Abstract
Background
Only limited evidence has been available to date on the accuracy of systematic low-dose chest computed tomography (LDCT) use in the diagnosis of COVID-19 in patients with non-specific clinical symptoms.
Methods
The COVID-19 Imaging Registry Study Aachen (COVID-19-Bildgebungs-Register Aachen, COBRA) collects data on imaging in patients with COVID-19. Two of the COBRA partner hospitals (RWTH Aachen University Hospital and Dueren Hospital) systematically perform reverse transcriptase polymerase chain reaction (RT-PCR) from nasopharyngeal swabs as well as LDCT in all patients presenting with manifestations that are compatible with COVID-19. In accordance with the COV-RADS protocol, the LDCT scans were prospectively evaluated before the RT-PCR findings were available in order to categorize the likelihood of COVID-19.
Results
From 18 March to 5 May 2020, 191 patients with COVID-19 manifestations (117 male, age 65 ± 16 years) underwent RT-PCR testing and LDCT. The mean time from the submission of the sample to the availability of the RT-PCR findings was 491 minutes (interquartile range [IQR: 276–1066]), while that from the performance of the CT to the availability of its findings was 9 minutes (IQR: 6–11). A diagnosis of COVID-19 was made in 75/191 patients (39%). The LDCT was positive in 71 of these 75 patients and negative in 106 of the 116 patients without COVID-19, corresponding to 94.7% sensitivity (95% confidence interval [86.9; 98.5]), 91.4% specificity [84.7; 95.8], positive and negative predictive values of 87.7% [78.5; 93.9] and 96.4% [91.1; 98.6], respectively, and an AUC (area under the curve) of 0.959 [0.930; 0.988]. The initial RT-PCR test results were falsely negative in six patients, yielding a sensitivity of 92.0% [83.4; 97.0]; these six patients had positive LDCT findings. 47.4% of the LDCTs that were negative for COVID-19 (55/116) exhibited pathological pulmonary changes, including infiltrates, that were correctly distinguished from SARS-CoV-2 related changes.
Conclusion
In patients with symptoms compatible with COVID-19, LDCT can esablish the diagnosis of COVID-19 with comparable sensitivity to RT-PCR testing. In addition, it offers a high specificity for distinguishing COVID-19 from other diseases associated with the same or similar clinical symptoms. We propose the systematic use of LDCT in addition to RT-PCR testing because it helps correct false-negative RT-PCR results, because its results are available much faster than those of RT-PCR-testing, and because it provides additional diagnostic information useful for treatment planning regardless of the type of the infectious agent.
The standard procedure for identifying coronavirus disease 2019 (COVID-19) in patients with clinical symptoms is a nasopharyngeal swab with subsequent reverse transcriptase–polymerase chain reaction (RT-PCR) for viral RNA identification (1, 2). RT-PCR analysis can identify virus material contained in a swab sample with almost complete certainty, allowing a SARS-CoV-2 infection to be specifically diagnosed (3). However, even if a swab is taken correctly, false-negative RT-PCR results can occur (4, 5). In the case of suggestive clinical findings, it is therefore common practice to repeat the swab testing.
Non-enhanced low-dose computed tomography (LDCT) of the chest was used early on to help guide management decisions in patients with COVID-19. COVID-19–associated pneumonia was found to often cause typical findings, such as relatively dense, peripheral, so-called ground glass opacities as well as focal consolidations and a “crazy paving” pattern (6, 7). A first observational study from Wuhan that compared the accuracy of LDCT versus RT-PCR testing in symptomatic patients found that LDCT identified patients with COVID-19 with a high sensitivity and helped to identify infected patients with initial or repetitively (false-) negative PCR results (8, 9). Furthermore, serial LDCT and swab examinations showed that LDCT identified positive patients up to five days faster than swab/RT-PCR for 60% to 93% of patients (8). Some facilities in Germany and Europe therefore use LDCT to complement RT-PCR testing for diagnosing COVID-19. However, this use of LDCT is controversial; some medical societies have explicitly advised against it, based on the lack of evidence from studies outside of China, among other reasons (10– 16).
As part of the COBRA study (COVID-19 Imaging Registry Study Aachen), the radiological departments of the facilities in the Aachen / Düren / Heinsberg area have joined forces to collect data on imaging in persons with suspected COVID-19. In two of the participating locations (namely, the RWTH Aachen University Hospital and Dueren Hospital), LDCT has been used in parallel to RT-PCR for the diagnosis of COVID-19. In the present work, we report the first experiences with this procedure.
Methods
The available data were collected between 18 March and 5 May, 2020, in the RWTH Aachen University Hospital (UKA, Universitätsklinikum Aachen) and Dueren Hospital (KHD, Krankenhaus Düren gGmbH) and made available for this analysis as part of the COBRA study (Ethics Committee Permission EK 097/20; German Clinical Trials Register DRKS00021740).
Data from patients who presented with clinical symptoms of COVID-19 and who received both RT-PCR testing and LDCT examinations within a 24-h time frame were included (see eMethods). Data from patients whose SARS-CoV-2 status had already been determined by RT-PCR analysis at the time of the CT examination were excluded from the analysis. For details on swab sampling and the CT technique, see eMethods. Non-enhanced LDCT of the chest was associated with an average radiation exposure of about 1.7 millisievert (mSv) for a 75-kg patient.
All LDCT examinations were prospectively assessed by one of six (UKA) or one of two (KHD) radiologists who were blinded to the RT-PCR results (which were not available at the time of CT diagnosis, anyway). All diagnoses were categorized according to a COV-RADS (COVID-19–Reporting and Data System) scheme (Table 1, eMethods).
Table 1. COV-RADS Scheme.
| Category | Description |
| COV-RADS 1 | Normal lung with no evidence of pneumonia or other pathology |
| COV-RADS 2 | Pathological CT finding of lungs, but no evidence of COVID-19* |
| COV-RADS 3 | CT findings that may be attributable to COVID-19 |
| COV-RADS 4 | CT findings that are suspicious of COVID-19 |
| COV-RADS 5 | CT findings that are typical of COVID-19 |
*This refers to findings that can mimic the clinical symptoms of COVID-19; for example, CT findings typical of bronchopneumonia, typical lobar pneumonia, or other viral pneumonia, etc.RADS, Reporting and Data System
As published data suggest a high rate of false-negative RT-PCR results (1, 2, 9), a composite standard of reference that took into account both the RT-PCR result and the further clinical course of a patient was used to determine the final diagnosis of “COVID-19 positive” or “COVID-19 negative” as the ground truth (eMethods).
The sensitivity, specificity, and negative predictive value (NPV) or positive predictive value (PPV) of the LDCT findings and the primary RT-PCT test result (that is, the RT-PCR test result of the swab taken at the same time as the LDCT) were determined on the basis of this reference standard. In addition, a receiver operating characteristic (ROC) analysis was carried out for the LDCT. For details on the radiological and statistical analyses of the LDCT findings, see eMethods.
Results
During the analysis period, 191 patients who presented at UKA (n= 145) or KHD (n= 46) with clinical symptoms compatible with COVID-19 underwent a nasopharyngeal swab with RT-PCR analysis as well as an LDCT of the thorax.
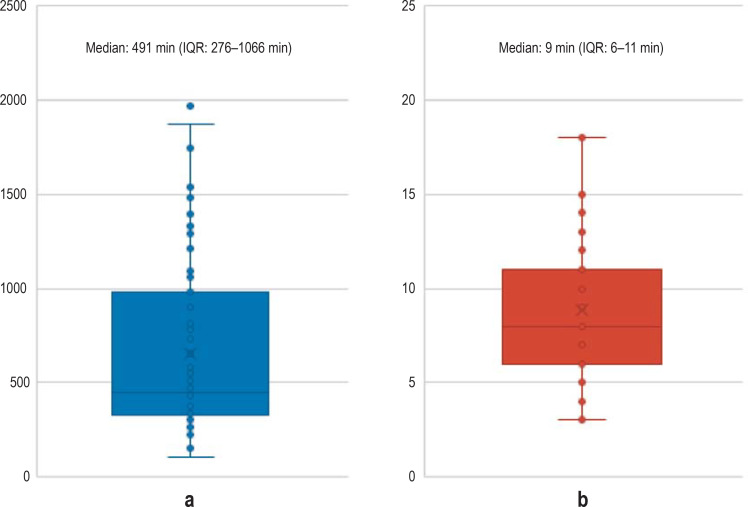
The median interval time between collecting the swab sample and performing an LDCT was 52 min (interquartile range [IQR], 0.3–3.3 h). The median time from sample receipt to availability of RT-PCR results was 491 min (IQR, 276 min to 1066 min). The median time from the LDCT examination to notification of LDCT results was 9 min (IQR, 6 min to 11 min). Thus, the median time required until the LDCT results were available was eight hours shorter than that for the RT-PCR results (IQR, 4.5–17.5 h) (eFigures 1, 2).
eFigure 1.
Time to availability of RT-PCR results (a) and time to availability of LDCT results (b) for the first 124 patients at Aachen University Hospital (UKA)
a) time from sample arrival at central laboratory of the UKA until availability of RT-PCR results
b) time from LDCT acquisition until availability of LDCT findings
IQR, interquartile range
Table 2 shows the relevant demographic data of the patients as well as the duration and type of clinical symptoms. The mean age was 64.9 ± 16.4 years; the symptoms had been present in the majority of patients (109/191) for less than a week at the time of examination. Fever (58%), cough (53%), and dyspnea (46%) were the most common symptoms.
Table 2. Demographic and clinical characteristics of the patient cohort.
| Total | COVID-19 present | COVID-19 absent | p | ||||
| Number | 191 | 100% | 75 | 39% | 116 | 61% | |
| Age (years) | 0.775 | ||||||
| Mean | 64.9 | 66.3 | 63.7 | ||||
| Median | 66 | 64 | 66 | ||||
| Range | 19–99 | 23–90 | 19–99 | ||||
| Sex | 0.508 | ||||||
| Male | 117 | 61% | 45 | 60% | 72 | 62% | |
| Female | 74 | 39% | 30 | 40% | 44 | 38% | |
| Time since onset of clinical symptoms | 0.006 | ||||||
| n/a (no symptoms) | 1 | 0.5% | 1 | 1% | 0 | 0% | |
| Acute (today) | 18 | 9% | 1 | 1% | 17 | 15% | |
| <1 week | 109 | 57% | 39 | 52% | 70 | 60% | |
| >1 <2 weeks | 33 | 17% | 25 | 33% | 8 | 7% | |
| >2 weeks | 30 | 16% | 9 | 12% | 21 | 18% | |
| Type of clinical symptoms | |||||||
| Fever/low fever | 110 | 58% | 51 | 68% | 59 | 51% | 0.056 |
| Cough | 102 | 53% | 43 | 57% | 59 | 51% | 0.356 |
| Shortness of breath/dyspnea | 88 | 46% | 32 | 43% | 56 | 48% | 0.685 |
| Gastrointest. symptoms | 24 | 13% | 18 | 24% | 11 | 9% | 0.194 |
| Fatigue | 37 | 19% | 13 | 17% | 19 | 16% | 0.183 |
A final diagnosis of “COVID-19 positive” was made for 75/191 patients (39.3%), and of “COVID-19 negative” for the remaining 116/191 (60.7%).
RT-PCR testing of the nasopharyngeal swabs was positive in 69/191 patients (36.1%) and negative in 122/191 (63.9%). LDCT findings were positive in 81/191 patients (42.4%), and negative in 110/191 (57.6%) (Figure 1).
Figure 1:
LDCT findings of two patients who each presented with fever and cough and with
a) COV-RADS 5 (COVID-19 typical finding)
b) COV-RADS 2 (pathological finding but without changes suspicious of COVID-19)
The PCR examination confirmed a SARS-COV-2 infection of patient (a) and did not result in virus detection in patient (b).
Of the 75 patients with a positive reference standard for COVID-19, 69 had positive RT-PCR results, and 71 had positive LDCT findings. All of the 116 patients who had a negative reference standard also received negative RT-PCR results, and 106 of these had negative LDCT findings (table 3).
Table 3. Four-field table and diagnostic indices of LDCT and RT-PCR.
| Four-field table: LDCT versus reference standard | |||
| COVID-19 present | COVID-19 absent | All patients | |
| CT positive | 71 | 10 | 81 |
| CT negative | 4 | 106 | 110 |
| Total | 75 | 116 | 191 |
| Four-field table: RT-PCR versus reference standard | |||
| PCR positive* | 69 | 0 | 69 |
| PCR negative | 6 | 116 | 122 |
| Total | 75 | 116 | 191 |
| * One patient had a positive swab/RT-PCR result only after re-testing; this was classified here as a positive PCR result | |||
| Diagnostic indices of LDCT | |||
| [95% CI] | |||
| Sensitivity | 71/75 | 94.7% | [86.9; 98.5] |
| Specificity | 106/116 | 91.4% | [84.7; 95.8] |
| PPV | 71/81 | 87.7% | [79.7; 92.8] |
| NPV | 106/110 | 96.4% | [91.1; 98.6] |
| Diagn. acc. | 177/191 | 92.7% | [88.0; 95.9] |
| LR+ | 11.0 | [6.1; 19.9] | |
| Diagnostic indices of RT-PCR testing | |||
| Sensitivity | 69/75 | 92.0% | [83.4; 97.0] |
| Specificity | 116/116 | 100.0% | [96.9; 100.0] |
| PPV | 69/69 | 100.0% | [94.8; 100.0] |
| NPV | 116/122 | 95.1% | [90.0; 97.7] |
| Diagn. acc. | 185/191 | 96.8% | [93.3; 98.8] |
| LR+ | maximum | ||
95% CI, 95% confidence interval; diagn. acc., diagnostic accuracy;
LR+, positive likelihood ratio; NPV, negative predictive value;
PPV, positive predictive value
For four patients with positive RT-PCR results, the LDCT findings were categorized as false negative; two of these patients exhibited normal lungs on LD-CT, and two had pathological findings that were deemed unrelated to COVID-19 (COV-RADS 2).
Sixteen patients with a negative RT-PCR result had a positive LDCT finding. Of these, the RT-PCR results of six patients were categorized as false-negative based on the further course of illness, as follows: a 47-year-old woman presenting with fever, dyspnea, and cough, and COV-RADS 5 results on LDCT had a negative swab result and only received a positive result when the test was repeated two days later. Of the remaining 15 patients with positive LDCT findings but negative RT-PCR results, repeated/repetitive RT-PCR testings remained negative; for five of these patients, COVID-19 was considered the most likely differential diagnosis based on the clinical course (table 4).
Table 4. Further clinical course of 16 patients with primary negative RT-PCR results and positive LDCT findings.
| Age, sex | Number of swabs | Clinical symptoms | Time since onset of symptoms | LDCT finding: COV-RADS | Further diagnoses/ known co-existing conditions/ course of illness | COVID-19 as final diagnosis |
| 47 years,female | 2 | Fever, cough, dyspnea | <1 week | 5 | None known; re-tested RT-PCR two days later → positive | |
| 59 years, male | 3 | Dyspnea, no fever | <1 week | 4 | COPD, TAA, cardiac decompensation, lupus erythematosus | |
| 83 years, female | 2 | Fever, cough, dyspnea, tachypnea | <1 week | 3 | Mitral regurgitation, cardiac decompensation, also infection → broad-spectrum antibiotic treatment, unsuccessful → Campylobacter enteritis confirmed → additionally given Clacid → slow recovery | |
| 81 years, female | 2 | Fever, respiratory distress syndrome with high oxygen demand, invasive ventilation | <1 week | 4 | None; no improvement despite invasive ventilation and broad-spectrum antibiotic treatment for 2 weeks; finally recovery and resolution of fever | |
| 59 years, male | 2 | Pronounced dyspnea, hypercapnia; severe septic shock with no evidence of microorganisms; invasive ventilation; patient died | Slightly more than a week | 3 | None; extremely rapid course with multi-organ failure; died after 4 days of intensive treatment | |
| 61 years, male | 5 | Fever, cough | More than 2 weeks | 3 | CMV pneumonia with ARDS | |
| 89 years, male | 2 | Fever, dyspnea; contact to SARS-CoV-2 virus carrier in long-term nursing home | <1 week | 3 | COPD | |
| 44 years, female | 2 | Fever, productive cough, dyspnea, vomiting | >2 weeks | 3 | Sepsis with Staphylococcus epidermidis | |
| 56 years, female | 2 | No fever, dry cough, exhaustion | >2 weeks | 3 | Bronchial carcinoma, treated previously | |
| 80 years, female | 1 | No fever, cough, dyspnea, peripheral edema | >2 weeks | 3 | Heart failure NYHA III – IV, cardiac decompensation | |
| 72 years, male | 1 | No fever, no cough, dyspnea, decompensated heart failure | <1 weeks | 4 | Acute posterior myocardial infarction with emergency coronary angioplasty | |
| 63 years, female | 2 | Fever with infection | 1–2 weeks | 3 | Unclear viral infection, immunosuppression after kidney transplant | |
| 79 years, male | 3 | Low fever, dry cough, fatigue | >2 weeks | 4 | No further conditions | |
| 70 years, female | 4 | Low fever, productive cough, fatigue | <1 weeks | 3 | Community-acquired pneumonia, pathogen unspecified | |
| 76 years, male | 2 | Fever, fatigue | Acute | 3 | Urinary tract infection | |
| 35 years, male | 2 | Fever, cough, loss of taste | <1 week | 3 | Community-acquired bacterial pneumonia |
![]() definitely;
definitely; ![]() unlikely;
unlikely; ![]() probably
probably
ARDS, acute respiratory distress syndrome; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; COV-RADS, COVID-19 Reporting and Data System; NYHA, New York Heart Association; TAA, tachyarrhythmia absoluta
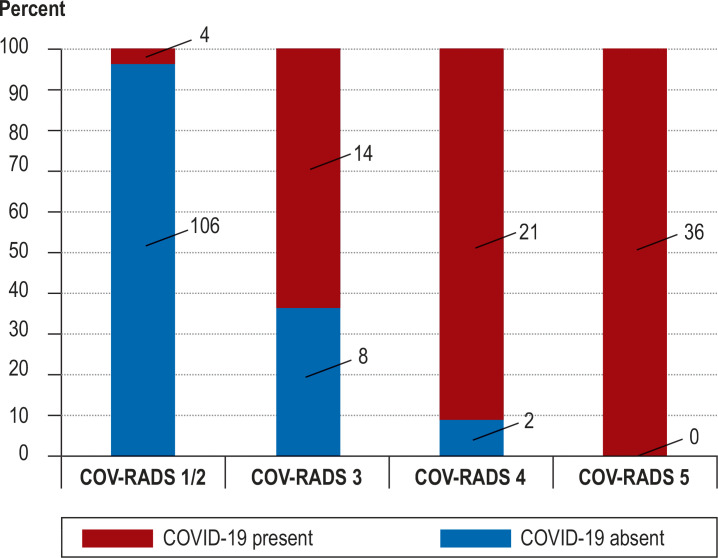
Table 3 provides the diagnostic indices (sensitivity, specificity, PPV, and NPV) of results from LDCT and RT-PCR in comparison with the reference standard; the frequency of COVID-19 depending on the COV-RADS category is shown in Figure 2.
Figure 2.
Likelihood of COVID-19 by COV-RADS category
COV-RADS, COVID-19 Reporting and Data System
The ROC analysis of the LDCT findings showed an area under the curve (AUC) of 0.959 (95% confidence interval [0.930; 0.988]) (efigure 3).
eFigure 3.
ROC curve of LDCT
Distinctions between four categories of increasing probability of COVID-19:
COV-RADS 1 and 2 (equally no evidience of COVID-19), COV-RADS 3 (possible),
COV-RADS-4 (probable), COV-RADS-5 (typical).
95% CI, 95% confidence interval; AUC, area under the curve; COV-RADS, COVID-19
Reporting and Data System; ROC, receiver operating characteristic
If RT-PCR is chosen as the reference standard, the positive predictive value of LDCT drops to around 80%, as expected (etable 1). When the centers (UKA, KHD) were differentiated, slight differences in test qualities became apparent (etable 2).
eTable 1. Diagnostic accuracy of LDCT compared to RT-PCR as the “reference standard”.
| LDCT versus RT-PCR as “reference standard” | |||
| PCR positive | PCR negative | All patients | |
| CT positive | 65 | 16 | 81 |
| CT negative | 4 | 106 | 110 |
| Total | 69 | 122 | 191 |
| * One patient had a positive swab/RT-PCR result only after re-testing; this was classified here as a positive PCR result | |||
| Diagnostic parameters of LDCT using RT-PCR results as the “reference standard” * | |||
| [95% CI] | |||
| Sensitivity | 65/69 | 94.2% | [85.8; 98.4] |
| Specificity | 106/122 | 86.9% | [79.6; 92.3] |
| PPV | 65/81 | 80.2% | [71.9; 86.6] |
| NPV | 106/110 | 96.4% | [91.1; 98.6] |
| Diagnostic accuracy | 171/191 | 89.5% | [84.3; 93.5] |
| LR+ | 7.18 | [4.53; 11.38] | |
*One patient had a positive swab/RT-PCR result only after re-testing; this was classified here as a positive RT-PCR result
95% CI, 95% confidence interval; LR+, positive likelihood ratio; NPV, negative predictive value; PPV, positive predictive value
eTable 2. Diagnostic accuracy of LDCT versus reference standard, per center.
| LDCT versus reference standard at Aachen University Hospital | ||||
| COVID-19 positive | COVID-19 negative | All patients | ||
| CT positive | 50 | 6 | 56 | |
| CT negative | 2 | 87 | 89 | |
| Total | 52 | 93 | 145 | |
| LDCT versus reference standard at Dueren Hospital | ||||
| CT positive | 21 | 4 | 25 | |
| CT negative | 2 | 19 | 21 | |
| Total | 23 | 23 | 46 | |
| Diagnostic parameters for LDCT in the individual centers | ||||
| Aachen University Hospital | Dueren Hospital | |||
| Sensitivity | 50/52 | 96.2% | 21/23 | 91.3% |
| Specificity | 87/93 | 93.5% | 19/23 | 82.6% |
| PPV | 50/56 | 89.3% | 21/25 | 84.0% |
| NPV | 87/89 | 97.8% | 19/21 | 90.5% |
| Diagn. acc. | 137/145 | 94.5% | 40/46 | 87.0% |
| LR+ | 14.90 | 5.02 | ||
Diagn. acc., diagnostic accuracy; LR+, positive likelihood ratio; NPV, negative predictive value; PPV, positive predictive value
The type and frequency distribution of the LDCT findings are shown in eTable 3. About half (55/116) of the patients who were correctly categorized as “test-negative” by LDCT did not have normal findings but rather exhibited pathological pulmonary findings on LDCT (Figure 1b) corresponding to ground glass opacities and/or consolidations of different severity. Thus, most CT imaging findings were, in principle, observable in both, patients with COVID-19 and those with lung disease attributable to other causes. Nonetheless, a distinction could be made with high accuracy between COVID-19 and non–COVID-19 diseases, based on the criteria described in eTable 3.
eTable 3. Prevalence and distribution of imaging findings.
| All patients(n = 191) | % | COVID-19 present(n = 75) | % | COVID-19 absent(n = 116) | % | p | |
| Ground glass opacity | 115 | 60% | 73 | 97% | 42 | 36% | <0.001 |
| Symmetry | 0.010 | ||||||
| – unilateral | 15 | 8% | 5 | 7% | 10 | 9% | |
| – bilateral | 100 | 52% | 68 | 91% | 32 | 28% | |
| Axial distribution | <0.001 | ||||||
| – central | 11 | 6% | 1 | 1% | 10 | 9% | |
| – peripheral | 57 | 30% | 43 | 57% | 14 | 12% | |
| – central + peripheral | 47 | 25% | 29 | 39% | 18 | 16% | |
| Cranio-caudal distribution | <0.001 | ||||||
| – apical predominance | 15 | 8% | 5 | 7% | 10 | 9% | |
| – basal predominance | 53 | 28% | 33 | 44% | 20 | 17% | |
| – uniform | 66 | 35% | 35 | 47% | 31 | 27% | |
| Severity | <0.001 | ||||||
| 1 | 25 | 13% | 7 | 9% | 18 | 16% | |
| 2 | 39 | 20% | 24 | 32% | 15 | 13% | |
| 3 | 31 | 16% | 27 | 36% | 4 | 3% | |
| 4 | 16 | 8% | 13 | 17% | 3 | 3% | |
| 5 | 4 | 2% | 2 | 3% | 2 | 2% | |
| Consolidations | 97 | 51% | 56 | 75% | 41 | 35% | <0.001 |
| Symmetry | 0.032 | ||||||
| – unilateral | 32 | 17% | 13 | 17% | 19 | 16% | |
| – bilateral | 65 | 34% | 43 | 57% | 22 | 19% | |
| Axial distribution | <0.001 | ||||||
| – central | 7 | 4% | 0 | 0% | 7 | 6% | |
| – peripheral | 57 | 30% | 41 | 55% | 16 | 14% | |
| – central + peripheral | 33 | 17% | 15 | 20% | 18 | 16% | |
| Severity | 0.778 | ||||||
| 1 | 28 | 15% | 15 | 20% | 13 | 11% | |
| 2 | 45 | 24% | 29 | 39% | 16 | 14% | |
| 3 | 19 | 10% | 9 | 12% | 10 | 9% | |
| 4 | 3 | 2% | 2 | 3% | 1 | 1% | |
| 5 | 2 | 1% | 1 | 1% | 1 | 1% | |
| Other signs of viral pneumonia | 37 | 19% | 28 | 37% | 9 | 8% | <0.001 |
| Crazy paving pattern | 31 | 16% | 23 | 31% | 8 | 7% | 0.001 |
| Atoll signs | 0 | 0% | 0 | 0% | 0 | 0% | 1 |
| Halo signs | 10 | 5% | 9 | 12% | 1 | 1% | 0.002 |
CT findings in COVID-19 versus non–COVID-19 associated pneumonia
Ground glass opacity (GGO) and consolidations were more common in patients who were positive for COVID-19 than those who were negative (97% vs. 36%, p <0.001; and 75% vs. 35%, p <0.001, respectively). GGO and consolidations were more often peripheral in COVID-19–positive versus COVID-19–negative patients (57% vs. 12%, and 55% vs. 14%, respectively). The extent of GGO lung involvement was also significantly higher in patients with COVID-19 than in patients without COVID-19 (median of 3 vs. 2; p = 0.001). No differences were seen in the extent of consolidation (2 vs. 2; p = 0.778). Other signs of viral pneumonia (crazy paving and halo signs) were rare overall but were more often present in patients with COVID-19 (31% vs. 7% for patients without COVID-19; p <0.001).
Discussion
This analysis of 191 patients who presented with clinical symptoms compatible with COVID-19 in the outpatient clinics of UKA (Aachen) or KHD (Düren) and who underwent both, swap sampling with RT-PCR testing and non-enhanced LDCT demonstrates that patients with COVID-19 can be identified by LDCT with high diagnostic accuracy. Non-enhanced LDCT was highly sensitive (94.7%) andallowed the distinction of COVID-19 from other diseases associated with similar clinical symptoms with high specificity (91.4%). Also of high clinical relevance is the high positive predictive value (87.7%) observed for LDCT.
Currently, medical societies either do not recommended the systematic use of LDCT for the diagnosis of COVID-19 or advise against its use for this purpose (10– 12, 17). This is mainly due to the lack of evidence for this approach (18); to our knowledge, prospective, systematically collected data such as ours have not been published outside of China.
In addition, reservations against the use of LDCT exist because allegedly, LDCT lacks the necessary specificity required to distinguish COVID-19 from other viral pneumonias (13, 14) or from other, non-infectious changes such as those secondary to drugs or inhalative toxins (15, 16).
In the publication from Wuhan, the specificity of LDCT testing was indeed reported to be as low as 25%. However, the authors argue that in almost half of the patients with negative RT-PCR testing and positive LDCT, COVID-19 was considered to be highly probable based on the patients’ respective clinical course, and thus the LDCT should have been regarded as true-positive (and the RT-PCT test as false-negative) rather than vice versa (9).
Therefore, the significantly higher specificity and PPV of LDCT observed in our cohort compared to the findings reported from Wuhan are probably best explained by the fact that we used a composite reference standard where not only RT-PCT test results but also the further clinical course and results of repeat RT-PCR testings were taken into account. Of note, compared to the results published in the Wuhan study, the RT-PCR results observed in our cohort were substantially less frequently “false-negative” when considering results from LDCT or the further clinical course. Yet the more accurate a reference standard works, the less often will a LDCT diagnosis be classified as “false positive” when it is, indeed, true-positive. The higher specificity of LDCT in our cohort could also be due to the fact that the data of our study were collected in spring rather than winter (as were the Chinese data); that is, at a time when the prevalence of other, more seasonal viruses (e.g., influenza or RSVs) decreases as a cause of pneumonic changes. However, we believe that such seasonal confounders cannot be the main reason for the observed higher specificity of LDCT in our hands. This is evidenced by the fact that almost half of our patients (55/116) who were classified as “COVID-19 negative” by LDCT did indeed have pathological pulmonary findings.
This in turn indicates that LDCT findings associated with COVID-19 can actually be distinguished from other pathologies, including other causes of viral pneumonia. The COVRADS classification presented here is also helpful for this purpose. Through designation of COV-RADS category 2, a radiologist is able to communicate that the CT study of a given patient is indeed abnormal, for instance due to pneumonic consolidations, but that these findings are attributable to other agents and not to SARS-COV-2. The rationale for systematically using both tests—RT-PCR and LDCT in parallel—in patients presenting with clinical symptoms that could be caused by COVID-19 was justified by the experiences published by the Wuhan group, who found that false-negative RT-PCR results were often corrected by positive CT diagnoses. And indeed, also in our cohort, both methods were complementary, even if to a much lesser extent than in the Chinese cohort: False-negative diagnoses were observed for RT-PCR and LDCT with similar frequency (in six and four patients, respectively), i.e. in a total of 10 patients, the correct diagnosis of COVID-19 was established by the respective other method. While the sensitivity of LDCT in our cohort was in good agreement with the results from Wuhan, this was not true for the sensitivity of the RT-PCR testing (9, 19). The reason for the significantly higher sensitivity of of RT-PCR testing observed in our study remains unclear; a more diligent technique of taking swab samples and/or a higher sensitivity of the PCR test kits used in Germany are possible explanations.
Thus, in summary, LDCT and RT-PCR offer an equivalent sensitivity for the identification of patients with COVID-19; the tests are complementary in only a small number of patients. This then raises the question of whether this justifies using both methods in parallel. In our opinion, the following aspects should be considered:
It is indisputable that a postive result from the RT-PCR test establishes a diagnosis of COVID-19 with absolute specificity/PPV. However, the advantage of LDCT is that its results are usually available much faster. Acquiring a non-enhanced LDCT study takes a few seconds; it took a median of nine minutes in our study until LDCT findings were available to the referring physician, whereas RT-PCR results were only available after a median of 8.3 hours. As a result, the median difference between time to CT findings and time to RT-PCR findings in the same patient was eight hours (4.5–17.5 h)—accordingly, LDCT diagnoses are available much faster than RT-PCR results. This time advantage is relevant in a pandemic situation in which infectious patients must be quickly identified and isolated.
Beyond such aspects of availability and time-to-diagnosis, an essential advantage of LDCT compared to pure RT-PCR testing is that it provides additional diagnostic information that can be essential for the appropriate management of patients whose nonspecific clinical symptoms may be attributable to a broad range of conditions—this is true for both, patients ultimatley found to be or not to be positive for COVID-19. In the former, LDCT can visualize and quantify the consequences of viral activity in the lung tissue. It can detect accompanying factors that can modulate a patient’s risk, such as pre-existing chronic obstructive pulmonary disease, emphysema, or pulmonary fibrosis. In the latter, LDCT can reveal or rule out alternative causes of clinical symptoms, such as bronchopneumonia, lobar pneumonia, etc., and thus enable an appropriate therapy to be initiated. Therefore, as long as this pandemic continues, we advocate the parallel use of LDCT and swab/RT-PCR in symptomatic patients.
Furthermore, it should be noted that even though there are sufficient RT-PCR test capacities for the time being, it cannot be ruled out that a second pandemic wave will follow, in which a considerably higher RT-PCR testing capacity and/or a faster availability of test results would be immediately required. If this happens, it might make sense to exploit the huge CT imaging capacities that exist, on a 24/7 basis, in every small hospital and many private practices throughout Germany, in order to offer LDCT for fast identification of COVID-19 in symptomatic patients.
The radiation exposure of LDCT is around 1.7 mSv, which is lower than the natural annual radiation exposure (2.1 mSv/year). Clinical symptoms (fever, cough) already justify the indication for imaging. However, a chest x-ray is far less powerful than LDCT when it comes to imaging changes associated with viral pneumonia (whether SARS-CoV-2 or others). Accordingly, the indication for LDCT is justified in patients for whom viral pneumonia is a clinical consideration (13, 20, 21).
For all patients with “COVID-19 typical” findings according to the COV-RADS 5 category, an infection was confirmed in the RT-PCR test; no false-positive COV-RADS 5 findings were observed. From this, we conclude that a SARS-CoV-2 infection should be assumed in a patient with a COV-RADS 5 finding until proven otherwise. If confirmed by further studies, our results indicate that a COVRADS category 5 finding in a patient with COVID-19–compatible clinical symptoms should be considered sufficient to justify reporting a “suspected illness” according to the German Coronavirus Reporting Ordinance—regardless of RT-PCR results.
The inter-observer variability of the CT findings was not examined in this registry study. However, as the LDCT results were prospectively assessed by different radiologists at two different locations, they reflect a certain cross-section of radiological expertise. Furthermore, there was no systematic follow-up of patients with concordant negative results in swab/RT-PCR and LDCT. In addition, the results of the method being tested (LDCT) are included in the composite reference standard. It is therefore possible that the observed specificity of LDCT, as well as the sensitivity of both methods (RT-PCR, LDCT), are overestimated.
Our results relate to the use of LDCT to establish the diagnosis of COVID-19 in symptomatic patients. Using LDCT for screening asymptomatic persons with the aim of detecting clinically occult or presymptomatic SARS-CoV-2 infections was not examined here. When considering the use of LDCT for screening, or in general in cohorts with a significantly lower prevalence of COVID-19, it should be noted that the positive predictive value (PPV) of diagnostic tests decrease with decreasing disease prevalence. However, a direct computational scaling of the predictive values of a diagnostic test to settings with lower disease prevalence is not possible, as radiologists take different levels of disease prevalence (that is, different pretest probabilities) into account when interpreting imaging studies. This is evidenced, for instance, by the PPVs associated with mammographic image interpretation of a screening vs. a diagnostic cohort: Despite a prevalence difference of approximately 1:100, the PPV in the screening situation is approximately the same as in the diagnostic situation (22, 23). Therefore, there is a need for prospective studies to establish the diagnostic indices of LDCT in cohorts with other disease prevalences.
Supplementary Material
eMethods
1. Determination of the time window to result availability
Information about the time at which low-dose CT (LDCT) scans were taken and swab samples were collected, and the release/transferal of CT findings and the release of PCR results, were taken from the electronic patient records and compared. Swab samples taken at Dueren Hospital (KHD) were sent to an external laboratory, with the turnaround time subject to various factors; therefore, analysis of time to results for swab examinations was limited to those tests performed at Aachen University Hospital (UKA) and included the first 124 patients.
2. Techniques for swab and CT acquisition
Swab sample were taken from the nasopharyngeal space, as recommended by the Robert Koch Institute, by employees of the UKA and KHD. After nucleic acid extraction from the swab sample, viral RNA was detected using reverse-transcriptase polymerase chain reaction (RT-PCR).
Non-enhanced LDCT scans of the thorax were taken using standard technology on two multi-slice spiral CT systems (Siemens AS-40 [UKA] and Canon Aquilion Lightning SP [KHD]). The following parameters were chosen: acquisition during breath hold; tube voltage, 80 kV; tube current, 35 milliamps (mA) with automatic dose modulation program; beam pitch, 1.5; reconstruction kernel, l70f and l30f; matrix, 512 x 512; field of view, 350 mm; acquisition slice thickness, 0.75 mm; reconstruction slice thickness, 3 mm and 1 mm; multiplanar reformations in the axial, coronary, and sagittal planes.
3. LDCT findings
Acquired images were transferred to the respective hospital picture archiving and communication systems (PACS). Images were interpreted immediately after examination by the responsible radiology specialist. The axial recordings with a 3-mm slice thickness were first examined in the so-called lung window (reconstruction kernel, l70f).
At UKA, COV-RADS categorization was carried out at the same time as the primary diagnosis; at KHD, it was carried out retrospectively for the first 19 patients based on the available written findings, and at the same time as the findings for the subsequent examinations (as done at UKA).
LDCT findings categorized as COV-RADS 3, 4, or 5 were assessed as test-positive, and findings categorized as COV-RADS 1 or 2, as test-negative.
If no pathological findings were present in the lungs, a COV-RADS 1 category was assigned.
If pathological findings were present in the lungs, a decision was made about whether they fit with the lung changes in CT that are seen with COVID-19.
If infiltrates were present, the type, distribution, and extent of infiltrates were assessed. The following lung findings were rated as suspicious for COVID-19–associated pneumonia: (1) bilateral, (2) relatively dense, (3) focal or wedge-shaped ground glass opacities, and (4) focal or linear consolidations, each with: (5) axial distribution pattern with peripheral predominance or peripheral and central predominance, (6) vertical distribution pattern with a basal predominance, and (7) crazy paving and/or halo sign.
If such changes were seen, a COV-RADS 3, 4, or 5 category was assigned. Categories 3 to 5 were differentiated according to the number of the above criteria and their degree of severity.
If none of the above-mentioned findings were present, and classic CT findings of bronchopneumonia, lobar pneumonia, excessive lung water due to cardiac congestion, etc., were found instead, the finding was categorized as COV-RADS 2.
The result (COV-RADS category) was communicated to the on-duty colleague of the emergency department by phone and stored as an annotation in the PACS (picture archiving and communication system). The final report was then written and released.
4. Establishment of the reference standard
Due to the published high rate of false-negative swab results, using RT-PCR is not a gold standard for establishing the final categorization of a patient as “COVID-19 positive” versus “COVID-19 negative”. Therefore, a composite standard of reference was used as ground truth. This was determined as follows:
5. Further analyses
A more extensive radiological analysis of the type, severity, and distribution of the CT image findings was carried out retrospectively by specialists (two at UKA, and two at KHD) who were blinded to the respective PCR results and the final diagnosis (e.g., COVID-19 positive/negative).
For this purpose, a binary survey was first carried out to determine whether ground glass opacities (GGO) and consolidations were present. The severity or, more specifically, the volume fraction of the affected lung tissue, was visually recorded on a five-point scale, whereby 1 corresponded to a very low level, and 5, to an almost complete involvement of lung tissue. Furthermore, assessments were made about the axial and cranio-caudal distribution patterns of the ground glass opacities and consolidations as well as whether one or both lung sides were affected. Any additional signs of atypical pneumonia were then recorded, including the presence of crazy paving (ground glass opacities with simultaneous thickening of the inter- and intralobular septa), halo sign (ground glass opacity that surrounds consolidation), an atoll sign or “reversed halo” (central ground glass opacities that is surrounded by consolidation).
6. Statistical analysis
In addition to the analysis of the diagnostic parameters of LDCT versus swab/RT-PCR in relation to the composite standard of reference, the relevant parameters of LDCT were also analyzed using the results of the swab/RT-PCR as a reference standard.
In addition, the diagnostic performance of LDCT was analyzed in relation to the composite standard of reference stratified according to the two participating centers (UKA and KHD).
The group of patients with positive COVID-19 results was compared with the group with negative COVID-19 results with regard to the distribution of demographic characteristics, clinical symptoms, and CT imaging findings. For all distributions, 95% confidence intervals according to Clopper–Pearson were calculated; continuous data were compared using the t-test for unconnected samples and categorical variables using the Mann–Whitney U test (SPSS version 25, IBM, Armonk, NY, USA).
Patients with a primary positive swab were categorized as “COVID-19 positive”.
For patients with a primary negative swab and a positive LDCT, the further clinical course was assessed in an interdisciplinary manner by emergency physicians and pneumologists by consensus. If a swab/RT-PCR result was positive during the course of illness and/or the further clinical course suggested COVID-19 (e.g., having suitable laboratory findings plus a lack of alternative pathogen detection), these patients were categorized as “COVID-19 present”; otherwise, they were categorized as “COVID-19 absent”.
Patients with a primary negative RT-PCR and negative LDCT, and with no other indications for COVID-19 in the further course of illness, were categorized as “COVID-19 absent”.
Key Messages.
Low-dose CT is a readily available, precise test for the diagnosis of COVID-19 that offers a sensitivity comparable to RT-PCR testing and can correct false-negative RT-PCR test results.
With high specificity and predictive value, LDCT can establish or refute a diagnosis of COVID-19 in patients presenting with non-specific clinical symptoms.
Our results support the use of LDCT in parallel with RT-PCR testing to detect COVID-19 in symptomatic patients.
Should RT-PCR testing capacities be lacking in a future pandemic wave, we should consider using LDCT to directly identify patients with COVID-19, especially as CT devices are almost ubiquitously available in Germany; this could ensure rapid identification of SARS-COV-2 infection in symptomatic patients.
eFigure 2.
Time interval between availability of CT findings and availability of RT-PCR results for the first 124 patients at Aachen University Hospital
Acknowledgments
Translated from the original German by Dr. Veronica A. Raker.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA wwwjamanetworkcom/journals/jama/fullarticle/2762997 (last accessed on 12 April 2020) 2020;323:1843–1844 . doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang F, Deng L, Zhang L, et al. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020,;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. 2000045. www.ncbi.nlm.nih.gov/pmc/articles/PMC6988269/ (last accessed on 12 April 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wikramaratna P, Paton RS, Ghafari M, Lourenco J. Estimating false-negative detection rate of SARS-CoV-2 by RT-PCR. medRxiv. wwwdoiorg/101101/2020040520053355 (last accessed on 13 May 2020) 2020 doi: 10.2807/1560-7917.ES.2020.25.50.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 doi: 10.1148/radiol.2020200463. 200463 epub ahead of print, 20 February 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. 200343 epub ahead of print, 12 February 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. 200642 epub ahead of print, 26 February 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection (last accessed on 12 April 2020) [Google Scholar]
- 11.Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Radiology. 2020 doi: 10.1148/radiol.2020201365. 201365 epub ahead of print, 7 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deutsche Röntgengesellschaft. COVID-19: Information der AG Thoraxdiagnostik der Deutschen Röntgengesellschaft. www.drg.de/de-DE/5995/covid-19/ (last accessed on 17 April 2020) doi: 10.1055/a-1112-8430. [DOI] [PubMed] [Google Scholar]
- 13.Franquet T. Imaging of pulmonary viral pneumonia. Radiology. 2011;260:18–39. doi: 10.1148/radiol.11092149. [DOI] [PubMed] [Google Scholar]
- 14.Hosseiny M, Kooraki S, Gholamrezanezhad A, Reddy S, Myers L. Radiology perspective of coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and Middle East respiratory syndrome. AJR Am J Roentgenol. 2020;214:1078–1082. doi: 10.2214/AJR.20.22969. [DOI] [PubMed] [Google Scholar]
- 15.Ellis SJ, Cleverley JR, Müller NL. Drug-induced lung disease: high-resolution CT findings. AJR Am J Roentgenol. 2000,;175:1019–1024. doi: 10.2214/ajr.175.4.1751019. [DOI] [PubMed] [Google Scholar]
- 16.Nishino M, Hatabu H, Hodi SF. Imaging of cancer immunotherapy: current approaches and future directions. Radiology. 2019;290:9–22. doi: 10.1148/radiol.2018181349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rüdiger Meyer (rme) SARS-CoV-2: Computertomografie nicht zum Screening geeignet. www.aerzteblatt.de/nachrichten/112080/SARS-CoV-2-Computertomografie-nicht-zum-Screening-geeignet (last accessed on 20 April 2020) [Google Scholar]
- 18.Raptis CA, Hammer MM, Short RG, et al. Chest CT and coronavirus disease (COVID-19): a critical review of the literature to date. AJR Am J Roentgenol. 2020,;16:1–4. doi: 10.2214/AJR.20.23202. [DOI] [PubMed] [Google Scholar]
- 19.Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020 doi: 10.1148/radiol.2020200432. 200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claessens Y-E, Debray M-P, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974–982. doi: 10.1164/rccm.201501-0017OC. [DOI] [PubMed] [Google Scholar]
- 21.Hyun JK, Soyeoun L, Jooae C, Sang-Ho C, Heungsup S, Kyung-Hyun D. Radiographic and CT features of viral pneumonia. Radiographics. 2018;38:719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 22.Jahresbericht Qualitätssicherung 2017. Ergebnisse des Deutschen Mammographie-Screening-Programms. Kooperationsgemeinschaft Mammographie, Berlin, Oktober 2019 [Google Scholar]
- 23.Carney PA, Parikh J, Sickles EA, et al. Diagnostic mammography: identifying minimally acceptable interpretive performance criteria. Radiology. 2013;267:359–367. doi: 10.1148/radiol.12121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
1. Determination of the time window to result availability
Information about the time at which low-dose CT (LDCT) scans were taken and swab samples were collected, and the release/transferal of CT findings and the release of PCR results, were taken from the electronic patient records and compared. Swab samples taken at Dueren Hospital (KHD) were sent to an external laboratory, with the turnaround time subject to various factors; therefore, analysis of time to results for swab examinations was limited to those tests performed at Aachen University Hospital (UKA) and included the first 124 patients.
2. Techniques for swab and CT acquisition
Swab sample were taken from the nasopharyngeal space, as recommended by the Robert Koch Institute, by employees of the UKA and KHD. After nucleic acid extraction from the swab sample, viral RNA was detected using reverse-transcriptase polymerase chain reaction (RT-PCR).
Non-enhanced LDCT scans of the thorax were taken using standard technology on two multi-slice spiral CT systems (Siemens AS-40 [UKA] and Canon Aquilion Lightning SP [KHD]). The following parameters were chosen: acquisition during breath hold; tube voltage, 80 kV; tube current, 35 milliamps (mA) with automatic dose modulation program; beam pitch, 1.5; reconstruction kernel, l70f and l30f; matrix, 512 x 512; field of view, 350 mm; acquisition slice thickness, 0.75 mm; reconstruction slice thickness, 3 mm and 1 mm; multiplanar reformations in the axial, coronary, and sagittal planes.
3. LDCT findings
Acquired images were transferred to the respective hospital picture archiving and communication systems (PACS). Images were interpreted immediately after examination by the responsible radiology specialist. The axial recordings with a 3-mm slice thickness were first examined in the so-called lung window (reconstruction kernel, l70f).
At UKA, COV-RADS categorization was carried out at the same time as the primary diagnosis; at KHD, it was carried out retrospectively for the first 19 patients based on the available written findings, and at the same time as the findings for the subsequent examinations (as done at UKA).
LDCT findings categorized as COV-RADS 3, 4, or 5 were assessed as test-positive, and findings categorized as COV-RADS 1 or 2, as test-negative.
If no pathological findings were present in the lungs, a COV-RADS 1 category was assigned.
If pathological findings were present in the lungs, a decision was made about whether they fit with the lung changes in CT that are seen with COVID-19.
If infiltrates were present, the type, distribution, and extent of infiltrates were assessed. The following lung findings were rated as suspicious for COVID-19–associated pneumonia: (1) bilateral, (2) relatively dense, (3) focal or wedge-shaped ground glass opacities, and (4) focal or linear consolidations, each with: (5) axial distribution pattern with peripheral predominance or peripheral and central predominance, (6) vertical distribution pattern with a basal predominance, and (7) crazy paving and/or halo sign.
If such changes were seen, a COV-RADS 3, 4, or 5 category was assigned. Categories 3 to 5 were differentiated according to the number of the above criteria and their degree of severity.
If none of the above-mentioned findings were present, and classic CT findings of bronchopneumonia, lobar pneumonia, excessive lung water due to cardiac congestion, etc., were found instead, the finding was categorized as COV-RADS 2.
The result (COV-RADS category) was communicated to the on-duty colleague of the emergency department by phone and stored as an annotation in the PACS (picture archiving and communication system). The final report was then written and released.
4. Establishment of the reference standard
Due to the published high rate of false-negative swab results, using RT-PCR is not a gold standard for establishing the final categorization of a patient as “COVID-19 positive” versus “COVID-19 negative”. Therefore, a composite standard of reference was used as ground truth. This was determined as follows:
5. Further analyses
A more extensive radiological analysis of the type, severity, and distribution of the CT image findings was carried out retrospectively by specialists (two at UKA, and two at KHD) who were blinded to the respective PCR results and the final diagnosis (e.g., COVID-19 positive/negative).
For this purpose, a binary survey was first carried out to determine whether ground glass opacities (GGO) and consolidations were present. The severity or, more specifically, the volume fraction of the affected lung tissue, was visually recorded on a five-point scale, whereby 1 corresponded to a very low level, and 5, to an almost complete involvement of lung tissue. Furthermore, assessments were made about the axial and cranio-caudal distribution patterns of the ground glass opacities and consolidations as well as whether one or both lung sides were affected. Any additional signs of atypical pneumonia were then recorded, including the presence of crazy paving (ground glass opacities with simultaneous thickening of the inter- and intralobular septa), halo sign (ground glass opacity that surrounds consolidation), an atoll sign or “reversed halo” (central ground glass opacities that is surrounded by consolidation).
6. Statistical analysis
In addition to the analysis of the diagnostic parameters of LDCT versus swab/RT-PCR in relation to the composite standard of reference, the relevant parameters of LDCT were also analyzed using the results of the swab/RT-PCR as a reference standard.
In addition, the diagnostic performance of LDCT was analyzed in relation to the composite standard of reference stratified according to the two participating centers (UKA and KHD).
The group of patients with positive COVID-19 results was compared with the group with negative COVID-19 results with regard to the distribution of demographic characteristics, clinical symptoms, and CT imaging findings. For all distributions, 95% confidence intervals according to Clopper–Pearson were calculated; continuous data were compared using the t-test for unconnected samples and categorical variables using the Mann–Whitney U test (SPSS version 25, IBM, Armonk, NY, USA).
Patients with a primary positive swab were categorized as “COVID-19 positive”.
For patients with a primary negative swab and a positive LDCT, the further clinical course was assessed in an interdisciplinary manner by emergency physicians and pneumologists by consensus. If a swab/RT-PCR result was positive during the course of illness and/or the further clinical course suggested COVID-19 (e.g., having suitable laboratory findings plus a lack of alternative pathogen detection), these patients were categorized as “COVID-19 present”; otherwise, they were categorized as “COVID-19 absent”.
Patients with a primary negative RT-PCR and negative LDCT, and with no other indications for COVID-19 in the further course of illness, were categorized as “COVID-19 absent”.