Abstract
Greigia sphacelata (Ruiz and Pav.) Regel (Bromeliaceae) is a Chilean endemic plant popularly known as “quiscal” and produces an edible fruit consumed by the local Mapuche communities named as “chupón”. In this study, several metabolites including phenolic acids, organic acids, sugar derivatives, catechins, proanthocyanidins, fatty acids, iridoids, coumarins, benzophenone, flavonoids, and terpenes were identified in G. sphacelata fruits using ultrahigh performance liquid chromatography-photodiode array detection coupled with a Orbitrap mass spectrometry (UHPLC-PDA-Orbitrap-MS) analysis for the first time. The fruits showed moderate antioxidant capacities (i.e., 487.11 ± 26.22 μmol TE/g dry weight) in the stable radical DPPH assay, 169.08 ± 9.81 TE/g dry weight in the ferric reducing power assay, 190.32 ± 6.23 TE/g dry weight in the ABTS assay, and 76.46 ± 3.18% inhibition in the superoxide anion scavenging assay. The cholinesterase inhibitory potential was evaluated against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). From the findings, promising results were observed for pulp and seeds. Our findings suggest that G. sphacelata fruits are a rich source of diverse secondary metabolites with antioxidant capacities. In addition, the inhibitory effects against AChE and BChE suggest that natural products or food supplements derived from G. sphacelata fruits are of interest for their neuroprotective potential.
Keywords: Greigia sphacelata, endemic fruits, UHPLC-PDA-Orbitrap-MS, metabolomic analysis, antioxidant, cholinesterase inhibition
1. Introduction
Plant-based foods, especially fruits, have a great impact on human health [1,2]. Indeed, there is a growing body of scientific literature describing curative effects of fruits against a broad spectrum of diseases including diabetes, obesity, neurodegenerative, gastric and cardiovascular disorders, and certain types of cancers [3,4,5,6]. Different types of secondary metabolites have been reported in fruits [4,7,8,9]. These compounds are in part responsible for their biological properties.
The genus Greigia (Bromeliaceae) comprise approximately 36 species in Central and South America and form an extraordinary disjunct distribution in humid habitats. Among Chilean Greigia, four endemic species have been described: G. pearcei (Mez), G. landbeckiia (Lechl. ex Phil.) F.Phil., G. berteroi (Skottsb), and G sphacelata (Ruiz and Pav.) Regel. Greigia sphacelata (Ruiz and Pav.) Regel (Bromeliaceae) (local name: Chupon and quiscal) is an endemic plant (Figure 1a,b) widely distributed in temperate zones of Central and Southern Chile [10].
Figure 1.
Greigia sphacelata (Ruiz and Pav.) Regel (Bromeliaceae). (a) Fruits, (b) plant.
The edible fruits of G. sphacelata are juicy, sweet, and with a similar flavor to apple and pineapple, and are eaten raw, or prepared as an infusion or as a fermented beverage. Few reports have investigated the chemical properties of G. sphacelata. From the aerial parts of G. sphacelata flavanones (5,7,3′-trihydroxy-6, 4′,5′-trimethoxyflavanone, and 5,3′-dihydroxy-6,7,4′,5′-tetramethoxyflavanone), glycerol derivatives (1-O-trans-p-coumaroylglycerol, 1,3-O-di-trans-p-coumaroylglycerol, 1-(ω-feruloyl-docosanoyl) glycerol, and 1-(ω-feruloyl-tetracosanoyl) glycerol), and trans-ferulic acid, arborinone, 22-hydroxydocosanoic acid ester, isoarborinol and arborinol have been isolated and characterized [11]. To our knowledge, there are no reports on the chemical characterization or antioxidant and enzyme inhibitory properties of the natural products obtained from G. sphacelata fruits.
HPLC or UHPLC coupled to mass spectrometry is a key technique for plant chemotaxonomy or comparative metabolic profiling. Q-Exactive type focus equipment uses a very rapid high-performance mass spectrometer for the detection of small organic molecules [12,13,14]. This is a dual high resolution with accurate mass (HRAM) spectrometer with an orbital trap (Orbitrap), a quadrupole (Q), and a high-performance collision cell (HCD), capable of producing high-resolution parent ions and diagnostic MS daughter fragments. The hyphenated ultrahigh performance liquid chromatography-photodiode array detection coupled with a Orbitrap mass spectrometry (UHPLC-PDA-Orbitrap-MS) approach is a key tool for the identification of secondary metabolites in plants and edible fruits [15,16,17].
In this work, we report the antioxidant activity, cholinesterase inhibitory potential plus the UHPLC-PDA-Orbitrap-MS fingerprinting from the endemic G. sphacelata fruits for the first time.
2. Results and Discussion
2.1. Metabolomic Analyses
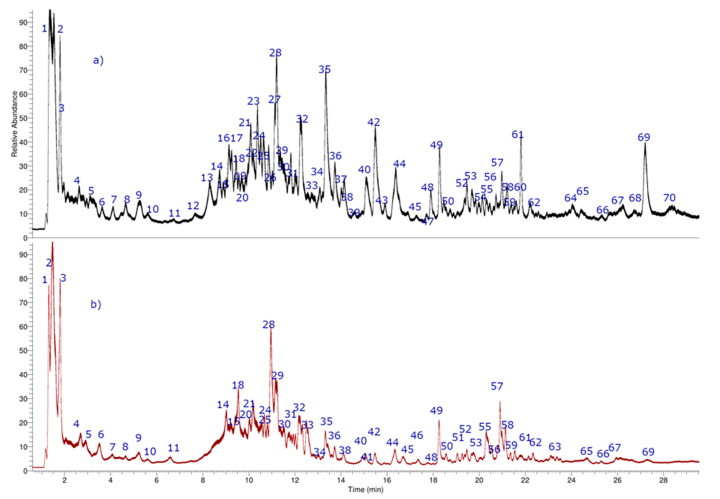
In this study, the fingerprint was generated using UHPLC-PDA-Orbitrap-MS (Figure 2) allowing the determination of several types of metabolites in the fruits of the Mapuche specie G. sphacelata (Table 1). The metabolites identified include: Eleven phenolic acids (peaks 1, 6, 8, 13, 14, 16, 21, 42, 47, 48, and 54); six organic acids (peaks 2–5, 7, and 10, including a vitamin peak 7); eleven sugar derivatives (peaks 12, 15, 19, 22–24, 26, 31, 39, 40, and 59); five catechins and/or proanthocyanidins (peaks 34, 37, 41, 44, and 49); thirteen fatty acids, (peaks 33, 50, 52, 55, 56, 58, 62–67, and 69); four iridoids (peaks 11, 17, 18, and 68); three coumarins (peaks 25, 27, and 30); one benzophenone (peak 57); ten flavonoids (peaks 20, 28, 29, 32, 35, 36, 38, and 43–46); five terpenes, (peaks 9, 51, 53, 60, and 61).
Figure 2.
UHPLC chromatograms of G. sphacelata (a) pulp, and (b) seeds.
Table 1.
Full metabolome identification in Greigia sphacelata by UHPLC-PDA-Orbitrap-MS.
| Peak | Tentative Identification | [M − H]− Ions |
Retention Time (min) |
UV Max (nm) |
Theoretical Mass (m/z) |
Measured Mass (m/z) |
Accuracy (ppm) |
Metabolite Type |
MS2 Ions (ppm) |
Fruit Part |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Syringaldehyde syringate | C18H17O9− | 1.36 | 315 | 377.08578 | 377.08671 | −2.44 | Pa | 215.03258 | P; S |
| 2 | Quinic acid | C7H11O6− | 1.45 | 191.05501 | 191.05621 | 3.16 | Oa | 135.02919 | P; S | |
| 3 | Citric acid | C6H7O7− | 1.52 | 187 | 191.01863 | 191.01915 | 3.12 | Oa | 162.89121, 111.00782 | P; S |
| 4 | Dehydroascorbic acid | C6H5O6− | 2.20 | 176 | 173.00806 | 173.00879 | 4.18 | Oa | P; S | |
| 5 | Isocitric acid | C6H7O7− | 2.74 | 191.01863 | 191.01923 | 3.76 | Oa | 162.89110, 111.00810 | P; S | |
| 6 | Diffutidin | C17H18O5− | 3.45 | 301.10705 | 301.10614 | −3.02 | Pa | 153.01875 | P; S | |
| 7 | Homocitric acid | C10H17O4− | 4.05 | 205.03542 | 205.03491 | −2.43 | Oa | P; S | ||
| 8 | Vanilloyl-glucoside | C14H17O9− | 4.34 | 287 | 329.08810 | 329.08671 | 4.24 | Pa | 151.03874, 123.02314 | P; S |
| 9 | Euonyminol | C15H25O10− | 5.26 | 365.14422 | 365.14560 | 3.76 | Te | P; S | ||
| 10 | Pantothenic acid | C9H16NO5− | 5.255 | 218.10230 | 218.10313 | 3.82 | Oa | 125.85231 | P; S | |
| 11 | Ebuloside | C21H31O10− | 6.50 | 175 | 443.19263 | 443.19117 | 3.27 | Ir | P; S | |
| 12 | Nonioside k | C17H29O11− | 7.85 | 409.17144 | 409.17191 | 3.58 | Sd | P | ||
| 13 | Glucosyringic acid | C15H19O10− | 8.56 | 325 | 359.09841 | 359.09824 | -0.47 | Pa | 197.0455 (syringic acid) | P |
| 14 | Acetylshikonin | C18H17O6− | 9.01 | 305 | 329.10162 | 329.10114 | −0.47 | Pa | 171.07063 | P; S |
| 15 | Allyl-sucrose | C15H25O11− | 9.12 | 381.14023 | 381.14066 | 2.86 | Sd | P; S | ||
| 16 | Feruloyl-O-galactarate | C16H17O11− | 9.25 | 246–310 | 385.07797 | 385.07654 | 3.64 | Pa | 194.0577, 133.8732 | P |
| 17 | Cinnamoyl catalpol | C24H27O11− | 9.29 | 198 | 491.15589 | 491.15479 | −1.74 | Ir | 175.06085 | P |
| 18 | Cinnamoyl catalpol isomer | C16H21O9− | 9.49 | 198 | 357.11801 | 357.11938 | −1.74 | Ir | 258.09830 | P; S |
| 19 | Linamarin | C10H15NO6− | 9.65 | 246.09821 | 246.09825 | 4.21 | Sd | 258.09830 | P | |
| 20 | Amurensin | C26H29O12− | 10.03 | 202–284 | 533.16431 | 533.16425 | −1.96 | Fl | 503.14331, 341.1015 | P; S |
| 21 | 2-caffeoylisocitric acid | C15H13O10− | 9.98 | 353.07032 | 353.05173 | 3.97 | Pa | 179.06425, 154.99808 | P; S | |
| 22 | Methyl 2,3,4-tris-O-[2-(carboxymethyl) ethyl]- glucose derivative | C26H31O13− | 10.12 | 479.17592 | 479.17749 | 3.27 | Sd | P | ||
| 23 | Methyl 2,3,4-tris-O-[2-(carboxymethyl) ethyl]-glucose | C19H31O12− | 10.43 | 451.18228 | 451.18100 | 2.83 | Sd | P | ||
| 24 | Dimethyl--hydroxyl-oct-2,7-die- noate-glucopyranosyl-glucose | C22H35O14− | 10.55 | 523.20213 | 523.20331 | 2.24 | Sd | 241.07164, 125.02764 | P; S | |
| 25 | Aesculetin-7-O-glucuronide | C15H13O10− | 10.62 | 232–279–329 | 353.05140 | 353.05130 | -0.28 | Co | 177.0193 (Aesculetin) | P; S |
| 26 | Hexamethyl-glucopiranuronosyl-glucoside methyl ester | C19H33O12− | 10.83 | 453.19800 | 453.19794 | 2.96 | Sd | P | ||
| 27 | 7-Hydroxycoumarin glucuronide | C15H13O9− | 10.95 | 255–355 | 337.05541 | 337.05661 | 3.56 | Co | P | |
| 28 | Quercetin-3-O-glucoside-acetate | C23H21O13− | 11.03 | 255–355 | 505.09767 | 505.09912 | 2.87 | Fl | 301.02755 (Quercetin) |
P; S |
| 29 | Tectoridin | C22H21O11− | 11.23 | 281 | 461.10894 | 461.10941 | 3.39 | Fl; Is | 300.2632 (tectorigenin) |
P; S |
| 30 | Escopoletin 7-O-glucuronide | C16H15O10− | 11.35 | 320 | 367.06597 | 367.06729 | 3.59 | Co | 171.06593 | P; S |
| 31 | Hexenyl-xylopiranosyl-glucose | C17H29O10− | 11.71 | 393.17706 | 393.17552 | 3.91 | Sd | P; S | ||
| 32 | Lupinisoflavone A | C16H15O9− | 12.12 | 285 | 351.07245 | 351.07106 | 3.96 | Fl; Is | 321.0763, 241.0502 | P; S |
| 33 | 2,2,3-Tris (2,3-dihydroxypropanoyl) decanoic acid | C19H31O11− | 12.43 | 435.18609 | 435.18750 | 3.23 | Fa | 287.15002 | P; S | |
| 34 | Trimeric proanthocyanidin C2 | C45H37O18− | 12.96 | 283 | 865.19859 | 865.19451 | −4.71 | Ca | 577.1342, (dimer), 289.0714 catechin, (C15H14O6−) | P; S |
| 35 | Genistein-7-O-di-glucoside | C27H29O15− | 13.20 | 230–238–333 | 593.15122 | 593.15033 | −1.65 | Fl; Is | 269.0452 (genistein) | P; S |
| 36 | Genistein-7-O-di-galactoside | C27H29O15− | 13.52 | 230–238–333 | 593.15120 | 593.15033 | −1.63 | Fl; Is | 269.0451 (genistein) | P; S |
| 37 | Procyanidin B1 | C30H23O12− | 13.83 | 285 | 577.13445 | 577.13373 | 1.16 | Ca | 289.0714 catechin, (C15H14O6−) | P |
| 38 | Genistein-7-O-glucoside | C21H19O10− | 14.03 | 230–238–333 | 431.09847 | 431.09824 | −0.53 | Fl; Is | 269.0452 (genistein) | P; S |
| 39 | Secolonitoside | C21H35O12− | 14.35 | 479.21230 | 479.21350 | 2.50 | Sd | P | ||
| 40 | Trehalose undecylenoate | C23H39O12− | 15.13 | 507.24360 | 507.24454 | 1.84 | Sd | 457.06708 | P; S | |
| 41 | Proanthocyanin tetramer | C60H49O24− | 15.32 | 285 | 1153.26191 | 1153.25146 | −9.05 | Ca | 577.1337 (dimer) | S |
| 42 | Bis-2-hydroxyethyl phtalate | C12H13O6− | 15.52 | 315 | 253.07173 | 253.07066 | 4.21 | Pa | P; S | |
| 43 | Daidzein-7-O-galactoside | C21H19O9− | 15.63 | 281 | 415.10236 | 415.10327 | 3.00 | Fl; Is | 253.05065 | P |
| 44 | Procyanidin A1 | C30H23O12− | 16.45 | 282 | 575.11958 | 575.11877 | −1.42 | Ca | 289.07157 (catechin) | P; Se |
| 45 | Daidzein-7-O-glucoside | C21H19O9− | 16.75 | 230–285–326 | 415.10236 | 415.10361 | 3.00 | Fl; Is | 273.04050 | P; S |
| 46 | Ononin (formononetin 7-O-glucoside) | C22H21O9− | 17.26 | 220–285–326 | 429.11917 | 429.11880 | −0.86 | Fl; Is | 267.06601, 251.0362 (formononetin) | P |
| 47 | 1-O-trans-p-coumaroylglycerol | C12H13O6− | 17.84 | 212–326 | 253.07184 | 253.07150 | −1.34 | Pa | 119.04922, 120.05251 | S |
| 48 | 1, 3-O-di-trans-p-coumaroylglycerol | C21H19O9− | 18.03 | 212–326 | 415.10355 | 415.10333 | 2.86 | Pa | 119.04920, 120.05248 | P; S |
| 49 | Catechin* | C15H13O6− | 18.32 | 285 | 289.07162 | 289.07164 | 0.06 | Fl; Ca | 260.06575, 245.08210 | P; S |
| 50 | Tetrahydroxy-tetradecadienoic acid | C14H23O6− | 18.45 | 265 | 287.15012 | 287.15001 | 4.50 | Fa | P; S | |
| 51 | Monic acid A | C17H27O7− | 19.03 | 215 | 343.17513 | 343.17657 | 4.21 | Te | P; S | |
| 52 | Pentahydroxy-octadecatetranoic acid | C18H27O7− | 19.47 | 189 | 355.17514 | 355.17648 | 3.81 | Fa | Pu; Se | |
| 53 | Dictamnoside N | C18H29O8− | 19.75 | 175 | 373.18569 | 373.18707 | 3.69 | Te | 217.88326 | P; S |
| 54 | Evodinnol | C14H16O4− | 19.83 | 325 | 247.09649 | 247.09760 | 4.49 | Pa | 133.02882 | P |
| 55 | Dihydroxy-pentadecanoic acid | C15H29O4− | 20.36 | 215 | 273.20724 | 273.20734 | 0.36 | Fa | P; S | |
| 56 | 12-hydroxyoctadecanoic acid | C18H36O3− | 20.52 | 225 | 299.25946 | 299.25807 | 4.64 | Fa | P; S | |
| 57 | Congestiflorone | C28H31O4− | 20.98 | 243 | 431.22169 | 431.22061 | −2.49 | Be | 365.17944, 269.17584, 152.99516 | P; S |
| 58 | Hydroxy-pentadecaenoic acid | C15H27O4− | 21.06 | 234 | 271.19144 | 271.19173 | 1.06 | Fa | P; S | |
| 59 | Oct-1-en-3-yl-arabinosyl-glucopyranoside | C19H33O10− | 21.57 | 421.20821 | 421.20816 | −0.11 | Sa | P; S | ||
| 60 | Marrubiin | C20H27O4− | 21.73 | 225 | 331.19196 | 331.19039 | 4.73 | Te | 207.19521 | P |
| 61 | Quillaic acid | C30H45O5− | 21.85 | 214 | 485.32788 | 485.32615 | 3.56 | Te | 405.32125, 249.03424 | P; S |
| 62 | Dihydroxy-octadecaenoic acid | C18H33O4− | 22.05 | 225 | 313.23883 | 313.23734 | −4.76 | Fa | P; S | |
| 63 | Pentadecanedioic acid | C15H27O4− | 23.25 | 235 | 271.19307 | 271.19165 | 4.66 | Fa | S | |
| 64 | Trihydroxy-octadecadienoic acid | C18H31O5− | 24.21 | 235 | 327.21770 | 327.21780 | 0.30 | Fa | P | |
| 65 | Trihydroxy-octadecaenoic acid | C18H33O5− | 24.68 | 222 | 329.23332 | 329.23341 | 0.27 | Fa | P; S | |
| 66 | 2,3-dihydroxypropyl dodecanoate | C15H29O4− | 25.36 | 198 | 273.20715 | 273.20724 | 4.42 | Fa | P; S | |
| 67 | Tetrahydroxy-heptadecatrienoic acid | C17H27O6− | 26.03 | 285 | 327.1813 | 327.18142 | 3.89 | Fa | P; S | |
| 68 | Jatamanvaltrate H | C22H33O9− | 26.19 | 215 | 441.21335 | 441.21329 | 3.26 | Ir | 389.62607, 311.22302, 135.04443 | P |
| 69 | Hydroxy-pentadecanoic acid | C15H29O3− | 27.45 | 235 | 257.21221 | 257.21222 | 0.03 | Fa | P; S | |
| 70 | Unknown | C14H29O8− | 28.33 | 220 | 325.18569 | 325.18442 | −3.92 | Fa | P |
Metabolite type: Be: Benzophenones; Ca: Catechins; Co: Coumarins; Fa: Fatty acids; Fl: Flavonoids; Ir: Iridoids; Is: Isoflavones; Oa: Organic acids; Pa: Phenolic acids; Sd: Sugar derivatives; and Tr: Terpenes. Fruit part: P: Pulp and S: Seed.
Examples of full MS spectra and structures of the compounds identified are displayed in Figure S1. The detailed identification is explained below:
2.1.1. Coumarins
Several metabolites were identified as coumarins, due to their characteristic UVmax spectra. Peak 25 with an anion [M − H]− (molecule without an hydrogen, charged negatively, formed by the heated electrospray device) at m/z (mass/charge ratio) 353.05130 was identified as aesculetin-7-O-glucuronide (C15H13O10−) and peak 27 with a [M − H]− ion at m/z 337.05643 was identified as 7-hydroxycoumarin-glucuronide (C15H13O9−). Peak 30, with a [M − H]− ion at m/z 367.06696 scopoletin 7-O-glucuronide (C16H17O10−).
2.1.2. Flavonoids
Peak 29 with a UVmax at 281 nm and with a parent ion at m/z 461.10894 was determined as tectoridin (C22H21O11−). Peak 32 with a similar UVmax and a MS ion at m/z 351.08591 was named as lupinisoflavone (C20H15O6−). Peak 35 with an anion [M − H]− at m/z 593.15024 was determined as genistein-7-O-di-glucoside (C27H29O15−) producing a genistein aglycone ion at m/z 269.0452 and peak 36 as a di-galactoside derivative. Peak 38 with a pseudo-molecular ion at m/z 431.09824 was named as genistein-7-O-glucoside (C21H19O10−) which produced a MS2 daughter ion at m/z 269.0452 (genistein). Peaks 43 and 45 with ions around m/z 415.10364 were identified as the isomers: Daidzein-7-O-galactoside and glucoside (C21H19O9−, respectively). Finally, peak 46 was named as ononin (formononetin 7-O-glucoside (C22H21O9−). Peak 20 with an anion [M − H]− at m/z 533.16435 was determined as amurensin (C26H29O12−), the tert-amyl alcoholic derivative of the flavonol kaempferol 7-O-glucoside, while peak 28 with a pseudo-molecular anion at m/z 505.09767 was determined as the related flavonol quercetin-3-O-glucoside-acetate (C23H21O13−).
2.1.3. Hydrocarbons and Saturated Organic Acids
Peak 2 was identified as quinic acid (C7H11O6−), Peaks 3 and 5 with ions around m/z 191.01933 were determined as citric acid and isocitric acid (C6H7O7−), respectively. Peak 4 with an ion [M − H]− at m/z 173.00879 was named as dehydroascorbic acid (C6H5O6−), while peak 7 with a [M − H]− ion at m/z 205.03492 was labeled as homocitric acid (C10H17O4−). Peak 10 was identified as pantothenic acid.
2.1.4. Oxylipins or Fatty Acids
Several metabolites were identified as polyhydroxylated unsaturated fatty acids known as the dietary antioxidants oxylipins. Accordingly, peak 33 was identified as 2,2,3-Tris(2,3-dihydroxypropanoyl) decanoic acid (C19H31O11−), peak 50 with an anion [M − H]− at m/z 287.15001 was determined as a tetrahydroxy-tetradecadienoic acid (C14H23O6−), peak 52 with a [M − H]− ion at m/z 355.17648 was determined as pentahydroxy-octadecatetraenoic acid (C18H27O7−), peak 55 with a [M − H]− ion at m/z 273.20734 was named as dihydroxy-pentadecanoic acid (C15H29O4−), peak 56 as 12-hydroxyoctadecanoic acid (C18H36O3−) and peak 58 as its monounsaturated derivative hydroxy-pentadecaenoic acid ion at m/z 271.19173 (C15H27O4−). Furthermore, peak 62 with an anion [M − H]− at m/z 313.23734 was reported as dihydroxy-octadecaenoic acid (C18H33O4−), peak 63 as pentadecanedioic acid (C15H27O4−) while peak 64 (ion at m/z 327.21780) was identified as trihydroxy-octadecadienoic acid (C18H31O5−), and peak 65 as trihydroxy-octadecaenoic acid while the related compound peak 67 with an ion at m/z 327.18142 was ascribed to tetrahydroxy-heptadecatrienoic acid (C17H27O6−). Peak 66 with an anion [M − H]− at m/z 273.20724 was identified as 2,3-dihydroxypropyl dodecanoate (C15H29O4−). Peak 65 with an anion [M − H]− at m/z 329.23341 was assigned to trihydroxy-octadecaenoic acid (C18H33O5−) as already reported by some of us from the fruits of the Gomortega keule (Molina) Baill. (Keule tree). Finally, peak 69 with an anion [M − H]− at m/z 257.21222 was assigned to hydroxy-pentadecanoic acid (C15H29O3−) and peak 70 unknown compound matches formula (C14H29O8−).
2.1.5. Iridoids
Three compounds were identified as iridoids, these compounds are very important because they have exhibited a wide range of bioactive effects, such as hypolipidemic, antioxidant, antimicrobic, hypoglycaemic, choleretic, antispasmodic, besides immuno-modulatory anti-inflammatory, neuroprotective, hepatoprotective, and cardioprotective effects [18]. Peak 11 with an ion [M − H]− at m/z 443.19117 was determined as the iridoid ebuloside (C21H31O10−). Peak 68 with an anion [M − H]− at m/z 441.21329 was identified as the acylated iridoid jatamanvaltrate H (C22H33O9−). Peak 17 with an anion [M − H]− at m/z 491.15479 was determined as cinnamoyl catalpol and peak 18 as its isomer (C24H27O11−).
2.1.6. Terpenes
Several compounds were ascribed as having the terpenoid (C-20 or C-30) skeleton. Peak 9 with an anion [M − H]− at m/z 365.14422 was tentatively determined as euonyminol (C15H25O10−). Peak 51 with a pseudo-molecular ion at m/z 343.17513 was identified as the terpenoid monic acid (C17H27O7−) and peak 53 as the sesquiterpene dictamnoside N [19]. Peaks 60 and 61 with ions at m/z 331.19039 and 485.32615 were identified as the diterpene marrubiin (C20H27O4−) and triterpene quillaic acid (C30H45O5−), respectively.
2.1.7. Benzophenones
Peak 57 with a pseudo-molecular anion at m/z 431.22061 was identified as the dibenzophenone derivative congestiflorone (C28H31O4−).
2.1.8. Phenolic Acids and Derivatives
Some eleven compounds were characterized as phenolic acids and derivatives. Peak 1 with an anion [M − H]− at m/z 377.08671 was determined as syringaldehyde syringate (C18H17O9−), peak 6 as diffutidin (C17H18O5−), Peak 8 with a [M − H]− ion at m/z 329.08671 was labeled as vanilloyl-glucoside (C14H17O9−), while peak 13 with a [M − H]− ion at m/z 359.09824 was assigned as glucosyringic acid (C15H19O10−). Peak 14 as acetylshikonin (C18H17O6−), peak 16 with a parent ion at m/z 385.07654 was assigned as O-feruloyl-galactarate (C16H17O11−). Peak 21 as 2-caffeoylisocitric acid (C15H13O10−), peak 42 with a [M − H]− ion at m/z 253.07066 was determined as bis-2-hydroxyethyl phtalate (C12H13O6−). Some two compounds were identified as phenolic acid glycerol derivatives. Peak 47 with an anion [M − H]− at m/z 253.07150 was resolved as 1-O-trans-p-coumaroylglycerol (C12H13O6−) while peak 48 with an anion [M − H]− at m/z 415.10236 was named as 1,-O-di-trans-p-coumaroylglycerol (C21H19O9−) and peak 54 as evodinnol (C14H16O4−).
2.1.9. Catechins and Proanthocyanidins
Peak 49 with a parent ion at m/z 289.07164 was determined as the flavanol catechin (C15H13O6−), peak 34 with a parent ion at m/z 865.19451 was resolved as a trimeric procyanidin (C45H37O18−), the fragmentation pattern is in concordance for the trimeric procyanidin C2, (C45H37O18−) isolated from Pinus radiata D. Don. Peak 37 with an ion [M − H]− at m/z 577.13512 was identified as procyanidin B1 (C30H23O12−), while peak 41 with a parent ion [M − H]− at m/z 1153.25147 was identified as proanthocyanin tetramer (C60H49O24−) and finally, peak 44 was resolved as procyanidin A1 (C30H23O12−).
2.1.10. Sugar Derivatives
The most abundant compounds in G. sphacelata fruits were the sugar derivatives. Peak 12 with an ion [M − H]− at m/z 409.17191 was resolved as the sugar derivative nonioside K (C17H29O11−), while peak 15 with a parent ion [M − H]− at m/z 381.13914 was determined as allyl-sucrose (C15H25O11−). Peak 22 with a [M − H]− ion at m/z 479.17749 was resolved as methyl 2,3,4-tris-O-[2-(carboxymethyl) ethyl]-glucose derivative (C26H31O13−) and peak 23 as methyl 2,3,4-tris-O-[2-(carboxymethyl) ethyl]-glucose (C19H31O12−). Peak 24 as (2E)-2,6-dimethyl-6-hydroxyl-oct-2,7-die-noate-2-O-β-d-glucopyranosyl-β-d-glucopyranoside (C22H35O14−). In addition, peak 26 with a parent ion [M − H]− at m/z 453.19665 was resolved as hexamethyl-glucopyranuronosyl-glucoside methyl ester (C19H33O12−) and peak 31 with a [M − H]− ion at m/z 393.17552 was determined as hexenyl-xylopyranosyl-glucose (C17H29O10−), peak 39 as secolonitoside (C21H35O12−). Finally, peak 59 with a parent ion [M − H]− at m/z 421.20816 was resolved as oct-1-en-3-yl-arabinosyl-glucopyranoside (C19H33O10−) and peak 40 as Trehalose undecylenoate (C23H39O12−). Peak 19 was identified as linamarin.
2.2. Total Phenolics, Flavonoid Contents, and Antioxidant Activity of G. sphacelata
The in vitro results of total phenolics, total flavonoid content, and antioxidant activity are summarized in Table 2. DPPH, ABTS, FRAP, and superoxide anion scavenging activity (O2−) were used to measure the antioxidant potential in pulp and seed of G. sphacelata fruits. These in vitro assays are very simple and widely employed to calculate the antioxidant activities of plant and fruit extracts. The results were compared with previous studies from our research as well as work on other Chilean fruits by other researchers [20,21]. The total phenolic contents of pulp of Chilean berries from G. sphacelata (45.44 ± 0.67 mg GAE/g dry weight, Table 2) was closer to previous studies reported for the blueberries high-bush type Vaccinium corimbosum L (45.86 ± 3.46 mg GAE/g) [20] and Chilean blackberries maqui (Aristotelia chilensis (Molina) Stuntz) (49.74 ± 0.57 mg GAE/g) [22]. In addition, our TPC values were almost twice the values for TPC (29 mg Q/g) of the Chilean blackberries Luma apiculata (DC.) Burret (commonly known as arrayán or palo colorado) [13]. The amount of TFC found, 35.57 ± 0.86 mg QE/g, was lower than those of Chilean berries Berberis microphilla G. Forst. (45.72 ± 2.68 mg QE/g) commonly known as calafate or michay [20]. In the DPPH assay, the trapping capacity of G. sphacelata (487.11 ± 26.22 μmol Trolox/g dry weight) was close to that obtained for the red Chilean berries Ugni molinae Turcz. (commonly known as murtilla, murta, murtillo, or uñi), (450 μmol Trolox equivalents, TE/g dry weight) which is considered as an average antioxidant level in the category of edible fruits [21]. The ABTS values (190.32 ± 6.23 μmol TE/g dry weight) were lower than those of numerous Latin American fruits, such as Chilean blackberries maqui with a trapping capacity of 254.8 ± 8.2 μmol TE/g dry weight, and Brazilian Acai (Euterpe oleraceae Mart.), with 211.0 ± 14 μmol TE/g dry weight [23], while the FRAP values (169.08 ± 9.81 μmol TE/g dry weight) were close to those of Acai (157.9 ± 8.7 μmol TE/g dry weight) [23], but lower than maqui (254.2 ± 2.6 mg TE/g dry weight) [22,23]. The superoxide anion scavenging activity (O2−) of G. sphacelata (76.46 ± 3.18%) was higher compared to those established for the blueberries growing in Chile Vaccinium corimbosum L. (72.61 ± 1.91%) [20]. These values can classify these G. sphacelata fruits as moderate to high antioxidant small fruits such as plum, cherries, and strawberries [23].
Table 2.
Total phenolic contents, total flavonoid contents, and antioxidant activities of G. sphacelata.
| Sample | TPC a | TFC b | DPPH c | ABTS d | FRAP e | O2− f (%) |
|---|---|---|---|---|---|---|
| Pulp | 45.44 ± 0.67 | 35.57 ± 0.86 | 487.11 ± 26.22 | 190.32 ± 6.23 | 169.08 ± 9.81 | 76.46 ± 3.18 b |
| Seeds | 37.21 ± 0.45 | 28.32 ± 0.35 | 354.51 ± 34.16 | 140.49 ± 3.58 | 147.84 ± 4.35 | 67.02 ± 2.23 b |
a Total phenolic content (TPC), expressed in mg of gallic acid equivalents per gram of dry plant. b Total flavonoid content (TFC), expressed in mg of quercetin equivalents per gram of dry plant. c 1,1-diphenyl-2-picrylhydrazyl radical (DPPH), as μmol of Trolox equivalent/g dry fruit, d ABTS as μmol of Trolox equivalent/g dry fruit. e Ferric reducing antioxidant power (FRAP), as μmol TE/g dry weight. f Superoxide anion scavenging activity (O2−) expressed in %. All values were expressed as means ± SD (n = 3). The results are statistically compared with a positive control. The results were analyzed using one-way analysis of variance (ANOVA) and Tuckey test statistical analysis (p-values < 0.05 were regarded as significant). Values in the same column marked with the same letter are not significantly different (at p < 0.05).
2.3. In Vitro Cholinesterase Inhibitory Assay
The inhibition of key enzymes, such as AChE and BChE (linked to Alzheimer’s disease), is an important metric for identifying novel and safe medicinal value from natural products, especially those found in edible fruits. Indeed, four of the approved anti-Alzheimer drugs are cholinesterase inhibitors. For this reason, we tested the cholinesterase inhibitory potential of G. sphacelata against AChE and BChE. The results are shown in Table 3 and expressed as IC50 values. Galantamine was used as a positive control (0.27 ± 0.03 μg/mL against AChE and 3.82 ± 0.02 μg/mL against BChE). G. sphacelata fruits showed promising results for both enzymes. In the AChE assay, the inhibition IC50 for pulp was 4.49 + 0.08 μg/mL and for seeds was 4.38 ± 0.04 μg/mL. As for the BChE assay, the inhibition IC50 for pulp was 73.86 ± 0.09 μg/mL and for seeds was 78.57 ± 0.06 μg/mL. No significant difference was observed.
Table 3.
Cholinesterase inhibitory activity of pulp and seed of G. sphacelata.
| Sample | AChE (IC50) | BChE (IC50) |
|---|---|---|
| Pulp | 4.49 ± 0.08 | 73.86 ± 0.09 |
| Seeds | 4.38 ± 0.04 | 78.57 ± 0.06 |
| Galantamine | 0.27 ± 0.03 | 3.82 ± 0.02 |
The results are compared with their positive control. Values correspond to the average of three experiments; all values were expressed as means ± SD. Units of concentrations are expressed as (μg/mL). The results were analyzed using one-way analysis of variance (ANOVA) and Tuckey test statistical analysis (p-values < 0.05 were regarded as significant).
Some metabolites contained in G. sphacelata have been linked to counteraction against Alzheimer’s disease in previous reports. The iridoid jatamanvaltrate H isolated from V. jatamansi showed moderate neuroprotective activity [24,25]. In the same way, the crude extract of V. jatamansi and its fractions have shown considerable activity against AChE [26]. Marrubiin exhibited potent antinociceptive effects in a dose-dependent manner [27]. Congestiflorone acetates, such as detected in this study, have shown significant inhibitory effects against AChE with an IC50 value at 20.25 ± 0.55 µM [28]. On the other hand, the phenolic glucosyringic acid, did not show significant inhibitory effect on BChE (IC50 > 100 µM) [29]. Catechin presents neuroprotective effects as evidenced by amyloid β-induced neurotoxicity by MTT, lactate dehydrogenase (LDH) release, and neutral red uptake assays [30], but insignificant inhibitory against AChE [31]. Recently, the inhibitory activity against BChE of a procyanidin B1 was reported [32]. To the best of our knowledge, this is the first scientific report regarding the cholinesterase inhibitory potential of G. sphacelata fruits.
2.4. Docking Studies
In order to get insights on the intermolecular interactions, the most abundant compounds according to the UHPLC chromatogram (Figure 2) obtained from the pulp and seeds of G. sphacelata as well as the known cholinesterase inhibitor galantamine, were subjected to docking assays into the TcAChE catalytic site and hBChE catalytic site. In order to rationalize their pharmacological results analyzing their protein molecular interactions in the light of their experimental inhibition, activities are shown in Table 3. The best docking binding energies expressed in kcal/mol of each compound are shown in Table 4.
Table 4.
Binding energies obtained from docking experiments of most abundant compounds in G. sphacelata’s fruit and the known cholinesterase inhibitor galantamine over acetylcholinesterase (TcAChE) and butyrylcholinesterase (hBChE).
| Compound | Binding Energy (kcal/mol) Acetylcholinesterase (TcAChE) |
Binding Energy (kcal/mol) Butyrylcholinesterase (hBChE) |
|---|---|---|
| Quercetin-3-O-glucoside-acetate | −9.46 | −8.31 |
| Lupinisoflavone | −9.36 | −7.99 |
| Genistein-7-O-di-glucoside | −9.18 | −6.89 |
| Ononin (formononetin 7-O-glucoside) | −7.45 | −6.44 |
| Genistein-7-O-glucoside | −7.24 | −5.86 |
| Aesculetin-7-O-glucuronide | −6.67 | −6.85 |
| Dihydroxy-octadecaenoic acid | −4.71 | −5.76 |
| Hydroxy-pentadecanoic acid | −4.81 | −4.87 |
| Galantamine | −11.81 | −9.5 |
2.4.1. Acetylcholinesterase (TcAChE) Docking Results
Table 3 showed that the flavonoid quercetin-3-O-glucoside-acetate, and the isoflavones lupinisoflavone and genistein-7-O-di-glucoside displayed the best binding energies of −9.46, −9.36, and −9.18 kcal/mol, respectively. These results suggest that the G. sphacelata pulp or seed extracts inhibitory activity over acetylcholinesterase are mainly due the compounds mentioned above, especially the flavonoid quercetin-3-O-glucoside-acetate.
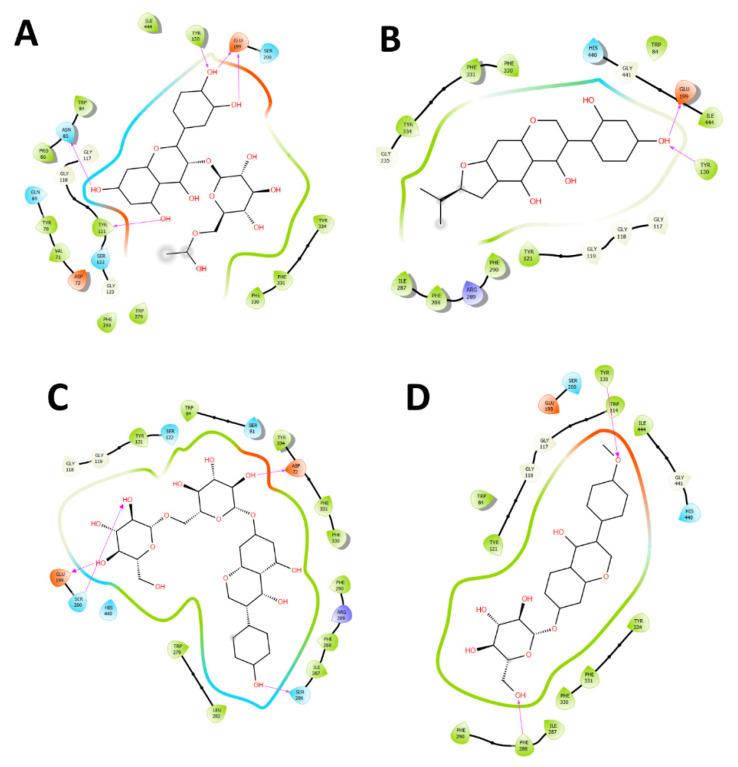
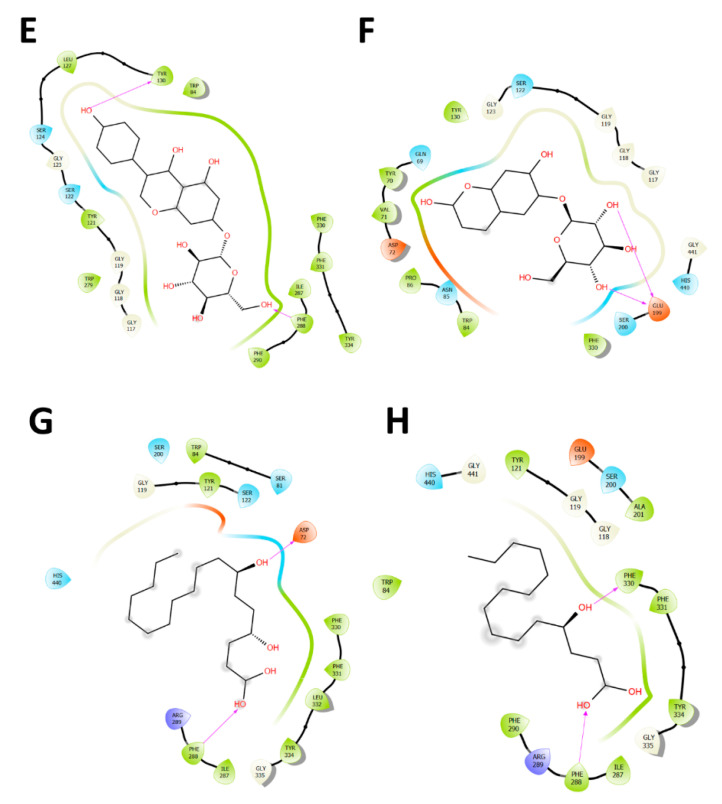
Pulp and seeds extract presented considerably abilities to exert an inhibitory potency over the TcAChE enzyme (IC50 = 4.49 ± 0.08 for pulp extract and IC50 = 4.38 ± 0.04 for seeds extract) considering the known cholinesterase inhibitor galantamine (see Table 3). In this sense, Figure 3 shows the hydrogen bond interactions in a two-dimensional diagram of each main and most abundant compounds determined from both extracts into the TcAChE catalytic site to summarize the information.
Figure 3.
Two-dimensional diagram of Torpedo californica acetylcholinesterase (TcAChE) catalytic site and hydrogen bond interactions of each main and most abundant compounds obtained from the pulp and seeds extracts. (A) Quercetin-3-O-glucoside-acetate (flavonoid); (B) Lupinisoflavone (isoflavone); (C) Genistein-7-O-di-glucoside (isoflavone); (D) Ononin (formononetin 7-O-glucoside) (isoflavone); (E) Genistein-7-O-glucoside (isoflavone); (F) Aesculetin-7-O-glucuronide (coumarin); (G) Dihydroxy-octadecaenoic acid (fatty acid); (H) Hydroxy-pentadecanoic acid (fatty acid).
2.4.2. Butyrylcholinesterase (hBuChE) Docking Results
All binding energies obtained from docking assays over butyrylcholinesterase (hBChE) of the most abundant compounds in the pulp and the seeds extracts were shown to be poorer compared to those in TcAChE. These results are consistent with the less inhibitory activity of the extracts over this enzyme shown in Table 2 (IC50 = 73.86 ± 0.09 for pulp extract and IC50 = 78.57 ± 0.06 for seeds extract).
Just like in TcAChE, the flavonoid quercetin-3-O-glucoside-acetate exhibited the best binding energy profile, suggesting that this derivative could be the main responsible for the inhibitory activity over the hBChE.
3. Materials and Methods
3.1. Chemicals and Plant Material
HPLC grade solvents (ethanol, formic acid, and acetonitrile) were obtained from Merck (Santiago, Chile). Ultrapure water was obtained from a water system of purification (Milli-Q Merck Millipore, Chile). Flavonol standards (catechin, isoflavones, and flavonoids, with a high purity: 95% by HPLC) were acquired from ChromaDex (Santa Ana, CA, USA), Sigma-Aldrich (Saint Louis, Mo, USA), or Extrasynthèse (Genay, France). Folin-Ciocalteu reagent, NaOH, Na2CO3, AlCl3, FeCl3, HCl, NaNO2, trichloroacetic acid, quercetin, Trolox sodium acetate, gallic acid, 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), nitroblue tetrazolium, xanthine oxidase, and DPPH (1,1-diphenyl-2-picrylhydrazyl radical), acetylcholinesterase (AChE) from electric eel (Torpedo californica), butylcholinesterase (BChE) from horse serum, 5,5′-dithiobis(2-nitrobenzoic) acid (DTNB), acetylthiocholine iodide, butyrylthiocholine chloride, and galantamine were purchased from Sigma-Aldrich Chemical Company (Santiago, Chile). G. sphacelata fruits were collected in November 2016 in Valdivia, XIV Region de Los Ríos, Chile. The fruits were identified by Jorge Macaya (Universidad de Chile) and a voucher specimen were kept at the Institute of Pharmacy of the Universidad Austral de Chile under number GS20161115.
3.2. Fruit Processing
Five g of pulp and seeds, separately, were chopped and lyophilized (FreeZone 2.5 L Labconco, USA). The material was extracted (20% w/v) with a mixture of ethanol-distilled water (1:1 v/v) as solvent (at 25 °C, for two h in an ultrasonic bath). The extract was filtered, and the solvent was evaporated under vacuum at 45 °C. The extract was frozen and lyophilized, until a yield of 327.3 and 125.8 mg of dark brown gums (6.54 and 2.51%) from pulp and seeds, respectively.
3.3. UHPLC-PDA-Orbitrap-MS
The Dionex Thermo Scientific Ultimate 3000 UHPLC system connected with a Thermo Q Exactive Focus machine was used as previously informed [33]. Samples were re-dissolved (2 mg/mL) in ethanol-distilled water (1:1 v/v) and 10 µL of filtered solution (PTFE filter) were injected in the instrument, as previously discussed [33].
3.4. LC Parameters and MS Parameters
Liquid chromatography (LC) was executed by a C-18 Acclaim UHPLC column (×4.6 mm ID 150 mm, 2.5 µm, Thermo Fisher Scientific, Bremen, Germany) set at 25 °C. The wavelength detection was set at: 280, 354, 254, and 330 nm, and DAD was attained from 200 to 800 nm for full characterization of peaks. Mobile phases employed were acetonitrile (B) and 1% formic aqueous solution (A) while the gradient program was: (0.00 min, 5% B); (5.00 min, 5% B); (10.00 min, 30% B); (15.00 min, 30% B); (20.00 min, 70% B); (25.00 min, 70% B); (35.00 min, 5% B); and 12 min for column equilibration before injections. The flow rate employed was 1.00 mL min−1, and the injection volume was 10 µL. Standards and the fruit extracts dissolved in methanol were maintained at 10 °C during storage in the auto-sampler. The HESI II and Orbitrap spectrometer parameters were set as informed previously [34].
3.5. Total Phenolic (TP) and Total Flavonoid (TF) Content
The total phenolic contents and total flavonoid content of the G. sphacelata fruits were measured using the Folin-Ciocalteu and FeCl3 method previously described by our work with some modifications [35]. For TP, the results were expressed as mg of gallic acid equivalents per gram of dry fruit. While for the TF content results were presented as mg of quercetin equivalents per gram of dry fruit. The determination was performed using a Synergy HTX microplate reader (Biotek, Winoosky, VT, USA), in triplicate and reported as the mean ± SD.
3.6. Antioxidant Assays
3.6.1. DPPH Cation Radical Discoloration Test
The method previously reported by Brand- Williams et al., was used to determine radical DPPH scavenging activity. Briefly, 2 mL of the DPPH solution was added in 400 μL of the extract (2 mg/mL) and mixed and 1.10 ± 0.02 at 517 nm absorbance was adjusted with methanol. This homogenized mixture of fruit extract and DPPH solution was then kept in a dark environment for a period of 20 min at room temperature [36]. Finally, the absorbance was calculated at 517 nm. The inhibition percentage was measured by a given formula:
| Percentage Inhibition = [1 − (S.A/B.A)] × 100 | (1) |
where S.A. is sample absorbance and B.A. is used for blank absorbance.
3.6.2. Bleaching Test with the Cationic Radical ABTS•+
The ABTS•+ radical capacity was evaluated using the decolorization method described by Kuskoski et al., 2004 [37]. The ABTS solution (7 mM) and potassium persulfate (2.45 mM) solution were prepared and mixed in a ratio of 1:1. The resultant solution was incubated for 16 h. Using 96-well plates, 275 µL of the solution (absorbance 0.7) was mixed with 25 µL of samples/standard. The absorbances were measured at 734 nm after 6 min of incubation period at 30 °C using a Synergy HTX microplate reader. Results were expressed as µmol Trolox per milliliters.
3.6.3. Ferric Reduction Ability-Antioxidant Power Test (FRAP)
The FRAP test was performed with the previously described protocol by Benzie and Strain, with a slight modification [38]. Briefly, the FRAP solution (2 mL) mixed with 200 μL of extract (2 mg/mL), was stirred and kept in the dark for 5 min. The absorbance was measured at 595 nm.
3.6.4. Superoxide Anion Scavenging Assay
The assay was carried using xanthine oxidase and hypoxanthine, and the absorbances were measured at 560 nm according to the reported with some modifications [13]. The production of the enzyme xanthine oxidase of superoxide anion radical (O2−) reduces the NBT (nitro blue tetrazolium) dye, producing a chromophore that absorbs at 520 nm. The superoxide anion trapping capacities of the extracts were measured using a Synergy HTX microplate reader (Biotek, CA, USA).
3.7. Cholinesterases (ChE) Inhibitory Activity
Ellman’s method was used to determinate the inhibitory activity of G. sphacelata fruits [39]. DTNB was dissolved in Tris HCl (pH 8) containing 0.02 M of MgCl2 and 0.1 M NaCl. The sample solution (50 mL dH2O, 2 mg L−1) was mixed with DTNB (125 mL), acetylcholinesterase (AChE; or BChE) (25 mL), while the blank sample as a control contained all the solutions except enzymes and was distributed in a 96-well microplate. The acetyl-thiocholine iodide (ATCI) or butyryl-thiocholine chloride (BTCl) (25 mL) was added to start the reaction. The reading was taken on a 405 nm absorbance after incubation at 25 °C for 15 min. The cholinesterase inhibitory activity was measured as IC50 (μg mL−1, concentration range 0.05 to 25 μg mL−1) by subtracting the absorbance of the sample from blank. Galantamine was used as a positive control. All data were collected in triplicate. In addition, 0.26 units/mL of each enzyme were used for the inhibition assays.
3.8. Docking Studies
The geometries and partial charges of several representative compounds contained in the extracts, as well as the known cholinesterases (TcAChE- hBChE) inhibitor Galantamine were fully optimized using the DFT method with the standard basis set PBEPBE/6-311 + g* [40,41]. All calculations were performed in the Gaussian 09W software.
3.9. Statistical Analysis
All the experiments were repeated five times to confirm the results and minimize the error and the data was presented as the mean of standard deviation. The results were analyzed using one-way analysis of variance (ANOVA) and Tuckey test statistical analysis (p-values < 0.05 were regarded as significant) using the origin Pro 9.0 software package (Origin lab Corporation, Northampton, MA, USA).
4. Conclusions
From the edible endemic Chilean G. sphacelata fruit seventy metabolites were detected using UHPLC-PDA-Orbitrap-MS analysis including phenolic acids, organic acids, sugar derivatives, catechins, proanthocyanidins, fatty acids, iridoids, coumarins, a benzophenone, flavonoids, and terpenes. G. sphacelata showed a good phenolic content and moderate antioxidant and enzyme inhibitory activities. This report could contribute for the better understanding of chemistry and biological activities in the genus Greigia. Furthermore, the list of compounds profiled with a cholinesterase inhibitory activity plus antioxidant potential make G. sphacelata fruits an interesting source of compounds with interesting properties to prepare functional foods or food derived supplements.
Acknowledgments
We acknowledge Milena Rios for her help in vitro microplate assays.
Supplementary Materials
The following are available online. Figure S1 (a–v): Full MS spectra and structures of compounds 1, 3, 7, 9, 12, 13, 15, 25, 27, 28, 29, 35, 37, 42, 43, 44, 46, 48, 51, 53, and 55; Table S1: Binding energies obtained from docking experiments of most abundant compounds in G. sphacelata’s fruit and the known cholinesterase inhibitor galantamine over acetylcholinesterase (TcAChE) and butyrylcholinesterase (hBuChE); Figure S2: Components of G. sphacelata fruits used in docking studies Figure S3: Predicted binding mode and predicted intermolecular interactions of all most abundant compounds in G. sphacelata pulp and seeds extracts and the residues of Torpedo californica acetylcholinesterase (TcAChE) catalytic site; Figure S4: Predicted binding mode and predicted intermolecular interactions of all most abundant compounds in G. sphacelata pulp and seeds extracts and the residues of human butyrylcholinesterase (hBuChE) catalytic site.
Author Contributions
R.E.B., S.A., and C.C. performed the LC-MS experiments and antioxidant and enzyme inhibitory assays; J.R.-P. performed the docking experiments; M.J.S., C.F.-G., S.A., and J.E. arranged the data and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funds from FONDECYT 1180059 and CONICYT PFCHA/beca doctorado nacional/2019-21191978. J.E. gratefully acknowledges funding from CONICYT (PAI/ACADEMIA No. 79160109). Postdoctorate grants 3190794 (C.F.-G.) and 3190572 (S.A.) are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Slavin J.L., Lloyd B. Health benefits of fruits and vegetables. Adv. Nutr. 2012;3:506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung A.W.K., Aggarwal B.B., Barreca D., Battino M., Belwal T., Horbańczuk O.K., Berindan-Neagoe I., Bishayee A., Daglia M., Devkota H.P., et al. Dietary natural products and their potential to influence health and disease including animal model studies. Anim. Sci. Pap. Rep. 2019;36:345–358. [Google Scholar]
- 3.Afrin S., Giampieri F., Gasparrini M., Forbes-Hernandez T.Y., Varela-López A., Quiles J.L., Mezzetti B., Battino M. Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules. 2016;21:169. doi: 10.3390/molecules21020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forino M., Tartaglione L., Dell’Aversano C., Ciminiello P. NMR-based identification of the phenolic profile of fruits of Lycium barbarum (goji berries). Isolation and structural determination of a novel N-feruloyl tyramine dimer as the most abundant antioxidant polyphenol of goji berries. Food Chem. 2016;194:1254–1259. doi: 10.1016/j.foodchem.2015.08.129. [DOI] [PubMed] [Google Scholar]
- 5.Belwal T., Bisht A., Devkota H.P., Ullah H., Khan H., Pandey A., Bhatt I.D., Echeverría J. Phytopharmacology and clinical updates of Berberis species against diabetes and other metabolic diseases. Front. Pharm. 2020;11:41. doi: 10.3389/fphar.2020.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Areche C., Hernandez M., Cano T., Ticona J., Cortes C., Simirgiotis M., Caceres F., Borquez J., Echeverría J., Sepulveda B. Corryocactus brevistylus (K. Schum. ex Vaupel) Britton & Rose (Cactaceae): Antioxidant, gastroprotective effects, and metabolomic profiling by ultrahigh-pressure liquid chromatography and electrospray high resolution orbitrap tandem mass spectrometry. Front. Pharm. 2020;11:417. doi: 10.3389/fphar.2020.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q., Chen W., Zhao J., Xi W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016;200:230–236. doi: 10.1016/j.foodchem.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 8.Simirgiotis M.J., Ramirez J.E., Schmeda Hirschmann G., Kennelly E.J. Bioactive coumarins and HPLC-PDA-ESI-ToF-MS metabolic profiling of edible queule fruits (Gomortega keule), an endangered endemic Chilean species. Food Res. Int. 2013;54:532–543. doi: 10.1016/j.foodres.2013.07.022. [DOI] [Google Scholar]
- 9.Li J., Yuan C., Pan L., Benatrehina P.A., Chai H., Keller W.J., Naman C.B., Kinghorn A.D. Bioassay-guided isolation of antioxidant and cytoprotective constituents from a maqui berry (Aristotelia chilensis) dietary supplement ingredient as markers for qualitative and quantitative analysis. J. Agric. Food Chem. 2017;65:8634–8642. doi: 10.1021/acs.jafc.7b03261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Will B., Zizka G.A. Review of the genus Greigia Regel (Bromeliaceae) in Chile. Harv. Pap. Bot. 1999;4:225–239. [Google Scholar]
- 11.Flagg M.L., Wächter G.A., Davis A.L., Montenegro G., Timmermann B.N. Two novel flavanones from Greigia sphacelata. J. Nat. Prod. 2000;63:1689–1691. doi: 10.1021/np0003387. [DOI] [PubMed] [Google Scholar]
- 12.Donno D., Beccaro G.L., Mellano M.G., Cerutti A.K., Bounous G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Foods. 2015;18:1070–1085. doi: 10.1016/j.jff.2014.05.020. [DOI] [Google Scholar]
- 13.Simirgiotis M.J., Bórquez J., Schmeda-Hirschmann G. Antioxidant capacity, polyphenolic content and tandem HPLC-DAD-ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. chequen. Food Chem. 2013;139:289–299. doi: 10.1016/j.foodchem.2013.01.089. [DOI] [PubMed] [Google Scholar]
- 14.Fu C., Liu M., Li Y., Wang K., Yang B., Deng L., Tian J., Yang G., Zheng G. UPLC-Q-exactive orbitrap MS analysis for identification of lipophilic components in citri sarcodactylis fructus from different origins in China using supercritical CO2 fluid extraction method. ACS Omega. 2020;5:11013–11023. doi: 10.1021/acsomega.0c00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrientos R., Fernández-Galleguillos C., Pastene E., Simirgiotis M., Romero-Parra J., Ahmed S., Echeverría J. Metabolomic analysis, fast isolation of phenolic compounds, and evaluation of biological activities of the bark from Weinmannia trichosperma Cav. (Cunoniaceae) Front. Pharm. 2020;11:780. doi: 10.3389/fphar.2020.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spínola V., Mendes B., Câmara J.S., Castilho P.C. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal. Bioanal. Chem. 2012;403:1049–1058. doi: 10.1007/s00216-011-5668-x. [DOI] [PubMed] [Google Scholar]
- 17.Lim V., Gorji S.G., Daygon V.D., Fitzgerald M. Untargeted and targeted metabolomic profiling of australian indigenous fruits. Metabolites. 2020;10:114. doi: 10.3390/metabo10030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinda B. Pharmacology of Iridoids. In: Dinda B., editor. Pharmacology and Applications of Naturally Occurring Iridoids. Springer International Publishing; Cham, Switzerland: 2019. pp. 145–254. [Google Scholar]
- 19.Chang J., Xuan L.J., Xu Y.M., Zhang J.S. Seven new sesquiterpene glycosides from the root bark of Dictamnus dasycarpus. J. Nat. Prod. 2001;64:935–938. doi: 10.1021/np000567t. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez J.E., Zambrano R., Sepúlveda B., Kennelly E.J., Simirgiotis M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC–HR-ESI-ToF-MS. Food Chem. 2015;176:106–114. doi: 10.1016/j.foodchem.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez K., Ah-Hen K., Vega-Gálvez A., López J., Quispe-Fuentes I., Lemus-Mondaca R., Gálvez-Ranilla L. Changes in bioactive compounds and antioxidant activity during convective drying of murta (Ugni molinae T.) berries. Int. J. Food Sci. Technol. 2014;49:990–1000. doi: 10.1111/ijfs.12392. [DOI] [Google Scholar]
- 22.Genskowsky E., Puente L.A., Pérez-Álvarez J.A., Fernández-López J., Muñoz L.A., Viuda-Martos M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016;96:4235–4242. doi: 10.1002/jsfa.7628. [DOI] [PubMed] [Google Scholar]
- 23.Gironés-Vilaplana A., Baenas N., Villaño D., Speisky H., García-Viguera C., Moreno D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods. 2014;7:599–608. doi: 10.1016/j.jff.2013.12.025. [DOI] [Google Scholar]
- 24.Xu J., Guo Y., Xie C., Jin D.-Q., Gao J., Gui L. Isolation and neuroprotective activities of acylated iridoids from Valeriana jatamansi. Chem. Biodivers. 2012;9:1382–1388. doi: 10.1002/cbdv.201100238. [DOI] [PubMed] [Google Scholar]
- 25.Xu J., Li Y., Guo Y., Guo P., Yamakuni T., Ohizumi Y. Isolation, structural elucidation, and neuroprotective effects of iridoids from Valeriana jatamansi. Biosci. Biotechnol. Biochem. 2012;76:1401–1403. doi: 10.1271/bbb.120097. [DOI] [PubMed] [Google Scholar]
- 26.Jugran A.K., Rawat S., Bhatt I.D., Rawal R.S. Valeriana jatamansi: An herbaceous plant with multiple medicinal uses. Phyther. Res. 2019;33:482–503. doi: 10.1002/ptr.6245. [DOI] [PubMed] [Google Scholar]
- 27.De Jesus R.A.P., Cechinel-Filho V., Oliveira A.E., Schlemper V. Analysis of the antinociceptive properties of marrubiin isolated from Marrubium vulgare. Phytomedicine. 2000;7:111–115. doi: 10.1016/S0944-7113(00)80082-3. [DOI] [PubMed] [Google Scholar]
- 28.Teh S.S., Ee G.C.L., Mah S.H., Ahmad Z. Structure–activity relationship study of secondary metabolites from Mesua beccariana, Mesua ferrea and Mesua congestiflora for anti-cholinesterase activity. Med. Chem. Res. 2016;25:819–823. doi: 10.1007/s00044-016-1531-0. [DOI] [Google Scholar]
- 29.Woo M.H., Nguyen D.H., Choi J.S., Park S.E., Thuong P.T., Min B.S., Le D.D. Chemical constituents from the roots of Kadsura coccinea with their protein tyrosine phosphatase 1B and acetylcholinesterase inhibitory activities. Arch. Pharm. Res. 2020;43:204–213. doi: 10.1007/s12272-020-01211-8. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.H., Choi G.N., Kwak J.H., Jeong H.R., Jeong C.-H., Heo H.J. Neuronal cell protection and acetylcholinesterase inhibitory effect of the phenolics in chestnut inner skin. Food Sci. Biotechnol. 2011;20:311–318. doi: 10.1007/s10068-011-0044-3. [DOI] [Google Scholar]
- 31.Gilani A.H., Ghayur M.N., Saify Z.S., Ahmed S.P., Choudhary M.I., Khalid A. Presence of cholinomimetic and acetylcholinesterase inhibitory constituents in betel nut. Life Sci. 2004;75:2377–2389. doi: 10.1016/j.lfs.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 32.Floris S., Fais A., Rosa A., Piras A., Marzouki H., Medda R., González-Paramás A.M., Kumar A., Santos-Buelga C., Era B. Phytochemical composition and the cholinesterase and xanthine oxidase inhibitory properties of seed extracts from the Washingtonia filifera palm fruit. RSC Adv. 2019;9:21278–21287. doi: 10.1039/C9RA02928A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simirgiotis J.M., Quispe C., Bórquez J., Areche C., Sepúlveda B. Fast detection of phenolic compounds in extracts of easter pears (Pyrus communis) from the Atacama Desert by ultrahigh-performance liquid chromatography and mass spectrometry (UHPLC–Q/Orbitrap/MS/MS) Molecules. 2016;21:92. doi: 10.3390/molecules21010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simirgiotis M.J., Quispe C., Bórquez J., Schmeda-Hirschmann G., Avendaño M., Sepúlveda B., Winterhalter P. Fast high resolution Orbitrap MS fingerprinting of the resin of Heliotropium taltalense Phil. from the Atacama Desert. Ind. Crop. Prod. 2016;85:159–166. doi: 10.1016/j.indcrop.2016.02.054. [DOI] [Google Scholar]
- 35.Quesada-Romero L., Fernández-Galleguillos C., Bergmann J., Amorós M.-E., Jiménez-Aspee F., González A., Simirgiotis M., Rossini C. Phenolic fingerprinting, antioxidant, and deterrent potentials of Persicaria maculosa Extracts. Molecules. 2020;25:3054. doi: 10.3390/molecules25133054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 37.Kuskoski E.M., Asuero A.G., García-Parilla M.C., Troncoso A.M., Fett R. Actividad antioxidante de pigmentos antociánicos. Ciênc. E Tecnol. Aliment. 2004;24:691–693. doi: 10.1590/S0101-20612004000400036. [DOI] [Google Scholar]
- 38.Benzie I., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 39.Mocan A., Moldovan C., Zengin G., Bender O., Locatelli M., Simirgiotis M., Atalay A., Vodnar D.C., Rohn S., Crișan G. UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory, and real-time cytotoxicological evaluation. Food Chem. Toxicol. 2018;115:414–424. doi: 10.1016/j.fct.2018.01.054. [DOI] [PubMed] [Google Scholar]
- 40.Adamo C., Barone V. Toward reliable density functional methods without adjustable parameters: The PBE0 model Seeking for parameter-free double-hybrid functionals: The PBE0-DH model accurate excitation energies from time-dependent density functional theory: Assessing the PBE0 model toward reliable density functional methods without adjustable parameters: The PBE0 model. Cit. J. Chem. Phys. 1999;110:2889. doi: 10.1063/1.478522. [DOI] [Google Scholar]
- 41.Petersson G.A., Bennett A., Tensfeldt T.G., Al-Laham M.A., Shirley W.A., Mantzaris J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988;89:2193–2218. doi: 10.1063/1.455064. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.