Abstract
Background
Colorectal cancer (CRC) screening with guaiac fecal occult blood test (gFOBT) reduces CRC-related death. Average risk individuals should be recalled for screening with gFOBT every 2 years in order to maximize effectiveness. However, adherence with repeated testing is often suboptimal. Our aim was to evaluate whether adding a gFOBT kit to a mailed recall letter improves participation compared with a mailed recall letter alone, among previous responders to a mailed invitation.
Methods
We conducted a cluster randomized controlled trial, with the primary care provider as the unit of randomization. Eligible patients had completed a gFOBT and tested negative in an earlier pilot study and were now due for recall. The intervention group received a mailed CRC screening recall letter from their primary care provider plus a gFOBT kit (n = 431) while the control group received a mailed CRC screening mailed recall letter alone (n = 452). The primary outcome was the uptake of gFOBT or colonoscopy within 6 months.
Results
gFOBT uptake was higher in the intervention group (61.3%, n = 264) compared with the control group (50.4%, n = 228) with an absolute difference between the two groups of 10.8% (95% confidence interval [CI]: 1.4 to 20.2%, P = <0.01). Patients in the intervention group were more likely to complete the gFOBT compared with the control group (odds ratio [OR] = 1.4; 95% CI: 1.1 to 1.9).
Conclusion
Our findings show that adding gFOBT kits to the mailed recall letter increased participation among persons recalled for screening. Nine gFOBT kits would have to be sent by mail in order to screen one additional person.
Keywords: Colorectal cancer, Fecal occult blood test, Randomized controlled trial, Screening
Regular screening for colorectal cancer (CRC) can reduce the incidence of and death from CRC (1–5). Noninvasive fecal occult blood tests (FOBT), such as the guaiac FOBT (gFOBT) and the fecal immunochemical test (FIT), are the most commonly used CRC screening methods worldwide (6), because they are inexpensive, safe and convenient. It is recommended that average risk individuals should be screened with FOBT every 1 to 2 years in order to maximize effectiveness (7,8). Stool tests are well suited to organized CRC screening programs as tests can be mailed directly to patients, samples are collected at home and returned by mail to a local laboratory (9).
The effectiveness of gFOBT depends on patients participating with repeated testing as the onetime sensitivity of this test is poor (10). In three landmark randomized controlled trials, all of which demonstrated that gFOBT reduced CRC-related mortality, testing was repeated either annually or biennially over 10 to 15 years (1–3). In these trials, 40 to 60% of persons participated with the recommended repeat test over the study period. In usual care, including in organized CRC screening programs, patient adherence to repeat testing may be even lower, potentially reducing the effectiveness of these programs.
In 2008, the province of Ontario launched Canada’s first organized CRC screening program, ColonCancerCheck (CCC) (11). In 2009, the CCC program conducted the CCC invitation pilot (‘the pilot’), which tested the technical feasibility of a centralized approach to large scale physician-linked mailed invitations for CRC screening using a convenience sample of approximately 11,000 eligible persons, aged 50 to 74 years (12). Invitations were issued by the program on behalf of participating primary care providers (PCPs) to their eligible associated patients who were due for CRC screening. Approximately, 11,000 eligible persons, aged 50 to 74 years, were sent mailed invitations, requesting them to visit their PCP to obtain a gFOBT kit or, if appropriate based on family history, a referral for colonoscopy.
Participation with repeated testing improves the effectiveness of CRC screening (13–15), but is often suboptimal (16,17). As most studies have focused on first time participation with a screening initiative, there is limited information on factors associated with participation with repeated CRC screening (18). While mail and telephone interventions have been shown to increase uptake of initial screening, little is known about the durability of their effect (19,20). As part of the CCC program, those who screen using the gFOBT receive recall letters 2 years after the initial screen, when they were due for repeat CRC screening.
In the current study, we evaluated whether adding a gFOBT kit to a recall letter was associated with increased participation with CRC screening compared with recall letter alone among eligible persons who had previously responded to a mailed invitation to complete a gFOBT.
METHODS
Study Design and Setting
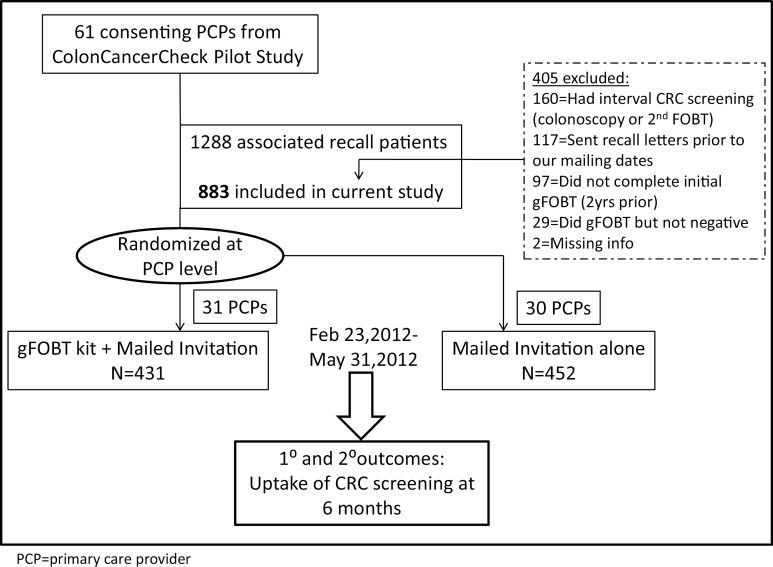
In this cluster randomized controlled trial, we tested the effect of adding a gFOBT kit to a recall letter among persons who had previously responded to a mailed invitation to complete a gFOBT in Ontario, Canada. Randomization was at the level of the PCP (Figure 1). This study was approved by the institutional review board at Sunnybrook Health Sciences Centre, Toronto, Canada (ClinicalTrials.gov: NCT01629004).
Figure 1.
Flow chart of study design, recruitment and randomization. PCP, Primary care provider.
Data Sources
We linked patient level information from Cancer Care Ontario’s (CCO) health administrative databases to the administrative databases held at the Institute for Clinical Evaluative Sciences (ICES) in Toronto. These datasets were linked using unique encoded identifiers and analyzed at ICES.
The Canadian Institute of Health Information (CIHI) databases, the Ontario Health Insurance Plan (OHIP) physician claims database, the Registered Persons Database (RPDB), the ICES Physician Database (IPDB) and the Client Agency Program Enrollment (CAPE) registry at ICES were used for the study. The CIHI, OHIP, RPDB and the IPDB have been previously described (21). The CAPE registry captures patients rostered to a specific provider in patient enrolled models (PEMs) of care (22).
The CCO databases used were the Laboratory Reporting Tool (LRT) and the Colonoscopy Interim Reporting Tool (CIRT). The CIRT includes data on all colonoscopies for all indications performed at participating CCC hospitals and the LRT comprises data related to the gFOBT kits administered by the CCC program, including the results of these tests.
Study Participants
The 102 PCPs who participated in the 2009 pilot study (12) were invited by mail to participate in the current trial. Nonresponders were then followed up by fax or email depending on available contact information. For PCPs who still did not respond to these invitations, study staff contacted them by phone to follow up on the invitation. Informed consent was obtained from 61 of the 102 PCPs.
All patients who were registered to the 61 PCPs, who had responded to the pilot study invitation in 2009 and had a negative gFOBT, were age eligible (50 to 74 years old), and were now due for repeat screening, were included. Patients were excluded if they had had a gFOBT or colonoscopy after the pilot study but before the mailing date of the current study, if they were inadvertently sent a routine CCC program mailed invitation for recall outside of our study, or if they were missing sociodemographic information in the health administrative databases.
Intervention + Control
Consenting PCPs were randomly allocated to one of the two study arms. Their eligible patients were: (a) mailed the PCP-endorsed CRC screening recall letter plus the gFOBT test (‘intervention’) OR (b) mailed the PCP-endorsed CRC screening recall letter alone (‘control’). The gFOBT test consisted of three cards, each with two windows; if one or more windows were positive for occult blood, then the test was considered abnormal and colonoscopy was recommended. An ICES analyst, blinded to all aspects of the study, performed randomization at the PCP level.
Patients assigned to the intervention arm received a package by mail containing (a) a PCP-endorsed recall letter from the CCC program, (b) a gFOBT kit prelabeled with their name and date of birth, (c) a laboratory requisition form signed by their PCP, (d) instructions on how to complete the test card and requisition form and (e) a prepaid envelope to return the completed gFOBT kit by post.
Patients assigned to the control arm received a PCP-endorsed recall letter by mail from the CCC program. The letter asked recipients to contact their PCP to set up an appointment to discuss their next CRC screening test. PCP contact information was provided.
Invitations were mailed to patients in both arms beginning in February 2012. The invitations were issued over a four month period, such that they were mailed approximately 2 years after the return of the initial gFOBT (see Supplementary Materials for study materials).
Outcome Measures
The primary and secondary outcomes were, respectively, the uptake of gFOBT alone and uptake of gFOBT or colonoscopy within 6 months of the mailing. We evaluated the uptake of colonoscopy in addition to the uptake of gFOBT because colonoscopy may be used for screening instead of gFOBT. In particular, the CCC program recommends those at increased risk (1+ first-degree relatives) to be screened with colonoscopy and as well, opportunistic screening with colonoscopy is readily available in Ontario for persons at average risk (i.e., irrespective of a positive gFOBT test result). We used the OHIP, LRT and CIRT databases to determine outcomes.
Patient and PCP Participant Factors
We characterized the patients by sex, age group, median neighborhood income (classified as rural or by urban income quintile), co-morbidity, health region (north versus south local health integration networks (LHINs)) (23) and gFOBT and colonoscopy use prior to the gFOBT completed during the pilot (i.e., >2 years prior to mailing). We measured co-morbidity using only major ADGs and categorized patients as having 0, 1, 2+ major ADGs using the number of major aggregated diagnosis groups (ADGs). Aggregated Diagnosis Groups are clinically meaningful groupings of diagnoses that have been shown to accurately predict mortality in a general population ambulatory cohort in Ontario (24). Diagnosis codes within the same ADG are similar in terms of both clinical criteria and expected need for healthcare resources. A patient can be assigned as few as none and as many as 32 ADGs. The system further classifies ADGs as ‘major’ or ‘minor’ (25). We retained only the major ADGs and categorized patients as having 0, 1, 2+ major ADGs. Statistics Canada census data, linked to patient postal code, was used to define median neighborhood income (26,27). We grouped the 14 LHINs in Ontario into northern (2 LHINs) and southern regions (12 LHINs).
We characterized the PCP participants by sex, age group, location of medical training (i.e., trained in Canada or outside Canada), years in practice and practice type. All PCPs in this study belonged to PEMs, practices that roster their patients. We categorized PCPs by PEM practice type as each differ in terms of organizational structure, services provided and reimbursement method (22). We used the following PEM practice categories: family health groups (FHGs, enhanced fee-for-service models), family health organizations (FHOs, a blended capitation model), family health networks (FHNs, work in interdisciplinary teams and have a slightly different blended capitation model) and other PEMs (28).
Statistical Analysis
We analyzed baseline demographic data by study arm and overall. Patients were excluded if they were missing sociodemographic information. The proportion of patients in each study arm who completed a gFOBT alone (primary outcome) and gFOBT and/or colonoscopy (secondary outcome) within 6 months of the mailing were compared using the adjusted Chi-square test to account for clustering of patients within PCPs (29). Logistic regression, using generalized estimating equations (GEE) methods (30) to account for the clustering of patients by PCP, was used to assess the effect of directly mailing the gFOBT on the odds of subsequent screening, after adjusting for patient and PCP baseline characteristics. The intraclass correlation coefficient (ICC) was estimated from the models for the primary and secondary outcomes and the results from the null models are reported. For all analyses, a P-value of less than 0.05 was considered significant.
Sample Size and Power
In the pilot study, 1950 patients tested negative with the gFOBT; these persons comprised the eligible population for the current study. Using Monte Carlo simulation methods, we determined the statistical power to detect a prespecified absolute difference in screening rates between the two arms of the study, assuming an ICC of 0.10 in the use of screening in PCP practices. We obtained the ICC estimate from an analysis of pilot study data on the rates of gFOBT use in the 2 years prior to the mailing among their eligible patients by the 118 pilot PCPs (16 dropped out of pilot study, leaving 102) (31).
We estimated our control rate and detectable difference on a prior study (31), assuming that there would be 59 clusters (PCP practices) per arm, 16 patients enrolled per PCP practice and that 8% of patients in the control arm would participate with recall screening. Under these assumptions, the study would have 86.7% power to detect an absolute difference of 7% between study arms.
RESULTS
The study cohort comprised 31 PCPs in the intervention arm and 30 in the control arm. Based on information contained in health administrative databases, a total of 1288 patients eligible for recall were identified. After excluding those who had had CRC test in the interval between the pilot and the current study (colonoscopy or second gFOBT) (n = 160), those inadvertently sent interval CCC program recall letters outside of our study (n = 117), those who did not complete an initial gFOBT (n = 97), those who completed a gFOBT but was not negative (n = 29) and patients with missing information (n=2), there were 431 patients in the intervention arm and 452 patients in the control arm (Figure 1).
Fifty-one per cent of the patients were female, 44% were 50 to 59 years of age, 64% had no major ADGs while 26% had one major ADG, and 85% lived in the southern part of Ontario. Six per cent of the patients completed a gFOBT in the 2 to 5 years prior to the pilot study. Just over two-third of the patients had a male PCP (Table 1). Fifty-four per cent of the PCP participants were male, 60% were over the age of 50, 92% were trained in Canada and 56% were in FHO model of care (data not shown).
Table 1.
Baseline patient characteristics overall and by study arm
| Characteristics | gFOBT + Mailed Invitation-for- Recall N = 431 | Mailed Invitation-for-Recall Alone N = 452 | Total N = 883 |
|---|---|---|---|
| Sex, No. (%) | |||
| Female | 207 (48%) | 239 (53%) | 446 (51%) |
| Male | 224 (52%) | 213 (47%) | 437 (49%) |
| Age group in years, No. (%) | |||
| 50–59 | 205 (48%) | 188 (42%) | 393 (44%) |
| 60–69 | 173 (40%) | 185 (41%) | 358 (41%) |
| 70+ | 53 (12%) | 79 (17%) | 132 (15%) |
| Co-morbidity*, sum of major ADGs, No. (%) | |||
| 0 | 277 (64%) | 286 (63%) | 563 (64%) |
| 1 | 111 (26%) | 119 (26%) | 230 (26%) |
| 2+ | 43 (10%) | 47 (11%) | 90 (10%) |
| Median neighbourhood income quintile**, No. (%) | |||
| Low Urban | 55 (13%) | 86 (19%) | 141 (16%) |
| 2 | 64 (15%) | 75 (18%) | 139 (16%) |
| 3 | 56 (13%) | 65 (14%) | 121 (14%) |
| 4 | 67 (16%) | 74 (16%) | 141 (16%) |
| High Urban | 92 (21%) | 79 (17%) | 171 (19%) |
| Rural | 97 (22%) | 73 (16%) | 170 (19%) |
| Health region, No. (%) | |||
| North regions | 46 (11%) | 87 (19%) | 133 (15%) |
| South regions | 385 (89%) | 365 (81%) | 750 (85%) |
| Prior FOBT use, No. (%) | |||
| 2–5 years prior to mailing | 23 (5%) | 30 (7%) | 53 (6%) |
| >5 years prior to mailing | 117 (27%) | 106 (24%) | 223 (25%) |
| 2–5 years and >5 years prior to mailing | 23 (5%) | 15 (3%) | 38 (4%) |
| None prior to pilot study | 268 (62%) | 301 (66.6%) | 569 (64%) |
| Prior colonoscopy, No. (%) | |||
| 2–5 years prior to mailing | 11 (3%) | 20 (4%) | 31 (4%) |
| 5–10 years prior to mailing | 13 (3%) | 28 (6%) | 41 (5%) |
| >10 years or never prior to mailing | 407 (94%) | 420 (93%) | 827 (94%) |
| Patients by PCP sex, No. (%) | |||
| Female | 159 (37%) | 126 (28%) | 285 (32%) |
| Male | 272 (63%) | 326 (72%) | 598 (68%) |
| Patients by PCP practice type, No. (%) | |||
| FHG | 64 (15%) | 240 (53%) | 304 (34%) |
| FHN | 60 (14%) | 34 (8%) | 94 (11%) |
| FHO | 297 (69%) | 157 (34%) | 454 (51%) |
| Other PEM | 10 (2%) | 21 (5%) | 31 (4%) |
*Co-morbidity scored using number of major Aggregated Diagnosis Groups (ADGs) using the Johns Hopkins Case Mix System.
**Patients with missing information were excluded.
FHG, Family health group; FHN, Family health networks; FHO, Family health organizations; FOBT, Fecal occult blood test; Other PEM, Other patient enrolled model of care; PCP, Primary care provider.
Two hundred and sixty-four of 431 patients in the intervention group (61.3%) completed a gFOBT within 6 months of the mailing compared with 228 of 452 patients in the control group (50.4%). The absolute difference in gFOBT completion between the two groups was 10.8% (95% confidence interval [CI]: 1.39 to 20.2%, P = <0.01). Therefore, in order to screen one additional person, approximately nine gFOBT kits would have to be sent directly by mail. Similarly, uptake of gFOBT or colonoscopy within 6 months of the mailing date was also higher in the intervention group (267 of 431, 61.9%) compared with the control group (230 of 452, 50.9%). The absolute difference between the two groups was 11.1% (95% CI: 1.8 to 20.3%, P = 0.008).
In the multivariable analyses, after adjusting for patient and PCP factors, patients in the intervention group were more likely to complete the gFOBT than those in the control group (odds ratio [OR] = 1.4, 95% CI: 1.1 to 1.9). The only other factor associated with gFOBT uptake was prior gFOBT use (2 to 5 years prior to initial mailing) (OR = 1.8, 95% CI: 1.1 to 3.0) (Table 2).
Table 2.
Association between intervention and uptake of gFOBT within 6 months, adjusted for patient and physician covariates using logistic regression with generalized estimating equations (GEE)
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Group | ||
| gFOBT + Mailed Recall Letter | 1.4 (1.1, 1.9) | 0.01 |
| Mailed Recall Letter | Reference | - |
| Patient sex | ||
| Male | 1.4 (0.8, 2.4) | 0.31 |
| Female | Reference | - |
| Patient age group, years | ||
| 50–59 | 0.7 (0.4,1.1) | 0.11 |
| 60–69 | 0.7 (0.4,1.1) | 0.16 |
| 70+ | Reference | - |
| Co-morbidity*, Sum of major ADGs | ||
| 0 | 0.8 (0.5,1.3) | 0.47 |
| 1 | 1.0 (0.6,1.7) | 0.85 |
| 2+ | Reference | - |
| Median neighbourhood income quintile | ||
| Low Urban | 0.8 (0.5, 1.2) | 0.33 |
| 2 | 1.0 (0.6, 1.4) | 0.85 |
| 3 | 0.7 (0.5, 1.0) | 0.05 |
| 4 | 0.7 (0.4, 1.0) | 0.07 |
| Rural | 0.9 (0.5, 1.5) | 0.62 |
| High Urban | Reference | - |
| Northern health regions | 0.7 (0.5, 1.1) | 0.09 |
| Southern health regions | Reference | - |
| FOBT use prior to 2009 pilot | ||
| 2–5 years prior to pilot | 1.8 (1.1,3.0) | 0.02 |
| >5 years prior to pilot | 1.2 (0.9, 1.7) | 0.27 |
| 2–5 years and >5 years prior to pilot | 2.2 (1.0,4.9) | 0.05 |
| No FOBT prior to pilot study | Reference | - |
| PCP sex | ||
| Male | 1.5 (1.0, 2.3) | 0.05 |
| Female | Reference | - |
| Type of practice | ||
| FHG | 1.1 (0.8, 1.6) | 0.50 |
| FHN | 1.4 (0.8, 2.4) | 0.24 |
| Other PEM | 0.5 (0.3, 1.0) | 0.04 |
| FHO | Reference | - |
| PCP age (by year) | 1.0 (1.0,1.0) | 0.34 |
*Co-morbidity scored using number of major Aggregated Diagnosis Groups (ADGs) using the Johns Hopkins Case Mix System.
FHG, Family health group; FHN, Family health networks; FHO, Family health organizations; FOBT, Fecal occult blood test; Other PEM, Other patient enrolled model of care; PCP, Primary care provider.
The ICC for the outcome of gFOBT was 0.039 and for the outcome of gFOBT or colonoscopy was 0.036, indicating that less than 4% of the variation in the outcomes was due to systematic differences between physicians.
Discussion
Strategies to promote participation in subsequent rounds of CRC screening using gFOBT have been infrequently reported in the literature. In the current study, we report that adding a gFOBT kit to a mailed recall increases the uptake of gFOBT in previous responders, compared with a mailed recall alone. In order to screen one additional recall patient, nine kits would have to be sent directly by mail. In addition to the intervention, we found that the factor most strongly associated with uptake was prior use of gFOBT (in the 2 to 5 years prior to the initial invitation), suggesting that those who engage in regular screening are likely to continue to do so.
Participation with annual or biennial gFOBT over time is associated with detection of CRC at an earlier stage (14,15). However, only a few studies have examined adherence to CRC screening among previous responders in a randomized controlled fashion. Giorgi Rossi et al. tested the addition of a gFOBT kit to mailed CRC screening invitation among previous respondents in three Italian centres. This randomized trial demonstrated an 11% increase in participation rate (32). Furthermore, a recent trial in the United States (33) randomized community health center patients who had previously completed an FOBT, to receive either a mailed reminder letter, a fecal immunochemical test (FIT) with instructions, postage-paid return envelope, automated text and telephone reminders and phone call from a navigator (intervention group) or to usual care. Over 82% of the intervention group completed FIT testing compared with 37.3% in the usual care group (33). The findings from this latter study suggest that additional strategies such as follow-up automated phone calls or use of a navigator could improve participation to an even greater extent.
Findings from several studies highlight more remote screening history as a major predictor of subsequent response to screening invitation, which is also consistent with findings from our study where we found that patients with prior gFOBT use (2 to 5 years prior to initial invitation) demonstrated higher uptake. In a recent randomized controlled trial, Green et al. evaluated the effect of continuing a centralized FOBT mailed program on screening adherence among patients in patient-centred medical home clinics. They found that patients adhering to FOBT screening in rounds 1 and 2 were significantly more likely to screen in round 3 (77%) compared with the patients completing only one FOBT in one of the prior two rounds (44.6%) (34). In England, Lo et al. examined gFOBT uptake over three biennial invitation rounds in the English bowel cancer screening program and found that participation in the third round was highest among individuals who had screened in both years 1 and 2 (94.5%) versus those who did not screen in either year (14.6%) (35). Results from organized screening programs seem to indicate that a high proportion of participation with fecal-based CRC testing beyond two rounds can be achieved (36,37). Although these findings are promising, further follow-up is needed to understand factors associated with long-term adherence rates over multiple rounds of CRC screening.
Our study has some limitations. Our sample size was smaller than projected, as delays initiating the study resulted in some participants already receiving standard programmatic communication about screening from the CCC program and/or undergoing interval CRC screening. Although the CCC program was not using FIT at the time of our study, as both gFOBT and FIT are noninvasive at home stool tests, we expect that our findings would be applicable to organized CRC screening programs that are using or considering FIT. An additional limitation is volunteer bias, that is, our PCP participants consisted of highly motivated PCPs who had previously participated in a pilot study.
In conclusion, our findings indicate that directly mailed gFOBT increases participation in persons due for recall screening; only nine additional kits would have to be mailed to screen one additional recall participant. Participation with repeated FOBT screening is vital to the success of organized CRC screening programs. Our findings are relevant to these programs as effective strategies to maximize continued screening participation are needed to fully realize the benefits of screening at the population level (14,15).
Supplementary Material
Acknowledgements
This study was conducted with the support of the Ontario Institute for Cancer Research (OICR) through funding provided by the Government of Ontario. This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC) and by Cancer Care Ontario (CCO). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES, the Ontario MOHLTC or CCO is intended or should be inferred. Parts of this material are based on data and information compiled and provided by Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI. P.C.A. is supported in part by a Career Investigator Award from the Heart and Stroke Foundation (Ontario Office).
References
- 1. Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med 2000;343(22):1603–7. [DOI] [PubMed] [Google Scholar]
- 2. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348(9040):1472–7. [DOI] [PubMed] [Google Scholar]
- 3. Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348(9040):1467–71. [DOI] [PubMed] [Google Scholar]
- 4. Atkin WS, Edwards R, Kralj-Hans I, et al. ; UK Flexible Sigmoidoscopy Trial Investigators Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: A multicentre randomised controlled trial. Lancet 2010;375(9726):1624–33. [DOI] [PubMed] [Google Scholar]
- 5. Segnan N, Armaroli P, Bonelli L, et al. ; SCORE Working Group Once-only sigmoidoscopy in colorectal cancer screening: Follow-up findings of the Italian randomized controlled trial–SCORE. J Natl Cancer Inst 2011;103(17):1310–22. [DOI] [PubMed] [Google Scholar]
- 6. Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: A global overview of existing programmes. Gut 2015;64(10):1637–49. [DOI] [PubMed] [Google Scholar]
- 7. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA 2016;315(23):2564–75. [DOI] [PubMed] [Google Scholar]
- 8. Canadian Task Force on Preventive Health Care. Recommendations on screening for colorectal cancer in primary care. Can Med Assoc J 2016;188(5):340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levin TR, Jamieson L, Burley DA, et al. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev 2011;33:101–10. [DOI] [PubMed] [Google Scholar]
- 10. Whitlock EP, Lin JS, Liles E, et al. Screening for colorectal cancer: A targeted, updated systematic review for the U.S. preventive services task force. Ann Intern Med 2008;149(9):638–58. [DOI] [PubMed] [Google Scholar]
- 11. ColonCancerCheck. ColonCancerCheck program: Ministry of health and long-term care, 2011. <http://health.gov.on.ca/en/public/programs/coloncancercheck/> (accessed August 12, 2011).
- 12. Tinmouth J, Baxter NN, Paszat LF, et al. Using physician-linked mailed invitations in an organised colorectal cancer screening programme: Effectiveness and factors associated with response. BMJ Open 2014;4(3):e004494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gellad ZF, Stechuchak KM, Fisher DA, et al. Longitudinal adherence to fecal occult blood testing impacts colorectal cancer screening quality. Am J Gastroenterol 2011;106(6):1125–34. [DOI] [PubMed] [Google Scholar]
- 14. Steele RJ, McClements P, Watling C, et al. Interval cancers in a FOBT-based colorectal cancer population screening programme: Implications for stage, gender and tumour site. Gut 2012;61(4):576–81. [DOI] [PubMed] [Google Scholar]
- 15. James PD, Rabeneck L, Yun L, et al. Repeated faecal occult blood testing is associated with decreased advanced colorectal cancer risk: A population-based study. J Med Screen 2017;25(3):141–8. doi:0969141317718860. [DOI] [PubMed] [Google Scholar]
- 16. Liang PS, Wheat CL, Abhat A, et al. Adherence to competing strategies for colorectal cancer screening over 3 years. Am J Gastroenterol 2016;111(1):105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steele RJ, Kostourou I, McClements P, et al. Effect of repeated invitations on uptake of colorectal cancer screening using faecal occult blood testing: Analysis of prevalence and incidence screening. BMJ 2010;341:c5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vernon SW. Participation in colorectal cancer screening: A review. J Natl Cancer Inst 1997;89(19):1406–22. [DOI] [PubMed] [Google Scholar]
- 19. Myers RE, Balshem AM, Wolf TA, et al. Adherence to continuous screening for colorectal neoplasia. Med Care 1993;31(6):508–19. [DOI] [PubMed] [Google Scholar]
- 20. Brouwers MC, De Vito C, Bahirathan L, et al. Effective interventions to facilitate the uptake of breast, cervical and colorectal cancer screening: An implementation* guideline. Implement Sci 2011;6:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alharbi O, Rabeneck L, Paszat LF, et al. A population-based analysis of outpatient colonoscopy in adults assisted by an anesthesiologist. Anesthesiology 2009;111(4):734–40. [DOI] [PubMed] [Google Scholar]
- 22. HealthForceOntario. Family Practice Models: Government of Ontario, 2013. <http://www.healthforceontario.ca/Work/OutsideOntario/PhysiciansOutsideOntario/PractisingInOntario/family_practice_models.aspx> (accessed August 13, 2011).
- 23. Anonymous. Ontario’s Local Health Integration Networks: Ontario Ministry of Health and Long Term Care, 2013. <http://www.lhins.on.ca/home.aspx> (accessed August 13, 2013).
- 24. Austin PC, van Walraven C, Wodchis WP, et al. Using the Johns Hopkins aggregated diagnosis groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care 2011;49(10):932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anonymous. The Johns Hopkins University ACG Case-Mix System: Johns Hopkins Bloomberg School of Public Health <http://www.acg.jhsph.edu/> (accessed August 13, 2013).
- 26. Alter DA, Naylor CD, Austin P, et al. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med 1999;341(18):1359–67. [DOI] [PubMed] [Google Scholar]
- 27. Singh SM, Paszat LF, Li C, et al. Association of socioeconomic status and receipt of colorectal cancer investigations: A population-based retrospective cohort study. CMAJ 2004;171(5):461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glazier RH, Klein-Geltink J, Kopp A, et al. Capitation and enhanced fee-for-service models for primary care reform: A population-based evaluation. CMAJ 2009;180(11):E72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donner A, Klar N.. Design and Analysis of Cluster Randomization Trials in Health Research. London: Arnold, 2000. [Google Scholar]
- 30. Liang K, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- 31. Tinmouth J, Patel J, Austin PC, et al. Increasing participation in colorectal cancer screening: Results from a cluster randomized trial of directly mailed gFOBT kits to previous nonresponders. Int J Cancer 2015;136(6):E697–703. [DOI] [PubMed] [Google Scholar]
- 32. Giorgi Rossi P, Grazzini G, Anti M, et al. Direct mailing of faecal occult blood tests for colorectal cancer screening: A randomized population study from Central Italy. J Med Screen 2011;18(3):121–7. [DOI] [PubMed] [Google Scholar]
- 33. Baker DW, Brown T, Buchanan DR, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: A randomized clinical trial. JAMA Intern Med 2014;174(8):1235–41. [DOI] [PubMed] [Google Scholar]
- 34. Green BB, Anderson ML, Chubak J, et al. Impact of continued mailed fecal tests in the patient-centered medical home: Year 3 of the systems of support to increase colon cancer screening and follow-up randomized trial. Cancer 2016;122(2):312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lo SH, Halloran S, Snowball J, Seaman H, Wardle J, von Wagner C. Colorectal cancer screening uptake over three biennial invitation rounds in the English bowel cancer screening programme. Gut:gutjnl-2013–306144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: A retrospective cohort study. Ann Intern Med 2016;164(7):456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Adherence to colorectal cancer screening: Four rounds of faecal immunochemical test-based screening. Br J Cancer 2017;116(1):44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.