Abstract
Identification of thioredoxin binding protein-2 (TBP-2), which is currently known as thioredoxin interacting protein (TXNIP), as an important binding partner for thioredoxin (TRX) revealed that an evolutionarily conserved reduction-oxidation (redox) signal complex plays an important role for pathophysiology. Due to the reducing activity of TRX, the TRX/TXNIP signal complex has been shown to be an important regulator for redox-related signal transduction in many types of cells in various species. In addition to its role in redox-dependent regulation, TXNIP has cellular functions that are performed in a redox-independent manner, which largely rely on their scaffolding function as an ancestral α-Arrestin family. Both the redox-dependent and -independent TXNIP functions serve as regulatory pathways in glucose metabolism. This review highlights the key advances in understanding TXNIP function as a master regulator for whole-body glucose homeostasis. The potential for therapeutic advantages of targeting TXNIP in diabetes and the future direction of the study are also discussed.
Keywords: TXNIP, glucose homeostasis, TBP-2
1. Introduction
Thioredoxin interacting protein (TXNIP) was first cloned as an up-regulated gene by 1,25-dihydroxy D3 (Vitamin D3) and named as Vitamin D3 upregulated protein 1 (VDUP1) in the human leukemia cell line (HL-60) derived from a patient with promyelocytic leukemia [1]. Subsequentially, TXNIP was identified as a thioredoxin (TRX) binding protein by yeast two-hybrid assay and named thioredoxin binding protein-2 (TBP-2) [2]. Later studies identified a TBP-2 nonsense mutation gene in HcB-19 mice that confers the feature of familial combined hyperlipidemia (FCHL), and VDUP1/TBP-2 was re-named TXNIP [3]. TXNIP was shown to lessen the reducing activity of TRX in an HL-60 cell line. Although a later study revealed no clear evidence of the vitamin D3 responsive element (VDRE) in the promoter of TXNIP and regulation of TXNIP expression by Vitamin D3 in other cell types is limited [4], the direct protein–protein binding of TXNIP to TRX as well as the responsiveness to extracellular stimulation makes it an interesting target for this study. The concept of the TRX–TXNIP complex acting as the redox sensitive signal transducer “redoxisome” was previously discussed [5]. The fundamental function of TXNIP is to act as the oxidative stress responsive signal transducer redoxisome, which explains the wide influence of its biological function. In addition to nuclear receptor (NR) signaling and redox regulation, physical binding of TXNIP to the NOD-like receptor protein 3 (NLRP3) inflammasome or E3-ubiquitin ligases sheds light on TXNIP function Figure 1. In this review, we summarize the decades of work analyzing TXNIP as a master regulator of glucose homeostasis by integrating its function, physiological role, and diabetes development.
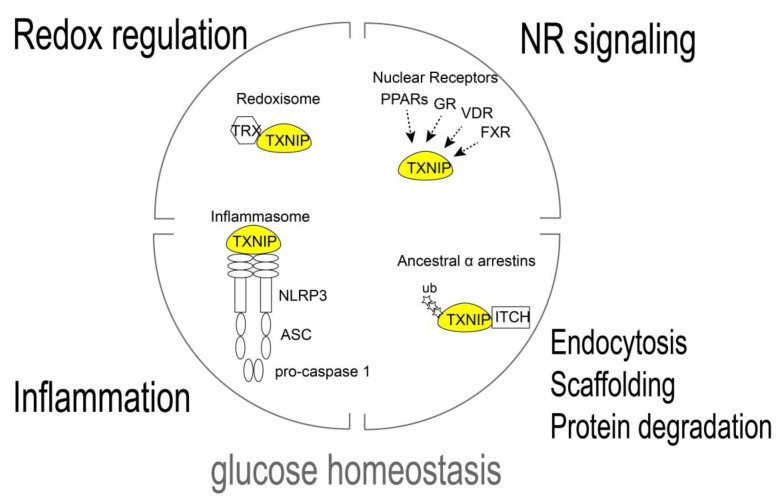
Figure 1.
TXNIP regulates glucose homeostasis as signal complex. The TRX/TXNIP signal complex, redoxisome, is the basis of TXNIP regulation of the reduction–oxidation (redox) response. TXNIP has been known to bind NOD-like receptor protein 3 (NLRP3) and activate the inflammasome. TXNIP is a member of the ancestral α-Arrestin family and TXNIP binds to the Itchy E3 Ubiquitin Protein Ligase (ITCH) and facilitates the ubiquitination of the substrates. TXNIP is transcriptionally regulated by nuclear receptors (NRs) such as peroxisome-proliferator activated receptors (PPARs), glucocorticoid receptor (GR), vitamin D receptor (VDR), and farnesoid X receptor (FXR) in a cell type specific manner. These signal complex/transducers are involved in the physiological functions of TXNIP, including the regulation of glucose homeostasis.
2. TXNIP/TBP-2 in Whole-Body Glucose–Lipid Metabolic Regulation
TXNIP has emerged as a master regulator for glucose and lipid metabolism, largely because of the finding of metabolic disordering phenotypes in TXNIP mutants or gene knockout (KO) mice. Earlier discovery of the nonsense mutation of TXNIP in HcB-19 mice revealed the important physiological function of TXNIP in glucose–lipid homeostasis [3,6]. HcB-19 mice were identified as a variant of the C3H strain, which exhibits a phenotype similar to familial combined hyperlipidemia (FCHL), such as elevated levels of plasma triglyceride, cholesterol, and free fatty acids [3,6]. In addition, HcB-19 mice have an abnormal glucose metabolic phenotype, including hyperinsulinemia, hypoglycemia, and ketosis during fasting [7,8,9]. Later studies revealed that many metabolic phenotypes identified in HcB-19 are reproducible in whole-body TXNIP knockout mice (TXNIP-WKO). With a genetic targeting strategy, we previously reported that TXNIP-WKO causes predisposition to death with hypoglycemia, hyperinsulinemia, ketosis, and abnormal liver steatosis during fasting [10,11]. Transcriptional dysregulation of feeding-fasting transition was observed in the liver of TXNIP-WKO, such as upregulation of the “fasting” signal, peroxisome proliferator activated receptor-alpha (PPAR-α), in the “feeding” status and upregulation of the feeding signal, sterol response element binding protein (SREBP), in the fasting status. Interestingly, genetic deletion or mutation of TXNIP in diabetic model mice such as ob/ob mice [12,13], STZ-induced diabetic [13,14] mice and HFD [15,16] mice exhibited improved glucose tolerance and remission of hyperglycemia. In addition, the correlation of the human insulin/glucose clamp study revealed that inversed correlation of TXNIP expression and insulin dependent glucose uptake in skeletal muscle and TXNIP expression are associated with the risk of the pathogenesis of type 2 diabetes (T2D) in humans [17]. These findings led to the idea that suppression of TXNIP in the prediabetic and diabetic conditions may be beneficial for treating human diabetes. TXNIP expression is sharply regulated in many tissues by physiological conditions, which limits the glucose metabolism such as fasting [11] and obesity-prediabetic condition [12]. Although cell type specificity of TXNIP expression has been observed, nutrients sensors, including (NRs) such as peroxisome proliferator activated receptors (PPARs) [11,18], vitamin D receptor (VDR) [1,2,18], and glucocorticoid receptor (GR) [19,20,21], also upregulate TXNIP transcriptional level and AMPK downregulates TXNIP by promoting protein degradation [22]. Glucose regulates TXNIP expression through transcriptional factor carbohydrate-response element-binding protein (ChREBP) in liver and β cells [23,24] and through MondoA/Mlx in skeletal muscle and the heart [25]. Insulin reciprocally suppresses TXNIP expression through insulin signaling in diverse tissues [11,12,13,16,26,27,28,29,30,31,32,33]. TXNIP is induced by hypoxic conditions by hypoxia-inducible factor 1 α (HIF-1α) in endothelial cells and various cancer cells [34,35,36,37,38,39,40,41]. HIF-1α is an important regulator of glycolysis, therefore local glucose metabolism may also be influenced by the HIF1-α/TXNIP axis under hypoxic conditions [42]. These findings suggest that the dynamic change of TXNIP expression is important to regulate nutritional and hormone sensing metabolic regulation Figure 2. Although the mutation or deletion of TXNIP shows that the imbalance of redox regulation, such as sulhydryl-redox or PTEN disulfide reduction, influences metabolic impairment in vivo [16,43], redox-independent, tissue specific TXNIP functions in metabolic regulation have also been widely observed. The specific role of TXNIP in metabolic tissues is discussed below.
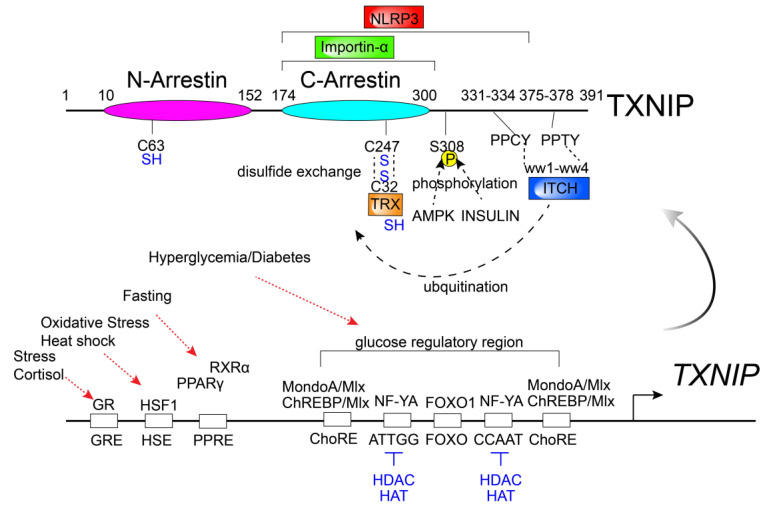
Figure 2.
Transcriptional and post-translational modification and protein interacting domains of TXNIP. TXNIP gene expression is regulated by various stimuli. Post-translational modifications and protein interactions of TXNIP influence its protein stability, localization and function in a cellular and context-dependent manner. Protein scaffolding and the biological function of TXNIP are biased in the C-arrestin domain. Two PPxY motifs (PPCY and PPTY) of Txnip bind to the four WW domains of E3 ubiquitin ligase, ITCH. Insulin and AMPK facilitate the protein degradation by serine 308 (S308) phosphorylation of TXNIP. TXNIP forms disulfide bonds with reduced TRX by disulfide exchange through its cysteine 247 (C247). Glucocorticoid–glucocorticoid responsive element (GR–GRE), heat shock factor 1–heat shock element (HSF1–HSE), peroxisome proliferator-activated receptor γ/retinoid X receptor α–peroxisome proliferator-activated receptor element (PPARγ/RXRα–PPRE), MLX interacting protein/Max-like protein X (MondoA/Mlx – ChoRE) or carbohydrate-responsive element-binding protein/Max-like protein X–carbohydrate response element (ChREBP/Mlx-ChoRE), nuclear transcription factor Y subunit α–CCAAT motif (NF-YA–ATTGG/CCAAT), Forkhead Box O1 (FOXO1)–putative FOXO1 binding site (FOXO).
2.1. TXNIP/TBP-2 in Immune Cells
TXNIP plays an important role in various types of immune cells. It was found that the number of Natural Killer (NK) cells is profoundly reduced in TXNIP-WKO, which was linked to poor ability of tumor rejection in TXNIP-WKO [44]. We previously reported that dendritic cells (DCs) derived from TXNIP are defective in inducing T-cell responses [45]. Although these results suggest that TXNIP is required for the maintenance of immune cell function in normal physiological conditions, in the tumorigenic condition, TXNIP expression shows cytotoxic effects in tumorigenic immune cells. For example, TXNIP expression is inversely correlated with hematopoietic malignancies, such as adult T-cell leukemia (ATL), and its responsiveness to glucocorticoids to induce apoptosis in T-cell lines infected with human T lymphotropic virus type-I (HTLV-I), the causative virus of adult T cell leukemia (ATL) [20,21]. It is still poorly understood how these TXNIP functions in immune cells affect metabolic phenotypes in TXNIP-WKO, while interestingly, we have previously found that lipopolysaccharide (LPS) injection in TXNIP-WKO unexpectedly exhibited dysregulation of the lipid and glucose metabolisms, such as hyperinsulinemia, hypoglycemia, fat deposition in the liver and kidney, organ injuries, glycogen depletion, and elevation of serum lipid derivatives such as free fatty acids, triglycerides, and cholesterol [46,47]. Glucose supplementation extended the survival in TXNIP-WKO under LPS challenge. These results suggest that hypoglycemia promoted by hyperinsulinemia may be a critical risk factor for mortality in circumstances in which fatty acid utilization is impaired during endotoxemia in TXNIP-WKO [46]. Notably, these metabolic disorder phenotypes resemble the phenotypes which TXNIP-WKO exhibit under fasting conditions [10,46]. These results suggest that defective immune response may contribute to the defect of metabolic regulation in TXNIP-WKO.
A remarkable feature of TXNIP in inflammatory signaling is the physical binding with NLRP3, a central component of inflammasome (Figure 2) [48]. The inflammasome activators, such as uric acid crystals, induce the dissociation of TXNIP from TRX in a reactive oxygen species (ROS)-sensitive manner and lead to the binding of NLRP3. TXNIP deficiency impaired the activation of the NLRP3 inflammasome and the subsequent secretion of interleukin-1β (IL-1β) in macrophages. Although TXNIP-NLRP3 inflammasome axis is independent with known NLRP3 activation by oligomers of islet amyloid polypeptides (IAPP) [49] (a protein that forms amyloid deposits that has been observed during type-2 diabetes in pancreatic β cells), TXNIP-NLRP3 inflammasome axis seems to be an important regulator for specific tissue inflammation in redox dependent and independent manners [48,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71].
2.2. TXNIP/TBP-2 in Pancreatic Islets
TXNIP is one of the genes most highly upregulated by high glucose stimulation in murine and human β cells [72,73]. Glucose sensing TXNIP induction is tissue specific, and in the case of β cells, the glucose sensor carbohydrate-response element-binding protein (ChREBP) directly binds to the promoter region of TXNIP and enhances the gene expression [72]. ChREBP is regulated by glucose metabolites such as glucose-6 phosphate (G6P), xylulose 5-phosphate (X5P), and fructose 2,6-biphosphate. The major regulator of glycolysis has been implicated to bind the glucose-response activation conserved element (GRACE) of ChREBP to activate cytosolic–nuclear translocation for further downstream regulation of β cell function including β cell proliferation/compensation and death [74]. Glucose responsiveness of TXNIP is linked to the high glucose-induced apoptosis induction. One of notable function of TXNIP in β cells is the induction of apoptosis in response to high glucose [72,75]. TXNIP is induced by various kinds of stimulation and links to the apoptosis induction by streptozotocin [14,76], ER-stress [77,78,79], dexamethasone/glucocorticoid [80], lipids [81], inflammation/cytokines [14,82], and oxidative stress [48,83]. The stress-induced upregulation of TXNIP is observed in the pancreatic islets during the progression of diabetes in both mice [12] and humans [17]. TXNIP deficiency is protective for β cells from mouse models of both type-1 (T1D) and type-2 diabetes (T2D) [12,13,77]. Mechanistically, TXNIP inhibits TRX (in the cytosol, nucleus) and thioredoxin-2 (TRX2, mitochondria) and enhances oxidative stresses. TXNIP is predominantly expressed in the cytosol and nucleus, while upon oxidative stress TXNIP shuttles to the mitochondria and interacts with TRX2 and releases the interaction between TRX2 and apoptosis signal regulating 1 protein (ASK1), which leads to the activation of ASK1 to induce β cell apoptosis [84,85]. TXNIP induces several microRNAs (miRNAs) to promote β cell apoptosis. TXNIP increases expression of pro-apoptotic miR-200, which inhibit zinc finger E-box-binding homeobox 1 (Zeb1) to promote apoptosis [86]. Although TXNIP undoubtedly controls β cell’s apoptosis, induction of apoptosis by TXNIP typically takes from 24 h to a few days by TXNIP overexpression [12]. Since the high glucose response TXNIP expression is rapid, TXNIP functions other than apoptotic regulation may be crucial for β cell function. TXNIP deficiency enhances glucose-stimulated insulin secretion (GSIS) under feeding-fasting nutritional regulation [11]. TXNIP deletion improves GSIS function in the T2D model ob/ob mice, which contributes the amelioration of hyperglycemia [12]. These results suggest that prior to the apoptotic induction, TXNIP acts as suppressor of GSIS, which may save glucose utilization under the fasting and/or stress induced conditions. In addition to the physiological regulation of insulin secretion and apoptosis, it has been reported that TXNIP mediates miR-204 induction and directly inhibits INSULIN transcription through down regulation of MAFA [87]. TXNIP has also been shown to mediate glucose dependent upregulation of islet amyloid polypeptide (IAPP) through miR-124a [88]. IAPP upregulation has been suggested as a marker for functional maturation during human β cells development [89]. Accumulation of IAPP is known to promote inflammation and β cell dysfunction [49]. These important gene regulatory functions of TXNIP have just started to be revealed. TXNIP may have differential functions under stress or healthy physiological conditions.
2.3. TXNIP/TBP-2 in Peripheral Tissues (Muscle, Adipose, Liver)
Regulation of glucose metabolism by TXNIP in muscle is highlighted by the human glucose/insulin physiological clamp study, which showed the dynamic regulation of TXNIP by glucose and insulin [17]. In addition, TXNIP expression is inversely correlated with glucose uptake in healthy humans and it is upregulated in skeletal muscle of prediabetic and diabetic T2D patients [17]. TXNIP expression in muscle cells, including vascular smooth muscle, skeletal muscle, and cardiomyocytes, is known to be regulated by MAPK signaling and PI3K/insulin signaling. Early studies identified that glucose-induced TXNIP expression is abolished by the P38 MAPK inhibitor PD169316 [43]. In addition, glucose-induced TXNIP expression is upregulated by phosphoinositide 3-kinase (PI3K) inhibitor wortmanin but not the ERK inhibitor U0126, Gi inhibition by the pertussis toxin, or protein kinase C (PKC) inhibition by GF109203X in human aortic smooth muscle cells (SMCs) [43]. PDGF-BB enhanced phosphorylation of PI3K/AKT in the central network of insulin signaling and reduced TXNIP transcription in both low and high glucose conditions in SMCs [43]. Serine 308 of TXNIP was shown to be targeted for the phosphorylation induced by insulin [32] or AMPK [22], which lead to the rapid degradation of TXNIP protein (Figure 2). TXNIP induction by glucose is regulated by MondoA, an analog of ChREBP, at a transcriptional level in skeletal muscle and the heart [25]. It has been reported that the mammalian target of rapamycin (mTOR), a downstream molecule of PI3K, physically binds to MondoA in the cytoplasm and prevents MondoA–Mlx complex formation and restricts MondoA’s nuclear entry and reduces transcriptional activation of TXNIP [25,90]. Overexpression of TXNIP suppresses glucose uptake while knockdown or knockout of TXNIP enhances glucose uptake in skeletal muscle and adipose in vitro and in vivo [12,16,17,32,91]. The amount of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) is regulated by the balance of PI3K and the phosphatidylinositol 3-phosphatase PTEN [92]. Oxidation of a small fraction of critical cysteine residues is regulated by TRX and inactivates PTEN; therefore, redox signaling influences insulin and growth factor receptor signaling via expansion of PIP3 accumulation, which activates the downstream signaling kinase Akt [93,94,95,96,97]. It has been shown that TXNIP regulates PTEN disulfide reduction via TRX and glucose uptake [16]. We have shown that TXNIP preserves almost full insulin sensitivity by increasing insulin responsiveness of AKT phosphorylation under severe insulin resistance conditions, such as ob/ob mice [12]. Regulation of whole-body insulin sensitivity by TXNIP is modulated in white adipose tissue. Larger adipose-size phenotypes in both TXNIP-WKO and adipose-specific TXNIP deletion (TXNIP-AKO) in control treatments and high-fat/diabetic conditions were observed, and this phenotype displayed larger capacity for the storage of nutrients, including glucose, compared wild type (WT) mice [12,15]. TXNIP also controls hepatic gluconeogenesis [98,99]. TXNIP-WKO exhibited abnormal liver steatosis and impaired gluconeogenesis during fasting, which may contribute the phenotype of hypoglycemia-induced predisposition for death [10,11]. Similarly, liver specific TXNIP deletion (TXNIP-LKO) caused hypoglycemia and ketoacidosis, which is consistent with the finding of poor glucose production and increased β-hydroxybutyrate release from isolated hepatocytes from TXNIP-LKO mice [99]. Forkhead box protein O1 (FOXO1), a master transcriptional factor for gluconeogenesis in the liver, directly regulates TXNIP expression through the binding of the promoter region of TXNIP which contains a conserved consensus sequence, ′GTAAACAA′, of the FOXO binding site [100,101]. Taken together, these early discoveries revealed the tight link between TXNIP expression and glucose or insulin signaling in peripheral tissues.
2.4. TXNIP/TBP-2 in Central Nervous System
Although the direct action of the hormones in peripheral tissues is sufficient to mediate the regulation of glucose/nutritional handling, the crucial role of the central nervous system (CNS) in glucose homeostasis [102] has been widely acknowledged. The mediobasal hypothalamic (MBH) TXNIP expression is changed depending on the nutritional condition. Fasting induces TXNIP expression, whereas refeeding, leptin infusion, or insulin infusion reduces TXNIP expression in the hypothalamus [103]. Experimental diabetes models induced by STZ, or polygenic models of obesity, adult onset T2D, diet-induced hyperglycemia and obesity, and NONcNZO10/LtJ mice all have MBH TXNIP expression increased at feeding status, suggesting that TXNIP is responsible for the nutritional sensing and pathophysiological conditions in MBH [103]. TXNIP overexpression but not C247S-mutated (binding site with TRX) TXNIP overexpression in MBH reduces the energy expenditure and causes glucose intolerance, evidence that TXNIP regulates energy homeostasis with TRX in a binding-dependent manner [103]. MBH comprises the arcuate nucleus of the hypothalamus (ARH) and the ventromedial nucleus of the hypothalamus, which includes agouti related peptide (AgRP) neurons. Later studies revealed that AgRP neuron specific TXNIP deletions generated by AgRP-Ires-Cre mice and TXNIP flox/flox mice exhibit mild lean phenotype [104]. In addition to nutritional sensing, the hypoxic–ischemia or excessive ROS-dependent neuron toxicity under normal or diabetic conditions is caused by TXNIP induction [33,53,105,106,107,108]. Although these phenotypes are caused by the CNS, it does not explain the remarkable glucose tolerance and glucose disposable phenotype of TXNIP-WKO. CNS TXNIP may be an important player to control whole-body energy expenditure and adiposity. Recent evidence indicates that Parkinson’s disease and diabetes, both age and environmental stress-related chronic diseases, share remarkably similar dysregulation pathways. TXNIP was linked with dysregulation in β-cells, peripheral tissues for diabetes, and dopaminergic neurons, especially under high glucose conditions.
3. TXNIP/TBP-2 in Molecular Functions
3.1. TXNIP/TBP-2 as α-Arrestin, a Scaffold Protein Family
Although the TRX/TXNIP redoxisome signal complex is the primary concept of the molecular basis of TXNIP function [5], several remarkable features of TXNIP may provide the molecular mechanism of TXNIP in glucose homeostasis beyond redox signaling. Arrestins are protein families of scaffolding proteins for signal transduction in organisms. For example, β-Arrestins such as β-Arrestin 1 and β-Arrestin 2 bind to the G-protein coupled receptors (GPCRs) and desensitize them by multiple strategies, including preventing further activation and promoting receptor internalization and degradation [109,110,111,112]. In addition, β-Arrestins bind to Smoothened and Frizzeled receptors, the known seven-transmembrane-receptors (7TMRs) that mediate Hedgehog and Wnt signaling. β-Arrestin 2 is an important regulator of insulin signaling by direct binding to the insulin receptor (IR) [113]. Inteestingly, β-Arrestin 2 knockout (β-Arrestin 2KO) mice showed insulin resistance when eating a normal control diet. Insulin resistance occurs in WT mice by failing to recruit the thyrosine-protein kinase Src and the serin/threonine protein kinase Akt to the IR/β-Arrestin 2 signal complex [113]. Another Arrestin family is called as the α-Arrestins, which consist of ARRDC1-5 and TXNIP. ARRDC3 (which is originally identified as thioredoxin-binding-protein-2-like inducible membrane protein/TLIMP, the target of PPARγ [18]) acts as a membrane scaffold protein like β-Arrestins to regulate glucose uptake in adipose tissues [114]. TXNIP may exert its regulatory effects through its actions in the cytosol, nucleus, and mitochondria as well as intracellular matrix. It has been shown that TXNIP translocate to the intracellular matrix to promote the internalization of GLUT1 and restrict glucose uptake and glycolysis [115]. This novel TXNIP function at the cellular level was linked to the extracellular remodeling during tumorigenesis and embryogenesis. At tissue level, TXNIP regulates glucose homeostasis not only by regulating insulin signaling in adipose or muscles, but also by regulating gluconeogenesis in the liver, insulin secretion in the pancreatic β cells, and whole-body glucose disposals through CNS. These insights suggest that TXNIP is a more dynamic regulator of glucose homeostasis than β-Arrestins. TXNIP directly binds to importin α, a nuclear transportation protein providing the mechanism of nuclear shuttling from the cytosol of TXNIP [116] (Figure 2). GFP-tagged TXNIP overexpression or HDAC inhibitor SAHA-induced TXNIP induction revealed that TXNIP mainly localizes in the nucleus [116], suggesting that TXNIP may act as the scaffold protein in the nucleus for regulation of gene expression. We have shown that TXNIP suppresses PPARs signaling and activates SREBP signaling in the liver [11]. Protein purification aimed to identify TXNIP interacting protein in the rat β cell line INS-1 revealed that the candidate nuclear proteins binding to TXNIP include Mybbp1a, DEAD (Asp-Glu-Ala-Asp) box polypeptide 5 (Ddx5), GCN1, and NoO/p54nrb homolog [12]. More recently, it has been identified that TXNIP forms a high molecular weight complex (1000–1300 kDa) in redox sensitive manners [117,118]. This finding suggests that the scaffolding function of TXNIP may be modified by oxidative stress and the physical interaction with TRX. The shuttling of TXNIP to the mitochondria was found by H2O2 treatment in INS-1 cells and forms the protein complex with TRX2 (mitochondrial TRX) [85]. Further identification of protein complexes of TXNIP in different cell type with different physiological conditions may facilitate understanding of TXNIP’s molecular function.
3.2. Protein Degradation of TXNIP/TBP-2
The α-Arrestins family of proteins includes TXNIP and conserves the PPXY sequences that are known binding motifs of the WW domain [119,120,121]. Yeast Arrestin-related trafficking adaptors (ARTs), which are the homologs of mammalian ARRDCs, have been identified to contain PPXY motifs that interact with Nedd4-like ubiquitin ligase, Rsp5, resulting in ubiquitination and regulating the internalization of plasma membrane proteins (cargos) and degradation in the lysosome [121]. In humans, the NEDD family has nine members: NEDD4, NEDD4L, WWP1, WWP2, ITCH, SMURF1, SMURF2, HECW1, and HECW2 [120]. TXNIP undergoes proteasomal degradation by polyubiquitination through the physical interaction with the HECT ubiquitin ligase ITCH [122,123,124,125]. In contrast, TXNIP stabilizes p53 expression by interacting with human ecdysoneless (hEcd), which is known for its role in stabilizing p53 protein expression [126]. TRX stabilizes TXNIP protein expression, possibly preventing the interaction between the PPXY motif of TXNIP and the WW domain of NEDD ubiquitin ligase families [127] (Figure 2). There is growing evidence that TXNIP may act as the scaffold for proteins when it should be undergoing proteasomal degradation when not bound with TRX. TXNIP stabilizes protein expression when bound with TRX in response to a variety of stress signaling in a redox dependent manner.
4. TXNIP/TBP-2 in Clinical Work and the Future
Given the remarkable function of TXNIP for glucose homeostasis, TXNIP has been recognized as an attractive target for treatment in both T1D and T2D. In addition, TXNIP possibly can be targeted to metabolic disease related complications such as cardio-vasculature disease [128,129,130,131] and kidney failure [132,133]. Verapamil was originally used for controlling ventricular rate in supraventricular tachycardia, migraine headache prevention, treatment of high blood pressure, and angina [134,135]. Recent studies revealed that Verapamil reduces TXNIP expression in multiple cell types in vivo and in vitro, including pancreatic β cells [76,136,137,138,139,140,141]. Since verapamil treatment in the multi low dose streptozotocin (MLD-STZ) induced mouse model of T1D and obese T2D model ob/ob mice preserved functional β cell mass and ameliorated hyperglycemia [76], a randomized-double blind, placebo controlled Phase 2 clinical trial with verapamil in adult subject recent-onset T1D was performed [140]. The study found once daily oral verapamil treatment for 12 month improves endogenous β cells insulin secretion function with a lower increase of insulin requirements and fewer hypoglycemic events in adult individuals with recent-onset T1D [140]. Interestingly, most recent study identified a small molecule that inhibit TXNIP expression and ameliorates hyperglycemia in both mice model of T2D (db/db) and T1D (STZ) [141]. It was shown that SRI-37330 treatments down-regulate TXNIP mRNA and protein level in rat β cell line as well as in mouse and human islets. The study also showed SRI-37330 reduces TXNIP-mediated glucagon secretion from α cells and suppress hepatic gluconeogenesis. Although the mechanism how SRI-37330 inhibits TXNIP expression, the specificity for targeting TXNIP and the molecular links between TXNIP and glucagon secretion is uncertain, these results encourage targeting TXNIP as promising anti-diabetic therapeutics (Figure 3). However, more tissue specific targeting of TXNIP is required for safer and efficient treatment of diabetes, since anti-oncogenic function of TXNIP has been well known [19,94,116,142,143,144,145,146,147,148,149,150,151,152,153], which suggests that chronic TXNIP inhibition in proliferative tissues such as the liver or intestine may increase the risk for tumorigenesis. Tissue specific physiological role of TXNIP give us a lesson to learn the targeting tissues for each specific condition in diabetes (Table 1). The specificity of TXNIP inhibition should be also considered since none of the known TXNIP modulators target only TXNIP, rather broadly affecting transcriptome [154]. In addition, although drug treatments such as insulin, glucagon-like peptide-1 (GLP-1/Exendin-4), thiazolidinediones (TZD), dipeptidyl peptidase 4 (DPP-IV) inhibitors, sodium–glucose co-transporter-2 (SGLT2) inhibitors, and metformin are successful to provide the therapeutics in T1D or T2D [155,156,157,158,159], none of these therapeutics provide a “functional cure” for the diabetes, which means life-long drug treatment to ameliorate diabetes is required for the patients. As a result, TXNIP targeting for diabetic therapeutics should be more specific rather than treatment with inhibitors to provide a functional cure for both T1D and T2D (Figure 3). Although, so far, the specific tissue or region targeting TXNIP therapeutics has not been demonstrated, one such idea is using gene delivery technology combined with genome engineering. Recent success of CRISPR associated protein 9 (Cas9) and targeted single guide RNA (sgRNA) delivery using adeno-associated virus (AAV) in selective regions of tissues in vivo provides evidence for the therapeutic utility of genome engineering technology [160,161]. Tissue specific TXNIP deletion in adipose or skeletal muscle exhibited powerful insulin sensitization with no evidence of tumorigenesis, therefore AAV-CRISPR mediated TXNIP deletion in those tissues would be beneficial for treating T2D. Using insulin promoter-driven Cas9 and TXNIP sgRNA expression in pancreatic β cell may also be effective to sustain the functional β cell mass in both T1D and T2D patients. Besides, direct gene editing of TXNIP in vivo, TXNIP modification in vitro may contribute for the advanced therapeutics in diabetes. For example, regulation of TXNIP expression may optimize the current protocol of human β-like cell generation from pluripotent stem cells. Although TXNIP-KO mice do not show any evidence for defects of β cell differentiation, TXNIP is one of the most highly responsive genes for high glucose and regulates both β cell function and survival. A recent study showed that in human pluripotent stem cells, derived insulin-producing β cells (sc-β cells) with or without gene collection of the pathogenic variant of Wolfram Syndrome (WS) revealed a 1.5–2-fold TXNIP gene induction by high glucose stimulation [162]. This is an encouraging observation; however, the induction rate of TXNIP genes in response to high glucose in human β cells lines or primary human islets are generally even higher (~10-fold) [12,72,73], suggesting that TXNIP signaling may not be fully activated in human pluripotent stem cell-derived insulin-producing β cells. Since TXNIP has been shown to regulate MAFA expression through miR-204 in murine and human islets [87], the current limited expression of MAFA in hPSC-derived insulin producing cells [89,163,164,165,166,167] might be potentially modified by TXNIP expression. Glucose responsiveness of TXNIP gene expression may provide the fine tuning of glucose responsiveness of human pluripotent stem cell-derived insulin-producing β cells.
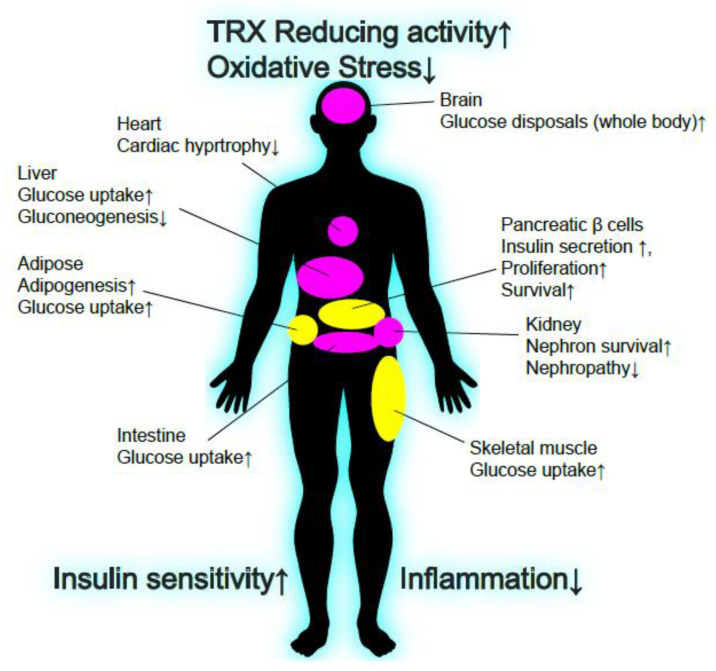
Figure 3.
Beneficial effects of TXNIP inhibition for glucose homeostasis. Known effects of TXNIP down-regulation in glucose homeostasis are shown. TXNIP is known to be up-regulated in many tissues of a variety of pathogenic conditions, including T1D and T2D. Broadly, TXNIP down-regulation enhances TRX reducing activity and protects from oxidative stress. TXNIP down-regulation also enhances insulin sensitivity and suppresses inflammation. Tissue specific TXNIP down-regulation may provide safer treatment for T1D and T2D (yellow), and since TXNIP has anti-oncogenic functions in several tissues, chronic whole-body TXNIP inhibition may cause serious issues for cancer development (pink).
Table 1.
Physiological role of TXNIP in normal, obese, STZ, endotoxin, ischemia, and diabetic conditions. The key physiological findings based on genetic knockout (KO) or mutated mice are summarized. ↑ Up-regulation/Enhanced function, ↓ Down-regulation/Reduced function.
| Tissue/Cell Type | TXNIP Function | Key Signal | TXNIP Whole Body KO/Mutant | TXNIP Tissue Specific KO/Mutant | Reference |
|---|---|---|---|---|---|
| β cells (Normal) |
Apoptosis↑ Glucose-stimulated insulin secretion (GSIS)↓ |
Mitochondria metabolism TRX/TRX2/ROS MAFA/miR-204 IAPP/miR-24a Zeb1/miR-200 |
GSIS↑ Hyperinsulinemia↑ |
β cell mass↑ Apoptosis↓ |
[11,12,41,86,87,88] |
| β cells (STZ/Obese/Diabetic) |
Apoptosis↑ Glucose-stimulated insulin secretion (GSIS)↓ β cells hypertrophy↓ |
Mitochondria metabolism/uncoupling AKT/Bcl-2 UPR/ER stress |
GSIS↑ Hyperinsulinemia↑ Apoptosis↓ |
Hyperinsulinemia↑ β cell mass↑ Apoptosis↓ |
[12,13,14,78,79] |
| Skeletal muscle (Normal) |
Insulin sensitivity/Glucose uptake↓ | AKT/GLUT4 PTEN AMPK |
Insulin Sensitivity↑ | Insulin Sensitivity↑ | [12,16,32,91,130] |
| Skeletal muscle (Obese/Diabetic) |
Insulin sensitivity/Glucose uptake↓ (Insulin Resistance↑) | AKT/GLUT4 PTEN |
Insulin Sensitivity↑ | [12,16] | |
| Adipose (Normal) |
Insulin sensitivity/Glucose uptake, Adipogenesis↓ | AKT/GLUT4 | Insulin Sensitivity↑ | [11,12,16,32] | |
| Adipose (Obese/Diabetic) |
Insulin sensitivity/Glucose uptake↓ (Insulin Resistance↑) | AKT/GLUT4 | Insulin Sensitivity↑ | Insulin Sensitivity↑ | [12,15] |
| Liver (Normal) |
Gluconeogenesis↑ Lipogenesis↓ |
FOXO1 SREBP PPARα AKT |
Abnormal steatosis in fasting | Normal glycemica, Hypoglycemia in fasting | [7,8,9,10,11,16,43,98,99] |
| Liver (Obese/Diabetic) |
Gluconeogenesis↑ Lipogenesis↓ |
PRMT1/PGC1a | Lipogenesis↓ | [12] | |
| Immune cells (Normal) |
Inflammation↓ Tumor rejection↑ Hematopoetic Stem Cells↓ |
NLRP3 inflammasome TRX/ROS P38 |
NK cells↓ T-cell response↓ |
[44,45,46,47,48] | |
| Immune cells (Obese/Diabetic/Endotoxin) |
Inflammation↓ | PI3K/ROS NO |
Resistant to P. aeruginosa-induced bacteremic shock Metabolic disordering by LPS |
[46,47] | |
| Brain (Normal) |
Glucose uptake↓ | TRX/ROS AKT/GLUT4 |
Glucose uptake↑ | [32] | |
| Brain (Obese/Diabetic) |
Energy expenditure↑ Adipogenesis↓ Body Weight↑ Insulin resistance↑ |
TRX/ROS | Hypothalamus:Body Weight↓ Insulin resistance↓ AgRP Neuron: Energy expenditure↓ Adipogenesis↓ |
[103,104,105] | |
| Heart (Normal) |
Fatty Acid oxidation↓ Glucose Oxidatation↑ |
miR33/AMPKα | Fatty Acid oxidation↑ Glucose Oxidatation↓ |
[131] | |
| Heart (Obese/Diabetic/Ischemia) |
Mitochondria↑ | Resistant for ischemia-reperfusion injury | [128,129,130] |
5. Conclusions
After the discovery that TXNIP/TBP-2 is a binding partner of the antioxidant TRX, the TRX/TXNIP signal complex in redox-dependent (redoxisome) and -independent pathways has been studied. Current emerging evidence clearly suggests that TXNIP is a central master regulator of whole-body glucose homeostasis in both rodents and humans. To further facilitate therapeutics to provide a functional cure for diabetes, more sophisticated TXNIP-targeting therapeutics should be developed using state-of-the-art biotechnology. We still do not fully understand the basic molecular mechanisms of how TXNIP interacts with other proteins, responding various stimuli in the different cell types and different cellular localizations. Of great interest, probably TXNIP function to inhibit TRX activity by direct binding is important not only for their redox sensitive regulation, but also for their redox independent function thorough structural changes of protein complex. Further investigation of redox-dependent and -independent scaffolding functions of TXNIP may give us deeper insights of the molecular functions of TXNIP in pathophysiology and future therapeutics for diabetes.
Acknowledgments
I apologize for those references I could not include due to space limitations. The author thanks Junji Yodoi and Hiroshi Masutani’s laboratory in Virus Research Institute at Kyoto University for the nurturing environment and mentorships to study TRX/TXNIP molecular pathophysiology in the glucose homeostasis, backing the time from 2006 to 2011. The author thanks Ronald Swerdloff for proof reading of the manuscript. Correspondence should be addressed to Eiji Yoshihara (eiji.yoshihara@lundquist.org).
Abbreviations
VDUP1: Vitamin D3 Upregulated protein; TBP-2, Thioredoxin Binding Protein-2, TXNIP, Thioredoxin-interacting Protein; TRX, Thioredoxin; NRs, nuclear receptors; PPARs, peroxisome-proliferator activated receptors; GR, glucocorticoid receptor, VDR, vitamin D receptor; FXR, farnesoid X receptor; TRX2, Thioredoxin-2; ASK1, Apoptosis signal regulating kinase 1; ChREBP, Carbohydrate response element binding protein; NLRP3, Nod-like receptor protein 3; ROS, Reactive oxygen species; T1D, Type-1 diabetes; T2D, Type-2 diabetes.
Funding
This research was partially supported by Mishima Kaiun Memorial foundation.
Conflicts of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
References
- 1.Chen K.S., DeLuca H.F. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim. Biophys. Acta. 1994;1219:26–32. doi: 10.1016/0167-4781(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 2.Nishiyama A., Matsui M., Iwata S., Hirota K., Masutani H., Nakamura H., Takagi Y., Sono H., Gon Y., Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 3.Bodnar J.S., Chatterjee A., Castellani L.W., Ross D.A., Ohmen J., Cavalcoli J., Wu C., Dains K.M., Catanese J., Chu M., et al. Positional cloning of the combined hyperlipidemia gene Hyplip1. Nat. Genet. 2002;30:110–116. doi: 10.1038/ng811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig D.L., Kotanides H., Le T., Chavkin D., Bohlen P., Witte L. Cloning, genetic characterization, and chromosomal mapping of the mouse VDUP1 gene. Gene. 2001;269:103–112. doi: 10.1016/S0378-1119(01)00455-3. [DOI] [PubMed] [Google Scholar]
- 5.Yoshihara E., Masaki S., Matsuo Y., Chen Z., Tian H., Yodoi J. Thioredoxin/Txnip: Redoxisome, as a redox switch for the pathogenesis of diseases. Front. Immunol. 2014;4:514. doi: 10.3389/fimmu.2013.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellani L.W., Weinreb A., Bodnar J., Goto A.M., Doolittle M., Mehrabian M., Demant P., Lusis A.J. Mapping a gene for combined hyperlipidaemia in a mutant mouse strain. Nat. Genet. 1998;18:374–377. doi: 10.1038/ng0498-374. [DOI] [PubMed] [Google Scholar]
- 7.Hui T.Y., Sheth S.S., Diffley J.M., Potter D.W., Lusis A.J., Attie A.D., Davis R.A. Mice lacking thioredoxin-interacting protein provide evidence linking cellular redox state to appropriate response to nutritional signals. J. Biol. Chem. 2004;279:24387–24393. doi: 10.1074/jbc.M401280200. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly K.L., Margosian M.R., Sheth S.S., Lusis A.J., Parks E.J. Increased lipogenesis and fatty acid reesterification contribute to hepatic triacylglycerol stores in hyperlipidemic Txnip-/-mice. J. Nutr. 2004;134:1475–1480. doi: 10.1093/jn/134.6.1475. [DOI] [PubMed] [Google Scholar]
- 9.Sheth S.S., Castellani L.W., Chari S., Wagg C., Thipphavong C.K., Bodnar J.S., Tontonoz P., Attie A.D., Lopaschuk G.D., Lusis A.J. Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J. Lipid Res. 2005;46:123–134. doi: 10.1194/jlr.M400341-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Oka S., Liu W., Masutani H., Hirata H., Shinkai Y., Yamada S., Yoshida T., Nakamura H., Yodoi J. Impaired fatty acid utilization in thioredoxin binding protein-2 (TBP-2)-deficient mice: A unique animal model of Reye syndrome. FASEB J. 2006;20:121–123. doi: 10.1096/fj.05-4439fje. [DOI] [PubMed] [Google Scholar]
- 11.Oka S., Yoshihara E., Bizen-Abe A., Liu W., Watanabe M., Yodoi J., Masutani H. Thioredoxin binding protein-2/thioredoxin-interacting protein is a critical regulator of insulin secretion and peroxisome proliferator-activated receptor function. Endocrinology. 2009;150:1225–1234. doi: 10.1210/en.2008-0646. [DOI] [PubMed] [Google Scholar]
- 12.Yoshihara E., Fujimoto S., Inagaki N., Okawa K., Masaki S., Yodoi J., Masutani H. Disruption of TBP-2 ameliorates insulin sensitivity and secretion without affecting obesity. Nat. Commun. 2010;1:127. doi: 10.1038/ncomms1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J., Hui S.T., Couto F.M., Mungrue I.N., Davis D.B., Attie A.D., Lusis A.J., Davis R.A., Shalev A. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J. 2008;22:3581–3594. doi: 10.1096/fj.08-111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masson E., Koren S., Razik F., Goldberg H., Kwan E.P., Sheu L., Gaisano H.Y., Fantus I.G. High beta-cell mass prevents streptozotocin-induced diabetes in thioredoxin-interacting protein-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2009;296:E1251–E1261. doi: 10.1152/ajpendo.90619.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chutkow W.A., Birkenfeld A.L., Brown J.D., Lee H.Y., Frederick D.W., Yoshioka J., Patwari P., Kursawe R., Cushman S.W., Plutzky J., et al. Deletion of the alpha-arrestin protein Txnip in mice promotes adiposity and adipogenesis while preserving insulin sensitivity. Diabetes. 2010;59:1424–1434. doi: 10.2337/db09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui S.T., Res A.M., Miller A.K., Spann N.J., Potter D.W., Post N.M., Chen A.Z., Sachithanantham S., Jung D.Y., Kim J.K., et al. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc. Natl. Acad. Sci. USA. 2008;105:3921–3926. doi: 10.1073/pnas.0800293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parikh H., Carlsson E., Chutkow W.A., Johansson L.E., Storgaard H., Poulsen P., Saxena R., Ladd C., Schulze P.C., Mazzini M.J., et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007;4:e158. doi: 10.1371/journal.pmed.0040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oka S., Masutani H., Liu W., Horita H., Wang D., Kizaka-Kondoh S., Yodoi J. Thioredoxin-binding protein-2-like inducible membrane protein is a novel vitamin D3 and peroxisome proliferator-activated receptor (PPAR)gamma ligand target protein that regulates PPARgamma signaling. Endocrinology. 2006;147:733–743. doi: 10.1210/en.2005-0679. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z., Rong Y.P., Malone M.H., Davis M.C., Zhong F., Distelhorst C.W. Thioredoxin-interacting protein (txnip) is a glucocorticoid-regulated primary response gene involved in mediating glucocorticoid-induced apoptosis. Oncogene. 2006;25:1903–1913. doi: 10.1038/sj.onc.1209218. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z., Yoshihara E., Son A., Matsuo Y., Masutani H., Sugie K., Maeda M., Yodoi J. Differential roles of Annexin A1 (ANXA1/lipocortin-1/lipomodulin) and thioredoxin binding protein-2 (TBP-2/VDUP1/TXNIP) in glucocorticoid signaling of HTLV-I-transformed T cells. Immunol. Lett. 2010;131:11–18. doi: 10.1016/j.imlet.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z., Lopez-Ramos D.A., Yoshihara E., Maeda Y., Masutani H., Sugie K., Maeda M., Yodoi J. Thioredoxin-binding protein-2 (TBP-2/VDUP1/TXNIP) regulates T-cell sensitivity to glucocorticoid during HTLV-I-induced transformation. Leukemia. 2011;25:440–448. doi: 10.1038/leu.2010.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu N., Zheng B., Shaywitz A., Dagon Y., Tower C., Bellinger G., Shen C.H., Wen J., Asara J., McGraw T.E., et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol. Cell. 2013;49:1167–1175. doi: 10.1016/j.molcel.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita H., Takenoshita M., Sakurai M., Bruick R.K., Henzel W.J., Shillinglaw W., Arnot D., Uyeda K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. USA. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cha-Molstad H., Saxena G., Chen J., Shalev A. Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300; histone H4 acetylation in pancreatic beta cells. J. Biol. Chem. 2009;284:16898–16905. doi: 10.1074/jbc.M109.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoltzman C.A., Peterson C.W., Breen K.T., Muoio D.M., Billin A.N., Ayer D.E. Glucose sensing by MondoA:Mlx complexes: A role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc. Natl. Acad. Sci. USA. 2008;105:6912–6917. doi: 10.1073/pnas.0712199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elgort M.G., O’Shea J.M., Jiang Y., Ayer D.E. Transcriptional and Translational Downregulation of Thioredoxin Interacting Protein Is Required for Metabolic Reprogramming during G(1) Genes Cancer. 2010;1:893–907. doi: 10.1177/1947601910389604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong S.Y., Yu F.X., Luo Y., Hagen T. Oncogenic activation of the PI3K/Akt pathway promotes cellular glucose uptake by downregulating the expression of thioredoxin-interacting protein. Cell Signal. 2016;28:377–383. doi: 10.1016/j.cellsig.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Du C., Wu M., Liu H., Ren Y., Du Y., Wu H., Wei J., Liu C., Yao F., Wang H., et al. Thioredoxin-interacting protein regulates lipid metabolism via Akt/mTOR pathway in diabetic kidney disease. Int. J. Biochem Cell Biol. 2016;79:1–13. doi: 10.1016/j.biocel.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Huy H., Song H.Y., Kim M.J., Kim W.S., Kim D.O., Byun J.E., Lee J., Park Y.J., Kim T.D., Yoon S.R., et al. TXNIP regulates AKT-mediated cellular senescence by direct interaction under glucose-mediated metabolic stress. Aging Cell. 2018;17:e12836. doi: 10.1111/acel.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panse M., Kluth O., Lorza-Gil E., Kaiser G., Muhlbauer E., Schurmann A., Haring H.U., Ullrich S., Gerst F. Palmitate and insulin counteract glucose-induced thioredoxin interacting protein (TXNIP) expression in insulin secreting cells via distinct mechanisms. PLoS ONE. 2018;13:e0198016. doi: 10.1371/journal.pone.0198016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan J., Du C., Shi Y., Liu D., Ma J. Thioredoxin-interacting protein deficiency ameliorates diabetic retinal angiogenesis. Int. J. Biochem. Cell Biol. 2018;94:61–70. doi: 10.1016/j.biocel.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Waldhart A.N., Dykstra H., Peck A.S., Boguslawski E.A., Madaj Z.B., Wen J., Veldkamp K., Hollowell M., Zheng B., Cantley L.C., et al. Phosphorylation of TXNIP by AKT Mediates Acute Influx of Glucose in Response to Insulin. Cell Rep. 2017;19:2005–2013. doi: 10.1016/j.celrep.2017.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaragoza-Campillo M.A., Moran J. Reactive Oxygen Species Evoked by Potassium Deprivation and Staurosporine Inactivate Akt and Induce the Expression of TXNIP in Cerebellar Granule Neurons. Oxid. Med. Cell. Longev. 2017;2017:8930406. doi: 10.1155/2017/8930406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Jan S., Le Meur N., Cazes A., Philippe J., Le Cunff M., Leger J., Corvol P., Germain S. Characterization of the expression of the hypoxia-induced genes neuritin, TXNIP and IGFBP3 in cancer. FEBS Lett. 2006;580:3395–3400. doi: 10.1016/j.febslet.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Baker A.F., Koh M.Y., Williams R.R., James B., Wang H., Tate W.R., Gallegos A., Von Hoff D.D., Han H., Powis G. Identification of thioredoxin-interacting protein 1 as a hypoxia-inducible factor 1alpha-induced gene in pancreatic cancer. Pancreas. 2008;36:178–186. doi: 10.1097/MPA.0b013e31815929fe. [DOI] [PubMed] [Google Scholar]
- 36.Wang J., Duhart H.M., Xu Z., Patterson T.A., Newport G.D., Ali S.F. Comparison of the time courses of selective gene expression and dopaminergic depletion induced by MPP+in MN9D cells. Neurochem. Int. 2008;52:1037–1043. doi: 10.1016/j.neuint.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Karar J., Dolt K.S., Mishra M.K., Arif E., Javed S., Pasha M.A. Expression and functional activity of pro-oxidants and antioxidants in murine heart exposed to acute hypobaric hypoxia. FEBS Lett. 2007;581:4577–4582. doi: 10.1016/j.febslet.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 38.Farrell M.R., Rogers L.K., Liu Y., Welty S.E., Tipple T.E. Thioredoxin-interacting protein inhibits hypoxia-inducible factor transcriptional activity. Free Radic. Biol. Med. 2010;49:1361–1367. doi: 10.1016/j.freeradbiomed.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong R.W., Hagen T. Mechanistic target of rapamycin (mTOR) dependent regulation of thioredoxin interacting protein (TXNIP) transcription in hypoxia. Biochem. Biophys. Res. Commun. 2013;433:40–46. doi: 10.1016/j.bbrc.2013.02.070. [DOI] [PubMed] [Google Scholar]
- 40.Abdelsaid M.A., Matragoon S., El-Remessy A.B. Thioredoxin-interacting protein expression is required for VEGF-mediated angiogenic signal in endothelial cells. Antioxid. Redox Signal. 2013;19:2199–2212. doi: 10.1089/ars.2012.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mogami H., Yura S., Kondoh E., Masutani H., Yodoi J., Konishi I. Differential expression of thioredoxin binding protein-2/Txnip in human placenta: Possible involvement of hypoxia in its suppression during early pregnancy. J. Obstet. Gynaecol. Res. 2017;43:50–56. doi: 10.1111/jog.13149. [DOI] [PubMed] [Google Scholar]
- 42.Gorgens S.W., Benninghoff T., Eckardt K., Springer C., Chadt A., Melior A., Wefers J., Cramer A., Jensen J., Birkeland K.I., et al. Hypoxia in Combination With Muscle Contraction Improves Insulin Action and Glucose Metabolism in Human Skeletal Muscle via the HIF-1alpha Pathway. Diabetes. 2017;66:2800–2807. doi: 10.2337/db16-1488. [DOI] [PubMed] [Google Scholar]
- 43.Schulze P.C., Yoshioka J., Takahashi T., He Z., King G.L., Lee R.T. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J. Biol. Chem. 2004;279:30369–30374. doi: 10.1074/jbc.M400549200. [DOI] [PubMed] [Google Scholar]
- 44.Lee K.N., Kang H.S., Jeon J.H., Kim E.M., Yoon S.R., Song H., Lyu C.Y., Piao Z.H., Kim S.U., Han Y.H., et al. VDUP1 is required for the development of natural killer cells. Immunity. 2005;22:195–208. doi: 10.1016/j.immuni.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Son A., Nakamura H., Okuyama H., Oka S., Yoshihara E., Liu W., Matsuo Y., Kondo N., Masutani H., Ishii Y., et al. Dendritic cells derived from TBP-2-deficient mice are defective in inducing T cell responses. Eur. J. Immunol. 2008;38:1358–1367. doi: 10.1002/eji.200737939. [DOI] [PubMed] [Google Scholar]
- 46.Oka S., Liu W., Yoshihara E., Ahsan M.K., Ramos D.A., Son A., Okuyama H., Zhang L., Masutani H., Nakamura H., et al. Thioredoxin binding protein-2 mediates metabolic adaptation in response to lipopolysaccharide in vivo. Crit. Care Med. 2010;38:2345–2351. doi: 10.1097/CCM.0b013e3181f85b2a. [DOI] [PubMed] [Google Scholar]
- 47.Park Y.J., Yoon S.J., Suh H.W., Kim D.O., Park J.R., Jung H., Kim T.D., Yoon S.R., Min J.K., Na H.J., et al. TXNIP deficiency exacerbates endotoxic shock via the induction of excessive nitric oxide synthesis. PLoS Pathog. 2013;9:e1003646. doi: 10.1371/journal.ppat.1003646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 49.Masters S.L., Dunne A., Subramanian S.L., Hull R.L., Tannahill G.M., Sharp F.A., Becker C., Franchi L., Yoshihara E., Chen Z., et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat. Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devi T.S., Lee I., Huttemann M., Kumar A., Nantwi K.D., Singh L.P. TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: Implications for diabetic retinopathy. Exp. Diabetes Res. 2012;2012:438238. doi: 10.1155/2012/438238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Q.Y., Pan Y., Wang R., Kang L.L., Xue Q.C., Wang X.N., Kong L.D. Quercetin inhibits AMPK/TXNIP activation and reduces inflammatory lesions to improve insulin signaling defect in the hypothalamus of high fructose-fed rats. J. Nutr. Biochem. 2014;25:420–428. doi: 10.1016/j.jnutbio.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Feng H., Gu J., Gou F., Huang W., Gao C., Chen G., Long Y., Zhou X., Yang M., Liu S., et al. High Glucose and Lipopolysaccharide Prime NLRP3 Inflammasome via ROS/TXNIP Pathway in Mesangial Cells. J. Diabetes Res. 2016;2016:6973175. doi: 10.1155/2016/6973175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X., Li R., Wang X., Fu Q., Ma S. Umbelliferone ameliorates cerebral ischemia-reperfusion injury via upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3 inflammasome. Neurosci. Lett. 2015;600:182–187. doi: 10.1016/j.neulet.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Li Y., Li J., Li S., Li Y., Wang X., Liu B., Fu Q., Ma S. Ilexgenin A inhibits endoplasmic reticulum stress and ameliorates endothelial dysfunction via suppression of TXNIP/NLRP3 inflammasome activation in an AMPK dependent manner. Pharmacol. Res. 2015;99:101–115. doi: 10.1016/j.phrs.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y., Li Q., Zhao W., Li J., Sun Y., Liu K., Liu B., Zhang N. Astragaloside IV and cycloastragenol are equally effective in inhibition of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in the endothelium. J. Ethnopharmacol. 2015;169:210–218. doi: 10.1016/j.jep.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 56.Liu W., Gu J., Qi J., Zeng X.N., Ji J., Chen Z.Z., Sun X.L. Lentinan exerts synergistic apoptotic effects with paclitaxel in A549 cells via activating ROS-TXNIP-NLRP3 inflammasome. J. Cell. Mol. Med. 2015;19:1949–1955. doi: 10.1111/jcmm.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y., Li J., Li S., Li Y., Wang X., Liu B., Fu Q., Ma S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015;286:53–63. doi: 10.1016/j.taap.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X., Zhang J.H., Chen X.Y., Hu Q.H., Wang M.X., Jin R., Zhang Q.Y., Wang W., Wang R., Kang L.L., et al. Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid. Redox Signal. 2015;22:848–870. doi: 10.1089/ars.2014.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J., Xu X., Li Y., Kou J., Huang F., Liu B., Liu K. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur J. Pharmacol. 2014;745:59–68. doi: 10.1016/j.ejphar.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 60.Abais J.M., Xia M., Li G., Chen Y., Conley S.M., Gehr T.W., Boini K.M., Li P.L. Nod-like receptor protein 3 (NLRP3) inflammasome activation and podocyte injury via thioredoxin-interacting protein (TXNIP) during hyperhomocysteinemia. J. Biol. Chem. 2014;289:27159–27168. doi: 10.1074/jbc.M114.567537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y., Lian K., Zhang L., Wang R., Yi F., Gao C., Xin C., Zhu D., Li Y., Yan W., et al. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res. Cardiol. 2014;109:415. doi: 10.1007/s00395-014-0415-z. [DOI] [PubMed] [Google Scholar]
- 62.Xiao J., Zhu Y., Liu Y., Tipoe G.L., Xing F., So K.F. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int. J. Biol. Macromol. 2014;69:73–78. doi: 10.1016/j.ijbiomac.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 63.Chen W., Zhao M., Zhao S., Lu Q., Ni L., Zou C., Lu L., Xu X., Guan H., Zheng Z., et al. Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: A novel inhibitory effect of minocycline. Inflamm. Res. 2017;66:157–166. doi: 10.1007/s00011-016-1002-6. [DOI] [PubMed] [Google Scholar]
- 64.Sun X., Jiao X., Ma Y., Liu Y., Zhang L., He Y., Chen Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016;481:63–70. doi: 10.1016/j.bbrc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 65.Tseng H.H., Vong C.T., Kwan Y.W., Lee S.M., Hoi M.P. TRPM2 regulates TXNIP-mediated NLRP3 inflammasome activation via interaction with p47 phox under high glucose in human monocytic cells. Sci. Rep. 2016;6:35016. doi: 10.1038/srep35016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao G., Jiang N., Hu Y., Zhang Y., Wang G., Yin M., Ma X., Zhou K., Qi J., Yu B., et al. Ruscogenin Attenuates Cerebral Ischemia-Induced Blood-Brain Barrier Dysfunction by Suppressing TXNIP/NLRP3 Inflammasome Activation and the MAPK Pathway. Int. J. Mol. Sci. 2016;17:1418. doi: 10.3390/ijms17091418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang L., Fei D., Gong R., Yang W., Yu W., Pan S., Zhao M., Zhao M. CORM-2 inhibits TXNIP/NLRP3 inflammasome pathway in LPS-induced acute lung injury. Inflamm. Res. 2016;65:905–915. doi: 10.1007/s00011-016-0973-7. [DOI] [PubMed] [Google Scholar]
- 68.Ding C., Zhao Y., Shi X., Zhang N., Zu G., Li Z., Zhou J., Gao D., Lv L., Tian X., et al. New insights into salvianolic acid A action: Regulation of the TXNIP/NLRP3 and TXNIP/ChREBP pathways ameliorates HFD-induced NAFLD in rats. Sci. Rep. 2016;6:28734. doi: 10.1038/srep28734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li X.F., Shen W.W., Sun Y.Y., Li W.X., Sun Z.H., Liu Y.H., Zhang L., Huang C., Meng X.M., Li J. MicroRNA-20a negatively regulates expression of NLRP3-inflammasome by targeting TXNIP in adjuvant-induced arthritis fibroblast-like synoviocytes. Joint Bone Spine. 2016;83:695–700. doi: 10.1016/j.jbspin.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 70.Elshaer S.L., Mohamed I.N., Coucha M., Altantawi S., Eldahshan W., Bartasi M.L., Shanab A.Y., Lorys R., El-Remessy A.B. Deletion of TXNIP Mitigates High-Fat Diet-Impaired Angiogenesis and Prevents Inflammation in a Mouse Model of Critical Limb Ischemia. Antioxidants (Basel) 2017;6:47. doi: 10.3390/antiox6030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Filhoulaud G., Benhamed F., Pagesy P., Bonner C., Fardini Y., Ilias A., Movassat J., Burnol A.F., Guilmeau S., Kerr-Conte J., et al. O-GlcNacylation Links TxNIP to Inflammasome Activation in Pancreatic beta Cells. Front. Endocrinol. (Lausanne) 2019;10:291. doi: 10.3389/fendo.2019.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minn A.H., Hafele C., Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology. 2005;146:2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- 73.Shalev A., Pise-Masison C.A., Radonovich M., Hoffmann S.C., Hirshberg B., Brady J.N., Harlan D.M. Oligonucleotide microarray analysis of intact human pancreatic islets: Identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology. 2002;143:3695–3698. doi: 10.1210/en.2002-220564. [DOI] [PubMed] [Google Scholar]
- 74.Abdul-Wahed A., Guilmeau S., Postic C. Sweet Sixteenth for ChREBP: Established Roles and Future Goals. Cell Metab. 2017;26:324–341. doi: 10.1016/j.cmet.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Shalev A. Lack of TXNIP protects beta-cells against glucotoxicity. Biochem. Soc. Trans. 2008;36:963–965. doi: 10.1042/BST0360963. [DOI] [PubMed] [Google Scholar]
- 76.Xu G., Chen J., Jing G., Shalev A. Preventing beta-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61:848–856. doi: 10.2337/db11-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen J., Fontes G., Saxena G., Poitout V., Shalev A. Lack of TXNIP protects against mitochondria-mediated apoptosis but not against fatty acid-induced ER stress-mediated beta-cell death. Diabetes. 2010;59:440–447. doi: 10.2337/db09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anthony T.G., Wek R.C. TXNIP switches tracks toward a terminal UPR. Cell Metab. 2012;16:135–137. doi: 10.1016/j.cmet.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 79.Oslowski C.M., Hara T., O’Sullivan-Murphy B., Kanekura K., Lu S., Hara M., Ishigaki S., Zhu L.J., Hayashi E., Hui S.T., et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab. 2012;16:265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reich E., Tamary A., Sionov R.V., Melloul D. Involvement of thioredoxin-interacting protein (TXNIP) in glucocorticoid-mediated beta cell death. Diabetologia. 2012;55:1048–1057. doi: 10.1007/s00125-011-2422-z. [DOI] [PubMed] [Google Scholar]
- 81.Karunakaran U., Moon J.S., Lee H.W., Won K.C. CD36 initiated signaling mediates ceramide-induced TXNIP expression in pancreatic beta-cells. Biochim. Biophys. Acta. 2015;1852:2414–2422. doi: 10.1016/j.bbadis.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 82.Hong K., Xu G., Grayson T.B., Shalev A. Cytokines Regulate beta-Cell Thioredoxin-interacting Protein (TXNIP) via Distinct Mechanisms and Pathways. J. Biol. Chem. 2016;291:8428–8439. doi: 10.1074/jbc.M115.698365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang J., Wang J., Wang J.J., Zhang W.F., Jiao X.Y. Role of autophagy in TXNIP overexpression-induced apoptosis of INS-1 islet cells. Sheng Li Xue Bao. 2017;69:445–451. [PubMed] [Google Scholar]
- 84.Lu J., Holmgren A. Thioredoxin system in cell death progression. Antioxid. Redox Signal. 2012;17:1738–1747. doi: 10.1089/ars.2012.4650. [DOI] [PubMed] [Google Scholar]
- 85.Saxena G., Chen J., Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Filios S.R., Xu G., Chen J., Hong K., Jing G., Shalev A. MicroRNA-200 is induced by thioredoxin-interacting protein and regulates Zeb1 protein signaling and beta cell apoptosis. J. Biol. Chem. 2014;289:36275–36283. doi: 10.1074/jbc.M114.592360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu G., Chen J., Jing G., Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat. Med. 2013;19:1141–1146. doi: 10.1038/nm.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jing G., Westwell-Roper C., Chen J., Xu G., Verchere C.B., Shalev A. Thioredoxin-interacting protein promotes islet amyloid polypeptide expression through miR-124a and FoxA2. J. Biol. Chem. 2014;289:11807–11815. doi: 10.1074/jbc.M113.525022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veres A., Faust A.L., Bushnell H.L., Engquist E.N., Kenty J.H., Harb G., Poh Y.C., Sintov E., Gurtler M., Pagliuca F.W., et al. Charting cellular identity during human in vitro beta-cell differentiation. Nature. 2019;569:368–373. doi: 10.1038/s41586-019-1168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaadige M.R., Yang J., Wilde B.R., Ayer D.E. MondoA-Mlx transcriptional activity is limited by mTOR-MondoA interaction. Mol. Cell. Biol. 2015;35:101–110. doi: 10.1128/MCB.00636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DeBalsi K.L., Wong K.E., Koves T.R., Slentz D.H., Seiler S.E., Wittmann A.H., Ilkayeva O.R., Stevens R.D., Perry C.G., Lark D.S., et al. Targeted metabolomics connects thioredoxin-interacting protein (TXNIP) to mitochondrial fuel selection and regulation of specific oxidoreductase enzymes in skeletal muscle. J. Biol. Chem. 2014;289:8106–8120. doi: 10.1074/jbc.M113.511535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cantley L.C. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 93.Song Z., Saghafi N., Gokhale V., Brabant M., Meuillet E.J. Regulation of the activity of the tumor suppressor PTEN by thioredoxin in Drosophila melanogaster. Exp. Cell Res. 2007;313:1161–1171. doi: 10.1016/j.yexcr.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meuillet E.J., Mahadevan D., Berggren M., Coon A., Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN’s lipid phosphatase activity and membrane binding: A mechanism for the functional loss of PTEN’s tumor suppressor activity. Arch. Biochem. Biophys. 2004;429:123–133. doi: 10.1016/j.abb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 95.Han S.J., Zhang Y., Kim I., Chay K.O., Yoon H.J., Jang D.I., Yang S.Y., Park J., Woo H.A., Park I., et al. Redox regulation of the tumor suppressor PTEN by the thioredoxin system and cumene hydroperoxide. Free Radic. Biol. Med. 2017;112:277–286. doi: 10.1016/j.freeradbiomed.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 96.Sadeghirizi A., Yazdanparast R., Aghazadeh S. Combating trastuzumab resistance by targeting thioredoxin-1/PTEN interaction. Tumour Biol. 2016;37:6737–6747. doi: 10.1007/s13277-015-4424-9. [DOI] [PubMed] [Google Scholar]
- 97.Schwertassek U., Haque A., Krishnan N., Greiner R., Weingarten L., Dick T.P., Tonks N.K. Reactivation of oxidized PTP1B and PTEN by thioredoxin 1. FEBS J. 2014;281:3545–3558. doi: 10.1111/febs.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jo S.H., Kim M.Y., Park J.M., Kim T.H., Ahn Y.H. Txnip contributes to impaired glucose tolerance by upregulating the expression of genes involved in hepatic gluconeogenesis in mice. Diabetologia. 2013;56:2723–2732. doi: 10.1007/s00125-013-3050-6. [DOI] [PubMed] [Google Scholar]
- 99.Chutkow W.A., Patwari P., Yoshioka J., Lee R.T. Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production. J. Biol. Chem. 2008;283:2397–2406. doi: 10.1074/jbc.M708169200. [DOI] [PubMed] [Google Scholar]
- 100.Yamaguchi F., Hirata Y., Akram H., Kamitori K., Dong Y., Sui L., Tokuda M. FOXO/TXNIP pathway is involved in the suppression of hepatocellular carcinoma growth by glutamate antagonist MK-801. BMC Cancer. 2013;13:468. doi: 10.1186/1471-2407-13-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Papadia S., Soriano F.X., Leveille F., Martel M.A., Dakin K.A., Hansen H.H., Kaindl A., Sifringer M., Fowler J., Stefovska V., et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat. Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myers M.G., Jr., Olson D.P. Central nervous system control of metabolism. Nature. 2012;491:357–363. doi: 10.1038/nature11705. [DOI] [PubMed] [Google Scholar]
- 103.Blouet C., Schwartz G.J. Nutrient-sensing hypothalamic TXNIP links nutrient excess to energy imbalance in mice. J. Neurosci. 2011;31:6019–6027. doi: 10.1523/JNEUROSCI.6498-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blouet C., Liu S.M., Jo Y.H., Chua S., Schwartz G.J. TXNIP in Agrp neurons regulates adiposity, energy expenditure, and central leptin sensitivity. J. Neurosci. 2012;32:9870–9877. doi: 10.1523/JNEUROSCI.0353-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tian Y., Su Y., Ye Q., Chen L., Yuan F., Wang Z. Silencing of TXNIP Alleviated Oxidative Stress Injury by Regulating MAPK-Nrf2 Axis in Ischemic Stroke. Neurochem. Res. 2020;45:428–436. doi: 10.1007/s11064-019-02933-y. [DOI] [PubMed] [Google Scholar]
- 106.Dafre A.L., Schmitz A.E., Maher P. Rapid and persistent loss of TXNIP in HT22 neuronal cells under carbonyl and hyperosmotic stress. Neurochem. Int. 2020;132:104585. doi: 10.1016/j.neuint.2019.104585. [DOI] [PubMed] [Google Scholar]
- 107.Gamdzyk M., Doycheva D.M., Malaguit J., Enkhjargal B., Tang J., Zhang J.H. Role of PPAR-beta/delta/miR-17/TXNIP pathway in neuronal apoptosis after neonatal hypoxic-ischemic injury in rats. Neuropharmacology. 2018;140:150–161. doi: 10.1016/j.neuropharm.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen D., Dixon B.J., Doycheva D.M., Li B., Zhang Y., Hu Q., He Y., Guo Z., Nowrangi D., Flores J., et al. IRE1alpha inhibition decreased TXNIP/NLRP3 inflammasome activation through miR-17-5p after neonatal hypoxic-ischemic brain injury in rats. J. Neuroinflammation. 2018;15:32. doi: 10.1186/s12974-018-1077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yarfitz S., Hurley J.B. Transduction mechanisms of vertebrate and invertebrate photoreceptors. J. Biol. Chem. 1994;269:14329–14332. [PubMed] [Google Scholar]
- 110.Gurevich V.V., Dion S.B., Onorato J.J., Ptasienski J., Kim C.M., Sterne-Marr R., Hosey M.M., Benovic J.L. Arrestin interactions with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, beta 2-adrenergic; m2 muscarinic cholinergic receptors. J. Biol. Chem. 1995;270:720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- 111.Lefkowitz R.J., Rajagopal K., Whalen E.J. New roles for beta-arrestins in cell signaling: Not just for seven-transmembrane receptors. Mol. Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 112.Patwari P., Lee R.T. An expanded family of arrestins regulate metabolism. Trends Endocrinol. Metab. 2012;23:216–222. doi: 10.1016/j.tem.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luan B., Zhao J., Wu H., Duan B., Shu G., Wang X., Li D., Jia W., Kang J., Pei G. Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457:1146–1149. doi: 10.1038/nature07617. [DOI] [PubMed] [Google Scholar]
- 114.Patwari P., Emilsson V., Schadt E.E., Chutkow W.A., Lee S., Marsili A., Zhang Y., Dobrin R., Cohen D.E., Larsen P.R., et al. The arrestin domain-containing 3 protein regulates body mass and energy expenditure. Cell Metab. 2011;14:671–683. doi: 10.1016/j.cmet.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sullivan W.J., Mullen P.J., Schmid E.W., Flores A., Momcilovic M., Sharpley M.S., Jelinek D., Whiteley A.E., Maxwell M.B., Wilde B.R., et al. Extracellular Matrix Remodeling Regulates Glucose Metabolism through TXNIP Destabilization. Cell. 2018;175:117–132.e121. doi: 10.1016/j.cell.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nishinaka Y., Masutani H., Oka S., Matsuo Y., Yamaguchi Y., Nishio K., Ishii Y., Yodoi J. Importin alpha1 (Rch1) mediates nuclear translocation of thioredoxin-binding protein-2/vitamin D(3)-up-regulated protein 1. J. Biol. Chem. 2004;279:37559–37565. doi: 10.1074/jbc.M405473200. [DOI] [PubMed] [Google Scholar]
- 117.Hirata C.L., Ito S., Masutani H. Dataset on the formation of Thioredoxin interacting protein (Txnip) containing redox sensitive high molecular weight nucleoprotein complexes. Data Brief. 2020;28:104893. doi: 10.1016/j.dib.2019.104893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hirata C.L., Ito S., Masutani H. Thioredoxin interacting protein (Txnip) forms redox sensitive high molecular weight nucleoprotein complexes. Arch. Biochem. Biophys. 2019;677:108159. doi: 10.1016/j.abb.2019.108159. [DOI] [PubMed] [Google Scholar]
- 119.Alvarez C.E. On the origins of arrestin and rhodopsin. BMC Evol. Biol. 2008;8:222. doi: 10.1186/1471-2148-8-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Masutani H., Yoshihara E., Masaki S., Chen Z., Yodoi J. Thioredoxin binding protein (TBP)-2/Txnip and alpha-arrestin proteins in cancer and diabetes mellitus. J. Clin. Biochem. Nutr. 2012;50:23–34. doi: 10.3164/jcbn.11-36SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin C.H., MacGurn J.A., Chu T., Stefan C.J., Emr S.D. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135:714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 122.Tseng P.C., Kuo C.F., Cheng M.H., Wan S.W., Lin C.F., Chang C.P., Lin Y.S., Wu J.J., Huang C.C., Chen C.L. HECT E3 Ubiquitin Ligase-Regulated Txnip Degradation Facilitates TLR2-Mediated Inflammation During Group A Streptococcal Infection. Front. Immunol. 2019;10:2147. doi: 10.3389/fimmu.2019.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu S., Wu X., Zong M., Tempel W., Loppnau P., Liu Y. Structural basis for a novel interaction between TXNIP and Vav2. FEBS Lett. 2016;590:857–865. doi: 10.1002/1873-3468.12110. [DOI] [PubMed] [Google Scholar]
- 124.Zhang P., Wang C., Gao K., Wang D., Mao J., An J., Xu C., Wu D., Yu H., Liu J.O., et al. The ubiquitin ligase itch regulates apoptosis by targeting thioredoxin-interacting protein for ubiquitin-dependent degradation. J. Biol. Chem. 2010;285:8869–8879. doi: 10.1074/jbc.M109.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohtake F., Tsuchiya H., Saeki Y., Tanaka K. K63 ubiquitylation triggers proteasomal degradation by seeding branched ubiquitin chains. Proc. Natl. Acad. Sci. USA. 2018;115:E1401–E1408. doi: 10.1073/pnas.1716673115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suh H.W., Yun S., Song H., Jung H., Park Y.J., Kim T.D., Yoon S.R., Choi I. TXNIP interacts with hEcd to increase p53 stability and activity. Biochem. Biophys. Res. Commun. 2013;438:264–269. doi: 10.1016/j.bbrc.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 127.Chutkow W.A., Lee R.T. Thioredoxin regulates adipogenesis through thioredoxin-interacting protein (Txnip) protein stability. J. Biol. Chem. 2011;286:29139–29145. doi: 10.1074/jbc.M111.267666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yoshioka J., Schulze P.C., Cupesi M., Sylvan J.D., MacGillivray C., Gannon J., Huang H., Lee R.T. Thioredoxin-interacting protein controls cardiac hypertrophy through regulation of thioredoxin activity. Circulation. 2004;109:2581–2586. doi: 10.1161/01.CIR.0000129771.32215.44. [DOI] [PubMed] [Google Scholar]
- 129.Yoshioka J., Chutkow W.A., Lee S., Kim J.B., Yan J., Tian R., Lindsey M.L., Feener E.P., Seidman C.E., Seidman J.G., et al. Deletion of thioredoxin-interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia-reperfusion injury. J. Clin. Investig. 2012;122:267–279. doi: 10.1172/JCI44927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoshioka J., Imahashi K., Gabel S.A., Chutkow W.A., Burds A.A., Gannon J., Schulze P.C., MacGillivray C., London R.E., Murphy E., et al. Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ. Res. 2007;101:1328–1338. doi: 10.1161/CIRCRESAHA.106.160515. [DOI] [PubMed] [Google Scholar]
- 131.Chen J., Young M.E., Chatham J.C., Crossman D.K., Dell’Italia L.J., Shalev A. TXNIP regulates myocardial fatty acid oxidation via miR-33a signaling. Am. J. Physiol. Heart Circ. Physiol. 2016;311:H64–H75. doi: 10.1152/ajpheart.00151.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Song S., Qiu D., Shi Y., Wang S., Zhou X., Chen N., Wei J., Wu M., Wu H., Duan H. Thioredoxin-interacting protein deficiency alleviates phenotypic alterations of podocytes via inhibition of mTOR activation in diabetic nephropathy. J. Cell. Physiol. 2019;234:16485–16502. doi: 10.1002/jcp.28317. [DOI] [PubMed] [Google Scholar]
- 133.Wu M., Li R., Hou Y., Song S., Han W., Chen N., Du Y., Ren Y., Shi Y. Thioredoxin-interacting protein deficiency ameliorates kidney inflammation and fibrosis in mice with unilateral ureteral obstruction. Lab. Investig. 2018;98:1211–1224. doi: 10.1038/s41374-018-0078-8. [DOI] [PubMed] [Google Scholar]
- 134.Sandler G., Clayton G.A., Thornicroft S.G. Clinical evaluation of verapamil in angina pectoris. Br. Med. J. 1968;3:224–227. doi: 10.1136/bmj.3.5612.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moiseev V.S., Moiseev S.V. Verapamil—On the 30th anniversary of its clinical use. Ter. Arkh. 1992;64:112–116. [PubMed] [Google Scholar]
- 136.Chen J., Cha-Molstad H., Szabo A., Shalev A. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am. J. Physiol. Endocrinol. Metab. 2009;296:E1133–E1139. doi: 10.1152/ajpendo.90944.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu L., Lin X., Guan M., Zeng Y., Liu Y. Verapamil Attenuated Prediabetic Neuropathy in High-Fat Diet-Fed Mice through Inhibiting TXNIP-Mediated Apoptosis and Inflammation. Oxid. Med. Cell. Longev. 2019;2019:1896041. doi: 10.1155/2019/1896041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Melone M.A.B., Dato C., Paladino S., Coppola C., Trebini C., Giordana M.T., Perrone L. Verapamil Inhibits Ser202/Thr205 Phosphorylation of Tau by Blocking TXNIP/ROS/p38 MAPK Pathway. Pharm. Res. 2018;35:44. doi: 10.1007/s11095-017-2276-2. [DOI] [PubMed] [Google Scholar]
- 139.Thielen L., Shalev A. Diabetes pathogenic mechanisms and potential new therapies based upon a novel target called TXNIP. Curr. Opin. Endocrinol. Diabetes Obes. 2018;25:75–80. doi: 10.1097/MED.0000000000000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ovalle F., Grimes T., Xu G., Patel A.J., Grayson T.B., Thielen L.A., Li P., Shalev A. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat. Med. 2018;24:1108–1112. doi: 10.1038/s41591-018-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thielen L.A., Chen J., Jing G., Moukha-Chafiq O., Xu G., Jo S., Grayson T.B., Lu B., Li P., Augelli-Szafran C.E., et al. Identification of an Anti-diabetic, Orally Available Small Molecule that Regulates TXNIP Expression and Glucagon Action. Cell Metab. 2020 doi: 10.1016/j.cmet.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou F., Zhang Y., Chen J., Hu Y., Xu Y. Verapamil Ameliorates Hepatic Metaflammation by Inhibiting Thioredoxin-Interacting Protein/NLRP3 Pathways. Front. Endocrinol. (Lausanne) 2018;9:640. doi: 10.3389/fendo.2018.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Masaki S., Masutani H., Yoshihara E., Yodoi J. Deficiency of thioredoxin binding protein-2 (TBP-2) enhances TGF-beta signaling and promotes epithelial to mesenchymal transition. PLoS ONE. 2012;7:e39900. doi: 10.1371/journal.pone.0039900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cheng G.C., Schulze P.C., Lee R.T., Sylvan J., Zetter B.R., Huang H. Oxidative stress and thioredoxin-interacting protein promote intravasation of melanoma cells. Exp. Cell Res. 2004;300:297–307. doi: 10.1016/j.yexcr.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 145.Goldberg S.F., Miele M.E., Hatta N., Takata M., Paquette-Straub C., Freedman L.P., Welch D.R. Melanoma metastasis suppression by chromosome 6: Evidence for a pathway regulated by CRSP3 and TXNIP. Cancer Res. 2003;63:432–440. [PubMed] [Google Scholar]
- 146.Ikarashi M., Takahashi Y., Ishii Y., Nagata T., Asai S., Ishikawa K. Vitamin D3 up-regulated protein 1 (VDUP1) expression in gastrointestinal cancer and its relation to stage of disease. Anticancer Res. 2002;22:4045–4048. [PubMed] [Google Scholar]
- 147.Sheth S.S., Bodnar J.S., Ghazalpour A., Thipphavong C.K., Tsutsumi S., Tward A.D., Demant P., Kodama T., Aburatani H., Lusis A.J. Hepatocellular carcinoma in Txnip-deficient mice. Oncogene. 2006;25:3528–3536. doi: 10.1038/sj.onc.1209394. [DOI] [PubMed] [Google Scholar]
- 148.Nakamura H., Masutani H., Yodoi J. Extracellular thioredoxin and thioredoxin-binding protein 2 in control of cancer. Semin. Cancer Biol. 2006;16:444–451. doi: 10.1016/j.semcancer.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 149.Jeon J.H., Lee K.N., Hwang C.Y., Kwon K.S., You K.H., Choi I. Tumor suppressor VDUP1 increases p27(kip1) stability by inhibiting JAB1. Cancer Res. 2005;65:4485–4489. doi: 10.1158/0008-5472.CAN-04-2271. [DOI] [PubMed] [Google Scholar]
- 150.Simmons D.G., Kennedy T.G. Rat endometrial Vdup1 expression: Changes related to sensitization for the decidual cell reaction and hormonal control. Reproduction. 2004;127:475–482. doi: 10.1530/rep.1.00029. [DOI] [PubMed] [Google Scholar]
- 151.Nishinaka Y., Nishiyama A., Masutani H., Oka S., Ahsan K.M., Nakayama Y., Ishii Y., Nakamura H., Maeda M., Yodoi J. Loss of thioredoxin-binding protein-2/vitamin D3 up-regulated protein 1 in human T-cell leukemia virus type I-dependent T-cell transformation: Implications for adult T-cell leukemia leukemogenesis. Cancer Res. 2004;64:1287–1292. doi: 10.1158/0008-5472.CAN-03-0908. [DOI] [PubMed] [Google Scholar]
- 152.Nishizawa K., Nishiyama H., Matsui Y., Kobayashi T., Saito R., Kotani H., Masutani H., Oishi S., Toda Y., Fujii N., et al. Thioredoxin-interacting protein suppresses bladder carcinogenesis. Carcinogenesis. 2011;32:1459–1466. doi: 10.1093/carcin/bgr137. [DOI] [PubMed] [Google Scholar]
- 153.Malone C.F., Emerson C., Ingraham R., Barbosa W., Guerra S., Yoon H., Liu L.L., Michor F., Haigis M., Macleod K.F., et al. mTOR and HDAC Inhibitors Converge on the TXNIP/Thioredoxin Pathway to Cause Catastrophic Oxidative Stress and Regression of RAS-Driven Tumors. Cancer Discov. 2017;7:1450–1463. doi: 10.1158/2159-8290.CD-17-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wondafrash D.Z., Nire’a A.T., Tafere G.G., Desta D.M., Berhe D.A., Zewdie K.A. Thioredoxin-Interacting Protein as a Novel Potential Therapeutic Target in Diabetes Mellitus and Its Underlying Complications. Diabetes Metab. Syndr. Obes. 2020;13:43–51. doi: 10.2147/DMSO.S232221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Foretz M., Guigas B., Bertrand L., Pollak M., Viollet B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 156.Soccio R.E., Chen E.R., Lazar M.A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20:573–591. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M., Evans R.M. PPARgamma signaling and metabolism: The good, the bad and the future. Nat. Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Campbell J.E., Drucker D.J. Pharmacology, physiology; mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 159.Drucker D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27:740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 160.Lau C.H., Suh Y. In vivo genome editing in animals using AAV-CRISPR system: Applications to translational research of human disease. F1000Research. 2017;6:2153. doi: 10.12688/f1000research.11243.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang D., Zhang F., Gao G. CRISPR-Based Therapeutic Genome Editing: Strategies and In Vivo Delivery by AAV Vectors. Cell. 2020;181:136–150. doi: 10.1016/j.cell.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maxwell K.G., Augsornworawat P., Velazco-Cruz L., Kim M.H., Asada R., Hogrebe N.J., Morikawa S., Urano F., Millman J.R. Gene-edited human stem cell-derived beta cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci. Transl. Med. 2020;12:eaax9106. doi: 10.1126/scitranslmed.aax9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Helman A., Melton D.A. A Stem Cell Approach to Cure Type 1 Diabetes. Cold Spring Harb. Perspect. Biol. 2020:a035741. doi: 10.1101/cshperspect.a035741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pagliuca F.W., Millman J.R., Gurtler M., Segel M., Van Dervort A., Ryu J.H., Peterson Q.P., Greiner D., Melton D.A. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yoshihara E., Wei Z., Lin C.S., Fang S., Ahmadian M., Kida Y., Tseng T., Dai Y., Yu R.T., Liddle C., et al. ERRgamma Is Required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive beta Cells. Cell Metab. 2016;23:622–634. doi: 10.1016/j.cmet.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A., O’Dwyer S., Quiskamp N., Mojibian M., Albrecht T., et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 167.Yoshihara E., Connor C.O., Gasser E., Wei Z., Oh T.G., Tseng T.W., Wang D., Cayabyab F., Dai Y., Yu R.T., et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature. 2020 doi: 10.1038/s41586-020-2631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]