Abstract
BACKGROUND:
The early diagnosis and treatment of depression are cancer care priorities. These priorities are critical for prostate cancer survivors because men rarely seek mental health care. However, little is known about the epidemiology of depression in this patient population. The goal of this study was to describe the prevalence and predictors of probable depression in prostate cancer survivors.
METHODS:
The data were from a population-based cohort of North Carolinian prostate cancer survivors who were enrolled from 2004 to 2007 in the North Carolina-Louisiana Prostate Cancer Project (n = 1031) and were prospectively followed annually from 2008 to 2011 in the Health Care Access and Prostate Cancer Treatment in North Carolina study (n = 805). Generalized estimating equations were used to evaluate an indicator of probable depression (Short Form 12 mental composite score ≤48.9; measured at enrollment and during the annual follow-up) as a function of individual-level characteristics within the longitudinal data set.
RESULTS:
The prevalence of probable depression fell from 38% in the year of the cancer diagnosis to 20% 6 to 7 years later. Risk factors for probable depression throughout the study were African American race, unemployment, low annual income, younger age, recency of cancer diagnosis, past depression, comorbidities, treatment decisional regret, and nonadherence to exercise recommendations.
CONCLUSIONS:
Depression is a major challenge for prostate cancer survivors, particularly in the first 5 years after the cancer diagnosis. To the authors’ knowledge, this is the first study to demonstrate an association between treatment decisional regret and probable depression.
Keywords: depression, health disparity, predictors, prostate cancer, risk factors
INTRODUCTION
The prevalence of common depressive disorders (major and persistent depressive disorders or depression) is up to 25% in cancer survivors (vs 5%-6% in noncancer controls).1,2 The causal pathway between cancer and depression remains unclear, but plausible explanations include biological factors (eg, cancer cells producing depression-inducing chemicals),3 psychological factors (eg, the trauma of a cancer diagnosis),4,5 environmental factors (eg, a side effect of chemotherapy),6 and behavioral factors (eg, depression hindering self-care abilities).7,8 Regardless of the causal mechanism, an elevated risk for depression persists at all times after a cancer diagnosis.9,10
Depression adversely affects the cost, quality, and duration of survivorship; hence, primary prevention and secondary prevention of depression are cancer care priorities. Prevention of depression is a cancer care priority because of the adverse effect that depression has on the cost, quality, and duration of survivorship.11–16 These priorities are critical to prostate cancer survivors because men are usually reluctant to report depressive symptoms or seek mental health care,17–19 and depression has been linked to certain prostate cancer treatment types (eg, androgen deprivation therapy) and complications (eg, erectile dysfunction).20,21 Approximately 750,000 prostate cancer survivors in the United States are depressed.22–24 However, little is known about the epidemiology of depression in this patient population. The goal of this study was to describe the prevalence and predictors of probable depression in prostate cancer survivors (we use the word probable because of our identifying strategy; more details are given later). The study was designed to motivate/support depression care recommendations in survivorship guidelines.22
The analytic approach was informed by Kinser and Lyon’s conceptual model for individual stress vulnerability, depression, and health outcomes.25 The authors of the conceptual model suggested that sociodemographic characteristics (eg, race, unemployment, and low income),26–32 lifestyle factors (eg, a lack of exercise),33,34 acute and chronic burdens (eg, treatment decisional regret), and interpersonal situations affect a person’s susceptibility to stress vulnerabilities that often precede depression.25 After reviewing evidence on stress vulnerabilities in other patient populations, we hypothesized that sociodemographic characteristics such as age, African American race, low education, rural residence, being unmarried, unemployment, and low income were positively associated with probable depression.22,26
MATERIALS AND METHODS
Study Population and Procedure
Panel data from a population-based cohort of North Carolinian prostate cancer survivors who were enrolled from 2004 to 2007 in the North Carolina–Louisiana Prostate Cancer Project (PCaP) were assessed (n = 1031).35,36 In brief, PCaP is a study of environmental, biological, and behavioral causes of racial differences in prostate cancer aggressiveness.36 North Carolinian participants received a prostate cancer diagnosis on or after July 1, 2004; they were identified with records from the North Carolina Central Cancer Registry. African American and white American survivors were enrolled in equal proportions (the sampling weight was 1:0.44, respectively).36 North Carolinian participants were enrolled between September 2004 and December 2007, and they provided questionnaire data, biological specimens, and permission to obtain medical records. Participants also had up to 3 annual follow-up interviews in the Health Care Access and Prostate Cancer Treatment in North Carolina (HCaP-NC) study (2008-2011; n = 805). Interview questionnaires were completed by regular mail or by phone interview during annual follow-up contacts (ie, September 2008 to August 2009 [first wave], September 2009 to August 2010 [second wave], and September 2010 to August 2011 [third wave]). Data from 1024 participants were analyzed, and this study was approved by the Office of Human Research Ethics of the University of North Carolina at Chapel Hill (study #17-0183).
Measures
Identifying probable depression
Short Form 12 (SF-12) is a validated 12-item self-reported questionnaire that measures generic health-related quality of life.37 SF-12 item response choices are on either a Likert or binary (yes/no) scale, and responses are scored, weighed, and summed to yield physical composite scores and mental composite scores (MCSs). Composite scores range from 0 to 100 (with higher scores indicating better health) and provide insight into physical and mental health aspects of health-related quality of life. The SF-12 MCS can be used to identify depressed adults in population studies.38–40 The credibility of this approach results from the SF-12 MCS’s high negative correlation with depression severity and SF-12 items that refer to symptoms in the diagnostic criteria for depression (eg, depressed mood).41–43 Vilagut et al38 have shown that an SF-12 MCS threshold score of 48.9 is 74% sensitive and 83% specific for depression occurring in the prior 12 months. The threshold score of 48.9 was used to create a binary indicator of probable depression for each participant at enrollment and during the 3 indicated annual follow-up contacts. The term probable depression is used throughout this text because the indicated SF-12 MCS threshold score is nondiagnostic.
Predictors
The key explanatory variables were age at enrollment, race, educational attainment (up to high school or beyond high school), rural or urban residence (according to the 2010 US Census classification),44 index marital status (currently married, previously married, or never married), index employment status (retired, employed, or unemployed), and index annual income (≤$20,000, $20,001-$40,000, $40,001-$70,000, or >$70,000). Control covariates included the time since the prostate cancer diagnosis (in years) as well as binary indicators of the following: prostate cancer stage at diagnosis (T1 vs T2/T3; see Table 1), self-reported clinical diagnosis of depression before enrollment, probable depression in any prior survey wave, Charlson Comorbidity Index score (0-1 vs ≥ 2),45 availability of social/emotional support at each survey contact, index tobacco use, index alcohol use, adherence to the exercise recommendations of the World Health Organization (WHO; ie, at least 600 metabolic equivalent minutes per week) in the 12 months preceding survey contact,46 and treatment decisional regret during follow-up (measured with Clark et al’s 2001 regret scale47 [specific to prostate cancer survivors], which is different from O’Connor et al’s 1996 decision regret scale48 [not specific to prostate cancer survivors]).
TABLE 1.
Baseline Characteristics of the Study Participants (n = 1024)
| Characteristic | No. (%) | Characteristic | No. (%) |
|---|---|---|---|
| Sample size | 1024 (100) | Age at enrollment | |
| 40-49 y | 51 (5) | ||
| Probable depression at enrollment | 50-59 y | 322 (31) | |
| No | 715 (70) | 60-69 y | 425 (42) |
| Yes | 305 (30) | 70-79 y | 226 (22) |
| Race | Time since prostate cancer diagnosis | ||
| African American | 525 (51) | 0-12 mo | 963 (94) |
| White American | 499 (49) | 13-24 mo | 58 (6) |
| Marital status | 25-36 mo | 3 (<1) | |
| Currently married | 776 (76) | Has health insurance | |
| Previously married | 188 (18) | No | 518 (51) |
| Never married | 59 (6) | Yes | 506 (49) |
| Educational attainment | Cancer stage at diagnosisa | ||
| High school or less than high school | 490 (48) | T1 | 609 (60) |
| More than high school | 533 (52) | T2/T3 | 408 (40) |
| Residence | Charlson Comorbidity Index | ||
| Urban | 781 (76) | 0-1 | 766 (75) |
| Rural | 243 (24) | ≥2 | 257 (25) |
| Employment status | Adheres to exercise recommendations | ||
| Retired | 480 (47) | No | 256 (25) |
| Employed/yet to retire | 485 (48) | Yes | 767 (75) |
| Unemployed | 56 (5) | Current tobacco use | |
| Annual income | No | 526 (76) | |
| >$70,000 | 311 (32) | Yes | 162 (24) |
| $40,001 -$70,000 | 240 (25) | Current alcohol use | |
| $20,001-$40,000 | 235 (24) | No | 407 (40) |
| <$20,000 | 182 (19) | Yes | 614 (60) |
The cancer stage at diagnosis was based on the size and extent of the primary tumor (see Prostate Cancer: Stages and Grades at https://www.cancer.net/cancer-types/prostate-cancer/stages-and-grades). At stage T1, the tumor is not detectable with a digital rectal examination or imaging but is found in prostate tissue from a biopsy or surgical treatment. At stage T2, the tumor is detectable with a digital rectal examination or imaging but is confined to the prostate. At stage T3, the cancer has grown outside the prostate and may have grown into the seminal vesicles. At stage T4, the cancer has grown into other nearby tissues, such as the urethral sphincter, rectum, bladder, or wall of the pelvis.
Not all groups of n values add up to 1024 due to missing observations.
Treatment decisional regret was assessed with the following 2 questions: whether the participant would have been better off with a different cancer treatment type (possible responses included definitely false, somewhat false, neither true nor false, somewhat true, and definitely true) and the amount of time that the participant spent wishing that he could change his mind about the cancer treatment type (possible responses included none of the time, rarely, neither a little nor a lot of the time, some of the time, and all of the time).47,48 A participant had treatment decisional regret if he definitely or somewhat agreed that he would have been better off choosing a different cancer treatment type or if he spent all or some of the time wishing that he could change his mind about the cancer treatment type.47,48 Treatment decisional regret was not assessed during enrollment. However, it was assumed that there was no regret at enrollment because 1) participants either were awaiting or had recently received cancer treatment and 2) available evidence suggests that treatment decisional regret is negligible in recently treated prostate cancer survivors.49,50 This assumption was examined with sensitivity analyses.
Statistical Analyses
Generalized estimating equations (GEEs) with a binomial family, logit link, and independent correlation (the correlation structure with the least quasi-likelihood under the independence model criterion [QIC]) were used to evaluate an indicator of probable depression as a function of indicated key explanatory variables and control covariates.51,52 The model was used to predict the average and annual prevalence of probable depression in the first 7 years after the cancer diagnosis. Survey sampling weights were applied, and an α value of .05 was used to determine statistical significance. Sensitivity analyses were performed with alternative GEE correlation structures (ie, unstructured and exchangeable) and an alternative assumption about treatment decisional regret during enrollment (ie, all participants had treatment decisional regret).
Dealing with missing data
Approximately 400 participants were lost to follow-up before the end of HCaP-NC (see Fig. 1). Chi-square and t-tests showed that participants lost to follow-up were more likely to be African American, uninsured, smokers, and low-income earners with a higher prostate cancer stage at diagnosis. Logit regression showed that loss to follow-up was random conditional on observed variables.53 Survey response rates were higher than 95% (with respect to analytic variables) during each survey contact. Missing observations from survey nonresponses occurred at random and were handled via multiple imputation (with 50 imputed data sets for explanatory variables only).54–56 Details of the imputation process (including specifications and diagnostics) are provided in the supplement.
Figure 1.
Schematic showing how study participation changed over time.
RESULTS
Descriptive Statistics
Baseline characteristics of study participants are presented in Table 1. Most participants were middle-aged or elderly, were urban residents, were previously or currently married, were retired or employed, and were enrolled within the 12 months after their prostate cancer diagnosis. Most participants had early-stage prostate cancer, had Charlson Comorbidity Index scores between 0 and 1, adhered to WHO’s exercise recommendations, and consumed alcoholic beverages but not tobacco-containing products.
Prevalence of Probable Depression
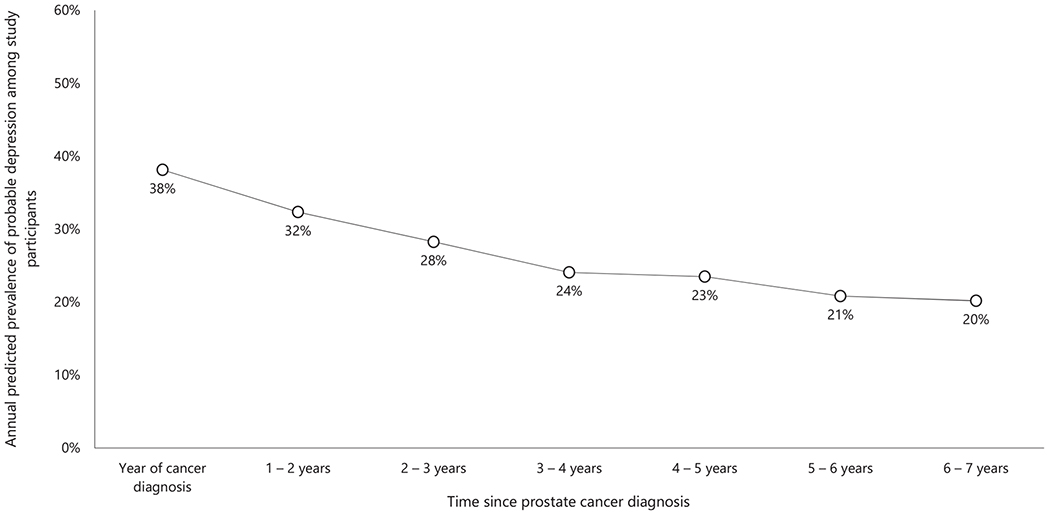
The average prevalence of probable depression was 28% over the study period. This prevalence had a temporal trend (Fig. 2): it was highest in the first 2 years after the cancer diagnosis (approximately 38%) before significantly declining to 20% in the seventh year (P < .01; Table 2).
Figure 2.
Temporal trend in the annual predicted prevalence of probable depression in study participants.
TABLE 2.
Factors Associated With Probable Depression
| Variable | Odds Ratio | Marginal Effect | Variable | Odds Ratio | Marginal Effect |
|---|---|---|---|---|---|
| Race | Educational attainment | ||||
| White American (referent) | – | – | High school or less | – | – |
| African American | 1.33a (1.07 to 1.66) | 0.05a (0.01 to 0.09) | More than high school | 0.95 (0.75 to 1.21) | −0.01 (−0.05 to 0.03) |
| Marital status | Residence | ||||
| Currently married (referent) | – | – | Mostly urban (referent) | – | – |
| Previously/never married | 1.19 (0.91 to 1.56) | 0.03 (−0.02 to 0.08) | Mostly rural | 0.88 (0.69 to 1.12) | −0.02 (−0.06 to 0.02) |
| Age at enrollment | 0.98a (0.97 to 0.99) | −0.003a (−0.01 to −0.001) | Has treatment decisional regret | ||
| No (referent) | – | – | |||
| Yes | 3.31b (2.23 to 4.92) | 0.23b (0.15 to 0.32) | |||
| Received a clinical diagnosis of depression before enrollment | Probable depression in any prior survey wave | ||||
| No (referent) | – | – | None (referent) | – | – |
| Yes | 2.44b (1.82 to 3.27) | 0.17b (0.11 to 0.23) | 1 or more | 4.37b (3.39 to 5.64) | 0.29b (0.24 to 0.34) |
| Time since cancer diagnosis | Employment status | ||||
| 0-12 mo (referent) | – | – | Retired (referent) | – | – |
| 13-24 mo | 0.73 (0.43 to 1.23) | −0.06 (−0.15 to 0.04) | Employed/yet to retire | 1.28 (1.00 to 1.64) | 0.04 (−0.001 to 0.08) |
| 25-36 mo | 0.57a (0.34 to 0.95) | −0.10a (−0.19 to −0.01) | Unemployed | 1.74b (1.18 to 2.56) | 0.10b (0.02 to 0.17) |
| 37-48 mo | 0.44b (0.27 to 0.69) | −0.14b (−0.22 to −0.06) | Annual income | ||
| $70,000 (referent) | – | – | |||
| 49-60 mo | 0.42b (0.26 to 0.67) | −0.15b (−0.22 to −0.07) | $40,001-$70,000 | 1.26 (0.96 to 1.65) | 0.04 (−0.01 to 0.08) |
| 61-72 mo | 0.34b (0.20 to 0.58) | −0.17b (−0.25 to −0.09) | $20,001-$40,000 | 1.28 (0.93 to 1.76) | 0.04 (−0.01 to 0.09) |
| 73-84 mo | 0.33b (0.16 to 0.66) | −0.18b (−0.28 to −0.08) | ≤$20,000 | 1.57a (1.03 to 2.39) | 0.08a (0.002 to 0.15) |
| Cancer stage at diagnosis | Charlson Comorbidity Index | ||||
| T1: a-c (referent) | – | – | 0-1 (referent) | – | – |
| T2/T3: a-c | 1.03 (0.84 to 1.26) | 0.01 (−0.03 to 0.04) | ≥2 | 1.59b (1.28 to 1.96) | 0.08b (0.04 to 0.12) |
| Has emotional support | Adherent to exercise recommendations | ||||
| No (referent) | – | – | No (referent) | – | – |
| Yes | 1.11 (0.73 to 1.68) | 0.02 (−0.05 to 0.09) | Yes | 0.67b (0.55 to 0.82) | −0.07b (−0.10 to −0.03) |
| Current tobacco use | Current alcohol use | ||||
| No (referent) | – | – | No (referent) | – | – |
| Yes | 1.13 (0.85 to 1.50) | 0.02 (−0.03 to 0.07) | Yes | 1.07 (0.87 to 1.33) | 0.01 (−0.02 to 0.05) |
There were 1024 participants and 3128 participant observations. Ninety-five percent confidence intervals are presented in parentheses. Fifty imputed data sets were used.
P ≤ .05.
P ≤ .01.
Predictors of Probable Depression
Variables associated with a higher risk of probable depression (ie, risk factors) throughout the study were African American race, unemployment, low income, past depressive episodes, a Charlson Comorbidity Index score of 2 or higher, and treatment decisional regret (Table 2). Variables associated with a lower risk of probable depression (ie, protective factors) throughout the study were age at enrollment, length of prostate cancer survivorship (ie, 3 or more years), and adherence to WHO’s exercise recommendations. No significant association was found between probable depression and any other model covariate.
Sensitivity Analyses
Study findings remained robust in GEEs with alternative correlation structures and under the assumption that all participants had treatment decisional regret during enrollment. Interaction terms (ie, between treatment decisional regret and cancer treatment type and between treatment decisional regret and cancer recurrence) were included as model covariates in separate regression models to examine whether the observed association between probable depression and treatment decisional regret varied by cancer treatment type or cancer recurrence. No significant difference was observed across categories of the interaction terms. In addition, we found no evidence of a significant association between probable depression and prostate cancer treatment type (and, by extension, side effects) or prostate cancer recurrence.
DISCUSSION
Study Implications
Unequal access to mental health care may explain the association between race and probable depression. Evidence from studies in the general population have shown that the incidence of depression is identical in African Americans and white Americans and that African Americans have poorer access to mental health care in comparison with white Americans.28,57–59 Appropriate depression care promotes recovery and prevents relapse/recurrence of depression60,61; thus, limited access to mental health care may make African American prostate cancer survivors more vulnerable to depression. However, little is known about access to mental health care among prostate cancer survivors, and this will be examined in another study.
Up to 2 in 5 participants experienced probable depression in the first 2 years after their cancer diagnosis. This is consistent with findings from studies on patients with other types of cancer and suggests a high need for depression care in recently diagnosed survivors.5,62 In addition, the annual prevalence of probable depression between the fifth and seventh years (ie, 20%; see Fig. 2) is similar to the post–cancer treatment prevalence of depression in the prostate cancer literature (ie, 18%; 95% confidence interval [CI], 15%-22%).23 This finding suggests that the prevalence of depression among prostate cancer survivors remains stable from 5 years after the cancer diagnosis. Moreover, the initial downward trend in the annual prevalence of probable depression may be explained by developing or peaked psychological resilience, which has been shown to protect prostate cancer survivors from depression.63
The association between adherence to WHO’s exercise recommendations and probable depression is consistent with the literature.33,34 The American Cancer Society’s prostate cancer survivorship guideline promotes regular exercise and lists benefits that are expected to improve the survivorship experience (eg, lower risks of prostate cancer recurrence, fatigue, and anxiety). The survivorship guideline recommends regular patient-provider conversations about exercise. However, available evidence suggests that many providers fail to discuss exercise with their patients,64,65 and this inaction among cancer care providers should be discouraged.
The American Cancer Society’s prostate cancer survivorship guideline also encourages providers to screen for depression in survivors at risk for depression. Indicated risk factors include being unmarried, low education, advanced prostate cancer, low physical or cognitive functioning, younger age, medical comorbidities, psychiatric history, and poor coping skills.22 This study presents supportive evidence for some indicated risk factors (ie, young age, medical comorbidities, and psychiatric history). However, other risk factors identified in this study (ie, African American race, unemployment, low annual income, treatment decisional regret, and nonadherence to WHO’s exercise recommendations) should be considered for inclusion in the guideline. An unemployed African American participant who earns less than $20,000 per year, has treatment decisional regret, and is nonadherent to exercise recommendations faces a 70% chance of probable depression (95% CI, 58%-80%) over a 12-month period. However, because of the low depression screening rate among men in the general population (4%-8%)66,67 and the rate of clinical recognition of depression among nonmental health providers (36%-47%),68,69 depression in this hypothetical participant is likely to remain undiagnosed.
Lastly, to the best of our knowledge, this study is the first to demonstrate an association between treatment decisional regret and depression.70 Treatment decisional regret affects 4% to 18% of prostate cancer survivors in the near term,48,71,72 and emerging evidence suggests that its association with depression is due to repetitive negative thinking.73,74 Available evidence also suggests that treatment decisional regret is likely to occur in prostate cancer survivors who assume a passive role in cancer treatment decision making.50,71,75 Hence, preventing future depression may be an additional motivating factor for active participation in cancer treatment decision making.
Strengths and Limitations
This study has several strengths. Several clinically relevant factors (eg, depression history, comorbidities, and cancer stage) were controlled in all regression models. In addition, the application of sampling weights makes study findings generalizable to prostate cancer survivors in North Carolina. However, the generalizability of study findings to all prostate cancer survivors in the United States remains uncertain. The distributions of prostate cancer survivors by age and race during enrollment in the Surveillance, Epidemiology and End Results program (2011-2015) and PCaP (2004-2007) are similar in the 2 data sets (Table 3). Any differences may be driven by the relative sample sizes or an earlier age at cancer diagnosis for PCaP participants.
TABLE 3.
Comparison of Prostate Cancer Survivors During Enrollment in PCaP and in SEER by Race and Age
| SEER (2011-2015) | PCaP (2004-2007) | P | |
|---|---|---|---|
| No. of African American enrollees | 7604 | 505 | – |
| Distribution of African | |||
| American enrollees by age, % | |||
| <40 y | 0 | 0 | .65 |
| 40-49 y | 4 | 6 | .07 |
| 50-59 y | 28 | 39 | <.05 |
| 60-69 y | 44 | 37 | <.05 |
| 70-79 y | 19 | 18 | .58 |
| ≥80 y | 5 | 0 | <.01 |
| No. of white American enrollees | 36,208 | 526 | |
| Distribution of white | |||
| American enrollees by age, % | |||
| <40 y | 0 | 0 | .47 |
| 40-49 y | 2 | 4 | <.05 |
| 50-59 y | 18 | 24 | <.05 |
| 60-69 y | 42 | 46 | .55 |
| 70-79 y | 28 | 26 | .42 |
| ɥ80 y | 10 | 0 | <.05 |
Abbreviations: PCaP, North Carolina-Louisiana Prostate Cancer Project; SEER, Surveillance, Epidemiology, and End Results.
P values were estimated with binomial tests of proportions.
The identification strategy for depression (SF-12 MCS ≤48.9) is imperfect (sensitivity, 74%; specificity, 83%). Hence, the false-positives and false-negatives in the data set may bias regression estimates toward the null or increase variances and risks of type II errors in explanatory variables. This risk of a type II error may affect the expected association between employment (vs retirement) and probable depression (odds ratio, 1.28; P = .052; see Table 2).76 However, study findings are likely to remain robust if a diagnostic instrument such as Patient Health Questionnaire 9 is used to identify depressed study participants (sensitivity, 80%; specificity, 92%).77,78
New episodes of probable depression could not be teased apart from recurrence/relapses, nor could anxiety disorders be isolated from probable depression. These limitations preclude accurate measurement of the annual incidence of depression in the sample. Also, the study sample did not include prostate cancer survivors with late-stage cancer, so the study findings do not extend to late-stage disease.
Lastly, the identification strategy for depression prevents the separation of anxiety disorders from probable depression or new cases from recurrences and relapses. These limitations prevent precise measurement of the annual incidence of probable depression among study participants, which could be used to simulate the natural history of depression in hypothetical prostate cancer survivors via Markov/microsimulation models. However, a conservative estimate was derived by the conversion of the 5-year cumulative incidence of probable depression between the third and seventh years after the prostate cancer diagnosis (ie, when the annual prevalence of probable depression appeared stable; see Fig. 2) into an annual incidence with a standard approach (ie, the proportion of incidental true-positive cases [n1 = 154] and incidental false-negative cases [n2 = 62] among at-risk study participants [N = 575] is (n1 + n2)/N or 216/575 or 37.6% over a 5-year period, which translates into 9.0% per year [95% CI, 7.9%-10.2%] under the constant incidence assumption).79,80 This conservative estimate of the annual incidence of probable depression may approximate the true annual incidence of depression because it is approximately 5 times the annual incidence of depression in Canadian men aged 65 years and older (ie, 1.8%),81 approximately 6 to 8 times the annual incidence of depression in Swedish men aged 70 to 85 years (ie, 1.2%),82 and consistent with the cancer literature (depression is up to 6 times more common in patients with cancer in comparison with the general population).1,2 However, the true annual incidence of depression in prostate cancer survivors may be lower than 9.0% because the estimated cumulative incidence may have inadvertently included a few recurrent cases.79 Conversely, the true annual incidence of depression may be higher than 9.0% because study participants who were lost to follow-up had fewer opportunities to be identified as true-positive cases. Nevertheless, 9.0% seems to be a more plausible estimate than 16% to 17%, which was obtained from inpatient samples of prostate cancer survivors with advanced disease.20,83
In conclusion, depression is a major challenge for prostate cancer survivors, particularly in the first 5 years after their cancer diagnosis. Risk factors for depression include African American race, unemployment, low annual income, relatively young age, recency of cancer diagnosis, past depression, comorbidities, treatment decisional regret, and nonadherence to WHO’s exercise recommendations.
Supplementary Material
Acknowledgments
We thank the staff, advisory committees, and research subjects that participated in the North Carolina–Louisiana Prostate Cancer Project and Health Care Access and Prostate Cancer Treatment in North Carolina studies for their important contributions. We also acknowledge contributions from the following individuals: Sally C. Stearns, PhD, George Pink, PhD, Marisa E. Domino, PhD, Antonia V. Bennet, PhD, Nkechi Conteh, MD, MPH, and Adrian Gerstel.
FUNDING SUPPORT
The North Carolina–Louisiana Prostate Cancer Project and the Health Care Access and Prostate Cancer Treatment in North Carolina study were supported by the Department of Defense (contract DAMD 17-03-2-0052) and the American Cancer Society (award RSGT-08-008-01-CPHPS).
CONFLICT OF INTEREST DISCLOSURES
Ronald C. Chen reports grants and personal fees from Accuray, Inc, and personal fees from Astellas/Medivation, Bayer, Inc, and Blue Earth outside the submitted work. The other authors made no disclosures.
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;32:57–71. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12:160–174. [DOI] [PubMed] [Google Scholar]
- 3.News BBC. Tumour make-up ‘fuels depression’. http://news.bbc.co.uk/2/hi/health/8055104.stm. Accessed August 20, 2017.
- 4.Edwards B, Clarke V. The psychological impact of a cancer diagnosis on families: the influence of family functioning and patients’ illness characteristics on depression and anxiety. Psychooncology. 2004;13:562–576. [DOI] [PubMed] [Google Scholar]
- 5.Linden W, Vodermaier A, MacKenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141:343–351. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. Mood changes. https://www.nccn.org/patients/resources/life_with_cancer/managing_symptoms/mood_changes.aspx. August 15, 2017.
- 7.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. [DOI] [PubMed] [Google Scholar]
- 8.Rost K, Zhang M, Fortney J, Smith J, Coyne J, Smith GR. Persistently poor outcomes of undetected major depression in primary care. Gen Hosp Psychiatry. 1998;20:12–20. [DOI] [PubMed] [Google Scholar]
- 9.Penn Medicine. Untreated late effects of breast cancer care increase depression and anxiety among survivors, Penn study shows. https://www.pennmedicine.org/news/news-releases/2016/december/untreated-late-effects-of-breast-cancer-care-increase-depression-and-anxiety-among-survivors. Published December 12, 2016 Accessed November 3, 2017.
- 10.Chochinov HM. Depression in cancer patients. Lancet Oncol. 2001; 2:499–505. [DOI] [PubMed] [Google Scholar]
- 11.Colleoni M, Mandala M, Peruzzotti G, Robertson C, Bredart A, Goldhirsch A. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet. 2000;356:1326–1327. [DOI] [PubMed] [Google Scholar]
- 12.Bui QUT, Ostir GV, Kuo YF, Freeman J, Goodwin JS. Relationship of depression to patient satisfaction: findings from the barriers to breast cancer study. Breast Cancer Res Treat. 2005;89:23–28. [DOI] [PubMed] [Google Scholar]
- 13.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Preventive Services Task Force. Screening for depression in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:784–792. [DOI] [PubMed] [Google Scholar]
- 15.Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32:1605–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bankhead C Depression in cancer common but untreated. https://www.medpagetoday.com/psychiatry/depression/47405 Published August 29, 2014. Accessed August 22, 2017.
- 17.Cochran SV, Rabinowitz FE. Men and Depression: Clinical and Empirical Perspectives. Amsterdam, the Netherlands: Elsevier; 1999. [Google Scholar]
- 18.Real T I Don’t Want to Talk About It: Overcoming the Secret Legacy of Male Depression. New York, NY: Simon and Schuster; 1998. [Google Scholar]
- 19.Addis ME, Mahalik JR. Men, masculinity, and the contexts of help seeking. Am Psychol. 2003;58:5–14. [DOI] [PubMed] [Google Scholar]
- 20.Pirl WF, Greer JA, Goode M, Smith MR. Prospective study of depression and fatigue in men with advanced prostate cancer receiving hormone therapy. Psychooncology. 2008;17:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson CJ, Mulhall JP, Roth AJ. The association between erectile dysfunction and depressive symptoms in men treated for prostate cancer. J Sex Med. 2011;8:560–566. [DOI] [PubMed] [Google Scholar]
- 22.Skolarus TA, Wolf A, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64:225–249. [DOI] [PubMed] [Google Scholar]
- 23.Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4:e003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett G, Badger TA. Depression in men with prostate cancer. Oncol Nurs Forum. 2005;32:545–556. [DOI] [PubMed] [Google Scholar]
- 25.Kinser PA, Lyon DE. A conceptual framework of stress vulnerability, depression, and health outcomes in women: potential uses in research on complementary therapies for depression. Brain Behav. 2014;4:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessler RC, Zhao S, Blazer DG, Swartz M. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. J Affect Disord. 1997;45:19–30. [DOI] [PubMed] [Google Scholar]
- 27.Riolo SA, Nguyen TA, Greden JF, King CA. Prevalence of depression by race/ethnicity: findings from the National Health and Nutrition Examination Survey III. Am J Public Health. 2005;95:998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams DR, Gonzalez HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305–315. [DOI] [PubMed] [Google Scholar]
- 29.Yoo KB, Park EC, Jang SY, et al. Association between employment status change and depression in Korean adults. BMJ Open. 2016;6:e008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knebelmann J, Prinz C. The Impact of Depression on Employment of Older Workers in Europe. https://www.oecd-ilibrary.org/employment/the-impact-of-depression-on-employment-of-older-workers-in-europe_106dbdae-en. Accessed September 15, 2017.
- 31.Hiswals AS, Walander A, Soares J, Macassa G. Employment status, anxiety and depression in a municipal context. Res Health Sci. 2017;2:12–23. [Google Scholar]
- 32.Garrard J, Rolnick SJ, Nitz NM, et al. Clinical detection of depression among community-based elderly people with self-reported symptoms of depression. J Gerontol A Biol Sci Med Sci. 1998;53:M92–M101. [DOI] [PubMed] [Google Scholar]
- 33.Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med. 2008;46:397–411. [DOI] [PubMed] [Google Scholar]
- 34.Mammen G, Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med. 2013;45:649–657. [DOI] [PubMed] [Google Scholar]
- 35.Holmes JA, Bensen JT, Mohler JL, Song L, Mishel MH, Chen RC. Quality of care received and patient-reported regret in prostate cancer: analysis of a population-based prospective cohort. Cancer. 2017;123:138–143. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder JC, Bensen JT, Su LJ, et al. The North Carolina-Louisiana Prostate Cancer Project (PCaP): methods and design of a multidisciplinary population-based cohort study of racial differences in prostate cancer outcomes. Prostate. 2006;66:1162–1176. [DOI] [PubMed] [Google Scholar]
- 37.Ware J Jr. SF-12 Health Survey (SF-12). Boston, MA: Medical Outcomes Trust; 1993. [Google Scholar]
- 38.Vilagut G, Forero CG, Pinto-Meza A, et al. The mental component of the Short-Form 12 Health Survey (SF-12) as a measure of depressive disorders in the general population: results with three alternative scoring methods. Value Health. 2013;16:564–573. [DOI] [PubMed] [Google Scholar]
- 39.Gill SC, Butterworth P, Rodgers B, Mackinnon A. Validity of the mental health component scale of the 12-item Short-Form Health Survey (MCS-12) as measure of common mental disorders in the general population. Psychiatry Res. 2007;152:63–71. [DOI] [PubMed] [Google Scholar]
- 40.Santos JF, Ramos-Cerqueira A, Furegato AF, Lebrao M, Duarte YO. The Short Form Health Survey as an instrument for the screening of depressive symptoms in the elderly population [abstract O2-3.5]. J Epidemiol Community Health. 2011;65(suppl 1):A24. [Google Scholar]
- 41.Lenert LA, Sherbourne CD, Sugar C, Wells KB. Estimation of utilities for the effects of depression from the SF-12. Med Care. 2000;38:763–770. [DOI] [PubMed] [Google Scholar]
- 42.Salyers MP, Bosworth HB, Swanson JW, Lamb-Pagone J, Osher FC. Reliability and validity of the SF-12 Health Survey among people with severe mental illness. Med Care. 2000;38:1141–1150. [DOI] [PubMed] [Google Scholar]
- 43.American Psychiatric Association; Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 44.Maske UE, Buttery AK, Beesdo-Baum K, Riedel-Heller S, Hapke U, Busch MA. Prevalence and correlates of DSM-IV-TR major depressive disorder, self-reported diagnosed depression and current depressive symptoms among adults in Germany. J Affect Disord. 2016;190:167–177. [DOI] [PubMed] [Google Scholar]
- 45.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. Global Recommendations on Physical Activity for Health. https://apps.who.int/iris/bitstream/handle/10665/44399/9789241599979_eng.pdf;jsessionid=B1D9EF9AC60BF810E05C56B258026063?sequence=1. Accessed October 5, 2017. [PubMed]
- 47.Clark JA, Wray NP, Ashton CM. Living with treatment decisions: regrets and quality of life among men treated for metastatic prostate cancer. J Clin Oncol. 2001;19:72–80. [DOI] [PubMed] [Google Scholar]
- 48.O’Connor AM. User manual — decision regret scale [document on the internet]. Ottawa: Ottawa Hospital Research Institute; © 1996 [modified 2003] 3 p. http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Regret_Scale.pdf [Google Scholar]
- 49.Hurwitz LM, Cullen J, Kim DJ, et al. Longitudinal regret after treatment for low- and intermediate-risk prostate cancer. Cancer. 2017;123:4252–4258. [DOI] [PubMed] [Google Scholar]
- 50.Davison B, Goldenberg S. Decisional regret and quality of life after participating in medical decision-making for early-stage prostate cancer. BJU Int. 2003;91:14–17. [DOI] [PubMed] [Google Scholar]
- 51.Pan W Model selection in estimating equations. Biometrics. 2001;57:529–534. [DOI] [PubMed] [Google Scholar]
- 52.Cui J QIC program and model selection in GEE analyses. Stata J. 2007;7:209–220. [Google Scholar]
- 53.Allison PD. Missing Data. Newbury Park, CA: Sage Publications; 2001. Quantitative Applications in the Social Sciences; vol 136. [Google Scholar]
- 54.Lin G, Rodriguez RN. Weighted methods for analyzing missing data with the GEE procedure. Paper presented at: SAS Global Forum; March 23-26, 2014; Washington, DC: Paper SAS166-2015. [Google Scholar]
- 55.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 56.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harman JS, Edlund MJ, Fortney JC. Disparities in the adequacy of depression treatment in the United States. Psychiatr Serv. 2004;55:1379–1385. [DOI] [PubMed] [Google Scholar]
- 58.Stockdale SE, Lagomasino IT, Siddique J, McGuire T, Miranda J. Racial and ethnic disparities in detection and treatment of depression and anxiety among psychiatric and primary health care visits, 1995-2005. Med Care. 2008;46:668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu CF, Campbell DG, Chaney EF, Li YF, McDonell M, Fihn SD. Depression diagnosis and antidepressant treatment among depressed VA primary care patients. Adm Policy Ment Health. 2006;33:331–341. [DOI] [PubMed] [Google Scholar]
- 60.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sim K, Lau WK, Sim J, Sum MY, Baldessarini RJ. Prevention of relapse and recurrence in adults with major depressive disorder: systematic review and meta-analyses of controlled trials. Int J Neuropsychopharmacol. 2016;19:pyv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korfage I, Essink-Bot ML, Janssens A, Schroder F, De Koning H. Anxiety and depression after prostate cancer diagnosis and treatment: 5-year follow-up. Br J Cancer. 2006;94:1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharpley CF, Wootten AC, Bitsika V, Christie DR. Variability over time-since-diagnosis in the protective effect of psychological resilience against depression in Australian prostate cancer patients: implications for patient treatment models. Am J Mens Health. 2013; 7:414–422. [DOI] [PubMed] [Google Scholar]
- 64.Carroll JK, Fiscella K, Meldrum SC, et al. Clinician-patient communication about physical activity in an underserved population. J Am Board Fam Med. 2008;21:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glasgow RE, Eakin EG, Fisher EB, Bacak SJ, Brownson RC. Physician advice and support for physical activity: results from a national survey. Am J Prev Med. 2001;21:189–196. [DOI] [PubMed] [Google Scholar]
- 66.Maimone RM, Marhatta A. The rate of depression screening at a federally qualified community health center. Health Serv Res Manag Epidemiol. 2015;2:2333392815613057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akincigil A, Matthews EB. National rates and patterns of depression screening in primary care: results from 2012 and 2013. Psychiatr Serv. 2017;68:660–666. [DOI] [PubMed] [Google Scholar]
- 68.Mitchell AJ, Vaze A, Rao S. Clinical diagnosis of depression in primary care: a meta-analysis. Lancet. 2009;374:609–619. [DOI] [PubMed] [Google Scholar]
- 69.Cepoiu M, McCusker J, Cole MG, Sewitch M, Belzile E, Ciampi A. Recognition of depression by non-psychiatric physicians—a systematic literature review and meta-analysis. J Gen Int Med. 2008;23:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kinlock BL, Parker LJ, Howard DL, Bowie JV, LaVeist TA, Thorpe RJ Jr. Prevalence and correlates of major depressive symptoms among black men with prostate cancer. Ethn Dis. 2017;27:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davison BJ, So AI, Goldenberg SL. Quality of life, sexual function and decisional regret at 1 year after surgical treatment for localized prostate cancer. BJU Int. 2007;100:780–785. [DOI] [PubMed] [Google Scholar]
- 72.Hu JC, Kwan L, Krupski TL, et al. Determinants of treatment regret in low-income, uninsured men with prostate cancer. Urology. 2008;72:1274–1279. [DOI] [PubMed] [Google Scholar]
- 73.Kertz SJ, Koran J, Stevens KT, Bjorgvinsson T. Repetitive negative thinking predicts depression and anxiety symptom improvement during brief cognitive behavioral therapy. Behav Res Ther. 2015;68:54–63. [DOI] [PubMed] [Google Scholar]
- 74.Roese NJ, Epstude K, Fessel F, et al. Repetitive regret, depression, and anxiety: findings from a nationally representative survey. J Soc Clin Psychol. 2009;28:671–688. [Google Scholar]
- 75.Hu JC, Kwan L, Saigal CS, Litwin MS. Regret in men treated for localized prostate cancer. J Urol. 2003;169:2279–2283. [DOI] [PubMed] [Google Scholar]
- 76.Fukuda Y, Hiyoshi A. Influences of income and employment on psychological distress and depression treatment in Japanese adults. Environ Health Prev Med. 2012;17:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509–515. [Google Scholar]
- 78.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Int Med. 2001; 16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vandenbroucke JP, Pearce N. Incidence rates in dynamic populations. Int J Epidemiol. 2012;41:1472–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics. 2007;25:3–6. [DOI] [PubMed] [Google Scholar]
- 81.Patten SB. Incidence of major depression in Canada. Can Med Assoc J. 2000;163:714–715. [PMC free article] [PubMed] [Google Scholar]
- 82.Palsson SP, Ostling S, Skoog I. The incidence of first-onset depression in a population followed from the age of 70 to 85. Psychol Med. 2001;31:1159–1168. [DOI] [PubMed] [Google Scholar]
- 83.Hervouet S, Savard J, Ivers H, Savard MH. Depression and androgen deprivation therapy for prostate cancer: a prospective controlled study. Health Psychol. 2013;32:675–684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.