Abstract
With this review, we provide the state of the art concerning brain metastases (BMs) from ovarian cancer (OC), a rare condition. Clinical, pathological, and molecular features, treatment options, and future perspectives are comprehensively discussed. Overall, a diagnosis of high-grade serous OC and an advanced disease stage are common features among patients who develop brain metastases. BRCA1 and BRCA2 gene mutations, as well as the expression of androgen receptors in the primary tumor, are emerging risk and prognostic factors which could allow one to identify categories of patients at greater risk of BMs, who could benefit from a tailored follow-up. Based on present data, a multidisciplinary approach combining surgery, radiotherapy, and chemotherapy seem to be the best approach for patients with good performance status, although the median overall survival (<1 year) remains largely disappointing. Hopefully, novel therapeutic avenues are being explored, like PARP inhibitors and immunotherapy, based on our improved knowledge regarding tumor biology, but further investigation is warranted.
Keywords: brain metastases, ovarian cancer, BRCA, treatment, management, pathology, diagnosis, radiotherapy, surgery
1. Introduction
Ovarian cancer (OC) is the current leading cause of gynecological cancer deaths. In the United States, over 20,000 new diagnoses of OC were estimated for 2020, and over 13,000 deaths due to this tumor type [1]. OC is characterized by a high frequency of loco-regional recurrence caused by the dissemination of neoplastic cells through peritoneal fluid or lymphatic drainage [2,3,4,5]. Despite sensitivity to chemotherapy, most tumor relapses are observed within three years from the end of adjuvant treatments [6]. The most common metastatic sites are peritoneum and omentum (86%), pelvic and/or para-aortic lymph nodes (70%), bowel (50%), and spleen (20%) [5].
Brain metastases (BMs) represent the most common adult intracranial malignancy, and it is estimated that approximately 20–30% of patients with a solid tumor will develop this complication during the disease course; moreover, data from autopsies of patients with malignant tumors showed an even higher incidence (up to 40%), suggesting that BMs can be frequently undetected [7,8]. Breast, lung, colorectal cancers, and melanoma are the neoplasms most frequently associated with the development of BMs [7,8,9,10], while the central nervous system (CNS) seeding from OC is more rarely observed. Within the last 30 years, excluding single case reports or small series (n < 10), only the outcomes of approximately 1100 patients have been described (Table 1) [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. OC cells can reach the CNS through the bloodstream or, more rarely, they may invade the meninges through direct invasion from a bone metastasis, or by lymphatic drainage [49]. The incidence of BMs in OC is estimated at 1.34%, ranging from 0.49% to 6.1% (Table 1) of cases. This heterogeneity can be partially explained by improvements in diagnostic procedures and treatments, which improved detection and outcome rates. Despite the available therapeutic options, such as surgery, radiotherapy, and chemotherapy, there are no established guidelines for the management of this severe complication and prognosis remains poor [5,49,50,51], with a median overall survival of 10.1 months (Table 1). The growing incidence of BMs from OC warrants specific attention and focused research as prompt treatment may influence the prognosis.
Table 1.
Clinico-pathological features, treatments and outcome data of patients with BMs from OC. Only series published since 1990 and with at least 10 cases were reported.
| Author | BM (N) | Incidence | Histotype (N, %) |
Grade (N, %) |
FIGO Stage at OC Diagnosis (N, %) |
Median Age at OC Diagnosis (yrs) |
Mean Interval to BMs (mo) |
Single vs. Multiple BMs (N, %) |
Most Common Symptoms (N, %) | Treatments | Median Survival (mo) from the Diagnosis of BM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LeRoux [11] 1990 |
14 | 1.1% | Serous (7, 50%) ED (2, 14%) CC (1, 7%) MC (2, 14%) Other (2, 14%) |
G1 (1, 7%) G2 (9, 64%) G3 (4, 28%) |
I (3, 21%) II (4, 29%) III (4, 29%) IV (3, (21%) |
52.5 | 14.5 | 9 (64%) vs. 5 (36%) | Motor Weakness (5, 36%) Seizure (5, 36%) Headache (2, 14%) Confusion (1, 7%) Speech disturbance (1, 7%) |
RT (8, 57%) Surg + RT (5, 36%) No treatment (1, 7%) |
3 |
| Rodriguez [12] 1992 |
15 | 1.9% | Serous (13, 87%) MC (1, 6.7%) Other (1, 6.7%) |
G1 (1, 6.7%) G2 (3, 20%) G3 (8, 53.3%) NA (3, 20%) |
I (2, 13.3%) III (8, 53%) IV (5, 33%) |
54.5 | 18.5 | 5 (33%) vs. 10 (67%) | Headache (6, 40%) Seizure (3, 20%) Aphasia (1, 6.7%) Decreased arm coordination (1, 6.7%) Vertigo (2, 13.3%) Personality change (1, 6.7%) Confusion (1, 6.7%) |
RT + CHT (6, 40%) RT (4, 26.7%) Surg + RT + CHT (3, 20%) Surg + RT (1, 6.7%) |
9 |
| Geisler [13] 1995 |
16 | 3.3% | Serous (16, 100%) | G1 (1, 6.25%) G2 (6, 37.5%) G3 (9, 56.25%) |
III (12, 75%) IV (4, 25%) |
56.6 | 19 | 8 (50%) vs. 8 (50%) | Headache (5, 31.2%) Seizure (2, 12.5% Vertigo (2, 12.5%) Paresis (2, 12.5%) Atassia (2, 12.5%) Aphasia (1, 6.25%) Syncope (1, 6.25%) Confusion (1, 6.25%) |
RT (8, 50%) Surg + RT (5, 31.25%) RT + CHT (2, 12.5%) No treatment (1, 6.25%) |
3 |
| Corn [14] 1995 |
32 | 0.8% | NA | NA | NA | 56 | 24 | 13 (40%) vs. 17 (53%) NA 2 (6%) |
Headache (12, 37,5%) Seizures (5, 16%) |
RT (27, 84%) RT + CHT (5, 16%) |
4 |
| Cormio [15] 1995 |
23 | NA | Serous (14, 61%) ED (6, 26%) CC (1, 4%) Other (2, 9%) |
G1 (2, 9%) G2 (4, 17%) G3 (17, 74%) |
I (3, 13%) III (17, 74%) IV (3, 13%) |
56 | 35 | 9 (41%) vs. 13 (59%) | NA | RT (14, 60.9%) Surg + RT (5, 21.7%) NA (4, 17.4) |
5 |
| Anupol [16] 2002 |
15 | 1.4 % | Serous (14, 93.3%) Other (1, 6.7%) |
G2 (1, 6.7%), G3 (12, 80%), NA (2, 13.3%) |
III (7, 47%) IV (8, 53%) |
58 | 22 | 7 (46.7%) vs. 7 (46.7%) NA: 1 (6.6%) |
Decreased mental status (7, 46%) Motor deficit (4, 20%) Headache (3, 20%) Visual disturbance (1, 6%) Seizure (1, 6%) |
RT + CHT + Surg (5, 33,3%) RT + CHT (4, 26,7%) RT (3, 20%) No treatment (2, 13,3%) RT + Surg (1, 6.7%) |
6 |
| Pothuri [17] 2002 |
14 | NA | Serous (9, 64%) ED (2, 14%) Other (3, 21%) |
G2 (4, 29%) G3 (10, 71%) |
II (1, 7%) III (12, 86%) IV (1, 7%) |
NA | 42 | 2 (14%) vs. 12 (86%) | Headache (6, 43%) Hemiparesis (4, 29%) |
Surg + CHT + RT (6, 43%) Surg + RT (5, 36%) Surg + CHT (2, 14%) Surg (1, 7%) |
18 |
| Sanderson [18] 2002 |
13 | 1.1% | Serous (3, 23%) CC (2, 15.4%) ED (1, 7.7%) Other (7, 53.8%) |
NA | III (6, 46.1%) IV (7, 53.9%) |
55 | 36 | NA | RT (7, 53.8%) Surg (3, 23.1%) CHT (2, 15.4%) No treatment (1, 7.7%) |
4 | |
| Kolomainen [19] 2002 |
18 | 0.49% | Serous (13, 72%) MC (1, 5.5%) ED (1, 5.5%) Other (3, 17%) |
G2 (8, 44%) G3 (9, 50%) NA (1, 6%) |
I (6, 33%) II (3, 17%) III (7, 39%) IV (2, 11%) |
52 | 46 | 9 (50%) vs. 9 (50%) | Motor weakness (6, 33.3%) Confusion (6, 33.3%) Headache (5, 27.8%) Seizures (3, 16.7%) |
CHT (18, 100%) | 7 |
| Cormio 2003 [20] |
22 | NA | Serous (15, 68%) ED (5, 22%) CC (1, 5%) Other (1, 5%) |
G1 (4, 17%) G2 (6, 28%) G3 (12, 55%) |
I (2, 8%) III (19, 88%) IV (1, 4%) |
54 | 29 | NA | NA | Surg + RT (17, 77%) Surg+ CHT (5, 23%) |
16 |
| Kumar [21] 2003 |
18 | 2.7% | Serous (13, 72%) MC (2, 11%) ED (1, 6%) Other (2, 11%) |
G1 (1, 6%) G2 (5, 28%) G3 (11, 61%) NA (1, 6%) |
I (1, 6%) III (13, 72%) IV (4, 22%) |
54 | 29 | 5 (28%) vs. 13 (72&%) | Headache (10, 56%) Weakness (9, 50%) Seizure (3, 17%) Vision problems (2, 11%) Tremors (2, 11%) Vertigo (2, 11%) Aphasia (2, 11%) Altered Consciousness (1, 6%) |
RT (8, 44%) CHT + RT (5, 28%) Surg + RT + CHT (4, 22%) No treatment (1, 6%) |
4 |
| Cohen [22] 2004 |
72 | 0.9% | Serous (14, 24%) MC (6, 10%) CC (2, 3%) ED (2, 3%) Other (35, 48%) NA (13, 18%) |
G1-G2 (11, 15%) G3 (52, 72%) NA (9, 13%) |
I–II (12, 16%) III–IV (52, 72%) NA (8, 11%) |
50.4 | 22 | 25 (35%) vs. 47 (65%) | Neurological deficit (41, 57%) Headache (18, 25%) Seizures (11, 15%) Dizziness (4, 6%) |
RT (36, 50%) Surg + RT (13, 18%) Surg (8, 12%) Steroids (8, 12%) CHT (3, 4%) NA (3, 4%) RT + CHT (1, 1%) |
6 |
| Pectasides [23] 2005 |
17 | 1.17% | Serous (12, 71%) MC (2, 12%) ED (2, 12%) Others (1, 6%) |
G2 (3, 18%) G3 (11, 65%) NA (3, 18%) |
II (2, 12%) III (12, 71%) IV (3, 18%) |
56 | 16 | 5 (29%) vs. 12 (71%) | NA | RT + CHT (6, 35%) No treatment (5, 29%) RT (4, 24%) Surg + CHT + RT (2, 12%) |
5.7 |
| D’Andrea [24] 2005 |
11 | NA | Serous (11, 100%) | NA | II (11, 100%) | 60.3 | 21 | 11 (100%) vs. 0 (0%) | Hemiparesis (8, 73%) | Surg + RT + CT (11, 100%) | 28 |
| Chen [25] 2005 |
19 | NA | Serous (7, 37%) CC (1, 5%) ED (1, 5%) Other (10, 53%) |
G3 (13, 68%) NA (6, 32%) |
I (1, 5%) III (13, 68%) IV (5, 26%) |
51 | 25 | 7 (37%) vs. 12 (63%) | Headache (12, 63%) Motor deficit (7, 37%) Mental status change (4, 21%) Seizure (3, 16%) Ataxia (2, 11%) Memory (3, 16%) Sensory deficit (1, 5%) Aphasia (1, 5%) |
RT (10, 53%) Surg + RT (8, 42 %) Surg (1, 5%) |
16 |
| Kim [26] 2007 |
13 | 2.7% | Serous (9, 69%) MC (1, 8%) ED (1, 8%) CC (1, 8%) Other (1, 8%) |
G1 (1, 8%) G2 (1, 8%) G3 (7, 54%) NA (4, 30%) |
I (1, 8%) III (9, 69%) IV (3, 23%) |
52 | 28 | 2 (15%) vs. 11 (84%) | Headache (9, 69%) Seizure (2, 15%) Weakness (2, 15%) |
RT (7, 54%) RT + CHT (4, 30%) RT + Surg (1, 8%) No treatment (1, 8%) |
7 |
| Gadducci [27] 2007 |
12 | 6.1% | Serous (8, 67%) ED (1, 8%) CC (1, 8%) Other (2, 17%) |
G2 (6, 50%) G3 (6, 50%) |
III (9, 75%) IV (3, 25%) |
59.5 | 33.5 | 6 (50%) vs. 6 (50%) | Headache (4, 33%) Weakness (3, 25%) Seizure (1, 8%) Impaired deambulation (3, 25%) |
RT (5, 42%) RT + CHT (3, 25%) No treatment (2, 17%) Surg (1, 8%) Surg + RT (1, 8%) |
8.3 |
| Lee [28] 2008 |
15 | 1.1% | Serous (8, 53%) ED (3, 20%) MC (1, 7%) Other (3, 20%) |
NA | I (1, 7%) III (9, 60%) IV (5, 33%) |
55 | 28 | 5 (33%) vs. 10 (67%) |
Headache (6, 40%) Loss of consciousness (1, 7%) Motor weakness (4, 27%) Nausea and vomiting (5, 15%) Hemiparesis (1, 7%) Atassia (1, 7%) |
RT + CHT (9, 60%) RT (6, 40%) |
14 |
| Ratner [29] 2009 |
24 | 1.1% | Serous (17, 71%) ED (3, 12%) Other (4, 17%) |
G2 (3, 12%) G3 (21, 88%) |
I (1, 4%) III (14, 58%) IV (5, 21%) NA (4, 17%) |
56 | 23 | 7 (29%) vs. 17 (71%) | Mental status change (4, 17%) Seizures (3, 13%) Hemiparesis (3, 13%) Dysphasia (3, 13%) Cerebrovascular accident (2, 8%) Diplopia (2, 8%) Headache (2, 8%) Gait disturbance (2, 8%) Neuropathy and incontinence (1, 4%) Loss peripheral visual field (1, 4% )Twitching hands (1, 4%) |
RT (18, 75%) Surge + RT (3, 13%) No treatment (2, 8%) Surg (1, 4%) |
8.5 |
| Sehouli [30] 2010 |
74 | 1.8% | Serous (53, 72%) Other (21, 28%) |
G1-G2 (31, 42%) G3 (43, 58%) |
I/II (12, 16%) III/IV (62, 84%) |
53.9 | 28.8 | 26 (35%) vs. 48 (65%) | Headache (25, 34%) Vomiting/nausea (16, 22%) Vertigo (13, 17.5%) Seizure (12, 16%) Impaired vision (11, 15%) Paralysis (9, 12%) |
Surg + RT + CHT (21, 28%) RT (20, 27%) Surg + RT (14, 19%) Surg (11, 15%) RT + CHT (6, 8%) Surg + CHT (2, 3%) |
6.2 |
| Chen [31] 2011 |
10 | 1.9% | Serous (7, 70%) MC (2, 20%) CC (1, 10%) |
G2 (4, 40%) G3 (6, 60%) |
II (1, 10%) III–IV (9, 90%) |
56.6 | 24.3 | 1 (10%) vs. 9 (90%) | Extremity weakness (2, 20%) Blurred vision (2, 20%) Headache (3, 30%) Dizziness (2, 20%) Consciousness disturbance (1, 10%) Seizure (1, 10%) |
RT + CHT (5, 50%) RT (3. 30%) Surg + RT (1, 10%) No treatment (1, 10%) |
3 |
| Cormio [32] 2011 |
20 | 5% | Serous (14, 70%) ED (3, 15%) Other (3, 15%) |
G1 (1. 5%) G2 (4, 20%) G3 (15, 75%) |
I (1, 5%) III (16, 80%) IV (3, 15%) |
55.5 | 32.7 | 11 (55%) vs. 9 (45%) | NA | Surg + RT+ CHT (3, 15%) CHT + RT (3, 15%) Surg + CT (3, 15%) RT (3, 15%) No treatment (3, 15%) Surg (2, 10%) Surg + RT (2, 10%) CHT (1, 5%) |
17.6 |
| Chiang [33] 2011 |
64 | 1.2% | Serous (32, 50%) MC (5, 8%) ED (7, 11%) CC (11, 17%) Others (9, 14%) |
G2 (11, 17%) G3 (33, 52%) NA (20, 31%) |
I (8, 13%) II (4, 6%) III (36, 56%) IV (16, 25%) |
52 | 22 | 18 (28%) vs. 46 (72%) | NA | RT + CHT (25, 39%) Surg + RT + CHT (12, 19%) RT (13, 20%) No treatment (7, 11%) Surg + RT (5, 8%) Surg (2, 3%) |
8 |
| Ogino [34] 2012 |
16 | NA | Serous (4, 25%) CC (2, 12%) NA (8, 50%) Others (2, 12%) |
NA | NA | 53 | 27.5 | 6 (37%) vs. 10 (63%) | Extremity weakness (5, 31%) Seizure (2, 12%) Aphasia (1, 6%) Headache (1, 6%) Dysarthria (1, 6%) Dizziness (1, 6%) |
RT (13, 81%) Surg + RT (3, 19%) |
12.5 |
| Nasu [35] 2013 |
56 | NA | Serous (33, 59%) CC (7, 12%) ED (6, 11%) MC (2, 4%) Others (4, 7%) NA (4, 7%) |
NA | I (7, 12%) II (1, 2%) III (32, 57%) IV (16, 29%) |
55.8 | 25.2 | 30 (54%) vs. 26 (46%) | NA | RT (21, 37%) RT + CHT (11, 20%) Surg+ RT (8, 14%) No treatment (6, 11%) Surg + RT + CHT (5, 9%) CHT (3, 5%) Surg + CHT (1, 2%) Surg (1, 2%) |
12.5 |
| Niu [36] 2013 |
12 | NA | Serous (10, 83%) Others (2, 17%) |
G2 (1, 8%) G3 (11, 92%) |
III (10, 83%) IV (2, 17%) |
56 | 19 | 4 (33%) vs. 8 (77%) | NA | RT (4, 33%) RT + CHT (3, 25%) Surg + RT +CHT (3, 25%) Surg + RT (1, 8%) No treatment (1, 8%) |
17 |
| Teckie [37] 2013 |
60 | NA | Serous (42, 70%) ED (8, 13%) CC (1, 2%) MC (1, 2%) Others (8, 13%) |
G1 (2, 3%) G2 (7, 12%) G3 (49, 82%) NA (2, 3%) |
I (3, 5%) II (4, 7%) III (40, 67%) IV (13, 21%) |
56 | 41 | 28 (47%) vs. 32 (53%) | NA | RT (38, 63%) Surg + RT (22, 37%) |
9.7 |
| Gressel [38] 2015 |
19 | NA | Serous (15, 79%) CC (2, 10,5%) NA (2, 10,5%) |
NA | I (1, 5%) II (2, 11%) III (6, 32%) IV (10, 52%) |
61 | 23 | 8 (42%) vs. 11 (58%) | NA | Surg + RT (9, 47%) RT (6, 32%) Surg (2, 10.5%) No treatment (2, 10.5%) |
9 |
| Gilani [39] 2016 |
13 | NA | Serous (5, 39%) Others (5, 39%) NA (3, 23%) |
NA | I (1, 8%) III (9, 69%) NA (3, 23%) |
60 | 31 | NA | NA | NA | 23 |
| Marchetti [40] 2016 |
174 | NA | Serous (135, 77.6%), ED (13, 7.5%), CC (9, 5.2), MC (1, 0.6%), others (14, 8.1%) NA (2, 1.1%) |
G1 (3, 1.7%), G2 (24, 13.8%) G3 (135, 77.6%), NA (12, 6.9%) |
I–II (8, 4.6%) III (134, 77%), IV (32, 18.4%) |
57 | 26 | 73 (42%) vs. 100 (57.4%) NA: 1 (0.6%) | Headache (58, 33.3%), Vertigo (40, 23%), Confusion (30, 17.2%), Nausea and vomiting (28, 16.1%), Seizures (17, 9.8%) |
RT + CT (55, 31.6%), RT + CHT + Surg (41, 23.7%), RT (38, 21.8%), RT + surg (11, 6.3%) No treatment (11, 6.3%) CHT (8, 4.6%), CHT + surg (6, 3.4%), Surg (4, 2.3%), |
12 |
| Matsunaga [41] 2016 |
33 | NA | Serous (23, 70%) MC (4, 12%) CC (3, 9%) ED (2, 6%) Others (1, 3%) |
NA | NA | 59 | 24 | 15 (45%) vs. 18 (55%) | NA | NA | 8 |
| Mittica [42] 2017 |
11 | NA | Serous (9, 82%) ED (2. 18%) |
G2 (1, 9%) G3 (10, 91%) |
II (2, 18%) III (6. 55%) IV (3, 27%) |
61 | 23 | 8 (73%) vs. 3 (27%) | Headache (2, 18%) Vertigo (2, 18%) Ataxia (2, 18%) Aphasia (1, 9%) Confusional state (1, 9%) NA (3, 27%) |
Surg + RT (5, 45%) Surg + RT + CHT (3, 27%) Surg (2, 18%) Surg + CHT (1, 9%) |
7 |
| Seber [43] 2017 |
33 | NA | NA | G2 (18, 55%) G3 (15, 45%) |
III (24, 73%) IV (9, 27%) |
57 | 22 | 15 (45%) vs. 18 (55%) | NA | CHT + Surg/RT (22, 67%) Surg +/- RT (19, 58%) Surg + RT + CHT (5, 4%) RT (2, 6%) |
15 |
| Kwon [44] 2018 |
56 | 2,8% | Serous (32, 57.2%) ED (5, 8.9%) Others 19 (33.9) |
NA | I–II (6, 10.7%) III (19, 34%) IV (26, 46.4%) NA (5, 8.9%) |
NA | 27 | 24 (42.9%) vs. 32 (57.1%) | NA | RT (33, 58.9%) Surg (5, 9%) No treatment (10. 17.8%) Surg + RT (8. 14.3%) |
NA |
| Wohl [45] 2019 |
25 | NA | Serous 11 (73.3%) | NA | NA | 58 | 42.3 | 18 (72%) vs. 7 (28%) | Headache (13, 53%) Motor deficit (8, 32%) Dysphasia (8, 32%) Seizure (3, 12%) |
Surg + RT (11, 44%) RT (9, 36%) Surg (5, 20%) |
35.8 |
| Da Costa [46] 2019 |
26 | 4.6% | Serous (17, 65.4%) ED (2, 7.7%) Others (7, 26.8%) |
NA | I–III (20, 76.9%) IV (5, 19.2%) NA (1, 3.8%) |
NA | 31.7 | 8 (30.7%) vs. 18 (69,3%) | NA | RT (13, 79.2%) Surg (8, 20.8%) |
10.8 |
| Keskin [47] 2019 |
21 | NA | Serous (12, 57%) ED (1, 5%) CC (1, 5%) Others (7, 33%) |
G2 3 (14)G3 18 (86) | I (1, 5%) III (17, 81%) IV (3, 14%) |
NA | 32 | 10 (48%) vs. 11 (52%) | NA | RT (8, 38%) RT + CHT (7, 34%) Surg (3, 14%) Surg + RT + CHT (3, 14%) |
9 |
| Mittica [48] 2020 |
29 | NA | Serous (25, 86%) CC (2, 7%) Other (2, 7%) |
G3 (29, 100%) | II (2, 7%) III (19, 65%) IV (8, 28%) |
57 | 25 | 12 (41.4%) vs. 17 (58.6%) | Headache (10, 34%) Motor deficit (8, 28%) Seizures (3, 10%) Aphasia (3, 10%) Vomiting (2, 7%) Vertigo (2, 7%) |
RT + CHT (7, 24%), Surg + RT (6, 21%) RT (6, 21%), RT + CHT + Surg (4, 14%) Surg (3, 10%), RT + surg (1, 3%) CHT + surg (1, 3%), No treatment (1, 3%) |
12 |
| Total | 1135 | 1,34% (0.49%-6.1%) | Serous (682, 63.7%) ED (80, 7.5%) MC (62, 5.8%) CC (49, 4.6%) Other (182, 17%) |
G1 (17, 2%) G2 (174, 21%) G3 (566, 69%) |
I–II (118, 12%) III–IV (865, 86%) |
55,9 (50.4–60.3) | 27,3 (14.5–46) | 450 (41,4%) vs. 632 (58, 1%) | Not Performed | Not Performed | 10.1 (3–35.8) |
OC: ovarian cancer, BM: brain metastasis, yrs: years, mo: months, NA: not available, ED: endometrioid, CC: clear cell, MC: mucinous, RT: radiotherapy, Surg: surgery, CHT: chemotherapy. The incidence of BMs was calculated only for studies in which the cohort of primary tumors was reported. The total numbers for ”histotype“, ”grade“ and ”FIGO Stage at OC diagnosis“ were provided only for studies in which these characteristics of primary tumors were reported.
The purpose of this review is to provide a comprehensive and up to date overview of risk and prognostic factors, clinical and pathological features, available treatments, and future therapeutic options for BMs from OC.
2. Risk and Prognostic Factors
2.1. Clinical Characteristics
Based on literature data, most patients who developed BMs had a high-grade serous OC as primary tumor (79%), followed by the endometrioid, mucinous and clear cell histotypes. Nevertheless, it should be noted that BMs can also occur in low-grade primary neoplasms (Table 1). Furthermore, 86% of patients had an advanced FIGO stage (III–IV) at OC diagnosis (Table 1).
Despite the rarity of BMs from OC, many prognostic factors have been identified. Patients who were <50 years at the primary tumor time of diagnosis [50] and with a Karnofsky performance status (KPS) ≥70 were associated with a better prognosis [35,37,44,49,50], while the number of previous extracranial recurrences before the diagnosis of BMs and the number of brain lesions (more than one) was reported as a significant unfavorable prognostic factor by several authors [30,33,35,40,45,49,50]. The prognostic significance of concomitant extracranial disease is controversial. Paknenshan et al. [50] found no relationship with survival, while Marchetti et al. [40] reported a worse survival rate. Similarly, the platinum sensitivity of the primary tumor has been linked to a better prognosis by Sehouli et al. [30] (HR 0.23, 95% CI 0.12–0.48), but this finding has not been confirmed by other authors [40,46]. The specific OC histotype, CA-125 levels and FIGO tumor stage at primary diagnosis and the residual tumor after surgery do not seem to affect the prognosis [35,50].
2.2. Hormone Receptors
Unfortunately, the implications of hormone [estrogen (ER), progesterone (PgR) and, androgen (AR)] receptors expression on the risk and prognosis of BMs from OC have been poorly investigated. Mittica et al. [42] compared the hormone receptors expression of 11 OC and their matched BMs with a control series of 22 OCs without brain involvement: BMs showed lower expression of ER and AR compared to the corresponding primary tumor. Furthermore, they also observed that the absence of AR expression in OC carries a 9.5-fold increased risk to develop BMs compared with AR-positive OC. This relationship between AR expression and risk of developing BMs has been confirmed by a second study of the same group [48], in an independent validation set of 19 new OC. In addition, a significantly worse survival outcome, both in terms of PFS (p = 0.005) and brain-specific PFS (p = 0.002), was observed in patients with low expression of AR (<10%). The group also investigated the role of HER-2 expression, but found no relationship with the risk of developing BMs or prognosis.
Despite the overall limitations in terms of available data, some information could be gathered from breast cancer, since this topic has been extensively investigated in this tumor type. A recent meta-analysis [52] suggested that ER negativity was an independent risk factor for BMs development, and that the triple-negative (ER, PgR and HER-2 negative) breast cancers showed shorter time to BMs development from the diagnosis of the primary tumor, compared to other breast cancer subtypes. Few data are available concerning AR expression, but overall it appears that this receptor is not related to the risk of developing distant metastases in triple-negative breast cancer [53]. Similar research efforts are warranted also for OC, to fully elucidate the role of hormone receptors as potential risk factors for BMs development.
2.3. BRCA Status
Germline mutations in the homologous DNA repair genes BRCA1 and BRCA2 account for about 5–10% of all breast cancers and for 10–18% of all OCs [54]. In particular, women who carry a BRCA1 or BRCA2 mutation have a cumulative lifetime risk of developing OC of 39–54% and 11–23%, respectively [55]. Furthermore, The Cancer Genome Atlas (TCGA) studies have shown that approximately half of the cases of serous OC harbor homologous recombination deficiency (HRD) [56]. Regarding the prognostic significance of HRD in OC, BRCA-deficient OC patients show higher survival rates and are more responsive to platinum-based chemotherapy [55,57,58,59]. A recent systematic review with meta-analysis [60] showed a benefit in PFS and overall survival (OS) for both BRCA1 (PFS HR: 0.68, 95% CI: 0.52–0.89; OS HR: 0.73, 95% CI: 0.63–0.86) and BRCA2 (PFS HR: 0.48, 95% CI: 0.30–0.75; OS HR: 0.57, 95% CI: 0.45–0.73) mutations’ carriers, compared with BRCA wild type patients. On the other hand, BRCA-mutated OC has a greater predisposition to develop visceral metastases outside the pelvis than non-mutated tumors [61]. A potential relationship between a positive family history for hereditary breast and OC and the risk of developing BMs in OC patients has been suggested by Jernigan et al. [62] However, few authors have extensively evaluated the role of HRD in BMs from OC. Ratner et al. [63], analyzing a cohort of 4515 OCs (473 BRCA mutated and 1679 BRCA wild type), found 46 patients who developed BMs (1%). Among the BRCA mutated patients, 3% (14/473) developed BMs, while within the BRCA wild type group, the BMs rate was 0.6% (10/1679). Moreover, BRCA mutated patients who developed BM had a lower mean age at primary tumor diagnosis (60 years vs. 63.5 years). The estimated HR for developing BMs in BRCA mutation carriers was 3.84 (95% CI: 1.60–9.22, p < 0.001), but no difference was observed between patients with or without BRCA mutations in terms of survival.
A recent study [64] explored the characteristics of 96 OC women who developed BMs, according to BRCA status (21 BRCA mutations carriers and 63 BRCA wild type patients). BRCA mutations carriers showed a better OS (29 months vs. 9 months), with an HR of 0.53 after stratifying for the presence of systemic disease (95% CI: 0.25–1.11, p = 0.09). The longer disease course of HRD patients may explain the higher predisposition to develop extra-abdominal disease, although in the previously reported study [63], patients with BRCA mutation developed BMs on average 8 months earlier than the BRCA wild type (median time 27 months versus 35 months) therefore, this assumption should be furtherly verified and the longer course of the disease in mutated patients may not be a relevant factor for the development of BMs.
3. Clinical Presentation and Diagnosis
The clinical presentation of BMs is variable, and depends on the location and the number of metastases. Overall, mild or severe headache is one of the most common symptoms and is present in up to 50% of cases. This finding typically occurs in patients with multiple metastases or with posterior fossa lesions, and can also be associated with papilledema (15–25%). Up to 40% of patients with BMs showed focal neurological deficits, while seizures occurred in 15–20% of cases. Confusion is also a common symptom, especially in patients with multiple metastases and/or in the case of intracranial hypertension [9,65].
Diagnosis of BMs from OC before or synchronous with the primary tumor is an extremely rare event [50], thus most diagnoses occur during the disease course. At presentation, most of the cases showed multiple metastatic lesions (58.1%) (Table 1), and were symptomatic at the time of BMs diagnosis. However, in one of the largest series reported so far, no symptoms were observed at diagnosis in about 26% of cases, [40] therefore, the diagnosis can also be incidental in a significant subgroup of patients. For this reason, some authors [40,48] have proposed to establish an active surveillance protocol aimed at high risk patients, as early detection may enable improved outcomes. The most commonly reported symptoms in BMs secondary to OC are headache, motor deficit/weakness, seizures, nausea and vomiting, dysphasia/aphasia, and vertigo (Table 1). The anatomical sites most frequently affected include cerebellum (30%) and frontal (20%), parietal (18%), and occipital (11%) lobes [50].
In patients with a history of OC, BMs can be suspected by consistent clinical and radiological (computed tomography—CT and/or magnetic resonance imaging—MRI) findings. For imaging, brain MRI is the technique of choice, because it has a higher resolution and allows one to study the posterior fossa and the presence of leptomeningeal disease more accurately than CT [9,26,66]. BMs from OC can also be occasionally detected by Positron emission tomography (PET)/PET-CT [67], but this technique does not provide a spatial resolution comparable with MRI.
Regarding circulating biomarkers, the role of CA-125 in the diagnosis and management of BMs from OC is unclear. CA-125, at the time of diagnosis of BMs, was positive in 58.5% of cases, but this marker is obviously affected by systemic disease status, and many patients with BMs also had an active extra-cranial disease, thus its real relevance to detect BMs cannot be inferred. Furthermore, CA-125 values were not found to be related to the time interval between the primary OC and the diagnosis of BMs. Other biomarkers, such as CA 72-4, lactate dehydrogenase (LDH), epithelial membrane antigen (EMA), chromogranin, CD-56, pan-CK, along with human chorionic gonadotropin (HCG) and alpha fetoprotein (AFP), have been rarely studied, and no correlation with BMs has been reported [50]. Finally, data on the role of human epidydimal protein 4 (HE4) are not available.
4. Pathology and Molecular Profiling
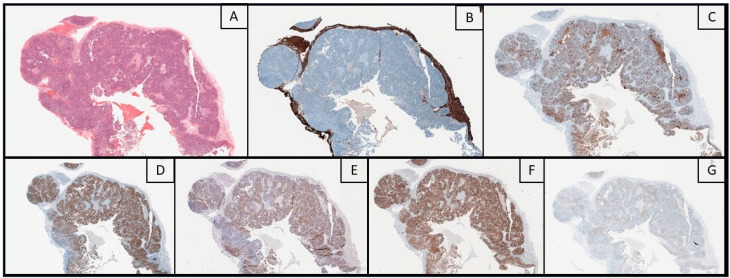
Histology is usually consistent with the primary tumor. In BMs from serous high-grade serous OC (HGSC), markedly atypical epithelial cells with pleomorphic hyperchromatic nuclei and prominent nucleoli can be observed within a papillary architecture [68], but, despite the presence of specific histopathological features, reaching a conclusive diagnosis can be challenging. For instance, in patients with multiple primary tumors, or if the primary OC is still undiagnosed, a careful evaluation is warranted, since morphological patterns and immunohistochemical (IHC) markers can overlap with other tumor entities. Nafisi et al. proposed a 6-step diagnostic flowchart, taking also into account the differential diagnosis between HGSC, clear cell (CCC) and endometrioid (EC) carcinoma. Other than clinical history, IHC positivity for CK7, ER and, paired box 8 (PAX-8) matched with negativity for CK20, support the Mullerian origin of the tumor. This pattern is reliable for any type of ovarian metastasis, although the expression of the single markers can vary among the different histotypes. Strong and diffuse positivity for Wilms tumor 1 (WT1), together with high expression of p53 and p16, point towards HGSC; conversely, WT1 negativity represents a clue for other tumor entities like CCC and EC, which are usually ER-negative and positive, respectively [69]. Moreover, it should be stressed that correlation with clinical and radiological findings is always extremely important to avoid diagnostic pitfalls, especially in patients without a previous oncological history. An example of histological features of BMs from OC is shown in Figure 1.
Figure 1.
Histological images (original magnification: 20X) of a brain metastasis from ovarian cancer. Hematoxylin-eosin stain (A) shows a papillary carcinoma with hyperchromic nuclei and prominent nucleoli, surrounded by GFAP-positive brain parenchyma (B). Immunohistochemistry showed a consistent profile: positive stainings for CA-125 (C), Estrogen Receptors (D), Wilms Tumor-1 (E) and PAX-8 (F) and, focally, for Androgen Receptors (G).
Regarding molecular profiling, Masoodi et al. evaluated the primary tumor and matched metastases of six patients with OC, showing branching evolution patterns among the primary tumor cells of high-grade serous OC, whereas linear and parallel metastatic progression was observed in 67.3% and 33.7% of cases, respectively [70]. Increased single nucleotide variations and loss of heterozygosity events and more extensive copy-number alterations were also reported in metastatic disease [70,71]. Concerning specific genes, BRCA1 resulted in being the most commonly altered gene in OC BMs [72]. A next-generation sequencing-based genomic analysis of eight OC BMs exhibited BRCA1/2 mutations in 7/8 cases, and all samples showed mutations in at least one gene involved in DNA repair (BRCA1/2, ATM, CHEK2) [72]. Furthermore, two authors demonstrated a high prevalence of BRCA1 protein loss in patients with BMs from OC [73,74].
Differences have been observed between primary tumors and metastatic lesions [75] in terms of transcriptomic profiles, but conflicting data have also been reported [76]. Regarding specific markers, Matsuo et al. found a higher expression of MDR1 in primitive ovarian cancers which then developed BMs and in the BMs themselves; this finding has therapeutic relevance, since a higher MDR1 expression is associated with resistance to chemotherapy drugs like paclitaxel [77]. Choi et al. reported a higher PD-L1 expression in brain metastasis compared to primary tumors [71] with potential implications for immunotherapy [78]. Indeed, the immune system seems to play a significant role in shaping OC metastasis development: a higher expression of IL7R, probably related to T cells activation and infiltration, was found in OC metastases, as well as differences in CALB2, CYP1B1, EFTUD1, RARRES2 and TIMP3 expressions [79]. Taken together, these genes have been suggested as a potential signature to distinguish metastatic lesions from primary cancers, as well as a potential tool to predict the metastatic risk. Furthermore, a higher expression of MYC, IRF1, BCL2L2, TNFSF10 was reported in metastasis, suggesting an anti-apoptotic and proliferative behavior. AXIN2, DKK2, NKD1/2 also resulted in being highly expressed [79], supporting the WNT-β-catenin pathway activation [80,81], and similar results were reported for the JAK-STAT and NOTCH pathways [79].
Finally, as previously anticipated, BMs show a reduction in the expression of hormonal receptors compared to the matched primary tumors, in particular PgR and AR. This finding suggests a potential "dedifferentiation" of neoplastic cells during the BMs development [42].
Proteomic analyses could also be exploited to identify novel potential therapeutic targets for OC BMs. For instance, Yoshida et al. identified a wide range of differentially expressed proteins between OC primary tumor and BMs. Among these, a strong signal was found for alpha-enolase, triosephosphate isomerase and transgelin-2 [82].
Lastly, considering the growing importance of extensive molecular profiling to tailor patients’ treatments, liquid biopsy approaches could be applied to overcome the limitations in sampling CNS lesions. Although the analysis of circulating tumor DNA (ctDNA) or of other nucleic acids it is not commonly used in clinical practice for sampling BMs from OC, multiple reports [83,84] showed the possibility to gather informative data in multiple types of primary and secondary CNS tumors; thus, this possibility could be investigated in future studies targeting OC BMs.
5. Treatments
5.1. Radiotherapy
Radiotherapy is one of the main treatment options for BMs. Whole brain radiotherapy (WBRT) was considered as the best approach for many years, since it allows one to target both macro- and microscopic lesions, allowing one to achieve good local disease control [9,85]. Nowadays, WBRT can still represent the first option for the treatment of BMs from OC in the presence of multiple lesions, with or without extra-cranial disease [50]. A recent study on 21 patients showed that WBRT alone had a positive effect on survival compared to regimens that did not include WBRT: 45 months (95% CI 35–54.9) versus 19 months (95% CI 11.1–26.8,) (p < 0.001), with an HR of 0.152 (95% CI 0.033–0.695, p = 0.015) [47]. In a large series of 72 patients, Cohen et al. [22] reported that WBRT in combination with surgery showed a more favorable survival (median 23 months) than WBRT (median 5.33 months) or surgery (median 6.9 months) alone (p < 0.01). However, WBRT is burdened by significant side effects, such as fatigue, somnolence, and memory impairments, and a literature review estimated a poor median survival with exclusive WBRT (3–6 months, range 1.5–27) [66].
Gamma knife radio surgery (GKRS) is an alternative option that provides satisfactory results, especially in patients with single metastases, severe comorbidity, or who cannot undergo surgical resection. Lee et al. [28] compared the outcomes of 7 patients treated with GKRS with 8 patients treated with WBRT as the primary treatment modality. GKRS-treated patients showed a better survival (median, 29 months) compared to WBRT (median, 6 months) (p = 0.00061), regardless of the number of metastases. Superior survival outcomes in patients treated with GKRS were also reported in a number of small retrospective series [26,34].
5.2. Surgery
Surgical excision of BMs can be considered as an alternative to radiotherapy and may be useful in selected patients, to control intracranial hypertension, symptoms, and improve survival. Furthermore, brain surgery allows one to obtain tissue for histopathological analysis and for the identification of prognostic and predictive markers [9].
Several authors reported better results in terms of local control and survival compared with chemotherapy and radiotherapy alone [20,25,49,50,86]. A literature review showed that surgery combined with radiotherapy +/− chemotherapy resulted in better outcomes (median OS 21.8 and 20.15 months) compared with surgery (6.5 months) or radiotherapy alone (5.4 months) [66].
More recently, a case series of 12 patients including an analysis of 20 studies conducted by Niu et al. [36] reported higher survival rates in patients treated with GKRS plus surgical excision, compared to those who did not receive this treatment (25 months vs. 6 months, p < 0.001).
Overall, patients with good general conditions (KPS > 70), a single BM and no extra-CNS localizations seem to be the best candidates for surgical therapy [9,20,87], but selected patients with extra-cranial disease or multiple brain metastasis can also benefit from surgery in terms of symptoms and quality of life improvements [66].
5.3. Chemotherapy with Cytotoxic Agents
Despite the significant advancements achieved in the treatment of solid malignancies and the advent of the precision medicine era, the cornerstone of neoadjuvant and adjuvant treatment of OC remains the intravenous administration of carboplatin plus paclitaxel every 3 weeks [88,89]. Regarding recurrent disease, in case of a ≥ 6 months therapy-free interval as defined by the Gynecologic Cancer Intergroup [90], a platinum-based re-challenge therapy can be proposed, or, in the case of platinum-refractory relapses, other cytotoxic agents such as pegylated liposomal doxorubicin (PLD), topotecan, gemcitabine, trabectedin or weekly paclitaxel, eventually with the addition of an anti-angiogenetic drug, can be considered [89,91,92].
The role of chemotherapy with cytotoxic agents in BMs treatment is controversial. Indeed, the major limitation to its use is linked to the difficulty of many drugs to cross the blood-brain barrier (BBB) and reach adequate therapeutic concentrations within the CNS. Furthermore, the addition of chemotherapy to WBRT did not always provide survival benefits in trials investigating this issue [93].
Platinum-based chemotherapy (carboplatin and cisplatin) represents the cornerstone of OC medical treatment and can be used in both primary and secondary brain tumors thanks to its ability to cross the BBB [94,95]. However, data about the role of chemotherapy on BMs from OC are scant. Some BMs remissions have been achieved in patients undergoing platinum-based regimen, with or without docetaxel, but these results remain anecdotal [15,96,97,98,99]. In addition, in patients treated with exclusive systemic chemotherapy, the median survival remains poor (2.5 to 7 months) [100]. As mentioned above, the prognostic role for BMs of previous platinum sensitivity is not yet defined, however, this aspect must be considered when attempting a re-challenge with platinum-based chemotherapy.
If platinum-based chemotherapy is not possible, other drugs capable of crossing the BBB, such as topotecan [101] and gemcitabine [102] (high concentration in the brain was reported, particularly after radiotherapy for glioblastoma or in combination with other chemotherapeutic agents), can be considered. Regarding anthracyclines, the glutathione PLD can reach a brain-to-blood ratio 4.8-fold higher compared to the uncoated PLD [103]. Conversely, the ability of trabectedin to cross the BBB is currently unknown.
Several new techniques are being proposed to increase the permeability of BBB and to selectively kill OC cells without affecting the surrounding healthy tissues: similar approaches could also be experimented to optimize treatment of BMs [104,105,106].
5.4. Trimodal Approach
Considering the overall advantages and disadvantages of the available treatment option, it should be noted that the best survival results for BMs from OC have been obtained with a trimodal therapy (radiotherapy, surgery, and chemotherapy), while monotherapy is associated with poor survival (HR: 2.57, 95% CI: 1.64–3.86) [40]. Pakneshan et al. [50] reported a significant improvement in survival (median OS 20.5 months) in patients treated with this approach. Similar results were observed by Anupol et al. [16] (median OS 20.5 months), Chiang et al. [33] (median OS 30 months), and Marchetti et al. [40] (median OS 24 months).
5.5. PARP Inhibitors
Poly (ADP) ribose polymerase (PARP) is a family of nuclear proteins involved in the repair of single-strand DNA breaks through a process known as PARylation. In particular, PARP-1 is the most common PARP isoform, and in the majority of tissues it accounts for about 90% of total cellular PARP activity [107,108]. Several inhibitors of the poly ADP ribose polymerase (PARPi) have allowed an increase in OC PFS, and have been approved by the Food and Drugs Administration (FDA) for treatment maintenance after adjuvant chemotherapy and platinum-sensitive recurrence. Such inhibitors include Olaparib for BRCA mutation carriers, Niraparib and Rucaparib for OC regardless the BRCA status [109,110,111,112]. PARPi have the ability to cross the BBB [113,114,115], and are currently under investigation for the treatment of BMs in triple-negative breast cancer [116,117]. PARPi could also play a role in the treatment of BMs from OC, and, despite the current lack of data in a large series of patients, three cases have been reported:
- a 61-year-old woman with BRCA2 mutation and diffuse leptomeningeal disease from a high-grade serous OC showed a good clinical response associated with improved symptoms and quality of life, after been treated with Olaparib. Disease progression was observed 12 months after starting the PARPi treatment [118].
- a 58-year-old woman with BRCA1 mutation and multiple BMs from high-grade serous OC achieved a complete response after a 21-month treatment with Olaparib [119].
- a 68-year-old woman with BM from high-grade serous OC was treated with Niraparib as maintenance therapy and remained free of disease progression for more than 17 months [120].
Although these cases remain anecdotal, the results are encouraging, and there is a strong preclinical rationale supporting the use of PARPi also in these patients, especially in BRCA mutated tumors.
5.6. Immunotherapy
It is well-known that the interactions between the tumor and the immune system play a fundamental role in cancer progression as well as treatment. In fact, the inhibition of T cell checkpoint molecules such as programmed cell death protein 1 (PD-1), its ligand (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) using monoclonal antibodies (immune checkpoint inhibitors (ICIs)) has changed the natural history of multiple solid malignancies, including melanoma, non-small cell lung cancer (NSCLC), and renal cell cancer [121]. However, initial data on the use of ICIs for OC treatment have shown disappointing results, with median response rates of 10–15% [122,123,124,125,126,127]. A study on NSCLC derived BMs suggested a higher expression of PD-L1 compared with the matched primary tumor [128]. Furthermore, a work of the same group investigating the role of tumor-infiltrating lymphocytes (TILs) in a series of BMs derived from melanoma, lung, breast and renal cancer showed that the presence of dense TILs infiltrates is common and related with survival [129]. These findings suggest a role of immunotherapy in the treatment of BMs [130]. Currently, the immunological profile of BMs from OC has been investigated in 2 cases only, and, as previously mentioned, these brain lesions showed a high mutational burden and increased PD-L1 expression compared with primary tumors [71]. In fact, some data suggested a role of features like the clear cell histotype, PD-L1 expression by cancer cells, BRCA status, microsatellite instability, and tumor mutational burden in predicting OC response to immunotherapy [131]. These markers could help to identify patients who might benefit from immunotherapy and thus be evaluated in future studies focused on OC BMs. Finally, there is a strong rationale supporting the use of radiotherapy to enhance the response to immune checkpoint inhibitors in OC treatment [132]. This combination showed synergism in the treatment of small series of patients affected by BMs from melanoma [133,134], suggesting a potential therapeutic efficacy also for brain lesions from OC.
6. Conclusions
BMs from OC remain a rare event, and the overall quality of current evidence is limited, since it is mainly based on heterogeneous retrospective series.
No specific follow-up strategy for early identification of BMs is currently recommended in patients with a history of OC, although, as mentioned above, up to 26% may be asymptomatic at diagnosis. In this context, women with BRCA mutation and low AR expression in primary cancer could benefit from a targeted follow-up, as they are at greater risk of developing BMs, but prospective studies are warranted to establish reliable risk and prognostic factors for OC BMs.
Concerning treatments, a multimodal strategy (including surgery, radiotherapy and chemotherapy), if feasible, seems to be the best way to improve survival rates compared to other therapeutic approaches. Novel avenues are becoming available based PARPi and immunotherapy, but further studies are needed.
Author Contributions
Conceptualization: F.B., L.B.; Methodology: F.B., L.B.; Resources: S.C., A.C., D.K., M.P., S.G.; Literature research F.B., L.B., A.M., A.G., M.B., G.V., G.S.; Data curation, F.B., L.B., A.M.; Interpretation of data: all authors. Writing—original draft preparation: F.B., L.B., A.M.; Writing—review and editing: all authors; Supervision: P.C., C.B.; Project administration, P.C., C.B.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
G. Valabrega has received personal fees from Roche, AstraZeneca, Tesaro, PharmaMar and Amgen. The remaining authors have no conflicts of interest to declare.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Lengyel E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wasnik A.P., Maturen K.E., Kaza R.K., Al-Hawary M.M., Francis I.R. Primary and secondary disease of the peritoneum and mesentery: Review of anatomy and imaging features. Abdom. Imaging. 2015;40:626–642. doi: 10.1007/s00261-014-0232-8. [DOI] [PubMed] [Google Scholar]
- 4.Van Baal J., van Noorden C.J.F., Nieuwland R., Van de Vijver K.K., Sturk A., van Driel W.J., Kenter G.G., Lok C.A.R. Development of peritoneal carcinomatosis in epithelial ovarian cancer: A Review. J. Histochem. Cytochem. 2018;66:67–83. doi: 10.1369/0022155417742897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomakos N., Diakosavvas M., Machairiotis N., Fasoulakis Z., Zarogoulidis P., Rodolakis A. Rare distant metastatic disease of ovarian and peritoneal carcinomatosis: A review of the literature. Cancers. 2019;11:1044. doi: 10.3390/cancers11081044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pogge von Strandmann E., Reinartz S., Wager U., Muller R. Tumor-host cell interactions in ovarian cancer: Pathways to therapy failure. Trends Cancer. 2017;3:137–148. doi: 10.1016/j.trecan.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Nayak L., Lee E.Q., Wen P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 8.Suh J.H., Kotecha R., Chao S.T., Ahluwalia M.S., Sahgal A., Chang E.L. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 2020;17:279–299. doi: 10.1038/s41571-019-0320-3. [DOI] [PubMed] [Google Scholar]
- 9.Achrol A.S., Rennert R.C., Anders C., Soffietti R., Ahluwalia M.S., Nayak L., Peters S., Arvold N.D., Harsh G.R., Steeg P.S., et al. Brain metastases. Nat. Rev. Dis. Primers. 2019;5:5. doi: 10.1038/s41572-018-0055-y. [DOI] [PubMed] [Google Scholar]
- 10.Berghoff A.S., Schur S., Fureder L.M., Gatterbauer B., Dieckmann K., Widhalm G., Hainfellner J., Zielinski C.C., Birner P., Bartsch R., et al. Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open. 2016;1:e000024. doi: 10.1136/esmoopen-2015-000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeRoux P.D., Berger M.S., Elliott J.P., Tamimi H.K. Cerebral metastases from ovarian carcinoma. Cancer. 1991;67:2194–2199. doi: 10.1002/1097-0142(19910415)67:8<2194::AID-CNCR2820670832>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez G.C., Soper J.T., Berchuck A., Oleson J., Dodge R., Montana G., Clarke-Pearson D.L. Improved palliation of cerebral metastases in epithelial ovarian cancer using a combined modality approach including radiation therapy, chemotherapy, and surgery. J. Clin. Oncol. 1992;10:1553–1560. doi: 10.1200/JCO.1992.10.10.1553. [DOI] [PubMed] [Google Scholar]
- 13.Geisler J.P., Geisler H.E. Brain metastases in epithelial ovarian carcinoma. Gynecol. Oncol. 1995;57:246–249. doi: 10.1006/gyno.1995.1134. [DOI] [PubMed] [Google Scholar]
- 14.Corn B.W., Greven K.M., Randall M.E., Wolfson A.H., Kim R.Y., Lanciano R.M. The efficacy of cranial irradiation in ovarian cancer metastatic to the brain: Analysis of 32 cases. Obstet. Gynecol. 1995;86:955–959. doi: 10.1016/0029-7844(95)00320-Q. [DOI] [PubMed] [Google Scholar]
- 15.Cormio G., Maneo A., Parma G., Pittelli M.R., Miceli M.D., Bonazzi C. Central nervous system metastases in patients with ovarian carcinoma. A report of 23 cases and a literature review. Ann. Oncol. 1995;6:571–574. doi: 10.1093/oxfordjournals.annonc.a059246. [DOI] [PubMed] [Google Scholar]
- 16.Anupol N., Ghamande S., Odunsi K., Driscoll D., Lele S. Evaluation of prognostic factors and treatment modalities in ovarian cancer patients with brain metastases. Gynecol. Oncol. 2002;85:487–492. doi: 10.1006/gyno.2002.6653. [DOI] [PubMed] [Google Scholar]
- 17.Pothuri B., Chi D.S., Reid T., Aghajanian C., Venkatraman E., Alektiar K., Bilsky M., Barakat R.R. Craniotomy for central nervous system metastases in epithelial ovarian carcinoma. Gynecol. Oncol. 2002;87:133–137. doi: 10.1006/gyno.2002.6792. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson A., Bonington S.C., Carrington B.M., Alison D.L., Spencer J.A. Cerebral metastasis and other cerebral events in women with ovarian cancer. Clin. Radiol. 2002;57:815–819. doi: 10.1053/crad.2001.0965. [DOI] [PubMed] [Google Scholar]
- 19.Kolomainen D.F., Larkin J.M., Badran M., A’Hern R.P., King D.M., Fisher C., Bridges J.E., Blake P.R., Barton D.P., Shepherd J.H., et al. Epithelial ovarian cancer metastasizing to the brain: A late manifestation of the disease with an increasing incidence. J. Clin. Oncol. 2002;20:982–986. doi: 10.1200/JCO.2002.20.4.982. [DOI] [PubMed] [Google Scholar]
- 20.Cormio G., Maneo A., Colamaria A., Loverro G., Lissoni A., Selvaggi L. Surgical resection of solitary brain metastasis from ovarian carcinoma: An analysis of 22 cases. Gynecol. Oncol. 2003;89:116–119. doi: 10.1016/S0090-8258(03)00060-X. [DOI] [PubMed] [Google Scholar]
- 21.Kumar L., Barge S., Mahapatra A.K., Thulkar S., Rath G.K., Kumar S., Mishra R., Dawar R., Singh R. Central nervous system metastases from primary epithelial ovarian cancer. Cancer Control. 2003;10:244–253. doi: 10.1177/107327480301000309. [DOI] [PubMed] [Google Scholar]
- 22.Cohen Z.R., Suki D., Weinberg J.S., Marmor E., Lang F.F., Gershenson D.M., Sawaya R. Brain metastases in patients with ovarian carcinoma: Prognostic factors and outcome. J. Neuro-Oncol. 2004;66:313–325. doi: 10.1023/B:NEON.0000014516.04943.38. [DOI] [PubMed] [Google Scholar]
- 23.Pectasides D., Aravantinos G., Fountzilas G., Kalofonos C., Efstathiou E., Karina M., Pavlidis N., Farmakis D., Economopoulos T., Dimopoulos M.A. Brain metastases from epithelial ovarian cancer. The Hellenic Cooperative Oncol.ogy Group (HeCOG) experience and review of the literature. Anticancer Res. 2005;25:3553–3558. [PubMed] [Google Scholar]
- 24.D’Andrea G., Roperto R., Dinia L., Caroli E., Salvati M., Ferrante L. Solitary cerebral metastases from ovarian epithelial carcinoma: 11 cases. Neurosurg. Rev. 2005;28:120–123. doi: 10.1007/s10143-004-0363-4. [DOI] [PubMed] [Google Scholar]
- 25.Chen P.G., Lee S.Y., Barnett G.H., Vogelbaum M.A., Saxton J.P., Fleming P.A., Suh J.H. Use of the Radiation Therapy Oncol.ogy Group recursive partitioning analysis classification system and predictors of survival in 19 women with brain metastases from ovarian carcinoma. Cancer. 2005;104:2174–2180. doi: 10.1002/cncr.21472. [DOI] [PubMed] [Google Scholar]
- 26.Kim T.J., Song S., Kim C.K., Kim W.Y., Choi C.H., Lee J.H., Lee J.W., Bae D.S., Kim B.G. Prognostic factors associated with brain metastases from epithelial ovarian carcinoma. Int. J. Gynecol. Cancer. 2007;17:1252–1257. doi: 10.1111/j.1525-1438.2007.00941.x. [DOI] [PubMed] [Google Scholar]
- 27.Gadducci A., Tana R., Teti G., Fanucchi A., Pasqualetti F., Cionini L., Genazzani A.R. Brain recurrences in patients with ovarian cancer: Report of 12 cases and review of the literature. Anticancer Res. 2007;27:4403–4409. [PubMed] [Google Scholar]
- 28.Lee Y.K., Park N.H., Kim J.W., Song Y.S., Kang S.B., Lee H.P. Gamma-knife radiosurgery as an optimal treatment modality for brain metastases from epithelial ovarian cancer. Gynecol. Oncol. 2008;108:505–509. doi: 10.1016/j.ygyno.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Ratner E.S., Toy E., O’Malley D.M., McAlpine J., Rutherford T.J., Azodi M., Higgins S.A., Schwartz P.E. Brain metastases in epithelial ovarian and primary peritoneal carcinoma. Int. J. Gynecol. Cancer. 2009;19:856–859. doi: 10.1111/IGC.0b013e3181a83301. [DOI] [PubMed] [Google Scholar]
- 30.Sehouli J., Pietzner K., Harter P., Munstedt K., Mahner S., Hasenburg A., Camara O., Wimberger P., Boehmer D., Buehling K.J., et al. Prognostic role of platinum sensitivity in patients with brain metastases from ovarian cancer: Results of a German multicenter study. Ann. Oncol. 2010;21:2201–2205. doi: 10.1093/annonc/mdq229. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y.L., Cheng W.F., Hsieh C.Y., Chen C.A. Brain metastasis as a late manifestation of ovarian carcinoma. Eur J. Cancer Care. 2011;20:44–49. doi: 10.1111/j.1365-2354.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 32.Cormio G., Loizzi V., Falagario M., Calace A., Colamaria A., De Tommasi A., Selvaggi L.E. Central nervous system metastases from epithelial ovarian cancer: Prognostic factors and outcomes. Int. J. Gynecol. Cancer. 2011;21:816–821. doi: 10.1097/IGC.0b013e318216cad0. [DOI] [PubMed] [Google Scholar]
- 33.Chiang Y.C., Qiu J.T., Chang C.L., Wang P.H., Ho C.M., Lin W.C., Huang Y.F., Lin H., Lu C.H., Chou C.Y. Brain metastases from epithelial ovarian carcinoma: Evaluation of prognosis and managements—A Taiwanese Gynecologic Oncology Group (TGOG) study. Gynecol. Oncol. 2012;125:37–41. doi: 10.1016/j.ygyno.2011.12.438. [DOI] [PubMed] [Google Scholar]
- 34.Ogino A., Hirai T., Fukushima T., Serizawa T., Watanabe T., Yoshino A., Katayama Y. Gamma knife surgery for brain metastases from ovarian cancer. Acta Neurochir. 2012;154:1669–1677. doi: 10.1007/s00701-012-1376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasu K., Satoh T., Nishio S., Nagai Y., Ito K., Otsuki T., Hongo A., Hirashima Y., Ogura T., Shimada M. Clinicopathologic features of brain metastases from Gynecologic malignancies: A retrospective study of 139 cases (KCOG-G1001s trial) Gynecol. Oncol. 2013;128:198–203. doi: 10.1016/j.ygyno.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Niu X., Rajanbabu A., Delisle M., Peng F., Vijaykumar D.K., Pavithran K., Feng Y., Lau S., Gotlieb W.H., Press J.Z. Brain metastases in women with epithelial ovarian cancer: Multimodal treatment including surgery or gamma-knife radiation is associated with prolonged survival. J. Obstet. Gynaecol. Can. 2013;35:816–822. doi: 10.1016/S1701-2163(15)30838-0. [DOI] [PubMed] [Google Scholar]
- 37.Teckie S., Makker V., Tabar V., Alektiar K., Aghajanian C., Hensley M., Beal K. Radiation therapy for epithelial ovarian cancer brain metastases: Clinical outcomes and predictors of survival. Radiat. Oncol. 2013;8:36. doi: 10.1186/1748-717X-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gressel G.M., Lundsberg L.S., Altwerger G., Katchi T., Azodi M., Schwartz P.E., Ratner E.S., Damast S. Factors predictive of improved survival in patients with brain metastases from gynecologic cancer: A single institution retrospective study of 47 cases and review of the literature. Int. J. Gynecol. Cancer. 2015;25:1711–1716. doi: 10.1097/IGC.0000000000000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilani M.A., Williams N.L., Giordano C., Rosenblum N., Shi W., Anne P., Schilder R.J. Brain Metastases in Patients with Gynecologic Cancers: A Single Institution Experience and Review of the Literature. Open J. Obstet. Gynecol. 2016;6:544–552. doi: 10.4236/ojog.2016.69070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marchetti C., Ferrandina G., Cormio G., Gambino A., Cecere S., Lorusso D., De Giorgi U., Bogliolo S., Fagotti A., Mammoliti S., et al. Brain metastases in patients with EOC: Clinico-pathological and prognostic factors. A multicentric retrospective analysis from the MITO group (MITO 19) Gynecol. Oncol. 2016;143:532–538. doi: 10.1016/j.ygyno.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 41.Matsunaga S., Shuto T., Sato M. Gamma Knife Surgery for Metastatic Brain Tumors from Gynecologic Cancer. World Neurosurg. 2016;89:455–463. doi: 10.1016/j.wneu.2016.01.062. [DOI] [PubMed] [Google Scholar]
- 42.Mittica G., Senetta R., Scotto G., Aglietta M., Maggiorotto F., Ghisoni E., Genta S., Boldorini R., Manini C., Morra I., et al. Androgen receptor status predicts development of brain metastases in ovarian cancers. Oncotarget. 2017;8:41143–41153. doi: 10.18632/oncotarget.17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seber S., Turkmen E., Harputoglu H., Yesil H., Arpaciota E., Menekse S., Pilanciota K., Oruc Z., Taskoylu B.Y., Gumusay O., et al. Central nervous system metastatic epithelial ovarian cancer. Clinical parameters and prognostic factors: A multicenter study of Anatolian Society of Medical Oncol.ogy. Eur. J. Gynaecol. Oncol. 2017;38:227–231. [PubMed] [Google Scholar]
- 44.Kwon J.W., Yoon J.H., Lim M.C., Joo J., Yoo H., Shin S.H., Park S.Y., Lee S.H., Kim Y.J., Kim J.Y., et al. Treatment results and prognostic factors of brain metastases from ovarian cancer: A single institutional experience of 56 patients. Int. J. Gynecol. Cancer. 2018;28:1631–1638. doi: 10.1097/IGC.0000000000001341. [DOI] [PubMed] [Google Scholar]
- 45.Wohl A., Kimchi G., Korach J., Perri T., Zach L., Zibly Z., Harel R., Nissim U., Spiegelmann R., Nass D., et al. Brain metastases from ovarian carcinoma: An evaluation of prognostic factors and treatment. Neurol. India. 2019;67:1431–1436. doi: 10.4103/0028-3886.273627. [DOI] [PubMed] [Google Scholar]
- 46.Da Costa A., Dos Santos E.S., Cotrim D.P., Pandolfi N.C., Cesca M.G., Mantoan H., Sanches S.M., Ribeiro A.R.G., de Brot L., Bonvolim G., et al. Prognostic impact of platinum sensitivity in ovarian carcinoma patients with brain metastasis. BMC Cancer. 2019;19:1194. doi: 10.1186/s12885-019-6382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keskin S., Kucucuk S., Ak N., Atalar B., Sari M., Sozen H., Ibis K., Topuz S., Saip P. Survival impact of optimal surgical cytoreduction in recurrent epithelial ovarian cancer with brain metastasis. Oncol. Res. Treat. 2019;42:101–106. doi: 10.1159/000494334. [DOI] [PubMed] [Google Scholar]
- 48.Mittica G., Goia M., Gambino A., Scotto G., Fonte M., Senetta R., Aglietta M., Borella F., Sapino A., Katsaros D., et al. Validation of Androgen Receptor loss as a risk factor for the development of brain metastases from ovarian cancers. J. Ovarian Res. 2020;13:53. doi: 10.1186/s13048-020-00655-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piura E., Piura B. Brain metastases from ovarian carcinoma. ISRN Oncol. 2011;2011:527453. doi: 10.5402/2011/527453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pakneshan S., Safarpour D., Tavassoli F., Jabbari B. Brain metastasis from ovarian cancer: A systematic review. J. Neuro-Oncol. 2014;119:1–6. doi: 10.1007/s11060-014-1447-9. [DOI] [PubMed] [Google Scholar]
- 51.Gardner A.B., Charo L.M., Mann A.K., Kapp D.S., Eskander R.N., Chan J.K. Ovarian, uterine, and cervical cancer patients with distant metastases at diagnosis: Most common locations and outcomes. Clin. Exp. Metastasis. 2020;37:107–113. doi: 10.1007/s10585-019-10007-0. [DOI] [PubMed] [Google Scholar]
- 52.Koniali L., Hadjisavvas A., Constantinidou A., Christodoulou K., Christou Y., Demetriou C., Panayides A.S., Pitris C., Pattichis C.S., Zamba-Papanicolaou E., et al. Risk factors for breast cancer brain metastases: A systematic review. Oncotarget. 2020;11:650–669. doi: 10.18632/oncotarget.27453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu M., Yuan Y., Yan P., Jiang J., Ma P., Niu X., Ma S., Cai H., Yang K. Prognostic significance of androgen receptor expression in triple negative breast cancer: A systematic review and meta-analysis. Clin. Breast Cancer. 2020 doi: 10.1016/j.clbc.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Walsh C.S. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol. Oncol. 2015;137:343–350. doi: 10.1016/j.ygyno.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 55.Yang D., Khan S., Sun Y., Hess K., Shmulevich I., Sood A.K., Zhang W. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–1565. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vencken P.M., Kriege M., Hoogwerf D., Beugelink S., van der Burg M.E., Hooning M.J., Berns E.M., Jager A., Collee M., Burger C.W., et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann. Oncol. 2011;22:1346–1352. doi: 10.1093/annonc/mdq628. [DOI] [PubMed] [Google Scholar]
- 58.Dann R.B., DeLoia J.A., Timms K.M., Zorn K.K., Potter J., Flake D.D., 2nd, Lanchbury J.S., Krivak T.C. BRCA1/2 mutations and expression: Response to platinum chemotherapy in patients with advanced stage epithelial ovarian cancer. Gynecol. Oncol. 2012;125:677–682. doi: 10.1016/j.ygyno.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Madariaga A., Lheureux S., Oza A.M. Tailoring ovarian cancer treatment: Implications of BRCA1/2 mutations. Cancers. 2019;11:416. doi: 10.3390/cancers11030416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu K., Yang S., Zhao Y. Prognostic significance of BRCA mutations in ovarian cancer: An updated systematic review with meta-analysis. Oncotarget. 2017;8:285–302. doi: 10.18632/oncotarget.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gourley C., Michie C.O., Roxburgh P., Yap T.A., Harden S., Paul J., Ragupathy K., Todd R., Petty R., Reed N., et al. Increased incidence of visceral metastases in scottish patients with BRCA1/2-defective ovarian cancer: An extension of the ovarian BRCAness phenotype. J. Clin. Oncol. 2010;28:2505–2511. doi: 10.1200/JCO.2009.25.1082. [DOI] [PubMed] [Google Scholar]
- 62.Jernigan A.M., Mahdi H., Rose P.G. Epithelial ovarian cancer metastatic to the central nervous system and a family history concerning for hereditary breast and ovarian cancer—A Potential Relationship. Int. J. Gynecol. Cancer. 2015;25:1232–1238. doi: 10.1097/IGC.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 63.Ratner E., Bala M., Louie-Gao M., Aydin E., Hazard S., Brastianos P.K. Increased risk of brain metastases in ovarian cancer patients with BRCA mutations. Gynecol. Oncol. 2019;153:568–573. doi: 10.1016/j.ygyno.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Stasenko M., Cybulska P., Feit N., Makker V., Konner J., O’Cearbhaill R.E., Alektiar K.M., Beal K., Gardner G.J., Long Roche K.C., et al. Brain metastasis in epithelial ovarian cancer by BRCA1/2 mutation status. Gynecol. Oncol. 2019;154:144–149. doi: 10.1016/j.ygyno.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soffietti R., Ducati A., Ruda R. Brain metastases. Handb. Clin. Neurol. 2012;105:747–755. doi: 10.1016/b978-0-444-53502-3.00021-5. [DOI] [PubMed] [Google Scholar]
- 66.Pectasides D., Pectasides M., Economopoulos T. Brain metastases from epithelial ovarian cancer: A review of the literature. Oncologist. 2006;11:252–260. doi: 10.1634/theoncologist.11-3-252. [DOI] [PubMed] [Google Scholar]
- 67.Cengiz A., Koc Z.P., Ozcan Kara P., Yurekli Y. The role of (18)F-FDG PET/CT in detecting ovarian cancer recurrence in patients with elevated CA-125 levels. Mol. Imaging Radionucl. Ther. 2019;28:8–14. doi: 10.4274/mirt.galenos.2018.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurman R.J., Hedrick Ellenson L., Ronnett B.M., editors. Blaustein’s Pathology of the Female Genital Tract. Springer; Cham, Switzerland: 2019. [Google Scholar]
- 69.Nafisi H., Cesari M., Karamchandani J., Balasubramaniam G., Keith J.L. Metastatic ovarian carcinoma to the brain: An approach to identification and classification for neuropathologists. Neuropathology. 2015;35:122–129. doi: 10.1111/neup.12172. [DOI] [PubMed] [Google Scholar]
- 70.Masoodi T., Siraj S., Siraj A.K., Azam S., Qadri Z., Parvathareddy S.K., Tulbah A., Al-Dayel F., AlHusaini H., AlOmar O., et al. Genetic heterogeneity and evolutionary history of high-grade ovarian carcinoma and matched distant metastases. Br. J. Cancer. 2020;122:1219–1230. doi: 10.1038/s41416-020-0763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi Y.J., Kim S.Y., Park H.C., Chung Y.J., Hur S.Y., Lee S.H. Integrative immunologic and genomic characterization of brain metastasis from ovarian/peritoneal cancer. Pathol. Res. Pract. 2019;215:152404. doi: 10.1016/j.prp.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 72.Balendran S., Liebmann-Reindl S., Berghoff A.S., Reischer T., Popitsch N., Geier C.B., Kenner L., Birner P., Streubel B., Preusser M. Next-Generation Sequencing-based genomic profiling of brain metastases of primary ovarian cancer identifies high number of BRCA-mutations. J. NeuroOncol. 2017;133:469–476. doi: 10.1007/s11060-017-2459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szarszewska M., Markowska A., Jach R., Marszalek A., Filas V., Bednarek W., Olejek A., Tomczak P., Sajdak S., Nowak-Markwitz E., et al. Significance of BRCA1 expression in breast and ovarian cancer patients with brain metastasis—A multicentre study. Adv. Med. Sci. 2019;64:235–240. doi: 10.1016/j.advms.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 74.Sekine M., Yoshihara K., Komata D., Haino K., Nishino K., Tanaka K. Increased incidence of brain metastases in BRCA1-related ovarian cancers. J. Obstet. Gynaecol. Res. 2013;39:292–296. doi: 10.1111/j.1447-0756.2012.01961.x. [DOI] [PubMed] [Google Scholar]
- 75.Lancaster J.M., Dressman H.K., Clarke J.P., Sayer R.A., Martino M.A., Cragun J.M., Henriott A.H., Gray J., Sutphen R., Elahi A., et al. Identification of genes associated with ovarian cancer metastasis using microarray expression analysis. Int. J. Gynecol. Cancer. 2006;16:1733–1745. doi: 10.1111/j.1525-1438.2006.00660.x. [DOI] [PubMed] [Google Scholar]
- 76.Adib T.R., Henderson S., Perrett C., Hewitt D., Bourmpoulia D., Ledermann J., Boshoff C. Predicting biomarkers for ovarian cancer using gene-expression microarrays. Br. J. Cancer. 2004;90:686–692. doi: 10.1038/sj.bjc.6601603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuo K., Eno M.L., Ahn E.H., Shahzad M.M., Im D.D., Rosenshein N.B., Sood A.K. Multidrug resistance gene (MDR-1) and risk of brain metastasis in epithelial ovarian, fallopian tube, and peritoneal cancer. Am. J. Clin. Oncol. 2011;34:488–493. doi: 10.1097/COC.0b013e3181ec5f4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun L., Zhang L., Yu J., Zhang Y., Pang X., Ma C., Shen M., Ruan S., Wasan H.S., Qiu S. Clinical efficacy and safety of anti-PD-1/PD-L1 inhibitors for the treatment of advanced or metastatic cancer: A systematic review and meta-analysis. Sci. Rep. 2020;10:2083. doi: 10.1038/s41598-020-58674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brodsky A.S., Fischer A., Miller D.H., Vang S., MacLaughlan S., Wu H.T., Yu J., Steinhoff M., Collins C., Smith P.J., et al. Expression profiling of primary and metastatic ovarian tumors reveals differences indicative of aggressive disease. PLoS ONE. 2014;9:e94476. doi: 10.1371/journal.pone.0094476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bitler B.G., Nicodemus J.P., Li H., Cai Q., Wu H., Hua X., Li T., Birrer M.J., Godwin A.K., Cairns P., et al. Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence. Cancer Res. 2011;71:6184–6194. doi: 10.1158/0008-5472.CAN-11-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burkhalter R.J., Symowicz J., Hudson L.G., Gottardi C.J., Stack M.S. Integrin regulation of beta-catenin signaling in ovarian carcinoma. J. Biol. Chem. 2011;286:23467–23475. doi: 10.1074/jbc.M110.199539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshida A., Okamoto N., Tozawa-Ono A., Koizumi H., Kiguchi K., Ishizuka B., Kumai T., Suzuki N. Proteomic analysis of differential protein expression by brain metastases of Gynecol.ogical malignancies. Hum. Cell. 2013;26:56–66. doi: 10.1007/s13577-012-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bertero L., Siravegna G., Ruda R., Soffietti R., Bardelli A., Cassoni P. Review: Peering through a keyhole: Liquid biopsy in primary and metastatic central nervous system tumours. Neuropathol. Appl. Neurobiol. 2019;45:655–670. doi: 10.1111/nan.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siravegna G., Geuna E., Mussolin B., Crisafulli G., Bartolini A., Galizia D., Casorzo L., Sarotto I., Scaltriti M., Sapino A., et al. Genotyping tumour DNA in cerebrospinal fluid and plasma of a HER2-positive breast cancer patient with brain metastases. ESMO Open. 2017;2:e000253. doi: 10.1136/esmoopen-2017-000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kann B.H., Park H.S., Johnson S.B., Chiang V.L., Yu J.B. Radiosurgery for brain metastases: Changing practice patterns and disparities in the United States. J. Natl. Compr. Cancer Netw. 2017;15:1494–1502. doi: 10.6004/jnccn.2017.7003. [DOI] [PubMed] [Google Scholar]
- 86.McMeekin D.S., Kamelle S.A., Vasilev S.A., Tillmanns T.D., Gould N.S., Scribner D.R., Gold M.A., Guruswamy S., Mannel R.S. Ovarian cancer metastatic to the brain: What is the optimal management? J. Surg. Oncol. 2001;78:194–200. doi: 10.1002/jso.1149. discussion 200-191. [DOI] [PubMed] [Google Scholar]
- 87.Hacker N.F., Rao A. Surgical management of lung, liver and brain metastases from Gynecol.ogical cancers: A literature review. Gynecol. Oncol. Res. Pract. 2016;3:7. doi: 10.1186/s40661-016-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karam A., Ledermann J.A., Kim J.W., Sehouli J., Lu K., Gourley C., Katsumata N., Burger R.A., Nam B.H., Bacon M., et al. Fifth ovarian cancer consensus conference of the gynecologic cancer intergroup: First-line interventions. Ann. Oncol. 2017;28:711–717. doi: 10.1093/annonc/mdx011. [DOI] [PubMed] [Google Scholar]
- 89.Lheureux S., Gourley C., Vergote I., Oza A.M. Epithelial ovarian cancer. Lancet. 2019;393:1240–1253. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 90.Wilson M.K., Pujade-Lauraine E., Aoki D., Mirza M.R., Lorusso D., Oza A.M., du Bois A., Vergote I., Reuss A., Bacon M., et al. Fifth ovarian cancer consensus conference of the gynecologic cancer intergroup: Recurrent disease. Ann. Oncol. 2017;28:727–732. doi: 10.1093/annonc/mdw663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DiSilvestro P., Alvarez Secord A. Maintenance treatment of recurrent ovarian cancer: Is it ready for prime time? Cancer Treat. Rev. 2018;69:53–65. doi: 10.1016/j.ctrv.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 92.Colombo N. When nonplatinum is the answer: The role of trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. Future Oncol. 2017;13:23–29. doi: 10.2217/fon-2017-0319. [DOI] [PubMed] [Google Scholar]
- 93.Mehta M.P., Paleologos N.A., Mikkelsen T., Robinson P.D., Ammirati M., Andrews D.W., Asher A.L., Burri S.H., Cobbs C.S., Gaspar L.E., et al. The role of chemotherapy in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neuro-Oncol. 2010;96:71–83. doi: 10.1007/s11060-009-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pitz M.W., Desai A., Grossman S.A., Blakeley J.O. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J. Neuro-Oncol. 2011;104:629–638. doi: 10.1007/s11060-011-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Drean A., Goldwirt L., Verreault M., Canney M., Schmitt C., Guehennec J., Delattre J.Y., Carpentier A., Idbaih A. Blood-brain barrier, cytotoxic chemotherapies and glioblastoma. Expert Rev. Neurother. 2016;16:1285–1300. doi: 10.1080/14737175.2016.1202761. [DOI] [PubMed] [Google Scholar]
- 96.Vlasveld L.T., Beynen J.H., Boogerd W., Ten Bokkel Huinink W.W., Rodenhuis S. Complete remission of brain metastases of ovarian cancer following high-dose carboplatin: A case report and pharmacokinetic study. Cancer Chemother. Pharmacol. 1990;25:382–383. doi: 10.1007/BF00686244. [DOI] [PubMed] [Google Scholar]
- 97.Cooper K.G., Kitchener H.C., Parkin D.E. Cerebral metastases from epithelial ovarian carcinoma treated with carboplatin. Gynecol. Oncol. 1994;55:318–323. doi: 10.1006/gyno.1994.1297. [DOI] [PubMed] [Google Scholar]
- 98.Cormio G., Gabriele A., Maneo A., Zanetta G., Bonazzi C., Landoni F. Complete remission of brain metastases from ovarian carcinoma with carboplatin. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;78:91–93. doi: 10.1016/S0301-2115(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 99.Watanabe A., Shimada M., Kigawa J., Iba T., Oishi T., Kanamori Y., Terakawa N. The benefit of chemotherapy in a patient with multiple brain metastases and meningitis carcinomatosa from ovarian cancer. Int. J. Clin. Oncol. 2005;10:69–71. doi: 10.1007/s10147-004-0437-x. [DOI] [PubMed] [Google Scholar]
- 100.Sadik Z.H.A., Beerepoot L.V., Hanssens P.E.J. Efficacy of gamma knife radiosurgery in brain metastases of primary Gynecol.ogical tumors. J. Neuro-Oncol. 2019;142:283–290. doi: 10.1007/s11060-019-03094-2. [DOI] [PubMed] [Google Scholar]
- 101.Wong E.T., Berkenblit A. The role of topotecan in the treatment of brain metastases. Oncologist. 2004;9:68–79. doi: 10.1634/theoncologist.9-1-68. [DOI] [PubMed] [Google Scholar]
- 102.Bastiancich C., Bastiat G., Lagarce F. Gemcitabine and glioblastoma: Challenges and current perspectives. Drug Discov. Today. 2018;23:416–423. doi: 10.1016/j.drudis.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 103.Birngruber T., Raml R., Gladdines W., Gatschelhofer C., Gander E., Ghosh A., Kroath T., Gaillard P.J., Pieber T.R., Sinner F. Enhanced doxorubicin delivery to the brain administered through glutathione PEGylated liposomal doxorubicin (2B3-101) as compared with generic Caelyx,((R))/Doxil((R))—A cerebral open flow microperfusion pilot study. J. Pharm. Sci. 2014;103:1945–1948. doi: 10.1002/jps.23994. [DOI] [PubMed] [Google Scholar]
- 104.Bonoiu A., Mahajan S.D., Ye L., Kumar R., Ding H., Yong K.T., Roy I., Aalinkeel R., Nair B., Reynolds J.L., et al. MMP-9 gene silencing by a quantum dot-siRNA nanoplex delivery to maintain the integrity of the blood brain barrier. Brain Res. 2009;1282:142–155. doi: 10.1016/j.brainres.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cao P., Mooney R., Tirughana R., Abidi W., Aramburo S., Flores L., Gilchrist M., Nwokafor U., Haber T., Tiet P., et al. Intraperitoneal administration of neural stem cell-nanoparticle conjugates targets chemotherapy to ovarian tumors. Bioconjug. Chem. 2017;28:1767–1776. doi: 10.1021/acs.bioconjchem.7b00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tiet P., Li J., Abidi W., Mooney R., Flores L., Aramburo S., Batalla-Covello J., Gonzaga J., Tsaturyan L., Kang Y., et al. Silica coated paclitaxel nanocrystals enable neural stem cell loading for treatment of ovarian cancer. Bioconjug. Chem. 2019;30:1415–1424. doi: 10.1021/acs.bioconjchem.9b00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaitanya G.V., Steven A.J., Babu P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010;8:31. doi: 10.1186/1478-811X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Min A., Im S.A. PARP inhibitors as therapeutics: Beyond modulation of PARylation. Cancers. 2020;12:394. doi: 10.3390/cancers12020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mirza M.R., Monk B.J., Herrstedt J., Oza A.M., Mahner S., Redondo A., Fabbro M., Ledermann J.A., Lorusso D., Vergote I., et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 110.Mittica G., Ghisoni E., Giannone G., Genta S., Aglietta M., Sapino A., Valabrega G. PARP Inhibitors in Ovarian Cancer. Recent Pat. Anticancer Drug Discov. 2018;13:392–410. doi: 10.2174/1574892813666180305165256. [DOI] [PubMed] [Google Scholar]
- 111.Boussios S., Karathanasi A., Cooke D., Neille C., Sadauskaite A., Moschetta M., Zakynthinakis-Kyriakou N., Pavlidis N. PARP inhibitors in ovarian cancer: The route to “Ithaca”. Diagnostics. 2019;9:55. doi: 10.3390/diagnostics9020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gonzalez-Martin A., Pothuri B., Vergote I., DePont Christensen R., Graybill W., Mirza M.R., McCormick C., Lorusso D., Hoskins P., Freyer G., et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2019;381:2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 113.Tentori L., Leonetti C., Scarsella M., D’Amati G., Vergati M., Portarena I., Xu W., Kalish V., Zupi G., Zhang J., et al. Systemic administration of GPI 15427, a novel poly(ADP-ribose) polymerase-1 inhibitor, increases the antitumor activity of temozolomide against intracranial melanoma, glioma, lymphoma. Clin. Cancer Res. 2003;9:5370–5379. [PubMed] [Google Scholar]
- 114.Karginova O., Siegel M.B., Van Swearingen A.E., Deal A.M., Adamo B., Sambade M.J., Bazyar S., Nikolaishvili-Feinberg N., Bash R., O’Neal S., et al. Efficacy of Carboplatin Alone and in Combination with ABT888 in Intracranial Murine Models of BRCA-Mutated and BRCA-Wild-Type Triple-Negative Breast Cancer. Mol. Cancer Ther. 2015;14:920–930. doi: 10.1158/1535-7163.MCT-14-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim M., Kizilbash S.H., Laramy J.K., Gampa G., Parrish K.E., Sarkaria J.N., Elmquist W.F. Barriers to Effective Drug Treatment for Brain Metastases: A Multifactorial Problem in the Delivery of Precision Medicine. Pharm Res. 2018;35:177. doi: 10.1007/s11095-018-2455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.O’Sullivan C.C., Davarpanah N.N., Abraham J., Bates S.E. Current challenges in the management of breast cancer brain metastases. Semin. Oncol. 2017;44:85–100. doi: 10.1053/j.seminoncol.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 117.Kotecki N., Lefranc F., Devriendt D., Awada A. Therapy of breast cancer brain metastases: Challenges, emerging treatments and perspectives. Ther. Adv. Med. Oncol. 2018;10:1758835918780312. doi: 10.1177/1758835918780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bangham M., Goldstein R., Walton H., Ledermann J.A. Olaparib treatment for BRCA-mutant ovarian cancer with leptomeningeal disease. Gynecol. Oncol. Rep. 2016;18:22–24. doi: 10.1016/j.gore.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sakamoto I., Hirotsu Y., Nakagomi H., Ikegami A., Teramoto K., Omata M. Durable response by olaparib for a Japanese patient with primary peritoneal cancer with multiple brain metastases: A case report. J. Obstet. Gynaecol. Res. 2019;45:743–747. doi: 10.1111/jog.13851. [DOI] [PubMed] [Google Scholar]
- 120.Gray S., Khor X.Y., Yiannakis D. Niraparib as maintenance therapy in a patient with ovarian cancer and brain metastases. BMJ Case Rep. 2019;12:e230738. doi: 10.1136/bcr-2019-230738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Christofi T., Baritaki S., Falzone L., Libra M., Zaravinos A. Current perspectives in cancer immunotherapy. Cancers. 2019;11:1472. doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hamanishi J., Mandai M., Ikeda T., Minami M., Kawaguchi A., Murayama T., Kanai M., Mori Y., Matsumoto S., Chikuma S., et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 2015;33:4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 123.Matulonis U.A., Shapira-Frommer R., Santin A.D., Lisyanskaya A.S., Pignata S., Vergote I., Raspagliesi F., Sonke G.S., Birrer M., Provencher D.M., et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann. Oncol. 2019;30:1080–1087. doi: 10.1093/annonc/mdz135. [DOI] [PubMed] [Google Scholar]
- 124.Varga A., Piha-Paul S., Ott P.A., Mehnert J.M., Berton-Rigaud D., Morosky A., Yang P., Ruman J., Matei D. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: Analysis of KEYNOTE-028. Gynecol. Oncol. 2019;152:243–250. doi: 10.1016/j.ygyno.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 125.Disis M.L., Taylor M.H., Kelly K., Beck J.T., Gordon M., Moore K.M., Patel M.R., Chaves J., Park H., Mita A.C., et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: Phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5:393–401. doi: 10.1001/jamaoncol.2018.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu J.F., Gordon M., Veneris J., Braiteh F., Balmanoukian A., Eder J.P., Oaknin A., Hamilton E., Wang Y., Sarkar I., et al. Safety, clinical activity and biomarker assessments of atezolizumab from a Phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol. Oncol. 2019;154:314–322. doi: 10.1016/j.ygyno.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 127.Gonzalez-Martin A., Sanchez-Lorenzo L. Immunotherapy with checkpoInt. inhibitors in patients with ovarian cancer: Still promising? Cancer. 2019;125:4616–4622. doi: 10.1002/cncr.32520. [DOI] [PubMed] [Google Scholar]
- 128.Berghoff A.S., Ricken G., Wilhelm D., Rajky O., Widhalm G., Dieckmann K., Birner P., Bartsch R., Preusser M. Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC) J. Neuro-Oncol. 2016;130:19–29. doi: 10.1007/s11060-016-2216-8. [DOI] [PubMed] [Google Scholar]
- 129.Berghoff A.S., Fuchs E., Ricken G., Mlecnik B., Bindea G., Spanberger T., Hackl M., Widhalm G., Dieckmann K., Prayer D., et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2016;5:e1057388. doi: 10.1080/2162402X.2015.1057388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jindal V., Gupta S. Expected Paradigm Shift in Brain Metastases Therapy-Immune Checkpoint Inhibitors. Mol. Neurobiol. 2018;55:7072–7078. doi: 10.1007/s12035-018-0905-3. [DOI] [PubMed] [Google Scholar]
- 131.Borella F., Ghisoni E., Giannone G., Cosma S., Benedetto C., Valabrega G., Katsaros D. Immune checkpoint. Inhibitors in epithelial ovarian cancer: An overview on efficacy and future perspectives. Diagnostics. 2020;10:146. doi: 10.3390/diagnostics10030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Herrera F.G., Irving M., Kandalaft L.E., Coukos G. Rational combinations of immunotherapy with radiotherapy in ovarian cancer. Lancet Oncol. 2019;20:e417–e433. doi: 10.1016/S1470-2045(19)30401-2. [DOI] [PubMed] [Google Scholar]
- 133.Nardin C., Mateus C., Texier M., Lanoy E., Hibat-Allah S., Ammari S., Robert C., Dhermain F. Tolerance and outcomes of stereotactic radiosurgery combined with anti-programmed cell death-1 (pembrolizumab) for melanoma brain metastases. Melanoma Res. 2018;28:111–119. doi: 10.1097/CMR.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 134.Anderson E.S., Postow M.A., Wolchok J.D., Young R.J., Ballangrud A., Chan T.A., Yamada Y., Beal K. Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J. Immunother. Cancer. 2017;5:76. doi: 10.1186/s40425-017-0282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]