Abstract
Meat products have been implicated in many listeriosis outbreaks globally, however there is a dearth of information on the diversity of L. monocytogenes isolates circulating in food products in South Africa. The aim of this study was to investigate the population structure of L. monocytogenes isolated in the meat value chain within the South African market. Based on whole-genome sequence analysis, a total of 217 isolates were classified into two main lineage groupings namely lineages I (n = 97; 44.7%) and II (n = 120; 55.3%). The lineage groups were further differentiated into IIa (n = 95, 43.8%), IVb (n = 69, 31.8%), IIb (n = 28, 12.9%), and IIc (n = 25, 11.5%) sero-groups. The most abundant sequence types (STs) were ST204 (n = 32, 14.7%), ST2 (n = 30, 13.8%), ST1 (n = 25, 11.5%), ST9 (n = 24, 11.1%), and ST321 (n = 21, 9.7%). In addition, 14 clonal complex (CCs) were identified with over-representation of CC1, CC3, and CC121 in “Processed Meat-Beef”, “RTE-Poultry”, and “Raw-Lamb” meat categories, respectively. Listeria pathogenic islands were present in 7.4% (LIPI-1), 21.7% (LIPI-3), and 1.8% (LIPI-4) of the isolates. Mutation leading to premature stop codons was detected in inlA virulence genes across isolates identified as ST121 and ST321. The findings of this study demonstrated a high-level of genomic diversity among L. monocytogenes isolates recovered across the meat value chain control points in South Africa.
Keywords: L. monocytogenes, subtyping, serogroups, sequence types, clone complexes, pathogenic islands, lineages, inlA, sequencing
1. Introduction
The consumption of meat and meat-based products has increased in the last few years in South Africa (SA) [1]. This increase is primarily linked to human population growth, urbanization, higher disposable income, and a change in eating patterns as many people are adopting diets that contain high-quality animal proteins [2]. However, the chemical composition of meat predisposes it to bacterial contamination and serves as a vector for transmission of foodborne bacteria that can cause infection in humans and result in economic losses [3]. Occurrence of foodborne bacteria on meat can be due to poor animal management, slaughter practices, processing, storage conditions, and lack of meat safety knowledge [4]. Consumers need to be protected and provided with safe and wholesome products of animal origin. This can be achieved by practicing good farm animal management, proper personal hygiene, and routine surveillance of food products within the meat value chain [2]. Safe handling of meat is paramount to circumvent potential devastating effects on the health and economy of populations.
Listeria monocytogenes is a zoonotic foodborne bacterium that is responsible for causing a rare but potentially fatal disease known as listeriosis in humans and animals [5]. Human listeriosis has become a priority and economically important disease that contributes to public health challenges in SA and globally [6,7,8,9]. Over the years, the number of human listeriosis outbreaks and sporadic cases that emanated from various sources such as unmarked potatoes [10] and polony (Bologna sausage) [8,9] have been documented in SA. Furthermore, several studies in different geographical areas of SA have reported the presence of L. monocytogenes in a variety of meat products [4,11,12,13]. These studies revealed health challenges associated with L. monocytogenes as a result of high occurrence in food products.
The epidemic and sporadic cases of human listeriosis are commonly associated with consumption of contaminated food, particularly ready to eat (RTE) products [14]. Despite the low overall incidence of human listeriosis, this disease is linked to a high case fatality rate (20–30%) and hospitalization rates [6,15]. There is also evidence to suggest higher case fatality rates in pregnant women and individuals with neurolisteriosis [16]. The clinical manifestations of human listeriosis can range from self-limiting gastroenteritis that last a few days to more severe invasive and systematic illnesses that might be fatal in high-risk groups such as the elderly, infants, and immunocompromised people [17]. Therefore, a high percentage of the South African population is at risk as the elderly and other immunocompromised individuals contribute significantly to the total population.
The pathogenicity of L. monocytogenes is based on the production of the virulence factors, susceptibility of the host organism, and the virulence of a particular strain; hence, the exact infective dose and the safety margin of L. monocytogenes sequences is not well defined [18,19]. Several studies have provided detailed insights into the global population distribution and virulence potential of L. monocytogenes strains as well as the sources associated with important clonal complexes (CCs) and sequence types (STs). These have indicated an over-representation of CC1, CC2, CC4, and CC6 in clinical cases and the predominance of CC121 and CC204 in food sources [20,21,22]. In addition, the genomic characterization of the L. monocytogenes invasion protein (InlA) has shown a reduced virulence potential of some strains globally due to mutations associated with premature stop codons (PMSCs) [22,23]. The primary sources of L. monocytogenes CCs in the meat value chain are not well understood and limited data are available on the distribution of meat-associated with CCs and their virulence potential in the SA agriculture and meat value chain.
Before the 2017–2018 outbreak of listeriosis in SA, the disease was not required to be reported and as such was not under surveillance in the country; however, a national surveillance system has since been implemented, and all isolates from human patients are analyzed by means of whole-genome sequencing (WGS) [9]. In comparison, comprehensive data on the genome characterization of L. monocytogenes in the food products of animal origin value chains is still lacking in SA. Matle and co-workers [13] performed an extensive national baseline survey involving nine provinces of SA to determine the occurrence of L. monocytogenes strains in meat and meat products in abattoirs, meat processing plants, and retail outlets. Although this study provided important information on the extent of meat contamination, the need still existed to further investigate the genomic characteristics of L. monocytogenes isolated from meat products using WGS in SA. The aim of this study was to subtype and characterize L. monocytogenes isolates recovered at selected control points in the meat value chain in SA by means of WGS.
2. Materials and Methods
2.1. Sample Information
The isolates used in this study were obtained from samples submitted between 2014 and 2019 at Agriculture Research Council-Onderstepoort Veterinary Research (ARC-OVR): Feed and Food laboratory, SA, as part of the Department of Agriculture, Land Reform, and Rural Development (DALRRD) Pathogen Profiling project number 21.1.1/VPH-01/OVI. The samples (n = 217) included raw meat (n = 55), processed meat (n = 126), RTE meat products (n = 15) and environmental samples collected from commercial pig farm environment during a listeriosis outbreak (n = 21). The samples originated from different animal protein sources such as beef, poultry, lamb, and pork and various food establishments (farm environments, butcheries, abattoirs, retail outlets and cold stores).
2.2. Isolates Categorisation
Considering the diversity of the samples from which the isolates originated, the isolates were grouped according to different categories based on the origin of the sample and the establishment of origin. The sample origin was defined as a concatenation of the type of meat product and the animal from which it was produced from. Samples collected from the farm environment were labelled as environmental samples. The number of isolates for each category is shown in Table 1.
Table 1.
Number of isolates for each category together with the different STs and serogroups found in each category.
| Establishment | Sample Origin | Number of Isolates | STs | CCs | Serogroups |
|---|---|---|---|---|---|
| Farm (n = 21) |
Piggery Environment samples | 21 | ST5, ST7, ST9, ST31, ST155, ST288 | CC5, CC7, CC9, CC31, CC155, CC288 | IIa, IIb, IIc |
| Abattoir (n = 3) |
Processed meat-Beef | 1 | ST9 | CC9 | IIc |
| Raw-Pork | 1 | ST122 | CC9 | IIc | |
| Raw-Poultry | 1 | ST204 | CC204 | IIa | |
| Butchery (n = 68) |
Processed meat-Beef | 53 | ST1, ST2, ST3, ST5, ST7, ST9, ST87, ST121, ST155, ST204, ST321, ST820, ST876, ST1428 | CC1, CC2, CC3, CC5, CC7, CC9, CC87, CC121, CC155, CC204, CC321 | IIa, IIb, IIc, IVb |
| Processed meat-Mixed | 1 | ST9 | CC9 | IIc | |
| Processed meat-Poultry | 3 | ST7, ST121, ST204 | CC7, CC121, CC204 | IIa | |
| Raw-Beef | 1 | ST378 | CC19 | IIa | |
| Raw-Pork | 1 | ST121 | CC121 | IIa | |
| Raw-Poultry | 3 | ST5, ST204, ST820 | CC5, CC204 | IIa, IIb | |
| 1 RTE-Beef | 6 | ST2, ST9, ST204 | CC2, CC9, CC204 | IIa, IIc, IVb | |
| Cold store (n = 19) |
Raw-Beef | 1 | ST9 | CC9 | IIc |
| Raw-Poultry | 18 | ST1, ST2, ST5, ST7, ST9, ST121, ST155, ST204 | CC1, CC2, CC5, CC7, CC9, CC121, CC155, CC204 | IIa, IIb, IIc, IVb | |
| Processing plant (n = 10) |
Processed meat-Beef | 2 | ST2, ST9 | CC2, CC9 | IIc, IVb |
| Processed meat-Pork | 2 | ST2, ST876 | CC1, CC2 | IVb | |
| Raw-Pork | 2 | ST2 | CC2 | IVb | |
| 1 RTE-Beef | 1 | ST204 | CC204 | IIa | |
| RTE-Pork | 2 | ST2, ST121 | CC2, CC121 | IIa, IVb | |
| 1 RTE-Poultry | 1 | ST3 | CC3 | IIb | |
| Retail (n = 96) |
Processed meat-Beef | 63 | ST1, ST2, ST5, ST7, ST9, ST121, ST204, ST321, ST876, ST1421, ST1428, ST1430 | CC1, CC2, CC5, CC7, CC9, CC121, CC204, CC321 | IIa, IIb, IIc, IVb |
| Processed meat-Mixed | 1 | ST204 | CC204 | IIa | |
| Raw-Beef | 2 | ST1, ST204 | CC1, CC204 | IIa, IVb | |
| Raw-Lamb | 2 | ST121, ST321 | CC121, CC321 | IIa | |
| Raw-Pork | 3 | ST9, ST155, ST321 | CC9, CC155, CC321 | IIa, IIc | |
| Raw-Poultry | 20 | ST1, ST5, ST9, ST121, ST155, ST204, ST321 | CC1, CC5, CC9, CC121, CC155, CC204, CC321 | IIa, IIb, IIc, IVb | |
| 1 RTE-Beef | 5 | ST1, ST2, ST121, ST204, ST876 | CC1, CC2, CC121, CC204 | IIa, IVb |
1 RTE—Ready to Eat.
2.3. Bacterial Strains and DNA Isolation
The isolates were preserved as lyophilized and were revived by inoculation into brain heart infusion (BHI) broth then incubated at 37 °C for 18–24 h. DNA was extracted from BHI broth culture using a High Pure PCR template preparation kit (Roche, Potsdam, Germany) according to manufacturer’s instructions.
2.4. Genome Sequencing, Quality Control and de novo Assembly
Whole-genome sequencing (WGS) of the isolates was performed at the Biotechnology Platform, Agricultural Research Council, Onderstepoort, SA. DNA libraries were prepared using TruSeq and Nextera DNA library preparation kits (Illumina, San Diego, CA, USA), followed by sequencing on HiSeq and MiSeq instruments (Illumina, San Diego, CA, USA). Quality control including adapter removal of the raw data was done using BBDuk (version 37.90; https://jgi.doe.gov/data-and-tools/bbtools/bb-tools-user-guide/bbduk-guide/). SPAdes v.3.12.0 [23] was used to create a de novo assembly of each isolate.
Multi locus sequence type (MLST) profiles were obtained from the Listeria database hosted by the Pasteur Institute, France (http://bigsdb.pasteur.fr/listeria/) [24]. The MLST database contains 7 loci with a total of 2069 different alleles. A k-mer based mapping tool, stringMLST [25], was used to align reads against these profiles to determine the MLST for each sequenced sample using k-mers of length 21 and 35. To validate the k-mer based predictions, all de novo assembled isolates were analyzed using MLST v.2.18.0 [26]. Serotype determination was done in silico using stringMLST and validated with blastn v.2.10.0+.
Genomes of which the in silico determined sequence type and serogroup correlated with L. monocytogenes were annotated using Prokka v.1.14.0 [27]. The pan-genome composition was extracted using Roary [28] and a core genome phylogenetic tree constructed with IQ-TREE v.1.6.6 [29]. Pan-genome clusters were defined as follows: Core—genes present in all isolates; soft core—genes present in at least 95% of isolates; shell-genes present between 15% and 95% of isolates; cloud-genes in less than 15% of isolates. The core genome phylogenetic tree was visualized using ggtree v1.16.6 [30].
2.5. Listeria Pathogenicity Islands
The presence of Listeria Pathogenicity Island (LIPI) in the de novo assemblies was determined for LIPI-1, LIPI-3, and LIPI-4 using blastn v.2.10.0+ with a minimum percent identity of 95% and an e-value of 1 × 10−30. All alleles for the abovementioned LIPI genes clusters were obtained from the Listeria database hosted by the Pasteur Institute, Paris, France (http://bigsdb.pasteur.fr/listeria/) [25].
2.6. Protein Sequence of inlA Genes
Protein sequences for the inlA genes were extracted from the annotated assemblies and aligned using all-versus-all blastp with an e-value of 1 × 10−30. The results were filtered for 99% identify and clustered using the Markov clustering algorithm (MCL) [31] with an inflation parameter of 1.8. Protein sequences were inspected for truncation based on the reference protein length of 800 amino acids (AAs).
2.7. Data Analysis
Analysis was done using R v.3.6.0 [32]. Proportion and association testing were done using Chi-Square tests and over-representation was indicated by a Pearson residual value of larger than 2. Diversity analysis according to ST occurrence within categories was done using the R package vegan v2.5-6 [33]. A distance matrix based on the ST count matrix was produced using vegan with the “bray” method invoked. Principle coordinate analysis was done using ape v5.3 [34] with the distance matrix as input.
3. Results
3.1. Typing Analysis
The isolates were grouped into different STs, 20 in total, and classified as either Lineage I or II (Figure 1). Eleven lineage I and nine lineage II STs were identified with lineage I accounting for 44.7% (n = 97) and lineage II accounting for 55.3% (n = 120) of the isolates. Five STs (25% of all STs) were found to be singularly represented in the isolates. The five most frequent STs were ST204 (n = 32, 14.7%), ST2 (n = 30, 13.8%), ST1 (n = 25, 11.5%), ST9 (n = 24, 11.1%), and ST321 (n = 21, 9.7%), respectively. The other identified STs are presented in Table 1. Fourteen CCs were identified of which six were in lineage I and eight in lineage II, with CC1 (n = 38, 17.5%) the most prevalent followed by CC204 (n = 32, 14.7%) and CC2 (n = 31, 14.3%). Four serogroups were identified in the 217 isolates, with serogroup IIa (n = 95, 43.8%) being the most prevalent, followed by IVb (n = 69, 31.8%), IIb (n = 28, 12.9%), and IIc (n = 25, 11.5%) (Figure 1).
Figure 1.
Lineage, serogroup, and sequence type distribution of Listeria monocytogenes isolates.
The distribution of lineages, serogroups, CCs, and STs among the 217 isolates were tested using a Chi-Square goodness of fit test. The test results indicated that the serogroups (p-value = 1.283 × 10−13), CCs (p-value = 5.761 × 10−24) and STs (p-value = 3.327 × 10−32) were not commonly distributed among the samples (Figure 2). In particular, serogroups IIa (lineage II) and IVb (lineage I) were found to be over-represented. The STs that exceeded the expected distribution were ST1, ST2, ST9, ST204, and ST321, which all belonged to serogroups IIa and IVb with the exception of ST9 in serogroup IIc.
Figure 2.
Hanging chi-gram indicating the deviation from expected occurrence across the Listeria monocytogenes isolates: (A) Sequence type; (B) clonal complex.
3.2. Samples Categories Analysis
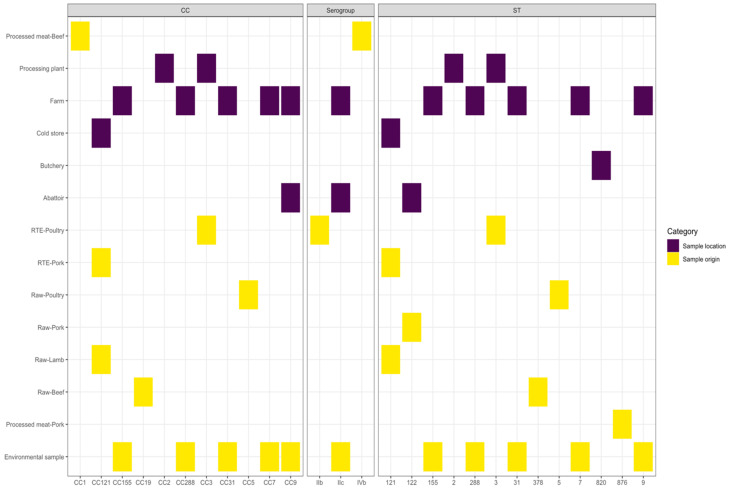
Sample origin contained 12 categories (Table 1) and a Chi-Square test of independence were used to identify significant associations between the categories and the isolate typing results. Serogroup IIb in the “RTE-Poultry”, IVb in the “Processed Meat-Beef”, and IIc in the “Environmental Sample”, “Abattoir” and “Farm” groups were found to be over-represented (p-value = 0.003). For the STs, over-representation was detected for “Processed Meat-Pork” (ST876), “Raw-Beef” (ST378), “Raw-Lamb” (ST121), “Raw-Pork” (ST122), “Raw-Poultry” (ST5), “RTE-Pork” (ST121), “RTE-Poultry” (ST3), and “Environmental Samples” (ST7, ST9, ST31, ST155, and ST288) (p-value = 1.771 × 10−11). In the Establishment category, serogroup IIc was found to be significantly over-represented in Abattoirs and Farms (p-value = 0.009). Abattoirs were further found to be significantly associated with ST122; “Butcheries” with ST820; “Cold Stores” with ST121; Farms with ST7, ST9, ST31, ST155, and ST288; and “Processing Plants” with ST2 and ST3 (p-value = 8.602 × 10−13).
Analysis of CCs and over-representation in the various categories indicated 11 CCs, which were deemed to be associated with a certain category. In the sample origin category, the “Environmental samples” group displayed over-representation of various CCs, which were CC7, CC9, CC31, CC155, and CC288 (p-value = 2.222 × 10−16). These CCs were further found to be over-represented in Farms (p-value = 2.658 × 10−8). The “Processed Meat-Beef” category had an over-representation of CC1; CC9 in Abattoirs; and in the “Processing Plant” establishment, CC2 was more than what was expected. In the “RTE-Poultry” and “Processing Plant” categories CC3 was over-represented, CC5 in the “Raw-Poultry” and C19 in “Raw-Beef” categories with CC121 significantly abundant in “Raw-Lamb”, “RTE-Pork”, and “Import Cold Stores”. Over-representation of serogroups, STs, and CCs, indicated by a Pearson residual value larger than 2, for all categories, is presented in Figure 3.
Figure 3.
Over-representation of serogroups, clonal complexes (CCs) and sequence types (STs) across all categories.
3.3. ST Diversity Analysis
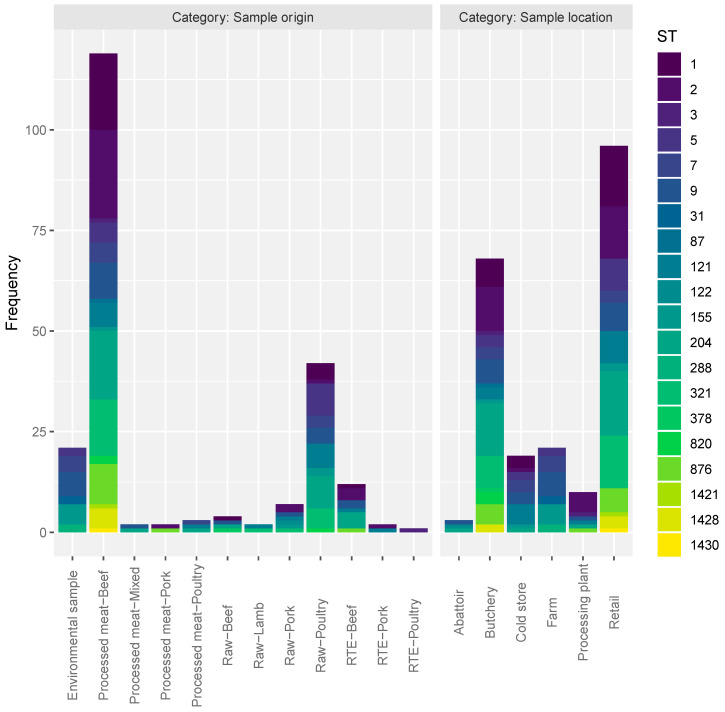
The ST results were transformed into a count matrix and diversity analysis done according to the sample collection categories. The frequency of STs per category is displayed in Figure 4. The values for four different diversity indices (Richness, Simpson, Shannon, and Inverse Simpson) are presented in Table 2 for both the categories “Sample origin” and “Sample location”. Samples from the “Processed meat-Beef” category displayed the highest ST diversity, as indicated by all the indices, with “Raw-Poultry” having the second highest diversity. With regards to the “Sample location” it was found that the “Butchery” category had the highest ST diversity, closely followed by the “Retail” category. Results of clustering analysis and Principle coordinates analysis (PCOA) of the sample categories and the ST occurrence are displayed in Figure 5. In general, the “Sample origin” categories “Processed Meat-Beef”, “Raw-Poultry”, and “Environmental sample” formed a cluster with “Processed meat-Mixed” and “Raw-Beef” grouping together. In the “Sample location” category, “Butchery” and “Retail” grouped closely together.
Figure 4.
Frequency of STs per sample collection category.
Table 2.
Diversity indices based on the occurrence of STs in the different categories.
| Category | Samples | Richness | Shannon | Simpson | Inverse Simpson |
|---|---|---|---|---|---|
| Sample origin | Processed meat-Beef | 16 | 2.357795795 | 0.884824518 | 8.682403433 |
| Processed meat-Mixed | 2 | 0.693147181 | 0.5 | 2 | |
| Processed meat-Pork | 2 | 0.693147181 | 0.5 | 2 | |
| Processed meat-Poultry | 3 | 1.098612289 | 0.666666667 | 3 | |
| Raw-Beef | 4 | 1.386294361 | 0.75 | 4 | |
| Raw-Lamb | 2 | 0.693147181 | 0.5 | 2 | |
| Raw-Pork | 6 | 1.747868097 | 0.816326531 | 5.444444444 | |
| Raw-Poultry | 10 | 2.122400638 | 0.866213152 | 7.474576271 | |
| 1 RTE-Beef | 6 | 1.632630927 | 0.777777778 | 4.5 | |
| RTE-Pork | 2 | 0.693147181 | 0.5 | 2 | |
| RTE-Poultry | 1 | 0 | 0 | 1 | |
| Environmental sample | 6 | 1.687293537 | 0.798185941 | 4.95505618 | |
| Sample location | Abattoir | 3 | 1.098612289 | 0.666666667 | 3 |
| Butchery | 15 | 2.405603569 | 0.890138408 | 9.102362205 | |
| Cold store | 8 | 1.927544531 | 0.836565097 | 6.118644068 | |
| Farm | 6 | 1.687293537 | 0.798185941 | 4.955056179 | |
| Processing plant | 6 | 1.497866137 | 0.7 | 3.333333333 | |
| Retail | 13 | 2.30089177 | 0.885416667 | 8.727272727 |
1 RTE—Ready to Eat.
Figure 5.
Hierarchical clustering dendograms and Principle coordinates analysis for the different sample categories.
3.4. Pathogenicity Islands
The actA, hly, and mpl genes, which form part of the LIPI-1 gene cluster were present in all the sequenced isolates. The complete gene cluster of LIPI-1 was present in 16 (7.4%) isolates all of which were found exclusively in “Raw-Poultry” and “Processed meat-Beef” categories obtained from “Butchery”, “Cold Stores”, and “Retail” Sample locations (Supplementary Table S1). These isolates presented CCs from lineage I (CC1, CC2 and CC5) and lineage II (CC9, CC121, CC155, and CC321). The complete LIPI-3 gene cluster was identified in 47 (21.7%) isolates (lineage I: 95.7%; lineage II: 4.3%), of which 35 (74.5%) originated from the “Processed meat-Beef” category. Four CCs (CC1, CC2, CC3, and CC288) belonged to lineage I and lineage II were represented only by CC204. A complete LIPI-4 gene cluster was detected in four isolates (1.8%) with the majority (75%) found in serogroup IVb (CC2 and CC87) and all in lineage I.
3.5. Protein Sequence of inlA
Eight different inlA groups (1–8) were identified from the 217 sequenced isolates in this study (Figure 6 and Supplementary Table S2). Group 1 (size = 100) and group 2 (size = 93), harbored diverse STs, which all belonged to lineage II and lineage I, respectively. A total of 18 InlA protein sequences were found to be truncated with lengths range from 491–699 AAs. All the proteins in cluster 3 (size = 14, ST121) and cluster 6 (size = 2, ST121) were found to be truncated as well as the proteins of the singleton clusters 7 (size = 1, ST121) and 8 (size= 1, ST321). All truncated proteins belonged to isolates from lineage II, serogroup IIa, which were obtained across different establishments (“Butchery”, “Retail”, “Processing Plant”, and “Cold Store”) and sample origin (“Raw-Pork”, “Raw-Lamb”, “Raw-Poultry”, “Processed Meat-Beef”, “Processed Meat-Poultry”, and “RTE-Beef”) categories.
Figure 6.
Phylogenetic analysis of inlA gene sequences obtained from isolates in this study.
3.6. Core Genome Phylogeny
In total, 22,790 genes were predicted across the 217 L. monocytogenes isolates. The partitioning of genes across the pan-genome was as follows: core – 1029 genes; soft core – 1141 genes; shell – 1711 genes; cloud – 18,909 genes. Phylogenetic analysis, based on the core genome, is displayed in Figure 7.
Figure 7.
Phylogenetic analysis of Listeria monocytogenes isolates based on the core genome.
4. Discussion
To have a better understanding of population structure and genomic diversity of L. monocytogenes isolates in SA, a total of 217 isolates representing different meat and meat products as well as environmental samples were characterized using WGS. WGS is a very powerful tool for the characterization of L. monocytogenes as it allows an unprecedented subtyping resolution by using the entire genome to determine strain diversity and virulence traits [35,36,37]. The findings of the present study give a detailed overview into the genomic diversity of L. monocytogenes in the meat value chain that can inform food safety risk-based decisions and risk assessment.
The primary and universally acceptable method for characterization of L. monocytogenes isolates has been serotyping [38]. Serotyping has been used as a rapid tool for epidemiological investigations of listeriosis outbreaks and to understand the importance of certain serotypes in causing listeriosis in humans [39]. Analysis of the serotypes in this study revealed that all isolates belonged to four major serogroups IIa, IVb, IIb, and IIc (43.8%, 31.8%, 12.9%, and 11.5%, respectively). This is in general agreement with observations made in other countries where serogroup IIa, IIb, and IVb isolates were found frequently while serogroup IIc isolates were rarely found [39,40,41]. In Ireland, O’Connor et al. [42] analyzed 5869 of L. monocytogenes isolates from different foods and found that the most common serogroup was IIa (43.9%), followed by IVb (27.5%), IIb (16.1%), and IIc (12.2%).
In a comparative analysis of serogroups, a hierarchy among isolates was observed with IIa and IVb found to be over-represented. The high presence of serogroup IIa in this study was expected, as IIa has been previously identified as over-represented in food sources and environmental samples in different countries [22,41,43]. Although serogroup IIa is highly associated with contamination of food, it is important to mention that they can cause human infection in certain countries with a high number of susceptible individuals such as SA [44]. Over-representation of serogroup IVb in the present study is concerning as more than 80% of human infections globally are caused by L. monocytogenes strains in this serogroup [22,45,46]. Further analysis of serogroup distribution based on sample origin indicated over-representation (p-value = 0.003) of IIb in “RTE-poultry”, IVb in “Processed meat”, and IIc in “Environmental samples”. This distribution provides critical information on the meat products that are prone to contamination by certain serogroups of L. monocytogenes and may subsequently help in good agricultural and hygiene practices, policy formation, and control measures of this bacterium in South Africa. For instance, implementing proper biosecurity and biosafety measures as good agriculture practice at farm level can play a critical role in minimizing the introduction and spread of different serogroups of L. monocytogenes on downstream processing steps across meat value chain.
MLST is a technique used to analyze nucleotide sequence data from a number of conserved (usually 7) housekeeping genes to derive a combination of alleles known as a ST. The application of this technique in the present study has served as tool also to determine the lineages and CCs of L. monocytogenes. CCs are defined as a group of STs differing by no more than one allele from at least one other ST in the group, regardless of its involvement in outbreaks [22]. Analysis of MLST data revealed the distribution of 20 different STs among all the sample isolates that belong to two main lineages, I or II. Similar descriptive differentiation of lineage I and lineage II isolates has been recorded in previous studies [22,47]. This suggests that lineage I and II isolates are important etiological agents common in the South African red meat and poultry value chain. The five largest ST groupings identified in this study, ST204 and ST321 (serogroup IIa), ST1 and ST2 (serogroup IVb), and ST9 (serogroup IIa), have previously been isolated from meat, meat products and production environments around the world [40,47,48,49]. However, this is the first detailed report on the distribution of STs along the livestock value chain in SA and as such it provides contemporary and applicable data. The predominant ST in the current study, ST204, has also been reported by Kwong et al. [50] and Ebner et al. [51], as the most common ST in meat-associated products in Australia and France. Other studies reported ST204 as a common persisting strain of L. monocytogenes that has been isolated from various sources such as food processing facilities [52], non-clinical isolates [22], and RTE food products [53]. ST1 and ST2 are regarded as the most common STs associated with food contamination and causing infection of humans and animal globally [54,55,56]. In a survey of food-producing facilities between 1996 and 2003 in Austria, ST1 and ST2 were the most predominant in meat-based products as cited by Ebner et al., [51]. Data on the occurrence and distribution of ST9 and ST321 in meat and meat products are lacking globally.
In comparison to the STs in the meat value chain, the non-ST6 sequence types reported from molecular epidemiology of human cases in South Africa are ST1, ST2, ST5, ST54, ST204, ST876, ST7, ST219, Unknown ST, Novel ST, ST101, ST1039, ST224, ST3, ST554, ST8, ST808, ST88, and ST87 in order of frequency [57]. ST6, ST132, ST155, ST2, ST204, ST3, ST5, ST533, ST602, and ST9 have been reported as the common environmental STs in SA can food production facilities [9,57]. Some of these STs (ST2, ST3, ST5, ST9, ST155, and ST204) have been reported in the present study and are induced with mechanisms that allow them to survive in food production environment and keep contaminating food products [5]. Therefore, there is need to link clinical isolates to food samples, since such epidemiological linkages are known to help further understand the key transmission routes and high-risk foods [22].
The absence of highly hypervirulent strains of L. monocytogenes ST6 was observed in the present study. In SA, ST6 strains have been associated with RTE products as samples cultured from a meat production facility’s food contact and non-contact environmental surfaces yielded ST6 isolates, which, together with the isolates from the human patients, belonged to the same core-genome MLST cluster with no more than four allelic differences [9]. The absence of ST6 in the current study and the rapid decline in the incidence of L. monocytogenes ST6 infections in humans soon after a recall of the implicated RTE processed meat products suggests that polony (Bologna sausage) produced at a single facility was highly likely to be the outbreak source with the primary contamination originating from a confined primary source [9].
Diversity analysis performed according to the sample collection categories (“Sample origin” and “Sample location”) showed that isolates from “Processed Meat-Beef” and “Butchery” categories harboured more heterogonous STs (ST1, ST2, ST3, ST5, ST7, ST9, ST87, ST121, ST155, ST204, ST321, ST820, ST876, and ST1428) of L. monocytogenes. It was also observed that isolates from “Raw-Poultry” and “Retail” categories harbored the second highest diversity of STs (ST1, ST5, ST9, ST155, ST204, and ST321). The clustering observed between “Processed Meat-Beef/Raw-Poultry” and “Butchery/Retail” categories based on the ST occurrence is highly comparative with several previous studies that recorded more diversity in isolates from RTE products [20,58]. Although, human infections caused by L. monocytogenes are commonly linked to RTE products, the findings of the present study are important in the South African context as raw-meat and processed-meat products are part of the raw materials for RTE.
The isolates in the present study were also classified into 14 CCs that represent two typical groups of L. monocytogenes CCs. The first group (infection-associated isolates) includes isolates (CC1, CC2, CC4, and CC6) that belong to lineage I and have a strong link to clinical cases (also known as hypervirulent strains) while the second group (food-associated isolates) represent isolates (CC7, CC9, CC121, CC155, and CC204), which belong to lineage II and are predominantly found in the food production environment [58]. The distribution of infection-associated isolates revealed the presence of CC1 and CC2, which were found to be over-represented in the “Raw-Beef” and “Processing Plant” categories, respectively. This over-representation of CC1 and CC2 clones in meat samples has been reported in different studies globally, which suggest their adaptation to diverse food products [25,59]. The distribution of food-associated isolates of L. monocytogenes CCs revealed a significant over-representation of CC7, CC155, CC9, CC121, and CC204, which all belong to lineage II. CC7 and CC155 were mostly found in isolates recovered from farm and environmental samples. CC7 isolates have been globally reported from diverse sources such as animal (wild, poultry, ruminants and fish), abattoir floor, compost, animal food products (milk, cheese, meat), and animal feeds (hay, silage) suggesting the possibility that it might persist in varying environments [39,60,61,62]. CC155 isolates were frequently detected in food samples in Eastern Asia [63], animals in Switzerland [64], and to a lesser extent in clinical cases in France, New Zealand, Greece, and Netherlands [65]. In the present study, CC9 and CC121 were significantly abundant in samples from “Raw-Lamb” and “Cold Stores (raw imported meat samples) categories and over-represented in “RTE-Pork” meat. Other studies also reported CC9 and CC121 as being significantly over-represented in food of animal origin and food-processing facilities around the world [47,52,66,67]; however, in this study, no significant association of CC204 isolates were observed with respect to environmental samples as well as meat and meat products isolates.
In the current study, although there was a bias towards specific lineages and CCs, there was considerable variation on pathogenic islands known to contribute to L. monocytogenes virulence among the isolates. The complete LIPI-1 gene cluster was detected in 16 isolates, recovered exclusively from “Raw-Poultry” and “Processed meat-Beef” categories. The presence of LIPI-1 in these meat products indicate an increased potential risk to cause infection in humans as LIPI-1 harbor a cluster of genes, prfA, plcA plcB, hly, mpl, and actA, that are very important in the infectious cycle of L. monocytogenes [68]. LIPI-3 consists of eight genes (llsAGHXBYDP) and this complete gene set was detected in 47 isolates, of which the majority (95.7%) were from lineage I (CC1, CC2, CC3, and CC228) and originating from the “Processed meat-Beef” category. This island encodes for hemolysin listeriolysin S, which is known to contribute to the survival of L. monocytogenes in human polymorphonuclear neutrophils [69]. Therefore, the presence of LIPI-1 and LIPI-3 islands in isolates from the “Processed meat-Beef” category, increases the risk for certain members of the population, such as the elderly, acquiring listeriosis in SA. LIPI-4 island is often implicated in placental and central nervouss system infections [69,70] and was found in four isolates in the present study. LIPI-4 has previously been identified mostly in CC4 isolates, however results of this study indicate its presence in CC2 and CC87 isolates, which is consistent with a recent report of this island in CC2 and CC87 isolates cultured in China [70]. The presence of LIPI-4 islands in hypervirulent CC2 and CC87 L. monocytogenes strains in beef and pork products must be a consideration in public health risk management.
The inlA gene encodes a surface protein that is responsible to facilitate the invasion of human intestinal epithelial cells by L. monocytogenes [71]. However, truncation of the inlA gene due to premature stop codons (PMSCs) has been associated with reduced invasiveness in some L. monocytogenes STs that possess them [71]. Analysis of the inlA protein sequence from isolates in this study identified 18 isolates, all from ST121 (n = 17) and ST321 (n = 1), having PMSCs, both of which were lineage II STs. Although the ST121 and ST321 PMSC mutation has previously been reported [22,64], this is the first report in SA.
5. Conclusions
Characterization of L. monocytogenes isolates from 2014–2019 using WGS has provided valuable insights into strain diversity and virulence potent of isolates found in meat products consumed in SA. This is also the largest study to report baseline data on the presence of L. monocytogenes serogroups, lineages, STs, and CCs across meat value chain in SA. This study confirmed the heterogeneous distribution of L. monocytogenes CCs across different meat and meat products with evidence of over-representation of certain CCs, which share similarities with those previously linked with human listeriosis outbreaks in other geographical areas. This study again illustrated meat products which are prone to contamination by diverse strains of L. monocytogenes within a specific point in the value chain. This study highlights the association of multiple STs of L. monocytogenes to different meat products in SA and identifies virulence traits as well as genetic mutations of certain subgroups found in food products. Therefore, the information generated here can be used in food safety risk assessment, management and protecting public health.
Future work is still required to compare the WGS dataset produced from this study with clinical isolates from the same timeframe and geographic regions, to identify clusters and determine potential linkages to human listeriosis cases and outbreaks, taking into consideration temporal, microbiological, and epidemiological evidence.
Acknowledgments
The following organisations and individuals are acknowledged for their contributions:
Department of Agriculture, Land Reform, and Rural Development—Directorate: Veterinary Public Health for project funding and the use of data for this study. The officials from the Department of Agriculture, Forestry, and Fisheries: Directorate:
Veterinary Public Health (Lizzy Molele, Pauline Modibane, Maphaseka Mosia, Mavis Phaswane, and Maruping Ntsatsi) for the field collection of samples for this study and Mphane Molefe for authorising funding allocation and the approval of the study.
The authors are grateful to the Agricultural Research Council: Onderstepoort Veterinary Research for providing all research facilities.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/8/1152/s1, Table S1: Listeria pathogenic islands metadata, Table S2: InlA protein sequence clusters metadata.
Author Contributions
Conceptualization, E.M., K.M.; supervision; E.M., K.R.M.; writing—original draft preparation, I.M.; methodology; I.M. writing—review and editing, K.R.M., T.M., R.P.; funding acquisition, K.M.; software, and formal analysis, R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Agriculture, Land Reform and Rural Development (DALRRD) under project number 21.1.1/VPH-01/OVI. The human resource capacity for sample collection was provided by the DALRRD—Directorate: Veterinary Public Health. Sample testing and DNA isolation was conducted by the Food and Feed Analysis and General Bacteriology Laboratories of the Agricultural Research Council: Onderstepoort Veterinary Institute.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.DAFF Abstract of Agricultural Statistics. Department of Agriculture, Forestry and Fisheries. [(accessed on 3 April 2020)];2017 Available online: https://www.daff.gov.za/Daffweb3/Portals/0/Statistics%20and%20Economic%20Analysis/Statistical%20Information/Abstract%202017.pdf.
- 2.Nyamakwere F. Ph.D. Thesis. University of Fort Hare; Fort Hare, South Africa: 2015. Microbiological Analyses of Beef Slaughtering Process and Meat Safety Knowledge of Handlers at Selected High and Low Throughput Abattoirs. [Google Scholar]
- 3.Nel H., Van Vuuren M., Swan G.E. Towards the establishment and standardization of a veterinary antimicrobial resistance surveillance and monitoring programme in South Africa. Onderstepoort. J. Vet. Res. 2004;71:239–246. doi: 10.4102/ojvr.v71i3.266. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz-Esser S., Maller A., Stessl B., Wagner M. Genomes of sequence type 121 Listeria monocytogenes strains harbor highly conserved plasmids and prophages. Front. Microbiol. 2015;6:380. doi: 10.3389/fmicb.2015.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maertens de Noordhout C., Devleesschauwer B., Angulo F.J., Verbeke G., Haagsma J., Kirk M., Havelaar A., Speybroeck N. The global burden of listeriosis: A systematic review and meta-analysis. Lancet. Infect. Dis. 2014;14:1073–1082. doi: 10.1016/S1473-3099(14)70870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camargo A.C., Woodward J.J., Call D.R., Nero L.A. Listeria monocytogenes in food-processing facilities, food contamination, and human listeriosis: The Brazilian scenario. Foodborne. Pathog. Dis. 2017;14:623–636. doi: 10.1089/fpd.2016.2274. [DOI] [PubMed] [Google Scholar]
- 7.Smith A.M., Tau N.P., Smouse S.L., Allam M., Ismail A., Ramalwa N.R., Disenyeng B., Ngomane M., Thomas J. Outbreak of Listeria monocytogenes in South Africa, 2017–2018: Laboratory Activities and Experiences Associated with Whole-Genome Sequencing Analysis of Isolates. Foodborne. Pathog. Dis. 2019;16:524–530. doi: 10.1089/fpd.2018.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas J., Govender N., McCarthy K.M., Erasmus L.K., Doyle T.J., Allam M., Ismail A., Ramalwa N., Sekwadi P., Ntshoe G., et al. Outbreak of listeriosis in South Africa associated with processed meat. N. Engl. J. Med. 2020;382:632–643. doi: 10.1056/NEJMoa1907462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Vollenhoven E. An outbreak of listeriosis in cattle and sheep ingesting potato offcuts. Tydskr. S. Afr. Vet. Ver. 1999;70:50–57. [Google Scholar]
- 10.Van Nierop W., Dusé A.G., Marais E., Aithma N., Thothobolo N., Kassel M., Stewart R., Potgieter A., Fernandes B., Galpin J.S., et al. Contamination of chicken carcasses in Gauteng, South Africa, by Salmonella, Listeria monocytogenes and Campylobacter. Int. J. Food. Microbiol. 2005;99:1–6. doi: 10.1016/j.ijfoodmicro.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Christison C.A., Lindsay D., von Holy A. Microbiological survey of ready-to-eat foods and associated preparation surfaces in retail delicatessens, Johannesburg, South Africa. Food. Control. 2008;19:727–733. doi: 10.1016/j.foodcont.2007.07.004. [DOI] [Google Scholar]
- 12.Plessis E.M.D., Govender S., Pillay B., Korsten L. Exploratory study into the microbiological quality of spinach and cabbage purchased from street vendors and retailers in Johannesburg, South Africa. J. Food Prot. 2017;80:1726–1733. doi: 10.4315/0362-028X.JFP-16-540. [DOI] [PubMed] [Google Scholar]
- 13.Matle I., Mbatha K.R., Lentsoane O., Magwedere K., Morey L., Madoroba E. Occurrence, serotypes, and characteristics of Listeria monocytogenes in meat and meat products in South Africa between 2014 and 2016. J. Food. Saf. 2019;39:1–14. doi: 10.1111/jfs.12629. [DOI] [Google Scholar]
- 14.Chen Y., Gonzalez-Escalona N., Hammack T.S., Allard M.W., Strain E.A., Brown E.W. Core genome multilocus sequence typing for identification of globally distributed clonal groups and differentiation of outbreak strains of Listeria monocytogenes. Appl. Env. Microbiol. 2016;82:6258–6272. doi: 10.1128/AEM.01532-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlier C., Perrodeau É., Leclercq A., Cazenave B., Pilmis B., Henry B., Lopes A., Maury M.M., Moura A., Goffinet F., et al. Clinical features and prognostic factors of listeriosis: The MONALISA national prospective cohort study. Lancet. Infect. Dis. 2017;17:510–519. doi: 10.1016/S1473-3099(16)30521-7. [DOI] [PubMed] [Google Scholar]
- 16.Zuber I., Lakicevic B., Pietzka A., Milanov D., Djordjevic V. Molecular characterization of Listeria monocytogenes isolates from a small- scale meat processor in Montenegro, 2011–2014. J. Food. Microbiol. 2019;79:116–122. doi: 10.1016/j.fm.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Mateus T., Silva J., Maia R.L., Teixeira P. Listeriosis during Pregnancy: A Public Health Concern. ISRN Obs. Gynecol. 2013;2013:1–6. doi: 10.1155/2013/851712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chlebicz A., Śliżewska K. Campylobacteriosis, Salmonellosis, Yersiniosis, and Listeriosis as Zoonotic Foodborne Diseases: A Review. Int. J. Env. Res. Public Health. 2018;15:863. doi: 10.3390/ijerph15050863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maury M.M., Tsai Y.H., Charlier C., Touchon M., Chenal-Francisque V., Leclercq A., Criscuolo A., Gaultier C., Roussel S., Brisabois A., et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016;48:308–313. doi: 10.1038/ng.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moura A., Criscuolo A., Pouseele H., Maury M.M., Leclercq A., Tarr C., Björkman J.T., Dallman T., Reimer A., Enouf V., et al. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbio. 2016;2:16185. doi: 10.1038/nmicrobiol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jennison A.V., Masson J.J., Fang N.X., Graham R.M., Bradbury M.I., Fegan N., Gobius K.S., Graham T.M., Guglielmino C.J., Brown J.L., et al. Analysis of the Listeria monocytogenes population structure among isolates from 1931 to 2015 in Australia. Front. Microbiol. 2017;8:1–13. doi: 10.3389/fmicb.2017.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S., Chen Y., Gorski L., Ward T.J., Osborne J., Kathariou S. Listeria monocytogenes source distribution analysis indicates regional heterogeneity and ecological niche preference among serotype 4b clones. mBio. 2018;9:1–12. doi: 10.1128/mBio.00396-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moura A., Tourdjman M., Leclercq A., Hamelin E., Laurent E., Fredriksen N., Van Cauteren D., Bracq-dieye H., Thouvenot P., Vales G., et al. Real-Time Whole-Genome Sequencing for Surveillance of Listeria monocytogenes, France. Emerg. Infect. Dis. 2017;23:1462–1470. doi: 10.3201/eid2309.170336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta A., Jordan I.K., Rishishwar L. stringMLST: A fast k-mer based tool for multilocus sequence typing. Bioinformatics. 2017;33:119–121. doi: 10.1093/bioinformatics/btw586. [DOI] [PubMed] [Google Scholar]
- 26.Seemann T. mlst Github. [(accessed on 9 April 2020)];2018 Available online: https://github.com/tseemann/mlst.
- 27.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 28.Ducey T.F., Page B., Usgaard T., Borucki M.K., Pupedis K., Ward T.J. A Single-Nucleotide-Polymorphism-Based Multilocus Genotyping Assay for Subtyping Lineage I Isolates of Listeria monocytogenes. Appl. Env. Microbiol. 2006;73:133–147. doi: 10.1128/AEM.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen L.T., Schmidt H.A., Von Haeseler A., Minh B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu G., Smith D.K., Zhu H., Guan Y., Lam T.T.Y. Ggtree: An R Package for Visualization and Annotation of Phylogenetic Trees With Their Covariates and Other Associated Data. Methods. Ecol. Evol. 2017;8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 31.Katoh K., Misawa K., Kuma K.I., Miyata T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic. Acids. Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: [(accessed on 9 April 2020)]. Available online: https://www.R-project.org/ [Google Scholar]
- 33.Oksanen A.J., Kindt R., Legendre P., Hara B.O., Simpson G.L., Stevens M.H.H., Wagner H. The vegan package. Community Ecol. Package. 2007;10:631–637. [Google Scholar]
- 34.Paradis E., Schliep K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2019;35:526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- 35.Ruppitsch W., Pietzka A., Prior K., Bletz S., Fernandez H.L., Allerberger F., Harmsen D., Mellmann A. Defining and evaluating a core genome multilocus sequence typing scheme for whole-genome sequence-based typing of Listeria monocytogenes. J. Clin. Microbiol. 2015;53:2869–2876. doi: 10.1128/JCM.01193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen E.M., Björkman J.T., Kiil K., Grant K., Dallman T., Painset A., Amar C., Roussel S., Guillier L., Félix B., et al. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready-to-eat (RTE) foods: Activity 3, the comparison of isolates from different compartments along the food chain, and from humans using whole genome sequencing (WGS) analysis. EFSA Support. Publ. 2017;14:1151E. doi: 10.2903/sp.efsa.2017.EN-1151. [DOI] [Google Scholar]
- 37.Van Walle I., Björkman J.T., Cormican M., Dallman T., Mossong J., Moura A., Pietzka A., Ruppitsch W., Takkinen J., Mattheus W., et al. Retrospective validation of whole genome sequencingenhanced surveillance of listeriosis in Europe, 2010 to 2015. Eurosurveillance. 2018;23:1–11. doi: 10.2807/1560-7917.ES.2018.23.33.1700798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeinali T., Jamshidi A., Ghasemi A., Mohammadi A. The effect of short-time microwave exposures on Salmonella typhimurium inoculated onto chicken drumettes. Iran. J. Vet. Res. 2009;10:378–382. [Google Scholar]
- 39.Chenal-Francisque V., Diancourt L., Cantinelli T., Passet V., Tran-Hykes C., Bracq-Dieye H., Leclercq A., Pourcel C., Lecuit M., Brissed S. Optimized multilocus variable-number tandem-repeat analysis assay and its complementarity with pulsed-field gel electrophoresis and multilocus sequence typing for Listeria monocytogenes clone identification and surveillance. J. Clin. Microbiol. 2013;51:1868–1880. doi: 10.1128/JCM.00606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orsi R.H., den Bakker H.C., Wiedmann M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011;301:79–96. doi: 10.1016/j.ijmm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Shimojima Y., Ida M., Nishino Y., Ishitsuka R., Kuroda S., Hirai A., Sadamasu K., Nakama A., Kai A. Multiplex PCR serogrouping of Listeria monocytogenes isolated in Japan. J. Vet. Med. Sci. 2016;78:477–479. doi: 10.1292/jvms.15-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Connor L., O’Leary M., Leonard N., Godinho M., O’Reilly C., Egan J., O’Mahony R. The characterization of Listeria spp. isolated from food products and the food-processing environment. Lett. Appl. Microbiol. 2010;51:490–498. doi: 10.1111/j.1472-765X.2010.02928.x. [DOI] [PubMed] [Google Scholar]
- 43.Braga V., Vázquez S., Vico V., Pastorino V., Mota M.I., Legnani M., Schelotto F., Lancibidad G., Varela G. Prevalence and serotype distribution of Listeria monocytogenes isolated from foods in Montevideo-Uruguay. Braz. J. Microbiol. 2017;48:689–694. doi: 10.1016/j.bjm.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leong D., Alvarez-Ordonez A., Zaouali S., Jordan K. Examination of Listeria monocytogenes in seafood processing facilities and smoked salmon in the Republic of Ireland. J. Food. Prot. 2015;78:2184–2190. doi: 10.4315/0362-028X.JFP-15-233. [DOI] [PubMed] [Google Scholar]
- 45.Jamali H., Paydar M., Ismail S., Looi C.Y., Wong W.F., Radmehr B., Abedini A. Prevalence, antimicrobial susceptibility and virulotyping of Listeria species and Listeria monocytogenes isolated from open-air fish markets. BMC Microbiol. 2015;15:144. doi: 10.1186/s12866-015-0476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nho S.W., Abdelhamed H., Reddy S., Karsi A., Lawrence M.L. Identification of high-risk Listeria monocytogenes serotypes in lineage I (serotype 1/2a, 1/2c, 3a and 3c) using multiplex PCR. J. Appl. Microbiol. 2015;119:845–852. doi: 10.1111/jam.12876. [DOI] [PubMed] [Google Scholar]
- 47.Haase J.K., Didelot X., Lecuit M., Korkeala H., Achtman M. The ubiquitous nature of Listeria monocytogenes clones: A large-scale Multilocus Sequence Typing study. Env. Microbiol. 2014;16:405–416. doi: 10.1111/1462-2920.12342. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Zhao A., Zhu R., Lan R., Jin D., Cui Z., Wang Y., Li Z., Wang Y., Xu J., et al. Genetic diversity and molecular typing of Listeria monocytogenes in China. BMC Microbiol. 2012;12:119. doi: 10.1186/1471-2180-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henri C., Leekitcharoenphon P., Carleton H.A., Radomski N., Kaas R.S., Mariet J.F., Felten A., Aarestrup F.M., Smidt P.G., Roussel S., et al. An assessment of different genomic approaches for inferring phylogeny of Listeria monocytogenes. Front. Microbiol. 2017;8:1–13. doi: 10.3389/fmicb.2017.02351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwong J.C., Mercoulia K., Tomita T., Easton M., Li H.Y., Bulach D.M., Stinear T.P., Seemann T., Howden B.P. Prospective whole-genome sequencing enhances national surveillance of Listeria monocytogenes. J. Clin. Microbiol. 2016;54:333–342. doi: 10.1128/JCM.02344-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebner R., Stephan R., Althaus D., Brisse S., Maury M., Tasara T. Phenotypic and genotypic characteristics of Listeria monocytogenes strains isolated during 2011–2014 from different food matrices in Switzerland. Food Control. 2015;57:321–326. doi: 10.1016/j.foodcont.2015.04.030. [DOI] [Google Scholar]
- 52.Stessl B., Fricker M., Fox E., Karpiskova R., Demnerova K., Jordan K., Ehling-Schulz M., Wagner M. Collaborative survey on the colonization of different types of cheese-processing facilities with Listeria monocytogenes. Foodborne Pathog. Dis. 2014;11:8–14. doi: 10.1089/fpd.2013.1578. [DOI] [PubMed] [Google Scholar]
- 53.Martín B., Perich A., Gómez D., Yangüela J., Rodríguez A., Garriga M., Aymerich T. Diversity and distribution of Listeria monocytogenes in meat processing plants. Food Microbiol. 2014;44:119–127. doi: 10.1016/j.fm.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 54.Esteban J.I., Oporto B., Aduriz G., Juste R.A., Hurtado A. A survey of food-borne pathogens in free-range poultry farms. Int. J. Food. Microbiol. 2008;123:177–182. doi: 10.1016/j.ijfoodmicro.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 55.Mammina C., Parisi A., Guaita A., Aleo A., Bonura C., Nastasi A., Pontello M. Enhanced surveillance of invasive listeriosis in the Lombardy region, Italy, in the years 2006–2010 reveals major clones and an increase in serotype 1/2a. BMC Infect. Dis. 2013;13:1. doi: 10.1186/1471-2334-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maury M.M., Bracq-Dieye H., Huang L., Vales G., Lavina M., Thouvenot P., Disson O., Leclercq A., Brisse S., Lecuit M. Hypervirulent Listeria monocytogenes clones’ adaption to mammalian gut accounts for their association with dairy products. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-019-10380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Institute for Communicable Diseases (NICD) Listeria monocytogenes Outbreak 2017/2018. [(accessed on 25 January 2018)]; Situational Report. Available online: https://www.bing.com/search?q=+listeria+monocytogenes+outbreak+2017%2F2018.+situational+report+-+25th+january+2018+.&qs=n&form=QBRE&sp=-1&pq=listeria+monocytogenes+outbreak+2017%2F2018.+situational+report+-+25th+january+2018+.&sc=0-83&sk=&cvid=1CE774E330C44606A150173989DF3CAA.
- 58.Wang Y., Luo L., Li Q., Wang H., Wang Y., Sun H., Xu J., Lan R., Ye C. Genomic dissection of the most prevalent Listeria monocytogenes clone, sequence type ST87, in China. BMC Genom. 2019;20:1–13. doi: 10.1186/s12864-019-6399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin Y., Tan W., Wang G., Kong S., Zhou X., Zhao D., Jia Y., Pan Z., Jiao X. Geographical and longitudinal analysis of Listeria monocytogenes genetic diversity reveals its correlation with virulence and unique evolution. Microbiol. Res. 2015;175:84–92. doi: 10.1016/j.micres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Nightingale K.K., Schukken Y.H., Nightingale C.R., Fortes E.D., Ho A.J., Her I.Z., Grohn Y.T., McDonough P.L., Wiedmann M. Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Appl. Environ. Microbiol. 2004;70:4458–4467. doi: 10.1128/AEM.70.8.4458-4467.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Den Bakker H.C., Desjardins C.A., Griggs A.D., Peters J.E., Zeng Q., Young S.K., Kodira C.D., Yandava C., Hepburn T.A., Haas B.J., et al. Evolutionary Dynamics of the Accessory Genome of Listeria monocytogenes. PLoS ONE. 2013;8:e67511. doi: 10.1371/journal.pone.0067511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim S.W., Haendiges J., Keller E.N., Myers R., Kim A., Lombard J.E., Karns J.S., Van Kessel J.A.S., Haley B.J. Genetic diversity and virulence profiles of Listeria monocytogenes recovered from bulk tank milk, milk filters, and milking equipment from dairies in the United States (2002 to 2014) PLoS ONE. 2018;13:1–17. doi: 10.1371/journal.pone.0197053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen M., Chen Y., Wu Q., Zhang J., Cheng J., Li F., Zeng H., Lei T., Pang R., Ye Q., et al. Genetic characteristics and virulence of Listeria monocytogenes isolated from fresh vegetables in China. BMC Microbiol. 2019;19:1–9. doi: 10.1186/s12866-019-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chenal-Francisque V., Lopez J., Cantinelli T., Caro V., Tran C., Leclercq A., Lecuit M., Brisse S. Worldwide distribution of major clones of Listeria monocytogenes. Emerg. Infect. Dis. 2011;17:1110–1112. doi: 10.3201/eid1706.101778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koopmans M.M., Engelen-Lee J.Y., Brouwer M.C., Jaspers V., Man W.K., Vall Seron M., van de Beek D. Characterization of a Listeria monocytogenes meningitis mouse model. J. Neuroinflammation. 2018;15:1–11. doi: 10.1186/s12974-018-1293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Unterholzner S.J., Poppenberger B., Rozhon W. Toxin–antitoxin systems. Mob. Genet. Elem. 2013;3:e26219. doi: 10.4161/mge.26219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hilliard A., Leong D., O’Callaghan A., Culligan E.P., Morgan C.A., Delappe N., Hill C., Jordan K., Cormican M., Gahan C.G.M. Genomic characterization of Listeria monocytogenes isolates associated with clinical listeriosis and the food production environment in Ireland. Genes. 2018;9:171. doi: 10.3390/genes9030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orsi R.H., Maron S.B., Nightingale K.K., Jerome M., Tabor H., Wiedmann M. Lineage specific recombination and positive selection in coding and intragenic regions contributed to evolution of the main Listeria monocytogenes virulence gene cluster. Infect. Genet. Evoi. 2008;8:566–576. doi: 10.1016/j.meegid.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H., Luo L., Zhang Z., Deng J., Wang Y., Miao Y., Zhang L., Chen X., Liu X., Sun S., et al. Prevalence and molecular characteristics of Listeria monocytogenes in cooked products and its comparison with isolates from listeriosis cases. Front. Med. 2018;12:104–112. doi: 10.1007/s11684-017-0593-9. [DOI] [PubMed] [Google Scholar]
- 70.Vázquez-Boland J., Kuhn M., Berche P., Chakraborty T., Domi G., González-zorn B., Wehland J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nightingale K.K., Windham K., Martin K.E., Yeung M., Wiedmann M. Select Listeria monocytogenes subtypes commonly found in foods carry distinct nonsense mutations in inlA. Appl. Env. Microbiol. 2005;71:8764–8772. doi: 10.1128/AEM.71.12.8764-8772.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.