We describe the history and our experience of introducing helmet continuous positive airway pressure (H-CPAP) to healthcare in the United States during the COVID-19 pandemic. We discuss the potential of this noninvasive ventilation (NIV) support method during this pandemic, the timeline of its introduction, and obstacles to its adoption.
European studies regarding helmet-based respiratory support were first published in the late 1990s. The transparent helmet covers the entire head with a collar neck seal and delivers air and oxygen either via wall flow meters, a high-flow device, or a ventilator. The helmet enclosure reduces aerosolization risk significantly compared to a high-flow nasal cannula or face mask, which have uncertain and uncontained aerosolization potential.1 Therefore, viral spread and transmission to healthcare workers is thought to be lessened with H-CPAP use.
Helmet technology received premarket US Food and Drug Administration approval for use in hyperbaric oxygen treatment. US companies involved in helmet manufacturing currently include Sea-Long Medical Systems, (Waxahachie, TX, USA), Amron International Inc., (Vista, CA, USA) and Lombardi Undersea LLC (Middletown, RI, USA). In 2016, a major American academic center demonstrated that helmet NIV decreased the need for intubation and intensive care unit length of stay in patients with mild to moderate adult respiratory distress syndrome (ARDS) compared with mask NIV.2,3 However, widespread adoption as a ventilation strategy for respiratory failure did not occur.
COVID-19 related acute respiratory distress syndrome (CARDS) is distinct from traditional ARDS, as CARDS is characterized by an intermediate stage with high lung compliance and a later stage with low lung compliance.4 The initial CARDS treatment strategy of early intubation and mechanical ventilation have now shifted towards avoidance of early intubation, with support of noninvasive techniques.
At our institution, anesthesiologist intensivists began regular teleconferences with their colleagues in Italy at the outset of the US COVID-19 pandemic escalation in late February 2020. It became clear that H-CPAP helped to avoid intubation in CARDS and was beneficial in decreasing resource utilization including ventilator demand. We published an institutional respiratory failure algorithm that recommended early helmet use for patients admitted to a COVID-19 critical care unit.
Recognizing the significant potential advantages of helmet use for COVID-19 affected patients in respiratory distress, we contacted the Veterans Administration (VA) National Anesthesia Service and the American Society of Anesthesiologists (ASA) leadership in mid-March 2020 to raise nationwide awareness about helmet technology and provide more information to clinicians. The ASA conducted a nationwide COVID-19 Town Hall Webinar on 19 March 2020, which included several talking points on H-CPAP. The ASA subsequently informed the Assistant Secretary for Preparedness and Response at the US Department of Health & Human Services, about this technology to further national dissemination of helmet technology knowledge. On 20 March 2020, the Society for Critical Care Medicine published guidelines that included H-CPAP as a ventilation strategy for COVID-19 patients.5
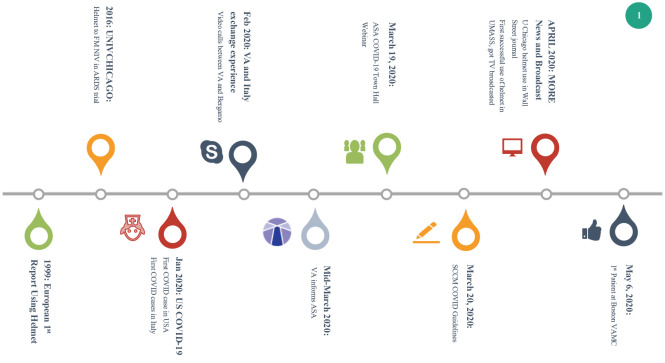
By the end of March 2020, the University of Chicago reported their successful H-CPAP use in COVID-19 patients in the Wall Street Journal. Additional VA centers across the country began ordering the devices. Towards the end of April, critical care teams at the University of Massachusetts Memorial Medical Center had begun a successful H-CPAP program for their patients (Figure 1). Several additional regional medical centers are now in possession of helmets and prepared to activate their respective programs.
Figure 1.
A timeline of helmet ventilation technology.
ASA, American Society of Anesthesiology; FM NIV, face mask noninvasive ventilation; SCCM, Society for Critical Care Medicine; UMASS, University of Massachusetts; VA, Veterans Affairs;
VAMC, Veterans Affairs Medical Center.
As a new treatment method, H-CPAP requires several champions and multiple service lines (nursing, respiratory, anesthesiology, critical care, others) to coordinate its use. Clinician concerns about the risk of viral transmission, the need for close supervision to detect or prevent anticipated device malfunction, and patient decompensation can be dispelled by education, scientific evidence, and experience.6 Lifelong learning, an open mind, and a positive attitude to new, lifesaving treatments remain cornerstones for successful new program implementation. We must accept a learning curve for the benefit of many patients.
Despite our intensivists’ early enthusiasm for H-CPAP, our introduction has been slower than in other environments. We conducted multiple educational and in-service sessions with nursing and respiratory services, residents, physician colleagues, and advanced practitioners. The University of Chicago program provides extensive online educational materials including video instruction, slide sets, infographics and manikin hands-on demonstrations, which were made available to our team. By early May 2020, the H-CPAP program was approved by our institutional leadership and in our collective experience of three early helmet-adopting institutions, we have treated a total of 60 patients with helmet CPAP. Approximately 30–50% of these patients were successfully managed with helmet respiratory support, not requiring escalation to intubation and mechanical ventilation. This may be an indication of the potential of H-CPAP to either obviate or delay (flatten the ventilator demand curve) the need for more invasive ventilation.
Supplemental Material
Supplemental material, Author_Response_1 for Helmet CPAP: how an unfamiliar respiratory tool is moving into treatment options during COVID-19 in the US by Houman Armirfarzan, Jessica L. Shanahan, Roman Schuman and Kay B. Leissner in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Helmet CPAP: how an unfamiliar respiratory tool is moving into treatment options during COVID-19 in the US by Houman Armirfarzan, Jessica L. Shanahan, Roman Schuman and Kay B. Leissner in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Helmet CPAP: how an unfamiliar respiratory tool is moving into treatment options during COVID-19 in the US by Houman Armirfarzan, Jessica L. Shanahan, Roman Schuman and Kay B. Leissner in Therapeutic Advances in Respiratory Disease
Footnotes
Author contribution(s): Houman Amirfarzan: Conceptualization; Investigation; Software; Supervision; Writing-original draft; Writing-review & editing.
Jessica L. Shanahan: Investigation; Writing-original draft; Writing-review & editing.
Roman Schumann: Investigation; Writing-review & editing.
Kay B. Leissner: Conceptualization; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: support was provided solely from institutional and/or department resources.
ORCID iD: Houman Armirfarzan  https://orcid.org/0000-0002-3843-2808
https://orcid.org/0000-0002-3843-2808
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Houman Armirfarzan, Tufts University School of Medicine, Boston, MA, USA; Anesthesiology and Perioperative Medicine, VA Boston Healthcare System, 1400 Vfw Pkwy, West Roxbury, MA 02132, USA.
Jessica L. Shanahan, Tufts University School of Medicine, Boston, MA, USA Anesthesiology and Perioperative Medicine, VA Boston Healthcare System, Boston, MA, USA.
Roman Schuman, Tufts University School of Medicine, Boston, MA, USA; Anesthesiology and Perioperative Medicine, VA Boston Healthcare System, Boston, MA, USA.
Kay B. Leissner, Harvard Medical School; Anesthesiology and Perioperative Medicine, VA Boston Healthcare System, Boston, MA, USA
References
- 1. Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest 2015; 147: 1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel BK, Wolfe KS, Pohlman AS, et al. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2016; 315: 2435–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mwenge GB, Rodenstein D. CPAP added to oxygen administration avoid intubation in acute respiratory distress in COVID-19 pneumonia. Case report. SN Compr Clin Med 2020; 18: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chiumello D, Caironi P, Busana M, et al. COVID-19 pneumonia: different respiratory treatment for different phenotypes? Intensive Care Med 2020; 46: 1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med 2020; 46: 854–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mojoli F, Iotti GA, Gerletti M, et al. Carbon dioxide rebreathing during non-invasive ventilation delivered by helmet: a bench study. Intensive Care Med 2008; 34: 1454–1460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Helmet CPAP: how an unfamiliar respiratory tool is moving into treatment options during COVID-19 in the US by Houman Armirfarzan, Jessica L. Shanahan, Roman Schuman and Kay B. Leissner in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Helmet CPAP: how an unfamiliar respiratory tool is moving into treatment options during COVID-19 in the US by Houman Armirfarzan, Jessica L. Shanahan, Roman Schuman and Kay B. Leissner in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Helmet CPAP: how an unfamiliar respiratory tool is moving into treatment options during COVID-19 in the US by Houman Armirfarzan, Jessica L. Shanahan, Roman Schuman and Kay B. Leissner in Therapeutic Advances in Respiratory Disease