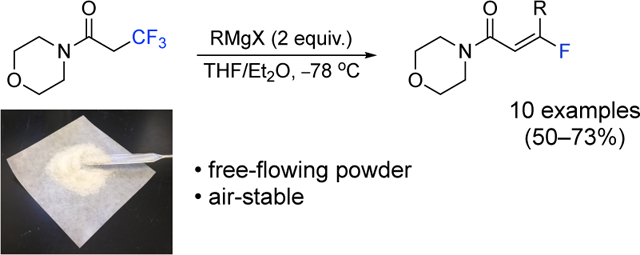
Abstract
Fluoroalkenes serve as bioisosteres to peptide bonds and are resistant to hydrolytic enzymes in vivo. Currently, α-fluoro-α,β-unsaturated carbonyl compounds are readily accessible by general synthetic methods; however, β-fluoro-α,β-unsaturated carbonyl groups are more challenging to construct. To address this need, we have designed a reagent, morpholine 3,3,3-trifluoropropanamide, that creates (E)-β-fluoro-α,β-unsaturated amides upon the addition of many commonly used Grignard reagents. Reactions with this reagent enable a high level of stereocontrol in the fluoroalkene product.
Graphical Abstract
The incorporation of fluorine into organic molecules can produce unique effects during drug discovery such as reducing metabolism, enhancing distribution, and increasing bioavailability.1 Fluoroalkenes are particularly valuable in the discovery process, because they can serve as bioisosteres of peptide bonds.2 Also, fluoroalkenes are resistant to the hydrolytic enzymes that easily cleave the amide bonds in many peptides. Fluoroalkenes have been used as surrogates for amide bonds in drug discovery, and for example, Pannecoucke and coworkers created a fluoroalkene analogue of the anti-hypertensive agent, enalapril (Figure 1).3 Other recent examples of this strategy have been reported by Altman,4 Augustyns,5 Welch,6 Leumann,7 and Fujii.8 Most of these cases also demonstrated the application of innovative synthetic methods to create the requisite fluoroalkene.9,10
Figure 1.
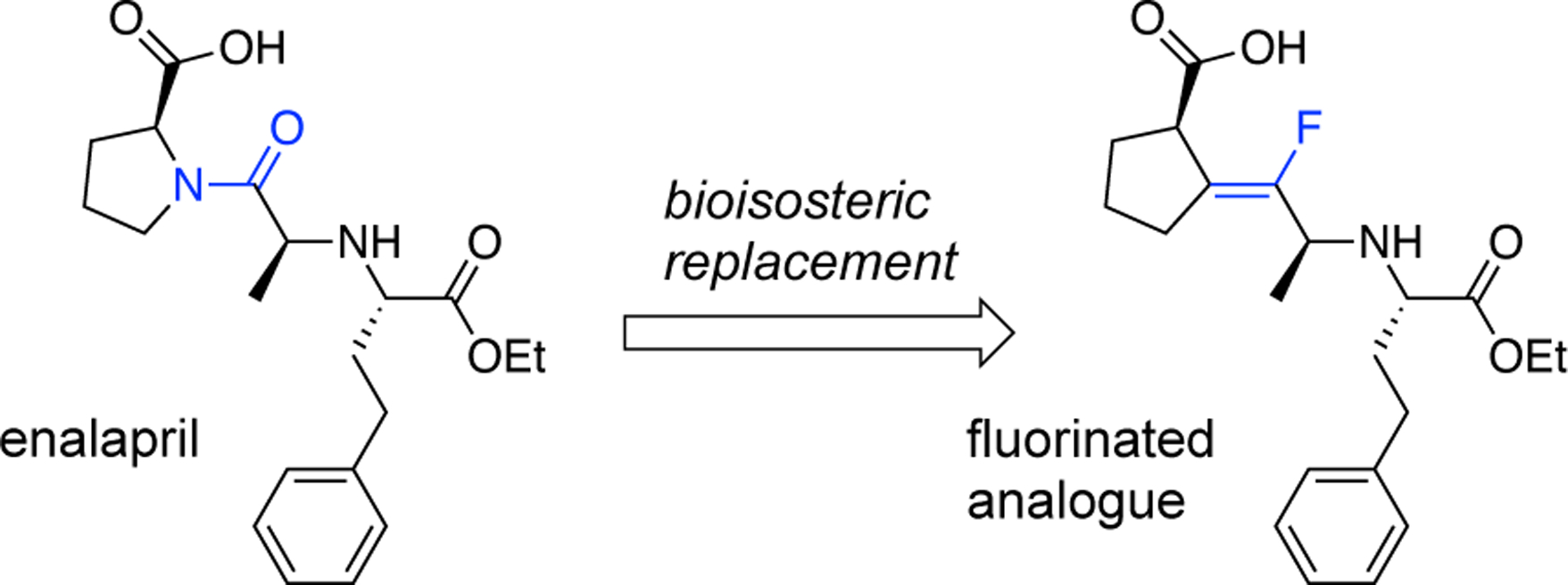
Enalapril and fluorinated analogue displaying a vinyl fluorine as a bioisostere of the amide bond.
Currently, there are general methods to synthesize α-fluoro-α,β-unsaturated carbonyl compounds,9,10 such as the Julia olefination,11 the Peterson olefination,12 the Horner-Wadsworth-Emmons reaction,3 and a Reformatsky addition/elimination process.13 Unfortunately, fewer methods exist for the preparation of β-fluoro-α,β-unsaturated carbonyl compounds, and most are limited by low yields and poor stereoselectivities.14–16 Notable recent exceptions are the hydrofluorination of alkynes using gold catalysts that provide access to (Z)-β-fluoro-α,β-un-saturated carbonyl compounds17 and the chromium-mediated reductive coupling of CBrF2-containing compounds with aldehydes to give (E)-β-fluoro-α,β-unsaturated amides18 (Scheme 1). The two shortcomings of the latter transformation are the stoichiometric amounts of chromium and the need of CBrF2-containing compounds, which are difficult to access. Herein, we report the design of a reagent that allows the creation of (E)-β-fluoro-α,β-unsaturated amides in a single step using commercially available Grignard reagents.
Scheme 1.
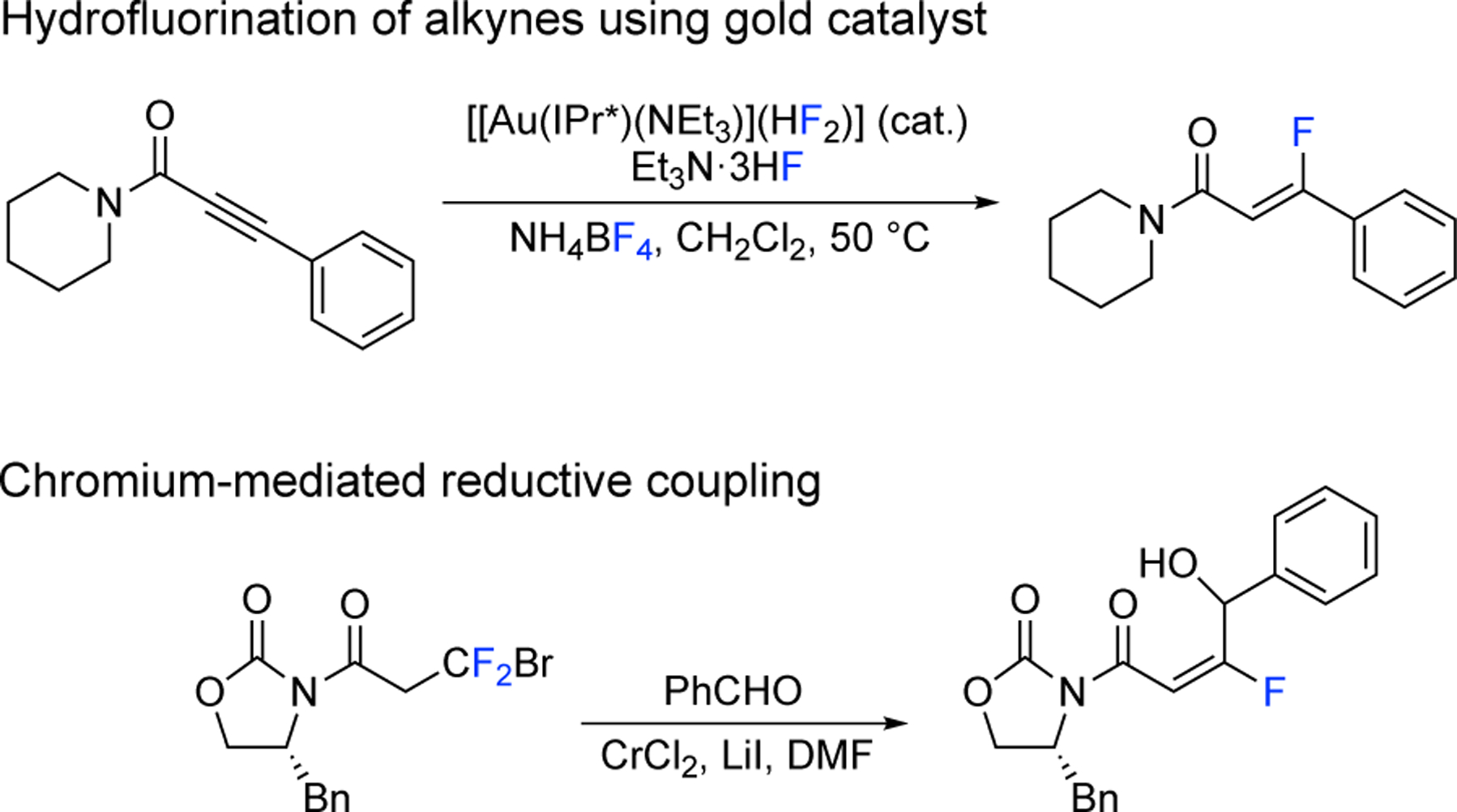
Two recent approaches to the synthesis of β-fluoro-α,β-unsaturated amides.
Initially, our plan was to synthesize a 3,3,3-trifluoropropanamide from N,O-dimethylhydroxylamine in order to create a Weinreb amide. Unfortunately, the coupling of 3,3,3-trifluoropropanoic acid with N,O-dimethylhydroxylamine produced low and inconsistent yields, varying from 0–33%. Although Weinreb amides are versatile synthetic intermediates, morpholine amides also participate in similar functional group transformations.19,20 Accordingly, morpholine 3,3,3-trifluoropropanamide (1) became the target and is synthesized in quantitative yield from morpholine and 3,3,3-trifluoropropanoic acid using EDCI, HOBT, and triethylamine (Figure 2). This preparation of 1 is an alternative to the previously reported syntheses that require N-pentafluoropropyl morpholines as starting materials.21,22 The morpholine 3,3,3-trifluoropropanamide 1 is a stable, anhydrous solid that can be freely weighed in the air. It was characterized by 1H, 13C, and 19F NMR, mass spectrometry, combustion analysis, and X-ray crystallography.23 Crystals of 1 from a 1:1 solution of hexanes and cyclohexanes were used for X-ray analysis, and data was collected at T=90K using Mo Kα radiation on a Bruker Kappa Apex-II DUO diffractometer. Also, 1 displays high solubility in common organic solvents such as THF, Et2O, EtOAc, CH3CN, benzene, toluene, and CH2Cl2, which makes it an ideal reagent for the subsequent production of fluoroalkenes.
Figure 2.
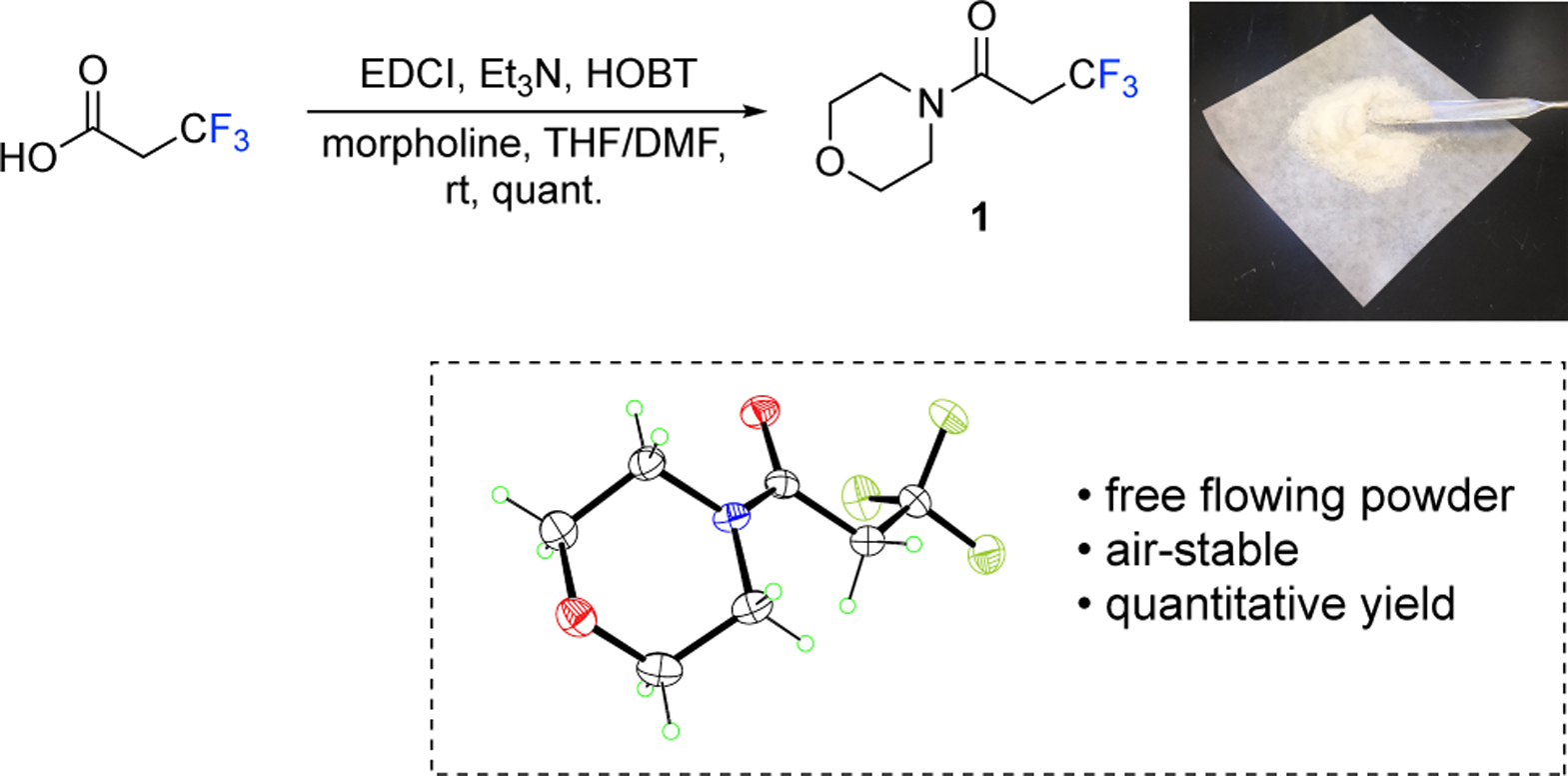
Preparation of morpholine 3,3,3-trifluoropropanamide (1).
The reagent 1 was treated with 2 equivalents of phenyl magnesium bromide in 1:1 THF/Et2O at −78 °C, and after 4 hours, the (E)-product 2 is obtained in 73% isolated yield (Figure 3). The stereochemical assignment was determined by the characteristic coupling constant between the vinyl proton and vinyl fluoride. In the case of 2, the JHF(cis) is 19.3 Hz which establishes the (E)-product.23,24 Other common phenyl Grignard reagents, such as tolyl, p-chlorophenyl, p-fluorophenyl, p-methoxyphenyl, and naphthyl, participate in a similar fashion and give (E)-products 3–7, respectively, in yields between 50–59%. Lithium salts (i.e., lithium chloride or lithium bromide) were added to the Grignard reagents to improve the isolated yields of the products 3, 5, 6, and 7. Knochel has previously reported the beneficial role of lithium salts in the reactivity of Grignard reagents,25 and an increase in yield was observed during optimization for this process. Although methyl magnesium chloride, ethyl magnesium bromide, vinyl magnesium bromide, and ethynyl magnesium bromide were also added to 1, only low yields of 12%, 10%, 9%, and 11% of volatile products were isolated, respectively. The isopropyl, cyclohexyl, and butyl Grignard reagents give products 8–10 with the expected (E)-stereoisomer as the major product in yields at 62–71%. The 1,3-dioxan-2-ylethyl magnesium bromide affords 11 in the yield of 73% and enabled the acquisition of its X-ray structure, confirming the (E)-fluoroalkene. The o-methylthiophenyl and 2-thienyl Grignard reagents give products 12 and 13, respectively, displaying compatibility with ortho-substituents and heteroaromatic rings. The ortho-product 12 was isolated as an inseparable 8:1 mixture of E/Z isomers. Lithium salts were added during the optimization of products, 8, 11, and 12. Overall, both alkyl and aryl Grignard reagents add to the reagent 1 to create the (E)-fluoroalkenes in modest to good yields.
Figure 3.
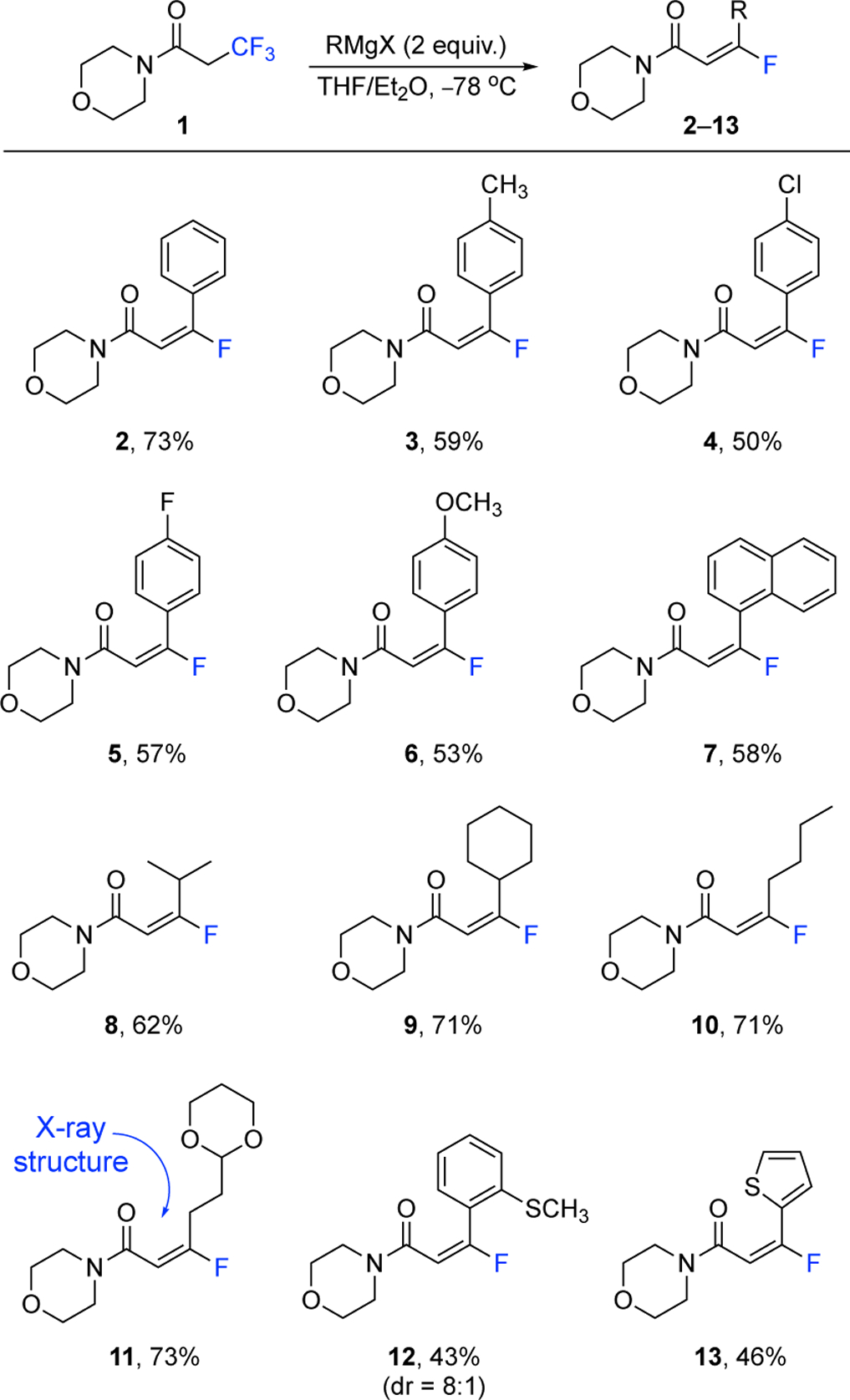
Synthesis of β-fluoro-α,β-unsaturated amides 2–13 using reagent 1 and Grignard reagents. Isolated yields are shown and see Supporting Information for details.
We conducted a brief mechanistic analysis to gain insight into the transformation of reagent 1 in the presence of the Grignard reagents.23 The reagent 1 was treated with one equivalent of isopropyl magnesium chloride in THF-d8 and observed by 19F NMR (Scheme 2). The β,β-difluoroacrylamide intermediate was observed by the presence of the diagnostic signals at −76.0 and −71.2 ppm. This intermediate is similar to the one proposed during the preparation of the CBrF2-containing compound depicted in Scheme 1.26 Following the addition of the second equivalent of isopropyl magnesium chloride, the (E)-fluoroalkene 8 is observed. The enolate intermediate is not observed by 19F NMR, suggesting the elimination of fluoride is instantaneous. The stereochemical outcome of the addition of nucleophiles to gem-difluoroalkenes is known in the literature to provide the (E)-isomer with high selectivity.27 These results apply to this case, because the electronic repulsion of the fluorine atoms control the transition state prior to the elimination and produce the (E)-fluoroalkene.
Scheme 2.
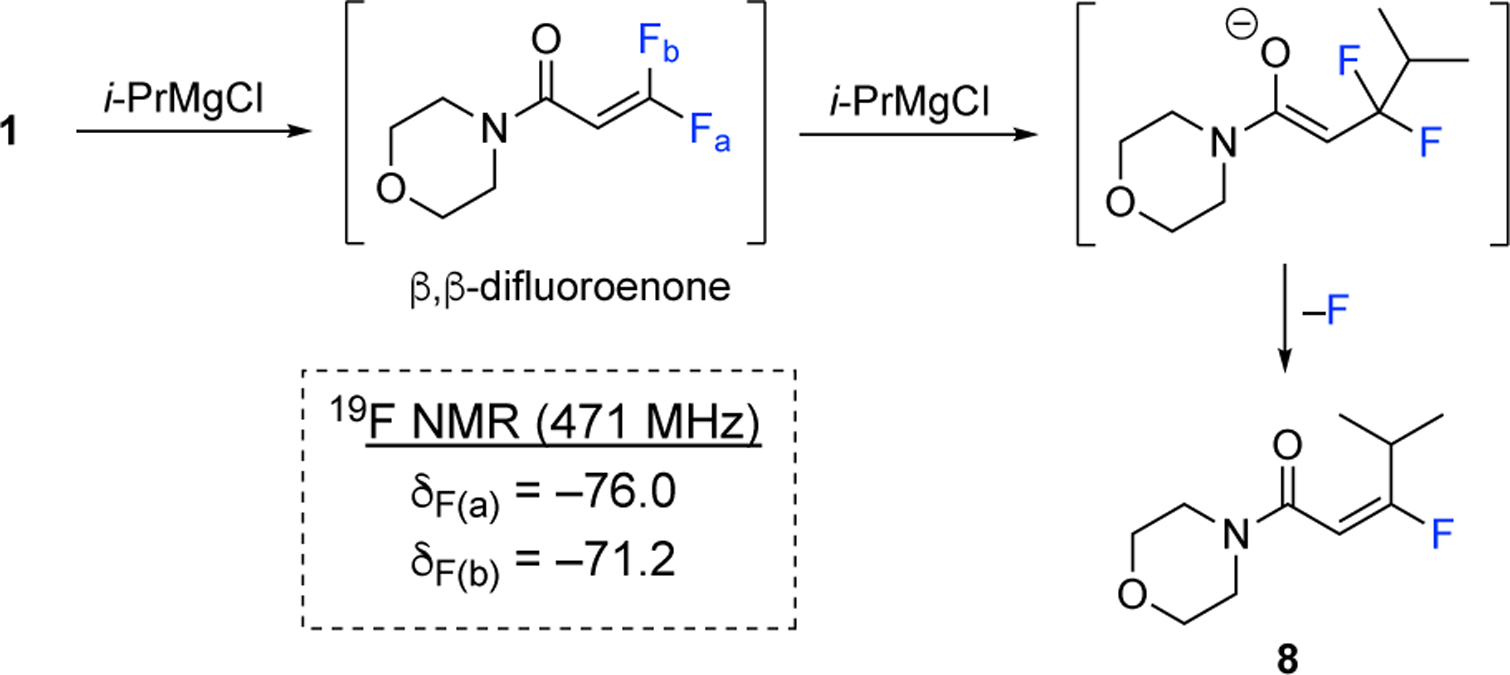
Proposed mechanism for the formation (E)-β-fluoro-α,β-unsaturated amide 8 from reagent 1 and two equivalents of i-PrMgCl. 19F NMR data for the β,β-difluoroacrylamide intermediate was obtained in THF-d8 at 471 Hz at rt.
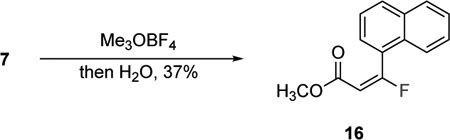
Morpholine amides are versatile groups that can be easily transformed into other functional groups.19,20 Usually, they provide access to ketones following the addition of a suitable nucleophile. Accordingly, the reagent 1 was treated with two equivalents of phenyl lithium in the presence of TMEDA, and the double addition product 12 was produced in a good 75% yield (eq 1). The ketone 14 was isolated exclusively as the (Z)-fluoroalkene; TMEDA improved the selectivity and yields. The characteristic JHF(trans) is 34.2 Hz and all characterization data for (Z)-14 matched the reported values.14 These data suggest that the transformation of the morpholine amide into to a ketone occurs with inversion of the stereochemistry of the fluoroalkene. These results broaden the potential utility of the reagent 1 in the stereoselective synthesis of fluoroalkenes and are currently under investigation. The morpholine amides 2 and 7 were transformed into the amine 15 and methyl ester 16, respectively (eq 2 and 3). The conversion of the morpholine amide 2 into amine 15 is accomplished by activation with trimethyloxonium tetrafluoroborate in the presence of 2,6-di-tert-butylpyridine followed by the addition of NaBH4 (eq 2), which are similar to the conditions reported by Toste and workers.17 The morpholine amide 7 was also activated with trimethyloxonium tetrafluoroborate and then treated with water to produce the methyl ester 16 (eq 3). Both of these reactions occur with complete retention of the (E)-fluoroalkene and demonstrate the potential uses of the products obtained from 1.
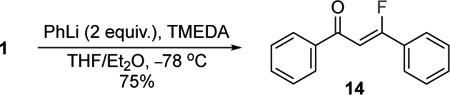 |
(1) |
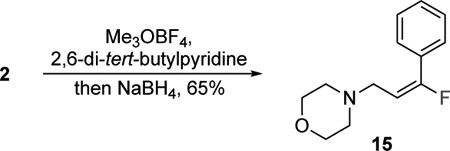 |
(2) |
 |
(3) |
In summary, morpholine 3,3,3-trifluoropropanamide (1), now commercially available,28 provides access to (E)-β-fluoro-α,β-unsaturated amides upon addition of two equivalents of common alkyl, aryl, and heteroaryl Grignard reagents. It avoids the use of toxic metals such as chromium. This one-pot transformation provides good yields and a high level of stereocontrol for the (E)-fluoroalkene. 19F NMR experiments demonstrate the formation of the β,β-difluoroacrylamide as the likely mechanistic intermediate. Also, the addition of an organolithium reagent provides the (Z)-β-fluoro-α,β-unsaturated ketone. The transformation of the morpholine amides into other functional groups is demonstrated. Overall, the reagent 1 offers a simple process for accessing fluoroalkenes, which have a growing role in medicinal chemistry and drug design.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the University of Mississippi and the National Institute of Drug Abuse (R15DA046795).
Footnotes
Supporting Information
Full experimental details, characterization data, and 1H, 13C, and 19F NMR spectra for all new compounds. The Supporting Information is available free of charge on the ACS Publications website.
The authors declare no competing financial interests.
REFERENCES
- (1).Gillis EP; Eastman KJ; Hill MD; Donnelly DJ; Mean-well NA Applications of Fluorine in Medicinal Chemistry. J. Med. Chem 2015, 58, 8315–8359. [DOI] [PubMed] [Google Scholar]
- (2).Couve-Bonnaire S; Cahard D; Pannecoucke X Chiral Dipeptide Mimics Possessing a Fluoroolefin Moiety: A Relevant Tool for Conformational and Medicinal Studies. Org. Biomol. Chem 2007, 5, 1151–1157. [DOI] [PubMed] [Google Scholar]
- (3).Villiers E; Couve-Bonnaire S; Cahard D; Pannecoucke X The Fluoroalkene Motif as a Surrogate of the Amide Bond: Syntheses of AA-Ψ[(Z) and (E)-CF=CH]-Pro Pseudodipeptides and an Enalapril Analog. Tetrahedron 2015, 71, 7054–7062. [Google Scholar]
- (4).Altman RA; Sharma KK; Rajewski LG; Toren PC; Baltezor MJ; Pal M; Karad SN Tyr1-ψ[(Z)CF=CH]-Gly2 Fluorinated Peptidomimetics Improves Distribution and Metabolism Properties of Leu-Eenkephalin. ACS Chem. Neurosci 2018, 9, 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Van der Veken P; Senten K; Kertesz I; De Meester I; Lambeir A-M; Maes M-B Scharpe S; Haemers A; Augustyns K Fluoro-Olefins as Peptidomimetic Inhibitors of Dipeptidyl Peptidases. J. Med. Chem 2005, 48, 1768–1780. [DOI] [PubMed] [Google Scholar]
- (6).Zhao K; Lim DS; Funaki T; Welch JT Inhibition of Dipeptidyl Peptidase IV (DPP IV) by 2-(2-Amino-1-fluoro-propylidene) -cyclopentanecarbonitrile, A Fluoroolefin Containing Peptidomimetic. Bioorg. Med. Chem 2003, 11, 207–215. [DOI] [PubMed] [Google Scholar]
- (7).Hollenstein M; Leumann CJ Fluorinated Olefinic Peptide Nucleic Acid: Synthesis and Pairing Properties with Complementary DNA. J. Org. Chem 2005, 70, 3205–3217. [DOI] [PubMed] [Google Scholar]
- (8).Niida A; Tomita K; Mizumoto M; Tanigaki H; Terada T; Oishi S; Otaka A; Inui K-I; Fujii N Unequivocal Synthesis of (Z) -Alkene and (E)-Fluoroalkene Dipeptide Isosteres To Probe Structural Requirements of the Peptide Transporter PEPT1. Org. Lett 2006, 8, 613–616 [DOI] [PubMed] [Google Scholar]
- (9).Drouin M; Hamel J-D; Paquin J-F Synthesis of Monofluoroalkenes: A Leap Forward. Synthesis, 2018, 50, 881–955. [Google Scholar]
- (10).Yanai H; Taguchi T Synthetic Methods for Fluorinated Ole-fins. Eur. J. Org. Chem 2011, 5939–5954. [Google Scholar]
- (11).Zajc R; Kake S Exceptionally Mild, High-Yield Synthesis of α-Fluoro Acrylates. Org. Lett 2006, 8, 4457–4460. [DOI] [PubMed] [Google Scholar]
- (12).Welch JT; Herbert RW The Stereoselective Construction of (Z)-3-Aryl-2-fluoroalkenoates. J. Org. Chem 1990, 55, 4782–4784. [Google Scholar]
- (13).Barma DK; Kundu A; Zhang H; Mioskowski C; Falck JR (Z)-α-Haloacrylates: An Exceptionally Stereoselective Preparation via Cr(II)-Mediated Olefination of Aldehydes with Trihaloacetates. J. Am. Chem. Soc 2003, 125, 3218–3219. [DOI] [PubMed] [Google Scholar]
- (14).Zhang J; Liu L; Duan J; Gu L; Chen B; Sun T; Gong Y Stereoselective One-Pot Sequential Dehydrochlorination/trans-Hydrofluorination Reaction of β-Chloro-α,β-unsaturated Aldehydes or Ketones: Facile Access to (Z)-β-Fluoro-β-arylenals/β-Fluoro-β-arylenones. Adv. Synth Catal 2017, 359, 4348–4358 [Google Scholar]
- (15).Sano K; Fukuhara T; Hara S Regioselective Synthesis of β-Fluoro-α,β-unsaturated Ketones by the Reaction of β-Diketones with DFMBA. J. Fluorine Chem 2009, 130, 708–713. [Google Scholar]
- (16).Krishnan G; Sampson P Synthesis of β-Fluoro-α,β-unsatu-rated Esters and Nitriles via a Fluoro-pummerer Rearrangement. Tetrahedron Lett. 1990, 31, 5609–5612. [Google Scholar]
- (17).O’Connor TJ; Toste FD Gold-Catalyzed Hydrofluorination of Electron-Deficient Alkynes: Stereoselective Synthesis of β-Fluoro Michael Acceptors. ACS Catal. 2018, 8, 5947–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Nihei T; Nishi Y; Ikeda N; Yokotani S; Arimitsu S; Konno T Highly Stereoselective Synthesis of Fluoroalkene Dipeptides via the Novel Chromium(II)-Mediated Carbon-Fluorine Bond Cleavage/New Carbon–Carbon Bond Formation. Synthesis 2016, 48, 865–881. [Google Scholar]
- (19).Dhoro F; Kristensen TE; Stockmann V; Yap GPA; Tius MA Asymmetric Amine-Intercepted Nazarov Cyclization. J. Am. Chem. Soc 2007, 129, 7256–7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Concellon JM; Rodriguez-Solla H; Diaz P Sequential Reactions Promoted by Manganese: Completely Stereoselective Synthesis of (E)-α,β-Unsaturated Amides, Ketones, Aldehydes, and Carboxylic Acids. J. Org. Chem 2007, 72, 7974–7979. [DOI] [PubMed] [Google Scholar]
- (21).Koroniak H; Walkowiak J; Grys K; Rajchel A; Alty A; Du Boisson R 1,1,3,3,3-Pentafluoropropene Secondary Amine Adducts, New Selective Fluorinating Agents. J. Fluor. Chem 2006, 127, 1245–1251. [Google Scholar]
- (22).Yamanaka H; Mantani T; Shiomi K; Ishihara T A New Practical and Convenient Access to the Synthesis of N,N-Dialkyl-3,3,3-trifluoropropanamides. Chem. Lett 1998, 27, 615–616. [Google Scholar]
- (23).See Supporting Information for details.
- (24).Dolbier WR Guide to Fluorine NMR for Organic Chemists; Second Edition; John Wiley and Sons, Inc: Hoboken, New Jersey, 2016. [Google Scholar]
- (25).Krasovskiy A; Knochel P A LiCl-Mediated Br/Mg Exchange Reaction for the Preparation of Functionalized Aryl- and Heteroaryl-magnesium Compounds from Organic Bromides. Angew. Chem. Int. Ed 2004, 43, 3333–3336. [DOI] [PubMed] [Google Scholar]
- (26).Shimada T; Konno T; Ishihara T A New Access to 3-Halo-3,3-difluoropropanoic Acid Derivatives via Fluorine-Halogen Exchange Reaction of Silyl Enolates of 3,3,3-Trifluoropropanoic Acid Derivatives. Chem. Lett 2007, 36, 636–637. [Google Scholar]
- (27).Zhang J; Xu C; Wu W; Cao S Mild and Copper-Free Stereoselective Cyanation of gem-Difluoroalkenes by Using Benzyl Nitrile as a Cyanating Reagent. Chem. Eur. J 2016, 22, 9902–9908. [DOI] [PubMed] [Google Scholar]
- (28).Morpholine 3,3,3-trifluoropropanamide is now available in the MilliporeSigma catalog (911933). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


