Abstract
Invasive species are considered as one of the major threats to ecosystems worldwide. Although invasive plants are regarded as a foe, they could be considered as natural resources for valuable bioactive compounds. The present study aimed to characterize the chemical composition of the essential oil (EO) from the invasive plant Argemone ochroleuca Sweet, collected from Saudi Arabia, as well as to evaluate its phytotoxic activity. Seventy-four compounds were characterized via GC-MS analysis of EO representing 98.75% of the overall mass. The oxygenated constituents (79.01%) were found as the main constituents, including mono- (43.27%), sesqui- (17.67%), and di-terpenes (0.53%), as well as hydrocarbons (16.81%) and carotenoids (0.73%). Additionally, 19.69% from the overall mass was characterized as non-oxygenated compounds with mono- (1.77%), sesquiterpenes (17.41%), and hydrocarbons (0.56%) as minors. From all identified constituents, trans-chrysanthenyl acetate (25.71%), γ-cadinene (11.70%), oleic acid, methyl ester (7.37%), terpinene-4-ol (4.77%), dihydromyrcenol (2.90%), α-muurolene (1.77%), and γ-himachalene (1.56%) were found as abundant. The EO of A. ochroleuca showed significant phytotoxic activity against the test plant Lactuca sativa and the noxious weed Peganum harmala. The EO attained IC50 values of 92.1, 128.6, and 131.6 µL L−1 for seedling root growth, germination, and shoot growth of L. sativa, respectively, while it had IC50 values of 134.8, 145.7, and 147.9 µL L−1, respectively, for P. harmala. Therefore, this EO could be used as a bioherbicide against weeds, while further study is recommended for the characterization of the authentic materials of the main compounds in the EO as well as for the evaluation of potency of this oil on a field scale and the determination of its biosafety.
Keywords: Argemone ochroleuca, essential oil, invasive plants, oxygenated terpenes, allelopathy
1. Introduction
Plants are promising natural resources for essential oils (EOs) with a complex mixture of secondary metabolites, including mono-, sesqui-, and di-terpenoids, in addition to hydrocarbons [1,2]. The chemical compounds of EOs were biosynthesized via the different isoprenoid pathways [3]. The EOs have been described as potent biological agents such as phytotoxic [4,5,6,7], antimicrobial [8], anti-inflammatory, antipyretic [9], antiulcer [10], and hepatoprotective [11]. The bioactivities of EOs are directly correlated and associated with their chemical constituents [5]. Additionally, EOs can be used widely in several industries as controlling agents for various harmful microorganisms that cause post-harvest diseases, like phytopathogenic and food-borne organisms [12].
Plants belonging to Argemone genus are important medicinal plants [13]. Several traditional uses were described from these plants, such as expectorant, demulcent, diuretic, emetic, and treatment in chronic skin diseases [14]. The oils from A. mexicana seeds were widely used in the treatment of several diseases like intestinal infections, ulcers, dysentery, asthma, and hypertension [15,16,17]. Leaves, seeds, and flowers of A. mexicana were stated to have numerous medicinal uses, such as coughs, and maintenance of blood cholesterol and normal circulation as well as anti-venom [18,19,20,21]. The chemical characterization of Argemone plant species afforded various metabolites, such as terpenoids, alkaloids, phenolics, and flavonoids [14].
In Saudi Arabia, A. mexicana and A. ochroleuca Sweet. were recorded, while the later was described as an abundant and invasive plant in several habitats such as roadsides and disturbed areas [22]. A. ochroleuca has been reported as a toxic plant to herbivores and as noxious and competitor weed for many crops due to its allelopathic effect [23]. Control of weeds in an environmentally friendly way is often considered a challenge in agricultural practices to avoid the harmful effects of synthetic herbicides. Therefore, many studies were devoted to finding alternative products derived from natural sources [24]. The EOs are described as promising natural compounds for controlling weeds [1,4,25].
Up to our knowledge, there are no studies concerning the chemical constituents of A. ochroleuca EO. We hypothesized that A. ochroleuca is a weed, but it may contain phytotoxic constituents such as EOs that could be used as bioherbicides against other weeds; thus, if EO of A. ochroleuca has potent herbicidal activity, it could be a possible way to integrate this oil as bioherbicide and make use of this plant. Therefore, the present work provided for the first time (i) the chemical profile of EO of A. ochroleuca as well as (ii) the potential phytotoxic activity of this EO against the test plant Lactuca sativa L. and the noxious weed Peganum harmala L.
2. Results and Discussion
2.1. EO Chemical Profile
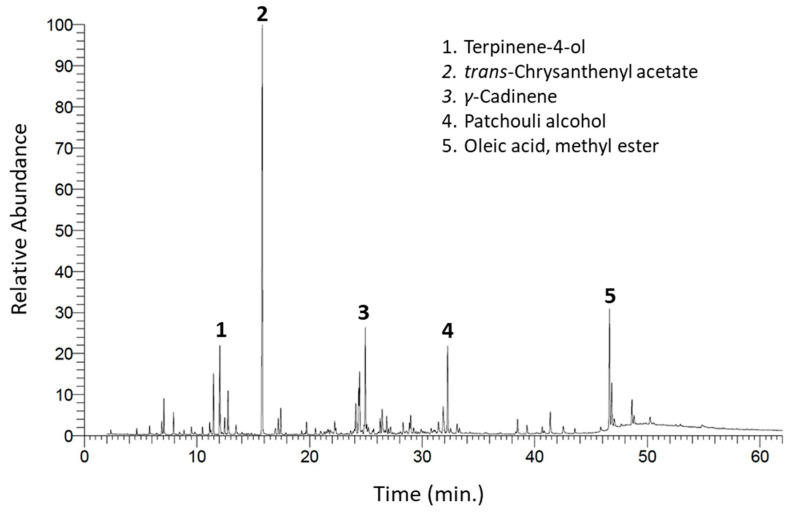
The hydrodistillation extraction of above-ground parts of A. ochroleuca provided 0.031 ± 0.001% (v/w) of a colorless oil. The GC-MS analysis of EO was performed, and the chromatogram, including representation of the main constituents over main peaks, is shown in Figure 1. The chemical profile of this plant was described here for the first time. The full chemical profile is presented in Table 1, which is composed of 70 compounds, representing 98.75% of the total mass. Among the overall identified mass, terpenes were found as the main compounds with a concentration of 80.65%, including mono-, sesqui-, and diterpenes.
Figure 1.
Gas chromatography-mass spectrometry (GC-MS) chromatogram of Argemone ochroleuca essential oil (EO). The major compounds’ peaks were numbered 1–5.
Table 1.
Essential oil constituents of above-ground parts of Argemone ochroleuca.
| No | Rt a | Conc. (%) b | Compound | KI c | Identification d | |
|---|---|---|---|---|---|---|
| Lit. | Exp. | |||||
| Monoterpenes hydrocarbons | ||||||
| 1 | 6.88 | 0.55 ± 0.02 | γ-Terpinene | 1062 | 1061 | MS, KI |
| 2 | 7.92 | 0.97 ± 0.02 | m-Cymene | 1082 | 1080 | MS, KI |
| 3 | 8.85 | 0.25 ± 0.01 | α-Terpinolene | 1088 | 1089 | MS, KI |
| Oxygenated Monoterpenes | ||||||
| 4 | 7.06 | 1.41 ± 0.04 | Eucalyptol | 1033 | 1035 | MS, KI |
| 5 | 8.46 | 0.14 ± 0.01 | cis-P-2-Menthen-1-ol | 1130 | 1129 | MS, KI |
| 6 | 9.51 | 0.42 ± 0.02 | cis-Verbenol | 1142 | 1141 | MS, KI |
| 7 | 10.49 | 1.57 ± 0.05 | Camphor | 1149 | 1151 | MS, KI |
| 8 | 11.14 | 0.63 ± 0.03 | Pinocarvone | 1158 | 1157 | MS, KI |
| 9 | 11.23 | 0.22 ± 0.02 | endo-Borneol | 1165 | 1166 | MS, KI |
| 10 | 11.47 | 2.90 ± 0.06 | Dihydromyrcenol | 1072 | 1172 | MS, KI |
| 11 | 12.03 | 4.77 ± 0.09 | Terpinene-4-ol | 1177 | 1178 | MS, KI |
| 12 | 12.46 | 0.90 ± 0.03 | p-Menth-1-en-4-ol | 1182 | 1181 | MS, KI |
| 13 | 12.76 | 2.23 ± 0.06 | α-Linalool | 1085 | 1186 | MS, KI |
| 14 | 14.01 | 0.16 ± 0.01 | α-Terpineol | 1189 | 1189 | MS, KI |
| 15 | 15.80 | 25.71 ± 0.21 | trans-Chrysanthenyl acetate | 1235 | 1237 | MS, KI |
| 16 | 17.45 | 1.45 ± 0.02 | α-Damascenone | 1391 | 1391 | MS, KI |
| 17 | 20.98 | 0.27 ± 0.01 | cis-Jasmone | 1394 | 1395 | MS, KI |
| 18 | 21.85 | 0.23 ± 0.01 | Dihydrojasmone | 1400 | 1402 | MS, KI |
| 19 | 23.65 | 0.26 ± 0.02 | α-Terpinyl propionate | 1747 | 1747 | MS, KI |
| Sesquiterpenes hydrocarbons | ||||||
| 20 | 20.52 | 0.38 ± 0.02 | α-Cubebene | 1351 | 1353 | MS, KI |
| 21 | 21.30 | 0.16 ± 0.01 | alfa.Copaene alfa.Copaene | 1376 | 1377 | MS, KI |
| 22 | 23.99 | 0.14 ± 0.01 | Isocaryophillene | 1413 | 1411 | MS, KI |
| 23 | 24.69 | 0.23 ± 0.02 | Aromandendrene | 1439 | 1438 | MS, KI |
| 24 | 25.69 | 0.34 ± 0.03 | Dehydroaromadendrene | 1466 | 1464 | MS, KI |
| 25 | 26.28 | 0.79 ± 0.03 | β-Cadinene | 1473 | 1472 | MS, KI |
| 26 | 26.45 | 1.56 ± 0.05 | γ-Himachalene | 1479 | 1479 | MS, KI |
| 27 | 30.80 | 0.34 ± 0.02 | γ-Muurolene | 1477 | 1478 | MS, KI |
| 28 | 24.11 | 1.77 ± 0.04 | α-Muurolene | 1499 | 1497 | MS, KI |
| 29 | 24.95 | 11.70 ± 0.08 | γ-Cadinene | 1513 | 1512 | MS, KI |
| Oxygenated sesquiterpenes | ||||||
| 30 | 19.30 | 0.22 ± 0.02 | Nerolidol | 1534 | 1535 | MS, KI |
| 31 | 21.70 | 0.18 ± 0.01 | Davana furan | 1399 | 1398 | MS, KI |
| 32 | 21.62 | 0.20 ± 0.01 | 2,6-Di-tert-butyl-4-hydroxy 4-methyl-2,5-cyclohexadien-1-one |
1478 | 1479 | MS, KI |
| 33 | 22.23 | 0.76 ± 0.02 | Davana ether | 1483 | 1483 | MS, KI |
| 34 | 22.34 | 0.32 ± 0.01 | 4-epi-cubedol | 1494 | 1496 | MS, KI |
| 35 | 25.23 | 0.86 ± 0.02 | 3-methyl-2-butenoic acid, 2,7-dimethyloct-7-en-5-yn-4-yl ester |
1521 | 1523 | MS, KI |
| 36 | 25.60 | 0.20 ± 0.01 | Isolongifolan-8-ol | 1531 | 1531 | MS, KI |
| 37 | 26.85 | 1.03 ± 0.03 | Epiglobulol | 1557 | 1558 | MS, KI |
| 38 | 28.31 | 0.71 ± 0.02 | Ledol | 1565 | 1566 | MS, KI |
| 39 | 28.87 | 0.63 ± 0.02 | Spathulenol | 1575 | 1574 | MS, KI |
| 40 | 28.99 | 1.11 ± 0.04 | Caryophyllene oxide | 1581 | 1583 | MS, KI |
| 41 | 29.24 | 0.34 ± 0.02 | Davanone | 1588 | 1589 | MS, KI |
| 42 | 29.46 | 0.14 ± 0.01 | Isoaromadendrene epoxide | 1594 | 1594 | MS, KI |
| 43 | 29.91 | 0.34 ± 0.02 | salvial-4(14)-en-1-one | 1595 | 1593 | MS, KI |
| 44 | 31.06 | 0.20 ± 0.01 | Widdrol | 1597 | 1598 | MS, KI |
| 45 | 31.16 | 0.17 ± 0.01 | Rosifoliol | 1613 | 1612 | MS, KI |
| 46 | 31.45 | 0.73 ± 0.02 | Fonenol | 1627 | 1625 | MS, KI |
| 47 | 31.87 | 2.00 ± 0.04 | Agarospirol | 1646 | 1647 | MS, KI |
| 48 | 32.26 | 5.25 ± 0.06 | Patchouli alcohol | 1659 | 1661 | MS, KI |
| 49 | 32.53 | 0.31 ± 0.02 | Longifolenaldehyde | 1668 | 1668 | MS, KI |
| 50 | 33.10 | 0.62 ± 0.02 | Juniper camphor | 1691 | 1690 | MS, KI |
| 51 | 33.31 | 0.33 ± 0.03 | Hexahydrofarnesyl acetone | 1845 | 1844 | MS, KI |
| 52 | 38.47 | 0.87 ± 0.03 | E, E-Farnesyl acetone | 1918 | 1920 | MS, KI |
| 53 | 40.83 | 0.15 ± 0.01 | α-Acorenol | 2135 | 2134 | MS, KI |
| Oxygenated diterpenes | ||||||
| 54 | 47.06 | 0.53 ± 0.03 | Phytol | 1942 | 1942 | MS, KI |
| Non-oxygenated hydrocarbons | ||||||
| 55 | 5.79 | 0.41 ± 0.02 | 2-n-Pentylfuran | 993 | 994 | MS, KI |
| 56 | 29.62 | 0.15 ± 0.01 | n-Hexadecane | 1600 | 1600 | MS, KI |
| Oxygenated hydrocarbons | ||||||
| 57 | 2.35 | 0.16 ± 0.01 | n-Hexanal | 800 | 801 | MS, KI |
| 58 | 4.66 | 0.21 ± 0.02 | n-Nonanal | 1098 | 1097 | MS, KI |
| 59 | 9.81 | 0.17 ± 0.01 | 5-Ethyl-3-hepten-2-one | 1124 | 1126 | MS, KI |
| 60 | 27.01 | 0.29 ± 0.02 | 2-Methoxy-1,4-benzenediol | 1473 | 1475 | MS, KI |
| 61 | 40.66 | 0.42 ± 0.02 | 7,9-Ditertbutyl-1-oxaspiro-(4,5)- deca-6,9-diene-2,8-dione |
1775 | 1775 | MS, KI |
| 62 | 41.38 | 1.34 ± 0.04 | Dibutyl phthalate | 1868 | 1869 | MS, KI |
| 63 | 42.53 | 0.48 ± 0.02 | Palmitic acid, methyl ester | 1926 | 1924 | MS, KI |
| 64 | 43.56 | 0.32 ± 0.02 | Ethyl palmitate | 1994 | 1995 | MS, KI |
| 65 | 45.86 | 0.39 ± 0.02 | Linoleic acid, methyl ester | 2092 | 2090 | MS, KI |
| 66 | 46.64 | 7.37 ± 0.11 | Oleic acid, methyl ester | 2108 | 2110 | MS, KI |
| 67 | 46.84 | 2.58 ± 0.09 | Gamolenic acid | 2143 | 2141 | MS, KI |
| 68 | 47.69 | 0.14 ± 0.01 | Ethyl oleate | 2161 | 2163 | MS, KI |
| 69 | 48.63 | 1.56 ± 0.05 | Oleic acid | 2179 | 2177 | MS, KI |
| 70 | 48.83 | 0.53 ± 0.02 | n-Nonadecanoic acid | 2236 | 2237 | MS, KI |
| 71 | 49.85 | 0.38 ± 0.01 | 18-Nonadecenoic acid | 2256 | 2256 | MS, KI |
| 72 | 50.24 | 0.47 ± 0.02 | Ethyl-9,12-octadecadienoate | 2527 | 2525 | MS, KI |
| Carotenoid-derived compounds | ||||||
| 73 | 16.98 | 0.49 ± 0.02 | Dihydroedulan II | 1284 | 1284 | MS, KI |
| 74 | 25.08 | 0.24 ± 0.01 | trans-α-Ionone | 1456 | 1458 | MS, KI |
a Rt: retention time, b compound concentration ± standard division; c KI: published Kovats retention indices (Lit); and experimental Kovats index (Exp.) relative to n-alkanes (C8–C28) (Exp); d EO constituents identification was performed via comparison of the mass spectral and Kovats indices (KI) with those of NIST Mass Spectral Library (2011) and Wiley Registry of Mass Spectral Data 8th edition and literature.
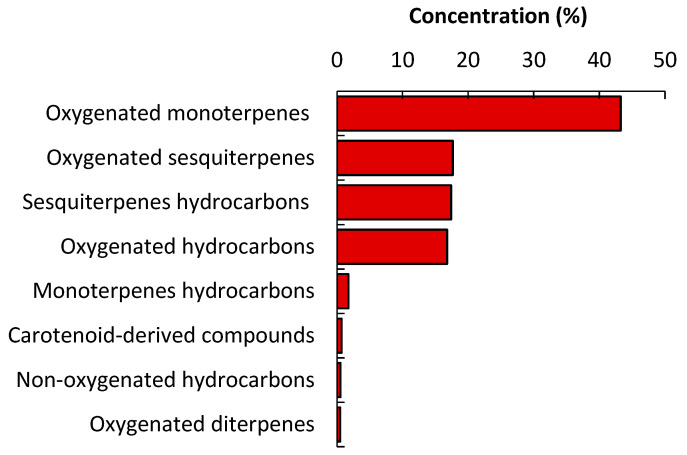
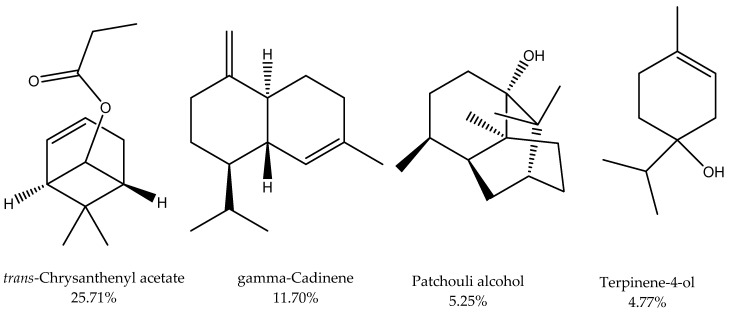
This result is in harmony with almost all of the described EOs derived from the plant kingdom [26]. The EOs derived from plants were characterized by the abundance of compounds structurally based on isoprene units, especially the terpenoids [3]. Monoterpenes were characterized as the major compounds (Figure 2), representing 45.05%, including oxygenated monoterpenes (43.27%) and monoterpenes hydrocarbons (1.77%). Fifteen monoterpenes in oxygenated forms were found to be the main class of compounds among all the identified constituents with an abundance of trans-chrysanthenyl acetate (25.71%, Figure 3), terpinene-4-ol (4.77%, Figure 3), and dihydromyrcenol (2.90%). However, cis-P-2-menthen-1-ol was represented as the minor compound from all the identified oxygenated compounds. On the other side, three monoterpene hydrocarbons were identified representing 1.77% of the whole mass including m-cymene (0.97%), γ-terpinene (0.55%), and α-terpinolene (0.25%). The trans-Chrysanthenyl acetate, a major monoterpene in our study, was already reported as the main compound in EOs of numerous plant species belonging to different families such as Artemisia absinthium [27], Artemisia herba-alba [28], Chrysanthemum coronarium [29]. Anthemis maritima [30], Tanacetum santolinoides [31], Bupleurum montanum, and B. plantagineum [32].
Figure 2.
Concentrations of different classes of the compounds in the EO of Argemone ochroleuca.
Figure 3.
Representative structures of the main compounds.
The sesquiterpenes were the second characteristic compounds in EO of A. ochroleuca, with a concentration of 35.08% comprising oxygenated (17.67%) and non-oxygenated compounds (17.41%). Out of twenty-three oxygenated sesquiterpenes, patchouli alcohol (5.25%, Figure 3) and agarospirol (2.00%) were identified as main compounds, while isoaromadendrene epoxide was found as a minor one. Furthermore, nine sesquiterpene hydrocarbons were identified, including γ-cadinene (11.70%, Figure 3), α-muurolene (1.77%), and γ-himachalene (1.56%), as majors, while isocaryophillene (0.14%) was identified as a minor one. Patchouli alcohol (Figure 3) has been described as a potent medicinal compound with various activities such as anti-influenza [33], anti-tumor [34], and anti-inflammatory [35]. Rifai and Soekamto [36] described the purification and abundance of patchouli alcohol in EO derived from Pogostemon cablin.
Diterpenes were rarely characterized in the EOs derived from the plant kingdom [37]. Phytol, a common oxygenated diterpenoid in EOs of plant species, was the only identified compound in EO of A. ochroleuca with a low concentration (0.53%). The identification of this compound in the EO of this plant was in agreement with that reported in A. mexicana [38].
Hydrocarbons (17.37%) represented remarkable constituents of the A. ochroleuca EO, including oxygenated (16.81%) and non-oxygenated compounds (0.56%) (Figure 2). Among all identified hydrocarbons, 16 oxygenated compounds were characterized, with abundance of oleic acid, methyl ester (7.37%), and gamolenic acid (2.58%), while ethyl oleate (0.14%) was detected as minor compound. Additionally, only two compounds (2-n-pentylfuran and n-hexadecane) were identified as non-oxygenated hydrocarbons. The carotenoids, including dihydroedulan II and trans-α-ionone, were characterized in the EO of A. ochroleuca with a concentration of 0.49% and 0.24%, respectively. It is pertinent to mention here that carotenoids (tetraterpenoids) are reported as abundant pigments in numerous EOs derived from wild plants, vegetables, and fruits [39].
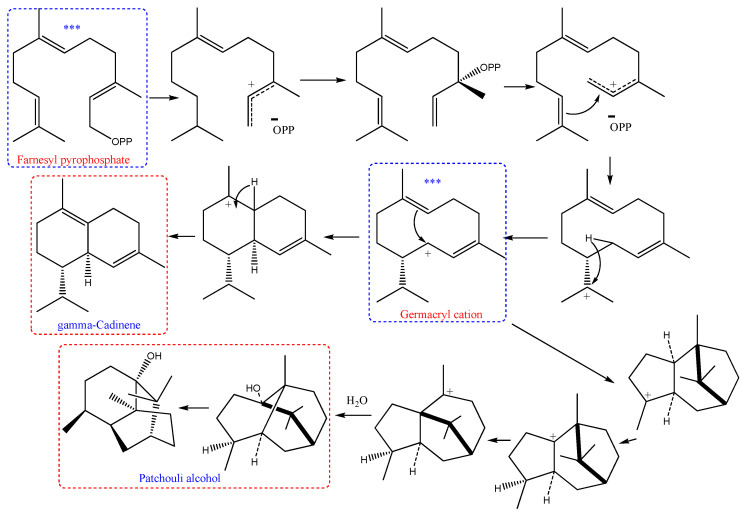
The biosynthetic pathways of the identified compounds might describe the relationship between all the constituents due to the similar starting and/or intermediate compounds as well as the pathway itself. The plausible biosynthetic pathways of the two major compounds, γ-cadinene, and patchouli alcohol, as examples, were united at the start with intermediate-compound, farnesyl pyrophosphate that biosynthetically transformed to germacryl cation [40,41] as described in Figure 4. From this brief overview, we can detect the theory that the biosynthetic of the constituents of EOs might establish the relationships between these constituents during the growth of the plant.
Figure 4.
Plausible biosynthetic pathway of gamma-cadinene [40] and patchouli alcohol [41]. *** the common starting and intermediate in biosynthetic of both compounds.
2.2. Phytotoxic Activity of the A. ochroleuca Essential Oil
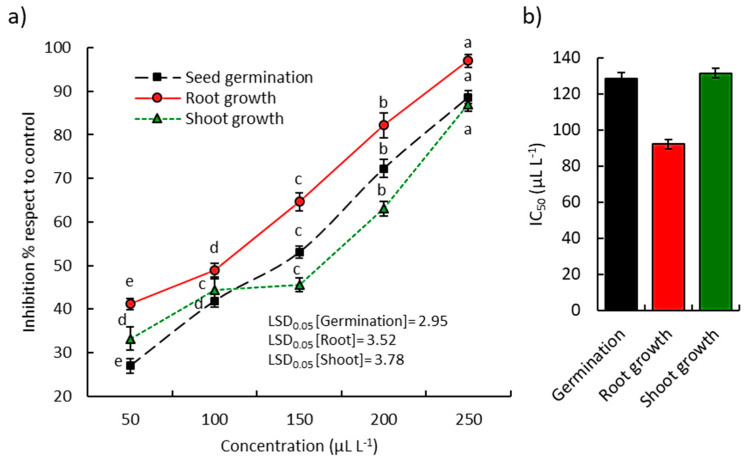
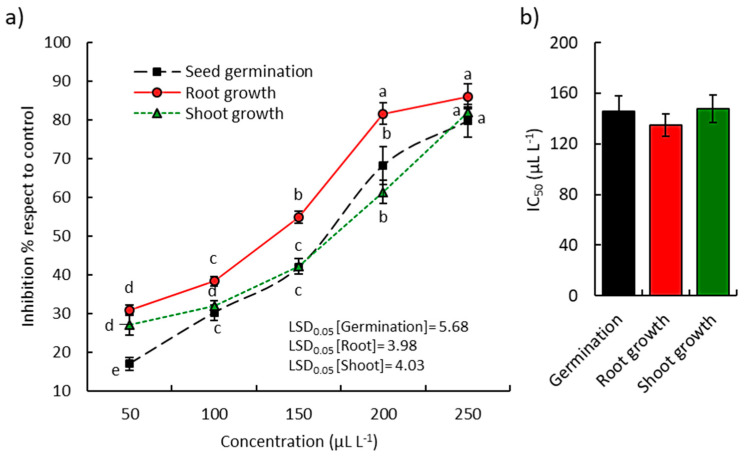
The EO from the above-ground parts of A. ochroleuca showed significant phytotoxic activity on seed germination and seedling development of L. sativa (Figure 5a) and P. harmala (Figure 6a).
Figure 5.
Phytotoxic activity of Argemone ochroleuca essential oil on the germination, root, and shoot growth of Lactuca sativa. (a) Effect of different concentrations and (b) the IC50 values. Different letters per each line indicate significant differences among treatments at p ≤ 0.05 (Tukey’s HSD test).
Figure 6.
Phytotoxic activity of Argemone ochroleuca essential oil on the germination, root, and shoot growth of Peeganum harmala. (a) Effect of different concentrations and (b) the IC50 values. Different letters per each line indicate significant differences among treatments at p ≤ 0.05 (Tukey’s HSD test).
At the highest concentration of the EO (250 µL L−1), the seed germination, shoot growth, and root growth of the L. sativa seedling were reduced by 88.6, 86.9, and 97.0%, respectively (Figure 5a). According to IC50 values, the root was the most inhibited with an IC50 value of 92.1 µL L−1 (Figure 5b), followed by germination (128.6 µL L−1) and finally the shoot (131.6 µL L−1). On the other hand, the EO of A. ochroleuca at the lowest concentrations (50, 100, and 150 µL L−1) revealed significant phytotoxic activity against the germination, shoot growth, and root growth of the noxious weed P. harmala, while at the highest concentrations (200 and 250 µL L−1) it revealed a sharp increase in the phytotoxicity (Figure 6). Based on the IC50, the root of P. harmala showed the lowest value (134.8 µL L−1), while the seed germination and shoot growth attained IC50 values of 145.7 and 147.9 µL L−1, respectively (Figure 6).
It is clear that the roots of both L. sativa and P. harmala seedling were more affected with the EO than shoots, and this could be attributed to the direct contact with the EO as well as the permeability of the root membrane [24,42]. In the present study, the potent phytotoxic activity of the A. ochroleuca EO could be ascribed to the presence of high oxygenated compounds, particularly the major compounds such as trans-chrysanthenyl acetate, γ-cadinene, oleic acid-methyl ester, patchouli alcohol, and terpinene-4-ol. The oxygenated compounds of the EOs were reported to have stronger biological activities than non-oxygenated ones [1,2,43,44]. The bicyclic sesquiterpene γ-cadinene was reported to have larvicidal activity against malaria, dengue, and filariasis mosquitoes [45], and it is also reported to have antimicrobial activity [46]. In addition, the EO of Annona salzmannii showed potent trypanocidal and antitumor activities due to its high content of γ-cadinene [47]. The fatty acid methyl esters have been reported to possess larvicidal activity [48] and antibacterial activity [49].
These compounds may act individually or in synergy as phytotoxic agents (allelochemicals). Compared to other reported EOs, the A. ochroleuca EO is more phytotoxic against lettuce (plant model) than the EO of Teucrium polium [50], Eucalyptus grandis and E. citriodora [51], Acacia cyanophylla [52], and Eremanthus erythropappus [53]. The most abundant compound, trans-chrysanthenyl acetate, has been reported as the main compound of other plants’ EO, with phytotoxic activity, such as Artemisia herba-alba [28] and Chrysanthemum coronarium [29]. In addition, the EO rich in trans-chrysanthenyl acetate has been reported to have antimicrobial and antioxidant activities [54,55].
The major compound γ-cadinene has been reported as major constituent (18.4%) in the essential oil of Eupatorium adenophorum, which showed a phytotoxic activity against Phalaris minor and Triticum aestivum [56]. Additionally, the EO of Schinus lentiscifolius showed a phytotoxic effect on lettuce due to the presence of high content of γ-cadinene [57]. Although the other major compounds have not been reported as allelochemicals, they could participate in the phytotoxic effect of the A. ochroleuca EO, particularly the patchouli alcohol, and terpinene-4-ol, which are oxygenated terpenes [58].
Several modes of action of EOs as allelochemicals were reported, including the inhibition of permeability, cell division, photosynthesis, respiration, enzyme activities, and genomic materials [59,60]. However, the specific mode(s) of action of the major identified terpene compounds in the present study, either alone or in combinations, needs further investigation.
It is worth mentioning that the weed P. harmala is considered a noxious weed in several countries, including Saudi Arabia, where it is widely distributed in the northern regions [61]. It is hard to control and needs powerful herbicides or manual uprooting; no reported biological control methods for the P. harmala is available [62]. In this context, the present study revealed the potentiality of A. ochroleuca EO to control P. harmala, this noxious weed, as an eco-friendly bioherbicide, where this oil showed strong phytotoxicity against this weed.
3. Material and Methods
3.1. Plant Materials Collection, Identification, and Preparation
The above-ground parts of A. ochroleuca were collected from a roadside habitat, Al Assir village, Taif, western Saudi Arabia (21°11′27.2″ N 40°40′05.9″ E). The plant specimen was identified according to Chaudhary [63] by Dr. Abdulaziz Assaeed, Professor of Range Ecology, Department of Plant Production, College of Sciences, King Saud University, Saudi Arabia. A voucher specimen of the collected plant is released in the herbarium of King Saud University, with code: KSU-0160115001. The above-ground parts of the healthy plants were collected in paper bags and transferred to the laboratory. The plant materials were dried in shade at room temperature (28 ± 3 °C) for two weeks (until complete dryness), ground into a fine powder, and packed in a paper bag.
3.2. Essential Oil Extraction, GC-MS Analysis, and Constituents’ Identification
The EOs were extracted by hydrodistillation from two samples of A. ochroleuca above-ground parts via a Clevenger-type apparatus for three hours. The oil layer was collected, and water was removed by 0.5 g of anhydrous Na2SO4; they were stored in a dark glass vial at 4 °C till further analysis. The yields of the extracted EOs were calculated via the equation 100× (V/W), where V: volume of extracted EO, and W: weight of the plant material used in extraction. The chemical composition of the EO samples was analyzed and identified separately by gas chromatography-mass spectrometry (GC-MS) as described in our previously documented work [2,4].
In brief, GC-MS analysis was carried out at the Department of Medicinal and Aromatic Plants Research, National Research Center, Giza, Egypt, using the GC-MS instrument which has TRACE GC Ultra Gas Chromatographs (THERMO Scientific™. Corporate, Waltham, MA, USA) and Thermo Scientific ISQ™ EC single quadrupole mass spectrometer. The GC-MS system is equipped with a TR-5 MS column with dimensions of 30 m × 0.32 mm i.d., 0.25 µm film thickness. At flow rate of 1.0 mL min−1, helium was used as carrier gas with split ratio of 1:10. The temperature program was 60 °C for 1 min, rising by 4.0 °C min−1 to 240 °C and held for 1 min. A diluted sample in hexane (1 µL) at a ratio of 1:10 (v/v) was injected, and the injector and detector were held at 210 °C. Mass spectra were recorded by electron ionization (EI) at 70 eV, using a spectral range of m/z 40–450. The identification of the chemical constituents of the EOs was achieved using Automated Mass spectral Deconvolution and Identification (AMDIS) software, Wiley spectral library collection, NIST library database, retention indices relative to n-alkanes (C8–C22), or appraisal of the mass spectrum with authentic standards.
3.3. Phytotoxic Bioassay of the A. ochroleuca Essential Oil
The phytotoxicity of the A. ochroleuca EO was performed against L. sativa L. as a standard test plant, where it is known to be very sensitive to allelochemicals [64] as well as the noxious weed P. harmala. In brief, the seeds of lettuce were purchased from the Agriculture Research Center, Cairo, Egypt, while the seeds of P. harmala were collected from Taif, southeast Saudi Arabia. Seeds with uniform size and color were carefully chosen and surface-sterilized via sodium hypochlorite (0.3%). Prior to the experiment, the viability of the seed was performed by the germination of seeds in the Petri plate lined with a filter paper (Whatman No. 1) using distilled water at 25 °C with adjusted light conditions of 16/8 h light/dark cycle. The germination percentage was 97.23 ± 0.5% for L. sativa and 90.12 ± 1.02% for P. harmala.
To assess the phytotoxicity of the EO, various concentrations (50, 100, 150, 200, and 250 µL L−1) were prepared by dilution using 1% Tween® 80 (Sigma-Aldrich, Darmstadt, Germany) as an emulsifying agent. In Petri plates, 20 sterilized seeds of either L. sativa or P. harmala were spread on sterilized filter paper (Whatman No. 1), 4 mL of each concentration or control (Tween® 80) was poured, and the plates were sealed with Parafilm® tape (Sigma, St. Louis, MO, USA). Five plates were prepared per each concentration, and the experiment was repeated three times. A total of 180 plates (2 plants × 6 treatments [5 concentrations + control] × 5 plates as replications × 3 times) were prepared and incubated in a growth chamber at 25 °C with adjusted light conditions of 16 h/8 h light/dark cycle. After 5 days of incubation for L. sativa and 7 days for P. harmala, the reduction in seed germination, shoot growth, and root growth of the seedlings were calculated based on the following equation:
| (1) |
where N is the number of germinated seeds and L is the length of seedling root or shoot.
3.4. Statistical Analysis
The experiment of bioassay was designed as a completely randomized design and repeated three times with five replications per each treatment. The data of seed germination and seedling growth inhibition were subjected to one-way ANOVA and followed by Duncan’s HSD post hoc test at a probability level of 0.05.
4. Conclusions
The chemical composition of A. ochroleuca EO was characterized with highly oxygenated constituents (79.01%), including mono-, sesqui-, di-terpenoids, carotenoids, and hydrocarbons. The trans-chrysanthenyl acetate, γ-cadinene, oleic acid, methyl ester, patchouli alcohol, and terpinene-4-ol were determined as the main compounds. The A. ochroleuca EO exhibited significant phytotoxic activity. The high oxygenation of the EOs constituents was deduced to be correlated with the increase of the phytotoxicity. Therefore, the substantial phytotoxicity of A. ochroleuca EO could be ascribed to its high content of the oxygenated compounds (78.28%), and thereby it could be used as an eco-friendly bioherbicide. However, further study is recommended for characterization of the main identified compounds, either singular or in combination at field scale level, as well as for the evaluation of their modes of action and biosafety.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1441-302). Further, the authors would like to sincerely thank the National Research Centre, and Department of Botany, Faculty of Science, Mansoura University, Egypt.
Author Contributions
Conceptualization, A.M.A.-E., A.M.A., and A.I.E.; Investigation, A.M.A.-E., A.E.-N.G.E.G., A.M.A., B.A.D. and A.I.E.; Methodology, A.M.A.-E., A.M.A., and A.I.E.; Validation, A.M.A.-E., A.M.A., S.L.A.-R., and A.I.E.; Funding acquisition, S.L.A.-R.; Writing—original draft, A.M.A.-E. and A.I.E.; Writing—review and editing, A.M.A.-E., A.E.-N.G.E.G., A.M.A., S.L.A.-R., E.A.O., B.A.D., W.A.A.-T., and A.I.E. All authors have read and agreed to the published version of the manuscript.
Funding
Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1441-302) and the APC was funded also by Deanship of Scientific Research at King Saud University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Assaeed A., Elshamy A., El Gendy A., Dar B., Al-Rowaily S., Abd-ElGawad A. Sesquiterpenes-rich essential oil from above ground parts of Pulicaria somalensis exhibited antioxidant activity and allelopathic effect on weeds. Agronomy. 2020;10:399. doi: 10.3390/agronomy10030399. [DOI] [Google Scholar]
- 2.Abd El-Gawad A.M., Elshamy A.I., El Gendy A.E.-N., Gaara A., Assaeed A.M. Volatiles profiling, allelopathic activity, and antioxidant potentiality of Xanthium strumarium leaves essential oil from Egypt: Evidence from chemometrics analysis. Molecules. 2019;24:584. doi: 10.3390/molecules24030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharifi-Rad J., Sureda A., Tenore G.C., Daglia M., Sharifi-Rad M., Valussi M., Tundis R., Sharifi-Rad M., Loizzo M.R., Ademiluyi A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules. 2017;22:70. doi: 10.3390/molecules22010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elshamy A., Abd-ElGawad A.M., El-Amier Y.A., El Gendy A., Al-Rowaily S. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019;34:316–328. doi: 10.1002/ffj.3512. [DOI] [Google Scholar]
- 5.Abd-ElGawad A.M., Elshamy A., Al-Rowaily S., El-Amier Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants. 2019;8:482. doi: 10.3390/plants8110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abd El-Gawad A.M., El-Amier Y.A., Bonanomi G. Essential oil composition, antioxidant and allelopathic activities of Cleome droserifolia (Forssk.) Delile. Chem. Biodivers. 2018;15:e1800392. doi: 10.1002/cbdv.201800392. [DOI] [PubMed] [Google Scholar]
- 7.Abd El-Gawad A.M., El-Amier Y.A., Bonanomi G. Allelopathic activity and chemical composition of Rhynchosia minima (L.) DC. essential oil from Egypt. Chem. Biodivers. 2018;15:e1700438. doi: 10.1002/cbdv.201700438. [DOI] [PubMed] [Google Scholar]
- 8.Deng W., Liu K., Cao S., Sun J., Zhong B., Chun J. Chemical composition, antimicrobial, antioxidant, and antiproliferative properties of grapefruit essential oil prepared by molecular distillation. Molecules. 2020;25:217. doi: 10.3390/molecules25010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elshamy A.I., Ammar N.M., Hassan H.A., Al-Rowaily S.L., Raga T.R., El Gendy A., Abd-ElGawad A.M. Essential oil and its nanoemulsion of Araucaria heterophylla resin: Chemical characterization, anti-inflammatory, and antipyretic activities. Ind. Crop. Prod. 2020;148:112272. doi: 10.1016/j.indcrop.2020.112272. [DOI] [Google Scholar]
- 10.Arunachalam K., Balogun S.O., Pavan E., de Almeida G.V.B., de Oliveira R.G., Wagner T., Cechinel Filho V., de Oliveira Martins D.T. Chemical characterization, toxicology and mechanism of gastric antiulcer action of essential oil from Gallesia integrifolia (Spreng.) Harms in the in vitro and in vivo experimental models. Biomed. Pharmacother. 2017;94:292–306. doi: 10.1016/j.biopha.2017.07.064. [DOI] [PubMed] [Google Scholar]
- 11.Damtie D., Braunberger C., Conrad J., Mekonnen Y., Beifuss U. Composition and hepatoprotective activity of essential oils from Ethiopian thyme species (Thymus serrulatus and Thymus schimperi) J. Essent. Oil Res. 2019;31:120–128. doi: 10.1080/10412905.2018.1512907. [DOI] [Google Scholar]
- 12.Mehdizadeh L., Moghaddam M. Essential oils: Biological activity and therapeutic potential. In: Alina A.G., Holban M., editors. Therapeutic, Probiotic, and Unconventional Foods. Academic Press, Elsevier; London, UK: 2018. pp. 167–179. [Google Scholar]
- 13.Sharma J., Gairola S., Gaur R., Painuli R. The treatment of jaundice with medicinal plants in indigenous communities of the Sub-Himalayan region of Uttarakhand, India. J. Ethnopharmacol. 2012;143:262–291. doi: 10.1016/j.jep.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 14.Brahmachari G., Gorai D., Roy R. Argemone mexicana: Chemical and pharmacological aspects. Rev. Bras. Farmacogn. 2013;23:559–567. doi: 10.1590/S0102-695X2013005000021. [DOI] [Google Scholar]
- 15.Prajapati N.D. A Handbook of Medicinal Plants: A Complete Source Book. Agrobios; Johdhpur, India: 2003. [Google Scholar]
- 16.Savithramma N., Sulochana C., Rao K. Ethnobotanical survey of plants used to treat asthma in Andhra Pradesh, India. J. Ethnopharmacol. 2007;113:54–61. doi: 10.1016/j.jep.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Bieski I.G.C., Rios Santos F., de Oliveira R.M., Espinosa M.M., Macedo M., Albuquerque U.P., de Oliveira Martins D.T. Ethnopharmacology of medicinal plants of the pantanal region (Mato Grosso, Brazil) Evid. Based Complementary Altern. Med. 2012;2012:1–36. doi: 10.1155/2012/272749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Albuquerque U.P., Monteiro J.M., Ramos M.A., de Amorim E.L.C. Medicinal and magic plants from a public market in northeastern Brazil. J. Ethnopharmacol. 2007;110:76–91. doi: 10.1016/j.jep.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Makhija I.K., Khamar D. Anti-snake venom properties of medicinal plants. Der Pharm. Lett. 2010;2:399–411. [Google Scholar]
- 20.Minu V., Harsh V., Ravikant T., Paridhi J., Noopur S. Medicinal plants of Chhattisgarh with anti-snake venom property. Int. J. Curr. Pharm. Res. 2012;3:1–10. [Google Scholar]
- 21.Brahmachari G., Roy R., Mandal L.C., Ghosh P.P., Gorai D. A new long-chain secondary alkanediol from the flowers of Argemone mexicana. J. Chem. Res. 2010;34:656–657. doi: 10.3184/030823410X12888074368680. [DOI] [Google Scholar]
- 22.Thomas J., El-Sheikh M.A., Alfarhan A.H., Alatar A.A., Sivadasan M., Basahi M., Al-Obaid S., Rajakrishnan R. Impact of alien invasive species on habitats and species richness in Saudi Arabia. J. Arid Environ. 2016;127:53–65. doi: 10.1016/j.jaridenv.2015.10.009. [DOI] [Google Scholar]
- 23.Dar B.A., Al-Rowaily S.L., Assaeed A.M., El-Bana M.I., Hegazy A.K., Malik J.A. Allelopathic potential of Argemone ochroleuca from different habitats on seed germination of native species and cultivated crops. Pak. J. Bot. 2017;49:1841–1848. [Google Scholar]
- 24.Abd El-Gawad A.M. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crops Prod. 2016;80:36–41. doi: 10.1016/j.indcrop.2015.10.054. [DOI] [Google Scholar]
- 25.Abd-ElGawad A.M., Elshamy A., El-Amier Y.A., El Gendy A., Al-Barati S., Dar B., Al-Rowaily S., Assaeed A. Chemical composition variations, allelopathic, and antioxidant activities of Symphyotrichum squamatum (Spreng.) Nesom essential oils growing in heterogeneous habitats. Arab. J. Chem. 2020;13:237–4245. doi: 10.1016/j.arabjc.2019.07.005. [DOI] [Google Scholar]
- 26.Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines. 2016;3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Judzentiene A., Budiene J. Compositional variation in essential oils of wild Artemisia absinthium from Lithuania. J. Essent. Oil Bear. Plants. 2010;13:275–285. doi: 10.1080/0972060X.2010.10643822. [DOI] [Google Scholar]
- 28.Arroyo A.I., Pueyo Y., Pellissier F., Ramos J., Espinosa-Ruiz A., Millery A., Alados C.L. Phytotoxic effects of volatile and water soluble chemicals of Artemisia herba-alba. J. Arid Environ. 2018;151:1–8. doi: 10.1016/j.jaridenv.2017.11.010. [DOI] [Google Scholar]
- 29.Hosni K., Hassen I., Sebei H., Casabianca H. Secondary metabolites from Chrysanthemum coronarium (Garland) flowerheads: Chemical composition and biological activities. Ind. Crop. Prod. 2013;44:263–271. doi: 10.1016/j.indcrop.2012.11.033. [DOI] [Google Scholar]
- 30.Ciccarelli D., Noccioli C., Pistelli L. Chemical composition of essential oils and aromatic waters from different Italian Anthemis maritima populations. Chem. Biodivers. 2013;10:1667–1682. doi: 10.1002/cbdv.201300006. [DOI] [PubMed] [Google Scholar]
- 31.El-Shazly A., Dorai G., Wink M. Composition and antimicrobial activity of essential oil and hexane-ether extract of Tanacetum santolinoides (DC.) Feinbr. and Fertig. Z. Naturforsch. C. 2002;57:620–623. doi: 10.1515/znc-2002-7-812. [DOI] [PubMed] [Google Scholar]
- 32.Laouer H., Hirèche-Adjal Y., Prado S., Boulaacheb N., Akkal S., Singh G., Singh P., Isidorov V.A., Szczepaniak L. Chemical composition and antimicrobial activity of essential oil of Bupleurum montanum and B. plantagineum. Nat. Prod. Commun. 2009;4:1605–1610. doi: 10.1177/1934578X0900401130. [DOI] [PubMed] [Google Scholar]
- 33.Kiyohara H., Ichino C., Kawamura Y., Nagai T., Sato N., Yamada H. Patchouli alcohol: In vitro direct anti-influenza virus sesquiterpene in Pogostemon cablin Benth. J. Nat. Med. 2012;66:55–61. doi: 10.1007/s11418-011-0550-x. [DOI] [PubMed] [Google Scholar]
- 34.Jeong J.B., Choi J., Lou Z., Jiang X., Lee S.-H. Patchouli alcohol, an essential oil of Pogostemon cablin, exhibits anti-tumorigenic activity in human colorectal cancer cells. Int. Immunopharmacol. 2013;16:184–190. doi: 10.1016/j.intimp.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Li Y.-C., Xian Y.-F., Ip S.-P., Su Z.-R., Su J.-Y., He J.-J., Xie Q.-F., Lai X.-P., Lin Z.-X. Anti-inflammatory activity of patchouli alcohol isolated from Pogostemonis Herba in animal models. Fitoterapia. 2011;82:1295–1301. doi: 10.1016/j.fitote.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Rifai A., Soekamto N. Purification and Analysis of Patchouli Alcohol from Patchouli Oil by Vacuum Fractionation Distillation. IOP Publishing; Bristol, UK: 2019. p. 052016. Journal of Physics: Conference Series. [DOI] [Google Scholar]
- 37.Abd-ElGawad A.M., Elshamy A.I., El-Nasser El Gendy A., Al-Rowaily S.L., Assaeed A.M. Preponderance of oxygenated sesquiterpenes and diterpenes in the volatile oil constituents of Lactuca serriola L. revealed antioxidant and allelopathic activity. Chem. Biodivers. 2019;16:e1900278. doi: 10.1002/cbdv.201900278. [DOI] [PubMed] [Google Scholar]
- 38.Chang Y.-C., Chang F.-R., Khalil A.T., Hsieh P.-W., Wu Y.-C. Cytotoxic benzophenanthridine and benzylisoquinoline alkaloids from Argemone mexicana. Z. Naturforsch. C. 2003;58:521–526. doi: 10.1515/znc-2003-7-813. [DOI] [PubMed] [Google Scholar]
- 39.Winterhalter P., Rouseff R. Carotenoid-Derived Aroma Compounds: An Introduction. In: Winterhalter P., Rouseff R., editors. Carotenoid-Derived Aroma Compounds. Volume 802. American Chemical Society; Washington, DC, USA: 2001. pp. 1–17. [Google Scholar]
- 40.Yoshikuni Y., Martin V.J., Ferrin T.E., Keasling J.D. Engineering cotton (+)-δ-cadinene synthase to an altered function: Germacrene D-4-ol synthase. Chem. Biol. 2006;13:91–98. doi: 10.1016/j.chembiol.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Li Z., Howell K., Fang Z., Zhang P. Sesquiterpenes in grapes and wines: Occurrence, biosynthesis, functionality, and influence of winemaking processes. Compr. Rev. Food Sci. Food Saf. 2020;19:247–281. doi: 10.1111/1541-4337.12516. [DOI] [PubMed] [Google Scholar]
- 42.El-Shora H.M., Abd El-Gawad A.M. Physiological and biochemical responses of Cucurbita pepo L. mediated by Portulaca oleracea L. allelopathy. Fresenius Environ. Bull. 2015;24:386–393. [Google Scholar]
- 43.Abd-ElGawad A., El Gendy A., El-Amier Y., Gaara A., Omer S., Al-Rowaily S., Assaeed A., Al-Rashed S., Elshamy A. Essential oil of Bassia muricata: Chemical characterization, antioxidant activity, and allelopathic effect on the weed Chenopodium murale. Saudi J. Biol. Sci. 2020;27:1900–1906. doi: 10.1016/j.sjbs.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elshamy A.I., Abd El-Gawad A.M., El Gendy A.G., Assaeed A.M. Chemical characterization of Euphorbia heterophylla L. essential oils and their antioxidant activity and allelopathic potential on Cenchrus echinatus L. Chem. Biodivers. 2019;16:e1900051. doi: 10.1002/cbdv.201900051. [DOI] [PubMed] [Google Scholar]
- 45.Govindarajan M., Rajeswary M., Benelli G. δ-Cadinene, calarene and δ-4-carene from Kadsura heteroclita essential oil as novel larvicides against malaria, dengue and filariasis mosquitoes. Comb. Chem. High Throughput Screen. 2016;19:565–571. doi: 10.2174/1386207319666160506123520. [DOI] [PubMed] [Google Scholar]
- 46.Mizushina Y., Kuriyama I. Cedar (Cryptomeria japonica) Oils. In: Preedy V.R., editor. Essential Oils in Food Preservation, Flavor and Safety. Elsevier; London, UK: 2016. pp. 317–324. [Google Scholar]
- 47.Costa E.V., Dutra L.M., Salvador M.J., Ribeiro L.H.G., Gadelha F.R., de Carvalho J.E. Chemical composition of the essential oils of Annona pickelii and Annona salzmannii (Annonaceae), and their antitumour and trypanocidal activities. Nat. Prod. Res. 2013;27:997–1001. doi: 10.1080/14786419.2012.686913. [DOI] [PubMed] [Google Scholar]
- 48.Kannathasan K., Senthilkumar A., Venkatesalu V., Chandrasekaran M. Larvicidal activity of fatty acid methyl esters of Vitex species against Culex quinquefasciatus. Parasitol. Res. 2008;103:999–1001. doi: 10.1007/s00436-008-1078-1. [DOI] [PubMed] [Google Scholar]
- 49.Chandrasekaran M., Kannathasan K., Venkatesalu V. Antimicrobial activity of fatty acid methyl esters of some members of Chenopodiaceae. Z. Naturforsch. C. 2008;63:331–336. doi: 10.1515/znc-2008-5-604. [DOI] [PubMed] [Google Scholar]
- 50.Saleh I., Abd-ElGawad A., El Gendy A.G., Abd El Aty A., Mohamed T., Kassem H., Aldosri F., Elshamy A., Hegazy M.-E.F. Phytotoxic and antimicrobial activities of Teucrium polium and Thymus decussatus essential oils extracted using hydrodistillation and microwave-assisted techniques. Plants. 2020;9:716. doi: 10.3390/plants9060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aragão F., Palmieri M., Ferreira A., Costa A., Queiroz V., Pinheiro P., Andrade-Vieira L. Phytotoxic and cytotoxic effects of Eucalyptus essential oil on lettuce (Lactuca sativa L.) Allelopath. J. 2015;35:259–272. [Google Scholar]
- 52.El Ayeb-Zakhama A., Sakka-Rouis L., Bergaoui A., Flamini G., Ben Jannet H., Harzallah-Skhiri F. Chemical composition and allelopathic potential of essential oils obtained from Acacia cyanophylla Lindl. cultivated in Tunisia. Chem. Biodivers. 2015;12:615–626. doi: 10.1002/cbdv.201400184. [DOI] [PubMed] [Google Scholar]
- 53.Pinto A.P.R., Seibert J.B., dos Santos O.D.H., Vieira Filho S.A., do Nascimento A.M. Chemical constituents and allelopathic activity of the essential oil from leaves of Eremanthus erythropappus. Aust. J. Bot. 2019;66:601–608. doi: 10.1071/BT18138. [DOI] [Google Scholar]
- 54.Karakaya S., Koca M., Simsek D., Bostanlik F.D., Özbek H., Kiliç C.S., Güvenalp Z., Demirci B., Altanlar N. Antioxidant, antimicrobial and anticholinesterase activities of Ferulago pauciradiata Boiss. & Heldr. growing in Turkey. J. Biol. Act. Prod. Nat. 2018;8:364–375. [Google Scholar]
- 55.Kürkçüoğlu M., İşcan G., Demirci F., Başer K., Malyer H., Erdoğan E. Composition and antibacterial activity of the essential oil of Ferulago confusa Velen. J. Essent. Oil Res. 2010;22:490–492. doi: 10.1080/10412905.2010.9700380. [DOI] [Google Scholar]
- 56.Ahluwalia V., Sisodia R., Walia S., Sati O.P., Kumar J., Kundu A. Chemical analysis of essential oils of Eupatorium adenophorum and their antimicrobial, antioxidant and phytotoxic properties. J. Pest Sci. 2014;87:341–349. doi: 10.1007/s10340-013-0542-6. [DOI] [Google Scholar]
- 57.Pawlowski Â., Kaltchuk-Santos E., Brasil M., Caramão E., Zini C., Soares G. Chemical composition of Schinus lentiscifolius March. essential oil and its phytotoxic and cytotoxic effects on lettuce and onion. S. Afr. J. Bot. 2013;88:198–203. doi: 10.1016/j.sajb.2013.07.026. [DOI] [Google Scholar]
- 58.Joshi R.K., Satyal P., Setzer W.N. Himalayan aromatic medicinal plants: A review of their ethnopharmacology, volatile phytochemistry, and biological activities. Medicines. 2016;3:6. doi: 10.3390/medicines3010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dudai N., Poljakoff-Mayber A., Mayer A., Putievsky E., Lerner H. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999;25:1079–1089. doi: 10.1023/A:1020881825669. [DOI] [Google Scholar]
- 60.El-Shora H.M., Abd El-Gawad A. Response of Cicer arietinum L. to allelopathic effect of Portulaca oleracea L. root extract. Phyton-Ann. Rei Bot. A. 2015;55:215–232. [Google Scholar]
- 61.Bukhari N.A., Al-Otaibi R.A., Ibhrahim M.M. Phytochemical and taxonomic evaluation of Rhazya stricta in Saudi Arabia. Saudi J. Biol. Sci. 2017;24:1513–1521. doi: 10.1016/j.sjbs.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mealor B.A., Collier T., Miller S.L., Burnett S. Wyoming Weed Watchlist: Field Guide. University of Wyoming Extension; Laramie, WY, USA: 2013. [Google Scholar]
- 63.Chaudhary S.A. Flora of the Kingdom of Saudi Arabia. vol. 1. Volume 1 Ministry of Agriculture and Water; Riyadh, Saudi Arabia: 1999. [Google Scholar]
- 64.Macías F.A., Oliveros-Bastidas A., Marín D., Carrera C., Chinchilla N., Molinillo J.M. Plant biocommunicators: Their phytotoxicity, degradation studies and potential use as herbicide models. Phytochem. Rev. 2008;7:179–194. doi: 10.1007/s11101-007-9062-4. [DOI] [Google Scholar]