Abstract
Porcine β-defensin 2 (PBD-2), expressed by different tissues of pigs, is a multifunctional cationic peptide with antimicrobial, immunomodulatory and growth-promoting abilities. As the latest generation of genome-editing tool, CRISPR/Cas9 system makes it possible to enhance the expression of PBD-2 in pigs by site-specific knock-in of pbd-2 gene into the pig genome. In this study, we aimed to generate marker-free pbd-2 knock-in pigs using the CRISPR/Cas9 and Cre/loxP systems. Two copies of pbd-2 gene linked by a T2A sequence were inserted into the porcine Rosa26 locus through CRISPR/Cas9-mediated homology-directed repair. The floxed selectable marker gene neoR, used for G418 screening of positive cell clones, was removed by cell-penetrating Cre recombinase with a recombination efficiency of 48.3%. Cloned piglets were produced via somatic cell nuclear transfer and correct insertion of pbd-2 genes was confirmed by PCR and Southern blot. Immunohistochemistry and immunofluorescence analyses indicated that expression levels of PBD-2 in different tissues of transgenic (TG) piglets were significantly higher than those of their wild-type (WT) littermates. Bactericidal assays demonstrated that there was a significant increase in the antimicrobial properties of the cell culture supernatants of porcine ear fibroblasts from the TG pigs in comparison to those from the WT pigs. Altogether, our study improved the protein expression level of PBD-2 in pigs by site-specific integration of pbd-2 into the pig genome, which not only provided an effective pig model to study the anti-infection mechanisms of PBD-2 but also a promising genetic material for the breeding of disease-resistant pigs.
Keywords: porcine β-defensin 2, CRISPR/Cas9, transgenic pigs, antimicrobial peptide, disease-resistant animals
1. Introduction
Different breeding techniques have been utilized to improve animal production traits including growth rate, milk yield and disease-resistance. With the rapid development of biotechnologies, transgenic (TG) techniques have been gradually applied in animal breeding. Notably, there are three major gene-editing tools used, named as zinc finger nuclease, transcription activator-like effector nuclease and CRISPR/Cas9 [1,2,3]. Events of using gene-editing tools to improve quantitative traits and welfare of animals, and to eliminate allergens in livestock products, have been well described. Previous studies showed that knock-out of myostatin gene in cattle, goats, pigs and rabbits greatly increased their muscle mass [4,5,6]. Hornless cattle were produced through integrating POLLED gene into the genome, which prevented the suffering of cattle caused by dehorning [7]. Eggs with low allergenicity were produced by gene disruptions of OVA and OVM genes in hens [8], while β-lactoglobulin-free goat milk was obtained by removing BLG gene in goats [9].
The gene-editing technologies have also been widely used to improve disease-resistance in pigs [10]. For instance, pigs expressing porcine reproductive and respiratory syndrome virus (PRRSV)-specific small interfering RNA (siRNA) displayed enhanced resistance to PRRSV infection [11]. Similarly, pigs resistant to foot-and-mouth disease and classical swine fever (CSF) were obtained by inserting a corresponding siRNA expression cassette into the pig genome, respectively [12,13]. The deletion of CD163 SRCR5 domain conferred resilience to PRRSV in pigs [14], while pigs lacking aminopeptidase N acquired insusceptibility to transmissible gastroenteritis virus infection [15]. In addition, it has been identified that overexpression of porcine β-defensin 2 (PBD-2) and histone deacetylase 6 can protect pigs from Actinobacillus pleuropneumoniae and PRRSV infection, respectively [16,17]. Fibroblasts isolated from pigs overexpressing Mx1 were less vulnerable to influenza A virus and CSF virus infection [18], and cells from MxA TG pigs could suppress CSF virus replication [19].
Mammal defensins are the major group of cationic host defense peptides which are classified as three subfamilies, α-, β- and θ-defensins, according to their intramolecular disulfide bond pattern [20]. In terms of defensins in pigs, β-defensin is the only subgroup that has been found, with 27 functional β-defensins identified [21,22]. PBD-2 was first discovered by sequence similarity analysis with the well characterized porcine β-defensin 1 [23] and was proved to exist in different organs in pigs [16]. It has been shown that PBD-2 possesses antimicrobial abilities against a broad range of bacteria, both Gram-positive and Gram-negative [24]. The antibacterial mechanism of PBD-2 was elucidated in Escherichia coli, being disruption of the membrane integrity and affecting the DNA transcription and translation when PBD-2 entered into the cytoplasm [25]. Previous studies described that PBD-2 could hamper PRRSV replication in vitro [26] and suppress proliferation of pseudorabies virus both in vitro and in TG mice [27]. Besides, PBD-2 has been identified to alleviated inflammation by binding toll-like receptor 4 and inhibiting the subsequent NF-κB activation [28]. Additionally, PBD-2 has been used as a feed additive to improve growth performance, and to prevent post-weaning diarrhea in piglets [29]. Taken together, the pbd-2 gene could be a promising candidate to generate disease-resistant TG animals.
Although pigs overexpressing PBD-2 have been produced by random gene integration in our previous study, a neomycin-resistance (neoR) gene has also been introduced into the pig genome [16]. Given that the CRISPR/Cas9 system is recognized as one of the most efficient tools for precise gene modifications in mammals [30,31], this study aimed to increase the expression level of PBD-2 in pigs by marker-free knock-in of pbd-2 gene into the porcine Rosa26 (pRosa26) locus, a safe harbor for ubiquitous expression of exogenous genes [32]. The resulting TG pigs would greatly improve resilience of pigs to infectious diseases, providing a potent genetic material for the breeding of disease-resistant pigs.
2. Materials and Methods
2.1. Cells, Bacterial Strains and Animals
Porcine fetal fibroblasts (PFFs) and porcine ear fibroblasts (PEFs) were grown in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 20% fetal bovine serum (FBS; Thermo Fisher Scientific), while PK-15 cells (ATCC Number: CCL-33) were maintained in DMEM supplemented with 10% FBS. Streptococcus suis strain SC19 was cultured in tryptic soy broth (TSB; BD, Franklin Lakes, NJ, USA) with 5% newborn calf serum (NBCS; TIANHANG, Huzhou, China) and on tryptic soy agar (TSA; BD) with 5% NBCS. A. pleuropneumoniae strain 4074 was grown in TSB with 5% NBCS and 10 μg/mL of nicotinamide adenine dinucleotide (NAD; Sigma-Aldrich, St. Louis, MO, USA) and on TSA with 5% NBCS and NAD. Experiments involving pigs were performed in accordance with the Hubei Regulations for the Administration of Affairs Concerning Experimental Animals and were approved by the Scientific Ethical Committee for Experimental Animals of Huazhong Agricultural University, Wuhan, China (HZAUSW-2019-010).
2.2. Plasmids
An efficient Kozak sequence (5′-GCCACC-3′) was added upstream of the ATG start codon of the pbd-2 gene in pcCAG-PBD2 vector constructed in our previous study [16], with the resulting vector named as pcCAG-nPBD2 (Figure S1A). To evaluate whether the 2A peptide system could be efficient in linking two copies of pbd-2 gene, pcCAG-PBD2-T2A-PBD2 was produced, containing two copies of pbd-2 gene linked by a T2A peptide sequence (Figure S1B). A 5′ homology arm (HA) of approximately 0.9 kb and a 3′ HA of approximately 0.76 kb was cloned into the SpeI and BstZ17I site of pcCAG-PBD2-T2A-PBD2, respectively. Subsequently, two loxP sites with the same orientation were inserted, flanking the selectable marker gene (SMG) neoR. The resulting vector was designated as pcCAG-R26-PBD2-T2A-PBD2 (Figure 1A). The pX330-pRosa26 vector (Figure S2) provided by Professor Bo Zuo (Huazhong Agricultural University) was used to express Cas9 and sgRNA targeting the pRosa26 locus (5′-GCTCCTTCTCGATTATGGGC-3′).
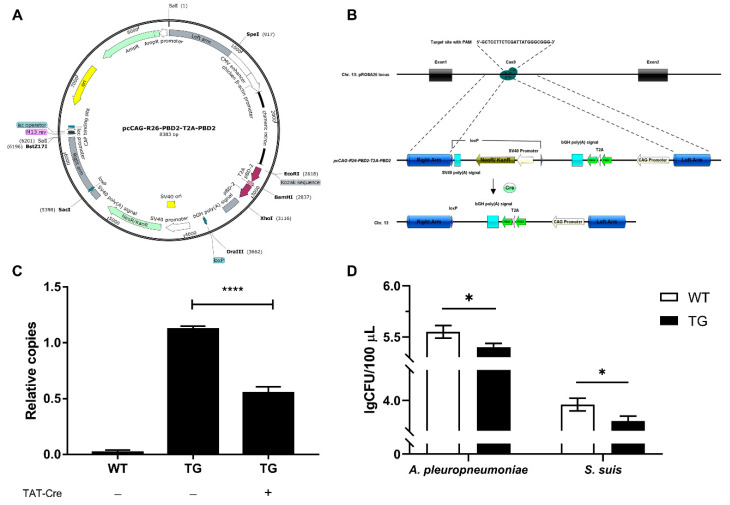
Figure 1.
Site-specific pbd-2 knock-in at the porcine Rosa26 (pRosa26) locus. (A) Map of the donor plasmid pcCAG-R26-PBD2-T2A-PBD2 for the site-specific pbd-2 knock-in. (B) Scheme for marker-free targeted pbd-2 integration in porcine fetal fibroblasts (PFFs) via CRISPR/Cas9-mediated homology-directed repair. The floxed selectable marker gene (SMG) was removed after treatment of Cre recombinase. (C) Relative copy number of the SMG neoR in wild-type (WT) PFFs, transgenic (TG) PFFs and Cre-recombinase-treated TG PFFs. (D) The bactericidal activities of cell culture supernatants of WT PFFs and TG PFFs on Actinobacillus pleuropneumoniae and Streptococcus suis quantified by bacterial counting. Data are presented as mean ± SD and are plotted from three independent experiments. * p < 0.05, **** p < 0.0001, unpaired one tailed Student′ s t-test.
2.3. Transfection of PK-15 Cells
At 80% confluency, PK-15 cells were respectively transfected with pcCAG-nPBD2 and pcCAG-PBD2-T2A-PBD2 using the Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s instruction. After 24 h the medium was replaced with fresh DMEM containing 1% FBS. The cell culture supernatant was collected 48 h later for the subsequent bactericidal assay against A. pleuoropneumoniae.
2.4. Bactericidal Assay
A 10 μL aliquot of S. suis (5000 CFU) or A. pleuropneumoniae (5000 CFU) was incubated with 90 μL of cell culture supernatants at 37 °C for 1 h, respectively. The mixture was then serially diluted (1:100 and 1:1000 dilution) in DPBS (Thermo Fisher Scientific) and spread onto TBA plates. The plates were left at 37 °C overnight and colonies were counted and analyzed. Bacteria incubated with DPBS was used as a blank control, while those incubated with 60 μg/mL of the synthetic mature PBD-2 (DHYICAKKGGTCNFSPCPLFNRIEGTCYSGKAKCCIR) (ChinaPeptides, Shanghai, China) diluted in DPBS served as a positive control.
2.5. Isolation of Genomic DNA
Cells or pig ear tissues were incubated in 500 μL of lysis buffer (10 mM Tris, pH 8.0; 10 mM NaCl; 10 mM EDTA, pH 8.0; 10% SDS; 400 μg/mL Proteinase K) at 56 °C for 8 h. After 200 μL of saturated NaCl was added, tubes were gently shaken for 5 min before centrifugation 13,000× g for 10 min. The supernatant was then transferred to a new tube and equal volume of isopropanol (chilled at −20 °C) was added. The mixture was incubated at −20 °C for another 2 h and was subjected to centrifugation 13,000× g for 10 min. The supernatant was discarded and the precipitate was washed with 70% ethanol. The tube was then centrifugated 13,000× g for another 10 min and the supernatant was removed. After air dry at room temperature (RT) for 10 min, the resulting DNA was resuspended in pre-heated ddH2O and the concentration was measured using a spectrophotometer (Thermo Fisher Scientific).
2.6. Electroporation and Selection of PFFs
PFFs were cultured in a 100-mm dish for two days to reach 80% confluency. After washing with DPBS twice, PFFs were detached from the dish using 0.25% Trypsin-EDTA (Thermo Fisher Scientific) and were centrifugated at 1000× g for 5 min. The cell pellet was suspended in 800 μL of Gene Pulser Electroporation Buffer (Bio-Rad, Hercules, CA, USA) and 32 μg of pcCAG-R26-PBD2-T2A-PBD2 and pX330-pRosa26 each were added and gently mixed. Cell suspension was added into a 0.2 cm-gap Gene Pulser Electroporation Cuvettes (200 μL) for the subsequent electroporation using the Gene Pulser Xcell system (Bio-Rad) and was left recovery for 24 h. Following selection in 400 μg/mL of G418 for two weeks, single colonies were picked and transferred onto a 48-well plate. As the cells reached confluency, half of them were subjected to genomic DNA isolation which was later used as the PCR template for genotyping. The site-specific knock-in events were identified by PCR using three primer pairs (Table S1) and the positive cell clone was then chosen for the removal of a SMG.
2.7. Removal of Selectable Marker
When cells reached about 60% confluency, cells were treated with 3 μM TAT-Cre recombinase (Merck Millipore, Burlington, MA, USA) in DMEM without the presence of antibiotics and FBS. After 3 h of incubation at 37 °C, the medium was removed and the cells were washed twice with PBS. The treated cells were grown in fresh medium and 50% of them were subjected to somatic cell nuclear transfer (SCNT). The remaining cells were used to determine the recombination efficiency by real-time quantitative PCR (RT-qPCR). Briefly, genomic DNA from wild-type (WT) cells, treated and untreated TG cells was extracted and subjected to RT-qPCR using SYBR® Green Realtime PCR Master Mix (TOYOBO, Osaka, Japan). The relative copy number of the SMG neoR was analyzed using the 2−ΔΔCt method [33], with β-actin as the reference gene. Specific primers for neoR are NeoR-F (5′-GCCCCATGGCTGACTAATTTTTTTT-3′) and NeoR-R (5′-CGATTGTCTGTTGTGCCCAGTC-3′), while primers for β-actin are ACTB-F (5′-GCCTCTCGTCTTGCTTGTTTTAAA-3′) and ACTB-R (5′-AGCAAGTGAGGGCGTATCCAG-3′). Recombination efficiency = (mean value of treated TG group − mean value of WT group)/(mean value of untreated TG group − mean value of WT group) × 100%. Meanwhile, the cell culture supernatants of WT cells and treated TG cells were collected to measure the bactericidal activity against A. pleuoropneumoniae and S. suis as described above.
2.8. Generation and Genotype Analysis of Cloned Piglets
After removal of the SMG, positive PFFs were subjected to the procedure of SCNT by ViaGen Animal Breeding Resources Development Company (Wuhan, China) in accordance with the protocol described elsewhere [34]. There were eight surrogate sows used and each was transferred with 250 reconstructed embryos.
The resulting piglets were identified by PCR. Genomic DNA was obtained from pig ear tissues of the newborn piglets as described above. Four pairs of primers (Table 1) were used to identify marker-free TG pigs and the PCR products were then sequenced for further confirmation. The Southern blot analysis was performed to confirm a site-specific transgene integration into the pig genome. Briefly, genomic DNA of high-quality was isolated from the PEFs using the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) and digested with XhoI. The digested DNA was electrophoretically separated on an agarose gel and then transferred onto a nylon membrane. The probe labeled with digoxigenin was hybridized to a single DNA fragment on the membrane, indicating a site-specific insertion of duplicate pbd-2 genes.
Table 1.
Information of primer pairs for genotype analysis of cloned pigs.
| Fragment | Primer | Sequence (5′-3′) | Length (bp) |
|---|---|---|---|
| PBD-2 | vTG-F | GCTGGTTGTTGTGCTGTCTCATCA | 547 |
| vTG-R | CCCTCTAGACTCGAGTCAGGGTCAGC | ||
| On-target | OT-F | CTTCCTTTCTCGCCACGTTC | 1235 |
| OT-R | TCGGTAAATAGCAATCAACTCAG | ||
| NeoR | NeoR-F | GCCCCATGGCTGACTAATTTTTTTT | 266 |
| NeoR-R | CGATTGTCTGTTGTGCCCAGTC | ||
| β-actin | Control-F | GCCTCTCGTCTTGCTTGTTTTAAA | 169 |
| Control-R | AGCAAGTGAGGGCGTATCCAG |
2.9. Immunofluorescent and Immunohistochemical Analysis for PBD-2
PEFs were isolated from the ear tissue of cloned pigs and were subjected to the subsequent immunofluorescent analysis. Same amounts of WT and TG PEFs (10,000 cells each) were firstly grown on coverslips for 24 h before being rinsed in PBS, then cells were incubated in chilled acetone (−20 °C) for 10 min. The coverslips were washed three times with ice-cold PBS before being blocked with 1% BSA, 22.52 mg/mL glycine in PBST (PBS+ 0.1% Tween 20) for 30 min. The PEFs were then incubated in self-made PBD-2 mouse monoclonal antibody [16] diluted in 1% BSA in PBST at RT for 1 h. Subsequently, cells were washed five times with PBST for 5 min each and were then incubated with FITC-labeled Goat Anti-Mouse IgG (H+L) (Abclonal, Wuhan, China) diluted at 1:100 in 1% BSA in PBST for 1 h without exposure to light. After that, cells were washed five times with PBST for 5 min each in the dark and then were stained with 4′,6-diamidino-2-phenylindole (Beyotime, Shanghai, China). The results were observed using the EVOS FL Auto Imaging System (Thermo Fisher Scientific).
Besides, a TG founder pig and a WT littermate were both sacrificed to harvest their heart, liver, spleen, lung, kidney, and brain tissues. Tissues were fixed in PBS-buffered 4% formaldehyde followed by paraffin embedding and sectioning. The sections were then subjected to immunohistochemical analysis to detect PBD-2 in different organs. Mouse anti-2A peptide monoclonal antibodies (Novus Biologicals, Littleton, CO, USA) and HRP-conjugated goat anti-mouse IgG were used as primary and secondary antibodies, respectively. PBD-2 was detected after diaminobenzidine staining as a brown coloration.
2.10. Transcriptional Analysis of pbd-2 Gene in TG Pigs Using RT-qPCR
Total RNA from heart, liver, spleen, lung, kidney and brain of the TG and WT pigs was extracted using RNAiso Plus (TAKARA, Dalian, China). The obtained RNA of 500 ng from different organs was then reverse-transcribed into cDNA for the subsequent RT-qPCR to measure the relative mRNA levels of pbd-2, with GAPDH as the reference gene. Specific primers for pbd-2 are tPBD2-F (5′-AGAGGGCAGAGGAAGTCTGCTAA-3′) and tPBD2-R (5′-TTTAAACGGGCCCTCTAGACTCGA-3′), while primers for GAPDH are GAPDH-F (5′-ACCCAGAAGACTGTGGATGGC-3′) and GAPDH-R (5′-AGCCAGAGGCAAAGTGATAGATA-3′).
2.11. Off-Target Analysis
Potential off-target sites in pig genome were predicted using an online tool Cas-OFFinder [35]. The maximal mismatch number was set as three, with PAM type being 5′-NGG-3′. After that, fragments containing predicted off-target sites were PCR amplified using primer pairs in Table S2 and subjected to Sanger sequencing for sequence alignment.
2.12. Statistical Analysis
Data were analyzed with GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA) using unpaired one-tailed Student’s t-test and shown as mean ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
3. Results
3.1. Enhanced Bactericidal Activity by T2A-Linked Dual pbd-2 Expression
To achieve simultaneous expression of two copies of pbd-2 gene, a T2A self-cleaving peptide sequence was put between two pbd-2 genes in pcCAG- PBD2-T2A-PBD2 (Figure S1B). Cell culture supernatants from PK-15 cells transfected with pcCAG-PBD2-T2A-PBD2 and the control vector pcCAG-nPBD2 carrying a single copy of pbd-2 (Figure S1A) were collected and incubated with the same number of A. pleuropneumoniae for 1 h, respectively. As shown in Figure S1C, the number of surviving bacteria after incubating with cell culture supernatant of cells transfected with pcCAG-PBD2-T2A-PBD2 was significantly less than that with pcCAG-nPBD2, indicating that T2A-mediated dual pbd-2 expression could significantly increase the bactericidal activity of cell culture supernatants.
3.2. CRISPR/Cas9 and Cre/loxP-Mediated Marker-Free Site-Specific Insertion of pbd-2 in PFFs
The pcCAG-R26-PBD2-T2A-PBD2 (Figure 1A) along with pX330-pRosa26 (Figure S2) were co-electroporated into PFFs to achieve the integration of dual pbd-2 genes, as well as a SMG neoR, into the pRosa26 site. After G418 screening, positive cell colonies was identified by PCR (Figure S3) and the treatment of TAT-Cre recombinase significantly decreased the copy number of neoR in TG cells (Figure 1B,C), reaching a recombination efficiency of 48.3% as calculated in accordance with the method described above. In the meantime, the cell culture supernatant of treated TG cells displayed significant increase in the bactericidal activity against both S. suis and A. pleuropneumoniae (Figure 1D). These indicated that 48.3% of the TG cells were marker-free and exerted enhanced antibacterial activity, suggesting an increase in PBD-2 expression in the treated TG cells, which could be used for the subsequent SCNT.
3.3. Genotyping of Cloned Piglets
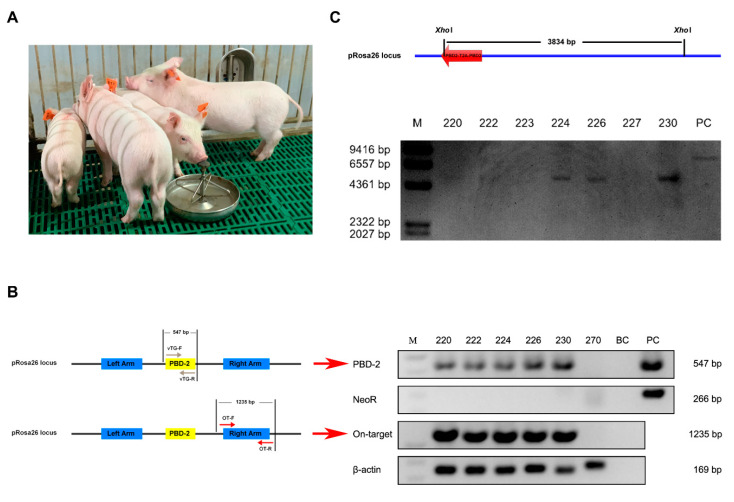
After transferring 250 reconstructed embryos into the uterus of each recipient, there were three surrogate sows successfully giving birth to seven piglets in total. All the cloned piglets had normal physical appearance when compared with WT pigs (Figure 2A). Regarding identification of TG pigs based on PCR results, the observed bands for the PBD-2 and On-target fragments represented a site-specific pbd-2 knock-in at the pRosa26 locus, while no band for the NeoR fragment indicated that the SMG neoR was not introduced into the pig genome. As shown in Figure 2B, there were five pigs harboring a site-specific pbd-2 at the pRosa26 locus, with no SMG detected. The on-target integration of pbd-2 in TG pigs was validated by Sanger sequencing analysis on the resulting PBD-2 and On-target fragments (Figure S4). The Southern blot analysis was carried out to further confirm a targeted pbd-2 insertion into the pig genome. Consistent with the PCR results, there were intended bands for pigs numbered as 220, 222, 224, 226 and 230 (Figure 2C), indicating a site-specific integration of duplicate pbd-2 genes in these pigs. Together, we obtained five marker-free pigs with the pbd-2 gene site-specifically incorporated at the pRosa26 locus.
Figure 2.
Genotyping of cloned piglets. (A) Physical appearance of cloned piglets. (B) PCR analysis to identify marker-free site-specific pbd-2 knock-in pigs. PBD-2: Amplification for the dual pbd-2 gene using primers vTG-F and vTG-R; NeoR: Amplification for the SMG neoR; Off-target: Amplification for the fragment which represents on-target insertion of the transgene using primers OT-F and OT-R; β-actin: Amplification for β-actin; Lane 220–227: Numbers for pigs; BC: Blank control; PC: Positive control. (C) Southern blot analysis for the identification of TG pigs. Genomic DNA from porcine ear fibroblasts (PEFs) was extracted and digested with XhoI, followed by Southern blot analysis using a digoxigenin-labeled pbd-2-specific probe. M: Molecular mass marker; Lane 220–230: Numbers for pigs; PC: Positive control.
3.4. Off-Target Analysis
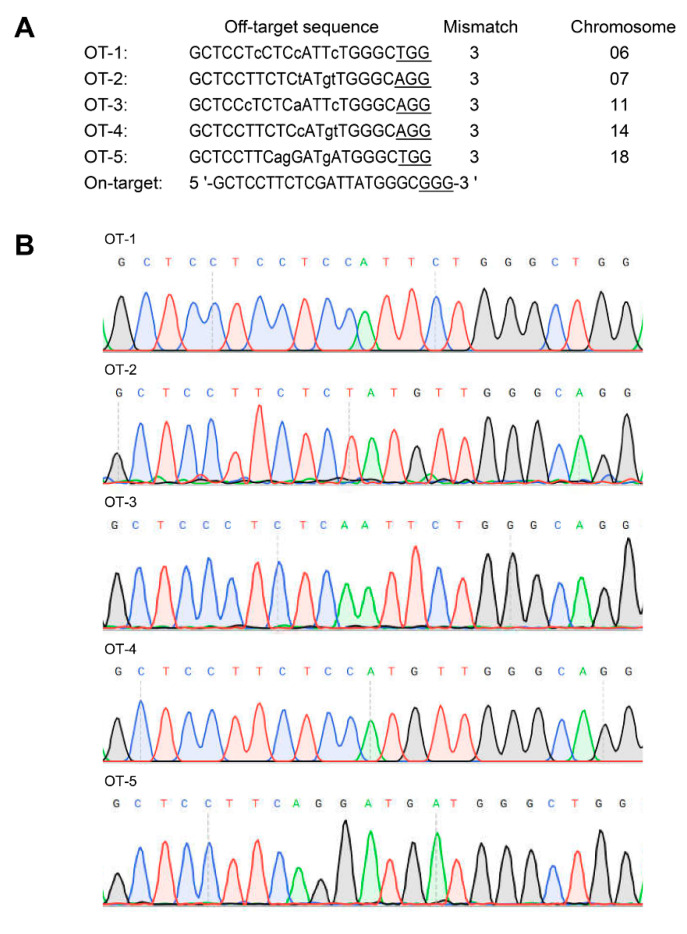
The potential off-target sites were predicted according to the target sequence (5′-GCTCCTTCTCGATTATGGGCGGG-3′) using Cas-OFFinder. The results showed that there were five potential off-target sites on chromosome 06, 07, 11, 14, 18, respectively (Figure 3A). The fragments containing all these potential off-targets loci were PCR amplified and sequenced. The Sanger sequencing results revealed that no insertions/deletions and site mutations were found within all these potential off-target sites (Figure 3B). These findings suggested no potential off-target effects were observed in TG pigs.
Figure 3.
Off-target analysis. (A) Predicted off-target sites. Lower case letters represent mismatched bases, while underlined letters represent the 3′ PAM of the target sequence. (B) Sanger sequencing results of the PCR products of potential off-target sites.
3.5. Characterization of PBD-2 Transcription and Translation
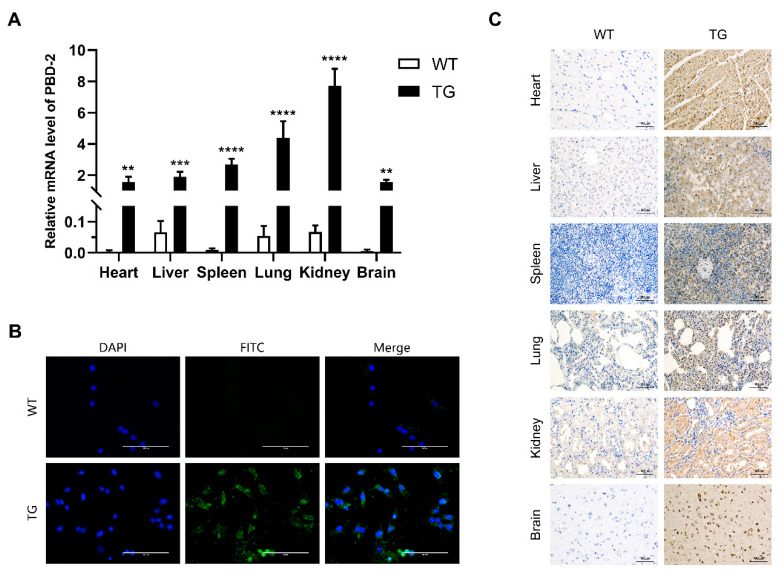
The transcriptional level of pbd-2 expression in different organs of TG pigs was quantified by RT-qPCR, with GAPDH serving as the reference gene. As shown in Figure 4A, the mRNA levels of pbd-2 in heart, liver, spleen, lung, kidney and brain tissues of TG pigs were significantly higher than that of their WT littermates. Besides, the kidney showed the highest mRNA level of pbd-2, while the transcriptional level of pbd-2 of lung and spleen ranked the second and the third, respectively.
Figure 4.
Transcriptional and translational analysis of pbd-2 in cloned pigs. (A) The mRNA expression level of pbd-2 in different organs. Total RNA in different organs of TG and WT pigs was extracted and then subjected to reverse transcription, followed by RT-qPCR to determine the mRNA level of pbd-2, GAPDH was used as an internal reference gene. Data are presented as mean ± SD and are plotted from three independent experiments. ** p < 0.01, *** p < 0.001, **** p < 0.0001, n = 3, unpaired one tailed Student′ s t-test. (B) Immunofluorescent analysis of the expression of porcine β-defensin 2 (PBD-2) using a 40× objective and a 100 ms exposure. The expression of PBD-2 in PEFs from TG pigs was detected by immunofluorescent analysis using mouse monoclonal anti-PBD2 antibody followed by FITC-labeled goat anti-mouse IgG. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole, the PEFs from WT pigs served as a negative control. Scale bar = 100 μm. (C) Immunohistochemical analysis of the expression of PBD-2 at a magnification of 200×, with an exposure time of 50 ms. Expression of PBD-2 in heart, liver, spleen, lung, kidney and brain tissues of WT and TG pigs was determined by immunohistochemistry using mouse monoclonal 2A peptide antibody. Brown color represents the detected 2A peptide, which is co-expressed with PBD-2. Scale bars = 100 μm.
To further determine whether the CRISPR/Cas9-mediated knock-in of pbd-2 could increase the expression of PBD-2, the immunofluorescent analysis was conducted to detect PBD-2 in PEFs isolated from WT and TG pigs, using the self-made mouse anti-PBD-2 monoclonal antibody. The results showed high expression of PBD-2 in TG PEFs, with strong green fluorescence. In contrast, little green signals could be seen in WT PEFs, indicating low expression of PBD-2 in WT pigs (Figure 4B). The immunohistochemical analysis was further performed to detect PBD-2 in different organs of both WT and TG pigs. To exclude the influence of endogenous expression of PBD-2, an anti-2A peptide antibody was used as the primary antibody for that 2A peptide and PBD-2 were co-translated. As was shown in Figure 4C, PBD-2 in different organs of TG pigs was highly expressed, appearing as brown signals, while no obvious brown coloration was observed in tissues of WT pigs, which was in good agreement with those results identified by immunofluorescence and RT-qPCR.
3.6. Verification of the Antibacterial Ability of Isolated TG PEFs
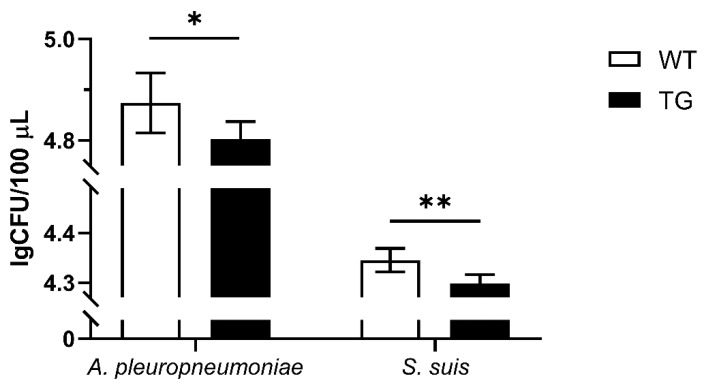
To investigate whether overexpression of PBD-2 could endow hosts with enhanced resistance to bacterial infections, PEFs were isolated from TG and WT pigs. Then cell culture supernatants of WT and TG PEFs were harvested to incubate with A. pleuoropneumoniae and S. suis, and the bactericidal activity of the supernatants was analyzed by bacterial counting. The surviving bacteria of the TG group were significantly less than that of the WT group (Figure 5), suggesting that the site-specific knock-in of pbd-2 successfully conferred an improved resistance against bacterial infections on TG pigs.
Figure 5.
Bactericidal activity of cell culture supernatant. The cell culture supernatant of PEFs from TG pigs was incubated with A. pleuropneumoniae and S. suis for 1 h and the surviving bacteria were then plated on agar for counting. Cell culture supernatant of PEFs from WT pigs was used as a negative control. Data are presented as mean ± SD and are plotted from three independent experiments. * p < 0.05, ** p < 0.01, unpaired one tailed Student′ s t-test.
4. Discussion
As a major group of cationic peptides widely existing in animals, defensins have been proven to possess broad antimicrobial activities against different pathogens including bacteria, fungi and viruses. Our previous in vivo studies showed that pigs overexpressing PBD-2 displayed increased resistance to A. pleuoropneumoniae infection [16], while mice expressing PBD-2 became more resistant to pseudorabies virus and Salmonella Typhimurium infections [27,28]. Given that the previous pbd-2 TG pigs were produced using random gene insertion, which also introduced a SMG, these would raise public concern on the biosafety of TG animals and hamper the commercialization of TG animals derived products. Therefore, this study achieved site-specific integration of pbd-2 into the pRosa26 locus through CRISPR/Cas9-mediated homology-directed repair and eliminated the SMG neoR using a cell-penetrating TAT-Cre recombinase. Five marker-free pbd-2 knock-in pigs were generated via SCNT, making it to the overexpression of PBD-2 in different organs of pigs with no off-target effects detected. Additionally, the cell culture supernatant of PEFs of TG pigs exhibited greater antibacterial activities against both Gram-negative (A. pleuoropneumoniae) and Gram-positive bacterium (S. suis).
The copy number of genes is positively correlated with corresponding protein levels [36]. Fellermann et al. claimed that people carried low copy number of human β-defensin 2 gene were more likely to develop Crohn’s disease [37], while a high copy number of β-defensin genes was considered the main reason why East Asians were more resistant to influenza infection [38]. As the 2A self-cleaving peptide has been widely used to generate multi-transgenic pigs [39], we applied T2A peptides, a 2A self-cleaving peptide found in Thosea asigna virus 2A [40], in expression of two copies of the pbd-2 gene in this study. Similar with other defensins, PBD-2 is firstly produced in the form of pro-peptide and then processed into active form when the N-terminal signal peptide is cleaved, followed by being transported out of cells [16]. Though de Felipe and Ryan claimed that proteins downstream of the 2A peptide could also occur on cell membrane or be secreted despite lack of a signal sequence when a protein upstream of the 2A peptide contained an N-terminal signal sequence [41], while Yan et al. argued that genes following the 2A peptide still demanded a signal sequence for successful secretion expression [42]. Thus, the signal sequence in the second pbd-2 gene was kept and the bactericidal assay showed that this design could efficiently improve the expression of PBD-2 without hampering its biological activity.
The random integration of exogenous genes has been found to lead to multiple undesired outcomes including gene silencing, unpredictable expression and activation of oncogene expression [43]. In addition to the pRosa26 locus described above, porcine H11 and GAPDH loci have been well characterized as safe harbors for targeted transgene integrations [44,45]. When introducing a foreign gene into the Rosa26 locus, higher expression could be achieved by positioning the transgene promoter in an opposite direction to the Rosa26 promoter [46]. Therefore, this study set pbd-2 gene in a reverse orientation to the Rosa26 promoter and achieved overexpression of PBD-2.
The unwanted effects of introducing a SMG neoR have been well investigated. Wang et al. argued that the neoR gene could inhibit the growth of Lactobacillus and Escherichia-Shigella-Hafnia in guts of TG pigs [47]. Meanwhile it was worth noting that the expression levels of metabolic genes varied between cells expressing exogenous neoR gene and control cells [48]. Also, the integration of neoR gene in target loci has been shown to exert a long-distance effect on the expression of downstream or upstream genes [49]. Another study revealed that removing the selectable marker increased the expression level of transgene when promoters of the Rosa26 and the transgene occurred in opposite orientations [46]. Bi et al. successfully excised the SMG in cells using Cre mRNA [4], while using cell-penetrating TAT-Cre recombinase to delete a SMG could reach a recombination efficiency of up to 55% in pig primary cells [50]. Alike, the use of commercialized TAT-Cre recombinase in this study achieved successful excision of neoR in 48.3% PFFs.
It has been quite controversial that whether CRISPR/Cas9-mediated gene-editing can cause genome-wide off-target mutations, while Zuo et al. created a large scale of screening system called GOTI and used to prove that classic CRISPR/Cas9 tool did not induce obvious off-target effects [51]. This study predicted five putative off-target loci using an online tool and the Sanger sequencing of the five sites in TG pigs revealed that no mutations were found among these sites. However, in-depth whole-genome sequencing of the TG pigs is still needed to exclude any off-target mutagenesis in the future.
Chen et al. found that, when compared with crossbred pigs, higher expression of β-defensins in Meishan pigs was one of the main reasons that Meishan pigs exhibited effective disease-resistance traits [52]. The RT-qPCR analysis combined with immunohistochemical and immunofluorescence assays had confirmed that PBD-2 was overexpressed in different organs of the marker-free pbd-2 knock-in pigs (Figure 4), while the bactericidal assay indicated that PEFs of TG pigs were more resistant to bacterial infections (Figure 5). In addition to the described functions of PBD-2 above, the in vitro studies have shown that PBD-2 could inhibit proliferations of PRRSV, Staphylococcus aureus and other pathogens [24,26]. Besides, PBD-2 could be used for growth-promotion among piglets [29,53]. Altogether these suggest excellent prospect for the application of marker-free site-specific pbd-2 knock-in pigs produced in this study.
In summary, this study generated marker-free site-specific pbd-2 knock-in pigs, achieving overexpression of PBD-2 in pigs, which provides a novel genetic material for the breeding of disease-resistant pigs. Given that PBD-2 displays multi-functions including antimicrobial and immunomodulatory activities, these pigs are supposed to be reliable animal models to study biofunctions of PBD-2 as well.
Acknowledgments
We thank Bo Zuo and Shuhong Zhao (Huazhong Agricultural University) for providing pX330-pRosa26 and PFFs, respectively.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/8/951/s1, Figure S1: Analysis for the T2A-mediated dual pbd-2 expression, Figure S2: Map of pX330-pRosa26, Figure S3: PCR analysis for the identification of site-specific pbd-2 knock-in PFFs, Figure S4: Sanger sequencing analyses to confirm on-target integration of pbd-2 in cloned pigs, Table S1: Information of primer pairs for identification of transgenic PFFs, Table S2: Information of primer pairs for amplification of predicted off-target loci.
Author Contributions
Conceptualization: J.H., R.Z. and L.L.; Methodology: J.H. and A.W.; Software: C.H., Y.S. and B.S.; Formal analysis: J.H., A.W., C.H., Y.S. and B.S.; Writing—original draft preparation: J.H., R.Z. and L.L.; supervision, R.Z. and L.L.; funding acquisition, R.Z. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by the National Transgenic Project of China (Grant No. 2016ZX08006003-004), National Key R & D Program of China (Grant No. 2017YFD0500201), and Hubei Province Natural Science Foundation for Innovative Research Groups (Grant No. 2016CFA015).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cermak T., Doyle E.L., Christian M., Wang L., Zhang Y., Schmidt C., Baller J.A., Somia N.V., Bogdanove A.J., Voytas D.F. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith J., Bibikova M., Whitby F.G., Reddy A.R., Chandrasegaran S., Carroll D. Requirements for double-strand cleavage by chimeric restriction enzymes with zinc finger DNA-recognition domains. Nucleic Acids Res. 2000;28:3361–3369. doi: 10.1093/nar/28.17.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi Y., Hua Z., Liu X., Hua W., Ren H., Xiao H., Zhang L., Li L., Wang Z., Laible G., et al. Isozygous and selectable marker-free MSTN knockout cloned pigs generated by the combined use of CRISPR/Cas9 and Cre/LoxP. Sci. Rep. 2016;6:31729. doi: 10.1038/srep31729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo R., Wan Y., Xu D., Cui L., Deng M., Zhang G., Jia R., Zhou W., Wang Z., Deng K., et al. Generation and evaluation of Myostatin knock-out rabbits and goats using CRISPR/Cas9 system. Sci. Rep. 2016;6:29855. doi: 10.1038/srep29855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J., Song Z., Yu S., Cui D., Wang B., Ding F., Li S., Dai Y., Li N. Efficient generation of myostatin (MSTN) biallelic mutations in cattle using zinc finger nucleases. PLoS ONE. 2014;9:e95225. doi: 10.1371/journal.pone.0095225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson D.F., Lancto C.A., Zang B., Kim E.S., Walton M., Oldeschulte D., Seabury C., Sonstegard T.S., Fahrenkrug S.C. Production of hornless dairy cattle from genome-edited cell lines. Nat. Biotechnol. 2016;34:479–481. doi: 10.1038/nbt.3560. [DOI] [PubMed] [Google Scholar]
- 8.Oishi I., Yoshii K., Miyahara D., Kagami H., Tagami T. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci. Rep. 2016;6:23980. doi: 10.1038/srep23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui C., Song Y., Liu J., Ge H., Li Q., Huang H., Hu L., Zhu H., Jin Y., Zhang Y. Gene targeting by TALEN-induced homologous recombination in goats directs production of β-lactoglobulin-free, high-human lactoferrin milk. Sci. Rep. 2015;5:10482. doi: 10.1038/srep10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proudfoot C., Lillico S., Tait-Burkard C. Genome editing for disease resistance in pigs and chickens. Anim. Front. 2019;9:6–12. doi: 10.1093/af/vfz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L., Li Q., Bao Y., Li J., Chen Z., Yu X., Zhao Y., Tian K., Li N. RNAi-based inhibition of porcine reproductive and respiratory syndrome virus replication in transgenic pigs. J. Biotechnol. 2014;171:17–24. doi: 10.1016/j.jbiotec.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu S., Qiao J., Fu Q., Chen C., Ni W., Wujiafu S., Ma S., Zhang H., Sheng J., Wang P., et al. Transgenic shRNA pigs reduce susceptibility to foot and mouth disease virus infection. Elife. 2015;4:e06951. doi: 10.7554/eLife.06951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Z., Pang D., Yuan H., Jiao H., Lu C., Wang K., Yang Q., Li M., Chen X., Yu T., et al. Genetically modified pigs are protected from classical swine fever virus. PLoS Pathog. 2018;14:e1007193. doi: 10.1371/journal.ppat.1007193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burkard C., Lillico S.G., Reid E., Jackson B., Mileham A.J., Ait-Ali T., Whitelaw C.B., Archibald A.L. Precision engineering for PRRSV resistance in pigs: Macrophages from genome edited pigs lacking CD163 SRCR5 domain are fully resistant to both PRRSV genotypes while maintaining biological function. PLoS Pathog. 2017;13:e1006206. doi: 10.1371/journal.ppat.1006206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitworth K.M., Rowland R.R.R., Petrovan V., Sheahan M., Cino-Ozuna A.G., Fang Y., Hesse R., Mileham A., Samuel M.S., Wells K.D., et al. Resistance to coronavirus infection in amino peptidase N-deficient pigs. Transgenic Res. 2019;28:21–32. doi: 10.1007/s11248-018-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X., Cheng Y.T., Tan M.F., Zhang H.W., Liu W.Q., Zou G., Zhang L.S., Zhang C.Y., Deng S.M., Yu L., et al. Overexpression of Porcine Β-Defensin 2 Enhances Resistance to Actinobacillus pleuropneumoniae Infection in Pigs. Infect. Immun. 2015;83:2836–2843. doi: 10.1128/IAI.03101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu T., Song Z., Li Q., Li Z., Wang M., Liu L., Tian K., Li N. Overexpression of Histone Deacetylase 6 Enhances Resistance to Porcine Reproductive and Respiratory Syndrome Virus in Pigs. PLoS ONE. 2017;12:e0169317. doi: 10.1371/journal.pone.0169317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Q., Yang H., Yang D., Zhao B., Ouyang Z., Liu Z., Fan N., Ouyang H., Gu W., Lai L. Production of transgenic pigs over-expressing the antiviral gene Mx1. Cell Regen. (Lond.) 2014;3:11. doi: 10.1186/2045-9769-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Wang T., Yao L., Liu B., Teng C., Ouyang H. Classical swine fever virus replicated poorly in cells from MxA transgenic pigs. BMC Vet. Res. 2016;12:169. doi: 10.1186/s12917-016-0794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattar E.H., Almehdar H.A., Yacoub H.A., Uversky V.N., Redwan E.M. Antimicrobial potentials and structural disorder of human and animal defensins. Cytokine Growth Factor Rev. 2016;28:95–111. doi: 10.1016/j.cytogfr.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Sang Y., Blecha F. Porcine host defense peptides: Expanding repertoire and functions. Dev. Comp. Immunol. 2009;33:334–343. doi: 10.1016/j.dci.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Choi M.K., Le M.T., Nguyen D.T., Choi H., Kim W., Kim J.H., Chun J., Hyeon J., Seo K., Park C. Genome-level identification, gene expression, and comparative analysis of porcine ss-defensin genes. BMC Genet. 2012;13:98. doi: 10.1186/1471-2156-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sang Y., Patil A.A., Zhang G., Ross C.R., Blecha F. Bioinformatic and expression analysis of novel porcine β-defensins. Mamm. Genome. 2006;17:332–339. doi: 10.1007/s00335-005-0158-0. [DOI] [PubMed] [Google Scholar]
- 24.Peng Z., Wang A., Feng Q., Wang Z., Ivanova I.V., He X., Zhang B., Song W. High-level expression, purification and characterisation of porcine β-defensin 2 in Pichia pastoris and its potential as a cost-efficient growth promoter in porcine feed. Appl. Microbiol. Biotechnol. 2014;98:5487–5497. doi: 10.1007/s00253-014-5560-7. [DOI] [PubMed] [Google Scholar]
- 25.Chen R.B., Zhang K., Zhang H., Gao C.Y., Li C.L. Analysis of the antimicrobial mechanism of porcine β defensin 2 against E. coli by electron microscopy and differentially expressed genes. Sci. Rep. 2018;8:14711. doi: 10.1038/s41598-018-32822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldhuizen E.J., Rijnders M., Claassen E.A., van Dijk A., Haagsman H.P. Porcine β-defensin 2 displays broad antimicrobial activity against pathogenic intestinal bacteria. Mol. Immunol. 2008;45:386–394. doi: 10.1016/j.molimm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Huang J., Qi Y., Wang A., Huang C., Liu X., Yang X., Li L., Zhou R. Porcine β-defensin 2 inhibits proliferation of pseudorabies virus in vitro and in transgenic mice. Virol. J. 2020;17:18. doi: 10.1186/s12985-020-1288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C., Yang X., Huang J., Liu X., Yang X., Jin H., Huang Q., Li L., Zhou R. Porcine Β-Defensin 2 Provides Protection Against Bacterial Infection by a Direct Bactericidal Activity and Alleviates Inflammation via Interference With the TLR4/NF-kappaB Pathway. Front. Immunol. 2019;10:1673. doi: 10.3389/fimmu.2019.01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Z., Wang A., Xie L., Song W., Wang J., Yin Z., Zhou D., Li F. Use of recombinant porcine β-defensin 2 as a medicated feed additive for weaned piglets. Sci. Rep. 2016;6:26790. doi: 10.1038/srep26790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M., Ouyang H., Yuan H., Li J., Xie Z., Wang K., Yu T., Liu M., Chen X., Tang X., et al. Site-Specific Fat-1 Knock-In Enables Significant Decrease of n-6PUFAs/n-3PUFAs Ratio in Pigs. G3 (Bethesda) 2018;8:1747–1754. doi: 10.1534/g3.118.200114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., Yang Y., Bu L., Guo X., Tang C., Song J., Fan N., Zhao B., Ouyang Z., Liu Z., et al. Rosa26-targeted swine models for stable gene over-expression and Cre-mediated lineage tracing. Cell Res. 2014;24:501–504. doi: 10.1038/cr.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Zhu X.X., Zhong Y.Z., Ge Y.W., Lu K.H., Lu S.S. CRISPR/Cas9-Mediated Generation of Guangxi Bama Minipigs Harboring Three Mutations in alpha-Synuclein Causing Parkinson’s Disease. Sci. Rep. 2018;8:12420. doi: 10.1038/s41598-018-30436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bae S., Park J., Kim J.S. Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics. 2014;30:1473–1475. doi: 10.1093/bioinformatics/btu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J., Sun Y., Carretero O.A., Zhu L., Harding P., Shesely E.G., Dai X., Rhaleb N.E., Peterson E., Yang X.P. Effects of cardiac overexpression of the angiotensin II type 2 receptor on remodeling and dysfunction in mice post-myocardial infarction. Hypertension. 2014;63:1251–1259. doi: 10.1161/HYPERTENSIONAHA.114.03247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fellermann K., Stange D.E., Schaeffeler E., Schmalzl H., Wehkamp J., Bevins C.L., Reinisch W., Teml A., Schwab M., Lichter P., et al. A chromosome 8 gene-cluster polymorphism with low human β-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am. J. Hum. Genet. 2006;79:439–448. doi: 10.1086/505915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardwick R.J., Machado L.R., Zuccherato L.W., Antolinos S., Xue Y., Shawa N., Gilman R.H., Cabrera L., Berg D.E., Tyler-Smith C., et al. A worldwide analysis of β-defensin copy number variation suggests recent selection of a high-expressing DEFB103 gene copy in East Asia. Hum. Mutat. 2011;32:743–750. doi: 10.1002/humu.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng W., Yang D., Zhao B., Ouyang Z., Song J., Fan N., Liu Z., Zhao Y., Wu Q., Nashun B., et al. Use of the 2A peptide for generation of multi-transgenic pigs through a single round of nuclear transfer. PLoS ONE. 2011;6:e19986. doi: 10.1371/journal.pone.0019986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z., Chen O., Wall J.B.J., Zheng M., Zhou Y., Wang L., Ruth Vaseghi H., Qian L., Liu J. Systematic comparison of 2A peptides for cloning multi-genes in a polycistronic vector. Sci. Rep. 2017;7:2193. doi: 10.1038/s41598-017-02460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Felipe P., Ryan M.D. Targeting of proteins derived from self-processing polyproteins containing multiple signal sequences. Traffic. 2004;5:616–626. doi: 10.1111/j.1398-9219.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- 42.Yan J., Wang H., Xu Q., Jain N., Toxavidis V., Tigges J., Yang H., Yue G., Gao W. Signal sequence is still required in genes downstream of “autocleaving” 2A peptide for secretary or membrane-anchored expression. Anal. Biochem. 2010;399:144–146. doi: 10.1016/j.ab.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 43.Ma L., Wang Y., Wang H., Hu Y., Chen J., Tan T., Hu M., Liu X., Zhang R., Xing Y., et al. Screen and Verification for Transgene Integration Sites in Pigs. Sci. Rep. 2018;8:7433. doi: 10.1038/s41598-018-24481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han X., Xiong Y., Zhao C., Xie S., Li C., Li X., Liu X., Li K., Zhao S., Ruan J. Identification of Glyceraldehyde-3-Phosphate Dehydrogenase Gene as an Alternative Safe Harbor Locus in Pig Genome. Genes. 2019;10:660. doi: 10.3390/genes10090660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruan J., Li H., Xu K., Wu T., Wei J., Zhou R., Liu Z., Mu Y., Yang S., Ouyang H., et al. Highly efficient CRISPR/Cas9-mediated transgene knockin at the H11 locus in pigs. Sci. Rep. 2015;5:14253. doi: 10.1038/srep14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strathdee D., Ibbotson H., Grant S.G. Expression of transgenes targeted to the Gt(ROSA)26Sor locus is orientation dependent. PLoS ONE. 2006;1:e4. doi: 10.1371/journal.pone.0000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q., Qian L., Jiang S., Cai C., Ma D., Gao P., Li H., Jiang K., Tang M., Hou J., et al. Safety Evaluation of Neo Transgenic Pigs by Studying Changes in Gut Microbiota Using High-Throughput Sequencing Technology. PLoS ONE. 2016;11:e0150937. doi: 10.1371/journal.pone.0150937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valera A., Perales J.C., Hatzoglou M., Bosch F. Expression of the neomycin-resistance (neo) gene induces alterations in gene expression and metabolism. Hum. Gene Ther. 1994;5:449–456. doi: 10.1089/hum.1994.5.4-449. [DOI] [PubMed] [Google Scholar]
- 49.Pham C.T., MacIvor D.M., Hug B.A., Heusel J.W., Ley T.J. Long-range disruption of gene expression by a selectable marker cassette. Proc. Natl. Acad. Sci. USA. 1996;93:13090–13095. doi: 10.1073/pnas.93.23.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang Q., Sun Z., Zou Z., Wang M., Li Q., Hu X., Li N. Cell-penetrating peptide-driven Cre recombination in porcine primary cells and generation of marker-free pigs. PLoS ONE. 2018;13:e0190690. doi: 10.1371/journal.pone.0190690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuo E., Sun Y., Wei W., Yuan T., Ying W., Sun H., Yuan L., Steinmetz L.M., Li Y., Yang H. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science. 2019;364:289–292. doi: 10.1126/science.aav9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J., Qi S., Guo R., Yu B., Chen D. Different messenger RNA expression for the antimicrobial peptides β-defensins between Meishan and crossbred pigs. Mol. Biol. Rep. 2010;37:1633–1639. doi: 10.1007/s11033-009-9576-5. [DOI] [PubMed] [Google Scholar]
- 53.Tang Z., Xu L., Shi B., Deng H., Lai X., Liu J., Sun Z. Oral administration of synthetic porcine β-defensin-2 improves growth performance and cecal microbial flora and down-regulates the expression of intestinal toll-like receptor-4 and inflammatory cytokines in weaned piglets challenged with enterotoxigenic Escherichia coli. Anim. Sci. J. 2016;87:1258–1266. doi: 10.1111/asj.12540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.