Abstract
Background: Exergame-induced changes in the volume of brain gray matter have not been studied in fibromyalgia (FM). This study evaluates the effects of a 24-week exergame-based intervention on the gray matter volume of different brain structures in patients with FM through magnetic resonance imaging (MRI). Methods: A total of 25 FM patients completed 24 weeks of intervention program, and another 25 FM patients did not receive any intervention. T1-weighted MRI was used to assess brain volume, and FreeSurfer software was used to segment the brain regions. Results: No significant effects on gray matter volume of different structures and total gray matter were found. Conclusions: FM patients did not show significant changes in gray matter brain volume between the control and experimental groups after 24 weeks. FM patients showed significant relationships between peak oxygen consumption (pVO2) and the left and right regions of the hippocampus and the left and right regions of the amygdala.
Keywords: MRI, physical exercise, pain, virtual reality
1. Introduction
Fibromyalgia (FM) is a chronic disease characterized by widespread pain associated with other symptoms such as sleep disorders, fatigue, anxiety, depression, stiffness, poor physical fitness, or cognitive dysfunction [1], among others. These symptoms lead to a reduction in the health-related quality of life [2], mainly in women between 40 and 59 years old [3].
Previous studies in FM have shown alterations in metabolic activity, functional connectivity, and regions involved in processing pain (i.e., insula, thalamus, amygdala, hippocampus, among others), known as the “pain matrix” [4], and brain structures [5,6,7,8,9,10,11]. Moreover, patients with FM have shown lower thresholds and higher pain ratios, as well as changes in brain activity [12,13] and brain morphology in gray matter [13]. For example, a study found that FM patients have an accelerated brain gray matter loss, and it was related to the duration of the disease [8]. The authors of another study concluded that there is evidence that volumetric changes of gray matter in the frontal-cingulate cortex and the amygdala might reflect both neurobiological preconditions for central sensitization in FM and plastic changes as consequences of chronic pain input [14].
Pharmacological and nonpharmacological therapies have been used in the management of FM symptoms [15]. In this regard, physical exercise is the one that has accumulated the highest level of evidence to date in reducing the symptoms of this disease. In addition, it has been considered a cheap and effective tool for enhancing pain relief, physical function, and well-being [16,17,18]. It is important to emphasize that exercise has a fundamental role in avoiding the physiological effects of aging, as well as promoting the increase of neurogenesis, angiogenesis, and synaptogenesis [19]. These effects are mainly induced by the brain-derived neurotrophic factor (BDNF), growth and differentiation factor 11 (GDF11), and the vascular endothelial growth factor (VEGF), which directly affect brain plasticity [20].
To our knowledge, no studies have evaluated the exercise-induced changes in brain volume in people with FM. Nevertheless, previous longitudinal studies on exercise-based interventions [21], as well as in other populations such as healthy subjects [22], cognitive impairment [23,24], older adults [24,25], multiple sclerosis [26], or schizophrenia [24,27], have shown volume increases in the hippocampus.
Virtual reality-based exercise (VRE), also known as exergames, has shown benefits in the different populations [28,29]. In patients with FM, this type of intervention has been previously used in a nonimmersive version, obtaining improvements in the overall quality of life, pain, disease, mobility, balance, and fear of falling [30,31]. Furthermore, the effects of exergame-based interventions on the brain dynamics of patients with fibromyalgia have previously been studied, finding changes in brain dynamics that could be related to increased cerebral blood flow [32]. On the other hand, two studies have assessed changes in brain structure after exergame training in older adults [33,34]. Therefore, the effects of exergames on the brain are of great interest to the field of neuroscience. However, the effects of exergame-based interventions on brain volume in patients with FM have not previously been studied. Thereby, this study aims (1) to evaluate the effects of a 24-week exergame-based intervention on the gray matter volume of different brain structures of women with FM by magnetic resonance imaging (MRI) analysis, and (2) to analyze the relationship between aerobic and cognitive capacities with the gray matter volume brain structures. As exercise-based interventions can produce changes in brain morphometry, it is hypothesized that participants would show increased gray matter volumes in the different brain structures after the exergame intervention.
2. Materials and Methods
2.1. Trial Design
This study was a single-blinded randomized controlled trial. Participants were randomly assigned numbers by one of the researchers who did not participate in the statistical analysis or data acquisition and they were randomly allocated into two groups: the control group (CG) and the exercise group (EG). All evaluations were performed by another researcher who was blinded to the grouping allocation. Participants were not blinded since they were informed of the procedures and knew whether or not they were performing the exercise intervention. All procedures were approved by the University Research Ethics Committee (62/2017). The trial was registered at the International Standard Randomized Controlled Trial Number Registry (ISRCTN65034180), and the protocol is available in BioMed Central website [35]. Participants gave their written consent for the procedures in the study.
Different articles focused on the primary outcomes (i.e., electroencephalography, physical fitness, and quality of life) of the trial have been recently published [32,36,37,38]. Nevertheless, the hypothesis in the present study is entirely novel (improvements in different volumetric brain structures after an exergame intervention) and significantly differs from the other articles. This enables us to deeply examine the findings in the brain volume of women with FM. Furthermore, the scope, audience, and research professionals that this article involves are different and complementary.
2.2. Participants
A total of 56 women from a local FM association were recruited for this study and fulfilled the following inclusion criteria: (1) female between 30 and 75 years old; (2) diagnosed with FM by a rheumatologist according to the 2010 criteria established by the American College of Rheumatology; (3) able to communicate with research staff; (4) have read and signed the written informed consent conforming to the updated Declaration of Helsinki. Moreover, participants were excluded if (1) they were pregnant, (2) they had any cerebral injury, (3) illegible MRI sequences were obtained, (4) they had contraindications for physical exercise, or (5) they had changed their usual care therapies during the intervention program. The intervention was carried out in the University facilities (Faculty of Sport Science, Caceres, Spain) from January 2018 to June 2018.
2.3. Interventions
The EG participants completed a 24-week training program, whereas the CG participants continued their daily routine. The intervention consisted of two sessions per week (60 min per session). All sessions were conducted in groups of two or three participants in the university facilities, and there were no important adverse events as a result of the intervention. A specialized physical therapist, who conducted participant evaluations, also supervised all the sessions.
The exercise intervention was based on an exergame called VirtualEx-FM that was created by the research group, which aims to improve the ability to develop activities of daily living as well as physical conditioning in FM patients. VirtualEx-FM meets the key points of VR rehabilitation therapy [39] and has been used previously [30,31]. The program focuses on balance, postural control, coordination, mobility, aerobic conditioning, and strengthening of the upper and lower body, providing visual feedback and maintaining the correct execution of movements [31].
A typical session contained the following parts: (1) a video warm-up, where an expert performs joint movements and participants imitate these movements. The speed could be controlled by the expert at 0.5×, 1×, 1.5×, and 2×. (2) An aerobic component through dance steps (Zumba) performed by a dance teacher. (3) A game in which participants have to reach an apple with an avatar, getting to work on postural control and coordination, which was controlled and modified by the physical therapist. (4) Walking training, where participants must complete a virtual trail of footprints on a virtual floor. The interface allows the selection of different types of steps (i.e., normal, heel walking, tiptoe, raised knees, and raised heels; see Collado-Mateo et al. [31] for further details).
2.4. Data Collection and Outcomes
All the tests were carried out in the laboratory of the research group. Thus, tests were implemented at the beginning and the end of the intervention program by the same researchers. The variables measured were anthropometric measures, disease impact, cognitive decline, aerobic domain, and gray matter brain volumes.
First, anthropometric measurements of the participants were taken to report the body mass index (BMI). Subsequently, participants completed the Spanish version of the Fibromyalgia Impact Questionnaire (FIQ), which evaluates the impact of symptoms of the disease from 0 to 100, indicating the minimum to maximum impacts, respectively. FIQ is an extensively validated fibromyalgia-specific tool that captures the overall effects of fibromyalgia symptomatology (i.e., pain, fatigue, rested, stiffness, anxiety, depression, physical impairment, feeling good, or work missed) [40,41,42]. After that, trained research staff administered the Mini-Mental State Examination (MMSE), previously used in patients with fibromyalgia [43,44]. MMSE is a widely used test of cognitive function; it includes tests of orientation, attention, memory, language, and visual–spatial skills. A higher score represents a better cognitive state.
The 6-min walk test is a reliable measure in people with fibromyalgia [45]. The results of the 6-min walk test were used to predict peak oxygen consumption (pVO2) [46] through an equation from the distance covered in 6 min [47]. The regression equation to predict pVO2 from 6-min walk distance and BMI is pVO2 (ml/Kg/min) = 21.48 + (−0.4316 × BMI) + (0.0304 × distance (m)).
2.5. Image Acquisition
T1-weighted structural MRI scans were acquired from a 3.0 Tesla (T) system (Achieva 3.0T TX, Philips Medical Systems, Best, Netherlands) with an 8-channel receiver head coil. For each T1-weighted structural scan, the parameters were set as follows: 196 slices were acquired; the turbo field echo (TFE) imaging sequence (time repetition/time to echo = 11.51/2.8 ms; matrix size = 256 × 256; flip angle = 10°; slice thickness = 0.9 mm; number of averages = 1) was used.
2.6. Image Processing
All T1-weighted images were processed using the FreeSurfer software 6.0 version, a program freely available for download (Laboratory for Computational Neuroimaging, Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, USA) [48]. Automated segmentation of the T1-weighted images was used employing the recon-all command [49] on a MacBook Pro (Version OS X 10.14, 8GB, 2.30 GHz, Intel Core i5).
Preprocessing data include the following steps: (1) motion correction and averaging [50]; (2) removal of nonbrain tissue [51]; (3) automated Talairach transformation [52]; (4) segmentation of subcortical white matter and deep gray matter structures [53]; (5) intensity normalization [54]; (6) tessellation of the gray matter and white matter boundary [55]; (7) topology correction; (8) surface deformation following intensity gradients to reconstruction [53]. Structures that are part of the “pain matrix” (left and right regions of the hippocampus, insula, thalamus, amygdala, and cerebellum, in addition to total gray matter) and have an interest in the study of FM [56,57] were selected.
2.7. Statistical Analyses
The SPSS statistical package version 24 (IBM Corp, Armonk, New York, United States) was used to analyze the data.
To conduct the intention-to-treat analysis by multiple imputations (MIs) of missing values, the data from all 55 participants were used following the Sterne et al. guidelines [58]. Our missing data were classified as missing at random. The SPSS software package was used for the MIs of data.
Nonparametric tests were conducted because the dataset was not large and the data were not always Gaussian. To explore the effectiveness of the exergame-based intervention, the Mann–Whitney U-test was conducted to examine the differences between groups for each variable. Moreover, within-group comparisons were conducted by the Wilcoxon signed-rank test.
The Benjamini–Hochberg false discovery rate correction for multiple comparisons was applied in each comparison to avoid Type I errors. The partial eta-squared effect size was reported for each statistical test [59]. According to Cohen [60], effect sizes could be classified as small (0.01 ≤ η² < 0.06), medium (0.06 ≤ η² < 0.14), and large (η² ≥ 0.14).
Furthermore, Spearman’s rho correlation analyses were used to evaluate the relationship between pVO2, MMSE, and brain structure volumes.
3. Results
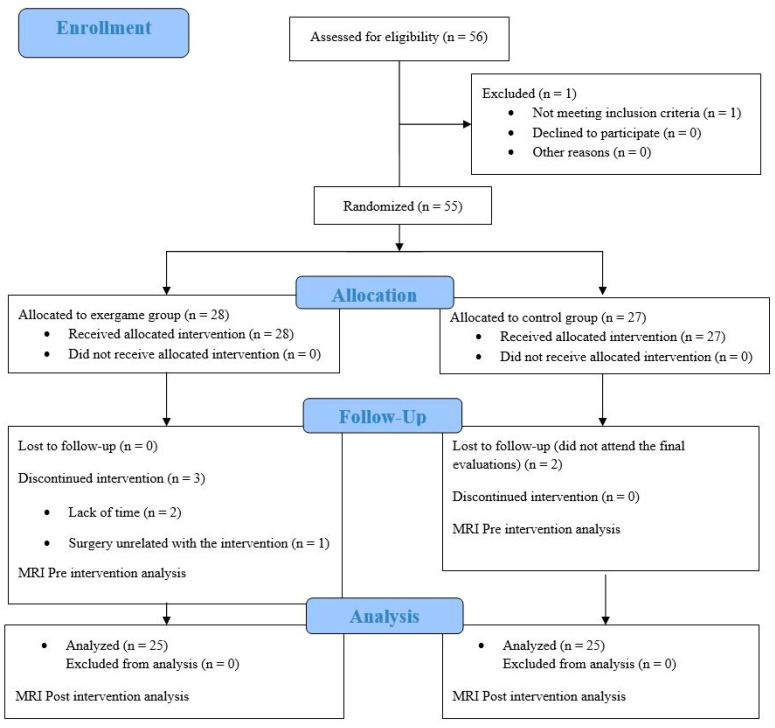
The flow diagram for participants is represented in Figure 1. A total of 56 women with FM were screened for eligibility. One participant was excluded for not meeting with the inclusion criteria. Therefore, 55 women were randomly allocated into two groups: CG and EG. Two women were not able to attend the final evaluations for CG. On the other hand, three women from EG were excluded by lack of time (n = 2) and a surgery unrelated to the exercise intervention (n = 1).
Figure 1.
Flow chart of participants.
Table 1 shows the baseline characteristics of the participants of both groups. Differences between EG and CG were not observed in age, the impact of the disease, years with FM symptoms, peak volume oxygen consumption, MMSE, anthropometric characteristics, and gray matter brain volumes (p-value > 0.05).
Table 1.
Descriptive characteristics of participants and differences between groups at the baseline of fibromyalgia patients.
| Variable | Exercise Group Median (IQR) | Control Group Median (IQR) | Value of the Contrast | p-Value |
|---|---|---|---|---|
| Sample size | 25 | 25 | ||
| Age (Years) | 54.00 (16.00) | 53.00 (13.00) | −0.351 | 0.800 |
| Height (cm) | 160.00 (11.00) | 159.00 (7.00) | −0.351 | 0.800 |
| Weight (Kg) | 69.30 (16.20) | 72.35 (19.40) | −0.470 | 0.800 |
| BMI (Kg/m²) | 27.00 (4.30) | 28.35 (7.40) | −0.650 | 0.800 |
| FIQ-100 | 57.58 (28.47) | 63.90 (23.56) | −0.490 | 0.800 |
| Years with FM | 8.50 (10.75) | 11.00 (10.25) | −0.308 | 0.800 |
| pVO2 (ml/Kg/min) | 23.77 (4.24) | 24.46 (5.38) | −0.019 | 0.985 |
| MMSE | 29.00 (1.00) | 28.50 (2.25) | −2.151 | 0.460 |
| Left Hippocampus | 3.04 (0.43) | 2.93 (0.36) | −1.660 | 0.460 |
| Right Hippocampus | 3.08 (0.31) | 3.07 (0.33) | −0.760 | 0.800 |
| Left Insula | 3.35 (2.06) | 3.34 (2.49) | −0.919 | 0.800 |
| Right Insula | 3.82 (1.70) | 4.28 (1.21) | −0.809 | 0.800 |
| Left Amygdala | 1.37 (0.19) | 1.41 (0.16) | −0.319 | 0.800 |
| Right Amygdala | 1.63 (0.18) | 1.64 (0.22) | −0.873 | 0.800 |
| Left Thalamus | 7.83 (0.94) | 7.51 (0.61) | −1.532 | 0.477 |
| Right Thalamus | 7.07 (0.81) | 6.81 (0.66) | −1.724 | 0.460 |
| Left Cerebellum | 48.37 (9.92) | 46.12 (8.09) | −0.958 | 0.800 |
| Right Cerebellum | 51.39 (13.14) | 49.04 (11.27) | −1.681 | 0.460 |
| Total Cerebral GM | 436.10 (72.50) | 428.35 (126.54) | −0.319 | 0.800 |
Abbreviations: IQR, interquartile range; BMI, body mass index; FIQ, fibromyalgia impact questionnaire; FM, fibromyalgia; pVO2, peak volume oxygen consumption; MMSE, mini-mental state examination; GM, gray matter.
Table 2 and Table 3 show the efficacy and the intent-to-treat analyses, respectively. Significant differences in the effect of the exergame-based intervention were not found for the gray matter volumes of the different brain structures, the total volume of gray matter, the pVO2, and the MMSE score.
Table 2.
Efficacy analysis of the effects of exergame intervention in patients with fibromyalgia on the different brain structures, the pVO2, and the MMSE.
| Variables | Between Group Comparison | Within Group Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain Areas (cm³) | Groups | Pre Median (IQR) | Post Median (IQR) | Value of the Contrast | p-Value | Effect Size | Value of the Contrast | p-Value | Effect Size |
| L. Hippocampus | EG | 3.04 (0.43) | 3.15 (0.22) | −0.342 | 0.925 | −0.020 | −2.738 | 0.016 | −0.323 |
| CG | 2.93 (0.36) | 3.07 (0.24) | −2.372 | 0.039 | −0.438 | ||||
| R. Hippocampus | EG | 3.08 (0.31) | 3.26 (0.36) | −0.075 | 0.925 | 0.071 | −3.011 | 0.013 | −0.354 |
| CG | 3.07 (0.33) | 3.15 (0.26) | −2.220 | 0.048 | −0.354 | ||||
| L. Insula | EG | 3.35 (2.06) | 5.08 (3.16) | 0.013 | 0.925 | 0.053 | −3.320 | 0.007 | −1.037 |
| CG | 3.34 (2.49) | 5.93 (1.12) | −2.896 | 0.016 | −1.245 | ||||
| R. Insula | EG | 3.82 (1.70) | 5.76 (2.41) | −0.450 | 0.925 | 0.000 | −2.516 | 0.022 | −0.578 |
| CG | 4.28 (1.21) | 5.87 (1.23) | −2.451 | 0.036 | −0.751 | ||||
| L. Amygdala | EG | 1.37 (0.19) | 1.39 (0.17) | −0.501 | 0.925 | -0.090 | −0.633 | 0.527 | 0.088 |
| CG | 1.41 (0.16) | 1.39 (0.11) | −0.087 | 0.931 | 0.003 | ||||
| R. Amygdala | EG | 1.63 (0.18) | 1.68 (0.27) | −0.103 | 0.925 | 0.197 | −2.776 | 0.016 | −0.329 |
| CG | 1.64 (0.22) | 1.65 (0.15) | −1.860 | 0.091 | −0.290 | ||||
| L. Thalamus | EG | 7.83 (0.94) | 7.72 (0.80) | −0.918 | 0.925 | −0.145 | −4.107 | 0.020 | 0.272 |
| CG | 7.51 (0.61) | 7.41 (0.65) | −4.074 | 0.075 | 0.208 | ||||
| R. Thalamus | EG | 7.07 (0.81) | 6.95 (0.73) | −1.599 | 0.371 | −0.338 | −1.834 | 0.097 | 0.153 |
| CG | 6.81 (0.66) | 6.74 (0.48) | −0.991 | 0.373 | −0.037 | ||||
| L. Cerebellum | EG | 48.37 (9.92) | 50.80 (8.43) | 1.601 | 0.706 | -0.397 | −1.705 | 0.114 | −0.158 |
| CG | 46.12 (8.09) | 48.27 (5.87) | −2.833 | 0.016 | −0.466 | ||||
| R. Cerebellum | EG | 51.39 (13.14) | 52.00 (6.91) | −1.483 | 0.377 | -0.489 | −1.282 | 0.236 | −0.093 |
| CG | 49.04 (11.27) | 50.36 (8.96) | −2.798 | 0.016 | −0.426 | ||||
| Total Cerebral GM | EG | 436.10 (72.50) | 507.24 (92.04) | −0.595 | 0.814 | -0.177 | −3.750 | < 0.001 | −0.939 |
| CG | 428.35 (126.54) | 521.54 (71.29) | −2.972 | 0.016 | −0.965 | ||||
| pVO2 (ml/Kg/min) | EG | 23.77 (4.24) | 24.51 (3.86) | −1.911 | 0.371 | 0.462 | −0.807 | 0.455 | −0.108 |
| CG | 23.62 (5.23) | 23.02 (5.52) | −1.601 | 0.142 | 0.132 | ||||
| MMSE | EG | 29.00 (1.00) | 29.00 (2.50) | −0.983 | 0.706 | −0.108 | −1.996 | 0.075 | 0.404 |
| CG | 28.00 (2.00) | 28.00 (4.00) | −0.945 | 0.373 | 0.248 | ||||
Abbreviations: IQR, interquartile range; GM, gray matter; pVO2, peak volume oxygen consumption; MMSE, mini-mental state examination; EG, exercise group; CG, control group; L, left; R, right.
Table 3.
Intent-to-treat analysis of the effects of exergame intervention on the different brain structures, the pVO2, and the MMSE.
| Variables | Between Group Comparison | Within Group Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain Areas (cm³) | Groups | Pre Median (IQR) | Post Median (IQR) | Value of the Contrast | p-Value | Effect Size | Value of the Contrast | p-Value | Effect Size |
| L. Hippocampus | EG (N = 28) | 3.10 (0.44) | 3.17 (0.27) | −0.511 | 0.853 | −0.057 | −2.846 | 0.011 | −0.304 |
| CG (N = 27) | 2.95 (0.37) | 3.07 (0.23) | −2.560 | 0.026 | −0.434 | ||||
| R. Hippocampus | EG (N = 28) | 3.11 (0.31) | 3.28 (0.36) | −0.435 | 0.853 | 0.086 | −3.155 | 0.009 | −0.348 |
| CG (N = 27) | 3.09 (0.35) | 3.16 (0.29) | −2.239 | 0.058 | −0.369 | ||||
| L. Insula | EG (N = 28) | 3.44 (1.98) | 5.20 (2.64) | −0.549 | 0.853 | 0.107 | −3.628 | < 0.001 | −1.031 |
| CG (N = 27) | 3.57 (2.10) | 5.76 (1.24) | −3.628 | < 0.001 | −1.283 | ||||
| R. Insula | EG (N = 28) | 4.00 (1.73) | 5.71 (2.26) | −0.261 | 0.853 | 0.023 | −2.951 | 0.011 | −0.611 |
| CG (N = 27) | 4.24 (1.66) | 5.78 (1.24) | −2.600 | 0.026 | −0.724 | ||||
| L. Amygdala | EG (N = 28) | 1.37 (0.19) | 1.39 (0.17) | −0.301 | 0.853 | −0.150 | −0.557 | 0.593 | 0.127 |
| CG (N = 27) | 1.39 (0.16) | 1.39 (0.13) | −0.237 | 0.816 | −0.025 | ||||
| R. Amygdala | EG (N = 28) | 1.64 (0.19) | 1.69 (0.27) | −0.187 | 0.853 | 0.155 | −2.900 | 0.011 | -0.302 |
| CG (N = 27) | 1.63 (0.22) | 1.65 (0.17) | −1.989 | 0.088 | −0.309 | ||||
| L. Thalamus | EG (N = 28) | 7.92 (0.97) | 7.73 (0.83) | −0.559 | 0.853 | −0.159 | −2.774 | 0.030 | 0.288 |
| CG (N = 27) | 7.53 (0.63) | 7.41 (0.66) | −2.050 | 0.088 | 0.222 | ||||
| R. Thalamus | EG (N = 28) | 7.09 (0.81) | 6.95 (0.75) | −1.832 | 0.355 | −0.330 | −1.957 | 0.082 | 0.161 |
| CG (N = 27) | 6.86 (0.68) | 6.74 (0.50) | −0.836 | 0.468 | −0.012 | ||||
| L. Cerebellum | EG (N = 28) | 48.18 (8.60) | 50.53 (8.12) | −1.138 | 0.712 | −0.368 | −1.878 | 0.098 | –1.169 |
| CG (N = 27) | 46.36 (7.46) | 49.01 (6.09) | −3.031 | 0.013 | −0.447 | ||||
| R. Cerebellum | EG (N = 28) | 52.25 (11.40) | 52.18 (7.39) | −1.851 | 0.355 | −0.514 | −1.087 | 0.360 | −0.066 |
| CG (N = 27) | 49.41 (10.03) | 50.36 (7.63) | −2.991 | 0.016 | −0.408 | ||||
| Total Cerebral GM | EG (N = 28) | 438.64 (80.28) | 498.36 (88.08) | −0.962 | 0.787 | −0.177 | −3.821 | < 0.001 | −0.852 |
| CG (N = 27) | 432.38 (125.40) | 520.53 (68.98) | −3.211 | 0.007 | −0.935 | ||||
| pVO2 (ml/Kg/min) | EG (N = 28) | 23.87 (4.28) | 24.62 (3.88) | −2.189 | 0.355 | 0.466 | −1.139 | 0.301 | −0.126 |
| CG (N = 27) | 24.08 (5.19) | 23.02 (5.37) | −1.685 | 0.126 | 0.134 | ||||
| MMSE | EG (N = 28) | 29.00 (2.00) | 29.00 (2.50) | −1.098 | 0.712 | -0.109 | −2.113 | 0.055 | 0.388 |
| CG (N = 27) | 29.00 (2.00) | 28.00 (4.00) | −0.945 | 0.407 | 0.231 | ||||
Abbreviations: IQR, interquartile range; GM, gray matter; pVO2, peak volume oxygen consumption; MMSE, mini-mental state examination; EG, exercise group; CG, control group; L, left; R, right.
Table 4 shows the relationship at baseline between the pVO2, the MMSE, and the gray matter brain volumes. The analyses revealed a relationship between the pVO2, the left and right regions of the hippocampus, and the left and right regions of the amygdala (p-value < 0.05).
Table 4.
Relationships between the pVO2, the MMSE, and the brain structure volumes.
| Variables | pVO2 | MMSE | |
|---|---|---|---|
| L. Hippocampus | Correlation coefficient | 0.349 | 0.229 |
| p-value | 0.017 | 0.125 | |
| R. Hippocampus | Correlation coefficient | 0.478 | 0.205 |
| p-value | 0.001 | 0.173 | |
| L. Insula | Correlation coefficient | 0.102 | 0.248 |
| p-value | 0.531 | 0.122 | |
| R. Insula | Correlation coefficient | 0.102 | −0.137 |
| p-value | 0.506 | 0.369 | |
| L. Amygdala | Correlation coefficient | 0.363 | 0.144 |
| p-value | 0.014 | 0.346 | |
| R. Amygdala | Correlation coefficient | 0.360 | 0.054 |
| p-value | 0.015 | 0.723 | |
| L. Thalamus | Correlation coefficient | 0.265 | 0.146 |
| p-value | 0.079 | 0.339 | |
| R. Thalamus | Correlation coefficient | 0.249 | 0.259 |
| p-value | 0.099 | 0.085 | |
| L. Cerebellum | Correlation coefficient | 0.214 | 0.113 |
| p-value | 0.158 | 0.458 | |
| R. Cerebellum | Correlation coefficient | 0.288 | 0.179 |
| p-value | 0.055 | 0.238 | |
| Total Cerebral GM | Correlation coefficient | 0.194 | 0.246 |
| p-value | 0.201 | 0.103 | |
Abbreviations: L, left; R, right; pVO2, peak volume oxygen consumption; GM, gray matter; MMSE, mini-mental state examination.
4. Discussion
This study is the first randomized controlled trial that examines the effects of an exergame-based intervention on the gray matter volume of different brain structures in patients with FM. The intervention did not show any overall effect in the volume of some brain structures involved in the “pain matrix” (hippocampus, amygdala, thalamus, insula, and cerebellum) as well as the total gray matter of the brain in the comparison of CG and EG.
Results showed that no statistically significant effects were found after 24 weeks of exergame intervention in the brain volumes studied. In line with our results, Firth et al. [24] reported in a review that aerobic exercise did not increase the hippocampus gray matter volumes, but produced retention of gray matter in the left hippocampus. However, this information should be taken with caution due to the heterogeneity of the groups and the programs included in this review. Along the same lines, a recent exergame-based study did not show increases in total gray matter and the hippocampus in older adults [61]. Regarding the thalamus, three studies did not show increases in volume after physical exercise interventions [25,26,62]. However, there are previous studies that have reported significant brain volume changes after exercise interventions. In this regard, Wittfeld et al. [63] found volumetric increases in the thalamus as well as in the cerebellum after an intervention with 2103 adults. Moreover, other previous studies have reported effects on the insula volume after aerobic [64] and dance interventions [65]. Considering the large variability of intensities, volumes, or types of training that have reported volumetric changes after physical exercise interventions, future research should elucidate how these variables (i.e., volume, intensity, and type of training) modulate brain volume changes. In addition, if dance interventions are performed, consideration should be given to whether the exercises performed are creative or repetitive, as they influence neuronal survival and brain volume changes [65].
The duration of the intervention might explain why significant results were not achieved. In this regard, previous studies have reported increases in different brain structures, mainly in the hippocampus, after twelve months of an intervention [25,66,67]. Furthermore, this hypothesis may be reinforced by the results of Nieman et al. [66], where they did not find volumetric changes after six months of intervention but did after 12 months. However, the 24-week exergame-based intervention has previously shown significant effects in physical fitness [36,38], quality of life and pain [36], autonomic modulation [37], and even in the EEG beta power spectrum [32] in women with FM. Interestingly, this could indicate that brain volume-related changes need more time to be achieved. Therefore, future studies should investigate the role of intervention duration in brain volume changes.
However, the within-group analysis showed significant changes in both intervention and control groups. An analysis of different brain volumes in the same group of patients may represent a critical step in elucidating the central mechanisms of FM. The significant changes within groups are in line with previous morphometric reports of changed gray matter volumes in FM patients [68]. Another study has reported volumetric changes to be more pronounced with longer exposure to FM pain. In that case, the authors explained that a longer FM duration reflects a compensatory mechanism in which the brain may attempt to prevent the negative effects of constant nociceptive input [69]. It is also possible that the morphometric changes reflect an increase in the affective–evaluative processing of pain, as has been suggested to occur in patients with chronic lower back pain [70].
The correlations of the present study provide interesting information regarding the significant relationships found between pVO2 and the right and left regions of the hippocampus and the right and left regions of the amygdala. Similar to our results, another study has indicated that higher levels of aerobic fitness are associated with higher hippocampal volumes in older humans [71]. Consequently, it is known that aerobic exercise increases cerebral blood volume in the dentate gyrus of adults, which is related to neurogenesis [72]. On the other hand, the significant relationships found between pVO2 and the right and left regions of the amygdala may be in line with the results of a study that analyzed neural activity and respiratory frequency on anticipation of anxiety. In the most anxious subject, electric current sources were found in the left amygdala. The activation of this area participates in the enhancement of respiratory frequency [73]. Thus, it could be interesting to study the relationship between the aerobic system and the amygdala, also taking into account that the population with FM is a population related to anxiety symptoms [74,75,76].
The present study has some limitations. First, we only included women with FM, so we cannot generalize the results to men with FM. In addition, the women of the present study have different ages, being an aspect to consider in gray matter studies [77]. Second, the effect of the exergame intervention cannot be isolated since no group performed traditional exercise training. Finally, the intensity of training was not specified since the personal conditions of the participants were changing due to the disease. However, this is the first study that evaluates the effects of an exergame tool on different gray matter volumes of brain structures in women with FM.
5. Conclusions
The exergame-based intervention did not induce significant changes in gray matter brain volume when comparing CG and EG of women with FM. The patients of the present study showed significant relationships between pVO2 with the left and right regions of the hippocampus and the left and right regions of the amygdala. Future research on the determinants of the sensitivity to exercise-specific brain changes are warranted.
Acknowledgments
We thank Jesús Morenas for help with FreeSurfer software. We are also grateful to the Extremadura Association of Fibromyalgia (AFIBROEX) in Cáceres for helping recruit the participants for this study. We thank the San Miguel clinic for obtaining the magnetic resonance images.
Author Contributions
Conceptualization, N.G., J.L.L.-L., and A.M.-G.; methodology, S.V., J.L.L.-L., and F.J.D.-M.; formal analysis, J.L.L.-L., S.V., and A.M.-G.; investigation, J.L.L.-L, S.V., and A.M.-G.; resources, F.J.D.-M., N.G., and S.V.; data curation, J.L.L.-L., S.V., and A.M.-G.; writing—original draft preparation, J.L.L.-L. and S.V.; writing—review and editing, A.M.-G. and N.G.; visualization, J.L.L.-L and S.V.; supervision, N.G.; project administration, N.G, S.V. and F.J.D.-M.; funding acquisition, N.G. and S.V. All authors have read and agreed to the published version of the manuscript.
Funding
In the framework of the Spanish National R + D + i Plan, the current study was cofunded by the Spanish Ministry of Economy and Competitiveness (MINECO; reference DEP2015-70356-R). This study was also funded by the Research Grant for Groups (GR18155) funded by Junta de Extremadura (Regional Government of Extremadura) and the European Regional Development Fund (ERDF/FEDER) “a way of doing Europe”). Moreover, this study was supported by the Biomedical Research Networking Center on Frailty and Healthy Aging (CIBERFES) and FEDER funds from the European Union (CB16/10/00477). The author J.L L.-L. was supported by a grant from the Spanish Ministry of Education, Culture, and Sport (FPU18/05655). The author S.V. was supported by a grant from the Regional Department of Economy and Infrastructure of the Government of Extremadura and the European Social Fund (PD16008). The author A.M-G. was supported by a grant from the Spanish Ministry of Education, Culture, and Sport (FPU17/031330). The funders played no role in the study design, the data collection, and analysis, the decision to publish, or the preparation of the manuscript.
Conflicts of Interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Wolfe F., Clauw D.J., Fitzcharles M.A., Goldenberg D.L., Katz R.S., Mease P., Russell A.S., Russell I.J., Winfield J.B., Yunus M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 2.Verbunt J.A., Pernot D.H., Smeets R.J. Disability and quality of life in patients with fibromyalgia. Health Qual. Life Outcomes. 2008;6:8. doi: 10.1186/1477-7525-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mas A., Carmona L., Valverde M., Ribas B. Prevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: Results from a nationwide study in Spain. Clin. Exp. Rheumatol. 2008;26:519. [PubMed] [Google Scholar]
- 4.Castillo D., Ernst T., Cunningham E., Chang L. Altered associations between pain symptoms and brain morphometry in the pain matrix of HIV-seropositive individuals. J. Neuroimmune Pharmacol. 2018;13:77–89. doi: 10.1007/s11481-017-9762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCrae C.S., O’Shea A.M., Boissoneault J., Vatthauer K.E., Robinson M.E., Staud R., Perlstein W.M., Craggs J.G. Fibromyalgia patients have reduced hippocampal volume compared with healthy controls. J. Pain Res. 2015;8:47. doi: 10.2147/JPR.S71959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson M.E., Craggs J.G., Price D.D., Perlstein W.M., Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J. Pain. 2011;12:436–443. doi: 10.1016/j.jpain.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris R.E., Sundgren P.C., Craig A.D., Kirshenbaum E., Sen A., Napadow V., Clauw D.J. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuchinad A., Schweinhardt P., Seminowicz D.A., Wood P.B., Chizh B.A., Bushnell M.C. Accelerated brain gray matter loss in fibromyalgia patients: Premature aging of the brain? J. Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pujol J., Macià D., Garcia-Fontanals A., Blanco-Hinojo L., López-Solà M., Garcia-Blanco S., Poca-Dias V., Harrison B.J., Contreras-Rodríguez O., Monfort J. The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. PAIN®. 2014;155:1492–1503. doi: 10.1016/j.pain.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Jensen K.B., Loitoile R., Kosek E., Petzke F., Carville S., Fransson P., Marcus H., Williams S.C.R., Choy E., Mainguy Y. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol. Pain. 2012;8:1744–8069. doi: 10.1186/1744-8069-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staud R. Brain imaging in fibromyalgia syndrome. Clin. Exp. Rheumatol. 2011;29:S109–S117. [PubMed] [Google Scholar]
- 12.Clauw D.J., Arnold L.M., McCarberg B.H. The Science of Fibromyalgia. Elsevier; Amsterdam, The Netherlands: 2011. pp. 907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawaddiruk P., Paiboonworachat S., Chattipakorn N., Chattipakorn S.C. Alterations of brain activity in fibromyalgia patients. J. Clin. Neurosci. 2017;38:13–22. doi: 10.1016/j.jocn.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Burgmer M., Gaubitz M., Konrad C., Wrenger M., Hilgart S., Heuft G., Pfleiderer B. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom. Med. 2009;71:566–573. doi: 10.1097/PSY.0b013e3181a32da0. [DOI] [PubMed] [Google Scholar]
- 15.Cohen H. Controversies and challenges in fibromyalgia: A review and a proposal. Ther. Adv. Musculoskelet. Dis. 2017;9:115–127. doi: 10.1177/1759720X17699199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bidonde J., Jean Busch A., Bath B., Milosavljevic S. Exercise for adults with fibromyalgia: An umbrella systematic review with synthesis of best evidence. Curr. Rheumatol. Rev. 2014;10:45–79. doi: 10.2174/1573403X10666140914155304. [DOI] [PubMed] [Google Scholar]
- 17.Andrade A., Dominski F.H., Sieczkowska S.M. Seminars in Arthritis and Rheumatism. WB Saunders; Philadelphia, PA, USA: 2020. What we already know about the effects of exercise in patients with fibromyalgia: An umbrella review. [DOI] [PubMed] [Google Scholar]
- 18.Macfarlane G.J., Kronisch C., Dean L.E., Atzeni F., Häuser W., Fluß E., Choy E., Kosek E., Amris K., Branco J. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017;76:318–328. doi: 10.1136/annrheumdis-2016-209724. [DOI] [PubMed] [Google Scholar]
- 19.Hillman C.H., Erickson K.I., Kramer A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 20.Di Liegro C.M., Schiera G., Proia P., Di Liegro I. Physical activity and brain health. Genes. 2019;10:720. doi: 10.3390/genes10090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voelcker-Rehage C., Niemann C. Structural and functional brain changes related to different types of physical activity across the life span. Neurosci. Biobehav. Rev. 2013;37:2268–2295. doi: 10.1016/j.neubiorev.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Killgore W.D.S., Olson E.A., Weber M. Physical exercise habits correlate with gray matter volume of the hippocampus in healthy adult humans. Sci. Rep. 2013;3:1–6. doi: 10.1038/srep03457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haeger A., Costa A.S., Schulz J.B., Reetz K. Cerebral changes improved by physical activity during cognitive decline: A systematic review on MRI studies. NeuroImage Clin. 2019:101933. doi: 10.1016/j.nicl.2019.101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firth J., Stubbs B., Vancampfort D., Schuch F., Lagopoulos J., Rosenbaum S., Ward P.B. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage. 2018;166:230–238. doi: 10.1016/j.neuroimage.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L., Kim J.S., Heo S., Alves H., White S.M. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motl R.W., Pilutti L.A., Hubbard E.A., Wetter N.C., Sosnoff J.J., Sutton B.P. Cardiorespiratory fitness and its association with thalamic, hippocampal, and basal ganglia volumes in multiple sclerosis. NeuroImage Clin. 2015;7:661–666. doi: 10.1016/j.nicl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vancampfort D., Probst M., De Hert M., Soundy A., Stubbs B., Stroobants M., De Herdt A. Neurobiological effects of physical exercise in schizophrenia: A systematic review. Disabil. Rehabil. 2014;36:1749–1754. doi: 10.3109/09638288.2013.874505. [DOI] [PubMed] [Google Scholar]
- 28.Costa M.T.S., Vieira L.P., de Oliveira Barbosa E., Oliveira L.M., Maillot P., Vaghetti C.A.O., Carta M.G., Machado S., Gatica-Rojas V., Monteiro-Junior R.S. Virtual reality-based exercise with exergames as medicine in different contexts: A short review. Clin. Pract. Epidemiol. Ment. Health CP EMH. 2019;15:15. doi: 10.2174/1745017901915010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park E.C., Kim S.G., Lee C.W. The effects of virtual reality game exercise on balance and gait of the elderly. J. Phys. Ther. Sci. 2015;27:1157–1159. doi: 10.1589/jpts.27.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collado-Mateo D., Dominguez-Muñoz F.J., Adsuar J.C., Garcia-Gordillo M.A., Gusi N. Effects of exergames on quality of life, pain, and disease effect in women with fibromyalgia: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2017;98:1725–1731. doi: 10.1016/j.apmr.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Collado-Mateo D., Dominguez-Muñoz F.J., Adsuar J.C., Merellano-Navarro E., Gusi N. Exergames for women with fibromyalgia: A randomised controlled trial to evaluate the effects on mobility skills, balance and fear of falling. PeerJ. 2017;5:e3211. doi: 10.7717/peerj.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villafaina S., Collado-Mateo D., Fuentes J.P., Rohlfs-Domínguez P., Gusi N. Effects of exergames on brain dynamics in women with fibromyalgia: A Randomized controlled trial. J. Clin. Med. 2019;8:1015. doi: 10.3390/jcm8071015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson-Hanley C., Barcelos N.M., Zimmerman E.A., Gillen R.W., Dunnam M., Cohen B.D., Yerokhin V., Miller K.E., Hayes D.J., Arciero P.J. The aerobic and cognitive exercise study (ACES) for community-dwelling older adults with or at-risk for mild cognitive impairment (MCI): Neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Front. Aging Neurosci. 2018;10:76. doi: 10.3389/fnagi.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji L., Zhang H., Potter G.G., Zang Y.F., Steffens D.C., Guo H., Wang L. Multiple neuroimaging measures for examining exercise-induced neuroplasticity in older adults: A quasi-experimental study. Front. Aging Neurosci. 2017;9:102. doi: 10.3389/fnagi.2017.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Central B. Effectivity of Virtual Reality Physical Exercise Program in Brain and Motor Aging in Fibromyalgia. [(accessed on 29 July 2020)]; Available online: http://www.isrctn.com/ISRCTN65034180.
- 36.Martín-Martínez J.P., Villafaina S., Collado-Mateo D., Pérez-Gómez J., Gusi N. Effects of 24-week exergame intervention on physical function under single-and dual-task conditions in fibromyalgia: A randomized controlled trial. Scand. J. Med. Sci. Sports. 2019;29:1610–1617. doi: 10.1111/sms.13502. [DOI] [PubMed] [Google Scholar]
- 37.Villafaina S., Collado-Mateo D., Domínguez-Muñoz F.J., Gusi N., Fuentes-Garcia J.P. Effects of exergames on heart rate variability of women with fibromyalgia: A randomized controlled trial. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-61617-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villafaina S., Borrega-Mouquinho Y., Fuentes-García J.P., Collado-Mateo D., Gusi N. Effect of exergame training and detraining on lower-body strength, agility, and cardiorespiratory fitness in women with fibromyalgia: Single-blinded randomized controlled trial. Int. J. Environ. Res. Public Health. 2020;17:161. doi: 10.3390/ijerph17010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis G.N., Rosie J.A. Virtual reality games for movement rehabilitation in neurological conditions: How do we meet the needs and expectations of the users? Disabil. Rehabil. 2012;34:1880–1886. doi: 10.3109/09638288.2012.670036. [DOI] [PubMed] [Google Scholar]
- 40.Burckhardt C.S., Clark S., Bennett R. Fibromyalgia and quality of life: A comparative analysis. J. Rheumatol. 1993;20:475–479. [PubMed] [Google Scholar]
- 41.Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): A review of its development, current version, operating characteristics and uses. Clin. Exp. Rheumatol. 2005;23:S154. [PubMed] [Google Scholar]
- 42.Esteve-Vives J., Redondo J.R., Salvat M.I.S., de Gracia Blanco M., de Miquele C.A. Proposal for a consensus version of the Fibromyalgia Impact Questionnaire (FIQ) for the Spanish population. Reumatología Clínica. 2007;3:21–24. doi: 10.1016/S1699-258X(07)73594-5. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Andreu J., Ibáñez-Bosch R., Portero-Vázquez A., Masramon X., Rejas J., Gálvez R. Cognitive impairment in patients with fibromyalgia syndrome as assessed by the mini-mental state examination. BMC Musculoskelet. Disord. 2009;10:162. doi: 10.1186/1471-2474-10-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segura-Jiménez V., Álvarez-Gallardo I.C., Carbonell-Baeza A., Aparicio V.A., Ortega F.B., Casimiro A.J., Delgado-Fernández M. Seminars in Arthritis and Rheumatism. WB Saunders; Philadelphia, PA, USA: 2015. Fibromyalgia has a larger impact on physical health than on psychological health, yet both are markedly affected: The al-Ándalus project; pp. 563–570. [DOI] [PubMed] [Google Scholar]
- 45.Pankoff B.A., Overend T.J., Lucy S.D., White K.P. Reliability of the six-minute walk test in people with fibromyalgia. Arthritis Care Res. 2000;13:291–295. doi: 10.1002/1529-0131(200010)13:5<291::AID-ANR8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 46.Cade W.T., Bohnert K.L., Reeds D.N., Peterson L.R., Bittel A.J., Bashir A., Byrne B.J., Taylor C.L. Peak oxygen uptake (VO2peak) across childhood, adolescence and young adulthood in Barth syndrome: Data from cross-sectional and longitudinal studies. PLoS ONE. 2018;13:e0197776. doi: 10.1371/journal.pone.0197776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King S., Wessel J., Bhambhani Y., Maikala R., Sholter D., Maksymowych W. Validity and reliability of the 6 minute walk in persons with fibromyalgia. J. Rheumatol. 1999;26:2233–2237. [PubMed] [Google Scholar]
- 48.Fischl B. FreeSurfer. [(accessed on 29 July 2020)]; Available online: http://surfer.nmr.mgh.harvard.edu/
- 49.Fischl B. FreeSurfer Recon-All. [(accessed on 29 July 2020)]; Available online: http://surfer.nmr.mgh.harvard.edu/fswiki/recon-all.
- 50.Reuter M., Rosas H.D., Fischl B. Highly accurate inverse consistent registration: A robust approach. Neuroimage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ségonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 52.Fischl B., Salat D.H., Van Der Kouwe A.J.W., Makris N., Ségonne F., Quinn B.T., Dale A.M. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 53.Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 55.Ségonne F., Pacheco J., Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med. Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 56.Murga I., Guillen V., Lafuente J.-V. Cambios en la resonancia magnética cerebral asociados al síndrome de fibromialgia. Med. Clin. (Barc.) 2017;148:511–516. doi: 10.1016/j.medcli.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 57.Gómez-Argüelles J.M., Maestú-Unturbe C., Gómez-Aguilera E.J. Neuroimagen en fibromialgia. Rev. Neurol. 2018;67:394–402. doi: 10.33588/rn.6710.2018050. [DOI] [PubMed] [Google Scholar]
- 58.Sterne J.A., White I.R., Carlin J.B., Spratt M., Royston P., Kenward M.G., Wood A.M., Carpenter J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fritz C.O., Morris P.E., Richler J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012;141:2. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 60.Cohen J. Statistical Power Analysis for the Social Sciences. Lawrence Erlbaum Associates; Mahwah, NJ, USA: 1988. [Google Scholar]
- 61.Adcock M., Fankhauser M., Post J., Lutz K., Zizlsperger L., Luft A.R., Guimarães V., Schättin A., de Bruin E.D. Effects of an in-home multicomponent exergame training on physical functions, cognition, and brain volume of older adults: A randomized controlled trial. Front. Med. 2020;6:321. doi: 10.3389/fmed.2019.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas A.G., Dennis A., Rawlings N.B., Stagg C.J., Matthews L., Morris M., Kolind S.H., Foxley S., Jenkinson M., Nichols T.E. Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. Neuroimage. 2016;131:162–170. doi: 10.1016/j.neuroimage.2015.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wittfeld K., Jochem C., Dörr M., Schminke U., Gläser S., Bahls M., Markus M.R.P., Felix S.B., Leitzmann M.F., Ewert R. Mayo Clinic Proceedings. Elsevier; Amsterdam, The Netherlands: 2020. Cardiorespiratory fitness and gray matter volume in the temporal, frontal, and cerebellar regions in the general population; pp. 44–56. [DOI] [PubMed] [Google Scholar]
- 64.Gondoh Y., Sensui H., Kinomura S., Fukuda H., Fujimoto T., Masud M., Nagamatsu T., Tamaki H., Takekura H. Effects of aerobic exercise training on brain structure and psychological well-being in young adults. J. Sports Med. Phys. Fit. 2009;49:129. [PubMed] [Google Scholar]
- 65.Rehfeld K., Lüders A., Hökelmann A., Lessmann V., Kaufmann J., Brigadski T., Müller P., Müller N.G. Dance training is superior to repetitive physical exercise in inducing brain plasticity in the elderly. PLoS ONE. 2018;13:e0196636. doi: 10.1371/journal.pone.0196636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niemann C., Godde B., Voelcker-Rehage C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front. Aging Neurosci. 2014;6:170. doi: 10.3389/fnagi.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erickson K.I., Leckie R.L., Weinstein A.M. Physical activity, fitness, and gray matter volume. Neurobiol. Aging. 2014;35:S20–S28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmidt-Wilcke T., Luerding R., Weigand T., Jürgens T., Schuierer G., Leinisch E., Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia—A voxel-based morphometry study. Pain. 2007;132:S109–S116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 69.Jensen K.B., Srinivasan P., Spaeth R., Tan Y., Kosek E., Petzke F., Carville S., Fransson P., Marcus H., Williams S.C.R. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum. 2013;65:3293–3303. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Apkarian A.V., Sosa Y., Krauss B.R., Thomas P.S., Fredrickson B.E., Levy R.E., Harden R.N., Chialvo D.R. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 71.Erickson K.I., Prakash R.S., Voss M.W., Chaddock L., Hu L., Morris K.S., White S.M., Wójcicki T.R., McAuley E., Kramer A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pereira A.C., Huddleston D.E., Brickman A.M., Sosunov A.A., Hen R., McKhann G.M., Sloan R., Gage F.H., Brown T.R., Small S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masaoka Y., Homma I. The source generator of respiratory-related anxiety potential in the human brain. Neurosci. Lett. 2000;283:21–24. doi: 10.1016/S0304-3940(00)00895-8. [DOI] [PubMed] [Google Scholar]
- 74.Thieme K., Turk D.C., Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: Relationship to somatic and psychosocial variables. Psychosom. Med. 2004;66:837–844. doi: 10.1097/01.psy.0000146329.63158.40. [DOI] [PubMed] [Google Scholar]
- 75.Gormsen L., Rosenberg R., Bach F.W., Jensen T.S. Depression, anxiety, health-related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur. J. Pain. 2010;14:e121–e127. doi: 10.1016/j.ejpain.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 76.Pagano T., Matsutani L.A., Ferreira E.A.G., Marques A.P., Pereira C.A.d.B. Assessment of anxiety and quality of life in fibromyalgia patients. São Paulo Med. J. 2004;122:252–258. doi: 10.1590/S1516-31802004000600005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taki Y., Kinomura S., Sato K., Goto R., Kawashima R., Fukuda H. A longitudinal study of gray matter volume decline with age and modifying factors. Neurobiol. Aging. 2011;32:907–915. doi: 10.1016/j.neurobiolaging.2009.05.003. [DOI] [PubMed] [Google Scholar]