Abstract
Pancreatic ductal adenocarcinoma (PDAC) is typically diagnosed at an advanced stage with systemic therapy being the mainstay of treatment. Survival continues to be limited, typically less than one year. The PDAC microenvironment is characterized by a paucity of malignant epithelial cells, abundant stroma with predominantly immunosuppressive T cells and myelosuppressive-type macrophages (M2), and hypovascularity. An improved understanding of PDAC pathobiology has triggered an exploration of potential breeches that can be targeted in this recalcitrant cancer. The current treatment options for metastatic PDAC are modified (m)FOLFIRINOX/FOLFIRINOX or nab-paclitaxel and gemcitabine in patients with good performance status (PS) (ECOG 0–1/KPS 70–100%) and gemcitabine with or without a second agent for those with ECOG PS 2–3.
New therapies are emerging, and the current guidelines endorse both germline and somatic testing in PDAC to evaluate actionable findings. Important themes related to new therapeutic approaches include DNA damage repair (DDR) strategies, immunotherapy, targeting the stroma and cancer cell metabolism.
Targeted therapy alone (outside small genomically defined subsets) or in combination with standard cytotoxic therapy, thus far, has proven disappointing in PDAC, however novel therapies are evolving with increased integration of genomic profiling along with a better understanding of the tumor microenvironment and immunology. A small but important sub-group of patients have some of these agents available in the clinics for use. Olaparib was recently FDA approved for maintenance therapy in germline BRCA1/2 mutated PDAC following demonstration of survival benefit in a phase 3 trial. Pembrolizumab is approved for patients with defects in mismatch repair/microsatellite instability. PDAC with wild-type KRAS represents a unique subgroup who have enrichment of potentially targetable oncogenic drivers, including ERBB inhibitors (e.g. afatinib, MCLA-128), TRK inhibitors (e.g. larotrectinib, entrectinib), ALK/ROS inhibitor (e.g. crizotinib), and BRAF/MEK inhibitors are in development. In a small subset of patients with KRASG12C mutation, KRASG12C inhibitor, AMG510 and other agents are being investigated.
Major efforts are underway to effectively target the tumor microenvironment and to integrate immunotherapy into the treatment of PDAC, although thus far the impact has been modest to ineffective, nonetheless, there is optimism that some of the challenges will be overcome.
1. Background
Pancreatic ductal adenocarcinoma (PDAC) continues to pose major therapeutic challenges with its relative refractoriness to current therapies and either de novo or early acquired development of resistance. While PDAC accounts for 3.2% (57600 estimated cases in 2020) of all new cancer cases it contributes to a much larger proportion (8% of all cancer deaths; 47050 estimated deaths in 2020) of total cancer deaths.[1] The obesity pandemic, diabetes mellitus and related dietary, lifestyle, environmental factors along with increasing life expectancy and rising cancer incidence in a younger population are likely responsible for a slow but steady rise in the incidence of pancreatic cancer (0.7% and 0.3% annually from 2007 to 2016, in whites and blacks respectively). This contrasts with the overall 27% decline in cancer incidence since 1991.[2, 1]
Early-onset pancreatic cancer (EOPC; age <50 years at diagnosis) accounts for 5.7–9.5% of PDAC with more patients being diagnosed as inoperable or metastatic disease.[3–6] However, these studies are all single center retrospective analyses. Of note, there was no significant difference in overall survival (OS) compared to patients who are diagnosed at a later age (typical onset). Furthermore, in some studies there was no difference in the risk factor profile between younger and older patients.[3–6] With the rising prevalence of obesity in younger adults it is anticipated that EOPC will be of increasing importance, and the above data suggests a trend towards disease presentation at more advanced stages. While it can be anticipated that these patients with disease onset at a younger age might have unique genomic alterations that predispose to neoplasia earlier in life there is a dearth of literature on genomic data in these patients. In a recently presented study, Varghese, et al identified that pathogenic germline mutations were present in 29% (33 of 114) of the EOPC patients who had undergone testing, and specifically mutations in BRCA1/2 (n=18), CHEK2 (n=3), PALB2 (n=3), ATM (n=2), MLH1 (n=1), and MSH3 (n=1) were observed. Somatic profiling data (N=132) identified 16% tumors to be KRAS wild-Type (KRASWT) with actionable mutations in a subset of the entire cohort (7 of 21 with KRASWT). Median OS in the entire cohort of 450 EOPC patients was 16 months and 11.3 months in patients with metastatic PDAC. [7]
There has been a small but definite improvement in the OS (5-year survival: 6% in 2014 to approximately 10% in 2020), however this remains poor for patients with metastatic disease (5-year survival: 3%) who comprise the majority (53%) of PDAC patients at the time of diagnosis.[8, 1, 9] Real world experience in treatment of metastatic PDAC suggests approximately three times improved survival with treatment compared to no treatment (median OS 8.1 versus 2.8 months, p=0.0001).[10] The improvement in survival of patients over the years is likely a result of overall improvement in supportive care, advancements in interventional biliary procedures, better treatment of infections, improvements in therapy and delivery of therapy to more patients. In addition, identification of actionable mutations in DNA damage repair (DDR) genes, some of the targetable somatic mutations along with a better understanding of the tumor microenvironment and the immune system pathophysiology in PDAC, have opened new possibilities for a subset of patients.
An understanding of PDAC pathobiology can explain therapeutic refractoriness, differences in survival and response to specific therapies. The development of PDAC has been conceptualized in three stages.[11] Initiation of the tumor led by a driver oncogene mutation is thought to precede phenotypic expression and diagnosis by at least two decades. This lag time has been partly attributed to the low proliferative rate of pancreatic cancer cells, and occurrence of somatic mutation.[12, 13] The second stage of clonal expansion is marked by the acquisition of added driver and passenger gene mutations in addition to division and continuous growth of the cell with the initiating mutation. The consequence is an increase in clonal heterogeneity.[14] The final stage is the introduction of tumor cells to a foreign microenvironment. Dissemination is a continuous process during tumor development and is likely restricted to a subpopulation of cells that are the most resilient.
Histopathologic study of localized and locally advanced PDAC’s has revealed three distinct subgroups based on local immune cell composition of the tumor microenvironment. The most common is the immune-escape subtype characterized by poor T- and B-cells and enriched regulatory T cells (FOXP3+ Tregs), followed by the immune-rich subtype with enriched T- and B-cells, and poor Tregs, while the immune-exhausted subtype is comprised of two subpopulations (one with high PD-L1 and other with microsatellite instability). The immune-rich subtype has a significantly better prognosis over the other two subtypes. Moreover, DDR gene mutations (ATM and STK11) are frequent in the subpopulation of immune-rich subtype providing opportunity for targeted and immune therapies.[15] Thus, dissection of the local immunophenotypic patterns in PDAC can provide insights into the relationship of molecular, morphologic and clinical characteristics with potential prognostic and therapeutic significance.
Somatic mutations in one or more of the four key genes, KRAS, cyclin dependent kinase Inhibitor 2A (CDKN2A), TP53 and SMAD4, characterize the genetic landscape of PDAC. KRAS, located on chromosome 12p is the most frequent and is mutated in 90–94% of PDAC, followed by mutations in TP53 which renders defective DNA repair, CDKN2A which encodes p16INK4A, p19ARF, and loss of SMAD4 resulting in altered TGF-b function. There is concurrent gene amplification in 4% of patients with KRAS mutation.[16–18] BRAF, the downstream signal to KRAS is mutated in 3–4% exclusive of KRAS.[19, 18] While most of the KRAS mutations result in constitutive activity, a small proportion of tumors with KRASG12C have nucleotide cycling activity which is potentially targetable.[20] ATM, a gene involved in TP53 phosphorylation and thus maintenance of genomic integrity is found to be mutated in some patients with familial PDAC.[21] Interestingly, SMAD4 and TP53 inactivation appear to be interdependent and TP53 mutant PDAC can be classified into two separate cohorts, one with SMAD4 inactivation and TP53 gain of function and other with wild-type SMAD4 and TP53 loss of function.[17]
The PDAC microenvironment is composed of stromal cells, dense extracellular matrix (ECM) and the immune cells. Myofibroblasts are metabolically active stromal cells derived from pancreatic stellate cells (PSC) and produce the dense ECM which forms a barricade for the tumor. Hyaluronic acid (HA), a large glycosaminoglycan is responsible for the high hydrostatic and interstitial pressure leading to vascular collapse and hypoperfusion.[22] This geographic isolation limits delivery of chemotherapeutic agents to the cancer cells. The intra-tumoral effector T cells which are inherent scavengers are rare in PDAC resulting in immune escape.[23] While they are not dysfunctional by themselves, accumulation of myeloid derived suppressor cells (MDSC), tumor associated macrophages (TAM), CD4+ regulatory T cells and fibroblasts (distinct from myofibroblasts) lead to suppression of cytotoxic T cells and altered immune surveillance.[23]
As noted above, the stroma forms a major part of PDAC and has been shown to comprise almost half of the tumor sample.[24] Tumor specific stromal subtypes have been identified in PDAC and can have prognostic significance. Moffitt, et al identified two subtypes, “classical” and “basal”. Patients with “basal” subtype stroma of PDAC had significantly worse median OS compared to those with “classical” stromal subtype (11 versus 19 months, p=0.007).[24] Earlier, Collison, et al found three transcriptional subtypes based on gene expression, and named them classic, quasi-mesenchymal and exocrine. Patients with classic subtype had better overall survival (HR 0.21; 95% CI 0.068–0.61) compared to quasi-mesenchymal while there was no significant difference between exocrine and quasi-mesenchymal (HR 0.66; 95% CI 0.31–3.32). Furthermore, study of human and mouse cancer cell lines identified only classical and quasi-mesenchymal cell lines raising question about existence of the exocrine subtype.[25] The two models of PDAC subtypes share most of the features, with “classical” subtypes probably representing similar characteristics and “basal” largely overlapping with quasi-mesenchymal.[26] While identification of PDAC subtypes can provide prognostic information and bears potential for subtype-targeted therapies laboratory models that identify each subtype need further prospective validation will be needed for routine integration into clinical practice.
The refractoriness to local or systemic therapies in PDAC may be partly attributed to the complex microenvironment dominated by dense stroma and the immune suppressive nature of this environment. Understanding of the tumor microenvironment has opened new prospects for therapeutic evaluation in PDAC. Thus far, we are yet to witness measurable clinical success with translating such approaches to the clinic. Herein, we aim to review the most pertinent developments in the treatment of metastatic PDAC (mPDAC) and discuss emerging therapies for this disease.
2.0. Standard of Care in Metastatic PDAC
2.1. American society of clinical oncology (ASCO), national comprehensive cancer network (NCCN) guidelines for metastatic PDAC: first- and second-line therapies
While we continue to find better treatment options to focus on longevity in advanced PDAC it is equally important to focus on goals of treatment, understand patient preferences and make available all supportive care options beyond cancer treatment per se. For patients with good performance status (PS) (European cooperative oncology group, ECOG 0 or 1) one of the regimens, gemcitabine plus nab-paclitaxel (GA) or a combination of FOLinic acid, 5-Flurouracil, IRINotecan, OXaliplatin (FOLFIRINOX) is recommended.[27] The recommendations are based on clear survival benefit of these regimens over gemcitabine, a previous standard of care in advanced PDAC. PRODIGE 4/ACCORD 11, a large randomized phase 3 trial established the efficacy of FOLFIRINOX (median OS 11.1 Vs 6.8 months with gemcitabine; 95% confidence interval [CI], 0.45 to 0.73; P<0.001) in treatment naïve metastatic PDAC patients.[28] Survival benefit of GA over gemcitabine was defined in the MPACT trial (a randomized phase 3 trial) which involved 842 patients with metastatic PDAC (median OS 8.5 Vs 6.7 months; 95% CI, 0.62 to 0.83; P<0.001).[29] Choice between the two regimens is largely based on physician and/or patient preference and toxicity profiles, with gastrointestinal toxicities, myelosuppression, fatigue and neuropathy being more common with FOLFIRINOX and alopecia more evident with GA. n
There is no head-to-head prospective trial comparing FOLFIRINOX with GA, however there is real-life evidence from multiple retrospective studies including a systematic review suggesting that there is no major significant difference in clinical outcomes apart from the toxicity profiles, although most of the data sets slightly trend in favor of the superiority of FOLFIRINOX.[30, 33, 32] One systematic review of 16 retrospective studies found no difference in the whole population OS (HR = 0.99, 95% CI 0.84(−)1.16; p = 0.9) or progression-free survival (PFS) between FOLFIRINOX or GA arms (HR = 0.88, 95% CI 0.71(−)1.1; p = 0.26), although there was slight weighted median OS favoring FOLFIRINOX (mean difference: 1.15, 95% CI 0.08(−)2.22, p = 0.03).[32] Nonetheless, no concrete conclusion can be drawn due to the retrospective non-randomized nature of these studies.
Modification of the standard FOLFIRINOX usually with omission of fluorouracil bolus (and/or dose adjustment of irinotecan or oxaliplatin), referred to as modified (m)FOLFIRINOX, was demonstrated in a randomized phase 2 trial (designed to evaluate the addition of PEGPH20) to provide the longest survival benefit (OS 14.4 months; 95% confidence interval [CI] 10.1–15.7) to date for treatment naïve metastatic PDAC.[34] In a recent retrospective analysis of 68 patients unresponsive to first-line GA, (m)FOLFIRINOX was shown to result in median OS of 13 months (95% CI, 7.0 – 18.0) following start of second-line treatment.[35] National guidelines recommend (m)FOLFIRINOX as a second-line therapy option for individuals with good performance status whose disease has progressed on first line GA.
Nanoliposomal irinotecan (nal-IRI) in combination with 5-FU and leucovorin (LV) is an established option in metastatic PDAC where resistance has developed to gemcitabine-based therapy. Nal-IRI alone has demonstrated modest anti-tumor activity as initially identified in a phase 2 trial (OS 5.2 months; progression free survival, PFS 2.4 months)[36], and is not recommended as a single agent. NAPOLI-1, a global randomized, open label, phase 3 trial compared the effectiveness of nal-IRI plus 5-FU/LV with 5-FU/LV or nal-IRI alone in metastatic PDAC patients previously treated with gemcitabine-based therapy. Median OS was 6.1 months (95% CI 4.8–8.9) in the combination arm vs 4.2 months (95% CI 3.3–5.3) with 5-FU/LV (HR 0.67, 95% CI 0.49–0.92; p=0.012). In the nal-IRI alone-arm median OS was 4.9 months (95% CI 4.2–5.6) which was not different than 5-FU/LV.[37]
Second line therapy with GA or gemcitabine is endorsed by current guidelines for patients who have received prior (m)FOLFIRINOX and continue to have good PS, and 5-FU plus nal-IRI for those with prior GA treatment. For patients with poor PS single agent gemcitabine or a fluropyrimidine based combination (e.g. 5-FU) can be considered.[27]
Major recent updates in national guidelines pertain to the integration of universal genetic testing as a standard practice for germline testing in 2019 and somatic testing in 2018. Routine testing of mPDAC patients for microsatellite instability (MSI) or defective mismatch repair (dMMR) is recommended in those who are candidates for checkpoint inhibitor therapy. Although a rare event in PDAC, patients with MSI-H tumors can have remarkable benefit from programmed cell death protein (PD)-1 inhibitor (pembrolizumab) and it is recommended as an option for second-line or later therapy.[38, 39] Current National Comprehensive Cancer Network (NCCN) guidelines recommend routine germline testing in all PDAC patients and additional molecular subtyping of the tumor tissue (somatic testing) in those with advanced disease.[40] These evaluations provide targeted therapeutic options for a small subset of the patients with specific genomic alterations and provides opportunity for clinical trial enrollment.
Figure 1 outlines the current treatment options for metastatic PDAC.
Figure 1. Outline of Current Treatment Options in Metastatic PDAC.
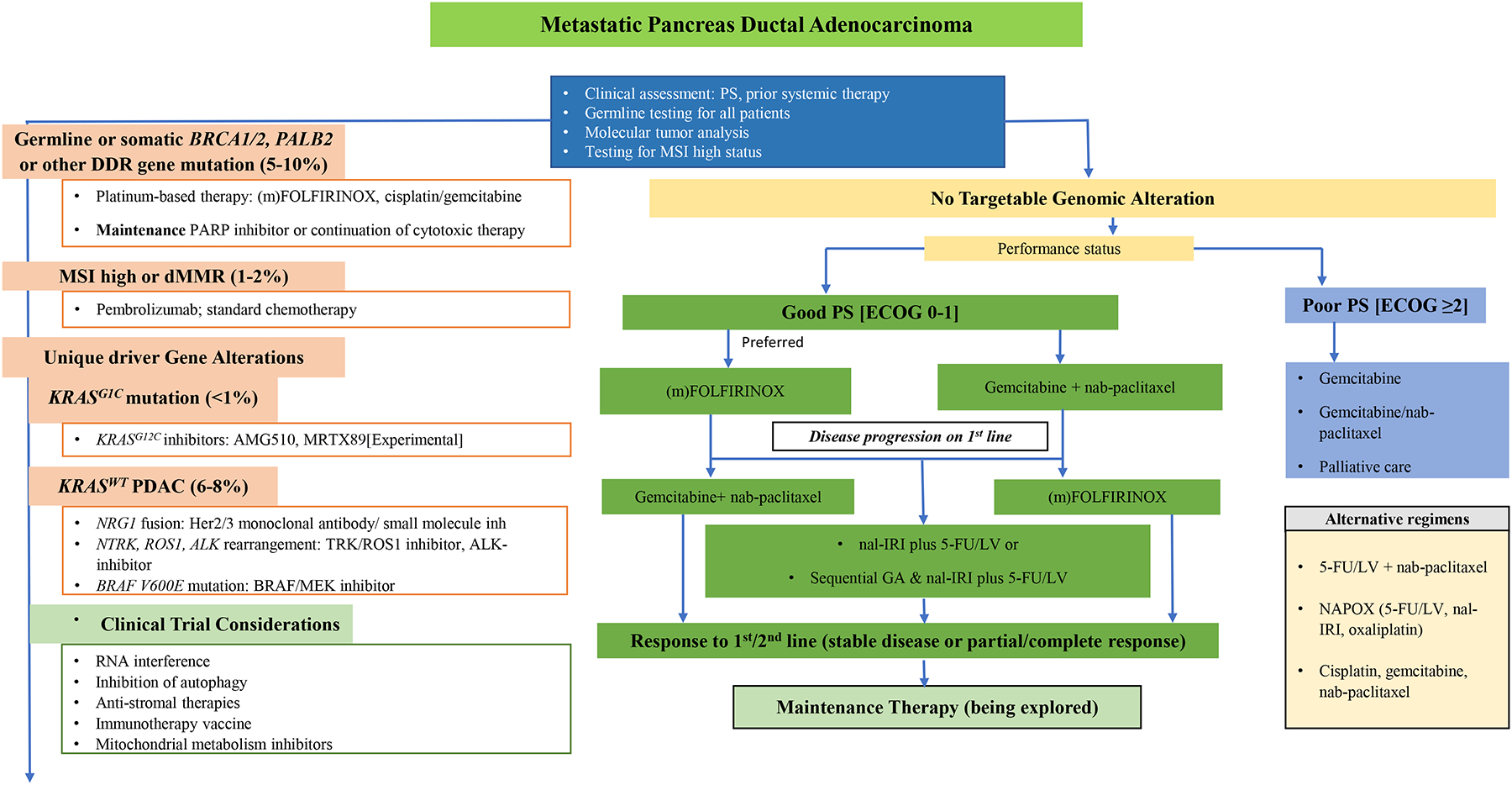
PS performance status; DDR DNA damage repair; MSI microsatellite instability; ECOG eastern cooperative oncology group; (m)FOLFIRINOX modified regimen of 5-fluorouracil/leucovorin (5-FU/LV), irinotecan and oxaliplatin; PARP poly(ADP-ribose) polymerase; dMMR defective mismatch repair; nal-IRI nanoliposomal irinotecan; GA Gemcitabine+ nab-paclitaxel.
2.2. Maintenance Therapy
Increasingly, maintenance therapy following initial combination cytotoxic therapy is being integrated as part of standard practice in PDAC for selected patients. The potential value of maintenance chemotherapy was tested in a randomized phase 2 trial by Dahan, et al. where patients with metastatic PDAC were randomized to one of three arms: arm A with six months of FOLFIRINOX, arm B with four months of FOLFIRINOX followed by maintenance therapy with 5-FU and leucovorin, and arm C with alternating gemcitabine and FOLFIRI (5-FU, folic acid and irinotecan) every two months. Six-month PFS was 47%, 44% and 34% and median OS was 10.1 months, 11.2 months and 7.3 months, respectively in arms A, B and C.[41] These data support the de-escalation of therapy for selected patients to maintenance 5-FU/LV and indicate no role for the alternating regimen (arm C) which was inferior to arms A and B. Likewise, the role of maintenance gemcitabine following three cycles of GA was studied in a prospective observational study of 36 elderly patients (>70 years) with locally advanced or metastatic PDAC whose disease did not progress on GA. Median PFS and median OS was 6.4 months (95% CI, 5.4–8.3) and 13.4 months (95% CI, 11.1–16.7), respectively. None of the patients experienced grade 3 neuropathy, although six (19%) experienced hematologic toxicity.[42]
Among patients with germline BReast CAncer gene (BRCA)1 or BRCA2 mutated metastatic PDAC single-agent oral olaparib is now established as a viable option for maintenance therapy as demonstrated in a phase 3 trial.[43] These results led to the FDA-approval of olaparib for maintenance therapy in late 2019, in deleterious or suspected deleterious germline BRCA mutated metastatic PDAC, representing validation of a biomarker based therapeutic strategy in this disease.
2.3. Emerging Cytotoxic Approaches in PDAC
A multicenter phase 2 study from France has evaluated the efficacy and safety of nab-paclitaxel with 5-fluorouracil (5-FU) and leucovorin in comparison with GA in previously untreated metastatic PDAC. The study met the primary endpoint of >50% (40/75) patients being progression free for at least four months, with an acceptable safety profile. At four months follow up, the treatment arms had similar PFS (56%; 90% CI 45–66 in 5-FU versus 54%; CI 40–68 in gemcitabine group). Interestingly, 90% CI was used. The total follow-up period was 13.1 months.[44] Results also demonstrated health-related quality of life deterioration-free survival in favor of 5-FU, leucovorin and nab-paclitaxel combination.[45]
Nal-IRI in combination 5-FU/LV and oxaliplatin (NAPOX, nalIRIFOX) was found to be well tolerated with good tumor response (disease control rate at 16 week of 71.9%) in a phase 1 trial comprising 25 patients.(NCT02551991). .[46] A phase 3, open label, randomized trial is underway to compare the efficacy and safety of the above regimen with GA in previously untreated metastatic PDAC.(NCT04083235) Nal-IRI is also being evaluated in an open label, multicenter, phase 3b trial for second-line treatment of patients whose disease has progressed on GA in advanced PDAC.(NCT03468335)
Jameson, et al conducted a single arm, open label, multicenter, phase 1b/2 trial to evaluate the response rate of adding cisplatin to GA in metastatic PDAC patients. Among 24 evaluable patients, the median OS was 16.4 months (95% CI, 10.2–25.3) and 16 patients (64%) were alive at one year. Overall response rate was 71%. However, the primary end point of 25% complete response rate was not reached.[47] Of additional note, there were two deaths on study. Further development of a related version of this regimen is underway in biliary cancers where the triplet is being compared to standard cisplatin/gemcitabine as front-line therapy in a North American Co-operative group trial (S1815, NCT03768414). This regimen is likely to be further explored in an adjuvant or neoadjuvant setting given high response rates and further may have a role in the setting of BRCA-related PDAC given the platinum agent.
A single-center, open label, phase 2, randomized trial demonstrated clinical efficacy of adding capecitabine and cisplatin to GA (PAXG) in previously untreated 83 metastatic PDAC patients. The primary endpoint was the rate of PFS at 6-month in the treatment arm. Among patients treated with PAXG 74% (95% CI 58–86) were progression free compared to 46% (95% CI 31–63) treated with GA.[48] The regimen was well tolerated and there was no death in the experimental arm.
2.4. Treatment of Metastatic PDAC in the Elderly
With the median age of 71 years at diagnosis, PDAC is a cancer of an older population. It is projected that by 2030 70% of PDAC will be represented by elderly patients (>70 years).[49] Despite contributing to the majority of PDAC elderly patients are consistently underrepresented in clinical trials. Treatment of this population of metastatic PDAC is always a challenge and the focus is more often on quality of life (QoL) over prolonging survival. Recent retrospective studies have shown survival advantage (9–13 months) with GA without compromising QoL. Some of the patients required dose modification without need for drug discontinuation.[50–53] In an analysis of 237 patients with metastatic PDAC aged 75 or more, those who received systemic therapy had significantly better survival (p< 0.01) compared to those who did not, with a mean OS difference of 5.6 months.[54] These findings concur with an earlier study of 80 years and older metastatic PDAC patients, where median OS (4.9 versus 1.7 months; HR = 0.41, p < 0.0001) was significantly better in those who received treatment.[55]
Fragile Patients with Advanced Pancreatic Cancer (FRAGANCE), a multicenter, randomized, open label, phase 1/2 trial assessed the safety and efficacy of GA in patients with poor performance status (ECOG PS 2). Patients were divided into four cohorts, however the ones with 3-weeks on and 1-week off gemcitabine (1000 mg/m2) and nab-paclitaxel (100 mg/m2 or 125 mg/m2) were selected for phase 2 based on better tolerability. The patients included in phase 2 part had a median age of 68–70 years with approximately 30% older than 75 years. Both the dose regimens were well tolerated with no difference in PFS (5.7 months and 6.7 months) or 6-month OS (63% and 69%).[56] The study provides evidence for utility of GA in metastatic PDAC patients with poor PS and older age.
A phase 4 multicenter trial is evaluating the use of GA or gemcitabine alone in elderly patients with metastatic PDAC based on comprehensive geriatric assessment.[57] The combination of gemcitabine with capecitabine is another well tolerated alternative in elderly patients with good PS.[58] A phase 3 trial is planned to recruit N= 184 older patients (aged 70 and older, ECOG 0–2) with a new diagnosis of metastatic PDAC who will be randomized to either GA or 5-FU/LV plus nal-IRI.(NCT04233866). Nonetheless, selection of therapy must be individualized and largely based on general health of the patient, nutritional status, comorbidities, PS and patient preferences at the time of treatment.
3.0. Individualized Therapy based on Genomics
3.1. Driver Mutations and Therapeutic Targets
Somatic driver mutations are universal in PDAC and are dominated by KRAS, P53, SMAD4, CDKN2A. Activating mutations in KRAS are detected in >90% of PDAC patients with codon 12 mutations being most frequent.[16, 17] While several agents targeting KRAS-downstream pathways (e.g. Mitogen-activated protein kinase or MEK inhibitors) have been tested alone or in combination with standard cytotoxic therapies and epidermal growth factor receptor (EGFR) inhibitors in advanced PDAC, there are no positive trials.[59] The combination of selumetinib, a MEK inhibitor and erlotinib, an EGFR inhibitor, demonstrated minimal anti-tumor activity in a single arm, phase 2 trial, with a PFS of 1.9 months (95% CI 1.4–3.3) and median OS of 7.3 months (95% CI, 5.2–8.0).[60] Likewise, in a phase 2 randomized trial the combination of selumetinib and MK2206 (AKT inhibitor) resulted in worse OS (3.9 vs 6.7 months; HR, 1.37; 95% CI, 0.90–2.08; P = .15) compared to standard of care modified FOLFOX.[61] The clinical ineffectiveness of these targeted therapies despite good biologic plausibility is likelycan be attributed to the adaptive reactivation of MAPK signaling and multiple pathway redundancies.
RNA interference (RNAi) mediated suppression of KRAS results in inhibition of tumor growth of PDAC tumor growth in preclinical models. Golan et al., in a phase 1/2a trial investigated the role of RNAi approach utilizing siG12D-LODERTM (Silenseed Ltd.) in patients with advanced PDAC. Fourteen patients were treated with standard cytotoxic therapy in combination with LODERTM implantation. The median OS was 15.1 months (95% CI 10.19–18.44), with survival of 27 months reported in the FOLFIRINOX (N=3) group. All patients received a single injection of LODERTM.[62] A randomized, phase 2 trial is currently evaluating the safety and efficacy of the combination of LODERTM with GA compared to chemotherapy alone in inoperable locally advanced PDAC, although it is likely to move forward with a single arm non-randomized design. (NCT01676259)
A small subgroup of patients with mutation in KRAS G12C may benefit from a novel inhibitor of KRASG12C, AMG510.[63] A phase 1 multicenter trial (NCT03600883) is currently evaluating AMG510 in non-small cell lung cancer and other solid tumors with early results demonstrating a favorable safety profile and evidence of clinical activity in NSCLC, colon and appendix cancers.[64] MRTX849, a potent and specific inhibitor of KRASG12C, was reported to cause marked tumor regressions in 65% (17 of 26) of cell lines.[65] Following demonstration of anti-tumor activity in mouse models, the drug was used to treat two patients with mPDAC whose disease was refractory to multiple lines of therapy. Both patients experienced a partial response with >33% tumor regression after 3 cycles of therapy. While this small molecular target may be of less significance in the Caucasian population with PDAC where G12C mutation is seen in 1–2%,[66, 67] it likely will be of particular importance for certain populations like Japanese in whom the mutation is seen in about 60% of PDAC.[68]
3.1.1. KRAS Wild-Type (KRASWT) PDAC and the Targetable Domains
Approximately 6–8% of patients with KRASWT tumor represent a distinct group of PDAC with enrichment of kinases, like neuregulin 1 (NRG1) rearrangements, NTRK fusion, anaplastic lymphoma kinase (ALK) rearrangements and ROS, all of which are therapeutically targetable. Most notably an NRG1 rearrangement confers susceptibility to ERBB inhibitors and anti-EGFR antibodies.[69, 70] Afatinib, a HER-family kinase inhibitor was found to be effective in three of 47 patients with KRASWT mPDAC whose disease had progressed on gemcitabine-based therapy. All three patients had an oncogenic NRG1 fusion and two were treated with afatinib and both had an objective response at 3 months follow up, although one was relatively short-lived. [71] Likewise, in a report by Heining, et al, two of the four patients with KRASWT mPDAC (following FOLFIRINOX and/or GA therapy) who had NRG1 réarrangements, exhibited a partial response to afatinib. Both tumors progressed at three months. Interestingly, in this study 23.5% of PDAC were KRASWT (4 of 17).[70] Zenocutuzumab (MCLA-128), a bispecific HER-2, −3 antibody was used to treat three patients with an NRG1 fusion, at a large academic cancer center, including two patients with metastatic PDAC and one with non-small cell lung cancer. Both patients with PDAC had an excellent and durable response with normalization of CA 19–9. One of them had approximately 50% reduction in tumor size while the other had 25% reduction, and both have continued therapy at the time of writing.[72] An ongoing single arm phase 2 trial is evaluating MCLA-128 in advanced solid tumors with documented NRG1 gene fusions, including a specific PDAC cohort.(NCT02912949).
Fusion of tropomyosin receptor kinase (TRK)-coding genes with other partner genes results in constitutive activation of RAS- and AKT-signaling pathways. NTRK fusion is rare in PDAC and is identified in less than 1% of tumors.[73] Pishvaian and colleagues reported a partial response (RECIST v1.1) to entrectinib, a potent TRK and ROS1 inhibitor in a subgroup of three advanced PDAC patients with the actionable gene rearrangement.[74] These patients were treated as part of a phase 2 multicenter trial involving advanced solid tumors with NTRK1/2/3, ROS1, or ALK Gene Rearrangements.(NCT02568267) Larotrectinib, a highly selective TRK inhibitor was shown to be effective in adults and children with TRK fusion-positive cancers. Although there was only one patient with PDAC, overall response rate in the 55 studied patients was 75% (95 CI 61–85) with 13% (N=7) complete responses and 62% (N=34) partial responses.[75] O’Reilly and colleagues reported a metastatic PDAC patient who was treated with larotrectinib following detection of CTRC-NTRK gene fusion on somatic profiling (refractory to two previous lines of systemic therapy). Tumor control was maintained for approximately 6 months, following which selitrectinib, a next generation TRK inhibitor, was attempted. Unfortunately, the disease quickly progressed.[76] Next generation NTRK inhibitors are being evaluated in solid tumors with an NTRK fusion. (NCT02576431; NCT03215511)
The monoclonal anti-EGFR antibody, nimotuzumab or placebo combined with gemcitabine was evaluated in a randomized phase 2b trial of 186 locally advanced or metastatic PDAC patients. One-year OS (HR 0.69; p=0.034) and PFS (HR 0.68; p 0.016) was significantly better for the nimotuzumab combination over gemcitabine plus placebo. Among 97 patients with known KRAS status 33 (34%) had KRASWT tumors, which was much higher than reported in other studies. Furthermore, KRASWT tumors responded significantly better to the combination (OS 11.6 versus 5.6 months; p=0.03) compared to those with mutant KRAS.[77]
ALK gene translocations have been reported in 0.16% of PDAC. Inhibitors of ALK, crizotinib, ceritinib and other agents, have shown efficacy in patients harboring this chromosomal rearrangement, mostly non-small cell lung cancer. In a comprehensive genomic profiling of over 3,000 PDAC patients, Singhi, et al detected an ALK gene translocation in five patients (0.16%). Four of the five patients were treated with one or more ALK-inhibitors and three demonstrated stable disease as the best outcome. The patients were previously treated with standard cytotoxic regimens including FOLFIRINOX and/or GA. Duration of survival in the patients was reported as 52 months (alive at the time of reporting), 20 months, 10 months and five months. [78, 79] In a case report of a patient with ALK rearranged locally advanced PDAC, crizotinib was reported to be effective following disease progression on FOLFIRINOX. The patient experienced a partial response with continued tumor regression resulting in consideration of surgical resection.[80]
As noted above, BRAF gene mutations are typically mutually exclusive of KRAS mutations representing another genetic alteration in the KRASWT cohort and account for approximately 3% of somatic mutations in PDAC.[81–83, 18] The effectiveness of BRAF- and combined BRAF/MEK-inhibition has led to approval in BRAF-mutant lung cancer, melanoma and thyroid cancers.[84] Guan, et al in their “know Your Tumor” program identified 18 BRAF-mutant PDAC patients and two of which were treated with a dabrafenib/trametinib (BRAF/MEK-inhibition) combination. Notably, 6% of BRAF mutated patients were also KRAS mutated. One of the patients with a BRAF V600E mutation had a sustained response to the combination, while the one individual with concurrent KRASG12A and BRAF K601N mutations failed to respond to the treatment.[83] Treatment response to dabrafenib has been reported in another BRAF mutated PDAC patient nonresponsive to multiple lines of systemic therapy, where an objective response was noted along with normalization of CA 19–9, however the disease progressed after four months.[85] Likewise, Aguirre, et al demonstrated MAPK sensitivity of BRAF altered PDAC. One of two patients with an in-frame BRAF deletion who was treated with trametinib had treatment response with a significant decrease in CA19–9 and evidence of partial response at an 8-week restaging scan, however subsequently the disease progressed after six months of treatment.[86]
3.1.2. Inhibition of Autophagy
KRAS mutations in PDAC have been shown to confer growth dependence on autophagy.[87] Hydroxychloroquine (HCQ), an indirect inhibitor of autophagy has been studied in combination with GA in an open label, randomized phase 2 trial involving 112 untreated metastatic PDAC patients. There was no advantage of adding HCQ to GA in the primary endpoint (median OS 11.1 Vs 12.1 months), however there was a better overall response rate (ORR 38% vs 21%) for the HCQ treated patients.[88] Previously treated metastatic PDAC patients with one or more prior regimens were enrolled in a phase 2 trial of single agent HCQ. The primary endpoint of PFS of 2 months was reached by only 2 patients with median PFS and median OS of 46.5 and 69 days, respectively.[89]
Autophagy has been shown to be deleterious to KRAS-mutant tumors. ERK1/ERK2 mitogen activated protein kinases (MAPK) are critical in sustaining growth in KRAS-mutant PDAC, and ERK inhibitors have been shown to increase dependency of KRAS-mutant PDAC on autophagy and inhibition of growth in combination with HCQs.[87] Thus, a combination of an inhibitor of autophagy with ERK- or MEK-inhibitor is appealing in KRAS-mutant PDAC. Evidence from preclinical evaluation suggests inhibition of tumor cell growth with a combination of MEK-inhibitor (trametinib) and chloroquine.[90] With this rationale, HCQ is being evaluated in combination with GA in patients with advanced PDAC in a phase 1/2 clinical trial, with a primary endpoint of OS.(NCT01506973) Binimetinib, a selective MEK inhibitor, and HCQ combination is being studied in a phase 1, open label trial in KRAS-mutant mPDAC with primary objective to find the maximum tolerated dose. The study also aims to determine the response rate and OS in the treated cohort.(NCT04132505) Likewise, trametinib plus HCQ is undergoing phase 1 evaluation to determine the recommended phase 2 dose in patients with advanced PDAC.(NCT03825289).
In addition, immunotherapy is being combined with autophagy inhibition. A randomized, phase 2 trial is evaluating the clinical benefit of adding avelumab (anti-PD-L1 monoclonal antibody with effect on both innate and adaptive immunity) to HCQ and GA in neoadjuvant setting for borderline resectable PDAC. However, the study has been suspended and results are pending. (NCT03344172) A non-randomized, phase 1/2 open label study is expected to soon begin recruitment of advanced KRAS-mutant cancers for treatment with a combination of cobimetinib (MEK inhibitor), atezolizumab (anti-PD-L1) and HCQ. Phase 1 part will determine maximum tolerated dose with a secondary goal to evaluate clinical efficacy (OS and PFS).(NCT04214418)
Despite the optimism and ongoing interest to inhibit autophagy by combining HCQ with cytotoxic and specific targeted therapies any added benefit from the drug has yet to be seen.
3.2. Germline Mutations in PDAC
Pathogenic germline mutations in BRCA1 or BRCA2 and related genes (ATM, PALB2, CHEK 1/2, ATR) are identified in 5–9% of PDAC.[91, 92] A systematic review from the authors identified 22 studies, including four phase 2 and a phase 3 trial that looked at DDR gene mutations and the corresponding therapeutic targets, particularly platinum agents and PARP inhibitors.[59] However, there are several more studies exploring the subject as noted below.
The safety and clinical efficacy of cisplatin, gemcitabine and veliparib combination was evaluated in a phase 1b trial of previously untreated advanced PDAC patients. Seventeen patients were evaluated as part of two cohorts, germline BRCA-mutant and BRCA-wildtype. BRCA-mutant patients experienced a high objective response in 78% (7 of 9) with improved survival (median OS 23 months [95% CI, 3.8–30.2] versus 11 months [95% CI, 1.5–12.1]) compared to the wild-type cohort.[93] A follow on multicenter, multinational, phase 2, randomized trial evaluated cisplatin and gemcitabine with or without veliparib in advanced PDAC patients with BRCA1/BRCA2 and/or PALB2 mutation. Primary endpoint of response rate (partial response 74% versus 65%, p=0.55), or survival rates were not statistically different between the groups (PFS 10.1 vs 9.7 months, p=0.73; median OS 15.5 vs 16.4 months, p=0.6). Moreover, the triple therapy group experienced greater hematologic toxicity. Nonetheless, the OS represents the highest survival reported in any advanced PDAC trial and thus these data establish cisplatin and gemcitabine as a standard of care option and provide the first prospective data set for the value of platinum therapy in germline BRCA1/2 and PALB2 mutated PDAC.[94] Earlier, in a retrospective analysis of 71 PDAC patients including 43 with advanced disease, Golan and colleagues demonstrated improved OS with platinum-based therapy compared to non-platinum therapies in germline BRCA mutated patients (22 vs 9 months; P=0.039).[95] Subsequently, another retrospective study reported a longer time to treatment failure (294 versus 52 days, p=0.027) with oxaliplatin-based therapy in advanced PDAC patients with BRCA and related gene (ATM, ATR, PALB2) mutations compared to those without these mutations.[96] Among advanced PDAC patients treated with platinum-based therapy the objective response rate was significantly better in those with germline BRCA1/BRCA2 or PALB2 mutation (58% versus 21%) compared to those without the mutations. However, there was no difference in outcome between various platinum regimens in the patients harboring the mutations.[97]
These results are early indicators of positive response to platinum-based therapy in PDAC patients with DDR gene mutations, although the collective data do not allow differentiation of the prognostic effect of having such a mutation from the predictive therapeutic effect. Summing up, either (m)FOLFIRINOX or cisplatin/gemcitabine are appropriate treatment options for this genomically-selected patient population with BRCA1/BRCA2 or PALB2 mutation.
The POLO (Pancreas OLaparib Ongoing) trial was a phase 3, randomized, double-blind, placebo-controlled trial that investigated the effectiveness of the PARP inhibitor olaparib as maintenance therapy in germline BRCA-mutated metastatic PDAC patients who had previously received platinum-based chemotherapy without disease progression. PFS was significantly improved with maintenance olaparib compared to placebo (7.4 months vs. 3.8 months; HR, Hazard ratio for disease progression or death, 0.53; 95% CI, 0.35 to 0.82; P = 0.004).[43] Health-related QoL was assessed in the patients randomized in POLO trial and was found to be preserved with no clinically meaningful deterioration.[98]
Single-agent PARP inhibitors have been evaluated in advanced PDAC patients with DDR gene mutations. In a single-arm non-randomized trial, single agent veliparib failed to demonstrate benefit in previously treated BRCA 1/2- or PALB2-mutated PDAC; however, all but one patient was platinum exposed/refractory likely in large part accounting for the relative lack of activity.[99] Rucaparib (an oral PARP-1 inhibitor) monotherapy was studied in a phase 2 multinational trial in advanced PDAC patients with deleterious germline or somatic BRCA 1/2 or PALB2 mutations. Further patient enrollment was stopped following limited response in the first 15 enrolled patients (overall response rate RR 15.8%, 3 of 19), however, three of the last four enrolled patients had a confirmed response.[100] Rucaparib is also being evaluated in another phase 2 trial involving similar cohort of patients.(NCT03140670) Maintenance rucaparib is being studied by Binder, et al in a single arm, phase 2 trial and results were presented in 2019 at an unplanned interim analysis. Advanced PDAC patients with BRCA 1/2 or PALB2 mutation whose disease had not progressed on platinum-based therapy were enrolled, and the result of 19 patients evaluable for PFS (primary endpoint) was reported. Median PFS was 9.1 months, with eight and two patients alive more than six months and a year respectively.[101]
Likewise, niraparib, a highly specific PARP-1, −2 inhibitor is currently being evaluated in a phase 2 trial in metastatic PDAC patients with somatic or germline defect in multiple DDR genes. (NCT03553004) PARPVAX, a randomized, phase 2 trial of niraparib with immune check point inhibitor, nivolumab (a monoclonal antibody that blocks PD-1 receptor) or ipilimumab (monoclonal antibody that activates CTLA-4 [cytotoxic T lymphocyte associated protein]) is being evaluated in a non-genomically selected patient population with advanced PDAC who have received platinum-based therapy for at least 16 weeks without progression.(NCT03404960) As noted, germline or somatic mutations in DDR genes was not an inclusion criteria with the hypothesis being that responsiveness to platinum agents can be a marker of defective DNA-repair with possible response to PARP inhibitor and immune checkpoint blockade in a broader patient population.
Somatic mutations in DDR genes have been identified in approximately 15% of PDAC.[102, 103] The benefit of platinum-based therapy or PARP inhibitors in tumors with DDR gene mutations beyond BRCA1/2 and PALB2 is not fully known as data is sparse and prospective evaluation is ongoing.[59]
In 2020, olaparib is being increasingly utilized as a maintenance therapy, as an alternative option to continuation of cytotoxic therapy in a germline selected population of patients with advanced PDAC and current clinical trials are looking to build on single agent PARP inhibitor in this disease setting.
4.0. Immunotherapy: Is there a Role in PDAC?
4.1. Mismatch Repair Deficient (dMMR)/MSI-H Tumors
MSI high (MSI-H) or dMMR is a rare occurrence in PDAC with a frequency of approximately 1% and most of these patients (83–100%; 5 of 6 and 7 of 7) are found to have Lynch syndrome.[104, 105] Tumors with dMMR have enhanced expression of mutation-associated neoantigens and strong expression of immune check-point ligands, and have been shown to benefit from immune checkpoint inhibitors (CPI), anti-PD-1 and anti-PD-L1, with improved survival.[106–109] dMMR is determined by immunohistochemistry of tumor samples to detect MLH1 and MSH2, less commonly MSH6 and PMS2.[104] Absence of nuclear staining in tumors along with retained staining in the surrounding benign tissue is interpreted as an MMR deficient tumor. Testing for MSI also involves PCR of microsatellite loci or MSI sensor analysis (bioinformatic algorithm from next generation sequencing (NGS)), and MSI-H is defined as microsatellite instability at two or more loci. Concurrent testing for MSI and MLH1/MSH2 can potentially optimize detection of MSI-H tumors.[110]
In an analysis of 833 PDAC patients who underwent NGS, seven were found to have dMMR (0.8%). Among five patients who received a PD-1 or PDL-1 inhibitor, four had stable disease or durable response.[104] The benefit of single-agent pembrolizumab, a PD-1 inhibitor was further evaluated in a phase 2 trial of dMMR solid tumors. The study included two PDAC patients.[108] These results have led to current recommendation by NCCN to use pembrolizumab as second line therapy in patients with dMMR or MSI-H PDAC. KEYNOTE-028, a large non-randomized, multicenter, phase 1b trial evaluated pembrolizumab in PD-L1 positive solid tumors (20 tumor types, N=475), including 24 patients with PDAC. The results were disappointing for the PDAC cohort with PFS and median OS of 1.7 (95% CI 1.5–2.9) and 3.9 months respectively, and not as encouraging compared to the entire cohort with overall PFS of 2.2 months (95% CI, 1.9 to 3.4 months).[111]
4.2. Tumor Microenvironment and Immunotherapy beyond dMMR/MSI-H
The microenvironment of PDAC is characterized by a paucity of epithelial malignant cells and an abundance of stroma. As noted earlier, the dense ECM forms a mechanical barrier potentially limiting the penetration of drugs. Potential strategies to disrupt the microenvironment include the use of cytotoxic treatment to kill tumor cells with release of tumor antigens, targeting suppressor myeloid cells to disinhibit the immunosuppressive environment, and augmenting an effector T-cell response. Clinical trials of immunotherapy approaches in PDAC have mostly focused on augmenting T-cell responses and overcoming the immune suppression inherent in this disease.
4.2.1. Immune Checkpoint Inhibitor Therapy
Current evidence on the use of PD-1 and PD-L1 inhibitors in PDAC patients beyond dMMR or MSI is disappointing. A recent multicenter, phase 2 randomized trial evaluated N= 65 previously treated (first-line gemcitabine- or fluoropyrimidine-based therapy) metastatic PDAC patients for the efficacy of durvalumab (anti-PD-L1 antibody) alone or in combination with anti-cytotoxic T-lymphocyte antigen (CTLA) 4 antibody, tremelimumab. The objective response rate was 3.1% (95% CI, 0.08–16.22) in the combination cohort and no responses were observed with monotherapy. The median PFS was the same in both arms, 1.5 months (95% CI, 1.2–1.5 and 1.3–1.5), as was median OS, 3.1 months (95% CI, 2.2–6.1) in the combination arm vs 3.6 months (95% CI, 2.7–6.1) with monotherapy. Interestingly, three patients survived longer and were alive at 61–65 weeks during data cut off.[112] Ipilimumab in combination with gemcitabine was found to be safe in a Phase 1b trial and there was a delayed response in one of the patients.[113] A randomized, phase 2 trial is underway to evaluate the efficacy of GA in combination with durvalumab and tremelimumab in treatment naïve metastatic PDAC patients compared to GA.(NCT02879318), and results are anticipated in 2020.
Le, et al provided data underscoring the challenge for immunotherapy in PDAC. In a phase 1b, open-label trial, 30 previously treated advanced PDAC patients were randomized either to treatment with ipilimumab (arm 1) or a combination of ipilimumab and GVAX (granulocyte-macrophage colony-stimulating factor gene transduced autologous pancreatic cancer vaccine) (arm 2). Best response was stable disease in two patients in arm 1 and three patients experienced prolonged stable disease with CA19–9 decline in arm 2. Median OS was 3.6 and 5.7 months (p=0.07) in the two arms.[114] In an earlier phase 2 trial by Royal, et al, single agent ipilimumab was found to be ineffective in treating advanced PDAC (N=27). However, one patient experienced delayed response with normalization of tumor markers and regression of liver metastases.[115] These studies provide further evidence that single or dual checkpoint inhibition in PDAC beyond MSI-H/dMMR is insufficient to overcome the immune suppression challenges of this disease, nonetheless these agents may benefit selective patients with advanced PDAC.
4.2.2. Chemotherapy and Immune Checkpoint Inhibitor and other Immune Therapy Combinations
Pembrolizumab was tested in combination with GA in a single arm non-randomized phase 1b/2 trial of treatment naïve metastatic PDAC patients with good PS. Although the study did not meet the primary endpoint of >15% complete response rate, the OS (15 months) and PFS (8.1 months) appeared to be better than typically reported for first-line GA in this population, accepting the caveats of the select centers, non-randomized design.[116] Further, the patients were younger than typically included in PDAC trials (usually mid 60’s) with a median age of 56 years.
Preclinical studies have suggested the plausibility of inhibiting granulocytic myeloid-derived suppressor cells (MDSC) and unmasking the endogenous T-cell response.[117] Additionally, a translational study has shown growth arrest or even regression of PDAC following depletion of suppressor myeloid cells is KRASG12D mutant PDAC.[118] CD40 has been identified as an interesting immunotherapy target in human cancers by virtue of its ability to stimulate helper T cell immune response and macrophage differentiation. CD40 ligand (CD40L) gene therapy utilizing trimerized CD40L has been shown to increase tumor infiltrating T cells in vivo and demonstrated an oncolytic effect.[119] Dendritic cells play an important role in initiating anti-tumor immune response, however they are often depleted in the PDAC microenvironment. CDX-1140, a CD40 agonist monoclonal antibody is being tested in a phase 1 dose escalation trial alone or in combination with CDX-301 (a dendritic cell growth factor) in solid tumors and non-Hodgkin lymphoma refractory to other therapies. Preliminary results suggest expected safety profile suggested from preclinical studies, and signs of biologic activity evidenced by activation of peripheral lymphocytes and enhanced cytokine activity.[120] A multicenter, open label, phase 1b trial was conducted to determine the recommended phase 2 dose of APX005M, a CD40 agonistic monoclonal antibody in combination with GA ± nivolumab. The authors concluded that the combination had manageable safety profile and there was demonstration of anti-tumor activity with 58% partial response (14/24) and 33% (8/24) stable disease during 32 weeks follow up.[121]. A randomized phase II trial evaluating 3 experimental arms, GA and nivolumab, GA and APX005M and GA with both APX005M and nivolumab, has completed and results are anticipated in 2020.(NCT03214250)
Anti-colony stimulating factor-1 receptor (anti-CSF-1R) antibody, cabiralizumab (targets macrophages) is being evaluated in combination with nivolumab and gemcitabine in an open label, phase 2, randomized trial of metastatic PDAC patients whose disease has progressed on first line chemotherapy. (NCT03697564) This follows phase 1 studies of anti-CSF-1R antibody in combination with anti-PDL1 (Durvalumab; NCT02777710) and anti-PD1 (Nivolumab; NCT02526017) in advanced PDAC and other solid tumors. These phase 1 trials are complete and the final reports are pending.
4.2.3. Oncolytic Virus Therapy
Another strategy for immunotherapy in PDAC is the use of oncolytic virus therapy which can induce local infiltration of T cells and upregulate PD-L1. Pelareorep, an oncolytic reovirus, and pembrolizumab was combined with chemotherapy (gemcitabine, 5-fluorouracil or irinotecan) in a phase 1b trial involving previously treated patients with PDAC. The combination was well tolerated and demonstrated some benefit (3/11 patients had PR or SD).[122] Further investigation is warranted.
4.2.4. Leveraging the Local Tumor-Immune System
Activation of tumor-specific T cells and their influx into the tumor microenvironment may be key to surmount innate immune suppression. CRS-207, a live-attenuated Listeria monocytogenes bacterial strain, has ability to promote an innate immune response and activate mesothelin specific cell mediated immunity. Sequential treatment with GVAX pancreas in combination with cyclophosphamide followed by CRS-207 resulted in an OS of 6.1 months in previously treated metastatic PDAC in a phase 2, randomized trial. In the subgroup of patients who had received two or more lines of therapy median OS was 5.7 months.[123] These results prompted a phase 2b, triple-arm, randomized trial comparing the combination of cyclophosphamide, GVAX and CRS-207, CRS-207 alone, and single-agent chemotherapy in metastatic PDAC who had previously received at least two lines of cytotoxic therapy including gemcitabine. However, the primary endpoint of OS in the three arms was not significantly different (3.4 Vs 5.4 Vs 4.6 months).[124] Negative results in the trial compared to the preceding one were attributed to the poor prognostic factors including higher CA 19–9 levels, more frequent poorly differentiated tumors and more liver metastasis.
Ibrutinib, a BTK- and an ITK-inhibitor, which is thought to reprogram the tumor immune-microenvironment, was studied in a randomized, phase 2/3, placebo-controlled trial involving 424 metastatic PDAC patients. There was no significant difference in median OS between the groups (9.7 vs 10.8 months; HR=1.1; P =0.32). Moreover, median PFS was numerically worse in the treatment arm compared to placebo (5.3 Vs 6 months; HR 1.5; p=<0.0001). [125] No further development of ibrutinib is planned in PDAC.
Napabucasin, an oral stemness inhibitor with antineoplastic activity (unknown exact target), was being studied in combination with weekly GA in a randomized, open-label, multi-center, phase 3 trial of previously untreated advanced PDAC patients (approximately 1,000 enrolled). (NCT02993731) The study has been discontinued per recommendation of an independent data and safety board, after the prespecified interim analysis of futility at 50% of total events was reached, according to the press release from the sponsors. (https://www.bostonbiomedical.com/news-and-media/20190701_boston-biomedical-inc-announces-update-canstem111p-study-following-interim-analysis/). Thus, no further development of napabucasin in PDAC is planned.
These preliminary results to target various immunotherapeutic pathways are interesting however, here-to-fore, none have proven effective in PDAC and strategies to overcome the inherent immune-resistance of this disease are needed.
5.0. Targeting the Stroma
Pancreatic stellate cells are activated to myofibroblasts which secrete ECM. HA is the major component of ECM which can be degraded by hyaluronidase. PEGylated recombinant hyaluronidase alfa (PEGH20) was tested in a randomized phase 2 trial, HALO 202[126], in combination with GA. In this mid phase study, there was an improvement in PFS in patients with treatment naïve metastatic PDAC (HR 0.73; 95% CI 0.53 to 1.00) without OS advantage. However, the results of HALO-301, a phase 3 randomized trial involving patients with previously untreated HA-high metastatic PDAC, are very disappointing. PEGPH20 with GA compared to GA alone, resulted in similar median OS (11.2 vs 11.5 months, HR 1.0, p=0.96), and the secondary endpoints of PFS or duration of response.[127] These results have recently led to the cessation of development of PEGPH20 in PDAC and other cancers.
5.1. Role of Vitamin D Analogues
A potential therapeutic target in the PDAC microenvironment is the vitamin D receptor which is thought to interfere with fibroblast activation and thus, inhibiting ECM deposition. PSC’s have abundant vitamin D receptors and thus blockade of the receptors inhibits production of stroma. Calcipotriol, a synthetic derivative of calcitriol in combination with gemcitabine was shown to reduce tumor volume and growth in 70% of mice.[128] Likewise, paricalcitol, a vitamin D analog, has been shown to promote breaches in the pancreatic cancer stroma.[128] Furthermore, PSC inactivation can potentially interfere with local tumor growth, angiogenesis and metastasis. An in vitro study has also shown antiproliferative effect via upregulation of cell cycle inhibitors (p17, p21).[129] Vitamin D analogs are being evaluated in combination with chemotherapy and/or immunotherapy.
Following demonstration of its effectiveness in preclinical set up,[129] paricalcitol is currently being evaluated in a double-blind, randomized, phase 2 trial in combination with pembrolizumab as maintenance therapy for patients with metastatic PDAC who have responded to standard cytotoxic therapy.(NCT03331562) A randomized, open label, placebo-controlled trial is evaluating the benefit of adding paricalcitol to GA in patients with metastatic PDAC.(NCT03520790) The addition of paricalcitol to the combination of cisplatin, gemcitabine and nab-paclitaxel is being studied in a phase 2, randomized, pilot study of untreated metastatic PDAC patients.(NCT03415854)
5.2. Connective tissue growth factor (CTGF) antagonism
PDAC is characterized by pronounced CTGF expression and has been shown to enhance tumor proliferation and invasiveness in preclinical models. Pamrevlumab (FG-3019), a human monoclonal antibody was shown to attenuate tumor growth and metastasis in mouse models of PDAC.[130] A recent phase 1/2, randomized trial examined pamrevlumab in combination with GA to treat locally advanced PDAC, and found it to be safe without increase in serious toxicity. Patients were assessed following six cycles of treatment, and significantly higher proportion were found to have resectable disease (70.8% versus 15.4%) and underwent surgery (33.3% versus 7.7%) in the pamrevlumab plus GA arm compared to GA. There was an indication of improved OS (27.73 versus 18.40 months, p=0.07) and PFS 16.39 versus 10.38 months, p=0.37), although statistically insignificant.[131] An ongoing phase 3, randomized, double-blind trial is recruiting patients with locally advanced, unresectable PDAC for treatment with a combination of GA plus pamrevlumab or placebo with a primary endpoint of OS and proportion of patients that undergo successful tumor resection.(NCT03941093)
5.3. Anti-Angiogenesis
PDAC is characterized by a paucity of microvasculature which is often typified during radiologic diagnosis (computed tomography, CT scans). Hypovascularity along with the dense stroma limits delivery of drugs into the tumor. A randomized, phase 3, placebo-controlled trial (CALGB 80303: cancer and leukemia group) demonstrated no survival benefit of adding bevacizumab to gemcitabine (median OS 5.9 Vs 5.8 months, p=0.95; PFS 3.8 Vs 2.9 months, p=0.07) in advanced PDAC.[132] Bevacizumab was studied in a phase I/II trial (FABLOx) in combination with 5-fluorouracil, leucovorin, nab-paclitaxel and oxaliplatin in patients with treatment naïve metastatic pancreatic cancer. The overall response rate was 33% (4/12 partial responses) with a PFS of 5.6 months and median OS of 9.9 months. The authors concluded that the combination was tolerable and a signal of interest was evident.[133] The benefit of adding bevacizumab to low-dose 5-fluorouracil, nab-paclitaxel and oxaliplatin was reported in a retrospective analysis of 65 advanced PDAC patients (70% metastatic) with objective response rate (ORR) of 71% and median survival of 27 months.[134]
While VEGF-inhibition in combination with cytotoxic therapy has thus far not yielded a positive result in PDAC, an area of interest is in combination with PARP inhibitor following encouraging results from a double blind, randomized, phase 3b trial that demonstrated significant survival benefit in advanced ovarian cancer. Bevacizumab plus olaparib resulted in PFS of 22 months compared to 16 months with bevacizumab alone (HR of disease progression or death 0.59; 95% CI, 0.49 to 0.72; P<0.001), interestingly regardless of BRCA mutation status. However, tumors with homologous recombination deficiency benefitted the most.[135] Future clinical trials are likely to target this novel combination in patients with advanced PDAC.
5.4. Targeting the Powerhouse: Metabolic Pathways
Devimistat (CPI-613) is a lipoate analog that interferes with mitochondrial metabolism (tricarboxylic acid cycle via pyruvate dehydrogenase regulatory kinases) and within tumor cells, results in cell death via multiple putatative pathways, including necrosis, apoptosis or autophagy. The agent has demonstrated antitumor activity in non-small cell lung cancer and pancreatic cancer cell-lines.[136] Devimistat was evaluated in a small single arm, non-randomized phase Ib trial in combination with modified FOLFIRINOX. Of 18 patients 11 (61%, 95% CI 36–83%) had an objective response with median PFS of 11.5 months (95% CI 133–560).[137] The encouraging preliminary results from this phase 1 trial have led to a phase 3 open label, multi-center trial that is recruiting patients to devimistat combined with (m) FOLFIRINOX compared to FOLFIRINOX with a goal to recruit 500 participants with untreated metastatic PDAC (AVENGER trial). The primary endpoints are the overall response rate (ORR) (complete or partial response) and PFS. (NCT03504423)
Ubidecarenone interferes with the mitochondrial bioenergetics causing generation of reactive oxygen molecules and consequent apoptosis. A phase 1 trial has provided initial safety data for ubidecarenone alone and in combination with gemcitabine which was found well tolerated with grade 1–2 toxicities (INR prolongation and thrombocytopenia). (NCT01957735).[138] BMP31510-IV (uses a nano-dispersion mechanism to deliver ubidecarenone preferentially into the cancer cells) is being evaluated in combination with gemcitabine in an open label, phase 2, multicenter trial. Forty-nine patients have been enrolled thus far and the primary endpoint is overall response rate. In addition, the study aims to perform multi-omic profiling to guide individualized therapy in the future.[139] The nano-dispersion technology to specifically target tumor cells appears to be an attractive model in PDAC, and awaits confirmation in later phase trials.
Lowery and colleagues conducted a phase 1/1b, open-label, non-randomized trial to determine the maximum tolerated dose and recommended phase 2 dose of pegylated arginine deiminase (thus, selective targeting of cancer cells which lack argininosynthetase succinate), ADI-PEG20 in combination with GA in metastatic PDAC patients who have not received more than one prior treatment. The regimen was found to be well tolerated with overall response rate of 45% in those at the recommended phase 2 dose. Median PFS was 6 months (95% CI 5.3–11.2 months) and median OS was 11 months (95% CI 6.7- not reached).[140]
Modulation of glutamine and asparagine levels make KRAS-driven PDAC cells vulnerable to cell death. Eryaspase (asparaginase encapsulated within erythrocytes) was studied in a multicenter, phase 2b, randomized trial as a second line therapy in combination with cytotoxic therapy (compared to chemotherapy alone), with primary endpoint of OS and PFS in patients with low asparaginase level. Patients treated with eryaspase plus chemotherapy (gemcitabine or combination of 5-FU/LV + oxaliplatin) experienced improved OS (6.0 months versus 4.4 months; HR, 0.60; p=0.008) and PFS (2.0 versus 1.6 months; HR, 0.56; p = 0.005).[141] Earlier, a phase 1 trial of patients with metastatic PDAC provided safety data and phase 2 dose of single agent eryaspase[142] An ongoing phase 3 trial is evaluating the efficacy of eryaspase in combination with GA or 5-FU/LV plus irinotecan as second line treatment of metastatic PDAC and has recently opened in the US.(NCT03665441)
Manipulation of the cancer cell metabolism via different mechanisms appears to be an exciting addition to the realm of targeted therapies in advanced PDAC, and it will be interesting to see the results of the ongoing clinical trials.
6.0. Other Prospective Targets
Androgens have been shown to promote PDAC growth. Enzalutamide, an androgen receptor (AR) antagonist, was combined with GA in AR+ metastatic PDAC in a phase 1 trial. There was no dose-limiting toxicity, although myelosuppression and nausea were common. The median OS and PFS was 9.7 and 7.5 months, respectively.[143] Olaratumab, a fully humanized monoclonal antibody against PDGFRα, was studied with GA in metastatic PDAC in a phase 1b dose-escalation trial. The combination was well tolerated with some clinical response (2/15 partial responses and 11 stable disease).[144] At this time, there is interest in further evaluation of TGF-b signaling inhibition therapies and several molecules are in early phase of clinical development.
The Axl pathway has been shown to mediate immune evasion and resistance to chemotherapeutic agents. Following encouraging results from a pre-clinical trial[145], bemcentinib (BGB324), an oral Axl inhibitor, is currently being tested in a multicenter, randomized, phase 1b/2 clinical trial in advanced PDAC. Patients are randomized to GA, cisplatin with or without bemcentinib. The primary outcome is to identify the complete response rate.[146]
Adenosine has been identified as an important regulator of tumor proliferation, survival and migration. Inhibition of adenosine receptor, A2AR has been shown to modulate immune response within the tumor microenvironment and thus, augmentation of anti-tumor effects.[147] Evidence from preclinical studies targeting adenosinergic pathways in combination with cytotoxic therapy and immunotherapy indicate antitumor effect[147] and have led to clinical trials targeting adenosine receptor, A2AR. Ciforadenant, an oral A2AR antagonist is being studied in a phase 1/1b, randomized, open-label trial in combination with anti-CD73 monoclonal antibody or pembrolizumab in patients with advanced solid cancers (including PDAC).(NCT03454451) NIR178, another experimental orally available A2AR antagonist is being evaluated in combination with PDR001 (anti-PD-1 monoclonal antibody) in a phase 2, nonrandomized trial involving solid tumors (including PDAC) and non-Hodgkin lymphoma.(NCT03207867) A phase 1/1b, non-randomized trial is assessing the safety and tolerability of TTX-030 (anti-CD39 antibody which can prevent adenosine mediated immunosuppression and leads to T cells activation in the TME) alone or in combination with pembrolizumab or chemotherapy in advanced cancers.(NCT03884556)
7.0. Thromboembolism and Management in PDAC
PDAC represents one of the select malignancies (others: lung, brain and colorectal cancers) at the highest risk of venous thromboembolism (VTE) and has been found in 23 to 36% of patients.[148, 149] PDAC’s express high levels of tissue factor (TF) and the expression correlates with histologic grade.[150] TF+ micro-vesicles have been found in all these cancers, however highest levels were identified in PDAC and this correlated with the risk of VTE.[151, 152] This pathophysiologic basis of VTE in PDAC has led to the exploration of novel targets (e.g. Phosphatidylethanolamine) for thromboprophylaxis.[153]
VTE has been associated with interruption in treatment and increased mortality in patients with PDAC (HR 2.6; 95% CI, 2.3–2.8; P < .01).[148] The CASSINI trial, a multicenter, randomized, double-blind, placebo-controlled phase 3b study involving ambulatory cancer patients at high risk (Khorana score 2 or more; a scale of 0–6 where higher score indicates an increased risk of thrombosis) of VTE did not find any significant benefit of prophylactic rivaroxaban, an oral factor Xa inhibitor over placebo (HR 0.66, 95% CI 0.40 to 1.09; P=0.10). However, during the intervention period rivaroxoban demonstrated reduction in the incidence of thromboembolism (HR 0.40; 95% CI, 0.20 to 0.80).[154] In a prespecified subgroup analysis of the CASSINI trial, the primary endpoint of major bleeding was not different in the two arms (HR 0.35; 95%CI 0.13–0.97; p = 0.03) during the treatment period.[155] Thus, there was reduced risk of thrombosis with no increase in major bleeding events during intervention period with rivaroxaban in advanced PDAC patients starting systemic therapy indicating potential benefit of prophylactic rivaroxaban in these patients.
Moreover, certain treatment regimens may pose a greater risk of VTE (e.g., PEGPH20) with chemotherapy. An ongoing pilot study is evaluating the safety and efficacy of rivaroxaban in combination with GA and PEGPH20.(NCT02921022) The initial data suggests rivaroxaban to be safe with a reduced incidence of VTE than historically reported.[156] https://doi.org/10.1111/j.1538-7836.2012.04754.xUpdated ASCO guidelines endorse offering thromboprophylaxis (with apixaban, rivaroxaban or low molecular weight heparin) for ambulatory cancer patients at high risk of VTE. [157] A prospective observational study of ambulatory cancer (gastrointestinal, thoracic, gynecologic and brain) patients at high risk of VTE is evaluating the rate of symptomatic and incidental thrombotic events in those who are on thromboprophylaxis based on current practice guidelines.(NCT03909399) Benefit of prolonged perioperative thromboprophylaxis with various agents is being assessed in multiple randomized trials in solid tumors (colorectal and ovarian cancer) at high risk of VTE.(NCT01455831, NCT02366871, NCT03532139) Further clinical trials utilizing risk stratification tools may provide better evidence to standardize use of thromboprophylaxis in a subgroup of ambulatory PDAC patients at the highest risk.
Table 1 lists the ongoing clinical trials exploring various targeted therapies in advanced PDAC.
Table 1.
Ongoing Clinical Trials Evaluating Selected Targets/Pathways in Advanced Pancreas Cancer.
| Target | Population | Drug | Additional therapy | Treatment setting | Ongoing clinical trials | Status¥ |
|---|---|---|---|---|---|---|
| Targeting the KRAS Pathway | ||||||
| HER2 | Advanced pancreatic, biliary cancers | Afatinib | Gemcitabine | Any line | Phase 1b NCT02451553 | Recruiting |
| Solid tumors with NRG1 fusion | Zenocutuzumab | None | Any line | Phase 1/2 NCT02912949 | Recruiting | |
| NTRK fusion | NTRK/ROS1/ALK gene rearranged advanced/metastatic solid tumors | Entrectinib | None | Any line | Phase 2 NCT02568267 | Recruiting |
| ALK, ROS1 gene translocations | ALK, ROS1 translocated solid tumors | Crizotinib | None | Any line | Phase 2 NCT02465060 (MATCH screening trial) | Recruiting |
| BRAF V600E mutation | BRAF V600E/R/K/D mutant solid tumors | Dabrafenib | Trametinib | Any line | Phase 2 NCT02465060 (MATCH screening trial) | Recruiting |
| KRASG12C | KRASG12C-mutant PDAC, solid tumors | AMG-510 | Alone or +MEK-inhibitor | ND | Phase 1b NCT04185883 | Recruiting |
| Alone | ND | Phase 1/2 NCT03600883 | Recruiting | |||
| MRTX849 | Alone | ND | Phase 1/2 NCT03785249 | |||
| Autophagy | KRAS-mutant PDAC | Hydroxychloroquine | GA | Any | Phase 1/2 NCT01506973 | Recruiting |
| MEK-inhibitors: Binimetinib, Trametinib | Any | Phase 1 NCT03825289, NCT04132505 | Recruiting | |||
| KRASG12D | KRASG12D-mutant mPDAC | KRASG12D siRNA | None | Second-line or more | Phase 1 NCT03608631 | Recruiting |
| Tumor Metabolism | ||||||
| Mitochondrial metabolism | mPDAC | Devimistat (CPI-613) | (m)FOLFIRINOX | First-line | Phase 3 NCT03504423 | Recruiting |
| Ubidecarenone (BMP31150) | Gemcitabine | Second- or third-line | Phase 2 NCT02650804 | Recruiting | ||
| Eryaspase | GA or 5-FU/LV plus Irinotecan | Second-line | Phase 3 NCT03665441 | Recruiting | ||
| Targeting the Tumor Microenvironment | ||||||
| Focal adhesion kinase (FAK) | Advanced solid tumors Advanced PDAC |
Defactinib | Gemcitabine, pembrolizumab | Second-line | Phase 1 NCT02546531 | Recruiting complete |
| Pembrolizumab | Exhausted standard CT | Phase 1/2 NCT02758587 | ||||
| GSK2256098 | Trametinib | Exhausted standard CT | Phase 2 NCT02428270 | Complete | ||
| Vitamin D pathway | mPDAC | Paricalcitol | Pembrolizumab | Maintenance Phase 2 NCT02428270 | Phase 2 NCT03331562, NCT03520790 | Complete |
| IDO1 inhibition | mPDAC | Epacadostat | Pembrolizumab, CRS-207 | Second-line | Phase 2 NCT03006302 | Recruiting |
| Immunotherapeutic strategies: Beyond MMR Gene Defects, MSI-H tumors | ||||||
| PD-1 | mPDAC, advanced PDAC | Pembrolizumab | PEGPH20 | Second-line | Phase 2 NCT04058964 | Not yet recruiting |
| Paricalcitol | Maintenance | Phase 2 NCT03331562 | Recruiting | |||
| Azacytidine | Second-line | Phase 2 NCT03264404 | Recruiting | |||
| BL-8040 | Second-line | Phase 2b NCT02907099 | Complete | |||
| Sonidegib | Second-line | Phase 1 (Part B) NCT04007744 | Recruiting | |||
| Nivolumab | Cabiralizumab+ gemcitabine/nab-paclitaxel | Second-line | Phase 2 NCT03697564 | Recruiting | ||
| PD-L1 | RAS-mutant solid tumors (mPDAC) | Avelumab | Binimetinib+ talazoparib | Second-line or later | Phase 1b/2 NCT03637491 | Recruiting |
| Oncolytic virus | Advanced PDAC | Pelareorep | Pembrolizumab | Second-line | Phase 2 NCT03723915 | Recruiting |
| Peptide vaccine | mPDAC, CRC | Tumor associated peptide vaccine | Personolized peptide vaccine | Imiquimod (topical) Pembrolizumab | Pilot NCT02600949 | Active not recruiting |
| T cells therapy | Metastatic GI cancers | Autologous CD 8+ T cells | Pembrolizumab | Second-line or more | Pilot NCT02757391 | Complete |
| CD40 | mPDAC | APX005M (CD40 agonist monoclonal Ab) | GA± nivolumab | First-line | Phase 1b/2 NCT03214250 | Active not recruiting |
| Combination immunotherapy | mPDAC | CRS-207, nivolumab, ipilimumab | ± GVAX | Second-line | Phase 2 NCT03190265 | Recruiting |
| Epacadostat | Pembrolizumab, CRS-207 | Second-line | Phase 2 NCT03006302 | Recruiting | ||
| Atezolizumab | CT, multiple immunotherapy (Emactuzumab, Selicrulumab, etc.) | Cohort 1: First-line; Cohort 2: second line | Phase 1b/2 NCT03193190 | Recruiting | ||
| mPDAC | Durvalumab+ tremelimumab | GA | First-line | Phase 2 NCT02879318 | Recruiting | |
| DDR Gene Mutation | ||||||
| DDR gene | Germline/somatic BRCA, PALB2 mutated mPDAC (not progressed on platinum-based therapy) | Rucaparib | None | Maintenance | Phase 2 NCT03140670 | Recruiting |
| Germline/somatic DDR gene mutated solid tumors | Rucaparib | None | Second-line | Phase 2 NCT04171700 | Recruiting | |
| mPDAC (other GI cancers) with DDR gene mutations | Rucaparib | 5-FU/LV + Irinotecan | First-line¶ | Phase 2 NCT03337087 | Recruiting | |
| mPDAC (±DDR gene mutation) | Rucaparib | FOLFIRI | Second-line | Phase 2 NCT02890355 | Active, not recruiting | |
| Advanced PDAC with BRCA1/2, PALB2, CHEK2 or ATM mutation | Niraparib | None | Second-line or more | Phase 2 NCT03601923 | Recruiting | |
| Phase 2 NCT03553004 | Recruiting | |||||
| mPDAC with DDR gene mutations | Niraparib | Nivolumab or ipilumumab (Parpvax) | Second-line or more | Phase 1b/2 NCT03404960 | Recruiting | |
| Other Approaches | ||||||
| Adenosine | Advanced solid cancers | Ciforadenant (A2AR antagonist) | CPI006, pembrolizumab | Second-line or more | Phase 1/1b NCT03454451 | Recruiting |
| NIR178 (A2AR antagonist) | PDR001 (anti-PD-1) | Second-line or more | Phase 2 (nonrandomized) NCT03207867 | Recruiting | ||
| TTX-030 (anti-CD39 antibody | Pembrolizumab or chemotherapy | ND | Phase 1/1b (nonrandomized) NCT03884556 | Recruiting | ||
| AXL pathway | mPDAC | Bemcentinib (BGB324) | GA | First-line | Phase 1b/2 NCT03649321 | Recruiting |
| RNAi | Locally advanced PDAC | siG12D-LODERTM | GA | First-line | Phase 2 NCT01676259† | Recruiting |
| PDGFR | mPDAC | Olaratumab | GA | First-line | Phase 1b/2 NCT03086369 | |
| Hedgehog pathway | Advanced solid tumors | Sonidegib | Pembrolizumab | Phase 1 NCT04007744 | Not yet recruiting | |
| Toll-like receptor | Refractory mPDAC | SD-101 | Nivolumab, radiation | Second-line or more | Pilot NCT04050085 | |
Date accessed 02/02/2020; § Trials of PEGPH20 has been negative so far;
Also includes phase 1 and phase 1b.
Part 1 and part 2;
Being amended to include (m)FOLFIRINOX.
mPDAC metastatic pancreatic ductal adenocarcinoma; GA gemcitabine plus nab-paclitaxel; mFOLFIRINOX modified regimen of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin; 5-FU/LV 5-fluorouracil plus leucovorin; HA hyaluronic acid; CT chemotherapy; IDO1 indoleamine 2,3-dioxygenase-1; CRS-207 Listeria monocytogenes expressing mesothelin; CRC colorectal cancer; GI gastrointestinal; ND not defined.
8.0. Conclusions
Progress is evident in the treatment of PDAC. Survival is steadily improving and there is much optimism that a deeper understanding of tumor pathobiology will lead to improved therapies and outcomes in this disease now and moving forward. Concrete recent developments include the importance and recognition of identifying patients with targetable germline and somatic alterations and integration of routine germline and somatic testing in daily practice. Proof of principle of this approach is established with the POLO trial and the recent FDA approval of olaparib as a maintenance therapy in PDAC, and next steps are to build on this signal in a broader population of patients with PDAC by combination and other approaches. There remains a very extensive therapeutic focus on the tumor microenvironment and immune strategies, nonetheless in light of key recent negative trials, it remains to be seen whether targeting these approaches will become integrated as part of standard therapy going forward given the several recent trial reports related to PEGPH20, ibrutinib and napabucasin. Recognition of the importance of metabolism as a central regulator in cell growth has led to a series of exciting trials targeting novel mitochondrial and other pathways. Looking forward, it is likely that stromal subtypes will help further refine treatment choices in PDAC and the expectation is that these descriptors will be proximately used in clinical practice.
Key points:
Pancreatic ductal adenocarcinoma (PDAC) is one of the most challenging malignancies with inherent therapy resistance and poor outcome.
Knowledge of PDAC biology is expanding leading to extensive research exploring targeted therapies and identification of prognostic sub-groups.
Targeting genomic alterations, tumor microenvironment and/or immunotherapy alongside cytotoxic therapy is anticipated to improve survival in select patients.
Funding Sources
David M. Rubenstein Center for Pancreatic Cancer Research
Cancer Center Support Grant P30 CA 008748
Footnotes
Conflict of Interest
EOR: Research funding to MSK: Genentech, Roche, Celgene/BMS, MabVax Therapeutics, ActaBiologica, AstraZeneca, Silenseed
Consulting/Advisory role: BioLineRx, Targovax, Celgene, Polaris, Sobi, Merck, Ipsen
Data Safety Monitoring Boards: CytomX Therapeutics, Rafael
RRS: No conflicts of interest
References
- 1.Facts & Figures 2020. American Cancer Society; Atlanta, GA: 2020. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Ansari D, Althini C, Ohlsson H, Andersson R. Early-onset pancreatic cancer: a population-based study using the SEER registry. Langenbeck’s archives of surgery. 2019;404(5):565–71. doi: 10.1007/s00423-019-01810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ntala C, Debernardi S, Feakins RM, Crnogorac-Jurcevic T. Demographic, clinical, and pathological features of early onset pancreatic cancer patients. BMC gastroenterology. 2018;18(1):139. doi: 10.1186/s12876-018-0866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piciucchi M, Capurso G, Valente R, Larghi A, Archibugi L, Signoretti M et al. Early onset pancreatic cancer: risk factors, presentation and outcome. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2015;15(2):151–5. doi: 10.1016/j.pan.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Tingstedt B, Weitkamper C, Andersson R. Early onset pancreatic cancer: a controlled trial. Annals of gastroenterology. 2011;24(3):206–12. [PMC free article] [PubMed] [Google Scholar]
- 7.Anna M Varghese IS, Singh Ritu Raj, Capanu Marinela, Chou Joanne F., Wong Winston, Stadler Zsofia Kinga, Salo-Mullen Erin E., Iacobuzio-Donahue Christine A, Kelsen David Paul, Park Wungki, Yu Kenneth H., O’Reilly Eileen Mary. Young-onset pancreas cancer (PC) in patients less than or equal to 50 years old at Memorial Sloan Kettering (MSK): Descriptors, genomics, and outcomes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020;38. [Google Scholar]
- 8.Facts & Figures 2014. American Cancer Society; Atlanta, GA: 2014. [Google Scholar]
- 9.National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer. Surveillance, Epidemiology and End Results Program. 2019. [Google Scholar]
- 10.Eileen Mary O’Reilly AS, Zheng Wu, Paul Cockrum. Real-world patterns of care among patients with metastatic pancreatic cancer (mPC). Gastrointestinal Cancers Symposium 2020; San Francisco: Journal of Clinical Oncology; 2020. [Google Scholar]
- 11.Vogelstein B, Kinzler KW. The Path to Cancer — Three Strikes and You’re Out. 2015;373(20):1895–8. doi: 10.1056/NEJMp1508811. [DOI] [PubMed] [Google Scholar]
- 12.Klein WM, Hruban RH, Klein-Szanto AJ, Wilentz RE. Direct correlation between proliferative activity and dysplasia in pancreatic intraepithelial neoplasia (PanIN): additional evidence for a recently proposed model of progression. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2002;15(4):441–7. doi: 10.1038/modpathol.3880544. [DOI] [PubMed] [Google Scholar]
- 13.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makohon-Moore A, Iacobuzio-Donahue CA. Pancreatic cancer biology and genetics from an evolutionary perspective. Nature reviews Cancer. 2016;16(9):553–65. doi: 10.1038/nrc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wartenberg M, Cibin S, Zlobec I, Vassella E, Eppenberger-Castori S, Terracciano L et al. Integrated Genomic and Immunophenotypic Classification of Pancreatic Cancer Reveals Three Distinct Subtypes with Prognostic/Predictive Significance. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018;24(18):4444–54. doi: 10.1158/1078-0432.Ccr-17-3401. [DOI] [PubMed] [Google Scholar]
- 16.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491(7424):399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yachida S, White CM, Naito Y, Zhong Y, Brosnan JA, Macgregor-Das AM et al. Clinical significance of the genetic landscape of pancreatic cancer and implications for identification of potential long-term survivors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(22):6339–47. doi: 10.1158/1078-0432.Ccr-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowery MA, Jordan EJ, Basturk O, Ptashkin RN, Zehir A, Berger MF et al. Real-Time Genomic Profiling of Pancreatic Ductal Adenocarcinoma: Potential Actionability and Correlation with Clinical Phenotype. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(20):6094–100. doi: 10.1158/1078-0432.Ccr-17-0899. [DOI] [PubMed] [Google Scholar]
- 19.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lito P, Solomon M, Li LS, Hansen R, Rosen N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science (New York, NY). 2016;351(6273):604–8. doi: 10.1126/science.aad6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts NJ, Jiao Y, Yu J, Kopelovich L, Petersen GM, Bondy ML et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer discovery. 2012;2(1):41–6. doi: 10.1158/2159-8290.Cd-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stromnes IM, DelGiorno KE, Greenberg PD, Hingorani SR. Stromal reengineering to treat pancreas cancer. Carcinogenesis. 2014;35(7):1451–60. doi: 10.1093/carcin/bgu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Current opinion in immunology. 2013;25(2):200–5. doi: 10.1016/j.coi.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47(10):1168–78. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nature medicine. 2011;17(4):500–3. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biankin AV, Maitra A. Subtyping Pancreatic Cancer. Cancer Cell. 2015;28(4):411–3. doi: 10.1016/j.ccell.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Sohal DPS, Kennedy EB, Khorana A, Copur MS, Crane CH, Garrido-Laguna I et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. 2018;36(24):2545–56. doi: 10.1200/jco.2018.78.9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364(19):1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 29.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. The New England journal of medicine. 2013;369(18):1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang J, Hwang I, Yoo C, Kim KP, Jeong JH, Chang HM et al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Investigational new drugs. 2018;36(4):732–41. doi: 10.1007/s10637-018-0598-5. [DOI] [PubMed] [Google Scholar]
- 31.Nakazawa J, Otsuka T, Shimokawa M, Koga F, Ueda Y, Otsu S et al. A multicenter retrospective study of gemcitabine plus nabpaclitaxel or FOLFIRINOX in metastatic pancreatic cancer: NAPOLEON study. Annals of Oncology. 2019;30 (Supplement 4):aa17–aa8. [Google Scholar]
- 32.Pusceddu S, Ghidini M, Torchio M, Corti F, Tomasello G, Niger M et al. Comparative Effectiveness of Gemcitabine plus Nab-Paclitaxel and FOLFIRINOX in the First-Line Setting of Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers. 2019;11(4). doi: 10.3390/cancers11040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakazawa J, Otsuka T, Shimokawa M, Koga F, Ueda Y, Otsu S et al. P-065A multicenter retrospective study of gemcitabine plus nab-paclitaxel or FOLFIRINOX in metastatic pancreatic cancer: NAPOLEON study. Annals of Oncology. 2019;30(Supplement_4). doi: 10.1093/annonc/mdz155.064. [DOI] [Google Scholar]
- 34.Ramanathan RK, McDonough SL, Philip PA, Hingorani SR, Lacy J, Kortmansky JS et al. Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients With Metastatic Pancreatic Adenocarcinoma: SWOG S1313. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019;37(13):1062–9. doi: 10.1200/jco.18.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das A, Dean A. Modified FOLFIRINOX as a second-line treatment in pancreatic adenocarcinoma following gemcitabine plus nab-paclitaxel failure in patients with performance status two or less. Annals of Oncology. 2019;30 (Supplement 4):aa83–aa4. [Google Scholar]
- 36.Ko AH, Tempero MA, Shan YS, Su WC, Lin YL, Dito E et al. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. British journal of cancer. 2013;109(4):920–5. doi: 10.1038/bjc.2013.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet (London, England). 2016;387(10018):545–57. doi: 10.1016/s0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- 38.Sohal DPS, Kennedy EB, Khorana A, Copur MS, Crane CH, Garrido-Laguna I et al. Metastatic pancreatic cancer: ASCO clinical practice guideline update. Journal of Clinical Oncology. 2018;36(24):2545–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020;38(1):1–10. doi: 10.1200/jco.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tempero MA. NCCN Guidelines Updates: Pancreatic Cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2019;17(5.5):603–5. doi: 10.6004/jnccn.2019.5007. [DOI] [PubMed] [Google Scholar]
- 41.Dahan L, Phelip JM, Malicot KL, Williet N, Desrame J, Volet J et al. FOLFIRINOX until progression, FOLFIRINOX with maintenance treatment, or sequential treatment with gemcitabine and FOLFIRI.3 for first-line treatment of metastatic pancreatic cancer: A randomized phase II trial (PRODIGE 35-PANOPTIMOX). 2018;36(15_suppl):4000-. doi: 10.1200/JCO.2018.36.15_suppl.4000. [DOI] [Google Scholar]
- 42.Petrioli R, Torre P, Pesola G, Paganini G, Paolelli L, Miano ST et al. Gemcitabine plus nab-paclitaxel followed by maintenance treatment with gemcitabine alone as first-line treatment for older adults with locally advanced or metastatic pancreatic cancer. Journal of Geriatric Oncology. 2019. [DOI] [PubMed] [Google Scholar]
- 43.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. New England Journal of Medicine. 2019;381(4):317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachet JB, Hammel P, Desrame J, Meurisse A, Chibaudel B, Andre T et al. Nab-paclitaxel plus either gemcitabine or simplified leucovorin and fluorouracil as first-line therapy for metastatic pancreatic adenocarcinoma (AFUGEM GERCOR): a non-comparative, multicentre, open-label, randomised phase 2 trial. The lancet Gastroenterology & hepatology. 2017;2(5):337–46. doi: 10.1016/s2468-1253(17)30046-8. [DOI] [PubMed] [Google Scholar]
- 45.Charton E, Bachet JB, Hammel P, Desrame J, Chibaudel B, Cohen R et al. Impact on health-related quality of life deterioration-free survival of a first-line therapy combining nab-paclitaxel plus either gemcitabine or simplified leucovorin and fluorouracil for patients with metastatic pancreatic cancer: Results of the randomized phase II AFUGEM GERCOR clinical trial. Cancer Medicine. 2019;8(11):5079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wainberg Z, Boland P, Lieu C, Dayyani F, Macarulla T, Zhang B et al. SO-005A phase 1/2, open-label, dose-expansion study of liposomal irinotecan (nal-IRI) plus 5-fluorouracil/leucovorin (5-FU/LV) and oxaliplatin (OX) in patients with previously untreated metastatic pancreatic cancer. Annals of Oncology. 2019;30(Supplement_4). doi: 10.1093/annonc/mdz157.004. [DOI] [Google Scholar]
- 47.Jameson GS, Borazanci E, Babiker HM, Poplin E, Niewiarowska AA, Gordon MS et al. Response Rate Following Albumin-Bound Paclitaxel Plus Gemcitabine Plus Cisplatin Treatment Among Patients With Advanced Pancreatic Cancer: A Phase 1b/2 Pilot Clinical Trial. JAMA Oncol. 2019. doi: 10.1001/jamaoncol.2019.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reni M, Zanon S, Peretti U, Chiaravalli M, Barone D, Pircher C et al. Nab-paclitaxel plus gemcitabine with or without capecitabine and cisplatin in metastatic pancreatic adenocarcinoma (PACT-19): a randomised phase 2 trial. The lancet Gastroenterology & hepatology. 2018;3(10):691–7. doi: 10.1016/s2468-1253(18)30196-1. [DOI] [PubMed] [Google Scholar]
- 49.Macchini M, Chiaravalli M, Zanon S, Peretti U, Mazza E, Gianni L et al. Chemotherapy in elderly patients with pancreatic cancer: Efficacy, feasibility and future perspectives. Cancer treatment reviews. 2019;72:1–6. doi: 10.1016/j.ctrv.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Martin JL, Sidhu S, Benhayoun N, Dedonno M, Brenner WS. Dosing modifications to increase tolerability of gemcitabine and nab-paclitaxel in treatment of pancreatic cancer in the elderly. Journal of Clinical Oncology Conference. 2019;37(Supplement 4). [Google Scholar]
- 51.Petrillo A, Pappalardo A, Calabrese F, Tirino G, Pompella L, Ventriglia J et al. First line nab-paclitaxel plus gemcitabine in elderly metastatic pancreatic patients: A good choice beyond age. Journal of Gastrointestinal Oncology. 2019;10(5):910–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michalaki V, Poydorou A, Frangulidis G, Vezakis A, Karvouni E, Papadimitriou C. Gemcitabine/nabpaclitaxel efficacy in elderly patients with metastatic or locally advanced pancreatic adenocarcinoma. Annals of Oncology. 2018;29 (Supplement 5):v14. [Google Scholar]
- 53.Ventriglia J, Laterza MM, Savastano B, Petrillo A, Tirino G, Pompella L et al. Safety and efficacy of gemcitabine/nabpaclitaxel in elderly patients with metastatic or locally advanced pancreatic adenocarcinoma: A retrospective analysis. Annals of Oncology. 2017;28 (Supplement 5):v259. [Google Scholar]
- 54.Li D, Capanu M, Yu KH, Lowery MA, Kelsen DP, O’Reilly EM. Treatment, Outcomes, and Clinical Trial Participation in Elderly Patients With Metastatic Pancreas Adenocarcinoma. Clin Colorectal Cancer. 2015;14(4):269–76.e1. doi: 10.1016/j.clcc.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aldoss IT, Tashi T, Gonsalves W, Kalaiah RK, Fang X, Silberstein P et al. Role of chemotherapy in the very elderly patients with metastatic pancreatic cancer — A Veterans Affairs Cancer Registry analysis. Journal of Geriatric Oncology. 2011;2(3):209–14. doi: 10.1016/j.jgo.2011.02.003. [DOI] [Google Scholar]
- 56.Macarulla T, Pazo-Cid R, Guillén-Ponce C, López R, Vera R, Reboredo M et al. Phase I/II Trial to Evaluate the Efficacy and Safety of Nanoparticle Albumin-Bound Paclitaxel in Combination With Gemcitabine in Patients With Pancreatic Cancer and an ECOG Performance Status of 2. Journal of Clinical Oncology. 2019;37(3):230–8. doi: 10.1200/jco.18.00089. [DOI] [PubMed] [Google Scholar]
- 57.Betge J, Chi-Kern J, Schulte N, Belle S, Gutting T, Burgermeister E et al. A multicenter phase 4 geriatric assessment directed trial to evaluate gemcitabine +/− nab-paclitaxel in elderly pancreatic cancer patients (GrantPax). BMC cancer. 2018;18 (1) (no pagination)(747). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vasiliki M, Andreas P, Antonios V, Georgios F, Theodosios T, Christos P. Gemcitabine plus capecitabine in elderly patients with advanced pancreatic cancer. Annals of Oncology. 2017;28 (Supplement 3):iii76. [Google Scholar]
- 59.Singh RR, Goldberg J, Varghese AM, Yu KH, Park W, O’Reilly EM. Genomic profiling in pancreatic ductal adenocarcinoma and a pathway towards therapy individualization: A scoping review. Cancer treatment reviews. 2019;75:27–38. doi: 10.1016/j.ctrv.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ko AH, Bekaii-Saab T, Van Ziffle J, Mirzoeva OM, Joseph NM, Talasaz A et al. A Multicenter, Open-Label Phase II Clinical Trial of Combined MEK plus EGFR Inhibition for Chemotherapy-Refractory Advanced Pancreatic Adenocarcinoma. 2016;22(1):61–8. doi: 10.1158/1078-0432.CCR-15-0979 %J Clinical Cancer Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung V, McDonough S, Philip PA, Cardin D, Wang-Gillam A, Hui L et al. Effect of Selumetinib and MK-2206 vs Oxaliplatin and Fluorouracil in Patients With Metastatic Pancreatic Cancer After Prior Therapy: SWOG S1115 Study Randomized Clinical Trial. JAMA Oncology. 2017;3(4):516–22. doi: 10.1001/jamaoncol.2016.5383 %J JAMA Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Golan T, Khvalevsky EZ, Hubert A, Gabai RM, Hen N, Segal A et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6(27):24560–70. doi: 10.18632/oncotarget.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–23. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 64.Fakih M, O’Neil B, Price TJ, Falchook GS, Desai J, Kuo J et al. Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. 2019;37(15_suppl):3003-. doi: 10.1200/JCO.2019.37.15_suppl.3003. [DOI] [Google Scholar]
- 65.Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM et al. The KRAS(G12C) Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer discovery. 2020;10(1):54–71. doi: 10.1158/2159-8290.Cd-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brauswetter D, Gurbi B, Varga A, Varkondi E, Schwab R, Banhegyi G et al. Molecular subtype specific efficacy of MEK inhibitors in pancreatic cancers. PloS one. 2017;12(9):e0185687. doi: 10.1371/journal.pone.0185687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(Database issue):D805–D11. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou L, Baba Y, Kitano Y, Miyake K, Zhang X, Yamamura K et al. KRAS, BRAF, and PIK3CA mutations, and patient prognosis in 126 pancreatic cancers: pyrosequencing technology and literature review. Medical oncology (Northwood, London, England). 2016;33(4):32. doi: 10.1007/s12032-016-0745-9. [DOI] [PubMed] [Google Scholar]
- 69.Boeck S, Jung A, Laubender RP, Neumann J, Egg R, Goritschan C et al. EGFR pathway biomarkers in erlotinib-treated patients with advanced pancreatic cancer: translational results from the randomised, crossover phase 3 trial AIO-PK0104. British journal of cancer. 2013;108(2):469–76. doi: 10.1038/bjc.2012.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heining C, Horak P, Uhrig S, Codo PL, Klink B, Hutter B et al. NRG1 Fusions in KRAS Wild-Type Pancreatic Cancer. Cancer discovery. 2018;8(9):1087–95. doi: 10.1158/2159-8290.Cd-18-0036. [DOI] [PubMed] [Google Scholar]
- 71.Jones MR, Williamson LM, Topham JT, Lee MKC, Goytain A, Ho J et al. NRG1 Gene Fusions Are Recurrent, Clinically Actionable Gene Rearrangements in KRAS Wild-Type Pancreatic Ductal Adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25(15):4674–81. doi: 10.1158/1078-0432.Ccr-19-0191. [DOI] [PubMed] [Google Scholar]
- 72.Alison M Schram EMOR, Somwar Romel, Benayed Ryma, Shameem Sara, Chauhan Thrusha, Torrisi Jean, Ford Jim, Maussang David, Wasserman Ernesto, Ladanyi Marc, Hyman David M, Sirulnik L Andres, Drilon Alexander E. Clinical proof-of-concept for MCLA-128, a bispecific HER2/3 antibody therapy, in NRG1 fusion-positive cancers. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics Octomber 26–0; Boston, MA 2019. [Google Scholar]
- 73.Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nature reviews Clinical oncology. 2019. doi: 10.1038/s41571-019-0281-6. [DOI] [PubMed] [Google Scholar]
- 74.Pishvaian MJ, Rolfo CD, Liu SV, Multani PS, Maneval EC, Garrido-Laguna I. Clinical benefit of entrectinib for patients with metastatic pancreatic cancer who harbor NTRK and ROS1 fusions. 2018;36(4_suppl):521-. doi: 10.1200/JCO.2018.36.4_suppl.521. [DOI] [Google Scholar]
- 75.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. The New England journal of medicine. 2018;378(8):731–9. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Reilly EM, Hechtman JF. Tumour response to TRK inhibition in a patient with pancreatic adenocarcinoma harbouring an NTRK gene fusion. Annals of oncology : official journal of the European Society for Medical Oncology. 2019;30(Supplement_8):viii36–viii40. doi: 10.1093/annonc/mdz385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schultheis B, Reuter D, Ebert MP, Siveke J, Kerkhoff A, Berdel WE et al. Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen in KRAS wildtype patients with locally advanced or metastatic pancreatic cancer: a multicenter, randomized phase IIb study. Annals of oncology : official journal of the European Society for Medical Oncology. 2017;28(10):2429–35. doi: 10.1093/annonc/mdx343. [DOI] [PubMed] [Google Scholar]
- 78.Aatur DS, Siraj MA, Jill L, Andrew H, Khanh N, Jamie K et al. Identification of Targetable ALK Rearrangements in Pancreatic Ductal Adenocarcinoma. Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw. 2017;15(5):555–62. doi: 10.6004/jnccn.2017.0058. [DOI] [PubMed] [Google Scholar]
- 79.Singhi AAS, Greenbowe J, Ross JS, Nguyen K, Nikiforova M, et al. A clinicopathologic study of ALK rearrangements in pancreatic ductal adenocarcinoma. . Lab Invest 2016;96:448A–9A. [Google Scholar]
- 80.Tuli R, Lo S, Koo J, Pishvaian M, Bender RJ, Petricoin E et al. Anaplastic Lymphoma Kinase Rearrangement and Response to Crizotinib in Pancreatic Ductal Adenocarcinoma. JCO Precision Oncology. 2017(1):1–5. doi: 10.1200/PO.17.00016. [DOI] [PubMed] [Google Scholar]
- 81.Foster SA, Whalen DM, Özen A, Wongchenko MJ, Yin J, Yen I et al. Activation Mechanism of Oncogenic Deletion Mutations in BRAF, EGFR, and HER2. Cancer Cell. 2016;29(4):477–93. doi: 10.1016/j.ccell.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 82.Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2017;32(2):185–203.e13. doi: 10.1016/j.ccell.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guan M, Bender RJ, Pishvaian MJ, Halverson DC, Tuli R, Klempner SJ et al. Molecular and clinical characterization of BRAF mutations in pancreatic ductal adenocarcinomas (PDACs). Journal of Clinical Oncology. 2018;36(4_suppl):214-. doi: 10.1200/JCO.2018.36.4_suppl.214. [DOI] [Google Scholar]
- 84.Ross KC, Andrews AJ, Marion CD, Yen TJ, Bhattacharjee V. Identification of the Serine Biosynthesis Pathway as a Critical Component of BRAF Inhibitor Resistance of Melanoma, Pancreatic, and Non-Small Cell Lung Cancer Cells. Molecular cancer therapeutics. 2017;16(8):1596–609. doi: 10.1158/1535-7163.Mct-16-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wrzeszczynski KO, Rahman S, Frank MO, Arora K, Shah M, Geiger H et al. Identification of targetable BRAF DeltaN486_P490 variant by whole-genome sequencing leading to dabrafenib-induced remission of a BRAF-mutant pancreatic adenocarcinoma. Cold Spring Harbor molecular case studies. 2019;5(6). doi: 10.1101/mcs.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aguirre AJ, Nowak JA, Camarda ND, Moffitt RA, Ghazani AA, Hazar-Rethinam M et al. Real-time Genomic Characterization of Advanced Pancreatic Cancer to Enable Precision Medicine. Cancer discovery. 2018;8(9):1096–111. doi: 10.1158/2159-8290.Cd-18-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bryant KL, Stalnecker CA, Zeitouni D, Klomp JE, Peng S, Tikunov AP et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nature medicine. 2019;25(4):628–40. doi: 10.1038/s41591-019-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karasic TB, O’Hara MH, Loaiza-Bonilla A, Reiss KA, Teitelbaum UR, Borazanci E et al. Effect of Gemcitabine and nab-Paclitaxel with or Without Hydroxychloroquine on Patients with Advanced Pancreatic Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncology. 2019;5(7):993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wolpin BM, Rubinson DA, Wang X, Chan JA, Cleary JM, Enzinger PC et al. Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinoma. The oncologist. 2014;19(6):637–8. doi: 10.1634/theoncologist.2014-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kinsey CG, Camolotto SA, Boespflug AM, Guillen KP, Foth M, Truong A et al. Protective autophagy elicited by RAF-->MEK-->ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nature medicine. 2019;25(4):620–7. doi: 10.1038/s41591-019-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Friedenson B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed : Medscape general medicine. 2005;7(2):60. [PMC free article] [PubMed] [Google Scholar]
- 92.Lynch HT, Deters CA, Snyder CL, Lynch JF, Villeneuve P, Silberstein J et al. BRCA1 and pancreatic cancer: pedigree findings and their causal relationships. Cancer genetics and cytogenetics. 2005;158(2):119–25. doi: 10.1016/j.cancergencyto.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 93.O’Reilly EM, Lee JW, Lowery MA, Capanu M, Stadler ZK, Moore MJ et al. Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: Germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. Cancer. 2018;124(7):1374–82. doi: 10.1002/cncr.31218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Reilly EM, Lee JW, Zalupski M, Capanu M, Park J, Golan T et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin With or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020:Jco1902931. doi: 10.1200/jco.19.02931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. British journal of cancer. 2014;111(6):1132–8. doi: 10.1038/bjc.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kondo T, Kanai M, Kou T, Sakuma T, Mochizuki H, Kamada M et al. Impact of BRCAness on the efficacy of oxaliplatin-based chemotherapy in patients with unresectable pancreatic cancer. Journal of Clinical Oncology. 2017;35(4_suppl):250-. doi: 10.1200/JCO.2017.35.4_suppl.250. [DOI] [Google Scholar]
- 97.Wattenberg MM, Asch D, Yu S, O’Dwyer PJ, Domchek SM, Nathanson KL et al. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. British journal of cancer. 2019. doi: 10.1038/s41416-019-0582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hammel P, Kindler HL, Reni M, Cutsem EV, Macarulla T, Hall MJ et al. Health-related quality of life in patients with a germline BRCA mutation and metastatic pancreatic cancer receiving maintenance olaparib. Annals of oncology : official journal of the European Society for Medical Oncology. 2019;28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lowery MA, Kelsen DP, Capanu M, Smith SC, Lee JW, Stadler ZK et al. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. European journal of cancer (Oxford, England : 1990). 2018;89:19–26. doi: 10.1016/j.ejca.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shroff RT, Hendifar A, McWilliams RR, Geva R, Epelbaum R, Rolfe L et al. Rucaparib Monotherapy in Patients With Pancreatic Cancer and a Known Deleterious BRCA Mutation. JCO Precis Oncol. 2018;2018. doi: 10.1200/po.17.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Binder KAR, Mick R, O’Hara M, Teitelbaum U, Karasic T, Schneider C et al. Abstract CT234: A Phase II, single arm study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic mutation in BRCA1, BRCA2 or PALB2. 2019;79(13 Supplement):CT234–CT. doi: 10.1158/1538-7445.AM2019-CT234 %J Cancer Research. [DOI] [Google Scholar]
- 102.Heeke AL, Pishvaian MJ, Lynce F, Xiu J, Brody JR, Chen WJ et al. Prevalence of Homologous Recombination-Related Gene Mutations Across Multiple Cancer Types. JCO Precis Oncol. 2018;2018. doi: 10.1200/po.17.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pishvaian MJ, Bender RJ, Halverson D, Rahib L, Hendifar AE, Mikhail S et al. Molecular Profiling of Patients with Pancreatic Cancer: Initial Results from the Know Your Tumor Initiative. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018;24(20):5018–27. doi: 10.1158/1078-0432.Ccr-18-0531. [DOI] [PubMed] [Google Scholar]
- 104.Hu ZI, Shia J, Stadler ZK, Varghese AM, Capanu M, Salo-Mullen E et al. Evaluating Mismatch Repair Deficiency in Pancreatic Adenocarcinoma: Challenges and Recommendations. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018;24(6):1326–36. doi: 10.1158/1078-0432.Ccr-17-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Latham A, Srinivasan P, Kemel Y, Shia J, Bandlamudi C, Mandelker D et al. Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019;37(4):286–95. doi: 10.1200/JCO.18.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cavalieri CC, Swanson E, Whisenant JR, Weis JR, Gilcrease GW, Stenehjem DD et al. Pembroliuzmab in gastrointestinal (GI) malignancies with defective DNA mismatch repair (dMMR): A single institution experience. 2017;35(4_suppl):792-. doi: 10.1200/JCO.2017.35.4_suppl.792. [DOI] [Google Scholar]
- 107.Humphris JL, Patch AM, Nones K, Bailey PJ, Johns AL, McKay S et al. Hypermutation In Pancreatic Cancer. Gastroenterology. 2017;152(1):68–74.e2. doi: 10.1053/j.gastro.2016.09.060. [DOI] [PubMed] [Google Scholar]
- 108.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (New York, NY). 2017;357(6349):409–13. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lupinacci RM, Goloudina A, Buhard O, Bachet JB, Marechal R, Demetter P et al. Prevalence of Microsatellite Instability in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Gastroenterology. 2018;154(4):1061–5. doi: 10.1053/j.gastro.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 110.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer research. 1998;58(22):5248–57. [PubMed] [Google Scholar]
- 111.Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC et al. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019;37(4):318–27. doi: 10.1200/jco.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- 112.O’Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun W et al. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019. doi: 10.1001/jamaoncol.2019.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kamath SD, Kalyan A, Kircher S, Nimeiri H, Fought AJ, Benson A 3rd et al. Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A Phase Ib Study. The oncologist. 2019. doi: 10.1634/theoncologist.2019-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36(7):382–9. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weiss GJ, Blaydorn L, Beck J, Bornemann-Kolatzki K, Urnovitz H, Schutz E et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Investigational new drugs. 2018;36(1):96–102. doi: 10.1007/s10637-017-0525-1. [DOI] [PubMed] [Google Scholar]
- 117.Stromnes IM, Brockenbrough JS, Izeradjene K, Carlson MA, Cuevas C, Simmons RM et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut. 2014;63(11):1769–81. doi: 10.1136/gutjnl-2013-306271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Y, Velez-Delgado A, Mathew E, Li D, Mendez FM, Flannagan K et al. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut. 2017;66(1):124–36. doi: 10.1136/gutjnl-2016-312078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eriksson E, Moreno R, Milenova I, Liljenfeldt L, Dieterich LC, Christiansson L et al. Activation of myeloid and endothelial cells by CD40L gene therapy supports T-cell expansion and migration into the tumor microenvironment. Gene therapy. 2017;24(2):92–103. doi: 10.1038/gt.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sanborn RE, Gabrail NY, Bhardwaj N, Gordon MS, O’Hara M, Khalil D et al. Abstract LB-194: First-in-human Phase I study of the CD40 agonist mAb CDX-1140 and in combination with CDX-301 (rhFLT3L) in patients with advanced cancers: Interim results. Cancer research. 2019;79(13 Supplement):LB-194–LB-. doi: 10.1158/1538-7445.Am2019-lb-194. [DOI] [Google Scholar]
- 121.O’Hara MH, O’Reilly EM, Rosemarie M, Varadhachary G, Wainberg ZA, Ko A et al. Abstract CT004: A Phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (Gem) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PDAC) patients. Cancer research. 2019;79(13 Supplement):CT004–CT. doi: 10.1158/1538-7445.Am2019-ct004. [DOI] [Google Scholar]
- 122.Mahalingam D, Wilkinson G, Eng KH, Fields P, Raber P, Moseley JL et al. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: a Phase 1b study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019. doi: 10.1158/1078-0432.Ccr-19-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(12):1325–33. doi: 10.1200/jco.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Le DT, Picozzi VJ, Ko AH, Wainberg ZA, Kindler H, Wang-Gillam A et al. Results from a phase IIb, randomized, multicenter study of GVAX pancreas and CRS-207 compared with chemotherapy in adults with previously treated metastatic pancreatic adenocarcinoma (ECLIPSE study). Clinical Cancer Research. 2019;25(18):5493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tempero M O D, Macarulla T, et al. Ibrutinib in combination with nab-paclitaxel and gemcitabine as first-line treatment for patients with metastatic pancreatic adenocarcinoma: results from the phase 3 RESOLVE study. ESMO 21st World Congress on Gastrointestinal Cancer. 2019:002. [Google Scholar]
- 126.Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS et al. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(4):359–66. doi: 10.1200/jco.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- 127.Margaret A Tempero EVC, Sigal Darren, Oh Do-Youn, Fazio Nicola, Macarulla Teresa, Hitre Erika, Hammel Pascal, Hendifar Andrew Eugene, Bates Susan Elaine, Li Chung-Pin, De La Fouchardiere Christelle, Heinemann Volker, Maraveyas Anthony, Bahary Nathan, Layos Laura, Sahai Vaibhav, Zheng Lei, Lacy Jill, Bullock Andrea J.. HALO 109–301: A randomized, double-blind, placebo-controlled, phase 3 study of pegvorhyaluronidase alfa (PEGPH20) + nab-paclitaxel/gemcitabine (AG) in patients (pts) with previously untreated hyaluronan (HA)-high metastatic pancreatic ductal adenocarcinoma (mPDA). Journal of Clinical Oncology. 2020;38:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159(1):80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schwartz GG, Eads D, Naczki C, Northrup S, Chen T, Koumenis C. 19-nor-1 alpha, 25-dihydroxyvitamin D2 (paricalcitol) inhibits the proliferation of human pancreatic cancer cells in vitro and in vivo. Cancer biology & therapy. 2008;7(3):430–6. doi: 10.4161/cbt.7.3.5418. [DOI] [PubMed] [Google Scholar]
- 130.Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Molecular cancer therapeutics. 2006;5(5):1108–16. doi: 10.1158/1535-7163.Mct-05-0516. [DOI] [PubMed] [Google Scholar]
- 131.Picozzi VJ, Pishvaian MJ, Mody K, Winter JM, Glaspy JA, Larson T et al. Effect of anti-CTGF human recombinant monoclonal antibody pamrevlumab on resectability and resection rate when combined with gemcitabine/nab-paclitaxel in phase 1/2 clinical study for the treatment of locally advanced pancreatic cancer patients. Journal of Clinical Oncology. 2018;36(15_suppl):4016-. doi: 10.1200/JCO.2018.36.15_suppl.4016. [DOI] [Google Scholar]
- 132.Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(22):3617–22. doi: 10.1200/jco.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sahai V, Saif MW, Kalyan A, Philip PA, Rocha-Lima CM, Ocean A et al. A Phase I/II Open-Label Multicenter Single-Arm Study of FABLOx (Metronomic 5-Fluorouracil Plus nab-Paclitaxel, Bevacizumab, Leucovorin, and Oxaliplatin) in Patients with Metastatic Pancreatic Cancer. Journal of Pancreatic Cancer. 2019;5(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Isacoff WH, Reber HA, Bedford R, Hoos W, Rahib L, Upfill-Brown A et al. Low-Dose Continuous 5-Fluorouracil Combined with Leucovorin, nab-Paclitaxel, Oxaliplatin, and Bevacizumab for Patients with Advanced Pancreatic Cancer: A Retrospective Analysis. Targeted oncology. 2018;13(4):461–8. doi: 10.1007/s11523-018-0572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. The New England journal of medicine. 2019;381(25):2416–28. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 136.Zachar Z, Marecek J, Maturo C, Gupta S, Stuart SD, Howell K et al. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. Journal of molecular medicine (Berlin, Germany). 2011;89(11):1137–48. doi: 10.1007/s00109-011-0785-8. [DOI] [PubMed] [Google Scholar]
- 137.Alistar A, Morris BB, Desnoyer R, Klepin HD, Hosseinzadeh K, Clark C et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. The Lancet Oncology. 2017;18(6):770–8. doi: 10.1016/s1470-2045(17)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shah MA, Yu P, Narain N, Sarangarajan R, Kiebish M, Vishnudas V et al. Abstract CT315: Phase I study of BPM 31510 (ubidecarenone) in patients with advanced solid tumors. Cancer research. 2015;75(15 Supplement):CT315–CT. doi: 10.1158/1538-7445.Am2015-ct315. [DOI] [Google Scholar]
- 139.Niewiarowska AA, Lucius DM, Sarangarajan R, Narain NR, Ramanathan R, Ritch P et al. A phase II clinical investigation of BPM31510-IV (ubidecarenone) in patients with advanced pancreatic cancer. Annals of Oncology. 2018;29 (Supplement 8):viii270. [Google Scholar]
- 140.Lowery MA, Yu KH, Kelsen DP, Harding JJ, Bomalaski JS, Glassman DC et al. A phase 1/1B trial of ADI-PEG 20 plus nab-paclitaxel and gemcitabine in patients with advanced pancreatic adenocarcinoma. Cancer. 2017;123(23):4556–65. doi: 10.1002/cncr.30897. [DOI] [PubMed] [Google Scholar]
- 141.Hammel P, Fabienne P, Mineur L, Metges JP, Andre T, De La Fouchardiere C et al. Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: An open-label, randomized Phase IIb trial. European journal of cancer (Oxford, England : 1990). 2020;124:91–101. doi: 10.1016/j.ejca.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 142.Bachet J-B, Gay F, Maréchal R, Galais M-P, Adenis A, MsC DS et al. Asparagine Synthetase Expression and Phase I Study With L-Asparaginase Encapsulated in Red Blood Cells in Patients With Pancreatic Adenocarcinoma. Pancreas. 2015;44(7):1141–7. doi: 10.1097/mpa.0000000000000394. [DOI] [PubMed] [Google Scholar]
- 143.Mahipal A, Tella SH, Kommalapati A, Goyal G, Soares H, Neuger A et al. Phase 1 trial of enzalutamide in combination with gemcitabine and nab-paclitaxel for the treatment of advanced pancreatic cancer. Investigational new drugs. 2019;37(3):473–81. doi: 10.1007/s10637-018-0676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pelzer U, Bendell JC, Womack MS, Bahary N, Macarulla T, Borazanci EH et al. A phase Ib study evaluating olaratumab in combination with nab-paclitaxel and gemcitabine in first-line treatment of metastatic pancreatic cancer. Journal of Clinical Oncology Conference. 2019;37(Supplement 4). [Google Scholar]
- 145.Ludwig KF, Du W, Sorrelle NB, Wnuk-Lipinska K, Topalovski M, Toombs JE et al. Small-Molecule Inhibition of Axl Targets Tumor Immune Suppression and Enhances Chemotherapy in Pancreatic Cancer. Cancer research. 2018;78(1):246–55. doi: 10.1158/0008-5472.Can-17-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Beg MS, Lowy AM, O’Dwyer PJ, Jameson GS, Borazanci EH, Patel H et al. A randomized clinical trial of chemotherapy with gemcitabine/cisplatin/nabpaclitaxel with or without the AXL inhibitor bemcentinib (BGB324) for patients with advanced pancreatic cancer. Journal of Clinical Oncology Conference. 2019;37(Supplement 4). [Google Scholar]
- 147.Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nature reviews Cancer. 2017;17(12):709–24. doi: 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 148.Epstein AS, Soff GA, Capanu M, Crosbie C, Shah MA, Kelsen DP et al. Analysis of incidence and clinical outcomes in patients with thromboembolic events and invasive exocrine pancreatic cancer. Cancer. 2012;118(12):3053–61. doi: 10.1002/cncr.26600. [DOI] [PubMed] [Google Scholar]
- 149.Menapace LA, Peterson DR, Berry A, Sousou T, Khorana AA. Symptomatic and incidental thromboembolism are both associated with mortality in pancreatic cancer. Thrombosis and haemostasis. 2011;106(2):371–8. doi: 10.1160/th10-12-0789. [DOI] [PubMed] [Google Scholar]
- 150.Kakkar AK, Lemoine NR, Scully MF, Tebbutt S, Williamson RCN. Tissue factor expression correlates with histological grade in human pancreatic cancer. BJS (British Journal of Surgery). 1995;82(8):1101–4. doi: 10.1002/bjs.1800820831. [DOI] [PubMed] [Google Scholar]
- 151.THALER J, AY C, MACKMAN N, BERTINA RM, KAIDER A, MAROSI C et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. Journal of Thrombosis and Haemostasis. 2012;10(7):1363–70. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- 152.Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC et al. Tissue Factor Expression, Angiogenesis, and Thrombosis in Pancreatic Cancer. Clinical Cancer Research. 2007;13(10):2870–5. doi: 10.1158/1078-0432.Ccr-06-2351. [DOI] [PubMed] [Google Scholar]
- 153.Stark K, Schubert I, Joshi U, Kilani B, Hoseinpour P, Thakur M et al. Distinct Pathogenesis of Pancreatic Cancer Microvesicle-Associated Venous Thrombosis Identifies New Antithrombotic Targets In Vivo. Arterioscler Thromb Vasc Biol. 2018;38(4):772–86. doi: 10.1161/atvbaha.117.310262. [DOI] [PubMed] [Google Scholar]
- 154.Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. The New England journal of medicine. 2019;380(8):720–8. doi: 10.1056/NEJMoa1814630. [DOI] [PubMed] [Google Scholar]
- 155.Vadhan-Raj S, McNamara MG, Venerito M, Riess H, O’Reilly EM, Overman MJ et al. Rivaroxaban thromboprohylaxis in ambulatory patients with pancreatic cancer: Results from a prespecified subgroup analysis of the CASSINI study. Journal of Clinical Oncology. 2019;37(15_suppl):4016-. doi: 10.1200/JCO.2019.37.15_suppl.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yu KH, Mantha S, Tjan C, Kaufmann ES, Brenner R, Lowery MA et al. Pilot study of gemcitabine, nab-paclitaxel, PEGPH20, and rivaroxaban for advanced pancreatic adenocarcinoma: An interim analysis. 2018;36(4_suppl):405-. doi: 10.1200/JCO.2018.36.4_suppl.405. [DOI] [Google Scholar]
- 157.Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019:Jco1901461. doi: 10.1200/jco.19.01461. [DOI] [PubMed] [Google Scholar]


