Abstract
Bone metastasis is a major cause of morbidity within solid tumours of the breast, prostate, lung and kidney. Metastasis to the skeleton is associated with a wide range of complications including bone fractures, spinal cord compression, hypercalcaemia and increased bone pain. Improved treatments for bone metastasis, such as the use of anti-bone resorptive bisphosphonate agents, within post-menopausal women have improved disease-free survival; however, these treatments are not without side effects. There is thus a need for biomarkers, which will predict the risk of developing the spread to bone within these cancers. The application of molecular profiling techniques, together with animal model systems and engineered cell-lines has enabled the identification of a series of potential bone-metastasis biomarker molecules predictive of bone metastasis risk. Some of these biomarker candidates have been validated within patient-derived samples providing a step towards clinical utility. Recent developments in multiplex biomarker quantification now enable the simultaneous measurement of up to 96 micro-RNA/protein molecules in a spatially defined manner with single-cell resolution, thus enabling the characterisation of the key molecules active at the sites of pre-metastatic niche formation as well as tumour-stroma signalling. These technologies have considerable potential to inform biomarker discovery. Additionally, a potential future extension of these discoveries could also be the identification of novel drug targets within cancer spread to bone. This chapter summarises recent findings in biomarker discovery within the key bone metastatic cancers (breast, prostate, lung and renal cell carcinoma). Tissue-based and circulating blood-based biomarkers are discussed from the fields of genomics, epigenetic regulation (micro-RNAs) and protein/cell-signalling together with a discussion of the potential future development of these markers towards clinical development.
Keywords: bone metastatic cancers, biomarkers, personalised medicine, micro-RNA, proteins, bone microenvironment
1. Introduction
Bone is the most common site of metastasis for many solid tumours (in particular breast and prostate cancer) [1]. Cancer spread to the skeleton is associated with a range of disease complications—termed skeletal-related events (SREs) including pathological fractures, spinal cord compression, severe bone pain and hypercalcaemia [1,2]. SREs cause a significant decrease in patient quality of life as well as resulting in a considerable economic burden on the healthcare system [3,4,5,6]—see Table 1.
Table 1.
Incidence of skeletal metastases within bone-metastatic cancers.
| Incidence of Skeletal Metastases from Autopsy Studies | |||
|---|---|---|---|
| Incidence of Bone Metastases (%) | |||
| Primary Tumour | Studies | Median | Range |
| Breast | 5 | 73 | 47–85 |
| Prostate | 6 | 68 | 33–85 |
| Thyroid | 4 | 42 | 28–60 |
| Kidney | 3 | 35 | 33–40 |
| Bronchus | 4 | 36 | 30–55 |
| Oesophagus | 3 | 6 | 5–7 |
| Gastrointestinal Tract | 4 | 5 | 3–11 |
| Rectum | 3 | 11 | 8–13 |
Modified from Coleman-RE [1].
Within breast cancer, metastases to the skeleton are detected in approximately 75% of patients with advanced breast cancer [7]. Despite improvements in the treatment for breast cancer, at the point of development of bone metastasis, breast cancer is currently incurable such that the median survival time following the diagnosis of bone metastasis ranges between 12 and 53 months [7].
Prostate cancer is the second most commonly diagnosed male cancer with 1.3 million cases reported in 2018 [8,9]. Mainly due to improvements in treatment, 84% of men with prostate cancer survive for 10 years or more. Metastasis to the skeleton is observed in up to 80% of advanced prostate cancer patients [10]. In contrast to bone lesions arising from breast cancer, which have mixed osteolytic and osteoblastic phenotypes, prostate cancer lesions are predominantly osteosclerotic. The new bone deposited in prostate cancer bone lesions has an unusual structure and is weaker than normal bone resulting in an increased rate of fractures and SREs. There is also an increased rate of bone resorption within prostate cancer bone lesions [11].
Lung cancer also metastasises to bone with the subtype of lung cancer being key at the time of primary diagnosis, such that the small-cell subtype has the highest incidence of skeletal involvement at diagnosis compared to squamous cell tumours. At the time of autopsy, the incidence of bone metastasis is 30% for all four subtypes of lung cancer. Bone metastases arising from lung cancer are predominantly osteolytic in nature. In contrast to metastases from breast and prostate cancer, the survival time of patients with bone metastases from lung cancer is relatively short with most patients dying within 12 to 18 months and a 5-year survival rate of only 10–20% [12,13].
Renal cell carcinoma (RCC) is responsible for over 200,000 new cancer diagnoses per year, with the incidence increasing at around 2% per year in Europe and North America [8,9]. Men have almost twice the incidence of renal cancer as women [14]. Renal cell carcinoma (RCC) accounts for 80–90% of all renal malignancies with a 5-year survival rate of approximately 45% [15,16,17]. Sub-divisions of RCC include clear cell, papillary and chromophobe, with clear cell disease being by far the most common. Although nephrectomy can be highly curable when RCC is detected at an early stage, between 20% and 50% of individuals (depending on the reference population and literature source) present with locally advanced or metastatic disease [16]. In a third of patients, metastatic disease appears later during disease progression following nephrectomy so that, overall, the majority of patients diagnosed with RCC will develop distant metastases.
There have been substantial recent advances in treatments for bone metastasis, such as the use of bisphosphonate anti-resorptive drugs and the anti-RANKL antibody therapeutic Denosumab for treatment of breast cancer bone spread, and these have improved patient survival; however, there is a strong need for the discovery of biomarkers predictive of bone metastasis risk within patients. This is particularly true for the administration of bisphosphonates, which can cause side effects including osteonecrosis of the jaw (ONJ). Biomarkers predictive of bone metastasis risk in the case of breast cancer will enable personalised medicine initiatives, whereby the molecular composition of a patient’s tumour determines the targeting and choice of treatments, minimising unwanted side effects. Biomarkers with potential to influence patient treatment decisions have arisen from genomic studies (including amplification of the MAF-gene [18,19]), protein-based biomarkers including increased expression of the proteins CAPG, GIPC1 and DOCK4 [20,21,22] and circulating products of extracellular matrix remodelling including the C-terminal propeptide of type-I collagen [11]. Recently interest has focussed upon the role of epigenetic regulators of the tumour and bone micro-environmental interactions, in particular, micro-RNAs within bone metastasis [23], and their potential as circulating markers of bone metastasis. We aim to summarise in this chapter the recent developments within the field of biomarker discovery for prediction of bone metastasis risk and their potential to influence personalised medicine initiatives.
2. Bone Homeostasis and the Vicious Cycle
Bone homeostasis is maintained by the actions of several cell-types, most notably bone-forming osteoblasts and bone-resorbing osteoclasts [24,25,26]. A wide variety of cytokines and growth factors regulate this process. In particular, pre-osteoclasts are activated to become active osteoclasts in response to the action of macrophage colony-stimulating factor (MCSF) and the receptor activator of nuclear factor-ĸB ligand (RANKL) [27]. Osteoclast activation is triggered by the binding of RANKL to the receptor activator of nuclear factor-ĸB (RANK). There are additional growth factors which mediate osteoclast activation including interleukin-11 (IL-11), prostaglandin-E2 and parathyroid hormone-related protein (PTHrP) [28].
Metastatic cancer cells alter the balance of osteoblast and osteoclast activation to alter normal bone homeostasis. Breast and lung cancer, as well as RCC, predominantly stimulates bone degradation (osteoclastic metastases), whilst prostate cancer is predominantly bone-forming (osteoblastic). Despite being predominantly bone-forming, prostate cancer also results in an increased risk of bone fractures as the new bone laid down has an unusual weaker structure than normal bone.
-
(i)
Osteolytic Metastases:
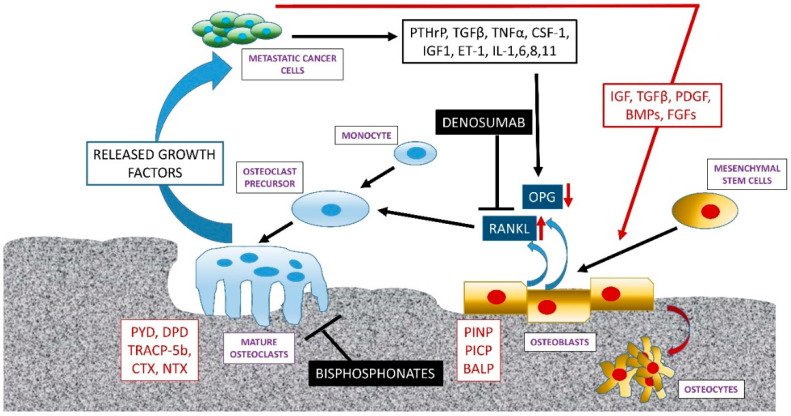
Upon arrival in bone, metastatic breast, lung or RCC cancer cells secrete PTHrP which acts on osteoblasts to trigger an increased release of RANKL and reduced expression of the RANKL-antagonist osteoprotegerin (OPG). PTHrP is not the only growth factor released by metastatic breast cancer cells, which cause this alteration in RANKL: OPG, Wnt-family members, transforming growth-factor-β (TGFβ), endothelin-1 (ET-1) and bone morphogenetic proteins (BMPs) also play a key role. The increased expression of RANKL and reduced expression of OPG results in osteoclast activation and the consequent degradation of the bone matrix. Bone degradation releases trapped growth factors which then act upon the cancer cells to stimulate their proliferation resulting in a “vicious cycle” of bone destruction [26]—see Figure 1.
Figure 1.
General mechanisms of altered bone homeostasis within bone metastatic cancers.
Bone turnover involves the action of bone absorbing osteoclasts and bone-forming osteoblasts. The actions of these two cell-types ensures normal bone homeostasis and continuous bone turnover. Mature osteoblasts are derived from mesenchymal stem cells, whilst mature active osteoclasts are derived from monocytes. Incoming metastatic cancer cells perturb this balance and alter bone turnover. Osteolytic cancers (breast, lung and RCC) secrete growth factors and cytokines (including PTHrP) which act on osteoblasts to increase their expression and secretion of RANKL, whilst decreasing their secretion of the RANKL decoy receptor OPG. The effect of this is to increase the binding of RANKL to RANK on osteoclast precursors and promote their activation. Mature, active osteoclasts degrade bone, releasing trapped growth factors, which then act on the metastatic cancer cells to promote their proliferation, in a “vicious cycle” of bone destruction. Osteoblastic cancers (prostate cancer) stimulate bone formation via promoting osteoblast differentiation via the release of growth factors and cytokines such as BMP, and TGFβ. Bone turnover results in the release of bone turnover markers (BTMs) including TRACP-5b, CTN and NTX (from bone degradation), and PINP, PICP and BALP from bone deposition. This system is the target of the current therapies bisphosphonates (targeting osteoclasts) and denosumab (binding and inhibiting RANKL).
Metastatic breast cancer cells extensively modify the extra-cellular matrix (ECM) in order to escape the primary tumour (extravasation), circulate in the bloodstream and eventually spread to bone. In order to do so, they actively secrete numerous matrix metalloproteinases (MMPs) and regulators of ECM remodelling. The diverse regulation of the MMP family of proteins within breast cancer bone metastasis is beyond the scope of this article, however, it has been reviewed extensively elsewhere [29,30].
In addition to the modification of the ECM, metastatic cancer cells also secrete other key regulators which play roles across multiple cancer types. Osteopontin (OPN) is one such regulator which plays a role in bone metastasis of both prostate and breast cancer. OPN (or secreted phosphoprotein-1) acts via binding to αvβ3 integrin and CD44 and plays a key role in both the metastatic spread as well as the drug-sensitivity of breast and prostate cancer. OPN is a promising target for therapeutic intervention within these cancer types [31].
-
(ii)
Osteoblastic Metastases:
Prostate cancer metastasis to bone can induce osteolytic as well as osteoblastic lesions, with the osteoblastic phenotype being the more prevalent outcome. There is evidence that most of the tumour-derived growth factors acting within breast cancer are also integral to prostate cancer signalling [32]. Osteoblastic lesions arise from cancer-induced signalling via bone morphogenetic proteins (BMPs), fibroblast growth factor and TGFβ resulting in osteoblast activation [24]. Expression of BMP-2, -4, -6 and -7 is elevated within bone metastases [33] within which BMPs can induce the osteogenic differentiation of osteoblasts [34].
In addition to the extensive interaction of metastatic cancer cells with the bone micro-environment via either autocrine or paracrine signalling events, several genetic alterations have been discovered within the metastatic cancer cells themselves which account for their metastatic phenotype. These will now be discussed.
2.1. Breast Cancer
Genes which predispose breast cancer cells towards metastasis to bone have been characterised by employing copy number analysis (CNA) within breast cancer cell lines (MCF7 and MDA-MB-231) and osteotropic variants of these cell lines, isolated within murine models of breast cancer bone homing. Copy number analysis identified the amplification of the 16q32 chromosomal region within the osteotropic cell line with an average 1.5-fold increase in the number of copies per cell [19]. Sequencing of the 16q32 region identified the v-maf avian musculoaponeurotic fibrosarcoma (MAF) oncogene homolog within this region. The MAF-transcription factor regulates the expression of proteins including PTHrP, involved in the vicious cycle of bone destruction, and MAF amplification within primary breast cancer tumours significantly correlates with bone metastasis risk (HR = 14.5, CI = 6.4 to 32.9, p < 0.001) [18,19,35].
The identification of gene expression profiles which predict the risk of bone metastasis has also recently focused upon gene expression databases resulting in a proposed predictive gene expression nomogram [36]. Measuring the level of five bone metastases-related genes (KRT23, REEP1, SPIB, ALDH3B2 and GLDC) was observed to predict bone metastasis risk within both patient training and testing sets [36].
2.2. Prostate Cancer
Studies performing genome-wide expression studies (GWAS) within prostate cancer bone metastases from patients who were either untreated or subjected to androgen-deprivation therapy (ADT) have identified three distinct molecular subtypes within bone lesions [37]. These three subtypes termed MetA-C display unique gene expression profiles. The MetA subtype was the most abundant gene expression profile within bone metastases being responsible for 71% of cases and showing increased expression of androgen-receptor-regulated genes (including prostate-specific antigen—PSA). The other molecular subtypes observed classified as MetB and MetC were responsible for 17% and 12% of cases, respectively. MetB bone metastases have alterations in genes associated with DNA damage repair and cell cycle regulation, whilst MetC metastases have enrichment for genes involved in stromal-epithelial cell interactions [37]. These genetic profiles within bone metastases were observed to correlate with different therapeutic outcomes, in particular, MetB bone metastases had the lowest levels of serum PSA as well as the poorest outcomes following ADT [37].
2.3. Lung Cancer
Genetic mutations which predispose lung cancer patients towards the development of bone metastasis have been studied by the application of next-generation sequencing (NGS). Exome sequencing conducted within eight primary tumours from NSCLC patients, compared to comparable sequencing within lung cancer patients with bone metastasis, focussed on 483 tumour-associated genes [38]. This study identified mutations, insertions and deletions within genes, with the top three genes observed to be frequently mutated being fibroblast growth factor receptor (FGFR), ataxia telangiectasia mutated (ATM), and cyclin-dependent kinase-12 (CDK12) [38]. In addition, mutations were detected within all patients sequenced for hepatocyte nuclear factor 1 alpha (HNF1α), adenomatous polyposis coli (APC), and CD22 with an over 50% mutation frequency (being 75%, 62.5%, and 50%, respectively) suggesting that these may be the genes with the greatest potential to account for bone metastasis within lung cancer patients [38].
2.4. Renal Cell Carcinoma
To date, few studies have identified genetic mutations specifically associated with the metastasis of RCC to bone sites. Next-generation sequencing has been applied to RCC primary tumours and samples isolated from metastatic sites demonstrating considerable genetic variation between the two within individual patients [39]. In this study which performed sequencing for mutations within 341 selected genes, there were no mutations detected that were enriched in either the primary or metastatic sites [39]. There was a higher incidence of genetic discordance between primary and metastatic site samples in patients harbouring mutations within the SETD2 (SET domain containing 2, histone lysine methyltransferase) gene, suggesting that this gene may regulate the rate of genetic variation within RCC cells enabling the generation of cell lines harbouring pro-metastatic mutations [39].
3. Treatment of Bone Metastases
The treatment of bone metastasis arising from breast or prostate cancer currently involves the use of anti-resorptive agents, such as bisphosphonates (i.e., zoledronic acid) [40,41], Denosumab [42] and (in the case of prostate cancer) 223Radium [43], and these agents have become a central part of treatments used to reduce the impact of SREs within cancer patients. A complete description of the treatment options for bone metastatic cancers is beyond the scope of this review but has been described elsewhere [42,44,45,46]. Two bone-targeted agents have gained widespread clinical applicability within breast cancer and their mechanisms of action are relevant as they both highlight the key importance of tumour-cell interactions within the bone micro-environment, as well as the case for predictive biomarkers is evident owing to side effects within the treatments. These concerns and mechanisms are highly relevant to the treatment of all bone metastatic cancers.
3.1. Bisphosphonates
The bisphosphonate class of drugs include the “non-nitrogen containing” bisphosphonates (etidronate and clodronate) and the “nitrogen-containing” bisphosphonates (risedronate, zoledronate, alendronate and ibandronate) [47]. The chemical structure of bisphosphonates, consisting of a P-C-P group (instead of a P-O-P group) ensures their rapid uptake by bone. Following the administration of oral bisphosphonates, approximately 50% of the dose is excreted by the kidneys, with the remaining bisphosphonate being taken up by bone resulting in an effective concentration of drug of up to 1000 mM within the resorption lacunae [48,49]. Bisphosphonates are currently used in the treatment of metastases from breast and prostate cancer, as well as in the treatment of multiple myeloma.
Bisphosphonates induce osteoclast apoptosis thus reducing osteoclast-mediated bone destruction. In a recent meta-analysis of data from the large international randomised phase-III AZURE trial, looking at the addition of bisphosphonates to standard adjuvant therapy in the treatment of patients with stage II/III breast cancer, bisphosphonate addition was observed to improve DFS and IDFS within postmenopausal women (HRDFS = 0.82, 95%CI = 0.67–1.00; HRIDFS = 0.78, 95%CI = 0.64–0.94) [35]. Despite this utility, bisphosphonates are not without side effects, most prominently osteonecrosis of the jaw (ONJ) [50]. Although the actions of bisphosphonates are mainly attributed to their induction of osteoclast apoptosis, increasing evidence suggests that bisphosphonates may also prevent apoptosis of osteoblasts and osteocytes, as well as promote the proliferation of osteoblasts [51].
3.2. Denosumab
Additional therapies have been devised for the treatment of bone metastasis including the humanised anti-RANKL monoclonal antibody agent Denosumab [42]. Within breast and prostate cancer denosumab was observed to be superior to zoledronic acid in preventing the development of first SREs [52,53] and denosumab is currently used in the clinic to treat bone metastases from breast and prostate cancer. Denosumab acts to prevent osteoclast maturation (in contrast to bisphosphonates, which induce osteoclast apoptosis) and act to reduce bone resorption. To date, there have been no effects reported of denosumab upon osteoblasts. Denosumab has side effects which are similar to those associated with the bisphosphonate class of drugs, namely: fatigue, nausea, diarrhoea, and osteonecrosis of the jaw, with the latter being an equally rare complication [54].
Biomarkers are thus urgently required to predict which patients are at highest risk of developing bone metastasis, enabling targeted treatment for higher-risk patients.
4. Biomarkers for Bone Metastasis
There is a pressing need for biomarkers predictive of bone metastasis. The development of SREs, results in release of molecular fragments derived from pro-collagen cleavage, in particular, pro-collagen type-I N-terminal pro-peptide (P1NP) and pro-collagen type I C-terminal pro-peptide (P1CP) as well as pyridinoline (PYD) and deoxypyridinoline (DPD). These protein fragments can be measured in serum and some are also detectable in urine [11]. A detailed summary of the use of bone turnover markers (BTMs) within the treatment of SREs is beyond the scope of this review, however, they are summarised in [55] and outlined in Table 2. Despite their utility, BTMs suffer several limitations including the wide range of hormone therapies used in cancer patients which interfere with BTM levels (as well as patient-related features-such as age, sex, presence of kidney and/or liver disease) which alter BTM levels [56].
Table 2.
Utility of bone turnover markers (BTMs) within bone metastasis.
| Bone Turnover Marker | Application | Reference |
|---|---|---|
| Bone Resorption Markers | ||
| N-Telopeptide of Type-I Collagen (NTX) | Diagnosis of bone metastasis—solid tumours | [57,58] |
| Prognostic role for SRE development within bone metastases from solid tumours | [11] | |
| Prediction of treatment response in prostate cancer and breast cancer | [59,60] | |
| Prognostic role within bone targeting agents’ treatment | [59,61,62,63] | |
| C-Telopeptide of Type-I Collagen (CTX) | Bone metastasis diagnosis within lung and prostate cancer | [57,58] |
| Tartrate Resistant Acid Phosphatase (TRACP) | Breast cancer bone metastasis—diagnosis of bone metastasis | [64,65] |
| Receptor Activator of Nuclear Factor ĸB-Ligand/Osteoprotegerin (RANK-L/OPG) | Breast cancer bone metastasis—diagnosis | [66] |
| Cross-Linked Carboxy-Terminal Telopeptide of Type-I Collagen (ICTP) | Lung cancer bone metastasis—diagnosis | [58] |
| Pyridinoline (PYD) | Breast cancer bone metastasis—diagnosis | [60] |
| Bone Formation Markers | ||
| Pro-Collagen-Type I N-Terminal Pro-Peptide (P1NP) | Prostate/breast cancer bone metastasis—diagnosis | [57,64,65,66,67,68] |
| Pro-Collagen-Type I C-Terminal Pro-Peptide (P1CP) | Breast cancer bone metastasis—diagnosis | [57] |
| Prostate cancer—prediction of treatment response | [60] | |
| Bone Alkaline Phosphatase (BALP) | Diagnosis of bone metastasis—solid tumours | [65,69] |
| Prognostic role for developing SRES within patients receiving bone targeting agents | [61,63,70] | |
| Prediction of treatment response in prostate cancer | [60] | |
| Prognostic role within bone metastases from solid tumours | [11] | |
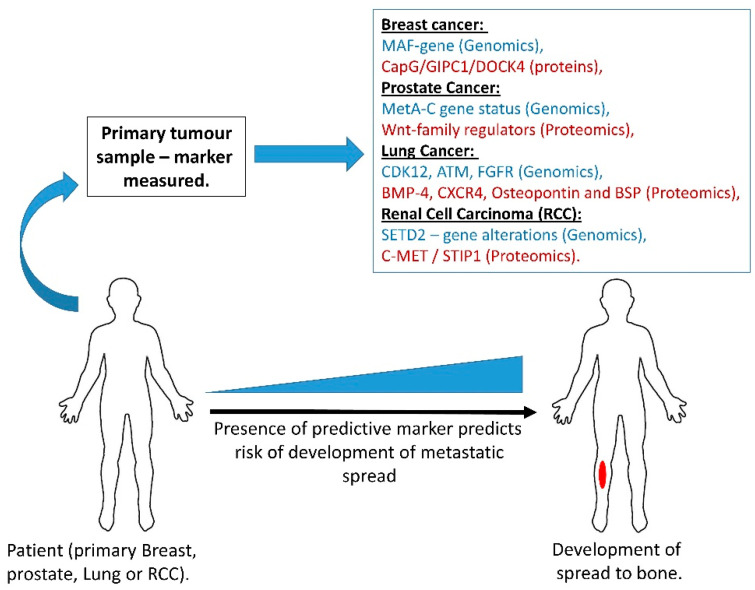
Biomarkers are thus urgently required in the diagnosis of bone metastasis as well as in identifying patients at high risk of developing spread to bone—see Figure 2.
Figure 2.
Tissue and potentially circulating biomarkers predictive of risk for cancer spread to bone.
Biomarkers are required to predict the risk of primary tumours spreading to bone. This will enable the targeting of current bone-directed therapies towards those patients who will benefit the most, thus sparing patients any unwanted side effects. These markers can have a genetic origin or be micro-RNAs or protein-based and studies have identified potential biomarkers in all of these classes. Molecules which are released from tumours into the bloodstream have the greatest utility for longitudinal monitoring of patients due to their ability to be sampled non-invasively. Tissue-based markers, cannot be sampled non-invasively, however, they have greatly revolutionised the treatment of patients with bone-metastatic cancers.
There is also a requirement for biomarkers which will predict response to therapies (prognostic markers). The following sections outline current biomarker discovery studies within genomics, epigenetics (at the level of regulatory micro-RNAs) and proteins. The utility of these markers varies from study to study, some biomarkers predict the risk of developing bone metastasis, some markers have additional utility within the diagnosis of bone metastasis as well as informing patient-treatment decisions. The potential applications of the individual markers are listed in each case.
4.1. Micro-RNAs as Key Regulators of Metastasis
An extensive body of research has highlighted the pivotal role small non-coding RNAs (micro-RNAs) play in the regulation of diverse cellular processes including cell growth, differentiation, programmed cell death, apoptosis and the cell cycle [71]. Micro-RNAs are 18–25 nucleotide long RNAs which bind to the 3′ or 5′ untranslated region (UTR) of mRNAs resulting mostly in the degradation of the mRNA or the regulation of protein translation [72]. Micro-RNAs act via complementary binding to regions of sequence homology, but may also bind to sequences with partial homology, with the thermodynamic stability of the micro-RNA-mRNA complex being pivotal [73]. Due to this mode of action, each individual micro-RNA can regulate multiple mRNAs, and each mRNA can be the target of multiple micro-RNAs. Numerous studies have highlighted the key role of micro-RNAs in the regulation of cancer and metastasis [74,75,76,77,78].
Micro-RNAs can be isolated and quantified from a wide variety of biological sources, including tissue samples, blood-based samples, urine and circulating and secreted exosomes [79,80]. Due to their role in the regulation of the steps of metastasis, and the relative ease of detecting and measuring these molecules within biological samples, there has been great interest in the role of micro-RNAs as circulating biomarkers within cancer metastasis to bone. The main marker candidates predictive of bone metastatic outcomes within patients arising from genomic and protein-based studies are summarised in Table S1
4.1.1. Breast Cancer
The absence of reliable biomarkers that predict the risk of developing bone metastasis within breast cancer patients has prompted considerable research into the potential of micro-RNAs as prognostic and diagnostic agents.
Studies conducted using both in vitro and in vivo approaches have identified the miR-30 family of micro-RNAs as being pivotal within bone metastasis of breast cancer. Metastatic spread of breast cancer cells to bone involves the increased expression of bone-related genes within the breast cancer cell. An integral player in this process of osteomimicry is the transcription factor RUNX2 [81]. Genes with increased expression mediated by RUNX2 including Collagen-α1, BSP, osteocalcin and osteopontin [82]. The expression of RUNX2 is regulated by a series of micro-RNAs including miR-30 family members (as well as miR-203 and miR-135a). Low expression of miR-30 within primary breast tumours is prognostic for poor relapse-free survival [83]. The role of miR-30 family members within bone metastasis was demonstrated by studies in which over-expression of miR-30 was observed to inhibit tumour growth in bone, reduce bone destruction and inhibit cancer cell invasion [83]. miR-30 levels correlated with ER/PR status, with ER/PR negative cells expressing lower levels of miR-30 family members than ER/PR positive cells [83]. The targets of RUNX2 which are responsible for osteomimicry are still being elucidated; however, studies have identified integrin-alpha-5 (ITGA5) and cadherin-11 (CDH11) as additional targets of miR-30 action, in addition to RUNX2.
Whilst studies have demonstrated the importance of micro-RNAs within bone metastasis, few have looked at the circulating levels of micro-RNAs within the bloodstream as a predictive tool. One such study is the elucidation of the role of miR-218 within breast cancer metastasis. Micro-RNA-218 levels are elevated within serum samples from patients with bone metastases compared to patients without metastatic spread [84]. Micro-RNA-218 is secreted by breast cancer cells within extracellular vesicles and mechanistically acts to inhibit expression of the proteins sFRP-2 and Sclerostin which are key inhibitors of Wnt-family signalling [85]. Activation of Wnt-family proteins occurs within breast cancer bone metastases, and key Wnt family targets involved in this process include PTHrP [85,86]. In this way, breast cancer secreted miR-218 can act to promote Wnt-mediated osteoclast differentiation and consequent bone destruction. Studies have demonstrated additional roles of miR-218 outside of promoting osteoclast activation, including uptake by osteoblasts inducing their reduced expression of collagen-I [84].
Several micro-RNAs have been implicated in the alterations in bone formation and breakdown induced by incoming metastatic breast cancer cells. The key role of miR-34a-5p within the inhibition of bone resorption has been demonstrated, and that this micro-RNA targets the pro-osteoclastogenic growth factor transforming growth factor-β-induced factor 2 (TGif2) [81]. Analysis within patient-derived samples demonstrated increased expression of miR-34a-5p within ductal carcinoma in situ (DCIS) samples compared to healthy mammary gland samples, as well as a greatly reduced expression within bone metastases [81].
4.1.2. Prostate Cancer
Micro-RNAs play a key role in the regulation of prostate cancer spread to bone and regulate all the key steps of metastatic dissemination.
Comparison of the micro-RNA expression profiles between bone-metastatic prostate cancer tissues and non-bone metastatic prostate cancer demonstrated that reduced miR-141-3p expression correlated with spread to bone [87]. The role of miR-141-3p in prostate cancer bone metastasis is complex with widespread effects upon numerous targets. Targets of miR-141-3p include tumour necrosis factor receptor-associated factor 5 (TRAF5) and 6 (TRAF6), which act to inhibit nuclear factor-ĸB (NF-ĸB) signalling resulting in increased bone metastasis. In addition, miR-141-3p has also been implicated as a key regulator of epithelial–mesenchymal transition (EMT) within prostate cancer. The over-expression of miR-141-3p within prostate cancer cells has been demonstrated to inhibit their invasive and migratory capabilities, whilst a reduction in miR-141-3p levels was observed to promote the spread of prostate cancer to bone [87].
A key pathway active in the bone metastatic spread of prostate cancer is TGFβ signalling. Studies have demonstrated that miR-19a-3p expression inhibits bone metastasis via targeting the TGFβ-signalling effectors SMAD2 and SMAD4 [87]. Studies demonstrated a reduced expression of miR-19a-3p as prostate cancer cells develop bone homing ability, as well as within prostate cancer tissues from patients with bone metastases.
Genetic studies have identified a cluster of micro-RNAs located on chromosome 14q32 implicated in prostate cancer bone metastasis, including the delta-like-1 homolog—deiodinase, iodothyronine 3 (DLK1-DIO3) region encompassing miR-154* (an additional micro-RNA from the miR-154 hairpin), miR-379 and miR-409-3p/-5p, all upregulated within prostate cancer and correlated with cancer spread to bone [88,89,90]. Increased expression of these micro-RNAs is observed within animal models of prostate cancer bone metastasis, and the central role of these micro-RNAs has been further validated within patient-derived samples [91,92,93]. Some of these markers have demonstrated ability to act as circulating markers of prostate cancer bone metastasis, in particular, miR-409-3p which has increased expression within the serum of patients with high-risk prostate cancer compared to low-risk patients [84]. In addition to displaying potential as circulating markers, these micro-RNAs also have potential to be tissue-based biomarkers, as demonstrated by miR-154* and miR-409-3p/-5p which display a higher level expression within prostate cancer bone metastatic samples in comparison to localised prostate cancer tissue samples [88,89].
Comparison of tissue samples from prostate cancer patients with bone metastases, and normal prostate tissue sections, as well as serum samples, identified a key role for miR-218-5p in the prediction of prostate cancer bone-metastasis-free survival [94]. Measurement of circulating serum miR-218-5p levels in patients demonstrated a ROC-curve area under curve (AUC) of 0.86 (95% confidence interval 0.80–0.92, p < 0.001) in studies of bone metastasis DFS [94]. Studies within prostate cancer cells demonstrated that miR-218-5p inhibited NF-κB signalling by targeting TRAF1, TRAF2 and TRAF5 resulting in reduced invasive and migration abilities [94].
4.1.3. Lung Cancer
Micro-RNAs have been implicated in the priming of bone sites for the arrival of metastatic lung cancer cells via promoting the conversion of mesenchymal stem cells (MSCs) into active osteoblasts. Exposure of MSCs to pro-osteogenic medium from NSCLC cells resulted in a decreased expression of miR-139-5p [95]. The action of miR-139-5p was demonstrated to occur via the downregulation of Notch-1 signalling within MSCs. Additionally, circulating serum levels of miR-139-5p were observed to be lower in lung cancer patients who developed bone metastases compared to patients with metastases to other sites [95].
Micro-RNAs have been implicated in the mode of action of genes, which act to regulate lung cancer metastasis. The gene Nm23-H1 is a demonstrated inhibitor of lung cancer metastasis to bone, and qRT-PCR-based studies demonstrated that Nm23-H1 suppresses the expression of micro-RNA miR-660-5p [96]. Micro-RNA miR-660-5p has demonstrated ability to promote bone metastasis of lung cancer via regulation of the transcriptional regulator SMARCA5 [96]. Elucidation of the function of miR-660-5p reveals a network of transcriptional regulation via micro-RNAs and transcription factors operating within the bone metastasis of lung cancer.
4.1.4. Renal Cell Carcinoma
Micro-RNAs play a key role in the development of RCC [97,98,99,100,101], however, despite this evidence, there have been few studies to date on micro-RNA expression specifically within bone metastasis of RCC. Micro-array-based quantification of micro-RNAs within matched primary RCC and bone metastatic samples from 57 patients identified a panel of 21 micro-RNAs, which demonstrated consistent down-regulation upon spread to bone [102]. The mechanism responsible for this micro-RNA down-regulation upon spread to bone appeared to be primarily epigenetic as treatment with trichostatin-A (a histone deacetylase—HDAC inhibitor) as well as 5-azacytosine (to reverse CpG methylation) was able to reverse these alterations in micro-RNA expression [102]. Predicted targets of this 21 micro-RNA panel were further validated and clustered within the “renal cell carcinoma” KEGG (Kyoto Encyclopaedia of Genes and Genomes) pathway [103].
4.2. Protein-Based Predictive Markers of Bone Metastasis
4.2.1. Breast Cancer
Several studies point to the fact that the molecular composition of breast cancer cells at the protein level influences their risk of spreading to bone. In a study of molecular subtypes within breast cancer, immunohistochemical (IHC) staining for Ki67 was combined with profiling for human epidermal growth factor 2 (HER2) and oestrogen receptor (ER) demonstrating a higher rate of bone metastasis (87.8%) within ER-positive HER2 negative tumours with a Ki67 score > 13% compared to other subtypes [104].
Proteomic techniques have recently been applied to the identification of protein signatures predictive of bone-metastasis risk within breast cancer. Repeated intra-cardiac injection of fluorescently labelled triple-negative breast cancer cells (MDA-MB-231) with subsequent isolation of breast cancer cells from bone was used to generate a bone-homing cell-line (BM1) which homes to bone upon tail vein injection [105]. Proteomic comparison of the bone homing cell line to the parental MDA-MB-231 cells has identified several proteins with potential to be markers of bone metastasis risk including macrophage actin-capping protein-1 (CAPG) and PDZ domain-containing protein-1 (GIPC1) [21] as well as dedicator of cytokinesis-4 (DOCK4) [20,22] as upregulated specifically within bone-homing breast cancer cells. Validation of these markers by IHC within primary tumour cores from the large randomised phase-III AZURE trial demonstrated that high-level expression of both CAPG and GIPC1 correlated with the development of bone metastasis (p < 0.001) as well as with the efficiency of zoledronic acid in the prevention of skeletal metastases (p < 0.008) [21]. In a parallel analysis of DOCK4 levels within the AZURE trial, patients’ high DOCK4 expression correlated with the risk of bone metastasis (HR 2.13, 95%CI 1.06–4.30, p = 0.034) [22]. These proteins have considerable potential to not only predict the risk of breast cancer spread to bone but also influence patient treatment decisions. The mechanistic role of these proteins within bone metastasis of breast cancer has recently been elucidated with the discovery that CAPG acts as an epigenetic regulator for the expression of a key pro-metastatic regulator [106].
Targeted approaches have also been applied to look at proteins selected for a potential role within the spread to bone. Based upon a review of previous data a multi-protein panel was measured within bone homing breast cancer cells (including osteopontin, bone sialoprotein, CXCR4 and cadherin-11). Such studies demonstrated that the level of the chemokine C-C ligand 2 (CCL2) was inversely correlated with the risk of developing metastatic spread to bone.
Several studies have implicated proteins secreted from breast cancer cells that alter the activity of Wnt-family signalling within metastasis to bone. The Wnt inhibitor sclerostin (SOST) is secreted by breast cancer cells and acts to inhibit the differentiation of osteoblasts thus promoting osteolysis [107]. Additional Wnt family regulators are also highly expressed within bone metastasis including the Wnt-antagonist Dickopf-1 (DKK1), which has increased expression in breast cancer metastasis and promotes apoptosis of osteoblasts [108,109]. DKK1 expression is inhibited by nitrogen-containing bisphosphonates albeit at supra-physiological concentrations of the drug. DKK1 is upregulated within bone metastases of breast cancer as well as within serum from patients with bone metastasis [110].
Studies of the cell biology of breast cancer metastasis have identified key regulators of bone metastasis including nuclear p21-activated kinase-4 (nPAK4) a repressor of ERα-transactivation [111]. nPAK4 inhibits the metastasis repressor LIFR and thus facilitates breast cancer metastasis (including to bone). nPAK4 translocates to the nucleus of breast cancer cells together with ERα to achieve its effects. In addition to cellular levels of nPAK4, breast cancer cells which express high levels of RANK have a poorer disease-free survival within univariate analysis (p = 0.04) and multivariate analysis (p = 0.02) [112]. In the same study, RANKL expression was associated with an improved bone-metastasis DFS in multivariate analysis (p = 0.03) [112].
Additional circulating effectors of breast cancer spread to bone have been discovered including prolactin (PRL). High-level expression of PRL receptor on breast cancer cells is associated with shorter times to relapse (including relapse to bone) and this effect has been demonstrated to occur via the increased differentiation of osteoclasts and resultant increased bone lysis with the release of growth factors [113]. The circulating markers influencing bone metastasis are summarised in Table 2.
4.2.2. Prostate Cancer
The high incidence of bone metastasis within advanced prostate cancer patients (occurring within 70–80% of patients with detectable metastases) has led to studies aiming to discover biomarkers predictive of bone spread. A systematic review of patient data (8644 patients) led to the proposal that a serum prostate-specific antigen (PSA) ≥ 20 ng/mL, a Gleason score ≥ 8 and the presence of locally advanced disease at the time of diagnosis was predictive of spread to bone [114]. The choice of these parameters is, however, the subject of debate and other published studies have contradicted these findings [115].
At the level of potential tissue-based markers of prostate cancer metastasis to bone, recent studies have compared the global proteome of bone metastatic prostate tumours post-surgical resection with primary prostate cancer tissue. These studies have identified molecular heterogeneity within prostate cancer bone metastases, with two predominant subgroups, one subtype expressing higher levels of targets of the androgen receptor (AR) as well as Golgi-resident and mitochondrial proteins, the other sub-group expressing high levels of proteins involved in DNA-repair and cell proliferation [116]. Expression of both of these molecular subtypes correlated with disease progression suggesting that this molecular heterogeneity should be considered when developing tissue-based biomarker panels predictive of bone metastasis risk [116].
Several studies have aimed to discover circulating biomarkers predictive for risk of bone metastasis in prostate cancer. Circulating tumour cells (CTCs) have become a key focus here with many studies seeking to address this correlation [117,118,119,120,121]. This has led to a definition of ≥5 CTC/7.5 mL as a cut-off above which men have an unfavourable prognosis. In patients, receiving abiraterone or chemotherapy a post-treatment decline in CTC count of ≥ 30% has been observed to correlate with better overall survival [122]. Molecular subtyping of prostate cancer CTCs has yielded information relevant to patient prognoses, including the observation that presence of epidermal growth factor receptor (EGFR) expressing CTCs in castration-resistant prostate cancer patients treated with docetaxel correlates with shorter overall survival (5.5 vs. 20.0 months for patients with no EGFR+ CTCs, p < 0.001) [123]. It has been observed that CTC counts are higher within patients with bone and visceral metastases (median 26, range 0–207), compared to patients with soft tissue lesions (median 0, range 0–28). Despite this, there is no correlation between CTC counts and parameters such as bone scan index (p = 0.81) and bone lesion count (p = 0.54) [124].
Secreted and circulating proteins predictive of prostate cancer bone metastasis have also been identified. The Wnt-inhibitor sclerostin is expressed at elevated levels within metastatic prostate cancer and appears to play a role in the promotion of osteolysis [125]. A significant positive relationship between sclerostin levels and bone turnover markers in metastatic PCa has been detected [126].
4.2.3. Lung Cancer
The discovery of tissue-based biomarkers predictive of lung cancer metastasis to bone has involved both proteomic and immunohistochemical studies.
Immunohistochemical analysis of primary non-small cell lung cancer (NSCLC) samples, comparing tumours with associated bone metastases, to tumours with visceral metastases and non-metastatic tumours for expression of bone sialoprotein (BSP) demonstrated a significant correlation between BSP expression and the development of bone metastases (p < 0.001) as well as worse disease outcomes (p < 0.02) [127]. BSP was a component of a subsequent immunohistochemical study within stage III NSCLC samples utilising a four protein panel (BMP-4, CXCR4, osteopontin and BSP) demonstrating a predictive specificity of 66.7% and a sensitivity of 85.7% for the development of bone metastasis [127].
Proteomic analysis of primary lung adenocarcinoma (n = 9 patients) and bone metastases (n = 28 patients) identified a series of proteins differentially expressed upon the development of spread to bone [128]. Increased expression of enolase 1 (ENO1) (HR = 1.67, logrank p = 1.9 × 10−5), NME/NM23 nucleoside diphosphate kinase 2 (NME1-NME2) (HR = 2.65, logrank p = 3.9 × 10−15) and ribosomal protein lateral stalk subunit P2 (RPLP2) (HR = 1.77, logrank p = 2.9 × 10−6) were observed, and all were significantly associated with poor patient survival (p < 0.05) [128]. IHC staining of patient tissue sections demonstrated that high ENO1 staining (odds ratio, O.R. = 7.5, p = 0.034) and low-level staining of calcyphosine-1 CAPS1 (O.R. = 0.01, p < 0.0001) were associated with development of bone metastasis [128].
The discovery of circulating biomarkers of lung cancer bone metastasis has involved both proteomic approaches and the specifically targeted measurement of cytokine levels using immunological methods. In a retrospective study of circulating levels of PTHrP within 1149 hypercalcaemic lung cancer patients, levels of circulating PTHrP > 150 pmol/L were found to be associated with decreased median survival (1.4 months vs. 5.4 months) and bone metastasis incidence (12.5% vs. 71.4%) [129]. Wnt family regulators are also involved in the regulation of lung cancer spread to bone. Sclerostin-domain containing protein-1 (SOSTDC1) is downregulated within bone metastatic NSCLC, and it has been demonstrated that SOSTDC1 acts to inhibit cell-proliferation, migration and invasion of NSCLC cells [130]. Serum levels of the Wnt-inhibitor DKK1 have also been identified as elevated within patients with bone metastasis from NSCLC [131].
4.2.4. Renal Cancer
There have been several studies aiming to identify the proteins driving the metastasis of RCC cells to bone. In one study, a bone-homing sub-clone of a clear cell RCC cell-line was generated by in vivo selection within a murine model [132,133]. Proteomic comparison of the osteotropic cell lines with the parental ccRCC cells identified 23 proteins which correlated with RCC metastasis and eight of these proteins were observed to be upregulated within bone metastasis tissue samples from RCC patients compared to primary RCC tumours [133]. These eight proteins were all involved in oxidative phosphorylation and mitochondrial function. Stress-induced phosphoprotein-1 (STIP1) was further characterised and found to exert two effects: (a) STIP1 promoted the proliferation and migration/invasion abilities of RCC tumour cells and (b) secreted STIP1 promoted the differentiation and activation of osteoclasts and the production of Cathepsin-K—a key enzyme involved in the degradation of the bone matrix [134].
Immunohistochemistry studies have demonstrated the key role of proteins such as the hepatocyte growth factor (HGF) receptor c-MET in the development of RCC bone metastases [135]. C-MET signalling has been implicated in the generation and maintenance of stem cells within bone metastases from RCC and use of a c-Met inhibitor was demonstrated to reduce the development of bone metastases within murine models and decrease the circulating serum level of the osteotropic factors IL-11 and CCL20. Inhibition of c-Met was accompanied by decreased osteoclast activity and an increase in osteoblast activity [135]. Circulating serum levels of CCL20 correlate with the development of bone metastases within RCC patients compared to metastasis to other sites [135].
4.3. Additional Sources of Circulating Markers
4.3.1. Exosomes
Exosomes are small lipid vesicles 30–100 nm in diameter, which are generated within the endosomal compartment of cells and released into the extracellular space and circulation. Increasing evidence points to the fact that tumour-released exosomes can re-program cells within the pre-metastatic niche and thus be mechanistic agents of metastasis organotropism [136]. The organotropic properties of exosomes are determined by the proteins they express, including specific integrins [137]. Exosomes contain both proteins and micro-RNAs and as such, they can be key sources of both of these types of circulating biomarkers. A complete description of how exosomes act within bone metastatic cancers is beyond the scope of this review but it has been extensively described elsewhere [138,139].
4.3.2. Circulating Tumour DNA (ctDNA)
Cancer cells are known to lyse and release DNA fragments into the circulation—termed circulating tumour DNA (ctDNA). Patients with metastatic tumours have higher levels of ctDNA than patients with localised tumours [140]. ctDNA has a wide variety of applications, and as an jkexample has been analysed within metastatic prostate cancer patients to reveal the development of key cancer driver mutations in a step towards personalised medicine [141]. ctDNA offers the possibility of a high sensitivity, PCR-based biomarker assay within bone metastasis (for a review see [142]).
4.3.3. Circulating Tumour Cells (CTCs)
In addition to ctDNA, tumours also release tumour cells (termed circulating tumour cells—CTCs) into the bloodstream. CTCs are detected and measured using methods including the FDA-approved CellSearch system [143]. CTC counting has widespread utility. In a study of 276 patients with mCRPC, before starting docetaxel chemotherapy patients were stratified into “favourable” and “unfavourable” categories on the basis of CTC counts (<5 or ≥5 CTCs per 7.5 mL blood, respectively). Patients with “unfavourable” CTC counts at baseline had a worse median overall survival (11.5 months) compared to 21.7 months in patients with “favourable” counts (p < 0.0001) [117]. In addition to CTC counts, the molecular composition of CTCs, i.e., gene expression patterns, may also be informative in monitoring cancer spread. As an example, studies have looked at the relationship between ER/PR and HER2 status of CTCs in the prediction of breast cancer outcomes [144,145]. For a more comprehensive review of the utility of CTCs see [142].
As well as CTCs, bone metastatic cancers are also characterised by the presence of disseminated tumour cells (DTCs) which are released cancer cells present within the bone marrow that have entered a state of dormancy [146]. DTCs can be counted and isolated and recent studies have demonstrated that they can be subjected to techniques such as RNA-seq as well as genomic sequencing to detect the presence of key genetic alterations with a proven role in cancer cell spread to bone [147,148,149].
5. Towards Clinical Utility of Bone Metastasis Markers—Biomarker Panels and Spatial Information
As previously outlined, there have been numerous studies discovering potential biomarkers with the power to inform patient treatment decisions. Many of these studies have involved pre-clinical model systems (animal models and cell-lines) with some studies incorporating validation within patient-derived samples. A biomarker-based test for use within the clinic must deal with tumour heterogeneity and the diverse molecular subtypes within any given cancer, and thus biomarker panels have greater potential utility than individual markers (for example see [150]).
The process of bone metastasis has a spatial dimension not tested within many biomarker studies. Experiments conducted within animal models have demonstrated that osteoclast and osteoblast proliferation and number depended strongly on whether the cells were in close proximity and direct contact with metastatic breast cancer cells [151]. In view of this research, it is becoming apparent that the clinical utility of biomarkers within bone metastasis may depend both upon their spatial location within the bone metastatic process and the timing of their alteration, with early changes having greater potential for informing more timely interventions. Considerable research interest currently focuses on the pre-metastatic niche, a spatially distinct region of bone (which overlaps with the haematopoietic stem cell niche) where the early events of bone homing occur [152].
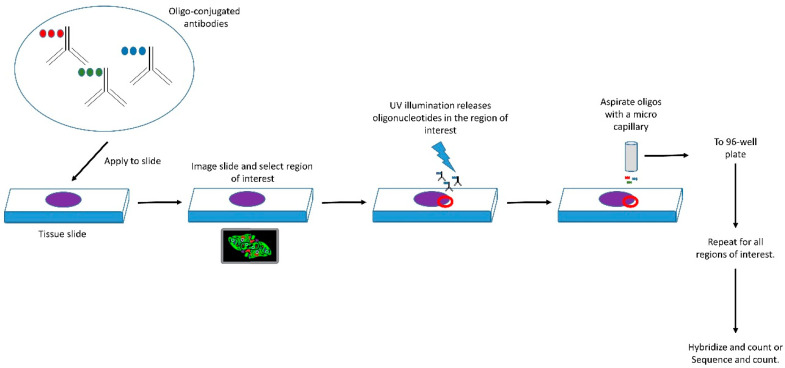
Recent advances in biomarker imaging and quantification, include the development of techniques such as digital spatial profiling (DSP) [153]. DSP involves the use of oligonucleotide-barcoded antibodies or complementary-RNAs, which are coupled together via a UV-cleavable linker. Following application of the barcoded reagents to samples (which can include FFPE-tissue sections), UV laser illumination allows release and quantification of the oligonucleotides within spatially defined regions of the sample (typically 50–100 µm in diameter) and the quantification of binding using techniques such as an nCounter machine—see Figure 3. DSP technology enables the quantification of up to 96 individual micro-RNAs or proteins in biological samples with single-cell resolution [153]. Data from DSP can be normalised via the incorporation of a positive control reagent within the analysis, a detectable molecule which is known to have a uniform and constitutive expression (pan nuclear antigens are an example of the latter). The results of DSP analysis of protein targets are antibody-dependent and to aid morphological analysis of tissue sections a panel of standard intracellular organelle marker antibodies has been developed. Data analysis by DSP is an evolving and recent application and still progressing.
Figure 3.
Digital spatial profiling (DSP) to reveal biomarker distribution within tissue sections.
Digital spatial profiling uses hybridisation reagents with individually bar-coded detection reagents to measure the levels of panels of potential markers (either proteins or micro-RNAs) within spatially defined regions of tissue sections. The detection reagents are then applied to the tissue sections and then following tissue imaging, defined regions of interest can be selected. UV laser illumination within these regions of interest releases the bound oligonucleotide bar-code reagents, which are removed using a micro-capillary. These barcode oligonucleotide reagents are then quantified using methods such as an nCounter machine. The repeating of this process across different regions of interest within the tissue section defines the spatial distribution of the panel of markers. Normalisation of data can be achieved by incorporation of positive control detection reagents within the procedure.
Digital spatial profiling is a recently developed technology, which is beginning to shed light on the cellular mechanisms of bone metastasis. In a recent study of prostate cancer bone metastasis, DSP analysis identified unique immune cell populations and signalling within lytic and blastic types of prostate cancer. Blastic lesions were populated with immune cells enriched for pSTAT3 as well as components of the JAK-STAT pathway. In contrast, within lytic-type lesions, immune cells were enriched for pAKT activity as well as components of the PI3K-AKT pathway [154]. Immune checkpoint proteins including PD-L1, B7-H4, OX40L and IDO-1 were identified within blastic prostate cancer. It is clear from these pioneering studies that biopsy samples could guide the selection of patients into appropriate therapeutic interventions based on protein levels and micro-RNA expression of desired targets [154]. Just as molecular pathology has complemented the diagnosis, treatment, and management of primary tumours it is thus possible that it could also be successfully extended to patients with metastatic lesions.
The discovery and application of biomarker panels within bone metastasis of cancers is thus at an exciting juncture at which insights from cellular and molecular biology, and the ability to measure multiple biomarkers in a spatially distinct manner, can provide complementary information. Techniques like digital spatial profiling have the ability to quantify distinct functionally defined subsets of molecules and thus identify information of relevance towards biomarker discovery. In addition, future extension of this work will enable the identification of potential therapeutic targets within these biomarkers.
6. Summary
Bone metastasis of breast, prostate, lung and kidney cancer is a very significant complication within these disease types contributing towards increased patient mortality and reduced patient quality of life. As treatments for these cancer types improve, and patient survival times increase, bone metastasis from these tumours is destined to become an ever-increasing area requiring effective prognosis, detection and treatment. Advances in molecular profiling techniques have identified numerous biomarkers, encompassing genetic mutations, regulatory micro-RNAs and proteins that can inform patient treatment potentially informing personalised medicine initiatives. The prediction of those patients at greatest risk of developing bone metastasis will enable more prompt and timely employment of bone-directed therapeutic agents. In the example of breast cancer, treatment of bone metastasis involves the use of bisphosphonate drugs that can cause side effects such as osteonecrosis of the jaw (ONJ). In view of these side effects, biomarkers of bone metastasis risk could enable targeting of bisphosphonates to those patients at greatest risk of developing skeletal complications.
Metastatic spread to bone is a spatially defined process involving the priming of a pre-metastatic niche and the migration of incoming cancer cells towards a growth-supporting bone micro-environment. Recent advances in biomarker quantification and spatial imaging open up the possibility to probe the molecular alterations occurring at the active interface between metastatic cancer cells and the reactive stroma of the bone microenvironment. Multiplex biomarker quantification at the site of active metastasis initiation within bone will enable identification of the pivotal molecules differentially expressed at the tumour–stromal interface informing personalised medicine. In addition, there is considerable potential that molecules involved at the tumour–stromal interface will have utility within drug development initiatives within bone metastasis.
7. Conclusions
Metastatic spread to bone is one of the most severe complications arising from cancers of the breast, prostate, lung and kidney. Bone metastasis greatly reduces patient quality of life and decreases patient life expectancy. The combination of well-defined cell-line and animal based models, with recent developments in the ability to identify and quantify molecules within biological samples in a robust and medium-to-high throughput manner, is beginning to shed light on the essential processes driving cancer spread to bone. The key regulators responsible for the steps of cancer cell spread to bone and bone colonization have been identified leading to the discovery of novel drug targets and potential biomarkers of cancer spread. The recent ability to measure large panels of molecules within spatially well-defined regions of tumors with single-cell resolution will enable a study of the molecular alterations occurring at the invasive front of tumor cell metastasis.
Translation of these findings into improved patient treatments will require clinical validation of potential biomarkers within well-defined patient cohorts and subsequent biomarker measurement within large patient groups to insure that developed biomarker tests are robust. High throughput methods of biomarker quantification and validation, including targeted mass spectrometry, are beginning to bridge this gap. There is thus considerable potential for molecular analysis methods to improve patient treatment outcomes in the future.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/8/2109/s1, Table S1: Genomic and protein markers predictive of bone metastatic outcomes within bone-metastatic cancers.
Funding
This work was funded by the University of Sheffield (S.L.W. and J.E.B.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Pelger R.C., Soerdjbalie-Maikoe V., Hamdy N.A. Strategies for management of prostate cancer-related bone pain. Drugs Aging. 2001;18:899–911. doi: 10.2165/00002512-200118120-00002. [DOI] [PubMed] [Google Scholar]
- 3.Costa L., Lipton A., Coleman R.E. Role of bisphosphonates for the management of skeletal complications and bone pain from skeletal metastases. Support. Cancer Ther. 2006;3:143–153. doi: 10.3816/SCT.2006.n.012. [DOI] [PubMed] [Google Scholar]
- 4.Delea T., McKiernan J., Brandman J., Edelsberg J., Sung J., Raut M., Oster G. Retrospective study of the effect of skeletal complications on total medical care costs in patients with bone metastases of breast cancer seen in typical clinical practice. J. Support. Oncol. 2006;4:341–347. [PubMed] [Google Scholar]
- 5.Delea T.E., McKiernan J., Brandman J., Edelsberg J., Sung J., Raut M., Oster G. Impact of skeletal complications on total medical care costs among patients with bone metastases of lung cancer. J. Thorac. Oncol. 2006;1:571–576. doi: 10.1097/01243894-200607000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Lage M.J., Barber B.L., Harrison D.J., Jun S. The cost of treating skeletal-related events in patients with prostate cancer. Am. J. Manag. Care. 2008;14:317–322. [PubMed] [Google Scholar]
- 7.Coleman R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 8.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 9.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Pineros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 10.Pros. [(accessed on 27 June 2020)]; Available online: https://www.healthline.com/health/prostate-cancer-prognosis-life-expectancy-bone-metastases.
- 11.Brown J.E., Cook R.J., Major P., Lipton A., Saad F., Smith M., Lee K.A., Zheng M., Hei Y.J., Coleman R.E. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J. Natl. Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 12.Stanley K.E. Prognostic factors for survival in patients with inoperable lung cancer. J. Natl. Cancer Inst. 1980;65:25–32. [PubMed] [Google Scholar]
- 13.Freise G., Gabler A., Liebig S. Bronchial carcinoma and long-term survival. Retrospective study of 433 patients who underwent resection. Thorax. 1978;33:228–234. doi: 10.1136/thx.33.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SEER. [(accessed on 29 June 2020)]; Available online: http://seer.cancer/gov/statfacts/index.html.
- 15.Zekri J., Ahmed N., Coleman R.E., Hancock B.W. The skeletal metastatic complications of renal cell carcinoma. Int. J. Oncol. 2001;19:379–382. doi: 10.3892/ijo.19.2.379. [DOI] [PubMed] [Google Scholar]
- 16.Lipton A., Colombo-Berra A., Bukowski R.M., Rosen L., Zheng M., Urbanowitz G. Skeletal complications in patients with bone metastases from renal cell carcinoma and therapeutic benefits of zoledronic acid. Clin. Cancer Res. 2004;10:6397S–6403S. doi: 10.1158/1078-0432.CCR-040030. [DOI] [PubMed] [Google Scholar]
- 17.Szendroi A., Dinya E., Kardos M., Szasz A.M., Nemeth Z., Ats K., Kiss J., Antal I., Romics I., Szendroi M. Prognostic factors and survival of renal clear cell carcinoma patients with bone metastases. Pathol. Oncol. Res. 2010;16:29–38. doi: 10.1007/s12253-009-9184-7. [DOI] [PubMed] [Google Scholar]
- 18.Coleman R., Hall A., Albanell J., Hanby A., Bell R., Cameron D., Dodwell D., Marshall H., Jean-Mairet J., Tercero J.C., et al. Effect of MAF amplification on treatment outcomes with adjuvant zoledronic acid in early breast cancer: A secondary analysis of the international, open-label, randomised, controlled, phase 3 AZURE (BIG 01/04) trial. Lancet Oncol. 2017;18:1543–1552. doi: 10.1016/S1470-2045(17)30603-4. [DOI] [PubMed] [Google Scholar]
- 19.Pavlovic M., Arnal-Estape A., Rojo F., Bellmunt A., Tarragona M., Guiu M., Planet E., Garcia-Albeniz X., Morales M., Urosevic J., et al. Enhanced MAF Oncogene Expression and Breast Cancer Bone Metastasis. J. Natl. Cancer Inst. 2015;107:djv256. doi: 10.1093/jnci/djv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown J.E., Westbrook J.A., Wood S.L. Dedicator of Cytokinesis 4: A Potential Prognostic and Predictive Biomarker Within the Metastatic Spread of Breast Cancer to Bone. Cancer Inform. 2019;18:1176935119866842. doi: 10.1177/1176935119866842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westbrook J.A., Cairns D.A., Peng J., Speirs V., Hanby A.M., Holen I., Wood S.L., Ottewell P.D., Marshall H., Banks R.E., et al. CAPG and GIPC1: Breast Cancer Biomarkers for Bone Metastasis Development and Treatment. J. Natl. Cancer Inst. 2016;108 doi: 10.1093/jnci/djv360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westbrook J.A., Wood S.L., Cairns D.A., McMahon K., Gahlaut R., Thygesen H., Shires M., Roberts S., Marshall H., Oliva M.R., et al. Identification and validation of DOCK4 as a potential biomarker for risk of bone metastasis development in patients with early breast cancer. J. Pathol. 2019;247:381–391. doi: 10.1002/path.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croset M., Kan C., Clezardin P. Tumour-derived miRNAs and bone metastasis. Bonekey Rep. 2015;4:688. doi: 10.1038/bonekey.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 25.Blonder J., Xiao Z., Veenstra T.D. Proteomic profiling of differentiating osteoblasts. Expert Rev. Proteom. 2006;3:483–496. doi: 10.1586/14789450.3.5.483. [DOI] [PubMed] [Google Scholar]
- 26.Guise T.A. Breaking down bone: New insight into site-specific mechanisms of breast cancer osteolysis mediated by metalloproteinases. Genes Dev. 2009;23:2117–2123. doi: 10.1101/gad.1854909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M.T., Martin T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 28.Sims N.A., Gooi J.H. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin. Cell Dev. Biol. 2008;19:444–451. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Artacho-Cordon F., Rios-Arrabal S., Lara P.C., Artacho-Cordon A., Calvente I., Nunez M.I. Matrix metalloproteinases: Potential therapy to prevent the development of second malignancies after breast radiotherapy. Surg. Oncol. 2012;21:e143–e151. doi: 10.1016/j.suronc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Tauro M., Lynch C.C. Cutting to the Chase: How Matrix Metalloproteinase-2 Activity Controls Breast-Cancer-to-Bone Metastasis. Cancers. 2018;10:185. doi: 10.3390/cancers10060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang X., Gong K., Zhang X., Wu S., Cui Y., Qian B.Z. Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharmacol. Res. 2019;144:235–244. doi: 10.1016/j.phrs.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 32.Wong S.K., Mohamad N.V., Giaze T.R., Chin K.Y., Mohamed N., Ima-Nirwana S. Prostate Cancer and Bone Metastases: The Underlying Mechanisms. Int. J. Mol. Sci. 2019;20:2587. doi: 10.3390/ijms20102587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spanjol J., Djordjevic G., Markic D., Klaric M., Fuckar D., Bobinac D. Role of bone morphogenetic proteins in human prostate cancer pathogenesis and development of bone metastases: Immunohistochemical study. Coll. Antropol. 2010;34:119–125. [PubMed] [Google Scholar]
- 34.Huntley R., Jensen E., Gopalakrishnan R., Mansky K.C. Bone morphogenetic proteins: Their role in regulating osteoclast differentiation. Bone Rep. 2019;10:100207. doi: 10.1016/j.bonr.2019.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coleman R.E., Collinson M., Gregory W., Marshall H., Bell R., Dodwell D., Keane M., Gil M., Barrett-Lee P., Ritchie D., et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04) J. Bone Oncol. 2018;13:123–135. doi: 10.1016/j.jbo.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao C., Lou Y., Wang Y., Wang D., Tang L., Gao X., Zhang K., Xu W., Liu T., Xiao J. A gene expression signature-based nomogram model in prediction of breast cancer bone metastases. Cancer Med. 2019;8:200–208. doi: 10.1002/cam4.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thysell E., Vidman L., Ylitalo E.B., Jernberg E., Crnalic S., Iglesias-Gato D., Flores-Morales A., Stattin P., Egevad L., Widmark A., et al. Gene expression profiles define molecular subtypes of prostate cancer bone metastases with different outcomes and morphology traceable back to the primary tumor. Mol. Oncol. 2019;13:1763–1777. doi: 10.1002/1878-0261.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang K., Zhang M., Zhu J., Hong W. Screening of gene mutations associated with bone metastasis in nonsmall cell lung cancer. J. Cancer Res. Ther. 2016;12:186–190. doi: 10.4103/0973-1482.200597. [DOI] [PubMed] [Google Scholar]
- 39.Becerra M.F., Reznik E., Redzematovic A., Tennenbaum D.M., Kashan M., Ghanaat M., Casuscelli J., Manley B., Jonsson P., DiNatale R.G., et al. Comparative Genomic Profiling of Matched Primary and Metastatic Tumors in Renal Cell Carcinoma. Eur. Urol. Focus. 2018;4:986–994. doi: 10.1016/j.euf.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong M.H., Stockler M.R., Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2012;2:CD003474. doi: 10.1002/14651858.CD003474.pub3. [DOI] [PubMed] [Google Scholar]
- 41.Coleman R.E., McCloskey E.V. Bisphosphonates in oncology. Bone. 2011;49:71–76. doi: 10.1016/j.bone.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Gul G., Sendur M.A., Aksoy S., Sever A.R., Altundag K. A comprehensive review of denosumab for bone metastasis in patients with solid tumors. Curr. Med. Res. Opin. 2016;32:133–145. doi: 10.1185/03007995.2015.1105795. [DOI] [PubMed] [Google Scholar]
- 43.Orcajo-Rincon J., Caresia-Aroztegui A.P., Del Puig Cozar-Santiago M., Garcia-Garzon J.R., De Arcocha-Torres M., Delgado-Bolton R.C., Garcia-Velloso M.J., Alvarez-Ruiz S., Garcia-Vicente A.M. Radium-223 in the treatment of bone metastasis in patients with castration-resistant prostate cancer. Review and procedure. Rev. Esp. Med. Nucl. Imagen Mol. 2018;37:330–337. doi: 10.1016/j.remn.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Litwin M.S., Tan H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA. 2017;317:2532–2542. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 45.Wu Z., Lu J. Advances in treatment of metastatic breast cancer with bone metastasis. Chin. Clin. Oncol. 2018;7:31. doi: 10.21037/cco.2018.06.05. [DOI] [PubMed] [Google Scholar]
- 46.LeVasseur N., Clemons M., Hutton B., Shorr R., Jacobs C. Bone-targeted therapy use in patients with bone metastases from lung cancer: A systematic review of randomized controlled trials. Cancer Treat. Rev. 2016;50:183–193. doi: 10.1016/j.ctrv.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Roelofs A.J., Thompson K., Ebetino F.H., Rogers M.J., Coxon F.P. Bisphosphonates: Molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr. Pharm. Des. 2010;16:2950–2960. doi: 10.2174/138161210793563635. [DOI] [PubMed] [Google Scholar]
- 48.Sousa S., Clezardin P. Bone-Targeted Therapies in Cancer-Induced Bone Disease. Calcif. Tissue Int. 2018;102:227–250. doi: 10.1007/s00223-017-0353-5. [DOI] [PubMed] [Google Scholar]
- 49.Cremers S., Drake M.T., Ebetino F.H., Bilezikian J.P., Russell R.G.G. Pharmacology of bisphosphonates. Br. J. Clin. Pharmacol. 2019;85:1052–1062. doi: 10.1111/bcp.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vescovi P., Nammour S. Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ) therapy. A critical review. Minerva Stomatol. 2010;59:181–203. [PubMed] [Google Scholar]
- 51.Maruotti N., Corrado A., Neve A., Cantatore F.P. Bisphosphonates: Effects on osteoblast. Eur. J. Clin. Pharmacol. 2012;68:1013–1018. doi: 10.1007/s00228-012-1216-7. [DOI] [PubMed] [Google Scholar]
- 52.Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., De Boer R.H., Lichinitser M., Fujiwara Y., Yardley D.A., Viniegra M., et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 53.Fizazi K., Carducci M., Smith M., Damiao R., Brown J., Karsh L., Milecki P., Shore N., Rader M., Wang H., et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ford J.A., Jones R., Elders A., Mulatero C., Royle P., Sharma P., Stewart F., Todd R., Mowatt G. Denosumab for treatment of bone metastases secondary to solid tumours: Systematic review and network meta-analysis. Eur. J. Cancer. 2013;49:416–430. doi: 10.1016/j.ejca.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 55.Ferreira A., Alho I., Casimiro S., Costa L. Bone remodeling markers and bone metastases: From cancer research to clinical implications. Bonekey Rep. 2015;4:668. doi: 10.1038/bonekey.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coleman R., Costa L., Saad F., Cook R., Hadji P., Terpos E., Garnero P., Brown J., Body J.J., Smith M., et al. Consensus on the utility of bone markers in the malignant bone disease setting. Crit. Rev. Oncol. Hematol. 2011;80:411–432. doi: 10.1016/j.critrevonc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Westerhuis L.W., Delaere K.P. Diagnostic value of some biochemical bone markers for the detection of bone metastases in prostate cancer. Eur. J. Clin. Chem. Clin. Biochem. 1997;35:89–94. doi: 10.1515/cclm.1997.35.2.89. [DOI] [PubMed] [Google Scholar]
- 58.Kong Q.Q., Sun T.W., Dou Q.Y., Li F., Tang Q., Pei F.X., Tu C.Q., Chen Z.Q. Beta-CTX and ICTP act as indicators of skeletal metastasis status in male patients with non-small cell lung cancer. Int. J. Biol. Markers. 2007;22:214–220. doi: 10.5301/JBM.2008.3777. [DOI] [PubMed] [Google Scholar]
- 59.Coleman R.E., Lipton A., Costa L., Cook R.J., Lee K.A., Saad F., Brown J.E., Terpos E., Major P.P., Kohno N., et al. Possible survival benefits from zoledronic acid treatment in patients with bone metastases from solid tumours and poor prognostic features-An exploratory analysis of placebo-controlled trials. J. Bone Oncol. 2013;2:70–76. doi: 10.1016/j.jbo.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lara P.N., Jr., Ely B., Quinn D.I., Mack P.C., Tangen C., Gertz E., Twardowski P.W., Goldkorn A., Hussain M., Vogelzang N.J., et al. Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: Results from SWOG 0421. J. Natl. Cancer Inst. 2014;106:dju013. doi: 10.1093/jnci/dju013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coleman R.E., Major P., Lipton A., Brown J.E., Lee K.A., Smith M., Saad F., Zheng M., Hei Y.J., Seaman J., et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J. Clin. Oncol. 2005;23:4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 62.Lipton A., Cook R., Saad F., Major P., Garnero P., Terpos E., Brown J.E., Coleman R.E. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 63.Lipton A., Smith M.R., Fizazi K., Stopeck A.T., Henry D., Brown J.E., Shore N.D., Saad F., Spencer A., Zhu L., et al. Changes in Bone Turnover Marker Levels and Clinical Outcomes in Patients with Advanced Cancer and Bone Metastases Treated with Bone Antiresorptive Agents. Clin. Cancer Res. 2016;22:5713–5721. doi: 10.1158/1078-0432.CCR-15-3086. [DOI] [PubMed] [Google Scholar]
- 64.Wada N., Fujisaki M., Ishii S., Ikeda T., Kitajima M. Evaluation of bone metabolic markers in breast cancer with bone metastasis. Breast Cancer. 2001;8:131–137. doi: 10.1007/BF02967492. [DOI] [PubMed] [Google Scholar]
- 65.Lumachi F., Basso S.M., Camozzi V., Tozzoli R., Spaziante R., Ermani M. Bone turnover markers in women with early stage breast cancer who developed bone metastases. A prospective study with multivariate logistic regression analysis of accuracy. Clin. Chim. Acta. 2016;460:227–230. doi: 10.1016/j.cca.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 66.Elfar G.A., Ebrahim M.A., Elsherbiny N.M., Eissa L.A. Validity of Osteoprotegerin and Receptor Activator of NF-kappaB Ligand for the Detection of Bone Metastasis in Breast Cancer. Oncol. Res. 2017;25:641–650. doi: 10.3727/096504016X14768398678750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oremek G., Sauer-Eppel H., Klepzig M. Total procollagen type 1 amino-terminal propeptide (total P1NP) as a bone metastasis marker in gynecological carcinomas. Anticancer Res. 2007;27:1961–1962. [PubMed] [Google Scholar]
- 68.Koopmans N., De Jong I.J., Breeuwsma A.J., Van der Veer E. Serum bone turnover markers (PINP and ICTP) for the early detection of bone metastases in patients with prostate cancer: A longitudinal approach. J. Urol. 2007;178:849–853. doi: 10.1016/j.juro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 69.Du W.X., Duan S.F., Chen J.J., Huang J.F., Yin L.M., Tong P.J. Serum bone-specific alkaline phosphatase as a biomarker for osseous metastases in patients with malignant carcinomas: A systematic review and meta-analysis. J. Cancer Res. Ther. 2014;10:140–143. doi: 10.4103/0973-1482.145842. [DOI] [PubMed] [Google Scholar]
- 70.Brown J.E., Cook R.J., Lipton A., Costa L., Coleman R.E. Prognostic factors for skeletal complications from metastatic bone disease in breast cancer. Breast Cancer Res. Treat. 2010;123:767–779. doi: 10.1007/s10549-010-0981-1. [DOI] [PubMed] [Google Scholar]
- 71.Gulyaeva L.F., Kushlinskiy N.E. Regulatory mechanisms of microRNA expression. J. Transl. Med. 2016;14:143. doi: 10.1186/s12967-016-0893-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deng X., Liu Y., Luo M., Wu J., Ma R., Wan Q., Wu J. Circulating miRNA-24 and its target YKL-40 as potential biomarkers in patients with coronary heart disease and type 2 diabetes mellitus. Oncotarget. 2017;8:63038–63046. doi: 10.18632/oncotarget.18593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 74.Calin G.A., Dumitru C.D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K., et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayashita Y., Osada H., Tatematsu Y., Yamada H., Yanagisawa K., Tomida S., Yatabe Y., Kawahara K., Sekido Y., Takahashi T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 76.Zhang L., Huang J., Yang N., Greshock J., Megraw M.S., Giannakakis A., Liang S., Naylor T.L., Barchetti A., Ward M.R., et al. microRNAs exhibit high frequency genomic alterations in human cancer. Proc. Natl. Acad. Sci. USA. 2006;103:9136–9141. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Esquela-Kerscher A., Slack F.J. Oncomirs—Micrornas with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 78.Peng Y., Croce C.M. The role of MicroRNAs in human cancer. Signal Transduct Target. Ther. 2016;1:1–9. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Christodoulatos G.S., Dalamaga M. Micro-RNAs as clinical biomarkers and therapeutic targets in breast cancer: Quo vadis? World J. Clin. Oncol. 2014;5:71–81. doi: 10.5306/wjco.v5.i2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weidle U.H., Epp A., Birzele F., Brinkmann U. The Functional Role of Prostate Cancer Metastasis-related Micro-RNAs. Cancer Genom. Proteom. 2019;16:1–19. doi: 10.21873/cgp.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krzeszinski J.Y., Wei W., Huynh H., Jin Z., Wang X., Chang T.C., Xie X.J., He L., Mangala L.S., Lopez-Berestein G., et al. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature. 2014;512:431–435. doi: 10.1038/nature13375. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Bruderer M., Richards R.G., Alini M., Stoddart M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014;28:269–286. doi: 10.22203/eCM.v028a19. [DOI] [PubMed] [Google Scholar]
- 83.Croset M., Pantano F., Kan C.W.S., Bonnelye E., Descotes F., Alix-Panabieres C., Lecellier C.H., Bachelier R., Allioli N., Hong S.S., et al. miRNA-30 Family Members Inhibit Breast Cancer Invasion, Osteomimicry, and Bone Destruction by Directly Targeting Multiple Bone Metastasis-Associated Genes. Cancer Res. 2018;78:5259–5273. doi: 10.1158/0008-5472.CAN-17-3058. [DOI] [PubMed] [Google Scholar]
- 84.Liu X., Cao M., Palomares M., Wu X., Li A., Yan W., Fong M.Y., Chan W.C., Wang S.E. Metastatic breast cancer cells overexpress and secrete miR-218 to regulate type I collagen deposition by osteoblasts. Breast Cancer Res. 2018;20:1–12. doi: 10.1186/s13058-018-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taipaleenmaki H., Farina N.H., Van Wijnen A.J., Stein J.L., Hesse E., Stein G.S., Lian J.B. Antagonizing miR-218-5p attenuates Wnt signaling and reduces metastatic bone disease of triple negative breast cancer cells. Oncotarget. 2016;7:79032–79046. doi: 10.18632/oncotarget.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson R.W., Merkel A.R., Page J.M., Ruppender N.S., Guelcher S.A., Sterling J.A. Wnt signaling induces gene expression of factors associated with bone destruction in lung and breast cancer. Clin. Exp. Metastasis. 2014;31:945–959. doi: 10.1007/s10585-014-9682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang S., Wa Q., Pan J., Peng X., Ren D., Huang Y., Chen X., Tang Y. Downregulation of miR-141-3p promotes bone metastasis via activating NF-kappaB signaling in prostate cancer. J. Exp. Clin. Cancer Res. 2017;36:1–13. doi: 10.1186/s13046-017-0645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gururajan M., Josson S., Chu G.C., Lu C.L., Lu Y.T., Haga C.L., Zhau H.E., Liu C., Lichterman J., Duan P., et al. miR-154* and miR-379 in the DLK1-DIO3 microRNA mega-cluster regulate epithelial to mesenchymal transition and bone metastasis of prostate cancer. Clin. Cancer Res. 2014;20:6559–6569. doi: 10.1158/1078-0432.CCR-14-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Josson S., Gururajan M., Hu P., Shao C., Chu G.Y., Zhau H.E., Liu C., Lao K., Lu C.L., Lu Y.T., et al. miR-409-3p/-5p promotes tumorigenesis, epithelial-to-mesenchymal transition, and bone metastasis of human prostate cancer. Clin. Cancer Res. 2014;20:4636–4646. doi: 10.1158/1078-0432.CCR-14-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Josson S., Gururajan M., Sung S.Y., Hu P., Shao C., Zhau H.E., Liu C., Lichterman J., Duan P., Li Q., et al. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene. 2015;34:2690–2699. doi: 10.1038/onc.2014.212. [DOI] [PubMed] [Google Scholar]
- 91.Hagan J.P., O’Neill B.L., Stewart C.L., Kozlov S.V., Croce C.M. At least ten genes define the imprinted Dlk1-Dio3 cluster on mouse chromosome 12qF1. PLoS ONE. 2009;4:e4352. doi: 10.1371/journal.pone.0004352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Formosa A., Markert E.K., Lena A.M., Italiano D., Finazzi-Agro E., Levine A.J., Bernardini S., Garabadgiu A.V., Melino G., Candi E. MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, mapped to the 14q32.31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene. 2014;33:5173–5182. doi: 10.1038/onc.2013.451. [DOI] [PubMed] [Google Scholar]
- 93.Liu L., Luo G.Z., Yang W., Zhao X., Zheng Q., Lv Z., Li W., Wu H.J., Wang L., Wang X.J., et al. Activation of the imprinted Dlk1-Dio3 region correlates with pluripotency levels of mouse stem cells. J. Biol. Chem. 2010;285:19483–19490. doi: 10.1074/jbc.M110.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng P., Chen T., Wang Q., Zhang Y., Zheng F., Huang S., Tang Y., Yang C., Ding W., Ren D., et al. Decreased miR-218-5p Levels as a Serum Biomarker in Bone Metastasis of Prostate Cancer. Oncol. Res. Treat. 2019;42:165–185. doi: 10.1159/000495473. [DOI] [PubMed] [Google Scholar]
- 95.Xu S., Yang F., Liu R., Li X., Fan H., Liu J., Wei S., Chen G., Chen J., Da Y. Serum microRNA-139-5p is downregulated in lung cancer patients with lytic bone metastasis. Oncol. Rep. 2018;39:2376–2384. doi: 10.3892/or.2018.6316. [DOI] [PubMed] [Google Scholar]
- 96.Ai C., Ma G., Deng Y., Zheng Q., Gen Y., Li W., Li Y., Zu L., Zhou Q. Nm23-H1 inhibits lung cancer bone-specific metastasis by upregulating miR-660-5p targeted SMARCA5. Thorac. Cancer. 2020;11:640–650. doi: 10.1111/1759-7714.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gottardo F., Liu C.G., Ferracin M., Calin G.A., Fassan M., Bassi P., Sevignani C., Byrne D., Negrini M., Pagano F., et al. Micro-RNA profiling in kidney and bladder cancers. Urol. Oncol. 2007;25:387–392. doi: 10.1016/j.urolonc.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 98.Nakada C., Matsuura K., Tsukamoto Y., Tanigawa M., Yoshimoto T., Narimatsu T., Nguyen L.T., Hijiya N., Uchida T., Sato F., et al. Genome-wide microRNA expression profiling in renal cell carcinoma: Significant down-regulation of miR-141 and miR-200c. J. Pathol. 2008;216:418–427. doi: 10.1002/path.2437. [DOI] [PubMed] [Google Scholar]
- 99.Petillo D., Kort E.J., Anema J., Furge K.A., Yang X.J., Teh B.T. MicroRNA profiling of human kidney cancer subtypes. Int. J. Oncol. 2009;35:109–114. doi: 10.3892/ijo_00000318. [DOI] [PubMed] [Google Scholar]
- 100.Jung M., Mollenkopf H.J., Grimm C., Wagner I., Albrecht M., Waller T., Pilarsky C., Johannsen M., Stephan C., Lehrach H., et al. MicroRNA profiling of clear cell renal cell cancer identifies a robust signature to define renal malignancy. J. Cell Mol. Med. 2009;13:3918–3928. doi: 10.1111/j.1582-4934.2009.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Juan D., Alexe G., Antes T., Liu H., Madabhushi A., Delisi C., Ganesan S., Bhanot G., Liou L.S. Identification of a microRNA panel for clear-cell kidney cancer. Urology. 2010;75:835–841. doi: 10.1016/j.urology.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 102.Wotschofsky Z., Liep J., Meyer H.A., Jung M., Wagner I., Disch A.C., Schaser K.D., Melcher I., Kilic E., Busch J., et al. Identification of metastamirs as metastasis-associated microRNAs in clear cell renal cell carcinomas. Int. J. Biol. Sci. 2012;8:1363–1374. doi: 10.7150/ijbs.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Papadopoulos G.L., Alexiou P., Maragkakis M., Reczko M., Hatzigeorgiou A.G. DIANA-mirPath: Integrating human and mouse microRNAs in pathways. Bioinformatics. 2009;25:1991–1993. doi: 10.1093/bioinformatics/btp299. [DOI] [PubMed] [Google Scholar]
- 104.Savci-Heijink C.D., Halfwerk H., Hooijer G.K., Horlings H.M., Wesseling J., Van de Vijver M.J. Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res. Treat. 2015;150:547–557. doi: 10.1007/s10549-015-3352-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kang Y., Siegel P.M., Shu W., Drobnjak M., Kakonen S.M., Cordon-Cardo C., Guise T.A., Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/S1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 106.Huang S., Chi Y., Qin Y., Wang Z., Xiu B., Su Y., Guo R., Guo L., Sun H., Zeng C., et al. CAPG enhances breast cancer metastasis by competing with PRMT5 to modulate STC-1 transcription. Theranostics. 2018;8:2549–2564. doi: 10.7150/thno.22523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hesse E., Schroder S., Brandt D., Pamperin J., Saito H., Taipaleenmaki H. Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight. 2019;5 doi: 10.1172/jci.insight.125543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rachner T.D., Gobel A., Benad-Mehner P., Hofbauer L.C., Rauner M. Dickkopf-1 as a mediator and novel target in malignant bone disease. Cancer Lett. 2014;346:172–177. doi: 10.1016/j.canlet.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 109.Rachner T.D., Gobel A., Thiele S., Rauner M., Benad-Mehner P., Hadji P., Bauer T., Muders M.H., Baretton G.B., Jakob F., et al. Dickkopf-1 is regulated by the mevalonate pathway in breast cancer. Breast Cancer Res. 2014;16:R20. doi: 10.1186/bcr3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kasoha M., Bohle R.M., Seibold A., Gerlinger C., Juhasz-Boss I., Solomayer E.F. Dickkopf-1 (Dkk1) protein expression in breast cancer with special reference to bone metastases. Clin. Exp. Metastasis. 2018;35:763–775. doi: 10.1007/s10585-018-9937-3. [DOI] [PubMed] [Google Scholar]
- 111.Li Y., Zhang H., Zhao Y., Wang C., Cheng Z., Tang L., Gao Y., Liu F., Li J., Li Y., et al. A mandatory role of nuclear PAK4-LIFR axis in breast-to-bone metastasis of ERalpha-positive breast cancer cells. Oncogene. 2019;38:808–821. doi: 10.1038/s41388-018-0456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park H.S., Lee A., Chae B.J., Bae J.S., Song B.J., Jung S.S. Expression of receptor activator of nuclear factor kappa-B as a poor prognostic marker in breast cancer. J. Surg. Oncol. 2014;110:807–812. doi: 10.1002/jso.23737. [DOI] [PubMed] [Google Scholar]
- 113.Sutherland A., Forsyth A., Cong Y., Grant L., Juan T.H., Lee J.K., Klimowicz A., Petrillo S.K., Hu J., Chan A., et al. The Role of Prolactin in Bone Metastasis and Breast Cancer Cell-Mediated Osteoclast Differentiation. J. Natl. Cancer Inst. 2016;108 doi: 10.1093/jnci/djv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abuzallouf S., Dayes I., Lukka H. Baseline staging of newly diagnosed prostate cancer: A summary of the literature. J. Urol. 2004;171:2122–2127. doi: 10.1097/01.ju.0000123981.03084.06. [DOI] [PubMed] [Google Scholar]
- 115.Briganti A., Suardi N., Gallina A., Abdollah F., Novara G., Ficarra V., Montorsi F. Predicting the risk of bone metastasis in prostate cancer. Cancer Treat. Rev. 2014;40:3–11. doi: 10.1016/j.ctrv.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 116.Iglesias-Gato D., Thysell E., Tyanova S., Crnalic S., Santos A., Lima T.S., Geiger T., Cox J., Widmark A., Bergh A., et al. The Proteome of Prostate Cancer Bone Metastasis Reveals Heterogeneity with Prognostic Implications. Clin. Cancer Res. 2018;24:5433–5444. doi: 10.1158/1078-0432.CCR-18-1229. [DOI] [PubMed] [Google Scholar]
- 117.De Bono J.S., Scher H.I., Montgomery R.B., Parker C., Miller M.C., Tissing H., Doyle G.V., Terstappen L.W., Pienta K.J., Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 118.Danila D.C., Heller G., Gignac G.A., Gonzalez-Espinoza R., Anand A., Tanaka E., Lilja H., Schwartz L., Larson S., Fleisher M., et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin. Cancer Res. 2007;13:7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 119.Olmos D., Arkenau H.T., Ang J.E., Ledaki I., Attard G., Carden C.P., Reid A.H., A’Hern R., Fong P.C., Oomen N.B., et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): A single-centre experience. Ann. Oncol. 2009;20:27–33. doi: 10.1093/annonc/mdn544. [DOI] [PubMed] [Google Scholar]
- 120.Scher H.I., Jia X., De Bono J.S., Fleisher M., Pienta K.J., Raghavan D., Heller G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–239. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goldkorn A., Ely B., Quinn D.I., Tangen C.M., Fink L.M., Xu T., Twardowski P., Van Veldhuizen P.J., Agarwal N., Carducci M.A., et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: A phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014;32:1136–1142. doi: 10.1200/JCO.2013.51.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lorente D., Olmos D., Mateo J., Bianchini D., Seed G., Fleisher M., Danila D.C., Flohr P., Crespo M., Figueiredo I., et al. Decline in Circulating Tumor Cell Count and Treatment Outcome in Advanced Prostate Cancer. Eur. Urol. 2016;70:985–992. doi: 10.1016/j.eururo.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Okegawa T., Itaya N., Hara H., Tambo M., Nutahara K. Epidermal Growth Factor Receptor Status in Circulating Tumor Cells as a Predictive Biomarker of Sensitivity in Castration-Resistant Prostate Cancer Patients Treated with Docetaxel Chemotherapy. Int. J. Mol. Sci. 2016;17:2008. doi: 10.3390/ijms17122008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thalgott M., Rack B., Maurer T., Souvatzoglou M., Eiber M., Kress V., Heck M.M., Andergassen U., Nawroth R., Gschwend J.E., et al. Detection of circulating tumor cells in different stages of prostate cancer. J. Cancer Res. Clin. Oncol. 2013;139:755–763. doi: 10.1007/s00432-013-1377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Larson S.R., Zhang X., Dumpit R., Coleman I., Lakely B., Roudier M., Higano C.S., True L.D., Lange P.H., Montgomery B., et al. Characterization of osteoblastic and osteolytic proteins in prostate cancer bone metastases. Prostate. 2013;73:932–940. doi: 10.1002/pros.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yavropoulou M.P., Van Lierop A.H., Hamdy N.A., Rizzoli R., Papapoulos S.E. Serum sclerostin levels in Paget’s disease and prostate cancer with bone metastases with a wide range of bone turnover. Bone. 2012;51:153–157. doi: 10.1016/j.bone.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 127.Papotti M., Kalebic T., Volante M., Chiusa L., Bacillo E., Cappia S., Lausi P., Novello S., Borasio P., Scagliotti G.V. Bone sialoprotein is predictive of bone metastases in resectable non-small-cell lung cancer: A retrospective case-control study. J. Clin. Oncol. 2006;24:4818–4824. doi: 10.1200/JCO.2006.06.1952. [DOI] [PubMed] [Google Scholar]
- 128.Yang M., Sun Y., Sun J., Wang Z., Zhou Y., Yao G., Gu Y., Zhang H., Zhao H. Differentially expressed and survival-related proteins of lung adenocarcinoma with bone metastasis. Cancer Med. 2018;7:1081–1092. doi: 10.1002/cam4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hiraki A., Ueoka H., Bessho A., Segawa Y., Takigawa N., Kiura K., Eguchi K., Yoneda T., Tanimoto M., Harada M. Parathyroid hormone-related protein measured at the time of first visit is an indicator of bone metastases and survival in lung carcinoma patients with hypercalcemia. Cancer. 2002;95:1706–1713. doi: 10.1002/cncr.10828. [DOI] [PubMed] [Google Scholar]
- 130.Chen G., Gong H., Wang T., Wang J., Han Z., Bai G., Han S., Yang X., Zhou W., Liu T., et al. SOSTDC1 inhibits bone metastasis in non-small cell lung cancer and may serve as a clinical therapeutic target. Int. J. Mol. Med. 2018;42:3424–3436. doi: 10.3892/ijmm.2018.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Qiao R., Zhong R., Chang Q., Teng J., Pei J., Han B., Chu T. Serum dickkopf-1 as a clinical and prognostic factor in non-small cell lung cancer patients with bone metastases. Oncotarget. 2017;8:79469–79479. doi: 10.18632/oncotarget.18446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J., Chen A., Yang C., Zeng H., Qi J., Guo F.J. A bone-seeking clone exhibits different biological properties from the ACHN parental human renal cell carcinoma in vivo and in vitro. Oncol. Rep. 2012;27:1104–1110. doi: 10.3892/or.2011.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang J., Zhao X., Qi J., Yang C., Cheng H., Ren Y., Huang L. Eight proteins play critical roles in RCC with bone metastasis via mitochondrial dysfunction. Clin. Exp. Metastasis. 2015;32:605–622. doi: 10.1007/s10585-015-9731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang J., You H., Qi J., Yang C., Ren Y., Cheng H. Autocrine and paracrine STIP1 signaling promote osteolytic bone metastasis in renal cell carcinoma. Oncotarget. 2017;8:17012–17026. doi: 10.18632/oncotarget.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.D’Amico L., Belisario D., Migliardi G., Grange C., Bussolati B., D’Amelio P., Perera T., Dalmasso E., Dalle Carbonare L., Godio L., et al. C-met inhibition blocks bone metastasis development induced by renal cancer stem cells. Oncotarget. 2016;7:45525–45537. doi: 10.18632/oncotarget.9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Myint P.K., Park E.J., Gaowa A., Kawamoto E., Shimaoka M. Targeted remodeling of breast cancer and immune cell homing niches by exosomal integrins. Diagn. Pathol. 2020;15:1–4. doi: 10.1186/s13000-020-00959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Mark M.T., Molina H., Kohsaka S., Di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wong G.L., Jalboush S.A., Lo H.W. Exosomal MicroRNAs and Organotropism in Breast Cancer Metastasis. Cancers. 2020;12:1827. doi: 10.3390/cancers12071827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ponzetti M., Rucci N. Switching Homes: How Cancer Moves to Bone. Int. J. Mol. Sci. 2020;21:4124. doi: 10.3390/ijms21114124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leon S.A., Shapiro B., Sklaroff D.M., Yaros M.J. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646–650. [PubMed] [Google Scholar]
- 141.Kumar A., Coleman I., Morrissey C., Zhang X., True L.D., Gulati R., Etzioni R., Bolouri H., Montgomery B., White T., et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 2016;22:369–378. doi: 10.1038/nm.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gourdin T., Sonpavde G. Utility of cell-free nucleic acid and circulating tumor cell analyses in prostate cancer. Asian J. Androl. 2018;20:230–237. doi: 10.4103/aja.aja_1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Riethdorf S., Fritsche H., Muller V., Rau T., Schindlbeck C., Rack B., Janni W., Coith C., Beck K., Janicke F., et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch system. Clin. Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 144.Pestrin M., Bessi S., Puglisi F., Minisini A.M., Masci G., Battelli N., Ravaioli A., Gianni L., Di Marsico R., Tondini C., et al. Final results of a multicenter phase II clinical trial evaluating the activity of single-agent lapatinib in patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells. A proof-of-concept study. Breast Cancer Res. Treat. 2012;134:283–289. doi: 10.1007/s10549-012-2045-1. [DOI] [PubMed] [Google Scholar]
- 145.Agelaki S., Kalykaki A., Markomanolaki H., Papadaki M.A., Kallergi G., Hatzidaki D., Kalbakis K., Mavroudis D., Georgoulias V. Efficacy of Lapatinib in Therapy-Resistant HER2-Positive Circulating Tumor Cells in Metastatic Breast Cancer. PLoS ONE. 2015;10:e0123683. doi: 10.1371/journal.pone.0123683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Summers M.A., McDonald M.M., Croucher P.I. Cancer Cell Dormancy in Metastasis. Cold Spring Harb. Perspect. Med. 2020;10 doi: 10.1101/cshperspect.a037556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cackowski F.C., Wang Y., Decker J.T., Sifuentes C., Weindorf S., Jung Y., Wang Y., Decker A.M., Yumoto K., Szerlip N., et al. Detection and isolation of disseminated tumor cells in bone marrow of patients with clinically localized prostate cancer. Prostate. 2019;79:1715–1727. doi: 10.1002/pros.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maheswaran S., Haber D.A. Circulating tumor cells: A window into cancer biology and metastasis. Curr. Opin. Genet. Dev. 2010;20:96–99. doi: 10.1016/j.gde.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li X.Y., Dong M., Zang X.Y., Li M.Y., Zhou J.Y., Ma J.J., Wang G.Y. The emerging role of circulating tumor cells in cancer management. Am. J. Transl. Res. 2020;12:332–342. [PMC free article] [PubMed] [Google Scholar]
- 150.Muinao T., Boruah H.P.D., Pal M. Multi-biomarker panel signature as the key to diagnosis of ovarian cancer. Heliyon. 2019;5:e02826. doi: 10.1016/j.heliyon.2019.e02826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brown H.K., Ottewell P.D., Evans C.A., Holen I. Location matters: Osteoblast and osteoclast distribution is modified by the presence and proximity to breast cancer cells in vivo. Clin. Exp. Metastasis. 2012;29:927–938. doi: 10.1007/s10585-012-9481-5. [DOI] [PubMed] [Google Scholar]
- 152.Liu Y., Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell. 2016;30:668–681. doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 153.DSP. [(accessed on 29 June 2020)]; Available online: https://www.nanostring.com/scientific-content/technology-overview/digital-spatial-profiling-technology.
- 154.Ihle C.L., Provera M.D., Straign D.M., Smith E.E., Edgerton S.M., Van Bokhoven A., Lucia M.S., Owens P. Distinct tumor microenvironments of lytic and blastic bone metastases in prostate cancer patients. J. Immunother. Cancer. 2019;7:293. doi: 10.1186/s40425-019-0753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.