Abstract
Plant endophytic fungi spend all or part of their lives inside host tissues without causing disease symptoms. They can colonize the plant to protect against predators, pathogens and abiotic stresses generated by drought, salinity, high concentrations of heavy metals, UV radiation and temperature fluctuations. They can also promote plant growth through the biosynthesis of phytohormones and nutrient acquisition. In recent years, the study of endophytic fungi for biological control of plant diseases and pests has been intensified to try to reduce the ecological and public health impacts due the use of chemicals and the emergence of fungicide resistance. In this review, we examine 185 patents related to endophytic fungi (from January 1988 to December 2019) and discuss their applicability for abiotic stress tolerance and growth promotion of plants, as agents for biocontrol of herbivores and plant pathogens and bio- and phytoremediation applications.
Keywords: endophytic fungi, patent, abiotic stress tolerance, biocontrol, bioremediation, phytoremediation
1. Introduction
An endophytic fungus is any organism inhabiting plant organs that, at certain point in its lifetime, can colonize tissues without causing apparent harm [1]. Endophytic fungi have been a proven source of secondary metabolites with potential uses as anticancer, antibiotics, antivirals, anti-inflammatories, antioxidants, neuroprotective agents, insecticides and antifungals, and have multiple applications in biotechnological developments in pharmaceutical, agriculture, cosmetic, food industry and environmental processes [2]. In the last decades, studies of endophytic fungi have resulted in a number of patents linked to the production of biologically active secondary metabolites and in biotransformation processes [3].
Moreover, interaction between fungi and their hosts drives changes in the host metabolism, altering the response to environmental stress and predator attack. Additionally, this interaction leads to the production of secondary metabolites by both the fungi and the host, which further enhance the capability to respond to the environment [4,5,6,7].
The use of endophytic fungi for environmental applications such as growth promotion, relief of abiotic stress, biocontrol of pest and plant pathogens and bio/phytoremediation has gained important attention in recent years due to the concern about global climate change and contamination in soils and natural sources that increases stress in crops, limiting and reducing the production [8,9,10,11]. Furthermore, basic and applied research has been conducted to develop processes, methodologies and technologies that resulted in a considerable number of patents with new proposals to overcome some of these challenges. Therefore, in this review, we cover patents on endophytic fungi applications related to (a) abiotic stress tolerance and growth promotion of plants; (b) biocontrol of herbivores and plant pathogens; (c) bio- or phytoremediation.
The highlighted topics in each of the patents, cited here, could inspire other researchers to take their investigation to the next level and contribute to overcome, in a more efficient way, some of the principal challenges of humanity today.
2. Materials and Methods
The present review was conducted mainly through searches in the Scifinder® and Google Patents databases. The search was initially conducted in Scifinder® using the terms “endophytic fungi” and “patents” covering the period from 1988 to 2019. 12,315 references were found. After removing duplicates (those describing the same patent/endophyte), we selected those related to the aim of this review, resulting in 185 documents. The patents covered in this study are described in five tables below.
3. Results
The description and analysis of patents was divided, considering the main objective of each one, into four sections; those associated to: (Section 3.1) abiotic stress tolerance and growth promotion of plants; (Section 3.2) biocontrol of herbivores and plant pathogens, and (Section 3.3) bio- and phytoremediation applications; (Section 3.4) patents where the endophyte has multiple applications. The information in tables describe the fungi, the host plant where they were isolated, and the main application of the patent. All endophytes, listed in the tables, have beneficial effects on plants, even though some of them could be considered as pathogens in previous reports.
3.1. Abiotic Stress Tolerance and Growth Promotion of Plants
The principal abiotic stress factors in plants include drought, salinity, high heavy metal concentrations, UV radiation and temperature fluctuations [12]. Abiotic stress affects the cellular pathways of plants, resulting in negative changes to their physiology and morphology [12]. Endophytic fungi have been shown to help their host plant to overcome abiotic stress and promote plant growth through the biosynthesis of phytohormones (indole-3-acetic-acid, gibberellins, cytokinins, ethylene, acetoin, 2, 3-butanediol) and nutrient absorption and uptake [12,13,14].
Plant endophytic fungi have been patented based on their ability to improve the following in plants: (a) root and seed development; (b) nutrient uptake or absorption; (c) photosynthesis promotion; (d) growth of biomass; (e) increase chlorophyll content; and (f) abiotic stress resistance. Numerous genera have been used for such purposes, including Acremonium, Alternaria, Aspergillus, Chaetomium, Fusarium, Penicillium, and others (Table 1 and Table 2). A specific area of application for which endophytic fungi have been widely used is in the growth promotion of medicinal plants; this includes such species as Acanthopanax senticosus [15], Salvia miltiorrhiza [16], Rumex gmelinii Turcz [17], Acacia confusa [18], Coix lacryma-jobi [19], Cynanchum acuminata [20], Huperzia serrata [21], Anoectochilus roxburghii [22], Arnebia sp. [23], Saussurea sp. [24], Rhizoma bletillae [25], Salvia miltiorrhiza [26,27], and Eucalyptus sp. [28,29,30]. Additionally, some endophytic fungi have been patented due to their capability to promote the growth of crop plants such as corn, tomato, soybean, rice, wheat, potato, and barley [31,32,33,34,35,36,37] as well as other useful plants such as Casuarina equisetifolia [38,39,40,41], fir [42,43,44,45], Aleurites montana [46,47,48,49,50,51], Dendrobium sp. [52,53,54], tobacco [55,56,57,58], Schima superba [59,60,61], Bletilla striata [62,63], and Paphiopedilum sp. [64].
Table 1.
Endophytic fungi applied to enhance the abiotic stress tolerance of plants.
| Patent No. | Endophyte | Host 1 | Patent Application | Ref. |
|---|---|---|---|---|
| CN104762216A | Arthrinium sp. | Salicornia bigelovii | Plant anti-salt stress. | [65] |
| WO2004000017A2 | Curvularia sp. | Dichanthelium languinosum | Conferring stress tolerance to inoculated plants (monocots and dicots). | [66] |
| WO2009012480A2 | Fusarium sp. | Leymus mollis | Conferring stress tolerance to inoculated plants (monocots and dicots). | [67] |
| CN105296359A | Lecanicillium sp. | Tobacco | Reducing the absorption of heavy metals in tobacco. | [58] |
| CN101314760A | Neotyphodium chisosum | Festuca arundinacea | Improving the stress tolerance to drought and diseases. | [68] |
| CN104004665A | Papulospora sp. | Fir roots | Relieving phosphorus stress in fir. | [43] |
| CN105002099A | Paraconiothyrium cyclothyrioides | Myricaria root | Reducing heavy metal pollution in plants. | [69] |
| CN101974437A | Penicillium sp. | Eucalyptus | Relieving aluminum toxicity in Eucalyptus. | [30] |
| CN102002463A | Penicillium sp. | Eucalyptus roots, stems, and leaves | Improving the cold resistance of Eucalyptus. | [28] |
| CN103865806A | Phialophora oryzae | Not disclosed | Reducing the absorption of heavy metals in tobacco | [57] |
| CN107926549A | Piriformospora indica | Not disclosed | Improving the resistance of plants to the herbicide bensulfuron-methyl. | [70] |
| CN103834578A | Pyrenochaeta sp. | Tobacco | Promoting plant growth and reducing the heavy metal content in tobacco. | [55] |
| CN105316240A | Rhizopycnis sp. | Tobacco | Reducing the absorption of heavy metals in tobacco. | [56] |
| US20150366217A1 | Group of several fungi 2 | Roots of Triticum turgidum L. | Improving seed vitality, biotic and abiotic stress resistance, and plant health and yield under both stressed and unstressed environmental conditions. | [71] |
1 Some patents just provided a common name for the host organism. 2 A list of the group of fungi is in Table S1.
Table 2.
Endophytic fungi applied for the growth promotion of plants.
| Patent No. | Endophyte | Host 1 | Patent Application | Ref. |
|---|---|---|---|---|
| CN105907648A | Acremonium sp. | Panax notoginseng | Root and seed development of different plants including Radix Ginseng, Oryza sativa L., Semen Maydis, Semen Tritici aestivi, Rhizoma Paridis, Rhizoma Solani tuberosi, etc. | [34] |
| CN108513990A | Alternaria alternata | Acanthopanax senticosus | Seedling-stage growth of A. senticosus. | [15] |
| CN104911108A | Alternaria sp. | Hippophae sp. | Drought resistance on turf grass. | [72] |
| CN104818218A | Alternaria sp. | Aleurites montana | Phosphorus uptake in A. montana. | [47] |
| CN102086439A | Alternaria tenuissima | Panax ginseng | Growth of corn plant. | [31] |
| CN103173362A | Aspergillus sp. | Casuarina sp. rhizosphere | Photosynthesis in C. equisetifolia. | [38] |
| CN103173361A | Aspergillus sp. | Casuarina sp. rhizosphere | Nutrient element absorption in Casuarina. | [39] |
| CN103173364A | Aspergillus sp. | Casuarina sp. rhizosphere | Casuarina biomass growth. | [41] |
| CN110343619A | Botryosphaeria sp. | Root of Schima superba | Schima superba seedling height and ground diameter under a low-phosphorus environment. | [61] |
| CN109456902A | Byssochlamys spectabilis | Rhizoma bletillae | The growth of R. bletillae. | [25] |
| CN106929436A | Cercosporella Sacc. | Rumex gmelini Turcz | Growth in R. gmelinii Turcz. | [17] |
| CN106801014A | Chaetomium globosum | Salvia miltiorrhiza | Radix root biomass, plant height, crown diameter in S. miltiorrhiza. | [73] |
| CN109628322A | Chaetomium nigricolor | Bletilla striata | The growth of B. striata. | [62] |
| CN110438011A | Cladosporium tenuissimum | Salvia miltiorrhiza | Synthesis of effective components (tanshinone and salvianolic acid substances) in the root system of Salvia miltiorrhiza. | [26] |
| CN104630073A | Claviceps sp. | Dendrobium officinale | Growth and yield in D. officinale. | [74] |
| CN104004664A | Colletotrichum sp. | Abies sp. roots | Photosynthesis of cedar. | [45] |
| CN106085872A | Colletotrichum sp./Fusarium sp. | Acacia sp. | Nutrient absorption in A. confusa. | [18] |
| CN104805019A | Coniothyrium sp. | Aleurites sp. | Nutrient element absorption in wood oil tree. | [75] |
| CN104004666A | Cylindrocarpon sp. | fir plant | Growth of fir. | [42] |
| CN110250210A | Darksidea sp. | Stipa capillata root | Rooting and growth of maize. | [36] |
| CN109504611A | Diaporthe spectabilis | Bletilla striata | Growth of B. striata. | [63] |
| CN103733829A | Emericella foeniculicola | Salvia miltiorrhizae | Growth of S. miltiorrhizae. | [76] |
| CN105624047A | Epichloë bromicola | Coix lacryma-jobi | Growth of Coix lacryma-jobi, Arabidopsis thaliana and other graminaceous plants. | [19] |
| CN105861334A | Filobasidium sp. | Acacia sp. | Taiwan Acacia biomass. | [77] |
| CN105861335A | Filobasidium sp. | Acacia sp. | Nutrient element absorption in Taiwan Acacia in a low-phosphorous environment. | [78] |
| CN106085873A | Filobasidium sp./Penicillium sp. | Acacia sp. | Phosphorous uptake in A. confusa under a low-phosphorus environment. | [79] |
| CN107432135A | Fusarium redolens | Not disclosed | Germination of Cynanchum acuminata seeds. | [20] |
| CN103173360A | Fusarium sp. | Casuarina equisetifolia | Chlorophyll content of C. equisetifolia. | [80] |
| CN110257259A | Fusarium sp. | Schima superba stems | Photosynthesis of Schima superba. | [59] |
| CN103114044A | Heterodera oryzae | rice | Plant growth regulation and/or plant pathogenicity. | [81] |
| CN103798293A | Hypha sp. | Salvia miltiorrhiza | The growth and improvement of S. miltiorrhiza hairy root tanshinone content. | [82] |
| CN1961631A | Mycocentrospora sp./Leptodontidium sp. | Saussurea involucrata | Saussurea sp. growth. | [24] |
| CN104593274A | Nectria sp. | Dendrobium officinale | Yield in Dendrobium artificial planting. | [83] |
| US20130104263A1 | Neotyphodium sp. | perennial ryegrass | Beneficial properties (phenotype) for plant. | [84] |
| CN104004667A | Paecilomyces sp. | Not disclosed | Phosphorus absorption in fir. | [44] |
| CN106010984A | Penicillium sp. | Acacia confusa | Plant biomass growth of Taiwan Acacia plant under low-phosphorus environment. | [85] |
| CN101974438A | Penicillium sp. | Eucalyptus | Phosphorus absorption in Eucalyptus. | [29] |
| CN104818219A | Penicillium sp. | Aleurites montana | Root growth of A. montana in a low-phosphorous environment. | [51] |
| CN104789481A | Penicillium sp. | Aleurites montana | Growth and photosynthesis enhancement of A. montana in a low-phosphorus environment. | [48] |
| CN104762219A | Penicillium sp. | Aleurites montana | Biomass growth of A. montana in a low-phosphorus environment. | [49] |
| CN110257258A | Penicillium sp. | Schima superba leaves | Phosphorus absorption of Schima superba. | [60] |
| WO2016210238A1 | Penicillium sp. | Not disclosed | Cultivation of agricultural plants, such as soybean and maize. | [33] |
| CN104818217A | Pestalotia sp. | Not disclosed | Biomass growth of A. montana. | [50] |
| CN105886405A | Pestalotiopsis sp. | Dendrobium officinale | Growth of D. officinale and change in metabolic components. | [54] |
| CN107988087A | Pezicula ericae | wild blueberry root | Growth effects. | [86] |
| CN109706084A | Phoma herbarum | Salvia miltiorrhiza | Growth of Salvia miltiorrhiza and synthesis of tanshinone compounds. | [27] |
| CN104593273A | Phyllachora sp. | Dendrobium officinale | Dendrobium yield. | [87] |
| CN103173363A | Phyllosticta sp. | Casuarina sp. | Photosynthesis of C. equisetifolia. | [40] |
| ES2500790A1 | Pochonia chlamydosporia | Not disclosed | Flowering and fruiting and increased yield in crops such as tomatoes. | [32] |
| WO2016038234A1 | Pochonia chlamydosporia | Meloidogyne spp. | Culture yield and reduction in flowering and fructification times. | [88] |
| CN105039172A | Pythium sp. | Huperzia serrata | Improved transplant survival rate of H. serrate. | [21] |
| CN108041078A | Rhizopycnis sp. | tobacco | Rice growth. | [89] |
| WO2019113255A1 | Serendipita vermifera ssp. bescii | Australian orchid | Enhancement of plant performance in combination with phosphite as a phosphorous source. | [90] |
| CN105420119A | Schizophyllum commune | Ginseng | Host tissue culture hairy root biomass and ingredients of ginseng saponins. | [91] |
| CN104774771A | Thermomyces sp. | Not disclosed | Photosynthesis of A. montana under a low-phosphorus environment. | [46] |
| CN107046965A | Trichoderma sp. | Anoectochilus formosanus | Seedling adaptation cultivation. | [92] |
| CN104745482A | Trichoderma sp. | Arnebia euchroma | Growth of Arnebia hairy roots and improved shikonin component content in hairy roots. | [23] |
| CN105969672A | Trichoderma sp. Fusarium sp. | Acacia sp. | Increase in the height and ground diameter of A. confusa seedlings. | [93] |
| CN110408551A | Tulasnella calospora | Roots of Paphiopedilum | Growth of aseptic seedlings of Paphiopedilum. | [64] |
| CN102876584A | Xylaria striata | Oryza meyeriana | Plant growth. | [94] |
| CN107460133A | Zasmidium sp. | mangrove | Growth and development of D. officinale. | [95] |
| WO2016179047A1 | Group of fungi | Not disclosed | Agronomic traits in plants. | [96] |
| CZ306950B6 | Group of fungi | Miscanthus sp. | Growth, especially of graminaceous and Miscanthus plants. | [97] |
| WO2017134664A1 | Acremonium sclerotigenum/Sarocladium implicatum | Set of grass relatives of wheat | Nutrient uptake. | [98] |
| US20150373993A1 | Group of several 2 fungi | A diverse type of wild relatives or ancestral landraces of maize, wheat, rice, and other seeds | Agronomic traits. | [99] |
| WO2018102733A1 | Group of several 2 fungi | Agricultural plants | Modulation of the nutritional quality traits in seeds | [100] |
1 Some patents just provided a common name for the host organism. 2 A list of the group of fungi is in Table S1.
3.2. Biocontrol of Herbivores and Plant Pathogens
Crop plant diseases represent a major threat in agriculture [101]. The number of chemicals that can be effectively used to control pathogens has been reduced due to the emergence of fungicide resistance along with an increased awareness of the negative associated ecological and public health impacts [101]. Due to these problems, study of the biological control of plant diseases with endophytes has intensified in recent years [101]. Endophytes have been shown to protect their hosts against diseases, reducing infection levels and inhibiting the growth of pathogens [102,103]. The proposed mechanisms used by endophytes are the production of antimicrobial and structural compounds, niche competition, and the induction of plant immunity [104].
Several patents describe the biocontrol of herbivores and plant pathogens using endophytic fungi (Table 3). Species of the genus Acremonium have been described to control Verticillium wilt [105]; Argentine stem weevil (Listronotus bonariensis) [106]; plant diseases caused by banana root nematode and different pathogenic microbes such as Bipolaris oryzae, Colletotrichum falcatum, Colletotrichum gloeosporioides, Corynespora cassiicola, Corynespora sp., Drechslera sp., Fusarium oxysporum, Gloeosporium musarum, and Magnaporthe grisea [107]; and to prevent fescue toxicosis [108]. Species of Alternaria can control the growth of different pathogens such as Rhizoctonia solani, Fusarium oxysporum, Botrytis cinerea, Phytophthora capsici, Pseudomonas aeruginosa, Proteus hauseri, and Plasmopara viticola [109,110,111,112,113,114,115]. Members of the genus Aspergillus have been applied to limit the growth of nematodes in soil [116]; the plant pathogenic fungi Sclerotinia sclerotiorum, Rhizoctonia solani, and Thanatephorus cucumeris [52,117,118]; as well as grass fungi [119]. Several strains of the genus Chaetomium have been reported to enhance plant disease resistance in Anoectochilus roxburghii cultivation [16], to control different plant pathogenic fungi [120,121,122], to inhibit Erwinia causing soft rot and Ralstonia solanacearum causing bacterial wilt [123], to inhibit anthracnose apple pathogens [124], in the preparation of an anti-plant pathogen fermentation liquid broth [125], and in the production of chaetoglobosin A with antagonistic activity against Exserohilum turcicum, Coniothyrium diplodiella, and Rhizopus stolonifer [126]. Species of Fusarium can prevent and treat black spot and fungal diseases in Panax notoginseng [127,128], control five plant pathogenic fungi (Fusarium oxysporum, Cytospora mandshurica, Colletotrichum gloeosporioides, Venturia pyrina, and Fusarium graminearum) [129], and control rice blast disease [130,131] and bacterial wilt of ginger [132]. Species of Neotyphodium can decrease the mildewing rate of Elymus sibiricus seeds at the germination stage [133] and improve fungicide and pest resistance in plants [134,135]. Species of Penicillium can restrain the effects of Panax notoginseng anthracnose, root rot [136,137,138], and Alternaria panax [139]; control different harmful pathogenic fungi [140,141] and litchi downy blight [142]; and prevent plant diseases such as Sclerotinia rot of colza and tobacco blackleg [53]. Species of Rhexocercosporidium can control the fungal pathogens Colletotrichum gloeosporioides, Fusarium solani, and Alternaria panax Whetzel on Panax notoginseng [143,144,145].
Table 3.
Endophytic fungi applied as biocontrol agents of herbivores and plant pathogens.
| Patent No. | Endophyte | Host 1 | Patent Application | Ref. |
|---|---|---|---|---|
| CN103897992A | Acremonium alternatum | cotton | Verticillium wilt. | [105] |
| US93951A0 | Acremonium coenophialum | Not disclosed | Fescue toxicosis. | [108] |
| AU639084B2 | Acremonium lolii | French perennial ryegrass ecotype | Argentine stem weevil (Listronotus bonariensis) by production of compound peramine. | [106] |
| CN101235355A | Acremonium strictum | Brachiaria brizantha | Banana root-knot nematode and different pathogenic microbes. | [107] |
| WO2012174585A1 | Acremonium sp. | Brachiaria/Urochloa | Fungal plant diseases. | [146] |
| CN108192832A | Acrocalymma sp. | Sinomenium acutum | Plant diseases caused by pathogenic bacteria. | [147] |
| CN108085259A | Arcopilus aureus | Dendrobium sp. | The plant pathogenic fungus Botrytis cinerea. | [148] |
| CN102204570A | Alternaria alternata | Cinnamomum camphora | Rhizoctonia solani, Fusarium oxysporum, and Botrytis cinerea. | [111] |
| CN102191184A | Alternaria alternata | Cinnamomum camphora | Plant pathogenic fungi such as Rhizoctonia solani, Fusarium oxysporum, and Botrytis cinerea. | [110] |
| CN110373331A | Alternaria alternata | Huperzia serrata | Gray mold of crops. | [115] |
| ES2696982A1 | Alternaria alternata and Fusarium acuminatum | Artemisia thuscula and Austrian Artemisia | Plant pathogenic fungi with the production of antifungal compounds. | [114] |
| CN103232942A | Alternaria sp. | Spiraea sp. | The plant pathogenic fungus Phytophthora capsici. | [112] |
| CN106520572A | Alternaria mali | Toona sinensis | The pathogens Pseudomonas aeruginosa or Proteus hauseri. | [113] |
| WO2008007251A2 | Alternaria alternata | Not disclosed | Plasmopara viticola. | [109] |
| CN108441426A | Aspergillus niger | Aquatic plant | Plant parasitic nematodes in soil. | [116] |
| CN104560735A | Aspergillus oryzae | Tephrosia purpurea | Plant pathogenic fungi such as Sclerotinia rot of colza and tobacco black shank disease. | [52] |
| CN102191185A | Aspergillus restrictus | Allium sativum | Plant pathogenic fungi such as Rhizoctonia solani and Thanatephorus cucumeris. | [117] |
| CN109504610A | Aspergillus sp. | Epiphyte | The pathogenic fungus rhizoctonia solani. | [118] |
| CN108342328A | Aspergillus versicolor | seaweed | Grass fungi. | [119] |
| US8709399B2 | Beauveria bassiana | maize stem borer Busseola fusca | Herbivorous insects and/or plant pathogens. | [149] |
| CN105462892A | Burkholderia sp. | Sophora tonkinensis | Panax notoginseng black spot. | [150] |
| CN105838613A | Chaetomium globosum | Cajanus cajan | Fungal plant diseases with the production of flavipin. | [151] |
| CN107475123A | Chaetomium globosum | Anoectochilus roxburghii | Plant disease in Anoectochilus roxburghii cultivation. | [16] |
| CN102742605A | Chaetomium globosum | Ginkgo biloba | Plant pathogenic fungi. | [122] |
| CN102690759A | Chaetomium globosum | Solidago canadensis | Plant pathogenic fungi propagation | [121] |
| CN101280320A | Chaetomium globosum | Not disclosed | Plant fungal diseases with the production of antibiotic substances | [120] |
| CN106754396A | Chaetomium globosum | Toona sinensis | Erwinia and Ralstonia solanacearum | [123] |
| CN104877919A | Chaetomium globosum | Phellopterus littoralis | Anthracnose pathogens of apples and certain inhibitory actions against other plant pathogens | [124] |
| CN103255065A | Chaetomium globosum | Camptotheca acuminata | Plant pathogens with broth culture of the endophytic fungi | [125] |
| CN102754652A | Chaetomium globosum | Ginkgo biloba | Exserohilum turcicum, Coniothyrium diplodiella, and Rhizopus stolonifer | [126] |
| CN105368720A | Chaetomium sp. | Healthy cotton plant | Cotton Verticillium wilt. | [152] |
| CN109749938A | Cladosporium tenuissimum | Healthy Panax notoginseng | Panax notoginseng rot. | [153] |
| CN110172408A | Clonostachys rosea | Podophyllum hexandrum | Diseases and pests of Podophyllum hexandrum. | [154] |
| CN110272829A | Colletotrichum boninense | Huperzia serrata | Sclerotinia sclerotiorum of crops. | [155] |
| WO2014136070A1 | Epichloë | Elymus mutabilis | Pests on Secale spp. plants. | [156] |
| CN105483022A | Fusarium solani | Sophora tonkinensis | Panax notoginseng black spot. | [127] |
| CN105483021A | Fusarium solani | Sophora tonkinensis | Panax notoginseng fungal diseases. | [128] |
| CN103194490A | Fusarium solani | Ginkgo biloba | Five plant pathogenic fungi. | [129] |
| CN105087386A | Fusarium sp. | Yinchuan Phragmites communis | Rice blast disease. | [130] |
| CN108624527A | Fusarium sp. | Ginkgo sp. | Bacterial wilt in ginger. | [132] |
| CN110558337A | Fusarium oxysporum | Ginkgo biloba | Rice blast disease. | [131] |
| CN102174416A | Fusella sp. | Angelica sinensis | Plant pathogenic bacteria. | [157] |
| WO2016034751A1 | Guignardia mangiferae | Persea indica | Phytopathogens and plant pests. | [158] |
| WO2013081448A2 | Hendersonia sp. | Not disclosed | Basal stem rot disease and Ganoderma disease in oil palms. | [159] |
| CN109536390A | Hypoxylon sp. nov | Midvein of citrus leaves | Citrus black spot disease. | [160] |
| CN103642704A | Leptosphaeria sp. | cotton | Cotton Verticillium wilt. | [161] |
| CN103289906A | Metarhizium sp. | Gentiana manshurica | G. manshurica leaf blight. | [162] |
| CN110229758A | Mortierella elongata | Atractylodes macrocephala | Atractylodes macrocephala root rot. | [163] |
| CN101691541A | Muscodor sp. | Not disclosed | Pathogenic fungi. | [164] |
| US20040141955A1 | Muscodor albus and Muscodor roseus | Not disclosed | Organisms such as microbes, insects, and nematodes with volatile compounds. | [165] |
| WO2002082898A1 | Muscodor albus and Muscodor roseus | Not disclosed | Plant pathogens, bacteria, nematodes, and insects with volatile antibiotics. | [166] |
| WO2010115156A2 | Muscodor strobelii | Not disclosed | Pests and pathogenic microbes, including Ganoderma boninense. | [167] |
| WO2004034785A2 | Muscodor vitigenus | Paullinia paullinioides | Insects with the production of repellents by a novel endophytic fungus. | [168] |
| CN106893678A | Myrothecium verrucaria | grapes | Grape gray mold. | [169] |
| CN104774768A | Nectria haematococca | Fritillaria wabuensis | Bacteria such as S. aureus and P. aeruginosa and pathogenic fungi. | [170] |
| CN106538108A | Neotyphodium sp. | gramineous plants | Mildewing rate of Elymus sibiricus seeds in the germination stage. | [133] |
| WO2007021200A1 | Neotyphodium sp. | Not disclosed | Plant pathogenic fungi. | [134] |
| CA2319847C | Neotyphodium sp. | Festuca arundinacea | Pests and reduce ergopeptine alkaloid levels. | [135] |
| CN102191186A | Nigrospora oryzae | Allium sativum | Plant pathogenic fungi such as Rhizoctonia solani, Colletotrichum lindemuthianum, and Botrytis cinerea. | [171] |
| CN104789482A | Nigrospora sp. | Magnolia officinalis | Wheat disease. | [172] |
| CN110178857A | Paecilomyces variotii | Hippophae rhamnoides | Plant virus. Induces plant endogenous salicylic acid accumulation and enhances the plant RNA silencing efficiency. | [173] |
| CN105462854A | Penicillium citrinum | Sophora tonkinensis | Panax notoginseng anthracnose. | [136] |
| CN105462850A | Penicillium citrinum | Sophora tonkinensis | Panax notoginseng root rot. | [137] |
| CN105462855A | Penicillium citrinum | Sophora tonkinensis Gagnep | Alternaria panax. | [139] |
| CN104531543A | Penicillium griseofulvum | Tephrosia purpurea | Plant diseases such as Sclerotinia rot of colza, tobacco blackleg, and others with a fermentation product. | [53] |
| CN105255742A | Penicillium sp. | Malus hupehensis | Harmful pathogens such as Fusarium solani, F. proliferatum, F. moniliforme, and F. oxysporum. | [140] |
| CN108546651A | Penicillium sp. | Kandelia candel | Plant pathogenic fungi such as Fusarium graminearum, Phytophthora sojae, and Colletotrichum musae with a fermentation product. | [141] |
| CN109112069A | Penicillium sp. | Panax notoginseng root | Panax notoginseng root rot. | [138] |
| CN103773699A | Penicillium purpurogenum | Litchi | Litchi downy blight. | [142] |
| CN103627643A | Penicillium simplicissimum | Healthy cotton plant | Cotton Verticillium wilt. | [174] |
| CN104161049A | Pestalotiopsis uvicola | Artemisia japonica | Kiwifruit Sclerotinia sclerotiorum, Phytophthora capsici, and other plant pathogenic fungi with a fermentation product. | [175] |
| CN110511878A | Pezicula neosporulosa | Fir | The pathogenic fungus Fusarium oxysporum. | [176] |
| CN109769535A | Phialophora oryzae | Wild rice root | Bacterial blight of rice. | [177] |
| CN102154116A | Phomopsis wenchengensis | Not disclosed | Plant pathogenic fungi by antifungal compounds. | [178] |
| CN105462853A | Rhexocercosporidium sp. | Sophora tonkinensis | Colletotrichum gloeosporioides on Panax notoginseng. | [143] |
| CN105462851A | Rhexocercosporidium sp. | Sophora tonkinensis | Fusarium solani on Panax notoginseng. | [144] |
| CN105462848A | Rhexocercosporidium sp. | Sophora tonkinensis | Alternaria panax Whetzel on Panax notoginseng. | [145] |
| CN102234618A | Rhizopus and Trichoderma | Not disclosed | Soft rot disease of the orchid family Dendrobium plants. | [179] |
| CN110452290A | Sarocladium brachiariae | Brachiaria brizantha | Plant disease and pests. | [180] |
| CN110468057A | Seimatosporium sp. | Rosa multiflora | Tobacco powdery mildew caused by Erysiphe cichoracearum. | [181] |
| CN106167767A | Schizothecium sp. | Not disclosed | Banana wilt. | [182] |
| CN110558336A | Spirillum roseum | Not disclosed | Lettuce sclerotinia rot. | [183] |
| CN103834580A | Talaromyces flavus | Not disclosed | Cotton Verticillium wilt | [184] |
| CN106119134A | Talaromyces flavus | Not disclosed | Fruit rot | [185] |
| CN109593658A | Talaromyces sp. | Fructus corni | Fungal diseases of wheat | [186] |
| CN105211105A | Trichothecium roseum | strawberries | Powdery mildew of wheat | [187] |
| US20120108425A1 | Trichoderma atroviride | healthy tea leaves | Foliar disease in tea plantations caused by Cercospora theae | [188] |
| CN108179115A | Zopfiella sp. | Chrysanthemum morifolium | Plant pathogens such as Fusarium moniliforme, F. oxysporum, Curvularia lunata, and Pythium | [189] |
| WO2018119419A1 | Group of several 2 fungi | cotton | Nematodes, aphids, flea hopper, lygus bug, stink bug, soy looper, cabbage looper, or fungi | [190] |
| US9469836B2 | Not disclosed | Pinus strobus | Pests in Pinus strobus | [191] |
1 Some patents just provided a common name for the host organism. 2 A list of the group of fungi is in Table S1.
Endophytic fungi of different genera such as Beauveria, Cladosporium, Metarhizium, Muscodor, Trichoderma, and others have also been described in patents to control pests or different plant diseases (Table 3).
3.3. Bio- and Phytoremediation
Bioremediation is a process that uses microorganisms, plants or enzymes to detoxify contamination in natural sources. In phytoremediation, plants and their own metabolic system can extract toxic chemicals from water, soil and air. This chemicals or contaminants include metals and metalloid pollutants, carcinogenic agents, industrial organic waste material, inorganic pesticides and herbicides, chlorinated products, excess nutrients and radionuclides [10,11,192].
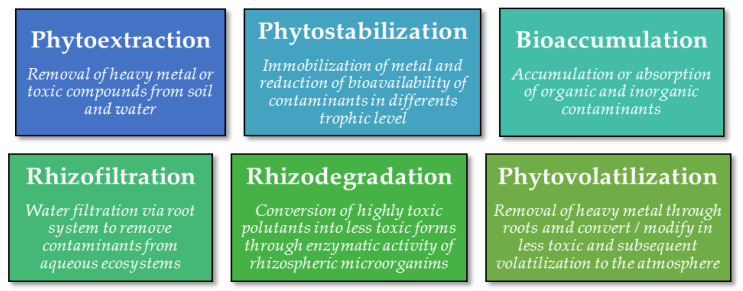
Endophytic fungi have the capability to degrade small and large organic compounds by enzymatic reactions, decompose environmental contaminants, and improve the soil microenvironment [193]. They can also increase the ability of host plants to remove contaminants from soil, water, sediment, and air [194], and to modulate morphological and physiological functions in the host plant improving its resistance to metals and providing different detoxification routes such as extracellular scavenging and complexation, compartmentalization and volatilization [14,195]. Figure 1 shows different bioremediation techniques involving endophytic fungi.
Figure 1.
Bio- and phytoremediation approaches involving endophytic fungi.
Some patents describe the use of endophytic fungi for bioremediation and phytoremediation (Table 4). Strains of the genus Fusarium have been reported to induce phytoremediation in heavy metal-contaminated soil [196], repair uranium-polluted water bodies [197], and decontaminate and decompose human and animal waste [198]. Additionally, the endophytic fungi Y2R14 and RWDL4-1 can be used to treat wastewater polluted by cadmium [199]. Heavy metals such as mercury, cadmium, arsenic, chromium, and lead are toxic at low concentrations. They can be accumulated in the ecosystem inside living organisms and are capable of entering the food chain [200]. The functions of several organs of the human body can be affected by heavy metals, and some of these substances can cause cancer by long-term exposure [200]. Uranium is a radioactive substance and is also harmful for the environment and human beings [197]. The use of microorganisms to repair large areas of farmland pollution can reduce costs, the use of large amounts of chemicals, and secondary pollution [196].
Table 4.
Endophytic fungi applied in bioremediation and phytoremediation.
| Patent No. | Endophyte | Host 1 | Patent Application | Ref. |
|---|---|---|---|---|
| CN105733958A | Fusarium oxysporum | Not disclosed | Phytoremediation of heavy metal-contaminated soil | [196] |
| CN106340337A | Fusarium sp. | mangrove | Repair of uranium-polluted water body | [197] |
| WO2005116272A2 | Fusarium culmorum and Muscodor albus | Not disclosed | Decontamination and decomposition of human and animal waste | [198] |
| CN106947697A | Phomopsis sp. | Not disclosed | Degradation of the herbicide MCPA (2-methyl-4-chlorophenoxyacetic acid) in water or soil | [201] |
| CN107177511A | Xylaria sp. | Not disclosed | Degradation of the herbicide MCPA in water and soil | [202] |
| CN107900098A | Group of several fungi 2 | Not disclosed | Production and application of a high-laccase content soil remediation agent | [203] |
| CN108751424A | Not disclosed | wild soybean | Treatment of wastewater polluted by the heavy metal cadmium | [199] |
1 Some patents just provided a common name for the host organism. 2 A list of the group of fungi is in Table S1.
Species of Phomopsis and Xylaria have been reported to degrade the herbicide MCPA (2-methyl-4-chlorophenoxyacetic acid) in water and soil [201,202]. Additionally, several genera of fungi can be used to produce high-laccase content for soil bioremediation [203].
3.4. Patents that Claim Multiple Applications
A small number of patents comprised more than one possible application (Table 5); this is the case of the applications for Neotyphodium uncinatum to induce insect resistant and drought tolerance in plants [204]; Phoma sp. can improve salt stress resistance, promote the growth and increase biomass in crop plants such as wheat and rice [205]; Clonostachys rosea promotes plant growth, stress resistance and reduces dependency on chemical pesticides [206,207]; Fusarium sp. stimulates plant growth and reduces heavy metal absorption in tobacco [208], and Rhizoctonia sp. fosters plant growth and stress resistance in Anoectochilus roxburghii [22].
Table 5.
Patents that claim multiple applications.
| Patent No. | Endophyte | Host 1 | Patent Application | Ref. |
|---|---|---|---|---|
| WO2000062600A1 | Neotyphodium uncinatum | meadow fescue | Import desired traits: include no adverse effects on herbivore, insect resistance, drought tolerance and improved persistence in the plants. | [204] |
| CN104293681A | Phoma sp. | Not disclosed | Improving salt stress resistance in rice and wheat. Promotion of growth in rice seedling, delaying salt damage of wheat in saline and alkaline land. Increasing biomass accumulation in wheat. |
[205] |
| US20160007613A1 | Clonostachys rosea | Not disclosed | Promotion of plant vigor, health, growth, yield, and resistance to competitive stress. | [206] |
| WO2007107000A1 | Clonostachys rosea | Not disclosed | Enhanced plant vigor, health, growth, yield, reducing environmental stress and reduction of dependency on chemical pesticides for pest control. | [207] |
| CN103849572A | Fusarium sp. | Not disclosed | Promoting plant growth and reduction of heavy metal absorption in tobacco. | [208] |
| CN101953261A | Rhizoctonia sp. | Anoectochilus roxburghii | Growth of A. roxburghii, improved the reproductive rate, survival rate and stress resistance. | [22] |
| WO2019115582A1 | Group of several fungi 2 | Hordeum murinum | Increased yield and biomass in cereal crops, and promotes biotic and abiotic stress resistance in cereal crops | [37] |
| WO2016030535A1 | Group of several fungi 2 | Hordeum murinum subsp. murinum | Improving dry shoot weight, mean dry grain weight and suppression of seed-borne infection in a cereal crop. | [35] |
1 Some patents just provided a common name for the host organism. 2 A list of the group of fungi is in Table S1.
We found two patents, whose applications implicated the use a plural number of fungi (genus/species); one of them claims the capability to increase biomass and promote biotic and abiotic stress resistance in cereal crops [37], the other claims to improve dry shoot weight, mean dry grain weight and suppression of seed-borne in cereal crops [35].
4. Discussion
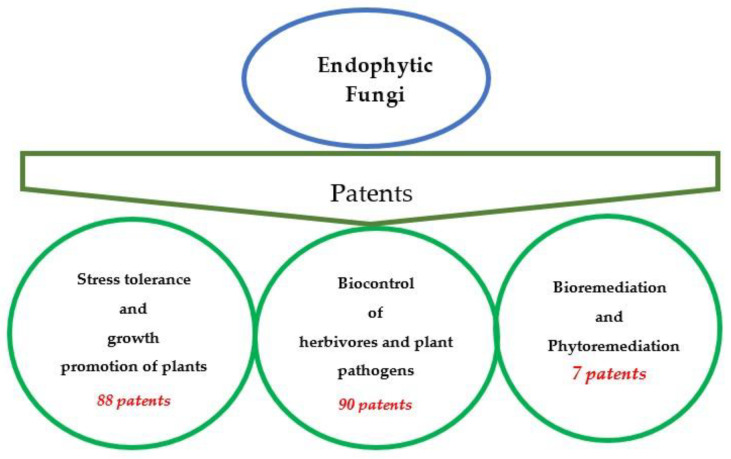
In the present review, we highlight a wide number of endophytic fungi that have been patented for developing processes, methodologies, or new techniques in applications that include but are not restricted to (a) alternatives to overcome biotic and abiotic stress and to reduce the use of chemicals associated with environmental toxicity in agricultural practices, (b) the degradation of harmful compounds, and (c) improvement in the ability of plants to remove contaminants from soil, water, and air. Abiotic stress tolerance and growth promotion of plants, and biocontrol of herbivores and plant pathogens, were the most patentable applications of endophytic fungi with 88 and 90 patents, respectively; concerning bio- and phytoremediation, 7 patents were recorded for the period 1988–2019 (Figure 2). The most representative genera of these applications belong to Alternaria, Aspergillus, Chaetomium, Fusarium, Penicillium and Muscodor.
Figure 2.
Total number of patents for area of application in the period 1988 to 2019.
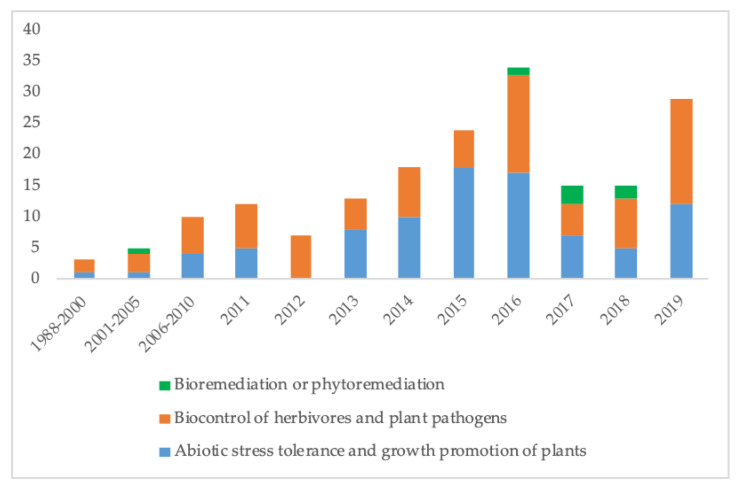
Studies of endophytic fungi ecology have allowed the understanding of the multiple interactions they develop with their host, other endophytes, as well with herbivores and pathogens that put the host under abiotic stress. Nonetheless, it is evident that one individual or group of endophytes can be used for mitigation stresses from different origins. Due to the concerns about global climate change and its implications in food security, there are an increased interest to develop applications for the use of endophytic fungi in abiotic stress tolerance and growth promotion of important food crops [209], as well as the use for biocontrol of herbivores and plant pathogens. This increment can be noted since 2011 as shown in Figure 3. The loss of growing areas due to contamination and the recovery of spaces contaminated by heavy metals, organic and inorganic compounds will lead the focus of research on endophytic fungi for bio- and phytoremediation applications.
Figure 3.
Patents of endophytic fungi for agricultural purposes and bio/phytoremediation registered from 1988 to 2019.
Considering the abundance of endophytic fungi under study, the development of patentable applications like those reviewed here, and other applications still unexplored like fungal pigments [210], has become a prominent research area for this class of microorganisms.
Future Perspectives
The use of endophytic fungi to improve the nutrients absorption in plants can change the optimum usage of organic and inorganic fertilizers [211]. The capability of endophytic fungi to increase biotic and abiotic stress tolerance in plant hosts is an unexplored area for agricultural purposes; the control of pests and diseases under climate change conditions [211]; studies in fungal species related to develop resistance to changes in their environment could lead their application in food production in limited resources areas and as an important alternative for crop production for human sustainability. Many endophytes are now often recognized as symbionts with unique and intimate interactions with the plant host [10]. The genetic engineering of fungi is an easier process than in plants. The genetic modification of endophytic fungi with useful genes could contribute, with new traits, to the inoculation of plants [212].
The use of endophytic fungi on remediation of contaminated ecosystems is an interesting prospect for further studies. Fungi that could increase the capacity of CO2 absorption by plants, degradation and biotransformation of waste, enhance food production without altering its quality or those that provided drought resistance/nutrient absorption capability to plant species related to human or animal feeding could be areas of significance to develop new applications and patents. The investigations applied in these fields are forwarded by the advance in the techniques used for the characterization of endophytic fungi and also by the technological advances in analytical techniques for carrying out studies of chemical processes at the cellular level.
Acknowledgments
The authors want to thank University of Sao Paulo, Brazil, for granted access to “Portal de Periodicos CAPES/MEC” and to Phyllis D. Coley for critical review of the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/8/1237/s1, Table S1: List of patens grounded in the use of several endophytic fungi to develop applications.
Author Contributions
H.E.O. and D.T.-M. performed the data search and organized and analyzed the data, visualized and wrote the manuscript; L.C.-R. conceptualized, visualized, supervised, wrote and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This project was supported by the National System of Research (SNI) and the National Secretariat for Science and Technology of Panama (SENACYT).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Petrini O. Fungal Endophytes of Tree Leaves. In: Andrews J.H., Hirano S.S., editors. Microbial Ecology of Leaves. Springer; New York, NY, USA: 1991. pp. 179–197. [DOI] [Google Scholar]
- 2.Tan R.X., Zou W.X. Endophytes: A Rich Source of Functional Metabolites. Nat. Prod. Rep. 2001;18:448–459. doi: 10.1039/b100918o. [DOI] [PubMed] [Google Scholar]
- 3.Torres-Mendoza D., Ortega H.E., Cubilla-Rios L. Patents on Endophytic Fungi Related to Secondary Metabolites and Biotransformation Applications. J. Fungi. 2020;6:58. doi: 10.3390/jof6020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll G. Fungal Endophytes in Stems and Leaves: From Latent Pathogen to Mutualistic Symbiont. Ecology. 1988;69:2–9. doi: 10.2307/1943154. [DOI] [Google Scholar]
- 5.Hallmann J., Sikora R.A. Toxicity of Fungal Endophyte Secondary Metabolites to Plant Parasitic Nematodes and Soil-Borne Plant Pathogenic Fungi. Eur. J. Plant Pathol. 1996;102:155–162. doi: 10.1007/BF01877102. [DOI] [Google Scholar]
- 6.Sturz A., Nowak J. Endophytic Communities of Rhizobacteria and the Strategies Required to Create Yield Enhancing Associations with Crops. Appl. Soil Ecol. 2000;15:183–190. doi: 10.1016/S0929-1393(00)00094-9. [DOI] [Google Scholar]
- 7.Azevedo J.L., Araujo W.L. Diversity and Applications of Endophytic Fungi Isolated from Tropical Plants. In: Ganguli B.N., Deshmukh S.K., editors. Fungi: Multifaceted Microbes. CRC Press; New Delhi, India: 2007. pp. 189–207. [Google Scholar]
- 8.Bamisile B.S., Dash C.K., Akutse K.S., Keppanan R., Afolabi O.G., Hussain M., Qasim M., Wang L. Prospects of Endophytic Fungal Entomopathogens as Biocontrol and Plant Growth Promoting Agents: An Insight on How Artificial Inoculation Methods Affect Endophytic Colonization of Host Plants. Microbiol. Res. 2018;217:34–50. doi: 10.1016/j.micres.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Bilal S., Shahzad R., Imran M., Jan R., Kim K.M., Lee I.J. Synergistic Association of Endophytic Fungi Enhances Glycine max L. Resilience to Combined Abiotic Stresses: Heavy Metals, High Temperature and Drought Stress. Ind. Crops Prod. 2020;143:111931. doi: 10.1016/j.indcrop.2019.111931. [DOI] [Google Scholar]
- 10.Deng Z., Cao L. Fungal Endophytes and Their Interactions with Plants in Phytoremediation: A Review. Chemosphere. 2017;168:1100–1106. doi: 10.1016/j.chemosphere.2016.10.097. [DOI] [PubMed] [Google Scholar]
- 11.Nandy S., Das T., Tudu C.K., Pandey D.K., Dey A., Ray P. Fungal Endophytes: Futuristic Tool in Recent Research Area of Phytoremediation. S. Afr. J. Bot. 2020 doi: 10.1016/j.sajb.2020.02.015. [DOI] [Google Scholar]
- 12.Yan L., Zhu J., Zhao X., Shi J., Jiang C., Shao D. Beneficial Effects of Endophytic Fungi Colonization on Plants. Appl. Microbiol. Biotechnol. 2019;103:3327–3340. doi: 10.1007/s00253-019-09713-2. [DOI] [PubMed] [Google Scholar]
- 13.Ripa F.A., Cao W., Tong S., Sun J. Assessment of Plant Growth Promoting and Abiotic Stress Tolerance Properties of Wheat Endophytic Fungi. Biomed. Res. Int. 2019;2019:1–12. doi: 10.1155/2019/6105865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He W., Megharaj M., Wu C.Y., Subashchandrabose S.R., Dai C.C. Endophyte-Assisted Phytoremediation: Mechanisms and Current Application Strategies for Soil Mixed Pollutants. Crit. Rev. Biotechnol. 2020;40:31–45. doi: 10.1080/07388551.2019.1675582. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z., Liu Y., Wang Q., Wang C., Shen H. A Kind of Method for Promoting the Growth of Manyprickle Acanthopanax Root at Seedling Stage. CN 108513990 A. 2018 Sep 11;
- 16.Zheng C., Wu J., Wu Y., Qin L., Ye B., Zhai X., Han T., Xin H. Anoectochilus Endophytic Fungi and Its Application. CN 107475123 A. 2017 Dec 15;
- 17.Ding C., Wang Q., Wang Z., Li J., Zhang S. Can Promote Hair Pulse Acid Mold Growth of Endophytic Fungi and Its Application. CN 106929436 A. 2017 Jul 7;
- 18.Lin H., Fan H., Hong T., Zhou Y., Wu C., Chen C. Mixed Endophyte Fungi Capable of Promoting Nutrient Absorption of Acacia confusa. CN 106085872 A. 2016 Nov 9;
- 19.Qin L., Jia M., Yang Y., Han T., Xin H., Zhang Q., Li Y., Kong Z. Coix lacryma-jobi Seed Endophytic Fungi and Its Application. CN 105624047 A. 2016 Jun 1;
- 20.Cui J., Zhang Y. By Fungi for Promoting Seed Germination Method for Cynomorii Herba. CN 107432135 A. 2017 Dec 5;
- 21.Guo B., He M., Chen X., He W., Wei Y. One Kind of Lycopodium serratum Endophytic Pythium and Its Application. CN 105039172 A. 2015 Nov 11;
- 22.Fang X., Jiang Q., Zheng G. Application of Endophytic Fungus Rhizoctonia in Raising Seedlings of Anoectochilus roxburghii. CN 101953261 A. 2011 Jan 26;
- 23.Han T., Qin L., Jia X., Zheng C., Jia M. A Lithospermum euchromum Endophytic Fungus and Its Application. CN 104745482 A. 2015 Jul 1;
- 24.Guo S., Wu L., Chen X., Wang C., Meng Z., Xiao P. Application of Dark Septate Endophyte in Culture of Saussurea. CN 1961631 A. 2007 May 16;
- 25.Li L., Chen J., Huang R. A Rhizoma bletillae Endophytic Fungus 1-N2 and Application Thereof. CN 109456902 A. 2019 Mar 12;
- 26.Chen H., Liang Z., Ma Y., Zhang H., Zhang X., Yang D., Hu X. Cladosporium tenuissimum and Application Thereof for Promoting Synthesis of Effective Components in Root System of Salvia miltiorrhiza. CN 110438011 A. 2019 Nov 12;
- 27.Chen H., Liang Z., Wu H., Yan B., Yu H., Zhang H., Hu X., Yang D. A Kind of Salvia miltiorrhiza Endophytic Fungi and Its Application in Promoting Growth and/or Effective Ingredient and Application in Synthesis. CN 109706084 A. 2019 May 3;
- 28.Hong W., Wu C., Xie A. Endophytic Fungus Penicillium sp. Strain 1 Capable of Improving Cold Resistance of Eucalyptus and Its Application. CN 102002463 A. 2011 Apr 6;
- 29.Hong W., Wu C., Xie A. Penicillium sp. Strain 1 as Endophytic Fungus of Eucalyptus and Its Application in Increasing Low-Phosphorus Stress Tolerance and Promoting Phosphorus Absorption of Eucalyptus. CN 101974438 A. 2011 Feb 16;
- 30.Hong W., Wu C., Xie A. Penicillium sp. Strain 2 as Endophytic Fungus of Eucalyptus, and Preparation Method of Its Microbial Solution and Its Application in Alleviating Aluminum Poisoning. CN 101974437 A. 2011 Feb 16;
- 31.Tao C., Yang T., Yang Z., Hu W., Ma X., Wang N., Xiao J., Wang H., Zhao Y., Chen X., et al. Endophytic Fungus of Panax ginseng for Promotion Growth of Corn. CN 102086439 A. 2011 Jun 8;
- 32.Lopez Llorca L.V., Zavala Gonzalez E.A., Ramirez Lepe M. Use of Pochonia chlamydosporia to Promote Flowering and Fruiting in Cultivated Crops. ES 2500790 A1. 2014 Sep 30;
- 33.Djonovic S., McKenzie E.A., Toledo G.V., Sadowski C., Von Maltzahn G., Ambrose K.V., Zhang X., Johnston D.M., Gulick T.A. Penicillium Endophyte Compositions and Methods for Improved Agronomic Traits in Plants. WO 2016210238 A1. 2016 Dec 29;
- 34.Du Y. Preparation Method and Application of Panax pseudoginseng and/or Panax notoginseng Endophytic Fungus Acremonium strictum. CN 105907648 A. 2016 Aug 31;
- 35.Murphy B., Hodkinson T., Doohan F. Fungal Endophytes for Improving the Mean Dry Shoot Weight, Mean Dry Grain Weight, and Suppressing Seed-Borne Infection in Barley. WO 2016030535 A1. 2016 Mar 3;
- 36.Bi Y., Xue Z., Quan W. Optimal Dse Strain for Promoting Corn Seed Soaking Rooting. CN 110250210 A. 2019 Sep 20;
- 37.Murphy B., Doohan F., Hodkinson T. Endophytes from Wild Populations of Barley Increase Crop Yield. WO 2019115582 A1. 2019 Jun 20;
- 38.Hong W., Wu C., Xie A., Lin Y., Li J. An Endophytic Fungus Capable of Promoting the Photosynthesis of Casuarina equisetifolia. CN 103173362 A. 2013 Jun 26;
- 39.Hong W., Wu C., Xie A., Lin Y., Lin H. Endophytic Fungus Capable of Promoting Nutrient Element Absorption of Casuarina. CN 103173361 A. 2013 Jun 26;
- 40.Wu C., Hong W., Xie A., Lin Y., Lin Y. Phyllosticta sp. for Promoting Photosynthesis of Casuarina equisetifolia. CN 103173363 A. 2013 Jun 26;
- 41.Xie A., Hong W., Wu C., Lin Y., Hong T. One Strain of Endophytic Fungus Which Can Promote Casuarina Biomass. CN 103173364 A. 2013 Jun 26;
- 42.Lin H., Xu C., Hong W., Wu C., Xie A., Li J. Endophytic Fungus Capable of Promoting Growth of Fir. CN 104004666 A. 2014 Aug 27;
- 43.Wu C., Xie A., Xu C., Hong W., Lin Y. Endophytic Fungus for Reliving Phosphorus Stress in Fir. CN 104004665 A. 2014 Aug 27;
- 44.Xie A., Hong W., Wu C., Xu C., Fan H. Endophytic Fungus Capable of Promoting Phosphorus Absorption of Fir. CN 104004667 A. 2014 Aug 27;
- 45.Xu C., Wu C., Xie A., Hong W., Hong T. Endophytic Fungus Capable of Promoting Photosynthesis of Fir. CN 104004664 A. 2014 Aug 27;
- 46.Hong T., Liu J., Wu C., Lin H., Xie A., Hong C. A Strain of Endophyte Fungi for Promoting Millennium Tong Photosynthesis under Low Phosphorus Environment. CN 104774771 A. 2015 Jul 15;
- 47.Hong C., Gong H., Lin H., Zhang G., Hong T., Wu C. An Endophytic Fungi for Promoting Phosphorus Uptake of Aleurites montana. CN 104818218 A. 2015 Aug 5;
- 48.Hong C., Lin H., Xie A., Hong T., Wu C., Hong W. Endophyte Fungi That Promotes Aleurites montana Growth and Photosynthesis at Low Phosphorus Environment. CN 104789481 A. 2015 Jul 22;
- 49.Lin H., Chen J., Hong T., Hong C., Xie A., Wu C. An Endophytic Fungus Strain for Promoting Aleurites montana Biomass Growth in Low-p Environment. CN 104762219 A. 2015 Jul 8;
- 50.Lin H., Hong C., Hong T., Su S., Wu C., Xie A. Endophyte That Can Promote the Growth of Biomass in Millennium Tong. CN 104818217 A. 2015 Aug 5;
- 51.Xie A., Hong T., Hong C., Lin H., Li J., Wu C. An Endophytic Fungus for Promoting the Growth of Aleurites montana Root System under Low Phosphorus Environment. CN 104818219 A. 2015 Aug 5;
- 52.Li Y., Luo Z., Ding W. Tephrosia purpurea Endophytic Fungi Tpl35 and Its Application in Preventing and Controlling Plant Diseases. CN 104560735 A. 2015 Apr 29;
- 53.Li Y., Luo Z., Ding W., Zou K. Herba Tephrosiae purpureae Endophytic Fungi Tpl25 and Its Application in Preventing and Controlling Plant Diseases. CN 104531543 A. 2015 Apr 22;
- 54.Wu L., Si J., Dong H., Han T., Zhu B. A Dendrobium officinale Endophytic Fungi and Its Application. CN 105886405 A. 2016 Aug 24;
- 55.Xia C., Xu Q., Zhou G., Jin H., Zhang Y., Wu J., Li Y., Zhang C., Lin F., Liu H., et al. Endophytic Fungi Strain Ycef193 and Its Use. CN 103834578 A. 2014 Jun 4;
- 56.Xia C., Xu Q., Cheng C., Jin H., Liu H., Zhang Y., Li Y., Zhang L., Li X., Zhang C., et al. An Endogenous Fungal Strain Nyn8g01 and Its Applications. CN 105316240 A. 2016 Feb 10;
- 57.Zhang C., Lin F., Liu H., Feng X. Endophytic Fungal Strain R5-6-1 Application. CN 103865806 A. 2014 Jun 18;
- 58.Jin H., Li X., Zhang C., Feng X., Liu H., Lin F., Xu Q., Cheng C., Xiang B., Li D., et al. An Endogenous Fungal Strain Nyn771c06 and Its Applications. CN 105296359 A. 2016 Feb 3;
- 59.Li J., Xu H., Wu C., Lin Y., Hong T., Lin H. Endophytic Fungus Capable of Improving Photosynthesis of Schima superba. CN 110257259 A. 2019 Sep 20;
- 60.Li J., Xu H., Wu C., Lin Y., Hong T., Lin H. Endophytic Fungus Capable of Promoting Phosphorus Absorption of Schima superba. CN 110257258 A. 2019 Sep 20;
- 61.Li J., Xu H., Wu C., Lin Y., Lin H., Hong T. Endophytic Fungus Capable of Promoting Growth of Schima superba Seedling Height and Ground Diameter under Low-Phosphorus Environment. CN 110343619 A. 2019 Oct 18;
- 62.Huang R., Tian P., Li L. Bletilla Striata Endophytic Fungus 3-G2 and Application Thereof. CN 109628322 A. 2019 Apr 16;
- 63.Li L., Tian P., Huang R. A Bletilla Striata Endophytic Fungi Strain 1-G1 and Application Thereof. CN 109504611 A. 2019 Mar 22;
- 64.Huang J., Zhang S., Zhu X., Qin J., Hu H. Tulasnella Calospora Qs104, Application Thereof and Method for Promoting Growth of Aseptic Seedling of Paphiopedilum. CN 110408551 A. 2019 Nov 5;
- 65.Zhou F., Huo G., Gu Z., Hua C. One Kind of Anti-Salt Stress Fungal Strain and Breeding Methods and Their Application. CN 104762216 A. 2015 Jul 8;
- 66.Henson J.M., Sheehan K.B., Rodriguez R.J., Redman R.S. The Use of Endophytic Fungi to Treat Plants. WO 2004000017 A2. 2003 Dec 31;
- 67.Redman R.S., Rodriguez R.J. Fungal Isolates and Their Use to Confer Salinity and Drought Tolerance in Plants. WO 2009012480 A2. 2009 Jan 22;
- 68.Ren A., Gao Y., Chen L., Zhao N., Wang Y., Xie F. Method for Transferring Fungal Endophyte of Wild Grasses to Turf Grasses for Improving Stress Tolerances to Drought and Diseases. CN 101314760 A. 2008 Dec 3;
- 69.An H., Luo X., Dong J. Heavy Metal-Resistant Endophytic Fungi Paraconiothyrium cyclothyrioides Mr2-1 and Application Thereof. CN 105002099 A. 2015 Oct 28;
- 70.Lv M., Zhou W., Wang J., Li L., Xu L., Bai Q., Song W., Zhu J. Method for Improving Crop Resistance to Herbicide Bensulfuron-Methyl Using Piriformospora indica. CN 107926549 A. 2018 Apr 20;
- 71.Vujanovic V., Germida J.J. Endophytic Microbial Symbionts in Plant Prenatal Care. US 20150366217 A1. 2015 Dec 24;
- 72.Zhao Y., Xiao J., Ma X., Wang H., Chen X., Yang T., Yang Z., Wang Y., Ren Z. Seabuckthorn Endogenous Fungi and Their Extracts for Use to Promote Drought Resistance of Lawn Grass. CN 104911108 A. 2015 Sep 16;
- 73.Zheng C., Qin L., Zhai X., Li X., Han T., Zhang Q., Jiang Y., Jia M. Improving Salviae miltiorrhizae radix Yield and Effective Ingredient Content of Endophytic Fungi and Its Application. CN 106801014 A. 2017 Jun 6;
- 74.Li W., Wang L., Ma Z., Tang C., Zhang C., Hu Z., Wang Z. Dendrobium officinale Endophytic Fungi Strains Nt66g01 and Its Applications. CN 104630073 A. 2015 May 20;
- 75.Wu C., Hong C., Hong T., Xiao Y., Lin H., Chen C. An Endophytic Fungi for Promoting Nutrient Absorption of Millennium Tung. CN 104805019 A. 2015 Jul 29;
- 76.Wei X., Wang F., Ma C., Wan S., Jing M., Liu Y., Wang Z. Method for Artificially Planting Salvia miltiorrhiza. CN 103733829 A. 2014 Apr 23;
- 77.Li J., Lin H., Zhou Y., Chen C., Xie A., Fan H. Can Promote the Growth of Taiwan Acacia Biomass Endophytic Fungi. CN 105861334 A. 2016 Aug 17;
- 78.Hong T., Lin Y., Lin H., Zhou Y., Xie A., Chen C. A under Low Phosphorus Environment Promoting Taiwan Acacia Nutrient Absorption of Endophytic Fungi. CN 105861335 A. 2016 Aug 17;
- 79.Wu C., Lin H., Zhou Y., Chen C., Xie A., Li J. Mixed Endophytic Fungi for Promoting Acacia confusa Phosphorus Uptake under Low Phosphorus Environment. CN 106085873 A. 2016 Nov 9;
- 80.Wu C., Hong W., Xie A., Lin Y., Fan H. One Endophytic Fungus Capable of Increaing the Chlorophyll Content of Casuarina. CN 103173360 A. 2013 Jun 26;
- 81.Zhang C., Feng X., Su Z., Lin F. Endophytic Fungi Strain RR21 and Its Application in Plant Growth Regulation and/or Plant Pathogenicity. CN 103114044 A. 2013 May 22;
- 82.Ming Q., Qin L., Han T., Zhang Q., Zheng C., Zhang H., Jia M. Preparation and Application of Water-Soluble Extract of Endophytic Fungus Hypha in Salvia miltiorrhiza and Part of the Extract. CN 103798293 A. 2014 May 21;
- 83.Li W., Wang L., Ma Z., Tang C., Zhang C., Hu Z., Wang Z. Dendrobium officinale Endophytic Fungi Strain Nt04y01 and Its Application. CN 104593274 A. 2015 May 6;
- 84.Spangenberg G.C., Guthridge K.M., Forster J.W., Sawbridge T.I., Ludlow E.J.I., Kaur J., Rochfort S.J., Rabinovich M.A., Ekanayake P. Neotyphodium Endophytes of Perrenial Ryegrass and Tall Fescue with Beneficial Properties for Plant Growth. US 20130104263 A1. 2013 Apr 25;
- 85.Xie A., Chen C., Lin H., Wu C., Hong T., Zhou Y. A under Low Phosphorus Environment for Promoting Growth of Taiwan Acacia Biomass Endophytic Fungi. CN 106010984 A. 2016 Oct 12;
- 86.Yao Q., Chen M., Zhou Y., Zhu H. Blueberry Endogenetic Fungus Strain with Growth-Promoting Function and Application Thereof. CN 107988087 A. 2018 May 4;
- 87.Li W., Wang L., Ma Z., Tang C., Zhang C., Hu Z., Wang Z. Dendrobium officinale Endophytic Fungi Strain Nt43j06 and Its Application. CN 104593273 A. 2015 May 6;
- 88.Lopez Llorca L.V., Zavala Gonzalez E., Ramirez Lepe M. Use of Pochonia chlamydosporia to Promote Flowering and Fruiting in Cultivated Crops. WO 2016038234 A1. 2016 Mar 17;
- 89.Mao L., Zhang C., Lin F., Feng X. Application of Endophytic Fungus Strain NYN8G01 in Promoting Rice Growth. CN 108041078 A. 2018 May 18;
- 90.Craven K., Ray P. A Symbiont Serendipita vermifera bescii for Enhancement of Plant Performance. WO 2019113255 A1. 2019 Jun 13;
- 91.Han T., Chen L., Qin L., Xin H., Zheng C., Jia M., Zhai X., Shen H., Yang Y. Radix ginseng Endophytic Fungi and Its Application. CN 105420119 A. 2016 Mar 23;
- 92.Zhang L. Anoectochilus formosanus Seedling Adaptation Cultivation Method. CN 107046965 A. 2017 Aug 18;
- 93.Zhou Y., Lin H., Chen C., Lin Y., Hong T., Wu C. Mixed Endophytic Fungi for Promoting Growth of Height and Ground Diameter of Acacia confusa Seedling. CN 105969672 A. 2016 Sep 28;
- 94.Zhang C., Lin F., Feng X., Li Y. Xylaria striata for Promoting Plant Growth and Increasing Plant Biomass. CN 102876584 A. 2013 Jan 16;
- 95.Lan T., Xie L., Zhang Y., Chen Y., Zhang W., Su Q., Qin L., Huang C., Lu J. Dark with Spacer Endophytic Fungi Hs40 Thereof in Herba Dendrobii Herba Production Application. CN 107460133 A. 2017 Dec 12;
- 96.Ambrose K.V., Boghigian B.A., Djonovic S., Gray P.A., Toledo G.V., Marquez L.M., Pelaez J.N., Von Maltzahn G. Designed Complex Endophyte Compositions and Methods for Improved Plant Traits. WO 2016179047 A1. 2016 Nov 10;
- 97.Mrnka L., Schmidt C.S., Frantik T., Vosatka M., Jandejsek Z., Fulin T., Kastanek P., Kronusova O., Lovecka P., Demnerova K. A Mixture of Endophytic Fungi for Increasing the Production of Biomass, the Method of Its Preparation and Its Use. CZ 306950 B6. 2017 Oct 4;
- 98.Sharon A., Gur Y., Ofek-Lalzar M., Llorens E., Sharon O. Fungal Endophyte Species for Agrochemical Uses. WO 2017134664 A1. 2017 Aug 10;
- 99.Von Maltzahn G., Flavell R.B., Toledo G.V., Leff J.W., Samayoa P., Marquez L.M., Johnston D.M., Djonovic S., Millet Y.A., Sadowski C., et al. Endophytes, Associated Compositions, and Methods of Use Thereof. US 20150373993 A1. 2015 Dec 31;
- 100.Riley R., Djonovic S., Vosnidou N., Bitas V. Novel Endophytic Microbes for Modulation of the Nutritional Quality Traits in Seeds. WO 2018102733 A1. 2018 Jun 7;
- 101.Latz M.A.C., Jensen B., Collinge D.B., Jørgensen H.J.L. Endophytic Fungi as Biocontrol Agents: Elucidating Mechanisms in Disease Suppression. Plant Ecol. Divers. 2018;11:555–567. doi: 10.1080/17550874.2018.1534146. [DOI] [Google Scholar]
- 102.Calvo-Polanco M., Sánchez-Romero B., Aroca R. Arbuscular mycorrhizal fungi and the tolerance of plants to drought and salinity. In: Aroca R., editor. Symbiotic Endophytes. Springer; Berlin/Heidelberg, Germany: 2013. pp. 271–288. [Google Scholar]
- 103.Bacon C.W., White J.F. Functions, Mechanisms and Regulation of Endophytic and Epiphytic Microbial Communities of Plants. Symbiosis. 2016;68:87–98. doi: 10.1007/s13199-015-0350-2. [DOI] [Google Scholar]
- 104.Pandey P.K., Samanta R., Yadav R.N.S. Inside the Plant: Addressing Bacterial Endophytes in Biotic Stress Alleviation. Arch. Microbiol. 2019;201:415–429. doi: 10.1007/s00203-019-01642-y. [DOI] [PubMed] [Google Scholar]
- 105.Feng Z., Zhu H., Li Z., Huang D., Wang L., Shi Y., Zhao L. A Kind of Endophytic Fungi Cef-193 of Cotton and Application Thereof. CN 103897992 A. 2014 Jul 2;
- 106.Latch G.C.M., Fletcher L.R., Rolston M.P., Easton H.S., Popay A.J., Tapper B.A., Rowan D.D., Christensen M.J. Endophytic Fungi: Acremonium lolii. AU 639084 B2. 1993 Jul 15;
- 107.Huang G., Guo Z., Cai J., Shi T., Liu X. Acremonium strictum HND5 Isolated from Leaf of Brachiaria sp. and Its Application in Biocontrol of Plant Diseases. CN 101235355 A. 2008 Aug 6;
- 108.Lipham L.B., Stuedemann J.A., Thompson F.N., Jr. Method and Compositions for Prevention and Treatment of Fescue Toxicosis Using Dopamine Antagonists Specific for D2 Receptors. US 93951 A0. 1988 Mar 1;
- 109.Musetti R., Borselli S., D’Ambrosio M. Antifungal Compositions Containing the Endophyte Fungus Alternaria alternata and/or Its Metabolites Belonging to the Family of Diketopiperazines, as Antagonist Agents of Plasmopara viticola. WO 2008007251 A2. 2008 Jan 17;
- 110.Shentu X., Yu X., Dong S., Hao P., Bian Y., Ma Z. Strain of Alternaria alternata 31 as Biocontrol Fungus. CN 102191184 A. 2011 Sep 21;
- 111.Yu X., Shentu X., Dong S., Hao P., Bian Y., Ma Z. Application of Metabolites of Alternaria alternata 31 in Preventing and Treating Rhizoctonia solani, Fusarium oxysporium, and Botrytis cinerea. CN 102204570 A. 2011 Oct 5;
- 112.Hua R., Xu Q., Bai Y., Zeng X., Cao H., Wu X. Endophytic Fungus of Spiraea for Biocontrol of Plant Pathogens. CN 103232942 A. 2013 Aug 7;
- 113.Wang Z., Wang X., Zhao H., Shi G., Zhao S., Yang H., Zhang Y. Toona sinensis Endophytic Fungus 56-50 (Alternaria mali), Its Secondary Metabolite, Preparation Method and Application in Degrading Pseudomonas aeruginosa or Proteus. CN 106520572 A. 2017 Mar 22;
- 114.Cosoveanu A., Cabrera Perez R. Extracts Obtained from Endophytic Fungi HTF58 Alternaria alternata and HRO8 Fusarium acuminatum of Artemisa thuscula and Austrian artemis as Antifungals for Agricultural Use. ES 2696982 A1. 2019 Jan 21;
- 115.Shu S., Cui L., Yan L. An Endophytic Fungus of Huperzia serrata Resistant to Gray Mold and Application Thereof. CN 110373331 A. 2019 Oct 25;
- 116.Yu Z., Qiao M. A Kind of Endophytic Bacterial for Preventing and Treating Plant Nematodes. CN 108441426 A. 2018 Aug 24;
- 117.Shentu X., Yu X., Dong S., Hao P., Bian Y., Ma Z. Strain of Aspergillus restrictus 28 as Biocontrol Fungus. CN 102191185 A. 2011 Sep 21;
- 118.Wang J., Zhang P., Wu P., Wang G., Wang C. Plant Endophytic Fungus Aspergillus sp. Mbl1612 Extract and Application Thereof. CN 109504610 A. 2019 Mar 22;
- 119.Han X., Ji J., Zhao J., Zhao D., Liu M., Zhang C., Peng Y., Li Y., Liu J., Zhang Z., et al. Herbicidal Fungus Screened from Seaweed and Its Extracts and Application in Weed Control of Farmland. CN 108342328 A. 2018 Jul 31;
- 120.Gao K., Liu X., He B., Li C. Method for Producing Antibiotic Substance from Plant Endophytic Fungus Chaetomium globosum ND35. CN 101280320 A. 2008 Oct 8;
- 121.Guo Z., Hua R., Bai Y., Cao H., Wu X., Li X., Tang J. Separating and Purifying Endogenous Endophytic Fungus of Solidago canadensis for Control of Phytopathogenic Fungi. CN 102690759 A. 2012 Sep 26;
- 122.Liu J., Zhang G., Pan H., Qin J., Qu X., Mo H., Zhang J., Zhang Y. Application of Chaetomium globosum NO.04 for Preventing and Treating Plant Pathogenic Fungi. CN 102742605 A. 2012 Oct 24;
- 123.Wang Z., Wang X., Zhang Y., Shi G., Zhao S., Zhao H., Yang H. Toona sinensis Endophytic Fungus TS8 and Its Secondary Metabolites, Preparation Method and Application Thereof. CN 106754396 A. 2017 May 31;
- 124.Zhang P., Mao Z., Cheng S. Chaetomium globosum and Its Application. CN 104877919 A. 2015 Sep 2;
- 125.Hua R., Zeng X., Bai Y., Xu Q., Cao H., Wu X. Endophytic Fungus of Camptotheca acuminata for Biocontrol of Phytopathogenic Fungi. CN 103255065 A. 2013 Aug 21;
- 126.Qin J., Zhang G., Pan H., Li X., Zhang Y., Mo H., Tian Y., Cheng H. Application of Chaetoglobosin A in Preparing Microbial Pesticide for Control of Northern Leaf Blight of Corn, Grape White Rot and Tomato Rhizopus Fruit Rot. CN 102754652 A. 2012 Oct 31;
- 127.Zhang P., Li L., Huang R., Yao Y. Sophora Tonkinensis Gagnep. Endogenetic Fungus TRXY-34-1 and Preparation Method of Metabolite Thereof for Preventing and Treating Black Spot of Radix notoginseng. CN 105483022 A. 2016 Apr 13;
- 128.Huang R., Lan F., Yao Y., Li L. Sophora tonkinensis gagnep. Endogenetic Fungus TRXY-34-1 and Preparation Method of Metabolite Thereof for Preventing and Treating Anthracnose of Radix notoginseng. CN 105483021 A. 2016 Apr 13;
- 129.Hua R., Bai Y., Cao H., Wu X., Xu Q., Zeng X. Method for Preparing Ginkgo biloba Endophytic Fungi Fermentation Broth as Fungicide against Plant Pathogen. CN 103194490 A. 2013 Jul 10;
- 130.Xiao J., Chen X., Wang H., Zhao Y., Gong N., Yang T., Yang Z., Li H. Endophytic Fungus of Phragmites communis, Preparation of Its Extract for Control of Rice Disease. CN 105087386 A. 2015 Nov 25;
- 131.He F., Yuan Z., Zhang B., Liu X., Zhang Z. Biocontrol Preparation for Preventing and Controlling Rice Blast and Preparation Method Thereof. CN 110558337 A. 2019 Dec 13;
- 132.Yuan Z., He F., Zhang B., Liu X., Zhang Z., Zhao Z., Pu X. Preventing Ginger Bacterial Wilt of Ginkgo Source Growth-Promoting Preparation. CN 108624527 A. 2018 Oct 9;
- 133.Li X., Li C., Wei X., Liu J. A Method for Reducing the Old of e.Sibiricus Seed Germination Period Rate Method for Mildew. CN 106538108 A. 2017 Mar 29;
- 134.Rolston M.P., Simpson W.R. Grass Endophyte with Enhanced Fungicide Resistance. WO 2007021200 A1. 2007 Feb 22;
- 135.Latch G.C.M., Christensen M.J., Tapper B.A., Easton H.S., Hume D.E., Fletcher L.R. Tall Fescue Endophytes Which Enhance Pest Resistance and Reduce Ergopeptine Alkaloid Levels. CA 2319847 C. 2012 Apr 10;
- 136.Huang R., Yao Y., Lan F., Li L. Application of Sophora tonkinensis Endophytic Fungi SDTE-P in Preventing and Controlling Anthracnose of Panax notoginseng. CN 105462854 A. 2016 Apr 6;
- 137.Huang R., Yao Y., Lan F., Li L. Application of Sophora tonkinensis Endophytic Fungus SDTE-P in Controlling Pseudo-Ginseng Root Rot. CN 105462850 A. 2016 Apr 6;
- 138.Zhang Y., Zhang M., Zhang T., Xie J., Hou S. A Biocontrol Endophytic Fungus and Application Thereof. CN 109112069 A. 2019 Jan 1;
- 139.Li L., Yao Y., Huang R., Lu X. Application of Sophora tonkinensis gagnep. Endophytic Fungi SDTE-P in Alternaria panax Control. CN 105462855 A. 2016 Apr 6;
- 140.Yin C., Xiang L., Mao Z., Zhang X., Wang G., Chen X. An Endophytic Fungus Resisting Four Kind of Fusarium Fungus and the Application Thereof. CN 105255742 A. 2016 Jan 20;
- 141.Ye Y., Dong G., Huang H., Fang Y. Kandelia Candel Endophytic Fungus Penicillium sp. 2cpe-1, Fermentation Broth and Application Thereof. CN 108546651 A. 2018 Sep 18;
- 142.Xi P., Jiang L., Xu D., Jiang Z., Xu Z., Chen J., Li M., Huang L., Zhong J. Penicillium purpurogenum Strain and Its Biological Preparation and Application in Controlling Litchi Downy Blight. CN 103773699 A. 2014 May 7;
- 143.Li L., Yao Y., Huang R., Lu X. Application of Rhexocercosporidium sp. TRXY-59-2 as Sophora tonkinensis Endophytic Fungi in Preventing and Controlling Panax notoginseng Anthracnose. CN 105462853 A. 2016 Apr 6;
- 144.Wei J., Yao Y., Huang R., Li L. Application of Sophora tonkinensis Endophytic Fungi TRXY-59-2 in Preventing and Treating Root Rot of Panax notoginseng. CN 105462851 A. 2016 Apr 6;
- 145.Yao Y., Huang R., Wu X., Li L. Application of Sophora tonkinensis gagnep Endophytic Fungus TRXY-59-2 to Prevent and Control Alternaria panax whetz. CN 105462848 A. 2016 Apr 6;
- 146.Spangenberg G.C., Guthridge K.M. Novel Fungal Endophytes from Brachiaria and Urochloa for Use in Resistance to Fungal Plant Diseases. WO 2012174585 A1. 2012 Dec 27;
- 147.Zu L., Xiao J. A New Strain of Endophytic Fungi Qty from Caulis sinomenii and Application in Biological Control Thereof. CN 108192832 A. 2018 Jun 22;
- 148.Zhang C., Zhang J., Dai D., Liu Y. Biocontrol Plant Endophytic Fungi and Application Thereof in Preventing Gray Mold of Cash Crop. CN 108085259 A. 2018 May 29;
- 149.Vidal S., Tefera T. Bio-Pesticide and Method for Pest Control. US 8709399 B2. 2014 Apr 29;
- 150.Li L., Yao Y., Lu X., Huang R. Application of Sophora tonkinensis Endophytic Fungus B21 in Preventing and Treating Panax notoginseng Black Spot. CN 105462892 A. 2016 Apr 6;
- 151.Fu Y., Yao M., Gao C., Sun J., Wang W., Zhao C., Gu C. A Pigeonpea Endophytic Fungi for High Yielding of Flavipin and Its Application. CN 105838613 A. 2016 Aug 10;
- 152.Zhu H., Zhang Y., Feng Z., Feng H., Li Z., Shi Y., Zhao L. The Cotton Endophytic Fungi Cef-082 and Its Application in the Cotton Verticillium Wilt. CN 105368720 A. 2016 Mar 2;
- 153.Zhang Y., Zhang T., Zhang M. Endophytic Fungus for Reducing the Incidence of Panax notoginseng Rot and Microbial Inoculum Thereof. CN 109749938 A. 2019 May 14;
- 154.Yang T., Wang Z., Wei Y., Li S., Li X., Fang Y. Endophytic Fungus of Podophyllum hexandrum and Application Thereof. CN 110172408 A. 2019 Aug 17;
- 155.Shu S., Cui L., Yan L. Sclerotinia sclerotiorum Resistant Huperzia serrata Endophytic Fungus and Application Thereof. CN 110272829 A. 2019 Sep 24;
- 156.Hume D.E., Johnson R.D., Simpson W.R., Card S.D. Improved Fungal Endophytes for Improved Pest Protection of Secale spp. Host Plant. WO 2014136070 A1. 2014 Sep 12;
- 157.Jiang S., Duan J., Qian D., Tao J. Fusella DG09 Having Activity in Resisting Plant Pathogens, and Its Fermentation Broth and Application. CN 102174416 A. 2011 Sep 7;
- 158.Gonzalez Coloma A., Diaz Hernandez C.E., Andres Yeves M., Fraga Gonzalez B.M., Bolanos Gonzalez P., Cabrera Perez R., Gimenez Marino C. Fungal Biocidal Products and Their Use for Control of Phytopathogens and Plant Pests. WO 2016034751 A1. 2016 Mar 10;
- 159.Seman I.A., Kushairi Din A., Moslim R., Ramli N.R., Ahmad Zairun M., Sebran N.H. Compositions for Controlling Ganoderma Disease in Plants and Method Thereof by Using Endophytic Fungus, Hendersonia gano EF1. WO 2013081448 A2. 2013 Jun 6;
- 160.Zhang L.A. Citrus Endophytic Fungi and Application Thereof. CN 109536390 A. 2019 Mar 29;
- 161.Zhu H., Feng Z., Li Z., Wang L., Zhao L., Shi Y., Zheng H. Cotton Endophytic Fungi Cef-714 and Its Application in Control of Cotton Verticillium Wilt. CN 103642704 A. 2014 Mar 19;
- 162.Du X., Zhou Y., Shang X., Liu D. An Endophytic Fungus Strain of Gentiana manshurica and Application Thereof. CN 103289906 A. 2013 Sep 11;
- 163.Zhu B., Qin L., Zhang Q., Yang K., Zhang W., Wu W., Lu J., Dong S. Atractylodes macrocephala Endophytic Fungus and Application Thereof in Preventing and Treating Root Rot of Atractylodes macrocephala. CN 110229758 A. 2019 Sep 13;
- 164.Lin F., Wang G., Zhang C., Mao L., Zhou Z. Plant Endophytic Fungus Muscodor sp. ZJLQ024, Application Thereof, and Antimicrobial Agent. CN 101691541 A. 2010 Apr 7;
- 165.Strobel G.A., Manker D.C., Mercier J., Jimenez J., Lin J., Thurston J., Kersting B. Biopesticidal Muscodor albus Formulations. US 20040141955 A1. 2004 Jul 22;
- 166.Strobel G.A., Manker D.C. Endophytic Fungi of the Genus Muscodor and Their Volatiles as Pesticides. WO 2002082898 A1. 2002 Oct 24;
- 167.Green W.A., Herrgard M.J., Kerovuo J.S., Lomelin D., Mathur E.J., Richarson T.H., Schwartz A.S., Strobel G.A. Endophytic Fungus Muscodor strobelii and Its Nucleic Acid and Polypeptide Sequences and Uses for Killing or Inhibiting Plant Pests or Pathogens. WO 2010115156 A2. 2010 Oct 7;
- 168.Strobel G.A., Daisy B. Naphthalene Insect Repellent from Muscodor vitigenus. WO 2004034785 A2. 2004 Apr 29;
- 169.Li Z., Wang X. A Kind of Grape Endophytic Fungi and Its Application for Preventing and Treating Grape Gray Mold. CN 106893678 A. 2017 Jun 27;
- 170.Wu W., Su X., Pan F., Chen A., Tang X. A Fritillaria wabuensis Endophytic Fungi Wbs003 and Its Application. CN 104774768 A. 2015 Jul 15;
- 171.Yu X., Shentu X., Dong S., Hao P., Bian Y., Ma Z. Strain of Nigrospora oryzae 46 as Biocontrol Fungus. CN 102191186 A. 2011 Sep 21;
- 172.Jiang H. Cortex Magnolia officinalis Endophytic Fungi Hpfj3 and Its Application in Preventing Wheat Take-All Disease. CN 104789482 A. 2015 Jul 22;
- 173.Zhu C., Peng C., Wang Q., Wang H., Geng Q., Kong B., Zhang A. Hippophae fructus Endophytic Fungi Strain Sj1 Fermentation Extract for Use. CN 110178857 A. 2019 Aug 30;
- 174.Zhu H., Li Z., Feng Z., Wang L., Zheng H., Shi Y., Zhao L. Cotton Endophytic Fungi Cef-818 and the Application in Cotton Verticillium Wilt Control Thereof. CN 103627643 A. 2014 Mar 12;
- 175.Qian Y., Kang J., Geng K. Application of Pestalotiopsis uvicola Metabolite in Prevention and Treatment of Kiwi Monilinia fructicola. CN 104161049 A. 2014 Nov 26;
- 176.Yang Y., Yuan Z., Wang X. Pezicula neosporulosa with Bacteriostasis and Lignocellulose Degradation Activity and Application Thereof. CN 110511878 A. 2019 Nov 29;
- 177.Su Z., Zhang C., Lin F., Feng X. Endophytic Fungi Strain R5-6-1 in Prevention and Treatment of Rice Bacterial Blight Application Of. CN 109769535 A. 2019 May 21;
- 178.Wang G., Zhang C., Lin F., Xia J., Zhou Z. Endophytic Fungus Phomopsis wenchengensis ZJWCF252 for Manufacture of Agricultural Fungicide 2,3-Dihydro-2-Hydroxy-2,4-Dimethyl-5-Trans-Propenylfuran-3-One. CN 102154116 A. 2011 Aug 17;
- 179.Guo S., Li X., Chen X., Wang H. Endophytic Fungi Rhizopus Dhs96 and Trichoderma Df188 Capable of Preventing Dendrobium Soft Rot. CN 102234618 A. 2011 Nov 9;
- 180.Yang Y., Cai J., Chen Y., Wang B., Xu C., Li B., Huang G. Application of Elicitor Protein Derived from Sarocladium brachiariae and Its Coding Gene in Vegetable Biocontrol. CN 110452290 A. 2019 Nov 15;
- 181.Li H., Zhao Y., Zhu Z., Tang W., Li S. Plant Endophytic Seimatosporium Fungus M7sb41 and Application Thereof. CN 110468057 A. 2019 Nov 19;
- 182.Xie L., Nong Q., Zhang W., Su Q., Chen Y., Lan T., Zhang Y., Qin L. Controlling Banana Blight Disease of Endophytic Fungi l—14 and Application Thereof. CN 106167767 A. 2016 Nov 30;
- 183.Yuan Z., Zhang B., Liu X., He F., Zhang Z. Biocontrol Preparation for Preventing and Treating Lettuce Sclerotinia and Preparation and Use Method Thereof. CN 110558336 A. 2019 Dec 13;
- 184.Li Z., Zhu H., Feng Z., Zhao L., Shi Y. Cotton Endophytic Fungus Cef-642 and Its Application. CN 103834580 A. 2014 Jun 4;
- 185.Liu P., Zhou S., Tang X., Zhu L., Ye Z., Jia B., Heng W., Liu L. Yellow Compacted Shaped Molded Y28 Thereof in Prevention and Treatment of Fruit Rot Application of. CN 106119134 A. 2016 Nov 16;
- 186.Zhao X., Hu Z., Lv S., Hou D., Song P. A Kind of Endophytic Fungi Zxl-Szy-r-9 with Antibiosis and Its Application. CN 109593658 A. 2019 Apr 9;
- 187.Yu D., Gong S., Xiang L., Yang L. Pink Trichothecenes and Its Fermented Products Used in the Control of Wheat Powdery Mildew. CN 105211105 A. 2016 Jan 6;
- 188.Gnanamangai B.M., Jr., Ponnusamy P.P., Sr. Biosynthesis of Gold and Silver Nanoparticles for Stability and Extended Shelf-Life of Antagonistic Activities. US 20120108425 A1. 2012 May 3;
- 189.Tang J., Wang G., Lu J., Tang J., Zhu Y., Li Q. An Endophytic Fungus of Chrysanthemum morifolium and Its Application. CN 108179115 A. 2018 Jun 19;
- 190.Sword G.A. Fungal Endophytes for Improved Crop Yields and Protection from Pests. WO 2018119419 A1. 2018 Jun 28;
- 191.Miller J.D., Adams G.W., Sumarah M. Antifungal Metabolites from Fungal Endophytes of Pinus strobus. US 9469836 B2. 2012 Jul 28;
- 192.Pietro-Souza W., de Campos Pereira F., Mello I.S., Stachack F.F.F., Terezo A.J., da Cunha C.N., White J.F., Li H., Soares M.A. Mercury Resistance and Bioremediation Mediated by Endophytic Fungi. Chemosphere. 2020;240:124874. doi: 10.1016/j.chemosphere.2019.124874. [DOI] [PubMed] [Google Scholar]
- 193.Krishnamurthy Y.L., Naik B.S. Endophytic Fungi Bioremediation. In: Maheshwari D.K., Annapurna K., editors. Endophytes: Crop Productivity and Protection. Volume 2. Springer; Cham, Switzerland: 2017. pp. 47–60. [DOI] [Google Scholar]
- 194.Li H.-Y., Wei D.-Q., Shen M., Zhou Z.-P. Endophytes and Their Role in Phytoremediation. Fungal Divers. 2012;54:11–18. doi: 10.1007/s13225-012-0165-x. [DOI] [Google Scholar]
- 195.Fomina M.A., Alexander I.J., Colpaert J.V., Gadd G.M. Solubilization of Toxic Metal Minerals and Metal Tolerance of Mycorrhizal Fungi. Soil Biol. Biochem. 2005;37:851–866. doi: 10.1016/j.soilbio.2004.10.013. [DOI] [Google Scholar]
- 196.Peng K., Wen T., Xiong R., Tian S., Chen C., Zhong Z., Jiang P., Peng Y., Wan Y. Fusarium Oxysporum and Its Application in Phytoremediation of Heavy Metal Contaminated Soil. CN 105733958 A. 2016 Jul 6;
- 197.Tan N., Hou D., Liao S., Yang X., Gao Y., Wang J., Nie C., Yan X., Wu Y., Jiang M., et al. A Method for Repairing Uranium-Polluted Water Body with Imprinted Material Prepared by Facultative Marine Fungi as Basal Body and Phytic Acid as Functional Monomer. CN 106340337 A. 2017 Jan 18;
- 198.Strobel G.A., Dirkse E., Ezra D., Castillo U., Phillips B. A Method of Using Endophytic Fungi to Decontaminate and Decompose Human and Animal Wastes. WO 2005116272 A2. 2005 Dec 8;
- 199.Xiao J., Zhang Q., Yin J., Sun J., Zhang L., Zhang Y., Yang H., Kong Q. A Method for by Use of Wild Soybean Endophytic Fungi Adsorption Water Body in Heavy Metal Cadmium Pollution. CN 108751424 A. 2018 Nov 6;
- 200.Verma S., Kuila A. Bioremediation of Heavy Metals by Microbial Process. Environ. Technol. Innov. 2019;14:100369. doi: 10.1016/j.eti.2019.100369. [DOI] [Google Scholar]
- 201.Wang Y., Zeng D., Feng G., Tang W., Li H. One Kind of Stem Point Enzyme E41 Metal Fungal Strain and Application Thereof. CN 106947697 A. 2017 Jul 14;
- 202.Wang Y., Feng G., Li H., Zeng D., Tang W. One Kind of Carbon Angle Bacterial Genus E68 Fungal Strain and Application Thereof. CN 107177511 A. 2017 Sep 19;
- 203.Dai C., Zhou J., Jia Y. Laccase-Containing Soil Remediation Agent, and Its Production Method and Application. CN 107900098 A. 2018 Apr 13;
- 204.Hignight K.W., Rush D.L. Enhancing Endophyte in Grass. WO 2000062600 A1. 2000 Oct 26;
- 205.Wang H., Chen Y., Lin F. Phoma Endophytic Fungi and Its Application. CN 104293681 A. 2015 Jan 21;
- 206.Brown W.G. Clonostachys rosea Inoculated Plant Materials with Fungicides and Adjuvants. US 20160007613 A1. 2016 Jan 14;
- 207.Stewart J.F., Brown W.G. The Production and Use of Endophytes as Novel Inoculants for Promoting Enhanced Plant Vigor, Health, Growth, Yield Reducing Environmental Stress and for Reducing Dependency on Chemical Pesticides for Pest Control. WO 2007107000 A1. 2007 Sep 27;
- 208.Xu Q., Xia C., Cheng C., Jin H., Xie Y., Liu H., Liao F., Zhang C., Lin F., Liu H., et al. Endophytic Fungal Strain Ycef005 and Its Use. CN 103849572 A. 2014 Jun 11;
- 209.Chakraborty S., Newton A.C. Climate Change, Plant Diseases and Food Security: An Overview. Plant. Pathol. 2011;60:2–14. doi: 10.1111/j.1365-3059.2010.02411.x. [DOI] [Google Scholar]
- 210.Venil C.K., Velmurugan P., Dufossé L., Devi P.R., Ravi A.V. Fungal Pigments: Potential Coloring Compounds for Wide Ranging Applications in Textile Dyeing. J. Fungi. 2020;6:68. doi: 10.3390/jof6020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bamisile B.S., Dash C.K., Akutse K.S., Keppanan R., Wang L. Fungal Endophytes: Beyond Herbivore Management. Front. Microbiol. 2018;9:544. doi: 10.3389/fmicb.2018.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mei C., Flinn B. The Use of Beneficial Microbial Endophytes for Plant Biomass and Stress Tolerance Improvement. Recent Pat. Biotechnol. 2010;4:81–95. doi: 10.2174/187220810790069523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.