Abstract
Bladder cancer (BC) is the 11th most common diagnosed cancer, and a number of factors including environmental and genetic ones participate in BC development. Metastasis of BC cells into neighboring and distant tissues significantly reduces overall survival of patients with this life-threatening disorder. Recently, studies have focused on revealing molecular pathways involved in metastasis of BC cells, and in this review, we focus on microRNAs (miRNAs) and their regulatory effect on epithelial-to-mesenchymal transition (EMT) mechanisms that can regulate metastasis. EMT is a vital process for migration of BC cells, and inhibition of this mechanism restricts invasion of BC cells. MiRNAs are endogenous non-coding RNAs with 19–24 nucleotides capable of regulating different cellular events, and EMT is one of them. In BC cells, miRNAs are able to both induce and/or inhibit EMT. For regulation of EMT, miRNAs affect different molecular pathways such as transforming growth factor-beta (TGF-β), Snail, Slug, ZEB1/2, CD44, NSBP1, which are, discussed in detail this review. Besides, miRNA/EMT axis can also be regulated by upstream mediators such as lncRNAs, circRNAs and targeted by diverse anti-tumor agents. These topics are also discussed here to reveal diverse molecular pathways involved in migration of BC cells and strategies to target them to develop effective therapeutics.
Keywords: bladder cancer, metastasis, epithelial-to-mesenchymal transition (EMT), microRNA (miRNA), cancer therapy
1. Introduction
Bladder cancer (BC) is among the most common cancers worldwide (11th most common diagnosed cancer) and its global incidence rate is higher in males compared to females (9 per 100,000 persons for males and 2.2 per 100,000 persons for females) [1,2]. According to estimates, incidence rate of BC is higher in European countries compared to the average worldwide incidence rate (19.1 for males and 4 for females) [3]. It seems that incidence and mortality of BC are different in various countries based on the risk factors, diagnostic tools and treatment availability as well as data collection strategies [4,5]. BC has a higher incidence rate in Southern and Western Europe and North America, as well in certain countries in Northern Africa or Western Asia [6].
Although etiology of BC is not completely understood, a number of factors are considered as risk factors for BC development. The most well-known risk factor is tobacco smoking which is responsible for development of 50% of BC cases [7]. Occupational exposure to agents such as aromatic amines, polycyclic aromatic hydrocarbons and chlorinated hydrocarbons can be considered as other factors involved in BC development. Genetic mutations are also involved in BC development by enhancing susceptibility into other risk factors [8,9,10]. Radiation exposure and chlorinating drinking water have been also reported to account for BC development [11,12].
BC is a heterogeneous group of tumors with up to 40 histological subgroups. Fortunately, novel therapeutics are evolving for BC therapy beyond chemotherapy, which includes immunotherapy and molecular targeted agents. Most of BC cases are urothelial carcinomas, and 10% of them are non-urothelial carcinomas [3,13]. For each of the aforementioned types of BC, there are different subtypes. For instance, urothelial carcinomas are divided into different subtypes based on histopathological profile such as conventional urothelial carcinoma, variant urothelial carcinoma and so on. The advantageous of this subtyping is of importance for precision therapy, and providing effective and separate diagnosis and treatment [14].
Although different subtypes have been developed for BC and much effort has been conducted for using chemotherapy and radiotherapy in BC therapy, the cure of BC patients remains one of the big challenges for scientists. Partly, this difficulty in treating BC is due to poor understanding of molecular profile of BC. In spite of experiments for revealing genetic factors and molecular pathways involved in BC progression and malignancy, there is still a long way, since cancer cells use dynamic and flexible molecular pathways for ensuring their proliferation and invasion. A group of these molecular signaling pathways involved in proliferation and migration of BC cells, and enhance their viability known as oncogenic pathways such as Wnt [15], STAT3 [16], Nrf2 [17], PI3K/Akt [18], ZEB [19], and oncogenic microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) [20,21]. Another group of molecular pathways include those which inhibit growth and metastasis of BC cells such as PTEN [22], AMPK [23], and onco-suppressor miRNAs and lncRNAs [24,25]. These molecular pathways have been extensively studied in BC cells and there are excellent reviews about them.
One of the issues in treatment of patients with BC is the high metastatic capability of BC cells that negatively affects overall survival of patients with this life-threatening disorder, and remarkably reduces efficacy of chemotherapy and radiotherapy, since cancer cells migrate in neighboring and distant tissues, providing problems with their effective eradication [26,27,28]. Several molecular pathways such as ZEB1 [19], EZH2 [29], PI3K/Akt [30], and so on have been identified as potential factors involved in metastasis and invasion of BC cells. Epithelial-to-mesenchymal transition (EMT) is one of the most important molecular pathways that enhanced migratory ability of cancer cells [31,32]. Interestingly, EMT has been recognized as a potential downstream target of miRNAs in cancer cells [33,34,35,36,37]. In the present review, we focus on metastatic BC cells, and the role of miRNA/EMT axis. This review will provide a comprehensive discussion about the role of miRNA/EMT in malignant behavior and strategies to target this axis in BC cells.
2. EMT Mechanism
The first definition of EMT is transformation of epithelial cells into mesenchymal ones that have migratory features and can be accompanied by downregulation of epithelial markers such as E-cadherin, and upregulation of mesenchymal markers such as N-cadherin and vimentin [38,39]. Epithelial cells have cell-cell junctions, apico-basal polarity and low potential in migration. The epithelial cells are detected via their cell surface markers including E cadherin (as the most important and well-known factor), cytokeratins, occluding and claudins [40,41,42,43]. However, these properties are reversed in mesenchymal cells during EMT. Mesenchymal cells have front-rear polarity and potential in migration. There are surface markers by which mesenchymal cells are recognized, including N-cadherin, fibronectin and vimentin [44,45,46,47,48]. The EMT process was first observed during embryonic development. It was found that EMT is a vital mechanism for mesoderm formation and neural crest delamination. Based on dynamic property of cell identity, different mechanisms may be involved in ensuring this feature and EMT is one of them. During EMT activation, cells lose their identity and morphology to become a new one with mesenchymal characteristics [49,50]. It is worth mentioning that EMT is a reversible mechanism and its converse route is known as mesenchymal-to-epithelial transition (MET) [51].
A variety of transcriptional and epigenetic factors can contribute to regulation of EMT and MET. Although EMT is vital for physiological conditions such as organismal development, tissue healing and homeostasis, cancer cells hijack EMT to ensure their migration [52,53,54,55,56]. In order to occupy and colonize in distant tissues, cancer cells use EMT mechanism to promote their migratory ability [57,58,59,60]. It has been noted that EMT mechanism not only ensures invasion of cancer cells, but also is involved in reducing sensitivity of cancer cells into apoptosis and stimulation of chemoresistance [61,62]. EMT is not a binary state procedure and has a dynamic spectrum in which cells can have both epithelial and mesenchymal features [63,64,65,66,67,68]. Moreover, the cells undergoing EMT have more tumor-initiating potential and are resistant to apoptosis [69,70]. Furthermore, cancer cells are required to alter their metabolism in order to meet their energy needs and synthesize biomolecules such as proteins, lipids, and nucleic acids [71,72]. The most important change in metabolism of cancer cells is shifting from oxidative phosphorylation into glycolysis to meet their needs into adenosine triphosphate (ATP). This is known as Warbrug effect [73]. There is a close relationship between EMT and glucose metabolism in which glycolysis, tricarboxylic acid cycle (TCA) cycle, lipid and amino acid metabolism participate in EMT induction and stimulating invasion and migration of cancer cells [74,75].
A number of published articles have investigated the role of molecular pathways in regulation of EMT in cancer cells [41,76]. Due to space limitations, it is impossible to discuss all the molecular pathways involved in EMT regulation in cancer cells. However, we briefly discuss upstream mediators of EMT in cancer cells. Increasing evidence demonstrates that tumorigenesis and EMT can be regulated by transcription factor STAT3 in cancer cells [77,78]. In a recently published article, it was found that phosphorylation of STAT3 at tyrosine705 is associated with an increase in metastasis and migration of cancer cells via EMT induction. Moreover, STAT3-mediated EMT stimulation mediates resistance of cancer cells into cisplatin chemotherapy [79]. In fact, relationship between STAT3 and EMT not only is beneficial for invasion of cancer cells, but can also trigger chemoresistance. In addition to STAT3, other molecular factors have been identified as regulators of EMT. It has been reported that non-muscle myosin IIA (NMIIA) can participate in regulation of EMT in cancer cells. NMIIA can play an important role in controlling cell cytokinesis and migration. NMIIA triggers Wnt/β-catenin signaling pathway, which in turn, stimulates EMT, leading to enhance metastasis and invasion of cancer cells [80]. EMT induction is mediated via upregulation of N-cadherin and downregulation of E-cadherin [81]. LncRNAs are also able to regulate EMT in cancer cells. LncRNA FLVCR1-AS1 can also elevate migration and metastasis of ovarian cancer cells via EMT induction [82]. These studies are in agreement with the fact that different molecular pathways contribute to EMT regulation. These pathways have been examined in different cancers and their further targeting can pave the way for effective suppression of cancer metastasis [83,84,85,86].
3. MicroRNAs and Their Role in Cancer Metastasis
MiRs are single stranded non-coding RNAs with a length of 19–24 nucleotides [86,87]. They can negatively affect expression of target gene via binding into 3/-untranslated region (3/-UTR) [88]. MiRs have been reported to be involved in regulation of various biological mechanisms such as differentiation, apoptosis, angiogenesis, proliferation, etc. and an impairment in their expression can provide conditions for development of diverse malignancies [89,90,91,92,93]. Accumulating data demonstrates that miRs are potential upstream regulators of EMT in cancer cells. The modulatory effect of miRs on EMT can regulate the processes of metastasis and invasion of cancer cells. For example, MiR-451a is considered an onco-suppressor miR in hepatocellular carcinoma cells. This miR is able to suppress metastasis of hepatocellular carcinoma cells, and thereby interfere with migration of cancer cells by promoting inhibition of EMT [33]. Transforming growth factor-beta (TGF-β) is involved in cancer progression via EMT induction [94]. TGF-β-mediated EMT can also be targeted by miRs. It has been reported that miR-455-3p can restrict migration of cancer cells via down-regulation of TGF-β, and subsequent inhibition of EMT [95]. In addition to onco-suppressor miRs, there are miRs that can significantly increase migratory capability of cancer cells such as miR-HCC2. It has been reported that miR-HCC2 can increase the migration and metastasis of cancer cells via EMT induction [96]. In EMT induction, oncogene miRs may also affect upstream mediators of EMT. For instance, it has been documented that miR-499a-5p facilitates metastasis of lung cancer cells via upregulation of mammalian target of rapamycin (mTOR), and subsequent stimulation of EMT [35].
4. MicroRNAs Inhibit EMT in Bladder Cancer Cells
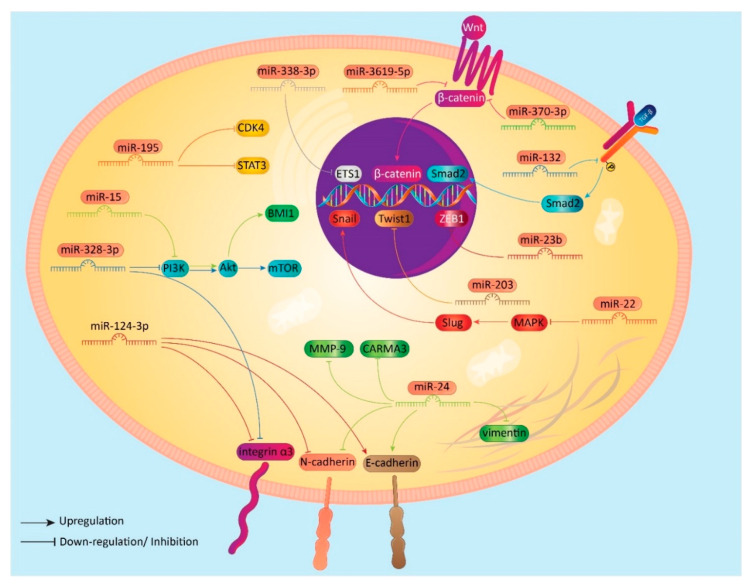
In the previous sections, we described EMT mechanism and its regulation by different molecular pathways with an emphasis on miRNAs. In this section, we specifically discuss the role of onco-suppressor miRNAs in inhibition of EMT in BC cells. Interestingly, expression analysis has revealed that onco-suppressor miRNAs undergo downregulation in BC cells and tissues. This occurs to ensure proliferation and migration of BC cells and is correlated with poor prognosis of patients with BC. miRNA-124-3p is an onco-suppressor miRNA and it appears that enhancing expression of this miRNA can disrupt migration of BC cells. In addition, miRNA-124-3p can inhibit EMT via E-cadherin upregulation and N-cadherin downregulation. Moreover, EMT inhibition by miRNA-124-3p relies on affecting integrin α3 (ITGA3). Increasing evidence demonstrates that ITGA3 is able to promote migration of cancer cells, and it is a downstream target of miRNAs [97,98]. In case of BC, miRNA-124-3p can target ITGA3 to suppress EMT, leading to a decrease in migration of BC cells [89]. Another study also emphasized the role of ITGA3 in metastasis of BC cells, and inhibitory effect of miRNA-328-3p on ITGA2 in suppressing BC migration [99].
4.1. MicroRNAs and PI3K/Akt/EMT Axis
PI3K/Akt signaling pathway can act as an inducer of metastasis in several malignancies including BC cells [100,101,102]. Anti-tumor agents such as leupaxin can suppress invasion of BC cells via PI3K/Akt downregulation [103]. miRNA-328-3p is considered as an onco-suppressor factor in BC cells. It has been found that miRNA-328-3p inhibits EMT via PI3K/Akt downregulation [99]. miRNAs are also able to affect downstream targets of PI3K/Akt signaling pathway in BC cells. B cell-specific Moloney murine leukemia virus integration site 1 (BMI1) is suggested to undergo upregulation in different cancers. BMI1 overexpression ensures tumor sphere formation of cancer cells, and its stability is a positive factor for tumorigenesis [104,105]. MiR-15 as an onco-suppressor factor has been reported to inhibit EMT in BC cells. It appears that miRNA-15 inhibits PI3K/Akt signaling pathway to suppress BMI1 expression, leading to EMT inhibition and decreased invasion of BC cells [106].
4.2. MicroRNAs and CARMA3/EMT Axis
An extensive amount of research has focused on various factors involved in metastasis of BC cells and a considerable role of EMT in malignant behavior and metastasis of these malignant cells has been established [107,108]. Interestingly, CARD-containing MAGUK 3 (CARMA3) is a scaffold with capability of stimulation of nuclear factor-kappa B (NF-κB) signaling pathway and enhancing tumor growth [109,110,111,112]. downregulation of CARMA3 is of interest in suppressing metastasis of BC cells, since CARMA3 is able to induce matrix metalloproteinase-2 (MMP-2), MMP-9 and c-Myc expression that are involved in the migration of BC cells. Besides, CARMA3 can reduce the levels of E-cadherin, whereas it increases β-catenin levels [113]. Moreover, miRNA-24 has gained much attention as a therapeutic target in BC therapy and it has been noted that that miRNA-24 can down-regulates the expression of CARMA3 to suppress EMT via increasing E-cadherin levels, and decreasing N-cadherin, vimentin and MMP-9 levels [114].
4.3. MicroRNAs and Wnt/EMT Axis
The Wnt/β-catenin signaling pathway has been under attention in recent years due to its role in tumorigenesis [115,116,117,118,119]. The tumor-promoting role of Wnt signaling pathway has been investigated in different cancers, including BC [120,121]. It has been found that Wnt signaling can act as an upstream mediator of EMT, so that after activation of Wnt signaling pathway by certain ligands of Wnt family, GSK-3β activity can be inhibited to facilitate the nuclear translocation of β-catenin. Thereafter, various downstream targets with stimulatory effect on proliferation and migration of cancer cells are activated [122,123,124,125]. EMT is a downstream effectt of Wnt activation in cancer progression [86,126]. miRNAs are able to regulate Wnt/EMT axis in BC cells. miRNA-3619-5p as an onco-suppressor factor in BC cells, is able to inhibit metastasis of BC cells. Moreover, examination of molecular pathways shows that miRNA-3619-5p can inhibit Wnt signaling pathway via suppressing nuclear translocation of β-catenin. As a consequence, a decrease occurs in EMT and mesenchymal markers causing an inhibition in invasion of BC cells [127]. Moreover, it has been demonstrated that Wnt7a is capable of induction of Wnt signaling pathway that in turn, enhances β-catenin levels to stimulate EMT, leading to increased migration and invasion of BC cells. miRNA-370-3p can suppress Wnt-mediated EMT via inhibition of EMT [115], thus making it an important therapeutic target for BC therapy.
4.4. MicroRNAs and EMT-Inducing Transcription Factors
TGF-β is another signaling pathway that plays a vital role in cancer malignancy [128]. Briefly, TGF-β induces the formation of Smad complex that can translocate into the nucleus and affect target genes [129,130]. TGF-β can promote migration and metastasis of cancer cells via EMT induction [131,132]. In BC cells, TGF-β1 induces nuclear translocation of Smad2, which in turn, stimulates EMT and can enhance BC migration. miRNA-132 inhibits TGF-β1/Smad2/EMT axis in suppressing BC invasion [133]. In addition to TGF-β, ZEB1 can also induce EMT. Both TGF-β and ZEB1 belong to EMT-inducing transcription factors (EMT-TFs). Similar to TGF-β, ZEB1 is able to stimulate EMT in promoting invasion and metastasis of cancer cells [134,135]. In BC cells, miRNAs are able to affect ZEB1 expression. For instance, miRNA-23b interferes with migration of BC cells into distant tissues by EMT inhibition via downregulation of ZEB1 [136]. Twist1 is another member of EMT-TFs. Twist1 is an enhancer of EMT in cancer cells and has been linked with invasion and migration [137,138]. miRNA-203 has demonstrated onco-suppressor role in BC cells by causing a downregulation of Twist1. Decreased expression of Twist1 is associated with a diminution in EMT and limited migration of BC cells [139]. Slug can also be affected by miRNAs in BC cells, which is able to induce EMT, and its activity can be regulated by MAPK [140,141]. MAPK/Slug/EMT axis is of importance in BC cells, and miRNAs have demonstrated great potential in its regulation. On the contrary, miRNA-22 as an onco-suppressor is able to inhibit MAPK signaling pathway [142,143]. A recently published study has examined the effect of miRNA-22 on MAPK/Slug/EMT axis in BC cells. It was found that miRNA-22 can bind to 3/UTR of MAPK to inhibit its activity. Consequently, MAPK-induced Slug expression can be reduced and a decrease occurs in the levels of vimentin, as a marker of mesenchymal cells. These effects result in inhibition of EMT in BC cells. Furthermore, miRNA-22 can suppress Snail in causing EMT inhibition in BC cells [144]. These studies are in agreement with the fact that miRNAs are able to target EMT-TFs in suppressing metastasis of BC cells. In addition, because of substantial capability of miRNAs in forming feedback loops among divesre oncogenic molecular pathways, it appears that anti-tumor agents or genetic tools applied for targeting aforementioned signaling pathways may not be effective enough to overcome cancer metastasis and EMT [145,146,147,148]. In fact, eradication of BC cells, and suppressing their migration may depend on using a combination of anti-tumor agents (poly-chemotherapy) capable of targeting various molecular pathways or using genetic tools for silencing various downstream targets of miRNAs that are involved in regulating EMT (Table 1, Figure 1).
Table 1.
MicroRNAs inhibit EMT in BC cells.
| MicroRNA | Downstream Target | Cell Line | Major Outcomes | Refs |
|---|---|---|---|---|
| miRNA-370 | SOX12 | Immortalized bladder cell line SV-HUC-1 (ATCC® CRL-9520™) and the human BC cell lines 5637 (ATCC® HTB-9™) and J82 (ATCC® HTB-1™) | miRNA-370 inhibits EMT via SOX12 downregulation, leading to a decreased metastasis of cancer cells | [149] |
| miRNA-34a | CD44 | Human bladder cancer cell lines (5637, T24, HT-1376, J82, SCABER and EJ) | Suppressing EMT via CD44 inhibition | [150] |
| miRNA-613 | SphK1 | Bladder cancer cell lines (J82, T24, UMUC3 and 5637) and a normal bladder cell line (SV-HUC-1) | Inhibition of SphK1 and reduced metastasis and EMT | [151] |
| miRNA-186 | NSBP1 | Human bladder cancer cell lines (J82, HT1376, RT4, T24 and TCCSUP) and immortalized human bladder epithelium (HCV29) cells | downregulation of NSBP1 and suppressed metastasis of cancer cells | [152] |
| miRNA-125a-5p | FUT4 | Bladder cancer cell lines (J82, T24, 5637 and BIU-87) and im-mortalized bladder cell line (SV-HUC-1) | miRNA-125a-5p inhibits invasion and EMT in BC cells via FUT4 downregulation | [153] |
| miRNA-203a | SIX4 | SV-HUC-1 human uro-epithelial cells and the bladder cancer cell lines T24, EJ, J8 and 5637 | There is a negative relationship between miRNA-203a and SIX4. This miRNA inhibits EMT via SIX4 downregulation | [154] |
| miRNA-22 | E2F3 | Human bladder cancer cell lines (5637 and T24) | Preventing expression of E2F3 and suppressed BC metastasis and EMT | [155] |
| miRNA-454-3p miRNA-374b-5p |
ZEB2 | SV-HUC, TCC, 253J, 5637, J82, T24, EJ, HEK-293T cells | Suppressing EMT via ZEB2 downregulation | [156] |
| miRNA-451 | - | T24, 5637 and J28 bladder cancer cell lines | Inhibiting EMT via E-cadherin upregulation, and N-cadherin and vimentin downregulation | [157] |
| miRNA-199a-5p | CCR7 | Human bladder cancer T24 cell line and human normal bladder epithelial cell line SV-HUC-1 | Interfering with metastasis of cancer cells by downregulation of CCR7, and subsequent inhibition of EMT | [158] |
| miRNA-7-5p | Gli3 | TCC, 253J, 5637, T24, EJ, J82 (BCa cell lines) and SV-HUC (human bladder epithelium immortalized cell) | miRNA-7-5p reduces expression of Gli3 as a member of Hedgehog signaling pathway to suppress EMT | [159] |
| miRNA-200 | - | UMUC series of urothelial carcinomas and 253J BV cells | miRNA-200 enhances E-cadherin levels, and reduces ZEB1 and ZEB2 levels to inhibit EMT, leading to a diminution in metastasis and enhanced sensitivity into chemotherapy | [160] |
| miRNA-200c | BMI1 E2F3 |
Human bladder cancer cell lines (UMUC-3 and T24) | Suppressing EMT and increasing E-cadherin levels via inhibition of BMI1 and E2F3 | [161] |
| miRNA-485-5p | HMGA2 | Human bladder cancer cell lines (SW780, T24, HT1376 and HT5637) and human bladder epithelial cell lines HU609 and HEK293 cell | Disrupting invasion of cancer cells by EMT inhibition via HMGA2 downregulation | [162] |
| miRNA-429 | ZEB1/2 β-catenin |
Human UCC cell lines, T24 | Enhancing E-cadherin level via ZEB1/2 downregulation and subsequent inhibition of EMT Suppressing nuclear translocation of β-catenin and its interaction with TCF/LEF1, resulting in EMT inhibition |
[163] |
| miRNA-381-3p | CCNA2 | T24, UM-UC3, and 5637 human BCa cell lines | Inhibition of EMT via CCNA2 downregulation | [164] |
Figure 1.
Inhibition of EMT in BC cells by miRNAs.
4.5. miRNA-200 Family and EMT
A well-known family of miRNAs that are capable of EMT regulation in different cancers is miRNA-200 family [165,166]. The EMT inhibition by miRNA-200 is a gateway for enhancing efficacy of chemotherapy [167]. ZEB proteins that can act as critical enhancers of EMT are often downregulated by miRNA-200 to suppress cancer migration (EMT) [168]. Noteworthy, two studies have evaluated role of miRNA-200 in affecting BC metastasis via EMT regulation. These studies are in accordance with our aforementioned discussions and show that EMT regulation by miRNA-200 leads to enhanced efficacy of chemotherapy and migration inhibition. EMT induction by ZEB proteins (ZEB1 and ZEB2) is a factor involved in resistance of BC cells into chemotherapy. Stable expression of miRNA-200 leads to inhibition of ZEB1 and ZEB2 that can lead to an increased levels of E-cadherin, thus causing a reduced migratory ability of BC cells [160]. This study highlights the fact that cancer may acquire chemoresistance, when they demonstrate malignant behaviors such as invasion. Therefore, EMT acts as a critical factor in cancer metastasis, and signaling pathways regulating this mechanism need to be identified in detail, and miRNAs are possibly one of them. Another study investigated the relationship between miRNA-200 and EMT in BC migration, while previous study demonstrated involvement of miRNA-200/EMT axis in BC chemoresistance. It has been reported that miRNA-200 can reduce expression of BMI and E2F3 as inducers of BC metastasis that results in EMT inhibition via E-cadherin upregulation [161]. Further studies can focus on identifying more downstream targets of miRNA-200 and how they can regulate both EMT and metastasis of BC cells.
4.6. Other microRNAs
In addition, clinical studies have shown that miRNA-195 undergoes downregulation in patients with BC, and is associated with undesirable prognosis [169]. This miRNA is able to suppress proliferation and progression of BC cells via STAT3 inhibition [170]. Moreover, a downregulation of miRNA-195 by lncRNA UCA1 can promote mitochondrial function and growth of BC cells [171]. miRNA-195 is also able to inhibit EMT in BC cells via CDK4 inhibition [172]. In addition, studies have demonstrated that different molecular pathways ensure progression and migration of BC cells. For example, E26 transformation specific-1 (ETS1) is an oncogene factor in BC cells, and its induction can elevate migration of BC cells [173,174]. There is a negative relationship between miRNA-338-3p and ETS1 in BC cells. Moreover, by causing a downregulation of ETS1, miRNA-338-3p can abrogate EMT in BC cells to suppress migration of BC cells [175].
5. MicroRNAs can Induce EMT in BC Cells
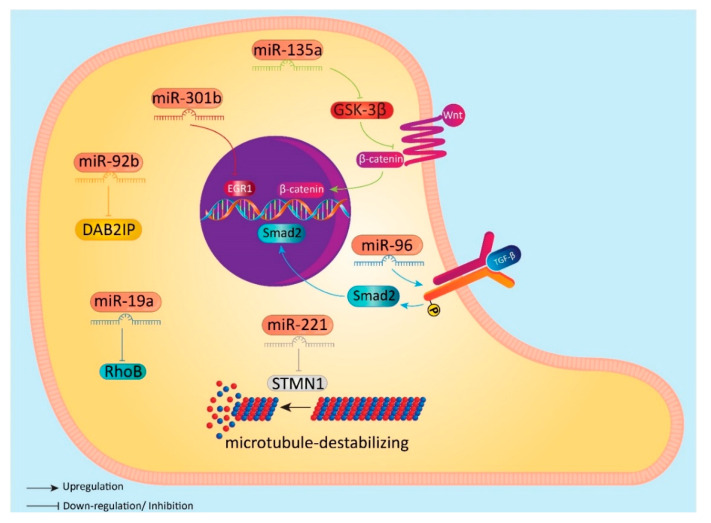
5.1. MicroRNAs and Wnt/EMT Axis
In previous section, we have highlighted that Wnt signaling pathway contributes to stimulation of EMT via β-catenin’s nuclear translocation. It was revealed that miRNA-3619-5p acts as an onco-suppressor, targets nuclear translocation of β-catenin. However, it has been reported that miRNAs can also target GSK-3β activity. miRNA-135a has demonstrated an oncogene role in BC cells. It appears that miRNA-135a induces EMT mechanism to promote metastasis of BC cells. Investigation of molecular pathways showed that miRNA-135a can induce Wnt/ β-catenin signaling pathway via inhibiting GSK-3β activity. Therefater, β-catenin undergoes nuclear translocation that activates EMT, leading to enhanced migratory ability of BC cells [176]. miRNA-135a/Wnt/EMT can therefore be targeted in further studies to inhibit the migration of BC cells.
5.2. MicroRNAs and EMT-Inducing Transcription Factors
Although onco-suppressor miRNAs reduce expression of EMT-TFs to suppress EMT in BC cells, there are oncogenic miRNAs capable of enhancing expression of EMT-TFs in EMT induction. TGF-β1 is able to induce EMT in BC cells and miRNA-96 as an oncogene factor upregulating the expression of TGF-β1 to stimulate EMT in BC cells and thus promoting their migration and invasion [177]. Stathmin 1 (STMN1) is a microtubule-destabilizing protein that plays a vital role in mitosis and cell cycle progression [178,179]. STMN1 is considered as an oncogenic protein, its induction enhances invasion and metastasis of cancer cells [180,181]. It has been reported that miRNA-221 can induce EMT in BC cells via induction of TGF-β. The stimulatory effect of miRNA-221 on TGF-β-mediated EMT relies primarily on inhibition of STMN1 [182].
5.3. MicroRNAs and EGR1/EMT Axis
In addition, early growth response gene 1 (EGR1) acts as a double-edged sword in cancer by displaying both tumor promoting and suppressing roles. EGR1 can reduce apoptosis of cancer cells and promote their migration and angiogenesis [183,184]. However, there are studies showing anti-tumor activity of EGR1 and its role in suppressing metastasis of cancer cells [185]. Moreover, miRNA-301b and EGR1 can act in conjunction to regulate EMT in BC cells. It was found that miRNA-301b has high expression in BC cells, while EGR1 undergoes downregulation in these malignant cells. Investigation of molecular pathways revealed that miRNA-301b can induce EMT in BC cells via EGR1 downregulation [186].
5.4. Other microRNAs
The difficulty in inhibiting metastasis of BC cells is due to involvement of different and complicated molecular pathways in progression of BC cells. RhoB is key member of Rho family and a GTP-binding protein. Increasing evidence demonstrates that RhoB undergoes downregulation in cancer cells and is correlated with poor prognosis [187,188]. RhoB has also been found to inhibit metastasis via EMT downregulation [189]. Based on inhibitory effect of RhoB on EMT, its activity and expression should be diminished by oncogenic miRNAs. Indeed, it was reported that miRNA-19a can promote metastasis and invasion of BC cells by EMT induction via reducing RhoB levels [190].
DAB2IP is a member of RAS-GTPase-activating protein family and its expression has been found to be downregulated in cancer cells [191]. It has been shown that inhibition of DAB2IP in BC cells can mediate both chemoresistance and metastasis [192,193]. Therefore, targeting DAB2IP is of interest for minimizing the migration of BC cells. In order to ensure invasion of BC cells, miRNA-92b can affects DAB2IP effectively. In fact, by causing an inhibition of DAB2IP, miRNA-92b induces EMT, leading to enhanced migration and invasion of BC cells [194]. These studies clearly establish that miRNAs are able to induce EMT in BC cells. However, most of the studies have focused on onco-suppressor miRNAs, and their capability in inhibition of EMT in BC cells. In addition, other studies have also paid attention into regulation of miRNA/EMT axis by other important molecular signaling pathways. Hence, more studies are needed to identify oncogenic miRNAs, which can induce EMT in BC cells to target them effectively for suppressing migration and metastasis of BC cells (Figure 2).
Figure 2.
Induction of EMT by miRNAs in BC cells.
6. Regulation of mciroRNA/EMT in Bladder Cancer Cells
6.1. LncRNAs as Main Regulators
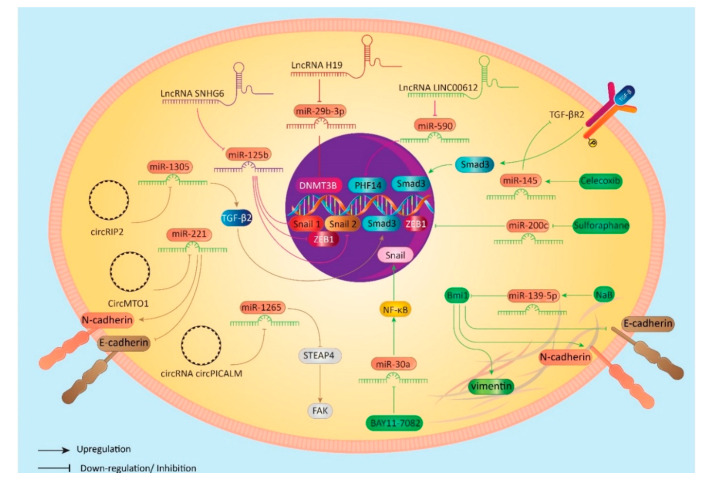
Although miRNAs are potential upstream modulators of EMT in BC cells, and according to previously reported findings, they can target EMT via affecting different molecular pathways. In addition, there are studies showing that several important molecular pathways can also influence miRNAs. This demonstrates the complexity of molecular pathways involved in regulating EMT in BC cells. Similar to miRNAs, lncRNAs are key members of non-coding RNA family that can participate in regulation of vital biological mechanisms such as cell proliferation and cell differentiation [183,195]. A number of studies have shown a significant role of lncRNAs in cancer cells, both as an oncogene or onco-suppressor [196,197]. Both miRNAs and lncRNAs can control the regulation of protein-coding genes [198]. LncRNAs are able to suppress interaction of miRNAs with their target via acting as molecular decoys and sequestering miRNAs [199,200]. Notably, miRNAs are downstream targets of lncRNAs in BC cells [201,202]. This dual relationship can be studied to draw a unique axis and potential therapeutic targeting in future studies. miRNAs are capaable to affect EMT-TFs involved in regulation of EMT in BC cells. LncRNA SNHG6 is an oncogene factor that can promote proliferation of cancer cells, and its downregulation can restrict malignant behavior of cancer cells [138]. Accumulating data demonstrates that SNHG6 can induce EMT in cancer cells via targeting EMT-TFs such as TGF-β and ZEB1 [203], making it an efficient target in suppressing metastasis of cancer cells.
For instance, SNHG6/miRNA-125b/EMT has been implicated in the migration of BC cells. It has been reported that SNHG6 can down-regulate the expression of miR-125b as an onco-suppressing factor. Therefore, an increase occurs in expression of Snail1/2 and ZEB1 to induce EMT, leading to an enhanced metastasis of BC cells [204]. LncRNA H19 is an enhancer of growth and migration in BC cells. Increasing evidence demonstrates that H19 is able to enhance metastasis of BC cells by reducing E-cadherin levels via EZH2 induction [205]. This inhibitory effect of lncRNA H19 on E-cadherin levels can provide condition for migration and invasion of BC cells and undesirable prognosis [206]. An experiment has examined the relationship between lncRNA H19 and miRNA-29b-3p in regulation of EMT in BC cells. This study found that LncRNA H19 can function as an inhibitor of miRNA-29b-3p by sponging. Consequently, an increase occurs in expression of DNMT3B, as a downstream target of miRNA-29b-3p. This provides condition for induction of EMT by H19/miRNA-29b-3p/DNMT3B axis. Inhibition of lncRNA H19 leads to inhibition of EMT and stimulation of MET to suppress metastasis of BC cells [207]. It is worth highlighting that lncRNAs may indirectly affect EMT and metastasis of BC cells. The final aim of targeting miRNAs by lncRNAs in BC cells is to modulate downstream targets of miRNAs and regulate EMT mechanism. For instance, lncRNA LINC00612 is able to induce PHF14 expression via miRNA-590 inhibition, leading to EMT induction and metastasis of BC cells [208]. These studies are in agreement with the fact that lncRNAs can substantially affect miRNA/EMT axis in BC cells. However, more studies are needed to examine the relationship between lncRNAs and miRNA/EMT in BC cells to unravel this intriguing association.
6.2. CircRNAs as Main Regulators
Circular RNAs (circRNAs) are another member of non-coding RNAs capable of regulating miRNAs in cancer cells [190,209]. CircRNAs can be divided into onco-suppressor and oncogenic in BC. They are able to target different molecular pathways in BC cells and miRNAs are their major downstream targets [210,211]. In BC cells, circRNA circPICALM functions as an onco-suppressor factor via causing downregulation of miRNA-1265. The expression of this circRNA undergoes downregulation in BC cells and tissues and has been correlated with poor prognosis. In addition, analysis of molecular pathways has demonstrated that circPICALM can reduce the expression of miRNA-1265. STEAP4, as a downstream target miRNA-1265 undergoes upregulation, which in turn, can inhibit phosphorylation of FAK at Y397, resulting in inhibition of EMT and metastasis of BC cells [212]. In addition to miRNA-1265, it appears that miRNA-221 may be affected by circRNAs in BC cells. For instance, miRNA-221 is an oncogene factor that can reduce the number of cancer cells undergoes apoptosis to ensure proliferation and survival of BC cells [213]. Besides, miRNA-221 has also demonstrated immunosuppressor activity and can facilitate immune evasion of BC cells [214]. So, targeting miRNA-221 is of importance in suppressing malignant behavior of BC cells. Another example is that of CircMTO1 that acts as an onco-suppressor to inhibit miRNA-221 in BC cells. It appears that inhibition of miRNA-221 by circMTO1 may be related to a decrease in migration of BC cells. This is due to inhibition of miRNA-221 and enhancement of E-cadherin/N-cadherin ratio, thereby leading to EMT and metastasis inhibition of BC cells [183]. It is also noteworthy that circRNAs are able to indirectly affect EMT-TFs in BC cells by targeting miRNAs. As it was mentioned earlier, TGF-β can act as a promoter of EMT in cancer cells and suppressing TGF-β ca be promising strategy in metastasis inhibition. Moreover, CircRIP2 can function as an oncogenic factor in BC cells that can trigger EMT to ensure metastasis and malignant behavior of tumor cells. Interestingly, investigation of molecular pathways indicated that circRIP2 could reduce the expression of miRNA-1305 that in turn, can inhibit TGF-β2 and its downstream target, Smad3. This eventually can lead to inhibition of EMT and decreased invasion of BC cells [215].
6.3. Anti-Tumor Compounds as Main Regulators
It is worth highlighting that pharmacologically active anti-tumor drugs are also able to target miRNAs for cancer therapy. Sulforaphane is a plant-derived natural compound exclusively found in cruciferous vegetables [216]. It has demonstrated high anti-tumor activity by induction of apoptosis and regulation of miRNAs, that can sensitize cancer cells to chemotherapy [217]. In BC cells, administration of sulforaphane has been suggested to be an ideal candidate for suppressing invasion and migration of cancer cells. In order to inhibit EMT in BC cells, sulforaphane can effectively target miRNA-200c/ZEB1 axis. miRNA-200c is an onco-suppressor factor and its upregulation by sulforaphane can lead to an inhibition of ZEB1 expression. This in turn can effectively suppress EMT and metastasis of BC cells [218]. Chronic inflammation acts as a positive factor for growth and malignancy of cancers, including BC [219,220]. Tumor-associated macrophages (TAMs) are key members of tumor microenvironment and their abundancy has been correlated with metastasis of cancer cells, and unfavorable prognosis [221,222]. BAY11-7082 as an inhibitor of oncogenic NF-κB signaling pathway [223,224,225,226] is able to inhibit stimulatory action of TAMs on EMT in BC cells. Normally, TAMs upregulate expression of miRNA-30a to induce NF-κB/Snail signaling pathway, leading to EMT and invasion of BC cells. BAY11-7082 can decrease expression of miRNA-30a to disrupt NF-βB/Snail axis, thereby restricting invasion of BC cells via inhibition of EMT [227]. Sodium butyrate (NaB) is a histone deacetylase inhibitor that is extensively applied in cancer therapy for inhibition of migration and induction of apoptosis via mitochondrial dysfunction [228,229,230,231]. Interestingly, administration of NaB has been found to be important in suppressing EMT in BC cells. It appears that NaB can enhance expression of miRNA-139-5p that in turn may reduce Bmi1 expression. Thus, miRNA-139-5p/Bmi1 axis cans lead to an increase in E-cadherin levels and a decrease in Snail, N-cadherin and vimentin levels to inhibit EMT in BC cells [232]. Celecoxib is another anti-tumor agent capable of suppressing cancer proliferation via induction of oxidative stress [233]. Celecoxib is able to suppress metastasis of cancer cells via EMT inhibition [234]. A recent study has shown that celecoxib is able to target miRNAs in regulation of EMT in BC cells. Administration of celecoxib enhanced the expression of miRNA-145 that in turn, inhibited TGFβR2. This leads to an inhibition of Smad3 and a decrease in migration and metastasis of BC cells via suppressing EMT [235]. These studies are in agreement with the fact that anti-tumor compounds can affect miRNAs in regulation of EMT in BC cells and more studies will be needed to discover novel anti-tumor drugs with modulatory impact on miRNA/EMT axis in BC cells. To date, a variety of studies have examined regulation of miRNA/EMT axis in BC cells. LncRNAs are major upstream regulators of miRNA/EMT axis in BC cells and experiments have extensively investigated their relationship with metastasis of BC cells. The p53 and KCNQ1OT1 are other upstream mediators capable of regulating miRNA/EMT axis in BC cells [236,237]. Overall, identification of these signaling pathways will enable us to target them in further studies and effectively inhibit metastasis of BC cells (Table 2, Figure 3).
Table 2.
Regulation of microRNA/EMT axis in BC cells.
| Upstream Regulator | MicroRNA | Downstream Target | Cell Line | Major Outcomes | Refs |
|---|---|---|---|---|---|
| LncRNA DANCR | miRNA-149 | MSI2 | Bladder cancer 5637, SW780, UM-UC-3, T24 and SV-HUC-1 cells | DANCR enhanced metastasis and invasion of cancer cells by downregulation of miRNA-149, and subsequent activation of MSI2, resulting in EMT | [238] |
| LncRNA ARSR | miRNA-129-5p | SOX4 | RT4 and 5637 cells | ARSR reduces expression of miRNA-129-5p via sponging to upregulate SOX4, leading to EMT | [239] |
| LncRNA XIST | miRNA-200c | - | Human bladder cancer cell lines 5637 and T24 | Stimulation of EMT by downregulation of miRNA-200c | [240] |
| LncRNA UCA1 | miRNA-143 | HMGB1 | Human bladder cancer cell lines (T24, 5637, J82, RT4 and HT1376) | UCA1 induces expression of HMGB1 via miRNA-143 downregulation, leading to EMT | [241] |
| LncRNA UCA1 | miRNA-145 | ZEB1/2 | Human bladder cancer cells 5637, T24, and UMUC2 | Stimulation of EMT by downregulation of miRNA-145 and subsequent activation of ZEB1/2 | [242] |
| LncRNA AC114812.8 | miRNA-371b-5p | FUT4 | Human BC cell lines T24, UM-UC-3, J82, and 5637, and the human immortalized normal urinary epithelial cell line SV-HUC-1 | Sponging of miiR-371b-5p by AC114812.8 leads to induction of FUT4, and EMT | [243] |
| LncRNA TUG1 | miRNA-145 | - | SV-HUC-1 cells | TUG1 induces EMT via downregulation of miRNA-145 | [244] |
Figure 3.
Regulation of miRNA/EMT axis in BC cells.
7. Conclusions and Future Directions
Based on the role of miRNAs in regulation of different cellular events, a disturbance in their expression leads to development of pathological events, particularly cancer. miRNAs have been under attention for regulating both proliferation and invasion of BC cells, and to date, a substantial number of studies have identified oncogene and onco-suppressor miRNAs in BC cells [22,25,211,245,246,247,248]. On the other hand, EMT can act as a downstream target of miRNAs in BC. EMT considerably enhances metastasis and migration of BC cells, and its targeting is of importance in suppressing invasion of BC cells and thereby providing desirable prognosis. In the present review, we have provided a comprehensive review about role of miRNAs in regulation of EMT in BC cells. We have divided discussion section into three parts including onco-suppressor miRNAs in EMT inhibition, oncogene miRNAs in EMT induction, and regulation of miRNAs by other molecular pathways such as lncRNAs, circRNAs, etc. Each of the above-mentioned section has investigated a unique signaling pathway in which miRNAs can function as key players regulating tumorigenesis. Targeting these molecular pathways can be considered as an efficient therapeutic strategy in suppressing EMT in BC cells and elevating overall survival of patients with BC.
Abbreviations
| BC | bladder cancer |
| miRNA | microRNA |
| lncRNA | long non-coding RNA |
| EMT | epithelial-to-mesenchymal transition |
| MET | mesenchymal-to-epithelial transition |
| NMIIA | non-muscle myosin IIA |
| 3/-UTR | 3/-untranslated region |
| TGF-β | transforming growth factor-β |
| mTOR | mammalian target of rapamycin |
| EMT-TFs | EMT-promoting transcription factors |
| ITGA3 | integrin α3 |
| ETS1 | E26 transformatin specific-1 |
| CARMA3 | CARD-cotnaining MAGUK 3 |
| NF-βB | nuclear factor-kappaB |
| MMP-2 | matrix metalloproteinase-2 |
| STMN1 | stathmin 1 |
| EGR1 | early growth response gene 1 |
| circRNAs | circular RNAs |
| TAMs | tumor-associated macrophages |
| NaB | sodium butyrate |
Funding
Conceptualization, A.Z. and G.S.; methodology, A.P.K.; software, M.A.; validation, A.Z., R.M.; M.E.A.; M.A. and G.S.; formal analysis, K.H.; investigation, M.H.; resources, M.S.; data curation, M.A.; writing—original draft preparation, M.A.; K.H.; writing—review and editing, P.K.; M.R.; visualization, L.K.; supervision, G.S.; project administration, M.A., M.N.; funding acquisition, G.S.; A.P.K. All authors have read and agreed to the published version of the manuscript.
Funding
A.P.K. is supported by the National Medical Research Council of Singapore. A.P.K is also supported by the National Medical Research Council of Singapore and the Singapore Ministry of Education under its Research Centres of Excellence initiative to Cancer Science Institute of Singapore, National University of Singapore. This work was also supported by National University Health System Seed Fund (R-184-000-301-114) to GS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Babjuk M., Burger M., Compérat E.M., Gontero P., Mostafid A.H., Palou J., van Rhijn B.W., Rouprêt M., Shariat S.F., Sylvester R. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 update. Eur. Urol. 2019;76 doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Alifrangis C., McGovern U., Freeman A., Powles T., Linch M. Molecular and histopathology directed therapy for advanced bladder cancer. Nat. Rev. Urol. 2019;16:465–483. doi: 10.1038/s41585-019-0208-0. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J., Soerjomataram I., Ervik M., Dikshit R., Eser S., Mathers C. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. IARC Publications; Lyon, France: 2013. [Google Scholar]
- 4.Burger M., Catto J.W., Dalbagni G., Grossman H.B., Herr H., Karakiewicz P., Kassouf W., Kiemeney L.A., La Vecchia C., Shariat S. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Chavan S., Bray F., Lortet-Tieulent J., Goodman M., Jemal A. International variations in bladder cancer incidence and mortality. Eur. Urol. 2014;66:59–73. doi: 10.1016/j.eururo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Ashrafizadeh M., Zarrabi A., Samarghandian S., Najafi M. PTEN: What we know of the function and regulation of this onco-suppressor factor in bladder cancer? Eur. J. Pharmacol. 2020;881:173226. doi: 10.1016/j.ejphar.2020.173226. [DOI] [PubMed] [Google Scholar]
- 7.Van Osch F.H., Jochems S.H., Van Schooten F.-J., Bryan R.T., Zeegers M.P. Quantified relations between exposure to tobacco smoking and bladder cancer risk: A meta-analysis of 89 observational studies. Int. J. Epidemiol. 2016;45:857–870. doi: 10.1093/ije/dyw044. [DOI] [PubMed] [Google Scholar]
- 8.Colt J.S., Friesen M.C., Stewart P.A., Donguk P., Johnson A., Schwenn M., Karagas M.R., Armenti K., Waddell R., Verrill C. A case-control study of occupational exposure to metalworking fluids and bladder cancer risk among men. Occup. Environ. Med. 2014;71:667–674. doi: 10.1136/oemed-2013-102056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egbers L., Grotenhuis A.J., Aben K.K., Alfred Witjes J., Kiemeney L.A., Vermeulen S.H. The prognostic value of family history among patients with urinary bladder cancer. Int. J. Cancer. 2015;136:1117–1124. doi: 10.1002/ijc.29062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Zalabani A.H., Stewart K.F., Wesselius A., Schols A.M., Zeegers M.P. Modifiable risk factors for the prevention of bladder cancer: A systematic review of meta-analyses. Eur. J. Epidemiol. 2016;31:811–851. doi: 10.1007/s10654-016-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinmaus C., Ferreccio C., Acevedo J., Yuan Y., Liaw J., Durán V., Cuevas S., García J., Meza R., Valdés R. Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol. Prev. Biomark. 2014;23:1529–1538. doi: 10.1158/1055-9965.EPI-14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuccori M., Filion K.B., Yin H., Oriana H.Y., Platt R.W., Azoulay L. Pioglitazone use and risk of bladder cancer: Population based cohort study. BMJ. 2016;352:i1541. doi: 10.1136/bmj.i1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alanee S., Alvarado-Cabrero I., Murugan P., Kumar R., Nepple K.G., Paner G.P., Patel M.I., Raspollini M.R., Lopez-Beltran A., Konety B.R. Update of the International Consultation on Urological Diseases on bladder cancer 2018: Non-urothelial cancers of the urinary bladder. World J. Urol. 2019;37:107–114. doi: 10.1007/s00345-018-2421-5. [DOI] [PubMed] [Google Scholar]
- 14.Humphrey P.A., Moch H., Cubilla A.L., Ulbright T.M., Reuter V.E. The 2016 WHO classification of tumours of the urinary system and male genital organs—Part B: Prostate and bladder tumours. Eur. Urol. 2016;70:106–119. doi: 10.1016/j.eururo.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Gong H., Chen W., Mi L., Wang D., Zhao Y., Yu C., Zhao A. Qici Sanling decoction suppresses bladder cancer growth by inhibiting the Wnt/Β-catenin pathway. Pharm. Biol. 2019;57:507–513. doi: 10.1080/13880209.2019.1626449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun N., Liang Y., Chen Y., Wang L., Li D., Liang Z., Sun L., Wang Y., Niu H. Glutamine affects T24 bladder cancer cell proliferation by activating STAT3 through ROS and glutaminolysis. Int. J. Mol. Med. 2019;44:2189–2200. doi: 10.3892/ijmm.2019.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li T., Jiang D., Wu K. p62 promotes bladder cancer cell growth by activating KEAP1/NRF2-dependent antioxidative response. Cancer Sci. 2020;111:1156–1164. doi: 10.1111/cas.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goan Y.G., Wu W.T., Liu C.I., Neoh C.A., Wu Y.J. Involvement of Mitochondrial Dysfunction, Endoplasmic Reticulum Stress, and the PI3K/AKT/mTOR Pathway in Nobiletin-Induced Apoptosis of Human Bladder Cancer Cells. Molecules. 2019;24 doi: 10.3390/molecules24162881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X., Wang D., Ding Y., Zhou J., Liu G., Ji Z. lncRNA ZEB1-AS1 promotes migration and metastasis of bladder cancer cells by post-transcriptional activation of ZEB1. Int. J. Mol. Med. 2019;44:196–206. doi: 10.3892/ijmm.2019.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai J., Xu J., Zhao J., Zhang R. Downregulation of lncRNA AWPPH inhibits colon cancer cell proliferation by downregulating GLUT-1. Oncology Lett. 2019;18:2007–2012. doi: 10.3892/ol.2019.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Q.L., Zhan D.M., Chong Y.K., Ding L., Yang Y.G. MiR-652-3p promotes bladder cancer migration and invasion by targeting KCNN3. Eur. Rev. Med. Pharm. Sci. 2019;23:8806–8812. doi: 10.26355/eurrev_201910_19275. [DOI] [PubMed] [Google Scholar]
- 22.Lu Q., Liu T., Feng H., Yang R., Zhao X., Chen W., Jiang B., Qin H., Guo X., Liu M., et al. Circular RNA circSLC8A1 acts as a sponge of miR-130b/miR-494 in suppressing bladder cancer progression via regulating PTEN. Mol. Cancer. 2019;18:111. doi: 10.1186/s12943-019-1040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F., Yang C., Zhang H.B., Ma J., Jia J., Tang X., Zeng J., Chong T., Wang X., He D., et al. BET inhibitor JQ1 suppresses cell proliferation via inducing autophagy and activating LKB1/AMPK in bladder cancer cells. Cancer Med. 2019;8:4792–4805. doi: 10.1002/cam4.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie H., Huang H., Huang W., Xie Z., Yang Y., Wang F. LncRNA miR143HG suppresses bladder cancer development through inactivating Wnt/β-catenin pathway by modulating miR-1275/AXIN2 axis. J. Cell. Physiol. 2019;234:11156–11164. doi: 10.1002/jcp.27764. [DOI] [PubMed] [Google Scholar]
- 25.Guo J., Zhao P., Liu Z., Li Z., Yuan Y., Zhang X., Yu Z., Fang J., Xiao K. MiR-204-3p Inhibited the Proliferation of Bladder Cancer Cells via Modulating Lactate Dehydrogenase-Mediated Glycolysis. Front. Oncol. 2019;9:1242. doi: 10.3389/fonc.2019.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C., Luo Y., He W., Zhao Y., Kong Y., Liu H., Zhong G., Li Y., Li J., Huang J., et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Investig. 2020;130:404–421. doi: 10.1172/JCI130892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida K., Tsuda M., Matsumoto R., Semba S., Wang L., Sugino H., Tanino M., Kondo T., Tanabe K., Tanaka S. Exosomes containing ErbB2/CRK induce vascular growth in premetastatic niches and promote metastasis of bladder cancer. Cancer Sci. 2019;110:2119–2132. doi: 10.1111/cas.14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun P., Wu T., Sun X., Cui Z., Zhang H., Xia Q., Zhang D. KMT2D inhibits the growth and metastasis of bladder Cancer cells by maintaining the tumor suppressor genes. Biomed. Pharm. 2019;115:108924. doi: 10.1016/j.biopha.2019.108924. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z., Du Y., Liu X., Chen H., Weng X., Guo J., Wang M., Wang X., Wang L. EZH2 inhibition suppresses bladder cancer cell growth and metastasis via the JAK2/STAT3 signaling pathway. Oncol. Lett. 2019;18:907–915. doi: 10.3892/ol.2019.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu J.J., Su Y.W., Wang C.J., Li D.F., Zhou L. Semaphorin 4D promotes the proliferation and metastasis of bladder cancer by activating the PI3K/AKT pathway. Tumori. 2019;105:231–242. doi: 10.1177/0300891618811280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Aznar E., Wiesmüller L., Sainz B., Jr., Hermann P.C. EMT and Stemness-Key Players in Pancreatic Cancer Stem Cells. Cancers. 2019;11 doi: 10.3390/cancers11081136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teeuwssen M., Fodde R. Wnt Signaling in Ovarian Cancer Stemness, EMT, and Therapy Resistance. J. Clin. Med. 2019;8 doi: 10.3390/jcm8101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei G.Y., Hu M., Zhao L., Guo W.S. MiR-451a suppresses cell proliferation, metastasis and EMT via targeting YWHAZ in hepatocellular carcinoma. Eur. Rev. Med. Pharm. Sci. 2019;23:5158–5167. doi: 10.26355/eurrev_201906_18180. [DOI] [PubMed] [Google Scholar]
- 34.Li H., Song H., Yuan X., Li J., Tang H. miR-30a reverses TGF-β2-induced migration and EMT in posterior capsular opacification by targeting Smad2. Mol. Biol. Rep. 2019;46:3899–3907. doi: 10.1007/s11033-019-04833-4. [DOI] [PubMed] [Google Scholar]
- 35.He S., Li Z., Yu Y., Zeng Q., Cheng Y., Ji W., Xia W., Lu S. Exosomal miR-499a-5p promotes cell proliferation, migration and EMT via mTOR signaling pathway in lung adenocarcinoma. Exp. Cell Res. 2019;379:203–213. doi: 10.1016/j.yexcr.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 36.Chi Y., Wang F., Zhang T., Xu H., Zhang Y., Shan Z., Wu S., Fan Q., Sun Y. miR-516a-3p inhibits breast cancer cell growth and EMT by blocking the Pygo2/Wnt signalling pathway. J. Cell. Mol. Med. 2019;23:6295–6307. doi: 10.1111/jcmm.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochoa A.E., Choi W., Su X., Siefker-Radtke A., Czerniak B., Dinney C., McConkey D.J. Specific micro-RNA expression patterns distinguish the basal and luminal subtypes of muscle-invasive bladder cancer. Oncotarget. 2016;7:80164–80174. doi: 10.18632/oncotarget.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Georgakopoulos-Soares I., Chartoumpekis D.V., Kyriazopoulou V., Zaravinos A. EMT Factors and Metabolic Pathways in Cancer. Front. Oncol. 2020;10:499. doi: 10.3389/fonc.2020.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko J.-H., Yang M.H., Baek S.H., Nam D., Jung S.H., Ahn K.S. Theacrine attenuates epithelial mesenchymal transition in human breast cancer MDA-MB-231 cells. Phytotherapy Research. 2019;33:1934–1942. doi: 10.1002/ptr.6389. [DOI] [PubMed] [Google Scholar]
- 40.Mohammadinejad R., Biagioni A., Arunkumar G., Shapiro R., Chang K.-C., Sedeeq M., Taiyab A., Hashemabadi M., Pardakhty A., Mandegary A., et al. EMT signaling: Potential contribution of CRISPR/Cas gene editing. Cell. Mol. Life Sci. 2020 doi: 10.1007/s00018-020-03449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loh C.-Y., Chai J.Y., Tang T.F., Wong W.F., Sethi G., Shanmugam M.K., Chong P.P., Looi C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells. 2019;8:1118. doi: 10.3390/cells8101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng J.T., Wang L., Wang H., Tang F.R., Cai W.Q., Sethi G., Xin H.W., Ma Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells. 2019;8 doi: 10.3390/cells8101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashrafizadeh M., Zarrabi A., Hushmandi K., Kalantari M., Mohammadinejad R., Javaheri T., Sethi G. Association of the Epithelial-Mesenchymal Transition (EMT) with Cisplatin Resistance. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Zeisberg M., Neilson E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin M., et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morel A.P., Lièvre M., Thomas C., Hinkal G., Ansieau S., Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tentler D., Lomert E., Novitskaya K., Barlev N.A. Role of ACTN4 in Tumorigenesis, Metastasis, and EMT. Cells. 2019;8 doi: 10.3390/cells8111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim H., Lee S., Shin E., Seong K.M., Jin Y.W., Youn H., Youn B. The Emerging Roles of Exosomes as EMT Regulators in Cancer. Cells. 2020;9 doi: 10.3390/cells9040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pattabiraman D.R., Bierie B., Kober K.I., Thiru P., Krall J.A., Zill C., Reinhardt F., Tam W.L., Weinberg R.A. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science. 2016;351:aad3680. doi: 10.1126/science.aad3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu X., Chen L., Liu L., Niu X. EMT-Mediated Acquired EGFR-TKI Resistance in NSCLC: Mechanisms and Strategies. Front. Oncol. 2019;9:1044. doi: 10.3389/fonc.2019.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Briem E., Ingthorsson S., Traustadottir G.A., Hilmarsdottir B., Gudjonsson T. Application of the D492 Cell Lines to Explore Breast Morphogenesis, EMT and Cancer Progression in 3D Culture. J. Mammary Gland Biol. Neoplasia. 2019;24:139–147. doi: 10.1007/s10911-018-09424-w. [DOI] [PubMed] [Google Scholar]
- 54.Wu H.T., Zhong H.T., Li G.W., Shen J.X., Ye Q.Q., Zhang M.L., Liu J. Oncogenic functions of the EMT-related transcription factor ZEB1 in breast cancer. J. Trans. Med. 2020;18:51. doi: 10.1186/s12967-020-02240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J.H., Chinnathambi A., Alharbi S.A., Shair O.H.M., Sethi G., Ahn K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharm. Res. 2019;150:104504. doi: 10.1016/j.phrs.2019.104504. [DOI] [PubMed] [Google Scholar]
- 56.Prasannan R., Kalesh K.A., Shanmugam M.K., Nachiyappan A., Ramachandran L., Nguyen A.H., Kumar A.P., Lakshmanan M., Ahn K.S., Sethi G. Key cell signaling pathways modulated by zerumbone: Role in the prevention and treatment of cancer. Biochem. Pharmacol. 2012;84:1268–1276. doi: 10.1016/j.bcp.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 57.Zhu J., Zheng Y., Zhang H., Liu Y., Sun H., Zhang P. Galectin-1 induces metastasis and epithelial-mesenchymal transition (EMT) in human ovarian cancer cells via activation of the MAPK JNK/p38 signalling pathway. Am. J. Trans. Res. 2019;11:3862–3878. [PMC free article] [PubMed] [Google Scholar]
- 58.Baek S.H., Ko J.H., Lee J.H., Kim C., Lee H., Nam D., Lee J., Lee S.G., Yang W.M., Um J.Y., et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-β-Induced EMT of Lung Cancer Cells Through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol. 2017;232:346–354. doi: 10.1002/jcp.25426. [DOI] [PubMed] [Google Scholar]
- 59.Ko J.-H., Nam D., Um J.-Y., Jung S.H., Sethi G., Ahn K.S. Bergamottin Suppresses Metastasis of Lung Cancer Cells through Abrogation of Diverse Oncogenic Signaling Cascades and Epithelial-to-Mesenchymal Transition. Molecules. 2018;23:1601. doi: 10.3390/molecules23071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steinbichler T.B., Dudás J., Riechelmann H., Skvortsova I.-I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017;44:170–181. doi: 10.1016/j.semcancer.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Li H., Li J., Chen L., Qi S., Yu S., Weng Z., Hu Z., Zhou Q., Xin Z., Shi L., et al. HERC3-Mediated SMAD7 Ubiquitination Degradation Promotes Autophagy-Induced EMT and Chemoresistance in Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019;25:3602–3616. doi: 10.1158/1078-0432.CCR-18-3791. [DOI] [PubMed] [Google Scholar]
- 62.Thege F.I., Gruber C.N., Cardle I.I., Cong S.H., Lannin T.B., Kirby B.J. anti-EGFR capture mitigates EMT- and chemoresistance-associated heterogeneity in a resistance-profiling CTC platform. Anal. Biochem. 2019;577:26–33. doi: 10.1016/j.ab.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Jordan N.V., Johnson G.L., Abell A.N. Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell Cycle. 2011;10:2865–2873. doi: 10.4161/cc.10.17.17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hong T., Watanabe K., Ta C.H., Villarreal-Ponce A., Nie Q., Dai X. An Ovol2-Zeb1 Mutual Inhibitory Circuit Governs Bidirectional and Multi-step Transition between Epithelial and Mesenchymal States. PLoS Comput. Biol. 2015;11:e1004569. doi: 10.1371/journal.pcbi.1004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nieto M.A., Huang R.Y., Jackson R.A., Thiery J.P. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 66.Lambert A.W., Pattabiraman D.R., Weinberg R.A. Emerging Biological Principles of Metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cook D.P., Vanderhyden B.C. Ovarian cancer and the evolution of subtype classifications using transcriptional profiling. Biol. Reprod. 2019;101:645–658. doi: 10.1093/biolre/ioz099. [DOI] [PubMed] [Google Scholar]
- 68.Kröger C., Afeyan A., Mraz J., Eaton E.N., Reinhardt F., Khodor Y.L., Thiru P., Bierie B., Ye X., Burge C.B., et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc. Natl. Acad. Sci. USA. 2019;116:7353–7362. doi: 10.1073/pnas.1812876116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jolly M.K., Boareto M., Huang B., Jia D., Lu M., Ben-Jacob E., Onuchic J.N., Levine H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front. Oncol. 2015;5:155. doi: 10.3389/fonc.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pastushenko I., Brisebarre A., Sifrim A., Fioramonti M., Revenco T., Boumahdi S., Van Keymeulen A., Brown D., Moers V., Lemaire S., et al. Identification of the tumour transition states occurring during EMT. Nature. 2018;556:463–468. doi: 10.1038/s41586-018-0040-3. [DOI] [PubMed] [Google Scholar]
- 71.Lazar M.A., Birnbaum M.J. Physiology. De-meaning of metabolism. Science. 2012;336:1651–1652. doi: 10.1126/science.1221834. [DOI] [PubMed] [Google Scholar]
- 72.Baenke F., Peck B., Miess H., Schulze A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Models Mech. 2013;6:1353–1363. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaupel P., Schmidberger H., Mayer A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019;95:912–919. doi: 10.1080/09553002.2019.1589653. [DOI] [PubMed] [Google Scholar]
- 74.Li L., Li W. Epithelial-mesenchymal transition in human cancer: Comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharm. Ther. 2015;150:33–46. doi: 10.1016/j.pharmthera.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 75.Bhowmik S.K., Ramirez-Peña E., Arnold J.M., Putluri V., Sphyris N., Michailidis G., Putluri N., Ambs S., Sreekumar A., Mani S.A. EMT-induced metabolite signature identifies poor clinical outcome. Oncotarget. 2015;6:42651–42660. doi: 10.18632/oncotarget.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen Y., Chen Q., Li L. Endostar regulates EMT, migration and invasion of lung cancer cells through the HGF-Met pathway. Mol. Cell. Probes. 2019;45:57–64. doi: 10.1016/j.mcp.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 77.Tan S.M., Li F., Rajendran P., Kumar A.P., Hui K.M., Sethi G. Identification of β-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2010;334:285–293. doi: 10.1124/jpet.110.165498. [DOI] [PubMed] [Google Scholar]
- 78.Kim C., Cho S.K., Kapoor S., Kumar A., Vali S., Abbasi T., Kim S.H., Sethi G., Ahn K.S. β-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 2014;53:793–806. doi: 10.1002/mc.22035. [DOI] [PubMed] [Google Scholar]
- 79.Liang F., Ren C., Wang J., Wang S., Yang L., Han X., Chen Y., Tong G., Yang G. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis. 2019;8:59. doi: 10.1038/s41389-019-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou P., Li Y., Li B., Zhang M., Liu Y., Yao Y., Li D. NMIIA promotes tumor growth and metastasis by activating the Wnt/β-catenin signaling pathway and EMT in pancreatic cancer. Oncogene. 2019;38:5500–5515. doi: 10.1038/s41388-019-0806-6. [DOI] [PubMed] [Google Scholar]
- 81.Meng H., Wu J., Huang Q., Yang X., Yang K., Qiu Y., Ren J., Shen R., Qi H. NEDD9 promotes invasion and migration of colorectal cancer cell line HCT116 via JNK/EMT. Oncol. Lett. 2019;18:4022–4029. doi: 10.3892/ol.2019.10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan H., Li H., Silva M.A., Guan Y., Yang L., Zhu L., Zhang Z., Li G., Ren C. LncRNA FLVCR1-AS1 mediates miR-513/YAP1 signaling to promote cell progression, migration, invasion and EMT process in ovarian cancer. J. Exp. Clin. Cancer Res. 2019;38:356. doi: 10.1186/s13046-019-1356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J., Chu D., Kawamura T., Tanaka K., He S. GRIM-19 repressed hypoxia-induced invasion and EMT of colorectal cancer by repressing autophagy through inactivation of STAT3/HIF-1α signaling axis. J. Cell. Physiol. 2019;234:12800–12808. doi: 10.1002/jcp.27914. [DOI] [PubMed] [Google Scholar]
- 84.Zhou J., Du Y., Lu Y., Luan B., Xu C., Yu Y., Zhao H. CD44 Expression Predicts Prognosis of Ovarian Cancer Patients Through Promoting Epithelial-Mesenchymal Transition (EMT) by Regulating Snail, ZEB1, and Caveolin-1. Front. Oncol. 2019;9:802. doi: 10.3389/fonc.2019.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tian S., Peng P., Li J., Deng H., Zhan N., Zeng Z., Dong W. SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/β-catenin signaling pathway. Aging. 2020;12:3574–3593. doi: 10.18632/aging.102831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J.R., Liu B., Zhou L., Huang Y.X. MicroRNA-124-3p suppresses cell migration and invasion by targeting ITGA3 signaling in bladder cancer. Cancer Biomark. Sect. A Dis. Mark. 2019;24:159–172. doi: 10.3233/CBM-182000. [DOI] [PubMed] [Google Scholar]
- 87.Wu Y., Hu G., Wu R., Gong N. High expression of miR-135b predicts malignant transformation and poor prognosis of gastric cancer. Life Sci. 2020;257:118133. doi: 10.1016/j.lfs.2020.118133. [DOI] [PubMed] [Google Scholar]
- 88.Ravegnini G., Cargnin S., Sammarini G., Zanotti F., Bermejo J.L., Hrelia P., Terrazzino S., Angelini S. Prognostic Role of miR-221 and miR-222 Expression in Cancer Patients: A Systematic Review and Meta-Analysis. Cancers. 2019;11:970. doi: 10.3390/cancers11070970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiao Y., Humphries B., Yang C., Wang Z. MiR-205 Dysregulations in Breast Cancer: The Complexity and Opportunities. Noncoding RNA. 2019;5:53. doi: 10.3390/ncrna5040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gulei D., Raduly L., Broseghini E., Ferracin M., Berindan-Neagoe I. The extensive role of miR-155 in malignant and non-malignant diseases. Mol. Asp. Med. 2019;70:33–56. doi: 10.1016/j.mam.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 91.Taddei M.L., Cavallini L., Ramazzotti M., Comito G., Pietrovito L., Morandi A., Giannoni E., Raugei G., Chiarugi P. Stromal-induced downregulation of miR-1247 promotes prostate cancer malignancy. J. Cell. Physiol. 2019;234:8274–8285. doi: 10.1002/jcp.27679. [DOI] [PubMed] [Google Scholar]
- 92.Pan Y.J., Wan J., Wang C.B. MiR-326: Promising Biomarker for Cancer. Cancer Manag. Res. 2019;11:10411–10418. doi: 10.2147/CMAR.S223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen W., Song J., Bian H., Yang X., Xie X., Zhu Q., Qin C., Qi J. The functions and targets of miR-212 as a potential biomarker of cancer diagnosis and therapy. J. Cell. Mol. Med. 2020;24:2392–2401. doi: 10.1111/jcmm.14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoshimoto S., Tanaka F., Morita H., Hiraki A., Hashimoto S. Hypoxia-induced HIF-1α and ZEB1 are critical for the malignant transformation of ameloblastoma via TGF-β-dependent EMT. Cancer Med. 2019;8:7822–7832. doi: 10.1002/cam4.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zeng Y., Gao T., Huang W., Yang Y., Qiu R., Hou Y., Yu W., Leng S., Feng D., Liu W., et al. MicroRNA-455-3p mediates GATA3 tumor suppression in mammary epithelial cells by inhibiting TGF-β signaling. J. Biol. Chem. 2019;294:15808–15825. doi: 10.1074/jbc.RA119.010800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yi J., Fan Y., Zhang L., Wang H., Mu T., Xie H., Gao H., Liu M., Li S., Tang H. MiR-HCC2 Up-regulates BAMBI and ELMO1 Expression to Facilitate the Proliferation and EMT of Hepatocellular Carcinoma Cells. J. Cancer. 2019;10:3407–3419. doi: 10.7150/jca.30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koshizuka K., Hanazawa T., Kikkawa N., Arai T., Okato A., Kurozumi A., Kato M., Katada K., Okamoto Y., Seki N. Regulation of ITGA3 by the anti-tumor miR-199 family inhibits cancer cell migration and invasion in head and neck cancer. Cancer Sci. 2017;108:1681–1692. doi: 10.1111/cas.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sa K.D., Zhang X., Li X.F., Gu Z.P., Yang A.G., Zhang R., Li J.P., Sun J.Y. A miR-124/ITGA3 axis contributes to colorectal cancer metastasis by regulating anoikis susceptibility. Biochem. Biophys. Res. Commun. 2018;501:758–764. doi: 10.1016/j.bbrc.2018.05.062. [DOI] [PubMed] [Google Scholar]
- 99.Yan T., Ye X.X. MicroRNA-328-3p inhibits the tumorigenesis of bladder cancer through targeting ITGA5 and inactivating PI3K/AKT pathway. Eur. Rev. Med. Pharm. Sci. 2019;23:5139–5148. doi: 10.26355/eurrev_201906_18178. [DOI] [PubMed] [Google Scholar]
- 100.Mohan C.D., Srinivasa V., Rangappa S., Mervin L., Mohan S., Paricharak S., Baday S., Li F., Shanmugam M.K., Chinnathambi A., et al. Trisubstituted-imidazoles induce apoptosis in human breast cancer cells by targeting the oncogenic PI3K/Akt/mTOR signaling pathway. PLoS ONE. 2016;11:e0153155. doi: 10.1371/journal.pone.0153155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ong P.S., Wang L.Z., Dai X., Tseng S.H., Loo S.J., Sethi G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016;7:395. doi: 10.3389/fphar.2016.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Siveen K.S., Ahn K.S., Ong T.H., Shanmugam M.K., Li F., Yap W.N., Kumar A.P., Fong C.W., Tergaonkar V., Hui K.M., et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget. 2014;5:1897–1911. doi: 10.18632/oncotarget.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hou T., Zhou L., Wang L., Kazobinka G., Chen Y., Zhang X., Chen Z. Leupaxin Promotes Bladder Cancer Proliferation, Metastasis, and Angiogenesis Through the PI3K/AKT Pathway. Cell. Physiol. Biochem. 2018;47:2250–2260. doi: 10.1159/000491536. [DOI] [PubMed] [Google Scholar]
- 104.Zhang L., Qiang J., Yang X., Wang D., Rehman A.U., He X., Chen W., Sheng D., Zhou L., Jiang Y.Z., et al. IL1R2 Blockade Suppresses Breast Tumorigenesis and Progression by Impairing USP15-Dependent BMI1 Stability. Adv. Sci. 2020;7:1901728. doi: 10.1002/advs.201901728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li K., Ma Y.B., Tian Y.H., Xu X.L., Gao Y., He Y.Q., Pan W.T., Zhang J.W., He C.J., Wei L. Silencing lncRNA SNHG6 suppresses proliferation and invasion of breast cancer cells through miR-26a/VASP axis. Pathol. Res. Pract. 2019;215:152575. doi: 10.1016/j.prp.2019.152575. [DOI] [PubMed] [Google Scholar]
- 106.Zhang L., Wang C.Z., Ma M., Shao G.F. MiR-15 suppressed the progression of bladder cancer by targeting BMI1 oncogene via PI3K/AKT signaling pathway. Eur. Rev. Med. Pharm. Sci. 2019;23:8813–8822. doi: 10.26355/eurrev_201910_19276. [DOI] [PubMed] [Google Scholar]
- 107.Guo C.C., Majewski T., Zhang L., Yao H., Bondaruk J., Wang Y., Zhang S., Wang Z., Lee J.G., Lee S., et al. Dysregulation of EMT Drives the Progression to Clinically Aggressive Sarcomatoid Bladder Cancer. Cell Rep. 2019;27:1781–1793. doi: 10.1016/j.celrep.2019.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu X., Xu X., Deng W., Huang M., Wu Y., Zhou Z., Zhu K., Wang Y., Cheng X., Zhou X., et al. CCL18 enhances migration, invasion and EMT by binding CCR8 in bladder cancer cells. Mol. Med. Rep. 2019;19:1678–1686. doi: 10.3892/mmr.2018.9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Banan A., Zhang L., Farhadi A., Fields J., Shaikh M., Keshavarzian A. PKC-β1 isoform activation is required for EGF-induced NF-κB inactivation and IκBα stabilization and protection of F-actin assembly and barrier function in enterocyte monolayers. Am. J. Physiol. Cell Physiol. 2004;286:C723–C738. doi: 10.1152/ajpcell.00329.2003. [DOI] [PubMed] [Google Scholar]
- 110.McAllister-Lucas L.M., Ruland J., Siu K., Jin X., Gu S., Kim D.S., Kuffa P., Kohrt D., Mak T.W., Nuñez G. CARMA3/Bcl10/MALT1-dependent NF-κB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc. Natl. Acad. Sci. USA. 2007;104:139–144. doi: 10.1073/pnas.0601947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grabiner B.C., Blonska M., Lin P.-C., You Y., Wang D., Sun J., Darnay B.G., Dong C., Lin X. CARMA3 deficiency abrogates G protein-coupled receptor-induced NF-κB activation. Genes Dev. 2007;21:984–996. doi: 10.1101/gad.1502507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang D., You Y., Lin P.-C., Xue L., Morris S.W., Zeng H., Wen R., Lin X. Bcl10 plays a critical role in NF-κB activation induced by G protein-coupled receptors. Proc. Natl. Acad. Sci. USA. 2007;104:145–150. doi: 10.1073/pnas.0601894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Man X., Liu T., Jiang Y., Zhang Z., Zhu Y., Li Z., Kong C., He J. Silencing of CARMA3 inhibits bladder cancer cell migration and invasion via deactivating β-catenin signaling pathway. Oncotarget. Ther. 2019;12:6309–6322. doi: 10.2147/OTT.S191502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang S., Zhang C., Liu W., Zheng W., Zhang Y., Wang S., Huang D., Liu X., Bai Z. MicroRNA-24 upregulation inhibits proliferation, metastasis and induces apoptosis in bladder cancer cells by targeting CARMA3. Int. J. Oncol. 2015;47:1351–1360. doi: 10.3892/ijo.2015.3117. [DOI] [PubMed] [Google Scholar]
- 115.Huang X., Zhu H., Gao Z., Li J., Zhuang J., Dong Y., Shen B., Li M., Zhou H., Guo H., et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J. Biol. Chem. 2018;293:6693–6706. doi: 10.1074/jbc.RA118.001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Z., Zhou L., Chen L., Xiong M., Kazobinka G., Pang Z., Hou T. RSPO3 promotes the aggressiveness of bladder cancer via Wnt/β-catenin and Hedgehog signaling pathways. Carcinogenesis. 2019;40:360–369. doi: 10.1093/carcin/bgy140. [DOI] [PubMed] [Google Scholar]
- 117.Bhuvanalakshmi G., Gamit N., Patil M., Arfuso F., Sethi G., Dharmarajan A., Kumar A.P., Warrier S. Stemness, Pluripotentiality, and Wnt Antagonism: sFRP4, a Wnt antagonist Mediates Pluripotency and Stemness in Glioblastoma. Cancers. 2018;11 doi: 10.3390/cancers11010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bhuvanalakshmi G., Basappa, Rangappa K.S., Dharmarajan A., Sethi G., Kumar A.P., Warrier S. Breast Cancer Stem-Like Cells Are Inhibited by Diosgenin, a Steroidal Saponin, by the Attenuation of the Wnt β-Catenin Signaling via the Wnt Antagonist Secreted Frizzled Related Protein-4. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ong M.S., Cai W., Yuan Y., Leong H.C., Tan T.Z., Mohammad A., You M.L., Arfuso F., Goh B.C., Warrier S., et al. ’Lnc’-ing Wnt in female reproductive cancers: Therapeutic potential of long non-coding RNAs in Wnt signalling. Br. J. Pharmacol. 2017;174:4684–4700. doi: 10.1111/bph.13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pang G., Xie Q., Yao J. Mitofusin 2 inhibits bladder cancer cell proliferation and invasion via the Wnt/β-catenin pathway. Oncol. Lett. 2019;18:2434–2442. doi: 10.3892/ol.2019.10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Garg M., Maurya N. WNT/β-catenin signaling in urothelial carcinoma of bladder. World J. Nephrol. 2019;8:83–94. doi: 10.5527/wjn.v8.i5.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang R., Song Y., Liu X., Wang Q., Wang Y., Li L., Kang C., Zhang Q. UBE2C induces EMT through Wnt/β-catenin and PI3K/Akt signaling pathways by regulating phosphorylation levels of Aurora-A. Int. J. Oncol. 2017;50:1116–1126. doi: 10.3892/ijo.2017.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu W., Wang Z., Zhang S., Lu X., Wu J., Yu K., Ji A., Lu W., Wang Z., Wu J., et al. IQGAP1 promotes pancreatic cancer progression and epithelial-mesenchymal transition (EMT) through Wnt/β-catenin signaling. Sci. Rep. 2019;9:7539. doi: 10.1038/s41598-019-44048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang J., Cai H., Sun L., Zhan P., Chen M., Zhang F., Ran Y., Wan J. LGR5, a novel functional glioma stem cell marker, promotes EMT by activating the Wnt/β-catenin pathway and predicts poor survival of glioma patients. J. Exp. Clin. Cancer Res. CR. 2018;37:225. doi: 10.1186/s13046-018-0864-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dai B.W., Yang Z.M., Deng P., Chen Y.R., He Z.J., Yang X., Zhang S., Wu H.J., Ren Z.H. HOXC10 promotes migration and invasion via the WNT-EMT signaling pathway in oral squamous cell carcinoma. J. Cancer. 2019;10:4540–4551. doi: 10.7150/jca.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wei C.Y., Zhu M.X., Yang Y.W., Zhang P.F., Yang X., Peng R., Gao C., Lu J.C., Wang L., Deng X.Y., et al. Downregulation of RNF128 activates Wnt/β-catenin signaling to induce cellular EMT and stemness via CD44 and CTTN ubiquitination in melanoma. J. Hematol. Oncol. 2019;12:21. doi: 10.1186/s13045-019-0711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Q., Miao S., Han X., Li C., Zhang M., Cui K., Xiong T., Chen Z., Wang C., Xu H. MicroRNA-3619-5p suppresses bladder carcinoma progression by directly targeting β-catenin and CDK2 and activating p21. Cell Death Dis. 2018;9:960. doi: 10.1038/s41419-018-0986-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hao Y., Baker D., Ten Dijke P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alvarez M.A., Freitas J.P., Mazher Hussain S., Glazer E.S. TGF-β Inhibitors in Metastatic Pancreatic Ductal Adenocarcinoma. J. Gastrointest. Cancer. 2019;50:207–213. doi: 10.1007/s12029-018-00195-5. [DOI] [PubMed] [Google Scholar]
- 130.Howley B.V., Howe P.H. TGF-beta signaling in cancer: Post-transcriptional regulation of EMT via hnRNP E1. Cytokine. 2019;118:19–26. doi: 10.1016/j.cyto.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang X., Zhang Z., Song C., Deng H., Yang R., Zhou L., Sun Y., Zhang Q. Glaucocalyxin A reverses EMT and TGF-β1-induced EMT by inhibiting TGF-β1/Smad2/3 signaling pathway in osteosarcoma. Chem. Biol. Interact. 2019;307:158–166. doi: 10.1016/j.cbi.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 132.Yao Y., Chen R., Wang G., Zhang Y., Liu F. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res. Ther. 2019;10:225. doi: 10.1186/s13287-019-1332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wei X.C., Lv Z.H. MicroRNA-132 inhibits migration, invasion and epithelial-mesenchymal transition via TGFβ1/Smad2 signaling pathway in human bladder cancer. Oncotarget. Ther. 2019;12:5937–5945. doi: 10.2147/OTT.S201731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen J., Hong L., Yu D., Cao T., Zhou Z., He S. LncRNA XIST promotes pancreatic cancer migration, invasion and EMT by sponging miR-429 to modulate ZEB1 expression. Int. J. Biochem. Cell Biol. 2019;113:17–26. doi: 10.1016/j.biocel.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 135.Xiao Y.Y., Lin L., Li Y.H., Jiang H.P., Zhu L.T., Deng Y.R., Lin D., Chen W., Zeng C.Y., Wang L.J., et al. ZEB1 promotes invasion and metastasis of endometrial cancer by interacting with HDGF and inducing its transcription. Am. J. Cancer Res. 2019;9:2314–2330. [PMC free article] [PubMed] [Google Scholar]
- 136.Majid S., Dar A.A., Saini S., Deng G., Chang I., Greene K., Tanaka Y., Dahiya R., Yamamura S. MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in bladder cancer. PLoS ONE. 2013;8:e67686. doi: 10.1371/journal.pone.0067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi D., Wu F., Mu S., Hu B., Zhong B., Gao F., Qing X., Liu J., Zhang Z., Shao Z. LncRNA AFAP1-AS1 promotes tumorigenesis and epithelial-mesenchymal transition of osteosarcoma through RhoC/ROCK1/p38MAPK/Twist1 signaling pathway. J. Exp. Clin. Cancer Res. 2019;38:375. doi: 10.1186/s13046-019-1363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yao N., Fu Y., Chen L., Liu Z., He J., Zhu Y., Xia T., Wang S. Long non-coding RNA NONHSAT101069 promotes epirubicin resistance, migration, and invasion of breast cancer cells through NONHSAT101069/miR-129-5p/Twist1 axis. Oncogene. 2019;38:7216–7233. doi: 10.1038/s41388-019-0904-5. [DOI] [PubMed] [Google Scholar]
- 139.Shen J., Zhang J., Xiao M., Yang J., Zhang N. miR-203 Suppresses Bladder Cancer Cell Growth and Targets Twist1. Oncol. Res. 2018;26:1155–1165. doi: 10.3727/096504017X15041934685237. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 140.Chien M.H., Lin Y.W., Wen Y.C., Yang Y.C., Hsiao M., Chang J.L., Huang H.C., Lee W.J. Targeting the SPOCK1-snail/slug axis-mediated epithelial-to-mesenchymal transition by apigenin contributes to repression of prostate cancer metastasis. J. Exp. Clin. Cancer Res. 2019;38:246. doi: 10.1186/s13046-019-1247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cai Y., Dong Z.Y., Wang J.Y. LncRNA NNT-AS1 is a major mediator of cisplatin chemoresistance in non-small cell lung cancer through MAPK/Slug pathway. Eur. Rev. Med. Pharm. Sci. 2018;22:4879–4887. doi: 10.26355/eurrev_201808_15624. [DOI] [PubMed] [Google Scholar]
- 142.Li W., He Q.Z., Wu C.Q., Pan X.Y., Wang J., Tan Y., Shan X.Y., Zeng H.C. PFOS Disturbs BDNF-ERK-CREB Signalling in Association with Increased MicroRNA-22 in SH-SY5Y Cells. Biomed. Res. Int. 2015;2015:302653. doi: 10.1155/2015/302653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang J., Fan Z., Yang J., Ding J., Yang C., Chen L. microRNA-22 attenuates myocardial ischemia-reperfusion injury via an anti-inflammatory mechanism in rats. Exp. Ther. Med. 2016;12:3249–3255. doi: 10.3892/etm.2016.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu M., Li J., Wang X., Meng S., Shen J., Wang S., Xu X., Xie B., Liu B., Xie L. MiR-22 suppresses epithelial-mesenchymal transition in bladder cancer by inhibiting Snail and MAPK1/Slug/vimentin feedback loop. Cell Death Dis. 2018;9:209. doi: 10.1038/s41419-017-0206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang X., Liang Z., Xu X., Li J., Zhu Y., Meng S., Li S., Wang S., Xie B., Ji A., et al. miR-148a-3p represses proliferation and EMT by establishing regulatory circuits between ERBB3/AKT2/c-myc and DNMT1 in bladder cancer. Cell Death Dis. 2016;7:e2503. doi: 10.1038/cddis.2016.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu X., Zhu Y., Liang Z., Li S., Xu X., Wang X., Wu J., Hu Z., Meng S., Liu B., et al. c-Met and CREB1 are involved in miR-433-mediated inhibition of the epithelial-mesenchymal transition in bladder cancer by regulating Akt/GSK-3β/Snail signaling. Cell Death Dis. 2016;7:e2088. doi: 10.1038/cddis.2015.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li J., Xu X., Meng S., Liang Z., Wang X., Xu M., Wang S., Li S., Zhu Y., Xie B., et al. MET/SMAD3/SNAIL circuit mediated by miR-323a-3p is involved in regulating epithelial-mesenchymal transition progression in bladder cancer. Cell Death Dis. 2017;8:e3010. doi: 10.1038/cddis.2017.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen M.F., Zeng F., Qi L., Zu X.B., Wang J., Liu L.F., Li Y. Transforming growth factor-β1 induces epithelial-mesenchymal transition and increased expression of matrix metalloproteinase-16 via miR-200b downregulation in bladder cancer cells. Mol. Med. Rep. 2014;10:1549–1554. doi: 10.3892/mmr.2014.2366. [DOI] [PubMed] [Google Scholar]
- 149.Wang Y., Ma D.L., Yu C.H., Sha K.F., Zhao M.J., Liu T.J. MicroRNA-370 suppresses SOX12 transcription and acts as a tumor suppressor in bladder cancer. Eur. Rev. Med. Pharm. Sci. 2020;24:2303–2312. doi: 10.26355/eurrev_202003_20496. [DOI] [PubMed] [Google Scholar]
- 150.Yu G., Yao W., Xiao W., Li H., Xu H., Lang B. MicroRNA-34a functions as an anti-metastatic microRNA and suppresses angiogenesis in bladder cancer by directly targeting CD44. J. Exp. Clin. Cancer Res. 2014;33:779. doi: 10.1186/s13046-014-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yu H., Duan P., Zhu H., Rao D. miR-613 inhibits bladder cancer proliferation and migration through targeting SphK1. Am. J. Trans. Res. 2017;9:1213–1221. [PMC free article] [PubMed] [Google Scholar]
- 152.Yao K., He L., Gan Y., Zeng Q., Dai Y., Tan J. MiR-186 suppresses the growth and metastasis of bladder cancer by targeting NSBP1. Diagn. Pathol. 2015;10:146. doi: 10.1186/s13000-015-0372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang Y., Zhang D., Lv J., Wang S., Zhang Q. MiR-125a-5p suppresses bladder cancer progression through targeting FUT4. Biomed. Pharm. 2018;108:1039–1047. doi: 10.1016/j.biopha.2018.09.100. [DOI] [PubMed] [Google Scholar]
- 154.Na X.Y., Shang X.S., Zhao Y., Ren P.P., Hu X.Q. MiR-203a functions as a tumor suppressor in bladder cancer by targeting SIX4. Neoplasma. 2019;66:211–221. doi: 10.4149/neo_2018_180512N312. [DOI] [PubMed] [Google Scholar]
- 155.Guo J., Zhang J., Yang T., Zhang W., Liu M. MiR-22 suppresses the growth and metastasis of bladder cancer cells by targeting E2F3. Int. J. Clin. Exp. Pathol. 2020;13:587–596. [PMC free article] [PubMed] [Google Scholar]
- 156.Wang S., Zhang G., Zheng W., Xue Q., Wei D., Zheng Y., Yuan J. MiR-454-3p and miR-374b-5p suppress migration and invasion of bladder cancer cells through targetting ZEB2. Biosci. Rep. 2018;38 doi: 10.1042/BSR20181436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zeng T., Peng L., Chao C., Fu B., Wang G., Wang Y., Zhu X. miR-451 inhibits invasion and proliferation of bladder cancer by regulating EMT. Int. J. Clin. Exp. Pathol. 2014;7:7653–7662. [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou M., Wang S., Hu L., Liu F., Zhang Q., Zhang D. miR-199a-5p suppresses human bladder cancer cell metastasis by targeting CCR7. BMC Urol. 2016;16:64. doi: 10.1186/s12894-016-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li J., Qiu M., An Y., Huang J., Gong C. miR-7-5p acts as a tumor suppressor in bladder cancer by regulating the hedgehog pathway factor Gli3. Biochem. Biophys. Res. Commun. 2018;503:2101–2107. doi: 10.1016/j.bbrc.2018.07.166. [DOI] [PubMed] [Google Scholar]
- 160.Adam L., Zhong M., Choi W., Qi W., Nicoloso M., Arora A., Calin G., Wang H., Siefker-Radtke A., McConkey D., et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009;15:5060–5072. doi: 10.1158/1078-0432.CCR-08-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu L., Qiu M., Tan G., Liang Z., Qin Y., Chen L., Chen H., Liu J. miR-200c inhibits invasion, migration and proliferation of bladder cancer cells through down-regulation of BMI-1 and E2F3. J. Trans. Med. 2014;12:305. doi: 10.1186/s12967-014-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen Z., Li Q., Wang S., Zhang J. miR-485-5p inhibits bladder cancer metastasis by targeting HMGA2. Int. J. Mol. Med. 2015;36:1136–1142. doi: 10.3892/ijmm.2015.2302. [DOI] [PubMed] [Google Scholar]
- 163.Wu C.L., Ho J.Y., Chou S.C., Yu D.S. MiR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget. 2016;7:26593–26603. doi: 10.18632/oncotarget.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li J., Ying Y., Xie H., Jin K., Yan H., Wang S., Xu M., Xu X., Wang X., Yang K., et al. Dual regulatory role of CCNA2 in modulating CDK6 and MET-mediated cell-cycle pathway and EMT progression is blocked by miR-381-3p in bladder cancer. FASEB J. 2019;33:1374–1388. doi: 10.1096/fj.201800667R. [DOI] [PubMed] [Google Scholar]
- 165.Guttilla I.K., Adams B.D., White B.A. ERα, microRNAs, and the epithelial-mesenchymal transition in breast cancer. Trends Endocrinol. Metab. 2012;23:73–82. doi: 10.1016/j.tem.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 166.Zhang H.F., Xu L.Y., Li E.M. A family of pleiotropically acting microRNAs in cancer progression, miR-200: Potential cancer therapeutic targets. Curr. Pharm. Des. 2014;20:1896–1903. doi: 10.2174/13816128113199990519. [DOI] [PubMed] [Google Scholar]
- 167.Gao Y., Zhang W., Liu C., Li G. miR-200 affects tamoxifen resistance in breast cancer cells through regulation of MYB. Sci. Rep. 2019;9:18844. doi: 10.1038/s41598-019-54289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gregory P.A., Bert A.G., Paterson E.L., Barry S.C., Tsykin A., Farshid G., Vadas M.A., Khew-Goodall Y., Goodall G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 169.Yang K., Tang H., Ding M., Guo Y., Kai K., Xiao J., Shen Y., Miao S., Zhou R. Expression of miR-195 and MEK1 in patients with bladder cancer and their relationship to prognosis. Int. J. Clin. Exp. Pathol. 2019;12:843–850. [PMC free article] [PubMed] [Google Scholar]
- 170.Zhao C., Qi L., Chen M., Liu L., Yan W., Tong S., Zu X. microRNA-195 inhibits cell proliferation in bladder cancer via inhibition of cell division control protein 42 homolog/signal transducer and activator of transcription-3 signaling. Exp. Ther. Med. 2015;10:1103–1108. doi: 10.3892/etm.2015.2633. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 171.Li H.J., Sun X.M., Li Z.K., Yin Q.W., Pang H., Pan J.J., Li X., Chen W. LncRNA UCA1 Promotes Mitochondrial Function of Bladder Cancer via the MiR-195/ARL2 Signaling Pathway. Cell. Physiol. Biochem. 2017;43:2548–2561. doi: 10.1159/000484507. [DOI] [PubMed] [Google Scholar]
- 172.Chen S., Wang W., Lin G., Zhong S. MicroRNA-195 inhibits epithelial-mesenchymal transition via downregulating CDK4 in bladder cancer. Int. J. Clin. Exp. Pathol. 2018;11:3891–3902. [PMC free article] [PubMed] [Google Scholar]
- 173.Liu L., Liu Y., Zhang X., Chen M., Wu H., Lin M., Zhan Y., Zhuang C., Lin J., Li J., et al. Inhibiting cell migration and cell invasion by silencing the transcription factor ETS-1 in human bladder cancer. Oncotarget. 2016;7:25125–25134. doi: 10.18632/oncotarget.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hui K., Wu S., Yue Y., Gu Y., Guan B., Wang X., Hsieh J.T., Chang L.S., He D., Wu K. RASAL2 inhibits tumor angiogenesis via p-AKT/ETS1 signaling in bladder cancer. Cell. Signal. 2018;48:38–44. doi: 10.1016/j.cellsig.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 175.Zhang L., Yan R., Zhang S.N., Zhang H.Z., Ruan X.J., Cao Z., Gu X.Z. MicroRNA-338-3p inhibits the progression of bladder cancer through regulating ETS1 expression. Eur. Rev. Med. Pharm. Sci. 2019;23:1986–1995. doi: 10.26355/eurrev_201903_17237. [DOI] [PubMed] [Google Scholar]
- 176.Mao X.W., Xiao J.Q., Li Z.Y., Zheng Y.C., Zhang N. Effects of microRNA-135a on the epithelial-mesenchymal transition, migration and invasion of bladder cancer cells by targeting GSK3β through the Wnt/β-catenin signaling pathway. Exp. Mol. Med. 2018;50:e429. doi: 10.1038/emm.2017.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.He C., Zhang Q., Gu R., Lou Y., Liu W. miR-96 regulates migration and invasion of bladder cancer through epithelial-mesenchymal transition in response to transforming growth factor-β1. J. Cell. Biochem. 2018;119:7807–7817. doi: 10.1002/jcb.27172. [DOI] [PubMed] [Google Scholar]
- 178.Baldassarre G., Belletti B., Nicoloso M.S., Schiappacassi M., Vecchione A., Spessotto P., Morrione A., Canzonieri V., Colombatti A. p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell. 2005;7:51–63. doi: 10.1016/j.ccr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 179.Rana S., Maples P.B., Senzer N., Nemunaitis J. Stathmin 1: A novel therapeutic target for anticancer activity. Exp. Rev. Anticancer Ther. 2008;8:1461–1470. doi: 10.1586/14737140.8.9.1461. [DOI] [PubMed] [Google Scholar]
- 180.Suzuki K., Watanabe A., Araki K., Yokobori T., Harimoto N., Gantumur D., Hagiwara K., Yamanaka T., Ishii N., Tsukagoshi M., et al. High STMN1 Expression Is Associated with Tumor Differentiation and Metastasis in Clinical Patients with Pancreatic Cancer. Anticancer Res. 2018;38:939–944. doi: 10.21873/anticanres.12307. [DOI] [PubMed] [Google Scholar]
- 181.Osone K., Yokobori T., Katayama C., Takahashi R., Kato R., Tatsuski H., Takada T., Yajima R., Motegi Y., Ogawa H., et al. STMN1 accumulation is associated with dysplastic and neoplastic lesions in patients with ulcerative colitis. Oncol. Lett. 2019;18:4712–4718. doi: 10.3892/ol.2019.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu J., Cao J., Zhao X. miR-221 facilitates the TGFbeta1-induced epithelial-mesenchymal transition in human bladder cancer cells by targeting STMN1. BMC Urol. 2015;15:36. doi: 10.1186/s12894-015-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang Y., Wu F., Zhang J., Sun R., Li F., Li Y., Chang S., Wang L., Wang X., Liu L., et al. EGR1 interacts with DNMT3L to inhibit the transcription of miR-195 and plays an anti-apoptotic role in the development of gastric cancer. J. Cell. Mol. Med. 2019;23:7372–7381. doi: 10.1111/jcmm.14597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li L., Ameri A.H., Wang S., Jansson K.H., Casey O.M., Yang Q., Beshiri M.L., Fang L., Lake R.G., Agarwal S., et al. EGR1 regulates angiogenic and osteoclastogenic factors in prostate cancer and promotes metastasis. Oncogene. 2019;38:6241–6255. doi: 10.1038/s41388-019-0873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhu W., Long J.L., Yin Y.T., Guo H.N., Jiang E.P., Li Y.L., He Q.L., Zeng C., Sun Y.Q. MicroRNA-34a suppresses the invasion and migration of colorectal cancer cells by enhancing EGR1 and inhibiting vimentin. Exp. Ther. Med. 2019;18:2459–2466. doi: 10.3892/etm.2019.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yan L., Wang Y., Liang J., Liu Z., Sun X., Cai K. MiR-301b promotes the proliferation, mobility, and epithelial-to-mesenchymal transition of bladder cancer cells by targeting EGR1. Biochem. Cell Biol. 2017;95:571–577. doi: 10.1139/bcb-2016-0232. [DOI] [PubMed] [Google Scholar]
- 187.Huang M., Prendergast G. RhoB in cancer suppression. Histol. Histopathol. 2006;21:6. doi: 10.14670/HH-21.213. [DOI] [PubMed] [Google Scholar]
- 188.Zhou J., Zhu Y., Zhang G., Liu N., Sun L., Liu M., Qiu M., Luo D., Tang Q., Liao Z. A distinct role of RhoB in gastric cancer suppression. Int. J. Cancer. 2011;128:1057–1068. doi: 10.1002/ijc.25445. [DOI] [PubMed] [Google Scholar]
- 189.Bousquet E., Calvayrac O., Mazieres J., Lajoie-Mazenc I., Boubekeur N., Favre G., Pradines A. RhoB loss induces Rac1-dependent mesenchymal cell invasion in lung cells through PP2A inhibition. Oncogene. 2016;35:1760–1769. doi: 10.1038/onc.2015.240. [DOI] [PubMed] [Google Scholar]
- 190.Bi J., Liu H., Dong W., Xie W., He Q., Cai Z., Huang J., Lin T. Circular RNA circ-ZKSCAN1 inhibits bladder cancer progression through miR-1178-3p/p21 axis and acts as a prognostic factor of recurrence. Mol. Cancer. 2019;18:133. doi: 10.1186/s12943-019-1060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen H., Toyooka S., Gazdar A.F., Hsieh J.-T. Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in prostate cancer cell lines. J. Biol. Chem. 2003;278:3121–3130. doi: 10.1074/jbc.M208230200. [DOI] [PubMed] [Google Scholar]
- 192.Shen Y.J., Kong Z.L., Wan F.N., Wang H.K., Bian X.J., Gan H.L., Wang C.F., Ye D.W. Downregulation of DAB2IP results in cell proliferation and invasion and contributes to unfavorable outcomes in bladder cancer. Cancer Sci. 2014;105:704–712. doi: 10.1111/cas.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wu K., Wang B., Chen Y., Zhou J., Huang J., Hui K., Zeng J., Zhu J., Zhang K., Li L., et al. DAB2IP regulates the chemoresistance to pirarubicin and tumor recurrence of non-muscle invasive bladder cancer through STAT3/Twist1/P-glycoprotein signaling. Cell. Signal. 2015;27:2515–2523. doi: 10.1016/j.cellsig.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 194.Huang J., Wang B., Hui K., Zeng J., Fan J., Wang X., Hsieh J.T., He D., Wu K. miR-92b targets DAB2IP to promote EMT in bladder cancer migration and invasion. Oncol. Rep. 2016;36:1693–1701. doi: 10.3892/or.2016.4940. [DOI] [PubMed] [Google Scholar]
- 195.Jiang M.C., Ni J.J., Cui W.Y., Wang B.Y., Zhuo W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am. J. Cancer Res. 2019;9:1354–1366. [PMC free article] [PubMed] [Google Scholar]
- 196.Liang Z.X., Liu H.S., Wang F.W., Xiong L., Zhou C., Hu T., He X.W., Wu X.J., Xie D., Wu X.R., et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis. 2019;10:829. doi: 10.1038/s41419-019-2077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yang W., Xu X., Hong L., Wang Q., Huang J., Jiang L. Upregulation of lncRNA GAS5 inhibits the growth and metastasis of cervical cancer cells. J. Cell. Physiol. 2019;234:23571–23580. doi: 10.1002/jcp.28926. [DOI] [PubMed] [Google Scholar]
- 198.López-Urrutia E., Montes L.P.B., de Guevara Cervantes D.L., Pérez-Plasencia C., Campos-Parra A.D. Crosstalk between long non-coding RNAs, micro-RNAs and mRNAs: Deciphering molecular mechanisms of master regulators in cancer. Front. Oncol. 2019;9 doi: 10.3389/fonc.2019.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wilusz J.E., Sunwoo H., Spector D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mercer T.R., Dinger M.E., Mattick J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 201.Feng C., Zhao Y., Li Y., Zhang T., Ma Y., Liu Y. LncRNA MALAT1 Promotes Lung Cancer Proliferation and Gefitinib Resistance by Acting as a miR-200a Sponge. Archivos de Bronconeumologia. 2019;55:627–633. doi: 10.1016/j.arbr.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 202.Zhao Y., Feng C., Li Y., Ma Y., Cai R. LncRNA H19 promotes lung cancer proliferation and metastasis by inhibiting miR-200a function. Mol. Cell. Biochem. 2019;460:1–8. doi: 10.1007/s11010-019-03564-1. [DOI] [PubMed] [Google Scholar]
- 203.Wang X., Lai Q., He J., Li Q., Ding J., Lan Z., Gu C., Yan Q., Fang Y., Zhao X., et al. LncRNA SNHG6 promotes proliferation, invasion and migration in colorectal cancer cells by activating TGF-β/Smad signaling pathway via targeting UPF1 and inducing EMT via regulation of ZEB1. Int. J. Med. Sci. 2019;16:51–59. doi: 10.7150/ijms.27359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang C., Tao W., Ni S., Chen Q. Upregulation of lncRNA snoRNA host gene 6 regulates NUAK family SnF1-like kinase-1 expression by competitively binding microRNA-125b and interacting with Snail1/2 in bladder cancer. J. Cell. Biochem. 2019;120:357–367. doi: 10.1002/jcb.27387. [DOI] [PubMed] [Google Scholar]
- 205.Luo M., Li Z., Wang W., Zeng Y., Liu Z., Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013;333:213–221. doi: 10.1016/j.canlet.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 206.Zhu Z., Xu L., Wan Y., Zhou J., Fu D., Chao H., Bao K., Zeng T. Inhibition of E-cadherin expression by lnc-RNA H19 to facilitate bladder cancer metastasis. Cancer Biomark. Sect. A Dis. Mark. 2018;22:275–281. doi: 10.3233/CBM-170998. [DOI] [PubMed] [Google Scholar]
- 207.Lv M., Zhong Z., Huang M., Tian Q., Jiang R., Chen J. lncRNA H19 regulates epithelial-mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochimica et Biophysica Acta Mol. Cell Res. 2017;1864:1887–1899. doi: 10.1016/j.bbamcr.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 208.Miao L., Liu H.Y., Zhou C., He X. LINC00612 enhances the proliferation and invasion ability of bladder cancer cells as ceRNA by sponging miR-590 to elevate expression of PHF14. J. Exp. Clin. Cancer Res. 2019;38:143. doi: 10.1186/s13046-019-1149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mao W., Huang X., Wang L., Zhang Z., Liu M., Li Y., Luo M., Yao X., Fan J., Geng J. Circular RNA hsa_circ_0068871 regulates FGFR3 expression and activates STAT3 by targeting miR-181a-5p to promote bladder cancer progression. J. Exp. Clin. Cancer Res. 2019;38:169. doi: 10.1186/s13046-019-1136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bi J., Liu H., Cai Z., Dong W., Jiang N., Yang M., Huang J., Lin T. Circ-BPTF promotes bladder cancer progression and recurrence through the miR-31-5p/RAB27A axis. Aging. 2018;10:1964–1976. doi: 10.18632/aging.101520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yang C., Yuan W., Yang X., Li P., Wang J., Han J., Tao J., Li P., Yang H., Lv Q., et al. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol. Cancer. 2018;17:19. doi: 10.1186/s12943-018-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yan D., Dong W., He Q., Yang M., Huang L., Kong J., Qin H., Lin T., Huang J. Circular RNA circPICALM sponges miR-1265 to inhibit bladder cancer metastasis and influence FAK phosphorylation. EBioMedicine. 2019;48:316–331. doi: 10.1016/j.ebiom.2019.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu H., Chang J.K., Hou J.Q., Zhao Z.H., Zhang L.D. Inhibition of miR-221 influences bladder cancer cell proliferation and apoptosis. Eur. Rev. Med. Pharm. Sci. 2017;21:3193–3199. [PubMed] [Google Scholar]
- 214.Fu B., Wang Y., Zhang X., Lang B., Zhou X., Xu X., Zeng T., Liu W., Zhang X., Guo J., et al. MiR-221-induced PUMA silencing mediates immune evasion of bladder cancer cells. Int. J. Oncol. 2015;46:1169–1180. doi: 10.3892/ijo.2015.2837. [DOI] [PubMed] [Google Scholar]
- 215.Su Y., Feng W., Shi J., Chen L., Huang J., Lin T. circRIP2 accelerates bladder cancer progression via miR-1305/Tgf-β2/smad3 pathway. Mol. Cancer. 2020;19:23. doi: 10.1186/s12943-019-1129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gong T.-T., Liu X.-D., Zhan Z.-P., Wu Q.-J. Sulforaphane enhances the cisplatin sensitivity through regulating DNA repair and accumulation of intracellular cisplatin in ovarian cancer cells. Exp. Cell Res. 2020;393:112061. doi: 10.1016/j.yexcr.2020.112061. [DOI] [PubMed] [Google Scholar]
- 217.Calcabrini C., Maffei F., Turrini E., Fimognari C. Sulforaphane Potentiates Anticancer Effects of Doxorubicin and Cisplatin and Mitigates Their Toxic Effects. Front. Pharmacol. 2020;11:567. doi: 10.3389/fphar.2020.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shan Y., Zhang L., Bao Y., Li B., He C., Gao M., Feng X., Xu W., Zhang X., Wang S. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J. Nutr. Biochem. 2013;24:1062–1069. doi: 10.1016/j.jnutbio.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 219.Gakis G. Inflammation and Cancer. Springer; New York, NY, USA: 2014. The role of inflammation in bladder cancer; pp. 183–196. [DOI] [PubMed] [Google Scholar]
- 220.Masson-Lecomte A., Rava M., Real F.X., Hartmann A., Allory Y., Malats N. Inflammatory biomarkers and bladder cancer prognosis: A systematic review. Eur. Urol. 2014;66:1078–1091. doi: 10.1016/j.eururo.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 221.Patel R., Baker S.S., Liu W., Desai S., Alkhouri R., Kozielski R., Mastrandrea L., Sarfraz A., Cai W., Vlassara H. Effect of dietary advanced glycation end products on mouse liver. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0035143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Galdiero M.R., Garlanda C., Jaillon S., Marone G., Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J. Cell. Physiol. 2013;228:1404–1412. doi: 10.1002/jcp.24260. [DOI] [PubMed] [Google Scholar]
- 223.Li F., Shanmugam M.K., Chen L., Chatterjee S., Basha J., Kumar A.P., Kundu T.K., Sethi G. Garcinol, a polyisoprenylated benzophenone modulates multiple proinflammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev. Res. 2013;6:843–854. doi: 10.1158/1940-6207.CAPR-13-0070. [DOI] [PubMed] [Google Scholar]
- 224.Sawhney M., Rohatgi N., Kaur J., Shishodia S., Sethi G., Gupta S.D., Deo S.V., Shukla N.K., Aggarwal B.B., Ralhan R. Expression of NF-kappaB parallels COX-2 expression in oral precancer and cancer: Association with smokeless tobacco. Int. J. Cancer. 2007;120:2545–2556. doi: 10.1002/ijc.22657. [DOI] [PubMed] [Google Scholar]
- 225.Ahn K.S., Sethi G., Jain A.K., Jaiswal A.K., Aggarwal B.B. Genetic deletion of NAD(P)H:Quinone oxidoreductase 1 abrogates activation of nuclear factor-κB, IκBα kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J. Biol. Chem. 2006;281:19798–19808. doi: 10.1074/jbc.M601162200. [DOI] [PubMed] [Google Scholar]
- 226.Siveen K.S., Mustafa N., Li F., Kannaiyan R., Ahn K.S., Kumar A.P., Chng W.J., Sethi G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-kappaB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget. 2014;5:634–648. doi: 10.18632/oncotarget.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhang Q., Mao Z., Sun J. NF-κB inhibitor, BAY11-7082, suppresses M2 tumor-associated macrophage induced EMT potential via miR-30a/NF-κB/Snail signaling in bladder cancer cells. Gene. 2019;710:91–97. doi: 10.1016/j.gene.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 228.Tailor D., Hahm E.-R., Kale R.K., Singh S.V., Singh R.P. Sodium butyrate induces DRP1-mediated mitochondrial fusion and apoptosis in human colorectal cancer cells. Mitochondrion. 2014;16:55–64. doi: 10.1016/j.mito.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Salimi V., Shahsavari Z., Safizadeh B., Hosseini A., Khademian N., Tavakoli-Yaraki M. Sodium butyrate promotes apoptosis in breast cancer cells through reactive oxygen species (ROS) formation and mitochondrial impairment. Lipids Health Dis. 2017;16:208. doi: 10.1186/s12944-017-0593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kuefer R., Hofer M., Altug V., Zorn C., Genze F., Kunzi-Rapp K., Hautmann R., Gschwend J. Sodium butyrate and tributyrin induce in vivo growth inhibition and apoptosis in human prostate cancer. Br. J. Cancer. 2004;90:535–541. doi: 10.1038/sj.bjc.6601510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Maruyama T., Yamamoto S., Qiu J., Ueda Y., Suzuki T., Nojima M., Shima H. Apoptosis of bladder cancer by sodium butyrate and cisplatin. J. Infect. Chemother. 2012;18:288–295. doi: 10.1007/s10156-011-0322-2. [DOI] [PubMed] [Google Scholar]
- 232.Wang F., Wu H., Fan M., Yu R., Zhang Y., Liu J., Zhou X., Cai Y., Huang S., Hu Z., et al. Sodium butyrate inhibits migration and induces AMPK-mTOR pathway-dependent autophagy and ROS-mediated apoptosis via the miR-139-5p/Bmi-1 axis in human bladder cancer cells. FASEB J. 2020;34:4266–4282. doi: 10.1096/fj.201902626R. [DOI] [PubMed] [Google Scholar]
- 233.Han Y., Chen P., Zhang Y., Lu W., Ding W., Luo Y., Wen S., Xu R., Liu P., Huang P. Synergy between Auranofin and Celecoxib against Colon Cancer In Vitro and In Vivo through a Novel Redox-Mediated Mechanism. Cancers. 2019;11 doi: 10.3390/cancers11070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Velmurugan B.K., Hua C.H., Tsai M.H., Lee C.P., Chung C.M., Ko Y.C. Combination of celecoxib and calyculin-A inhibits epithelial-mesenchymal transition in human oral cancer cells. Biotech. Histochem. 2020;95:1–8. doi: 10.1080/10520295.2019.1700429. [DOI] [PubMed] [Google Scholar]
- 235.Liu X., Wu Y., Zhou Z., Huang M., Deng W., Wang Y., Zhou X., Chen L., Li Y., Zeng T., et al. Celecoxib inhibits the epithelial-to-mesenchymal transition in bladder cancer via the miRNA-145/TGFBR2/Smad3 axis. Int. J. Mol. Med. 2019;44:683–693. doi: 10.3892/ijmm.2019.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tran M.N., Choi W., Wszolek M.F., Navai N., Lee I.L., Nitti G., Wen S., Flores E.R., Siefker-Radtke A., Czerniak B., et al. The p63 protein isoform ΔNp63α inhibits epithelial-mesenchymal transition in human bladder cancer cells: Role of MIR-205. J. Biol. Chem. 2013;288:3275–3288. doi: 10.1074/jbc.M112.408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang J., Zhang H., Situ J., Li M., Sun H. KCNQ1OT1 aggravates cell proliferation and migration in bladder cancer through modulating miR-145-5p/PCBP2 axis. Cancer Cell Int. 2019;19:325. doi: 10.1186/s12935-019-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhan Y., Chen Z., Li Y., He A., He S., Gong Y., Li X., Zhou L. Long non-coding RNA DANCR promotes malignant phenotypes of bladder cancer cells by modulating the miR-149/MSI2 axis as a ceRNA. J. Exp. Clin. Cancer Res. 2018;37:273. doi: 10.1186/s13046-018-0921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Liao C., Long Z., Zhang X., Cheng J., Qi F., Wu S., Huang T. LncARSR sponges miR-129-5p to promote proliferation and metastasis of bladder cancer cells through increasing SOX4 expression. Int. J. Biol. Sci. 2020;16:1–11. doi: 10.7150/ijbs.39461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Xu R., Zhu X., Chen F., Huang C., Ai K., Wu H., Zhang L., Zhao X. LncRNA XIST/miR-200c regulates the stemness properties and tumourigenicity of human bladder cancer stem cell-like cells. Cancer Cell Int. 2018;18:41. doi: 10.1186/s12935-018-0540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Luo J., Chen J., Li H., Yang Y., Yun H., Yang S., Mao X. LncRNA UCA1 promotes the invasion and EMT of bladder cancer cells by regulating the miR-143/HMGB1 pathway. Oncol. Lett. 2017;14:5556–5562. doi: 10.3892/ol.2017.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xue M., Pang H., Li X., Li H., Pan J., Chen W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016;107:18–27. doi: 10.1111/cas.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Li W., Li Y., Ma W., Zhou J., Sun Z., Yan X. Long noncoding RNA AC114812.8 promotes the progression of bladder cancer through miR-371b-5p/FUT4 axis. Biomed. Pharm. 2020;121:109605. doi: 10.1016/j.biopha.2019.109605. [DOI] [PubMed] [Google Scholar]
- 244.Tan J., Qiu K., Li M., Liang Y. Double-negative feedback loop between long non-coding RNA TUG1 and miR-145 promotes epithelial to mesenchymal transition and radioresistance in human bladder cancer cells. FEBS Lett. 2015;589:3175–3181. doi: 10.1016/j.febslet.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 245.Li Z., Hong S., Liu Z. LncRNA LINC00641 predicts prognosis and inhibits bladder cancer progression through miR-197-3p/KLF10/PTEN/PI3K/AKT cascade. Biochem. Biophys. Res. Commun. 2018;503:1825–1829. doi: 10.1016/j.bbrc.2018.07.120. [DOI] [PubMed] [Google Scholar]
- 246.Wu X., Chen B., Shi H., Zhou J., Zhou F., Cao J., Sun X. miR-758-3p suppresses human bladder cancer cell proliferation, migration and invasion by targeting NOTCH2. Exp. Ther. Med. 2019;17:4273–4278. doi: 10.3892/etm.2019.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhang Z., Qin H., Jiang B., Chen W., Cao W., Zhao X., Yuan H., Qi W., Zhuo D., Guo H. miR-30e-5p suppresses cell proliferation and migration in bladder cancer through regulating metadherin. J. Cell. Biochem. 2019;120:15924–15932. doi: 10.1002/jcb.28866. [DOI] [PubMed] [Google Scholar]
- 248.Liu Y., Liu T., Jin H., Yin L., Yu H., Bi J. MiR-411 suppresses the development of bladder cancer by regulating ZnT1. Oncotarget. Ther. 2018;11:8695–8704. doi: 10.2147/OTT.S173750. [DOI] [PMC free article] [PubMed] [Google Scholar]