Abstract
This study examined the effect of Fe3O4 nanoparticles on boar semen. Beltsville thawing solution without antibiotics was used to extend ejaculates from 5 boars (4 ejaculates/boar). Semen samples of control group (C) and group with Fe3O4 (Fe; 0.192 mg/mL semen) were incubated under routine boar semen storage temperature (17 °C) for 0.5 h and nanoparticles were removed by a magnetic field. Before and after treatment, aliquots of all groups were cultured using standard microbiological methods. The samples after treatment were stored (17 °C) for 48 h and sperm parameters (computer-assisted sperm analyzer (CASA) variables; morphology; viability; hypo-osmotic swelling test (HOST); DNA integrity) were evaluated at storage times 0, 24, 48 h. Semen data were analyzed by a repeated measures mixed model and microbial data with Student’s t-test for paired samples. Regarding CASA parameters, Fe group did not differ from C at any time point. In group C, total motility after 24 h and progressive motility after 48 h of storage decreased significantly compared to 0 h. In group Fe, linearity (LIN) after 48 h and head abnormalities after 24 h of storage increased significantly compared to 0 h. The microbiological results revealed a significant reduction of the bacterial load in group Fe compared to control at both 24 and 48 h. In conclusion, the use of Fe3O4 nanoparticles during semen processing provided a slight anti-microbiological effect with no adverse effects on sperm characteristics.
Keywords: bacterial resistance, boar, microbiological analyses, nanoparticles, semen
1. Introduction
The performance of artificial insemination (AI) is the most acknowledged method worldwide to fertilize sows with liquid-extended boar semen. Bacterial contamination affects semen’s qualitative characteristics and fertilizing capacity and induces a potential health risk to the females after AI. Previous studies reported that bacteriospermia results in higher estrus returns, early embryo death, endometritis and specific infections in pigs [1,2,3]. Porcine semen usually contains two to three species of bacteria, most commonly Staphylococci, Streptococci and Pseudomonas [4]. In this aspect, the antibiotics have been main constituents of semen extenders to control the bacterial growth during the storage time of boar liquid semen. However, Althouse and Lu [5] found bacterial occurrence in one third of the produced boar sperm doses, with bacteria largely resistant to antibiotics such as amoxycillin, gentamycin, lincomycin and tylosin. This fact has led the scientific community to explore alternative strategies to minimize the development of antibiotic resistance. Thus, methods of physically removing bacteria by centrifugation in colloidal solutions [6] and the addition of natural or synthetic peptides with antimicrobial activity to the diluents have been reported in boar semen processing [7,8]. Nanoparticles (NPs) of size 40–60 nm, expressed significant antimicrobial capacity against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus [9]. Li et al. [10], investigating the mechanism of action of Ag NPs, found that 10 μg Ag NPs/mL can completely restrain the growth of 107 cfu/mL of E. coli cells, by damaging cell membrane structure, limiting the activity of enzymes and inducing bacterial death. The results of the study of Shahverdi et al. [11], where silver NPs significantly increased the antimicrobial activity of vancomycin, amoxycillin and penicillin against S. aureus are remarkable. Moreover, iron oxide magnetic NPs provided significant antibacterial properties against p. aeruginosa and S. aureus, due to the reactive oxygen species (ROS) generation [12,13]. Although the scientific community continuously seeks alternative approaches to the use of antibiotics in semen processing, some studies have reported toxic effects of NPs on live cells. Sahu et al. [14] found that nanotoxicity could be dependent on the type of the cell in terms of a different sensitivity response. For this reason, our research team was the first to investigate the appropriate effective antibacterial concentration and co-incubation time of silver Ag/Fe and Fe3O4 NPs in semen [15]. In this study, Ag/Fe NPs demonstrated a detrimental effect on boar spermatozoa. Conversely, Fe3O4 NPs at a minimum inhibitory concentration (0.192 mg/mL semen) had no negative effect on computer-assisted sperm analyzer (CASA) motility parameters of boar sperm after 30 min of co-incubation [15]. Therefore, further research on their application for semen handling is necessary. The aim of this research was to extend our knowledge regarding an alternative methodology in order to control the microbial load of boar semen without having detrimental effects on its quality, using iron oxide NPs.
2. Materials and Methods
The semen samples used in the present study were commercially available. No operations on research animals were carried out and no approval by the Ethics Committee on Animal Use of Aristotle University of Thessaloniki (Greece) was necessary.
2.1. Reagents and Media
All the reagents and chemicals used were purchased from Sigma Aldrich, Seelze, Germany, unless otherwise stated. Semen samples were extended with laboratory-produced Beltsville thawing solution (BTS: 205 mM glucose, 20.4 mM 112 sodium citrate, 10.0 mM KCl, 15.0 mM NaHCO3, 3.6 mM ethylenediaminetetraacetic acid (EDTA); pH 7.2–7.4; 290–300 mOsmol/kg) without antibiotics.
2.2. Synthesis and Dispersions of Fe3O4 NPs
2.2.1. Synthesis
Magnetite nanoparticles were synthesized by the oxidative precipitation of FeSO4 in an ethanol/water mixture and NaNO3 and NaOH were added as mild oxidant and acidity controller, respectively [16,17]. A solution of 1 M FeSO4·7H2O was prepared by the reagent’s dissolution in 350 mL of 0.01 M H2SO4 solution. Another solution with dissolved NaNO3 (0.25 M) and NaOH (0.52 M) in a 30% ethanol mixture in distilled water was prepared to a total volume of 1400 mL. The two solutions were mixed under intense stirring to form green rust. After agitating the mixture for 15 min, it was transferred to a water bath regulated at 90 °C and allowed for 6 h to ageing of green rust and to the formation of magnetite nanoparticles. When cooled to room temperature, the dispersion was washed/centrifuged several times with distilled water to remove any residuals. The Fe3O4 NPs were sterilized before co-incubation with semen by heating in an autoclave (steam sterilization, 121–124 °C, 3 atm, 20 min).
2.2.2. Characterization
The identification of the structural phases appearing in the nanoparticles was carried out by powder X-ray diffractometry (XRD) using an Ultima+ diffractometer, Rigaku, Sendagaya, Japan, operating with CuKα radiation at 40 kV/30 mA, 0.05° for step size and 3 s as step time. The diffraction diagrams were evaluated after comparison with the Powder Diffraction Files (PDF) database [18]. Scanning electron microscopy (SEM) images were taken by a Quanta 200 ESEM FEG instrument (FEI, Hillsboro, OR, USA) with a field-emission gun adjusted to 30 kV. Magnetic measurements of the nanoparticles were performed in a MPMS XL SQUID magnetometer (Quantum Design, San Diego, CA, USA) at room temperature.
To determine the percentage of bivalent iron in the nanoparticles (Fe2+/Fe3+ ratio) as an indicator of Fe3O4 formation, the dried sample (0.1 g) was digested in 50 mL 7 M H2SO4 under heating and then, titrated by a 0.05 M KMnO4 solution. The appearance of pink color signified the complete reduction of MnO4− ions and the end of the titration. The sum of iron in the sample was defined by graphite furnace atomic absorption spectrophotometry (Perkin Elmer AAnalyst 800, Perkin Elmer, Waltham, MA, USA) after dissolving a weighted quantity in HCl.
2.3. Animals, Semen Samples Collection and Dilution
The semen samples were collected from 5 crossbred boars (2–2.5 years of age) from a commercial pig farm with capacity of 700 sows. In total, 20 ejaculates (4 ejaculates/boar) were collected by the gloved hand technique and the gelatinous portion was discarded using a gauze. Two ejaculates were collected on a weekly basis, pooled, and transported in an isothermal vessel (37 °C) to the farm laboratory. The ejaculates were assessed for the basic quality parameters [volume, concentration (SDM1, Minitube®, Tiefenbach, Germany) and motility (subjective microscopic evaluation by a phase contrast microscope, Zeiss, Oberkochen, Germany)], and those with volume >200 mL, concentration >200 × 106 sperm/mL, total number of spermatozoa/ejaculate >40 × 109, and gross motility >70% were further processed.
Semen samples of good quality were extended in BTS without antibiotics (30 × 106 spermatozoa/mL) and re-evaluated microscopically for motility. Extended pooled semen samples with motility >70% were transported (17 °C) within 60 min into a portable semen storage unit (Minitube®, Tiefenbach, Germany) to the Unit of Biotechnology of Reproduction, Clinic of Farm Animals, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki.
2.4. Experimental Design
2.4.1. Semen Processing with Nanoparticles (NPs)
Upon arrival at the Unit of Biotechnology of Reproduction, each semen sample was separated in 2 aliquots and the following two experimental groups were prepared: (1) control group (C): extended semen without any treatment; (2) iron oxide group (Fe): extended semen with Fe3O4 NPs (0.192 mg Fe3O4/mL semen).
2.4.2. Trial 1: Determination of Non-Detrimental Co-Incubation Time of Semen with NPs
The beneficial/detrimental co-incubation period and the appropriate antibacterial concentration of iron oxide NPs for boar semen handling, was evaluated in a previous trial of our laboratory [15]. Briefly, semen samples were cultured for the detection of bacterial pathogens. Plates containing sheep blood agar and plate count agar (PCA, Oxoid, Thermo Scientific, Lenexa, KS, USA) were inoculated with 100 μL aliquot of extended semen without antibiotics and incubated at 37 °C to estimate the microbial load and select strains for the antibacterial assay. Isolation and identification of picked colonies from blood agar was performed after incubation in 10 mL of tryptone soya broth (TS broth, Oxoid, Thermo Scientific, Lenexa, KS, USA), (37 °C for 24 h) and the conduction of conventional laboratory procedures. Pseudomonas, Staphylococcus and Streptococcus strains were finally selected for further investigation of the antimicrobial activity of nanoparticles. The selected strains were tested against several concentrations of the examined iron oxide NPs using a standard two-fold broth dilution method [19]. Dilutions of the Fe3O4 NPs were prepared and dispensed in tubes. Each tube was inoculated with the relevant microbial inoculum (adjusted to 0.5 McFarland scale) of each of the selected strains. Tubes were incubated at 37 °C aerobically and 100 μL were spread on blood agar at time 0, 30 min, 60 min and 24 h of incubation. The results demonstrated that the concentration of iron oxide NPs that prevented the growth of the selected bacteria was different for each strain. The minimum inhibitory concentration (MIC) of NPs for the bacterial growth was defined as the lowest concentration of NPs, which inhibited bacterial growth.
Concentrations of iron oxide NPs were re-assessed and finally selected so as not to be harmful for semen samples. Considering the MIC (0.192 mg/mL semen) of the examined NPs after in vitro antimicrobial activity assessment, they were dissolved in distilled water to prepare a stock solution of 19.2 mg Fe3O4 NPs/mL distilled water. A fresh stock solution of NPs was prepared every week. Prior to its use, the NPs solution was sonicated for 20 min to improve its dispersion stability. The C and NPs groups were incubated at 17 °C (appropriate storage temperature of extended boar semen) for 30, 45 and 60 min and afterwards the NPs were removed with a magnetic field (as it is described in Trial 2). Total motility and progressive movement spermatozoa were assessed by the CASA. The incubation period of 45 and 60 min were excluded as the values of the examined CASA parameters were significantly decreased in the NPs groups compared to the control group. The co-incubation period of 30 min had no adverse effects on the evaluated CASA parameters and was selected for further research.
2.4.3. Trial 2: Investigation of the Effect of Iron Oxide (Fe3O4) NPs on Boar Semen Quality
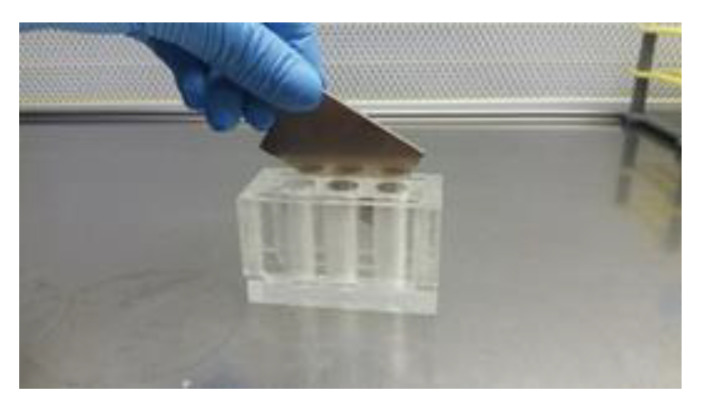
The C and Fe groups were incubated (17 °C) for 30 min after iron oxide NPs addition to group Fe. Subsequently NPs were removed with the help of a magnetic field. To achieve that, the tubes were placed in a plexiglass acrylic rack equipped with commercial NdFeB permanent magnets, remained in vertical position for at least 5 min and the post-treated semen was transferred to a new tube, while the NPs were discarded (Figure 1). Three repetitions of this process were applied to completely remove the NPs. Then the control and the post-treatment NPs samples were stored at 17 °C for 48 h. Semen quality and functionality as well as the microbiological tests were performed at 0 (time of NPs removal), 24 and 48 h post treatment.
Figure 1.
Plexiglass acrylic rack with 6 Eppendorf tubes’ places, equipped with commercial NdFeB permanent magnets.
2.5. Sperm Kinetics/Motility
The Sperm Class Analyser (SCA®, Microptic S.L., Barcelona, Spain) CASA system, a microscope (AXIO Scope A1, Zeiss, Oberkochen, Germany) with a heating stage (37 °C) and a camera (Basler scA780 54fc, Ahrensburg, Germany) were used for the evaluation of sperm motility and kinetics. The analysis by SCA® software (v.6.3.) was performed with the following configurations: 4–6 fields were recorded (×100) for each sample, >500 spermatozoa, 25 frames/s, region of particle control 10–18 microns, progressive movement of >45% of the parameter straightness (STR), circumferential movement <50% linearity (LIN), depth of field 10, and temperature of the microscope plate 37 °C. The debris incorrectly classified as spermatozoa were manually deleted.
A semen sample volume of 10 μL was placed on Makler counting chamber (Makler®, 10 μm, Sefi Medical Instruments, Haifa, Israel), which was preheated at 37 °C, and the following parameters were evaluated: (1) total and progressive motility (%), (2) spermatozoa with slow/medium/rapid movement (10 < slow < 25 < medium < 45 < rapid μm/s; %), (3) straight line velocity (VSL; μm/s), (4) curvilinear velocity (VCL; μm/s), (5) average path velocity (VAP; μm/s), (6) linearity (LIN; VSL/VCL × 100), (7) straightness (STR; VSL/VAP × 100), (8) wobble (WOB; VAP/VCL × 100), (9) amplitude of lateral head displacement (ALH; μm), (10) beat cross-frequency (BCF; Hz), and (11) hyperactivation (LIN < 0.32, VSL > 97 μm/s, ALH > 3.5 μm; %).
2.6. Sperm Viability
Sperm viability was evaluated applying the eosin-nigrosine staining protocol in one step [20]. Two hundred spermatozoa were estimated in of an optical microscope (×1000; Zeiss, Oberkochen, Germany), to calculate the ratio (%) of live-dead cells.
2.7. Sperm Morphology
According to the manufacturer’s instructions, the SpermBlue staining method (SpermBlue®, Microptic S.L., Barcelona, Spain) was applied for the assessment of sperm morphology. For each semen sample, 200 spermatozoa were counted microscopically (×400; Zeiss, Oberkochen, Germany) and the results were described as percentage of spermatozoa with normal morphology or with morphological abnormalities including head and integrity of acrosome membrane, midpiece, tail, and cytoplasmic droplets.
2.8. Sperm Membrane Functionality
For the sperm membranes functionality, the hypo-osmotic swelling test (HOST) was applied according to Vazquez et al. [21] after modification. The HOST solution was prepared (75 mmol/L fructose; 32 mmol/L sodium citrate) and the osmolarity was adjusted to 150 mosm/kg (OSMOMAT® 030, Gonotec, Berlin, Germany). Briefly, for each group, a semen sample of 100 μL was mixed with 1 mL of HOST solution and incubated (37 °C) for 1 h. Finally, 200 spermatozoa per sample were evaluated microscopically (×400; Zeiss, Oberkochen, Germany) and the results were indicated as spermatozoa with functional membrane that is with swollen tails (%).
2.9. Sperm DNA Integrity
Sperm DNA integrity was assessed by the acridine orange test [22], which quantifies the metachromatic shift of acridine orange fluorescence [23]. Specifically, spermatozoa with compact chromatin structure fluoresced green, while those with damaged chromatin integrity fluoresced red. For each semen sample, 200 spermatozoa were counted under a fluorescence microscope (×1000; Zeiss, Oberkochen, Germany) and the results were expressed as spermatozoa with damaged DNA (%).
2.10. Microbiological Analysis
Samples from C and Fe experimental groups were subjected to microbiological analysis for bacterial counts and culture using standard protocols. Preparations of all culture media were made according to the manufacturer’s recommendations. Samples were diluted up to 10−6 in 0.9% normal saline and 100 μL of each dilution were spread into plate count agar (OXOID) and incubated at 37 °C. The plates were read after 24 and 48 h and the number of colonies formed was reported as colony forming units per mL (cfu/mL). For the detection of frequently isolated bacteria in semen such as Staphylococcus spp., Streptococcus spp., Enterobacter spp., Bacillus spp., Proteus spp., Escherichia coli, Pseudomonas aeruginosa [4], 100 μL of each dilution was spread on sheep blood agar (OXOID), MacConkey agar (OXOID), Baird Parker medium with egg yolk tellurite emulsion (OXOID), kanamycin aesculin azide agar (OXOID) and incubated at 37 °C. Pseudomonas agar base containing CN selective supplement (OXOID) plates were incubated at 25 °C. Growth of bacterial colonies on plates was monitored and recorded after 24 and 48 h of incubation. Bacterial isolates were then identified using standard microbiological procedures, considering production of haemolysin, culture and colonial characteristics, Gram staining, oxidase- and catalase- reaction, coagulase testing and other conventional biochemical tests when needed.
2.11. Statistical Analysis
Statistical Analysis Systems version 9.3 (SAS Institute Inc., 1996, Cary, NC, USA) was used for the performance of the statistical analysis. The Shapiro-Wilk Test (PROC UNIVARIATE) was applied to test the normality of the data. The parameters Head, Midpiece, Tail abnormalities and Cytoplasmic droplets did not follow a normal distribution and were normalized by square root transformation. For reasons of clarity, the means and SEM of the not transformed data are presented. To conduct the statistical analysis a repeated measures mixed model (PROC MIXED) was applied. The model included group, time and their interaction as fixed effects and boar as a random effect. Semen sample was defined as the subject of the repeated observations. Covariance structure was chosen based on the values of the Akaike information criterion (AIC). Six models were run with different structures (variance components, compound symmetry, unstructured, first-order autoregressive, first-order ante dependence and Toeplitz) and the model with the least AIC was chosen. Pairwise comparisons where performed with the PDIFF command incorporating the Tukey adjustment. Regarding microbiological data-paired differences between the control and Fe group were calculated for every variable and time point by extracting the values of group Fe from control. The normality of the differences was tested using the Shapiro–Wilk test and normality was evident in all cases. A paired t-test was applied to examine the null hypothesis that the true mean was zero. Statistically significant difference was defined as p < 0.05.
3. Results
3.1. Nanoparticles’ Characteristics
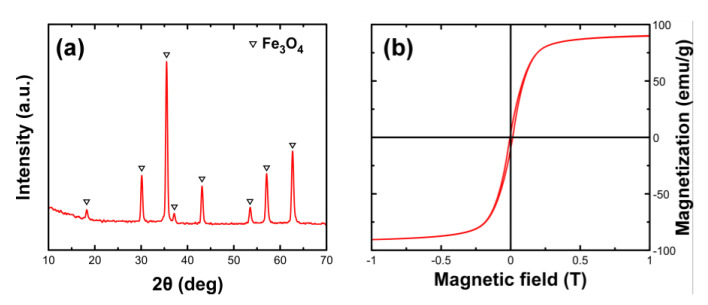
The obtained nanoparticles following the described methodology were identified to be iron oxides with inverse spinel structure according to the XRD diagram (Figure 2). However, the saturation magnetization value which approaches 90 emu/g, stands very close to the expected value for magnetite, indicating Fe3O4 as the dominant phase (Figure 2b). In addition, chemical analysis and specifically the determination of Fe2+/Fe3+ ratio provides further evidence of the Fe3O4 presence, which was roughly 43% with the ideal case of Fe3O4 stoichiometry being 50%. SEM imaging was used to find the geometrical characteristics of the sample (Figure 2a). Nanoparticles appear to show a narrow size distribution with the average diameter of the observed spheres estimated around 42 nm.
Figure 2.
X-ray diffraction (XRD) diagram of the synthesized Fe3O4 nanoparticles (a) and corresponding magnetic hysteresis loop at room temperature (b).
3.2. Efficiency of the Experimental Process
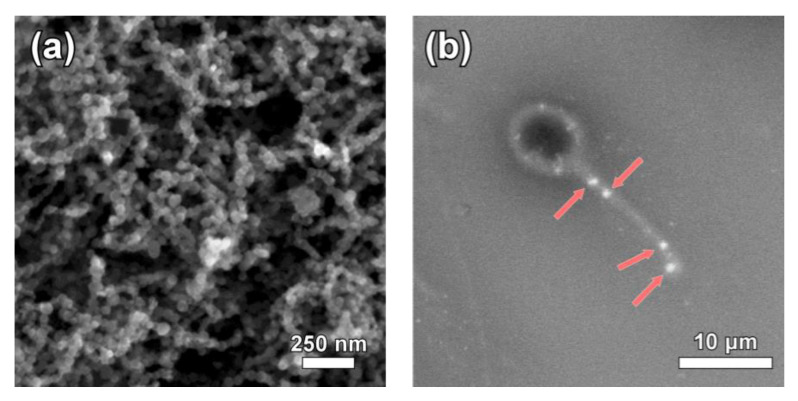
The efficiency of incubation procedure is illustrated by the attachment of magnetic nanoparticles onto the semen. Figure 3b indicates a representative case were nanoparticles aggregates were located in the tail of an isolated spermatozoon.
Figure 3.
Scanning electron microscope (SEM) image of the synthesized Fe3O4 nanoparticles (a) and representative picture of nanoparticles attachment on a spermatozoon after incubation (b).
3.3. Semen Variables’ Assessment
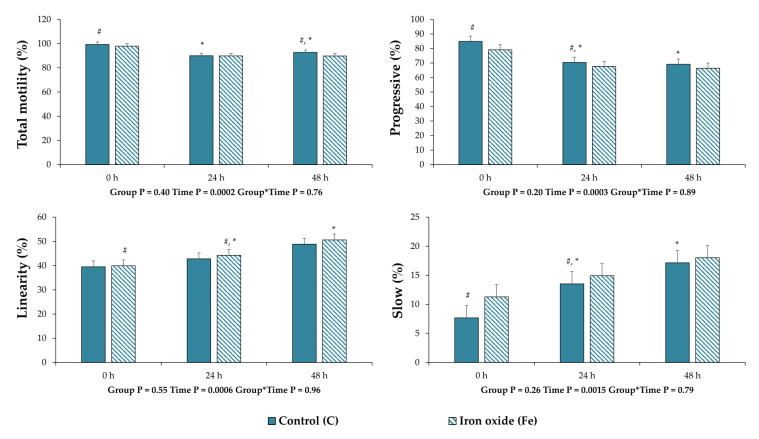
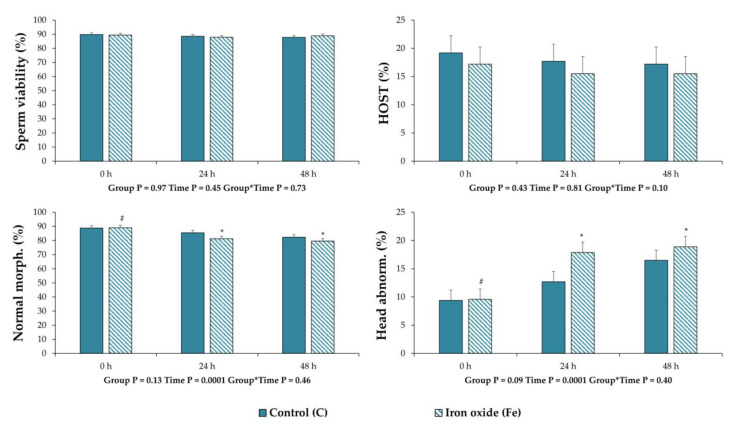
No differences (p > 0.05) were observed between the experimental groups in the mean values for all the variables that were assessed in this experiment (Figure 4 and Figure 5, Table 1). However, regarding CASA motility and kinetic parameters, the percentage of total motility (p = 0.03) and progressive movement spermatozoa (p = 0.03) were less after 24 and 48 h of storage post treatment (0 h) in group C, respectively (Figure 4). For the same group, the percentage of slow movement spermatozoa increased (p = 0.03) after 48 h of storage compared to 0 h (Figure 4). In the Fe group, only the LIN decreased (p = 0.03) after 48 h of storage compared to 0 h (Figure 4). For the remaining sperm quality and function variables (Table 1 and Figure 5), there were statistical differences only for sperm morphology. Specifically, in the Fe group, the values of spermatozoa with normal morphology decreased (p = 0.0001) along the storage period (Figure 5). Regarding the statistical analysis for each category of morphological abnormalities, it was revealed that the deterioration of sperm morphology corresponds only to an increase (p = 0.0001) of spermatozoa with head abnormalities in terms of acrosome reacted membrane (Figure 5). Finally, regarding all samples the percentage of DNA fragmentation was 0–1% and no differences were observed between treatments.
Figure 4.
Sperm motility and kinematic parameters of extended boar semen samples at 0, 24 and 48 h of storage post treatment with Fe3O4 nanoparticles (NPs). Control group (C): extended boar semen samples without any treatment; Iron oxide group (Fe): extended semen with Fe3O4 NPs (0.192 mg Fe3O4/mL semen). All the values are expressed as mean ± standard error of the mean (SEM). Different symbols (#, *) denote significant differences between evaluation times within each experimental group.
Figure 5.
Sperm quality and function variables of extended boar semen samples at 0, 24 and 48 h of storage post treatment with Fe3O4 nanoparticles (NPs). Control group (C): extended boar semen samples without any treatment; Iron oxide group (Fe): extended semen with Fe3O4 NPs (0.192 mg Fe3O4/mL semen). All the values are expressed as mean ± standard error of the mean (SEM). Different symbols (#, *) denote significant differences between evaluation times within each experimental group.
Table 1.
Computer-assisted sperm analyzer (CASA) kinematic parameters (mean ± SEM) of extended boar semen samples at 0, 24 h and 48 h of storage post treatment with Fe3O4 NPs.
| Variable | Group C | Group Fe | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 0 h | 24 h | 48 h | Group | Time | G*T | |
| Rapid (%) | 68.83 ± 4.68 | 57.95 ± 4.68 | 52.17 ± 4.68 | 64.57 ± 4.68 | 51.26 ± 4.68 | 50.19 ± 4.68 | 0.26 | 0.0042 | 0.88 |
| Medium (%) | 22.85 ± 2.01 | 18.60 ± 2.01 | 23.44 ± 2.01 | 22.36 ± 2.01 | 18.72 ± 2.01 | 21.60 ± 2.01 | 0.65 | 0.09 | 0.88 |
| VCL (μm/sec) | 64.65 ± 3.14 | 62.66 ± 3.14 | 55.62 ± 3.14 | 61.57 ± 3.14 | 58.14 ± 3.14 | 55.51 ± 3.14 | 0.32 | 0.06 | 0.77 |
| VSL (μm/sec) | 25.02 ± 2.52 | 28.39 ± 2.52 | 30.39 ± 2.52 | 24.04 ± 2.52 | 27.07 ± 2.52 | 29.66 ± 2.52 | 0.63 | 0.10 | 0.99 |
| VAP (μm/sec) | 47.48 ± 3.09 | 43.32 ± 3.09 | 36.72 ± 3.09 | 46.42 ± 3.09 | 41.82 ± 3.09 | 37.78 ± 3.09 | 0.84 | 0.0107 | 0.91 |
| ALH (μm) | 2.75 ± 0.16 | 2.52 ± 0.16 | 2.29 ± 0.16 | 2.56 ± 0.16 | 2.30 ± 0.16 | 2.28 ± 0.16 | 0.29 | 0.07 | 0.80 |
| BCF (Hz) | 5.49 ± 2.45 | 5.57 ± 2.45 | 5.32 ± 2.45 | 5.26 ± 2.45 | 5.52 ± 2.45 | 5.14 ± 2.45 | 0.43 | 0.45 | 0.93 |
| STR (%) | 49.07 ± 2.85 | 49.76 ± 2.85 | 53.84 ± 2.85 | 52.81 ± 2.85 | 51.12 ± 2.85 | 55.69 ± 2.85 | 0.32 | 0.26 | 0.91 |
| WOB (%) | 72.90 ± 1.77 | 75.10 ± 1.77 | 75.77 ± 1.77 | 74.39 ± 1.77 | 76.87 ± 1.77 | 76.27 ± 1.77 | 0.39 | 0.31 | 0.93 |
| Hyper (%) | 0.74 ± 0.43 | 1.08 ± 0.43 | 0.84 ± 0.43 | 0.93 ± 0.43 | 0.99 ± 0.43 | 1.31 ± 0.43 | 0.39 | 0.16 | 0.53 |
Group C: untreated extended semen sample, and Group Fe: extended semen sample treated with Fe nanoparticles. Time points 0, 24 and 48 h: storage time post removal of Fe3O4 NPs. Rapid Medium: rapid, medium movement spermatozoa (%; 25<medium<45<rapid μm/sec); VCL: curvilinear velocity (μm/sec); VSL: straight line velocity (μm/sec); VAP: average path velocity (μm/sec); STR: straightness (VSL/VAP × 100); WOB: wobble (VAP/VCL × 100); ALH: amplitude of lateral head displacement (μm); BCF: beat/cross-frequency (Hz); Hyper.: hyperactive spermatozoa (%). G*T: Group*Time interaction.
3.4. Microbiological Results
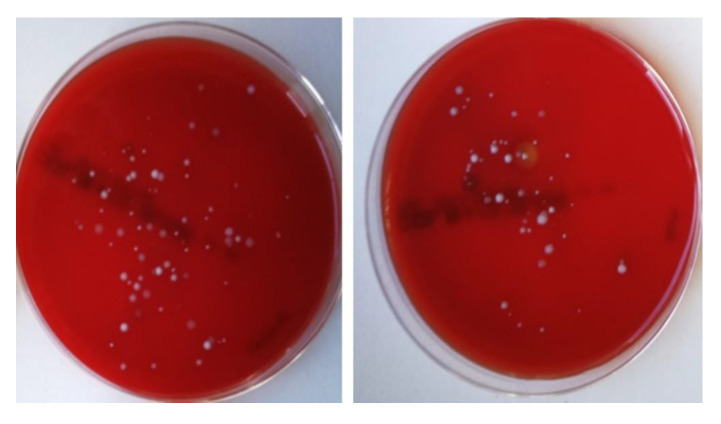
Bacterial growth was present in all semen samples. However, the microbial load varied. The most prevalent bacteria belonged to the Enterobacteriaceae family, Staphylococcus spp., Enterococcus spp. and Pseudomonas spp. The latter was found not to be affected by the examined NPs when detected at the specific concentration (data not shown). Total bacterial count in boar semen was respectively low and the number of cfu/mL demonstrated a wide range of microbial load among samples (from 45 to 1855, min–max, respectively). Treatment with Fe3O4 NPs did not eliminate bacterial content (Figure 6). However, a statistically significant reduction of the microbial load of semen was evident (p = 0.03) (Table 2). Among the other detected bacteria staphylococci tended to be less on Fe group compared to control, while Enterobacteriaceae (Enterobacter spp., E. coli, Proteus spp) and Enterococcus spp. had no significant difference from the control group (Table 2).
Figure 6.
Culture on sheep blood agar [a: control group (C), b: iron oxide group (Fe)]. Reduction of the microbial load after 24 h of incubation (37 °C).
Table 2.
Microorganisms (cfu/mL) isolated from boar semen samples 24 h and 48 h after incubation on blood agar and selective culture media.
| Variable | Control | Fe | Difference (Control-Fe) | p-Value |
|---|---|---|---|---|
| Blood Agar 24 h | 558 ± 455 | 443 ± 381 | 115 ± 103 | 0.03 |
| Blood Agar 48 h | 779 ± 651 | 616 ± 515 | 164 ± 150 | 0.03 |
| Staphylococcus spp | 314 ± 411 | 251 ± 380 | 63 ± 92 | 0.06 |
| Enterococcus spp | 5.4 ± 7.6 | 4.5 ± 8.7 | 0.9 ± 7.9 | 0.72 |
| Enterobacteriaceae | 69.9 ± 106.6 | 60.1 ± 86.5 | 9.8 ± 27.5 | 0.29 |
4. Discussion
Despite the potential biological benefits of NPs, nanotoxicity and its impact on cells’ health is a concern [14]. Previous studies reported that Fe3O4 NPs affect rainbow trout sperm [24], whilst titanium dioxide TiO2 NPs negatively affect mouse gene expression of Leydig cells, as well as semen quality parameters [25]. No studies were found to report the effects of Fe3O4 NPs on boar semen. Thus, the first aim of this study was to perform a full laboratory assessment of boar semen processing with iron oxide NPs. This study was based on previous findings of our research team [15], that explored the minimum inhibitory concentration of Fe3O4 NPs (0.192 mg/mL semen) and the appropriate co-incubation time of semen with NPs (30 min). However, the present study assessed the full profile regarding boar sperm characteristics.
The process of fertilization is a complex of sequencing events, involving the normal movement of the spermatozoa to reach the oviduct, the approach to oocyte, the sperm acrosome reaction, the sperm penetration into the ooplasm, the merging of the gametes, the fusion of the pronuclei and the intermingling of the paternal and maternal chromosomes. Low semen quality, as expressed by variables from semen analysis, is a common cause of subfertility or infertility [24]. This study provided a protocol for co-incubation of sperm with NPs, regarding both time and concentration, which had no detrimental effect on semen parameters. No effects were observed regarding sperm viability, morphology, membrane functionality, DNA integrity and CASA analyzed kinematics. This is an important finding, as it realizes the use of Fe3O4 NPs in boar semen handling with no toxicity. The sperm parameters analyzed in this study are of paramount importance for the fertilizing capacity of boar semen. Many researchers highlighted that motility is better correlated to field fertility compared to other kinetic parameters [26,27]. Moreover, Broekhuijse et al. [28,29] showed that CASA parameters, like progressive motility, BCF and VCL, could be related to farrowing rate, while the total number of born piglets could be affected by total motility, ALH, VSL and VAP [26,27]. In accordance with these findings, Holt et al. [30] showed that the VSL could positively affect the litter size. In vitro fertility of boar sperm has been positively correlated with progressive motility, VAP and VSL, and negatively correlated with STR, LIN and ALH [31]. Also, it is well accepted that the more diagnostic tests performed (such as the assessment of sperm morphology, motility and chromatin integrity), the better the prediction of in vitro fertility that can be achieved [32,33]. None of the above-mentioned sperm parameters were affected in our study. It seems that the restricted period of sperm co-incubation with Fe3O4 NPs and the gentle removal of them with a magnetic field, protected boar spermatozoa from NPs toxicity. This is in agreement with previous reports, that suggest a hypothesis that the time of interaction between semen and NPs can be crucial regarding their potential toxic activity [34,35]. In accordance with this scientific hypothesis, a significant decrease of VCL, VSL and VAP was observed after a prolonged (24 h) incubation of rainbow trout semen with Fe3O4 NPs [24].
In the Fe group, the value of spermatozoa with acrosome-reacted membrane was increased during storage time, which was not the case for the control group. However, even if this effect was statistically significant, the reported numerical values are not indicative of an important biological consequence. This finding could be attributed to the presence of the examined NPs. It is known that NPs penetrate the cells’ membranes affecting their physiology [36]. Although it was not within the purposes of the present study, according to the literature, the most prevalent mechanism of action of nanoparticles is related to the induction of oxidative stress [37]. Subsequently, the increased production of ROS can irreversibly affect the membranes of spermatozoa and can be involved in sperm capacitation [38], leading to the perturbation of the acrosome membrane’s integrity [39]. Therefore, the reported increase in spermatozoa with reacted acrosome membrane could be an undesirable characteristic for extended liquid semen used in AI programs, but it could be a perspective for IVF protocols, in which the induction of acrosome reaction is a prerequisite.
Semen extenders are cell culture media and thus an ideal environment for bacteria proliferation. There are directives from the European Union [40] and from national governments that specify the antibiotics’ category and dose as semen extenders’ additives. A variety of antibiotic compounds have been used to control microbial contamination in extenders. In farm animals’ AI, including boars, streptomycin and penicillin are the most widely used antibiotics [41]. Additionally, antibiotics, like gentamicin, linco-spectin and clindamycin have been successfully implemented in different semen extenders [42,43]. During the last decades, the bacterial resistance to antibiotics has been a serious problem to humans’ as well as animals’ health. Moreover, some antibiotics, at certain concentrations, may have a direct detrimental effect on spermatozoa [44]. Contemporary studies have proved that some antimicrobials may have a deleterious effect on bull [45] and equine [46] spermatozoa. In this aspect, NPs have been an interesting alternative for the scientists. In the present study, the examined iron oxide NPs had no toxic effect on boar spermatozoa and showed a slight antibacterial effect, although not for all bacteria species. Boar semen contaminants were present in all samples of the study, but the microbial load varied between them. Total bacterial count for aerobic mesophiles was up to 1.8 × 103, while Gączarzewicz et al. [4] reported findings up to 360 × 106. Despite the different degrees of contamination, a significant reduction regarding the total microbial count was observed in the presence of Fe3O4 NPs compared to control after 24 and 48 h of incubation. Given the initial low microbial load, this reduction suggests a promising result for the use of Fe NPs in heavier bacterial contaminations. After culture on selective media, the antimicrobial activity of Fe3O4 NPs demonstrated variation depending on the strain. The predominant bacteria on the samples were Staphylococci and Enterobacteriaceae, similar to studies previously reported [4,47]. Treatment with Fe3O4 NPs reduced the number of viable Staphylococci, though had a minimum effect on the Gram negative Enterobacteriaceae. In this field, reports demonstrate the bactericidal properties of iron oxide NPs (50 and 100 μg/mL) against Shigella dysentery and Escherichia coli [48] as well as their antimicrobial effects on antibiotic-resistant strains of E. coli [49]. Others report that iron NPs have only moderate antimicrobial action against Escherichia coli and Bacillus subtilis but remain potentially useful for application in the pharmaceutical and biomedical industry [50]. Studies have reported NPs to have excellent antimicrobial resistance properties and the ability to inhibit the formation of bacterial biofilms [51,52], which favor their use in drug-delivery systems. Concerning their mechanism of action, under specific conditions iron oxide nanoparticles may provide significant antimicrobial activity in contact with common bacteria. The interaction is stronger when nanoparticles’ surface is positively charged and when it involves the presence of both Fe2+ and Fe3+. Both properties are met in uncoated Fe3O4 nanoparticles distributed in the pH of biological environment. It has been reported that positively-charged Fe3O4 nanoparticles indicate stronger interaction with bacteria while the addition of surfactants counterbalances surface charge and limits such tendency [53]. An occurring mechanism involves the attachment of Fe3O4 nanoparticles on the bacteria membrane, and the dissolution of Fe2+ and Fe3+ at their interface which initiates the generation of reactive oxygen species. This triggers hydrogen peroxide release and production of free radicals through a Fenton reaction:
| Fe3+ + H2O2 → Fe2+ + OH− + OH• |
| Fe2+ + H2O2 → Fe3+ + HO2• + H+ |
The presence of toxic free radicals on bacteria membrane causes its electrostatic modification inducing chemical stress that causes the damage of the bacteria unit. The antimicrobial potential of Fe3O4 nanoparticles is preserved until their surface gets fully oxidized to γ-Fe2O3. Armijo et al. [54] findings suggest that Fe3O4 NPs are potential alternatives to silver NPs in several antibacterial applications minimizing the cost and enhancing microbial inactivation and elimination. The results of our research are aligned with these findings, reinforcing the future utilization of the antibacterial activity of Fe3O4 NPs as a new perspective to prevent bacteria in semen.
In conclusion, the Fe3O4 NPs examined are a potential useful and effective semen supplementation with antibacterial properties. Moreover, the combination of NPs with conventional antibiotics could enhance their antibacterial action and thus reduce the dose demanded. It is suggested that further studies regarding the oxidative status of boar semen treated with Fe3O4 NPs should be carried out to investigate the mechanism of action on boar spermatozoa.
Acknowledgments
The authors are grateful to “Karanikas farm” for kindly providing the semen samples and to Theofanis Avramidis for his contribution to semen doses preparation. Also, authors would like to thank Anna Esther Carrillo and Bernat Bozzo for the experimental assistance during SEM observations and SQUID measurements.
Author Contributions
Conceptualization, I.A.T., T.S. and C.M.B.; methodology, I.A.T., T.S., C.M.B. and G.T.; formal analysis, G.T.; investigation, I.A.T., T.S., S.A., A.B., A.N., I.M., K.S., I.K., G.T., M.A. and C.M.B.; data curation, S.A., A.B., A.N., I.M., K.S. and I.K.; writing—original draft preparation, I.A.T., S.A., K.S. and A.B.; writing—review and editing, C.M.B., T.S. and G.T.; visualization, I.A.T., G.T., S.A., A.B. and K.S.; supervision, C.M.B., I.A.T. and T.S.; project administration, C.M.B.; funding acquisition, C.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by Greece and the European Union (European Social Fund—ESF) through the Operational Programme Human Resources Development, Education and Lifelong Learning 2014–2020 in the context of the project “Investigation of nanotechnological applications on farm animals” semen processing “(MIS 5004681)”. Part of the measurements received funding from the EU H2020 research and innovation program under grant agreement No. 654360, having benefitted from the access provided by ICMAB-CSIC and Universitat Autonoma de Barcelona in Bellaterra-Barcelona within the framework of the NFFA Europe Transnational Access Activity.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Goldberg A.M., Argenti L.E., Faccin J.E., Linck L., Santi M., Bernardi M.L., Cardoso M.R.I., Wentz I., Bortolozzo F.P. Risk factors for bacterial contamination during boar semen collection. Res. Vet. Sci. 2013;95:362–367. doi: 10.1016/j.rvsc.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Kuster C.E., Althouse G.C. The impact of bacteriospermia on boar sperm storage and reproductive performance. Theriogenology. 2016;85:21–26. doi: 10.1016/j.theriogenology.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 3.Maes D., Nauwynck H., Rijsselaere T., Mateusen B., Vyt P., de Kruif A., Soom A.V. Diseases in swine transmitted by artificial insemination: An overview. Theriogenology. 2008;70:1337–1345. doi: 10.1016/j.theriogenology.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Gączarzewicz D., Udała J., Piasecka M., Błaszczyk B., Stankiewicz T. Bacterial contamination of boar semen and its relationship to sperm quality preserved in commercial extender containing gentamicin sulfate. Pol. J. Vet. Sci. 2016;19:451–459. doi: 10.1515/pjvs-2016-0057. [DOI] [PubMed] [Google Scholar]
- 5.Althouse G.C., Lu K.G. Bacteriospermia in extended porcine semen. Theriogenology. 2005;63:573–584. doi: 10.1016/j.theriogenology.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Morrell J., Wallgren M. Alternatives to antibiotics in semen extenders: A review. Pathogens. 2014;3:934–946. doi: 10.3390/pathogens3040934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulze M., Dathe M., Waberski D., Müller K. Liquid storage of boar semen: Current and future perspectives on the use of cationic antimicrobial peptides to replace antibiotics in semen extenders. Theriogenology. 2016;85:39–46. doi: 10.1016/j.theriogenology.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Speck S., Courtiol A., Junkes C., Dathe M., Müller K., Schulze M. Cationic synthetic peptides: Assessment of their antimicrobial potency in liquid preserved boar semen. PLoS ONE. 2014;9:e105949. doi: 10.1371/journal.pone.0105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman M., Dille J., Godet S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012;8:37–45. doi: 10.1016/j.nano.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Li W.R., Xie X.B., Shi Q.S., Zeng H.Y., Ouyang Y.S., Chen Y.B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010;85:1115–1122. doi: 10.1007/s00253-009-2159-5. [DOI] [PubMed] [Google Scholar]
- 11.Shahverdi A.R., Fakhimi A., Shahverdi H.R., Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007;3:168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Irshad R., Tahir K., Li B., Ahmad A.R., Siddiqui A., Nazir S. Antibacterial activity of biochemically capped iron oxide nanoparticles: A view towards green chemistry. J. Photochem. Photobiol. B Biol. 2017;170:241–246. doi: 10.1016/j.jphotobiol.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Toledo L.D.A.S., Rosseto H.C., Bruschi M.L. Iron oxide magnetic nanoparticles as antimicrobials for therapeutics. Pharmaceut. Dev. Technol. 2018;23:316–323. doi: 10.1080/10837450.2017.1337793. [DOI] [PubMed] [Google Scholar]
- 14.Sahu D., Kannan G.M., Tailang M., Vijayaraghavan R. In vitro cytotoxicity of nanoparticles: A comparison between particle size and cell type. J. Nanosci. 2016:4023852. doi: 10.1155/2016/4023852. [DOI] [Google Scholar]
- 15.Basioura A., Michos I., Ntemka A., Karagiannis I., Boscos C.M. Effect of iron oxide and silver nanoparticles on boar semen CASA motility and kinetics. J. Hellenic Vet. Med. Soc. 2020 accepted for publication. [Google Scholar]
- 16.Asimakidou T., Makridis A., Veintemillas-Verdaguer S., Morales M.P., Kellartzis I., Mitrakas M., Vourlias G., Angelakeris M., Simeonidis K. Continuous production of magnetic iron oxide nanocrystals by oxidative precipitation. Chem. Eng. J. 2020;393:124593. doi: 10.1016/j.cej.2020.124593. [DOI] [Google Scholar]
- 17.Luengo Y., Morales M.P., Gutiérrez L., Verdaguer S.V. Counterion and solvent effects on the size of magnetite nanocrystals obtained by oxidative precipitation. J. Mater. Chem. 2016;4:9482–9488. doi: 10.1039/C6TC03567A. [DOI] [Google Scholar]
- 18.Powder Diffraction File. International Centre for Diffraction Data; Newtown Square, PA, USA: 2004. Joint center for powder diffraction studies. [Google Scholar]
- 19.M100-S25 Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute (CLSI); Wayne, PA, USA: 2015. Twenty-fifth informational supplement. [Google Scholar]
- 20.World Health Organization (WHO) Laboratory Manual for the Examination of Human Sperm and Semen-Cervical Mucus Interaction. 5th ed. WHO Press; Geneva, Switzerland: 2010. [Google Scholar]
- 21.Vazquez J.M., Martinez E.A., Martinez P., Artiga C.G., Roca J. Hypoosmotic swelling of boar spermatozoa compared to other methods for analysing the sperm membrane. Theriogenology. 1997;47:913–922. doi: 10.1016/S0093-691X(97)00046-0. [DOI] [PubMed] [Google Scholar]
- 22.Tsakmakidis I.A., Lymberopoulos A.G., Khalifa T.A.A., Boscos C.M., Saratsi A., Alexopoulos C. Evaluation of zearalenone and α-zearalenol toxicity on boar sperm DNA integrity. J. Appl. Toxicol. 2008;28:681–688. doi: 10.1002/jat.1322. [DOI] [PubMed] [Google Scholar]
- 23.Silva P.F.N., Gadella B.M. Detection of damage in mammalian sperm cells. Theriogenology. 2006;65:958–978. doi: 10.1016/j.theriogenology.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Özgür M.E., Ulu A., Balcıoğlu S., Özcan I., Köytepe S., Ates B. The toxicity assessment of iron oxide (Fe3O4) nanoparticles on physical and biochemical quality of rainbow trout spermatozoon. Toxics. 2018;6:62. doi: 10.3390/toxics6040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komatsu T., Tabata M., Irie M.K., Shimizu T., Suzuki K.I., Nihei Y., Takeda K. The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol. Vitr. 2008;22:1825–1831. doi: 10.1016/j.tiv.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Blondin P., Beaulieu M., Fournier V., Morin N., Crawford L., Madan P., King W.A. Analysis of bovine sexed sperm for IVF from sorting to the embryo. Theriogenology. 2009;71:30–38. doi: 10.1016/j.theriogenology.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Tardif S., Laforest J.P., Cormier N., Bailey J.L. The importance of porcine sperm parameters on fertility in vivo. Theriogenology. 1999;52:447–459. doi: 10.1016/S0093-691X(99)00142-9. [DOI] [PubMed] [Google Scholar]
- 28.Broekhuijse M.L., Šoštarić E., Feitsma H., Gadella B.M. Application of computer-assisted semen analysis to explain variations in pig fertility. J. Anim. Sci. 2012;90:779–789. doi: 10.2527/jas.2011-4311. [DOI] [PubMed] [Google Scholar]
- 29.Broekhuijse M.L., Feitsma H., Gadella B.M. Field data analysis of boar semen quality. Reprod. Domest. Anim. 2011;46:59–63. doi: 10.1111/j.1439-0531.2011.01861.x. [DOI] [PubMed] [Google Scholar]
- 30.Holt C., Holt W.V., Moore H.D.M., Reed H.C.B., Curnock R.M. Objectivily measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: Results of two fertility trials. J. Androl. 1997;18:312–323. doi: 10.1002/j.1939-4640.1997.tb01925.x. [DOI] [PubMed] [Google Scholar]
- 31.Kathiravan P., Kalatharan J., Karthikeya G., Rengarajan K., Kadirvel G. Objective sperm motion analysis to assess dairy bull fertility using computer-aided system-a review. Reprod. Domest. Anim. 2011;46:165–172. doi: 10.1111/j.1439-0531.2010.01603.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsakmakidis I.A., Lymberopoulos A.G., Khalifa T.A.A. Relationship between sperm quality traits and field-fertility of porcine semen. J. Vet. Sci. 2010;11:151–154. doi: 10.4142/jvs.2010.11.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillan L., Kroetsch T., Chis Maxwell W.M., Evans G. Assessment of in vitro sperm characteristics in relation to fertility in dairy bulls. Anim. Reprod. Sci. 2008;103:201–214. doi: 10.1016/j.anireprosci.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Feugang J.M., Shengfa F.L., Crenshaw M.A., Clemente H., Willard S.T., Ryan P.L. Lectin-functionalized magnetic iron oxide nanoparticles for reproductive improvement. JFIV Reprod. Med. Genet. 2014;03:1–5. doi: 10.4172/2375-4508.1000145. [DOI] [Google Scholar]
- 35.Odhiambo J.F., DeJarnette J.M., Geary T.W., Kennedy C.E., Suarez S.S., Sutovsky M., Sutovsky P. Increased conception rates in beef cattle inseminated with nanopurified bull semen1. Biol. Reprod. 2014;91:1–10. doi: 10.1095/biolreprod.114.121897. [DOI] [PubMed] [Google Scholar]
- 36.Brooking J., Davis S.S., Illum L. Transport of nanoparticles across the rat nasal mucosa. J. Drug Target. 2001;9:267–279. doi: 10.3109/10611860108997935. [DOI] [PubMed] [Google Scholar]
- 37.Wang R., Song B., Wu J., Zhang Y., Chen A., Shao L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018;13:8487–8506. doi: 10.2147/IJN.S170723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamirande E.D., Tsai C., Harakat A., Gagnon C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J. Androl. 1998;19:585–594. doi: 10.1002/J.1939-4640.1998.TB02061.X. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida Y., Itoh N., Saito Y., Hayakawa M., Niki E. Application of water-soluble radical initiator, 2,2′-azobis-[2-(2-imidazolin-2-yl)propane] dihydrochloride, to a study of oxidative stress. Free Radic. Res. 2004;38:375–384. doi: 10.1080/1071576042000191763. [DOI] [PubMed] [Google Scholar]
- 40.Council Directive, European Union, 90/429/EEC. [(accessed on 28 October 2014)]; Available online: http://faolex.fao.org/docs/pdf/eur110397.pdf.
- 41.Salamon S., Maxwell W.M. Storage of ram semen. Anim. Reprod. Sci. 2000;62:77–111. doi: 10.1016/S0378-4320(00)00155-X. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad K., Foote R.H., Kaproth M. Antibiotics for bull semen frozen in milk and egg yolk extenders. J. Dairy Sci. 1987;70:2439–2443. doi: 10.3168/jds.S0022-0302(87)80306-5. [DOI] [PubMed] [Google Scholar]
- 43.Gadea J. Review: Semen extenders used in the artificial inseminarion of swine. Span. J. Agric. Res. 2003;1:17. doi: 10.5424/sjar/2003012-17. [DOI] [Google Scholar]
- 44.Lorton S.P., Sullivan J.J., Bean B., Kaproth M., Kellgren H., Marshall C. A new antibiotic combination for frozen bovine semen. 2. Evaluation of seminal quality. Theriogenology. 1988;29:593–607. doi: 10.1016/S0093-691X(88)80007-4. [DOI] [PubMed] [Google Scholar]
- 45.Azawi O.I., Ismaeel M.A. Influence of addition of different antibiotics in semen diluent on viable bacterial count and spermatozoal viability of awassi ram semen. Vet. World. 2012;5:75–79. doi: 10.5455/vetworld.2012.75-79. [DOI] [Google Scholar]
- 46.Varner D.D., Scanlan C.M., Thompson J.A., Brumbaugh G.W., Blanchard T.L., Carlton C.M., Johnson L. Bacteriology of preserved stallion semen and antibiotics in semen extenders. Theriogenology. 1998;50:559–573. doi: 10.1016/S0093-691X(98)00161-7. [DOI] [PubMed] [Google Scholar]
- 47.Oehler C., Janett F., Schmitt S., Malama E., Bollwein H. Development of a flow cytometric assay to assess the bacterial count in boar semen. Theriogenology. 2019;133:125–134. doi: 10.1016/j.theriogenology.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 48.Saqib S., Munis M.F.H., Zaman W., Ullah F., Shah S.N., Ayaz A., Farooq M., Bahadur S. Synthesis, characterization and use of iron oxide nano particles for antibacterial activity. Microsc. Res. Tech. 2019;82:415–420. doi: 10.1002/jemt.23182. [DOI] [PubMed] [Google Scholar]
- 49.Gabrielyan L., Hakobyan L., Hovhannisyan A., Trchounian A. Effects of iron oxide (Fe3O4) nanoparticles on Escherichia coli antibiotic-resistant strains. J. Appl. Microbiol. 2019;126:1108–1116. doi: 10.1111/jam.14214. [DOI] [PubMed] [Google Scholar]
- 50.Margabandhu M., Sendhilnathan S., Maragathavalli S., Karthikeyan V., Annadurai B. Synthesis characterization and antibacterial activity of iron oxide nanoparticles. Global. J. Biosci. Biotechnol. 2015;4:335–341. [Google Scholar]
- 51.Wang L., Hu C., Shao L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramos M.A.D.S., Silva P.B.D., Spósito L., Toledo L.G.D., Bonifácio B.V., Rodero C.F., Santos K.C.D., Chorilli M., Bauab T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018;13:1179–1213. doi: 10.2147/IJN.S146195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arakha M., Pal S., Samantarrai D., Panigrahi T.K., Mallick B.C., Pramanik K., Mallick B., Jha S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015;5:14813. doi: 10.1038/srep14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Armijo L.M., Wawrzyniec S.J., Kopciuch M., Brandt Y.I., Rivera A.C., Withers N.J., Cook N.C., Huber D.L., Monson T.C., Smyth H.D.C., et al. Antibacterial activity of iron oxide, iron nitride, and tobramycin conjugated nanoparticles against pseudomonas aeruginosa biofilms. J. Nanobiotechnol. 2020;18:35. doi: 10.1186/s12951-020-0588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]