Abstract
DNA methyltransferases are an essential class of modifiers in epigenetics. In mammals, DNMT1, DNMT3A and DNMT3B participate in DNA methylation to regulate normal biological functions, such as embryo development, cell differentiation and gene transcription. Aberrant functions of DNMTs are frequently associated with tumorigenesis. DNMT aberrations usually affect tumor-related factors, such as hypermethylated suppressor genes and genomic instability, which increase the malignancy of tumors, worsen the prognosis for patients, and greatly increase the difficulty of cancer therapy. However, the impact of DNMTs on tumors is still controversial, and therapeutic approaches targeting DNMTs are still under exploration. Here, we summarize the biological functions and paradoxes associated with DNMTs and we discuss some emerging strategies for targeting DNMTs in tumors, which may provide novel ideas for cancer therapy.
Keywords: DNA methyltransferases, cancer, DNMT aberrations, therapy
1. Introduction
Regulation of gene expression is an area of intense research interest. In particular, great attention has been paid to the epigenetic phenomenon where heritable changes in gene expression occur with no alteration of the DNA sequence [1]. The discipline of epigenetics is defined as the study of molecular processes that affect information flow between the same DNA sequence and variable gene expression patterns. At present, epigenetics spans many research fields, such as oncology and immunology [2]. Aberrant epigenetic processes can lead to changes in gene function and transformation of malignant cells. Global alterations in epigenetic modifications are a hallmark of cancer. Cancer has traditionally been regarded as a hereditary disease, and is now recognized as resulting from epigenetic abnormalities related to genetic changes. In the rapidly developing field of cancer epigenetics, the relevant epigenetic modifications mainly include DNA methylation, histone modification and RNA regulation [3].
DNA methylation is one of the earliest discovered and most deeply studied epigenetic regulatory mechanisms [4]. It is a major form of epigenetic modification of genomic DNA and an important means of regulating genomic function. The methylation status of the genome is mainly the result of joint regulation by DNA methyltransferases (DNMTs) and DNA demethylases [5]. Using S-adenosylmethionine (SAM) as the methyl donor, DNMTs add a methyl group (CH3) to the C5 position of the cytosine ring in a cytosine-phosphate-guanine (CpG) dinucleotide pair to yield 5′-methyl-cytosine [6]. DNMTs play a crucial role in many biological processes, such as embryo development, cell differentiation and gene transcription [7]. In mammalian genomic DNA, 3% to 5% of cytosine exists in the form of 5-methylcytosine, and 70% of 5-methylcytosine is located within CpG sequences. Non-methylated CpG sequences are associated with housekeeping genes and tissue-specifically expressed genes, which suggests that CpG methylation plays an important role in gene expression [8]. Genes are located on chromosomes in the context of chromatin, so histone modifications are also indispensable in the process of gene regulation. Histone modification refers to the methylation and acetylation of histone proteins under the action of related enzymes such as histone methyltransferases, histone acetyltransferases, histone deacetylases, etc. Histone methylation mainly occurs on lysine and arginine residues of histones H3 and H4, histone methyltransferase can selectively methylate lysines and arginines in the tails of histones, and synergistically induce transcriptional activity. Moreover, when histone acetylation in chromatin is in a loose state, which is conducive to gene expression [9,10]. In addition to the coding part of nucleic acid plays an important role in gene expression, and functional non-coding RNA also plays an important role in gene expression. There are long and small non-coding RNAs. Long non-coding RNA plays a cis-regulatory role at the gene and even the entire chromosome level, and small non-coding RNA regulates gene expression at the genomic level. Small non-coding RNA can mediate the degradation of mRNA, induce changes of chromatin structure, and determine how cells differentiate. Small non-coding RNA can also degrade foreign nucleic acid sequences to protect the cell’s genome [11,12].
This review focuses on induction of DNA methylation by DNMTs and specifically introduces this process in the context of tumor research [3]. A large number of studies have shown that aberrant DNMTs can cause tumorigenesis [13,14]. Therefore, we describe the phenomena associated with DNMT alterations, discuss current therapies that target DNMTs, and provide more ideas for targeting DNMTs in cancer.
2. DNMTs
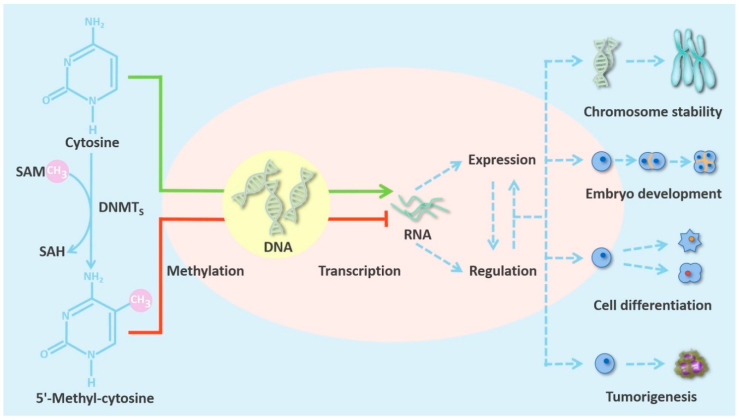
DNMTs are an extremely important class of epigenetic regulatory enzymes. They catalyze DNA methylation, a normal endogenous modification of eukaryotic DNA, which is vital for life [15]. This process, which is illustrated in Figure 1, can cause alterations in gene expression, but does not change the sequence of the DNA or of the gene product [16].
Figure 1.
The characteristics of DNMTs in mammalian cells. DNMTs catalyze DNA methylation, which alters patterns of gene regulation and expression. This in turn affects chromosome stability, embryo development and cell differentiation. Aberrant DNMTs can induce tumorigenesis.
2.1. Family Members
The first eukaryotic DNMT was successfully cloned in 1988 [17]. The DNMT family has five members, which are DNMT1, DNMT2, DNMT3A, DNMT3B, and DNMT3L. The main function of DNMT1 is to maintain the methylation status after DNA synthesis and DNMT2 is an RNA methyltransferase, which methylates multiple tRNAs [18]. DNMT3A and DNMT3B are involved in the de novo methylation of DNA [19], while DNMT3L is a DNMT3-like protein that lacks a catalytic domain. DNMT3L can interact with DNMT3A and DNMT3B to improve their catalytic activity and positively regulate DNA de novo methylation [20]. DNMT3A and DNMT3B catalyze the methylation of different genomic DNA regions in vivo. DNMT3A has higher methylation activity on naked DNA and the naked part of nucleosomal DNA than DNMT3B. DNMT3A hardly methylates DNA in the core region of nucleosomes, while DNMT3B significantly methylates this DNA, although the activity is low. Studies have shown that the preferential methylation activity of DNMT3A on naked nucleosomal DNA and the significant methylation activity of DNMT3B on the DNA in the nucleosome core contribute to their significant methylation of genomic DNA in vivo. The expression of DNMTs at different times during embryonic development may be one of the reasons why these enzymes methylate different DNA regions [21].
In mammals, DNA methylation is mainly mediated by three proteins, DNMT1, DNMT3A, and DNMT3B, which are localized in the nucleus [22]. This review will focus on these three main enzymes.
2.2. Locations and Domains
The human DNMT1 gene, which is located on chromosome 19, has a total protein coding region of 4851 bp and encodes a protein of about 183 kDa. It is generally believed that the DNMT1 protein consists of three domains, namely the N-terminal regulatory domain, the C-terminal catalytic domain, and a central junction region [23]. The N-terminal regulatory domain is responsible for localizing the protein to the nucleus. Most of the C-terminal catalytic domain is evolutionarily conserved, with binding sites for various substrates such as cytosine. The central junction region, which connects the other two domains, contains repeated glycine-lysine dipeptide sequences [17].
The human DNMT3A gene, located on chromosome 2, has a total protein coding region of 2172 bp and encodes a protein of about 130 kDa, while the DNMT3B gene, on chromosome 20, has a total protein coding region of 2538 bp and encodes a protein of about 95 kDa [24]. DNMT3A and DNMT3B have similar structures, consisting of three domains: the N-terminal proline-tryptophan-tryptophan-proline (PWWP) domain [25], the cysteine-enriched ATRX-DNMT3-DNMT3L (ADD) zinc-binding region and the C-terminal methyltransferase catalytic region. The PWWP domain mediates the specific binding of DNA. The cysteine-enriched zinc-binding region interacts with unmodified histones to enhance methyltransferase activity. The C-terminal catalytic region maintains the stable function of the enzyme and catalyzes DNA methylation [26].
The N-terminal regulatory domain of DNMT1, DNMT3A and DNMT3B comprises a proliferating cell nuclear antigen binding domain (PBD), a nuclear localization signal (NLS), a cytosine-rich zinc finger DNA binding motif (ATRX), a polybromo homology domain (PHD), and a PWWP tetrapeptide chromatin binding domain. The C-terminal catalytic domain contains 10 different characteristic motifs, of which 6 motifs (I, IV, VI, VIII, IX and X) are evolutionarily conserved in mammals. The methylation reaction is initiated by conserved cysteine residues in motif IV, which mediate nucleophilic attack on the cytosine ring [27].
2.3. Normal Biological Characteristics
In general, DNA methyltransferases are involved in important biological activities such as preserving chromosome stability and genome integrity, embryo development, cell differentiation, and growth of organisms [7]. DNMT1, which maintains methylation, is believed to have a high affinity for hemimethylated DNA templates. DNMT3A and DNMT3B are considered to be the main de novo methyltransferases that change the methylation status of unmethylated CpG sites [28]. De novo methyltransferases are mainly active in the development of embryonic stem cells, where they identify unmethylated cytosines in DNA and establish a new methylation pattern. Moreover, DNMT3B is highly expressed in totipotent embryonic stem cells in the early developmental stage, when DNA methylation occurs. After that, DNMT3A is widely expressed in mesenchymal cells, and the expression level of DNMT3B is one order of magnitude higher than that of the widely expressed DNMT3A [21]. Both DNMT3A and DNMT3B are downregulated in most differentiated somatic cells [29]. The maintenance methyltransferase DNMT1 is associated with early embryo implantation in the endometrium, where it is an important component of the DNA replication complex. After identifying the methylation site, it catalyzes the methylation of DNA hemimethylation sites through nucleophilic attack to maintain the established methylation pattern by de novo methyltransferases [30]. Table 1 summarizes information from the Uniprot portal (www.uniprot.org) to show the expression levels of DNMTs in normal tissues.
Table 1.
Expression levels of DNMTs in normal tissues and deregulation of DNMTs in cancers.
| DNMT | High Level in Normal Tissues | Low Level in Normal Tissues | Cancers with Deregulated DNMTs | Nature of Deregulation in Cancers | Result |
|---|---|---|---|---|---|
| DNMT1 | Fetal tissues, heart, kidney, placenta, peripheral blood mononuclear cells | Spleen, lung, brain, small intestine, colon, liver, skeletal muscle | Pituitary adenoma | Overexpression | Silenced RASSF1A, CDH13, CDH1, and p16 [31] |
| Colorectal cancer | Mutation | Alteration of global DNA methylation status [32] | |||
| Pancreatic cancer | Overexpression | Transcriptional repression of SOCS3 in abnormal activation of the IL-6/STAT3 pathway [33] | |||
| Gastric cancer | Overexpression | Hypermethylated CpG islands of the p16, hMLH1, E-cadherin and THBS-1 [34] | |||
| Lung cancer | Overexpression | Hypermethylation of p16, RARβ, FHIT, RASSF1A and hRAB37 with aberrant p53/Sp1 axis [35] | |||
| Thyroid cancer | Overexpression | Downregulation of PTEN by DNA methylation in PI3K/AKT signal pathway [36] | |||
| DNMT3A | Fetal tissues, skeletal muscle, heart, peripheral blood mononuclear cells, kidney | Placenta, brain, liver, colon, spleen, small intestine, lung | Vulvar squamous cell carcinoma | Overexpression | The absence of p16 expression by promoter hypermethylation [37] |
| Pituitary adenoma | Overexpression | Silenced RASSF1A, CDH13, CDH1, and p16 [31] | |||
| Acute myeloid leukemia | Mutation | DNA hypomethylation by DNMT3A R882 mutation [38] | |||
| Overexpression | Transcription silencing of BASP1 in A/E positive AML [39] | ||||
| DNMT3B | Fetal liver, heart, kidney, placenta | Spleen, colon, brain, liver, small intestine, lung, peripheral blood mononuclear cells, skeletal muscle | Lung cancer | Overexpression | Highly methylation of RASSF1A promoter [40] |
| Hepatocellular carcinoma | Overexpression | Increased expression of the oncogene OCT4 through the IL-6/STAT3 pathway [41] | |||
| Ovarian cancer | Overexpression | Hypermethylation of hMLH1, p16 and p53 [42] | |||
| Breast cancer | Overexpression | Highly methylation of multiple genes (CDH1, CEACAM6, CST6, ESR1, LCN2 and SCNN1A) [43] |
The DNMT-mediated methylation of CpG sites can make chromatin structure highly spiral, prevent the activation of promoters, and facilitate interaction with methylated CpG-binding proteins such as MeCP2, which recruits histone deacetylase [44]. These changes can inhibit gene expression. Methylated DNA can also directly inhibit the binding of methylation-sensitive transcription factors, such as E2F, CREB, etc., so that transcription is blocked [45,46]. At the same time, histones can also affect DNA methylation. The PWWP motif in DNMT3A recognizes the modification H3K36me3 (trimethlyation of lysine 36 in histone H3). The interactions between the PWWP domain and the H3K36me3 modification can increase the ability of DNMT3A to methylate nucleosome DNA [47]. Studies have shown that DNMT3B, not DNMT3A, is responsible for methylating the bodies of transcribed genes, and this function is also realized by PWWP recognizing the H3K36me modification [48]. DNA methylation has been proved to overlap with H3K27me3 at multiple sites throughout the genome, and unmethylated CpG sites play a role in guiding polycomb repressive complex 2 (PRC2) to bind to its target genes [49].
DNMTs are also closely linked with the function of the nervous system. Many studies have found that DNA methylation is related to transcriptional silencing. For example, memory formation during fear conditioning involves methylation and silencing of the memory suppressor gene PP1. The inhibition of DNMTs prevents the increase of PP1 methylation, resulting in abnormal gene transcription during the memory consolidation period [50]. DNMT activity is also involved in the process of cellular aging, and there is a widespread decline in DNA methylation in many aging tissues [51].
2.4. Pathological Characteristics
Genetic mutations caused by chemical carcinogens or pathological conditions, may directly affect the functions or levels of DNMTs, which may in turn cause the genome-wide methylation profile alterations and cancer-promoting changes in gene expression, such as increasing genomic instability and the expression of oncogenes and decreasing the expression of tumor suppressor genes [52,53,54].
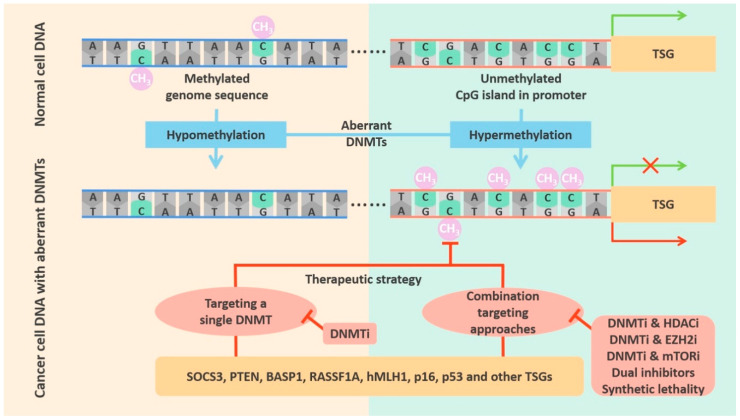
Recent studies have found that gene expression abnormalities caused by alterations in DNMT activity and function are closely connected with the occurrence and development of various cancers [55,56]. In mammals, some CpG dinucleotides are dispersed in the genome, while others occur in dense clusters called CpG islands. Dispersed CpGs are hypermethylated; in contrast, CpG islands are usually unmethylated and are often located in gene promoter regions that regulate transcriptional activation and repression. In normal cells, CpG islands in the promoters of tumor suppressor genes (TSGs) are unmethylated, and in these regions chromatin is in an open conformation, so gene transcription can proceed normally [57]. However, genome-wide hypomethylation and promoter hypermethylation are hallmarks of many cancers and are associated with tumorigenesis. Genome-wide hypomethylation can lead to increased genomic instability [58,59]. More importantly, hypermethylation of the CpG islands in gene promoters can silence TSGs and affect key cellular processes such as the cell cycle, apoptosis, DNA repair, cell interactions, and angiogenesis [60]. Tumor therapy focuses on restoring transcription of TSGs which is inhibited by CpG island methylation. Therefore, it is particularly important to explore aberrations in DNMT activity. The essential processes described above are shown in Figure 1 and Table 1.
Furthermore, in addition to investigating DNMT alterations at the cellular level, the researchers also established DNMT animal models so as to study DNMT pathological characteristics in vivo and in vitro. Belinsky et al. utilized a mouse model containing an allele with the DNMT1 gene disrupted to study the functional role of DNMT in tumor formation [61]. The reduction of DNMT1 led to a decrease in the incidence of lung cancer induced by tobacco carcinogens, and DNMT activity was also reduced in pneumocytes that could give rise to lung cancer. Moreover, it has been reported that DNMT3A may directly inhibit the upregulation of DNMT3B in lymphoma in animal models. Inactivation of the DNMT3B gene accelerated the development of lymphoma in DNMT3A-deficient mice, which indicates that the upregulation of DNMT3B inhibits disease progression in DNMT3A-deficient lymphoma [62]. In mice treated with skin cancer-inducing chemicals, deletion of DNMT3A increases the number of carcinogen-induced squamous cell carcinomas without affecting tumor progression. When DNMT3A and DNMT3B are both deleted, the squamous cell carcinomas become more invasive and metastatic [63]. These studies show that loss of DNMTs has a major impact on organisms.
2.5. The Paradox of DNMTs
An interesting phenomenon was found when the effects of aberrant DNMTs were characterized in tumors. Some studies showed that overexpression of the DNMT3A protein in vulvar squamous cell carcinomas and pituitary adenomas is associated with malignant characteristics such as high invasiveness and recurrence [31,37]. Similarly, increased expression of DNMT3A in lung cancer leads to the proliferation and metastasis of tumor cells. Yan et al. showed that microRNA-101 (miR-101) expressed significantly inhibited DNMT3A activity corresponding to miR-101 binding site. While in the presence of miR-101, the expression of DNMT1 and DNMT3B did not change significantly, indicating that DNMT3A is a direct target of the miR-101 in lung cancer cells. Therefore, expression of miR-101 will reduce the expression of endogenous DNMT3A, which will decrease the overall DNA methylation. Cadherin-1 (CDH1) is a known inhibitor of lung metastasis. The methylation status of CpG sites in the CDH1 promoter was analyzed in miR-101-transfected lung cancer cells by bisulfite analysis. The proportion of unmethylated sites was much higher in the miR-101-transfected cells than in control cells, which indicates that CDH1 is re-expressed via promoter hypomethylation [64]. Similarly, miR-708-5p can directly inhibit the translation of DNMT3A, resulting in a significant reduction in promoter DNA methylation levels and upregulation of the tumor suppressor gene CDH1. Upregulation of CDH1 reduces the activity of Wnt/β-catenin signaling in non-small cell lung cancer cells, thereby impairing the stem cell characteristics of NSCLC cells [65].
In complete contrast, Husni et al. pointed out that the lack of DNMT3A facilitate tumor progression [66]. Gao et al. used a mouse lung cancer model to verify that DNMT3A deficiency can promote tumor growth and progression and detected no significant difference in the abundance of DNMT1 or DNMT3B mRNAs in DNMT3A-deficient and WT tumors [67]. Raddatz et al. proved that DNMT3A is necessary to effectively maintain the methylation of active chromosome domains and DNMT3A-deficient tumors showed moderate gene deregulation in these domains. Through overall methylation analysis, it was found that the proportion of fully methylated CpGs in DNMT3A knockout tumors decreased significantly, while the proportion of partially methylated CpGs increased accordingly. This study revealed the conserved features of genomic methylation in cancer cells, DNMT3A deficiency increased the instability of the whole genome, confirming the role of DNMT3A in maintaining the methylation pattern of cancer DNA [68].
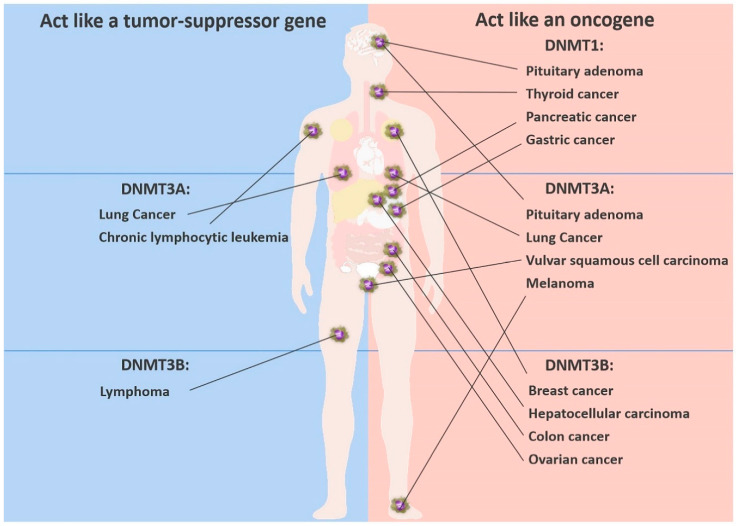
These two completely opposite findings suggest that DNMT3A can act both as an oncogene and as a tumor suppressor gene in lung cancer (Figure 2). At present, it is less clear whether the paradox applies to the other DNMTs in other cancers. The paradoxical effect of DNMT3A led us to ask the following questions: How do the alterations in DNMTs affect the tumor? And what strategies can be used to target DNMTs in tumors?
Figure 2.
The DNMT paradox and the different roles of DNMTs in various cancers in mammals. DNMT3A can act both as an oncogene and as a tumor suppressor gene in lung cancer. Other DNMTs play different roles in other cancers.
In the next sections, we systematically introduce the role of DNMTs in different tumors as well as the treatment approaches. We believe that the impact of DNMT alterations on cancers needs to be further investigated to provide potential routes to cancer therapy. Moreover, understanding the molecular roles of aberrant DNMTs in tumorigenesis will lay the theoretical foundations for clinical research and development of new drugs.
3. DNMT Aberrations in Cancer
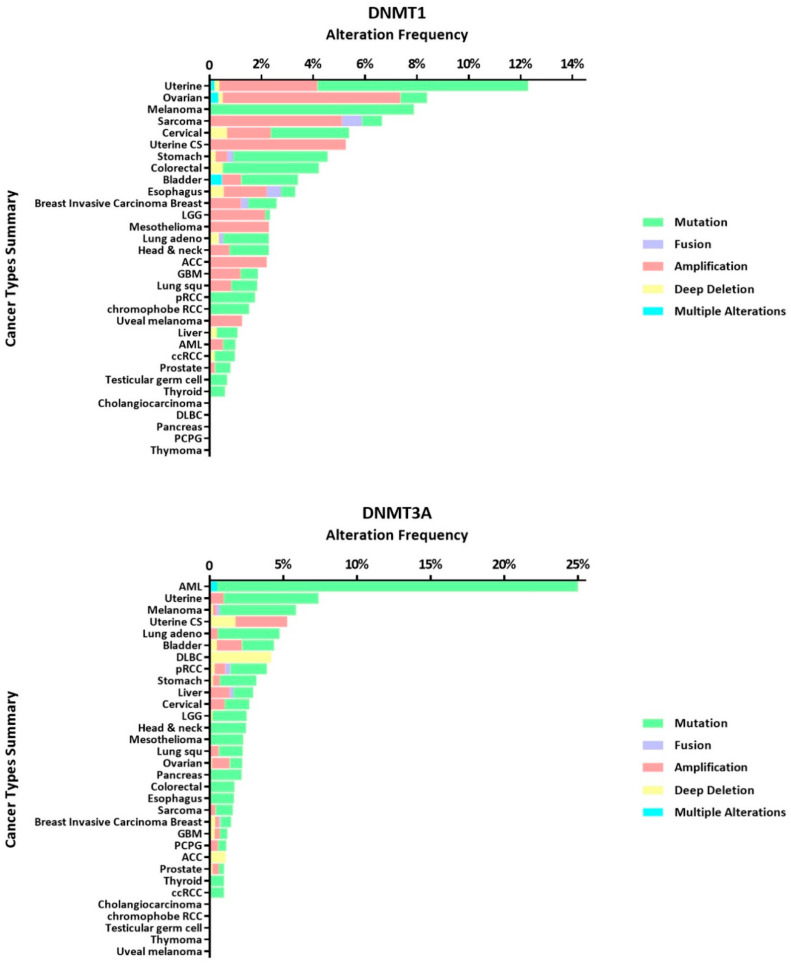
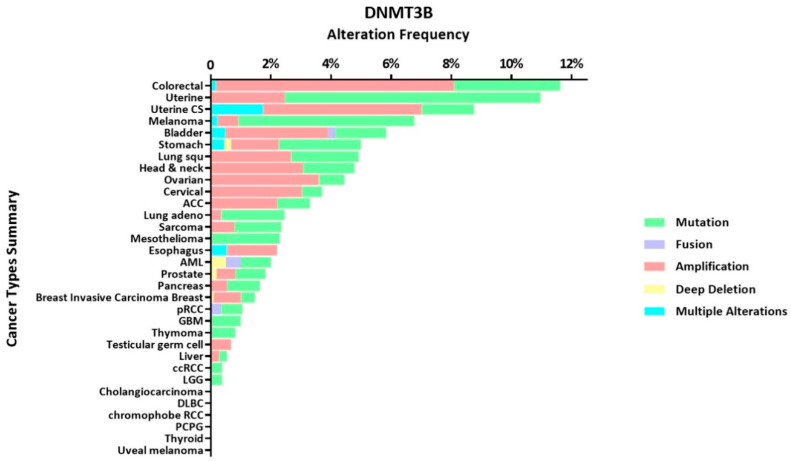
Carcinogenesis of normal cells is often related to changes in the genetic material [69,70]. DNMT alterations have been frequently observed by many researchers in various types of tumors, which indicates that they often accompany the occurrence and development of tumors. We utilize publicly available datasets (TCGA PanCancer Atlas Studies) from cBioPortal for Cancer Genomics (www.cbioportal.org) to better present DNMT alternation frequency in variety cancer types (see Figure 3). Therefore, analysis of DNMT abnormalities in tumors may uncover a way to restore DNA stability, which may lead to new methods to treat tumors. The aberrations mentioned here mainly refer to mutations, high expression and low expression.
Figure 3.
DNMT alteration frequency in cancer types summary. DNMT1, DNMT3A, DNMT3B occur mutation, fusion, amplification, deep deletion and multiple alterations in variety cancer types from cBioPortal datasets.
3.1. DNMT1 Aberrations
Mutations in DNMT1 generally cause neurological diseases [71]. In addition, Kanai et al. found that the coding exons of the DNMT1 gene were mutated in 7% of human colorectal cancers tested, which was the first evidence that DNMT1 is mutated in human cancer [32].
High expression of DNMT1 has been observed in a variety of tumors and is a characteristic change of tumor cells. Li et al. analyzed 20 pancreatic cancer cell lines and found that 16 of them had DNMT1 expression more than twice that of normal cells [72]. Etoh et al. found that DNMT1 could not be detected in normal epithelium, but was detected in most gastric cancers. Furthermore, the overexpression of DNMT1 protein in gastric cancer tissues is significantly related to decreased E-cadherin expression, which indicates that an increase in DNMT1 expression will enhance the migration ability of the cancer cells. Researchers have investigated the relationship between the high expression of DNMT1 protein and the DNA methylation status of multiple CpG islands (p16, hMLH1, E-cadherin and THBS-1) [34]. For patients who show DNA methylation of a CpG island in both non-cancerous mucosa and cancer, the signal intensity of the product of methylated DNA is increased in the cancer cells compared with the corresponding non-cancerous mucosa.
Belinsky’s team discovered that in lung cancer, in addition to affecting the methylation status of cytosine, DNMTs can also bind to histone deacetylase (HDAC) and mediate the formation of heterochromatin around abnormally methylated promoters [61]. They also found that a 2-fold overexpression of the DNMT1 gene increased DNA methylation and tumorigenic transformation in NIH3T3 cells. Similarly, the work of Lin et al. indicates that overexpression of DNMT1 causes abnormal regulation of the p53/Sp1 pathway, resulting in hypermethylation of multiple TSGs (p16, RARβ, FHIT, RASSF1A and hRAB37), and eventually leading to the occurrence of lung cancer with poor prognosis [35].
3.2. DNMT3A Aberrations
Among DNMT1, DNMT3A and DNMT3B, mutations in DNMT3A have been reported most frequently in cancer. DNMT3A mutation is one of the earliest that occurs during the development of hematopoietic malignancies. Most researchers believe that DNMT3A mutation indicates a poor prognosis in patients with AML. The R882 site of DNMT3A is the most likely to mutate. The most common mutation is R882H, and the others are R882C, R882S, and R882P [73]. Mutation of the R882 site will alter the activity of DNMT3A, which then affects cell cycle regulation to promote the development of myeloid leukemia. DNMT3A mutations occur in about one fifth of acute myeloid leukemia (AML) cases, and these mutations are associated with reduced enzymatic activity and genomic methylation in the leukemia cells [74]. DNMT3A mutation often occurs simultaneously with the mutation of other genes, the effects of DNMT3A mutation on the methylation of the whole genome are often different. Yan et al. found some differences in DNA methylation pattern in patients with and without the DNMT3A R882 mutation [38]. In individuals with the DNMT3A R882 mutation, hypomethylation of CpG islands is detected in the HOXB locus and genes in the HOXB cluster are extensively upregulated at the mRNA level. HOX family proteins play an important role in regulating normal hematopoiesis [75]. In addition, expression of the DNA demethylase IDH1 increased and genome demethylation increased in individuals with the DNMT3A R882 mutation.
DNMT3A was found to be slightly overexpressed in other human cancers [22]. We speculate that this may be related to hypermethylation of TSGs. Deng et al. found that in a mouse model, silencing of DNMT3A with RNA interference inhibited melanoma growth and metastasis [76]. Deng’s group evaluated the role of DNA methylation in regulating gene expression. MHC class II transactivator (Ciita), which is essential for T cell-mediated immunity, was upregulated after silencing of DNMT3A. Bisulfite sequencing revealed that most CpG sites in the Ciita promoter were significantly demethylated, indicating that the deletion of DNMT3A directly mediates the upregulation of Ciita and enhances the immune response. This work also shows that DNMT3A is required for melanoma growth in vivo. Leonard et al. also showed that DNMT3A overexpression in vulvar squamous cell carcinomas increases tumor invasion, and may be useful for predicting the risk of relapse [37].
However, Gao et al. analyzed the role of DNMT3A in lung cancer in genetically engineered mice, and found that deletion of DNMT3A significantly promotes oncogenesis [67]. This suggests that in normal cells, the enzyme may act as a TSG to inhibit tumor development by stabilizing DNA methylation patterns, and its deficiency may play a key role in lung cancer malignancies [66,68]. Haney et al. reported that loss of one copy of the DNMT3A gene in mice cannot induce the transformation of hematopoietic cells immediately, but can cause chronic lymphocytic leukemia (CLL)-like symptoms in more than half of 16-month-old mice [77]. Global methylation profiling showed that most gene promoters in normal spleen cells are highly methylated and silent, while the loss of one copy of DNMT3A induced promoter hypomethylation, leading to overexpression of putative oncogenic drivers. This suggests that DNMT3A is a tumor suppressor in CLL.
3.3. DNMT3B Aberrations
There are very few reports about DNMT3B mutations. Mutations in the human DNMT3B gene cause a rare autosomal recessive genetic disease called immunodeficiency-centromeric instability-facial anomalies syndrome 1 (ICF) [78]. Although there is not much evidence for DNMT3B mutations in cancer, increased expression of DNMT3B is found in some tumors, which suggests that abnormal expression of DNMT3B may silence TSGs. Robertson et al. reported that DNMT3B was significantly overexpressed in human bladder and pancreatic cancer tissues [22]. It was found that DNMT3B methylates genes that are usually silenced in human colon cancer, this process promotes the formation of intestinal tumors in mouse models [79,80]. Lin et al. investigated the transition of microadenomas (considered to be the earliest stage of intestinal tumor formation) to macroscopic colon tumors in a mouse model. They demonstrated that DNMT3B plays a role in the transitional process [81]. DNMT3B deficiency can apparently reduce the formation of macroscopic adenomas, but it does not reduce microadenoma formation.
Hlady et al. specifically knocked out DNMT3B in T cells in a mouse model of MYC-induced lymphomagenesis, thereby increasing cell proliferation and accelerating lymphoma development [82]. Global methylation analysis shows that many gene promoters are potential targets for DNMT3B activity. These promoters are demethylated in DNMT3B-deficient lymphoma, but not demethylated in DNMT3B-deficient precancerous thymocytes, which indicates that DNMT3B maintains cytosine methylation in cancer cells. This work suggests that DNMT3B has an anti-tumor effect in T cells.
4. Targeting DNMTs
Since DNMTs are frequently overexpressed in some cancers, DNMTs are excellent targets for treating cancer. As described above, TSG promoters are often hypermethylated in cancer cells. DNMT inhibitors (DNMTis) generally demethylate TSG promoters to facilitate expression of TSGs. Here we introduce different types of DNMTis that inhibit various TSGs and provide a wide range of treatment methods. Depending on the type of cancer, agents that target a single DNMT or multiple DNMTs can be selected. Treatment approaches are mostly divided into two types: the first mainly acts on a single DNMT target, while the second targets DNMTs in combination with other key enzymes, which will greatly improve the efficacy and the likelihood of patient recovery. The two methods are described below in detail.
4.1. Targeting a Single DNMT
4.1.1. Targeting of DNMT1 with Decitabine/Shikonin/RG108/DC_05
When a DNMTi is mentioned, the first thought is decitabine (5-aza-2′-deoxycytidine). The cytosine analog 5-aza-2′-deoxycytidine has been approved by the FDA for the treatment of hematological malignancies. When DNA replicates, 5-aza-2′-deoxycytidine is incorporated into the DNA. It can then trap DNMTs and effectively prevent them from methylating the DNA [83]. Hypermethylated TSGs will demethylate and restore the expression of TSGs. The nucleoside drug has a good anti-cancer effect, especially in hematological malignancies, and it also has a good effect in solid tumors. It is reported that aberrant methylation can lead to pancreatic cancer, and overexpression of DNMT1 may be a crucial factor leading to the increased DNA methylation in cancer cells, resulting in abnormal activation of transcription and inhibition of protein degradation. Thus, there is a very urgent need for drugs to treat pancreatic cancer [84]. Epigenetic silencing of SOCS3 (suppressor of cytokine signaling 3) has a carcinogenic effect and SOCS3 promoter methylation has been observed in pancreatic cancer. Wang et al. evaluated the methylation status of SOCS3 gene in 36 patients with pancreatic cancer by MSP analysis. The results showed that SOCS3 promoter was hypermethylated in one third of the patients, which was closely related to tumor size, tumor differentiation degree and TNM stage. It indicates that SOCS3 methylation is related to the malignant degree of pancreatic cancer [85]. Huang et al. demonstrated that abnormal activation of the IL-6 (interleukin-6)/STAT3 (signal transducer and activator of transcription 3) signaling pathway promotes transcriptional repression of SOCS3 by increasing DNMT1 activity, which can lead to the growth and metastasis of pancreatic cancer. The use of decitabine to target DNMT1 has delivered a breakthrough in therapy [72]. Decitabine can reverse IL-6 mediated downregulation of SOCS3 by preventing the hypermethylation of the SOCS3 promoter and increasing its transcriptional activity [33].
Shikonin is a naturally occurring naphthoquinone isolated from the herb purple gromwell (Lithospermum erythrorhizon) [86]. It has been reported to show anti-tumor activity in some human cancers [87,88]. Yang et al. noted that shikonin can inhibit thyroid cancer cell growth [89]. Zhang et al. explored the effect of shikonin on DNMT1 in thyroid cancer cells and found that DNMT1 is usually highly expressed and expression of PTEN is low in thyroid cancer. PTEN is a classic TSG which negatively regulates the PI3K/AKT signal pathway. It is a lipid phosphatase that dephosphorylates PIP3 (phosphatidylinositol (3,4,5)-trisphosphate) to PIP2 (phosphatidylinositol (4,5)-bisphosphate), This process inhibits AKT activity, thereby downregulating the signaling pathway and inhibiting tumor development [90]. Hypermethylation of the PTEN promoter in somatic cells downregulates PTEN expression. The DNMT1 gene acts upstream of PTEN and downregulates PTEN via promoter hypermethylation, which promotes the metastasis of human papillary thyroid cancer (TPC-1). Therefore, inhibiting the expression of DNMT1 is a potential therapy for thyroid cancer. It has been experimentally proven that shikonin can inhibit the expression of DNMT1, reduce PTEN gene methylation, and increase the expression of PTEN protein, which inhibits the migration of TPC-1 cells. Zhang et al. discovered that shikonin has an inhibitory ability on DNMT1. However, it is not clear how shikonin specifically inhibits DNMT1. It may still be an effective agent to treat thyroid cancer in the future [36].
The non-nucleoside inhibitor RG108 (N-phthaloyl-l-tryptophan 1) and selective non-nucleoside DNMT1 inhibitors in the DC_05 series of compounds (DC_501 and DC_517) can also play an anti-cancer role by inducing DNA hypomethylation to restore the expression of TSGs [91,92].
4.1.2. Targeting of DNMT3A with Decitabine/SGI-110/MC3353
Hyperactivity of DNMT3A often occurs in hematological tumors and other tumors. Therefore finding treatments that target DNMT3A has become a research hot spot [93]. Decitabine is one of the most commonly used classical drugs for treating leukemia. It directly inhibits DNA methyltransferase activity to reduce DNA methylation, thereby further inhibiting the proliferation of tumor cells and preventing the occurrence of drug resistance. It is the most powerful known specific DNMT inhibitor. Zhou et al. reported that the gene BASP1 (brain acid-soluble protein 1) plays a role as a TSG in tumors. Specific downregulation of the BASP1 gene and methylation of the promoter were observed in AML. The new fusion protein AML1-ETO (A/E) is considered as a possible cause of AML, and the A/E fusion protein can recruit DNMT3A to the BASP1 promoter region. DNMT3A methylates the BASP1 promoter sequence and silences BASP1 expression. Bisulfite sequencing analysis also showed that CpG islands were highly methylated in A/E positive AML cell lines, but not in A/E negative AML cell lines. It suggested that DNA methylation may be involved in transcription silencing of BASP1 in A/E positive AML. Upregulation of BASP1 gene expression by decitabine treatment can induce AML cell death [39]. BASP1, as a transcriptional regulator of WT1 (Wilms’ tumor 1) protein, participates in the regulation of many genes related to apoptosis, such as the BCL-2 family, c-Myc and p21 [94]. Cashen et al. also reported that a low-dose 5-day decitabine regimen in elderly AML patients has a good early therapeutic effect, less toxicity, and lower 30-day mortality [95].
SGI-110 (guadecitabine) is a second-generation hypomethylating compound, which is a dinucleotide of decitabine and deoxyguanosine linked by a phosphodiester bond. It prolongs the exposure time of the active product decitabine in vivo, thus producing a better effect [96]. It is also commonly used to treat AML diseases. As well as demethylating CpG-island promoters, it can also enhance the sensitivity of tumor cells to some anticancer drugs [97]. In hepatocarcinoma, Jueliger et al. used Luminometric Methylation Assay to analyze global DNA methylation, and found that the whole genome methylation levels of HepG2 and Huh-7 cells were similar. The promoters of the tumor suppressor genes MTS1 (multiple tumor suppressor 1 or p16), DLEC1 (Deleted in lung and oesophageal cancer 1) and RUNX3 (RUNX family transcription factor 3) are hypermethylated. Jueliger et al. discovered that SGI-110 demethylates the promoters of the tumor suppressor genes MTS1, DLEC1 and RUNX3 and achieves an anticancer effect [98]. Similarly, a new quinoline-based molecule, MC3353, can also inhibit the activity of DNMT1 and DNMT3A to reverse hypermethylation caused by cancer [99].
4.1.3. Targeting of DNMT3B with Nanaomycin A
There are many non-selective inhibitors that can inhibit DNMT3B along with other DNMTs, but here we introduce a rare selective DNMT3B inhibitor. Nanaomycin A is the first selective DNMT3B inhibitor which can induce genomic demethylation. It interacts with amino acid residues in DNMT3B which are involved in methylation, so that DNMT3B cannot participate in normal DNA methylation [100]. Nanaomycin A demethylated the DNA and reactivated the transcription and expression of the gene RASSF1A (ras association domain family 1 isoform A). RASSF1A is a TSG frequently silenced by promoter hypermethylation in cancer [101]. Kuck et al. noted that in lung cancer cells, the promoter region of RASSF1A was sequenced by bisulfite sequence, which confirmed that RASSF1A promoter was highly methylated in A549 cells [40]. Hepatocellular carcinoma (HCC) is a cancer with a high mortality rate worldwide, and patients are prone to drug resistance and poor prognosis in treatment [102]. This is mainly because increased expression of DNMT3B mediates increased expression of the OCT4 gene (octamer-binding transcription factor 4) through the IL-6/STAT3 pathway in HCC cells. Lai et al. found that the activation of p-STAT3 increased the resistance of HCC cells to sorafenib and explored whether nanaomycin A also achieves a good therapeutic effect in HCC. They demonstrated that nanaomycin A targeted DNMT3B to inhibit OCT4 expression and increase the sensitivity of drug-resistant cells. The OCT4 promoter is typically hypermethylated in normal liver cells. However, the methylation status of oncogene OCT4 during carcinogenesis still remains unclear. Perhaps there are other mediators between DNMT3B and OCT4 [41].
4.2. Combination Targeting Approaches
4.2.1. Combination of Decitabine and HDACi
The decline in the efficacy of decitabine alone has led to the emergence of combination therapies. The effect of decitabine combined with a histone deacetylase inhibitor (HDACi) has been gradually verified in different tumors, such as AML [103]. As DNMTis are common drugs for the treatment of hematological malignancies, we will not introduce more leukemia-related applications here. Instead, we want to present more extensive applications, so we will now focus on solid tumors. Lung cancer is one of the most frequently occurring malignancies in the world, and its incidence rate and mortality rate are the highest of all cancers [104]. In general, drugs are the first choice for treating lung cancer. However, in cases where the expression of DNMT genes is altered, the effect of drug treatment is reduced and the condition becomes worse.
As described above, evidence suggests that overexpression of DNMTs induces DNA methylation and silencing of TSGs, leading to malignant lung cancer. Forced expression of DNMT1 or DNMT3A increases DNA methylation, while elimination of DNMT expression by small molecule inhibitors reduces DNA methylation of the entire genome and specific genes. More importantly, it restores the expression of TSGs. Their silencing predicts poor results by promoter hypermethylation. In order to change this hypermethylation state, Belinsky et al. proposed that combining decitabine with an HDACi (sodium phenylbutyrate) would restore the normal methylation state in murine lung cancer, including the re-expression of TSGs that are densely hypermethylated and transcriptionally silenced [61]. Similarly, Vendetti et al. proposed epigenetic treatments that target DNMTs and HDACs in non-small cell lung cancer [105].
Among malignant tumors of the female genital tract, ovarian cancer is the third in terms of incidence and mortality. Thus, it is essential to understand the molecular mechanisms underlying ovarian cancer and find effective drugs for the treatment of this highly metastatic and deadly disease [106]. Zhao et al. found that the increased expression of DNMT3B in ovarian cancer cells increased the methylation level of the promoter regions of anti-oncogenes such as hMLH1 (mismatch repair gene human mutL homolog-1), p16 and p53. These methylation changes decreased the expression of the hMLH1, p16 and p53 genes and promoted tumor development [42,107]. As mentioned above, decitabine is a specific DNMT inhibitor, which can partially reverse DNA methylation. Steele et al. noted that the effect of decitabine on demethylation is limited by the dose and the eventual remethylation of the gene. They also noted that histone acetylation is related to chromatin opening, which helps to promote gene transcription and is closely involved in the proliferation, differentiation and progress of tumor cells. Therefore, they used decitabine with a clinically effective inhibitor of histone deacetylase activity (belinostat), and found that this combination restored the expression of epigenetically silenced genes [108].
4.2.2. Combination of Decitabine and GSK126
EZH2 is the catalytic subunit of the polycomb repressive complex 2 (PRC2), which regulates histone methylation (H3K27me3) [109]. EZH2 is often dysregulated in melanoma, the deadliest skin cancer, and it has been shown to interact with DNMT and cause hypermethylation of TSG promoters, resulting in silencing of TSGs [110]. Tiffen et al. found DNA methylation and H3K27me3 on some EZH2 target genes. Moreover, treatment of melanoma cell lines with GSK126 alone did not restore the expression of EZH2 target genes, and there was no significant change in DNA methylation in TSG promoter regions, which proves that DNA hypermethylation inhibits the expression of EZH2 target genes in melanomas. Tiffen et al. speculated that the combination of decitabine and GSK126 would result in a more effective upregulation of EZH2 target genes compared to either inhibitor alone. After the combined treatment, it was found that two EZH2 target genes were upregulated, namely the tumor suppressor gene RASSF5 (ras association domain family member 5) and ITGB2 (integrin subunit Beta 2), which is associated with anti-tumor immunity [111,112]. The results showed that EZH2-driven hypermethylation silenced these two target genes. This may be due to the direct interaction between DNMTs and EZH2 protein domains, or to the recruitment of EZH2-containing PRC2 complex to unmethylated CpG islands. The combined use of a DNMTi and an EZH2 inhibitor (EZH2i) may lead to expression of tumor suppressor genes and increased immune responses to effectively treat melanoma [49].
4.2.3. Combination of AZA and RPM
DNMT-mediated abnormal DNA methylation is a common epigenetic change in the early stages of tumorigenesis and is closely related to the development of colorectal cancer (CRC), the third most frequently diagnosed malignancy worldwide. Most colorectal cancers have no obvious symptoms in the early stage and are difficult to treat in the later stage [113]. Rodriguez et al. found that DNMT suppression activates gene promoter regions (especially TSG promoters) after demethylation, thereby inhibiting cancer growth [114].
Zhang et al. noted that mammalian target of rapamycin (mTOR) plays a critical role in cell growth and homeostasis, and it is often altered in cancers. Based on these reasons, mTOR is currently being studied as a potential target for anti-cancer therapy. Rapamycin (RPM) is an mTOR inhibitor (mTORi) and has been proved to have anti-tumor activity. Zhang et al. investigated whether a combination of RPM and other inhibitors has additive effects on tumor growth. They found that combining RPM with 5-aza-deoxycytidine (AZA) effectively eliminated the downstream effects of mTOR activation in colorectal cancer cell lines. The mechanism may relate to the regulation of tumor suppressor factors, such as PTEN, which play a role upstream of the PI3K/AKT/mTOR signaling pathway, leading to significant apoptosis as well as cell cycle arrest. In addition, in a mouse CRC model, the combination of AZA and RPM reduced the incidence of CRC and the tumor volume, and decreased the activity of the mTOR signaling pathway. The results showed that a DNMTi combined with an mTORi had inhibitory effects on colorectal cancer cells in vivo and in vitro. Such combinations may help to optimize the overall benefits associated with molecularly targeted anti-cancer drugs [115].
4.2.4. Dual Inhibitors
Combining the effects of two inhibitors in the same compound will achieve better efficacy. C02S is such a drug and may be used to treat breast cancer, which is the second most common cause of cancer-related deaths in women [116]. Most breast cancers have a poor prognosis, are prone to drug resistance, and lack targeted agents [117]. Some breast cancer cells exhibit a genomic hypermethylation phenotype, which is caused by the abnormal activity of DNMTs. High expression of DNMT3B protein is related to the overall increase in DNMT activity, thus inducing highly methylation of multiple genes (CDH1, CEACAM6, CST6, ESR1, LCN2 and SCNN1A) [43]. It has also been reported that overexpression of DNMT3B increases the recurrence of breast cancer in patients treated with tamoxifen [118]. Yuan et al. noted that DNMTs and HDACs respectively catalyze the methylation of CpG islands in DNA and the deacetylation of histones and other substrate proteins. Abnormalities in these enzymes are related to the reduced expression of TSGs (p16, p21 and TIMP3), and their interaction reinforces the silencing effect of TSGs. Yuan et al. found that the compound C02S, which acts as a dual DNMTi and HDACi, was effective in breast cancer cells and downregulated multiple cancer-related processes. These results suggest that C02S may be a means of treating breast cancer in the future [116].
In addition, CM-272 is a potent dual inhibitor of G9a (a type of histone methyltransferase) and DNMTs for the treatment of hematological malignancies [119]. G9a is responsible for methylating lysine 9 of histone 3 (H3K9), and overexpression of G9a leads to increased tumor progression in some tumors [120,121]. It is involved in the transcriptional repression of the tumor suppressor gene PTEN, and upregulation of the G9a gene and downregulation of the PTEN gene can predict poor overall survival [122]. Decreasing the methylation level of DNA and H3K9 can cause reactivation of PTEN and inhibit cancer cell proliferation. San et al. synthesized a compound, CM-272, to simultaneously target G9a and DNMTs as an improved approach for cancer treatment. They demonstrated that CM-272 inhibited the proliferation of different hematological cancer cells and promoted cancer cell apoptosis. Dual inhibition of G9a/DNMTs represents a novel method for safely and effectively targeting cancer [119].
5. Conclusions and Perspectives
It is known that DNA methyltransferases play an essential role in life and participate in the development of many cancers. This review has systematically introduced the characteristics of DNMTs, the DNMT3A paradox and the different roles of DNMTs in cancers, and the strategies that are available for targeting DNMTs in cancer therapy.
We propose that the DNMT3A paradox may result from the different characteristics of DNMT3A in different processes. On the one hand, DNMT3A plays a critical role in promoting tumorigenesis by hypermethylation of certain regions, such as the promoters of TSGs. Decreased activity of DNMT3A causes DNA demethylation, and TSGs may be re-expressed to suppress tumors via an increase of microRNAs [64,123]. On the other hand, DNMT3A guards the stability of the genome by maintaining global genomic hypermethylation. In this respect, it is equivalent to a tumor suppressor gene. When the DNMT3A gene is deleted by experimental means, the methylation level decreases and the CpG sites originally modified by methylation are hypomethylated. Thus, the chromatin structure opens up, which makes the genome unstable and susceptible to alteration. The degree of malignancy will increase [66,67,68]. In addition, we suspect that the paradox is maybe due to various types of lung cancer cells with different inherent mutations, such as EGFR mutation, KRAS mutation, TP53 mutation, etc [124]. It is worth considering whether DNMT genes and these genes will produce synergistic or antagonistic effects, which will lead to distinct functions of DNMT.
Since DNMT overexpression often occurs in cancer, many researchers have tried to use drugs to specifically target DNMTs for therapeutic purposes. The whole-genome hypomethylation in cancer cells can be restored to normal levels by targeting DNMTs in a variety of tumors. Targeting of DNMTs can also reverse the hypermethylation of TSG promoter regions which is seen in many cancers. This targeted therapy is powerful and widely applicable to different cancers. In addition to targeting DNMTs alone, it is possible to use combined treatments to improve the effect (see Figure 4).
Figure 4.
Strategies for targeting DNMTs in cancers. In normal cells, the genome sequence is usually methylated, and the promoter regions of TSGs are unmethylated. In tumor cells, DNMT aberrations cause hypomethylation of the genome sequence and hypermethylation of TSG promoters. There are two therapeutic strategies: one is to use a DNMTi that targets a single DNMT, and the other is to use a combinatorial approach, such as DNMTi + HDACi, DNMTi + EZH2i, DNMTi + mTORi, dual inhibitors, or synthetic lethality.
However, there are some potential drawbacks of using DNMTi. On the one hand, clinical trials and clinically approved DNMTis are mostly applicable to hematological tumors. In addition to decitabine, which is often mentioned above, there is guadecitabine for the treatment of relapsed/refractory AML [125], and the combination of DNMTi CC-486 and HDACi romidepsin for the treatment of advanced solid tumors [126]. These drugs are also very promising in clinical applications. In our review, for some cancers, targeted DNMT agents may not be the most suitable and some drugs can inhibit multiple DNMT members simultaneously. However, we want to provide a novel idea to treat multiple cancers in order to increase the diversity of therapies. On the other hand, this method only targets the particular tumors of overexpressed DNMTs and can prevent hypermethylation of TSGs promoter. It is not suitable to use DNMT inhibitors to treat the cancers caused by decreased DNMTs activity, and the therapies for this type of cancers need to be further explored.
The previous results from our group have demonstrated that DNMT3A acts as a tumor suppressor gene in lung cancer. We knocked out DNMT3A using the CRISPR-Cas9 system and found that it participates in cell proliferation, migration and invasion. However, the high mutation rate of DNMT3A always results in loss of DNMT3A function, and it is difficult to target loss-of-function mutations. Furthermore, although DNMT inhibitors have some effect in solid tumors, they are not as effective as in hematological malignancies [61,127], and therefore we still do not have an effective cure for lung cancer. We are looking forward to identifying some effective molecular targets and extending them to the therapy of other types of cancer. We speculate that it will be possible to develop new therapeutic approaches, such as synthetic lethality [128]. We can use the CRISPR-Cas9 system, shRNA technology or inhibitor libraries for high-throughput screening [129,130], and identifies multiple epigenetic-related synthetic lethal pairs [131,132,133]. Cancer cells with “undruggable” mutant genes can be selectively killed by specifically targeting the function of a synthetic lethal partner [134]. Perhaps we can find new DNMT-targeted tumor treatments through a synthetic lethality strategy (see Figure 4).
In summary, this review presents the biological characteristics of DNMTs, their alterations in different malignancies, and therapies that target them. In addition, we have proposed more ideas for targeting DNMTs, which may provide the foundation for future cancer treatments.
Author Contributions
J.Z. and C.Y. contributed to drafting the manuscript and analyzing the study; L.W., W.C. and C.W. contributed to designing and drafting the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the National Natural Science Foundation of China (No. 81773216, 81673652, and 81973365), the Natural Science Foundation of Liaoning Province (No. 20170540841, and 20180550076), the “Xingliao Talents” Program of Liaoning Province (No. XLYC1902008), and the Youth Science and Technology Innovation Leader Program of Shenyang (No.RC190457).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Egger G., Liang G., Aparicio A., Jones P. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 2.Arrowsmith C.H., Bountra C., Fish P.V., Lee K., Schapira M. Epigenetic protein families: A new frontier for drug discovery. Nat. Rev. Drug Discov. 2012;11:384–400. doi: 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- 3.Jones P.A., Baylin S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 4.Holliday R., Pugh J.E. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–232. doi: 10.1126/science.1111098. [DOI] [PubMed] [Google Scholar]
- 5.Feng S., Jacobsen S.E., Reik W. Epigenetic reprogramming in plant and animal development. Science. 2010;330:622–627. doi: 10.1126/science.1190614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okano M., Xie S., Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat. Genet. 1998;19:219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 7.Turek-Plewa J., Jagodziński P.P. The role of mammalian DNA methyltransferases in the regulation of gene expression. Cell. Mol. Biol. Lett. 2005;10:631–647. [PubMed] [Google Scholar]
- 8.Kulis M., Esteller M. DNA methylation and cancer. Adv. Genet. 2010;70:27–56. doi: 10.1016/B978-0-12-380866-0.60002-2. [DOI] [PubMed] [Google Scholar]
- 9.Jezek M., Green E.M. Histone Modifications and the Maintenance of Telomere Integrity. Cells. 2019;8:199. doi: 10.3390/cells8020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Mattick J.S., Makunin I.V. Non-coding RNA. Hum. Mol. Genet. 2006;15:R17–R29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Liu C. Coding or Noncoding, the Converging Concepts of RNAs. Front. Genet. 2019;10:496. doi: 10.3389/fgene.2019.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laird P.W., Jaenisch R. The role of DNA methylation in cancer genetic and epigenetics. Annu. Rev. Genet. 1996;30:441–464. doi: 10.1146/annurev.genet.30.1.441. [DOI] [PubMed] [Google Scholar]
- 14.Jones P.A. Epigenetics in carcinogenesis and cancer prevention. Ann. N. Y. Acad. Sci. 2003;983:213–219. doi: 10.1111/j.1749-6632.2003.tb05976.x. [DOI] [PubMed] [Google Scholar]
- 15.Hermann A., Gowher H., Jeltsch A. Biochemistry and biology of mammalian DNA methyltransferases. Cell. Mol. Life Sci. 2004;61:2571–2587. doi: 10.1007/s00018-004-4201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byun D.S., Lee M.G., Chae K.S., Ryu B.G., Chi S.G. Frequent epigenetic inactivation of RASSF1A by aberrant promoter hypermethylation in human gastric adenocarcinoma. Cancer Res. 2001;61:7034–7038. [PubMed] [Google Scholar]
- 17.Bestor T., Laudano A., Mattaliano R., Ingram V. Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. The carboxyl-terminal domain of the mammalian enzymes is related to bacterial restriction methyltransferases. J. Mol. Biol. 1988;203:971–983. doi: 10.1016/0022-2836(88)90122-2. [DOI] [PubMed] [Google Scholar]
- 18.Tuorto F., Herbst F., Alerasool N., Bender S., Popp O., Federico G., Reitter S., Liebers R., Stoecklin G., Grone H.J., et al. The tRNA methyltransferase Dnmt2 is required for accurate polypeptide synthesis during haematopoiesis. EMBO J. 2015;34:2350–2362. doi: 10.15252/embj.201591382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hervouet E., Peixoto P., Delage-Mourroux R., Boyer-Guittaut M., Cartron P.F. Specific or not specific recruitment of DNMTs for DNA methylation, an epigenetic dilemma. Clin. Epigenet. 2018;10:17. doi: 10.1186/s13148-018-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurkowska R.Z., Anspach N., Urbanke C., Jia D., Reinhardt R., Nellen W., Cheng X., Jeltsch A. Formation of nucleoprotein filaments by mammalian DNA methyltransferase Dnmt3a in complex with regulator Dnmt3L. Nucleic Acids Res. 2008;36:6656–6663. doi: 10.1093/nar/gkn747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeshima H., Suetake I., Shimahara H., Ura K., Tate S., Tajima S. Distinct DNA methylation activity of Dnmt3a and Dnmt3b towards naked and nucleosomal DNA. J. Biochem. 2006;139:503–515. doi: 10.1093/jb/mvj044. [DOI] [PubMed] [Google Scholar]
- 22.Robertson K.D., Uzvolgyi E., Liang G., Talmadge C., Sumegi J., Gonzales F.A., Jones P.A. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: Coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res. 1999;27:2291–2298. doi: 10.1093/nar/27.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araujo F.D., Croteau S., Slack A.D., Milutinovic S., Bigey P., Price G.B., Zannis-Hadjopoulos M., Szyf M. The DNMT1 target recognition domain resides in the N terminus. J. Biol. Chem. 2001;276:6930–6936. doi: 10.1074/jbc.M009037200. [DOI] [PubMed] [Google Scholar]
- 24.Xie S., Wang Z., Okano M., Nogami M., Li Y., He W.W., Okumura K., Li E. Cloning, expression and chromosome locations of the human DNMT3 gene family. Gene. 1999;236:87–95. doi: 10.1016/S0378-1119(99)00252-8. [DOI] [PubMed] [Google Scholar]
- 25.Chen T., Tsujimoto N., Li E. The PWWP domain of Dnmt3a and Dnmt3b is required for directing DNA methylation to the major satellite repeats at pericentric heterochromatin. Mol. Cell. Biol. 2004;24:9048–9058. doi: 10.1128/MCB.24.20.9048-9058.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo X., Wang L., Li J., Ding Z., Xiao J., Yin X., He S., Shi P., Dong L., Li G., et al. Structural insight into autoinhibition and histone H3-induced activation of DNMT3A. Nature. 2015;517:640–644. doi: 10.1038/nature13899. [DOI] [PubMed] [Google Scholar]
- 27.Uysal F., Ozturk S. DNA Methyltransferases in Mammalian Oocytes. Results Probl. Cell Differ. 2017;63:211–222. doi: 10.1007/978-3-319-60855-6_10. [DOI] [PubMed] [Google Scholar]
- 28.Okano M., Bell D.W., Haber D.A., Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z., Zhang Y. Role of Mammalian DNA Methyltransferases in Development. Annu. Rev. Biochem. 2019 doi: 10.1146/annurev-biochem-103019-102815. [DOI] [PubMed] [Google Scholar]
- 30.Smith S.S., Kaplan B.E., Sowers L.C., Newman E.M. Mechanism of human methyl-directed DNA methyltransferase and the fidelity of cytosine methylation. Proc. Natl. Acad. Sci. USA. 1992;89:4744–4748. doi: 10.1073/pnas.89.10.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma H.S., Wang E.L., Xu W.F., Yamada S., Yoshimoto K., Qian Z.R., Shi L., Liu L.L., Li X.H. Overexpression of DNA (Cytosine-5)-Methyltransferase 1 (DNMT1) And DNA (Cytosine-5)-Methyltransferase 3A (DNMT3A) Is Associated with Aggressive Behavior and Hypermethylation of Tumor Suppressor Genes in Human Pituitary Adenomas. Med. Sci. Monit. 2018;24:4841–4850. doi: 10.12659/MSM.910608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanai Y., Ushijima S., Nakanishi Y., Sakamoto M., Hirohashi S. Mutation of the DNA methyltransferase (DNMT) 1 gene in human colorectal cancers. Cancer Lett. 2003;192:75–82. doi: 10.1016/S0304-3835(02)00689-4. [DOI] [PubMed] [Google Scholar]
- 33.Huang L., Hu B., Ni J., Wu J., Jiang W., Chen C., Yang L., Zeng Y., Wan R., Hu G., et al. Transcriptional repression of SOCS3 mediated by IL-6/STAT3 signaling via DNMT1 promotes pancreatic cancer growth and metastasis. J. Exp. Clin. Cancer Res. 2016;35:27. doi: 10.1186/s13046-016-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Etoh T., Kanai Y., Ushijima S., Nakagawa T., Nakanishi Y., Sasako M., Kitano S., Hirohashi S. Increased DNA Methyltransferase 1 (DNMT1) Protein Expression Correlates Significantly with Poorer Tumor Differentiation and Frequent DNA Hypermethylation of Multiple CpG Islands in Gastric Cancers. Am. J. Pathol. 2004;164:689–699. doi: 10.1016/S0002-9440(10)63156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin R.K., Wu C.Y., Chang J.W., Juan L.J., Hsu H.S., Chen C.Y., Lu Y.Y., Tang Y.A., Yang Y.C., Yang P.C., et al. Dysregulation of p53/Sp1 control leads to DNA methyltransferase-1 overexpression in lung cancer. Cancer Res. 2010;70:5807–5817. doi: 10.1158/0008-5472.CAN-09-4161. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y., Sun B., Huang Z., Zhao D.W., Zeng Q. Shikonin Inhibites Migration and Invasion of Thyroid Cancer Cells by Downregulating DNMT1. Med. Sci. Monit. 2018;24:661–670. doi: 10.12659/MSM.908381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard S., Pereira M., Fox R., Gordon N., Yap J., Kehoe S., Luesley D., Woodman C., Ganesan R. Over-expression of DNMT3A predicts the risk of recurrent vulvar squamous cell carcinomas. Gynecol. Oncol. 2016;143:414–420. doi: 10.1016/j.ygyno.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Yan X.J., Xu J., Gu Z.H., Pan C.M., Lu G., Shen Y., Shi J.Y., Zhu Y.M., Tang L., Zhang X.W., et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat. Genet. 2011;43:309–315. doi: 10.1038/ng.788. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L., Fu L., Lv N., Liu J., Li Y., Chen X., Xu Q., Chen G., Pang B., Wang L., et al. Methylation-associated silencing of BASP1 contributes to leukemogenesis in t(8;21) acute myeloid leukemia. Exp. Mol. Med. 2018;50:44. doi: 10.1038/s12276-018-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuck D., Caulfield T., Lyko F., Medina-Franco J.L. Nanaomycin A selectively inhibits DNMT3B and reactivates silenced tumor suppressor genes in human cancer cells. Mol. Cancer Ther. 2010;9:3015–3023. doi: 10.1158/1535-7163.MCT-10-0609. [DOI] [PubMed] [Google Scholar]
- 41.Lai S.C., Su Y.T., Chi C.C., Kuo Y.C., Lee K.F., Wu Y.C., Lan P.C., Yang M.H., Chang T.S., Huang Y.H. DNMT3b/OCT4 expression confers sorafenib resistance and poor prognosis of hepatocellular carcinoma through IL-6/STAT3 regulation. J. Exp. Clin. Cancer Res. 2019;38:474. doi: 10.1186/s13046-019-1442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao L., Shou H., Chen L., Gao W., Fang C., Zhang P. Effects of ginsenoside Rg3 on epigenetic modification in ovarian cancer cells. Oncol. Rep. 2019;41:3209–3218. doi: 10.3892/or.2019.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roll J.D., Rivenbark A.G., Jones W.D., Coleman W.B. DNMT3b overexpression contributes to a hypermethylator phenotype in human breast cancer cell lines. Mol. Cancer. 2008;7:15. doi: 10.1186/1476-4598-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei Y., Huang Y.H., Goodell M.A. DNA methylation and de-methylation using hybrid site-targeting proteins. Genome Biol. 2018;19:187. doi: 10.1186/s13059-018-1566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poirier J.T., Gardner E.E., Connis N., Moreira A.L., de Stanchina E., Hann C.L., Rudin C.M. DNA methylation in small cell lung cancer defines distinct disease subtypes and correlates with high expression of EZH2. Oncogene. 2015;34:5869–5878. doi: 10.1038/onc.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabatucci A., Berchet V., Bellia F., Maccarrone M., Dainese E., D’Addario C., Pucci M. A new methodological approach for in vitro determination of the role of DNA methylation on transcription factor binding using AlphaScreen® analysis: Focus on CREB1 binding at hBDNF promoter IV. J. Neurosci. Methods. 2020;341:108720. doi: 10.1016/j.jneumeth.2020.108720. [DOI] [PubMed] [Google Scholar]
- 47.Dhayalan A., Rajavelu A., Rathert P., Tamas R., Jurkowska R.Z., Ragozin S., Jeltsch A. The Dnmt3a PWWP domain reads histone 3 lysine 36 trimethylation and guides DNA methylation. J. Biol. Chem. 2010;285:26114–26120. doi: 10.1074/jbc.M109.089433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baubec T., Colombo D.F., Wirbelauer C., Schmidt J., Burger L., Krebs A.R., Akalin A., Schubeler D. Genomic profiling of DNA methyltransferases reveals a role for DNMT3B in genic methylation. Nature. 2015;520:243–247. doi: 10.1038/nature14176. [DOI] [PubMed] [Google Scholar]
- 49.Tiffen J., Gallagher S., Filipp F., Gunatilake D., Al Emran A., Cullinane C., Dutton-Register K., Aoude L., Hayward N., Chatterjee A., et al. EZH2 cooperates with DNA methylation to downregulate key tumour suppressors and interferon gene signatures in melanoma. J. Investig. Dermatol. 2020 doi: 10.1016/j.jid.2020.02.042. [DOI] [PubMed] [Google Scholar]
- 50.Miller C.A., Sweatt J.D. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Morgan A.E., Davies T.J., Mc Auley M.T. The role of DNA methylation in ageing and cancer. Proc. Nutr. Soc. 2018;77:412–422. doi: 10.1017/S0029665118000150. [DOI] [PubMed] [Google Scholar]
- 52.Valencia A.M., Kadoch C. Chromatin regulatory mechanisms and therapeutic opportunities in cancer. Nat. Cell Biol. 2019;21:152–161. doi: 10.1038/s41556-018-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y., Zhang L., Li R., Chang D.W., Ye Y., Minna J.D., Roth J.A., Han B., Wu X. Genetic variations in cancer-related significantly mutated genes and lung cancer susceptibility. Ann. Oncol. 2017;28:1625–1630. doi: 10.1093/annonc/mdx161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han M., Jia L., Lv W., Wang L., Cui W. Epigenetic Enzyme Mutations: Role in Tumorigenesis and Molecular Inhibitors. Front. Oncol. 2019;9:194. doi: 10.3389/fonc.2019.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao J., Wang L., Xu J., Zheng J., Man X., Wu H., Jin J., Wang K., Xiao H., Li S., et al. Aberrant DNA Methyltransferase Expression in Pancreatic Ductal Adenocarcinoma Development and Progression. J. Exp. Clin. Cancer Res. 2013;32:86. doi: 10.1186/1756-9966-32-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Micevic G., Theodosakis N., Bosenberg M. Aberrant DNA methylation in melanoma: Biomarker and therapeutic opportunities. Clin. Epigenet. 2017;9:34. doi: 10.1186/s13148-017-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erdmann A., Halby L., Fahy J., Arimondo P.B. Targeting DNA methylation with small molecules: What’s next? J. Med. Chem. 2015;58:2569–2583. doi: 10.1021/jm500843d. [DOI] [PubMed] [Google Scholar]
- 58.Jung H., Kim H.S., Kim J.Y., Sun J.M., Ahn J.S., Ahn M.J., Park K., Esteller M., Lee S.H., Choi J.K. DNA methylation loss promotes immune evasion of tumours with high mutation and copy number load. Nat. Commun. 2019;10:4278. doi: 10.1038/s41467-019-12159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheaffer K.L., Elliott E.N., Kaestner K.H. DNA Hypomethylation Contributes to Genomic Instability and Intestinal Cancer Initiation. Cancer Prev. Res. 2016;9:534–546. doi: 10.1158/1940-6207.CAPR-15-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma S., Kelly T.K., Jones P.A. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belinsky S.A., Klinge D.M., Stidley C.A., Issa J.-P., Herman J.G., March T.H., Baylin S.B. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 2003;63:7089–7093. [PubMed] [Google Scholar]
- 62.Haney S.L., Hlady R.A., Opavska J., Klinkebiel D., Pirruccello S.J., Dutta S., Datta K., Simpson M.A., Wu L., Opavsky R. Methylation-independent repression of Dnmt3b contributes to oncogenic activity of Dnmt3a in mouse MYC-induced T-cell lymphomagenesis. Oncogene. 2015;34:5436–5446. doi: 10.1038/onc.2014.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rinaldi L., Avgustinova A., Martin M., Datta D., Solanas G., Prats N., Benitah S.A. Loss of Dnmt3a and Dnmt3b does not affect epidermal homeostasis but promotes squamous transformation through PPAR-gamma. eLife. 2017;6:e21697. doi: 10.7554/eLife.21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan F., Shen N., Pang J., Xie D., Deng B., Molina J.R., Yang P., Liu S. Restoration of miR-101 suppresses lung tumorigenesis through inhibition of DNMT3a-dependent DNA methylation. Cell Death Dis. 2014;5:e1413. doi: 10.1038/cddis.2014.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu T., Wu X., Chen T., Luo Z., Hu X. Downregulation of DNMT3A by miR-708-5p Inhibits Lung Cancer Stem Cell-like Phenotypes through Repressing Wnt/beta-catenin Signaling. Clin. Cancer Res. 2018;24:1748–1760. doi: 10.1158/1078-0432.CCR-17-1169. [DOI] [PubMed] [Google Scholar]
- 66.Husni R.E., Shiba-Ishii A., Iiyama S., Shiozawa T., Kim Y., Nakagawa T., Sato T., Kano J., Minami Y., Noguchi M. DNMT3a expression pattern and its prognostic value in lung adenocarcinoma. Lung Cancer. 2016;97:59–65. doi: 10.1016/j.lungcan.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 67.Gao Q., Steine E.J., Barrasa M.I., Hockemeyer D., Pawlak M., Fu D., Reddy S., Bell G.W., Jaenisch R. Deletion of the de novo DNA methyltransferase Dnmt3a promotes lung tumor progression. Proc. Natl. Acad. Sci. USA. 2011;108:18061–18066. doi: 10.1073/pnas.1114946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raddatz G., Gao Q., Bender S., Jaenisch R., Lyko F. Dnmt3a protects active chromosome domains against cancer-associated hypomethylation. PLoS Genet. 2012;8:e1003146. doi: 10.1371/journal.pgen.1003146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seton-Rogers S. The mechanics of tumour formation. Nat. Rev. Cancer. 2019;19:183. doi: 10.1038/s41568-019-0124-x. [DOI] [PubMed] [Google Scholar]
- 70.Dart A. Tumour microenvironment: Radical changes. Nat. Rev. Cancer. 2018;18:65. doi: 10.1038/nrc.2018.4. [DOI] [PubMed] [Google Scholar]
- 71.Klein C.J., Botuyan M.V., Wu Y., Ward C.J., Nicholson G.A., Hammans S., Hojo K., Yamanishi H., Karpf A.R., Wallace D.C., et al. Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss. Nat. Genet. 2011;43:595–600. doi: 10.1038/ng.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li A., Omura N., Hong S.M., Goggins M. Pancreatic cancer DNMT1 expression and sensitivity to DNMT1 inhibitors. Cancer Biol. Ther. 2010;9:321–329. doi: 10.4161/cbt.9.4.10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Emperle M., Adam S., Kunert S., Dukatz M., Baude A., Plass C., Rathert P., Bashtrykov P., Jeltsch A. Mutations of R882 change flanking sequence preferences of the DNA methyltransferase DNMT3A and cellular methylation patterns. Nucleic Acids Res. 2019;47:11355–11367. doi: 10.1093/nar/gkz911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Im A.P., Sehgal A.R., Carroll M.P., Smith B.D., Tefferi A., Johnson D.E., Boyiadzis M. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid malignancies: Associations with prognosis and potential treatment strategies. Leukemia. 2014;28:1774–1783. doi: 10.1038/leu.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eklund E.A. The role of HOX genes in malignant myeloid disease. Curr. Opin. Hematol. 2007;14:85–89. doi: 10.1097/MOH.0b013e32801684b6. [DOI] [PubMed] [Google Scholar]
- 76.Deng T., Kuang Y., Wang L., Li J., Wang Z., Fei J. An essential role for DNA methyltransferase 3a in melanoma tumorigenesis. BioChem. Biophys. Res. Commun. 2009;387:611–616. doi: 10.1016/j.bbrc.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 77.Haney S.L., Upchurch G.M., Opavska J., Klinkebiel D., Hlady R.A., Suresh A., Pirruccello S.J., Shukla V., Lu R., Costinean S., et al. Promoter Hypomethylation and Expression Is Conserved in Mouse Chronic Lymphocytic Leukemia Induced by Decreased or Inactivated Dnmt3a. Cell Rep. 2016;15:1190–1201. doi: 10.1016/j.celrep.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hansen R., Wijmenga C., Luo P., Stanek A., Canfield T., Weemaes C., Gartler S. The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proc. Natl. Acad. Sci. USA. 1999;96:14412–14417. doi: 10.1073/pnas.96.25.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Linhart H.G., Lin H., Yamada Y., Moran E., Steine E.J., Gokhale S., Lo G., Cantu E., Ehrich M., He T., et al. Dnmt3b promotes tumorigenesis in vivo by gene-specific de novo methylation and transcriptional silencing. Genes Dev. 2007;21:3110–3122. doi: 10.1101/gad.1594007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steine E.J., Ehrich M., Bell G.W., Raj A., Reddy S., van Oudenaarden A., Jaenisch R., Linhart H.G. Genes methylated by DNA methyltransferase 3b are similar in mouse intestine and human colon cancer. J. Clin. Investig. 2011;121:1748–1752. doi: 10.1172/JCI43169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin H., Yamada Y., Nguyen S., Linhart H., Jackson-Grusby L., Meissner A., Meletis K., Lo G., Jaenisch R. Suppression of intestinal neoplasia by deletion of Dnmt3b. Mol. Cell. Biol. 2006;26:2976–2983. doi: 10.1128/MCB.26.8.2976-2983.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hlady R.A., Novakova S., Opavska J., Klinkebiel D., Peters S.L., Bies J., Hannah J., Iqbal J., Anderson K.M., Siebler H.M., et al. Loss of Dnmt3b function upregulates the tumor modifier Ment and accelerates mouse lymphomagenesis. J. Clin. Investig. 2012;122:163–177. doi: 10.1172/JCI57292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Orta M.L., Calderon-Montano J.M., Dominguez I., Pastor N., Burgos-Moron E., Lopez-Lazaro M., Cortes F., Mateos S., Helleday T. 5-Aza-2′-deoxycytidine causes replication lesions that require Fanconi anemia-dependent homologous recombination for repair. Nucleic Acids Res. 2013;41:5827–5836. doi: 10.1093/nar/gkt270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 85.Wang J., Zhou H., Han Y., Liu X., Wang M., Wang X., Yin G., Li X., Xiang M. SOCS3 methylation in synergy with Reg3A overexpression promotes cell growth in pancreatic cancer. J. Mol. Med. 2014;92:1257–1269. doi: 10.1007/s00109-014-1184-8. [DOI] [PubMed] [Google Scholar]
- 86.Jang S.Y., Hong D., Jeong S.Y., Kim J.H. Shikonin causes apoptosis by up-regulating p73 and down-regulating ICBP90 in human cancer cells. Biochem. Biophys. Res. Commun. 2015;465:71–76. doi: 10.1016/j.bbrc.2015.07.131. [DOI] [PubMed] [Google Scholar]
- 87.Boulos J.C., Rahama M., Hegazy M.F., Efferth T. Shikonin derivatives for cancer prevention and therapy. Cancer Lett. 2019;459:248–267. doi: 10.1016/j.canlet.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 88.Gara R.K., Srivastava V.K., Duggal S., Bagga J.K., Bhatt M., Sanyal S., Mishra D.P. Shikonin selectively induces apoptosis in human prostate cancer cells through the endoplasmic reticulum stress and mitochondrial apoptotic pathway. J. Biomed. Sci. 2015;22:26. doi: 10.1186/s12929-015-0127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Q., Ji M., Guan H., Shi B., Hou P. Shikonin inhibits thyroid cancer cell growth and invasiveness through targeting major signaling pathways. J. Clin. Endocrinol. Metab. 2013;98:E1909–E1917. doi: 10.1210/jc.2013-2583. [DOI] [PubMed] [Google Scholar]
- 90.Bazzichetto C., Conciatori F., Pallocca M., Falcone I., Fanciulli M., Cognetti F., Milella M., Ciuffreda L. PTEN as a Prognostic/Predictive Biomarker in Cancer: An Unfulfilled Promise? Cancers. 2019;11:435. doi: 10.3390/cancers11040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Asgatay S., Champion C., Marloie G., Drujon T., Senamaud-Beaufort C., Ceccaldi A., Erdmann A., Rajavelu A., Schambel P., Jeltsch A., et al. Synthesis and evaluation of analogues of N-phthaloyl-l-tryptophan (RG108) as inhibitors of DNA methyltransferase 1. J. Med. Chem. 2014;57:421–434. doi: 10.1021/jm401419p. [DOI] [PubMed] [Google Scholar]
- 92.Chen S., Wang Y., Zhou W., Li S., Peng J., Shi Z., Hu J., Liu Y.C., Ding H., Lin Y., et al. Identifying novel selective non-nucleoside DNA methyltransferase 1 inhibitors through docking-based virtual screening. J. Med. Chem. 2014;57:9028–9041. doi: 10.1021/jm501134e. [DOI] [PubMed] [Google Scholar]
- 93.Shivarov V., Gueorguieva R., Stoimenov A., Tiu R. DNMT3A mutation is a poor prognosis biomarker in AML: Results of a meta-analysis of 4500 AML patients. Leuk. Res. 2013;37:1445–1450. doi: 10.1016/j.leukres.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 94.Green L.M., Wagner K.J., Campbell H.A., Addison K., Roberts S.G. Dynamic interaction between WT1 and BASP1 in transcriptional regulation during differentiation. Nucleic Acids Res. 2009;37:431–440. doi: 10.1093/nar/gkn955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cashen A.F., Schiller G.J., O’Donnell M.R., DiPersio J.F. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J. Clin. Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 96.Roboz G.J., Kantarjian H.M., Yee K.W.L., Kropf P.L., O’Connell C.L., Griffiths E.A., Stock W., Daver N.G., Jabbour E., Ritchie E.K., et al. Dose, schedule, safety, and efficacy of guadecitabine in relapsed or refractory acute myeloid leukemia. Cancer. 2018;124:325–334. doi: 10.1002/cncr.31138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dittmann J., Haydn T., Metzger P., Ward G.A., Boerries M., Vogler M., Fulda S. Next-generation hypomethylating agent SGI-110 primes acute myeloid leukemia cells to IAP antagonist by activating extrinsic and intrinsic apoptosis pathways. Cell Death Differ. 2019;27:1878–1895. doi: 10.1038/s41418-019-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jueliger S., Lyons J., Cannito S., Pata I., Pata P., Shkolnaya M., Lo Re O., Peyrou M., Villarroya F., Pazienza V., et al. Efficacy and epigenetic interactions of novel DNA hypomethylating agent guadecitabine (SGI-110) in preclinical models of hepatocellular carcinoma. Epigenetics. 2016;11:709–720. doi: 10.1080/15592294.2016.1214781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zwergel C., Schnekenburger M., Sarno F., Battistelli C., Manara M.C., Stazi G., Mazzone R., Fioravanti R., Gros C., Ausseil F., et al. Identification of a novel quinoline-based DNA demethylating compound highly potent in cancer cells. Clin. Epigenet. 2019;11:68. doi: 10.1186/s13148-019-0663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caulfield T., Medina-Franco J.L. Molecular dynamics simulations of human DNA methyltransferase 3B with selective inhibitor nanaomycin A. J. Struct. Biol. 2011;176:185–191. doi: 10.1016/j.jsb.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 101.Chatzifrangkeskou M., Pefani D.E., Eyres M., Vendrell I., Fischer R., Pankova D., O’Neill E. RASSF1A is required for the maintenance of nuclear actin levels. EMBO J. 2019;38:e101168. doi: 10.15252/embj.2018101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neumann O., Kesselmeier M., Geffers R., Pellegrino R., Radlwimmer B., Hoffmann K., Ehemann V., Schemmer P., Schirmacher P., Lorenzo Bermejo J., et al. Methylome analysis and integrative profiling of human HCCs identify novel protumorigenic factors. Hepatology. 2012;56:1817–1827. doi: 10.1002/hep.25870. [DOI] [PubMed] [Google Scholar]
- 103.Blagitko-Dorfs N., Schlosser P., Greve G., Pfeifer D., Meier R., Baude A., Brocks D., Plass C., Lubbert M. Combination treatment of acute myeloid leukemia cells with DNMT and HDAC inhibitors: Predominant synergistic gene downregulation associated with gene body demethylation. Leukemia. 2019;33:945–956. doi: 10.1038/s41375-018-0293-8. [DOI] [PubMed] [Google Scholar]
- 104.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 105.Vendetti F., Rudin C. Epigenetic therapy in non-small-cell lung cancer: Targeting DNA methyltransferases and histone deacetylases. Expert Opin. Biol. Ther. 2013;13:1273–1285. doi: 10.1517/14712598.2013.819337. [DOI] [PubMed] [Google Scholar]
- 106.Henderson J.T., Webber E.M., Sawaya G.F. Screening for Ovarian Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018;319:595–606. doi: 10.1001/jama.2017.21421. [DOI] [PubMed] [Google Scholar]
- 107.Choi J.A., Kwon H., Cho H., Chung J.Y., Hewitt S.M., Kim J.H. ALDH1A2 Is a Candidate Tumor Suppressor Gene in Ovarian Cancer. Cancers. 2019;11:1553. doi: 10.3390/cancers11101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Steele N., Finn P., Brown R., Plumb J.A. Combined inhibition of DNA methylation and histone acetylation enhances gene re-expression and drug sensitivity in vivo. Br. J. Cancer. 2009;100:758–763. doi: 10.1038/sj.bjc.6604932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamagishi M., Uchimaru K. Targeting EZH2 in cancer therapy. Curr. Opin. Oncol. 2017;29:375–381. doi: 10.1097/CCO.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 110.Emran A.A., Chatterjee A., Rodger E.J., Tiffen J.C., Gallagher S.J., Eccles M.R., Hersey P. Targeting DNA Methylation and EZH2 Activity to Overcome Melanoma Resistance to Immunotherapy. Trends Immunol. 2019;40:328–344. doi: 10.1016/j.it.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 111.Li S., Teng J., Li H., Chen F., Zheng J. The Emerging Roles of RASSF5 in Human Malignancy. Anti-Cancer Agents Med. Chem. 2018;18:314–322. doi: 10.2174/1871520617666170327120747. [DOI] [PubMed] [Google Scholar]
- 112.Wang B., Lim J.H., Kajikawa T., Li X., Vallance B.A., Moutsopoulos N.M., Chavakis T., Hajishengallis G. Macrophage beta2-Integrins Regulate IL-22 by ILC3s and Protect from Lethal Citrobacter rodentium-Induced Colitis. Cell Rep. 2019;26:1614–1626.e5. doi: 10.1016/j.celrep.2019.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siegel R.L., Torre L.A., Soerjomataram I., Hayes R.B., Bray F., Weber T.K., Jemal A. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68:2179–2185. doi: 10.1136/gutjnl-2019-319511. [DOI] [PubMed] [Google Scholar]
- 114.Rodriguez J., Frigola J., Vendrell E., Risques R.A., Fraga M.F., Morales C., Moreno V., Esteller M., Capella G., Ribas M., et al. Chromosomal instability correlates with genome-wide DNA demethylation in human primary colorectal cancers. Cancer Res. 2006;66:8462–9468. doi: 10.1158/0008-5472.CAN-06-0293. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Y.J., Zhao S.L., Tian X.Q., Sun D.F., Xiong H., Dai Q., Li X.Q., Fang J.Y. Combined inhibition of Dnmt and mTOR signaling inhibits formation and growth of colorectal cancer. Int. J. Colorectal. Dis. 2009;24:629–639. doi: 10.1007/s00384-009-0664-8. [DOI] [PubMed] [Google Scholar]
- 116.Yuan Z., Chen S., Gao C., Dai Q., Zhang C., Sun Q., Lin J.S., Guo C., Chen Y., Jiang Y. Development of a versatile DNMT and HDAC inhibitor C02S modulating multiple cancer hallmarks for breast cancer therapy. Bioorg. Chem. 2019;87:200–208. doi: 10.1016/j.bioorg.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 117.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 118.Jahangiri R., Mosaffa F., Emami Razavi A., Teimoori-Toolabi L., Jamialahmadi K. Altered DNA methyltransferases promoter methylation and mRNA expression are associated with tamoxifen response in breast tumors. J. Cell. Physiol. 2018;233:7305–7319. doi: 10.1002/jcp.26562. [DOI] [PubMed] [Google Scholar]
- 119.San Jose-Eneriz E., Agirre X., Rabal O., Vilas-Zornoza A., Sanchez-Arias J.A., Miranda E., Ugarte A., Roa S., Paiva B., Estella-Hermoso de Mendoza A., et al. Discovery of first-in-class reversible dual small molecule inhibitors against G9a and DNMTs in hematological malignancies. Nat. Commun. 2017;8:15424. doi: 10.1038/ncomms15424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhong X., Chen X., Guan X., Zhang H., Ma Y., Zhang S., Wang E., Zhang L., Han Y. Overexpression of G9a and MCM7 in oesophageal squamous cell carcinoma is associated with poor prognosis. Histopathology. 2015;66:192–200. doi: 10.1111/his.12456. [DOI] [PubMed] [Google Scholar]
- 121.Dang N.-N., Jiao J., Meng X., An Y., Han C., Huang S. Abnormal overexpression of G9a in melanoma cells promotes cancer progression via upregulation of the Notch1 signaling pathway. Aging. 2020;12:2393–2407. doi: 10.18632/aging.102750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang L., Dong X., Ren Y., Luo J., Liu P., Su D., Yang X. Targeting EHMT2 reverses EGFR-TKI resistance in NSCLC by epigenetically regulating the PTEN/AKT signaling pathway. Cell Death Dis. 2018;9:129. doi: 10.1038/s41419-017-0120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu J., Eckert M.A., Harada B.T., Liu S.M., Lu Z., Yu K., Tienda S.M., Chryplewicz A., Zhu A.C., Yang Y., et al. m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat. Cell. Biol. 2018;20:1074–1083. doi: 10.1038/s41556-018-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ozaki Y., Muto S., Takagi H., Watanabe M., Inoue T., Fukuhara M., Yamaura T., Okabe N., Matsumura Y., Hasegawa T., et al. Tumor mutation burden and immunological, genomic, and clinicopathological factors as biomarkers for checkpoint inhibitor treatment of patients with non-small-cell lung cancer. Cancer Immunol. Immuno. Ther. 2020;69:127–134. doi: 10.1007/s00262-019-02446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chung W., Kelly A.D., Kropf P., Fung H., Jelinek J., Su X.Y., Roboz G.J., Kantarjian H.M., Azab M., Issa J.J. Genomic and epigenomic predictors of response to guadecitabine in relapsed/refractory acute myelogenous leukemia. Clin. Epigenet. 2019;11:106. doi: 10.1186/s13148-019-0704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gaillard S.L., Zahurak M., Sharma A., Durham J.N., Reiss K.A., Sartorius-Mergenthaler S., Downs M., Anders N.M., Ahuja N., Rudek M.A., et al. A phase 1 trial of the oral DNA methyltransferase inhibitor CC-486 and the histone deacetylase inhibitor romidepsin in advanced solid tumors. Cancer. 2019;125:2837–2845. doi: 10.1002/cncr.32138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zeng H., Qu J., Jin N., Xu J., Lin C., Chen Y., Yang X., He X., Tang S., Lan X., et al. Feedback Activation of Leukemia Inhibitory Factor Receptor Limits Response to Histone Deacetylase Inhibitors in Breast Cancer. Cancer Cell. 2016;30:459–473. doi: 10.1016/j.ccell.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 128.Kaelin W.G. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 129.McDonald E.R., de Weck A., Schlabach M.R., Billy E., Mavrakis K.J., Hoffman G.R., Belur D., Castelletti D., Frias E., Gampa K., et al. Project DRIVE: A Compendium of Cancer Dependencies and Synthetic Lethal Relationships Uncovered by Large-Scale, Deep RNAi Screening. Cell. 2017;170 doi: 10.1016/j.cell.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 130.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu C., Lyu J., Yang E.J., Liu Y., Zhang B., Shim J.S. Targeting AURKA-CDC25C axis to induce synthetic lethality in ARID1A-deficient colorectal cancer cells. Nat. Commun. 2018;9:3212. doi: 10.1038/s41467-018-05694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fleury H., Malaquin N., Tu V., Gilbert S., Martinez A., Olivier M.-A., Sauriol A., Communal L., Leclerc-Desaulniers K., Carmona E., et al. Exploiting interconnected synthetic lethal interactions between PARP inhibition and cancer cell reversible senescence. Nat. Commun. 2019;10:2556. doi: 10.1038/s41467-019-10460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chan E.M., Shibue T., McFarland J.M., Gaeta B., Ghandi M., Dumont N., Gonzalez A., McPartlan J.S., Li T., Zhang Y., et al. WRN helicase is a synthetic lethal target in microsatellite unstable cancers. Nature. 2019;568:551–556. doi: 10.1038/s41586-019-1102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang H., Cui W., Wang L. Epigenetic synthetic lethality approaches in cancer therapy. Clin. Epigenet. 2019;11:136. doi: 10.1186/s13148-019-0734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]