Abstract
Neurogenic orthostatic hypotension (nOH) is a subtype of orthostatic hypotension in which patients have impaired regulation of standing blood pressure due to autonomic dysfunction. Several primary and secondary causes of this disease exist. Patients may present with an array of symptoms making diagnosis difficult. This review article addresses the epidemiology, pathophysiology, causes, clinical features, and management of nOH. We highlight various pharmacological and non-pharmacological approaches to treatment, and review the recent guidelines and our approach to nOH.
Keywords: Neurogenic OH, autonomic dysfunction
Introduction
Orthostatic hypotension (OH) is defined as a fall in systolic blood pressure (SBP) of ⩾20 mm Hg or diastolic blood pressure (DBP) of ⩾10 mm Hg, within 3 minutes of standing.1 This definition was updated in 2011 to include a fall in SBP of ⩾30 mm Hg for patients with an elevated baseline BP including those with supine hypertension (SH).2 In patients with Multiple System Atrophy (MSA), a fall of ⩾30 mmHg in SBP or ⩾15 mmHg in DBP is used to define OH.3,4 Delayed OH is when orthostatic symptoms take longer than 3 minutes to emerge.5 This indicates an early form of sympathetic adrenergic failure—more than 50% of such patients will develop classic OH over the next decade.5 These differences in diagnostic criteria create an area of some uncertainty regarding optimal definitions.
Non-neurogenic OH is caused by reduced cardiac output and/or impaired vasoconstriction without a primary autonomic disorder. On the other hand, neurogenic OH (nOH) typically results from inadequate vasomotor sympathetic release of norepinephrine due to autonomic dysfunction.6 This reduction in sympathetic innervation also causes the heart rate (HR) to increase less than expected.7 nOH is not solely a disease of low BP but also of high BP (ie, supine hypertension) such that the narrow range of BP normality is perturbed, and patients often display both OH and SH at differing times. This dysfunction can arise from impaired central neural pathways that regulate sympathetic control, or from deficient activation of vascular adrenoceptors due to degenerative postganglionic sympathetic neurons.6 nOH is a debilitating disorder that carries significant morbidity and is an independent risk factor for mortality.8-10
Methods
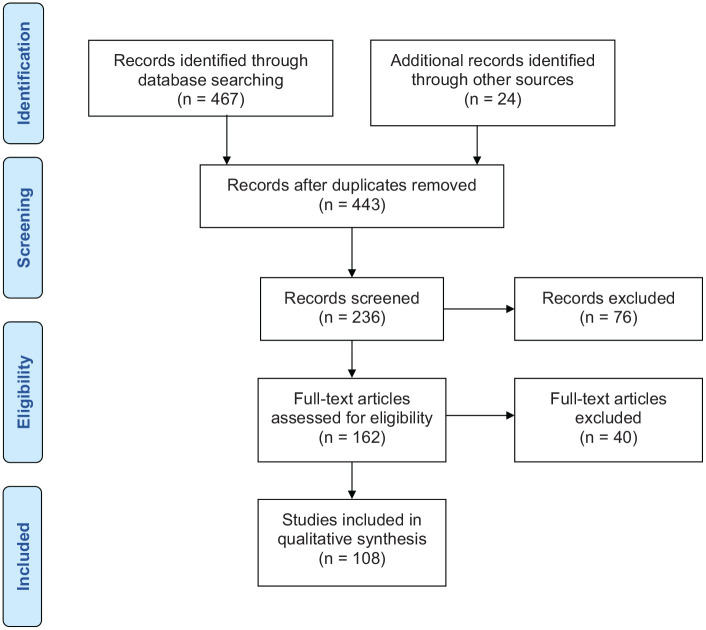
We searched PubMed CENTRAL, MEDLINE, Embase, and Web of Science in July 2020. We included all English language studies on nOH reporting diagnosis and therapies. We included reviews, controlled trials, cohort studies or case-control studies. Inclusion was considered irrespective of prospective or retrospective recruitment or publication date. The key terms searched were neurogenic orthostatic hypotension, expert documents, guidelines, and practice recommendations. The reference lists of all relevant articles were scrutinized for relevant studies. Full texts of all eligible studies were retrieved. Data from the included studies were extracted and collated using a standardized extraction form. The selection process was recorded in a PRISMA flow diagram (Figure 1). This systematic literature search identified 491 citations. Deduplication left 443 citations, of which 281 were excluded because they did not contain relevant data on therapy, were case reports, or not about nOH. Full texts of 162 articles were retrieved and 54 were excluded because they did not report enough detail or were conference abstracts. Thus, 108 studies were included.
Figure 1.
PRISMA flow chart.
Epidemiology and causes
The cross-sectional prevalence of OH in unselected patients aged 65 years or older is between 5% and 30%.8,11 OH is an independent risk factor for mortality, stroke, and adverse cardiovascular (CV) outcomes including coronary artery disease and myocardial infarction.8,10,12-18 The increase in age related prevalence may be attributed to the decrease in the effectiveness of the counter-regulatory mechanisms including reduced vasomotor tone, decreased baroreflex sensitivity, decreased ß-adrenoreceptor mediated responses, reduced cardiac compliance, increased arterial stiffness, and attenuation of the vestibulosympathetic reflex.12,19,20
Although OH is a frequent problem encountered in primary care, nOH is relatively uncommon and is more often diagnosed by neurologists and cardiologists. As previously mentioned, when OH occurs either because of reduced norepinephrine release from postganglionic sympathetic nerves, or due to central autonomic neurodegeneration leading to defective vasoconstriction in the upright posture, it is referred to as nOH.7 nOH is best understood as a neurotransmitter disorder and has been classified as an orphan disease (ie, one which affects fewer than 200 000 people in the United States7,11,21), however the true prevalence of nOH is unknown and is likely underestimated.7
Among the primary causes of nOH are a group of neurodegenerative diseases characterized pathologically by the deposition of the protein α-synuclein in the central or peripheral nervous system, including Parkinson’s Disease (PD), MSA, Pure Autonomic Failure (PAF), and Dementia with Lewy Bodies (DLB). These 4 conditions are thus called α-synucleinopathies and have nOH as a common manifestation. Despite clinical similarities, there are major pathological differences; thus, they are further subdivided into 2 phenotypes: Lewy Body Diseases (PD, PAF, and DLB), and MSA on basis of the neuronal cell type involved and degree of neuronal loss.22,23 In a large meta-analysis of 25 studies, the prevalence of nOH in PD was 30%.24 In another prospective cohort study of 175 patients with MSA, the prevalence of nOH was 78%. About 50% of patients with DLB have nOH.25
There are also a variety of metabolic, autoimmune, and neoplastic conditions that produce secondary nOH7,26-28 (Figure 2). Amongst these, diabetes is one of the most common causes and about 8% of diabetics develop nOH.29
Figure 2.
Classification of causes of nOH.
Clinical presentation and severity
The majority of patients with OH do not have dizziness12—in one study, only 2% of participants had symptoms, whereas 16.2% were asymptomatic.13 In another study of 12 433 patients, symptoms were not significantly different in patients with or without OH (11.3% vs 9.4%).15 Similarly, patients with nOH may or may not experience symptoms. The chronic nature of nOH allows remarkable adaptive changes in cerebral autoregulatory mechanisms. Thus, patients, are frequently able to tolerate wide swings in BPs and remain conscious at pressures that would otherwise induce syncope in healthy subjects.30 In a study of 1125 patients with PD, only 18% reported any OH symptoms.31 In contrast, nOH symptoms were much more frequently seen in patients with MSA (81%) and DLB (31%).
Symptoms also vary according to the location of the lesion, especially the presence of Lewy bodies in locations such as locus ceruleus, sympathetic ganglia, and parasympathetic plexus which may be responsible for autonomic, axial, or cognitive symptoms.32 A recent study of 363 nOH patients with PD, MSA, or PAF reported lightheadedness as the most common symptom followed by several others (Table 1).33 Additional symptoms of autonomic failure and neurological symptoms can help differentiate them from patients with non-neurogenic OH (Table 2).30,34 Common symptoms include dizziness, blurring of vision/tunnel vision, impaired cognition, syncope and rarely, seizures.34 Symptoms related to muscle hypoperfusion are coat hanger ache (pain in the suboccipital, paracervical, and shoulder muscles), lower back pain or calf claudication.34-37 Symptoms such as angina due to cardiac hypoperfusion, orthostatic dyspnea due to lower perfusion of the lung apices, and oliguria due to renal hypoperfusion may occur.20,34,38 Neurologic symptoms may include pill rolling tremor, rigidity, bradykinesia and postural instability in PD.39 MSA is characterized by parkinsonism, autonomic dysfunction and cerebellar signs such as ataxia and dysarthria.1 Additional features may include an action tremor with superimposed jerks (rather than the “pill-rolling” PD tremor), dystonia, camptocormia (severe anterior flexion of the spine) and dysphagia20,39,40 PAF is diagnosed based on a history of symptomatic OH and low serum norepinephrine levels during supine rest. Signs of central neurodegeneration are typically absent.20,41 Some of these same symptoms such as lightheadedness, blurring of vision, weakness or fatigue may also be seen in patients with postural orthostatic tachycardia syndrome, however the latter can usually be differentiated from nOH by a sustained HR increase of 30 beats per minute within 10 minutes of standing in the absence of OH.2
Table 1.
Symptom burden in patients with nOH.29
| Symptoms | Percentage of respondents reporting symptoms multiple times a day |
|---|---|
| 1. Dizziness or lightheadedness | 29 |
| 2. Fatigue when standing | 28 |
| 3. Difficulty walking | 26 |
| 4. Blurry vision | 19 |
| 5. Pain running down neck and across shoulders | 17 |
| 6. Confused, foggy, inability to think clearly | 16 |
| 7. Feeling faint | 11 |
| 8. Difficulty breathing | 10 |
Table 2.
Symptoms of autonomic failure and neurological deficits indicative of nOH.
| Symptoms of autonomic failure | Urinary symptoms | Increased frequency, retention, incontinence |
| Gastrointestinal | Constipation, nocturnal diarrhea, gastroparesis, dry mouth | |
| Vision problems | Blurring of vision, inability of pupils to react to light | |
| Genitourinary | Erectile dysfunction, retrograde ejaculation | |
| Symptoms of neurological deficits | Cognitive impairment | Forgetfulness, poor judgment, depression, irritability |
| Parkinsonian symptoms | Pill rolling tremor, rigidity, bradykinesia, postural instability | |
| Cerebellar signs | Ataxia, dysarthria, dysmetria, dysdiadochokinesia | |
| Sensory neuropathy | Numbness, tingling, burning pain |
A study by Merola et al in 121 patients with PD showed that patients with asymptomatic OH had similar impairment in activities of daily living, quality of life and falls as compared to patients with symptomatic OH.32 In another study by Freeman et al in 89 patients with OH, no correlation was found between the magnitude of SBP drop and symptoms. Absence of warning symptoms may place these patients at a higher risk of complications.42 Thus, screening all patients with α-synucleinopathies for orthostatic BP drop may help in better detection of patients with asymptomatic OH. Symptoms may occur after stressors such as meals, standing or just after waking up, coughing, warm weather, and hot baths.34,43
Morbidity and mortality
The debilitating symptoms of nOH make it difficult for patients to complete simple activities of daily living as they live in fear of falling. Syncope, head trauma, and fractures were higher in patients with autonomic failure.44 In one study, 87% of patients with nOH reported that it had a negative impact on their ability to perform everyday activities and their quality of life (59%) and robbed them of their independence (42%).33 In a prospective study of 844 nursing home patients (age > 60 years), those with OH had a 2.6 fold higher risk of recurrent falls.45 Limitation of physical activities frequently leads to debilitation.46 Similarly, in a community based middle age population, Juraschek et al reported that OH was an independent risk factor for falls over 20 years of follow-up.47 Patients with nOH have a 3-fold increased risk of mortality compared to age matched controls, with highest the mortality being in patients with MSA followed by PD and PAF. The median survival in MSA is 7 to 9.5 years compared to PD and PAF where it is 10 to 15 years.8
Pathophysiology
A complex interplay between blood volume, reflex and humoral systems, vascular tone and capacitance of the striated muscle bed, splanchnic-mesenteric bed, and cerebrovascular beds helps maintain postural normotension.43 Upon standing, 500 to 1000 mL of blood pools in the lower extremities and splanchnic circulation. This leads to decreased venous return, reduced ventricular filling, diminished cardiac output, and a drop in BP (Figure 3). In an individual with an intact autonomic nervous system, baroreceptors sense this drop in the carotid sinus and aortic arch. An afferent signal is thus sent via fibers of the glossopharyngeal and vagus nerves to the nucleus tractus solitarus in the dorsomedial medulla.20 A compensatory neural response follows in which there is efferent parasympathetic inhibition20,43 (Figure 3). Parasympathetic inhibition in the nucleus solitarius and nucleus ambiguus also results in sinus node acceleration. There is also a concomitant increase in sympathetic outflow from the medulla via the thoracic spinal cord to the heart and peripheral vasculature.20,43 In this sympathetic efferent pathway, norepinephrine is the major regulatory neurotransmitter and its levels promptly increase. The net effect of this increased sympathetic and decreased parasympathetic tone is peripheral vasoconstriction and a small increase in HR and cardiac contractility. This results in an increase in the venous return and cardiac output which helps blunt the BP drop.36 In nOH, central or peripheral autonomic lesions reduce the effects of these compensatory mechanisms28 (Figure 4) and patients experience a sustained drop in BP. Inadequate release of peripheral norepinephrine upon orthostatic stress is thus a common feature in most conditions that produce nOH.
Figure 3.

Schematic showing normal baroreflex mediated maintenance of BP and physiologic principles behind pharmacotherapy in patients with nOH (highlighted in gray boxes). (The arrow does not denote any hierarchy in treatment.)
Figure 4.
Pathway for autoregulation of blood pressure and conditions that cause nOH.
In the Parkinsonian diseases, this results from both sympathetic neurocirculatory failure (with or without noradrenergic denervation) and impaired baroreflex cardiovagal activity. Central autonomic lesions are most commonly seen in MSA (the peripheral noradrenergic system may or may not be intact), whereas PD and PAF cause predominant peripheral noradrenergic sympathetic denervation (but may have contributions from central denervation as well).48-50 In PD, 3 processes contributing to catecholaminergic deficiency have been described. First, failure of arterial baroreflex can impair homeostatic functions including HR adjustment. Second, central and peripheral norepinephrine deficiency causes sympathetic denervation impairing vasoconstriction. Third, cardiac noradrenergic sympathetic denervation decreases the heart’s ability to increase cardiac output. Post-mortem histological studies in PD patients have confirmed loss of sympathetic noradrenergic nerves in the heart, that is, there is a profound loss of tyrosine hydroxylase immunoreactive cardiac innervation.51 Another study in patients with PD and nOH reported that there was a loss of noradrenergic nerves in extra cardiac tissues as well.48
The pathophysiology of SH is more complex and stems from an impaired baroreflex mechanism and a chronically activated renin-angiotensin system.52 Impaired baroreflex buffering of the BP, inappropriate natriuresis, higher blood volume, and residual sympathetic tone acting on hypersensitive postsynaptic adrenoreceptors in nOH, all play an important role.53 During the day, while patients spend most of their time in a seated or upright position and blood pressure is low, sodium excretion is decreased, and blood volume is increased.54 Moving from standing to a supine position at night increases the venous return and thus increases cardiac output and blood pressure. nOH patients with an impaired baroreflex do not appropriately compensate for this shift and thus develop SH.52 These patients also have chronically activated renin-angiotensin system that exacerbates this problem. In this feedback loop, SH causes nocturnal pressure-diuresis and hypovolemia leading to worsening of early morning orthostatic symptoms.43 A consensus statement in 2018 defined SH as mild (SBP 140-159 mm Hg or DBP 90-99 mm Hg), moderate (SBP 160-179 mm Hg or DBP 100-109 mm Hg), and severe (SBP ⩾ 180 mm Hg or DBP ⩾ 110 mm Hg).55
Screening and diagnosis
The American Autonomic Society and the National Parkinson Foundation guideline recommends that nOH screening start with questions to identify OH symptoms followed by orthostatic BP measurement in the office to diagnose it.26 It describes 5 high risk patient groups who are suitable for such screening (Table 3) and enumerates questions to be asked to elicit these symptoms (Table 4). A positive response to any question warrants additional testing.26 Our 4-step approach to distinguish nOH from OH is described below.
Table 3.
Categories of patients who should be routinely screened for OH.19
| 1. Patients with suspected or diagnosed with any neurodegenerative disorder associated with autonomic dysfunction, including PD, MSA, PAF, DLB |
| 2. Patients who have reported an unexplained fall or have had an episode of syncope |
| 3. Patients with peripheral neuropathies known to be associated with autonomic dysfunction |
| 4. Patients who are elderly (70 years and older) and frail or on multiple medications |
| 5. Patients with postural dizziness or non-specific symptoms that only occur on standing |
Table 4.
Screening questions for nOH.
| 1. Have you fainted/blacked out recently? |
| 2. Do you feel dizzy or lightheaded upon standing? |
| 3. Do you have vision disturbances when standing? |
| 4. Do you have difficulty breathing when standing? |
| 5. Do you have leg buckling of leg weakness when standing? |
| 6. Do you ever experience neck pain or aching when standing? |
| 7. Do the above symptoms improve or disappear when you sit or lay down? |
| 8. Are the above symptoms worse in the morning or after meals? |
| 9. Have you experienced a fall recently? |
| 10. Are there any other symptoms you commonly experience when you stand up or within 3 to 5 min of standing and get better when you sit or lay down? |
A detailed history and physical examination are vital. This helps differentiate nOH from other causes of light headedness (eg, vasovagal, hypoglycemia, obstructive cardiac lesions etc.). Symptoms which point towards a diagnosis of nOH include symptoms of autonomic failure and neurological deficits (Table 2).
BP monitoring should not stop at 3 minutes and may be extended to 10 minutes as some patients will have delayed OH.2,56 If the history is suggestive of OH, but orthostatic testing in the office is negative, a Head Up Tilt (HUT) test (with a tilt of at least 60°) should be considered. This test is also useful in patients with physical limitations who are unable to remain standing for orthostatic testing in the office.26,56 As the symptoms of OH can fluctuate widely throughout the day and office BP readings may not be able to recapitulate the BP readings that occur at the time that the patient experiences symptoms, Ambulatory Blood Pressure Monitoring (ABPM) can be considered to detect such BP drops with meals, exercise, and upon getting out of bed in the morning. ABPM is thus a very useful tool to diagnose covert nOH.7,57 Additionally, one can use the mean BP to ascertain the diagnosis. Palma et al in their study of 210 patients with PD, showed that an upright mean BP < 75 mm Hg is an important marker for detecting symptomatic OH.58
HR response is an important marker to differentiate between neurogenic and non-neurogenic causes as patients with nOH have a blunted HR response. If a patient develops OH upon standing, an increase in HR < 15 bpm suggests a diagnosis of nOH, whereas patients with non-neurogenic OH will typically demonstrate an increase in HR of 15 bpm within 3 minutes of standing.26 Data from Norcliffe-Kaufmann et al.’s study of 402 patients suggested the use of a quantitative tool to assess this feature—they showed that a ΔHR/ΔSBP ratio <0.5 bpm/mm Hg could be used as a diagnostic marker of nOH indicating a diminished HR increase.59
A comprehensive review of medications is important as polypharmacy in elderly patients is common.60 Offending drugs include diuretics, alpha1-antagonists, antidepressants, antipsychotics, and levodopa (Table 6).60 Numerous medications can also diminish the compensatory increase in HR (eg, beta blockers, non-dihydropyridine calcium channel blockers, central alpha-2 agonists, and antiarrhythmic agents).26
Table 6.
Drugs that cause OH.
| Medication class | Offending drugs |
|---|---|
| Alpha-1 antagonists | Doxazosin, prazosin, tamsulosin, terazosin |
| Diuretics | Furosemide, torsemide, hydrochlorothiazide, acetazolamide, spironolactone |
| Nitrates | Nitroprusside, isosorbide dinitrate, nitroglycerin |
| Beta-blockers | Propranolol, metoprolol, atenolol, bisoprolol, carvedilol, labetalol (the last 2 also have α-1 antagonist properties) |
| Tricylic antidepressants | Amitriptyline, nortriptyline, imipramine, desipramine |
| Phosphodiesterase inhibitors | Sildenafil, vardenafil, tadalafil |
| Alpha-2 agonists | Clonidine, guanfacine |
One should ensure the patient is intravascularly volume repleted and should tailor further testing according to the suspected cause (Table 5). For example, a cardiac murmur of aortic or mitral stenosis or hypertrophic cardiomyopathy should point away from nOH and toward a cardiac etiology for OH. Similarly, neurologic symptoms (Figure 2) should raise suspicion of nOH. For example, a pill rolling tremor, rigidity, and bradykinesia indicates PD, whereas, cool skin, stocking-glove sensory loss, and extremity numbness points toward diabetes causing nOH.27,34,61 Nonetheless, even after extensive evaluation, 10% to 20% of patients will have no identifiable cause initially but will eventually get diagnosed as OH or nOH as symptoms appear over the course of their disease.
Table 5.
Testing for nOH.
| Initial testing | Utility in diagnosis |
|---|---|
| Complete blood count | To rule out anemia, infection |
| Comprehensive metabolic panel | To assess volume status, electrolyte abnormalities, kidney dysfunction, intravascular volume (albumin), hypoglycemia |
| Electrocardiogram | To identify cardiac etiology |
| CT or MRI head/spine | To rule out structural central neurologic problems |
| Autonomic testing | To identify a specific etiology |
| Secondary testing | |
| Vitamin B12, methyl malonic acid, fasting plasma glucose, glycosylated hemoglobin | To screen for peripheral neuropathies |
| Morning cortisol, thyroid stimulating hormone | To rule out endocrine abnormalities |
| Paraneoplastic panel | To assess autoimmune etiologies |
| Serum/urine electrophoresis and further cardiac imaging (eg, pyrophosphate scan) | To identify monoclonal gammopathy and amyloidosis |
Various techniques can be used to study adrenergic failure in patients with nOH including serum levels of catecholamines and their metabolites, neuropharmacological tests, and cardiac neural imaging.34,61 Baroreflex-cardiovagal integrity can be measured from the slope of the cardiac interbeat interval (with 1 beat delay) to SBP during Phase II of the Valsalva maneuver or by analyzing the ratio between HR increase and BP drop from lying to standing position.59 Cholinergic system testing includes thermoregulatory sweat response and quantitative sudomotor axon reflex test.20 Neuropharmacological tests assess hemodynamic and neurochemical responses to various stimulants such as isoproterenol, tyramine, edrophonium, and glucagon.27 Plasma norepinephrine dihydroxyphenylglycol levels, and neuroimaging tests such as 6-[18F] fluorodopamine positron emission tomographic (PET) scanning and [123I] metaiodobenzylguanidine (MIBG) scintigraphy can be used to localize sympathetic noradrenergic denervation.27 In nOH patients without central neurodegeneration and normal peripheral noradrenergic innervation, the diagnosis of autoimmune autonomic ganglionopathy should be considered and one should test for circulating antibodies to the neuronal nicotinic receptor (nAChR).27
Management
Goals of treatment
The usual outcome measures in nOH studies can be objective (BP goals) or subjective (symptom control) although both these outcome measures are imperfect and pose interpretative challenges. As mentioned previously, the severity of nOH symptoms often varies day-to-day or throughout the day and can be affected by ambient temperature, physical exertion, and food and fluid intake. In a similar way BP values obtained during clinic evaluation may not recapitulate BP values when the patient experiences symptoms.62 The goals of treatment should be to reduce the burden of symptoms, allow the patient to be able to stand for longer periods of time, reduce the risk of falls and improve physical performance to restore independence in activities of daily living. Thus, solely fixing orthostatic BP abnormalities or normalizing standing BP should not be the primary goal as changes in postural BP are not always correlated with symptoms.26,63,64 Furthermore, it is currently not known whether treatment prevents long term mortality associated with OH, although intuitively it makes sense to prevent falls related to OH. Current treatment recommendations are based mostly on studies in small numbers of patients with primary forms of autonomic failure and severe OH, with limited evidence of long-term efficacy from randomized controlled clinical trials.
Various consensus statements from The American Autonomic Society and the National Parkinson Foundation,26 European Society of Cardiology (ESC) 2018 guidelines for diagnosis and management of syncope,65 European Academy of Neurology,56 and American College of Cardiology (ACC) 2017 guidelines for patients with syncope66 provide broad strategies for the management of OH patients.
We employ a 4-step approach to management of nOH: eliminate offending drugs, use conservative/non-pharmacological measures, then use pharmacologic therapy starting with a single agent and finally if needed, use drugs in combination (Figure 5). These steps are not hierarchical, for example, conservative measures should continue to be the backbone of therapy even in patients who are being treated with multiple drugs.26
Figure 5.
Approach to the management of nOH.
Medication review
A list of common offending agents is listed in Table 6. In patients with mild symptoms, minimizing or eliminating these medications is often enough. This may require collaboration with other prescribers to prevent exacerbation of other conditions like depression, hypertension, urinary retention, etc.
Non-pharmacological measures
This includes lifestyle modifications, physical counter-pressure maneuvers, and external wearables.
- Lifestyle modifications: older patients are more symptomatic after prolonged bed rest and should use a gradual, staged transition from a supine position to reduce symptoms.43,65
- a. Adequate hydration: many patients with nOH are often blood volume depleted due to inadequate oral fluid intake.67 Maintaining adequate hydration is a first step in both the ESC and ACC guidelines.65,66 A daily fluid intake of 2 L is recommended, but if cardiac status allows this can be as high as 3 L, especially in warmer weather.26
- e. Physical conditioning: patients who are deconditioned experience larger BP falls.26 Thus, lower body strength training and non-gravitation exercises such as stationary bicycle, rowing machines and water exercises are recommended. Contrarily, upright posture exercises such as running on a treadmill should be avoided.7,26
Physical counter-pressure maneuvers: patients who have warning signs upon standing can use certain physical counter-pressure maneuvers.65,66 Examples include leg crossing, squatting, tiptoeing, lower body muscle tensing (thigh and leg muscle or buttock clenching), bending forward, and hand grip. These maneuvers work by increasing the venous return and increasing cardiac output.7,69,70 Breathing-related counter-maneuvers such as slow, deep breathing and the creation of inspiratory resistance through the use of an impedance threshold device, inspiratory sniffing work by their effect on the respiratory pump to facilitate venous return to the heart from the abdomen and upper extremities.68 Patients should avoid Valsalva like maneuvers such as straining during bowel movements which will lower the BP.7
External wearables: compression garments such as waist high compression stockings and abdominal binders can improve orthostatic symptoms. They work by preventing the gravitational pooling of venous blood but are effective only when tight fitting.26,65,66 A randomized trial in nOH patients who wore an automated inflatable abdominal binder (40 mm Hg compression) was shown to be as effective as midodrine in reducing orthostatic symptoms.71,72
Pharmacotherapy
For patients with severe symptoms (falls, syncope) or whose symptoms are not controlled by non-pharmacologic measures, we use pharmacotherapy (Table 7). Until 2014, the 2 drugs primarily used were fludrocortisone and midodrine, with only midodrine having FDA approval for OH. However, in 2014 droxidopa was the first drug to receive FDA approval specifically for the treatment of nOH in the US.66
Table 7.
Drugs used to treat OH.
| Medication | Mechanism of action | Dosages | Adverse effects | Comments |
|---|---|---|---|---|
| Midodrine | α-1 adrenoreceptor agonist leading to vasoconstriction | 2.5-15 mg TID | Supine hypertension, piloerection, scalp itching, and urinary retention | FDA approved. ACC/AHA IIa. Single agent or combination therapy |
| Droxidopa | Pro-drug converted to norepinephrine; stimulates adrenergic receptors | 100-600 mg TID | Supine hypertension, headache, dizziness, nausea, fatigue | FDA approved, ACC/AHA IIa. Single agent or combination therapy |
| Fludrocortisone | Synthetic mineralocorticoid, acts as an aldosterone agonist leading to volume expansion | 0.1-0.3 mg QAM | Supine hypertension, hypokalemia, hypomagnesemia, peripheral edema | ACC/AHA IIa. Single agent or combination therapy |
| Pyridostigmine | Acetylcholinesterase inhibitor, enhances sympathetic activity | 30-60 mg, QD to TID | GI upset, sialorrhea, excessive sweating, and urinary incontinence | ACC/AHA IIb. Combination therapy or refractory cases. Likely Inferior to fludrocortisone. Minimal supine hypertension |
Midodrine
Midodrine has been shown to improve symptoms in patients with nOH.65,66 It is an oral prodrug whose active metabolite, desglymidodrine, acts as an alpha 1-adrenoreceptor agonist that increases arteriolar and venous vascular resistance by causing vasoconstriction thus elevating SBP and DBP.73 Jankovic et al in 1993 evaluated midodrine in a 4-week, double-blind, placebo-controlled study of 97 patients with OH—it increased standing SBP by 22 mm Hg and reduced orthostatic symptoms compared to placebo.74,75 Peak effect occurs in 1 hour.76 It received FDA approval in 1996 for the treatment of symptomatic OH. Typical doses of midodrine range from 2.5 to 15 mg 1 to 3 times a day, with dosing being titrated up for symptom relief. It has a short half-life and the effect lasts for 2 to 4 hours, hence it can be taken every 4 to 6 hours.20,75 The most important side effect is the SH that occurs in up to 25% of patients77 and can limit its use. Thus patients should not take it within 5 hours of bedtime and omit a dose if BP is ⩾180/110 mm Hg.74-77 Other significant side-effects include piloerection, pruritus, paresthesia, chills, and urinary retention (especially in elderly men).77
Droxidopa
Droxidopa, also known as l-DOPS (l-threo-dihydroxyphenylserine, NORTHERA; Lundbeck), is a synthetic precursor of norepinephrine and the newest drug available for nOH treatment.66 It is an oral pro-drug that is converted both peripherally and centrally to norepinephrine by decarboxylation.78 Droxidopa can exert a pressor effect in 3 ways: as a central stimulator of sympathetic activity, as a peripheral sympathetic neurotransmitter and as a circulating hormone.79 Kaufman et al evaluated the use of droxidopa in 162 patients with symptomatic nOH due to PD, MSA, PAF, or nondiabetic autonomic neuropathy. The primary efficacy endpoint was patient self-ratings on the OH questionnaire (OHQ). At the end of 7 days, patients in droxidopa group reported significantly better composite OHQ scores compared to placebo.62,80-82 An integrated analysis of 3 studies evaluated OHQ scores from baseline to 1 week of droxidopa treatment. And showed a significant unit change of in the OHQ composite score compared with placebo (−2.68 ± 2.20 vs −1.82 ± 2.34; P < .001).83 Based on these studies, it received FDA approval in February 2014 for nOH stemming from primary autonomic neuropathies (PD, MSA, PAF, dopamine beta hydroxylase deficiency, and non-diabetic neuropathy). A more recent study also provided longer follow up data (12 months) in 102 patients. nOH symptom severity and impact on daily activities improvements exceeded 50% and were maintained throughout the 12-months.84
Typical dosages range from 100 to 600 mg TID, starting at 100 mg TID and upward titration every 24 to 48 hours until symptom relief or intolerable hypertension occurs. Droxidopa levels peak at 3 hours; half-life is 2 to 3 hours. However due to decarboxylation of droxidopa within cellular sites, plasma norepinephrine levels peak at 6 hours and remain elevated for 46 hours.85 The major route of elimination is renal. It should also be avoided within 5 hours of bedtime to avoid SH.
In patients with PD being treated with levodopa/carbidopa, droxidopa may not be effective as carbidopa inhibits its conversion of norepinephrine.85 However, co-administration of the peripheral catechol-o-methyl transferase inhibitor, entacapone, with droxidopa did not affect plasma droxidopa concentrations or the droxidopa-induced increase in plasma norepinephrine levels or SBP.86 Potential adverse events (AEs) include headache, dizziness, decreased appetite, fatigue, urinary tract infections, and SH.83,87
Droxidopa can exacerbate conditions such as ischemic heart disease, arrhythmias, and congestive heart failure.78 In the short term studies, were no CV events; in the intermediate term studies, CV event rates were 4.4% and 1.8% (droxidopa vs placebo).88 In long-term studies, CV events occurred in 10.8% of droxidopa patients (open-label studies, no placebo arm was available for comparison). Most events were minor atrial arrythmias and none were major adverse CV events.88
Some studies suggest that droxidopa may also have a role in reducing intradialytic hypotension89 in hypotensive individuals with spinal cord injury.90,91 Although there have been no head to head trials comparing midodrine with droxidopa, a Bayesian meta-analysis to compare outcomes of standing and supine BP outcomes reported a significant difference between the 2, with midodrine showing a greater mean change in standing SBP. There was also a higher risk for SH with midodrine (midodrine RR = 5.1 [95% CI = 1.6-24] vs droxidopa RR = 1.4 [95% CI = 0.7-2.7]).92
Off-label medications
Fludrocortisone
Fludrocortisone is a synthetic mineralocorticoid used off-label for the treatment of nOH that acts as an aldosterone receptor agonist to increase renal sodium reabsorption and thus increase plasma volume. Other possible mechanisms include sensitization of the vasculature to circulating catecholamines such as norepinephrine and angiotensin II.93 Even though the strength of data supporting use of fludrocortisone is limited, ACC guidelines give it a IIa recommendation as its benefits outweigh the risks.66 Treatment dosages range from 0.1 to 0.3 mg/day with the onset of action between 3 to 7 days.26 The main side effects of fludrocortisone include SH, hypokalemia, edema. More serious side effects such as hypothalamic-pituitary-adrenal axis suppression and immunosuppression occur mainly with doses >0.3 mg daily.26 Hypokalemia is dose dependent and is seen in 25% of patients.78 Chronic use can cause cardiac hypertrophy and worsening heart failure. It increases the warfarin effect and may require a dose reduction of concomitant warfarin therapy.78
Pyridostigmine
Pyridostigmine is an acetylcholinesterase inhibitor used to treat myasthenia gravis. It increases sympathetic activity via nicotinic acetylcholine receptors and thus raises peripheral vascular resistance.26 It has the advantage of not producing SH.94-96 It is used off label for nOH/OH, usually as adjunctive therapy in refractory cases. Small studies have reported a modest improvement in orthostatic symptoms.94-96 A recent phase II non-inferiority study compared pyridostigmine to fludrocortisone in PD and found pyridostigmine to be inferior.66,97 Typical dosing is 30 to 60 mg once to 3 times per day. Adverse effects are related to cholinergic stimulation, including nausea, vomiting, abdominal cramps, diarrhea, sialorrhea, excessive sweating, and urinary incontinence.26
The aforementioned 4 drugs mentioned for nOH are labeled pregnancy category C by the FDA. Other medications infrequently used to treat nOH include dihydroergotamine, indomethacin,70 intranasal desmopressin, and erythropoietin.20,70 Little data exists to determine efficacy and safety of different combinations compared to monotherapy for nOH. Studies evaluating the outcomes of droxidopa have reported a larger proportion of subjects taking fludrocortisone in the droxidopa group.82 Occasional case reports and anecdotal use of triple therapy are available. Limited date shows that addition of as-needed midodrine to combination therapy of fludrocortisone and droxidopa may help refractory patients.98 Similarly, safety data of combination therapy is scarce.26 The selection of 1 drug over the other is driven by clinician preference and side effect profile. There have been no head-to-head comparison studies to guide the initial medication choice in nOH. We recommend home blood pressure diaries or AMBP readings and the OHQ to assess treatment response. If symptoms do not improve after reaching the maximum labeled dose, we switch to another single agent or add a second drug. Caution should be used with in patients with congestive heart failure, aortic disease, cerebrovascular disease, chronic renal failure, and SH.26
Worsening SH is a concern during treatment. SH may lead to hypertensive emergencies and acute ischemic and aortic syndromes, however data is currently not robust enough to define cut-off values as to when to treat it.55 Prevention of short term immediate complications (eg, falls) from OH may take precedence over the longer term complications of SH, especially in elderly patients with limited life expectancy.99 To counteract SH, patients should not take their last dose of pressor medication within 5 hours of bedtime, should sleep in a head-up position (30° or more), and omit a dose if supine or sitting BP is ⩾180/110 mm Hg (ie, severe SH). When these conservative measures are unable to control SH, and if it is in the severe range persistently or frequently, small doses of short acting anti-hypertensives can be used at bedtime26 (eg, hydralazine [25-50 mg], captopril [6.25-12.5 mg]).
Conclusion
nOH is a debilitating form of OH caused by autonomic dysfunction. Screening appropriate patients in the clinic with orthostatic BP testing and utilizing an orthostatic symptom questionnaire is helpful in not missing patients, and in identifying the severity of disease, and evaluating treatment success. The first step in managing a nOH patient involves review of their current medications and eliminating drugs that can cause OH. Second, conservative measures such as patient education, physical counter-maneuvers, and lifestyle changes are often enough to treat mild symptoms. Next, pharmacotherapy is used in those with residual or severe symptoms. Future research should be aimed at multicenter studies with larger cohorts with longer follow up and head-to-head comparisons of the various therapeutic agents.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors contributed to this article.
ORCID iDs: Dinesh K Kalra  https://orcid.org/0000-0001-5254-4067
https://orcid.org/0000-0001-5254-4067
Sumit Sohal  https://orcid.org/0000-0002-0176-258X
https://orcid.org/0000-0002-0176-258X
References
- 1. Schatz IJ, Bannister R, Freeman RL, et al. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470 [DOI] [PubMed] [Google Scholar]
- 2. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72. doi: 10.1007/s10286-011-0119-5 [DOI] [PubMed] [Google Scholar]
- 3. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670-676. doi: 10.1212/01.wnl.0000324625.00404.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roncevic D, Palma J-A, Martinez J, Goulding N, Norcliffe-Kaufmann L, Kaufmann H. Cerebellar and parkinsonian phenotypes in multiple system atrophy: similarities, differences and survival. J Neural Transm. 2014;121:507-512. doi: 10.1007/s00702-013-1133-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology. 2006;67:28-32. doi: 10.1212/01.wnl.0000223828.28215.0b [DOI] [PubMed] [Google Scholar]
- 6. Keating GM. Droxidopa: a review of its use in symptomatic neurogenic orthostatic hypotension. Drugs. 2015;75:197-206. doi: 10.1007/s40265-014-0342-1 [DOI] [PubMed] [Google Scholar]
- 7. Palma J-A, Kaufmann H. Epidemiology, diagnosis, and management of neurogenic orthostatic hypotension. Mov Disord Clin Pract. 2017;4:298-308. doi: 10.1002/mdc3.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Low PA. Prevalence of orthostatic hypotension. Clin Auton Res. 2008;18(S1):8-13. doi: 10.1007/s10286-007-1001-3 [DOI] [PubMed] [Google Scholar]
- 9. Maule S, Milazzo V, Maule MM, Di Stefano C, Milan A, Veglio F. Mortality and prognosis in patients with neurogenic orthostatic hypotension. Funct Neurol. 2012;27:101. [PMC free article] [PubMed] [Google Scholar]
- 10. Shibao C, Grijalva CG, Raj SR, Biaggioni I, Griffin MR. Orthostatic hypotension-related hospitalizations in the United States. Am J Med. 2007;120:975-980. doi: 10.1016/j.amjmed.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 11. Low PA, Tomalia VA. Orthostatic hypotension: mechanisms, causes, management. J Clin Neurol. 2015;11:220-226. doi: 10.3988/jcn.2015.11.3.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benvenuto LJJ, Krakoff LRR. Morbidity and mortality of orthostatic hypotension: implications for management of cardiovascular disease. Am J Hypertens. 2011;24:135-144. doi: 10.1038/ajh.2010.146 [DOI] [PubMed] [Google Scholar]
- 13. Rutan G, Hermanson B, Bild D, Kittner S, Labaw F, Tell G. Orthostatic hypotension in older adults: the cardiovascular health study. Hypertension. 1992;19:508-519. [DOI] [PubMed] [Google Scholar]
- 14. Verwoert GC, Mattace-Raso FUS, Hofman A, et al. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc. 2008;56:1816-1820. doi: 10.1111/j.1532-5415.2008.01946.x [DOI] [PubMed] [Google Scholar]
- 15. Rose KM, Tyroler HA, Nardo CJ, et al. Orthostatic hypotension and the incidence of coronary heart disease: the atherosclerosis risk in communities study. Am J Hypertens. 2000;13:571-578. doi: 10.1016/s0895-7061(99)00257-5 [DOI] [PubMed] [Google Scholar]
- 16. Masaki KH, Schatz IJ, Burchfiel CM, et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98:2290-2295. [DOI] [PubMed] [Google Scholar]
- 17. Luukinen H, Koski K, Laippala P, Airaksinen KEJ. Orthostatic hypotension and the risk of myocardial infarction in the home-dwelling elderly. J Intern Med. 2004;255:486-493. doi: 10.1111/j.1365-2796.2004.01313.x [DOI] [PubMed] [Google Scholar]
- 18. Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J. 2010;31:85-91. doi: 10.1093/eurheartj/ehp329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lipsitz LA. Orthostatic hypotension in the elderly. N Engl J Med. 1989;321:952-957. doi: 10.1056/NEJM198910053211407 [DOI] [PubMed] [Google Scholar]
- 20. Freeman R. Neurogenic orthostatic hypotension. N Engl J Med. 2008;358:615-624. doi: 10.1056/nejmcp074189 [DOI] [PubMed] [Google Scholar]
- 21. National Organization for Rare Diseases. Orthostatic hypotension. https://rarediseases.org/rare-diseases/orthostatic-hypotension/. Accessed July 20, 2020.
- 22. Merola A, Espay AJ, Zibetti M, et al. Pure autonomic failure versus prodromal dysautonomia in Parkinson’s disease: insights from the bedside. Mov Disord Clin Pract. 2017;4:141-144. doi: 10.1002/mdc3.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCann H, Stevens CH, Cartwright H, Halliday GM. α-Synucleinopathy phenotypes. Parkinsonism Relat Disord. 2014;20:S62-S67. doi: 10.1016/S1353-8020(13)70017-8 [DOI] [PubMed] [Google Scholar]
- 24. Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RMA. Prevalence of orthostatic hypotension in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2011;17:724-729. doi: 10.1016/j.parkreldis.2011.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thaisetthawatkul P, Boeve BF, Benarroch EE, et al. Autonomic dysfunction in dementia with Lewy bodies. Neurology. 2004;62:1804-1809. doi: 10.1212/01.wnl.0000125192.69777.6d [DOI] [PubMed] [Google Scholar]
- 26. Gibbons CH, Schmidt P, Biaggioni I, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264:1567-1582. doi: 10.1007/s00415-016-8375-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldstein DS, Sharabi Y. Neurogenic orthostatic hypotension: a pathophysiological approach. Circulation. 2009;119:139-146. doi: 10.1161/CIRCULATIONAHA.108.805887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berger MJ, Kimpinski K. A practical guide to the treatment of neurogenic orthostatic hypotension. Can J Neurol Sci/J Can des Sci Neurol. 2014;41:156-163. doi: 10.1017/s0317167100016528 [DOI] [PubMed] [Google Scholar]
- 29. Low PAA, Benrud-Larson LMM, Sletten DMM, et al. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care. 2004;27:2942-2947. doi: 10.2337/diacare.27.12.2942 [DOI] [PubMed] [Google Scholar]
- 30. Kaufmann H, Palma J-A. Neurogenic orthostatic hypotension: the very basics. Clin Auton Res. 2017;27:39-43. doi: 10.1007/s10286-017-0437-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ha AD, Brown CH, York MK, Jankovic J. The prevalence of symptomatic orthostatic hypotension in patients with Parkinson’s disease and atypical parkinsonism. Parkinsonism Relat Disord. 2011;17:625-628. doi: 10.1016/j.parkreldis.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 32. Merola A, Romagnolo A, Rosso M, et al. Orthostatic hypotension in Parkinson’s disease: does it matter if asymptomatic? Parkinsonism Relat Disord. 2016;33:65-71. doi: 10.1016/j.parkreldis.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 33. Gupta D, Nair MD. Neurogenic orthostatic hypotension: chasing “the fall.” Postgrad Med J. 2008;84:6-14. doi: 10.1136/pgmj.2007.062075 [DOI] [PubMed] [Google Scholar]
- 34. Low PA, Opfer-Gehrking TL, McPhee BR, et al. Prospective evaluation of clinical characteristics of orthostatic hypotension. Mayo Clin Proc. 1995;70:617-622. doi: 10.4065/70.7.617 [DOI] [PubMed] [Google Scholar]
- 35. Guaraldi P, Poda R, Calandra-Buonaura G, et al. Cognitive function in peripheral autonomic disorders. PLoS ONE. 2014;9:e85020. doi: 10.1371/journal.pone.0085020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Centi J, Freeman R, Gibbons CH, Neargarder S, Canova AO, Cronin-Golomb A. Effects of orthostatic hypotension on cognition in Parkinson disease. Neurology. 2017;88:17-24. doi: 10.1212/WNL.0000000000003452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robertson D, Kincaid DW, Haile V, Robertson RM. The head and neck discomfort of autonomic failure: an unrecognized aetiology of headache. Clin Auton Res. 1994;4:99-103. doi: 10.1007/BF01845772 [DOI] [PubMed] [Google Scholar]
- 38. Claassen DO, Adler CH, Hewitt LA, Gibbons C. Characterization of the symptoms of neurogenic orthostatic hypotension and their impact from a survey of patients and caregivers. BMC Neurol. 2018;18:125. doi: 10.1186/s12883-018-1129-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008. doi: 10.1136/jnnp.2007.131045 [DOI] [PubMed] [Google Scholar]
- 40. Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med. 2015. doi: 10.1056/NEJMra1311488 [DOI] [PubMed] [Google Scholar]
- 41. Schatz IJ, Bannister R, Freeman RL, et al. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125-126. doi: 10.1007/BF02291236 [DOI] [PubMed] [Google Scholar]
- 42. Freeman R, Illigens BMW, Lapusca, et al. Symptom recognition is impaired in patients with orthostatic hypotension. Hypertension. 2020;75:1325-1332. doi: 10.1161/HYPERTENSIONAHA.119.13619 [DOI] [PubMed] [Google Scholar]
- 43. Low PA, Singer W. Management of neurogenic orthostatic hypotension: an update. Lancet Neurol. 2008;7:451-458. doi: 10.1016/S1474-4422(08)70088-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pathak A, Lapeyre-Mestre M, Montastruc J, Senard J. Heat-related morbidity in patients with orthostatic hypotension and primary autonomic failure. Mov Disord. 2005;20:1213-1219. doi: 10.1002/mds.20571 [DOI] [PubMed] [Google Scholar]
- 45. Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. 2000;108:106-111. doi: 10.1016/s0002-9343(99)00425-8 [DOI] [PubMed] [Google Scholar]
- 46. Low PA. Neurogenic orthostatic hypotension: pathophysiology and diagnosis. Am J Manag Care. 2015;21:248. [PubMed] [Google Scholar]
- 47. Juraschek SP, Daya N, Appel LJ, et al. Orthostatic hypotension in middle-age and risk of falls. Am J Hypertens. 2017;30:188-195. doi: 10.1093/ajh/hpw108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sharabi Y, Imrich R, Holmes C, Pechnik S, Goldstein DS. Generalized and neurotransmitter-selective noradrenergic denervation in Parkinson’s disease with orthostatic hypotension. Mov Disord. 2008;23:1725-1732. doi: 10.1002/mds.22226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goldstein DS, Holmes C, Cannon RO, Eisenhofer G, Kopin IJ. Sympathetic cardioneuropathy in dysautonomias. N Engl J Med. 1997;336:696-702. [DOI] [PubMed] [Google Scholar]
- 50. Braune S, Reinhardt M, Schnitzer R, Riedel A, Lucking CH. Cardiac uptake of [123I]MIBG separates Parkinson’s disease from multiple system atrophy. Neurology. 1999;53:1020. doi: 10.1212/wnl.53.5.1020 [DOI] [PubMed] [Google Scholar]
- 51. Orimo S, Oka T, Miura H, et al. Sympathetic cardiac denervation in Parkinson’s disease and pure autonomic failure but not in multiple system atrophy. J Neurol Neurosurg Psychiatry. 2002;73:776-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. In Sinn D, Gibbons CH. Pathophysiology and treatment of orthostatic hypotension in Parkinsonian disorders. Curr Treat Options Neurol. 2016;18:28. doi: 10.1007/s11940-016-0410-9 [DOI] [PubMed] [Google Scholar]
- 53. Naschitz JE, Slobodin G, Elias N, Rosner I. The patient with supine hypertension and orthostatic hypotension: a clinical dilemma. Postgrad Med J. 2006;82:246-253. doi: 10.1136/pgmj.2005.037457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jordan J, Biaggioni I. Diagnosis and treatment of supine hypertension in autonomic failure patients with orthostatic hypotension. J Clin Hypertens. 2002;4:139-145. doi: 10.1111/j.1524-6175.2001.00516.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fanciulli A, Jordan J, Biaggioni I, et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS): endorsed by the European Academy of Neurology. Clin Auton Res. 2018;28:355-362. doi: 10.1007/s10286-018-0529-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pavy-Le Traon A. How to manage a patient with orthostatic hypotension. In: Proceedings of the 3rd Congress of the European Academy of Neurology, Amsterdam, The Netherlands, 2017, pp. 24–27. https://www.ean.org. Accessed July 10, 2020. [Google Scholar]
- 57. Norcliffe-Kaufmann L, Kaufmann H. Is ambulatory blood pressure monitoring useful in patients with chronic autonomic failure? Clin Auton Res. 2014;24:189-192. doi: 10.1007/s10286-014-0229-y [DOI] [PubMed] [Google Scholar]
- 58. Palma J-A, Gomez-Esteban JC, Norcliffe-Kaufmann L, et al. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Mov Disord. 2015;30:639-645. doi: 10.1002/mds.26079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Norcliffe-Kaufmann L, Kaufmann H, Palma J-A, et al. Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann Neurol. 2018;83:522-531. doi: 10.1002/ana.25170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Isaacson SH. Managed care approach to the treatment of neurogenic orthostatic hypotension. Am J Manag Care. 2015;21:258. [PubMed] [Google Scholar]
- 61. Jones PK, Shaw BH, Raj SR. Orthostatic hypotension: managing a difficult problem. Expert Rev Cardiovasc Ther. 2015;13:1263-1276. doi: 10.1586/14779072.2015.1095090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kaufmann H, Freeman R, Biaggioni I, et al. Droxidopa for neurogenic orthostatic hypotension. Neurology. 2014;83:328-335. doi: 10.1212/WNL.0000000000000615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Logan IC, Witham MD. Efficacy of treatments for orthostatic hypotension: a systematic review. Age Ageing. 2012;41:587-594. doi: 10.1093/ageing/afs061 [DOI] [PubMed] [Google Scholar]
- 64. Shibao C, Lipsitz LA, Biaggioni I. Evaluation and treatment of orthostatic hypotension. J Am Soc Hypertens. 2013;7:317-324. doi: 10.1016/j.jash.2013.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brignole M, Moya A, de Lange FJ, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:1883-1948. doi: 10.1093/eurheartj/ehy037 [DOI] [PubMed] [Google Scholar]
- 66. Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol. 2017;70:e39-e110. doi: 10.1016/j.jacc.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 67. Weinberg ADD, Minaker KL. Dehydration. Evaluation and management in older adults. Council on Scientific Affairs, American Medical Association. JAMA. 1995;274:1552-1556. doi: 10.1001/jama.274.19.1552 [DOI] [PubMed] [Google Scholar]
- 68. Arnold AC, Satish RR. Orthostatic hypotension—a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tutaj M, Marthol H, Berlin D, Brown CM, Axelrod FB, Hilz MJ. Effect of physical countermaneuvers on orthostatic hypotension in familial dysautonomia. J Neurol. 2005;253:65-72. doi: 10.1007/s00415-005-0928-3 [DOI] [PubMed] [Google Scholar]
- 70. Eschlböck S, Wenning G, Fanciulli A. Evidence-based treatment of neurogenic orthostatic hypotension and related symptoms. J Neural Transm. 2017;124:1567-1605. doi: 10.1007/s00702-017-1791-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Okamoto LE, Diedrich A, Baudenbacher FJ, et al. Efficacy of servo-controlled splanchnic venous compression in the treatment of orthostatic hypotension: a randomized comparison with midodrine. Hypertension. 2016;68:418-426. doi: 10.1161/HYPERTENSIONAHA.116.07199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fanciulli A, Goebel G, Metzler B, et al. Elastic abdominal binders attenuate orthostatic hypotension in Parkinson’s disease. Mov Disord Clin Pract. 2015;3:156-160. doi: 10.1002/mdc3.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Doyle JF. Midodrine: use and current status in the treatment of hypotension. Br J Cardiol. 2012;19:34-37. doi: 10.5837/bjc.2012.007 [DOI] [Google Scholar]
- 74. Jankovic J, Gilden JL, Hiner BC, et al. Neurogenic orthostatic hypotension: a double-blind, placebo-controlled study with midodrine. Am J Med. 1993;95:38-48. doi: 10.1016/0002-9343(93)90230-m [DOI] [PubMed] [Google Scholar]
- 75. Low PA, Gilden JL, Freeman R, Sheng K-N, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension: a randomized, double-blind multicenter study. JAMA. 1997;277:1046-1051. doi: 10.1001/jama.1997.03540370036033 [DOI] [PubMed] [Google Scholar]
- 76. Wright RA, Kaufmann HC, Perera R, et al. A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology. 1998;51:120-124. doi: 10.1212/wnl.51.1.120 [DOI] [PubMed] [Google Scholar]
- 77. McClellan KJ, Wiseman LR, Wilde MI. Midodrine: a review of its therapeutic use in the management of orthostatic hypotension. Drugs Aging. 1998;12:75-86. doi: 10.2165/00002512-199812010-00007 [DOI] [PubMed] [Google Scholar]
- 78. Vijayan J, Sharma VK. Neurogenic orthostatic hypotension—management update and role of droxidopa. Ther Clin Risk Manag. 2015;11:915-923. doi: 10.2147/TCRM.S68439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kaufmann H. L-dihydroxyphenylserine (Droxidopa): a new therapy for neurogenic orthostatic hypotension. Clin Auton Res. 2008;18:19-24. doi: 10.1007/s10286-007-1002-2 [DOI] [PubMed] [Google Scholar]
- 80. Biaggioni I, Freeman R, Mathias CJ, et al. Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa. Hypertension. 2015;65:101-107. doi: 10.1161/HYPERTENSIONAHA.114.04035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hauser RA, Hewitt LA, Isaacson S. Droxidopa in patients with neurogenic orthostatic hypotension associated with Parkinson’s disease (NOH306A). J Parkinsons Dis. 2014;4:57-65. doi: 10.3233/jpd-130259 [DOI] [PubMed] [Google Scholar]
- 82. Hauser RAA, Isaacson S, Lisk JPP, Hewitt LAA, Rowse G. Droxidopa for the short-term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson’s disease (nOH306B). Mov Disord. 2014;30:646-654. doi: 10.1002/mds.26086 [DOI] [PubMed] [Google Scholar]
- 83. Biaggioni I, Arthur Hewitt L, Rowse GJ, Kaufmann H. Integrated analysis of droxidopa trials for neurogenic orthostatic hypotension. BMC Neurol. 2017;17:90. doi: 10.1186/s12883-017-0867-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Isaacson S, Shill HA, Vernino S, Ziemann A, Rowse GJ. Safety and durability of effect with long-term, open-label droxidopa treatment in patients with symptomatic neurogenic orthostatic hypotension (NOH303). J Parkinsons Dis. 2016;6:751-759. doi: 10.3233/jpd-160860 [DOI] [PubMed] [Google Scholar]
- 85. Kaufmann H, Saadia D, Voustianiouk A, et al. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation. 2003;108:724-728. doi: 10.1161/01.cir.0000083721.49847.d7 [DOI] [PubMed] [Google Scholar]
- 86. Goldstein D, Holmes C, Sewell L, Pechnik S, Kopin I. Effects of carbidopa and entacapone on the metabolic fate of the norepinephrine prodrug L-DOPS. J Clin Pharmacol. 2011;51:66-74. doi: 10.1177/0091270010363476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Isaacson S, Vernino S, Ziemann A, Rowse GJ, Kalu U, White WB. Long-term safety of droxidopa in patients with symptomatic neurogenic orthostatic hypotension. J Am Soc Hypertens. 2016;10:755-762. doi: 10.1016/j.jash.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 88. White WB, Hauser RA, Rowse GJ, Ziemann A, Hewitt LA. Cardiovascular safety of droxidopa in patients with symptomatic neurogenic orthostatic hypotension. Am J Cardiol. 2017;119:1111-1115. doi: 10.1016/j.amjcard.2016.11.066 [DOI] [PubMed] [Google Scholar]
- 89. Vannorsdall MD, Hariachar S, Hewitt LA. A randomized, placebo-controlled, phase 2 study of the efficacy and safety of droxidopa in patients with intradialytic hypotension. Postgrad Med. 2015;127:133-143. doi: 10.1080/00325481.2015.1015393 [DOI] [PubMed] [Google Scholar]
- 90. Wecht JM, Rosado-Rivera D, Weir JP, Ivan A, Yen C, Bauman WA. Hemodynamic effects of l-threo-3,4-dihydroxyphenylserine (droxidopa) in hypotensive individuals with spinal cord injury. Arch Phys Med Rehabil. 2013;94:2006-2012. doi: 10.1016/j.apmr.2013.03.028 [DOI] [PubMed] [Google Scholar]
- 91. Coll M, Rodriguez S, Raurell I, et al. Droxidopa, an oral norepinephrine precursor, improves hemodynamic and renal alterations of portal hypertensive rats. Hepatology. 2012;56:1849-1860. doi: 10.1002/hep.25845 [DOI] [PubMed] [Google Scholar]
- 92. Chen JJ, Han Y, Tang J, Portillo I, Hauser RA, Dashtipour K. Standing and supine blood pressure outcomes associated with droxidopa and midodrine in patients with neurogenic orthostatic hypotension: a Bayesian meta-analysis and mixed treatment comparison of randomized trials. Ann Pharmacother. 2018;52:1182-1194. doi: 10.1177/1060028018786954 [DOI] [PubMed] [Google Scholar]
- 93. van Lieshout JJ, ten Harkel AD, Wieling W. Fludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failure. Clin Auton Res. 2000;10:35-42. doi: 10.1007/BF02291388 [DOI] [PubMed] [Google Scholar]
- 94. Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatry. 2003;74:1294-1298. doi: 10.1136/jnnp.74.9.1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Singer W, Opfer-Gehrking TL, Nickander KK, Hines SM, Low PA. Acetylcholinesterase inhibition in patients with orthostatic intolerance. J Clin Neurophysiol. 2006;23:477-482. doi: 10.1097/01.wnp.0000229946.01494.4c [DOI] [PubMed] [Google Scholar]
- 96. Singer W, Sandroni P, Opfer-Gehrking TL, et al. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol. 2006;63:513. doi: 10.1001/archneur.63.4.noc50340 [DOI] [PubMed] [Google Scholar]
- 97. Schreglmann SR, Büchele F, Sommerauer M, et al. Pyridostigmine bromide versus fludrocortisone in the treatment of orthostatic hypotension in Parkinson’s disease—a randomized controlled trial. Eur J Neurol. 2017;24:545-551. doi: 10.1111/ene.13260 [DOI] [PubMed] [Google Scholar]
- 98. Kremens D, Lew M, Claassen D, Goodman BP. Adding droxidopa to fludrocortisone or midodrine in a patient with neurogenic orthostatic hypotension and Parkinson disease. Clin Auton Res. 2017;27:29-31. doi: 10.1007/s10286-017-0434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE. Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol. 2016;15:954-966. doi: 10.1016/S1474-4422(16)30079-5 [DOI] [PubMed] [Google Scholar]