Abstract
Context
The ultradistal (UD) radius is rich in trabecular bone and is easily measured by dual energy X-ray absorptiometry (DXA). UD radius areal bone mineral density (aBMD) may help identify trabecular bone deficits, but reference data are needed for research and clinical interpretation of this measure.
Objective
We developed age-, sex-, and population ancestry-specific reference ranges for UD radius aBMD assessed by DXA and calculated Z-scores. We examined tracking of UD radius aBMD Z-scores over 6 years and determined associations between UD radius aBMD Z-scores and other bone measures by DXA and peripheral quantitative computed tomography.
Design
Multicenter longitudinal study.
Participants
A total of 2014 (922 males, 22% African American) children ages 5 to 19 years at enrollment who participated in the Bone Mineral Density in Childhood Study.
Main Outcome Measure
UD radius aBMD.
Results
UD radius aBMD increased nonlinearly with age (P < 0.001) and tended to be greater in males versus females (P = 0.054). Age-, sex-, and ancestry-specific UD radius aBMD reference curves were constructed. UD radius aBMD Z-scores positively associated with Z-scores at other skeletal sites (r = 0.54-0.64, all P < 0.001) and peripheral quantitative computed tomography measures of distal radius total volumetric BMD (r = 0.68, P < 0.001) and trabecular volumetric BMD (r = 0.70, P < 0.001), and was weakly associated with height Z-score (r = 0.09, P = 0.015). UD radius aBMD Z-scores tracked strongly over 6 years, regardless of pubertal stage (r = 0.66-0.69; all P < 0.05).
Conclusion
UD radius aBMD Z-scores strongly associated with distal radius trabecular bone density, with marginal confounding by stature. These reference data may provide a valuable resource for bone health assessment in children.
Keywords: DXA, pediatric, reference, aBMD, ultradistal radius
The International Society for Clinical Densitometry (ISCD) recommends using scans of the total body and lumbar spine for bone health assessment by dual-energy X-ray absorptiometry (DXA) in children and adolescents (1, 2). However, these scan regions might not be appropriate or feasible for certain patient groups, as in the case of obesity, mobility constraints, or surgical implants. Therefore, the ISCD recently recognized the importance of DXA assessment at alternative skeletal sites (3).
The forearm represents an alternative DXA scan site for patient groups for whom standard scan sites are not practical or appropriate. An advantage of the forearm measurement is that the radius scan encompasses the distal one-third (1/3 radius) region, which is primarily cortical bone, and the ultradistal (UD) region, rich in trabecular bone (4). The forearm is an upper body appendicular skeletal site and is not often subjected to weight bearing, and patient positioning is less burdensome compared to other DXA scan locations. Furthermore, forearm fractures are common during childhood (5), so bone density assessment at the UD radius may have clinical value. However, because of the paucity of robust pediatric reference data, the clinical utility of the UD radius areal bone mineral density (aBMD) measure is unknown (2).
The Bone Mineral Density in Childhood Study (BMDCS) was a large prospective, multisite, multiethnic study of children 5 to 19 years of age (6, 7). The BMDCS has been critical in developing pediatric DXA reference data and characterizing bone accrual in childhood. For the current study, we used BMDCS data to develop age-specific reference ranges for the UD radius aBMD measure for African American and non-African American males and females. To assess how UD radius aBMD compares to other bone density outcomes, we also examined the relationships between UD radius aBMD Z-scores and Z-scores for other DXA-derived aBMD measures and height; the association of forearm aBMD measures (UD radius and 1/3 radius) with radius cortical and trabecular volumetric bone density, geometry, and estimated strength; and the tracking (or stability) of UD radius aBMD over time.
Methods
Study population
Detailed descriptions of the BMDCS study have been reported previously (6-8). The BMDCS included 2014 healthy children from 5 clinical centers across the United States. At baseline, potential participants were excluded if they had a height, weight, or body mass index percentile below the 3rd or above the 97th; delayed or advanced maturation; premature birth; scoliosis; were currently using medications known to influence bone metabolism; or were previously diagnosed with a medical condition known to threaten normal bone accretion. Further, children younger or older than 10 years of age were excluded for having experienced ≥2 fractures and ≥3 fractures, respectively.
Participants younger than 18 years of age provided assent, and a parent/legal guardian provided written informed consent. Written informed consent was provided by participants 18 years of age or older. The institutional review board for Human Subjects at each clinical center approved the study protocols and procedures.
Demographics, anthropometrics, and sexual maturation
Self-reported population ancestry and ethnicity were determined via questionnaire using National Institutes of Health classification criteria. Height and weight were measured while participants were dressed in light clothing and without shoes. Using the Centers for Disease Control and Prevention 2000 growth charts, Z-scores for height, weight, and body mass index were calculated (9). Forearm length was measured using a sliding caliper (Rosscroft, Surrey, BC, Canada) from the head of the radius to the ulnar styloid process. Sexual maturity stage was assessed via physical examination by an experienced physician or nurse practitioner and categorized according to Tanner (10).
Bone densitometry
Whole body (less head), lumbar spine, total hip, femoral neck, and radius DXA scans were acquired by trained technicians using a standard protocol on Hologic devices (QDR4500A, QDR4500W, and Delphi A models; Hologic Inc., Bedford, MA). Forearm DXA scans were acquired on the nondominant arm and were analyzed using instrument and software-specific procedures. Fig. 1 displays representative forearm scans and analyses for 3 subjects ages 5, 10, and 15 years. The overall forearm analysis consists of specific regions of interest for the UD, Mid, and 1/3 regions of the forearm. The distal line of the global region of interest was manually placed at the ulnar styloid process. The distal line of the UD region of interest was manually placed at the proximal border of the distal radius growth plate if the growth plate was open or at the proximal border of the end plate if the growth plate was fused.
Figure 1.
Representative forearm DXA scans from three subjects that were ages 5 (top; UD radius aBMD Z-score = -0.3), 10 (middle; UD radius aBMD Z-score = 0.9), and 15 (bottom; UD radius aBMD Z-score = 0.8) years. For each respective subject, the forearm DXA scan image and analyzed image are displayed. The distal line of the total analysis region was manually placed at the ulnar styloid process (arrow 1). The distal line of the UD region of interest was manually placed at the medial proximal border of the distal growth plate (arrow 2) if the growth plate was open or at the medial proximal boarder of the growth plate (arrow 3) remnant if the growth plate was fused. aBMD, areal bone mineral density; DXA, dual energy X-ray absorptiometry; UD, ultradistal.
All DXA scans were analyzed centrally at the DXA Core Laboratory at the University of California, San Francisco (San Francisco, CA). Baseline measures were analyzed using the Hologic software version Discovery 12.3 and follow-up measurements were analyzed using the Apex 2.1 software and the “compare” feature. Z-scores were computed for all DXA aBMD measures for which reference ranges were available (6, 7, 11), and for lumbar spine bone mineral apparent density (BMAD) (12).
Peripheral quantitative computed tomography (pQCT, Stratec XCT-2000; Orthometrix, Inc., White Plains, NY) scans were acquired on a subset of study participants at baseline, year 1, and year 4. After performing a scout view scan, the reference line was placed at the medial proximal border of the distal growth plate or the medial proximal border of the endplate if the growth plate was fused. Scans were acquired on the left radius at relative distances from the reference line (3% and 30% sites) with a voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 25 mm/s. Measurements at the 3% site included trabecular volumetric BMD (Tb.vBMD; mg/cm3), total vBMD (mg/cm3), and total bone area (mm2), and measurements at the radius 30% site included cortical vBMD (Ct.vBMD; mg/mm3), cortical thickness (mm), and polar strength strain index (mm3).
Statistical methods
Participants >20 years of age were excluded from all statistical analyses unless otherwise indicated. The relationships between UD radius aBMD and age, sex, and ancestry, including main effects and interaction effects, were evaluated using mixed effects regression to account for the multiple observations per subject. Polynomial age terms (age2, age3, and age4) were used to capture the nonlinearity of the relationship.
The LMS approach was used to generate age-, sex-, and population ancestry-specific UD radius aBMD reference curves using the “gamlss” package in RStudio (version 1.1.463). Children who, after the baseline visit, acquired medical conditions or were using medications known to affect bone accretion at the time of the study visit were excluded from the reference curve development. Participants older than 20 years were included in the creation of the reference curves to provide stability in the curves at the older age ranges. However, because there were few observations in that age range, the reference curves are presented for ages 5 to 20.
The LMS approach generates age-specific median (M, mu), coefficient of variation (S, sigma), and Box-Cox power transformation (L, lambda) values, which are then used for the construction of centile curves. Z-scores are calculated for a given measure (X) using the age-specific L, M, and S parameters and the following equation (Equation 1).
| (1) |
Relationships between UD radius aBMD Z-score and other DXA aBMD Z-scores were assessed in the total cohort using mixed effects regression to account for the multiple observations per subject. Similar analyses were performed to assess relationships between UD radius aBMD Z-score and height Z-score in the total cohort and in groups stratified by age (5-9.9 years, 10–14.9 years, and > 15 years). For each mixed effects regression analysis, UD radius aBMD Z-score was the outcome and the other DXA aBMD Z-score/height Z-score was the predictor. The correlation coefficient (i.e., r) was calculated as the square root of the R2.
We used hierarchical regression (“nestreg” command in STATA) to assess the relationships between forearm aBMD Z-scores (UD radius and 1/3 radius) and pQCT-derived bone measurements. For these analyses, the pQCT bone measurement was the outcome variable. The first step of the regression procedure fitted African American ancestry, sex, age, and forearm length, and the second step added forearm aBMD Z-score. The change in R2 between the first and second steps was evaluated to determine the relationship between DXA aBMD Z-score and each pQCT measures.
The tracking, or consistency, of UD radius aBMD Z-scores over time was investigated using Pearson’s correlations between the baseline and 6-year measures. Fisher’s z transformation was used to generate 95% confidence intervals for these correlation coefficients (“corcci” command in STATA). Tracking correlations were determined in the total cohort, and in groups stratified by sexual maturity at the baseline time point (Tanner stage 1 vs. stages 2-4 vs. stage 5, representing early, mid, and late pubertal maturation, respectively).
In a subset of our subjects (n = 154), repeat scans were performed at either the baseline or year 1 study visits to examine precision error of UD radius aBMD (13). Percent coefficient of variation was calculated in groups stratified by age and in all children combined. Statistical analyses were performed using STATA (version 15.1), and P values < 0.05 were considered statistically significant.
Results
Sample description
Ultradistal radius DXA scans were available from 2013 participants for a total of 9965 measurements. Six hundred and thirty-four UD radius aBMD measurements were excluded from all analyses because they were acquired when study participants were not in a usual state of health or because of excessive movement or interfering objects. Descriptive characteristics of our study participants are presented in Table 1.
Table 1.
Description of Sample for Creating Reference Curves
| Non-African American Males | African American Males | Non-African American Females | African American Females | |||||
|---|---|---|---|---|---|---|---|---|
| Number of participants | 746 | 246 | 788 | 233 | ||||
| Number of observations | 3777 | 1096 | 3970 | 1122 | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age, years | 13.73 | 4.26 | 13.88 | 4.31 | 13.45 | 4.16 | 13.37 | 3.86 |
| Height (Z-score) | 0.13 | 0.83 | 0.32 | 0.86 | 0.14 | 0.86 | 0.21 | 0.87 |
| Weight (Z-score) | 0.28 | 0.82 | 0.50 | 0.84 | 0.30 | 0.82 | 0.60 | 0.76 |
| BMI (Z-score) | 0.24 | 0.90 | 0.40 | 0.87 | 0.29 | 0.84 | 0.59 | 0.82 |
Abbreviations: BMI, body mass index; SD, standard deviation.
Age, sex, and ancestry effects on UD radius aBMD
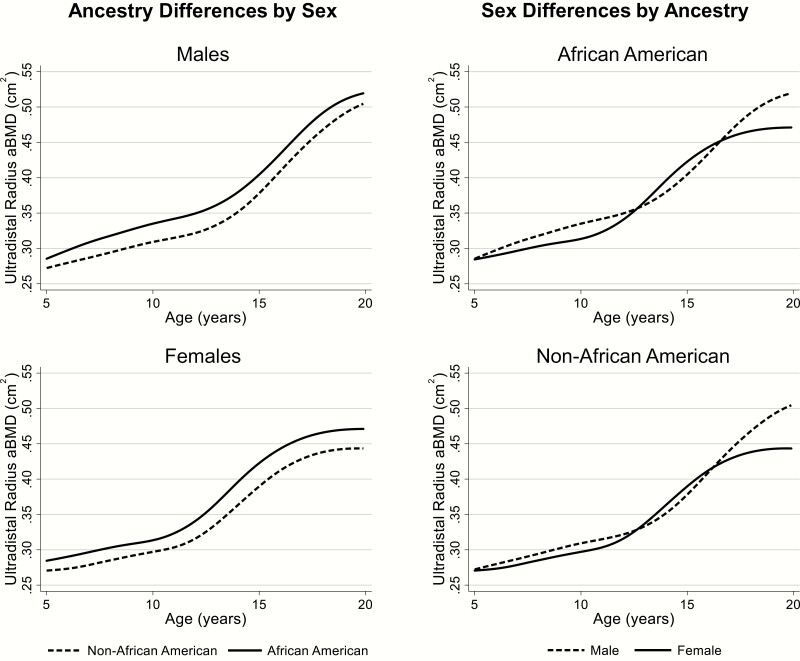
In Fig. 2, median UD radius aBMD values are presented relative to age in African American and non-African American males and females. Longitudinal mixed effects analysis revealed nonlinear increases in UD radius aBMD with age (age, age2, age3, and age4; P < 0.001), as well as a significant age4 by sex interaction (P = 0.008) and a marginal main effect of sex (P = 0.054), suggesting slightly greater aBMD values in males versus females.
Figure 2.
Median UD radius aBMD values in African American and non-African American males and females. Population ancestry-related differences by sex (left; dashed line for non-African Americans; solid line for African Americans), as well as sex-related differences by population ancestry (right; dashed line for males and solid line for females) are presented. aBMD, areal bone mineral density; UD, ultradistal.
UD radius aBMD reference curves
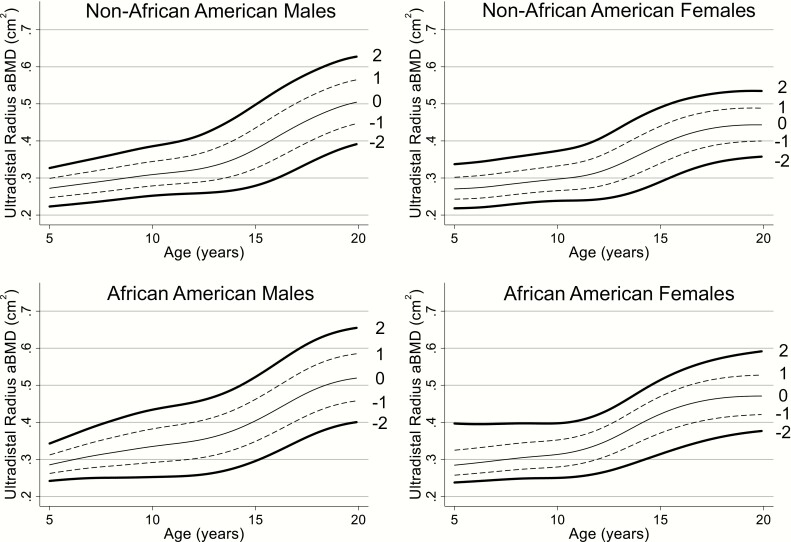
Reference curves for UD radius aBMD are presented in Fig. 3. Age-, sex-, and ancestry-specific UD radius aBMD reference percentiles, including L, M, and S values, are displayed in Table 2 and Supplemental Table 1 (14).
Figure 3.
UD radius aBMD reference curves for non-African American males, non-African American females, African American males, and African American females. Within each figure, the 2 (solid line), 1 (dashed line), 0 (solid line), -1 (dashed line), and -2 (solid line) SD curves are displayed. aBMD, areal bone mineral density; UD, ultradistal.
Table 2.
UD Radius aBMD LMS Values and Reference Percentiles
| UD Radius aBMD | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | Black Females | Black Males | ||||||||||||
| L | S | M | L | S | M | |||||||||
| 3rd | 10th | 50th | 90th | 97th | 3rd | 10th | 50th | 90th | 97th | |||||
| 5 | -2.494 | 0.113 | 0.238 | 0.251 | 0.284 | 0.341 | 0.397 | -0.592 | 0.087 | 0.242 | 0.256 | 0.285 | 0.320 | 0.343 |
| 6 | -2.094 | 0.114 | 0.241 | 0.256 | 0.290 | 0.345 | 0.395 | -0.401 | 0.097 | 0.247 | 0.263 | 0.297 | 0.338 | 0.364 |
| 7 | -1.687 | 0.114 | 0.245 | 0.260 | 0.297 | 0.351 | 0.396 | -0.211 | 0.108 | 0.250 | 0.269 | 0.309 | 0.355 | 0.385 |
| 8 | -1.251 | 0.115 | 0.248 | 0.265 | 0.303 | 0.357 | 0.397 | -0.025 | 0.119 | 0.251 | 0.273 | 0.318 | 0.371 | 0.404 |
| 9 | -0.755 | 0.115 | 0.249 | 0.268 | 0.308 | 0.361 | 0.397 | 0.155 | 0.129 | 0.251 | 0.276 | 0.327 | 0.385 | 0.421 |
| 10 | -0.217 | 0.116 | 0.250 | 0.271 | 0.313 | 0.364 | 0.397 | 0.320 | 0.135 | 0.252 | 0.280 | 0.335 | 0.397 | 0.434 |
| 11 | 0.334 | 0.116 | 0.254 | 0.278 | 0.323 | 0.374 | 0.405 | 0.462 | 0.139 | 0.254 | 0.284 | 0.342 | 0.406 | 0.444 |
| 12 | 0.850 | 0.117 | 0.263 | 0.290 | 0.341 | 0.392 | 0.422 | 0.574 | 0.141 | 0.257 | 0.289 | 0.350 | 0.415 | 0.454 |
| 13 | 1.290 | 0.117 | 0.277 | 0.310 | 0.366 | 0.420 | 0.449 | 0.651 | 0.142 | 0.264 | 0.298 | 0.361 | 0.429 | 0.469 |
| 14 | 1.597 | 0.117 | 0.295 | 0.334 | 0.396 | 0.453 | 0.483 | 0.691 | 0.142 | 0.277 | 0.313 | 0.380 | 0.451 | 0.492 |
| 15 | 1.679 | 0.116 | 0.314 | 0.356 | 0.423 | 0.483 | 0.514 | 0.696 | 0.140 | 0.296 | 0.334 | 0.404 | 0.479 | 0.523 |
| 16 | 1.545 | 0.116 | 0.333 | 0.374 | 0.443 | 0.506 | 0.540 | 0.676 | 0.137 | 0.320 | 0.360 | 0.434 | 0.513 | 0.558 |
| 17 | 1.259 | 0.115 | 0.349 | 0.388 | 0.457 | 0.524 | 0.560 | 0.640 | 0.133 | 0.347 | 0.388 | 0.465 | 0.547 | 0.595 |
| 18 | 0.852 | 0.114 | 0.361 | 0.398 | 0.466 | 0.535 | 0.574 | 0.589 | 0.129 | 0.372 | 0.414 | 0.492 | 0.576 | 0.625 |
| 19 | 0.370 | 0.114 | 0.371 | 0.405 | 0.470 | 0.542 | 0.584 | 0.527 | 0.125 | 0.391 | 0.432 | 0.510 | 0.595 | 0.645 |
| 20 | -0.137 | 0.113 | 0.377 | 0.408 | 0.471 | 0.545 | 0.592 | 0.458 | 0.122 | 0.401 | 0.442 | 0.520 | 0.605 | 0.656 |
| Non-Black Females | Non-Black Males | |||||||||||||
| L | S | M | L | S | M | |||||||||
| Age, y | 3rd | 10th | 50th | 90th | 97th | 3rd | 10th | 50th | 90th | 97th | ||||
| 5 | -0.110 | 0.109 | 0.218 | 0.236 | 0.271 | 0.311 | 0.337 | 0.452 | 0.095 | 0.223 | 0.240 | 0.272 | 0.307 | 0.327 |
| 6 | -0.163 | 0.111 | 0.220 | 0.238 | 0.273 | 0.315 | 0.342 | 0.278 | 0.098 | 0.228 | 0.246 | 0.280 | 0.317 | 0.339 |
| 7 | -0.207 | 0.111 | 0.224 | 0.242 | 0.279 | 0.322 | 0.349 | 0.094 | 0.101 | 0.234 | 0.252 | 0.287 | 0.326 | 0.350 |
| 8 | -0.249 | 0.110 | 0.230 | 0.248 | 0.285 | 0.329 | 0.357 | -0.096 | 0.103 | 0.240 | 0.258 | 0.294 | 0.336 | 0.362 |
| 9 | -0.246 | 0.110 | 0.235 | 0.254 | 0.291 | 0.336 | 0.365 | -0.269 | 0.105 | 0.246 | 0.265 | 0.302 | 0.346 | 0.375 |
| 10 | -0.161 | 0.112 | 0.238 | 0.258 | 0.297 | 0.343 | 0.373 | -0.381 | 0.106 | 0.252 | 0.271 | 0.309 | 0.355 | 0.386 |
| 11 | 0.016 | 0.118 | 0.239 | 0.261 | 0.303 | 0.353 | 0.384 | -0.419 | 0.108 | 0.256 | 0.275 | 0.315 | 0.364 | 0.395 |
| 12 | 0.242 | 0.128 | 0.243 | 0.267 | 0.316 | 0.371 | 0.405 | -0.392 | 0.115 | 0.258 | 0.279 | 0.322 | 0.375 | 0.409 |
| 13 | 0.499 | 0.136 | 0.252 | 0.281 | 0.337 | 0.398 | 0.435 | -0.260 | 0.126 | 0.261 | 0.285 | 0.333 | 0.393 | 0.432 |
| 14 | 0.756 | 0.136 | 0.268 | 0.301 | 0.363 | 0.428 | 0.465 | 0.002 | 0.137 | 0.267 | 0.295 | 0.352 | 0.419 | 0.462 |
| 15 | 0.958 | 0.129 | 0.290 | 0.326 | 0.390 | 0.454 | 0.491 | 0.330 | 0.144 | 0.279 | 0.312 | 0.378 | 0.452 | 0.498 |
| 16 | 1.079 | 0.119 | 0.313 | 0.349 | 0.412 | 0.475 | 0.510 | 0.603 | 0.144 | 0.299 | 0.337 | 0.410 | 0.488 | 0.534 |
| 17 | 1.104 | 0.111 | 0.332 | 0.367 | 0.428 | 0.489 | 0.522 | 0.725 | 0.137 | 0.324 | 0.365 | 0.441 | 0.520 | 0.566 |
| 18 | 1.026 | 0.106 | 0.345 | 0.379 | 0.438 | 0.497 | 0.531 | 0.728 | 0.129 | 0.352 | 0.392 | 0.468 | 0.547 | 0.593 |
| 19 | 0.872 | 0.102 | 0.353 | 0.385 | 0.443 | 0.501 | 0.535 | 0.684 | 0.122 | 0.375 | 0.416 | 0.490 | 0.569 | 0.615 |
| 20 | 0.666 | 0.100 | 0.358 | 0.388 | 0.443 | 0.501 | 0.535 | 0.655 | 0.117 | 0.393 | 0.432 | 0.506 | 0.583 | 0.628 |
Abbreviations: aBMD, areal bone mineral density; L, lambda (Box-Cox power transformation); M, mu (median); S, sigma (coefficient of variation); UD, ultradistal.
Relationships between UD radius aBMD, other DXA aBMD measures, and height Z-scores
Linear mixed effects regression was used to assess the relationships between UD radius aBMD Z-score with other DXA aBMD Z-scores (Table 3) and height Z-score (Table 4), accounting for the multiple observations per participant. Ultradistal radius aBMD was positively and significantly associated with aBMD at the distal 1/3 radius, femoral neck, total hip, lumbar spine, and total body, as well as lumbar spine BMAD (all P < 0.001). Overall, UD radius aBMD Z-score and 1/3 radius aBMD Z-score were positively associated with height Z-score, but these relationships were considerably weaker for UD radius versus 1/3 radius.
Table 3.
Relationship Between Z-scores for UD Radius aBMD and Other DXA-derived aBMD Measures
| Beta Coefficient |
SE | P | r a | |
|---|---|---|---|---|
| Total body aBMD | 0.34 | 0.01 | < 0.001 | 0.63 |
| Lumbar spine aBMD | 0.33 | 0.01 | < 0.001 | 0.59 |
| Lumbar spine BMAD | 0.29 | 0.01 | < 0.001 | 0.54 |
| Total hip aBMD | 0.32 | 0.01 | < 0.001 | 0.64 |
| Femoral neck aBMD | 0.35 | 0.01 | < 0.001 | 0.64 |
| Distal 1/3 radius aBMD | 0.36 | 0.01 | < 0.001 | 0.56 |
Abbreviations: aBMD, areal bone mineral density; BMAD, bone mineral apparent density; DXA, dual-energy X-ray absorptiometry; UD, ultradistal.
aCalculated as square root of R2.
Table 4.
Relationship Between Radius aBMD Z-scores and Height Z-scores in the Total Cohort and Age Groups
| UD Radius aBMD Z-score | 1/3 Radius aBMD Z-score | |||||||
|---|---|---|---|---|---|---|---|---|
| Beta Coefficient | SE | P | r a | Beta Coefficient | SE | P | r a | |
| Total cohort | 0.03 | 0.01 | 0.015 | 0.09 | 0.34 | 0.01 | <0.001 | 0.24 |
| 5-9.9 years | 0.23 | 0.04 | <0.001 | 0.21 | 0.38 | 0.03 | <0.001 | 0.36 |
| 10-14.9 years | 0.02 | 0.02 | 0.302 | 0.16 | 0.39 | 0.02 | <0.001 | 0.34 |
| > 15 years | 0.10 | 0.03 | <0.001 | 0.05 | 0.30 | 0.03 | <0.001 | 0.11 |
Abbreviations: aBMD, areal bone mineral density; UD, ultradistal
aCalculated as square root of R2.
Relationship between radius aBMD from DXA and bone geometry and volumetric density from pQCT
Hierarchical regression was used to assess the relationships between forearm aBMD Z-score from DXA and cortical and trabecular bone from pQCT (Table 5). These analyses were restricted to the subset of participants in which pQCT data were available (398 measurements from 162 subjects, 42% African American, 50% female).
Table 5.
Relationships Between Radius Trabecular and Cortical Bone Measures From pQCT and Radius aBMD Z-scores From DXA
| Base Model (Population Ancestry, Sex, Age, Limb Length) | Base Model + UD Radius aBMD Z-score | Base Model + 1/3 Radius aBMD Z-score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | R 2 | P | R 2 | R 2 Change | r a | P | R 2 | R 2 Change | r a | |
| 3% site | ||||||||||
| Tb.vBMD | <0.001 | 0.11 | <0.001 | 0.60 | 0.49 | 0.70 | <0.001 | 0.16 | 0.05 | 0.23 |
| Tot.vBMD | <0.001 | 0.14 | <0.001 | 0.61 | 0.46 | 0.68 | <0.001 | 0.21 | 0.07 | 0.26 |
| Tot.Ar | <0.001 | 0.71 | <0.001 | 0.72 | 0.01 | 0.09 | <0.001 | 0.73 | 0.01 | 0.12 |
| 30% site | ||||||||||
| Ct.vBMD | <0.001 | 0.61 | 0.067 | 0.62 | 0.00 | 0.06 | <0.001 | 0.71 | 0.10 | 0.32 |
| Ct.Th | <0.001 | 0.71 | <0.001 | 0.75 | 0.03 | 0.18 | <0.001 | 0.87 | 0.15 | 0.39 |
| pSSI | <0.001 | 0.74 | <0.001 | 0.78 | 0.03 | 0.18 | <0.001 | 0.78 | 0.04 | 0.20 |
For hierarchical regression analyses, the respective pQCT measure was the outcome variable. In the first step of this procedure, the base model (population ancestry, sex, age, and forearm length) was included, followed by the respective radius aBMD measure (UD radius aBMD Z-score or 1/3 radius aBMD Z-score).
aCalculated as square root of R2 change.
Abbreviations: aBMD, areal bone mineral density; Ct.Th, cortical thickness; Ct.vBMD, cortical volumetric bone mineral density; pQCT, peripheral quantitative computed tomography; pSSI, polar strength strain index; Tot.Ar, total bone area; Tb.vBMD, trabecular volumetric bone mineral density; UD, ultradistal.
For all regression analyses, the base model included African American ancestry, sex, age, and forearm length, which accounted for 11% (Tb.vBMD) to 74% (polar strength strain index) of the explained variability in pQCT bone measures (all P < 0.001). Overall, adding UD radius aBMD Z-score or 1/3 radius aBMD Z-score to the regression model resulted in a significant increase in explained variability for most pQCT measures. However, the strength of these relationships varied considerably (r = 0.09 to 0.70, all P < 0.001), with associations between UD radius aBMD Z-score and measures of distal radius bone density (i.e., Tb.vBMD and total vBMD) being the strongest. For example, after accounting for covariates, UD radius aBMD Z-score accounted for 49% of the explained variability in distal radius Tb.vBMD, but 1/3 radius aBMD accounted for only 10% of the explained variability in distal radius Ct.vBMD (both P < 0.001).
Tracking of UD radius aBMD over time
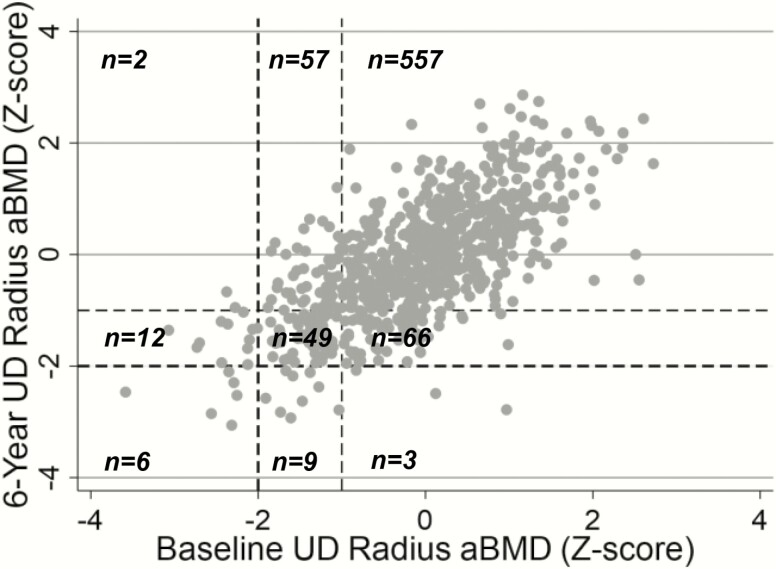
The correlation between baseline and year 6 values was assessed to estimate the tracking of UD radius aBMD Z-scores over time. These analyses were restricted to the 761 participants with usable data at both the baseline and 6-year time points. Fig. 4 presents the tracking relationship in the total cohort, as well as the manner in which Z-scores changed between designated cutoffs (< -2.0 vs. -2.0 to -1.0 vs. > -1.0) from baseline to the 6-year time point. In the total cohort, UD radius aBMD Z-scores tracked strongly from baseline to the 6-year time point (P < 0.05). The majority of children with a lower UD radius aBMD Z-score at baseline had a lower Z-score at the 6-year time point. For instance, 20 children (2.6%) had a UD radius aBMD Z-score < -2.0 at baseline, and 18 of these 20 individuals (90%) had a Z-score < -1.0 at the 6-year follow up. Tracking relationships were also assessed in groups stratified by baseline sexual maturity (Table 6). At baseline, 55% of participants were at maturation stage 1, 39% were at maturation stages 2 through 4, and 6% were at maturation stage 5. The tracking correlations within the 3 sexual maturity groups were all strong and significant (all P < 0.05). The 95% confidence intervals for the correlation coefficients within each of the maturation groups overlapped, indicating that the strength of tracking did not differ across maturity stages.
Figure 4.
Scatter plot depicting the tracking of UD radius aBMD from baseline to the 6-year time point in the total cohort. Dotted lines represent the -1.0 and -2.0 Z-score cutoffs at baseline (vertical lines) and 6 years (horizontal lines). The number of participants with aBMD Z-scores within the given ranges are displayed. aBMD, areal bone mineral density; UD, ultradistal.
Table 6.
Tracking of UD Radius aBMD Z-scores Between the Baseline and 6-year Time Points in the Total Cohort and in Groups Based on Sexual Maturation Rating Stage at Baseline
| r | 95% CI | ||
|---|---|---|---|
| Total cohort | 0.69 | 0.65 | 0.72 |
| Maturation stage 1 | 0.69 | 0.64 | 0.74 |
| Maturation stages 2-4 | 0.69 | 0.62 | 0.74 |
| Maturation stage 5 | 0.66 | 0.46 | 0.79 |
Abbreviations: aBMD, areal bone mineral density; UD, ultradistal.
Precision error of UD radius aBMD
Percent coefficient of variation for UD radius aBMD was 2.65% for 6.9 to 9.9 year olds (n = 50), 2.33% for 10.0 to 13.9 year olds (n = 53), 2.01% for 14.0 to 17.0 year olds (n = 51), and 2.34% for all children combined.
Discussion
This study provides the first pediatric reference ranges for UD radius aBMD, thus addressing a critical need for the use of UD radius aBMD in the pediatric clinical setting (2). We showed that UD radius aBMD increased nonlinearly with age and was greater in males and African Americans. Further, UD radius aBMD correlated with other DXA-derived measures of bone density, was strongly reflective of distal radius trabecular volumetric density, was only marginally associated with stature, and tracked significantly from childhood to adolescence. These are importance characteristics for selecting a skeletal site for use in monitoring bone health in the clinical setting. Additional studies are warranted to elucidate the utility of UD radius aBMD in monitoring childhood bone health and fracture prediction.
The whole body and lumbar spine are the preferred regions for pediatric DXA examinations, but the most recent ISCD Pediatric Position statement recognized the clinical applicability of forearm DXA scans. Most available pediatric reference data for forearm DXA measures are for the distal 1/3 radius site and reference data for the UD radius site are lacking. The large, multisite, longitudinal BMDCS has been an important resource in the development of pediatric bone density reference data, and the reference ranges presented here complement the previously published reference data for aBMD and BMC of the distal 1/3 radius, total body, lumbar spine, hip, lateral distal femur, and femoral neck (6, 7, 15), as well as lumbar spine bone mineral apparent density (11). Similar to bone density at other skeletal sites, UD radius aBMD increased nonlinearly with age and tended to be greater in males versus females. These results support the need for sex- and population ancestry-specific reference data, which we present here.
It is generally assumed that the UD radius and 1/3 radius aBMD measures from DXA are primarily reflective of trabecular and cortical bone, respectively. This is considered a key advantage of forearm DXA assessment, but it is unknown whether these forearm aBMD measures are indeed reflective of specific bone compartments. Thus, we compared UD radius and 1/3 radius aBMD Z-scores from DXA to indices of trabecular and cortical bone geometry, volumetric density, and estimated strength assessed using pQCT. Whereas aBMD at the 1/3 and UD radius regions were associated with distal radius cortical and trabecular bone outcomes from pQCT, respectively, relationships involving UD radius aBMD and trabecular bone volumetric density were stronger. This suggests that UD radius aBMD might be a more accurate reflection of trabecular bone density than the 1/3 radius aBMD measure is reflective of cortical bone density. These results help guide interpretation of clinical forearm DXA measurements and support the use of the UD radius aBMD measure while monitoring bone health in interventions or chronic health conditions that are understood to preferentially affect trabecular bone.
Similar to the UD radius, the lumbar spine and femoral neck also largely comprise trabecular bone, so this characteristic is not exclusive to the UD radius. Bone density at the UD radius was positively associated with aBMD at the spine and hip. However, the observation that these measures were not perfectly correlated indicates that UD radius aBMD likely provides unique insight regarding trabecular bone health. Compared with the hip and lumbar spine, the forearm is not often subjected to compression loading. This may be an advantage for monitoring treatment effectiveness in patients during immobilization or prolonged bed rest, as the confounding of unloading-related reductions in bone density might be mitigated. Because forearm scans are often less burdensome to acquire compared with other DXA measures, radius aBMD might be more feasible to attain in patients with positioning constraints compared with other measures such as the total body, lumbar spine, and hip. Furthermore, the forearm is the most common fracture site during childhood (5), so the UD radius represents an anatomic location with strong clinical relevance. In 1 earlier study, UD radius aBMD was lower in girls with previous forearm fractures (16). Additional studies are warranted to further define the relationships between UD radius aBMD and fracture among children who are prone to fracture.
The tracking, or consistency, of a given bone density measure over time provides valuable insight regarding its utility in monitoring patients. In a recent 17-year prospective cohort study, Yang et al. reported strong tracking of bone density at multiple sites from ages 8 to 25 years in both males and females (17). Strong tracking of bone density Z-scores over shorter periods (3 and 6 years) at the total body, lumbar spine (aBMD and BMAD), total hip, femoral neck, and distal 1/3 radius was reported using data from the BMDCS (11, 18, 19). The tracking coefficients were weaker during mid-puberty for some of these measures, but in the current study, UD radius aBMD Z-scores tracked strongly and consistently regardless of maturation which is similar to lumbar spine BMAD. As the reference range for UD radius BMD was based on the BMDCS, a cohort of exclusively healthy children, relatively few exhibited low bone density for age (UD radius aBMD Z-score < -2.0) at the baseline time point. Nonetheless, from the 20 children with a UD radius aBMD Z-score < -2.0 at baseline, 18 (90%) had a Z-score < -1.0 at the 6-year time point. Kalkwarf et al reported similar findings for other bone density measures previously, such that 72% to 87% of children with an aBMD Z-score < -1.5 at baseline had a Z-score < -1.0 at year 3 (19). Together, these findings support the utility of UD radius aBMD in tracking childhood bone health over time, especially in those at greatest risk for suboptimal bone accrual and potentially fracture.
Bone density by DXA at most skeletal sites associates with height, so it is recommended that DXA aBMD measures in individuals with shorter stature or growth delay are adjusted for stature (2, 8). Accordingly, Zemel et al reported a method to adjust DXA bone Z-scores for height Z-score to account for this confounding (8). Overall, UD radius and 1/3 radius aBMD Z-scores were significantly associated with height Z-scores, but relationships were substantially weaker for UD radius aBMD and not considered clinically meaningful. These findings are consistent with the correspondence between UD radius and the pQCT measure of trabecular vBMD; when using pQCT outcomes, measures of cortical dimensions and structural strength, but not trabecular density, are adjusted for limb length (20, 21). The minimal stature-related confounding on trabecular bone density at the UD radius supports the utility of this measure in patients with clinical conditions affecting linear growth.
There are strengths and limitations of this study. The multisite, mixed-longitudinal design of the BMDCS was a major strength, yielding valuable data that are generalizable to healthy children in the United States. All BMDCS study sites used standard DXA scan acquisition and patient positioning procedures, and all scans were analyzed at a central location by trained researchers. These are important points to recognize when considering forearm DXA scan analysis because reference line placement is dependent on the location of the ulnar styloid process as well as the presence/absence of an open growth plate. Researchers and clinicians intending to use these reference data should be mindful of proper reference line placement, especially in actively growing children. Additionally, because aBMD data for both the radius and ulna are generated through the scan analysis, aBMD values from the radius only, and not the ulna or ulna + radius, should be used. Unfortunately, pQCT measurements were obtained on a subset of the BMDCS sample, and only acquired at 2 clinical sites, so these results may not be generalizable to the entire cohort. Finally, we observed favorable precision error for UD radius aBMD that was in line with other DXA measurements (lumbar spine, total body less head, total hip, femoral neck, and 1/3 radius) from the BMDCS (13). This information should help guide clinical determination of whether observed changes between serial measurements are suspected to be genuine or rather attributed to measurement error.
Conclusions
This study fills an important need highlighted by the ISCD by providing the first pediatric reference dataset for UD radius aBMD (1, 2). These newly developed reference data are appropriate for use in children ranging in age from 5 to 20 years. Bone density Z-scores at the UD radius correlated with Z-scores at other skeletal sites and were only marginally confounded by stature. Compared with pQCT-derived measures, UD radius aBMD was more closely related to trabecular bone density than 1/3 radius aBMD was related to cortical bone density. Low UD radius bone density Z-scores in early childhood persisted into adolescence and young adulthood, suggesting that this measure might be useful in monitoring childhood bone health over time and identifying at-risk individuals with respect to fracture and osteoporosis later in life. Because traditional DXA scan sites (i.e., the total body and lumbar spine) might not be feasible for certain clinical populations and the distal radius is a common fracture site in growing children, the UD radius represents a promising alternative skeletal site for pediatric bone health assessment.
Acknowledgments
We are grateful for the pediatric endocrine divisions of each BMDCS clinical center, the study staff, and the participants and their families. We also acknowledge the guidance and advice from the Data Safety and Monitoring Board members, including Drs. Clifford Rosen, Ralph D’Agostino, Ingrid Holms, James Reynolds, and Reginald Tsang.
Financial Support : J.M.K. is funded through the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number TL1TR001880, the American Diabetes Association (ADA) under award number 1-19-PDF-129, and the Maternal & Child health Bureau (MCHB) under award number T71MC30798. This study was funded by the National Institute of Child Health and Human Development contracts NO1-HD-1-3228, -3329, -3330, -3331, -3332, and -3333 and the Clinical and Translational Research Center Grant 5-MO1-RR-000240 and UL1 RR-026314. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, ADA, or MCHB.
Glossary
Abbreviations
- aBMD
areal bone mineral density
- BMAD
bone mineral apparent density
- BMDCS
Bone Mineral Density in Childhood Study
- Ct.vBMD
cortical volumetric bone mineral density
- DXA
dual energy X-ray absorptiometry
- ISCD
International Society for Clinical Densitometry
- L
lambda
- M
mu
- pQCT
peripheral quantitative computed tomography
- S
sigma
- Tb.vBMD
volumetric bone mineral density
- UD
ultradistal.
Additional Information
Disclosure Summary : The authors have nothing to disclose.
Data Availability
The source data from the National Institute of Child Health and Human Development Bone Mineral Density in Childhood Study is available upon request from the NICHD Data and Specimen Hub (DASH).
References
- 1. Crabtree NJ, Arabi A, Bachrach LK, et al. ; International Society for Clinical Densitometry . Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17(2):225-242. [DOI] [PubMed] [Google Scholar]
- 2. Gordon CM, Leonard MB, Zemel BS; International Society for Clinical Densitometry . 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom. 2014;17(2):219-224. [DOI] [PubMed] [Google Scholar]
- 3. Weber DR, Boyce A, Gordon C, et al. The utility of DXA assessment at the forearm, proximal femur, and lateral distal femur, and vertebral fracture assessment in the pediatric population: 2019 ISCD official position. J Clin Densitom. 2019;22(4):567-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dual energy X ray absorptiometry for bone mineral density and body composition assessment. Vienna: International Atomic Energy Agency; 2010. [Google Scholar]
- 5. Moon RJ, Harvey NC, Curtis EM, de Vries F, van Staa T, Cooper C. Ethnic and geographic variations in the epidemiology of childhood fractures in the United Kingdom. Bone. 2016;85:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92(6):2087-2099. [DOI] [PubMed] [Google Scholar]
- 7. Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95(3):1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000(314):1–27. [PubMed] [Google Scholar]
- 10. Tanner J. Growth and adolescence. 2nd ed. Oxford, UK: Blackwell Scientific Publications; 1962. [Google Scholar]
- 11. Kindler JM, Lappe JM, Gilsanz V, et al. Lumbar spine bone mineral apparent density in children: results from the bone mineral density in childhood study. J Clin Endocrinol Metab. 2019;104(4):1283-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7(2):137-145. [DOI] [PubMed] [Google Scholar]
- 13. Shepherd JA, Wang L, Fan B, et al. Optimal monitoring time interval between DXA measures in children. J Bone Miner Res. 2011;26(11):2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kindler JM KH, Lappe JM, Gilsanz V, et al. Data from: reference ranges for ultradistal radius bone density during childhood: results from the bone mineral density in Childhood study. Deposited 21 May 2020; 10.6084/m9.figshare.12075312. [DOI] [PMC free article] [PubMed]
- 15. Zemel BS, Stallings VA, Leonard MB, et al. Revised pediatric reference data for the lateral distal femur measured by Hologic Discovery/Delphi dual-energy X-ray absorptiometry. J Clin Densitom. 2009;12(2):207-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones IE, Taylor RW, Williams SM, Manning PJ, Goulding A. Four-year gain in bone mineral in girls with and without past forearm fractures: a DXA study. Dual energy X-ray absorptiometry. J Bone Miner Res. 2002;17(6):1065-1072. [DOI] [PubMed] [Google Scholar]
- 17. Yang Y, Wu F, Winzenberg T, Jones G. Tracking of areal bone mineral density from age eight to young adulthood and factors associated with deviation from tracking: a 17-year prospective cohort study. J Bone Miner Res. 2018;33(5): 832-839. [DOI] [PubMed] [Google Scholar]
- 18. Wren TA, Kalkwarf HJ, Zemel BS, et al. ; Bone Mineral Density in Childhood Study Group . Longitudinal tracking of dual-energy X-ray absorptiometry bone measures over 6 years in children and adolescents: persistence of low bone mass to maturity. J Pediatr. 2014;164(6):1280-5.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalkwarf HJ, Gilsanz V, Lappe JM, et al. Tracking of bone mass and density during childhood and adolescence. J Clin Endocrinol Metab. 2010;95(4):1690-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leonard MB, Zemel BS, Wrotniak BH, et al. Tibia and radius bone geometry and volumetric density in obese compared to non-obese adolescents. Bone. 2015;73:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ward LM, Ma J, Rauch F, et al. Musculoskeletal health in newly diagnosed children with Crohn’s disease. Osteoporos Int. 2017;28(11):3169-3177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The source data from the National Institute of Child Health and Human Development Bone Mineral Density in Childhood Study is available upon request from the NICHD Data and Specimen Hub (DASH).