Abstract
Background: Numerous studies have revealed that statins have antitumor effects in vivo and in vitro. However, few studies have explored the relationship between statin use and the mortality of gastric cancer (GC) patients after treatments. This study examines the relationship between statin use and the overall survival (OS) of GC patients after surgery and adjuvant chemotherapy, using data from the nationwide cohort database of Taiwan. Methods: All patients newly diagnosed with GC from 1999 to 2008 in Taiwan were identified from the Registry of Catastrophic Illness Patients Database. Through propensity score matching, statin users were matched to statin non-users at a 1:4 ratio. The relationship between statin use and the OS of patients with GC was estimated through Cox regression models. Results: The study cohort included 1835 patients with GC who had received therapies during the study period. The death numbers among statin users (defined as those who used more than 28 cumulative defined daily doses (cDDDs)) and statin non-users were 138 and 895, respectively. A dose–response association was noted between statin use and the OS of patients with GC after treatments. The adjusted hazard ratios were 0.62 (95% confidence intervals (CI), 0.50–0.78) and 0.34 (95% CI, 0.26–0.45) for statin users administered 28–167 cDDDs and >168 cDDDs, respectively, compared with no statin use (<28 cDDDs). Conclusions: This study highlights that statin use may dose-dependently improve the OS of patients with GC after surgery and adjuvant chemotherapy in Taiwan. Additional studies are required to confirm the efficacy and safety of statin use.
Keywords: statin, gastric cancer, overall survival, National Health Insurance Research Database, Registry of Catastrophic Illness Patients Database
1. Introduction
Gastric cancer (GC), the third leading cause of cancer death globally [1] and the seventh leading cause of cancer death in Taiwan in 2018, is one of the most widespread cancers, particularly in East Asia [2]. Although the incidence of GC has been declining, it remains a fatal disease. Recently, there has been an upward trend of GC incidence among young patients, especially in the Chinese population [3], and a less remarkable decline among women than in men [4]. Each year, approximately 4000 people are diagnosed with GC, and the standard incidence rate is 9.5 cases per 100,000 person-years in Taiwan [5].
Approximately 85–90% of GC cases are due to adenocarcinomas [6]. The etiology of GC is related to diet, Helicobacter pylori infection, and the environment [7]. According to the American Joint Committee on Cancer (AJCC), GC is staged into four groups based on tumor, node, and metastasis (TNM) classification. The cancer stage determines the treatment protocol and prognosis. GC is difficult to detect in the early stages because the disease usually progresses gradually in the beginning; thus, there is often a delay in the diagnosis [8].
Surgery, chemotherapy, and radiotherapy are the conventional treatments for GC. Surgical resection and chemotherapy are the first-line therapies for GC. Radiotherapy, immunotherapy, target therapy, and hyperthermic intraperitoneal chemotherapy (HIPEC) are often administered as adjuvant therapy in the advanced stage [9]. The treatment of GC stages Ib, II, and III mainly comprises first-line surgery and subsequent adjuvant chemotherapy. Stage Ia with favorable prognosis can be treated simply with surgery without any other treatment, whereas stage IV with very poor prognosis is treated with chemotherapy as a first-line treatment, usually accompanied by other neoadjuvant or adjuvant therapies [10,11]. More than half of patients with GC are at stages Ib, II, and III, and surgery and chemotherapy are the most common treatments in Taiwan [5]. The recurrence rate after GC surgery is 40%, which is reduced to 13% when accompanied by chemotherapy. The five-year survival rate for patients with GC varies based on the cancer stage and population characteristics [12]. It is less than 40% in most countries and 60–69% in Japan and South Korea [13]. Generally, the five-year survival rate after treatment is 55% in Taiwan [5]. Therefore, developing strategies to effectively enhance the survival of patients with GC is critical.
Statins, which can significantly decrease plasma cholesterol levels, are 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors. It has been confirmed that statins can lower mortality and morbidity in cardiovascular diseases, and they are often used to treat hypercholesterolemia [14]. The anticancer mechanisms of statins involve the inhibition of angiogenesis, inflammation, immunomodulation, and others [9]. In GC cell lines, statins can decrease the level of cellular cholesterol [15], suppress genes involved in cell division, and activate apoptosis [16]. Additionally, animal model studies have shown that statins, combined with radiotherapy or chemotherapy, can reduce tumor volumes [17]. However, clinical studies on this topic are limited. Some previous case–control studies have verified that patients who used statins exhibited a reduced risk of GC [15,18]. Few studies have investigated how statin use improves survival and outcomes after GC diagnosis. One study observed a 17% decrease in the cancer-related death in the United Kingdom [19], and another study revealed an 83% decrease in all-cause mortality after six months of statin use in Korea [20].
Studies have assessed the antitumor and chemopreventive role of statins; nevertheless, the effects of statin use on mortality in patients with GC after treatments remain unclear. This nationwide population-based research investigates the relationship between statin use and the overall survival (OS) of patients with GC after surgery and adjuvant chemotherapy in Taiwan.
2. Results
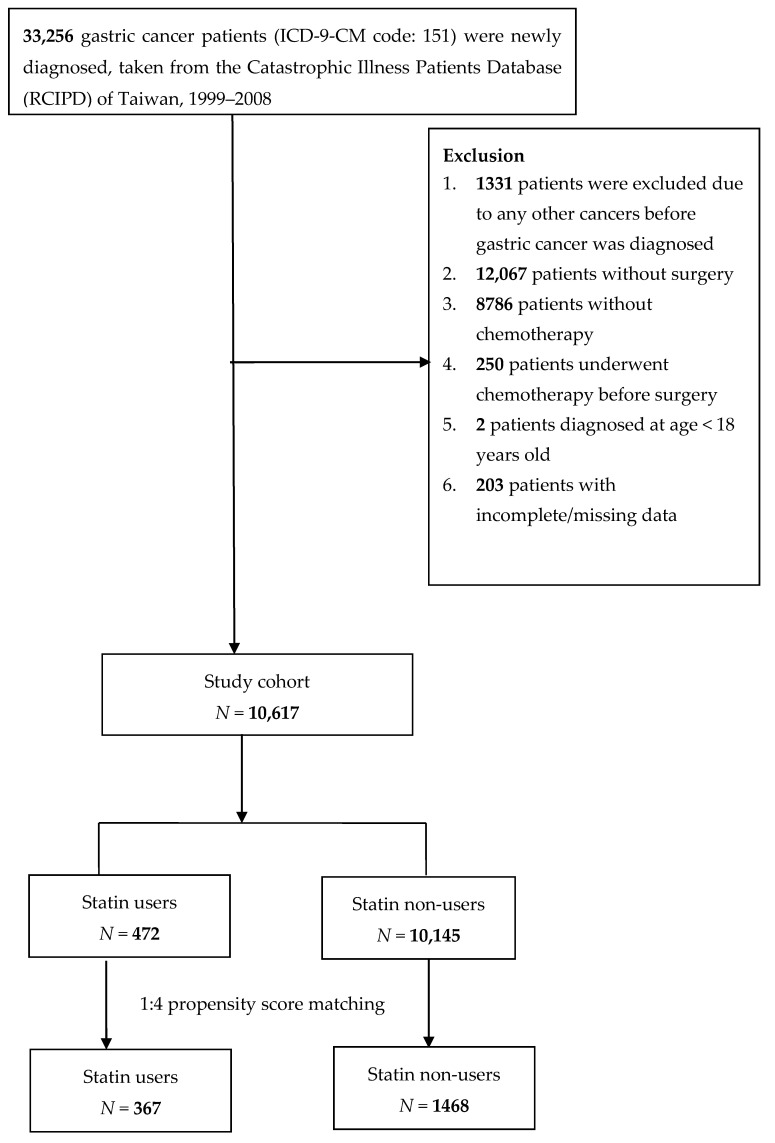
In this analysis, a total of 10,617 patients with GC were included out of the one million randomly sampled patients. We identified 472 (4.4%) patients who used statins and 10,145 (95.6%) patients who were statin non-users during 1999–2008. After propensity score (PS) matching at a ratio of 1:4, there were 367 and 1468 statin users and non-users, respectively (Figure 1).
Figure 1.
Flowchart of the patient enrollment process of the study cohort and matched cohort.
The characteristics of the study cohort are summarized in Table 1. The mean age of both statin users and non-users was 64 years. No significant difference was observed between the baseline characteristics of statin users and non-users, except that statin non-users received more triglyceride-lowering drugs. The number of GC patients that died with and without statin use was 138 (37.6%) and 895 (61.0%), respectively.
Table 1.
Demographic characteristics of statin users and statin non-users among gastric cancer patients after surgery and adjuvant chemotherapy in Taiwan during 1999–2008 in the matched cohort.
| Variables | Statin Users (N = 367) |
Statin Non-Users (N = 1468) |
p-Value * | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Gender | 0.5543 | ||||
| Female | 159 | 43.3 | 611 | 41.6 | |
| Male | 208 | 56.7 | 857 | 58.4 | |
| Age at Surgery | 0.4388 | ||||
| 18–64 | 172 | 46.9 | 655 | 44.6 | |
| ≥65 | 195 | 53.1 | 813 | 55.4 | |
| Mean (SD) | 64.3 (10.5) | 64.1 (12.6) | 0.758 | ||
| Insured Salaries (NTD$/month) a | |||||
| 0+ | 70 | 19.1 | 277 | 18.9 | |
| 1–15,840 | 38 | 10.4 | 154 | 10.5 | |
| 15,841–25,000 | 185 | 50.4 | 733 | 49.9 | |
| >25,000 | 74 | 20.2 | 304 | 20.7 | |
| Urbanization Level | 0.5232 | ||||
| Very High | 106 | 28.9 | 395 | 26.9 | |
| High | 170 | 46.3 | 737 | 50.2 | |
| Moderate | 62 | 16.9 | 241 | 16.4 | |
| Low | 29 | 7.9 | 95 | 6.5 | |
| Comorbidities | |||||
| Hypertension | 214 | 58.3 | 878 | 59.8 | 0.6009 |
| Diabetes Mellitus | 122 | 33.2 | 467 | 31.8 | 0.5996 |
| Alcoholism | 8 | 2.2 | 23 | 1.6 | 0.4150 |
| Smoking-related disorder | 40 | 10.9 | 166 | 11.3 | 0.8244 |
| Chronic kidney disease | 13 | 3.5 | 64 | 4.4 | 0.4848 |
| Liver cirrhosis | 14 | 3.8 | 61 | 4.2 | 0.7682 |
| Chemotherapy Regimen | 0.9425 | ||||
| Group 1 (epirubicin-based) | 29 | 7.9 | 105 | 7.2 | |
| Group 2 (mitomycin-based) | 49 | 13.4 | 205 | 14.0 | |
| Group 3 (taxanes) | 2 | 0.5 | 10 | 0.7 | |
| Group 4 (others) b | 287 | 78.2 | 1148 | 78.2 | |
| Medication | |||||
| Triglyceride-Lowering Drugs | 0.0046 | ||||
| User | 19 | 5.2 | 35 | 2.4 | |
| Non-user | 348 | 94.8 | 1433 | 97.6 | |
| Non-Statin Lipid-Lowering Drugs | 0.4756 | ||||
| User | 8 | 2.2 | 24 | 1.6 | |
| Non-user | 359 | 97.8 | 1444 | 98.4 | |
| ACE Inhibitors | 0.9579 | ||||
| User | 98 | 26.7 | 390 | 26.6 | |
| Non-user | 269 | 73.3 | 1078 | 73.4 | |
| Aspirin | 0.3669 | ||||
| User | 123 | 33.5 | 529 | 36.0 | |
| Non-user | 244 | 66.5 | 939 | 64.0 | |
| NSAID | 0.7487 | ||||
| User | 291 | 79.3 | 1175 | 80.0 | |
| Non-user | 76 | 20.7 | 293 | 20.0 | |
| Death | 138 | 37.6 | 895 | 61.0 | |
* Pearson’s chi-square test for categorical variables and t-test for continuous variables. a 1 USD = 32.3 New Taiwan Dollars (NTD) in the year 2008. b Others: cisplatin, carboplatin, oxaliplatin, 5-FU (fluorouracil), capecitabine, TS-1 (tegafur/gimeracil/oteracil), and tegafur.
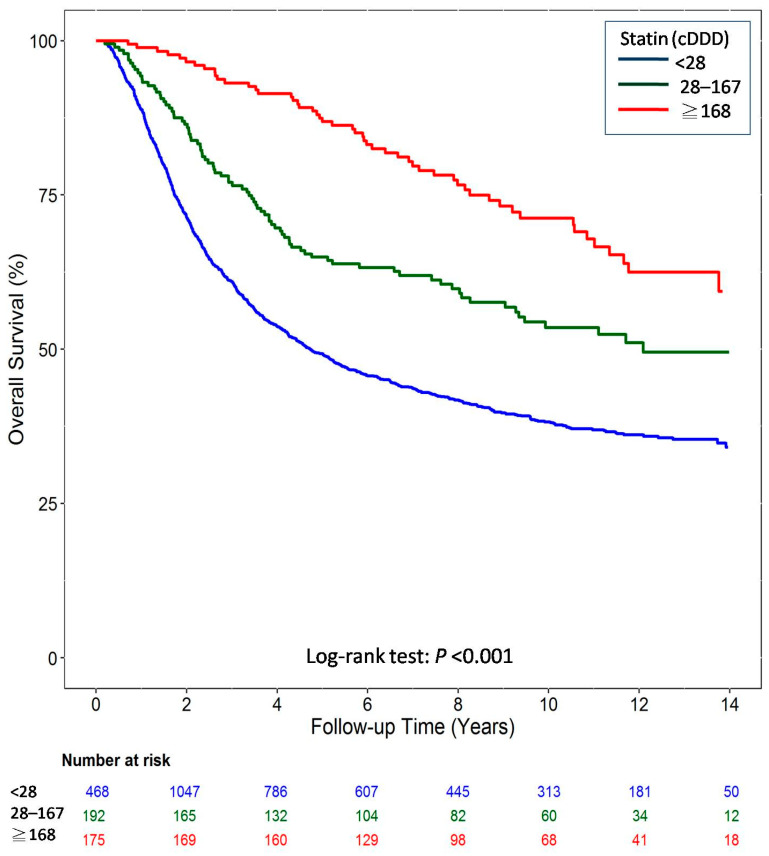
Figure 2 illustrates the outcomes of the Kaplan–Meier analysis for the matched cohort. The OS improvement showed a progressive dose–response relationship in the matched cohort. The log-rank test revealed a significant increase in the OS (p < 0.001, as per the Kaplan–Meier curve) of patients with GC.
Figure 2.
Overall survival of gastric cancer patients after surgery and adjuvant chemotherapy by cumulative defined daily dose (cDDD) of statin use during the follow-up period from the matched cohort.
Table 2 indicates the dose–response relationship between statin use and the OS of patients with GC after surgery and adjuvant chemotherapy. The adjusted hazard ratios (HRs) were 0.62 (95% confidence intervals (CI), 0.50–0.78) and 0.34 (95% CI, 0.26–0.45) for GC patients with a statin use of 28–167 cumulative defined daily doses (cDDDs) and >168 cDDDs, respectively. Sensitivity analysis showed that statin use had a small effect on the OS of patients with GC in different models with related comorbidities, chemotherapy regimen, and other medicines. This outcome was found to be consistent after excluding some factors causing significant heterogeneity. Sensitivity analysis also demonstrated that the dose–response association between statin use and OS remained in different subgroups of gender and age.
Table 2.
Adjusted hazard ratios (HRs) of overall survival of gastric cancer patients after surgery and adjuvant chemotherapy associated with statin use after surgery during the follow-up period in the matched cohort.
| Variables | 28–167 cDDD | ≥168 cDDD | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |||
| Main Model * | 0.62 | 0.50 | 0.78 | <0.0001 | 0.34 | 0.26 | 0.45 | <0.0001 |
| Additional Covariates † | ||||||||
| Main model + Diabetes mellitus | 0.64 | 0.51 | 0.80 | <0.0001 | 0.33 | 0.25 | 0.44 | <0.0001 |
| Main model + Hypertension | 0.62 | 0.50 | 0.78 | <0.0001 | 0.34 | 0.26 | 0.45 | <0.0001 |
| Main model + Alcoholism | 0.62 | 0.50 | 0.78 | <0.0001 | 0.34 | 0.26 | 0.45 | <0.0001 |
| Main model + Smoking-related disorder | 0.62 | 0.50 | 0.78 | <0.0001 | 0.34 | 0.26 | 0.45 | <0.0001 |
| Main model + Chronic renal failure | 0.62 | 0.50 | 0.78 | <0.0001 | 0.34 | 0.26 | 0.45 | <0.0001 |
| Main model + Liver cirrhosis | 0.62 | 0.50 | 0.77 | <0.0001 | 0.34 | 0.26 | 0.45 | <0.0001 |
| Main model + Chemotherapy regimen | 0.63 | 0.50 | 0.78 | <0.0001 | 0.33 | 0.25 | 0.44 | <0.0001 |
| Main model + Triglyceride-lowering drugs | 0.62 | 0.50 | 0.78 | <0.0001 | 0.35 | 0.26 | 0.46 | <0.0001 |
| Main model + Non-statin lipid-lowering drugs | 0.62 | 0.50 | 0.78 | <0.0001 | 0.34 | 0.26 | 0.45 | <0.0001 |
| Main model + ACE inhibitors | 0.61 | 0.49 | 0.76 | <0.0001 | 0.34 | 0.26 | 0.45 | <0.0001 |
| Main model + Aspirin | 0.60 | 0.48 | 0.75 | <0.0001 | 0.33 | 0.25 | 0.44 | <0.0001 |
| Main model + NSAID | 0.60 | 0.48 | 0.75 | <0.0001 | 0.32 | 0.24 | 0.43 | <0.0001 |
| Subgroup Effects | ||||||||
| Sex | ||||||||
| Male | 0.76 | 0.57 | 1.02 | 0.0674 | 0.41 | 0.30 | 0.57 | <0.0001 |
| Female | 0.49 | 0.35 | 0.70 | <0.0001 | 0.23 | 0.14 | 0.40 | <0.0001 |
| Age at Surgery | ||||||||
| 18–64 | 0.46 | 0.31 | 0.67 | <0.0001 | 0.24 | 0.14 | 0.41 | <0.0001 |
| ≥65 | 0.75 | 0.57 | 0.99 | 0.0402 | 0.40 | 0.29 | 0.55 | <0.0001 |
* Main model was adjusted for sex, age, urbanization, and income. † The models were adjusted for covariates in the main model as well as each additional listed covariate.
3. Discussion
To the best of our knowledge, our study is the first broad random nationwide research to examine the dose–response relationship between statin use and the OS of patients with GC after surgery and adjuvant chemotherapy in Taiwan. This study was controlled for the confounding effects of age, gender, urbanization, income, comorbidities, chemotherapy regimen, and other medicines. The study period was 1999–2008, and the follow-up time was until 31 December 2013. Our subpopulation analysis revealed that the use of statins reduced the mortality in these cohorts. The risk of the death for statin users and statin non-users was 37.6% and 61.0%, respectively, during the study period. After controlling for potential confounders, as the cumulative dose of statins increased a significant tendency towards reducing GC mortality was observed.
We identified the cohort from a population-based and high-quality historical computerized database, which included all the GC patients’ demographic and medical information during the study period. Thus, the possibility of selection and recall bias was eliminated. The study also had a substantial cohort size and a long follow-up period. Moreover, to examine whether the outcomes were consistent, sensitivity analyses were performed with stratification to clarify potential confounders. Especially comorbidities and chemotherapy regimen, which may affect the outcome and prognosis of GC [20], were considered important confounders and examined in this study. No significant changes were observed in the HRs of our measured outcomes in different subgroups.
Statins, which mainly prevent the risk of cardiovascular and cerebrovascular diseases and reduce serum cholesterol levels clinically, are inhibitors of HMG-CoA reductase [14]. Various previous in vitro and in vivo studies have confirmed the antitumor effects of statins on GC. Several potential anticancer mechanisms have been investigated. The basic mechanism of the anticancer effect of statins involves the rate-limiting enzyme in mevalonate synthesis and the inhibition of HMG-CoA reductase, leading to decreased activity of the RAS protein involved in cellular proliferation, differentiation, angiogenesis, and anti-apoptosis [21,22]. An experimental study showed that the use of simvastatin decreased cholesterol levels in the epithelial cells of the stomach and reduced the translocation and phosphorylation of H. pylori cytotoxin-associated gene A (CagA), which is considered to play a main role in GC development [15]. Moreover, Histone deacetylase 2 (HDAC2) was proven to be overexpressed in GC. Recent studies have demonstrated that lovastatin can suppress HDAC2 expression by binding to areas near HDAC2 active sites, and HDAC2 inhibition induces the apoptosis of GC cells. Lovastatin has also been found to inhibit the growth of GC cells in vitro in a dose-dependent manner [23]. The results of our research are consistent with the hypothesis concerning the actions of statins.
The chemopreventive effects of statins on GC have been widely discussed in many countries, especially in East Asian countries such as Japan, Korea, and Taiwan, where the GC incidence rate is the highest. Several studies have shown 30–35% reductions in GC risk with statin use [15,18,22,24]. However, only a few observational and clinical research studies have investigated the survival rate of GC patients using statins. Little evidence of progression-free survival or OS difference between statin users and placebo users was noticed [20,25,26]. Poor prognosis of the study-selected advanced GC patients may be the reason why slight significant differences were observed between the overall survival of statin users and statin non-users. A Korean study that examined all-cause mortality and recurrence-free survival in 241 patients with stage II and III GC undergoing radical gastrectomy has provided some evidence. All-cause mortality (HR 0.17; 95% CI, 0.03–0.88) and recurrence-free survival (HR 0.37; 95% CI, 0.10–1.37) in long-term statin users who had been administered statins for more than 6 months were more positive than in short-term users (<6 months) [20]. Another independent UK cohort study demonstrated decreased cancer-specific mortality (adjusted HR 0.83; 95% CI, 0.74–0.92) in GC patients with statin use [19]. In our study, a notable dose–response relationship was observed between statin use and the OS of patients with GC post surgery and adjuvant chemotherapy. The adjusted HRs were 0.62 (95% CI, 0.50–0.78) and 0.34 (95% CI, 0.26–0.45) for statin users with 28–167 cDDDs and >168 cDDDs, respectively. The difference may have been due to practical issues such as different stages of GC, varied patient populations, uneven research duration, or categories of statin exposure [27]. The sample size and the duration of follow-up affected the outcomes. Nonetheless, the survival benefits of statin use for patients with GC after surgery and adjuvant chemotherapy were confirmed in Taiwan, consistent with the result in Asian and Western populations.
This study also has several limitations. First, statin users might not have fully followed the prescribed dosage; however, improvements in the OS of patients with GC following statin use were observed. Second, National Health Insurance (NHI) does not reimburse over-the-counter statin prescriptions, which might have resulted in an underestimation. However, this influence was minimal because only a small number of patients purchase this medicine by themselves. Third, because only the date of death, but not the cause of death, was recorded by the Registry of Catastrophic Illness Patients Database (RCIPD) of National Health Insurance Research Database (NHIRD), the effects of statins on patients with GC who died of a different cause could not be analyzed. Fourth, some potential confounders, including body mass index, smoking and drinking status, which might be associated with the survival of GC patients, were not included in our database. We used alcoholism, smoking-related disorder, diabetes mellitus, hypertension, chronic kidney disease, and liver cirrhosis as additional covariates in the sensitivity analyses. No obvious confounding effects were found because the estimates did not change significantly. Finally, we could not determine the stage of GC from the NHIRD, but we could speculate the stage of GC patients from their treatment protocol. The clinical practice guideline was made by medical experts, according to Taiwan NHI payment regulations.
4. Materials and Methods
4.1. Data Source
The current research employed the Taiwan NHIRD, which included the RCIPD. By the end of 2010, nearly 23.7 million Taiwanese people (i.e., 99% of the country’s population) were insured under the NHI Program implemented in 1995 [28]. The RCIPD enrolls every patient affected by catastrophic illness that was confirmed through imaging, laboratory, pathology, and clinical diagnosis by NHI Administration experts. The dataset comprises the medical records and information of patients, such as age, gender, date of birth, medical care facilities and specialties, date of outpatient clinical visits or admission, management, procedures and treatment, prescription drugs (name, dosage, and duration), identification number of transfer, and three major diagnoses according to International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Therefore, the NHIRD provides the best platform for epidemiologic research.
Electronic information or patient identity and organization, was encrypted to protect patient privacy. Thus, the informed consent record of patients were not required in our study. The Ethics Review Board of Chang Gung Memorial Hospital, Chia-Yi Branch, Taiwan (201901743B0C501), approved this study on 9 April 2020.
4.2. Study Population
Patients newly diagnosed with GC (ICD-9-CM code 151) from 1999 to 2008 who were older than 18 years from RCIPD of Taiwan comprised the study cohort. Patients with GC who had not undergone surgery, chemotherapy, or chemotherapy before surgery were excluded from our cohort. We tracked the study cohort until 31 December 2013, or the date of death. Patients with any other cancer diagnosed before GC and those with incomplete data were also excluded.
The statin prescriptions for this study cohort were collected from RCIPD from the surgery date of patients newly diagnosed with GC during the study period, to the date of death or the end of follow-up. The total exposed dosage, cDDD [29], was used to compare the sum of dispensed DDD of statin usage with the OS of patients with GC. In the study population, patients treated with more than 28 cDDDs of statins after the date of the GC surgery were defined as statin users, whereas those treated with less than 28 cDDDs were defined as statin non-users. We also classified statin users into two groups (28–67 cDDDs and >167 cDDDs) to observe the dose–effect relationship in comparison with statin non-users.
4.3. Study Variables
The demographic characteristics of patients were studied to identify the major variables affecting statin use in this GC cohort. In addition to stratification by gender and age, analyses were stratified by the urban levels of NHI registration location, monthly insurance income, and some clinical comorbidities, which were the variables. Comorbidities included diabetes mellitus (ICD-9-CM codes 249–250), hypertension (ICD-9-CM codes 401–405), alcoholism (ICD-9-CM code 303), smoking-related disorders (ICD-9-CM codes 305.1, 491.2, 492.8, 496, 523.6, and V15.82), chronic kidney disease (ICD-9-CM code 585), and liver cirrhosis (ICD-9-CM codes 571.2, 571.5, and 571.6). Chemotherapy regimens (epirubicin-based, mitomycin-based, and taxanes) related to GC and exposure information of other medicine such as triglyceride-lowering drugs, non-statin lipid-lowering drugs, angiotensin-converting enzyme (ACE) inhibitors, aspirin, and non-steroidal anti-inflammatory drugs (NSAIDs) were collected and identified as potential confounders in this study.
4.4. Propensity Score Matching
Propensity score (PS) matching was applied to reduce the confounding effects in the two groups [30]. Thus, based on clinical variables comprising age, gender, level of urbanization, monthly insurance income, comorbidities, chemotherapy regimen, and other medicines mentioned above, we used PS to assess the probability of assigning patients as statin users to examine the effect of statin use. Statin users and statin non-users were matched by using PS at a ratio of 1:4.
4.5. Sensitivity Analyses
Sensitivity analysis was applied to assess the consistency between statin use and GC mortality. We performed analysis stratified by groups with and without the use of triglyceride-lowering drugs, non-statin lipid-lowering drugs, ACE inhibitors, aspirin, and NSAIDs; and the diseases of diabetes mellitus, hypertension, alcoholism, smoking-related disorder, chronic kidney disease, and liver cirrhosis. We also performed analysis stratified by gender and age from the date of surgery.
4.6. Statistical Analysis
Data analysis of descriptive statistics was performed to compare statin users with statin non-users stratified by patient demographics and comorbidities. We performed Pearson’s chi-square test for categorical variables and t-test for continuous variables. The Kaplan–Meier method was used to appraise the accumulative probability of OS for statin users and non-users. We used the log-rank test to compare OS curves between the groups. The HRs with 95% CIs were computed using the Cox proportional hazard model adjusted for age, gender, urban level, monthly insurance income, comorbidities, chemotherapy regimen, and other medicines. A two-tailed p < 0.05 indicated a significant difference. Data processing and analysis were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
5. Conclusions
Statin use may improve OS and reduce mortality in patients with GC after surgery and adjuvant chemotherapy in a dose-dependent manner. Further research must be conducted to improve the clinical evidence on the efficacy and safety of statin use in patients with GC.
Acknowledgments
The authors would like to thank the Health Information and Epidemiology Laboratory, Chang Gung Memorial Hospital, Chia-Yi Branch (CFRPG6K0021) for their comments and help with the data analysis. This study was based on data from RCIPD of NHIRD, which was provided by the Central Bureau of NHI and the Department of Health and was managed by the National Health Research Institutes (NHRI). The explanations and conclusions presented herein do not represent those of the Bureau of NHI, the Department of Health, or the NHRI.
Abbreviations
| cDDDs | Cumulative defined daily doses |
| GC | gastric cancer |
| CI | confidence interval |
| H. pylori | Helicobacter pylori |
| HMG-CoA | 3-hydroxy-3-methylglutaryl-coenzyme A |
| OS | overall survival |
| NHIRD | National Health Insurance Research Database |
| RCIPD | Registry of Catastrophic Illness Patients Database |
| ICD-9-CM | International Classification of Diseases, Ninth Revision, Clinical Modification |
| ACE | angiotensin-converting enzyme |
| NSAID | non-steroidal anti-inflammatory drug |
| PS | propensity score |
| HR | hazard ratio |
| Cag A | cytotoxin-associated gene A |
| HDAC2 | histone deacetylase 2 |
Author Contributions
All authors designed and conceived the study. P.-R.Y., Y.-Y.T., K.-J.C., and Y.-H.Y. analyzed and interpreted the data. P.-R.Y. and W.-T.S. conducted the study. All authors approved the final version of the submitted manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- 1.Irino T., Takeuchi H., Terashima M., Wakai T., Kitagawa Y. Gastric Cancer in Asia: Unique Features and Management. Am. Soc. Clin. Oncol. Annu. Meet. 2017;37:279–291. doi: 10.14694/EDBK_175228. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer; Lyon, France: 2018. [Google Scholar]
- 3.Correa P. Gastric cancer: Two epidemics? Dig. Dis. Sci. 2011;56:1585–1586. doi: 10.1007/s10620-011-1642-x. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA: Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 5.Health Promotion Administration Ministry of Health and Welfare Cancer Registry Report. [(accessed on 25 December 2018)];2016 Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=119.
- 6.Waddell T., Verheij M., Allum W., Cunningham D., Cervantes A., Arnold D. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2014;40:584–591. doi: 10.1016/j.ejso.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Hung K.F., Hsu C.P., Chiang J.H., Lin H.J., Kuo Y.T., Sun M.F., Yen H.R. Complementary Chinese herbal medicine therapy improves survival of patients with gastric cancer in Taiwan: A nationwide retrospective matched-cohort study. J. Ethnopharmacol. 2017;199:168–174. doi: 10.1016/j.jep.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Patel T.N., Roy S., Ravi R. Gastric cancer and related epigenetic alterations. Ecancermedicalscience. 2017;11:714. doi: 10.3332/ecancer.2017.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Cutsem E., Sagaert X., Topal B., Haustermans K., Prenen H. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 10.Coburn N., Cosby R., Klein L., Knight G., Malthaner R., Mamazza J., Mercer C.D., Ringash J. Staging and surgical approaches in gastric cancer: A systematic review. Cancer Treat. Rev. 2018;63:104–115. doi: 10.1016/j.ctrv.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Association Japan Gasoline Corporation Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roukos D.H., Kappas A.M. Perspectives in the treatment of gastric cancer. Nat. Clin. Pract. Oncol. 2005;2:98–107. doi: 10.1038/ncponc0099. [DOI] [PubMed] [Google Scholar]
- 13.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Niksic M., Bonaventure A., Valkov M., Johnson C.J., Esteve J., et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baigent C., Keech A., Kearney P.M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 15.Lin C.J., Liao W.C., Lin H.J., Hsu Y.M., Lin C.L., Chen Y.A., Feng C.L., Chen C.J., Kao M.C., Lai C.H., et al. Statins Attenuate Helicobacter pylori CagA Translocation and Reduce Incidence of Gastric Cancer: In Vitro and Population-Based Case-Control Studies. PLoS ONE. 2016;11:e0146432. doi: 10.1371/journal.pone.0146432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katoh Y., Katoh M. Hedgehog signaling pathway and gastric cancer. Cancer Biol. Ther. 2005;4:1050–1054. doi: 10.4161/cbt.4.10.2184. [DOI] [PubMed] [Google Scholar]
- 17.Manu K.A., Shanmugam M.K., Li F., Chen L., Siveen K.S., Ahn K.S., Kumar A.P., Sethi G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014;92:267–276. doi: 10.1007/s00109-013-1095-0. [DOI] [PubMed] [Google Scholar]
- 18.Chiu H.F., Ho S.C., Chang C.C., Wu T.N., Yang C.Y. Statins are associated with a reduced risk of gastric cancer: A population-based case-control study. Am. J. Gastroenterol. 2011;106:2098–2103. doi: 10.1038/ajg.2011.277. [DOI] [PubMed] [Google Scholar]
- 19.Spence A.D., Busby J., Hughes C.M., Johnston B.T., Coleman H.G., Cardwell C.R. Statin use and survival in patients with gastric cancer in two independent population-based cohorts. Pharmacoepidemiol. Drug Saf. 2019;28:460–470. doi: 10.1002/pds.4688. [DOI] [PubMed] [Google Scholar]
- 20.Nam D.H., Lee H., Park J.C., Shin S.K., Lee S.K., Hyung W.J., Lee Y.C., Kang M.W., Noh S.H. Long-term statin therapy improves oncological outcome after radical gastrectomy for stage II and III gastric cancer. Anticancer Res. 2014;34:355–361. [PubMed] [Google Scholar]
- 21.Demierre M.F., Higgins P.D., Gruber S.B., Hawk E., Lippman S.M. Statins and cancer prevention. Nat. Rev. Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 22.Joo M.K., Park J.J., Chun H.J. Additional Benefits of Routine Drugs on Gastrointestinal Cancer: Statins, Metformin, and Proton Pump Inhibitors. Dig. Dis. 2018;36:1–14. doi: 10.1159/000480149. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L., Kang W., Lu X., Ma S., Dong L., Zou B. Weighted gene co-expression network analysis and connectivity map identifies lovastatin as a treatment option of gastric cancer by inhibiting HDAC2. Gene. 2019;681:15–25. doi: 10.1016/j.gene.2018.09.040. [DOI] [PubMed] [Google Scholar]
- 24.Singh P.P., Singh S. Statins are associated with reduced risk of gastric cancer: A systematic review and meta-analysis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013;24:1721–1730. doi: 10.1093/annonc/mdt150. [DOI] [PubMed] [Google Scholar]
- 25.Kim S.T., Kang J.H., Lee J., Park S.H., Park J.O., Park Y.S., Lim H.Y., Hwang I.G., Lee S.C., Park K.W., et al. Simvastatin plus capecitabine-cisplatin versus placebo plus capecitabine-cisplatin in patients with previously untreated advanced gastric cancer: A double-blind randomised phase 3 study. Eur. J. Cancer. 2014;50:2822–2830. doi: 10.1016/j.ejca.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Bujanda L., Rodriguez-Gonzalez A., Sarasqueta C., Eizaguirre E., Hijona E., Marin J.J., Perugorria M.J., Banales J.M., Cosme A. Effect of pravastatin on the survival of patients with advanced gastric cancer. Oncotarget. 2016;7:4379–4384. doi: 10.18632/oncotarget.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friis S., Poulsen A.H., Johnsen S.P., McLaughlin J.K., Fryzek J.P., Dalton S.O., Sorensen H.T., Olsen J.H. Cancer risk among statin users: A population-based cohort study. Int. J. cancer. 2005;114:643–647. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 28.Insurance BoNH . The National Health Insurance Annual Statistical Report. Bureau of National Health Insurance; Taipei, Taiwan: 2010. [Google Scholar]
- 29.World Health Organization World Health Organization collaborating centre for drug Stat Methodology. [(accessed on 11 June 2019)]; Available online: https://www.whocc.no/atc_ddd_methodology/who_collaborating_centre/
- 30.Austin P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]