Abstract
Rationale: Millions of workers around the world are exposed to respirable crystalline silica. Although silica is a confirmed human lung carcinogen, little is known regarding the cancer risks associated with low levels of exposure and risks by cancer subtype. However, little is known regarding the disease risks associated with low levels of exposure and risks by cancer subtype.
Objectives: We aimed to address current knowledge gaps in lung cancer risks associated with low levels of occupational silica exposure and the joint effects of smoking and silica exposure on lung cancer risks.
Methods: Subjects from 14 case–control studies from Europe and Canada with detailed smoking and occupational histories were pooled. A quantitative job-exposure matrix was used to estimate silica exposure by occupation, time period, and geographical region. Logistic regression models were used to estimate exposure–disease associations and the joint effects of silica exposure and smoking on risk of lung cancer. Stratified analyses by smoking history and cancer subtypes were also performed.
Measurements and Main Results: Our study included 16,901 cases and 20,965 control subjects. Lung cancer odds ratios ranged from 1.15 (95% confidence interval, 1.04–1.27) to 1.45 (95% confidence interval, 1.31–1.60) for groups with the lowest and highest cumulative exposure, respectively. Increasing cumulative silica exposure was associated (P trend < 0.01) with increasing lung cancer risks in nonsilicotics and in current, former, and never-smokers. Increasing exposure was also associated (P trend ≤ 0.01) with increasing risks of lung adenocarcinoma, squamous cell carcinoma, and small cell carcinoma. Supermultiplicative interaction of silica exposure and smoking was observed on overall lung cancer risks; superadditive effects were observed in risks of lung cancer and all three included subtypes.
Conclusions: Silica exposure is associated with lung cancer at low exposure levels. An exposure–response relationship was robust and present regardless of smoking, silicosis status, and cancer subtype.
Keywords: lung cancer, crystalline silica, occupational exposure
At a Glance Commentary
Scientific Knowledge on the Subject
Occupational silica exposure is linked to human lung cancer. Studies have reported that lung cancer risks increase monotonically with increasing cumulative silica exposure.
What This Study Adds to the Field
We provide new insight on the quantification and characterization of the exposure–response relationships between occupational silica exposure and lung cancer subtypes. We further explore silica-related lung cancer risks in important subgroups, including subjects with different smoking histories and subjects with low levels of exposure, as well as subjects without silicosis.
Occupational exposure to respirable crystalline silica (silica hereafter) occurs in tens of millions of workers globally in a wide range of industries, including construction, mining, and quarrying, as well as manufacturing of bricks, ceramics, and metal products (1, 2). Silica is classified as a human lung carcinogen by the International Agency for Research on Cancer (IARC), the U.S. National Institute for Occupational Safety and Health, and the U.S. National Toxicology Program (3–5). A pooled analysis of 1,072 lung cancer cases from 10 industry-based studies showed that the risk of cancer increased monotonically with increases in cumulative silica exposure (6). Additional evidence of an exposure–response relationship between silica and lung cancer was observed in different industrial cohorts (7, 8) as well as in case–control studies in different countries (9–11).
Despite the strong epidemiologic evidence of an exposure–response relationship between silica and lung cancer, questions still remain regarding certain aspects of the carcinogenicity of silica, including the role of cigarette smoking as a potential confounder and effect modifier (12), whether an exposure threshold exists for silica-related lung cancer (13), whether silicosis is a prerequisite for developing silica-related lung cancer (14, 15), the effect of silica exposure on risks of different histological subtypes of lung cancer (9, 10), and the joint effect of exposure to silica and smoking on risk of lung cancer and its subtypes (7, 9).
In the current study we present findings from the Pooled Analysis of Case-Control Studies on the Joint Effects of Occupational Carcinogens in the Development of Lung Cancer (SYNERGY) project, which is a pooled analysis of lung cancer case-control studies from Europe and Canada (16). Occupational exposure to quartz silica was estimated via a quantitative general population job-exposure matrix (SYN-JEM) (17). The aims of our work were to assess 1) the risks of lung cancer in relation to various indices of occupational silica exposure by cancer subtype, smoking status, and silicosis status; 2) the interaction of silica exposure and smoking on the risk of lung cancer and its major subtypes on the additive and multiplicative scale; and 3) the excess lifetime risks (ELRs) of lung cancer associated with different levels of occupational silica exposure.
Methods
Study Population
The SYNERGY project is a pooled analysis of 14 population- and hospital-based lung cancer case–control studies in 13 European countries and Canada (see Table E1 in the online supplement). Detailed description of the population was presented elsewhere (16). Lifetime occupational and smoking histories were available for all subjects. Self-reports of physician-diagnosed silicosis were collected in the AUT-Munich (Arbeit und Technik-Munich), EAGLE (Environment and Genetics in Lung Cancer Etiology), HdA (Humanisierung des Arbeitslebens), and INCO (International Agency for Research on Cancer Multicenter Case-Control Study of Occupation, Environment, and Lung Cancer in Central and Eastern Europe) studies by in-person or next-of-kin interview (full silicosis questions available in Table E1). Ethical approvals for the SYNERGY project were obtained from all participating countries, as well as the IARC institutional review board. More information about the project is available at http://synergy.iarc.fr.
Exposure Assessment
The elaborated SYN-JEM and the underlying models for exposure to quartz silica have been described in detail elsewhere (17–19). Briefly, 23,640 historical personal respirable quartz measurements were combined with exposure ratings from a general population JEM, the Domtoren-JEM (20). Quantitative quartz silica exposure estimates (in milligram per cubic meter) representing annual average exposure levels were derived for each job title, region, and year combination. Silica concentrations before 1960 were assumed to be the same as those in 1960. JEM linkage to the population was performed via the International Standard Classification of Occupations (version 1968, or ISCO-68) (21). Cumulative exposure (in milligram per cubic meter years) was calculated as the sum of the products of modeled exposure intensities and years of employment for all jobs over a subject’s entire working history.
Statistical Analysis
The overall analysis protocol for silica is similar to those previously applied to characterize lung cancer risks for exposure to diesel engine exhaust and asbestos in the SYNERGY study (16, 22). Odds ratios (ORs) and 95% confidence intervals (CIs) for lung cancer associated with various categorical indices of occupational silica exposure were calculated using unconditional logistic regression models. Trend analysis P values were obtained by including the various indices of exposure as continuous variables in models for all subjects and for exposed subjects only. In our main categorical models, lung cancer risks were calculated for the following silica exposure metrics: ever/never exposure, duration of exposure (1–9, 10–19, 20–29, and >29 yr), time since last exposure (<5, 5–9, 10–19, 20–29, 30–39, and >39 yr), and cumulative exposure (quartiles of exposure distribution among control subjects: >0–0.39, 0.4–1.09, 1.1–2.39, and ≥2.4 mg/m3-years). Adjustments were made for age group (<45, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, and >74 yr), sex, study, smoking (log [cigarette pack-years + 1]), smoking cessation since interview/diagnosis (current smokers: >0–7, 8–15, 16–25, and >25 yr; never-smokers), and ever employment in “list A jobs.” List A jobs are occupations with known occupational lung cancer risks (e.g., welders, long-distance truck drivers, or boiler operators), and their inclusion in the model served as an adjustment for exposures to other occupational lung carcinogens. The list was first published in 1982, then updated in 1995 and 2000 to include exposures reviewed by the IARC up to volume 75 of the IARC Monographs on the Identification of Carcinogenic Hazards to Humans (23, 24). We defined smokers as subjects who smoked more than one cigarette per day for more than one year; pack-years was calculated as the sum of the products of smoking duration in years and average smoking of 20-cigarette packs per day.
Various silica cumulative exposure lag times (0, 5, 10, 15, and 20 yr) were applied in the main models, but only results with zero lag are presented because models with no lag had the best model fit according to minimized Akaike information criterion values. Stratified analyses for cancer risks associated with cumulative exposure categories were also calculated for subjects with different major cancer subtypes, without reported silicosis, and with different smoking habits.
For analyses of silica exposure as a continuous variable, both untransformed and natural log-transformed cumulative exposure were used. For the model with log-transformed exposure, nonexposed subjects were assigned two-thirds of the lowest cumulative exposure value among the exposed group (0.0036 mg/m3-years). To further explore the shape of the exposure–response relationship, we performed thin-plate regression spline analyses as implemented in the R package mgcv (25), with relative maximum likelihood selected as the method for smoothing parameter estimation and total number of basis functions limited to three. The 95% CIs around the splines were based on simulations from posterior distributions of model coefficients with random draws from a multivariate normal distribution parameterized by the estimated mean vector and covariance matrix of the model coefficients. All splines were truncated at the 99th percentile to focus on results that were the most relevant and best supported by our exposure data.
Multiplicative interactions between silica exposure and smoking on risks of overall lung cancer and major cancer subtypes were assessed using P values from the cross-product interaction terms between silica exposure and smoking in the logistic models. For additive interactions, relative excess risks due to interaction (RERI) were calculated using ORs from the adjusted logistic models as defined by Rothman and Greenland (26) and implemented in the R package epiR (27).
The ELRs of lung cancer at age 80 years associated with 45 years of occupational silica exposure at various concentrations were calculated according to life table methods described by Vermeulen and colleagues (28) with all-cause and lung cancer mortality rates from the European Union in 2008 as the referent (29). Silica exposure levels for our ELR calculations were selected based on the current recommended 8-hour threshold limit value by the American Conference of Governmental Industrial Hygienists at 0.025 mg/m3 (30), the recently updated 0.05 mg/m3 permissible exposure limit from U.S. Occupational Safety and Health Administration (31), and the exposure limit of 0.1 mg/m3 in the latest European Union directive (2019/130) on the protection of workers from carcinogens (32).
Statistical analyses were conducted using SAS (version 9.3; SAS Institute) and R (version 3.5) (33).
Results
After excluding participants with incomplete information on covariates (804 cases and 848 control subjects), 16,901 lung cancer cases (4,752 adenocarcinomas, 6,503 squamous cell carcinomas, 2,730 small cell carcinomas, 2,822 other/unspecified lung cancers, and 94 not available) and 20,965 control subjects remained for our main analyses (Table 1). Silicosis status was available in 50% of the study population (n = 18,931), among which 108 cases of silicosis were reported. Occupations with the highest modeled silica exposure concentrations in SYN-JEM were chimney bricklayers, stone cutters/carvers, and hand monument carvers; the most frequently reported exposed job titles among the control subjects in our population were farm helpers, general farmers, and construction bricklayers (more occupations in these categories are available in Table E2).
Table 1.
Selected Study Population Characteristics by Lung Cancer Status and Silica Exposure
| Characteristic | Ever Exposed to Silica |
Never Exposed to Silica |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (n) | % | Control Subjects (n) | % | Cases (n) | % | Control Subjects (n) | % | ||
| Sex | |||||||||
| M | 4,649 | 94.4 | 4,140 | 92.2 | 8,956 | 74.8 | 12,311 | 74.7 | |
| F | 274 | 5.6 | 348 | 7.8 | 3,022 | 25.2 | 4,166 | 25.3 | |
| Age group | |||||||||
| <45 yr | 142 | 2.9 | 194 | 4.3 | 573 | 4.8 | 1,177 | 7.1 | |
| 45–64 yr | 2,503 | 50.8 | 2,055 | 45.8 | 6,260 | 52.3 | 8,299 | 50.4 | |
| >64 yr | 2,278 | 46.3 | 2,239 | 49.9 | 5,145 | 43.0 | 7,001 | 42.5 | |
| Smoking status | |||||||||
| Never-smoker | 248 | 5.0 | 1,253 | 27.9 | 1,121 | 9.4 | 5,900 | 35.8 | |
| Former smoker | 1,736 | 35.3 | 2,010 | 44.8 | 3,696 | 30.9 | 6,210 | 37.7 | |
| Current smoker | 2,939 | 59.7 | 1,225 | 27.3 | 7,161 | 59.8 | 4,367 | 26.5 | |
| Smoking pack-years | |||||||||
| Never-smoker | 248 | 5.0 | 1,253 | 27.9 | 1,121 | 9.4 | 5,900 | 35.8 | |
| <10 | 227 | 4.6 | 683 | 15.2 | 582 | 4.9 | 2,386 | 14.5 | |
| 10–19 | 475 | 9.6 | 598 | 13.3 | 1,127 | 9.4 | 2,264 | 13.7 | |
| >19 | 3,973 | 80.7 | 1,954 | 43.5 | 9,148 | 76.4 | 5,927 | 36.0 | |
| Years since quitting smoking | |||||||||
| Never-smoker | 248 | 5.0 | 1,253 | 27.9 | 1,121 | 9.4 | 5,900 | 35.8 | |
| >0–7 yr | 638 | 13.0 | 317 | 7.1 | 1,388 | 11.6 | 1,105 | 6.7 | |
| 8–15 yr | 494 | 10.0 | 461 | 10.3 | 1,037 | 8.7 | 1,437 | 8.7 | |
| 16–25 yr | 379 | 7.7 | 590 | 13.1 | 792 | 6.6 | 1,756 | 10.7 | |
| >25 yr | 225 | 4.6 | 642 | 14.3 | 479 | 4.0 | 1,912 | 11.6 | |
| Current smoker | 2,939 | 59.7 | 1,225 | 27.3 | 7,161 | 59.8 | 4,367 | 26.5 | |
| List A job | |||||||||
| Ever employment | 829 | 16.8 | 597 | 13.3 | 958 | 8.0 | 767 | 4.7 | |
| Never employment | 4,094 | 83.2 | 3,891 | 86.7 | 10,905 | 92.0 | 15,563 | 95.3 | |
| Silicosis | |||||||||
| Reported silicosis | 57 | 1.2 | 33 | 0.7 | 13 | 0.1 | 5 | 0 | |
| No reported silicosis | 2,882 | 58.5 | 2,311 | 51.5 | 6,091 | 50.9 | 7,539 | 45.8 | |
| Unknown | 1,984 | 40.3 | 2,144 | 47.8 | 5,874 | 49.0 | 8,933 | 54.2 | |
| Lung cancer subtype | |||||||||
| Adenocarcinoma | 1,069 | 21.7 | — | — | 3,683 | 30.7 | — | — | |
| Small cell carcinoma | 869 | 17.7 | — | — | 1,861 | 15.5 | — | — | |
| Squamous cell carcinoma | 2,251 | 45.7 | — | — | 4,252 | 35.5 | — | — | |
| Other/unspecified | 711 | 14.4 | — | — | 2,111 | 17.6 | — | — | |
| Not available | 23 | 0.5 | — | — | 71 | 0.6 | — | — | |
Increased overall lung cancer risks were observed for silica-exposed versus nonexposed subjects across three occupational exposure metrics, including ever exposure (OR, 1.30 [95% CI, 1.23–1.38]), longer exposure duration (longest category: >29 yr; OR, 1.48 [95% CI, 1.34–1.63]), and higher cumulative exposure (highest category: >2.4 mg/m3-years; OR, 1.45 [95% CI, 1.31–1.60]) (Table 2). Elevated lung cancer risk increases were found for groups with the lowest exposure duration and cumulative exposure; ORs were 1.22 (95% CI, 1.12–1.31) and 1.15 (95% CI, 1.04–1.27) for subjects with exposure duration of one to nine years and cumulative exposure <0.4 mg/m3-years, respectively. Increasing cancer risk trends were also associated (P trends < 0.01) with both increasing exposure duration and increasing cumulative exposure. Lung cancer risks for those who were more recently exposed also tended to be higher than for those who were last exposed a longer time ago, but confidence in this risk trend is lower (P trend = 0.10 among exposed subjects). Results for analyses restricted to subjects who did not report silicosis were similar to those from the main analyses (Table 3).
Table 2.
Lung Cancer Odds Ratios Associated with Various Indices of Occupational Silica Exposure
| Occupational Silica Exposure | Cases (n) | % | Control Subjects (n) | % | OR* | 95% CI |
|---|---|---|---|---|---|---|
| Never | 11,978 | 70.9 | 16,477 | 78.6 | 1.0 | Referent |
| Ever exposure | 4,923 | 29.1 | 4,488 | 21.4 | 1.30 | 1.23–1.38 |
| Duration, yr | ||||||
| 1–9 | 2,035 | 12.0 | 1,936 | 9.2 | 1.22 | 1.12–1.31 |
| 10–19 | 926 | 5.5 | 905 | 4.3 | 1.20 | 1.08–1.34 |
| 20–29 | 635 | 3.8 | 519 | 2.5 | 1.45 | 1.26–1.66 |
| >29 | 1,327 | 7.9 | 1,128 | 5.5 | 1.48 | 1.34–1.63 |
| Test for trend, P value | <0.01 | |||||
| P value excluding never exposed | <0.01 | |||||
| Cumulative exposure, mg/m3-years | ||||||
| >0–0.39 | 1,113 | 6.6 | 1,128 | 5.4 | 1.15 | 1.04–1.27 |
| 0.4–1.09 | 1,221 | 7.2 | 1,120 | 5.3 | 1.33 | 1.21–1.47 |
| 1.1–2.39 | 1,231 | 7.3 | 1,122 | 5.4 | 1.29 | 1.17–1.42 |
| ≥2.4 | 1,358 | 8.0 | 1,118 | 5.3 | 1.45 | 1.31–1.60 |
| Test for trend, P value | <0.01 | |||||
| P value excluding never exposed | <0.01 | |||||
| Time since last exposure†, yr | ||||||
| <5 | 934 | 5.5 | 815 | 3.9 | 1.43 | 1.18–1.73 |
| 5–9 | 462 | 2.7 | 351 | 1.7 | 1.43 | 1.15–1.77 |
| 10–19 | 679 | 4.0 | 569 | 2.7 | 1.36 | 1.13–1.63 |
| 20–29 | 617 | 3.7 | 536 | 2.6 | 1.26 | 1.08–1.47 |
| 30–39 | 931 | 5.5 | 812 | 3.9 | 1.30 | 1.15–1.47 |
| >39 | 1,300 | 7.7 | 1,405 | 6.7 | 1.09 | 0.99–1.20 |
| Test for trend, P value | — | |||||
| P value excluding never exposed | 0.10 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
OR adjusted for study, age group, sex, smoking (pack-years, time since quitting smoking), and list A jobs.
OR in “time since last exposure” is additionally adjusted for duration (continuous) of silica exposure. Trend test limited to exposed subjects.
Table 3.
Lung Cancer Odds Ratios Associated with Cumulative Occupational Silica Exposure in Subjects without Silicosis
| Cumulative Silica Exposure (mg/m3-years) | Cases (n) | OR* | 95% CI |
|---|---|---|---|
| Never | 6,091 | 1.0 | Referent |
| >0–0.39 | 665 | 1.22 | 1.07–1.40 |
| 0.4–1.09 | 720 | 1.50 | 1.31–1.71 |
| 1.1–2.39 | 757 | 1.48 | 1.30–1.69 |
| ≥2.4 | 740 | 1.42 | 1.25–1.63 |
| Test for trend, P value | <0.01 | ||
| P value excluding never exposed | <0.01 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
OR adjusted for study, age group, sex, smoking (pack-years, time since quitting smoking), and list A jobs.
Increasing risks of all three included lung cancer subtypes were observed with increasing silica cumulative exposure (Table 4). We observed elevated risks of squamous cell carcinoma for all cumulative exposure groups, including the lowest (OR, 1.22 [95% CI, 1.06–1.39]). Clear increased risks of small cell carcinoma were found for groups with cumulative exposures ≥0.4 mg/m3-years, with an OR of 1.70 (95% CI, 1.43–2.02) for the highest exposed group. Adenocarcinoma risks were generally lower than those observed in small cell and squamous cell carcinomas; adenocarcinoma OR for the highest exposed group was 1.17 (95% CI, 1.00–1.37).
Table 4.
Lung Cancer Major Subtype Risks Associated with Cumulative Occupational Silica Exposure
| Cumulative Exposure (mg/m3-years) | Adenocarcinoma |
Squamous Cell Carcinoma |
Small Cell Carcinoma |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (n) | OR* | 95% CI | Cases (n) | OR* | 95% CI | Cases (n) | OR* | 95% CI | |
| Never | 3,683 | 1.0 | Referent | 4,252 | 1.0 | Referent | 1,861 | 1.0 | Referent |
| >0–0.39 | 283 | 1.14 | 0.98–1.33 | 455 | 1.22 | 1.06–1.39 | 194 | 1.07 | 0.89–1.28 |
| 0.4–1.09 | 282 | 1.18 | 1.02–1.37 | 557 | 1.51 | 1.33–1.71 | 204 | 1.41 | 1.17–1.68 |
| 1.1–2.39 | 240 | 1.03 | 0.88–1.20 | 593 | 1.46 | 1.29–1.65 | 229 | 1.48 | 1.25–1.76 |
| ≥2.4 | 264 | 1.17 | 1.00–1.37 | 646 | 1.55 | 1.37–1.76 | 242 | 1.70 | 1.43–2.02 |
| Test for trend, P value | 0.01 | <0.01 | <0.01 | ||||||
| P value excluding never exposed | 0.02 | <0.01 | <0.01 | ||||||
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
OR adjusted for study, age group, sex, smoking (pack-years, time since quitting smoking), and list A jobs.
The continuous model with untransformed exposure showed that every 1 mg/m3-year increase in cumulative silica exposure increased lung cancer risk by a factor of 1.06 (95% CI, 1.04–1.08). In the model with log-transformed exposure, lung cancer risk increased by a factor of 1.05 (95% CI, 1.04–1.06) for every unit increase in log-cumulative exposure. Nonparametric spline analysis showed monotonic increases in risks of overall lung cancer and its subtypes for both untransformed and log-transformed silica cumulative exposure (Figure 1). Individual splines for overall lung cancer and all subtypes with corresponding 95% CI are available in Figures E1 and E2.
Figure 1.
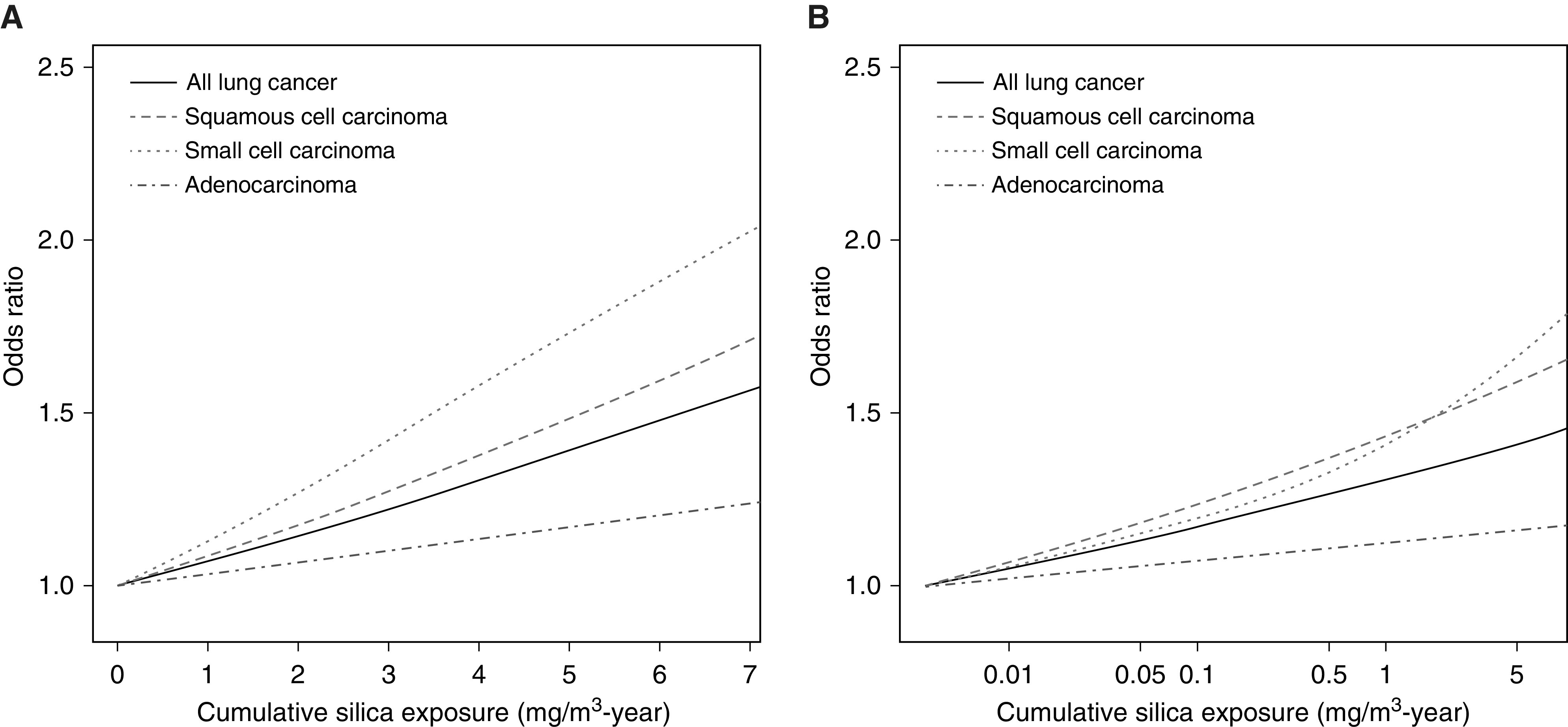
Spline analyses results on exposure–response relationships between lung cancer with (A) cumulative exposure and (B) natural log-transformed cumulative exposure.
Stratified analyses showed that, regardless of smoking status, increasing cumulative silica exposure was associated (P trends for all subjects < 0.01) with increasing lung cancer risks (Table 5). Risks of lung cancer for different silica exposure groups were similar for former and current smokers, with ORs of 1.47 (95% CI, 1.27–1.70) and 1.39 (95% CI, 1.20–1.62) for the highest exposed group, respectively. For never-smokers, the OR point estimates for all silica cumulative exposure categories were above 1, with the highest exposed group having an OR of 1.40 (95% CI, 1.03–1.86).
Table 5.
Lung Cancer Risks Associated with Cumulative Occupational Silica Exposure by Smoking Status
| Cumulative exposure (mg/m3-years) | Never-Smokers |
Former Smokers |
Current Smokers |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (n) | OR* | 95% CI | Cases (n) | OR† | 95% CI | Cases (n) | OR‡ | 95% CI | |
| Never | 1,121 | 1.0 | Referent | 3,696 | 1.0 | Referent | 7,161 | 1.0 | Referent |
| >0–0.39 | 60 | 1.17 | 0.85–1.57 | 366 | 1.07 | 0.92–1.25 | 687 | 1.19 | 1.03–1.39 |
| 0.4–1.09 | 59 | 1.07 | 0.78–1.43 | 433 | 1.37 | 1.18–1.59 | 729 | 1.33 | 1.15–1.55 |
| 1.1–2.39 | 60 | 1.02 | 0.75–1.36 | 441 | 1.35 | 1.16–1.57 | 730 | 1.29 | 1.11–1.50 |
| ≥2.4 | 69 | 1.40 | 1.03–1.86 | 496 | 1.47 | 1.27–1.70 | 793 | 1.39 | 1.20–1.62 |
| Test for trend, P value | <0.01 | <0.01 | <0.01 | ||||||
| P value excluding never exposed | 0.02 | <0.01 | 0.07 | ||||||
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
OR adjusted for sex, study, age group, and list A jobs.
OR adjusted for sex, study, age group, list A jobs, pack-years, and time since quitting smoking.
OR adjusted for sex, study, age group, list A jobs, and pack-years.
Interactions beyond the additive model between smoking and occupational silica exposure were observed for overall lung cancer (RERI = 2.34 [95% CI, 1.85–2.83]), adenocarcinoma (RERI = 0.70 [95% CI, 0.26–1.15]), squamous cell carcinoma (RERI = 4.86 [95% CI, 3.63–6.09]), and small cell carcinoma (RERI = 5.13 [95% CI, 3.03–7.23]) (Tables 6 and 7). Supermultiplicative joint effect of smoking and silica exposure was observed on overall lung cancer risk (P < 0.01). OR point estimates also suggest supermultiplicative interactions for risks of adenocarcinoma and squamous cell carcinoma, though these effect estimates were associated with higher uncertainties (P = 0.17 and P = 0.23, respectively) because of smaller sample sizes.
Table 6.
Interactions between Occupational Silica Exposure and Smoking for All Lung Cancers
| Exposure Status | All Lung Cancers |
|||
|---|---|---|---|---|
| Control Subjects (n) | Cases (n) | OR* | 95% CI | |
| Never-smoker and never silica | 5,900 | 1,121 | 1.0 | Referent |
| Never-smoker and ever silica | 1,253 | 248 | 1.02 | 0.87–1.19 |
| Ever-smoker and never silica | 10,577 | 10,857 | 6.37 | 5.91–6.87 |
| Ever-smoker and ever silica | 3,235 | 4,675 | 8.72 | 8.0–9.52 |
| P value multiplicative interaction | <0.01 | |||
| RERI | 2.34 | 1.85–2.83 | ||
Definition of abbreviations: CI = confidence interval; OR = odds ratio; RERI = relative excess risks due to interaction.
OR adjusted for sex, study, age group, and list A jobs.
Table 7.
Interactions between Occupational Silica Exposure and Smoking for Major Lung Cancer Subtypes
| Exposure Status | Adenocarcinoma |
Squamous Cell Carcinoma |
Small Cell Carcinoma |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (n) | OR* | 95% CI | Cases (n) | OR* | 95% CI | Cases (n) | OR* | 95% CI | |
| Never-smoker and never silica | 589 | 1.0 | Referent | 195 | 1.0 | Referent | 82 | 1.0 | Referent |
| Never-smoker and ever silica | 111 | 1.01 | 0.81–1.24 | 62 | 1.22 | 0.90–1.62 | 29 | 1.49 | 0.96–2.27 |
| Ever-smoker and never silica | 3,094 | 3.90 | 3.52–4.32 | 4,057 | 11.0 | 9.47–12.8 | 1,779 | 13.6 | 10.9–17.3 |
| Ever-smoker and ever silica | 958 | 4.61 | 4.06–5.23 | 2,189 | 16.1 | 13.7–18.9 | 840 | 19.2 | 15.3–24.7 |
| P value multiplicative interaction | 0.17 | 0.23 | 0.80 | ||||||
| RERI | 0.70 | 0.26–1.15 | 4.86 | 3.63–6.09 | 5.13 | 3.03–7.23 | |||
For definition of abbreviations, see Table 6.
OR adjusted for sex, study, age group, and list A jobs.
Lung cancer ELRs were 0.22%, 0.45%, and 0.96% for workers exposed to 0.025, 0.05, and 0.1 mg/m3 of silica, respectively.
Discussion
In a large, international, pooled case–control study with more than 16,000 lung cancer cases, we found increases in lung cancer risks associated with continuous silica cumulative exposure as well as different categorical exposure metrics, including ever exposure, longer exposure duration, and higher cumulative exposure.
Positive associations between occupational silica exposure and lung cancer have been reported mainly in industrial cohorts. In a pooled analysis of 10 silica-exposed industrial cohorts, Steenland and colleagues reported a lung cancer risk increase of 1.07 for every unit increase in log-transformed cumulative silica exposure in milligram per cubic meter years with zero lag (6). The corresponding risk increase reported by Liu and colleagues in a cohort of 34,018 workers in China was 1.06 (7). These estimates were very similar to the result from our analysis with log-cumulative exposure (OR, 1.05). Results from our corresponding spline analyses were consistent with the exposure–response relationships observed in the linear cumulative exposure logistic models; monotonic risk increases were observed for lung cancer and its subtypes.
Our results showed that silica is associated with lung cancer at very low cumulative exposures with no apparent threshold at concentrations investigated. ORs were 1.15 and 1.33 for our two lowest exposed groups, which had median cumulative exposures of 0.22 and 0.73 mg/m3-years, respectively. Few other studies quantified lung cancer risks at levels near or below 1 mg/m3-year. A meta-analysis with data from 19 studies calculated a pooled risk estimate of 1.19 (95% CI, 1.01–1.39) for workers with a median cumulative exposure of 0.42 mg/m3-years (34). Liu and colleagues reported an OR point estimate of 1.12 (1.26 with 25 yr lag) for Chinese workers in the lowest exposed group with median exposure of 0.56 mg/m3-years (7). However, results by Sogl and coworkers, who assessed silica exposure in German uranium mines using a measurement-based JEM, observed no lung cancer effects below cumulative silica exposures of 10 mg/m3-years (8).
For some carcinogens and related cancers, there is good evidence that disease relative risks after cessation of exposure are below unity when compared with groups with continued exposure (e.g., cigarette smoking) (35, 36). We tested whether such a pattern was present in our population using time-since-exposure categories. We observed results suggesting that higher lung cancer risks were associated with more recent silica exposure. To our knowledge, this is the only study that included this metric for silica exposure and more evidence is needed to support this finding.
Whether silicosis is a prerequisite for silica-related lung cancer had been a topic of debate, primarily because results from earlier studies failed to support a consistent association between silica and lung cancer after excluding subjects with silicosis (14, 15). A number of more recent studies set up analyses specifically to address this issue and reported evidence of a positive relationship between silica exposure and lung cancer without clinical silicosis (7, 8, 10, 34, 37, 38). Results from our restricted analysis of subjects without silicosis similarly support a direct association between silica and lung cancer. Although underreporting of silicosis owing to self-reports by the index subject or proxy was possible in our study, the effects observed were unlikely to be caused solely by the misclassification of silicosis owing to the rarity of the condition in the general population.
Our findings suggest that lung squamous cell and small cell carcinomas are more strongly associated with silica exposure than lung adenocarcinoma. Research on lung cancer subtypes related specifically to silica exposure is rare. Two other large case–control studies in Europe and Canada similarly reported increased risks for all three major subtypes in relation to silica exposure, with the strongest association observed in squamous cell carcinoma (9, 11). A large case–control study in Italy found elevated risk only for squamous cell and small cell carcinomas but not for adenocarcinoma (10). Most subjects in the three aforementioned studies, however, were also included in the current study and represented approximately 35% of our total participants.
Increases in overall lung cancer risk with increasing cumulative exposure were found regardless of smoking status. Our findings are in accordance with those from Liu and colleagues, in which never-smokers with cumulative silica exposure >1.12 mg/m3-years had a lung cancer hazard ratio of 1.60 (95% CI, 1.01–2.55) (7). Ours is the first study to report an exposure–response between cumulative exposure to silica and lung cancer among never-smokers. Superadditive interactions of silica exposure and cigarette smoking were observed for overall lung cancer as well as all three major subtypes. Supermultiplicative interaction was also observed for all lung cancers combined. One other study reported a superadditive joint effect of silica exposure and smoking on lung cancer (7), and one reported no evidence for a joint effect beyond the multiplicative model (9).
Our study population comprised a large number of cases exposed to silica (n = 4,923) and allowed for stratification and interaction analyses for different cancer subtypes and risk factors. Despite having a large study population, our power to investigate silica exposure-related cancer risks in women were limited. This is because the number of exposed cases in women (n = 274) was much smaller than those in men (n = 4,649). Analyses restricted to females showed imprecise results with OR point estimates that were generally >1 (see Table E3a). Male-specific results are also available in Table E3b.
We performed quantitative exposure assessment specific for exposure to quartz silica, which allowed for quantification of exposure–disease risks and exploration of the shape of the exposure–response curves in a population-based case–control setting. However, our estimates of silica exposure may be affected by exposure misclassification and less accurate than some industrial cohort–based studies, particularly those with detailed work history and extensive historical silica measurements. This misclassification was likely to be nondifferential with respect to case status and would result in a bias of risk estimates toward the null. Owing to sparse measurement data for years before 1960 in our JEM, we assumed in backward extrapolation that silica exposure did not further increase in years before 1960. In a previous publication we have explored different time-trend assumptions in our exposure model (19). Naturally, the assigned silica exposures in the population (and the slope of the exposure–response) would vary if different time-trend assumptions were made, but these changes have little effect on the exposure status and ranking of cumulative exposure among our study population. When we restricted our categorical exposure model to include only subjects who started work after 1960 (see Table E4.2), the silica lung cancer exposure–response in general and, more specifically, elevated lung cancer risks associated with lower categories of cumulative silica exposure were still observed.
Our study included more complete information on individual covariates than most industry-based studies, which allowed for the control of important potential confounders such as smoking and exposures to other occupational lung carcinogens in our models. As an alternative to adjusting for coexposures to other lung carcinogens with ever employment in list A jobs, we performed a sensitivity analysis controlling for Domtoren-JEM–assessed ever exposure to diesel engine exhaust, hexavalent chromium, asbestos, and polycyclic aromatic hydrocarbons in our categorical exposure model. Results of this analysis (see Table E4.4) were very similar compared with our main results. The associations we observed between silica and lung cancer were also robust in other sensitivity analyses with different subgroups (see online supplement Methods, Results, and Tables E4.1–4.5).
Current definitions of “tolerable” ELR owing to occupational exposure to carcinogens vary by jurisdiction, ranging from the 0.4% in the Netherlands and Germany to 0.1% generally accepted by the U.S. Occupational Safety and Health Administration (39–41). According to our calculations, lifetime occupational silica exposure at 0.05 and 0.1 mg/m3 would result in respective lung cancer ELRs of 0.45% and 0.96%, which clearly exceed this range of tolerable risks. Lifetime silica exposure at 0.025 mg/m3 would result in approximately two lung cancers in 1,000 workers, which falls below the Dutch/German limit of 0.4% but above the U.S. limit of 0.1%. Other studies have estimated similar lung cancer ELRs at low levels of silica exposure, with one study estimating an ELR of 0.23% to 0.48% for workers exposed to 0.07 mg/m3 of silica and another estimating an ELR of 0.2% to 0.3% for workers with an exposure level of 0.01 mg/m (3, 6, 7). The ELR findings from other studies and ours suggest that lower occupational silica exposure limits may be considered to protect exposed workers from excess lung cancer risks. Lastly, because our exposure assessment was specific for quartz silica and did not include other forms of silica, our ELR may not reflect risks from exposures to other forms of crystalline silica such as cristobalite and tridymite. However, because quartz is by far the most common form of crystalline silica, exposure prevalence and disease burden associated with other crystalline silica polymorphs are likely to be much smaller than those associated with quartz exposure (42).
Conclusions
In a large pooled analysis of lung cancer case–control studies, we observed a positive association and exposure–response relationship between occupational silica exposure and lung cancer. The exposure–disease association was consistent regardless of tobacco smoking history or silicosis status. Silica-exposed workers had higher risks for all investigated lung cancer subtypes; risks were higher for squamous cell and small cell carcinomas than for adenocarcinoma. Our findings support efforts to further reduce occupational exposure to silica for the protection of exposed workers against risks of developing lung cancer.
Footnotes
Supported by the German Social Accident Insurance (DGUV). The project is coordinated by the International Agency for Research on Cancer (IARC), the Institute for Prevention and Occupational Medicine of the DGUV, Institute of the Ruhr-University Bochum (IPA), and the Institute for Risk Assessment Sciences (IRAS) at Utrecht University. Where authors are identified as personnel of the IARC/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the IARC/World Health Organization. V.H. is grateful for support provided by the Cancer Research Society, Fonds de Recherche du Québec–Santé (FRQS), and Ministère de l'Économie, de la Science et de l'Innovation du Québec (MESI). H.K. has received funding from the Industrial Minerals Association (IMA)-Europe since 2006 to maintain and analyze the IMA-Dust Monitoring Program (DMP) exposure database. H.K. also recently received funding through an EU grant to work on a standard monitoring protocol for the European Network for Silica (NEPSI). Neither the IMA-DMP data nor NEPSI collaboration were involved in or influenced the design, analysis, and result interpretation of the current project. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Author Contributions: C.G., S.P., A.O., L.P., J. Schüz, J.A., K.S., H.K., and R.V. contributed significantly in data analysis, results interpretation, and original drafting of the work. T. Brüning, K.S., H.K., and R.V. contributed to the original conception of the project and secured project funding. T. Behrens, B.P., B.K., W.A., V.B., S.B., P.B., B.B.-d.-M., N.C., D.C., P.D., E.F., G.F.-T., J.F., F.F., L.F., P. Guénel, P. Gustavsson, V.H., V.J., K.-H.J., S.K., M.T.L., J.L., D.L., D. Mates, J.M., F.M., D. Mirabelli, N.P., H.P., L.R., P.R., J. Siemiatycki, B.Ś., A.T., H.-E.W., D.Z., T. Brüning, K.S., H.K., and R.V. participated in data acquisition and data analysis design of the project. All authors participated in critical revision of the manuscript and provided approval of the finalized submitted version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201910-1926OC on April 24, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. World Health Organization. Elimination of silicosis. The Global Occupational Health Network Newsletter. Geneva, Switzerland: World Health Organization Department of Public Health and Environment, Occupational and Environmental Health Programme; 2007. Issue No. 12–2007.
- 2.Rushton L. Chronic obstructive pulmonary disease and occupational exposure to silica. Rev Environ Health. 2007;22:255–272. doi: 10.1515/reveh.2007.22.4.255. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. Silica dust, crystalline, in the form of quartz or cristobalite. Lyon, France: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100C. International Agency for Research on Cancer; 2012 [accessed 2020 Jul 7]. Available from: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100C-14.pdf.
- 4.U.S. National Toxicological Program. Silica, crystalline (respirable size): report on carcinogens, 14th ed. Research Triangle Park, NC.: National Toxicology Program; 2016. [Google Scholar]
- 5.U.S. National Institute for Occupational Safety and Health. Health effects of occupational exposure to respirable crystalline silica. NIOSH Hazard Review. Cincinnati, OH: NIOSH—Publications Dissemination; 2002. DHHS (NIOSH) Publication No. 2002–129.
- 6.Steenland K, Mannetje A, Boffetta P, Stayner L, Attfield M, Chen J, et al. International Agency for Research on Cancer. Pooled exposure-response analyses and risk assessment for lung cancer in 10 cohorts of silica-exposed workers: an IARC multicentre study. Cancer Causes Control. 2001;12:773–784. doi: 10.1023/a:1012214102061. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Steenland K, Rong Y, Hnizdo E, Huang X, Zhang H, et al. Exposure-response analysis and risk assessment for lung cancer in relationship to silica exposure: a 44-year cohort study of 34,018 workers. Am J Epidemiol. 2013;178:1424–1433. doi: 10.1093/aje/kwt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sogl M, Taeger D, Pallapies D, Brüning T, Dufey F, Schnelzer M, et al. Quantitative relationship between silica exposure and lung cancer mortality in German uranium miners, 1946-2003. Br J Cancer. 2012;107:1188–1194. doi: 10.1038/bjc.2012.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassidy A, ’t Mannetje A, van Tongeren M, Field JK, Zaridze D, Szeszenia-Dabrowska N, et al. Occupational exposure to crystalline silica and risk of lung cancer: a multicenter case-control study in Europe. Epidemiology. 2007;18:36–43. doi: 10.1097/01.ede.0000248515.28903.3c. [DOI] [PubMed] [Google Scholar]
- 10.De Matteis S, Consonni D, Lubin JH, Tucker M, Peters S, Vermeulen RCh, et al. Impact of occupational carcinogens on lung cancer risk in a general population. Int J Epidemiol. 2012;41:711–721. doi: 10.1093/ije/dys042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vida S, Pintos J, Parent M-E, Lavoué J, Siemiatycki J. Occupational exposure to silica and lung cancer: pooled analysis of two case-control studies in Montreal, Canada. Cancer Epidemiol Biomarkers Prev. 2010;19:1602–1611. doi: 10.1158/1055-9965.EPI-10-0015. [DOI] [PubMed] [Google Scholar]
- 12.Steenland K, Ward E. Silica: a lung carcinogen. CA Cancer J Clin. 2014;64:63–69. doi: 10.3322/caac.21214. [DOI] [PubMed] [Google Scholar]
- 13.Manno M, Levy L, Johanson G, Cocco P. Silica, silicosis and lung cancer: what level of exposure is acceptable? Med Lav. 2018;109:478–480. doi: 10.23749/mdl.v109i6.7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Checkoway H, Franzblau A. Is silicosis required for silica-associated lung cancer? Am J Ind Med. 2000;37:252–259. doi: 10.1002/(sici)1097-0274(200003)37:3<252::aid-ajim2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Kurihara N, Wada O. Silicosis and smoking strongly increase lung cancer risk in silica-exposed workers. Ind Health. 2004;42:303–314. doi: 10.2486/indhealth.42.303. [DOI] [PubMed] [Google Scholar]
- 16.Olsson AC, Gustavsson P, Kromhout H, Peters S, Vermeulen R, Brüske I, et al. Exposure to diesel motor exhaust and lung cancer risk in a pooled analysis from case-control studies in Europe and Canada. Am J Respir Crit Care Med. 2011;183:941–948. doi: 10.1164/rccm.201006-0940OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters S, Vermeulen R, Portengen L, Olsson A, Kendzia B, Vincent R, et al. SYN-JEM: a quantitative job-exposure matrix for five lung carcinogens. Ann Occup Hyg. 2016;60:795–811. doi: 10.1093/annhyg/mew034. [DOI] [PubMed] [Google Scholar]
- 18.Peters S, Vermeulen R, Portengen L, Olsson A, Kendzia B, Vincent R, et al. Modelling of occupational respirable crystalline silica exposure for quantitative exposure assessment in community-based case-control studies. J Environ Monit. 2011;13:3262–3268. doi: 10.1039/c1em10628g. [DOI] [PubMed] [Google Scholar]
- 19.Peters S, Kromhout H, Portengen L, Olsson A, Kendzia B, Vincent R, et al. Sensitivity analyses of exposure estimates from a quantitative job-exposure matrix (SYN-JEM) for use in community-based studies. Ann Occup Hyg. 2013;57:98–106. doi: 10.1093/annhyg/mes045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters S, Vermeulen R, Cassidy A, Mannetje A, van Tongeren M, Boffetta P, et al. INCO Group. Comparison of exposure assessment methods for occupational carcinogens in a multi-centre lung cancer case-control study. Occup Environ Med. 2011;68:148–153. doi: 10.1136/oem.2010.055608. [DOI] [PubMed] [Google Scholar]
- 21.International Labour Organization. ISCO-International standard classification of occupations: brief history. 2010 [accessed 2018 Jul 20] Available from: http://www.ilo.org/public/english/bureau/stat/isco/intro2.htm.
- 22.Olsson AC, Vermeulen R, Schüz J, Kromhout H, Pesch B, Peters S, et al. Exposure-response analyses of asbestos and lung cancer subtypes in a pooled analysis of case-control studies. Epidemiology. 2017;28:288–299. doi: 10.1097/EDE.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahrens W, Merletti F. A standard tool for the analysis of occupational lung cancer in epidemiologic studies. Int J Occup Environ Health. 1998;4:236–240. doi: 10.1179/oeh.1998.4.4.236. [DOI] [PubMed] [Google Scholar]
- 24.Mirabelli D, Chiusolo M, Calisti R, Massacesi S, Richiardi L, Nesti M, et al. Database of occupations and industrial activities that involve the risk of pulmonary tumors [in Italian] Epidemiol Prev. 2001;25:215–221. [PubMed] [Google Scholar]
- 25.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J Royal Stat Soc (B) 2011;73:3–36. [Google Scholar]
- 26.Rothman K, Greenland S. Modern epidemiology. Philadelphia, PA: Lippincott - Raven; 1998. [Google Scholar]
- 27.Stevenson M, Nunes T, Heuer C, Marshall J, Sanchez J, Thornton R, et al. epiR: tools for the analysis of epidemiological data. R package version 1.0-10. 2019 [accessed 2020 Jul 7]. Available from: https://CRAN.R-project.org/package=epiR.
- 28.Vermeulen R, Silverman DT, Garshick E, Vlaanderen J, Portengen L, Steenland K. Exposure-response estimates for diesel engine exhaust and lung cancer mortality based on data from three occupational cohorts. Environ Health Perspect. 2014;122:172–177. doi: 10.1289/ehp.1306880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Commission. Eurostat 2008 dataset on all causes and lung cancer mortality in European Union countries [assessed 2012 Jun 1]. Available from: https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=hlth_cd_aro&lang=en.
- 30.ACGIH. Silica, crystalline: alpha-quartz and cristobalite: TLV(R) chemical substances 7th edition documentation. Cincinnati, OH: ACGIH; 2010. [Google Scholar]
- 31.U.S. Occupational Safety and Health Administration. Small entity compliance guide for the respirable crystalline silica standard for general industry and maritime. Washington, DC: OSHA; 2017. Publication No. OSHA 3911-07 2017.
- 32.EU Parliament and Council. Brussels: European Union; 2019. Directive (EU) 2019/130 of the European Parliament and of the Council of 16 January 2019 amending directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. [Google Scholar]
- 33.R: R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019 [accessed 2020 Jul 7]. Available from: https://www.R-project.org/
- 34.Poinen-Rughooputh S, Rughooputh MS, Guo Y, Rong Y, Chen W. Occupational exposure to silica dust and risk of lung cancer: an updated meta-analysis of epidemiological studies. BMC Public Health. 2016;16:1137. doi: 10.1186/s12889-016-3791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Agency for Research on Cancer. Tobacco control: reversal of risk after quitting smoking. IARC Handbooks of Cancer Prevention Volume 11. Lyon, France: IARC; 2007.
- 36.Vlaanderen J, Portengen L, Schüz J, Olsson A, Pesch B, Kendzia B, et al. Effect modification of the association of cumulative exposure and cancer risk by intensity of exposure and time since exposure cessation: a flexible method applied to cigarette smoking and lung cancer in the SYNERGY Study. Am J Epidemiol. 2014;179:290–298. doi: 10.1093/aje/kwt273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Checkoway H, Hughes JM, Weill H, Seixas NS, Demers PA. Crystalline silica exposure, radiological silicosis, and lung cancer mortality in diatomaceous earth industry workers. Thorax. 1999;54:56–59. doi: 10.1136/thx.54.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taeger D, Krahn U, Wiethege T, Ickstadt K, Johnen G, Eisenmenger A, et al. A study on lung cancer mortality related to radon, quartz, and arsenic exposures in German uranium miners. J Toxicol Environ Health A. 2008;71:859–865. doi: 10.1080/15287390801987972. [DOI] [PubMed] [Google Scholar]
- 39.Health Council of the Netherlands. Diesel engine exhaust: health-based recommended occupational exposure limit. The Hague, the Netherlands.: Health Council of the Netherlands; 2019. Publication No. 2019/02 [accessed 2019 Jul 2] Available from: https://www.gezondheidsraad.nl/binaries/gezondheidsraad/documenten/adviezen/2019/03/13/dieselmotoremissie/Diesel+Engine+Exhaust.pdf.
- 40.Rodricks JV, Brett SM, Wrenn GC. Significant risk decisions in federal regulatory agencies. Regul Toxicol Pharmacol. 1987;7:307–320. doi: 10.1016/0273-2300(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 41.Ausschuss für Gefahrstoffe. TRGS 910 Risikobezogenes Maßnahmenkonzept für Tätigkeiten mit krebserzeugenden Gefahrstoffen (technical rules for hazardous substances 910: risk-based action plan for activities with carcinogenic hazardous substances [in German]). 2019 [accessed 2019 Jul 2] Available from: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/TRGS/pdf/TRGS-910.pdf?__blob=publicationFile&v=4.
- 42.International Agency for Research on Cancer. Silica, some silicates, coal dust and para-aramid fibrils. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 68. Lyon, France: International Agency for Research on Cancer; 1997 [accessed 2020 Jul 7]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK410047/


