Abstract
Objective
To assess medical resource utilization associated with Prader-Willi syndrome (PWS) in the US, hypothesized to be greater relative to a matched control group without PWS.
Study design
We used a retrospective case-matched control design and longitudinal US administrative claims data (MarketScan) during a 5-year enrollment period (2009–2014). Patients with PWS were identified by Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code 759.81. Controls were matched on age, sex, and payer type. Outcomes included total, outpatient, inpatient and prescription costs.
Results
After matching and application of inclusion/exclusion criteria, we identified 2030 patients with PWS (1161 commercial, 38 Medicare supplemental, and 831 Medicaid). Commercially insured patients with PWS (median age 10 years) had 8.8-times greater total annual direct medical costs than their counterparts without PWS (median age 10 years: median costs $14 907 vs $819; P < .0001; mean costs: $28 712 vs $3246). Outpatient care comprised the largest portion of medical resource utilization for enrollees with and without PWS (median $5605 vs $675; P < .0001; mean $11 032 vs $1804), followed by mean annual inpatient and medication costs, which were $10 879 vs $1015 (P < .001) and $6801 vs $428 (P < .001), respectively. Total annual direct medical costs were ~42% greater for Medicaid-insured patients with PWS than their commercially insured counterparts, an increase partly explained by claims for Medicaid Waiver day and residential habilitation.
Conclusion
Direct medical resource utilization was considerably greater among patients with PWS than members without the condition. This study provides a first step toward quantifying the financial burden of PWS posed to individuals, families, and society.
Prader-Willi syndrome (PWS) is a complex genetic, chronic, life-threatening disorder presenting in childhood with a prevalence at live birth estimated to range from 1 in 10 000 to 1 in 30 000.1–3 Individuals born with PWS experience a wide variety of medical challenges throughout their lifetime, generating a burden that is likely considerable and spread across medical, nonmedical, productivity, and intangible costs.3,4
One of the hallmarks of PWS is hyperphagia, which typically presents as an overriding physiological drive to eat and results in potentially fatal food-seeking behaviors. If unchecked, it may lead to a variety of sequelae and comorbidities.5
In addition to hyperphagia, patients with PWS also experience serious physiological and developmental deficiencies.4,6–9 Infants classically have hypotonia and poor suck, which may warrant the placement of a nasogastric tube along with additional perinatal follow-up care. Severe cases may be cared for in the neonatal intensive care unit for weeks to months.1 Hypogonadism and growth hormone deficiency may lead to poor skeletal growth, short stature, immature appearance, and osteoporosis. Although recombinant human growth hormone (rhGH) replacement therapy may improve lean body mass and linear growth, it does not have any clinical impact on the hyperphagia in PWS.10 Other medical issues that frequently arise include dental anomalies, scoliosis, skin picking, strabismus, sleep disturbances and apnea, and psychiatric disturbances.
We sought to understand the impact of PWS on direct medical costs in the US. Using a nationally representative US administrative health care claims database, we estimated the medical resource utilization for individuals in the US with a medical diagnosis of PWS compared with individuals without PWS.
Methods
We used commercial, Medicare supplemental, and Medicaid data from the MarketScan Research Databases (Truven Health Analytics, Ann Arbor, Michigan) for the years 2009–2014. These data provide a cross-sectional and longitudinal view of health care utilization, expenditures, demographics, and enrollment in the US. Specifically, the databases contain deidentified health insurance enrollment information and fully adjudicated claims data for inpatient and outpatient medical services as well as outpatient medications. The commercial database includes claims from individuals covered by employer-sponsored private health insurance. The Medicare database contains data from retirees with Medicare supplemental insurance paid by employers.
Several characteristics set the MarketScan Databases apart from other similar datasets. The core MarketScan Databases contain more than 180 million patients since 1995 and include private sector health data from approximately 100 payers. Moreover, the MarketScan Medicaid Database contains the pooled health care experience of approximately 6 million Medicaid enrollees from multiple states. Historically, more than 500 million claim records are available in the MarketScan Databases.
This study used a case-matched control design to quantify health care utilization among patients with PWS compared with those without the condition. We used the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code (759.81), which is specific to PWS, to identify the PWS case cohort, and conversely, the absence of the code to define control cohort without PWS. Patients with PWS initially were identified as having at least 1 matching ICD-9-CM code 759.81 within the given study period. Matching controls were matched at a 5:1 ratio to cases with PWS, on the variables of sex, age, and payer type (commercial, Medicare supplemental, and Medicaid). After the initial data extract, we applied additional criteria to increase the robustness of our sample.
To account for extraneous coding errors, we included only cases for which there were at least 2 PWS diagnoses on separate dates. Even though the MarketScan Lab Database includes laboratory test results that could ideally be used to validate diagnosis, these tests are only captured within the patients’ enrollment timeframe in our study (maximum 5 years, 2009–2014) and would therefore exclude the patients who became enrolled after their primary diagnosis occurred and would have significantly limited our study population. Finally, we also required patients to be continuously enrolled for at least 12 months within the 5-year study window, which helped to ensure that measured healthcare utilization was not affected by gaps in insurance coverage.
Statistical Analyses
Outpatient service frequency was calculated on the basis of the number of claims reported; outpatient provider visits, as well as emergency department visits, and other billable services were included in this variable. Average component costs associated with inpatient admissions, outpatient services, and prescription medication claims were calculated for individuals with and without PWS. To account for bias in the results caused by differences in lengths of continuous enrollment, utilization statistics were calculated on a per-person per-year (PPPY) basis. Mean and median costs and cost ratios were derived for both patients with PWS and their matched controls. Median costs reflect a typical or standard patient, whereas mean costs may be more relevant for assessments of total costs. Because payers are responsible for paying claims for both typical and outlier cases, we have primarily reported mean costs here.
Statistical tests were performed on costs with the Wilcoxon rank-sum test. Statistics on demographics results in the Table were run with χ2 (categorical variables) and t tests (continuous variables). To study the baseline level of comorbidity in each of the study cohorts, we used a modified Elixhauser methodology to produce a composite comorbidity score from a list of 31 chronic diseases identified by ICD-9-CM codes.11,12 Statistical analysis was performed with SAS version 9.4 (SAS Institute, Cary, North Carolina).
Table.
Subject demographics after matching and application of exclusion criteria (at least 2 ICD-9-CM codes for PWS and 12 months continuous enrollment)
| Commercial and medicare supplemental | Medicaid | |||||
|---|---|---|---|---|---|---|
| PWS | Non-PWS | PWS | Non-PWS | |||
| n | 1199 | 3945 | P value | 831 | 2592 | P value |
| Sex | ||||||
| Male | 49.9% | 49.7% | .920 | 52.8% | 53.5% | .731 |
| Female | 50.1% | 50.3% | 47.2% | 46.5% | ||
| Age distribution, y | ||||||
| 0–1 | 12.7% | 11.0% | .694 | 14.1% | 13.2% | .003 |
| 2–4 | 13.6% | 13.2% | 8.5% | 10.5% | ||
| 5–11 | 26.8% | 28.2% | 17.6% | 19.3% | ||
| 12–17 | 16.5% | 16.1% | 12.2% | 14.4% | ||
| 18–25 | 12.9% | 13.4% | 16.2% | 11.5% | ||
| 26–40 | 9.0% | 8.6% | 19.6% | 17.5% | ||
| 41–64 | 5.8% | 6.4% | 10.5% | 11.3% | ||
| 65+ | 2.8% | 3.2% | 1.3% | 2.3% | ||
| Age, y, at start of continuous enrollment | ||||||
| Mean (SD) | 15.6 (16.8) | 16.3 (17.7) | .209 | 19.4 (16.6) | 19.3 (17.7) | .924 |
| Median (Min, Max) | 10 (0, 92) | 11 (0, 95) | 17 (0, 90) | 14 (0, 90) | ||
| Number of continuously enrolled months | ||||||
| Mean (SD) | 36.1 (16.3) | 30.8 (15.4) | <.001 | 48.2 (17.0) | 31.5 (17.8) | <.001 |
| Median (min, max) | 36 (12, 60) | 27 (12, 60) | 60 (12, 60) | 25 (12, 60) | ||
Individuals were dispersed evenly across US regions: 23% northeast, 24% north-central, 30% south, 21% west, and 2% unknown (commercial/Medicare analysis) and matched very closely in the Medicaid analysis (1%−2% variation per geography).
Results
We identified 2030 individuals with PWS who met the dual PWS diagnosis code and 12-month continuous enrollment inclusion criteria (1161 commercial, 38 Medicare supplemental, and 831 Medicaid). Because of the low number of patients with PWS in the dataset with Medicare supplemental insurance, for these analyses we combined them with the commercial plan patients in the 65+ age cohort. After we matched and applied continuous enrollment criteria, our dataset included 6537 controls without PWS (3945 commercial/Medicare supplement and 2592 Medicaid). Sample demographics are shown after matching and exclusion criteria were applied (Table).
Very little variation was observed between the demographic makeup of cohorts with and without PWS, demonstrating effective matching. Of note, 69% of patients with PWS were younger than the age of 18 years (mean: 16 years, commercial/Medicare supplemental; mean: 19 years, Medicaid). Additionally, patients with PWS had longer continuous enrollment duration vs controls without PWS (mean 3.0 vs 2.6 years, commercial/Medicare supplemental; and mean: 4.0 vs 2.6 years, Medicaid). The mean modified Elixhauser composite score was 2.38 for PWS and 0.62 for subjects without PWS with commercial insurance. For the population insured by Medicaid, these scores were 3.92 for subjects with PWS and 1.34 for subjects without PWS.
Prescription medication costs were 15.9 times greater for commercially insured patients with PWS and 7.6 times greater for Medicaid-insured patients with PWS (all-age, ratio of PWS to without PWS). The mean PPPY cost for prescription medications for individuals with PWS varied by age-cohort from ~$1000 to ~$10 000, with an all-age mean of $6801 for commercial/Medicare supplemental and $4220 for Medicaid (Figure 1).
Figure 1.
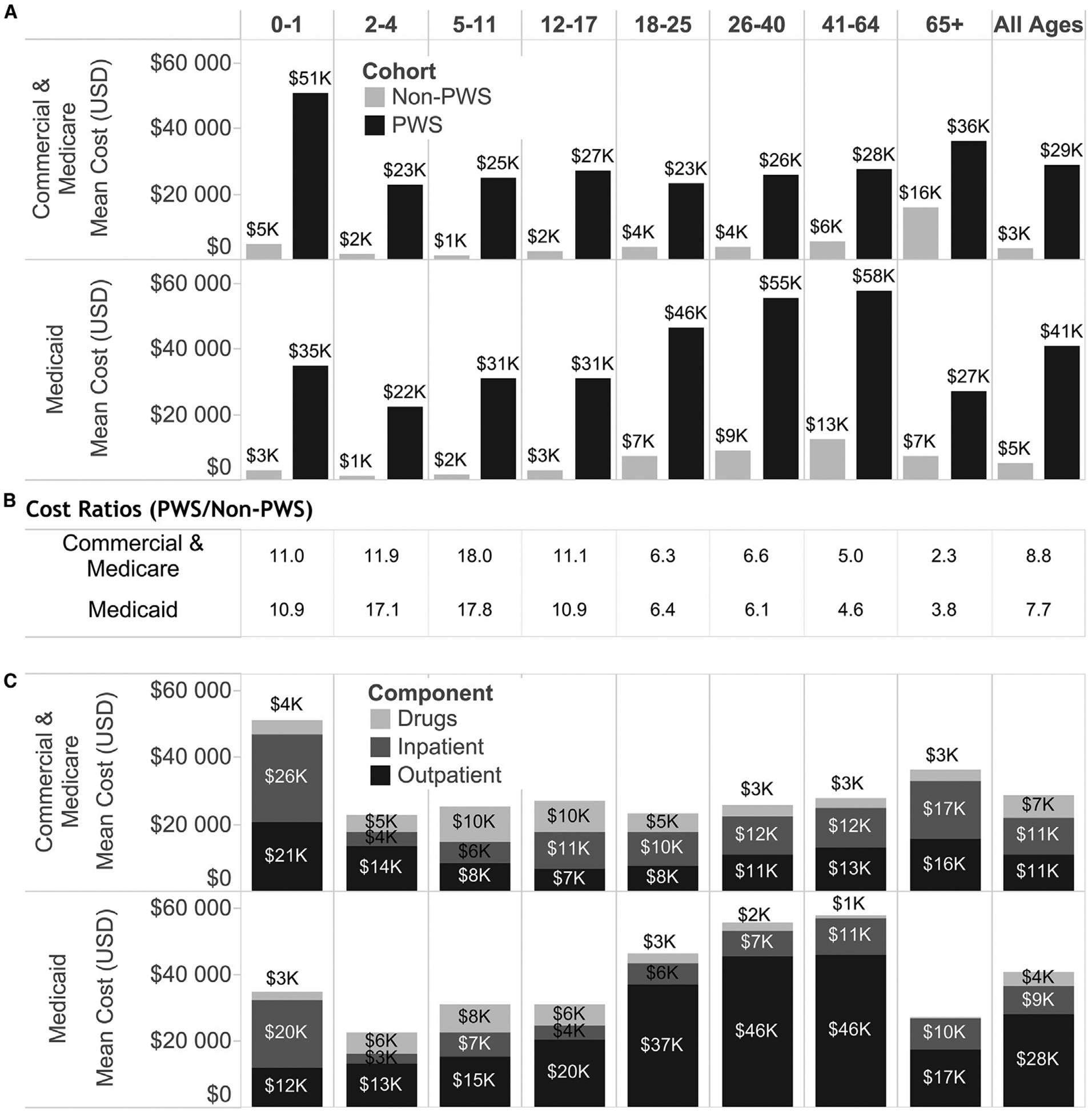
A, Mean total costs for individuals with and without PWS by payer type and age cohort. Differences are statistically significant (P < .0001) for all comparisons of subjects with and without PWS. B, High cost ratios demonstrate the high disparity between health care costs of subjects with and without PWS. The greatest disparity occurs between ages 2–17 years, as patients with PWS incur heavy healthcare utilization, particularly compared with their controls, composed largely of individuals without PWS or other comorbidities. C, Mean component costs for patients with PWS by payer type and age cohort. Outpatient claims comprise the largest cost driver overall (“All Ages”) and are especially pronounced in Medicaid patients older than 18 years of age. Inpatient claims are the largest contributor to costs for individuals with PWS age 0–1 year of age. Medication costs are at their greatest level for individuals between ages 5–17 years of age.
Patients with PWS ages 5–17 years had the greatest medication costs, due largely to the use of rhGH therapy. The top 5 therapeutic medication categories most frequently prescribed to individuals with PWS included: (1) hormones and synthetic hormone substitutes; (2) central nervous system agents; (3) anti-infective agents; (4) cardiovascular agents; and (5) gastrointestinal agents. Hormones and synthetic hormone substitutes constituted the vast majority of the costs (78% commercial/Medicare supplement, 54% Medicaid) and decreased slightly in prescription frequency with increasing age cohort (peak utilization ages 5–11 years). Central nervous system and cardiovascular agents were used with increasing frequency in older PWS (and without PWS) age cohorts (Figure 2).
Figure 2.
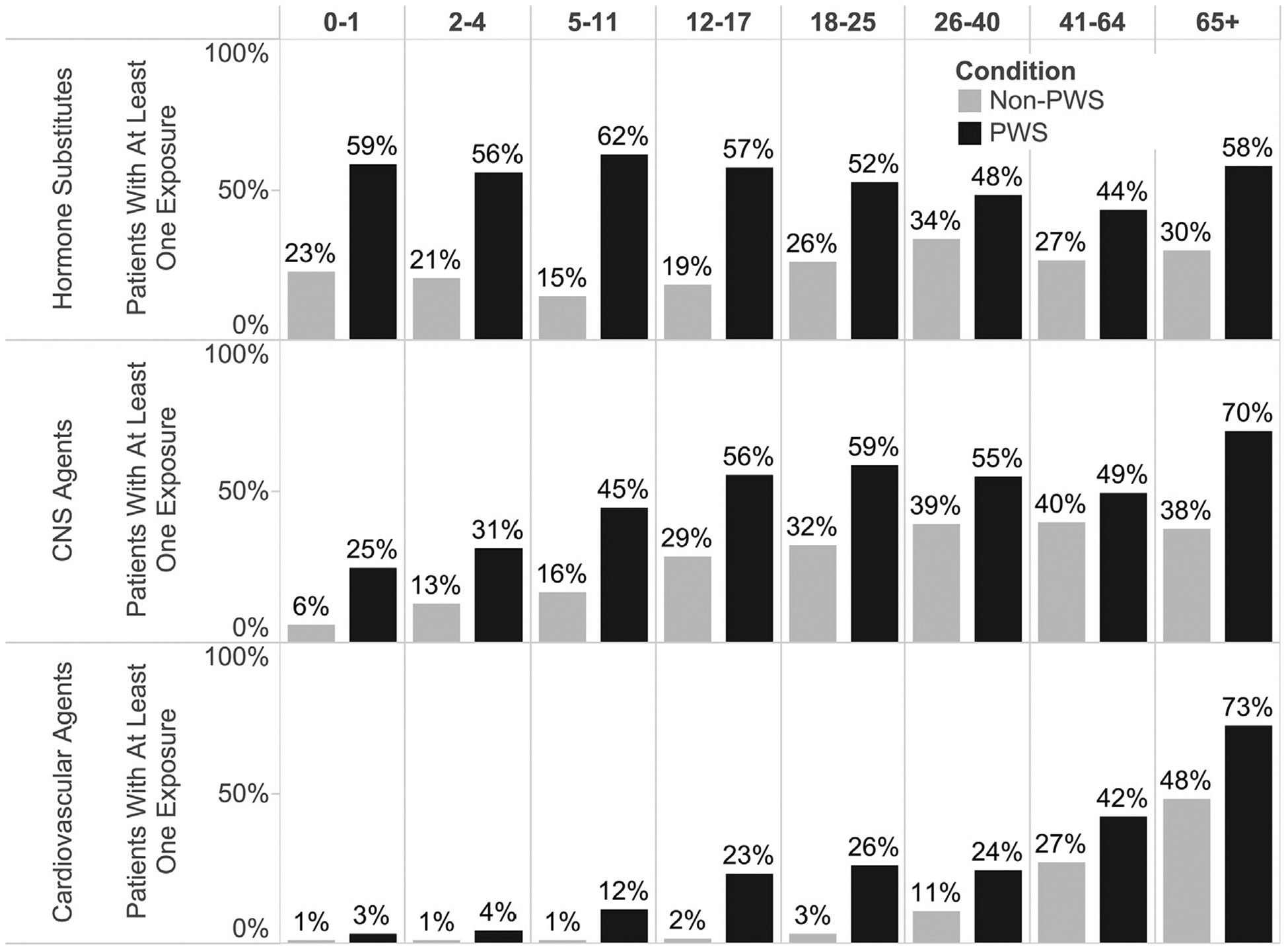
More than one-half of individuals with PWS had at least one claim for a hormone agent, a number that declined slightly with increasing age cohort. CNS and cardiovascular agents were used with increasing frequency by older age cohorts. The difference in all-age drug exposure frequency between individuals with and without PWS and was statistically significant for each class of agents shown (hormone, CNS agents, and cardiovascular agents), P < .0001. CNS, central nervous system.
Inpatient care costs across all ages were 10.7 times greater for commercially insured patients with PWS and 4.8 times greater for Medicaid-insured patients with PWS compared with individuals without PWS. The mean cost for inpatient admissions varied by age cohort from ~$3000 to ~$26 000 PPPY, with an all-age mean of $10 879 for commercial and $8727 for Medicaid insured patients (Figure 1). The PWS 0–1 years of age cohort had the greatest inpatient admission costs (mean $26 270 and $20 291 for commercial and Medicaid patients with PWS, respectively), likely due to complicated neonatal care.
Driving these costs, inpatient admission frequency and length of stay (LOS) were greater in the population with PWS compared with the population without PWS. Commercially insured patients with and without PWS had 0.28 and 0.05 inpatient admissions PPPY, with a mean LOS of 2.73 and 0.23 days PPPY, respectively. Medicaid-insured patients with and without PWS had 0.33 and 0.16 inpatient admissions PPPY, with a mean LOS of 3.62 and 0.86 days PPPY, respectively.
Mean PPPY costs for outpatient care (eg, office visits, outpatient procedures, therapy, and some medical equipment and supplies) were 6.1 times greater ($11 032 vs $1804) for commercially insured patients and 9.5 times greater ($27 921 vs $2928) for Medicaid-insured patients (all-age, ratio of PWS to without PWS). These costs varied by age cohort (Figure 1). In the commercially insured population, outpatient costs were greatest in both young childhood and adult age cohorts (0–4 years and 26+ years). In the Medicaid-insured population, outpatient costs rose precipitously with increasing age cohort, to greater than $35 000 for patient cohorts older than 18 years of age.
Of note, the frequency of Medicaid outpatient encounters for patients with and without PWS increased significantly in older age cohorts (18+). On investigating this trend further, we found that the increase was attributable primarily to day and residential habilitation Medicaid waivers, accounting for ~50% of Medicaid outpatient costs for individuals over 18 years (Figure 3).
Figure 3.
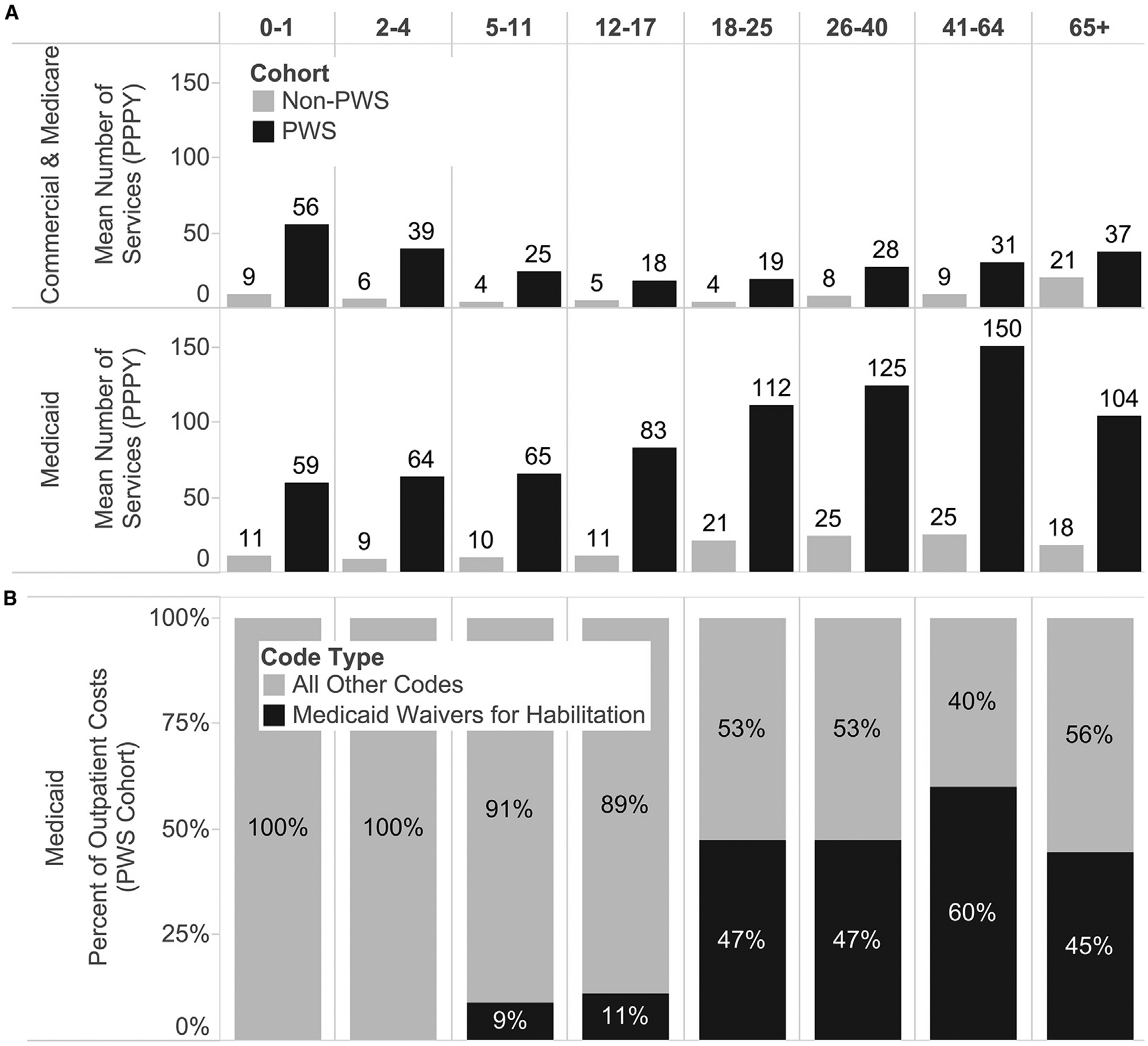
A, Frequency of outpatient services (PPPY). The number of claims for Medicaid outpatient services increased drastically for individuals in older age cohorts. B, Four Healthcare Common Procedure Coding System codes (T2014, T2016, T2020, T2021) used for Medicaid habilitation waivers comprised a large percentage of Medicaid outpatient costs.
Mean all-cause total care costs were 8.8 times greater ($28 712 vs $3246) for commercially insured patients and 7.7 times greater ($40 868 vs $5306) for Medicaid-insured patients (all-age, ratio of PWS to without PWS). The mean total cost varied by age cohort between ~$22 000 and ~$55 000 PPPY (Figure 1). This finding is in stark contrast to the population without PWS with mean total costs ranging from under ~$1000 in younger age cohorts, to a maximum value of just over ~$10 000 in the 41+ age cohort. PWS vs without PWS comparisons for all-cause total costs of care were statistically significant (P < .0001) for all age cohorts. The greatest cost disparity (as demonstrated by PWS to without PWS cost ratios) occurs in the younger age populations (Figure 3).
Discussion
Results from this analysis of US commercial, Medicare supplemental, and Medicaid administrative claims suggest that total, medication, inpatient, and outpatient medical resource use was considerably greater among patients with PWS than their matched controls without PWS. Specifically, we found that synthetic hormones, predominantly rhGH, were a leading driver of medication costs in the population with PWS, particularly in the 5–17 years of age cohort. We also found that costs for inpatient admissions (relatively infrequent for patients with and without PWS) were explained by a few individual admissions or episodes of care and were especially pronounced in the 0–1 years of age neonatal cohort. Outpatient care costs contributed the largest portion of total direct medical costs for patients with PWS. Although a portion of outpatient care costs were attributable to planned procedures such as spinal fusion (scoliosis) surgery, particularly high costs also were observed for unplanned admissions, for example, for respiratory distress (tracheostomies with mechanical ventilation). Finally, our analyses indicated that outpatient care costs were disproportionately high for Medicaid patients with PWS compared with patients with PWS with commercial or Medicare supplemental insurance. The high rate of Medicaid outpatient costs was attributable primarily to day and residential habilitation Medicaid waivers that accounted for ~50% of Medicaid outpatient costs for individuals with PWS older than 18 years of age.
Heavy healthcare utilization is well-recognized in certain genetic disorders, such as Down syndrome. In a previous study of health care costs in a pediatric (age 0–4 years) Down syndrome population, average annual inpatient, outpatient, and prescription medication costs were $21 842, $13 594, and $927 respectively.13 These high costs are driven primarily by congenital heart disorders that commonly manifest in medical resource utilization in the first years of life. Although much less recognized as a high burden condition, patients with PWS ages 0–4 years accrue costs of similar magnitude.13
In a Dutch study on use of medical care and prevalence of serious illness in adult cohort with PWS, the authors reported similar types of medical utilization with regard to hospital admissions and medication use; however, costs were not captured, and the sample was limited to 102 individuals with PWS.14 In another study of fragile X syndrome, Sacco et al15 also observed greater Medicaid outpatient costs (mean $12 608) compared with commercial/Medicare costs ($4643). In their case, claims for case management services appeared to be driving the discrepancy.15 Mean total costs they presented for individuals with fragile X syndrome (~$5700-$8800 commercial/Medicare, ~$10 500-$23 000 Medicaid, 2004–2009 dollars) were comparable with those we presented for individuals with PWS.
This study has a number of important limitations. First, the identification of patients in administrative claims studies relies on the association of ICD-9-CM codes used for billing of services. As a result, the disproportionately small number of older adult patients with PWS (>41 years of age) observed in this dataset was likely due to PWS coding behavior rather than actual trends in utilization or population composition. We surmise that the further a patient is from his/her original PWS diagnosis, the less likely that his/her medical record will reflect that diagnosis. Of note, the same age distribution trend was observed in a similar study of fragile X syndrome, a disorder also primarily diagnosed in infancy.15
Second, we observed a longer continuous enrollment period in the group with PWS compared with the group without PWS, which may reflect the fact that patients with chronic conditions retain their health insurance carrier longer. To adjust for differing patient follow-up lengths of time and to produce equally weighted metrics, analyses were calculated on a PPPY basis.
Finally, administrative claims only reflect billed health care services and thus do not capture innumerable nonmedical and indirect services or resources consumed by patients and caregivers. Health care workers and caregivers that manage PWS recognize it as a highly burdensome condition, encompassing direct nonmedical costs (eg, food access prevention, family care), productivity costs (eg, reduced earning potential of patient and caregiver), and psychosocial costs (eg, social isolation, stress).6,7,16 A broader look at all cost categories is warranted in future research, but because our results report specifically on the direct medical costs associated with PWS, they likely vastly understate the overall societal burden of the disorder.
Interestingly, our analysis yielded lower medication costs than expected. At time of publication, an average wholesale price for rhGH was ~$100 per milligram.17 At doses ranging from 0.16 to 0.24 mg/kg-week (assuming a body weight of 35 kg), the expected costs are in the range of $29 120 to $43 680 for individuals receiving this medication, which is significantly greater than our mean prescription medication cost findings. On further investigation, we discovered that only ~50% of patients had at least 1 claim for a growth hormone or other synthetic hormone substitute. This rate is low compared with published guidelines.18 There may be many important clinical factors influencing the apparently low rate of rhGH prescriptions, including proper prescribing, dosing, and usage of rhGH in this population with PWS, as well as patient compliance and adherence, which would be reflected in data captured here. Other explanations for the discrepancy, however, also may be related to medications being covered by other pharmacy “carve-out” benefits and/or dual coverage from multiple payers (eg, some patients may receive both commercial and Medicaid benefits) that are not captured in this dataset.
Because PWS is rare, its overall financial impact is likely modest compared with other conditions. That PWS-related costs are so drastically and disproportionately high compared with individuals without PWS, however, speaks to the need for effective treatments to improve patient survival and quality of life and to reduce the financial burden on patients and families.
Acknowledgments
Funded by Zafgen, Boston, MA, a company that is developing a product to treat individuals with Prader-Willi syndrome. A.S., J.G., and D.D. are employed by Health Advances, LLC, a company that has received consulting fees from Zafgen. N.K. is employed by Zafgen. The other authors declare no conflicts of interest.
Glossary
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- LOS
Length of stay
- PPPY
Per-person per-year
- PWS
Prader-Willi syndrome
- rhGH
Recombinant human growth hormone
Footnotes
Portions of the study were presentedat the PWS Association Scientific Day, Orlando, FL, November 5, 2015, and JP Morgan Healthcare Conference, San Fransisco, CA, January 14, 2016.
References
- 1.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med 2012;14:10–26. [DOI] [PubMed] [Google Scholar]
- 2.Yearwood EL, McCulloch MR, Tucker ML, Riley JB. Care of the patient with Prader-Willi syndrome. Medsurg Nurs 2011;20: 113–22. [PubMed] [Google Scholar]
- 3.Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest 2015;38: 1249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elena G, Bruna C, Benedetta M, Stefania DC, Giuseppe C. Prader-Willi syndrome: clinical aspects. J Obes 2012;2012:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller JL, Lynn CH, Driscoll DCJ, Goldstone AP, Gold JA, Kimonis V, et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet A 2011;155:1040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazaheri MM, Rae-Seebach RD, Preston HE, Schmidt M, Kountz-Edwards S, Field N, et al. The impact of Prader-Willi syndrome on the family’s quality of life and caregiving, and the unaffected siblings’ psychosocial adjustment. J Intellect Disabil Res 2013;57: 861–73. [DOI] [PubMed] [Google Scholar]
- 7.Hodapp RM, Dykens EM, Masino LL. Families of children with Prader-Willi syndrome: stress-support and relations to child characteristics. J Autism Dev Disord 1997;27:11–24. [DOI] [PubMed] [Google Scholar]
- 8.Angelis A, Tordrup D, Kanavos P. Socio-economic burden of rare diseases: a systematic review of cost of illness evidence. Health Policy 2015;119:964–79. [DOI] [PubMed] [Google Scholar]
- 9.Eiholzer U, Whitman B. A comprehensive team approach to the management of Prader-Willi syndrome. J Pediatr Endocrinol Metab 2004; 17:1153–75. [DOI] [PubMed] [Google Scholar]
- 10.Wolfgram PM, Carrel AL, Allen DB. Long-term effects of recombinant human growth hormone therapy in children with Prader-Willi syndrome. Curr Opin Pediatr 2013;25:509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 12.Garland A, Fransoo R, Olafson K, Ramsey C, Yogendren M, Chateau D, et al. The Epidemiology and Outcomes of Critical Illness in Manitoba. Winnipeg (MB): Manitoba Centre for Health Policy; 2012. [Google Scholar]
- 13.Boulet SL, Molinari N-A, Grosse SD, Honein MA, Correa-Villaseñor A. Health care expenditures for infants and young children with Down syndrome in a privately insured population. J Pediatr 2008;153:241–6. [DOI] [PubMed] [Google Scholar]
- 14.Sinnema M, Maaskant MA, van Schrojenstein Lantman-de Valk HMJ, Boer H, Curfs LMG, Schrander-Stumpel CTRM. The use of medical care and the prevalence of serious illness in an adult Prader-Willi syndrome cohort. Eur J Med Genet 2013;56:397–403. [DOI] [PubMed] [Google Scholar]
- 15.Sacco P, Capkun-Niggli G, Zhang X, Jose R. The economic burden of fragile X syndrome: healthcare resource utilization in the United States. Am Health Drug Benefits 2013;5:73–83. [PMC free article] [PubMed] [Google Scholar]
- 16.Allen K Managing Prader-Willi syndrome in families: an embodied exploration. Soc Sci Med 2011;72:460–8. [DOI] [PubMed] [Google Scholar]
- 17.Red Book. Greenwood Village (CO): Truven Health Analytics, Inc; 2015. [Google Scholar]
- 18.Deal CL, Tony M, Höybye C, Allen DB, Tauber M, Christiansen JS. GrowthHormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab 2013;98:E1072–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


