Abstract
Interleukin 15 (IL-15) has shown remarkable biological properties of promoting NK- and T-cell activation and proliferation, as well as enhancing antitumor immunity of CD8+ T cells in preclinical models. Here, we report the development of an E. coli cell line to express recombinant human Interleukin-15 (rhIL-15) for clinical manufacturing. Human IL-15 cDNA sequence was inserted into a pET28b plasmid and expressed in several E. coli BL21 strains. Through product quality comparisons among several E. coli strains, including E. coli BL21(DE3), BL21(DE3)pLysS, BLR(DE3)pLysS, and BL21-AI, E. coli BL21-AI was selected for clinical manufacturing. Expression optimization was carried out at shake flask and 20-L fermenter scales, and the product was expressed as inclusion bodies that were solubilized, refolded, and purified to yield active rhIL-15. Stop codons of the expression construct were further investigated after 15–20% of the purified rhIL-15 showed an extraneous peak corresponding to an extra tryptophan residue based on peptide mapping and mass spectrometry analysis. It was determined that the presence of an extra tryptophan was due to a stop codon wobble effect, which could be eliminated by replacing TGA (opal) stop codon with TAA (ochre). As a novel strategy, a simple method of demonstrating lack of tRNA suppressors in the production host cells was developed to validate the cells in this study. The E. coli BL21-AI cells containing the rhIL-15 coding sequence with a triplet stop codon TAATAATGA were banked for further clinical manufacturing.
Keywords: interleukin 15, protein expression, stop codon, clinical manufacturing, E. coli
Introduction
Interleukin 15 (IL-15) was originally discovered as a T-cell stimulatory agent present in the culture supernatants of a simian kidney epithelial cell line and an HTLV-1-associated adult T-cell leukemia line.1,2 IL-15 is a member of the four α-helix bundle family of cytokines that shares a number of biological activities with IL-2. IL-15 is dedicated to the prolonged maintenance of memory T-cell responses to invading pathogens.3 IL-15 has been shown to enhance the antitumor immunity of CD8+ T cells in preclinical models.4,5 Zhang et al.6 demonstrated a connection between the IL-15 receptor and CD40, which provides the scientific basis for human clinical trials for the treatment of patients with metastatic malignant melanoma or renal cell cancer via a synergistic antitumor activity by administering agonistic antibodies to CD40 in combination with IL-15. Animal toxicology studies with monkeys showed7 that 10–50 μg/kg of IL-15 administered intravenously daily for 12 days to rhesus macaques has both short- and long-lasting effects on T-cell homeostasis. Peripheral blood lymphopenia preceded a dramatic expansion of NK cells and memory CD8 T cells in circulation, particularly a fourfold expansion of central memory CD8 T cells and a sixfold expansion of effector memory CD8 T cells. IL-15 generates a dramatic expansion of short-lived memory CD8 T cells and NK cells in immune-competent macaques, and has long-term effects on the balance of CD4+ and CD8+ T cells. The biological function of the cytokine will encourage the development of novel therapies for malignancy and autoimmune diseases, as well as the design of vaccines against infectious diseases.3
IL-15 has been investigated as an adjuvant for antibody-dependent response to vaccines.8 A significant IL-15 dose-dependent increase in antigen-specific antibody was observed after coadministration of IL-15 with a recombinant staphylococcal enterotoxin B vaccine (STEBVax) and an aluminium-based adjuvant (alhydrogel).8 Furthermore, the coadministration also protected mice from lethal toxic shock above the levels obtained without IL-15 treatment. IL-15 may represent a potential candidate for cytokine treatment in combination with highly active antiretroviral therapy during HIV infection.9 A plasmid encoding IL-15 as a DNA vaccine adjuvant was delivered for the induction of improved Ag-specific CD8+ T cellular immune responses.10 Coinjection of IL-15-and IL-15 receptor alpha-expressing plasmids into mice led to significantly increased levels of the cytokine in serum and increased biological activity of IL-15.11
Based on the extraordinary biological functions discovered in academic laboratories, translational development of recombinant human Interleukin-15 (rhIL-15) has been ranked number one as a potential immunotherapeutic drug by representatives from academia, industry, the National Cancer Institute, and the Food and Drug Administration.12 Accordingly recombinant IL-15 has been expressed in several systems, including mammalian cells,13 yeast,14–16 and E. coli.17 Fusions of human IL-15 with the sushi domain of IL-15R α have been expressed in a baculovirus expression system.18 Efficient transient expression vectors for IL-15 were developed combining RNA/codon optimization and modification of the IL-15 native long signal peptide.13 These changes resulted in elevated cytoplasmic levels of the optimized mRNA and more than 100-fold improved production of secreted human IL-15 protein. Other attempts in different expression systems were mainly for laboratory research purposes, which were not suited to large-scale clinical manufacturing. Ward et al.17 reported that human IL-15 with an N-terminal (His)6-tag was expressed in E. coli as an insoluble protein with a fermentation titer of ~ 60 mg/L. The expressed IL-15 was purified from other cellular proteins by dissolution in 6 M guanidine HCl, followed by Ni-NTA chromatography in a buffer containing 8 M urea. The method was scaled up to produce milligram quantities of folded material in its native conformation. Mature IL-15 was generated by cleavage with recombinant enterokinase, and the final purification yield was 1.7%.
Recombinant IL-15 may be overexpressed as a stable complex in the presence of its high affinity receptor, IL-15R alpha16; expressed as a fusion protein with IL-15R α-sushi domain18; a fusion protein with Fc domain of IgG19; or coexpressed with IL-15 R alpha in HEK293.10,11 Hanicket found that free IL-15 is highly prone to aggregation, and they were able to achieve high-level expression of IL-15 in yeast by addition of its high affinity alpha receptor during expression.16 IL-15 receptor α-sushi fused with N-terminal of IL-15 was expressed in baculovirus system and showed higher biological activity in TF-1β cell apoptosis induced by cytokine deprivation.18 IL-15 expressed in HEK 293 was not stable and degraded immediately. Coexpression of IL-15 and IL-15R α in the same cell resulted in increased stability and secretion of both molecules as a complex.11
It is highly desirable to develop a scalable and reproducible process from expression to final high-quality product for clinical application of IL-15. However, prior to this report, human IL-15 has not been manufactured at clinical grade in any recombinant protein production system, due partly to low expression level, instability, and insufficient product quality for use in clinical trials. In this report, the authors describe the development of an E. coli system with a good expression level of ~ 100 mg/L that is suitable for clinical manufacture. Following this report, the authors will present development of a complete production process with stringent quality control in the manufacturing of clinical grade rhIL-15. The rhIL-15 manufactured using the procedure described in this report and other reports20,21 from our group has been used in a phase I clinical trial to evaluate the safety, dosing, and antitumor efficacy of rhIL-15 in patients at the National Institutes of Health.22
Materials and Methods
E. coli strain and cell banks
Several E. coli strains were used in this study either for expressing protein or for amplifying plasmid DNA. The strains are listed in Table 1. E. coli cells were normally kept frozen at −70°C. Strains carrying the expression plasmid were kept at ≤−70°C as cell banks. The master cell bank (MCB) was manufactured under current Good Manufacturing Practice (cGMP) conditions for producing clinical grade material. Production of the MCB was initiated from one frozen vial of cells from an IL-15 accession cell bank (ACB). Two hundred-fifty microliter of inoculum from the ACB were inoculated to a 500 mL flask containing 150 mL medium (sodium chloride 10 g/L, soytone 10 g/L, yeast extract 5 g/L, and kanamycin 0.05 g/L) and the culture was grown for 14 h at 37 ± 1°C. The cells were mixed with a 60% (w/v) glycerol solution to yield a final glycerol concentration of 20% (w/v) and aliquoted to 1 mL/vial. The vials were placed into controlled storage at ⩽−70C.
Table 1.
E. coli Strains used in the Clinical Production Cell Line Development Study
| Strain | Description | Purpose |
|---|---|---|
| E. coli XL-10 Gold | Used for transformation of the plasmid pET28b-rhIL-15ochre | Plasmid amplification |
| E. coli BLR (DE3) pLysS | Novagen Cat# 69956-4 | Product expression |
| E. coli BL21(DE3) pLysS | Invitrogen Cat# 440034 | Product expression |
| E. coli BL21(DE3) | Invitrogen Cat# 440048 | Product expression |
| E. coli BL21-AI | Invitrogen Cct#6070-03 | Product expression |
| E. coli DE04 | E. coli BL21(DE3) transformed with pRARE plasmid | GFP expression |
E. coli XL-10 Gold was used for plasmid amplification, while other strains were used for expression of recombinant protein. All strains were purchased from commercial sources, except E. coli DE 04, which was further transformed with plasmid pRARE.
Reference IL-15
A recombinant IL-15 reference material was purchased from Peprotech Cat# 200–15, Lot# 1205B24). The reference material was used for analytical comparisons described in this report.
Plasmid vectors
Plasmid pET 28b (Novagen Cat# 69865; Lot N44268–10) was used for expressing the IL-15 coding sequence. Plasmid pEL 115 was used for studying stop codon UGA (opal) suppressor tRNA. pEL 115, a derivative of the Gateway Entry clone, was generated by PCR amplification of the eGFP gene from pIRES2-eGFP (Clontech), using a 5’ primer containing the epsilon-SD ribosome binding site (TCATTTAACTTTAAGAAGGAGATATATACC), preceded by a Gateway attB1 sequence and a 3’ primer containing the Gateway attB2 sequence. After 20 cycles of PCR using Platinum Taq Hi Fidelity Supermix (Invitrogen) with an extension time of 30 s, the PCR product was cleaned using the Qiaquick PCR Purification Kit (Qiagen) and recombined with pDonr223 (Invitrogen) to generate the Gateway Entry clone. pDest-521 is a gateway att1/att2 destination vector with a T7 promoter and pBR origin constructed by the Protein Expression Laboratory of SAIC-Frederick, NCI-Frederick.23
Construction of plasmids for IL-15 expression
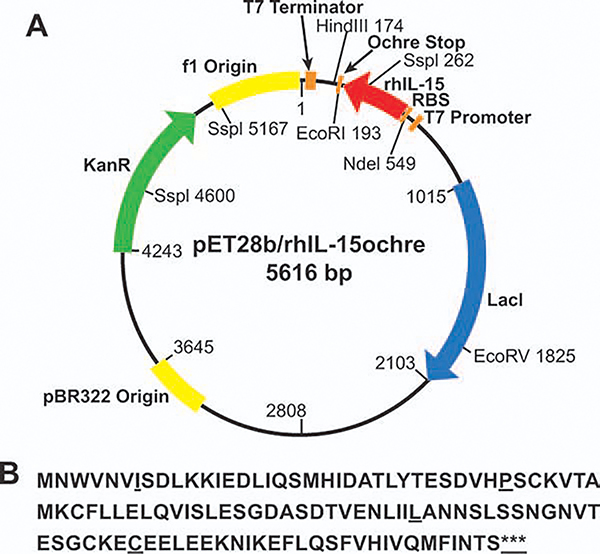
The plasmid for the expression of rhIL-15 in E. coli is shown in Figure 1A. The product with the 115 amino acid1 coding sequence of the pET28b/IL-15 cistron is listed in Figure 1B. The plasmid vector pET28b carrying human IL-15 cDNA (GenBank# NM_172174 and NM_000585) was used in two constructs with different stop codons. Translation of expression was stopped by either of the two triplet stop codons, TGATAATGA or TAATAATGA.
Figure 1. Genetic information of rhIL-15 expression in E. coli.
A: Plasmid pET28b/rhIL-15ochre map. The construct was used in expression of rhIL-15 in E. coli BL21-AI. The plasmid was transformed to E. coli BL21-AI for expression of rhIL-15. B: It is the human interleukin-15 amino acid sequence as expressed in E. coli BL21-AI. The triple stop codon sequences TAATAATGA and TGATAATGA are represented by “***”.
Product expression and fermentation
The plasmid carrying the IL-15 coding sequence was transformed into E. coli BL21-AI-competent cells.24 Transformants were picked and grown in soytone LB medium [soytone (Difco 212488) 10 g/L, yeast extract (BD 212,730) 5 g/L, sodium chloride (J.T. Baker 3629–07) 10 g/L, supplemented with kanamycin (Sigma K4000, 50 mg/L)]. The overnight culture was used as a seed culture, and 0.5 mL was inoculated to a 250 mL flask containing 20 mL soytone LB medium to grow the cells, which was then induced at OD600 = 0.6 by the addition of 1.0 mM IPTG and 0.2% arabinose for 3 h. Cells were harvested and lysed for SDS PAGE and Western blotting.
A 20 L fermenter (Bioflo IV, New Brunswick Scientific, NJ) was prepared for the fermentation by batching the fermenter with 10 L of production medium (soytone 12 g/L, yeast extract 24 g/L, glycerol 15.1 g/L, potassium phosphate dibasic 12.5 g/L, potassium phosphate monobasic 3.8 g/L, and P2000 antifoam 0.1 mL/L). The production medium sterilized in situ was then supplemented with a sterile solution of magnesium sulfate heptahydrate and kanamycin to a final concentration of 0.4 and 0.05 g/L, respectively. The culture parameters were set as follows: temperature 37°C, pH 7.0, aeration 0.5–1.0 VVM, and dissolved oxygen higher than 30% of saturation. Seed culture was grown in a flask overnight and inoculated into the fermenter at a final concentration of 2% (v/v). Once the cell density reached OD600 4–10, IPTG and arabinose were added to induce expression. Three hours after the induction, cells were harvested by centrifugation, and cell paste was stored at −70°C until it was further processed.
rhIL-15 recovery and purification
Cell paste was resuspended into a buffer containing 50 mM Tris, 20 mM EDTA, and 150 mM NaCl, pH 7.4, and lyzed using a homogenizer (GEA Niro Soavi-Panda, NS1001L2K). The inclusion body in the cell lysis was washed using buffers with or without detergent Triton X-100 and recovered by centrifugation (~ 16,000g for 30–45 min). The inclusion body was purified by S-200 column chromatography and refolded by dilution using a refolding buffer containing the redox pair, oxidized, and reduced glutathione, at 4°C for 2–5 h.20 Refolded rhIL-15 was purified by hydrophobic interaction, ion exchange, and size-exclusion chromatographic steps.20
Construction of plasmids to verify lack of tRNA suppressor in the E. coli host strains
Three Gateway Entry clones were generated by mutagenesis of a plasmid pEL115 that contained eGFP. This clone contained the eGFP gene flanked by a TAA (Ochre) stop codon at the 3’ end and a strong Shine-Dalgarno translation initiation site at the 5’ end. Mutants were made by converting the single tryptophan residue (W58) of eGFP to each of the three stop codons as follows:
4872-E1: Amber mutant (TGG–>TAG)
4872-E2: Opal mutant (TGG–>TGA)
4873-E3: Ochre mutant (TGG–>TAA)
Mutants were made using the QuickChange site-directed mutagenesis and were sequence-verified to ensure that the correct mutations were made and no additional mutations were introduced. The three mutant entry clones and the wild-type pEL115 were transferred to a native T7 E. coli expression vector called pDest-521 to make the following expression clones:
4872-X1–521: eGFP–Amber
4872-X2–521: eGFP–Opal
4872-X3–521: eGFP–Ochre
pEL115–521: eGFP–wild type
These expression clones can be propagated in any E. coli strain using 100 μg/mL ampicillin to maintain the plasmid. For expression purposes, they were introduced into E. coli BL21-AI and E. coli DE04 strains that contain the T7 RNA polymerase gene.
GFP expression to verify lack of tRNA suppressor in the E. coli host strains
Vectors described above were transformed into two strains: E. coli DE04 and BL21-AI. The BL21-AI strain is BL21 with the T7 polymerase under the control of the arabinose inducible pAraBAD promoter. To test growth, cells were grown at 37°C until an OD600 of 0.5, and were then induced at 30°C for 18–20 h. Induction was carried out with 0.5 mM Isopropyl-β-D-thiogalactoside (IPTG) in the case of the DE04 cells, or 0.5 mM IPTG and 0.2% L-arabinose for the BL21-AI cells. After induction, three OD units of cells were centrifuged (~ 10,000g) and resuspended in 200 μL 1 x PBS. One hundred microliter of each cell suspension was transferred to a Greiner Microclear 96-well plate, and the samples were analyzed for fluorescence using the FLUOstar Optima plate reader. In addition, SDS-PAGE gels were run on whole-cell samples to verify rhIL-15 expression.
Cell banking for clinical manufacturing
An E. coli BL21-AI pET28b/rhIL-15 ochre MCB, Lot L0704014, was manufactured under cGMP conditions. The production of the MCB was initiated from one frozen vial of cells from the ACB. Each of three 500 mL flasks was batched with 150 mL sterile seed culture medium. Each of the three flasks was aseptically inoculated using 250 ± 10 μL of inoculum from the thawed ACB culture vial. The three inoculated flasks were incubated for ~ 14 h at 140–160 RPM and 36–38°C. The contents of Flask 2 were mixed with a 60% (w/v) glycerol solution to yield a final glycerol concentration of 20% (w/v). One hundred-sixty cryovials were filled with a 1 mL volume of the culture/glycerol mixture; the vials were then placed into controlled storage at ≤−70°C.
SDS-PAGE and Western blotting
Assessment of approximate molecular weight (MW) and identity of rhIL-15 was performed by SDS-PAGE and Western blotting. Samples were analyzed under reducing and non-reducing conditions, using Novex® Xcell Mini Cell Electrophoresis Apparatus and NuPAGE™ 4–12% bis-tris precast gels. Reduced and nonreduced samples were heated to 70°C for 10 min in 2 x Sample Buffer (NuPAGE™ LDS). Electrophoresis was performed at a constant 200 volts for 35 min. A MW marker (Invitrogen LC5925 Mark 12 or Invitrogen IM5677G) was run concurrently with the rhIL-15 test samples. Following electrophoresis, proteins were visualized by Coomassie Blue or SYPRO® Ruby staining. Densitometry of the visualized protein was performed using the Hewlett Packard ScanJet 5P and Gel Pro-4 Analyzer Software (Media Cybernetics). Coomassie or SYPRO® Ruby stained gels were scanned and quantitative analysis of the area percentage of the stained proteins, as well as the MW of the protein bands, were performed. Estimates of product purity based on gel densitometry were also verified by HPLC-SEC.
For Western blots, mouse antihuman IL-15 monoclonal antibody was obtained from R&D Systems (Cat# MAB247, Lot# SA07). The procedure from the Western Breeze™ Kit (Invitrogen Cat# WB7104) was followed, including the following steps: 30 min blocking with Western Breeze™ blocking buffer at room temperature; two water washes; incubation with the antihuman IL-15 antibody overnight at 4°C with rocking; incubation with the antihuman IL-15 antibody for 1 h with shaking. The protein was detected with chemiluminescence and exposed to Kodak X-ray film.
rhIL-15 characterization by peptide mapping and mass spectrometry
For peptide mapping, samples were reduced with 10 mM DTT and alkylated with 50 mM iodoacetic acid. They were then buffer-exchanged into 0.1 M Tris pH 8.0 and digested overnight with modified trypsin (Promega, Madison, WI). On-line LC-MS and MS/MS analyses used a Thermo Finnigan (Waltham, MA) LCQDECA ion-trap MS interfaced with a Thermo Finnigan Surveyor™ HPLC. A Grace Vydac (Deerfield, IL) C18 Mass Spec™ HPLC column was used with a gradient of 0.1% formic acid in acetonitrile for separation and elution of samples into the MS. Analysis of intact rhIL-15 employed the same instrument configuration.
CTLL-2 cell proliferation assay
The potency of rhIL-15 was determined using a colorimetric cell proliferation assay, which quantifies the IL-15-dependent CTLL-2 cell proliferation activity. Detailed method development has been described by Soman et al.25 Briefly, CTLL-2 cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 200 U/mL IL-2. Reference material, positive control, and test samples were diluted to an initial concentration of 2 ng/mL (20 IU/mL for NIBSC standard) in the assay medium, followed by serial twofold dilutions (additional points 0.4 and 0.2 ng/mL were also included). The diluted samples were added in triplicate to individual wells on the 96-well tissue culture plate containing 100 μL of the assay medium. After 4 h incubation, the prepared cell suspension was transferred to a sterile reservoir and seeded immediately in the wells of a 96-well plate containing 100 μL of rhIL-15 at different concentrations to yield a final cell density of 5 × 104 cells/well. After a 48 h incubation period, CellTiter96® aqueous one solution was added (20 μL/well) and incubated for another 4 h then 25 μL/well of 10% SDS was added to stop the reaction. The plate was then read at 490 nm and the data were analyzed using a four-parameter curve fit (SoftMax® Pro from Molecular Devices).
Results and Discussion
IL-15 expression and host selection
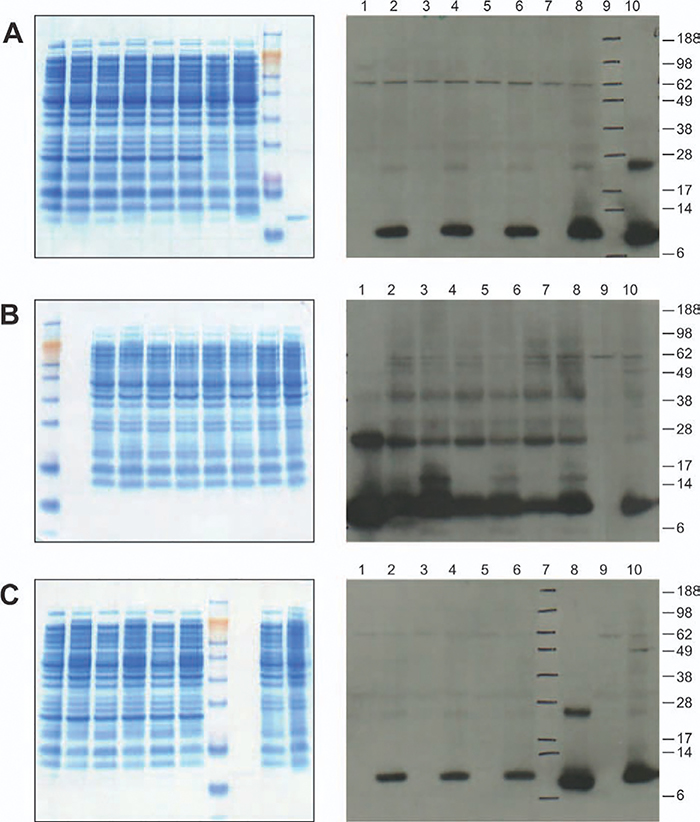
The coding sequences for human IL-15 were cloned into the parent vector pET28b containing a T7 promoter, so that the expression of rhIL-15 was driven by the T7 promoter under the induction regulation system in an E. coli host cell containing T7 RNA polymerase. The expression plasmid pET28/rhIL-15 (Figure 1A) was transformed into the E. coli hosts BL21(DE3), BL21(DE3)pLysS, BLR(DE3)pLysS, and BL21-AI (Table 1) to evaluate the expression of human IL-15 (Figure 1B). Three clones were picked from each transformation to assess expression by SDS-PAGE and Western blot. Colonies were expanded in culture to make frozen vials for further comparison studies. All studies conducted at this stage were in shake flasks with a volume of 25–100 mL. Although results showed that E. coli BL21(DE3) was the highest producer, followed by BL21-AI (Figure 2), it was decided not to use this strain for further high density scaleup studies in the fermenter as the BL21 (DE3) strain was not tightly regulated and IL-15 was expressed in small amounts even before IPTG induction; this would hamper cell growth during the preinduction phase of growth in the fermenter.
Figure 2. Comparison of rhIL-15 expressed in different E. coli hosts: Left panels of A, B, and C are SDS-PAGEs and right panels of A, B, and C are Western blots.
A: rhIL-15 expression in E. coli BLR(DE3) pLysS and E. coli BL21-AI. Lanes 1–6 correspond to BLR(DE3) pLysS Colony A before induction; Colony A 3 h post-induction; Colony B before induction; Colony B 3 h post-induction; Colony C before induction; Colony C 3 h post-induction; Lanes 7–8 correspond to BL21-AI Colony C before induction; Colony C 3 h post-induction; Lane 9: NuPAGE SeeBlue Plus2 MW marker; and Lane 10: IL-15 Peprotech Reference Standard. B: rhIL-15 expression in E. coli BL21(DE3) and E. coli BL21-AI. Lane1 NuPAGE® SeeBlue® Plus2 MW marker; Lane 2: IL-15 Peprotech Reference Standard; Lanes 3–8 correspond to BL21(DE3) Colony A before induction; Colony A 3 h post-induction; Colony B before induction; Colony B 3 h post-induction; Colony C before induction; Colony C 3 h post-induction; Lanes 9–10: BL21-AI Colony A before induction; and 3 h post-induction. C: rhIL-15 expression in E. coli BL21(DE3) pLysS and E. coli BL21-AI. Lanes 1–6 correspond to: BL21(DE3) pLysS Colony A before induction; Colony A 3 h post-induction; Colony B before induction; Colony B 3 h post-induction; Colony C before induction; Colony C 3 h post-induction, Lane 7: NuPAGE® SeeBlue® Plus2 MW marker; Lane 8: IL-15 Peprotech Reference Standard; Lanes 9–10: BL21-AI Colony B before induction; and 3 h post-induction.
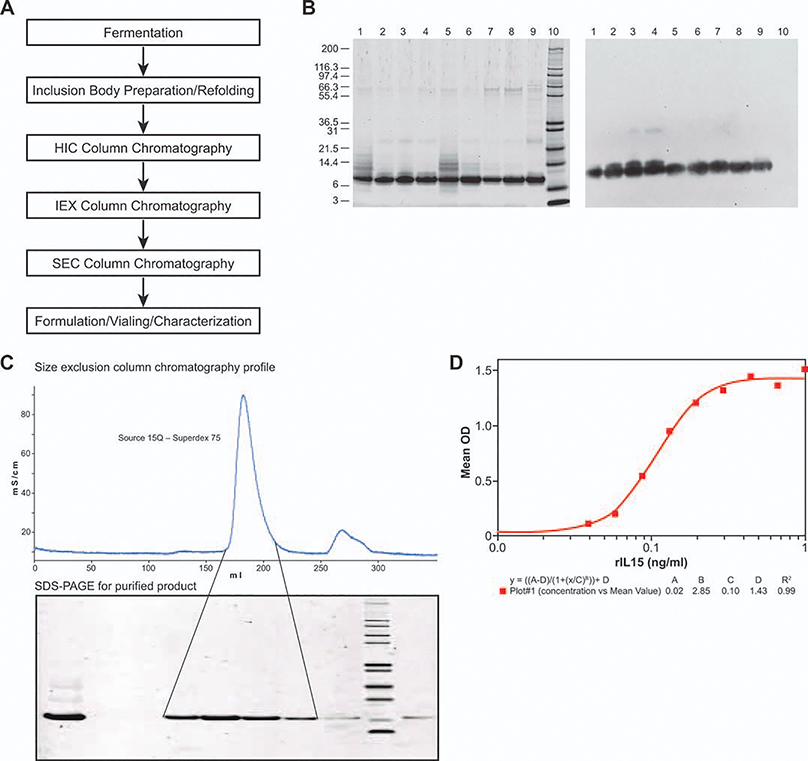
E. coli BL21(DE3) and E. coli BL21-AI were further compared through the entire production process (Figure 3A) at 10 L fermentation and 1 g protein purification scales. After fermentation, cells were harvested and lysed to recover inclusion bodies containing rhIL-15. Inclusion bodies were dissolved and purified through a size exclusion column to collect fractions containing IL-15, which was monitored by SDS-PAGE (data not shown) prior to pooling. Purified inclusion bodies were diluted for renaturation, and refolded IL-15 was purified to yield the final product that was biologically active (Average 0.09 ng/mL ED50) in the CTLL-2 cell proliferation assay (Figure 3D). Samples from each step were analyzed by SDS-PAGE stained by SYPRO® Ruby and immunoblotted by probed anti-IL15 antibody. SDS-PAGE and Western blot results are shown in Figure 3B. Process yield data are listed in Table 2. Overall, during fermentation, the BL21(DE3) strain was observed to be 50% higher in volumetric expression compared with the BL21-AI strain (~ 100 mg/L broth). After purification, the recovered yield IL-15 final product from the BL21(DE3) strain was 1.74-fold higher than that from the BL21-AI strain (Table 2). However, the final product obtained from BL21-AI was purer, as shown on both SDS-PAGE and Western blot (Figure 3B). Lanes 4 and 8 on both panels of Figure 3B are final samples either from BL21(DE3) or BL21-AI™, and lane 8 shows a purer product profile than lane 4 on both panels. The large MW band observed in lane 8 of the SDS-PAGE is easily removed through a polishing chromatographic step. E. coli BL21-AI was selected as the production strain for further process development, because of its final product quality. The final product quality was given the highest priority over other considerations, especially since the rhIL-15 produced by this process was to be used in clinical trials as a therapeutic cytokine. In addition, although the E. coli BL21(DE3) host appeared to produce relatively higher levels of IL-15 in the whole cell lysate preparations, the other hosts seemed to be better regulated in expression (Figure 2), which has potential implications for subsequent higher cell density fermentations in the future.
Figure 3. rhIL-15 production process and product comparison expressing from two E. coli BL21 (DE3) and E. coli BL21-AI, respectively.
A: Schematic diagram showing rhIL-15 expression and purification process. Conditions were briefly described in the Materials and Methods section. B: SDS-PAGE and Western blot analysis of IL-15 purification stage pools expressed from E.coli BL21 (DE3) and BL21-AI, respectively. Reducing SDS-PAGE gel stained with SYPRO® Ruby (left panel); Western blot probed with anti-IL-15 antibody (right panel); Lanes 1–4 from DE3 strain and Lanes 5–8 from AI strain; Lanes 1 and 5, Load of Source 15Q; Lanes 2 and 6, Main peak of Source 15Q; Lanes 3 and 7, Main Peak of Q-Sepharose XL; Lanes 4 and 8, Main peak of Superdex 75; Lane 9, IL-15 reference standard from Peprotech (3.25 μg/lane); Lane 10, Mark 12 MW standard from Invitrogen (10 μg/lane). C: Final step of purification of rhIL-15 Size-exclusion column chromatography profile (upper panel) and SDS-PAGE analysis of the SEC fractions stained with SYPRO® Ruby. Lane 1, Load; Lanes 2 and 3, Prepeak; Lanes 4, 5, 6, and 7, Main peak; lane 8, Tail peak; lane 9, Mark 12 MW standard; lane 10, Reference standard from Preprotech Inc. D: Recombinant human IL-15 mediated proliferation of CTLL2 cells.
Table 2.
Host Cell E. coli BL21-AI and BL21 (DE3) Comparison of Expression and Productivity Through Production Process
| Unit Operation | Host: BL21-AI Recombinant hIL-15 | Host: BL21 (DE3) Recombinant hIL-15 |
|---|---|---|
| Fermentation (10L) | ∼ 1 g | ∼ 1.5 g |
| Inclusion Body Purification | ∼ 480 mg | ∼ 750 mg |
| Source 15 Q Chromatography | ∼ 270 mg | ∼ 505 mg |
| Q XL Chromatography | NA* | ∼ 363 mg |
| S-75 SEC Chromatography | ∼ 196 mg | ∼ 342 mg |
NA: Data is not available. Two strains were compared: E. coli BL21-AI™ and E. coli BL21(DE3). The comparison was carried out through the entire process, from fermentation and inclusion body preparation to downstream chromatographic steps.
The plasmid constructs used in the study were amplified by the E. coli XL-10 Top strain, which is a nonexpression host. The established plasmids were transformed into a host bearing the T7 RNA polymerase for expression of IL-15. The E. coli BLR strain is a recA-derivative of BL21(DE3) that may improve plasmid stability.26 pLysS is a plasmid that contains the T7 lysozyme gene (LysS), a natural inhibitor of T7 RNA polymerase, to reduce T7-driven transcription in uninduced cells. When IPTG is added, the amount of T7 RNA polymerase increases and overcomes the inhibition by LysS (www.invitrogen.com). In our study, expression was tightly controlled in both cases with pLysS strains; however, expression levels were somewhat inhibited, as compared to the BL21-AI strain (Figure 2A,C). The E. coli BL21-AI strain was constructed by inserting a chromosomal copy of the T7 RNA polymerase gene (T7 RNAP) under control of the arabinose-inducible araBAD promoter. The araBAD promoter tightly regulates expression of the T7 RNAP and hence expression of the gene of interest in T7-mediated expression systems without DE3 lysogen (www.invitrogen.com). In addition, BL21-AI reduces the risk of low-level λ phage contamination that was occasionally observed in our E. coli strain-testing program with the BL-21(DE3) strain (Data not shown).
Expression optimization
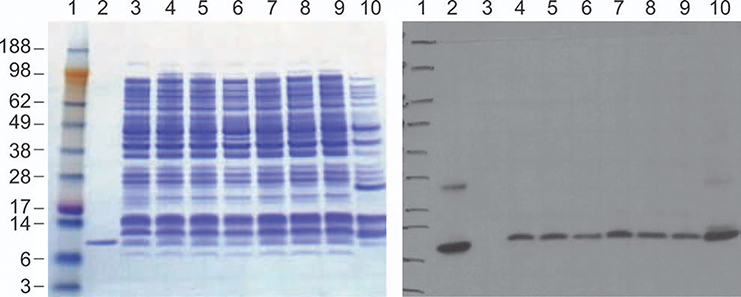
Several transformed colonies were evaluated for expression, as shown in Figure 2. Induction conditions were optimized by varying inducer concentration ranging from 0.2 to 2.0 mM of IPTG and 0.2 to 0.5% of arabinose in shake flasks (Figure 4). No significant difference was observed with using either a low IPTG concentration of 0.2 vs. 2.0 mM (Figure 4). Likewise, no significant increase or decrease was observed as arabinose concentration was increased from 0.2 to 0.5% for induction. Considering bioreactor cell density will be high, a final concentration of 1 mM IPTG with 0.5% arabinose was selected for production to provide a margin of safety.
Figure 4. Optimization of rhIL-15 expression in E. coli BL21-AI/pET28b/rhIL-15 with different IPTG and arabinose concentrations. Lane 1: MW Marker; Lane 2: IL-15 reference standard from Peprotech; Lane 3: before induction; Lanes 4–6 3 h after induction with 0.5, 1.0, or 2.0 mM IPTG plus 0.2% of arabinose; Lanes 7–9: induction with 0.5, 1.0, or 2.0 mM IPTG plus 0.5% of arabinose; Lane 10: Control vector (with ampicillin-resistance marker) expression 1 mM IPTG.
All lanes were loaded 19.5 μL, except MW marker for 12 μL.
Expression, purification, and characterization of rhIL-15
To characterize extensively, the final product expressed by this system, a large-scale fermentation with gram-scale purification was performed to generate high-quality reference material (Figure 3A). Cells were cultured following the methodology described in the Materials and Methods section. One millimolar IPTG and 0.5% arabinose were added to the fermenter after the OD600 reached between 5 and 7 to induce expression. The culture was grown for a further 3 h prior to harvest. Expression titer was 100 mg/L, as estimated by SDS-PAGE/Western blot image scanning and densitometry results as compared with the standard from Peprotech (see Materials and Methods). Recombinant human IL-15 expressed as inclusion bodies was recovered and purified through S-200 chromatography prior to refolding. Refolding was carried out by dilution using a refolding buffer containing the redox pair, oxidized and reduced glutathione, at 4°C for 2–5 h. Refolded rhIL-15 went through purification steps as shown in Figure 3A; detailed development of the purification process is described by Vyas et al.20 The final purification step was Sepharose-75 size-exclusion chromatography, which resulted in a single peak (Figure 3C) and a single band on the SDS-PAGE (Figure 3C).
Purified rhIL-15 was characterized by a panel of assays, including N-terminal amino acid sequencing, peptide mapping, mass spectrometry, C-terminal amino acid sequencing, and CTLL cell proliferation bioactivity. N-terminal sequencing showed the peptide starts with a methionine, as normally observed in recombinant proteins expressed in E. coli, and the first 15 amino acids were identical to the published sequence (Figure 1B).1 While almost all characterization tests confirmed the quality of the product, peptide-mapping data showed that an extra peak was associated with the construct that has an initial TGA (opal) stop codon. This observation was further verified by mass spectroscopy of the intact molecule. A thorough investigation was performed following the observation of the abnormal peak and results are shown in the following section.
Observation of stop codon wobble
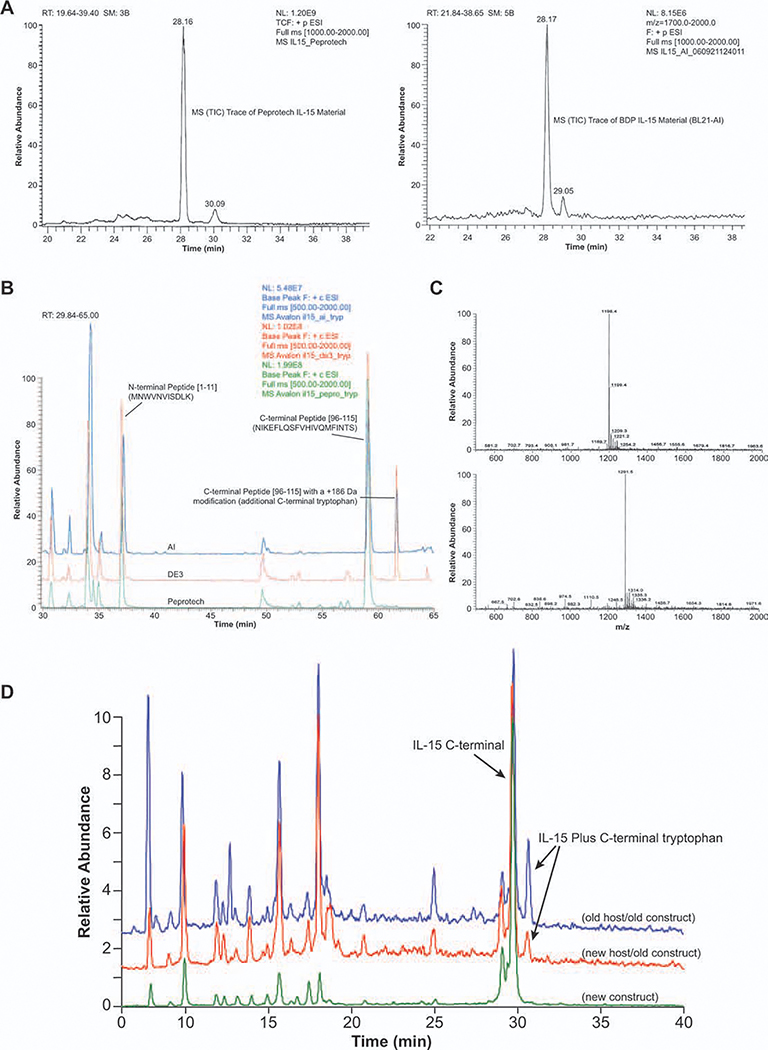
It was observed that 15–20% of purified rhIL-15 expressed in E. coli BL21-AI and BL21(DE3) strains had an extra tryptophan (Trp) at the C-terminus, as revealed by mass spectrometry, reverse phase HPLC, and peptide mapping (Figure 5). The extra C-terminal Trp was associated with the initial plasmid construct with expression termination by the triplet stop codons TGATAATGA.
Figure 5. Structure analysis and characterization of rhIL-15 by peptide mapping and LC/MS. Purified rhIL-15 was analyzed and characterized by using peptide mapping, reverse phase HPLC, and mass spectroscopy.
A: Characterized by RP-HPLC. Comparison of reference standard from Peprotech (left) with product from E. coli BL21-AI/pET 28b/rhIL-15. Reverse phase HPLC with UV detector was used. B: Characterized by peptide mapping. Base peak overlays of trypsin-digested product from E. coli BL21-AI, BL21(DE3) and from Peprotech. C: Full scan MS of C-terminal tryptic peptide (top) and C-terminal peptide with a +186 Da modification (bottom). The mass difference is m/z 93.1 (M + 2H)+2, which is +186.2 Da (average mass of a tryptophan residue is 186.21). D: Characterized by MS. Peptide mapping of tryptic-digested IL-15 to show existence of C-terminal Trp.
Reversed-phase liquid chromatography mass spectrometry (LC/MS) data showed a major component of ~ 12900.5 Da [rhIL-15 theoretical MW is 12900.7 Da, assuming two disulfide bonds] for rhIL-15 produced by BL21-AI™ and BL21(DE3) strains, as well as by the reference material from Peprotech (Materials and Methods). However, there was an additional component with MW = 13086.3 Da in the purified rhIL-15 from both BL-21 strains (Figure 5A, retention time 29.05 min). The mass difference between the two components corresponds to the residue MW of Trp. Comparison of the tryptic peptide maps of rhIL-15 from the BL-21 strains with rhIL-15 from Peprotech indicated the presence of C-terminal Trp peptides were only present in the rhIL-15 purified from the BL-21 strains. The additional Trp residue present in rhIL-15 from the BL-21 strains was identified as the C-terminal residue by MS/MS of the peptide and insource fragmentation of the intact molecule. The presence of an additional Trp residue at the carboxy terminus of rhIL-15 suggested that the first stop codon (TGA) of the expression cassette was misread 15–20% of the time, due to wobble translation of TGA to TGG (Trp).
Stop codons normally signal termination of translation. However, translation may not be terminated due to a tRNA wobble effect, reading-through TGA,27,28 or because of the existence of a specific tRNA stop suppressor in the E. coli cells.29,30 In either circumstance, translated protein may end up with an undesired C-terminal sequence modification.
For clinical manufacturing, it is not acceptable to produce rhIL-15 with ~ 20% of the product containing a C-terminal Trp residue (rhIL-15-Trp) in the final product. Eliminating the 20% of rhIL-15-Trp by chromatographic separation of the product-related impurity rhIL-15-Trp from rhIL-15 would be a challenging task in downstream processing. A genetic modification of the plasmid would be a better approach, including optimization of stop codon and verification that the host cell lacks tRNA suppressors.
Stop codon optimization
A second expression plasmid was constructed to replace the original triplet stop codon sequence TGATAATGA with TAATAATGA and transformed into E. coli BL21-AI cells. The same procedures for fermentation, recovery, and purification were followed to generate purified product, followed by peptide mapping and LC/MS characterization. Results clearly showed that the ~ 20% “extra” rhIL-15-Trp peak was eliminated by the revised stop codon sequence (Figure 4D).
The original cDNA was cloned from the human IL-15 gene where the native stop codon TGA (opal) is the most frequent in the human genome, while in E. coli TAA (ocher) is the most common stop codon (up to 92%), particularly in highly expressed genes.30 The original expression plasmid (pET28/IL-15) contained unmodified cDNA from human gene without species codon optimization. While amino acid codons may have an impact on translation efficiency in E. coli, use of an infrequent used stop codon may increase the probability for the wobble mistranslation, which leads to the synthesis of one or more extra C-terminal amino acids.
Demonstration of lack of tRNA suppressors in cell lines
To further evaluate the E. coli production cell line, another crucial study was to demonstrate that the host cells lack tRNA suppressors. A simple experiment was designed and developed to demonstrate if the host E. coli BL21-AI contains the tRNA suppressor. A vector pEL115 containing eGFP was mutated to convert the single Trp residue (W58) of eGFP to three stop codons TAA, TGA, and TAG. Expression of these three vectors, plus wild type, was compared in two strains: E. coli DE04 and E. coli BL21-AI.
In this design, the fluorescence assay was used to monitor the cells that read the stop codon. Table 3 shows the relative amount of expressed eGFP detected by fluorescence assay in various samples after induction. Raw values are fluorescence intensities based on a gain setting of 80% of the maximum linear value of the instrument. At this level, samples with values from 128 to 55,000 are within the linear range of the instrument.
Table 3.
Colony Counts of Two Strains in Three Constructs Expressed eGFP as Detected by Fluorescence Assay after Induction
| DE04 (raw) | BL21-AI (raw) | DE04 (%) | BL21-AI (%) | |
|---|---|---|---|---|
| Amber | 0 | 23 | 0 | 0 |
| Opal | 5,500 | 4,400 | 14 | 8 |
| Ochre | 26 | 0 | 0 | 0 |
| WT | 38,420 | 52,154 | 100 | 100 |
Raw fluorescence values of two strains containing one of four constructs expressing eGFP as detected by fluorescence assay. E. coli BL21-AI and E. coli DE04 were transformed by plasmid pEL115 containing eGFP that was mutated to convert the single tryptophan residue (W58) of eGFP to three stop codons: TAA, TGA, and TAG. One hundred microliter of each cell suspension was transferred to a Greiner Microclear 96-well plate, and using the FLUOstar Optima plate reader, the samples were analyzed for fluorescence. Raw values are fluorescence intensities based on a gain setting of 80% of the maximum linear value of the instrument. At this level, samples with values from 128 to 55,000 are within the linear range of the instrument.
No induced protein or background was seen in the Amber (TAG) or Ochre (TAA) mutants. The difference between the DE04 and BL21-AI read-through percentages is probably not specific (Table 3). The BL21-AI cells grew at a higher rate and were in stationary phase longer than the DE04 cells, and this may have had an effect on the amounts of eGFP produced. Interestingly, the data showed that the Opal (TGA) mutant showed 8% of florescence intensity or read-through, compared with the wild type, which was supported by another E. coli strain, DE04, with 14% (Table 3). The 8–14% is quite compatible to our observation of 15–20% read-through with the E. coli BL21-AI expressing rhIL-15.
To validate the eGFP assay results, SDS-PAGE was performed to verify the expressed product. The data (not shown) showed similar results to the fluorescence data—bands are clearly present in the wild-type construct in both strains, and additional faint bands are visible in the Opal mutants.
The results of these experiments confirm literature references that indicate that Opal codon read-through occurs in E. coli even in the absence of stop codon suppressors.26 Although no literature on BL21 read-through was found, several E. coli cell lines, including YN3230, have been shown to read-through Opal codons at a rate of 10–15%.31 Many of these strains have mutations in the release factor, RF2, and it is possible that BL21 and its derivatives harbor a similar mutation. Literature reports32 also indicate that lowering expression temperature can dramatically reduce read-through rates.
Here, we have developed a novel and simple method to evaluate if an E. coli cell line contains tRNA suppressors. It is critical to validate a production strain that is reliable in protein translation and express correct biomolecule for clinical use. Based on these results from E. coli BL21 strains, the authors would expect that all BL21-derived strains likely will exhibit the same effect, and believe alteration of TGA stop codons to TAG or TAA is the simplest approach to avoid the read-through problem.
Master cell banking and testing
Newly purchased cells from Invitrogen (Invitrogen, CA) were tested for the lack of tRNA suppressors, as previously described. Further, the cells were transformed with a plasmid carrying human interleukin-15 cDNA with the stop codon “TAATAATGA” Several colonies that positively expressed rhIL-15 were picked to assess expression levels. One colony was selected as a candidate for making a MCB. The candidate colony was evaluated through fermentation and purification, following the same procedure previously described (Materials and Methods). The purified rhIL-15 product was analyzed by mass spectrometry and compared with the original purified rhIL-15 material, as shown in Figure 5D, which clearly showed that the extra C-terminal rhIL-15-Trp peak was not present as the result of the stop codon modification. It was further demonstrated that when the original construct was transformed into the newly procured cells, the extra rhIL-15-Trp form (Figure 5D) reappeared.
The cells containing the plasmid with the corrected stop codon were then expanded and an E. coli BL21-AI pET28b/rhIL-15ochre MCB was made (Lot L0704014; Materials and Methods). To qualify the MCB for clinical manufacturing, tests were performed, including full-length plasmid sequencing and restriction mapping, plasmid copy number and stability, host cell identity and purity, as well as toxin and fimbrial gene detection. The results were satisfactory (data not shown) for further CGMP use.
Conclusion
Human IL-15 cDNA was expressed in E. coli BL21 strains. After evaluating E. coli strains BL21(DE3), BL21(DE3)pLysS, BLR(DE3)pLysS, and BL21-AI for expression levels and product quality, E. coli BL21-AI was selected for further clinical manufacturing based on higher initial product purity. Expression optimization was carried out at shake flask and 20 L fermenter scales, and the recombinant product was expressed as inclusion bodies that were solubilized, refolded, and purified to yield active rhIL-15 as demonstrated in CTLL-2 cell proliferation assays. Biochemical and biophysical characterization of rhIL-15 was carried out to assess product quality. Stop codons of the expression construct were further investigated, since 15–20% of the purified rhIL-15 showed an extraneous peak corresponding to an extra C-terminal rhIL-15-Trp residue, based on peptide mapping and mass spectrometry. It was determined by a simple novel method that the presence of extra rhIL-15-Trp was due to a stop codon wobble effect, which was eliminated by replacement of the TGA (opal) stop codon with TAA (ocher). The E. coli BL21-AI cells containing the rhIL-15 coding sequence with the modified stop codon TAATAATGA were shown to have eliminated the C-terminal rhIL-15-Trp variant but still have the same product quality attributes as the original plasmid construct. BL-21-AI cells containing the modified stop codon were isolated, characterized, and banked for clinical manufacturing.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contracts N01-CO-12400 and #HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mentioning of trade names, commercial products, or organizations imply endorsement by the U.S. government.
The authors gratefully acknowledge technical assistance from Ms. Jane Wu for her outstanding contribution in fermentation, selection of production strain, and part of downstream processing. They also acknowledge biological assay support by the group led by Dr. Gopalan Soman. Particularly, they would like to acknowledge operational support from the Biopharmaceutical Development Program in GMP production of the MCB by the Late Process Sciences Department; analytical support from the Quality Control and Process Analytical Sciences Departments, and quality assurance support from the Quality Assurance and Regulatory Affairs Departments.
Notation
- ACB
accession cell bank
- BDP
biopharmaceutical development program
- cGMP
current good manufacturing practice
- DTT
dithiothreitol
- ED50
effective dose at 50% response
- FBS
fetal bovine serum
- GFP
green florescence protein
- HPLC-SEC
high-pressure liquid chromatography size-exclusive chromatography
- IAA
iodoacetic acid
- IPTG
isopropyl-β-D-1-thiogalactopyranoside
- LC/MS
liquid chromatography/mass spectrometry
- MCB
master cell bank
- OD
optical density
- rhIL-15
recombinant human Interleukin-15
- SDS
sodium dodecyl sulfate
- Trp
tryptophan
- VVM
volume/volume/min
Contributor Information
Vinay V. Vyas, Biopharmaceutical Development Program, SAIC-Frederick Inc., National Cancer Institute at Frederick (NCI-Frederick), Frederick, MD 21702
Dominic Esposito, Protein Expression Laboratory, Advanced Technology Program, SAIC-Frederick, NCI-Frederick, Frederick, MD 21702.
Terry L. Sumpter, Biopharmaceutical Development Program, SAIC-Frederick Inc., National Cancer Institute at Frederick (NCI-Frederick), Frederick, MD 21702
Trevor L. Broadt, Biopharmaceutical Development Program, SAIC-Frederick Inc., National Cancer Institute at Frederick (NCI-Frederick), Frederick, MD 21702
James Hartley, Protein Expression Laboratory, Advanced Technology Program, SAIC-Frederick Inc., NCI-Frederick, Frederick, MD 21702.
George C. Knapp, IV, Biopharmaceutical Development Program, SAIC-Frederick Inc., NCI-Frederick, Frederick, MD 21702.
Wei Cheng, Biopharmaceutical Development Program, SAIC-Frederick Inc., NCI-Frederick, Frederick, MD 21702.
Man-Shiow Jiang, Biopharmaceutical Development Program, SAIC-Frederick Inc., NCI-Frederick, Frederick, MD 21702.
John M. Roach, Biopharmaceutical Development Program, SAIC-Frederick Inc., NCI-Frederick, Frederick, MD 21702
Xiaoyi Yang, Biopharmaceutical Development Program, SAIC-Frederick Inc., NCI-Frederick, Frederick, MD 21702.
Steven L. Giardina, Biopharmaceutical Development Program, SAIC-Frederick Inc., NCI-Frederick, Frederick, MD 21702
George Mitra, Biopharmaceutical Development Program, SAIC-Frederick Inc., NCI-Frederick, Frederick, MD 21702.
Jason L. Yovandich, Protein Expression Laboratory, Advanced Technology Program, SAIC-Frederick, NCI-Frederick, Frederick, MD 21702
Stephen P. Creekmore, Protein Expression Laboratory, Advanced Technology Program, SAIC-Frederick, NCI-Frederick, Frederick, MD 21702
Thomas A. Waldmann, Metabolism Branch, Center for Cancer Research, National Cancer Institute, Bethesda, MD 20892
Jianwei Zhu, Biopharmaceutical Development Program, SAIC-Frederick Inc., NCI-Frederick, Frederick, MD 21702.
Literature Cited
- 1.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, Johnson L, Alderson MR, Watson JD, Anderson DM, Giri JG. Cloning of a T-cell growth factor that interacts with the b chain of the interleukin-2 receptor. Science. 1994;264:965–968. [DOI] [PubMed] [Google Scholar]
- 2.Burton JD, Bamford RN, Peters C, Grant AJ, Kurys G, Goldman CK, Roessler E, Waldmann TA. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4935–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. [DOI] [PubMed] [Google Scholar]
- 4.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML,Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlén C, Greenberg PD. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–341. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M, Yao Z, Dubois S, Ju W, Müller JR, Waldmann TA. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci USA.2009;5:106:7513–7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lugli F, Goldman CK, Perera LP, Smedley J, Pung R, Yovandich JL, Creekmore SP, Waldmann TA, Roederer M. Transient and persistent effects of IL15 on lymphocyte homeostasis in nonhuman primates. Blood. 2010;116:3238–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saikh KU, Kissner TL, Nystrom S, Ruthel G, Ulrich RG. Interleukin-15 increases vaccine efficacy through a mechanism linked to dendritic cell maturation and enhanced antibody titers. Clin Vaccine Immunol. 2008;15:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.d’Ettorre G, Forcina G, Lichtner M, Mengoni F, D’Agostino C, Massetti AP, Mastroianni CM, Vullo V. Interleukin-15 in HIV infection: immunological and virological interactions in antiretroviral-naive and -treated patients. AIDS. 2002;16:181–188. [DOI] [PubMed] [Google Scholar]
- 10.Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, Choe PY, Ramanathan MP, Parkinson R, Kudchodkar S, Tamura Y, Sidhu M, Roopchand V, Kim JJ|, Pavlakis PN, Felber BK, Waldmann TA, Boyer JD, Weiner DB. Co-immunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T-cell help. J Immunol. 2005;175:112–123. [DOI] [PubMed] [Google Scholar]
- 11.Bergamaschi C, Jalah R, Kulkarni V, Rosati M, Zhang GM, Alicea C, Zolotukhin AS, Felber BK, Pavlakis GN. Secretion and biological activity of short signal peptide IL-15 is chaperoned by IL-15 receptor alpha in vivo. J Immunol. 2009;183:3064–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. [DOI] [PubMed] [Google Scholar]
- 13.Jalah R, Rosati M, Kulkarni V, Patel V, Bergamaschi C, Valentin A, Zhang GM, Sidhu MK, Eldridge JH, Weiner DB, Pavlakis GN, Felber BK. Efficient systemic expression of bioactive IL-15 in mice upon delivery of optimized DNA expression plasmids. DNA Cell Biol. 2007;26:827–840. [DOI] [PubMed] [Google Scholar]
- 14.Pettit DK, Bonnert TP, Eisenman J, Srinivasan S, Paxton R, Beers C, Lynchi D, Milleri B, Yost J, Grabstein KH, Gombotz WR. Structure–function studies of interleukin 15 using site-specific mutagenesis polyethylene glycol conjugation, and homology modeling. J Biol Chem. 1997;272:2312–2318. [DOI] [PubMed] [Google Scholar]
- 15.WHO. WHO reference reagent Interleukin-15 (Human rDNA-derived) NIBSC code :95/554 instruction for use (Version 4.0, Dated 30/01/2008). Available at: http://www.who.int/biologicals/en.
- 16.Hanick NA, Rickert M, Varani L, Bankovich AJ, Cochran JR, Kim DM, Surh CD, Garcia KC. Elucidation of the interleukin-15 binding site on its alpha receptor by NMR. Biochemistry. 2007;46:9453–9461. [DOI] [PubMed] [Google Scholar]
- 17.Ward A, Anderson M, Craggs RI, Maltby J, Grahames C, Davies RA, Finch D, Pattison D, Oakes H, Mallinder PR. E. coli expression and purification of human and cynomolgus IL-15. Protein Expr Purif. 2009;68:42–48. [DOI] [PubMed] [Google Scholar]
- 18.Mortier E, Quéméner A, Vusio P, Lorenzen I, Boublik Y, Grötzinger J, Plet A, Jacques Y. Soluble interleukin-15 receptorα (IL-15Rα)-sushi as a selective and potent agonist of IL-15 action through IL-15R β/γ. J Biol Chem. 2006;281:1612–1619. [DOI] [PubMed] [Google Scholar]
- 19.Xia ZJ, Kong XL, Zhang P. In vivo effect of recombined IL-15/Fc fusion protein on EAU. Sichuan Da Xue Xue Bao Yi Xue Ban. 2008;39:944–949. [PubMed] [Google Scholar]
- 20.Vyas VV. Clinical manufacturing of recombinant human Interleukin 15. Biotech Progress. To be submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nellis D Characterization of deamidation of recombinant IL-15. Pharm Res. In press. DOI: 10.1007/s11095-011-0597-0. [DOI] [PubMed] [Google Scholar]
- 22.NCI. A phase I study of intravenous recombinant human IL-15 in adults with refractory metastatic malignant melanoma and metastatic renal cell cancer. 2009. Available at:http://clinicaltrials.gov/ct2/show/NCT01021059 Last retrieve date: Nov 29, 2011. [Google Scholar]
- 23.Esposito D, Garvey LA, Chakiath CS. Gateway cloning for protein expression In: Sharon AD, editor. Method in Molecular Biology: High-throughput protein expression and purification. Totowa, NJ: Humana Press; 2009: 31–54. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Russell D. Molecular cloning: A laboratory manual, 3rd ed. Cold Spring Harbor, New York: Cold Spring Harbor Laborator Press;2001 [Google Scholar]
- 25.Soman G, Yang X, Jiang H, Giardina S, Vyas V, Mitra G, Yovandich J, Creekmore SP, Waldmann TA, Quinones O, Alvord WG. MTS dye-based colorimetric CTLL-2 cell proliferation assay for product release and stability monitoring of Interleukin-15: Assay qualification, standardization, and statistical analysis. J Immonol Methods. 2009;348:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacBeath G, Kast P. UGA read-through artifacts—when popular gene expression systems need a pATCH. BioTechniques. 1998;24:789–794. [DOI] [PubMed] [Google Scholar]
- 27.Geller AI, Rich AP. A UGA termination suppression tRNATrp active in rabbit reticulocytes. Nature. 1980;283:41–46. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Rice C. The signal for translational read-through of a UGA codon in sindbis virus RNA involves a single cytidine residue immediately downstream of the termination codon. J Virol. 1993;67:5062–5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatfield DL, Smith DWE, Lee BJ, Worland PJ, Oroszlan S. Structure and function of suppressor tRNAs in higher eukaryotes. Crit Rev Biochem Mol Biol. 1990;25:71–96. [DOI] [PubMed] [Google Scholar]
- 30.Brown CM, Stockwell PA, Trotman CAN, Tate WP. Sequence analysis suggests that tetra-nucleotides signal the termination of protein synthesis in eukaryotes. Nucleic Acid Res. 1990;18:6339–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopelowitz J, Hampe C, Goldman R, Reches M, Engelberg-Kulka H. Influence of codon context on UGA suppression and readthrough. J Mol Biol. 1992;225:261–269. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez B, Ceciliani F, Galizzi A. Growth at low temperature suppresses read-through of the UGA stop codon during the expression of Bacillus subtillis flgM gene in Escherichia coli. J Biotechnol. 2003;101:173–180. [DOI] [PubMed] [Google Scholar]