Abstract
Fatty acid-binding proteins (Fabps) make up a family of widely distributed cytoplasmic lipid-binding proteins. The small intestine contains three predominant Fabp species, Fabp1, Fabp2, and Fabp6. Our previous studies showed that Fabp2 and Fabp6 gene-disrupted mice exhibited sexually dimorphic phenotypes. In this study, we carried out a systematic comparative analysis of the small intestinal transcriptomes of 10 week-old wild-type (WT) and Fabp gene-disrupted male and female mice. We found that the small intestinal transcriptome of male and female mice showed key differences in the gene expression profiles that affect major biological processes. The deletion of specific Fabp genes induced unique and sex-specific changes in the gene expression program, although some differentially expressed genes in certain genotypes were common to both sexes. Functional annotation and interaction network analyses revealed that the number and type of affected pathways, as well as the sets of interacting nodes in each of the Fabp genotypes, are partitioned by sex. To our knowledge, this is the first time that sex differences were identified and categorized at the transcriptome level in mice lacking different intestinal Fabps. The distinctive transcriptome profiles of WT male and female small intestine may predetermine the nature of transcriptional reprogramming that manifests as sexually dimorphic responses to the ablation of intestinal Fabp genes.
Keywords: sex differences, transcriptome, fatty acid-binding proteins, Fabp, microarray analysis, nutrient metabolism, small intestine
1. Introduction
Fatty acid-binding proteins (Fabps) are highly abundant cytoplasmic proteins that are found in several mammalian tissues [1]. The function of these proteins are not fully known but they are thought to participate in the maintenance of intracellular lipid homeostasis [1,2]. The small intestine contains three types of Fabps, namely Fabp1 [3,4], Fabp2 [5], and Fabp6 [6,7].
Fabp1 (also known as L-FABP) was first found in the liver [4] but is also present throughout the small intestine with the highest abundance occurring in the proximal portion of the organ [8]. It can bind fatty acids with a preference for unsaturated long-chain fatty acids as well as an assortment of other hydrophobic molecules including bile acids and fibrates [9,10]. Studies have demonstrated a direct interaction between Fabp1 and peroxisome proliferator-activated receptors (PPAR), suggesting a role of Fabp1 in delivering PPAR ligands to the nucleus which in turn can lead to the expression modulation of PPAR target genes [11,12]. Fabp1 gene ablation results in a variety of metabolic defects, including dysregulated hepatic lipid metabolism [13,14] and gallstone susceptibility [15]. A T94A variant of the human FABP1 gene has been found to be associated with reduced body weight as well as altered glucose metabolic response to lipid challenge [16]. It can also influence the efficacy of lipid-lowering therapies [17]. Interestingly, the association between the T94A variant and increased fasting triacylglycerols and low-density lipoprotein (LDL)-cholesterol levels was only observed in females [18].
Fabp2 (also known as I-FABP) is restricted to the small intestine but distributed throughout the organ with its maximum abundance occurring after the midpoint of the organ [8]. Fabp2 shows a preference for saturated long chain fatty acids [9,10]. In vitro studies suggest that Fabp2 and Fabp1 enable the transfer of fatty acids from donor to acceptor membranes via different mechanisms: Fabp1 transfers fatty acids via an aqueous diffusion-mediated process whereas Fabp2 transfers fatty acids by direct collisional interaction with membranes [19]. The deletion of the Fabp2 gene in mice did not prevent dietary fat assimilation but influenced adiposity and glucose tolerance in a sex-dependent manner [20,21]. Studies on humans have found a potential association between the A54T human FABP2 gene variant and insulin resistance, obesity as well as dyslipidemias in specific sexes in certain populations [22,23,24,25].
Fabp6 (also known as ILBP and I-BABP) is abundant in the distal region of the small intestine and displays a clear preference for bile acids although it is capable of binding fatty acids at lower affinity [8,9,26]. The targeted disruption of the Fabp6 gene in mice demonstrated that Fabp6 is important in the intracellular transport of bile acids in enterocytes and the maintenance of the bile acid pool in the enterohepatic circulation [27]. Interestingly, Fabp6 is also found in the ovaries but not in the testes, and female mice lacking Fabp6 display a reduced ovulation rate compared to female wild-type (WT) mice [28]. Moreover, Fabp6 was found to be associated with the function of farnesoid X receptor (FXR) [29,30], suggesting a potential role of Fabp6 in modulating the activity of FXR in the nucleus. In humans, the T79M variant of human FABP6 is associated with some protective effect on type 2 diabetes in obese subjects [31].
The targeted ablation of Fabp1, Fabp2, or Fabp6 genes have all resulted in sexually dimorphic phenotypes [14,20,21,27,32]. Here, we compared the transcriptomes of small intestines from mice lacking either Fabp2 or Fabp6, or both of these Fabps to gain a comprehensive insight into the changes in the gene expression programs that occur in both sexes following the loss of these Fabps.
2. Materials and Methods
2.1. Mice
Mice (n = 5 per cage) were housed in a temperature-controlled specific-pathogen-free facility and fed a standard lab diet (Purina 5001). Fabp2–/– [21] and Fabp6–/– [27] mice were maintained on the C57BL/6J background, which included one cross with a male C57BL/6J mouse, and interbred to generate the Fabp2–/–;Fabp6–/– line. The use of mice was approved by the institutional animal welfare and policy committees in accordance with the policies of the Canadian Council on Animal Care (McGill University AUP5350; this project was initiated at the University of Alberta under Animal Protocol 270).
2.2. Preparation of RNA and Hybridization to DNA Microarray
The small intestine, starting from the base of the stomach and ending just before the cecum, was excised from fasted 10-week old mice littermates and then flushed with ice-cold saline prior to tissue homogenization. RNA was extracted from homogenates using Trizol (Invitrogen) and assessed for integrity (RIN > 7) prior to use in microarray analysis. The total RNA sample from 4 mice of each sex (male or female) and genotype (wild-type C57BL/6J, Fabp2–/–, Fabp6–/–, Fabp2–/–;Fabp6–/–) was analyzed as 2 biological replicates (RNA from 2 mice were pooled and sequenced as 1 sample). Fluorescently labeled cDNA probes generated from RNA samples were hybridized to Affymetrix 430A and 430B chips (total of 32, 16 per chip type) and processed as specified by the manufacturer.
2.3. Analysis of DNA Microarray Data
2.3.1. Processing of Raw DNA Microarray Data
The microarray data were processed and analyzed using the R statistical environment and Bioconductor software [33] as illustrated in Supplementary Figure S1. Raw intensities from each gene chip were transformed to a log2 scale and quantile normalized using the Robust Multichip Average (RMA) algorithm with background correction [34]. The quality assessments were done by comparing the intensity between the biological replicates (See Supplementary Figure S2) and technical replicates (See Supplementary Figure S3). Boxplots were also used to evaluate the data quality across the chips (See Supplementary Figure S4). To improve the statistical power, the data were filtered based on their inter-quartile range and Entrez annotations [35]. The limFit function (limma package) [36] was applied to fit data into linear models to identify the genes with a differential expression between the male and female of the same genotypes and also between each of the Fabp gene-disrupted genotypes and wild-type mice of the same sex. The genes were screened with volcano plots (Supplementary Figure S5) generated using the EnhancedVolcano R package [37]. Genes with a false discovery rate <0.2 and an absolute log2 fold change > 0.5 were considered statistically significantly different. The Venn diagram plots were generated using the BioVenn website to present common and sex-specific differentially expressed (DE) genes induced by the deletion of Fabp genes [38]. See Supplementary Figure S1 for a more detailed description of the workflow and software resources used in the analyses.
2.3.2. Gene Annotation and Functional Enrichment Analysis
The murine gene ID conversion and functional annotation of identified sex-biased genes and DE genes were done using DAVID Bioinformatics Resources (version 6.8) [39] with the default setting. The gene ontology (GO) terms in the biological process (BP) domains were extracted. The chromosomal locations of DE genes were determined from the Mouse Genome Database [40]. To display the metabolic patterns comprehensively, the relevant biological processes were grouped into four categories of interest in this study, namely lipid metabolism, carbohydrate metabolism, protein metabolism and sterol metabolism (complete lists are shown in Supplementary Table S2), and the number of unique DE genes in each category for each sex and genotype were counted and presented.
2.3.3. Protein–Protein Interaction Network Analysis
In order to reveal sexually dimorphic protein interaction patterns, the corresponding proteins of the DE genes that were affected by the disruption of Fabp genes in each sex were used as seed proteins to identify the interacting proteins based on the literature-curated interactions in the NetworkAnalyst’s protein–protein interaction database (accessed on May 2020) [41]. Generic protein–protein interaction analysis was conducted using the International Molecular Exchange consortium (IMEx) interactome database using the default setting. The seed proteins were selected by the NetworkAnalyst algorithm based on the submitted gene lists. Subsequently, these seed proteins were used as the starting points to predict the functional protein network.
2.3.4. Availability of DNA Microarray Data
The NCBI GEO accession number for the microarray data used in this study is GSE128862.
3. Results
3.1. Gene Expression Patterns in Male and Female Murine Small Intestine Are Distinct
To address the difference in the gene expression in the small intestine between male and female mice with or without Fabps, transcriptome profiling was performed using microarrays in our study (see Supplementary Figure S1 for the description of the analysis workflow). Sex-biased genes were identified in all genotypes (Figure 1a). A total of 67 sex-biased genes were found in the WT mice. This number was reduced in the Fabp2–/– and Fabp2–/–;Fabp6–/– mice whereas the Fabp6 gene deletion increased the number of sex-biased genes (Figure 1a), suggesting that the deletion of the Fabp2 gene or both Fabp2 and Fabp6 genes made the intestinal gene expression of males and females more similar, while the disruption of the Fabp6 gene increased the sexual dimorphism at the transcriptome level.
Figure 1.
Sex-biased genes and functional annotation (gene ontology: biological process, GO: BP). (a) Comparison of the number of sex-biased genes identified in wild-type (WT), Fabp2–/–, Fabp6–/–, and Fabp2–/–;Fabp6–/– mice. (b) The number of male- and female-biased genes in the small intestinal transcriptome across all genotypes. (c) Comparison of the biological processes enriched (p-value < 0.05) using sex-biased genes. The numbers of genes involved in each biological process are represented by the green color, darker shades indicate a greater number of genes. See Supplementary Table S1 for a complete list of sex-biased genes.
The genes that were identified as sex-biased were then further classified as either female-biased or male-biased. The results show that the number of female-biased genes, the sex-biased genes that have higher expression in females, is greater than male-biased ones in WT mice (Figure 1b), and this is consistent with previous findings [42,43]. The deletion of Fabp genes increased the proportion of male-biased genes, which might play roles in determining the male-specific phenotypes in Fabp gene-disrupted mice. In addition, most of the sex-biased genes reside on autosomal chromosomes (See Supplementary Figure S6).
The gene ontology-enriched analysis was applied to identify the biological pathways involving sex-biased genes. Many of the genes that show sexually dimorphic expression in WT small intestine are involved in nutrient and drug metabolism (Figure 1c), which is in accordance with other studies [44,45]. Some of the sexually dimorphic pathways in the small intestine that pre-existed in WT male and female mice were retained in mice lacking only one Fabp, i.e., either Fabp2 or Fabp6, whereas two pathways remained identifiable when mice were lacking both Fabps (Figure 1c). In general, the deletion of Fabp6 resulted in a greater number of sex dimorphic pathways compared to Fabp2, while the deletion of both Fabps resulted in the least number of sexually dimorphic pathways.
Thus, pre-existing differences in the gene expression program, as illustrated in the small intestinal transcriptome of WT male and female mice, may predetermine the intestinal gene expression program in response to the disruption of specific intestinal Fabp genes.
3.2. Genomic Responses to Ablation of Specific Fabp Genes Are Sexually Dimorphic
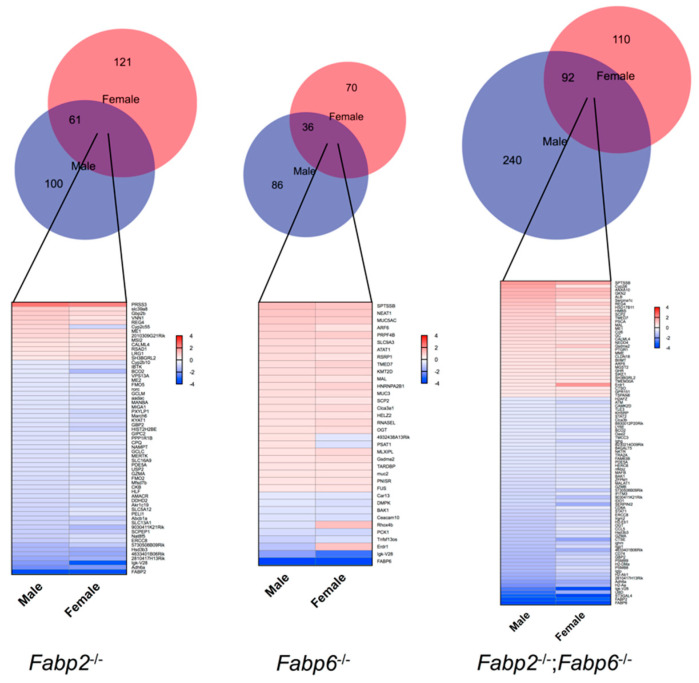
We further evaluated the genomic responses to Fabp2 and/or Fabp6 gene disruption in male and female mice. DE genes were identified by comparing the transcript abundance between WT and Fabp gene-disrupted mice of each sex. In general, less than 50% of DE genes induced by Fabp gene ablations are shared by male and female mice (Figure 2, top, overlap of red and blue circles). Many of the shared DE genes (Figure 2, bottom, gene list) had distinct alterations in male and female mice. Some shared genes altered the gene expression in the opposite direction in males and females in response to the same Fabp gene disruption, such as Cyp2c55 in Fabp2–/– mice and Psat1 in Fabp6–/– mice. Notably, the numbers of DE genes identified in the small intestine of Fabp2–/–;Fabp6–/– mice were much higher than the single Fabp gene-disrupted mice in the same sex. Fabp2–/–;Fabp6–/– mice also have different sets and total number of DE genes compared to either single Fabp gene disruption regardless of the sex (See Supplementary Figure S7), implying a greater effect of the combined Fabp2 and Fabp6 deficiency on the overall gene expression program in the small intestine. Moreover, like sex-biased genes (See Supplementary Figure S6), most of the DE genes identified also reside on autosomal chromosomes (See Supplementary Figure S8).
Figure 2.
The comparison of the differentially expressed (DE) genes in different Fabp gene-disrupted male and female mice. The Venn Diagram of the common and sex-specific DE genes induced by the deletion of Fabp genes (top). The heatmap comparing the altered expression of the common DE genes shared by males and females (bottom). See Supplementary Table S1 for a complete list of DE genes.
To gain insight into the metabolic pathways differentially affected in males and females by Fabp gene ablations, gene ontology analysis using DE genes grouped by sex for each genotype was carried out. The top 10 biological processes having a p-value < 0.05 are shown in Table 1. Two groups of biological processes, namely metabolism-related and immune-related processes, comprise the major proportion of affected processes. Specifically, DE genes influenced by Fabp2 or Fabp6 gene single deletions are involved in metabolism-related biological processes, whereas combined Fabp2 and Fabp6 gene deletions mainly influenced immune-related biological processes. Furthermore, the males and females displayed distinct alterations of biological processes in response to the same Fabp gene ablation, which is concordant with previously reported changes observed in Fabp2 gene-disrupted mice [46].
Table 1.
Gene ontology analysis for the DE genes induced in different genotypes. The top 10 biological processes that are enriched in each genotype are shown, as are the number of genes that fall within each process (count) and the p-values obtained.
| Fabp2–/– (M) | Count | p-Value | Fabp2–/– (F) | Count | p-Value |
|---|---|---|---|---|---|
| GO:0055114~oxidation-reduction process | 22 | 6.42 × 10−8 | GO:0006749~glutathione metabolic process | 5 | 6.03 × 10−4 |
| GO:0006629~lipid metabolic process | 17 | 6.02 × 10−7 | GO:0006805~xenobiotic metabolic process | 4 | 8.68 × 10−4 |
| GO:0032922~circadian regulation of gene expression | 7 | 7.89 × 10−6 | GO:0008152~metabolic process | 12 | 0.001148 |
| GO:0008202~steroid metabolic process | 7 | 4.98 × 10−5 | GO:0042130~negative regulation of T cell proliferation | 4 | 0.003863 |
| GO:0048511~rhythmic process | 8 | 6.51 × 10−5 | GO:0035458~cellular response to interferon-β | 4 | 0.004144 |
| GO:0006631~fatty acid metabolic process | 8 | 2.24 × 10−4 | GO:0035729~cellular response to hepatocyte growth factor stimulus | 3 | 0.00693 |
| GO:0006805~xenobiotic metabolic process | 4 | 8.33 × 10−4 | GO:0055085~transmembrane transport | 9 | 0.00846 |
| GO:0006694~steroid biosynthetic process | 5 | 0.001392 | GO:0017144~drug metabolic process | 3 | 0.008746 |
| GO:0007623~circadian rhythm | 6 | 0.001573 | GO:0032922~circadian regulation of gene expression | 4 | 0.012478 |
| GO:0006641~triglyceride metabolic process | 4 | 0.003454 | GO:0006807~nitrogen compound metabolic process | 3 | 0.012935 |
| Fabp6–/– (M) | Fabp6–/– (F) | ||||
| GO:0045944~positive regulation of transcription from RNA polymerase II promoter | 14 | 0.002734 | GO:0006915~apoptotic process | 10 | 0.001953 |
| GO:0006814~sodium ion transport | 5 | 0.004634 | GO:0045779~negative regulation of bone resorption | 3 | 0.002748 |
| GO:0048511~rhythmic process | 5 | 0.0051837 | GO:0006397~mRNA processing | 7 | 0.005133 |
| GO:0006351~transcription, DNA-templated | 20 | 0.005575 | GO:0008652~cellular amino acid biosynthetic process | 3 | 0.006677 |
| GO:0033137~negative regulation of peptidyl-serine phosphorylation | 3 | 0.008042 | GO:0033137~negative regulation of peptidyl-serine phosphorylation | 3 | 0.006677 |
| GO:0008652~cellular amino acid biosynthetic process | 3 | 0.008042 | GO:0006094~gluconeogenesis | 3 | 0.00721 |
| GO:0051726~regulation of cell cycle | 4 | 0.023003 | GO:0061430~bone trabecula morphogenesis | 2 | 0.014694 |
| GO:0035020~regulation of Rac protein signal transduction | 2 | 0.032085 | GO:0051726~regulation of cell cycle | 4 | 0.017872 |
| GO:0006915~apoptotic process | 8 | 0.03525 | GO:0006564~L-serine biosynthetic process | 2 | 0.019545 |
| GO:0006810~transport | 17 | 0.035583 | GO:0045944~positive regulation of transcription from RNA polymerase II promoter | 11 | 0.024183 |
| Fabp2–/–;Fabp6–/– (M) | Fabp2–/–;Fabp6–/– (F) | ||||
| GO:0002376~immune system process | 26 | 5.43 × 10−9 | GO:0019886~antigen processing and presentation of exogenous peptide antigen via MHC class II | 6 | 9.87 × 10−8 |
| GO:0034341~response to interferon-γ | 8 | 1.55 × 10−7 | GO:0002376~immune system process | 17 | 3.32 × 10−7 |
| GO:0019882~antigen processing and presentation | 10 | 2.21 × 10−7 | GO:0019882~antigen processing and presentation | 8 | 4.85 × 10−7 |
| GO:0055114~oxidation-reduction process | 32 | 2.85 × 10−7 | GO:0034341~response to interferon-γ | 6 | 2.97 × 10−6 |
| GO:0035458~cellular response to interferon-β | 9 | 2.95 × 10−7 | GO:0035458~cellular response to interferon-β | 6 | 3.04 × 10−5 |
| GO:0042572~retinol metabolic process | 7 | 2.00 × 10−6 | GO:0006955~immune response | 12 | 3.31 × 10−5 |
| GO:0019886~antigen processing and presentation of exogenous peptide antigen via MHC class II | 6 | 2.08 × 10−6 | GO:0002504~antigen processing and presentation of peptide or polysaccharide antigen via MHC class II | 4 | 5.60 × 10−5 |
| GO:0006955~immune response | 17 | 1.23 × 10−5 | GO:0042130~negative regulation of T cell proliferation | 5 | 4.31 × 10−4 |
| GO:0007584~response to nutrient | 9 | 2.63 × 10−5 | GO:0060337~type I interferon signaling pathway | 3 | 0.001154 |
| GO:0006629~lipid metabolic process | 22 | 2.64 × 10−5 | GO:0031175~neuron projection development | 7 | 0.00154 |
Together, the data show that Fabp gene deletions, either separately or combined, resulted in the sex-specific modification of gene expression in the small intestine. The identity of genes with altered expression in Fabp2–/– and Fabp6–/– mice are different from those in Fabp2–/–;Fabp6–/– mice, suggesting that the biological processes that are affected when both Fabp2 and Fabp6 genes are missing are different from those when only one of these genes is absent.
3.3. Predicted Nutrient Metabolism Processes in the Small Intestine of Fabp Gene Ablated Mice Are Sex Biased
Since the small intestine is the frontline for nutrient acquisition, transport, metabolism, as well as signaling, and where sex differences are also known to be pre-existing [47,48,49,50], we asked whether the genes involved in nutrient metabolism were influenced differently in male and female mice by the loss of specific Fabps. The affected biological process (GO:BP) identified was categorized according to macromolecule/macronutrient metabolism (carbohydrate, protein and lipid metabolism) and sterol/bile acid metabolism, and then the number of unique DE genes belonging to these categories was stratified according to genotype and sex. As shown in Figure 3, Fabp2–/– and Fabp2–/–;Fabp6–/– mice displayed similar metabolism patterns partitioned by sex. Specifically, all four categories of metabolism were affected more in males than in females, and the lipid and protein metabolism were influenced the most in both sexes. However, this pattern was not evident in the Fabp6–/– mice, where the total numbers of DE genes involved in macronutrient metabolism were similar in male and female mice. In these mice, protein metabolism was influenced the most in males while lipid metabolism was greatly influenced in females. Surprisingly, only a few genes involved in sterol/bile acid metabolism were identified in both male and female Fabp6–/– mice (Figure 3). For Fabp2–/–;Fabp6–/– mice, there was a substantially higher number of DE genes involved in macronutrient and sterol metabolism compared to mice with single Fabp gene disruption.
Figure 3.
The comparison of the numbers of DE genes involved in key nutrient metabolism processes. The biological processes that comprise the nutrient metabolism categories are listed in the Supplementary Table S2.
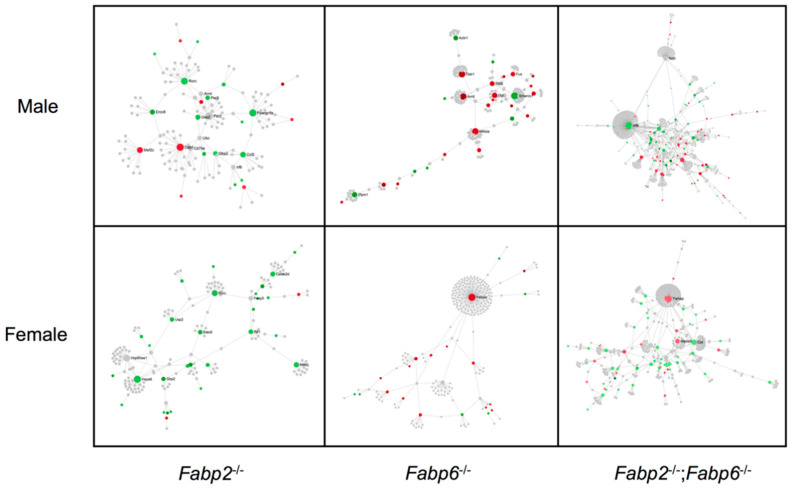
We used the interaction network analysis to gain insight into the possible functions of DE genes based on the predicted interactions among their encoded proteins. Generally, males and females had very different network patterns in response to same Fabp gene ablation, suggesting sex-dependent biological responses, even though some nodes of the networks are shared (Figure 4). Specifically, the first-order networks of Fabp6–/– and Fabp2–/–;Fabp6–/– males contain more seed proteins, which are starting-point proteins extracted by the NetworkAnalyst to build networks, than females (28 for Fabp6–/– and 90 for Fabp2–/–; Fabp6–/– males; 24 for Fabp6–/– and 66 for Fabp2–/–;Fabp6–/– females) whereas the network of Fabp2–/– males contains fewer seed proteins (25 for Fabp2–/– males and 33 for Fabp2–/– females) (Figure 4). When comparing the nodes that have more than one interacting protein, all mice (all genotypes, both sexes) have one node in common, forkhead box P3 (Foxp3), whereas only male mice share NF-KB (Nfkb1) and Sirtuin-1 (Sirt1). Interestingly, when both Fabp2 and Fabp6 are missing, Nfkb1, but not Sirt1, appear only in the network map of female mice. Fabp2–/–;Fabp6–/– mice have a greater number of nodes in both sexes than the combined number of nodes in Fabp2–/– and Fabp6–/– mice, suggesting that a larger and more complex interaction network was affected when both Fabp2 and Fabp6 are missing.
Figure 4.
The protein–protein interaction networks of DE genes in different Fabp gene-disrupted genotypes (Fabp2–/–, Fabp6–/–, and Fabp2–/–;Fabp6–/–). Three types of proteins (nodes) are presented in the networks: seed proteins represented in green are encoded by downregulated genes and those represented in red are encoded by upregulated genes. Only the shortest-path first-order networks from the seed proteins are shown. Proteins represented in grey are known to directly interact with the seed proteins. The nodes are listed in the Supplementary Table S3.
4. Discussion
The existence of sexual dimorphism in lipid metabolism in the intestine has been described in both humans and mice [45,51,52]. As the most abundant cytoplasmic proteins that play pivotal roles in lipid metabolism in the small intestine, intestinal Fabps have been shown to exhibit sexually dimorphic expression in the small intestine of mice [20,27]. As for human intestinal FABPs [53], less is known about sex differences in the expression of their genes owing to the difficulty in obtaining samples for study. The targeted disruption of intestinal Fabp genes in mice also results in sexually dimorphic effects. The ablation of the Fabp2 gene causes a much larger degree of metabolic disturbance in male Fabp2–/– mice than female Fabp2–/– mice [21,54]. Similarly, the ablation of the Fabp6 gene induced differential alterations in male and female mice regarding bile acid metabolism [27]. The whole-body deficiency of the Fabp1 has also been shown to induce sexually dimorphic phenotypes [32,55] but since the Fabp1 gene is expressed in many tissues, particularly in the liver, the metabolic consequences of intestine-specific deficiency of Fabp1 is not readily apparent from currently available Fabp1–/– mouse models. It is clear from available studies that the ablation of genes encoding intestinal Fabps impacts on the expression of genes in a variety of tissues, in addition to the small intestine, and alter whole-body metabolism in a sex-dimorphic manner [27,54,55].
The sex dimorphic transcriptome is determined by many factors. Despite the males and females sharing a common autosomal genome, it is estimated that the sex-biased expression of genes in specific tissues ranges from less than 1% to 30%, depending on the sequencing techniques and statistical cut-offs used in the analyses [56,57]. Indeed, sex-dependent gene expression patterns are evident in the brain, liver, muscle, adipose tissue, and intestines of mice [43,58,59]. Sex differences in the transcriptome of a specific tissue are likely framed by the combined effects of biological sex as dictated by sex chromosomes, sex hormones or the sex-specific modification of the epigenome [60,61]. For example, substantial sex-biased gene expression is clearly evident in the small intestine of prepubescent mice and even as early as during embryo development [43,62]. In our study, nearly all the of DE genes detected in the intestinal transcriptome of all Fabp gene-disrupted genotypes were resident on autosomal chromosomes and very few were involved in sex hormonal-related processes. Moreover, protein–protein interaction analysis revealed that the major networks involving the DE genes in the majority of Fabp gene-disrupted genotypes included transcriptional signaling processes related to PPAR and FXR. It has been shown that PPAR and FXR themselves manifest a sexually dimorphic expression [63,64,65]. Given the fact that Fabps share the many ligands with these nuclear receptors, the loss of specific Fabps could further alter the ability of these transcription factors to regulate gene expression in a sex-dependent manner. All these findings might suggest the existence of sex-biased regulatory networks, which are constructed by proteins and RNAs with sex-biased abundance, including Fabps. Such networks, in turn, influence the internal availability of bioactive substrates such as nutrients and xenobiotics. Consequently, biological sex modifies the physiological responses to extrinsic interventions and differentiates the onset, development, and outcome of diseases in males and females [44,66].
This study provides insights into the potential biological roles and functional relationships of the intestinal Fabps. It was previously suggested that multiple Fabps in the small intestine might share some functions to ensure fatty acid and bile acid metabolism [20]. Fabp1 and Fabp2 show preference for binding fatty acids whereas Fabp6 prefers bile acids [9,26]. On the other hand, Fabp1 and Fabp6 can bind bile acids and fatty acids, respectively, at lower affinities [8,26]. Indeed, we found that mice lacking both Fabp2 and Fabp6 were viable. Interestingly, the network analysis revealed the loss of both Fabps affected a much larger number of processes in male mice than in female mice, similar to Fabp2–/– mice. Future studies will uncover how these changes manifest at the organismal level. In addition, different regions of the small intestine have specialized metabolic and immune functions [67,68]. It would also be interesting to determine the nature of the changes in the gene expression program at these regions of the small intestine in males and females.
It should be noted that the findings of our analyses are applicable to a population of mice with the same age, fed the standard murine laboratory diet and housed under standard vivarium environmental conditions. Thus, our study does not inform on how the gene expression programs of male and female small intestine might evolve as a function of aging, in response to specific dietary challenges, changes in environmental conditions, nor does it provide information on changes in protein abundance or activity. Nevertheless, our findings provide a reference point for future studies to understand how biological sex impacts on the genomic responses associated with metabolic pathways involved in nutrient processing by the intestines, overall nutrient metabolism and susceptibility to various diet-induced metabolic diseases.
In conclusion, our study shows that sex is an important determinant of the intestinal transcriptome. Moreover, the pre-existing differences between males and females may govern the distinct alterations of the intestinal gene expression program manifested by males and females in response to the targeted inactivation of genes encoding the intestinal Fabps.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/8/943/s1. Supplementary Figure S1: Flowchart illustrating the microarray data analysis workflow. Supplementary Figure S2: Assessment of the quality of data by comparing signal intensity from the biological replicates using linear correlation (x axis, replicate 1 and y axis, replicate (2) after robust multichip average (RMA) processing. Supplementary Figure S3: Assessment of the quality of the data by comparing signal intensities of shared probes on Chip 430A (x axis) and Chip 430B (y axis) for each biological replicate using the linear correlation after a robust multichip average (RMA) processing. Supplementary Figure S4: Assessment of the quality of microarray data using boxplot after robust multichip average processing. Supplementary Figure S5. The volcano plots of differentially expressed genes in different Fabp gene-disrupted male and female mice. Supplementary Figure S6: Chromosomal location of sex-biased genes identified in wild-type (WT) and Fabp gene-disrupted mice. Supplementary Figure S7: Venn diagrams and heatmaps showing the number of differentially expressed genes in male and female intestinal transcriptomes of different Fabp gene-disrupted genotypes. Supplementary Figure S8: Chromosomal location of differentially expressed (DE) genes identified in male and female intestinal transcriptomes of different Fabp gene-disrupted genotypes. Supplementary Table S1: Complete lists of sex-biased genes and differentially expressed (DE) genes. Supplementary Table S2: List of the biological processes cited in Figure 3 categorized under lipid metabolism, carbohydrate metabolism, protein metabolism and sterol metabolism. Supplementary Table S3: List of nodes, edges and seeds identified in the networks shown in Figure 4.
Author Contributions
Y.C. conducted the analysis and interpreted the results. L.B.A. designed the study and interpreted the results. Y.C. and L.B.A. wrote, edited, and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research. The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Furuhashi M., Hotamisligil G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storch J., Córsico B. The Emerging Functions and Mechanisms of Mammalian Fatty Acid–Binding Proteins. Annu. Rev. Nutr. 2008;28:73–95. doi: 10.1146/annurev.nutr.27.061406.093710. [DOI] [PubMed] [Google Scholar]
- 3.Lowe J.B., Boguski M.S., Sweetser D.A., Elshourbagy N., Taylor J.M., Gordon J.I. Human liver fatty acid binding protein. Isolation of a full length cDNA and comparative sequence analyses of orthologous and paralogous proteins. J. Biol. Chem. 1985;260:3413–3417. [PubMed] [Google Scholar]
- 4.Ockner R.K., A Manning J., Kane J.P. Fatty acid binding protein. Isolation from rat liver, characterization, and immunochemical quantification. J. Biol. Chem. 1982;257:7872–7878. [PubMed] [Google Scholar]
- 5.Sweetser D.A., Birkenmeier E.H., Klisak I.J., Zollman S., Sparkes R.S., Mohandas T., Lusis A.J., I Gordon J. The human and rodent intestinal fatty acid binding protein genes. A comparative analysis of their structure, expression, and linkage relationships. J. Biol. Chem. 1987;262:16060–16071. [PubMed] [Google Scholar]
- 6.Walz D.A., Wider M.D., Snow J.W., Dass C., Desiderio D.M. The complete amino acid sequence of porcine gastrotropin, an ileal protein which stimulates gastric acid and pepsinogen secretion. J. Biol. Chem. 1988;263:14189–14195. [PubMed] [Google Scholar]
- 7.Gong Y.Z., Everett E.T., Schwartz D.A., Norris J.S., Wilson F.A. Molecular cloning, tissue distribution, and expression of a 14-kDa bile acid-binding protein from rat ileal cytosol. Proc. Natl. Acad. Sci. USA. 1994;91:4741–4745. doi: 10.1073/pnas.91.11.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agellon L.B., Toth M.J., Thomson A.B. Intracellular lipid binding proteins of the small intestine. Mol. Cell. Biochem. 2002;239:79–82. doi: 10.1023/A:1020520521025. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerman A.W., Van Moerkerk H.T., Veerkamp J.H. Ligand specificity and conformational stability of human fatty acid-binding proteins. Int. J. Biochem. Cell Biol. 2001;33:865–876. doi: 10.1016/S1357-2725(01)00070-X. [DOI] [PubMed] [Google Scholar]
- 10.Richieri G.V., Ogata R.T., Zimmerman A.W., Veerkamp J.H., Kleinfeld A.M. Fatty acid binding proteins from different tissues show distinct patterns of fatty acid interactions. Biochemistry. 2000;39:7197–7204. doi: 10.1021/bi000314z. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder F., Petrescu A.D., Huang H., Atshaves B.P., McIntosh A.L., Martin G.G., Hostetler H.A., Vespa A., Landrock D., Landrock K.K., et al. Role of Fatty Acid Binding Proteins and Long Chain Fatty Acids in Modulating Nuclear Receptors and Gene Transcription. Lipids. 2007;43:1–17. doi: 10.1007/s11745-007-3111-z. [DOI] [PubMed] [Google Scholar]
- 12.Wolfrum C., Borrmann C.M., Börchers T., Spener F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors α- and γ-mediated gene expression via liver fatty acid binding protein: A signaling path to the nucleus. Proc. Natl. Acad. Sci. USA. 2001;98:2323–2328. doi: 10.1073/pnas.051619898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newberry E.P., Xie Y., Kennedy S.M., Han X., Buhman K.K., Luo J., Gross R.W., Davidson N.O. Decreased Hepatic Triglyceride Accumulation and Altered Fatty Acid Uptake in Mice with Deletion of the Liver Fatty Acid-binding Protein Gene. J. Biol. Chem. 2003;278:51664–51672. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 14.Martin G.G., Danneberg H., Kumar L.S., Atshaves B.P., Erol E., Bader M., Schroeder F., Binas B. Decreased Liver Fatty Acid Binding Capacity and Altered Liver Lipid Distribution in Mice Lacking the Liver Fatty Acid-binding Protein Gene. J. Biol. Chem. 2003;278:21429–21438. doi: 10.1074/jbc.M300287200. [DOI] [PubMed] [Google Scholar]
- 15.Xie Y., Newberry E.P., Kennedy S.M., Luo J., Davidson N.O. Increased susceptibility to diet-induced gallstones in liver fatty acid binding protein knockout mice. J. Lipid Res. 2009;50:977–987. doi: 10.1194/jlr.M800645-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weickert M.O., Loeffelholz C.V., Roden M., Chandramouli V., Brehm A., Nowotny P., Osterhoff M.A., Isken F., Spranger J., Landau B.R., et al. A Thr94Ala mutation in human liver fatty acid-binding protein contributes to reduced hepatic glycogenolysis and blunted elevation of plasma glucose levels in lipid-exposed subjects. Am. J. Physiol. Metab. 2007;293:E1078–E1084. doi: 10.1152/ajpendo.00337.2007. [DOI] [PubMed] [Google Scholar]
- 17.Brouillette C., Bossé Y., Pérusse L., Gaudet D., Vohl M.-C. Effect of liver fatty acid binding protein (FABP) T94A missense mutation on plasma lipoprotein responsiveness to treatment with fenofibrate. J. Hum. Genet. 2004;49:424–432. doi: 10.1007/s10038-004-0171-2. [DOI] [PubMed] [Google Scholar]
- 18.Fisher E., Weikert C., Klapper M., Lindner I., Möhlig M., Spranger J., Boeing H., Schrezenmeir J., Döring F. L-FABP T94A is associated with fasting triglycerides and LDL-cholesterol in women. Mol. Genet. Metab. 2007;91:278–284. doi: 10.1016/j.ymgme.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Hsu K.T., Storch J. Fatty Acid Transfer from Liver and Intestinal Fatty Acid-binding Proteins to Membranes Occurs by Different Mechanisms. J. Biol. Chem. 1996;271:13317–13323. doi: 10.1074/jbc.271.23.13317. [DOI] [PubMed] [Google Scholar]
- 20.Agellon L.B., Li L., Luong L., Uwiera R.R.E. Adaptations to the loss of intestinal fatty acid binding protein in mice. Mol. Cell. Biochem. 2006;284:159–166. doi: 10.1007/s11010-005-9042-1. [DOI] [PubMed] [Google Scholar]
- 21.Vassileva G., Huwyler L., Poirier K., Agellon L.B., Toth M.J. The intestinal fatty acid binding protein is not essential for dietary fat absorption in mice. FASEB J. 2000;14:2040–2046. doi: 10.1096/fj.99-0959com. [DOI] [PubMed] [Google Scholar]
- 22.Hasnain S. The fatty acid binding protein 2 (FABP2) polymorphism Ala54Thr and obesity in Pakistan: A population based study and a systematic meta-analysis. Gene. 2015;574:106–111. doi: 10.1016/j.gene.2015.07.087. [DOI] [PubMed] [Google Scholar]
- 23.Tavridou A., Arvanitidis K.I., Tiptiri-Kourpeti A., Petridis I., Ragia G., Kyroglou S., Christakidis D., Manolopoulos V.G. Thr54 allele of fatty-acid binding protein 2 gene is associated with obesity but not type 2 diabetes mellitus in a Caucasian population. Diabetes Res. Clin. Pract. 2009;84:132–137. doi: 10.1016/j.diabres.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Alharbi K.K., Khan I.A., Bazzi M.D., Al-Daghri N.M., Hasan T.N., Al-Nbaheen M.S., Alharbi F.K., Al-Sheikh Y.A., Syed R., Aboul-Soud M.A. A54T polymorphism in the fatty acid binding protein 2 studies in a Saudi population with type 2 diabetes mellitus. Lipids Health Dis. 2014;13:61. doi: 10.1186/1476-511X-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damcott C.M., Feingold E., Moffett S.P., Barmada M.M., Marshall J.A., Hamman R.F., Ferrell R.E. Variation in the FABP2 promoter alters transcriptional activity and is associated with body composition and plasma lipid levels. Hum. Genet. 2003;112:610–616. doi: 10.1007/s00439-003-0937-1. [DOI] [PubMed] [Google Scholar]
- 26.Labonté E.D., Li Q., Kay C.M., Agellon L.B. The relative ligand binding preference of the murine ileal lipid binding protein. Protein Expr. Purif. 2003;28:25–33. doi: 10.1016/S1046-5928(02)00561-2. [DOI] [PubMed] [Google Scholar]
- 27.Praslickova D., Torchia E.C., Sugiyama M.G., Magrane E.J., Zwicker B.L., Kolodzieyski L., Agellon L.B. The Ileal Lipid Binding Protein Is Required for Efficient Absorption and Transport of Bile Acids in the Distal Portion of the Murine Small Intestine. PLoS ONE. 2012;7:e50810. doi: 10.1371/journal.pone.0050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duggavathi R., Siddappa D., Schuermann Y., Pansera M., Menard I.J., Praslickova D., Agellon L.B. The fatty acid binding protein 6 gene (Fabp6) is expressed in murine granulosa cells and is involved in ovulatory response to superstimulation. J. Reprod. Dev. 2015;61:237–240. doi: 10.1262/jrd.2014-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakahara M., Furuya N., Takagaki K., Sugaya T., Hirota K., Fukamizu A., Kanda T., Fujii H., Sato R. Ileal Bile Acid-binding Protein, Functionally Associated with the Farnesoid X Receptor or the Ileal Bile Acid Transporter, Regulates Bile Acid Activity in the Small Intestine. J. Biol. Chem. 2005;280:42283–42289. doi: 10.1074/jbc.M507454200. [DOI] [PubMed] [Google Scholar]
- 30.Landrier J.F., Grober J., Zaghini I., Besnard P. Regulation of the ileal bile acid-binding protein gene: An approach to determine its physiological function(s) Mol. Cell. Biochem. 2002;239:149–155. doi: 10.1023/A:1020557502795. [DOI] [PubMed] [Google Scholar]
- 31.Fisher E., Grallert H., Klapper M., Pfäfflin A., Schrezenmeir J., Illig T., Boeing H., Döring F. Evidence for the Thr79Met polymorphism of the ileal fatty acid binding protein (FABP6) to be associated with type 2 diabetes in obese individuals. Mol. Genet. Metab. 2009;98:400–405. doi: 10.1016/j.ymgme.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Martin G.G., Atshaves B.P., McIntosh A.L., Mackie J.T., Kier A.B., Schroeder F. Liver fatty-acid-binding protein (L-FABP) gene ablation alters liver bile acid metabolism in male mice. Biochem. J. 2005;391:549–560. doi: 10.1042/BJ20050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gentleman R.C., Carey V.J., Bates D.M., Bolstad B., Dettling M., Dudoit S., Ellis B., Gautier L., Ge Y., Gentry J., et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U., Speed T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 35.Bourgon R., Gentleman R., Huber W. Independent filtering increases detection power for high-throughput experiments. Proc. Natl. Acad. Sci. USA. 2010;107:9546–9551. doi: 10.1073/pnas.0914005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smyth G.K. Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. Stat. Appl. Genet. Mol. Biol. 2004;3:1–25. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 37.Blighe K., Rana S., Lewis M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. [(accessed on 31 July 2020)]; Available online: https://github.com/kevinblighe/EnhancedVolcano.
- 38.Hulsen T., De Vlieg J., Alkema W. BioVenn–A web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genom. 2008;9:488. doi: 10.1186/1471-2164-9-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 40.Smith C.L., Blake J.A., Kadin J.A., Richardson J.E., Bult C.J. Mouse Genome Database Group Mouse Genome Database (MGD)-2018: Knowledgebase for the laboratory mouse. Nucleic Acids Res. 2018;46:D836–D842. doi: 10.1093/nar/gkx1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia J., Benner M.J., Hancock R.E.W. NetworkAnalyst-integrative approaches for protein—protein interaction network analysis and visual exploration. Nucleic Acids Res. 2014;42:W167–W174. doi: 10.1093/nar/gku443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B., Qing T., Zhu J., Wen Z., Yu Y., Fukumura R., Zheng Y., Gondo Y., Shi L. A Comprehensive Mouse Transcriptomic BodyMap across 17 Tissues by RNA-seq. Sci. Rep. 2017;7:4200. doi: 10.1038/s41598-017-04520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steegenga W.T., Mischke M., Lute C., Boekschoten M.V., Pruis M.G., Lendvai A., Verkade H.J., Boekhorst J., Timmerman H.M., Plosch T., et al. Sexually dimorphic characteristics of the small intestine and colon of prepubescent C57BL/6 mice. Biol. Sex Differ. 2014;5:11. doi: 10.1186/s13293-014-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soldin O.P., Mattison D.W. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009;48:143–157. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugiyama M.G., Agellon L.B. Sex differences in lipid metabolism and metabolic disease risk. Biochem. Cell Biol. 2012;90:124–141. doi: 10.1139/o11-067. [DOI] [PubMed] [Google Scholar]
- 46.Sugiyama M.G., Hobson L., Agellon A.B., Agellon L.B. Visualization of Sex-Dimorphic Changes in the Intestinal Transcriptome of Fabp2 Gene-Ablated Mice. Lifestyle Genom. 2012;5:45–55. doi: 10.1159/000337193. [DOI] [PubMed] [Google Scholar]
- 47.Burton-Freeman B., Davis P.A., Schneeman B.O. Interaction of fat availability and sex on postprandial satiety and cholecystokinin after mixed-food meals. Am. J. Clin. Nutr. 2004;80:1207–1214. doi: 10.1093/ajcn/80.5.1207. [DOI] [PubMed] [Google Scholar]
- 48.Knuth N.D., Horowitz J.F. The Elevation of Ingested Lipids within Plasma Chylomicrons Is Prolonged in Men Compared with Women. J. Nutr. 2006;136:1498–1503. doi: 10.1093/jn/136.6.1498. [DOI] [PubMed] [Google Scholar]
- 49.Klaassen C.D., Aleksunes L.M. Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacol. Rev. 2010;62:1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pédrono F., Blanchard H., Kloareg M., D’Andréa S., Daval S., Rioux V., Legrand P. The fatty acid desaturase 3 gene encodes for different FADS3 protein isoforms in mammalian tissues. J. Lipid Res. 2009;51:472–479. doi: 10.1194/jlr.M000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.France M., Skorich E., Kadrofske M., Swain G.M., Galligan J.J. Sex-related differences in small intestinal transit and serotonin dynamics in high-fat-diet-induced obesity in mice. Exp. Physiol. 2015;101:81–99. doi: 10.1113/EP085427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Priego T., Sanchez J., Picó C., Palou A. Sex-differential Expression of Metabolism-related Genes in Response to a High-fat Diet. Obesity. 2008;16:819–826. doi: 10.1038/oby.2007.117. [DOI] [PubMed] [Google Scholar]
- 53.Pelsers M.M., Namiot Z., Kisielewski W., Namiot A., Januszkiewicz M., Hermens W.T., Glatz J.F.C. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin. Biochem. 2003;36:529–535. doi: 10.1016/S0009-9120(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 54.Agellon L.B., Drozdowski L., Li L., Iordache C., Luong L., Clandinin M.T., Uwiera R.R., Toth M.J., Thomson A.B. Loss of intestinal fatty acid binding protein increases the susceptibility of male mice to high fat diet-induced fatty liver. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids. 2007;1771:1283–1288. doi: 10.1016/j.bbalip.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Martin G.G., Landrock D., Chung S., Dangott L.J., Seeger D.R., Murphy E.J., Golovko M.Y., Kier A.B., Schroeder F. Fabp1gene ablation inhibits high-fat diet-induced increase in brain endocannabinoids. J. Neurochem. 2016;140:294–306. doi: 10.1111/jnc.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Melé M., Ferreira P.G., Reverter F., DeLuca D.S., Monlong J., Sammeth M., Young T.R., Goldmann J.M., Pervouchine D.D., Sullivan T.J., et al. The human transcriptome across tissues and individuals. Science. 2015;348:660–665. doi: 10.1126/science.aaa0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gershoni M., Pietrokovski S. The landscape of sex-differential transcriptome and its consequent selection in human adults. BMC Biol. 2017;15:7. doi: 10.1186/s12915-017-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang X., Schadt E.E., Wang S., Wang H., Arnold A.P., Ingram-Drake L., Drake T.A., Lusis A.J. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naqvi S., Godfrey A.K., Hughes J.F., Goodheart M.L., Mitchell R.N., Page D.C. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science. 2019;365:eaaw7317. doi: 10.1126/science.aaw7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grath S., Parsch J. Sex-Biased Gene Expression. Annu. Rev. Genet. 2016;50:29–44. doi: 10.1146/annurev-genet-120215-035429. [DOI] [PubMed] [Google Scholar]
- 61.Rinn J.L., Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005;21:298–305. doi: 10.1016/j.tig.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Bermejo-Álvarez P., Rizos D., Lonergan P., Gutiérrez-Adán A. Transcriptional sexual dimorphism during preimplantation embryo development and its consequences for developmental competence and adult health and disease. Reproduction. 2011;141:563–570. doi: 10.1530/REP-10-0482. [DOI] [PubMed] [Google Scholar]
- 63.Jalouli M., Carlsson L., Améen C., Lindén D., Ljungberg A., Michalik L., Edén S., Wahli W., Oscarsson J. Sex Difference in Hepatic Peroxisome Proliferator-Activated Receptor α Expression: Influence of Pituitary and Gonadal Hormones. Endocrinology. 2003;144:101–109. doi: 10.1210/en.2002-220630. [DOI] [PubMed] [Google Scholar]
- 64.Selwyn F.P., Csanaky I.L., Zhang Y., Klaassen C.D. Importance of Large Intestine in Regulating Bile Acids and Glucagon-Like Peptide-1 in Germ-Free Mice. Drug Metab. Dispos. 2015;43:1544–1556. doi: 10.1124/dmd.115.065276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheng L., Jena P.K., Liu H.-X., Kalanetra K.M., Gonzalez F.J., French S.W., Krishnan V.V., Mills D.A., Wan Y.-J.Y. Gender Differences in Bile Acids and Microbiota in Relationship with Gender Dissimilarity in Steatosis Induced by Diet and FXR Inactivation. Sci. Rep. 2017;7:1748. doi: 10.1038/s41598-017-01576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y., Michalak M., Agellon L.B. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J. Biol. Med. 2018;91:95–103. [PMC free article] [PubMed] [Google Scholar]
- 67.Mowat A.M., Agace W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014;14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 68.Altmann S.W., Davis H.R., Zhu L.J., Yao X., Hoos L.M., Tetzloff G., Iyer S.P.N., Maguire M., Golovko A., Zeng M., et al. Niemann-Pick C1 Like 1 Protein Is Critical for Intestinal Cholesterol Absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.