Abstract
Although hemp seed (HS) oil is characterized by more than 80% polyunsaturated fatty acids (PUFAs), a very high ω-6-to-ω-3 ratio is not a popular commodity. The aim of this work was to provide useful data about the bioactive components and cannabidiolic acid content in thirteen different commercial hemp seed oils. The investigated HS oils showed a good ω-6/ω-3 ratio, ranging from 1.71 to 2.27, massively differed in their chlorophylls (0.041–2.64 µg/g) and carotenoids contents (0.29–1.73 µg/g), as well as in total phenols (22.1–160.8 mg Gallic Acid Equivalents (GAE)/g) and tocopherols (3.47–13.25 mg/100 g). Since the high content of PUFAs in HS oils, photo-oxidative stability was investigated by determining the Thiobarbituric Acid Reactive Substances (TBARS) assay and extinction coefficient K232 and K270 after the photo-oxidative test. The percentage of increase in K232 and K270 ranged from 1.2 to 8.5% and from 3.7 to 26.0%, respectively, indicating good oxidative stability, but TBARS showed a 1.5- to 2.5-fold increase in oxidative behavior when compared to the initial values. Therefore, the diversity in bioactive compounds in HS oils, and their high nutritional value, suggest the need for a disciplinary booklet that well defines agronomic and post-harvest management conditions for achieving a good food objective.
Keywords: hemp seed oil, bioactive compounds, polyphenols, ω-6/ω-3 ratio, tocopherols, chlorophylls, carotenoids, cannabidiolic acid
1. Introduction
Industrial hemp (Cannabis sativa L.) is an annual herbaceous plant original from Central Asia but is widely spread around different geographical zones through its ability to adapt and respond to climate changes [1]. Consequently, this plant can be found in many different forms according to the resultant genetic variability. It has been long used since ancient times with medicinal and nutritional purposes, among others, due to its rich chemical content [2]. More than 500 different compounds have been characterized in C. sativa, with phytocannabinoids, flavonoids, terpenoids and fatty acids being the most relevant families [3]. Nevertheless, those compounds are not equally distributed throughout the plant, so the chemical content can drastically vary [4].
Currently, the EU Approved Common Catalogue of Cultivars includes some C. sativa cultivars with a total content of ∆9-tetrahydrocannabinol (∆9-THC) below 0.2% [5]. On the other hand, these cannabis cultivars popularly called “industrial hemp” generally contain a high concentration of the acidic precursor of cannabidiol (CBDA) [6,7]. The latter compound is known to have a wide range of important biological properties including anticonvulsive, anti-epileptic, and antimicrobial activities, and is also used as supplements in the treatment of osteoarthritis and for musculoskeletal disease [8].
In this line, the seed of hemp stands as a great source of nutrients and no-nutrients, containing 25–35% lipid, 20–25% protein and 30% carbohydrates [9]. Nowadays, the use of hemp for nutrition purposes is focused on the oilseed, mainly due to a rich content of polyunsaturated fatty acids (PUFAs) and a high ω-6 to ω-3 ratio of fatty acids, that reaches a 3:1 proportion. This value is considered as optimal for human health, with a positive influence in health condition by reducing the risk of suffering from cardiovascular diseases [10].
However, the high content of PUFAs in hemp seed oil makes it highly susceptible to lipid oxidation [11]. Oxidative instability is one of the most important factors responsible for reducing oil quality and shelf life [12]. This process in edible oils affects nutrition, toxicity, color and aroma, leading to the development of various off-flavors and an unpleasant taste, important reasons for consumers’ rejection [13]. Furthermore, it has been reported that the hemp seed oil contains a huge amount of chlorophylls that are able to interfere with oxidative stability and rancidity [14]. These natural pigments act as powerful prooxidants, increasing the susceptibility to light-induced oxidation or photo-oxidation of the edible oils when exposed to light and promote change from the intensive dark green color to yellow [15,16]. Several refining processes are needed during the hemp seed oil production to reduce the chlorophyll content and other minor components such as metals and phospholipids that could affect the oil quality.
Moreover, the hemp seed oil contains other minor constituents such as polyphenols, carotenoids, and tocopherols, all involved in antioxidant processes, which could play an important role in the protection of edible oils against lipid oxidation [17,18,19]. In humans, all these compounds can display important biological properties such as antioxidant and anti-inflammatory effects [20,21,22]. Because of this, hemp seed oil could become an alternative for other oil typologies in current diets, and would also be suitable for vegetarian, vegan or gluten-free diets even more considering their uprising trends. Although several hemp-based oily products are commercially available, the legal frame for marketing this kind of product in the European Union is still ambiguous. In fact, despite being classified as a novel food, no quality controls are established for marketed hemp seed oil, whereas olive oil has to fit several quality requirements set by the authorities [23]. Acidity, peroxide index, absorbance characteristics through K232 and K270 measurements and chemical composition, are the parameters that commonly qualify an olive oil as suitable for marketing, but their applicative definition for hemp seed oils could be limiting. Hemp seed oil quality should be guaranteed by more restrictive parameters that take into account its chemical complexity and its differentiation by means of several factors such as genetic factors (variety), or methods used for its obtainment (e.g., pressing and solvent extraction), as well as the refining and bleaching processes [24]. The development of shared standards to improve hemp seed oil quality management at a national level should be deeply pursued through the definition of a special disciplinary booklet. Although some studies have examined the chemical composition of hemp seed and hemp seed oils, little is known about the characteristics of commercial hemp seed oil that actually reaches the consumers [6,25,26]. Therefore, this study aims to provide useful data regarding the chemical composition as well as quality parameters of thirteen commercial hemp seed oils available in the Italian market in order to evaluate their biochemical characteristic variability and propose their introduction in the habitual human diet. The oil oxidation stability, evaluated by measuring the primary and secondary oxidation products during accelerated photo-oxidation tests, was also investigated.
2. Results and Discussion
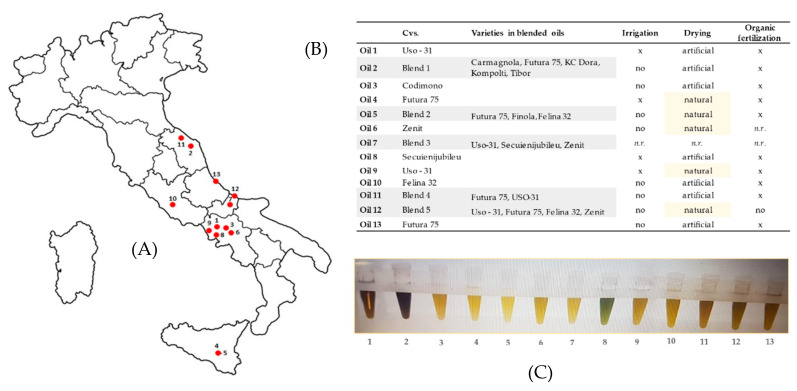
Thirteen commercial hemp seed (HS) oils, which were from different industrial hemp varieties cultivated in Italy (Figure 1A), and produced as monovarietal oil or blended oil (Figure 1B), were investigated. HS oils, all obtained by cold-pressing extraction, are from different geographical origins, and are characterized by a large color variability (from light yellow to dark green) (Figure 1C), which could be related to a diverse content of chlorophylls, and other pigments such as carotenoids and polyphenols. The content of these latter compounds, together with that of other antioxidants in hemp seeds such as tocopherols, was valued. Furthermore, ω-6/ω-3 fatty acids ratio was assessed, and different conditions and parameters allowed oxidative stability to be assessed. Moreover, cannabidiolic acid amount was estimated in each analyzed hemp seed oil.
Figure 1.
Cold-pressed hemp seed oils from different Italian locations. Main cultural and post-harvest techniques applied are indicated. n.r. = not reported. (A) Graphic distribution of hemp seed oils in the various Italian regions; (B) Main characteristics of hemp seed oils: cultivars, varieties, irrigation, drying and organic fertilization; (C) Visual representation of analyzed hemp seed oils.
2.1. Polyphenols Determination of Hempseed Oil
Recently, hydroxycinnamoyl amides, lignanamides, and flavonol glycosides were identified as minor constituents of hemp seed [18,27], and their presence is favorably revealed in hemp seed meal, whereas their content is highly variable in hemp seed oil, being strongly dependent on the extraction method applied, and/or cultivar considered for oil obtainment [28]. Smeriglio et al. [29] also characterized the polyphenolic compounds of cold-pressed seed oil from Finola cultivar, finding a Total Phenol Content (TPC) equal to 267.5 mg Gallic Acid Equivalents (GAE)/100 g. The cultivar identity could also affect TPC content, as a value 10-fold lower (2.1 mg GAE/100 g) was estimated for hemp seed oil from Fedora cultivar by Siano et al. [26], who monitored the distribution of hemp seed components in the flour and oil after fractionation by cold-pressing. In the study conducted by Yu et al. [30] who evaluated the bioactivity of cold-pressed hemp seed oils for their potential application in the prevention of oxidative stress related diseases, a content of 44 mg GAE/100 g was reported. Moreover, Teh et al. [31] who studied the physicochemical and quality characteristics of cold-pressed New Zealand hemp seed oil, reported a TPC of 188.23 mg GAE/100 g. However, while considering the high variability in TPC values, and that total polyphenols by Folin–Ciocalteu (FC) method might be biased by several interfering components, including sugars and free amino acids [26], FC assay is rapid, applicable in routine laboratory use, and advantageously utilizable for screening antioxidant activity. This latter is the most known property attributed to phenolic compounds, which are also responsible for other relevant effects including nutritional characteristics, stability and the preventive effect against quenching radical reactions. Thus, investigated hemp seeds oils were properly fractionated, and total phenols content was determined on polyphenols enriched fractions obtained by means of FC method. Data acquired highlighted that TPC ranged from 36.1 to 160.8 mg GAE/g (Table 1), and strong differences were found taking into account oils obtained also from the same cultivar, cultivated and harvested in different geographical areas. This is the case of USO 31 cv. -based oils 1 and 9, as well as Futura 75 cv. oils 4 and 13. In particular, it was observed that TPC was 1.47-fold higher in oil 1 than in oil 9, whereas Futura 75 cv. oil 13 contained more than double TPC in respect to oil 4. The results suggest that, beyond the cultivar, geographical localization could massively affect total phenol content, whereas oils from the innermost areas show a lower content. Furthermore, drying methods (artificial or natural), and drought stress could benefit polyphenols enrichment. This is particularly true considering samples 4 and 13, the first of which was from irrigated fields-derived hemp seeds, which were then naturally dried. According to Leonard et al. [32], hemp seeds’ total phenolic content varies depending on the cultivar, and within the seed itself differs from one fraction to another. Based on seed growth location, seed maturity, extraction conditions, resulting oil could have a strong and pungent flavor. Postharvest management practices could also affect compounds formation and consequentially the taste. It seems that cold-pressed hemp seed has the best quality almost compared to walnuts and sunflower seeds [33].
Table 1.
Total Polyphenol Content (TPC) evaluated for hemp seed oil samples. Values are reported as mean ± SD of three independent measurements, and are expressed as mg Gallic Acid Equivalents (GAE) per g of oil.
| Sample | Cvs. | TPC mg GAE/g |
|---|---|---|
| Oil 1 | Uso-31 | 32.5 ± 4.9 |
| Oil 2 | Blend 1 | 160.8 ± 3.7 |
| Oil 3 | Codimono | 48.9 ± 1.2 |
| Oil 4 | Futura 75 | 69.2 ± 8.6 |
| Oil 5 | Blend 2 | 58.2 ± 8.4 |
| Oil 6 | Zenit | 36.7 ± 7.1 |
| Oil 7 | Blend 3 | 36.1 ± 2.3 |
| Oil 8 | Secuieni jubileu | 108.0 ± 0.1 |
| Oil 9 | Uso-31 | 22.1 ± 0.3 |
| Oil 10 | Felina 32 | 124.3 ± 18.4 |
| Oil 11 | Blend 4 | 36.3 ± 4.8 |
| Oil 12 | Blend 5 | 105.0 ± 11.4 |
| Oil 13 | Futura 75 | 139.7 ± 14.8 |
| Skewness | 0.6 | |
| Kurtosis | −2.2 |
2.2. Tocopherols Determination in Investigated Hemp Seed Oils
Hemp seeds count in their antioxidant baggage vitamin E vitamers such as tocopherols, and tocotrienols, which are minor components of the hempseed oily fraction. These compounds are known to preserve the oxidative stability of oils acting as chain-breakers, and slowing-down lipo-peroxidation. Thus, these constituents are able to prevent the oxidation of PUFAs-rich oils [28] positively affecting their storage [34]. Among vitamin E vitamers, γ-Tocopherol is found to be the most abundant, followed by α-, and δ-tocopherols [35]. The assessment of tocopherols quantification in the different hemp seed oils is carried data, and data are reported in Table 2. Total tocopherols content is in the range of 3.47–13.25 mg/100 g. These results are in accordance with Smeriglio et al. [29] who reported a total tocopherol content in hemp oil after cold-pressing of Finola seed corresponding to 11.40 mg/100 g. In another study conducted by Anwar et al. [36] a detailed analysis of hemp seed oil native to three agro-ecological zones in Pakistan was carried out. The reported content of total tocopherol was in the range of 63.03–85 mg/100 g. Similar results were obtained by The et al. [31] who found a total tocopherol value of 59.16 mg/100 g. Moreover, Aladić et al. [37] studied the various processes in supercritical conditions in order to determine fatty acids, tocopherols and pigment content. Results highlighted an amount of α-tocopherol ranging from 3.71 to 11.06 mg/100 g and a quantity of γ-tocopherol content 2–3 folds higher, correlated on applied process conditions. Loss of tocopherols content in vegetable oils was attributed to similar factors to those which influence lipid oxidation, such as storage time, oxygen exposure and temperature [38].
Table 2.
Tocopherols determination evaluated for hemp seed oil samples. Results are expressed as mg 100/g ± SD from three independent measurements.
| Sample | Variety | Tocopherols (mg/100 g) |
|---|---|---|
| Oil 1 | Uso-31 | 7.23 ± 0.47 |
| Oil 2 | Blend 1 | 13.25 ± 0.18 |
| Oil 3 | Codimono | 7.29 ± 0.20 |
| Oil 4 | Futura 75 | 6.55 ± 0.50 |
| Oil 5 | Blend 2 | 5.83 ± 0.34 |
| Oil 6 | Zenit | 7.39 ± 0.02 |
| Oil 7 | Blend 3 | 6.46 ± 0.58 |
| Oil 8 | Secuieni jubileu | 3.47 ± 0.32 |
| Oil 9 | Uso-31 | 6.25 ± 0.01 |
| Oil 10 | Felina 32 | 6.54 ± 0.22 |
| Oil 11 | Blend 4 | 7.42 ± 0.45 |
| Oil 12 | Blend 5 | 7.84 ± 0.20 |
| Oil 13 | Futura 75 | 8.23 ± 0.13 |
| Skewness | 1.6 | |
| Kurtosis | 5.7 |
2.3. Essential Polyunsaturated Fatty Acids Ratio
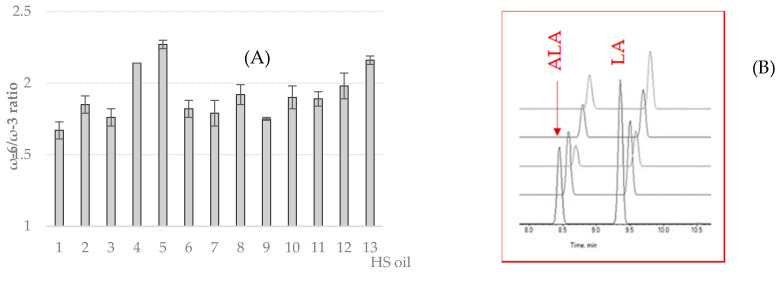
HS oil is a rare fount of nourishment for human diet, in particular for vegetarians, because of their unique reported ratio in ω-6/ω-3, most represented by linoleic acid (LA; 18:2, n-6) and α-linolenic acid (ALA; 18:3, n-3) [39]. The optimum ratio is approximately 2.5:1–3:1, also recommended for human nutrition [40]. Herein, the UHPLC analysis, combined with the quadrupole-time hybrid mass spectrometer (QTOF) high resolution, was utilized to detect and quantify fatty acids in analyzed oils. Taking into account relative areas of ω-6 and ω-3 fatty acids, their dietary ratio was calculated and data, which were in the range of 1.71–2.27, are listed in Figure 2. Several factors could affect fatty acids content and modify oil characters. Cultivars, climatic, farming and light conditions could strongly influence the ratio of ω6/ω3, in particular during seed development leading to the different content of acids in plants. Possessing high levels of unsaturated fatty acids protects the seeds from frost during the cold months of the year [36,41,42].
Figure 2.
Fatty acids ratio ω-6/ω-3 evaluated on the different cultivars of hemp seed oils (A). Results are expressed as mean of three independent measurements ± SD. (B) A representative total ion current TIC’s enlarged part, underlining α-linolenic acid (ALA) and linolenic acid (LA) separation, is reported in the red square.
As reported by Abdollahi et al. [43] who studied cultivars of Fedora 17 and seeds of two native populations from Iran planted in three different regions, fatty acid composition is influenced by the origin of seeds. Their results confirm the variability on ratio ω6/ω3 reporting a value between 2.59 and 7.88.
Moreover, Devi et al. [39] investigated six different extraction processes for hemp oil comparing resulting physicochemical properties. The process that led to the best-suited optimum ω6/ω3 ratio was offered by Soxhlet treatment. Results on ratio ω6/ω3 of other treatments were in the range of 1.99–9.24.
Uston-Argon et al. [44] evaluated fatty acid compositions of cold-pressed hemp seed oil from Turkey. Results showed that linoleic/α-linolenic acid ratios were between 2.96–3.27 confirming the optimal level for a healthy daily diet.
In a study, conducted by Porto et al. [24] the optimization of the extraction process for obtaining high-quality hemp seed oil from Felina cultivar was performed. Soxhlet and SC-CO2 extraction processes were evaluated and no significant differences were found. Results on ratio ω-6/ω-3 were in the range of 3.25–3.31. Soxhlet extraction process is considered a prevalent method with relatively low cost and high oil extraction efficiency, which at the same time requires much more time to extract the oil and necessitates the use of hazardous chemical solvents [35].
However, Anwar et al. [36] evaluated hemp seed oil from three different agro-ecological zones of Pakistan. Results showed that the fatty acid composition of hemp seed oil native to Pakistan falls in the recommended nutritional ratio of 18:2 n-6 to 18:3 n-3 [40]. The wild hemp that grows over vast areas of the north of Pakistan appears to be a potentially valuable crop from which products with nutraceutical value could be derived. In fact, comparing the typical unsaturated fatty acid profiles of common food oils (rape, soybean, and linseed), hemp seed oil appears to have the major beneficial results according to Apostol et al. [45].
As evidenced by several numbers of studies above-reported, hemp seed oil fatty acid composition is characterized by an optimum ratio ω-6/ω-3. Polyunsaturated fatty acids are able to affect several biological activities and the optimal proportion ω-6/ω-3 of 3:1 has been claimed to have medicinal effects on the human body such as reducing cholesterol and high blood pressure, providing an anti-inflammatory effect and immune support effects, on the skin, diabetes and cardiovascular health [35,45].
2.4. Chlorophylls and Carotenoids in HS Oils under Study
The content of total chlorophylls and carotenoids in the different commercial hemp seed oil samples have been quantified as shown in Table 3. Total content of chlorophylls found in the different types of commercial hemp seed oil samples presented a wide range, from 0.41 (blend 2 and 3) up to 4.81 mg/kg (blend 1), with a mean value of 1.46 mg/kg for all samples. Our results are in accordance with data reported by Saastamoinen et al. [46] who analyzed the chemical composition of Finola hemp oil seeds cultivated in south-western part of Finland. Results showed a great difference in chlorophyll content among studied cultivars: 0–6.75 mg/kg. Instead, other studies have reported higher chlorophyll content in cold-pressed hemp seed oil up to 98.6 mg/Kg [47,48]. The natural chlorophyll content present in the samples analyzed here was effectively removed by conventional refining and bleaching processes in order to minimize the negative effects of high chlorophyll content in edible oils. Chlorophylls are undesirable, among minor components retained in cold-pressing oils. Their presence affects negatively the oils appearance with color from dark to light green, as well as oxidative stability. In fact, chlorophylls are photosensitizer and pro-oxidant species, able to induce PUFAs oxidation and to reduce, in turn, the hempseed oil shelf-life [49].
Table 3.
Chlorophylls and carotenoids content in the analyzed oils (µg/g ± SD).
| Sample | Variety | Chlorophylls a + b (µg/g ± SD) |
Carotenoids (µg/g ± SD) |
|---|---|---|---|
| Oil 1 | Uso-31 | 2.52 ± 0.27 | 0.82 ± 0.09 |
| Oil 2 | Blend 1 | 4.81 ± 0.12 | 1.73 ± 0.04 |
| Oil 3 | Codimono | 0.97 ± 0.08 | 0.30 ± 0.02 |
| Oil 4 | Futura 75 | 0.45 ± 0.02 | 0.19 ± 0.00 |
| Oil 5 | Blend 2 | 0.41 ± 0.02 | 0.15 ± 0.01 |
| Oil 6 | Zenit | 0.85 ± 0.01 | 0.30 ± 0.01 |
| Oil 7 | Blend 3 | 0.41 ± 0.06 | 0.18 ± 0.02 |
| Oil 8 | Secuieni jubileu | 2.64 ± 0.76 | 0.69 ± 0.10 |
| Oil 9 | Uso-31 | 0.78 ± 0.03 | 0.29 ± 0.01 |
| Oil 10 | Felina 32 | 1.05 ± 0.07 | 0.44 ± 0.01 |
| Oil 11 | Blend 4 | 0.86 ± 0.08 | 0.40 ± 0.03 |
| Oil 12 | Blend 5 | 1.70 ± 0.19 | 0.61 ± 0.02 |
| Oil 13 | Futura 75 | 0.97 ± 0.01 | 0.49 ± 0.01 |
| Skewness | 1.9 | 2.3 | |
| Kurtosis | 3.9 | 6.2 |
Carotenoids can be extracted along with chlorophylls by methanol, diethyl ether or other organic solvents, and determined by spectrophotometer at wavelength between 400 and 500 nm. The presence of carotenoids in hemp seed oil can protect chlorophylls from degradation and prevent any color change during storage [50]. Thus, to avoid photo-oxidation, minimizing chlorophylls’ content is mandatory. Furthermore, carotenoids were detected in a concentration range between 0.18 (blend 2) and 1.73 mg/kg (blend 1), with a mean value of 0.52 mg/kg for all samples. High levels of carotenoids have been previously reported in commercial hemp seed oils from Canada, at concentrations ten-fold greater when compared to the levels found in the assayed samples [51]. The wide difference in the content of the carotenoids in edible seed oils may be summarized in the variety and degree of seed maturity, climate characteristics during the plant growth, and the intensity of the bleaching process [52].
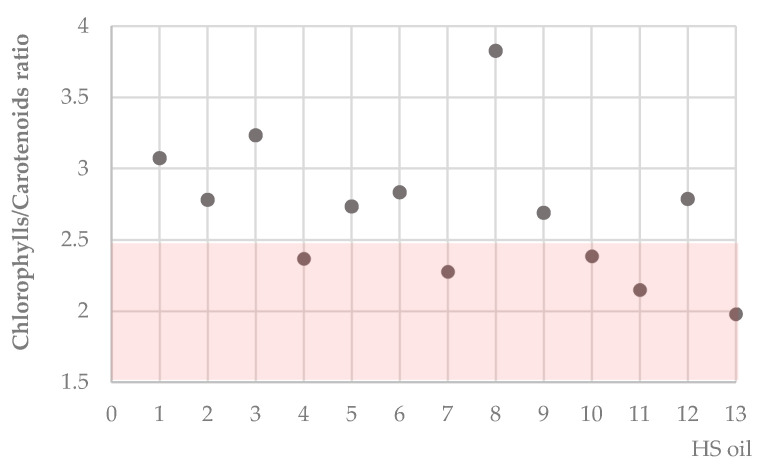
The ratio between the chlorophyll fraction and the carotenoid fraction greatly differed, demonstrating that the green and yellow fractions were not in balance (Figure 3). For other edible oils such as olive virgin oils, the ratio between the two isochromic fractions of pigments appeared to be constant at a value close to unity, independent of the variety [53]. There was a large variability for chlorophylls/carotenoids ratio within the hemp seed oil under study. In particular, hemp seed oil 8 was found to show the highest chlorophylls/carotenoids ratio, whereas only four samples, HS oils 4, 7, 11, and 13, appeared to contain a good carotenoids amount and a ratio less than 2.5. Among these latter oils, although no conclusions are drawn, HS oils 4 and 13 are obtained from Futura 75 cv. seeds, and HS oil 11 is a blend in which 2/3 seeds were from Futura 75 variety. The chlorophylls/carotenoids ratio is able to indicate phenology and physiology of plants. Different varieties from different sources, harvested in a similar ripeness state, possess different chlorophyll and carotenoid pigment profiles and content. Different chlorophyll a/b content and chlorophyll/carotenoid ratios caused by stress, damage, and senescence, impact the normal course of plant biological processes. Even though there is not a restrict regulation on the ratio between chlorophylls and carotenoids, it tends to remain more or less constant whatever the variety of sources, in a concentration range of 2.5 to 3.7 mg total chlorophyll/mg total carotenoid [53].
Figure 3.
Chlorophylls/carotenoids ratio of the investigated hemp seed oils.
2.5. Cannabidiolic Acid Content of Investigated Hemp Seed Oils
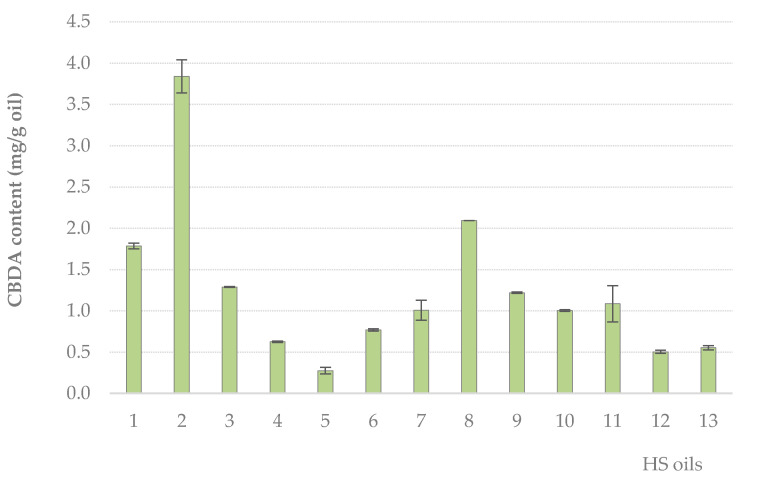
Cannabidiolic acid, the main phytocannabinoid compound in fiber and seed-oil plants, was detected and quantified by means of its HPLC-UV features [54]. Although the nutraceutical value of this compound is still far from being achieved, its high content in hemp varieties cultivated for food purposes, suggests the need for a deeper understanding of its detection and quantification, and to address its content assessment as a quality feature of hemp seed oil. Data acquired are presented in Figure 4. HS oil 5 showed the lowest CBDA content (0.28 mg per g of oil), where the blend 1 (HS oil 2) was 14-fold more abundant. As for TPC value, it was observed that each oil could massively differ from the others according to the CBDA rate.
Figure 4.
Cannabidiolic acid (CBDA) content (mg per g of oil) of the analyzed hemp seed oils, calculated by means of the calibration curve of a home-made isolated CBDA. Values are the mean of three independent measurements ± SD.
Our results are in accordance with Citti et al. [7] who analyzed cannabinoids contents in 13 different commercial Italian hemp seed oils. Results showed a concentration range between 2.265 and 233.8 mg/kg. Even though hemp seeds do not contain cannabinoids, their presence in them could be caused by direct contact with the resin secreted by the epidermal glands situated on flowers and leaves. So, cannabinoids represent “impurities” of the hemp seed oil probably deriving from the cleaning process of the seed. Moreover, the concentrations of cannabinoids can be highly changeable among different oil varieties.
To date, several countries including Austria, Finland, UK, Italy, and Germany allowed foods containing hemp products but lack or uncertainty regulation generating confusion in others. CBD food products may rely on Regulation (EU) 2015/2283 [55] in which CBD food products are considered as “Novel Food”. To comply with the new novel food guidelines, a concentration in CBD food products intended for internal use below 5% CBD is recommended.
2.6. Quality Parameters Determination
Acidity index, also indicated as acidity value, is an important indicator of vegetable oil quality and is expressed as the amount of potassium hydroxide (KOH) necessary to neutralize free fatty acids contained in 1 g of oil.
The AI values for investigated oils (Table 4) are not in line with the maximum oleic acidity permitted for alimentary oils, which is estimated equal to 2%. Indeed, drying and storage are factors that could affect this value [56]. In particular, drying is reported to increase the index by 0.1%, whereas storage increases it by 0.05% per month. Taking into account that the main goal should be to commercialize hempseed oils with AI values below 2%, with the only exception of HS oils 5 and 12, the oils studied did not fully reach the desired objective. In fact, acidity index varied from 1.3 to 8.05%, but considering that Pharmacopoeia establishes a maximum value of 6% and 10% for hemp oil acidity and peroxide [57], most of the samples showed satisfactory results, with values fitting the above-mentioned requirements. In fact, when peroxide index, which is still the most common chemical method of measuring oxidative deterioration of oils, was evaluated, it was found in the range of 1.75–7.62 meq O2/kg for the analyzed samples.
Table 4.
Quality parameters in terms of acidity (%), peroxide index (meq O2/kg) and Delta-k spectrophotometric analysis measured for the hemp seed oils under study. Results are expressed as mean of three independent measurements ± SD.
| Sample | Variety | Acidity (%) | Peroxide Value (meq O2/kg) |
Delta-k |
|---|---|---|---|---|
| Oil 1 | Uso-31 | 9.9 ± 0.08 | 6.5 ± 0.07 | 0.015± 0.003 |
| Oil 2 | Blend 1 | 7.9 ± 0.01 | 4.2 ± 0.01 | 0.029 ± 0.002 |
| Oil 3 | Codimono | 5.1 ± 0.09 | 1.8 ± 0.15 | 0.022 ± 0.001 |
| Oil 4 | Futura 75 | 2.8 ± 0.06 | 6.8 ± 0.003 | 0.019 ± 0.001 |
| Oil 5 | Blend 2 | 1.3 ± 0.01 | 5.3 ± 0.003 | 0.013 ± 0.003 |
| Oil 6 | Zenit | 5.1 ± 0.01 | 6.3 ± 0.75 | 0.019 ± 0.002 |
| Oil 7 | Blend 3 | 6.8 ± 0.08 | 4.8 ± 0.20 | 0.027 ± 0.001 |
| Oil 8 | Secuieni jubileu | 4.9 ± 0.20 | 5.3 ± 0.50 | 0.029 ± 0.003 |
| Oil 9 | Uso-31 | 6.2 ± 0.03 | 8.8 ± 0.07 | 0.020 ± 0.002 |
| Oil 10 | Felina 32 | 2.1 ± 0.09 | 4.2 ± 0.30 | 0.047 ± 0.003 |
| Oil 11 | Blend 4 | 8.0 ± 0.07 | 3.8 ± 0.25 | 0.023 ± 0.001 |
| Oil 12 | Blend 5 | 1.7 ± 0.09 | 4.8 ± 0.15 | 0.024 ± 0.002 |
| Oil 13 | Futura 75 | 3.0 ± 0.06 | 3.0 ± 0.08 | 0.020 ± 0.003 |
| Skewness | 0.3 | 0.3 | 1.7 | |
| Kurtosis | −1.9 | 0.7 | 4.4 |
As the formation of PUFAs hydroperoxide derivatives, which could occur in the early stages of an oxidation process, may result in the synthesis of conjugated dienic and trienic systems, spectrophotometric analyses were carried out to calculate Delta-k value (Table 4), which was less than 0.047 for all analyzed samples.
Quality parameters of hemp seed oil were evaluated by Jourdi et al. [58] who studied the physicochemical characterization of the fatty oils obtained by cold pressing of three hemp seeds harvested in ecological crops from Romania. Peroxide results were in the range of 0.6–1.2 meq O2/kg and acidity index was from 0.5 to 0.75%. In another study, [31] reported values for hemp seed oil of 1.94 meq O2/kg and 1.76% for peroxide and acidity index, respectively. As reported by Aladić et al. [37] who evaluated the extraction process from Croatian hemp seed oil by cold pressing, followed by extraction with supercritical CO2, methodology and the effects of temperature influenced quality parameters.
Although the quality parameters could not be compared with extra virgin oil [59], and the high acidity could impact on sensory characteristics of oil, such as flavor, the collateral characteristics make it still a very valuable oil. These oil benefits encourage the chemical industries versus the optimization of extraction process in order to accomplish the quality of the product [39].
2.7. Accelerated Photo-Oxidation Tests
Lipid oxidation is the main reaction responsible for reducing the nutritional value and shelf life of edible oils by the formation of oxidation products [60]. In accelerated photo-oxidation tests, the level of primary oxidation was evaluated by spectrophotometric absorbance at 232 nm which provides information on the conjugated dienes, and formation of products such as hydroperoxides [61]. Secondary lipid oxidation products such as aldehydes and ketones were monitored by absorbance of 270 nm, indicating the level of conjugated trienes as well as the content of carbonyl compounds [62]. Moreover, malondialdehyde (MDA) one of the main secondary oxidation products linked to the production of off-flavor and rancidity taste in edible oils was monitored by Thiobarbituric Acid Reactive Substances (TBARS) method [63]. Table 5 shows changes in K232- and K270-specific coefficients during the accelerated photo-oxidation tests. As far as the initial UV extinction coefficient was concerned, the K232 values of the commercial hemp seed oils were quantified at concentrations ranging from 0.81 (HS oil 10-Felina 32) up to 0.86 units (HS oil 11-Blend 4), with a mean value of 0.83 units for all samples. These levels were similar to the data previously reported in hemp seed oils [31,51]. On the other hand, the initial K270 monitored in different oil samples ranged from 0.34 (HS oil 5-Blend 2) to 0.73 units (HS oil 2-Blend 1), with a mean value of 0.51 units for all samples. All studied samples exceeded the limit of 0.25 units which has been established by the Commission Regulation (EC) for virgin olive oil [64].
Table 5.
Extinction coefficient at 232 and 270 nm in initial oils and after 7 days of accelerated photo-oxidation tests. Values are the mean of three independent measurements ± SD.
| Sample | t0 | t7 | ||
|---|---|---|---|---|
| K232 | K270 | K232 | K270 | |
| Oil 1 | 0.86 ± 0.08 | 0.69 ± 0.07 | 0.86 ± 0.03 | 0.79 ± 0.05 |
| Oil 2 | 0.84 ± 0.07 | 0.73 ± 0.04 | 0.88 ± 0.04 | 0.81 ± 0.06 |
| Oil 3 | 0.84 ± 0.03 | 0.51 ± 0.02 | 0.88 ± 0.04 | 0.54 ± 0.04 |
| Oil 4 | 0.84 ± 0.01 | 0.39 ± 0.05 | 0.87 ± 0.02 | 0.42 ± 0.05 |
| Oil 5 | 0.82 ± 0.08 | 0.34 ± 0.01 | 0.82 ± 0.03 | 0.38 ± 0.02 * |
| Oil 6 | 0.83 ± 0.03 | 0.40 ± 0.04 | 0.87 ± 0.02 | 0.44 ± 0.03 |
| Oil 7 | 0.85 ± 0.04 | 0.54 ± 0.07 | 0.85 ± 0.03 | 0.56 ± 0.04 |
| Oil 8 | 0.83 ± 0.03 | 0.65 ± 0.06 | 0.82 ± 0.04 | 0.70 ± 0.03 |
| Oil 9 | 0.81 ± 0.02 | 0.46 ± 0.05 | 0.83 ± 0.03 | 0.47 ± 0.02 |
| Oil 10 | 0.81 ± 0.05 | 0.50 ± 0.05 | 0.86 ± 0.03 | 0.63 ± 0.02 * |
| Oil 11 | 0.86 ± 0.02 | 0.50 ± 0.04 | 0.87 ± 0.02 | 0.60 ± 0.02 * |
| Oil 12 | 0.82 ± 0.01 | 0.47 ± 0.04 | 0.89 ± 0.04 * | 0.54 ± 0.03 |
| Oil 13 | 0.83 ± 0.08 | 0.36 ± 0.02 | 0.88 ± 0.07 | 0.43 ± 0.01 * |
Statistical significance is calculated by Student’s t-test analysis: * p < 0.05 Extinction coefficient (232 or 270 nm) in initial oils vs. after 7 days of accelerated photo-oxidation tests.
After accelerated photo-oxidation tests, the percentage of increase in K232 and K270 ranged from 1.2 to 8.5%, and from 3.7 to 26.0%, respectively. In particular, the percentage of increase in K232 was significantly increased compared to initial oils samples (p < 0.05) only in HS oil 12. However, a significant increase in K270-specific coefficient during the accelerated photo-oxidation test was observed in four samples (Table 5). The results of this study indicated that the commercial hemp seed oils were resistant to photo-oxidation, which may be due to the presence of bioactive compounds such as polyphenols and carotenoids that play an important role in the oxidative stability [65].
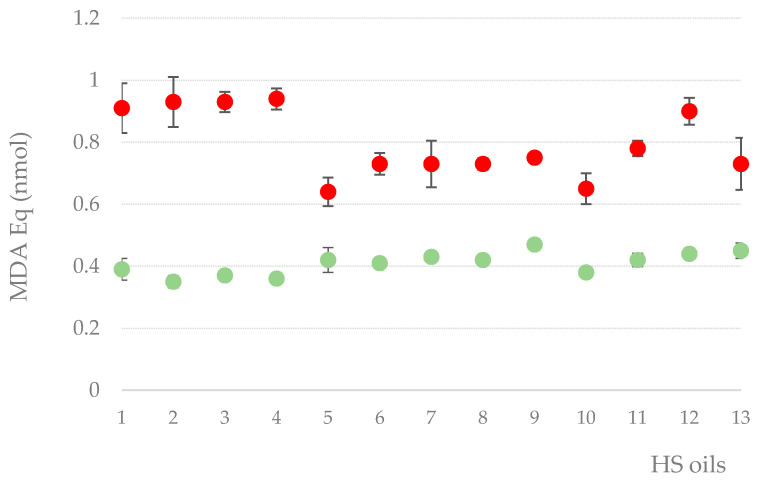
On the other hand, the secondary oxidation of oils was also determined by the TBARs assay, as shown in Figure 5. The initial TBARS value was quantified at a concentration ranging from 0.35 up to 0.44 mmol MDA/kg. Concerning the occurrence of MDA in the oils after accelerated photo-oxidation tests, the levels found in assayed samples showed a 1.5- to 2.5-fold increase when compared to the initial values. Photo-oxidation susceptibility of the hemp seed oils may be due to the high content in unsaturated fatty acids recognized as vulnerable by oxygen, especially α-linolenic acid (ALA), that contributes the most to the degree of lipid peroxidation [66]. Pigments present in edible oil are known to play an important role in oxidative stability [67]. A weak linear relationship existed between the carotenoid levels and increased Thiobarbituric Acid Reactive Substances (TBARS) values after the accelerated photo-oxidation test (R2 = 0.43), but not with chlorophylls, suggesting that these pigments could provide some oxidative stability to oils. The significant increase in secondary products of lipid peroxidation showed after accelerated photo-oxidation tests limit the nutritive value as well as customer satisfaction [68]. Therefore, in order to protect the beneficial lipids in hemp oil, it is significantly important to promote better oxidative stability of hemp seed oils by the use of the dark opaque package, improving the lipid stability adding antioxidant compounds or develop a suitable formulation to encapsulate the hemp oil.
Figure 5.
Thiobarbituric Acid Reactive Substances (TBARS) values, expressed as nmol of malondialdehyde (MDA) equivalents, in initial oils (●), and after 7 days accelerated photo-oxidation tests (●). Values are the mean of three independent measurements ± SD.
3. Materials and Methods
3.1. Reagents and Materials
All solvents, water (LC-MS grade), chloroform, methanol, n-hexane, iso-octane (2,2,4 trimethylpentane), and diethyl ether were acquired from Carlo Erba reagents (Milan, Italy), whereas ethanol absolute (≥99.8%), hydrochloric acid and formic acid (mass spectrometry grade) were purchased from VWR Chemicals (Milan, Italy).
Trichloroacetic acid (TCA, C2HCl3O2), 2-thiobarbituric acid (TBA, C4H4N2O2S), sodium thiosulphate (Na2S2O3), sodium carbonate (Na2CO3), potassium iodide (KI), Folin–Ciocalteu reagent, gallic acid, anhydrous sodium sulfate (Na2SO4) anhydrous sodium sulfate (Na2SO4) and phenolphthalein solution were obtained from Sigma Aldrich (Milan, Italy).
All standards (purity > 98%), namely malondialdehyde (MDA), butylated hydroxytoluene (BHT), gallic acid, α-tocopherol, oleic acid and α-tocopherol were acquired from Sigma Aldrich (Milan, Italy). Chemicals and reagents were of analytical grade.
3.2. Sampling
A total of thirteen samples of commercial hemp (Cannabis sativa L.) seed oil of different origins (Center and Southern Italy) were purchased from the Italian local market. Eight samples were monovarietal, including Codimono (n = 1), Felina 32 (n = 1), Futura 75 (n = 2), Secuieni jubileu (n = 1), Uso – 31 (n = 2) and Zenit (n = 1), whereas five oil samples were the result of a blend composed of several varieties such as Blend 1 (Carmagnola, Futura 75, KC Dora, Kompolti and Tibor); Blend 2 (Futura 75, Finola and Felina 32), Blend 3 (Uso-31, Secuieni jubileu and Zenit); Blend 4 (Futura 75 and USO-31); and Blend 5 (Uso-31, Futura 75, Felina 32 and Zenit). The percentages of each variety were not declared in labels from samples. All the samples were stored in dark and cool conditions until further analysis.
3.3. Polyphenols and Fatty Acids Extraction
Polyphenols extraction was performed in accordance with the procedure reported by Moccia et al. [19], with modifications. Briefly, 5 g of oil, previously dissolved in 5 mL of n-hexane, were extracted with 50 mL of MeOH:Me2CO:H2O (7:7:6, v:v:v) solution. The mixture was intensively vortexed for 3 min and sonicated for 30 min in an ultrasonic bath. Then, the mixture was centrifuged 5000× g at 4 °C for 10 min. The lower phases from three consecutive extractions were combined, filtered through 0.45 mm vacuum membranes (Millipore, Billerica, MA, USA) and concentrated using rotary evaporation in a water bath at 30 °C, and analyzed for their total phenolic content (please see Section 3.4). The upper phases were washed three times with MeOH:H2O (4:1, v:v) solution to remove non-lipid substances, anhydrified on Na2SO4 and then investigated for their polyunsaturated fatty acids (please see Section 3.6).
3.4. Determination of Total Phenolic Content (TPC)
Total phenolic content was determined based on Folin Ciocalteu method [69]. In short, 0.125 mL of extract was diluted in 0.5 mL of deionized water and reacted with 0.125 mL of Folin–Ciocalteu reagent for 6 min in dark conditions at room temperature. Then, 1.25 mL of 7.5% of sodium carbonate solution and 1 mL of deionized water were added to reach a final volume of 3 mL. The absorbance at 760 nm after 90 min of incubation in the dark was determined using an UV/Vis Spectrophotometer (DU 730, Beckman Coulter, Brea, CA, USA). Results was expressed as mg of gallic acid equivalents GAE/g of sample.
3.5. α-Tocopherol Extraction and Determination
α-Tocopherol extraction was performed according to the method of Mallek-Ayadi et al. [70], with slight modifications. In short, 0.1 mL of oil was diluted in 0.9 mL of n-hexane contained in an Eppendorf tube. From this mixture, 0.2 mL was transferred in an Eppendorf tube in which 0.8 mL of methanol was present. The mixture was vortexed for 3 min and centrifuged at 1008× g for 5 min at 15 °C. The supernatant was filtered through 0.45 mm vacuum membranes (Millipore, Billerica, MA) and kept at 4 °C until HPLC analysis.
α-tocopherol was assessed by HPLC/diode-array detector (DAD) analysis, performed using an HPLC system Jasco Extrema LC-4000 system (Jasco Inc., Easton, MD, USA) fitted with an autosampler, a binary solvent pump, and a diode-array detector (DAD), according to Chen et al. 2010. The separation and quantification were carried out using Prodigy C18 column (250 × 4.6 mm, 5 µm particle size; Phenomenex, Castel Maggiore, Italy) preceded by a security guard cartridge. The column temperature was set at 30 °C. The photometric diode array (PDA) acquisition wavelength was set in the range between 200 and 400 nm. The mobile phase consisted of isocratic CH3OH:H2O (98:2 v/v) with a flow rate of 1 mL/min. The injection volume was set at 20 µL. α-tocopherol was identified by comparison with a pure standard (Sigma Chemicals, Milan, Italy); the calibration curve was obtained by measuring absorbance at 290 nm, over the concentration range of 0.5–500 mg/kg.
3.6. UHPLC-ESI-QqTOF-MS/MS Analysis for PUFAs Determination
Hexane fractions from liquid–liquid extraction (please see Section 3.3) were analyzed for PUFA content [28,71]. For this purpose, a Shimadzu NEXERA UHPLC system was used with a Luna® Omega Polar C18 column (1.6 μm particle size, 150 × 2.1 mm i.d., Phenomenex, Torrance, CA, USA). Separation was achieved with a linear gradient of water (A) and acetonitrile (B), both with 0.1% formic acid. Gradient conditions were as follows: 0–5 min, linear from 5 to 55% B; 5–10 min, linear from 55 to 75% B; 10–11 min, from 75 to 95% B; 11–12 min, isocratic 95% B. Then, at 12.01 min the starting conditions were restored and the column was allowed to re-equilibrate for 2 min. The total run time was 14 min, with a flow rate of 0.5 mL/min and an injection volume of 2.0 μL. MS analysis was performed using a hybrid QqTOF MS instrument, the AB SCIEX TripleTOF® 4600 (AB Sciex, Concord, ON, Canada), equipped with a DuoSprayTM ion source (consisting of both electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) probes), which was operated in the negative ESI mode. The APCI probe was used for automated mass calibration using the Calibrant Delivery System (CDS). The CDS injects a calibration solution matching the polarity of ionization, and calibrates the mass axis of the TripleTOF® system in all scan functions used (MS and/or MS/MS). The QqTOF HRMS method, which combines TOF-MS and MS/MS with Information Dependent Acquisition (IDA) for identifying non-targeted and unexpected compounds, consisted of a full scan TOF survey (dwell time 100 ms, 100–1000 Da) and a maximum number of eight IDA MS/MS scans (dwell time 50 ms, 80–85 Da)]. Quantitation is carried out taking into account peak area from selected ion chromatograms (three independent measurements were performed). In particular, the [M − H]− ion for α-linolenic acid was at m/z 277.2173, according to the molecular formula C18H30O2 (calcd. 277.2176 error ppm 1.4, unsaturation degree 4) and TOF-MS/MS fragment ions were at m/z 259.2072 ([M−H-H2O]−), 233.2275 ([M − H-CO2]−). The [M − H]− ion for linoleic acid was at m/z 279.2330, according to the molecular formula C18H30O2 (calcd. 279.2331 error ppm 0.5, unsaturation degree 3) and TOF-MS/MS fragment ion was at m/z 261.2225 ([M − H-H2O]−).
3.7. Oil Pigment Determination
Chlorophyll and carotenoid content of the hemp seed oil samples were assessed based on the method reported by Isabel Minguez-Mosquera et al. [72] with slight modifications. Briefly, each oil sample (0.100 ± 0.001 g) was placed in a tube and the volume was made up to 3 mL using absolute diethyl ether. The solution was vortexed thoroughly and sonicated for 1 min. The absorbance of the solution was measured in the wavelength range 250–750 nm by UV-1700 UV/Vis spectrophotometer (Shimadzu, Kyoto, Japan) against a blank. Oil pigment content (µg/mL) was calculated according to the formula:
| (1) |
| (2) |
| (3) |
| (4) |
3.8. Cannabidiolic Content Determination
Cannabidiolic acid (CBDA) content was estimated by HPLC-UV-DAD analysis according to Formato et al. [54]. To this purpose, the HPLC 1260 INFINITY II system (Agilent, Santa Clara, CA, USA) was utilized, equipped with an Agilent G7129A autosampler, an Agilent GY115A DAD-UV-visible detector and a Quaternary pump Agilent G711A. The analysis was carried out using the Luna® Phenyl-Hexyl column (150 × 2 mm, 3 µm). The mobile phase consisted of a binary solution A: 0.1% HCOOH in H2O, B: 0.1% HCOOH in CH3CN. A linear gradient was started at 55% B, held for 1.5 min, and linearly ramped to 95% B in 6.50 min. The mobile phase composition was maintained at 95% B for another 2 min, then returned to the starting conditions and allowed to re-equilibrate for 3 min. The total analysis time was 13.00 min. The analyses were carried out in three independent measurements and the results were expressed as mean values ± Standard Deation (SD).
3.9. Quality Parameters Determination
Quality parameters of oil were evaluated according to the procedures indicated in Commission regulation (EU) No. 2016/1227 [73], No. 2016/1784 [74], No. 2015/1833 [75], amending Regulation (EEC) No. 2568/91 [23].
3.9.1. Determination of Free Fatty Acids
An aliquot of 2.5 g of hemp oil sample was dissolved in 100 mL of a mixture of diethyl ether and ethanol (1:1 v/v). Phenolphthalein solution (1% in ethanol) was used as indicator. The free fatty acids present were neutralized using potassium hydroxide solution (0.1 mol/L) until the indicator color changes and persists for at least 10 s. Results were expressed in % of oleic acid, and calculated with the following formula:
| (5) |
where: V = the volume potassium hydroxide solution used in the titrated (mL); c = the exact concentration in mol/L of potassium hydroxide solution; M = 282 g/mol, the mar mass of oleic acid; m = the mass of the sample (g).
3.9.2. Determination of Peroxide Value
An aliquot of 2 g of hemp oil sample was dissolved in 25 mL of a mixture of acetic acid: chloroform (3:2 v/v) stirred until complete solubilization. Then, 1 mL of saturated KI solution was added and the flask was immediately closed and stirred for 2 min in the dark, the necessary time for the oxidation of the iodide to iodine from part of hydroperoxides. After, 75 mL of distilled water and 1 mL of starch solution were added before the titration with thiosulphate solution 0.01 N until the color blue-violet disappeared. The peroxide value, expressed in meq O2/kg, is given by:
| (6) |
where: V = the volume of thiosulphate solution 0.01 N used in the titrated (mL); T = the exact concentration in mol/L of thiosulphate solution; m = the mass of the sample (g).
3.9.3. Spectrophotometric Investigation in the Ultraviolet
An aliquot of 0.25 g of hemp oil sample, weighted into a 25-mL graduated flask, was dissolved in 25 mL iso-octane. After exhaustive shaking, the solution was read at the wavelengths of 232, 270, 264, 268 and 272 nm by using a spectrophotometer instrument. Quartz cuvettes were used for the described test. The variation of the absolute value of the extinction () is given by:
| (7) |
where Km is the specific extinction at the wavelength for maximum absorption at 268 nm in iso-octane.
3.10. Preparation of Samples for Accelerated Photo-Oxidation Tests
The photo-oxidation procedure proposed by Abuzaytoun et al. [76] was selected as a starting point and then slightly modified. A 15 mL PTFE tube was filled with the oil so that the headspace was ∼1% of the volume. The test tube was placed in a thermo block (70 cm length × 35 cm width × 25 cm height) with fluorescent radiation of 2650 lux and an internal temperature of 24 °C. After incubation of 7 days, the commercial hemp seed oil samples were recovered from the oven and kept at −20 °C until analysis.
3.10.1. Determination of the Lipid Photostability
Lipid photo-oxidation of the commercial hemp seed oils obtained during the accelerated photo-oxidation tests and non- oxidized samples was assessed and compared by TBARS test and UV–spectrophotometric analyses.
3.10.2. Thiobarbituric Acid Reactive Substances (TBARS) Determination
The determination of TBARS was carried out according to the procedure described by Maqsood et al. [77] with minor modifications. Thiobarbituric acid (TBA) reagent was prepared as follows: for reagent A, TBA (375.0 mg) and tannic acid (30.0 mg) were dissolved in hot water (30.0 mL), for reagent B, trichloroacetic acid (15 g) was dissolved in an aqueous hydrogen chloride solution (0.30 M, 70.0 mL). Then, reagent A was mixed with reagent B. Hemp seed oil sample (0.25 g) was emulsified with Tween-40 (15.6 mg) initially dissolved in Tris-HCl buffer (0.2 M, 2.0 mL, pH 7.4). After addition of the TBA reagent (1.0 mL), all test tubes were placed in a boiling water bath for 15 min. Then, 500.0 μL of n-butanol was added, and centrifuged at 252× g for 3 min. The absorbance of supernatant was measured at 532 nm. Inhibition of lipid peroxidation was recorded as percentage vs. a blank containing no test sample [62]. Results were expressed as nmol of MDA equivalents/kg sample, by interpolation from the MDA standard curve.
3.10.3. UV-Pectrophotometric Analyses
The photo-oxidation was evaluated monitoring the spectrophotometric absorbance at 232 and 270 nm of the samples after accelerated photo-oxidation tests compared to their respective non-oxidized samples. The results were expressed as K270 and K232.
3.11. Statistical Analysis
All experiments were conducted in triplicate and the results expressed as the average values ± standard deviation (SD). The differences between average values were evaluated by using the Student t-test at a significance level of 0.05. Correlation coefficients between the different experimental data were determined using Pearson’s test. Statistical analysis was carried out using STATA 12 (STATACorp LP, College Station, TX, USA).
4. Conclusions
Nowadays, consumers are constantly looking for useful natural products to supplement the human diet in order to prevent or treat human diseases. Despite some reticence towards this matrix, hemp seed oil has been heavily studied in recent decades. So, several studies reported the beneficial effect derived from oil extracted from hemp seed. Indeed, although the use of hemp seed in Italy, as part of human diet as it is or as its by-product (e.g., oil), has been legislated since 2009 [78], little is known about its chemical composition and the variability of this. This can have several primary consequences, among which is the marketing of highly dissimilar products from an analytical-quantitative point of view. The uniqueness of the hemp seed oil products must be the subject/object of greater attention. Data herein reported showed that investigated HS oils markedly differ in the content of some bioactive compounds, suggesting the need for a disciplinary booklet that well defines agronomic and post-harvest management conditions for achieving a good food objective.
Acknowledgments
Authors acknowledge Nicomede Di Michele (J.D.), President of the Fracta Sativa UniCanapa Association, for supplying the oil samples and also Claudia Comune for her technical support.
Author Contributions
Conceptualization, L.I., S.P. (Severina Pacifico) and A.R.; methodology, L.I., S.P. (Simona Piccolella); formal analysis, L.I.; investigation, L.I., L.C. and A.N.; resources, A.R.; writing, original draft preparation, L.I., L.C. and A.N.; writing, review and editing, S.P. (Severina Pacifico); supervision, M.G., S.P. (Severina Pacifico) and A.R.; project administration, A.R.; funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Bonini S.A., Premoli M., Tambaro S., Kumar A., Maccarinelli G., Memo M., Mastinu A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharm. 2018;227:300–315. doi: 10.1016/j.jep.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Andre C.M., Hausman J.-F., Guerriero G. Cannabis sativa: The Plant of the thousand and One Molecules. Front. Plant. Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves P., Amaral C., Teixeira N., Correia-da-Silva G. Cannabis sativa: Much more beyond Δ9-tetrahydrocannabinol. Pharmacol. Res. 2020;157:104822. doi: 10.1016/j.phrs.2020.104822. [DOI] [PubMed] [Google Scholar]
- 4.Izzo L., Castaldo L., Narváez A., Graziani G., Gaspari A., Rodríguez-Carrasco Y., Ritieni A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules. 2020;25:631. doi: 10.3390/molecules25030631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavlovic R., Panseri S., Giupponi L., Leoni V., Citti C., Cattaneo C., Cavaletto M., Giorgi A. Phytochemical and ecological analysis of two varieties of hemp (Cannabis sativa L.) grown in a mountain environment of Italian Alps. Front. Plant. Sci. 2019;10:1265. doi: 10.3389/fpls.2019.01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Citti C., Linciano P., Panseri S., Vezzalini F., Forni F., Vandelli M.A., Cannazza G. Cannabinoid profiling of hemp seed oil by liquid chromatography coupled to high-resolution mass spectrometry. Front. Plant. Sci. 2019;10:120. doi: 10.3389/fpls.2019.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Citti C., Pacchetti B., Vandelli M.A., Forni F., Cannazza G. Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of cannabidiolic acid (CBDA) J. Pharm. Biomed. Anal. 2018;149:532–540. doi: 10.1016/j.jpba.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Leizer C., Ribnicky D., Poulev A., Dushenkov S., Raskin I. The composition of hemp seed oil and its potential as an important source of nutrition. J. Nutraceuticals Funct. Med. Foods. 2000;2:35–53. doi: 10.1300/J133v02n04_04. [DOI] [Google Scholar]
- 9.Deferne J.-L., Pate D.W. Hemp seed oil: A source of valuable essential fatty acids. J. Int. Hemp Assoc. 1996;3:4–7. [Google Scholar]
- 10.Kang J.X. The Importance of Omega-6/Omega-3 Fatty Acid Ratio in Cell Function. Karger; Basel, Swizerland: 2003. [DOI] [PubMed] [Google Scholar]
- 11.Callaway J.C., Pate D.W. Gourmet and Health-Promoting Specialty Oils. AOCS PRESS; Urbana, IL, USA: 2009. Hempseed oil; pp. 185–213. [Google Scholar]
- 12.Sharma S., Cheng S.-F., Bhattacharya B., Chakkaravarthi S. Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nanoemulsion-based encapsulation. Trends Food Sci. Tech. 2019;91:305–318. doi: 10.1016/j.tifs.2019.07.030. [DOI] [Google Scholar]
- 13.Amaral A.B., Silva M.V.d.S., Lannes S.C.d.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Tech. 2018;38:1–15. doi: 10.1590/fst.32518. [DOI] [Google Scholar]
- 14.Aladić K., Jarni K., Barbir T., Vidović S., Vladić J., Bilić M., Jokić S. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind. Crops Prod. 2015;76:472–478. doi: 10.1016/j.indcrop.2015.07.016. [DOI] [Google Scholar]
- 15.Matthäus B., Brühl L. Virgin hemp seed oil: An interesting niche product. Eur. J. Lipid. Sci. Tech. 2008;110:655–661. doi: 10.1002/ejlt.200700311. [DOI] [Google Scholar]
- 16.Tarchoune I., Sgherri C., Eddouzi J., Zinnai A., Quartacci M.F., Zarrouk M. Olive leaf addition increases olive oil nutraceutical properties. Molecules. 2019;24:545. doi: 10.3390/molecules24030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frassinetti S., Moccia E., Caltavuturo L., Gabriele M., Longo V., Bellani L., Giorgi G., Giorgetti L. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018;262:56–66. doi: 10.1016/j.foodchem.2018.04.078. [DOI] [PubMed] [Google Scholar]
- 18.Faugno S., Piccolella S., Sannino M., Principio L., Crescente G., Baldi G.M., Fiorentino N., Pacifico S. Can agronomic practices and cold-pressing extraction parameters affect phenols and polyphenols content in hempseed oils? Ind. Crops Prod. 2019;130:511–519. doi: 10.1016/j.indcrop.2018.12.084. [DOI] [Google Scholar]
- 19.Moccia S., Siano F., Russo G.L., Volpe M.G., La Cara F., Pacifico S., Piccolella S., Picariello G. Antiproliferative and antioxidant effect of polar hemp extracts (Cannabis sativa L., Fedora cv.) in human colorectal cell lines. Int. J. Food Sci. Nutr. 2019;71:1–14. doi: 10.1080/09637486.2019.1666804. [DOI] [PubMed] [Google Scholar]
- 20.Castaldo L., Narváez A., Izzo L., Graziani G., Gaspari A., Di Minno G., Ritieni A. Red Wine Consumption and Cardiovascular Health. Molecules. 2019;24:3626. doi: 10.3390/molecules24193626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azzi A. Molecular mechanism of α-tocopherol action. Free Radic. Biol. Med. 2007;43:16–21. doi: 10.1016/j.freeradbiomed.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Li G., Lee M.-J., Liu A.B., Yang Z., Lin Y., Shih W.J., Yang C.S. The antioxidant and anti-inflammatory activities of tocopherols are independent of Nrf2 in mice. Free Radic. Biol. Med. 2012;52:1151–1158. doi: 10.1016/j.freeradbiomed.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 23.European Commission (EC) European Union Commission. Regulation EEC 2568/91. On the characteristics of olive oil and olive pomace and their analytical methods. J. Off. J. Euro. Comm. L. 1991;248:1–81. [Google Scholar]
- 24.Da Porto C., Decorti D., Tubaro F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind. Crops Prod. 2012;36:401–404. doi: 10.1016/j.indcrop.2011.09.015. [DOI] [Google Scholar]
- 25.Montserrat-de la Paz S., Marín-Aguilar F., García-Giménez M.D., Fernández-Arche M.A. Hemp (Cannabis sativa L.) Seed Oil: Analytical and Phytochemical Characterization of the Unsaponifiable Fraction. J. Agric. Food Chem. 2014;62:1105–1110. doi: 10.1021/jf404278q. [DOI] [PubMed] [Google Scholar]
- 26.Siano F., Moccia S., Picariello G., Russo G.L., Sorrentino G., Di Stasio M., La Cara F., Volpe M.G. Comparative study of chemical, biochemical characteristic and ATR-FTIR analysis of seeds, oil and flour of the edible Fedora cultivar hemp (Cannabis sativa L.) Molecules. 2019;24:83. doi: 10.3390/molecules24010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nigro E., Crescente G., Formato M., Pecoraro M.T., Mallardo M., Piccolella S., Daniele A., Pacifico S. Hempseed lignanamides rich-fraction: Chemical Investigation and cytotoxicity towards U-87 glioblastoma cells. Molecules. 2020;25:1049. doi: 10.3390/molecules25051049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crescente G., Piccolella S., Esposito A., Scognamiglio M., Fiorentino A., Pacifico S. Chemical composition and nutraceutical properties of hempseed: An ancient food with actual functional value. Phytochem. Rev. 2018;17:733–749. doi: 10.1007/s11101-018-9556-2. [DOI] [Google Scholar]
- 29.Smeriglio A., Galati E.M., Monforte M.T., Lanuzza F., D’Angelo V., Circosta C. Polyphenolic Compounds and Antioxidant Activity of Cold-Pressed Seed Oil from Finola Cultivar of Cannabis sativa L. Phytother. Res. 2016;30:1298–1307. doi: 10.1002/ptr.5623. [DOI] [PubMed] [Google Scholar]
- 30.Yu L.L., Zhou K.K., Parry J. Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chem. 2005;91:723–729. doi: 10.1016/j.foodchem.2004.06.044. [DOI] [Google Scholar]
- 31.Teh S.-S., Birch J. Physicochemical and quality characteristics of cold-pressed hemp, flax and canola seed oils. J. Food Compos. Anal. 2013;30:26–31. doi: 10.1016/j.jfca.2013.01.004. [DOI] [Google Scholar]
- 32.Leonard W., Zhang P., Ying D., Fang Z. Hempseed in food industry: Nutritional value, health benefits, and industrial applications. Compr. Rev. Food sci. F. 2020;19:282–308. doi: 10.1111/1541-4337.12517. [DOI] [PubMed] [Google Scholar]
- 33.Dunford N.T. Gourmet and Specialty Oils. [(accessed on 28 May 2020)];Food Technol. 2017 Available online: https://shareok.org/bitstream/handle/11244/316238/oksd_fapc_211_2017-12.pdf?sequence=1. [Google Scholar]
- 34.Chen T., He J., Zhang J., Zhang H., Qian P., Hao J., Li L. Analytical characterization of hempseed (seed of Cannabis sativa L.) oil from eight regions in China. J. Diet. Suppl. 2010;7:117–129. doi: 10.3109/19390211003781669. [DOI] [PubMed] [Google Scholar]
- 35.Rezvankhah A., Emam-Djomeh Z., Safari M., Askari G., Salami M. Microwave-assisted extraction of hempseed oil: Studying and comparing of fatty acid composition, antioxidant activity, physiochemical and thermal properties with Soxhlet extraction. J. Food Sci. Tech. 2019;56:4198–4210. doi: 10.1007/s13197-019-03890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anwar F., Latif S., Ashraf M. Analytical characterization of hemp (Cannabis sativa) seed oil from different agro-ecological zones of Pakistan. J. Am. Oil Chem. Soc. 2006;83:323–329. doi: 10.1007/s11746-006-1207-x. [DOI] [Google Scholar]
- 37.Aladić K., Jokić S., Moslavac T., Tomas S., Vidović S., Vladić J., Šubarić D. Cold pressing and supercritical CO2 extraction of hemp (Cannabis sativa) seed oil. Chem. Biochem. Eng. Q. 2014;28:481–490. doi: 10.15255/CABEQ.2013.1895. [DOI] [Google Scholar]
- 38.Blade S.F., Ampong-Nyarko K., Przybylski R. Fatty acid and tocopherol profiles of industrial hemp cultivars grown in the high latitude prairie region of Canada. J. Ind. Hemp. 2006;10:33–43. doi: 10.1300/J237v10n02_04. [DOI] [Google Scholar]
- 39.Devi V., Khanam S. Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects. J. Clean. Prod. 2019;207:645–657. doi: 10.1016/j.jclepro.2018.10.036. [DOI] [Google Scholar]
- 40.Yang L.G., Song Z.X., Yin H., Wang Y.Y., Shu G.F., Lu H.X., Wang S.K., Sun G.J. Low n-6/n-3 PUFA ratio improves lipid metabolism, inflammation, oxidative stress and endothelial function in rats using plant oils as n-3 fatty acid source. Lipids. 2016;51:49–59. doi: 10.1007/s11745-015-4091-z. [DOI] [PubMed] [Google Scholar]
- 41.Baldini M., Ferfuia C., Piani B., Sepulcri A., Dorigo G., Zuliani F., Danuso F., Cattivello C. The performance and potentiality of monoecious hemp (Cannabis sativa L.) cultivars as a multipurpose crop. Agronomy. 2018;8:162. doi: 10.3390/agronomy8090162. [DOI] [Google Scholar]
- 42.Kiralan M., Gül V., Kara S.M. Fatty acid composition of hempseed oils from different locatins in Turkey. Span. J. Agric. Res. 2010:385–390. doi: 10.5424/sjar/2010082-1220. [DOI] [Google Scholar]
- 43.Abdollahi M., Sefidkon F., Calagari M., Mousavi A., Mahomoodally M.F. A comparative study of seed yield and oil composition of four cultivars of Hemp (Cannabis sativa L.) grown from three regions in northern Iran. Ind. Crops Prod. 2020;152:112397. doi: 10.1016/j.indcrop.2020.112397. [DOI] [Google Scholar]
- 44.Ustun-Argon Z. Phenolic Compounds, Antioxidant Activity and Fatty Acid Compositions of Commercial Cold-Pressed Hemp Seed (Cannabis Sativa L) Oils From Turkey. Int. J. Sci. Eng. Res. 2019;10:166–173. [Google Scholar]
- 45.Apostol L. Studies on using hemp seed as functional ingredient in the production of functional food products. J. Ecoagritourism. 2017;13:12–17. [Google Scholar]
- 46.Saastamoinen M., Eurola M., Hietaniemi V. Oil, protein, chlorophyll, cadmium and lead contents of seeds in oil and fiber flax (Linum usitatissimum L.) cultivars and in oil hemp (Cannabis sativa L.) cultivar Finola cultivated in south-western part of Finland. J. Food Chem. Nanotechnol. 2016;2:73–76. doi: 10.17756/jfcn.2016-013. [DOI] [Google Scholar]
- 47.Liang J., Appukuttan Aachary A., Thiyam-Holländer U. Hemp seed oil: Minor components and oil quality. Lipid Tech. 2015;27:231–233. doi: 10.1002/lite.201500050. [DOI] [Google Scholar]
- 48.Aachary A.A., Liang J., Hydamaka A., Eskin N.M., Thiyam-Holländer U. A new ultrasound-assisted bleaching technique for impacting chlorophyll content of cold-pressed hempseed oil. LWT-Food Sci. Tech. 2016;72:439–446. doi: 10.1016/j.lwt.2016.05.011. [DOI] [Google Scholar]
- 49.Li X., Yang R., Lv C., Chen L., Zhang L., Ding X., Zhang W., Zhang Q., Hu C., Li P. Effect of chlorophyll on lipid oxidation of rapeseed oil. Eur. J. Lipid. Sci. Tech. 2019;121:1800078. doi: 10.1002/ejlt.201800078. [DOI] [Google Scholar]
- 50.Borges T.H., Pereira J.A., Cabrera-Vique C., Lara L., Oliveira A.F., Seiquer I. Characterization of Arbequina virgin olive oils produced in different regions of Brazil and Spain: Physicochemical properties, oxidative stability and fatty acid profile. Food Chem. 2017;215:454–462. doi: 10.1016/j.foodchem.2016.07.162. [DOI] [PubMed] [Google Scholar]
- 51.Oomah B.D., Busson M., Godfrey D.V., Drover J.C. Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chem. 2002;76:33–43. doi: 10.1016/S0308-8146(01)00245-X. [DOI] [Google Scholar]
- 52.Ratusz K., Symoniuk E., Wroniak M., Rudzińska M. Bioactive Compounds, nutritional quality and oxidative stability of cold-pressed Camelina (Camelina sativa L.) oils. Appl. Sci. 2018;8:2606. doi: 10.3390/app8122606. [DOI] [Google Scholar]
- 53.Roca M. Change in the natural ratio between chlorophylls and carotenoids in olive fruit during processing for virgin olive oil. J. Am. Oil Chem. Soc. 2001;78:133–138. doi: 10.1007/s11746-001-0233-z. [DOI] [Google Scholar]
- 54.Formato M., Crescente G., Scognamiglio M., Fiorentino A., Pecoraro M.T., Piccolella S., Catauro M., Pacifico S. (−)-Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules. 2020;25:2638. doi: 10.3390/molecules25112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.European Commission (EC) Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods. Volume 327. European Union Commission; Brussels, Belgium: 2015. [(accessed on 28 May 2020)]. pp. 1–21. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32015R2283. [Google Scholar]
- 56.Prescha A., Grajzer M., Dedyk M., Grajeta H. The antioxidant activity and oxidative stability of cold-pressed oils. J. Am. Oil Chem. Soc. 2014;91:1291–1301. doi: 10.1007/s11746-014-2479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.European Pharmacopoeia. 9th ed. Council of Europe; Strasbourg, France: 2016. [Google Scholar]
- 58.Al Jourdi H., Popescu C., Udeanu D.I., Arsene A., Sevastre A., Velescu B.S., Lupuliasa D. Comparative study regarding the physico-chemical properties and microbiological activities of olea europaea l. Oil and cannabis sativa l. Seed oil obtained by cold pressing. Farmacia. 2019;67:759–763. doi: 10.31925/farmacia.2019.5.2. [DOI] [Google Scholar]
- 59.Grossi M., Palagano R., Bendini A., Riccò B., Servili M., García-González D.L., Toschi T.G. Design and in-house validation of a portable system for the determination of free acidity in virgin olive oil. Food Cont. 2019;104:208–216. doi: 10.1016/j.foodcont.2019.04.019. [DOI] [Google Scholar]
- 60.Muik B., Lendl B., Molina-Díaz A., Ayora-Cañada M.J. Direct monitoring of lipid oxidation in edible oils by Fourier transform Raman spectroscopy. Chem. Phys. Lipids. 2005;134:173–182. doi: 10.1016/j.chemphyslip.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Melo T., Maciel E., Reis A., Domingues P., Domingues M.R.M. Lipidomics. Humana Press; New York, NY, USA: 2017. Mass Spectrometric Analysis of Lipid Hydroperoxides; pp. 133–146. [Google Scholar]
- 62.Guillaume C., De Alzaa F., Ravetti L. Evaluation of chemical and physical changes in different commercial oils during heating. Acta Sci. Nutr. Health. 2018;2:2–11. [Google Scholar]
- 63.Mei W.S.C., Ismail A., Esa N.M., Akowuah G.A., Wai H.C., Seng Y.H. The effectiveness of rambutan (Nephelium lappaceum L.) extract in stabilization of sunflower oil under accelerated conditions. Antioxidants. 2014;3:371–386. doi: 10.3390/antiox3020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.European Commission (EC) Commission Regulation (EC) no. 1989/2003, 6 November, Amending Regulation (EEC) no. 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis. Off. J. Eur. Union. 2003;295:57–77. [Google Scholar]
- 65.Ayadi M., Grati-Kamoun N., Attia H. Physico-chemical change and heat stability of extra virgin olive oils flavoured by selected Tunisian aromatic plants. Food Chem. Toxic. 2009;47:2613–2619. doi: 10.1016/j.fct.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 66.Shi X.-C., Jin A., Sun J., Yang Z., Tian J.-J., Ji H., Yu H.-B., Li Y., Zhou J.-S., Du Z.-Y. α-lipoic acid ameliorates n-3 highly-unsaturated fatty acids induced lipid peroxidation via regulating antioxidant defenses in grass carp (Ctenopharyngodon idellus) Fish Shellfish. Immunol. 2017;67:359–367. doi: 10.1016/j.fsi.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 67.Sotomayor-Gerding D., Oomah B.D., Acevedo F., Morales E., Bustamante M., Shene C., Rubilar M. High carotenoid bioaccessibility through linseed oil nanoemulsions with enhanced physical and oxidative stability. Food Chem. 2016;199:463–470. doi: 10.1016/j.foodchem.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 68.Unicomb A. Master’s Thesis. Dalhousie University; Halifax, Nova Scotia: Aug, 2017. Storage and Thermal Effects on the Oxidative Stability and Emulsion Characteristics of Hemp (Cannabis sativa L.) Oil-in-Water Emulsions. [Google Scholar]
- 69.Tenore G.C., Campiglia P., Ciampaglia R., Izzo L., Novellino E. Antioxidant and antimicrobial properties of traditional green and purple “Napoletano” basil cultivars (Ocimum basilicum L.) from Campania region (Italy) Nat. Prod. Res. 2017;31:2067–2071. doi: 10.1080/14786419.2016.1269103. [DOI] [PubMed] [Google Scholar]
- 70.Mallek-Ayadi S., Bahloul N., Kechaou N. Chemical composition and bioactive compounds of Cucumis melo L. seeds: Potential source for new trends of plant oils. Process. Saf. Environ. 2018;113:68–77. doi: 10.1016/j.psep.2017.09.016. [DOI] [Google Scholar]
- 71.Kumar S.J., Prasad S.R., Banerjee R., Agarwal D.K., Kulkarni K.S., Ramesh K. Green solvents and technologies for oil extraction from oilseeds. Chem. Cent. J. 2017;11:9. doi: 10.1186/s13065-017-0238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Isabel Minguez-Mosquera M., Rejano-Navarro L., Gandul-Rojas B., SanchezGomez A.H., Garrido-Fernandez J. Color-pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc. 1991;68:332–336. doi: 10.1007/BF02657688. [DOI] [Google Scholar]
- 73.European Commission (EC) European Commission implementing Regulation 2016/1227 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union. 2016;202:7–13. [Google Scholar]
- 74.European Commission (EC) Commission Implementing Regulation (EU) 2016/1784 of 30 September 2016 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis C/2016/6207. Off. J. Eur. Union. 2016;273:5–9. [Google Scholar]
- 75.European Commission (EC) European Commission implementing Regulation 2015/1833 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union. 2015;26:29–49. [Google Scholar]
- 76.Abuzaytoun R., Shahidi F. Oxidative stability of flax and hemp oils. J. Am. Oil Chem. Soc. 2006;83:855–861. doi: 10.1007/s11746-006-5037-7. [DOI] [Google Scholar]
- 77.Maqsood S., Benjakul S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010;119:123–132. doi: 10.1016/j.foodchem.2009.06.004. [DOI] [Google Scholar]
- 78.Ministerial Circular Foods based on hemp. [(accessed on 28 May 2020)];Minist. Health. 2009 2 Available online: http://www.federcanapa.it/wp-content/uploads/2016/07/Circolare_Miistero_della_Salute_22_maggio_2009___Produzione_e_commercializzazione_di_prodotti_a_base_di_semi_di_canapa_per_l__utilizzo_nei_settori_dell__alimentazione_umana-4.pdf. [Google Scholar]