Abstract
Objectives:
This review aimed to systematically evaluate the characteristics of technology-based interventions (TBIs) and their effectiveness on anxiety, depression, and health-related quality of life (HRQoL) among patients with prostate cancer.
Methods:
We identified eligible research reports published in English language between January 1, 2000, and September 15, 2018, from CINAHL; Embase; “Library and Information Science Abstracts”; “Library, Information Science and Technology Abstracts”; “Library and Information Science Source”; PsychINFO; and PubMed. We abstracted randomized control trials and quasi-experimental studies that measured the outcomes related to anxiety, depression, or HRQoL. We extracted the data using the Cochrane Handbook for Systematic Reviews of Interventions guideline.
Results:
Among the six randomized control trials and four quasi-experimental studies that met our inclusion criteria, the TBIs aimed to provide informational, psychosocial, self-care management, and communication support. About 60-92% of the participants in six studies completed all required contents and 77-94% of the participants in four studies logged onto the TBI platform. Compared with the patients in usual care, the TBI users reported a significant reduction in anxiety (N=1 study) and depression (N=2 studies) and improvement in HRQoL (N=2 studies). We also identified the limitations of the existing TBI trials.
Conclusions:
We found insufficient evidence to support the effectiveness of TBIs in improving health outcomes (anxiety, depression, and HRQOL) among patients with prostate cancer. Future research needs to (1) use rigorous randomized control trials, (2) be sufficiently powered to examine the effects of TBIs, and (3) examine how the effect of TBIs on health outcomes vary by actual intervention use, intervention components, and duration.
Keywords: anxiety, depression, intervention, prostate cancer, quality of life, systematic review, technology
1 |. BACKGROUND
Approximately 18 to 27% of patients with prostate cancer experience depression, anxiety, and reduced health-related quality of life (HRQoL).1,2 Health care providers often fail to meet the psychosocial care needs for prostate cancer patients3 due to health care costs and insufficient health care resources.4–6 National guidelines on cancer survivorship from the American Cancer Society has called for programs to better address the unmet care needs for prostate cancer patients and their families. Although in-person interventions such as nurse-led group counseling have been effective in reducing depressive symptoms among prostate cancer patients, they are expensive and inconvenient because of the time and travel required.7
Psychosocial and behavioral care delivered using technology-based interventions (TBIs) has the potential to provide cost-effective psychosocial care services to a large number of cancer patients and their families, to facilitate engagement in survivorship care, and to reduce the fear of stigma related to attending mental health services.8,9 Frequently used TBIs include web-based programs, mobile apps, telehealth communication, and monitoring sensors to enhance individualized health care.10 Although TBIs can be scalable to improve the psychological well-being and HRQoL among prostate cancer patients and meet their survivorship care needs,11–13 little is known about the characteristics and effectiveness of TBIs on anxiety, depression, and HRQoL among patients with prostate cancer.8 The purpose of this systematic review is to evaluate the characteristics of TBIs and their effectiveness on anxiety, depression, and HRQoL among patients with prostate cancer. The findings from this review will provide evidence integral to designing effective TBIs enhancing supportive care for prostate cancer patients.
2 |. METHODS
2.1 |. Identification of studies
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to guide the systematic review process (Figure 1). A research librarian developed the search strategy and performed the search to identify articles reporting the effectiveness of TBIs on anxiety, depression, and HRQoL among prostate cancer patients. For the purposes of this review, we defined TBIs as health management or behavioral change interventions implemented via digital platforms, such as mobile apps, web-based programs, multimedia applications, or delivered through electronic devices, including smartphones, tablets, and computers.14
FIGURE 1.
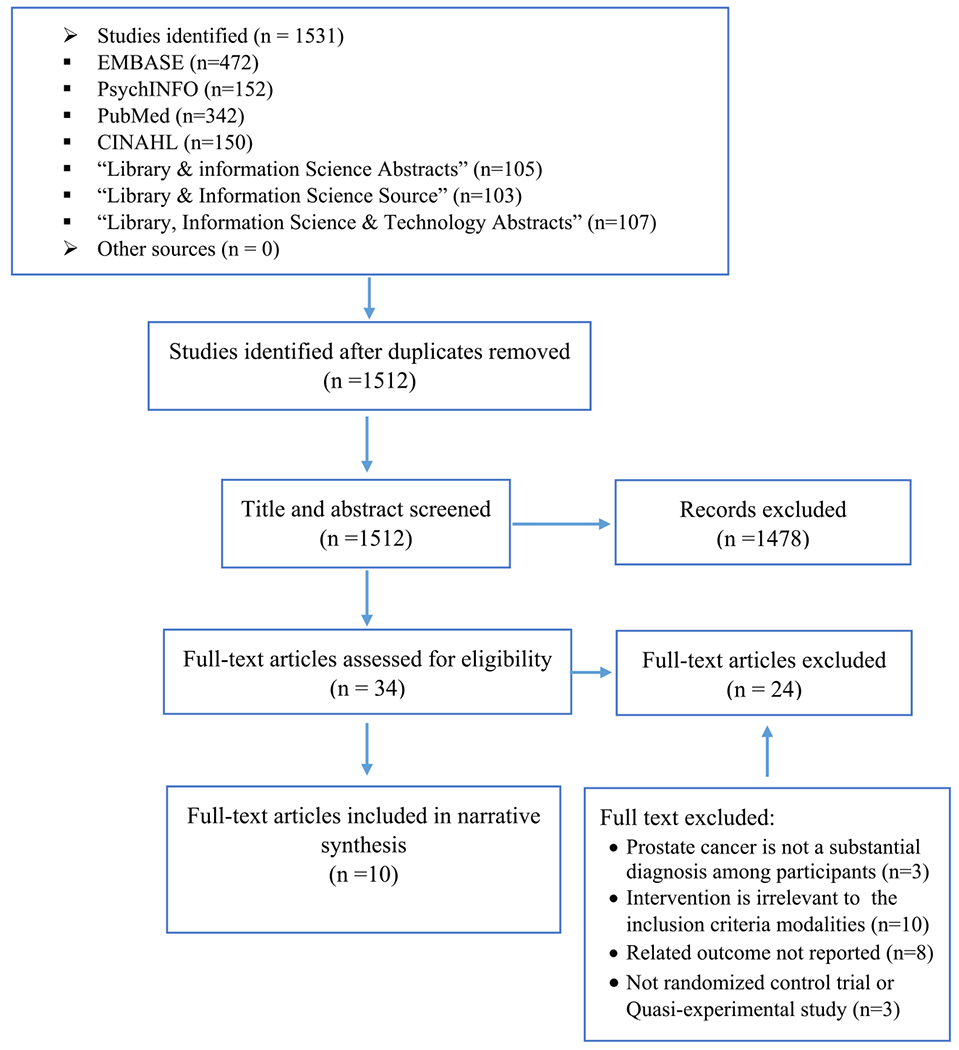
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of study identification and selection
We identified eligible research articles from the following electronic databases: CINAHL; Embase; “Library and Information Science Abstracts”; “Library, Information Science and Technology Abstracts”; “Library and Information Science Source”; PsychINFO; and PubMed. We adjusted these keywords with vocabulary and syntax across databases: (eHealth OR “digital health” OR mHealth OR multimedia OR web* OR app OR apps OR “mobile technology” OR internet OR online OR “mobile phone” OR smartphone OR tablet OR computer* OR application* OR “mobile applications”) AND English[lang] AND (“prostate neoplasm*” OR “prostate cancer” OR “prostate cancers” OR “prostate tumour” OR “prostate tumours” OR “prostate tumor*”) AND (depress* OR anx* OR “quality of life” OR “health related quality of life” OR QOL OR HRQOL).
To meet inclusion criteria, the studies (1) targeted patients with prostate cancer at different stages; (2) included TBIs for health management or behavioral change; (3) measured health outcomes of anxiety, depression, or HRQoL; (4) used a randomized control trial (RCT) or quasi-experimental research design; and (5) were published between January 1, 2000, and September 15, 2018 (to maximize the number of studies). Quasi-experimental studies were included in this review because of the lack of published RCTs in this area. Studies were also excluded if they (1) included only telemonitoring (eg, telephone calls, video calls, or other approaches for self-monitoring) of chronic illness and/or symptoms, (2) were published in a language other than English, and (3) only included abstracts but full articles were unavailable. Two investigators independently screened and evaluated the abstracts to determine if the studies met inclusion criteria, and then reviewed and assessed the full reports of those meeting inclusion criteria. Disagreements were resolved among members of the research team.
2.2 |. Data extraction
We used the Cochrane Handbook for Systematic Reviews of Interventions as a guide to extract from the eligible studies.15 The extracted data included study design, aims, participant demographics, sample size, retention rate, TBI characteristics, TBI use, and health outcomes. If research projects assessed multiple outcomes, our reviewers extracted the outcomes related to anxiety, depression, and HRQoL. Thereafter, we developed a preliminary synthesis using the textual description as recommended in “The Guidance on the Conduct of Narrative Synthesis in Systematic Reviews”16 (see Table S1 in the Supporting Information). The textual description is a preliminary starting point in the synthesis to describe the studies in more depth than can be given in tables.16
The Cochrane risk of bias criteria were used to evaluate whether the risk of bias was low, unclear, or high risk.17 We rated studies as having a low risk of bias if all eight Cochrane criteria were met (see Table S2). Studies not meeting any of the Cochrane criteria were rated as high risk of bias. If a study omitted mention of Cochrane criteria, the study was rated as having an unclear risk of bias (Higgins et al, 2011).
3 |. RESULTS
3.1 |. Study characteristics
The initial search yielded 1,512 publications (Figure 1). After removal of duplicate papers and ineligible studies, 10 studies met our inclusion criteria, and thus, were included in this review. These studies included randomized control trials (N=6),12,18–22 and quasi-experimental studies (N=4: three “2-group pretest-posttest” studies and one “1-group pretest-posttest” study).23–26 These studies were conducted in Australia, Canada, Germany, Netherlands, Norway, Sweden, the United Kingdom, and the United States (Table 1).
TABLE 1.
Characteristics of included studies and technology-based interventions (TBIs)
| First Author, Year, Design, and Country | Study Aims | Sample Size (n); Setting and Severity of illness | Method of Recruitment | Control | TBI Name | TBI Modality and Platform | TBI Content | TBI Duration and Sessions Time | Interventionist |
|---|---|---|---|---|---|---|---|---|---|
| Cockle-Hearne et al 2018; One group pretest-posttest; UK | Assess feasibility of online CBT among men experiencing mild to moderate distress after prostate cancer treatment. | (n=16) Outpatient diagnosed with localized prostate cancer within the last 3.5 years | Physician and nurse consultant invitation | N/A | Getting Down to Coping | Online CBT support website | CBT supplemented by peers’ support and chat room to encourage self-management. Self-monitor, improve cognitive, behavioral, and emotional responses necessary to maintain psychological wellbeing | 4 weekly sessions lasting one hour per session | Psychological practitioner, and oncology nurse |
| Hawkins et al 2017; RCT; USA | Examine computer-based support to improve HRQoL | (n=310) Outpatient newly diagnosed with prostate cancer (Stage I or II) | Hospital and cancer centers | Cancer mentor | CHESS | Interactive online support website | Address patient needs for information, communication and support, build skills, decision map tool, and a module on managing sexual problems | 6 months (as needed) | N/A (self-guided) |
| Lange et al 2017; Two group pretest-posttest; Germany | Examine the effectiveness of a guided chat groups in psychosocial aftercare | (n=143) Outpatient postprostatectomy; prostate cancer (Stage I to IV) | Prostate cancer clinic | Usual care | Web-based chat | Online chat group support website | Exchange concerns, problems and support with peers about incontinence, fear of progression, sexuality, communication, occupational reintegration, resource orientation, and coping | 1 to 2 months (Weekly group session lasting 60 to 90 min) | Psycho-therapist |
| Loiselle et al 2010; Two group pretest-posttest; Canada | Examine the impact of a multimedia informational system on health-related outcomes | (n=45) Outpatient newly diagnosed with breast or prostate cancer; (Stage I or II) | Oncology clinics | Usual care | Oncology Interactive Educational Series | Cancer related multimedia information (websites and CD-ROM) | Information about cancer prevention, detection, symptoms, diagnosis, treatment, nutrition, pain, psychosocial care, community support, and answers to frequently asked questions | 8 weeks (as needed) | N/A (self-guided) |
| Osei et al 2013; RCT; USA | Examine the effect of an online support system on HRQoL | (n=40) Outpatient diagnosed with prostate cancer within the last 5 years | Mailing | Prostate Cancer Resource kit | Us TOO | International online support group website | Offers information about prostate cancer, symptoms and side effects management, informed decisions, active surveillance, and treatment options | 6 weeks (at least three times per week) | N/A (self-guided) |
| Ruland et al 2013; RCT; Norway | Examine the effects of online support system on symptom distress, depression, self-efficacy, HRQoL, and social support | (n=136) Outpatient undergoing treatment of breast or prostate cancer (Stage I to IV) | Advertising and mailing | Public cancer website | WebChoice | Online interactive health communication website | Enhanced symptoms monitoring, information and self-management, e-communication with health professionals, and e-forum group with peers. |
1 year (as needed) | N/A (self-guided) |
| Sundberg et al 2017; Two group pretest-posttest; Sweden | Examine the effect of a smartphone application on symptom burden and HRQoL | (n=130) Outpatient undergoing radiotherapy for localized prostate cancer | Hospitals | Usual Care | Interaktor | Interactive smartphone application for detecting, reporting and managing of symptoms | Selfcare advice, symptom history graphs, symptom assessment for bladder, bowel function, fatigue, pain, anxiety, distress, sleep, and flushing, and risk assessment model based on symptoms with alert notification. | 5 to 11 weeks (as needed) | Clinical nurses |
| Van De Wal et al 2017; RCT; Netherland | Examine the efficacy of blended CBT for high fear of cancer recurrence | (n=88) Outpatient survivors 6 months to 5 years posttreatment of breast, prostate, or colorectal cancer | Hospitals and mailing | Usual Care | bCBT | Blended CBT (website, face to face, and telephone call) | Psychoeducation, cognitive restructuring, and behavioral modification modules delivered as face-to-face and e-consultations or may be replaced with telephone consultations along with a workbook. |
3 months (8 sessions total: five 1-h face-to-face sessions and three 15 minutes online consultation) | Oncology psychologist |
| Wootten et al 2015; RCT; Australia | Examine the efficacy of an online intervention to reduce participants’ psychological distress | (n=142) Outpatient undergoing treatment or posttreatment of localized prostate cancer within 5 years | Advertising, mailing, and urologist invitation | Moderated forum | My Road Ahead | Self-guided online psychological CBT website | Psycho-education, interactive exercises, automated feedback. The topics about the emotional impact of illness, cognitive strategies, communication, coping, and planning. | 10 weeks (as needed; recommended weekly) | N/A (self-guided) |
| Yanez et al 2015; RCT; USA | Examine the feasibility, acceptability, and efficacy of a technology assisted psychosocial program | (n=74) Outpatient with advanced prostate cancer (Stage III or IV) | Hospitals | Health promotion | CBSM | Online cognitive behavioral stress management website | Stress management, education about (impotence, incontinence, intimacy, acceptance, existential concerns, and life narratives), stress reduction/relaxation technique, coping skills, interpersonal skills, and social networks. | 10 Weekly group sessions: 30 min of relaxation and 60 min of stress management. | Masters level therapist |
Abbreviations: bCBT: blended cognitive behavioral therapy; CBSM: cognitive-behavioral stress management; CBT: cognitive behavioral therapy; CHESS: Comprehensive Health Enhancement Support System; HRQoL: health-related quality of life; N/A: not applicable; RCT: randomized controlled trial.
3.2 |. Characteristics of research participants
The study sample sizes ranged from 16 to 310 participants (Table 2) recruited from outpatient settings in hospitals, cancer centers, and prostate cancer clinics through advertisements, mailings, or clinical provider’s invitation. Seven studies targeted only prostate cancer patients,12,18,19,22,23,25,26 whereas three studies targeted breast and colorectal cancer patients in addition to prostate cancer patients.20,21,24 The cancer patients were in remission after being diagnosed with localized cancer or at varying stages of cancer (Table 1). The average age of participants across studies ranged from 57 to 69 years. Study participants were mostly White (57-100%), married (65-87%), and retired (30-77%). Participants reported educational levels ranging from elementary to graduate school, and most had post-secondary school degrees (73-89%). Two studies reported participants’ locations of residence,12,23 and 73% and 89% of these participants were urban residents, respectively.
TABLE 2.
Effect of technology-based interventions on anxiety, depression, and health-related quality of life
| Study | Assessment Time Point | Outcomes | Study Measures | Between Group Difference* | Within Group Difference (Intervention)* | TBI Use | Retention Rate (By End of Follow-up) |
|---|---|---|---|---|---|---|---|
| Cockle-Hearne et al 2018 | Baseline, and end of intervention | Anxiety Depression |
General Health Questionnaire-28 | N/A | Positive change in anxiety between baseline and post-intervention (A Wilcoxon signed-rank test: (z= −3.466, r= −.613, p=.001) NS |
92%a | 80% |
| Hawkins et al | Baseline, 2, 6, 12 and 24 weeks | HRQoL | WHOQOL | NS | NS | 78% | 94% |
| Lange et al | Baseline and follow up | Anxiety Depression HRQoL |
ET-5 and MAX-PC ET-5 SF-8 |
NS NS NS |
N/A N/A N/A |
N/A | 31% |
| Loiselle et al | Baseline, end of intervention, and 3-month postintervention | Anxiety Depression HRQoL |
STAI CESD SF-36 |
NS NS NS |
NS NS NS |
94% | 93% |
| Osei et al | Baseline, 6 and 8 weeks | HRQoL | EPIC-26 | NS | Improved HRQoL (p =.036) from baseline to 6 weeks | N/A | N/A |
| Ruland et al | Baseline, 3, 6, 9, and 12 months | Depression HRQoL |
Epidemiological Studies Depression Scale 15-D HRQoL |
NS NS |
Improved depression over study period (mixed effect model: slope estimate= −0.41, CI= −0.71 to −0.11, t= −2.71; p= .007) NS |
77% | 75% |
| Sundberg et al | Baseline (T1), end of treatment (T2), and 3-month posttreatment (T3) | HRQoL | EORTC QLQ-C30 | Interaktor users reported improved HRQoL at T2 and T3(p <.05) | Improved HRQoL (p<.05) over study period (baseline to T3) | N/A | 88% |
| Van De Wal et al | Baseline and 3 months | Anxiety Depression HRQoL |
HADS EORTC QLQC30 |
bCBT users reported decreased anxiety (ANCOVA: CI= −4.131 to −2.763, ES “Cohen’s d”= .67, p≤.001) bCBT users reported decreased depression (ANCOVA: CI= −2.950 to −0.781, ES Cohen’s d=.56, P≤.001) bCBT users reported improved HRQoL (ANCOVA: CI= 5.441 to 19.325, ES Cohen’s d=.64, p=.001) |
N/A N/A |
66%a | 93% |
| Wootten et al | Baseline, 5 weeks, 10 weeks, 3 months and 6 months | Anxiety Depression |
DASS-21 | NS NS |
NS NS |
60% | 73% |
| Yanez et al | Baseline and 6 months | Depression HRQoL |
PROMIS depression item bank CAT FACT-G |
CBSM users reported improved PROMIS depression (ANCOVA: F= 5.11, ES Cohen’s d= 0.69, p=.03) NS |
N/A N/A |
70 %a | 82% |
Abbreviations: 15-D HRQoL, 15 dimensions HRQoL instrument; ANCOVA: analysis of covariance CESD, Center for Epidemiological Studies Depression; DASS-21, 21 items depression, anxiety, and stress scale; EPIC-26, 26 items Expanded Prostate Cancer Index Composite; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of life Questionnaire Core 30; ET-5, The Emotion Thermometers; FACT-G, The Functional Assessment of Cancer Therapy–General; HADS, Hospital Anxiety and Depression Scale; HRQoL, health-related quality of life; MAX-PC, Memorial Anxiety Scale for Prostate Cancer; N/A: no available data; NS: no significant difference; PROMIS, Patient-Reported Outcomes Measurement Information System; SF-36, 36-Item Short Form Health Survey; SF-8, Short Form survey 8 items of health outcomes; STAI, State-Trait Anxiety Inventory; TBI: technology-based intervention; WHOQOL, World health Organization Quality of Life; depression item bank CAT, Adult Depression computerized adaptive testing; T1, 2, 3= time 1, 2, 3.
Completed TBI contents.
Logged onto TBI.
Statistical significance at p ≤.5.
3.3 |. Characteristics of the TBIs
The intervention characteristics are displayed in Table 1. The TBIs aimed to provide informational, psychosocial, self-care management, or communication support. These studies used self-guided websites (N=5) 12,18–20,24; web-based programs supported by medical professionals (N=4)21–23,26; and a mobile health app with clinical nurse supervision (N=1).25
The intervention duration of eight studies ranged from 4 weeks to 3 months,12,19,21–26 and two studies continued the interventions for 6-12 months.18,20 Six studies specified that participants needed to complete 4 to 18 TBI sessions,12,19,21–23,26 whereas participants in four studies used the TBI programs as needed.18,20,24,25 Four studies reported that the length of each session ranged from 15 to 90 minutes.21–23,26 Seven studies provided technical assistance, IT training, or TBI user manuals to the participants.18,20,22–26
We defined platform use as how often the study participants logged onto the TBIs or completed all TBI content.27 Seven studies (Table 2) reported varied platform use, ranging from 60-92% of the participants completing all required TBI contents (N=4 studies),12,21,22,26 to between 77 and 94% of the participants logging onto TBI sessions (N=3 studies).18,20,24
3.4 |. Contents of the TBIs
The contents of the TBIs in the studies under review are outlined in Table 1. However, these studies have not determined which components of the TBIs are more or less effective and whether a combination of different TBI components or a particular component are the most effective. The TBI components can be categorized as (a) informational, (b) psychosocial, (c) self-care management, and (d) communication support. Informational components included patient needs in cancer,18 information about cancer illness,12,19,20,22,24,25 cancer prevention,24 cancer management,19,23–25 and decision making skills.18,19 Psychosocial components involved psychological wellbeing and social integration contents including psychoeducation,12,21 Cognitive Behavioral Therapy support,12,21,22,26 stress management skills,22 behavioral modification strategies,21,26 coping skills,12,22,23 peer support,20,23,26 community support,24 social networks enhancement,22 occupational reintegration,23 and interpersonal skills.22 Self-care management components included self-monitoring skills,20,24–26 symptoms history graph,25 symptoms management skills,20,24,26 and self-care advice.25 Communication support components included e-forum group discussion with other patients,20,23,26 e-communication with health professional,18,20,21,23 risk assessment alert notification,25 and interactive exercises with automated feedback.12
3.5 |. The risk of bias
The studies under review demonstrated various levels of risk of bias in different domains as measured using the criteria established in the Cochrane risk of bias assessment tool (Table 3). All RCTs maintained a random sequence generation of group assignments using computer-generated lists; however, none included information about the process of concealment of allocation of group assignments and blindness. No study reported strategies to maintain intervention fidelity (eg, consistent intervention use among participants). Three studies used an intention-to-treat approach to examine the intervention effects.12,20,21 Two RCTs reported unbalanced baseline measures among the study populations19,23; these studies also lacked population diversity in terms of demographical characteristics among the participants, which limited the generalizability of the study results.
TABLE 3.
Quality of study assessment tool
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Deferential Intervention use | Baseline Imbalance | Level of Risk |
|---|---|---|---|---|---|---|---|---|---|
| Cockle-Hearne et al | H | H | H | H | L | L | L | H | H |
| Hawkins et al | L | U | U | U | L | L | H | L | H |
| Lange et al | H | H | H | U | L | L | U | H | H |
| Loiselle et al | H | H | H | U | L | L | H | L | H |
| Osei et al | L | U | U | U | L | L | U | H | H |
| Ruland et al | L | H | H | U | L | L | H | U | H |
| Sundberg et al | H | H | H | H | L | U | U | U | H |
| Van De Wal et al | L | H | U | U | L | L | H | L | H |
| Wootten et al | L | U | U | U | L | H | H | L | H |
| Yanez et al | L | U | U | U | L | L | H | L | H |
Abbreviations: L: low risk; H: high risk; U: unclear.
Two RCT studies18,21 and one quasi-experimental study25 reported having sufficient power (>0.80) to detect medium intervention effect size, whereas the remaining studies reported that the estimated sample sizes were underpowered. The participant retention rates between baseline and at the end of follow-up assessments ranged from 73 to 94% in eight studies.12,18,20–22,24–26 One quasi-experimental study reported a retention rate of 31%,23 and one RCT included all of the randomized participants in the analysis and did not report information about participants dropout between the baseline and follow up points.19
3.6 |. Effect of TBIs on anxiety, depression, and HRQoL
Five studies examined the effects of TBIs on anxiety.12,21,23,24,26 Among these studies, one study reported a significant reduction in anxiety among TBI users (N = 45) compared with patients receiving usual care (N = 43),21 and one study reported a within-group reduction in anxiety in the TBI group only26 (see Table 2).
Seven studies reported the TBI effects on depression.12,20–24,26 Among these studies, two reported a significant improvement in depression in the TBI users compared with the participants in control conditions.21,22 Ruland and colleagues also showed improvement in depression in the TBI group during the study period.20
Eight studies reported on the effects of TBIs on HRQoL,18–25 with three studies indicating significant improvement in HRQoL among TBI users.19,21,25 Sundberg and colleagues showed within and between groups improvement in HRQoL among participants using smartphone applications for symptom management.25 Van De Wal and colleagues reported significant improvement in HRQoL among online cognitive behavioral therapy users, but not those in face-to-face psychological support groups.21 In their quasi-experimental study, Osei and colleagues reported improvement in HRQoL within online informational and educational support group subjects over time.19
4 |. DISCUSSION
We systematically reviewed TBI characteristics and effectiveness of TBIs on anxiety, depression, and HRQoL among patients with prostate cancer. We found limited evidence of TBI effectiveness in reducing anxiety and depression and improving HRQoL, which may be explained by the modest number of studies available for the review, the varied and underpowered sample sizes, and the lack of randomization, allocation concealment, and blinding in a number of studies. The significant heterogeneity in the study design, intervention use, outcome measurements, timing of follow-up, and the TBI modalities, components, duration and frequency of sessions, and availability of professional facilitation and technical support, has made the TBI effectiveness comparison across studies challenging. Furthermore, the homogeneity in study participants’ demographic variables (eg, older age, White, and married), although reduced the baseline imbalance in some studies, has limited the generalizability of the study findings among cancer patients with different backgrounds.
Our review, different from previous reviews of TBIs in other cancer populations, has shown that TBIs have the potential to reduce anxiety and depressive symptoms and improve HRQoL among prostate cancer patients.19–22,25,26 Web-based survivorship interventions have been shown to reduce anxiety and improving HRQoL among breast cancer patients.28 Fridriksdottir et al reported in their review that web-based interventions positively affected anxiety, depression, and HRQoL for cancer patients who mostly were breast or female cancer survivors.9 Previous studies have indicated that website attributes, Internet usage patterns, and habits are different across genders.29–31 The differences in the findings of our review and those from the other reviews suggest that the effects of TBIs may also vary by gender. Future research needs to explore the potential gender difference in TBI effects and the causes of these gender differences.
The studies in this review varied significantly in the TBI characteristics. For example, the TBI components included information and psychosocial support for symptom management; the TBIs also varied in whether they were self-guided by participants or involved professionals’ guidance and control. There was significant variation in TBI dosages in terms of total program duration, length of each session, and frequency of the TBI implementation. Nonetheless, we found limited evidence about how these TBI characteristics (eg, components, formats, and TBI dosage) influenced TBI effects on anxiety, depression, and HRQoL because of the limited number of studies available for this review, and insufficient data about how the TBI components and format contributed to the health outcomes. However, in their review, Talboom-Kamp and colleagues (2018) suggested that a combination of TBI components would show more promising effects than single component TBIs. Furthermore, although no studies in our review have reported whether and how the TBI duration and frequency changed the outcomes, previous reviews did not find evidence that TBIs lasting longer than 3 months, compared with those lasting less than 3 months, achieved a greater reduction in anxiety and depression or improvement in HRQoL among breast cancer patients 28,32. Likewise, Lee and colleagues found in another systematic review that positive health outcomes were not directly related to the length of the TBIs.33 Future research needs to examine rigorously whether and how the effects of TBIs on health outcomes vary by the TBI characteristics.
Neither did we find evidence about participants’ health outcomes being related to their use of TBIs (eg, completed all TBI sessions, discontinued use, or completed sessions over time). Our findings are consistent with the results from a previous systematic review of TBIs that showed that little studies monitored intervention dose (number of log on and website exploration) and its influence on HRQoL among breast and prostate cancer patients.8 Maintaining intervention use over time during study is a major moderator of TBI effectiveness34 and is likely to influence participants’ long-term health outcomes.28,35,36 In this review, participant retention rates between baseline and the end of follow-up were considered high, ranging from 73 to 94%.37 The retention rate, however, reflects the percentage of participants retained for postintervention assessments, but not necessarily the actual intervention uses among participants. For example, Van De Wal and colleagues reported that 93% of the participants completed the follow-up assessments, indicating a high retention rate; yet only 66% of the participants completed all the required TBI sessions. In the meanwhile, although seven studies reported the actual intervention use over time, none reported how TBI use affected the study results.
Amid growing concerns regarding barriers to continuous TBI use among patients with chronic illness patients and how to overcome these barriers,36 investigators have commented on the importance of developing assessment tools to explore facilitators and barriers to maintaining TBIs engagement.38,39 Furthermore, the number of times a participant logs into the website may not reflect the time of TBI use (eg, completing sessions).40 Future research, thus, should not only consider the benefits of TBI on participants’ health but also closely monitor, analyze, and report TBI use by participants.
Next, our findings have shown that half of the TBIs offered professional support to users, yet they did not achieve better outcomes than the self-guided interventions. Ugalde and colleagues (2018) recommended that further rigorous evidence is needed to understand the effect of self-guided interventions compared with the role of professional support or interventionists. Professional support in TBIs could provide information, guidance, and assistance for users regarding their symptoms management.41 The lack of guidance and assistance by health professionals is a substantial barrier to the use of TBIs among elderly people (eg, prostate cancer patients),35 while health care providers’ guidance and assistance have resulted in more enhanced self-management in patients with chronic conditions than TBI access alone.33 Similarly, Seiler and colleagues found in a systematic review that therapist-guided TBIs were more efficacious than self-guided interventions.42 In addition to health professionals’ support, we found that more than half of the studies provided participants with technical support such as user manuals, technical assistance, and technology training, which can reduce technical problems related to TBI use and enhance intervention fidelity in studies that require regular monitoring and attention.36
Finally, all TBIs in this review were web-based, except the smartphone app in a quasi-experimental study (Sundberg et al, 2017), a finding similar to a previous report that TBIs were exclusively delivered via online web-based programs for health promotion among elderly people.35 Smartphone apps, on the other hand, have been the most common type of TBI for health behavioral change among people with different health problems.43,44 More user-friendly and straightforward web-based platforms are needed to deliver complex and advanced content consistent with illness severity and needs for patients with chronic illness (eg, prostate cancer patients) who are elderly, have cognitive deficits and comorbid conditions, and lack of technology skills.43,44 These physical, individual, and social challenges coupled with the technical challenges in using mobile apps (small screen and font size) may mean that web-based interactive and comprehensive health interventions are more preferable.42,45–47 However, new smartphone technologies have advanced features such as interactive mode, augmented reality, flexible screen, and voice recognition that might improve the usability of smartphone apps for complex health issues for older patients.47,48
4.1 |. Limitations
Our selection criteria intentionally included TBIs that focused specifically on prostate cancer patients because of the unique challenges that prostate cancer survivor face. However, our review included three studies that also targeted breast and colorectal cancer patients, in addition to prostate cancer patients.20,21,24 Among these studies, Loiselle and colleagues conducted subgroup analyses for breast and prostate cancer participants, whereas the other two studies combined all cancer conditions in the same analysis. In addition to HRQoL, we only assessed the effectiveness of TBIs on anxiety and depression since they are the major psychological symptoms among cancer patients, and they are with a clear definition, measurements, and diagnostic criteria.49 Future studies need to assess the effects of TBIs on other psychological outcomes, such as psychological distress and cancer-related stress.
4.2 |. Implications in research and practice
The high risk of bias and underpowered sample sizes suggest that future research will require robust designs to examine the effectiveness of TBIs on health-related outcomes among prostate cancer patients. Randomization, blinding, consistent intervention use, and sufficient sample size should be considered in future research to minimize the risk of bias and enhance the study quality. Future studies must also include analysis of the impact of TBI characteristics such as components, format, duration and frequency of TBI sessions and the role of interventionists on the health outcomes. The variability of outcome measures and the timing of follow-up assessments have made it difficult and sometimes impossible to compare across studies and draw definitive conclusions. Therefore, researchers need to adopt standardized outcome measures such as the common data elements including PROMIS measures in future research and also to set up standardized follow-up assessment periods.
There have been a growing number of studies that develop and evaluate TBIs to improve the health outcomes of prostate cancer patients. Different research studies tend to lack the theoretical frameworks that lead the TBI development and evaluation process. Future research should integrate evidence-based and theory-guided TBIs for health management and behavioral change to determine what components, format, and dose are appropriate for prostate cancer conditions and needs, as well as how to consider biopsychosocial barriers in the use of TBIs.
5 |. CONCLUSION
Our review has found insufficient evidence to support the effectiveness of TBIs in improving health outcomes such as anxiety, depression, and HRQoL among patients with prostate cancer. However, to improve the methodological quality of this line of research, future research needs rigorous designs such as randomized control trials and sufficient sample size and power to examine the effects of TBIs on the health outcomes among patients with prostate cancer. Moreover, future research needs to examine the impact of the actual TBI use, components, sessions duration, and frequencies, as well as involvement of interventionists on patient outcomes.
ACKNOWLEDGMENTS
This project is partially supported by the R01 NR016990-01A1 (PI: Song) and the UNC Lineberger Comprehensive Cancer Center University Cancer Research Fund (PI: Song).
Funding information
UNC Lineberger Comprehensive Cancer Center
Footnotes
CONFLICTS OF INTEREST
None declared.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Chambers SK, Ng SK, Baade P, et al. Trajectories of quality of life, life satisfaction, and psychological adjustment after prostate cancer. Psychooncology. 2017;26(10):1576–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4(3):e003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim. Measurement of quality of life in patients with end-stage cancer. Cancer Nurs. 2014;37(1):44–49. [DOI] [PubMed] [Google Scholar]
- 4.Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17(8):1117–1128. [DOI] [PubMed] [Google Scholar]
- 5.Puts MT, Papoutsis A, Springall E, Tourangeau AE. A systematic review of unmet needs of newly diagnosed older cancer patients undergoing active cancer treatment. Support Care Cancer. 2012;20(7):1377–1394. [DOI] [PubMed] [Google Scholar]
- 6.Chambers SK, Hyde MK, Smith DP, et al. New challenges in psychooncology research III: a systematic review of psychological intervenetions for prostate cancer survivors and their partners: clinical and research implications. Psychooncology. 2017;26(7):873–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schofield P, Gough K, Lotfi-Jam K, et al. Nurse-led group consultation intervention reduces depressive symptoms in men with localised prostate cancer: a cluster randomised controlled trial. BMC Cancer. 2016;16(1):637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rincon E, Monteiro-Guerra F, Rivera-Romero D-ZE, Sanchez-Bocanegra CL, Gabarron E. Mobile apps for quality of life and well-being assessment in breast and prostate cancer patients: Systematic Review. JMIR Mhealth Uhealth. 2017;5(12):e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridriksdottir N, Gunnarsdottir S, Zoëga S, Ingadottir B, Hafsteinsdottir EJG. Effects of web-based interventions on cancer patients’ symptoms: review of randomized trials. Support Care Cancer. 2018;26(2):337–351. [DOI] [PubMed] [Google Scholar]
- 10.Widmer RJ, Collins NM, Collins CS, West CP, Lerman LO, Lerman A. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta-analysis. Mayo Clin Proc. 2015;90(4):469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leykin Y, Thekdi SM, Shumay DM, Muñoz RF, Riba M, Dunn LB. Internet interventions for improving psychological well-being in psychooncology: review and recommendations. Psychooncology. 2012;21(9):1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wootten AC, Abbott JA, Meyer D, et al. Preliminary results of a randomised controlled trial of an online psychological intervention to reduce distress in men treated for localised prostate cancer. Eur Urol. 2015;68(3):471–479. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Koh PW, Ritter PL, Lorig K, Bantum EO, Saria S. Dissecting an online intervention for cancer survivors: four exploratory analyses of internet engagement and its effects on health status and health behaviors. Health Educ Behav. 2015;42(1):32–45. [DOI] [PubMed] [Google Scholar]
- 14.Murray E, Hekler EB, Andersson G, et al. Evaluating digital health interventions. Am J Prev Med. 2016;51(5):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochrane CCG. Available from: http://www.cochrane.org/training/cochrane-handbook. 2011. [Google Scholar]
- 16.Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, Britten N, Roen K, Duffy S Guidance on the conduct of narrative synthesis in systematic reviews. A Product from the ESRC methods programme Version. 2006;1:b92. [Google Scholar]
- 17.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins RP, Pingree S, van Bogaert D, et al. The impact of combining human and online supportive resources for prostate cancer patients. J Comm Support Oncol. 2017;15(6):E321–E329. [Google Scholar]
- 19.Osei DK, Lee JW, Modest NN, Pothier PK. Effects of an online support group for prostate cancer survivors: a randomized trial. Urol Nurs. 2013;33(3):123–133. [PubMed] [Google Scholar]
- 20.Ruland CM, Andersen T, Jeneson A, et al. Effects of an internet support system to assist cancer patients in reducing symptom distress: a randomized controlled trial. Cancer Nurs. 2013;36(1):6–17. [DOI] [PubMed] [Google Scholar]
- 21.van de Wal M, Thewes B, Gielissen M, Speckens A, Prins J. Efficacy of blended cognitive behavior therapy for high fear of recurrence in breast, prostate, and colorectal cancer survivors: the SWORD study, a randomized controlled trial. J Clin Oncol. 2017;35(19):2173–2183. [DOI] [PubMed] [Google Scholar]
- 22.Yanez B, McGinty HL, Mohr DC, et al. Feasibility, acceptability, and preliminary efficacy of a technology-assisted psychosocial intervention for racially diverse men with advanced prostate cancer. Cancer. 2015;121(24):4407–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange L, Fink J, Bleich C, Graefen M, Schulz H. Effectiveness, acceptance and satisfaction of guided chat groups in psychosocial aftercare for outpatients with prostate cancer after prostatectomy. Internet Interv. 2017;9:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loiselle CG, Edgar L, Batist G, Lu J, Lauzier S. The impact of a multimedia informational intervention on psychosocial adjustment among individuals with newly diagnosed breast or prostate cancer: a feasibility study. Patient Educ Couns. 2010;80(1):48–55. [DOI] [PubMed] [Google Scholar]
- 25.Sundberg K, Wengström Y, Blomberg K, Hälleberg-Nyman M, Frank C, Langius-Eklöf A. Early detection and management of symptoms using an interactive smartphone application (Interaktor) during radiotherapy for prostate cancer. Support Care Cancer. 2017;25(7):2195–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cockle-Hearne J, Barnett D, Hicks J, Simpson M, White I, Faithfull S. A web-based intervention to reduce distress after prostate cancer treatment: development and feasibility of the getting down to coping program in two different clinical settings. JMIR cancer. 2018;4(1):e8–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venkatesh V, Morris MG, Davis GB, Davis FD. User acceptance of information technology: toward a unified view. MIS Q. 2003;27(3):425–478. [Google Scholar]
- 28.Cheng KKF, Lim YTE, Koh ZM, Tam WWS. Home-based multidimensional survivorship programmes for breast cancer survivors. Cochrane Database Syst Rev. 2017;8:Cd011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumann E, Czerwinski F, Reifegerste D. Gender-specific determinants and patterns of online health information seeking: results from a representative German health survey. J Med Internet Res. 2017;19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bidmon S, Terlutter R. Gender differences in searching for health information on the Internet and the virtual patient-physician relationship in Germany: Exploratory results on how men and women differ and why. J Med Internet Res. 2015;17(6):e156–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penard T, Poussing N, Mukoko B, Piaptie GBT. Internet adoption and usage patterns in Africa: evidence from Cameroon. Technol Soc. 2015;42:71–80. [Google Scholar]
- 32.Post KE, Flanagan J. Web based survivorship interventions for women with breast cancer: an integrative review. Eur J Oncol Nurs. 2016;25:90–99. [DOI] [PubMed] [Google Scholar]
- 33.Lee J- A, Choi M, Lee SA, Jiang N. Effective behavioral intervention strategies using mobile health applications for chronic disease management: a systematic review. BMC Med Inform Decis Mak. 2018;18(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beatty L, Binnion C. A systematic review of predictors of, and reasons for, adherence to online psychological interventions. Int J Behav Med. 2016;23(6):776–794. [DOI] [PubMed] [Google Scholar]
- 35.Kampmeijer R, Pavlova M, Tambor M, Golinowska S, Groot W. The use of e-health and m-health tools in health promotion and primary prevention among older adults: a systematic literature review. BMC Health Serv Res. 2016;16(Suppl 5):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitehead L, Seaton P. The effectiveness of self-management mobile phone and tablet apps in long-term condition management: a systematic review. J Med Internet Res. 2016;18(5):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amico KR. Percent total attrition: a poor metric for study rigor in hosted intervention designs. Am J Public Health. 2009;99(9):1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharpe EE, Karasouli E, Meyer C. Examining factors of engagement with digital interventions for weight management: rapid review. JMIR Res Protoc. 2017;6(10):e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velu AV, van Beukering MD, Schaafsma FG, et al. Barriers and facilitators for the use of a medical mobile app to prevent work-related risks in pregnancy: A Qualitative Analysis. JMIR Res Protoc. 2017;6(8):e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vollmer Dahlke D, Fair K, Hong YA, Beaudoin CE, Pulczinski J, Ory MG. Apps seeking theories: results of a study on the use of health behavior change theories in cancer survivorship mobile apps. JMIR Mhealth Uhealth. 2015;3(1):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dennison L, Morrison L, Conway G, Yardley L. Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. J Med Internet Res. 2013;15(4):e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seiler A, Klaas V, Troster G, Fagundes CP. eHealth and mHealth interventions in the treatment of fatigued cancer survivors: a systematic review and meta-analysis. Psychooncology. 2017;26(9):1239–1253. [DOI] [PubMed] [Google Scholar]
- 43.McKay FH, Cheng C, Wright A, Shill J, Stephens H, Uccellini M. Evaluating mobile phone applications for health behaviour change: a systematic review. J Telemed Telecare. 2016;1357633X16673538. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y, Yao X, Vespasiani G, et al. Mobile app-based interventions to support diabetes self-management: A systematic review of randomized controlled trials to identify functions associated with glycemic efficacy. JMIR Mhealth Uhealth. 2017;5(3):e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smits R, Bryant J, Sanson-Fisher R, et al. Tailored and integrated web-based tools for improving psychosocial outcomes of cancer patients: The DoTTI Development Framework. J Med Internet Res. 2014;16(3):e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borgmann H, Wolm JH, Vallo S, et al. Prostate cancer on the web-expedient tool for patients’ decision-making? J Cancer Educ. 2017;32(1):135–140. [DOI] [PubMed] [Google Scholar]
- 47.Levy D, Simonovsky E. Keeping in touch: mobile apps use by older adults. Paper presented at: International Conference on Human Aspects of IT for the Aged Population2018. [Google Scholar]
- 48.Kim J, Kam HJ, Park YR, et al. Enchanted life space: adding value to smart health by integrating human desires. Healthcare Informatics Res. 2018;24(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. [DOI] [PubMed] [Google Scholar]


