Abstract
The effects of various forms of ionising radiation are known to be mediated by interactions with cellular and molecular targets in irradiated and in some cases non-targeted tissue volumes. Despite major advances in advanced conformal delivery techniques, the probability of normal tissue complication (NTCP) remains the major dose-limiting factor in escalating total dose delivered during treatment. Potential strategies that have shown promise as novel delivery methods in achieving effective tumour control whilst sparing organs at risk involve the modulation of critical dose delivery parameters. This has led to the development of techniques using high dose spatial fractionation (GRID) and ultra-high dose rate (FLASH) which have translated to the clinic. The current review discusses the historical development and biological basis of GRID, microbeam and FLASH radiotherapy as advanced delivery modalities that have major potential for widespread implementation in the clinic in future years.
Introduction
Radiotherapy remains the most effective non-surgical cancer treatment modality.1 The speciality has undergone an unparalleled period of development over the past two decades due to major technical advances in treatment planning, delivery and understanding of radiobiological response at the cell, tissue and whole organism levels.2–6 This has enabled the implementation of advanced delivery modalities into routine clinical practice including intensity-modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT). However, these approaches remain limited by dose constraints in surrounding organs at risk (OARs). This has led to the development of novel delivery modalities that aim to exploit physical beam delivery parameters for biologically optimised treatments.
The goal of this review of spatial fractionation and ultra-high dose rate radiotherapy for the 125th anniversary issue of the BJR is to refresh our current perspectives on the potential of increasing therapeutic efficacy through innovative local radiation exposures, and to explore the potential underlying biological mechanisms that can be exploited for patient benefit.
Spatially fractionated radiotherapy (GRID)
Spatially fractionated radiotherapy challenges the conventional radiotherapy paradigm by aiming to deliver a highly heterogeneous dose to the tumour volume. The concept of GRID was first demonstrated over a century ago by Alban Köhler7 and was developed as a means of reducing skin injury with low energy X-rays by placing an attenuating grid directly on the skin of the patient to reduce skin dose directly under the grid. At a depth of several millimetres, the uniform dose distribution is lost and so does not affect the dose distribution in the tumour.8 Evidence from both clinical and preclinical studies has clearly demonstrated that dividing the target volume into discrete subvolumes can limit normal tissue damage from orthovoltage sources which have been recently been reviewed.9,10 The possible biological mechanisms and extent to which GRID contributes to overall tumour control in the context of modern-day chemoradiation regimens is not clearly understood.
Interestingly, several in vitro and in vivo studies have implicated radiation-induced abscopal effects as potential mediators of response to GRID.11–14 The first introduction to the concept of abscopal effects was made in a report published in the British Journal of Radiology which for the first time suggested that radiation responses may occur remotely following local or whole body irradiation scenarios.15,16 In the later half-century and beyond, there have been a growing numbers of reports describing observations of the abscopal effect both experimentally and in the clinic.17–25 Futhermore, the prospect that GRID can increase total tumour dose and possibly activate beneficial bystander or abscopal immune effects whilst minimising effects to normal tissues offers intriguing opportunities for further investigation.
Whilst the abscopal effect remains a controversial and poorly controlled phenomenon, accurate assessment of tumour and immune response through circulating biomarkers may enable more widespread integration of GRID in the clinic. As advanced imaging and radiotherapy technologies continue to develop, the ability to deliver GRID has also greatly improved. Whether or not the advanced technology and treatment planning available for new beam geometries can finally unlock the full potential of abscopal effects remains an open question. Ultimately, the answer may require integrating knowledge of complex radiobiological mechanisms driving these responses with innovative technological solutions.
Ultrahigh dose rate applications (FLASH and microbeam):
In addition to optimised spatial delivery of radiation beams, there is currently major interest in exploiting unique radiobiological responses occurring at mean dose rates in excess >40 Gy/s, delivered as a single or a few pulses over intervals of milliseconds, known as FLASH-RT. It is important to note that ultrahigh dose rates have been used for decades in spatial fractionation applications known as microbeam radiation therapy (MRT). However, recent work and technological advances in radiation delivery and control have allowed open field FLASH research which has succesfully translated to treatment of a single patient with multiresistant T-cell cutaneous lymphoma.26 FLASH-RT has the potential to deliver a paradigm shift in curative radiotherapy, yet the mechanistic underpinnings of response remain largely unknown but are thought to be due radiochemical depletion of oxygen at ultrahigh dose rates which affects radioresistance conferred on the irradiated normal tissue more than on tumor tissues.27–30
On a basic level, both FLASH and GRID represent alternative dose delivery modalities that can exploit certain differences between normal tissue and tumours. These techniques have resulted in unexpected beneficial effects that could not be predicted based on classical understanding of dose rate effects and targeted responses. Understanding the biological underpinnings and the optimum physical parameters of these techniques will require significant multidisciplinary effort to drive further biologically based clinical trials in the future.29
Classifications and delivery of major forms of SFRT
Spatially fractionated conventional radiotherapy (GRID):
The field of GRID is the closest manifestation of the early work by Kohler and many others using relatively low energy photons and a screen or patterned blocking sheet to divide the exposure into high and low exposure segments. As the development of higher voltage sources occurred, interest began in re-evaluating the original GRID concept to avoid normal tissue morbidities at depth. Mohuiddin and associates were the pioneers of these studies which began in the 1980’s and continue today.31–33 A number of other centres have also joined efforts in the hope of providing patients with a boost of antitumour control whilst keeping normal tissue responses at an acceptable level. For example, as demonstrated by Peñagarícano and colleagues in a small series of patients.34 The approach was initially implemented in three-dimensional plans with one or two fields created by MLC shaping, cerrobend blocks or customised blocks that can be placed in the linear accelerator head.35,36 MLC or cerrobend-based GRID makes the implementation straightforward. This has the potential benefit of implementing GRID in developing countries in which financial constraints limit the use of advanced GRID techniques. After initial studies over the years suggested that patients may benefit from GRID applications to their tumour, several new strategies for applying GRID with photons in IMRT plans or with protons have emerged with advancing technological capabilities.36–38 These advanced GRID techniques allow for the delivery of spatial fractionation to deep seated bulky tumours and dose sparing of normal tissues. However, it remains to be seen if the dose distribution is sharp enough to retain optimal/effective peak to valley ratios related to the biological mechanisms of action (Figure 1). Nonetheless, these additional options expand the possibilities of utilising spatial fractionation in a wide variety of patients in some indications where therapeutic gain could be achieved in deep seated tumours.39 Even after more than a century of clinical and research experience, the use of GRID remains a simple strategy to avoid normal tissue damage whilst increasing the dose to the tumour (Figure 2). Advanced mechanisms of action and potential synergism with cutting-edge drug or antibody therapies are still to be fully exploited, which is a current inspiration of a number of centres around the world.
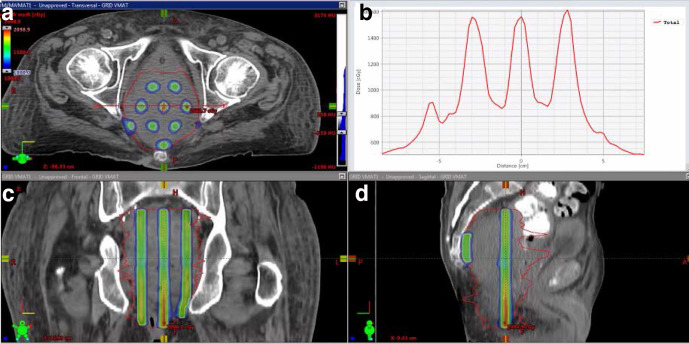
Figure 1.
Application of spatially fractionated dose distributions to a deep seated pelvic tumour using an IMRT based method. (A, C, D) GTV iso-dose peak and valley dose distribution in axial, coronal, and sagittal views and (B) dose profile across the axial view. Note the variable dose-gradient slope in the valley dose regions, as well as the difference in peak dose regions due to anatomic placing of the spatially fractionated dose. These variations and valley doses are characteristic features of clinically delivered spatial fractionation that need to be recognised in interpreting mechanisms and outcomes. GTV, gross tumour volume;IMRT, intensity modulated radiotherapy.
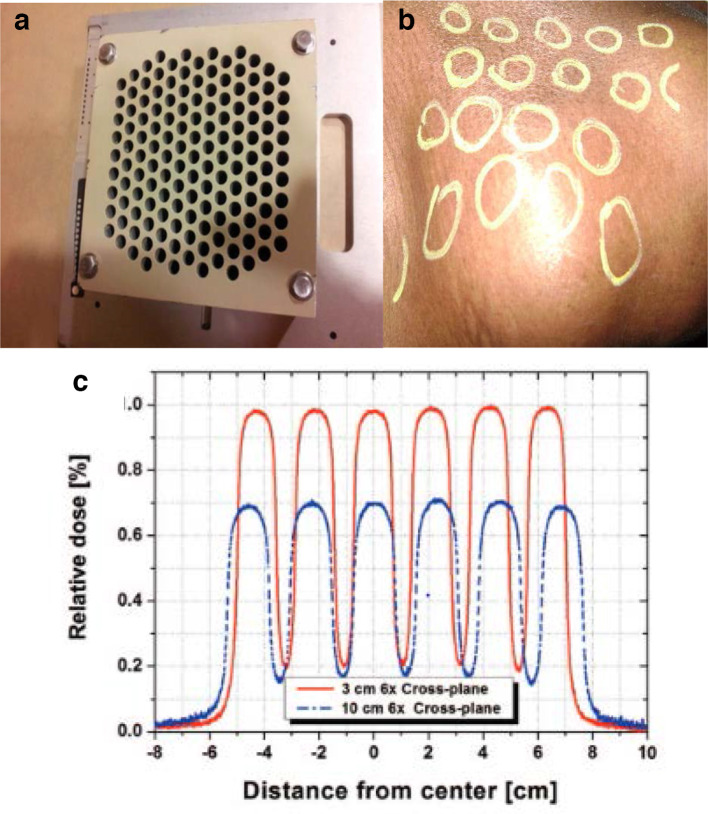
Figure 2.
Example application of spatially fractionated dose in a patient with bulky mass in the neck from carcinoma of the head and neck using a GRID collimator designed to fit in a linear accelerator. Panel A: Commercially available GRID block (Radiation Products Design, Inc.). Panel B: Hand drawing of the GRID field as indicated by light guides from linear accelerator on patient that received GRID radiotherapy for a large neck nodal lesion. Panel C: Expected dose at 3 and 10 cm in the gross tumour volume using a 6 MV linear accelerator. Note the peak and valley doses are more consistent across the field when using the collimator in comparison to the IMRT application in Figure 1. Prescription was 20 Gy in one fraction. This patient proceeded to have a full course of adjuvant chemoradiotherapy (66 Gy in 30 fractions) after completing GRID therapy. IMRT, intensitymodulated radiotherapy.
Microbeam (MRT with photons or protons):
Very high dose rates of the order of up to 100 Gy/s or higher have been investigated for decades in a spatial fraction format using synchrotron generation and known as microbeam therapy.40,41 There is much to learn from the early work done by a number of groups that employed the synchrotron-produced beams at Brookhaven National Laboratory in New York, and the European Synchrotron Research Facility in Grenoble, France among others.42–44 One of the original questions was, what width of beam in a repeating pattern could be used without increasing normal tissue damage. It was found that beam widths approaching 0.5 mm or higher became quite destructive to tissues, especially when repeated at centre to centre distances of the same width, while below this distance relative tissue sparing was observed, even when the peak doses were in the 100's of Gy. Part of this result may have to do with the unique way in which normal vs tumour cells react to ultrahigh dose rates, and part of the explanation lies in the preservation of tissue stem cell/functional units at a level and distribution that allows tissue repair and function to resume quickly after exposure.
Ultrahigh dose rate radiotherapy (FLASH with electrons, photons or protons):
The concept of ultrahigh dose rate applications in radiotherapy has experienced a recent explosion in interest from all three of the major disciplines that make up the field of radiotherapy: clinical, biology and physics. The interest is driven by several recent publications that have demonstrated an enhanced therapeutic ratio when ultrahigh does rate (typically characterised by 40 Gy/s or greater) are applied to model tumour systems in several animal models.29,45–47 As well as dose-rate, it is clear that other parameters such as the intrapulse dose rate, frequency (Hz), overall time of exposure, oxygenation and the target volume are also important.27,48 The majority of the FLASH experiments and clinical applications in certain isolated cases have been with electron beams which necessitate a superficial target and can be applied to large areas, if not volumes. However, there are demonstrations in the literature that FLASH is possible with both photons, protons or other heavy ions, and some biological advantages have been demonstrated using modifications of existing radiotherapy equipment, heavy ion or proton facilities.49–52 Although there are distinct differences in the concept of spatial and FLASH radiotherapy applications, high doses and dose-rates are a constant requirement and the dependency of biological responses to different beam sizes and dose rate combinations achievable with different modalities requires further investigation (Figure 3).
Figure 3.
Schematic representation of dose rate and beam sizes for different clinical and experimental radiotherapy techniques.
Biological mechanisms of response
Bystander effects
One of the biological phenomena associated with response to spatial fractionation has been the bystander effect. Original bystander work was largely performed in vitro and mostly with low dose exposures.53–56 The potential clinical impact of th bystander effect remains to be fully elucidated.57 However, a number of groups have demonstrated bystander effects, including cell killing, following high dose spatial fractionation in vitro and in vivo.14,58,59 Furthermore, evidence of adaptive/bystander like responses has been observed within and between modulated fields, which would also have special relevance to the application of GRID therapy approaches.60,61
Vascular/angiogenic effects
The use of stereotactic, high dose radiotherapy has brought the potential impact of vascular damage to the front line in explaining why many tumours are controlled at total doses that would not be mathematically predicted to kill all cells.62,63 It follows that vascular damage is likely important in both conventional X-ray spatial fractionation as well as ultra high dose, spatially fractionated microbeam applications. There have been a number of studies published with a focus on vascular perturbations, damage and inflammation induced by spatial fractionation.40,64–66 In addition, a general response to vascular stress or damage is an angiogenic response similar to wound healing have been demonstrated in a number of preclinical studies of spatially fractionated radiotherapy with obvious implications for tumour control.67
Immune effects; abscopal influence of local/spatial exposures
The immune response is closely related to vascular damage and plays a central role in responses to high dose spatial and ultrahigh dose rate exposures. This response may be distinctly different in magnitude and outcomes following spatial fractionation. In whole tumour exposures, the amount of abscopal effect may be driven by extensive vascular damage and inflammation across the entire tumour volume.25 Constrastingly, GRID and microbeam treatment may allow a more intact physiological response to radiation fields as the low dose volumes retain viable vasculature and immune cells. Supporting this hypothesis, a number of studies have found that spatial fractionation induces a more beneficial overall immune reaction than other approaches.11,12 It follows that the production or expression of an abscopal response against remotely located tumours could be enhanced since the irradiated tumour has retained a greater, more functional ability to recruit immune cells, or to allow antigens to interact with the immune system and enhance a systemic response.
Oxygen availability and radiochemistry considerations
Recent advances concerning the intracellular mechanisms of response to FLASH have postulated that oxygenation radical dynamics/depletion are responsible for the observed differences between tumour and normal tissues.68,69 In addition, an important role for differential redox biology in tumours relative to normal tissues has been proposed.28 There are now reports emerging in different model systems suggesting in certain tissues or cells that the FLASH effect may not always be protective following electron or proton irradiation.70,71 Whether or not whole tissue exposures to ultrahigh dose rates will ultimately be of clinical benefit, there are also indications that spatial fractionation promotes the reoxygenation and revascularisation of residual tumour or normal tissue.40,67,72,73 Detailed understanding of the dose response relationship and distribution of radiation exposure to physiological changes within targets tissue will greatly facilitate the development of these approaches towards viable clinical treatment options in certain tumour types.
Outstanding challenges for spatial fractionation and ultrahigh dose rate approaches
Some promising results have been acheived in the clinc using GRID followed by conventional radiation or chemoradiation.33,34,38,39,74 In addition, initial clinical experience with FLASH has been promising. The idea of using radiation as a tool as a part of optimised sequencing with chemotherapy, targeted agents or immunotherapy continues to gain credibility and as the immunotherapy field matures, and it may well be highly influential in determining how GRID, microbeam & FLASH can be optimsed for patient benefit. A closely related issue is the possible sparing of immune cells during spatial fractionation as a reduction in immune cell killing that meaningfully contributes to tumour control could provide even greater rationale for implementation of these approaches. In addition, a few cytokine biomarkers have been studied in clinical and preclinical studies (e.g. TNF-α and IL-2), but these are yet to be validated in large patient populations. As is typical for promising preclinical therapy approaches, the use of any of these concepts needs further preclinical and clinical study results to validate their potential for widespread translation.
Current outlook
GRID, microbeam and FLASH radiotherapy are intriguing strategies that aim to optimise therapeutic index in radiotherapy. Further benefits may be derived using charged particles to create much more conformal dose distributions and radiobiological advantages, yet these remain to be fully demonstrated.
Contributor Information
Robert J Griffin, Email: rjgriffin@uams.edu.
Kevin M Prise, Email: k.prise@qub.ac.uk.
Stephen J McMahon, Email: s.mcmahon@qub.ac.uk.
Xin Zhang, Email: xinzhang2003@gmail.com.
Jose Penagaricano, Email: Jose.Penagaricano@moffitt.org.
Karl T Butterworth, Email: k.butterworth@qub.ac.uk.
REFERENCES
- 1.Citrin DE. Recent developments in radiotherapy. N Engl J Med Overseas Ed 2017; 377: 1065–75. doi: 10.1056/NEJMra1608986 [DOI] [PubMed] [Google Scholar]
- 2.Coleman CN, Ahmed MM. Implementation of New Biology-Based Radiation Therapy Technology: When Is It Ready So “Perfect Makes Practice?”. Int J Radiat Oncol Biol Phys 2019; 105: 934–7. doi: 10.1016/j.ijrobp.2019.08.013 [DOI] [PubMed] [Google Scholar]
- 3.Kunos CA, Coleman CN. Current and future initiatives for radiation oncology at the National cancer Institute in the era of precision medicine. Int J Radiat Oncol Biol Phys 2018; 102: 18–25. doi: 10.1016/j.ijrobp.2017.02.225 [DOI] [PubMed] [Google Scholar]
- 4.Hall WA, Bergom C, Thompson RF, Baschnagel AM, Vijayakumar S, Willers H, et al. Precision oncology and genomically guided radiation therapy: a report from the American Society for radiation Oncology/American association of physicists in Medicine/National cancer Institute precision medicine conference. Int J Radiat Oncol Biol Phys 2018; 101: 274–84. doi: 10.1016/j.ijrobp.2017.05.044 [DOI] [PubMed] [Google Scholar]
- 5.Vikram B, Coleman CN, Deye JA. Current status and future potential of advanced technologies in radiation oncology. Part 2. State of the science by anatomic site. Oncology 2009; 23: 380–5. [PubMed] [Google Scholar]
- 6.Vikram B, Coleman CN, Deye JA. Current status and future potential of advanced technologies in radiation oncology. Part 1. Challenges and resources. Oncology 2009; 23: 279–83. [PubMed] [Google Scholar]
- 7.Laissue JA, Blattmann H, Slatkin DN, Kohler A. 1874-1947): Inventor of grid therapy. Z Med Phys 2012; 22: 90–9. [DOI] [PubMed] [Google Scholar]
- 8.Shirato H, Gupta NK, Jordan TJ, Hendry JH. Lack of late skin necrosis in man after high-dose irradiation using small field sizes: experiences of grid therapy. Br J Radiol 1990; 63: 871–4. doi: 10.1259/0007-1285-63-755-871 [DOI] [PubMed] [Google Scholar]
- 9.Billena C, Khan AJ. A current review of spatial fractionation: back to the future? Int J Radiat Oncol Biol Phys 2019; 104: 177–87. doi: 10.1016/j.ijrobp.2019.01.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan W, Khan MK, Wu X, Simone CB. 2Nd, FAN J, Gressen E, et al. spatially fractionated radiation therapy: history, present and the future. Clin Transl Radiat Oncol 2020; 20: 30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smilowitz HM, Blattmann H, Bräuer-Krisch E, Bravin A, Michiel MD, Gebbers J-O, et al. Synergy of gene-mediated immunoprophylaxis and microbeam radiation therapy for advanced intracerebral rat 9L gliosarcomas. J Neurooncol 2006; 78: 135–43. doi: 10.1007/s11060-005-9094-9 [DOI] [PubMed] [Google Scholar]
- 12.Kanagavelu S, Gupta S, Wu X, Philip S, Wattenberg MM, Hodge JW, et al. In Vivo Effects of Lattice Radiation Therapy on Local and Distant Lung Cancer: Potential Role of Immunomodulation. Radiat Res 2014; 182: 149–62. doi: 10.1667/RR3819.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathishkumar S, Dey S, Meigooni AS, Regine WF, Kudrimoti M, Ahmed MM, et al. The impact of TNF-α induction on therapeutic efficacy following high dose spatially fractionated (grid) radiation. Technol Cancer Res Treat 2002; 1: 141–7. doi: 10.1177/153303460200100207 [DOI] [PubMed] [Google Scholar]
- 14.Asur R, Butterworth KT, Penagaricano JA, Prise KM, Griffin RJ. High dose bystander effects in spatially fractionated radiation therapy. Cancer Lett 2015; 356: 52–7. doi: 10.1016/j.canlet.2013.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mole RH. Whole body irradiation and the idea of stress. Br J Exp Pathol 1956; 37: 528–31. [PMC free article] [PubMed] [Google Scholar]
- 16.Mole RH. Whole body Irradiation—Radiobiology or medicine? Br J Radiol 1953; 26: 234–41. doi: 10.1259/0007-1285-26-305-234 [DOI] [PubMed] [Google Scholar]
- 17.D’Andrea MA, Reddy GK. Systemic antitumor effects and Abscopal responses in melanoma patients receiving radiation therapy. Oncology 2020; 98: 202–15. doi: 10.1159/000505487 [DOI] [PubMed] [Google Scholar]
- 18.Choi JS, Sansoni ER, Lovin BD, Lindquist NR, Phan J, Mayo LL, et al. Abscopal effect following immunotherapy and combined stereotactic body radiation therapy in recurrent metastatic head and neck squamous cell carcinoma: a report of two cases and literature review. Ann Otol Rhinol Laryngol 2019; 3489419896602. [DOI] [PubMed] [Google Scholar]
- 19.Chicas-Sett R, Morales-Orue I, Rodriguez-Abreu D, Lara-Jimenez P. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: a systematic review. Clinical and Translational Radiation Oncology 2018; 9: 5–11. doi: 10.1016/j.ctro.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett 2015; 356: 82–90. doi: 10.1016/j.canlet.2013.09.018 [DOI] [PubMed] [Google Scholar]
- 21.Cotter SE, et al. Abscopal effect in a patient with metastatic Merkel cell carcinoma following radiation therapy: potential role of induced antitumor immunity. Arch Dermatol 2011; 147: 870–2. doi: 10.1001/archdermatol.2011.176 [DOI] [PubMed] [Google Scholar]
- 22.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58: 862–70. doi: 10.1016/j.ijrobp.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 23.Ehlers G, Fridman M. Abscopal effect of radiation in papillary adenocarcinoma. Br J Radiol 1973; 46: 220–2. doi: 10.1259/0007-1285-46-543-220 [DOI] [PubMed] [Google Scholar]
- 24.Law AW, Mole RH. Direct and Abscopal effects of x-radiation on the thymus of the weanling rat. Int J Radiat Biol Relat Stud Phys Chem Med 1961; 3: 233–48. doi: 10.1080/09553006114551161 [DOI] [PubMed] [Google Scholar]
- 25.Demaria S, Formenti SC. The abscopal effect 67 years later: from a side story to center stage. Br J Radiol 2020; 93: 20200042. doi: 10.1259/bjr.20200042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, et al. Treatment of a first patient with FLASH-radiotherapy. Radiotherapy and Oncology 2019; 139: 18–22. doi: 10.1016/j.radonc.2019.06.019 [DOI] [PubMed] [Google Scholar]
- 27.Adrian G, Konradsson E, Lempart M, Bäck S, Ceberg C, Petersson K. The flash effect depends on oxygen concentration. Br J Radiol 2020; 93: 20190702. doi: 10.1259/bjr.20190702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spitz DR, Buettner GR, Petronek MS, St-Aubin JJ, Flynn RT, Waldron TJ, et al. An integrated physico-chemical approach for explaining the differential impact of flash versus conventional dose rate irradiation on cancer and normal tissue responses. Radiotherapy and Oncology 2019; 139: 23–7. doi: 10.1016/j.radonc.2019.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin RJ, Ahmed MM, Amendola B, Belyakov O, Bentzen SM, Butterworth KT, et al. Understanding high-dose, ultra-high dose-rate and, spatially fractionated radiotherapy. Int J Radiat Oncol Biol Phys 2020;. [DOI] [PubMed] [Google Scholar]
- 30.Petersson K, Adrian G, Butterworth K, McMahon SJ. A quantitative analysis of the role of oxygen tension in flash radiation therapy. Int J Radiat Oncol Biol Phys 2020; 107: 539–47. doi: 10.1016/j.ijrobp.2020.02.634 [DOI] [PubMed] [Google Scholar]
- 31.Nobah A, Mohiuddin M, Devic S, Moftah B. Effective spatially fractionated grid radiation treatment planning for a passive grid block. Br J Radiol 2015; 88: 20140363. doi: 10.1259/bjr.20140363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huhn JL, Regine WF, Valentino JP, Meigooni AS, Kudrimoti M, Mohiuddin M. Spatially fractionated grid radiation treatment of advanced neck disease associated with head and neck cancer. Technol Cancer Res Treat 2006; 5: 607–12. doi: 10.1177/153303460600500608 [DOI] [PubMed] [Google Scholar]
- 33.Mohiuddin M, Fujita M, Regine WF, Megooni AS, Ibbott GS, Ahmed MM, et al. Grid): a new paradigm in the management of advanced cancers. Int J Radiat Oncol Biol Phys 1999; 45: 721–7. [DOI] [PubMed] [Google Scholar]
- 34.Peñagarícano JA, Moros EG, Ratanatharathorn V, Yan Y, Corry P. Evaluation of spatially fractionated radiotherapy (grid) and definitive chemoradiotherapy with curative intent for locally advanced squamous cell carcinoma of the head and neck: initial response rates and toxicity. Int J Radiat Oncol Biol Phys 2010; 76: 1369–75. doi: 10.1016/j.ijrobp.2009.03.030 [DOI] [PubMed] [Google Scholar]
- 35.Neuner G, Mohiuddin MM, Vander Walde N, Goloubeva O, Ha J, Yu CX, et al. High-Dose spatially fractionated grid radiation therapy (SFGRT): a comparison of treatment outcomes with Cerrobend vs. MLC SFGRT. Int J Radiat Oncol Biol Phys 2012; 82: 1642–9. doi: 10.1016/j.ijrobp.2011.01.065 [DOI] [PubMed] [Google Scholar]
- 36.Trapp JV, Warrington AP, Partridge M, Philps A, Glees J, Tait D, et al. Measurement of the three-dimensional distribution of radiation dose in grid therapy. Phys Med Biol 2004; 49: N317–23. doi: 10.1088/0031-9155/49/19/N01 [DOI] [PubMed] [Google Scholar]
- 37.Mohiuddin M, Lynch C, Gao M, Hartsell W. Early clinical results of proton spatially fractionated grid radiation therapy (SFGRT. Br J Radiol 2019;: 20190572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Penagaricano J, Yan Y, Sharma S, Griffin RJ, Hardee M, et al. Application of spatially fractionated radiation (grid) to helical tomotherapy using a novel TOMOGRID template. Technol Cancer Res Treat 2016; 15: 91–100. doi: 10.7785/tcrtexpress.2013.600261 [DOI] [PubMed] [Google Scholar]
- 39.Narayanasamy G, Zhang X, Meigooni A, Paudel N, Morrill S, Maraboyina S, et al. Therapeutic benefits in grid irradiation on tomotherapy for bulky, radiation-resistant tumors. Acta Oncol 2017; 56: 1043–7. doi: 10.1080/0284186X.2017.1299219 [DOI] [PubMed] [Google Scholar]
- 40.Griffin RJ, Koonce NA, Dings RPM, Siegel E, Moros EG, Bräuer-Krisch E, et al. Microbeam radiation therapy alters vascular architecture and tumor oxygenation and is enhanced by a galectin-1 targeted anti-angiogenic peptide. Radiat Res 2012; 177: 804–12. doi: 10.1667/RR2784.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouchet A, Lemasson B, Le Duc G, Maisin C, Bräuer-Krisch E, Siegbahn EA, et al. Preferential effect of synchrotron microbeam radiation therapy on intracerebral 9L gliosarcoma vascular networks. Int J Radiat Oncol Biol Phys 2010; 78: 1503–12. doi: 10.1016/j.ijrobp.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 42.Slatkin DN, Spanne P, Dilmanian FA, Sandborg M. Microbeam radiation therapy. Med Phys 1992; 19: 1395–400. doi: 10.1118/1.596771 [DOI] [PubMed] [Google Scholar]
- 43.Crosbie JC, Anderson RL, Rothkamm K, Restall CM, Cann L, Ruwanpura S, et al. Tumor cell response to synchrotron microbeam radiation therapy differs markedly from cells in normal tissues. Int J Radiat Oncol Biol Phys 2010; 77: 886–94. doi: 10.1016/j.ijrobp.2010.01.035 [DOI] [PubMed] [Google Scholar]
- 44.Laissue JA, Blattmann H, Wagner HP, Grotzer MA, Slatkin DN. Prospects for microbeam radiation therapy of brain tumours in children to reduce neurological sequelae. Dev Med Child Neurol 2007; 49: 577–81. doi: 10.1111/j.1469-8749.2007.00577.x [DOI] [PubMed] [Google Scholar]
- 45.Vozenin M-C, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond J-F, et al. The advantage of flash radiotherapy confirmed in mini-pig and Cat-cancer patients. Clin Cancer Res 2019; 25: 35–42. doi: 10.1158/1078-0432.CCR-17-3375 [DOI] [PubMed] [Google Scholar]
- 46.Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate flash irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 2014; 6: 245ra93245ra93. doi: 10.1126/scitranslmed.3008973 [DOI] [PubMed] [Google Scholar]
- 47.Wilson JD, Hammond EM, Higgins GS, Petersson K, Rate U-HD. Flash) radiotherapy: silver bullet or Fool's gold? Front Oncol 2019; 9: 1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hendry J. Taking care with flash radiation therapy. Int J Radiat Oncol Biol Phys 2020; 107: 239–42. doi: 10.1016/j.ijrobp.2020.01.029 [DOI] [PubMed] [Google Scholar]
- 49.Vozenin M-C, Baumann M, Coppes RP, Bourhis J. Flash radiotherapy International workshop. Radiotherapy and Oncology 2019; 139: 1–3. doi: 10.1016/j.radonc.2019.07.020 [DOI] [PubMed] [Google Scholar]
- 50.Montay-Gruel P, Bouchet A, Jaccard M, Patin D, Serduc R, Aim W, et al. X-Rays can trigger the flash effect: ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiotherapy and Oncology 2018; 129: 582–8. doi: 10.1016/j.radonc.2018.08.016 [DOI] [PubMed] [Google Scholar]
- 51.Colangelo NW, Azzam EI. The importance and clinical implications of flash ultra-high dose-rate studies for proton and heavy ion radiotherapy. Radiat Res 2019;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Marlen P, Dahele M, Folkerts M, Abel E, Slotman BJ, Verbakel W. Bringing flash to the clinic: treatment planning considerations for ultrahigh dose-rate proton beams. Int J Radiat Oncol Biol Phys 2019;. [DOI] [PubMed] [Google Scholar]
- 53.Mothersill C, Seymour C. Radiation-Induced bystander effects: past history and future directions. Radiat Res 2001; 155: 759–67. doi: 10.1667/0033-7587(2001)155[0759:RIBEPH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 54.Seymour CB, Mothersill C. Relative contribution of bystander and targeted cell killing to the low-dose region of the radiation Dose–Response curve. Radiat Res 2000; 153(5 Pt 1): 508–11. doi: 10.1667/0033-7587(2000)153[0508:RCOBAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 55.Mothersill C, Seymour CB. Cell-Cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res 1998; 149: 256–62. doi: 10.2307/3579958 [DOI] [PubMed] [Google Scholar]
- 56.Brenner DJ, Sachs RK. Do low dose-rate bystander effects influence domestic radon risks? Int J Radiat Biol 2002; 78: 593–604. doi: 10.1080/09553000210121740 [DOI] [PubMed] [Google Scholar]
- 57.Prise KM, O'Sullivan JM. Radiation-Induced bystander signalling in cancer therapy. Nat Rev Cancer 2009; 9: 351–60. doi: 10.1038/nrc2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asur RS, Sharma S, Chang C-W, Penagaricano J, Kommuru IM, Moros EG, et al. Spatially fractionated radiation induces cytotoxicity and changes in gene expression in bystander and radiation adjacent murine carcinoma cells. Radiat Res 2012; 177: 751–65. doi: 10.1667/RR2780.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Autsavapromporn N, Suzuki M, Funayama T, Usami N, Plante I, Yokota Y, et al. Gap junction communication and the propagation of bystander effects induced by microbeam irradiation in human fibroblast cultures: the impact of radiation quality. Radiat Res 2013; 180: 367–75. doi: 10.1667/RR3111.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trainor C, Butterworth KT, McGarry CK, Liberante F, O'Sullivan JM, Hounsell AR, et al. Cell survival responses after exposure to modulated radiation fields. Radiat Res 2012; 177: 44–51. doi: 10.1667/RR2656.1 [DOI] [PubMed] [Google Scholar]
- 61.Butterworth KT, McGarry CK, Trainor C, O’Sullivan JM, Hounsell AR, Prise KM. Out-of-field cell survival following exposure to intensity-modulated radiation fields. Int J Radiat Oncol Biol Phys 2011; 79: 1516–22. doi: 10.1016/j.ijrobp.2010.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song CW, Griffin RJ, Lee Y-J, Cho H, Seo J, Park I, et al. Reoxygenation and repopulation of tumor cells after ablative Hypofractionated radiotherapy (SBRT and SRS) in murine tumors. Radiat Res 2019; 192: 159–68. doi: 10.1667/RR15346.1 [DOI] [PubMed] [Google Scholar]
- 63.Song CW, Lee Y-J, Griffin RJ, Park I, Koonce NA, Hui S, et al. Indirect Tumor Cell Death After High-Dose Hypofractionated Irradiation: Implications for Stereotactic Body Radiation Therapy and Stereotactic Radiation Surgery. Int J Radiat Oncol Biol Phys 2015; 93: 166–72. doi: 10.1016/j.ijrobp.2015.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lemasson B, Serduc R, Maisin C, Bouchet A, Coquery N, Robert P, et al. Monitoring blood-brain barrier status in a rat model of glioma receiving therapy: dual injection of low-molecular-weight and macromolecular Mr contrast media. Radiology 2010; 257: 342–52. doi: 10.1148/radiol.10092343 [DOI] [PubMed] [Google Scholar]
- 65.Serduc R, Christen T, Laissue J, Farion R, Bouchet A, Sanden Bvander, Sanden B, et al. Brain tumor vessel response to synchrotron microbeam radiation therapy: a short-term in vivo study. Phys Med Biol 2008; 53: 3609–22. doi: 10.1088/0031-9155/53/13/015 [DOI] [PubMed] [Google Scholar]
- 66.Serduc R, van de Looij Y, Francony G, Verdonck O, van der Sanden B, Laissue J, et al. Characterization and quantification of cerebral edema induced by synchrotron X-ray microbeam radiation therapy. Phys Med Biol 2008; 53: 1153–66. doi: 10.1088/0031-9155/53/5/001 [DOI] [PubMed] [Google Scholar]
- 67.Fontanella AN, Boss M-K, Hadsell M, Zhang J, Schroeder T, Berman KG, et al. Effects of high-dose microbeam irradiation on tumor microvascular function and angiogenesis. Radiat Res 2015; 183: 147–58. doi: 10.1667/RR13712.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adrian G, Konradsson E, Lempart M, Back S, Ceberg C, Petersson K. The flash effect depends on oxygen concentration. Br J Radiol 2019;: 20190702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, et al. Long-Term neurocognitive benefits of flash radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci U S A 2019; 116: 10943–51. doi: 10.1073/pnas.1901777116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Venkatesulu BP, Sharma A, Pollard-Larkin JM, Sadagopan R, Symons J, Neri S, et al. Ultra high dose rate (35 Gy/sec) radiation does not spare the normal tissue in cardiac and splenic models of lymphopenia and gastrointestinal syndrome. Sci Rep 2019; 9: 17180. doi: 10.1038/s41598-019-53562-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buonanno M, Grilj V, Brenner DJ. Biological effects in normal cells exposed to flash dose rate protons. Radiotherapy and Oncology 2019; 139: 51–5. doi: 10.1016/j.radonc.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bouchet A, Potez M, Coquery N, Rome C, Lemasson B, Bräuer-Krisch E, et al. Permeability of brain tumor vessels induced by uniform or spatially Microfractionated synchrotron radiation therapies. Int J Radiat Oncol Biol Phys 2017; 98: 1174–82. doi: 10.1016/j.ijrobp.2017.03.025 [DOI] [PubMed] [Google Scholar]
- 73.Bouchet A, Serduc R, Laissue JA, Djonov V. Effects of microbeam radiation therapy on normal and tumoral blood vessels. Physica Medica 2015; 31: 634–41. doi: 10.1016/j.ejmp.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 74.Zwicker RD, Meigooni A, Mohiuddin M. Therapeutic advantage of grid irradiation for large single fractions. Int J Radiat Oncol Biol Phys 2004; 58: 1309–15. doi: 10.1016/j.ijrobp.2003.07.003 [DOI] [PubMed] [Google Scholar]