Abstract
Background: Anacardium occidentale L. is a medicinal plant with powerful anti-oxidative and anti-inflammatory properties. Acute inflammatory events cause tissue alterations, decrease of anti-oxidative endogenous enzymes such as superoxide dismutase, catalase and glutathione, neutrophils infiltration, increase in the activities of myeloperoxidase, malondialdehyde, and pro-inflammatory release. Methods: Paw edema was induced by subplantar injection of carrageenan into the right hind paw in rats, but 30 min before a group of animals were orally treated with 100 mg/kg of cashew nuts to evaluate the anti-inflammatory and anti-oxidative response. Results: In the present work, we found that (1) cashew nuts reduced the development of carrageenan-induced paw edema limiting the formation of edema and pain; (2) cashew nuts ameliorated the diminutions of the anti-oxidative enzymes caused by carrageenan injection; (3) cashew nuts decreased myeloperoxidase malondialdehyde activity induced by carrageenan; and (4) cashew nuts acted by blocking pro-inflammatory cytokines response and nitrate/nitrite formation stimulated by carrageenan injection. Conclusions: The mechanisms of anti-inflammatory and analgesic effects exerted by cashew nuts were relevant to oxygen free radical scavenging, anti-lipid peroxidation, and inhibition of the formation of inflammatory cytokines.
Keywords: paw edema, cashew nuts, antioxidant, inflammation, polyphenols, analgesic
1. Introduction
Inflammation is the first physiological response to tissue injury involving a complex cascade of reactions which can be provoked by numerous agents such as toxic compounds, microbes, etc., [1,2]. The changes that happen during acute inflammatory event have physiologically functions in controlling infection and restoring tissue to its normal state. The acute inflammatory state is generally composed of four sub-events distinctly into: (1) Exudation of fluid that helps deliver plasma proteins to sites of damage; (2) infiltration of neutrophils that leads to remove pathogens and cellular fragments; (3) vasodilation that the delivery of necessary proteins and cells (like exudation) and increasing tissue temperature; (4) pain and loss of function help to enforce rest and lower the risk of further tissue damage [3].
When acute inflammatory response was controlled, the result is the elimination of the infectious agents followed by a resolution and repair phase [3]. However, when the inflammation is uncontrolled it can be harmful to health [4,5,6]. Main events during the inflammatory state involve nitric oxide (NO) imbalance, lipid peroxidation, cytokines release, and maybe the most important, neutrophil-derived reactive oxygen species (ROS) formation [7].
The instability of free radicals is fundamentally the result of the loss of an electron that leads to an heightened reactivity and to a constantly “steal” electrons from other molecules starting a dangerous chain reaction called “free radical damage” [8]. Principal targets of these “steal” are proteins, lipids, and DNA/RNA, and all these modifications in different and several molecules may increase the chances of mutagenesis. In fact, ROS/RNS overproduction over a prolonged period of time can cause serious injury of the cellular structure and functions. For this reason, it is mandatory to remove it quickly [9]. Free radicals are important mediators that initiate inflammatory processes and, consequently, their neutralization by antioxidants and radical scavengers can attenuate inflammation. In order to minimize the damage caused by free radicals, the organism utilizes several enzyme such as superoxide dismutase (SOD) and catalase (CAT) and cofactor such as glutathione (GSH) [10,11]. Often, however, the physiological endogenous response put in place by the antioxidant enzymes may not be sufficient to limit ROS production [12].
Actually, the primary treatment during acute inflammatory event is the use nonsteroidal anti-inflammatory drugs (NSAIDs), in particular to limit neutrophil migration and oxygen free-radical generation, but several studies demonstrated that long-term use could lead to a lot of side effects, such as cardiovascular and gastrointestinal complications [13,14].
For this reason, it is mandatory to find new molecules to counteract novel drugs for treatment of acute inflammation and pain.
In the past decades, there has been a growing interest in studying and quantifying the antioxidant and anti-inflammatory constituents of vegetables in terms of their potential health functionality through action against inflammatory conditions [15,16]. The use of plant with known anti-inflammatory and/or antioxidant properties can be of great significance in therapeutic anti-inflammatory treatments. In particular, plants or fruit or nuts, rich in phenolic compounds are known for their wide ranges of biological activities, including anticancer, antibacterial, antioxidant, antidiabetic, and anti-inflammatory properties which could constitute an alternative in therapeutics [17].
Numerous studies, has been carried out on nuts demonstrating that a diet enriched with walnuts decreases serum cholesterol levels compared to a standard healthy diet [18]. By definition, tree nuts are dry fruits with one seed in which the ovary wall becomes hard at maturity. One of the most popular edible tree nuts is cashews (Anacardium occidentale L.) [18].
Cashew nuts are rich of unsaturated fatty acids (UFAs) such as oleic (ω-9) and linoleic (ω-6) acid, flavonoids, anthocyanins and tannins, fiber, folate and tocopherols [19,20,21,22,23].
The proposal of nuts as cardio-protective foods was supported from both epidemiological observations suggesting a consistent inverse association between nut intake and development of heart disease and numerous short-term clinical trials that showed beneficial effects of nut intake on the lipid profile [24,25,26,27,28]. Additionally, recent studies proved that the use of cashew nuts (Anacardium occidentale L.) can modulate the effects of several chronic inflammatory state such as colitis, degenerative joint disease, dyslipidemia, and others [29,30,31,32,33,34].
Its anti-inflammatory and anti-oxidative activities is probably due to the inhibition of the biosynthesis of inflammatory mediators by blocking the activities of 5-lipoxygenase (5-LOX) or cyclooxygenase 2 (Cox-2) which makes it a promising treatment for different inflammatory diseases [35,36].
However, until today, nobody evaluated its effects during acute inflammatory events.
Carrageenan-induced paw edema is a common murine experimental model used for the study of new compounds during the acute phase of inflammation [37]. As well-known, following injury induced by carrageenan, there is cell infiltration, mainly neutrophils, that contributes to the inflammatory response by producing myeloperoxidase and pro-inflammatory cytokines [4,6,30,38,39]. Moreover, a critical role during the development of the inflammatory state is the lipid peroxidation and the imbalance between ROS production and anti-oxidant enzyme activities [4,6,30,38,39]. With this background in mind, we used this consolidated experimental model to evaluate for the first time the analgesic, anti-inflammatory, and anti-oxidant effects of cashew nuts.
2. Materials and Methods
2.1. Animals
Male rats (Sprague-Dawley (200–230 g, Envigo, Milan, Italy)) were used throughout. The University of Messina Review Board for animal care (OPBA) approved the study. All animal experiments agree with the new Italian regulations (D. Lgs 2014/26), EU regulations (EU Directive 2010/63), and the ARRIVE guidelines.
2.2. Carrageenan-Induced Paw Edema
After anesthesia with 5.0% isoflurane in 100% O2 rats were subjected to a subplantar injection of CAR (0.1 mL/rat of a 1% suspension in saline) with a 27-gauge needle into the right hind paw, as described previously by Morris and Britti [40,41]. The animals were sacrificed after 6 h post CAR-injection by isoflurane overdose. All analyses were performed in a blinded manner of experimental groups [42].
2.3. Experimental Groups
Rats were randomly divided into the following groups:
(1) CAR + vehicle (saline): rats were subjected to CAR-induced paw edema (n = 10);
(2) CAR + cashew nuts (100 mg/kg): rats were subjected to CAR-induced paw edema and cashew nuts (100 mg/kg) was administered 30 min before CAR (n = 10);
(3) The sham-operated group underwent the same surgical procedures as the CAR group, except that saline or drugs were administered instead of CAR (n = 10 for all experimental groups).
The tested dose was chosen based on previous studies performed in our laboratories [30]. After sacrifice, paw tissue and blood were collected for histological and biochemical analysis.
In another sets of experiments (n = 6 for each group) we analyzed ROS production and 5-LOX/COX pathways.
2.4. Assessment of CAR-Induced Paw Edema
Edema was assessed as previously described [40]. In short, the volume of the paw was measured with a plethysmometer (Ugo Basile, Comerio, Italy) immediately before carrageenan was injected and for 6 h at hourly intervals subsequently. For each animal, edema was expressed as increase in paw volume (mL) after CAR injection relative to pre-injection value.
2.5. Pain-Related Behavioral Analysis in the CAR-Induced Inflammation
To evaluate the analgesic effects of cashew nuts we made a plantar and Von Frey tests. Briefly, during the plantar test we analyzed the hyperalgesic response to heat at different time point using a Basile Plantar Test (Ugo Basile, Varese, Italy) with a cut-off latency of twenty seconds to prevent tissue injury. A mobile unit containing a high-intensity projector bulb was located to carry on a thermal stimulus directly to a single hind paw from beneath the chamber. The withdrawal latency period of injected paws was determined with an electronic clock circuit and thermocouple. Results are expressed as paw withdrawal latencies [41,43]. Additionally, Von Frey test (BIO-EVF4, Bioseb, Vitrolles, France) was made. The device encloses a force transducer furnished with a plastic tip. The force applied was measured when pressure is applied to the tip. The tip was applied to the hind leg’s plantar region, and an increasing force was exerted upwards before the paw was extracted. The withdrawal threshold was defined as the force, expressed in grams, at which the rats removed the paw [41,44,45].
2.6. Myeloperoxidase (MPO) and Malonaldehyde (MDA) Activity
As previously described for MPO evaluation, paw tissues were homogenized in 0.5 percent hexadecyltrimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7.0) and centrifuged at 20,000× g at 4 °C for 30 min. A supernatant aliquot had been allowed to react with a 1.6 mM tetramethylbenzidine/0.1 mM H2O2 solution. The rate of absorbance shift was measured at 650 nm, using a spectrophotometer. MPO activity was defined as the amount of enzyme degrading 1 mM of peroxide at 37 °C within 1 min, and expressed in units per gram of wet tissue weight [46,47]. Additionally, for MDA analysis, paw tissues, collected at the end of experiment, were homogenized in 1.15% KCl solution. An aliquot of the homogenate was added to a reaction mixture containing sodium dodecyl sulfate (SDS), acetic acid (pH 3.5), thiobarbituric acid, and distilled water. Samples were then boiled and centrifuged. The supernatant’s absorbance was measured at 650 nm using spectrophotometry [46,47,48].
2.7. Determination of Nitrite/Nitrate Concentration in Paw
Levels of nitrite/nitrate production in the paw tissue were determined as previously described by Costantino et al. [47]. Briefly, at the end of experiment, paws were cut and centrifuged to recover a sample of the edematous fluid. Blood was separated from the fluid sample and nitrite + nitrate (NOx) production, an indicator of NO synthesis, was measured [47]. Concentrations of nitrate were determined by comparison of regular sodium nitrate solutions prepared in saline solution at the OD550.
2.8. Evaluation of Cytokines and Antioxidant Enzymes in Blood
TNF-α, IL-6, IL-1β, and IL-10 levels from each sample were measured in duplicate with highly sensitive rat Elisa kit according to manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA) [49]. Additionally, also the levels of SOD, GSH, and CAT were assayed in blood according to manufacturer’s instructions (Cusabio Biotech Co., Ltd, Wuhan, Hubei, China) [50,51,52,53].
2.9. Histological Examination of the CAR-Inflamed Hind Paw
For histological examination hematoxylin/eosin (H/E) was made and observed blinded to the treatment protocol. Briefly, paw tissues were taken at the end of experiment, and were dehydrated, embedded in Paraplast, and cut into sections of 7 μm and observed under microscopy (Leica DM7, Milan, Italy). The gradation of inflammation was estimated according a score based on 5 point: none, mild, mild/moderate, moderate, moderate/severe, and severe inflammation [54,55].
2.10. Cashew Nuts Nutritional Composition
Nutritional composition of Cashew kernel samples (Anacardium occidentale L.) obtained from Burkina Faso, a landlocked country in West Africa, was previously detected [30]. Briefly, 100 g of cashew kernel samples containing moisture 4.86 g, protein 21.01 g, lipids (total) 44.70 g, dietary fiber (total) 3.86 g, sugars (total) 32.80 g, ash 2.68 g, and total phenols 69.64 mg.
2.11. Estimation of Oxidant Levels
At the end of the experiment, through the dichlorodihydrofluoresceindiacetate (H2DCFDA) staining method we measured the intracellular oxidant levels as previously described [56,57]. Briefly, we dissolved H2DCFDA probes (Invitrogen Corporation; Carlsbad, CA, USA) in a solution of ethanol with a final concentration of 12.5 mM and we kept it at −80 °C in the dark. Before use, the solution was diluted with potassium phosphate buffer with a final concentration of 125 μM. To obtain the fluorescence reactions, 96-well black microplates were loaded with potassium phosphate buffer to a concentration of 152 μM/well. Then 8 μL diluted tissue homogenate and 40 μL (152 μM dye) were added to get a final concentration of 25 μM. The variation in fluorescence intensity was monitored every 5 min for 30 min with excitation and emission wavelengths set at 485 nm and 538 nm [58].
2.12. Western Blots Analysis for 5-LOX and Cox-2
Western blot examination on cytosolic fraction of the paw tissue was prepared as previously described [59]. Membranes were incubated with anti-5-LOX (1:1000) (Santa Cruz Biotechnology, Heidelberg, Germany), anti-Cox-2 (1:1000) (Santa Cruz Biotechnology, Heidelberg, Germany), and β-actin (1:500) (Santa Cruz Biotechnology, Heidelberg, Germany) for the standardization. Signals were identified with enhanced chemiluminescence (ECL) detection system reagent and the relative expression of the protein bands was measured by densitometry with BIORAD ChemiDocTM XRS+software (Bio-rad, Milan, Italy). A representation of blot signals were imported to analysis software (Image Quant TL, v2003).
2.13. Reagents
All other materials were purchased from Sigma-Aldrich Co. Stock solutions were prepared in nonpyrogenic saline (0.9% NaCl, Baxter Healthcare Ltd., Thetford, Norfolk, UK).
2.14. Data Analysis
All values are expressed as mean ± standard error of the mean of N observations. For in vivo experiments, N represents the number of animals. For experiments involving histology, the photos shown are demonstrative at least three experiments performed on different experimental days on tissue sections collected from all animals in each group. The results were analyzed by two-way ANOVA when the effect of the treatment was investigated in time-dependent mode or by one-way ANOVA when the means of two or more samples were analyzed. All analysis were followed by a Bonferroni post-hoc test for multiple comparisons. In all statistical studies GraphPad Software Prism 8 (La Jolla, CA, USA) was used. A p value of less than 0.05 was considered significant. # p < 0.05 vs CAR; ## p < 0.01 vs CAR; ** p < 0.01 vs sham; *** p < 0.001 vs sham.
3. Results
3.1. Effect of Cashew Nuts on CAR-Induced Inflammation and Pain
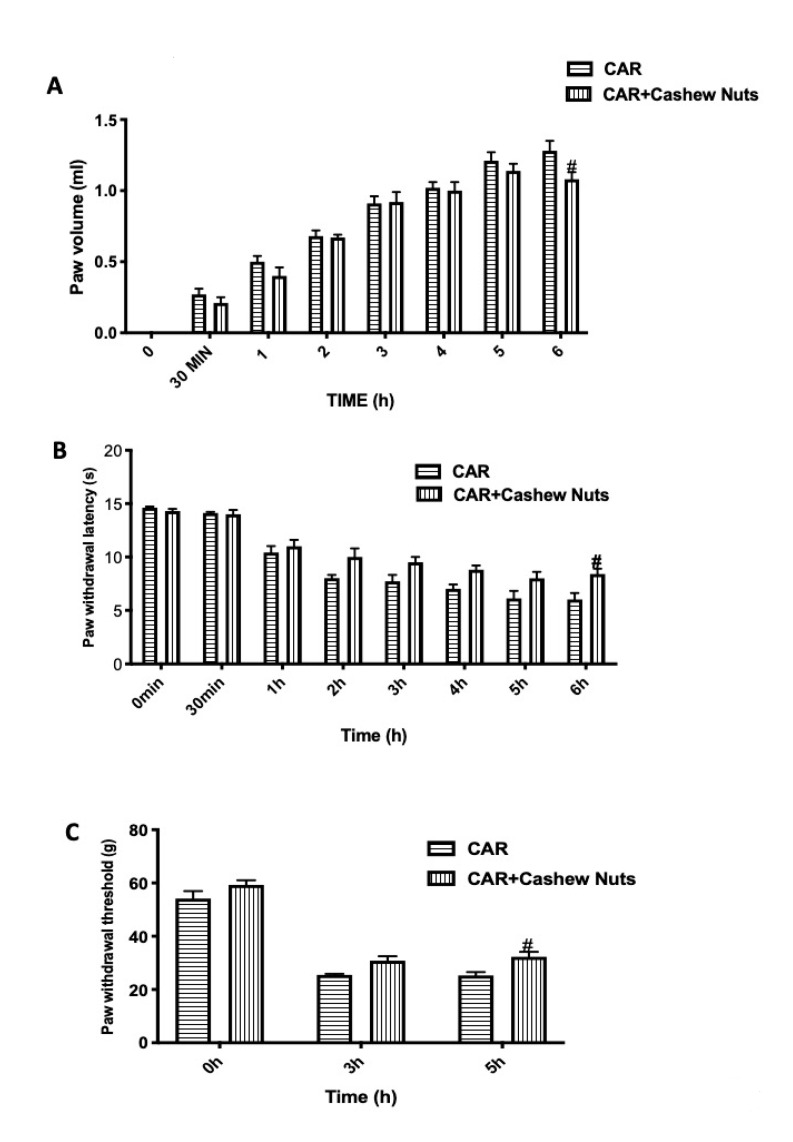
One of the first sign of intraplantar injection of CAR, was the increase in paw volume in a time-dependent way (Figure 1A) measured at 8 different set point from 0 (time when the experiment started) to 6 h (time when experiment ended). The increase in paw volume leads to pain that was assessed by the development of thermal hyperalgesia (Figure 1B) and mechanical allodynia (Figure 1C). In our study, we found that oral treatment with Cashew nuts at the dose of 100 mg/kg given 30 min before CAR, showed a reduction of the volume of rat paw significantly at 6 h post-CAR as well as a decrease in pain showing an inflammatory activity and algesic response.
Figure 1.
Evaluation of the effects of cashew nuts on carrageenan (CAR)-induced inflammation and pain. Paw edema was induced by subplantar injection of CAR. Paw volume was measured before the subplantar injection and hourly to 6 h. The edema volume is the difference in the paw volume at each time-point and the basal paw volume. Hyperalgesia and mechanical allodynia were assessed at the time points indicated with plantar and Von Frey tests. Cashew nuts administration shows significant improvement in the treatment of inflammation and pain. See materials and methods for further details. Paw volume (A); plantar test (B); Von Frey test (C). # p < 0.05 vs. CAR.
3.2. Effects of Cashew Nuts on Histological Alteration after CAR Injection
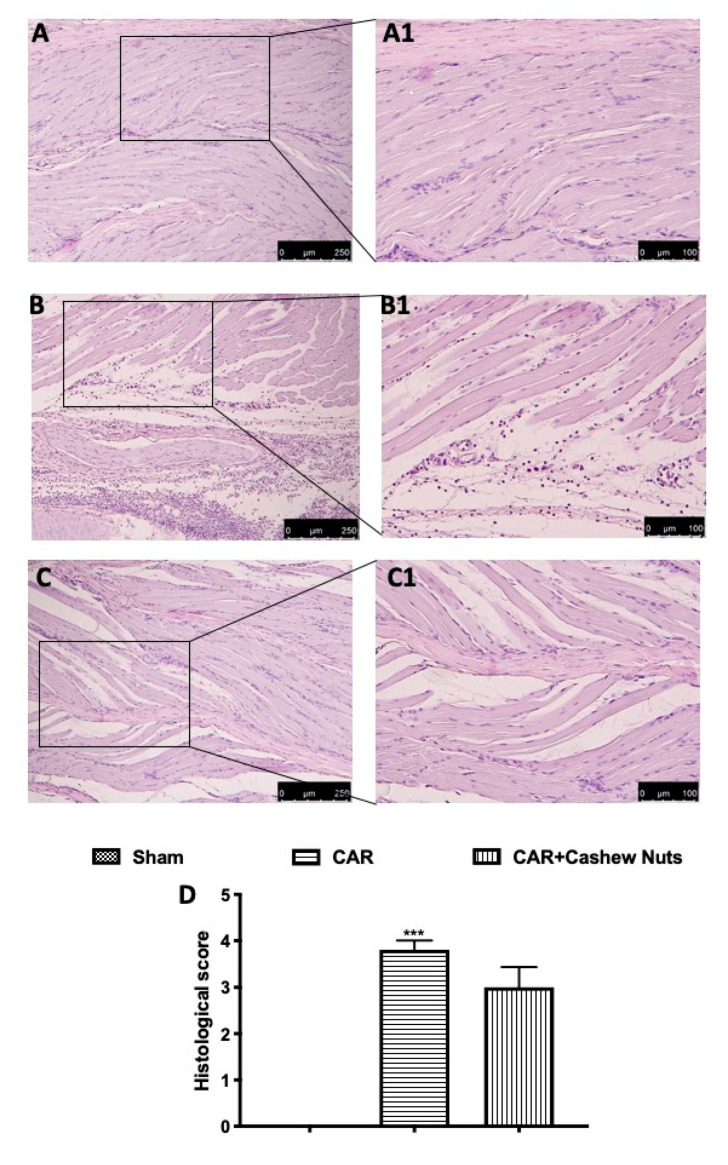
At the end of experiment, a histopathological study was made in paw tissue by H/E examination. A microscopic study of the paw biopsies in CAR group showed edema formation and cellular diffuses infiltration with serious alteration in tissue architecture (Figure 2B and inset B1, see histological score D). Cashew nuts administration, at the dose of 100 mg/kg, was able to slightly reduce histological injury in paw tissues of rats (Figure 3C, and inset C1, see histological score D) counteracting both cellular infiltration and edema formation. Sham rats showed a normal architecture of paw tissue (Figure 2A and inset A1).
Figure 2.
Histological evaluation of paw tissue after cashew nuts treatment following CAR-injection. Investigation of tissue injury of the animals subjected to CAR, was made by H/E staining. CAR group shows a loss of the physiological architecture compared to sham. Cashew nuts administered 30 min before CAR-injection shows reduction in infiltrating cells and edema formation compared to vehicle. Histological score (D). See materials and methods for further details. Sham (A,A1); CAR (B,B1); cashew nuts (C,C1). *** p < 0.001 vs. sham.
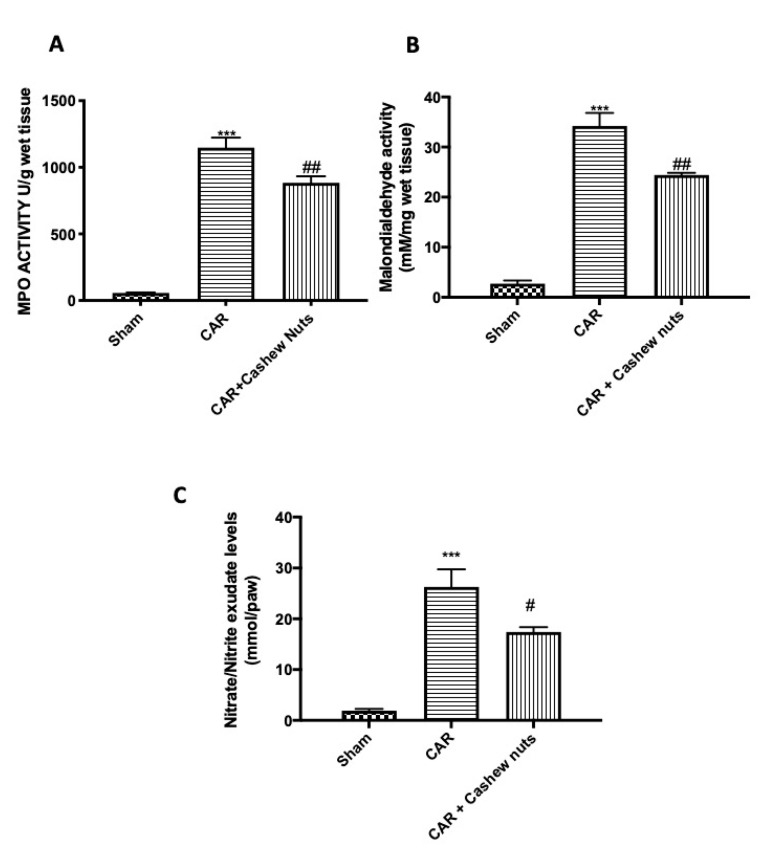
Figure 3.
Effects of cashew nuts on nitrate/nitrite, MPO, and MDA activity after CAR-injection. As a consequence of neutrophils infiltration and lipid peroxidation MPO and MDA were assessed. Additionally, considering the key role played by NO during inflammatory events, we analyzed also levels in nitrite/nitrate in paw exudate. Carrageenan induces a significant increase in all parameter taken in consideration. On the other hands, oral treatment with cashew nuts at the dose of 100 mg/kg significantly reduced MPO, MDA, and nitrate/nitrite CAR-induction. MPO (A); MDA (B); nitrate/nitrite (C). See materials and methods for further details. # p < 0.05 vs. CAR; ## p < 0.01 vs. CAR; *** p < 0.001 vs. sham.
3.3. Effects of Cashew Nuts on Nitrate/Nitrite, MPO, and MDA Activity in CAR-Injured Rats
The development of histological damage was associated with a statistically significant increase in MPO activity (Figure 3A), as an indicator of neutrophil infiltration, and MDA (Figure 3B), marker of lipid peroxidation. In our study, we found that cashew nuts administered 30 min before CAR injection was able to reduce not only MPO activity by inhibiting neutrophil recruitment but also MDA levels.
Additionally, during inflammatory events, NO played a critical role in tissue injury [60]. For this reason, nitrite/nitrate levels were measured in exudate of paw tissues to regulate the expression of nitric oxide (Figure 3C). Oral treatment with cashew nuts at the doses of 100 mg/kg were able to significantly decrease also nitrite/nitrate levels.
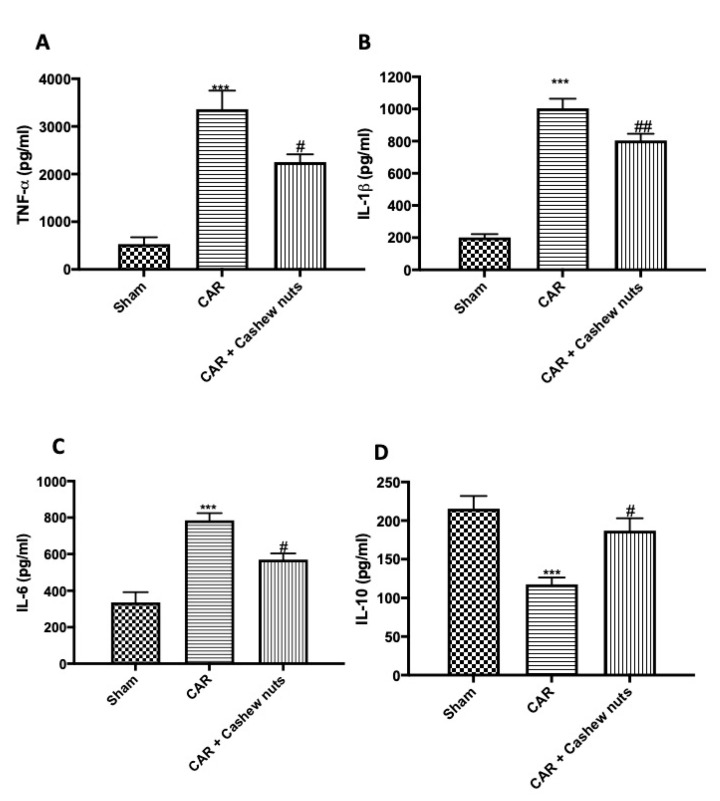
3.4. Effects of Anacardium occidentale L. on Cytokines Production
As well-know, cytokines exert important effects during inflammatory events, for this reason they can be used as biomarkers in indicating or monitoring inflammation and its progress [61]. In our study, we found a significant increase compared to sham animals in serum pro-inflammatory cytokine levels in the group subjected to CAR (Figure 4A–C) as well as, a significant decrease in IL-10 production was detected (Figure 4D). Cashew nuts administration given 30 min before CAR-injection at the dose of 100 mg/kg was able to significantly decrease pro-inflammatory cytokines production, and on the other hand, significantly increase IL-10 release.
Figure 4.
Effects of cashew nuts on cytokines production CAR-induced. Investigation of cytokines is mandatory when talking of inflammatory condition. As predicted, during CAR-caused inflammation, we observed a significant increase in TNF-α, IL-1β, IL-6 levels; vice versa we observed a significant decrease in IL-10 production. Cashew nuts administration 30 min before CAR-injection at the dose of 100 mg/kg significantly reduced pro-inflammatory cytokines expression and, vice versa, increased anti-inflammatory expression of IL-10. TNF-α (A), IL-1β (B), IL-6 (C), and IL-10 (D). See materials and methods for further details. # p < 0.05 vs. CAR; ## p < 0.01 vs. CAR; *** p < 0.001 vs. sham.
3.5. Effect of Cashew Nuts on CAR-Induced Oxidative Stress
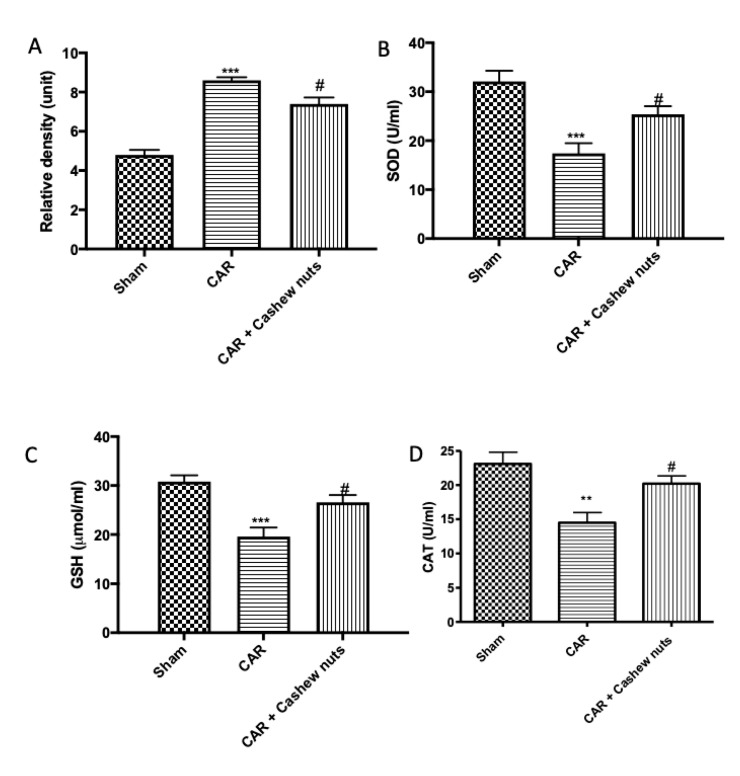
Considering a variety of oxidants and free radicals that are implicated in the pathogenesis of inflammatory process and considering that dietary components also may contribute to the antioxidant defense either by providing redox active compounds that can directly scavenge or neutralize free radicals, we investigated the oxidative stress through H2DCFDA probes and ELISA kits. As supposed, after CAR-injection, we observed a very important increase of ROS production (Figure 5A) and, on the other hand, a decrease in SOD (Figure 5B), GSH (Figure 5C), and CAT (Figure 5D) activity compared to sham animals. After cashew nuts treatment, decrease in oxidative stress and increase in the activity of SOD, GSH, and CAT were observed.
Figure 5.
Anti-oxidant effects of cashew nuts after CAR-induction. Oxidative stress is a major component of acute inflammatory condition. First line of defense against free radical production (A) is represented by SOD (B), CAT (C), and GSH (D). Administration of cashew nuts at the dose of 100 mg/kg significantly increased the activity of the anti-oxidant enzymes SOD, CAT, and GSH which had been significantly reduced by carrageenan injection. SOD (A), CAT (B), and GSH (C). See materials and methods for further details. # p < 0.05 vs. CAR; ** p < 0.01 vs. sham; *** p < 0.001 vs. sham.
3.6. Effect of Cashew Nuts on CAR-Induced 5-LOX and Cox-2 Expressions
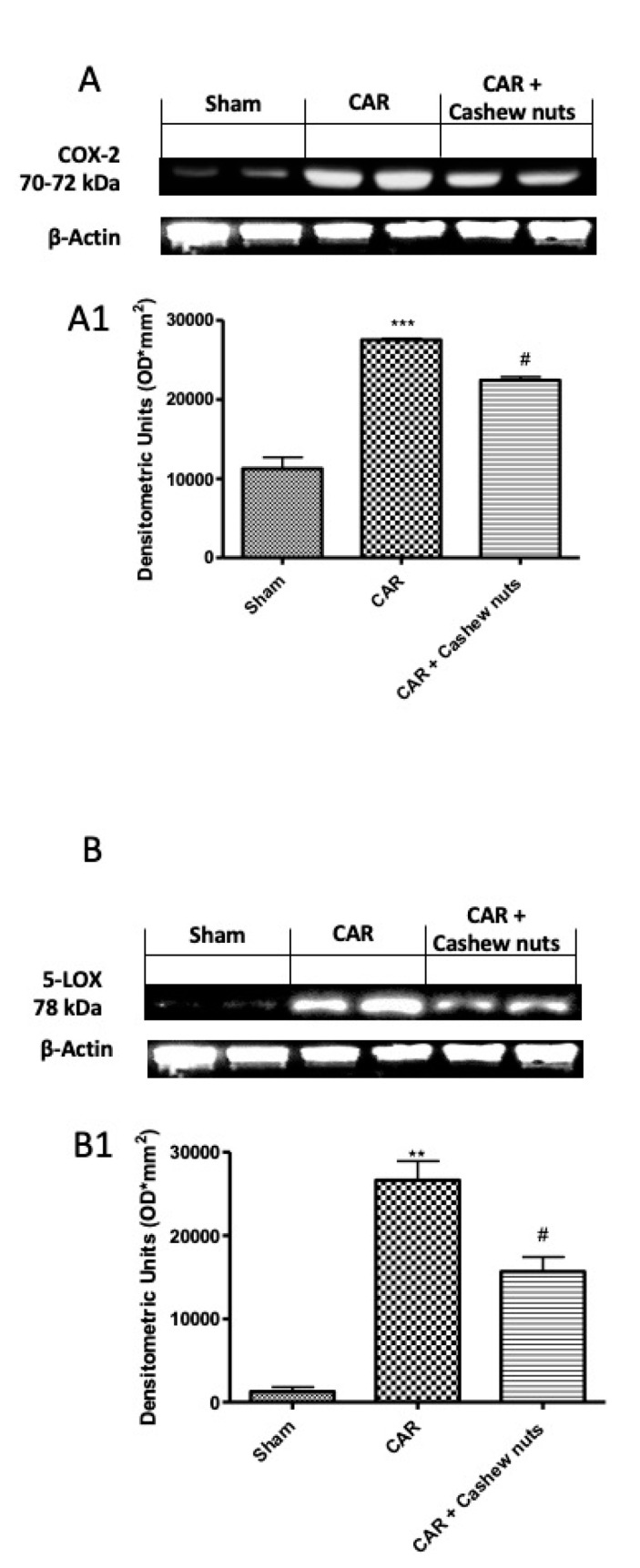
One of the most important role, during inflammatory events, is done by the mediators of the arachidonic acid cascade from COX and LOX pathways, that, as well-known, are modulated by flavonoids [62]. For better understanding the molecular mechanism of cashew nut, we investigate by Western blots, 5-LOX and Cox-2 expressions. As speculated, after CAR injection we found a significant increase in both expression, compared to sham animals. After oral treatment with cashew nuts at the dose of 100 mg/kg, we found a significant decrease in both (Figure 6A,B).
Figure 6.
Effects of cashew nuts on 5-LOX and Cox-2 expressions. The inhibition of the biosynthesis of inflammatory mediators through the modulation of the activities of 5-LOX and/or Cox-2 considered as a promising approach to treat inflammatory diseases. For these reason we investigated the effect of cashew nuts treatment by Western blots on both enzymes. We found a significant increase after CAR injection, compared to sham, that was significantly decreased in 5-LOX as well as in Cox-2 expression. Representative western blot for Cox-2 (A) and LOX-5 (B) and respectively densitometric analysis (A1,B1). See materials and methods for further details. # p < 0.05 vs. CAR; ** p < 0.01 vs. sham.
4. Discussion
Inflammatory condition are universally identified as one of the most important causes of co-morbidity across the population [63]. When under control, inflammation is a defensive response of a body against invasion by the foreign bodies [64]. An acute inflammatory response is, for definition, represented by redness, heat, swelling, pain, and the loss of function [65,66]. The protective effects of inflammatory cascade and potential for tissue destruction are usually balanced in normal state, whereas, when uncontrolled, inflammation may arise in numerous diseased states like rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, and many others [4,67,68,69,70].
Considering doubts about the side effects of repeated use of synthetic chemicals, there is growing interest in the medicinal uses of natural chemicals and their derivatives as healthier replacements, such as functional products or as nutraceuticals [71].
Until today, the most advantageous therapies for the management of inflammatory state is based on the use of NSAIDs. Unfortunately, the chronic use of NSAIDs is connected with a broad spectrum of side effects ranging from gastrointestinal problems to kidney toxicity [64]. Toxicity and reappearance of signs is a major problem related to currently available synthetic drugs [64]. For these reasons the development of safer anti-inflammatory agents remains to be a subject of great interest [16]. Improvement of anti-inflammatory drugs derived from natural sources is the rational and productive strategy toward the cure of inflammatory ailments [72,73].
The search for natural molecules with antioxidant and anti-inflammatory activities has increased extremely over the past decades because the natural products are safe, efficacious, biocompatible, and cost-effective alternatives to treat inflammatory diseases [64].
In particular, researchers focused the attention on the possibilities that dietary daily intake of sources of antioxidants may offer a cost-effective approach to treating most linked pathways associated with inflammation: the oxidative stress [62,63,64].
Nuts would be a promising alternative in reducing oxidative damage, owing to their secondary metabolites richness such as polyphenols, flavonoids, tannins, terpenoids, and anthraquinones [74,75,76,77,78]. The most accredited hypothesis may be that polyphenolic components of dietary plants may increase the endogenous antioxidant defense potential and thus modulate cellular redox state, additionally, and sequentially is apt to consider how polyphenols may modulate the redox system and its components in a cell during normal and pathophysiological conditions [78].
Anacardium occidentale L. is a Brazilian plant that is usually consumed in nature and used in folk medicine with high value edible nut and a source of carbohydrates, proteins, phosphorous, iron, zinc, magnesium, fibers, and fatty acids [79]. Actually, it is officially listed in the National System of Medicinal Plants and Herbal Medicine of the specific Italian health system for medicinal purposes [30]. Also, it is a tree rich in anthocyanins, carotenoids, flavonoids, and other polyphenols as well as mineral components [30]. In recent years, it was used for its antioxidant, antigenotoxic, antimutagenic, antiulcerogenic, anti-inflammatory, antibacterial, antifungal, and larvicides activities [30,80,81,82,83,84,85,86,87]. In another studies made in our laboratory we demonstrated, in a murine model of colitis, that cashew nuts treatment, was able to alleviate the clinical signs of colon damage as well as oxidative stress, inflammation, and iNOS, ICAM-1, and P-selectin expressions [31].
Until today, nobody demonstrated the effect of cashew nuts treatment in an acute inflammatory model.
Carrageenan-induced paw edema is a very sensitive and reproducible test used in the screening of new molecules with anti-inflammatory activities [88]. Carrageenan-induced inflammation causes an acute and local inflammatory response that is advantageous for detecting orally active anti-inflammatory agents; therefore, it has significant prognostic value for anti-inflammatory agents acting through mediators of acute inflammation [88].
First step of acute inflammatory response is characterized by edema often formed because of exudation of fluid and plasma proteins [89]. In our work we found that edema formation was reduced significantly at 6 h post-CAR.
Additionally, carrageenan-induced paw edema leads to sensitization of primary sensory neurons, essentially event to inflammatory pain [90]. In humans, this nociceptor sensitization usually leads to clinical conditions known as hyperalgesia defined as an increased response to a painful stimulus or allodynia described as pain evoked by non-noxious stimuli. In our study, we have proven that oral administration of cashew nuts 30 min before CAR, was in grade to reduce hyperalgesia and allodynia significantly at 6 h post-CAR.
Edema and pain in the hind paw of animals as a result of CAR-induced inflammation usually limit their motility and cause trouble in using their hind paw.
During a CAR-induced acute inflammation event, paw tissue loses normal muscle architecture and shows important amassing of infiltrating inflammatory cells and increased inter-fiber space during microscopic observation [6]. During our study, we found that oral administration of cashew nuts decreased infiltrating inflammatory cell as also demonstrated by the significant decrease of MPO assay.
One of the most dangerous consequences of uncontrolled oxidative is cell injury caused by ROS [91]. Since it is complex measuring the free radicals directly in vivo, it is common in use to carry out the quantification of different molecules which can react with these free radicals, such as for example lipids [92]. Considering that lipid peroxides are very reactive compounds, they appear to quickly degrade in a range of sub-products. MDA is one of the most known secondary products of lipid peroxidation, and it is the most used as marker of cell membrane injury [92]. Additionally, another important biomarker, in the pathogenesis of inflammation, is NO that is produced by inducible nitric oxide synthase during the formation of l-citrulline from l-arginine [93]. In our studies we found that cashew nuts at the dose of 100 mg/kg was in grade to significantly diminished lipid peroxidation and NO formation.
Conversion of arachidonic acid to biologically active leukotrienes, a potent mediators of inflammatory reactions is a key point when looking for new molecules that can inhibit inflammatory events. Plant domain is a valuable source for new 5-LOX and dual 5-LOX/COX-inhibitors, because they are fundamentally rich in flavonoids compound [36]. In past, Wagner and colleagues demonstrate that Anacardium occidentale L. strongly inhibited prostaglandin synthase, but nobody evaluated these activities in vivo [94]. Our results demonstrate that cashew nuts decrease inflammation probably across to the modulation of 5-LOX and Cox-2.
Inflammatory cascade activates cells and induce production of inflammatory cytokines, such as IL-1β, IL-6, and TNF-α. These molecules can potentially serve as biomarkers for diseases diagnosis, prognosis, and therapeutic management [95,96,97]. In these work we demonstrated that cashew nuts could inhibit the production of the cytokines involved in carrageenan-induced paw edema.
To counteract ROS formation, cells have developed a complex antioxidant defense system that consists of several enzyme systems involved in the conversion of ROS to less reactive molecules such as O2 and water [98,99]. The first line of antioxidants defense was composed by SOD, CAT, and GSH.
SOD, is the most powerful antioxidant enzyme in the cell that catalytically converts superoxide radical or singlet oxygen into hydrogen peroxide and molecular oxygen; CAT catalyzes the degradation or reduction of hydrogen peroxide to water and molecular oxygen, completing the detoxification process initiated by SOD, and GSH is the most abundant intracellular non-protein thiol in cells with the functions of removing potentially toxic electrophiles and metals protecting cells from toxic oxygen products [100,101].
In our studies, carrageenan significantly increased ROS production and reduced GSH, SOD, and CAT levels, but this increase/decrease was counteracted by the oral treatment of cashew nuts, suggesting that the inhibition of carrageenan-induced oxidative stress may also explain the analgesic effect.
5. Conclusions
Inflammation studies have been one of the main hubs of global science study. The inflammation is known to be correlated with oxidative processes, mainly because they share some common pathways. Since oxidative stress is common in several degenerative disease, it has been supposed that dietary antioxidants may explain a very important protective effect. Nuts are a main source of antioxidants in the diets worldwide. Nuts are high in antioxidant, in fiber, and in beneficial unsaturated fats and low in saturated fats. Nuts are usually eaten as a snack or added to food to provide both nutrients and bioactive antioxidants. In conclusion, in our work, we demonstrated for the first time that cashew nuts consumption not only brings benefits in experimental mouse models of chronic inflammation, but also in acute inflammation events. In particular was in grade to significantly counteract edema formation and consequently carrageenan-related pain. In addition, oral treatment with 100 mg/kg of cashew nuts significantly decreased MPO and MDA activity as well as nitrate/nitrite formation. Moreover, in agreement with our previous study, we demonstrated for the first time, that cashew nuts administration was able to significantly improve endogenous antioxidant activity, limiting pro-inflammatory cytokines release. Its beneficial effect is probably due to the high content of phenols that mediate activation of 5-LOX COX pathways. Considering all the benefits brought by cashew nuts, its usual consumption in the diet could be considered in order to reduce the events of cellular oxidative stress. Taken together, our result fits with previous study in which it was demonstrated that cashew nuts possess interesting anti-inflammatory, anti-oxidative, and analgesic activities that will be of interest for further investigation.
Acknowledgments
We would like to thank Salma Seetaroo from Ivorienne de Noix de Cajou S.A. of Cote d’Ivoire for providing the cashew kernel samples from West Africa.
Author Contributions
Conceptualization: M.C.; formal analysis: T.G. and R.C.; investigation: A.F.P.; methodology: R.S. and D.I.; project administration: S.C.; supervision: R.D.P.; validation: E.G. and M.S.; writing—original draft, R.F. and R.D.; writing—review and editing: G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adegbaju O.D., Otunola G.A., Afolayan A.J. Anti-inflammatory and cytotoxic evaluation of extracts from the flowering stage of Celosia argentea. BMC Complement Med. Ther. 2020;20:152. doi: 10.1186/s12906-020-02941-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 4.Cordaro M., Siracusa R., Impellizzeri D., R D.A., Peritore A.F., Crupi R., Gugliandolo E., Fusco R., Di Paola R., Schievano C., et al. Safety and efficacy of a new micronized formulation of the ALIAmide palmitoylglucosamine in preclinical models of inflammation and osteoarthritis pain. Arthritis Res. Ther. 2019;21:254. doi: 10.1186/s13075-019-2048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peritore A.F., Siracusa R., Crupi R., Cuzzocrea S. Therapeutic efficacy of palmitoylethanolamide and its new formulations in synergy with different antioxidant molecules present in diets. Nutrients. 2019;11:2175. doi: 10.3390/nu11092175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paterniti I., Impellizzeri D., Cordaro M., Siracusa R., Bisignano C., Gugliandolo E., Carughi A., Esposito E., Mandalari G., Cuzzocrea S. The Anti-inflammatory and antioxidant potential of pistachios (Pistacia vera L.) in vitro and in vivo. Nutrients. 2017;9:915. doi: 10.3390/nu9080915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad T., Shinkafi T.S., Routray I., Mahmood A., Ali S. Aqueous extract of dried flower buds of Syzygium aromaticum inhibits inflammation and oxidative stress. J. Basic Clin. Pharm. 2012;3:323–327. doi: 10.4103/0976-0105.103813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao B.G., Kiran P.M., Raju A.D.V. Investigation of antioxidant and anti-inflammatory activity of leaves of Dalbergia paniculata (Roxb) Asian Pac. J. Trop. Med. 2012;5:455–458. doi: 10.1016/S1995-7645(12)60077-7. [DOI] [PubMed] [Google Scholar]
- 9.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C., Rahu N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubrano V., Balzan S. Enzymatic antioxidant system in vascular inflammation and coronary artery disease. World J. Exp. Med. 2015;5:218–224. doi: 10.5493/wjem.v5.i4.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Torres M., Perez-Campo R., Rojas C., Cadenas S., Barja G. Maximum life span in vertebrates: Relationship with liver antioxidant enzymes, glutathione system, ascorbate, urate, sensitivity to peroxidation, true malondialdehyde, in vivo H2O2, and basal and maximum aerobic capacity. Mech. Ageing Dev. 1993;70:177–199. doi: 10.1016/0047-6374(93)90047-U. [DOI] [PubMed] [Google Scholar]
- 12.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 13.Wu C.X., Liu Y., Zhang J.C. Chronic intermittent hypoxia and hypertension: A review of systemic inflammation and Chinese medicine. Chin. J. Integr. Med. 2013;19:394–400. doi: 10.1007/s11655-013-1459-x. [DOI] [PubMed] [Google Scholar]
- 14.Sun K., Song X., Jia R., Yin Z., Zou Y., Li L., Yin L., He C., Liang X., Yue G., et al. Evaluation of analgesic and anti-inflammatory activities of water extract of Galla chinensis in vivo models. Evid. Based Complement Alternat. Med. 2018;2018:6784032. doi: 10.1155/2018/6784032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menichini F., Tundis R., Bonesi M., Loizzo M.R., Conforti F., Statti G., De Cindio B., Houghton P.J., Menichini F. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv habanero. Food Chem. 2009;114:553–560. doi: 10.1016/j.foodchem.2008.09.086. [DOI] [Google Scholar]
- 16.Mueller M., Hobiger S., Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010;122:987–996. doi: 10.1016/j.foodchem.2010.03.041. [DOI] [Google Scholar]
- 17.Boutennoun H., Boussouf L., Kebieche M., Al-Qaoud K., Madani K. In vivo analgesic, anti-inflammatory and antioxidant potentials of Achillea odorata from north Algeria. S. Afr. J. Bot. 2017;112:307–313. doi: 10.1016/j.sajb.2017.06.004. [DOI] [Google Scholar]
- 18.Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652–682. doi: 10.3390/nu2070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agila A., Barringer S.A. Volatile profile of cashews (Anacardium occidentale L.) from different geographical origins during roasting. J. Food Sci. 2011;76:C768–C774. doi: 10.1111/j.1750-3841.2011.02180.x. [DOI] [PubMed] [Google Scholar]
- 20.de Melo M., Pereira D.E., Sousa M.M., Medeiros D.M.F., Lemos L.T.M., Madruga M.S., Santos N.M., de Oliveira M.E.G., de Menezes C.C., Soares J.K.B. Maternal intake of cashew nuts accelerates reflex maturation and facilitates memory in the offspring. Int. J. Dev. Neurosci. 2017;61:58–67. doi: 10.1016/j.ijdevneu.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Baptista A., Goncalves R.V., Bressan J., Peluzio M. Antioxidant and antimicrobial activities of crude extracts and fractions of cashew (Anacardium occidentale L.), cajui (Anacardium microcarpum), and pequi (Caryocar brasiliense C.): A systematic review. Oxid. Med. Cell Longev. 2018;2018:3753562. doi: 10.1155/2018/3753562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexiadou K., Katsilambros N. Nuts: Anti-atherogenic food? Eur. J. Intern. Med. 2011;22:141–146. doi: 10.1016/j.ejim.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Caravaca A.M., Verardo V., Caboni M.F. Chromatographic techniques for the determination of alkyl-phenols, tocopherols and other minor polar compounds in raw and roasted cold pressed cashew nut oils. J. Chromatogr. A. 2010;1217:7411–7417. doi: 10.1016/j.chroma.2010.09.054. [DOI] [PubMed] [Google Scholar]
- 24.Stewart R.A.H. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018;379:1388. doi: 10.1056/NEJMc1809971. [DOI] [PubMed] [Google Scholar]
- 25.What you eat significantly impacts your heart health. A low-fat diet, plus more fruits, grains, nuts, fish and poultry instead of red meat, yields cardiovascular health benefits. Duke Med. Health News. 2011;17:4–5. [PubMed] [Google Scholar]
- 26.Nash S.D., Nash D.T. Nuts as part of a healthy cardiovascular diet. Curr. Atheroscler. Rep. 2008;10:529–535. doi: 10.1007/s11883-008-0082-3. [DOI] [PubMed] [Google Scholar]
- 27.Mukuddem-Petersen J., Oosthuizen W., Jerling J.C. A systematic review of the effects of nuts on blood lipid profiles in humans. J. Nutr. 2005;135:2082–2089. doi: 10.1093/jn/135.9.2082. [DOI] [PubMed] [Google Scholar]
- 28.Ros E. Nuts and novel biomarkers of cardiovascular disease. Am. J. Clin. Nutr. 2009;89:1649S–1656S. doi: 10.3945/ajcn.2009.26736R. [DOI] [PubMed] [Google Scholar]
- 29.Liu C.M., Peng Q., Zhong J.Z., Liu W., Zhong Y.J., Wang F. Molecular and functional properties of protein fractions and isolate from cashew nut (Anacardium occidentale L.) Molecules. 2018;23:393. doi: 10.3390/molecules23020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siracusa R., Fusco R., Peritore A.F., Cordaro M., D’Amico R., Genovese T., Gugliandolo E., Crupi R., Smeriglio A., Mandalari G., et al. The antioxidant and anti-inflammatory properties of Anacardium occidentale L. cashew nuts in a mouse model of colitis. Nutrients. 2020;12:834. doi: 10.3390/nu12030834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fusco R., Siracusa R., Peritore A.F., Gugliandolo E., Genovese T., D’Amico R., Cordaro M., Crupi R., Mandalari G., Impellizzeri D., et al. The role of cashew (Anacardium occidentale L.) nuts on an experimental model of painful degenerative joint disease. Antioxidants. 2020;9:511. doi: 10.3390/antiox9060511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batista K.S., Alves A.F., Lima M.D.S., da Silva L.A., Lins P.P., de Sousa Gomes J.A., Silva A.S., Toscano L.T., de Albuquerque Meireles B.R.L., de Magalhaes Cordeiro A.M.T., et al. Beneficial effects of consumption of acerola, cashew or guava processing by-products on intestinal health and lipid metabolism in dyslipidaemic female Wistar rats. Br. J. Nutr. 2018;119:30–41. doi: 10.1017/S0007114517003282. [DOI] [PubMed] [Google Scholar]
- 33.Dias C.C.Q., Madruga M.S., Pintado M.M.E., Almeida G.H.O., Alves A.P.V., Dantas F.A., Bezerra J.K.G., de Melo M., Viera V.B., Soares J.K.B. Cashew nuts (Anacardium occidentale L.) decrease visceral fat, yet augment glucose in dyslipidemic rats. PLoS ONE. 2019;14:e0225736. doi: 10.1371/journal.pone.0225736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira A.S., Nascimento J.R., Trovao L.O., Alves P.C.S., Maciel M.C.G., Silva L.D.M., Marques A.A., Santos A., Silva L.A., Nascimento F.R.F., et al. The anti-inflammatory activity of Anacardium occidentale L. increases the lifespan of diabetic mice with lethal sepsis. J. Ethnopharmacol. 2019;236:345–353. doi: 10.1016/j.jep.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Schneider I., Bucar F. Lipoxygenase inhibitors from natural plant sources. Part 2: Medicinal plants with inhibitory activity on arachidonate 12-lipoxygenase, 15-lipoxygenase and leukotriene receptor antagonists. Phytother. Res. 2005;19:263–272. doi: 10.1002/ptr.1604. [DOI] [PubMed] [Google Scholar]
- 36.Schneider I., Bucar F. Lipoxygenase inhibitors from natural plant sources. Part 1: Medicinal plants with inhibitory activity on arachidonate 5-lipoxygenase and 5-lipoxygenase[sol]cyclooxygenase. Phytother. Res. 2005;19:81–102. doi: 10.1002/ptr.1603. [DOI] [PubMed] [Google Scholar]
- 37.Halici Z., Dengiz G.O., Odabasoglu F., Suleyman H., Cadirci E., Halici M. Amiodarone has anti-inflammatory and anti-oxidative properties: An experimental study in rats with carrageenan-induced paw edema. Eur. J. Pharmacol. 2007;566:215–221. doi: 10.1016/j.ejphar.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 38.Gugliandolo E., D’Amico R., Cordaro M., Fusco R., Siracusa R., Crupi R., Impellizzeri D., Cuzzocrea S., Di Paola R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflamm. 2018;15:264. doi: 10.1186/s12974-018-1303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrosino S., Cordaro M., Verde R., Schiano Moriello A., Marcolongo G., Schievano C., Siracusa R., Piscitelli F., Peritore A.F., Crupi R., et al. Oral Ultramicronized palmitoylethanolamide: Plasma and tissue levels and spinal anti-hyperalgesic effect. Front. Pharmacol. 2018;9:249. doi: 10.3389/fphar.2018.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris C.J. Carrageenan-induced paw edema in the rat and mouse. Methods Mol. Biol. 2003;225:115–121. doi: 10.1385/1-59259-374-7:115. [DOI] [PubMed] [Google Scholar]
- 41.Britti D., Crupi R., Impellizzeri D., Gugliandolo E., Fusco R., Schievano C., Morittu V.M., Evangelista M., Di Paola R., Cuzzocrea S. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet. Res. 2017;13:229. doi: 10.1186/s12917-017-1151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salvemini D., Wang Z.Q., Wyatt P.S., Bourdon D.M., Marino M.H., Manning P.T., Currie M.G. Nitric oxide: A key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hargreaves K., Dubner R., Brown F., Flores C., Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 44.Peritore A.F., Siracusa R., Fusco R., Gugliandolo E., D’Amico R., Cordaro M., Crupi R., Genovese T., Impellizzeri D., Cuzzocrea S., et al. Ultramicronized palmitoylethanolamide and paracetamol, a new association to relieve hyperalgesia and pain in a sciatic nerve injury model in rat. Int. J. Mol. Sci. 2020;21:509. doi: 10.3390/ijms21103509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fusco R., Siracusa R., D’Amico R., Peritore A.F., Cordaro M., Gugliandolo E., Crupi R., Impellizzeri D., Cuzzocrea S., Di Paola R. Melatonin plus folic acid treatment ameliorates reserpine-induced fibromyalgia: An evaluation of pain, oxidative stress, and inflammation. Antioxidants. 2019;8:628. doi: 10.3390/antiox8120628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cuzzocrea S., Mazzon E., Esposito E., Muia C., Abdelrahman M., Di Paola R., Crisafulli C., Bramanti P., Thiemermann C. Glycogen synthase kinase-3beta inhibition attenuates the development of ischaemia/reperfusion injury of the gut. Intensive Care Med. 2007;33:880–893. doi: 10.1007/s00134-007-0595-1. [DOI] [PubMed] [Google Scholar]
- 47.Costantino G., Cuzzocrea S., Mazzon E., Caputi A.P. Protective effects of melatonin in zymosan-activated plasma-induced paw inflammation. Eur. J. Pharmacol. 1998;363:57–63. doi: 10.1016/S0014-2999(98)00673-6. [DOI] [PubMed] [Google Scholar]
- 48.Impellizzeri D., Esposito E., Di Paola R., Ahmad A., Campolo M., Peli A., Morittu V.M., Britti D., Cuzzocrea S. Palmitoylethanolamide and luteolin ameliorate development of arthritis caused by injection of collagen type II in mice. Arthritis Res. Ther. 2013;15:R192. doi: 10.1186/ar4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nithya S. Anti-inflammatory effect of Elettaria cardamom oil on carrageenan-induced paw edema using rats based on tumor necrosis factor α, interleukin 6, and interleukin 1 levels in serum. Asian J. Pharm. Clin. Res. 2018;11:207. doi: 10.22159/ajpcr.2018.v11i2.20434. [DOI] [Google Scholar]
- 50.Shukla A., Shukla R., Rai G. Preliminary investigations on Lens culinaris Med. Seeds for anti-inflammatory and antioxidant properties. Asian J. Pharm. Pharmacol. 2017;3:29–31. [Google Scholar]
- 51.Parlar A., Arslan S.O., Dogan M.F., Cam S.A., Yalcin A., Elibol E., Ozer M.K., Uckardes F., Kara H. The exogenous administration of CB2 specific agonist, GW405833, inhibits inflammation by reducing cytokine production and oxidative stress. Exp. Ther. Med. 2018;16:4900–4908. doi: 10.3892/etm.2018.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Sayed el S.M., Mansour A.M., Nady M.E. Protective effects of pterostilbene against acetaminophen-induced hepatotoxicity in rats. J. Biochem. Mol. Toxicol. 2015;29:35–42. doi: 10.1002/jbt.21604. [DOI] [PubMed] [Google Scholar]
- 53.Vajic U.J., Grujic-Milanovic J., Miloradovic Z., Jovovic D., Ivanov M., Karanovic D., Savikin K., Bugarski B., Mihailovic-Stanojevic N. Urtica dioica L. leaf extract modulates blood pressure and oxidative stress in spontaneously hypertensive rats. Phytomedicine. 2018;46:39–45. doi: 10.1016/j.phymed.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 54.Bang J.S., Oh da H., Choi H.M., Sur B.J., Lim S.J., Kim J.Y., Yang H.I., Yoo M.C., Hahm D.H., Kim K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009;11:R49. doi: 10.1186/ar2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coura C.O., Souza R.B., Rodrigues J.A., Vanderlei Ede S., de Araujo I.W., Ribeiro N.A., Frota A.F., Ribeiro K.A., Chaves H.V., Pereira K.M., et al. Mechanisms involved in the anti-inflammatory action of a polysulfated fraction from Gracilaria cornea in rats. PLoS ONE. 2015;10:e0119319. doi: 10.1371/journal.pone.0119319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalyanaraman B., Darley-Usmar V., Davies K.J.A., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Rad. Biol. Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radak Z., Naito H., Kaneko T., Tahara S., Nakamoto H., Takahashi R., Cardozo-Pelaez F., Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. Eur. J. Physiol. 2002;445:273–278. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- 58.Ziaaldini M.M., Koltai E., Csende Z., Goto S., Boldogh I., Taylor A., Radak Z. Exercise training increases anabolic and attenuates catabolic and apoptotic processes in aged skeletal muscle of male rats. Exp. Gerontol. 2015;67:9–14. doi: 10.1016/j.exger.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 59.Gugliandolo E., Fusco R., D’Amico R., Militi A., Oteri G., Wallace J.L., Di Paola R., Cuzzocrea S. Anti-inflammatory effect of ATB-352, a H2S-releasing ketoprofen derivative, on lipopolysaccharide-induced periodontitis in rats. Pharmacol. Res. 2018;132:220–231. doi: 10.1016/j.phrs.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 60.Waltz P., Escobar D., Botero A.M., Zuckerbraun B.S. Nitrate/nitrite as critical mediators to limit oxidative injury and inflammation. Antioxid. Redox Signal. 2015;23:328–339. doi: 10.1089/ars.2015.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Omoboyowa D.A., Nwodo O.F.C., Joshua P.E. Anti-diarrhoeal activity of chloroform-ethanol extracts of cashew (Anacardium occidentale) kernel. J. Nat. Prod. 2013;6:109–117. [Google Scholar]
- 63.Dewanjee S., Dua T.K., Sahu R. Potential anti-inflammatory effect of Leea macrophylla Roxb. leaves: A wild edible plant. Food Chem. Toxicol. 2013;59:514–520. doi: 10.1016/j.fct.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 64.Patil K.R., Mahajan U.B., Unger B.S., Goyal S.N., Belemkar S., Surana S.J., Ojha S., Patil C.R. Animal models of inflammation for screening of anti-inflammatory drugs: Implications for the discovery and development of phytopharmaceuticals. Int. J. Mol. Sci. 2019;20:4367. doi: 10.3390/ijms20184367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calixto J.B., Otuki M.F., Santos A.R. Anti-inflammatory compounds of plant origin. Part I. Action on arachidonic acid pathway, nitric oxide and nuclear factor kappa B (NF-kappaB) Planta Med. 2003;69:973–983. doi: 10.1055/s-2003-45141. [DOI] [PubMed] [Google Scholar]
- 66.Chung H.J., Lee H.S., Shin J.S., Lee S.H., Park B.M., Youn Y.S., Lee S.K. Modulation of acute and chronic inflammatory processes by a traditional medicine preparation GCSB-5 both in vitro and in vivo animal models. J. Ethnopharmacol. 2010;130:450–459. doi: 10.1016/j.jep.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 67.Simmons D.L. What makes a good anti-inflammatory drug target? Drug Discov. Today. 2006;11:210–219. doi: 10.1016/S1359-6446(05)03721-9. [DOI] [PubMed] [Google Scholar]
- 68.Debnath S., Ghosh S., Hazra B. Inhibitory effect of Nymphaea pubescens Willd. flower extract on carrageenan-induced inflammation and CCl(4)-induced hepatotoxicity in rats. Food Chem. Toxicol. 2013;59:485–491. doi: 10.1016/j.fct.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 69.Fangkrathok N., Junlatat J., Sripanidkulchai B. In vivo and in vitro anti-inflammatory activity of Lentinus polychrous extract. J. Ethnopharmacol. 2013;147:631–637. doi: 10.1016/j.jep.2013.03.055. [DOI] [PubMed] [Google Scholar]
- 70.Gualillo O., Eiras S., Lago F., Dieguez C., Casanueva F.F. Elevated serum leptin concentrations induced by experimental acute inflammation. Life Sci. 2000;67:2433–2441. doi: 10.1016/S0024-3205(00)00827-4. [DOI] [PubMed] [Google Scholar]
- 71.Ben Khedir S., Mzid M., Bardaa S., Moalla D., Sahnoun Z., Rebai T. In vivo evaluation of the anti-inflammatory effect of Pistacia lentiscus fruit oil and its effects on oxidative stress. Evid. Based Complement Alternat. Med. 2016;2016:6108203. doi: 10.1155/2016/6108203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sofidiya M.O., Imeh E., Ezeani C., Aigbe F.R., Akindele A.J. Antinociceptive and anti-inflammatory activities of ethanolic extract of Alafia barteri. Rev. Bras. Farmacogn. 2014;24:348–354. doi: 10.1016/j.bjp.2014.07.013. [DOI] [Google Scholar]
- 73.Uddin G., Rauf A., Siddiqui B.S., Muhammad N., Khan A., Shah S.U.A. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus L. Phytomedicine. 2014;21:954–959. doi: 10.1016/j.phymed.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 74.Meagher E.A., Barry O.P., Lawson J.A., Rokach J., FitzGerald G.A. Effects of vitamin E on lipid peroxidation in healthy persons. JAMA. 2001;285:1178–1182. doi: 10.1001/jama.285.9.1178. [DOI] [PubMed] [Google Scholar]
- 75.Vincent H.K., Bourguignon C.M., Vincent K.R., Weltman A.L., Bryant M., Taylor A.G. Antioxidant supplementation lowers exercise-induced oxidative stress in young overweight adults. Obesity. 2006;14:2224–2235. doi: 10.1038/oby.2006.261. [DOI] [PubMed] [Google Scholar]
- 76.Hogan S., Canning C., Sun S., Sun X., Zhou K. Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J. Agric. Food Chem. 2010;58:11250–11256. doi: 10.1021/jf102759e. [DOI] [PubMed] [Google Scholar]
- 77.Sacchet C., Mocelin R., Sachett A., Bevilaqua F., Chitolina R., Kuhn F., Boligon A.A., Athayde M.L., Roman Junior W.A., Rosemberg D.B., et al. Antidepressant-like and antioxidant effects of Plinia trunciflora in mice. Evid. Based Complement. Alternat. Med. 2015;2015:601503. doi: 10.1155/2015/601503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rahman I., Biswas S.K., Kirkham P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 79.Pereira de Jesus Costa A.C., Kelly Dos Santos Silva M., Batista de Oliveira S., Silva L.L., Silva A.C., Barroso R.B., Macedo Costa J.R., Lima Hunaldo V.K., Neto M.S., Pascoal L.M., et al. Effects of cashew nut (Anacardium occidentale L.) seed flour in moderately malnourished children: Randomized clinical trial. J. Nutr. Metab. 2020;2020:6980754. doi: 10.1155/2020/6980754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Melo-Cavalcante A.A., Dantas S.M., Leite Ade S., Matos L.A., e Sousa J.M., Picada J.N., da Silva J. In vivo antigenotoxic and anticlastogenic effects of fresh and processed cashew (Anacardium occidentale) apple juices. J. Med. Food. 2011;14:792–798. doi: 10.1089/jmf.2010.0153. [DOI] [PubMed] [Google Scholar]
- 81.Melo Cavalcante A.A., Rubensam G., Picada J.N., Gomes da Silva E., Fonseca Moreira J.C., Henriques J.A. Mutagenicity, antioxidant potential, and antimutagenic activity against hydrogen peroxide of cashew (Anacardium occidentale) apple juice and cajuina. Environ. Mol. Mutagen. 2003;41:360–369. doi: 10.1002/em.10158. [DOI] [PubMed] [Google Scholar]
- 82.Behravan E., Heidari M.R., Heidari M., Fatemi G., Etemad L., Taghipour G., Abbasifard M. Comparison of gastric ulcerogenicity of percolated extract of Anacardium occidentale (cashew nut) with indomethacin in rats. Pak. J. Pharm. Sci. 2012;25:111–115. [PubMed] [Google Scholar]
- 83.Olajide O.A., Aderogba M.A., Adedapo A.D., Makinde J.M. Effects of Anacardium occidentale stem bark extract on in vivo inflammatory models. J. Ethnopharmacol. 2004;95:139–142. doi: 10.1016/j.jep.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 84.Carvalho N.S., Silva M.M., Silva R.O., Nicolau L.A., Sousa F.B., Damasceno S.R., Silva D.A., Barbosa A.L., Leite J.R., Medeiros J.V. Gastroprotective properties of cashew gum, a complex heteropolysaccharide of Anacardium occidentale, in naproxen-induced gastrointestinal damage in rats. Drug Dev. Res. 2015;76:143–151. doi: 10.1002/ddr.21250. [DOI] [PubMed] [Google Scholar]
- 85.Vilar M.S., de Souza G.L., Vilar Dde A., Leite J.A., Raffin F.N., Barbosa-Filho J.M., Nogueira F.H., Rodrigues-Mascarenhas S., Moura T.F. Assessment of phenolic compounds and anti-inflammatory activity of ethyl acetate phase of Anacardium occidentale L. bark. Molecules. 2016;21:1087. doi: 10.3390/molecules21081087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.da Silveira Vasconcelos M., Gomes-Rochette N.F., de Oliveira M.L., Nunes-Pinheiro D.C., Tome A.R., Maia de Sousa F.Y., Pinheiro F.G., Moura C.F., Miranda M.R., Mota E.F., et al. Anti-inflammatory and wound healing potential of cashew apple juice (Anacardium occidentale L.) in mice. Exp. Biol. Med. 2015;240:1648–1655. doi: 10.1177/1535370215576299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khan H.B., Vinayagam K.S., Moorthy B.T., Palanivelu S., Panchanatham S. Anti-inflammatory and anti-hyperlipidemic effect of Semecarpus anacardium in a high fat diet: STZ-induced type 2 diabetic rat model. Inflammopharmacology. 2013;21:37–46. doi: 10.1007/s10787-011-0109-1. [DOI] [PubMed] [Google Scholar]
- 88.Dzoyem J., McGaw L., Kuete V., Bakowsky U. Medicinal Spices and Vegetables from Africa. Academic Press; Cambridge, MA, USA: 2017. Anti-inflammatory and anti-nociceptive activities of African medicinal spices and vegetables; pp. 239–270. [Google Scholar]
- 89.Mansouri M.T., Hemmati A.A., Naghizadeh B., Mard S.A., Rezaie A., Ghorbanzadeh B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015;47:292–298. doi: 10.4103/0253-7613.157127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gill N., Bijjem K.R., Sharma P.L. Anti-inflammatory and anti-hyperalgesic effect of all-trans retinoic acid in carrageenan-induced paw edema in Wistar rats: Involvement of peroxisome proliferator-activated receptor-beta/delta receptors. Indian J. Pharmacol. 2013;45:278–282. doi: 10.4103/0253-7613.111944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ayala A., Munoz M.F., Arguelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grotto D., Maria L.S., Valentini J., Paniz C., Schmitt G., Garcia S.C., Pomblum V.J., Rocha J.B.T., Farina M. Importance of the lipid peroxidation biomarkers and methodological aspects FOR malondialdehyde quantification. Química Nova. 2009;32:169–174. doi: 10.1590/S0100-40422009000100032. [DOI] [Google Scholar]
- 93.Tsai D.S., Huang M.H., Tsai J.C., Chang Y.S., Chiu Y.J., Lin Y.C., Wu L.Y., Peng W.H. Analgesic and anti-inflammatory activities of Rosa taiwanensis nakai in mice. J. Med. Food. 2015;18:592–600. doi: 10.1089/jmf.2014.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wagner H., Breu W., Willer F., Wierer M., Remiger P., Schwenker G. In vitro inhibition of arachidonate metabolism by some alkamides and prenylated phenols. Planta Med. 1989;55:566–567. doi: 10.1055/s-2006-962097. [DOI] [PubMed] [Google Scholar]
- 95.Goldstein B.I., Kemp D.E., Soczynska J.K., McIntyre R.S. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: A systematic review of the literature. J. Clin. Psychiatry. 2009;70:1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- 96.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 97.Di Rosa M., Giroud J.P., Willoughby D.A. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J. Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- 98.Valerio D.A., Cunha T.M., Arakawa N.S., Lemos H.P., Da Costa F.B., Parada C.A., Ferreira S.H., Cunha F.Q., Verri W.A., Jr. Anti-inflammatory and analgesic effects of the sesquiterpene lactone budlein A in mice: Inhibition of cytokine production-dependent mechanism. Eur. J. Pharmacol. 2007;562:155–163. doi: 10.1016/j.ejphar.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 99.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ighodaro O., Akinloye O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018;54:287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- 101.Du Z.X., Zhang H.Y., Meng X., Guan Y., Wang H.Q. Role of oxidative stress and intracellular glutathione in the sensitivity to apoptosis induced by proteasome inhibitor in thyroid cancer cells. BMC Cancer. 2009;9:56. doi: 10.1186/1471-2407-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]