Summary
Background
Although a positive association has been established, it is unclear whether lower respiratory tract infections (LRTIs) with respiratory syncytial virus (RSV) cause chronic wheezing illnesses. If RSV-LRTI were causal, we would expect RSV-LRTI prevention to reduce the incidence of chronic wheezing illnesses in addition to reducing acute disease. We aimed to evaluate the strength of evidence for a causal effect of RSV-LRTI on subsequent chronic wheezing illness to inform public health expectations for RSV vaccines.
Methods
We did a systematic review and meta-analysis of observational studies evaluating the association between RSV-LRTI and subsequent wheezing illness (exposure studies) and studies evaluating the association between RSV immunoprophylaxis and subsequent wheezing illness (immunoprophylaxis studies). Exposure studies were included if the exposure group members had an LRTI with laboratory-confirmed RSV and if the exposure ascertainment period began before 2 years of age and ended before 5 years of age. We required a wash-out period of more than 30 days between the index RSV-LRTI and the outcome measurement to allow for resolution of the acute illness. Comparisons between RSV-LRTI and non-RSV-LRTI were not included. Immunoprophylaxis studies were included if they measured the association with subsequent wheezing illness relative to a control group, either in a randomised controlled trial (RCT) or an observational design. For the immunoprophylaxis drugs in question, we required evidence of efficacy in targeting RSV-LRTI from at least one RCT to ensure biological plausibility. All variations of wheezing illness were combined into a single outcome that refers broadly to asthma or any other respiratory illness with wheezing symptoms. Ovid MEDLINE and Embase databases were searched from inception up to Aug 28, 2018. We evaluated whether data from exposure studies could provide evidence against the most viable non-causal theory that RSV-LRTI is a marker of respiratory illness susceptibility rather than a causal factor. Additionally, we tested whether RSV immunoprophylaxis reduces the odds of subsequent wheezing illnesses. We used a random-effects modelling framework and, to accommodate studies providing multiple correlated estimates, robust variance estimation meta-regressions. Meta-regression coefficients (b) quantify differences between exposure and comparator groups on the loge odds ratio (loge OR) scale.
Findings
From 14 235 records we identified 57 eligible articles that described 42 studies and provided 153 effect estimates. 35 studies estimated the direct effect of RSV-LRTI on wheezing illnesses (exposure studies) and eight evaluated the effect of RSV immunoprophylaxis (immunoprophylaxis studies). Exposure studies that adjusted for genetic influences yielded a smaller mean adjusted OR estimate (aOR+ 2·45, 95% CI 1·23–4·88) compared with those that did not (4·17, 2·36–7·37), a significant difference (b 0·53, 95% CI 0·04–1·02). Infants who were not protected with RSV immunoprophylaxis tended to have higher odds of subsequent wheezing illness, as we would expect if RSV-LRTI were causal, but the effect was not significant (OR+ 1·21, 95% CI 0·73–1·99). There was generally a high threat of confounding bias in the observational studies. Additionally, in both the observational studies and immunoprophylaxis RCTs, there was high risk of bias due to missing outcome data.
Interpretation
Our findings, limited to exposure and immunoprophylaxis studies, do not support basing policy decisions on an assumption that prevention of RSV-LRTI will reduce recurrent chronic wheezing illnesses.
Funding
Bill & Melinda Gates Foundation.
Introduction
Lower respiratory tract infections (LRTIs) caused by the respiratory syncytial virus (RSV) contribute substantially to infant (aged 0–1 years) morbidity and mortality,1, 2 making RSV prevention a global health priority.3 With policy makers committed to support the introduction of future licensed RSV vaccines,3, 4 there is a pressing need to estimate the full range of public health benefits. Although there is a well established positive association between RSV-LRTI and subsequent wheezing illness,5, 6, 7 it is unclear whether the association is causal.8 If the association were causal, efficacious RSV-LRTI prevention would probably reduce the burden of chronic wheezing illnesses in addition to acute disease, substantially increasing vaccine health benefits.9 In this Article, we evaluate the extent to which existing research supports a causal effect of RSV-LRTI on subsequent wheezing illness in young children. Although meta-analyses cannot resolve the question of causality, they can help appraise the strength of evidence for causality, which is critical for policy makers.
Research in context.
Evidence before this study
Although there is a well established positive association between respiratory syncytial virus lower respiratory tract infections (RSV-LRTIs) and subsequent wheezing illnesses, it is unclear whether RSV-LRTI is a causal factor, a marker of susceptibility to respiratory illness, or both. Before doing the literature search for this Article, we searched PubMed and Google Scholar for articles published from inception until May 12, 2018. The full list of search terms is available in the appendix (pp 28–37). We identified two relevant meta-analytic reviews: the first reported a positive mean association (odds ratio [OR] 3·84) between RSV hospitalisation and subsequent asthma or wheezing in 15 studies; and the second also reported a significant mean association (OR 4·03) between RSV-bronchiolitis and asthma. In terms of causal evidence, a population-based birth cohort study showed that asthma risk was temporally associated with birth timing in close proximity to winter viral bronchiolitis epidemics, consistent with a causal effect. In contrast, twin-registry studies suggested that RSV-LRTI is more likely a marker of genetic risk than a causal contributor to asthma. One of the two randomised controlled trials of RSV immunoprophylaxis that evaluated effects on wheezing illness showed a reduction in parent-reported wheezing outcomes in those aged between 1 and 6 years, but neither trial found evidence of reductions in medically-attended wheezing illness.
Added value of this study
Although previous meta-analyses provided estimates of the association between RSV-LRTI and recurring wheezing illness, this Article was, to our knowledge, unique in that it had the explicit goal of appraising the strength of evidence for a causal effect. We could have increased confidence in a causal effect by providing evidence against the most plausible non-causal theory (ie, that RSV-LRTI is a marker of shared susceptibility to lung disease) and showing that RSV immunoprophylaxis protects against childhood wheezing illness. However, our findings did not support either of these conditions. These findings do not rule out a causal effect but suggest that the current evidence does not support basing policy decisions on an assumption that prevention of RSV-LRTI will reduce recurrent wheezing illnesses.
Implications of all the available evidence
Our findings, in combination with previous RSV twin studies, are consistent with the hypothesis that shared genetic predisposition accounts for a substantial proportion of the association between RSV-LRTI and subsequent wheezing. Individual studies and meta-analyses that do not account for shared genetic risk could substantially overestimate the effect of RSV-LRTI on recurring wheezing illness. Additionally, we found insufficient evidence that RSV immunoprophylaxis prevents wheezing illness, which we would expect if RSV-LRTI were causal. Long-term follow-up data from ongoing trials are needed before assuming RSV-LRTI prevention might lead to reduction in recurrent wheeze or asthma. Additionally, more research is needed to assess the potential effects of RSV infection without LRTI and potential host–viral interactions.
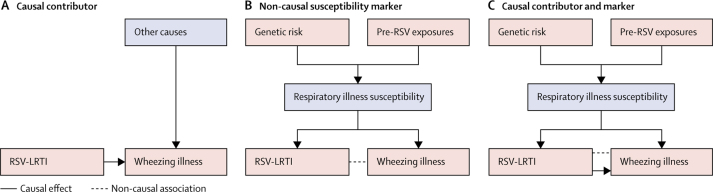
We present three possible models for the established association between RSV-LRTI and wheezing illness (figure 1).10, 11 In the first model, RSV-LRTI is one of several causal contributors to subsequent wheezing illness (figure 1A). The potential mechanisms by which RSV infection could contribute to recurrent wheezing illness (eg, by degrading airway epithelial barriers and altering functioning of regulatory T cells) have been reviewed in other papers.11 The second is a non-causal model, in which a pre-existing susceptibility to respiratory illness causes both the RSV-LRTI and subsequent wheezing illness (ie, confounding; figure 1B).10, 12, 13 In this model, the pre-existing respiratory illness susceptibility is attributable to heritable factors and early environmental insults. Past research has shown that infants who develop RSV-LRTI have poorer pre-existing lung function14 (a highly heritable trait),15 which could make them susceptible to both severe illness in response to RSV infection (ie, RSV-LRTI) and recurrent wheezing illness.12, 13, 16 In the third and final model, the association is attributable partly to a causal effect and partly to the confounding influence of pre-existing respiratory illness susceptibility (figure 1C).11
Figure 1.
Three potential models to explain the observed association between RSV-LRTI and subsequent wheezing illness
(A) RSV-LRTI as one of multiple causal contributors to wheezing illness. There is a directed solid arrow (representing a causal effect) connecting RSV-LRTI and wheezing illness. (B) A non-causal model in which the positive association between RSV-LRTI and subsequent wheezing illness is confounded by a pre-existing susceptibility to respiratory illnesses. According to this model, this pre-existing susceptibility is driven by genetics and early environmental insults that precede RSV-LRTI. The dotted, non-directional line connecting RSV-LRTI and wheezing illness represents a non-causal association. (C) The association between RSV-LRTI and subsequent wheezing illness is due partly to the confounding influence of pre-existing respiratory illness susceptibility and partly to a causal effect of RSV-LRTI. RSV-LRTI=respiratory syncytial virus lower respiratory tract infections.
In this Article, we use meta-regression to consider both causal and non-causal models of the association between RSV-LRTI and subsequent wheezing illness and evaluate whether their unique implications (ie, what must be true if the models were correct) are consistent with empirical data.17 The non-causal model in figure 1B has testable implications17 for studies evaluating the association between early life RSV-LRTI and subsequent wheezing illness (exposure studies): studies adjusting for contributors to, or markers of, pre-existing respiratory susceptibility should yield smaller effect estimates.18 If they do not, this would provide evidence against the non-causal explanation, increasing the plausibility of a causal effect.19
Studies evaluating the association between RSV immunoprophylaxis and subsequent wheezing illness (immunoprophylaxis studies) can also help to appraise the strength of evidence for a causal effect (figure 1A). If RSV-LRTI were a causal factor, then efficacious immunoprophylaxis would most likely reduce the risk of wheezing illness. Meta-analysis could therefore be used to test whether RSV immunoprophylaxis is associated with a decreased risk of subsequent wheezing illness.18 In standard care, infants receiving RSV immunoprophylaxis tend to have poorer baseline health status than those who do not, even within groups at high risk.20, 21 Consequently, observational RSV immunoprophylaxis studies require particular care to limit confounding by indication,22 in addition to other biases.18 Properly done immunoprophylaxis randomised controlled trials (RCTs) eliminate confounding by indication.18, 23 However, drawing causal inferences about the effect of RSV-LRTI on wheezing illness in these RCTs requires the assumption that treatment assignment (eg, immunoprophylaxis vs placebo) affects risk for wheezing illness only by limiting the severity of RSV-LRTI exposure and not through any other mechanisms.24, 25
We were commissioned by WHO to do a systematic review and meta-analysis to assess the evidence for a causal effect of RSV-LRTI on subsequent wheezing illness to inform public health expectations for RSV vaccines.
Methods
Search strategy and selection criteria
The protocol for this systematic review and meta-analysis provides complete methodological details (appendix pp 3–27). As there was no involvement of human participants or identifiable information, institutional review board approval was not required. We have described the two types of published peer-reviewed studies included in the analysis and all the inclusion criteria (panel, table 1). RSV-LRTI exposure studies measured the direct association between RSV-LRTI exposure and subsequent wheezing illness. Immunoprophylaxis studies measured the association between RSV immunoprophylaxis and subsequent wheezing illness.
Panel. Research designs of relevant studies.
Respiratory syncytial virus (RSV)-associated lower respiratory tract infection (LRTI) exposure studies
Evaluate the direct association between RSV-LRTI and subsequent wheezing illness using an observational design
Viral surveillance studies
Do viral surveillance over a discrete exposure ascertainment period: those developing RSV-LRTI during this period form the exposure group and those with no LRTI form the comparison group
Medical event studies
Compare children (aged 0–5 years) with an RSV-LRTI medical event (eg, hospital admission, acute medical visit, etc) during the exposure ascertainment period to those without an LRTI-related medical event (comparator group)
RSV immunoprophylaxis studies
Evaluate the association between receipt of RSV immunoprophylaxis with established efficacy and subsequent wheezing illness
Randomised controlled trial
Randomly assign infants (aged 0–1 years) to receive either RSV immunoprophylaxis or placebo
Observational (non-randomised) study
Compare infants (aged 0–1 year) receiving RSV immunoprophylaxis in their normal clinical care to those not receiving immunoprophylaxis (ie, intervention not determined by study procedures)
Table 1.
Summary of inclusion and exclusion criteria and PICOS literature search framework
| RSV-LRTI exposure studies | RSV immunoprophylaxis studies | |
|---|---|---|
| Population characteristics | Human participants | Human participants |
| Intervention or exposure* | RSV-LRTI during a period beginning <2 years of age and fully contained within ages 0–5 years (operationalised as an exposure or mediator variable) | RSV immunoprophylaxis with established efficacy (either from the trial in question or past RCTs) in preventing or mitigating RSV-LRTI |
| Comparator | LRTI absent or undetected during the exposure period | RSV immunoprophylaxis not received during the exposure period |
| Outcome | Wheezing illness measured subsequent† to the index RSV-LRTI illness that defines inclusion in the exposure vs comparator groups | Wheezing illness subsequent to study intervention protection period |
| Study design | Study analysed quantitative data and was published (including online only publication ahead of print) in English language in a peer-reviewed journal before the final search date; exposure and comparator groups sampled from the same population; method of ascertaining exposure and outcomes were the same for exposure and comparator groups | As for exposure studies |
PICOS=population, intervention or exposure, comparator, outcome, study design. RSV=respiratory syncytial virus. LRTI=lower respiratory tract infection. RCT=randomised controlled trial.
Clinical trials might estimate the effect of RSV-LRTI on asthma or wheezing outcomes indirectly by reporting the effect of immunoprophylaxis on asthma or wheezing outcomes and report the direct association between RSV-LRTI and asthma or wheezing outcomes.
Defined as occurring >30 days after the index RSV-LRTI.
RSV-LRTI exposure studies were included if the exposure group members had an LRTI with laboratory-confirmed RSV. LRTI was considered present if children were diagnosed with relevant illnesses (eg, bronchiolitis or pneumonia), had relevant clinical indications (eg, wheeze), or received treatment in hospital for RSV-related illnesses. As we were interested in early life RSV-LRTI, the exposure ascertainment period had to begin before 2 years of age and end before 5 years. These exposure studies could be further categorised by study design. Some exposure studies determined RSV-LRTI status by doing viral surveillance over a defined ascertainment period (surveillance studies), whereas others compared individuals with an RSV-LRTI medical event (eg, treatment in hospital) to those without an LRTI medical event (medical event studies). We required a wash-out period of more than 30 days26 between the index RSV-LRTI and the outcome measurement to allow for resolution of the acute illness. Comparisons between RSV-LRTI and non-RSV-LRTI were not included because they do not directly address the causal question of interest, which pertains to RSV-LRTI in particular and not LRTI more generally.
We included RSV immunoprophylaxis studies if they measured the association with subsequent wheezing illness relative to a control group, either in an RCT or an observational design. For the immunoprophylaxis drugs in question, we required evidence of efficacy in targeting RSV-LRTI from at least one RCT27, 28 to ensure biological plausibility. Because studies did not use consistent definitions for asthma and other wheezing illnesses (eg, what some authors termed asthma, others labelled recurrent wheeze), all variations of wheezing illness were combined into a single outcome. Wheezing illness, therefore, refers broadly to asthma or any other respiratory illness with wheezing symptoms. To evaluate whether associations were driven by transient wheezing illnesses, we did a sensitivity analysis including only outcomes described as asthma measured at 6 years or older, when asthma can be diagnosed reliably.29
An expert systematic review information specialist did a literature search of Ovid MEDLINE and Embase databases using the population, intervention or exposure, comparator, outcome, study design framework,30 in English (table 1). The original search strategy was reviewed by an independent information specialist using the Peer Review of Electronic Search Strategies checklist,31 evaluated against a test set of scholarly articles with known relevance, and subsequently refined to ensure comprehensiveness. The final search was done on Aug 28, 2018, with no restriction on publication dates, and the search terms can be found in the appendix (pp 28–37).
Four study investigators (AJD, BMS, JRO, and SMB) with doctoral degrees in health-care sciences completed record reviews and data abstraction. Literature search records were reviewed in two stages. Stage 1 eliminated records that were irrelevant based on the study abstracts alone. Two of these investigators evaluated each abstract independently, blinded to the others' ratings. Any article deemed potentially relevant by either reviewer was retained. In stage 2, two investigators independently and redundantly reviewed the full text of the articles that passed stage 1 to determine whether the full inclusion criteria were met.
For each article, one of the four investigators abstracted relevant data and a different investigator did quality assurance reviews, resolving discrepancies by consensus. For estimates from RSV-LRTI exposure studies, we noted whether or not analyses controlled for genetic predisposition to wheezing illness and neonatal health proxies that might be markers of respiratory illness predisposition (eg, preterm birth). As RSV often co-occurs with other respiratory infections that are also positively associated with subsequent wheezing illness,32 it is plausible that the effect of these co-infections could be misattributed to RSV. Therefore, we also coded whether studies adjusted for non-RSV viral or bacterial respiratory co-infections. Additionally, we extracted data on the following key study features (table 2): country income level, asthma risk-based versus non-risk-based enrolment, age at outcome ascertainment (preschool 0–4 years; school 5–12 years; adolescence 13–18 years; or adulthood ≥19 years old), and timing of exposure ascertainment (limited to the first year of life or not). Finally, we coded whether immunoprophylaxis studies were RCTs or observational.
Table 2.
Study characteristics
| Countries | Country income level* | Exposure ascertainment† | Estimates included‡ | Measured asthma (age ≥6 years)§ | Outcomes age groups¶ | Enrolment strategy‖ | ||
|---|---|---|---|---|---|---|---|---|
| RSV immunoprophylaxis studies | ||||||||
| Randomised controlled trials | ||||||||
| Blanken et al (2013);33 Scheltema et al (2018)34 | Netherlands | High | Limited to first year of life | 6 | Yes | Preschool and primary school | Risk based: preterm birth | |
| O'Brien et al (2015)35 | USA | High | Limited to first year of life | 3 | No | Preschool | Risk based: ethnic groups at high risk | |
| Observational studies | ||||||||
| Carroll et al (2017)20 | USA | High | Limited to first year of life | 3 | No | Primary school | Risk based: chronic lung disease or preterm birth | |
| Yoshihara et al (2013);36 Mochizuki et al (2017)37 | Japan | High | Limited to first year of life | 3 | Yes | Preschool | Risk based: preterm birth | |
| Simoes et al (2007)38 | Spain, Germany, Netherlands, Canada, Poland, and Sweden | High | Limited to first year of life | 2 | No | Preschool | Risk based: preterm birth | |
| Prais et al (2016)39 | Israel | High | Limited to first year of life | 3 | No | Preschool | Risk based: preterm birth | |
| Haerskjold et al (2017)21 | Denmark and Sweden | High | Extends beyond first year of life | 4 | No | Preschool | Not risk based | |
| dos Santos Simões et al (2019)40 | Brazil | Upper-middle | Extends beyond first year of life | 1 | No | Preschool | Risk based: preterm birth and referred for RSV immunoprophylaxis | |
| RSV-LRTI exposure studies | ||||||||
| Medical event studies | ||||||||
| Ruotsalainen et al (2013);41 Backman et al (2018)42 | Finland | High | Extends beyond first year of life | 3 | Yes | Adolescence | Not risk based | |
| Korppi et al (1994);43 Korppi et al (2004);44 Ruotsalainen et al (2010);45, 46 Backman et al (2014)47 | Finland | High | Extends beyond first year of life | 11 | Yes | Primary school, adolescence, and adulthood | Not risk based | |
| Sigurs et al (1995, 2000, 2005, 2010)48, 49, 50, 51 | Sweden | High | Limited to first year of life | 10 | Yes | Preschool, primary school, and adolescence | Not risk based | |
| Poorisrisak et al (2010)16 | Denmark | High | Extends beyond first year of life | 1 | Yes | Primary school | Not risk based | |
| Fjaerli et al (2005)52 | Norway | High | Limited to first year of life | 2 | Yes | Primary school | Not risk based | |
| Henderson et al (2005)53 | UK | High | Limited to first year of life | 3 | Yes | Preschool and primary school | Not risk based | |
| Stensballe et al (2018)54 | Denmark | High | Extends beyond first year of life | 2 | No | Preschool | Not risk based | |
| Carbonell-Estrany et al (2015)55 | Spain | High | Limited to first year of life | 3 | No | Preschool | Risk based: preterm birth | |
| Escobar et al (2013)56 | USA | High | Limited to first year of life | 12 | No | Preschool | Not risk based | |
| Kim et al (2013)57 | South Korea | High | Extends beyond first year of life | 1 | No | Preschool | Not risk based | |
| Palmer et al (2011)58 | USA | High | Limited to first year of life | 4 | No | Preschool | Risk based: preterm birth | |
| Blanken et al (2016)59 | Netherlands | High | Limited to first year of life | 1 | No | Preschool | Risk based: preterm birth | |
| Bloemers et al (2010)60 | Netherlands | High | Extends beyond first year of life | 3 | No | Preschool | Risk based: Down syndrome | |
| García-García et al (2007)61 | Spain | High | Extends beyond first year of life | 3 | No | Preschool | Not risk based | |
| Mikalsen et al (2012)62 | Norway | High | Limited to first year of life | 1 | Yes | Primary school | Not risk based | |
| Osundwa et al (1993)63 | Qatar | High | Limited to first year of life | 1 | No | Preschool | Not risk based | |
| Palmer et al (2010)64 | USA | High | Limited to first year of life | 3 | No | Preschool | Not risk based | |
| Weber et al (1999)65 | The Gambia | Low | Limited to first year of life | 1 | No | Preschool | Not risk based | |
| Fauroux et al (2014)66 | France | High | Limited to first year of life | 2 | No | Preschool | Risk based: preterm birth | |
| Sims et al (1978)67 | UK | High | Limited to first year of life | 1 | No | Primary school | Not risk based | |
| Pullan & Hey (1982)68 | UK | High | Limited to first year of life | 1 | No | Primary school | Not risk based | |
| Juntti et al (2003)69 | Finland | High | Limited to first year of life | 3 | Yes | Primary school | Not risk based | |
| Bertrand et al (2015)70 | Chile | High | Limited to first year of life | 2 | No | Preschool | Not risk based | |
| Singleton et al (2003)71 | USA | High | Extends beyond first year of life | 4 | No | Preschool and primary school | Risk based: ethnic group at high risk | |
| Schauer et al (2002)72 | Germany | High | Limited to first year of life | 2 | No | Preschool | Not risk based | |
| Stensballe et al (2009)73 | Denmark | High | Extends beyond first year of life | 7 | No | Preschool | Not risk based | |
| Munywoki et al (2013)74 | Kenya | Lower-middle | Limited to first year of life | 1 | No | Preschool | Not risk based | |
| Viral surveillance studies | ||||||||
| Kusel et al (2007, 2012)32, 75 | Australia | High | Limited to first year of life | 10 | Yes | Preschool and primary school | Risk based: family history of asthma or atopy | |
| Calişkan et al (2013);76 Bønnelykke et al (2015)77 | Denmark | High | Extends beyond first year of life | 3 | Yes | Primary school | Risk based: family history of asthma or atopy | |
| Calişkan et al (2013);76 Lemanske et al (2005);78 Jackson et al (2008);79 Rubner et al (2017)80 | USA | High | Extends beyond first year of life | 11 | Yes | Preschool or primary school | Risk based: family history of asthma or atopy | |
| Stein et al (1999);81 Voraphani et al (2014)82 | USA | High | Extends beyond first year of life | 10 | Yes | Primary school and adulthood | Not risk based | |
| Drysdale et al (2015)83 | UK | High | Limited to first year of life | 2 | No | Preschool | Risk based: preterm birth | |
| Zomer-Kooijker et al (2014)84 | Netherlands | High | Limited to first year of life | 3 | Yes | Primary school | Not risk based | |
| Broughton et al (2007)85 | UK | High | Limited to first year of life | 1 | No | Preschool | Risk based: preterm birth | |
RSV=respiratory syncytial virus. LRTI=lower respiratory tract infection.
Determined using the World Bank classifications.86
Was the exposure ascertainment period limited to the first year of life or did it extend beyond?
Number of effect size estimates that contributed to the primary analysis.
At least one outcome was described as asthma and measured at ≥6 years of age.
Age category when outcomes measured: preschool 0–4 years; primary school 5–12 years; adolescence 13–18 years; and adulthood ≥19 years.
Was enrolment limited to individuals with known risk for wheezing illness other than early life LRTI?
Data analysis
We quantified differences between exposure and comparator groups using the loge odds ratio (logeOR). Whenever possible, for RSV-LRTI exposure studies and non-randomised immunoprophylaxis studies, we included estimates from analyses in which there was explicit effort to adjust for confounders, as these estimates were generally expected to be less biased than unadjusted estimates. Some studies reported other adjusted ratio-based effect estimates (eg, adjusted risk ratios). Rather than excluding these estimates or replacing them with unadjusted ORs, we included them in our analyses as the best-available estimates. 17% of estimates (26 of 153) used non-OR ratio estimates as stand ins for OR estimates, which pulls the estimated mean OR systematically toward the null.87 We used unadjusted estimates for the effect of RSV immunoprophylaxis on wheezing illness in RCTs. Most studies provided multiple correlated estimates. To make optimal use of the available data,88 we included all relevant estimates that provided at least some unique information.
To accommodate studies providing multiple correlated estimates, we did robust variance estimation (RVE) meta-regressions89 using robumeta version 2.090 in R version 3.5.0. RVE allows studies to contribute multiple correlated estimates while providing accurate point estimates and confidence intervals.88, 89 For exposure studies, we regressed effect estimates on study-level covariates selected a priori to test study hypotheses and account for variability in effect estimates across studies. Meta-regression coefficient estimates (b) are reported to quantify covariate effects on the logeOR scale.
We used the Newcastle-Ottawa Scale91 for observational studies and the Cochrane Risk of Bias Tool92 for RCTs, to identify potential biases in the extant literature and corresponding priorities for improving future study design.
Role of the funding source
This study was commissioned by WHO through a grant from the Bill & Melinda Gates Foundation. A WHO RSV expert (DRF) contributed to the study conceptual isation, protocol and search strategy development, interpretation of results, and manuscript editing. AJD was supported by the National Institute of Health (NIH). The NIH had no role in the study design, data collection, analysis, interpretation of data, writing of the report, or in the decision to submit the paper for publication. The Gates Foundation had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
From 14 235 initial records, we identified 57 eligible articles, describing 42 studies, and providing 153 effect estimates (table 2 and figure 2). In addition to the 34 RSV-LRTI exposure studies (table 2), one of the immunoprophylaxis studies (Scheltema et al)34 provided an estimate of the direct effect of RSV-LRTI on wheezing illnesses. Therefore, 35 studies estimated the direct effect of RSV-LRTI on wheezing illnesses and eight studies evaluated the effect of immunoprophylaxis on wheezing illnesses. All but two studies were done in high-income countries (table 2). A common potential source of bias, among both RSV-LRTI exposure studies and immunoprophylaxis studies, was that missing outcome data were treated as completely random without clear justification (appendix pp 40–48).93 It is unclear whether the suboptimal treatment of missing data is more likely to bias towards a larger or smaller estimate of the effect of RSV-LRTI on subsequent wheezing illness. Additionally, it was often impossible to know with certainty, particularly in medical event studies, whether RSV-LRTI preceded early wheezing illness manifestations that might have gone undetected. This would probably result in inflated effect estimates as the exposure cannot follow the outcome it purportedly causes.94
Figure 2.
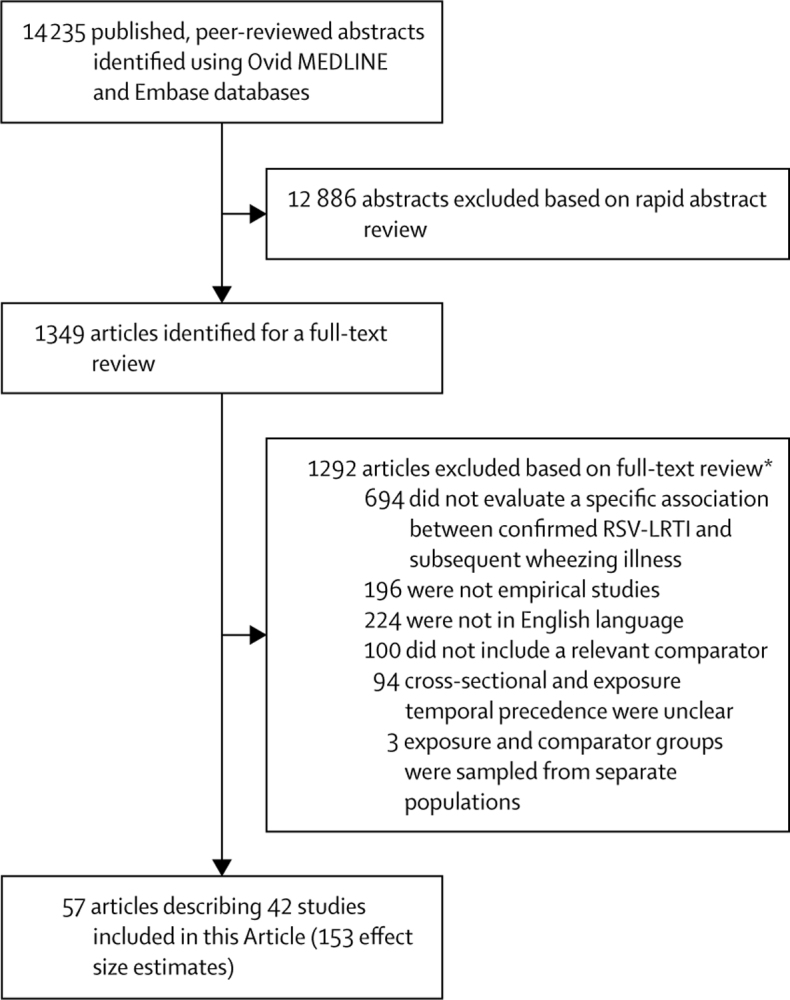
Study selection
RSV-LRTI=respiratory syncytial virus lower respiratory tract infections. *Reviewers sometimes selected multiple inclusion criteria that were not met; hence, the numbers associated with specific reasons for exclusion do not add up to the total number of articles excluded.
Among RSV-LRTI exposure studies, the unconditional mean OR (OR+) indicated that children exposed to RSV-LRTI had a 3·39 times increase in odds of subsequent wheezing illness (95% CI 2·72–4·24). OR+ remained positive when limiting the analysis to effect estimates of RSV-LRTI on asthma outcomes measured at age 6 years and older (41 estimates from 14 studies, OR+ 2·64, 95% CI 1·75–3·98).
In our primary model, effect estimates for the association between RSV-LRTI and wheezing illness differed depending on whether estimates were adjusted for genetic influences (b 0·53, 95% CI 0·04–1·02). As shown in figure 3, the adjusted mean OR (aOR+) was considerably smaller when estimates accounted for genetic influences (n=77, aOR+ 2·45, 95% CI 1·23–4·88) relative to those that did not (n=52, aOR+ 4·17, 2·36–7·37). The one study16 that eliminated genetic influences by comparing wheezing illness outcomes in monozygotic twins with discordant RSV-LRTI status had a point estimate (OR 1·21) smaller than 88% (68 of 77) of estimates in which genetic influences were only partly controlled. However, uncertainty was high in this study with 95% CI values ranging from highly protective to highly damaging (0·36–4·00).16 There was no evidence that effect estimates varied depending on whether they controlled for differences in neonatal health or co-infections. The effect of controlling for genetic influences was robust when simultaneously removing all unadjusted estimates (b 0·63, 95% CI 0·05–1·20). However, when sequentially removing one study at a time (and all its estimates) from the analysis, eight studies32, 54, 58, 59, 64, 69, 73, 85 had enough influence that their removal nullified the effect of controlling for genetic influences (appendix p 49).
Figure 3.
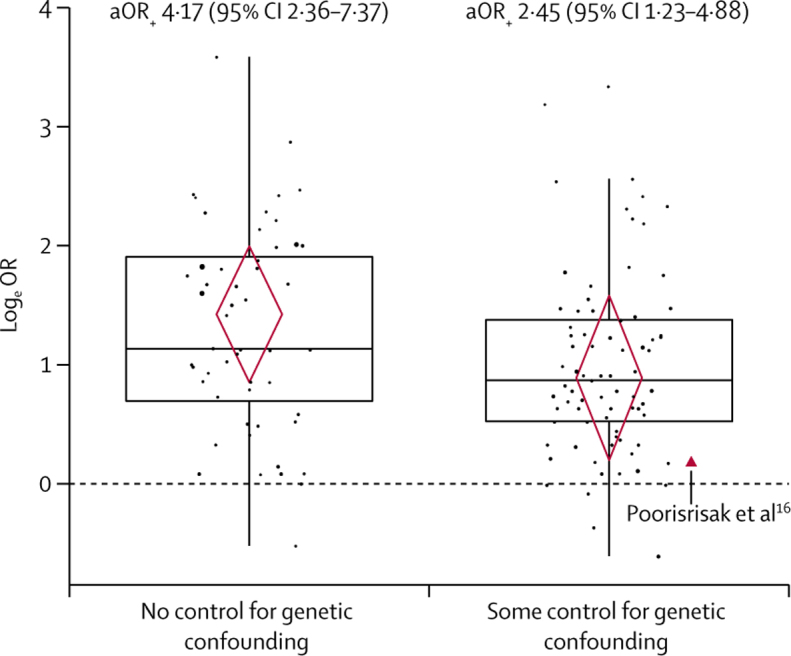
Observed effect size distributions and conditional mean effect sizes for studies that did and did not control for genetic confounding
Effect estimates from respiratory syncytial virus lower respiratory tract infections exposure studies controlling for potential genetic influences (n=77) were smaller, on average, than those that did not control for potential genetic influences (n=52). The y-axis represents logeOR estimates, with 0 indicating no effect (dashed line). Points represent observed individual effect estimates and their size is proportional to their inverse-variance weights (ie, more precise estimates have larger points). The estimate provided by Poorisrisak and colleagues16 is displayed as a red triangle and annotated as it is the only estimate in which genetic influences were eliminated completely. The boxplots display characteristics of the distributions of the observed effect estimates (eg, medians and IQRs). The centres of the red diamonds represent the conditional mean logeOR estimates based on the primary meta-regression model. The bottom and top points of the diamonds represent, respectively, the lower and upper bounds of the 95% CIs. Mean aOR+ and 95% Cls based on the primary meta-regression model are provided for each group. LogeOR=loge odds ratio. aOR+=adjusted OR estimates.
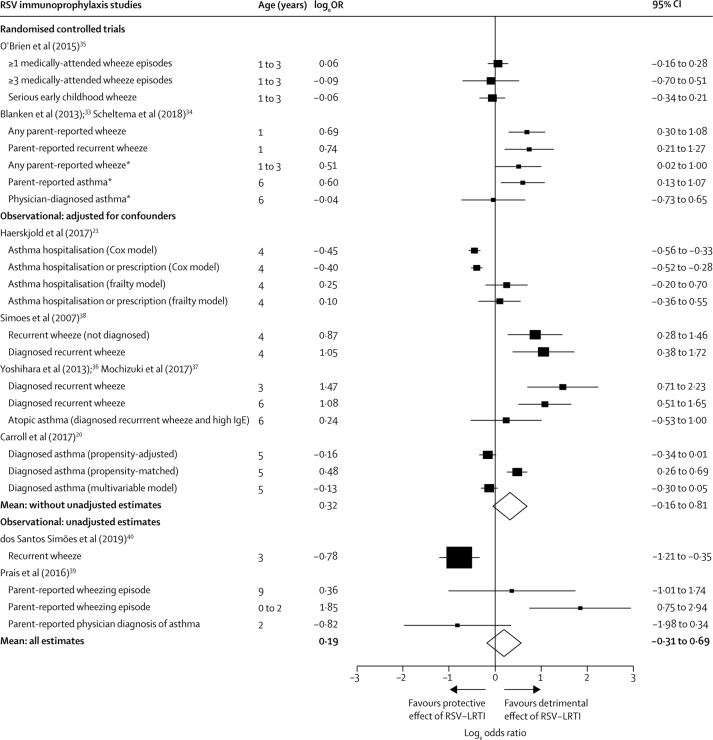
In our primary model of RSV immunoprophylaxis studies, the mean effect estimate was positive (OR+ 1·21), indicating that those not receiving immunoprophylaxis (with presumably greater risk of RSV-LRTI) tended to have higher odds of subsequent wheezing illness, but the 95% CI (0·73–1·99) included the null (figure 4). The mean effect size was slightly larger but remained non-statistically significant when removing two studies39, 40 that did not adjust for confounders (OR+ 1·38, 95% CI 0·85–2·24). Owing to the small sample size (eight estimates), there was considerable uncertainty around the mean estimate among the two RCTs and no evidence of increased odds of wheezing illness among children who did not receive RSV immunoprophylaxis (OR+ 1·24, 0·04–36·27).
Figure 4.
Forest plot evaluating whether infants who did not receive RSV immunoprophylaxis had increased odds of subsequent wheezing illness
If RSV-LRTI were a cause of subsequent wheezing illness, then we would expect infants (aged 0-1 years) not receiving RSV immunoprophylaxis to have greater odds of developing subsequent wheezing illness compared with infants at similar risk who do receive RSV immunoprophylaxis. Our analyses from RSV immunoprophylaxis studies provided insufficient evidence for this hypothesis. In the figure, logeOR >0 indicate greater odds of subsequent wheezing illness in children who did not receive immunoprophylaxis. The higher of the two diamonds depicts the weighted mean logeOR for randomised trials and observational studies that adjusted for confounders. The lower of the two diamonds depicts the weighted mean logeOR across all estimates, including those from observational studies that did not adjust for confounders. Neither mean estimate was significantly greater than 0. RSV-LRTI=respiratory syncytial virus lower respiratory tract infection. LogeOR= loge odds ratios. *Estimates from the Blanken et al33 and Scheltema et al34 were based on outcomes measured after the blinding of study participants had been broken at 1 year of age, although assessors were blinded throughout the study.
Discussion
Although we cannot rule out a causal effect of RSV-LRTI on subsequent wheezing illness, neither of our two primary findings support the case for causality. First, RSV-LRTI exposure studies controlling for genetic influences produced smaller effect estimates, consistent with what we would expect if RSV-LRTI were at least partly a marker of genetic susceptibility rather than a purely causal association. Although this finding was not fully robust in a leave-one-out sensitivity analysis, all but one16 of the studies controlling for genetic influences measured genetic risk using imperfect proxies (eg, familial asthma history) and could only partly remove heritable influences. Consequently, our models probably underestimated the influence of adjusting for genetics.95, 96 In sum, the mean effect estimate for exposure studies was positive and significant even among studies that adjusted for proxies of genetic risk; however, it is possible that some or much of the effect is attributable to residual heritable influences that were not accounted for by adjusting for imperfect proxy variables.
Second, existing immunoprophylaxis studies did not provide compelling evidence that RSV immunoprophylaxis protects against subsequent wheezing illness. As there were only eight studies (24 estimates) contributing to the model, our primary estimate of the effect of immunoprophylaxis on wheezing illness had a wide 95% CI (0·73–1·99), indicative of insufficient evidence for benefit or harm. Additional, preferably large, RCTs would improve precision.97 In sum, this study, in combination with previous analyses,12, 13 suggests that the evidence for a causal effect of RSV-LRTI on subsequent wheezing illness is not well supported by the existing data. Further, the current evidence does not support the assumption that effective RSV-LRTI prevention strategies would reduce subsequent wheezing illnesses.
Future observational studies evaluating the association between RSV-LRTI and wheezing illness are unlikely to be helpful in resolving the question of causality unless they accurately account for genetic influences. Additional twin registry studies would be valuable, particularly if the data could be combined to improve precision. Although we have focused on genetic risk as a potential confounder, future studies should also evaluate potential gene and RSV interactions.11, 98, 99 Studies measuring strong genetic markers (eg, 17q21 genotypes)100 could help to determine whether RSV has any meaningful causal effect, either independently or in combination with specific genotypes.
Regarding RSV immunoprophylaxis studies, RCTs are likely to provide less biased estimates than observational studies.33, 35 However, RCTs should evaluate the plausibility that randomised treatment assignment is a valid instrumental variable when drawing inferences about the causal effect of RSV-LRTI on wheezing illness.24 The case for randomised treatment assignment as an instrumental variable is predicated on making a compelling argument that RSV immunoprophylaxis only affects one's risk of wheezing illness by reducing the severity of RSV infection and not through any other mechanisms.24, 101 Powering a single RCT to detect effects on asthma in those aged 6 years or older will be challenging, owing to sample size requirements;97 however, combining data across multiple RCTs could improve precision. Standardisation of outcome measures should therefore be advocated when designing new RCTs.
Ultimately, evidence of causality in epidemiological research is always uncertain.18 However, studies can reduce uncertainty about whether RSV-LRTI causes wheezing illness by employing designs that minimise the influence of the most likely confounders102 and capitalising on causal modelling strategies.17, 103, 104 Uncertainty can also be reduced by use of objective markers of airway inflammation and disease (eg, lung function).105
The primary limitation of this Article is that it did not address all the evidence relevant to assessing whether RSV-LRTI causes wheezing illness. Several important studies did not meet our inclusion criteria. Notably, in a study of monozygotic and dizygotic twins, Thomsen and colleagues12 found that RSV-LRTI requiring treatment in hospital was more plausibly a marker of genetic risk rather than a cause of asthma, but the study was eliminated from our analysis because the RSV-LRTI exposure did not clearly precede the wheezing illness outcome.12 Wu and colleagues8 found that rates of asthma were correlated with birth near the peak of the winter RSV bronchiolitis season, consistent with a causal effect; however, this study was eliminated because it did not confirm RSV infection.106 Further, we did not review animal or human tissue studies experimentally evaluating mechanisms of RSV's effect on wheezing illness,11 nor did we include non-English language articles, which could have resulted in selection bias. Our findings should be interpreted in combination with these other important sources of evidence. The fact that all wheezing-related illnesses were combined into a single outcome in our analyses is another limitation, which could obscure effects on specific wheezing outcomes (eg, physician-diagnosed wheezing illness). Additionally, all RSV immunoprophylaxis studies have been done in high-risk populations, which could limit generalisability. Finally, nearly all the included data came from high-income countries; therefore, the findings might not be representative of low-income and middle-income countries.
This study had notable strengths. We used RVE meta-regression allowing for inclusion of multiple correlated estimates from the same study. Additionally, our search strategy was peer-reviewed by an expert information specialist. Finally, we provided all analytic code, allowing for replication and extension of our findings. Our assessment of findings from observational studies and immunoprophylaxis RCTs could neither discount a plausible non-causal model of the association between RSV-LRTI and subsequent wheezing illness, nor confirm that RSV immunoprophylaxis protects against wheezing illness. Consequently, this study does not provide compelling support for a causal effect of RSV-LRTI on subsequent wheezing illness. RSV-LRTI prevention will probably have a substantial public health effect by reducing complications associated with the acute infection;107 however, it remains uncertain whether there would be added value due to the prevention of recurrent wheezing illnesses.
Data sharing
All analyses and R code are available at https://brunwasser.github.io/whorsv.github.io/.
Acknowledgments
Acknowledgments
This study was commissioned by WHO through a grant from the Bill & Melinda Gates Foundation (Global Health Grant OPP1114766). AJD was supported by NIH (T32 AI007524).
Contributors
DRF, BMS, SMB, AJD, JRO, and TVH conceived of the study. All authors developed the study protocol. BMS, SMB, AJD, and JRO screened the articles for inclusion. BMS, SMB, and AJD extracted data from the included studies. BMS, SMB, AJD, JRO, and TVH analysed the data. All authors interpreted the results. BMS, SMB, AJD, JRO, and TVH drafted the manuscript. All authors critically reviewed all drafts of the manuscript. BMS, SMB, AJD, JRO, TVH, BS, DBF, and DRF developed the search strategy. BS did the literature search. All authors have seen and approved the final version of this manuscript.
Declaration of interests
SMB reports grants from WHO during the conduct of the study. BMS and AJD report grants from the National Institutes of Health (NIH) during the conduct of the study. TVH reports grants from WHO during the conduct of the study; and grants from NIH and personal fees from Pfizer, outside the submitted work. WHO received a grant from the Bill & Melinda Gates Foundation to support work related to RSV infection and asthma. WHO funded this work. DRF works for WHO but has no personal conflicts of interest in this topic. HJZ reports grants from the Bill & Melinda Gates Foundation (OPP 1017641), Novavax, Medimmune, South African Medical Research Council, National Research Foundation, outside the submitted work. LJB has regular interaction with pharmaceutical and other industrial partners and has not received personal fees or other personal benefits. LJB's institution University Medical Centre Utrecht (UMCU) has received major funding (>€100 000 per industrial partner) for investigator-initiated studies from AbbVie, MedImmune, Janssen, the Bill & Melinda Gates Foundation, Nutricia (Danone), and MeMed Diagnostics. UMCU has received major cash or in-kind funding as part of the public private partnership funded by the Innovative Medicine Initiative's REspiratory Syncytial virus Consortium in EUrope project from GlaxoSmithKline, Novavax, Janssen, AstraZeneca, Pfizer, and Sanofi. UMCU has received major funding by Julius Clinical for participating in the INFORM study sponsored by MedImmune. UMCU has received minor funding for participation in trials by Regeneron and Janssen from 2015–17 (total annual estimate <€20 000). UMCU received minor funding for consultation and invited lectures by AbbVie, MedImmune, Ablynx, Bavaria Nordic, MabXience, Novavax, Pfizer, Janssen (total annual estimate less than €20 000). LJB is the founding chairman of the ReSViNET Foundation. DBF, DAS, BS, NB, WDD, PW, TG, PGH, and JRO declare no competing interests.
Supplementary Material
References
- 1.Scheltema NM, Gentile A, Lucion F. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health. 2017;5:e984–e991. doi: 10.1016/S2214-109X(17)30344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi T, McAllister DA, O'Brien KL. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO RSV vaccine research and development technology roadmap: priority activities for development, testing, licensure and global use of RSV vaccines, with a specific focus on the medical need for young children in low- and middle-income countries. 2017. https://apps.who.int/iris/bitstream/handle/10665/258706/WHO-IVB-17·12-eng.jsessionid=BE971870FE08AB07D48E2D8177DFDC35?sequence=1
- 4.Gavi. The Vaccine Alliance Vaccine investment strategy. 2019. https://www.gavi.org/about/strategy/vaccine-investment-strategy
- 5.Kabego L, de Beer C. Association between respiratory syncytial virus infection in infancy and subsequent asthma: a meta-analysis of observational studies. JSM Allergy and Asthma. 2017;2 [Google Scholar]
- 6.Régnier SA, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta-analysis. Pediatr Infect Dis J. 2013;32:820–826. doi: 10.1097/INF.0b013e31829061e8. [DOI] [PubMed] [Google Scholar]
- 7.Shi T, Ooi Y, Zaw EM. Association between respiratory syncytial virus-associated acute lower respiratory infection in early life and recurrent wheeze and asthma in later childhood. J Infect Dis. 2019 doi: 10.1093/infdis/jiz311. published online Aug 1. [DOI] [PubMed] [Google Scholar]
- 8.Wu P, Dupont WD, Griffin MR. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis. 2014;18:1269–1278. doi: 10.5588/ijtld.14.0170. [DOI] [PubMed] [Google Scholar]
- 10.Groves HE, Shields MD. Respiratory syncytial virus and asthma inception: cause and effect, or shared susceptibility? J Infect Dis. 2019;220:547–549. doi: 10.1093/infdis/jiy672. [DOI] [PubMed] [Google Scholar]
- 11.Feldman AS, He Y, Moore ML, Hershenson MB, Hartert TV. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med. 2015;191:34–44. doi: 10.1164/rccm.201405-0901PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomsen SF, van der Sluis S, Stensballe LG. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med. 2009;179:1091–1097. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 13.Thomsen SF, Stensballe LG. Respiratory syncytial virus and asthma in twin children: lessons from the population-based Danish registries. Curr Respir Med Rev. 2011;7:167–171. [Google Scholar]
- 14.Chawes BLK, Poorisrisak P, Johnston SL, Bisgaard H. Neonatal bronchial hyperresponsiveness precedes acute severe viral bronchiolitis in infants. J Allergy Clin Immunol. 2012;130:354. doi: 10.1016/j.jaci.2012.04.045. 61.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Repapi E, Sayers I, Wain LV. Genome-wide association study identifies five loci associated with lung function. Nat Genet. 2010;42:36–44. doi: 10.1038/ng.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poorisrisak P, Halkjaer LB, Thomsen SF. Causal direction between respiratory syncytial virus bronchiolitis and asthma studied in monozygotic twins. Chest. 2010;138:338–344. doi: 10.1378/chest.10-0365. [DOI] [PubMed] [Google Scholar]
- 17.Pearl J. Causal inference in statistics: an overview. Stat Surv. 2009;3:96–146. [Google Scholar]
- 18.Savitz DA, Wellenius GA. 2nd edn. Oxford University Press; New York, NY: 2016. Interpreting epidemiologic evidence: connecting research to applications. [Google Scholar]
- 19.Pearl J, Glymour M, Jewell N. John Wiley & Sons; West Sussex, UK: 2016. Causal inference in statistics: a primer. [Google Scholar]
- 20.Carroll KN, Gebretsadik T, Escobar GJ. Respiratory syncytial virus immunoprophylaxis in high-risk infants and development of childhood asthma. J Allergy Clin Immunol. 2017;139:66–71. doi: 10.1016/j.jaci.2016.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haerskjold A, Stokholm L, Linder M. Palivizumab exposure and the risk of atopic dermatitis, asthma and allergic rhinoconjunctivitis: a cross-national, population-based cohort study. Paediatr Drugs. 2017;19:155–164. doi: 10.1007/s40272-017-0215-7. [DOI] [PubMed] [Google Scholar]
- 22.Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA. 2016;316:1818–1819. doi: 10.1001/jama.2016.16435. [DOI] [PubMed] [Google Scholar]
- 23.Dupont W, Wu P, Gebretsadik T, Hartert T. RSV prevention in infancy and asthma in later life. Lancet Respir Med. 2018;6:e32. doi: 10.1016/S2213-2600(18)30231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;91:444–455. [Google Scholar]
- 25.Achten NB, Wu P, Bont L. Interference between respiratory syncytial virus and human rhinovirus infection in infancy. J Infect Dis. 2017;215:1102–1106. doi: 10.1093/infdis/jix031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall CB, Douglas RG, Jr, Geiman JM. Respiratory syncytial virus infections in infants: quantitation and duration of shedding. J Pediatr. 1976;89:11–15. doi: 10.1016/s0022-3476(76)80918-3. [DOI] [PubMed] [Google Scholar]
- 27.Feltes TF, Sondheimer HM, Tulloh RMR. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatr Res. 2011;70:186–191. doi: 10.1203/PDR.0b013e318220a553. [DOI] [PubMed] [Google Scholar]
- 28.Gutfraind A, Galvani AP, Meyers LA. Efficacy and optimization of palivizumab injection regimens against respiratory syncytial virus infection. JAMA Pediatr. 2015;169:341–348. doi: 10.1001/jamapediatrics.2014.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Aalderen WM. Childhood asthma: diagnosis and treatment. Scientifica. 2012 doi: 10.6064/2012/674204. published online Sept 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Kusel MMH, de Klerk NH, Kebadze T. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanken MO, Rovers MM, Molenaar JM. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 34.Scheltema NM, Nibbelke EE, Pouw J. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. Lancet Respir Med. 2018;6:257–264. doi: 10.1016/S2213-2600(18)30055-9. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien KL, Chandran A, Weatherholtz R. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis. 2015;15:1398–1408. doi: 10.1016/S1473-3099(15)00247-9. [DOI] [PubMed] [Google Scholar]
- 36.Yoshihara S, Kusuda S, Mochizuki H, Okada K, Nishima S, Simões EA. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics. 2013;132:811–818. doi: 10.1542/peds.2013-0982. [DOI] [PubMed] [Google Scholar]
- 37.Mochizuki H, Kusuda S, Okada K. Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. six-year follow-up study. Am J Respir Crit Care Med. 2017;196:29–38. doi: 10.1164/rccm.201609-1812OC. [DOI] [PubMed] [Google Scholar]
- 38.Simoes EAF, Groothuis JR, Carbonell-Estrany X. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 39.Prais D, Kaplan E, Klinger G. Short- and long-term pulmonary outcome of palivizumab in children born extremely prematurely. Chest. 2016;149:801–808. doi: 10.1378/chest.15-0328. [DOI] [PubMed] [Google Scholar]
- 40.dos Santos Simões MCR, Inoue Y, Matsunaga NY. Recurrent wheezing in preterm infants: prevalence and risk factors. J Pediatr (Rio J) 2019;95:720–727. doi: 10.1016/j.jped.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Ruotsalainen M, Hyvärinen MK, Piippo-Savolainen E, Korppi M. Adolescent asthma after rhinovirus and respiratory syncytial virus bronchiolitis. Pediatr Pulmonol. 2013;48:633–639. doi: 10.1002/ppul.22692. [DOI] [PubMed] [Google Scholar]
- 42.Backman K, Ollikainen H, Piippo-Savolainen E, Nuolivirta K, Korppi M. Asthma and lung function in adulthood after a viral wheezing episode in early childhood. Clin Exp Allergy. 2018;48:138–146. doi: 10.1111/cea.13062. [DOI] [PubMed] [Google Scholar]
- 43.Korppi M, Kuikka L, Reijonen T, Remes K, Juntunen-Backman K, Launiala K. Bronchial asthma and hyperreactivity after early childhood bronchiolitis or pneumonia. An 8-year follow-up study. Arch Pediatr Adolesc Med. 1994;148:1079–1084. doi: 10.1001/archpedi.1994.02170100077015. [DOI] [PubMed] [Google Scholar]
- 44.Korppi M, Piippo-Savolainen E, Korhonen K, Remes S. Respiratory morbidity 20 years after RSV infection in infancy. Pediatr Pulmonol. 2004;38:155–160. doi: 10.1002/ppul.20058. [DOI] [PubMed] [Google Scholar]
- 45.Ruotsalainen M, Piippo-Savolainen E, Hyvärinen MK, Korppi M. Adulthood asthma after wheezing in infancy: a questionnaire study at 27 years of age. Allergy. 2010;65:503–509. doi: 10.1111/j.1398-9995.2009.02212.x. [DOI] [PubMed] [Google Scholar]
- 46.Ruotsalainen M, Piippo-Savolainen E, Hyvärinen MK, Korppi M. Respiratory morbidity in adulthood after respiratory syncytial virus hospitalization in infancy. Pediatr Infect Dis J. 2010;29:872–874. doi: 10.1097/inf.0b013e3181dea5de. [DOI] [PubMed] [Google Scholar]
- 47.Backman K, Piippo-Savolainen E, Ollikainen H, Koskela H, Korppi M. Adults face increased asthma risk after infant RSV bronchiolitis and reduced respiratory health-related quality of life after RSV pneumonia. Acta Paediatr. 2014;103:850–855. doi: 10.1111/apa.12662. [DOI] [PubMed] [Google Scholar]
- 48.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Björkstén B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 49.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 50.Sigurs N, Gustafsson PM, Bjarnason R. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 51.Sigurs N, Aljassim F, Kjellman B. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 52.Fjaerli HO, Farstad T, Rød G, Ufert GK, Gulbrandsen P, Nakstad B. Acute bronchiolitis in infancy as risk factor for wheezing and reduced pulmonary function by seven years in Akershus County, Norway. BMC Pediatr. 2005;5:31. doi: 10.1186/1471-2431-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16:386–392. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 54.Stensballe LG, Simonsen J, Breindahl M, Winding L, Kofoed P-E. Wheeze after hospitalization for respiratory syncytial virus infection in children. J Pediatr Infect Dis. 2018;13:46–50. [Google Scholar]
- 55.Carbonell-Estrany X, Pérez-Yarza EG, García LS, Guzmán Cabañas JM, Bòria EV, Atienza BB. Long-term burden and respiratory effects of respiratory syncytial virus hospitalization in preterm infants—the SPRING study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Escobar GJ, Masaquel AS, Li SX, Walsh EM, Kipnis P. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013;13:97. doi: 10.1186/1471-2431-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim C-K, Seo JK, Ban SH, Fujisawa T, Kim DW, Callaway Z. Eosinophil-derived neurotoxin levels at 3 months post-respiratory syncytial virus bronchiolitis are a predictive biomarker of recurrent wheezing. Biomarkers. 2013;18:230–235. doi: 10.3109/1354750X.2013.773078. [DOI] [PubMed] [Google Scholar]
- 58.Palmer L, Hall CB, Katkin JP. Respiratory outcomes, utilization and costs 12 months following a respiratory syncytial virus diagnosis among commercially insured late-preterm infants. Curr Med Res Opin. 2011;27:403–412. doi: 10.1185/03007995.2010.542744. [DOI] [PubMed] [Google Scholar]
- 59.Blanken MO, Korsten K, Achten NB. Population-attributable risk of risk factors for recurrent wheezing in moderate preterm infants during the first year of life. Paediatr Perinat Epidemiol. 2016;30:376–385. doi: 10.1111/ppe.12295. [DOI] [PubMed] [Google Scholar]
- 60.Bloemers BLP, van Furth AM, Weijerman ME. High incidence of recurrent wheeze in children with Down syndrome with and without previous respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2010;29:39–42. doi: 10.1097/INF.0b013e3181b34e52. [DOI] [PubMed] [Google Scholar]
- 61.García-García ML, Calvo C, Casas I. Human metapneumovirus bronchiolitis in infancy is an important risk factor for asthma at age 5. Pediatr Pulmonol. 2007;42:458–464. doi: 10.1002/ppul.20597. [DOI] [PubMed] [Google Scholar]
- 62.Mikalsen IB, Halvorsen T, Øymar K. The outcome after severe bronchiolitis is related to gender and virus. Pediatr Allergy Immunol. 2012;23:391–398. doi: 10.1111/j.1399-3038.2012.01283.x. [DOI] [PubMed] [Google Scholar]
- 63.Osundwa VM, Dawod ST, Ehlayel M. Recurrent wheezing in children with respiratory syncytial virus (RSV) bronchiolitis in Qatar. Eur J Pediatr. 1993;152:1001–1003. doi: 10.1007/BF01957225. [DOI] [PubMed] [Google Scholar]
- 64.Palmer L, Hall CB, Katkin JP. Healthcare costs within a year of respiratory syncytial virus among Medicaid infants. Pediatr Pulmonol. 2010;45:772–781. doi: 10.1002/ppul.21244. [DOI] [PubMed] [Google Scholar]
- 65.Weber MW, Milligan P, Giadom B. Respiratory illness after severe respiratory syncytial virus disease in infancy in The Gambia. J Pediatr. 1999;135:683–688. doi: 10.1016/s0022-3476(99)70085-5. [DOI] [PubMed] [Google Scholar]
- 66.Fauroux B, Gouyon J-B, Roze J-C. Respiratory morbidity of preterm infants of less than 33 weeks gestation without bronchopulmonary dysplasia: a 12-month follow-up of the CASTOR study cohort. Epidemiol Infect. 2014;142:1362–1374. doi: 10.1017/S0950268813001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sims DG, Downham MA, Gardner PS, Webb JK, Weightman D. Study of 8-year-old children with a history of respiratory syncytial virus bronchiolitis in infancy. BMJ. 1978;1:11–14. doi: 10.1136/bmj.1.6104.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pullan CR, Hey EN. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed) 1982;284:1665–1669. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Juntti H, Kokkonen J, Dunder T, Renko M, Niinimäki A, Uhari M. Association of an early respiratory syncytial virus infection and atopic allergy. Allergy. 2003;58:878–884. doi: 10.1034/j.1398-9995.2003.00233.x. [DOI] [PubMed] [Google Scholar]
- 70.Bertrand P, Lay MK, Piedimonte G. Elevated IL-3 and IL-12p40 levels in the lower airway of infants with RSV-induced bronchiolitis correlate with recurrent wheezing. Cytokine. 2015;76:417–423. doi: 10.1016/j.cyto.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 71.Singleton RJ, Redding GJ, Lewis TC. Sequelae of severe respiratory syncytial virus infection in infancy and early childhood among Alaska Native children. Pediatrics. 2003;112:285–290. doi: 10.1542/peds.112.2.285. [DOI] [PubMed] [Google Scholar]
- 72.Schauer U, Hoffjan S, Bittscheidt J. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J. 2002;20:1277–1283. doi: 10.1183/09031936.02.00019902. [DOI] [PubMed] [Google Scholar]
- 73.Stensballe LG, Simonsen JB, Thomsen SF. The causal direction in the association between respiratory syncytial virus hospitalization and asthma. J Allergy Clin Immunol. 2009;123:131–137. doi: 10.1016/j.jaci.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 74.Munywoki PK, Ohuma EO, Ngama M, Bauni E, Scott JAG, Nokes DJ. Severe lower respiratory tract infection in early infancy and pneumonia hospitalizations among children, Kenya. Emerg Infect Dis. 2013;19:223–229. doi: 10.3201/eid1902.120940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kusel MMH, Kebadze T, Johnston SL, Holt PG, Sly PD. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur Respir J. 2012;39:876–882. doi: 10.1183/09031936.00193310. [DOI] [PubMed] [Google Scholar]
- 76.Calişkan M, Bochkov YA, Kreiner-Møller E. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bønnelykke K, Vissing NH, Sevelsted A, Johnston SL, Bisgaard H. Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol. 2015;136:81–86. doi: 10.1016/j.jaci.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lemanske RF, Jr, Jackson DJ, Gangnon RE. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 79.Jackson DJ, Gangnon RE, Evans MD. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubner FJ, Jackson DJ, Evans MD. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol. 2017;139:501–507. doi: 10.1016/j.jaci.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stein RT, Sherrill D, Morgan WJ. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 82.Voraphani N, Stern DA, Wright AL, Guerra S, Morgan WJ, Martinez FD. Risk of current asthma among adult smokers with respiratory syncytial virus illnesses in early life. Am J Respir Crit Care Med. 2014;190:392–398. doi: 10.1164/rccm.201311-2095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drysdale SB, Alcazar-Paris M, Wilson T. Viral lower respiratory tract infections and preterm infants' healthcare utilisation. Eur J Pediatr. 2015;174:209–215. doi: 10.1007/s00431-014-2380-9. [DOI] [PubMed] [Google Scholar]
- 84.Zomer-Kooijker K, van der Ent CK, Ermers MJJ, Uiterwaal CS, Rovers MM, Bont LJ. Increased risk of wheeze and decreased lung function after respiratory syncytial virus infection. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Broughton S, Sylvester KP, Fox G. Lung function in prematurely born infants after viral lower respiratory tract infections. Pediatr Infect Dis J. 2007;26:1019–1024. doi: 10.1097/INF.0b013e318126bbb9. [DOI] [PubMed] [Google Scholar]
- 86.The World Bank Data: World Bank country and lending groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 87.Cook TD. Advanced statistics: up with odds ratios! A case for odds ratios when outcomes are common. Acad Emerg Med. 2002;9:1430–1434. doi: 10.1111/j.1553-2712.2002.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 88.Tanner-Smith EE, Tipton E, Polanin JR. Handling complex meta-analytic data structures using robust variance estimates: a tutorial in R. J Dev Life Course Criminol. 2016;2:85–112. [Google Scholar]
- 89.Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Res Synth Methods. 2010;1:39–65. doi: 10.1002/jrsm.5. [DOI] [PubMed] [Google Scholar]
- 90.Fisher Z, Tipton E. Robumeta: an R-package for robust variance estimation in meta-analysis. aRxiv. 2015 published online March 7. https://doi.org/1503.02220v1. [Google Scholar]
- 91.Wells GA, Shea B, O'Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2019. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 92.Higgins JPT, Altman DG, Gøtzsche PC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- 94.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fewell Z, Davey Smith G, Sterne JAC. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol. 2007;166:646–655. doi: 10.1093/aje/kwm165. [DOI] [PubMed] [Google Scholar]
- 96.Ogburn EL, VanderWeele TJ. On the nondifferential misclassification of a binary confounder. Epidemiology. 2012;23:433–439. doi: 10.1097/EDE.0b013e31824d1f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Riddell CA, Bhat N, Bont LJ. Informing randomized clinical trials of respiratory syncytial virus vaccination during pregnancy to prevent recurrent childhood wheezing: a sample size analysis. Vaccine. 2018;36:8100–8109. doi: 10.1016/j.vaccine.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Larkin EK, Hartert TV. Genes associated with RSV lower respiratory tract infection and asthma: the application of genetic epidemiological methods to understand causality. Future Virol. 2015;10:883–897. doi: 10.2217/fvl.15.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Calışkan M, Bochkov YA, Kreiner-Møller E. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baiocchi M, Cheng J, Small DS. Instrumental variable methods for causal inference. Stat Med. 2014;33:2297–2340. doi: 10.1002/sim.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Savitz DA, Wellenius GA, Trikalinos TA. The problem with mechanistic risk of bias assessments in evidence synthesis of observational studies and a practical alternative: assessing the impact of specific sources of potential bias. Am J Epidemiol. 2019;188:1581–1585. doi: 10.1093/aje/kwz131. [DOI] [PubMed] [Google Scholar]
- 103.VanderWeele T. Oxford University Press; Oxford, UK: 2015. Explanation in causal inference: methods for mediation and interaction. [Google Scholar]
- 104.Rubin D. Causal inference using potential outcomes. J Am Stat Assoc. 2005;100:322–331. [Google Scholar]
- 105.Karron RA, Zar HJ. Determining the outcomes of interventions to prevent respiratory syncytial virus disease in children: what to measure? Lancet Respir Med. 2018;6:65–74. doi: 10.1016/S2213-2600(17)30303-X. [DOI] [PubMed] [Google Scholar]
- 106.Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Review of Anti-infective Therapy. 2011;9:731–745. doi: 10.1586/eri.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nair H, Nokes DJ, Gessner BD. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All analyses and R code are available at https://brunwasser.github.io/whorsv.github.io/.