Abstract
Polypropylene (PP) is a commodity plastic known for high rigidity and crystallinity, which is suitable for a wide range of applications. However, high flammability of PP has always been noticed by users as a constraint; therefore, a variety of additives has been examined to make PP flame-retardant. In this work, research papers on the flame retardancy of PP have been comprehensively reviewed, classified in terms of flame retardancy, and evaluated based on the universal dimensionless criterion of Flame Retardancy Index (FRI). The classification of additives of well-known families, i.e., phosphorus-based, nitrogen-based, mineral, carbon-based, bio-based, and hybrid flame retardants composed of two or more additives, was reflected in FRI mirror calculated from cone calorimetry data, whatever heat flux and sample thickness in a given series of samples. PP composites were categorized in terms of flame retardancy performance as Poor, Good, or Excellent cases. It also attempted to correlate other criteria like UL-94 and limiting oxygen index (LOI) with FRI values, giving a broad view of flame retardancy performance of PP composites. The collected data and the conclusions presented in this survey should help researchers working in the field to select the best additives among possibilities for making the PP sufficiently flame-retardant for advanced applications.
Keywords: flame retardancy, polypropylene, Flame Retardancy Index (FRI), cone calorimetry, flame retardants
1. Introduction
Polymers are building blocks of advanced materials and systems, but their flammability has been a serious constraint in their usage in advanced applications [1,2,3]. Polypropylene (PP) is a commodity plastic widely used in a variety of applications, particularly in the form of composites in load-bearing uses due to its high rigidity and crystallinity [4]. By the end of 2020, the PP market size is expected to reach $112 billion, and it is estimated to reach $155 billion by 2026 [5,6]. Its global production was 56.0 million metric tons in 2018, and it is estimated to reach around 88.0 million metric tons by 2026. This growing demand reflects the importance of PP for applications where low density, hardness, high flexural modulus, and chemical resistance are needed [7,8]. Moreover, PP is a low-cost plastic capable of being processed with various methods, e.g., extrusion, thermoforming, and injection molding [9,10]. Therefore, a huge number of PP products, including fibers, films, sheets, textiles, pipes, and profiles, have been developed and used in the automotive, electrical and electronic, packaging, and construction industries [11,12,13,14]. On the other hand, due to the inherent flammability, the use of flame-retardant additives in PP is necessary to minimize the risk of fire [15]. Different types of flame retardants have been used in PP including minerals, phosphorus-based, nitrogen-based, and intumescent [16,17,18]. It was recognized that additive selection plays a crucial role in achieving acceptable flame retardancy [19], where the type, the size, and the loading percentage of flame retardants control the fire behavior of PP matrix.
A diversity of additives are used in PP to make it flame retardant. There is a need for a comprehensive survey to classify PP composites in terms of flame retardancy. In the present paper, several families of flame retardants examined in PP have been identified and categorized to evaluate their flame retardancy performance in terms of Flame Retardancy Index (FRI) [19,20]. FRI is a universal dimensionless index that takes into account well-known parameters obtained from cone calorimeter test (peak of heat release rate (pHRR), the total heat release (THR), and the time to ignition (TTI)). FRI can be simply calculated using Equation (1):
| (1) |
Basically, the use of FRI makes it possible to semi-qualitatively classify polymer composites by labeling them as Poor, Good, or Excellent flame retardancy performance and thus enables evaluation of the efficiency of the incorporated flame retardant (FR). There has always been a need for fast-tracking and classifying polymers for their flame retardant performance. The use of FRI made possible classifying polymers and polymer composites in terms of flame retardancy in a simple manner. For FRI values below 100 obtained by the use of Equation (1), we have the case (namely Poor) where the addition of FR adversely affects flame retardancy of polymer. When FRI takes values in the range of 100–101, we name it Good flame retardancy performance, such that addition of FR enhances the resistance of polymer against fire. For FRI values above 101, which is rare in practical cases, we have an Excellent case, where FR significantly improves flame retardancy. It is worth mentioning that some important parameters of testing such as irradiance and sample thickness as well as sample weight can be neglected due to the fact that, in the FRI formula, the parameters related to the neat polymer are divided by those of polymer/FR composite. Thus, the dimensionless value obtained can be used as a reliable measure of the efficiency of FR in polymer. In this survey, the data from the literature were extracted first, and five families of flame retardants that served as PP were considered including phosphorus-based, nitrogen-based, mineral, carbon-based, and bio-based flame retardants, and hybrid cases composed of the aforementioned five categories were distinguished. The main aim of the present survey is to give the readers a broad view of FR systems used in PP via FRI classification method. Certainly, this classification is not a precise and unique data set for FR selection for PP, but it can be considered as a database to compare different systems. The focus of this work was particularly placed on the reports in which cone calorimetry test was carried out. However, some other parameters such as smoke quantity or the percentage of FR elements (phosphorus, nitrogen, …) were not systematically given in this research paper due to the lack of data, which could lead to unreliable judgments. For some papers, limiting oxygen index (LOI) and UL-94 data were also available, which were used in finding possible correlations between the FRI variation and other criteria.
2. Phosphorus-Based Flame Retardants
Various types of phosphorus-based flame retardants have been incorporated into PP to make it flame-retardant [21,22,23]. Table 1 reviews the names and the percentages of these flame retardants incorporated into PP. Moreover, the values obtained from cone calorimetry such as the peak of heat release rate (pHRR), the total heat release (THR), and the time to ignition (TTI) are summarized in this Table. The FRI value, calculated from cone calorimetry parameters, as well as the LOI and UL-94 values, are also presented in Table 1. In some cases, if LOI and/or UL-94 values were not available, the sign “―” was used.
Table 1.
Flame-retardant PP materials containing phosphorus-based (P) flame retardants. Data are extracted from the literature: cone calorimetry parameters (TTI, pHRR, THR), LOI, and UL-94 values. The FRI values were calculated by authors of the present review. The name and the percentage of flame retardants are provided in separate columns. “wt.%” was used for loading level of additives, while “―” stands for the systems free of additive or the neat PP. * FR means flame retardant. Since all comparisons were made in terms of FRI, classification of polymers in terms of their flame retardant properties was not surveyed based on the chemistry of additives, heat flux, sample thickness, etc.
| PP Containing Phosphorus-Based (P) FR * | wt.% | TTI (s) | pHRR (kW·m−2) |
THR (MJ·m−2) |
Irradiance (kW·m−2) |
Sample Thickness (mm) | FRI | LOI | UL-94 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| ― | 14 | 1104 | 106 | 35 | 0.4 | ― | ― | ― | [24] | |
| Ammonium polyphosphate (APP) | 10 | 24 | 925 | 92 | 35 | 0.4 | 2.35 | ― | ― | [24] |
| ― | 54 | 1610 | 106 | 35 | 3 | ― | 20.8 | NR | [25] | |
| APP | 12 | 37 | 510 | 97 | 35 | 3 | 2.36 | 22.3 | V-2 | [25] |
| APP | 15 | 27 | 339 | 89 | 35 | 3 | 2.82 | 25.4 | V-0 | [25] |
| ― | 34 | 1294 | 154.2 | 50 | 4 | ― | 19 | NR | [26] | |
| APP | 20 | 21 | 306 | 141.6 | 50 | 4 | 2.84 | 27 | NR | [26] |
| ― | 48 | 1351 | 107 | 35 | 3.2 | ― | 18.5 | NR | [27] | |
| APP | 20 | 40 | 787 | 92 | 35 | 3.2 | 1.66 | 20.5 | NR | [27] |
| ― | 24.3 | 1388.3 | 80.3 | 50 | 2.4 | ― | ― | NR | [28] | |
| APP | 20 | 19.3 | 254.8 | 54.5 | 50 | 2.4 | 6.37 | ― | V-0 | [28] |
| ― | 66 | 633 | 44.2 | 35 | 3 | ― | 17 | NR | [23] | |
| APP | 20 | 31 | 424 | 38.6 | 35 | 3 | 0.80 | 21 | NR | [23] |
| ― | 18 | 1457 | 156 | 50 | 3 | ― | 19 | NR | [29] | |
| APP | 25 | 20 | 1455 | 148 | 50 | 3 | 1.17 | 21.9 | V-2 | [29] |
| ― | 25 | 981 | 147 | 50 | ― | ― | 17.6 | NR | [30] | |
| APP | 25 | 18 | 579 | 109 | 50 | ― | 1.64 | 23.2 | NR | [30] |
| ― | 48 | 988 | 88.3 | 35 | 3.2 | ― | 17 | NR | [31] | |
| APP | 25 | 43 | 652 | 80 | 35 | 3.2 | 1.49 | 21 | NR | [31] |
| ― | 20 | 809 | 96 | 50 | 3 | ― | 17.6 | NR | [32] | |
| APP | 25 | 11 | 397 | 87 | 50 | 3 | 1.23 | 20.6 | NR | [32] |
| ― | 21 | 1242 | 111 | 50 | 3.2 | ― | 18.6 | NR | [33] | |
| APP | 25 | 21 | 979 | 107 | 50 | 3.2 | 1.31 | 21.7 | NR | [33] |
| ― | 35 | 1203 | 197.6 | 50 | 6 | ― | 18.2 | NR | [34] | |
| APP | 25 | 33 | 390.8 | 196 | 50 | 6 | 2.92 | 20.9 | NR | [34] |
| ― | 25 | 841.6 | 89.1 | 50 | 3 | ― | 18 | NR | [35] | |
| APP | 25 | 13 | 473.3 | 90.2 | 50 | 3 | 0.91 | 20 | NR | [35] |
| Piperazine-modified APP (m-APP) | 25 | 17 | 162.6 | 84.5 | 50 | 3 | 3.71 | 32.5 | V-0 | [35] |
| ― | 33 | 1416 | 219 | 50 | 6 | ― | 17 | NR | [36] | |
| APP | 25 | 19 | 526 | 180 | 50 | 6 | 1.88 | 19.6 | NR | [36] |
| Polysiloxane shell-coated APP (mc-APP) | 25 | 19 | 214 | 137 | 50 | 6 | 6.08 | 25 | NR | [36] |
| ― | 45 | 759.2 | 98.8 | 35 | 3 | ― | 17 | NR | [37] | |
| Melamine and phytic acid-modified APP (m-APP) | 25 | 33 | 218.1 | 80.6 | 35 | 3 | 3.12 | 22.5 | V-2 | [37] |
| ― | 37 | 1284 | 121 | 50 | 3 | ― | ― | ― | [38] | |
| APP | 30 | 22 | 767 | 111 | 50 | 3 | 1.08 | 21.7 | NR | [38] |
| ― | 48 | 988 | 88.3 | 35 | 3 | ― | 17 | NR | [39] | |
| APP | 30 | 32 | 459 | 77.6 | 35 | 3 | 1.63 | 22 | NR | [39] |
| ― | 50 | 1350 | 91.2 | 35 | 3 | ― | 17 | NR | [40] | |
| APP | 30 | 58 | 851 | 74.4 | 35 | 3 | 2.25 | 22 | NR | [40] |
| ― | 33 | 1238 | 123.7 | 50 | 3 | ― | 17.8 | NR | [41] | |
| Melamine-formaldehyde-tris(2-hydroxyethyl) isocyanurate resin microencapsulated APP (mc-APP) | 30 | 24 | 375 | 116.4 | 50 | 3 | 2.55 | 32 | V-0 | [41] |
| ― | 44 | 831 | 158 | 35 | 3 | ― | 17.5 | NR | [42] | |
| APP | 30 | 30 | 432 | 114 | 35 | 3 | 1.81 | 22 | NR | [42] |
| Dipentaerythritol and 4,4′ diphenylmethanediisocyanate and melamine microencapsulated APP (mc-APP) | 30 | 27 | 300 | 100 | 35 | 3 | 2.68 | 32.1 | V-0 | [42] |
| ― | 29 | 1186 | 215 | 50 | 6 | ― | 17 | NR | [43] | |
| APP | 30 | 18 | 543 | 180 | 50 | 6 | 1.61 | 20.1 | NR | [43] |
| Epoxy acrylate microencapsulated APP (mc-APP) | 30 | 13 | 332 | 149 | 50 | 6 | 2.31 | 24.8 | NR | [43] |
| ― | 40 | 1174.7 | 102.2 | 35 | 3 | ― | 17 | NR | [44] | |
| APP | 30 | 38 | 526.5 | 80.6 | 35 | 3 | 2.68 | 20 | NR | [44] |
| 4,4′-diphenylmethane diisocyanate and melamine and pentaerythritol microencapsulated APP (mc-APP) | 30 | 30 | 301.8 | 65.1 | 35 | 3 | 4.58 | 25 | V-1 | [44] |
| ― | 68 | 577.5 | 82.7 | 35 | 3 | ― | 18.2 | NR | [45] | |
| APP | 30 | 41 | 201.1 | 44.5 | 35 | 3 | 3.21 | 20.1 | NR | [45] |
| Thermoplastic polyurethane microencapsulated APP (mc-APP) | 5 | 57 | 395.4 | 67.2 | 35 | 3 | 1.50 | 18.7 | NR | [45] |
| Thermoplastic polyurethane microencapsulated APP (mc-APP) | 10 | 42 | 282.5 | 63.7 | 35 | 3 | 1.63 | 19.6 | NR | [45] |
| Thermoplastic polyurethane microencapsulated APP (mc-APP) | 15 | 40 | 214.9 | 59.9 | 35 | 3 | 2.18 | 20 | NR | [45] |
| Thermoplastic polyurethane microencapsulated APP (mc-APP) | 20 | 32 | 193.6 | 57.3 | 35 | 3 | 2.02 | 20.3 | NR | [45] |
| Thermoplastic polyurethane microencapsulated APP (mc-APP) | 25 | 30 | 145.4 | 64.1 | 35 | 3 | 2.26 | 22.2 | NR | [45] |
| Thermoplastic polyurethane microencapsulated APP (mc-APP) | 30 | 31 | 140.6 | 41.8 | 35 | 3 | 3.70 | 22.9 | NR | [45] |
| ― | 25 | 841.6 | 89.1 | 50 | 3 | ― | 18 | NR | [46] | |
| APP | 35 | 11 | 435.9 | 83.9 | 50 | 3 | 0.90 | 20.4 | NR | [46] |
| Ethylenediamine-modified APP (m-APP) | 35 | 11 | 156.1 | 60.5 | 50 | 3 | 3.49 | 30.5 | V-0 | [46] |
| ― | 25 | 841.6 | 89.1 | 50 | 3 | ― | 18 | NR | [47] | |
| APP | 35 | 11 | 435.9 | 83.9 | 50 | 3 | 0.90 | 20.4 | NR | [47] |
| Ethanolamine-modified APP (m-APP) | 35 | 18 | 96.6 | 22.6 | 50 | 3 | 24.73 | 35 | V-0 | [47] |
| ― | 33 | 837 | 212 | 50 | 6 | ― | 17 | NR | [48] | |
| APP | 40 | 30 | 440 | 186 | 50 | 6 | 1.97 | 20.8 | NR | [48] |
| Pentaerythritol triacrylate microencapsulated APP (mc-APP) | 40 | 32 | 214 | 183 | 50 | 6 | 4.39 | 30.5 | V-0 | [48] |
| ― | 38 | 1284 | 214 | 50 | 6 | ― | 18.2 | NR | [49] | |
| APP | 25 | 34 | 537 | 177 | 50 | 6 | 2.58 | 20.9 | NR | [49] |
| Phosphorus-based charring agent: 3,9-Bis-(1-oxo-2,6,7-trioxa-1-phospha-bicyclo[2,2,2]oct-4-ylmethoxy)-2,4,8,10-tetraoxa-3,9 diphospha-spiro[5.5]undecane 3,9-dioxide (P-CA) | 25 | 35 | 480 | 168 | 50 | 6 | 3.13 | 22.6 | NR | [49] |
| ― | 42 | 831 | 112 | 35 | 3 | ― | 18 | NR | [50] | |
| APP | 25 | 36.4 | 578 | 83 | 35 | 3 | 1.68 | 21 | NR | [50] |
| ― | 36 | 1373 | 174.8 | 50 | 3 | ― | 18.5 | NR | [51] | |
| APP-based intumescent flame retardant (APP-IFR) | 20 | 22 | 326 | 149.9 | 50 | 3 | 3.00 | 29.5 | V-0 | [51] |
| ― | 28 | 865 | 30.7 | 35 | 5 | ― | 18.4 | NR | [52] | |
| Phosphorus-based IFR: Six(1-oxo-2,6,7-trioxa-1-phosphabicyclo[2,2,2]octane-4-methyl) cyclotriphosphazene (P-IFR) | 10 | 28 | 595 | 28.2 | 35 | 5 | 1.58 | 19.7 | NR | [52] |
| Phosphorus-based IFR: Six(1-oxo-2,6,7-trioxa-1-phosphabicyclo[2,2,2]octane-4-methyl) cyclotriphosphazene (P-IFR) | 15 | 30 | 515 | 25.8 | 35 | 5 | 2.14 | 22.8 | NR | [52] |
| Phosphorus-based IFR: Six(1-oxo-2,6,7-trioxa-1-phosphabicyclo[2,2,2]octane-4-methyl) cyclotriphosphazene (P-IFR) | 20 | 33 | 433 | 23 | 35 | 5 | 3.14 | 26.1 | V-2 | [52] |
| Phosphorus-based IFR: Six(1-oxo-2,6,7-trioxa-1-phosphabicyclo[2,2,2]octane-4-methyl) cyclotriphosphazene (P-IFR) | 25 | 35 | 407 | 19.5 | 35 | 5 | 4.18 | 29.4 | V-0 | [52] |
| ― | 30 | 390 | 44 | 35 | 1.6 | ― | 17.4 | ― | [53] | |
| Phosphorus-based IFR: Poly (4,4-diamino diphenyl methane Obicyclicpentaerythritol phosphate-phosphate) (P-IFR) | 20 | 24 | 224 | 27 | 35 | 1.6 | 2.26 | 25 | ― | [53] |
| ― | 37 | 363 | 56 | 35 | 3 | ― | ― | ― | [54] | |
| Phosphorus-based IFR: compound containing Phosphorus(22%) and Nitrogene(18%) (P-IFR) | 28 | 33 | 62 | 24 | 35 | 3 | 12.18 | ― | ― | [54] |
| ― | 37 | 363 | 56 | 35 | 3 | ― | ― | ― | [55] | |
| Phosphorus-based IFR: compound containing Phosphorus(22%) and Nitrogene(18%) (P-IFR) | 28 | 33 | 62 | 24 | 35 | 3 | 12.18 | ― | ― | [55] |
| ― | 29 | 980 | 136 | 50 | ― | ― | 18.5 | ― | [56] | |
| Phosphorus and Nitrogene-based IFR | 30 | 22 | 229 | 93 | 50 | ― | 4.74 | 36.3 | ― | [56] |
| ― | 65 | 1416.6 | 128.5 | 35 | 3 | ― | ― | NR | [57] | |
| Phosphorus-based flame retardant: Tri (1-oxo-2,6,7-trioxa-1-phosphabicyclo [2,2,2] octane-methyl) phosphate (P-FR) | 30 | 38 | 640.2 | 104.8 | 35 | 3 | 1.58 | ― | NR | [57] |
| ― | 54 | 1199 | 97.8 | 35 | ― | ― | ― | ― | [58] | |
| Phosphorus-based FR: Poly(4,4-diaminodiphenyl methane spirocyclicpentaerythritol bisphosphonate) (P-FR) | 20 | 69 | 620 | 78.5 | 35 | ― | 3.07 | ― | ― | [58] |
| ― | 61 | 1026 | 166 | 35 | 4 | ― | ― | ― | [15] | |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 10 | 60 | 648 | 141 | 35 | 4 | 1.83 | ― | ― | [15] |
| ― | 84 | 1000 | 96 | 35 | 3 | ― | ― | ― | [59] | |
| Tetraethyl orthosilicate microencapsulated bisphenol-A bis (diphenyl phosphate) (mc-BDP) | 10 | 57 | 808 | 101 | 35 | 3 | 0.79 | ― | ― | [59] |
| Tetraethyl orthosilicate microencapsulated BDP (mc-BDP) | 20 | 60 | 932 | 93 | 35 | 3 | 0.79 | ― | ― | [59] |
| ― | 44 | 1172 | 87.1 | 35 | 2.5 | ― | 18.1 | NR | [60] | |
| Organic phosphinate (OP) | 20 | 46 | 1052 | 84.2 | 35 | 2.5 | 1.20 | 20.1 | V-2 | [60] |
| ― | 34 | 1052.4 | 90.8 | 50 | 3 | ― | 17.5 | NR | [61] | |
| Aluminum hypophosphite (AHP) | 24 | 23 | 267.1 | 77.3 | 50 | 3 | 3.13 | ― | ― | [61] |
| ― | 66 | 480 | 93 | 35 | 3 | ― | 17 | ― | [62] | |
| Aluminium phosphinate (ALPi) | 30 | 73 | 524 | 89.8 | 35 | 3 | 1.04 | 26 | ― | [62] |
| ― | 44 | 1175 | 106 | 35 | 3 | ― | ― | ― | [63] | |
| Pentaerythritol phosphate (PEPA) | 40 | 35 | 776 | 81 | 35 | 3 | 1.57 | ― | ― | [63] |
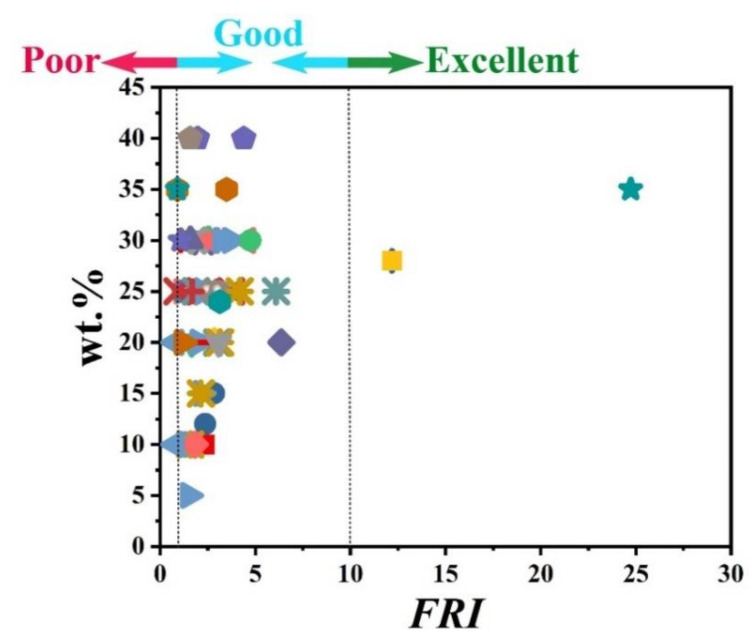
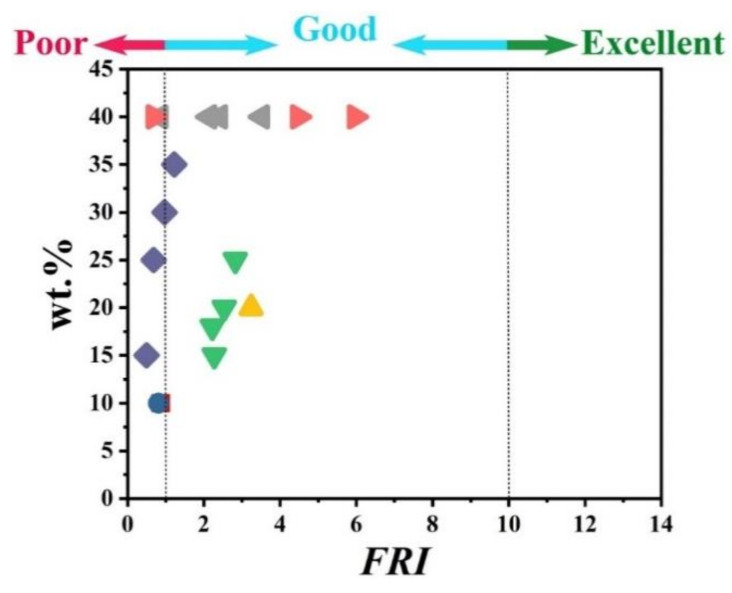
The information provided in Table 1 clearly reveals that APP is quite frequently used as a major phosphorus flame retardant in PP matrix. The percentage of incorporation of phosphorus flame retardants was variable from 10 to 40 wt.%. Figure 1 displays the FRI as a function of wt.% phosphorus-based FR in PP systems. The name/type of each phosphorus flame retardant is provided in the caption of Figure 1. Three formulations reached the Excellent level of flame retardancy, which is quite rare among such data pool. The loading percentage of FR in these formulations varied from 28 to 35 wt.%. Many additives were modified APP and modified phosphorus-nitrogen flame retardants. It can also be speculated that a high loading percentage cannot necessarily guarantee the Excellent level of flame retardancy; besides, the type of phosphorus FR is also an important parameter. Figure 1 also reveals that the majority of points are located in the Good zone of FRI. Therefore, it can be concluded that phosphorus-based flame retardants have quite satisfactorily reinforced PP against flame.
Figure 1.
Flame Retardancy Index (FRI) values as a function of phosphorus flame retardant (FR) type and content. Symbols are indicative of different types of phosphorus flame retardant used. Here: ■ APP-10 [24],  APP-12, APP-15 [25],
APP-12, APP-15 [25],  APP-20 [26],
APP-20 [26],
 APP-20 [27],
APP-20 [27],
 APP-20 [28],
APP-20 [28],
 APP-20 [23],
APP-20 [23],
 APP-25 [29],
APP-25 [29],
 APP-25 [30],
APP-25 [30],
 APP-25 [31],
APP-25 [31],
 APP-25 [32],
APP-25 [32],
 APP-25 [33],
APP-25 [33],
 APP-25 [34],
APP-25 [34],
 APP-25, m-APP-25 [35],
APP-25, m-APP-25 [35],  APP-25, mc-APP-25 [36],
APP-25, mc-APP-25 [36],  m-APP-25 [37],
m-APP-25 [37],
 APP-30 [38],
APP-30 [38],
 APP-30 [39],
APP-30 [39],
 APP-30 [40],
APP-30 [40],
 mc-APP-30 [41],
mc-APP-30 [41],
 APP-30, mc-APP-30 [42],
APP-30, mc-APP-30 [42],
 APP-30, mc-APP-30 [43],
APP-30, mc-APP-30 [43],
 APP-30, mc-APP-30 [44],
APP-30, mc-APP-30 [44],
 APP-30, mc-APP-5, mc-APP-10, mc-APP-15, mc-APP-20, mc-APP-25, mc-APP-30 [45],
APP-30, mc-APP-5, mc-APP-10, mc-APP-15, mc-APP-20, mc-APP-25, mc-APP-30 [45],  APP-35, m-APP-35 [46],
APP-35, m-APP-35 [46],  APP-35, m-APP-35 [47],
APP-35, m-APP-35 [47],  APP-40, mc-APP-40 [48],
APP-40, mc-APP-40 [48],  APP-25, P-CA-25 [49],
APP-25, P-CA-25 [49],  APP-25 [50],
APP-25 [50],
 APP-IFR-20 [51],
APP-IFR-20 [51],
 P-IFR-10, P-IFR-15, P-IFR-20, P-IFR-25 [52],
P-IFR-10, P-IFR-15, P-IFR-20, P-IFR-25 [52],
 P-IFR-20 [53],
P-IFR-20 [53],
 P-IFR-28 [54],
P-IFR-28 [54],
 P-IFR-28 [55],
P-IFR-28 [55],
 PN-IFR-30 [56],
PN-IFR-30 [56],
 P-FR-30 [57],
P-FR-30 [57],
 P-FR-20 [58],
P-FR-20 [58],
 DOPO-10 [15],
DOPO-10 [15],
 mc-BDP-10, mc-BDP-20 [59],
mc-BDP-10, mc-BDP-20 [59],
 OP-20 [60],
OP-20 [60],
 AHP-24 [61],
AHP-24 [61],
 ALPi-30 [62],
ALPi-30 [62],
 PEPA-40 [63].
PEPA-40 [63].
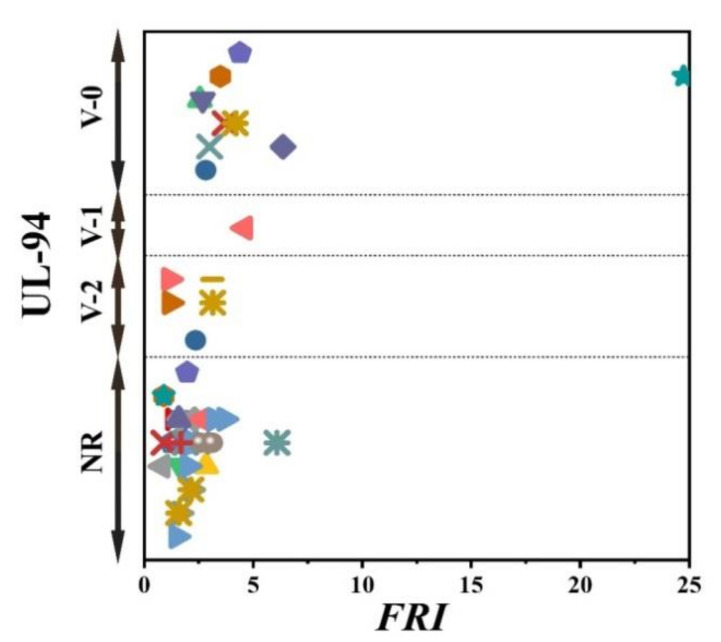
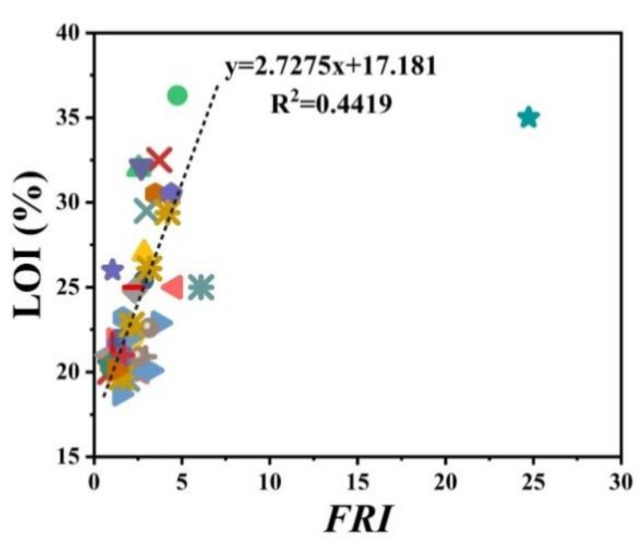
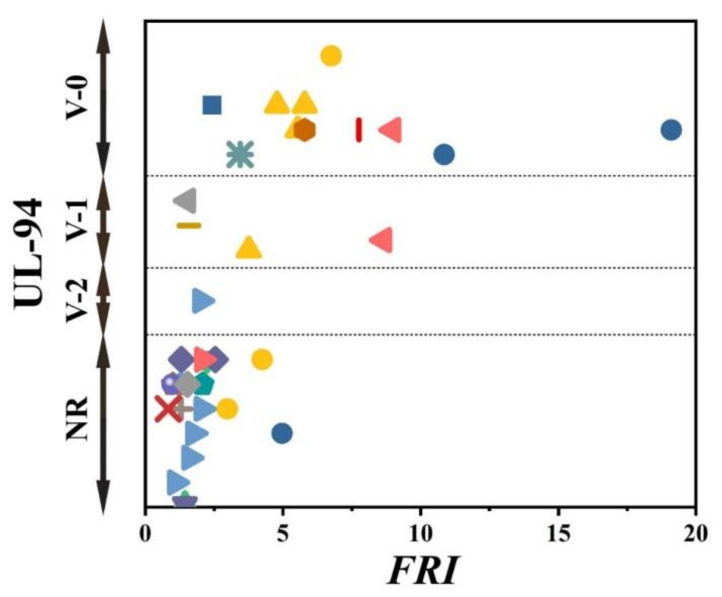
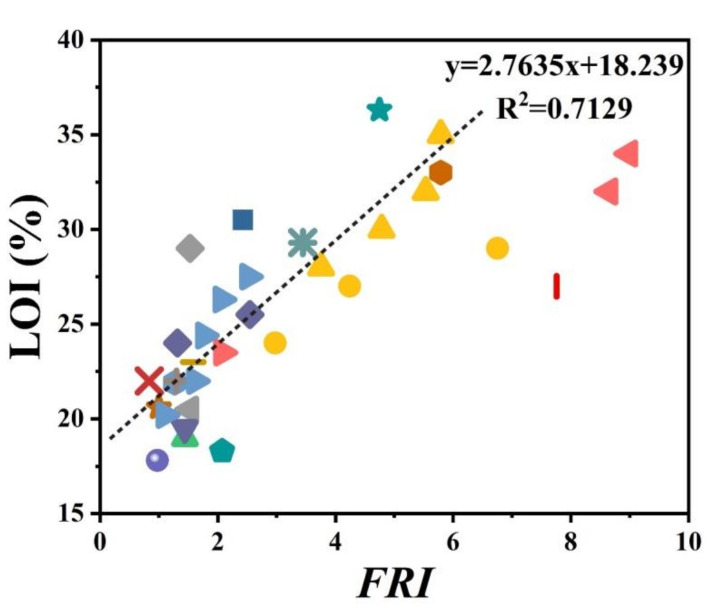
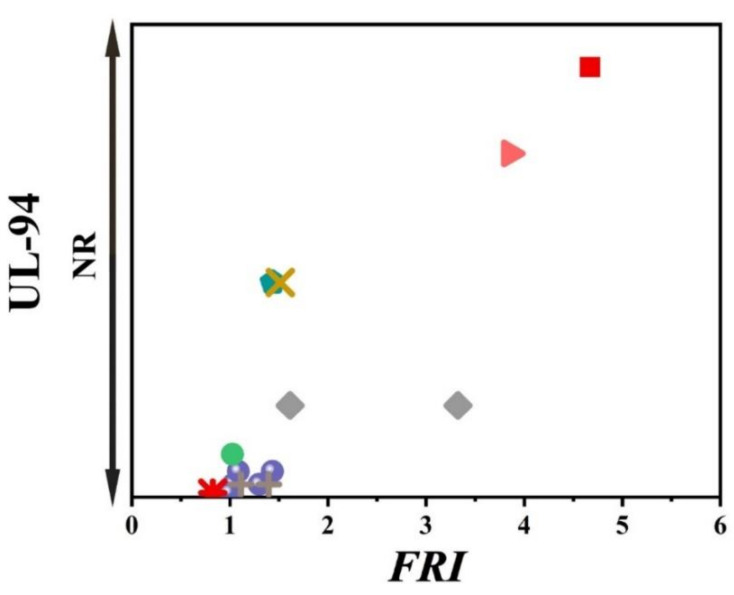
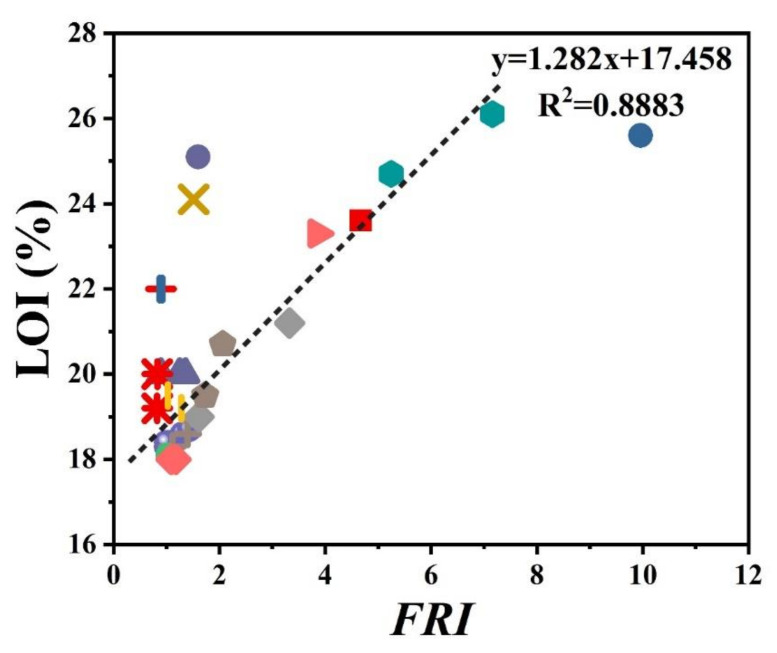
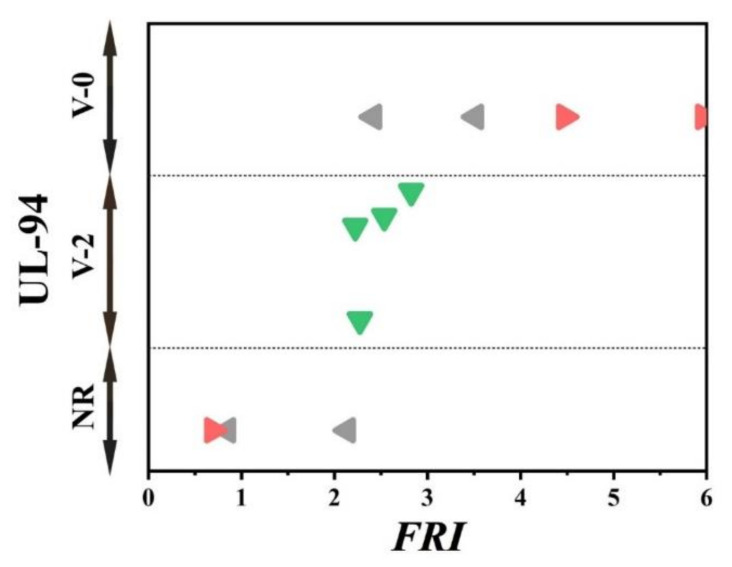
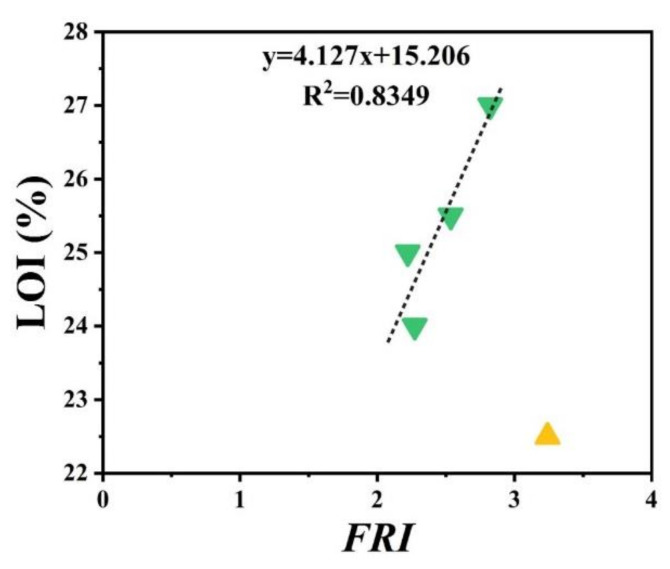
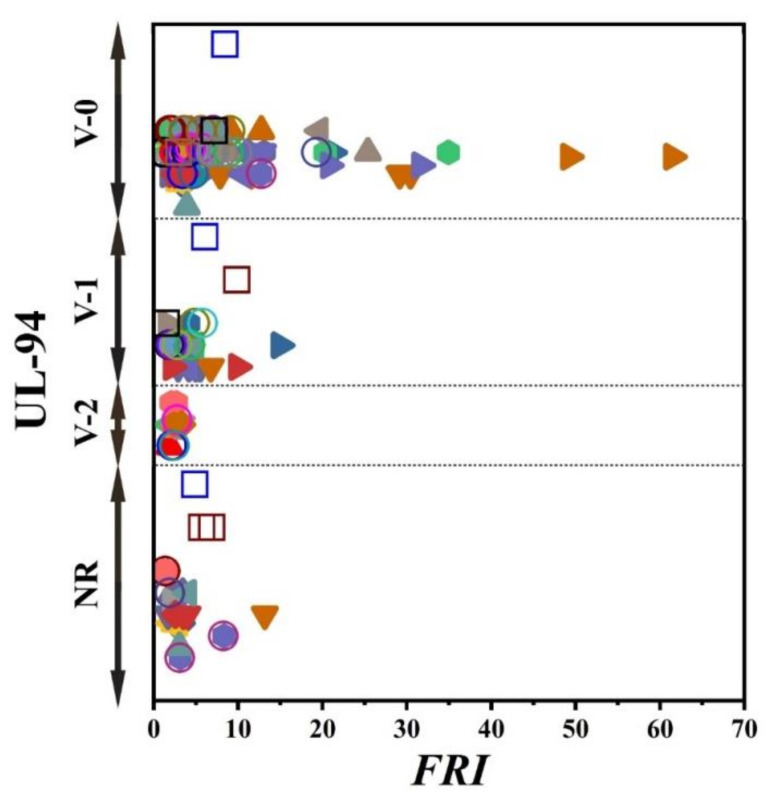
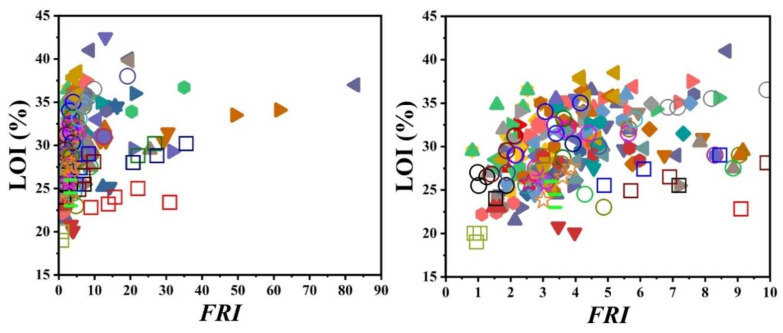
There has always been interest in exploring possible correlations between the data collected from different analyses made on PP materials. Figure 2 shows the flame retardancy performance of phosphorus FR-containing PP in terms of FRI versus the corresponding UL-94 test outcomes. From these data, it is evident that no specified correlation exists between the qualitative results collected from UL-94 and the quantitative ones obtained in cone calorimeter measurements. However, in the case of LOI results, Figure 3 suggests a meaningful relationship can be drawn among data achieved from the calculated FRI and the LOI test results. The LOI value for pure PP is around 17; however, it is increased by addition of flame retardant up to 36, more than a two-fold rise.
Figure 2.
FRI values versus UL-94 test results. Symbols are indicative of different types of phosphorus flame retardant (FR) used. The vertical intervals in each category, i.e., V-0, V-1, V-2, and NR, are schematically representative of the amount of additive used. For example, two data distinguished by different symbols having the same or very close FRI values (horizontal quantity) in a given category (e.g., V-1) may have different vertical quantities, e.g., both reveal V-1 behavior in the UL-94 test, but the upper contains more FR in Polypropylene (PP).
Figure 3.
FRI values of PP as a function of limiting oxygen index (LOI) test results. Symbols are indicative of different types of phosphorus flame retardant used.
3. Nitrogen-Based Flame Retardants
Nitrogen-based flame retardants have also been used in PP to make it resistant against fire. Table 2 gives the names and the percentages of incorporation of these flame retardants, where the data were obtained in cone calorimetry (pHRR, THR, and TTI), FRI calculated from cone calorimetry parameters, as well as LOI and UL-94 values. Some of the nitrogen-based FRs listed in Table 2 also contain a phosphorus element. However, the percentage of nitrogen is more important, and therefore these FRs are listed in this Table.
Table 2.
Flame retardant PP materials containing nitrogen-based (N) flame retardants. Data are extracted from the literature: cone calorimetry parameters (TTI, pHRR, THR), LOI, and UL-94 values. The FRI values were calculated by authors of the present review. The name and the percentage of flame retardants are provided in separate columns. “wt.%” was used for loading level of additives, while “―” stands for the systems free of additive or the neat PP. * FR means flame retardant. Since all comparisons were made in terms of FRI, classification of polymers in terms of their flame-retardant properties was not surveyed based on the chemistry of additives, heat flux, sample thickness, etc.
| PP Containing Nitrogen-Based (N) FR * | wt.% | TTI (s) |
pHRR (kW·m−2) |
THR (MJ·m−2) |
Irradiance (kW·m−2) |
Sample Thickness (mm) | FRI | LOI | UL-94 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| ― | 44 | 1175 | 106 | 35 | 3 | ― | ― | ― | [63] | |
| Melamine phosphate (MP) | 40 | 39 | 296 | 78 | 35 | 3 | 4.78 | ― | ― | [63] |
| ― | 54 | 930 | 140 | 35 | 4 | ― | ― | NR | [64] | |
| Melamine salt of pentaerythritol phosphate kaolinite (MPPK) | 15 | 30 | 208 | 70 | 35 | 4 | 4.96 | ― | NR | [64] |
| MPPK | 20 | 28 | 148 | 42 | 35 | 4 | 10.86 | ― | V-0 | [64] |
| MPPK | 25 | 34 | 130 | 33 | 35 | 4 | 19.10 | ― | V-0 | [64] |
| ― | 30 | 929 | 134 | 50 | 3 | ― | 17 | NR | [65] | |
| Melamine salt of tripentaerythriol phosphate (MTP) | 15 | 22 | 480 | 101 | 50 | 3 | 1.88 | ― | ― | [65] |
| MTP | 20 | 22 | 267 | 91 | 50 | 3 | 3.75 | 28 | V-1 | [65] |
| MTP | 25 | 22 | 226 | 73 | 50 | 3 | 5.53 | 32 | V-0 | [65] |
| MTP | 30 | 22 | 219 | 72 | 50 | 3 | 5.78 | 35 | V-0 | [65] |
| Methyl hydrogen siloxane modified MTP (m-MTP) | 30 | 21 | 253 | 72 | 50 | 3 | 4.78 | 30 | V-0 | [65] |
| ― | 65 | 1417 | 128.5 | 35 | 3 | ― | ― | NR | [57] | |
| Melamine pyrophosphate (MPyP) | 30 | 36 | 437 | 103.1 | 35 | 3 | 2.23 | ― | NR | [57] |
| ― | 34 | 1727 | 112 | 35 | 3 | ― | 17 | NR | [66] | |
| MPyP | 30 | 19 | 511 | 83 | 35 | 3 | 2.54 | 25.5 | NR | [66] |
| Triazine-based charring foaming agent: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine (TA-CFA) | 30 | 13 | 584 | 96 | 35 | 3 | 1.31 | 24 | NR | [66] |
| ― | 48 | 988 | 88.3 | 35 | 3 | ― | 17 | NR | [39] | |
| Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine (TA-CFA) | 30 | 34 | 468 | 86.6 | 35 | 3 | 1.52 | 20.5 | V-1 | [39] |
| ― | 50 | 1350 | 91.2 | 35 | 3 | ― | 17 | NR | [40] | |
| Triazine-based CFA: synthesized by polycondensation of 2-chloro-4,6-di-(2-hydroxyethylamino)-s-triazine (TA-CFA) | 30 | 38 | 518 | 86.7 | 35 | 3 | 2.08 | 23.5 | NR | [40] |
| ― | 45 | 759.2 | 98.8 | 35 | 3 | ― | 17 | NR | [37] | |
| Triazine-based CFA: synthesized from a macromolecular triazine derivative containing hydroxyethylamino and triazine rings and ethylenediamino groups (TA-CFA) | 25 | 34 | 487.4 | 91.6 | 35 | 3 | 1.26 | 21.9 | NR | [37] |
| ― | 20 | 809 | 96 | 50 | 3 | ― | 17.6 | NR | [32] | |
| Triazine-based CFA: Poly[N4-bis(ethylenediamino)-phenyl phosphonic-N2, N6-bis(ethylenediamino)-1,3,5-triazine-N-phenyl (TA-CFA) | 25 | 12 | 529 | 88 | 50 | 3 | 1.00 | 20.6 | NR | [32] |
| ― | 18 | 1457 | 156 | 50 | 3 | ― | 19 | NR | [29] | |
| Triazin-based CA—Zinc oxide (TA-CA-ZnO) | 25 | 17 | 694 | 149 | 50 | 3 | 2.07 | 18.3 | NR | [29] |
| ― | 41 | 840.3 | 115.7 | 35 | 3 | ― | 16.4 | NR | [67] | |
| Triazin-based CA: Poly(ethanediamine-1,3,5-triazine-p-4-amino-2,2,6,6-tetramethylpiperidine) (TA-CA) | 25 | 30 | 684 | 106.7 | 35 | 3 | 0.97 | 17.8 | NR | [67] |
| ― | 48 | 1351 | 107 | 35 | 3.2 | ― | 18.5 | NR | [27] | |
| Triazin-based CA: compound containing pentaerythritol and triazine structure (TA-CA) | 20 | 42 | 994 | 98 | 35 | 3.2 | 1.29 | 22 | NR | [27] |
| ― | 66 | 633 | 44.2 | 35 | 3 | ― | 17 | NR | [23] | |
|
Triazin-based CA: synthesized by
reaction of tris (2-hydrooxyethyl) isocyanurate and 2-carboxyethyl (phenyl) phosphinic acid (TA-CA) |
20 | 31 | 417 | 37.6 | 35 | 3 | 0.83 | 22 | NR | [23] |
| ― | 31 | 1239 | 123.6 | 50 | 3 | ― | 18.5 | NR | [68] | |
|
Triazin-based IFR: synthesized by
reaction of tris(2-hydroxyethyl) isocyanurate and polyphosphoric acid and melamine (TA-IFR) |
20 | 18 | 289.9 | 89 | 50 | 3 | 3.44 | 29.3 | V-0 | [68] |
| ― | 48 | 988 | 88.3 | 35 | 3.2 | ― | 17 | NR | [31] | |
|
Triazin-based IFR: synthesized by
reaction of cyanuric chloride and N-amino ethylpiperazine (TA-IFR) |
25 | 38 | 504 | 86.6 | 35 | 3.2 | 1.58 | 23 | V-1 | [31] |
| ― | 20 | 904.4 | 126.2 | 50 | 3 | ― | 18 | NR | [69] | |
|
Piperazine-based FR: synthesized by
reaction of diphenylphosphinyl chloride and piperazine (PI-FR) |
25 | 58 | 487.7 | 87.5 | 50 | 3 | 7.75 | 27 | V-0 | [69] |
| ― | 45 | 1269 | 146.4 | 50 | 3 | ― | 17.5 | NR | [70] | |
| Piperazine-based IFR: Piperazine spirocyclic phosphoramidate (PI-IFR) | 30 | 17 | 240.4 | 120.2 | 50 | 3 | 2.42 | 30.5 | V-0 | [70] |
| ― | 47 | 802 | 104 | 35 | 3 | ― | 18 | NR | [71] | |
|
Piperazine-based IFR: synthesized by
reaction of phosphorus chloride and 2,6,7-trioxa-l-phosphabicyclo[2,2,2]-octane-4-methanol and anhydrous piperazine (PI-IFR) |
20 | 36 | 275 | 78 | 35 | 3 | 2.97 | 24 | NR | [71] |
|
Piperazine-based IFR: synthesized by
reaction of phosphorus chloride and 2,6,7-trioxa-l-phosphabicyclo[2,2,2]-octane-4-methanol and anhydrous piperazine (PI-IFR) |
30 | 37 | 209 | 74 | 35 | 3 | 4.24 | 27 | NR | [71] |
|
Piperazine-based IFR: synthesized by
reaction of phosphorus chloride and 2,6,7-trioxa-l-phosphabicyclo[2,2,2]-octane-4-methanol and anhydrous piperazine (PI-IFR) |
40 | 37 | 162 | 60 | 35 | 3 | 6.75 | 29 | V-0 | [71] |
| ― | 36 | 799.3 | 170.9 | 35 | 4 | ― | 18 | NR | [72] | |
| N-alkoxy hindered amine (NOR116) | 0.5 | 44 | 738.8 | 156.5 | 35 | 4 | 1.44 | 19 | NR | [72] |
| ― | 36 | 799 | 170 | 35 | 4 | ― | 17.5 | NR | [73] | |
| NOR116 | 0.3 | 44 | 738 | 156 | 35 | 4 | 1.44 | 19.5 | NR | [73] |
| ― | 42 | 831 | 112 | 35 | 3 | ― | 18 | NR | [50] | |
| Polyurethane containing Phosphorus-based CA (PPU-CA) | 25 | 27.3 | 475 | 83 | 35 | 3 | 1.53 | 29 | NR | [50] |
| ― | 42 | 1025 | 137.7 | 35 | 4 | ― | ― | ― | [74] | |
| Nitrogen-based FR: compound containing Nitrogen (27.5.wt.%) and Phosphorus (15.6 wt.%) (N-FR) | 22 | 22 | 170 | 50.3 | 35 | 4 | 8.64 | 32 | V-1 | [74] |
| Nitrogen-based FR: compound containing Nitrogen (27.5 wt.%) and Phosphorus (15.6 wt.%) (N-FR) | 25 | 21 | 160 | 49.1 | 35 | 4 | 8.98 | 34 | V-0 | [74] |
| ― | 30 | 1093 | 108.2 | 50 | 3 | ― | 18 | NR | [75] | |
| Nitrogen-based IFR: Poly (diallyldimethylammonium) and polyphosphate polyelectrolyte complexe-based IFR (N-IFR) | 5 | 28 | 968.5 | 103.4 | 50 | 3 | 1.10 | 20.2 | NR | [75] |
| Nitrogen-based IFR: Poly (diallyldimethylammonium) and polyphosphate polyelectrolyte complexe-based IFR (N-IFR) | 10 | 25 | 626.2 | 97.1 | 50 | 3 | 1.62 | 22 | NR | [75] |
| Nitrogen-based IFR: Poly (diallyldimethylammonium) and polyphosphate polyelectrolyte complexe-based IFR (N-IFR) | 15 | 23 | 543.1 | 94.3 | 50 | 3 | 1.76 | 24.4 | NR | [75] |
| Nitrogen-based IFR: Poly (diallyldimethylammonium) and polyphosphate polyelectrolyte complexe-based IFR (N-IFR) | 20 | 21 | 443.9 | 90.1 | 50 | 3 | 2.06 | 26.3 | NR | [75] |
| Nitrogen-based IFR: Poly (diallyldimethylammonium) and polyphosphate polyelectrolyte complexe-based IFR (N-IFR) | 25 | 18 | 335.3 | 83.9 | 50 | 3 | 2.52 | 27.5 | V-2 | [75] |
| ― | 25 | 874.1 | 89.3 | 50 | 3 | ― | 18 | NR | [76] | |
| Nitrogen-based IFR: compound containing Nitrogen (23%) and Phosphorus (21%) (N-IFR) | 25 | 12 | 94.9 | 68.2 | 50 | 3 | 5.78 | 33 | V-0 | [76] |
| ― | 29 | 980 | 136 | 50 | ― | ― | 18.5 | ― | [56] | |
| Phosphorus and Nitrogene based IFR | 30 | 22 | 229 | 93 | 50 | ― | 4.74 | 36.3 | ― | [56] |
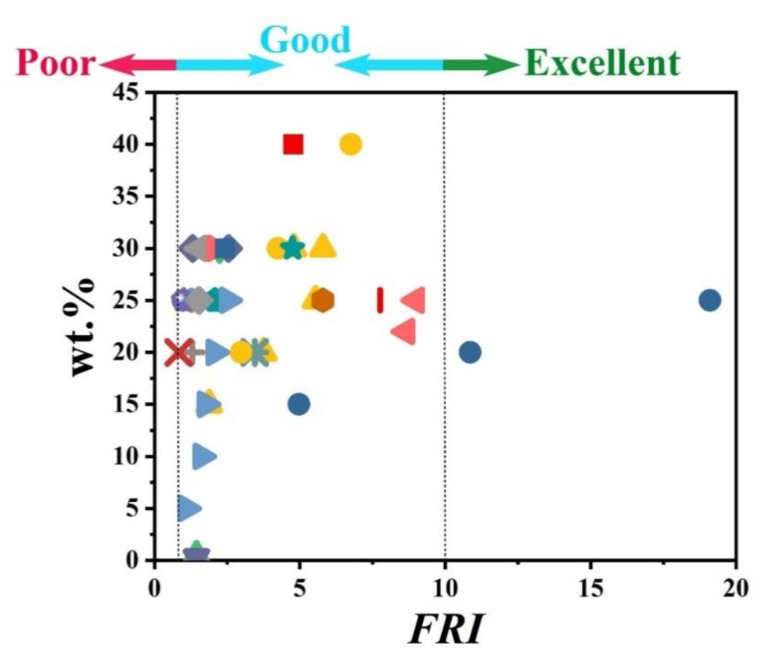
To give a bright view of the variation trend, Figure 4 illustrates the FRI values as a function of wt.% of nitrogen-based flame retardants incorporated into the PP. The percentage of incorporation was changed from 15 to 40 wt.%. Of note, all points are located in the Good zone of FRI, except two points remarked as Excellent. These two points correspond to a kaolinite additive modified with nitrogen and phosphorus agents. A very noticeable point to be considered is that increasing the amount of diallyldimethylammonium (nominated with the  symbol in Figure 4) from 5 to 25 has no serious effect on the value of FRI, so that they are aligned vertically around FRI values between 1.0 and 2.5. Overall, like what happened to other polymers [77,78], combinatorial flame retardants may be the solution to flammability reduction of PP materials.
symbol in Figure 4) from 5 to 25 has no serious effect on the value of FRI, so that they are aligned vertically around FRI values between 1.0 and 2.5. Overall, like what happened to other polymers [77,78], combinatorial flame retardants may be the solution to flammability reduction of PP materials.
Figure 4.
FRI values as a function of nitrogen FR type and content. Symbols are indicative of different types of nitrogen flame retardant used. Here: ■ MP-40 [63],  MPPK-15, MPPK-20, MPPK-25 [64],
MPPK-15, MPPK-20, MPPK-25 [64],  MTP-15, MTP-20, MTP-25, MTP-30, m-MTP-30 [65],
MTP-15, MTP-20, MTP-25, MTP-30, m-MTP-30 [65],  MPyP-30 [57],
MPyP-30 [57],  MPyP-30, TA-CFA-30 [66],
MPyP-30, TA-CFA-30 [66],  TA-CFA-30 [39],
TA-CFA-30 [39],
 TA-CFA-30 [40],
TA-CFA-30 [40],  TA-CFA-25 [37],
TA-CFA-25 [37],
 TA-CFA-25 [32],
TA-CFA-25 [32],  TA-CA-ZnO-25 [29],
TA-CA-ZnO-25 [29],
 TA-CA-25 [67],
TA-CA-25 [67],
 TA-CA-20 [27],
TA-CA-20 [27],
 TA-CA-20 [23],
TA-CA-20 [23],
 TA-IFR-20 [68],
TA-IFR-20 [68],  TA-IFR-25 [31],
TA-IFR-25 [31],
 PI-FR-25 [69],
PI-FR-25 [69],
 PI-IFR-30 [70],
PI-IFR-30 [70],
 PI-IFR-20, PI-IFR-30, PI-IFR-40 [71],
PI-IFR-20, PI-IFR-30, PI-IFR-40 [71],
 NOR116-0.5 [72],
NOR116-0.5 [72],
 NOR116-0.3 [73],
NOR116-0.3 [73],
 PPU-CA-25 [50],
PPU-CA-25 [50],
 N-FR-22, N-FR-25 [74],
N-FR-22, N-FR-25 [74],
 N-IFR-5, N-IFR-10, N-IFR-15, N-IFR-20, N-IFR-25 [75],
N-IFR-5, N-IFR-10, N-IFR-15, N-IFR-20, N-IFR-25 [75],
 N-IFR-25 [76],
N-IFR-25 [76],
 PN-IFR-30 [56].
PN-IFR-30 [56].
Figure 5 patterns UL-94 results as a function of FRI for nitrogen-based flame retardant in PP. It can be observed that even at small quantities of FRI, V0 in UL-94 was achieved. The diversity of data in Figure 5 can be taken as a signature of sensitivity of UL-94 to FRI. Figure 6 shows LOI values as a function of FRI. There is a quite reasonable correlation between the LOI and FRI values, up to FRI value of 6.
Figure 5.
FRI values versus UL-94 test results. Symbols are indicative of different types of nitrogen flame retardant (FR) used. The vertical intervals in each category, i.e., V-0, V-1, V-2, and NR, are schematically representative of the amount of additive used. For example, two data distinguished by different symbols having the same or very close FRI values (horizontal quantity) in a given category (e.g., V-1), may have different vertical quantities; e.g., both reveal V-1 behavior in UL-94 test, but the upper contains more FR in PP.
Figure 6.
FRI values of PP as a function of LOI test results. Symbols are indicative of different types of nitrogen flame retardant used.
4. Mineral-Based Flame Retardants
Mineral additives have been widely used in polymers for their acceptable cost and properties [79]. Mineral-based flame retardants including clays are widely used in PP due to their low cost and acceptable thermal resistance. In this family, the most used flame retardants in volume were aluminum trihydroxide (ATH) and magnesium dihydroxide (MDH). However, due to their low efficiency, a high percentage of loading was necessary for achieving an acceptable level of flame retardancy of polymers. The name and the percentage of the used mineral-based flame retardants in PP are listed in Table 3. Cone calorimetry data, FRI, LOI, and UL-94 values are also given so as to make possible a detailed view on the status of flame retardant efficiency of PP materials.
Table 3.
Flame-retardant PP materials containing mineral-based (M) flame retardants. Data are extracted from the literature: cone calorimetry parameters (TTI, pHRR, THR), LOI, and UL-94 values. The FRI values were calculated by authors of the present review. The name and the percentage of flame retardants are provided in separate columns. “wt.%” was used for loading level of additives, while “―” stands for the systems free of additive or the neat PP. * FR means flame retardant. Since all comparisons were made in terms of FRI, classification of polymers in terms of their flame-retardant properties was not surveyed based on the chemistry of additives, heat flux, sample thickness, etc.
| PP Containing Mineral-Based (M) FR * | wt.% | TTI (s) |
pHRR (kW·m−2) |
THR (MJ·m−2) | Irradiance (kW·m−2) |
Sample Thickness (mm) | FRI | LOI | UL-94 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| ― | 37 | 1425 | 121.4 | 50 | 3 | ― | 17.3 | NR | [80] | |
| Aluminum trihydroxide (ATH) | 50 | 52 | 539 | 96.6 | 50 | 3 | 4.66 | 23.6 | NR | [80] |
| ― | 32 | 1470 | 175 | 50 | 4 | ― | 18 | ― | [81] | |
| ATH | 60 | 34 | 280 | 98 | 50 | 4 | 9.96 | 25.6 | ― | [81] |
| ― | 26 | 1967 | 112 | 50 | 3 | ― | ― | ― | [82] | |
| ATH | 20 | 27 | 817 | 90 | 50 | 3 | 3.11 | ― | ― | [82] |
| ATH | 40 | 28 | 467 | 70 | 50 | 3 | 7.25 | ― | ― | [82] |
| Magnesium dihydroxide (MDH) | 20 | 31 | 1000 | 98 | 50 | 3 | 2.68 | ― | ― | [82] |
| MDH | 40 | 34 | 433 | 75 | 50 | 3 | 8.87 | ― | ― | [82] |
| ― | 30 | 1684 | 89 | 50 | 3 | ― | ― | ― | [82] | |
| MDH | 40 | 3 | 377 | 71 | 50 | 3 | 0.55 | ― | ― | [82] |
| MDH | 60 | 29 | 228 | 51 | 50 | 3 | 12.46 | ― | ― | [82] |
| ― | 63.2 | 521.35 | 49.8 | 30 | ― | ― | ― | ― | [83] | |
| MDH | 62.5 | 81.1 | 115.5 | 75.7 | 30 | ― | 3.81 | ― | ― | [83] |
| ― | 71 | 2283 | 218 | 35 | 1 | ― | ― | ― | [84] | |
| MDH | 50 | 97 | 789 | 238 | 35 | 1 | 3.62 | ― | ― | [84] |
| ― | 38 | 1425 | 121.4 | 50 | 3 | ― | 17.5 | NR | [85] | |
| MDH | 40 | 46 | 548 | 99.1 | 50 | 3 | 3.85 | 23.3 | NR | [85] |
| ― | 29 | 1660 | 33.4 | 35 | 1 | ― | ― | ― | [86] | |
| MDH | 30 | 39 | 989 | 28.3 | 35 | 1 | 2.66 | ― | ― | [86] |
| Dodecanoic acid-treated MDH (m-MDH) | 30 | 32 | 882 | 28.7 | 35 | 1 | 2.41 | ― | ― | [86] |
| Dodecylphosphate treated MDH (m-MDH) | 30 | 29 | 651 | 28.8 | 35 | 1 | 2.95 | ― | ― | [86] |
| ― | 37 | 584 | 75.6 | 50 | 3 | ― | ― | ― | [87] | |
| MDH | 10 | 33 | 471 | 65.9 | 50 | 3 | 1.26 | ― | ― | [87] |
| MDH | 15 | 31 | 381 | 61.2 | 50 | 3 | 1.58 | ― | ― | [87] |
| ― | 54 | 930 | 140 | 35 | 4 | ― | ― | NR | [64] | |
| Kaolinite (Kaol) | 25 | 32 | 463 | 116 | 35 | 4 | 1.43 | ― | NR | [64] |
| ― | 29 | 1474 | 142 | 50 | 3 | ― | 18 | NR | [88] | |
| Kaol | 0.5 | 28 | 1429 | 142 | 50 | 3 | 0.99 | 18.3 | NR | [88] |
| Kaol | 1.5 | 27 | 1346 | 140 | 50 | 3 | 1.03 | 18.3 | NR | [88] |
| Kaol | 3 | 26 | 1279 | 135 | 50 | 3 | 1.08 | 18.4 | NR | [88] |
|
Ammonium sulfamate intercalated kaol
(m-Kaol) |
0.5 | 27 | 1389 | 141 | 50 | 3 | 0.99 | 18.4 | NR | [88] |
|
Ammonium sulfamate intercalated kaol
(m-Kaol) |
1.5 | 28 | 1169 | 133 | 50 | 3 | 1.29 | 18.6 | NR | [88] |
|
Ammonium sulfamate intercalated kaol
(m-Kaol) |
3 | 27 | 1079 | 126 | 50 | 3 | 1.43 | 18.7 | NR | [88] |
| ― | 27 | 1474 | 142 | 50 | ― | ― | 18 | NR | [89] | |
| Kaol | 1.5 | 27 | 1346 | 140 | 50 | ― | 1.11 | 18.3 | NR | [89] |
|
Ammonium sulfamate intercalated Kaol
(m-Kaol) |
1.5 | 28 | 1169 | 133 | 50 | ― | 1.39 | 18.6 | NR | [89] |
| ― | 44 | 1000 | 145 | 50 | ― | ― | ― | ― | [90] | |
| Kaol | 10 | 35 | 634 | 144 | 50 | ― | 1.26 | ― | ― | [90] |
| Kaol | 20 | 38 | 396 | 136 | 50 | ― | 2.33 | ― | ― | [90] |
| Kaol | 30 | 41 | 348 | 126 | 50 | ― | 3.08 | ― | ― | [90] |
| Trisilanolisooctyl polyhedral oligomeric silsesquioxane modified kaol (m-Kaol) | 10 | 35 | 850 | 140 | 50 | ― | 0.96 | ― | ― | [90] |
| Trisilanolisooctyl polyhedral oligomeric silsesquioxane modified kaol (m-Kaol) | 20 | 38 | 650 | 141 | 50 | ― | 1.36 | ― | ― | [90] |
| Trisilanolisooctyl polyhedral oligomeric silsesquioxane modified kaol (m-Kaol) | 30 | 50 | 430 | 137 | 50 | ― | 2.79 | ― | ― | [90] |
| Talc (TC) | 10 | 49 | 377 | 128 | 50 | ― | 3.34 | ― | ― | [90] |
| TC | 20 | 56 | 341 | 118 | 50 | ― | 4.58 | ― | ― | [90] |
| TC | 30 | 50 | 295 | 112 | 50 | ― | 4.98 | ― | ― | [90] |
| ― | 45 | 1831.96 | 110.8 | 50 | ― | ― | ― | ― | [91] | |
| Ni-Al layered double hydroxide (LDH) | 0.5 | 53 | 1635.53 | 106.8 | 50 | ― | 1.36 | ― | ― | [91] |
| Ni-Al LDH (LDH) | 1 | 92 | 1430.59 | 117.8 | 50 | ― | 2.46 | ― | ― | [91] |
| Ni-Al LDH (LDH) | 1.5 | 41 | 1266.66 | 129.1 | 50 | ― | 1.13 | ― | ― | [91] |
| Organically modified Ni-Al LDH (m-LDH) | 0.5 | 59 | 1116.37 | 70.2 | 50 | ― | 3.39 | ― | ― | [91] |
| Organically modified Ni-Al LDH (m-LDH) | 1 | 45 | 1026.86 | 81.24 | 50 | ― | 2.43 | ― | ― | [91] |
| Organically modified Ni-Al LDH (m-LDH) | 1.5 | 49 | 1254.95 | 111.1 | 50 | ― | 1.58 | ― | ― | [91] |
| Cu-Al LDH (LDH) | 0.5 | 45 | 1026.86 | 81.2 | 50 | ― | 2.43 | ― | ― | [91] |
| Cu-Al LDH (LDH) | 1 | 57 | 1276.46 | 123 | 50 | ― | 1.63 | ― | ― | [91] |
| Cu-Al LDH (LDH) | 1.5 | 50 | 1449.98 | 121.8 | 50 | ― | 1.27 | ― | ― | [91] |
| Organically modified Cu-Al LDH (m-LDH) | 0.5 | 69 | 985.91 | 120 | 50 | ― | 2.63 | ― | ― | [91] |
| Organically modified Cu-Al LDH (m-LDH) | 1 | 54 | 1175.99 | 121.6 | 50 | ― | 1.70 | ― | ― | [91] |
| Organically modified Cu-Al LDH (m-LDH) | 1.5 | 54 | 1345.14 | 114.3 | 50 | ― | 1.58 | ― | ― | [91] |
| ― | 20 | 1849 | 121 | 50 | 3 | ― | ― | ― | [92] | |
| Mg-Al LDH with mole ratio: Zn:Mg:Al/0:2:1 (A-LDH) | 1 | 15 | 1981 | 141 | 50 | 3 | 0.60 | ― | ― | [92] |
| A-LDH | 2 | 16 | 1764 | 139 | 50 | 3 | 0.73 | ― | ― | [92] |
| Zn-Mg-Al LDH with mole ratio: Zn:Mg:Al/0.5:1.5:1 (B-LDH) | 1 | 14 | 1997 | 136 | 50 | 3 | 0.57 | ― | ― | [92] |
| B-LDH | 2 | 14 | 1512 | 133 | 50 | 3 | 0.77 | ― | ― | [92] |
| B-LDH | 4 | 13 | 1153 | 128 | 50 | 3 | 0.98 | ― | ― | [92] |
| Zn-Mg-Al LDH with mole ratio: Zn:Mg:Al/1:1:1 (C-LDH) | 1 | 18 | 2004 | 135 | 50 | 3 | 0.74 | ― | ― | [92] |
| C-LDH | 2 | 14 | 1546 | 132 | 50 | 3 | 0.76 | ― | ― | [92] |
| C-LDH | 4 | 12 | 1225 | 125 | 50 | 3 | 0.87 | ― | ― | [92] |
| Zn-Mg-Al LDH with mole ratio: Zn:Mg:Al/1.5:0.5:1 (D-LDH) | 1 | 18 | 1938 | 135 | 50 | 3 | 0.76 | ― | ― | [92] |
| D-LDH | 2 | 15 | 1656 | 130 | 50 | 3 | 0.77 | ― | ― | [92] |
| D-LDH | 4 | 13 | 1294 | 123 | 50 | 3 | 0.91 | ― | ― | [92] |
| Zn-Al LDH with mole ratio: Zn:Mg:Al/2:0:1 (E-LDH) | 1 | 16 | 1977 | 136 | 50 | 3 | 0.66 | ― | ― | [92] |
| E-LDH | 2 | 17 | 1543 | 113 | 50 | 3 | 1.09 | ― | ― | [92] |
| E-LDH | 4 | 14 | 1382 | 126 | 50 | 3 | 0.89 | ― | ― | [92] |
| ― | 17 | 2380 | 140 | 50 | 3 | ― | ― | ― | [92] | |
| A-LDH | 1 | 20 | 1906 | 135 | 50 | 3 | 1.52 | ― | ― | [92] |
| A-LDH | 4 | 16 | 1137 | 129 | 50 | 3 | 2.13 | ― | ― | [92] |
| B-LDH | 1 | 17 | 1715 | 134 | 50 | 3 | 1.44 | ― | ― | [92] |
| B-LDH | 4 | 17 | 1025 | 124 | 50 | 3 | 2.62 | ― | ― | [92] |
| C-LDH | 1 | 16 | 1875 | 130 | 50 | 3 | 1.28 | ― | ― | [92] |
| C-LDH | 4 | 14 | 992 | 125 | 50 | 3 | 2.21 | ― | ― | [92] |
| D-LDH | 1 | 15 | 2008 | 135 | 50 | 3 | 1.08 | ― | ― | [92] |
| D-LDH | 4 | 16 | 997 | 126 | 50 | 3 | 2.49 | ― | ― | [92] |
| E-LDH | 1 | 17 | 1796 | 13 | 50 | 3 | 14.27 | ― | ― | [92] |
| E-LDH | 4 | 16 | 757 | 125 | 50 | 3 | 3.31 | ― | ― | [92] |
| ― | 26 | 1975 | 125 | 50 | 3 | ― | ― | ― | [92] | |
| A-LDH | 1 | 21 | 1831 | 149 | 50 | 3 | 0.73 | ― | ― | [92] |
| A-LDH | 4 | 23 | 1274 | 127 | 50 | 3 | 1.34 | ― | ― | [92] |
| B-LDH | 1 | 23 | 1838 | 135 | 50 | 3 | 0.88 | ― | ― | [92] |
| B-LDH | 4 | 18 | 1017 | 126 | 50 | 3 | 1.33 | ― | ― | [92] |
| C-LDH | 1 | 20 | 1676 | 137 | 50 | 3 | 0.82 | ― | ― | [92] |
| C-LDH | 4 | 17 | 981 | 124 | 50 | 3 | 1.32 | ― | ― | [92] |
| D-LDH | 1 | 19 | 1833 | 136 | 50 | 3 | 0.72 | ― | ― | [92] |
| D-LDH | 4 | 15 | 1061 | 126 | 50 | 3 | 1.06 | ― | ― | [92] |
| E-LDH | 1 | 18 | 1966 | 136 | 50 | 3 | 0.63 | ― | ― | [92] |
| E-LDH | 4 | 17 | 965 | 126 | 50 | 3 | 1.32 | ― | ― | [92] |
| ― | 23 | 1726 | 133 | 50 | 3 | ― | ― | ― | [92] | |
| A-LDH | 1 | 22 | 1763 | 121 | 50 | 3 | 1.02 | ― | ― | [92] |
| A-LDH | 4 | 20 | 1283 | 125 | 50 | 3 | 1.24 | ― | ― | [92] |
| C-LDH | 1 | 19 | 1795 | 131 | 50 | 3 | 0.80 | ― | ― | [92] |
| C-LDH | 4 | 16 | 897 | 121 | 50 | 3 | 1.47 | ― | ― | [92] |
| E-LDH | 1 | 21 | 1845 | 130 | 50 | 3 | 0.87 | ― | ― | [92] |
| E-LDH | 4 | 18 | 750 | 122 | 50 | 3 | 1.96 | ― | ― | [92] |
| ― | 16 | 1443 | 141 | 50 | 3 | ― | — | — | [93] | |
| Sodium dodecyl sulphate modified Ni-Al LDH (m-LDH) | 1 | 30 | 1100 | 122 | 50 | 3 | 2.84 | ― | ― | [93] |
| Sodium dodecyl sulphate modified Ni-Al LDH (m-LDH) | 3 | 32 | 1040 | 120 | 50 | 3 | 3.26 | ― | ― | [93] |
| Sodium dodecyl sulphate modified Ni-Al LDH (m-LDH) | 5 | 36 | 975 | 113 | 50 | 3 | 4.15 | ― | ― | [93] |
| ― | 44 | 1443 | 158 | 35 | 3 | ― | ― | ― | [94] | |
| Undecenoate modified Mg-Al LDH (m-LDH) | 3 | 40 | 1627 | 174 | 35 | 3 | 0.73 | ― | ― | [94] |
| Undecenoate modified Mg-Al LDH (m-LDH) | 5 | 32 | 1629 | 167 | 35 | 3 | 0.60 | ― | ― | [94] |
| Undecenoate modified Mg-Al LDH (m-LDH) | 10 | 42 | 1339 | 157 | 35 | 3 | 1.03 | ― | ― | [94] |
| ― | 31 | 1302 | 114 | 50 | 4 | ― | 17.8 | NR | [95] | |
| Carbonate intercalated Mg-Al LDH (LDH) | 10.7 | 22 | 837 | 78 | 50 | 4 | 1.61 | 19 | NR | [95] |
| Dihydrogen phosphate intercalated Mg-Al LDH (m-LDH) | 10.7 | 23 | 534 | 62 | 50 | 4 | 3.32 | 21.2 | NR | [95] |
| ― | 52 | 1792 | 219.4 | 35 | ― | ― | ― | ― | [96] | |
| Octadecyltrimethyl ammonium chloride (alkyl-NH4Cl) | 1.2 | 53 | 1463 | 215.6 | 35 | ― | 1.27 | ― | ― | [96] |
| Montmorillonite (MMT) | 5 | 45 | 1196 | 216.7 | 35 | ― | 1.31 | ― | ― | [96] |
| Protoned MMT (H-MMT) | 5 | 42 | 1000 | 211.4 | 35 | ― | 1.50 | ― | ― | [96] |
| Dioctadecyldimethyl ammonium chloride modified MMT (m-MMT) | 5 | 43 | 996 | 210.8 | 35 | ― | 1.54 | ― | ― | [96] |
| ― | 55 | 1740 | 219.8 | 35 | ― | ― | ― | ― | [96] | |
| Dioctadecyldimethyl ammonium chloride modified MMT (m-MMT) | 5 | 50 | 982 | 208.6 | 35 | ― | 1.69 | ― | ― | [96] |
| ― | 37 | 2655 | 131 | 35 | 3 | ― | ― | ― | [97] | |
| Methyl tallow bis(2-hydroxyethyl) ammonium modified MMT (m-MMT) | 4.75 | 27 | 1365 | 123 | 35 | 3 | 1.51 | ― | ― | [97] |
| Silica pillared methyl tallow bis(2-hydroxyethyl) ammonium modified MMT (m-MMT) | 4.75 | 22 | 2585 | 132 | 35 | 3 | 0.60 | ― | ― | [97] |
| Silica pillared methyl tallow bis(2-hydroxyethyl) ammonium modified MMT powder supported with CuO (m-MMT) | 4.75 | 24 | 2315 | 132 | 35 | 3 | 0.73 | ― | ― | [97] |
| ― | 14 | 1104 | 106 | 35 | 0.4 | ― | ― | ― | [24] | |
| MMT | 10 | 18 | 1005 | 99 | 35 | 0.4 | 1.51 | ― | ― | [24] |
| Modified MMT (m-MMT) | 10 | 19 | 925 | 98 | 35 | 0.4 | 1.75 | ― | ― | [24] |
| ― | 53 | 1896 | 102 | 35 | 3 | ― | ― | ― | [98] | |
| Alkylstyrene surfactant modified MMT (m-MMT) | 3 | 50 | 1502 | 99 | 35 | 3 | 1.22 | ― | ― | [98] |
| Alkylstyrene surfactant modified MMT (m-MMT) | 10 | 50 | 1200 | 94 | 35 | 3 | 1.61 | ― | ― | [98] |
| Alkylstyrene surfactant modified MMT (m-MMT) | 16 | 51 | 882 | 95 | 35 | 3 | 2.22 | ― | ― | [98] |
| ― | 60 | 1136 | 296 | 35 | ― | ― | ― | ― | [99] | |
| MMT | 2 | 51 | 633 | 295 | 35 | ― | 1.53 | ― | ― | [99] |
| Alkylammonium modified MMT (m-MMT) | 2 | 58 | 870 | 297 | 35 | ― | 1.25 | ― | ― | [99] |
| Alkylammonium modified MMT (m-MMT) | 5 | 55 | 459 | 295 | 35 | ― | 2.27 | ― | ― | [99] |
| Alkylammonium modified MMT (m-MMT) | 10 | 56 | 357 | 293 | 35 | ― | 3.00 | ― | ― | [99] |
| ― | 52 | 1897 | 101 | 35 | 3 | ― | ― | ― | [100] | |
| Ammonium salt of an oligomer modified MMT (m-MMT) | 3 | 48 | 1577 | 95 | 35 | 3 | 1.18 | ― | ― | [100] |
| Ammonium salt of an oligomer modified MMT (m-MMT) | 8 | 49 | 1309 | 97 | 35 | 3 | 1.42 | ― | ― | [100] |
| Ammonium salt of an oligomer modified MMT (m-MMT) | 12 | 52 | 1160 | 93 | 35 | 3 | 1.77 | ― | ― | [100] |
| ― | 43 | 1845 | 118 | 50 OR 35 | — | ― | — | — | [101] | |
| Styrene-vinylbenzyl chloride copolymer modified MMT (m-MMT) | 2.5 | 47 | 1953 | 114 | 50 OR 35 | — | 1.06 | ― | ― | [101] |
| Styrene-vinylbenzyl chloride copolymer modified MMT (m-MMT) | 5 | 45 | 1889 | 111 | 50 OR 35 | — | 1.08 | ― | ― | [101] |
| Styrene-vinylbenzyl chloride copolymer modified MMT (m-MMT) | 15 | 37 | 1448 | 108 | 50 OR 35 | — | 1.19 | ― | ― | [101] |
| Styrene-vinylbenzyl chloride copolymer modified MMT (m-MMT) | 25 | 38 | 1191 | 102 | 50 OR 35 | — | 1.58 | ― | ― | [101] |
| Methyl methacrylate-vinylbenzyl chloride copolymer modified MMT (m-MMT) | 2.5 | 44 | 2025 | 123 | 50 OR 35 | — | 0.89 | ― | ― | [101] |
| Methyl methacrylate-vinylbenzyl chloride copolymer modified MMT (m-MMT) | 5 | 42 | 1738 | 120 | 50 OR 35 | — | 1.01 | ― | ― | [101] |
| Methyl methacrylate-vinylbenzyl chloride copolymer modified MMT (m-MMT) | 15 | 39 | 1651 | 115 | 50 OR 35 | — | 1.04 | ― | ― | [101] |
| Methyl methacrylate-vinylbenzyl chloride copolymer modified MMT (m-MMT) | 25 | 41 | 1139 | 105 | 50 OR 35 | — | 1.73 | ― | ― | [101] |
| ― | 55 | 1586 | 113 | 35 | — | ― | — | — | [102] | |
|
Methyl methacrylate modified MMT
(m-MMT) |
1 | 66 | 1108 | 104 | 35 | ― | 1.86 | ― | ― | [102] |
|
Methyl methacrylate modified MMT
(m-MMT) |
3 | 44 | 839 | 87 | 35 | ― | 1.96 | ― | ― | [102] |
|
Methyl methacrylate modified MMT
(m-MMT) |
5 | 35 | 557 | 77 | 35 | ― | 2.65 | ― | ― | [102] |
| ― | 50.2 | 789 | 156.6 | 35 | ― | ― | 17.5 | ― | [103] | |
| Nanofil (Nf) | 5 | 48 | 739 | 173.4 | 35 | ― | 0.92 | 22 | ― | [103] |
| Organically modified bentonite (m-BT) | 5 | 45.6 | 774 | 166.6 | 35 | ― | 0.87 | 22 | ― | [103] |
| ― | 33 | 847 | 159.8 | 50 | ― | ― | 17.5 | ― | [103] | |
| Nf | 5 | 37 | 1047 | 174 | 50 | ― | 0.83 | 22 | ― | [103] |
| m-BT | 5 | 36 | 1093 | 164 | 50 | ― | 0.82 | 22 | ― | [103] |
| ― | 35 | 1622 | 103 | 35 | 3 | ― | ― | ― | [104] | |
| Cloisite 20A: Dimethyl, dihydrogenated tallow ammonium modified MMT (C20A) | 1 | 33 | 1751 | 105 | 35 | 3 | 0.85 | ― | ― | [104] |
| C20A | 3 | 34 | 1874 | 107 | 35 | 3 | 0.80 | ― | ― | [104] |
| C20A | 5 | 39 | 1487 | 105 | 35 | 3 | 1.19 | ― | ― | [104] |
| 44 | 1172 | 87.1 | 35 | 2.5 | ― | 18.1 | NR | [60] | ||
| Cloisite 15A: dimethyl dehydrogenated tallow ammonium cation modified sodium MMT (C15A) | 5 | 41 | 1050 | 88.2 | 35 | 2.5 | 1.02 | 18.1 | NR | [60] |
| ― | 88 | 565.9 | 71.9 | 35 | 3 | ― | ― | ― | [105] | |
| C20A | 5 | 76 | 518.2 | 75.9 | 35 | 3 | 0.89 | 20 | ― | [105] |
| C20A | 5 | 89 | 415.6 | 73 | 35 | 3 | 1.35 | 20 | ― | [105] |
| Titanium dioxide (TiO2) | 0.5 | 99 | 488.1 | 75 | 35 | 3 | 1.25 | 20 | ― | [105] |
| ― | 49 | 1247 | 114.2 | 35 | ― | ― | ― | ― | [106] | |
| Activated alumina (Al2O3) | 2 | 35 | 943 | 108.2 | 35 | ― | 0.99 | ― | ― | [106] |
| ― | 28 | 1633 | 132 | 50 | 4 | ― | 18 | ― | [107] | |
| NiFeO | 2 | 27 | 1372 | 129 | 50 | 4 | 1.17 | 18 | ― | [107] |
| CoFeO | 2 | 24 | 1335 | 127 | 50 | 4 | 1.08 | 18 | ― | [107] |
| ― | 38 | 1284 | 241 | 50 | 6 | ― | ― | ― | [108] | |
| Ni2O3 | 7.5 | 53 | 655 | 161 | 50 | 6 | 4.09 | ― | ― | [108] |
| ― | 64 | 1909 | 254 | 50 | 3 | ― | ― | ― | [109] | |
| Mo/Mg/Ni/O catalysts (Nmm-cat) | 1 | 62 | 490 | 205 | 50 | 3 | 4.67 | ― | ― | [109] |
| Nmm-cat | 2 | 63 | 292 | 168 | 50 | 3 | 9.72 | ― | ― | [109] |
| Nmm-cat | 3 | 60 | 275 | 149 | 50 | 3 | 11.09 | ― | ― | [109] |
| ― | 52 | 915.7 | 112.5 | 50 | 3 | ― | 18 | ― | [110] | |
| Magnesium oxysulfate whisker (MOSw) | 30 | 62 | 259.1 | 90.4 | 50 | 3 | 5.24 | 24.7 | ― | [110] |
| Dodecyl dihydrogen phosphate modified MOSw (m-MOSw) | 30 | 64 | 243.3 | 72.8 | 50 | 3 | 7.15 | 26.1 | ― | [110] |
| ― | 48 | 195.5 | 28.6 | 35 | 2 | ― | ― | ― | [111] | |
| Manganese oxide (MnO) | 10 | 54 | 233.7 | 31.8 | 35 | 2 | 0.84 | ― | ― | [111] |
| Manganese oxide (Mn2O3) | 10 | 48 | 271.3 | 31.5 | 35 | 2 | 0.65 | ― | ― | [111] |
| Manganese oxalate (MnC2O4) | 10 | 50 | 281.6 | 29.3 | 35 | 2 | 0.70 | ― | ― | [111] |
| ― | 30 | 390 | 44 | 35 | 1.6 | ― | 17.4 | ― | [53] | |
| Zinc acetyl acetonate (Znacac) | 1 | 31 | 366 | 28 | 35 | 1.6 | 1.73 | 19.5 | ― | [53] |
| Chromium acetyl acetonate (Cracac) | 1 | 31 | 307 | 28 | 35 | 1.6 | 2.06 | 20.7 | ― | [53] |
| ― | 51 | 1053 | 117.6 | 35 | 3 | ― | ― | ― | [112] | |
| Zirconium phenylphosphonate (ZrPP) | 2 | 34 | 754 | 99.4 | 35 | 3 | 1.10 | ― | ― | [112] |
| ― | 40 | 364 | 40 | 35 | 2 | ― | ― | ― | [113] | |
| Siloxane silsesquioxane resin (S4SQH) | 1 | 41 | 354 | 47 | 35 | 2 | 0.89 | ― | ― | [113] |
| S4SQH | 5 | 21 | 500 | 44 | 35 | 2 | 0.34 | ― | ― | [113] |
| S4SQH | 10 | 19 | 445 | 44 | 35 | 2 | 0.35 | ― | ― | [113] |
| n-octyl functionalized S4SQH (m-S4SQH) | 1 | 43 | 227 | 29 | 35 | 2 | 2.37 | ― | ― | [113] |
| n-octyl functionalized S4SQH (m-S4SQH) | 5 | 40 | 481 | 48 | 35 | 2 | 0.63 | ― | ― | [113] |
| n-octadecyl functionalized S4SQH (m-S4SQH) | 1 | 40 | 168 | 22 | 35 | 2 | 3.93 | ― | ― | [113] |
| n-octadecyl functionalized S4SQH (m-S4SQH) | 5 | 43 | 328 | 42 | 35 | 2 | 1.13 | ― | ― | [113] |
| n-octadecyl functionalized S4SQH (m-S4SQH) | 10 | 47 | 391 | 47 | 35 | 2 | 0.93 | ― | ― | [113] |
| ― | 25 | 981 | 147 | 50 | ― | ― | 17.6 | NR | [30] | |
| Polysiloxane based FR (Si-FR) | 25 | 18 | 624 | 110 | 50 | ― | 1.51 | 24.1 | NR | [30] |
| ― | 54 | 1610 | 106 | 35 | 3 | ― | 20.8 | NR | [25] | |
| Sepiolite (SEP) | 0.5 | 48 | 1701 | 108 | 35 | 3 | 0.82 | 20 | NR | [25] |
| Organically modified SEP (m-SEP) | 0.5 | 46 | 1665 | 106 | 35 | 3 | 0.82 | 19.2 | NR | [25] |
| ― | 37 | 584 | 75.6 | 50 | 3 | ― | ― | ― | [87] | |
| SEP | 5 | 24 | 533 | 68.1 | 50 | 3 | 0.78 | ― | ― | [87] |
| Organically treated SEP (m-SEP) | 5 | 23 | 515 | 66.1 | 50 | 3 | 0.80 | ― | ― | [87] |
| ― | 60 | 968 | 100 | 35 | 3 | ― | 19.2 | ― | [114] | |
| Methyl polyhedral oligomeric silsesquioxane (me-POSS) | 1.95 | 54 | 1023 | 100 | 35 | 3 | 0.85 | – | ― | [114] |
| me-POSS | 6.5 | 60 | 786 | 96 | 35 | 3 | 1.28 | 19.2 | ― | [114] |
| Phenyl POSS (ph-POSS) | 3.75 | 61 | 858 | 98 | 35 | 3 | 1.17 | – | ― | [114] |
| ph-POSS | 12.5 | 53 | 872 | 96 | 35 | 3 | 1.02 | 19.5 | ― | [114] |
| ― | 56 | 1103 | 111 | 35 | 3 | ― | ― | ― | [115] | |
| Octaisobutyl POSS (T8-POSS) | 10 | 50 | 1325 | 112 | 35 | 3 | 0.73 | ― | ― | [115] |
| Al-POSS | 10 | 37 | 624 | 98 | 35 | 3 | 1.32 | ― | ― | [115] |
| Zn-POSS | 10 | 54 | 1069 | 108 | 35 | 3 | 1.02 | ― | ― | [115] |
| ― | 54 | 1242 | 221 | 35 | 6 | ― | 17.5 | ― | [5] | |
| Silica aerogel (SA) | 10 | 57 | 892 | 203 | 35 | 6 | 1.60 | 25.1 | ― | [5] |
| ― | 49.5 | 622 | 74.5 | 50 | 3 | ― | ― | ― | [116] | |
| Halloysite nanotube (HNT) | 8 | 44 | 495 | 68.5 | 50 | 3 | 1.21 | ― | ― | [116] |
| HNT-Water injection (HNT-W) | 8 | 45.5 | 451 | 66.5 | 50 | 3 | 1.42 | ― | ― | [116] |
| ― | 52.5 | 620 | 70.5 | 50 | 3 | ― | ― | ― | [116] | |
| HNT | 8 | 48 | 495 | 67 | 50 | 3 | 1.20 | ― | ― | [116] |
| HNT-W | 4 | 49 | 507 | 66.5 | 50 | 3 | 1.21 | ― | ― | [116] |
| HNT-W | 8 | 46 | 367 | 60.5 | 50 | 3 | 1.72 | ― | ― | [116] |
| HNT-W | 16 | 42.5 | 219 | 55 | 50 | 3 | 2.93 | ― | ― | [116] |
| ― | 35 | 749 | 90.1 | 50 | 3 | ― | ― | ― | [117] | |
| HNT | 5 | 34 | 936.7 | 98 | 50 | 3 | 0.71 | ― | ― | [117] |
| HNT | 10 | 31 | 773.6 | 99.3 | 50 | 3 | 0.77 | ― | ― | [117] |
| HNT | 15 | 32 | 557.9 | 91.9 | 50 | 3 | 1.20 | ― | ― | [117] |
|
Melamine and phytic acid modified HNT
(m-HNT) |
5 | 30 | 713.2 | 89.4 | 50 | 3 | 0.90 | ― | ― | [117] |
|
Melamine and phytic acid modified HNT
(m-HNT) |
10 | 28 | 708.4 | 93.4 | 50 | 3 | 0.81 | ― | ― | [117] |
|
Melamine and phytic acid modified HNT
(m-HNT) |
15 | 27 | 678.8 | 89.3 | 50 | 3 | 0.85 | ― | ― | [117] |
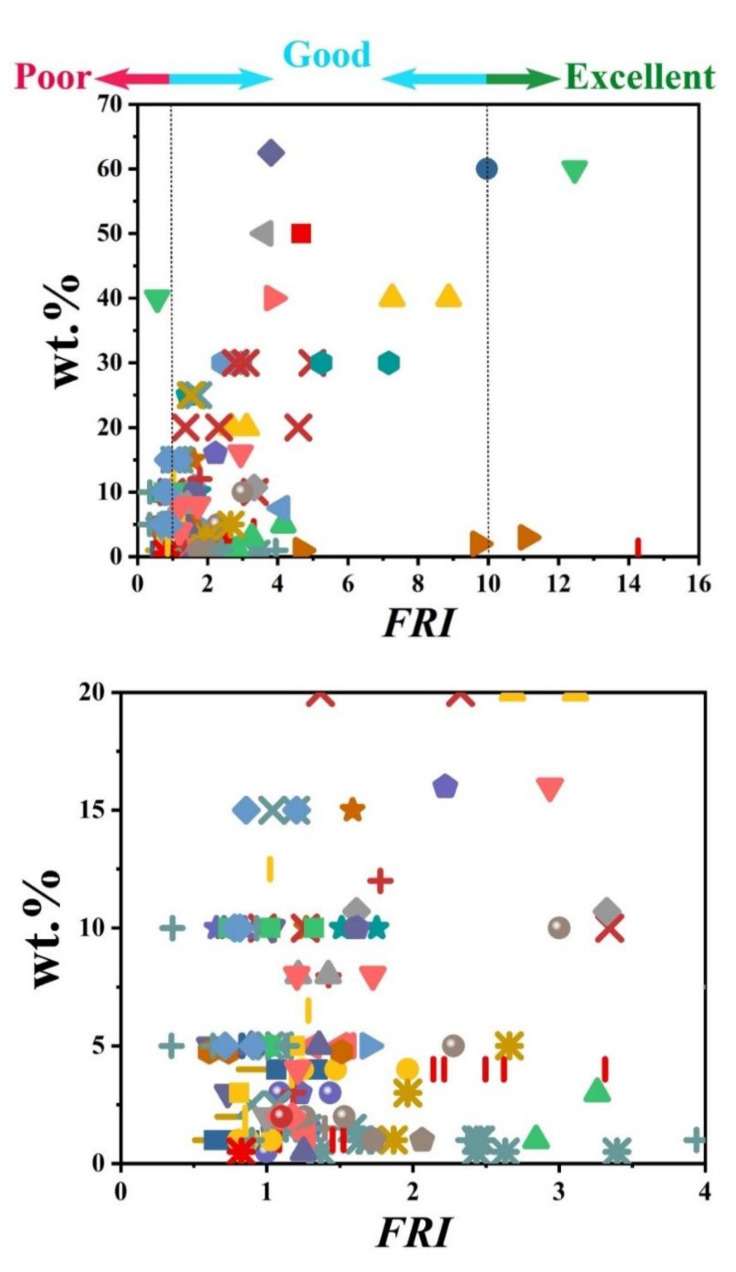
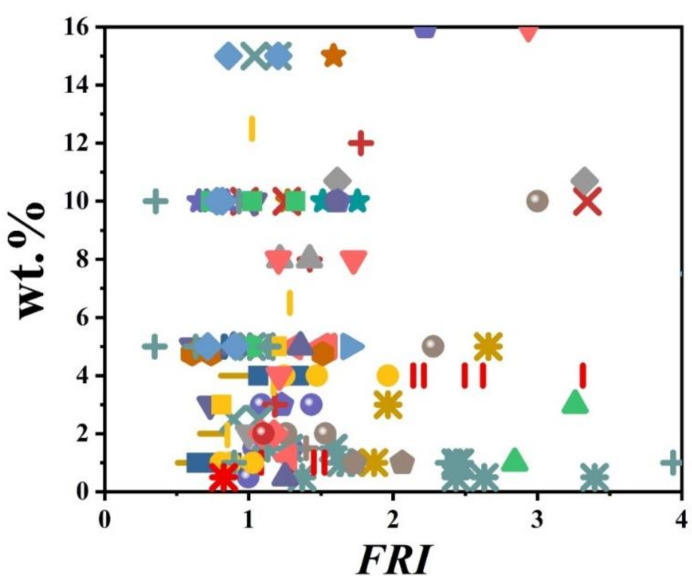
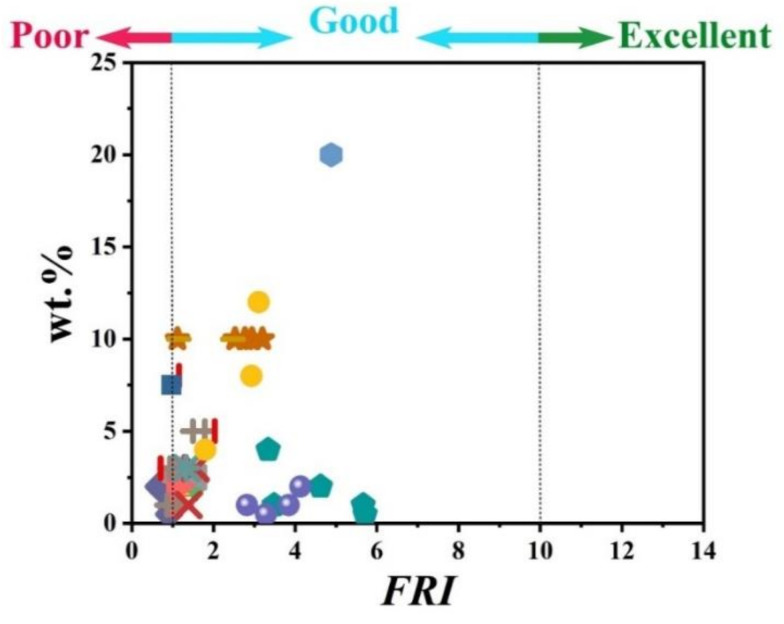
Figure 7 visualizes the variation of FRI value as a function of flame retardant loading in PP systems (for the convenience of readers, two figures are added for giving a better zoom on data points). This figure clearly shows that even at low loading percentages, it is possible to achieve a relatively high FRI value depending on the type of mineral. There is no denying that some parameters such as the state of dispersion and size of particles are important factors affecting the flame retardant properties.
Figure 7.
FRI values as a function of the mineral FR type and content from close-up and long-shot views. Symbols are indicative of different types of mineral flame retardant used. The diversity and abundance of data were reasons why such different scales were provided for detection of behavior of PP against flame. Here: ■ ATH-50 [80],  ATH-60 [81],
ATH-60 [81],
 ATH-20, ATH-40, MDH-20, MDH-40 [82],
ATH-20, ATH-40, MDH-20, MDH-40 [82],
 MDH-40, MDH-60 [82],
MDH-40, MDH-60 [82],  MDH-62.5 [83],
MDH-62.5 [83],
 MDH-50 [84],
MDH-50 [84],
 MDH-40 [85],
MDH-40 [85],
 MDH-30, m-MDH-30, m-MDH-30 [86],
MDH-30, m-MDH-30, m-MDH-30 [86],
 MDH-10, MDH-15 [87],
MDH-10, MDH-15 [87],
 Kaol-25 [64],
Kaol-25 [64],
 Kaol-0.5, Kaol-1.5, Kaol-3, m-Kaol-0.5, m-Kaol-1.5, m-Kaol-3 [88],
Kaol-0.5, Kaol-1.5, Kaol-3, m-Kaol-0.5, m-Kaol-1.5, m-Kaol-3 [88],
 Kaol-1.5, m-Kaol-1.5 [89],
Kaol-1.5, m-Kaol-1.5 [89],
 Kaol-10, Kaol-20, Kaol-30, m-Kaol-10, m-Kaol-20, m-Kaol-30, TC-10, TC-20, TC-30 [90],
Kaol-10, Kaol-20, Kaol-30, m-Kaol-10, m-Kaol-20, m-Kaol-30, TC-10, TC-20, TC-30 [90],
 LDH-0.5, LDH-1, LDH-1.5, m-LDH-0.5, m-LDH-1, m-LDH-1.5, LDH-0.5, LDH-1, LDH-1.5, m-LDH-0.5, m-LDH-1, m-LDH-1.5 [91],
LDH-0.5, LDH-1, LDH-1.5, m-LDH-0.5, m-LDH-1, m-LDH-1.5, LDH-0.5, LDH-1, LDH-1.5, m-LDH-0.5, m-LDH-1, m-LDH-1.5 [91],
 A-LDH-1, A-LDH-2, B-LDH-1, B-LDH-2, B-LDH-4, C-LDH-1, C-LDH-2, C-LDH-4, D-LDH-1, D-LDH-2, D-LDH-4, E-LDH-1, E-LDH-2, E-LDH-4 [92],
A-LDH-1, A-LDH-2, B-LDH-1, B-LDH-2, B-LDH-4, C-LDH-1, C-LDH-2, C-LDH-4, D-LDH-1, D-LDH-2, D-LDH-4, E-LDH-1, E-LDH-2, E-LDH-4 [92],
 A-LDH-1, A-LDH-4, B-LDH-1, B-LDH-4, C-LDH-1, C-LDH-4, D-LDH-1, D-LDH-4, E-LDH-1, E-LDH-4 [92],
A-LDH-1, A-LDH-4, B-LDH-1, B-LDH-4, C-LDH-1, C-LDH-4, D-LDH-1, D-LDH-4, E-LDH-1, E-LDH-4 [92],
 A-LDH-1, A-LDH-4, B-LDH-1, B-LDH-4, C-LDH-1, C-LDH-4, D-LDH-1, D-LDH-4, E-LDH-1, E-LDH-4 [92],
A-LDH-1, A-LDH-4, B-LDH-1, B-LDH-4, C-LDH-1, C-LDH-4, D-LDH-1, D-LDH-4, E-LDH-1, E-LDH-4 [92],
 A-LDH-1, A-LDH-4, C-LDH-1, C-LDH-4, E-LDH-1, E-LDH-4 [92],
A-LDH-1, A-LDH-4, C-LDH-1, C-LDH-4, E-LDH-1, E-LDH-4 [92],
 m-LDH-1, m-LDH-3, m-LDH-5 [93],
m-LDH-1, m-LDH-3, m-LDH-5 [93],
 m-LDH-3, m-LDH-5, m-LDH-10 [94],
m-LDH-3, m-LDH-5, m-LDH-10 [94],
 LDH-10.7, m-LDH-10.7 [95],
LDH-10.7, m-LDH-10.7 [95],
 alkyl-NH4Cl-1.2, MMT-5, H-MMT-5, m-MMT-5 [96],
alkyl-NH4Cl-1.2, MMT-5, H-MMT-5, m-MMT-5 [96],
 m-MMT-5 [96],
m-MMT-5 [96],
 m-MMT-4.75, m-MMT-4.75, m-MMT-4.75 [97],
m-MMT-4.75, m-MMT-4.75, m-MMT-4.75 [97],
 MMT-10, m-MMT-10 [24],
MMT-10, m-MMT-10 [24],
 m-MMT-3, m-MMT-10, m-MMT-16 [98],
m-MMT-3, m-MMT-10, m-MMT-16 [98],
 MMT-2, m-MMT-2, m-MMT-5, m-MMT-10 [99],
MMT-2, m-MMT-2, m-MMT-5, m-MMT-10 [99],
 m-MMT-3, m-MMT-8, m-MMT-12 [100],
m-MMT-3, m-MMT-8, m-MMT-12 [100],
 m-MMT-2.5, m-MMT-5, m-MMT-15, m-MMT-25, m-MMT-2.5, m-MMT-5, m-MMT-15, m-MMT-25 [101],
m-MMT-2.5, m-MMT-5, m-MMT-15, m-MMT-25, m-MMT-2.5, m-MMT-5, m-MMT-15, m-MMT-25 [101],
 m-MMT-1, m-MMT-3, m-MMT-5 [102],
m-MMT-1, m-MMT-3, m-MMT-5 [102],
 Nf-5, m-BT-5 [103],
Nf-5, m-BT-5 [103],
 Nf-5, m-BT-5 [103],
Nf-5, m-BT-5 [103],
 C20A-1, C20A-3, C20A-5 [104],
C20A-1, C20A-3, C20A-5 [104],
 C15A-5 [60],
C15A-5 [60],
 C20A-5, C20A-5, TiO2-0.5 [105],
C20A-5, C20A-5, TiO2-0.5 [105],
 Al2O3-2 [106],
Al2O3-2 [106],
 NiFeO-2, CoFeO-2 [107],
NiFeO-2, CoFeO-2 [107],
 Ni2O3-7.5 [108],
Ni2O3-7.5 [108],
 Nmm-cat-1, Nmm-cat-2, Nmm-cat-3 [109],
Nmm-cat-1, Nmm-cat-2, Nmm-cat-3 [109],
 MOSw-30, m-MOSw-30 [110],
MOSw-30, m-MOSw-30 [110],
 MnO-10, Mn2O3-10, MnC2O4-10 [111],
MnO-10, Mn2O3-10, MnC2O4-10 [111],
 Znacac-1, Cracac-1 [53],
Znacac-1, Cracac-1 [53],
 ZrPP-2 [112],
ZrPP-2 [112],
 S4SQH-1, S4SQH-5, S4SQH-10, m-S4SQH-1, m-S4SQH-5, m-S4SQH-1, m-S4SQH-5, m-S4SQH-10 [113],
S4SQH-1, S4SQH-5, S4SQH-10, m-S4SQH-1, m-S4SQH-5, m-S4SQH-1, m-S4SQH-5, m-S4SQH-10 [113],
 Si-FR-25 [30],
Si-FR-25 [30],
 SEP-0.5, m-SEP-0.5 [25],
SEP-0.5, m-SEP-0.5 [25],  SEP-5, m-SEP-5 [87],
SEP-5, m-SEP-5 [87],
 me-POSS-1.95, me-POSS-6.5, ph-POSS-3.75, ph-POSS-12.5 [114],
me-POSS-1.95, me-POSS-6.5, ph-POSS-3.75, ph-POSS-12.5 [114],
 T8-POSS-10, Al-POSS-10, Zn-POSS-10 [115],
T8-POSS-10, Al-POSS-10, Zn-POSS-10 [115],
 SA-10 [5],
SA-10 [5],
 HNT-8, HNT-W-8 [116],
HNT-8, HNT-W-8 [116],
 HNT-8, HNT-W-4, HNT-W-8, HNT-W-16 [116],
HNT-8, HNT-W-4, HNT-W-8, HNT-W-16 [116],
 HNT-5, HNT-10, HNT-15, m-HNT-5, m-HNT-10, m-HNT-15 [117].
HNT-5, HNT-10, HNT-15, m-HNT-5, m-HNT-10, m-HNT-15 [117].
Unfortunately, the number of papers in which cone calorimetry, UL-94, and LOI values were studied was indeed limited, but the ones available are used plotting Figure 8. It should be noted that no formulation among studied ones is rated at V0. In conclusion, it is quite difficult to find a correlation between quantitative and qualitative parameters based on such a tiny set of data. In regard to the relationship between LOI and FRI, a meaningful trend can still be seen in Figure 9.
Figure 8.
FRI values versus UL-94 test results. Symbols are indicative of different types of mineral flame retardant (FR) used. The vertical intervals in each category, i.e., V-0, V-1, V-2, and NR, are schematically representative of the amount of additive used. For example, two data distinguished by different symbols having the same or very close FRI values (horizontal quantity) in a given category (e.g., V-1), may have different vertical quantities, e.g., both reveal V-1 behavior in UL-94 test, but the upper contains more FR in PP.
Figure 9.
FRI values of PP as a function of LOI test results. Symbols are indicative of different types of mineral flame retardant used.
5. Carbon-Based Flame Retardants
Carbon-based additives have been widely used in developing polymer composites and nanocomposites [118,119,120,121]. However, due to expense and limited interaction with PP, a few works based on carbon-based flame retardants have been reported on flame-retardant PP materials. Table 4 summarizes all information available on the flame-retardant PP materials containing carbon-based additives.
Table 4.
Flame-retardant PP materials containing carbon-based (C) flame retardants. Data are extracted from the literature: cone calorimetry parameters (TTI, pHRR, THR), LOI, and UL-94 values. The FRI values were calculated by authors of the present review. The name and the percentage of flame retardants are provided in separate columns. “wt.%” was used for loading level of additives, while “―” stands for the systems free of additive or the neat PP. * FR means flame retardant. Since all comparisons were made in terms of FRI, classification of polymers in terms of their flame-retardant properties was not surveyed based on the chemistry of additives, heat flux, sample thickness, etc.
| PP Containing Carbon-Based (C) FR * | wt.% | TTI (s) |
pHRR (kW·m−2) |
THR (MJ·m−2) |
Irradiance (kW·m−2) |
Sample Thickness (mm) | FRI | LOI | UL-94 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| ― | 49 | 1247 | 114.2 | 35 | ― | ― | ― | ― | [106] | |
| Graphene (GN) | 2 | 35 | 989 | 107.9 | 35 | ― | 0.95 | ― | ― | [106] |
| Activated alumina decorated GN (m-GN) | 2 | 41 | 866 | 110.5 | 35 | ― | 1.24 | ― | ― | [106] |
| ― | 51 | 1053 | 118.4 | 35 | 3 | ― | ― | ― | [122] | |
| P-phenylenediamine modified reduced graphene oxide (m–rGNO) | 2 | 33 | 928 | 104 | 35 | 3 | 0.83 | ― | ― | [122] |
|
Polyaniline nanofiber modified rGNO
(m–rGNO) |
2 | 27 | 763 | 98.4 | 35 | 3 | 0.87 | ― | ― | [122] |
| ― | 45 | 1230 | 113.6 | 35 | 3 | ― | ― | ― | [123] | |
| rGNO | 2 | 30 | 1105 | 97.5 | 35 | 3 | 0.86 | ― | ― | [123] |
| Hexachlorocyclotriphosphazene modified rGNO (m–rGNO) | 2 | 27 | 967 | 112.9 | 35 | 3 | 0.76 | ― | ― | [123] |
|
Hexachlorocyclotriphosphazene modified rGNO decoration with Ni(OH)2 nanosheet
(m–rGNO) |
2 | 35 | 829 | 92 | 35 | 3 | 1.42 | ― | ― | [123] |
| ― | 51 | 1053 | 117.6 | 35 | 3 | ― | ― | ― | [112] | |
| rGNO | 2 | 31 | 835 | 98.3 | 35 | 3 | 0.91 | ― | ― | [112] |
| Zirconium phenylphosphonate decorated rGNO (m-rGNO) | 2 | 39 | 676 | 89.8 | 35 | 3 | 1.55 | ― | ― | [112] |
| ― | 50 | 1044 | 101.4 | 35 | 3 | ― | ― | ― | [124] | |
| Graphene oxide (GNO) | 2 | 33 | 979 | 108.2 | 35 | 3 | 0.65 | ― | ― | [124] |
| Melamine modified GNO (m-GNO) | 0.5 | 40 | 892 | 104.1 | 35 | 3 | 0.91 | ― | ― | [124] |
| Melamine modified GNO (m-GNO) | 1 | 37 | 834 | 100.6 | 35 | 3 | 0.93 | ― | ― | [124] |
| Melamine modified GNO (m-GNO) | 2 | 33 | 739 | 98.7 | 35 | 3 | 0.95 | ― | ― | [124] |
| ― | 38 | 1526 | 47.4 | 35 | ― | ― | ― | ― | [125] | |
| GN | 2.5 | 39 | 1279 | 58.8 | 35 | ― | 0.98 | ― | ― | [125] |
| GN-Nickel oxide (GN-NiO) | 2.5 | 35 | 1110 | 45.4 | 35 | ― | 1.32 | ― | ― | [125] |
| GN and Ni–Ce mixed oxide (GN-NiCexOy) | 2.5 | 32 | 956 | 39.2 | 35 | ― | 1.62 | ― | ― | [125] |
| ― | 32 | 909 | 45.8 | 35 | ― | ― | ― | ― | [126] | |
| rGNO | 2 | 28 | 778 | 40 | 35 | ― | 1.17 | ― | ― | [126] |
|
Phosphomolybdic acid modified rGNO
(m-rGNO) |
1 | 27 | 773 | 39.6 | 35 | ― | 1.14 | ― | ― | [126] |
|
Phosphomolybdic acid modified rGNO
(m-rGNO) |
2 | 23 | 737 | 38.4 | 35 | ― | 1.05 | ― | ― | [126] |
|
Phosphomolybdic acid modified rGNO
(m-rGNO) |
3 | 25 | 700 | 38.4 | 35 | ― | 1.21 | ― | ― | [126] |
| ― | 54 | 1199 | 97.8 | 35 | ― | ― | ― | ― | [58] | |
| Poly(4,4-diaminodiphenyl methane spirocyclicpentaerythritol bisphosphonate)-4,4-diaminodiphenyl methane modified rGNO (m-rGNO) | 20 | 66 | 397 | 73.9 | 35 | ― | 4.88 | ― | ― | [58] |
| ― | 66 | 383 | 76 | 35 | 3 | ― | ― | ― | [127] | |
| Expandable graphite with commercial name ES 350 F5 (EG(ES 350 F5)) | 10 | 32 | 91 | 56 | 35 | 3 | 2.76 | ― | ― | [127] |
| EG with commercial name ES 700 F5 (EG(ES 700 F5)) | 10 | 35 | 92 | 57 | 35 | 3 | 2.94 | ― | ― | [127] |
| EG with commercial name Nyagraph FP (EG(Nyagraph FP)) | 10 | 44 | 92 | 66 | 35 | 3 | 3.19 | ― | ― | [127] |
| EG with commercial name TEG 315 (EG(TEG 315)) | 10 | 53 | 134 | 69 | 35 | 3 | 2.52 | ― | ― | [127] |
| EG with commercial name Nyagraph KP251 (EG(Nyagraph KP251)) | 10 | 54 | 308 | 69 | 35 | 3 | 1.12 | ― | ― | [127] |
| ― | 38 | 1361 | 85 | 35 | 3 | ― | ― | ― | [128] | |
| Carbon nanotube (CNT) | 1 | 37 | 431 | 75 | 35 | 3 | 3.48 | ― | ― | [128] |
| Modofied CNT (m-CNT) | 0.5 | 42 | 361 | 62 | 35 | 3 | 5.71 | ― | ― | [128] |
| Modofied CNT (m-CNT) | 1 | 42 | 342 | 66 | 35 | 3 | 5.66 | ― | ― | [128] |
| Modofied CNT (m-CNT) | 2 | 41 | 386 | 70 | 35 | 3 | 4.61 | ― | ― | [128] |
| Modofied CNT (m-CNT) | 4 | 39 | 450 | 79 | 35 | 3 | 3.33 | ― | ― | [128] |
| ― | 40 | 1360 | 80 | 35 | 3 | ― | ― | ― | [129] | |
| CNT | 1 | 35 | 462 | 73 | 35 | 3 | 2.82 | ― | ― | [129] |
| Fullerene C60 decorated CNT (m-CNT) | 0.5 | 38 | 443 | 71 | 35 | 3 | 3.28 | ― | ― | [129] |
| Fullerene C60 decorated CNT (m-CNT) | 1 | 39 | 400 | 69 | 35 | 3 | 3.84 | ― | ― | [129] |
| Fullerene C60 decorated CNT (m-CNT) | 2 | 38 | 385 | 65 | 35 | 3 | 4.13 | ― | ― | [129] |
| ― | 35 | 1203 | 208 | 50 | 6 | ― | 18.2 | ― | [130] | |
| Multiwall carbon nanotube (MWCNT) | 1 | 24 | 945 | 211 | 50 | 6 | 0.86 | 19.3 | ― | [130] |
| MWCNT | 3 | 23 | 845 | 208 | 50 | 6 | 0.93 | 21.8 | ― | [130] |
| MWCNT | 5 | 23 | 553 | 199 | 50 | 6 | 1.49 | 23.4 | ― | [130] |
| Modified MWCNT (m-MWCNT) | 1 | 25 | 775 | 208 | 50 | 6 | 1.10 | 20 | ― | [130] |
| Modified MWCNT (m-MWCNT) | 3 | 24 | 670 | 200 | 50 | 6 | 1.28 | 22.6 | ― | [130] |
| Modified MWCNT (m-MWCNT) | 5 | 24 | 485 | 198 | 50 | 6 | 1.78 | 24.1 | ― | [130] |
| ― | 30 | 1261 | 208 | 50 | 6 | ― | 18 | ― | [131] | |
| MWCNT | 1 | 21 | 678 | 195 | 50 | 6 | 1.38 | 19.5 | ― | [131] |
| MWCNT | 3 | 20 | 584 | 192 | 50 | 6 | 1.55 | 22.6 | ― | [131] |
| ― | 24 | 1620 | 110 | 50 | 3 | ― | ― | ― | [132] | |
| MWCNT | 3 | 17 | 931 | 102 | 50 | 3 | 1.32 | ― | ― | [132] |
| ― | 38 | 1284 | 214 | 50 | 6 | ― | 18.2 | ― | [133] | |
| MWCNT | 10 | 25 | 367 | 199 | 50 | 6 | 2.47 | 24.6 | ― | [133] |
| Carbon fiber (CF) | 10 | 30 | 915 | 207 | 50 | 6 | 1.14 | 20.6 | ― | [133] |
| ― | 35 | 1212 | 198 | 50 | 6 | ― | 18.2 | ― | [134] | |
| CF | 3 | 25 | 1203 | 203 | 50 | 6 | 0.70 | 19.9 | ― | [134] |
| CF | 8 | 26 | 777 | 198 | 50 | 6 | 1.15 | 20.2 | ― | [134] |
| Carbon black (CB) | 5 | 23 | 417 | 186 | 50 | 6 | 2.03 | 24.6 | ― | [134] |
| ― | 38 | 1284 | 241 | 50 | 6 | ― | ― | ― | [108] | |
| Activated carbon (AC) | 7.5 | 15 | 682 | 185 | 50 | 6 | 0.96 | ― | ― | [108] |
| ― | 48 | 1518 | 112.4 | 35 | 3.1 | ― | ― | ― | [6] | |
| Vapor grown carbon nanofiber (VGCNF) | 4 | 35 | 610 | 113.1 | 35 | 3.1 | 1.80 | ― | ― | [6] |
| VGCNF | 8 | 47 | 525 | 108.6 | 35 | 3.1 | 2.93 | ― | ― | [6] |
| VGCNF | 12 | 49 | 547 | 102.4 | 35 | 2.9 | 3.10 | ― | ― | [6] |
Figure 10 shows that with low loading percentage (1 wt.%) of carbon nanotubes, it is possible to achieve the Good FRI. No data were available for UL-94 tests. Comparison between Figure 7 and Figure 10 also suggests that low-cost minerals were used at higher loadings, while carbon-based additives were used almost at loadings below 10 wt.%. A limited number of data have also been reported on LOI values. These points are plotted as a function of FRI in Figure 11, where a good correlation can be established between FRI and LOI values. Deeper understanding of the mechanism behind such correlation requires a detailed view of the origin of tests as well as the chemical structure of additives and possible interaction between the PP and additives.
Figure 10.
FRI values as a function of carbonaceous FR type and content. Symbols are indicative of different types of carbonaceous flame retardant used. Here: ■ GN-2, m-GN-2 [106],
 m–rGNO-2, m–rGNO-2 [122],
m–rGNO-2, m–rGNO-2 [122],
 rGNO-2, m-rGNO-2, m-rGNO-2 [123],
rGNO-2, m-rGNO-2, m-rGNO-2 [123],
 rGNO-2, m-rGNO-2 [112],
rGNO-2, m-rGNO-2 [112],
 GNO-2, m-GNO-0.5, m-GNO-1, m-GNO-2 [124],
GNO-2, m-GNO-0.5, m-GNO-1, m-GNO-2 [124],
 GN-2.5, GN-NiO-2.5, GN-NiCexOy-2.5 [125],
GN-2.5, GN-NiO-2.5, GN-NiCexOy-2.5 [125],
 rGNO-2, m-rGNO-1, m-rGNO-2, m-rGNO-3 [126],
rGNO-2, m-rGNO-1, m-rGNO-2, m-rGNO-3 [126],
 m-rGNO-20 [58],
m-rGNO-20 [58],
 EG(ES 350 F5)-10, EG(ES 700 F5)-10, EG(Nyagraph FP)-10, EG(TEG 315)-10, EG(Nyagraph KP251)-10 [127],
EG(ES 350 F5)-10, EG(ES 700 F5)-10, EG(Nyagraph FP)-10, EG(TEG 315)-10, EG(Nyagraph KP251)-10 [127],
 CNT-1, m-CNT-0.5, m-CNT-1, m-CNT-2, m-CNT-4 [128],
CNT-1, m-CNT-0.5, m-CNT-1, m-CNT-2, m-CNT-4 [128],
 CNT-1, m-CNT-0.5, m-CNT-1, m-CNT-2 [129],
CNT-1, m-CNT-0.5, m-CNT-1, m-CNT-2 [129],
 MWCNT-1, MWCNT-3, MWCNT-5, m-MWCNT-1, m-MWCNT-3, m-MWCNT-5 [130],
MWCNT-1, MWCNT-3, MWCNT-5, m-MWCNT-1, m-MWCNT-3, m-MWCNT-5 [130],
 MWCNT-1, MWCNT-3 [131],
MWCNT-1, MWCNT-3 [131],
 MWCNT-3 [132],
MWCNT-3 [132],
 MWCNT-10, CF-10 [133],
MWCNT-10, CF-10 [133],
 CF-3, CF-8, CB-5 [134],
CF-3, CF-8, CB-5 [134],
 AC-7.5 [108],
AC-7.5 [108],
 VGCNF-4, VGCNF-8, VGCNF-12 [6].
VGCNF-4, VGCNF-8, VGCNF-12 [6].
Figure 11.
FRI values of PP as a function of LOI test results. Symbols are indicative of different types of carbon-based flame retardant used.
6. Bio-Based Flame Retardants
In recent years, due to sustainability issues, the use of bio-based additives has also been investigated in PP. However, the number of research papers is limited on this subject. Table 5 gives the name and loading percentage of these bio-based FR. The obtained results from cone calorimetry, LOI, and UL-94 tests are also listed in Table 5. Figure 12 and Figure 13 display UL-94 and LOI results as a function of FRI for bio-based flame retardant in PP, respectively.
Table 5.
Flame-retardant PP materials containing bio-based (Bio) flame retardants. Data are extracted from the literature: cone calorimetry parameters (TTI, pHRR, THR), LOI, and UL-94 values. The FRI values were calculated by authors of the present review. The name and the percentage of flame retardants are provided in separate columns. “wt.%” was used for loading level of additives, while “―” stands for the systems free of additive or the neat PP. * FR means flame retardant. Since all comparisons were made in terms of FRI, classification of polymers in terms of their flame-retardant properties was not surveyed based on the chemistry of additives, heat flux, sample thickness, etc.
| PP Containing Bio-Based (Bio) FR * | wt.% | TTI (s) | pHRR (kW·m−2) | THR (MJ·m−2) | Irradiance (kW·m−2) | Sample Thickness (mm) | FRI | LOI | UL-94 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| ― | 46 | 1541 | 90 | 35 | 3 | ― | ― | ― | [135] | |
| Cyclodextrin nanosponge (CD) | 10 | 34 | 1462 | 80 | 35 | 3 | 0.87 | ― | ― | [135] |
| ― | 61 | 1026 | 166 | 35 | 4 | ― | ― | ― | [15] | |
| Hydroxyapatite and Cyclodextrin-based FR (HAandCD-FR) | 10 | 32 | 708 | 156 | 35 | 4 | 0.80 | ― | ― | [15] |
| Propylene-block-ethylene copolymer | ― | 49 | 1350 | 87.3 | 35 | 3 | ― | 17.5 | ― | [136] |
| Phosphorus and nitrogen elements modified lignin (m-lig) | 20 | 38 | 380 | 74.2 | 35 | 3 | 3.24 | 22.5 | ― | [136] |
| ― | 22.5 | 1004.7 | 122.6 | 50 | 3.2 | ― | 18 | NR | [137] | |
| Phytic acid and Piperazine-based FR (PHPI-FR) | 15 | 17.5 | 388.5 | 108.5 | 50 | 3.2 | 2.27 | 24 | V-2 | [137] |
| PHPI-FR | 18 | 17 | 386.2 | 108.4 | 50 | 3.2 | 2.22 | 25 | V-0 | [137] |
| PHPI-FR | 20 | 17 | 346 | 106.1 | 50 | 3.2 | 2.53 | 25.5 | V-0 | [137] |
| PHPI-FR | 25 | 16.5 | 303.4 | 105.4 | 50 | 3.2 | 2.82 | 27 | V-0 | [137] |
| ― | 29 | 1054 | 97 | 50 | ― | ― | ― | ― | [138] | |
| Biochar (BC) | 15 | 12 | 753.01 | 112.68 | 50 | ― | 0.49 | ― | ― | [138] |
| BC | 25 | 13.3 | 616.31 | 111.26 | 51 | ― | 0.68 | ― | ― | [138] |
| BC | 30 | 15 | 539.34 | 101.2 | 52 | ― | 0.96 | ― | ― | [138] |
| BC | 35 | 16.3 | 477.22 | 98.31 | 53 | ― | 1.22 | ― | ― | [138] |
| ― | 24.3 | 1388.3 | 80.3 | 50 | 2.4 | ― | ― | NR | [28] | |
| Wool | 40 | 12.3 | 858.7 | 77.3 | 50 | 2.4 | 0.85 | ― | NR | [28] |
| Phosphoric acid-treated wool fiber (m-wool) | 40 | 14.3 | 426.7 | 72 | 50 | 2.4 | 2.13 | ― | NR | [28] |
| Phosphoric acid-treated wool fiber (m-wool) | 40 | 15 | 436.3 | 65.3 | 50 | 2.4 | 2.41 | ― | V-0 | [28] |
|
Phosphoric acid-treated chicken feather
(m-CF) |
40 | 14.7 | 336.7 | 57 | 50 | 2.4 | 3.51 | ― | V-0 | [28] |
| ― | 24.7 | 1198.2 | 78.7 | 50 | 2.4 | ― | ― | NR | [139] | |
| Chicken feather (CF) | 40 | 17 | 1234.1 | 76.1 | 50 | 2.4 | 0.69 | ― | NR | [139] |
| Phosphoric acid and ethylenediamine treated chicken feather (m-CF) | 40 | 19.3 | 280.5 | 58.7 | 50 | 2.4 | 4.47 | ― | V-0 | [139] |
| Phosphoric acid and ethylenediamine treated chicken feather (m-CF) | 40 | 17.7 | 216.1 | 52.4 | 50 | 2.4 | 5.96 | ― | V-0 | [139] |
Figure 12.
FRI values versus UL-94 test results. Symbols are indicative of different types of bio-based flame retardant (FR) used. The vertical intervals in each category, i.e., V-0, V-1, V-2, and NR, are schematically representative of the amount of additive used. For example, two data distinguished by different symbols having the same or very close FRI values (horizontal quantity) in a given category (e.g., V-1) may have different vertical quantities, e.g., both reveal V-1 behavior in UL-94 test, but the upper contains more FR in PP.
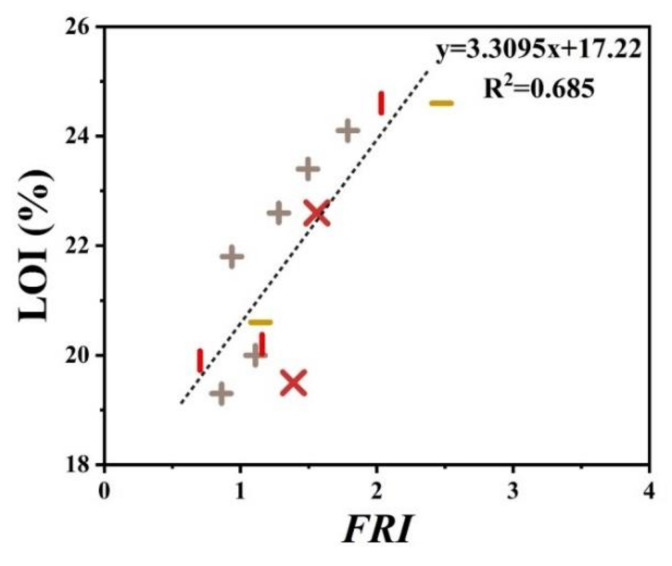
Figure 13.
FRI values of PP as a function of LOI test results. Symbols are indicative of different types of bio-based flame retardant used. The green triangles are related to a mixture of phytic acid and piperazine-based FR. The increase of LOI is directly related to the percentage of FR loading, 15, 18, 20, and 25 wt.%.
FRI values are plotted as a function of loading percentage of bio-based FR in Figure 14. It can be observed that a high quantity of bio-based FR, 40 wt.% is needed to achieve FRI equal to 6.
Figure 14.
FRI values as a function of bio-based FR type and content. Symbols are indicative of different types of bio-based flame retardant used. Here: ■ CD-10 [135],  HAandCD-FR-10 [15],
HAandCD-FR-10 [15],
 m-lig-20 [136],
m-lig-20 [136],
 PHPI-FR-15, PHPI-FR-18, PHPI-FR-20, PHPI-FR-25 [137],
PHPI-FR-15, PHPI-FR-18, PHPI-FR-20, PHPI-FR-25 [137],
 BC-15, BC-25, BC-30, BC-35 [138],
BC-15, BC-25, BC-30, BC-35 [138],
 Wool-40, m-wool-40, m-wool-40, m-CF-40 [28],
Wool-40, m-wool-40, m-wool-40, m-CF-40 [28],
 CF-40, m-CF-40, m-CF-40 [139].
CF-40, m-CF-40, m-CF-40 [139].
7. Combination of Flame Retardants
As observed in previous sections, using an additive alone can to a limited extent improve flame-retardant properties of PP. Combination of flame retardants is a strategy to improve further the flame retardancy via synergism between various flame retardants [140,141,142]. Moreover, the quantity of the used flame retardant can be reduced in polymer so as to prevent mechanical properties deterioration. Different combinative additive systems were considered in PP. The corresponded data are collected and summarized in Table 6. The third column gives the ratio between flame retardants.
Table 6.
The flame retardancy performance of PP containing various combinations of flame retardants in terms of FRI (* the name and percentage of incorporated flame retardants are given after PP). P = phosphorus FR, Np = non-phosphorus FR, N = nitrogen FR, nN = non-nitrogen-based FR, M = mineral FR, Bio = bio-based FR, nBio = non bio-based FR (one can also consider some nitrogen-based FRs containing phosphorus element as the combination of phosphorus and nitrogen resulting in synergism, Table 2). Since all comparisons were made in terms of FRI, classification of polymers in terms of their flame-retardant properties was not surveyed based on the chemistry of additives, heat flux, sample thickness, etc.
| Name | wt.% | Type of FR | TTI (s) |
pHRR (kW·m−2) |
THR (MJ·m−2) |
Irradiance (kW·m−2) |
Sample Thickness (mm) | FRI | LOI | UL-94 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ― | ― | 31 | 1239 | 123.6 | 50 | 3 | ― | 18.5 | NR | [68] | |
| APP/Pentaerythritol (APP/PER) | 20 | P:nP 2:1 |
18 | 514.7 | 92.6 | 50 | 3 | 1.86 | 27.6 | V-2 | [68] |
| ― | ― | 25 | 1239 | 123.6 | 50 | 3 | ― | 18.5 | NR | [143] | |
| APP/PER | 25 | P:nP 2:1 |
17 | 442.3 | 98.8 | 50 | 3 | 2.38 | 30.1 | V-0 | [143] |
| ― | ― | 41 | 840.3 | 115.7 | 35 | 3 | ― | 16.4 | NR | [67] | |
| APP/PER | 25 | P:nP 3:1 |
32 | 354.7 | 82 | 35 | 3 | 2.60 | 24.7 | V-1 | [67] |
| ― | ― | 34 | 1727 | 112 | 35 | 3 | ― | 18 | NR | [144] | |
| APP/PER | 30 | P:nP 3:1 |
13 | 392 | 80 | 35 | 3 | 2.35 | 31 | V-0 | [144] |
| Hydroxyl silicone oil co-microencapsulated APP and PER (mc-(APPandPER)) | 30 | P:nP 3:1 |
10 | 325 | 78 | 35 | 3 | 2.24 | 32.5 | V-0 | [144] |
| ― | ― | 166 | 412 | 105 | 25 | 3 | ― | ― | ― | [145] | |
| APP/PER /Melamine (APP/PER/MEL) | 29.4 | P:nP:nP 1.07:1:0.92 |
175 | 76.4 | 55 | 25 | 3 | 10.85 | ― | ― | [145] |
| APP/PER/MEL | 33.2 | P:nP:nP 1.64:1:0.94 |
188 | 65 | 46.3 | 25 | 3 | 16.27 | ― | ― | [145] |
| APP/PER/MEL | 36.2 | P:nP:nP 2.14:1:0.92 |
180 | 68 | 49.4 | 25 | 3 | 13.96 | ― | ― | [145] |
| ― | ― | 24 | 687 | 119 | 50 | 3 | ― | ― | ― | [145] | |
| APP/PER/MEL | 29.4 | P:nP:nP 1.07:1:0.92 |
33 | 158 | 72.5 | 50 | 3 | 9.81 | ― | ― | [145] |
| APP/PER/MEL | 33.2 | P:nP:nP 1.64:1:0.94 |
36 | 115 | 67.8 | 50 | 3 | 15.72 | ― | ― | [145] |
| APP/PER/MEL | 36.2 | P:nP:nP 2.14:1:0.92 |
30 | 133 | 73.2 | 50 | 3 | 10.49 | ― | ― | [145] |
| ― | ― | 24 | 687 | 119 | 25 | 3 | ― | 17.8 | ― | [146] | |
| APP/PER/MEL | 33.2 | P:nP:nP 1.64:1:0.93 |
36 | 115 | 67.8 | 25 | 3 | 15.72 | 34.5 | ― | [146] |
| APP/PER/MEL/MDH | 37.3 | P:nP:nP:nP 1.64:1:0.9:0.7 |
36 | 156 | 63.9 | 25 | 3 | 12.30 | 25.2 | ― | [146] |
| ― | ― | 166 | 412 | 105 | 50 | 3 | ― | 17.8 | [146] | ||
| APP/PER/MEL | 33.2 | P:nP:nP 1.64:1:0.93 |
188 | 65 | 46.3 | 50 | 3 | 16.27 | 34.5 | ― | [146] |
| APP/PER/MEL/MDH | 37.3 | P:nP:nP:nP 1.64:1:0.9:0.7 |
196 | 79.5 | 45.1 | 50 | 3 | 14.24 | 25.2 | ― | [146] |
| ― | ― | 35 | 1622 | 103 | 35 | 3 | ― | ― | ― | [104] | |
| APP/PER/MEL/C20A | 21 | P:nP:nP:nP 3:1:1:0.25 |
25 | 463 | 89 | 35 | 3 | 2.89 | ― | ― | [104] |
| APP/PER/MEL/C20A | 23 | P:nP:nP:nP 3:1:1:0.75 |
26 | 430 | 91 | 35 | 3 | 3.17 | ― | ― | [104] |
| APP and MMT/PER/MEL/C20A | 21 | P:nP:nP:nP 3:1:1:0.25 |
24 | 306 | 81 | 35 | 3 | 4.62 | ― | ― | [104] |
| APP and MMT/PER/MEL/C20A | 23 | P:nP:nP:nP 3:1:1:0.75 |
21 | 344 | 80 | 35 | 3 | 3.64 | ― | ― | [104] |
| ― | ― | 48 | 662 | 83.3 | 35 | 4 | ― | 17.6 | NR | [147] | |
|
γ-aminopropyltriethoxysilane modified APP/Dipentaerythritol/MEL
(m-APP/DPER/MEL) |
25 | P:nP:nP 4:1:1 |
30 | 71 | 32.5 | 35 | 4 | 14.93 | 34.4 | V-1 | [147] |
|
γ-aminopropyltriethoxysilane modified APP/ DPER/MEL/SEP
(m-APP-/DPER/MEL/SEP) |
25 | P:nP:nP:nP 4:1:1:0.25 |
29 | 51 | 30.8 | 35 | 4 | 21.20 | 36 | V-0 | [147] |
| ― | ― | 48 | 1007 | 126.3 | 35 | 3.2 | ― | 17.5 | NR | [148] | |
| APP/PER | 18 | P:nP 3:1 |
45 | 423.5 | 112 | 35 | 3.2 | 2.51 | 24.6 | NR | [148] |
| APP/PER/Melamine fomaldehyde (APP/PER/MF) | 18 | P:nP:nP 3:1:0.2 |
48 | 352.3 | 110.4 | 35 | 3.2 | 3.27 | 26.3 | NR | [148] |
| APP/PER/Adenosine monophosphate embedded Melamine fomaldehyde (APP/PER/MFA) | 18 | P:nP:nP 3:1:0.2 |
49 | 355.1 | 108.1 | 35 | 3.2 | 3.38 | 27 | V-0 | [148] |
| ― | ― | 70 | 1267 | 147 | 50 | 3 | ― | 17.5 | NR | [149] | |
| APP/PER | 30 | P:nP 3:1 |
40 | 321 | 80 | 50 | 3 | 4.14 | 29.5 | V-1 | [149] |
| ― | ― | 47 | 991.4 | 140.8 | 35 | 3.2 | ― | 18.4 | NR | [150] | |
| APP/PER | 18 | P:nP 2.4:1 |
44 | 434.1 | 123.8 | 35 | 3.2 | 2.43 | 26.2 | NR | [150] |
| APP/PER /Triazine-based FR: N, N′, N″-1, 3, 5-triazine-2, 4, 6-triyltris-glycine (APP/PER/TA-FR) | 18 | P:nP:nP 2.4:1:0.2 |
42 | 323.3 | 126 | 35 | 3.2 | 3.06 | 29.5 | V-0 | [150] |
| ― | ― | 36 | 799.3 | 170.9 | 35 | 4 | ― | 18 | NR | [72] | |
| APP/PER | 25 | P:nP 2:1 |
23 | 322 | 144.5 | 35 | 4 | 1.87 | 31 | V-1 | [72] |
| APP/PER/NOR116 | 25 | P:nP:nP 2:1:0.06 |
33 | 313.8 | 136.5 | 35 | 4 | 2.92 | 35 | V-0 | [72] |
| ― | ― | 51 | 888 | 125 | 35 | 3.2 | ― | 17.5 | NR | [151] | |
| APP/PER | 18 | P:nP 3:1 |
45 | 439 | 111 | 35 | 3.2 | 2.00 | 24.6 | NR | [151] |
| APP/PER/Guanine: nitrogenous bases (APP/PER/G-bases) | 18 | P:nP:nP 3:1:0.3 |
46 | 324 | 105 | 35 | 3.2 | 2.94 | 27.6 | NR | [151] |
| APP/PER/Uracil: nitrogenous bases (APP/PER/U-bases) | 18 | P:nP:nP 3:1:0.3 |
46 | 293 | 105 | 35 | 3.2 | 3.25 | 28.7 | V-0 | [151] |
| ― | ― | 38 | 886 | 144 | 50 | 3 | ― | 17.6 | NR | [152] | |
| APP/DPER | 25 | P:nP 2.2:1 |
30 | 386 | 117 | 50 | 3 | 2.23 | 26.8 | V-1 | [152] |
| Aluminum chloride modified APP/ DPER (m-APP/DPER) | 25 | P:nP 2.2:1 |
25 | 226 | 104 | 50 | 3 | 3.57 | 32.1 | V-0 | [152] |
| APP/DPER/ATH | 25 | P:nP:nP 2:1:0.1 |
28 | 381 | 108 | 50 | 3 | 2.28 | 28.7 | V-0 | [152] |
| ― | ― | 31 | 1002 | 114 | 50 | 4 | ― | 17.8 | NR | [70] | |
| APP/PER | 28.5 | P:nP 3:1 |
24 | 318 | 122 | 50 | 4 | 2.27 | 30 | V-0 | [70] |
| APP/PER/Kaol | 28.5 | P:nP:nP 3:1:0.2 |
22 | 222 | 131 | 50 | 4 | 2.78 | 33 | V-0 | [70] |
| ― | ― | 70 | 415.6 | 99.6 | 35 | 3 | ― | 17 | NR | [153] | |
| APP/PER | 29 | P:nP 3:1 |
21 | 160.7 | 94.2 | 35 | 3 | 0.82 | 29.5 | V-0 | [153] |
| APP/PER/MMT | 29 | P:nP:nP 3:1:0.46 |
42 | 149.8 | 69.5 | 35 | 3 | 2.38 | 34.5 | V-0 | [153] |
| APP/PER/Melamine modified MMT (APP/PER/m-MMT) | 29 | P:nP:nP 3:1:0.46 |
37 | 157.9 | 55.1 | 35 | 3 | 2.51 | 36.5 | V-0 | [153] |
| ― | ― | 70 | 415.6 | 99.6 | 35 | 3 | ― | 17 | NR | [154] | |
| APP/PER | 29 | P:nP 3:1 |
21 | 160.7 | 94.2 | 35 | 3 | 0.82 | 29.5 | V-0 | [154] |
| APP/PER/MMT | 29 | P:nP:nP 3:1:0.46 |
42 | 149.8 | 69.5 | 35 | 3 | 2.38 | 34.5 | V-0 | [154] |
| APP/PER/Melamine modified MMT (APP/PER/m-MMT) | 29 | P:nP:nP 3:1:0.46 |
37 | 157.9 | 55.1 | 35 | 3 | 2.51 | 36.5 | V-0 | [154] |
| APP/PER/Triphenylphonium modified MMT (APP/PER/m-MMT) | 29 | P:nP:nP 3:1:0.46 |
38 | 168.2 | 84.7 | 35 | 3 | 1.57 | 34.8 | V-0 | [154] |
| ― | ― | 50 | 720 | 136 | 35 | 4 | ― | 17 | NR | [155] | |
| APP/PER | 25 | P:nP 3:1 |
39 | 267 | 111 | 35 | 4 | 2.57 | 26.3 | V-1 | [155] |
| APP/PER/Zn-Ni-Al LDH(APP/PER/LDH) | 25 | P:nP:nP 3:1:0.16 |
35 | 296 | 109 | 35 | 4 | 2.12 | 27 | V-1 | [155] |
|
APP/PER/Azobenzene-4,4′-dicarboxylic acid modified Ni-Zn-Al LDH
(APP/PER/m-LDH) |
25 | P:nP:nP 3:1:0.16 |
39 | 271 | 102 | 35 | 4 | 2.76 | 29.3 | V-0 | [155] |
| ― | ― | 53 | 655 | 108.1 | 35 | 3 | ― | 17.1 | NR | [156] | |
| APP/PER | 25 | P:nP 3:1 |
32 | 261 | 93.4 | 35 | 3 | 1.75 | 28.9 | V-2 | [156] |
| APP/PER/Acid-treated waste silicon rubber composite insulator (APP/PER/m-SiR) | 25 | P:nP:nP 3:1:0.16 |
29 | 273 | 92.6 | 35 | 3 | 1.53 | 28.5 | V-0 | [156] |
| APP/PER/Acid and N2 plasma-treated SiR (APP/PER/m-SiR) | 25 | P:nP:nP 3:1:0.16 |
29 | 205 | 91.8 | 35 | 3 | 2.05 | 30.9 | V-0 | [156] |
| APP/PER/Acid and N2 plasma-treated SiR (APP/PER/m-SiR) | 25 | P:nP:nP 3:1:0.16 |
24 | 208 | 97.5 | 35 | 3 | 1.58 | 27.4 | V-0 | [156] |
| ― | ― | 30 | 930 | 135 | 50 | 3 | ― | 17 | NR | [157] | |
| Methyl hydrogen siloxane-treated APP/DPER (m-APP/DPER) | 25 | P:nP 2:1 |
19 | 347 | 113 | 50 | 3 | 2.02 | 32.5 | V-0 | [157] |
| Methyl hydrogen siloxane-treated APP/DPER/Zeolite (m-APP/DPER/Z) | 26 | P:nP:nP 2:1:0.1 |
21 | 209 | 50 | 50 | 3 | 8.41 | 35.6 | V-0 | [157] |
|
Methyl hydrogen siloxane-treated APP/DPER/ Z/MWCNT
(m-APP/DPER/Z/MWCNT) |
26.1 | P:nP:nP:nP 2:1:0.1:0.01 |
21 | 226 | 60 | 50 | 3 | 6.48 | 34.3 | V-0 | [157] |
| ― | ― | 76 | 590 | 93 | 35 | 3 | ― | 18 | NR | [158] | |
| APP/PER | 25 | P:nP 3:1 |
55 | 200 | 74 | 35 | 3 | 2.68 | 27 | V-1 | [158] |
| APP/PER/Allophane:hydrated aluminosilicate (APP/PER/ALL) | 27 | P:nP:nP 3:1:0.3 |
53 | 149 | 68 | 35 | 3 | 3.77 | 35 | V-0 | [158] |
| ― | ― | 28 | 1547 | 123 | 50 | 3 | ― | 17.7 | NR | [159] | |
| APP/PER | 25 | P:nP 2:1 |
18 | 436 | 123 | 50 | 3 | 2.28 | 27.6 | V-1 | [159] |
| APP/PER/Mesoporous aluminosilicate oxide (APP/PER/MAO) | 25 | P:nP:nP 2:1:0.33 |
31 | 188 | 55 | 50 | 3 | 20.37 | 33.9 | V-0 | [159] |
| APP/PER/Zn-MAO | 25 | P:nP:nP 2:1:0.33 |
28 | 136 | 40 | 50 | 3 | 34.97 | 36.7 | V-0 | [159] |
| ― | ― | 38 | 1060 | 151.1 | 50 | 4 | ― | 18.5 | NR | [160] | |
| APP/PER | 25 | P:nP 2:1 |
24 | 263 | 134.6 | 50 | 4 | 2.85 | 28.8 | V-1 | [160] |
| APP/PER/Organo modified SEP (APP/PER/m-SEP) | 25 | P:nP:nP 2:1:0.125 |
27 | 223 | 105.6 | 50 | 4 | 4.83 | 30 | V-0 | [160] |
| APP/PER/Organo modified SEP (APP/PER/m-SEP) | 25 | P:nP:nP 2:1:0.25 |
30 | 247 | 114.1 | 50 | 4 | 4.48 | 36.5 | V-0 | [160] |
| APP/PER/Organo modified SEP (APP/PER/m-SEP) | 25 | P:nP:nP 2:1:0.41 |
31 | 237 | 104.3 | 50 | 4 | 5.28 | 35 | V-0 | [160] |
| APP/PER/Organo modified SEP (APP/PER/m-SEP) | 25 | P:nP:nP 2:1:0.57 |
29 | 263 | 135.8 | 50 | 4 | 3.42 | 24.5 | NR | [160] |
| APP/PER/Organo modified SEP (APP/PER/m-SEP) | 25 | P:nP:nP 2:1:0.74 |
29 | 362 | 158.4 | 2.13 | 21.5 | NR | [160] | ||
| ― | ― | 43 | 460 | 58 | 35 | 3 | ― | 17.7 | NR | [161] | |
| APP/PER | 20 | P:nP 3:1 |
27.2 | 229 | 44 | 35 | 3 | 1.67 | 24.5 | NR | [161] |
|
APP/PER/Octaphenyl POSS
(APP/PER/OP-POSS) |
20 | P:nP:nP 3:1:0.2 |
33.2 | 199 | 36 | 35 | 3 | 2.87 | 26 | V-1 | [161] |
| APP/PER/Aminopropyl isobutyl-octaphenyl POSS (APP/PER/A-POSS) | 20 | P:nP:nP 3:1:0.2 |
32.3 | 178 | 27 | 35 | 3 | 4.17 | 28.1 | V-1 | [161] |
| APP/PER/Octaammonium POSS (APP/PER/OA-POSS) | 20 | P:nP:nP 3:1:0.2 |
37.7 | 164 | 26 | 35 | 3 | 5.48 | 29.7 | V-1 | [161] |
| APP/PER/Trissulfonic acid propyl POSS (APP/PER/TS-POSS) | 20 | P:nP:nP 3:1:0.2 |
35.4 | 153 | 29 | 35 | 3 | 4.95 | 32.4 | V-1 | [161] |
| ― | ― | 59 | 347.1 | 80.97 | 35 | 3 | ― | 17 | ― | [162] | |
| APP/PER | 30 | P:nP 2:1 |
52.5 | 70.43 | 49.96 | 35 | 3 | 7.10 | 29 | ― | [162] |
| APP/PER/Thermally-treated solid waste (APP/PER/T-RS) | 33.5 | P:nP:nP 2:1:0.5 |
48 | 65.71 | 40.21 | 35 | 3 | 8.65 | 41 | ― | [162] |
| APP/PER/Volcanic ash (APP/PER/CV) | 33.5 | P:nP:nP 2:1:0.5 |
101 | 19.73 | 29.48 | 35 | 3 | 82.72 | 37 | ― | [162] |
| APP/PER/Rice husk ash (APP/PER/CR) | 33.5 | P:nP:nP 2:1:0.5 |
62 | 48.16 | 31.36 | 35 | 3 | 19.55 | 40 | [162] | |
| ― | ― | 65 | 920 | 145 | 35 | 4 | ― | 17.5 | NR | [163] | |
| APP/PER | 20 | P:nP 3:1 |
32 | 305 | 93 | 35 | 4 | 2.31 | 23 | NR | [163] |
| APP/PER/Zinc borate (APP/PER/ZnB) | 20 | P:nP:nP 3:1:0.2 |
37 | 330 | 125 | 35 | 4 | 1.84 | 29.5 | V-0 | [163] |
| APP/PER/Borophosphate (APP/PER/BPO4) | 20 | P:nP:nP 3:1:0.2 |
33 | 226 | 53 | 35 | 4 | 5.65 | 30 | V-0 | [163] |
| APP/PER/Boron silicon containing preceramic oligomer (APP/PER/Bsi) | 20 | P:nP:nP 3:1:0.2 |
34 | 255 | 70 | 35 | 4 | 3.90 | 25.5 | V-0 | [163] |
| APP/PER/Lanthanum borate (APP/PER/LaB) | 20 | P:nP:nP 3:1:0.2 |
43 | 260 | 97 | 35 | 4 | 3.49 | 27 | V-0 | [163] |
| ― | ― | 28 | 1633 | 132 | 50 | 4 | ― | 18 | ― | [107] | |
| APP/PER | 25 | P:nP 2:1 |
21 | 483 | 116 | 50 | 4 | 2.88 | 27.5 | ― | [107] |
| APP/PER/NiFeO | 25 | P:nP:nP 2:1:0.35 |
20 | 425 | 107 | 50 | 4 | 3.38 | 34.6 | ― | [107] |
| APP/PER/CoFeO | 25 | P:nP:nP 2:1:0.35 |
19 | 323 | 124 | 50 | 4 | 3.65 | 35 | ― | [107] |
| ― | ― | 28 | 1337 | 95.1 | 35 | 3 | ― | 18 | NR | [164] | |
| APP/PER | 25 | P:nP 2:1 |
41 | 588.8 | 88.4 | 35 | 3 | 3.57 | 28 | V-0 | [164] |
| APP/PER/Nickel phosphide nanocrystalline (APP/PER/Ni12P5) | 25 | P:nP:P 2:1:0.26 |
54 | 363.2 | 88.2 | 35 | 3 | 7.65 | 36 | V-0 | [164] |
| APP/PER/Cobaltous phosphide nanocrystalline (APP/PER/Co2P) | 25 | P:nP:P 2:1:0.26 |
79 | 306.6 | 89.1 | 35 | 3 | 13.12 | 34 | V-0 | [164] |
| APP/PER/Cupric phosphide nanocrystalline (APP/PER/Cu3P) | 25 | P:nP:P 2:1:0.26 |
42 | 562.4 | 93.2 | 35 | 3 | 3.63 | 31.5 | V-1 | [164] |
| ― | ― | 75 | 471 | 102 | 50 | 3 | ― | 18 | NR | [165] | |
| APP/PER | 25 | P:nP 3:1 |
45 | 265 | 83 | 50 | 3 | 1.31 | 27 | V-1 | [165] |
| APP/PER/Zinc hydroxystannate (APP/PER/ZHS) | 25 | P:nP:nP 3:1:0.16 |
40 | 193 | 75 | 50 | 3 | 1.77 | 32 | V-0 | [165] |
| ― | ― | 24 | 1361 | 107.5 | 50 | 3 | ― | ― | ― | [166] | |
| APP/PER | 25 | P:nP 3:1 |
21 | 455 | 85.4 | 50 | 3 | 3.29 | ― | V-2 | [166] |
| APP/PER/Manganese acetate (APP/PER/MnAc) | 26 | P:nP:nP 3:1:0.16 |
23 | 372 | 75.2 | 50 | 3 | 5.01 | ― | V-0 | [166] |
| APP/PER/MnAc | 27 | P:nP:nP 3:1:0.32 |
19 | 366 | 74.1 | 50 | 3 | 4.27 | ― | V-0 | [166] |
| APP/PER/MnAc | 28 | P:nP:nP 3:1:0.48 |
19 | 383 | 83.6 | 50 | 3 | 3.61 | ― | V-0 | [166] |
| APP/PER/MnAc | 29 | P:nP:nP 3:1:0.64 |
18 | 369 | 96.1 | 50 | 3 | 3.09 | ― | V-0 | [166] |
| ― | ― | 15 | 782 | 230 | 35 | 4 | ― | ― | ― | [167] | |
| APP/DPER/phosphorylated sodium alginate (APP/DPER/m-SA) | 35 | P:nP:nP 3:1:1 |
27 | 335 | 128 | 35 | 4 | 7.55 | ― | ― | [167] |
| ― | ― | 46 | 631.6 | 135.4 | 35 | 4 | ― | 19 | NR | [168] | |
| APP/PEPA | 23 | P:nP 2:1 |
38 | 297.9 | 113.8 | 35 | 4 | 2.08 | 30.5 | NR | [168] |
| APP/PEPA/NOR116 | 25 | P:nP:nP 2:1:0.26 |
36 | 260.3 | 112.5 | 35 | 4 | 2.28 | 34 | V-2 | [168] |
| APP/PEPA/Zirconium phosphate (APP/PEPA/ZrP) | 25 | P:nP:nP 2:1:0.26 |
41 | 221.8 | 112.5 | 35 | 4 | 3.05 | 31.5 | V-2 | [168] |
| APP/PEPA/Macromolecular N-alkoxy hindered amine functionalized ZrP (APP/PEPA/m-ZrP) | 25 | P:nP:nP 2:1:0.26 |
40 | 157 | 112.2 | 35 | 4 | 4.22 | 33 | V-0 | [168] |
| ― | ― | 27 | 1474 | 142 | 50 | 3 | ― | 18 | NR | [169] | |
| MCAPP/PEPA | 25 | P:P 2:1 |
18 | 438 | 123 | 50 | 3 | 2.59 | 31.1 | V-2 | [169] |
| MCAPP/PEPA/Kaol | 25 | P:P:nP 2:1:0.2 |
17 | 373 | 123 | 50 | 3 | 2.87 | 32.5 | V-0 | [169] |
| MCAPP/PEPA/Acidically modified kaol (MCAPP/PEPA/m-Kaol) | 25 | P:P:nP 2:1:0.2 |
20 | 233 | 105 | 50 | 3 | 6.33 | 34.9 | V-0 | [169] |
| ― | ― | 27 | 1474 | 142 | 50 | 3 | ― | 18.1 | NR | [170] | |
| MCAPP/PEPA | 25 | P:P 2:1 |
18 | 438 | 123 | 50 | 3 | 2.59 | 31.1 | V-2 | [170] |
| MCAPP/PEPA/Kaol | 25 | P:P:nP 2:1:0.2 |
17 | 372 | 123 | 50 | 3 | 2.88 | 32.5 | V-0 | [170] |
| MCAPP/PEPA/Thiourea modified kaol (MCAPP/PEPA/m-Kaol) | 25 | P:P:nP 2:1:0.2 |
21 | 291 | 103 | 50 | 3 | 5.43 | 35.4 | V-0 | [170] |
| ― | ― | 27 | 1474 | 142 | 50 | 3 | ― | 18 | NR | [171] | |
| MCAPP/PEPA | 25 | P:P 2:1 |
18 | 438 | 123 | 50 | 3 | 2.59 | 31.1 | V-2 | [171] |
| MCAPP/PEPA/Kaol | 25 | P:P:nP 2:1:0.2 |
17 | 373 | 123 | 50 | 3 | 2.87 | 32.5 | V-0 | [171] |
| MCAPP/PEPA/Kaol nanoroll (MCAPP/PEPA/Kaol nanoroll) | 25 | P:P:nP 2:1:0.2 |
19 | 269 | 120 | 50 | 3 | 4.56 | 34.5 | V-0 | [171] |
| ― | ― | 27 | 1474 | 142 | 50 | ― | ― | 18 | NR | [89] | |
| MCAPP/PEPA | 25 | P:nP 2:1 |
18 | 438 | 123 | 50 | ― | 2.59 | 31.1 | V-2 | [89] |
| MCAPP/PEPA/Kaol | 25 | P:P:nP 2:1:0.2 |
17 | 373 | 123 | 50 | ― | 2.87 | 32.5 | V-0 | [89] |
| MCAPP/PEPA/Ammonium sulfamate intercalated kaol (MCAPP/PEPA/m-Kaol) | 25 | P:P:nP 2:1:0.2 |
18 | 309 | 125 | 50 | ― | 3.61 | 35.3 | V-0 | [89] |
| ― | ― | 27 | 1474 | 142 | 50 | 3 | ― | 18 | NR | [172] | |
|
Microcapsulated APP/PEPA
(mc-APP/PEPA) |
25 | P:P 2:1 |
18 | 438 | 123 | 50 | 3 | 2.59 | 31 | V-2 | [172] |
|
Microcapsulated APP/PEPA/Kaol
(mc-APP/PEPA/Kaol) |
25 | P:P:nP 2:1:0.2 |
17 | 373 | 123 | 50 | 3 | 2.87 | 32.5 | V-0 | [172] |
|
Microcapsulated APP/PEPA/HNT
(mc-APP/PEPA/HNT) |
25 | P:P:nP 2:1:0.2 |
18 | 341 | 109 | 50 | 3 | 3.75 | 35.2 | V-0 | [172] |
| Microcapsulated APP/PEPA/Kaol/HNT (mc-APP/PEPA/Kaol/HNT) | 25 | P:P:nP:nP 2:1:0.18:0.2 |
19 | 263 | 97 | 50 | 3 | 5.77 | 36.9 | V-0 | [172] |
| ― | ― | 27 | 1474 | 142 | 50 | 3 | ― | 18 | NR | [173] | |
|
Microcapsulated APP/PEPA
(mc-APP/PEPA) |
25 | P:P 2:1 |
18 | 436 | 123 | 50 | 3 | 2.60 | 31.2 | V-2 | [173] |
|
Microcapsulated APP/PEPA/Kaol
(mc-APP/PEPA/Kaol) |
25 | P:P:nP 2:1:0.2 |
17 | 374 | 122 | 50 | 3 | 2.88 | 32.5 | V-2 | [173] |
|
Microcapsulated APP/PEPA/HSA-A
(mc-APP/PEPA/HSA-A) |
25 | P:P:nP 2:1:0.2 |
23 | 299 | 106 | 50 | 3 | 5.62 | 34.1 | V-0 | [173] |
|
Microcapsulated APP/PEPA/HSA-P
(mc-APP/PEPA/HSA-P) |
25 | P:P:nP 2:1:0.2 |
20 | 257 | 84 | 50 | 3 | 7.18 | 35.1 | V-0 | [173] |
| Microcapsulated APP/PEPA/ HSA-A-La (mc-APP/PEPA/HSA-A-La) | 25 | P:P:nP 2:1:0.2 |
16 | 248 | 103 | 50 | 3 | 4.85 | 35.5 | V-0 | [173] |
|
Microcapsulated APP/PEPA/HSA-P-La
(mc-APP/PEPA/HSA-P-La) |
25 | P:P:nP 2:1:0.2 |
17 | 212 | 82 | 50 | 3 | 7.58 | 37.5 | V-0 | [173] |
| ― | ― | 38 | 1284 | 214 | 50 | 6 | ― | 18.2 | NR | [49] | |
|
APP/Phosphorus based CA: 3,9-Bis-(1-oxo-2,6,7-trioxa-1-phospha-bicyclo[2,2,2]oct-4-ylmethoxy)-2,4,8,10-tetraoxa-3,9 diphospha-spiro[5.5]undecane 3,9-dioxide
(APP/P-CA) |
25 | P:P 1:1 |
31 | 318 | 161 | 50 | 6 | 4.37 | 30.5 | V-0 | [49] |
| ― | ― | 37 | 1284 | 121 | 50 | 3 | ― | ― | ― | [38] | |
| APP/Phosphorus-based FR: Cyclotriphosphazene containing six (aminopropyl)-triethoxysilicone groups (APP/P-FR) | 30 | P:P 14:1 |
18 | 596 | 114 | 50 | 3 | 1.11 | 22.2 | NR | [38] |
| APP/Phosphorus-based FR: Cyclotriphosphazene containing six (aminopropyl)-triethoxysilicone groups (APP/P-FR) | 30 | P:P 6.5:1 |
17 | 420 | 109 | 50 | 3 | 1.55 | 22.4 | NR | [38] |
| APP/Phosphorus-based FR: Cyclotriphosphazene containing six (aminopropyl)-triethoxysilicone groups (APP/P-FR) | 30 | P:P 4:1 |
18 | 382 | 95 | 50 | 3 | 2.08 | 23.5 | V-2 | [38] |
| APP/Phosphorus based FR: Cyclotriphosphazene containing six (aminopropyl)-triethoxysilicone groups (APP/P-FR) | 30 | P:P 2.751 |
17 | 282 | 95 | 50 | 3 | 2.66 | 26.5 | V-2 | [38] |
| ― | ― | 33 | 1238 | 123.7 | 50 | 3 | ― | 17.8 | NR | [41] | |
|
Melamine-formaldehyde-tris(2-hydroxyethyl) isocyanurate resin microencapsulated APP/Tris(2-hydroxyethyl) isocyanurate
(mc-APP/THEIC) |
30 | P:nP 3:1 |
28 | 232 | 100.7 | 50 | 3 | 5.56 | 36 | V-0 | [41] |
| ― | ― | 42 | 831 | 112 | 35 | 3 | ― | 18 | NR | [50] | |
| APP/Polyurethane containing phosphorus-based CA (APP/PPU-CA) | 25 | P:nP 2:1 |
19.8 | 232 | 69 | 35 | 3 | 2.74 | 24.5 | V-2 | [50] |
| APP/PPU-CA | 25 | P:N 1:1 |
17.1 | 288 | 70 | 35 | 3 | 1.87 | 25.5 | V-1 | [50] |
| ― | ― | 37 | 1677 | 184.4 | 50 | 4 | ― | 17 | NR | [95] | |
| APP/Triazine-based CFA (APP/TA-CFA) | 22 | P:nP 4:1 |
21 | 397.3 | 161.1 | 50 | 4 | 2.74 | 30.4 | V-0 | [95] |
|
(3-Aminopropyl) triethoxysilane modified APP microcapsulated with methylpolysiloxane/Triazine-based CFA
(m-APP/TA-CFA) |
22 | P:nP 4:1 |
16 | 271.7 | 140.8 | 50 | 4 | 3.49 | 31.7 | V-0 | [95] |
| ― | ― | 20 | 809 | 96 | 50 | 3 | ― | 17.6 | NR | [32] | |
| APP/Triazine based CFA: Poly[N4-bis(ethylenediamino)-phenyl phosphonic-N2, N6-bis(ethylenediamino)-1,3,5-triazine-N-phenyl (APP/TA-CFA) | 25 | P:nP 2:1 |
11 | 121 | 81 | 50 | 3 | 4.35 | 34 | V-0 | [32] |
| ― | ― | 45 | 759.2 | 98.8 | 35 | 3 | ― | 17 | NR | [37] | |
|
APP/Triazine based CFA: synthesized from a macromolecular triazine derivative containing hydroxyethylamino and triazine rings and ethylenediamino groups
(APP/TA-CFA) |
25 | P:nP 4:1 |
40 | 167.6 | 82.5 | 35 | 3 | 4.82 | 34 | V-0 | [37] |
|
Melamine and phytic acid modified APP/Triazine-based CFA: synthesized from a macromolecular triazine derivative containing hydroxyethylamino and triazine rings and ethylenediamino groups
(m-APP/TA-CFA) |
25 | P:nP 4:1 |
43 | 115.6 | 82.3 | 35 | 3 | 7.53 | 35 | V-0 | [37] |
| ― | ― | 50 | 1350 | 91.2 | 35 | 3 | ― | 17 | NR | [40] | |
|
APP/Triazine-based CFA: synthesized by polycondensation of 2-chloro-4,6-di-(2-hydroxyethylamino)-s-triazine
(APP/TA-CFA) |
30 | P:nP 2:1 |
56 | 422 | 70.7 | 35 | 3 | 4.62 | 32.5 | V-0 | [40] |
|
APP/Triazine-based CFA: synthesized by polycondensation of 2-chloro-4,6-di-(2-hydroxyethylamino)-s-triazine
(APP/TA-CFA) |
30 | P:nP 3:1 |
48 | 316 | 68.8 | 35 | 3 | 5.43 | 33 | V-0 | [40] |
|
APP/Triazine-based CFA: synthesized by polycondensation of 2-chloro-4,6-di-(2-hydroxyethylamino)-s-triazine
(APP/TA-CFA) |
30 | P:nP 4:1 |
52 | 414 | 71.1 | 35 | 3 | 4.35 | 31.5 | V-0 | [40] |
| ― | ― | 48 | 988 | 88.3 | 35 | 3 | ― | 17 | NR | [39] | |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine (APP/TA-CFA) | 30 | P:N 1:1 |
32 | 82.4 | 77.9 | 35 | 3 | 9.06 | 29 | V-0 | [39] |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine (APP/TA-CFA) | 30 | P:nP 2:1 |
52 | 94.2 | 78.4 | 35 | 3 | 12.79 | 32 | V-0 | [39] |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine (APP/TA-CFA) | 30 | P:nP 3:1 |
34 | 167 | 83.4 | 35 | 3 | 4.43 | 34 | V-0 | [39] |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine (APP/TA-CFA) | 30 | P:nP 4:1 |
56 | 163.6 | 67.9 | 35 | 3 | 9.16 | 29.5 | V-0 | [39] |
| ― | ― | 48 | 906 | 90.3 | 35 | 3.2 | ― | 17 | NR | [174] | |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine (APP/TA-CFA) | 20 | P:nP 3:1 |
34 | 143 | 60.1 | 35 | 3.2 | 6.74 | 29 | V-1 | [174] |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine /hexadecyl trimethyl ammonium bromide modified MMT (APP/TA-CFA/m-MMT) | 20 | P:nP:nP 3:1:0.1 |
36 | 132 | 58.7 | 35 | 3.2 | 7.91 | 31 | V-0 | [174] |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine /hexadecyl trimethyl ammonium bromide modified MMT (APP/TA-CFA/m-MMT) | 20 | P:nP:nP 3:1:0.2 |
36 | 90 | 58.6 | 35 | 3.2 | 11.63 | 30.5 | V-0 | [174] |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine /hexadecyl trimethyl ammonium bromide modified MMT (APP/TA-CFA/m-MMT) | 20 | P:nP:nP 3:1:0.3 |
32 | 52.6 | 35.5 | 35 | 3.2 | 29.20 | 30.5 | V-0 | [174] |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine /hexadecyl trimethyl ammonium bromide modified MMT (APP/TA-CFA/m-MMT) | 20 | P:nP:nP 3:1:0.5 |
38 | 55.2 | 38.5 | 35 | 3.2 | 30.47 | 31.5 | V-0 | [174] |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine /hexadecyl trimethyl ammonium bromide modified MMT (APP/TA-CFA/m-MMT) | 20 | P:nP:nP 3:1:0.7 |
36 | 112.7 | 41.4 | 35 | 3.2 | 13.15 | 30.5 | NR | [174] |
| ― | ― | 34 | 1052 | 90.8 | 50 | 3 | ― | 17.5 | NR | [61] | |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine/Silicon dioxide (APP/TA-CFA/SiO2) | 24 | P:nP:nP 4:1:0.26 |
30 | 236.6 | 84.7 | 50 | 3 | 4.20 | 36.4 | V-1 | [61] |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine/AHP/SiO2 (APP/TA-CFA/AHP/SiO2) | 24 | P:nP:nP:nP 4:1:0.86:0.2 |
33 | 221 | 83.2 | 50 | 3 | 5.04 | 35.8 | V-0 | [61] |
| ― | ― | 36 | 1153 | 130 | 50 | ― | ― | 17.5 | NR | [175] | |
| APP/Triazine-based CFA: N-ethyl triazineepiperazine copolymer/SiO2 (APP/TA-CFA/SiO2) | 24 | P:nP:nP 4:1:0.26 |
26 | 88 | 20 | 50 | ― | 61.50 | 34.1 | V-0 | [175] |
| APP/Triazine-based CFA: N-ethyl triazineepiperazine copolymer/SiO2 (APP/TA-CFA/SiO2) | 24 | P:nP:nP 4:1:0.26 |
27 | 95 | 24 | 50 | ― | 49.30 | 33.5 | V-0 | [175] |
| ― | ― | 50 | 1025 | 110.8 | 35 | 3 | ― | 17 | NR | [176] | |
|
APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine
(APP/TA-CFA) |
25 | P:nP 4:1 |
36 | 213 | 90.5 | 35 | 3 | 4.24 | 34 | V-0 | [176] |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine/rGNO (APP/TA-CFA/rGNO) | 25 | P:nP:nP 4:1:0.1 |
35 | 140 | 90.4 | 35 | 3 | 6.28 | 32 | V-0 | [176] |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine/rGNO (APP/TA-CFA/rGNO) | 25 | P:nP:nP 4:1:0.2 |
34 | 156 | 86 | 35 | 3 | 5.75 | 28 | V-0 | [176] |
| APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine/rGNO (APP/TA-CFA/rGNO) | 25 | P:nP:nP 4:1:0.4 |
36 | 262 | 94.4 | 35 | 3 | 3.30 | 25 | V-2 | [176] |
| ― | ― | 45 | 1456 | 139.1 | 35 | 3 | ― | 17 | NR | [177] | |
| Piperazine modified APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine (m-APP/TA-CFA) | 25 | P:nP 4:1 |
41 | 501 | 126 | 35 | 3 | 2.92 | 35 | V-0 | [177] |
|
Piperazine modified APP/Triazine based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine/rGNO
(m-APP/TA-CFA/rGNO) |
25 | P:nP:nP 4:1:0.1 |
39 | 434 | 125.1 | 35 | 3 | 3.23 | 34 | V-0 | [177] |
|
Piperazine modified APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine/rGNO
(m-APP/TA-CFA/rGNO) |
25 | P:nP:nP 4:1:0.2 |
37 | 350 | 123.9 | 35 | 3 | 3.84 | 32 | V-0 | [177] |
|
Piperazine modified APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine/rGNO
(m-APP/TA-CFA/rGNO) |
25 | P:nP:nP 4:1:0.4 |
37 | 397 | 125.4 | 35 | 3 | 3.34 | 30 | V-0 | [177] |
|
Piperazine modified APP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine/Piperazine modified APP attached with rGNO
(m-APP/TA-CFA/m-APP@rGNO) |
25 | P:nP:nP 3.6:1:0.5 |
38 | 401 | 117.3 | 35 | 3 | 3.63 | 33 | V-0 | [177] |
|
Piperazine modified APP/Piperazine modified APP attached with rGNO/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine
(m-APP/m-APP@rGNO/TA-CFA) |
25 | P:nP:nP 3.04:1:0.96 |
34 | 290 | 117.1 | 35 | 3 | 4.50 | 30.5 | V-0 | [177] |
|
Piperazine modified APP/Piperazine modified APP attached with rGNO/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine
(m-APP/m-APP@rGNO/TA-CFA) |
25 | P:nP:nP 1.04:1:0.46 |
31 | 464 | 125.8 | 35 | 3 | 2.39 | 26.5 | V-2 | [177] |
| ― | ― | 66 | 633 | 44.2 | 35 | 3 | ― | 17 | NR | [23] | |
| APP/Triazine-based CA: synthesized by reaction of 2-carboxyethyl (phenyl) phosphinic acid and tris (2-hydrooxyethyl) isocyanurate (APP/TA-CA) | 20 | P:N 1:1 |
38 | 83 | 41 | 35 | 3 | 4.73 | 30 | V-0 | [23] |
| ― | ― | 48 | 1351 | 107 | 35 | 3.2 | ― | 18.5 | NR | [27] | |
| APP/Triazine-based CA: synthesized by reaction of cyanuric chloride, 2,6,7-trioxa-1-phosphabicyclo [2,2,2]octane-4-methanol and piperazine (APP/TA-CA) | 20 | P:nP 2:1 |
39 | 255 | 101 | 35 | 3.2 | 4.56 | 27.5 | V-0 | [27] |
| APP/Triazine-based CA: synthesized by reaction of cyanuric chloride, 2,6,7-trioxa-1-phosphabicyclo [2,2,2]octane-4-methanol and piperazine (APP/TA-CA) | 20 | P:nP 3:1 |
37 | 253 | 98 | 35 | 3.2 | 4.49 | 28 | V-0 | [27] |
| ― | ― | 41 | 840.3 | 115.7 | 35 | 3 | ― | 16.4 | NR | [67] | |
| APP/Triazine-based CA: Poly(ethanediamine-1,3,5-triazine-p-4-amino-2,2,6,6-tetramethylpiperidine) (APP/TA-CA) | 25 | P:nP 2:1 |
28 | 227.9 | 62 | 35 | 3 | 4.69 | 30.3 | V-0 | [67] |
| ― | ― | 33 | 1416 | 219 | 50 | 6 | ― | 17 | NR | [36] | |
| APP/Triazin-based CA: poly(1,3,5-triazin-2-aminoethanol diethylenetriamine) (APP/TA-CA) | 25 | P:nP 3:1 |
17 | 219 | 165 | 50 | 6 | 4.42 | 32.7 | V-0 | [36] |
| Polysiloxane shell-coated APP/Triazin-based CA: poly(1,3,5-triazin-2-aminoethanol diethylenetriamine) (mc-APP/TA-CA) | 25 | P:nP 3:1 |
18 | 176 | 82 | 50 | 6 | 11.72 | 35 | V-0 | [36] |
| ― | ― | 70 | 1267 | 147 | 50 | 3 | ― | 17.5 | NR | [149] | |
| APP/Triazine based CA: synthesized by reaction of cyanuric trichloride and diphenylamine and ethylenediamine (APP/TA-CA) | 30 | P:nP 2:1 |
35 | 187 | 68 | 50 | 3 | 7.32 | 31.5 | V-0 | [149] |
| ― | ― | 25 | 1239 | 123.6 | 50 | 3 | ― | 18.5 | NR | [178] | |
| APP/Triazine-based CA: Tris(2-hydroxyethyl) isocyanurate (APP/TA-CA) | 30 | P:nP 2:1 |
18 | 253.7 | 91.2 | 50 | 3 | 4.76 | 32.8 | V-0 | [178] |
| APP/Triazine-based CA: Tris(2-hydroxyethyl) isocyanurate homopolymer (APP/Homo-TA-CA) | 30 | P:nP 2:1 |
18 | 349.9 | 91.3 | 50 | 3 | 3.45 | 34.6 | V-0 | [178] |
| ― | ― | 47 | 860.6 | 110.3 | 35 | 3 | ― | 18 | NR | [179] | |
| APP/Triazine-based CA: synthesized by reaction of cyanuric chloride and 2,6,7-trioxa-l-phosphabicyclo[2,2,2]octane-4-methanol and diethylenetriamine (APP/TA-CA) | 30 | P:N 1:1 |
31 | 136.5 | 81 | 35 | 3 | 5.66 | 32 | V-0 | [179] |
| APP/Triazine-based CA: synthesized by reaction of cyanuric chloride and 2,6,7-trioxa-l-phosphabicyclo[2,2,2]octane-4-methanol and diethylenetriamine (APP/TA-CA) | 30 | P:nP 4:1 |
35 | 257.6 | 80.8 | 35 | 3 | 3.39 | 35.5 | V-0 | [179] |
| ― | ― | 36 | 799 | 170 | 35 | 4 | ― | 17.5 | NR | [73] | |
| APP/Triazine-based CA: synthesized by reaction of Cyanuric chloride and Ethanedi-amine and γ-Aminopropyl triethoxysilane (APP/TA-CA) | 25 | P:nP 2:1 |
25 | 180 | 131 | 35 | 4 | 4.00 | 35.5 | V-0 | [73] |
| APP/Triazine-based CA: synthesized by reaction of Cyanuric chloride and Ethanedi-amine and γ-Aminopropyl triethoxysilane/ NOR116 (APP/TA-CA/NOR116) | 25 | P:nP:nP 2:1:0.03 |
32 | 76 | 122 | 35 | 4 | 13.02 | 42.5 | V-0 | [73] |
| ― | ― | 55 | 818 | 213 | 35 | 6 | ― | ― | ― | [180] | |
|
APP/Triazine-based CA: synthesized by polycondensation of 2-amino-4,6-dichloro-s-triazines and diethylenetriamine
(APP/TA-CA) |
20 | P:nP 2.8:1 |
35 | 218 | 126 | 35 | 6 | 4.03 | 30.8 | V-1 | [180] |
| APP/Triazine-based CA: synthesized by polycondensation of 2-amino-4,6-dichloro-s-triazines and diethylenetriamine/Organically modified MMT (APP/TA-CA/m-MMT) | 20 | P:nP:nP 2.8:1:0.2 |
34 | 159 | 64 | 35 | 6 | 10.58 | 33 | V-0 | [180] |
| APP/Triazine-based CA: synthesized by polycondensation of 2-amino-4,6-dichloro-s-triazines and diethylenetriamine/Organically modified MMT (APP/TA-CA/m-MMT) | 20 | P:nP:nP 2.8:1:0.6 |
37 | 270 | 156 | 35 | 6 | 2.78 | 28.9 | NR | [180] |
| ― | ― | 29 | 1603 | 133 | 50 | 4 | ― | 17.5 | NR | [181] | |
| APP/Triazine-based CFA: N-methyl triazineethylenediamine copolymer/SiO2 (APP/TA-CFA/SiO2) | 22 | P:nP:nP 4:1:0.26 |
16 | 91 | 62 | 50 | 4 | 20.84 | 29.6 | V-0 | [181] |
| APP/Triazine-based CFA: N-methyl triazineethylenediamine copolymer/SiO2 (APP/TA-CFA/SiO2) | 22 | P:nP:nP 4:1:0.26 |
22 | 74 | 69 | 50 | 4 | 31.67 | 29.3 | V-0 | [181] |
| ― | ― | 18 | 1457 | 156 | 50 | 3 | ― | 19 | NR | [29] | |
| APP/Triazine-based CA: synthesized by reaction of cyanuric chloride and γ-aminopropyltriethoxy silane and trimethylamine and ethylenediamine-Zinc oxide (APP/TA-CA-ZnO) | 25 | P:nP 2:1 |
19 | 191 | 112 | 50 | 3 | 11.21 | 31.1 | V-0 | [29] |
| APP/Triazine-based CA: synthesized by reaction of cyanuric chloride and γ-aminopropyltriethoxy silane and trimethylamine and ethylenediamine/ZnO (APP/TA-CA/ZnO) | 25 | P:nP:nP 2.25:1:0.12 |
18 | 430 | 132 | 50 | 3 | 4.00 | 26.1 | V-1 | [29] |
| ― | ― | 62 | 1221 | 265 | 35 | 6 | ― | 19 | NR | [182] | |
| APP and Triazine-based IFR (APPandTA-IFR) | 10 | P:N ― |
44 | 313 | 235 | 35 | 6 | 3.12 | 26 | NR | [182] |
| APP and Triazine-based IFR (APPandTA-IFR) | 15 | P:N ― |
45 | 148 | 191 | 35 | 6 | 8.30 | 29 | NR | [182] |
| APP and Triazine-based IFR (APPandTA-IFR) | 20 | P:N ― |
43 | 115 | 153 | 35 | 6 | 12.75 | 31 | V-0 | [182] |
| ― | ― | 48 | 988 | 88.3 | 35 | 3.2 | ― | 17 | NR | [31] | |
|
APP/Triazin-based IFR: synthesized by reaction of cyanuric chloride
and N-amino ethylpiperazine (APP/TA-IFR) |
25 | P:N 1:1 |
44 | 123 | 73.3 | 35 | 3.2 | 8.86 | 27.5 | V-0 | [31] |
|
APP/Triazin-based IFR: synthesized by reaction of cyanuric chloride
and N-amino ethylpiperazine (APP/TA-IFR) |
25 | P:nP 2:1 |
38 | 117 | 23.2 | 35 | 3.2 | 25.44 | 29.5 | V-0 | [31] |
|
APP/Triazin-based IFR: synthesized by reaction of cyanuric chloride
and N-amino ethylpiperazine (APP/TA-IFR) |
25 | P:nP 3:1 |
36 | 113 | 73.9 | 35 | 3.2 | 7.83 | 30.5 | V-0 | [31] |
| ― | ― | 21 | 1242 | 111 | 50 | 3.2 | ― | 18.6 | NR | [33] | |
| APP/Piperazine-Triazine-based CA: synthesized by reaction of cyanuric chloride and anhydrous piperazine (APP/PI-TI-CA) | 25 | P:nP 4:1 |
22 | 330 | 103 | 50 | 3.2 | 4.24 | 34.1 | V-0 | [33] |
| APP/Piperazine-Triazine-based CA cluster: synthesized by reaction of cyanuric chloride and anhydrous piperazine (APP/PI-TI-CA) | 25 | P:nP 4:1 |
21 | 242 | 96 | 50 | 3.2 | 5.93 | 33.9 | V-0 | [33] |
| ― | ― | 45 | 1269 | 146.35 | 50 | 3 | ― | 17.5 | NR | [70] | |
| APP/Piperazine-based IFR: Piperazine spirocyclic phosphoramidate (APP/PI-IFR) | 30 | P:nP 3:1 |
23 | 208.8 | 81.41 | 50 | 3 | 5.58 | 32.5 | V-0 | [70] |
| APP/Piperazine-based IFR: Piperazine spirocyclic phosphoramidate/Triazine based CFA (APP/PI-IFR/TA-CFA) | 30 | P:nP:nP 2:1:0.65 |
23 | 116.1 | 41.57 | 50 | 3 | 19.66 | 39.8 | V-0 | [70] |
| ― | ― | 76 | 455 | 102 | 35 | 3 | ― | 17 | NR | [183] | |
| APP/ATH | 30 | P:M 1:1 |
40 | 210 | 75 | 35 | 3 | 1.55 | 24 | V-1 | [183] |
|
4,4′-diphenylmethane diisocyanateandmelamine co-microencapsulated APP and ATH
(mc-(APPandATH)) |
30 | P:M 1:1 |
75 | 120 | 53 | 35 | 3 | 7.20 | 25.5 | V-0 | [183] |
| ― | ― | 14 | 1104 | 106 | 35 | 0.4 | ― | ― | ― | [24] | |
| APP/MMT | 10 | P:nP 4:1 |
25 | 769 | 66 | 35 | 0.4 | 4.11 | ― | ― | [24] |
| APP/MMT | 10 | P:nP 1.5:1 |
27 | 765 | 65 | 35 | 0.4 | 4.53 | ― | ― | [24] |
| APP/Modified MMT (APP/m-MMT) | 10 | P:nP 4:1 |
29 | 715 | 64 | 35 | 0.4 | 5.29 | ― | ― | [24] |
| APP/Modified MMT (APP/m-MMT) | 10 | P:nP 1.5:1 |
30 | 619 | 63 | 35 | 0.4 | 6.43 | ― | ― | [24] |
| ― | ― | 50.2 | 789 | 156.6 | 35 | ― | ― | 17.5 | ― | [103] | |
| APP/Nf | 20 | P:nP 3:1 |
40.8 | 399 | 167.9 | 35 | ― | 1.49 | 23 | ― | [103] |
| APP/organically modified BT (APP/m-BT) | 20 | P:nP 3:1 |
42.6 | 386 | 155.1 | 35 | ― | 1.75 | 23 | ― | [103] |
| ― | ― | 33 | 847 | 159.8 | 50 | ― | ― | 17.5 | ― | [103] | |
| APP/Nf | 20 | P:nP 3:1 |
29 | 426 | 168.2 | 50 | ― | 1.66 | 23 | ― | [103] |
| APP/organically modified BT (APP/m-BT) | 20 | P:nP 3:1 |
24 | 445 | 150 | 50 | ― | 1.47 | 23 | ― | [103] |
| ― | ― | 44 | 1172 | 87.1 | 35 | 2.5 | ― | 18.1 | NR | [60] | |
| APP/C15A | 20 | P:nP 3:1 |
61 | 490 | 72.7 | 35 | 2.5 | 3.97 | 20.1 | NR | [60] |
| OP/C15A | 20 | P:nP 3:1 |
50 | 400 | 83.6 | 35 | 2.5 | 3.46 | 20.8 | NR | [60] |
| ― | ― | 35 | 1622 | 103 | 35 | 3 | ― | ― | ― | [104] | |
| APP/C20A/PER/MEL | 25 | P:nP:nP:nP 2.4:1:0.8:0.8 |
26 | 403 | 93 | 35 | 3 | 3.31 | ― | ― | [104] |
| APPandMMT/C20A/PER/MEL | 25 | P:nP:nP:nP 2.4:1:0.8:0.8 |
23 | 385 | 80 | 35 | 3 | 3.56 | ― | ― | [104] |
| APPandMMT/C20A/PER/MEL | 15 | P:nP:nP:nP 1.2:1:0.4:0.4 |
18 | 460 | 86 | 35 | 3 | 2.17 | ― | ― | [104] |
| APPandMMT/C20A/PER/MEL | 20 | P:nP:nP:nP 1.8:1:0.6:0.6 |
18 | 411 | 86 | 35 | 3 | 2.43 | ― | ― | [104] |
| ― | ― | 34 | 1294 | 154.2 | 50 | 4 | ― | 19 | NR | [26] | |
|
APP/Phytic acid modified LDH
(APP/m-LDH) |
20 | P:nP 19:1 |
20 | 200 | 59.3 | 50 | 4 | 9.89 | ― | V-1 | [26] |
|
APP/Phytic acid modified LDH
(APP/m-LDH) |
20 | P:nP 9:1 |
19 | 291 | 144.3 | 50 | 4 | 2.65 | ― | V-0 | [26] |
|
APP/Phytic acid modified LDH
(APP/m-LDH) |
20 | P:nP 5.6:1 |
18 | 327 | 150.8 | 50 | 4 | 2.14 | ― | V-1 | [26] |
| ― | ― | 36 | 1373 | 174.8 | 50 | 3 | ― | 18.5 | NR | [51] | |
| APP based IFR/Mg-Al LDH (APP-IFR/LDH) | 20 | P:nP 9:1 |
18 | 286 | 108.8 | 50 | 3 | 3.85 | 32.5 | V-0 | [51] |
| APP-based IFR/Mg-Al LDH (APP-IFR/LDH) | 20 | P:nP 4:1 |
15 | 326 | 120.3 | 50 | 3 | 2.54 | ― | NR | [51] |
|
APP-based IFR/Mg-Zn-Al LDH
(APP-IFR/LDH) |
20 | P:nP 9:1 |
16 | 306 | 136 | 50 | 3 | 2.56 | ― | V-0 | [51] |
|
APP-based IFR/Mg-Zn-Al LDH
(APP-IFR/LDH) |
20 | P:nP 4:1 |
14 | 265 | 90.4 | 50 | 3 | 3.89 | ― | V-0 | [51] |
| ― | ― | 25 | 981 | 147 | 50 | ― | ― | 17.6 | NR | [30] | |
| APP/Polysiloxane-based FR (APP/Si-FR) | 25 | P:M 1:1 |
14 | 277 | 97 | 50 | ― | 3.00 | 28.9 | V-0 | [30] |
| APP/Polysiloxane-based FR (APP/Si-FR) | 25 | P:nP 1.5:1 |
15 | 168 | 91 | 50 | ― | 5.65 | 29.8 | V-0 | [30] |
| APP/Polysiloxane-based FR (APP/Si-FR) | 25 | P:nP 1.5:1 |
14 | 238 | 98 | 50 | ― | 3.46 | 28.2 | V-0 | [30] |
| APP/Polysiloxane-based FR (APP/Si-FR) | 25 | P:nP 2:1 |
18 | 245 | 93 | 50 | ― | 4.55 | 29.1 | V-0 | [30] |
| APP/Polysiloxane-based FR (APP/Si-FR) | 25 | P:nP 3:1 |
20 | 197 | 98 | 50 | ― | 5.97 | 28.4 | V-0 | [30] |
| ― | ― | 54 | 1610 | 106 | 35 | 3 | ― | 20.8 | NR | [25] | |
| APP/SEP | 12.5 | P:nP 24:1 |
27 | 320 | 88 | 35 | 3 | 3.03 | 25.7 | NR | [25] |
|
APP/Organically modified SEP
(APP/m-SEP) |
12.5 | P:nP 24:1 |
28 | 241 | 92 | 35 | 3 | 3.99 | 26.3 | V-0 | [25] |
| ― | ― | 36 | 1153 | 130 | 50 | ― | ― | 17.5 | NR | [175] | |
| APP/SiO2 | 24 | P:nP 19:1 |
30 | 502 | 151 | 50 | ― | 1.64 | ― | ― | [175] |
| ― | ― | 35 | 1203 | 197.6 | 50 | 6 | ― | 18.2 | NR | [34] | |
| APP/CB | 25 | P:nP 7.3:1 |
32 | 343.6 | 157.2 | 50 | 6 | 4.02 | 28.3 | NR | [34] |
| APP/CB | 25 | P:nP 4:1 |
31 | 316.2 | 136.9 | 50 | 6 | 4.86 | 29.1 | V-1 | [34] |
| APP/CB | 25 | P:nP 2.5:1 |
33 | 302.1 | 121.4 | 50 | 6 | 6.11 | 29.8 | V-0 | [34] |
| ― | ― | 46 | 1541 | 90 | 35 | 3 | ― | ― | ― | [135] | |
| APP/CD | 15 | P:Bio 1:1 |
24 | 910 | 89 | 35 | 3 | 0.89 | ― | ― | [135] |
| ― | ― | 38 | 1284 | 214 | 50 | 6 | ― | 18.2 | NR | [184] | |
| Phosphorus based CA: 3,9-Bis-(1-oxo-2,6,7-trioxa-1-phospha-bicyclo[2,2,2]oct-4-ylmethoxy)- 2,4,8,10-tetraoxa-3,9-diphospha-spiro[5.5]undecane 3, 9-dioxide/MEL (P-CA/MEL) | 30 | P:nP 4:1 |
18 | 198 | 175 | 50 | 6 | 3.75 | 31.6 | V-0 | [184] |
| ― | ― | 65 | 1417 | 128.5 | 35 | 3 | ― | ― | NR | [57] | |
| Phosphorus based FR: Tri (1-oxo-2,6,7-trioxa-1-phosphabicyclo [2,2,2] octane-methyl) phosphate/MPyP (P-FR/MPyP) | 30 | P:N 1:1 |
40 | 175.2 | 90.6 | 35 | 3 | 7.05 | ― | V-0 | [57] |
| ― | ― | 54 | 1199 | 97.8 | 35 | ― | ― | ― | ― | [58] | |
| Phosphorus-based FR: Poly(4,4-diaminodiphenyl methane spirocyclicpentaerythritol bisphosphonate)/GNO (P-FR/GNO) | 20 | P:nP 10:1 |
64 | 473 | 79 | 35 | ― | 3.71 | ― | ― | [58] |
| ― | ― | 30 | 390 | 44 | 35 | 1.6 | ― | 17.4 | ― | [53] | |
| Phosphorus-based IFR: Poly (4,4-diamino diphenyl methane Obicyclicpentaerythritol phosphate-phosphate)/Znacac (P-IFR/Znacac) | 20 | P:nP 19:1 |
23 | 175 | 25 | 35 | 1.6 | 3.00 | 27.4 | ― | [53] |
|
Phosphorus-based IFR: Poly (4,4-diamino diphenyl methane Obicyclicpentaerythritol phosphate-phosphate)/Cracac
(P-IFR/Cracac) |
20 | P:nP 19:1 |
24 | 184 | 24 | 35 | 1.6 | 3.10 | 28.2 | ― | [53] |
| ― | ― | 29 | 980 | 136 | 50 | ― | ― | 18.5 | ― | [56] | |
|
Phosphorus and Nitrogene-based IFR: compound containing Phosphorus and Nitrogen /Dioctadecyl dimethyl ammonium chloride modified MMT
(PN-IFR/m-MMT) |
30 | P:nP 59:1 |
20 | 173 | 102 | 50 | ― | 5.20 | 38.5 | ― | [56] |
|
Phosphorus and Nitrogene-based IFR: compound containing Phosphorus and Nitrogen /Dioctadecyl dimethyl ammonium chloride modified MMT
(PN-IFR/m-MMT) |
30 | P:nP 29:1 |
22 | 192 | 127 | 50 | ― | 4.14 | 38 | ― | [56] |
|
Phosphorus and Nitrogene-based IFR: compound containing Phosphorus and Nitrogen /Dioctadecyl dimethyl ammonium chloride modified MMT
(PN-IFR/m-MMT) |
30 | P:nP 19:1 |
21 | 173 | 134 | 50 | ― | 4.16 | 37.7 | ― | [56] |
|
Phosphorus and Nitrogene-based IFR: compound containing Phosphorus and Nitrogen /Dioctadecyl dimethyl ammonium chloride modified MMT
(PN-IFR/m-MMT) |
30 | P:nP 14:1 |
22 | 176 | 133 | 50 | ― | 4.31 | 35.7 | ― | [56] |
|
Phosphorus and Nitrogene-based IFR: compound containing Phosphorus and Nitrogen /Dioctadecyl dimethyl ammonium chloride modified MMT
(PN-IFR/m-MMT) |
30 | P:nP 11:1 |
20 | 135 | 131 | 50 | ― | 5.19 | 35.8 | ― | [56] |
|
Phosphorus and Nitrogene-based IFR: compound containing Phosphorus and Nitrogen /Dioctadecyl dimethyl ammonium chloride modified MMT
(PN-IFR/m-MMT) |
30 | P:nP 9:1 |
21 | 155 | 134 | 50 | ― | 4.64 | 31 | ― | [56] |
|
Phosphorus and Nitrogene-based IFR: compound containing Phosphorus and Nitrogen /Dioctadecyl dimethyl ammonium chloride modified MMT
(PN-IFR/m-MMT) |
30 | P:n P6.5:1 |
10 | 202 | 132 | 50 | ― | 1.72 | 28.5 | ― | [56] |
|
Phosphorus and Nitrogene-based IFR: compound containing Phosphorus and Nitrogen /Dioctadecyl dimethyl ammonium chloride modified MMT
(PN-IFR/m-MMT) |
30 | P:nP 5:1 |
19 | 202 | 133 | 50 | ― | 3.25 | 25.5 | ― | [56] |
| ― | ― | 37 | 363 | 56 | 35 | 3 | ― | ― | ― | [55] | |
|
Phosphorus-based IFR: compound containing Phosphorus(22%) and Nitrogene(18%)/Octadecyl trimethyl ammonium bromide modified MMT
(P-IFR/m-MMT) |
28 | P:nP 10.2:1 |
31 | 45 | 18 | 35 | 3 | 21.02 | ― | ― | [55] |
| Phosphorus-based IFR: compound containing Phosphorus(22%) and Nitrogene(18%)/Sodium dodecyl sulfonate intercalated Ni-Al LDH (P-IFR/m-LDH) | 28 | P:nP 10.2:1 |
30 | 64 | 20 | 35 | 3 | 12.87 | ― | ― | [55] |
| Phosphorus-based IFR: compound containing Phosphorus(22%) and Nitrogene(18%)/A-POSS (P-IFR/A-POSS) | 28 | P:nP 10.2:1 |
32 | 55 | 16 | 35 | 3 | 19.97 | ― | ― | [55] |
| Phosphorus-based IFR: compound containing Phosphorus(22%) and Nitrogene(18%)/MWCNT (P-IFR/MWCNT) | 28 | P:nP 10.2:1 |
33 | 145 | 54 | 35 | 3 | 2.31 | ― | ― | [55] |
| ― | ― | 37 | 363 | 56 | 35 | 3 | ― | ― | ― | [54] | |
| Phosphorus-based IFR: compound containing Phosphorus(22%) and Nitrogene(18%)/MWCNT (P-IFR/MWCNT) | 28 | P:nP 17.6:1 |
33 | 225 | 73 | 35 | 3 | 1.10 | ― | ― | [54] |
| Phosphorus-based IFR: compound containing Phosphorus(22%) and Nitrogene(18%)/MWCNT (P-IFR/MWCNT) | 28 | P:nP 10.2:1 |
33 | 145 | 54 | 35 | 3 | 2.31 | ― | ― | [54] |
| Phosphorus-based IFR: compound containing Phosphorus(22%) and Nitrogene(18%)/MWCNT (P-IFR/MWCNT) | 28 | P:nP 7:1 |
31 | 140 | 79 | 35 | 3 | 1.53 | ― | ― | [54] |
| ― | ― | 24.7 | 1198.2 | 78.7 | 50 | 2.4 | ― | ― | NR | [139] | |
| Phosphoric acid/ethylenediamine (PA/EDA) | 25 | P:nP 1.7:1 |
12.8 | 263.1 | 57.1 | 50 | 2.4 | 3.25 | ― | V-0 | [139] |
| ― | ― | 42 | 831 | 112 | 35 | 3 | ― | 18 | NR | [50] | |
| PPU-CA/APP | 25 | N:P 1:1 |
17.1 | 288 | 70 | 35 | 3 | 1.87 | 25.5 | V-1 | [50] |
| PPU-CA/APP | 25 | N:nN 2:1 |
19.7 | 568 | 75 | 35 | 3 | 1.02 | 25.5 | V-0 | [50] |
| PPU-CA/APP | 25 | N:nN 3:1 |
26.8 | 605 | 77 | 35 | 3 | 1.27 | 26.5 | V-0 | [50] |
| PPU-CA/APP | 25 | N:nN 4:1 |
22.2 | 642 | 77 | 35 | 3 | 0.99 | 27 | V-0 | [50] |
| ― | ― | 54 | 930 | 140 | 35 | 4 | ― | ― | NR | [64] | |
| MP/PER | 25 | N:nN 1.5:1 |
28 | 250 | 99 | 35 | 4 | 2.72 | ― | V-0 | [64] |
| MP/PER/Kaol | 25 | N:nN:nN 1.66:1:0.11 |
35 | 305 | 110 | 35 | 4 | 2.51 | ― | V-0 | [64] |
| MP/PER/Kaol | 25 | N:nN:nN 1.87:1:0.25 |
37 | 400 | 114 | 35 | 4 | 1.95 | ― | NR | [64] |
| ― | ― | 42 | 1290 | 228 | 50 | 6 | ― | 18.1 | NR | [185] | |
| MP/PER | 20 | N:nN 1.6:2 |
25 | 380 | 212 | 50 | 6 | 2.17 | 29 | V-2 | [185] |
| MP/PER | 25 | N:nN 1.6:2 |
24 | 265 | 206 | 50 | 6 | 3.07 | 34 | V-0 | [185] |
| MP/PER/Organically modified MMT (MP/PER/m-MMT) | 20 | N:nN:nN 1.6:2:0.13 |
23 | 228 | 207 | 50 | 6 | 3.41 | 31.5 | V-0 | [185] |
| MP/PER/Organically modified MMT (MP/PER/m-MMT) | 25 | N:nN:nN 1.6:2:0.1 |
24 | 197 | 205 | 50 | 6 | 4.16 | 35 | V-0 | [185] |
| ― | ― | 51 | 903 | 119.6 | 35 | 3 | ― | 17.5 | NR | [186] | |
| MMP/PER | 26 | N:nN 1.7:1 |
40 | 308 | 99 | 35 | 3 | 2.77 | 27.5 | V-2 | [186] |
| MMP/PER/Lanthanum oxide (MMP/PER/La2O3) | 26 | N:nN:nN 1.7:1:0.1 |
45 | 271 | 94.3 | 35 | 3 | 3.72 | 32 | V-0 | [186] |
| MMP/PER/La2O3 | 26 | N:nN:nN 1.7:1:0.23 |
47 | 247 | 91.4 | 35 | 3 | 4.40 | 31.5 | V-0 | [186] |
| MMP/PER/La2O3 | 26 | N:nN:nN 1.7:1:0.36 |
50 | 221 | 85 | 35 | 3 | 5.63 | 31.5 | V-0 | [186] |
| ― | ― | 38 | 1166 | 89.1 | 35 | 3 | ― | 17 | NR | [187] | |
| MPP/DPER | 30 | N:nN 3:1 |
45 | 427.6 | 79.4 | 35 | 3 | 3.62 | 28.7 | V-0 | [187] |
| MPP/EG/DPER | 30 | N:nN:nN 1.5:1:0.5 |
20 | 218.2 | 68.7 | 35 | 3 | 3.64 | 33.2 | V-0 | [187] |
| ― | ― | 25 | 1239 | 123.6 | 50 | 3 | ― | 18.5 | NR | [143] | |
| Amino trimethylene phosphonic acid melamine salt/PER (MATMP/PER) | 25 | N:nN 2:1 |
17 | 256.4 | 103.2 | 50 | 3 | 3.93 | 30.3 | V-0 | [143] |
| ― | ― | 54 | 961 | 175 | 35 | 4 | ― | ― | ― | [188] | |
| MPyP/PER | 25 | N:nN 3:1 |
32 | 343 | 136 | 35 | 4 | 2.13 | 29 | V-1 | [188] |
|
MPyP/PER/Epoxy crosslinked β-cyclodextrin nanosponge
(MPyP/PER/m-CD) |
25 | N:nN:nN 3:1:0.35 |
30 | 235 | 118 | 35 | 4 | 3.36 | 32.5 | V-0 | [188] |
| ― | ― | 65 | 1417 | 128.5 | 35 | 3 | ― | ― | NR | [57] | |
| MPyP/Phosphorus-based FR: Tri (1-oxo-2,6,7-trioxa-1-phosphabicyclo [2,2,2] octane-methyl) phosphate (MPyP/P-FR) | 30 | N:P 1:1 |
40 | 175.2 | 90.6 | 35 | 3 | 7.05 | ― | V-0 | [57] |
| ― | ― | 34 | 1727 | 112 | 35 | 3 | ― | 17 | NR | [66] | |
|
MPyP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine
(MPyP/TA-CFA) |
30 | N:N 3:1 |
12 | 431 | 84 | 35 | 3 | 1.88 | 29.5 | V-0 | [66] |
|
MPyP/Triazine-based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine
(MPyP/TA-CFA) |
30 | N:N 1:1 |
15 | 469 | 85 | 35 | 3 | 2.14 | 31.2 | V-0 | [66] |
|
MPyP/Triazine based CFA: synthesized by reaction of cyanuric chloride and ethanolamine and ethylenediamine
(MPyP/TA-CFA) |
30 | N:N 3:1 |
12 | 525 | 92 | 35 | 3 | 1.41 | 26.8 | NR | [66] |
| ― | ― | 48 | 988 | 88.3 | 35 | 3 | ― | 17 | NR | [39] | |
|
Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine/APP (TA-CFA/APP)
|
30 | N:P 1:1 |
32 | 82.4 | 77.9 | 35 | 3 | 9.06 | 29 | V-0 | [39] |
| Triazine-based CFA: synthesized by reaction of cyanuric chloride and piperazine/APP (TA-CFA/APP) | 30 | N:nN 2:1 |
52 | 247 | 78.4 | 35 | 3 | 4.88 | 23 | V-1 | [39] |
| ― | ― | 66 | 633 | 44.2 | 35 | 3 | ― | 17 | NR | [23] | |
| Triazine-based CA: synthesized by reaction of 2-carboxyethyl (phenyl) phosphinic acid and tris (2-hydrooxyethyl) isocyanurate/APP (TA-CA/APP) | 20 | N:P 1:1 |
38 | 83 | 41 | 35 | 3 | 4.73 | 30 | V-0 | [23] |
| ― | ― | 48 | 1351 | 107 | 35 | 3.2 | ― | 18.5 | NR | [27] | |
| Triazine-based CA: synthesized by reaction of cyanuric chloride, 2,6,7-trioxa-1-phosphabicyclo [2,2,2]octane-4-methanol and piperazine/APP (TA-CA/APP) | 20 | N:nN 2:1 |
36 | 456 | 96 | 35 | 3.2 | 2.47 | 25.5 | V-2 | [27] |
| ― | ― | 47 | 860.6 | 110.3 | 35 | 3 | ― | 18 | NR | [179] | |
| Triazine-based CA: synthesized by reaction of cyanuric chloride and 2,6,7-trioxa-l-phosphabicyclo[2,2,2]octane-4-methanol and diethylenetriamine/APP (TA-CA/APP) | 30 | N:nN 2:1 |
32 | 166.8 | 108.2 | 35 | 3 | 3.58 | 28 | V-0 | [179] |
| Triazine-based CA: synthesized by reaction of cyanuric chloride and 2,6,7-trioxa-l-phosphabicyclo[2,2,2]octane-4-methanol and diethylenetriamine/APP (TA-CA/APP) | 30 | N:P 1:1 |
31 | 136.5 | 81 | 35 | 3 | 5.66 | 32 | V-0 | [179] |
| ― | ― | 48 | 988 | 88.3 | 35 | 3.2 | ― | 17 | NR | [31] | |
| Triazin-based IFR: synthesized by reaction of cyanuric chloride and N-amino ethylpiperazine/APP (TA-IFR/APP) | 25 | N:P 1:1 |
44 | 123 | 73.3 | 35 | 3.2 | 8.86 | 27.5 | V-0 | [31] |
| Triazin-based IFR: synthesized by reaction of cyanuric chloride and N-amino ethylpiperazine/APP (TA-IFR/APP) | 25 | N:nN 2:1 |
44 | 241 | 77.2 | 35 | 3.2 | 4.29 | 24.5 | V-1 | [31] |
| ― | ― | 62 | 1221 | 265 | 35 | 6 | ― | 19 | NR | [182] | |
| Triazine-based IFR and APP (TAandAPP-IFR) | 10 | N:P ― |
44 | 313 | 235 | 35 | 6 | 3.12 | 26 | NR | [182] |
| Triazine-based IFR and APP (TAandAPP-IFR) | 15 | N:P ― |
45 | 148 | 191 | 35 | 6 | 8.30 | 29 | NR | [182] |
| Triazine-based IFR and APP (TAandAPP-IFR) | 20 | N:P ― |
43 | 115 | 153 | 35 | 6 | 12.75 | 31 | V-0 | [182] |
| ― | ― | 45 | 1269 | 146.35 | 50 | 3 | ― | 17.5 | NR | [70] | |
| Piperazine-based IFR: Piperazine spirocyclic phosphoramidate/APP (PI-IFR/APP) | 30 | N:nN 2:1 |
20 | 189.2 | 74.85 | 50 | 3 | 5.82 | 33.1 | V-1 | [70] |
| ― | ― | 42 | 1025 | 137.7 | 35 | 4 | ― | ― | ― | [74] | |
| Nitrogen-based FR: compound containing nitrogen (27.5 wt.%) and Phosphorus (15.6 wt.%)/Fumed silica (NP-FR/SiO2) | 25 | N:nN 49:1 |
25 | 124 | 35.1 | 35 | 4 | 19.30 | 38 | V-0 | [74] |
|
Nitrogen-based FR: compound containing nitrogen (27.5 wt.%) and Phosphorus
(15.6 wt.%)/Fumed silica (NP-FR/SiO2) |
25 | N:nN 7.3:1 |
17 | 341 | 87.9 | 35 | 4 | 1.90 | 27 | NR | [74] |
| ― | ― | 30 | 1093 | 108.2 | 50 | 3 | ― | 18 | NR | [75] | |
| Nitrogen-based IFR: Poly (diallyldimethylammonium) and polyphosphate polyelectrolyte complexe/Polyamide-6 (N-IFR/PA6) | 25 | N:nN 4:1 |
17 | 295.2 | 80.5 | 50 | 3 | 2.81 | 27.3 | V-1 | [75] |
| ― | ― | 25 | 874.1 | 89.3 | 50 | 3 | ― | 18 | NR | [76] | |
| Nitrogen-based IFR: compound containing nitrogen (23%) and Phosphorus (21%)/Hollow glass microsphere (N-IFR/HGM) | 25 | N:nN 49:1 |
16 | 93.8 | 74.4 | 50 | 3 | 7.15 | 34.5 | V-0 | [76] |
| Nitrogen (23%) and Phosphorus (21%)-based intumescent flame retardant/Hollow glass microsphere (N-IFR/HGM) | 25 | N:nN 24:1 |
17 | 78.8 | 68 | 50 | 3 | 9.90 | 36.5 | V-0 | [76] |
| Nitrogen-based IFR: compound containing nitrogen (23%) and Phosphorus (21%)/HGM (N-IFR/HGM) | 25 | N:nN 11.5:1 |
12 | 61.6 | 74.2 | 50 | 3 | 8.19 | 35.5 | V-0 | [76] |
| Nitrogen-based IFR: compound containing nitrogen (23%) and Phosphorus (21%)/HGM (N-IFR/HGM) | 25 | N:nN 5.25:1 |
13 | 81.6 | 72.5 | 50 | 3 | 6.86 | 34.5 | V-0 | [76] |
| ― | ― | 76 | 455 | 102 | 35 | 3 | ― | 17 | NR | [183] | |
| ATH/APP | 30 | M:P 1:1 |
40 | 210 | 75 | 35 | 3 | 1.55 | 24 | V-1 | [183] |
|
4,4′-diphenylmethane diisocyanateandmelamine co-microencapsulated ATH and APP
(mc-(ATHandAPP)) |
30 | M:P 1:1 |
75 | 120 | 53 | 35 | 3 | 7.20 | 25.5 | V-0 | [183] |
| ― | ― | 32 | 1470 | 175 | 50 | 4 | ― | 18 | ― | [81] | |
| ATH/Glass Bubble (ATH/GB) | 60 | M:M 11:1 |
31 | 212 | 53 | 50 | 4 | 22.17 | 25 | ― | [81] |
| ATH/GB | 60 | M:M 5:1 |
36 | 190 | 49 | 50 | 4 | 31.08 | 23.4 | ― | [81] |
| ATH/GB/Octacedylamine modified ZrP (ATH/GB/m-ZrP) | 60 | M:M:M 4.7:1:0.3 |
24 | 136 | 90 | 50 | 4 | 15.76 | 24 | ― | [81] |
| ATH/GB/Octacedylamine modified ZrP (ATH/GB/m-ZrP) | 60 | M:M:M 4.4:1:0.6 |
24 | 152 | 91 | 50 | 4 | 13.94 | 23.2 | ― | [81] |
| ATH/GB/Octacedylamine modified ZrP (ATH/GB/m-ZrP) | 60 | M:M:M 4.1:1:0.9 |
21 | 189 | 98 | 50 | 4 | 9.11 | 22.8 | ― | [81] |
| ― | ― | 37 | 1425 | 121.4 | 50 | 3 | ― | 17.3 | NR | [80] | |
| ATH/Cetyltrimethyl ammonium bromide modified Fe MMT (ATH/m-MMT) | 50 | M:M 49:1 |
48 | 482 | 95.1 | 50 | 3 | 4.89 | 25.5 | NR | [80] |
| ATH/Cetyltrimethyl ammonium bromide modified Fe MMT (ATH/m-MMT) | 50 | M:M 15.6:1 |
49 | 412 | 90.9 | 50 | 3 | 6.11 | 27.4 | V-1 | [80] |
| ATH/Cetyltrimethyl ammonium bromide modified Fe MMT (ATH/m-MMT) | 50 | M:M 9:1 |
53 | 329 | 89 | 50 | 3 | 8.46 | 29 | V-0 | [80] |
| ― | ― | 26 | 1967 | 112 | 50 | 3 | ― | ― | ― | [82] | |
| ATH/Styrene-co-vinylbenzyl chloride modified MMT (ATH/m-MMT) | 23 | M:M 6.6:1 |
21 | 677 | 84 | 50 | 3 | 3.12 | ― | ― | [82] |
| ATH/Styrene-co-vinylbenzyl chloride modified MMT (ATH/m-MMT) | 30 | M:M 2:1 |
20 | 592 | 77 | 50 | 3 | 3.71 | ― | ― | [82] |
| ATH/Styrene-co-vinylbenzyl chloride modified MMT (ATH/m-MMT) | 37 | M:M 1.17:1 |
18 | 536 | 74 | 50 | 3 | 3.84 | ― | ― | [82] |
| ― | ― | 24 | 687 | 119 | 25 | 3 | ― | 17.8 | ― | [146] | |
| MDH/APP/PER/MEL | 44.2 | M:nM:nM:nM 1.3:1:0.6:0.56 |
43 | 121 | 58.2 | 25 | 3 | 20.79 | 28 | ― | [146] |
| MDH/APP/PER/MEL | 50 | M:nM:nM:nM 2.1:1:0.6:0.56 |
43 | 121 | 54.5 | 25 | 3 | 22.21 | 28.8 | ― | [146] |
| MDH/APP/PER/MEL | 54.4 | M:nM:nM:nM 3:1:0.6:0.56 |
44 | 104 | 53.6 | 25 | 3 | 26.88 | 30.2 | ― | [146] |
| ― | ― | 166 | 412 | 105 | 50 | 3 | ― | 17.8 | [146] | ||
| MDH/APP/PER/MEL | 44.2 | M:nM:nM:nM 1.3:1:0.6:0.56 |
217 | 68.8 | 39.3 | 50 | 3 | 20.91 | 28 | ― | [146] |
| MDH/APP/PER/MEL | 50 | M:nM:nM:nM 2.1:1:0.6:0.56 |
220 | 57.3 | 36.4 | 50 | 3 | 27.48 | 28.8 | ― | [146] |
| MDH/APP/PER/MEL | 54.4 | M:nM:nM:nM 3:1:0.6:0.56 |
232 | 54.3 | 31.2 | 50 | 3 | 35.68 | 30.2 | ― | [146] |
| ― | ― | 26 | 1967 | 112 | 50 | 3 | ― | ― | ― | [82] | |
| MDH/Styrene-co-vinylbenzyl chloride modified MMT (MDH/m-MMT) | 37 | M:M 1.17:1 |
24 | 476 | 70 | 50 | 3 | 6.10 | ― | ― | [82] |
| ― | ― | 30 | 1684 | 89 | 50 | 3 | ― | ― | ― | [82] | |
| MDH/Styrene-co-vinylbenzyl chloride modified MMT (MDH/m-MMT) | 40 | M:M 3:1 |
24 | 471 | 80 | 50 | 3 | 3.18 | ― | ― | [82] |
| MDH/Styrene-co-vinylbenzyl chloride modified MMT (MDH/m-MMT) | 50 | M:M 4:1 |
23 | 385 | 69 | 50 | 3 | 4.32 | ― | ― | [82] |
| MDH/Styrene-co-vinylbenzyl chloride modified MMT (MDH/m-MMT) | 60 | M:M 5:1 |
22 | 304 | 59 | 50 | 3 | 6.12 | ― | ― | [82] |
| ― | ― | 38 | 1425 | 121.4 | 50 | 3 | ― | 17.5 | NR | [85] | |
| MDH/Cetyltrimeyhyl ammonium bromide modified Fe MMT (MDH/m-MMT) | 40 | M:M 39:1 |
52 | 422 | 98.1 | 50 | 3 | 5.71 | 24.9 | NR | [85] |
| MDH/Cetyltrimeyhyl ammonium bromide modified Fe MMT (MDH/m-MMT) | 40 | M:M 12.3:1 |
56 | 378 | 97.5 | 50 | 3 | 6.91 | 26.5 | NR | [85] |
| MDH/Cetyltrimeyhyl ammonium bromide modified Fe MMT (MDH/m-MMT) | 40 | M:M 7:1 |
63 | 329 | 87.9 | 50 | 3 | 9.91 | 28.1 | V-1 | [85] |
| ― | ― | 71 | 2283 | 218 | 35 | 1 | ― | ― | ― | [84] | |
| MDH/4,4′-bis (acrylamido) diphenylsulfone crosslinked N-(4-methyl phenyl) acrylamide monomer (MDH/Cobalt chelate) | 50 | M:M 9:1 |
72 | 619 | 306 | 35 | 1 | 2.66 | ― | ― | [84] |
| MDH/Cobalt chelate | 50 | M:M 4:1 |
63 | 618 | 277 | 35 | 1 | 2.57 | ― | ― | [84] |
| MDH/Cobalt chelate | 50 | M:M 2.3:1 |
53 | 776 | 236 | 35 | 1 | 2.02 | ― | ― | [84] |
| MDH/Cobalt chelate | 50 | M:M 1.5:1 |
56 | 780 | 222 | 35 | 1 | 2.26 | ― | ― | [84] |
| ― | ― | 37 | 584 | 75.6 | 50 | 3 | ― | ― | ― | [87] | |
| Cetyltrimethylammonium bromide modified MMT/SEP (m-MMT/SEP) | 5 | M:M 1:1 |
62 | 417 | 63.7 | 50 | 3 | 2.78 | ― | ― | [87] |
|
MDH/Organically treated SEP
(MDH/m-SEP) |
15 | M:M 2:1 |
29 | 325 | 62.1 | 50 | 3 | 1.71 | ― | ― | [87] |
|
MDH/Organically treated SEP
(MDH/m-SEP) |
20 | M:M 3:1 |
26 | 205 | 53.5 | 50 | 3 | 2.82 | ― | ― | [87] |
| MDH/cetyltrimethylammonium bromide modified MMT/SEP (MDH/m-MMT/SEP) | 15 | M:M:M 4:1:1 |
54 | 246 | 56.3 | 50 | 3 | 4.65 | ― | ― | [87] |
| MDH/cetyltrimethylammonium bromide modified MMT/SEP (MDH/m-MMT/SEP) | 20 | M:M:M 4:1:1 |
50 | 209 | 50.1 | 50 | 3 | 5.69 | ― | ― | [87] |
| ― | ― | 14 | 1104 | 106 | 35 | 0.4 | ― | ― | ― | [24] | |
| MMT/APP | 10 | M:nM 1.5:1 |
28 | 764 | 64 | 35 | 0.4 | 4.78 | ― | ― | [24] |
| MMT/APP | 10 | M:nM 4:1 |
29 | 751 | 62 | 35 | 0.4 | 5.20 | ― | ― | [24] |
| Modified MMT/APP (m-MMT/APP) | 10 | M:nM 1.5:1 |
30 | 599 | 57 | 35 | 0.4 | 7.34 | ― | ― | [24] |
| Modified MMT/APP (m-MMT/APP) | 10 | M:nM 4:1 |
31 | 575 | 56 | 35 | 0.4 | 8.04 | ― | ― | [24] |
| ― | ― | 25 | 981 | 147 | 50 | ― | ― | 17.6 | NR | [30] | |
| Polysiloxane based FR/APP (Si-FR/APP) | 25 | M:P 1:1 |
14 | 277 | 97 | 50 | ― | 3.00 | 28.9 | V-0 | [30] |
| ― | ― | 38 | 1284 | 241 | 50 | 6 | ― | ― | ― | [108] | |
| Ni2O3/AC | 15 | M:C 1:1 |
18 | 385 | 132 | 50 | 6 | 2.88 | ― | ― | [108] |
| ― | ― | 47 | 1933 | 176 | 50 | 5 | ― | ― | ― | [189] | |
| SEP/MWCNT | 12 | M:nM 5:1 |
32 | 355 | 241 | 50 | 5 | 2.70 | ― | ― | [189] |
| ― | ― | 62 | 1378 | 332 | 35 | ― | ― | ― | ― | [190] | |
| Silicon/Stannous chloride (Si/SnCl2) | 5 | M:M 1.5:1 |
91 | 860.1 | 193.7 | 35 | ― | 4.03 | ― | ― | [190] |
| ― | ― | 88 | 565.9 | 71.9 | 35 | 3 | ― | ― | ― | [105] | |
| C20A/TiO2 | 5.5 | M:M 10:1 |
83 | 458.9 | 78.1 | 35 | 3 | 1.07 | 20 | ― | [105] |
| Ethylene glycol methacrylate phosphate modified C20A/TiO2 (m-C20A/TiO2) | 5.5 | M:M 10:1 |
78 | 498.2 | 75.2 | 35 | 3 | 0.96 | 19 | ― | [105] |
| Ethylene glycol methacrylate phosphate modified C20A/TiO2 (m-C20A/TiO2) | 10.5 | M:M 20:1 |
61 | 424.7 | 74.8 | 35 | 3 | 0.88 | 20 | ― | [105] |
| ― | ― | 38 | 1284 | 214 | 50 | 6 | ― | 18.2 | ― | [133] | |
| CF/MWCNT | 10 | C:C 1:1 |
25 | 364 | 194 | 50 | 6 | 2.55 | 25.8 | ― | [133] |
| ― | ― | 30 | 1261 | 208 | 50 | 6 | ― | 18 | ― | [131] | |
| CB/MWCNT | 4 | C:C 3:1 |
26 | 402 | 187 | 50 | 6 | 3.02 | 23.8 | ― | [131] |
| CB/MWCNT | 6 | C:C 5:1 |
27 | 353 | 185 | 50 | 6 | 3.61 | 26.5 | ― | [131] |
| CB/MWCNT | 8 | C:C 1.6:1 |
25 | 314 | 180 | 50 | 6 | 3.86 | 27.6 | ― | [131] |
| ― | ― | 35 | 1212 | 198 | 50 | 6 | ― | 18.2 | ― | [134] | |
| CB/CF | 8 | C:C 1.66:1 |
27 | 361 | 166 | 50 | 6 | 3.08 | 25.7 | ― | [134] |
| ― | ― | 38 | 1284 | 241 | 50 | 6 | ― | ― | ― | [108] | |
| AC/Ni2O3 | 15 | C:M 1:1 |
18 | 385 | 132 | 50 | 6 | 2.88 | ― | ― | [108] |
| ― | ― | 166 | 412 | 105 | 25 | 3 | ― | ― | ― | [145] | |
| PER/MEL/APP | 25.3 | C:nC:nC 1.07:1:0.53 |
170 | 140 | 61.1 | 25 | 3 | 5.17 | ― | ― | [145] |
| ― | ― | 24 | 687 | 119 | 50 | 3 | ― | ― | ― | [145] | |
| PER/MEL/APP | 25.3 | C:nC:nC 1.07:1:0.53 |
32 | 198 | 79 | 50 | 3 | 6.96 | ― | ― | [145] |
| ― | ― | 46 | 1541 | 90 | 35 | 3 | ― | ― | ― | [135] | |
| Cyclodextrin nanosponge/Triethylphosphate (CD/TEP) | 10 | Bio:nBio 2.3:1 |
30 | 1529 | 93 | 35 | 3 | 0.63 | ― | ― | [135] |
| CD/TEP | 15 | Bio:nBio 2:1 |
26 | 839 | 90 | 35 | 3 | 1.03 | ― | ― | [135] |
| CD/APP | 15 | Bio:P 1:1 |
24 | 910 | 89 | 35 | 3 | 0.89 | ― | ― | [135] |
| ― | ― | 49 | 1350 | 87.3 | 35 | 3 | ― | 17.5 | ― | [136] | |
| Phosphorus and Nitrogen elements modified lignin/Nickel acetate (m-lig/Ni(Ac)2) | 20 | Bio:nBio 9:1 |
31 | 330 | 69.5 | 35 | 3 | 3.25 | 26 | ― | [136] |
| Phosphorus and Nitrogen elements modified lignin/Cobalt acetate (m-lig/Co(Ac)2) | 20 | Bio:nBio 9:1 |
37 | 362 | 72.8 | 35 | 3 | 3.37 | 24.5 | ― | [136] |
| Phosphorus and Nitrogen elements modified lignin/Zinc acetate (m-lig/Zn(Ac)2) | 20 | Bio:nBio 9:1 |
38 | 368 | 73.5 | 35 | 3 | 3.37 | 23 | ― | [136] |
Figure 15 displays the performance of different combinatorial additive systems used for PP. It can be clearly observed from the left-hand side figure that cases with FRI values above 10 (Excellent zone) are more frequent compared to all previous cases in which only one additive was used. More interestingly, the combination of additives appeared a useful strategy where very high FRI values (event more than 50) took place at intermediate loadings (25–30 wt.%). For achieving a high FRI value, the combination of several types of flame retardants is needed, for example, phosphorus, intumescent, and mineral flame retardants [150] or phosphorus, nitrogen, and mineral flame retardants [164].
Figure 15.
FRI values as a function of combinatorial FR additives and their content in PP in long-shot (left-hand figure) and close-up (right-hand figure) views. Symbols are indicative of different types of combinatorial flame retardant used. Here: ▲ APP-13.2/PER-6.8 [68], ▼
APP-16.7/PER-8.3 [143],  APP-18.7/PER-6.3 [67],
APP-18.7/PER-6.3 [67],  APP-22.5/PER-7.5, mc-(APP-22.5&PER-7.5) [144],
APP-22.5/PER-7.5, mc-(APP-22.5&PER-7.5) [144],
 APP-10.5/PER-9.8/MEL-9.1, APP-15.3/PER-9.3/MEL-8.8, APP-19.1/PER-8.9/MEL-8.2 [145],
APP-10.5/PER-9.8/MEL-9.1, APP-15.3/PER-9.3/MEL-8.8, APP-19.1/PER-8.9/MEL-8.2 [145],
 APP-10.5/PER-9.8/MEL-9.1, APP-15.3/PER-9.3/MEL-8.8, APP-19.1/PER-8.9/MEL-8.2 [145],
APP-10.5/PER-9.8/MEL-9.1, APP-15.3/PER-9.3/MEL-8.8, APP-19.1/PER-8.9/MEL-8.2 [145],
 APP-15.3/PER-9.3/MEL-8.6, APP-14.3/PER-8.7/MEL-8.1/MDH-6.2 [146],
APP-15.3/PER-9.3/MEL-8.6, APP-14.3/PER-8.7/MEL-8.1/MDH-6.2 [146],
 APP-15.3/PER-9.3/MEL-8.6, APP-14.3/PER-8.7/MEL-8.1/MDH-6.2 [146],
APP-15.3/PER-9.3/MEL-8.6, APP-14.3/PER-8.7/MEL-8.1/MDH-6.2 [146],  APP-12/PER-4/MEL-4/C20A-1, APP-12/PER-4/MEL-4/C20A-3, APP&MMT-12/PER-4/MEL-4/C20A-1, APP&MMT-12/PER-4/MEL-4/C20A-3 [104]
APP-12/PER-4/MEL-4/C20A-1, APP-12/PER-4/MEL-4/C20A-3, APP&MMT-12/PER-4/MEL-4/C20A-1, APP&MMT-12/PER-4/MEL-4/C20A-3 [104]  m-APP-16.6/DPER-4.2/MEL-4.2, m-APP-16/DPER-4/MEL-4/SEP-1 [147],
m-APP-16.6/DPER-4.2/MEL-4.2, m-APP-16/DPER-4/MEL-4/SEP-1 [147],  APP-13.5/PER-4.5, APP-12.75/PER-4.25/MF-1, APP-12.75/PER-4.25/MFA-1 [148],
APP-13.5/PER-4.5, APP-12.75/PER-4.25/MF-1, APP-12.75/PER-4.25/MFA-1 [148],
 APP-22.5/PER-7.5 [149],
APP-22.5/PER-7.5 [149],
 APP-12.7/PER-5.3, APP-12/PER-5/TA-FR-1 [150],
APP-12.7/PER-5.3, APP-12/PER-5/TA-FR-1 [150],
 APP-16.67/PER-8.33, APP-16.33/PER-8.17/NOR116-0.5 [72],
APP-16.67/PER-8.33, APP-16.33/PER-8.17/NOR116-0.5 [72],
 APP-13.5/PER-4.5, APP-12.75/PER-4.25/G-bases-1, APP-12.75/PER-4.25/U-bases-1 [151],
APP-13.5/PER-4.5, APP-12.75/PER-4.25/G-bases-1, APP-12.75/PER-4.25/U-bases-1 [151],
 APP-17.2/DPER-7.8, m-APP-17.2/DPER-7.8, APP-16.2/DPER-7.8/ATH-1 [152],
APP-17.2/DPER-7.8, m-APP-17.2/DPER-7.8, APP-16.2/DPER-7.8/ATH-1 [152],
 APP-21.4/PER-7.1, APP-20.3/PER-6.8/Kaol-1.4 [70],
APP-21.4/PER-7.1, APP-20.3/PER-6.8/Kaol-1.4 [70],
 APP-21.75/PER-7.25, APP-19.5/PER-6.5/MMT-3, APP-19.5/PER-6.5/m-MMT-3 [153],
APP-21.75/PER-7.25, APP-19.5/PER-6.5/MMT-3, APP-19.5/PER-6.5/m-MMT-3 [153],
 APP-21.75/PER-7.25, APP-19.5/PER-6.5/MMT-3, APP-19.5/PER-6.5/m-MMT-3, APP-19.5/PER-6.5/m-MMT-3 [154],
APP-21.75/PER-7.25, APP-19.5/PER-6.5/MMT-3, APP-19.5/PER-6.5/m-MMT-3, APP-19.5/PER-6.5/m-MMT-3 [154],
 APP-18.75/PER-6.25, APP-18/PER-6/LDH-1, APP-18/PER-6/m-LDH-1 [155],
APP-18.75/PER-6.25, APP-18/PER-6/LDH-1, APP-18/PER-6/m-LDH-1 [155],
 APP-18.75/PER-6.25, APP-18/PER-6/m-SiR-1, APP-18/PER-6/m-SiR-1, APP-16.5/PER-5.5/m-SiR-3 [156],
APP-18.75/PER-6.25, APP-18/PER-6/m-SiR-1, APP-18/PER-6/m-SiR-1, APP-16.5/PER-5.5/m-SiR-3 [156],
 m-APP-16.7/DPER-8.3, m-APP-16.7/DPER-8.3/Z-1, m-APP-16.7/DPER-8.3/Z-1/MWCNT-0.1 [157],
m-APP-16.7/DPER-8.3, m-APP-16.7/DPER-8.3/Z-1, m-APP-16.7/DPER-8.3/Z-1/MWCNT-0.1 [157],
 APP-18.75/PER-6.25, APP-18.75/PER-6.25/ALL-2 [158],
APP-18.75/PER-6.25, APP-18.75/PER-6.25/ALL-2 [158],
 APP-16.7/PER-8.3, APP-15/PER-7.5/MAO-2.5, APP-15/PER-7.5/Zn-MAO-2.5 [159],
APP-16.7/PER-8.3, APP-15/PER-7.5/MAO-2.5, APP-15/PER-7.5/Zn-MAO-2.5 [159],
 APP-16.7/PER-8.3, APP-16/PER-8/m-SEP-1, APP-15.3/PER-7.7/m-SEP-2, APP-14.7/PER-7.3/m-SEP-3, APP-14/PER-7/m-SEP-4, APP-13.3/PER-6.7/m-SEP-5 [160],
APP-16.7/PER-8.3, APP-16/PER-8/m-SEP-1, APP-15.3/PER-7.7/m-SEP-2, APP-14.7/PER-7.3/m-SEP-3, APP-14/PER-7/m-SEP-4, APP-13.3/PER-6.7/m-SEP-5 [160],
 APP-15/PER-5, APP-14.25/PER-4.75/OP-POSS-1, APP-14.25/PER-4.75/A-POSS-1, APP-14.25/PER-4.75/OA-POSS-1, APP-14.25/PER-4.75/TS-POSS-1 [161],
APP-15/PER-5, APP-14.25/PER-4.75/OP-POSS-1, APP-14.25/PER-4.75/A-POSS-1, APP-14.25/PER-4.75/OA-POSS-1, APP-14.25/PER-4.75/TS-POSS-1 [161],  APP-20/PER-10¸ APP-19/PER-9.5/T-RS-5, APP-19/PER-9.5/CV-5, APP-19/PER-9.5/CR-5 [162],
APP-20/PER-10¸ APP-19/PER-9.5/T-RS-5, APP-19/PER-9.5/CV-5, APP-19/PER-9.5/CR-5 [162],  APP-15/PER-5, APP-14.25/PER-4.75/ZnB-1, APP-14.25/PER-4.75/BPO4-1, APP-14.25/PER-4.75/Bsi-1, APP-14.25/PER-4.75/LaB-1 [163],
APP-15/PER-5, APP-14.25/PER-4.75/ZnB-1, APP-14.25/PER-4.75/BPO4-1, APP-14.25/PER-4.75/Bsi-1, APP-14.25/PER-4.75/LaB-1 [163],
 APP-18.75/PER-6.25, APP-17.25/PER-5.75/NiFeO-2, APP-17.25/PER-5.75/CoFeO-2 [107],
APP-18.75/PER-6.25, APP-17.25/PER-5.75/NiFeO-2, APP-17.25/PER-5.75/CoFeO-2 [107],
 APP-16.67/PER-8.33, APP-15.33/PER-7.67/Ni12P5-2, APP-15.33/PER-7.67/Co2P-2, APP-15.33/PER-7.67/Cu3P-2 [164],
APP-16.67/PER-8.33, APP-15.33/PER-7.67/Ni12P5-2, APP-15.33/PER-7.67/Co2P-2, APP-15.33/PER-7.67/Cu3P-2 [164],
 APP-18.75/PER-6.25, APP-18/PER-6/ZHS-1 [165],
APP-18.75/PER-6.25, APP-18/PER-6/ZHS-1 [165],
 APP-18.75/PER-6.25, APP-18.75/PER-6.25/MnAc-1, APP-18.75/PER-6.25/MnAc-2, APP-18.75/PER-6.25/MnAc-3, APP-18.75/PER-6.25/MnAc-4 [166],
APP-18.75/PER-6.25, APP-18.75/PER-6.25/MnAc-1, APP-18.75/PER-6.25/MnAc-2, APP-18.75/PER-6.25/MnAc-3, APP-18.75/PER-6.25/MnAc-4 [166],  APP-21/DPER-7/m-SA-7 [167],
APP-21/DPER-7/m-SA-7 [167],  APP-15.4/PEPA-7.6, APP-15.4/PEPA-7.6/NOR116-2, APP-15.4/PEPA-7.6/ZrP-2, APP-15.4/PEPA-7.6/m-ZrP-2 [168],
APP-15.4/PEPA-7.6, APP-15.4/PEPA-7.6/NOR116-2, APP-15.4/PEPA-7.6/ZrP-2, APP-15.4/PEPA-7.6/m-ZrP-2 [168],  MCAPP-16.7/PEPA-8.3, MCAPP-15.7/PEPA-7.8/Kaol-1.5, MCAPP-15.7/PEPA-7.8/m-Kaol-1.5 [169],
MCAPP-16.7/PEPA-8.3, MCAPP-15.7/PEPA-7.8/Kaol-1.5, MCAPP-15.7/PEPA-7.8/m-Kaol-1.5 [169],
 MCAPP-16.7/PEPA-8.3, MCAPP-15.7/PEPA-7.8/Kaol-1.5, MCAPP-15.7/PEPA-7.8/m-Kaol-1.5 [170],
MCAPP-16.7/PEPA-8.3, MCAPP-15.7/PEPA-7.8/Kaol-1.5, MCAPP-15.7/PEPA-7.8/m-Kaol-1.5 [170],  MCAPP-16.7/PEPA-8.3, MCAPP-15.7/PEPA-7.8/Kaol-1.5, MCAPP-15.7/PEPA-7.8/Kaol nanoroll-1.5 [171],
MCAPP-16.7/PEPA-8.3, MCAPP-15.7/PEPA-7.8/Kaol-1.5, MCAPP-15.7/PEPA-7.8/Kaol nanoroll-1.5 [171],
 MCAPP-16.7/PEPA-8.3, MCAPP-15.7/PEPA-7.8/Kaol-1.5, MCAPP-15.7/PEPA-7.8/m-Kaol-1.5 [89],
MCAPP-16.7/PEPA-8.3, MCAPP-15.7/PEPA-7.8/Kaol-1.5, MCAPP-15.7/PEPA-7.8/m-Kaol-1.5 [89],
 mc-APP-16.7/PEPA-8.3, mc-APP-15.7/PEPA-7.8/Kaol-1.5, mc-APP-15.7/PEPA-7.8/HNT-1.5, mc-APP-15.7/PEPA-7.8/Kaol-1.35/HNT-0.15 [172],
mc-APP-16.7/PEPA-8.3, mc-APP-15.7/PEPA-7.8/Kaol-1.5, mc-APP-15.7/PEPA-7.8/HNT-1.5, mc-APP-15.7/PEPA-7.8/Kaol-1.35/HNT-0.15 [172],
 mc-APP-16.7/PEPA-8.3, mc-APP-15.7/PEPA-7.8/Kaol-1.5, mc-APP-15.7/PEPA-7.8/HSA-A-1.5, mc-APP-15.7/PEPA-7.8/HSA-P-1.5, mc-APP-15.7/PEPA-7.8/HSA-A-La-1.5, mc-APP-15.7/PEPA-7.8/HSA-P-La-1.5 [173],
mc-APP-16.7/PEPA-8.3, mc-APP-15.7/PEPA-7.8/Kaol-1.5, mc-APP-15.7/PEPA-7.8/HSA-A-1.5, mc-APP-15.7/PEPA-7.8/HSA-P-1.5, mc-APP-15.7/PEPA-7.8/HSA-A-La-1.5, mc-APP-15.7/PEPA-7.8/HSA-P-La-1.5 [173],
 APP-12.5/P-CA-12.5 [49],
APP-12.5/P-CA-12.5 [49],
 APP-28/PhZ-FR-2, APP-26/PhZ-FR-4, APP-24/PhZ-FR-6, APP-22/PhZ-FR-8 [38],
APP-28/PhZ-FR-2, APP-26/PhZ-FR-4, APP-24/PhZ-FR-6, APP-22/PhZ-FR-8 [38],
 mc-APP-22.5/THEIC-7.5 [41],
mc-APP-22.5/THEIC-7.5 [41],
 APP-16.67/PPU-CA-8.33, APP-12.5/PPU-CA-12.5 [50],
APP-16.67/PPU-CA-8.33, APP-12.5/PPU-CA-12.5 [50],
 APP-17.6/TA-CFA-4.4, m-APP-17.6/TA-CFA-4.4 [95],
APP-17.6/TA-CFA-4.4, m-APP-17.6/TA-CFA-4.4 [95],
 APP-16.7/TA-CFA-8.3 [32],
APP-16.7/TA-CFA-8.3 [32],
 APP-20/TA-CFA-5, m-APP-20/TA-CFA-5 [37],
APP-20/TA-CFA-5, m-APP-20/TA-CFA-5 [37],
 APP-20/TA-CFA-10, APP-22.5/TA-CFA-7.5, APP-24/TA-CFA-6 [40],
APP-20/TA-CFA-10, APP-22.5/TA-CFA-7.5, APP-24/TA-CFA-6 [40],
 APP-15/TA-CFA-15, APP-20/TA-CFA-10, APP-22.5/TA-CFA-7.5, APP-24/TA-CFA-6 [39],
APP-15/TA-CFA-15, APP-20/TA-CFA-10, APP-22.5/TA-CFA-7.5, APP-24/TA-CFA-6 [39],
 APP-15/TA-CFA-5, APP-14.63/TA-CFA-4.87/m-MMT-0.5, APP-14.25/TA-CFA-4.75/m-MMT-1, APP-13.87/TA-CFA-4.63/m-MMT-1.5, APP-13.5/TA-CFA-4.5/m-MMT-2, APP-12.75/TA-CFA-4.25/m-MMT-3 [174],
APP-15/TA-CFA-5, APP-14.63/TA-CFA-4.87/m-MMT-0.5, APP-14.25/TA-CFA-4.75/m-MMT-1, APP-13.87/TA-CFA-4.63/m-MMT-1.5, APP-13.5/TA-CFA-4.5/m-MMT-2, APP-12.75/TA-CFA-4.25/m-MMT-3 [174],
 APP-18.24/TA-CFA-4.56/SiO2-1.2, APP-15.66/TA-CFA-3.91/AHP-3.4/SiO2-1.03 [61],
APP-18.24/TA-CFA-4.56/SiO2-1.2, APP-15.66/TA-CFA-3.91/AHP-3.4/SiO2-1.03 [61],
 APP-18.24/TA-CFA-4.56/SiO2-1.2, APP-18.24/TA-CFA-4.56/SiO2-1.2 [175],
APP-18.24/TA-CFA-4.56/SiO2-1.2, APP-18.24/TA-CFA-4.56/SiO2-1.2 [175],  APP-20/TA-CFA-5, APP-19.6/TA-CFA-4.9/rGNO-0.5, APP-19.2/TA-CFA-4.8/rGNO-1, APP-18.4/TA-CFA-4.6/rGNO-2 [176],
APP-20/TA-CFA-5, APP-19.6/TA-CFA-4.9/rGNO-0.5, APP-19.2/TA-CFA-4.8/rGNO-1, APP-18.4/TA-CFA-4.6/rGNO-2 [176],  m-APP-20/TA-CFA-5, m-APP-19.6/TA-CFA-4.9/rGNO-0.5, m-APP-19.2/TA-CFA-4.8/rGNO-1, m-APP-18.4/TA-CFA-4.6/rGNO-2, m-APP-17.6/TA-CFA-4.9/m-APP@rGNO-2.5, m-APP-15.2/m-APP@rGNO-5/TA-CFA-4.8, m-APP-10.4/m-APP@rGNO-10/TA-CFA-4.6 [177],
m-APP-20/TA-CFA-5, m-APP-19.6/TA-CFA-4.9/rGNO-0.5, m-APP-19.2/TA-CFA-4.8/rGNO-1, m-APP-18.4/TA-CFA-4.6/rGNO-2, m-APP-17.6/TA-CFA-4.9/m-APP@rGNO-2.5, m-APP-15.2/m-APP@rGNO-5/TA-CFA-4.8, m-APP-10.4/m-APP@rGNO-10/TA-CFA-4.6 [177],
 APP-10/TA-CA-10 [23],
APP-10/TA-CA-10 [23],
 APP-13.33/TA-CA-6.67, APP-15/TA-CA-5 [27],
APP-13.33/TA-CA-6.67, APP-15/TA-CA-5 [27],
 APP-16.7/TA-CA-8.3 [67],
APP-16.7/TA-CA-8.3 [67],  APP-18.75/TA-CA-6.25, mc-APP-18.75/TA-CA-6.25 [36],
APP-18.75/TA-CA-6.25, mc-APP-18.75/TA-CA-6.25 [36],
 APP-13.33/TA-CA-6.67 [149],
APP-13.33/TA-CA-6.67 [149],
 APP-20/TA-CA-10, APP-20/Homo-TA-CA-10 [178], ▲ APP-15/TA-CA-15, APP-24/TA-CA-6 [179], ▼ APP-16.7/TA-CA-8.3, APP-16.5/TA-CA-8.2/NOR116-0.3 [73], ◄ APP-14.7/TA-CA-5.3, APP-14/TA-CA-5/m-MMT-1, APP-12.5/TA-CA-4.5/m-MMT-3 [180], ► APP-16.72/TA-CFA-4.18/SiO2-1.1, APP-16.72/TA-CFA-4.18/SiO2-1.1 [181], ♦ APP-16.7/TA-CA-ZnO-8.3 [29],
APP-20/TA-CA-10, APP-20/Homo-TA-CA-10 [178], ▲ APP-15/TA-CA-15, APP-24/TA-CA-6 [179], ▼ APP-16.7/TA-CA-8.3, APP-16.5/TA-CA-8.2/NOR116-0.3 [73], ◄ APP-14.7/TA-CA-5.3, APP-14/TA-CA-5/m-MMT-1, APP-12.5/TA-CA-4.5/m-MMT-3 [180], ► APP-16.72/TA-CFA-4.18/SiO2-1.1, APP-16.72/TA-CFA-4.18/SiO2-1.1 [181], ♦ APP-16.7/TA-CA-ZnO-8.3 [29],  APP&TA-IFR-10, APP&TA-IFR-15, APP&TA-IFR-20 [182],
APP&TA-IFR-10, APP&TA-IFR-15, APP&TA-IFR-20 [182],  APP-12.5/TA-IFR-12.5, APP-16.67/TA-IFR-8.33, APP-18.75/TA-IFR-6.25 [31],
APP-12.5/TA-IFR-12.5, APP-16.67/TA-IFR-8.33, APP-18.75/TA-IFR-6.25 [31],
 APP-20/PI-TA-CA-5, APP-20/PI-TA-CA-5 [33],
APP-20/PI-TA-CA-5, APP-20/PI-TA-CA-5 [33],
 APP-22.5/PI-IFR-7.5, APP-16.4/PI-IFR-8.2/TA-CFA-5.4 [70],
APP-22.5/PI-IFR-7.5, APP-16.4/PI-IFR-8.2/TA-CFA-5.4 [70],
 APP-15/ATH-15, mc-(APP-15&ATH-15) [183],
APP-15/ATH-15, mc-(APP-15&ATH-15) [183],
 APP-8/MMT-2, APP-6/MMT-4, APP-8/m-MMT-2, APP-6/m-MMT-4 [24],
APP-8/MMT-2, APP-6/MMT-4, APP-8/m-MMT-2, APP-6/m-MMT-4 [24],
 APP-15/Nf-5, APP-15/m-BT-5 [103],
APP-15/Nf-5, APP-15/m-BT-5 [103],
 APP-15/Nf-5, APP-15/m-BT-5 [103],
APP-15/Nf-5, APP-15/m-BT-5 [103],
 APP-15/C15A-5, OP-15/C15A-5 [60],
APP-15/C15A-5, OP-15/C15A-5 [60],
 APP-12/C20A-5/PER-4/MEL-4, APP&MMT-12/C20A-5/PER-4/MEL-4, APP&MMT-6/C20A-5/PER-2/MEL-2, APP&MMT-9/C20A-5/PER-3/MEL-3 [104],
APP-12/C20A-5/PER-4/MEL-4, APP&MMT-12/C20A-5/PER-4/MEL-4, APP&MMT-6/C20A-5/PER-2/MEL-2, APP&MMT-9/C20A-5/PER-3/MEL-3 [104],
 APP-19/m-LDH-1, APP-18/m-LDH-2, APP-17/m-LDH-3 [26],
APP-19/m-LDH-1, APP-18/m-LDH-2, APP-17/m-LDH-3 [26],
 APP-IFR-18/LDH-2, APP-IFR-16/LDH-4, APP-IFR-18/LDH-2, APP-IFR-16/LDH-4 [51],
APP-IFR-18/LDH-2, APP-IFR-16/LDH-4, APP-IFR-18/LDH-2, APP-IFR-16/LDH-4 [51],
 APP-12.5/Si-FR-12.5, APP-15/Si-FR-10, APP-13.8/Si-FR-9.2, APP-16.67/Si-FR-8.33, APP-18.75/Si-FR-6.25 [30],
APP-12.5/Si-FR-12.5, APP-15/Si-FR-10, APP-13.8/Si-FR-9.2, APP-16.67/Si-FR-8.33, APP-18.75/Si-FR-6.25 [30],
 APP-12/SEP-0.5, APP-12/m-SEP-0.5 [25],
APP-12/SEP-0.5, APP-12/m-SEP-0.5 [25],
 APP-22.8/SiO2-1.2 [175],
APP-22.8/SiO2-1.2 [175],
 APP-22/CB-3, APP-20/CB-5, APP-18/CB-7 [34],
APP-22/CB-3, APP-20/CB-5, APP-18/CB-7 [34],
 APP-7.5/CD-7.5 [135],
APP-7.5/CD-7.5 [135],
 P-CA-24/MEL-6 [184],
P-CA-24/MEL-6 [184],
 P-FR-15/MPyP-15 [57],
P-FR-15/MPyP-15 [57],
 P-FR-20/GNO-2 [58],
P-FR-20/GNO-2 [58],
 P-IFR-19/Znacac-1, P-IFR-19/Cracac-1 [53],
P-IFR-19/Znacac-1, P-IFR-19/Cracac-1 [53],
 PN-IFR-29.5/m-MMT-0.5, PN-IFR-29/m-MMT-1, PN-IFR-28.5/m-MMT-1.5, PN-IFR-28/m-MMT-2, PN-IFR-27.5/m-MMT-2.5, PN-IFR-27/m-MMT-3, PN-IFR-26/m-MMT-4, PN-IFR-25/m-MMT-5 [56],
PN-IFR-29.5/m-MMT-0.5, PN-IFR-29/m-MMT-1, PN-IFR-28.5/m-MMT-1.5, PN-IFR-28/m-MMT-2, PN-IFR-27.5/m-MMT-2.5, PN-IFR-27/m-MMT-3, PN-IFR-26/m-MMT-4, PN-IFR-25/m-MMT-5 [56],
 P-IFR-25.5/m-MMT-2.5, P-IFR-25.5/m-LDH-2.5, P-IFR-25.5/A-POSS-2.5, P-IFR-25.5/MWCNT-2.5 [55],
P-IFR-25.5/m-MMT-2.5, P-IFR-25.5/m-LDH-2.5, P-IFR-25.5/A-POSS-2.5, P-IFR-25.5/MWCNT-2.5 [55],
 P-IFR-26.5/MWCNT-1.5, P-IFR-25.5/MWCNT-2.5, P-IFR-24.5/MWCNT-3.5 [54],
P-IFR-26.5/MWCNT-1.5, P-IFR-25.5/MWCNT-2.5, P-IFR-24.5/MWCNT-3.5 [54],
 PA-16/EDA-9 [139],
PA-16/EDA-9 [139],
 PPU-CA-12.5/APP-12.5, PPU-CA-16.67/APP-8.33, PPU-CA-18.75/APP-6.25, PPU-CA-20/APP-5 [50],
PPU-CA-12.5/APP-12.5, PPU-CA-16.67/APP-8.33, PPU-CA-18.75/APP-6.25, PPU-CA-20/APP-5 [50],
 MP-15/PER-10, MP-15/PER-9/Kaol-1, MP-15/PER-8/Kaol-2 [64],
MP-15/PER-10, MP-15/PER-9/Kaol-1, MP-15/PER-8/Kaol-2 [64],
 MP-12.3/PER-7.7, MP-15.4/PER-9.6, MP-11.7/PER-7.3/m-MMT-1, MP-14.7/PER-9.3/m-MMT-1 [185],
MP-12.3/PER-7.7, MP-15.4/PER-9.6, MP-11.7/PER-7.3/m-MMT-1, MP-14.7/PER-9.3/m-MMT-1 [185],
 MMP-16.64/PER-9.36, MMP-16/PER-9/La2O3-1, MMP-15.36/PER-8.64/La2O3-2, MMP-14.72/PER-8.28/La2O3-3 [186],
MMP-16.64/PER-9.36, MMP-16/PER-9/La2O3-1, MMP-15.36/PER-8.64/La2O3-2, MMP-14.72/PER-8.28/La2O3-3 [186],
 MPP-22.5/DPER-7.5, MPP-15/EG-10/DPER-5 [187],
MPP-22.5/DPER-7.5, MPP-15/EG-10/DPER-5 [187],
 MATMP-16.7/PER-8.3 [143],
MATMP-16.7/PER-8.3 [143],
 MPyP-18.75/PER-6.25, MPyP-17.25/PER-5.75/m-CD-2 [188],
MPyP-18.75/PER-6.25, MPyP-17.25/PER-5.75/m-CD-2 [188],
 MPyP-15/P-FR-15 [57],
MPyP-15/P-FR-15 [57],
 MPyP-22.5/TA-CFA-7.5, MPyP-15/TA-CFA-15, TA-CFA-22.5/MPyP-7.5 [66],
MPyP-22.5/TA-CFA-7.5, MPyP-15/TA-CFA-15, TA-CFA-22.5/MPyP-7.5 [66],
 TA-CFA-15/APP-15, TA-CFA-20/APP-10 [39],
TA-CFA-15/APP-15, TA-CFA-20/APP-10 [39],
 TA-CA-10/APP-10 [23],
TA-CA-10/APP-10 [23],
 TA-CA-13.33/APP-6.67 [27],
TA-CA-13.33/APP-6.67 [27],  TA-CA-20/APP-10, TA-CA-15/APP-15 [179],
TA-CA-20/APP-10, TA-CA-15/APP-15 [179],
 TA-IFR-12.5/APP-12.5, TA-IFR-16.67/APP-8.33 [31],
TA-IFR-12.5/APP-12.5, TA-IFR-16.67/APP-8.33 [31],
 TA&APP-IFR-10, TA&APP-IFR-15, TA&APP-IFR-20 [182],
TA&APP-IFR-10, TA&APP-IFR-15, TA&APP-IFR-20 [182],
 PI-IFR-20/APP-10 [70],
PI-IFR-20/APP-10 [70],
 N-FR-24.5/SiO2-0.5, N-FR-22/SiO2-3 [74],
N-FR-24.5/SiO2-0.5, N-FR-22/SiO2-3 [74],
 N-IFR-20/PA6-5 [75],
N-IFR-20/PA6-5 [75],
 N-IFR-24.5/HGM-0.5, N-IFR-24/HGM-1, N-IFR-23/HGM-2, N-IFR-22/HGM-3 [76],
N-IFR-24.5/HGM-0.5, N-IFR-24/HGM-1, N-IFR-23/HGM-2, N-IFR-22/HGM-3 [76],
 ATH-15/APP-15, mc-(ATH-15&APP-15)
[183],
ATH-15/APP-15, mc-(ATH-15&APP-15)
[183],
 ATH-55/GB-5, ATH-50/GB-10, ATH-47/GB-10/m-ZrP-3, ATH-44/GB-10/m-ZrP-6, ATH-41/GB-10/m-ZrP-9 [81],
ATH-55/GB-5, ATH-50/GB-10, ATH-47/GB-10/m-ZrP-3, ATH-44/GB-10/m-ZrP-6, ATH-41/GB-10/m-ZrP-9 [81],
 ATH-49/m-MMT-1, ATH-47/m-MMT-3, ATH-45/m-MMT-5 [80],
ATH-49/m-MMT-1, ATH-47/m-MMT-3, ATH-45/m-MMT-5 [80],
 ATH-20/m-MMT-3, ATH-20/m-MMT-10, ATH-20/m-MMT-17 [82],
ATH-20/m-MMT-3, ATH-20/m-MMT-10, ATH-20/m-MMT-17 [82],
 MDH-16.6/APP-12.7/PER-7.7/MEL-7.2, MDH-25/APP-11.5/PER-7/MEL-6.5, MDH-31.8/APP-10.4/PER-6.3/MEL-5.9 [146],
MDH-16.6/APP-12.7/PER-7.7/MEL-7.2, MDH-25/APP-11.5/PER-7/MEL-6.5, MDH-31.8/APP-10.4/PER-6.3/MEL-5.9 [146],
 MDH-16.6/APP-12.7/PER-7.7/MEL-7.2, MDH-25/APP-11.5/PER-7/MEL-6.5, MDH-31.8/APP-10.4/PER-6.3/MEL-5.9 [146],
MDH-16.6/APP-12.7/PER-7.7/MEL-7.2, MDH-25/APP-11.5/PER-7/MEL-6.5, MDH-31.8/APP-10.4/PER-6.3/MEL-5.9 [146],
 MDH-20/m-MMT-17 [82],
MDH-20/m-MMT-17 [82],
 MDH-30/m-MMT-10, MDH-40/m-MMT-10, MDH-50/m-MMT-10 [82],
MDH-30/m-MMT-10, MDH-40/m-MMT-10, MDH-50/m-MMT-10 [82],
 MDH-39/m-MMT-1, MDH-37/m-MMT-3, MDH-35/m-MMT-5 [85],
MDH-39/m-MMT-1, MDH-37/m-MMT-3, MDH-35/m-MMT-5 [85],
 MDH-45/Cobalt chelate-5, MDH-40/Cobalt chelate-10, MDH-35/Cobalt chelate-15, MDH-30/Cobalt chelate-20 [84],
MDH-45/Cobalt chelate-5, MDH-40/Cobalt chelate-10, MDH-35/Cobalt chelate-15, MDH-30/Cobalt chelate-20 [84],
 m-MMT-2.5/SEP-2.5, MDH-10/m-SEP-5, MDH-15/m-SEP-5, MDH-10/m-MMT-2.5/SEP-2.5, MDH-15/m-MMT-2.5/SEP-2.5 [87],
m-MMT-2.5/SEP-2.5, MDH-10/m-SEP-5, MDH-15/m-SEP-5, MDH-10/m-MMT-2.5/SEP-2.5, MDH-15/m-MMT-2.5/SEP-2.5 [87],
 MMT-6/APP-4, MMT-8/APP-2, m-MMT-6/APP-4, m-MMT-8/APP-2 [24],
MMT-6/APP-4, MMT-8/APP-2, m-MMT-6/APP-4, m-MMT-8/APP-2 [24],
 Si-FR-12.5/APP-12.5 [30],
Si-FR-12.5/APP-12.5 [30],
 Ni2O3-7.5/AC-7.5 [108],
Ni2O3-7.5/AC-7.5 [108],
 SEP-10/MWCNT-2 [189],
SEP-10/MWCNT-2 [189],
 C30B-3/ACPB-3, C30B-3/BUPB-3, C30B-3/MEPB-3, C30B-3/PBPA-3 [191],
C30B-3/ACPB-3, C30B-3/BUPB-3, C30B-3/MEPB-3, C30B-3/PBPA-3 [191],
 Si-3/SnCl2-2 [190],
Si-3/SnCl2-2 [190],
 C20A-5/TiO2-0.5, m-C20A-5/TiO2-0.5, m-C20A-10/TiO2-0.5 [105],
C20A-5/TiO2-0.5, m-C20A-5/TiO2-0.5, m-C20A-10/TiO2-0.5 [105],
 CF-5/MWCNT-5 [133],
CF-5/MWCNT-5 [133],
 CB-3/MWCNT-1, CB-5/MWCNT-1, CB-5/MWCNT-3 [131],
CB-3/MWCNT-1, CB-5/MWCNT-1, CB-5/MWCNT-3 [131],
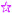 CB-5/CF-3 [134],
CB-5/CF-3 [134],
 AC-7.5/Ni2O3-7.5 [108],
AC-7.5/Ni2O3-7.5 [108],
 PER-10.4/MEL-9.7/APP-5.2 [145],
PER-10.4/MEL-9.7/APP-5.2 [145],
 PER-10.4/MEL-9.7/APP-5.2 [145],
PER-10.4/MEL-9.7/APP-5.2 [145],
 CD-7/TEP-3, CD-10/TEP-5, CD-7.5/APP-7.5 [135],
CD-7/TEP-3, CD-10/TEP-5, CD-7.5/APP-7.5 [135],
 m-lig-18/Ni(Ac)2-2, m-lig-18/Co- (Ac)2-2, m-lig-18/Zn(Ac)2-2 [136].
m-lig-18/Ni(Ac)2-2, m-lig-18/Co- (Ac)2-2, m-lig-18/Zn(Ac)2-2 [136].
Figure 16 shows that V-0 level in UL-94 is automatically obtained in the case of combined flame retardant systems used in PP regardless of the FRI value. However, no correlation exists between the FRI and LOI (Figure 17). The complexity of polymer–filler interaction can be considered as the main reason for diversity of properties.
Figure 16.
FRI values versus UL-94 test results. Symbols are indicative of combination of flame retardant (FR) additives used in PP. The vertical intervals in each category, i.e., V-0, V-1, V-2, and NR, are schematically representative of the amount of additive used. For example, two data distinguished by different symbols having the same or very close FRI values (horizontal quantity) in a given category (e.g., V-1), may have different vertical quantities, e.g., both reveal V-1 behavior in UL-94 test, but the upper contains more FR in PP.
Figure 17.
FRI values of PP as a function of LOI test results in long-shot (left-hand figure) and close-up (right-hand figure). Symbols are indicative of different types of blend flame retardants used. The left-side plot reveals that FRI values above 10 (Excellent zone) took place in several cases, which is in contradiction with all previous cases in which only one additive was used.
8. Conclusions and Future Perspective
This work opens new avenues to the experts working on “flame retardant polyolefins”, the title of a Special Issue entitled “Flame Retardant Polyolefins” in Polymers journal for which this work is designed and carried out. In this work, more than 150 research papers from the literature dealing with the flame retardancy of PP were analyzed, classified, and discussed in terms of flame retardancy performance. From the selected papers were extracted cone calorimetry data to calculate Flame Retardancy Index (FRI) as a measure or label of flame retardant performance. To have a comprehensive overview of flame retardant PP materials, works on PP flame retardancy were categorized in terms of additives used in classes including: phosphorus-based, nitrogen-based, mineral, carbon-based, bio-based, and hybrid combinatorial flame retardants composed of two or more additives. The analysis of efficiency of flame retardancy was performed in terms of the FRI variation as a function of wt.% of additives used. The analysis quite obviously unveiled the superiority of the combination of additives over the use of each one separately. In addition, the UL-94 and LOI values available in each class of additives were plotted in terms of the FRI so as to find possible correlation between analyses made in the literature. This work provided a pool of data on flame-retardant PP materials for future research on PP materials. It was elucidated that FRI can satisfactorily make possible classification of PP materials in terms of flame retardancy performance. The present work provides those research works that claim achieving synergistic effect of two or more flame retardants with a clear measure of flame retardant performance as Poor, Good, and Excellent labels assigned to PP materials, based on cone calorimetry data. Moreover, future works on LOI and UL-94 tests can be added to the data used here so as to draw a more detailed picture of flame retardancy behavior of PP materials. The approach can be used to make judgement about other flame retardant polyolefins. Moreover, we believe that the mechanical properties of FR polymers should also be considered in the future, but it is pertinent to the completeness of data in the literature. The importance of mechanical properties springs from the fact that highly loaded systems are prone to mechanical failure as a consequence of stress concentration. All in all, the type and the percentage of FRs in polymers affect both the mechanical and flame retardant properties of polymers; therefore, optimization of both properties is of importance.
Acknowledgments
The authors would like to acknowledge Reza Sheibani, the Head of Research & Development Center, Marun Petrochemical Company, Mahshahr, Iran, for providing E.M. with an opportunity to visit petrochemical plant, discussing practical processing criticisms in developing flame-retardant PP, and giving advice on concerns of engineers working on petrochemical plants who intend to make PP flame retardant.
Author Contributions
Conceptualization, H.V. and M.R.S.; methodology, H.V.; validation, H.V. and M.R.S.; formal analysis, F.S., V.A. and E.M.; investigation, F.D., G.N., V.A. and R.S.; data curation, H.V.; writing—original draft preparation, F.S. and E.M.; writing—review and editing, H.V. and M.R.S.; visualization, H.V. and M.R.S.; supervision, H.V. and M.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Laoutid F., Bonnaud L., Alexandre M., Lopez-Cuesta J.-M., Dubois P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009;63:100–125. doi: 10.1016/j.mser.2008.09.002. [DOI] [Google Scholar]
- 2.Vahabi H., Jouyandeh M., Cochez M., Khalili R., Vagner C., Ferriol M., Movahedifar E., Ramezanzadeh B., Rostami M., Ranjbar Z. Short-lasting fire in partially and completely cured epoxy coatings containing expandable graphite and halloysite nanotube additives. Prog. Org. Coat. 2018;123:160–167. doi: 10.1016/j.porgcoat.2018.07.014. [DOI] [Google Scholar]
- 3.Rad E.R., Vahabi H., de Anda A.R., Saeb M.R., Thomas S. Bio-epoxy resins with inherent flame retardancy. Prog. Org. Coat. 2019;135:608–612. doi: 10.1016/j.porgcoat.2019.05.046. [DOI] [Google Scholar]
- 4.Tripathi D. Practical Guide to Polypropylene. iSmithers Rapra Publishing; Shawbury Shewsbury Shropshire, UK: 2002. [Google Scholar]
- 5.Motahari S., Motlagh G.H., Moharramzadeh A. Thermal and flammability properties of polypropylene/silica aerogel composites. J. Macromol. Sci. Part B. 2015;54:1081–1091. doi: 10.1080/00222348.2015.1078619. [DOI] [Google Scholar]
- 6.Morgan A.B., Liu W. Flammability of thermoplastic carbon nanofiber nanocomposites. Fire Mater. 2011;35:43–60. doi: 10.1002/fam.1034. [DOI] [Google Scholar]
- 7.Maddah H.A. Polypropylene as a promising plastic: A review. Am. J. Polym. Sci. 2016;6:1–11. [Google Scholar]
- 8.Shubhra Q.T., Alam A., Quaiyyum M. Mechanical properties of polypropylene composites: A review. J. Thermoplast Compos. Mater. 2013;26:362–391. doi: 10.1177/0892705711428659. [DOI] [Google Scholar]
- 9.Spoerk M., Holzer C., Gonzalez-Gutierrez J. Material extrusion-based additive manufacturing of polypropylene: A review on how to improve dimensional inaccuracy and warpage. J. Appl. Polym. Sci. 2020;137:48545. doi: 10.1002/app.48545. [DOI] [Google Scholar]
- 10.Dabrowska I., Fambri L., Pegoretti A., Slouf M., Vackova T., Kolarik J. Spinning, drawing and physical properties of polypropylene nanocomposite fibers with fumed nanosilica. Express Polym. Lett. 2015;9:277–290. doi: 10.3144/expresspolymlett.2015.25. [DOI] [Google Scholar]
- 11.Allahvaisi S. Polypropylene in the Industry of Food Packaging. IN TECH; Janeza, Croatia: 2012. [Google Scholar]
- 12.Yusuf M. A Review on Flame Retardant Textile Finishing: Current and Future Trends. Curr. Smart Mater. 2018;3:99–108. doi: 10.2174/2405465803666180703110858. [DOI] [Google Scholar]
- 13.Gulrez S.K., Ali Mohsin M., Shaikh H., Anis A., Pulose A.M., Yadav M.K., Qua E.H.P., Al-Zahrani S. A review on electrically conductive polypropylene and polyethylene. Polym. Compos. 2014;35:900–914. doi: 10.1002/pc.22734. [DOI] [Google Scholar]
- 14.Patil A., Patel A., Purohit R. An overview of polymeric materials for automotive applications. Mater. Today Proc. 2017;4:3807–3815. doi: 10.1016/j.matpr.2017.02.278. [DOI] [Google Scholar]
- 15.Vahabi H., Paran S.M.R., Shabanian M., Dumazert L., Sonnier R., Movahedifar E., Zarrintaj P., Saeb M.R. Triple-faced polypropylene: Fire retardant, thermally stable, and antioxidative. J. Vinyl Addit. Technol. 2019;25:366–376. doi: 10.1002/vnl.21705. [DOI] [Google Scholar]
- 16.Vahabi H., Dumazert L., Khalili R., Saeb M.R., Cuesta J.-M.L. Flame retardant PP/PA6 blends: A recipe for recycled wastes. Flame Retard. Therm. Stab. Mater. 2019;2:1–8. doi: 10.1515/flret-2019-0001. [DOI] [Google Scholar]
- 17.Hajibeygi M., Mousavi M., Shabanian M., Vahabi H. The effect of phosphorus based melamine-terephthaldehyde resin and Mg-Al layered double hydroxide on the thermal stability, flame retardancy and mechanical properties of polypropylene MgO composites. Mater. Today Commun. 2020;23:100880. doi: 10.1016/j.mtcomm.2019.100880. [DOI] [Google Scholar]
- 18.Pani B., Sidhaarath S., Dhirendra S. Studies on the effects of various flame retardants on polypropylene. Am. J. Polym. Sci. 2013;3:63–69. [Google Scholar]
- 19.Vahabi H., Kandola B.K., Saeb M.R. Flame retardancy index for thermoplastic composites. Polymers. 2019;11:407. doi: 10.3390/polym11030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Movahedifar E., Vahabi H., Saeb M.R., Thomas S. Flame retardant epoxy composites on the road of innovation: An analysis with flame retardancy index for future development. Molecules. 2019;24:3964. doi: 10.3390/molecules24213964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S., Horrocks A.R. A review of flame retardant polypropylene fibres. Prog. Polym. Sci. 2003;28:1517–1538. doi: 10.1016/j.progpolymsci.2003.09.001. [DOI] [Google Scholar]
- 22.Li Q., Jiang P., Su Z., Wei P., Wang G., Tang X. Synergistic effect of phosphorus, nitrogen, and silicon on flame-retardant properties and char yield in polypropylene. J. Appl. Polym. Sci. 2005;96:854–860. doi: 10.1002/app.21522. [DOI] [Google Scholar]
- 23.Duan L., Yang H., Song L., Hou Y., Wang W., Gui Z., Hu Y. Hyperbranched phosphorus/nitrogen-containing polymer in combination with ammonium polyphosphate as a novel flame retardant system for polypropylene. Polym. Degrad. Stab. 2016;134:179–185. doi: 10.1016/j.polymdegradstab.2016.10.004. [DOI] [Google Scholar]
- 24.Hanna A., Nour M., Souaya E., Abdelmoaty A. Studies on the flammability of polypropylene/ammonium polyphosphate and montmorillonite by using the cone calorimeter test. Open Chem. 2018;16:108–115. doi: 10.1515/chem-2018-0013. [DOI] [Google Scholar]
- 25.Pappalardo S., Russo P., Acierno D., Rabe S., Schartel B. The synergistic effect of organically modified sepiolite in intumescent flame retardant polypropylene. Eur. Polym. J. 2016;76:196–207. doi: 10.1016/j.eurpolymj.2016.01.041. [DOI] [Google Scholar]
- 26.Kalali E.N., Montes A., Wang X., Zhang L., Shabestari M.E., Li Z., Wang D.Y. Effect of phytic acid–modified layered double hydroxide on flammability and mechanical properties of intumescent flame retardant polypropylene system. Fire Mater. 2018;42:213–220. doi: 10.1002/fam.2482. [DOI] [Google Scholar]
- 27.Wang W., Wen P., Zhan J., Hong N., Cai W., Gui Z., Hu Y. Synthesis of a novel charring agent containing pentaerythritol and triazine structure and its intumescent flame retardant performance for polypropylene. Polym. Degrad. Stab. 2017;144:454–463. doi: 10.1016/j.polymdegradstab.2017.09.011. [DOI] [Google Scholar]
- 28.Jung D., Bhattacharyya D. Keratinous fiber based intumescent flame retardant with controllable functional compound loading. ACS Sustain. Chem. Eng. 2018;6:13177–13184. doi: 10.1021/acssuschemeng.8b02756. [DOI] [Google Scholar]
- 29.Xu B., Wu X., Ma W., Qian L., Xin F., Qiu Y. Synthesis and characterization of a novel organic-inorganic hybrid char-forming agent and its flame-retardant application in polypropylene composites. J. Anal. Appl. Pyrolysis. 2018;134:231–242. doi: 10.1016/j.jaap.2018.06.013. [DOI] [Google Scholar]
- 30.Zhao Z., Jin Q., Zhang N., Guo X., Yan H. Preparation of a novel polysiloxane and its synergistic effect with ammonium polyphosphate on the flame retardancy of polypropylene. Polym. Degrad. Stab. 2018;150:73–85. doi: 10.1016/j.polymdegradstab.2018.02.007. [DOI] [Google Scholar]
- 31.Wen P., Feng X., Kan Y., Hu Y., Yuen R.K. Synthesis of a novel triazine-based polymeric flame retardant and its application in polypropylene. Polym. Degrad. Stab. 2016;134:202–210. doi: 10.1016/j.polymdegradstab.2016.10.003. [DOI] [Google Scholar]
- 32.Xu Z.-Z., Huang J.-Q., Chen M.-J., Tan Y., Wang Y.-Z. Flame retardant mechanism of an efficient flame-retardant polymeric synergist with ammonium polyphosphate for polypropylene. Polym. Degrad. Stab. 2013;98:2011–2020. doi: 10.1016/j.polymdegradstab.2013.07.010. [DOI] [Google Scholar]
- 33.Tang W., Qian L., Chen Y., Qiu Y., Xu B. Intumescent flame retardant behavior of charring agents with different aggregation of piperazine/triazine groups in polypropylene. Polym. Degrad. Stab. 2019;169:108982. doi: 10.1016/j.polymdegradstab.2019.108982. [DOI] [Google Scholar]
- 34.Yang H., Guan Y., Ye L., Wang S., Li S., Wen X., Chen X., Mijowska E., Tang T. Synergistic effect of nanoscale carbon black and ammonium polyphosphate on improving thermal stability and flame retardancy of polypropylene: A reactive network for strengthening carbon layer. Compos. Part B Eng. 2019;174:107038. doi: 10.1016/j.compositesb.2019.107038. [DOI] [Google Scholar]
- 35.Shao Z.-B., Deng C., Tan Y., Chen M.-J., Chen L., Wang Y.-Z. An efficient mono-component polymeric intumescent flame retardant for polypropylene: Preparation and application. ACS Appl. Mater. Interfaces. 2014;6:7363–7370. doi: 10.1021/am500789q. [DOI] [PubMed] [Google Scholar]
- 36.Deng C.-L., Du S.-L., Zhao J., Shen Z.-Q., Deng C., Wang Y.-Z. An intumescent flame retardant polypropylene system with simultaneously improved flame retardancy and water resistance. Polym. Degrad. Stab. 2014;108:97–107. doi: 10.1016/j.polymdegradstab.2014.06.008. [DOI] [Google Scholar]
- 37.Sun Y., Yuan B., Shang S., Zhang H., Shi Y., Yu B., Qi C., Dong H., Chen X., Yang X. Surface modification of ammonium polyphosphate by supramolecular assembly for enhancing fire safety properties of polypropylene. Compos. Part B Eng. 2020;181:107588. doi: 10.1016/j.compositesb.2019.107588. [DOI] [Google Scholar]
- 38.Qin Z., Li D., Lan Y., Li Q., Yang R. Ammonium polyphosphate and silicon-containing cyclotriphosphazene: Synergistic effect in flame-retarded polypropylene. Ind. Eng. Chem. Res. 2015;54:10707–10713. doi: 10.1021/acs.iecr.5b02343. [DOI] [Google Scholar]
- 39.Wen P., Wang X., Wang B., Yuan B., Zhou K., Song L., Hu Y., Yuen R.K. One-pot synthesis of a novel s-triazine-based hyperbranched charring foaming agent and its enhancement on flame retardancy and water resistance of polypropylene. Polym. Degrad. Stab. 2014;110:165–174. doi: 10.1016/j.polymdegradstab.2014.08.019. [DOI] [Google Scholar]
- 40.Wen P., Wang X., Xing W., Feng X., Yu B., Shi Y., Tang G., Song L., Hu Y., Yuen R.K. Synthesis of a novel triazine-based hyperbranched char foaming agent and the study of its enhancement on flame retardancy and thermal stability of polypropylene. Ind. Eng. Chem. Res. 2013;52:17015–17022. doi: 10.1021/ie401955n. [DOI] [Google Scholar]
- 41.Jiang Z., Liu G. Microencapsulation of ammonium polyphosphate with melamine-formaldehyde-tris (2-hydroxyethyl) isocyanurate resin and its flame retardancy in polypropylene. Rsc Adv. 2015;5:88445–88455. doi: 10.1039/C5RA14586D. [DOI] [Google Scholar]
- 42.Yu S., Xiao S., Zhao Z., Huo X., Wei J. Microencapsulated ammonium polyphosphate by polyurethane with segment of dipentaerythritol and its application in flame retardant polypropylene. Chin. J. Chem. Eng. 2019;27:1735–1743. doi: 10.1016/j.cjche.2019.04.023. [DOI] [Google Scholar]
- 43.Deng C.L., Deng C., Zhao J., Fang W.H., Lin L., Wang Y.Z. Water resistance, thermal stability, and flame retardation of polypropylene composites containing a novel ammonium polyphosphate microencapsulated by UV-curable epoxy acrylate resin. Polym. Adv. Technol. 2014;25:861–871. doi: 10.1002/pat.3319. [DOI] [Google Scholar]
- 44.Zheng Z., Qiang L., Yang T., Wang B., Cui X., Wang H. Preparation of microencapsulated ammonium polyphosphate with carbon source-and blowing agent-containing shell and its flame retardance in polypropylene. J. Polym. Res. 2014;21:443. doi: 10.1007/s10965-014-0443-2. [DOI] [Google Scholar]
- 45.Chen M., Xu Y., Chen X., Ma Y., He W., Yu J., Zhang Z. Thermal stability and combustion behavior of flame-retardant polypropylene with thermoplastic polyurethane-microencapsulated ammonium polyphosphate. High Perform. Polym. 2014;26:445–454. doi: 10.1177/0954008313517910. [DOI] [Google Scholar]
- 46.Shao Z.-B., Deng C., Tan Y., Chen M.-J., Chen L., Wang Y.-Z. Flame retardation of polypropylene via a novel intumescent flame retardant: Ethylenediamine-modified ammonium polyphosphate. Polym. Degrad. Stab. 2014;106:88–96. doi: 10.1016/j.polymdegradstab.2013.10.005. [DOI] [Google Scholar]
- 47.Shao Z.-B., Deng C., Tan Y., Yu L., Chen M.-J., Chen L., Wang Y.-Z. Ammonium polyphosphate chemically-modified with ethanolamine as an efficient intumescent flame retardant for polypropylene. J. Mater. Chem. A. 2014;2:13955–13965. doi: 10.1039/C4TA02778G. [DOI] [Google Scholar]
- 48.Deng C.-l., Deng C., Zhao J., Li R.-m., Fang W.-h., Wang Y.-z. Simultaneous improvement in the flame retardancy and water resistance of PP/APP through coating UV-curable pentaerythritol triacrylate onto APP. Chin. J. Polym. Sci. 2015;33:203–214. doi: 10.1007/s10118-015-1575-5. [DOI] [Google Scholar]
- 49.Tian N., Wen X., Jiang Z., Gong J., Wang Y., Xue J., Tang T. Synergistic effect between a novel char forming agent and ammonium polyphosphate on flame retardancy and thermal properties of polypropylene. Ind. Eng. Chem. Res. 2013;52:10905–10915. doi: 10.1021/ie401058u. [DOI] [Google Scholar]
- 50.Zhang T., Tao Y., Zhou F., Sheng H., Qiu S., Ma C., Hu Y. Synthesis of a hyperbranched phosphorus-containing polyurethane as char forming agent combined with ammonium polyphosphate for reducing fire hazard of polypropylene. Polym. Degrad. Stab. 2019;165:207–219. doi: 10.1016/j.polymdegradstab.2019.05.003. [DOI] [Google Scholar]
- 51.Wang X., Spörer Y., Leuteritz A., Kuehnert I., Wagenknecht U., Heinrich G., Wang D.-Y. Comparative study of the synergistic effect of binary and ternary LDH with intumescent flame retardant on the properties of polypropylene composites. RSC Adv. 2015;5:78979–78985. doi: 10.1039/C5RA15565G. [DOI] [Google Scholar]
- 52.Zhang C., Guo X., Ma S., Zheng Y., Xu J., Ma H. Synthesis of a novel branched cyclophosphazene-PEPA flame retardant and its application on polypropylene. J. Therm. Anal. Calorim. 2019;137:33–42. doi: 10.1007/s10973-018-7943-y. [DOI] [Google Scholar]
- 53.Song P., Fang Z., Tong L., Jin Y., Lu F. Effects of metal chelates on a novel oligomeric intumescent flame retardant system for polypropylene. J. Anal. Appl. Pyrolysis. 2008;82:286–291. doi: 10.1016/j.jaap.2008.04.008. [DOI] [Google Scholar]
- 54.Du B., Fang Z. Effects of carbon nanotubes on the thermal stability and flame retardancy of intumescent flame-retarded polypropylene. Polym. Degrad. Stab. 2011;96:1725–1731. doi: 10.1016/j.polymdegradstab.2011.08.002. [DOI] [Google Scholar]
- 55.Du B., Ma H., Fang Z. How nano-fillers affect thermal stability and flame retardancy of intumescent flame retarded polypropylene. Polym. Adv. Technol. 2011;22:1139–1146. doi: 10.1002/pat.1914. [DOI] [Google Scholar]
- 56.Chen Y.J., Wang C., Yang C.Z. Synergism between Intumescent Flame Retardant and Organo-Montmorillonite in Polypropylene Nanocomposites. Trans Tech Publications; Bäch, Switzerland: 2013. pp. 502–507. Advanced Materials Research. [DOI] [Google Scholar]
- 57.He Q., Lu H., Song L., Hu Y., Chen L. Flammability and thermal properties of a novel intumescent flame retardant polypropylene. J. Fire Sci. 2009;27:303–321. [Google Scholar]
- 58.Yu B., Wang X., Qian X., Xing W., Yang H., Ma L., Lin Y., Jiang S., Song L., Hu Y. Functionalized graphene oxide/phosphoramide oligomer hybrids flame retardant prepared via in situ polymerization for improving the fire safety of polypropylene. Rsc Adv. 2014;4:31782–31794. doi: 10.1039/C3RA45945D. [DOI] [Google Scholar]
- 59.Salaün F., Creach G., Rault F., Giraud S. Microencapsulation of bisphenol-A bis (diphenyl phosphate) and influence of particle loading on thermal and fire properties of polypropylene and polyethylene terephtalate. Polym. Degrad. Stab. 2013;98:2663–2671. doi: 10.1016/j.polymdegradstab.2013.09.030. [DOI] [Google Scholar]
- 60.Dahiya J.B., Kumar N., Bockhorn H. Fire performance and thermal stability of polypropylene nanocomposites containing organic phosphinate and ammonium polyphosphate additives. Fire Mater. 2014;38:1–12. doi: 10.1002/fam.2151. [DOI] [Google Scholar]
- 61.Xu M.-j., Wang J., Ding Y.-h., Li B. Synergistic effects of aluminum hypophosphite on intumescent flame retardant polypropylene system. Chin. J. Polym. Sci. 2015;33:318–328. doi: 10.1007/s10118-015-1588-0. [DOI] [Google Scholar]
- 62.Li H., Ning N., Zhang L., Wang Y., Liang W., Tian M. Different flame retardancy effects and mechanisms of aluminium phosphinate in PPO, TPU and PP. Polym. Degrad. Stab. 2014;105:86–95. doi: 10.1016/j.polymdegradstab.2014.03.032. [DOI] [Google Scholar]
- 63.Zhou S., Song L., Wang Z., Hu Y., Xing W. Flame retardation and char formation mechanism of intumescent flame retarded polypropylene composites containing melamine phosphate and pentaerythritol phosphate. Polym. Degrad. Stab. 2008;93:1799–1806. doi: 10.1016/j.polymdegradstab.2008.07.012. [DOI] [Google Scholar]
- 64.Abdelkhalik A., Makhlouf G., Hassan M.A. Manufacturing, thermal stability, and flammability properties of polypropylene containing new single molecule intumescent flame retardant. Polym. Adv. Technol. 2019;30:1403–1414. doi: 10.1002/pat.4573. [DOI] [Google Scholar]
- 65.Wang X., Li Y., Liao W., Gu J., Li D. A new intumescent flame-retardant: Preparation, surface modification, and its application in polypropylene. Polym. Adv. Technol. 2008;19:1055–1061. doi: 10.1002/pat.1077. [DOI] [Google Scholar]
- 66.Chen X., Jiao C. Flame retardancy and thermal Degradation of intumescent flame retardant polypropylene material. Polym. Adv. Technol. 2011;22:817–821. doi: 10.1002/pat.1583. [DOI] [Google Scholar]
- 67.Chen H., Wang J., Ni A., Ding A., Han X., Sun Z. The effects of a macromolecular charring agent with gas phase and condense phase synergistic flame retardant capability on the properties of PP/IFR composites. Materials. 2018;11:111. doi: 10.3390/ma11010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao S., Zhao X., Liu G. Synthesis of an integrated intumescent flame retardant and its flame retardancy properties for polypropylene. Polym. Degrad. Stab. 2017;138:106–114. doi: 10.1016/j.polymdegradstab.2016.05.007. [DOI] [Google Scholar]
- 69.Shao Z.-B., Zhang M.-X., Han Y., Yang X.-D., Jin J., Jian R.-K. A highly efficient gas-dominated and water-resistant flame retardant for non-charring polypropylene. RSC Adv. 2017;7:51919–51927. doi: 10.1039/C7RA09868E. [DOI] [Google Scholar]
- 70.Li B., Zhan Z., Zhang H., Sun C. Flame retardancy and thermal performance of polypropylene treated with the intumescent flame retardant, piperazine spirocyclic phosphoramidate. J. Vinyl Addit. Technol. 2014;20:10–15. doi: 10.1002/vnl.21337. [DOI] [Google Scholar]
- 71.Yang R., Ma B., Zhang X., Li J. Fire retardance and smoke suppression of polypropylene with a macromolecular intumescent flame retardant containing caged bicyclic phosphate and piperazine. J. Appl. Polym. Sci. 2019;136:47593. doi: 10.1002/app.47593. [DOI] [Google Scholar]
- 72.Xie H., Lai X., Zhou R., Li H., Zhang Y., Zeng X., Guo J. Effect and mechanism of N-alkoxy hindered amine on the flame retardancy, UV aging resistance and thermal degradation of intumescent flame retardant polypropylene. Polym. Degrad. Stab. 2015;118:167–177. doi: 10.1016/j.polymdegradstab.2015.04.022. [DOI] [Google Scholar]
- 73.Lai X., Qiu J., Li H., Zhou R., Xie H., Zeng X. Thermal degradation and combustion behavior of novel intumescent flame retardant polypropylene with N-alkoxy hindered amine. J. Anal. Appl. Pyrolysis. 2016;120:361–370. doi: 10.1016/j.jaap.2016.06.004. [DOI] [Google Scholar]
- 74.Ye L., Wu Q., Qu B. Synergistic effects of fumed silica on intumescent flame-retardant polypropylene. J. Appl. Polym. Sci. 2010;115:3508–3515. doi: 10.1002/app.30585. [DOI] [Google Scholar]
- 75.Zhang L., Yi D., Hao J. Poly (diallyldimethylammonium) and polyphosphate polyelectrolyte complexes as an all-in-one flame retardant for polypropylene. Polym. Adv. Technol. 2019;31:260–272. doi: 10.1002/pat.4766. [DOI] [Google Scholar]
- 76.Kang B.-h., Yang X.-y., Lu X. Effect of hollow glass microsphere on the flame retardancy and combustion behavior of intumescent flame retardant polypropylene composites. Polym. Bull. 2019;77:1–18. doi: 10.1007/s00289-019-02953-2. [DOI] [Google Scholar]
- 77.Vahabi H., Raveshtian A., Fasihi M., Sonnier R., Saeb M.R., Dumazert L., Kandola B.K. Competitiveness and synergy between three flame retardants in poly (ethylene-co-vinyl acetate) Polym. Degrad. Stab. 2017;143:164–175. doi: 10.1016/j.polymdegradstab.2017.07.005. [DOI] [Google Scholar]
- 78.Vahabi H., Shabanian M., Aryanasab F., Laoutid F., Benali S., Saeb M.R., Seidi F., Kandola B.K. Three in one: β-cyclodextrin, nanohydroxyapatite, and a nitrogen-rich polymer integrated into a new flame retardant for poly (lactic acid) Fire Mater. 2018;42:593–602. doi: 10.1002/fam.2513. [DOI] [Google Scholar]
- 79.Vahabi H., Sonnier R., Taguet A., Otazaghine B., Saeb M.R., Beyer G. Clay Nanoparticles. Elsevier; Amsterdam, The Netherlands: 2020. Halloysite nanotubes (HNTs)/polymer nanocomposites: Thermal degradation and flame retardancy; pp. 67–93. [DOI] [Google Scholar]
- 80.Liu L., Zhang H., Sun L., Kong Q., Zhang J. Flame-retardant effect of montmorillonite intercalation iron compounds in polypropylene/aluminum hydroxide composites system. J. Therm. Anal. Calorim. 2016;124:807–814. doi: 10.1007/s10973-015-5213-9. [DOI] [Google Scholar]
- 81.Pérez N., Qi X.-L., Nie S., Acuña P., Chen M.-J., Wang D.-Y. Flame retardant polypropylene composites with low densities. Materials. 2019;12:152. doi: 10.3390/ma12010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J., Wilkie C.A. Fire Retardancy of Polypropylene-Metal Hydroxide Nanocomposites. ACS Publications; Washington, DC, USA: 2006. [Google Scholar]
- 83.Molesky F., Falk D.P. Comparison of Smoke Measurements of the Cone Calorimeter and ASTM E-662 Smoke Chamber in Flame Retardant Polypropylene. J. Fire Sci. 1991;9:60–68. doi: 10.1177/073490419100900103. [DOI] [Google Scholar]
- 84.Shehata A. A new cobalt chelate as flame retardant for polypropylene filled with magnesium hydroxide. Polym. Degrad. Stab. 2004;85:577–582. doi: 10.1016/j.polymdegradstab.2004.01.019. [DOI] [Google Scholar]
- 85.Kong Q., Wu H., Zhang H., Zhang X., Zhao W., Zhang J. Effect of Fe-montmorillonite on flammability behavior in polypropylene/magnesium hydroxide composites. J. Nanosci. Nanotechnol. 2016;16:8287–8293. doi: 10.1166/jnn.2016.11615. [DOI] [Google Scholar]
- 86.Oyama H.T., Sekikawa M., Ikezawa Y. Influence of the polymer/inorganic filler interface on the mechanical, thermal, and flame retardant properties of polypropylene/magnesium hydroxide composites. J. Macromol. Sci. Part B. 2011;50:463–483. doi: 10.1080/00222341003780996. [DOI] [Google Scholar]
- 87.Marosfoi B., Garas S., Bodzay B., Zubonyai F., Marosi G. Flame retardancy study on magnesium hydroxide associated with clays of different morphology in polypropylene matrix. Polym. Adv. Technol. 2008;19:693–700. doi: 10.1002/pat.1153. [DOI] [Google Scholar]
- 88.Tang W., Li H., Zhang S., Sun J., Gu X. The intercalation of ammonium sulfamate into kaolinite and its effect on the fire performance of polypropylene. J. Thermoplast. Compos. Mater. 2018;31:1352–1370. doi: 10.1177/0892705717738291. [DOI] [Google Scholar]
- 89.Tang W., Zhang S., Sun J., Gu X. Flame retardancy and thermal stability of polypropylene composite containing ammonium sulfamate intercalated kaolinite. Ind. Eng. Chem. Res. 2016;55:7669–7678. doi: 10.1021/acs.iecr.6b01722. [DOI] [Google Scholar]
- 90.Batistella M., Otazaghine B., Sonnier R., Petter C., Lopez-Cuesta J.-M. Fire retardancy of polypropylene/kaolinite composites. Polym. Degrad. Stab. 2016;129:260–267. doi: 10.1016/j.polymdegradstab.2016.05.003. [DOI] [Google Scholar]
- 91.Zoromba M.S., Nour M.A., Eltamimy H.E., El-Maksoud S.A.A. Effect of modified layered double hydroxide on the flammability and mechanical properties of polypropylene. Sci. Eng. Compos. Mater. 2018;25:101–108. doi: 10.1515/secm-2016-0050. [DOI] [Google Scholar]
- 92.Manzi-Nshuti C., Songtipya P., Manias E., del Mar Jimenez-Gasco M., Hossenlopp J.M., Wilkie C.A. Polymer nanocomposites using zinc aluminum and magnesium aluminum oleate layered double hydroxides: Effects of the polymeric compatibilizer and of composition on the thermal and fire properties of PP/LDH nanocomposites. Polym. Degrad. Stab. 2009;94:2042–2054. doi: 10.1016/j.polymdegradstab.2009.07.013. [DOI] [Google Scholar]
- 93.Wu T., Kong Q., Zhang H., Zhang J. Thermal stability and flame retardancy of polypropylene/NiAl layered double hydroxide nanocomposites. J. Nanosci. Nanotechnol. 2018;18:1051–1056. doi: 10.1166/jnn.2018.14107. [DOI] [PubMed] [Google Scholar]
- 94.Nyambo C., Wang D., Wilkie C.A. Will layered double hydroxides give nanocomposites with polar or non-polar polymers? Polym. Adv. Technol. 2009;20:332–340. doi: 10.1002/pat.1272. [DOI] [Google Scholar]
- 95.Zhang S., Liu X., Gu X., Jiang P., Sun J. Flammability and thermal behavior of polypropylene composites containing dihydrogen phosphate anion-intercalated layered double hydroxides. Polym. Compos. 2015;36:2230–2237. doi: 10.1002/pc.23135. [DOI] [Google Scholar]
- 96.Qin H., Zhang S., Zhao C., Hu G., Yang M. Flame retardant mechanism of polymer/clay nanocomposites based on polypropylene. Polymer. 2005;46:8386–8395. doi: 10.1016/j.polymer.2005.07.019. [DOI] [Google Scholar]
- 97.Zhu F., Liu D., Cai G., Tan X., Wang J., Lu H., Wilkie C.A. Thermal stability and flammability performance of polypropylene composites with silica pillared montmorillonites. Polym. Adv. Technol. 2014;25:211–216. doi: 10.1002/pat.3225. [DOI] [Google Scholar]
- 98.Zhang J., Wilkie C.A. Polyethylene and polypropylene nanocomposites based on polymerically-modified clay containing alkylstyrene units. Polymer. 2006;47:5736–5743. doi: 10.1016/j.polymer.2006.06.018. [DOI] [Google Scholar]
- 99.Qin H., Zhang S., Zhao C., Feng M., Yang M., Shu Z., Yang S. Thermal stability and flammability of polypropylene/montmorillonite composites. Polym. Degrad. Stab. 2004;85:807–813. doi: 10.1016/j.polymdegradstab.2004.03.014. [DOI] [Google Scholar]
- 100.Zhang J., Jiang D.D., Wilkie C.A. Thermal and flame properties of polyethylene and polypropylene nanocomposites based on an oligomerically-modified clay. Polym. Degrad. Stab. 2006;91:298–304. doi: 10.1016/j.polymdegradstab.2005.05.006. [DOI] [Google Scholar]
- 101.Su S., Jiang D.D., Wilkie C.A. Poly (methyl methacrylate), polypropylene and polyethylene nanocomposite formation by melt blending using novel polymerically-modified clays. Polym. Degrad. Stab. 2004;83:321–331. doi: 10.1016/S0141-3910(03)00277-5. [DOI] [Google Scholar]
- 102.Zheng X., Jiang D.D., Wang D., Wilkie C.A. Flammability of styrenic polymer clay nanocomposites based on a methyl methacrylate oligomerically-modified clay. Polym. Degrad. Stab. 2006;91:289–297. doi: 10.1016/j.polymdegradstab.2005.05.007. [DOI] [Google Scholar]
- 103.Szustakiewicz K., Kiersnowski A., Gazińska M., Bujnowicz K., Pigłowski J. Flammability, structure and mechanical properties of PP/OMMT nanocomposites. Polym. Degrad. Stab. 2011;96:291–294. doi: 10.1016/j.polymdegradstab.2010.11.001. [DOI] [Google Scholar]
- 104.Yi D., Yang R., Wilkie C.A. Full scale nanocomposites: Clay in fire retardant and polymer. Polym. Degrad. Stab. 2014;105:31–41. doi: 10.1016/j.polymdegradstab.2014.03.042. [DOI] [Google Scholar]
- 105.Alsharif M.A., Zadhoush A., Mortazavi S.M. Thermal Degradation and Flammability Properties of Polypropylene Nanocomposite Using Organoclay-graft-poly (Ethylene Glycol Methacrylate Phosphate) Adv. Polym. Technol. 2014;33 doi: 10.1002/adv.21386. [DOI] [Google Scholar]
- 106.Yuan B., Chen G., Zou Y., Shang S., Sun Y., Yu B., He S., Chen X. Alumina nanoflake-coated graphene nanohybrid as a novel flame retardant filler for polypropylene. Polym. Adv. Technol. 2019;30:2153–2158. doi: 10.1002/pat.4689. [DOI] [Google Scholar]
- 107.Dong M., Gu X., Zhang S. Effects of compound oxides on the fire performance of polypropylene composite. Ind. Eng. Chem. Res. 2014;53:8062–8068. doi: 10.1021/ie500178u. [DOI] [Google Scholar]
- 108.Gong J., Tian N., Liu J., Yao K., Jiang Z., Chen X., Wen X., Mijowska E., Tang T. Synergistic effect of activated carbon and Ni2O3 in promoting the thermal stability and flame retardancy of polypropylene. Polym. Degrad. Stab. 2014;99:18–26. doi: 10.1016/j.polymdegradstab.2013.12.016. [DOI] [Google Scholar]
- 109.Song R., Fu Y., Li B. Transferring noncharring polyolefins to charring polymers with the presence of Mo/Mg/Ni/O catalysts and the application in flame retardancy. J. Appl. Polym. Sci. 2013;129:138–144. doi: 10.1002/app.38717. [DOI] [Google Scholar]
- 110.Dang L., Nai X., Dong Y., Li W. Functional group effect on flame retardancy, thermal, and mechanical properties of organophosphorus-based magnesium oxysulfate whiskers as a flame retardant in polypropylene. RSC Adv. 2017;7:21655–21665. doi: 10.1039/C7RA02863F. [DOI] [Google Scholar]
- 111.Rault F., Pleyber E., Campagne C., Rochery M., Giraud S., Bourbigot S., Devaux E. Effect of manganese nanoparticles on the mechanical, thermal and fire properties of polypropylene multifilament yarn. Polym. Degrad. Stab. 2009;94:955–964. doi: 10.1016/j.polymdegradstab.2009.03.012. [DOI] [Google Scholar]
- 112.Chen X., Yun Y., Fan A., Yuan B., Shang S., He S. The assembly nanohybrid of graphene with lamellar zirconium phenylphosphonate for improving flame retardancy and mechanical properties of polypropylene. Polym. Compos. 2019;40(Suppl. S2):E1757–E1765. doi: 10.1002/pc.25149. [DOI] [Google Scholar]
- 113.Niemczyk A., Dziubek K., Sacher-Majewska B., Czaja K., Czech-Polak J., Oliwa R., Lenża J., Szołyga M. Thermal stability and flame retardancy of polypropylene composites containing siloxane-silsesquioxane resins. Polymers. 2018;10:1019. doi: 10.3390/polym10091019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fina A., Tabuani D., Camino G. Polypropylene–polysilsesquioxane blends. Eur. Polym. J. 2010;46:14–23. doi: 10.1016/j.eurpolymj.2009.07.019. [DOI] [Google Scholar]
- 115.Fina A., Abbenhuis H.C., Tabuani D., Camino G. Metal functionalized POSS as fire retardants in polypropylene. Polym. Degrad. Stab. 2006;91:2275–2281. doi: 10.1016/j.polymdegradstab.2006.04.014. [DOI] [Google Scholar]
- 116.Lecouvet B., Sclavons M., Bourbigot S., Devaux J., Bailly C. Water-assisted extrusion as a novel processing route to prepare polypropylene/halloysite nanotube nanocomposites: Structure and properties. Polymer. 2011;52:4284–4295. doi: 10.1016/j.polymer.2011.07.021. [DOI] [Google Scholar]
- 117.Shang S., Ma X., Yuan B., Chen G., Sun Y., Huang C., He S., Dai H., Chen X. Modification of halloysite nanotubes with supramolecular self-assembly aggregates for reducing smoke release and fire hazard of polypropylene. Compos. Part B Eng. 2019;177:107371. doi: 10.1016/j.compositesb.2019.107371. [DOI] [Google Scholar]
- 118.Nonahal M., Rastin H., Saeb M.R., Sari M.G., Moghadam M.H., Zarrintaj P., Ramezanzadeh B. Epoxy/PAMAM dendrimer-modified graphene oxide nanocomposite coatings: Nonisothermal cure kinetics study. Prog. Org. Coat. 2018;114:233–243. doi: 10.1016/j.porgcoat.2017.10.023. [DOI] [Google Scholar]
- 119.Yarahmadi E., Didehban K., Sari M.G., Saeb M.R., Shabanian M., Aryanasab F., Zarrintaj P., Paran S.M.R., Mozafari M., Rallini M. Development and curing potential of epoxy/starch-functionalized graphene oxide nanocomposite coatings. Prog. Org. Coat. 2018;119:194–202. doi: 10.1016/j.porgcoat.2018.03.001. [DOI] [Google Scholar]
- 120.Choolaei M., Goodarzi V., Khonakdar H.A., Jafari S.H., Seyfi J., Saeb M.R., Häußler L., Boldt R. Influence of graphene oxide on crystallization behavior and chain folding surface free energy of poly (vinylidenefluoride-co-hexafluoropropylene) Macromol. Chem. Phys. 2017;218:1700103. doi: 10.1002/macp.201700103. [DOI] [Google Scholar]
- 121.Saeb M.R., Najafi F., Bakhshandeh E., Khonakdar H.A., Mostafaiyan M., Simon F., Scheffler C., Mäder E. Highly curable epoxy/MWCNTs nanocomposites: An effective approach to functionalization of carbon nanotubes. Chem. Eng. J. 2015;259:117–125. doi: 10.1016/j.cej.2014.07.116. [DOI] [Google Scholar]
- 122.Yuan B., Wang B., Hu Y., Mu X., Hong N., Liew K.M., Hu Y. Electrical conductive and graphitizable polymer nanofibers grafted on graphene nanosheets: Improving electrical conductivity and flame retardancy of polypropylene. Compos. Part A Appl. Sci. Manuf. 2016;84:76–86. doi: 10.1016/j.compositesa.2016.01.003. [DOI] [Google Scholar]
- 123.Yuan B., Hu Y., Chen X., Shi Y., Niu Y., Zhang Y., He S., Dai H. Dual modification of graphene by polymeric flame retardant and Ni (OH) 2 nanosheets for improving flame retardancy of polypropylene. Compos. Part A Appl. Sci. Manuf. 2017;100:106–117. doi: 10.1016/j.compositesa.2017.04.012. [DOI] [Google Scholar]
- 124.Yuan B., Sheng H., Mu X., Song L., Tai Q., Shi Y., Liew K.M., Hu Y. Enhanced flame retardancy of polypropylene by melamine-modified graphene oxide. J. Mater. Sci. 2015;50:5389–5401. doi: 10.1007/s10853-015-9083-0. [DOI] [Google Scholar]
- 125.Hong N., Pan Y., Zhan J., Wang B., Zhou K., Song L., Hu Y. Fabrication of graphene/Ni–Ce mixed oxide with excellent performance for reducing fire hazard of polypropylene. Rsc Adv. 2013;3:16440–16448. doi: 10.1039/c3ra42095g. [DOI] [Google Scholar]
- 126.Yuan B., Song L., Liew K.M., Hu Y. Solid acid-reduced graphene oxide nanohybrid for enhancing thermal stability, mechanical property and flame retardancy of polypropylene. Rsc Adv. 2015;5:41307–41316. doi: 10.1039/C5RA04699H. [DOI] [Google Scholar]
- 127.Bensabath T., Sarazin J., Jimenez M., Samyn F., Bourbigot S. Intumescent polypropylene: Interactions between physical and chemical expansion. Fire Mater. 2019 doi: 10.1002/fam.2790. [DOI] [Google Scholar]
- 128.Xu L., Guo Z., Zhang Y., Fang Z. Flame-retardant-wrapped carbon nanotubes for simultaneously improving the flame retardancy and mechanical properties of polypropylene. J. Mater. Chem. 2008;18:5083–5091. [Google Scholar]
- 129.Song P., Zhao L., Cao Z., Fang Z. Polypropylene nanocomposites based on C 60-decorated carbon nanotubes: Thermal properties, flammability, and mechanical properties. J. Mater. Chem. 2011;21:7782–7788. doi: 10.1039/c1jm10395d. [DOI] [Google Scholar]
- 130.Yang H., Ye L., Gong J., Li M., Jiang Z., Wen X., Chen H., Tian N., Tang T. Simultaneously improving the mechanical properties and flame retardancy of polypropylene using functionalized carbon nanotubes by covalently wrapping flame retardants followed by linking polypropylene. Mater. Chem. Front. 2017;1:716–726. doi: 10.1039/C6QM00172F. [DOI] [Google Scholar]
- 131.Wen X., Tian N., Gong J., Chen Q., Qi Y., Liu Z., Liu J., Jiang Z., Chen X., Tang T. Effect of nanosized carbon black on thermal stability and flame retardancy of polypropylene/carbon nanotubes nanocomposites. Polym. Adv. Technol. 2013;24:971–977. doi: 10.1002/pat.3172. [DOI] [Google Scholar]
- 132.Fina A., Bocchini S., Camino G. Catalytic fire retardant nanocomposites. Polym. Degrad. Stab. 2008;93:1647–1655. doi: 10.1016/j.polymdegradstab.2008.05.027. [DOI] [Google Scholar]
- 133.Gong J., Niu R., Wen X., Yang H., Liu J., Chen X., Sun Z.-Y., Mijowska E., Tang T. Synergistic effect of carbon fibers and carbon nanotubes on improving thermal stability and flame retardancy of polypropylene: A combination of a physical network and chemical crosslinking. RSC Adv. 2014;5:5484–5493. doi: 10.1039/C4RA11591K. [DOI] [Google Scholar]
- 134.Yang H., Gong J., Wen X., Xue J., Chen Q., Jiang Z., Tian N., Tang T. Effect of carbon black on improving thermal stability, flame retardancy and electrical conductivity of polypropylene/carbon fiber composites. Compos. Sci. Technol. 2015;113:31–37. doi: 10.1016/j.compscitech.2015.03.013. [DOI] [Google Scholar]
- 135.Alongi J., Poskovic M., Visakh P., Frache A., Malucelli G. Cyclodextrin nanosponges as novel green flame retardants for PP, LLDPE and PA6. Carbohydr. Polym. 2012;88:1387–1394. doi: 10.1016/j.carbpol.2012.02.038. [DOI] [Google Scholar]
- 136.Yu Y., Song P.a., Jin C., Fu S., Zhao L., Wu Q., Ye J. Catalytic effects of nickel (cobalt or zinc) acetates on thermal and flammability properties of polypropylene-modified lignin composites. Ind. Eng. Chem. Res. 2012;51:12367–12374. doi: 10.1021/ie301953x. [DOI] [Google Scholar]
- 137.Gao Y.-Y., Deng C., Du Y.-Y., Huang S.-C., Wang Y.-Z. A novel bio-based flame retardant for polypropylene from phytic acid. Polym. Degrad. Stab. 2019;161:298–308. doi: 10.1016/j.polymdegradstab.2019.02.005. [DOI] [Google Scholar]
- 138.Das O., Bhattacharyya D., Hui D., Lau K.-T. Mechanical and flammability characterisations of biochar/polypropylene biocomposites. Compos. Part B Eng. 2016;106:120–128. doi: 10.1016/j.compositesb.2016.09.020. [DOI] [Google Scholar]
- 139.Jung D., Persi I., Bhattacharyya D. Synergistic Effects of Feather Fibers and Phosphorus Compound on Chemically Modified Chicken Feather/Polypropylene Composites. ACS Sustain. Chem. Eng. 2019;7:19072–19080. doi: 10.1021/acssuschemeng.9b04894. [DOI] [Google Scholar]
- 140.Tang Y., Hu Y., Wang S., Gui Z., Chen Z., Fan W. Intumescent flame retardant–montmorillonite synergism in polypropylene-layered silicate nanocomposites. Polym. Int. 2003;52:1396–1400. doi: 10.1002/pi.1270. [DOI] [Google Scholar]
- 141.Vahabi H., Ferry L., Longuet C., Otazaghine B., Negrell-Guirao C., David G., Lopez-Cuesta J.-M. Combination effect of polyhedral oligomeric silsesquioxane (POSS) and a phosphorus modified PMMA, flammability and thermal stability properties. Mater. Chem. Phys. 2012;136:762–770. doi: 10.1016/j.matchemphys.2012.07.053. [DOI] [Google Scholar]
- 142.Vahabi H., Laoutid F., Movahedifar E., Khalili R., Rahmati N., Vagner C., Cochez M., Brison L., Ducos F., Ganjali M.R. Description of complementary actions of mineral and organic additives in thermoplastic polymer composites by Flame Retardancy Index. Polym. Adv. Technol. 2019;30:2056–2066. doi: 10.1002/pat.4638. [DOI] [Google Scholar]
- 143.Gao S., Liu G. Synthesis of amino trimethylene phosphonic acid melamine salt and its application in flame-retarded polypropylene. J. Appl. Polym. Sci. 2018;135:46274. doi: 10.1002/app.46274. [DOI] [Google Scholar]
- 144.Chen X., Jiao C. Study on flame retardance of co-microencapsulated ammonium polyphosphate and pentaerythritol in polypropylene. J. Fire Sci. 2010;28:509–521. doi: 10.1177/0734904110363795. [DOI] [Google Scholar]
- 145.Chiu S.-H., Wang W.-K. Dynamic flame retardancy of polypropylene filled with ammonium polyphosphate, pentaerythritol and melamine additives. Polymer. 1998;39:1951–1955. doi: 10.1016/S0032-3861(97)00492-8. [DOI] [Google Scholar]
- 146.Chiu S.H., Wang W.K. The dynamic flammability and toxicity of magnesium hydroxide filled intumescent fire retardant polypropylene. J. Appl. Polym. Sci. 1998;67:989–995. doi: 10.1002/(SICI)1097-4628(19980207)67:6<989::AID-APP4>3.0.CO;2-I. [DOI] [Google Scholar]
- 147.Huang N., Chen Z., Wang J., Wei P. Synergistic effects of sepiolite on intumescent flame retardant polypropylene. Express Polym. Lett. 2010;4:743–752. doi: 10.3144/expresspolymlett.2010.90. [DOI] [Google Scholar]
- 148.Wang Z., Liu Y., Li J. Preparation of nucleotide-based microsphere and its application in intumescent flame retardant polypropylene. J. Anal. Appl. Pyrolysis. 2016;121:394–402. doi: 10.1016/j.jaap.2016.09.003. [DOI] [Google Scholar]
- 149.Su X., Yi Y., Tao J., Qi H., Li D. Synergistic effect between a novel triazine charring agent and ammonium polyphosphate on flame retardancy and thermal behavior of polypropylene. Polym. Degrad. Stab. 2014;105:12–20. doi: 10.1016/j.polymdegradstab.2014.03.041. [DOI] [Google Scholar]
- 150.Wang X., Wang Z., Li J. Effects of a semi-bio-based triazine derivative on intumescent flame-retardant polypropylene. Polym. Adv. Technol. 2019;30:1259–1268. doi: 10.1002/pat.4559. [DOI] [Google Scholar]
- 151.Wang Z., Liu Y., Li J. Regulating effects of nitrogenous bases on the char structure and flame retardancy of polypropylene/intumescent flame retardant composites. ACS Sustain. Chem. Eng. 2017;5:2375–2383. doi: 10.1021/acssuschemeng.6b02712. [DOI] [Google Scholar]
- 152.Qin Z., Li D., Yang R. Study on inorganic modified ammonium polyphosphate with precipitation method and its effect in flame retardant polypropylene. Polym. Degrad. Stab. 2016;126:117–124. doi: 10.1016/j.polymdegradstab.2016.01.022. [DOI] [Google Scholar]
- 153.Zhu H.F., Li J., Xu L., Tao K., Xue L.X., Fan X.Y. Synergistic Effect between Montmorillonite Intercalated by Melamine and Intumescent Flame Retardant (IFR) on Polypropylene. Trans Tech Publications; Zurich, Switzerland: 2011. pp. 315–318. Advanced Materials Research. [DOI] [Google Scholar]
- 154.Zhu H., Li J., Zhu Y., Chen S. Roles of organic intercalation agent with flame retardant groups in montmorillonite (MMT) in properties of polypropylene composites. Polym. Adv. Technol. 2014;25:872–880. doi: 10.1002/pat.3320. [DOI] [Google Scholar]
- 155.Wang P.-J., Hu X.-P., Liao D.-J., Wen Y., Hull T.R., Miao F., Zhang Q.-T. Dual fire retardant action: The combined gas and condensed phase effects of azo-modified NiZnAl layered double hydroxide on intumescent polypropylene. Ind. Eng. Chem. Res. 2017;56:920–932. doi: 10.1021/acs.iecr.6b03953. [DOI] [Google Scholar]
- 156.Zhang Y., He S., Wu J., Ma J., Shao S., He L., Li X., Fang Z., Cao H., Xi Z. Application of waste silicon rubber composite treated by N2 plasma in the flame-retardant polypropylene. J. Appl. Polym. Sci. 2019;136:48187. doi: 10.1002/app.48187. [DOI] [Google Scholar]
- 157.Zhao Q., Hu Y., Wang X. Mechanical performance and flame retardancy of polypropylene composites containing zeolite and multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2016;133 doi: 10.1002/app.42875. [DOI] [Google Scholar]
- 158.Su X., Li D., Tao J., Dai Q. Synergistic effect of allophane with intumescent flame retardants on thermal behavior and fire retardancy of polypropylene. Polym. Bull. 2015;72:2089–2104. doi: 10.1007/s00289-015-1391-7. [DOI] [Google Scholar]
- 159.Zhang S., Tang W., Li L., Li H., Sun J., Gu X., Chen S., Peng X., Bourbigot S. Fabrication of fly ash-based mesoporous aluminosilicate oxides loaded with zinc and its synergistic fire resistancy in polypropylene. J. Vinyl Addit. Technol. 2020;26:135–143. doi: 10.1002/vnl.21726. [DOI] [Google Scholar]
- 160.de Juan S., Zhang J., Acuña P., Nie S., Liu Z., Zhang W., Luisa Puertas M., Esteban-Cubillo A., Santarén J., Wang D.Y. An efficient approach to improving fire retardancy and smoke suppression for intumescent flame-retardant polypropylene composites via incorporating organo-modified sepiolite. Fire Mater. 2019;43:961–970. doi: 10.1002/fam.2757. [DOI] [Google Scholar]
- 161.Turgut G., Dogan M., Tayfun U., Ozkoc G. The effects of POSS particles on the flame retardancy of intumescent polypropylene composites and the structure-property relationship. Polym. Degrad. Stab. 2018;149:96–111. doi: 10.1016/j.polymdegradstab.2018.01.025. [DOI] [Google Scholar]
- 162.Almirón J., Roudet F., Duquesne S. Influence of volcanic ash, rice husk ash, and solid residue of catalytic pyrolysis on the flame-retardant properties of polypropylene composites. J. Fire Sci. 2019;37:434–451. doi: 10.1177/0734904119867912. [DOI] [Google Scholar]
- 163.Doğan M., Yılmaz A., Bayramlı E. Synergistic effect of boron containing substances on flame retardancy and thermal stability of intumescent polypropylene composites. Polym. Degrad. Stab. 2010;95:2584–2588. doi: 10.1016/j.polymdegradstab.2010.07.033. [DOI] [Google Scholar]
- 164.Zhou K., Jiang S., Wang B., Shi Y., Liu J., Hong N., Hu Y., Gui Z. Combined effect of transition metal phosphide (MxPy, M= Ni, Co, and Cu) and intumescent flame retardant system on polypropylene. Polym. Adv. Technol. 2014;25:701–710. doi: 10.1002/pat.3273. [DOI] [Google Scholar]
- 165.Su X., Yi Y., Tao J., Qi H. Synergistic effect of zinc hydroxystannate with intumescent flame-retardants on fire retardancy and thermal behavior of polypropylene. Polym. Degrad. Stab. 2012;97:2128–2135. doi: 10.1016/j.polymdegradstab.2012.08.017. [DOI] [Google Scholar]
- 166.Zhang Y., Li X., Fang Z., Hull T.R., Kelarakis A., Stec A.A. Mechanism of enhancement of intumescent fire retardancy by metal acetates in polypropylene. Polym. Degrad. Stab. 2017;136:139–145. doi: 10.1016/j.polymdegradstab.2016.12.018. [DOI] [Google Scholar]
- 167.Pallmann J., Ren Y.L., Mahltig B., Huo T.G. Phosphorylated sodium alginate/APP/DPER intumescent flame retardant used for polypropylene. J. Appl. Polym. Sci. 2019;136:47794. doi: 10.1002/app.47794. [DOI] [Google Scholar]
- 168.Li J., Lai X., Li H., Zeng X., Liu Y., Zeng Y., Jiang C. Functionalized ZrP nanosheet with free-radical quenching capability and its synergism in intumescent flame-retardant polypropylene. Polym. Adv. Technol. 2019;31:602–615. doi: 10.1002/pat.4801. [DOI] [Google Scholar]
- 169.Tang W., Zhang S., Sun J., Li H., Liu X., Gu X. Effects of surface acid-activated kaolinite on the fire performance of polypropylene composite. Thermochim. Acta. 2017;648:1–12. doi: 10.1016/j.tca.2016.12.007. [DOI] [Google Scholar]
- 170.Tang W., Song L., Zhang S., Li H., Sun J., Gu X. Preparation of thiourea-intercalated kaolinite and its influence on thermostability and flammability of polypropylene composite. J. Mater. Sci. 2017;52:208–217. doi: 10.1007/s10853-016-0323-8. [DOI] [Google Scholar]
- 171.Tang W., Zhang S., Gu X., Sun J., Jin X., Li H. Effects of kaolinite nanoroll on the flammability of polypropylene nanocomposites. Appl. Clay Sci. 2016;132:579–588. doi: 10.1016/j.clay.2016.08.008. [DOI] [Google Scholar]
- 172.Sun W., Tang W., Gu X., Zhang S., Sun J., Li H., Liu X. Synergistic effect of kaolinite/halloysite on the flammability and thermostability of polypropylene. J. Appl. Polym. Sci. 2018;135:46507. doi: 10.1002/app.46507. [DOI] [Google Scholar]
- 173.Zhang S., Tang W., Guo J., Jin X., Li H., Gu X., Sun J. Improvement of flame retardancy and thermal stability of polypropylene by P-type hydrated silica aluminate containing lanthanum. Polym. Degrad. Stab. 2018;154:276–284. doi: 10.1016/j.polymdegradstab.2018.06.014. [DOI] [Google Scholar]
- 174.Wen P., Wang D., Liu J., Zhan J., Hu Y., Yuen R.K. Organically modified montmorillonite as a synergist for intumescent flame retardant against the flammable polypropylene. Polym. Adv. Technol. 2017;28:679–685. doi: 10.1002/pat.3967. [DOI] [Google Scholar]
- 175.Yang K., Xu M.-J., Li B. Synthesis of N-ethyl triazine–piperazine copolymer and flame retardancy and water resistance of intumescent flame retardant polypropylene. Polym. Degrad. Stab. 2013;98:1397–1406. doi: 10.1016/j.polymdegradstab.2013.03.023. [DOI] [Google Scholar]
- 176.Yuan B., Fan A., Yang M., Chen X., Hu Y., Bao C., Jiang S., Niu Y., Zhang Y., He S. The effects of graphene on the flammability and fire behavior of intumescent flame retardant polypropylene composites at different flame scenarios. Polym. Degrad. Stab. 2017;143:42–56. doi: 10.1016/j.polymdegradstab.2017.06.015. [DOI] [Google Scholar]
- 177.Yuan B., Sun Y., Chen X., Shi Y., Dai H., He S. Poorly-/well-dispersed graphene: Abnormal influence on flammability and fire behavior of intumescent flame retardant. Compos. Part A Appl. Sci. Manuf. 2018;109:345–354. doi: 10.1016/j.compositesa.2018.03.022. [DOI] [Google Scholar]
- 178.Gao S., Zhao X., Liu G. Synthesis of tris (2-hydroxyethyl) isocyanurate homopolymer and its application in intumescent flame retarded polypropylene. J. Appl. Polym. Sci. 2017;134 doi: 10.1002/app.44663. [DOI] [Google Scholar]
- 179.Yang R., Ma B., Zhao H., Li J. Preparation, thermal degradation, and fire behaviors of intumescent flame retardant polypropylene with a charring agent containing pentaerythritol and triazine. Ind. Eng. Chem. Res. 2016;55:5298–5305. doi: 10.1021/acs.iecr.6b00204. [DOI] [Google Scholar]
- 180.Liu Y., Wang J.S., Deng C.L., Wang D.Y., Song Y.P., Wang Y.Z. The synergistic flame-retardant effect of O-MMT on the intumescent flame-retardant PP/CA/APP systems. Polym. Adv. Technol. 2010;21:789–796. doi: 10.1002/pat.1502. [DOI] [Google Scholar]
- 181.Wang Y., Xu M.-J., Li B. Synthesis of N-methyl triazine-ethylenediamine copolymer charring foaming agent and its enhancement on flame retardancy and water resistance for polypropylene composites. Polym. Degrad. Stab. 2016;131:20–29. doi: 10.1016/j.polymdegradstab.2016.07.003. [DOI] [Google Scholar]
- 182.Enescu D., Frache A., Lavaselli M., Monticelli O., Marino F. Novel phosphorous–nitrogen intumescent flame retardant system. Its effects on flame retardancy and thermal properties of polypropylene. Polym. Degrad. Stab. 2013;98:297–305. doi: 10.1016/j.polymdegradstab.2012.09.012. [DOI] [Google Scholar]
- 183.Zheng Z., Sun H., Li W., Zhong S., Yan J., Cui X., Wang H. Co-microencapsulation of ammonium polyphosphate and aluminum hydroxide in halogen-free and intumescent flame retarding polypropylene. Polym. Compos. 2014;35:715–729. doi: 10.1002/pc.22715. [DOI] [Google Scholar]
- 184.Tian N., Wen X., Gong J., Ma L., Xue J., Tang T. Synthesis and characterization of a novel organophosphorus flame retardant and its application in polypropylene. Polym. Adv. Technol. 2013;24:653–659. doi: 10.1002/pat.3129. [DOI] [Google Scholar]
- 185.Wang J.-S., Wang G.-H., Liu Y., Jiao Y.-H., Liu D. Thermal stability, combustion behavior, and toxic gases in fire effluents of an intumescent flame-retarded polypropylene system. Ind. Eng. Chem. Res. 2014;53:6978–6984. doi: 10.1021/ie500262w. [DOI] [Google Scholar]
- 186.Wu J., Hu Y., Song L., Kang W. Synergistic effect of lanthanum oxide on intumescent flame-retardant polypropylene-based formulations. J. Fire Sci. 2008;26:399–414. [Google Scholar]
- 187.Zheng Z., Liu Y., Zhang L., Wang H. Synergistic effect of expandable graphite and intumescent flame retardants on the flame retardancy and thermal stability of polypropylene. J. Mater. Sci. 2016;51:5857–5871. doi: 10.1007/s10853-016-9887-6. [DOI] [Google Scholar]
- 188.Lai X., Zeng X., Li H., Yin C., Zhang H., Liao F. Synergistic effect of phosphorus-containing nanosponges on intumescent flame-retardant polypropylene. J. Appl. Polym. Sci. 2012;125:1758–1765. doi: 10.1002/app.35646. [DOI] [Google Scholar]
- 189.Hapuarachchi T.D., Peijs T., Bilotti E. Thermal degradation and flammability behavior of polypropylene/clay/carbon nanotube composite systems. Polym. Adv. Technol. 2013;24:331–338. doi: 10.1002/pat.3087. [DOI] [Google Scholar]
- 190.Zaikov G.E., Lomakin S.M. Polymer flame retardancy: A new approach. J. Appl. Polym. Sci. 1998;68:715–725. doi: 10.1002/(SICI)1097-4628(19980502)68:5<715::AID-APP4>3.0.CO;2-R. [DOI] [Google Scholar]
- 191.Wang D., Echols K., Wilkie C.A. Cone calorimetric and thermogravimetric analysis evaluation of halogen-containing polymer nanocomposites. Fire Mater. Int. J. 2005;29:283–294. doi: 10.1002/fam.885. [DOI] [Google Scholar]