Abstract
Objective: To describe postprandial lipemia in patients with rheumatoid arthritis (RA) and to analyze its association with subclinical atherosclerosis measured as carotid intima-media thickness (cIMT). Methods: We performed an observational study of 40 patients with RA and 40 sex and age-matched controls. Patients with dyslipidemia were excluded. Pathologically increased cIMT was defined as a carotid thickness greater than the 90th percentile (>p90) for age and sex. Fasting and postprandial plasma lipids, cholesterol, triglycerides, apolipoprotein B48 (ApoB48), and total ApoB were evaluated. The other variables included were clinical and laboratory values, Framingham score, and the 28-joint Disease Activity Score (DAS28). Two multivariate models were constructed to identify factors associated with pathologic cIMT in patients with RA. Results: Fasting lipid values were similar in patients with RA and controls, although those of postprandial ApoB48 were higher (median (IQR), 14.4 (10.8–12.1) vs. 12.1 (2.3–9,8); p = 0.042). Pathologic cIMT was recorded in 10 patients with RA (25%) and nine controls (22.5%). In patients with RA, pathologic cIMT was associated with postprandial ApoB48 (OR (95% CI), 1.15 (1.0–1.3)) and total ApoB (OR [95% CI], 1.12 [1.1–1.2]). The second model revealed a mean increase of 0.256 mm for cIMT in patients with elevated anticitrullinated protein antibodies (ACPAs). Conclusion: Postprandial ApoB48 levels in patients with RA are higher than in controls. Postprandial ApoB48 and total ApoB levels and markers of severity, such as ACPAs, are associated with pathologic cIMT in patients with RA. Our findings could indicate that these atherogenic particles have a negative effect on the endothelium.
Keywords: rheumatoid arthritis, postprandial lipemia, apolipoprotein B48, subclinical atherosclerosis
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by persistent synovitis, bone erosions, and functional disability. It is associated with premature death and multiple morbidities [1], mainly because the cardiovascular risk of affected patients is similar to that of patients with type 2 diabetes mellitus [2]. Accelerated atherosclerosis in patients with RA is due to both the presence of traditional cardiovascular risk factors and to nontraditional cardiovascular risk factors, including systemic inflammation and dyslipidemia [3,4,5,6,7].
Ultrasound measurement of intima-media thickness (IMT) and the presence of plaques in the carotid artery has been reported to be a useful test for detecting subclinical atherosclerosis [8,9,10]. It has also been considered valid for confirming accelerated atherogenesis in persons with RA. In a study of a cohort of patients with chronic RA and no traditional cardiovascular risk factors or previous cardiovascular events, carotid IMT (cIMT) revealed abnormally high values compared with healthy controls [11].
More than half of patients with RA have dyslipidemia [12]—which is one of the reported factors that contributes to increased cardiovascular risk [13]. Park et al. [14] reported that in RA patients, levels of apolipoprotein A (ApoA) and HDL cholesterol were significantly lower than in controls. Similarly, lipoprotein levels, the ApoB/ApoA ratio, total cholesterol/HDL ratio, and LDL/HDL ratio were greater in untreated RA patients. In contrast with total cholesterol, LDL cholesterol, and HDL cholesterol, which are associated with cardiovascular risk, the importance of triglycerides as a cause of premature coronary disease is less clear [15,16]. Interest in triglycerides as a cardiovascular risk factor is growing because recent studies show that increased postprandial lipemia is an independent predictor of the risk of atherosclerosis in the general population [15,17]. Intolerance to triglycerides arises from the inability to process triglyceride-loaded lipoproteins, which in turn increases the risk of atherosclerosis [18,19]. For example, an association has been reported between higher ApoB48 levels and coronary atherosclerosis in patients with type 2 diabetes mellitus [20,21].
Few data have been published to date on subclinical atherosclerosis measured based on cIMT and its association with postprandial lipemia in patients with RA [7]. The objective of the present study was to analyze the association between postprandial lipid values (i.e., ApoB48 levels after a mixed breakfast) and subclinical atherosclerosis measured as cIMT and plaque presence in patients with RA. The results were compared with those of controls.
2. Patients and Methods
2.1. Design
We performed a controlled observational cross-sectional study of a cohort of patients with established RA and controls. The study was carried out at The Institute of Biomedical Research in Malaga (IBIMA) in the Rheumatology Department of Hospital Regional Universitario de Málaga (HRUM), in the Lipid and Atherosclerosis Laboratory of Universidad de Málaga (UMA) and the Center for Medical and Health Research (CIMES), Spain. The study was approved by the local Clinical Research Ethics Committee (CEIC) on 16/11/2018 (Code 1787-N-18). All participants gave their written informed consent.
2.2. Patients
Patients with RA were recruited consecutively between January and October 2019 from an initial cohort established between 2007 and 2012. The inclusion criteria were a diagnosis of RA (following the 2010 criteria of the American College of Rheumatology/European League Against Rheumatism [22]), onset after age 16 years, and being in prospective follow-up in the cohort at the cut-off date. The exclusion criteria were inflammatory diseases other than RA (except secondary Sjögren syndrome), diagnosis of dyslipidemia, lipid-lowering treatment, active infection, and pregnancy.
2.3. Controls
The controls were volunteers aged more than 16 years with no inflammatory or autoimmune diseases or symptoms that would lead us to suspect these diseases. The exclusion criteria were the same as for the patients. The controls were volunteers aged more than 16 years with no inflammatory or autoimmune diseases or symptoms that would lead us to suspect these diseases (i.e., joint inflammation, inflammatory pain, morning stiffness, etc.). The exclusion criteria were the same as for the patients. The controls were selected at random from a health center in the catchment area of the hospital. Controls and patients were matched by age and sex.
2.4. Protocol
A fasting preprandial blood sample was taken from all patients at 8:30 AM. The study population then had a mixed breakfast in the hospital cafeteria (milk, cured ham, cheese, olive oil, and bread). The nutritional information for breakfast was as follows—775 kcal, 50 g of fat (53% saturated fatty acids, 41% monounsaturated fatty acids, 6% polyunsaturated fatty acids), and 40 g of carbohydrates. During the postprandial period, the participants could only take their habitual medication (if they had it to hand) and water on demand. They had to rest and refrain from smoking. Four hours later, a second blood sample was taken and processed at the local laboratory, except for the lipid analysis sample, which was frozen immediately and then analyzed at the CIMES. On the same day, all participants underwent a physical examination, and their clinical history was taken according to a pre-established protocol. An ultrasound scan of the carotid artery was also taken.
2.5. Main Outcome Measure
The main outcomes measures were ApoB48 postprandial lipid values and cIMT assessed using ultrasound. Pathologic cIMT was defined as a carotid thickness greater than the 90th percentile (>p90) for age and sex according to reference data for the Spanish population [23]. Atheromatous plaque was defined by consensus [24] as (1) focal thickening of the arterial wall protruding toward the lumen and measuring >0.5 mm, (2) more than 50% of the neighboring cIMT, or (3) cIMT >1.5 mm. The ultrasound scans of the carotid were taken using the ART.LAB system (ESAOTE, Barcelona, Spain) by a trained ultrasound specialist with experience in the technique.
Patients were classified into two groups based on the presence or absence of carotid plaque and/or pathologic cIMT.
2.6. Laboratory Measures
Plasma lipids, cholesterol, and triglycerides [25] were determined using enzymatic techniques (SPINREACT, Barcelona, Spain) in a Mindray BS 380 autoanalyzer (MINDRAY, Shenzhen, China). Chylomicrons (cholesterol and triglycerides) and very low-density lipoprotein (VLDL) were measured using sequential ultracentrifugation with the same enzymatic kits. HDL cholesterol was determined using a homogeneous assay (SIEMENS, Erlangen, Germany). LDL cholesterol was calculated based on the Friedewald formula. ApoB48 and total ApoB levels were measured in plasma after 8 h fasting and 4 h after the meal using a commercially available ELISA approach (Shibayagi Co Ltd., Ishihara, Japan) [20] and immunoturbidimetry (BIOSYSTEMS, Barcelona, Spain), respectively. Serum levels of high sensitivity C-reactive protein (hsCRP) were measured using turbidimetry (BIOSYSTEMS, Barcelona, Spain).
2.7. Other Variables
The other variables collected on the cut-off date included epidemiological variables (sex, race, body mass index (BMI), weight/height in m2) waist circumference (cm), hip circumference (cm), and the waist-hip ratio (quotient of waist circumference/hip circumference in cm)). We also collected conventional cardiovascular risk–related variables, as follows: Smoking (active, exsmoker, never), obesity (BMI > 30), arterial hypertension ≥140/90 mmHg or current antihypertensive medication [26], diabetes diagnosed according to the criteria of the American Diabetes Association [27], a personal history of cardiovascular disease, family history of coronary disease defined as a first-degree relative with myocardial infarction or stroke (<55 years for men and <60 years for women), Framingham score indicating risk of coronary heart disease at 10 years expressed as a percentage [28] (>20%, high-risk; 10–20%, intermediate risk; and <10%, low risk), and a validated questionnaire on adherence to a Mediterranean diet (MEDAS, 14 items [29], with ≥9 items considered adherent and <9 items considered nonadherent). The activity was assessed using the International Physical Activity Questionnaire (IPAQ) score [30], expressed as metabolic equivalent of task (MET) minutes, as follows: Low/sedentary or insufficient level of physical activity for the healthy activity recommendations, <600 MET minutes in the previous week; or moderate/high/fulfills the criteria for moderate levels, >600 MET minutes in the previous week.
The clinical-laboratory and therapeutic values recorded included rheumatoid factor (RF), which was considered positive if >10 IU/mL, and anticitrullinated protein antibody (ACPA), which was considered positive if >20 IU/mL. Inflammatory activity was evaluated in patients using the 28-joint Disease Activity Score with erythrocyte sedimentation rate (DAS28-ESR) (continuous, range, 0–9.4) [31] recorded at the visit [32]. According to the DAS28-ESR, activity was considered high with a value >5.1; moderate with 3.2–5.1; low with 2.6–3.2; and remission with ≤2.6. We also took into account severity variables, such as the presence of radiologic erosions and the score on the Health Assessment Questionnaire (HAQ) [32]. Treatment with conventional synthetic disease-modifying antirheumatic drugs (DMARDs) and biologic DMARDs was recorded.
2.8. Statistical Analysis
We used summary statistics to describe the participants and the χ2 and t or Mann-Whitney or Wilcoxon test to refute differences between them, as applicable.
We used summary statistics to describe the different variables. Normality was confirmed using the Kolmogorov-Smirnov test. The χ2 and t-test or Mann-Whitney test were used to compare the main characteristics between patients and controls, as well as between patients with RA and a normal and pathologic cIMT. Mean ranges for the baseline and postprandial values of ApoB48 and other lipid variables were compared between associated variables using the Wilcoxon test. Finally, we constructed two multivariate models: A backward binary logistic regression analysis (Wald) (dependent variable (DV): pathologic cIMT) and linear regression (DV: cIMT) to explore the variables that were independently associated with cIMT in patients with RA. The variables selected for the multivariate analysis that were significant in the bivariate analysis and those that were of clinical interest. Statistical significance was set at p < 0.05. The analyses were performed using R 2.4–0.
3. Results
3.1. Baseline Characteristics
The study population was comprised of 80 participants (40 patients with RA and 40 controls). Table 1 shows the baseline characteristics of both patients and controls. Most participants were women (85%), with a median age of around 56 years. Both groups were well balanced in terms of epidemiological characteristics, comorbidities, and cardiovascular risk factors. However, physical activity in MET minutes was better in the controls (median (interquartile range [IQR]) = 893 (280.0–1188.0) vs. 495.0 (70.0–990.0); p = 0.008)), who also had higher acute phase reactant and antibody levels.
Table 1.
Baseline characteristics of 40 patients with RA and 40 controls.
| Variable | Patients n = 40 | Controls n = 40 | p Value |
|---|---|---|---|
| Epidemiological Characteristics | |||
| Age in Years, Median (IQR) | 55.7 (52.9–61.7) | 57.0 (52.7–61.1) | 0.662 |
| Female Sex; n (%) | 35 (87.5) | 34 (85.0) | 0.745 |
| Smoking | 0.181 | ||
| Never Smoked, n (%) | 15 (37.5) | 21 (52.5) | |
| Exsmoker, n (%) | 19 (47.5) | 11 (27.5) | |
| Active Smoker, n (%) | 6 (15.0) | 8 (20.0) | |
| Comorbidities | |||
| Arterial Hypertension, n (%) | 10 (25.0) | 9 (22.5) | 0.792 |
| Diabetes Mellitus, n (%) | 2 (5.0) | 4 (10.0) | 0.395 |
| Cardiovascular Disease, n (%) | 3 (7.5) | 2 (5.0) | 0.644 |
| Family History of Coronary Artery Disease, n (%) | 13 (32.5) | 7 (17.5) | 0.121 |
| Anthropometric Characteristics | |||
| BMI (kg/m2), Median (IQR) | 26.7 (24.5–31.0) | 27.2 (24.4–30.8) | 0.758 |
| Obesity, n (%) | 10 (26.3) | 10 (27.0) | 0.944 |
| Waist Circumference, (cm), Median (IQR) | 91.5 (83.0–108.5) | 91 (84.0–102.0) | 0.361 |
| Hip Circumference (cm), Median (IQR) | 106.5 (103.5–112.3) | 105.0 (100.0–114.0) | 0.308 |
| Waist-Hip Ratio, Median (IQR) | 0.86 (0.8–0.9) | 0.86 (0.8–0.9) | 0.828 |
| MET-Minute, Median (IQR) | 495.0 (70.0–990.0) | 893.0 (280.5–1188.0) | 0.008 |
| Total MEDAS Score, Median (IQR) | 10.0 (8.0–11.0) | 9.0 (8.0–11.0) | 0.184 |
| Framingham %, Median (IQR) | 2.6 (0.9–4.1) | 1.8 (0.7–4.6) | 0.501 |
| High Risk, n (%) | 0 (0.0) | 0 (0.0) | 1.000 |
| Intermediate Risk, n (%) | 6 (16.2) | 3 (8.1) | 0.286 |
| Low Risk, n (%) | 31 (83.8) | 34 (91.9) | 0.286 |
| Clinical-Laboratory Characteristics | |||
| Progression of RA, Months, Median (IQR) | 119 (81.2–167.9) | - | - |
| Diagnostic delay, months, median (IQR) | 8.1 (5.6–16.7) | - | - |
| Erosions, n (%) | 16 (40.0) | - | - |
| RF > 10, n (%) | 26 (65.0) | 0 (0.0) | <0.001 |
| ACPA > 20, n (%) | 31 (77.5) | 0 (0.0) | <0.001 |
| High-Sensitivity CRP (mg/dL), Median (IQR) | 4.2 (2.7–7.4) | 1.7 (0.8–3.1) | 0.002 |
| ESR (mm/h), Median (IQR) | 15 (9.0–26.5) | 11 (6.6–18.5) | 0.016 |
| DAS28 at Protocol, Median (IQR) | 3.06 (2.5–4.2) | - | - |
| Remission-Low Activity, n (%) | 21 (53.8) | - | - |
| Moderate-High Activity, n (%) | 18 (46.1) | - | - |
| HAQ, Median (IQR) | 0.9 (0.2–1.6) | - | - |
| Synthetic DMARDs, n (%) | 31 (77.5) | - | - |
| Methotrexate, n (%) | 23 (62.2) | - | - |
| Leflunomide, n (%) | 3 (8.1) | - | - |
| Sulfasalazine, n (%) | 3 (8.1) | - | - |
| Hydroxychloroquine, n (%) | 2 (5.4) | ||
| Biologic DMARDs, n (%) | 21 (52.5) | - | - |
| Anti TNF-α, n (%) | 17 (45.9) | - | - |
| Jak Inhibitor, n (%) | 1 (2.7) | - | - |
| Anti-IL-6, n (%) | 3 (8.1) | - | - |
| Glucocorticoid at Protocol, n (%) | 13 (32.5) | - | - |
| Glucocorticoid Dose at Protocol, Median (IQR) | 5 (5.0–5.0) | - | - |
| Other Treatments | |||
| Antihypertensive Drugs | 10 (25.0) | 9 (22.5) | 0.792 |
| ACEIs, n (%) | 7 (17.5) | 7 (17.5) | 0.778 |
| ARAIIs, n (%) | 3 (7.5) | 2 (5.0) | 0.462 |
| Diuretics, n (%) | 5 (12.5) | 8 (20.0) | 0.370 |
| Metformin, n (%) | 2 (5.0) | 3 (7.5) | 0.320 |
| Insulin, n (%) | 0 (0.0) | 1 (2.5) | 0.320 |
| Other Oral Antidiabetic Agents, n (%) | 0 (0.0) | 1 (2.5) | 0.320 |
Abbreviations: RA, rheumatoid arthritis; IQR, interquartile range; ACPA, anti-citrullinated peptide antibodies; RF, rheumatoid factor; SD, standard deviation; MEDAS, Mediterranean Diet Adherence Survey; DAS28, 28-joint Disease Activity Score; HAQ, Health Assessment Questionnaire; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; DMARD, disease-modifying antirheumatic drug; IL-6, interleukin 6; Anti TNF, anti–tumor necrosis factor ACEI, angiotensin-converting enzyme inhibitor; ARAII, angiotensin II receptor antagonists.
Most patients were women with established RA (median (IQR) time since onset, 119 (81.2–167.9) months); 16/40 (40%) had erosive disease, and more than half were in remission or with low disease activity. A total of 31/40 (77.5%) were taking a synthetic DMARD, mainly methotrexate, and 21/40 (52.5%) were taking a biologic DMARD, mainly a tumor necrosis factor alpha (anti-TNF-α) drug. The median dose of corticosteroids at the date of the protocol was 5 mg of prednisone equivalents in 13/40 (32.5%) who were taking corticosteroids.
3.2. Study of Pre- and Postprandial Blood Lipid Values and cIMT in Patients and Controls
Table 2 shows the baseline and postprandial data for the lipid profile in patients and controls, as well as the findings for the carotid ultrasound.
Table 2.
Lipid profile and carotid ultrasound in 40 patients with RA and 40 controls.
| Variable | RA n = 40 | Controls n = 40 | RA vs. Controls p | |||
|---|---|---|---|---|---|---|
| Fasting | Postprandial | Fasting | Postprandial | Fasting | Postprandial | |
| Fasting Lipid Profile | ||||||
| Total Cholesterol (mg/dL), Median (IQR) | 212.1 (187.0–234.2) | 202.0 (178.0–226.2) | 200.2 (176.0–227.2) | 201.0 (168.1–220.5) | 0.148 | 0.222 |
| LDL Cholesterol (mg/dL), Median (IQR) | 127.0 (107.1–140.0) | 110.3 (98.5–130.0) | 116.5 (95.7–140.5) | 108.0 (83.1–128.6) | 0.229 | 0.203 |
| HDL Cholesterol (mg/dL), Median (IQR) | 62.5 (54.7–76.5) | 62.2 (52.7–72.2) | 59.5 (47.7–71) | 57.1 (46.2–67.2) | 0.162 | 0.063 |
| Triglycerides (mg/dL), Median (IQR) | 82.5 (66.7–113.5) | 130.0 (91.7–185.0) * | 88.5 (64.5–125.7) | 132.5 (108.2–210.4) * | 0.823 | 0.913 |
| Chylomicrons (Triglycerides), Median (IQR) | 14.7 (10.3–27.4) | 42.3 (22.1–81.3) * | 16.4 (7.5–39.8) | 43.7 (31.9–84.7) * | 0.644 | 0.225 |
| Chylomicrons (Cholesterol), Median (IQR) | 9.2 (6.8–13.5) | 9.2 (6.8–13.5) * | 12.3 (5.3–21.9) | 12.3 (5.3–21.9) * | 0.544 | 0.613 |
| VLDL (Triglycerides), Median (IQR) | 16.0 (10.1–27.7) | 29.6 (15.5–41.1) * | 21.0 (9.8–31.5) | 24.6 (17.6–38.7) * | 0.758 | 0.859 |
| VLDL (Cholesterol), Median (IQR) | 3.6 (2.3–6.1) | 3.6 (2.3–6.1) * | 5.8 (2.7–9.8) | 5.8 (2.7–9.8) * | 0.087 | 0.083 |
| ApoB48, Median (IQR) | 7.4 (6.2–10.5) | 14.4 (10.8–23.2) * | 7.7 (5.5–10.3) | 12.1 (10.9–16.2) * | 0.874 | 0.042 |
| ApoB Total, Median (IQR) | 96.1 (84.9–104.9) | 92.4 (80–103.4) | 98.0 (84.3–108.3) | 93.2 (77.3–102.9) | 0.950 | 0.517 |
| TG/HDL Ratio, Median (IQR) | 1.2 (0.8–2.0) | 2.1 (1.3–3.5) | 1.4 (0.8–2.9) | 2.5 (1.6–4.1) | 0.597 | 0.574 |
| ApoB48/TG Ratio, Median (IQR) | 0.09 (0.07–0.1) | 0.1 (0.7–0.1) | 0.08 (0.1–0.13) | 0.09 (0.7–0.1) | 0.985 | 0.326 |
| Fasting Carbohydrate Profile | ||||||
| Baseline Blood Sugar (mg/dL), Median (IQR) | 78.0 (74.7–83) | 80.0 (72.7–88.2) | 0.843 | 0.277 | ||
| Homocysteine, Median (IQR) | 14.4 (12.8–18) | 13.5 (11.4–16.8) | 0.494 | |||
| Carotid Ultrasound | ||||||
| Pathologic cIMT >p90, n (%) | 10 (25.0) | 9 (22.5) | 0.555 | |||
| Right cIMT (mm), Median (IQR) | 0.7 (0.6–0.8) | 0.7 (0.7–1.0) | 0.652 | |||
| Left cIMT (mm), Median (IQR) | 0.66 (0.6–0.7) | 0.7 (0.64–0.78) | 0.353 | |||
| Patients with Atheromatous Plaques, n (%) | 7 (18.4) | 8 (20.0) | 0.481 | |||
* p < 0.005 fasting vs. postprandial value. Abbreviations IQR, interquartile range; cIMT, carotid intima media thickness; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; VLDL, very-low-density lipoproteins.
In general, there were no differences between patients and controls with respect to fasting and postprandial blood lipid values in most readings. As for the postprandial increase in lipid values, a significant increase was recorded in triglycerides, chylomicrons (triglycerides), very-low-density lipoprotein (triglycerides), and ApoB48. The increase in ApoB48 was significantly greater in patients than in controls (median (IQR), 14.4 (10.8–23.2) vs. 12.1 (10.9–16.2); p = 0.042).
Ten patients with RA (25%) and nine controls (22.5%) had pathologic cIMT, although there were no significant differences in cIMT or in the number of plaques.
3.3. Study of pre- and Postprandial Blood Lipids and Baseline Characteristics of Patients with RA According to cIMT
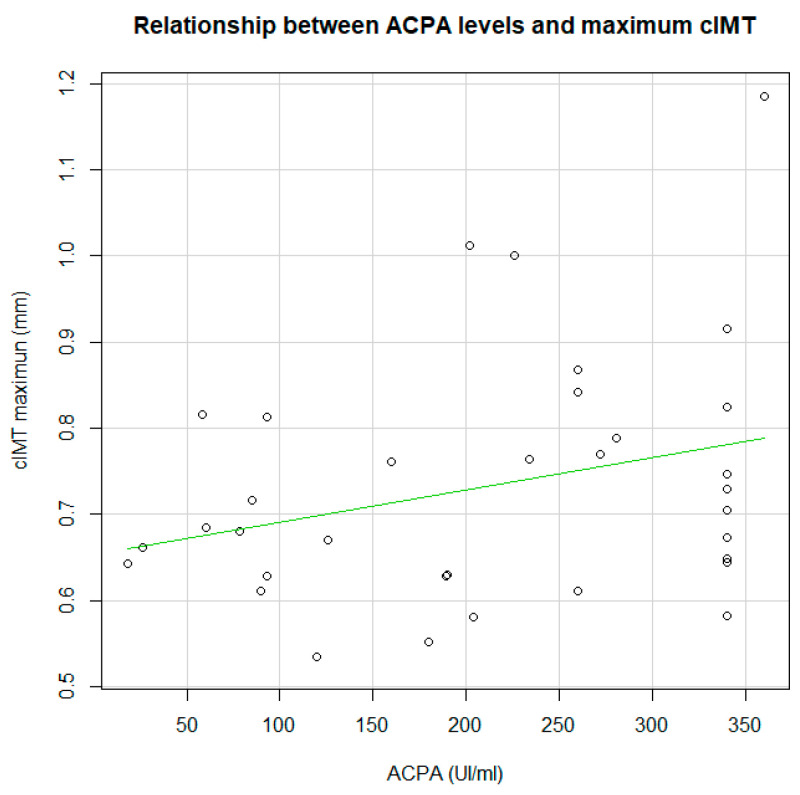
As shown in Table 3, 10 of the 40 patients with RA (25%) had pathologic cIMT. There were fewer women in this group (p = 0.002), and the median waist-hip ratio was greater (p = 0.048), as was the median Framingham score (p = 0.039). There were no differences in the clinical and laboratory data, except that ACPAs were more frequently positive and with higher titters (>340) in patients with pathologic cIMT (p = 0.036). The correlation analysis did not show significant differences, although it shows a positive correlation with a tend statistical significance (r = 0.290; p = 0.096) (Figure 1). There were no differences in DMARDs, although patients with pathologic cIMT more frequently took glucocorticoids (p = 0.032).
Table 3.
Baseline characteristics of patients with RA according to cIMT.
| Variable | RA with IMT >p90 n = 10 | RA with IMT ≤p90 n = 30 | p Value |
|---|---|---|---|
| Age, Years, Median (IQR) | 55.3 (48.6–68.3) | 55.7 (53.3–61.6) | 0.900 |
| Female Sex; n (%) | 6 (60.0) | 29 (96.7) | 0.002 |
| Smoking | 0.818 | ||
| Never, n (%) | 4 (40.0) | 11 (36.7) | |
| Exsmoker, n (%) | 4 (40.0) | 15 (50.0) | |
| Active Smoker, n (%) | 2 (20.0) | 4 (13.3) | |
| Comorbidities | |||
| Arterial Hypertension, n (%) | 3 (30.0) | 7 (23.3) | 0.673 |
| Diabetes Mellitus, n (%) | 1 (10.0) | 1 (3.3) | 0.442 |
| Cardiovascular Disease, n (%) | 0 (0.0) | 3 (10.0) | 0.298 |
| Anthropometric Characteristics | |||
| BMI (kg/m2), Median (IQR) | 27.1 (24.4–32.2) | 26.6 (24.6–29.5) | 0.700 |
| Obesity, n (%) | |||
| Waist Circumference, (cm), Median (IQR) | 106.5 (87–110.7) | 89 (83–103) | 0.212 |
| Hip Circumference (cm), Median (IQR) | 106 (103.2–109.7) | 106.5 (102.2–112.2) | 0.941 |
| Waist-Hip Index, Median (IQR) | 0.92 (0.83–1) | 0.85 (0.81–0.91) | 0.048 |
| MET-Minute, Median (IQR) | 247.5 (70.0–618.7) | 594.0 (84.0–1064.0) | 0.164 |
| Total MEDAS, Median (IQR) | 10. (8.7–11.0) | 10.0 (8.0–11.0) | 0.824 |
| Framingham %, Median (IQR) | 4.6 (1.5–13.8) | 1.2 (0.6–3.8) | 0.039 |
| Clinical-Laboratory Characteristics | |||
| Time Since Diagnosis of RA, Months, Median (IQR) | 140 (93–214.4) | 113 (80–166.2) | 0.138 |
| Diagnostic Delay, Months, Median (IQR) | 9.9 (5.5–18.5) | 6.9 (5.3–12.0) | 0.414 |
| Erosions, n (%) | 4 (40.0) | 12 (40.0) | 0.473 |
| RF > 10, n (%) | 7 (70.0) | 21 (70.0) | 1.000 |
| ACPA > 20, n (%) | 8 (80.0) | 22 (73.3) | 0.473 |
| High ACPA (>340), n (%) | 6 (60.0) | 10 (33.3) | 0.036 |
| High-Sensitivity CRP (mg/dL), Median (IQR) | 4.4 (3.2–8.7) | 3.8 (2.5–7.5) | 0.221 |
| ESR (mm/h), Median (IQR) | 12.0 (7.7–37.2) | 15.0 (9.0–26.0) | 0.839 |
| DAS28 at Protocol, Median (IQR) | 3.3 (2.5–3.9) | 2.9 (2.5–4.2) | 0.644 |
| HAQ, Median (IQR) | 1.3 (0.7–1.7) | 0.8 (0.2–1.6) | 0.544 |
| Synthetic DMARDs, n (%) | 9 (90.0) | 22 (73.0) | 0.174 |
| Methotrexate, n (%) | 5 (50.0) | 18 (60.0) | 0.580 |
| Biologic DMARDs, n (%) | 4 (40.0) | 18 (60.0) | 0.271 |
| Corticosteroids, n (%) | 6 (60.0) | 7 (23.3) | 0.032 |
Abbreviations: cIMT, carotid intima media thickness; IQR, interquartile range; BMI, body mass index.
Figure 1.
Correlation between anti-citrullinated peptide antibodies (ACPA)titters and carotid intima media thickness (cIMT) maximum.
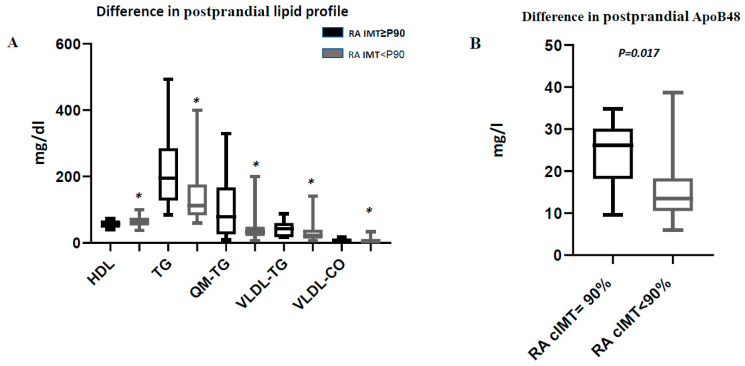
With respect to preprandial blood lipids, patients with pathologic cIMT had higher levels of HDL cholesterol (p = 0.045), and triglycerides (p = 0.014) and higher levels of VLDL (triglycerides) (p = 0.022), and total cholesterol (p = 0.028) than patients with normal cIMT. Similar results were recorded for postprandial blood lipids, with lower levels of HDL cholesterol (p = 0.042) and higher levels of triglycerides (p = 0.033), chylomicrons (triglycerides) (p = 0.045), VLDL (triglycerides) (p = 0.036), and VLDL (cholsterol) (p = 0.046) in patients with pathologic cIMT (Figure 2). There were no preprandial differences in apolipoproteins, although postprandial ApoB48 was higher in patients with pathologic cIMT (p = 0.017). Furthermore, after a mixed breakfast, the increase in ApoB48 and chylomicrons (triglyceride) was significantly greater in patients with pathologic cIMT (p = 0.002 and p = 0.045, respectively) (Table 4). These differences were not found in the levels of blood lipids and ApoB48 between control group with pathologic cIMT and control group with normal cIMT (Supplementary Material-Table S1).
Figure 2.
Postprandial lipid profile amongRA patients with cIMT≥90% and cIMT<90%. (A) Difference in postprandial lipid profile. * Indicates significant differences (p-value ≤ 0.05). (B) Difference in postprandial ApoB48. Abreviature; RA: Rheumatoid arthritis; cIMT:carotidintima-media thickness; HDL:high-densitylipoproteincholesterol; TG:Triglyceride; QM-TG: Triglyceridechylomicrons; VLDL-TG: VeryLow DensityLipoprotein-Triglyceride; VLDL-CO: VeryLow DensityLipoprotein-cholesterol.
Table 4.
Lipid profile and carotid ultrasound in RA patients according to cIMT.
| Variable | RA with IMT >p90 n = 10 | RA with IMT ≤p90 n = 30 | RA with IMT >p90 vs. RA with IMT ≤p90 p | |||
|---|---|---|---|---|---|---|
| Fasting | Postprandial | Fasting | Postprandial | Fasting | Postprandial | |
| Fasting Lipid Profile | ||||||
| Total Cholesterol (mg/dL), Median (IQR) | 226.0 (189.5–243.2) | 204.1 (184–237.2) | 210.2 (187.2–225.7) | 200.0 (179.2–222.2) | 0.471 | 0.573 |
| LDL Cholesterol (mg/dL), Median (IQR) | 138.2 (118.7–152.0) | 110.0 (107.2–130.5) | 123.5 (105.5–139.0) | 110.0 (95.5–129.7) | 0.266 | 0.647 |
| HDL Cholesterol (mg/dL), Median (IQR) | 61.0 (51.2–69.7) | 57.5 (49.0–65.7) | 66.5 (56.5–77.5) | 63.5 (53.7–73.7) | 0.045 | 0.042 |
| Triglycerides (mg/dL), Median (IQR) | 112.0 (82.7–17.6) | 195.0 (127.5–285.2) | 77.5 (863.5–107.2) | 116.0 (83.5–176.2) | 0.014 | 0.033 |
| Chylomicrons (Triglycerides), Median (IQR) | 33.8 (14.2–57.0) * | 79.1 (25.6–167.1) * | 14.1 (9.3–23.2) | 32.7 (21.8–54.7) | 0.066 | 0.045 |
| Chylomicrons (Cholesterol), Median (IQR) | 12.3 (8.6–16.1) | 15.6 (5.7–25.0) | 8.7 (6.0–11.6) | 15.3 (7.3–24.5) | 0.089 | 0.770 |
| VLDL (Triglycerides), Median (IQR) | 24.2 (21.9–39.5) | 42.6 (17.3–60.0) | 14.4 (9.8–26.4) | 23.1 (13.1–39.7) | 0.022 | 0.036 |
| VLDL (Cholesterol), Median (IQR) | 5.3 (3.9–9.4) | 8.2 (3.7–12.7) | 2.8 (1.9–6.0) | 3.9 (2.7–8.9) | 0.028 | 0.046 |
| ApoB48, Median (IQR) | 8.5 (5.9–13.0) * | 24.3 (15.1–27.1) * | 7.7 (5.5–10.3) | 13.5 (10.5–18.2) | 0.186 | 0.017 |
| ApoB Total, Median (IQR) | 102.7 (94.4–115.1) | 98.0 (87–110.8) | 94 (82.6–104.3) | 91.7 (76.1–102.6) | 0.141 | 0.100 |
| Increased Postprandial Blood Lipids | ||||||
| Triglycerides (mg/dL), Median (IQR) | 73.2 (24.0–134.5) | 39.9 (17.2–68.0) | 0.122 | |||
| Chylomicrons (Triglycerides), Median (IQR) | 47.4 (14.6–124.1) | 21.5 (10.2–37.7) | 0.045 | |||
| VLDL (Triglycerides), Median (IQR) | 12.9 (6.4–20.7) | 8.0 (3.0–16.2) | 0.424 | |||
| ApoB48, median (IQR) | 12.3 (10.8–14.3) | 6.7 (3.4–8.6) | 0.002 | |||
* p < 0.005 fasting vs. postprandial value. Abbreviations: cIMT, carotid intima media thickness; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; VLDL, very-low-density lipoprotein.
3.4. Multivariate Analysis
Table 5 shows the results of the multivariate logistic regression analysis (DV: pathologic cIMT) in patients with RA. Total ApoB and postprandial ApoB48 were independently associated with pathologic cIMT in patients with RA, whereas female sex was a protective factor. Similarly, Table 6 shows an alternative multivariate linear regression analysis (DV: cIMT). In this model, cIMT continued to be independently associated with sex, total ApoB and postprandial ApoB48, and high ACPA titers (>340).
Table 5.
Logistic regression of characteristics associated with pathologic cIMT (p > 90) in patients with rheumatoid arthritis.
| Predictor | OR | 95% CI | p Value |
|---|---|---|---|
| Female Sex | 0.010 | 0.000–0.381 | 0.014 |
| Postprandial ApoB48 * | 1.159 | 1.021–1.315 | 0.023 |
| Total ApoB | 1.121 | 1.109–1.259 | 0.046 |
Nagelkerke R2 = 0.450, Variables not included in the equation: age, HDL post, TG post, ACPA >340, Framingham, smoking, corticosteroids * ApoB48 log transformed. Abbreviations; CI, confidence interval.
Table 6.
Multiple linear regression of characteristics associated with cIMT in patients with RA.
| Dependent Variable | Predictor | B | 95% CI for B | p Value |
|---|---|---|---|---|
| Pathologic cIMT | Female Sex | −0.607 | −0.306 to −0.151 | <0.001 |
| Postprandial ApoB48 | 0.285 | 0.002 to 0.013 | 0.002 | |
| Total ApoB | 0.239 | 0.001 to 0.005 | 0.047 | |
| ACPA≥340 | 0.256 | 0.018 to 0.137 | 0.018 |
Nagelkerke R2 = 0,520, Variables not included in the equation: age, HDL post, TG post, Framingham, smoking, corticosteroids. Abbreviations; CI, confidence interval; B, beta coefficient.
4. Discussion
Cardiovascular morbidity and mortality are high in patients with RA owing to accelerated atherosclerosis [33,34]. Hyperlipidemia is an increasingly interesting cardiovascular risk factor given that postprandial triglycerides are similar to and even better than fasting triglycerides as a predictor of cardiovascular disease, with the practical advantage that the patient is not required to fast [15,35,36]. Postprandial blood lipids have received little attention in patients with RA [37]. Therefore, we gave patients with RA and controls a mixed breakfast containing 50 g of different types of fat to determine the potential association with subclinical atherosclerosis measured using cIMT.
Consistent with this approach, we observed that fasting triglyceride and ApoB48 levels were similar in patients with RA and controls, although postprandial ApoB48 levels were higher. Burggraaf et al. [33] showed that patients with RA had higher levels of baseline ApoB48 than controls and that the accumulation of atherogenic chylomicron remnants could contribute to the high risk of cardiovascular disease in these patients. Similarly, the authors did not find differences in triglyceride levels between patients and controls, suggesting that lipolysis of chylomicrons could be normal in patients with RA and that perhaps the abnormality lies in the catabolism of chylomicron remnants in the liver.
Furthermore, we found that increased postprandial blood lipids in patients with RA was associated with pathologic cIMT. When both groups of patients were compared, we found that patients with pathologic cIMT had higher postprandial values for triglycerides, chylomicrons (triglycerides), VLDL (triglycerides), and ApoB48 than patients with normal cIMT. However, while baseline ApoB48 values were also higher in patients with pathologic cIMT, the difference was not statistically significant. This finding differs from those of a study of postprandial blood lipids in patients with type 2 diabetes mellitus [38], where higher postprandial and fasting ApoB48 levels were observed only in the subgroup with subclinical peripheral arterial disease. Our postprandial findings could be explained, as reported elsewhere, by the fact that postprandial triglycerides and ApoB48 are better associated with subclinical atherosclerosis in the carotid arteries [39] and femoral arteries [40]. Valero et al. [41] attempted to determine whether fasting ApoB48 level could replace postprandial blood lipids as a marker for evaluating the risk of coronary disease and found that fasting ApoB48 did not predict the risk of coronary disease, in contrast with postprandial levels.
An increasing number of studies show that patients with untreated active RA have lower levels of total cholesterol, LDL cholesterol, and HDL cholesterol [42]. This paradoxical association between lipids and RA has been associated with a greater risk of cardiovascular disease owing to current inflammation. Moreover, control of inflammation can increase these lipid values in serum [43]. In our study, we found no differences in cholesterol levels between patients and controls. However, while we found no differences in total cholesterol and LDL cholesterol between patients with pathologic or normal cIMT, the HDL value was lower in patients with pathologic cIMT. In this sense, some authors report an inverse relationship between HDL cholesterol and the presence of carotid plaque, but not cIMT [44], and attribute this relationship to an alteration in the cholesterol efflux capacity of HDL, thus potentially modifying inverse transport of cholesterol. Similarly, we did not find an association between HDL and pathologic cIMT, since HDL was eliminated from the multivariate model when we included sex, owing to the weight of this factor and to the higher number of men with pathologic cIMT (Supplementary Material-Table S2). The differences found in apob48 between individuals with pathological and non-pathological cIMT were only found in the patient group and not in the control group, so this difference may be more related to the disease itself and not to other frequently associated factors with the lipids level.
After controlling for confounders in a multivariate logistic regression analysis, ApoB48, total ApoB, and sex were independent predictors of pathologic cIMT in patients with RA. As we have seen, some studies associate postprandial ApoB48 with pathologic cIMT. Postprandial chylomicrons and their remnants may have a direct effect on the development of atherosclerosis and an indirect effect by stimulating inflammation through activation of circulating leukocytes and the formation of foam cells [20,45,46,47]. We also found total ApoB to be a predictor of subclinical atherosclerosis in RA; this observation was to be expected, since all atherogenic lipoproteins carry ApoB100. Also, as expected, the multivariate analysis showed that men with RA had a greater risk of pathologic cIMT. This finding is consistent with the data reported by van Breukelen et al. [47], possibly because estrogens suppress progression of atherosclerosis and because other genetic and hormonal factors in men contribute to these differences [48].
The linear regression analysis also showed that postprandial ApoB48 and total ApoB levels, male sex, and high ACPA titers were associated with pathologic cIMT. This association with high levels of ACPA has been reported elsewhere [49,50,51]. Vázquez-Del Mercado et al. [51] demonstrated an independent association between ACPA values and cIMT, suggesting a possible role for ACPA in the pathogenesis of atherosclerosis in RA.
Our study is subject to a series of limitations. First, it is noteworthy that the cIMT values and the number of plaques were similar between patients with RA and controls, even though other studies have shown an increase in cIMT in patients with RA compared with controls, even at the onset of the disease [47]. This may be because our sample was not sufficiently large to show these differences and because of a bias resulting from the voluntary participation of the controls. It could also be due to the relatively low inflammatory activity of the disease in patients with RA, which may have had a beneficial effect on cIMT. Recent studies have shown a lower cardiovascular risk in RA cohorts, where a treat-to-target strategy, has been applied [52]. Furthermore, postprandial blood lipids are generally evaluated as an increase in the area under the curve for triglycerides after fat loading [53]; we measured ApoB48 as a measure of postprandial blood lipids. Nevertheless, we believe that our findings are reliable, because other studies have shown that measurement of ApoB48 4 h after a mixed breakfast correlates well with the area under the curve for triglycerides after 8 h [54]. In addition, the controls in our study were not completely healthy, since they had comorbidities that were common in the general population, such as arterial hypertension, although they did not have inflammatory disease or dyslipidemia and were not taking statins. However, this may make them similar to the study patients in terms of comorbidities not linked to RA itself. Guclocorticosteroids treatment was higher in patients with pathologic cIMT. However, this variable was eliminated from the multivariate model, probably due to the weight of lipid effect. In this sense, some authors have reported a relationship between glucocorticosteroids and cardiovascular events by increasing the risk, due to deleterious effects on lipids, among other causes [55,56]. Finally, although differences in postprandial ApoB48 between patients and controls were significative according to the design of our study, the weak significance of the results should be taken into account.
In conclusion, postprandial ApoB48 levels are higher in patients with RA than in controls. Similarly, our results support that postprandial levels of ApoB48, together with total ApoB levels, male sex, and factors affecting severity (e.g., ACPA), are associated with pathologic cIMT in patients with RA. While these findings should be compared in specific studies, they could point to delayed catabolism of chylomicrons, delayed uptake of chylomicrons in the liver, or more marked synthesis in patients with RA leading to a greater risk of atherosclerosis.
Acknowledgments
To the Spanish Rheumatology Society (SER) for the translation of the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/8/2483/s1, Table S1: Lipid profile and carotid ultrasound in control group according to cIMT, Table S2: Lipid profile and carotid ultrasound in RA patients according to cIMT excluded the men.
Author Contributions
N.M.-V. and M.R.-G. participated in the design of the study, carried out patient recruitment and data collection and they were a major contributor in writing the manuscript. F.G.J.N. performed the ultrasound scans of the carotid. S.M.-A. and I.U. were a contributor in including patients and interpreting the patient data. J.R. and P.R.-L. were a major contributor in performing laboratory determination and contributor in interpreting laboratory data. M.C.-C. and P.V. were contributor in analyzing and interpreting the patient data. A.F.-N. participated in the design of the study, interpreting the patient data and major contributor in writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Grant for Medical Researchers from “Fundación Española de Reumatología” 2019. Grant from “Fundación Española de Reumatología” 2018 for non-funded projects.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Goodson N., Marks J., Lunt M., Symmons D. Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Ann. Rheum. Dis. 2005;64:1595–1601. doi: 10.1136/ard.2004.034777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agca R., Rollefstad S., Södergren A., Semb A.G., Kitas G.D., Sattar N., Nurmohamed M.T. Response to: “Influence of changes in cholesterol levels and disease activity on the 10-year cardiovascular risk estimated with different algorithms in rheumatoid arthritis patients” by Fornaro et al. Ann. Rheum. Dis. 2019 doi: 10.1136/annrheumdis-2019-215748. [DOI] [PubMed] [Google Scholar]
- 3.Dessein P.H., Joffe B.I., Stanwix A.E. Inflammation, insulin resistance, and aberrant lipid metabolism as cardiovascular risk factors in rheumatoid arthritis. J. Rheumatol. 2003;30:1403–1405. [PubMed] [Google Scholar]
- 4.Sattar N., McCarey D.W., Capell H., McInnes I.B. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–2963. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- 5.Del Rincon I.D., Williams K., Stern M.P., Freeman G.L., Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.La Montagna G., Cacciapuoti F., Buono R., Manzella D., Mennillo G.A., Arciello A., Valentini G., Paolisso G. Insulin resistance is an independent risk factor for atherosclerosis in rheumatoid arthritis. Diab. Vasc. Dis. Res. 2007;24:130–135. doi: 10.3132/dvdr.2007.031. [DOI] [PubMed] [Google Scholar]
- 7.Burggraaf B., Stoep D.F.V.B.-V.D., De Vries M.A., Klop B., Liem A.H., Van De Geijn G.-J.M., Van Der Meulen N., Birnie E., Van Der Zwan E.M., Van Zeben J., et al. Effect of a treat-to-target intervention of cardiovascular risk factors on subclinical and clinical atherosclerosis in rheumatoid arthritis: A randomised clinical trial. Ann. Rheum. Dis. 2019;78:335–341. doi: 10.1136/annrheumdis-2018-214075. [DOI] [PubMed] [Google Scholar]
- 8.Kisiel B., Kruszewski R., Juszkiewicz A., Raczkiewicz A., Bachta A., Kłos K., Duda K., Maliborski A., Szymański K., Ploski R., et al. Common atherosclerosis genetic risk factors and subclinical atherosclerosis in rheumatoid arthritis: The relevance of disease duration. Rheumatol. Int. 2019;39:327–336. doi: 10.1007/s00296-018-4186-y. [DOI] [PubMed] [Google Scholar]
- 9.Catapano A.L., Graham I., De Backer G., Wiklund O., Chapman M.J., Drexel H., Hoes A.W., Jennings C.S., Landmesser U., Pedersen T.R., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 10.Gepner A.D., Young R., Delaney J.A., Budoff M.J., Polak J.F., Blaha M.J., Post W.S., Michos E.D., Kaufman J.D., Stein J.H. Comparison of Carotid Plaque Score and Coronary Artery Calcium Score for Predicting Cardiovascular Disease Events: The Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2017;6:e005179. doi: 10.1161/JAHA.116.005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Juanatey C., Testa A., Garcia-Castelo A., Garcia-Porrua C., Llorca J., Ollier W.E., González-Gay M. Echocardiographic and Doppler findings in long-term treated rheumatoid arthritis patients without clinically evident cardiovascular disease. Semin. Arthritis Rheum. 2004;33:231–238. doi: 10.1053/j.semarthrit.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Toms T.E., Panoulas V.F., Douglas K.M.J., Griffiths H., Sattar N., Smith J.P., Symmons D.P.M., Nightingale P., Metsios G.S., Kitas G.D. Statin use in rheumatoid arthritis in relation to actual cardiovascular risk: Evidence for substantial undertreatment of lipid-associated cardiovascular risk? Ann. Rheum. Dis. 2010;69:683–688. doi: 10.1136/ard.2009.115717. [DOI] [PubMed] [Google Scholar]
- 13.Kim S.-H., Lee C.-K., Lee E.Y., Park S.Y., Cho Y.S., Yoo B., Moon H.-B. Serum oxidized low-density lipoproteins in rheumatoid arthritis. Rheumatol. Int. 2004;24:230–233. doi: 10.1007/s00296-003-0358-4. [DOI] [PubMed] [Google Scholar]
- 14.Park Y.B., Lee S.K., Lee W.K., Suh C.H., Lee C.W., Song C., Lee J. Lipid profiles in untreated patients with rheumatoid arthritis. J. Rheumatol. 1999;26:1701–1704. [PubMed] [Google Scholar]
- 15.Bansal S., Buring J.E., Rifai N., Mora S., Sacks F.M., Ridker P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 16.Brunzell J.D. Clinical practice. Hypertriglyceridemia. N. Engl. J. Med. 2007;357:1009–1017. doi: 10.1056/NEJMcp070061. [DOI] [PubMed] [Google Scholar]
- 17.Nordestgaard B.G., Benn M., Schnohr P., Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 18.Kolovou G.D., Anagnostopoulou K.K., Daskalopoulou S.S., Mikhailidis D.P., Cokkinos D.V. Clinical relevance of postprandial lipaemia. Curr. Med. Chem. 2005;12:1931–1945. doi: 10.2174/0929867054546609. [DOI] [PubMed] [Google Scholar]
- 19.Stalenhoef A.F., de Graaf J. Association of fasting and nonfasting serum triglycerides with cardiovascular disease and the role of remnant-like lipoproteins and small dense LDL. Curr. Opin. Lipidol. 2008;19:355–361. doi: 10.1097/MOL.0b013e328304b63c. [DOI] [PubMed] [Google Scholar]
- 20.Alipour A., Valdivielso P., Elte J.W.F., Janssen H.W., Rioja J., Van Der Meulen N., Van Mechelen R., Njo T.L., Gonzalez-Santos P., Rietveld A.P., et al. Exploring the value of apoB48 as a marker for atherosclerosis in clinical practice. Eur. J. Clin. Investig. 2012;42:702–708. doi: 10.1111/j.1365-2362.2011.02635.x. [DOI] [PubMed] [Google Scholar]
- 21.Masuda D., Sugimoto T., Tsujii K.-I., Inagaki M., Nakatani K., Yuasa-Kawase M., Tsubakio-Yamamoto K., Ohama T., Nishida M., Ishigami M., et al. Correlation of fasting serum apolipoprotein B-48 with coronary artery disease prevalence. Eur. J. Clin. Investig. 2012;42:992–999. doi: 10.1111/j.1365-2362.2012.02687.x. [DOI] [PubMed] [Google Scholar]
- 22.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., III, Birnbaum N.S., Burmester G.R., Bykerk V.P., Cohen M.D., et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Hervas S., Bauer-Izquierdo S.I., Priego M.A., Real J.T., Carmena R., Ascaso J.F. Grosor Íntima-Media Carotídeo y Frecuencia de Placas de Ateroma en Población Española sin Factores de Riesgo Cardiovascular. [(accessed on 1 August 2012)];2012 Available online: https://www.myendnoteweb.com/EndNoteWeb.html.
- 24.Stein J.H., Korcarz C.E., Post W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: Summary and discussion of the American Society of Echocardiography consensus statement. Prev. Cardiol. 2009;12:34–38. doi: 10.1111/j.1751-7141.2008.00021.x. [DOI] [PubMed] [Google Scholar]
- 25.Cleeman J.I., Grundy S.M., Becker D., Clark L. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 26.Mancia G., De Backer G., Dominiczak A., Cifkova R., Fagard R., Germano G., Grassi G., Heagerty A.M., Kjeldsen S.E., Laurent S., et al. ESH/ESC 2007 Guidelines for the management of arterial hypertension. Rev Esp Cardiol. 2007;60:968–994. doi: 10.1097/HJH.0b013e3282f857e7. [DOI] [PubMed] [Google Scholar]
- 27.Canivell S., Gomis R. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson P.W., D’Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.CIR.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 29.Estruch R., Ros E., Salas-Salvadó J., Covas M.-I., Corella D., Arós F., Gomez-Gracia E., Ruiz-Gutierrez V., Fiol M., Lapetra J., et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. New Engl. J. Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 30.Craig C.L., Marshall A.L., Sjöström M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F., et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 31.Iwamoto N., Kawakami A., Fujikawa K., Aramaki T., Kawashiri S.Y., Tamai M., Arima K., Ichinose K., Kamachi M., Yamasaki S., et al. Prediction of DAS28-ESR remission at 6 months by baseline variables in patients with rheumatoid arthritis treated with etanercept in Japanese population. Mod. Rheumatol. 2009;19:488–492. doi: 10.3109/s10165-009-0187-8. [DOI] [PubMed] [Google Scholar]
- 32.Moyano S., Scolnik M., Vergara F., Garcia M.V., Sabelli M.R., Rosa J.E., Catoggio L.J., Soriano E.R. Evaluation of Learned Helplessness, Perceived Self-efficacy, and Functional Capacity in Patients With Fibromyalgia and Rheumatoid Arthritis. J. Clin. Rheumatol. 2018 doi: 10.1097/RHU.0000000000000769. [DOI] [PubMed] [Google Scholar]
- 33.Burggraaf B., Stoep D.F.V.B.-V.D., Van Zeben J., Van Der Meulen N., Van De Geijn G.-J.M., Liem A., Valdivielso P., Villodres J.R., Ramírez-Bollero J., Van Der Zwan E., et al. Evidence for increased chylomicron remnants in rheumatoid arthritis. Eur. J. Clin. Investig. 2018;48 doi: 10.1111/eci.12873. [DOI] [PubMed] [Google Scholar]
- 34.Van Breukelen-van der Stoep D.F., Klop B., van Zeben D., Hazes J.M., Castro Cabezas M. Cardiovascular risk in rheumatoid arthritis: How to lower the risk? Atherosclerosis. 2013;231:163–172. doi: 10.1016/j.atherosclerosis.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Van Wijk J.P., Halkes C.J., Erkelens D.W., Castro Cabezas M. Fasting and daylong triglycerides in obesity with and without type 2 diabetes. Metabolism. 2003;52:1043–1049. doi: 10.1016/S0026-0495(03)00106-9. [DOI] [PubMed] [Google Scholar]
- 36.Masuda D., Nishida M., Arai T., Hanada H., Yoshida H., Yamauchi-Takihara K., Moriyama T., Tada N., Yamashita S. Reference interval for the apolipoprotein B-48 concentration in healthy Japanese individuals. J. Atheroscler. Thromb. 2014;21:618–627. doi: 10.5551/jat.22558. [DOI] [PubMed] [Google Scholar]
- 37.Strang A.C., Bisoendial R.J., Kootte R.S., Schulte D., Dallinga-Thie G.M., Levels J.H., Kok M., Vos K., Tas S.W., Tietge U.J., et al. Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis. 2013;229:174–181. doi: 10.1016/j.atherosclerosis.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 38.Valdivielso P., Puerta S., Rioja J., Alonso I., Ariza M., Sánchez-Chaparro M.-Á., Palacios R., Gonzalez-Santos P. Postprandial apolipoprotein B48 is associated with asymptomatic peripheral arterial disease: A study in patients with type 2 diabetes and controls. Clin. Chim. Acta. 2010;411:433–437. doi: 10.1016/j.cca.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Teno S., Uto Y., Nagashima H., Endoh Y., Iwamoto Y., Omori Y., Takizawa T. Association of postprandial hypertriglyceridemia and carotid intima-media thickness in patients with type 2 diabetes. Diabetes Care. 2000;23:1401–1406. doi: 10.2337/diacare.23.9.1401. [DOI] [PubMed] [Google Scholar]
- 40.Valdivielso P., Hidalgo A., Rioja J., Aguilar I., Ariza M.J., González-Alegre T., Gonzalez-Santos P. Smoking and postprandial triglycerides are associated with vascular disease in patients with type 2 diabetes. Atherosclerosis. 2007;194:391–396. doi: 10.1016/j.atherosclerosis.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Valéro R., Lorec A.-M., Paganelli F., Beliard S., Atlan C., Lairon D., Vialettes B., Portugal H. Fasting apoprotein B-48 level and coronary artery disease in a population without frank fasting hypertriglyceridemia. Metabolism. 2005;54:1442–1447. doi: 10.1016/j.metabol.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Choy E., Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: A challenge to conventional cardiovascular risk actions. Ann. Rheum. Dis. 2009;68:460–469. doi: 10.1136/ard.2008.101964. [DOI] [PubMed] [Google Scholar]
- 43.Steiner G., Urowitz M.B. Lipid profiles in patients with rheumatoid arthritis: Mechanisms and the impact of treatment. Semin. Arthritis Rheum. 2009;38:372–381. doi: 10.1016/j.semarthrit.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Segura B.T., Macía-Díaz M., Machado J.D., De Vera-González A.M., Dopico J.A.G., Olmos J.M., Hernandez J.L., Díaz-González F., González-Gay M.A., Ferraz-Amaro I. HDL cholesterol efflux capacity in rheumatoid arthritis patients: Contributing factors and relationship with subclinical atherosclerosis. Arthritis Res. Ther. 2017;19:113. doi: 10.1186/s13075-017-1311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Vries M.A., Klop B., Alipour A., Van De Geijn G.-J.M., Prinzen L., Liem A.H., Valdivielso P., Villodres J.R., Ramírez-Bollero J., Cabezas M.C. In vivo evidence for chylomicrons as mediators of postprandial inflammation. Atherosclerosis. 2015;243:540–545. doi: 10.1016/j.atherosclerosis.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 46.Valdivielso P., Ramirez-Bollero J., Perez-Lopez C. Peripheral arterial disease, type 2 diabetes and postprandial lipidaemia: Is there a link? World J. Diabetes. 2014;5:577–585. doi: 10.4239/wjd.v5.i5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoep D.F.V.B.D., Van Zeben D., Klop B., Van De Geijn G.-J.M., Janssen H.J.W., Hazes M.J.M.W., Birnie E., Van Der Meulen N., De Vries M.A., Cabezas M.C. Association of Cardiovascular Risk Factors with Carotid Intima Media Thickness in Patients with Rheumatoid Arthritis with Low Disease Activity Compared to Controls: A Cross-Sectional Study. PLoS ONE. 2015;10:e0140844. doi: 10.1371/journal.pone.0140844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan T.Y., Lu C.H., Lin T.K., Liou C.W., Chuang Y.C., Schminke U. Factors associated with gender difference in the intima-media thickness of the common carotid artery. Clin. Radiol. 2009;64:1097–1103. doi: 10.1016/j.crad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Spinelli F.R., Pecani A., Ciciarello F., Colasanti T., Di Franco M., Miranda F., Conti F., Valesini G., Alessandri C. Association between antibodies to carbamylated proteins and subclinical atherosclerosis in rheumatoid arthritis patients. BMC Musculoskelet. Disord. 2017;18:214. doi: 10.1186/s12891-017-1563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerli R., Bocci E.B., Sherer Y., Vaudo G., Moscatelli S., Shoenfeld Y. Association of anti-cyclic citrullinated peptide antibodies with subclinical atherosclerosis in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2008;67:724–725. doi: 10.1136/ard.2007.073718. [DOI] [PubMed] [Google Scholar]
- 51.Mercado M.V.-D., Nuñez-Atahualpa L., Figueroa-Sanchez M., Gómez-Bañuelos E., Rocha-Muñoz A.D., Martín-Márquez B.T., Corona-Sanchez E.G., Martínez-García E.A., Macías-Reyes H., Gonzalez-Lopez L., et al. Serum Levels of Anticyclic Citrullinated Peptide Antibodies, Interleukin-6, Tumor Necrosis Factor-α, and C-Reactive Protein Are Associated with Increased Carotid Intima-Media Thickness: A Cross-Sectional Analysis of a Cohort of Rheumatoid Arthritis Patients without Cardiovascular Risk Factors. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/342649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Provan S.A., Lillegraven S., Sexton J., Angel K., Austad C., Haavardsholm E.A., Kvien T.K., Uhlig T. Trends in all-cause and cardiovascular mortality in patients with incident rheumatoid arthritis: A 20-year follow-up matched case-cohort study. Rheumatology. 2020;59:505–512. doi: 10.1093/rheumatology/kez371. [DOI] [PubMed] [Google Scholar]
- 53.Su J.W., Nzekwu M.M., Cabezas M.C., Redgrave T., Proctor S.D. Methods to assess impaired post-prandial metabolism and the impact for early detection of cardiovascular disease risk. Eur. J. Clin. Investig. 2009;39:741–754. doi: 10.1111/j.1365-2362.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- 54.Karamanos B.G., Thanopoulou A.C., Roussi-Penesi D.P. Maximal post-prandial triglyceride increase reflects post-prandial hypertriglyceridaemia and is associated with the insulin resistance syndrome. Diabet. Med. 2001;18:32–39. doi: 10.1046/j.1464-5491.2001.00386.x. [DOI] [PubMed] [Google Scholar]
- 55.Roubille C., Richer V., Starnino T., McCourt C., McFarlane A., Fleming P., Siu S., Kraft J., Lynde C., Pope J., et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015;74:480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panoulas V.F., Douglas K.M.J., Metsios G., Kita M.D., Elisaf M., Stavropoulos-Kalinoglou A., Nightingale P., Kitas G.D. Long-term exposure to medium-dose glucocorticoid therapy associates with hypertension in patients with rheumatoid arthritis. Rheumatology. 2008;47:72–75. doi: 10.1093/rheumatology/kem311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.