed by mulberry leaf polyphenol extract in hepatocellular carcinomathrough inhibition of COX-2. Antioxidants. 2020;9:26. doi: 10.3390/antiox9010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negro C., Aprile A., De Bellis L., Miceli A. Nutraceutical properties of mulberries grown in southern Italy (Apulia) Antioxidants. 2019;8:223. doi: 10.3390/antiox8070223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeszka-Skowron M., Flaczyk E., Kobus-Cisowska J., Kośmider A., Górecka D. Optymalizacja procesu ekstrakcji związków fenolowych o aktywności przeciwrodnikowej z liści morwy białej za pomocą metody płaszczyzny odpowiedzi (RSM) Żywn. Nauk. Technoligia Jakość. 2014;1:148–159. doi: 10.15193/zntj/2014/92/148-159. [DOI] [Google Scholar]
- 5.Nakamura S., Hashiguchi M., Yamaguchi Y., Oku T. Hypoglycemic effects of Morus alba leaf extract on postprandial glucose and insulin levels in patients with type 2 diabetes treated with sulfonylurea hypoglycemic agents. J. Diabetes Metab. 2011;2:158. doi: 10.4172/2155-6156.1000158. [DOI] [Google Scholar]
- 6.Kujawska M., Ewertowska M., Adamska T., Ignatowicz E., Flaczyk E., Przeor M., Kurpik M., Jodynis-Liebert J. Protective effect of Morus alba leaf extract on N-nitrosodiethylamine-induced hepatocarcinogenesis in rats. In Vivo. 2016;30:807–812. doi: 10.21873/invivo.10998. [DOI] [PubMed] [Google Scholar]
- 7.Peng C.-H., Lin H.-T., Chung D.-J., Huang C.-N., Wang C.-J. Mulberry Leaf Extracts prevent obesity-induced NAFLD with regulating adipocytokines, inflammation and oxidative stress. J. Food Drug Anal. 2018;26:778–787. doi: 10.1016/j.jfda.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szczepaniak O., Kobus-Cisowska J., Kusek W., Przeor M. Functional properties of Cornelian cherry (Cornus mas L.): A comprehensive review. Eur. Food Res. Technol. 2019;245:2071–2087. doi: 10.1007/s00217-019-03313-0. [DOI] [Google Scholar]
- 9.Butt M.S., Nazir A., Sultan M.T., Schroën K. Morus alba L. nature’s functional tonic. Trends Food Sci Tech. 2008;19:505–512. doi: 10.1016/j.tifs.2008.06.002. [DOI] [Google Scholar]
- 10.Gültekin-Özgüven M., Karadağ A., Duman Ş., Özkal B., Özçelik B. Fortification of dark chocolate with spray dried black mulberry (Morus nigra) waste extract encapsulated in chitosan-coated liposomes and bioaccessability studies. Food Chem. 2016;201:205–212. doi: 10.1016/j.foodchem.2016.01.091. [DOI] [PubMed] [Google Scholar]
- 11.Przeor M., Flaczyk E. Antioxidant properties of Paratha type flat bread enriched with white mulberry leaf extract. Indian J. Tradit. Knowl. 2016;15:237–244. [Google Scholar]
- 12.Gramza A., Korczak J. Tea constituents (Camellia sinensis L.) as antioxidants in lipid systems. Trends Food Sci. Technol. 2005;16:351–358. doi: 10.1016/j.tifs.2005.02.004. [DOI] [Google Scholar]
- 13.Przeor M., Flaczyk E. Wpływ temperatury suszenia pędów i liści morwy białej (Morus alba) na aktywność przeciwutleniającą. Zesz. Probl. Postęp. Nauk Rol. 2011;569:277–283. [Google Scholar]
- 14.Chon S.-U., Kim Y.-M., Park Y.-J., Heo B.-G., Park Y.-S., Gorinstein S. Antioxidant and antiproliferative effects of methanol extracts from raw and fermented parts of mulberry plant (Morus alba L.) Eur. Food Res. Technol. 2009;230:231–237. doi: 10.1007/s00217-009-1165-2. [DOI] [Google Scholar]
- 15.Jeszka-Skowron M., Flaczyk E., Jeszka J., Krejpcio Z., Król E., Buchowski M. Mulberry leaf extract intake reduces hyperglycaemia in streptozotocin (STZ)-induced diabetic rats fed high-fat diet. J. Funct. Foods. 2014;8:9–17. doi: 10.1016/j.jff.2014.02.018. [DOI] [Google Scholar]
- 16.AOAC . AOAC Official Methods of Analysis. AOAC; Rockville, Maryland: 2000. Methods of Food Analysis. [Google Scholar]
- 17.Dziedzic K., Górecka D., Szwengiel A., Sulewska H., Kreft I., Gujska E., Walkowiak J. The Content of Dietary Fibre and Polyphenols in Morphological Parts of Buckwheat (Fagopyrum tataricum) Plant Foods Hum. Nutr. 2018;73:82–88. doi: 10.1007/s11130-018-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AOCS . AOCS Official Method. American Oil Chemists’ Society; Champaign, IL, USA: 2009. [Google Scholar]
- 19.Kmiecik D., Kobus-Cisowska J., Korczak J. The content of anti-nutritional components in frozen fried-potato products. LWT-Food Sci. Technol. 2017;85:275–282. doi: 10.1016/j.lwt.2017.07.030. [DOI] [Google Scholar]
- 20.AOAC . AOAC Official Methods of Analysis. AOAC; Rockville, Maryland: 2000. Amino Acids in Feeds. [Google Scholar]
- 21.AOAC . AOAC Offiicial Methods of Analysis. AOAC; Rockville, Maryland: 2014. Standard Method Performance Requirements for Determination of Amino Acids in Infant Formula and Adult/Pediatric Nutritional Formula. [Google Scholar]
- 22.Bader N.R. Sample preparation for flame atomic absorption spectroscopy: An overview. Rasayan J. Chem. 2011;4:49–55. [Google Scholar]
- 23.Eder R. Pigments. In: Nolle L., editor. Handbook of Food Analysis. Marcel Dekker Inc.; New York, NY, USA: 2004. pp. 805–879. [Google Scholar]
- 24.Siger A., Nogala-Kałucka M., Lampart-Szczapa E., Hoffmann A. Zawartość związków fenolowych w nowych odmianach rzepaku. Rośl. Oleiste. 2004;25:263–274. [Google Scholar]
- 25.Kobus J., Flaczyk E., Siger A., Nogala-Kałucka A., Korczak J., Pegg R. Phenolic compounds and antioxidant activity of extracts of Ginkgo leaves. Eur. J. Lipid Sci. Technol. 2009;111:1140–1150. doi: 10.1002/ejlt.200800299. [DOI] [Google Scholar]
- 26.Cheung L., Cheung P., Ooi V. Antioxidant activity and total phenolics of epigallocatechin gallate in the NBT-II bladder tumour cell line. Biol. Pharm. Bull. 2003;18:1676–1680. [Google Scholar]
- 27.Kim J.W., Kim S.U., Lee H.S., Kim I., Ahn M.Y., Ryu K.S. Determination of 1-deoxynojirimycin in Morus alba L. leaves by derivatization with 9-fluorenylmethyl chloroformate followed by reversed-phase high-performance liquid chromatography. J. Chromatogr. A. 2003;1002:93–99. doi: 10.1016/S0021-9673(03)00728-3. [DOI] [PubMed] [Google Scholar]
- 28.Amarowicz R., Pegg R., Bautista D. Antibacterial activity of green tea polyhenols against Escherichia coli K12. Nahrung. 2000;44:60–62. doi: 10.1002/(SICI)1521-3803(20000101)44:1<60::AID-FOOD60>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 29.Re R., Pellegrinia N., Proteggentea A., Pannalaa A., Yanga M., Rice-Evansa C. Antioxidant activity an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 30.Tang S., Joe K., Sheehan D., Buckley D. Antioxidative mechanisms of tea catechins in chicken meat system. Food Chem. 2002;76:45–51. doi: 10.1016/S0308-8146(01)00248-5. [DOI] [Google Scholar]
- 31.Benzie I., Strain J. Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In: Abelson J., Simon M., editors. Methods in Enzymology. Academic Press; Cambridge, MA, USA: 1999. pp. 15–27. [DOI] [PubMed] [Google Scholar]
- 32.Łochyńska M. Energy and Nutritional Properties of the White Mulberry (Morus alba L.) J. Agric. Sci. Technol. A. 2015;5:709–716. doi: 10.17265/2161-6256/2015.09.001. [DOI] [Google Scholar]
- 33.Przeor M., Flaczyk E. Morwa biała-nieocenione znaczenie zdrowotne. Przem. Spoż. 2016;5:33–35. doi: 10.15199/65.2016.5.7. [DOI] [Google Scholar]
- 34.Iqbal S., Younas U., Uddin S., Chan K.W., Sarfraz R.A., Uddin K. Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): A comparative study. Int. J. Mol. Sci. 2012;13:6651–6664. doi: 10.3390/ijms13066651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao J., Yan B., Wang Y., Zhang Y. Nutritional evaluation of mulberry leaves as feeds for ruminants. Livest. Res. Rural Dev. 2000;12:9–16. [Google Scholar]
- 36.Radojković M., Zeković Z., Mašković P., Vidović S., Mandić A., Mišan A., Đurović S. Biological activities and chemical composition of Morus leaves extracts obtained by maceration and supercritical fluid extraction. J. Supercrit. Fluids. 2016;117:50–58. doi: 10.1016/j.supflu.2016.05.004. [DOI] [Google Scholar]
- 37.Fedko M., Kmiecik D., Siger A., Kulczyński B., Przeor M., Kobus-Cisowska J. Comparative characteristics of oil composition in seeds of 31 Cucurbita varieties. J. Food Meas. Charact. 2020;14:894–904. doi: 10.1007/s11694-019-00339-6. [DOI] [Google Scholar]
- 38.Sánchez-Salcedo E.M., Sendra E., Carbonell-Barrachina Á.A., Martínez J.J., Hernández F. Fatty acids composition of Spanish black (Morus nigra L.) and white (Morus alba L.) mulberries. Food Chem. 2016;190:566–571. doi: 10.1016/j.foodchem.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Nookabkaew S., Rangkadilok N., Satayavivad J. Determination of Trace Elements in Herbal Tea Products and Their Infusions Consumed in Thailand. J. Agric. Food Chem. 2006;54:6939–6944. doi: 10.1021/jf060571w. [DOI] [PubMed] [Google Scholar]
- 40.Radojković M.M., Zeković Z.P., Dojinović B.P., Stojanović Z.S., Cvetanović A.D., Manojlović D.D. Characterization of Morus species in respect to micro, macro, and toxic elements. Acta Period. Technol. 2014;45:229–237. doi: 10.2298/APT1445229R. [DOI] [Google Scholar]
- 41.Srivastava S., Kapoor R., Thathola A., Srivastava R.P. Nutritional quality of leaves of some genotypes of mulberry (Morus alba) Int. J. Food Sci. Nutr. 2006;57:305–313. doi: 10.1080/09637480600801837. [DOI] [PubMed] [Google Scholar]
- 42.Kim G.-N., Jang H.-D. Flavonol Content in the Water Extract of the Mulberry (Morus alba L.) Leaf and Their Antioxidant Capacities. J. Food Sci. 2011;76:C869–C873. doi: 10.1111/j.1750-3841.2011.02262.x. [DOI] [PubMed] [Google Scholar]
- 43.Sánchez-Salcedo E., Mena P., García-Viguera C., Hernández F., Martínez J.J. (Poly)phenolic compounds and antioxidant activity of white (Morus alba) and black (Morus nigra) mulberry leaves: Their potential for new products rich in phytochemicals. J. Funct. Foods. 2015;18:1039–1046. doi: 10.1016/j.jff.2015.03.053. [DOI] [Google Scholar]
- 44.Arabshahi-Delouee S., Urooj A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem. 2007;102:1233–1240. doi: 10.1016/j.foodchem.2006.07.013. [DOI] [Google Scholar]
- 45.Tajner-Czopek A., Gertchen M., Rytel E., Kita A., Kucharska A.Z., Sokół-Łętowska A. Study of antioxidant activity of some medicinal plants having high content of caffeic acid derivates. Antioxidants. 2020;9:412. doi: 10.3390/antiox9050412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wanyo P., Siriamornpun S., Meeso N. Improvement of quality and antioxidant properties of dried mulberry leaves with combined far-infrared radiation and air convection in Thai tea process. Food Bioprod. Process. 2011;89:22–30. doi: 10.1016/j.fbp.2010.03.005. [DOI] [Google Scholar]
- 47.Zhang D.-Y., Wan Y., Hao J.-Y., Hu R.-Z., Chen C., Yao X.-H., Zhao W.-G., Liu Z.-Y., Li L. Evaluation of the alkaloid, polyphenols, and antioxidant contents of various mulberry cultivars from different planting areas in eastern China. Ind. Crops Prod. 2018;122:298–307. doi: 10.1016/j.indcrop.2018.05.065. [DOI] [Google Scholar]
- 48.Lee W.J., Choi S.W. Quantitative Changes of Polyphenolic Compounds in Mulberry (Morus alba L.) Leaves in Relation to Varieties, Harvest Period, and Heat Processing. Prev. Nutr. food Sci. 2012;17:280–285. doi: 10.3746/pnf.2012.17.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flaczyk E., Kobus-Cisowska J., Przeor M., Korczak J., Remiszewski M., Korbas E., Buchowski M. Chemical characterization and antioxidative properties of Polish variety of Morus alba L. leaf aqueous extracts from the laboratory and pilot-scale processes. Agric. Sci. 2013;04:141–147. doi: 10.4236/as.2013.45B026. [DOI] [Google Scholar]