Abstract
Objective:
To investigate the diagnostic performance of deep learning (DL)-based vascular extraction and stenosis detection technology in assessing coronary artery disease (CAD).
Methods:
The diagnostic performance of DL technology was evaluated by retrospective analysis of coronary computed tomography angiography in 124 suspected CAD patients, using invasive coronary angiography as reference standard. Lumen diameter stenosis ≥50% was considered obstructive, and the diagnostic performances were evaluated at per-patient, per-vessel and per-segment levels. The diagnostic performances between DL model and reader model were compared by the areas under the receiver operating characteristics curves (AUCs).
Results:
In patient-based analysis, AUC of 0.78 was obtained by DL model to detect obstructive CAD [sensitivity of 94%, specificity of 63%, positive predictive value of 94%, and negative predictive value of 59%], While AUC by reader model was 0.74 (sensitivity of 97%, specificity of 50%, positive predictive value of 93%, negative predictive value of 73%). In vessel-based analysis, the AUCs of DL model and reader model were 0.87 and 0.89 respectively. In segment-based analysis, the AUCs of 0.84 and 0.89 were obtained by DL model and reader model respectively. It took 0.47 min to analyze all segments per patient by DL model, which is significantly less than reader model (29.65 min) (p < 0.001).
Conclusion:
The DL technology can accurately and effectively identify obstructive CAD, with less time-consuming, and it could be a reliable diagnostic tool to detect CAD.
Advances in knowledge:
The DL technology has valuable prospect with the diagnostic ability to detect CAD.
Introduction
Coronary artery disease (CAD) is the leading cause of death in the world.1,2 In symptomatic patients, the diagnosis of the presence and severity of CAD is critical for determining appropriate clinical management.3 At present, the assessment of CAD mainly depends on coronary computed tomographic angiography (CCTA) and invasive coronary angiography (ICA). Despite as the well-established gold-standard for making the diagnosis of CAD,4 ICA is not a good choice in some cases due to its invasive nature and the risk of complications, i.e. arrhythmia, myocardial infarction, stroke etc.5
CCTA is a widely adopted non-invasive imaging technique to detect or rule out obstructive CAD and has made substantial progress since the utility of 64-slice CT scanners approximately 10 years ago.6 Previous studies4,7–11 showed that CCTA could accurately identify the presence of obstructive CAD, but often overestimated the severity of disease. Meanwhile, CCTA also benefits the prognostic evaluation. In the prospective large-scale studies,12,13 CCTA in patients with stable chest pain provided better prognostic information than standard care alone or functional testing. CCTA-derived plaque features increased the ability for major adverse cardiovascular events (MACEs) prognostication significantly compared to the use of clinical risk factors alone.14 The recently introduced CT-derived fractional flow reserve (CT-FFR) can assess coronary hemodynamics and is highly consistent with invasive FFR.15 A prospective study16 proposed CT-FFR value of 0.8 or less was a predictor of long-term outcomes.
In the past few years, there were several advances in application of deep learning (DL) for medical imaging interpretation tasks, including identifying and grading diabetic retinopathy17 and classifying skin lesions as benign and malignant.18. In cardiac image, DL has been reported for automatic coronary artery calcium scoring,19,20 CCTA-derived plaque quantification,21 detecting arterial lesions with stenosis,22 identifying significant functional coronary artery stenosis by analysis of left ventricular myocardium,23 reclassifying hemodynamically non-significant stenosis with CT-FFR.24 With rapid development of DL, vascular extraction and stenosis detection technology-based artificial intelligence (AI) is emerging as a fast and efficient diagnostic tool to detect CAD. However, to date, the diagnostic performance of DL technology for CAD remains uncertain and controversial.
Recently, a DL-based end-to-end solution including automatic vascular extraction and stenosis detection for CCTA was introduced by ShuKun technology. To our knowledge, the diagnostic performance of this technology hasn’t been reported. In this study, the diagnostic performance of DL model in the assessment of CCTA was retrospectively investigated, compared with reader model and using ICA as reference standard.
Methods
Population
The study was approved by the Institutional Human Research Ethics Committee. From January 2017 to December 2018, 432 patients with suspected CAD including stable and unstable angina pectoris who underwent CCTA were initially enrolled. Patient with stable angina pectoris was categorized as having chest discomfort inducible by exercise, emotion or other stress and reproducible.25 Unstable angina pectoris was diagnosed in patients with unstable chest discomfort (rest, new onset, or worsening of angina) and the presence of cardiac troponin I values ≤30 ng l−1.26 To avoid radiation exposure in young patients, patients over 40 years old were enrolled in the study. The time interval of ICA and standardized CCTA scan was no more than 1 month.
Patients were excluded from the study cohort if one of the following criteria was met: (1) patients had history of cardiac surgery, including percutaneous coronary intervention or coronary artery bypass grafting, (2) patients with coronary malformation or aneurysm, (3) patients with calcium scores of more than 600, (4) image quality did not meet diagnostic request. The image requests for diagnosis included: (1) score of any segment was three or above on a 4-point Likert scale,11 (2) completed range of cardiac scan, (3) CT value of aortic root was 300 Hounsfield inits above.
Imaging protocols and reconstructions
All image acquisitions were performed with second-generation dual-source CT system (SOMATOM Definition Flash, Siemens Healthineers, Forchheim, Germany) or 256-row CT (Revolution CT, GE Healthcare, Milwaukee). Patients with heart rate over 70 beats/min received oral beta-blockers. Before CCTA scan, each patient received sublingual nitroglycerin. All patients underwent non-enhanced calcium score scan and CCTA scan. Scan parameters of different CT scanners were shown in Table 1. Adaptive auto electrocardiograph (ECG) gating and smart arrhythmia management were applied in CCTA scan. A bolus-tracking technology with a threshold of 220 Hounsfield unit and a region of interest in the ascending aorta was used. 45 or 60 ml of iodinated contrast material were administered with a concentration of 320 or 370 mg I/mL at a flow rate of 5 ml s−1 and following 40 ml saline flush by using dual-barrel power injector (Ulrich, Germany). Contrast material included Iodixanol (Nycomed, Norway) or Ultravist (Bayer, Germany).
Table 1.
Scan parameters of the different CT scanners
| Scan parameters | Siemens dual-source CT | GE Revolution CT |
|---|---|---|
| CCTA scan | ||
| Tube voltage (kV) | 80–120 | 100–120 |
| Tube current (mAs) | 593–1210 | 416–719 |
| Slice thickness (mm) | 0.6 | 0.625 |
| Slice gap (mm) | 0.6 | 0.625 |
| FOV (cm) | 21.2 | 21.2 |
| Retrospective gating | Yes | Yes |
| Scan trigger mode | Bolus tracking | Bolus tracking |
| Contrast material | Iodixanol or Ultravist | Iodixanol or Ultravist |
| Volume (mL) | 60 | 45 |
| Iodine flux (g/s) | 1.6–1.85 | 1.6–1.85 |
| CT value of aortic root (HU) | 598.40 ± 134.21 | 504.95 ± 96.04 |
| Estimated effective dose (mSv) | 6.07 ± 2.11 | 4.20 ± 1.37 |
| Calcium score scan | ||
| Tube voltage (kV) | 120 | 120 |
| Tube current (mAs) | 52–211 | 114–619 |
| Estimated effective dose (mSv) | 1.51 ± 0.47 | 0.51 ± 0.19 |
CCTA, coronary computed tomographic angiography; FOV, field of view; HU, Hounsfield unit.
To obtain optimal images, data sets were reconstructed retrospectively with iterative reconstruction and ECG editing was used when necessary. Percentage technique was used to reconstruct CCTA images of GE Revolution CT, allowing for multiphasic reconstruction every five percentages. CCTA images of Siemens dual-source CT were reconstructed during the diastolic phase (60%–70% of the R–R interval) and the systolic phase (30%–40% of the R–R interval). The phase with optimal image quality was used for CCTA analysis.
The effective doses of the calcium score scan and the CCTA scan were estimated by using the dose–length product (DLP) multiplied with a conversion coefficient [k = 0.014 mSv/(mGy ×cm)] for the chest as the investigated anatomical region.10
Imaging analysis and interpretation
The image quality of CCTA image was assessed on a per-segment level with a 4-point Likert scale,11 and classified as 1 (poor, presence of severe image artifacts, non-diagnostic), 2 (insufficient, presence of moderate artifacts, but diagnosis with low confidence), 3 (good, presence of mild artifacts and diagnosis with high confidence), 4 (excellent, absence of any artifacts).
Coronary arteries were evaluated using the Society of Cardiovascular Computed Tomography coronary 18-segmentation classification.27 The segments with vessel diameter less than 1.5 mm were excluded. Lumen diameter with 50% stenosis or more was considered obstructive. The diagnostic performances were assessed on per-patient, per-vessel and per-segment levels respectively. Vessel level analysis included left main coronary artery (LM), left anterior descending artery (LAD), left circumflex artery (LCX) and right coronary artery (RCA). Small vessels represented the branches of LAD, LCX and RCA. Segments distal to a total occlusion were excluded.
CCTA images were processed by a software (CoronaryDoc, ShuKun Techonolgy, Beijing) using DL technology for vascular extraction, stenosis detection and generating multiple planner reformat, curve plannar reformat, maximum intensity projection and volume rendering images. Vascular extraction was obtained with an improved three-dimensional (3D) U-Net architecture added a Bottle-Neck model and a growing iterative prediction network model, then a 3D segmentation neural network and a one-dimensional sequence checking hybrid technique applied to multiple planner reformat and curve plannar reformat images to detect stenosis.
Two radiologists (5 and 16 years of experience in cardiac imaging diagnosis) evaluated the CCTA images independently on post-processing workstation (Advantage workstation v. 4.7, GE Healthcare, Milwaukee, Wisconsin, USA or Syngo Via, Siemens Healthineers, Forchheim, Germany), and finally results were obtained by consensus if disagreements existed. The readers were ignorant of the ICA results when evaluating the CCTA image.
ICA was performed with standard procedure and two different views at least were acquired for each vessel. All segmental angiograms were evaluated by an experienced interventional cardiologist who was blinded to CCTA images and patient characteristics. The 18-segment model was in accord with CCTA images and diameter stenosis ≥50% was defined as obstructive CAD.
Statistical analysis
Statistical analysis was performed using SPSS (v. 19.0, SPSS Inc, Chicago, IL) at a significance level of p < 0.05. Categorical demographics were compared using χ2 tests, while continuous variables were compared using independent-sample t-test or Mann–Whitney U test depending on their distribution (Kolmogorov–Smirnov Test). Diagnostic parameters of DL model or reader model were determined with sensitivity, specificity, positive predictive value and negative predictive value (NPV) and their corresponding 95% confidence intervals. The diagnostic performances of DL model and reader model for obstructive CAD were evaluated by receive operating characteristic curves and were quantitatively expressed with areas under the curve (AUCs).
Results
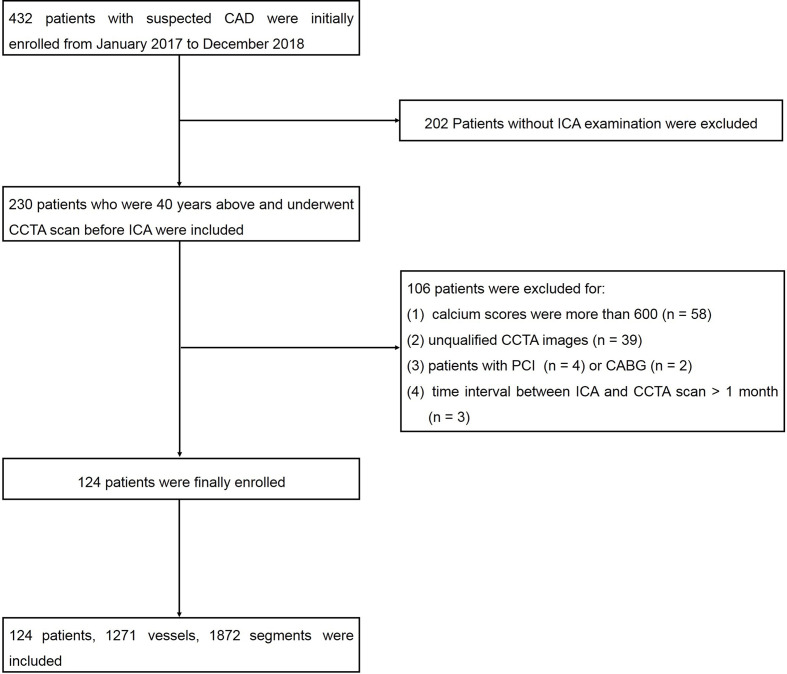
Finally, 124 patients, 1271 vessels and 1872 segments were included (Figure 1). The demographics characteristics were shown in Table 2. The prevalence of obstructive CAD was 87%. Patients with obstructive CAD had a higher prevalence of hypercholesterolemia (p = 0.042) and diabetes mellitus (p = 0.021), while smoking, drinking, hypertension, and family history of CAD all had no statistical significance.
Figure 1.
Flow diagram showed inclusion and exclusion criteria of the study. CAD,coronary artery disease; CCTA, coronary computed tomographic angiography; ICA, invasivecoronary angiography; PCI, percutaneous coronary intervention.
Table 2.
Patient demographics characteristics
| Characteristics | Value |
|---|---|
| Age (years) | 67 ± 9 |
| Males - no. (%) | 92 (74) |
| Clinical presentation - no. (%) | |
| Stable angina | 73 (59) |
| Unstable angina | 51 (41) |
| Risk factors - no. (%) | |
| Hypertension | 87 (70) |
| Hypercholesterolemia | 48 (39) |
| Diabetes mellitus | 45 (36) |
| Family history of CAD | 53 (43) |
| Previous myocardial infarction | 3 (2) |
| Smoking | 46 (37) |
| Drinking | 26 (21) |
| Invasive coronary angiography - no. (%) | |
| Prevalence of obstructive CAD | 108 (87) |
| Absence of obstructive CAD | 16 |
| Left main coronary artery disease | 9 |
| One vessel | 44 |
| Two vessels | 27 |
| Three vessels | 23 |
| Small vessels | 5 |
| Calcium score - no. (%) | |
| Calcium score ≤200 | 49 (40) |
| 200<Calcium score ≤400 | 21 (17) |
| 400<Calcium score ≤600 | 54 (44) |
CAD, coronary artery disease.
Radiation doses were 1.10 ± 0.59 mSv for calcium score scan and 5.61 ± 2.03 mSv for CCTA scan respectively. Within 2 weeks after ICA, 58 patients underwent percutaneous coronary intervention and 3 patients for coronary artery bypass grafting.
Patient-based analysis
The diagnostic parameters of CCTA assessed by DL model and reader model for detecting significant stenosis on patient-based analysis were comparatively shown in Table 3. 94% (101 of 108) obstructive CAD confirmed by ICA were identified by DL model (Figure 2) and 7 patients (6 patients with 1-vessel disease and 1 patient with 2-vessel disease) were missed. Among them, six patients with nonobstructive CAD were misdiagnosed as obstructive CAD by DL model (Figure 3) and all the patients had 1-vessel disease. On patient-based comparison, the diagnostic performance of DL model was slightly better than reader model.
Table 3.
The diagnostic parameters of DL model and reader model on patient-, vessel-, segment-based analyses
| Accuracy parameters | Patient-based analysis | Vessel-based analysis | Segment-based analysis | |||
|---|---|---|---|---|---|---|
| No. (n) | 124 | 496 | 1872 | |||
| Prevalence of stenosis n (%) |
108 (87) | 198 (40) | 307 (16) | |||
| DL model | Reader model | DL model | Reader model | DL model | Reader model | |
| TP (n) | 101 | 105 | 168 | 180 | 224 | 257 |
| FP (n) | 6 | 8 | 30 | 39 | 66 | 82 |
| FN (n) | 7 | 3 | 30 | 18 | 83 | 50 |
| TN (n) | 10 | 8 | 268 | 259 | 1499 | 1483 |
| Sensitivity % (95% CI) |
94 (87-97) |
97 (92-99) |
85 (79-89) |
91 (86-94) |
73 (68-78) |
84 (79-88) |
| Specificity % (95% CI) |
63 (36-84) |
50 (26-74) |
90 (86-93) |
87 (82-90) |
96 (95-97) |
95 (94-96) |
| PPV % (95% CI) |
94 (88-98) |
93 (86-97) |
85 (79-89) |
82 (76-87) |
77 (72-82) |
76 (71-80) |
| NPV % (95% CI) |
59 (33-81) |
73 (39-93) |
90 (86-93) |
94 (90-96) |
95 (94-96) |
97 (96-98) |
| AUC (95% CI) |
0.78 (0.63–0.93) |
0.74 (0.58–0.90) |
0.87 (0.84–0.91) |
0.89 (0.86–0.92) |
0.84 (0.81–0.87) |
0.89 (0.87–0.92) |
AUC, area under the curve; CI, confidence interval; DL, deep learning; FN, false negative; FP, false positive; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive.
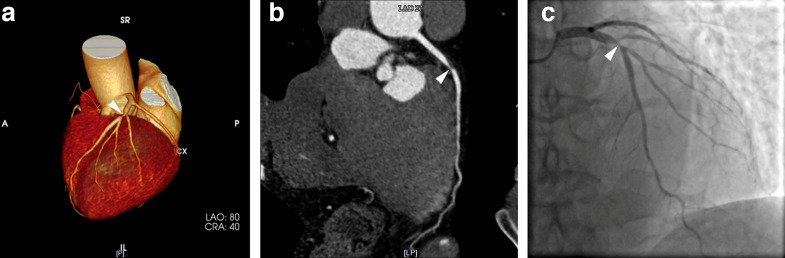
Figure 2.
LAD stenosis in a 79-year-old male patient, was correctly depicted by DL technique, including VR (2A, arrowhead) and CPR (2B, arrowhead) provided by CoronaryDoc software, which was in accord with ICA (2C, arrowhead). CPR, curve plannar reformat; DL, deep learning; ICA, invasive coronary angiography; LAD, left anterior descending artery; VR, volume rendering.
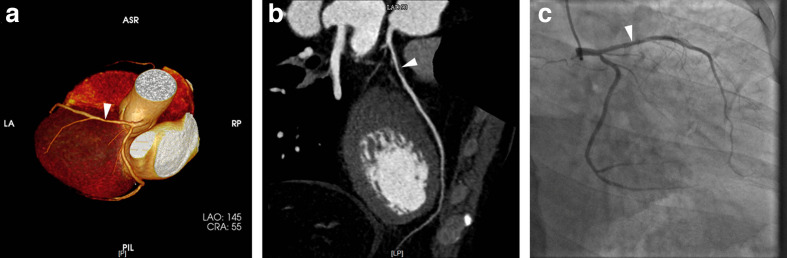
Figure 3.
LAD stenosis in a 43-year-old male patient which was chose to the 50% cut-off point, was incorrectly diagnosed obstructive CAD in VR (3A, arrowhead) and CPR (3B, arrowhead) provided by CoronaryDoc software. The ICA (3C, arrowhead) showed non-obstructive stenosis. CAD, coronary artery disease; CPR, curve plannar reformat; ICA, invasive coronaryangiography; LAD, left anterior descending artery; VR, volume rendering.
Vessel-based analysis
The diagnostic parameters of CCTA assessed by DL model and reader model for detecting significant stenosis on vessel-based analysis were shown comparatively in Tables 3–4. Significant stenosis was more often occurred in LAD, followed by RCA, LCX, LM. The diagnostic performances for the above vessels (AUCs were 0.87 for DL model and 0.89 for reader model) were better than other small vessels (AUCs were 0.74 for DL model, 0.88 for reader model).
Table 4.
The detailed parameters of DL model and reader model on vessel-based analysis
| Accuracy parameters | RCA | LM | LAD | LCX | Small vessels | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. (n) | 124 | 124 | 124 | 124 | 775 | |||||
| Prevalence of stenosis n (%) |
52 (42) | 8 (6) | 87 (70) | 51 (41) | 46 (6) | |||||
| DL model | Reader model | DL model | Reader model | DL model | Reader model | DL model | Reader model | DL model | Reader model | |
| TP (n) | 45 | 47 | 6 | 7 | 78 | 85 | 39 | 41 | 23 | 36 |
| FP (n) | 9 | 12 | 2 | 2 | 11 | 15 | 8 | 10 | 19 | 22 |
| FN (n) | 7 | 5 | 2 | 1 | 9 | 2 | 12 | 10 | 23 | 10 |
| TN (n) | 63 | 60 | 114 | 114 | 26 | 22 | 65 | 63 | 710 | 707 |
| Sensitivity % (95% CI) |
87 (74–94) |
90 (78–96) |
75 (36-96) |
88 (47-99) |
90 (81-95) |
98 (91-100) |
76 (62-87) |
80 (66-90) |
50 (35-65) |
78 (63-89) |
| Specificity % (95% CI) |
88 (77-94) |
83 (72-91) |
98 (93-100) |
98 (93-100) |
70 (53-84) |
59 (42-75) |
89 (79-95) |
86 (76-93) |
97 (96-98) |
97 (95-98) |
| PPV % (95% CI) |
83 (70-92) |
80 (67-89) |
75 (36-96) |
78 (40-96) |
88 (79-93) |
85 (76-91) |
83 (69-92) |
80 (66-90) |
55 (39-70) |
62 (48-74) |
| NPV % (95% CI) |
90 (80-96) |
92 (82-97) |
98 (93-100) |
99 (95-100) |
74 (56-87) |
92 (72-99) |
84 (74-91) |
86 (76-93) |
97 (95-98) |
99 (97-99) |
| AUC (95% CI) |
0.87 (0.80–0.94) |
0.87 (0.80–0.94) |
0.87 (0.69–1.00) |
0.93 (0.79–1.00) |
0.80 (0.70–0.90) |
0.79 (0.68–0.89) |
0.83 (0.75–0.91) |
0.83 (0.76–0.91) |
0.74 (0.64–0.83) |
0.88 (0.81–0.95) |
AUC, area under the curve; CI, confidence interval; DL, deep learning; FN, false negative; FP, false positive; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main coronary artery; RCA, right coronary artery; TN, true negative; TP, true positive.
Segment-based analysis
The diagnostic parameters of CCTA assessed by DL model and reader model for detecting significant stenosis on segment-based analysis were comparatively shown in Table 3. 1872 of 2108 potentially available segments were included for comparison with ICA. Unavailable segments included 190 anatomically absent segments on ICA and 46 segments distal to an occluded coronary segment. On either vessel or segment-based comparisons, the diagnostic performances of reader model were slightly better than DL model.
Comparison of diagnostic time between DL model and reader model
The median diagnostic time for all segments per patient by DL model was 0.47 (interquartile range: 0.43–0.48) min, while the average diagnostic time by reader model was 29.65 ± 2.15 min (Z = −9.663, p < 0.001).
Discussion
This study aims to explore the diagnostic performance of DL based vascular extraction and stenosis detection technology for CAD evaluation. The results demonstrated that DL technology could accurately and effectively identify obstructive CAD, with less time-consuming. Thus, it has the potential to be a reliable diagnostic tool to detect or rule out obstructive CAD.
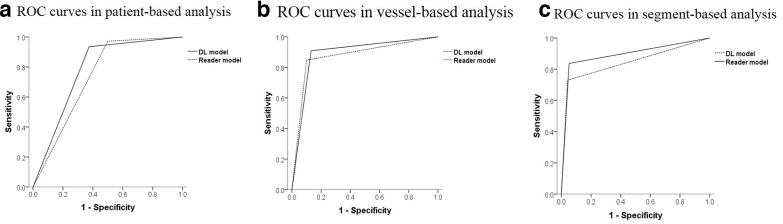
Figure 4.
ROC curves in patient, vessel and segment-based analyses.
The present study revealed that DL technique has preferable accuracy and robustness in the diagnostic performance to detect CAD, which is in consistent with earlier multicenter studies4,28,29 and meta analysis.30 In this study, the diagnostic performance of DL technology is similar to the intermediate and senior radiologist. However, the doctors for post-processing and reporting are usually junior radiologists in practice. The efficiency of DL technology in the post-processing and reporting times is exciting, as the post-processing time is limited to half a minute and the report is generated automatically. The average post-processing time and reporting time could be reached to 13.6 and 18.0 min respectively.31 DL technology can obviously reduce the heavy burden of radiologists in CCTA diagnosis, which may be a promising diagnostic tool in future large-scale application.
The DL technology showed excellent sensitivity and accuracy to detect CAD at patient and vessel levels in this preliminary study. Moreover, ﹥90 NPV of DL technology at vessel and segment levels demonstrates a highly effective alternative to rule out obstructive CAD. Further, DL technology for CCTA evaluation is preferentially used in low to intermediate risk patients without massive calcifications. Compared with previous studies,7–11,30 the specificity and NPV of this study were lower in detecting obstructive CAD, which might be related with high positive rate of CAD in the included subjects and two-fifths presence of patients with calcium scores > 400. It was reported that the specificity of CCTA diagnosed CAD was reduced in patients with calcium scores > 400 vs ≤400 Agatston units (52.6% vs 86.3%).29
Compared with ICA, CCTA might overestimate the extent of stenosis4,29 because of high number of false-positive cases resulting in low specificity. DL technology can improve the specificity in diagnosing CAD compared with reader model. International multicenter prospective researches32–34 showed CT-FFR provided high diagnostic accuracy and discrimination for the diagnosis of hemodynamically significant CAD and led to a marked increase in specificity compared with CCTA. CT-FFR has the advantage of evaluating coronary hemodynamics for diagnosis and management of patients with suspected CAD,35 while the “gray value” (0.75–0.80) of CT-FFR value has poor discrimination for CAD.36 Combination of DL technology and CT-FFR may provide intelligent and efficient diagnostic tools for patients with suspected CAD.
The diagnostic performances of DL technology for small vessels were significantly lower than those of the LM and 3-vessel. Previous relevant studies4,28 also focused on the LM and 3-vessel, rather than referring to small vessels, which might be related with limited diagnostic capability. With the decrease of coronary artery diameter, the resolution ratio and diagnostic ability of DL technology decrease obviously. Fortunately, most of the offending vessels of CAD are LM and 3-vessel that are easy to be detected accurately by DL technology. The current study also showed that significant coronary stenosis was more often occurred in LAD.
Exposure to radiation remains a major concern in CCTA scan. High tube voltage with adaptive tube and retrospective ECG-triggered scan mode were used for CCTA scan in this study to guarantee image quality. The mean estimated effective dose is 5.61 mSv. In addition, there was a cause for concern about selection bias, which inevitably resulted in the high positive rate of obstructive CAD. 202 patients without ICA examination were excluded and it would be good to know the outcomes in those patients.
The study had several limitations. First, the study sample was relatively small, which did not allow performing further subanalyses according to demographics parameters. Multicenter and multivendor studies should be further performed to establish the diagnostic robustness of DL technology. Second, two different CT scanners were used in the study, resulting in the measurement bias, which was an issue to be concerned. Third, a positive rate of 87% CAD in the included subjects was rather high in view of the recommendation for a preferential use of CCTA in low to intermediate CAD prevalence. Last, the radiation dose was relatively high in current study because of retrospective scan and high tube voltage. The new generation of CCTA scan has prompted efforts to reduce the associated radiation dose below 1 mSv via a number of approaches, such as prospective ECG triggering, low tube voltage, anatomy-based tube current modulation and iterative reconstruction.7,9
Conclusion
This study demonstrated that DL technology could accurately and effectively identify obstructive CAD, with less time-consuming, and it could be a reliable diagnostic tool to detect CAD.
Footnotes
Funding: This work was supported by the National Key Research and Development Program of China (No. 2017YFC0114300), National Natural Science Foundation of China (No. 81771885) and Suzhou Science and Technology Development Project (No. SZS201818).
The authors Meng Chen and Ximing Wang contributed equally to the work and should be regarded as co-first authors.
Contributor Information
Meng Chen, Email: chenmeng9595@126.com.
Ximing Wang, Email: wangximing1998@163.com.
Guangyu Hao, Email: hgy80801@126.com.
Xujie Cheng, Email: 2310418837@qq.com.
Chune Ma, Email: anne@shukun.net.
Ning Guo, Email: guoning@shukun.net.
Su Hu, Email: husu1022@126.com.
Qing Tao, Email: tq162102@163.com.
Feirong Yao, Email: 67898526@163.com.
Chunhong Hu, Email: sdhuchunhong@sina.com.
REFERENCES
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007; 115: e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918 [DOI] [PubMed] [Google Scholar]
- 2.Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2019; 394: 1145–58. doi: 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the coronary artery bypass graft surgery Trialists collaboration. Lancet 1994; 344: 563–70. doi: 10.1016/S0140-6736(94)91963-1 [DOI] [PubMed] [Google Scholar]
- 4.Meijboom WB, Meijs MFL, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CAG, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008; 52: 2135–44. doi: 10.1016/j.jacc.2008.08.058 [DOI] [PubMed] [Google Scholar]
- 5.Sajjadieh A, Hekmatnia A, Keivani M, Asoodeh A, Pourmoghaddas M, Sanei H. Diagnostic performance of 64-row coronary CT angiography in detecting significant stenosis as compared with conventional invasive coronary angiography. ARYA Atheroscler 2013; 9: 157–63. [PMC free article] [PubMed] [Google Scholar]
- 6.Cury RC. President's page: ten years of innovation in cardiac CT. J Cardiovasc Comput Tomogr 2014; 8: 338–9. doi: 10.1016/j.jcct.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 7.Stehli J, Fuchs TA, Bull S, Clerc OF, Possner M, Buechel RR, et al. Accuracy of coronary CT angiography using a submillisievert fraction of radiation exposure: comparison with invasive coronary angiography. J Am Coll Cardiol 2014; 64: 772–80. doi: 10.1016/j.jacc.2014.04.079 [DOI] [PubMed] [Google Scholar]
- 8.Layritz C, Schmid J, Achenbach S, Ulzheimer S, Wuest W, May M, et al. Accuracy of prospectively ECG-triggered very low-dose coronary dual-source CT angiography using iterative reconstruction for the detection of coronary artery stenosis: comparison with invasive catheterization. Eur Heart J Cardiovasc Imaging 2014; 15: 1238–45. doi: 10.1093/ehjci/jeu113 [DOI] [PubMed] [Google Scholar]
- 9.Yin W-H, Lu B, Hou Z-H, Li N, Han L, Wu Y-J, et al. Detection of coronary artery stenosis with sub-milliSievert radiation dose by prospectively ECG-triggered high-pitch spiral CT angiography and iterative reconstruction. Eur Radiol 2013; 23: 2927–33. doi: 10.1007/s00330-013-2920-0 [DOI] [PubMed] [Google Scholar]
- 10.Albrecht MH, Nance JW, Schoepf UJ, Jacobs BE, Bayer RR, Litwin SE, et al. Diagnostic accuracy of low and high tube voltage coronary CT angiography using an X-ray tube potential-tailored contrast medium injection protocol. Eur Radiol 2018; 28: 2134–42. doi: 10.1007/s00330-017-5150-z [DOI] [PubMed] [Google Scholar]
- 11.Dai X, Yu M, Pan J, Lu Z, Shen C, Wang Y, et al. Image quality and diagnostic accuracy of coronary CT angiography derived from low-dose dynamic CT myocardial perfusion: a feasibility study with comparison to invasive coronary angiography. Eur Radiol 2019; 29: 4349–56. doi: 10.1007/s00330-018-5777-4 [DOI] [PubMed] [Google Scholar]
- 12.Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018; 379: 924–33. doi: 10.1056/NEJMoa1805971 [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the promise trial (prospective multicenter imaging study for evaluation of chest pain. Circulation 2017; 135: 2320–32. doi: 10.1161/CIRCULATIONAHA.116.024360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Assen M, Varga-Szemes A, Schoepf UJ, Duguay TM, Hudson HT, Egorova S, et al. Automated plaque analysis for the prognostication of major adverse cardiac events. Eur J Radiol 2019; 116: 76–83. doi: 10.1016/j.ejrad.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 15.Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013; 61: 2233–41. doi: 10.1016/j.jacc.2012.11.083 [DOI] [PubMed] [Google Scholar]
- 16.Ihdayhid AR, Norgaard BL, Gaur S, Leipsic J, Nerlekar N, Osawa K, et al. Prognostic value and risk continuum of noninvasive fractional flow reserve derived from coronary CT angiography. Radiology 2019; 292: 343–51. doi: 10.1148/radiol.2019182264 [DOI] [PubMed] [Google Scholar]
- 17.Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus Photographs. JAMA 2016; 316: 2402–10. doi: 10.1001/jama.2016.17216 [DOI] [PubMed] [Google Scholar]
- 18.Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017; 542: 115–8. doi: 10.1038/nature21056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cano-Espinosa C, González G, Washko GR, Cazorla M, Estépar RSJ. Automated Agatston score computation in non-ECG gated CT scans using deep learning. Proc SPIE Int Soc Opt Eng 2018; 1057402 03 2018. doi: 10.1117/12.2293681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Datong C, Minghui L, Cheng J, Yue S, Dongbin X, Yueming L. Coronary calcium detection based on improved deep residual network in mimics. J Med Syst 2019; 43: 119. doi: 10.1007/s10916-019-1218-4 [DOI] [PubMed] [Google Scholar]
- 21.von Knebel Doeberitz PL, De Cecco CN, Schoepf UJ, Duguay TM, Albrecht MH, van Assen M, et al. Coronary CT angiography-derived plaque quantification with artificial intelligence CT fractional flow reserve for the identification of lesion-specific ischemia. Eur Radiol 2019; 29: 2378–87. doi: 10.1007/s00330-018-5834-z [DOI] [PubMed] [Google Scholar]
- 22.Kang D, Dey D, Slomka PJ, Arsanjani R, Nakazato R, Ko H, et al. Structured learning algorithm for detection of nonobstructive and obstructive coronary plaque lesions from computed tomography angiography. J Med Imaging 2015; 2: 014003. doi: 10.1117/1.JMI.2.1.014003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Hamersvelt RW, Zreik M, Voskuil M, Viergever MA, Išgum I, Leiner T. Deep learning analysis of left ventricular myocardium in CT angiographic intermediate-degree coronary stenosis improves the diagnostic accuracy for identification of functionally significant stenosis. Eur Radiol 2019; 29: 2350–9. doi: 10.1007/s00330-018-5822-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coenen A, Kim Y-H, Kruk M, Tesche C, De Geer J, Kurata A, et al. Diagnostic accuracy of a Machine-Learning approach to coronary computed tomographic Angiography-Based fractional flow reserve: result from the machine Consortium. Circ Cardiovasc Imaging 2018; 11: e007217. doi: 10.1161/CIRCIMAGING.117.007217 [DOI] [PubMed] [Google Scholar]
- 25.Ambrosio G, Mugelli A, Lopez-Sendón J, Tamargo J, Camm J. Management of stable angina: a commentary on the European Society of cardiology guidelines. Eur J Prev Cardiol 2016; 23: 1401–12. doi: 10.1177/2047487316648475 [DOI] [PubMed] [Google Scholar]
- 26.D'Souza M, Sarkisian L, Saaby L, Poulsen TS, Gerke O, Larsen TB, et al. Diagnosis of unstable angina pectoris has declined markedly with the advent of more sensitive troponin assays. Am J Med 2015; 128: 852–60. doi: 10.1016/j.amjmed.2015.01.044 [DOI] [PubMed] [Google Scholar]
- 27.Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of cardiovascular computed tomography guidelines Committee. J Cardiovasc Comput Tomogr 2014; 8: 342–58. doi: 10.1016/j.jcct.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 28.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008; 359: 2324–36. doi: 10.1056/NEJMoa0806576 [DOI] [PubMed] [Google Scholar]
- 29.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter accuracy (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J Am Coll Cardiol 2008; 52: 1724–32. doi: 10.1016/j.jacc.2008.07.031 [DOI] [PubMed] [Google Scholar]
- 30.Menke J, Unterberg-Buchwald C, Staab W, Sohns JM, Seif Amir Hosseini A, Schwarz A. Head-To-Head comparison of prospectively triggered vs retrospectively gated coronary computed tomography angiography: meta-analysis of diagnostic accuracy, image quality, and radiation dose. Am Heart J 2013; 165: 165: e153–163.e3. doi: 10.1016/j.ahj.2012.10.026 [DOI] [PubMed] [Google Scholar]
- 31.Liu K, Hsieh C, Zhuang N, Gao Y, Li Z, Ren X, et al. Current utilization of cardiac computed tomography in mainland China: a national survey. J Cardiovasc Comput Tomogr 2016; 10: 76–81. doi: 10.1016/j.jcct.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 32.Koo B-K, Erglis A, Doh J-H, Daniels DV, Jegere S, Kim H-S, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. results from the prospective multicenter DISCOVER-FLOW (diagnosis of Ischemia-Causing stenoses obtained via noninvasive fractional flow reserve) study. J Am Coll Cardiol 2011; 58: 1989–97. doi: 10.1016/j.jacc.2011.06.066 [DOI] [PubMed] [Google Scholar]
- 33.Min JK, Leipsic J, Pencina MJ, Berman DS, Koo B-K, van Mieghem C, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012; 308: 1237–45. doi: 10.1001/2012.jama.11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (analysis of coronary blood flow using CT angiography: next steps. J Am Coll Cardiol 2014; 63: 1145–55. doi: 10.1016/j.jacc.2013.11.043 [DOI] [PubMed] [Google Scholar]
- 35.Benton SM, Tesche C, De Cecco CN, Duguay TM, Schoepf UJ, Bayer RR, Bayer RR. Noninvasive derivation of fractional flow reserve from coronary computed tomographic angiography: a review. J Thorac Imaging 2018; 33: 88–96. doi: 10.1097/RTI.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 36.Tanaka K, Bezerra HG, Gaur S, Attizzani GF, Bøtker HE, Costa MA, et al. Comparison between non-invasive (coronary computed tomography angiography derived) and Invasive-Fractional flow reserve in patients with serial stenoses within one coronary artery: a NXT trial substudy. Ann Biomed Eng 2016; 44: 580–9. doi: 10.1007/s10439-015-1436-y [DOI] [PubMed] [Google Scholar]