Abstract
Recently, the stabilization of the endothelium has been explicitly identified as a therapeutic goal in coronavirus disease 2019 (COVID-19). Adrecizumab (HAM8101) is a first-in-class humanized monoclonal anti-Adrenomedullin (anti-ADM) antibody, targeting the sepsis- and inflammation-based vascular and capillary leakage. Within a “treatment on a named-patient basis” approach, Adrecizumab was administered to eight extreme-critically ill COVID-19 patients with acute respiratory distress syndrome (ARDS). The patients received a single dose of Adrecizumab, which was administered between 1 and 3 days after the initiation of mechanical ventilation. The SOFA (median 12.5) and SAPS-II (median 39) scores clearly documented the population at highest risk. Moreover, six of the patients suffered from acute renal failure, of whom five needed renal replacement therapy. The length of follow-up ranged between 13 and 27 days. Following the Adrecizumab administration, one patient in the low-dose group died at day 4 due to fulminant pulmonary embolism, while four were in stable condition, and three were discharged from the intensive care unit (ICU). Within 12 days, the SOFA score, as well as the disease severity score (range 0–16, mirroring critical resources in the ICU, with higher scores indicating more severe illness), decreased in five out of the seven surviving patients (in all high-dose patients). The PaO2/FiO2 increased within 12 days, while the inflammatory parameters C-reactive protein, procalcitonin, and interleukin-6 decreased. Importantly, the mortality was lower than expected and calculated by the SOFA score. In conclusion, in this preliminary uncontrolled case series of eight shock patients with life-threatening COVID-19 and ARDS, the administration of Adrecizumab was followed by a favorable outcome. Although the non-controlled design and the small sample size preclude any definitive statement about the potential efficacy of Adrecizumab in critically ill COVID-19 patients, the results of this case series are encouraging.
Keywords: COVID-19, Adrecizumab, HAM 8101, adrenomedullin, endothelial function
1. Introduction
Beginning in December 2019, a novel coronavirus, designated SARS-CoV-2, caused an international outbreak of a respiratory illness termed COVID-19 [1,2]. Its full spectrum ranges from a mild, self-limiting respiratory tract illness to severe progressive pneumonia, multi-organ failure, and death [3] Thus far, there are no specific therapeutic agents for coronavirus infections, and a causal therapy beyond supportive measures is not available [4]. The main reason for admission to the ICU is acute hypoxemic respiratory failure, which often requires mechanical ventilation, with a high mortality [5].
Adrenomedullin is described as a key player in the (dys-) regulation of endothelial function and vascular integrity [6]. Adrecizumab is a first-in-class humanized monoclonal anti-ADM antibody that only marginally inhibits ADM activity but enhances its half-life and thus acts as a long-lasting plasma ADM enhancer, stabilizing and maintaining the endothelial barrier function [7]. By increasing the functional plasma ADM levels, Adrecizumab is hypothesized to target the sepsis- and inflammation-based vascular and capillary leakage. The latter leads to the deterioration of severe COVID-19 to septic shock and ARDS; a recent study used electron microscopy examinations in the autopsy lung tissues of COVID-19 patients, and found the diffuse loosening of the inter-endothelial junctional complex [8]. The rationale for the use of Adrecizumab is derived from the biomarker-guided phase-2 sepsis trial AdrenOSS-2, which just announced positive top-line results [9,10]. Moreover, very recently, the stabilization of the endothelium has been explicitly identified as a therapeutic goal in COVID-19 [11]. As derived from the AdrenOSS-1 and the AdrenOSS-2 studies, the administration of Adrecizumab is conducted in a biomarker-guided way, indicated by an elevated “do-treat-if high” biomarker bio-ADM (bioactive ADM) and a low-to-normal “do-not-treat-if-high” biomarker DPP3 [9,10,12,13,14]. Bio-ADM is a biomarker for endothelial function; it shows an inverse relationship with blood pressure and subsequently a direct relationship with vasopressor demand [9]. DPP3 is an amino dipeptidase involved in the degradation process of renin-angiotensin system (RAS) peptides, especially angiotensin II [12]. The DPP3 blood levels in septic and cardiogenic shock patients at admission are associated with severe organ dysfunction, refractory shock, and high short-term mortality [12,13]. Furthermore, a reduction in the DPP3 levels within 24 h of admission was associated with improved outcomes in these patient populations, and, similar to the SARS-CoV-2 receptor ACE2, DPP3 mediates the degradation of Ang II [14,15]. Accordingly, these assumptions strengthen the hypothesis that improving endothelial function by the modification of the ADM homeostasis might improve the prognosis and outcomes in severely affected COVID-19 patients.
So far, several experimental therapies have been evaluated in the critically ill, while data on experimental therapies in extreme-critically ill patients is sparse [1]. Here, we describe the first clinical experience with Adrecizumab in extreme-critically ill patients with COVID-19.
2. Materials and Methods
2.1. Regulatory
The treatment was conducted at the Department of Intensive Care Medicine, University Medical Center Hamburg-Eppendorf in Hamburg, Germany. The clinical outcomes were compared before and after the application of Adrecizumab. The treatment on a named-patient basis approach is based on article 37 of the Declaration of Helsinki and article 41 of the German Pharmaceuticals Act [16,17]. Accordingly, while ethics committee approval and approval by regulatory bodies are not applicable for this approach, both institutions were notified, and informed consent was obtained from the patients, their relatives, or legal representatives.
2.2. Patients
Patients with laboratory-confirmed COVID-19, classified as critically ill, were assessed for Adrecizumab treatment when meeting the following criteria: (i) ARDS, (ii) PaO2/FiO2 ≤ 220 (PaO2 measured in mmHg and FiO2 measured as the fraction of inspired oxygen), (iii) mechanical ventilation, (iv) hypotension with vasopressors required to maintain a mean arterial pressure of ≥65 mmHg, (v) acute clinical deterioration, (vi) a circulating bio-ADM plasma level of ≥60 pg/mL or a ≥25% relative increase within 24 h, and (vii) circulating DPP3 plasma levels of ≤50 ng/mL.
2.3. Laboratory Diagnostics
Lactate, C-reactive protein (CRP), procalcitonin, and interleukin-6 were determined in the routine lab. SARS-CoV-2 was detected on a high-throughput platform, the Roche Cobas 6800 (Roche Diagnostics, Basel, Switzerland), using the “open channel” for the integration of a laboratory-developed assay, as recently published [18]. DPP-3 was measured using the IB10 sphingotest® DPP3, a commercial point-of-care immunoassay from SphingoTec GmbH/4TEEN4 Pharmaceuticals (Hennigsdorf, Germany). Bio-ADM was measured in plasma using a commercial immunoassay from SphingoTec GmbH (sphingotest® bio-ADM, Hennigsdorf, Germany) [9,10].
2.4. Administration of Adrecizumab
Patients received a single dose of Adrecizumab at a dose of 4 mg/kg (2 patients) or 8 mg/kg (6 patients) body weight over a 1-h period. The dose selection was based on the baseline bio-ADM levels, the clinical course within the last few hours, and acute deterioration. The dose range was between 400 mg and 1000 mg. Adrecizumab was administered between 1 and 3 days after the initiation of mechanical ventilation, separately from any concomitant drugs using a dedicated lumen of a central venous catheter or a separate peripheral line.
2.5. Disease Severity Classification
Disease severity was classified using the SOFA and SAPS-II scores. The dedicated disease severity score aims to mirror critical resources on ICU, and therefore considers the following 4 dimensions: (i) vital and hospitalization status, (ii) circulation status, (iii) ventilation status, and (iv) the PaO2/FiO2-index. The maximum score is 16, while the minimum is 0, with higher scores indicating a more severe illness.
2.6. Clinical Information
Clinical information for the 8 patients before and after the Adrecizumab administration was obtained from the hospital information system and included the following: demographic data, including anamnesis and physical examination; treatment and therapies, including mechanical ventilation and antiviral therapies; clinical data, including PaO2/FiO2, the SOFA score (range 0–24, with higher scores indicating a more severe illness), and the SAPS-II score (higher scores indicating a more severe illness); and laboratory data, including bio-ADM, DPP3, the white blood cell count, lactate, the liver and kidney function, and inflammatory factors (CRP, procalcitonin, and interleukin-6).
3. Results
The clinical characteristics of the patients are summarized in Table 1. A total of eight patients were treated with Adrecizumab—seven male patients and one female patient. The patients’ age range was between 31 and 76 years. All eight patients had pre-existing conditions, with six having type 2 diabetes, and seven suffering from hypertension. All the patients were in critical condition, with ARDS and shock. Six out of eight were suffering from renal failure, with five of them in need of renal replacement therapy, and one out of eight suffering from liver failure.
Table 1.
Clinical characteristics of the 8 treated patients.
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| Sex | Male | Male | Male | Male | Male | Male | Male | Female |
| Age | 54 | 61 | 73 | 71 | 31 | 76 | 58 | 68 |
| BMI | 29.2 | 31.9 | 26.4 | 32.7 | 41.4 | 30.9 | 27.7 | 39.1 |
| SAPS II score on admission to ICU | 32 | 55 | 37 | 43 | 20 | 64 | 40 | 35 |
| Chronic diseases |
|
|
|
|
|
|
|
|
| Pre-existing RAS-I medication | ACE-inhibitor | ACE-inhibitor | ACE-inhibitor | Angiotensin receptor blocker | None | Angiotensin receptor blocker | ACE-inhibitor | ACE-inhibitor |
| Complication prior to Adrecizumab therapy |
|
|
|
|
|
|
|
|
| Days between symptoms onset and positive testing | 2 | 5 | 14 | 4 | 4 | 2 | 1 | |
| Days between symptom onset and admission | 9 | 5 | 15 | 3 | 5 | 3 | 15 | 3 |
| Days between ICU admission and Adrecizumab therapy | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 3 |
| Most severe disease classification | Critical | Critical | Critical | Critical | Critical | Critical | Critical | Critical |
| Prior CPR | No | No | No | No | No | No | Yes | No |
| Prior treatment with antivirals | Lopinavir/ritonavir | Lopinavir/ritonavir | None | Lopinavir/ritonavir | None | None | None | None |
| Other experimental therapies | None | CytoSorb® | None | CytoSorb® | None | None | None | None |
BMI = body-mass-index; T2DM = type 2 diabetes mellitus; RAS = renin-angiotensin-system; ICU = intensive care unit; CPR = cardiopulmonary resuscitation.
Table 2 shows the dose of Adrecizumab administered and the course of the therapy-guiding biomarkers bio-ADM and DPP3. All eight patients received a single dose of Adrecizumab at a dose of 4 mg/kg (two patients) or 8 mg/kg (six patients) body weight. Dose selection was based on the baseline bio-ADM levels, the clinical course within the last few hours, and the acute deterioration. The dose range was between 400 mg and 1000 mg. The therapy was well tolerated in all patients, and no immediate adverse reactions were noted. The median bio-ADM at baseline was 77.8 (IQR 63.9:102.6) pg/mL, while the median DPP-3 was 18.7 [(QR 17.9:19.0) ng/mL. Upon the Adrecizumab administration, the bio-ADM levels strongly increased (median 383.0 (IQR 264.5:403.5) pg/mL).
Table 2.
Dose of Adrecizumab administered and course of therapy-guiding biomarkers bio-ADM and DPP-3.
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| Body weight [kg] | 100 | 100 | 75 | 100 | 125 | 98 | 100 | 100 |
| Dosing group | high-dose [8 mg/kg bw] |
high-dose [8 mg/kg bw] |
high-dose [8 mg/kg bw] |
low-dose [4 mg/kg bw] |
high-dose [8 mg/kg bw] |
low-dose [4 mg/kg bw] |
high-dose [8 mg/kg bw] |
high-dose [8 mg/kg bw] |
| Dose Adrecizumab [mg] | 800 | 800 | 600 | 400 | 1000 | 400 | 800 | 800 |
| Bio-ADM [pg/mL] before Adrecizumab |
63.9 | 64.9 | 53.9 | 53.0 | 102.6 | 191.3 | 90.7 | 170.3 |
| day 1 | 264.5 | 377.2 | 403.9 | 239.4 | 226.1 | 678.9 | 388.8 | 515.0 |
| day 2 | 244.6 | n/a | 281.5 | 312.3 | 271.1 | 383.8 | 257.6 | n/a |
| day 3 | 235.6 | 274.7 | 200.2 | 171.8 | 239.8 | 209.9 | 231.1 | 224.8 |
| day 5 | 123.5 | 194.9 | 157.7 | n/a | 246.1 | 130.3 | 167.9 | n/a |
| day 7 | 63.9 | 113.8 | 143.0 | n/a | 155.0 | 118.9 | n/a | n/a |
| day 10–2 | 42.3 | 130.6 | 53.8 | n/a | 134.0 | 170.3 | 92.0 | n/a |
| DPP-3 [ng/mL] before Adrecizumab |
6.92 | 19.3 | 17.9 | 19.0 | 19.0 | 23.5 | 18.3 | 12.5 |
| day 1 | 7.38 | 18.9 | 12.8 | 17.7 | n/a | n/a | n/a | n/a |
| day 3 | 13.22 | 7.84 | n/a | 135.7 | n/a | n/a | n/a | n/a |
| day 5–10 | 9.90 | n/a | n/a | >150 | n/a | 23.5 | n/a | n/a |
Bio-ADM = bioactive adrenomedullin; DPP-3 = dipeptidyl peptidase-3; kg = kilogram; bw = body weight.
Table 3 shows the course of clinical parameters and scores before and after the administration of Adrecizumab. Upon the administration of Adrecizumab, the PaO2/FiO2 and SOFA score improved within 12 days in five patients.
Table 3.
Comparison of clinical parameters and score before and after the Adrecizumab administration.
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| Days of follow-up | 29 | 27 | 24 | 4 | 21 | 21 | 20 | 13 |
| Current status as of 15 April 2020 | Transferred to normal ward | Alive; still receiving mechanical ventilation |
Transferred to normal ward | Deceased–81 h after intervention | Alive; still receiving mechanical ventilation |
Alive; still receiving mechanical ventilation |
Transferred to normal ward | Alive; still receiving mechanical ventilation |
| Mechanical ventilation | ||||||||
| Onset days before Adrecizumab | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 |
| Status | Extubated | De-escalation from BIPAP to intermittent CPAP | Extubated | Deceased | De-escalation from BIPAP to intermittent CPAP | De-escalation from BIPAP to intermittent CPAP | Extubated | De-escalation from BIPAP to intermittent CPAP |
| ECMO | ||||||||
| Onset before/after Adrecizumab | Not received | Same day | Not received | Not received | Same day | Not received | Same day | Not received |
| Removal, days after Adrecizumab | n/a | Still in use | n/a | n/a | 19 | n/a | 6 | n/a |
| PaO2/FiO2 | ||||||||
| before Adrecizumab | 126 | 90 | 181 | 108 | 107 | 213 | 75 | 160 |
| best value within 12 h | 244 | 127 | 224 | 122 | 133 | 215 | 113 | 241 |
| mean value day 1 | 204 | 105 | 184 | 142 | 81 | 171 | 132 | 168 |
| mean value day 2 | 186 | 96 | 161 | 116 | 87 | 162 | 163 | 166 |
| mean value day 3 | 218 | 106 | 177 | 93 | 78 | 165 | 155 | 185 |
| mean value day 5 | 216 | 122 | 190 | n/a | 171 | 181 | 192 | 196 |
| mean value day 7 | 225 | 98 | 209 | n/a | 162 | 97 | 316 | 220 |
| mean value day 10 | 259 | 78 | 226 | n/a | 168 | 134 | 348 | 236 |
| mean value day 12 | 249 | 84 | 240 | n/a | 176 | 157 | 295 | 230 |
| Noradrenaline [µg/kg/min] | ||||||||
| before Adrecizumab | 0.02 | 0.20 | 0.14 | 0.14 | 0.11 | 0.16 | 0.52 | 0.06 |
| highest dose day 1 | 0.10 | 0.96 | 0.17 | 0.40 | 0.11 | 0.30 | 0.33 | 0.07 |
| highest dose day 2 | 0.14 | 0.48 | 0.16 | 1.60 | 0.11 | 0.11 | 0.28 | 0.04 |
| highest dose day 3 | 0.12 | 0.48 | 0.15 | 4.00 | 0.13 | 0.07 | 0.09 | 0.06 |
| highest dose day 5 | 0 | 0.44 | 0.26 | n/a | 0.10 | 0.09 | 0.10 | 0.08 |
| highest dose day 7 | 0 | 0.20 | 0.16 | n/a | 0.09 | 0.20 | 0.11 | 0.02 |
| highest dose day 10 | 0.25 | 0.44 | 0 | n/a | 0.23 | 0.31 | 0 | 0 |
| highest dose day 12 | 0 | 0.40 | 0 | n/a | 0.25 | 0.37 | 0 | 0 |
| SOFA score | ||||||||
| before Adrecizumab | 12 | 14 | 12 | 12 | 16 | 12 | 13 | 14 |
| day 1 | 11 | 16 | 12 | 16 | 16 | 12 | 12 | 14 |
| day 2 | 10 | 17 | 11 | 18 | 16 | 12 | 14 | 13 |
| day 3 | 10 | 17 | 11 | n/a | 15 | 11 | 14 | 13 |
| day 5 | 10 | 18 | 10 | n/a | 14 | 13 | 11 | 12 |
| day 7 | 6 | 17 | 10 | n/a | 14 | 12 | 13 | 13 |
| day 10 | 7 | 19 | 6 | n/a | 12 | 13 | 10 | 4 |
| day 12 | 9 | 18 | 6 | n/a | 14 | 13 | 5 | 7 |
| C-reactive protein [mg/L]; Reference value < 5 mg/L | ||||||||
| before Adrecizumab | 105 | 186 | 86 | 304 | 72 | 284 | 137 | 37 |
| day 1 | 151 | 277 | 186 | 339 | 80 | 213 | 160 | 24 |
| day 2 | 203 | 286 | 279 | 334 | 42 | 239 | 136 | 47 |
| day 3 | 210 | 189 | 307 | 266 | 27 | 224 | 122 | 43 |
| day 5 | 82 | 61 | 296 | n/a | 45 | 100 | 91 | 89 |
| day 7 | 61 | 108 | 261 | n/a | 20 | 292 | 91 | 205 |
| day 10 | 27 | 213 | 97 | n/a | 22 | 286 | 56 | 150 |
| day 12 | 29 | 141 | 51 | n/a | 14 | 122 | 35 | 72 |
| Procalcitonin [µg/L]; Reference value < 0.5µg/L | ||||||||
| before Adrecizumab | 0.47 | 2.56 | n/a | 1297 | 2.29 | n/a | 2.76 | 0.15 |
| day 1 | 0.55 | 17.33 | 0.49 | n/a | n/a | 4.08 | 2.37 | 0.25 |
| day 2 | 0.49 | 14.8 | n/a | 456.8 | 1.51 | 9.86 | 1.64 | 0.32 |
| day 3 | 0.6 | 9.33 | 0.48 | 413.1 | n/a | 7.74 | 1.38 | 0.22 |
| day 5 | 0.35 | 4.96 | n/a | n/a | 0.79 | 4.02 | 0.77 | 0.25 |
| day 7 | 0.23 | 2.27 | n/a | n/a | 0.51 | 3.08 | 0.51 | 0.39 |
| day 10 | 0.1 | 1.87 | 0.23 | n/a | 0.67 | 4.21 | 0.26 | 0.36 |
| day 12 | 0.12 | 1.75 | 0.20 | n/a | 0.86 | 3.35 | 0.20 | 0.20 |
| Interleukin-6 [ng/L]; Reference value < 7 ng/L | ||||||||
| before Adrecizumab | 129 | 18,825 | n/a | 1297 | 304 | n/a | 364 | 33 |
| day 1 | 426 | 781 | 1052 | n/a | 50 | n/a | 427 | 93 |
| day 2 | 132 | 106 | n/a | 457 | 46 | n/a | 232 | 79 |
| day 3 | 179 | 37 | 421 | 413 | 41 | n/a | 173 | 101 |
| day 5 | 62 | 104 | 382 | n/a | 43 | n/a | 131 | 41 |
| day 7 | 62 | 132 | 192 | n/a | 15 | 1078 | 84 | 67 |
| day 10 | 33 | 129 | 34 | n/a | 36 | 335 | 32 | 35 |
| day 12 | 24 | 94 | 63 | n/a | 49 | 58 | 21 | 15 |
| Lactate [mmol/L]; Reference value 0.5–2.2 mmol/L | ||||||||
| before Adrecizumab | 1.1 | 3.4 | 0.9 | 1.3 | 2.3 | 3.0 | 2.2 | 0.7 |
| day 1 | 1.4 | 3.6 | 1.0 | 2.5 | 2.4 | 1.9 | 1.5 | 0.7 |
| day 2 | 1.2 | 3.0 | 1.4 | 7.0 | 1.8 | 2.1 | 1.0 | 0.6 |
| day 3 | 1.3 | 2.2 | 1.5 | 12.0 | 2.2 | 1.3 | 0.9 | 1.0 |
| day 5 | 1.4 | 1.7 | 1.3 | n/a | 1.5 | 2.0 | 1.0 | 0.8 |
| day 7 | 0.9 | 1.5 | 1.3 | n/a | 1.2 | 1.3 | 1.0 | 0.8 |
| day 10 | n/a | 1.8 | 1.2 | n/a | 1.6 | 1.6 | 1.0 | 0.4 |
| day 12 | n/a | 1.8 | 1.1 | n/a | 1.4 | 1.1 | 1.2 | 0.5 |
ECMO = extracorporeal membrane oxygenation; SOFA = sequential organ failure assessment; kg = kilogram; h = hours.
The course of the disease severity score before and after the Adrecizumab administration is shown in Table 4 and Figure 1. Upon therapy, a normalization was seen in three patients, while two others showed a marked decrease. One patient without improvement, as well as the deceased patient, were in the low-dose group. This is an important finding, since this score aims to mirror the critical resources in the ICU.
Table 4.
Comparison of the disease severity score before and after the Adrecizumab administration.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Vital and hospitalization status | ||||||||
| Before Adrecizumab | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| day 1 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| day 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| day 7 | 3 | 3 | 3 | 5 | 3 | 3 | 3 | 3 |
| day 12 | 3 | 3 | 3 | n/a | 3 | 3 | 3 | 3 |
| last day of follow-up | 1 | 3 | 1 | n/a | 3 | 3 | 1 | 3 |
| Circulation status | ||||||||
| Before Adrecizumab | 1 | 2 | 2 | 2 | 2 | 2 | 3 | 1 |
| day 1 | 1 | 3 | 2 | 2 | 2 | 2 | 2 | 1 |
| day 3 | 2 | 3 | 2 | 3 | 2 | 1 | 1 | 1 |
| day 7 | 0 | 2 | 2 | 3 | 1 | 2 | 2 | 1 |
| day 12 | 0 | 2 | 0 | n/a | 2 | 2 | 0 | 0 |
| last day of follow-up | 0 | 3 | 0 | n/a | 0 | 3 | 0 | 0 |
| Ventilation status | ||||||||
| Before Adrecizumab | 3 | 3 | 3 | 3 | 4 | 3 | 4 | 2 |
| day 1 | 3 | 4 | 2 | 3 | 4 | 3 | 4 | 2 |
| day 3 | 3 | 4 | 3 | 3 | 4 | 3 | 4 | 2 |
| day 7 | 3 | 4 | 3 | 3 | 4 | 3 | 2 | 2 |
| day 12 | 1 | 4 | 0 | n/a | 4 | 3 | 0 | 2 |
| last day of follow-up | 0 | 4 | 0 | n/a | 2 | 2 | 0 | 2 |
| Mean PaO2/FiO2 | ||||||||
| Before Adrecizumab | 2 | 3 | 2 | 2 | 2 | 1 | 3 | 2 |
| day 1 | 1 | 2 | 2 | 2 | 3 | 2 | 2 | 2 |
| day 3 | 1 | 2 | 2 | 3 | 3 | 2 | 2 | 2 |
| day 7 | 1 | 3 | 1 | 3 | 2 | 3 | 0 | 1 |
| day 12 | 1 | 3 | 1 | n/a | 2 | 2 | 1 | 1 |
| last day of follow-up | 0 | 3 | 0 | n/a | 1 | 2 | 0 | 0 |
| Total score | ||||||||
| Before Adrecizumab | 9 | 11 | 10 | 10 | 11 | 9 | 13 | 8 |
| day 1 | 8 | 12 | 9 | 10 | 12 | 10 | 11 | 8 |
| day 3 | 9 | 12 | 10 | 12 | 12 | 9 | 10 | 8 |
| day 7 | 7 | 12 | 9 | 14 | 10 | 11 | 7 | 7 |
| day 12 | 5 | 12 | 4 | n/a | 11 | 10 | 4 | 6 |
| last day of follow-up | 1 | 13 | 1 | n/a | 6 | 10 | 1 | 5 |
Scoring system: maximum 16, minimum 0, with higher scores indicating more severe illness. Vital and hospitalization status: deceased (5), on ICU ward (3), on normal ward (1), discharged (0). Circulation: VA-ECMO (4), max. noradrenaline > 0.40 µg/kg/min (3), max. noradrenaline 0.40–0.10 µg/kg/min (2), max. noradrenaline < 0.10 µg/kg/min (1), no vasopressor (0). Ventilation status: VV-ECMO (4), mechanical ventilation BIPAP (3), intermittent mechanical ventilation CPAP (2), non-invasive ventilation (1), no non-invasive ventilation (0). Mean PaO2/FiO2: ≤ 100 (3), 101–200 (2), 201–300 (1), ≥300 (0).
Figure 1.
Change in the clinical severity score during follow-up. FU = follow-up; ICU = intensive care unit; VA = veno-arterial; VV =veno-venous. Scoring system: maximum 16, minimum 0-with higher scores indicating more severe illness. Vital and hospitalization status: deceased (5), on ICU ward (3), on normal ward (1), discharged (0). Circulation: VA-ECMO (4), max. noradrenaline > 0.40 µg/kg/min (3), max. noradrenaline 0.40–0.10 µg/kg/min (2), max. noradrenaline < 0.10 µg/kg/min (1), no vasopressor (0). Ventilation status: VV-ECMO (4), mechanical ventilation BIPAP (3), intermittent mechanical ventilation CPAP (2), non-invasive ventilation (1), no ventilation at all (invasive/non-invasive) (0). Mean PaO2/FiO2: ≤100 (3), 101–200 (2), 201–300 (1), >300 (0).
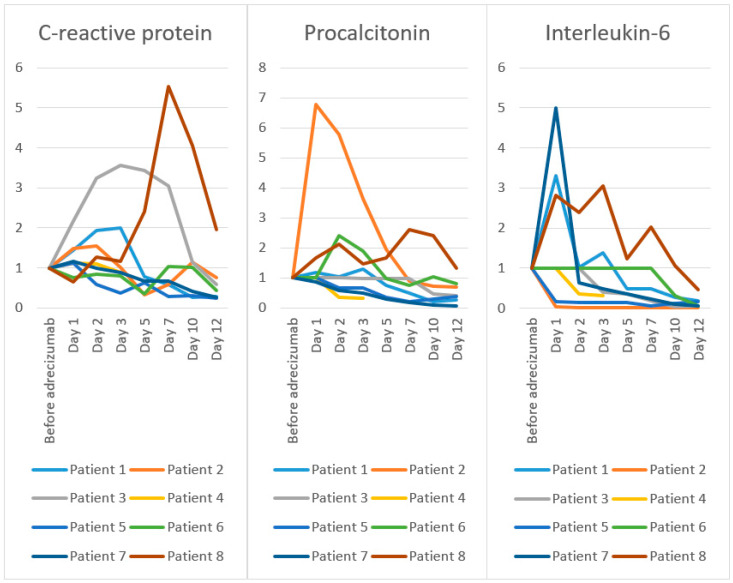
Figure 2 shows the course of the inflammatory parameters CRP, procalcitonin, and interleukin-6 normalized to the value 1.0 at baseline. While a marked decrease in the interleukin-6 levels was noted in all seven surviving patients, a relevant decrease in the CRP and procalcitonin within 12 days following the Adrecizumab administration was seen in six out of seven patients.
Figure 2.
Change in inflammatory parameters (baseline levels normalized to 1) during follow-up.
4. Discussion
4.1. Adrecizumab in COVID-19
Within this case series, eight extreme-critically ill COVID-19 patients were treated with the monoclonal antibody Adrecizumab.
All the patients were mechanically ventilated and needed vasopressors to maintain a mean arterial pressure (MAP) ≥ 65 mmHg. To our knowledge, this is the first case series evaluating an experimental therapy in extreme-critically ill COVID-19 patients. The therapy was well tolerated in all the patients, and no immediate adverse reactions were observed. Upon the Adrecizumab administration, the bio-ADM levels significantly increased, clearly documenting efficacy in all the patients. In summary, the administration of the non-neutralizing anti-ADM antibody Adrecizumab was followed by a favorable outcome. Within a short follow-up period of 13 to 27 days, four patients were in stable condition, and three were transferred to a normal ward. Moreover, an encouraging outcome was also seen with regard to the SOFA and disease severity scores, which decreased in five of the seven surviving patients. In addition, the course of PaO2/FiO2 showed a beneficial effect, and the inflammatory parameters showed a marked decrease within 12 days.
One patient in the low-dose group died at day 4 due to fulminant pulmonary embolism—presumably because of disseminated intravascular coagulation, since the repetitive clotting of the hemofiltration lines was observed previous to the patient´s death. The mortality (one in eight) was lower than was expected and calculated by the SOFA score. Other sources report higher mortality rates: (i) the ICNARC (intensive care national audit and research center) covers 5578 patients from the United Kingdom and reported a mortality rate of 67.4% in 1795 patients receiving advanced respiratory support, and a mortality rate of 80.1% in 558 patients receiving any renal support [19]. (ii) An early Chinese study reported a mortality rate of 97% (31 out of 32 patients) in those with invasive mechanical ventilation, based on a total of 191 patients [20]. (iii) Another Chinese study involved 710 COVID-19 patients, of whom 52 were admitted to an ICU and 22 eventually required mechanical ventilation. Of these, 19 (86%) died [21]. (iv) The very first data from the US-24 patients admitted to the ICU at nine Seattle-area hospitals, of whom 18 required mechanical ventilation, showed a mortality rate of 50% (12 out of 24) [22]. (v) The most recent data from the New York City area, including 5700 patients, showed a mortality of 88% in those who were mechanically ventilated [23]. At the ICU of the University Medical Center Hamburg-Eppendorf, 53 patients fulfilled similar criteria as in this case series—a diagnosis of COVID-19, mechanical ventilation, and hypotension with vasopressors required to maintain a mean arterial pressure of ≥65 mmHg. A total of 22 out of these 53 patients died during the hospital stay, corresponding to a mortality rate of 41.5%. Regarding only those patients with acute renal failure in need of dialysis (as five out of eight patients in this case series), the rate increased to 60.6%. Of interest, a steep increase in the DPP3 levels in deceased patient 4 was observed at day 3. DPP-3 was introduced as an independent prognostic marker which is not altered or influenced by Adrecizumab. Just recently, Mebazaa and coworkers have associated high DPP3 levels in critically ill patients with sepsis and cardiogenic shock with reduced cardiac output and low left ventricular function, a high SOFA and liver SOFA score, and short-term mortality [12,13,14,15]. The authors assume that DPP3-mediated impaired prognosis is an independent disease mechanism which cannot be targeted with Adrecizumab or other supportive therapies.
The dosing of the antibody was applied according to the absolute value and the dynamics of bio-ADM in longitudinal measurements. It remains unclear whether the outcomes in the two patients of the low-dose (4 mg/kg) group (including the deceased patient) would differ if they had received the regular dose. It also remains unclear whether the high prevalence of pre-existing RAS-inhibiting therapy (in seven out of eight patients) is an incidental finding.
4.2. Other Case Series in Critically Ill COVID-19 Patients
Just weeks ago, a Chinese group reported a case series using convalescent plasma in five critically ill patients with COVID-19 [1]. While their findings were promising, and we follow a similar line of analysis, a direct comparison between both case series falls short. Disease severity in this report was much more pronounced; all eight patients had extensive pre-existing conditions (see Table 1), while within the other report four out of five patients had no co-existing disease at all. This is also mirrored in the median baseline SOFA score, which was 3 in the Chinese report but 12.5 in our report. Moreover, PaO2/FiO2 was lower in our patients, and all our patients were in shock and had vasopressor demand. In addition, six of our patients had renal failure (five with renal replacement therapy) and one patient had liver failure, while these conditions were not seen prior to experimental therapy in the other report.
4.3. Adrecizumab and Shock
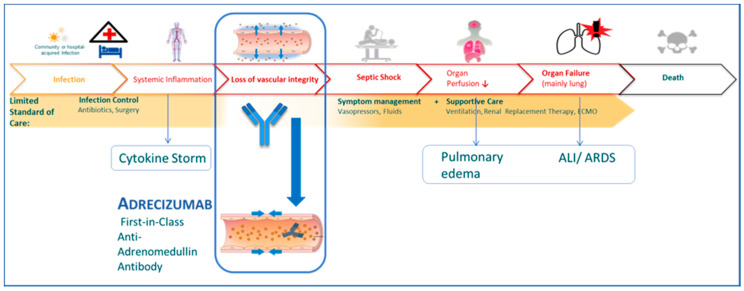
The loss of vascular integrity plays a pivotal role in the development of vascular leakage and organ dysfunction leading to septic shock [24,25,26]. Several animal studies have proven that ADM shows strong anti-inflammatory properties, improves the cardiomyocyte survival in myocardial ischemia, and has a marked anti-apoptotic effect on cardiomyocytes [27,28]. Moreover, it was demonstrated that ADM infusion attenuates ventilator-induced lung injury by reducing lung hyperpermeability, leucocyte recruitment to the alveolar space, and the deterioration of the systemic microcirculation [29]. While it was recognized that the therapeutic administration of adrenomedullin is not feasible, Adrecizumab acts as a long-lasting plasma ADM enhancer [7,30,31,32,33,34]. In preclinical studies, Adrecizumab reduced the mortality from sepsis and positively impacted the vasoactive adrenomedullin system, leading to the stabilization of blood pressure and renal function and improved catecholamine responsiveness, while the results of the phase 2 trial are still to be reported [7,9,24,25]. As idealized in Figure 3, we hypothesize that the loss of vascular integrity precedes septic shock in COVID-19, and that Adrecizumab is capable to improve endothelial function and vascular integrity in critically ill patients with COVID-19. Importantly, Adrecizumab was claimed to have a long half-life of 14 days, so the therapeutic effect may last up to 8 weeks. Nevertheless, in most of the patients the bio-ADM levels returned to the baseline values within 12 days, which might indicate a shorter duration of effect in septic shock.
Figure 3.
Hypothesis of the loss of vascular integrity preceding septic shock, and Adrecizumab improving endothelial function and vascular integrity in critically ill patients with COVID-19. COVID-19 = coronavirus disease 2019; ALI = acute lung injury; ARDS = acute respiratory distress syndrome.
4.4. Study Limitations
Our report has some limitations that need to be addressed. As a case series, it included no controls, so definite statements regarding the efficacy of Adrecizumab need to be demonstrated in a randomized trial. Furthermore, it remains unclear if the seven surviving patients would have improved without Adrecizumab, although the rapid improvement in PaO2/FiO2 in six of the eight patients and the rapid improvement in the disease severity score in seven of the eight patients, is an encouraging finding. Finally, the influence of the other experimental therapies—lopinavir/ritonavir and CytoSorb®—remain unclear. While two patients received both therapies, including the deceased patient 4, another patient received lopinavir/ritonavir alone.
5. Conclusions
In this preliminary uncontrolled case series of eight extreme-critically ill patients with COVID-19 and ARDS, the administration of the non-neutralizing anti-ADM antibody Adrecizumab was followed by a favorable outcome. Although the non-controlled design and the small sample size preclude any definitive statement about the potential efficacy of Adrecizumab in critically ill COVID-19 patients, the result of this case series is encouraging.
Acknowledgments
We thank Adrenomed AG for free provision of Adrecizumab.
Author Contributions
Conceptualization, M.K., S.K., and M.M.A.; methodology, M.K., S.K., and M.M.A.; formal analysis, D.J., M.B., and K.R.; investigation, all authors; resources, all authors.; data curation, all authors; writing—original draft preparation, M.K., D.J., S.K.; writing—review and editing, all authors; visualization, M.K.; supervision, S.K. and M.K.; funding acquisition, n/a. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financed by own means. Adrecizumab was provided by Adrenomed AG.
Conflicts of Interest
M.K. reports grant support from Adrenomed and Vifor and honoraria within the last 5 years from Adrenomed, Sphingotec, Vifor, Amgen, Bayer, 4TEEN4, Astra-Zeneca, Roche Diagnostics, and Sanofi. D.J., M.B., K.R., M.M.A., and M.L. do not report any conflicts of interest. F.H., A.B., and J.Z. are employed by Adrenomed and hold shares in Adrenomed AG. A.B. is further employed at Sphingotec and 4TEEN4 Pharmaceuticals and holds shares in both companies. TPS received lecture fees, honoraria, and travel reimbursement from Adrenomed AG., Sphingotec A.G., BBraun A.G., and Biotest A.G. G.M. received research support by BBraun, Adrenomed, and Biotest; lecture honorarium from BBraun and Philips; and consultant honorarium from Adrenomed, BBraun, and 4teen4. A.N. reports honoraria and travel reimbursement from Thermo Fisher. S.K. received research support by Ambu, E.T.View Ltd., Fisher & Paykel, Pfizer, and Xenios; lecture honorarium from ArjoHuntleigh, Astellas, Astra, Basilea, Bard, Baxter, Biotest, CSL Behring, Cytosorbents, Fresenius, Gilead, MSD, Orion, Pfizer, Philips, Sedana, Sorin, Xenios, and Zoll; and consultant honorarium from AMOMED, Astellas, Baxter, Bayer, Fresenius, Gilead, MSD, Pfizer, and Xenios. No other potential conflict of interest relevant to this article was reported.
References
- 1.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Novel Coronavirus (COVID-19) [(accessed on 20 April 2020)]; Available online: https://who.sprinklr.com/
- 3.Poston J.T., Patel B.K., Davis A.M. Management of critically ill adults with COVID-19. JAMA. 2020;323:1839–1841. doi: 10.1001/jama.2020.4914. [DOI] [PubMed] [Google Scholar]
- 4.Kluge S., Janssens U., Welte T., Weber-Carstens S., Marx G., Karagiannidis C. Recommendations for critically ill patients with COVID-19. Med. Klin. Intensivmed. Notfmed. 2020;115:175–177. doi: 10.1007/s00063-020-00674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voors A.A., Kremer D., Geven C., Ter Maaten J.M., Struck J., Bergmann A., Pickkers P., Metra M., Mebazaa A., Düngen H.D., et al. Adrenomedullin in heart failure: Pathophysiology and therapeutic application. Eur. J. Heart Fail. 2019;21:163–171. doi: 10.1002/ejhf.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blet A., Deniau B., Geven C., Sadoune M., Caillard A., Kounde P.R., Polidano E., Pickkers P., Samuel J.L., Mebazaa A. Adrecizumab, a non-neutralizing anti-adrenomedullin antibody, improves haemodynamics and attenuates myocardial oxidative stress in septic rats. Intensive Care Med. Exp. 2019;7:25. doi: 10.1186/s40635-019-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu A.M., Fossali T., Pandolfi L., Carsana L., Ottolina D., Frangipane V., Rech R., Tosoni A., Agarossi A., Cogliati C., et al. COVID-19: The key role of pulmonary capillary leakage. An observational cohort study. medRxiv. 2020 doi: 10.1101/2020.05.17.20104877. [DOI] [PubMed] [Google Scholar]
- 9.Geven C., Blet A., Kox M., Hartmann O., Scigalla P., Zimmermann J., Marx G., Laterre P.F., Mebazaa A., Pickkers P. A double-blind, placebo-controlled, randomised, multicentre, proof-of-concept and dose-finding phase II clinical trial to investigate the safety, tolerability and efficacy of adrecizumab in patients with septic shock and elevated adrenomedullin concentration (AdrenOSS-2) BMJ Open. 2019;9:e024475. doi: 10.1136/bmjopen-2018-024475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adrenomed Announces Positive Top-Line AdrenOSS-2 Phase II Results with Adrecizumab in Septic Shock. [(accessed on 21 April 2020)]; Available online: https://adrenomed.com/adrenomed-announces-positive-top-line-adrenoss-2-phase-ii-results-with-adrecizumab-in-septic-shock/
- 11.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deniau B., Rehfeld L., Santos K., Dienelt A., Azibani F., Sadoune M., Kounde P.R., Samuel J.L., Tolpannen H., Lassus J., et al. Circulating dipeptidyl peptidase 3 is a myocardial depressant factor: Dipeptidyl peptidase 3 inhibition rapidly and sustainably improves haemodynamics. Eur. J. Heart Fail. 2020;22:290–299. doi: 10.1002/ejhf.1601. [DOI] [PubMed] [Google Scholar]
- 13.Takagi K., Blet A., Levy B., Deniau B., Azibani F., Feliot E., Bergmann A., Santos K., Hartmann O., Gayat E., et al. Circulating dipeptidyl peptidase 3 and alteration in haemodynamics in cardiogenic shock: Results from the OptimaCC trial. Eur. J. Heart Fail. 2020;22:279–286. doi: 10.1002/ejhf.1600. [DOI] [PubMed] [Google Scholar]
- 14.Magliocca A., Omland T., Latini R. Dipeptidyl peptidase 3, a biomarker in cardiogenic shock and hopefully much more. Eur. J. Heart Fail. 2020;22:300–302. doi: 10.1002/ejhf.1649. [DOI] [PubMed] [Google Scholar]
- 15.Ocaranza M.P., Jalil J.E. On Endogenous Angiotensin II Antagonism in Hypertension: The Role of Dipeptidyl Peptidase III. Hypertension. 2016;68:552–554. doi: 10.1161/HYPERTENSIONAHA.116.07471. [DOI] [PubMed] [Google Scholar]
- 16.World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 17.Nickel L., Seibel Y., Frech M., Sudhop T. Changes in the German Medicinal Product Act imposed by the EU regulation on clinical trials. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2017;60:804–811. doi: 10.1007/s00103-017-2574-1. [DOI] [PubMed] [Google Scholar]
- 18.Pfefferle S., Reucher S., Nörz D., Lütgehetmann M. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. Eur. Surveill. 2020;25:2000152. doi: 10.2807/1560-7917.ES.2020.25.9.2000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Intensive Care National Audit & Research Centre [(accessed on 21 April 2020)]; Available online: https://www.icnarc.org/
- 20.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., Greninger A.L., Pipavath S., Wurfel M.M., Evans L., et al. Covid-19 in critically ill patients in the Seattle region—Case series. N. Engl. J. Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., The Northwell COVID-19 Research Consortium. Barnaby D.P., Becker L.B., Chelico J.D., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geven C., van Lier D., Blet A., Peelen R., Elzen B.T., Mebazaa A., Kox M., Pickkers P. Safety, tolerability and pharmacokinetics/pharmacodynamics of the adrenomedullin antibody adrecizumab in a first-in-human study and during experimental human endotoxaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018;84:2129–2141. doi: 10.1111/bcp.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geven C., Bergmann A., Kox M., Pickkers P. Vascular effects of adrenomedullin and the anti-adrenomedullin antibody adrecizumab in sepsis. Shock. 2018;50:132–140. doi: 10.1097/SHK.0000000000001103. [DOI] [PubMed] [Google Scholar]
- 26.Tolppanen H., Rivas-Lasarte M., Lassus J., Sans-Roselló J., Hartmann O., Lindholm M., Arrigo M., Tarvasmäki T., Köber L., Thiele H., et al. Adrenomedullin: A marker of impaired hemodynamics, organ dysfunction, and poor prognosis in cardiogenic shock. Ann. Intensive Care. 2017;7:6. doi: 10.1186/s13613-016-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trincot C.E., Xu W., Zhang H., Kulikauskas M.R., Caranasos T.G., Jensen B.C., Sabine A., Petrova T.V., Caron K.M. Adrenomedullin induces cardiac lymphangiogenesis after myocardial infarction and regulates cardiac edema via connexin 43. Circ. Res. 2019;124:101–113. doi: 10.1161/CIRCRESAHA.118.313835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinrichs S., Scherschel K., Krüger S., Neumann J.T., Schwarzl M., Yan I., Warnke S., Ojeda F.M., Zeller T., Karakas M., et al. Precursor proadrenomedullin influences cardiomyocyte survival and local inflammation related to myocardial infarction. Proc. Natl. Acad. Sci. USA. 2018;115:E8727–E8736. doi: 10.1073/pnas.1721635115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller H.C., Witzenrath M., Tschernig T., Gutbier B., Hippenstiel S., Santel A., Suttorp N., Rosseau S. Adrenomedullin attenuates ventilator-induced lung injury in mice. Thorax. 2010;65:1077–1084. doi: 10.1136/thx.2010.135996. [DOI] [PubMed] [Google Scholar]
- 30.Nagaya N., Goto Y., Satoh T., Sumida H., Kojima S., Miyatake K., Kangawa K. Intravenous adrenomedullin in myocardial function and energy metabolism in patients after myocardial infarction. J. Cardiovasc. Pharmacol. 2002;39:754–760. doi: 10.1097/00005344-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura R., Kato J., Kitamura K., Onitsuka H., Imamura T., Marutsuka K., Asada Y., Kangawa K., Eto T. Beneficial effects of adrenomedullin on left ventricular remodeling after myocardial infarction in rats. Cardiovasc. Res. 2002;56:373–380. doi: 10.1016/S0008-6363(02)00594-1. [DOI] [PubMed] [Google Scholar]
- 32.Okumura H., Nagaya N., Itoh T., Okano I., Hino J., Mori K., Tsukamoto Y., Ishibashi-Ueda H., Miwa S., Tambara K., et al. Adrenomedullin infusion attenuates myocardial ischemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway. Circulation. 2004;109:242–248. doi: 10.1161/01.CIR.0000109214.30211.7C. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura R., Kato J., Kitamura K., Onitsuka H., Imamura T., Cao Y., Marutsuka K., Asada Y., Kangawa K., Eto T. Adrenomedullin administration immediately after myocardial infarction ameliorates progression of heart failure in rats. Circulation. 2004;110:426–431. doi: 10.1161/01.CIR.0000136085.34185.83. [DOI] [PubMed] [Google Scholar]
- 34.Hamid S.A., Baxter G.F. A critical cytoprotective role of endogenous adrenomedullin in acute myocardial infarction. J. Mol. Cell. Cardiol. 2006;41:360–363. doi: 10.1016/j.yjmcc.2006.05.017. [DOI] [PubMed] [Google Scholar]