Abstract
The normal colon epithelium is transformed into its neoplastic counterpart through a series of genetic alterations in driver genes including activating mutations in PIK3CA. Treatment often involves surgery followed by 5-fluorouracil (5-FU) based therapy, which has limited efficiency and serious side effects. We sought to determine whether fisetin, a dietary flavonoid, alone or in combination with 5-FU affected tumorigenesis in the mammalian intestine. We first determined the effect of fisetin, 5-FU or their combination on PIK3CA-mutant and PIK3CA wild-type colon cancer cells by assessing cell viability, colony formation, apoptosis and effects on PI3K/AKT/mTOR signaling. Treatment of PIK3CA-mutant cells with fisetin and 5-FU reduced the expression of PI3K, phosphorylation of AKT, mTOR, its target proteins, constituents of mTOR signaling complex and this treatment increased the phosphorylation of AMPKα. We then determined whether fisetin and 5-FU together or singly affected tumorigenesis in ApcMin/+ mice that also express constitutively active PI3K in the distal small intestine and colon. Tumor incidence was markedly lower in fisetin-treated FC13K1ApcMin/+ mice that also express constitutively active PI3K in distal small intestine and colon, as compared to control animals, indicating that fisetin is a strong preventive agent. In addition, the combination of fisetin and 5-FU also reduced the total number of intestinal tumors. Fisetin could be used as a preventive agent plus an adjuvant with 5-FU for the treatment of PIK3CA-mutant colorectal cancer.
Keywords: colorectal cancer, PIK3CA, 5-fluorouracil, PI3K/AKT/mTOR signaling
Introduction
Colorectal cancer (CRC) is the third most common cause of cancer-related deaths in the United States and is anticipated to result in 145,600 new cases and 51,020 deaths in 2019.1 After surgical intervention, 5-fluorouracil (5-FU) is the most commonly used chemotherapeutic drug for the treatment of colon cancer, however, it also causes serious toxicities in patients.2–4 Thus, the development of new approaches to decrease toxicity and overcome resistance to 5-FU is urgently desired.
CRC arises as a consequence of mutations in several driver genes including phosphatidylinositol-4,5-bisphosphonate-3-kinase, catalytic subunit alpha (PIK3CA) which encodes phosphatidylinositol-4,5-bisphosphate-3-kinase (PI3K). This kinase is one of the critical kinases in the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway responsible for cellular growth, proliferation and survival of multiple solid tumors.5,6 PIK3CA is one of the most frequently mutated genes in CRC with about 15–20% of advanced colorectal cancers harboring activating mutations in PIK3CA exon 9, exon 20 or both.7,8 It has been reported that patients with PIK3CA-mutated tumors experienced an increase in colon cancer-specific mortality compared to patients with PIK3CA wild-type tumors.7,9 Leystra et al. have shown that a dominant active form of PI3K is able to initiate the development of flat mucinous invasive adenocarcinomas in the proximal colon via a noncanonical mechanism of tumorigenesis.10 Recently, we generated a new murine model by crossing the females carrying a Fabp1-Cre transgene (FC) that expresses CRE recombinase in the distal small intestine and colon to males carrying ApcMin and Pik3ca* transgene (3K) that encodes a Cre-dependent activating mutation in Pik3ca (see Methods for complete genotypes).11 In these resulting progeny (FC13K1ApcMin/+; 1 denotes that the animal carries one copy of the transgene), tumors forming in the distal small intestine and colon have lost APC activity and express constitutively active PI3K as often occurs in humans.11 The presence of a dominant active PI3K in the setting of allelic loss of APC increased tumor multiplicity and size with tumors being more aggressive and less differentiated. This phenotype was related to increased activation of AKT and phosphorylation of downstream targets.11
Natural compounds have shown promise as preventive and therapeutic agents against CRC. Fisetin (3,3′,4′,7-tetrahydroxyflavone), a naturally occurring flavonoid, is present in fruits and vegetables such as strawberry, apple, persimmon, grape, onion and cucumber.12–16 Our earlier studies have demonstrated that fisetin inhibited the PI3K/AKT and mTOR pathway and induces autophagy in human cancer cells.17,18 In our study, we report that dietary flavonoid fisetin raises the possibility for improving the window of opportunity for the use of 5-FU for the prevention of CRC. We provide evidence that this approach is very effective in PIK3CA-mutant human colon cancer cells, and we also provide preclinical evidence on colorectal tumorigenesis in FC13K1ApcMin/+ mice. This is the first study which shows the effectiveness of a natural dietary agent as an adjuvant for the prevention and treatment of PIK3CA-mutant CRC.
Materials and Methods
Chemicals, reagents and antibodies
PI3 Kinase (p85), PI3 Kinase (p110), p-AKTSer473, p-AKTThr308, p-mTORSer2448, p-4EBP1Ser65, p-eIF4ESer209, p-p70S6K, p-AMPKαThr172, p-PRAS40Thr246, Rictor, Raptor, GβL and vinculin antibodies were obtained from Cell Signaling Technology (Danvers, MA). Antimouse and antirabbit secondary antibodies conjugated to horseradish peroxidase were obtained from Amersham Life Science Inc. (Arlington Height, IL). Fisetin and 5-FU were purchased from Sigma Chemical Co. (St. Louis, MO). Novex precast Tris-glycine gels were from BioRad (Hercules, CA). BCA Protein assay kit was obtained from Pierce (Rockford, IL). The Annexin-V-FLUOS staining kit was purchased from Roche Diagnostics GmbH, Mannheim, Germany.
Cell culture and treatment
The cell-lines (SW480, HCT116 and HT29) were obtained from American Type Culture Collection (ATCC; Manassas, VA). These cells were tested by ATCC for postfreeze viability, growth properties, morphology, mycoplasma contamination, species determination (cytochrome c oxidase I assay and short tandem repeat analysis), sterility test and human pathogenic viruses. The cell-lines were immediately resuscitated upon receipt and frozen in aliquots in liquid nitrogen. Cells were cultured within 6 months and were regularly tested for mycoplasma contamination using MycoAlert Mycoplasma Detection Kit from Lonza (Basel, Switzerland). The HCT116 and HT29 cells were maintained in McCoy’s 5A medium (ATCC; Manassas, VA). supplemented with 10% fetal bovine serum (Sigma Chemical Co., St. Louis, MO), whereas SW480 cells were maintained in Leibovitz’s L-15 medium (ATCC; Manassas, VA) supplemented with 10% fetal bovine serum from Sigma Chemical Co. (St. Louis, MO). SW480, HCT116 and HT29 cells were tested by ATCC for postfreeze viability, growth properties, morphology, mycoplasma contamination and species determination (COI assay and STR analysis). The cells were maintained under standard cell culture conditions at 37·C and 5% CO2 in a humid environment. Cells were treated with fisetin (30–90 μM), 5-FU (50 μM) and their combination in dimethyl sulfoxide (DMSO) for 48 hr for all biochemical assays.
Cell viability
The effect of fisetin (30–90 μM), 5-FU (50 μM) and their combination on the viability of cells was determined by 3-[4,−5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay.19,20 SW480, HCT116 and HT29 cells were plated at 1 × 104 cells per well in 200 μl of complete culture medium containing fisetin, 5-FU or their combination for 48 hr. After incubation at 37·C in a humidified incubator, MTT (5 mg/ml in PBS) was added to each well and incubated for 2 hr, after which the plate was centrifuged at 1,800g for 5 min at 4·C. The supernatant was discarded and the pellet dissolved in 200 μl of DMSO and absorbance was recorded on a microplate reader at the wavelength of 540 nm. The effect of fisetin, 5-FU or their combination on growth inhibition was assessed as percent cell viability.
Colony formation assay
Human colon cancer cells were plated in six-well plates at a very low density and treated with fisetin (30–90 μM), 5-FU (50 μM) or their combination and maintained at 37·C in a humidified 10% CO2 atmosphere. Cells were allowed to grow for 14 days and fresh media containing fisetin, 5-FU and their combination was replaced every 3 days. At the conclusion of the study, colonies were rinsed with 1× phosphate-buffered saline and stained with crystal violet according to our published protocol.20
Apoptosis assessment by Annexin-V-FLUOS staining
The Annexin V-FITC early apoptosis detection kit (Cell Signaling Technology, Danvers, MA) was used to identify apoptotic cells within a cell population. The human colon cancer cells were grown to ~70% confluence and treated with fisetin (30–90 μM), 5-FU (50 μM) or their combination for 48 hr. The cells were washed, centrifuged and the cell pellet was resuspended in Annexin-V-FLUOS labeling solution (prepared by prediluting Annexin-V-FLUOS labeling reagent in incubation buffer and adding propidium iodide solution). The data was collected on a Becton–Dickinson FACSCalibur (San Jose, CA) and analyzed in FlowJo, Version 9.7 (FLowJo, LLC, Ashland, OR).
Protein extraction and immunoblotting
This was done according to our previously published protocols.17,19–21 After the treatment of cells with fisetin, 5-FU or their combination, the media was aspirated, the cells were washed with cold PBS (pH 7.4) and were incubated in ice-cold lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 20 mM NaF, 100 mM Na3VO4, 0.5% NP-40, 1% Triton X-100, 1 mM PMSF [pH 7.4]) with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Set III, Calbiochem, La Jolla, CA) over ice for 30 min. The cells were scraped and the lysate was collected in a microfuge tube and passed through needle to break up the cell aggregates. The lysate was cleared by centrifugation at 14,000g for 15 min at 4·C and the supernatant (whole cell lysate) was used or immediately stored at −80·C.
For immunoblotting, 30–50 μg protein was resolved over 8–12% polyacrylamide gels and transferred to a nitrocellulose membrane. The blot was blocked in blocking buffer (5% nonfat dry milk/1% Tween 20, in 20 mM TBS, pH 7.6) for 1 hr at room temperature, incubated with appropriate monoclonal or polyclonal primary antibody in blocking buffer for 1.5 hr to overnight at 4·C, followed by incubation with antimouse or antirabbit secondary antibody horseradish peroxidase conjugate obtained from Amersham Life Science Inc. (Arlington Height, IL) and detected by chemiluminescence and autoradiography using Bio-Rad Gel- Doc (Bio-Rad Laboratories Inc., Hercules, CA).
Animals
All animal studies were conducted under protocols approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison, following the guidelines of the American Association for the Assessment and Accreditation of Laboratory Animal Care. FC1 mice (FVB/N-Tg(Fabp1-Cre)1Jig; NCI Mouse Repository; Strain number 01XD8), 3K1 mice (C57BL/6-Gt(ROSA) 26Sortm7(Pik3ca*,EGFP)Rsky/J; The Jackson Laboratory; Stock Number-012343) and ApcMin/+ males (C57BL/6J ApcMin/J; The Jackson Laboratory; Stock number 002020) were maintained as described previously.22 3K1 females were crossed with ApcMin/+ males to generate 3K1ApcMin/+ mice. FC1 obtained from the repository were backcrossed for 10 generations to generate B6 congenic strain. FC1 females were then crossed with mice 3K1ApcMin/+ males to generate B6 FC13K1ApcMin/+ experimental mice and controls. Mice were genotyped for FC, 3K, ApcMin/+ and Min as described earlier.23,24
Treatment of animals
All mice were fed AIN-76A diet and water ad libitum, weighed weekly to monitor for weight loss, and examined at least once daily to identify signs of distress (i.e., hunched posture, bloating, pallor, etc.). FC13K1ApcMin/+ mice were randomly distributed into four groups containing at least 15 mice in each group. Since FC13K1ApcMin/+ mice develop adenomas around 1 month of age, the treatment with fisetin and 5-FU by intraperitoneal injection was started between 26 and 30 days (Supporting Information Fig. S1). The first group of animals received i.p. injection of DMSO (30 μl) and served as control. Mice in Group 2 were treated with 5-FU (40 mg/kg body weight) in PBS, twice a week. Mice in Group 3 were treated with fisetin (1 mg/animal) in 30 μl of DMSO, twice a week. Animals in Group 4 were administered combination of fisetin and 5-FU, twice a week. This treatment schedule was followed for 3 weeks (Supporting Information Fig. S1). Colonic tumors were monitored at regular intervals employing colonoscopy.25 The colon was flushed with warm PBS to clear any fecal material. The endoscope was inserted about four centimeters and withdrawn. The colon was insufflated to carefully examine the epithelial lining. Video of the entire procedure and still images of each tumor were collected. The volume of each tumor was estimated by determining what proportion of the lumen was occluded by the tumor. This approach allows changes in tumor volume as small as 20% to be readily detected. Mice were euthanized when moribund or when treatment was complete to score tumors. Tumors were scored using a dissection microscope by investigator (RBH) who was blind to treatment information as described previously.26
To determine whether the drugs affected more advanced tumors, the treatment of a second set of experimental mice was delayed, starting at 46–50 days of age. These mice received up to five rounds of treatment. Colonic tumors were monitored and tumor size was calculated from images in the same manner as first group. Mice were euthanized when moribund or when the treatment was complete.
Statistical analysis
Results were analyzed using a two-tailed Student’s t-test to assess statistical significance using Graph Pad Prism 6.0 software and p values <0.05 were considered significant.
Results
Effect of fisetin, 5-FU or their combination on cell viability and colony formation in PIK3CA-mutant and PIK3CA wild- type human colon cancer cells
Treatment of PIK3CA-mutant HCT116 and HT29 colon cancer cells with 5-FU caused 48 and 41% decreases in cell viability, while there were 35–46 and 32–41% decreases with fisetin noting that the maximum effect was observed at the highest dose with both lines and 51–60 and 47–55% decreases with the combination of fisetin and 5-FU, respectively. In PIK3CA wild-type SW480 colon cancer cells, there was a 33% decrease in cell viability on treatment with 5-FU, a 24–34% decrease with fisetin and a 39–44% decrease with combination of fisetin and 5-FU, respectively (Fig. 1 a). In accordance with the results of the cell viability assay, treatment with fisetin or 5-FU caused decreases in the number of colonies in both HCT116 (Fig. 1 b) and HT29 (Fig. 1 c) colon cancer and a more striking decrease was observed with the combination of fisetin and 5-FU in a dose-dependent manner with almost complete inhibition still evident at 14 days (Fig. 1 b and 1 c).
Figure 1.
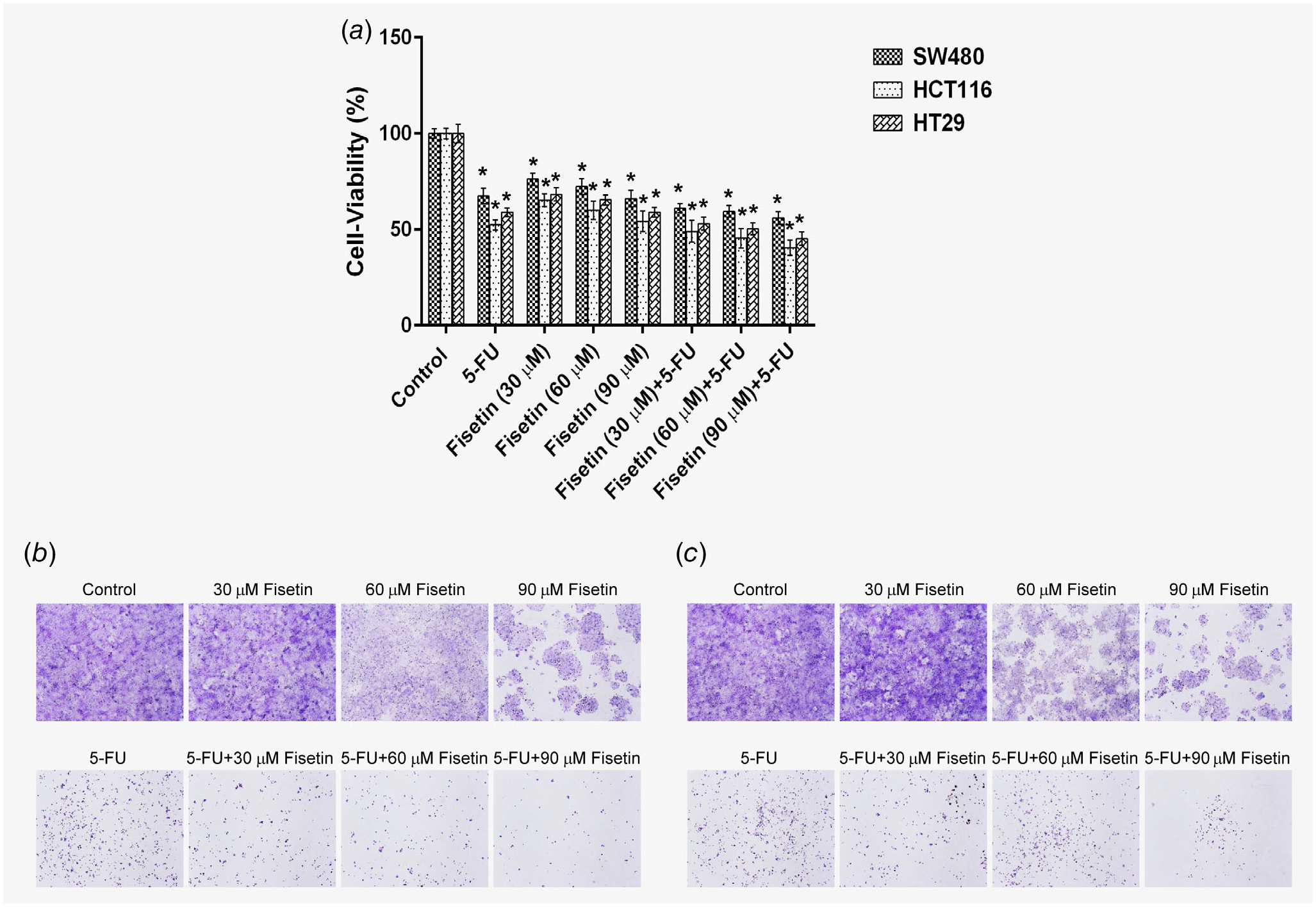
Effect of fisetin, 5-FU or their combination on cell growth and colony formation in human colon cancer cells. (a) Effect of fisetin, 5-FU or their combination on cell growth. As described in Materials and Methods, SW480, HCT116 and HT29 cells were treated with fisetin (30–90 μM), 5-FU (50 μM) and their combination for 48 hr and the viability of cells was determined by the MTT assay. The data is expressed as the percentage of cell viability and represent the mean ± SEM of three experiments in which each treatment was performed in multiple wells. Treatment groups of each cell line were compared to the respective control group of each cell line (*p < 0.0001). Effect of fisetin, 5-FU and their combination on colony formation in (b) HCT116 and (c) HT29 cells. The cells were seeded in six-well plates and treated with fisetin (30–90 μM), 5-FU (50 μM) or their combination as described in Materials and Methods. At the end of the experiment (14 days), colonies were washed with 1× phosphate-buffered saline, stained with crystal violet and photographed. [Color figure can be viewed at wileyonlinelibrary.com]
Effect of fisetin, 5-FU or their combination on induction of apoptosis in PIK3CA-mutant human colon cancer cells
To determine whether apoptosis is involved in the cell growth inhibition of colon cancer cells, we determined the effect of fisetin, 5-FU or their combination on the induction of apoptosis. A well- established early feature of apoptosis is the externalization of phosphatidyl serine (PS) from the inner to outer membrane. Early apoptotic cells are detectible by flow cytometry because fluorescently labeled annexin V binds externalized PS. These cells can be discriminated from late apoptotic cells because they still exclude dyes like propidium iodine that are internalized once membrane integrity is compromised. In HCT116 cells, the late apoptosis was11.5–12.7% in fisetin, 17.4% in 5-FU and 13.7–26.3% in combination of fisetin and 5-FU treated groups as compared to 2.0% in control group, whereas the early apoptosis was 5.7–16.9% in fisetin, 8.8% in 5-FU and 19.2–25.5% in combination of fisetin and 5-FU treated groups as compared to 5.8% in control group (Fig. 2 a and Supporting Information Table S1a). Similarly, in HT29 cells, the late apoptosis was 4.6–19.9% in fisetin, 5.3% in 5-FU and 13.7–23.0% in combination of fisetin and 5-FU treated groups as compared to 1.3% in control group, whereas the early apoptosis was 8.3–11.3% in fisetin, 5.3% in 5-FU and 13.7–23.0% in combination of fisetin and 5-FU treated groups as compared to 3.7% in control group (Fig. 2 b and Supporting Information Table S1b). These results clearly establish that apoptotic process is induced on treatment of both HCT116 and HT29 colon cancer cells.
Figure 2.
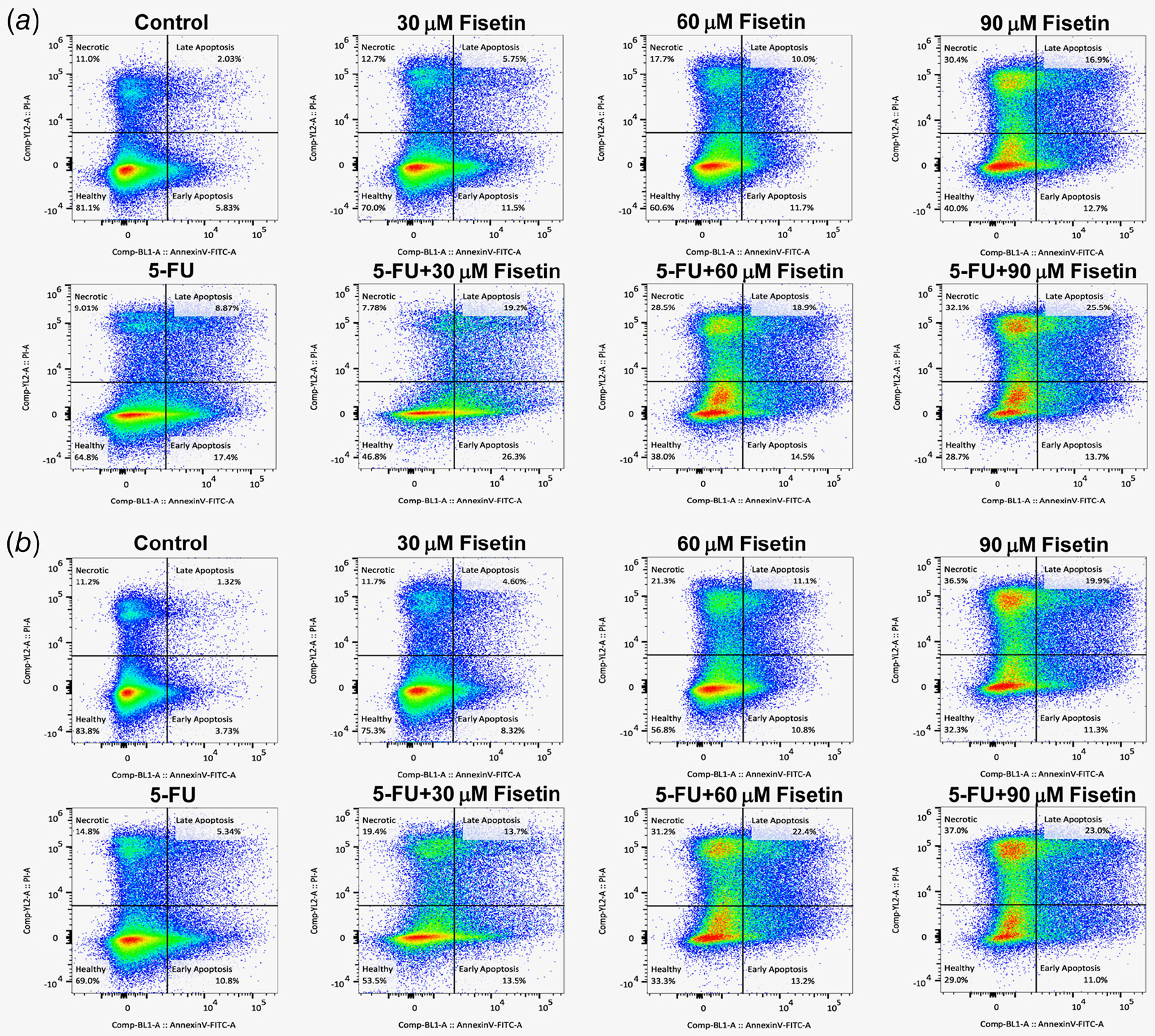
Effect of fisetin, 5-FU or their combination on apoptosis by annexin-V-FLUOS staining in (a) HCT116 cells and (b) HT29 cells. The cells were treated with fisetin (30–90 μM), 5-FU (50 μM) or their combination and resuspended in Annexin-V-FLUOS labeling solution. The data was collected on a Becton-Dickinson FACSCalibur (San Jose, CA) and analyzed in FlowJo, Version 9.7 (FLowJo, LLC, Ashland, OR). [Color figure can be viewed at wileyonlinelibrary.com]
Effect of fisetin, 5-FU or their combination on PI3K and phosphorylation of AKT in PIK3CA-mutant human colon cancer cells
The PI3K/AKT pathway is frequently activated in CRC leading to tumorigenesis and the resistance to chemotherapy.27,28 Cellular signaling stimulates cancer cell growth, survival and metabolism which protect the cells from apoptosis and is initiated by catalytic (p110) subunit of PI3K.27,29,30 Treatment of PIK3CA-mutant HCT116 and HT29 colon cancer cells with fisetin or 5-FU caused decrease in the expression of catalytic (p110) subunit of PI3K (Figs. 3 a and 3b) and inhibition in the phosphorylation of AKT at Ser473, however, the inhibition of both proteins was more striking when cells were treated with combination of both fisetin and 5-FU (Figs. 3 a and 3b).
Figure 3.
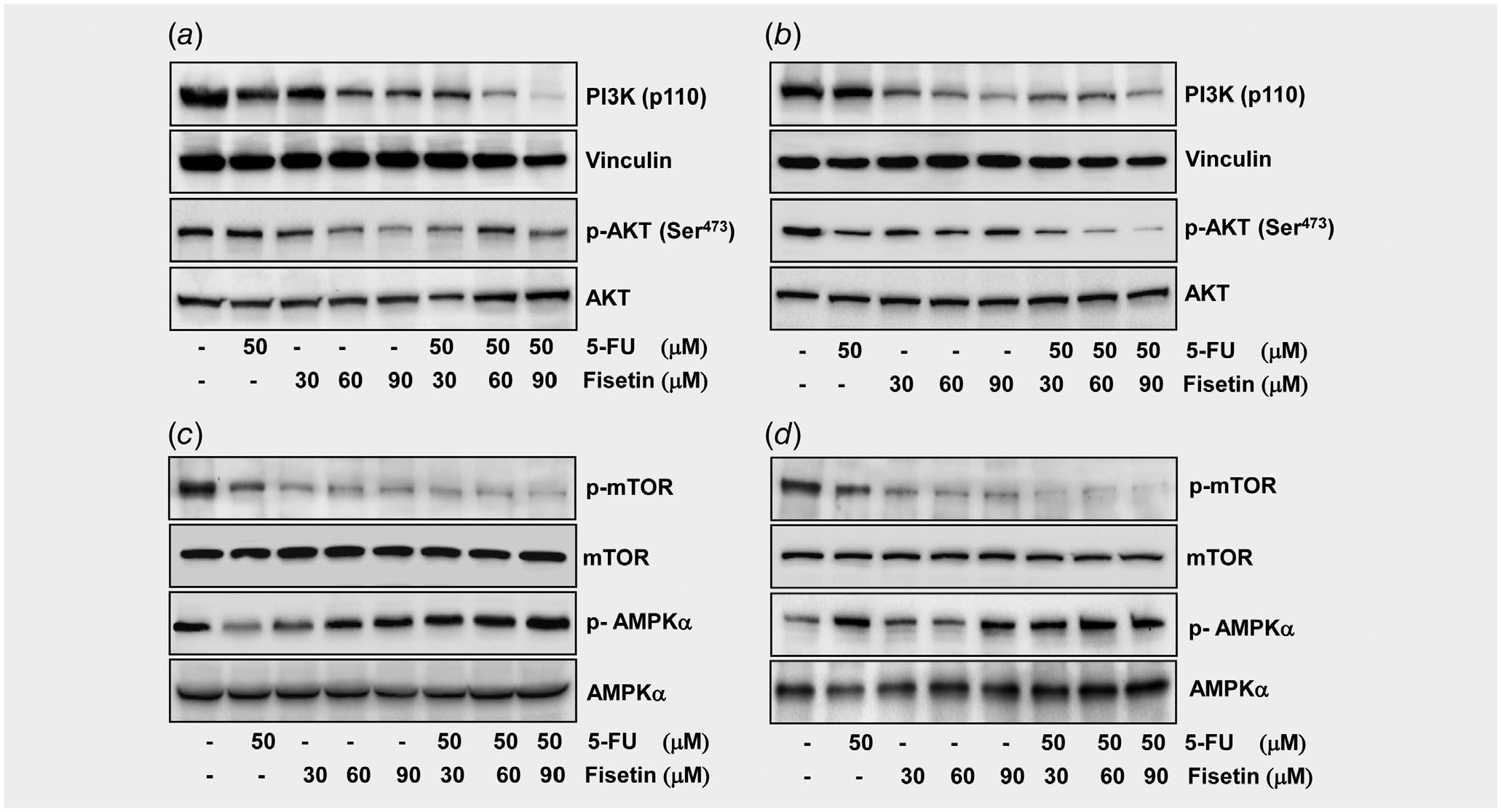
Effect of fisetin, 5-FU or their combination on the protein expression of PI3K, phosphorylation of AKT, mTOR and AMPKα in PIK3CA-mutant human colon cancer cells. (a) Effect of fisetin, 5-FU or their combination on the protein expression of PI3K and phosphorylation of AKT in HCT116 cells, and (b) HT29 cells. (c) Effect of fisetin, 5-FU or their combination on the phosphorylation of mTOR and AMPKα in HCT116 cells, and (d) HT29 cells. As described in the in Materials and Methods, the cells were treated with fisetin (30–90 μM), 5-FU (50 μM) and their combination for 48 hr and then harvested. Total cell lysates were prepared and 40 μg protein was subjected to SDS-PAGE followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for vinculin.
Effect of fisetin, 5-FU or their combination on the phosphorylation of mTOR and AMPKα in PIK3CA-mutant human colon cancer cells
The protein mTOR has a central role in the regulatory network sensing nutrition and growth signals, coordinating cell growth and proliferation. It has been proposed that mTOR inhibitors may be successful for the prevention and treatment of colorectal cancer.31–33 AMP-activated protein kinase (AMPK) regulates the PI3K/AKT/mTOR pathway and its activation causes cell cycle arrest and tumor growth inhibition, consequently playing a critical role in cancer prevention.34 Treatment with combination of fisetin and 5-FU led to a stronger decrease in mTOR and a remarkable increase in AMPKα in HCT116 (Fig. 3 c) and HT29 cells (Fig. 3 d) than either agent alone.
Effect of fisetin, 5-FU or their combination on mTOR target proteins, mTORC1 and mTORC2 in PIK3CA-mutant human colon cancer cells
mTOR is a critical component in two functionally varied protein complexes, mTOR complex 1 and 2 (mTORC1 and mTORC2).35 mTORC1 consists of regulatory-associated protein of mTOR (Raptor), mammalian lethal with Sec13 protein 8 (mLST8)/G-protein β-subunit-like protein (GβL) and DEP-domain-containing partner of mTOR (Deptor).
mTORC1 activity is regulated in part by proline-rich Akt substrate of 40 kDa (PRAS40).36 Activation of mTORC1 leads to phosphorylation of two downstream effectors, ribosomal S6 kinase (p70S6K) and the eukaryotic translation initiation factor 4E (eIF4E) binding protein 1 (4E-BP1),37 which inhibits the initiation of protein translation by binding and inactivating eIF4E. The phosphorylation of 4E-BP1 promotes dissociation of 4E-BP1 from eIF4E and increases the cap-dependent translation of mRNAs.38 mTORC2 consists of rapamycin-insensitive companion of mTOR (Rictor), mammalian stress-activated protein kinase interacting protein (mSIN1), mLST8/GβL, protein observed with Rictor-1 (Protor-1), and Deptor.39
The level of proteins in both mTORC1 and mTORC2 in HCT116 (Fig. 4 c) and HT29 (Fig. 4 d) was impacted after drug treatment albeit the effect was stronger with the former than the latter. The amount of Raptor, Rictor and GβL decreased significantly when cells were exposed to intermediary concentrations (60 and 90 μM) of fisetin but not a low concentration of fisetin (30 μM) or intermediary concentration of 5-FU (50 μM). The reduction was even more significant when fisetin and 5-FU were combined. The amount of p-PRAS40 decreased significantly with either fisetin or 5-FU. Consistently, the level of downstream effectors of mTORC1 in HCT116 (Fig. 4 a) and HT29 (Fig. 4 b) was also impacted by fisetin. The amount of 4E- BP1, eIF4E and p70S6K decreased after treatment with a low or intermediary dose of fisetin. Thus, PI3K signaling is clearly impacted by fisetin and the effect appears to be further enhanced by 5-FU.
Figure 4.
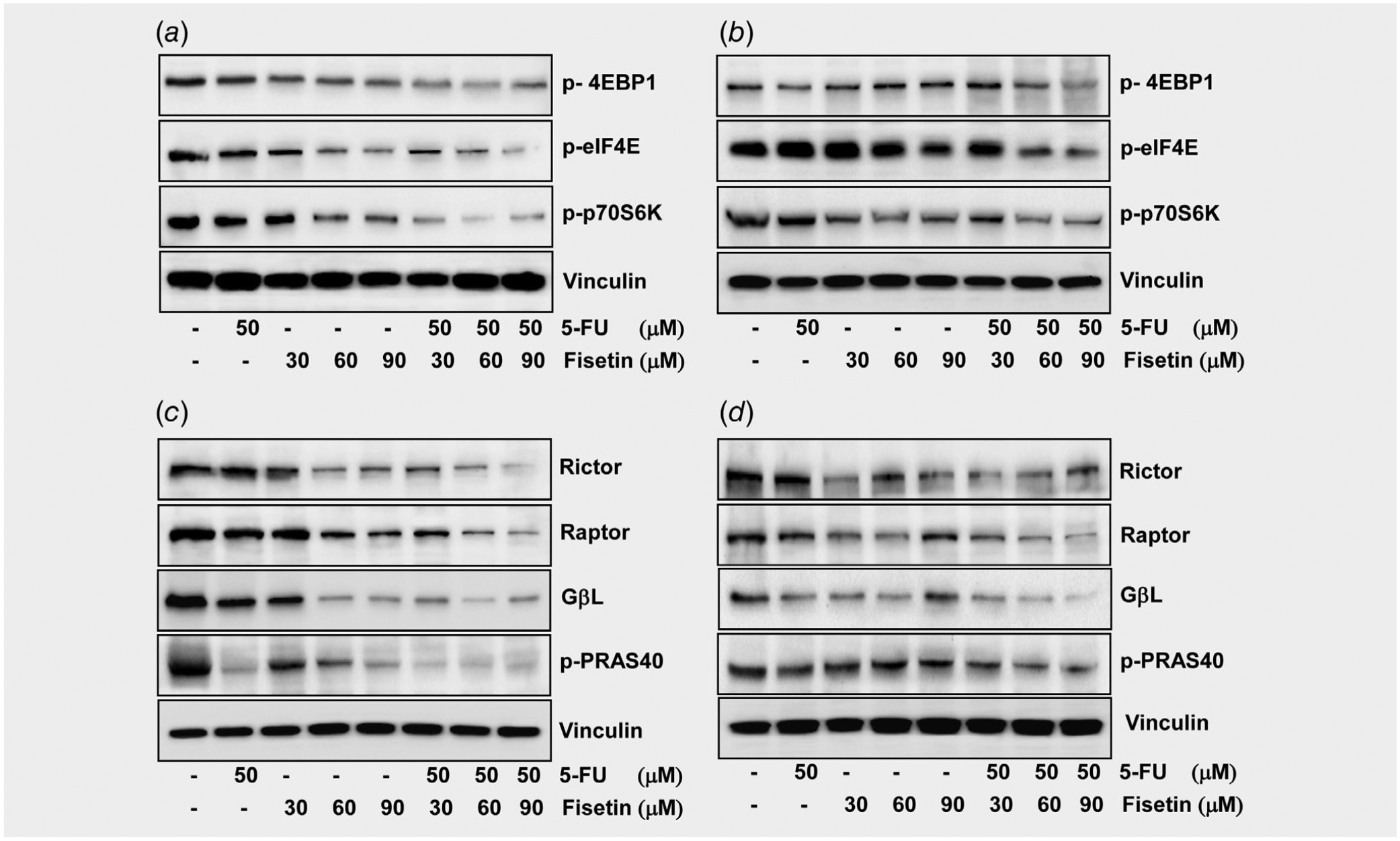
Effect of fisetin, 5-FU or their combination on the phosphorylation of mTOR target proteins and constituents of the mTOR signaling complex in PIK3CA-mutant human colon cancer cells. (a) Effect of fisetin, 5-FU or their combination on the phosphorylation of mTOR target proteins in HCT116 cells, and (b) HT29 cells. (c) Effect of fisetin, 5-FU or their combination on the protein expression of constituents of the mTOR signaling complex in HCT116 cells, and (d) HT29 cells. As described in the Materials and Methods, the cells were treated with fisetin (30–90 μM), 5-FU (50 μM) and their combination for 48 hr and then harvested. Total cell lysates were prepared and 40 μg proteins was subjected to SDS-PAGE followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for vinculin.
Effect of the treatment of fisetin, 5-FU and their combination on the incidence and multiplicity of colonic tumors in FC13K1ApcMin/+ mice
Colonoscopy was performed on experimental mice before, during, and after treatment with fisetin, 5-FU, or the combination of fisetin and 5-FU to assess tumor development and growth in the colon. Briefly, the endoscope was inserted into the anus and withdrawn. Video was collected during the entire procedure and still images were collected of each tumor. At the initial colonoscopy visit prior to treatment, some mice were tumor-free whereas others were tumor-bearing. One set of experimental mice started treatment between 26 and 30 days of age and received three rounds of drugs (Supporting Information Fig. S1). The mice that were tumor-free tended to remain tumor-free throughout the study if they were treated with fisetin alone or the combination (Fig. 5a, p < 0.05). Only 8 of the 20 mice that were treated with fisetin and only 6 out of 17 mice that were treated with the combination had an observed colon tumor at the end of the study. In contrast, 15 out of 21 controls had an observed colon tumor. This observation indicates that fisetin prevents tumor formation.
Figure 5.
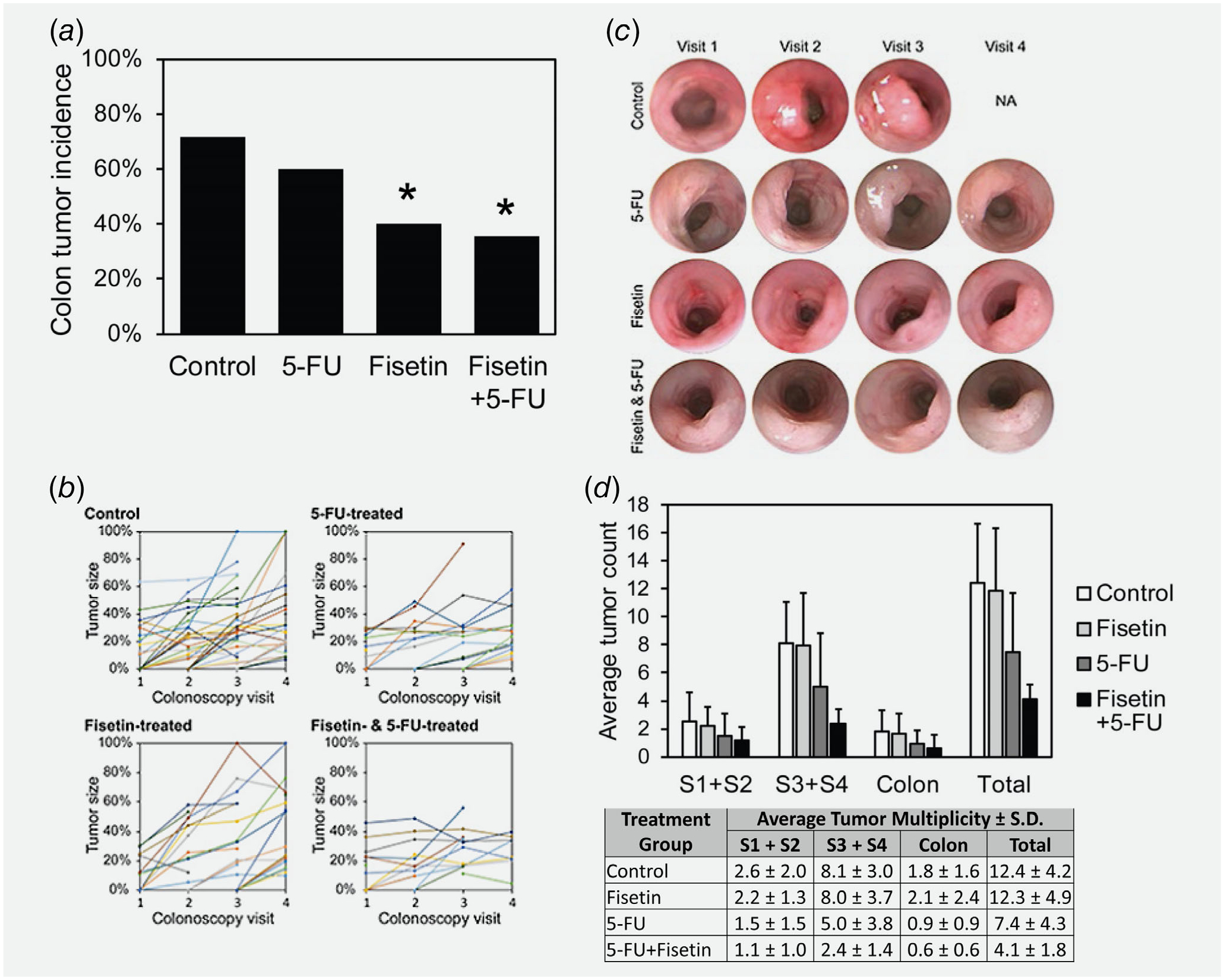
Effect of the treatment of fisetin, 5-FU or their combination on colorectal tumorigenesis in FC13K1ApcMin/+ mice. (a) Effect of the treatment of fisetin, 5-FU or their combination on colon tumor incidence in FC13K1ApcMin/+ mice that had no visible tumors at the beginning of treatment. (b) Effect of the treatment of fisetin, 5-FU or their combination on tumor growth (early) in FC13K1ApcMin/+ mice. The size of each tumor at each colonoscopy visit was calculated by determining what percentage of the fully inflated lumen was occluded by the tumor and plotted with each different colored line representing a distinct tumor. (c) The calculation was made from still images of each tumor that were collected during each colonoscopy visit. NA, not available, because the control became moribund and was euthanized prior to the fourth colonoscopy visit. (d) Effect of the treatment of fisetin, 5-FU or their combination on tumor multiplicity in FC13K1ApcMin/+ mice. When mice became moribund or the study ended, experimental mice in all groups were euthanized and necropsied to score the number of intestinal tumors. The small intestine was divided into four equal segments with S1 being closest to the stomach and S4 being closest to the cecum; S1 was combined with S2 and S3 was combined with S4 because the FC transgene is expressed only in S3, S4 and the colon. [Color figure can be viewed at wileyonlinelibrary.com]
Treatment with 5-FU alone or in combination with fisetin appeared to affect tumor growth. Representative images of a tumors in a mice from the control and each treatment groups are shown (Fig. 5 b). Tumor size was estimated from still images by calculating the percentage of the lumen excluded as described previously.40 Estimates of tumor size from still images collected before and during treatment were plotted to determine the growth rate of the tumor. 5-FU slowed the rate of growth. This result was consistent with a previous study with a different mouse model.25
The combination of fisetin and 5-FU affected total tumor multiplicity. After treatment was completed, mice were euthanized and necropsied to score the number of tumors throughout their entire intestinal tract. Experimental mice treated with the combination developed on average 4 ± 2 tumors, whereas controls developed 12 ± 4 tumors (Fig. 5 d; p < 0.001). The reduction in tumor multiplicity was apparent along the entire length of the intestinal tract.
In the long-lived mice, the group treated with combination of fisetin and 5-FU was the only one which lived past 4 weeks and therefore, there was increase in survival of mice as comspared to other groups, but it was not statistically significant (Fig. 6). Therefore, much larger studies are to be done to be confident in this effect.
Figure 6.
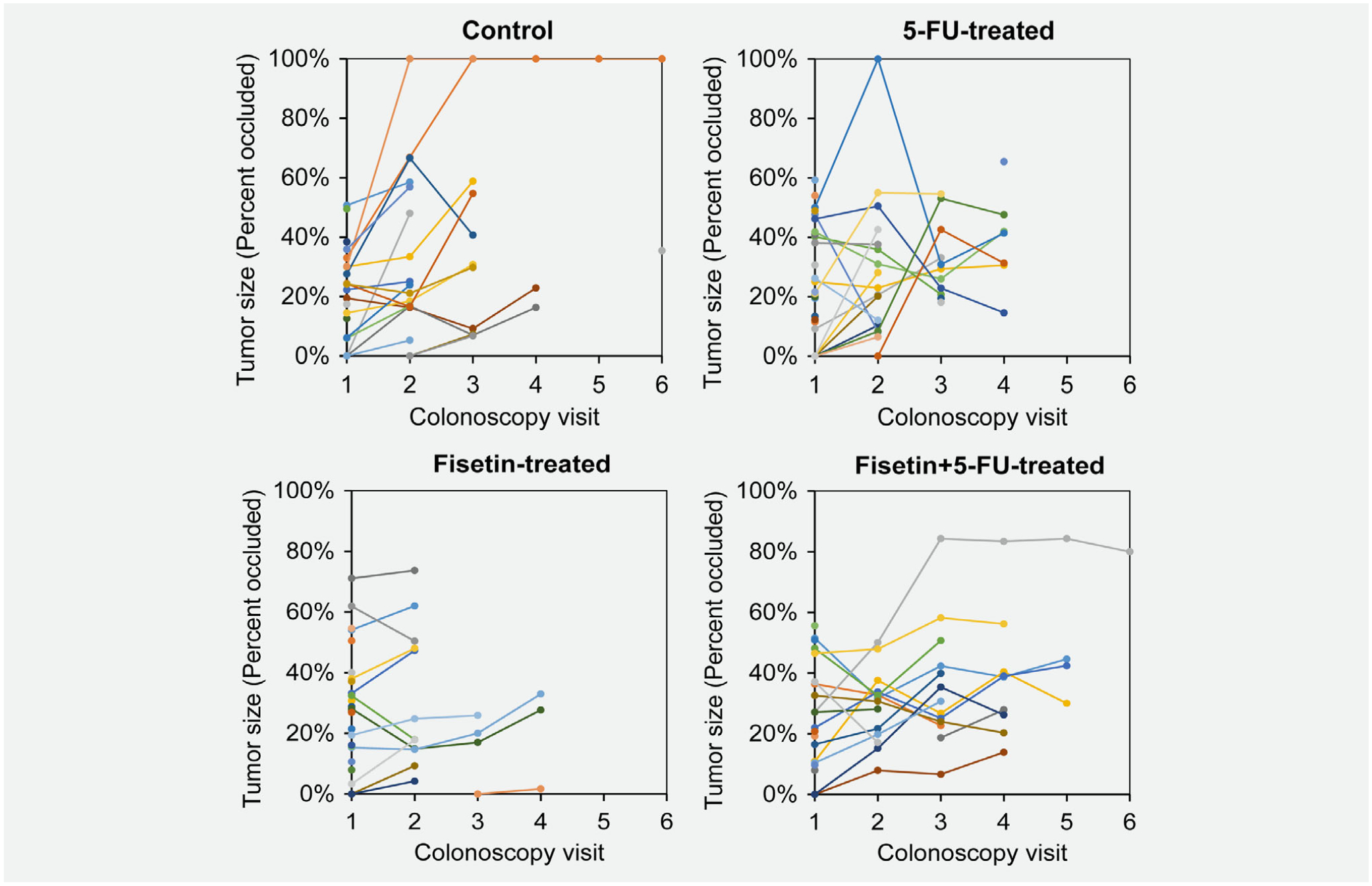
Effect of the treatment of fisetin, 5-FU or their combination on tumor growth (late) in FC13K1ApcMin/+ mice. Treatment with fisetin and 5-FU seemed to extend the life of experimental animals. To determine whether the drugs were effective on tumors that were more advanced, a second set of experimental mice received drugs starting between 45 and 53 days of age. Mice were given up to five rounds of treatment and euthanized when moribund or when the study was completed. Tumor growth was monitored by colonoscopy and plotted. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
About 15–20% of colorectal cancers harbor activating mutations in PIK3CA, which has been recognized as a significant oncogene in numerous cancers. Hence, modeling this mutation in the mammalian colon is imperative. The phosphatidylinositide-3-kinase (PI3K)/AKT/mTOR signaling cascade has been identified as favorable target for the development of new agents for chemoprevention and therapy.41,42 5-FU is the most commonly used chemotherapeutic agent after surgical intervention in CRC; however, it has serious side effects. Therefore, a therapeutic association which could improve the effect of this agent and mean- while reduce its adverse reactions is greatly required.
Several murine models of human CRC have developed in the last decade, with some limitations.43,44 These animal models have become the basic source of research to develop new chemopreventive/chemotherapeutic agents and to understand the pathogenesis of disease. It has been shown that there was inhibition of the formation of aberrant crypt foci in the colon of azoxymethane-treated F344 rats on dietary supplementation of polyphenon E.45 Treatment of human CRC cells with curcumin (the primary component of turmeric), blocked Wnt/β-catenin pathway-mediated c-myc expression, disrupted cell–cell adhesion in CRC cells, and caused G2/M phase cell- cycle arrest and induction of apoptosis.46 The present study is the first to describe the effect of a dietary agent (fisetin), as an adjuvant for the prevention and treatment of colon cancer and for the management of PIK3CA-mutant CRC.
In the current study, we investigated whether the combination of fisetin and 5-FU at low doses produces higher inhibitory activity than fisetin or 5-FU alone against CRC cells. We found that treatment of PIK3CA-mutant colon cancer cells and PIK3CA wild-type colon cancer cells with fisetin or 5-FU caused a significant decrease in their viability which was further enhanced with the combination of fisetin and 5-FU.
The PI3K/AKT pathway is frequently activated in colorectal cancer leading to tumorigenesis and resistance to chemotherapy. Treatment of PIK3CA-mutant CRC cells with fisetin and 5-FU caused decreased expression of PI3K and inhibition in the phosphorylation of AKT. Treatment with fisetin or 5-FU also led to an increase in the phosphorylation of AMPKα, and decrease in the phosphorylation of mTOR target proteins: the combination of fisetin and 5-FU led to more remarkable increase in p-AMPKα and stronger decrease in mTOR target proteins in PIK3CA-mutant CRC cells.
Treatment of cells with combination of fisetin and 5-FU led to dose-dependent robust inhibition of both mTOR complexes with inhibition of Raptor (mTORC1), Rictor (mTORC2) and inhibition of the phosphorylation of PRAS40 and GβL. The in vitro data is very encouraging and the combination of fisetin and 5-FU proved to be more effective than either agent alone. Therefore, we conducted in vivo studies to determine the effect of the treatment of combination of fisetin and 5-FU on the multiplicity of colonic tumors in FC13K1ApcMin/+ mice.
The tumors in the colon of FC13K1ApcMin/+ mice were longitudinally monitored during and after treatment at regular intervals with colonoscopy to identify mice bearing measurable tumors and to study the natural progression of tumors. We found that only 40% of fisetin treated mice had at least one tumor in the distal colon as compared to 71% of the control mice. This difference was statistically significant. Interestingly, the effect of fisetin was much stronger than that of 5-FU and comparable to the fisetin and 5-FU combination. Both fisetin alone and combination of fisetin and 5-FU treatment groups had significantly lower incidence relative to the control group. These results indicate that fisetin is a strong preventive agent that suppresses the development of tumors in the distal colon. The combination of fisetin and 5-FU also reduced the number of intestinal tumors in experimental mice, which usually quickly succumb to the disease because the tumors lack APC activity and express activated PI3K, which is strong driver of tumor progression.
The combination of fisetin and 5-FU prevents intestinal tumors from forming in the laboratory mouse. A critical step in the development of intestinal tumors appears to be the establishment of a large field of APC-deficient cells.47 Such a field can presumably form through the expansion of an existing field or else the coalescence of smaller fields. Activation of PI3K in APC-deficient cells would accelerate the rate of cell division while decelerating the rate of apoptosis thereby rapidly expanding a field of APC-deficient. Consistent with this notion, mice carrying mutations in Apc and Pik3ca develop more intestinal tumors than mice carrying mutations in only Apc. In this context, the combination of fisetin and 5-FU should have an additive effect. Fisetin blocks PI3K signaling (Fig. 4) so it should decelerate the rate of cell division and accelerate the rate of apoptosis (Fig. 2). Moreover, 5-FU triggers apoptosis (Fig. 2). Recently, others demonstrated that PI3K signaling had to be blocked in HER2-mutant gastric cancers for 5-FU to trigger apoptosis. Thus, the combination of fisetin and 5-FU might impede the expansion of a field of APC-deficient cells and consequently prevent tumors from becoming established.
Once a tumor was established, fisetin, 5-FU or the combination failed to cause substantial tumor regression. In a previous study, we demonstrated that 5-FU alone caused small colon tumors to grow more slowly and large colon tumors to regress in mice carrying only alterations in Apc. In our study, 5-FU still causes colon tumors to grow more slowing (Figs. 5 and 6). This effect is unchanged by the addition of fisetin. It is unclear why treatment would not trigger apoptosis and tumor regression. Possibly, the rapid growth of tumors owing to the activation of PI3K signaling led to more spontaneous mutations occurring and consequently a more diverse population of cells in each tumor. Colorectal cancers in humans with PIK3CA mutations are more resistant to 5-FU based therapies than those lacking PIK3CA mutations. This difference could be because of tumor heterogeneity and intrinsic resistance.
Fisetin is a nontoxic, inexpensive, dietary agent which possesses antiproliferative properties against several cancers.14 Therefore, it can be effectively used in cancer patients without damaging side effects, which are the main drawback of most chemotherapeutic drugs. It has also been earlier reported that fisetin targets specifically the cancer cells, and the normal cells were unaffected or had very minimal effect.17,48
We anticipate that these findings will establish fisetin as an adjuvant for the prevention and treatment of PIK3CA- mutant CRC.
Supplementary Material
What’s new?
Activating mutations in PIK3CA occur in 15 to 20% of advanced colorectal cancers (CRCs) and are associated with increased CRC-specific mortality. Hence, therapeutic inhibition of PIK3CA is a potential strategy for improving outcome in some CRC patients. Here, the dietary flavonoid fisetin, an inhibitor of the PI3K/AKT and mTOR pathways, when given in combination with 5-fluorouracil (5-FU), was found to significantly enhance apoptosis in PIK3CA-mutant CRC cells. Fisetin further prevented tumor formation in mice, and together with 5-FU reduced the total number of intestinal tumors. The data suggest that fisetin can broaden opportunities for 5-FU use against PIK3CA-mutant CRC.
Acknowledgements
NK (PhD) is supported by a Research Scholar Grant RSG-15-013-01-CNE from the American Cancer Society and University of Wisconsin Carbone Cancer Center (UWCCC) Support Grant P30 CA014520. RH (PhD) received support from the Wisconsin Dual Sport Riders. The authors would like to thank the staff of the UWCCC Flow Cytometry core for assistance in determining the number of apoptotic cells and the UWCCC Experimental Pathology Core for preparing histologic slides of tumors from mice.
Abbreviations:
- AMPK
AMP-activated protein kinase
- ATCC
American Type Culture Collection
- CRC
colorectal cancer
- DMSO
dimethyl sulfoxide
- eIF4E
eukaryotic initiation factor-4E
- 4E-BP1
4E-binding protein1
- 5-FU
5-fluorouracil
- mTOR
mammalian target of rapamycin
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
- PI3K
phosphatidylinositol 3-kinase
- PIK3CA
phosphatidylinositol-4,5-bisphosphonate-3-kinase, catalytic subunit alpha
- PRAS40
proline-rich Akt substrate of 40 kilodaltons Additional Supporting Information may be found in the online version of this article
Footnotes
Conflict of interest: None declared.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Garg MB, Lincz LF, Adler K, et al. Predicting 5-fluorouracil toxicity in colorectal cancer patients from peripheral blood cell telomere length: a multivariate analysis. Br J Cancer 2012;107:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsalic M, Bar-Sela G, Beny A, et al. Severe toxicity related to the 5-fluorouracil/leucovorin combination (the Mayo Clinic regimen): a prospective study in colorectal cancer patients. Am J Clin Oncol 2003;26:103–6. [DOI] [PubMed] [Google Scholar]
- 4.Patel K, Anthoney DA, Crellin AM, et al. Weekly 5-fluorouracil and leucovorin: achieving lower toxicity with higher dose-intensity in adjuvant chemotherapy after colorectal cancer resection. Ann Oncol 2004;15:568–73. [DOI] [PubMed] [Google Scholar]
- 5.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006; 441:424–30. [DOI] [PubMed] [Google Scholar]
- 6.Samuels Y, Diaz LA Jr., Schmidt-Kittler O, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell 2005;7: 561–73. [DOI] [PubMed] [Google Scholar]
- 7.Ogino S, Nosho K, Kirkner GJ, et al. PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol 2009;27:1477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nosho K, Kawasaki T, Ohnishi M, et al. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia 2008; 10:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao X, Morikawa T, Lochhead P, et al. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res 2012;18:2257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leystra AA, Deming DA, Zahm CD, et al. Mice expressing activated PI3K rapidly develop advanced colon cancer. Cancer Res 2012;72: 2931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deming DA, Leystra AA, Nettekoven L, et al. PIK3CA and APC mutations are synergistic in the development of intestinal cancers. Oncogene 2014; 33:2245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arai Y, Watanabe S, Kimira M, et al. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr 2000;130:2243–50. [DOI] [PubMed] [Google Scholar]
- 13.Kimira M, Arai Y, Shimoi K, et al. Japanese intake of flavonoids and isoflavonoids from foods. J Epidemiol 1998;8:168–75. [DOI] [PubMed] [Google Scholar]
- 14.Khan N, Syed DN, Ahmad N, et al. Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal 2013;19:151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adhami VM, Syed D, Khan N, et al. Dietary flavonoid fisetin: a novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem Pharmacol 2012;84:1277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Syed DN, Adhami VM, Khan N, et al. Exploring the molecular targets of dietary flavonoid fisetin in cancer. Semin Cancer Biol 2016;40–41:130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan N, Afaq F, Khusro FH, et al. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int J Cancer 2012;130:1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh Y, Afaq F, Khan N, et al. Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis 2010;31:1424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan N, Hadi N, Afaq F, et al. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis 2007;28: 163–73. [DOI] [PubMed] [Google Scholar]
- 20.Khan N, Jajeh F, Khan MI, et al. Sestrin-3 modulation is essential for therapeutic efficacy of cucurbitacin B in lung cancer cells. Carcinogenesis 2017;38:184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan N, Afaq F, Kweon MH, et al. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res 2007;67:3475–82. [DOI] [PubMed] [Google Scholar]
- 22.Su LK, Kinzler KW, Vogelstein B, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 1992; 256:668–70. [DOI] [PubMed] [Google Scholar]
- 23.Hung KE, Maricevich MA, Richard LG, et al. Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proc Natl Acad Sci USA 2010;107: 1565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saam JR, Gordon JI. Inducible gene knockouts in the small intestinal and colonic epithelium. J Biol Chem 1999;274:38071–82. [DOI] [PubMed] [Google Scholar]
- 25.Durkee BY, Shinki K, Newton MA, et al. Longitudinal assessment of colonic tumor fate in mice by computed tomography and optical colonoscopy. Acad Radiol 2009;16:1475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halberg RB, Chen X, Amos-Landgraf JM, et al. The pleiotropic phenotype of Apc mutations in the mouse: allele specificity and effects of the genetic background. Genetics 2008;180:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 2009;9:550–62. [DOI] [PubMed] [Google Scholar]
- 28.Danielsen SA, Eide PW, Nesbakken A, et al. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim Biophys Acta 2015;1855:104–21. [DOI] [PubMed] [Google Scholar]
- 29.Rychahou PG, Murillo CA, Evers BM. Targeted RNA interference of PI3K pathway components sensitizes colon cancer cells to TNF-related apoptosis-inducing ligand (TRAIL). Surgery 2005; 138:391–7. [DOI] [PubMed] [Google Scholar]
- 30.Lee YC, Lin HH, Hsu CH, et al. Inhibitory effects of and rographolide on migration and invasion in human non-small cell lung cancer A549 cells via down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol 2010;632:23–32. [DOI] [PubMed] [Google Scholar]
- 31.Tsang CK, Qi H, Liu LF, et al. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today 2007;12:112–24. [DOI] [PubMed] [Google Scholar]
- 32.Zhang YJ, Dai Q, Sun DF, et al. mTOR signaling pathway is a target for the treatment of colorectal cancer. Ann Surg Oncol 2009;16:2617–28. [DOI] [PubMed] [Google Scholar]
- 33.Brown EJ, Albers MW, Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin- receptor complex. Nature 1994;369:756–8. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Saud SM, Young MR, et al. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015;6:7365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loewith R, Jacinto E, Wullschleger S, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 2002;10:457–68. [DOI] [PubMed] [Google Scholar]
- 36.Kovacina KS, Park GY, Bae SS, et al. Identification of a proline-rich Akt substrate as a 14–3-3 binding partner. J Biol Chem 2003;278:10189–94. [DOI] [PubMed] [Google Scholar]
- 37.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004;4: 335–48. [DOI] [PubMed] [Google Scholar]
- 38.Sonenberg N, Gingras AC. The mRNA 5′capbinding protein eIF4E and control of cell growth. Curr Opin Cell Biol 1998;10:268–75. [DOI] [PubMed] [Google Scholar]
- 39.Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr Opin Cell Biol 2009; 21:209–18. [DOI] [PubMed] [Google Scholar]
- 40.Hadac JN, Leystra AA, Paul Olson TJ, et al. Colon tumors with the simultaneous induction of driver mutations in APC, KRAS, and PIK3CA still Progress through the adenoma-to-carcinoma sequence. Cancer Prev Res (Phila) 2015;8:952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hay N The Akt-mTOR tango and its relevance to cancer. Cancer Cell 2005;8:179–83. [DOI] [PubMed] [Google Scholar]
- 42.Ghayad SE, Cohen PA. Inhibitors of the PI3K/ Akt/mTOR pathway: new hope for breast cancer patients. Recent Pat Anticancer Drug Discov 2010;5: 29–57. [DOI] [PubMed] [Google Scholar]
- 43.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 1990;247:322–4. [DOI] [PubMed] [Google Scholar]
- 44.Mittal VK, Bhullar JS, Jayant K. Animal models of human colorectal cancer: current status, uses and limitations. World J Gastroenterol 2015;21:11854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao X, Xiao H, Ju J, et al. Green tea polyphenols inhibit colorectal tumorigenesis in azoxymethane- treated F344 rats. Nutr Cancer 2017;69:623–31. [DOI] [PubMed] [Google Scholar]
- 46.Jaiswal AS, Marlow BP, Gupta N, et al. Betacatenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 2002;21:8414–27. [DOI] [PubMed] [Google Scholar]
- 47.Fischer JM, Schepers AG, Clevers H, et al. Occult progression by Apc-deficient intestinal crypts as a target for chemoprevention. Carcinogenesis 2014; 35:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan N, Afaq F, Syed DN, et al. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis 2008;29:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


