Abstract
Lycopene, belonging to the carotenoids, is a tetraterpene compound abundantly found in tomato and tomato-based products. It is fundamentally recognized as a potent antioxidant and a non-pro-vitamin A carotenoid. Lycopene has been found to be efficient in ameliorating cancer insurgences, diabetes mellitus, cardiac complications, oxidative stress-mediated malfunctions, inflammatory events, skin and bone diseases, hepatic, neural and reproductive disorders. This review summarizes information regarding its sources and uses amongst different societies, its biochemistry aspects, and the potential utilization of lycopene and possible mechanisms involved in alleviating the abovementioned disorders. Furthermore, future directions with the possible use of this nutraceutical against lifestyle-related disorders are emphasized. Its protective effects against recommended doses of toxic agents and toxicity and safety are also discussed.
Keywords: lycopene, antioxidants, oxidative stress, cancer, diabetes, cardiovascular diseases, skin disorders
1. Introduction
Lycopene is a phytochemical mainly found in tomato and tomato-based products. It is a tetraterpene compound consisting of eight isoprene units and 11 double linear bonds. Lycopene is a non-pro-vitamin A carotenoid [1,2]. However, it is mentioned as an intermediate of carotenoid synthesis in plants [3]. Some fruits’ and vegetables’ red and orange coloration is attributed to this liposoluble pigment [2]. Despite this, it is found in some non-red or non-orange plants, such as asparagus and parsley [4]. It should be noted that lycopene cannot be synthesized in the human body. Therefore, it must be consumed in a daily diet [5]. The absorbed lycopene is mostly stored in the liver, adrenals, and prostate. Moreover, it can be found in other body parts (e.g., brain and skin) in a lower concentration [6]. Lycopene bioavailability can be decreased by ageing, and some of the pathological states, such as cardiovascular diseases (CVDs) [7]. Therefore, its supplementation—through various means, such as pasteurized watermelon juice—has been suggested to increase its circulating serum level in populations in need [8,9].
Interestingly, carotenoids have the highest concentration in the serum and tissues between different natural antioxidants. In addition, according to one study on a United States population, lycopene is the leading carotenoid in plasma and tissues [10]. Furthermore, second to astaxanthin (amongst different carotenoids), lycopene is the most potent antioxidant. It is an important deactivator of reactive oxygen species (ROS). For instance, it can remove singlet oxygen two and ten times more than beta-carotene and alpha-tocopherol, respectively [11]. In recent years, there has been an ever-increasing interest in lycopene’s health benefits. It is not only a potent antioxidant; its beneficial effects in the prevention and treatment of a wide variety of diseases have been assessed and approved by many systematic reviews and meta-analysis studies [12].
For instance, it has been shown that a higher dietary intake and circulating concentration of lycopene have protective effects against prostate cancer (PCa), in a dose-dependent way [13]. The findings approve this effect for the whole tomato, cooked tomato, and sauces consumption [14]. In addition, lycopene’s inhibitory effect on the proliferation and progression of the colorectal cancer cells has been investigated [15]. Moreover, the protective role of lycopene in metabolic syndrome has been well-studied. It seems that different metabolic syndrome components can be improved by lycopene supplementation “rather than demonstrating consistent improvement in a single component” [16]. Lycopene can be used in different CVDs, too [17]. It can improve ventricular remodeling, and vascular and endothelial function. In addition, it is useful in the reduction in atherosclerotic plaque size, and arterial stiffness. Therefore, it can be concluded that this nutraceutical has a vital role in the primary and secondary prevention of CVDs [3]. In addition, lycopene is a natural neuroprotective agent. It seems that this carotenoid contributes to cognitive longevity [18] and the treatment of several neuronal diseases, including cerebral ischemia, Parkinson’s disease (PD), Alzheimer’s disease (AD), subarachnoid haemorrhage, epilepsy, Huntington’s disease, and depression [19]. There are several other clinical applications for this available phytochemical. It can be used in different skin [20], oral, and dental diseases, too [21]. This paper aims to make a comprehensive review on lycopene, from its sources and uses amongst different societies, to its biochemistry and its different biological effects. Furthermore, its protective effect against various toxins, its recommended dose, and toxicity and side effects were discussed separately.
2. Natural Sources of Lycopene and Its Use Amongst Different Societies
Tomato and tomato-based products are the major dietary sources of lycopene and account for approximately 80% of the consumption of lycopene in western countries. It is also present in a high amount in watermelon, guava, pink grapefruit, rosehips, papaya, and apricot [22]. Table 1 shows its amount in different sources [23,24,25]. Lycopene contents significantly differ in diverse varieties of tomato and other fruits and vegetables. Its content depends on several factors: 1, the degree of maturity; 2, the weather temperature (lycopene is transformed to beta-carotene at temperatures higher than 35 °C); and 3, the soil quality (conditioners including necessary microorganisms may cause lycopene content to be increased about 36%) [26].
Table 1.
Lycopene content in different sources.
| Food Sources | Contents (mg/100 g) |
|---|---|
| Fresh tomatoes | 0.72–4.2 |
| Cooked tomatoes | 3.70 |
| Tomato sauce | 6.20 |
| Tomato paste | 5.40–150 |
| Ketchup | 9.90–13.44 |
| Pumpkin | 0.38–0.46 |
| Sweet potato | 0.02–0.11 |
| Pink grapefruit | 0.35–3.36 |
| Carrot | 0.65–0.78 |
| Pink guava | 5.23–5.5 |
| Watermelon | 2.30–7.20 |
| Apricot | 0.01–0.05 |
| Papaya | 0.11–5.3 |
| Rosehip | 0.68–0.71 |
Lycopene intake is also varied among different people in different regions [25]. In the population of European countries, lycopene consumption from natural sources is varied from approximately 0.5 to 5 mg/day. Nevertheless, variation in lycopene consumption was reported, where adults in Spain consume less lycopene (1.64 mg/day) compared to adults from France, and the UK, where the intake was from 4.43 to 5.01 mg/day [27,28]. In the United States, daily intake is more than 7 mg/day [29]. Higher utilization of fruits and vegetables, especially tomato-based products, may result in occasional intakes of 20 mg lycopene/day. Approximately 50–65% of the total exposure to lycopene was produced from natural sources. Some of the important daily sources of lycopene are tomatoes, soups, pasta dishes, tomato sauces and ketchup [30].
3. Biochemistry of Lycopene
In nature, there are more than 600 carotenoids which are mostly colored, produced by plants, fungi and bacteria. Carotenoids have two main groups: (i) the highly unsaturated hydrocarbons (α, β-, and γ-carotene, lycopene), (ii) xanthophylls (lutein, β-cryptoxanthin, and zeaxanthin). Xanthophylls possess a minimum of an oxygenated group on their end rings, while unsaturated hydrocarbon carotenoids contain just carbon and hydrogen atoms with no oxygen [31]. Modified carotenoid structures, such as the cyclization of terminal groups and inserting oxygen, are linked with diverse forms of carotenoids resulting in color alterations and also various antioxidant properties [32].
Lycopene (C40H56), as a hydrocarbon carotenoid, includes an acyclic open chain structure containing 13 double bonds that are subjected to isomerization and differed cis isomers, such as 5, 9, 13, and 15 observed in plants and blood plasma [33]. Naturally, lycopene can be found in all the trans isomers, but the cis isomers are the most typical type in tissue and plasma [34]. This takes place during food pretreatment, preparation, processing, storage and transportation, as well as during metabolism in the body [35]. The conversion of cis to trans isomerization takes place in enterocytes, liver and stomach [36,37]. The absorption of lycopene in the intestine is aided by scavenger receptor CD36 and B1 [38,39]. Partial metabolism can be seen in the enterocyte assisted by two enzymes: 15′-oxygenase-1, β-carotene 15, which is linked with blood lycopene level, and 10′ oxygenase-2, β-carotene-9 [40,41].
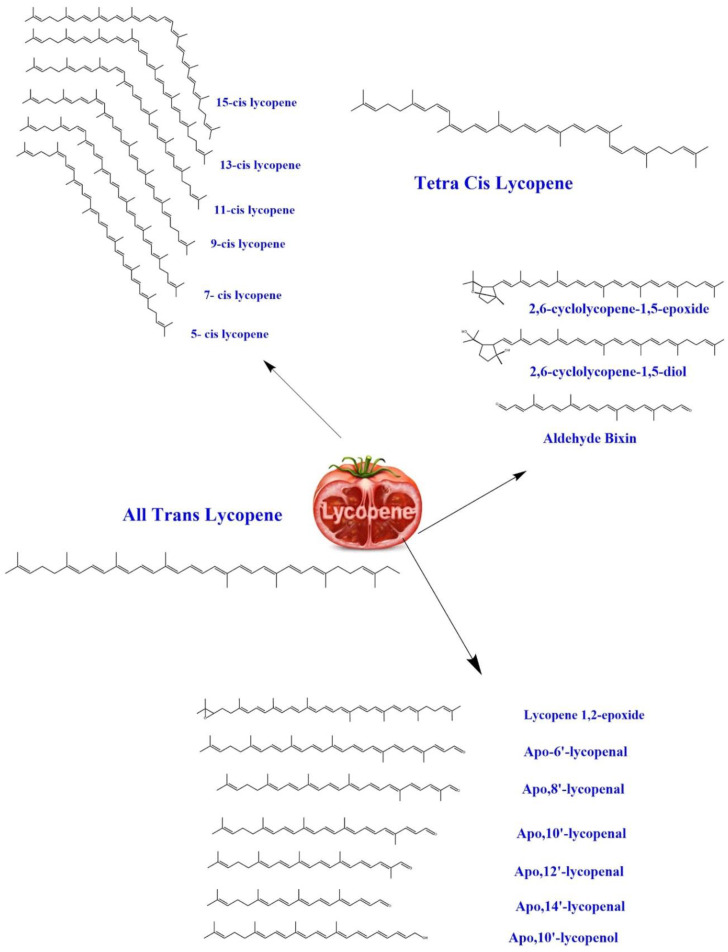
Furthermore, there is limited research on labelled lycopene molecules. For instance, a study conducted by Ross et al. on 14C-labelled lycopene (92% trans-lycopene) revealed that the trans-lycopene was completely isomerized (5-cis, 9-cis, 13-cis, and 15-cis lycopene isomers) following dosing and quickly metabolized into polar metabolites entered in the urine [42]. The quick exclusion of 14CO2 indicated sufficient oxidization of the ingested lycopene. In the compartmental model research, 13C-labelled lycopene was used. No significant differences were found in the bioavailability of cis- and trans-lycopene (24.5 vs. 23.2%, respectively). Moreover, it was confirmed that post-absorptive trans-to-cis isomerization affects tissue and plasma isomeric profiles [43]. The most thermodynamical lycopene firm configuration has shown the all-trans-isomer. Light, heat, or many chemical reactions may cause isomerization from the trans-isomer toward several mono- or poly-cis types, of which two types are non-conjugated. In comparison, 11 types are conjugated double bonds, forming a chromophore involved in visible ruby color, as well as lycopene antioxidant activities [23]. Figure 1 shows the molecular structures of lycopene and its derivatives and isomers.
Figure 1.
The molecular structures of lycopene and its derivatives and isomers.
4. Biological Effects
There is a wide variety of pharmacological actions and clinical applications which have been attributed to the lycopene by a chain of researches worldwide. Table 2 summarizes these insights by discussing different mechanisms of action briefly.
Table 2.
Various mechanisms for lycopene’s biological effects.
| Biological Effect | Mechanisms of Action | References |
|---|---|---|
| Anticancer | Reduced nuclear factor-kappa B (NF-κB) expression, serum level of oxidative stress marker malondialdehyde (MDA) Improved nuclear factor erythroid 2 expressions Reduced the expression of signal transducer and activator of transcription 3 (STAT3) Induced the protein inhibitor of activated STAT3 expression |
[44,45] |
| Induced cell cycle arrest and modified the potential of mitochondrial membrane, DNA fragmentation | [46,47] | |
| Treatment of HGC-27 cells with lycopene exhibited significant improvement in LC3-I, Phosphorylated Extracellular Signal-Regulated Kinase (p-ERK) proteins expressions | [48] | |
| Reduced NADPH oxidase (NOX) 4 activity Lowered invasion, migration, and adhesion, NOX activity, matrix metalloproteinase (MMP)-9, MMP-2 activities and NOX4 protein expression |
[49] | |
| Reduced metastatic load and inhibited cancer antigen 125 (CA125) expression Down-regulated expression of Integrin beta-1 (ITGB1), MMP9, Integrin alpha-5 (ITGA5), Focal Adhesion Kinase (FAK), integrin-linked kinase (ILK) and EMT markers Decreased activity of mitogen-activated protein kinase (MAPK) and inhibited integrin α5 protein expression |
[50] | |
| Antidiabetic | Lowered MDA levels, and increased antioxidant enzyme activities | [51] |
| Decreased the glycated haemoglobin (HbA1c) and C-reactive protein (CRP) | [52,53] | |
| Enhanced antioxidant enzymes activities (i.e., superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), glutathione-S-transferase) | [54] | |
| Lowered the expression of BCL2-associated X protein (BAX), but improved B-cell lymphoma-extra large (Bcl-xL) levels and B-cell lymphoma 2 (Bcl-2) | [55] | |
| Downregulated RAGE expression and declined NF-κB and MMP-2 expressions | [56] | |
| Reduced levels of serum nitrate-nitrite level | [57] | |
| Cardioprotective | Decreased low-density lipoprotein-cholesterol (LDL), total cholesterol (TC), and thiobarbituric acid-reacting substances | [58] |
| Lowered very-low-density lipoprotein-cholesterol (VLDL), triglycerides (TG) and increased high-density lipoprotein-cholesterol (HDL) level | [59] | |
| Decreased inflammatory factors (CRP, interleukin (IL)-6), pulse wave velocity, adhesion molecules and endothelial function | [60] | |
| Lowered the expression of Rho-associated protein kinase (ROCK)1, Ki-67, intercellular adhesion molecule-1 (ICAM-1) and ROCK2 Improved the expression of endothelial nitric oxide synthase (eNOS) implanted arteries and cGMP plasma concentration | [61] | |
| Antioxidant | Increased CAT, glutathione (GSH) and SOD activities and mRNAs antioxidant enzyme Reduced age-related neuroinflammatory disorders by decreasing microgliosis (Ionized calcium-binding adaptor protein-1 (IBA-1)) Down regulate the accumulation of amyloid beta (Aβ) 1-42 in the old CD-1 mice’s brain. |
[62] |
| Decreased Aβ accumulation, amyloid precursor protein (APP) Reduced the expression of neuronal β-secretase (BACE)1 Improved the expressions of α-secretase A Disintegrin And Metalloproteinase (ADAM)10 Suppressed IBA-1 (a marker of microglial activation) expression, inflammatory mediators Inhibited NF-κB, phosphorylation of MAPKs and Nuclear factor erythroid 2-related factor 2 (Nrf2) activity |
[63] | |
| Decreased myeloperoxidase (MPO) activity, tumor necrosis factor-alpha (TNF-α) Down-regulated gene expression of inducible nitric oxide synthase (iNOS) | [64] | |
| Decreased inflammatory cytokines Improved antioxidant enzymes NAD(P)H: quinone oxidoreductase (NQO1) and heme oxygenase-1 (HO-1) mRNA expressions Downregulated inflammatory cytokines TNF-α and IL-1β Improved the glial cells inflammatory markers glial fibrillary acidic protein (GFAP) and IBA-1 expression |
[65] | |
| Antiinflammatory | Up-regulated the HO-1 mRNA Gene expression suppression of the TNF-α and lipopolysaccharide (LPS)-stimulated cyclooxygenase-2 (COX-2), nitric oxide synthase 2 (NOS2) |
[66] |
| Lowered the IL-1β, TNF-α, IL-6β serum level and increased the NF-κB p65 mRNA, Toll-like receptor 4 (TLR4) and protein expressions | [67] | |
| Inhibited COX-2, iNOS and NF-κB Reduced the leukocytes migration |
[68] | |
| Hepatoprotective | Lowered aspartate aminotransferase, alanine aminotransferase, free fatty acid, MDA and LDL Increased the SOD, GSH condensation Down-regulated the expression of Cytochrome P450 2E1 (CYP2E1), TNF-α |
[69] |
| Enhanced the S-adenosyl-homocysteine hydrolase, hepatic cystathionine beta-synthase activities | [70] | |
| Reduced nitric oxide (NO) and MDA levels | [71] | |
| Against dermatologic diseases | Decreased Ultraviolet (UV) B-induced cell growth and increased apoptosis Prevented from Forkhead box O3 (FOXO3) phosphorylation when exposing to UVB radiation and cytoplasm sequestering Decreased cyclin-dependent kinase 4 (CDK4) and CDK2 Increased in the expression of Poly (ADP-ribose) polymerase (PARP), BAX and cell apoptosis Inhibited UVA1- and UVA/B-induced upregulation of HO-1 |
[72] |
| Neuroprotective | Suppressing 4 aminopyridines (4-AP) evoked glutamate release and elevated intrasynaptosomal Ca2+ level Suppressing release of 4-AP-evoked glutamate |
[73] |
| Improved mitochondrial enzymatic activities, kindling score, oxidative stress | [74] | |
| Decreased impairment in biochemical, behavioral, neuroinflammatory and neurochemical markers | [75] | |
| Boneprotective | Osteoblasts differentiation improved | [76] |
| Modified the biomarkers of serum osteocalcin, bone metabolism, crosslinked carboxyterminal telopeptides and N-terminal propeptide of type 1 collagen in serum Downregulated the osteoclast differentiation concurrent Up-regulated the osteoblasts alongside GPx, SOD, and CAT activities |
[77] | |
| Targeting reproductive disorders | Reduced lipid peroxidation and sperm DNA fragmentation | [78] |
| Improved the sperm motility and sperm count Improved mitochondrial enzymatic activity, i.e., CAT, SOD, glucocorticoid receptor (GR), GPx and alcohol dehydrogenase (ADH)) and non-enzymatic antioxidant level (GSH and ascorbate) |
[79] |
4.1. Anticancer
Inflammation is known as one of the most important key points in cancer. Therefore, lycopene, as one of the most potent antiinflammatory nutraceuticals, is under research in many preclinical and clinical cancer studies. Epidemiological studies showed a reverse relation between its serum level and cancer occurrence [80]. In addition, increased consumption of lycopene (from each source) has been reported to be associated with a decreased risk of a wide variety of cancers, such as breast, lung, ovary, prostate, stomach, and ovary [81].
In laying hens, supplementation (200 to 400 mg lycopene/kg diet) significantly reduced ovarian tumor occurrence, and also the tumors’ size and number. Moreover, supplementation with lycopene reduced the STAT3 expression in ovarian tissues via the induction of the protein inhibitor of activated STAT3 expression [44,45]. The lycopene extract (dose: 400 and 800 μg/mL) reduced the risk of human breast adenocarcinoma cells (MCF-7). It could modify the mitochondrial membrane potential, DNA fragmentation and also affected the granularity and size of the MCF-7 cell. Additionally, it does not result in noticeable damage to the cell membrane, necrosis and regular apoptosis [46,47]. Treatment of HGC-27 cells with lycopene exhibited significant improvement in LC3-I, p-ERK proteins expressions [48].
Moreover, in vitro and in vivo investigations exhibited that lycopene (dose: 0.1–5 μM) affected human liver adenocarcinoma (SK-Hep-1) cells and indicated a substantial reduction in NOX activity. Moreover, it inhibits the protein expression of NOX4, NOX4 mRNA and ROS intracellular amounts. The transforming growth factor-β (TGF-β)-related effects were antagonized via the lycopene (2.5 μM) incubation with SK-Hep-1 cells. Through small interfering RNA (siRNA) transient transfection against NOX4, the results reveal that NOX4 downregulation could mimic lycopene via the inhibition of cell migration and MMP-9 and MMP-2 activities in incubating with/without TGF-β on SK-Hep-1 cells [49].
In another study, lycopene consumption markedly decreased the metastatic load in a tumor-bearing rat model of ovarian carcinoma. Lycopene has anti-tumorigenic effects on paclitaxel and carboplatin. Its consumption further inhibits biomarkers for ovarian cancer, such as CA125 expression. The anti-proliferative and anti-metastatic impacts were complemented via ITGB1, MMP9, ITGA5, FAK, ILK and EMT markers’ down-regulated expression, decreasing the MAPK activity and inhibiting integrin α5 protein expression [50].
FOXO3a is crucial to modify cell death genes expression. Treatment with lycopene reduced cell hyperproliferation induced by UVB and ultimately promoted apoptosis and reduced CDK2 and CDK4 complex in SKH-1 hairless mice and human keratinocytes. It is sequestered in cytoplasm and can phosphorylate against UVB irradiation, although pretreatment with lycopene reduced all these activities. FOXO3a gene ablation reduced lycopene-caused attenuation in cell hyper-proliferation, CDK2, and CDK4 complex. FOXO3a siRNA transfection prevents lycopene-related elevation in cell apoptosis, cleaved PARP expression and BAX. Furthermore, protein kinase B (AKT) loss can induce more lycopene-caused FOXO3a dephosphorylation, whereas losing the mechanistic target of rapamycin complex 2 (mTORC2) via transfection with a rapamycin-insensitive companion of mammalian target of rapamycin (RICTOR) siRNA induces AKT phosphorylation to amounts like the levels gained by lycopene. On the other hand, AKT/mTORC2 overexpression decreases the lycopene impact on the FOXO3a expression and AKT phosphorylation, indicating the link between lycopene and the mTORC2/AKT signaling negative modulation [82]. Interestingly, lycopene treatment for human breast carcinoma cell line MCF-7 in vitro, leading to cell shrinkage and breakage, is dependent on the dose and time. Moreover, lycopene treatment up-regulates Bax mRNAs and p53 expression [83].
Lycopene doses of 0, 10, 20, and 30 µM were used to treat human colorectal cancer cells. Lycopene’s effect on cell proliferation was evaluated through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method. Prostaglandin E2 (PGE2), and NO levels declined after lycopene administration [84].
Treatment of human PCa cells was done with lycopene extracts (5 mg/mL) and indicated a significant drop in cell viability and apoptotic cell population [85]. In addition, lycopene nanoparticles (in lipid-based wall material in MCF-7 cells), in a time- and concentration-dependent fashion, significantly reduced cell viability and cell survival [86]. Regarding human trails, in head and neck squamous cell carcinoma, a lycopene concentration of >10 µM for a period of 24 h prevents Cal27 and FaDu cell growth, dependent on the dose and time. Lycopene doses of 25 µM decrease invasion abilities and have significant inhibitory effects. Moreover, lycopene consumption up-regulates B cell lymphoma linked with X protein, a pro-apoptotic protein, and results in inhibiting mitogen-activated and B-protein kinase signaling route [87,88].
In another study on PCa, lycopene, or its metabolite (i.e., apo-10-lycopene), enhanced β, β-carotene-9′, 10′-oxygenase 2 (BCO2) expression, while decreasing cell proliferation in androgen-sensitive cells. However, lycopene cannot modify BCO2 expression and cell development in androgen-resistant cells. The mutant (enzymatically inactive)/wild-type BCO2 exogenous expression in PCa cells decreased NF-κB function, DNA binding and reduced NF-κB translocation in the nucleus [89]. In hepatocellular carcinoma, lycopene supplementation (lycopene dose: 6.6 mg/kg BW/day) inhibited NNK-related α7 nicotinic acetylcholine receptor expression (lung), NF-κB and CYP2E1 (liver) and reduced NNK-related death and pathological abrasions in ferrets’ livers and lungs [90]. However, cell invasion, proliferation and migration showed no alteration in a dose-dependent fashion. In another research, lycopene intake is protective against PCa in 46,719 men. It seems that this effect is via decreasing the gene fusion transmembrane protease, serine 2 (TMPRSS2): v-ets avian erythroblastosis virus E26 oncogene homolog (ERG) [91].
4.2. Antidiabetic
There is scientific evidence which supports the beneficial role of lycopene against diabetes. Regarding animal studies and epidemiological surveys, it can be used for both the prevention and treatment of diabetes [92]. In a diabetic rat model (streptozotocin (STZ)-induced), lycopene decreased diabetes-associated pancreas injury and urine and blood glucose levels. In addition, it increased serum insulin levels [93]. The interactions between advanced glycation end products (AGEs) and their receptors (RAGEs) induce oxidative stress-like conditions. Moreover, in vitro and in vivo investigations revealed that lycopene slows down ribose-related forming of AGE in HK-2 cells, while in vivo studies showed that in kidneys, it down-regulates RAGE expression. HK-2 cells with reduced amounts of RAGE indicated reduced NF-κB and MMP 2 expressions [56]. Lycopene supplementation significantly reduced serum nitrate–nitrite levels in diabetic Wistar rats [57].
In addition, lycopene exhibited some effects on histological alterations and also Bcl-2 family gene expression in the STZ-induced diabetic rats’ hippocampus. Lycopene (dose: 4 mg/day/kg) decreased the Bax expression. However, it improved Bcl-xL amounts and Bcl-2 [55].
In addition, lycopene considerably controlled high-fat diet (HFD)-related elevation of glucose, insulin level, fasting blood glucose, insulin intolerance and decrease hepatic glycogen level. Lycopene decreases the fatty acid synthase (FAS), sterol regulatory element-binding protein 1c (SREBP-1c), and Acetyl-CoA carboxylase (ACC1) expression in HFD mice. The consumption of lycopene considerably decreases the phosphorylation and STAT3 expression in livers in HFD mice. The adenovirus treatment with lycopene noticeably inhibited a reduction in SREBP-1c expression. Moreover, an increase in STAT3 motioning via adenovirus noticeably blocked a decrease in fasting blood insulin as well as glucose amount [94].
4.3. Cardioprotective
Lycopene is a cardioprotective nutraceutical. Different research showed a protective effect against atherosclerosis and several CVDs [58,95]. It can scavenge some of the potent oxidants that are known to be associated with atherosclerosis. Moreover, lycopene reduces oxidation of cholesterols. Therefore, the early steps in atherosclerosis are prohibited [96]. In hypercholesteremic rats, lycopene administration (50 mg/kg) significantly decreased low-density lipoprotein-cholesterol (LDL), total cholesterol (TC), thiobarbituric acid reactive substances (TBARS), very low-density lipoprotein-cholesterol (VLDL), triglycerides (TG), and increased high-density lipoprotein-cholesterol (HDL) level [59,97]. Furthermore, lycopene was studied for its positive health benefits on some important inflammatory factors (e.g., CRP, IL-6), lowering blood pressure, pulse wave velocity, adhesion molecules (ICAM-1) and endothelial function (flow-mediated dilation) [60]. Moreover, lycopene was found to protect grafted vessels by regulating the expression of key proteins which are essential in arteriosclerosis. It remarkably lessened the expression of ROCK1, Ki-67, ICAM-1 and ROCK2, whereas it improved the expression of eNOS-implanted arteries and cGMP plasma concentration. In summary, lycopene is able to improve the vascular arteriosclerosis in the case of allograft transplantation by downregulating the expression of Rho-related kinases and by regulation of the NO/cGMP pathways expression, as well [61].
Importantly, lycopene consumption protects the heart from Atrazine (ATZ) exposure and also prevents ATZ-induced damage [98]. The results reveal that oral intake of lycopene also prevents myocardial ischemia-reperfusion (I/R) injury. Lycopene use (1 μM) prior to the incidence of re-oxygenation significantly prevents hypoxia/reoxygenation (H/R)-induced cardiomyocyte death. Lycopene (1 μM, intravenous) considerably inhibited myocardial infarction (MI), production of ROS and c-Jun N-terminal Kinase (JNK) phosphorylation in I/R mice [7,99]. Moreover, lycopene has a protective effect against the apoptosis mediated by endoplasmic reticulum stress (ERS), which is due to H/R in H9C2 cardiomyocytes. In I/R myocardium, lycopene dose (10 μM) diminished the lactic dehydrogenase, apoptosis ratio and protein expression of 78 kDa glucose-regulated protein (GRP78). It can be associated with phosphorylated JNK (pJNK), C/EBP homologous protein (CHOP) and Caspase-12 routes [100].
It also improved ERS caused by apoptosis that can be observed via decreasing the expression of CHOP/growth arrest and DNA damage-inducible gene (GADD153), Bax/Bcl-2, caspase-3, caspase-12 activity in H/R-treated cardiomyocytes. It was also reported that lycopene is capable of preventing thapsigargin (THG)-related ERS through a reduction in the GRP78 and CHOP/GADD153 protein expression in the THG group, considerably enhancing cell viability which is caused by THG. It also inhibited apoptosis in THG-treated cardiomyocytes [35,101,102]. Another study reported that lycopene (dose: 10 mg/day/kg) in mice with post-MI ventricular remodeling notably reduced the expression of TGF-β1, collagen III, collagen I, IL-1β, TNF-α, caspase-3, caspase-8 and caspase-9, and reduced NF-κB activity. The administration of lycopene also decreased the level of ventricular remodeling post-MI [103]. Moreover, lycopene supplementation improves the endothelial function in patients with CVD diseases [104].
4.4. Antioxidative
Lycopene is a well-known antioxidant. It can protect DNA, proteins, and lipids against oxidation. In addition, “lycopene can act on other free radicals such as hydrogen peroxide, nitrogen dioxide and hydroxyl radicals” [105].
Oxidative stress, as well as inflammation, is essential in acute pancreatitis (AP) pathogenesis. Lycopene (50 mg/kg) significantly prevented AP by a substantial decrease in MPO activity, TNF-α and down-regulating iNOS gene expression along with decreasing NO level, increasing pancreatic glutathione (GSH), decreasing serum α-amylase and lipase functions in Wistar rats [64]. Fluorosis can induce oxidative stress by activating MAPK cascade that can cause cell apoptosis. The combination of vitamin E and lycopene prevented fluoride-induced spermatogenic cell apoptosis in rats. Both decreased the expression of rescued clustering and toxicity induced by fluorosis. As well as this, it also decreased the improved JNK and also phosphorylation of the ERK [106].
Another study showed the lycopene role via down-regulating the expression rate of the proprotein convertase subtilisin/kexin type-9 (PCSK-9) by inhibiting hepatocyte nuclear factor-1α, improving LDL-receptor, and sterol regulatory element-binding protein-2. Lycopene reduces Apo-CIII to assemble with lipoprotein lipase (LPL) bonding, too. Likewise, lycopene improved LPS-induced oxidative stress by increasing total antioxidant and HDL-related PON-1 function as well as down-regulating the plasma level and inflammatory mediators expression [107].
Interestingly, lycopene intake (50 mg/kg BW/day) improved d-galactose in CD-1 male mice having cognitive defects. It also improved histopathological injury and repaired brain-derived neurotrophic factor (BDNF) amounts in mice’s hippocampal area. Lycopene considerably increased antioxidant enzymatic activity and decreased inflammatory cytokines in d-galactose-administrated mice serum. Furthermore, lycopene supplementation improves mRNA expressions of the NQO-1 and HO-1 as antioxidant enzymes. It was also found to downregulate inflammatory cytokines (i.e., TNF-α, and IL-1β) in the hippocampus of the mice. Lycopene noticeably improves the GFAP and Iba-1 expression as the glial cells’ inflammatory makers. Lycopene decreased neuronal oxidative damage by activating Nrf2, as well as by inactivating NF-κB translocation in H2O2-related SH-SY5Y cell model [65]. Moreover, in a rat model of colitis, it significantly reduced the malondialdehyde (MDA), overall sialic acid, DNA fragmentation concentration, and improved the function of the antioxidant enzyme [108]. In addition, lycopene has significant effects against pathological findings caused by carbofuran on oxidative and biochemical stress biomarkers. It (18 mg/kg BW) markedly improved the serum acetylcholinesterase, albumin, protein and lipids. Furthermore, it improved the catalase (CAT), superoxide dismutase (SOD), and GSH levels, and antioxidant capacity [109].
Lycopene administration considerably improved cognitive defects, noticeably reduced MDA levels and elevated GSH-Px activity, and remarkably reduced tau (or τ proteins) hyperphosphorylation at Thr231/Ser235, Ser262, and Ser396 in P301L transgenic mice’s brains [109] (104). In a study conducted on an animal model, in which male mice (C57BL/6) were orally administrated (lycopene dose; 10 or 100 mg/kg), the results reveal that lycopene significantly reduced ROS synthesis in SK-Hep-1 cells while inhibiting NADPH oxidase, which was carried by the protein kinase C (PKC) pathway. Lycopene was also found to be effective against hepatotoxicity by acting as an antioxidant, regulating total glutathione (tGSH) and CAT concentrations, reducing glutathione disulfide (GSSG) and oxidative damage via reducing protein carbonylation. It also elevated MMP-2 down-regulation [110]. Regarding regulator of calcineurin 1 (RCAN1), lycopene reduced mitochondrial ROS and intracellular concentrations, NF-κB activity, Nucling expression, while decreasing the respiration per mitochondrion, MMP and glycolytic activity in RCAN1-overexpressing cells. It suppressed cell death, caspase-3 activation, DNA fragmentation, and cytochrome-c secretion in RCAN1-overexpressing cell lines [111].
The antioxidative role of lycopene was tested in the field of nephrology, also. In gentamicin-induced nephrotoxicity, intake of lycopene and rosmarinic acid could reduce the elevated level of blood urea N2, serum creatinine, renal MDA and immune expression autophagic marker protein (LC3/B), proapoptotic protein (Bax), autophagic marker protein (LC3/B), iNOS and autophagic marker protein (LC3/B). They also increased lower SOD level, GSH, GPx, and antiapoptotic protein (Bcl-2) immunoexpression [112]. In addition, previous researchers found that lycopene (10 mg/kg) attenuated fluoride toxicity via decreasing caspase-3 and caspase-9, and Bax gene expression functions, while improving the activities of GPX and SOD and Bcl-2 gene expression in rats. Ferrous ascorbate administration decreased spermatozoa mobility, viability and spermatozoa antioxidant capacity. It enhanced superoxide production, ROS generation and lipid peroxidation (ferrous ascorbate (FeAA)). On the other hand, the administration of lycopene prevented these changes and has significant antioxidant properties and ROS-scavenging activity in the case of male reproductive cells [113].
It is also able to reduce Aβ-induced oxidative stress due to the lower production of intracellular ROS and superoxide obtained by mitochondria. Moreover, it showed improved Aβ-related mitochondrial morphological modifications, releasing cytochrome-c and opening of mitochondrial transition pores. Lycopene was also found to be effective in restoring ATP in Aβ-treated neurons and improved mitochondrial complex activities. In vivo studies also revealed that in mitochondria, lycopene significantly prevented DNA damage and enhanced transcription factor A level [114].
4.5. Anti-Inflammatory Activity
Lycopene not only quenches singlet ROS, but also prevents lipid peroxidation, too. It has been shown that lycopene (50 and 100 μM) upregulated the HO-1 mRNA. However, it cannot regulate the NOS2 mRNA and COX-2 expression. Such concentrations also suppressed the LPS-stimulated COX-2, NOS2 and TNF-α gene expression in RAW264.7 cells [66]. Moreover, lycopene has a protective role in β-amyloid-induced inflammation. β-amyloid increased the serum IL-1β, TNF-α, IL-6β levels, and upregulated the expressions of NF-κB p65 mRNA, TLR4 and protein at the choroid plexus. Lycopene supplementation decreased the inflammatory cytokines and reversed the Aβ1-42-related expression and up-regulation of NF-κB p65 mRNA, TLR4 and protein at the choroid plexus [67].
Lycopene supplementation (12.5 mg/kg BW) in carrageenan-induced inflammation considerably decreased edema in Swiss mice via various phlogistic compounds and immunostaining for COX-2, iNOS and NF-κB. It also decreased the migration of leukocytes in paw tissue and peritoneal cavity, lowered the concentration of MPO, while increasing the GSH levels [68]. Furthermore, lycopene has been investigated in different doses (i.e., 0.5, 1.0, 2.0, 4.0, 8.0, 10.0 and 25 μM) for the prevention of cigarette smoking-induced inflammation. Lycopene inhibited the increase in interferon-γ, TNF-α and interleukin-10 concentrations [115]. In a recent study, induction of endotoxin-induced uveitis was performed in Sprague–Dawley rats by LPS single injection (200 μg). The lycopene intraperitoneal administration (10 mg/kg) noticeably lowered the elevated levels of infiltrating cell number, total protein concentration, and NO, TNF-α, and IL-6 induced via LPS [116].
Lycopene is also effective against the treatment of mitochondrial dysfunctioning caused by Aβ1-42 accompanied by TGF-β, increased proinflammatory cytokines, TNF-α and IL-1β and also NF-κB and caspase-3 function in the brain of rats [117]. Lycopene from tomato juice significantly lowered the ICAM-1, and vascular cell adhesion molecule 1 (VCAM-1) level [118]. Lycopene also protects rats against acute lung injury due to LPS by reducing the IL-6 and TNF-α level, suppressing MAPK activity and NF-κB transcription factor. As a result, normal metabolism is retrieved [119]. In LPS-challenge inflammation and depression, lycopene treatment (60 mg/kg/day, orally) for 7 days and also an intraperitoneal injection of LPS (1 mg/kg) was effective. In addition, lycopene improved neuronal cell damage in the hippocampal CA1 region. It reduced HO-1 and IL-1β LPS-related expression in the hippocampus as well as reducing TNF-α and IL-6 in the plasma [120,121].
In the case of THP1 cells, lycopene (2 μM for 2 h) strengthened pro-inflammatory gene expression. The effectiveness of lycopene dose is associated with using a pro-inflammatory stimulus (i.e., LPS, PMA or TNF). At higher concentrations (>2 Μm), lycopene improved metalloprotease secretion, which is a c-AMP-dependent process, by reducing the production of ROS. Cell culture media, PMA-treated monocytes and moved on CaCo-2 epithelial cells could induce proinflammation in these cells. Such inflammation was overcome by treating the cells with lycopene within 12 h. At a lower level of lycopene (<2 μM), it promotes an inflammatory condition not associated with the modulation of ROS. When the concentration of lycopene increases (5 to 20 μM), it was noted that ROS production decreases and consequently, an antiinflammatory effect was produced [122].
4.6. Hepatoprotective
Functional mitochondria perturbation is related to fulminant hepatic failure. Recently, in a study conducted on animal models, d-GalN/LPS (dose: 300 mg/kg and 30 μg/kg BW) induced several instabilities in mitochondrial functioning by increasing H2O2 and lipid peroxide levels, decreasing the antioxidant activities of mitochondria, disturbing enzymatic functions of the electron transport chain, tricarboxylic acid cycle and adenosine triphosphate content in cells. Lycopene (10 mg/kg BW, through 6 days) reduced lipid peroxidation and limited excessive H2O2 production. Lycopene consumption also regulated d-GalN/LPS-related disturbance in ATP synthesis and elevated enzymatic functions [123]. In addition, pre-treatment with lycopene (5, 10, and 20 mg/kg) has a protective role in a rat model of non-alcoholic fatty liver disease through lowering the liver enzymes levels, like aspartate transaminase (AST), alanine transaminase (ALT), LDL, free fatty acid, and MDA. Furthermore, its role was conducted via an increase in the SOD and GSH concentrations in liver tissue, down-regulating the CYP2E1 and TNF-α expression and reducing the penetration of liver fats [69]. In another study by Yefsah-Idres et al. (2016), it was shown that lycopene administration ameliorated liver injury. It decreased the levels of ALT, serum homocysteine, and AST. In addition, its hepatoprotective effect was performed by enhancing the S-adenosyl-homocysteine hydrolase, hepatic cystathionine beta-synthase activities and decreasing the level of hepatic MDA [70]. Moreover, tomato powder has been shown to have a protective agent against alcohol-induced hepatic injury by inducing cytochrome p450 2E1 [80]. The lycopene treatment considerably improved liver functioning in case of rats with bile duct ligation (BDL). It reduced NO and MDA levels and improved reduced enzymatic level (i.e., CAT, GSTs, GSH, and SOD) in the BDL rat. In addition, lycopene decreased DNA damage [71].
The efficacy of lycopene (40 mg/kg) with proanthocyanidins (450 mg/kg) against mercuric chloride-induced hepatotoxicity was assessed in an animal model. It has been revealed that they inhibited ROS production, protected antioxidant enzymes, and reversed hepatotoxicity in rats’ liver [124]. In another study, carbon tetrachloride-caused hepatotoxicity was done in male Wistar rats via intraperitoneal injection (dose; 0.1 mL/kg BW, 14 days). Then, the Portulaca oleracea aqueous extract with lycopene (dose; 50 mg/kg body weight) exhibited a hepatoprotective effect against carbon tetrachloride-induced hepatotoxicity. The results also reveal that lycopene significantly restores serum enzyme level to the normal amount in rats [125].
In a study on methotrexate-induced liver injury (20 mg/kg), histopathologic examination of the rats showed that inflammatory cell infiltration, sinusoidal dilatation and congestion were improved significantly by lycopene consumption (10 mg/kg). According to the results, IL-1β, and TNF-α concentrations in the liver tissue were reduced significantly compared to the control group. At the same time, a reduction in oxidative stress index (OSI) was non-significant [126]. In another study on acetaminophen-induced liver damage in C57BL/6 mice, lycopene treatment improved redox imbalance, decreased IL-1β expression, thiobarbituric acid reactive species level and MMP-2 activity [127]. Another study on the hepatoprotective role of lycopene focused on ATZ-induced hepatotoxicity. ATZ (50 and 200 mg/kg) induced hepatotoxicity, whereas lycopene (5 mg/kg) significantly reduced the total cytochrome b5 (Cyt b5) and CYP450 contents. In addition, it prevented from CYP450s stimulation activity (erythromycin N-demethylase (ERND), aniline-4-hydroxylase (AH), aminopyrine N-demethylase (APND), and NADPH-cytochrome c reductase (NCR)) in microsomes of the liver. Lycopene administration effectively modulates CYP450s activities and contents and also normalizes the four CYP450s genes expression, including CYP2a4, CYP2E1, CYP1b1, and 4A14 [128,129].
Furthermore, lycopene (10 mg/kg BW/day, intraperitoneal administration) markedly prevented oxidative damage in experimental hepatitis due to d-galactosamine/LPS. It improved the enzymatic antioxidants levels (i.e., CAT, GPx, SOD, and GSTs) as well as non-enzymatic antioxidants (i.e., vitamin C and E, and GHS). Moreover, lycopene reduced the high level of lipid peroxides and decreased the DNA strand breaks [130]. Additionally, d-Gal/LPS could induce hepatitis in experimental rats, and lycopene significantly reduced the lipid metabolizing enzymatic activity (i.e., LPL, lecithin-cholesterol acyltransferase (LCAT), hepatic triglyceride lipase (HTGL), and increase in the HDL level [131]).
4.7. Against Dermatologic Diseases
Treatment with lycopene decreased UVB-caused cell proliferation while increasing apoptosis via declining CDK2 and CDK4 in hairless SKH-1 mice and human keratinocytes [82]. Moreover, the study conducted on volunteers revealed that lycopene decreased the expression of UVA1 radiation-inducible genes HO-1 upregulation caused by UVA1 and UVA/B [72].
4.8. Neuroprotective
The lycopene consumption relieved cognitive defects, age-related memory loss, neuronal damage, and synaptic dysfunction of the brain. Furthermore, lycopene consumption considerably reduced age-related neuroinflammatory disorders by decreasing microgliosis (IBA-1), as well as down-regulating inflammatory mediators. In addition, a study revealed that lycopene down-regulates the Aβ1-42 accumulation in the brains of aged CD-1 mice [62]. Similarly, lycopene (0.03% w/w) has been found to inhibit the LPS (0.25 mg/kg)-induced memory loss in 3-month old male C57BL/6J mice by reducing Aβ accumulation, APP, reducing the expression of neuronal β-secretase beta-site amyloid precursor protein cleaving enzyme 1 (BACE1), and improving the α-secretase A disintegrin and metalloproteinase 10 (ADAM10) expressions. In addition, it suppressed IBA-1 expression as the marker of microglia activation, inflammatory mediators and also decreased oxidative stress (among mice treated with LPS). Furthermore, lycopene inhibited NF-κB, MAPKs and Nrf2 activity phosphorylation in BV2 microglial cells (LPS treated) [63].
Lycopene suppressed the 4-AP-invoked release of glutamate and elevated intra-synaptosomal Ca2+ level. The studies revealed that the inhibitory impact of lycopene on 4-AP-evoked glutamate secretion decreased the presence of Cav2.1 (P/Q-type) and Cav2.2 (N-type) channel blocker ω-conotoxin MVIIC noticeably. However, it did not affect the inhibitors of CGP37157 and intracellular Ca2+ release. Moreover, lycopene’s effect on evoked glutamate secretion was protected via PKC inhibitors Go6976 and GF109203X [73]. Wang et al. reported that rats that were fed on HFD with lycopene (4 mg/kg, oral administration) had considerably reduced cognitive defects and improved learning abilities through the prevention of a decrease in dendritic spine density [132]. In another study, lycopene administration (5, 10 mg/kg, oral) significantly decreased impairment in biochemical, behavioral, neuroinflammatory and neurochemical markers in rats with haloperidol-induced orofacial dyskinesia [75].
Lycopene’s neuroprotective effect was also studied for pentylenetetrazol (PTZ)-induced kindling epilepsy in rats treated with lycopene at the concentrations of 2.5, 5, and 10 mg/kg, sodium valproate through 29 days, and 40 mg/kg of PTZ (intraperitoneal) with alternate days. Rats were assessed for various behavioral and biochemical parameters (SOD, lipid peroxidation, CAT, decreased GSH and nitrite) and activities of the enzymes of mitochondria (I, II, and IV) in the brain. Based on the findings, lycopene (5, 10 mg/kg) considerably improved mitochondrial enzymatic activities, kindling score, and oxidative stress as compared to control [74]. The neuroprotective role of lycopene was also studied in a male C57BL/6 mice model of bilateral common carotid artery occlusion (BCCAO). The Nrf2/HO 1 signaling route function via increasing the Nrf2 HO 1 expression was also seen. The results indicate that the Nrf2/HO 1 signaling route is associated with lycopene’s neuroprotective impact [133].
Regarding AD, lycopene considerably prevents paralysis in Aβ1-42-transgenic Caenorhabditis elegans strain GMC101. Treating with lycopene inhibited Aβ1-42 release in SH-SY5Y cells by the overexpression of the Swedish mutant type of human APP (APPsw). Additionally, lycopene can effectively down-regulate APP expression in APPsw cells. Furthermore, treating with lycopene did not affect endogenous ROS amount and apoptosis in case of APPsw cells. It significantly improved the neurological scores in BCCAO mouse. It reduced neuronal apoptosis via TUNEL staining and decreased oxidative stress due to global brain ischemia [134]. Interestingly, it has a neuroprotective effect against PD caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mice. Treating with lycopene (5, 10 and 20 mg/kg/day, oral administration) prevented PD by MPTP in a concentration-dependent fashion. It also reduced MPTP-caused motor abnormalities and oxidative stress [135]. Chronic consumption of lycopene can effectively improve cognitive abilities, decrease mitochondrial-oxidative damage, inhibit neuroinflammation and repair BDNF concentration in β-A1-42-treated rats [136].
The lycopene neuroprotective effect on oxidative stress, as well as neurobehavioral disorders in PD of adult C57BL/6 mice due to rotenone, was studied in a recent study. Lycopene treatment (10 mg/kg BW, oral administration) in the rotenone-treated animals enhanced GSH-Px, SOD and CAT activities and reduced MDA concentrations. It also elevated the number of tyrosine hydroxylase-, enhanced alpha-synuclein (alpha-SYN)- and microtubule-associated protein 3 light chain (LC3-B)-positive neurons [137]. Lycopene provides protection against neurotoxicity through methylmercury (MeHg) induced in cultured rat cerebellar granule neurons (CGNs). Lycopene prevented the loss of cell viability and release of LDH. Lycopene also protected from the inhibition of mitochondrial complexes III and IV enzyme activities and decreased the production of ATP and mtDNA (copy, transcript levels) [138].
There is an in vivo study on rat’s treatment using rotenone (3 mg/kg BW, intraperitoneal) for 30 days. NADH dehydrogenase as a marker of rotenone action was markedly suppressed (35%) in treated animals’ striatum. On the other hand, oral administration of lycopene (10 mg/kg) to the rotenone-treated animals for 30 days elevated the activity by 39% compared to the animals treated with rotenone. Rotenone treatment enhanced the MDA concentrations (75.15%) in the striatum, while lycopene treatment reduced its concentrations by 24.33%. This was associated with cognitive and motor impairments in rotenone-treated animals that were reversed on lycopene administration. Finally, lycopene administration inhibited the cytochrome c release from mitochondria [139].
4.9. Bone Protective
Lycopene has several molecular and cellular effects on human osteoblasts and osteoclasts. It reduced osteoclast differentiation, whereas it did not change cell survival/cell density; calcium-phosphate resorbing was also reduced. In addition, osteoblast proliferation (reduction on apoptosis) and differentiation were improved by lycopene [76,140]. Moreover, lycopene supplementation has an effective role in postmenopausal osteoporosis [80]. Lycopene administration inhibited the ovariectomized (OVX)-related increase in bone turnover, such as modification in biomarkers of serum osteocalcin, serum cross-linked carboxyterminal telopeptides, bone metabolism, serum N-terminal propeptide of type 1 collagen, and urinary deoxypyridinoline. Remarkable upgrading in OVX-related loss of bone mass, microarchitectural deterioration and bone strength was witnessed in lycopene-administrated OVX animals. These observations were more prominent in areas full of trabecular bone and low level of cortical bone. In these six-week-old Sprague–Dawley female rats, the bone mineral density of the tibial proximal metaphysis and lumbar spine increased via lycopene treatment dependent on the dose. Lycopene administration also down-regulated the osteoclast differentiation along with up-regulation of the osteoblast and GPx CAT and SOD activities [77,141]. Daily lycopene consumption reduces the risk of bone resorption in postmenopausal female cases by oxidation prevention [142].
4.10. Targeting Reproductive Disorders
Lycopene can decrease sperm DNA fragmentation, as well as lipid peroxidation by its antioxidant activity in normospermic infertile men [78,105]. It (4 mg/kg/day, orally) improved the sperm count and motility by decreasing H2O2 and lipid peroxidation, and improving mitochondrial enzymatic activity (i.e., CAT, SOD, GR, GPx, and ADH) and non-enzymatic antioxidant level (GSH and ascorbate). In addition, lycopene showed a significant improvement in the testicular mitochondria’s enzymatic activities of tricarboxylic (i.e., isocitrate dehydrogenase, succinate dehydrogenase, fumarase and malate dehydrogenase) [79].
Lycopene (4 mg/kg) ameliorated adriamycin (ADR) (10 mg/kg)-induced reductions in Sprague–Dawley rats’ epididymis and testes weights. The sperm morphology and motility were noticeably normalized by lycopene treatment. While the testosterone amount was reduced in the ADR group, no significant effect was found in the treated group. Interestingly, pretreatment with lycopene significantly reformed MDA and decreased GSH levels. Moreover, ADR-induced histopathological changes were reversed by the lycopene treatment [143].
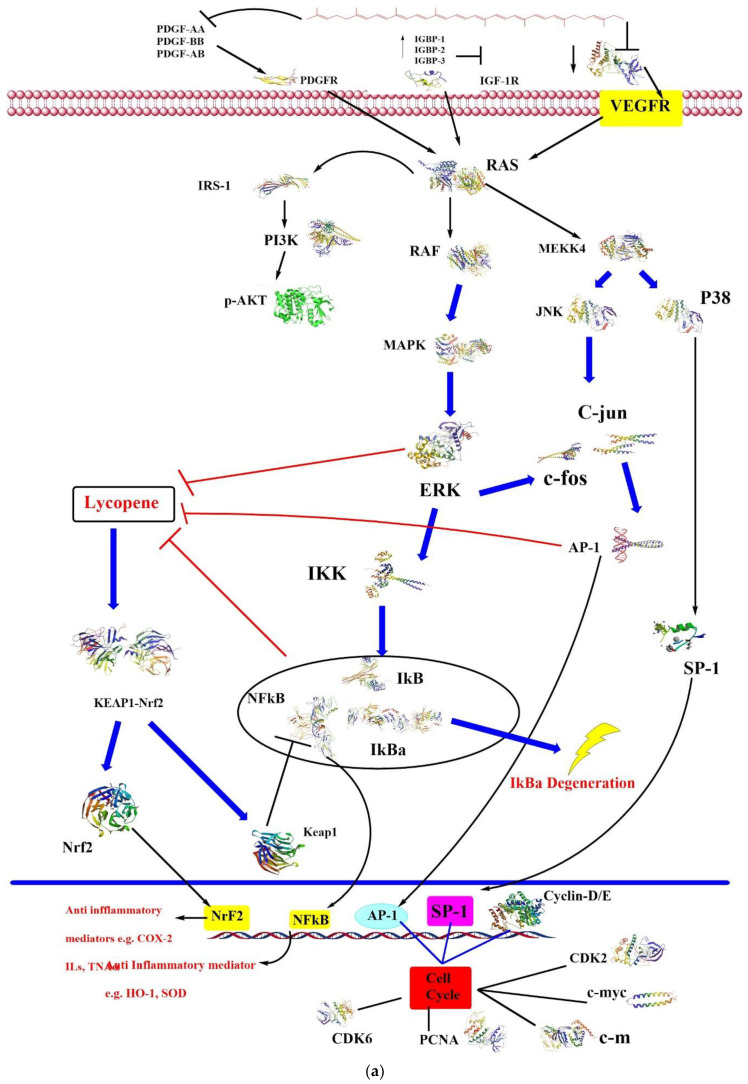
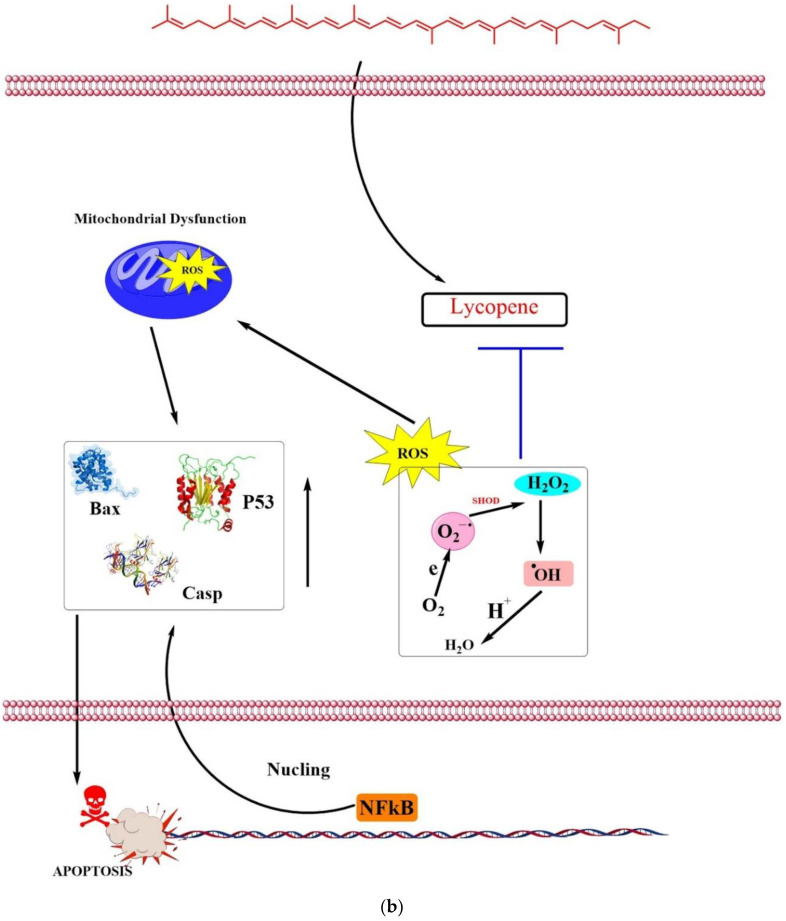
Lycopene has protective effects against cisplatin-induced spermiotoxicity, too. Lycopene (4 mg/kg) showed significant results in decreasing total abnormal sperms, improving sperm viability and motility. In addition, lycopene decreased testicular MDA concentration and increased GPx activity [144]. Another study revealed a protective effect of lycopene (1.5 mg/0.5 mL Tween; 80/100 g BW) in cyproterone acetate-induced infertility in rats. Lycopene made a significant retrieval effect on sperm count, viability, and motility, hypo-osmotic swelling tail to coil spermatozoa, testicular functions of 17β- hydroxysteroid dehydrogenase (HSD), CAT, 3β-HSD and SOD, conjugated diene level, MDA, serum testosterone, and testicular cholesterol [145]. Figure 2 illustrates some of the common signaling pathways which are mentioned in this section. The figure was designed and modified based on Trejo-Solís and co-authors’ paper [146].
Figure 2.
(a). Effect of lycopene on PDGFR-, IGF-IR-, and VEGFR-mediated signal pathways. (b) Effect of Lycopene on Reactive Oxygen Species (modified from [146]).
VEGF: vascular endothelial growth factor; AP-1: activator protein-1; CASP: caspase; ERK1/2: kinase regulated by extracellular signals; IGF: insulin-like growth factor; IKK: IκB kinase; IRS: insulin-1 receptor substrate; Keap1: Kelch-like ECH-associated protein 1; PCNA: proliferating cell nuclear antigen; PDGF: platelet-derived growth factor; PI3K: phosphoinositide 3-kinase; RAF: proto-oncogene serine/threonine-protein kinase; SP-1: specificity protein 1.
5. Protective Effects of Lycopene Against Different Toxins
Besides the aforementioned anti-toxicity effects of lycopene, this action should be briefly discussed. Previous studies suggested its significant protection against a wide variety of natural and chemical toxins. Different chemical agents with known neurotoxicity, hepatotoxicity, nephrotoxicity, and cardiotoxicity are inhibited by lycopene. Lycopene’s main properties which are believed to be necessary for this action are anti-oxidative, chelating, free-radical scavenging and antiapoptotic activities. This miracle pigment prevents bacterial toxins, mycotoxins, fluoride, metals, and pesticides from exerting their toxicities against the human body [4].
6. Recommended Dose
It should be noted that there is no ideal dose for daily lycopene consumption. Although previous studies only give ideas about lycopene intake at a different level, that would be helpful. For example, an in vivo study revealed that lycopene (6.5 mg/day) was effective against cancer in men [147]. However, lycopene dose should be increased up to 10 mg/day, in the case of advanced PCa. In another study, lycopene supplementation (15 mg/day, for 12 weeks) in an old aged population improved immune function through increasing natural killer cell activity by 28% [148]. Therefore, it seems that different lycopene doses and duration of supplementation can be suggested for various health purposes. Finally, according to different epidemiological studies, daily lycopene intake can be suggested to be 2 to 20 mg per day [10].
7. Safety and Toxicity
Safety monitoring should be considered as an important issue about medicinal plants and plant-derived phytochemicals [149,150]. There are several in vitro and in vivo studies on the possible toxicity of lycopene. For instance, it has been shown that lycopene (up to 10 μM) has no toxicity on the rat’s cultured cerebellar granule neurons viability [138]. Another research on cultured rat hippocampal neurons showed no significant toxic effects of lycopene when it was applied to these cells [151], even though it seems that carotenoids in some certain settings at high tissue concentrations may show a pro-oxidant effect [152]. A toxicological study on rats showed the no-observed-adverse-effect level at the highest examined dose (i.e., 1.0% in the diet) [153]. It should be noted that different lycopene forms (i.e., lycopene extracted from tomato, synthetic lycopene, and its crystallized extract) are generally recognized as safe when used in different food products [154]. There are no reports on adverse events from lycopene use at normal and ordinary doses [155]. Human studies suggested the no-observed-adverse-effect level for lycopene as 3 g per day/kg of body weight. Daily lycopene intake has been estimated as being significantly less than this level. Even the 99th percentile for its intake is 123 mg per day [156]. It seems that the expression of cytochrome P450 2E1 can be induced by high doses of lycopene and alcohol. Therefore, their high dose of concomitant use should be avoided [157]. In addition, regarding lycopene’s potent antioxidative effect, precautions should be kept in mind for patients under chemo and radiation therapy [158]. A case report described lycopenemia in a woman who had ingested about 2 liters of tomato juice every day and for several years. She had lycopene deposits in the liver (with no evidence of hepatic dysfunction) and deep orange discoloration of the skin. The lycopenodermia disappeared 3 weeks after stopping tomato juice consumption [159]. In addition, there is a chain of studies in which the lycopene mutagenicity was assessed. The results indicate that formulated lycopene has no mutagenic effects, although crystalline lycopene showed some mutagenic activity when degraded under exposure to light and air [160].
8. Conclusions
Lycopene possesses potent anticancer, antioxidant, antiinflammatory, and antidiabetic potential. In addition, it is a nutraceutical which protects against a wide variety of heart, liver, bone, skin, nervous, and reproductive systems diseases, as evident from numerous studies. However, further investigations are necessary to unveil the underlying mechanisms of actions, with a special emphasis on gene expression studies. Additionally, the recommended and effective doses of this functional food need to be further investigated. Safety concerns about its genotoxicity, maternal toxicity, and teratogenic effects should also be inquired.
Author Contributions
M.I., F.G., I.U.-H., H.U.-R., F.A., M.A.S., and M.H.H. conceived the review idea and focus, drafted the article, and critically revised the article. M.R., M.H., E.O., Z.Y. and M.T. were involved in the reference collection, writing the article. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by the KU Research Professor Program of Konkuk University, Seoul, South Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pennathur S., Maitra D., Byun J., Sliskovic I., Abdulhamid I., Saed G.M., Diamond M.P., Abu-Soud H.M. Potent antioxidative activity of lycopene: A potential role in scavenging hypochlorous acid. Free Radic. Biol. Med. 2010;49:205–213. doi: 10.1016/j.freeradbiomed.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin Y., Zheng Z., Jiang Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed. Pharmacother. 2019;109:2070–2077. doi: 10.1016/j.biopha.2018.07.100. [DOI] [PubMed] [Google Scholar]
- 3.Mozos I., Stoian D., Caraba A., Malainer C., Horbańczuk J.O., Atanasov A.G. Lycopene and vascular health. Front. Pharmacol. 2018;9:521. doi: 10.3389/fphar.2018.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedayati N., Naeini M.B., Nezami A., Hosseinzadeh H., Wallace Hayes A., Hosseini S., Imenshahidi M., Karimi G. Protective effect of lycopene against chemical and natural toxins: A review. BioFactors. 2019;45:5–23. doi: 10.1002/biof.1458. [DOI] [PubMed] [Google Scholar]
- 5.Woodside J.V., McGrath A.J., Lyner N., McKinley M.C. Carotenoids and health in older people. Maturitas. 2015;80:63–68. doi: 10.1016/j.maturitas.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Moran N.E., Erdman J.W., Jr., Clinton S.K. Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Arch. Biochem. Biophys. 2013;539:171–180. doi: 10.1016/j.abb.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petyaev I.M. Lycopene deficiency in ageing and cardiovascular disease. Oxidative Med. Cell. Longev. 2016;2016:3218605. doi: 10.1155/2016/3218605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis A.C., Dudenbostel T., Crowe-White K. Watermelon juice: A novel functional food to increase circulating lycopene in older adult women. Plant Foods Hum. Nutr. 2019;74:200–203. doi: 10.1007/s11130-019-00719-9. [DOI] [PubMed] [Google Scholar]
- 9.Naviglio D., Sapio L., Langilla C., Ragone A., Illiano M., Naviglio S., Gallo M. Beneficial Effects and Perspective Strategies for Lycopene Food Enrichment: A Systematic Review. Syst. Rev. Pharm. 2019;10:383–392. [Google Scholar]
- 10.Saini R.K., Rengasamy K.R., Mahomoodally F.M., Keum Y.-S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020;155:104730. doi: 10.1016/j.phrs.2020.104730. [DOI] [PubMed] [Google Scholar]
- 11.Przybylska S. Lycopene–a bioactive carotenoid offering multiple health benefits: A review. Int. J. Food Sci. Technol. 2020;55:11–32. doi: 10.1111/ijfs.14260. [DOI] [Google Scholar]
- 12.Joshi B., Kar S.K., Yadav P.K., Yadav S., Shrestha L., Bera T.K. Therapeutic and medicinal uses of lycopene: A systematic review. Int. J. Res. Med Sci. 2020;8:1195. doi: 10.18203/2320-6012.ijrms20200804. [DOI] [Google Scholar]
- 13.Rowles J., Ranard K., Smith J., An R., Erdman J. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017;20:361–377. doi: 10.1038/pcan.2017.25. [DOI] [PubMed] [Google Scholar]
- 14.Rowles J.L., Ranard K.M., Applegate C.C., Jeon S., An R., Erdman J.W. Processed and raw tomato consumption and risk of prostate cancer: A systematic review and dose–response meta-analysis. Prostate Cancer Prostatic Dis. 2018;21:319–336. doi: 10.1038/s41391-017-0005-x. [DOI] [PubMed] [Google Scholar]
- 15.Carini F., David S., Tomasello G., Mazzola M., Damiani P., Rappa F., Battaglia L., Cappello F., Jurjus A., Geagea A.G. Colorectal cancer: An update on the effects of lycopene on tumor progression and cell proliferation. J. Biol. Regul. Homeost. Agents. 2017;31:769–774. [PubMed] [Google Scholar]
- 16.Senkus K.E., Tan L., Crowe-White K.M. Lycopene and metabolic syndrome: A systematic review of the literature. Adv. Nutr. 2019;10:19–29. doi: 10.1093/advances/nmy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwatra B. A review on potential properties and therapeutic applications of lycopene. Int. J. Med Biomed. Stud. 2020;4 doi: 10.32553/ijmbs.v4i4.1081. [DOI] [Google Scholar]
- 18.Crowe-White K.M., Phillips T.A., Ellis A.C. Lycopene and cognitive function. J. Nutr. Sci. 2019;8 doi: 10.1017/jns.2019.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen D., Huang C., Chen Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019;111:791–801. doi: 10.1016/j.biopha.2018.12.151. [DOI] [PubMed] [Google Scholar]
- 20.Chernyshova M.P., Pristenskiy D.V., Lozbiakova M.V., Chalyk N.E., Bandaletova T.Y., Petyaev I.M. Systemic and skin-targeting beneficial effects of lycopene-enriched ice cream: A pilot study. J. Dairy Sci. 2019;102:14–25. doi: 10.3168/jds.2018-15282. [DOI] [PubMed] [Google Scholar]
- 21.Salehi B., Lopez-Jornet P., Pons-Fuster López E., Calina D., Sharifi-Rad M., Ramírez-Alarcón K., Forman K., Fernández M., Martorell M., Setzer W.N. Plant-derived bioactives in oral mucosal lesions: A key emphasis to curcumin, lycopene, chamomile, aloe vera, green tea and coffee properties. Biomolecules. 2019;9:106. doi: 10.3390/biom9030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maiani G., Periago Castón M.J., Catasta G., Toti E., Cambrodón I.G., Bysted A., Granado-Lorencio F., Olmedilla-Alonso B., Knuthsen P., Valoti M. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009;53:S194–S218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- 23.Shi J., Maguer M.L. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Food Sci. Nutr. 2000;40:1–42. doi: 10.1080/10408690091189275. [DOI] [PubMed] [Google Scholar]
- 24.Bramley P.M. Is lycopene beneficial to human health? Phytochemistry. 2000;54:233–236. doi: 10.1016/S0031-9422(00)00103-5. [DOI] [PubMed] [Google Scholar]
- 25.Barber N., Barber J. Lycopene and prostate cancer. Prostate Cancer Prostatic Dis. 2002;5:6–12. doi: 10.1038/sj.pcan.4500560. [DOI] [PubMed] [Google Scholar]
- 26.Grabowska M., Wawrzyniak D., Rolle K., Chomczyński P., Oziewicz S., Jurga S., Barciszewski J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019;10:3090–3102. doi: 10.1039/C9FO00580C. [DOI] [PubMed] [Google Scholar]
- 27.O’Neill M., Carroll Y., Corridan B., Olmedilla B., Granado F., Blanco I., Van den Berg H., Hininger I., Rousell A.-M., Chopra M. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. Br. J. Nutr. 2001;85:499–507. doi: 10.1079/BJN2000284. [DOI] [PubMed] [Google Scholar]
- 28.Porrini M., Riso P. What are typical lycopene intakes? J. Nutr. 2005;135:2042S–2045S. doi: 10.1093/jn/135.8.2042S. [DOI] [PubMed] [Google Scholar]
- 29.Jacques P.F., Lyass A., Massaro J.M., Vasan R.S., D’Agostino Sr R.B. Relationship of lycopene intake and consumption of tomato products to incident CVD. Br. J. Nutr. 2013;110:545–551. doi: 10.1017/S0007114512005417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Authority E.F.S. Revised exposure assessment for lycopene as a food colour. EFSA J. 2010;8:1444. doi: 10.2903/j.efsa.2010.1444. [DOI] [Google Scholar]
- 31.Holzapfel N.P., Holzapfel B.M., Champ S., Feldthusen J., Clements J., Hutmacher D.W. The potential role of lycopene for the prevention and therapy of prostate cancer: From molecular mechanisms to clinical evidence. Int. J. Mol. Sci. 2013;14:14620–14646. doi: 10.3390/ijms140714620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal S., Rao A. Carotenoids and chronic diseases. Drug Metab. Drug Interact. 2000;17:189–210. doi: 10.1515/DMDI.2000.17.1-4.189. [DOI] [PubMed] [Google Scholar]
- 33.Canene-Adams K., Campbell J.K., Zaripheh S., Jeffery E.H., Erdman J.W., Jr. The tomato as a functional food. J. Nutr. 2005;135:1226–1230. doi: 10.1093/jn/135.5.1226. [DOI] [PubMed] [Google Scholar]
- 34.Walfisch Y., Walfisch S., Agbaria R., Levy J., Sharoni Y. Lycopene in serum, skin and adipose tissues after tomato-oleoresin supplementation in patients undergoing haemorrhoidectomy or peri-anal fistulotomy. Br. J. Nutr. 2003;90:759–766. doi: 10.1079/BJN2003955. [DOI] [PubMed] [Google Scholar]
- 35.Burton-Freeman B.M., Sesso H.D. Whole food versus supplement: Comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv. Nutr. 2014;5:457–485. doi: 10.3945/an.114.005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richelle M., Sanchez B., Tavazzi I., Lambelet P., Bortlik K., Williamson G. Lycopene isomerisation takes place within enterocytes during absorption in human subjects. Br. J. Nutr. 2010;103:1800–1807. doi: 10.1017/S0007114510000103. [DOI] [PubMed] [Google Scholar]
- 37.Teodoro A.J., Perrone D., Martucci R.B., Borojevic R. Lycopene isomerisation and storage in an in vitro model of murine hepatic stellate cells. Eur. J. Nutr. 2009;48:261–268. doi: 10.1007/s00394-009-0001-6. [DOI] [PubMed] [Google Scholar]
- 38.Moussa M., Landrier J.-F., Reboul E., Ghiringhelli O., Coméra C., Collet X., Fröhlich K., Böhm V., Borel P. Lycopene absorption in human intestinal cells and in mice involves scavenger receptor class B type I but not Niemann-Pick C1-like 1. J. Nutr. 2008;138:1432–1436. doi: 10.1093/jn/138.8.1432. [DOI] [PubMed] [Google Scholar]
- 39.Moussa M., Gouranton E., Gleize B., Yazidi C.E., Niot I., Besnard P., Borel P., Landrier J.F. CD36 is involved in lycopene and lutein uptake by adipocytes and adipose tissue cultures. Mol. Nutr. Food Res. 2011;55:578–584. doi: 10.1002/mnfr.201000399. [DOI] [PubMed] [Google Scholar]
- 40.Ferrucci L., Perry J.R., Matteini A., Perola M., Tanaka T., Silander K., Rice N., Melzer D., Murray A., Cluett C. Common variation in the β-carotene 15, 15′-monooxygenase 1 gene affects circulating levels of carotenoids: A genome-wide association study. Am. J. Hum. Genet. 2009;84:123–133. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindshield B.L., Canene-Adams K., Erdman J.W., Jr. Lycopenoids: Are lycopene metabolites bioactive? Arch. Biochem. Biophys. 2007;458:136–140. doi: 10.1016/j.abb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Ross A.B., Vuong L.T., Ruckle J., Synal H.A., Schulze-König T., Wertz K., Rümbeli R., Liberman R.G., Skipper P.L., Tannenbaum S.R. Lycopene bioavailability and metabolism in humans: An accelerator mass spectrometry study. Am. J. Clin. Nutr. 2011;93:1263–1273. doi: 10.3945/ajcn.110.008375. [DOI] [PubMed] [Google Scholar]
- 43.Moran N.E., Cichon M.J., Riedl K.M., Grainger E.M., Schwartz S.J., Novotny J.A., Erdman J.W., Jr., Clinton S.K. Compartmental and noncompartmental modeling of 13C-lycopene absorption, isomerization, and distribution kinetics in healthy adults. Am. J. Clin. Nutr. 2015;102:1436–1449. doi: 10.3945/ajcn.114.103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahin K., Yenice E., Tuzcu M., Orhan C., Mizrak C., Ozercan I.H., Sahin N., Yilmaz B., Bilir B., Ozpolat B. Lycopene protects against spontaneous ovarian cancer formation in laying hens. J. Cancer Prev. 2018;23:25. doi: 10.15430/JCP.2018.23.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cataño J.G., Trujillo C.G., Caicedo J.I., Bravo-Balado A., Robledo D., Mariño-Alvarez A.M., Pedraza A., Arcila M.J., Plata M. Efficacy of lycopene intake in primary prevention of prostate cancer: A systematic review of the literature and meta-analysis. Arch. Esp. Urol. 2018;71:187–197. [PubMed] [Google Scholar]
- 46.Dos Santos R.C., Ombredane A.S., Souza J.M.T., Vasconcelos A.G., Plácido A., das GN Amorim A., Barbosa E.A., Lima F.C., Ropke C.D., Alves M.M. Lycopene-rich extract from red guava (Psidium guajava L.) displays cytotoxic effect against human breast adenocarcinoma cell line MCF-7 via an apoptotic-like pathway. Food Res. Int. 2018;105:184–196. doi: 10.1016/j.foodres.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 47.Morgia G., Voce S., Palmieri F., Gentile M., Iapicca G., Giannantoni A., Blefari F., Carini M., Vespasiani G., Santelli G. Association between selenium and lycopene supplementation and incidence of prostate cancer: Results from the post-hoc analysis of the procomb trial. Phytomedicine. 2017;34:1–5. doi: 10.1016/j.phymed.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Zhou S., Zhang R., Bi T., Lu Y., Jiang L. Inhibitory effect of lycopene against the growth of human gastric cancer cells. Afr. J. Tradit. Complement. Altern. Med. 2016;13:184–190. doi: 10.21010/ajtcam.v13i4.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jhou B.-Y., Song T.-Y., Lee I., Hu M.-L., Yang N.-C. Lycopene inhibits metastasis of human liver adenocarcinoma SK-Hep-1 cells by downregulation of NADPH oxidase 4 protein expression. J. Agric. Food Chem. 2017;65:6893–6903. doi: 10.1021/acs.jafc.7b03036. [DOI] [PubMed] [Google Scholar]
- 50.Holzapfel N.P., Shokoohmand A., Wagner F., Landgraf M., Champ S., Holzapfel B.M., Clements J.A., Hutmacher D.W., Loessner D. Lycopene reduces ovarian tumor growth and intraperitoneal metastatic load. Am. J. Cancer Res. 2017;7:1322. [PMC free article] [PubMed] [Google Scholar]
- 51.Baş H., Pandır D. Protective effects of lycopene on furantreated diabetic and non-diabetic rat lung. Biomed. Environ. Sci. 2016;29:143–147. doi: 10.3967/bes2016.016. [DOI] [PubMed] [Google Scholar]
- 52.Reddy P.V.N., Ambati M., Koduganti R. Systemic lycopene as an adjunct to scaling and root planing in chronic periodontitis patients with type 2 diabetes mellitus. J. Int. Soc. Prev. Community Dent. 2015;5:S25. doi: 10.4103/2231-0762.156520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandikci M., Karagenc L., Yildiz M. Changes in the Pancreas in Experimental Diabetes and the Effect of Lycopene on These Changes: Proliferating, Apoptotic, and Estrogen Receptor α Positive Cells. Anat. Rec. 2017;300:2000–2007. doi: 10.1002/ar.23641. [DOI] [PubMed] [Google Scholar]
- 54.Uçar S., Pandir D. Furan induced ovarian damage in non-diabetic and diabetic rats and cellular protective role of lycopene. Arch. Gynaecol. Obstet. 2017;296:1027–1037. doi: 10.1007/s00404-017-4521-7. [DOI] [PubMed] [Google Scholar]
- 55.Soleymaninejad M., Joursaraei S.G., Feizi F., Jafari Anarkooli I. The effects of lycopene and insulin on histological changes and the expression level of Bcl-2 family genes in the hippocampus of streptozotocin-induced diabetic rats. J. Diabetes Res. 2017;2017:4650939. doi: 10.1155/2017/4650939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tabrez S., Al-Shali K.Z., Ahmad S. Lycopene powers the inhibition of glycation-induced diabetic nephropathy: A novel approach to halt the AGE-RAGE axis menace. Biofactors. 2015;41:372–381. doi: 10.1002/biof.1238. [DOI] [PubMed] [Google Scholar]
- 57.Yegın S.Ç., Yur F., Çetın S., Güder A. Effect of lycopene on serum nitrite-nitrate levels in diabetic rats. Indian J. Pharm. Sci. 2015;77:357. doi: 10.4103/0250-474X.159676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costa-Rodrigues J., Pinho O., Monteiro P. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018;245:1148–1153. doi: 10.1016/j.foodchem.2017.11.055. [DOI] [PubMed] [Google Scholar]
- 59.Kumar R., Salwe K.J., Kumarappan M. Evaluation of antioxidant, hypolipidemic, and antiatherogenic property of lycopene and astaxanthin in atherosclerosis-induced rats. Pharmacogn. Res. 2017;9:161. doi: 10.4103/0974-8490.204654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng H.M., Koutsidis G., Lodge J.K., Ashor A., Siervo M., Lara J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis. 2017;257:100–108. doi: 10.1016/j.atherosclerosis.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 61.He Y., Xia P., Jin H., Zhang Y., Chen B., Xu Z. Lycopene Ameliorates Transplant Arteriosclerosis in Vascular Allograft Transplantation by Regulating the NO/cGMP Pathways and Rho-Associated Kinases Expression. Oxidative Med. Cell. Longev. 2016;2016:3128280. doi: 10.1155/2016/3128280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao B., Liu H., Wang J., Liu P., Tan X., Ren B., Liu Z., Liu X. Lycopene supplementation attenuates oxidative stress, neuroinflammation, and cognitive impairment in aged CD-1 mice. J. Agric. Food Chem. 2018;66:3127–3136. doi: 10.1021/acs.jafc.7b05770. [DOI] [PubMed] [Google Scholar]
- 63.Wang J., Li L., Wang Z., Cui Y., Tan X., Yuan T., Liu Q., Liu Z., Liu X. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J. Nutr. Biochem. 2018;56:16–25. doi: 10.1016/j.jnutbio.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 64.El-Ashmawy N.E., Khedr N.F., El-Bahrawy H.A., Hamada O.B. Suppression of inducible nitric oxide synthase and tumor necrosis factor-alpha level by lycopene is comparable to methylprednisolone in acute pancreatitis. Dig. Liver Dis. 2018;50:601–607. doi: 10.1016/j.dld.2018.01.131. [DOI] [PubMed] [Google Scholar]
- 65.Zhao B., Ren B., Guo R., Zhang W., Ma S., Yao Y., Yuan T., Liu Z., Liu X. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem. Toxicol. 2017;109:505–516. doi: 10.1016/j.fct.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 66.Kawata A., Murakami Y., Suzuki S., Fujisawa S. Anti-inflammatory activity of β-Carotene, lycopene and tri-n-butylborane, a scavenger of reactive oxygen species. Vivo. 2018;32:255–264. doi: 10.21873/invivo.11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu C.-B., Wang R., Yi Y.-F., Gao Z., Chen Y.-Z. Lycopene mitigates β-amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer’s disease rats. J. Nutr. Biochem. 2018;53:66–71. doi: 10.1016/j.jnutbio.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 68.Vasconcelos A.G., das GN Amorim A., dos Santos R.C., Souza J.M.T., de Souza L.K.M., de SL Araújo T., Nicolau L.A.D., de Lima Carvalho L., de Aquino P.E.A., da Silva Martins C. Lycopene rich extract from red guava (Psidium guajava L.) displays anti-inflammatory and antioxidant profile by reducing suggestive hallmarks of acute inflammatory response in mice. Food Res. Int. 2017;99:959–968. doi: 10.1016/j.foodres.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 69.Jiang W., Guo M.-H., Hai X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 2016;22:10180. doi: 10.3748/wjg.v22.i46.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yefsah-Idres A., Benazzoug Y., Otman A., Latour A., Middendorp S., Janel N. Hepatoprotective effects of lycopene on liver enzymes involved in methionine and xenobiotic metabolism in hyperhomocysteinemic rats. Food Funct. 2016;7:2862–2869. doi: 10.1039/C6FO00095A. [DOI] [PubMed] [Google Scholar]
- 71.Tokac M., Aydin S., Taner G., Özkardeş A.B., Taşlipinar M.Y., DoĞan M., Dündar H.Z., Kilic M., Başaran A.A., Başaran A.N. Hepatoprotective and antioxidant effects of lycopene in acute cholestasis. Turk. J. Med Sci. 2015;45:857–864. doi: 10.3906/sag-1404-57. [DOI] [PubMed] [Google Scholar]
- 72.Grether-Beck S., Marini A., Jaenicke T., Stahl W., Krutmann J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: Results from a double-blinded, placebo-controlled, crossover study. Br. J. Dermatol. 2017;176:1231–1240. doi: 10.1111/bjd.15080. [DOI] [PubMed] [Google Scholar]
- 73.Lu C.-W., Hung C.-F., Jean W.-H., Lin T.-Y., Huang S.-K., Wang S.-J. Lycopene depresses glutamate release through inhibition of voltage-dependent Ca2+ entry and protein kinase C in rat cerebrocortical nerve terminals. Can. J. Physiol. Pharmacol. 2017;96:479–484. doi: 10.1139/cjpp-2017-0520. [DOI] [PubMed] [Google Scholar]
- 74.Bhardwaj M., Kumar A. Neuroprotective effect of lycopene against PTZ-induced kindling seizures in mice: Possible behavioural, biochemical and mitochondrial dysfunction. Phytother. Res. 2016;30:306–313. doi: 10.1002/ptr.5533. [DOI] [PubMed] [Google Scholar]
- 75.Datta S., Jamwal S., Deshmukh R., Kumar P. Beneficial effects of lycopene against haloperidol induced orofacial dyskinesia in rats: Possible neurotransmitters and neuroinflammation modulation. Eur. J. Pharmacol. 2016;771:229–235. doi: 10.1016/j.ejphar.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 76.Costa-Rodrigues J., Fernandes M.H., Pinho O., Monteiro P.R.R. Modulation of human osteoclastogenesis and osteoblastogenesis by lycopene. J. Nutr. Biochem. 2018;57:26–34. doi: 10.1016/j.jnutbio.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Ardawi M.-S.M., Badawoud M.H., Hassan S.M., Rouzi A.A., Ardawi J.M., AlNosani N.M., Qari M.H., Mousa S.A. Lycopene treatment against loss of bone mass, microarchitecture and strength in relation to regulatory mechanisms in a postmenopausal osteoporosis model. Bone. 2016;83:127–140. doi: 10.1016/j.bone.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 78.Ghyasvand T., Goodarzi M.T., Amiri I., Karimi J., Ghorbani M. Serum levels of lycopene, beta-carotene, and retinol and their correlation with sperm DNA damage in normospermic and infertile men. Int. J. Reprod. Biomed. 2015;13:787. doi: 10.29252/ijrm.13.12.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aly H.A., El-Beshbishy H.A., Banjar Z.M. Mitochondrial dysfunction induced impairment of spermatogenesis in LPS-treated rats: Modulatory role of lycopene. Eur. J. Pharmacol. 2012;677:31–38. doi: 10.1016/j.ejphar.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 80.Nedamani A.R., Nedamani E.R., Salimi A. The role of lycopene in human health as a natural colorant. Nutr. Food Sci. 2019;49:284–298. [Google Scholar]
- 81.Ghadage S., Mane K., Agrawal R., Pawar V. Tomato lycopene: Potential health benefits. Pharma Innov. J. 2019;8:1245–1248. [Google Scholar]
- 82.Chen P., Xu S., Qu J. Lycopene protects keratinocytes against UVB radiation-induced carcinogenesis via negative regulation of FOXO3a through the mTORC2/AKT signaling pathway. J. Cell. Biochem. 2018;119:366–377. doi: 10.1002/jcb.26189. [DOI] [PubMed] [Google Scholar]
- 83.Peng S., Li J., Zhou Y., Tuo M., Qin X., Yu Q., Cheng H., Li Y. In vitro effects and mechanisms of lycopene in MCF-7 human breast cancer cells. Genet. Mol. Res. 2017;16:13. doi: 10.4238/gmr16029434. [DOI] [PubMed] [Google Scholar]
- 84.Cha J.H., Kim W.K., Ha A.W., Kim M.H., Chang M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017;11:90–96. doi: 10.4162/nrp.2017.11.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Soares N.D.C.P., Machado C.L., Trindade B.B., do Canto Lima I.C., Gimba E.R.P., Teodoro A.J., Takiya C., Borojevic R. Lycopene extracts from different tomato-based food products induce apoptosis in cultured human primary prostate cancer cells and regulate TP53, Bax and Bcl-2 transcript expression. Asian Pac. J. Cancer Prev. APJCP. 2017;18:339. doi: 10.22034/APJCP.2017.18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jain A., Sharma G., Kushwah V., Thakur K., Ghoshal G., Singh B., Jain S., Shivhare U., Katare O. Fabrication and functional attributes of lipidic nanoconstructs of lycopene: An innovative endeavour for enhanced cytotoxicity in MCF-7 breast cancer cells. Colloids Surf. B Biointerfaces. 2017;152:482–491. doi: 10.1016/j.colsurfb.2017.01.050. [DOI] [PubMed] [Google Scholar]
- 87.Ye M., Wu Q., Zhang M., Huang J. Lycopene inhibits the cell proliferation and invasion of human head and neck squamous cell carcinoma. Mol. Med. Rep. 2016;14:2953–2958. doi: 10.3892/mmr.2016.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X., Yang H.-H., Liu Y., Zhou Q., Chen Z.-H. Lycopene consumption and risk of colorectal cancer: A meta-analysis of observational studies. Nutr. Cancer. 2016;68:1083–1096. doi: 10.1080/01635581.2016.1206579. [DOI] [PubMed] [Google Scholar]
- 89.Gong X., Marisiddaiah R., Zaripheh S., Wiener D., Rubin L.P. Mitochondrial β-carotene 9′, 10′ oxygenase modulates prostate cancer growth via NF-κB inhibition: A lycopene-independent function. Mol. Cancer Res. 2016;14:966–975. doi: 10.1158/1541-7786.MCR-16-0075. [DOI] [PubMed] [Google Scholar]
- 90.Aizawa K., Liu C., Tang S., Veeramachaneni S., Hu K.Q., Smith D.E., Wang X.D. Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation. Int. J. Cancer. 2016;139:1171–1181. doi: 10.1002/ijc.30161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Graff R.E., Pettersson A., Lis R.T., Ahearn T.U., Markt S.C., Wilson K.M., Rider J.R., Fiorentino M., Finn S., Kenfield S.A. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am. J. Clin. Nutr. 2016;103:851–860. doi: 10.3945/ajcn.115.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu R., Chen B., Bai Y., Miao T., Rui L., Zhang H., Xia B., Li Y., Gao S., Wang X.-D. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020;159:104966. doi: 10.1016/j.phrs.2020.104966. [DOI] [PubMed] [Google Scholar]
- 93.Ozmen O., Topsakal S., Haligur M., Aydogan A., Dincoglu D. Effects of caffeine and lycopene in experimentally induced diabetes mellitus. Pancreas. 2016;45:579–583. doi: 10.1097/MPA.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 94.Zeng Y.-C., Peng L.-S., Zou L., Huang S.-F., Xie Y., Mu G.-P., Zeng X.-H., Zhou X.-L., Zeng Y.-C. Protective effect and mechanism of lycopene on endothelial progenitor cells (EPCs) from type 2 diabetes mellitus rats. Biomed. Pharmacother. 2017;92:86–94. doi: 10.1016/j.biopha.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 95.Hasan T., Sultana M. Lycopene and Cardiovascular Diseases: A Review of the Literature. Int. J. Res. Rev. 2017;4:73–86. [Google Scholar]
- 96.Sen S. The chemistry and biology of lycopene: Antioxidant for human health. Int. J. Adv. Life Sci. Res. 2019;2:8–14. doi: 10.31632/ijalsr.2019v02i04.002. [DOI] [Google Scholar]
- 97.Song B., Liu K., Gao Y., Zhao L., Fang H., Li Y., Pei L., Xu Y. Lycopene and risk of cardiovascular diseases: A meta-analysis of observational studies. Mol. Nutr. Food Res. 2017;61:1601009. doi: 10.1002/mnfr.201601009. [DOI] [PubMed] [Google Scholar]
- 98.Lin J., Li H.-X., Xia J., Li X.-N., Jiang X.-Q., Zhu S.-Y., Ge J., Li J.-L. The chemopreventive potential of lycopene against atrazine-induced cardiotoxicity: Modulation of ionic homeostasis. Sci. Rep. 2016;6:24855. doi: 10.1038/srep24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tong C., Peng C., Wang L., Zhang L., Yang X., Xu P., Li J., Delplancke T., Zhang H., Qi H. Intravenous administration of lycopene, a tomato extract, protects against myocardial ischemia-reperfusion injury. Nutrients. 2016;8:138. doi: 10.3390/nu8030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao Y., Jia P., Shu W., Jia D. The protective effect of lycopene on hypoxia/reoxygenation-induced endoplasmic reticulum stress in H9C2 cardiomyocytes. Eur. J. Pharmacol. 2016;774:71–79. doi: 10.1016/j.ejphar.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 101.Müller L., Caris-Veyrat C., Lowe G., Böhm V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—A critical review. Crit. Rev. Food Sci. Nutr. 2016;56:1868–1879. doi: 10.1080/10408398.2013.801827. [DOI] [PubMed] [Google Scholar]
- 102.Xu J., Hu H., Chen B., Yue R., Zhou Z., Liu Y., Zhang S., Xu L., Wang H., Yu Z. Lycopene protects against hypoxia/reoxygenation injury by alleviating ER stress induced apoptosis in neonatal mouse cardiomyocytes. PLoS ONE. 2015;10:e0136443. doi: 10.1371/journal.pone.0136443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He Q., Zhou W., Xiong C., Tan G., Chen M. Lycopene attenuates inflammation and apoptosis in post-myocardial infarction remodeling by inhibiting the nuclear factor-κB signaling pathway. Mol. Med. Rep. 2015;11:374–378. doi: 10.3892/mmr.2014.2676. [DOI] [PubMed] [Google Scholar]
- 104.Gajendragadkar P.R., Hubsch A., Mäki-Petäjä K.M., Serg M., Wilkinson I.B., Cheriyan J. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: A randomised controlled trial. PLoS ONE. 2014;9:e99070. doi: 10.1371/journal.pone.0099070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Caseiro M., Ascenso A., Costa A., Creagh-Flynn J., Johnson M., Simões S. Lycopene in human health. LWT. 2020;127:109323. doi: 10.1016/j.lwt.2020.109323. [DOI] [Google Scholar]
- 106.Tian Y., Xiao Y., Wang B., Sun C., Tang K., Sun F. Vitamin E and lycopene reduce coal burning fluorosis-induced spermatogenic cell apoptosis via oxidative stress-mediated JNK and ERK signaling pathways. Biosci. Rep. 2018:38. doi: 10.1042/BSR20171003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alvi S.S., Ansari I.A., Ahmad M.K., Iqbal J., Khan M.S. Lycopene amends LPS induced oxidative stress and hypertriglyceridemia via modulating PCSK-9 expression and Apo-CIII mediated lipoprotein lipase activity. Biomed. Pharmacother. 2017;96:1082–1093. doi: 10.1016/j.biopha.2017.11.116. [DOI] [PubMed] [Google Scholar]
- 108.Baykalir B.G., Aksit D., Dogru M.S., Yay A.H., Aksit H., Seyrek K., Atessahin A. Lycopene ameliorates experimental colitis in rats via reducing apoptosis and oxidative stress. Int. J. Vitam Nutr. Res. 2016;86:27–35. doi: 10.1024/0300-9831/a000280. [DOI] [PubMed] [Google Scholar]
- 109.Yu L., Wang W., Pang W., Xiao Z., Jiang Y., Hong Y. Dietary lycopene supplementation improves cognitive performances in tau transgenic mice expressing P301L mutation via inhibiting oxidative stress and tau hyperphosphorylation. J. Alzheimer’s Dis. 2017;57:475–482. doi: 10.3233/JAD-161216. [DOI] [PubMed] [Google Scholar]
- 110.Bandeira A.C.B., da Silva T.P., de Araujo G.R., Araujo C.M., da Silva R.C., Lima W.G., Bezerra F.S., Costa D.C. Lycopene inhibits reactive oxygen species production in SK-Hep-1 cells and attenuates acetaminophen-induced liver injury in C57BL/6 mice. Chem. -Biol. Interact. 2017;263:7–17. doi: 10.1016/j.cbi.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 111.Lim S., Hwang S., Yu J.H., Lim J.W., Kim H. Lycopene inhibits regulator of calcineurin 1-mediated apoptosis by reducing oxidative stress and down-regulating Nucling in neuronal cells. Mol. Nutr. Food Res. 2017;61:1600530. doi: 10.1002/mnfr.201600530. [DOI] [PubMed] [Google Scholar]
- 112.Bayomy N.A., Elbakary R.H., Ibrahim M.A., Abdelaziz E.Z. Effect of lycopene and rosmarinic acid on gentamicin induced renal cortical oxidative stress, apoptosis, and autophagy in adult male albino rat. Anat. Rec. 2017;300:1137–1149. doi: 10.1002/ar.23525. [DOI] [PubMed] [Google Scholar]
- 113.Tvrdá E., Kováčik A., Tušimová E., Paál D., Mackovich A., Alimov J., Lukáč N. Antioxidant efficiency of lycopene on oxidative stress-induced damage in bovine spermatozoa. J. Anim. Sci. Biotechnol. 2016;7:50. doi: 10.1186/s40104-016-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qu M., Jiang Z., Liao Y., Song Z., Nan X. Lycopene prevents amyloid [beta]-induced mitochondrial oxidative stress and dysfunctions in cultured rat cortical neurons. Neurochem. Res. 2016;41:1354–1364. doi: 10.1007/s11064-016-1837-9. [DOI] [PubMed] [Google Scholar]
- 115.Campos K.K.D., Araújo G.R., Martins T.L., Bandeira A.C.B., de Paula Costa G., Talvani A., Garcia C.C.M., Oliveira L.A.M., Costa D.C., Bezerra F.S. The antioxidant and anti-inflammatory properties of lycopene in mice lungs exposed to cigarette smoke. J. Nutr. Biochem. 2017;48:9–20. doi: 10.1016/j.jnutbio.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 116.Göncü T., Oğuz E., Sezen H., Koçarslan S., Oğuz H., Akal A., Adıbelli F.M., Çakmak S., Aksoy N. Anti-inflammatory effect of lycopene on endotoxin-induced uveitis in rats. Arq. Bras. Oftalmol. 2016;79:357–362. doi: 10.5935/0004-2749.20160102. [DOI] [PubMed] [Google Scholar]
- 117.Sachdeva A.K., Chopra K. Lycopene abrogates Aβ(1-42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer’s disease. J. Nutr. Biochem. 2015;26:736–744. doi: 10.1016/j.jnutbio.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 118.Colmán-Martínez M., Martínez-Huélamo M., Valderas-Martínez P., Arranz-Martínez S., Almanza-Aguilera E., Corella D., Estruch R., Lamuela-Raventós R.M. trans-Lycopene from tomato juice attenuates inflammatory biomarkers in human plasma samples: An intervention trial. Mol. Nutr. Food Res. 2017;61:1600993. doi: 10.1002/mnfr.201600993. [DOI] [PubMed] [Google Scholar]
- 119.Liu T.Y., Chen S.B. Sarcandra glabra combined with lycopene protect rats from lipopolysaccharide induced acute lung injury via reducing inflammatory response. Biomed. Pharmacother. 2016;84:34–41. doi: 10.1016/j.biopha.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 120.Li Y.F., Chang Y.Y., Huang H.C., Wu Y.C., Yang M.D., Chao P.M. Tomato juice supplementation in young women reduces inflammatory adipokine levels independently of body fat reduction. Nutrition. 2015;31:691–696. doi: 10.1016/j.nut.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 121.Zhang F., Fu Y., Zhou X., Pan W., Shi Y., Wang M., Zhang X., Qi D., Li L., Ma K., et al. Depression-like behaviors and heme oxygenase-1 are regulated by lycopene in lipopolysaccharide-induced neuroinflammation. J. Neuroimmunol. 2016;298:1–8. doi: 10.1016/j.jneuroim.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 122.Makon-Sébastien N., Francis F., Eric S., Henri V.P., François L.J., Laurent P., Yves B., Serge C. Lycopene modulates THP1 and Caco2 cells inflammatory state through transcriptional and nontranscriptional processes. Mediat. Inflamm. 2014;2014:507272. doi: 10.1155/2014/507272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sheriff S.A., Shaik Ibrahim S., Devaki T., Chakraborty S., Agarwal S., Pérez-Sánchez H. Lycopene Prevents mitochondrial dysfunction during d-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in albino rats. J. Proteome Res. 2017;16:3190–3199. doi: 10.1021/acs.jproteome.7b00176. [DOI] [PubMed] [Google Scholar]
- 124.Deng Y., Xu Z., Liu W., Yang H., Xu B., Wei Y. Effects of lycopene and proanthocyanidins on hepatotoxicity induced by mercuric chloride in rats. Biol. Trace Elem. Res. 2012;146:213–223. doi: 10.1007/s12011-011-9242-3. [DOI] [PubMed] [Google Scholar]
- 125.Anusha M., Venkateswarlu M., Prabhakaran V., Taj S.S., Kumari B.P., Ranganayakulu D. Hepatoprotective activity of aqueous extract of Portulaca oleracea in combination with lycopene in rats. Indian J. Pharmacol. 2011;43:563–567. doi: 10.4103/0253-7613.84973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yucel Y., Oguz E., Kocarslan S., Tatli F., Gozeneli O., Seker A., Sezen H., Buyukaslan H., Aktumen A., Ozgonul A., et al. The effects of lycopene on methotrexate-induced liver injury in rats. Bratisl. Lek. Listy. 2017;118:212–216. doi: 10.4149/bll_2017_042. [DOI] [PubMed] [Google Scholar]
- 127.Bandeira A.C.B., da Silva R.C., Rossoni J.V.J., Figueiredo V.P., Talvani A., Cangussú S.D., Bezerra F.S., Costa D.C. Lycopene pretreatment improves hepatotoxicity induced by acetaminophen in C57BL/6 mice. Bioorg. Med. Chem. 2017;25:1057–1065. doi: 10.1016/j.bmc.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 128.Bayramoglu G., Bayramoglu A., Altuner Y., Uyanoglu M., Colak S. The effects of lycopene on hepatic ischemia/reperfusion injury in rats. Cytotechnology. 2015;67:487–491. doi: 10.1007/s10616-014-9706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xia J., Lin J., Zhu S.Y., Du Z.H., Guo J.A., Han Z.X., Li J.L., Zhang Y. Lycopene protects against atrazine-induced hepatotoxicity through modifications of cytochrome P450 enzyme system in microsomes. Exp. Toxicol. Pathol. 2016;68:223–231. doi: 10.1016/j.etp.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 130.Sheik Abdulazeez S., Thiruvengadam D. Effect of lycopene on oxidative stress induced during D-galactosamine/lipopolysaccharide-sensitized liver injury in rats. Pharm. Biol. 2013;51:1592–1599. doi: 10.3109/13880209.2013.803579. [DOI] [PubMed] [Google Scholar]
- 131.Sheriff S.A., Devaki T. Lycopene stabilizes lipoprotein levels during D-galactosamine/lipopolysaccharide induced hepatitis in experimental rats. Asian Pac. J. Trop. Biomed. 2012;2:975–980. doi: 10.1016/S2221-1691(13)60009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Z., Fan J., Wang J., Li Y., Xiao L., Duan D., Wang Q. Protective effect of lycopene on high-fat diet-induced cognitive impairment in rats. Neurosci. Lett. 2016;627:185–191. doi: 10.1016/j.neulet.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 133.Lei X., Lei L., Zhang Z., Cheng Y. Neuroprotective effects of lycopene pretreatment on transient global cerebral ischemia-reperfusion in rats: The role of the Nrf2/HO-1 signaling pathway. Mol. Med. Rep. 2016;13:412–418. doi: 10.3892/mmr.2015.4534. [DOI] [PubMed] [Google Scholar]
- 134.Chen W., Mao L., Xing H., Xu L., Fu X., Huang L., Huang D., Pu Z., Li Q. Lycopene attenuates Aβ1-42 secretion and its toxicity in human cell and Caenorhabditis elegans models of Alzheimer disease. Neurosci. Lett. 2015;608:28–33. doi: 10.1016/j.neulet.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 135.Prema A., Janakiraman U., Manivasagam T., Thenmozhi A.J. Neuroprotective effect of lycopene against MPTP induced experimental Parkinson’s disease in mice. Neurosci. Lett. 2015;599:12–19. doi: 10.1016/j.neulet.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 136.Prakash A., Kumar A. Implicating the role of lycopene in restoration of mitochondrial enzymes and BDNF levels in β-amyloid induced Alzheimer’s disease. Eur. J. Pharm. 2014;741:104–111. doi: 10.1016/j.ejphar.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 137.Liu C.B., Wang R., Pan H.B., Ding Q.F., Lu F.B. Effect of lycopene on oxidative stress and behavioral deficits in rotenone induced model of Parkinson’s disease. Zhongguo Ying Yong Sheng Li Xue Za Zhi = Zhongguo Yingyong Shenglixue Zazhi = Chin. J. Appl. Physiol. 2013;29:380–384. [PubMed] [Google Scholar]
- 138.Qu M., Nan X., Gao Z., Guo B., Liu B., Chen Z. Protective effects of lycopene against methylmercury-induced neurotoxicity in cultured rat cerebellar granule neurons. Brain Res. 2013;1540:92–102. doi: 10.1016/j.brainres.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 139.Kaur H., Chauhan S., Sandhir R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson’s disease. Neurochem. Res. 2011;36:1435–1443. doi: 10.1007/s11064-011-0469-3. [DOI] [PubMed] [Google Scholar]
- 140.Hayhoe R.P.G., Lentjes M.A.H., Mulligan A.A., Luben R.N., Khaw K.T., Welch A.A. Carotenoid dietary intakes and plasma concentrations are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Br. J. Nutr. 2017;117:1439–1453. doi: 10.1017/S0007114517001180. [DOI] [PubMed] [Google Scholar]
- 141.Sahni S., Hannan M.T., Blumberg J., Cupples L.A., Kiel D.P., Tucker K.L. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: A 17-year follow-up from the Framingham Osteoporosis Study. J. Bone Miner. Res. 2009;24:1086–1096. doi: 10.1359/jbmr.090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mackinnon E.S., Rao A.V., Rao L.G. Dietary restriction of lycopene for a period of one month resulted in significantly increased biomarkers of oxidative stress and bone resorption in postmenopausal women. J. Nutr. Health Aging. 2011;15:133–138. doi: 10.1007/s12603-011-0026-4. [DOI] [PubMed] [Google Scholar]
- 143.Ateşşahin A., Türk G., Karahan I., Yilmaz S., Ceribaşi A.O., Bulmuş O. Lycopene prevents adriamycin-induced testicular toxicity in rats. Fertil. Steril. 2006;85 (Suppl. 1):1216–1222. doi: 10.1016/j.fertnstert.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 144.Ateşşahin A., Karahan I., Türk G., Gür S., Yilmaz S., Ceribaşi A.O. Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod. Toxicol. 2006;21:42–47. doi: 10.1016/j.reprotox.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 145.Tripathy A., Ghosh A., Dey A., Pakhira B.P., Ghosh D. Attenuation of the cyproterone acetate-induced testicular hypofunction by a novel nutraceutical lycopene: A genomic approach. Andrologia. 2017;49:e12709. doi: 10.1111/and.12709. [DOI] [PubMed] [Google Scholar]
- 146.Trejo-Solís C., Pedraza-Chaverrí J., Torres-Ramos M., Jiménez-Farfán D., Cruz Salgado A., Serrano-García N., Osorio-Rico L., Sotelo J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid. -Based Complement. Altern. Med. eCAM. 2013;2013:705152. doi: 10.1155/2013/705121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Giovannucci E., Ascherio A., Rimm E.B., Stampfer M.J., Colditz G.A., Willett W.C. Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995;87:1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 148.Corridan B., O’Donohue M., Morrissey P. Proceedings of Proceedings-Nutrition Society of London. Cambridge University Press; Cambridge, UK: 1998. Carotenoids and immune response in elderly people; p. 4A. [Google Scholar]
- 149.Khiveh A., Hashempur M.H., Shakiba M., Lotfi M.H., Shakeri A., Kazemeini S., Mousavi Z., Jabbari M., Kamalinejad M., Emtiazy M. Effects of rhubarb (Rheum ribes L.) syrup on dysenteric diarrhea in children: A randomized, double-blind, placebo-controlled trial. J. Integr. Med. 2017;15:365–372. doi: 10.1016/S2095-4964(17)60344-3. [DOI] [PubMed] [Google Scholar]
- 150.Shakeri A., Hashempur M.H., Mojibian M., Aliasl F., Bioos S., Nejatbakhsh F. A comparative study of ranitidine and quince (Cydonia oblonga Mill) sauce on gastroesophageal reflux disease (GERD) in pregnancy: A randomised, open-label, active-controlled clinical trial. J. Obstet. Gynaecol. 2018;38:899–905. doi: 10.1080/01443615.2018.1431210. [DOI] [PubMed] [Google Scholar]
- 151.Qu M., Zhou Z., Chen C., Li M., Pei L., Chu F., Yang J., Wang Y., Li L., Liu C., et al. Lycopene protects against trimethyltin-induced neurotoxicity in primary cultured rat hippocampal neurons by inhibiting the mitochondrial apoptotic pathway. Neurochem. Int. 2011;59:1095–1103. doi: 10.1016/j.neuint.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 152.Wang X.-D. Carotenoid Oxidative/Degradative Products and Their Biological Activities. Marcel Dekker; New York, NY, USA: 2004. [Google Scholar]
- 153.Jonker D., Kuper C.F., Fraile N., Estrella A., Rodríguez Otero C. Ninety-day oral toxicity study of lycopene from Blakeslea trispora in rats. Regul. Toxicol. Pharmacol. RTP. 2003;37:396–406. doi: 10.1016/S0273-2300(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 154.Trumbo P.R. Are there adverse effects of lycopene exposure? J. Nutr. 2005;135:2060s–2061s. doi: 10.1093/jn/135.8.2060S. [DOI] [PubMed] [Google Scholar]
- 155.Krinsky N.I., Beecher G., Burk R., Chan A., Erdman j.J., Jacob R., Jialal I., Kolonel L., Marshall J., Taylor Mayne P.R. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Inst. Med. 2000 doi: 10.17226/9810. [DOI] [Google Scholar]
- 156.Trumbo P., Yates A.A., Schlicker S., Poos M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Acad. Nutr. Diet. 2001;101:294. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 157.Veeramachaneni S., Ausman L.M., Choi S.W., Russell R.M., Wang X.D. High dose lycopene supplementation increases hepatic cytochrome P4502E1 protein and inflammation in alcohol-fed rats. J. Nutr. 2008;138:1329–1335. doi: 10.1093/jn/138.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cassileth B. Lycopene. Oncology. 2010;24:296. [PubMed] [Google Scholar]
- 159.Reich P., Shwachman H., Craig J.M. Lycopenemia: A variant of carotenemia. N. Engl. J. Med. 1960;262:263–269. doi: 10.1056/NEJM196002112620601. [DOI] [PubMed] [Google Scholar]
- 160.Michael McClain R., Bausch J. Summary of safety studies conducted with synthetic lycopene. Regul. Toxicol. Pharmacol. RTP. 2003;37:274–285. doi: 10.1016/S0273-2300(03)00004-7. [DOI] [PubMed] [Google Scholar]