Abstract
Introduction:
Oxidative stress is a key contributor to aging and age-related diseases. In the present study, we examine the protective effects of PFT, a novel kefir product, against age-associated oxidative stress using aged (10-month-old) mice.
Methods:
Mice were treated with PFT orally at a daily dose of 2 mg/kg body weight over 6 weeks, and antioxidant status, protein oxidation, and lipid peroxidation were studied in the brain, liver, and blood.
Results:
PFT supplementation significantly reduced the oxidative stress biomarkers malondialdehyde (MDA) and nitric oxide; reversed the reductions in glutathione (GSH) levels, total antioxidant capacity (TAC), and anti-hydroxyl radical (AHR) content; enhanced the antioxidant enzyme activities of glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD); inhibited the liver enzyme levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT); significantly reduced triglyceride (TG), total cholesterol (TC), and low density lipoprotein (LDL) levels; and significantly elevated high density lipoprotein (HDL) levels. Interestingly, PFT supplementation reversed the oxidative changes associated with aging, thus bringing levels to within the limits of the young control mice in the brain, liver, and blood. We also note that PFT affects the redox homeostasis of young mice and that it is corrected post-treatment with PFT.
Conclusion:
Our findings show the effectiveness of dietary PFT supplementation in modulating age-associated oxidative stress in mice and motivate further studies of PFT’s effects in reducing age-associated disorders where free radicals and oxidative stress are the major cause.
Keywords: aging, antioxidants, oxidative stress, PFT
Introduction
The processes involved with aging have been closely linked with oxidative stress, which in turn is thought to be tightly linked to the production of reactive oxygen species (ROS).1–3 ROS are highly reactive and can oxidatively damage a wide range of biological macromolecules, including lipids, proteins, and nucleic acids.4–6 This damage may lead to cellular senescence and genetic mutation.7 Studies of aged rats have found them to possess higher free radical levels2,8 and attributed this to reduced levels of antioxidants. Indeed, the decline of antioxidant function with age has been well documented in rats and humans,2,3,7,9–13 and it has been associated with decreasing levels of enzymatic antioxidant mRNA levels for glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD).14
Diet may be an important modifier of the cellular redox state that can change during aging. For example, iron deficiency can induce oxidative stress.15 In studies of endothelial cells and of murine and human leukemia cells, ferritin has been shown to protect against oxidative damage.16,17 Our previous studies of murine splenic cells in vitro and in vivo have also shown that MRN-100, an iron-based hydro-ferrate fluid derived from bivalent and trivalent ferrates, can protect cells from oxidative stress-induced apoptosis.18,19
Lactic acid bacteria (LAB) are another natural dietary-related agent that for over 100 years have been considered to potentially increase the human life span.20 LAB are frequently utilized in fermented milk and food product production, and LAB have been shown to help reduce pathogenic bacteria in the gut while maintaining a healthy balance of probiotic bacteria.21,22 Furthermore, LAB have been found to have beneficial effects for a range of diseases that include rheumatoid arthritis, Crohn’s disease, and cancer.23–27 LAB also improve lactose digestion and tolerance28 and its kefiran component has been found to reduce serum cholesterol levels and to lower high blood pressure in rats.29
Considering that aging is linked with increases in oxidative stress, we chose to examine anti-oxidative stress in aged mice using a novel kefir product called PFT (Probiotics Fermentation Technology). PFT is a kefir grain product that primarily contains the unique LAB strain named Lactobacillus kefiri P-IF (L. kefiri P-IF) and a small amount of various yeasts. The primary LAB strain of PFT has a unique DNA sequence and shows a 99.6% homology with regular kefiries and a similar 16S ribosome sequence compared with other L. kefiri strains. Previous work has shown that P-IF is effective against cancer, potentially due to its many distinct features.27,30,31 In contrast with other strains of L. kefiri, P-IF utilizes galactose as a carbon source, and when its growth medium is agitated it generates carbonic acid. In addition, P-IF grows three dimensionally in contrast to the lengthwise-dimenisonal growth pattern of most L. kefiri strains, an attribute which may be due to the unique carbohydrate chains on P-IF’s surface.32 Results here demonstrate that PFT can reverse age-associated oxidative stress in mice. This study further indicates PFT’s potential benefit in treating age-associated disorders that are primarily caused by oxidative stress and free radicals.
Materials and methods
Probiotics Fermentation Technology kefir grain product
Probiotics Fermentation Technology (PFT) is a mixture that contains primarily (~90%) a heat-killed freeze-dried form of L. kefiri P-IF. PFT also consists of ~2–3% of the following: one bacterial strain L. kefiri P-B1, and yeast strains Kazachstania turicensis, Kazachstania unispora, and Kluyveromyces marxianus. P-IF is a specific LAB strain with a unique DNA sequence, and PET scans show a 99.6% homology with regular kefiries. The characteristics of P-IF have been previously reported27,32; the exact chemical composition is under active investigation. Unlike other L. kefiri strains, P-IF utilizes galactose as a carbon source and produces carbonic acid when its growth medium is agitated. While most L. kefiri strains grow in a lengthwise-dimensional pattern, P-IF grows three-dimensionally, which is attributed to the unique carbohydrate chains on its surface.32 The yeast strains are not intentionally added, but rather are present in large amounts when obtaining the product from the Caucasus mountains and are filtered out in order to maximize the kefiri levels. PFT was provided by Paitos Co., Ltd., Yokohama, Kanagawa, Japan.
Animals
Thirty two male Swiss albino mice were used in this study. Sixteen older mice aged 10 months (23–28 g) and 16 young mice aged 2 months (weighed from 11–22 g) were obtained from the animal house of Faculty of Medicine, Alexandria University, Egypt. Mice arrived in solid-bottom, transparent polycarbonate cages with stainless-steel wire lids. All animal studies were performed according to the animal protocols approved by the Institutional Ethics Committee of Faculty of Science, Alexandria University, Egypt, in accordance with the ethical standards (AU 04190824101). The mice were housed (four mice/cage) and maintained at 23°C–25°C with a 12-h light/dark cycle. Food and water were available ad libitum. Food consisted of pellets that were 54% carbohydrate, 3% fat, 26% protein, and 17% vitamins and minerals, with 3.5 Kcal/g. We calculated the sample sizes in our study by power analysis33,34 and used the free G Power software for sample size calculation and justification.35
Experimental design
Since this study seeks to examine PFT’s effect on age-related changes in mice, we used four groups of mice with eight mice/group as follows: Group 1: the untreated young control mice (Young-control); Group 2: the PFT-treated young mice (Young-PFT); Group 3: the untreated aged mice (Aged-control); and Group 4: the PFT-treated aged mice (Aged-PFT). The Young-PFT and Aged-PFT groups received PFT orally at a dose of 2 mg/kg body weight daily for 6 weeks with free access to water. In a preliminary study, we examined the dose effect of PFT and found a dose of 2 mg/kg body weight sufficient to induce anti-oxidative changes in aged mice. The dose was also deemed safe to mice, as it was lower than the dose utilized in previous work by Furukawa et al.36 who administered PFT orally to mice at 2 g/kg/day. PFT was supplemented at the same time every day after breakfast and the solution of PFT was prepared by dissolving the appropriate dose of PFT in normal saline solution according to the body weight of each mouse and then ingested by oral gavage to mouse. Age-related changes in oxidative stress parameters could be determined by comparing the measurements of the Aged-control group versus the Young-control group, while the effects of PFT on the age-related changes could then be determined by the measured values of the Aged-PFT group relative to the two control groups. After 6 weeks of treatment, animals were fasted overnight and weighed. They were then anesthetized and blood was collected using a syringe puncture from the abdominal aorta and serum was isolated. Following euthanization, the liver and brain tissues were rapidly excised, washed with ice-cold 0.9% NaCl, frozen in liquid nitrogen, and stored at −80°C for subsequent analyses. Figure 1 shows a graphical summary of the experimental design along with a brief summary of the investigated results.
Figure 1.
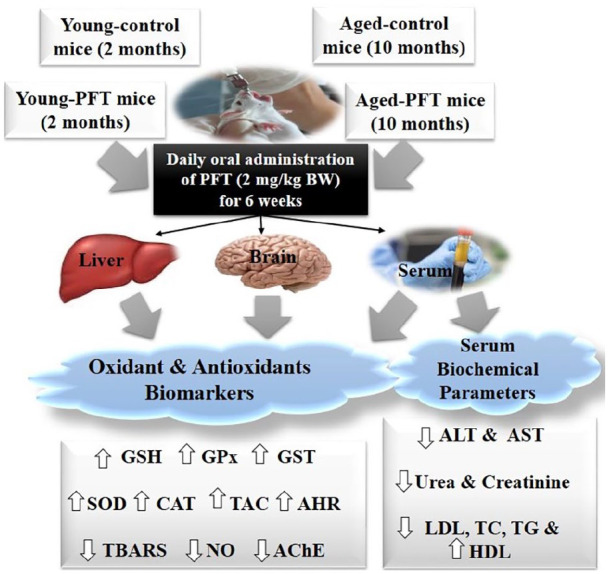
Experimental design and brief summary of results.
Biochemical analyses
Assays of the liver function were analyzed by measuring enzymes such as ALT (EC 2.6.1.2) and AST (EC 2.6.1.1) as well as total protein. The serum lipid profile was investigated by analyzing concentrations of total cholesterol (TC), triglyceride (TG), low density lipoprotein (LDL), and high density lipoprotein (HDL). The biochemical serum parameters were analyzed using the method described in the commercial kits following the manufacturer’s instructions.
Preparation of liver and brain homogenates
Frozen brain and liver tissues were homogenized on lysis buffer (150 mM NaCl, 1% Triton X-100, 10 mM Tris solution, pH 7.4) containing protease inhibitor, using SCILOGEX Homogenizer, on ice. The homogenate was centrifuged at 10,000 g for 10 min at 4°C and the supernatant was collected for measurement of parameters. The total protein content of the liver homogenate was analyzed by the standard protocol.37
Determination of lipid peroxidation
The malondialdehyde (MDA) content was assayed in the form of thiobarbituric acid-reactive substances (TBARS) in liver and brain homogenate as well as serum according to the method described previously.38 Briefly, 500 µl of sample was added to 1 ml of trichloroacetic acid (TCA, 15%) and centrifuged at 3000 rpm for 10 min. 1 ml of supernatant was mixed with 500 µl of thiobarbituric acid (TBA, 0.7%), heated in boiling water bath for 10 min, and cooled, and the color was read at 532 nm. The TBARS level was calculated against control according to the following equation: TBARS level (nmol/ml) = Absorbance/0.156. The majority of TBARS are MDA, and thus the concentration of MDA in the sample homogenate was expressed as nmol MDA/mg protein. The results were calculated using an index of absorption for MDA by using molar extinction coefficient 1.56 × 105/M/cm.
Nitric oxide assay
Nitric oxide (NO) was determined using Griess reaction in which 100 μl of sample was added to 100 μl acidic Griess reagent (1% sulfanilamide and 0.1% naphthlethylenediamine dihydrochloride in 2.5% phosphoric acid). The absorbance was read at 540 nm against blank.39 The NO level was calculated with the following equation: NO level (μM) = Absorbance of test/Absorbance of standard times concentration of standard, then expressed as μM/mg protein.
Determination of endogenous antioxidant activities
Glutathione (GSH) level: GSH was assayed by a method previously described.40 100 µl sample (test), distilled water (Blank), and GSH (standard) were mixed with 100 µl of sulphosalicylic acid (4%), kept at 4°C for at least 1 h, and then centrifuged at 1200 g for 10 min at 4°C. 100 µl supernatant was then mixed with 2.7 ml phosphate buffer (0.1 M, pH 7.4) and 0.2 ml DTNB (5,5’-dithiobis-(2-nitrobenzoic acid)) and incubated for 5 min. The produced yellow color was measured immediately at 412 nm. A standard curve was constructed using standard GSH. Finally, GSH content was expressed as mg/mg protein.
Glutathione S-transferase (GST) activity (EC 2.5.1.18): GST was measured as previously described.41 In brief, 100 µl GSH (5 mM), 10 µl p-nitrobenzyl chloride (1 mM in ethanol), and 25 µl sample were added to 1.365 ml phosphate buffer (0.1M) at pH 6.5 and vortexed, followed by incubation for 20 min at room temperature. The absorbance of sample was measured against air at 310 nm using the following equation: GST activity (μmol/min/mg protein) = Absorbance of sample/(1.9 × time × mg protein).
Superoxide dismutase (SOD) activity (EC 1.15.1.1): In the assay of SOD, 20 µl of sample (test) or buffer and 10 µl of pyrogallol (20 mM in 10 mM HCl) were added to 1 ml buffer solution.42 The absorbance of test (At) or reference (Ar) was measured at 420 nm against air after 30 and 90 s. The percentage inhibition of pyrogallol autoxidation was calculated according to the following equation: The percentage inhibition = [100–(At/min/ml sample)/(Ar/min/ml)] × 100. From the standard curve of SOD, it was found that one unit equals 153 ng. The sample enzyme activity in U/mg protein was obtained by dividing specific activity by 153.
Catalase (CAT) activity (EC 1.11.1.6): Catalase enzyme activity was determined following the method of Aebi.43 In the sample cuvette, 0.1 ml of sample was mixed with 0.5 ml of 0.2 M sodium phosphate buffer at pH 7.6 and 0.3 ml of 0.5% H2O2. The mixture was brought to a final volume of 3 ml with distilled water. The breakdown of H2O2 was recorded by measuring the absorbance at 240 nm and the enzyme activity was calculated as the change in absorbance per minute.
Glutathione peroxidase (GPx) activity (EC 1.11.1.9): GPx was assayed and calculated according to the previous method.44 The assay mixture consisted of 100 µl GSH, 100 µl cummen H2O2, 750 µl Tris-HCl, pH 7.6 and 50 µl of sample and the control consisted of 100 µl GSH, 750 µl Tris-HCl and 50 µl of sample. The reaction was started by incubation of sample and control at 37°C for 10 min, then 1 ml of (TCA, 15%) was added to test and control and 100 µl cummen H2O2 was added to control. Both were then centrifuged at 3000 rpm for 20 min. Finally, 100 µl of DTNB was added to 1 ml of supernatant and the absorbance of test and control were read at 412 nm. The GPx activity (mol/min/mg protein) = E × 6.2 × 100/13.1 × 0.05 × 10, where E = Absorbance of sample – Absorbance of control.
Total antioxidant capacity (TAC) assay: The TAC assay was determined by a standard method of Umamaheswari and Chatterjee.45 1 ml of sample was added to 1 ml of the solution containing sulphuric acid (0.6 M), sodium phosphate (28 mmol), and ammonium molybdate (4.0 mmol). The mixtures were incubated for 90 min at 95°C. The absorbance was measured after cooling at 695 nm. The calculation of TAC was carried out using the following formula: % scavenging =, where A is the absorbance of the control and At is the absorbance of the test sample.
Anti-hydroxyl radical (AHR) activity: Anti-hydroxyl radical activity was determined according to a modified method.46 The reaction mixture contained 100 μl H2O2, 100 μl FeSO4, 100 μl 2-deoxyribose-D-ribose, 2.7 ml of phosphate buffer pH 7.4, and 10 μl of the homogenates and serum. After incubation (60 min at 37°C), 0.2 ml of EDTA and 2 ml of TBA reagent (5.2 ml perchloric acid, 1.5 g thiobarbituric acid, and 60 g of trichloroacetic acid) were added. Afterwards, the absorbance of a characteristic pink complex was measured at 532 nm. Results are expressed in U/mg protein.
Determination of serum triacylglycerol
Triacylglycerol (TG) was measured by the method described in the commercial triacylglycerol kit following the manufacturer’s instructions.47 Briefly, triacylglycerol standard or serum sample (10 µl) were pipetted to 1 ml reagent (Good’s Buffer, 100 mmol/l, Magnesium Chloride, 15 mmol/l, ATP (Adenosina-5-Triphosphate), 4 mmol/l, 4-AAP (4-Aminoantipyrine), 1 mmol/l, 4-Chlorophenol, 0.1 mmol/l, LPL (Lipoprotein Lipase), 2500 U/l, GK (Glycerol Chinasi), 1000 U/l, GPO (Glycerol-3-phosphate oxidase), 5500 U/l and POD (Peroxidase), 1800 U/l). Tubes were mixed and incubated at 37°C for 5 min. Finally, absorbance of standard and sample were recorded against blank at 525 nm. The concentration of triglyceride was calculated by using the following formula: (mg/dl) =
Estimation of serum total cholesterol
The assay for serum total cholesterol (TC) was carried out according to the method of Watson using a commercial cholesterol kit following the manufacturer’s instructions.48 Briefly, cholesterol standard or serum sample (10 µl) were pipetted to 1 ml reagent (Good’s Buffer, 100 mmol/l, Cholesterol esterase, 300 U/l, Cholesterol oxidase Peroxidase, 1500 U/l, 4-AAP, 5500 U/l, Phenol derivates 1 mmol/l). Tubes were mixed and incubated at 37°C for 10 min to form a red dye compound. Finally, absorbance of standard and sample were recorded against blank at 546 nm. The concentration of total cholesterol was calculated by using the following formula: concentration of TC (mg/dl) =
Estimation of high and low density lipoproteins
Low density lipoproteins (LDL) and high density lipoproteins (HDL) were assayed using the commercial kit following the manufacturer’s instructions.49,50 Briefly, sample (200 µl) was added to precipitating reagent (20 µl), vortexed, let stand for 10 min, centrifuged for 15 min at 3000 rpm. Then cholesterol standard or sample supernatant (50 µl) were pipetted to 1 ml reagent (Good’s Buffer, 100 mmol/l, Cholesterol esterase, 300 U/l, Cholesterol oxidase Peroxidase, 1500 U/l, 4-AAP, 5500 U/l, Phenol derivates, 1 mmol/l). Tubes were mixed and incubated at 37°C for 10 min to form a red dye compound. Finally, absorbance of standard and sample were recorded against blank at 500 nm. The concentration of HDL was calculated by using the following formula: Concentration of HDL (mg/dl) = × Standard concentration. LDL can be calculated by: LDL cholesterol (mg/dl) = total cholesterol–HDL cholesterol–(triglycerides/5).
Statistical analysis
Measured data are reported as mean ± SEM (standard error of the mean) for eight mice in each group. One-way analysis of variance (ANOVA) was used to determine the significance of differences between mean values. Significance is noted at values of P < 0.05. Data were analyzed using SAS (version 9.4, Cary, NC). Shapiro Wilk was used for normality test, and Levene’s test was used to assess homogeneity of variance.
Results
General animal information
General information about the mice was examined, including food and water consumption, energy intake, tissue weight, and protein content. For all of these parameters, as well as for measurements of the liver and brain indices, we found insignificant changes in the PFT-treated mice in comparison with the controls (data not shown).
Effect of PFT on body weight
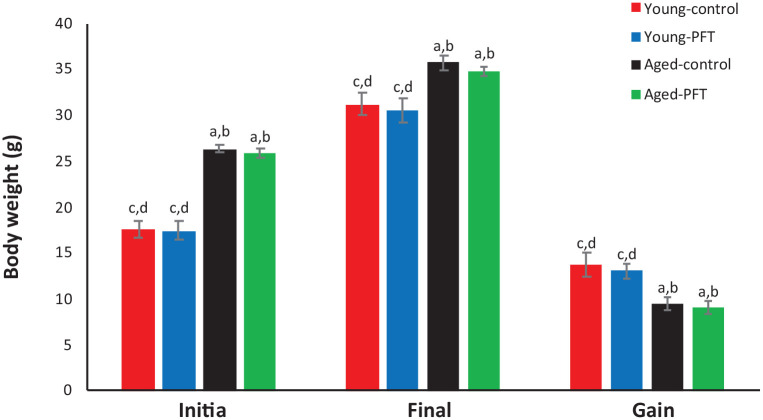
Body weight gain was measured as the difference between final and initial body weights. Data in Figure 2 show that treatment with PFT has no significant effect on body weight gain in comparison with control aged mice. Similar results were also observed in the young mice.
Figure 2.
Effect of PFT administration on body weight of young and aged mice. Each value represents the mean ± SEM for eight animals per group.
aSignificantly different from Young-control at P < 0.05.
bSignificantly different from Young-PFT at P < 0.05.
cSignificantly different from Aged-control at P < 0.05.
dSignificantly different from Aged-PFT at P < 0.05.
Effect of PFT on lipid peroxidation and NO
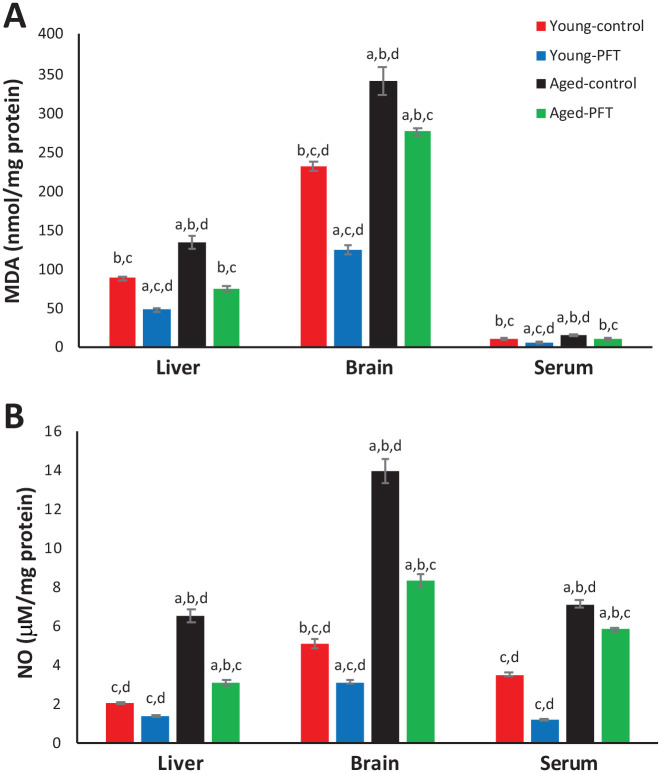
MDA content was assayed in the form of thiobarbituric acid-reactive substances in liver, brain, and serum. Data in Figure 3 show control aged mice demonstrated significantly higher MDA levels in liver, brain and serum as compared with young control mice. However, treatment with PFT significantly reduced the mean MDA values (P < 0.001) in the liver (–45%), brain (–19%), and plasma (–27%) as compared to control aged mice. Figure 3 also shows that the aged mice NO levels are significantly larger in comparison with young control mice. The mean NO values were significantly lower in PFT-treated mice than those of aged controls by −53%, −40%, and −18% for NO levels of the liver, brain, and plasma of the aged controls, respectively. PFT counteracted the increase in NO and MDA levels related to aging. It can also be noted that young mice experienced the same trend.
Figure 3.
Effect of PFT administration on MDA and NO level. Each value represents the mean ± SEM for eight animals per group.
aSignificantly different from Young-control at P < 0.05.
bSignificantly different from Young-PFT at P < 0.05.
cSignificantly different from Aged-control at P < 0.05.
dSignificantly different from Aged-PFT at P < 0.05.
Effect of PFT on GSH and GST level
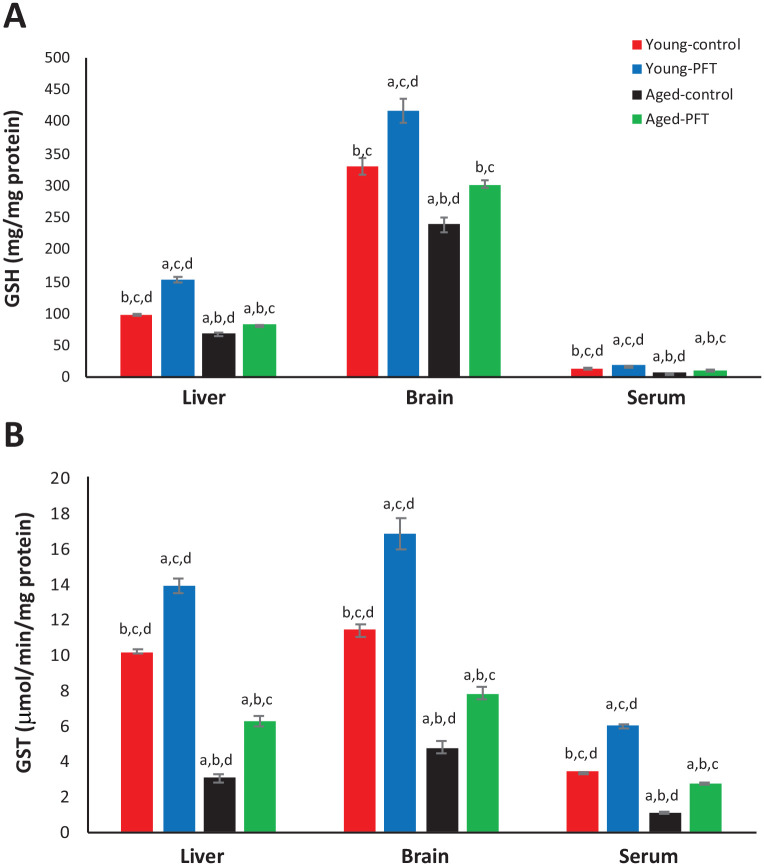
The effect of PFT administration on GSH and GST levels was examined in different tissues. Aged mice had a highly significant depletion (P < 0.05) of GSH levels in the liver, brain, and serum in comparison with young mice (Figure 4), but PFT treatment significantly increased the GSH level in aged mice as follows: liver 22%, brain 26%, and serum 67%. A similar trend in results was obtained in GST activity in the liver, brain, and serum of aged mice (Figure 4). Results in Figure 4 also showed similar effects for young mice.
Figure 4.
Effect of PFT administration on GSH and GST levels. Each value represents the mean ± SEM for eight animals per group.
aSignificantly different from Young-control at P < 0.05.
bSignificantly different from Young-PFT at P < 0.05.
cSignificantly different from Aged-control at P < 0.05.
dSignificantly different from Aged-PFT at P < 0.05.
Effect of PFT on the antioxidant enzymes GPx, CAT, and SOD
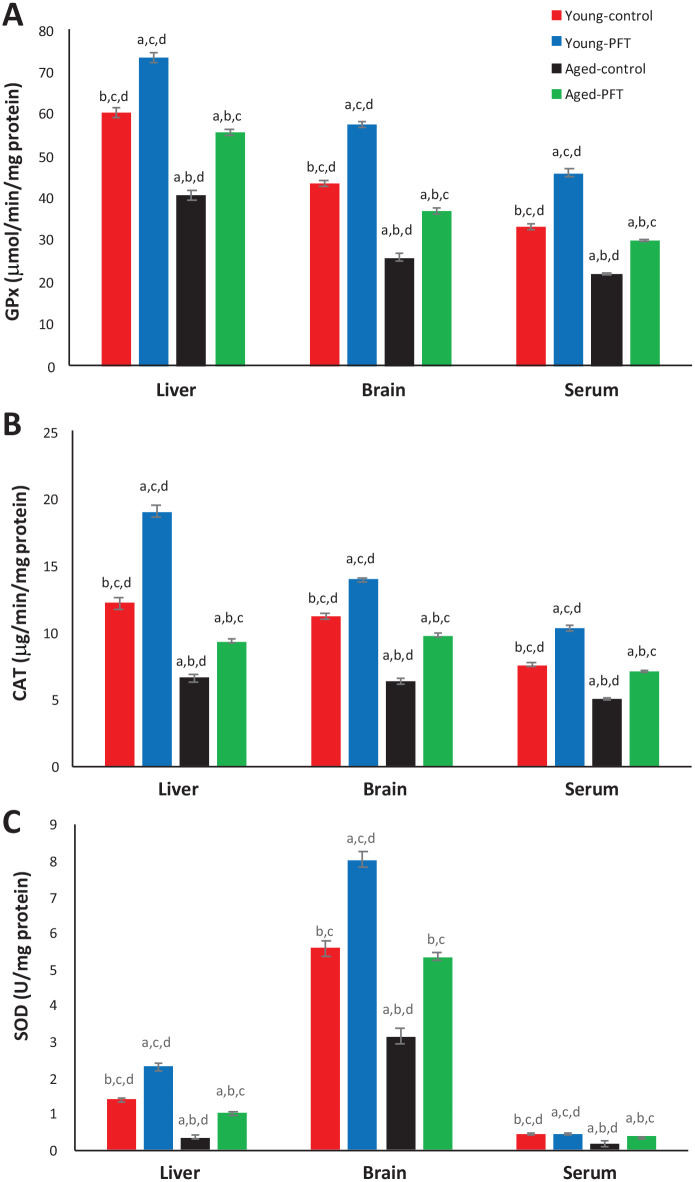
The effect of PFT on GPx, CAT, and SOD in different tissues of aged mice in the presence and absence of PFT is shown in Figure 5. GPx levels in control untreated aged mice was significantly decreased (P < 0.05) in different tissues in comparison with control young mice. On the other hand, PFT treatment of aged mice resulted in a significant increase in GPx levels in comparison with aged untreated mice and led to levels similar to those seen in the young groups. Liver, brain, and serum tissues of aged mice showed increases of 36%, 42%, and 35%, respectively. Figure 5 also shows a highly significant increase (P < 0.05) in CAT and SOD activities in PFT treated mice in comparison to the control aged mice. Interestingly, young mice demonstrated the same trend.
Figure 5.
Effect of PFT on GPx, CAT, and SOD in different tissues of young and aged mice. Each value represents the mean ± SEM for eight animals per group.
aSignificantly different from Young-control at P < 0.05.
bSignificantly different from Young-PFT at P < 0.05.
cSignificantly different from Aged-control at P < 0.05.
dSignificantly different from Aged-PFT at P < 0.05.
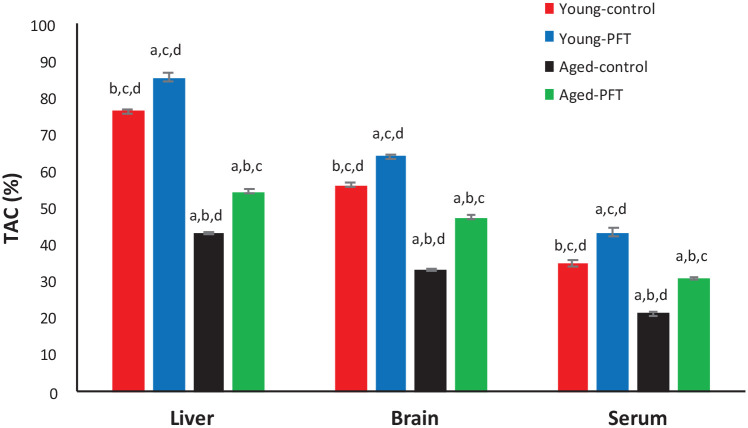
Effect of PFT on total antioxidant capacity
TAC levels measured in different tissues are presented in Figure 6. TAC levels of aged mice are significantly lower in the liver, brain, and serum (P < 0.05) as compared to control young mice. Aged mice treated with PFT, however, had significantly higher levels of TAC in the liver (26%), brain (42%), and serum (44%) as compared to control aged animals. PFT prevented the age-associated decrease in TAC levels. The TAC was shown to exhibit a similar pattern in young mice.
Figure 6.
Effect of PFT administration on TAC level in different tissues of young and aged mice. Each value represents the mean ± SEM for eight animals per group.
aSignificantly different from Young-control at P < 0.05.
bSignificantly different from Young-PFT at P < 0.05.
cSignificantly different from Aged-control at P < 0.05.
dSignificantly different from Aged-PFT at P < 0.05.
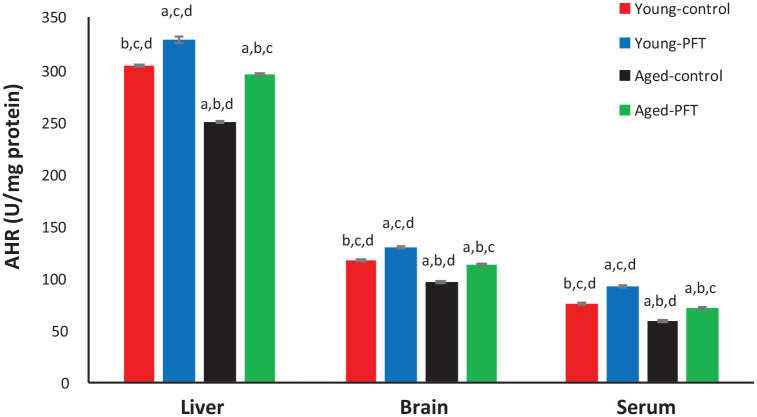
Effect of PFT on anti-hydroxyl radical activity
Control aged mice demonstrated a significant decline in the a AHR activity in different tissues relative to young control mice (Figure 7), but PFT administration in aged mice increased AHR levels in the liver (19%), brain (18%), and serum (19%). These results suggested that PFT could inhibit oxygen radical generation of aged mice. It is noteworthy that young mice showed a similar trend.
Figure 7.
Effect of PFT administration on AHR level of young and aged mice. Each value represents the mean ± SEM for eight animals per group.
aSignificantly different from Young-control at P < 0.05.
bSignificantly different from Young-PFT at P < 0.05.
cSignificantly different from Aged-control at P < 0.05.
dSignificantly different from Aged-PFT at P < 0.05.
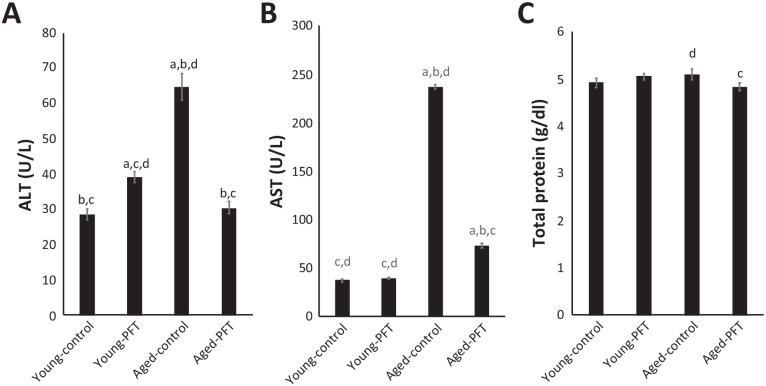
Effect of PFT on liver function and total protein levels
PFT’s effect was measured on the liver enzymes ALT and AST and on total protein levels. Figure 8 shows that ALT, AST, and total protein were significantly higher in control aged mice relative to control young mice. However, supplementation with PFT resulted in a significant decrease in ALT (–53%) and AST (–69%) activities relative to untreated aged control mice. Results in Figure 8 also show an insignificant difference in young mice with respect to the levels of AST and total protein.
Figure 8.
Effect of PFT on liver function and total protein levels in the serum of young and aged mice. Each value represents the mean ± SEM for eight animals per group.
aSignificantly different from Young-control at P < 0.05.
bSignificantly different from Young-PFT at P < 0.05.
cSignificantly different from Aged-control at P < 0.05.
dSignificantly different from Aged-PFT at P < 0.05.
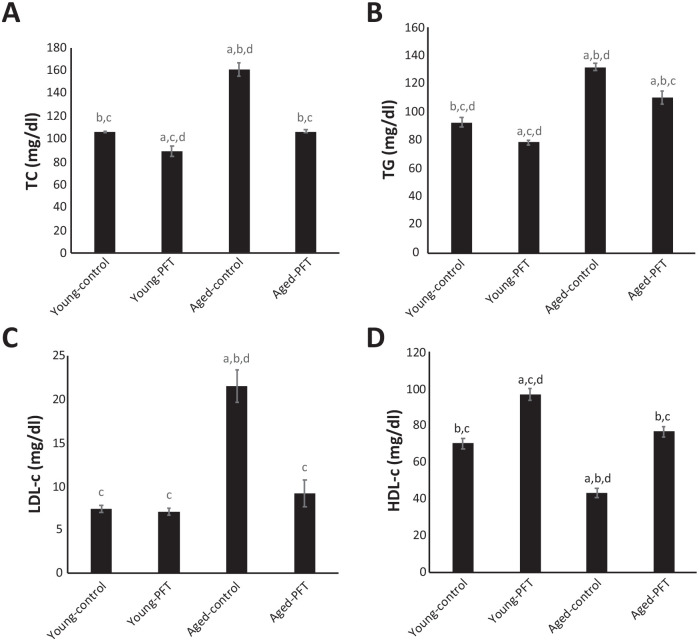
Effect of PFT on lipid levels
Figure 9 shows the lipid profile levels of TC, TG, LDL, and HDL. Levels of TC, TG, and LDL dramatically increased in control aged mice, while HDL level decreased, as compared with young mice. On the other hand, PFT-treated aged mice exhibited a significant decline in the lipid profile for TC (–34%), TG (–16%), and LDL (–57%) relative to untreated aged control mice, thus bringing it close to the levels of control young mice. It is of interest to note that HDL concentrations increase by 77% in PFT-treated aged mice. PFT administration to young mice resulted in a significant decrease in serum TC and TG with a significant elevation in serum HDL levels compared to control young mice, while serum LDL levels were not affected by PFT supplementation to young mice.
Figure 9.
Effect of PFT on lipid levels in the serum of young and aged mice. Each value represents the mean ± SEM for eight animals per group.
aSignificantly different from Young-control at P < 0.05.
bSignificantly different from Young-PFT at P < 0.05.
cSignificantly different from Aged-control at P < 0.05.
dSignificantly different from Aged-PFT at P < 0.05.
Discussion
A major cause of age-related diseases is oxidation caused by reactive oxygen species (ROS). Therefore, increasing evidence has been mounting of the potential for nutraceuticals to counter-act age-associated oxidative stress and molecular alterations. The present study shows that PFT, a novel kefir product, has the ability to reverse age-associated oxidative stress in mice by inhibiting oxygen radical generation; increasing GSH, TAC, AHR, and enzymatic antioxidant capacity; and decreasing the levels of MDA and NO of aged mice. The levels of lipid peroxidation and antioxidant capacity of PFT-treated aged mice were comparable to that of young mice. Recent studies showed that other probiotics have the ability in mice to improve biomarkers’ expression related to aging and alleviate oxidative stress, including the probiotic Dahi (containing Bifidobacterium bifidum and Lactobacillus acidophilus)51 and Lactobacillus plantarum AR113 and AR501.52 Other LABs have also been shown to relieve oxidative stress in other models.53,54 Furthermore, we have previously shown that MRN-100, an iron-based dietary supplement, can reverse age-related oxidative stress.18,19 Finally, other dietary components that exhibit antioxidant properties, such as selenium and vitamins A, C, and E, can ameliorate oxidative damage.55,56
The mechanisms underlying PFT’s effect may include its enhancement of antioxidant defense systems, both enzymatic and non-enzymatic. Our results show that aged mice have significantly lower levels of antioxidant enzymes (GPx, CAT, and SOD) in comparison with young mice. These lower levels are observed in the brain, liver, and blood of untreated aged mice and agree with observations noted in other earlier studies.9,13,14,57 PFT supplementation for aged mice dramatically increased these antioxidant enzymes’ activities to levels within range of the young mice’s enzyme activity. Enzyme activation induced by PFT may increase the hydrogen peroxide to water conversion rate mediated by GPx and CAT as well as the superoxide to hydrogen peroxide conversion rate mediated by SOD.58,59
With regard to the non-enzymatic antioxidant defense system, PFT’s effect may include its action relevant to GSH. GSH is a protective endogenous antioxidant that is important in combating free radicals and other oxidants, and it has been linked with inflammatory responses and immune modulation.60 One potential method to protect cells from damage caused by ROS is to restore intracellular levels of GSH by exogenous administration of GSH or its precursors, a method that has therapeutic potential for protecting lungs against injury, inflammation, and oxidative stress.61–63 Our observed depletion of GSH levels in aged mice agrees well with other studies,2,64 while we also observed that GSH levels for PFT-treated aged mice were restored to levels similar to those of young control mice. Altogether, the present results indicate that PFT has a free radical-scavenging effect in vivo.
The correlation between age-related changes in lipid metabolism and oxidative stress has recently been of great interest.65–67 Increased lipid content is thought to result in decreased antioxidant enzyme expression, increased NADPH oxidase expression, and increased reactive oxygen species concentrations.68 This may represent an additional mechanism by which PFT is able to exert antioxidant capabilities. In this study, PFT supplementation in aged mice exhibited significant decreases in the concentrations of TC (–34%) and TG (–16%) relative to untreated aged control mice, as shown in Figure 9. Importantly, the concentration of the “bad cholesterol” LDL, problematic for its role in distributing cholesterol onto the arterial walls of the heart, also decreased significantly (–57%) in the PFT-treated group relative to aged control. In conjunction with the decrease in LDL, HDL concentrations were shown to increase by 77% in PFT-treated aged mice relative to aged control. Treatment with PFT in aged mice returned levels of TC, TG, LDL, and HDL to near that of the young control mice. These data suggest that another mechanism by which PFT may reduce oxidative stress is through changes in the lipid profile. This data is in accordance with earlier findings that show how detrimental changes to the lipid profile result in increased oxidative stress.68,69 However, there is inconsistency regarding elevated LDL/reduced HDL for aged versus young individuals. There are plenty of inconclusive studies about how this behaves, with some showing increases in LDL up to middle-age and then a flat-line or reduction, and similarly for HDL, a decrease to a certain point followed by flat-line or small increase.70,71
Additionally, oxidative stress in tissues also leads to damage of hepatocytes. One of the most common indicators of the damage to liver cells is the release of intracellular transaminase enzymes in the circulation.72,73 In the current study, AST and ALT activities were raised in aged mice, indicating that the hepatic function was affected by age and oxidative stress. Figure 8 shows that supplementation with PFT resulted in a significant decrease in ALT (–53%) and AST (–69%) activities relative to untreated aged control mice. Based on this result, we argue that PFT may have hepato-protective effect.
The mechanisms by which PFT induces an antioxidant effect in non-GI tissues such as the blood, liver, and brain are not fully understood. The effect might be due to the presence of bioactive molecules produced by probiotics. Other LAB studies have reported bioactive molecules such parasporin-2Aa1 (from Bacillus thuringiensis strain A1547),74 epsilon-poly-L-lysine (from marine Bacillus subtilis SDNS),75 and polyphosphate (poly P) (from L. brevis SBL8803).76 PFT, which is mainly constituted of L. kefiri P-IF, does have unique characteristics among LAB, namely that 1) due to surface carbohydrate chains, P-IF can grow three-dimensionally whereas other L. kefiri strains grow lengthwise, and 2) P-IF can produce carbonic acid by using galactose as a carbon source.32 Our earlier studies showed that PFT induces apoptosis in human multidrug-resistant (MDR) myeloid leukemia (HL60/AR) cells27 and it inhibits Ehrlich Ascites Carcinoma in mice via induction of apoptosis and immunomodulation.77 These could potentially indicate other channels of activity for PFT’s effects.
In addition to studying aged mice, it has been interesting to compare the effects of PFT on the redox homeostasis of juvenile mice. Results of the current study show that young mice are also subjected to oxidative stress, and furthermore that treatment with PFT was able to correct the parameters under investigation. The oxidative stress theory of aging offers the best mechanistic elucidation of the aging phenomenon and other age-related diseases. The age associated decline in the efficiency of the antioxidant system and oxidative damage to proteins and lipids is evident in the current study on mice as well as in our earlier study on rats.19 A similar finding has also been reported in humans by Maciejczyk et al78 who showed that elderly subjects (65–85 years old) demonstrated a significant decrease in the efficiency of both enzymatic and non-enzymatic antioxidant systems as compared with adults (25–45 years old). However, when compared to children (2–14 years), aged subjects did not show a significant difference in the antioxidant enzymes. Maciejczyk et al suggested that it is mainly non-enzymatic antioxidants (GSH, uric acid) that are responsible for differences in the redox status of aged subjects. In this study, we did not observe such a dissociation between enzymatic and non-enzymatic antioxidants between young (2 months old) and aged mice (10 month old). This discrepancy may be related to differences in physiological and growth changes in mice and humans.
PFT has been shown to be non-toxic in several studies. In a study of human PBMCs treated with 5.0 mg/ml of PFT for 3 days, no significant change in apoptosis percentage was found.30 Mice that were orally administered PFT for 30 days showed normal behavior and feeding activity, and they maintained normal health levels without any side effects.77 Additionally, in earlier in vivo studies, mice treated with PFT had no histopathological or macroscopic abnormalities in different organs and no change in body weight.79
Results of the current study have focused on biomarkers, but the evaluation of oxidative damage to proteins and DNA can also give important insights into the dynamics of oxidative stress and should be considered in future studies. In addition, while we have shown that the ratio between antioxidants increased and pro-oxidants decreased, suggesting that PFT promotes antioxidant mechanisms under the dose and conditions in this study, we cannot exclude the pro-oxidant effect of PFT. Other antioxidants such as vitamin C and flavonoids, though known as potent antioxidants, have been shown in other studies to exert pro-oxidant effects.80,81 This should be explored further. Finally, our results indicate the effectiveness of dietary PFT supplementation in modulating oxidative stress in mice. Whether supplementation with antioxidative PFT can change oxidative stress in aged humans remains to be evaluated.
Conclusion
In conclusion, we have shown that PFT, a novel kefir product, can alleviate age-inflicted oxidative stress. PFT reduces oxidative stress biomarkers and augments endogenous antioxidant enzymes in aged mice. Daily supplementation with PFT may protect against age-associated oxidative stress.
Acknowledgments
The authors would like to thank Paitos Co., Ltd., Yokohama, Kanagawa, Japan; grant no. T0099108.
Footnotes
Animal welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
Availability of data and materials: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was partially supported by the National Institutes of Health-National Institute on Minority Health and Health Disparities (grant nos. U54MD007598 and S21MD000103).
Ethics approval: Animal protocols were in compliance with the Guide for the Care and Use of Institutional Ethics Committee of Faculty of Science, Alexandria University, Egypt and the experiments were approved by the University (AU 04190824101).
ORCID iD: Mamdooh Ghoneum  https://orcid.org/0000-0002-1087-7127
https://orcid.org/0000-0002-1087-7127
References
- 1. Harman D. (1984) Free radical theory of aging: The “free radical” diseases. AGE 7(4): 111–131. [Google Scholar]
- 2. Skrzydlewska E, Augustyniak A, Michalak K, et al. (2005) Green tea supplementation in rats of different ages mitigates ethanol-induced changes in brain antioxidant abilities. Alcohol 37(2): 89–98. [DOI] [PubMed] [Google Scholar]
- 3. Murali G, Panneerselvam KS, Panneerselvam C. (2008) Age-associated alterations of lipofuscin, membrane-bound ATPases and intracellular calcium in cortex, striatum and hippocampus of rat brain: Protective role of glutathione monoester. International Journal of Developmental Neuroscience 26(2): 211–215. [DOI] [PubMed] [Google Scholar]
- 4. Cutler RG. (1991) Human longevity and aging: Possible role of reactive oxygen species. Annals of the New York Academy of Sciences 621(1): 1–28. [DOI] [PubMed] [Google Scholar]
- 5. Weyemi U, Lagente-Chevalier O, Boufraqech M, et al. (2012) ROS-generaing NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene 31: 1117–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schieber M, Chandel NS. (2014) ROS function in redox signaling and oxidative stress. Current Biology 24(10): R453–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harman D. (1992) Free radical theory of aging. Mutation Research 275(3–6): 257–266. [DOI] [PubMed] [Google Scholar]
- 8. Sawada M, Carlson JC. (1987) Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mechanisms of Ageing and Development 41(1–2): 125–137. [DOI] [PubMed] [Google Scholar]
- 9. Siqueira IR, Fochesatto C, Andrade AD, et al. (2005) Total antioxidant capacity is impaired in different structures from aged rat brain. International Journal of Developmental Neuroscience 23(8): 663–671. [DOI] [PubMed] [Google Scholar]
- 10. Pansarasa O, Bertorelli L, Vecchiet J, et al. (1999) Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free Radical Biology and Medicine 27(5–6): 617–622. [DOI] [PubMed] [Google Scholar]
- 11. Wei YH, Lu CY, Lee HC, et al. (1998) Oxidative damage and mutation to mitochondrial DNA and age-dependent decline of mitochondrial respiratory function. Annals of the New York Academy of Sciences 854: 155–170. [DOI] [PubMed] [Google Scholar]
- 12. Rizvi SI, Maurya PK. (2007) Alterations in antioxidant enzymes during aging in humans. Molecular Biotechnology 37: 58–61. [DOI] [PubMed] [Google Scholar]
- 13. Akila VP, Harishchandra H, D’souza V, et al. (2007) Age related changes in lipid peroxidation and antioxidants in elderly people. Indian Journal of Clinical Biochemistry 22: 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rao G, Xia E, Richardson A. (1990) Effect of age on the expression of antioxidant enzymes in male Fischer F344 rats. Mechanisms of Ageing and Development 53(1): 49–60. [DOI] [PubMed] [Google Scholar]
- 15. Koskenkorva-Frank TS, Weiss G, Koppenol WH, et al. (2013) The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radical Biology and Medicine 65: 1174–1194. [DOI] [PubMed] [Google Scholar]
- 16. Balla G, Jacob HS, Balla J, et al. (1992) Ferritin: A cytoprotective antioxidant stratagem of endothelium. Journal of Biological Chemistry 267: 18148–18153. [PubMed] [Google Scholar]
- 17. Lin F, Girotti AW. (1997) Elevated ferritin production, iron containment, and oxidant resistance in hemin-treated leukemia cells. Archives of Biochemistry and Biophysics 346(1): 131–141. [DOI] [PubMed] [Google Scholar]
- 18. Ghoneum M, Matsuura M, Gollapudi S. (2009) An iron-based beverage, HydroFerrate fluid (MRN-100), alleviates oxidative stress in murine lymphocytes in vitro. Nutrition Journal 8(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Badr El-Din NK, Noaman E, Fattah SM, et al. (2010) Reversal of age-associated oxidative stress in rats by MRN-100, a hydro-ferrate fluid. In Vivo 24(4): 525–533. [PubMed] [Google Scholar]
- 20. Metchinkoff E, Metchinkoff II. (1908) The Prolongation of Life: Optimistic Studies. New York, NY: Putnam, 109–132. [Google Scholar]
- 21. Ljungh A, Wadstrom T. (2006) Lactic acid bacteria as probiotics. Current Issues in Intestinal Microbiology 7(2): 73–89. [PubMed] [Google Scholar]
- 22. Kanmani P, Satish Kumar R, Yuvaraj N, et al. (2013) Probiotics and its functionally valuable products - a review. Critical Reviews in Food Science and Nutrition 53: 641–658. [DOI] [PubMed] [Google Scholar]
- 23. Marco ML, Pavan S, Kleerebezem M. (2006) Towards understanding molecular modes of probiotic action. Current Opinion in Biotechnology 17: 204–210. [DOI] [PubMed] [Google Scholar]
- 24. Mandel DR, Eichas K, Holmes J. (2010) Bacillus coagulans: A viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complementary and Alternative Medicine 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LeBlanc JG, del Carmen S, Miyoshi A, et al. (2011) Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. Journal of Biotechnology 151: 287–293. [DOI] [PubMed] [Google Scholar]
- 26. Van’t Veer P, van Leer EM, Rietdijk A, et al. (1991) Combination of dietary factors in relation to breast-cancer occurrence. International Journal of Cancer 47: 649–653. [DOI] [PubMed] [Google Scholar]
- 27. Ghoneum M, Gimzewski J. (2014) Apoptotic effect of a novel kefir product, PFT, on multidrug-resistant myeloid leukemia cells via a hole-piercing mechanism. International Journal of Oncology 44: 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hertzler SR, Clancy SM. (2003) Kefir improves lactose digestion and tolerance in adults with lactose maldigestion. Journal of the American Dietetic Association 103: 582–587. [DOI] [PubMed] [Google Scholar]
- 29. Maeda H, Zhu X, Omura K, et al. (2004) Effects of an exopolysaccharide (kefiran) on lipids, blood pressure, blood glucose, and constipation. Biofactors 22: 197–200. [DOI] [PubMed] [Google Scholar]
- 30. Ghoneum M, Felo N. (2015) Selective induction of apoptosis in human gastric cancer cells by Lactobacillus kefiri (PFT), a novel kefir product. Oncology Reports 34(4): 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghoneum M, Felo N, Agrawal S, et al. (2015) A novel kefir product (PFT) activates dendritic cells to induce CD4+T and CD8+T cell responses in vitro. International Journal of Immunopathology and Pharmacology 28(4): 488–496. [DOI] [PubMed] [Google Scholar]
- 32. Suzuki K, Tani H, Yabumoto T, et al. (2011) Novel fermented milk product and use thereof. US Patent No. US 20110123640 A1. May 2011. [Google Scholar]
- 33. Festing MF, Altman DG. (2002) Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR Journal 43: 244–258. [DOI] [PubMed] [Google Scholar]
- 34. Festing MF. 3Rs-Reduction.co.uk. Available at: http://www.3rs-reduction.co.uk/ (accessed 11 May 2020.
- 35. Faul F, Erdfelder E, Lang AG, et al. (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 39: 175–91. [DOI] [PubMed] [Google Scholar]
- 36. Furukawa N, Matsuoka A, Yamanaka Y. (1990) Effects of orally administered yogurt and kefir on tumor growth in mice. Nippon Eiyo Shokuryo Gakkaishi (Journal of Japan Society of Nutrition and Food) 43: 450–453. [Google Scholar]
- 37. Lowry OH, Rosebrough NJ, Farr AL, et al. (1951) Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 193: 265–275. [PubMed] [Google Scholar]
- 38. Tappel A, Zalkin H. (1959) Lipide peroxidation in isolated mitochondria. Archives of Biochemistry and Biophysics 80: 326–332. [DOI] [PubMed] [Google Scholar]
- 39. Sosroseno N, Sugiatno E, Samsudin AR, et al. (2008) The role of nitric oxide on the proliferation of a human osteoblast cell line stimulated with hydroxyapatite. Journal of Oral Implantology 34: 196–202. [DOI] [PubMed] [Google Scholar]
- 40. Jollow D, Mitchell J, Zampaglione N, et al. (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11: 151–169. [DOI] [PubMed] [Google Scholar]
- 41. Habig W, Pabst M, Jakoby W. (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry 249: 7130–7139. [PubMed] [Google Scholar]
- 42. Marklund S, Marklund G. (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry 47: 469–474. [DOI] [PubMed] [Google Scholar]
- 43. Aebi H. (1984) Catalase in vitro. Methods in Enzymology 105: 121–126. [DOI] [PubMed] [Google Scholar]
- 44. Paglia D, Valentine W. (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine 70: 158–169. [PubMed] [Google Scholar]
- 45. Umamaheswari M, Chatterjee TK. (2008) In vitro antioxidant activities of the fractions of Coccinnia grandis L. leaf extract. African Journal of Traditional, Complementary and Alternative Medicines 5: 61–73. [PMC free article] [PubMed] [Google Scholar]
- 46. Halliwell B, Gutteridge JMC, Aruoma OI. (1987) The deoxyribose method: A simple test tube assay for determination of rate constants for reaction of hydroxyl radical. Analytical Biochemistry 165: 215–219. [DOI] [PubMed] [Google Scholar]
- 47. Fossati P, Prencipe L. (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clinical Chemistry 28(10): 2077–2080. [PubMed] [Google Scholar]
- 48. Watson D. (1960) A simple method for the determination of serum cholesterol. Clinica Chimica Acta 5(5): 637–643. [DOI] [PubMed] [Google Scholar]
- 49. Friedewald WT, Levy RI, Fredrickson DS. (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry 18(6): 499–502. [PubMed] [Google Scholar]
- 50. Lopes-Virella MF, Stone P, Ellis S, et al. (1977) Cholesterol determination inHDL separated by three different methods. Clinical Chemistry 23(5): 882–884. [PubMed] [Google Scholar]
- 51. Kaushal D, Kansal VK. (2012) Probiotic Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum alleviates age-inflicted oxidative stress and improves expression of biomarkers of ageing in mice. Molecular Biology Reports 39(2): 1791–1799. [DOI] [PubMed] [Google Scholar]
- 52. Lin X, Xia Y, Wang G, et al. (2018) Lactobacillus plantarum AR501 alleviates the oxidative stress of D-galactose-induced aging mice liver by upregulation of Nrf2-mediated antioxidant enzyme expression. Journal of Food Science 83(7): 1990–1998. [DOI] [PubMed] [Google Scholar]
- 53. Hou Y, Li X, Liu X, et al. (2019) Transcriptomic responses of Caco-2 cells to Lactobacillus rhamnosus GG and Lactobacillus plantarum J26 against oxidative stress. Journal of Dairy Science 102(9): 7684–7696. [DOI] [PubMed] [Google Scholar]
- 54. Valcarce DG, Genovés S, Riesco MF, et al. (2017) Probiotic administration improves sperm quality in asthenozoospermic human donors. Beneficial Microbes 8(2): 193–206. [DOI] [PubMed] [Google Scholar]
- 55. Dragsted LO. (2008) Biomarkers of exposure to vitamins A, C, and E and their relation to lipid and protein oxidation markers. European Journal of Nutrition 47(Suppl. 2): 3–18. [DOI] [PubMed] [Google Scholar]
- 56. Battin EE, Brumaghim JL. (2009) Antioxidant activity of sulfur and selenium: A review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochemistry and Biophysics 55: 1–23. [DOI] [PubMed] [Google Scholar]
- 57. Rho KA, Kim MK. (2006) Effects of different grape formulations on antioxidative capacity, lipid peroxidation and oxidative DNA damage in aged rats. Journal of Nutritional Science and Vitaminology 52: 33–46. [DOI] [PubMed] [Google Scholar]
- 58. Aoyama K, Nakak T. (2012) Inhibition of GTRAP3-18 may increase neuroprotective glutathione (GSH) synthesis. International Journal of Molecular Sciences 13: 12017–12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang Y, Branicky R, Noe A, et al. (2018) Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. Journal of Cell Biology 217: 1915–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ghezzi P. (2011) Role of glutathione in immunity and inflammation in the lung. International Journal of General Medicine 4: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Borok Z, Buhl R, Grimes GJ, et al. (1991) Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet 338: 215–216. [DOI] [PubMed] [Google Scholar]
- 62. Roum JH, Borok Z, McElvaney NG, et al. (1999) Glutathione aerosol suppresses lung epithelial surface inflammatory cell derived oxidants in cystic fibrosis. Journal of Applied Physiology 87: 438–443. [DOI] [PubMed] [Google Scholar]
- 63. Rahman I, MacNee W. (2000) Oxidative stress and regulation of glutathione in lung inflammation. European Respiratory Journal 16: 534–554. [DOI] [PubMed] [Google Scholar]
- 64. Rikans LE, Hornbrook KR. (1997) Lipid peroxidation, antioxidant protection and aging. Biochim Biophys Acta 1362: 116–127. [DOI] [PubMed] [Google Scholar]
- 65. Ming M, Guanhua L, Zhanhai Y, et al. (2008) Effect of the Lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chemistry 113: 872–877. [Google Scholar]
- 66. Bonomini F, Rodella LF, Rezzani R. (2015) Metabolic syndrome, aging and involvement of oxidative stress. Aging and Disease 6(2): 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dominguez LJ, Barbagallo M. (2016) The biology of the metabolic syndrome and aging. Current Opinion in Clinical Nutrition and Metabolic Care 19(1): 5–11. [DOI] [PubMed] [Google Scholar]
- 68. Furukawa S, Fujita T, Shimabukuro M, et al. (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. Journal of Clinical Investigation 114(12): 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wonisch W, Falk A, Sundl I, et al. (2012) Oxidative stress increases continuously with BMI and age with unfavourable profiles in males. The Aging Male 15(3): 159–165. [DOI] [PubMed] [Google Scholar]
- 70. Murakata Y, Fujimaki T, Yamada Y. (2015) Age-related changes in clinical parameters and their associations with common complex diseases. Biomedical Reports 3: 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Loh TP, Ma S, Heng D, et al. (2016) Age-related changes in the cardiometabolic profiles in Singapore resident adult population: Findings from the national health survey 2010. PLOS ONE 11(8): e0162102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pereira DI, Gibson GR. (2002) Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Critical Reviews in Biochemistry and Molecular Biology 37: 259–281. [DOI] [PubMed] [Google Scholar]
- 73. Lee DK, Jang S, Baek EH, et al. (2009) Lactic acid bacteria affect serum cholesterol levels, harmful fecal enzyme activity, and fecal water content. Lipids in Health and Disease 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brasseur K, Auger P, Asselin E, et al. (2015) Parasporin-2 from a new Bacillus thuringiensis 4R2 strain induces caspase activation and apoptosis in human cancer cells. PLOS ONE 10: e0135106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. El-Sersy NA, Abdelwahab AE, Abouelkhiir SS, et al. (2012) Antibacterial and anticancer activity of epsilon-poly-L-lysine (epsilon-PL) produced by a marine Bacillus subtilis sp. Journal of Basic Microbiology 52: 513–522. [DOI] [PubMed] [Google Scholar]
- 76. Sakatani A, Fujiya M, Ueno N, et al. (2016) Polyphosphate derived from Lactobacillus brevis inhibits colon cancer progression through induction of cell apoptosis. Anticancer Research 36: 591–598. [PubMed] [Google Scholar]
- 77. Badr El-Din NK, Shabana S, Abdulmajeed BA, et al. (2020) Anticancer effect by Lactobacillus kefiri (PFT), a novel kefir product, in animals bearing Ehrlich ascites carcinoma. BMC Complementary Medicine and Therapies 20: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Maciejczyk M, Zalewska A, Ładny JR. (2019) Salivary antioxidant barrier, redox status, and oxidative damage to proteins and lipids in healthy children, adults, and the elderly. Oxidative Medicine and Cellular Longevity 2019: 4393460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Paitos Co., Ltd. Yokohama, Kanagawa, Japan. Increase the good bacteria held by nature, ideal AH21 is a functional food, consider preventive medicine and food. Available at: http://www.bio-j.net/ken00.html (accessed 14 June 2013).
- 80. Pawlowska E, Szczepanska J, Blasiak J. (2019) Pro- and antioxidant effects of vitamin C in cancer in correspondence to its dietary and pharmacological concentrations. Oxidative Medicine and Cellular Longevity 2019: 7286737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cotelle N. (2001) Role of flavonoids in oxidative stress. Current Topics in Medicinal Chemistry 1: 569–590. [DOI] [PubMed] [Google Scholar]