Abstract
Positron emission tomography (PET) with 18F-sodium fluoride (18F-NaF) has emerged as a promising non-invasive imaging modality to identify high-risk and ruptured atherosclerotic plaques. By visualizing microcalcification, 18F-NaF PET holds clinical promise in refining how we evaluate coronary artery disease, shifting our focus from assessing disease burden to atherosclerosis activity. In this review, we provide an overview of studies that have utilized 18F-NaF PET for imaging atherosclerosis. We discuss the associations between traditional coronary artery disease measures (risk factors) and 18F-NaF plaque activity. We also present the data on the histological validation as well as show how 18F-NaF uptake is associated with plaque morphology on intravascular and CT imaging. Finally, we discuss the technical challenges associated with 18F-NaF coronary PET highlighting recent advances in this area.
Introduction
Despite improvements in therapies for atherosclerotic disease, myocardial infarction (MI) remains the leading cause of death worldwide.1 The underlying etiology of MI is most commonly rupture of a high-risk plaque.2 3 While standard non-invasive tests can detect coronary artery disease (CAD) and assess ischemia or high-risk plaque features, they fail to identify the biologic activity of CAD, potentially limiting the discrimination of stable from high-risk disease which may result in imprecision in assigning patients for intensive therapies. A reliable warning of an acute coronary syndrome after initial testing could facilitate highly targeted application of more intensive treatments in a well-defined highest risk group.
Recently, 18F-sodium fluoride positron emission tomography (18F-NaF PET) has shown promise in imaging the activity of calcification processes in vivo.4 In a landmark study, Joshi et al showed that intense ¹⁸F-NaF uptake localizes to recent plaque rupture in patients with acute MI and in those with symptomatic carotid disease.5 Moreover, in patients with stable CAD, ¹⁸F-NaF uptake identified coronary plaques with high-risk features on intravascular ultrasound.
18F-sodium fluoride in nuclear medicine
18F-NaF has been used for decades in nuclear medicine to image bone malignancies.6 In the skeleton, fluoride binding is facilitated through a chemical reaction with hydroxyapatite, a crystalline structure that is a key component of both bone and vascular calcification.7,8 In an initial retrospective feasibility study, Derlin et al showed that arterial wall ¹⁸F-NaF distribution is consistent with established atherosclerotic topography, with increased uptake in the thoracic aorta and at the carotid bifurcation.9 In a second study, Derlin et al demonstrated a correlation of ¹⁸F-NaF uptake in calcified carotid plaque with conventional cardiovascular risk factors in a large cohort of asymptomatic individuals who were investigated for non-cardiovascular indications.10 The authors concluded that ¹⁸F-NaF PET can potentially have a role as a tool for visualizing calcification activity the carotids and other major arteries.
Dedicated 18F-NaF cardiovascular PET
The feasibility of 18F-NaF for imaging calcification activity was based on retrospective analyses of ¹⁸F-NaF PET studies acquired for oncological purposes.8–10 In 2012, a dedicated cardiac ¹⁸F-NaF approach for assessing coronary activity was introduced. In a cohort of 106 patients with aortic stenosis and concomitant coronary atherosclerosis, Dweck et al performed 18F-NaF PET of the thorax with ECG-gated breath-hold non-contrast CT performed for calcium scoring as anatomical reference.11 The authors described 18F-NaF uptake in the coronary arteries with PET/CT. They demonstrated that this technique is both feasible and reproducible and that it can provide key insights into coronary artery plaque biology. The effective radiation dose associated with 18F-NaF PET ranges from 3.5 to 4 miniSieverts.
Association of 18F-NaF uptake with traditional risk factors
Considered together, the early literature provided clear evidence that 18F-NaF PET uptake is associated with the presence and extent of atherosclerosis and cardiovascular risk factors.8,11,12 The latter included associations with age, male sex, hypertension, hypercholesterolemia and cumulative smoking exposure. Of interest, the prevalence of radiotracer uptake was strongly associated with the number of atherogenic risk factors.10 Dweck et al made the distinction between patients with increased tracer uptake (active calcification) and those with inactive calcification by using a threshold equating to the highest 18F-NaF uptake value reported in a control group without coronary artery calcification on CT.11 In their study, those with active calcification (38%) were more likely to have clinically significant CAD, a higher incidence of previous major adverse cardiovascular events, lower serum high-density lipoprotein cholesterol concentrations, and higher Framingham risk prediction scores. Since the authors performed longer single bed position acquisitions than in oncological studies (10 vs 3 min) and used non-contrast CT for anatomical reference, they observed localization of the 18F-NaF signal to specific coronary territories. Further, the authors reported that although 18F-NaF activity was most commonly observed overlying existing calcium and a strong correlation was observed with coronary calcium scores, 41% of patients with scores > 1000 had no significant 18F-NaF uptake and that areas of increased tracer uptake were also found in regions remote from established calcium. They speculated that increased tracer activity potentially relates to developing microcalcification that is below the resolution of CT. Microcalcification in coronary plaques is believed to be associated with increased mechanical stress and risk of future cardiovascular events.13–19 Accordingly, Dweck et al suggested that 18F-NaF might to be able to distinguish patients with dormant calcific disease, established many months or years previously, and subjects with metabolically active disease where the calcification processes are ongoing.11 Importantly, this distinction showed clinical potential as those with increased coronary 18F-NaF uptake demonstrated higher rates of anginal symptoms, prior MACE events, and cardiovascular risk factor scores.
18F-NaF uptake in patients with stable and unstable CAD
In an elegant study, using coronary 18F-NaF assessments performed alongside coronary CT angiography, Joshi et al studied coronary 18F-NaF activity in 40 patients with recent acute MI and 40 patients with stable angina undergoing elective invasive coronary angiography.5 Increased 18F-NaF uptake was found in 37/40 (93%) of patients with recent infarction, with uptake localizing to the culprit plaque that had ruptured and caused the infarct, independent of stenting (Figure 1). Further, increased 18F-NaF uptake was observed in 45% of the stable patients and was associated with multiple adverse plaque features (e.g. positive remodeling, macrophage burden, necrotic core, microcalcification) on intravascular ultrasound and on coronary CT angiography. Thus, it appears that 18F-NaF can identify high-risk and culprit coronary atherosclerotic plaques, providing hope that that this novel method could aid in the prediction of heart attacks.
Figure 1.
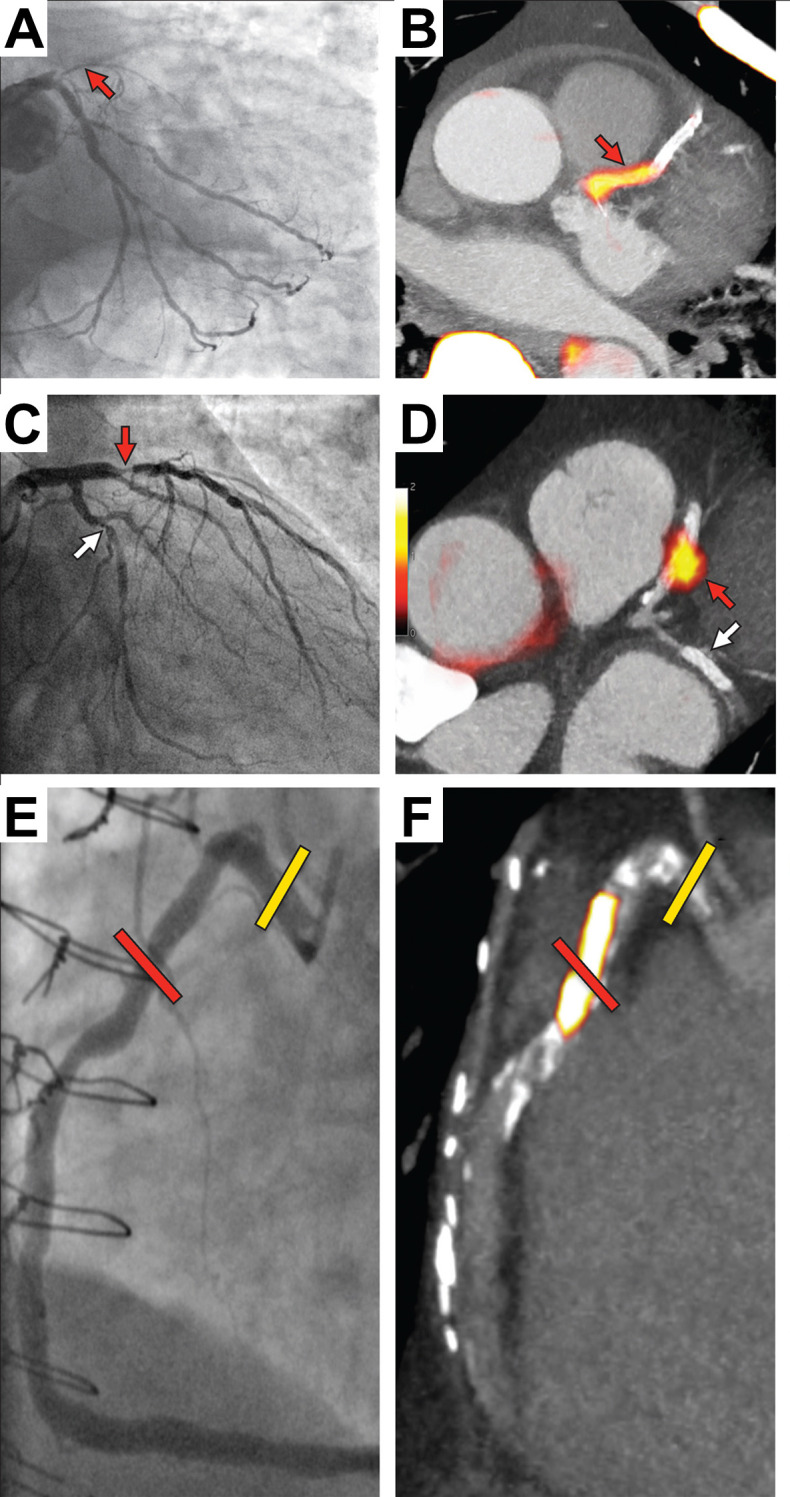
Focal 18F-NaF in patients with myocardial infarction and stable angina. Patient with acute ST-segment elevation myocardial infarction with (A) proximal occlusion (red arrow) of the left anterior descending artery on invasive coronary angiography and (B) intense focal 18F-NaF [ tissue-to-background ratios, culprit 2.27 vs reference segment 1.09 (108% increase)] uptake (yellow-red) at the site of the culprit plaque (red arrow) on the combined PET and CT. Patient with anterior non-ST-segment elevation MI with (C) culprit (red arrow; left anterior descending artery) and bystander non-culprit (white arrow; circumflex artery) lesions on invasive coronary angiography that were both stented during the index admission. Only the culprit lesion had increased 18F-NaF uptake [tissue-to-background ratios, culprit 2.03 vs reference segment 1.08 (88% increase)] on PET-CT (D) after percutaneous coronary intervention. In a patient with stable angina with previous coronary artery bypass grafting, invasive coronary angiography (E) showed non-obstructive disease in the right coronary artery. Corresponding PET-CT scan (F) showed a region of increased 18F-NaF activity (positive lesion, red line) in the mid-right coronary artery (tissue-to-background ratio, 3.13) and a region without increased uptake in the proximal vessel (negative lesion, yellow line). This figure was originally published in the Lancet under the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). (Data from Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014 383:705–713.5 18F-NaF, 18F-sodium fluoride; PET, positron emission tomography.
In a subsequent study, Lee et al sought to evaluate the association between 18F-NaF uptake and adverse plaque features on intravascular ultrasound and optical coherence tomography.20 Again, increased ¹⁸F-NaF uptake was associated with greater positive remodeling, more microcalcification, larger necrotic cores, higher maximum lipid arc and prominent microvessels compared with 18F-NaF–negative plaques. Importantly, such differences were not observed in relation to the lumen area or coronary calcium.
Histological validation of 18F-NaF coronary uptake
In the context of cardiovascular imaging, histological validation of 18F-NaF PET has been provided by investigation of the carotid plaques excised after endarterectomy using electron microscopy, autoradiography, and μPET/CT. These have consistently demonstrated that 18F-NaF binds preferentially to microcalcification beyond the resolution of μCT and co-localizes to regions of active mineralization within vascular tissue.21 The preferential binding to microcalcification is likely to be due to the high surface area of hydroxyapatite in regions where this nanocrystal builds up.22,23 These findings have been recently corroborated in study by Creager et al.24 By employing near-infrared fluorescence molecular imaging, histology and ex vivo human atherosclerotic plaque specimens, the authors provided evidence that regions identified as PET-positive and CT-negative with 18F-fluoride PET/CT imaging are the result of the presence of developing microcalcifications within atherosclerotic plaques.
18F-NaF activity in high-risk plaques on CT angiography
Pathological studies have identified the adverse histological plaque features that are associated with culprit coronary lesions. These vulnerable or adverse plaque features include a large lipid core, spotty calcification, positive remodeling, and inflammatory cell infiltration.25–30
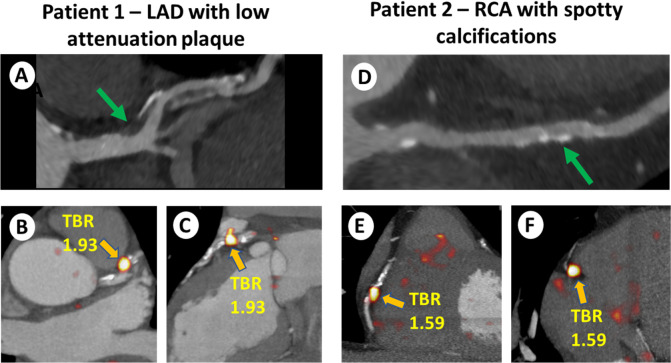
To date, several studies have addressed the relationship between coronary 18F-NaF uptake and the presence of adverse coronary plaque features (APF) in a wide range of clinical settings. Kitagawa et al studied the relationship of coronary CT angiography findings and 18F-NaF PET in a cohort of 41 patients with stable CAD of whom 26 presented with positive remodeling and low attenuation plaque.31 The authors demonstrated that these plaque features are more prevalent in patients with coronary 18F-NaF target to background ratios (TBR) >1.28, but provided only an analysis of combined adverse features (both low attenuation plaque and positive remodeling) but not on distinct associations of each APF with 18F-NaF uptake.32 In a recent study, we have shown that almost two-thirds of stable CAD patients with high-risk plaques on coronary CT angiography (defined as a simultaneous presence of a large plaque volume, over 50% lesion stenosis and at least one adverse plaque feature) have abnormal 18F-NaF uptake.33,34 We demonstrated that low attenuation plaque is closely associated with increased tracer activity with over 90% of such lesions having increased 18F-NaF uptake (Figure 2). Of lesions with positive remodeling and spotty calcifications, however, only 55 and 57% presented with increased 18F-NaF. While low attenuation plaque was strongly associated with 18F-NaF, a substantial proportion of the plaques with increased 18F-NaF uptake did not have low attenuation plaque. Jointly, these studies suggest that there is substantial discordance in morphological plaques characteristics (the presence of adverse plaque characteristics) and the activity of the disease (measured with 18F-NaF PET) indicating that the two imaging modalities provide complementary information. To this end, we have recently demonstrated a relationship between coronary 18F-NaF uptake and increased attenuation in pericoronary adipose tissue, which has been proposed as a method for examining coronary plaque inflammation.34 This observation provides further evidence that both imaging techniques contribute unique assessments of high-risk atherosclerotic plaques.
Figure 2.
Examples of 18F-NaF uptake in coronary lesions with adverse plaque features. Patient 1: 67-year-old male with a LAD plaque with low attenuation plaque (green arrow) (A) and focal 18F-NaF uptake (orange arrow) with increased TBR of 1.93 (B, C). Patient 2: 58-year-old male with a RCA lesion with spotty calcifications (green arrow) (D), and focal 18F-NaF uptake (orange arrow) with increased TBR of 1.59 (E, F). Reprinted by permission from Oxford University Press: European Heart Journal – Cardiovascular Imaging, Predictors of 18F-sodium fluoride uptake in patients with stable coronary artery disease and adverse plaque features on computed tomography angiography, Jacek Kwiecinski, Damini Dey, Sebastien Cadet et al, 2019. LAD, left anterior descending; RCA, right coronary artery; TBR, target to background ratio.
Technical challenges for 18F-NaF coronary PET
Despite the promising results of initial studies, widespread dissemination of 18F-NaF PET is hampered by the inherent complexities of this imaging modality.35,36 The measured uptake is adversely affected by blurring of the tracer signal in target lesions by cardiorespiratory motion which occurs as a result of physiological tidal breathing and heart contractions. Additionally, due to the small target lesions size, the measured uptake is prone to partial volume effects. Moreover, to ensure adequate count statistics, 18F-NaF coronary PET requires long emission scanning. The duration of the acquisition has evolved from 90 s per bed position in the initial retrospective analysis by Derlin et al to 10 min in the first cardiac specific study by Dweck et al to 30 min in currently ongoing prospective trials.9,11 Such long emission scanning times increase the risk of interscan patient motion as subjects may find it difficult to remain still over the entire length of the acquisition.37 Finally, for evaluating uptake 18F-NaF PET anatomical reference for attributing foci of increased tracer activity to coronary arteries is required. In view of the small target lesion sizes and the combined effects of cardiorespiratory motion, 18F-NaF PET would undoubtedly benefit from refinement of image acquisition, reconstruction and analysis methods.
Motion
The quality of coronary PET and the accuracy of uptake measurements are degraded by cardiorespiratory and patient motion during the 30 min scans. It has been shown that physiological tidal breathing alone causes the heart to move more than 1 cm.38 The amplitude of coronary artery motion during the cardiac cycle is about 8–26 mm, depending on the artery and location, with the highest motion in the RCA.39 Further, typical gross patient motion (other than cardiorespiratory motion) results in repositioning of the heart, typically by 5–15 mm, during a 30 min scan.37
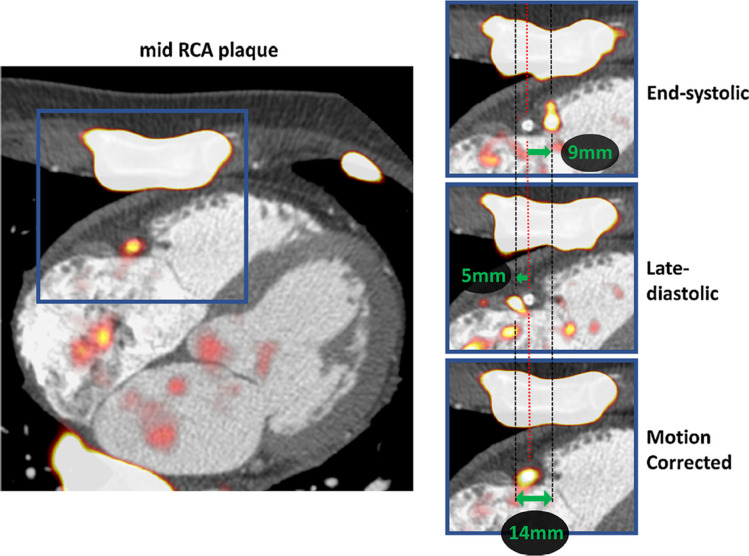
To mitigate the effect of motion associated with heart contraction, ECG gating has been employed. In many studies to date, only the end-diastolic phase has been utilized because coronary motion is much lower during this phase of the cardiac cycle. Unfortunately, this approach uses only 25% of PET counts, consequently increasing image noise.40 Recent studies have reduced the risks of potential false-negative findings by correcting for cardiac motion. Rubeaux et al have developed a correction method to tackle coronary motion in 18F-NaF PET imaging data by applying level-set based non-linear PET registration of individual PET time-bins to the end-diastolic position.41 The proposed registration was constrained to the coronary regions extracted from the co-registered coronary CT angiography to avoid registration to spurious noise signal outside of the arteries in question. As compared to the diastolic imaging, they have demonstrated increased uptake values, while drastically reducing noise (Figure 3). According to this study, best results can be obtained with 10-bin motion corrected data as it provides the best time-resolved motion correction. The same group has recently evaluated frequency and relative effect of gross patient motion (repositioning during coronary PET emission scanning) and developed a method for compensation of such events.42 According to the study, patient repositioning events occur approximately 3–4 times during the 30 min long acquisition. The proposed compensation approach can increase the uptake measurements by up to 30% potentially limiting the number on false negative findings on 18F-NaF PET. Importantly, this simple, but effective automated data-driven patient motion compensation technique, which utilizes exclusively list-mode data, does not require any additional hardware during image acquisition, and can be applied retrospectively to data obtained with standard coronary PET protocols.
Figure 3.
Motion-correction of physiological motion of the right coronary artery. Systolic excursion of the tricuspid annular plane leads to displacement of the PET signal during the cardiac cycle (zoomed area of interest in blue squares). The difference in the shift of PET from reference is shown on the end-systolic (top-right) and late-diastolic (mid-right) images. Green arrows represent the vectors of mid-RCA motion. By co-registration of all PET data to the reference end-diastolic gate, the final motion-corrected image is corrected for the 14 mm mid RCA motion (bottom-right). Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Journal of Nuclear Cardiology, Optimization of reconstruction and quantification of motion-corrected coronary PET-CT, Mhairi K. Doris, Yuka Otaki, Sandeep K. Krishnan et al, 2018. RCA, rightcoronary artery; PET, positron emission tomography.
Small target lesion size
Due to the small volume of coronary plaques (usually below 200 mm3 and only rarely exceeding 300 mm3), coronary 18F-NaF PET is prone to partial volume effects. State-of-the-art PET scanners ameliorate this issue to some degree with resolution recovery. However, since size of the plaques of interest is at best comparable to the minimum spatial resolution of PET, further corrections for partial volume effects are needed. In a recent study, Cal-Gonzalez et al employed a local projection method which was previously validated in the context of single photon emission CT and preclinical PET imaging.43 By applying this elaborate compensation technique, the authors were able to enhance the carotid plaque uptake values by up to 227%.44 Such impressive results were achieved by means of a single post-processing step which results in a partial-volume-corrected image and can in principle be easily disseminated, as the proposed method does not require projection data nor any upgrade of the existing reconstruction algorithms.
Uncertainty regarding reconstruction parameters
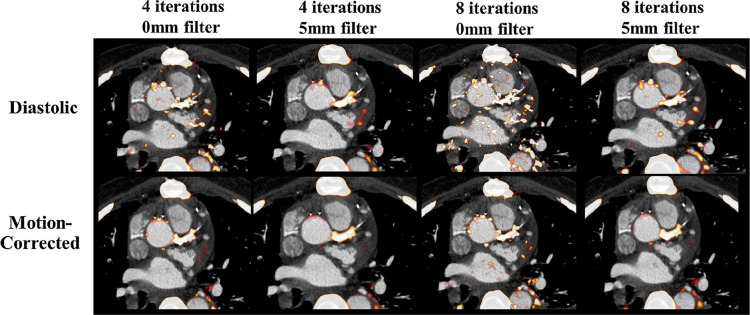
Another potential aspect of 18F-NaF PET imaging which requires attention is image reconstruction.45 The number of iterations, the use of filtering, applying point spread functions as well as taking full advantage of the time of flight and resolution recovery techniques, all have a profound impact on the uptake values.46 In a recent study, Doris et al showed how particular settings affect the signal to noise ratio and influence image quality.40 The authors demonstrated that while more iterations and no post-filtering result in higher uptake values, such images are excessively noisy and difficult to interpret (Figure 4). It appears that standardization of reconstruction parameters would be an important step in disseminating 18F-NaF PET as it would enable making direct comparisons between centers and vendors.
Figure 4.
The impact of different PET reconstructions on visual image quality in diastolic and motion-corrected images in a patient with a positive culprit lesion in the left main coronary artery. The PET reconstruction using 4 iterations and 5 mm post-filtering was considered to provide superior image quality (TBR = 1.92 for motion-corrected image). Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Journal of Nuclear Cardiology, Optimization of reconstruction and quantification of motion-corrected coronary PET-CT, Mhairi K. Doris, Yuka Otaki, Sandeep K. Krishnan et al, 2018. PET, positron emission tomography; TBR, target to background ratio.
Standardization of the uptake measurement utilized would be beneficial. While TBR remain the most widely used measure, it was pointed out that despite the broad application of TBR in the literature, particularly in the field of atherosclerosis PET imaging, its biologic significance and physiologic or mathematic explanation have not been provided.47 Huet et al argued that blood activity adds to plaque activity because of the spatial blurring of PET images (which occurs as a result of the imperfect spatial resolution).45 It was suggested that to correct for blood uptake, subtraction of venous standardized uptake value (SUV) from arterial SUV would be a more rational solution than dividing the two measured values. The rationale supporting this approach is the fact that the clearance of PET tracers from the venous blood pool can be quite variable from subject to subject, as it depends on multiple factors. As a result, dividing the vascular wall SUV with the venous blood pool SUV may introduce more variability and confusion to the TBR measurement.47
Only modest difference in uptake between culprit and non-culprit plaques
The relatively modest TBR increases in culprit versus non-culprit plaques (~34% higher) reported by Joshi et al may hamper the clinical implementation of coronary 18F-NaF PET.5 It is however important to realize that the coronary 18F-NaF TBR relies on the background bloodpool activity (as it is calculated by dividing the maximum SUV of the tracer in the plaque by the mean SUVs of the background blood pool measured in the atria). These associations mandate performing image acquisition with relatively long injection to acquisition intervals as with time the tracer activity in the bloodpool decreases.
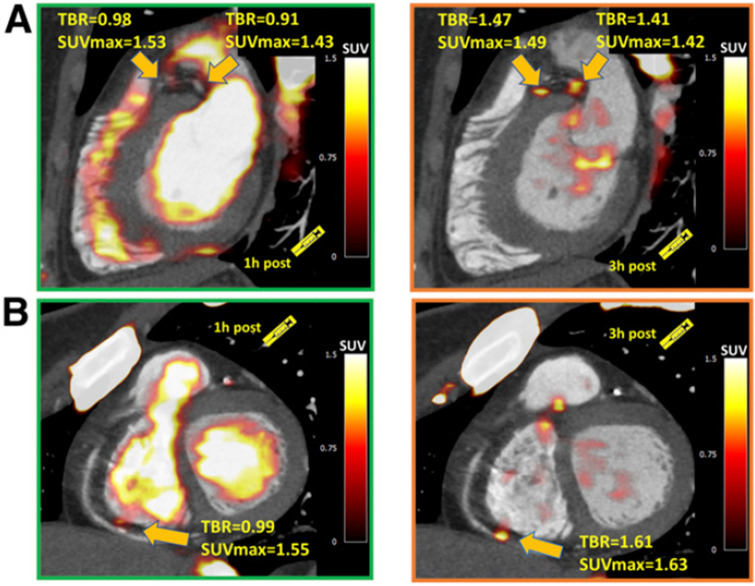
In cardiovascular PET, most studies to date have assessed 18F-NaF uptake in atherosclerotic plaques 1 h after tracer administration.9–11,19 Although semi-quantitative analysis is feasible with 1 h images, it can be difficult to discriminate plaques with 18F-NaF uptake from noise. In fact, compared to uptake in the bony skeleton, 18F-NaF uptake due to active microcalcification formation in coronary plaque is very small. In one of the early studies, it was speculated that the optimal time for atherosclerotic plaque imaging with 18F-NaF might be longer than the 1 h post-injection time point used for bone imaging.10 Subsequently, longer delays for cardiovascular 18F-NaF PET imaging were evaluated by two studies to date. Blomberg et al concluded that delayed 18F-NaF PET imaging does not improve quantification of vascular calcification.48 However, the authors measured only overall heart 18F-NaF activity (including blood pool) by placing regions of interest around the entire cardiac silhouette on ungated PET/CT images (excluding 18F-NaF activity originating from bones and cardiac valves) and averaged the SUV values derived from all slice. More recently, utilizing a dedicated coronary PET protocol, we showed that that prolonging the time from tracer injection to PET acquisition can facilitate image analysis and provide lower background activity and higher TBR values than the currently used 1 h post-injection protocol.49 We demonstrated that by delaying image acquisition to 3 h, new lesions, categorized as false-negative on the 1 h PET, are readily identified (Figure 5). We reported a TBR increase of 80% in 3 h compared to 1 h imaging, providing evidence that delayed PET might be an important step in refining coronary PET imaging with 18F-NaF.
Figure 5.
Examples of coronary plaques with significant uptake on 3 h PET and low tracer activity of 1 h post injection imaging. Short axis images of proximal left anterior descending, proximal circumflex (A) and distal right coronary artery (B) plaques (arrows) which had a TBR <1.0 on 1 h PET (left column) and showed uptake exceeding the 1.25 TBR threshold at 3 h. This research was originally published in JNM. Kwiecinski J, Berman DS, Lee SE, Dey D, Cadet S, Lassen ML, Germano G, Jansen MA, Dweck MR, Newby DE, Chang HJ, Yun M, Slomka PJ. Three-Hour Delayed Imaging Improves Assessment of Coronary 18F-Sodium Fluoride PET. J Nucl Med. 2019 Apr;6025 :530–535. doi: 10.2967/jnumed.118.217885. PET, positron emission tomography; SUV, standardized uptake value; TBR, target to background ratio.
High quality anatomical reference
As outlined above for coronary 18F-NaF PET image analysis, anatomical reference is essential: there is a need for exact registration of the 18F-NaF signal and the associated coronary artery. While initial studies utilized low dose CT images acquired for attenuation correction or coronary calcium scoring purposes to localize increased 18F-NaF activity to atherosclerotic plaques, this approach has several limitations. For instance, when assessing 18F-NaF activity in the left main or mid-left anterior descending artery uptake originating from the ascending aorta and main pulmonary artery can be erroneously assigned to the coronaries. Likewise, extra coronary foci of uptake (in patients who suffered from pericardial, aortic, or mitral disease which resulted in calcification outside of coronary arteries) can be problematic (Figure 6). Further, clear delineation of the coronary arteries is not possible in a large proportion of the coronary artery tree.
Figure 6.
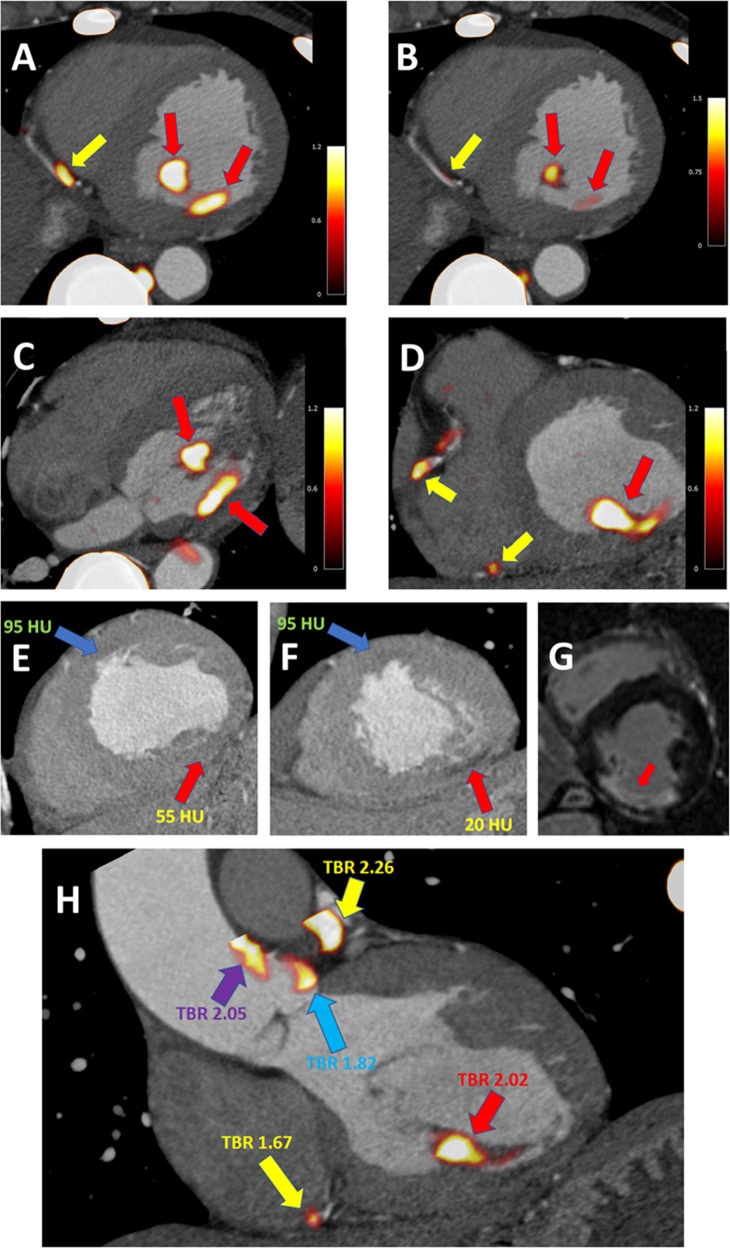
Case example of coronary and non-coronary 18F-NaF uptake. A 67-year-old-male with uptake in the distal RCA (yellow arrow) and non-coronary uptake in the inferomedial papillary muscle (red arrow) (A). The uptake in the papillary muscle is more pronounced than in the coronary plaque (B). In the transverse plane the extra coronary foci of uptake can be easily seen (red arrows) (C) and have higher activity than the RCA plaques (yellow arrows) (D). Taking advantage of the CT angiography, which was used for anatomical reference, careful inspection of the dataset revealed an area of low attenuation within the inferior LV wall and the postero medial papillary muscle (red arrow) as compared to the LV anteroseptum (blue arrow) (E&F). Such low attenuation can be seen in infarcted myocardium—which might, in the process of healing, develop foci of microcalcification (which attract 18F-NaF). Without the reference of a CT angiography the foci of pronounced 18F-NaF uptake could potentially be erroneously attributed to plaques in the PDA or the PLV artery. Due to the findings on the PET study the patient underwent cardiac magnetic resonance imaging which revealed scarring of the inferior wall and postero medial papillary muscle (area of delayed enhancement [white] as opposed to the remote myocardium [black]) (G). While uptake situated within the myocardium is a rare finding, the same patient showed non-coronary 18F-NaF activity in the aorta—which without anatomical reference could be easily attributed to one of the left coronary proximal plaques (H). Yellow arrows indicate coronary plaque uptake (in the distal RCA and proximal LAD). The red arrow highlights the infarcted postero medial papillary muscle. The purple arrow indicates increased PET tracer activity in the ascending aorta and the blue arrow highlights uptake immediately adjacent to the left coronary cusp of the aortic valve. Reprinted by permission from Wolters Kluwer Health: Circulation: Cardiovascular Imaging, Feasibility of coronary 18F-sodium fluoride positron-emission tomography assessment with the utilization of previously acquired computed tomography angiography, Jacek Kwiecinski, Philip D Adamson, Martin Lassen et al, 2018, Volume 11, Issue 12, e0008325, https://www.ahajournals.org/journal/circimaging. LAD, left anterior descending; PDA, posterior descending artery; PET, positron emission tomography; PLV, posterior left ventricular; RCA, right coronary artery; RV, right ventricle; TBR, target to background ratio.
These caveats of image analysis have been addressed by employing coronary CT angiography as the anatomical reference. This approach enables localizing increased tracer to activity to individual coronary plaques. Studies to date have utilized CT angiography acquired using hybrid PET/CT scanner during the same imaging session.5,31,50,51 This approach, however, does not lend itself to patient selection for imaging based on prior CT angiography findings. Most importantly, if the 18F-NaF imaging becomes part of a clinical assessment strategy, it is likely that patients would be selected for 18F-NaF PET based on the findings of a CT angiography performed for clinical purposes. Moreover, the single-session hybrid PET/CT protocol does not allow for the use of optimal CT equipment for CT angiography, which may only be available on standalone CT scanners. Finally, the use of hybrid PET/CT angiography requires multifaceted staff expertise in both PET and coronary CT angiography during a single session.
We have recently shown that these limitations can be eliminated by employing a prior coronary CT angiography approach with a subsequent standalone PET-only coronary scan can be used for the evaluation of coronary 18F-NaF PET uptake.52 Such a protocol enables equivalent categorization of plaques with increased 18F-NaF activity and accurate quantification of PET uptake. As the clinical application of 18F-NaF evolves, a coronary CT angiography performed clinically will be the most common basis for selection of patients who might benefit from assessment of disease activity with coronary PET imaging.53 In this scenario, the requirement for a second CT angiography obtained at the time of coronary PET imaging would not be practical or economically feasible and would incur unnecessary additional radiation exposure. The coronary CT angiography first approach facilitates the selection of high-risk patients for PET-only 18F-NaF PET imaging, based on results from prior CT angiography scan. It enables taking full advantage of dedicated state-of-the-art CT scanners which provide superior image quality compared to hybrid PET/CT machines. Finally, given the simplicity of the prior CT angiography and subsequent PET-only acquisition protocol, this approach could facilitate dissemination of 18F-NaF coronary uptake imaging beyond experienced academic institutions.
Awaiting outcome
Over the last decade, extensive information has been gathered regarding the biologic mechanisms of increased coronary 18F-NaF uptake. Promising findings have been reported regarding the relationship of 18F-NaF uptake to adverse coronary plaque features and to culprit coronary plaques in patients with acute MI. Further, a great deal of progress has been made in optimizing coronary 18F-NaF acquisitions and measurements. This work has led to great interest in the potential of this approach to provide an important dimension beyond anatomy in guiding the management of patients with CAD. However, to date there is only sparse information regarding the prognostic implications of coronary 18F-NaF uptake.
Recently, one cohort of 32 patients with known or suspected CAD who were followed for 2 years for major adverse cardiac events demonstrated that higher coronary 18F-NaF uptake predicts adverse events.31 The study also confirmed previously reported findings regarding the added value of 18F-NaF PET over CT angiography alone and the associations between the presence of adverse plaque features and tracer activity. Of interest, in this small study, the 1.25 TBR threshold initially proposed by Joshi et al and subsequently utilized in multiple studies has been to some degree validated.34,40,42 When applied in a Kaplan–Meier analysis, patients with TBR ≥ 1.28 had a higher risk of earlier coronary events than those with lower values. Finally, in the study by Kitagawa et al on a multivariate Cox proportional analysis adjusted for age, sex, presence of coronary risk factors, and statin use, this TBR threshold remained a significant predictor of adverse coronary events.31
A large multicenter study assessing the relationship between coronary 18F-NaF uptake and cardiac outcomes is currently underway: Prediction of Recurrent Events with 18F-Fluoride to Identify Ruptured and High-Risk Coronary Artery Plaques in Patients with Myocardial Infarction (PRE18FFIR) Study (NCT02278211). PRE18FFIR is an observational study that is following 700 high risk patients with CAD to determine whether baseline 18F-NaF PET can identify subjects who go on to sustain MI.54 This study will provide important information regarding the potential of 18F-NaF to become an integral part of the routine clinical practice. Ultimately, if increased 18F-NaF activity emerges as a predictor of adverse events it could then serve as a risk stratification tool for targeting more aggressive therapies (such as PCSK9 inhibitors and interleukin 1-β inhibitors) to patients at greatest risk of adverse cardiovascular events.
Conclusions
The way calcification in atherosclerosis is perceived has recently evolved. Once regarded as a passive and degenerative phenomenon, it is now believed to be a highly dynamic and regulated process which plays a key role in plaque biology and vulnerability. 18F-NaF PET has emerged as potential tool for measuring disease activity in coronary atheroma and identifying high-risk vulnerable plaques. Further studies are now required to assess whether this imaging technique can prospectively identify patients at increased risk of MI and add prognostic data over and beyond currently used approaches. If future studies confirm 18F-NaF PET clinical potential, this imaging approach might revolutionize risk evaluation in CAD patients.
Footnotes
Acknowledgements: This research was supported in part by grants R01HL135557 from the National Heart, Lung, and Blood Institute/National Institute of Health (NHLBI/NIH) and by a grant from Dr. Miriam and Sheldon G. Adelson Medical research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. DEN (CH/09/002, RE/13/3/30183, RG/16/10/32375) and MRD (FS/14/78/31020) are supported by the British Heart Foundation. DEN is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA) and MRD of Sir Jules Thorn Award for Biomedical Research Award (2015).
Contributor Information
Jacek Kwiecinski, Email: jkwiecinski@ikard.pl.
Piotr J Slomka, Email: Piotr.Slomka@cshs.org.
Marc R Dweck, Email: marc.dweck@ed.ac.uk.
David E Newby, Email: d.e.newby@ed.ac.uk.
Daniel S Berman, Email: Daniel.Berman@cshs.org.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, AS G, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update: a report from the American heart association. Circulation 2015; 131: e29–322. [DOI] [PubMed] [Google Scholar]
- 2.Muller JE, Kaufmann PG, Luepker RV, Weisfeldt ML, Deedwania PC, Willerson JT. Mechanisms precipitating acute cardiac events: review and recommendations of an NHLBI workshop. National heart, lung, and blood Institute. mechanisms precipitating acute cardiac events participants. Circulation 1997; 96: 3233–9. [DOI] [PubMed] [Google Scholar]
- 3.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006; 47(8 Suppl): C13–18. doi: 10.1016/j.jacc.2005.10.065 [DOI] [PubMed] [Google Scholar]
- 4.Adamson PD, Vesey AT, Joshi NV, Newby DE, Dweck MR. Salt in the wound: (18)F-fluoride positron emission tomography for identification of vulnerable coronary plaques. Cardiovasc Diagn Ther 2015; 5: 150–5. doi: 10.3978/j.issn.2223-3652.2015.03.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi NV, Vesey AT, Williams MC, Shah ASV, Calvert PA, Craighead FHM, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. The Lancet 2014; 383: 705–13. doi: 10.1016/S0140-6736(13)61754-7 [DOI] [PubMed] [Google Scholar]
- 6.Jadvar H, Desai B, Conti PS. Sodium 18F-fluoride PET/CT of bone, joint, and other disorders. Semin Nucl Med 2015; 45: 58–65. doi: 10.1053/j.semnuclmed.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blau M, Ganatra R, Bender MA. 18 F-fluoride for bone imaging. Semin Nucl Med 1972; 2: 31–7. doi: 10.1016/S0001-2998(72)80005-9 [DOI] [PubMed] [Google Scholar]
- 8.Fiz F, Morbelli S, Piccardo A, Bauckneht M, Ferrarazzo G, Pestarino E, et al. ¹⁸F-NaF uptake by atherosclerotic plaque on PET/CT imaging: inverse correlation between calcification density and mineral metabolic activity. J Nucl Med 2015; 56: 1019–23. doi: 10.2967/jnumed.115.154229 [DOI] [PubMed] [Google Scholar]
- 9.Derlin T, Richter U, Bannas P, Begemann P, Buchert R, Mester J, et al. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med 2010; 51: 862–5. doi: 10.2967/jnumed.110.076471 [DOI] [PubMed] [Google Scholar]
- 10.Derlin T, Wisotzki C, Richter U, Apostolova I, Bannas P, Weber C, et al. In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: correlation with atherogenic risk factors. J Nucl Med 2011; 52: 362–8. doi: 10.2967/jnumed.110.081208 [DOI] [PubMed] [Google Scholar]
- 11.Dweck MR, Chow MWL, Joshi NV, Williams MC, Jones C, Fletcher AM, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol 2012; 59: 1539–48. doi: 10.1016/j.jacc.2011.12.037 [DOI] [PubMed] [Google Scholar]
- 12.Blomberg BA, de Jong PA, Thomassen A, Lam MGE, Vach W, Olsen MH, et al. Thoracic aorta calcification but not inflammation is associated with increased cardiovascular disease risk: results of the CAMONA study. Eur J Nucl Med Mol Imaging 2017; 44: 249–58. doi: 10.1007/s00259-016-3552-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques circulation. 2001; 103: 1051–6. [DOI] [PubMed] [Google Scholar]
- 14.Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation 2004; 110: 3424–9. doi: 10.1161/01.CIR.0000148131.41425.E9 [DOI] [PubMed] [Google Scholar]
- 15.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, et al. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A 2006; 103: 14678–83. doi: 10.1073/pnas.0606310103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutcheson JD, Maldonado N, Aikawa E. Small entities with large impact: microcalcifications and atherosclerotic plaque vulnerability. Curr Opin Lipidol 2014; 25: 327–32. doi: 10.1097/MOL.0000000000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke AP, Taylor A, Farb A, Malcolm GT, Virmani R, et al. Coronary calcification: insights from sudden coronary death victims. Zeitschrift fr Kardiologie 2000; 89(Suppl 2): S049–53. doi: 10.1007/s003920070099 [DOI] [PubMed] [Google Scholar]
- 18.Kelly-Arnold A, Maldonado N, Laudier D, Aikawa E, Cardoso L, Weinbaum S. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc Natl Acad Sci U S A 2013; 110: 10741–6. doi: 10.1073/pnas.1308814110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenney-Drake ML, Territo PR, Salavati A, Houshmand S, Persohn S, Liang Y, et al. 18)F-NaF PET Imaging of Early Coronary Artery Calcification. JACC Cardiovasc Imaging 2016; 9: 627–8. doi: 10.1016/j.jcmg.2015.02.026 [DOI] [PubMed] [Google Scholar]
- 20.Lee JM, Bang J-I, Koo B-K, Hwang D, Park J, Zhang J, et al. Clinical Relevance of 18 F-Sodium Fluoride Positron-Emission Tomography in Noninvasive Identification of High-Risk Plaque in Patients With Coronary Artery Disease. Circulation 2017; 10: pii: e006704. doi: 10.1161/CIRCIMAGING.117.006704 [DOI] [PubMed] [Google Scholar]
- 21.Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JLE, Dweck MR, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun 2015; 6: 7495. doi: 10.1038/ncomms8495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasser P, Voegel JC, Gramain P. Surface reactions on hydroxyapatite in the presence of fluoride ions 1. saturated and congruent conditions. Colloids and Surfaces A: Physicochemical and Engineering Aspects 1993; 74(2-3): 275–86. doi: 10.1016/0927-7757(93)80271-F [DOI] [Google Scholar]
- 23.Lin J, Raghavan S, Fuerstenau DW. The adsorption of fluoride ions by hydroxyapatite from aqueous solution. Colloids and Surfaces 1981; 3: 357–70. doi: 10.1016/0166-6622(81)80062-5 [DOI] [Google Scholar]
- 24.Creager MD, Hohl T, Hutcheson JD, Moss AJ, Schlotter F, Blaser MC, et al. 18F-Fluoride Signal Amplification Identifies Microcalcifications Associated With Atherosclerotic Plaque Instability in Positron Emission Tomography/Computed Tomography Images. Circ Cardiovasc Imaging 2019; 12: e007835. doi: 10.1161/CIRCIMAGING.118.007835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narula J, Nakano M, Virmani R, Kolodgie FD, Petersen R, Newcomb R, et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol 2013; 61: 1041–51. doi: 10.1016/j.jacc.2012.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000; 20: 1262–75. doi: 10.1161/01.atv.20.5.1262 [DOI] [PubMed] [Google Scholar]
- 27.Ferencik M, Mayrhofer T, Bittner DO, Emami H, Puchner SB, Lu MT, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the promise randomized clinical trial. JAMA Cardiol 2018; 3: 144–52. doi: 10.1001/jamacardio.2017.4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol 2015; 66: 337–46. doi: 10.1016/j.jacc.2015.05.069 [DOI] [PubMed] [Google Scholar]
- 29.Nerlekar N, FJ H, Cheshire C, Rashid H, Cameron JD, Wong DT, et al. Computed tomographic coronary angiography-derived plaque characteristics predict major adverse cardiovascular events. Circ Cardiovasc Imaging 2017; 10: 50: e006973. [DOI] [PubMed] [Google Scholar]
- 30.Ferencik M, Hoffmann U. High-Risk coronary plaque on computed tomography angiography: time to recognize a new imaging risk factor. Circ Cardiovasc Imaging 2018; 11: e007288. doi: 10.1161/CIRCIMAGING.117.007288 [DOI] [PubMed] [Google Scholar]
- 31.Kitagawa T, Yamamoto H, Nakamoto Y, Sasaki K, Toshimitsu S, Tatsugami F, et al. Predictive Value of 18F-Sodium Fluoride Positron Emission Tomography in Detecting High-Risk Coronary Artery Disease in Combination With Computed Tomography. J Am Heart Assoc 2018; 7: e010224. doi: 10.1161/JAHA.118.010224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitagawa T, Yamamoto H, Ohhashi N, Okimoto T, Horiguchi J, Hirai N, et al. Comprehensive evaluation of noncalcified coronary plaque characteristics detected using 64-slice computed tomography in patients with proven or suspected coronary artery disease. Am Heart J 2007; 154: 1191–8. doi: 10.1016/j.ahj.2007.07.020 [DOI] [PubMed] [Google Scholar]
- 33.Kwiecinski J, Dey D, Cadet S, Lee SE, Tamarappoo B, Otaki Y, et al. Predictors of 18F-sodium fluoride uptake in patients with stable coronary artery disease and adverse plaque features on computed tomography angiography. Eur Heart J Cardiovasc Imaging 2019;: pii: jez152: pii: jez152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwiecinski J, Dey D, Cadet S, Lee S-E, Otaki Y, Huynh PT, et al. Peri-Coronary Adipose Tissue Density Is Associated With 18F-Sodium Fluoride Coronary Uptake in Stable Patients With High-Risk Plaques. JACC Cardiovasc Imaging 2019; 12: 2000–10. doi: 10.1016/j.jcmg.2018.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blomberg BA, Thomassen A, de Jong PA, Simonsen JA, Lam MGEH, Nielsen AL, et al. Impact of personal characteristics and technical factors on quantification of sodium 18F-fluoride uptake in human arteries: prospective evaluation of healthy subjects. J Nucl Med 2015; 56: 1534–40. doi: 10.2967/jnumed.115.159798 [DOI] [PubMed] [Google Scholar]
- 36.Thomas GS, Haraszti RA. A new frontier in atherosclerotic coronary imaging. The Lancet 2014; 383: 674–5. doi: 10.1016/S0140-6736(13)61911-X [DOI] [PubMed] [Google Scholar]
- 37.Woo J, Tamarappoo B, Dey D, Nakazato R, Le Meunier L, Ramesh A, et al. Automatic 3D registration of dynamic stress and rest (82)Rb and flurpiridaz F 18 myocardial perfusion PET data for patient motion detection and correction. Med Phys 2011; 38: 6313–26. doi: 10.1118/1.3656951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dawood M, Büther F, Stegger L, Jiang X, Schober O, Schäfers M, et al. Optimal number of respiratory gates in positron emission tomography: a cardiac patient study. Med Phys 2009; 36: 1775–84. doi: 10.1118/1.3112422 [DOI] [PubMed] [Google Scholar]
- 39.Shechter G, Resar JR, McVeigh ER. Displacement and velocity of the coronary arteries: cardiac and respiratory motion. IEEE Trans Med Imaging 2006; 25: 369–75. doi: 10.1109/TMI.2005.862752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doris MK, Otaki Y, Krishnan SK, et al. Optimization of reconstruction and quantification of motion-corrected coronary PET-CT. J Nucl Cardiol 2018;: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubeaux M, Joshi NV, Dweck MR, Fletcher A, Motwani M, Thomson LE, et al. Motion correction of 18F-NaF PET for imaging coronary atherosclerotic plaques. J Nucl Med 2016; 57: 54–9. doi: 10.2967/jnumed.115.162990 [DOI] [PubMed] [Google Scholar]
- 42.Lassen ML, Kwiecinski J, Cadet S, et al. Data driven gross patient motion detection and compensation: implications for coronary (18)F-NaF PET imaging. JNuclMed 2018;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cal-González J, Moore SC, Park M-A, Herraiz JL, Vaquero JJ, Desco M, et al. Improved quantification for local regions of interest in preclinical PET imaging. Phys Med Biol 2015; 60: 7127–49. doi: 10.1088/0031-9155/60/18/7127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cal-Gonzalez J, Li X, Heber D, Rausch I, Moore SC, Schäfers K, et al. Partial volume correction for improved PET quantification in 18F-NaF imaging of atherosclerotic plaques. J Nucl Cardiol 2018; 25: 1742–56. doi: 10.1007/s12350-017-0778-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huet P, Burg S, Le Guludec D, Hyafil F, Buvat I. Variability and uncertainty of 18F-FDG PET imaging protocols for assessing inflammation in atherosclerosis: suggestions for improvement. J Nucl Med 2015; 56: 552–9. doi: 10.2967/jnumed.114.142596 [DOI] [PubMed] [Google Scholar]
- 46.Jaskowiak CJ, Bianco JA, Perlman SB, Fine JP. Influence of reconstruction iterations on 18F-FDG PET/CT standardized uptake values. J Nucl Med 2005; 46: 424–8. [PubMed] [Google Scholar]
- 47.Chen W, Dilsizian V. Targeted PET/CT imaging of vulnerable atherosclerotic plaques: microcalcification with sodium fluoride and inflammation with fluorodeoxyglucose. Curr Cardiol Rep 2013; 15: 364. doi: 10.1007/s11886-013-0364-4 [DOI] [PubMed] [Google Scholar]
- 48.Blomberg BA, Thomassen A, Takx RAP, Vilstrup MH, Hess S, Nielsen AL, et al. Delayed sodium 18F-fluoride PET/CT imaging does not improve quantification of vascular calcification metabolism: results from the CAMONA study. J Nucl Cardiol 2014; 21: 293–304. doi: 10.1007/s12350-013-9829-5 [DOI] [PubMed] [Google Scholar]
- 49.Kwiecinski J, Berman DS, Lee S-E, Dey D, Cadet S, Lassen ML, et al. Three-Hour Delayed Imaging Improves Assessment of Coronary 18F-Sodium Fluoride PET. J Nucl Med 2019; 60: 530–5. doi: 10.2967/jnumed.118.217885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira-Santos Mde, Castelo-Branco M, Silva R, Gomes A, Chichorro N, Abrunhosa A, et al. Atherosclerotic plaque metabolism in high cardiovascular risk subjects - A subclinical atherosclerosis imaging study with 18F-NaF PET-CT. Atherosclerosis 2017; 260: 41–6. doi: 10.1016/j.atherosclerosis.2017.03.014 [DOI] [PubMed] [Google Scholar]
- 51.Vesey AT, Jenkins WSA, Irkle A, Moss A, Sng G, Forsythe RO, et al. 18F-Fluoride and 18F-Fluorodeoxyglucose Positron Emission Tomography After Transient Ischemic Attack or Minor Ischemic Stroke: Case-Control Study. Circ Cardiovasc Imaging 2017; 10: e004976. doi: 10.1161/CIRCIMAGING.116.004976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwiecinski J, Adamson PD, Lassen ML, Doris MK, Moss AJ, et al. Feasibility of coronary 18F-sodium fluoride PET assessment with the utilization of previously acquired CT angiography. Circ Cardiovasc Imaging 2018;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raggi P, Pontone G, Andreini D. Role of new imaging modalities in pursuit of the vulnerable plaque and the vulnerable patient. Int J Cardiol 2018; 250: 278–83. doi: 10.1016/j.ijcard.2017.10.046 [DOI] [PubMed] [Google Scholar]
- 54.ClinicalTrials.gov. National Library of Medicine (U.S.) Study prediction of recurrent events with 18F-Fluoride..