Abstract
Multiple dysregulated signaling pathways are implicated in the pathogenesis of cancer. The conventional therapies used in cancer prevention/treatment suffer from low efficacy, considerable toxicity, and high cost. Hence, the discovery and development of novel multi-targeted agents to attenuate the dysregulated signaling in cancer is of great importance. In recent decades, phytochemicals from dietary and medicinal plants have been successfully introduced as alternative anticancer agents due to their ability to modulate numerous oncogenic and oncosuppressive signaling pathways. Rutin (also known as rutoside, quercetin-3-O-rutinoside and sophorin) is an active plant-derived flavonoid that is widely distributed in various vegetables, fruits, and medicinal plants, including asparagus, buckwheat, apricots, apples, cherries, grapes, grapefruit, plums, oranges, and tea. Rutin has been shown to target various inflammatory, apoptotic, autophagic, and angiogenic signaling mediators, including nuclear factor-κB, tumor necrosis factor-α, interleukins, light chain 3/Beclin, B cell lymphoma 2 (Bcl-2), Bcl-2 associated X protein, caspases, and vascular endothelial growth factor. A comprehensive and critical analysis of the anticancer potential of rutin and associated molecular targets amongst various cancer types has not been performed previously. Accordingly, the purpose of this review is to present an up-to-date and critical evaluation of multiple cellular and molecular mechanisms through which the anticancer effects of rutin are known to be exerted. The current challenges and limitations as well as future directions of research are also discussed.
Keywords: rutin, cancer, signaling pathways, therapeutic targets, pharmacology, drug delivery system
1. Introduction
Cancer is a complex and multifaceted disease that is characterized by the unlimited proliferation of abnormal cells with the ability to attack or spread to the whole body [1]. This disease arises as a result of homeostasis imbalance between cell survival and cell death [2]. Multiple signaling pathways involved in the pathogenesis of cancer bolster the need for further research [3,4]. Despite the progress in cancer research, providing more involved pathways and molecular targets is of great importance. Disturbance in the expression of tumor suppressor genes, oncogenes, and apoptotic genes play a key role in the pathophysiological mechanisms of cancer [5,6]. In addition, several inflammatory, oxidative stress, autophagy, and apoptotic dysregulated pathways are involved in the initiation and development of cancer [7,8,9]. A wide variety of intracellular molecules have been identified to provoke the uncontrolled proliferation of cancer cells. For instance, in malignant cells, the upregulation of cyclin-dependent kinase (CDK) and downregulation of tumor suppressor proteins (p53), CDK inhibitors, p21, p27, and p57 have been identified [10]. The inappropriate regulation of signaling proteins, including phosphoinositide 3-kinase (PI3K), protein kinase B (PKB, also known as Akt), mammalian target of rapamycin (mTOR), and mitogen-activated protein kinases (MAPK) as well as the altered expression of various pro-inflammatory transcription factors, including nuclear factor-κB (NF-κB), activating protein-1 (AP-1), hypoxia-inducible factor 1 (HIF-1) and signal transducers and activators of transcription (STAT) families have been reported in tumor cells [11,12,13,14,15]. Chronic inflammation is considered a key driver of both the initiation and progression of tumorigenesis [16]. Therefore, targeting the key aberrant proteins and pathways represents a desirable approach to cancer therapy.
Common forms of cancer treatment include surgery, radiotherapy, stem cell therapy, photodynamic therapy, and chemo/immunotherapies [17,18]. Despite the efficiency of chemotherapy, the demerits associated with classical cytotoxic treatments, including multiple drug resistance (MDR), high financial costs, and severe adverse effects, cause a major hurdle in its clinical application [19]. Thus, there is a dire need to discover new, safe, and more efficacious treatment options to achieve ideal results. Plant-derived natural products have attained great attention in drug discovery programs. Numerous drugs used for cancer therapy, including doxorubicin, vinblastine, paclitaxel, and camptothecin, have been obtained from natural sources [20]. The use of chemo-herbal combination therapy has been found to increase the anticancer effects of chemotherapeutic agents and to ameliorate drug resistance and chemotherapy-related adverse effects [21,22]. Natural secondary metabolites have shown pleiotropic effects and target various cancer hallmarks, including inflammation, cancer cell proliferation, migration, invasion, angiogenesis, and metastasis [23].
As natural compounds are potential multi-targeted agents in combating cancer, they are of great interest to prevent associated side effects in treating cancer [24]. Oxidative stress and inflammation associated with synthetic anticancer agents are implicated in high levels of toxicity, host tissue damage, and even manifestation of secondary tumors [25]. Growing evidence demonstrates that cytostatic effects of natural products are derived from their potential in modulating oxidative stress, inflammation, autophagy and apoptosis, thereby leading to the prevention/reduction of their associated toxicity [26,27]. Indeed, free radical generation and pro-oxidant properties of natural agents seem to underlie their direct toxicity towards tumor cells. At the same time, antioxidant properties of naturally occurring agents contribute to their cancer preventive ability and lower toxicity compared to synthesized anticancer drugs [25].
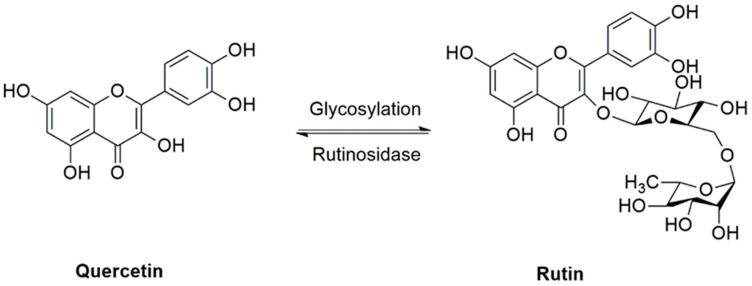
Rutin, also known as rutoside, quercetin-3-O-rutinoside and sophorin, is a glycoside consisting of the flavonol quercetin and the disaccharide rutinose. Rutin has also been called vitamin P, as it is widely distributed in various plants, from vegetables and fruits to medicinal plants, including asparagus, buckwheat, apricots, apples, cherries, grapes, grapefruit, plums, oranges, and tea. Rutin has shown ubiquitous pharmacological properties, including antioxidant, anti-inflammatory, antiangiogenic, pro-apoptotic, and antiproliferative activities, all of which may participate in the prevention and treatment of cancer [28,29,30,31,32,33,34]. Figure 1 displays the chemical structure of rutin and its reversible deglycosylation to produce quercetin. Amongst previous reviews, Prasad et al. [35] and Ganeshpurkar et al. [36] described the pharmacological activities of rutin in combating several diseases, with very limited information related to cancer studies. In another study, Perk et al. [37] reviewed the anticancer effect of rutin without a specific focus on cancer types. A comprehensive and critical analysis of the anticancer effects of rutin and associated molecular targets amongst various cancer types has not been performed before. Therefore, the purpose of this review is to present an up-to-date and critical evaluation of multiple cellular and molecular mechanisms through which the anticancer effects of rutin are known to be exerted. The current challenges and limitations, as well as future directions of research, are also discussed.
Figure 1.
Rutin, a glycoside from quercetin flavonoid.
2. Role of Inflammation, Oxidative Stress, Apoptosis, and Autophagy in Cancer Progression
Prevailing studies are revealing the critical roles of inflammation, oxidative stress, apoptosis, and autophagy in cancer progression. A growing body of evidence has shown that inflammatory responses are key components of tumorigenesis and cancer promotion [38]. Several inflammatory mediators, including cytokines, such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), chemokines, growth factors, and reactive oxygen species (ROS) contribute to the proliferation, metastasis, angiogenesis, and chemoresistance of cancer cells by activation of transcription factors like MAPKs, NF-κB, and mTOR [39]. Aberrant regulation of the molecular pathways involved in inflammation displays a close association with cancer [40]. Targeting impaired inflammatory molecules represents an attractive approach for cancer therapy [41]. Upon exposure to stressful stimuli, the upregulation of the aforementioned intracellular signaling pathways triggers the synthesis and release of inflammatory cytokines, oxidative stress, and carcinogenesis [42]. Constitutive activation of MAPKs and NF-κB signaling pathways have been reported in several types of cancers [41]. MAPKs include a family of protein serine/threonine kinases, which are classified into three main subfamilies, including c-Jun NH2-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38 [43]. The MAPKs signaling cascade plays a critical role in inflammation-associated cancers, participating in cell proliferation, differentiation, and apoptosis [13]. As a parallel pathway to MAPKs, and an upstream signaling pathway of NF-κB, the PI3K/Akt/mTOR signaling pathway is also linked with the regulation of inflammation and cancer cells survival [44]. Accumulating data suggest that there is crosstalk between mTOR activation and inflammatory response, which contributes to the coupling of cell survival and proliferation in response to environmental stimuli [45]. Accordingly, mTOR interacts with the upstream molecule mesenchymal–epithelial transition factor (c-met) to regulate tumor progression. Upregulation of c-met and its ligand, hepatocyte growth factor (HGF), provokes PI3K/Akt/mTOR, Ras/Raf/mitogen-activated protein kinase kinase (MEK)/ERK/MAPK, paxillin/Ras-related C3 botulinum toxin substrate 1 (Rac-1), and STATs signaling cascades, thereby causing inflammation, proliferation, migration, angiogenesis, and metastasis [46,47]. Furthermore, malignant cells activate PI3K/Akt/mTOR, Ras/Raf/MEK/ERK/MAPK, and AP-1/vascular endothelial growth factor (VEGF) pathways via growth factor binding to their putative receptors such as insulin-like growth factor receptor (IGFR), platelet-derived growth factor receptor (PDGFR), and epidermal growth factor receptor (EGFR) [48].
Compelling studies have also demonstrated that ROS play a fundamental role in crosstalk between autophagy and apoptosis [49,50]. Oxidative stress, resulting from an imbalance between ROS production and elimination by enzymatic/non-enzymatic antioxidants including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione (GSH), promotes tumor cell proliferation, angiogenesis, and metastasis. Surprisingly, new evidence indicates that ROS are not only able to induce tumorigenesis but also possess tumor-suppressive properties [51]. Considering the dual role of ROS in cancer, nuclear factor erythroid 2–related factor 2 (Nrf2)-mediated antioxidant response may act as an anti- or as a pro-tumorigenesis [52]. It has been reported that ROS amplify transcription factor AP-1 which, in turn, augments VEGF expression, a trigger of the angiogenesis cascade.
Apoptosis or type I programmed cell death plays a central role in the pathogenesis of cancer. As a hallmark of cancer, apoptosis resistance leads to uncontrolled proliferation, cancer cells survival under hypoxic conditions, and resistance to chemotherapeutic drugs [53]. The underlying mechanisms by which cancer cells evade apoptosis encompasses the downregulation of pro-apoptotic proteins, upregulation of anti-apoptotic proteins, and the dysregulation of death receptors and p53-related signaling pathways [54]. Apoptosis can occur through two well-known apoptotic pathways, including the mitochondrial pathway (intrinsic) and the death receptor pathway (extrinsic) [55]. The intrinsic and extrinsic pathways are associated with caspase-9 and caspase-8, respectively. The extrinsic pathway is initiated through the occupation of cell surface death receptors of the TNF receptor family and the intrinsic pathway is triggered by cellular stresses [56,57]. Tumor suppressor p53 can regulate the intrinsic pathway of apoptosis through controlling the B cell lymphoma 2 (Bcl-2) proteins family [58]. Induction of pro-apoptotic BH3-only proteins suppresses the pro-survival Bcl-2 proteins, thereby leading to the upregulation of pro-apoptotic proteins BH3-interacting domain death agonist (Bid), Bcl-2 antagonist killer (BAK), and Bcl-2 associated X protein (Bax) which, in turn, cause the outer mitochondrial membrane to become permeable and the release of cytochrome c from the mitochondria [59]. Cytochrome c then activates cysteine protease enzymes called caspases that are responsible for cleaving vital cellular proteins [60].
On the other hand, p53 possesses the ability to control the expression of components of the death receptors pathway. P53 activates the death receptors tumor necrosis factor receptor 1 (TNFR1) and fatty acid synthase (FAS) to sensitize cells to death ligands TNF-α, Fas ligand (FasL), and TNF-related apoptosis-inducing ligand (TRAIL), thus facilitating apoptosis [61]. Interestingly, MAPK kinase 4 (MKK4), selective for JNK activation, couples oncogenic stimuli to p53 activation which, in turn, leads to p21-mediated cell-cycle arrest and/or Bax-mediated apoptosis [62]. TNF-α not only participates in fostering tumor growth through chronic inflammation but also amplifies apoptosis through activating the extrinsic pathway. NF-κB also serves as a key factor in inducing apoptosis mediated by TRAIL or TNF-α [63]. Therefore, impaired activation of NF-κB expedites resistance to apoptosis. TNF-α also promotes poly (ADP ribose) polymerase (PARP) activation [64], an important enzyme in DNA repair and programmed cell death. PARP inhibitors expedite ROS production, DNA damage, and programmed cell death [59]. Phosphatases and tensin homolog (PTEN) is an important tumor suppressor gene that negatively regulates the PI3K/Akt/mTOR anti-apoptotic pathway. Impairment of the PTEN/ PI3K/Akt/mTOR pathway represses apoptosis and promotes tumorigenesis [65].
Autophagy (programmed cell death type II) is an intracellular regulated process that plays a vital role in the maintenance of cellular homeostasis by eliminating malformed and unwanted proteins [66]. Aberrant regulation of autophagy contributes toward tumorigenesis. Autophagy acts as a double edged sword, containing both tumor suppression and tumor promotion characteristics [67]. This dual role of autophagy poses a great challenge in the development of efficient anticancer drugs. As a homeostasis control process, autophagy displays cytoprotective properties through the degradation of misfolded proteins and the clearing of ROS. As a tumor promoter, the stress-hindering activities of autophagy protect malignant cells from necrosis caused by metabolic stress. Autophagy also supplies the elevated energy demands of tumor cells, which is necessary for tumor cell survival and proliferation [68]. Mechanistically, JNK, p38MAPK, and ERK signaling pathways positively regulate autophagy in malignant cells. Upregulation of these pathways putatively activates autophagy associated proteins like autophagy-related protein (Atg), Beclin1, and light chain 3 (LC3B) [69,70,71]. MTOR is a well-known inhibitor of autophagy. As upstream regulators of mTOR, Akt and Forkhead box O3 (FoxO3) play key roles in the positive regulation of mTOR and inhibition of autophagy [72].
In summary, there are numerous altered signaling pathways identified across several cancer types. Targeting cross-linked intracellular signaling pathways that are associated with dysregulated proliferation and cell survival by utilizing multi-targeted agents is an attractive strategy to combat cancer.
3. Rutin: Sources and Pharmacological Effects
Rutin (3, 3′, 4′, 5, 7-pentahydroxyflavone-3-rhamnoglucoside) is a flavonol glycoside found in a wide variety of vegetables, fruits, and beverages, including passionflower, grapes, green asparagus, apples, tea, and wine. A large number of medicinal plants also contain rutin, such as Buckwheat (Fagopyrum esculentum Moench), Ruta graveolens L., Sophora japonica L., Maranta leuconeura E. Morren., and Eucalyptus spp. [73,74,75], with the former being the most significant source of natural rutin [76]. Rutin has been also isolated from several herbal families, including Polygonaceae, Rutaceae, Fabaceae, Marantaceae, and Myrtaceae [75,77]. It has been reported that the concentration of rutin varies within the different parts of plants, becoming elevated after UV-B exposure to protect against radiation [78,79]. Rutin is also called vitamin P or rutoside and contains extensive pharmacological properties, including neuroprotective [30], hepatoprotective [80,81], cardioprotective [82], and anticarcinogenic activities [37]. Rutin has also been demonstrated to hamper inflammation, oxidative insults, and platelet aggregation [83]. The insulin-sensitizing and lipid-lowering properties of rutin support the beneficial effects of this agent in diabetes mellitus, hyperlipidemia, and cardiovascular disease. The underlying mechanisms by which rutin counteracts diabetes and its complications include the suppression of gluconeogenesis, increased glucose uptake, and the abrogation of intestinal glucose absorption [84]. Rutin also reverses endothelial dysfunction through enhancing nitric oxide production and repressing ROS responsive nucleotide-binding domain-like receptor 3 (NLRP3) [85,86], thereby decreasing the risk of cardiovascular disease. Rutin has been also reported to combat neurodegenerative diseases by abrogating neuroinflammation, abnormal protein accumulation, and apoptosis, as well as regulating microglia and astrocyte activation [87,88,89]. It has been documented that rutin possesses promising nephroprotective effects against nephrotoxins, such as cisplatin, vancomycin, and mercuric chloride, via mitigating inflammation, oxidative damage, apoptosis, and enhancing aquaporin 1 level [90,91,92]. From another mechanistic perspective, rutin also targets several inflammatory mediators such as NF-κB and TNF-α, thereby counteracting inflammation-driven disease. The hepatoprotective properties of rutin in animal models of non-alcoholic fatty liver disease include its ability to mitigate autophagy corroborated by abrogating key autophagy biomarkers and modulating the expression of lipolytic and lipogenic genes [93]. In various preclinical models, rutin has been also shown to elevate Nrf2 accompanied by an increase in enzymatic/non-enzymatic antioxidant activities, including SOD, CAT, and GPx, thereby alleviating the aforementioned diseases.
Rutin can, overall, be regarded as a promising multi-targeted nutraceutical agent that elicits several health benefits.
4. Methodology for Literature Search on Rutin and Cancer
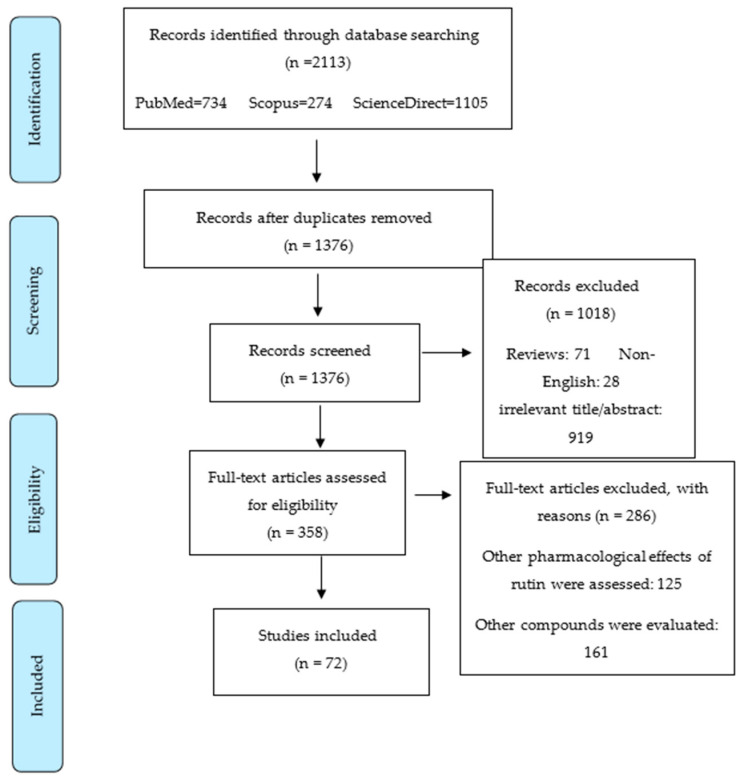
The present systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) criteria. PRISMA statement is useful for improving the reporting of systematic reviews and meta-analyses [94]. A systematic literature search was performed using the scholarly electronic database, including PubMed, Science Direct, and Scopus. The last search was made in June 2020. The systematic search in databases was conducted using the following keywords: “Rutin” and (“cancer” OR “neoplasm” OR “malignancy” OR “carcinoma” OR “melanoma” OR “leukemia” OR “tumor”) [full text]. It should be mentioned that in the Scopus database, the aforementioned keywords were found in [title/abstract/keywords]. Out of the initial 2113 articles that were obtained by electronic search, 737 were excluded due to duplicated results, 71 were excluded because they were reviews, and 919 were irrelevant based on title and/or abstract information. Additionally, 28 were omitted since they were not in English. Among 358 retrieved articles, 125 were excluded as they evaluated other pharmacological effects of rutin rather than anticancer effects and 161 were ruled out since they focused on other compounds, not rutin. Finally, 72 reports were included in this review, as shown in a summary of results in Figure 2.
Figure 2.
Flow diagram related to selection process of articles.
5. Anticancer Activities of Rutin
Rutin has been found to counteract several types of cancer through various mechanisms, e.g., inhibition of malignant cell growth, induction of cell cycle arrest and apoptosis, and modulation of angiogenesis, inflammation, and oxidative stress, all of which are mediated by regulating multiple cellular signaling pathways. The pharmacological activities and basic antitumor mechanisms of rutin in several cancer types are discussed below.
5.1. Rutin and Breast Cancer
Breast cancer is a multifaceted and heterogeneous disease [95]. Based on the presence or absence of three molecular biomarkers, estrogen receptor-α (ER-α), progesterone receptor (PR), and human epidermal growth factor-2 (HER2), breast cancer is classified into five distinct molecular subtypes: (a) luminal A (positive for ER-α and/or PR while negative for HER-2); (b) luminal B (positive for ER-α and/or PR as well as HER2); (c) HER-2 overexpressing; (d) triple-negative; and (e) normal breast-like tumors [96,97]. Triple-negative breast cancer (TNBC) is a heterogenetic and aggressive subtype of breast cancer that is negative for the expression of ER-α, PR, and HER2 [98]. TNBC represents poor prognosis and outcome due to the lack of ideal target options [99]. Therefore, there exists a dire need to discover new targeted therapies for counteracting TNBC. Overactivation of c-met and its ligand, HGF, plays a key role in the initiation and/or progression of TNBC [100]. It has been reported that c-met/HGF is involved in inducing several downstream effectors of different signaling pathways such as Ras/Raf/MEK/ERK/MAPK, PI3K/Akt/mTOR, and Rac-1 [46,47]. Targeting c-met/HGF signaling with novel inhibitory agents is an innovative strategy to combat TNBC. Rutin exhibits anticancer effects on TNBC cell lines through abrogating c-met/HGF axis and its downstream cascades, including paxillin, Rac-1, mTOR, and Akt [101] (Table 1). Additionally, rutin was capable of decreasing the average tumor volume of the TNBC in nude mice [101]. Rutin is therefore a promising c-met inhibitor that may serve as a suitable option to hamper c-met-dependent malignancies.
Table 1.
Potential anticancer effects and mechanisms of action of rutin based on in vitro and in vivo studies.
| Type of Cancer | Type of Study | Cell Type/Animal Model | Anticancer Effects | References |
|---|---|---|---|---|
| Breast | In vitro In vivo |
Human TNBC cells (MDA-MB-231 and MDA-MB-468) Female athymic Foxn1nu/Foxn1C mice |
↓c-met/ HGF, ↓paxillin, ↓Rac-1 ↓mTOR, ↓Akt, ↓tumor volume | [101] |
| Breast | In vitro | Human breast cancer cells (MCF-7) | ↓Proliferation, ↑apoptosis, ↑cell cycle arrest, ↑PTEN, ↑p53 ↑p21 |
[102] |
| Breast | In vitro In vivo |
Human breast cancer cells (MCF-7) Female Swiss albino mice |
↑Apoptosis, ↓tumor volume, ↓CEA, ↓cholesterol, ↓FAS, ↓MDA, ↑GSH, ↑caspase-3, ↑caspase-7 | [103] |
| Breast | In vitro | Human TNBC cells (MDA-MB-231) and breast cancer cells (MCF-7) | ↑Chemosensitivity, ↓MDR, ↓P-gp ↓BCRP | [104] |
| Breast | In vitro | Human breast cancer cells (MCF-7) | ↑Chemosensitivity | [105] |
| Breast | In vitro | Human TNBC cells (MDA-MB-231) | ↓VEGF, ↓angiogenesis | [106] |
| Breast | In vitro | Human breast cancer cells (MCF-7) | ↑Apoptosis, ↑cell cycle arrest | [107] |
| Breast | In vitro | Human TNBC cells (MDA-MB-231) and Human breast cancer cells (MCF-7) | ↑Apoptosis, ↑p53, ↑Bax, ↓Bcl-2, ↓VEGF | [108] |
| Breast | In vitro | Human TNBC cells (MDA-MB-231) and human breast cancer cells (MCF-7) | ↓Proliferation, ↑apoptosis, ↑ROS | [109] |
| Lung | In vitro | Human lung cancer cells (A549) | ↑Cytotoxicity, ↑GSK-3β, ↑TNF-α | [110] |
| Lung | In vitro | Human lung cancer cells (A549) | ↓Migration, ↓fibronectin, ↓collagen type I and IV, ↑ROS, ↓superoxide | [29] |
| Lung | In vitro | Human lung cancer cells (A549) | ↓Single strand DNA break, ↓ROS | [111] |
| Lung | In vivo | C57BL/6 female mice | ↓Lung tumor nodules, ↑life span | [112] |
| Lung | In vivo | Albino Swiss mice | ↓Lung tumor nodules, ↓growth, ↓invasion index | [113] |
| Lung | In vitro | Human lung cancer cells (A549) | ↑Autophagy, ↑Beclin1, ↑Atg5/12, ↑LC3-II, ↓NF-κB, ↓TNF-α | [114] |
| Lung | In vitro | Human lung cancer cells | ↓Proliferation ↓cell cycle, ↓NF-κB, ↓p38 | [115] |
| Colon | In vitro | Human colon cancer cells (HT-29) | ↑Apoptosis, ↑caspase-3, ↑caspase-8, ↑caspase-9 ↑PARP, ↓Bcl-2, ↑Bax |
[116] |
| Colon | In vitro In vivo |
Human colon cancer cells (SW480) nu/nu mice |
↓Tumor growth ↓angiogenesis, ↓VEGF | [117] |
| Colon | In vitro | Human colon cancer cells (HT-29) | ↑Cytotoxicity, ↓mitochondrial membrane potential, ↑lipid peroxidation, ↓SOD ↓CAT ↓GPx |
[118] |
| Colon | In vitro | Human colon cancer cells (HT-29) | ↓Adhesion, ↓migration, ↑ROS, ↓superoxide | [29] |
| Colon | In vitro | Human colon cancer cells (HT-29) | ↑Apoptosis, ↓Bcl-2, ↑Bax, ↑caspase-3, ↑caspases-8, ↑caspase-9, ↑p53, ↓NF-kB, ↓IKK-α, ↓IKK-β, ↓MAPK | [119] |
| Colon | In vitro | Human colon cancer cells (SW480) | ↑Apoptosis, ↑cell cycle arrest, ↓metabolism | [120] |
| Colon | In vitro | Human colon cancer cells (HT-29) | ↓ cell viability | [121] |
| Colon | In vitro | Human colon cancer cells (LoVo) | ↑Apoptosis, ↑cell cycle arrest | [107] |
| Colon | In vitro | Human colon cancer cells (Caco2) | ↓DNA damage | [122] |
| Colon | In vitro | Human colon cancer cells (Caco2) | No effect on DNA repair | [123] |
| Colon | In vivo | Female CF1 mice | ↓Focal areas of dysplasia, ↓hyperproliferation | [124] |
| Colon | In vivo | Male F344 rats | ↓Aberrant crypt foci, ↑apoptosis | [125] |
| Colon | In vivo | Male F344 rats | No effect | [126] |
| Colon | In vitro | Human colon cancer cells (HCT-8) | No effect | [127] |
| Colon | In vivo | Male F344 rats | No effect | [128] |
| Brain | In vitro | Human glioblastoma cell line (GL-15) |
↓Proliferation, ↑apoptosis, ↓ERK ↑GFAP | [129] |
| Brain | In vitro | Human glioblastoma cell line (GL-15) |
↓Invasion, ↓angiogenesis, ↓VEGF, ↓TGF-β1 | [130] |
| Brain | In vitro | Human glioblastoma cell line (GL-15) |
↓Proliferation, ↓invasion, ↓MMP-2, ↑fibronectin, ↑laminin | [131] |
| Brain | In vitro | Human neuroblastoma cells (LAN-5) | ↑Apoptosis, ↓cell cycle, ↓TNF-α, ↓Bcl-2, ↑Bax |
[132] |
| Brain | In vitro | Human glioma cells (CHME) | ↑p53, ↑caspase-3, ↑caspase-9, ↑cytochrome c, ↑Bax, ↓Bcl-2, ↑ROS ↓mitochondrial membrane potential | [133] |
| Brain | In vitro In vivo |
Human glioblastoma cells (U87-MG, D54-MG, and U251-MG) BALB/c athymic mice |
↑Cytotoxicity, ↑apoptosis, ↓ JNK, ↓autophagy, ↑caspase-3, | [134] |
| Leukemia | In vitro | Human leukemic cells (U937, HL-60, KG1, and KG1a) |
↑Cytotoxicity, ↑apoptosis, ↓GSK-3β, ↑Akt | [135] |
| Leukemia | In vivo | Human leukemia HL-60 cells induced leukemia in BALB/c mice | ↓Tumor weight, ↓tumor volume | [136] |
| Leukemia | In vivo | Murine leukemia WEHI-3 cells induced leukemia in BALB/c mice | ↓Proliferation, ↓macrophage phagocytosis | [137] |
| Leukemia | In vitro | Human leukemic cells (THP-1) | ↑Autophagy, ↓NF-κB, ↓TNF-α | [114] |
| Leukemia | In vitro | Human promyelocytic leukemia cells (HL-60) | ↓Angiogenesis, ↓VEGF, ↓AP-1, ↓IGF-1R/IRS-1 | [138] |
| Leukemia | In vitro | Human acute myeloid leukemia cells (KG1) |
↑Cytotoxicity, ↑antioxidant activity | [139] |
| Leukemia | In vitro | human myelogenous leukemia cells (K562) | ↓Single strand DNA break, ↓ROS |
[140] |
| Leukemia | In vitro | human myelogenous leukemia cells (K562) | ↑Apoptosis | [141] |
| Leukemia | In vitro | human promyeloleukemic cells (HL-60) | No effect | [142] |
| Leukemia | In vitro | Murine leukemia cells (L1210) | No effect | [143] |
| Multiple myeloma | In vitro | Human multiple myeloma cells (RPMI8226) | ↑Cytotoxicity, ↑antioxidant activity | [139] |
| Multiple myeloma | In vitro | Human multiple myeloma cells (ARH–77) | ↑Cytotoxicity, ↓mitochondrial and lysosomal activity | [144] |
| Lymphoma | In vitro | Dalton’s lymphoma cells | ↑Apoptosis, ↓Bcl-xL, ↓c-FLIP, ↓GST, ↓GR | [145] |
| Liver | In vitro | Rat hepatoma cells (HTC) | ↓Proliferation, ↓cell viability | [146] |
| Liver | In vitro | Human liver cancer cells (HEPG2) | ↓Proliferation, ↑apoptosis, ↓CYP3A4, ↓CYP1A1, ↑NQO1, ↑GSTP1 | [147] |
| Liver | In vitro | human hepatoma cell line (HepG2) | ↓ROS, ↓MDA | [148] |
| Liver | In vitro | human hepatoma cell line (HepG2) | ↓GSH | [149] |
| Liver | In vitro | human hepatoma cell line (HepG2) | ↑Cytotoxicity | [150] |
| Liver | In vitro | human hepatoma cell line (HepG2) | ↓DNA damage | [122] |
| Liver | In vitro | human hepatoma cell line (HepG2) | No effect | [123] |
| Liver | In vivo | Wistar albino rats | ↑Membrane bound ATPases | [151] |
| Liver | In vivo | Wistar rats | ↓PARP, ↓DNA polymerase β, ↓DNA ligase | [152] |
| Gastric | In vitro | Human gastric cancer cells (SGC-7901) | ↑Apoptosis, ↑caspase-3, ↑caspase-7, ↑caspase-9, ↓Bcl-2/Bax, ↑p38MAPK, ↑G0/G1 arrest | [153] |
| Prostate | In vitro | Human prostatic cancer cells (PC3) | ↓Proliferation, ↑apoptosis, ↓Bcl-2, ↑p53 | [28] |
| Prostate | In vitro | Human prostate cancer cells (LNCaP) |
No effect | [154] |
| Oral | In vitro | Drug resistance oral carcinoma cells (KBCHR8–5) | ↓Wnt/GSK-3β/β-catenin pathway, ↓P-gp | [105] |
| Cervical | In vitro | cervical cancer cells (HeLa) | ↓Proliferation, ↓growth | [155] |
| Ovarian | In vitro | ovarian cancer cells (OVCAR-3) | ↓Proliferation, ↓VEGF | [156] |
| Melanoma | In vitro | melanoma cells (B16F-10) | ↓Angiogenesis, ↓VEGF, ↓IL-1β, ↑TNF-α | [157] |
Abbreviations: Akt, protein kinase B; AP-1, activating protein-1; Atg5/12, autophagy related 5/12; Bax, Bcl-2 associated X protein; Bcl-2, B cell lymphoma 2; BCRP, breast cancer resistance protein; CAT, catalase; CEA, carcinoembryonic antigen; c-FLIP, cellular FLICE-inhibitory protein; C-met, mesenchymal–epithelial transition factor; CYPs, cytochrome P450s; FAS, fatty acid synthase; GFAP, glial fibrillary acidic protein; GPx, glutathione peroxidase; GR, glutathione reductase; GSK-3β, glycogen synthase kinase; GST, glutathione S-transferase; GSTP1, glutathione S-transferase Pi 1; HGF, hepatocyte growth factor; IGF-1R, insulin-like growth factor-1 receptor; IKK, IκB kinase; IL, interleukin; IRS-1; insulin receptor substrate-1; JNK, Jun N-terminal Kinase; LC3-II, light chain 3; MAPK, mitogen-activated protein kinase; MDR, multidrug resistance; MMP-2, metalloproteinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; NQO1, NADPH quinone oxidoreductase 1; PARP, poly (ADP ribose) polymerase; P-gp, P-glycoprotein; PTEN, Phosphatases and tensin homolog; Rac-1, Ras-related C3 botulinum toxin substrate 1; ROS, reactive oxygen species; SOD, superoxide dismutase; TGF-β, transforming growth factor-β; TNBC, triple-negative breast cancer; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor; Wnt, wingless/integrated.
P53 is a well-known tumor suppressor gene that participates in the induction of cell cycle arrest and apoptosis [158]. Upregulation of p53 promotes p21 activation and subsequently leads to the abrogation of a myriad of cell cycle proteins, including CDK6, CDK2, CDK4, and cyclin B1 [159,160]. Rutin promotes cell cycle arrest at the G2/M phase through interfering with p53- and p21-dependent pathways in ER-α positive-breast cancer MCF-7 cells (luminal A subtype). Additionally, rutin markedly induces apoptosis through enhancing p53 and PTEN. Rutin synergistically increases the antiproliferative effect of tamoxifen on ER-α positive-breast cancer MCF-7 cells [102]. Therefore, rutin may be considered a promising adjuvant agent to increase tamoxifen efficacy in ER-α positive-breast cancer.
From another mechanistic point of view, hypercholesterolemia plays a key role in the progression of breast cancer [161]. Elevated cholesterol levels are associated with uncontrolled cell growth and a worse breast cancer prognosis [162]. The upregulation of FAS participates in tumorigenesis by hampering apoptosis [163]. Rutin abates FAS, elevates antioxidants, and causes cytotoxicity in MCF-7 cells by inducing caspase-dependent apoptosis [103]. Additionally, rutin illustrates anticancer effects against Ehrlich ascites carcinoma, an animal model of breast cancer, as observed by mitigating carcinoembryonic antigen, tumor volume, and cholesterol levels [103].
Prolonged chemotherapy often leads to MDR, which is implicated in the failure of conventional chemotherapeutic agents [164]. MDR occurs due to the upregulation of several drug efflux transporters and the failure of apoptotic pathways. Targeting adenosine triphosphate-binding cassette (ABC) transporters including P-glycoprotein (P-gp/ABCB1), breast cancer resistance protein (BCRP/ABCG2), and multidrug resistance-associated protein-1 (MRP1/ABCC1) by natural products has been a critical approach to reverse MDR and restore chemosensitization [165,166]. Chemoresistance to anticancer therapy is the main cause of tumor recurrence [167]. Therefore, abrogation of chemoresistance can mitigate the relapsed tumor. As a chemosensitizing agent, rutin can be considered as a promising nutraceutical agent to alleviate relapsed tumors. It has been found that the formulations containing rutin and other compounds (arctigenin, arctiin, berberine, berbamine, sanguinarine, and chelerythrine) can successfully inhibit the tumor resistance to chemotherapy, thereby preventing tumor recurrence [168]. Hydrolyzed rutin, a compound modified via rutin deglycosylation, displayed antiproliferative effects and diminished anaplasia in a mouse model with recurrent glioblastoma [169]. Interestingly, rutin amplifies chemosensitivity to cyclophosphamide and methotrexate while reversing MDR by suppressing P-gp and BCRP pumps in MB-MDA-231 and MCF-7 cell lines using well-characterized models of TNBC and HER2-negative breast cancer, respectively. From a different anticancer mechanistic perspective, rutin arrests the cell cycle at G2/M and G0/G1 phases, thereby inducing cell apoptosis [104]. Rutin diminished the resistance to doxorubicin in MCF-7/ADR cells [105]. In addition, rutin showed the potential to suppress angiogenesis, VEGF synthesis and expression in MDA-MB-231 breast cancer cells [106]. Interestingly, this phytochemical depicted antitumor effect via cell cycle arrest at S phase and ROS-mediated apoptosis in MCF-7 cells [107]. Rutin-vanadium complex successfully provoked apoptosis through interfering with p53, Bax, Bcl-2 and abated VEGF expression in both MCF-7 and MDA-MB-231 cells [108]. Further research is needed to confirm the potential of rutin as an adjuvant or synergistic agent in breast cancer therapy.
Controlled release systems are a promising strategy to decrease the fluctuation of drug concentration, enhance treatment efficacy, and diminish side effects [170]. Fabrication of hydrogels of both natural and synthetic polymers offers various advantages, as they supply controlled release and targeting, protect incorporated drugs from degradation and metabolism, and exhibit good biocompatibility and biodegradable properties [171,172]. The pH-responsive hydrogels incorporated with rutin and 5-fluorouracil were successfully formulated using natural water-insoluble polymer (Zein) with the synthetic monomer (acrylic acid). The anticancer effect was evaluated against MDA-MB-231 and MCF-7 breast cancer cell lines. Rutin and 5-fluorouracil loaded pH-sensitive Zein-co-acrylic acid hydrogels demonstrate a controlled release manner and augment anticancer effects by inducing apoptosis and ROS generation [109]. Based on these results, pH-sensitive hydrogels may be a suitable formulation for oral delivery of anticancer drugs with the intent of attaining the tumor site-responsive controlled release and thereby decreasing undesirable toxic effects in normal tissues.
5.2. Rutin and Lung Cancer
Lung cancer is the most frequent leading cause of cancer-related death worldwide [173]. Distant metastasis, resistance to the chemotherapeutic regimens, and the cytotoxicity of the drugs are common causes of death amongst lung cancer patients [174]. Therefore, there exists an urgent need to discover non-toxic alternative treatments for chemotherapy responsive lung cancer. In this regard, Wu et al. [110] revealed that rutin exhibits cytotoxicity against A549 human lung cancer cells through modulating TNF-α and glycogen synthase kinase-3β (GSK-3β) expression. GSK-3β participates in numerous cellular processes including proliferation, the cell cycle, and apoptosis [175]. Fibronectin and collagen type I and IV play an important role in the formation of the extracellular matrix, which controls adhesion and migration of cancerous cells [176]. Rutin hampers the adhesion of A549 cells to Fibronectin and collagen type I and IV, thereby inhibiting the migration of lung cancer cells. Additionally, rutin enhances ROS generation and alleviates superoxide production in A549 cells [29]. Rutin hindered the increased effect of β-carotene on single-strand DNA break induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A549 cells. This effect can be ascribed to its antioxidant properties since it abolished ROS level [111]. Rutin repressed lung metastasis induced by B16FlO melanoma cells in mice as observed by decreasing the lung tumor nodules and enhancing the life span of mice [112]. Similarly, in another in vivo study, rutin diminished the number of metastatic nodules, growth, and invasion index, thereby ameliorated lung metastasis induced by B16FlO melanoma cells in mice [113]. From another mechanistic point of view, rutin induces autophagy in A549 cells corroborated by elevating Beclin1, Atg5/12, and LC3-II expression. Additionally, rutin mitigates the expression of NF-κB and TNF-α, acting as a modulator of tumorigenesis [114]. Several transcription factors, namely NF-κB and STAT, have been identified as direct targets of p38 [177]. P38 is then phosphorylated and activated by MKK3 and MKK6 [178], inducing inflammation by producing various pro-inflammatory mediators, such as IL-1β, TNF-α, cyclooxygenase (COX)-2, and inducible nitric oxide synthase (iNOS) [177]. The majority of existing data suggests opposing activity of the p38 signaling pathway with respect to apoptosis and cell cycle modulation [179]. In an in vitro study, rutin prevented the development of lung cancer by diminishing NF-κB and p38 expression, arresting the cell cycle [115]. Additional experiments on lung cancer cell lines and in vivo tumor models should be conducted to further evaluate the beneficial effects of rutin against lung cancer.
5.3. Rutin and Colon Cancer
Colorectal cancer results from various risk factors, such as inflammatory bowel disease, obesity, and smoking [180]. Dietary rutin considerably abolishes the viability of human colon adenocarcinoma HT 29 cells in a concentration-dependent manner. Rutin-mediated inhibition of HT 29 cells is achieved by augmentation of cleaved caspase-3, caspase-8, caspase-9, and PARP. PARP is an important enzyme in the detection of DNA damage and programmed cell death [181]. Additionally, rutin upregulates Bax and downregulates Bcl-2. These findings illustrate that rutin induces apoptosis in HT 29 colon cancer cells through concomitant activation of the death receptors and mitochondrial pathways [116].
VEGF is considered a key factor in angiogenesis and tumor growth promotion. Therapeutic intervention involving the inhibition of VEGF has become an innovative strategy for abrogating tumor metastasis [182]. Rutin exerts cytotoxic effects against SW480 colon cancer cells in vitro, markedly suppressing tumor growth and diminishing the expression of VEGF in vivo [117]. In another study, combined treatment of rutin and irradiation sensitized the HT-29 cells to irradiation. Further, concurrent rutin treatment enhanced apoptotic cells, DNA damage, and lipid peroxidative markers. The antioxidant performance elicited by concurrent rutin treatment was reduced by inhibiting antioxidant enzymes (SOD and CAT) and decreasing the mitochondrial membrane potential as cell survival and apoptosis factor [118]. Therefore, rutin is a suitable candidate to increase the radiotherapy response to colon cancer. In an in vitro study, rutin depicted cytotoxic activity on HT-29 cells via increasing ROS generation, ameliorating superoxide production, impairing cell adhesion, and mitigating migration [29].
There is increasing evidence that inflammation is implicated in the proliferation, survival, invasion, angiogenesis, and metastasis of tumor cells [183,184]. Targeting the inflammatory signaling pathway by rutin provides an attractive strategy for cancer prevention and treatment. Rutin effectively ameliorates the expression of biomarkers of the NF-κB inflammatory pathway, including NF-κB, IκB kinase (IKK)-α, and IKK-β in HT-29 colon cancer cells [119]. This indicates that rutin may play a critical role in the prevention of inflammation-mediated cancers. MAPKs are involved in modulating numerous cellular activities related to cancer progression, including inflammatory cascades, proliferation, differentiation, and apoptosis [13], supporting the use of potent MAPKs inhibitor agents. Rutin hinders tumor growth in vitro through interfering with p38MAPK and MAPK activated protein kinase 2 (MK-2). Moreover, rutin ameliorates apoptosis by targeting apoptosis-related proteins, including caspase-3, caspase-8, caspase-9, Bax, Bcl-2, and p53 [119].
Dysregulated metabolism contributes to tumor initiation and progression [26,185]; therefore, their regulation in cancer is of great importance. Rutin ameliorates the metabolism of colon cancer SW480 cells, increases apoptosis, and arrests the cell cycle at the sub-G1 phase. Analysis of microRNAs, long noncoding RNAs, messenger RNAs, and transcription factors revealed that these promising effects were associated with the modulation of dysregulated intracellular signaling pathways involved in glucose, lipid, and protein metabolism, extrinsic and intrinsic apoptosis, reticulum stress responses, and cell cycle stages [120]. Future studies should investigate the proposed panel in other cancer models. Rutin encapsulated in low methoxyl pectin beads abolished cell viability of HT-29 colon cancer cells [121]. It presented antitumor effects via cell cycle arrest at S phase and ROS-mediated apoptosis in LoVo colon cancer cells [107]. Rutin also protected colon cancer Caco2 cells against hydrogen peroxide-induced DNA damage; however, it did not enhance the DNA repair process [122,123]. Deschner et al. [124] indicated the potential of rutin in repressing azoxymethanol (AOM)-induced colonic neoplasia as seen by decreasing focal areas of dysplasia and abrogating hyperproliferation of colonic epithelial cells. In another in vivo study, rutin hindered aberrant crypt foci and induced apoptosis in AOM-induced rat colon cancer [125]; however, Dihal et al. [126] showed that rutin in contrast to its aglycone, quercetin, exerted no protective effect against AOM-induced colorectal carcinogenesis in rats. In this line, rutin did not hamper methylcholantrene (MCH)-mediated CYP1A1 activation, as an enzyme metabolizing precarcinogenic agents, participates in carcinogenesis of intestinal cells (HCT-8) [127]. In another study, rutin could not hinder the development of AOM-induced rat colon cancer and augmented tissue inhibitor of metalloproteinase 1 (TIMP-1) expression, a biomarker of colorectal cancer progression [128]. Overall, further biological and biochemical effects of rutin in colon cancer are needed in-depth clarification in future studies.
5.4. Rutin and Brain Cancer
Due to low targeting and negligible permeability of anticancer agents through the blood–brain barrier, brain cancer is an aggressive and devastating neoplasm that is difficult to treat [186]. As a part of the MAPK family, ERK is aberrantly upregulated in cancers expediting the survival, proliferation, and migration of cancer cells [187]. Additionally, ERK participates in crosstalk between programmed cell death and autophagy [188]. ERK plays a key role in TNF-induced autophagy, inhibition of which enhances cellular sensitivity to TNF-induced apoptosis [189]. Discovering and developing new agents to hinder ERK activity is a promising anticancer strategy. Rutin displays pro-apoptotic and antiproliferative effects on human glioblastoma cell lines (GL-15) by diminishing the level of ERK1/2 phosphorylation. Additionally, rutin stops the cell cycle at the G2 stage and stimulates differentiation of GL-15 cells towards an astroglial phenotype, characterized by the upregulation of glial fibrillary acidic protein (GFAP), an astrocyte neurobiomarker [129]. Rutin inhibited the invasion and angiogenesis of GL-15 cells corroborated by mitigating the VEGF and transforming growth factor (TGF)-β1 [130]. In another study, it also exerted an antiproliferative effect on GL-15 cells accompanied by an anti-invasive activity with regard to the potential of this nutraceutical agent in decreasing metalloproteinase (MMP-2) expression, as well as enhancing the expression of extracellular matrix proteins including fibronectin and laminin [131].
MYCN oncogene is a characteristic feature of an advanced and aggressive neuroblastoma stage, representing a poor prognosis [190]. MYCN is a desired target for counteracting neuroblastoma. Rutin obviously abrogates MYCN expression and suppresses the migration and invasion of human neuroblastoma cells, LAN-5. Rutin promotes apoptosis corroborated by a decrease of Bcl-2 expression and Bcl-2/Bax ratio. Additionally, rutin blocks cell cycle progression at the G2/M stage and ameliorates inflammation through the attenuation of TNF-α secretion [132]. Therefore, rutin may be considered a suitable candidate for the treatment of MYCN-dependent tumors.
Rutin exhibits an apoptotic effect on human glioma CHME cells through inducing ROS generation and abating mitochondrial membrane potential. A promising apoptotic effect of rutin was further corroborated by the upregulation of p53, caspase-3, caspase-9, cytochrome c, and Bax as well as the downregulation of Bcl-2 [133]. In addition to the critical role of apoptosis in preventing cancer, autophagy plays an important role in the maintenance of cellular homeostasis and metabolism management [191]. Autophagy can represent both oncogenic and cancer suppressive features, thereby acting as a double-edged sword in cancer cells [192]. Considering the dual function of autophagy in tumorigenesis, both suppression and promotion of this pathway have attracted attention as a promising cancer treatment. The JNK pathway exhibits a multifaceted role in regulating autophagy, apoptosis, and DNA damage [193]. Rutin interestingly mitigates JNK activity both in vitro and in vivo, thereby amplifying the cytotoxic effect of temozolomide through blocking JNK-mediated autophagy. Rutin reinforces the apoptosis effect of temozolomide corroborated by the overexpression of cleaved caspase-3 [134].
5.5. Rutin and Leukemia/Multiple Myeloma/Lymphoma
Acute myeloid leukemia is a heterogeneous and aggressive malignancy characterized by the accumulation of immature myeloid hematopoietic cells [194]. The pivotal role of GSK-3β in preserving quiescent hematopoietic stem cells makes it a promising therapeutic target in acute human leukemia [195]. Rutin triggers apoptosis of leukemic cells and promotes cell quiescence through activating Akt and inhibiting GSK-3β. Rutin suppresses the survival of adherent leukemic cells, thus it may be considered as a promising therapeutic agent to combat cell adhesion-mediated drug resistance in acute myeloid leukemia [135]. Rutin decreases tumor weight and volume in human leukemia HL-60 cells in a murine xenograft animal model [136]. In another in vivo study, rutin reduces liver/spleen weight, abolishes proliferation, and augments the activity of macrophage phagocytosis, thereby inducing an immune response in WEHI-3-induced leukemia model in BALB/c mice [137]. Interestingly, rutin exhibits anticancer effects on the leukemia THP-1 cells by promoting autophagy and diminishing inflammation corroborated by decreasing NF-κB and TNF-α [114]. Belonging to the c-Jun subfamily, AP-1 is a well-known transcription factor that plays a critical role in the positive regulation of VEGF [196]. AP-1 activity can be regulated by transcription factors, such as ERK, p38, and JNK [197]. ROS promotes VEGF as a trigger of the angiogenesis cascade [198]. On the other hand, activation of insulin-like growth factor 1 receptor (IGF-1R)/insulin receptor substrate-1 (IRS-1) signaling pathway amplifies the activity of AP-1, which, in turn, stimulates VEGF expression [199]. Targeting the VEGF signaling pathway by naturally occurring compounds appears to be a promising antiangiogenic approach to combat tumor growth. Rutin and vitamin E synergistically suppress VEGF in HL-60 cells. This beneficial effect was mainly attributed to the downregulation of AP-1 and IGF-1R/IRS-1. Antioxidant activity from a combined treatment of rutin and vitamin E (confirmed by decreasing ROS generation) plays a partial role in the decrease of VEGF secretion [138]. Rutin-zinc complex contains antioxidant and cytotoxicity activity against leukemia (KG1) and multiple myeloma (RPMI8226) cell lines [139]. ROS scavenging properties of rutin caused a protective effect of this compound against hydrogen peroxide-induced single-strand DNA break in human myelogenous leukemia cells (K562) [140]. Rutin exhibited anticancer effect through reinforcing susceptibility of K562 cells to natural killer cell-mediated apoptosis [141]. However, Shen et al. [142] revealed that rutin exhibited no apoptosis effect in human promyeloleukemic HL-60 cells compared to its aglycone, quercetin. On the other hand, rutin combined with cytarabine decreased the antiproliferative effect of cytarabine in L1210 leukemia cells [143]. In another study, rutin caused a cytotoxicity in ARH–77 multiple myeloma cell line and mitigated mitochondrial and lysosomal activity [144]. Rutin promotes apoptosis and abrogates GSH levels in Dalton’s lymphoma cells. According to a molecular docking study, rutin acts as a potential suppressor of anti-apoptotic proteins, namely Bcl-xL and cellular FLICE-inhibitory protein (c-FLIP), and antioxidant enzymes, such as GST and glutathione reductase [145]. Further studies should be performed to verify the in silico results.
5.6. Rutin and Liver Cancer
Chronic liver diseases, including persistent viral hepatitis and alcoholic and nonalcoholic fatty liver disease are common causes of liver cancer [200]. Approximately 90% of liver cancers are recognized as hepatocellular carcinomas (HCCs) and 10% are cholangiocarcinomas (CCAs) [200]. Due to the asymptomatic feature of this disease, a diagnosis is made at an advanced stage and therefore therapeutic approaches remain ineffective [201]. A deeper exploration of the biology of HCC and CCA, regarding the development of potential therapies, is desperately needed. Rutin induces DNA damage, suppresses uncontrolled proliferation, and decreases cell viability in HTC hepatic cells. Additionally, rutin exhibits a protective effect against the procarcinogenic agent, benzopyrene, through decreasing DNA damage [146]. In another study, rutin dramatically promoted early/late-stage apoptosis and mitigated proliferation, invasion, and colony formation of HEPG2 cells [147]. An imbalance between phase 1 and phase 2 metabolism implicates toxicity through oxidative insults. Agents that hinder phase 1 metabolism, such as cytochrome P450-produced reactive intermediates, or that augment phase 2 metabolism, such as antioxidant enzymes, are considered potential protective agents against chemical carcinogenesis [202]. Treatment by rutin abrogates cytochrome P450-dependent CYP3A4 and CYP1A1 enzymes in addition to enhancing the antioxidant enzymes NADPH Quinone Dehydrogenase1 (NQO1) and glutathione S-transferase Pi 1 (GSTP1) [147]. Rutin favorably augmented antioxidant performance by mitigating ROS generation and malondialdehyde concentration in HepG2 cells [148]. In contrast, prolonged treatment of rutin caused a depletion of GSH in HepG2 cells and acted as a pro-oxidant, resulting in cell death [149]. Interestingly, rutin caused a significant cytotoxic effect on HepG2 cancer cells [150]. Rutin protected HepG2 cells against hydrogen peroxide induced DNA damage; however, it did not enhance the DNA repair process [122,123]. In an in vivo study, rutin hampered liver tumor markers, including α-fetoprotein and carcinoembryonic antigens, in nitrosodiethylamine and phenobarbital administered rats. Additionally, rutin enhances the declined level of membrane bound ATPases [151]. Na+/K+, Ca2+, and Mg2+ ATPases play a key role in the transportation of the electrolytes sodium, potassium, calcium, and magnesium across membranes [203]. The lipid peroxidation activity, which is often raised when in a cancerous state, plays a deleterious effect on ATPase activities and electrolyte levels [204], while electrolyte imbalance contributes to cancer progression [205]. Rutin reverses common electrolyte abnormalities, including hyperkalemia, hyponatremia, hypercalcemia, and hypomagnesemia in hepatocellular carcinoma-bearing rats [151]. Upregulation of enzymes involved in repairing DNA damage, including PARP, DNA polymerase β, and DNA ligase participate in tumorigenesis [206,207,208]. Modulation of these parameters is a promising way of controlling cancer. In an in vivo experiment, rutin interestingly hampered DNA damage and the activity of repair enzymes induced by hepatocarcinogens, namely aflatoxin B1 and N-nitrosodimethylamine [152].
5.7. Rutin and Gastric Cancer
Gastric cancer is a result of various genetic and environmental factors. Helicobacter pylori infection, smoking, dietary habits, and obesity are important risk factors influencing the development of gastric cancer [209]. According to the World Health Organization, gastric cancer is classified into three categories, such as adenocarcinoma, signet ring-cell carcinoma, and undifferentiated carcinoma [210]. Another most common classification system, the Laurén classification, categorized gastric cancer into two groups, namely intestinal and diffuse types [211]. Despite a multitude of advances achieved in treatment of gastric cancer, adverse effects and resistance to chemotherapeutic agents limit their therapeutic efficacy [212]. Therefore, new alternative strategies to overcome these challenges and the design of novel drugs for targeting gastric cancer therapy are needed. P38MAPK is a key factor in modulating various functions of tumor cells, including differentiation, invasion, proliferation, and apoptosis [213], attracting further interest as an auspicious therapeutic target for cancer therapy [214]. Rutin mitigates the proliferation of human gastric adenocarcinoma SGC-7901 cells, arrests tumor cells at G0/G1, upregulates caspase-3, caspase-7, and caspase-9, and lowers the Bcl-2/Bax ratio. These effects are attributed to the upregulation of the p38 signaling pathway. Concomitant treatment with rutin and oxaliplatin displays synergistic anticancer effects, allowing a decrease in the dose of oxaliplatin, thus decreasing toxicity [153].
5.8. Rutin and Prostate Cancer
Prostate cancer represents the second most commonly diagnosed cancer among men [215]. Prostate cancer is a result of both genetic and environmental factors; however, the main etiology is still unclear [216]. The combined use of chemotherapeutic drugs and nutraceutical agents is a promising solution for enhancing anticancer effects, as well as ameliorating drug resistance and chemotherapy adverse effects [217]. A combination of 5-fluorouracil (5-FU) and rutin synergistically acts as a potential cytotoxic agent against PC3 prostate cancer cells. Furthermore, combined treatment hampers cell proliferation, augments apoptosis, downregulates Bcl-2 signaling protein, and upregulates p53 expression [28]. Overactivation of Bcl-2 proto-oncogene plays a critical role in abrogating cell apoptosis and tumor suppressor protein p53 activity [61]. Further investigations should be conducted to evaluate the combined effects of rutin and 5-FU in the regulation of other pro-apoptotic signaling pathways. Voltage gated K+ channels (IK) participates in modulating numerous cellular activities related to cancer progression [218]. IK current inhibitors may be considered as suitable target of cancer therapy; however, George et al. [154] demonstrated that rutin presented no modulatory effect on IK current in human prostate cancer cell line (LNCaP). Future studies should be performed to evaluate the influence of novel anticancer compounds on IK current.
5.9. Rutin and Other Cancers
It has been well-stablished that augmentation of wingless/integrated (Wnt)/GSK-3β/β-catenin signaling pathway plays a key role in upregulation of P-gp in various cancer types [219]. Rutin enhanced doxorubicin-mediated cell cycle arrest at G2/M phase through interfering with Wnt/GSK-3β/β-catenin signaling pathway, thereby alleviated the overexpression of P-gp in drug resistant oral carcinoma KB cells [105]. Rutin-Cu (II) complex suppressed the growth and proliferation of cervical cancer cells (HeLa) in a time- and concentration-dependent manner [155]. Ovarian cancer is considered a second leading cause of gynecologic cancer death among women [220]. Although the chemotherapy and surgical procedures are applied in ovarian cancer therapy, the five-year survival rate is poor, less than 50% [221]. Rutin exerted an acceptable potential in abrogation of cell proliferation and VEGF expression of ovarian cancer OVCAR-3 cells [156]. Rutin also demonstrated antiangiogenic effects against B16F-10 melanoma cell-induced capillary formation in an animal model. In addition, rutin downregulated the expression of VEGF, IL-1β and enhanced the expression of TNF-α in tumor associated macrophage. Therefore, antiangiogenic activity of rutin can be attributed to the modulation of these cytokines and growth factors [157].
6. Nanostructured Formulations of Rutin in Combating Cancer
Despite the encouraging anticancer properties of rutin in preclinical studies, there are certainly obstacles in its clinical transition. Rutin has poor solubility, high metabolism, low gastrointestinal absorption, and limited bioavailability, therefore limiting the capability to achieve effective concentrations in tumor tissues [32,222]. A promising way to overcome these challenges is encapsulating the agent into various forms of nanosized delivery vehicles. Nanotechnology offers the potential to deliver bioactive phytochemicals and nutraceutical agents directly to the desired locations, such as tumor tissues, thereby providing maximum therapeutic activities of these compounds [19,223,224,225,226]. Nanostructured carriers can passively accumulate in solid tumors by an enhanced permeability retention effect [227,228]. Active targeting is attained by attachment of a targeting ligand to the nanoparticles (NPs) surface that binds to its receptor expressed on tumor cells, thereby increasing site-specificity and controlled drug delivery to the cancer tissue [229].
Rutin encapsulated in folic acid conjugated keratin NPs promotes cell death in MCF-7 breast cancer cells while exhibiting less toxicity in healthy cells [230] (Table 2). Additionally, the actively targeted nanoformulation decreases tumor cell migration, elevates rutin uptake in cancer cells, and boosts apoptosis through ROS production and mitochondrial potential loss. An in vitro study indicated that the nanoformulation selectively targets breast cancer cells [230]. However, in vivo studies are needed to confirm the active targeted delivery of folic acid conjugated keratin NPs.
Table 2.
Rutin based nanoscale drug delivery systems for counteracting several types of cancer.
| Nanoformulation Model | Type of Cancer | Type of Study | Cell Type/Animal Model | Outcomes | References |
|---|---|---|---|---|---|
| Folic acid-conjugated keratin NPs | Breast | In vitro | Huma breast cancer cells (MCF-7) | ↑Apoptosis, ↓migration, ↑ROS, ↓mitochondrial membrane potential | [230] |
| Nanoemulsions | Prostate | In vitro | Human prostatic cancer cells (PC3) | ↑Apoptosis, ↑ROS | [233] |
| Ionic liquids-NPs | Renal | In vitro | Human renal cancer cells (786-O) | ↑Cytotoxicity, ↑sub-G1 population, ↑solubility | [234] |
| Eudragit S100 nanospheres | Colon | In vitro | Human colon cancer cells (HCT 116) | ↑Cytotoxicity ↑solubility | [235] |
| Keratin NPs | Cervical | In vitro | Human cervical cancer cells (Hela) | ↑Cytotoxicity | [236] |
| PLGA NPs | Liver | In vivo | Albino male Wistar rats | ↓IL-1β, ↓TNF-α, ↓IL-6 ↓NF-κB, ↑SOD, ↑CAT, ↑GSH, ↑GPx, ↑membrane-bound enzymes |
[237] |
| PLGA nanospheres | Breast | In vitro | Human TNBC cells (MDA-MB-231) | ↓Proliferation, ↑apoptosis, ↑ROS | [238] |
| Chitosan NPs | Breast | In vitro | Human TNBC cells (MDA-MB-231) | ↑Apoptosis, ↑cell cycle arrest | [239] |
| ZnO NPs | Breast | In vitro | Human breast cancer cells (MCF-7) | ↑Cytotoxicity | [240] |
| Chitosan/copper oxide nanocomposites | Lung | In vitro | Human lung cancer cells (A549) | ↑Cytotoxicity, ↑apoptosis | [241] |
| Fucoidan NPs |
Cervical | In vitro | Human cervical cancer cells (Hela) | ↑DNA fragmentation, ↑cell cycle arrest, ↑ROS, ↓mitochondrial membrane potential | [242] |
| Chitosan/poly (acrylic acid) nanogel | Liver | In vivo | Male albino rats | ↓proliferation, ↓angiogenesis, ↓VEGF, ↑Bax, ↓Bcl-2, ↑p53, ↑caspase-3 | [243] |
| Nanosized polymeric micelles | Leukemia | In vitro | human myelogenous leukemia cells (K562) | Low cytotoxicity | [244] |
Abbreviations: Bax, Bcl-2 associated X protein; Bcl-2, B cell lymphoma 2; CAT, catalase; GPx, glutathione peroxidase; GSH, glutathione; IL-6, interleukin-6; NF-κB, nuclear factor-κB; NPs, nanoparticles; PLGA, poly (lactic co-glycolic acid); ROS, reactive oxygen species; SOD, superoxide dismutase; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
Nanoemulsions are thermodynamically stable systems that are favorable vehicles for enhancing solubility, intestinal uptake, and bioavailability of lipophilic drugs [231,232]. Rutin-based nanoemulsion dramatically promotes cytotoxicity in PC3 prostatic cancer cells through inducing ROS and apoptosis. The rutin nanoemulsion is more effective against prostate cancer when compared to rutin suspension. Optimized rutin nanoemulsion exhibits thermodynamic stability and an efficient drug release profile [233]. Rutin nanoemulsion may be a suitable candidate to be evaluated in in vivo models of prostate cancer.
Ionic liquids are salts with a melting point below 100 °C which enhance the solubility of poorly water-soluble drugs [245,246]. Ionic liquids are composed of organic cations, such as imidazolium, pyrrolidinium, pyridinium, tetraalkylammonium, or tetraalkylphosphonium, along with organic or inorganic anions, including tetrafluoroborate, hexafluorophosphate, and bromide [247]. Interestingly, hybrid ionic liquids contain active pharmaceutical ingredients and ionic liquids, which are promising strategies to improve their solubility, bioavailability, and biological effects. For instance, ionic liquid-based formulations enhanced the solubility and anticancer activities of several compounds, such as curcumin and paclitaxel [248,249]. Rutin-loaded ionic liquid–NPs were fabricated by a double-emulsion method and were found to exhibit cytotoxic effects against 786-O human renal cancer cells through amplifying sub-G1 population. Ionic liquids increased the solubility of rutin and enhanced its incorporation into water/oil/water emulsion, thereby providing a controlled delivery system [234].
The development of stimuli-responsive nanocarriers is another promising solution for the targeted delivery and site-specific triggering of the release of anticancer agents [250,251]. Stimuli-sensitive nanocarriers rapidly release anticancer drugs in response to environmental stimuli, such as pH, temperature, redux, and enzymes [252,253]. Eudragit S100 is a pH-sensitive copolymer that dissolves at colon pH and is extensively engaged for drug targeting to the colon [254,255]. Rutin-loaded eudragit S100 nanospheres display pH-sensitive activity that can effectively achieve rutin into the colon. The pH-sensitive nanospheres significantly increase the solubility of rutin and provoke its cytotoxic activity against human colon cancer HCT 116 cells vs. rutin suspension [235]. Biodistribution and in vivo studies should be conducted to better understand the anticancer potential of rutin-loaded pH-sensitive nanospheres.
As another delivery system, protein-based NPs possess certain advantages, since they are inherently biocompatible, stable, and have a potential for surface functionalization and covalent attachment of ligands for targeted drug delivery [256]. Keratin is a natural protein that is abundantly found in human hair [257]. Biocompatible and stable keratin NPs incorporated with dual phytocompounds, rutin and quercetin, were successfully fabricated. According to an in silico study, the keratin-based NPs eagerly dock into binding pockets of H-Ras P21 proto-oncogene. This report has been supported by an in vitro study in which the nanoformulation caused significant cytotoxicity in Hela cervical cancer cells [236].
Consistently, poly (lactic-co-glycolic acid) (PLGA) NPs also attain special attention in biomedical applications as they represent desirable features, including biocompatibility, surface modifiability, controlled delivery, and targeting [258]. Oral administration of rutin-loaded PLGA NPs ameliorates diethylnitrosamine-induced HCC. This beneficial effect is mediated by decreasing pro-inflammatory cytokines, including IL-1β, TNF-α, and IL-6, as well as abrogating the NF-κB inflammatory cascade. From another mechanistic point of view, the nanoformulation restores membrane-bound enzymes and mitigates the enhanced level of hepatic enzymatic and α-glutamyl transferase (GGT). PLGA NPs also enhance endogenous antioxidant activity (confirmed by increasing the content of SOD, CAT, GSH, and GPx), suggesting its protective effect against HCC [237].
In addition to the previous goals, nanocarriers offer a potential strategy for efficient delivery of combination anticancer drugs to overcome MDR and decrease the frequency of drug administration during combination therapy of anticancer agents [259,260]. The co-delivery of benzamide along with rutin through PLGA nanospheres synergistically suppresses the proliferation of MDA-MB-231 cells in the G0/G1 phase through empowering apoptosis and ROS generation. The polymeric nanospheres provide a sustained release of chemotherapeutic agents, augment therapeutic efficiency, and target a MDR associated phenotype TNBC [238]. Chang et al. [239] revealed that rutin–chitosan nanoconjugates could promisingly induce apoptosis and cell cycle arrest in TNBC. The fabricated nanoconjugates markedly stop TNBC growth at a concentration of 12.5 µg/mL.
In recent years, metal-based biocompatible NPs have attracted scientific interest as they are cost-effective, eco-friendly, easy to synthesize, and simple to modify and functionalize the surface [261,262]. Metal NPs possess various applications in the treatment of several diseases, including cancer [263]. Biosynthesized zinc oxide (ZnO) NPs using rutin display higher cytotoxic effect against MCF-7 breast cancer cells vs. rutin alone [240]. In another study, chitosan functionalized copper oxide (CuO) nanocomposites were biosynthesized using rutin and exhibited antiproliferative activity, provoking apoptosis in the human lung cancer cell line A549 [241]. Further mechanistic studies are needed to confirm the promising effects of rutin-loaded metal NPs in the treatment of cancer.
Fucoidan, a natural sulfated polysaccharide, possesses widespread applications in the treatment of cancer, inflammatory disease, and bacterial infections [264,265,266]. Fucoidan is able to form complexes with different drugs using reactive functional groups to increase their solubility, absorption, and bioavailability [267,268]. The nanosized rutin-fucoidan complex is biocompatible in normal cells and provides sustained release of compounds from the mixture at a pH of 5.5. Additionally, the complex synergistically boosts growth effects, arrests the cell cycle, and enhances apoptosis through ROS production, mitochondrial potential loss, and DNA fragmentation in HeLa cervical cancer cells [242]. The development of such complex formulations can be considered as a promising solution to counteract cervical cancer, amongst other cancer types. Rutin-loaded chitosan/poly (acrylic acid) nanogel enhanced bioavailability of rutin and significantly reinforced antiproliferative, antiangiogenic (by reducing VEGF), and apoptotic effects (by increasing p53, caspase-3, and Bax as well as mitigating Bcl-2), indicating potential antitumor activity of the nanoformolation against diethylnitrosamine (DENA)/carbon tetrachloride (CCl4)-induced hepatocarcinoma in rats [243]. However, both free rutin and rutin-loaded nanosized polymeric micelles displayed low cytotoxicity in sensitive K562 and resistant K562/ADR cells [244].
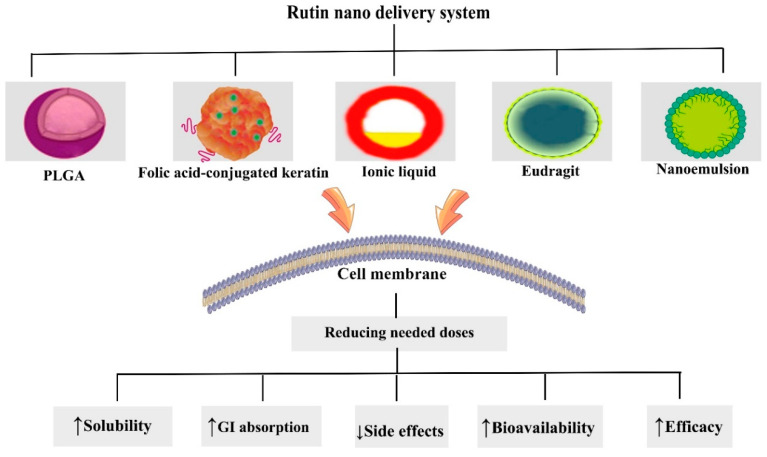
Overall, experimental results demonstrate that the properties of nano drug delivery systems have been able to overcome pharmacokinetic limitations of rutin, underscoring its promising effects in chemotherapy. Further research needs to be performed to design surface-modified nanoformulations of rutin to attain optimized drug delivery systems. Various novel drug delivery systems of rutin and their effects on improving pharmacokinetic limitations are depicted in Figure 3.
Figure 3.
Nanoformulations of rutin used to combat cancer. GI, gastrointestinal; PLGA, poly (lactic co-glycolic acid).
7. Conclusions
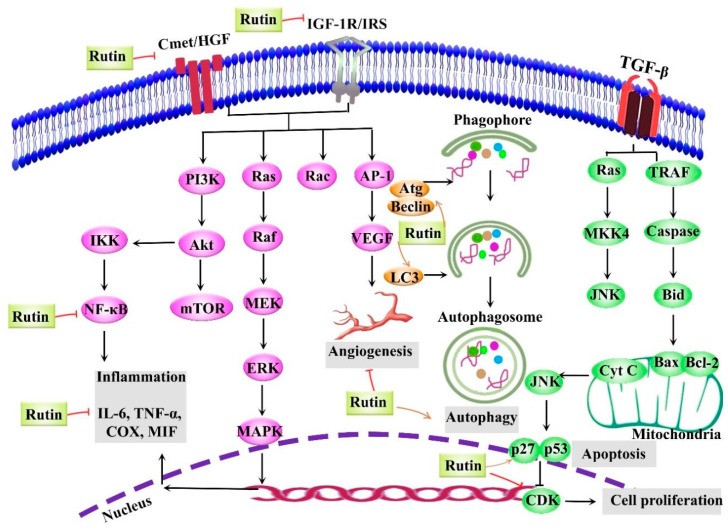
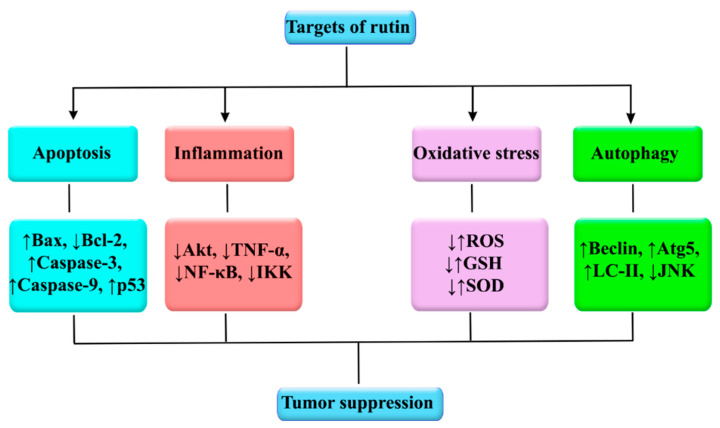
The plant kingdom offers a tremendous source of alternative anticancer drugs. Among natural entities, rutin, a glycosylated flavonoid, possesses several significant biological activities with the prevailing evidence now being focused on its anticancer effects. Rutin has been shown to employ multiple mechanisms to impede cancer initiation and progression by modulating various dysregulated signaling pathways implicated in inflammation, apoptosis, autophagy, and angiogenesis (Figure 4). Specifically, the tumor-inhibitory effects of rutin have been shown to be exerted through the regulation of various signaling pathways, such as PI3K/Akt/mTOR, NF-κB, Nrf2, ERK, p38 MAPK, and JNK. This bioactive natural agent potentially interferes with several intracellular signaling molecules, including TNF-α, ILs, LC3/Beclin, Bax, Bcl-2, caspases, and VEGF. In particular, extensive studies have revealed that rutin targets various therapeutically important molecules, such as p53, Bax, Bcl-2, caspase-3, caspase-9, NF-κB, Akt, TNF-α, Atg5, Beclin, GSH, and SOD (Figure 5). Several cancer types, including breast cancer, glioblastoma, prostate cancer, lung adenocarcinoma, gastric cancer, hepatocellular carcinoma, leukemia, and colon cancer, are impacted by rutin. Most of the current anticancer evidence of rutin is focused on in vitro models of cancer, with very limited in vivo studies. Despite various preclinical mechanistic studies on the anticancer effects of rutin, lack of well-designed randomized clinical trials on the therapeutic activities and safety of rutin escalates the need toward more clinical investigations. The possible pharmacokinetic limitations of rutin underscore the need for developing appropriate delivery systems. Additional studies and engineering methods are required to design surface modified nanostructures of rutin to achieve targeted drug delivery systems against cancer. A further area of research on novel molecular targets and signaling pathways of rutin, as well as providing well-controlled clinical trials, will develop its clinical applications in the prevention and treatment of several cancer types.
Figure 4.
Numerous dysregulated mechanisms and therapeutic targets implicated in the anticancer effects of rutin. Akt, protein kinase B; Atg, autophagy-related gene; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; CDK, cyclin-dependent kinase; C-met/HGF, mesenchymal–epithelial transition factor/hepatocyte growth factor; COX, cyclooxygenase; Cyt C, cytochrome c; ERK, extracellular signal-regulated kinase; IGF-1R/IRS-1, insulin-like growth factor 1 receptor /insulin receptor substrate-1; IKK, IκB kinase; IL-6, interleukin-6; JNK, Jun N-terminal Kinase; LC3, light chain 3; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase kinase; MIF, macrophage migration inhibitory factor; MKK, MAPK kinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; PI3K, phosphatidylinositol 3-Kinase; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; TRAF, tumor necrosis factor receptor-associated factor; VEGF, vascular endothelial growth factor.
Figure 5.
Main molecular targets influenced by rutin in cancer. Akt, protein kinase B; Atg, autophagy-related gene; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; GSH, glutathione; IKK, IκB kinase; JNK, Jun N-terminal Kinase; LC, light chain; NF-κB, nuclear factor-κB; ROS, reactive oxygen species; SOD, superoxide dismutase; TNF-α, tumor necrosis factor-α.
Author Contributions
Conceptualization, Z.N. and M.H.F.; writing—original draft preparation, Z.N., S.F., K.N., and M.H.F.; Software, Z.N., and S.F.; writing—review and editing, Z.N., S.F., M.H.F., C.E.W., and A.B; revising, Z.N., S.F., M.H.F., and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium Pan-cancer analysis of whole genomes. Nature. 2020;578:82–93. doi: 10.1038/s41586-020-1969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y., Henson E.S., Xiao W., Huang D., McMillan-Ward E.M., Israels S.J., Gibson S.B. Tyrosine kinase receptor EGFR regulates the switch in cancer cells between cell survival and cell death induced by autophagy in hypoxia. Autophagy. 2016;12:1029–1046. doi: 10.1080/15548627.2016.1164357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakhri S., Abbaszadeh F., Jorjani M., Pourgholami M.H. The effects of anticancer medicinal herbs on vascular endothelial growth factor based on pharmacological aspects: A review study. Nutr. Cancer. 2019:1–15. doi: 10.1080/01635581.2019.1673451. [DOI] [PubMed] [Google Scholar]
- 4.Ochwang’i D.O., Kimwele C.N., Oduma J.A., Gathumbi P.K., Mbaria J.M., Kiama S.G. Medicinal plants used in treatment and management of cancer in Kakamega County, Kenya. J. Ethnopharmacol. 2014;151:1040–1055. doi: 10.1016/j.jep.2013.11.051. [DOI] [PubMed] [Google Scholar]
- 5.Slattery M.L., Herrick J.S., Mullany L.E., Samowitz W.S., Sevens J.R., Sakoda L., Wolff R.K. The co-regulatory networks of tumor suppressor genes, oncogenes, and miRNAs in colorectal cancer. Genes Chromosomes Cancer. 2017;56:769–787. doi: 10.1002/gcc.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croce C.M., Reed J.C. Finally, an apoptosis-targeting therapeutic for cancer. Cancer Res. 2016;76:5914–5920. doi: 10.1158/0008-5472.CAN-16-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monkkonen T., Debnath J. Inflammatory signaling cascades and autophagy in cancer. Autophagy. 2018;14:190–198. doi: 10.1080/15548627.2017.1345412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postovit L., Widmann C., Huang P., Gibson S.B. Harnessing oxidative stress as an innovative target for cancer therapy. Hindawi. 2018 doi: 10.1155/2018/6135739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mileo A.M., Miccadei S. Polyphenols as modulator of oxidative stress in cancer disease: New therapeutic strategies. Oxid. Med. Cell Longev. 2016;2016 doi: 10.1155/2016/6475624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams G.H., Stoeber K. The cell cycle and cancer. J. Pathol. 2012;226:352–364. doi: 10.1002/path.3022. [DOI] [PubMed] [Google Scholar]
- 11.Huang J., Gao L., Li B., Liu C., Hong S., Min J., Hong L. Knockdown of hypoxia-inducible factor 1α (HIF-1α) promotes autophagy and inhibits Phosphatidylinositol 3-Kinase (PI3K)/AKT/Mammalian target of rapamycin (mTOR) signaling pathway in ovarian cancer cells. Med. Sci. Monit. 2019;25:4250–4263. doi: 10.12659/MSM.915730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amani H., Ajami M., Maleki S.N., Pazoki-Toroudi H., Daglia M., Sokeng A.J.T., Di Lorenzo A., Nabavi S.F., Devi K.P., Nabavi S.M. Targeting signal transducers and activators of transcription (STAT) in human cancer by dietary polyphenolic antioxidants. Biochimie. 2017;142:63–79. doi: 10.1016/j.biochi.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Peluso I., Yarla N.S., Ambra R., Pastore G., Perry G. MAPK signalling pathway in cancers: Olive products as cancer preventive and therapeutic agents. Semin. Cancer Biol. 2019;56:185–195. doi: 10.1016/j.semcancer.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Park M.H., Hong J.T. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5:15. doi: 10.3390/cells5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atsaves V., Leventaki V., Rassidakis G.Z., Claret F.X. AP-1 transcription factors as regulators of immune responses in cancer. Cancers. 2019;11:1037. doi: 10.3390/cancers11071037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marelli G., Sica A., Vannucci L., Allavena P. Inflammation as target in cancer therapy. Curr. Opin. Pharmacol. 2017;35:57–65. doi: 10.1016/j.coph.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi M., Jaafari M., Mirzaei H., Mirzaei H. Mesenchymal stem cell: A new horizon in cancer gene therapy. Cancer Gene Ther. 2016;23:285–286. doi: 10.1038/cgt.2016.35. [DOI] [PubMed] [Google Scholar]
- 18.Fujishiro T., Nonoguchi N., Pavliukov M., Ohmura N., Kawabata S., Park Y., Kajimoto Y., Ishikawa T., Nakano I., Kuroiwa T. 5-Aminolevulinic acid-mediated photodynamic therapy can target human glioma stem-like cells refractory to antineoplastic agents. Photodiagnosis Photodyn. Ther. 2018;24:58–68. doi: 10.1016/j.pdpdt.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Davatgaran-Taghipour Y., Masoomzadeh S., Farzaei M.H., Bahramsoltani R., Karimi-Soureh Z., Rahimi R., Abdollahi M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017;12:2689. doi: 10.2147/IJN.S131973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cragg G.M., Pezzuto J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016;25:41–59. doi: 10.1159/000443404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo R.R., Colilla M., Vallet-Regí M. Advances in mesoporous silica-based nanocarriers for co-delivery and combination therapy against cancer. Expert Opin. Drug Deliv. 2017;14:229–243. doi: 10.1080/17425247.2016.1211637. [DOI] [PubMed] [Google Scholar]
- 22.Khurana R.K., Jain A., Jain A., Sharma T., Singh B., Kesharwani P. Administration of antioxidants in cancer: Debate of the decade. Drug Discov. Today. 2018;23:763–770. doi: 10.1016/j.drudis.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Bishayee A., Sethi G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016;40–41:1–3. doi: 10.1016/j.semcancer.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Bordoloi D., Roy N.K., Monisha J., Padmavathi G., Kunnumakkara A. Multi-targeted agents in cancer cell chemosensitization: What we learnt from curcumin thus far. Recent Pat. Anticancer Drug Discov. 2016;11:67–97. doi: 10.2174/1574892810666151020101706. [DOI] [PubMed] [Google Scholar]
- 25.Korkina L., De Luca C., Kostyuk V., Pastore S. Plant polyphenols and tumors: From mechanisms to therapies, prevention, and protection against toxicity of anti-cancer treatments. Curr. Med. Chem. 2009;16:3943–3965. doi: 10.2174/092986709789352312. [DOI] [PubMed] [Google Scholar]
- 26.Fakhri S., Khodamorady M., Naseri M., Farzaei M.H., Khan H. The ameliorating effects of anthocyanins on the cross-linked signaling pathways of cancer dysregulated metabolism. Pharmacol. Res. 2020;159:104895. doi: 10.1016/j.phrs.2020.104895. [DOI] [PubMed] [Google Scholar]
- 27.Braicu C., Zanoaga O., Zimta A.-A., Tigu A.B., Kilpatrick K.L., Bishayee A., Nabavi S.M., Berindan-Neagoe I. Natural compounds modulate the crosstalk between apoptosis-and autophagy-regulated signaling pathways: Controlling the uncontrolled expansion of tumor cells. Semin. Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.05.015. in press. [DOI] [PubMed] [Google Scholar]
- 28.Satari A., Amini S.A., Raeisi E., Lemoigne Y., Heidarian E. Synergetic impact of combined 5-fluorouracil and rutin on apoptosis in PC3 cancer cells through the modulation of P53 gene expression. Adv. Pharm. Bull. 2019;9:462. doi: 10.15171/apb.2019.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ben Sghaier M., Pagano A., Mousslim M., Ammari Y., Kovacic H., Luis J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed. Pharmacother. 2016;84:1972–1978. doi: 10.1016/j.biopha.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Song H.-l., Zhang X., Wang W.-z., Liu R.-h., Zhao K., Liu M.-y., Gong W.-m., Ning B. Neuroprotective mechanisms of rutin for spinal cord injury through anti-oxidation and anti-inflammation and inhibition of p38 mitogen activated protein kinase pathway. Neural Regen Res. 2018;13:128. doi: 10.4103/1673-5374.217349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautam R., Singh M., Gautam S., Rawat J.K., Saraf S.A., Kaithwas G. Rutin attenuates intestinal toxicity induced by Methotrexate linked with anti-oxidative and anti-inflammatory effects. BMC Complement. Altern. Med. 2016;16:99. doi: 10.1186/s12906-016-1069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang C.-Y., Hsiu S.-L., Wen K.-C., Lin S.-P., Tsai S.-Y. Bioavailability and metabolic pharmacokinetics of rutin and quercetin in rats. J. Food Drug Anal. 2005;13 doi: 10.38212/2224-6614.2517. [DOI] [Google Scholar]
- 33.Zheng Y., Zhao Z., Fan L., Meng S., Song C., Qiu L., Xu P., Chen J. Dietary supplementation with rutin has pro-/anti-inflammatory effects in the liver of juvenile GIFT tilapia, Oreochromis niloticus. Fish. Shellfish Immunol. 2017;64:49–55. doi: 10.1016/j.fsi.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Khajevand-Khazaei M.-R., Mohseni-Moghaddam P., Hosseini M., Gholami L., Baluchnejadmojarad T., Roghani M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57BL/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT1. Eur. J. Pharmacol. 2018;833:307–313. doi: 10.1016/j.ejphar.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Prasad R., Prasad S.B. A review on the chemistry and biological properties of Rutin, a promising nutraceutical agent. Asian J. Pharm. Pharmacol. 2019;5:1–20. doi: 10.31024/ajpp.2019.5.s1.1. [DOI] [Google Scholar]
- 36.Ganeshpurkar A., Saluja A.K. The pharmacological potential of rutin. Saudi. Pharm. J. 2017;25:149–164. doi: 10.1016/j.jsps.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perk A.A., Shatynska-Mytsyk I., Gerçek Y.C., Boztaş K., Yazgan M., Fayyaz S., Farooqi A.A. Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell Int. 2014;14:1–5. doi: 10.1186/s12935-014-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greten F.R., Grivennikov S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantovani A. Molecular pathways linking inflammation and cancer. Curr. Mol. Med. 2010;10:369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 40.Siveen K.S., Sikka S., Surana R., Dai X., Zhang J., Kumar A.P., Tan B.K., Sethi G., Bishayee A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Biophys. Acta Rev. Cancer. 2014;1845:136–154. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Tsao S.-m., Hsia T.-c., Yin M.-c. Protocatechuic acid inhibits lung cancer cells by modulating FAK, MAPK, and NF-κB pathways. Nutr. Cancer. 2014;66:1331–1341. doi: 10.1080/01635581.2014.956259. [DOI] [PubMed] [Google Scholar]
- 42.de Pasquali M.A.B., Gelain D.P., Zeidán-Chuliá F., Pires A.S., Gasparotto J., Terra S.R., Moreira J.C.F. Vitamin A (retinol) downregulates the receptor for advanced glycation endproducts (RAGE) by oxidant-dependent activation of p38 MAPK and NF-kB in human lung cancer A549 cells. Cell Signal. 2013;25:939–954. doi: 10.1016/j.cellsig.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y., Zhang L., Zhang M., Li R., Li Y., Hu X., Wang S., Bao Z. Characterization of three mitogen-activated protein kinases (MAPK) genes reveals involvement of ERK and JNK, not p38 in defense against bacterial infection in Yesso scallop Patinopecten yessoensis. Fish Shellfish Immunol. 2016;54:507–515. doi: 10.1016/j.fsi.2016.04.139. [DOI] [PubMed] [Google Scholar]
- 44.Ye J., Piao H., Jiang J., Jin G., Zheng M., Yang J., Jin X., Sun T., Choi Y.H., Li L. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-κB and Nrf2/HO-1 pathways. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-12252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jhaveri K., Teplinsky E., Silvera D., Valeta-Magara A., Arju R., Giashuddin S., Sarfraz Y., Alexander M., Darvishian F., Levine P.H. Hyperactivated mTOR and JAK2/STAT3 pathways: Molecular drivers and potential therapeutic targets of inflammatory and invasive ductal breast cancers after neoadjuvant chemotherapy. Clin. Breast Cancer. 2016;16:113–122.e1. doi: 10.1016/j.clbc.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ebrahim H.Y., Elsayed H.E., Mohyeldin M.M., Akl M.R., Bhattacharjee J., Egbert S., Sayed K.A.E. Norstictic acid inhibits breast cancer cell proliferation, migration, invasion, and in vivo invasive growth through targeting C-Met. Phytother. Res. 2016;30:557–566. doi: 10.1002/ptr.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pothula S.P., Xu Z., Goldstein D., Merrett N., Pirola R.C., Wilson J.S., Apte M.V. Targeting the HGF/c-MET pathway: Stromal remodelling in pancreatic cancer. Oncotarget. 2017;8:76722. doi: 10.18632/oncotarget.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vahidnezhad H., Youssefian L., Uitto J. Molecular genetics of the PI3K-AKT-mTOR pathway in genodermatoses: Diagnostic implications and treatment opportunities. J. Invest. Dermatol. 2016;136:15–23. doi: 10.1038/JID.2015.331. [DOI] [PubMed] [Google Scholar]
- 49.Kim K.-Y., Yu S.-N., Lee S.-Y., Chun S.-S., Choi Y.-L., Park Y.-M., Song C.S., Chatterjee B., Ahn S.-C. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem. Biophys. Res. Commun. 2011;413:80–86. doi: 10.1016/j.bbrc.2011.08.054. [DOI] [PubMed] [Google Scholar]
- 50.Kongara S., Karantza V. The interplay between autophagy and ROS in tumorigenesis. Front. Oncol. 2012;2:171. doi: 10.3389/fonc.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmed A.M. Oxidative Stress in Lung Diseases. Springer; Berlin/Heidelberg, Germany: 2019. The dual role of oxidative stress in lung cancer; pp. 99–113. [Google Scholar]
- 52.Menegon S., Columbano A., Giordano S. The dual roles of NRF2 in cancer. Trends Mol. Med. 2016;22:578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Matsuura K., Canfield K., Feng W., Kurokawa M. Metabolic regulation of apoptosis in cancer. Int. Rev. Cell Mol. Biol. 2016;327:43–87. doi: 10.1016/bs.ircmb.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Safa A.R. Oncogenomics. Elsevier; Amsterdam, The Netherlands: 2019. Cancer stem cells, apoptosis pathways and mechanisms of death resistance; pp. 89–101. [Google Scholar]
- 55.Hongmei Z. Apoptosis and Medicine. InTechOpen; London, UK: 2012. Extrinsic and intrinsic apoptosis signal pathway review. [Google Scholar]
- 56.Adams J.M., Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25:27–36. doi: 10.1038/cdd.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akl M.R., Elsayed H.E., Ebrahim H.Y., Haggag E.G., Kamal A.M., Sayed K.A.E. 3-O-[N-(p-fluorobenzenesulfonyl)-carbamoyl]-oleanolic acid, a semisynthetic analog of oleanolic acid, induces apoptosis in breast cancer cells. Eur. J. Pharmacol. 2014;740:209–217. doi: 10.1016/j.ejphar.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 58.Fridman J.S., Lowe S.W. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 59.Nikoletopoulou V., Markaki M., Palikaras K., Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta Mol. Cell Res. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Elena-Real C.A., Díaz-Quintana A., González-Arzola K., Velázquez-Campoy A., Orzáez M., López-Rivas A., Gil-Caballero S., Miguel Á., Díaz-Moreno I. Cytochrome c speeds up caspase cascade activation by blocking 14-3-3ε-dependent Apaf-1 inhibition. Cell Death Dis. 2018;9:1–12. doi: 10.1038/s41419-018-0408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aubrey B.J., Kelly G.L., Janic A., Herold M.J., Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018;25:104–113. doi: 10.1038/cdd.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu G.S. The functional interactions between the MAPK and p53 signaling pathways. Cancer Biol. Ther. 2004;3:156–161. doi: 10.4161/cbt.3.2.614. [DOI] [PubMed] [Google Scholar]
- 63.Qin J.-Z., Bacon P., Chaturvedi V., Nickoloff B.J. Role of NF-κB activity in apoptotic response of keratinocytes mediated by interferon-γ, tumor necrosis factor-α, and tumor-necrosis-factor-related apoptosis-inducing ligand. J. Invest. Dermatol. 2001;117:898–907. doi: 10.1046/j.0022-202x.2001.01477.x. [DOI] [PubMed] [Google Scholar]
- 64.Los M., Mozoluk M., Ferrari D., Stepczynska A., Stroh C., Renz A., Herceg Z., Wang Z.-Q., Schulze-Osthoff K. Activation and caspase-mediated inhibition of PARP: A molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell. 2002;13:978–988. doi: 10.1091/mbc.01-05-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y., Chen B., Wang Z., Zhang W., Hao K., Chen Y., Li K., Wang T., Xie Y., Huang Z. Marsdenia tenacissimae extraction (MTE) inhibits the proliferation and induces the apoptosis of human acute T cell leukemia cells through inactivating PI3K/AKT/mTOR signaling pathway via PTEN enhancement. Oncotarget. 2016;7:82851. doi: 10.18632/oncotarget.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yun C.W., Lee S.H. The roles of autophagy in cancer. Int. J. Mol. Sci. 2018;19:3466. doi: 10.3390/ijms19113466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rao S., Tortola L., Perlot T., Wirnsberger G., Novatchkova M., Nitsch R., Sykacek P., Frank L., Schramek D., Komnenovic V. A dual role for autophagy in a murine model of lung cancer. Nat. Commun. 2014;5:1–15. doi: 10.1038/ncomms4056. [DOI] [PubMed] [Google Scholar]
- 68.Singh S.S., Vats S., Chia A.Y.-Q., Tan T.Z., Deng S., Ong M.S., Arfuso F., Yap C.T., Goh B.C., Sethi G. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37:1142–1158. doi: 10.1038/s41388-017-0046-6. [DOI] [PubMed] [Google Scholar]
- 69.Dower C.M., Wills C.A., Frisch S.M., Wang H.-G. Mechanisms and context underlying the role of autophagy in cancer metastasis. Autophagy. 2018;14:1110–1128. doi: 10.1080/15548627.2018.1450020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu L., Zhang X., Li Y., Lu S., Lu S., Li J., Wang Y., Tian X., Wei J.-j., Shao C. Neferine induces autophagy of human ovarian cancer cells via p38 MAPK/JNK activation. Tumor. Biol. 2016;37:8721–8729. doi: 10.1007/s13277-015-4737-8. [DOI] [PubMed] [Google Scholar]
- 71.Kim K.-Y., Park K.-I., Kim S.-H., Yu S.-N., Park S.-G., Kim Y.W., Seo Y.-K., Ma J.-Y., Ahn S.-C. Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int. J. Mol. Sci. 2017;18:1088. doi: 10.3390/ijms18051088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jung C.H., Ro S.-H., Cao J., Otto N.M., Kim D.-H. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kreft I., Fabjan N., Yasumoto K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006;98:508–512. doi: 10.1016/j.foodchem.2005.05.081. [DOI] [Google Scholar]
- 74.Patel K., Patel D.K. Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases. Elsevier; Amsterdam, The Netherlands: 2019. The beneficial role of rutin, a naturally occurring flavonoid in health promotion and disease prevention: A systematic review and update; pp. 457–479. [Google Scholar]
- 75.Chua L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013;150:805–817. doi: 10.1016/j.jep.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 76.Gullón B., Lú-Chau T.A., Moreira M.T., Lema J.M., Eibes G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017;67:220–235. doi: 10.1016/j.tifs.2017.07.008. [DOI] [Google Scholar]
- 77.Abdullah Y., Schneider B., Petersen M. Occurrence of rosmarinic acid, chlorogenic acid and rutin in Marantaceae species. Phytochem. Lett. 2008;1:199–203. doi: 10.1016/j.phytol.2008.09.010. [DOI] [Google Scholar]
- 78.Kreft S., Štrukelj B., Gaberščik A., Kreft I. Rutin in buckwheat herbs grown at different UV-B radiation levels: Comparison of two UV spectrophotometric and an HPLC method. J. Exp. Bot. 2002;53:1801–1804. doi: 10.1093/jxb/erf032. [DOI] [PubMed] [Google Scholar]
- 79.Harborne J.B., Williams C.A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/S0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 80.Abdel-Ghaffar O., Mahmoud S.T., Said A.A., Sanad F.A.-A.Y. Hepatoprotective effect of rutin against oxidative stress of Isoniazid in albino rats. Int. J. Pharmacol. 2017;13:516–528. [Google Scholar]
- 81.Domitrović R., Jakovac H., Marchesi V.V., Vladimir-Knežević S., Cvijanović O., Tadić Ž., Romić Ž., Rahelić D. Differential hepatoprotective mechanisms of rutin and quercetin in CCl 4-intoxicated BALB/cN mice. Acta Pharmacol. Sin. 2012;33:1260–1270. doi: 10.1038/aps.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin Q., Chen X.-Y., Zhang J., Yuan Y.-L., Zhao W., Wei B. Upregulation of SIRT1 contributes to the cardioprotective effect of Rutin against myocardial ischemia-reperfusion injury in rats. J. Funct. Foods. 2018;46:227–236. doi: 10.1016/j.jff.2018.05.007. [DOI] [Google Scholar]
- 83.Sheu J.-R., Hsiao G., Chou P.-H., Shen M.-Y., Chou D.-S. Mechanisms involved in the antiplatelet activity of rutin, a glycoside of the flavonol quercetin, in human platelets. J. Agric. Food Chem. 2004;52:4414–4418. doi: 10.1021/jf040059f. [DOI] [PubMed] [Google Scholar]
- 84.Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017;96:305–312. doi: 10.1016/j.biopha.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Wang W., Wu Q.-h., Sui Y., Wang Y., Qiu X. Rutin protects endothelial dysfunction by disturbing Nox4 and ROS-sensitive NLRP3 inflammasome. Biomed. Pharmacother. 2017;86:32–40. doi: 10.1016/j.biopha.2016.11.134. [DOI] [PubMed] [Google Scholar]
- 86.Ugusman A., Zakaria Z., Chua K.H., Nordin N.A.M.M., Mahdy Z.A. Role of rutin on nitric oxide synthesis in human umbilical vein endothelial cells. Sci. World J. 2014;2014 doi: 10.1155/2014/169370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu J., Maoqiang L., Fan H., Zhenyu B., Qifang H., Xuepeng W., Liulong Z. Rutin attenuates neuroinflammation in spinal cord injury rats. J. Surg. Res. 2016;203:331–337. doi: 10.1016/j.jss.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 88.Enogieru A.B., Haylett W., Hiss D.C., Bardien S., Ekpo O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell Longev. 2018;2018 doi: 10.1155/2018/6241017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Budzynska B., Faggio C., Kruk-Slomka M., Samec D., Nabavi S.F., Sureda A., Devi K.P., Nabavi S.M. Rutin as neuroprotective agent: From bench to bedside. Curr. Med. Chem. 2019;26:5152–5164. doi: 10.2174/0929867324666171003114154. [DOI] [PubMed] [Google Scholar]
- 90.Radwan R.R., Fattah S.M.A. Mechanisms involved in the possible nephroprotective effect of rutin and low dose γ irradiation against cisplatin-induced nephropathy in rats. J. Photochem. Photobiol. B. 2017;169:56–62. doi: 10.1016/j.jphotobiol.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 91.Qu S., Dai C., Lang F., Hu L., Tang Q., Wang H., Zhang Y., Hao Z. Rutin attenuates vancomycin-induced nephrotoxicity by ameliorating oxidative stress, apoptosis, and inflammation in rats. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.01545-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caglayan C., Kandemir F.M., Yildirim S., Kucukler S., Eser G. Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J. Trace Elem. Med. Biol. 2019;54:69–78. doi: 10.1016/j.jtemb.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Liu Q., Pan R., Ding L., Zhang F., Hu L., Ding B., Zhu L., Xia Y., Dou X. Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. Int. Immunopharmacol. 2017;49:132–141. doi: 10.1016/j.intimp.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 94.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kravchenko J., Akushevich I., Seewaldt V.L., Abernethy A.P., Lyerly H.K. Breast cancer as heterogeneous disease: Contributing factors and carcinogenesis mechanisms. Breast Cancer Res. Treat. 2011;128:483–493. doi: 10.1007/s10549-011-1347-z. [DOI] [PubMed] [Google Scholar]
- 96.Sinha D., Biswas J., Nabavi S.M., Bishayee A. Tea phytochemicals for breast cancer prevention and intervention: From bench to bedside and beyond. Semin. Cancer Biol. 2017;46:33–54. doi: 10.1016/j.semcancer.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Telang N.T., Li G., Katdare M., Sepkovic D.W., Bradlow H.L., Wong G.Y. The nutritional herb Epimedium grandiflorum inhibits the growth in a model for the Luminal A molecular subtype of breast cancer. Oncol. Lett. 2017;13:2477–2482. doi: 10.3892/ol.2017.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Agelaki S., Dragolia M., Markonanolaki H., Alkahtani S., Stournaras C., Georgoulias V., Kallergi G. Phenotypic characterization of circulating tumor cells in triple negative breast cancer patients. Oncotarget. 2017;8:5309. doi: 10.18632/oncotarget.14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bianchini G., Balko J.M., Mayer I.A., Sanders M.E., Gianni L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016;13:674. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohyeldin M.M., Akl M.R., Ebrahim H.Y., Dragoi A.M., Dykes S., Cardelli J.A., Sayed K.A.E. The oleocanthal-based homovanillyl sinapate as a novel c-Met inhibitor. Oncotarget. 2016;7:32247. doi: 10.18632/oncotarget.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elsayed H.E., Ebrahim H.Y., Mohyeldin M.M., Siddique A.B., Kamal A.M., Haggag E.G., El Sayed K.A. Rutin as a novel c-Met inhibitory lead for the control of triple negative breast malignancies. Nutr. Cancer. 2017;69:1256–1271. doi: 10.1080/01635581.2017.1367936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hasani N.A.H., Amin I.M., Kamaludin R., Rosdyd N.M.M.N.M., Ibahim M.J., Kadir S.H.S.A. P53 and cyclin B1 mediate apoptotic effects of apigenin and rutin in ERα+-breast cancer MCF-7 cells. J. Teknol. 2018;80 doi: 10.11113/jt.v80.10704. [DOI] [Google Scholar]
- 103.Saleh A., ElFayoumi H.M., Youns M., Barakat W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn Schmiedeberg’s Arch. Pharmacol. 2019;392:165–175. doi: 10.1007/s00210-018-1579-0. [DOI] [PubMed] [Google Scholar]
- 104.Iriti M., Kubina R., Cochis A., Sorrentino R., Varoni E.M., Kabała-Dzik A., Azzimonti B., Dziedzic A., Rimondini L., Wojtyczka R.D. Rutin, a quercetin glycoside, restores chemosensitivity in human breast cancer cells. Phytother. Res. 2017;31:1529–1538. doi: 10.1002/ptr.5878. [DOI] [PubMed] [Google Scholar]
- 105.Mohana S., Ganesan M., Rajendra Prasad N., Ananthakrishnan D., Velmurugan D. Flavonoids modulate multidrug resistance through wnt signaling in P-glycoprotein overexpressing cell lines. BMC Cancer. 2018;18:1168. doi: 10.1186/s12885-018-5103-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Schindler R., Mentlein R. Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J. Nutr. 2006;136:1477–1482. doi: 10.1093/jn/136.6.1477. [DOI] [PubMed] [Google Scholar]
- 107.Yang S., Zhang H., Yang X., Zhao H., Zhu Y. Evaluation of antiproliferative activities of rutin on human colon cancer lovo cells and breast cancer MCF-7 cells. Anal. Quant. Cytopathol. Histopathol. 2017;39:99–107. [Google Scholar]
- 108.Zhu H., Wang Y., Liu D., Sun X., Wang F. Vanadium-rutin complex sensitizes breast cancer cells via modulation of p53/BAx/BCl2/VEGF correlated with apoptotic events. Acta Pol. Pharm. Drug Res. 2020;77:89–98. doi: 10.32383/appdr/115158. [DOI] [Google Scholar]
- 109.Kunjiappan S., Theivendran P., Baskararaj S., Sankaranarayanan B., Palanisamy P., Saravanan G., Arunachalam S., Sankaranarayanan M., Natarajan J., Somasundaram B. Modeling a pH-sensitive Zein-co-acrylic acid hybrid hydrogels loaded 5-fluorouracil and rutin for enhanced anticancer efficacy by oral delivery. Biotechnology. 2019;9:185. doi: 10.1007/s13205-019-1720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu F., Chen J., Fan L.M., Liu K., Zhang N., Li S.W., Zhu H., Gao H.C. Analysis of the effect of rutin on GSK-3β and TNF-α expression in lung cancer. Exp. Ther. Med. 2017;14:127–130. doi: 10.3892/etm.2017.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yeh S.L., Wang W.Y., Huang C.S., Hu M.L. Flavonoids suppresses the enhancing effect of beta-carotene on DNA damage induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A549 cells. Chem. Biol. Interact. 2006;160:175–182. doi: 10.1016/j.cbi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 112.Menon L.G., Kuttan R., Kuttan G. Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer lett. 1995;95:221–225. doi: 10.1016/0304-3835(95)03887-3. [DOI] [PubMed] [Google Scholar]
- 113.Conesa C.M., Ortega V.V., Yáñez Gascón M.J., Baños M.A., Jordana M.C., Benavente-García O., Castillo J. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J. Agric. Food Chem. 2005;53:6791–6797. doi: 10.1021/jf058050g. [DOI] [PubMed] [Google Scholar]
- 114.Park M.H., Kim S., Song Y.-R., Kim S., Kim H.-J., Na H.S., Chung J. Rutin induces autophagy in cancer cells. Int. J. Oral Biol. 2016;41:45–51. doi: 10.11620/IJOB.2016.41.1.045. [DOI] [Google Scholar]
- 115.Li X.-H., Liu Z.-Y., Gu Y., Lv Z., Chen Y., Gao H.-C. Expression of NF-kappaB and p38 under intervention of rutin in lung cancer therapy. Biomed Res. 2017;14:2344–2347. [Google Scholar]
- 116.Guon T.E., Chung H.S. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol. Lett. 2016;11:2463–2470. doi: 10.3892/ol.2016.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alonso-Castro A.J., Domínguez F., García-Carrancá A. Rutin exerts antitumor effects on nude mice bearing SW480 tumor. Arch. Med. Res. 2013;44:346–351. doi: 10.1016/j.arcmed.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 118.Vijay M., Sivagami G., Thayalan K., Nalini N. Radiosensitizing potential of rutin against human colon adenocarcinoma HT-29 cells. Bratisl. Lek. Listy. 2016;117:171–178. doi: 10.4149/BLL_2016_033. [DOI] [PubMed] [Google Scholar]
- 119.Nafees S., Mehdi S.H., Zafaryab M., Zeya B., Sarwar T., Rizvi M.A. Synergistic interaction of rutin and silibinin on human colon cancer cell line. Arch. Med. Res. 2018;49:226–234. doi: 10.1016/j.arcmed.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 120.Nasrabadi P.N., Zareian S., Nayeri Z., Salmanipour R., Parsafar S., Gharib E., Aghdaei H.A., Zali M.R. A detailed image of rutin underlying intracellular signaling pathways in human SW480 colorectal cancer cells based on miRNAs-lncRNAs-mRNAs-TFs interactions. J. Cell Physiol. 2019;234:15570–15580. doi: 10.1002/jcp.28204. [DOI] [PubMed] [Google Scholar]
- 121.Jantrawut P., Akazawa H., Ruksiriwanich W. Anti-cancer activity of rutin encapsulated in low methoxyl pectin beads. Int. J. Pharmcy Pharm. Sci. 2014;6:199–202. [Google Scholar]
- 122.Aherne S.A., O’Brien N.M. Protection by the flavonoids myricetin, quercetin, and rutin against hydrogen peroxide-induced DNA damage in Caco-2 and Hep G2 cells. Nutr. Cancer. 1999;34:160–166. doi: 10.1207/S15327914NC3402_6. [DOI] [PubMed] [Google Scholar]
- 123.Aherne S.A., O’Brien N.M. Lack of effect of the flavonoids, myricetin, quercetin, and rutin, on repair of H2O2-induced DNA single-strand breaks in Caco-2, Hep G2, and V79 cells. Nutr. Cancer. 2000;38:106–115. doi: 10.1207/S15327914NC381_15. [DOI] [PubMed] [Google Scholar]
- 124.Deschner E.E., Ruperto J., Wong G., Newmark H.L. Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis. 1991;12:1193–1196. doi: 10.1093/carcin/12.7.1193. [DOI] [PubMed] [Google Scholar]
- 125.Volate S.R., Davenport D.M., Muga S.J., Wargovich M.J. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin) Carcinogenesis. 2005;26:1450–1456. doi: 10.1093/carcin/bgi089. [DOI] [PubMed] [Google Scholar]
- 126.Dihal A.A., de Boer V.C., van der Woude H., Tilburgs C., Bruijntjes J.P., Alink G.M., Rietjens I.M., Woutersen R.A., Stierum R.H. Quercetin, but not its glycosidated conjugate rutin, inhibits azoxymethane-induced colorectal carcinogenesis in F344 rats. J. Nutr. 2006;136:2862–2867. doi: 10.1093/jn/136.11.2862. [DOI] [PubMed] [Google Scholar]
- 127.Volková M., Forstová-Křížová V., Skálová L., Trejtnar F. Modulatory effects of quercetin and rutin on the activity, expression and inducibility of CYP1A1 in intestinal HCT-8 cells. Phytother. Res. 2013;27:1889–1893. doi: 10.1002/ptr.4992. [DOI] [PubMed] [Google Scholar]
- 128.Wijnands M.V.W., Van Erk M.J., Doornbos R.P., Krul C.A.M., Woutersen R.A. Do aberrant crypt foci have predictive value for the occurrence of colorectal tumours? Potential of gene expression profiling in tumours. Food Chem. Toxicol. 2004;42:1629–1639. doi: 10.1016/j.fct.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 129.Santos B., Silva A., Pitanga B., Sousa C., Grangeiro M., Fragomeni B., Coelho P., Oliveira M., Menezes-Filho N., Costa M.F.D., et al. Antiproliferative, proapoptotic and morphogenic effects of the flavonoid rutin on human glioblastoma cells. Food Chem. 2011;127:404–411. doi: 10.1016/j.foodchem.2010.12.131. [DOI] [PubMed] [Google Scholar]
- 130.Freitas S., Costa S., Azevedo C., Carvalho G., Freire S., Barbosa P., Velozo E., Schaer R., Tardy M., Meyer R., et al. Flavonoids inhibit angiogenic cytokine production by human glioma cells. Phytother. Res. 2011;25:916–921. doi: 10.1002/ptr.3338. [DOI] [PubMed] [Google Scholar]
- 131.Santos B.L., Oliveira M.N., Coelho P.L.C., Pitanga B.P.S., da Silva A.B., Adelita T., Silva V.D.A., Costa M.F.D., El-Bachá R.S., Tardy M., et al. Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression. Chem. Biol. Interact. 2015;242:123–138. doi: 10.1016/j.cbi.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 132.Chen H., Miao Q., Geng M., Liu J., Hu Y., Tian L., Pan J., Yang Y. Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Sci. World J. 2013;2013 doi: 10.1155/2013/269165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yan X., Hao Y., Chen S., Jia G., Guo Y., Zhang G., Wang C., Cheng R., Hu T., Zhang X. Rutin induces apoptosis via P53 up-regulation in human glioma CHME cells. Transl. Cancer Res. 2019;8:2005–2013. doi: 10.21037/tcr.2019.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang P., Sun S., Li N., Ho A.S.W., Kiang K.M.Y., Zhang X., Cheng Y.S., Poon M.W., Lee D., Pu J.K.S. Rutin increases the cytotoxicity of temozolomide in glioblastoma via autophagy inhibition. J. Neurooncol. 2017;132:393–400. doi: 10.1007/s11060-017-2387-y. [DOI] [PubMed] [Google Scholar]
- 135.Bourogaa E., Bertrand J., Despeaux M., Jarraya R., Fabre N., Payrastre L., Demur C., Fournié J.-J., Damak M., Feki A.E. Hammada scoparia flavonoids and rutin kill adherent and chemoresistant leukemic cells. Leuk. Res. 2011;35:1093–1101. doi: 10.1016/j.leukres.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 136.Lin J.P., Yang J.S., Lin J.J., Lai K.C., Lu H.F., Ma C.Y., Sai-Chuen Wu R., Wu K.C., Chueh F.S., Wood W.G. Rutin inhibits human leukemia tumor growth in a murine xenograft model in vivo. Environ. Toxicol. 2012;27:480–484. doi: 10.1002/tox.20662. [DOI] [PubMed] [Google Scholar]
- 137.Lin J.-P., Yang J.-S., Lu C.-C., Chiang J.-H., Wu C.-L., Lin J.-J., Lin H.-L., Yang M.-D., Liu K.-C., Chiu T.-H. Rutin inhibits the proliferation of murine leukemia WEHI-3 cells in vivo and promotes immune response in vivo. Leuk. Res. 2009;33:823–828. doi: 10.1016/j.leukres.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 138.Chuang C.-H., Huang C.-S., Hu M.-L. Vitamin E and rutin synergistically inhibit expression of vascular endothelial growth factor through down-regulation of binding activity of activator protein-1 in human promyelocytic leukemia (HL-60) cells. Chem. Biol. Interact. 2010;183:434–441. doi: 10.1016/j.cbi.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 139.Ikeda N.E.A., Novak E.M., Maria D.A., Velosa A.S., Pereira R.M.S. Synthesis, characterization and biological evaluation of Rutin–zinc (II) flavonoid-metal complex. Chem. Biol. Interact. 2015;239:184–191. doi: 10.1016/j.cbi.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 140.Horváthová K., Novotný L., Tóthová D., Vachálková A. Determination of free radical scavenging activity of quercetin, rutin, luteolin and apigenin in H2O2-treated human ML cells K562. Neoplasma. 2004;51:395–399. [PubMed] [Google Scholar]
- 141.Dedoussis G.V.Z., Kaliora A.C., Andrikopoulos N.K. Effect of phenols on natural killer (NK) cell-mediated death in the K562 human leukemic cell line. Cell Biol. Int. 2005;29:884–889. doi: 10.1016/j.cellbi.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 142.Shen S.C., Chen Y.C., Hsu F.L., Lee W.R. Differential apoptosis-inducing effect of quercetin and its glycosides in human promyeloleukemic HL-60 cells by alternative activation of the caspase 3 cascade. J. Cell Biochem. 2003;89:1044–1055. doi: 10.1002/jcb.10559. [DOI] [PubMed] [Google Scholar]
- 143.Nadova S., Miadokova E., Cipak L. Flavonoids potentiate the efficacy of cytarabine through modulation of drug-induced apoptosis. Neoplasma. 2007;54:202–206. [PubMed] [Google Scholar]
- 144.Canturk Z., Dikmen M., Artagan O., Ozarda M.G., Ozturk N. Cytotoxic effects of resveratrol, rutin and rosmarinic acid on ARH–77 human (multiple myeloma) cell line. Nat. Prod. Commun. 2016;11:1441–1444. doi: 10.1177/1934578X1601101007. [DOI] [PubMed] [Google Scholar]
- 145.Prasad R., Banerjee S., Kharshiing C., Bhattacharjee A., Prasad S. Rutin-mediated apoptosis and glutathione changes in ascites daltons lymphoma cells: In silico analysis of rutin interactions with some antiapoptotic and glutathione-related proteins. Indian J. Pharm. Sci. 2019;81:720–728. doi: 10.36468/pharmaceutical-sciences.563. [DOI] [Google Scholar]
- 146.Marcarini J.C., Tsuboy M.S.F., Luiz R.C., Ribeiro L.R., Hoffmann-Campo C.B., Mantovani M.S. Investigation of cytotoxic, apoptosis-inducing, genotoxic and protective effects of the flavonoid rutin in HTC hepatic cells. Exp. Toxicol. Pathol. 2011;63:459–465. doi: 10.1016/j.etp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 147.Karakurt S. Modulatory effects of rutin on the expression of cytochrome P450s and antioxidant enzymes in human hepatoma cells. Acta Pharm. 2016;66:491–502. doi: 10.1515/acph-2016-0046. [DOI] [PubMed] [Google Scholar]
- 148.Alía M., Mateos R., Ramos S., Lecumberri E., Bravo L., Goya L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2) Eur. J. Nutr. 2006;45:19–28. doi: 10.1007/s00394-005-0558-7. [DOI] [PubMed] [Google Scholar]
- 149.Kim G.N., Jang H.D. Protective mechanism of quercetin and rutin using glutathione metabolism on HO-induced oxidative stress in HepG2 cells. Ann. N. Y. Acad. Sci. 2009;1171:530–537. doi: 10.1111/j.1749-6632.2009.04690.x. [DOI] [PubMed] [Google Scholar]
- 150.Labh A.K., Priya V.V., Gayathri R. Cytotoxic action of rutin isolated from Morinda citrifolia against hepatic carcinoma cell lines. Drug Invent. Today. 2019;12:1904–1907. [Google Scholar]
- 151.Chandra Y.P., Viswanathswamy A. Chemopreventive effect of Rutin against N-nitrosodiethylamine-induced and phenobarbital-promoted hepatocellular carcinoma in Wistar rats. Indian J. Pharm. Educ. Res. 2018;52:78–86. doi: 10.5530/ijper.52.1.9. [DOI] [Google Scholar]
- 152.Webster R., Gawde M., Bhattacharya R. Protective effect of rutin, a flavonol glycoside, on the carcinogen-induced DNA damage and repair enzymes in rats. Cancer Lett. 1996;109:185–191. doi: 10.1016/S0304-3835(96)04443-6. [DOI] [PubMed] [Google Scholar]
- 153.Li Q., Ren L., Zhang Y., Gu Z., Tan Q., Zhang T., Qin M., Chen S. P38 signal transduction pathway has more cofactors on apoptosis of SGC-7901 gastric cancer cells induced by combination of rutin and oxaliplatin. Biomed. Res. Int. 2019;2019 doi: 10.1155/2019/6407210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.George K., Malathi R. Influence of the novel anticancer agents on the activity of outward rectifier potassium currents in human prostate cancer cell line-LNCaP. Asian J. Pharm. 2017;11:S603–S608. [Google Scholar]
- 155.Roy A.S., Tripathy D.R., Samanta S., Ghosh S.K., Dasgupta S. DNA damaging, cell cytotoxicity and serum albumin binding efficacy of the rutin-Cu(ii) complex. Mol. Biosyst. 2016;12:1687–1701. doi: 10.1039/C6MB00161K. [DOI] [PubMed] [Google Scholar]
- 156.Luo H., Jiang B.H., King S.M., Chen Y.C. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr. Cancer. 2008;60:800–809. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- 157.Guruvayoorappan C., Kuttan G. Antiangiogenic effect of rutin and its regulatory effect on the production of VEGF, IL-1β and TNF-α in tumor associated macrophages. J. Biol. Sci. 2007;7:1511–1519. doi: 10.3923/jbs.2007.1511.1519. [DOI] [Google Scholar]
- 158.Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb. Perspect. Med. 2016;6:a026104. doi: 10.1101/cshperspect.a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu B., Li M., Yu Y., He J., Hu S., Pan M., Lu S., Liao K., Pan Z., Zhou Y. Effects of harmaline on cell growth of human liver cancer through the p53/p21 and Fas/FasL signaling pathways. Oncol. Lett. 2018;15:1931–1936. doi: 10.3892/ol.2017.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Keay S., Nallar S.C., Gade P., Zhang C.-O., Kalvakolanu D.V. Oncosuppressor protein p53 and cyclin-dependent kinase inhibitor p21 regulate interstitial cystitis associated gene expression. Cytokine. 2018;110:110–115. doi: 10.1016/j.cyto.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 161.Munir M.T., Ponce C., Powell C.A., Tarafdar K., Yanagita T., Choudhury M., Gollahon L.S., Rahman S.M. The contribution of cholesterol and epigenetic changes to the pathophysiology of breast cancer. J. Steroid Biochem. Mol. Biol. 2018;183:1–9. doi: 10.1016/j.jsbmb.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 162.Garcia-Estevez L., Moreno-Bueno G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 2019;21:35. doi: 10.1186/s13058-019-1124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xiao F., Wang C., Yin H., Yu J., Chen S., Fang J., Guo F. Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget. 2016;7:63679. doi: 10.18632/oncotarget.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang J., Seebacher N., Shi H., Kan Q., Duan Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget. 2017;8:84559. doi: 10.18632/oncotarget.19187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Saraswathy M., Gong S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013;31:1397–1407. doi: 10.1016/j.biotechadv.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 166.Whitlock B.D., Leslie E.M. Drug Efflux Pumps in Cancer Resistance Pathways: From Molecular Recognition and Characterization to Possible Inhibition Strategies in Chemotherapy. Elsevier; Amsterdam, The Netherlands: 2020. Efflux transporters in anti-cancer drug resistance: Molecular and functional identification and characterization of multidrug resistance proteins (MRPs/ABCCs) pp. 31–65. [Google Scholar]
- 167.Das M., Law S. Role of tumor microenvironment in cancer stem cell chemoresistance and recurrence. Int. J. Biochem. Cell Biol. 2018;103:115–124. doi: 10.1016/j.biocel.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 168.Frascini F., Iriti M., Maestri P., Rimondini L., Catalano E., Megna S. Compositions Comprising Rutin Useful for the Treatment of Tumors Resistant to Chemotherapy. CN Patent Application No. CN105611945A. 2016 May 25;
- 169.de Oliveira C.T., Colenci R., Pacheco C.C., Mariano P.M., do Prado P.R., Mamprin G.P.R., Santana M.G., Gambero A., de Carvalho P.O., Priolli D.G. Hydrolyzed rutin decreases worsening of anaplasia in glioblastoma relapse. CNS Neurol. Disord. Drug Targets. 2019;18:405–412. doi: 10.2174/1871527318666190314103104. [DOI] [PubMed] [Google Scholar]
- 170.Moghanjoughi A.A., Khoshnevis D., Zarrabi A. A concise review on smart polymers for controlled drug release. Drug Deliv. Transl. Res. 2016;6:333–340. doi: 10.1007/s13346-015-0274-7. [DOI] [PubMed] [Google Scholar]
- 171.Gyles D.A., Castro L.D., Silva Jr J.O.C., Ribeiro-Costa R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017;88:373–392. doi: 10.1016/j.eurpolymj.2017.01.027. [DOI] [Google Scholar]
- 172.Caló E., Khutoryanskiy V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015;65:252–267. doi: 10.1016/j.eurpolymj.2014.11.024. [DOI] [Google Scholar]
- 173.Judd J., Borghaei H. Combining immunotherapy and chemotherapy for non–small cell lung cancer. Thorac. Surg. Clin. 2020;30:199–206. doi: 10.1016/j.thorsurg.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 174.Lee K.Y., Shueng P.W., Chou C.M., Lin B.X., Lin M.H., Kuo D.Y., Tsai I.L., Wu S.M., Lin C.W. Elevation of CD109 promotes metastasis and drug resistance in lung cancer via activation of EGFR-AKT-mTOR signaling. Cancer Sci. 2020;111:1652. doi: 10.1111/cas.14373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gao Y., Liu Z., Zhang X., He J., Pan Y., Hao F., Xie L., Li Q., Qiu X., Wang E. Inhibition of cytoplasmic GSK-3β increases cisplatin resistance through activation of Wnt/β-catenin signaling in A549/DDP cells. Cancer Lett. 2013;336:231–239. doi: 10.1016/j.canlet.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 176.Filla M.S., Dimeo K.D., Tong T., Peters D.M. Disruption of fibronectin matrix affects type IV collagen, fibrillin and laminin deposition into extracellular matrix of human trabecular meshwork (HTM) cells. Exp. Eye Res. 2017;165:7–19. doi: 10.1016/j.exer.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee J.K., Kim N.-J. Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules. 2017;22:1287. doi: 10.3390/molecules22081287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stramucci L., Pranteda A., Bossi G. Insights of crosstalk between p53 protein and the MKK3/MKK6/p38 MAPK signaling pathway in cancer. Cancers. 2018;10:131. doi: 10.3390/cancers10050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bradham C., McClay D.R. p38 MAPK in development and cancer. Cell Cycle. 2006;5:824–828. doi: 10.4161/cc.5.8.2685. [DOI] [PubMed] [Google Scholar]
- 180.Johnson C.M., Wei C., Ensor J.E., Smolenski D.J., Amos C.I., Levin B., Berry D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24:1207–1222. doi: 10.1007/s10552-013-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhou L., Wang S., Cao L., Ren X., Li Y., Shao J., Xu L. Lead acetate induces apoptosis in Leydig cells by activating PPARγ/caspase-3/PARP pathway. Int. J. Environ. Health Res. 2019:1–11. doi: 10.1080/09603123.2019.1625034. [DOI] [PubMed] [Google Scholar]
- 182.Qi X., Du L., Chen X., Chen L., Yi T., Chen X., Wen Y., Wei Y., Zhao X. VEGF-D-enhanced lymph node metastasis of ovarian cancer is reversed by vesicular stomatitis virus matrix protein. Int. J. Oncol. 2016;49:123–132. doi: 10.3892/ijo.2016.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Todoric J., Antonucci L., Karin M. Targeting inflammation in cancer prevention and therapy. Cancer Prev. Res. 2016;9:895–905. doi: 10.1158/1940-6207.CAPR-16-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jiangjiang Q., Wei W., Zhang R. Novel natural product therapeutics targeting both inflammation and cancer. Chin. J. Nat. Med. 2017;15:401–416. doi: 10.1016/S1875-5364(17)30062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fakhri S., Moradi S.Z., Farzaei M.H., Bishayee A. Modulation of dysregulated cancer metabolism by plant secondary metabolites: A mechanistic review. Semin. Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.02.007. in press. [DOI] [PubMed] [Google Scholar]
- 186.Su S., Wang J., Vargas E., Wei J., Martínez-Zaguilán R., Sennoune S.R., Pantoya M.L., Wang S., Chaudhuri J., Qiu J. Porphyrin immobilized nanographene oxide for enhanced and targeted photothermal therapy of brain cancer. ACS Biomater. Sci. Eng. 2016;2:1357–1366. doi: 10.1021/acsbiomaterials.6b00290. [DOI] [PubMed] [Google Scholar]
- 187.Lewinska A., Adamczyk-Grochala J., Kwasniewicz E., Deregowska A., Wnuk M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017;265:117–130. doi: 10.1016/j.toxlet.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 188.Cagnol S., Chambard J.C. ERK and cell death: Mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 189.Sivaprasad U., Basu A. Inhibition of ERK attenuates autophagy and potentiates tumour necrosis factor-α-induced cell death in MCF-7 cells. J. Cell Mol. Med. 2008;12:1265–1271. doi: 10.1111/j.1582-4934.2008.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zeid R., Lawlor M.A., Poon E., Reyes J.M., Fulciniti M., Lopez M.A., Scott T.G., Nabet B., Erb M.A., Winter G.E. Enhancer invasion shapes MYCN-dependent transcriptional amplification in neuroblastoma. Nat. Genet. 2018;50:515–523. doi: 10.1038/s41588-018-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Moosavi M.A., Haghi A., Rahmati M., Taniguchi H., Mocan A., Echeverría J., Gupta V.K., Tzvetkov N.T., Atanasov A.G. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett. 2018;424:46–69. doi: 10.1016/j.canlet.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 192.Fulda S. Autophagy in cancer therapy. Front. Oncol. 2017;7:128. doi: 10.3389/fonc.2017.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vasilevskaya I.A., Selvakumaran M., Roberts D., O’Dwyer P.J. JNK1 inhibition attenuates hypoxia-induced autophagy and sensitizes to chemotherapy. Mol. Cancer Res. 2016;14:753–763. doi: 10.1158/1541-7786.MCR-16-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Saultz J.N., Garzon R. Acute myeloid leukemia: A concise review. J. Clin. Med. 2016;5:33. doi: 10.3390/jcm5030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Reddiconto G., Toto C., Palamà I., De Leo S., De Luca E., De Matteis S., Dini L., Passerini C.G., Di Renzo N., Maffia M. Targeting of GSK3β promotes imatinib-mediated apoptosis in quiescent CD34+ chronic myeloid leukemia progenitors, preserving normal stem cells. Blood. 2012;119:2335–2345. doi: 10.1182/blood-2011-06-361261. [DOI] [PubMed] [Google Scholar]
- 196.Wang Z., Jin C., Li X., Ding K. Sulfated polysaccharide JCS1S2 inhibits angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Carbohydr. Polym. 2019;207:502–509. doi: 10.1016/j.carbpol.2018.11.091. [DOI] [PubMed] [Google Scholar]
- 197.Eferl R., Wagner E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 198.Cheng J., Yang H.L., Gu C.J., Liu Y.K., Shao J., Zhu R., He Y.Y., Zhu X.Y., Li M.Q. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 2019;43:945–955. doi: 10.3892/ijmm.2018.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lai J., Chen F., Chen J., Ruan G., He M., Chen C., Tang J., Wang D.W. Overexpression of decorin promoted angiogenesis in diabetic cardiomyopathy via IGF1R-AKT-VEGF signaling. Sci. Rep. 2017;7:1–11. doi: 10.1038/srep44473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Affo S., Yu L.-X., Schwabe R.F. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev. Pathol. 2017;12:153–186. doi: 10.1146/annurev-pathol-052016-100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Abbasi B.H., Nazir M., Muhammad W., Hashmi S.S., Abbasi R., Rahman L., Hano C. A comparative evaluation of the antiproliferative activity against Hepg2 liver carcinoma cells of plant-derived silver nanoparticles from basil extracts with contrasting anthocyanin contents. Biomolecules. 2019;9:320. doi: 10.3390/biom9080320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang Q.-L., Wu Q., Tao Y.-Y., Liu C.-H., El-Nezami H. Salvianolic acid B modulates the expression of drug-metabolizing enzymes in HepG2 cells. Hepatobiliary Pancreat. Dis. Int. 2011;10:502–508. doi: 10.1016/S1499-3872(11)60085-4. [DOI] [PubMed] [Google Scholar]
- 203.Aloud A.A., Veeramani C., Govindasamy C., Alsaif M.A., Al-Numair K.S. Galangin ameliorates changes of membrane–bound enzymes in rats with streptozotocin–induced hyperglycemia. Asian Pac. J. Trop. Biomed. 2019;9:284. [Google Scholar]
- 204.Rajamanickam E., Gurudeeban S., Satyavani K., Ramanathan T. Chemopreventive effect of Acanthus ilicifolius extract on modulating antioxidants, lipid peroxidation and membrane bound enzymes in diethyl nitrosamine induced liver carcinogenesis. Int. J. Cancer Res. 2016;12:1–16. [Google Scholar]
- 205.Nriagu J., Darroudi F., Shomar B. Health effects of desalinated water: Role of electrolyte disturbance in cancer development. Environ. Res. 2016;150:191–204. doi: 10.1016/j.envres.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 206.Wang Y., Chen X., Sun Q., Zang W., Li M., Dong Z., Zhao G. Retraction note: Overexpression of A613T and G462T variants of DNA polymerase β weakens chemotherapy sensitivity in esophageal cancer cell lines. Cancer Cell Int. 2019;19:19. doi: 10.1186/s12935-019-0814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sallmyr A., Matsumoto Y., Roginskaya V., Van Houten B., Tomkinson A.E. Inhibiting mitochondrial DNA ligase IIIα activates caspase 1–Dependent apoptosis in cancer cells. Cancer Res. 2016;76:5431–5441. doi: 10.1158/0008-5472.CAN-15-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lin K.Y., Kraus W.L. PARP inhibitors for cancer therapy. Cell. 2017;169:183. doi: 10.1016/j.cell.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 209.Haghi A., Azimi H., Rahimi R. A comprehensive review on pharmacotherapeutics of three phytochemicals, curcumin, quercetin, and allicin, in the treatment of gastric cancer. J. Gastrointest. Cancer. 2017;48:314–320. doi: 10.1007/s12029-017-9997-7. [DOI] [PubMed] [Google Scholar]
- 210.Berlth F., Bollschweiler E., Drebber U., Hoelscher A.H., Moenig S. Pathohistological classification systems in gastric cancer: Diagnostic relevance and prognostic value. World J. Gastroenterol. 2014;20:5679. doi: 10.3748/wjg.v20.i19.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sitarz R., Skierucha M., Mielko J., Offerhaus G.J.A., Maciejewski R., Polkowski W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018;10:239. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ren L.-Q., Li Q., Zhang Y. Luteolin suppresses the proliferation of gastric cancer cells and acts in synergy with oxaliplatin. Biomed. Res. Int. 2020;2020 doi: 10.1155/2020/9396512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zou X., Blank M. Targeting p38 MAP kinase signaling in cancer through post-translational modifications. Cancer Lett. 2017;384:19–26. doi: 10.1016/j.canlet.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 214.Martínez-Limón A., Joaquin M., Caballero M., Posas F., de Nadal E. The p38 pathway: From biology to cancer therapy. Int. J. Mol. Sci. 2020;21:1913. doi: 10.3390/ijms21061913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kawan M.A., Kyrou I., Ramanjaneya M., Williams K., Jeyaneethi J., Randeva H.S., Karteris E. Involvement of the glutamine RF-amide peptide and its cognate receptor GPR103 in prostate cancer. Oncol. Rep. 2019;41:1140–1150. doi: 10.3892/or.2018.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Abidi S.H., Bilwani F., Ghias K., Abbas F. Viral etiology of prostate cancer: Genetic alterations and immune response. A literature review. Int. J. Surg. 2018;52:136–140. doi: 10.1016/j.ijsu.2018.02.050. [DOI] [PubMed] [Google Scholar]
- 217.de Júnior R.G.O., Adrielly A.F.C., da Almeida J.R.G.S., Grougnet R., Thiéry V., Picot L. Sensitization of tumor cells to chemotherapy by natural products: A systematic review of preclinical data and molecular mechanisms. Fitoterapia. 2018;129:383–400. doi: 10.1016/j.fitote.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 218.Abdul M., Hoosein N. Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett. 2002;186:99–105. doi: 10.1016/S0304-3835(02)00348-8. [DOI] [PubMed] [Google Scholar]
- 219.Flahaut M., Meier R., Coulon A., Nardou K., Niggli F., Martinet D., Beckmann J., Joseph J., Mühlethaler-Mottet A., Gross N. The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/β-catenin pathway. Oncogene. 2009;28:2245–2256. doi: 10.1038/onc.2009.80. [DOI] [PubMed] [Google Scholar]
- 220.Lheureux S., Braunstein M., Oza A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019;69:280–304. doi: 10.3322/caac.21559. [DOI] [PubMed] [Google Scholar]
- 221.Pashaei-Asl F., Pashaei-Asl R., Khodadadi K., Akbarzadeh A., Ebrahimie E., Pashaiasl M. Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artif. Cells Nanomed. Biotechnol. 2018;46:1483–1487. doi: 10.1080/21691401.2017.1374281. [DOI] [PubMed] [Google Scholar]
- 222.Miyake K., Arima H., Hirayama F., Yamamoto M., Horikawa T., Sumiyoshi H., Noda S., Uekama K. Improvement of solubility and oral bioavailability of rutin by complexation with 2-hydroxypropyl-β-cyclodextrin. Pharm. Dev. Technol. 2000;5:399–407. doi: 10.1081/PDT-100100556. [DOI] [PubMed] [Google Scholar]
- 223.Arora D., Jaglan S. Nanocarriers based delivery of nutraceuticals for cancer prevention and treatment: A review of recent research developments. Trends Food Sci Technol. 2016;54:114–126. doi: 10.1016/j.tifs.2016.06.003. [DOI] [Google Scholar]
- 224.Kashyap D., Tuli H.S., Yerer M.B., Sharma A., Sak K., Srivastava S., Pandey A., Garg V.K., Sethi G., Bishayee A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2019 doi: 10.1016/j.semcancer.2019.08.014. in press. [DOI] [PubMed] [Google Scholar]
- 225.Lagoa R., Silva J., Rodrigues J.R., Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020;38:107382. doi: 10.1016/j.biotechadv.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 226.McClements D.J. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnol. Adv. 2020;38:107287. doi: 10.1016/j.biotechadv.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 227.Nel A., Ruoslahti E., Meng H. New insights into “permeability” as in the enhanced permeability and retention effect of cancer nanotherapeutics. ACS Nano. 2017 doi: 10.1021/acsnano.7b07214. [DOI] [PubMed] [Google Scholar]
- 228.Kalyane D., Raval N., Maheshwari R., Tambe V., Kalia K., Tekade R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C. 2019;98:1252–1276. doi: 10.1016/j.msec.2019.01.066. [DOI] [PubMed] [Google Scholar]
- 229.Muhamad N., Plengsuriyakarn T., Na-Bangchang K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018;13:3921. doi: 10.2147/IJN.S165210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kunjiappan S., Panneerselvam T., Govindaraj S., Parasuraman P., Baskararaj S., Sankaranarayanan M., Arunachalam S., Babkiewicz E., Jeyakumar A., Lakshmanan M. Design, in silico modelling, and functionality theory of novel folate receptor targeted rutin encapsulated folic acid conjugated keratin nanoparticles for effective cancer treatment. Anticancer Agents Med. Chem. 2019;19:1966–1982. doi: 10.2174/1871520619666190702145609. [DOI] [PubMed] [Google Scholar]
- 231.Ge L., He X., Zhang Y., Zhang Y., Chai F., Jiang L., Webster T.J., Zheng C. A dabigatran etexilate phospholipid complex nanoemulsion system for further oral bioavailability by reducing drug-leakage in the gastrointestinal tract. Nanomedicine. 2018;14:1455–1464. doi: 10.1016/j.nano.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 232.Khan W., Ansari V.A., Hussain Z., Siddique N.F. Nanoemulsion: A Droplet Nanocarrier System for Enhancing Bioavailability of Poorly Water Soluble Drugs. Res. J. Pharm. Technol. 2018;11:5191–5196. doi: 10.5958/0974-360X.2018.00948.4. [DOI] [Google Scholar]
- 233.Ahmad M., Sahabjada J.A., Hussain A., Badaruddeen M.A., Mishra A. Development of a new rutin nanoemulsion and its application on prostate carcinoma PC3 cell line. Excli. J. 2017;16:810. doi: 10.17179/excli2016-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Caparica R., Júlio A., Araújo M.E.M., Baby A.R., Fonte P., Costa J.G., Santos de Almeida T. Anticancer Activity of Rutin and Its Combination with Ionic Liquids on Renal Cells. Biomolecules. 2020;10:233. doi: 10.3390/biom10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Asfour M.H., Mohsen A.M. Formulation and evaluation of pH-sensitive rutin nanospheres against colon carcinoma using HCT-116 cell line. J. Adv. Res. 2018;9:17–26. doi: 10.1016/j.jare.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kunjiappan S., Panneerselvam T., Somasundaram B., Sankaranarayanan M., Chowdhury R., Chowdhury A., Bhattacharjee C. Design, in silico modeling, biodistribution study of rutin and quercetin loaded stable human hair keratin nanoparticles intended for anticancer drug delivery. Biomed. Phys. Eng. Express. 2018;4:025019. doi: 10.1088/2057-1976/aaa1cf. [DOI] [Google Scholar]
- 237.Pandey P., Rahman M., Bhatt P.C., Beg S., Paul B., Hafeez A., Al-Abbasi F.A., Nadeem M.S., Baothman O., Anwar F. Implication of nano-antioxidant therapy for treatment of hepatocellular carcinoma using PLGA nanoparticles of rutin. Nanomedicine. 2018;13:849–870. doi: 10.2217/nnm-2017-0306. [DOI] [PubMed] [Google Scholar]
- 238.Deepika M.S., Thangam R., Sheena T.S., Vimala R., Sivasubramanian S., Jeganathan K., Thirumurugan R. Dual drug loaded PLGA nanospheres for synergistic efficacy in breast cancer therapy. Mater. Sci. Eng. C. 2019;103:109716. doi: 10.1016/j.msec.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 239.Chang C., Zhang L., Miao Y., Fang B., Yang Z. Anticancer and apoptotic-inducing effects of rutin-chitosan nanoconjugates in triple negative breast cancer cells. J. Clust. Sci. 2020:1–10. doi: 10.1007/s10876-020-01792-w. [DOI] [Google Scholar]
- 240.Bharathi D., Bhuvaneshwari V. Synthesis of zinc oxide nanoparticles (ZnO NPs) using pure bioflavonoid rutin and their biomedical applications: Antibacterial, antioxidant and cytotoxic activities. Res. Chem. Intermediat. 2019;45:2065–2078. doi: 10.1007/s11164-018-03717-9. [DOI] [Google Scholar]
- 241.Bharathi D., Ranjithkumar R., Chandarshekar B., Bhuvaneshwari V. Bio-inspired synthesis of chitosan/copper oxide nanocomposite using rutin and their anti-proliferative activity in human lung cancer cells. Int. J. Biol. Macromol. 2019;141:476–483. doi: 10.1016/j.ijbiomac.2019.08.235. [DOI] [PubMed] [Google Scholar]
- 242.Deepika M.S., Thangam R., Sheena T.S., Sasirekha R., Sivasubramanian S., Babu M.D., Jeganathan K., Thirumurugan R. A novel rutin-fucoidan complex based phytotherapy for cervical cancer through achieving enhanced bioavailability and cancer cell apoptosis. Biomed. Pharmacother. 2019;109:1181–1195. doi: 10.1016/j.biopha.2018.10.178. [DOI] [PubMed] [Google Scholar]
- 243.Radwan R.R., Ali H.E. Radiation-synthesis of chitosan/poly (acrylic acid) nanogel for improving the antitumor potential of rutin in hepatocellular carcinoma. Drug Deliv. Transl. Res. 2020 doi: 10.1007/s13346-020-00792-7. [DOI] [PubMed] [Google Scholar]
- 244.Khonkarn R., Daowtak K., Okonogi S. Chemotherapeutic efficacy enhancement in P-gp-Overexpressing cancer cells by flavonoid-loaded polymeric micelles. AAPS Pharm. Sci. Tech. 2020;21:121. doi: 10.1208/s12249-020-01657-5. [DOI] [PubMed] [Google Scholar]
- 245.Júlio A., Antunes C., Mineiro R., Raposo M., Caparica R., Araújo M., Rosado C., Fonte P., Santos de Almeida T. Influence of two choline-based ionic liquids on the solubility of caffeine. J. Biomed. Biopharm. Res. 2018;15:96–102. doi: 10.19277/BBR.15.1.178. [DOI] [Google Scholar]
- 246.Caparica R., Júlio A., Rosado C., Santos de Almeida T. Applicability of ionic liquids in topical drug delivery systems: A mini review. J. Pharmacol. Clin. Res. 2018;4:555649–555655. doi: 10.19080/JPCR.2018.04.555649. [DOI] [Google Scholar]
- 247.Khezeli T., Ghaedi M., Daneshfar A., Bahrani S., Asfaram A., Soylak M. New Generation Green Solvents for Separation and Preconcentration of Organic and Inorganic Species. Elsevier; Amsterdam, The Netherlands: 2020. Ionic liquids in separation and preconcentration of organic and inorganic species; pp. 267–318. [Google Scholar]
- 248.Kumar S.S.D., Surianarayanan M., Vijayaraghavan R., Mandal A.B., Macfarlane D.R. Curcumin loaded poly (2-hydroxyethyl methacrylate) nanoparticles from gelled ionic liquid–In vitro cytotoxicity and anti-cancer activity in SKOV-3 cells. Eur. J. Pharm. Sci. 2014;51:34–44. doi: 10.1016/j.ejps.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 249.Chowdhury M.R., Moshikur R.M., Wakabayashi R., Tahara Y., Kamiya N., Moniruzzaman M., Goto M. Ionic-liquid-based paclitaxel preparation: A new potential formulation for cancer treatment. Mol. Pharm. 2018;15:2484–2488. doi: 10.1021/acs.molpharmaceut.8b00305. [DOI] [PubMed] [Google Scholar]
- 250.Karimi M., Eslami M., Sahandi-Zangabad P., Mirab F., Farajisafiloo N., Shafaei Z., Ghosh D., Bozorgomid M., Dashkhaneh F., Hamblin M.R. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol. 2016;8:696–716. doi: 10.1002/wnan.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cheng R., Meng F., Deng C., Klok H.-A., Zhong Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34:3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 252.Liu M., Du H., Zhang W., Zhai G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications. Mater. Sci. Eng. C. 2017;71:1267–1280. doi: 10.1016/j.msec.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 253.Fathi M., Zangabad P.S., Majidi S., Barar J., Erfan-Niya H., Omidi Y. Stimuli-responsive chitosan-based nanocarriers for cancer therapy. BioImpacts. 2017;7:269. doi: 10.15171/bi.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chawla A., Sharma P., Pawar P. Eudragit S-100 coated sodium alginate microspheres of naproxen sodium: Formulation, optimization and in vitro evaluation/Alginatne mikrosfere naproksen natrija obložene Eudragitom S-100: Priprava, optimizacija i in vitro vrednovanje. Acta Pharm. 2012;62:529–545. doi: 10.2478/v10007-012-0034-x. [DOI] [PubMed] [Google Scholar]
- 255.Madhavi M., Madhavi K., Jithan A. Preparation and in vitro/in vivo characterization of curcumin microspheres intended to treat colon cancer. J. Pharm. Bioallied Sci. 2012;4:164. doi: 10.4103/0975-7406.94825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tarhini M., Greige-Gerges H., Elaissari A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. Int. J. Pharm. 2017;522:172–197. doi: 10.1016/j.ijpharm.2017.01.067. [DOI] [PubMed] [Google Scholar]
- 257.Sharma S., Gupta A. Sustainable management of keratin waste biomass: Applications and future perspectives. Braz. Arch. Biol. Technol. 2016;59 doi: 10.1590/1678-4324-2016150684. [DOI] [Google Scholar]
- 258.Lee Y.-H., Lai Y.-H. Synthesis, characterization, and biological evaluation of anti-her2 indocyanine green-encapsulated peg-coated plga nanoparticles for targeted phototherapy of breast cancer cells. PLoS ONE. 2016;11:e0168192. doi: 10.1371/journal.pone.0168192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wang H., Zhao Y., Wu Y., Hu Y.-l., Nan K., Nie G., Chen H. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials. 2011;32:8281–8290. doi: 10.1016/j.biomaterials.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 260.Song X., Zhao Y., Hou S., Xu F., Zhao R., He J., Cai Z., Li Y., Chen Q. Dual agents loaded PLGA nanoparticles: Systematic study of particle size and drug entrapment efficiency. Eur. J. Pharm. Biopharm. 2008;69:445–453. doi: 10.1016/j.ejpb.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 261.Mugaka B.P., Hu Y., Ma Y., Ding Y. Surface Modification of Nanoparticles for Targeted Drug Delivery. Springer; Berlin/Heidelberg, Germany: 2019. Surface modification of gold nanoparticles for targeted drug delivery; pp. 391–403. [Google Scholar]
- 262.Ovais M., Khalil A.T., Raza A., Khan M.A., Ahmad I., Islam N.U., Saravanan M., Ubaid M.F., Ali M., Shinwari Z.K. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine. 2016;12:3157–3177. doi: 10.2217/nnm-2016-0279. [DOI] [PubMed] [Google Scholar]
- 263.Sharma H., Mishra P.K., Talegaonkar S., Vaidya B. Metal nanoparticles: A theranostic nanotool against cancer. Drug Discov. Today. 2015;20:1143–1151. doi: 10.1016/j.drudis.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 264.Wu L., Sun J., Su X., Yu Q., Yu Q., Zhang P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016;154:96–111. doi: 10.1016/j.carbpol.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 265.Chmit M., Kanaan H., Habib J., Abbass M., Mcheik A., Chokr A. Antibacterial and antibiofilm activities of polysaccharides, essential oil, and fatty oil extracted from Laurus nobilis growing in Lebanon. Asian Pac. J. Trop Med. 2014;7:S546–S552. doi: 10.1016/S1995-7645(14)60288-1. [DOI] [PubMed] [Google Scholar]
- 266.Bartlett M.R., Warren H.S., Cowden W.B., Parish C.R. Effects of the anti-inflammatory compounds castanospermine, mannose-6-phosphate and fucoidan on allograft rejection and elicited peritoneal exudates. Immunol. Cell Biol. 1994;72:367–374. doi: 10.1038/icb.1994.55. [DOI] [PubMed] [Google Scholar]
- 267.Phan N.H., Ly T.T., Pham M.N., Luu T.D., Vo T.V., Tran P.H., Tran T.T. A comparison of fucoidan conjugated to paclitaxel and curcumin for the dual delivery of cancer therapeutic agents. Anticancer Agents Med. Chem. 2018;18:1349–1355. doi: 10.2174/1871520617666171121125845. [DOI] [PubMed] [Google Scholar]
- 268.Lu K.-Y., Li R., Hsu C.-H., Lin C.-W., Chou S.-C., Tsai M.-L., Mi F.-L. Development of a new type of multifunctional fucoidan-based nanoparticles for anticancer drug delivery. Carbohydr. Polym. 2017;165:410–420. doi: 10.1016/j.carbpol.2017.02.065. [DOI] [PubMed] [Google Scholar]